

R&D Spotlight: CLYM116 and the IgAN Opportunity SEPTEMBER 29, 2025 Exhibit 99.2

Forward Looking Statements This presentation contains “forward-looking statements” within the meaning of the Private Securities Litigation Reform Act of 1995, including without limitation statements regarding: future expectations, plans and prospects for Climb Bio, Inc. (“Climb Bio”); expectations regarding the therapeutic benefits, clinical potential and clinical development of budoprutug and CLYM116; the trial design for the planned clinical trials of budoprutug and CLYM116; the anticipated timelines for initiating clinical trials of budoprutug for primary membranous nephropathy and CLYM116 for IgA nephropathy; plans to optimize the administration of budoprutug; the anticipated benefits of Climb Bio’s license agreement with Beijing Mabworks Biotech Co., Ltd. (“Mabworks”); expectations regarding the timing of an investigational new drug application or clinical trial application submission for CLYM116; anticipated timelines for announcing data from Climb Bio’s ongoing and planned clinical trials; the sufficiency of Climb Bio’s cash resources for the period anticipated and other statements containing the words “anticipate,” “believe,” “continue,” “could,” “estimate,” “expect,” “intend,” “may,” “plan,” “potential,” “predict,” “project,” “should,” “target,” “would,” “will,” “working” and similar expressions. Forward-looking statements are based on management’s current expectations of future events and are subject to a number of risks and uncertainties that could cause actual results to differ materially and adversely from those set forth in, or implied by, such forward-looking statements. Climb Bio may not actually achieve the plans, intentions or expectations disclosed in these forward-looking statements, and you should not place undue reliance on these forward-looking statements. These risks and uncertainties include, but are not limited to, important risks and uncertainties associated with: the ability of Climb Bio to timely and successfully achieve or recognize the anticipated benefits of its acquisition of Tenet Medicines, Inc. and its license agreement with Mabworks; changes in applicable laws or regulation; the possibility that Climb Bio may be adversely affected by other economic, business and/or competitive factors; Climb Bio’s ability to advance budoprutug and CLYM116 on the timelines expected or at all and to obtain and maintain necessary approvals from the U.S. Food and Drug Administration and other regulatory authorities; obtaining and maintaining the necessary approvals from investigational review boards at clinical trial sites and independent data safety monitoring boards; replicating in clinical trials positive results found in early-stage clinical trials and preclinical studies; competing successfully with other companies that are seeking to develop treatments for primary membranous nephropathy, immune thrombocytopenia, systemic lupus erythematosus, IgA nephropathy and other immune-mediated diseases; maintaining or protecting intellectual property rights related to budoprutug, CLYM116 and/or its other product candidates; managing expenses; and raising the substantial additional capital needed, on the timeline necessary, to continue development of budoprutug, CLYM116 and any other product candidates Climb Bio may develop. For a discussion of other risks and uncertainties, and other important factors, any of which could cause Climb Bio’s actual results to differ materially from those contained in the forward-looking statements, see the “Risk Factors” section, as well as discussions of potential risks, uncertainties and other important factors, in Climb Bio’s most recent filings with the U.S. Securities and Exchange Commission. In addition, the forward-looking statements included in this presentation represent Climb Bio’s views as of the date hereof and should not be relied upon as representing Climb Bio’s views as of any date subsequent to the date hereof. Climb Bio anticipates that subsequent events and developments will cause Climb Bio’s views to change. However, while Climb Bio may elect to update these forward-looking statements at some point in the future, Climb Bio specifically disclaims any obligation to do so, except as required by law.

