

Leaders in Developing Allogeneic γδ1 CAR-T Cell Therapies to Fight Autoimmune Diseases and Cancer γδ= Gamma delta; CAR= Chimeric antigen receptor

Forward-Looking Statements This presentation contains "forward-looking statements" of Adicet Bio, Inc. (Adicet) within the meaning of the Private Securities Litigation Reform Act of 1995 relating to business and operations of Adicet. These forward-looking statements include, but are not limited to, express or implied statements regarding: preclinical and clinical development of Adicet's product candidates, including future plans or expectations for ADI-001 and ADI-212 and the potential safety, durability, tolerability and efficacy of these product candidates; the expected timing, success and progress of the Phase 1 clinical trial of ADI -001, including initiation of a pivotal study; and potential for clinical updates for ADI-001 and ADI-212 in 2026. Any forward-looking statements in this presentation are based on management’s current expectations and beliefs of future events, and are subject to a number of risks and uncertainties that could cause actual results to differ materially and adversely from those set forth in or implied by such forward-looking statements, including without limitation, the effect of global economic conditions and public health emergencies on Adicet’s business and financial results, including with respect to disruptions to our preclinical and clinical studies, business operations, employee hiring and retention, and ability to raise additional capital; Adicet’s ability to execute on its strategy including obtaining the requisite regulatory approvals on the expected timeline, if at all; that positive results, including interim results, from a preclinical or clinical study may not necessarily be predictive of the results of future or ongoing studies; clinical studies may fail to demonstrate adequate safety and efficacy of Adicet’s product candidates, which would prevent, delay, or limit the scope of regulatory approval and commercialization; and regulatory approval processes of the FDA and comparable foreign regulatory authorities are lengthy, time-consuming, and inherently unpredictable; and Adicet’s ability to meet production and product release expectations. For a discussion of these and other risks and uncertainties, and other important factors, any of which could cause Adicet’s actual results to differ from those contained in the forward-looking statements, see the section entitled “Risk Factors” in Adicet’s most recent annual report on Form 10-K and our periodic reports on Form 10-Q and Form 8-K filed with the U.S. Securities and Exchange Commission (SEC), as well as discussions of potential risks, uncertainties, and other important factors in Adicet’s other filings with the SEC. All information in this presentation is as of the date of the presentation, and Adicet undertakes no duty to update this information unless required by law. Industry and Market Information Information regarding market share, market position and industry data pertaining to Adicet’s business contained in this presentation consists of estimates based on data and reports compiled by industry professional organizations and analysts and Adicet’s knowledge of their industry. Although Adicet believes the industry and market data to be reliable, this information could prove to be inaccurate. You should carefully consider the inherent risks and uncertainties associated with the market and other industry data contained in this presentation. Forward-looking information obtained from third-party sources is subject to the same qualifications and the additional uncertainties as the other forward-looking statements in this presentation.
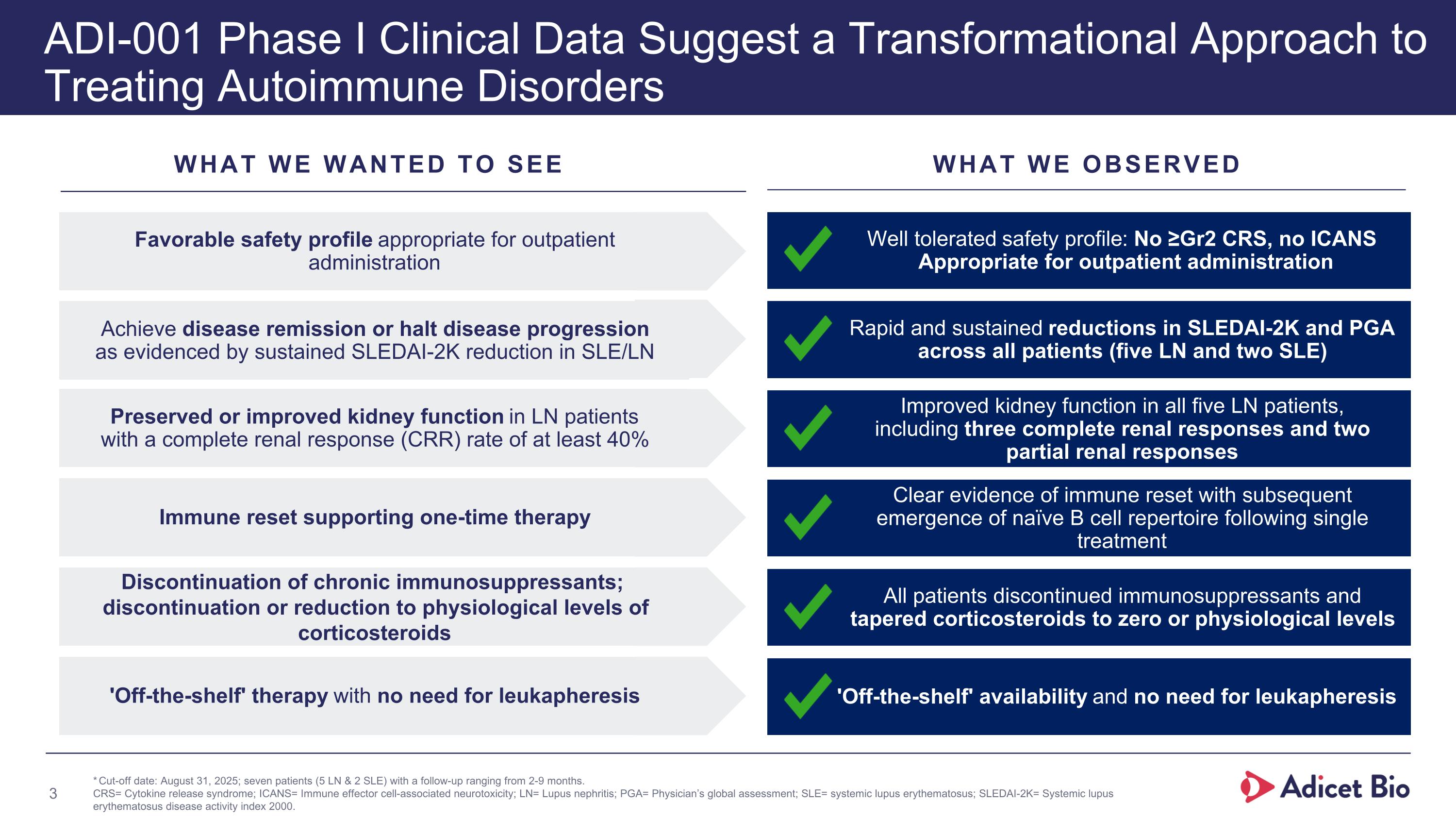
WHAT WE WANTED TO SEE WHAT WE OBSERVED 'Off-the-shelf' therapy with no need for leukapheresis 'Off-the-shelf' availability and no need for leukapheresis Favorable safety profile appropriate for outpatient administration Well tolerated safety profile: No ≥Gr2 CRS, no ICANS Appropriate for outpatient administration Achieve disease remission or halt disease progression as evidenced by sustained SLEDAI-2K reduction in SLE/LN Rapid and sustained reductions in SLEDAI-2K and PGA across all patients (five LN and two SLE) Preserved or improved kidney function in LN patients with a complete renal response (CRR) rate of at least 40% Improved kidney function in all five LN patients, including three complete renal responses and two partial renal responses Immune reset supporting one-time therapy Clear evidence of immune reset with subsequent emergence of naïve B cell repertoire following single treatment Discontinuation of chronic immunosuppressants; discontinuation or reduction to physiological levels of corticosteroids All patients discontinued immunosuppressants and tapered corticosteroids to zero or physiological levels new ADI-001 Phase I Clinical Data Suggest a Transformational Approach to Treating Autoimmune Disorders *Cut-off date: August 31, 2025; seven patients (5 LN & 2 SLE) with a follow-up ranging from 2-9 months. CRS= Cytokine release syndrome; ICANS= Immune effector cell-associated neurotoxicity; LN= Lupus nephritis; PGA= Physician’s global assessment; SLE= systemic lupus erythematosus; SLEDAI-2K= Systemic lupus erythematosus disease activity index 2000.
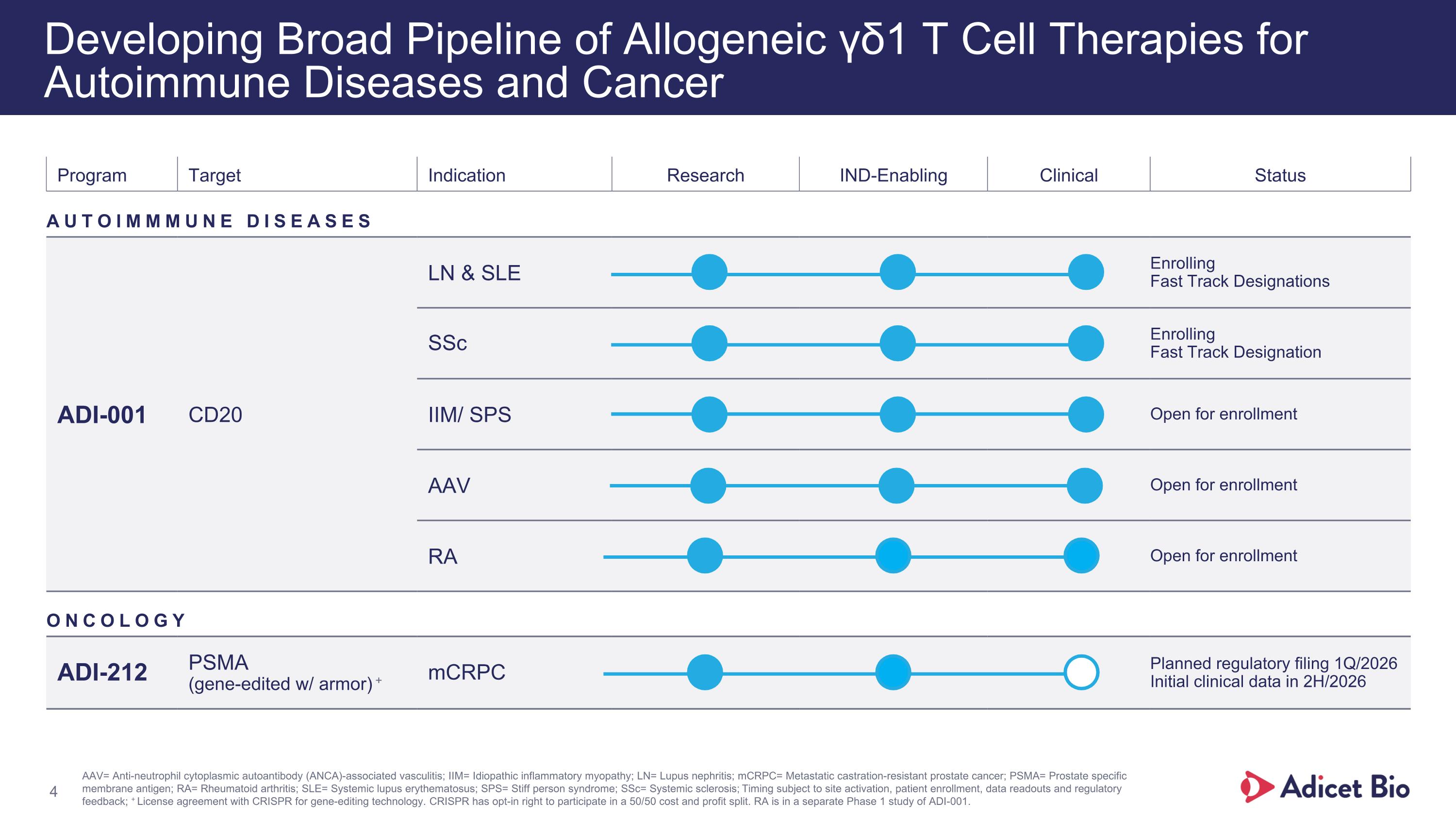
Program Target Indication Research IND-Enabling Clinical Status AUTOIMMMUNE DISEASES ADI-001 CD20 LN & SLE Enrolling Fast Track Designations SSc Enrolling Fast Track Designation IIM/ SPS Open for enrollment AAV Open for enrollment RA Open for enrollment ONCOLOGY ADI-212 PSMA (gene-edited w/ armor) + mCRPC Planned regulatory filing 1Q/2026 Initial clinical data in 2H/2026 Developing Broad Pipeline of Allogeneic γδ1 T Cell Therapies for Autoimmune Diseases and Cancer AAV= Anti-neutrophil cytoplasmic autoantibody (ANCA)-associated vasculitis; IIM= Idiopathic inflammatory myopathy; LN= Lupus nephritis; mCRPC= Metastatic castration-resistant prostate cancer; PSMA= Prostate specific membrane antigen; RA= Rheumatoid arthritis; SLE= Systemic lupus erythematosus; SPS= Stiff person syndrome; SSc= Systemic sclerosis; Timing subject to site activation, patient enrollment, data readouts and regulatory feedback; + License agreement with CRISPR for gene-editing technology. CRISPR has opt-in right to participate in a 50/50 cost and profit split. RA is in a separate Phase 1 study of ADI-001.
