
Inspire Medical Systems, Inc. February 2026 NYSE: INSP

© Inspire Medical Systems, Inc. All Rights Reserved. Disclaimer This presentation contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. All statements other than statements of historical facts are forward-looking statements, including, without limitation, statements regarding potential impacts to our business associated with Inspire V reimbursement, our plans to obtain a long-term solution that would support appropriate reimbursement for Inspire V, and our expectations regarding our full year 2026 financial outlook (including without limitation expectations for the impacts of coding uncertainty and the range of outcomes from applying a CPT code 64582 with a modifier and other aspects to reimbursement, revenue, expected growth, adjusted operating margin, net income per diluted share, adjusted net income per diluted share, effective tax rate, adjusted effective tax rate, weighted average diluted shares outstanding and capital expenditures). In some cases, you can identify forward-looking statements by terms such as ‘‘may,’’ ‘‘will,’’ ‘‘should,’’ ‘‘expect,’’ ‘‘plan,’’ ‘‘anticipate,’’ ‘‘could,’’ “future,” “outlook,” “guidance,” ‘‘intend,’’ ‘‘target,’’ ‘‘project,’’ ‘‘contemplate,’’ ‘‘believe,’’ ‘‘estimate,’’ ‘‘predict,’’ ‘‘potential,’’ ‘‘continue,’’ or the negative of these terms or other similar expressions, although not all forward-looking statements contain these words. These forward-looking statements are based on management’s current expectations and involve known and unknown risks and uncertainties that may cause our actual results, performance, or achievements to be materially different from any future results, performance, or achievements expressed or implied by the forward-looking statements. Such risks and uncertainties include, among others: there may be uncertainty in, or changes to, billing codes to be used for our Inspire therapy, which could impact reimbursement rates and physician usage; our financial results may fluctuate significantly and may not fully reflect the underlying performance of our business; our history of operating losses and dependency on our Inspire therapy for revenues; commercial success and market acceptance of our Inspire therapy; our ability to achieve and maintain adequate and clear levels of coverage or reimbursement for our Inspire therapy or any future products we may seek to commercialize; competitive companies, technologies and pharmaceuticals in our industry; our involvement in current or future legal disputes or regulatory proceedings; our ability to expand our indications and develop and commercialize additional products and enhancements to our Inspire therapy; future results of operations, financial position, research and development costs, capital requirements and our needs for additional financing; our ability to accurately forecast customer demand for our Inspire therapy and manage our inventory; our dependence on third-party suppliers, vendors, and contract manufacturers; consolidation in the healthcare industry; our ability to expand, manage and maintain our direct sales and marketing organization, and to market and sell our Inspire therapy in markets outside of the U.S.; our ability to manage our growth; our ability to hire and retain our senior management and other highly qualified personnel; risk related to product liability claims and warranty claims; our ability to address quality issues that may arise with our Inspire therapy; our ability to successfully integrate any acquired business, products, or technologies; changes in global macroeconomic trends; our business model and strategic plans for products, technologies and business, including our implementation thereof; the impact of glucagon-like peptide 1 class of drugs on demand for our Inspire therapy; risks related to information technology and cybersecurity; our ability to commercialize or obtain regulatory approvals for our Inspire therapy, or the effect of delays in commercializing or obtaining regulatory approvals; and FDA or other U.S. or foreign regulatory actions affecting us or the healthcare industry generally. Other important factors that could cause actual results, performance or achievements to differ materially from those contemplated in this presentation can be found under the captions “Risk Factors” and "Management's Discussion and Analysis of Financial Condition and Results of Operations“ in our Annual Report on Form 10-K for the fiscal year ended December 31, 2024, as updated in our Quarterly Report on Form 10-Q for the quarter ended September 