
Exhibit 99.2 Phase 2 Trial of VTX3232 in Participants with Obesity and Cardiovascular Risk Factors Topline Results October 22, 2025

Forward Looking Statements Ventyx Biosciences, Inc. (“Ventyx” or the “Company”) cautions you that statements contained in this presentation regarding matters that are not historical facts are forward-looking statements. These statements are based on the Company’s current beliefs and expectations. Such forward-looking statements include, but are not limited to, statements regarding: the potential of VTX3232 as a therapy for cardiovascular disease, including the potential of meaningful value creation through past and future clinical trial results, the ability of VTX3232 to become a new generation of oral anti-inflammatory therapy, the potential for the combination of VTX3232 and semaglutide to serve as a powerful adjunct therapy to GLP-1 in appropriate patients, VTX3232 having an optimal benefit-risk profile, the potential for VTX3232 to emerge as a best-in-class NLRP3 inhibitor, management’s plans with respect to further development of VTX3232, and management’s plans with respect to future presentations of VTX3232 data. The inclusion of forward-looking statements should not be regarded as a representation by Ventyx that any of its plans will be achieved. Actual results may differ from those set forth in this press release due to the risks and uncertainties inherent in Ventyx’s business, including, without limitation: potential delays in the commencement, enrollment and completion of clinical trials; Ventyx’s dependence on third parties in connection with product manufacturing, research and preclinical and clinical testing; disruptions in the supply chain, including raw materials needed for manufacturing and animals used in research, delays in site activations and enrollment of clinical trials; the results of preclinical studies and clinical trials; early clinical trials not necessarily being predictive of future results; interim results not necessarily being predictive of final results; the potential of one or more outcomes to materially change as a trial continues and more patient data become available, and following more comprehensive audit and verification procedures; regulatory developments in the United States and foreign countries; economic uncertainty in global markets caused by, among other things, geopolitical conditions, tariffs, military conflicts, and inflation volatility; unexpected adverse side effects or inadequate efficacy of Ventyx’s product candidates that may limit their development, regulatory approval and/or commercialization, or may result in recalls or product liability claims; Ventyx’s ability to obtain and maintain intellectual property protection for its product candidates; the use of capital resources by Ventyx sooner than expected; and other risks described in Ventyx’s prior press releases and Ventyx’s filings with the Securities and Exchange Commission (SEC), including in Part II, Item 1A (Risk Factors) of Ventyx’s Quarterly Report on Form 10-Q for the quarter year ended June 30, 2025, filed on August 7, 2025, and Ventyx’s subsequent filings with the SEC. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date hereof, and Ventyx undertakes no obligation to update such statements to reflect events that occur or circumstances that exist after the date hereof. All forward-looking statements are qualified in their entirety by this cautionary statement, which is made under the safe harbor provisions of the Private Securities Litigation Reform Act of 1995 This presentation includes statistical and other industry and market data that we obtained from industry publications and research, surveys and studies conducted by third parties as well as our own estimates of potential market opportunities. Industry publications and third-party research, surveys and studies generally indicate that their information has been obtained from sources believed to be reliable, although they do not guarantee the accuracy or completeness of such information. Our estimates of the potential market opportunities for our products include several key assumptions based on our industry knowledge, industry publications, third-party research and other surveys, which may be based on a small sample size and may fail to accurately reflect market opportunities. While we believe that our internal assumptions are reliable, such assumptions have not been verified by any third party. The industry in which we operate is subject to a high degree of uncertainty and risk due to a variety of important factors that could cause results to differ materially from those expressed in the estimates made by third parties and by us. Trademarks in this presentation are the property of their respective owners and used for informational and education purposes only. 2