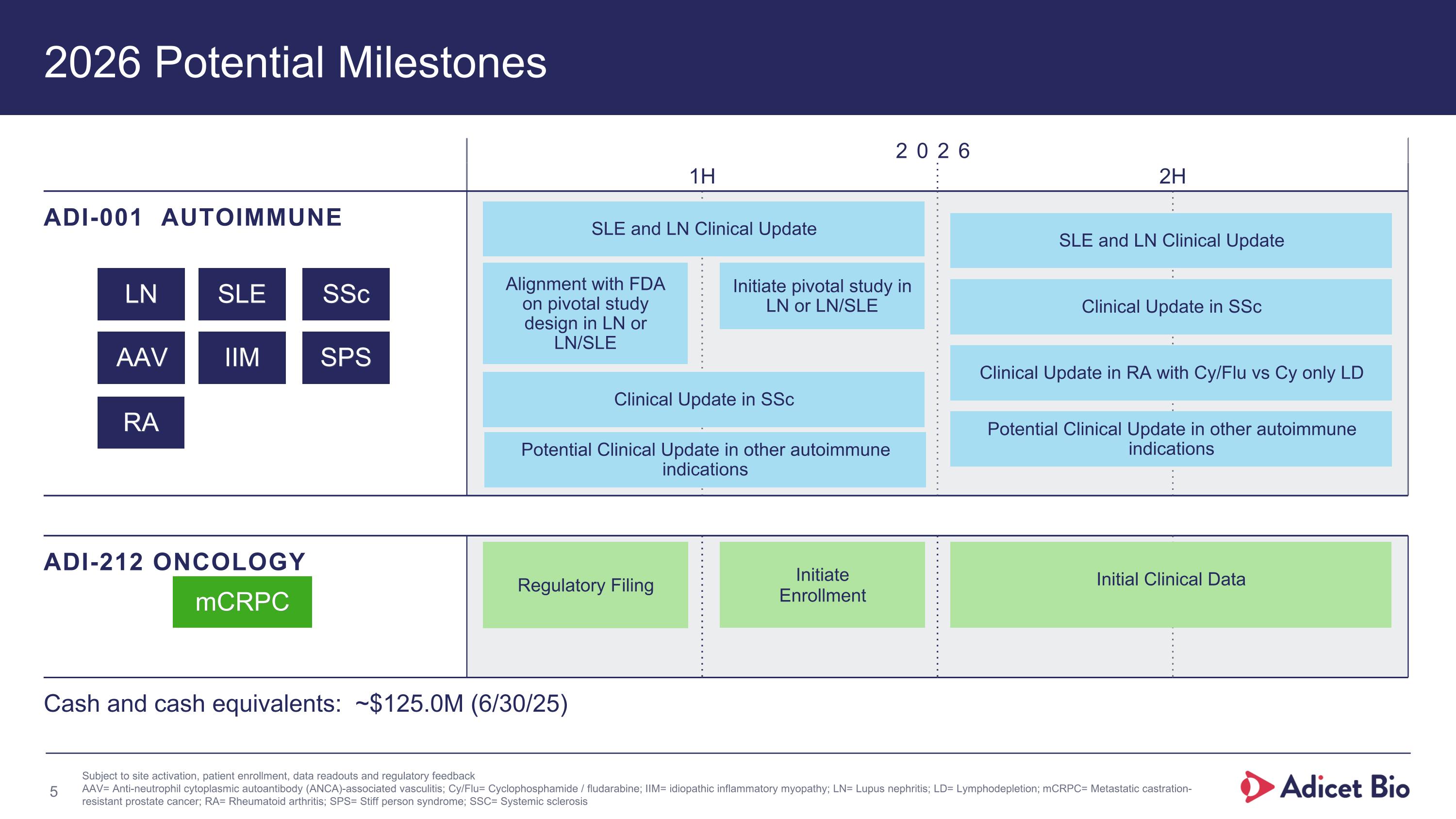
2026 Potential Milestones 2026 1H 2H ADI-001 AUTOIMMUNE ADI-212 ONCOLOGY mCRPC Cash and cash equivalents: ~$125.0M (6/30/25) Subject to site activation, patient enrollment, data readouts and regulatory feedback AAV= Anti-neutrophil cytoplasmic autoantibody (ANCA)-associated vasculitis; Cy/Flu= Cyclophosphamide / fludarabine; IIM= idiopathic inflammatory myopathy; LN= Lupus nephritis; LD= Lymphodepletion; mCRPC= Metastatic castration-resistant prostate cancer; RA= Rheumatoid arthritis; SPS= Stiff person syndrome; SSC= Systemic sclerosis LN SLE SSc AAV IIM SPS Initial Clinical Data SLE and LN Clinical Update Initiate pivotal study in LN or LN/SLE Alignment with FDA on pivotal study design in LN or LN/SLE SLE and LN Clinical Update Clinical Update in SSc Clinical Update in SSc Clinical Update in RA with Cy/Flu vs Cy only LD Potential Clinical Update in other autoimmune indications Regulatory Filing Initiate Enrollment Potential Clinical Update in other autoimmune indications RA
Adicet Bio Leadership Team Julie Maltzman, M.D. Chief Medical Officer Chen Schor President and CEO Nick Harvey Chief Financial Officer Don Healey, Ph.D. Chief Technology Officer Blake Aftab, Ph.D. Chief Scientific Officer Amy Locke Head of Human Resources

ADI-001 A Transformational Approach to Treating Autoimmune Disorders
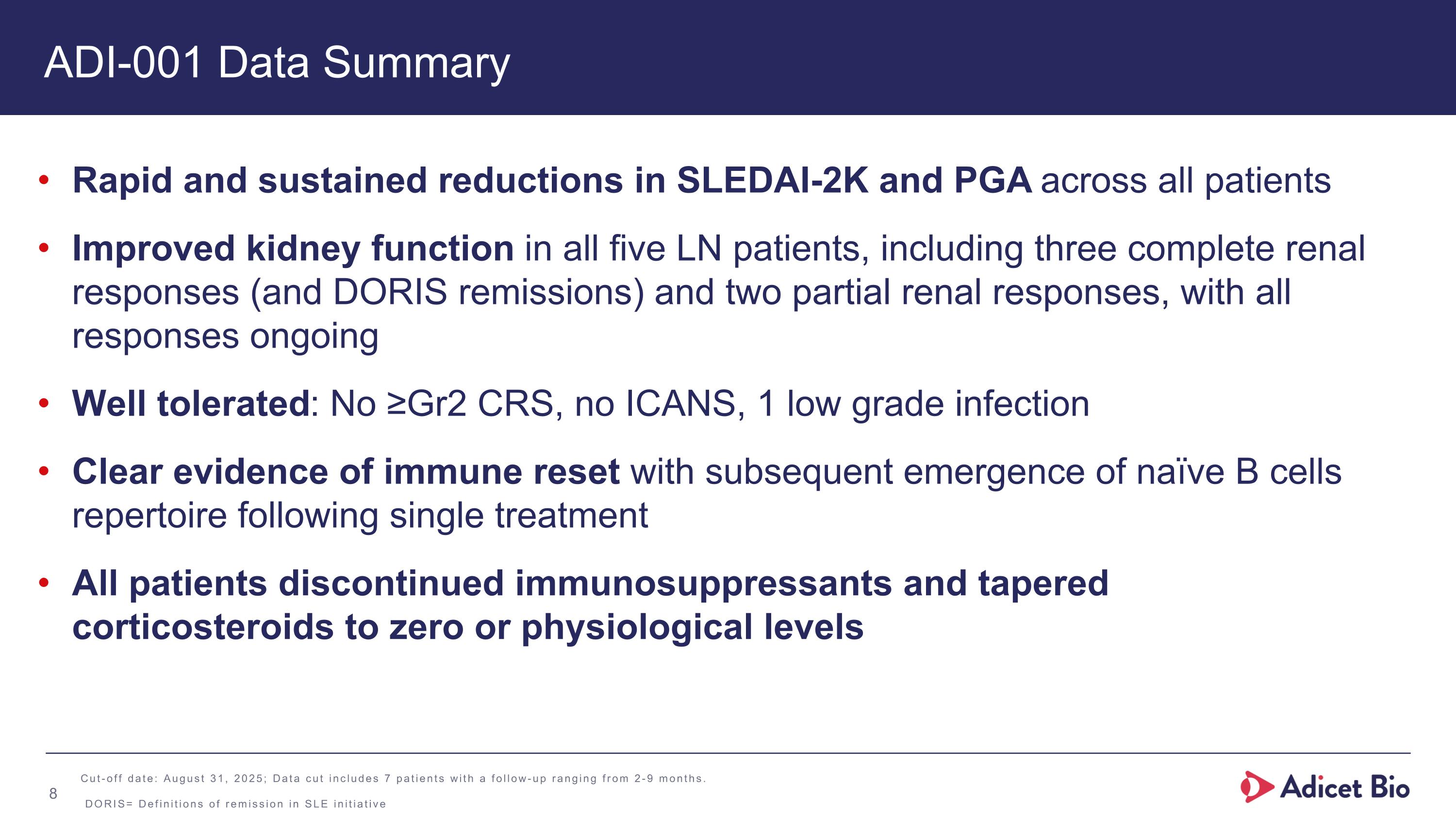
ADI-001 Data Summary Rapid and sustained reductions in SLEDAI-2K and PGA across all patients Improved kidney function in all five LN patients, including three complete renal responses (and DORIS remissions) and two partial renal responses, with all responses ongoing Well tolerated: No ≥Gr2 CRS, no ICANS, 1 low grade infection Clear evidence of immune reset with subsequent emergence of naïve B cells repertoire following single treatment All patients discontinued immunosuppressants and tapered corticosteroids to zero or physiological levels Cut-off date: August 31, 2025; Data cut includes 7 patients with a follow-up ranging from 2-9 months. DORIS= Definitions of remission in SLE initiative
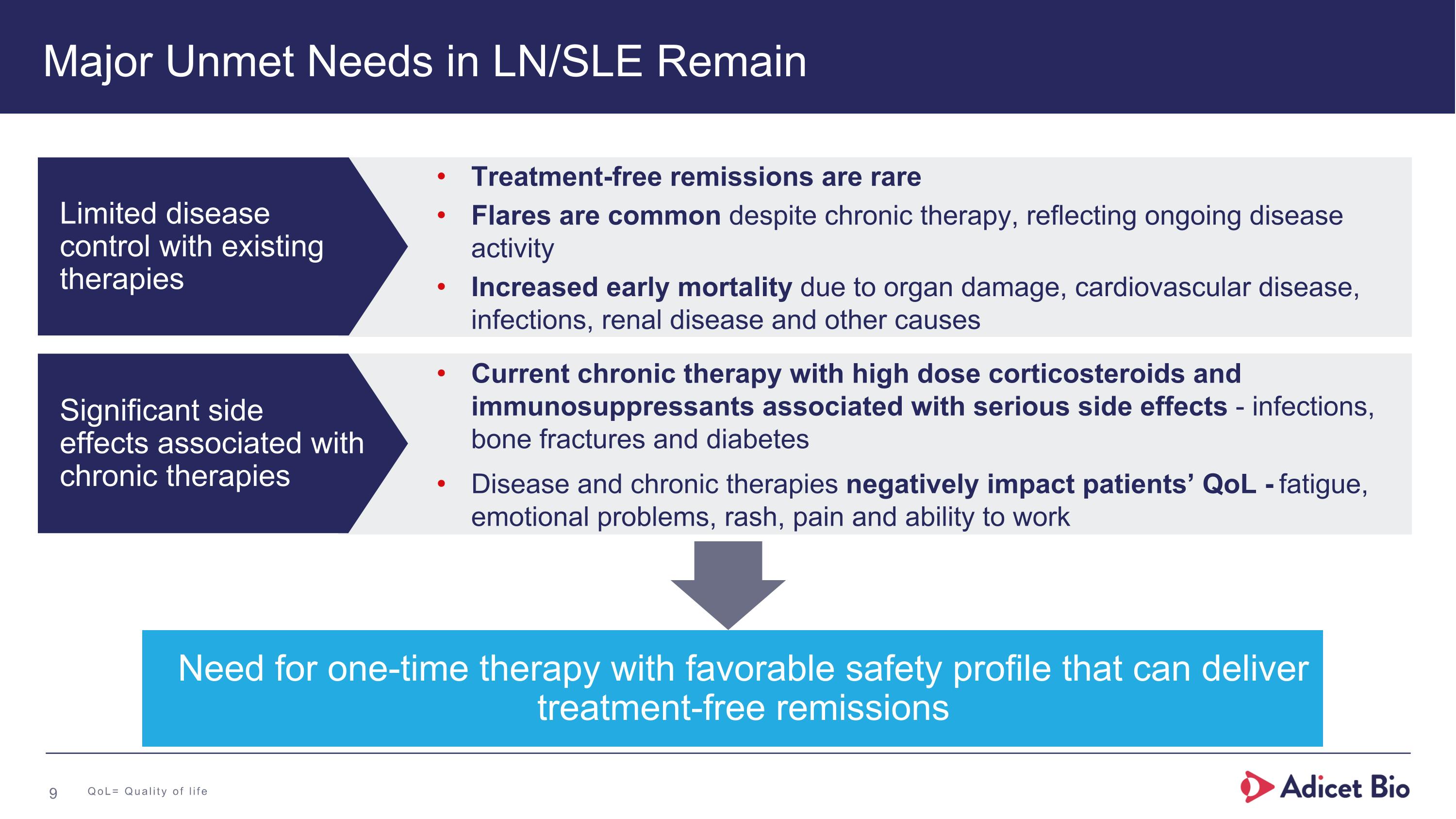
Major Unmet Needs in LN/SLE Remain Need for one-time therapy with favorable safety profile that can deliver treatment-free remissions Treatment-free remissions are rare Flares are common despite chronic therapy, reflecting ongoing disease activity Increased early mortality due to organ damage, cardiovascular disease, infections, renal disease and other causes Current chronic therapy with high dose corticosteroids and immunosuppressants associated with serious side effects - infections, bone fractures and diabetes Disease and chronic therapies negatively impact patients’ QoL - fatigue, emotional problems, rash, pain and ability to work Limited disease control with existing therapies Significant side effects associated with chronic therapies QoL= Quality of life