30, 2025 and as will be further updated in our Annual Report on Form 10-K for the fiscal year ended December 31, 2025, as such factors may be updated from time to time in our other filings with the SEC, which are accessible on the SEC’s website at www.sec.gov and the Investors page of our website at www.inspiresleep.com. These and other important factors could cause actual results to differ materially from those indicated by the forward-looking statements made in this presentation. Any such forward-looking statements represent management’s estimates as of the date of this presentation. While we may elect to update such forward-looking statements at some point in the future, unless required by applicable law, we disclaim any obligation to do so, even if subsequent events cause our views to change. Thus, one should not assume that our silence over time means that actual events are bearing out as expressed or implied in such forward-looking statements. These forward-looking statements should not be relied upon as representing our views as of any date after the date of this presentation. This presentation contains trademarks, trade names and service marks of other companies, which are the property of their respective owners. We do not intend our use or display of other parties' trademarks, trade names or service marks to imply, and such use or display should not be construed to imply, a relationship with, or endorsement or sponsorship of us by, these other parties. 2
© Inspire Medical Systems, Inc. All Rights Reserved. Use of Non-GAAP Measures This presentation includes non-GAAP financial measures. Reconciliations of such non-GAAP financial measures to the most directly comparable GAAP financial measure have been provided along with the presentation. These non-GAAP financial measures are presented because we believe they are useful indicators of our operating performance and facilitate a more meaningful trend analysis without the distortion of various adjustment items. Management uses these measures principally as measures of our underlying operating performance, trends and for planning purposes, including the preparation of our annual operating plan and financial projections. We believe these measures are useful to investors as supplemental information and because they are frequently used by analysts, investors and other interested parties to evaluate companies in our industry. We also believe these non-GAAP financial measures are useful to our management and investors as a measure of comparative operating performance from period to period. These non-GAAP financial measures should not be considered as an alternative to, or superior to, the most directly comparable GAAP financial measures, as measures of financial performance or cash flows from operations, as a measure of liquidity, or any other performance measure derived in accordance with GAAP, and they should not be construed to imply that our future results will be unaffected by unusual or non-recurring items. In evaluating our non-GAAP financial measures, you should be aware that in the future we may incur expenses that are the same as or similar to some of the adjustments in this presentation. Our presentation of non-GAAP financial measures should not be construed to imply that our future results will be unaffected by any such adjustments. Management compensates for these limitations by primarily relying on our GAAP results in addition to using non-GAAP financial measures on a supplemental basis. These measures and their definitions are discussed in more detail in the presentation, and our definition of these non-GAAP financial measures is not necessarily comparable to other similarly titled captions of other companies due to different methods of calculating. 3

enhancing patient lives through sleep innovation I t a l l s tarts and ends wi th our mission We are a medical technology company committed to
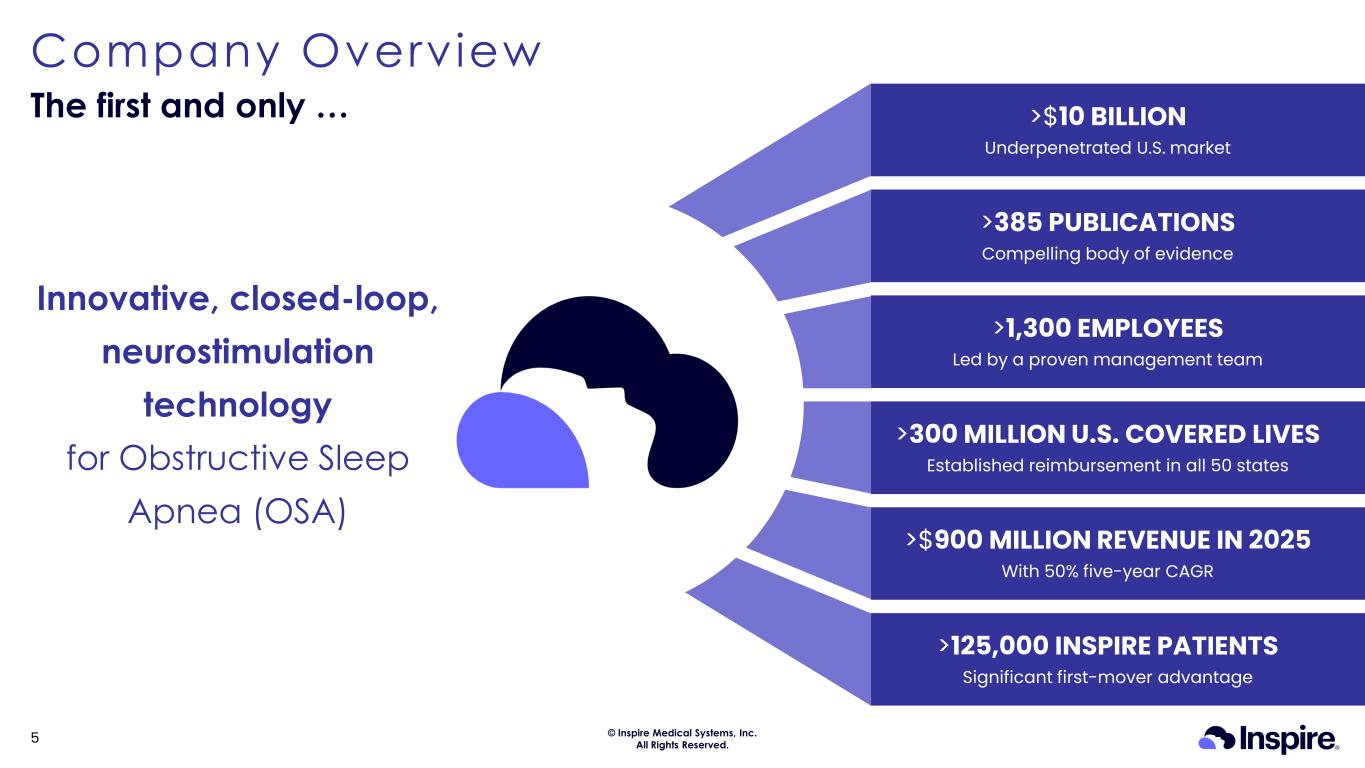
© Inspire Medical Systems, Inc. All Rights Reserved. Company Overview The first and only … 5 >385 PUBLICATIONS Compelling body of evidence >300 MILLION U.S. COVERED LIVES Established reimbursement in all 50 states >1,300 EMPLOYEES Led by a proven management team >$900 MILLION REVENUE IN 2025 With 50% five-year CAGR >125,000 INSPIRE PATIENTS Significant first-mover advantage >$10 BILLION Underpenetrated U.S. market Innovative, closed-loop, neurostimulation technology for Obstructive Sleep Apnea (OSA)