Today’s Presenters Ventyx Management Team Guest Speakers and KOLs Raju Mohan, PhD Mark Forman, MD, PhD Matthew Moore Peter Libby, MD Antonio Abbate, MD, PhD CHIEF EXECUTIVE OFFICER, CHIEF MEDICAL OFFICER CHIEF OPERATING OFFICER MASS GENERAL BRIGHAM UNIVERSITY OF VIRGINIA, FOUNDER HEART & VASCULAR INSTITUTE SCHOOL OF MEDICINE 3

VTX3232 Phase 2 Trial Top-Line Results and Key Takeaways • Rapid and sustained reductions in hsCRP with VTX3232 vs. placebo • Statistically significant decrease in hsCRP of up to 80% within first week of treatment • Majority of participants achieved hsCRP levels of <2 mg/L at Week 12, a critical threshold for 1 determining CV risk 2 • Statistically significant reductions in IL-6 to levels below threshold for CV risk • Statistically significant reductions in Lipoprotein(a) • Statistically significant reduction in liver inflammation as measured with cT1 MRI • Clinically meaningful benefit on inflammation as add-on to semaglutide • VTX3232 was safe and well tolerated in this study • Rates of adverse events comparable to placebo (monotherapy and as add-on to semaglutide) • No effect on weight loss either as monotherapy or as add-on to semaglutide 1 2 Ridker P.M. et. al. Inflammation and cholesterol as predictors of cardiovascular events. Lancet 2023; 401:1293-301; Ridker P.M. et. al. Modulation of the interleukin-6 signalling pathway and incidence rates of ASCVD and all-cause 4 mortality, Eur Heart J,2018. CV, cardiovascular; cT1, corrected T1 imaging; MRI, magnetic resonance imaging; hsCRP, high-sensitivity C-reactive protein;
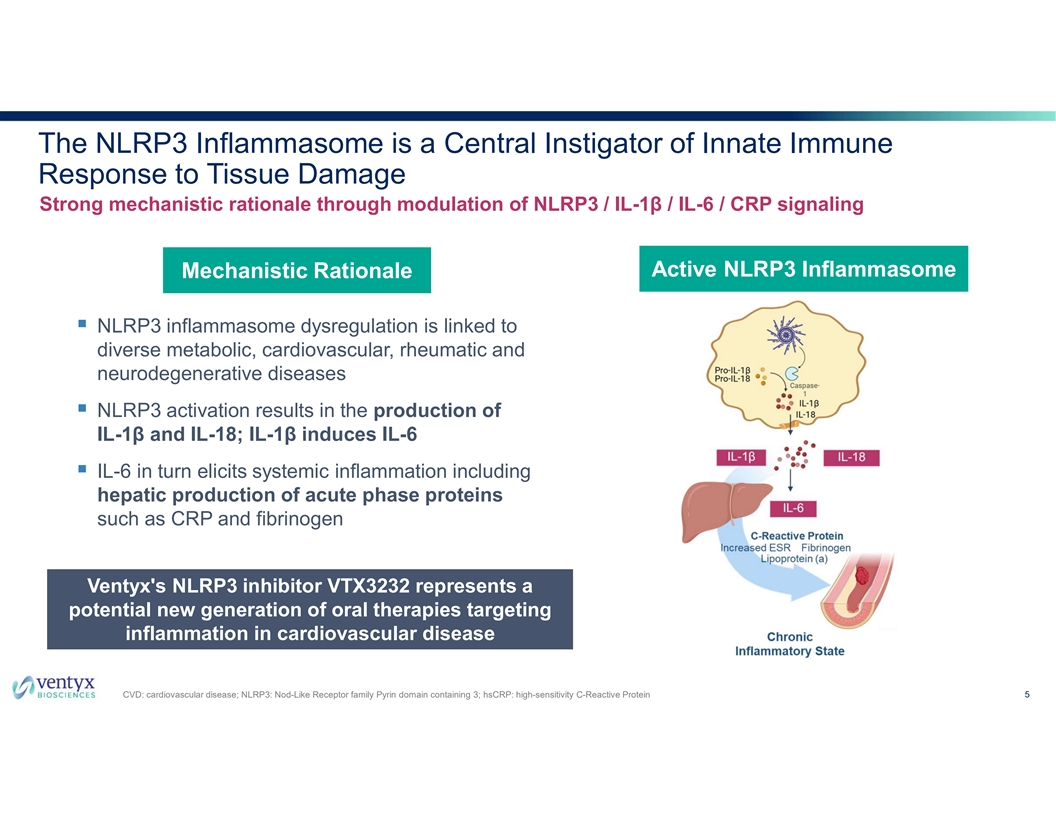
The NLRP3 Inflammasome is a Central Instigator of Innate Immune Response to Tissue Damage Strong mechanistic rationale through modulation of NLRP3 / IL-1β / IL-6 / CRP signaling Active NLRP3 Inflammasome Mechanistic Rationale § NLRP3 inflammasome dysregulation is linked to diverse metabolic, cardiovascular, rheumatic and neurodegenerative diseases § NLRP3 activation results in the production of IL-1β and IL-18; IL-1β induces IL-6 § IL-6 in turn elicits systemic inflammation including hepatic production of acute phase proteins such as CRP and fibrinogen Ventyx's NLRP3 inhibitor VTX3232 represents a potential new generation of oral therapies targeting inflammation in cardiovascular disease CVD: cardiovascular disease; NLRP3: Nod-Like Receptor family Pyrin domain containing 3; hsCRP: high-sensitivity C-Reactive Protein 5

Mark Forman, MD, PhD Chief Medical Officer
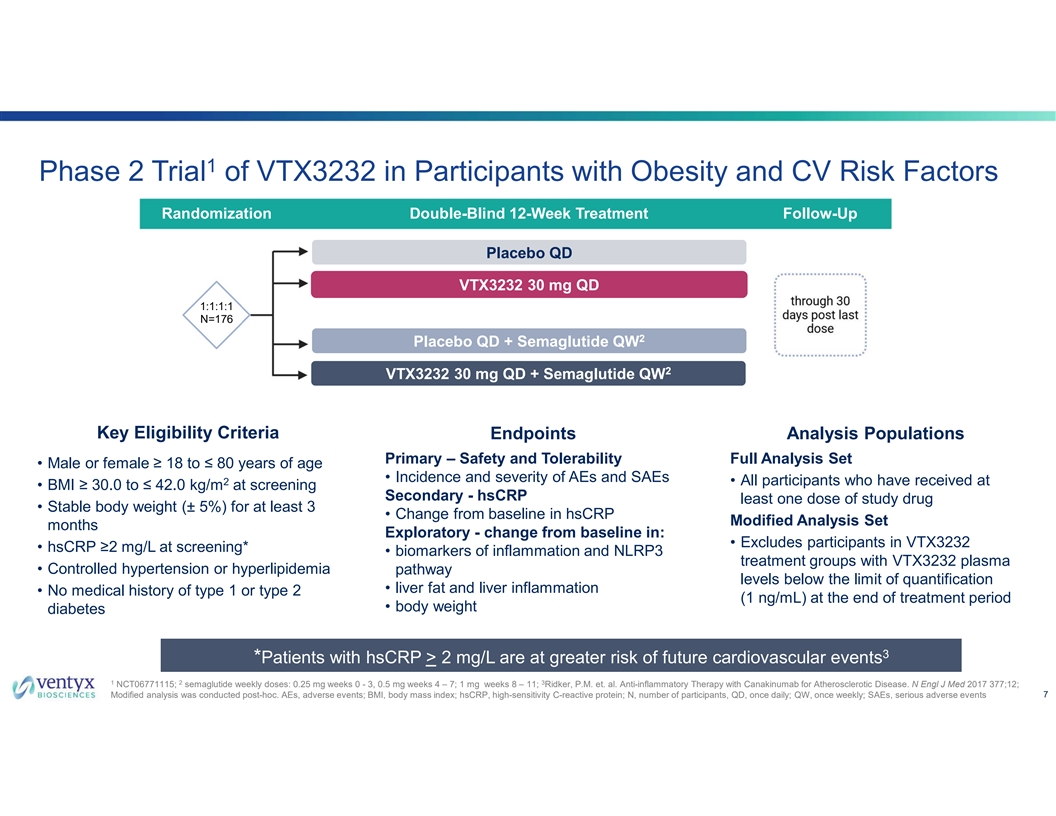
1 Phase 2 Trial of VTX3232 in Participants with Obesity and CV Risk Factors Randomization Double-Blind 12-Week Treatment Follow-Up Placebo QD VTX3232 30 mg QD 1:1:1:1 N=176 * 2 Placebo QD + Semaglutide QW * * 2 VTX3232 30 mg QD + Semaglutide QW * Key Eligibility Criteria Endpoints Analysis Populations Primary – Safety and Tolerability Full Analysis Set • Male or female ≥ 18 to ≤ 80 years of age • Incidence and severity of AEs and SAEs 2 • All participants who have received at • BMI ≥ 30.0 to ≤ 42.0 kg/m at screening Secondary - hsCRP least one dose of study drug • Stable body weight (± 5%) for at least 3 • Change from baseline in hsCRP Modified Analysis Set months Exploratory - change from baseline in: • Excludes participants in VTX3232 • hsCRP ≥2 mg/L at screening* • biomarkers of inflammation and NLRP3 treatment groups with VTX3232 plasma • Controlled hypertension or hyperlipidemia pathway levels below the limit of quantification • liver fat and liver inflammation • No medical history of type 1 or type 2 (1 ng/mL) at the end of treatment period • body weight diabetes 3 *Patients with hsCRP > 2 mg/L are at greater risk of future cardiovascular events 1 2 3 NCT06771115; semaglutide weekly doses: 0.25 mg weeks 0 - 3, 0.5 mg weeks 4 – 7; 1 mg weeks 8 – 11; Ridker, P.M. et. al. Anti-inflammatory Therapy with Canakinumab for Atherosclerotic Disease. N Engl J Med 2017 377;12; 7 Modified analysis was conducted post-hoc. AEs, adverse events; BMI, body mass index; hsCRP, high-sensitivity C-reactive protein; N, number of participants, QD, once daily; QW, once weekly; SAEs, serious adverse events