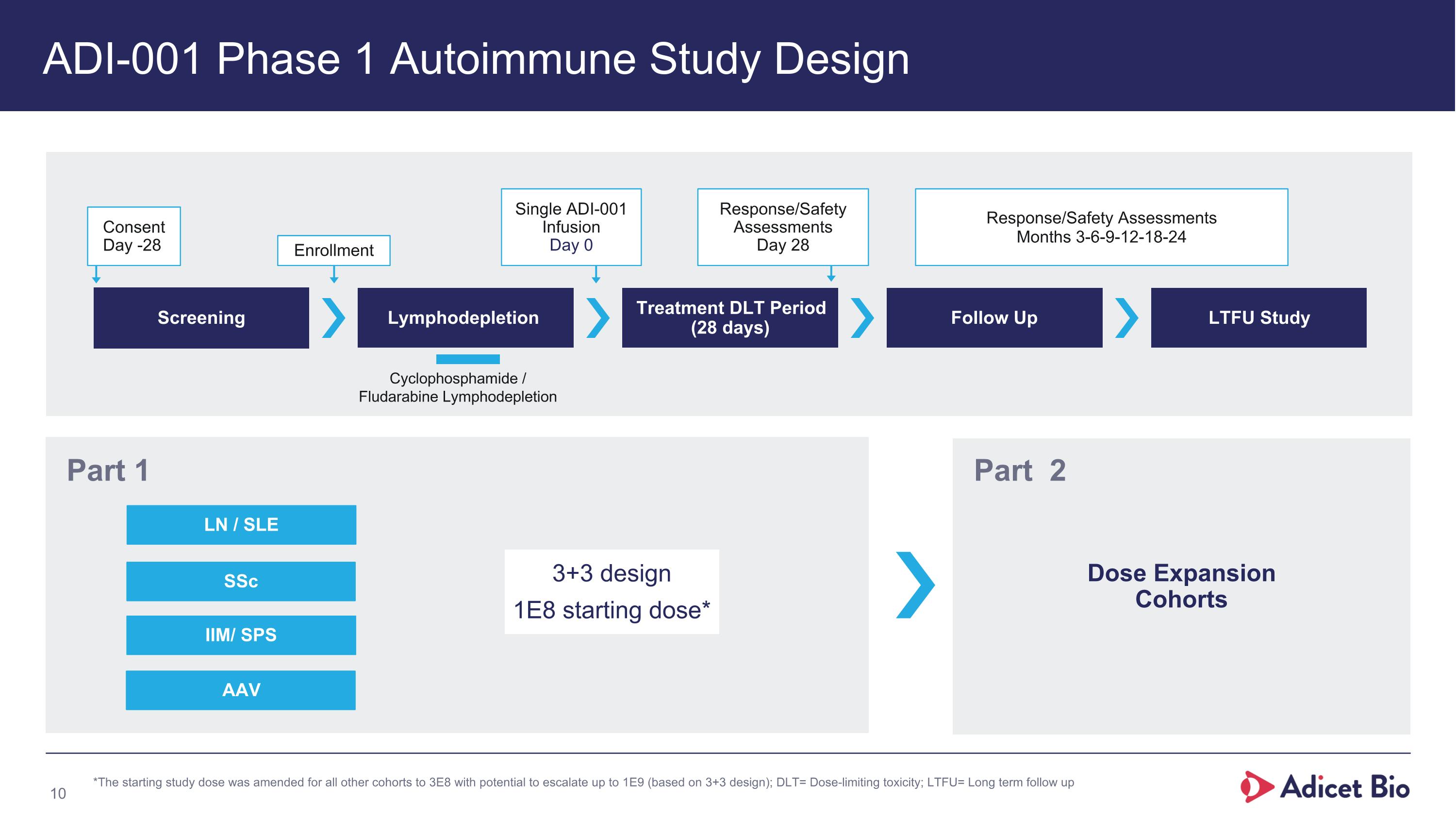
ADI-001 Phase 1 Autoimmune Study Design Screening Lymphodepletion Treatment DLT Period (28 days) Follow Up LTFU Study Consent Day -28 Enrollment Single ADI-001 Infusion Day 0 Response/Safety Assessments Day 28 Response/Safety Assessments Months 3-6-9-12-18-24 Cyclophosphamide / Fludarabine Lymphodepletion SSc LN / SLE Part 1 Dose Expansion Cohorts Part 2 *The starting study dose was amended for all other cohorts to 3E8 with potential to escalate up to 1E9 (based on 3+3 design); DLT= Dose-limiting toxicity; LTFU= Long term follow up 3+3 design 1E8 starting dose* IIM/ SPS AAV
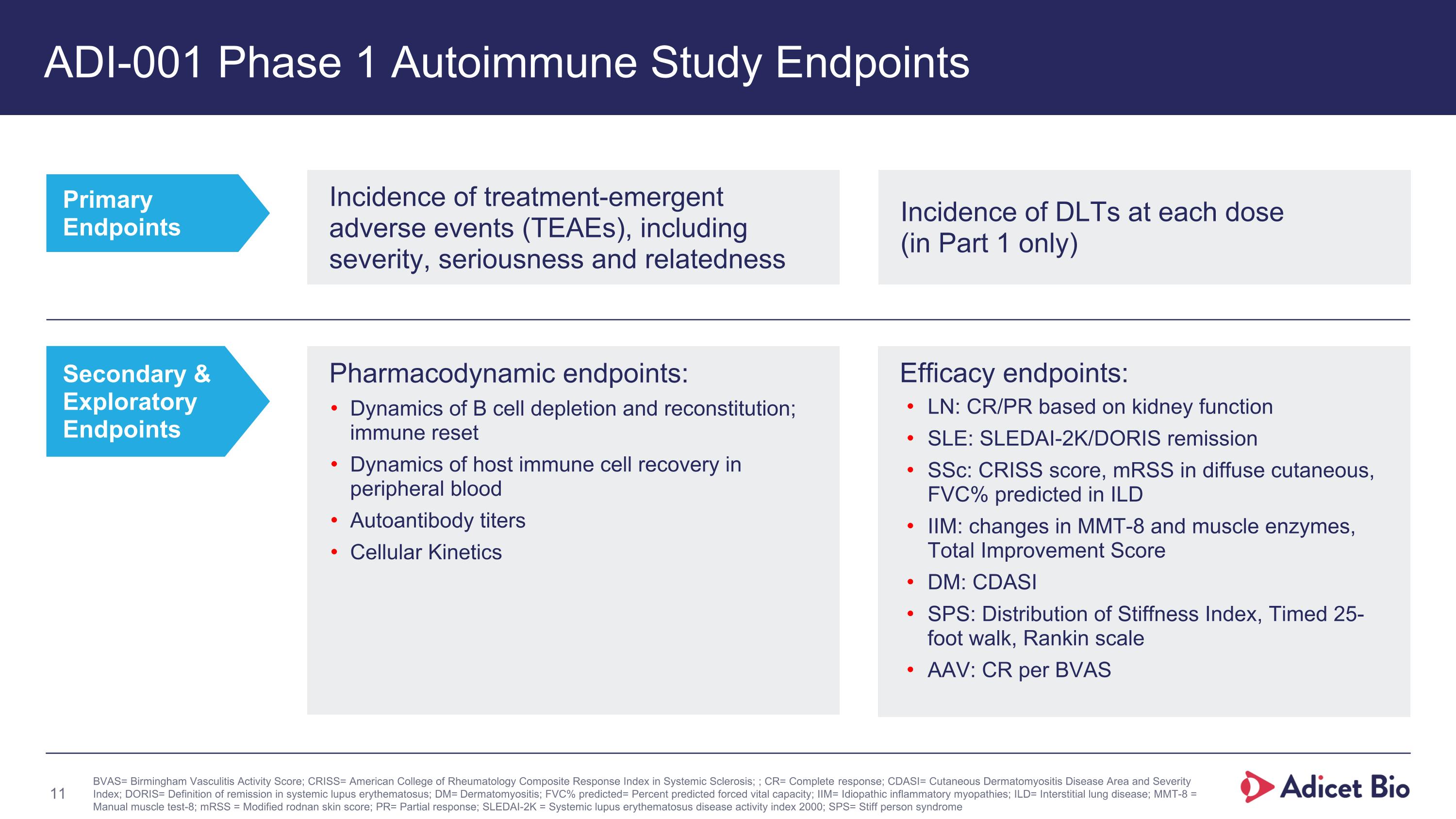
ADI-001 Phase 1 Autoimmune Study Endpoints Primary Endpoints Secondary & Exploratory Endpoints BVAS= Birmingham Vasculitis Activity Score; CRISS= American College of Rheumatology Composite Response Index in Systemic Sclerosis; ; CR= Complete response; CDASI= Cutaneous Dermatomyositis Disease Area and Severity Index; DORIS= Definition of remission in systemic lupus erythematosus; DM= Dermatomyositis; FVC% predicted= Percent predicted forced vital capacity; IIM= Idiopathic inflammatory myopathies; ILD= Interstitial lung disease; MMT-8 = Manual muscle test-8; mRSS = Modified rodnan skin score; PR= Partial response; SLEDAI-2K = Systemic lupus erythematosus disease activity index 2000; SPS= Stiff person syndrome Efficacy endpoints: LN: CR/PR based on kidney function SLE: SLEDAI-2K/DORIS remission SSc: CRISS score, mRSS in diffuse cutaneous, FVC% predicted in ILD IIM: changes in MMT-8 and muscle enzymes, Total Improvement Score DM: CDASI SPS: Distribution of Stiffness Index, Timed 25- foot walk, Rankin scale AAV: CR per BVAS Incidence of treatment-emergent adverse events (TEAEs), including severity, seriousness and relatedness Incidence of DLTs at each dose (in Part 1 only) Pharmacodynamic endpoints: Dynamics of B cell depletion and reconstitution; immune reset Dynamics of host immune cell recovery in peripheral blood Autoantibody titers Cellular Kinetics
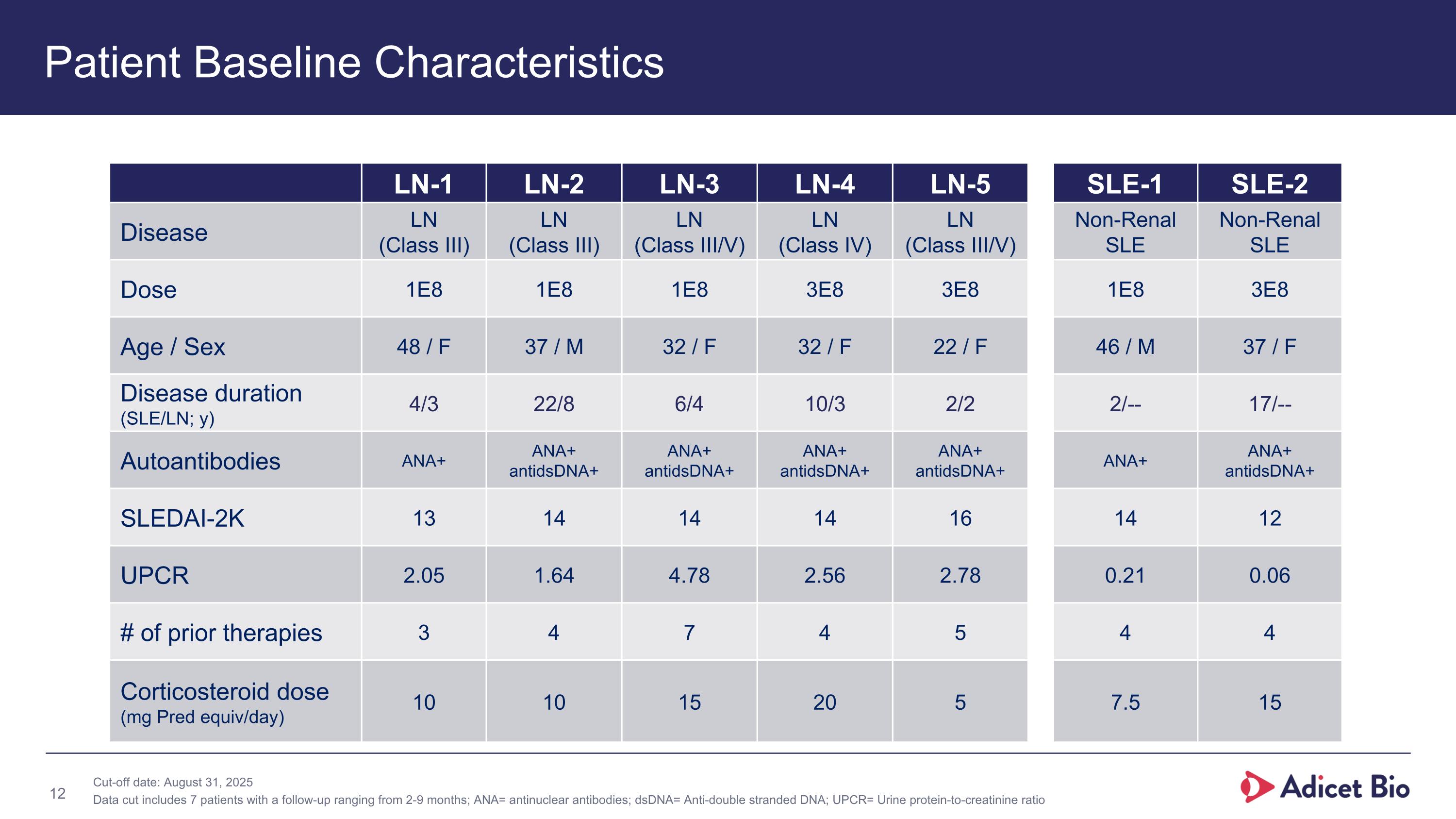
LN-1 LN-2 LN-3 LN-4 LN-5 SLE-1 SLE-2 Disease LN (Class III) LN (Class III) LN (Class III/V) LN (Class IV) LN (Class III/V) Non-Renal SLE Non-Renal SLE Dose 1E8 1E8 1E8 3E8 3E8 1E8 3E8 Age / Sex 48 / F 37 / M 32 / F 32 / F 22 / F 46 / M 37 / F Disease duration (SLE/LN; y) 4/3 22/8 6/4 10/3 2/2 2/-- 17/-- Autoantibodies ANA+ ANA+ antidsDNA+ ANA+ antidsDNA+ ANA+ antidsDNA+ ANA+ antidsDNA+ ANA+ ANA+ antidsDNA+ SLEDAI-2K 13 14 14 14 16 14 12 UPCR 2.05 1.64 4.78 2.56 2.78 0.21 0.06 # of prior therapies 3 4 7 4 5 4 4 Corticosteroid dose (mg Pred equiv/day) 10 10 15 20 5 7.5 15 Patient Baseline Characteristics Cut-off date: August 31, 2025 Data cut includes 7 patients with a follow-up ranging from 2-9 months; ANA= antinuclear antibodies; dsDNA= Anti-double stranded DNA; UPCR= Urine protein-to-creatinine ratio