From our entrepreneurial beginnings, and with a focus on delivering life-changing outcomes, we’ve been enhancing the lives of patients for over 18 years… >125,000 Patients receiving Inspire >$900M Revenue >1,500 Implanters Founded 2007 IPO 2018 Today 4,000 Patients receiving Inspire $50M Revenue 200 Implanters With new innovations on the horizon and a big blue ocean of opportunity in front of us! … and we are still just getting started
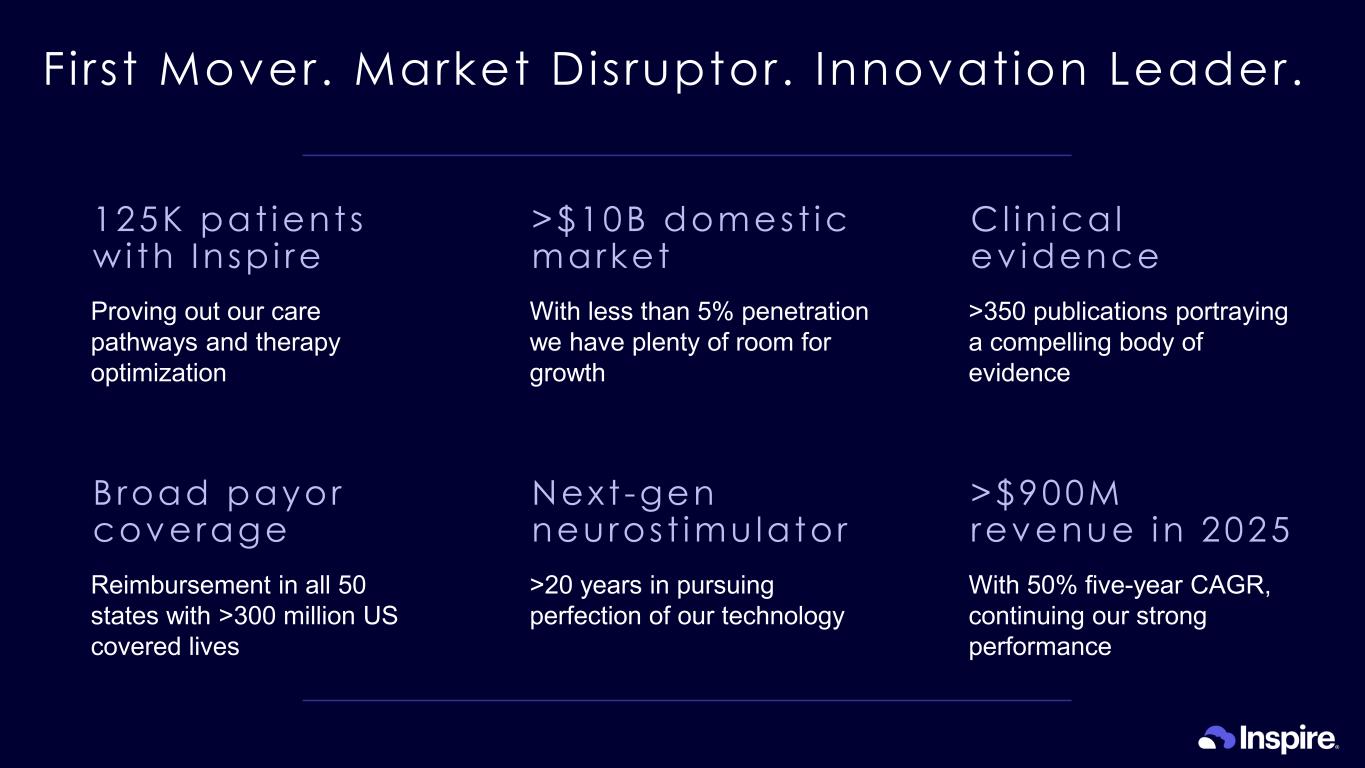
First Mover. Market Disruptor. Innovation Leader. 125K pat ient s w i th Insp i re Proving out our care pathways and therapy optimization Cl in ica l ev idence >350 publications portraying a compelling body of evidence >$900M revenue in 2025 With 50% five-year CAGR, continuing our strong performance Next -gen neuros t imu lator >20 years in pursuing perfection of our technology Broad payor coverage Reimbursement in all 50 states with >300 million US covered lives >$10B domest ic market With less than 5% penetration we have plenty of room for growth
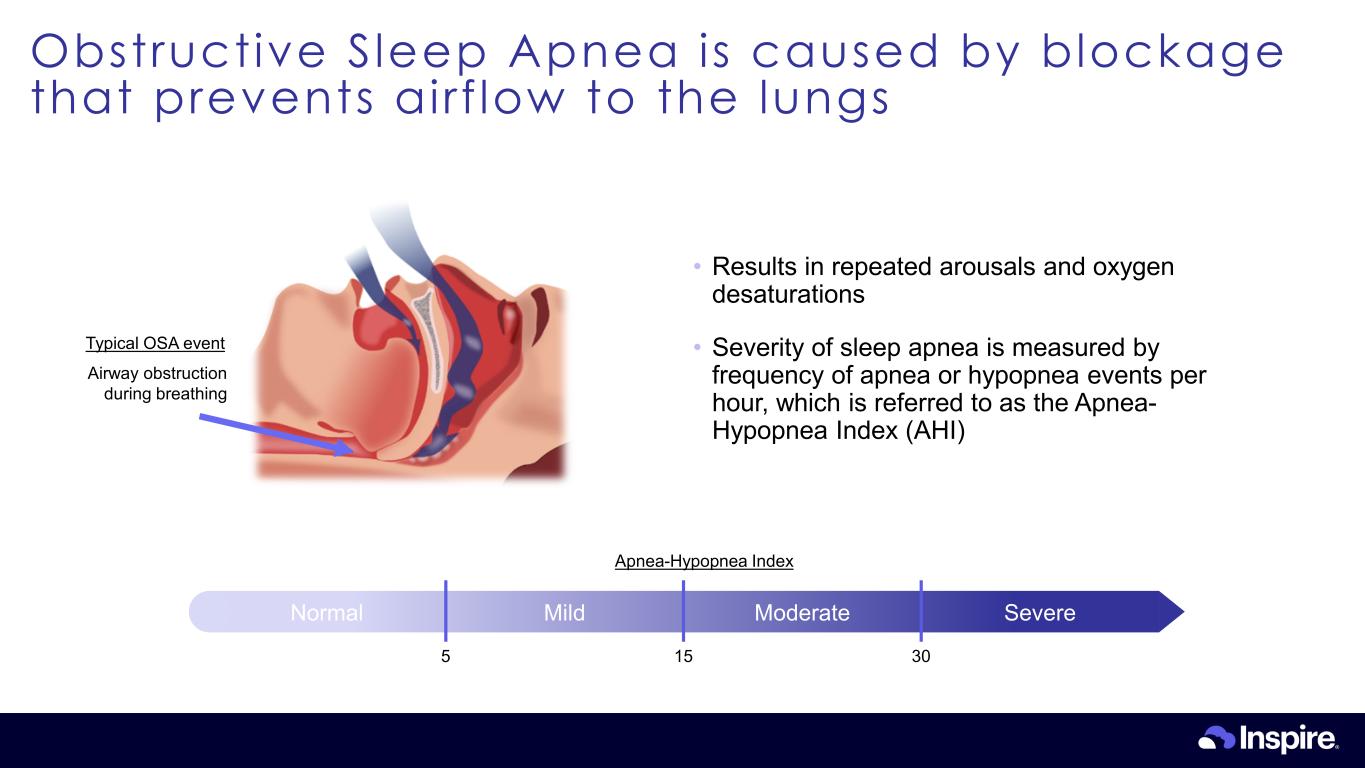
Obstructive Sleep Apnea is caused by blockage that prevents airf low to the lungs 8 Airway obstruction during breathing Typical OSA event • Results in repeated arousals and oxygen desaturations • Severity of sleep apnea is measured by frequency of apnea or hypopnea events per hour, which is referred to as the Apnea- Hypopnea Index (AHI) Normal Mild Moderate Severe 5 15 30 Apnea-Hypopnea Index
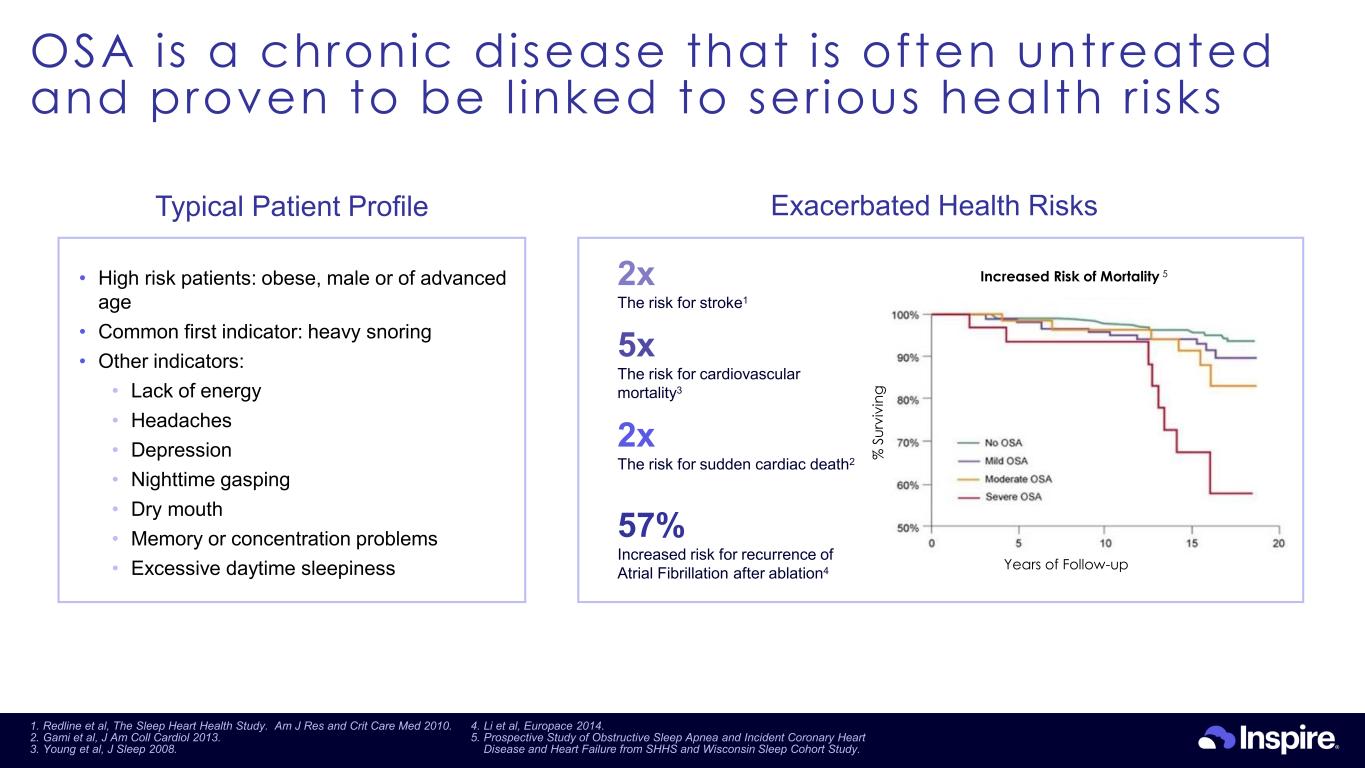
OSA is a chronic disease that is often untreated and proven to be l inked to ser ious health r isks 9 Exacerbated Health Risks • High risk patients: obese, male or of advanced age • Common first indicator: heavy snoring • Other indicators: • Lack of energy • Headaches • Depression • Nighttime gasping • Dry mouth • Memory or concentration problems • Excessive daytime sleepiness 2x The risk for stroke1 2x The risk for sudden cardiac death2 57% Increased risk for recurrence of Atrial Fibrillation after ablation4 5x The risk for cardiovascular mortality3 Years of Follow-up % S ur vi vi ng Increased Risk of Mortality 5 Typical Patient Profile 1. Redline et al, The Sleep Heart Health Study. Am J Res and Crit Care Med 2010. 2. Gami et al, J Am Coll Cardiol 2013. 3. Young et al, J Sleep 2008. 4. Li et al, Europace 2014. 5. Prospective Study of Obstructive Sleep Apnea and Incident Coronary Heart Disease and Heart Failure from SHHS and Wisconsin Sleep Cohort Study.
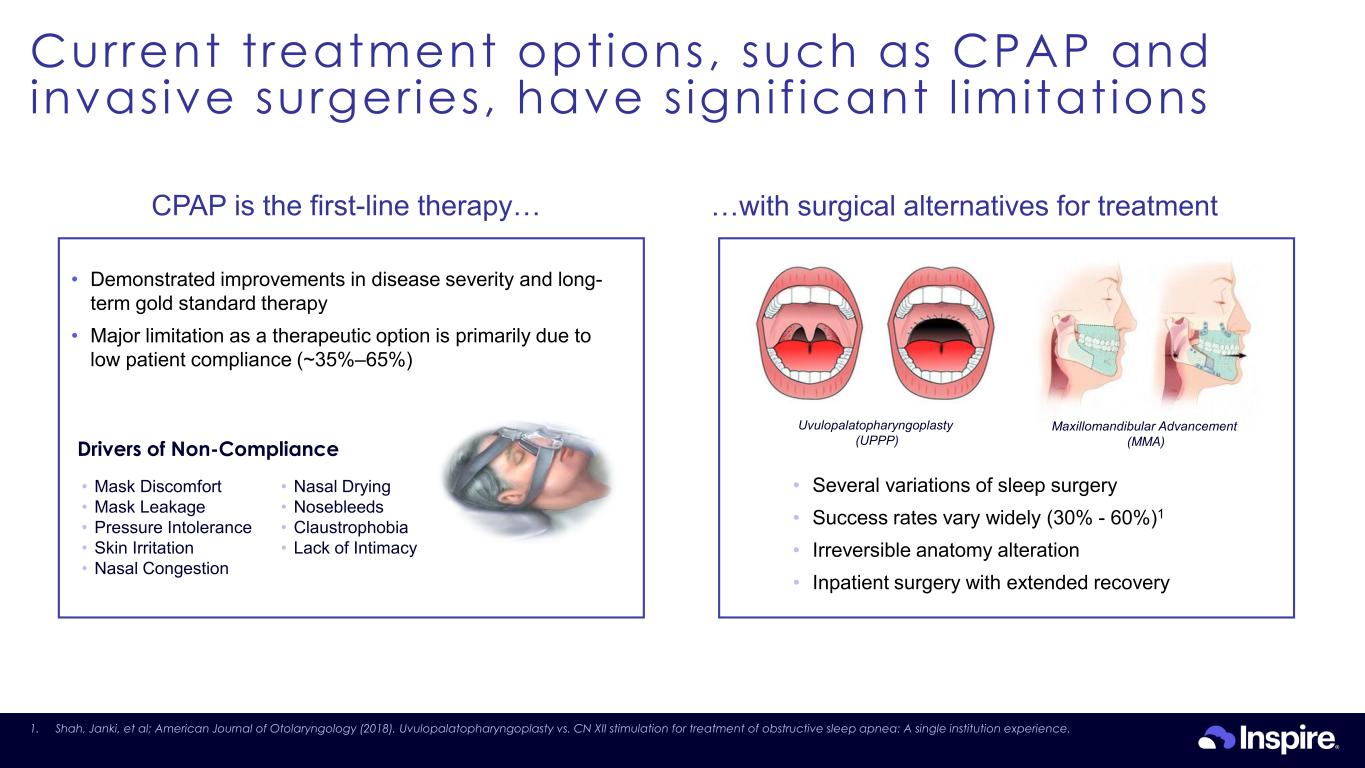
Current treatment options, such as CPAP and invasive surgeries, have s ignif icant l imitations 10 InaUvulopalatopharyngoplasty (UPPP) Maxillomandibular Advancement (MMA) • Several variations of sleep surgery • Success rates vary widely (30% - 60%)1 • Irreversible anatomy alteration • Inpatient surgery with extended recovery …with surgical alternatives for treatmentCPAP is the first-line therapy… Drivers of Non-Compliance • Demonstrated improvements in disease severity and long- term gold standard therapy • Major limitation as a therapeutic option is primarily due to low patient compliance (~35%–65%) • Mask Discomfort • Mask Leakage • Pressure Intolerance • Skin Irritation • Nasal Congestion • Nasal Drying • Nosebleeds • Claustrophobia • Lack of Intimacy 1. Shah, Janki, et al; American Journal of Otolaryngology (2018). Uvulopalatopharyngoplasty vs. CN XII stimulation for treatment of obstructive sleep apnea: A single institution experience.

CPAP prescriptions annually ~2,000,000 CPAP non-compliant ~700,000 Inspire eligible ~500,000 Adults with moderate to severe OSA ~23,000,000 >$10B opportuni ty The domestic OSA market is huge… Internal estimates
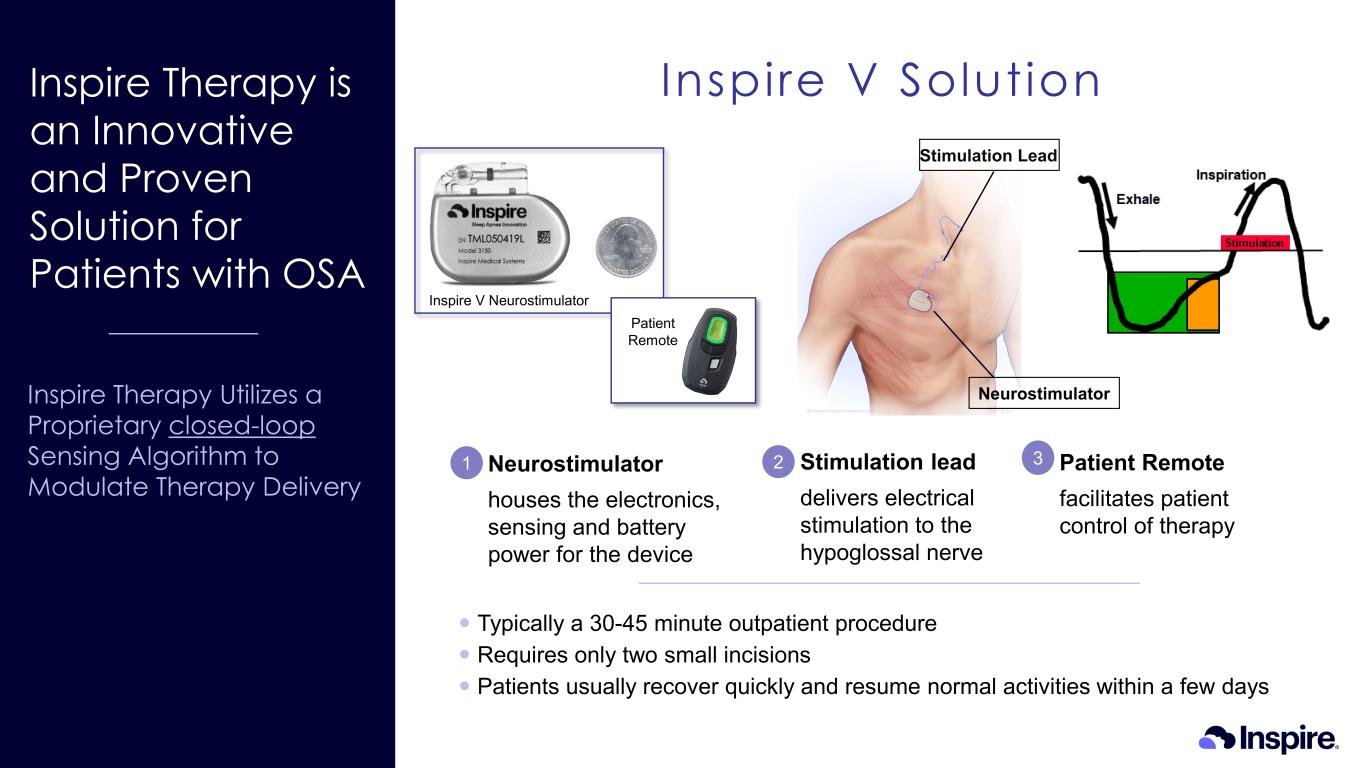
Inspire Therapy is an Innovative and Proven Solution for Patients with OSA Inspire Therapy Utilizes a Proprietary closed-loop Sensing Algorithm to Modulate Therapy Delivery Inspire V Solution 2 Typically a 30-45 minute outpatient procedure Requires only two small incisions Patients usually recover quickly and resume normal activities within a few days 31 Neurostimulator houses the electronics, sensing and battery power for the device Patient Remote facilitates patient control of therapy Stimulation lead delivers electrical stimulation to the hypoglossal nerve Stimulation Lead Neurostimulator Inspire V Neurostimulator Patient Remote
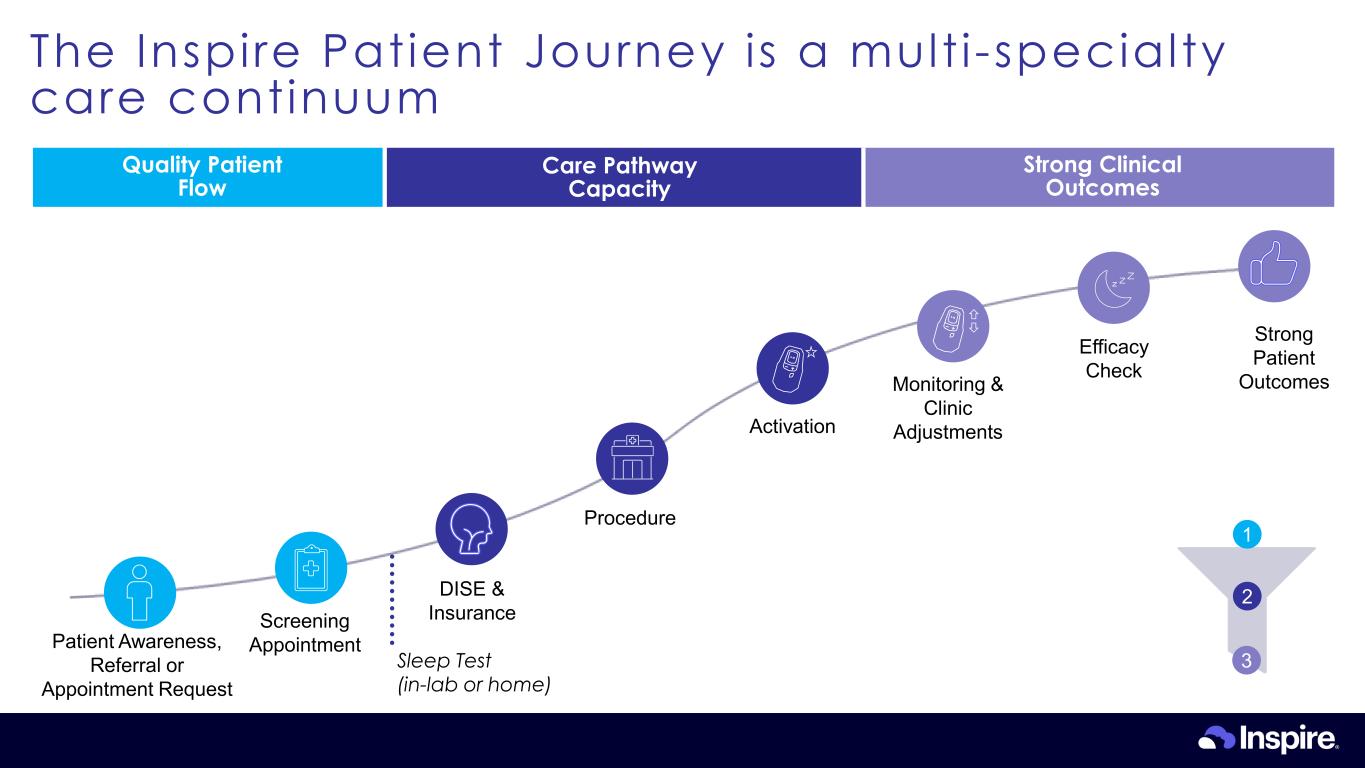
The Inspire Patient Journey is a mult i-specialty care continuum Quality Patient Flow Care Pathway Capacity Strong Clinical Outcomes Sleep Test (in-lab or home) Activation Procedure DISE & Insurance Strong Patient Outcomes Patient Awareness, Referral or Appointment Request Screening Appointment Efficacy Check Monitoring & Clinic Adjustments 1 2 3