Webcast Agenda Corporate Strategy & CLYM116 Opportunity Aoife Brennan, M.B., Ch.B. President and CEO, Climb Bio IgAN Disease Overview & Evolving Treatment Landscape Craig Gordon, M.D., M.S. Nephrologist; Professor, Tufts University School of Medicine Co-director, Evidence Review Team for KDIGO clinical practice guideline for the treatment of IgAN CLYM116 Preclinical Data & Development Plan Edgar Charles, M.D. Chief Medical Officer, Climb Bio Q&A session including Dr. Gordon and management

Deliver high impact, disease-modifying medicines for individuals living with immune-mediated diseases Scaling New Heights in the Development of Transformative Immune Medicines OUR MISSION
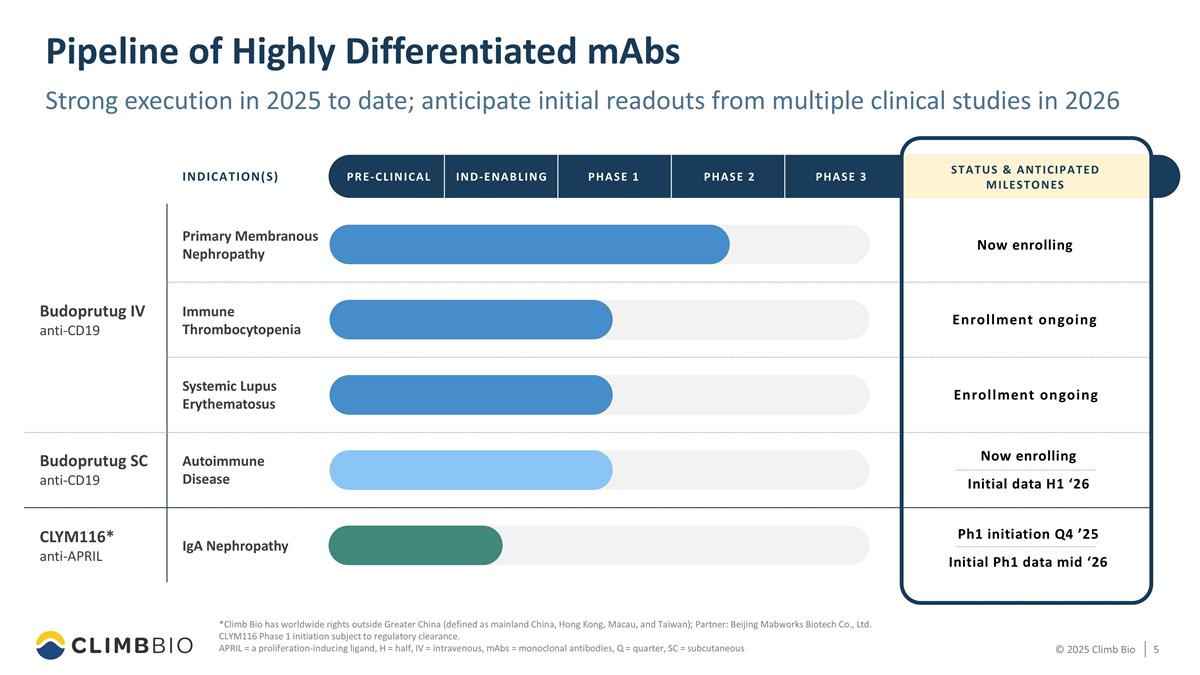
Pipeline of Highly Differentiated mAbs *Climb Bio has worldwide rights outside Greater China (defined as mainland China, Hong Kong, Macau, and Taiwan); Partner: Beijing Mabworks Biotech Co., Ltd. CLYM116 Phase 1 initiation subject to regulatory clearance. APRIL = a proliferation-inducing ligand, H = half, IV = intravenous, mAbs = monoclonal antibodies, Q = quarter, SC = subcutaneous Budoprutug IV anti-CD19 INDICATION(S) Primary Membranous Nephropathy Systemic Lupus