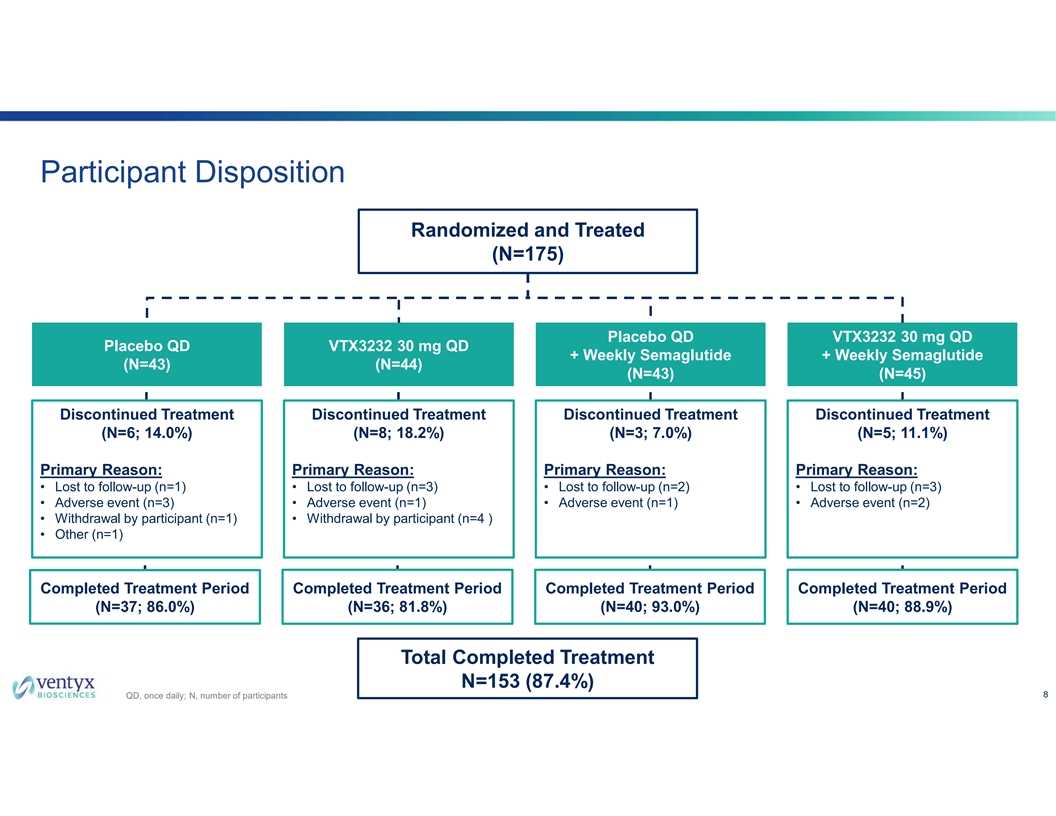
Participant Disposition Randomized and Treated (N=175) P Plla ac ce eb bo o Q QD D V VT TX X3 32 23 32 2 3 30 0 m mg g Q QD D P Plla ac ce eb bo o Q QD D V VT TX X3 32 23 32 2 3 30 0 m mg g Q QD D + + W We ee ek klly y S Se em ma ag gllu ut tiid de e + + W We ee ek klly y S Se em ma ag gllu ut tiid de e ( (N N= =4 43 3) ) ( (N N= =4 44 4) ) ( (N N= =4 43 3) ) ( (N N= =4 45 5) ) Discontinued Treatment Discontinued Treatment Discontinued Treatment Discontinued Treatment (N=6; 14.0%) (N=8; 18.2%) (N=3; 7.0%) (N=5; 11.1%) Primary Reason: Primary Reason: Primary Reason: Primary Reason: • Lost to follow-up (n=1) • Lost to follow-up (n=3) • Lost to follow-up (n=2) • Lost to follow-up (n=3) • Adverse event (n=3) • Adverse event (n=1) • Adverse event (n=1) • Adverse event (n=2) • Withdrawal by participant (n=1) • Withdrawal by participant (n=4 ) • Other (n=1) Completed Treatment Period Completed Treatment Period Completed Treatment Period Completed Treatment Period (N=37; 86.0%) (N=36; 81.8%) (N=40; 93.0%) (N=40; 88.9%) Total Completed Treatment N=153 (87.4%) 8 QD, once daily; N, number of participants
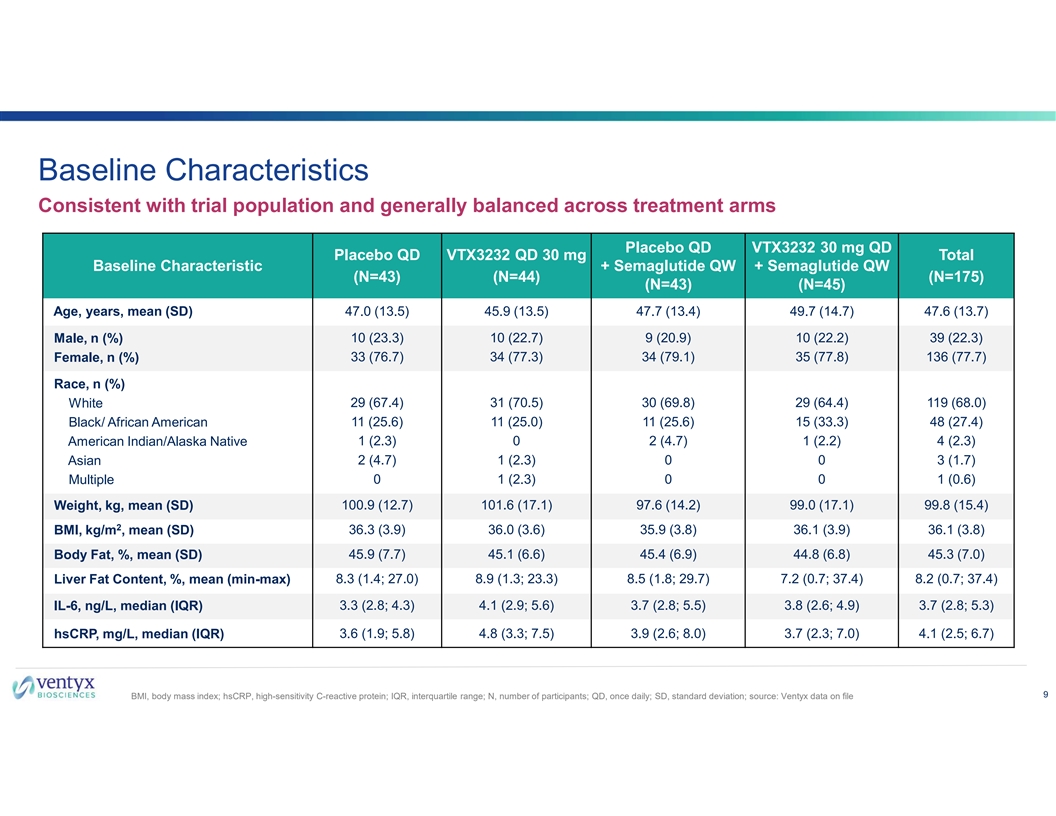
Baseline Characteristics Consistent with trial population and generally balanced across treatment arms Placebo QD VTX3232 30 mg QD Placebo QD VTX3232 QD 30 mg Total Baseline Characteristic + Semaglutide QW + Semaglutide QW (N=43) (N=44) (N=175) (N=43) (N=45) Age, years, mean (SD) 47.0 (13.5) 45.9 (13.5) 47.7 (13.4) 49.7 (14.7) 47.6 (13.7) Male, n (%) 10 (23.3) 10 (22.7) 9 (20.9) 10 (22.2) 39 (22.3) 33 (76.7) 34 (77.3) 34 (79.1) 35 (77.8) 136 (77.7) Female, n (%) Race, n (%) White 29 (67.4) 31 (70.5) 30 (69.8) 29 (64.4) 119 (68.0) Black/ African American 11 (25.6) 11 (25.0) 11 (25.6) 15 (33.3) 48 (27.4) 1 (2.3) 0 2 (4.7) 1 (2.2) 4 (2.3) American Indian/Alaska Native Asian 2 (4.7) 1 (2.3) 0 0 3 (1.7) Multiple 0 1 (2.3) 0 0 1 (0.6) 100.9 (12.7) 101.6 (17.1) 97.6 (14.2) 99.0 (17.1) 99.8 (15.4) Weight, kg, mean (SD) 2 BMI, kg/m , mean (SD) 36.3 (3.9) 36.0 (3.6) 35.9 (3.8) 36.1 (3.9) 36.1 (3.8) 45.9 (7.7) 45.1 (6.6) 45.4 (6.9) 44.8 (6.8) 45.3 (7.0) Body Fat, %, mean (SD) Liver Fat Content, %, mean (min-max) 8.3 (1.4; 27.0) 8.9 (1.3; 23.3) 8.5 (1.8; 29.7) 7.2 (0.7; 37.4) 8.2 (0.7; 37.4) IL-6, ng/L, median (IQR) 3.3 (2.8; 4.3) 4.1 (2.9; 5.6) 3.7 (2.8; 5.5) 3.8 (2.6; 4.9) 3.7 (2.8; 5.3) hsCRP, mg/L, median (IQR) 3.6 (1.9; 5.8) 4.8 (3.3; 7.5) 3.9 (2.6; 8.0) 3.7 (2.3; 7.0) 4.1 (2.5; 6.7) 9 BMI, body mass index; hsCRP, high-sensitivity C-reactive protein; IQR, interquartile range; N, number of participants; QD, once daily; SD, standard deviation; source: Ventyx data on file
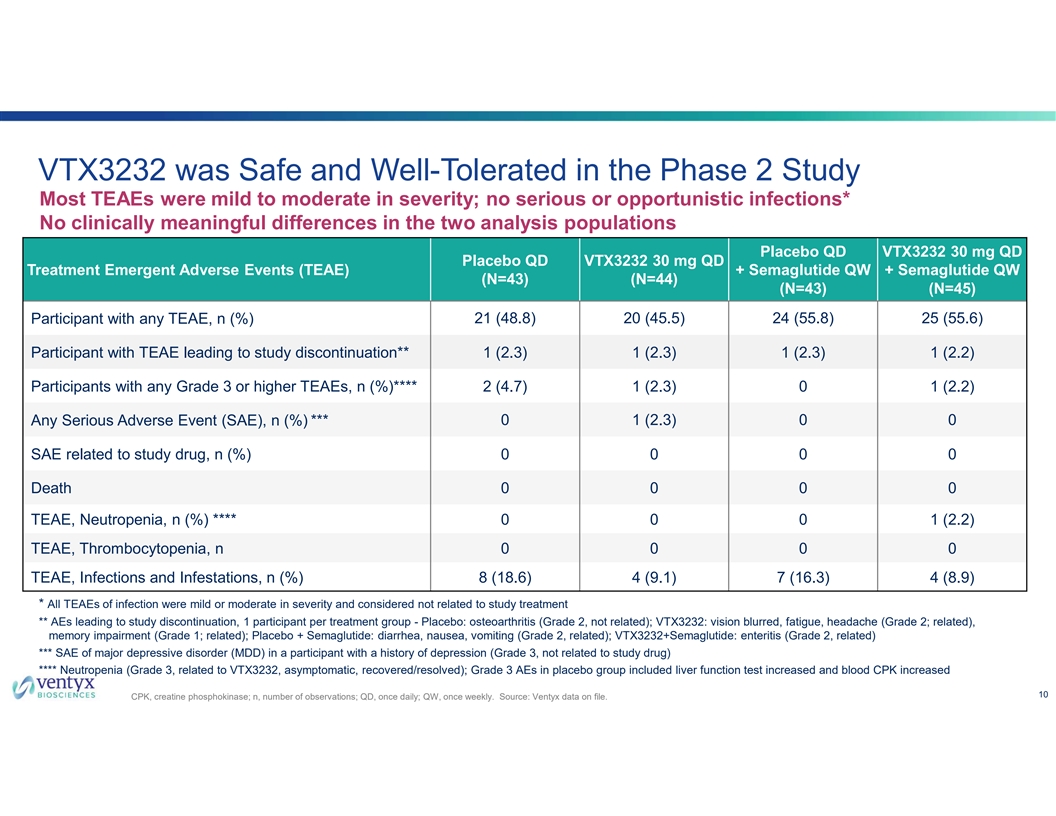
VTX3232 was Safe and Well-Tolerated in the Phase 2 Study Most TEAEs were mild to moderate in severity; no serious or opportunistic infections* No clinically meaningful differences in the two analysis populations Placebo QD VTX3232 30 mg QD Placebo QD VTX3232 30 mg QD Treatment Emergent Adverse Events (TEAE) + Semaglutide QW + Semaglutide QW (N=43) (N=44) (N=43) (N=45) Participant with any TEAE, n (%) 21 (48.8) 20 (45.5) 24 (55.8) 25 (55.6) Participant with TEAE leading to study discontinuation** 1 (2.3) 1 (2.3) 1 (2.3) 1 (2.2) Participants with any Grade 3 or higher TEAEs, n (%)**** 2 (4.7) 1 (2.3) 0 1 (2.2) Any Serious Adverse Event (SAE), n (%) *** 0 1 (2.3) 0 0 SAE related to study drug, n (%) 0 0 0 0 Death 0 0 0 0 TEAE, Neutropenia, n (%) **** 0 0 0 1 (2.2) TEAE, Thrombocytopenia, n 0 0 0 0 TEAE, Infections and Infestations, n (%) 8 (18.6) 4 (9.1) 7 (16.3) 4 (8.9) * All TEAEs of infection were mild or moderate in severity and considered not related to study treatment ** AEs leading to study discontinuation, 1 participant per treatment group - Placebo: osteoarthritis (Grade 2, not related); VTX3232: vision blurred, fatigue, headache (Grade 2; related), memory impairment (Grade 1; related); Placebo + Semaglutide: diarrhea, nausea, vomiting (Grade 2, related); VTX3232+Semaglutide: enteritis (Grade 2, related) *** SAE of major depressive disorder (MDD) in a participant with a history of depression (Grade 3, not related to study drug) **** Neutropenia (Grade 3, related to VTX3232, asymptomatic, recovered/resolved); Grade 3 AEs in placebo group included liver function test increased and blood CPK increased 10 CPK, creatine phosphokinase; n, number of observations; QD, once daily; QW, once weekly. Source: Ventyx data on file.