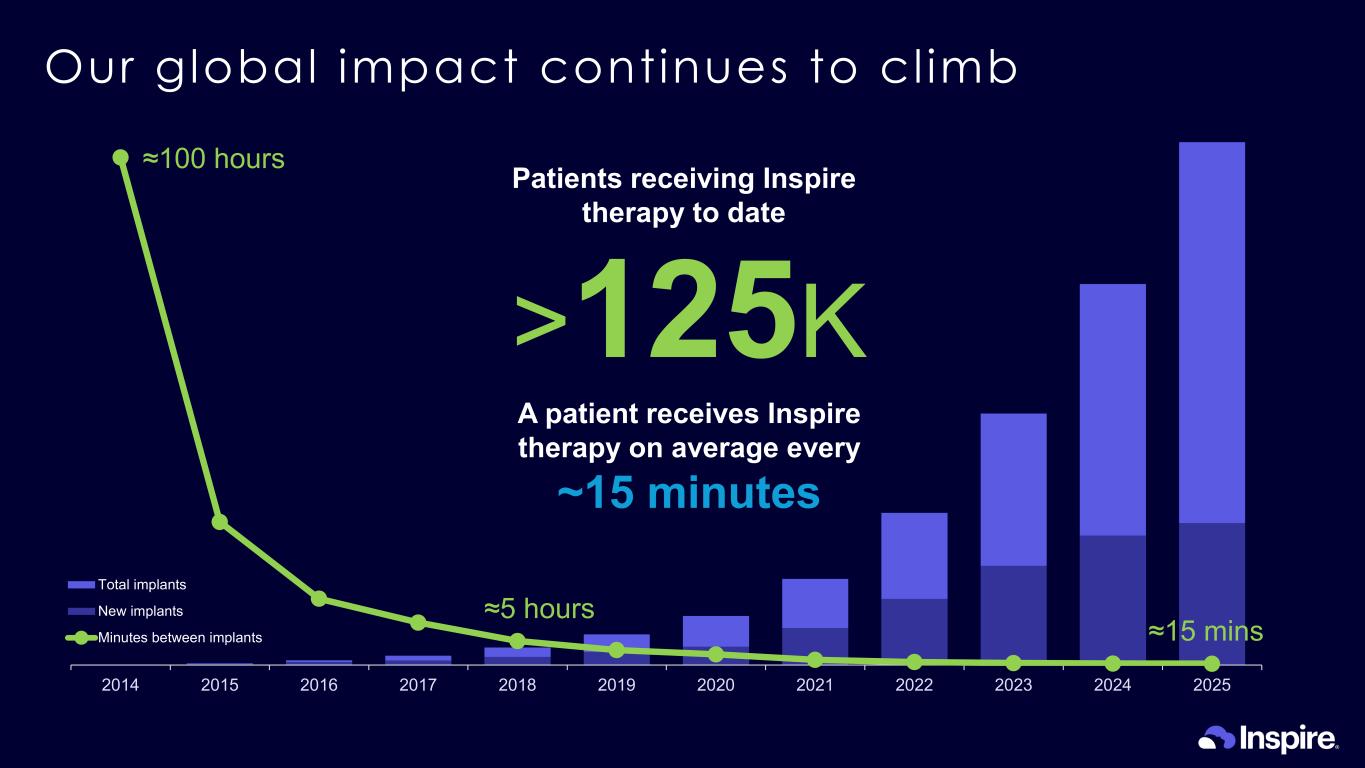
- 20,000 40,000 60,000 80,000 100,000 120,000 2014 2015 2016 2017 2018 2019 2020 2021 2022 2023 2024 2025 Total implants New implants Minutes between implants Our global impact continues to cl imb >125K Patients receiving Inspire therapy to date A patient receives Inspire therapy on average every ~15 minutes ≈100 hours ≈5 hours ≈15 mins
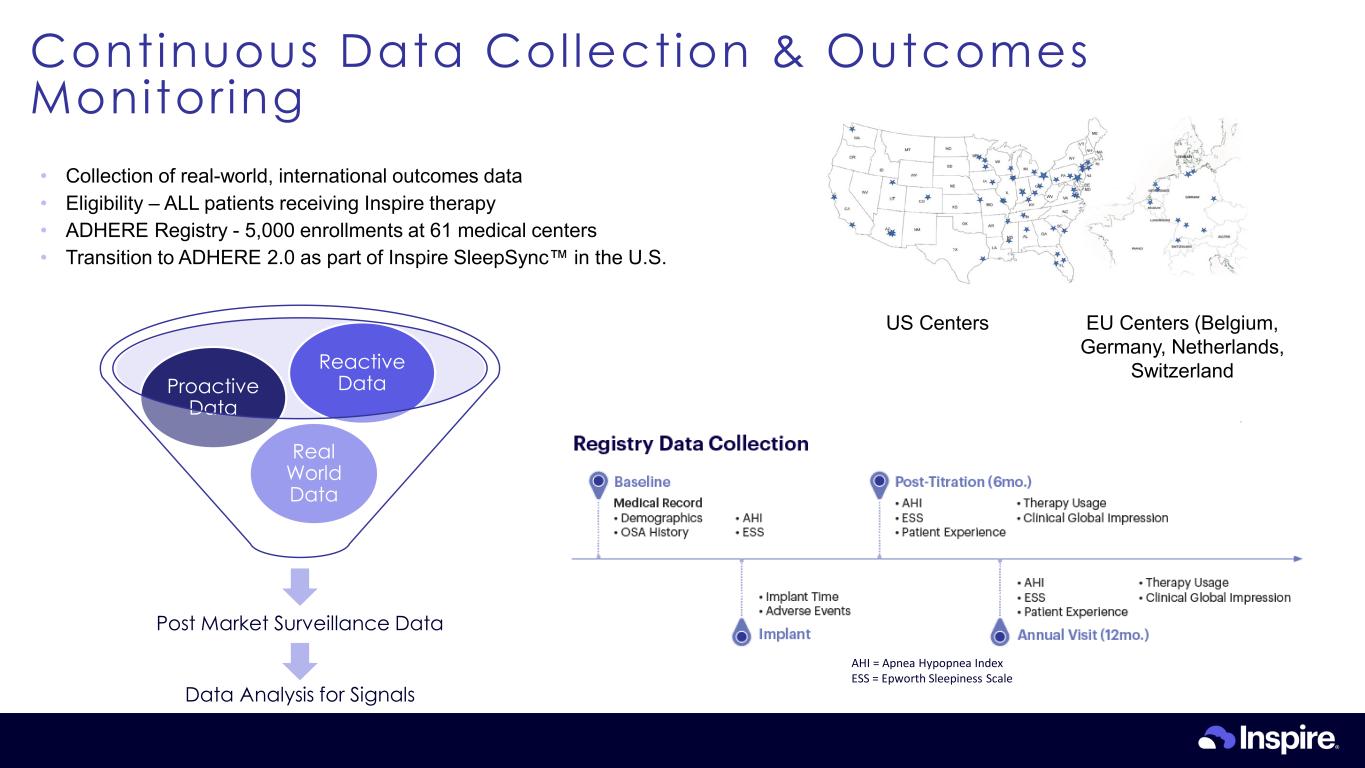
Continuous Data Collection & Outcomes Monitor ing 15 AHI = Apnea Hypopnea Index ESS = Epworth Sleepiness Scale Post Market Surveillance Data Real World Data Proactive Data Reactive Data Data Analysis for Signals US Centers EU Centers (Belgium, Germany, Netherlands, Switzerland • Collection of real-world, international outcomes data • Eligibility – ALL patients receiving Inspire therapy • ADHERE Registry - 5,000 enrollments at 61 medical centers • Transition to ADHERE 2.0 as part of Inspire SleepSync in the U.S.
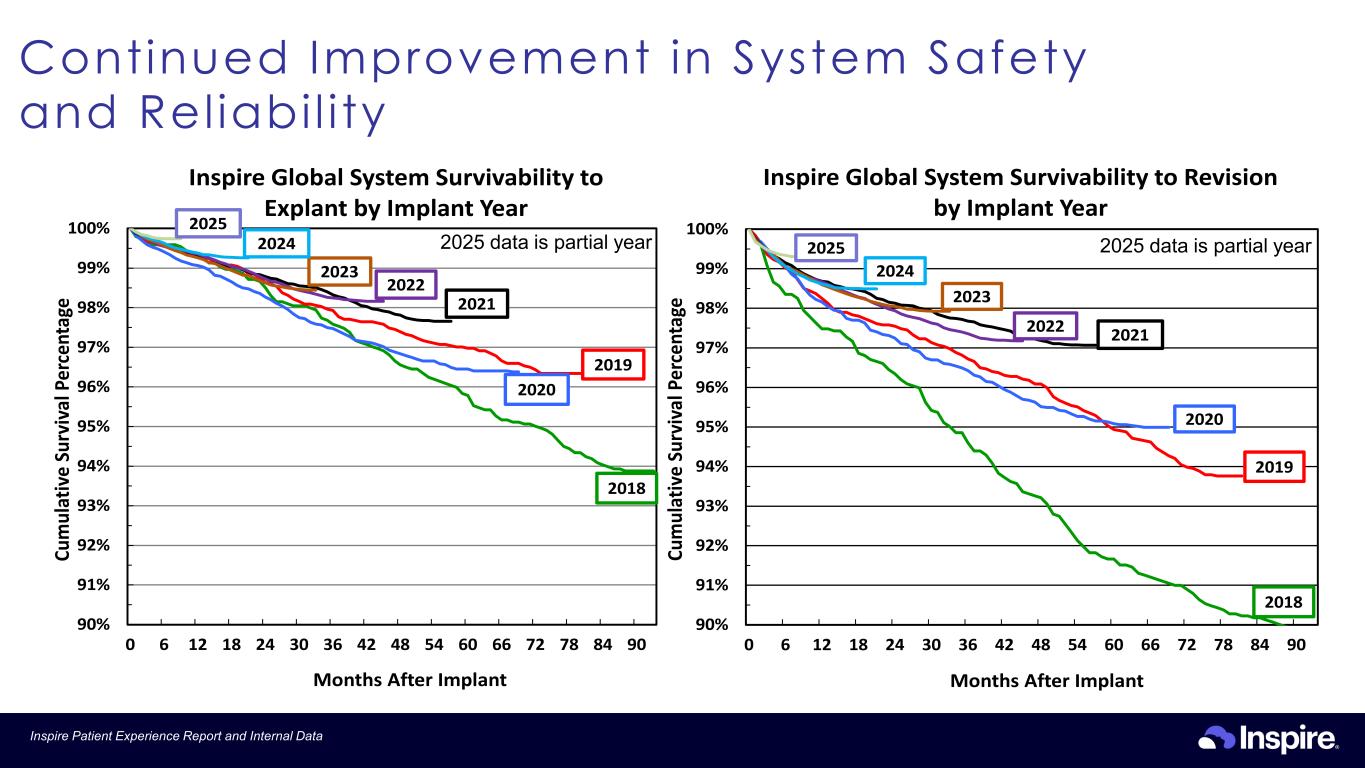
16Inspire Patient Experience Report and Internal Data 90% 91% 92% 93% 94% 95% 96% 97% 98% 99% 100% 0 6 12 18 24 30 36 42 48 54 60 66 72 78 84 90 Cu m ul at iv e Su rv iv al P er ce nt ag e Months After Implant Inspire Global System Survivability to Revision by Implant Year 2018 2020 2019 20212022 2023 2024 2025 90% 91% 92% 93% 94% 95% 96% 97% 98% 99% 100% 0 6 12 18 24 30 36 42 48 54 60 66 72 78 84 90 Cu m ul at iv e Su rv iv al P er ce nt ag e Months After Implant Inspire Global System Survivability to Explant by Implant Year 2018 2020 2019 2021 2022 2023 2024 2025 2025 data is partial year 2025 data is partial year Continued Improvement in System Safety and Rel iabi l i ty
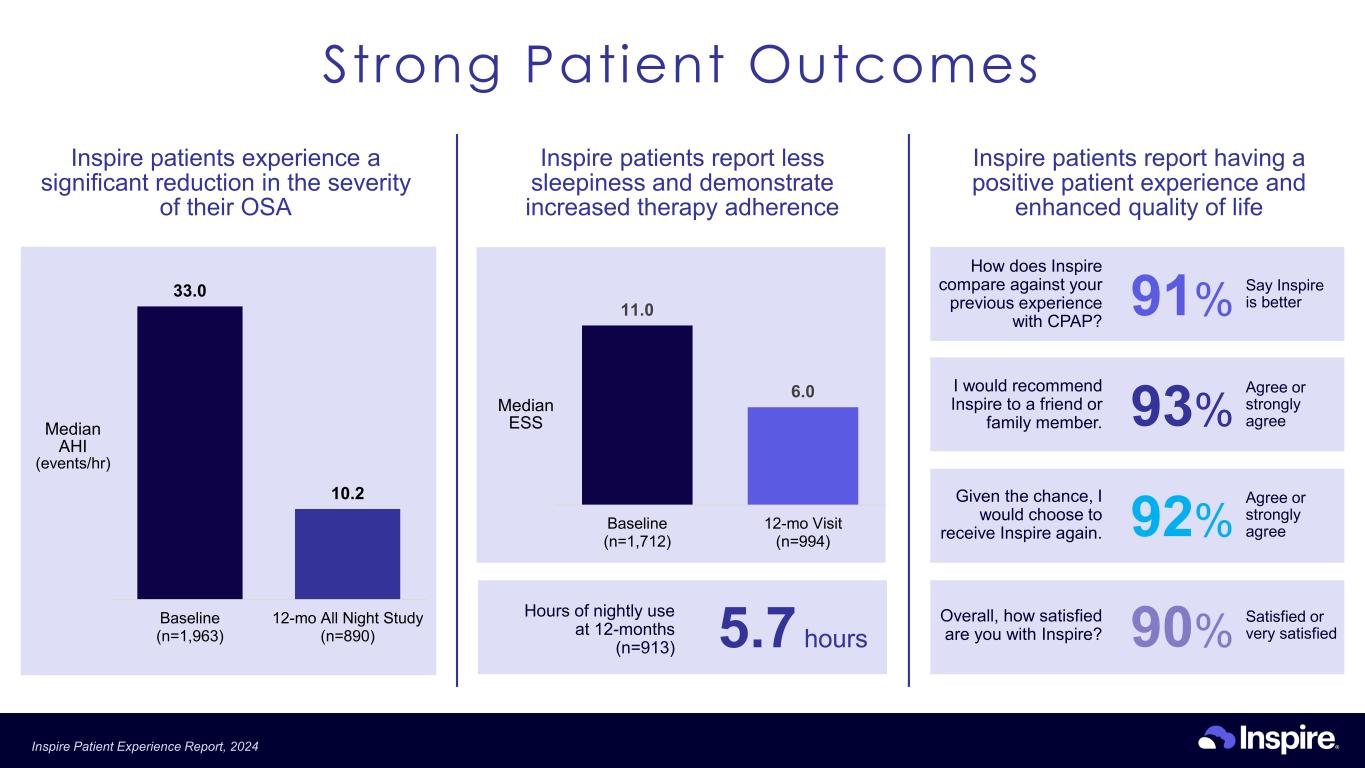
How does Inspire compare against your previous experience with CPAP? 91% Say Inspire is better I would recommend Inspire to a friend or family member. 93% Agree or strongly agree Overall, how satisfied are you with Inspire? 90% Satisfied or very satisfied Given the chance, I would choose to receive Inspire again. 92% Agree or strongly agree Inspire patients experience a significant reduction in the severity of their OSA 33.0 10.2 Baseline (n=1,963) 12-mo All Night Study (n=890) Median AHI (events/hr) Inspire patients report less sleepiness and demonstrate increased therapy adherence 11.0 6.0 Baseline (n=1,712) 12-mo Visit (n=994) Median ESS Hours of nightly use at 12-months (n=913) 5.7 hours Inspire patients report having a positive patient experience and enhanced quality of life Strong Patient Outcomes Inspire Patient Experience Report, 2024
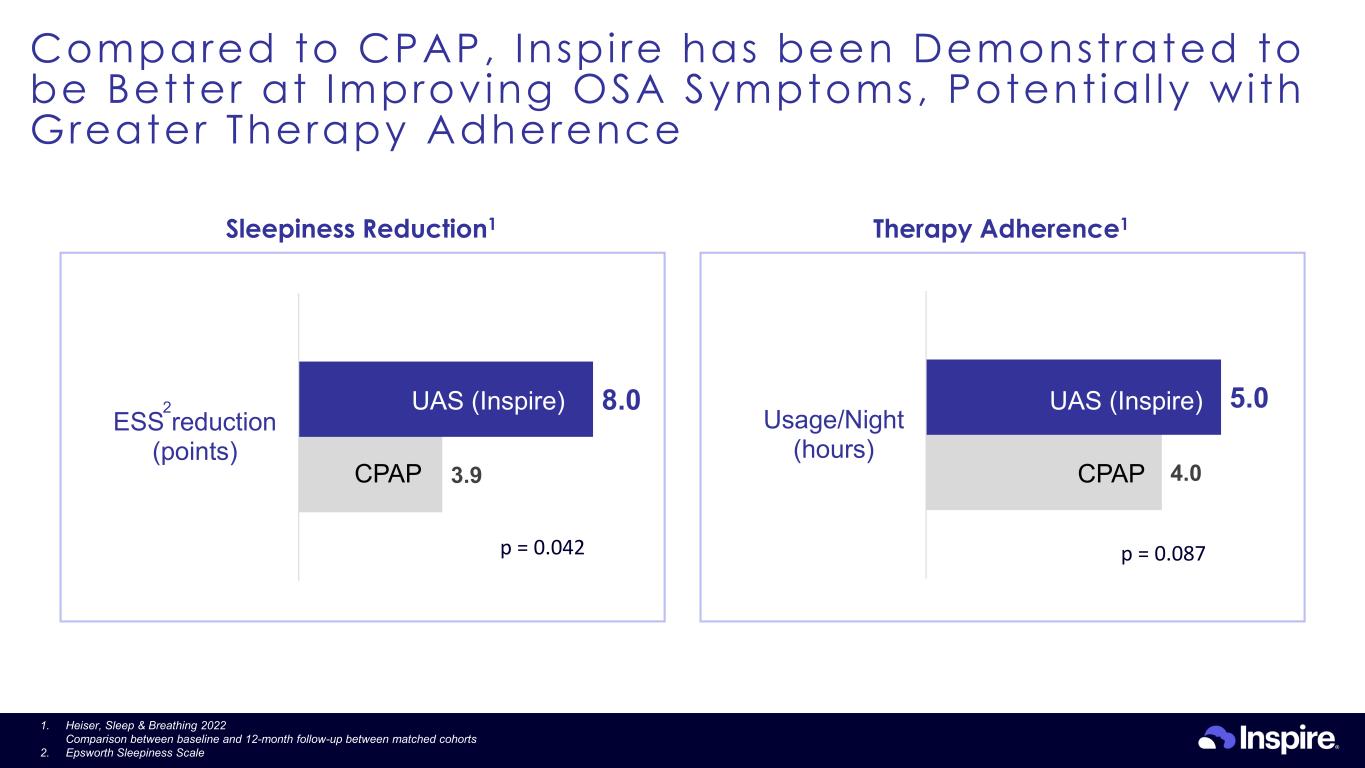
Compared to CPAP, Inspi re has been Demonstrated to be Better at Improving OSA Symptoms, Potent ia l ly wi th Greater Therapy Adherence 18 Therapy Adherence1 4.0 5.0 Usage/Night (hours) Sleepiness Reduction1 3.9 8.0 ESS reduction (points) 2 p = 0.042 p = 0.087 CPAP CPAP UAS (Inspire) UAS (Inspire) 1. Heiser, Sleep & Breathing 2022 Comparison between baseline and 12-month follow-up between matched cohorts 2. Epsworth Sleepiness Scale
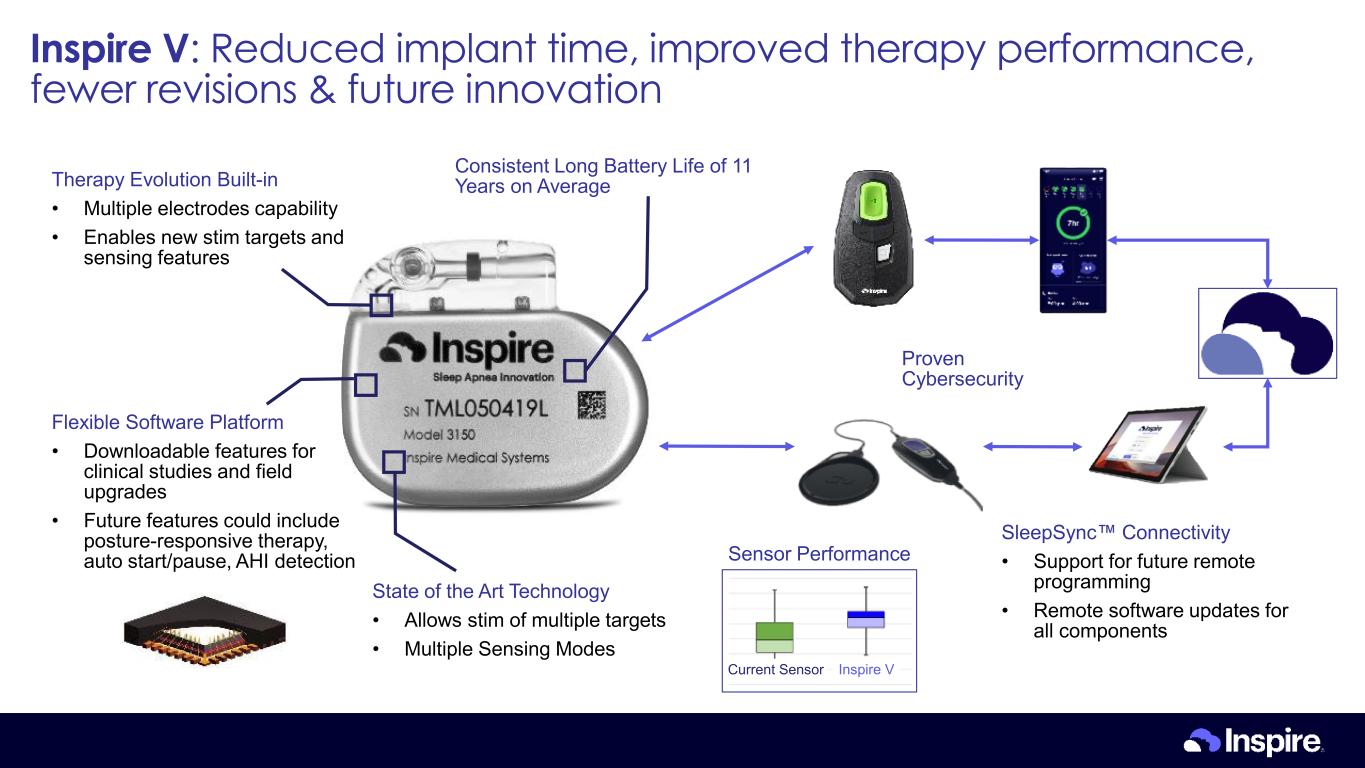
Current Sensor Inspire V Sensor Performance Therapy Evolution Built-in • Multiple electrodes capability • Enables new stim targets and sensing features Flexible Software Platform • Downloadable features for clinical studies and field upgrades • Future features could include posture-responsive therapy, auto start/pause, AHI detection State of the Art Technology • Allows stim of multiple targets • Multiple Sensing Modes Proven Cybersecurity Consistent Long Battery Life of 11 Years on Average SleepSync Connectivity • Support for future remote programming • Remote software updates for all components Inspire V: Reduced implant time, improved therapy performance, fewer revisions & future innovation
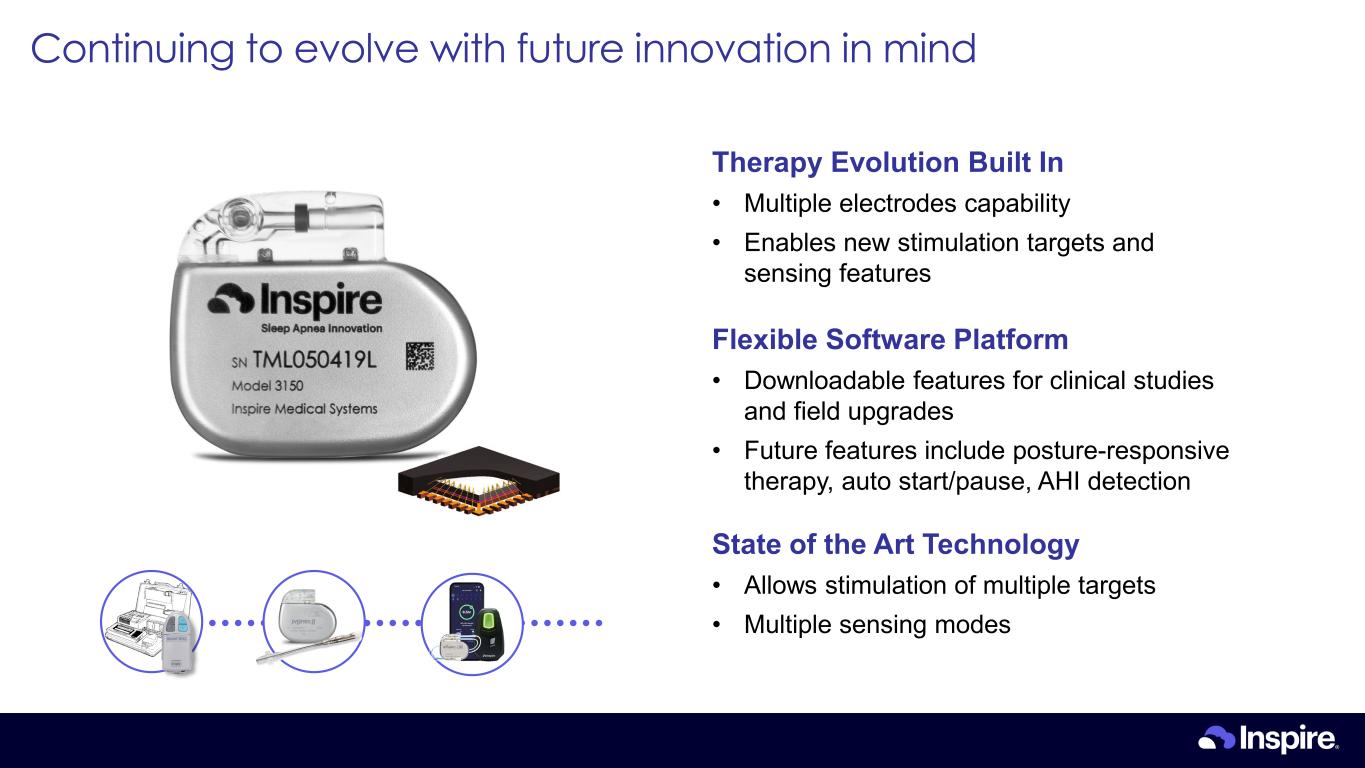
Therapy Evolution Built In • Multiple electrodes capability • Enables new stimulation targets and sensing features Flexible Software Platform • Downloadable features for clinical studies and field upgrades • Future features include posture-responsive therapy, auto start/pause, AHI detection State of the Art Technology • Allows stimulation of multiple targets • Multiple sensing modes Continuing to evolve with future innovation in mind
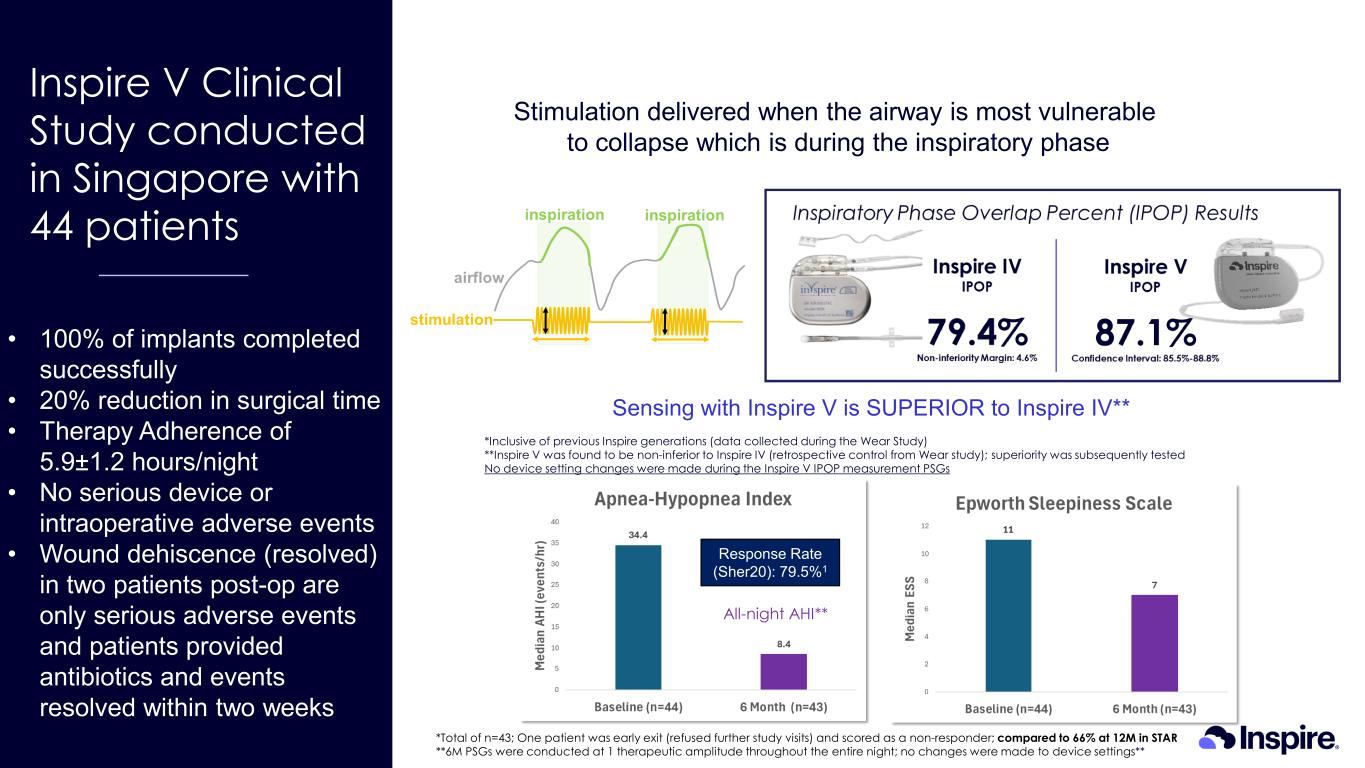
