

THE SCIENCE TO OVERCOME INFLAMMATION OCTOBER 20, 2025 Phase 2 Topline Results of RPT904 (JYB1904) in Chronic Spontaneous Urticaria

Disclaimer Statements in this Presentation that are not statements of historical fact are forward-looking statements. Such forward-looking statements include, without limitation, statements regarding the development of RPT904, including the expected timing of clinical trials and the availability of data therefrom; expectations regarding regulatory interactions; the therapeutic and commercial potential of RPT904, including as compared to omalizumab; and the ability to obtain necessary regulatory approvals. Words such as "believe," "anticipate," "plan," "expect," "will," "may," “could,” "upcoming,“ “projected,” "milestone," "potential," "target" or the negative of these terms or similar expressions are intended to identify forward-looking statements, though not all forward-looking statements necessarily contain these identifying words. These forward-looking statements are based on the current beliefs of the Company's management with respect to future events and trends and are subject to known and unknown risks and uncertainties that may cause our actual performance or achievements to be materially different from any future performance or achievements expressed or implied by the forward-looking statements in this Presentation. Risks and uncertainties that may cause actual results to differ materially include: risks inherent in the initiation, progress and completion of clinical trials and clinical development of our product candidates; the risk that clinical trials may have unsatisfactory outcomes; risks associated with preclinical development of product candidates; regulatory authorities, including the U.S. Food and Drug Administration (FDA) may not agree with our interpretation of the data from clinical trials of our drug candidates; we may decide, or regulatory authorities may require us, to conduct additional clinical trials or to modify our ongoing clinical trials; we may experience delays in the commencement, enrollment, completion or analysis of clinical testing for our drug candidates, or significant issues regarding the adequacy of our clinical trial designs or the execution of our clinical trials may arise, which could result in increased costs and delays, or limit our ability to obtain regulatory approval; our drug candidates may not receive regulatory approval or be successfully commercialized; unexpected adverse side effects or inadequate therapeutic efficacy of our drug candidates could delay or prevent regulatory approval or commercialization; uncertainties inherent in the conduct of clinical trials, reliance on our partners and third parties over which we may not always have full control; our ability to enter into strategic partnerships on commercially reasonable terms; our ability to obtain additional financing; the uncertainty regarding the macroeconomic environment and other risks and uncertainties that are described in the “Risk Factors” section of our most recent Form 10-Q filed with the Securities and Exchange Commission, and any current and periodic reports filed thereafter. These forward-looking statements should not be taken as