Erythematosus Immune Thrombocytopenia Autoimmune Disease CLYM116* anti-APRIL IgA Nephropathy Budoprutug SC anti-CD19 PRE-CLINICAL PHASE 1 PHASE 2 PHASE 3 IND-ENABLING Now enrolling Enrollment ongoing Enrollment ongoing Now enrolling Initial data H1 ‘26 Ph1 initiation Q4 ’25 Initial Ph1 data mid ‘26 STATUS & ANTICIPATED MILESTONES Strong execution in 2025 to date; anticipate initial readouts from multiple clinical studies in 2026
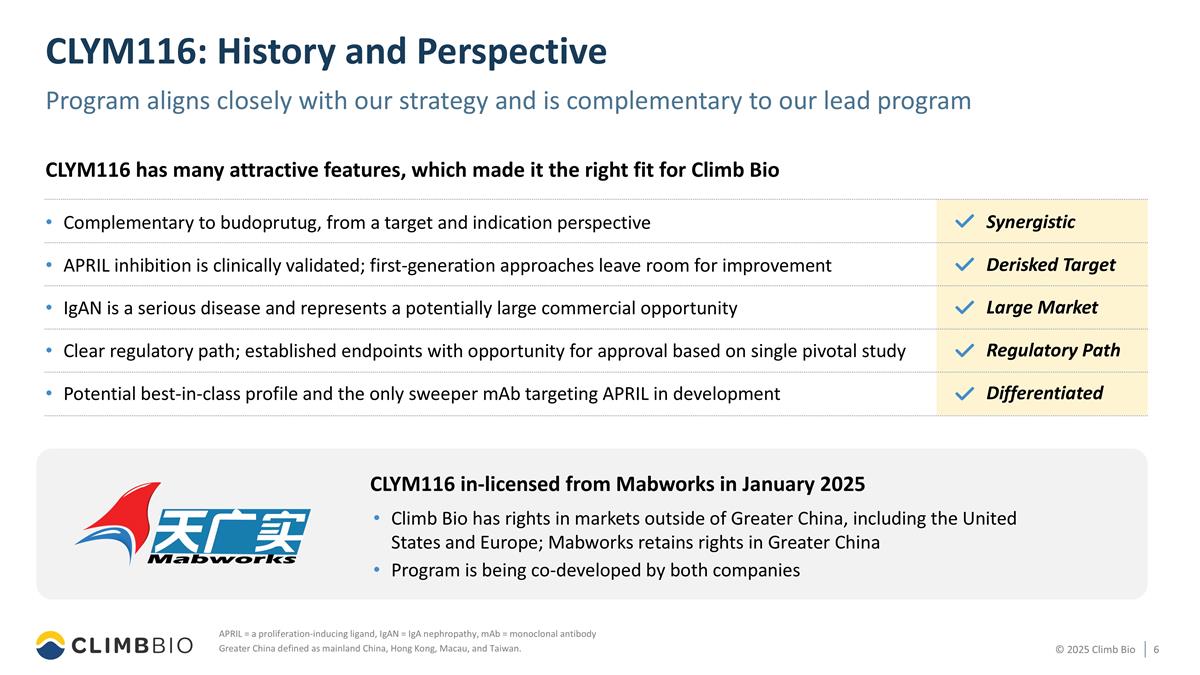
CLYM116: History and Perspective Program aligns closely with our strategy and is complementary to our lead program APRIL = a proliferation-inducing ligand, IgAN = IgA nephropathy, mAb = monoclonal antibody Greater China defined as mainland China, Hong Kong, Macau, and Taiwan. Complementary to budoprutug, from a target and indication perspective APRIL inhibition is clinically validated; first-generation approaches leave room for improvement IgAN is a serious disease and represents a potentially large commercial opportunity Clear regulatory path; established endpoints with opportunity for approval based on single pivotal study Potential best-in-class profile and the only sweeper mAb targeting APRIL in development