• 100% of implants completed successfully • 20% reduction in surgical time • Therapy Adherence of 5.9±1.2 hours/night • No serious device or intraoperative adverse events • Wound dehiscence (resolved) in two patients post-op are only serious adverse events and patients provided antibiotics and events resolved within two weeks Inspire V Clinical Study conducted in Singapore with 44 patients *Inclusive of previous Inspire generations (data collected during the Wear Study) **Inspire V was found to be non-inferior to Inspire IV (retrospective control from Wear study); superiority was subsequently tested No device setting changes were made during the Inspire V IPOP measurement PSGs *Total of n=43; One patient was early exit (refused further study visits) and scored as a non-responder; compared to 66% at 12M in STAR **6M PSGs were conducted at 1 therapeutic amplitude throughout the entire night; no changes were made to device settings** All-night AHI** Stimulation delivered when the airway is most vulnerable to collapse which is during the inspiratory phase Response Rate (Sher20): 79.5%1 Sensing with Inspire V is SUPERIOR to Inspire IV**
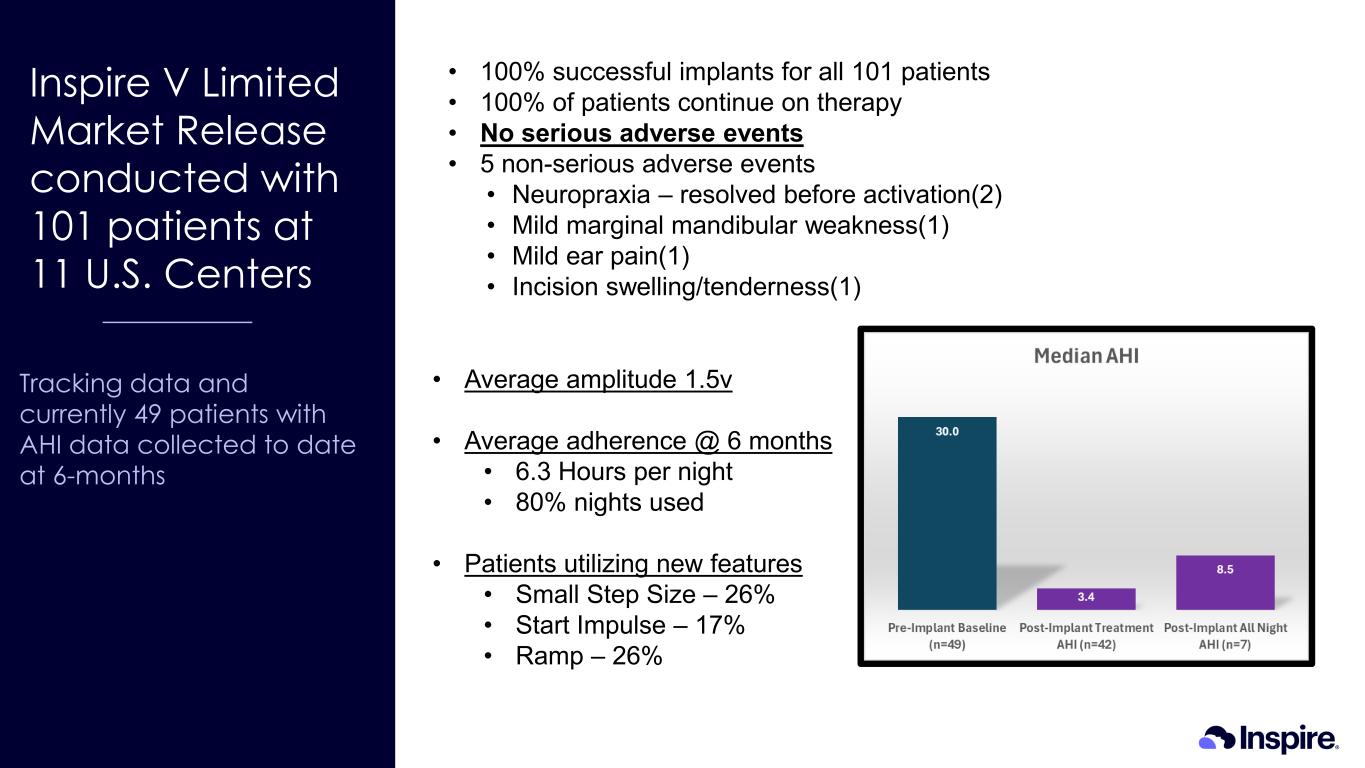
Inspire V Limited Market Release conducted with 101 patients at 11 U.S. Centers Tracking data and currently 49 patients with AHI data collected to date at 6-months • 100% successful implants for all 101 patients • 100% of patients continue on therapy • No serious adverse events • 5 non-serious adverse events • Neuropraxia – resolved before activation(2) • Mild marginal mandibular weakness(1) • Mild ear pain(1) • Incision swelling/tenderness(1) • Average amplitude 1.5v • Average adherence @ 6 months • 6.3 Hours per night • 80% nights used • Patients utilizing new features • Small Step Size – 26% • Start Impulse – 17% • Ramp – 26%
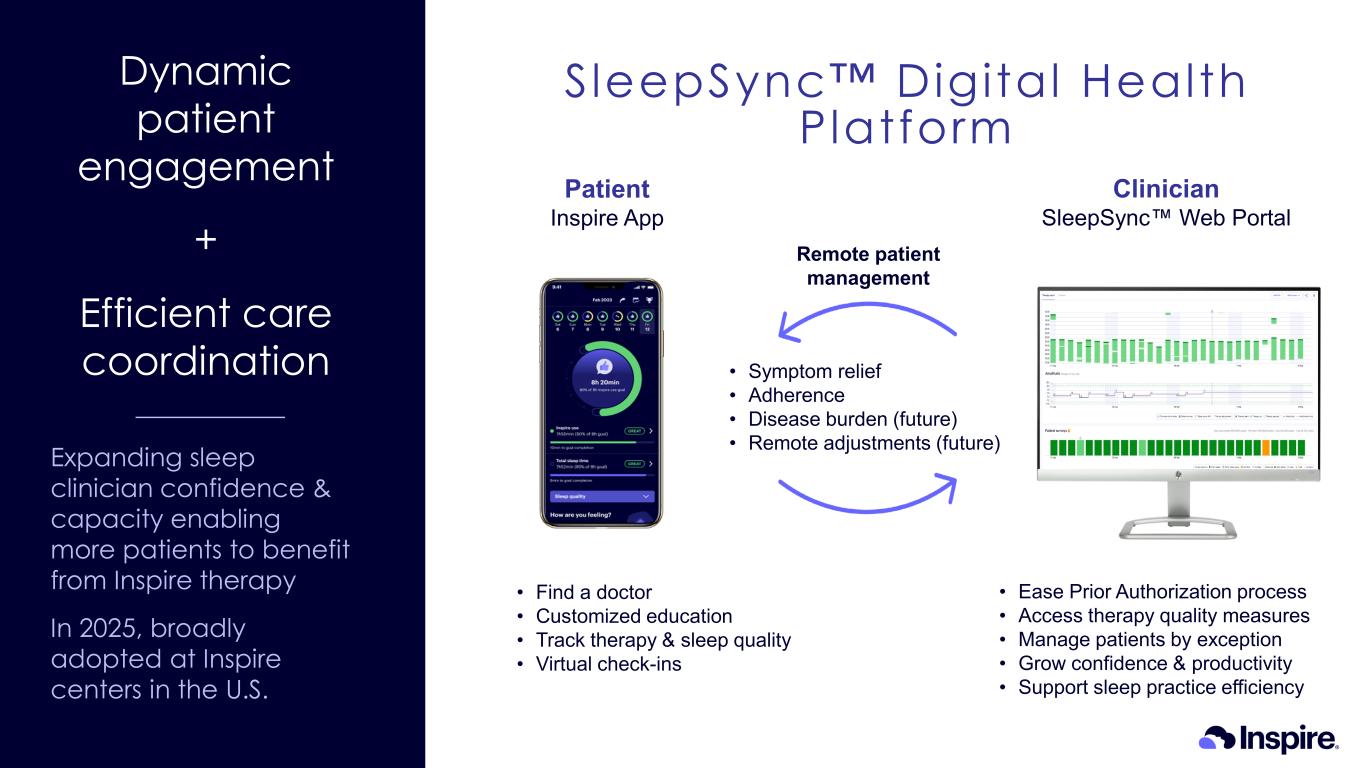
Dynamic patient engagement + Efficient care coordination Expanding sleep clinician confidence & capacity enabling more patients to benefit from Inspire therapy In 2025, broadly adopted at Inspire centers in the U.S. Remote patient management Patient Inspire App Clinician SleepSync Web Portal • Find a doctor • Customized education • Track therapy & sleep quality • Virtual check-ins • Ease Prior Authorization process • Access therapy quality measures • Manage patients by exception • Grow confidence & productivity • Support sleep practice efficiency • Symptom relief • Adherence • Disease burden (future) • Remote adjustments (future) SleepSync Digital Health Platform
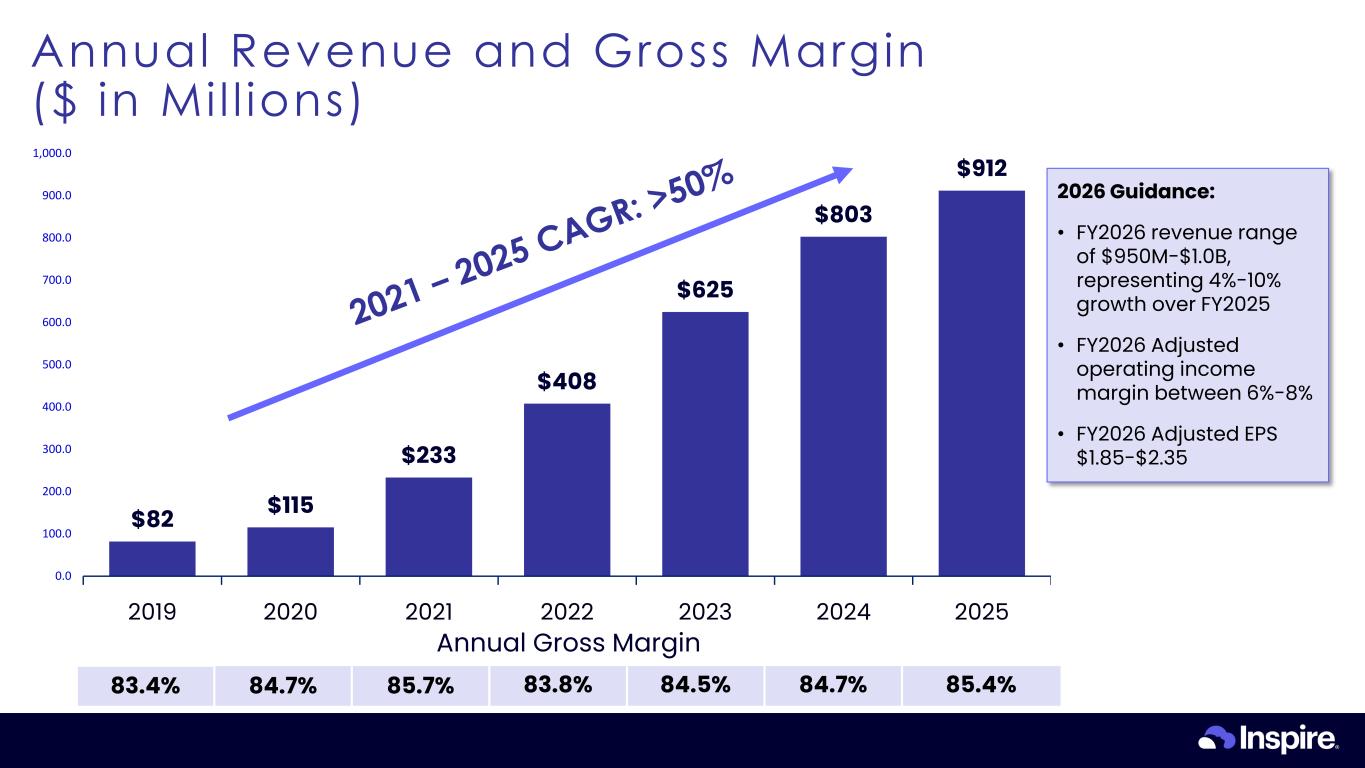
© Inspire Medical Systems, Inc. All Rights Reserved. $82 $115 $233 $408 $625 $803 $912 0.0 100.0 200.0 300.0 400.0 500.0 600.0 700.0 800.0 900.0 1,000.0 2019 2020 2021 2022 2023 2024 2025 24 Annual Revenue and Gross Margin ($ in Mil l ions) Annual Gross Margin 83.4% 84.7% 85.7% 83.8% 84.5% 84.7% 85.4% 2026 Guidance: • FY2026 revenue range of $950M-$1.0B, representing 4%-10% growth over FY2025 • FY2026 Adjusted operating income margin between 6%-8% • FY2026 Adjusted EPS $1.85-$2.35
© Inspire Medical Systems, Inc. All Rights Reserved.25 Recent Business Highlights • Made steady progress on the full launch of the Inspire V system in the U.S. • Presented Inspire V safety and efficacy data at AAO-HNS / ISSS meetings Continued Commercial Expansion Financial Performance • Generated $269.1 million of revenue in the fourth quarter, a 12% increase over the same quarter last year • Achieved gross margin of 86.6% in the fourth quarter • Reported net income per diluted share of $4.66 in the fourth quarter or $1.65 adjusted • Generated $53 million in operating cash flow for the fourth quarter; $117 million full year • Completed $175 million in share repurchase in 2025
© Inspire Medical Systems, Inc. All Rights Reserved. Our Growth Strategy 26 1 Through planned and controlled market expansion and robust physician training Ensure Strong Clinical Outcomes 2 By enhancing interconnectivity, simplifying the care pathway, and closely tracking outcomes Improve the Customer Experience 3 Amongst patients, ENT/Sleep physicians, and general practitioners Promote Widespread Consumer Awareness 4 Commensurate with new center additions and leveraging consumer outreach programs Drive Continued Commercial Scale 5 Driving breakthrough technology innovation and expanded indications Invest in Research & Development 6 Further penetrating existing markets and entering into new geographical locations Facilitate International Market Expansion

Inspire Way We are a medical technology company committed to enhancing patient lives through sleep innovation “Put the patient first and you will never lose your way.” Demonstrate Operational Excellence Drive Therapy Adoption Strengthen Organizational Culture Focused on Outcomes. Fueled by Innovation. Grounded in Integrity. Committed to Compliance. Leading with Respect. Positively Persistent.