forecasts or promises nor should they be taken as implying any indication, assurance or guarantee that any assumptions on which such forward-looking statements have been made are correct or exhaustive or, in the case of such assumptions, fully stated in the Presentation. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date this Presentation is given. Although we believe that the beliefs and assumptions reflected in the forward-looking statements are reasonable, we cannot guarantee future performance or achievements. Except as required by law, we undertake no obligation to update publicly any forward-looking statements for any reason after the date of this Presentation. This Presentation discusses drug candidates that are under clinical study and which have not yet been approved for marketing by the U.S. FDA. No representation is made as to the safety or effectiveness of any drug candidates for any use for which such drug candidates are being studied.
Summary of RPT904 Phase 2 Results in CSU RPT904 at both Q8W and Q12W dosing showed comparable efficacy and safety to omalizumab at Q4W dosing Both RPT904 arms showed numerically superior efficacy to omalizumab arm on UAS7 and UAS7=0 (complete response) across all timepoints* Sustained efficacy out to 16 weeks after a single 300 mg dose underscores durability RPT904 was well tolerated with no drug related SAEs or discontinuations and no AEs of special interest (i.e. anaphylaxis) We believe results support moving to pivotal Phase 3 studies in CSU Jeyou and RAPT to approach regulatory agencies in respective territories to discuss potential registrational paths to approval Additional efficacy and safety data from this trial be presented at future medical conferences *Study was not a formal non-inferiority study; no statistical hypothesis was tested