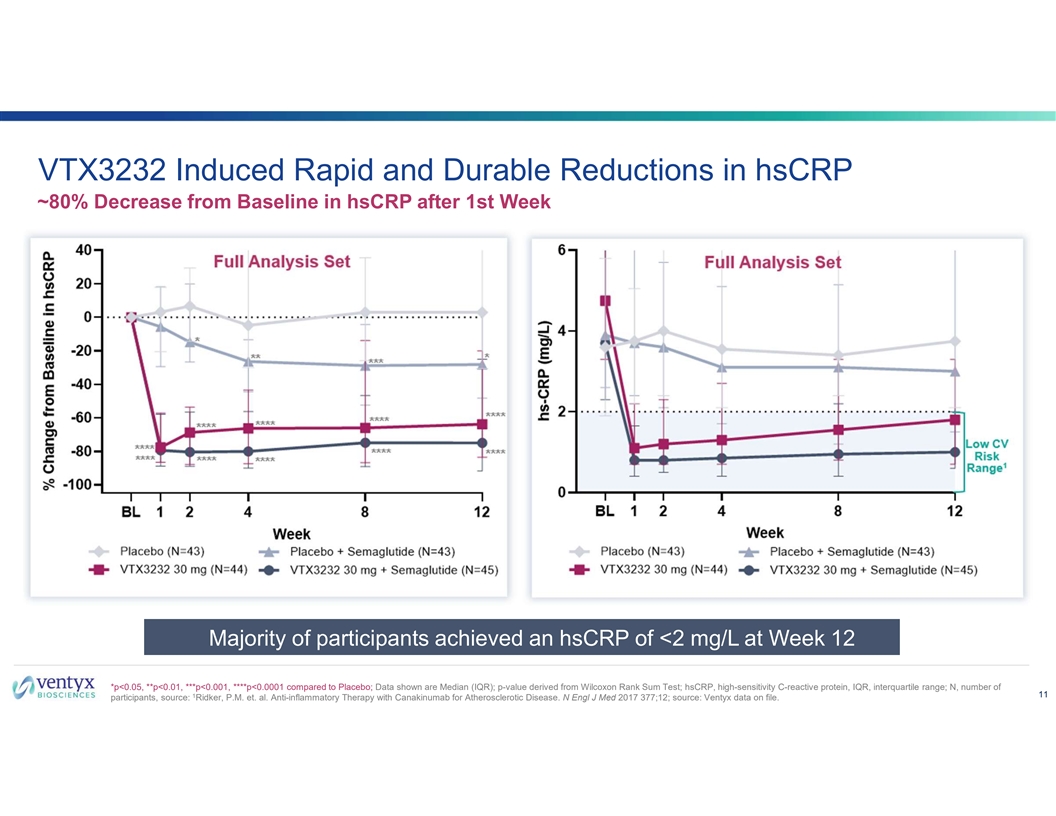
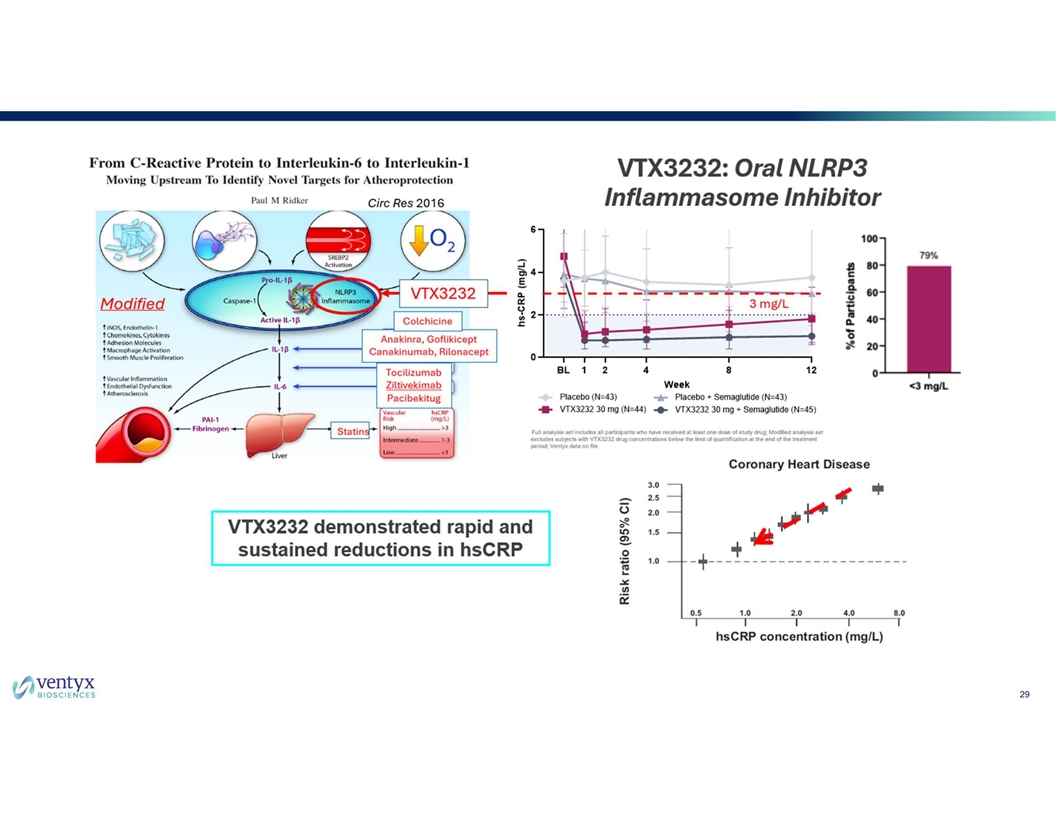
VTX3232 Induced Rapid and Durable Reductions in hsCRP ~80% Decrease from Baseline in hsCRP after 1st Week Majority of participants achieved an hsCRP of <2 mg/L at Week 12 *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001 compared to Placebo; Data shown are Median (IQR); p-value derived from Wilcoxon Rank Sum Test; hsCRP, high-sensitivity C-reactive protein, IQR, interquartile range; N, number of 11 1 participants, source: Ridker, P.M. et. al. Anti-inflammatory Therapy with Canakinumab for Atherosclerotic Disease. N Engl J Med 2017 377;12; source: Ventyx data on file.
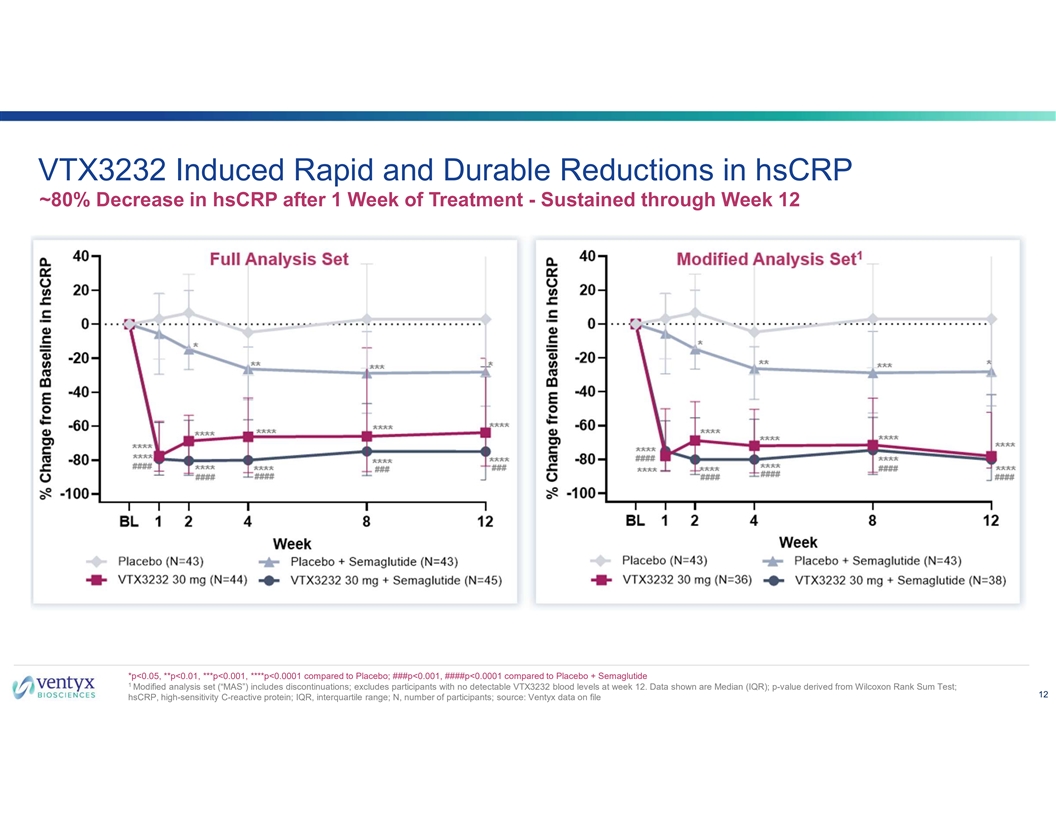
VTX3232 Induced Rapid and Durable Reductions in hsCRP ~80% Decrease in hsCRP after 1 Week of Treatment - Sustained through Week 12 *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001 compared to Placebo; ###p<0.001, ####p<0.0001 compared to Placebo + Semaglutide 1 Modified analysis set (“MAS”) includes discontinuations; excludes participants with no detectable VTX3232 blood levels at week 12. Data shown are Median (IQR); p-value derived from Wilcoxon Rank Sum Test; 12 hsCRP, high-sensitivity C-reactive protein; IQR, interquartile range; N, number of participants; source: Ventyx data on file
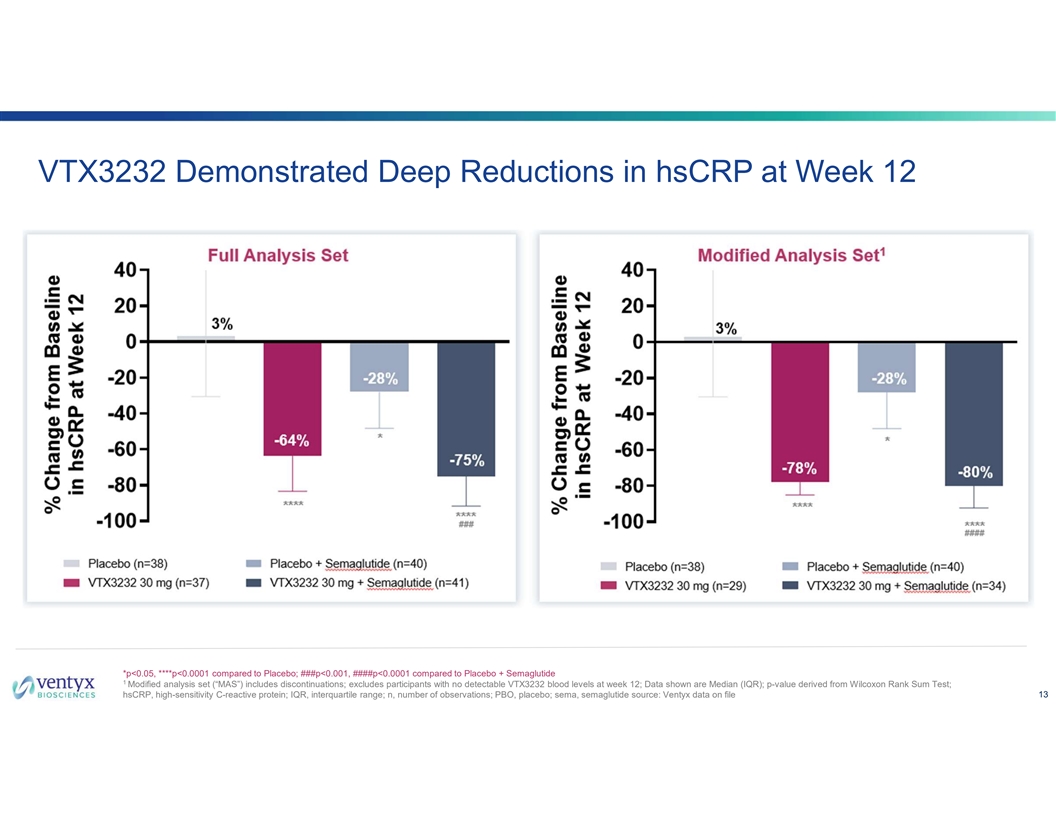
VTX3232 Demonstrated Deep Reductions in hsCRP at Week 12 *p<0.05, ****p<0.0001 compared to Placebo; ###p<0.001, ####p<0.0001 compared to Placebo + Semaglutide 1 Modified analysis set (“MAS”) includes discontinuations; excludes participants with no detectable VTX3232 blood levels at week 12; Data shown are Median (IQR); p-value derived from Wilcoxon Rank Sum Test; hsCRP, high-sensitivity C-reactive protein; IQR, interquartile range; n, number of observations; PBO, placebo; sema, semaglutide source: Ventyx data on file 13
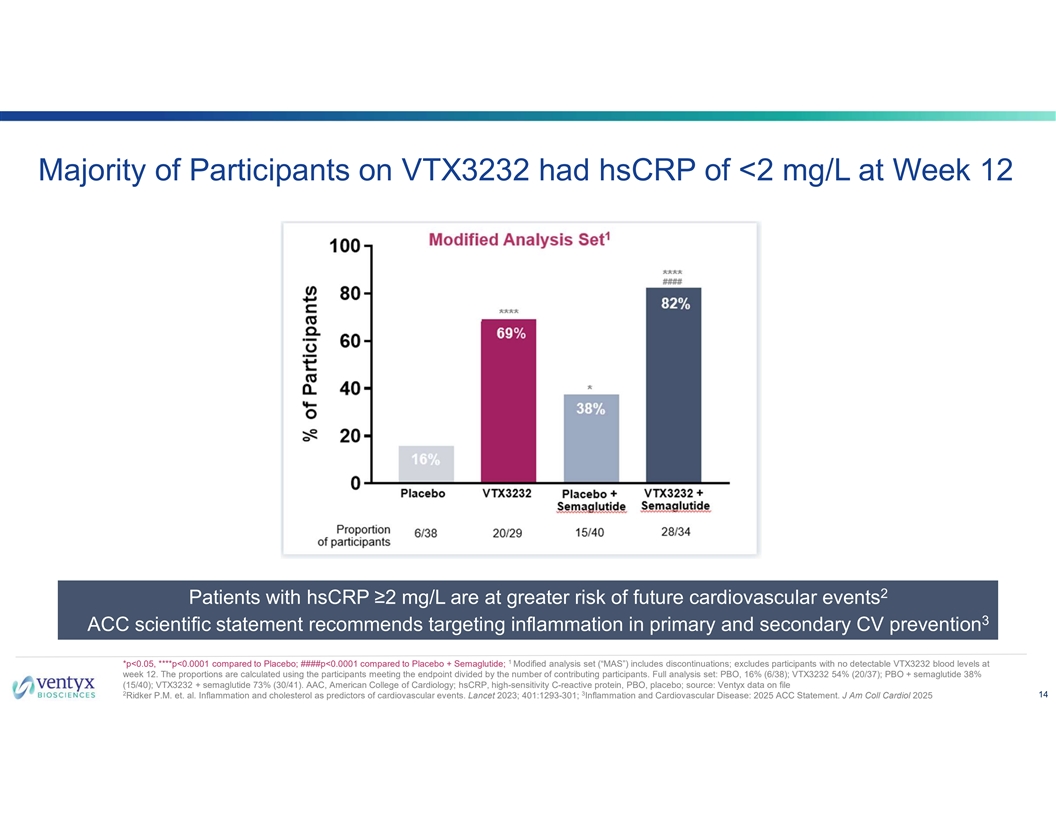