CLYM116 has many attractive features, which made it the right fit for Climb Bio Synergistic Derisked Target Large Market Regulatory Path Differentiated CLYM116 in-licensed from Mabworks in January 2025 Climb Bio has rights in markets outside of Greater China, including the United States and Europe; Mabworks retains rights in Greater China Program is being co-developed by both companies
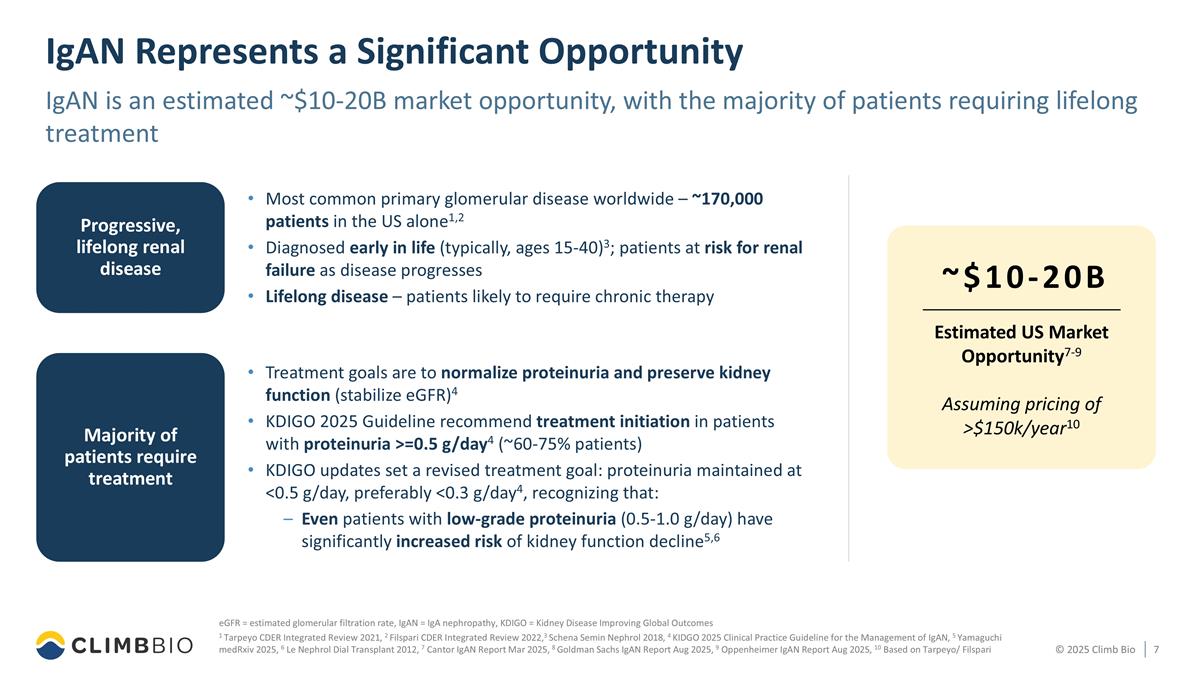
Most common primary glomerular disease worldwide – ~170,000 patients in the US alone1,2 Diagnosed early in life (typically, ages 15-40)3; patients at risk for renal failure as disease progresses Lifelong disease – patients likely to require chronic therapy Progressive, lifelong renal disease IgAN Represents a Significant Opportunity IgAN is an estimated ~$10-20B market opportunity, with the majority of patients requiring lifelong treatment eGFR = estimated glomerular filtration rate, IgAN = IgA nephropathy, KDIGO = Kidney Disease Improving Global Outcomes 1 Tarpeyo CDER Integrated Review 2021, 2 Filspari CDER Integrated Review 2022,3 Schena Semin Nephrol 2018, 4 KIDGO 2025 Clinical Practice Guideline for the Management of IgAN, 5 Yamaguchi medRxiv 