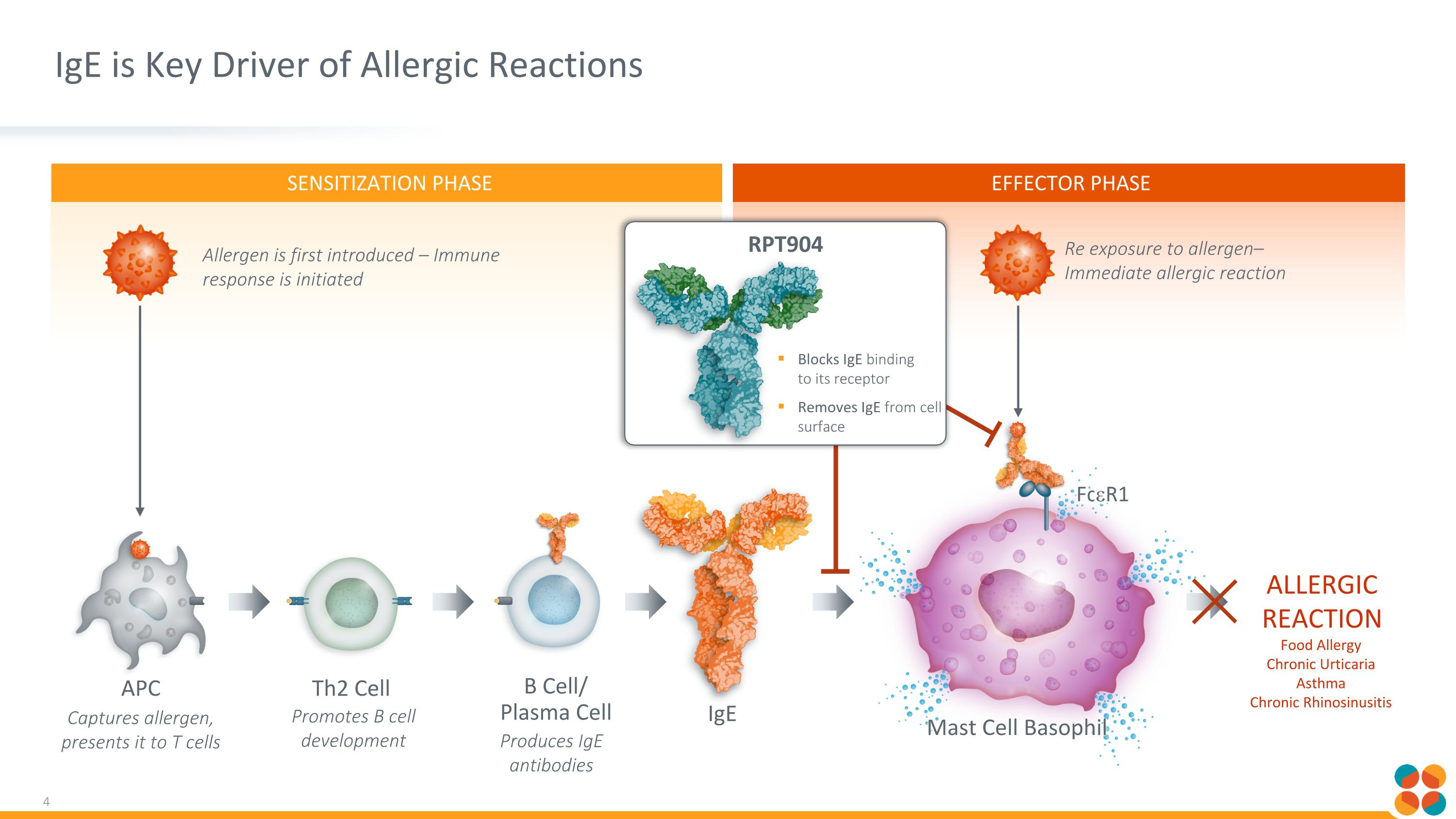
SENSITIZATION PHASE EFFECTOR PHASE IgE is Key Driver of Allergic Reactions Allergen is first introduced – Immune response is initiated Captures allergen, presents it to T cells Promotes B cell development Produces IgE antibodies Th2 Cell B Cell/ Plasma Cell APC ALLERGIC REACTION Food Allergy Chronic Urticaria Asthma Chronic Rhinosinusitis FceR1 Re exposure to allergen– Immediate allergic reaction Mast Cell Basophil IgE Blocks IgE binding to its receptor Removes IgE from cell surface RPT904
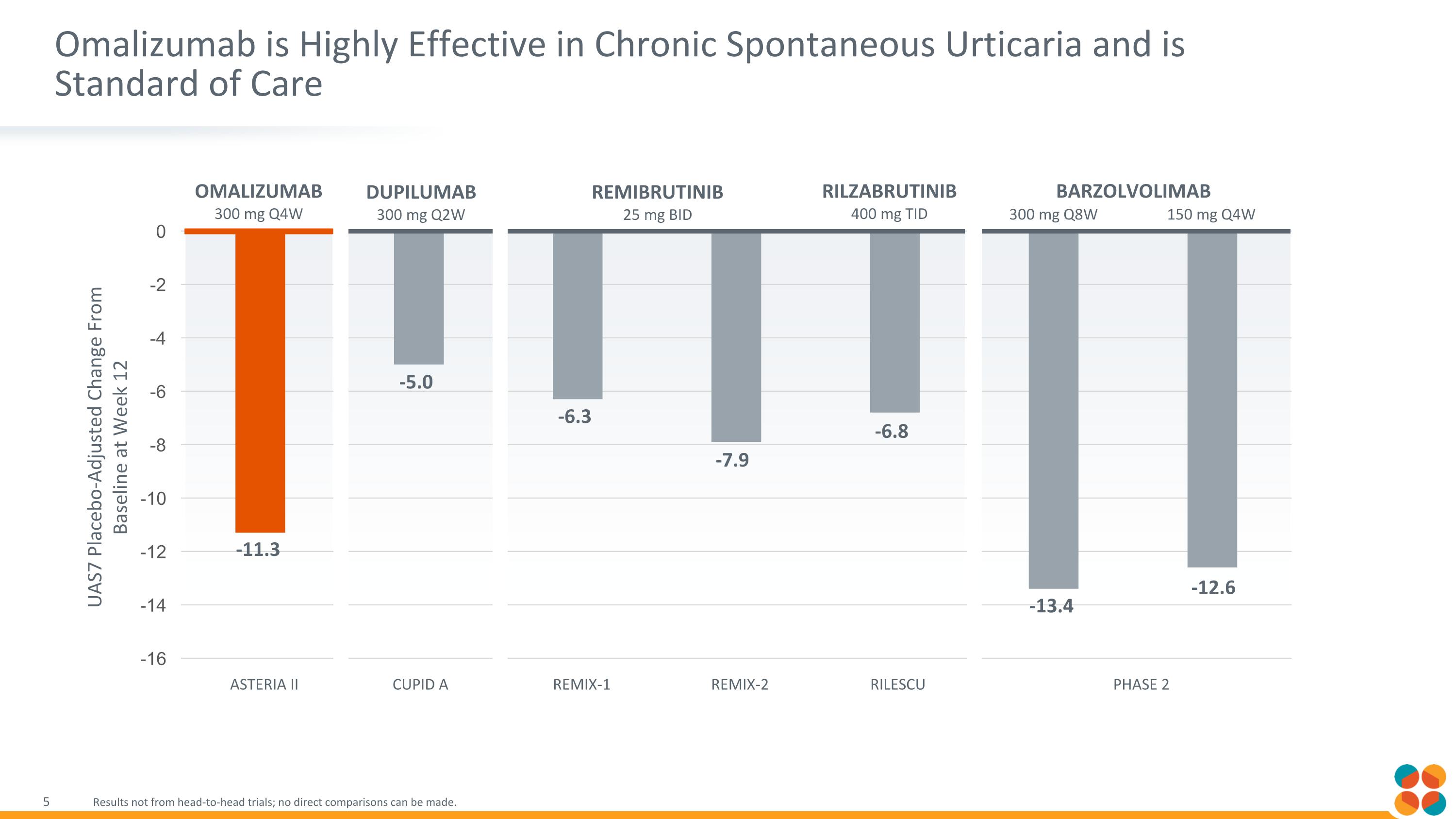
Omalizumab is Highly Effective in Chronic Spontaneous Urticaria and is Standard of Care Results not from head-to-head trials; no direct comparisons can be made. Omalizumab 300 mg Q4W REMIBRUTINIB 25 mg BID DUPILUMAB 300 mg Q2W -11.3 -5.0 -6.3 -7.9 -6.8 -13.4 -12.6 REMIX-1 REMIX-2 RILZABRUTINIB 400 mg TID BARZOLVOLIMAB 300 mg Q8W 150 mg Q4W ASTERIA II CUPID A RILESCU PHASE 2 UAS7 Placebo-Adjusted Change From Baseline at Week 12
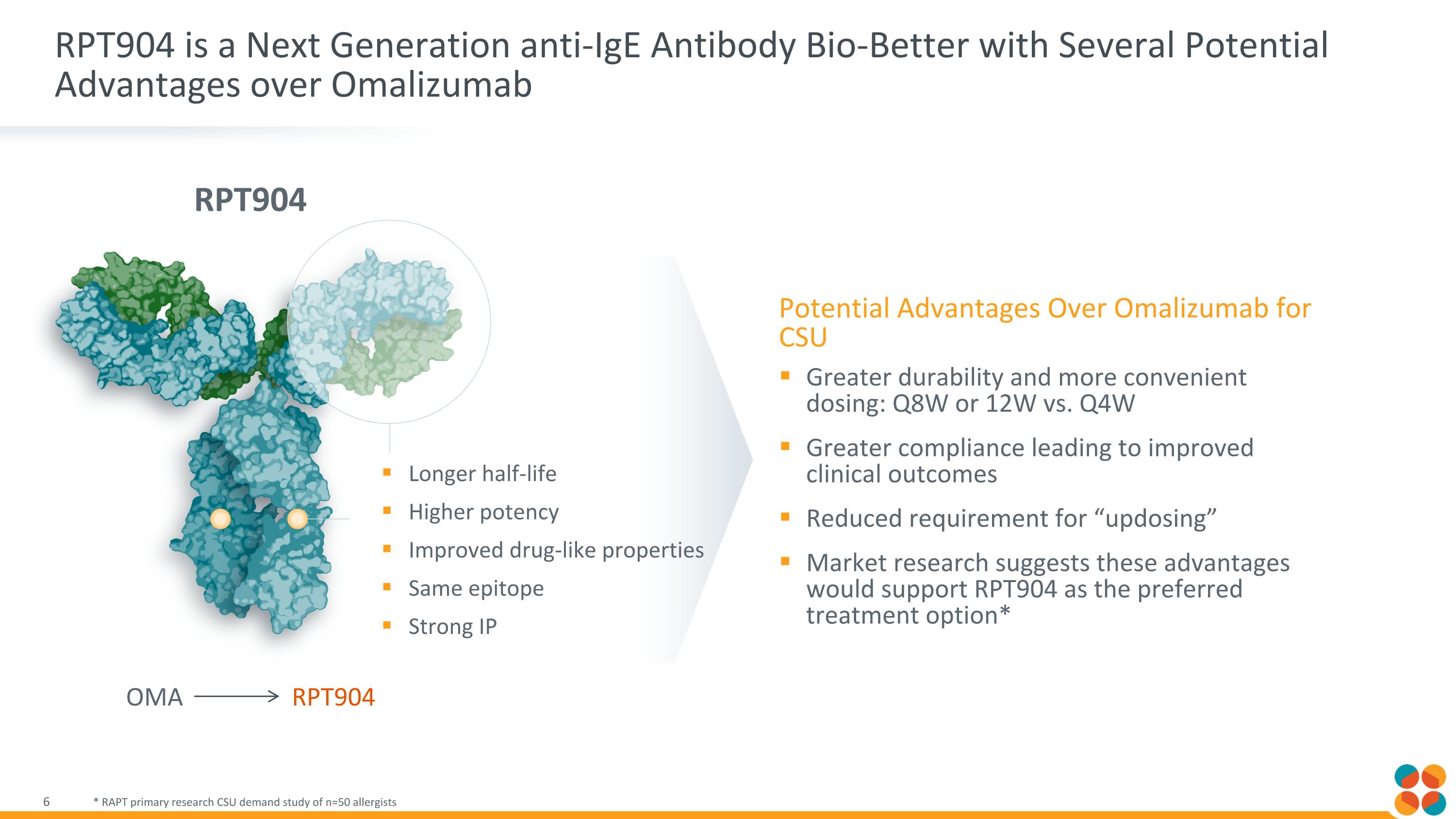
RPT904 is a Next Generation anti-IgE Antibody Bio-Better with Several Potential Advantages over Omalizumab * RAPT primary research CSU demand study of n=50 allergists Longer half-life Higher potency Improved drug-like properties Same epitope Strong IP RPT904 OMA RPT904 Potential Advantages Over Omalizumab for CSU Greater durability and more convenient dosing: Q8W or 12W vs. Q4W Greater compliance leading to improved clinical outcomes Reduced requirement for “updosing” Market research suggests these advantages would support RPT904 as the preferred treatment option*