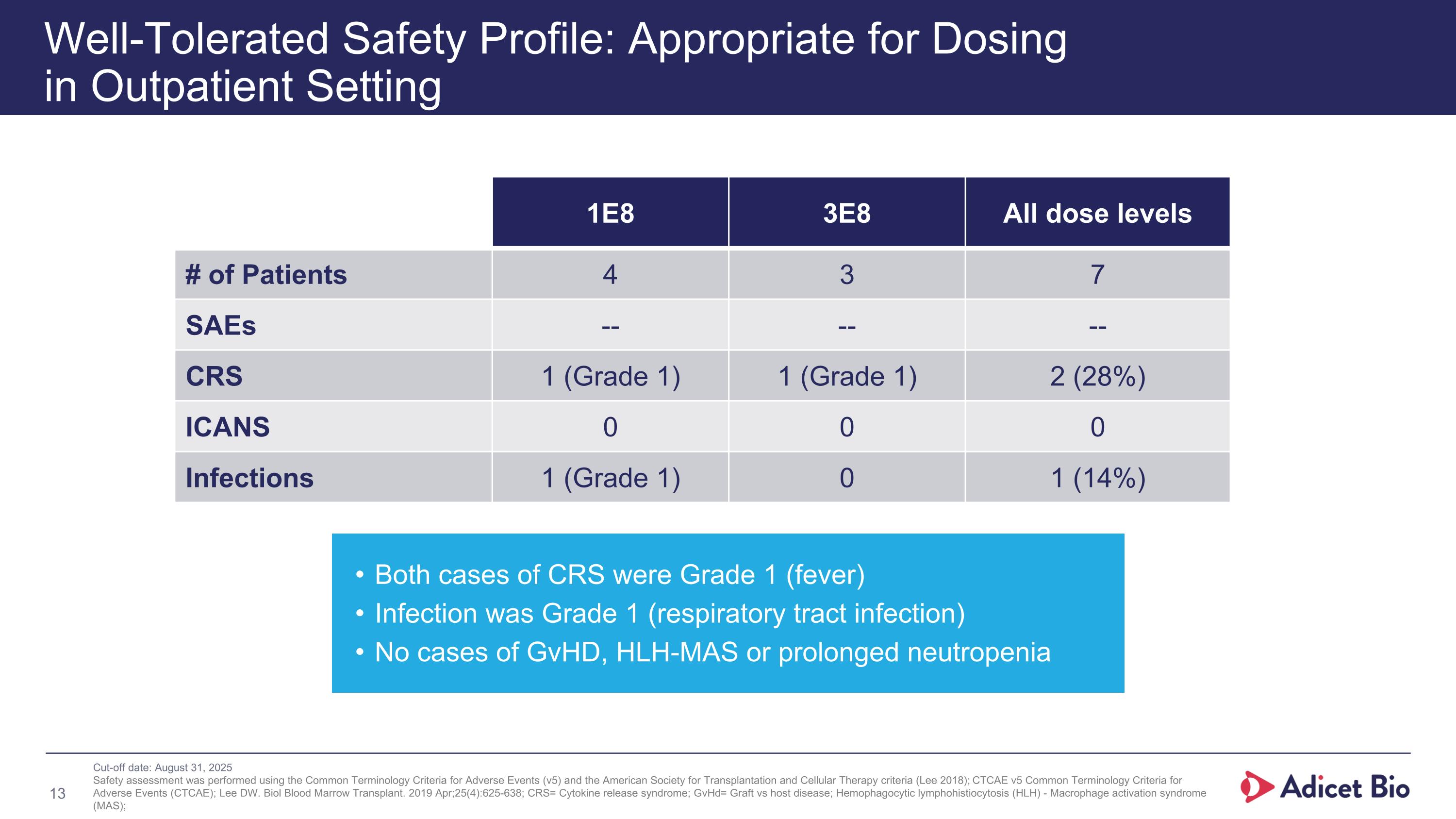
Well-Tolerated Safety Profile: Appropriate for Dosing in Outpatient Setting 1E8 3E8 All dose levels # of Patients 4 3 7 SAEs -- -- -- CRS 1 (Grade 1) 1 (Grade 1) 2 (28%) ICANS 0 0 0 Infections 1 (Grade 1) 0 1 (14%) Both cases of CRS were Grade 1 (fever) Infection was Grade 1 (respiratory tract infection) No cases of GvHD, HLH-MAS or prolonged neutropenia Cut-off date: August 31, 2025 Safety assessment was performed using the Common Terminology Criteria for Adverse Events (v5) and the American Society for Transplantation and Cellular Therapy criteria (Lee 2018); CTCAE v5 Common Terminology Criteria for Adverse Events (CTCAE); Lee DW. Biol Blood Marrow Transplant. 2019 Apr;25(4):625-638; CRS= Cytokine release syndrome; GvHd= Graft vs host disease; Hemophagocytic lymphohistiocytosis (HLH) - Macrophage activation syndrome (MAS); Well-Tolerated Safety Profile: Appropriate for Dosing in Outpatient Setting
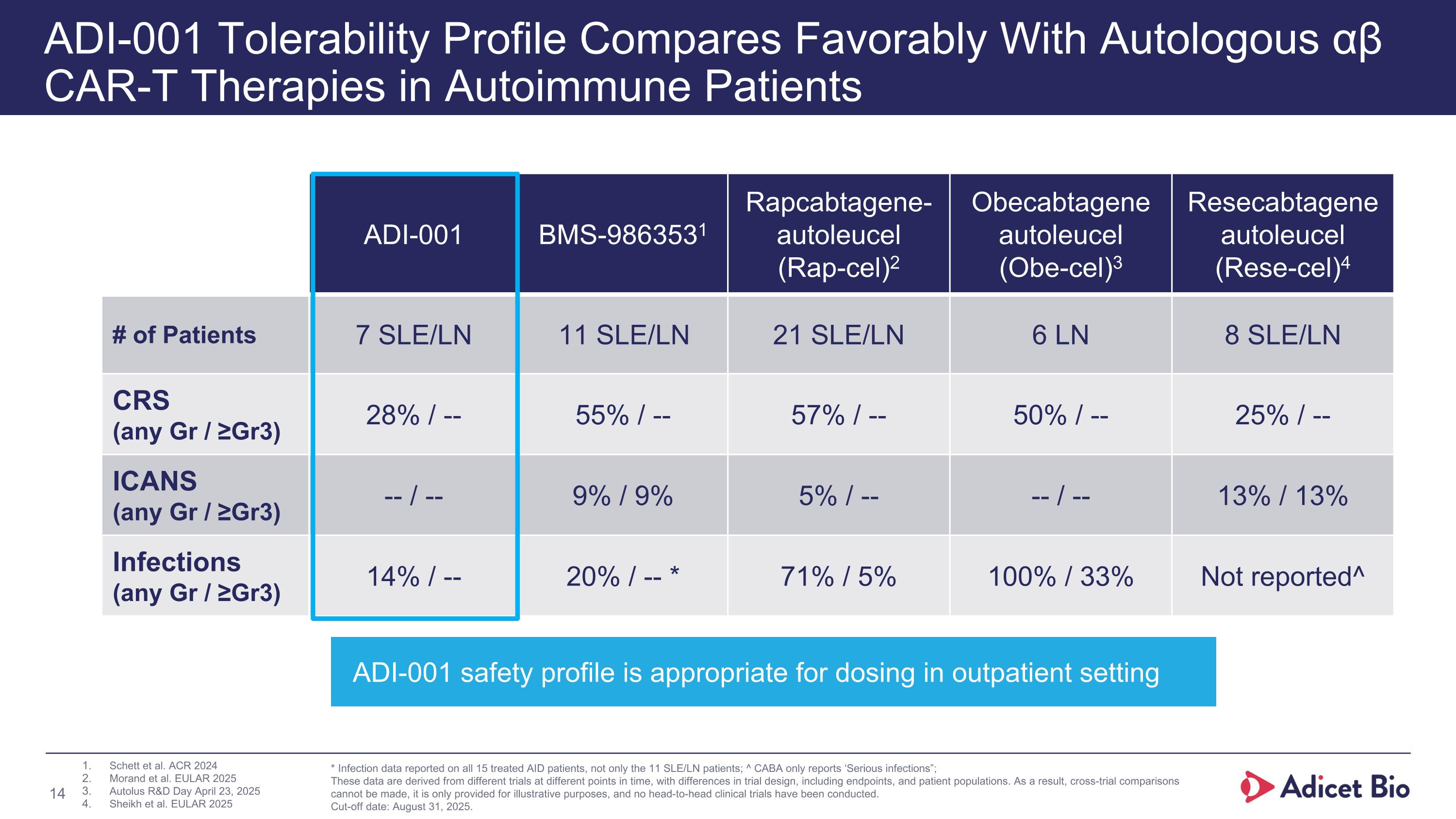
ADI-001 Tolerability Profile Compares Favorably With Autologous αβ CAR-T Therapies in Autoimmune Patients ADI-001 BMS-9863531 Rapcabtagene-autoleucel (Rap-cel)2 Obecabtagene autoleucel (Obe-cel)3 Resecabtagene autoleucel (Rese-cel)4 # of Patients 7 SLE/LN 11 SLE/LN 21 SLE/LN 6 LN 8 SLE/LN CRS (any Gr / ≥Gr3) 28% / -- 55% / -- 57% / -- 50% / -- 25% / -- ICANS (any Gr / ≥Gr3) -- / -- 9% / 9% 5% / -- -- / -- 13% / 13% Infections (any Gr / ≥Gr3) 14% / -- 20% / -- * 71% / 5% 100% / 33% Not reported^ Schett et al. ACR 2024 Morand et al. EULAR 2025 Autolus R&D Day April 23, 2025 Sheikh et al. EULAR 2025 * Infection data reported on all 15 treated AID patients, not only the 11 SLE/LN patients; ^ CABA only reports ‘Serious infections”; These data are derived from different trials at different points in time, with differences in trial design, including endpoints, and patient populations. As a result, cross-trial comparisons cannot be made, it is only provided for illustrative purposes, and no head-to-head clinical trials have been conducted. Cut-off date: August 31, 2025. ADI-001 safety profile is appropriate for dosing in outpatient setting
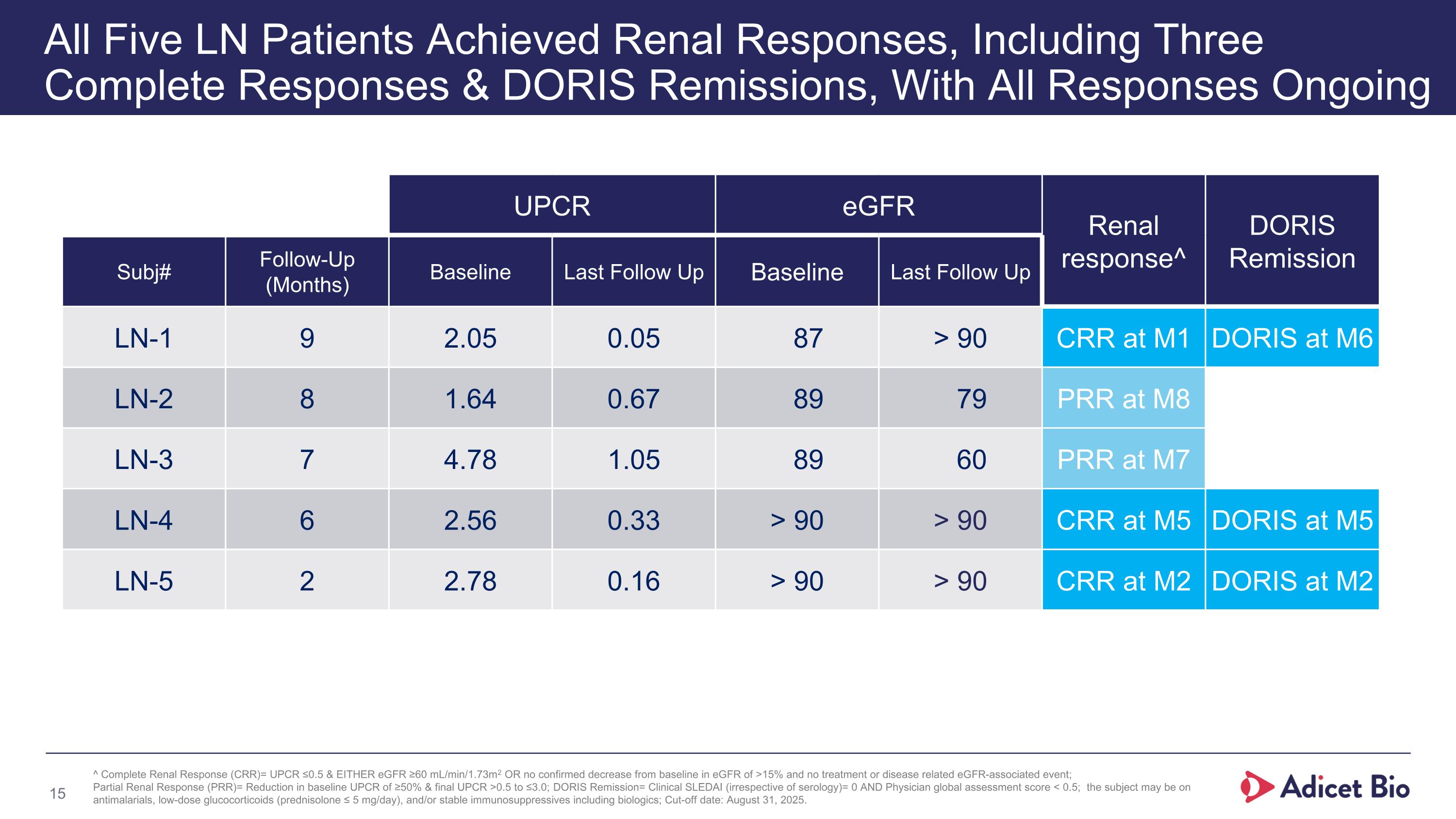