No mask. No hose. Just sleep. INSPIRE CONFIDENTIAL. Inspire is a public company and has an Insider Trading Policy. The content in this deck is not to be shared with anybody outside of Inspire Medical Systems, Inc. It is for internal review and discussion only www.inspiresleep.com ®

© Inspire Medical Systems, Inc. All Rights Reserved. Appendix 29
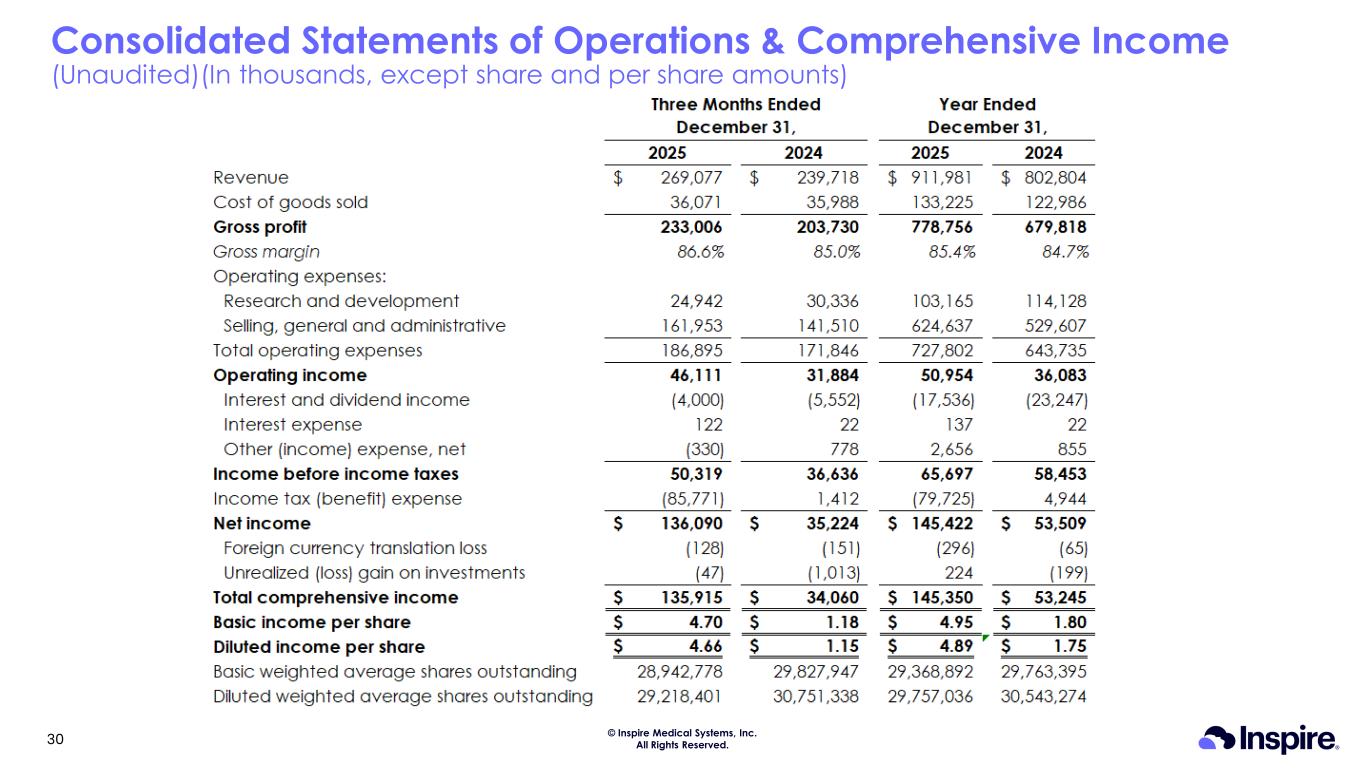
© Inspire Medical Systems, Inc. All Rights Reserved. Consolidated Statements of Operations & Comprehensive Income (Unaudited)(In thousands, except share and per share amounts) 30
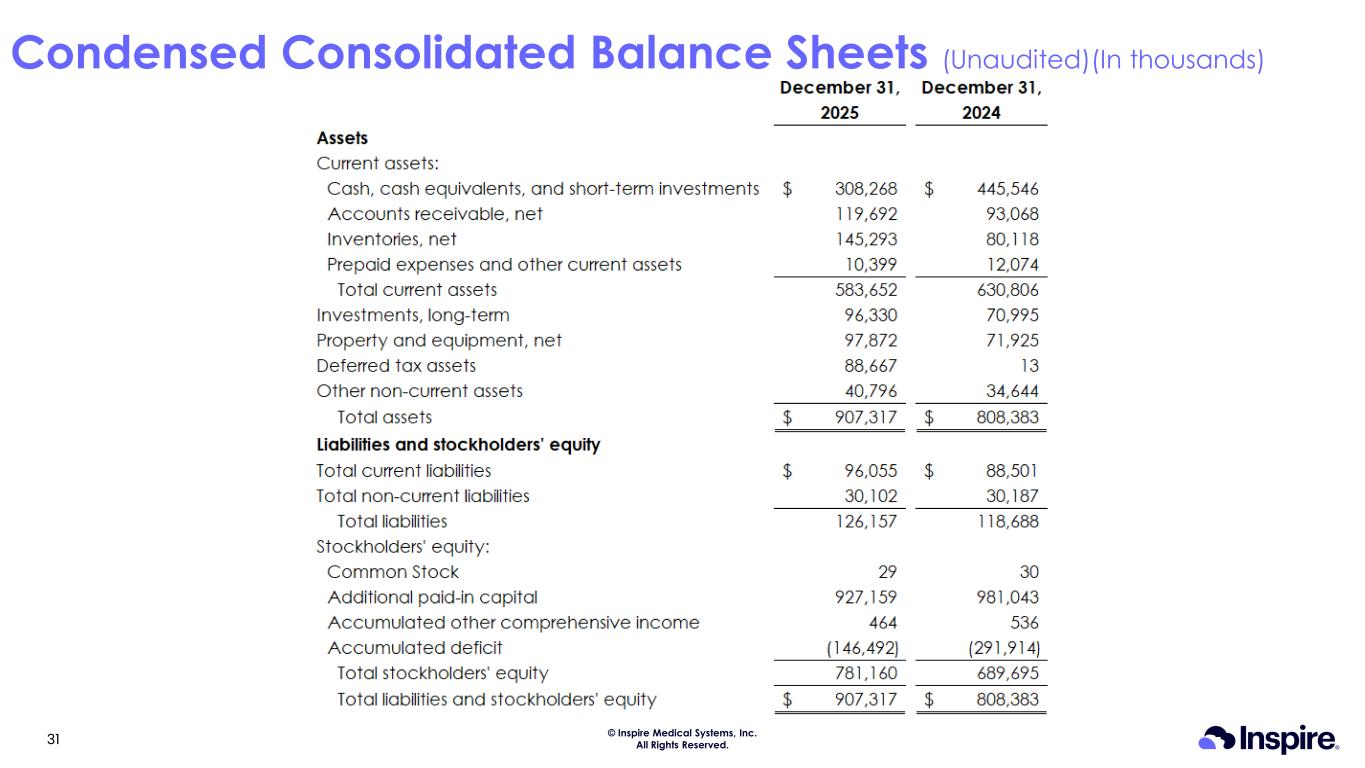
© Inspire Medical Systems, Inc. All Rights Reserved. Condensed Consolidated Balance Sheets (Unaudited)(In thousands) 31
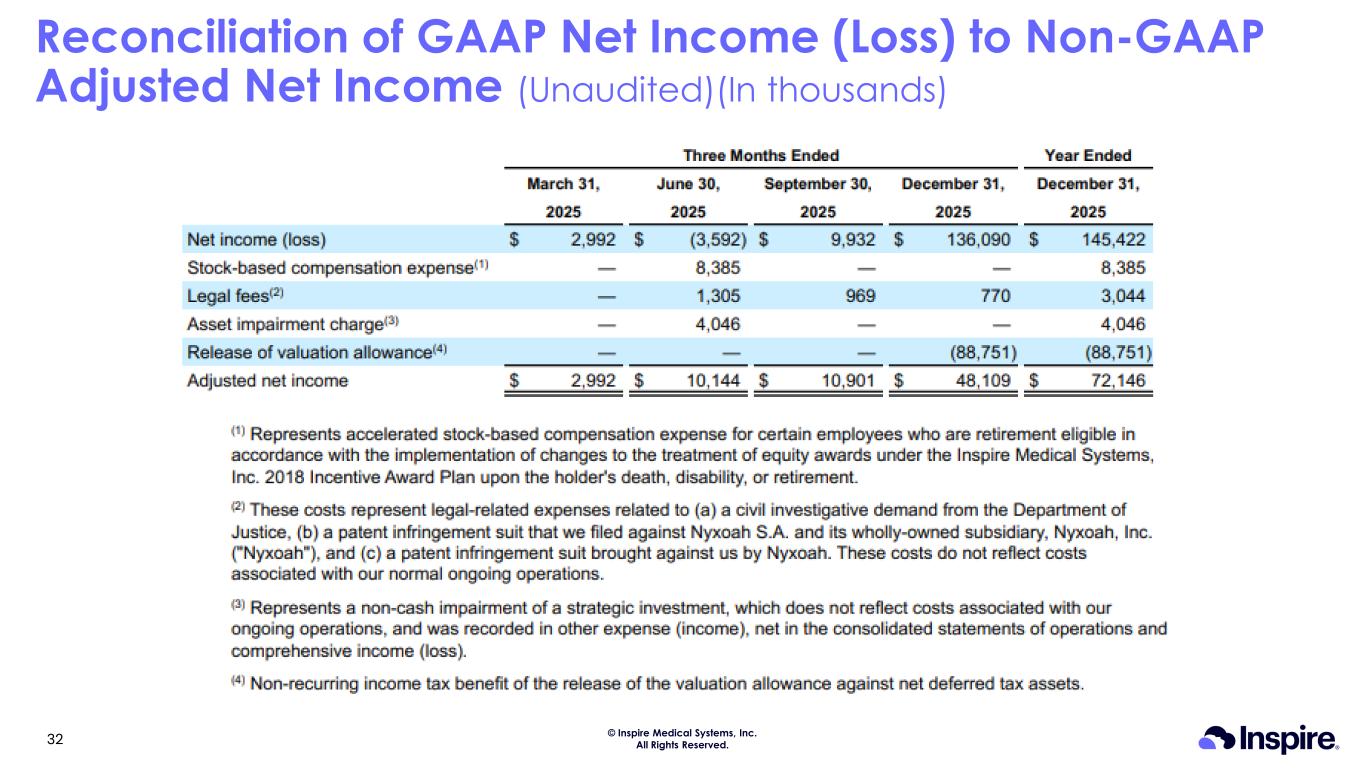
© Inspire Medical Systems, Inc. All Rights Reserved. Reconciliation of GAAP Net Income (Loss) to Non-GAAP Adjusted Net Income (Unaudited)(In thousands) 32
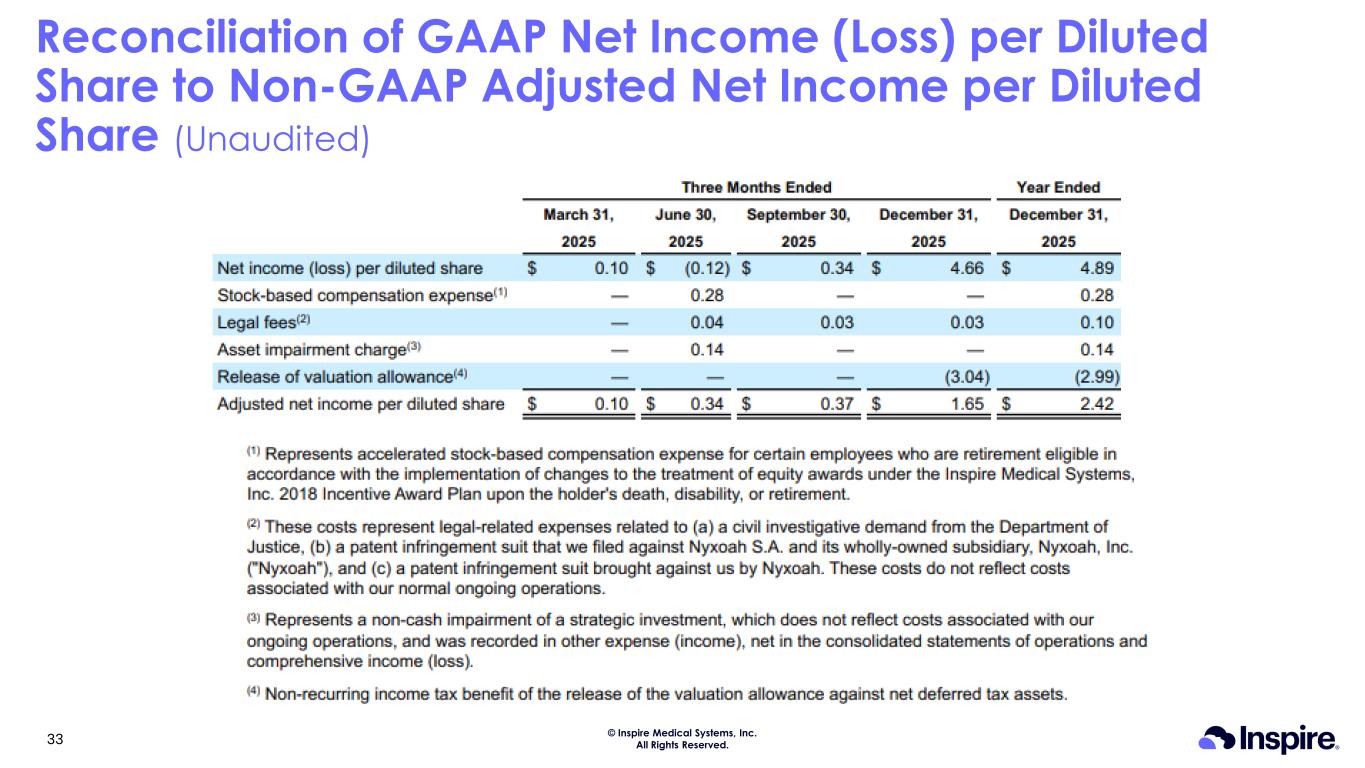
© Inspire Medical Systems, Inc. All Rights Reserved. Reconciliation of GAAP Net Income (Loss) per Diluted Share to Non-GAAP Adjusted Net Income per Diluted Share (Unaudited) 33
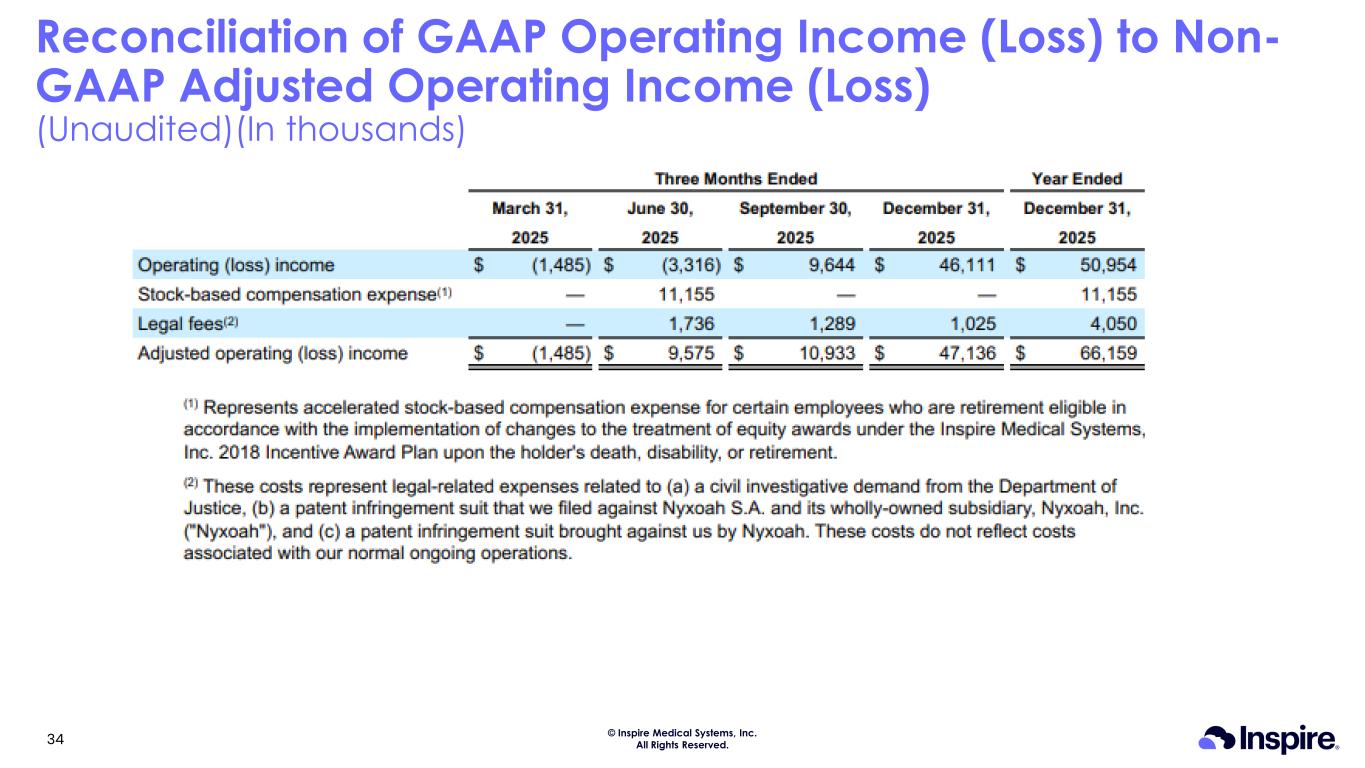
© Inspire Medical Systems, Inc. All Rights Reserved. Reconciliation of GAAP Operating Income (Loss) to Non- GAAP Adjusted Operating Income (Loss) (Unaudited)(In thousands) 34
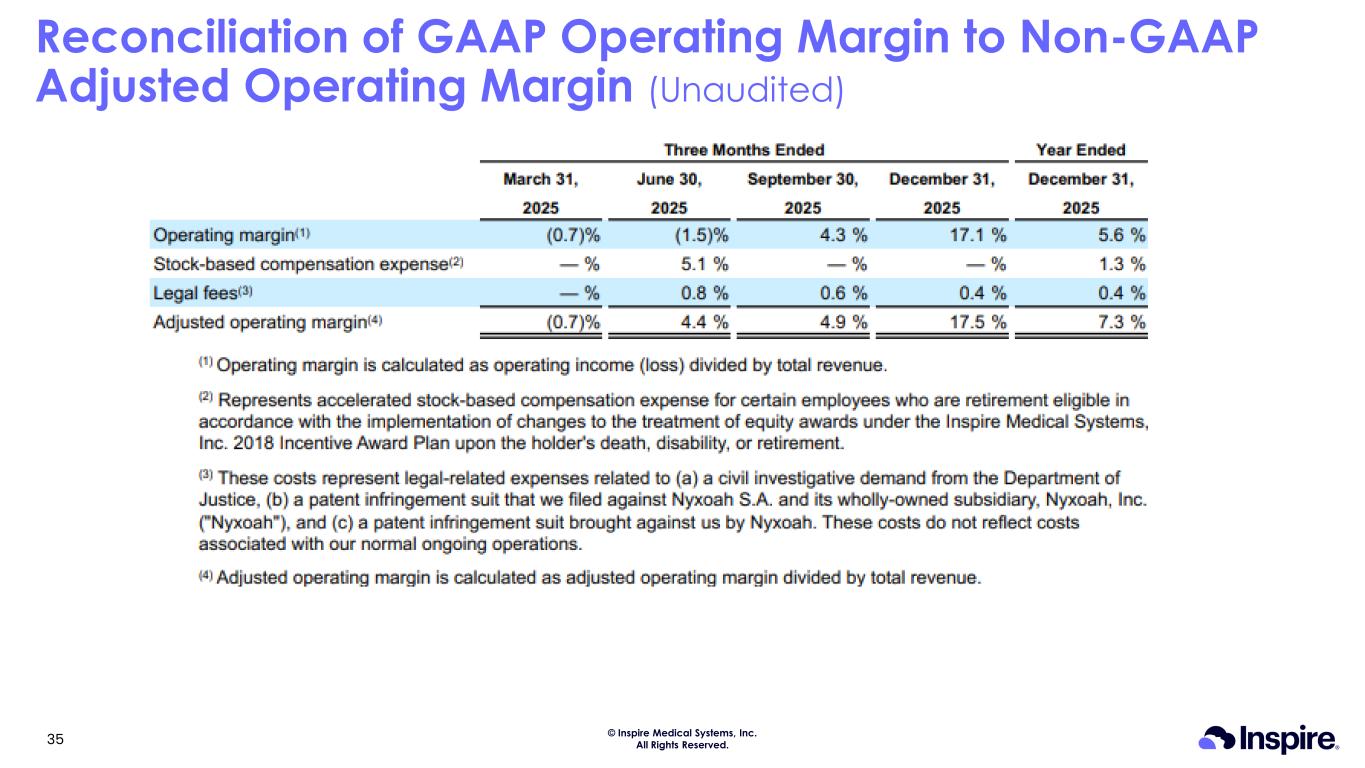
© Inspire Medical Systems, Inc. All Rights Reserved. Reconciliation of GAAP Operating Margin to Non-GAAP Adjusted Operating Margin (Unaudited) 35
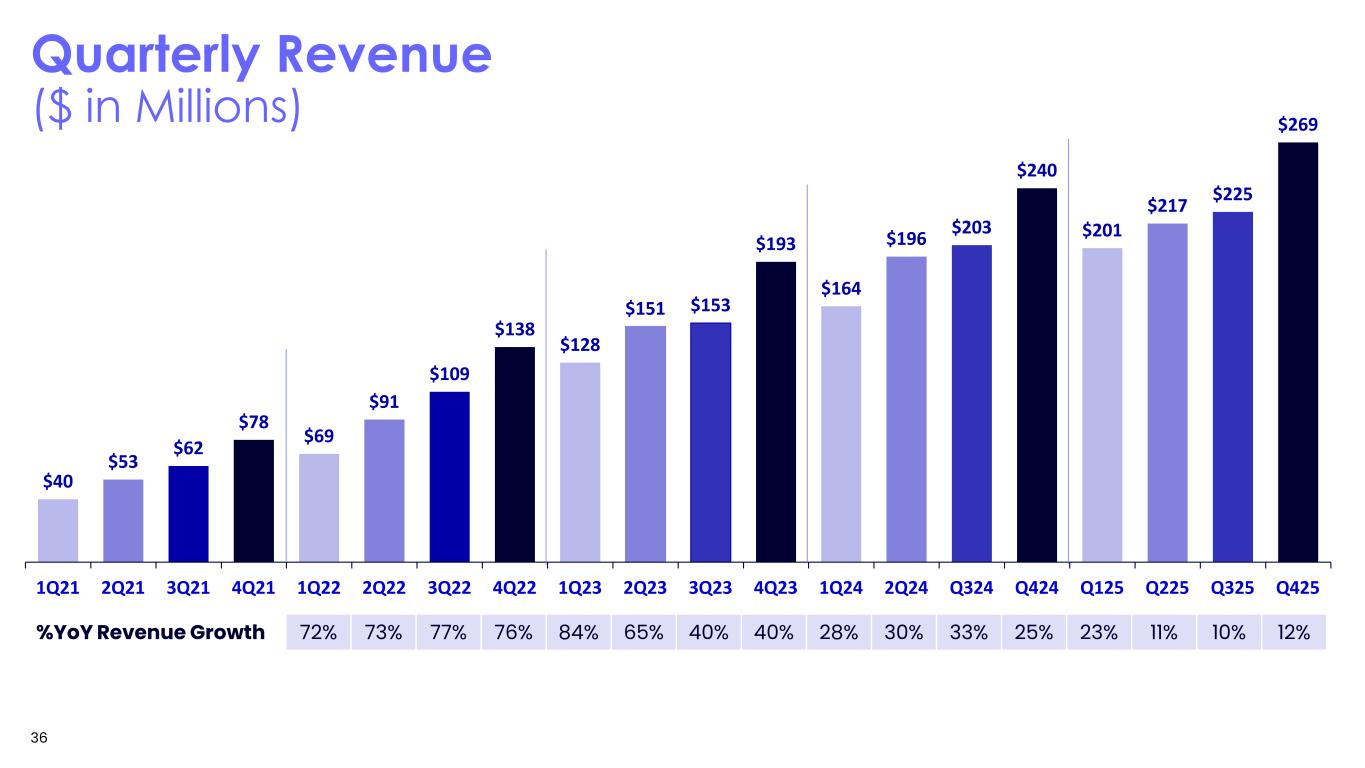
$40 $53 $62 $78 $69 $91 $109 $138 $128 $151 $153 $193 $164 $196 $203 $240 $201 $217 $225 $269 1Q21 2Q21 3Q21 4Q21 1Q22 2Q22 3Q22 4Q22 1Q23 2Q23 3Q23 4Q23 1Q24 2Q24 Q324 Q424 Q125 Q225 Q325 Q425 36 Quarterly Revenue ($ in Millions) %YoY Revenue Growth 72% 73% 77% 76% 84% 65% 40% 40% 28% 30% 33% 25% 23% 11% 10% 12%
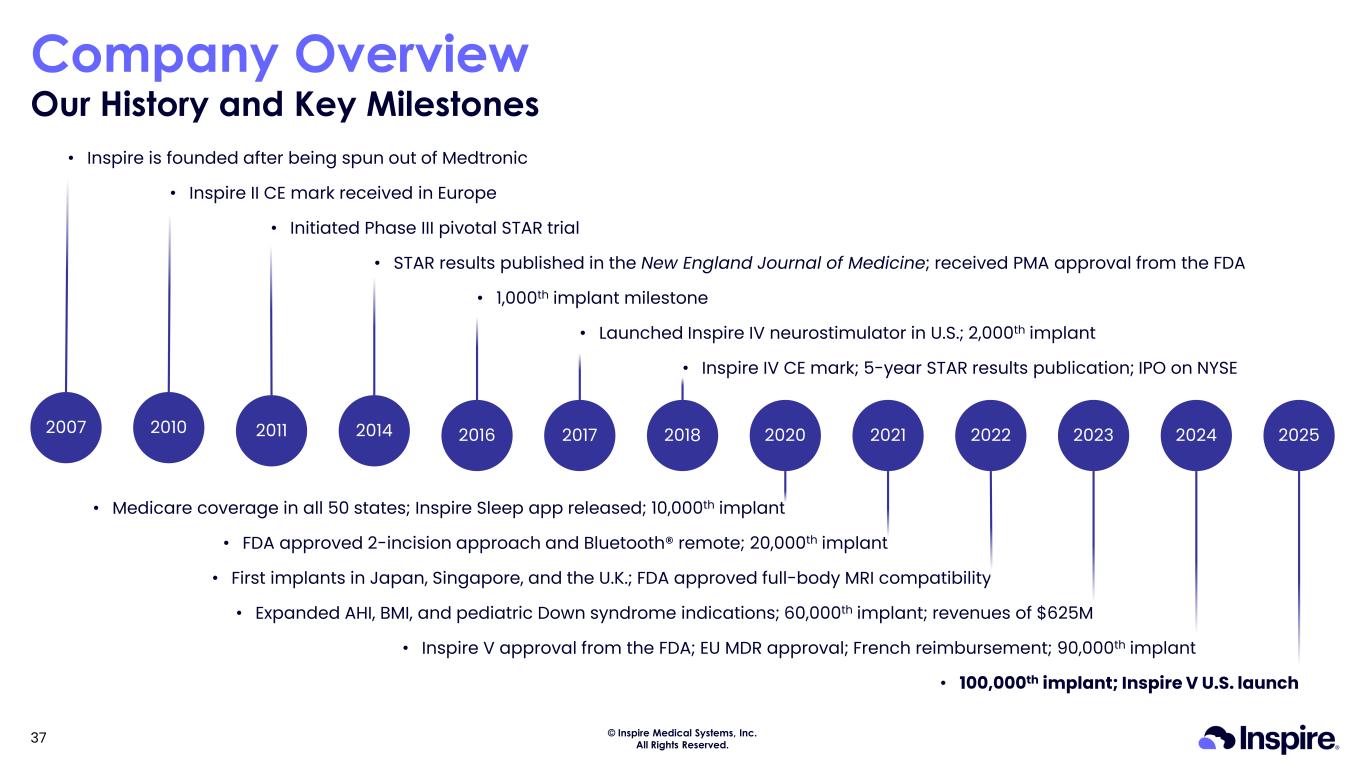
© Inspire Medical Systems, Inc. All Rights Reserved. Company Overview Our History and Key Milestones 37 20222016 20202007 2014 2018 20232017 20212011 • Inspire is founded after being spun out of Medtronic • Initiated Phase III pivotal STAR trial • STAR results published in the New England Journal of Medicine; received PMA approval from the FDA • 1,000th implant milestone • Launched Inspire IV neurostimulator in U.S.; 2,000th implant • Inspire IV CE mark; 5-year STAR results publication; IPO on NYSE • Medicare coverage in all 50 states; Inspire Sleep app released; 10,000th implant • FDA approved 2-incision approach and Bluetooth® remote; 20,000th implant • First implants in Japan, Singapore, and the U.K.; FDA approved full-body MRI compatibility • Expanded AHI, BMI, and pediatric Down syndrome indications; 60,000th implant; revenues of $625M 2010 • Inspire II CE mark received in Europe 2024 • Inspire V approval from the FDA; EU MDR approval; French reimbursement; 90,000th implant 2025 • 100,000th implant; Inspire V U.S. launch

Proven management team that is grounded in integrity, fueled by innovation, and devoted to delivering on the promise of our mission Randy Ban Executive Vice President, Patient Access & Therapy Development Joined 2009 Bryan Phillips SVP, General Counsel & Chief Compliance Officer Joined 2021 Tim Herbert Chair, President & Chief Executive Officer Joined 2007 Ezgi Yagci Vice President, Investor Relations Joined 2022 Matt Osberg Chief Financial Officer Joined 2026 John Rondoni Chief Product & Innovation Officer Joined 2008 Carlton Weatherby Chief Strategy & Growth Officer Joined 2023 Jason Kelly Chief Manufacturing & Quality Officer Joined 2025 Melissa Mann Chief People Officer Joined 2024