2025, 6 Le Nephrol Dial Transplant 2012, 7 Cantor IgAN Report Mar 2025, 8 Goldman Sachs IgAN Report Aug 2025, 9 Oppenheimer IgAN Report Aug 2025, 10 Based on Tarpeyo/ Filspari Majority of patients require treatment ~$10-20B Estimated US Market Opportunity7-9 Assuming pricing of >$150k/year10 Treatment goals are to normalize proteinuria and preserve kidney function (stabilize eGFR)4 KDIGO 2025 Guideline recommend treatment initiation in patients with proteinuria >=0.5 g/day4 (~60-75% patients) KDIGO updates set a revised treatment goal: proteinuria maintained at <0.5 g/day, preferably <0.3 g/day4, recognizing that: Even patients with low-grade proteinuria (0.5-1.0 g/day) have significantly increased risk of kidney function decline5,6
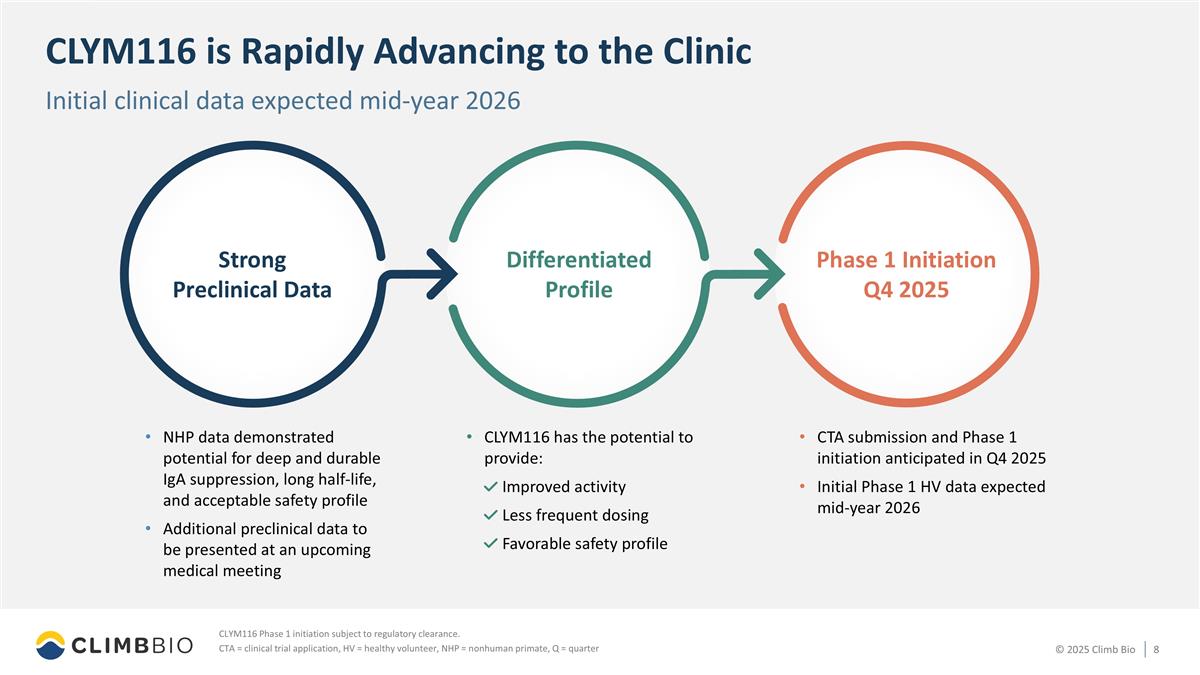
CLYM116 is Rapidly Advancing to the Clinic CLYM116 Phase 1 initiation subject to regulatory clearance. CTA = clinical trial application, HV = healthy volunteer, NHP = nonhuman primate, Q = quarter Initial clinical data expected mid-year 2026 NHP data demonstrated potential for deep and durable IgA suppression, long half-life, and acceptable safety profile Additional preclinical data to be presented at an