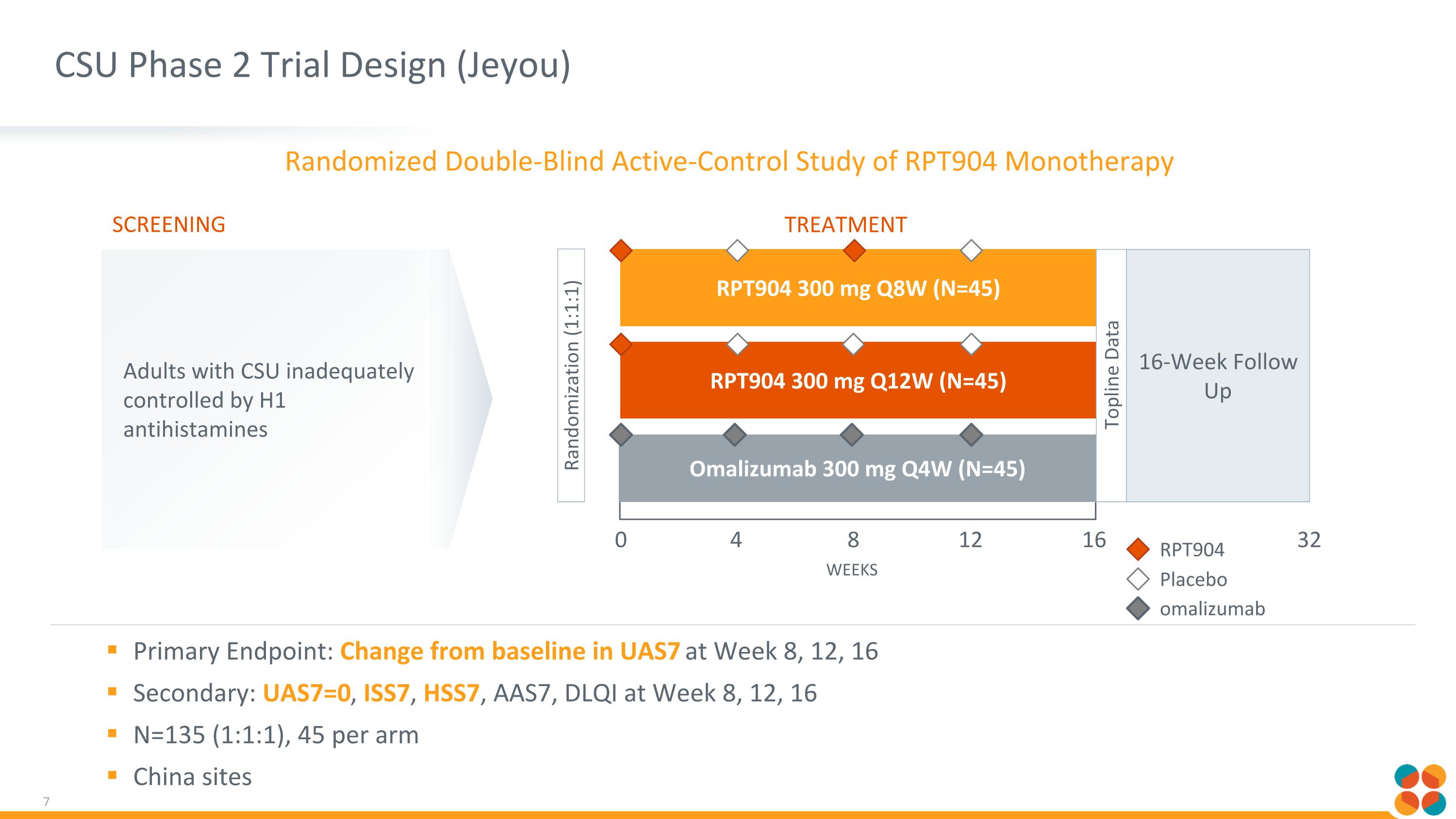
CSU Phase 2 Trial Design (Jeyou) Primary Endpoint: Change from baseline in UAS7 at Week 8, 12, 16 Secondary: UAS7=0, ISS7, HSS7, AAS7, DLQI at Week 8, 12, 16 N=135 (1:1:1), 45 per arm China sites 16-Week Follow Up RPT904 300 mg Q12W (N=45) RPT904 300 mg Q8W (N=45) Omalizumab 300 mg Q4W (N=45) Randomization (1:1:1) 0 WEEKS 16 4 8 12 32 Topline Data RPT904 Placebo omalizumab Screening Treatment Adults with CSU inadequately controlled by H1 antihistamines Randomized Double-Blind Active-Control Study of RPT904 Monotherapy
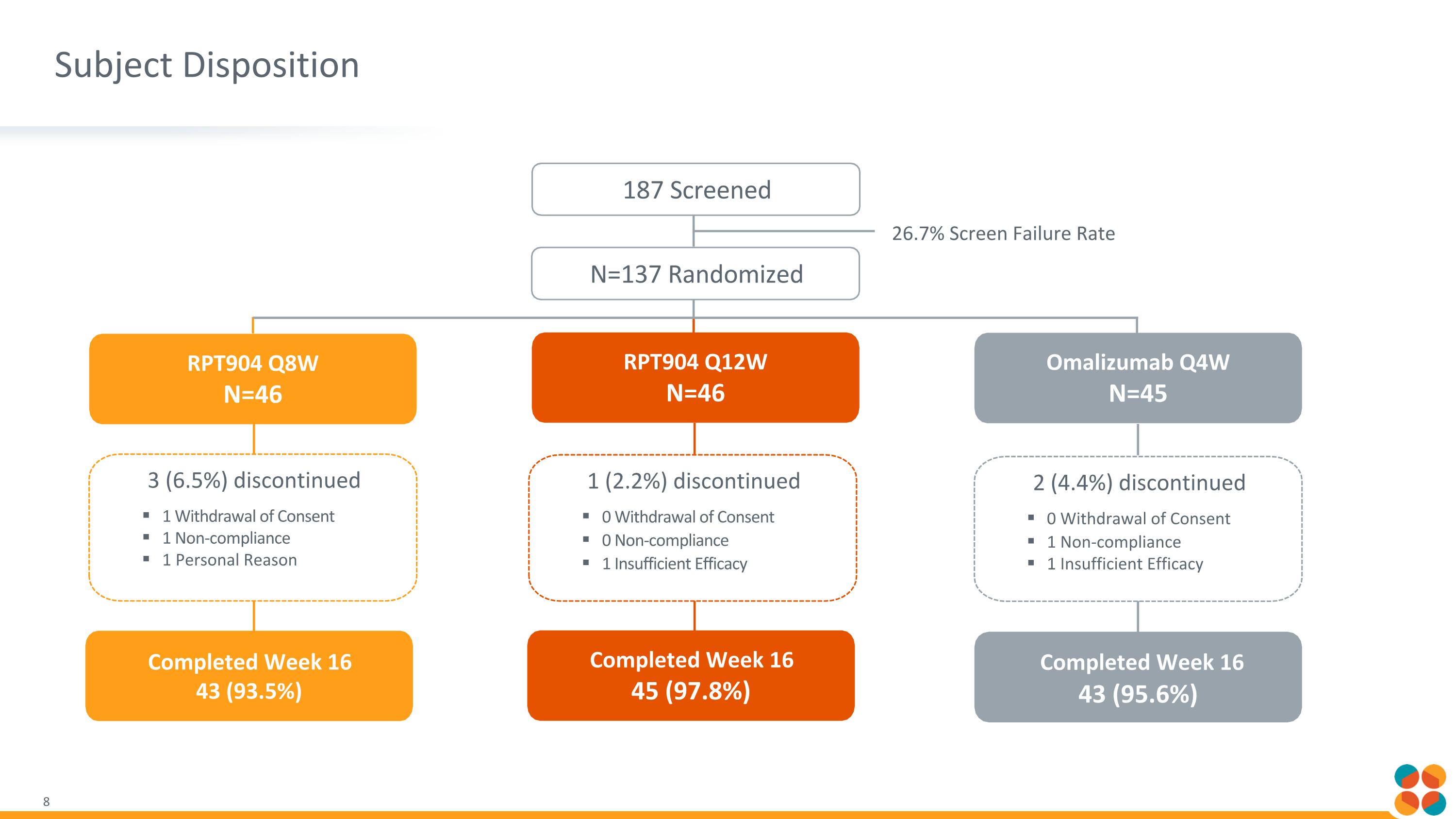
Subject Disposition 187 Screened Omalizumab Q4W N=45 Completed Week 16 43 (95.6%) N=137 Randomized RPT904 Q8W N=46 3 (6.5%) discontinued 1 Withdrawal of Consent 1 Non-compliance 1 Personal Reason 2 (4.4%) discontinued 0 Withdrawal of Consent 1 Non-compliance 1 Insufficient Efficacy Completed Week 16 43 (93.5%) RPT904 Q12W N=46 1 (2.2%) discontinued 0 Withdrawal of Consent 0 Non-compliance 1 Insufficient Efficacy Completed Week 16 45 (97.8%) 26.7% Screen Failure Rate
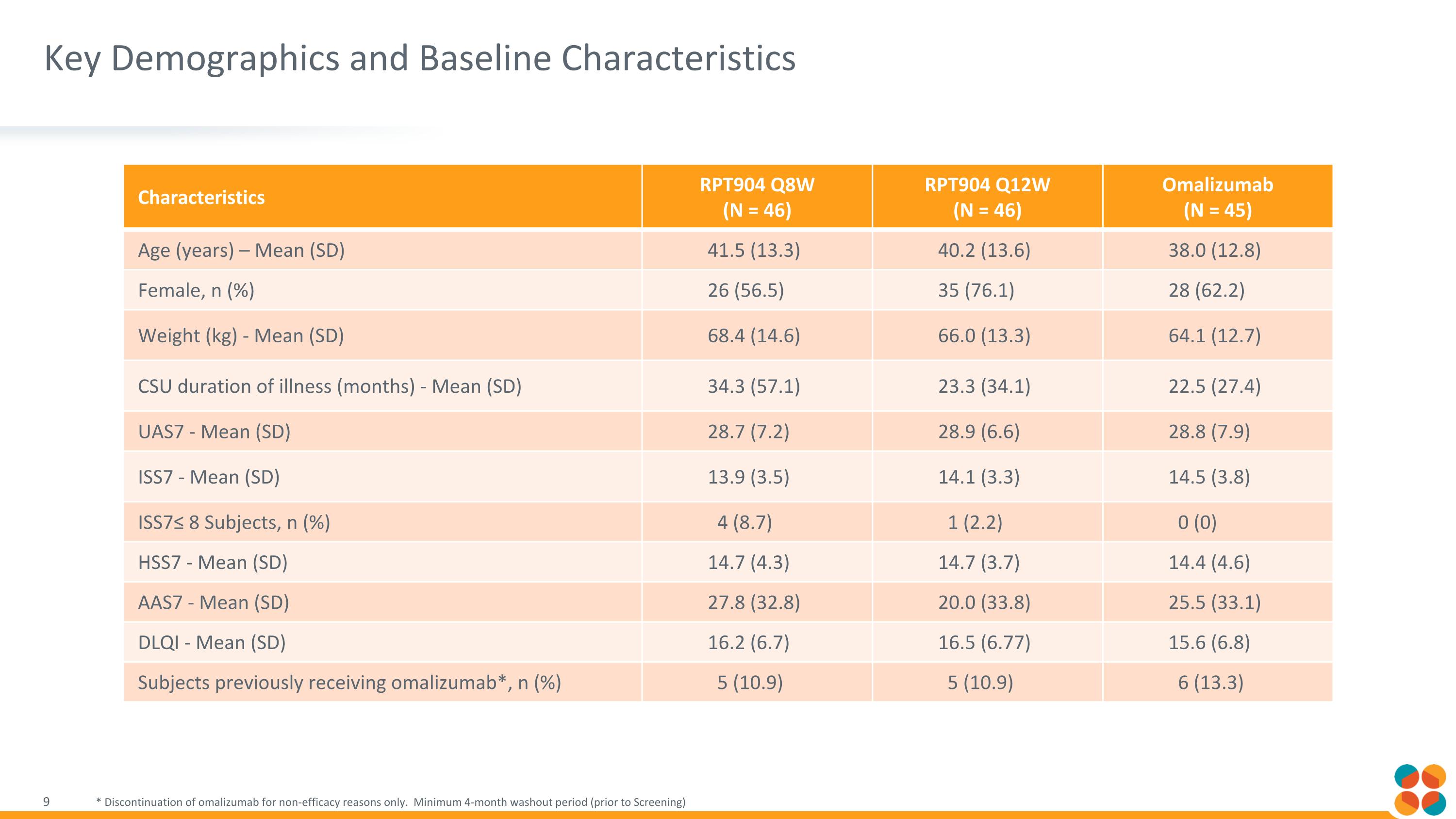
Key Demographics and Baseline Characteristics * Discontinuation of omalizumab for non-efficacy reasons only. Minimum 4-month washout period (prior to Screening) Characteristics RPT904 Q8W (N = 46) RPT904 Q12W (N = 46) Omalizumab (N = 45) Age (years) – Mean (SD) 41.5 (13.3) 40.2 (13.6) 38.0 (12.8) Female, n (%) 26 (56.5) 35 (76.1) 28 (62.2) Weight (kg) - Mean (SD) 68.4 (14.6) 66.0 (13.3) 64.1 (12.7) CSU duration of illness (months) - Mean (SD) 34.3 (57.1) 23.3 (34.1) 22.5 (27.4) UAS7 - Mean (SD) 28.7 (7.2) 28.9 (6.6) 28.8 (7.9) ISS7 - Mean (SD) 13.9 (3.5) 14.1 (3.3) 14.5 (3.8) ISS7≤ 8 Subjects, n (%) 4 (8.7) 1 (2.2) 0 (0) HSS7 - Mean (SD) 14.7 (4.3) 14.7 (3.7) 14.4 (4.6) AAS7 - Mean (SD) 27.8 (32.8) 20.0 (33.8) 25.5 (33.1) DLQI - Mean (SD) 16.2 (6.7) 16.5 (6.77) 15.6 (6.8) Subjects previously receiving omalizumab*, n (%) 5 (10.9) 5 (10.9) 6 (13.3)
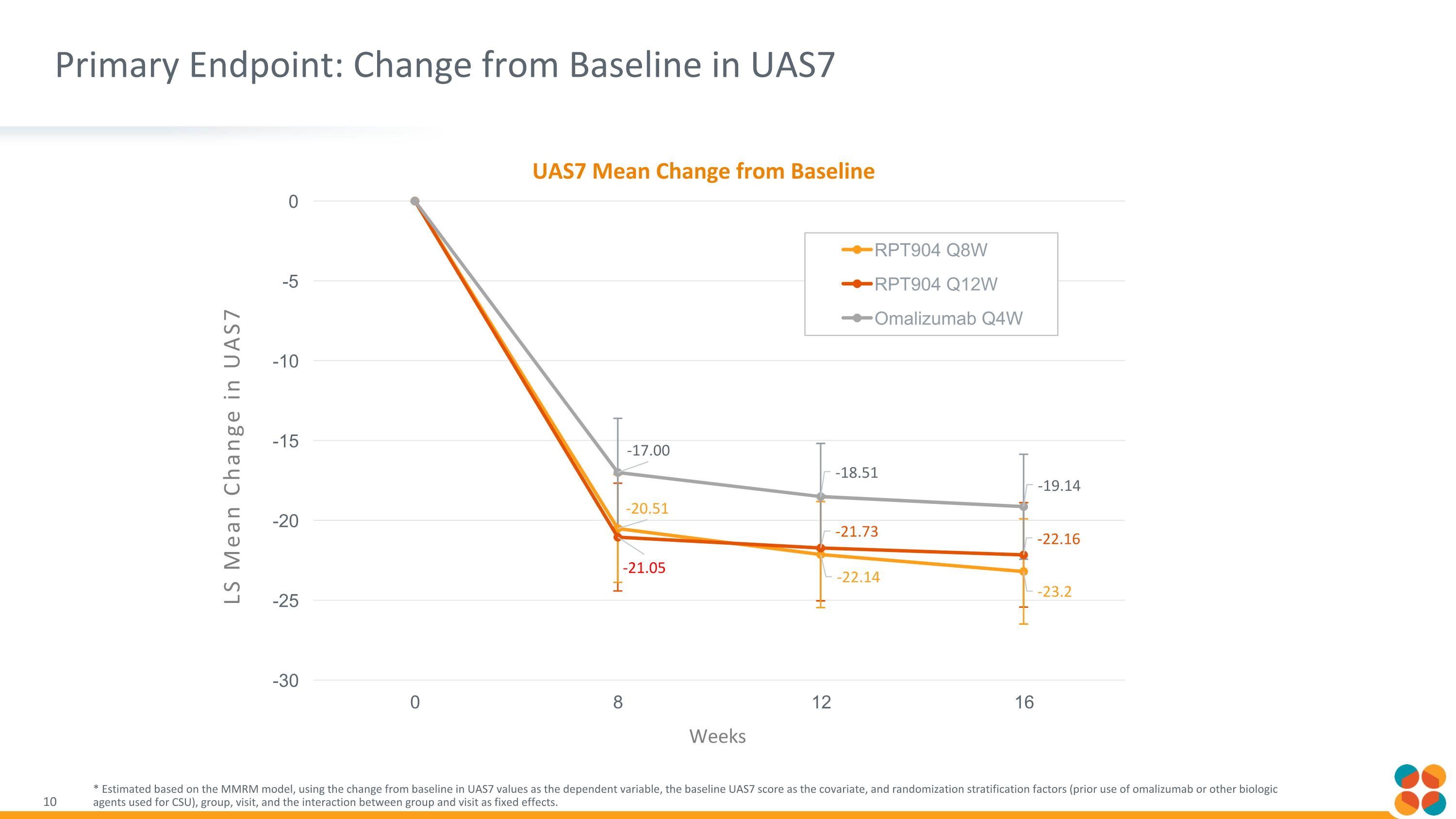
Primary Endpoint: Change from Baseline in UAS7 * Estimated based on the MMRM model, using the change from baseline in UAS7 values as the dependent variable, the baseline UAS7 score as the covariate, and randomization stratification factors (prior use of omalizumab or other biologic agents used for CSU), group, visit, and the interaction between group and visit as fixed effects. LS Mean Change in UAS7 Weeks UAS7 Mean Change from Baseline
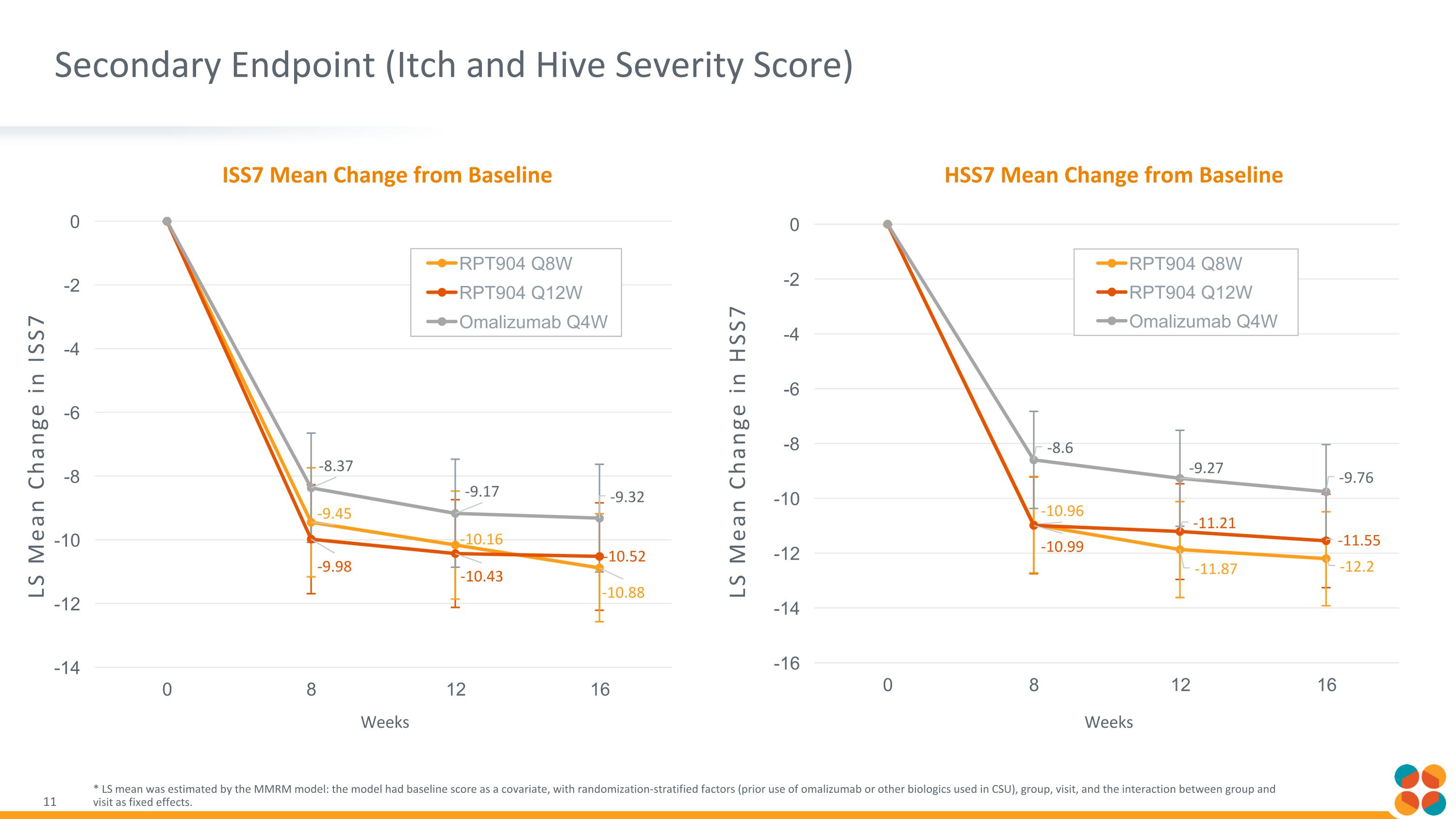
Secondary Endpoint (Itch and Hive Severity Score) * LS mean was estimated by the MMRM model: the model had baseline score as a covariate, with randomization-stratified factors (prior use of omalizumab or other biologics used in CSU), group, visit, and the interaction between group and visit as fixed effects. ISS7 Mean Change from Baseline HSS7 Mean Change from Baseline Weeks Weeks LS Mean Change in HSS7 LS Mean Change in ISS7
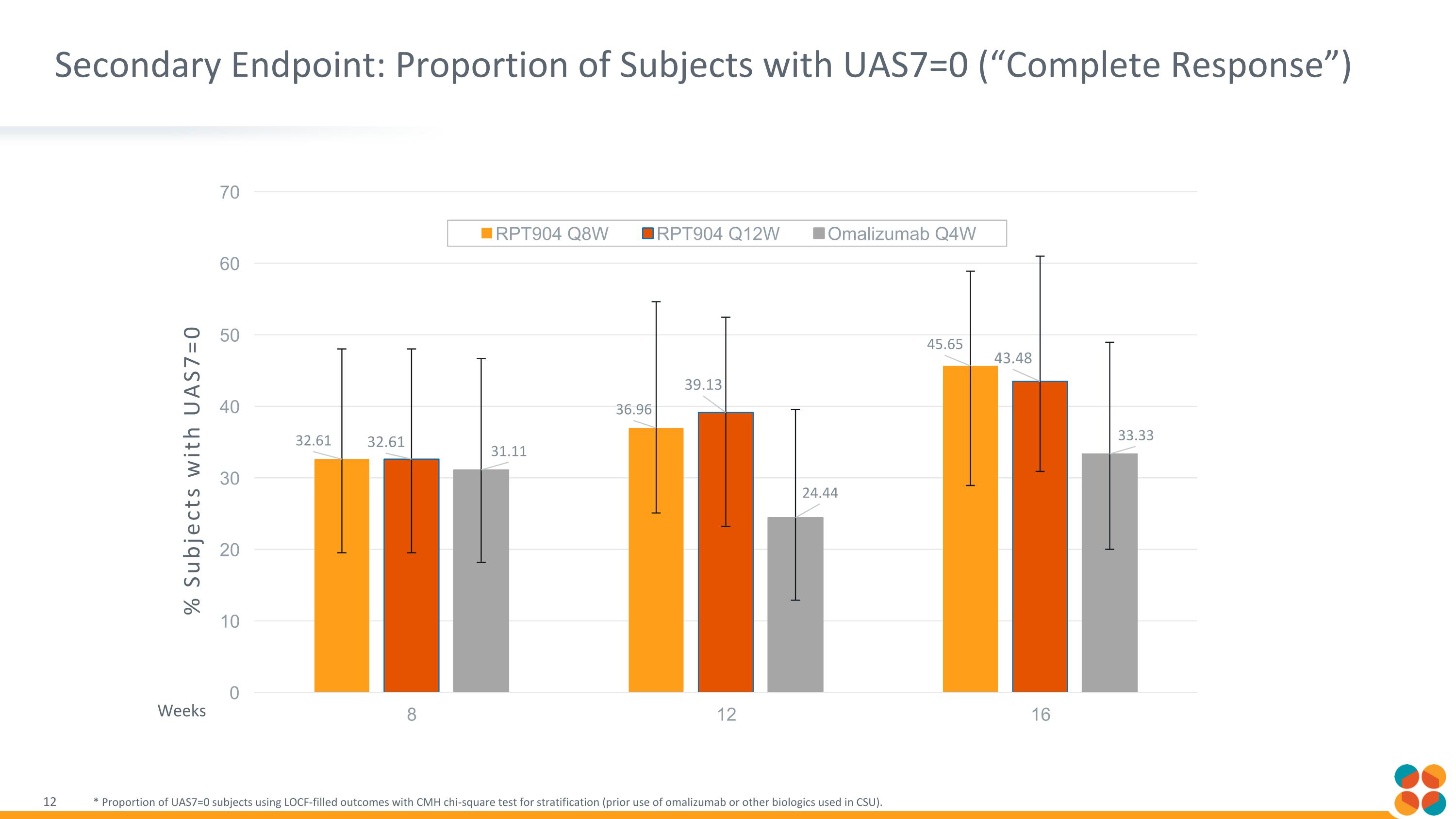