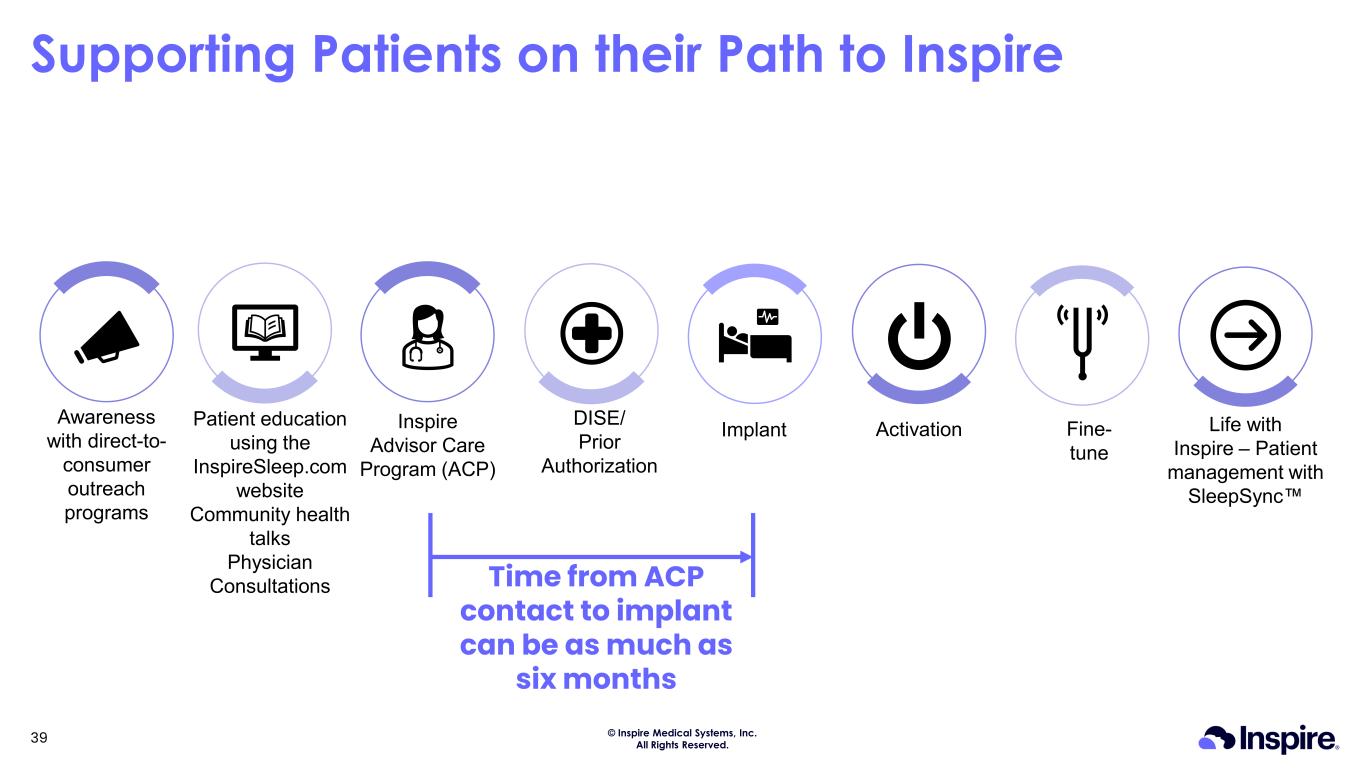
© Inspire Medical Systems, Inc. All Rights Reserved. Supporting Patients on their Path to Inspire Implant Fine- tune ActivationInspire Advisor Care Program (ACP) DISE/ Prior Authorization Patient education using the InspireSleep.com website Community health talks Physician Consultations Awareness with direct-to- consumer outreach programs Life with Inspire – Patient management with SleepSync Time from ACP contact to implant can be as much as six months 39
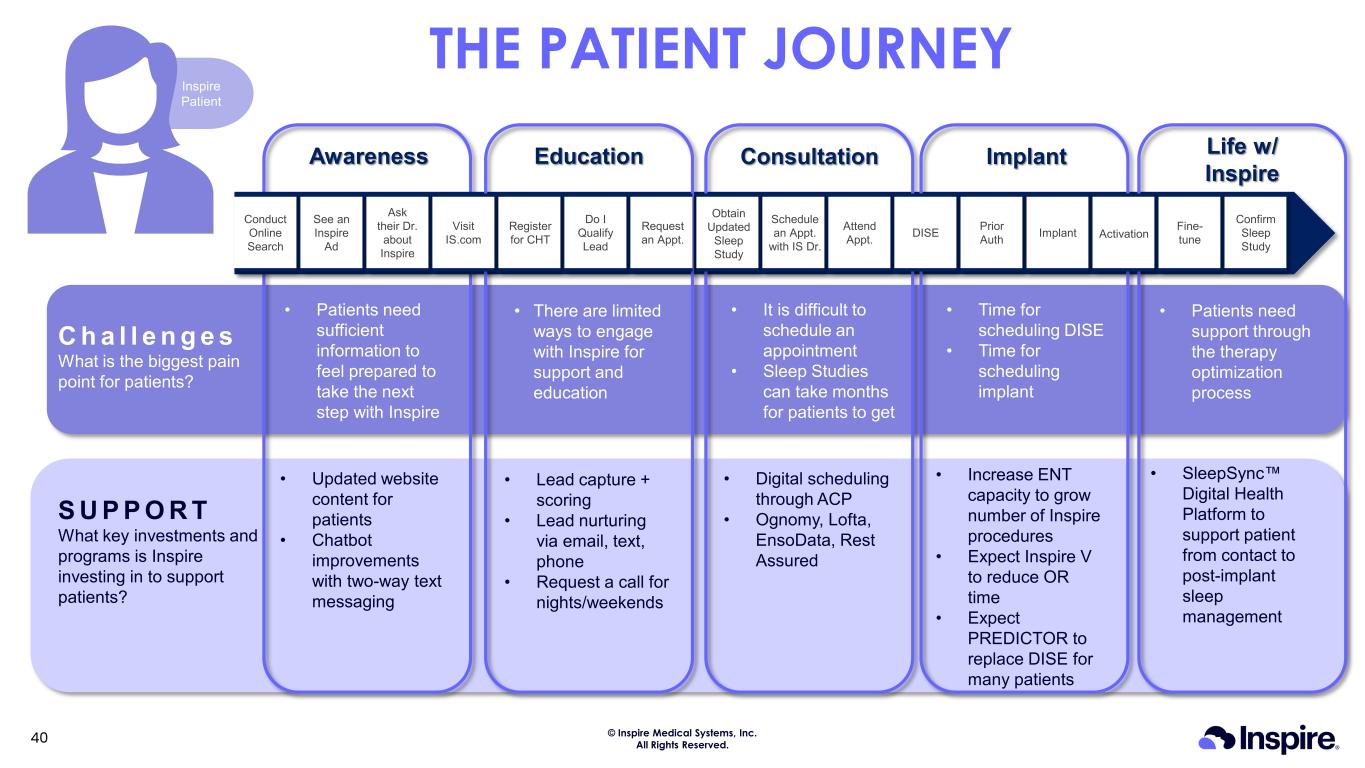
© Inspire Medical Systems, Inc. All Rights Reserved. Inspire Patient THE PATIENT JOURNEY Awareness Education Consultation Implant Life w/ Inspire Confirm Sleep Study Fine- tuneActivationImplantPrior AuthDISE Conduct Online Search Attend Appt. Schedule an Appt. with IS Dr. Obtain Updated Sleep Study Request an Appt. Do I Qualify Lead Register for CHT Visit IS.com Ask their Dr. about Inspire See an Inspire Ad C h a l l e n g e s What is the biggest pain point for patients? S U P P O R T What key investments and programs is Inspire investing in to support patients? • Patients need sufficient information to feel prepared to take the next step with Inspire • There are limited ways to engage with Inspire for support and education • It is difficult to schedule an appointment • Sleep Studies can take months for patients to get • Time for scheduling DISE • Time for scheduling implant • Patients need support through the therapy optimization process • Lead capture + scoring • Lead nurturing via email, text, phone • Request a call for nights/weekends • Updated website content for patients • Chatbot improvements with two-way text messaging • Digital scheduling through ACP • Ognomy, Lofta, EnsoData, Rest Assured • Increase ENT capacity to grow number of Inspire procedures • Expect Inspire V to reduce OR time • Expect PREDICTOR to replace DISE for many patients • SleepSync Digital Health Platform to support patient from contact to post-implant sleep management 40
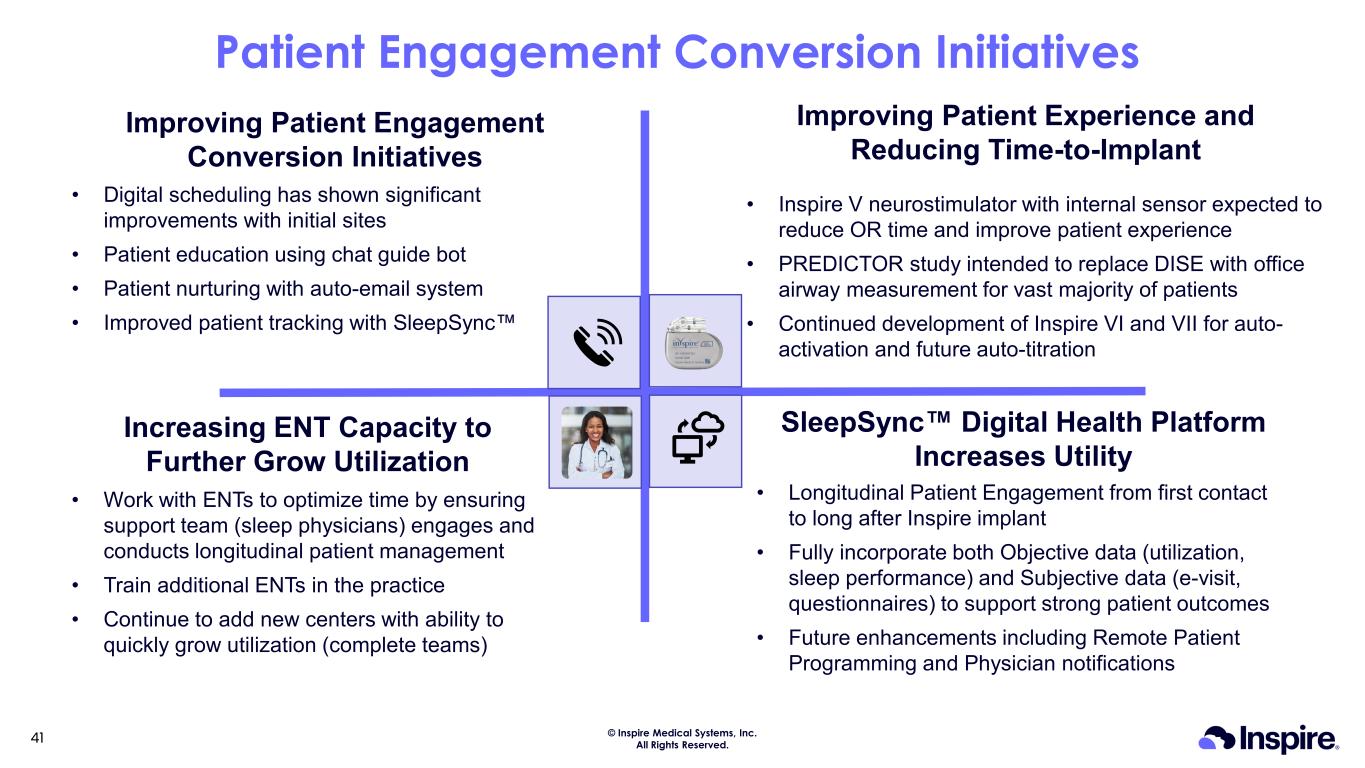
© Inspire Medical Systems, Inc. All Rights Reserved. Patient Engagement Conversion Initiatives Improving Patient Engagement Conversion Initiatives Increasing ENT Capacity to Further Grow Utilization SleepSync Digital Health Platform Increases Utility • Digital scheduling has shown significant improvements with initial sites • Patient education using chat guide bot • Patient nurturing with auto-email system • Improved patient tracking with SleepSync • Work with ENTs to optimize time by ensuring support team (sleep physicians) engages and conducts longitudinal patient management • Train additional ENTs in the practice • Continue to add new centers with ability to quickly grow utilization (complete teams) • Longitudinal Patient Engagement from first contact to long after Inspire implant • Fully incorporate both Objective data (utilization, sleep performance) and Subjective data (e-visit, questionnaires) to support strong patient outcomes • Future enhancements including Remote Patient Programming and Physician notifications Improving Patient Experience and Reducing Time-to-Implant • Inspire V neurostimulator with internal sensor expected to reduce OR time and improve patient experience • PREDICTOR study intended to replace DISE with office airway measurement for vast majority of patients • Continued development of Inspire VI and VII for auto- activation and future auto-titration 41