upcoming medical meeting CTA submission and Phase 1 initiation anticipated in Q4 2025 Initial Phase 1 HV data expected mid-year 2026 CLYM116 has the potential to provide: Improved activity Less frequent dosing Favorable safety profile Strong Preclinical Data Differentiated Profile Phase 1 Initiation Q4 2025

IgA nephropathy – an overview of pathogenesis and treatment Craig E. Gordon, MD MS, FASN Professor of Medicine Tufts University School of Medicine
IgA nephropathy Common condition compared with other glomerular diseases Incidence: >2.5 per 100,000 individuals Higher in Asian Pacific populations Median age of diagnosis: 40 years of age UK National Registry of Rare Kidney Diseases (RaDaR) Median kidney survival time of 11.4 y (10.5-12.5y) Consequently, IgA nephropathy is a major cause of kidney failure Can we do better than ACE-I/ARB? The answer is an emphatic “yes”… with so many new treatment options available… Hemodynamic treatments like SGLT2i, dual endothelin angiotensin receptor antagonists (DEARA), endothelin receptor antagonists Immunosuppressive treatments like corticosteroids, TRF budesonide, complement blockade (iptacopan), or BAFF/APRIL antagonists The question remains which combinations? Hemodynamic and immunosuppressive treatments? What about multiple immunomodulating treatments? And for which patients? And when in the disease course? KDIGO 2025 CPG on IgA; Pitcher, CJASN 2023
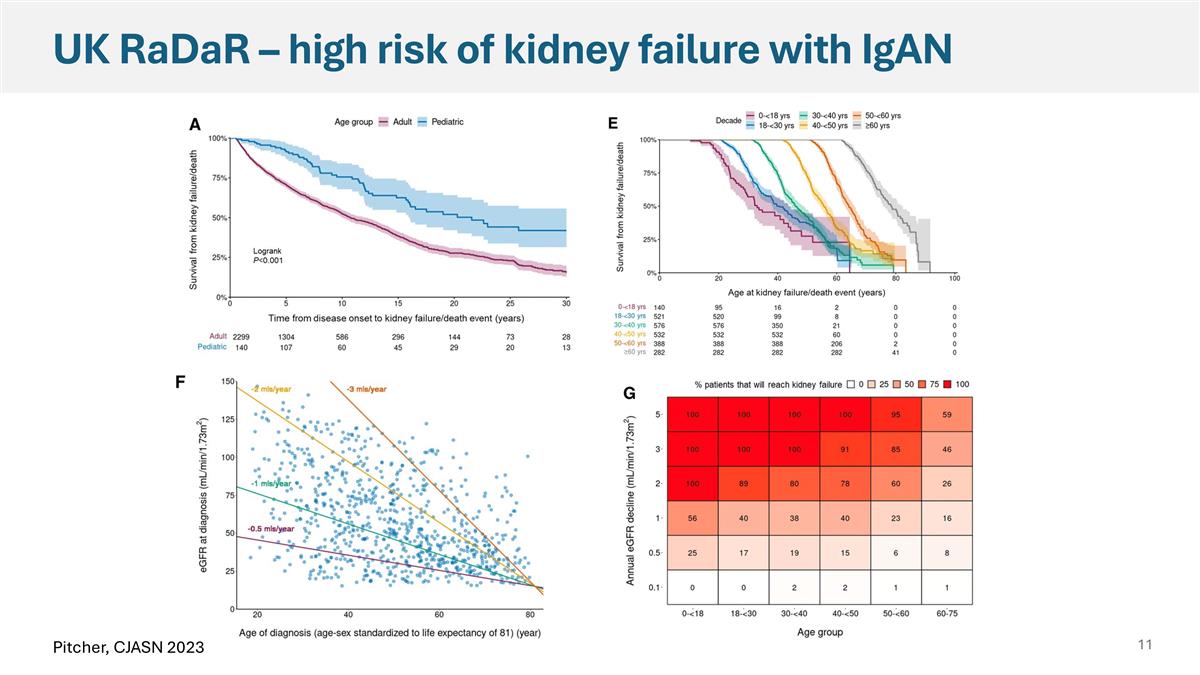
UK RaDaR – high risk of kidney failure with IgAN Pitcher, CJASN 2023
A patient case to highlight the challenges… 46 yo M referred for hematuria, proteinuria, decreased GFR, no symptoms Blood pressure 138/84 mmHg Urinalysis revealed 3+ blood, 3+ protein UPCR 2.9 g/g eGFR 58 ml/min/1.73m2 Note the disease progression (i.e. decreased GFR) in absence of symptoms Underwent kidney biopsy which showed changes c/w IgA nephropathy Oxford classification (MEST-C): M1 EO S1 T0 C0 Mesangial hypercellularity and Segmental sclerosis were present but Endocapillary hypercellularity and Tubular atrophy were absent Started on ARB Discussions held with patient re: treatment options
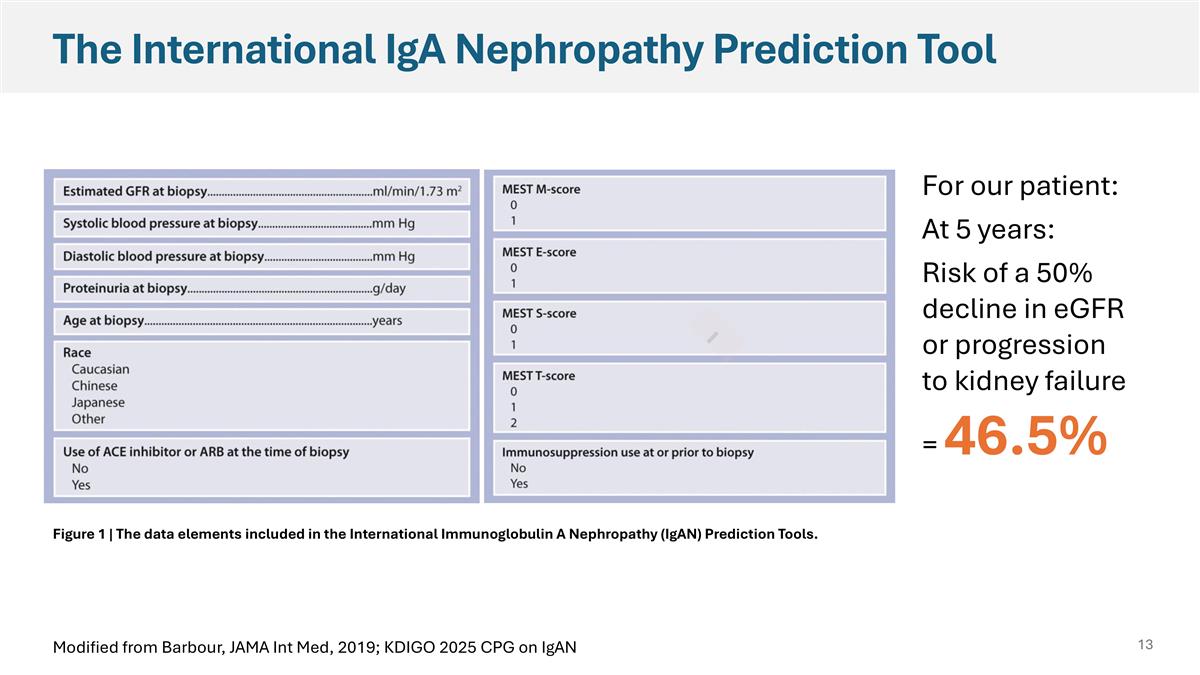
The International IgA Nephropathy Prediction Tool For our patient: At 5 years: Risk of a 50% decline in eGFR or progression to kidney failure = 46.5% Figure 1 | The data elements included in the International Immunoglobulin A Nephropathy (IgAN) Prediction Tools. Modified from Barbour, JAMA Int Med, 2019; KDIGO 2025 CPG on IgAN