Majority of Participants on VTX3232 had hsCRP of <2 mg/L at Week 12 2 Patients with hsCRP ≥2 mg/L are at greater risk of future cardiovascular events 3 ACC scientific statement recommends targeting inflammation in primary and secondary CV prevention 1 *p<0.05, ****p<0.0001 compared to Placebo; ####p<0.0001 compared to Placebo + Semaglutide; Modified analysis set (“MAS”) includes discontinuations; excludes participants with no detectable VTX3232 blood levels at week 12. The proportions are calculated using the participants meeting the endpoint divided by the number of contributing participants. Full analysis set: PBO, 16% (6/38); VTX3232 54% (20/37); PBO + semaglutide 38% (15/40); VTX3232 + semaglutide 73% (30/41). AAC, American College of Cardiology; hsCRP, high-sensitivity C-reactive protein, PBO, placebo; source: Ventyx data on file 2 3 14 Ridker P.M. et. al. Inflammation and cholesterol as predictors of cardiovascular events. Lancet 2023; 401:1293-301; Inflammation and Cardiovascular Disease: 2025 ACC Statement. J Am Coll Cardiol 2025
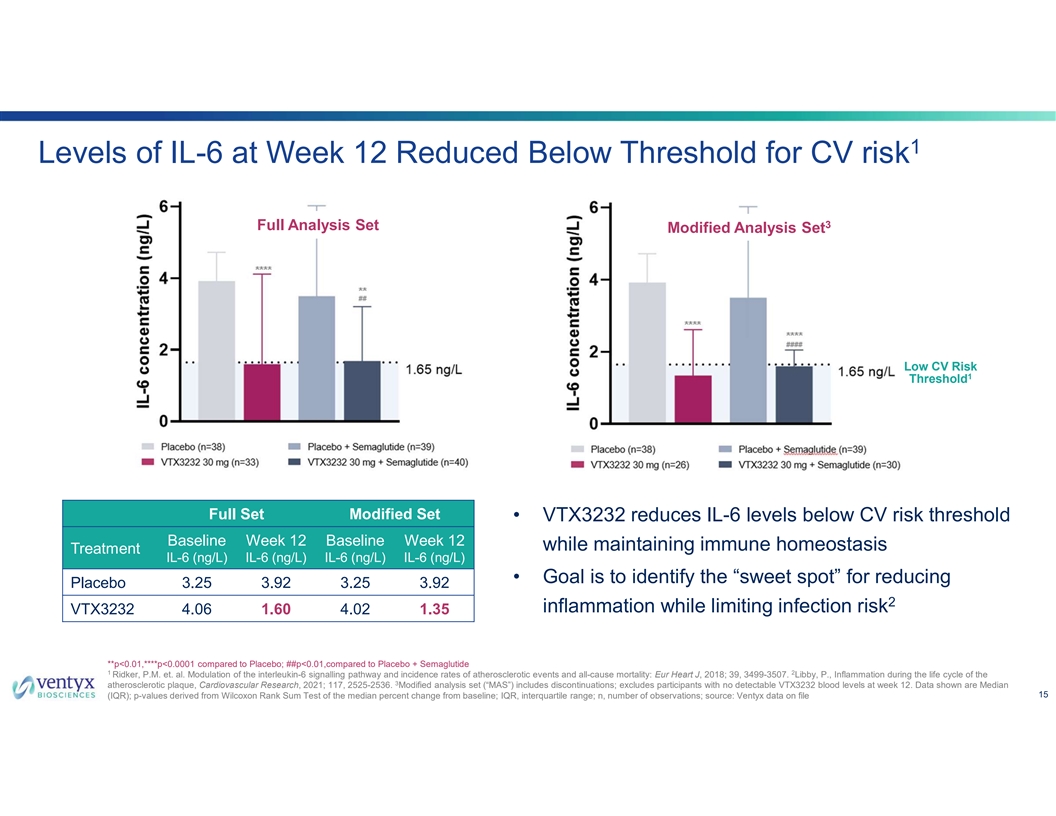
1 Levels of IL-6 at Week 12 Reduced Below Threshold for CV risk 3 Full Analysis Set Modified Analysis Set Low CV Risk 1 Threshold Full Set Modified Set • VTX3232 reduces IL-6 levels below CV risk threshold Baseline Week 12 Baseline Week 12 while maintaining immune homeostasis Treatment IL-6 (ng/L) IL-6 (ng/L) IL-6 (ng/L) IL-6 (ng/L) • Goal is to identify the “sweet spot” for reducing Placebo 3.25 3.92 3.25 3.92 2 inflammation while limiting infection risk VTX3232 4.06 1.60 4.02 1.35 **p<0.01,****p<0.0001 compared to Placebo; ##p<0.01,compared to Placebo + Semaglutide 1 2 Ridker, P.M. et. al. Modulation of the interleukin-6 signalling pathway and incidence rates of atherosclerotic events and all-cause mortality: Eur Heart J, 2018; 39, 3499-3507. Libby, P., Inflammation during the life cycle of the 3 atherosclerotic plaque, Cardiovascular Research, 2021; 117, 2525-2536. Modified analysis set (“MAS”) includes discontinuations; excludes participants with no detectable VTX3232 blood levels at week 12. Data shown are Median 15 (IQR); p-values derived from Wilcoxon Rank Sum Test of the median percent change from baseline; IQR, interquartile range; n, number of observations; source: Ventyx data on file