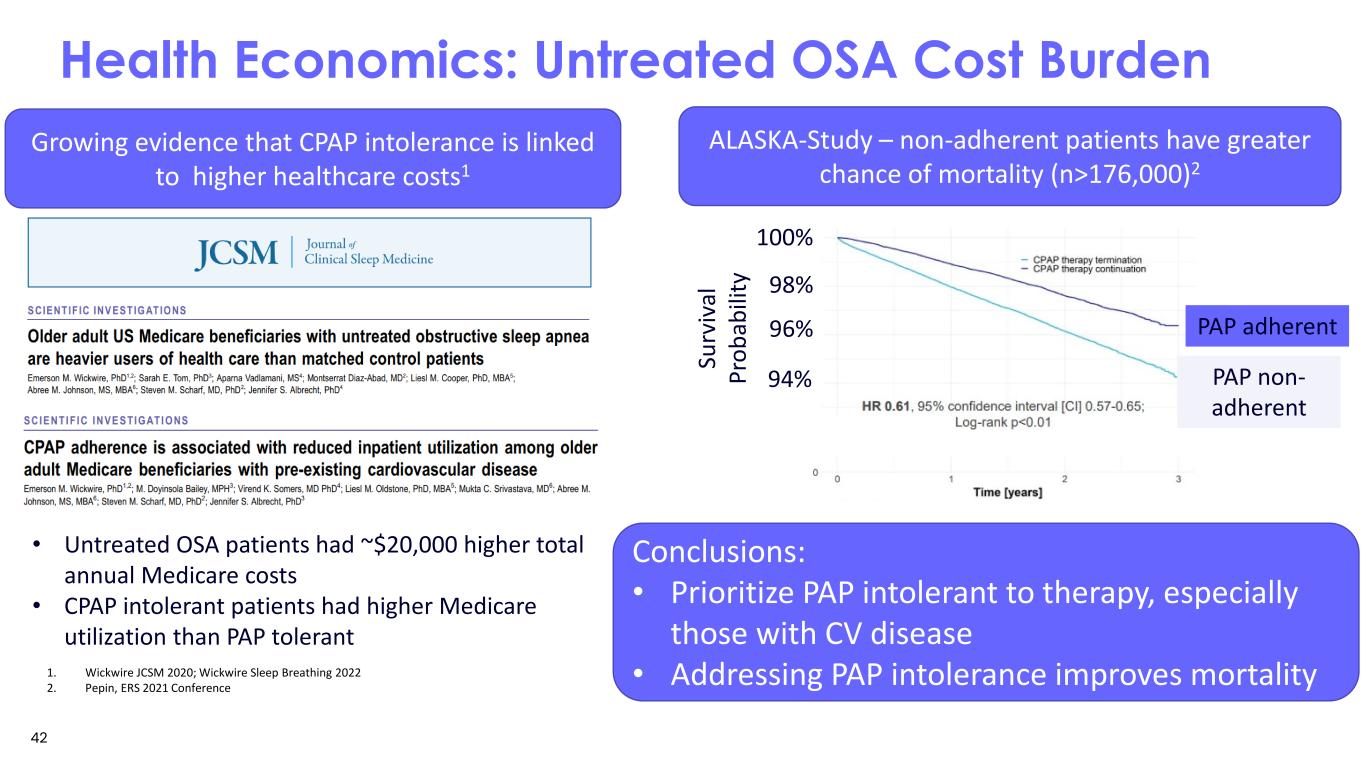
Health Economics: Untreated OSA Cost Burden • Untreated OSA patients had ~$20,000 higher total annual Medicare costs • CPAP intolerant patients had higher Medicare utilization than PAP tolerant ALASKA-Study – non-adherent patients have greater chance of mortality (n>176,000)2 PAP non- adherent PAP adherent96% 98% 100% Su rv iv al Pr ob ab ili ty Conclusions: • Prioritize PAP intolerant to therapy, especially those with CV disease • Addressing PAP intolerance improves mortality1. Wickwire JCSM 2020; Wickwire Sleep Breathing 2022 2. Pepin, ERS 2021 Conference Growing evidence that CPAP intolerance is linked to higher healthcare costs1 94% 42

Sustainability at Inspire Committed to improving the economic, social, and environmental impacts that our business has on the communities in which we operate, as well as our customers, business partners, suppliers, employees, and stockholders. E N V I R O N M E N TA L We work to operate our business responsibly and reduce our impact on the environment wherever feasible. • Our Board and executive officers are responsible for oversight, identification, and communication of climate-related risks and opportunities. • We are focused on building out foundational programmatic elements and oversight that enable meaningful future reductions in our environmental impact. S O C I A L Product safety and quality are of the utmost importance at Inspire. We also pride ourselves on our innovative and collaborative work environment, which we believe has driven our success and which we seek to uphold through an inclusive workforce, generous compensation and benefits, open communication, a focus on employee health, well- being and engagement, and robust training and development programs. • Our company’s success is built on our enduring commitment to product quality and patient outcomes. • InspireGives is our community outreach program and the foundation of our charitable giving and volunteer efforts. • We aim to foster a culture of continuous learning with significant investments in our people through programs focused on leadership and professional development. G O V E R N A N C E We strive to maintain strong governance practices and high standards of ethics, compliance, and accountability designed to provide long-term value creation opportunities. • Our governance practices include regular consideration and assessment of our governance structure, board and committee function, and board and management succession. • Our strong and diverse Board collectively possesses a range of qualifications, skills, and experiences that align with our long-term strategy and business needs. • Sustainability matters are overseen by our Board, executive leadership, and cross- functional team.

© Inspire Medical Systems, Inc. All Rights Reserved. Our Intellectual Property Portfolio (as of December 31, 2025) Covers aspects of our current Inspire system and future product concepts 119 issued U.S. patents (expiring between 2029 and 2043) and 73 pending U.S. patent applications 83 issued foreign patents and 73 pending foreign patent applications 178 pending and registered trademark filings worldwide Competitive position enhanced by trade secrets, proprietary know-how and continuing technological innovation Entered into an agreement with Medtronic in 2007 to make, use, import, and sell products and practice methods in the field of electrical stimulation of the upper airway for the treatment of OSA Royalty-free license agreement Perpetual license (no right of termination) 44