UPCR eGFR Renal response^ DORIS Remission Subj# Follow-Up (Months) Baseline Last Follow Up Baseline Last Follow Up LN-1 9 2.05 0.05 87 > 90 CRR at M1 DORIS at M6 LN-2 8 1.64 0.67 89 79 PRR at M8 LN-3 7 4.78 1.05 89 60 PRR at M7 LN-4 6 2.56 0.33 > 90 > 90 CRR at M5 DORIS at M5 LN-5 2 2.78 0.16 > 90 > 90 CRR at M2 DORIS at M2 All Five LN Patients Achieved Renal Responses, Including Three Complete Responses & DORIS Remissions, With All Responses Ongoing ^ Complete Renal Response (CRR)= UPCR ≤0.5 & EITHER eGFR ≥60 mL/min/1.73m2 OR no confirmed decrease from baseline in eGFR of >15% and no treatment or disease related eGFR-associated event; Partial Renal Response (PRR)= Reduction in baseline UPCR of ≥50% & final UPCR >0.5 to ≤3.0; DORIS Remission= Clinical SLEDAI (irrespective of serology)= 0 AND Physician global assessment score < 0.5; the subject may be on antimalarials, low-dose glucocorticoids (prednisolone ≤ 5 mg/day), and/or stable immunosuppressives including biologics; Cut-off date: August 31, 2025.
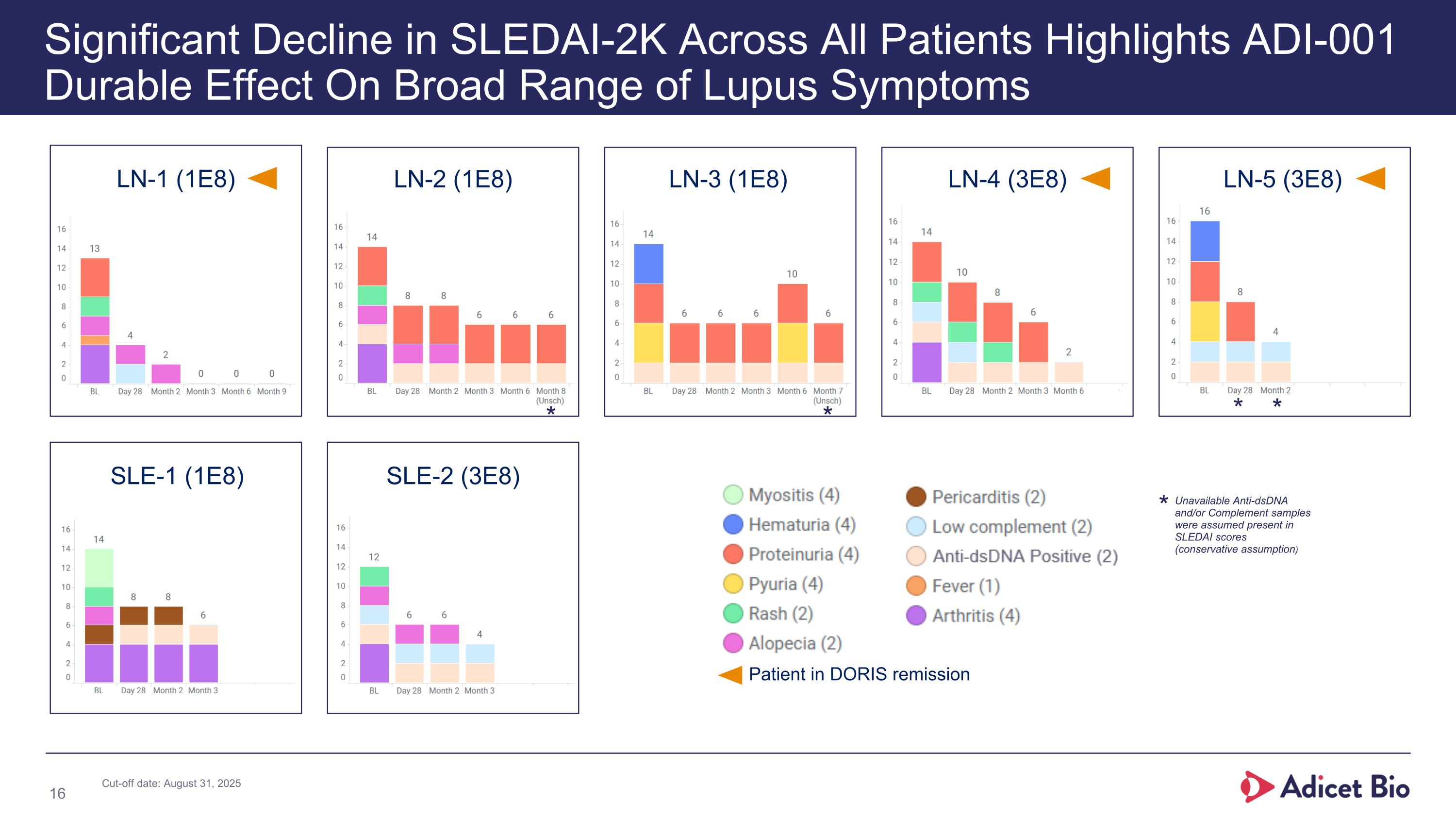
LN-1 (1E8) LN-2 (1E8) LN-3 (1E8) LN-4 (3E8) LN-5 (3E8) SLE-1 (1E8) SLE-2 (3E8) Significant Decline in SLEDAI-2K Across All Patients Highlights ADI-001 Durable Effect On Broad Range of Lupus Symptoms Patient in DORIS remission * * * * Unavailable Anti-dsDNA and/or Complement samples were assumed present in SLEDAI scores (conservative assumption) * Cut-off date: August 31, 2025
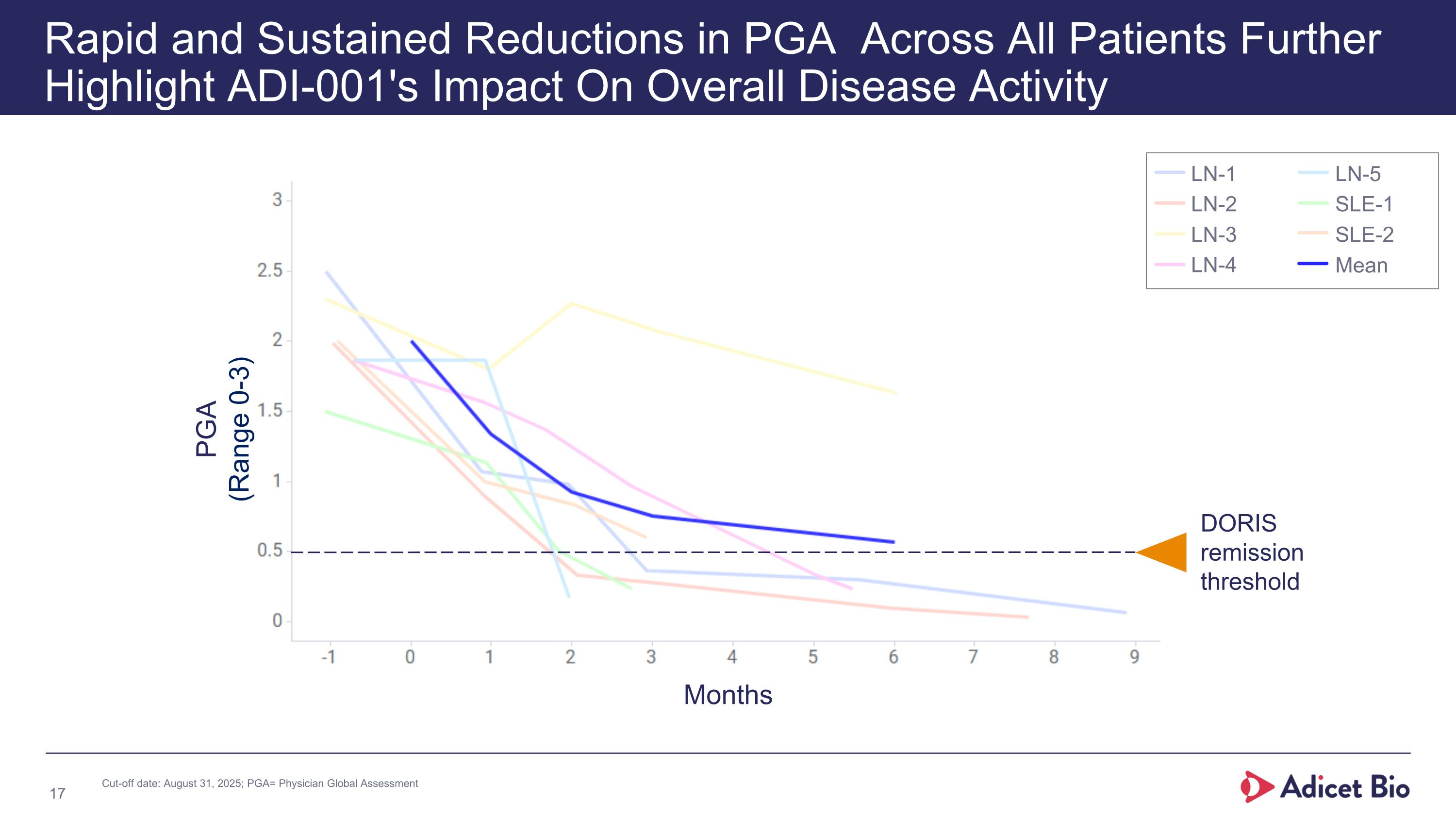
Rapid and Sustained Reductions in PGA Across All Patients Further Highlight ADI-001's Impact On Overall Disease Activity PGA (Range 0-3) DORIS remission threshold LN-1 LN-2 LN-3 LN-4 LN-5 SLE-1 SLE-2 Mean Months Cut-off date: August 31, 2025; PGA= Physician Global Assessment
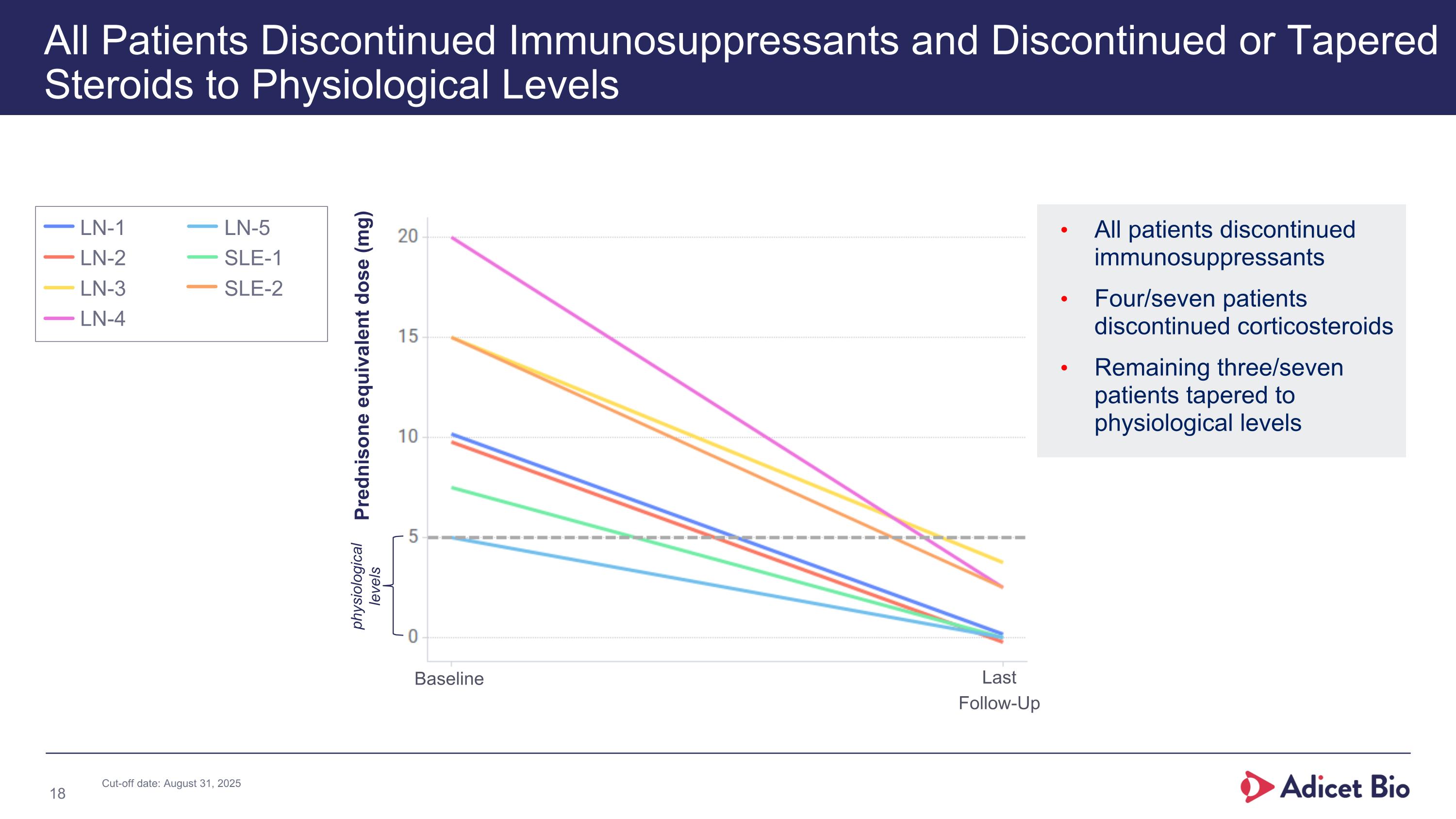
All patients discontinued immunosuppressants Four/seven patients discontinued corticosteroids Remaining three/seven patients tapered to physiological levels All Patients Discontinued Immunosuppressants and Discontinued or Tapered Steroids to Physiological Levels Prednisone equivalent dose (mg) physiological levels Baseline Last Follow-Up LN-1 LN-2 LN-3 LN-4 LN-5 SLE-1 SLE-2 Cut-off date: August 31, 2025
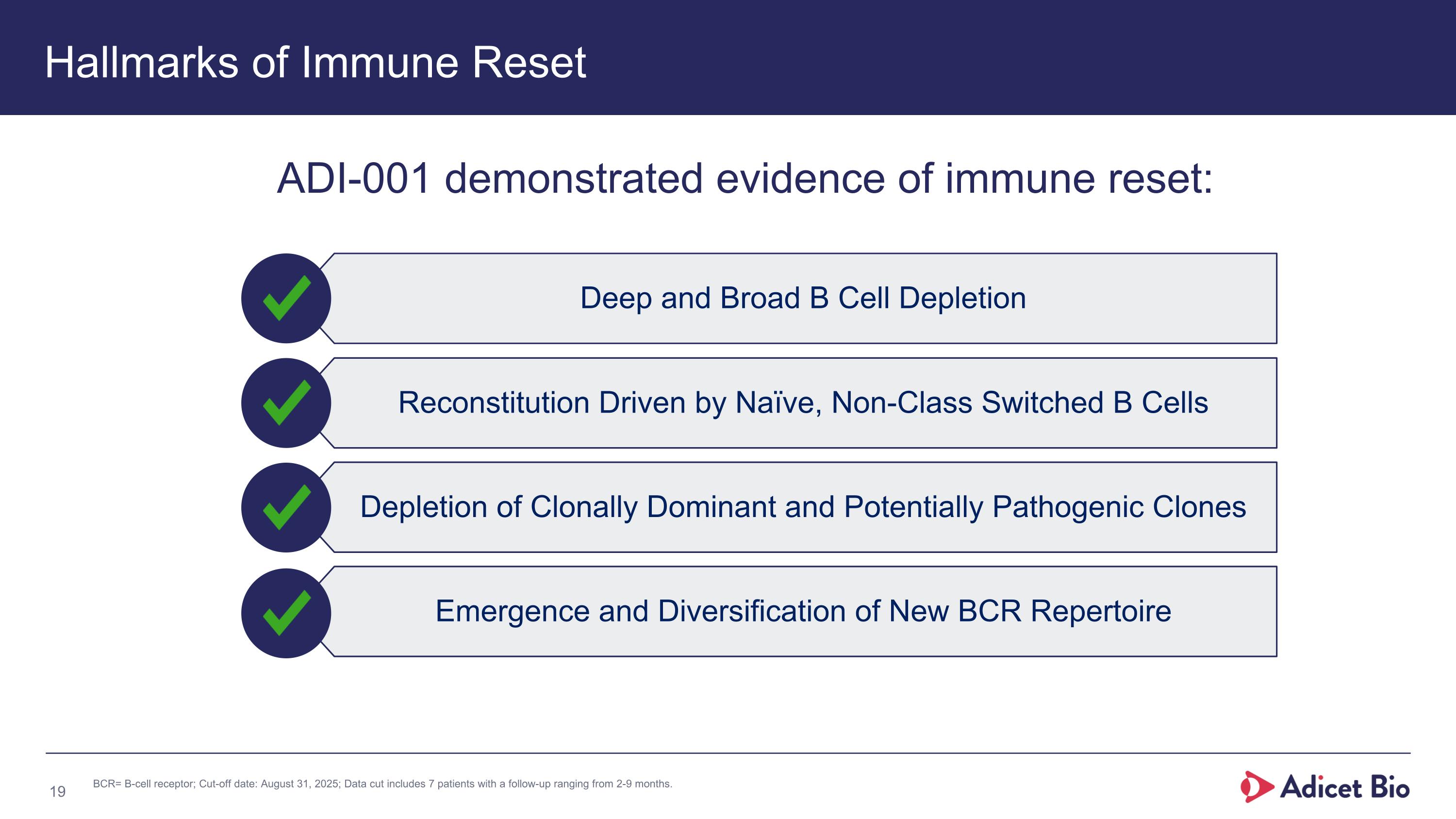
Deep and Broad B Cell Depletion Reconstitution Driven by Naïve, Non-Class Switched B Cells Depletion of Clonally Dominant and Potentially Pathogenic Clones Emergence and Diversification of New BCR Repertoire Hallmarks of Immune Reset ADI-001 demonstrated evidence of immune reset: BCR= B-cell receptor; Cut-off date: August 31, 2025; Data cut includes 7 patients with a follow-up ranging from 2-9 months.
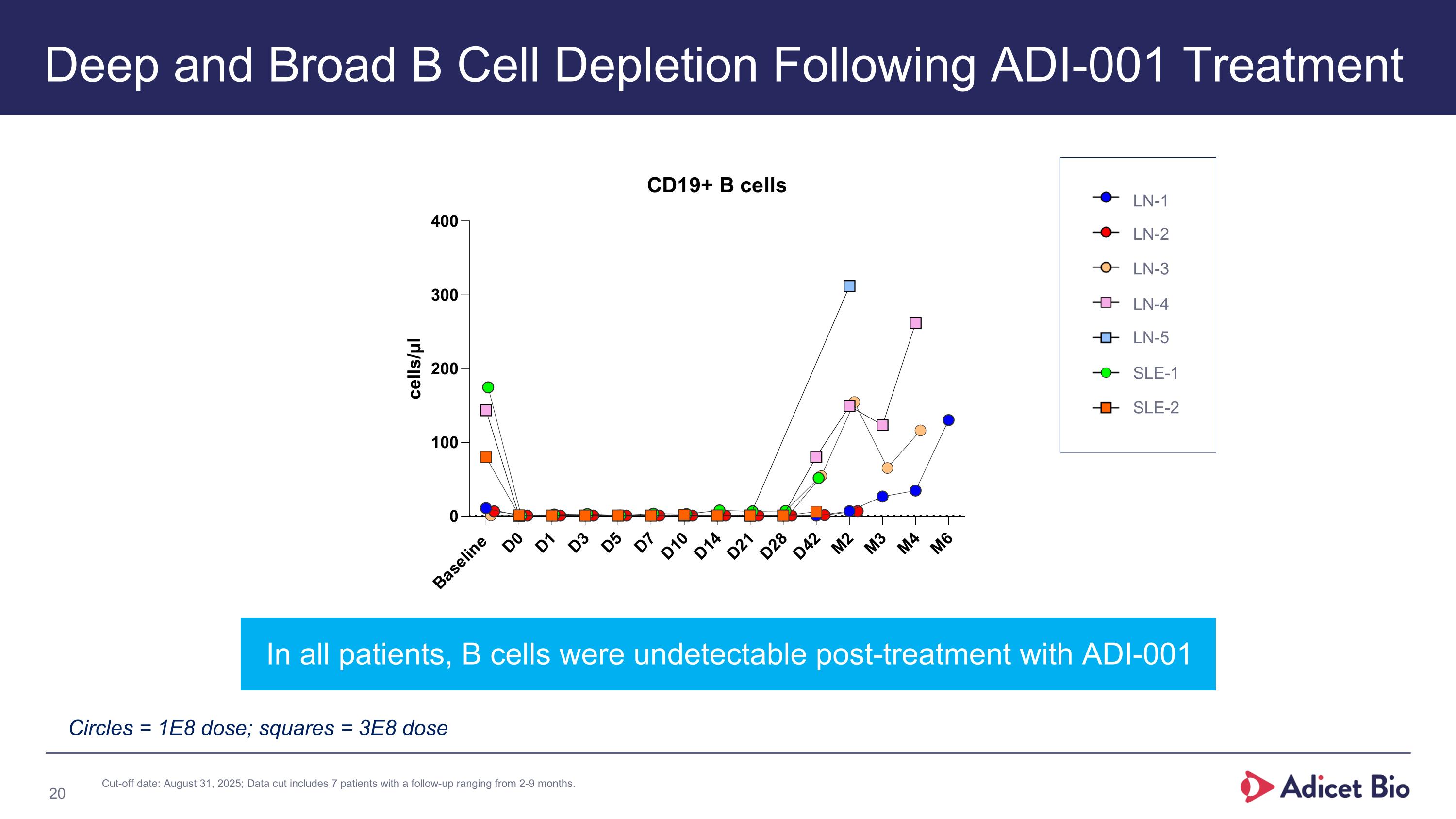
Deep and Broad B Cell Depletion Following ADI-001 Treatment In all patients, B cells were undetectable post-treatment with ADI-001 Circles = 1E8 dose; squares = 3E8 dose LN-1 LN-2 LN-3 LN-4 LN-5 SLE-1 SLE-2 Cut-off date: August 31, 2025; Data cut includes 7 patients with a follow-up ranging from 2-9 months.