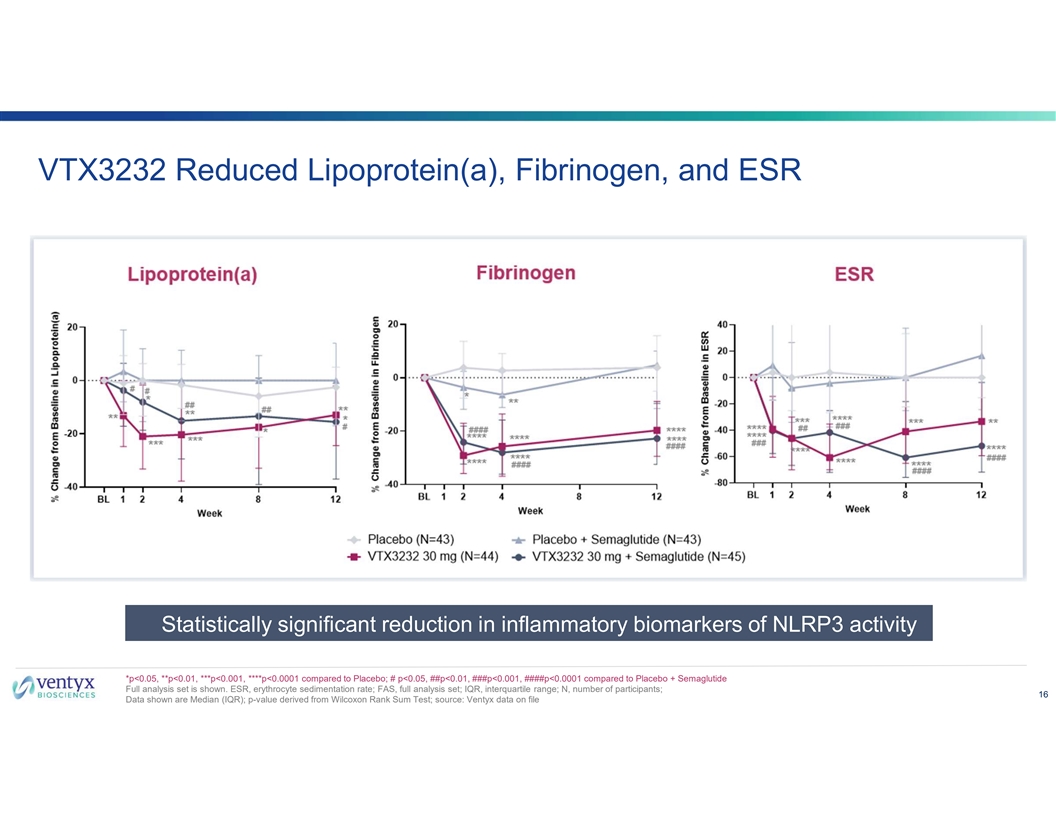
VTX3232 Reduced Lipoprotein(a), Fibrinogen, and ESR Statistically significant reduction in inflammatory biomarkers of NLRP3 activity *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001 compared to Placebo; # p<0.05, ##p<0.01, ###p<0.001, ####p<0.0001 compared to Placebo + Semaglutide Full analysis set is shown. ESR, erythrocyte sedimentation rate; FAS, full analysis set; IQR, interquartile range; N, number of participants; 16 Data shown are Median (IQR); p-value derived from Wilcoxon Rank Sum Test; source: Ventyx data on file
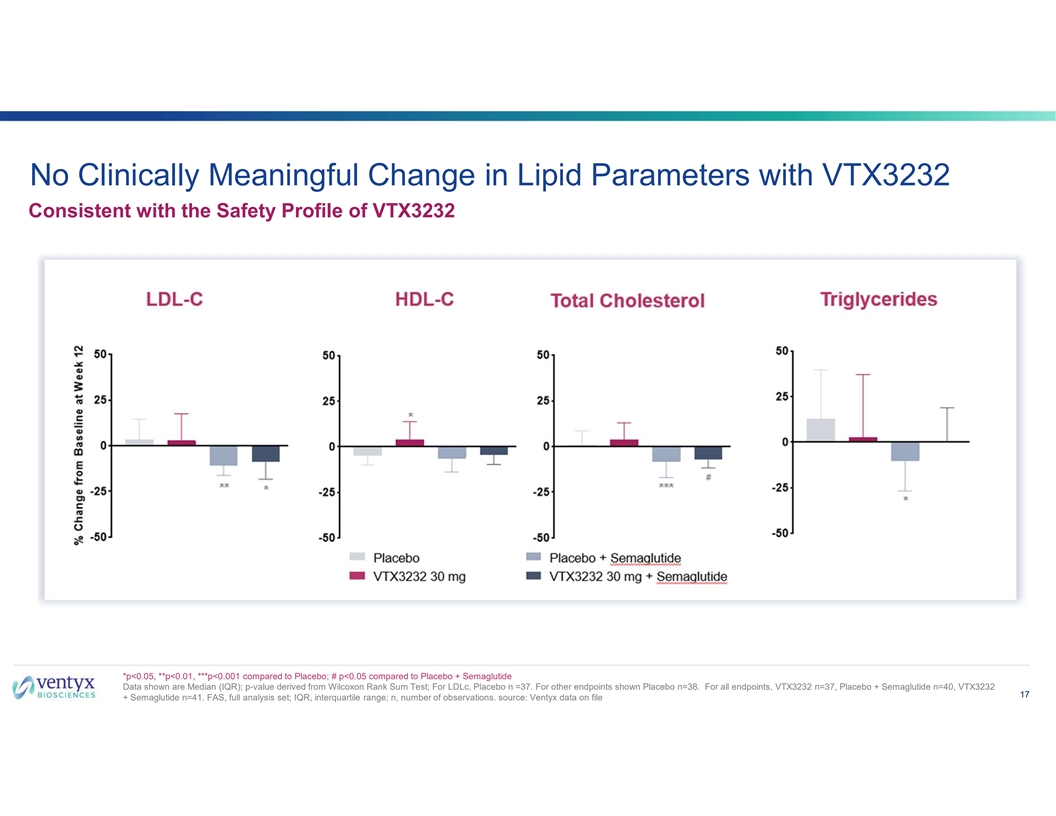
No Clinically Meaningful Change in Lipid Parameters with VTX3232 Consistent with the Safety Profile of VTX3232 *p<0.05, **p<0.01, ***p<0.001 compared to Placebo; # p<0.05 compared to Placebo + Semaglutide Data shown are Median (IQR); p-value derived from Wilcoxon Rank Sum Test; For LDLc, Placebo n =37. For other endpoints shown Placebo n=38. For all endpoints, VTX3232 n=37, Placebo + Semaglutide n=40, VTX3232 17 + Semaglutide n=41. FAS, full analysis set; IQR, interquartile range; n, number of observations. source: Ventyx data on file
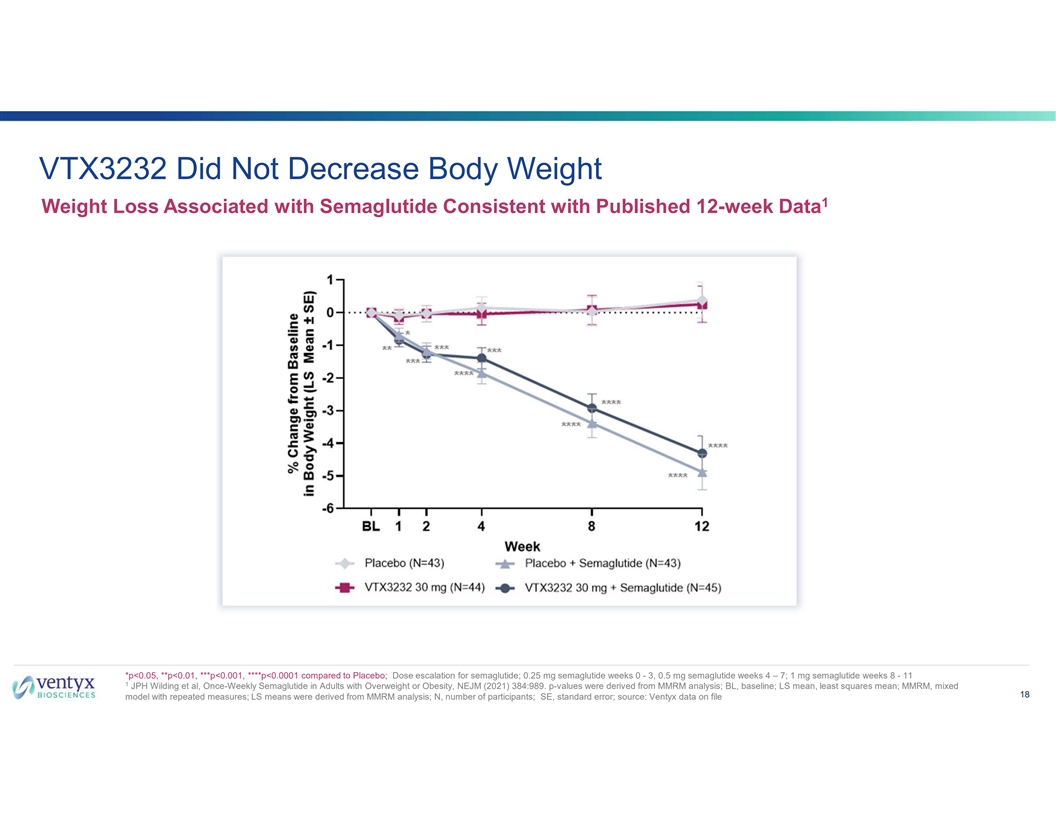
VTX3232 Did Not Decrease Body Weight 1 Weight Loss Associated with Semaglutide Consistent with Published 12-week Data *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001 compared to Placebo; Dose escalation for semaglutide; 0.25 mg semaglutide weeks 0 - 3, 0.5 mg semaglutide weeks 4 – 7; 1 mg semaglutide weeks 8 - 11 1 JPH Wilding et al, Once-Weekly Semaglutide in Adults with Overweight or Obesity, NEJM (2021) 384:989. p-values were derived from MMRM analysis; BL, baseline; LS mean, least squares mean; MMRM, mixed 18 model with repeated measures; LS means were derived from MMRM analysis; N, number of participants; SE, standard error; source: Ventyx data on file
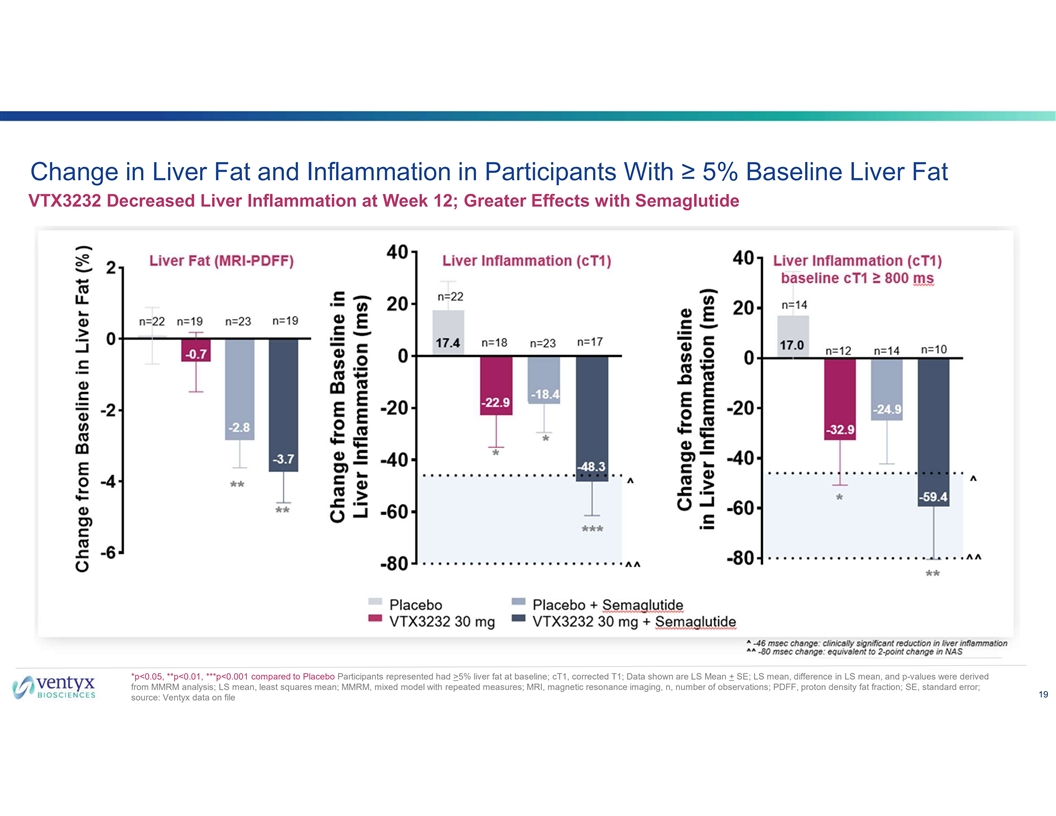