Secondary Endpoint: Proportion of Subjects with UAS7=0 (“Complete Response”) * Proportion of UAS7=0 subjects using LOCF-filled outcomes with CMH chi-square test for stratification (prior use of omalizumab or other biologics used in CSU). % Subjects with UAS7=0 Weeks
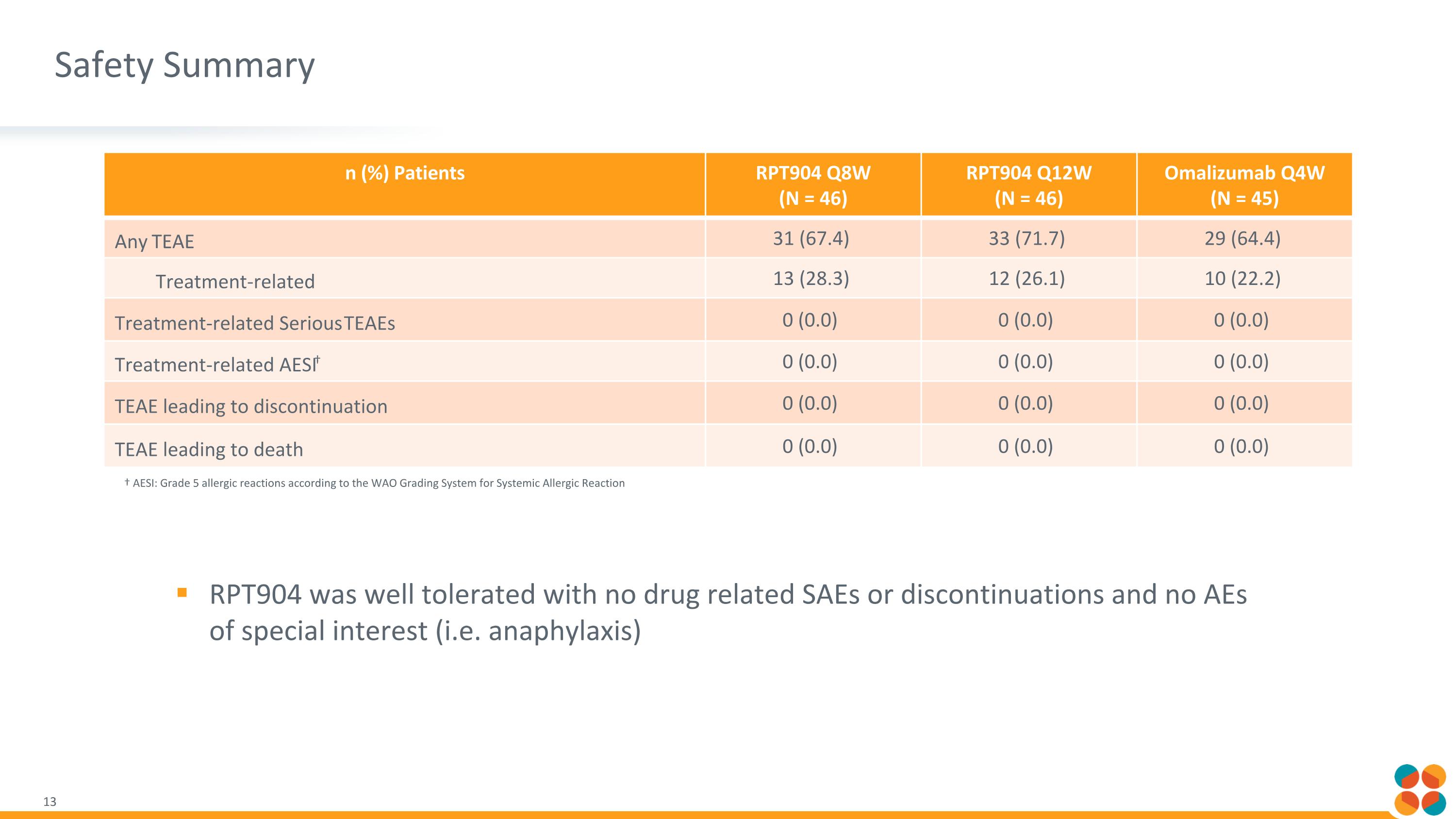
Safety Summary † AESI: Grade 5 allergic reactions according to the WAO Grading System for Systemic Allergic Reaction n (%) Patients RPT904 Q8W (N = 46) RPT904 Q12W (N = 46) Omalizumab Q4W (N = 45) Any TEAE 31 (67.4) 33 (71.7) 29 (64.4) Treatment-related 13 (28.3) 12 (26.1) 10 (22.2) Treatment-related Serious TEAEs 0 (0.0) 0 (0.0) 0 (0.0) Treatment-related AESI† 0 (0.0) 0 (0.0) 0 (0.0) TEAE leading to discontinuation 0 (0.0) 0 (0.0) 0 (0.0) TEAE leading to death 0 (0.0) 0 (0.0) 0 (0.0) RPT904 was well tolerated with no drug related SAEs or discontinuations and no AEs of special interest (i.e. anaphylaxis)

Comments from Dr. Ana Maria Giménez-Arnau, MD PhD Dr. Gimenez-Arnau is a consultant to RAPT. Professor at the Hospital del Mar Research Institute (IMIM) and Associate Professor of Dermatology at Universitat Pompeu Fabra in Barcelona Specialist in immunoallergic skin diseases More than 300 international scientific publications and 250 national publications, including textbooks on inflammatory skin diseases Co-author and investigator for key CSU trials, including remibrutinib (REMIX 1 and 2), dupilumab (LIBERTY-CSU) and ligelizumab (PEARL 1 and 2)