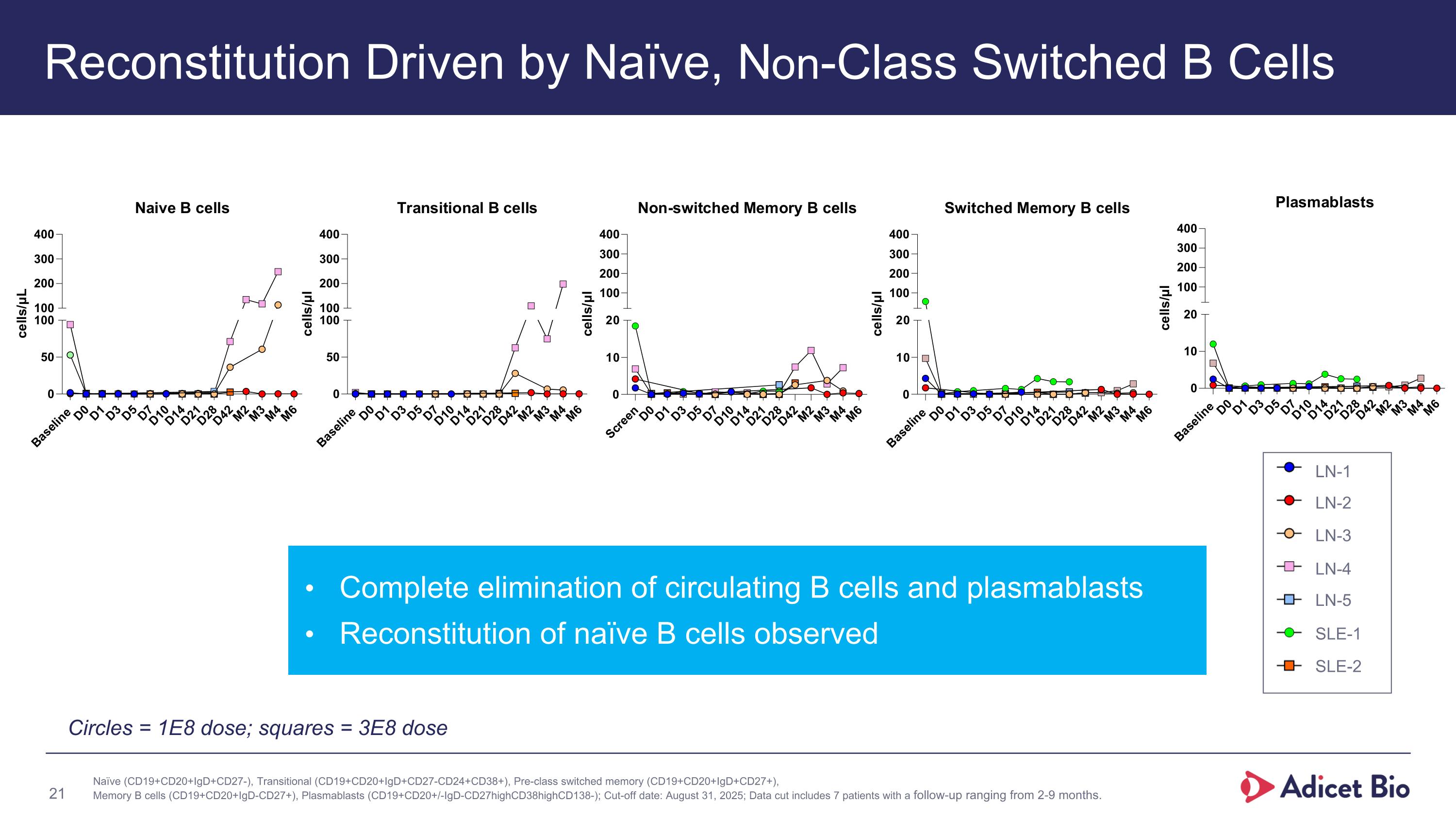
Reconstitution Driven by Naïve, Non-Class Switched B Cells Naïve (CD19+CD20+IgD+CD27-), Transitional (CD19+CD20+IgD+CD27-CD24+CD38+), Pre-class switched memory (CD19+CD20+IgD+CD27+), Memory B cells (CD19+CD20+IgD-CD27+), Plasmablasts (CD19+CD20+/-IgD-CD27highCD38highCD138-); Cut-off date: August 31, 2025; Data cut includes 7 patients with a follow-up ranging from 2-9 months. Complete elimination of circulating B cells and plasmablasts Reconstitution of naïve B cells observed Circles = 1E8 dose; squares = 3E8 dose LN-1 LN-2 LN-3 LN-4 LN-5 SLE-1 SLE-2
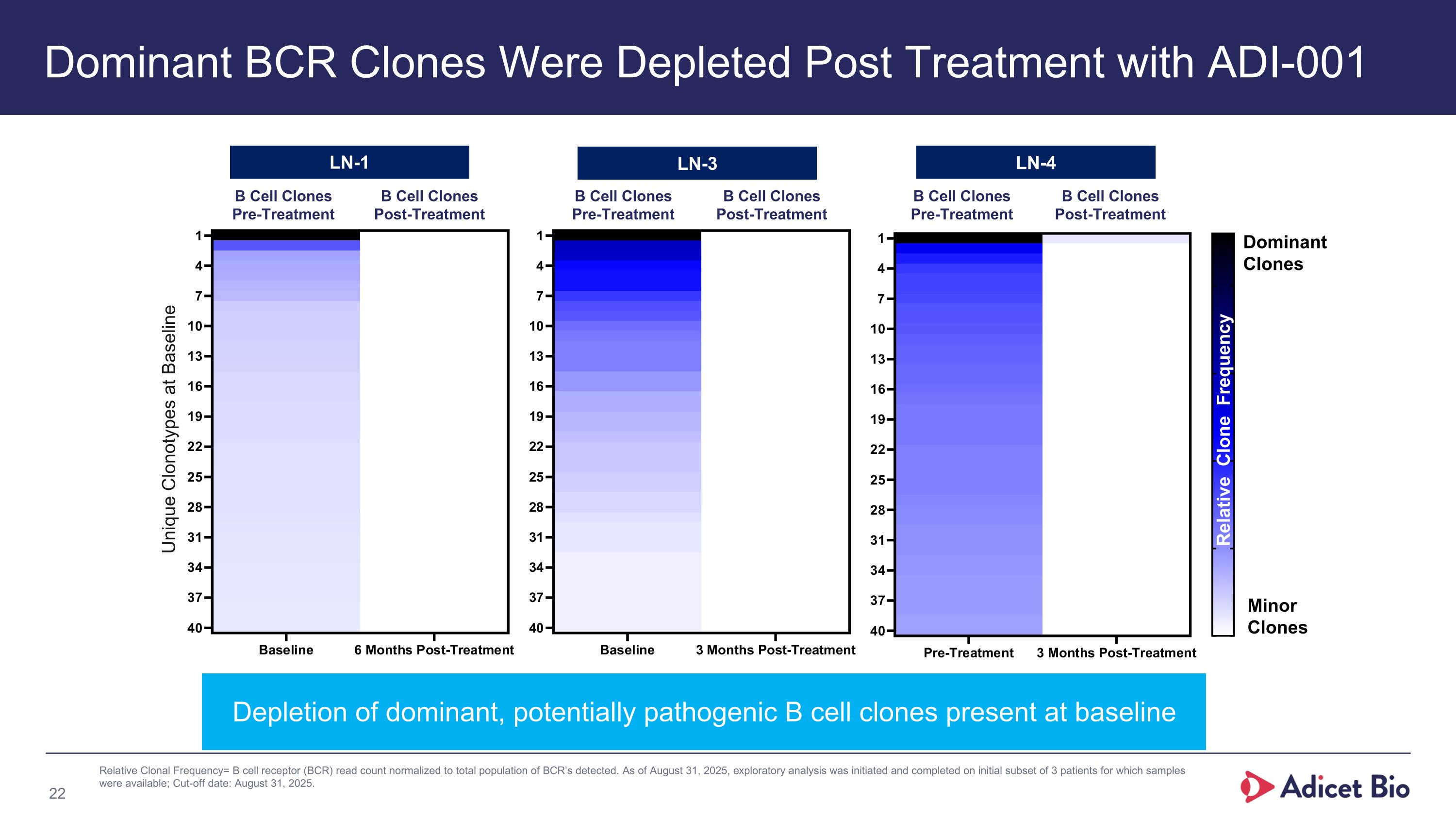
Dominant BCR Clones Were Depleted Post Treatment with ADI-001 Depletion of dominant, potentially pathogenic B cell clones present at baseline Dominant Clones Minor Clones Relative Clone Frequency Relative Clonal Frequency= B cell receptor (BCR) read count normalized to total population of BCR’s detected. As of August 31, 2025, exploratory analysis was initiated and completed on initial subset of 3 patients for which samples were available; Cut-off date: August 31, 2025. B Cell Clones Pre-Treatment B Cell Clones Post-Treatment LN-1 LN-3 LN-4 B Cell Clones Pre-Treatment B Cell Clones Post-Treatment B Cell Clones Pre-Treatment B Cell Clones Post-Treatment Unique Clonotypes at Baseline
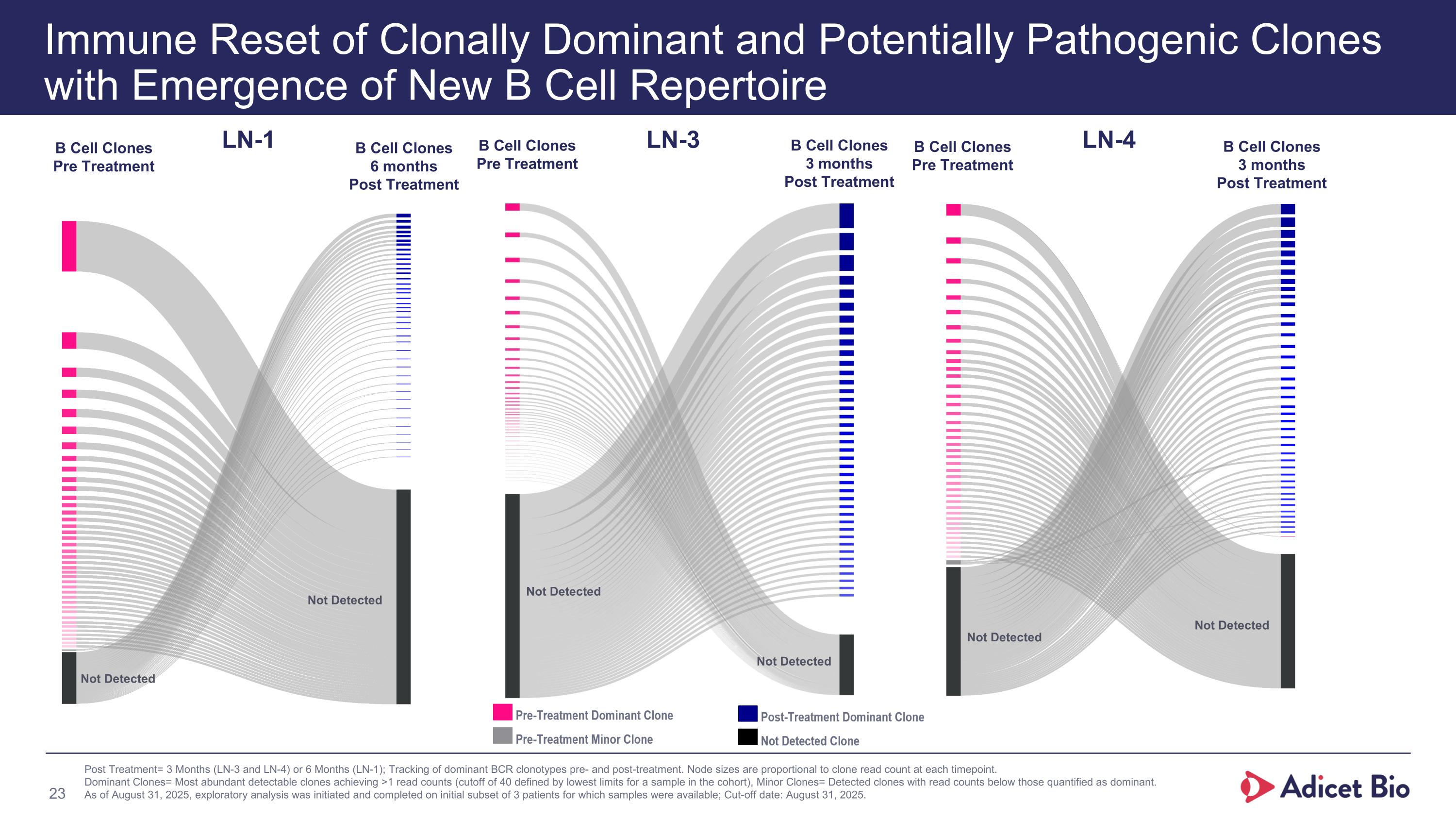
Immune Reset of Clonally Dominant and Potentially Pathogenic Clones with Emergence of New B Cell Repertoire Post Treatment= 3 Months (LN-3 and LN-4) or 6 Months (LN-1); Tracking of dominant BCR clonotypes pre- and post-treatment. Node sizes are proportional to clone read count at each timepoint. Dominant Clones= Most abundant detectable clones achieving >1 read counts (cutoff of 40 defined by lowest limits for a sample in the cohort), Minor Clones= Detected clones with read counts below those quantified as dominant. As of August 31, 2025, exploratory analysis was initiated and completed on initial subset of 3 patients for which samples were available; Cut-off date: August 31, 2025. LN-3 B Cell Clones Pre Treatment B Cell Clones 3 months Post Treatment Not Detected Not Detected LN-1 B Cell Clones Pre Treatment B Cell Clones 6 months Post Treatment Not Detected Not Detected LN-4 B Cell Clones Pre Treatment B Cell Clones 3 months Post Treatment Not Detected Not Detected
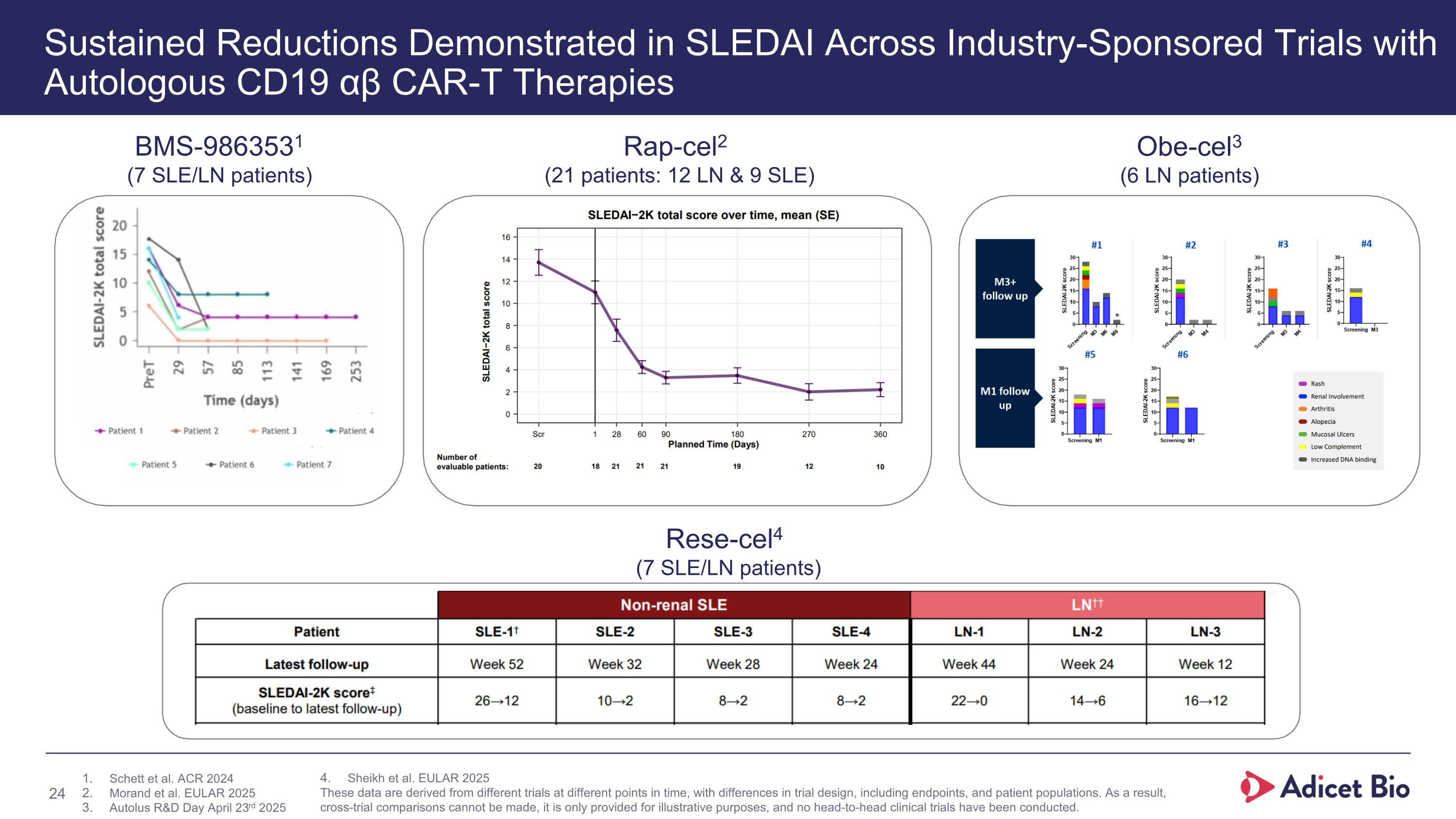
Sustained Reductions Demonstrated in SLEDAI Across Industry-Sponsored Trials with Autologous CD19 αβ CAR-T Therapies BMS-9863531 (7 SLE/LN patients) Rap-cel2 (21 patients: 12 LN & 9 SLE) Obe-cel3 (6 LN patients) Rese-cel4 (7 SLE/LN patients) Schett et al. ACR 2024 Morand et al. EULAR 2025 Autolus R&D Day April 23rd 2025 Sheikh et al. EULAR 2025 These data are derived from different trials at different points in time, with differences in trial design, including endpoints, and patient populations. As a result, cross-trial comparisons cannot be made, it is only provided for illustrative purposes, and no head-to-head clinical trials have been conducted.