Change in Liver Fat and Inflammation in Participants With ≥ 5% Baseline Liver Fat VTX3232 Decreased Liver Inflammation at Week 12; Greater Effects with Semaglutide *p<0.05, **p<0.01, ***p<0.001 compared to Placebo Participants represented had >5% liver fat at baseline; cT1, corrected T1; Data shown are LS Mean + SE; LS mean, difference in LS mean, and p-values were derived from MMRM analysis; LS mean, least squares mean; MMRM, mixed model with repeated measures; MRI, magnetic resonance imaging, n, number of observations; PDFF, proton density fat fraction; SE, standard error; 19 source: Ventyx data on file
VTX3232: Targeting Inflammation in CV Disease with Optimal Benefit-Risk Profile • Rapid and sustained reductions in hsCRP vs. placebo § Decrease in hsCRP of ~80% within first week of treatment § Achieves target levels of hsCRP reduction in ~70% of participants • Comprehensive inhibition of NLRP3 pathway § Reductions in IL-6 to levels below threshold for cardiovascular risk § Rapid and sustained reductions in acute phase reactants and biomarkers of systemic inflammation § Reduction in liver inflammation assessed with cT1 imaging • Clinically meaningful benefit on inflammation as add-on to semaglutide • VTX3232 was safe and well tolerated in this study § Rates of adverse events comparable to placebo § No evidence of increased risk of infection 20 hsCRP, high sensitivity C-reactive protein;

Raju Mohan, PhD Chief Executive Officer
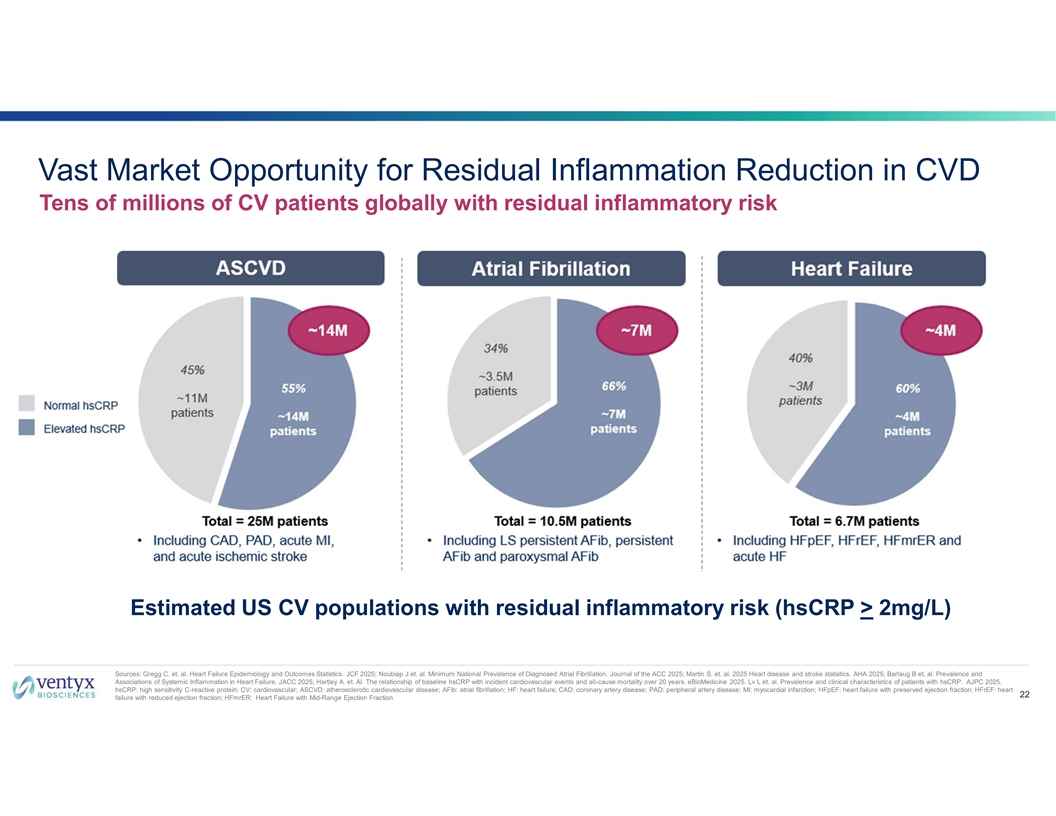
Vast Market Opportunity for Residual Inflammation Reduction in CVD Tens of millions of CV patients globally with residual inflammatory risk Estimated US CV populations with residual inflammatory risk (hsCRP > 2mg/L) Sources: Gregg C. et. al. Heart Failure Epidemiology and Outcomes Statistics. JCF 2025; Noubiap J et. al. Minimum National Prevalence of Diagnosed Atrial Fibrillation. Journal of the ACC 2025; Martin S. et. al. 2025 Heart disease and stroke statistics. AHA 2025; Barlaug B et. al. Prevalence and Associations of Systemic Inflammation in Heart Failure. JACC 2025; Hartley A. et. Al. The relationship of baseline hsCRP with incident cardiovascular