Results from this randomized double-blind phase 2 study show that extended half life and pharmacodynamic effects of RPT904 translate to durable clinical benefit A single 300 mg dose of RPT904 showed numerically superior efficacy to four 300 mg doses of omalizumab at week 16* Data support promise of best-in-class profile across multiple indications We believe results support moving to pivotal Phase 3 studies in CSU Jeyou and RAPT to approach regulatory agencies in respective territories to discuss potential registrational paths to approval RAPT’s Phase 2b in food allergy is on track to start before end of year with topline data expected 1H 2027 *Study was not a formal non-inferiority study; no statistical hypothesis was tested Conclusions
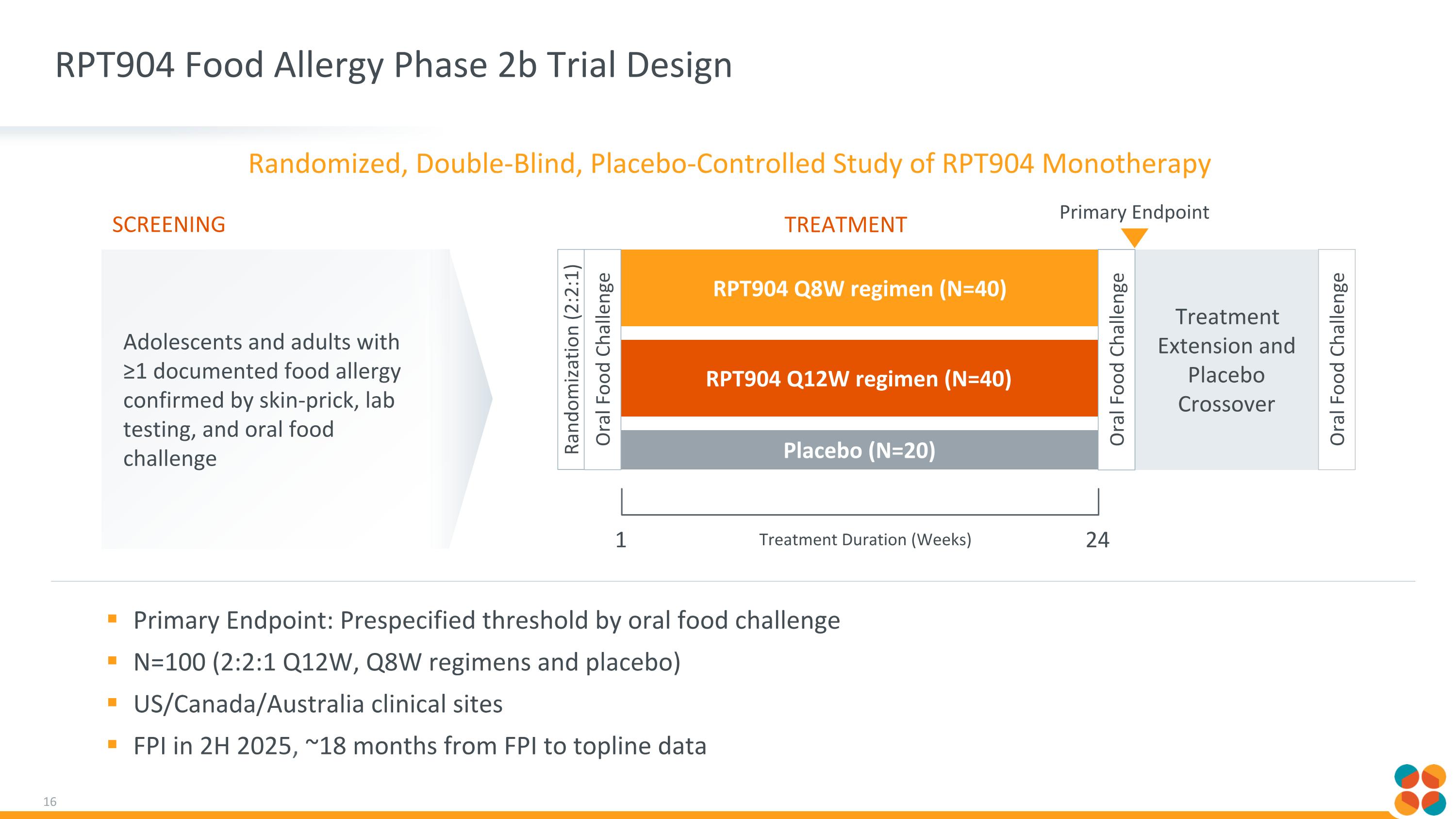
RPT904 Food Allergy Phase 2b Trial Design Primary Endpoint: Prespecified threshold by oral food challenge N=100 (2:2:1 Q12W, Q8W regimens and placebo) US/Canada/Australia clinical sites FPI in 2H 2025, ~18 months from FPI to topline data Treatment Extension and Placebo Crossover RPT904 Q12W regimen (N=40) Adolescents and adults with ≥1 documented food allergy confirmed by skin-prick, lab testing, and oral food challenge Screening RPT904 Q8W regimen (N=40) Placebo (N=20) Randomization (2:2:1) Treatment 1 Treatment Duration (Weeks) Oral Food Challenge 24 Oral Food Challenge Oral Food Challenge Primary Endpoint Randomized, Double-Blind, Placebo-Controlled Study of RPT904 Monotherapy
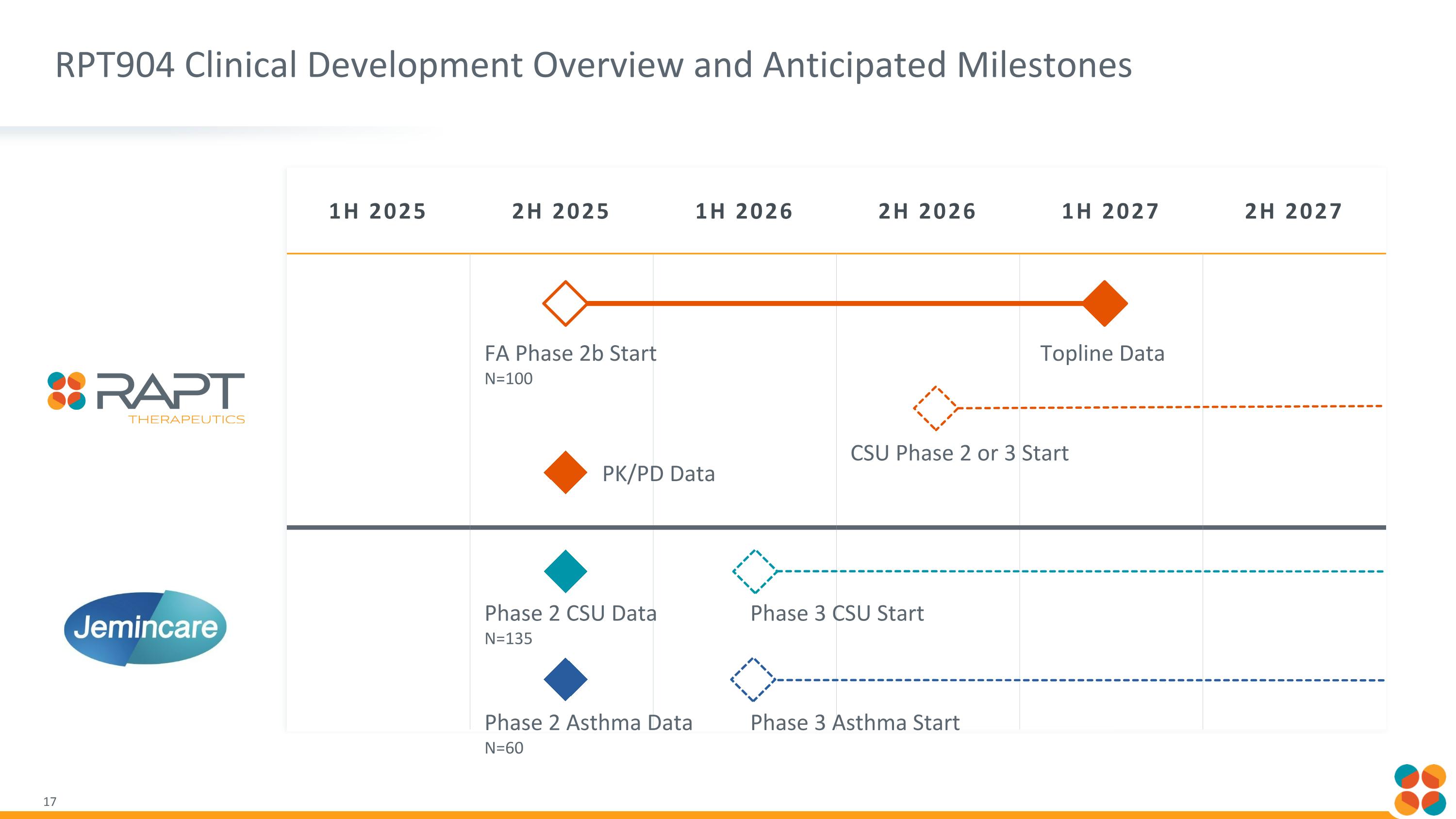
1H 2025 2H 2025 1H 2026 2H 2026 1H 2027 2H 2027 FA Phase 2b Start N=100 Topline Data CSU Phase 2 or 3 Start Phase 2 CSU Data N=135 RPT904 Clinical Development Overview and Anticipated Milestones Phase 2 Asthma Data N=60 Phase 3 CSU Start Phase 3 Asthma Start PK/PD Data

Thank You Please visit www.rapt.com