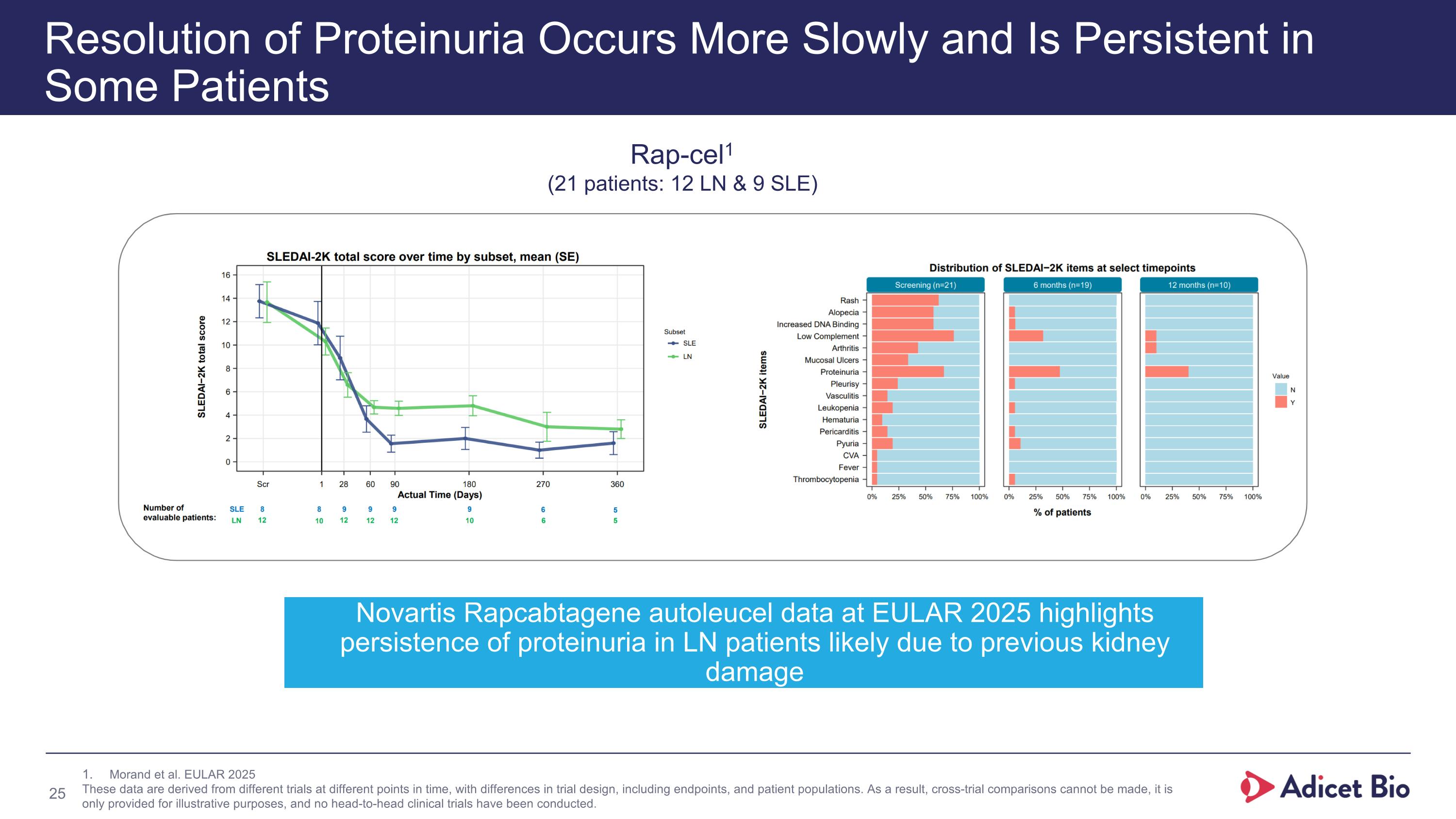
Resolution of Proteinuria Occurs More Slowly and Is Persistent in Some Patients Rap-cel1 (21 patients: 12 LN & 9 SLE) Morand et al. EULAR 2025 These data are derived from different trials at different points in time, with differences in trial design, including endpoints, and patient populations. As a result, cross-trial comparisons cannot be made, it is only provided for illustrative purposes, and no head-to-head clinical trials have been conducted. Novartis Rapcabtagene autoleucel data at EULAR 2025 highlights persistence of proteinuria in LN patients likely due to previous kidney damage
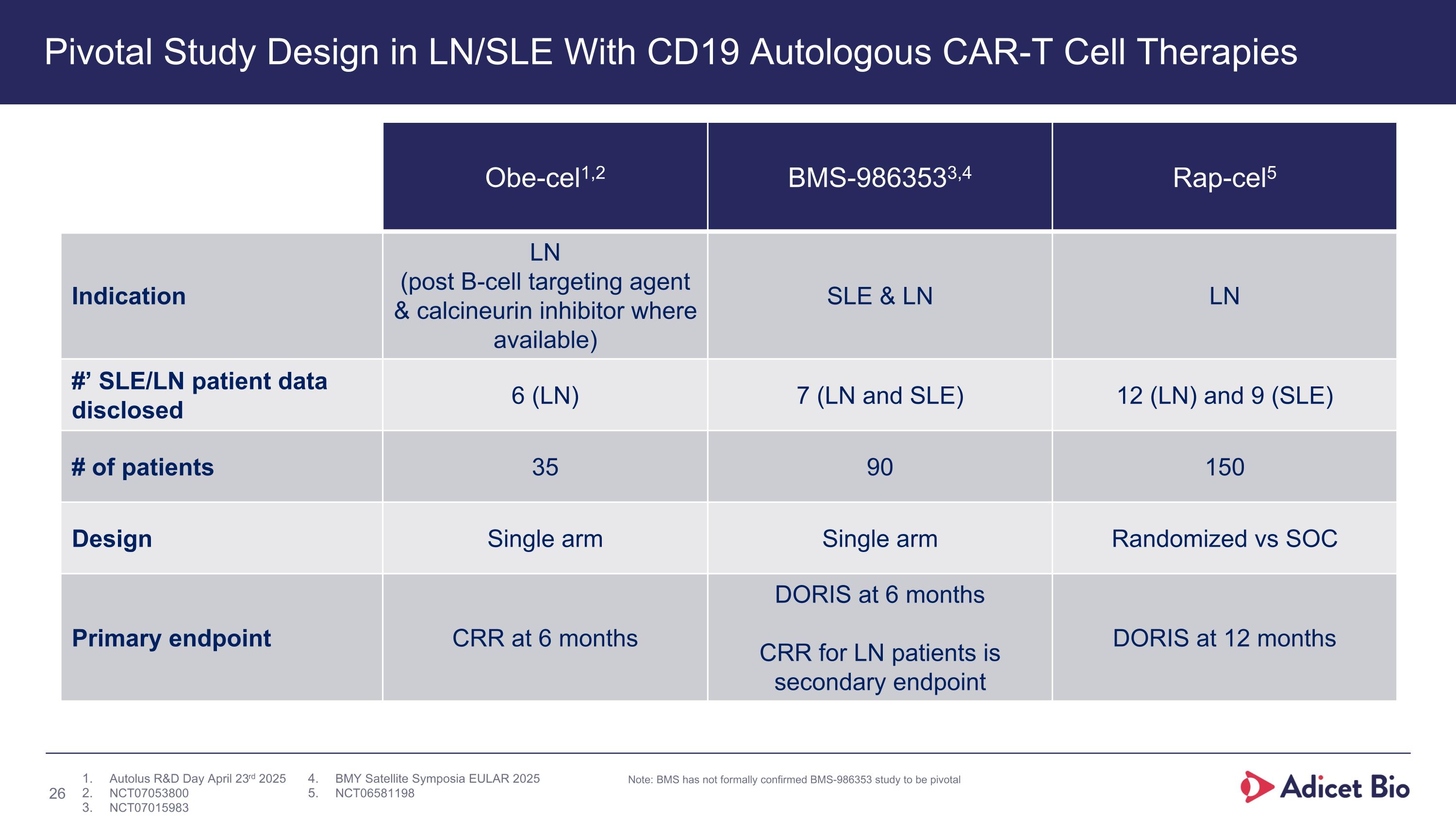
Pivotal Study Design in LN/SLE With CD19 Autologous CAR-T Cell Therapies Obe-cel1,2 BMS-9863533,4 Rap-cel5 Indication LN (post B-cell targeting agent & calcineurin inhibitor where available) SLE & LN LN #’ SLE/LN patient data disclosed 6 (LN) 7 (LN and SLE) 12 (LN) and 9 (SLE) # of patients 35 90 150 Design Single arm Single arm Randomized vs SOC Primary endpoint CRR at 6 months DORIS at 6 months CRR for LN patients is secondary endpoint DORIS at 12 months Note: BMS has not formally confirmed BMS-986353 study to be pivotal Autolus R&D Day April 23rd 2025 NCT07053800 NCT07015983 BMY Satellite Symposia EULAR 2025 NCT06581198
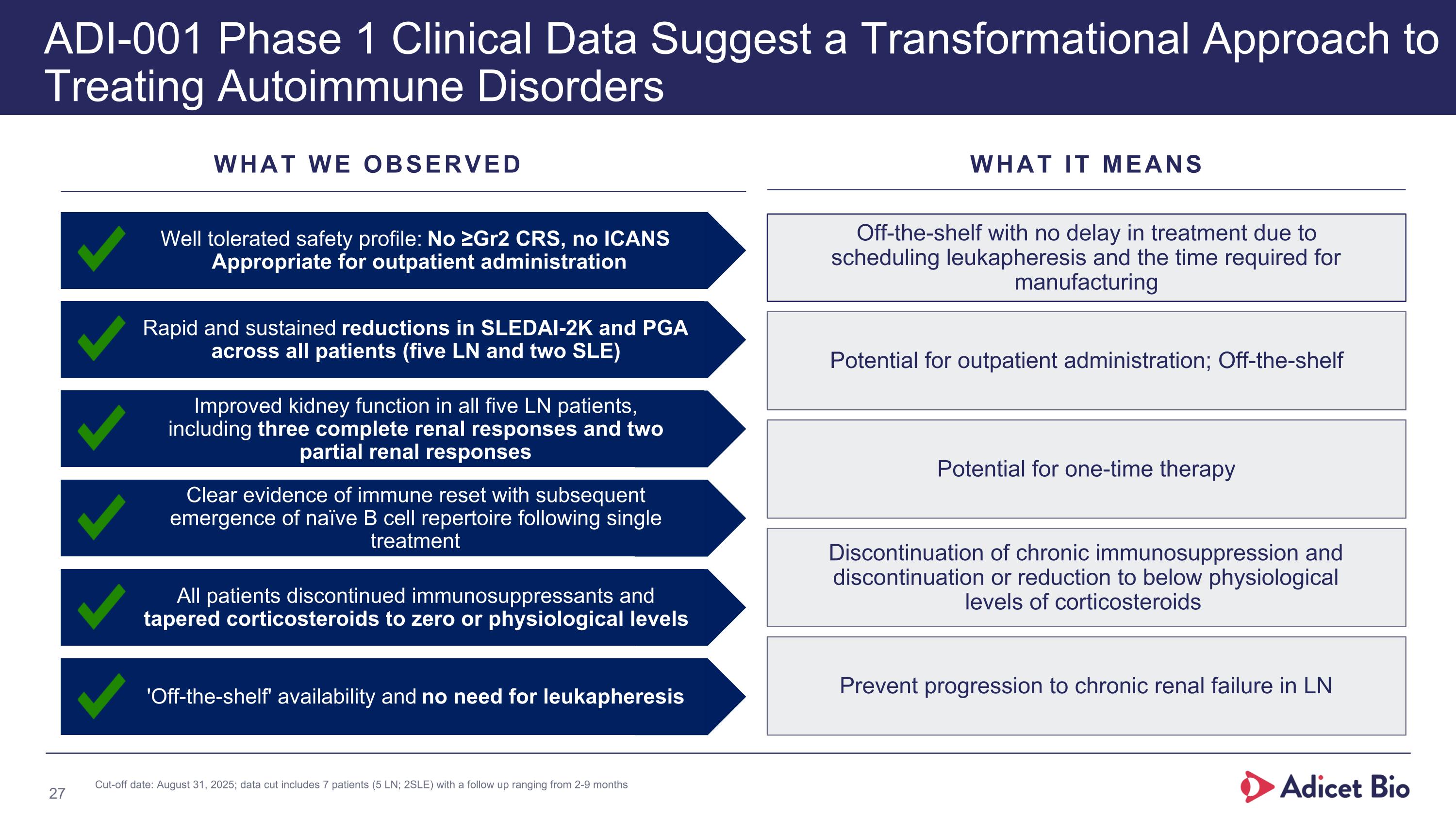
WHAT WE OBSERVED WHAT IT MEANS new ADI-001 Phase 1 Clinical Data Suggest a Transformational Approach to Treating Autoimmune Disorders Off-the-shelf with no delay in treatment due to scheduling leukapheresis and the time required for manufacturing Potential for outpatient administration; Off-the-shelf Discontinuation of chronic immunosuppression and discontinuation or reduction to below physiological levels of corticosteroids Prevent progression to chronic renal failure in LN Potential for one-time therapy 'Off-the-shelf' availability and no need for leukapheresis Well tolerated safety profile: No ≥Gr2 CRS, no ICANS Appropriate for outpatient administration Rapid and sustained reductions in SLEDAI-2K and PGA across all patients (five LN and two SLE) Improved kidney function in all five LN patients, including three complete renal responses and two partial renal responses Clear evidence of immune reset with subsequent emergence of naïve B cell repertoire following single treatment All patients discontinued immunosuppressants and tapered corticosteroids to zero or physiological levels Cut-off date: August 31, 2025; data cut includes 7 patients (5 LN; 2SLE) with a follow up ranging from 2-9 months