events and all-cause mortality over 20 years. eBioMedicine 2025. Lv L et. al. Prevalence and clinical characteristics of patients with hsCRP. AJPC 2025. hsCRP: high sensitivity C-reactive protein; CV: cardiovascular; ASCVD: atherosclerotic cardiovascular disease; AFib: atrial fibrillation; HF: heart failure; CAD: coronary artery disease; PAD: peripheral artery disease; MI: myocardial infarction; HFpEF: heart failure with preserved ejection fraction; HFrEF: heart 22 failure with reduced ejection fraction; HFmrER: Heart Failure with Mid-Range Ejection Fraction
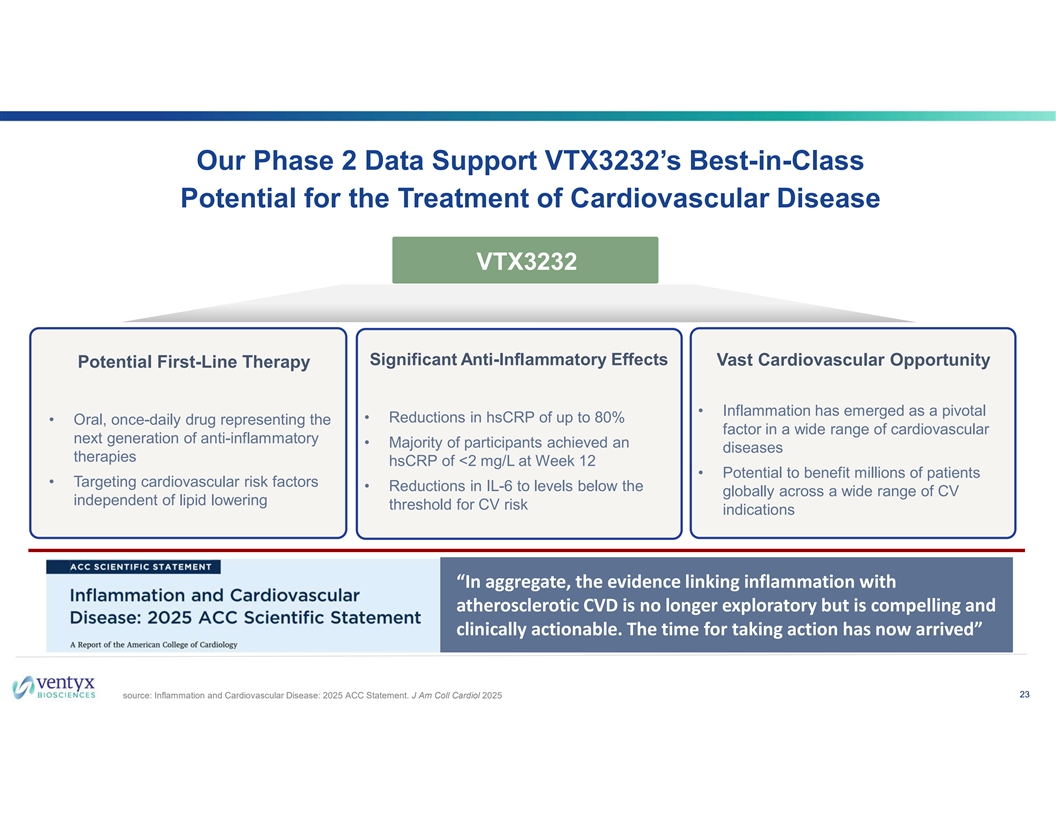
Our Phase 2 Data Support VTX3232’s Best-in-Class Potential for the Treatment of Cardiovascular Disease VTX3232 Significant Anti-Inflammatory Effects Vast Cardiovascular Opportunity Potential First-Line Therapy • Inflammation has emerged as a pivotal • Reductions in hsCRP of up to 80% • Oral, once-daily drug representing the factor in a wide range of cardiovascular next generation of anti-inflammatory • Majority of participants achieved an diseases therapies hsCRP of <2 mg/L at Week 12 • Potential to benefit millions of patients • Targeting cardiovascular risk factors • Reductions in IL-6 to levels below the globally across a wide range of CV independent of lipid lowering threshold for CV risk indications “In aggregate, the evidence linking inflammation with atherosclerotic CVD is no longer exploratory but is compelling and clinically actionable. The time for taking action has now arrived” 23 source: Inflammation and Cardiovascular Disease: 2025 ACC Statement. J Am Coll Cardiol 2025

Expert Perspective

Expert Perspectives on Inflammation and Cardiovascular Risk Peter Libby, MD Antonio Abbate, MD, PhD Ruth C. Heede Professor of Cardiology, Cardiovascular Specialist at Mass General Brigham Heart & Vascular Institute University of Virginia, School of Medicine Immediate Past President of the International Atherosclerosis Society 25

26
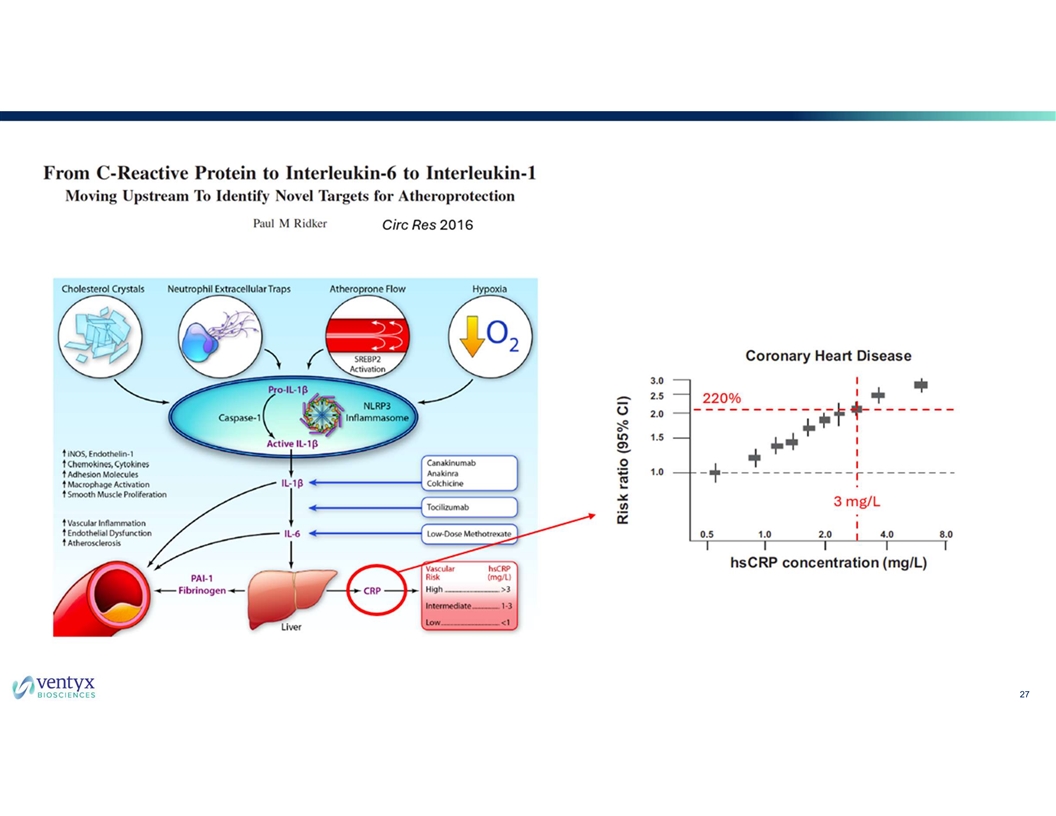
27
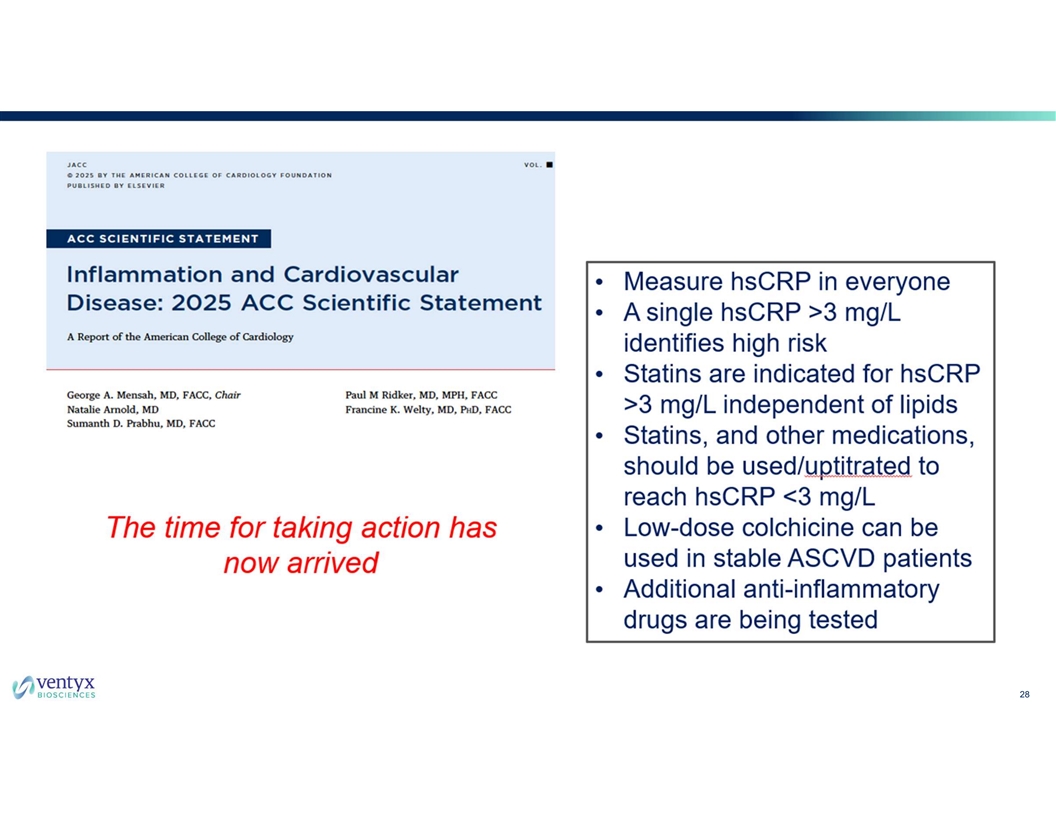
28
29

Question & Answer