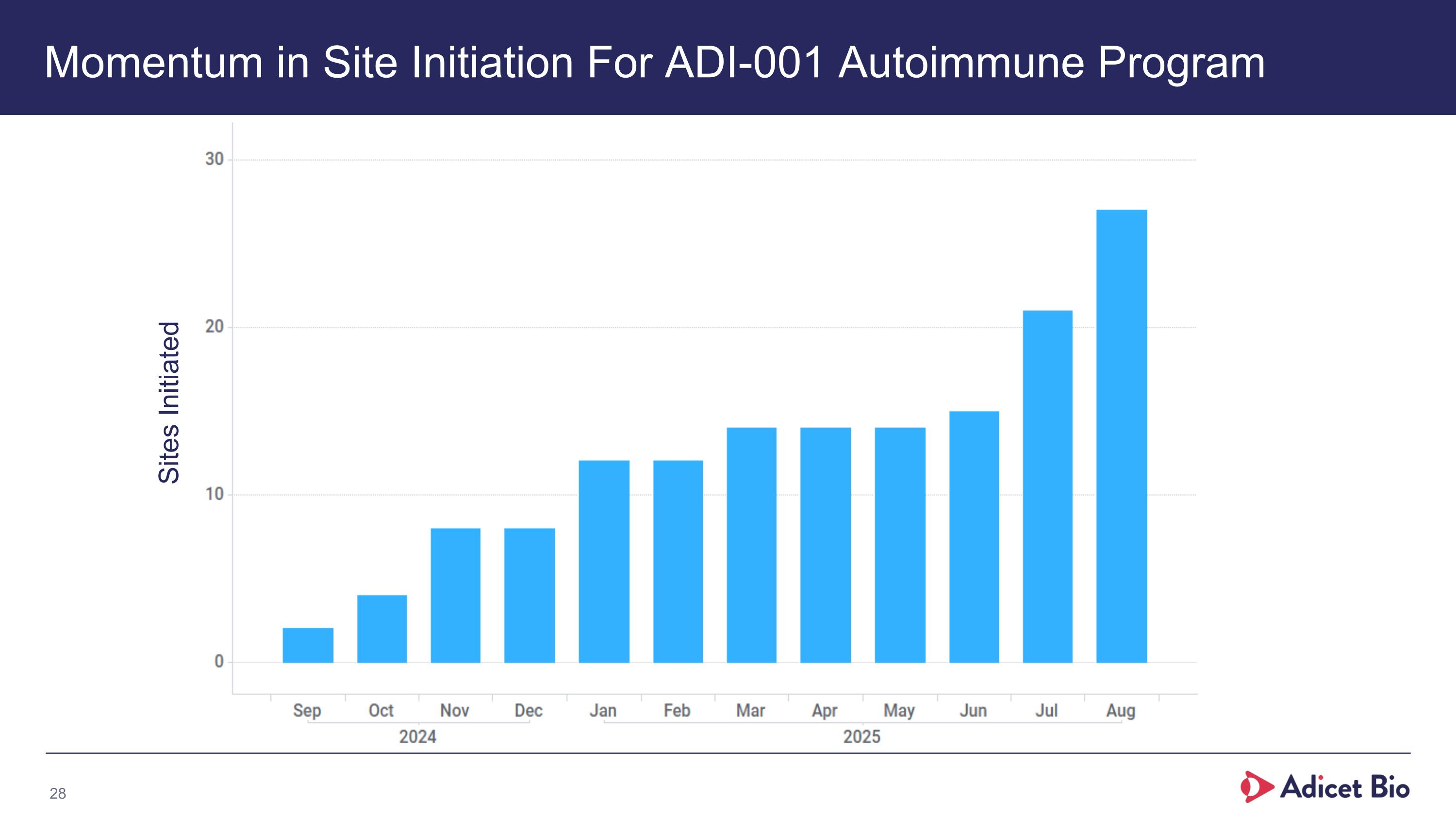
Momentum in Site Initiation For ADI-001 Autoimmune Program Sites Initiated
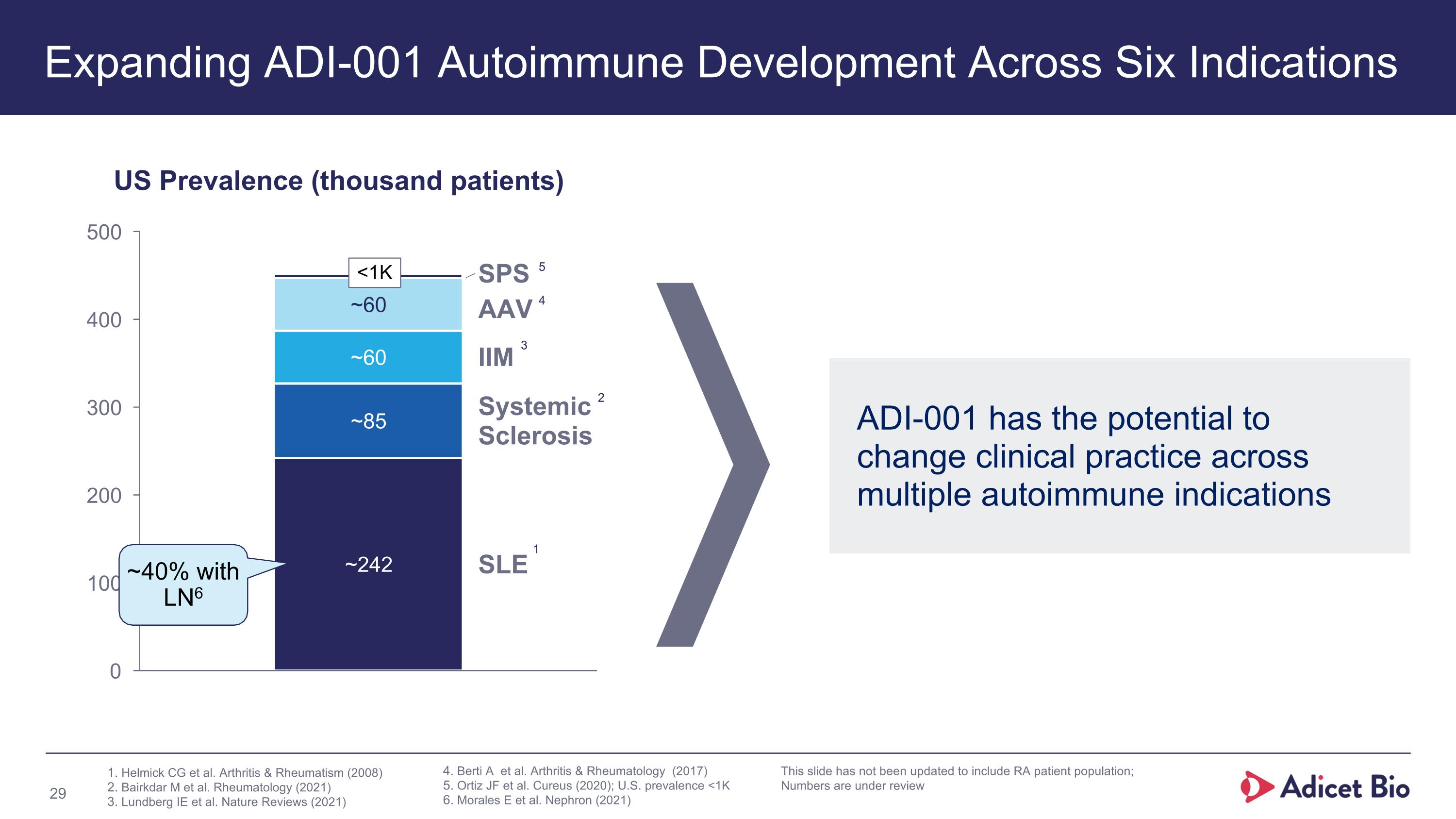
Expanding ADI-001 Autoimmune Development Across Six Indications SPS AAV IIM Systemic
Sclerosis SLE US Prevalence (thousand patients) ~40% with LN6 ADI-001 has the potential to change clinical practice across multiple autoimmune indications Helmick CG et al. Arthritis & Rheumatism (2008) Bairkdar M et al. Rheumatology (2021) Lundberg IE et al. Nature Reviews (2021) 1 2 3 Berti A et al. Arthritis & Rheumatology (2017) Ortiz JF et al. Cureus (2020); U.S. prevalence <1K Morales E et al. Nephron (2021) 4 5 This slide has not been updated to include RA patient population; Numbers are under review <1K
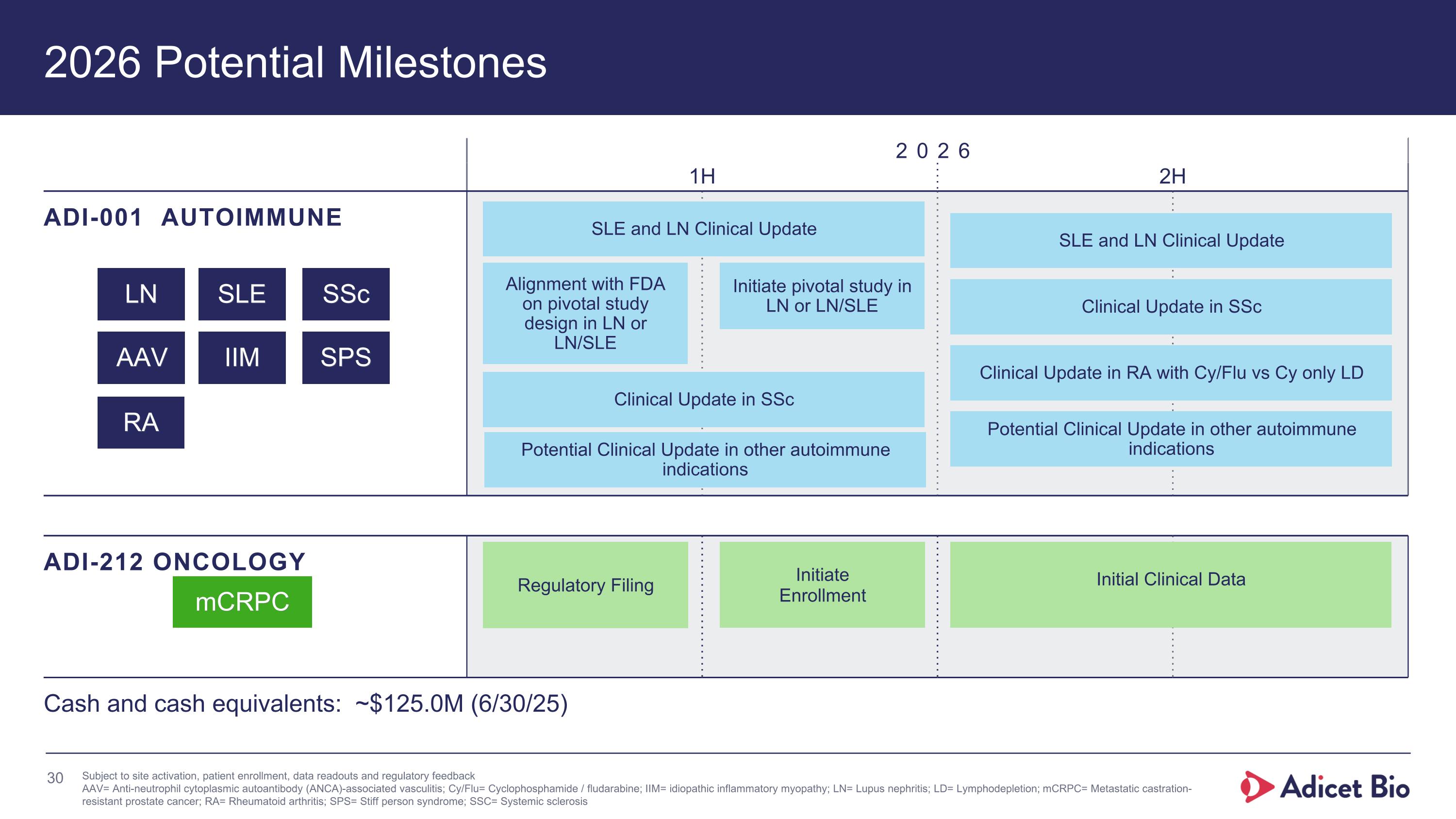
2026 Potential Milestones 2026 1H 2H ADI-001 AUTOIMMUNE ADI-212 ONCOLOGY mCRPC Cash and cash equivalents: ~$125.0M (6/30/25) Subject to site activation, patient enrollment, data readouts and regulatory feedback AAV= Anti-neutrophil cytoplasmic autoantibody (ANCA)-associated vasculitis; Cy/Flu= Cyclophosphamide / fludarabine; IIM= idiopathic inflammatory myopathy; LN= Lupus nephritis; LD= Lymphodepletion; mCRPC= Metastatic castration-resistant prostate cancer; RA= Rheumatoid arthritis; SPS= Stiff person syndrome; SSC= Systemic sclerosis LN SLE SSc AAV IIM SPS Initial Clinical Data SLE and LN Clinical Update Initiate pivotal study in LN or LN/SLE Alignment with FDA on pivotal study design in LN or LN/SLE SLE and LN Clinical Update Clinical Update in SSc Clinical Update in SSc Clinical Update in RA with Cy/Flu vs Cy only LD Potential Clinical Update in other autoimmune indications Regulatory Filing Initiate Enrollment Potential Clinical Update in other autoimmune indications RA

Leaders in Developing Allogeneic γδ1 CAR-T Cell Therapies to Fight Autoimmune Diseases and Cancer γδ= Gamma delta; CAR= Chimeric antigen receptor