Pathogenesis of IgAN Current disease model in 2025
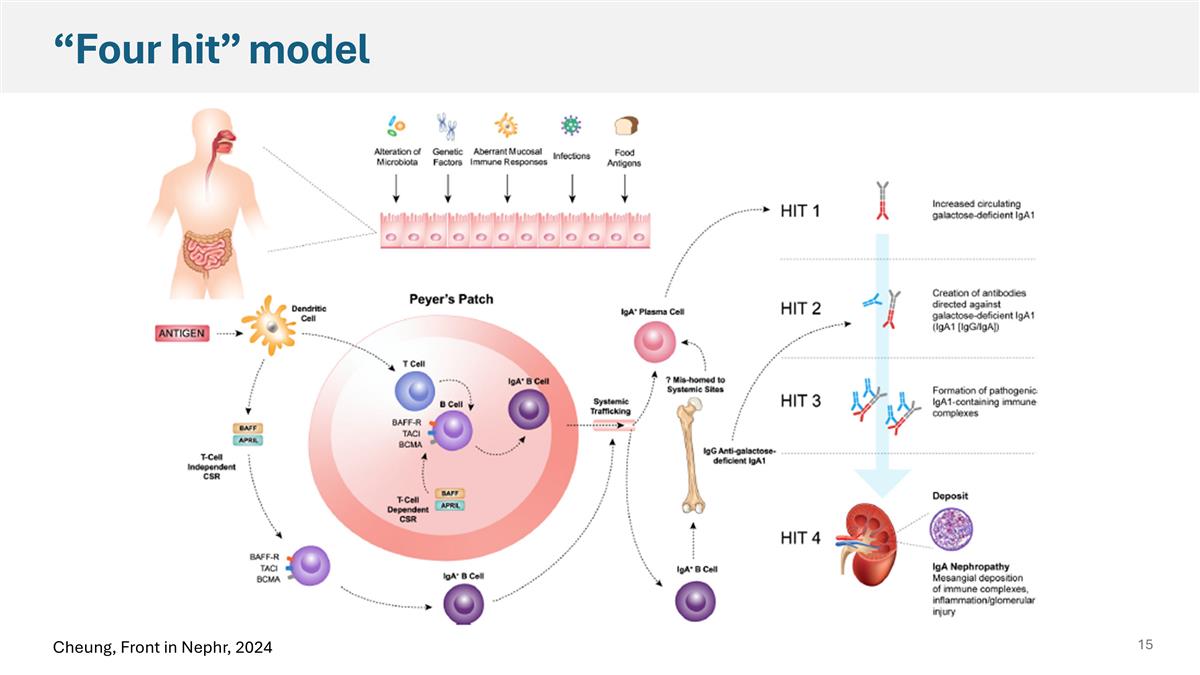
Cheung, Front in Nephr, 2024 “Four hit” model
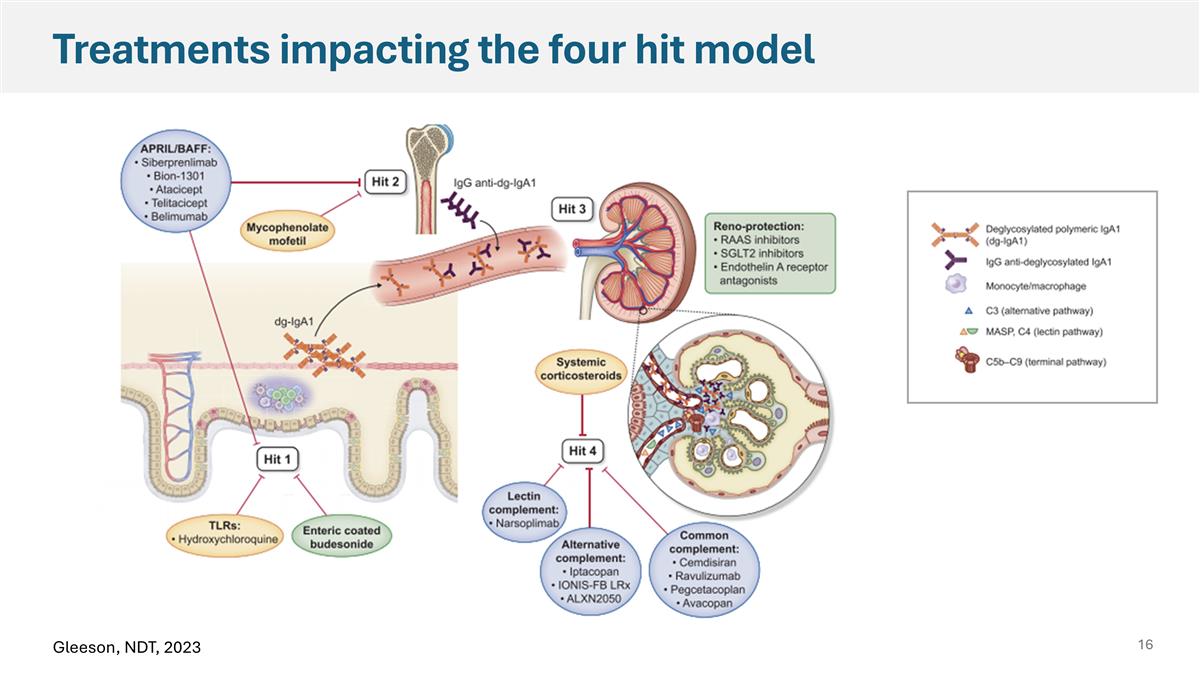
Treatments impacting the four hit model Gleeson, NDT, 2023

Surrogate outcomes in IgA nephropathy FDA recognized need for surrogate outcomes in studies of IgA nephropathy (slowly progressive disease) Recent accelerated approval by FDA of treatments based on improvement in proteinuria True of TRF-budesonide, sparsentan, iptacopan Presumably will be the pathway for all new agents studied in IgAN Full FDA approval based on improvement kidney function (eGFR) Outcomes of interest scientifically may include decreases in: gd-IgA1 APRIL Others?

Executive summary of the KDIGO 2025 Clinical Practice Guideline for the Management of Immunoglobulin A Nephropathy (IgAN) and Immunoglobulin A Vasculitis (IgAV)
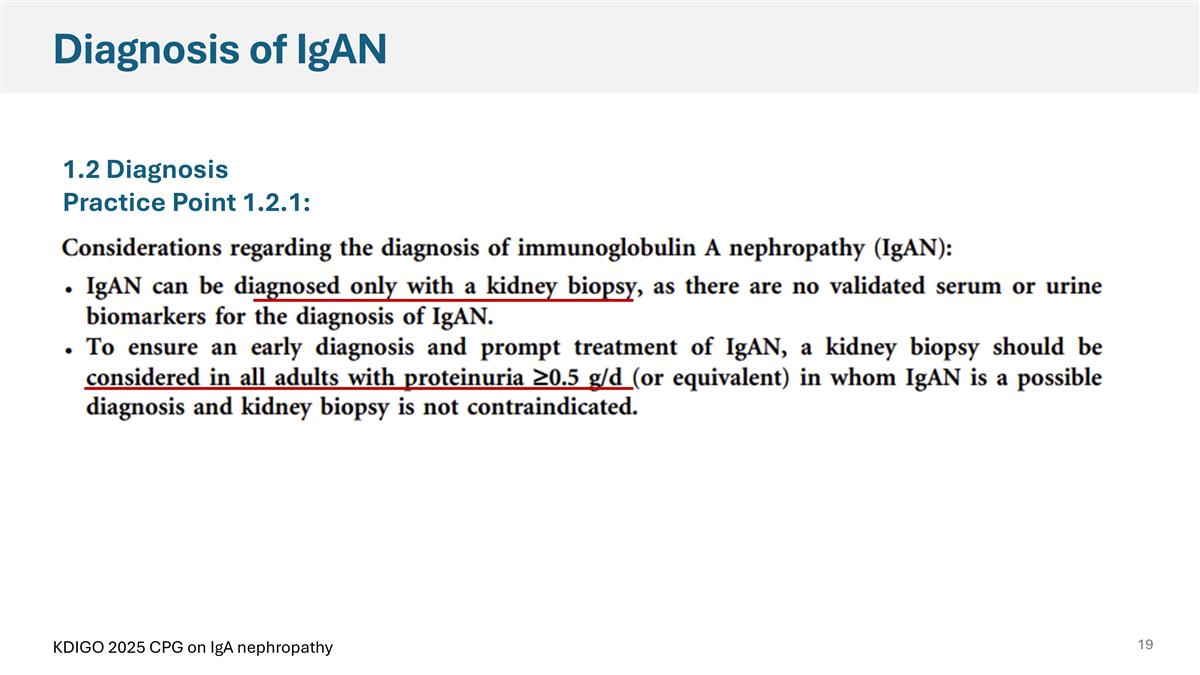
Diagnosis of IgAN 1.2 Diagnosis Practice Point 1.2.1: KDIGO 2025 CPG on IgA nephropathy

Treatment of IgAN 1.4 Treatment KDIGO 2025 CPG on IgA nephropathy
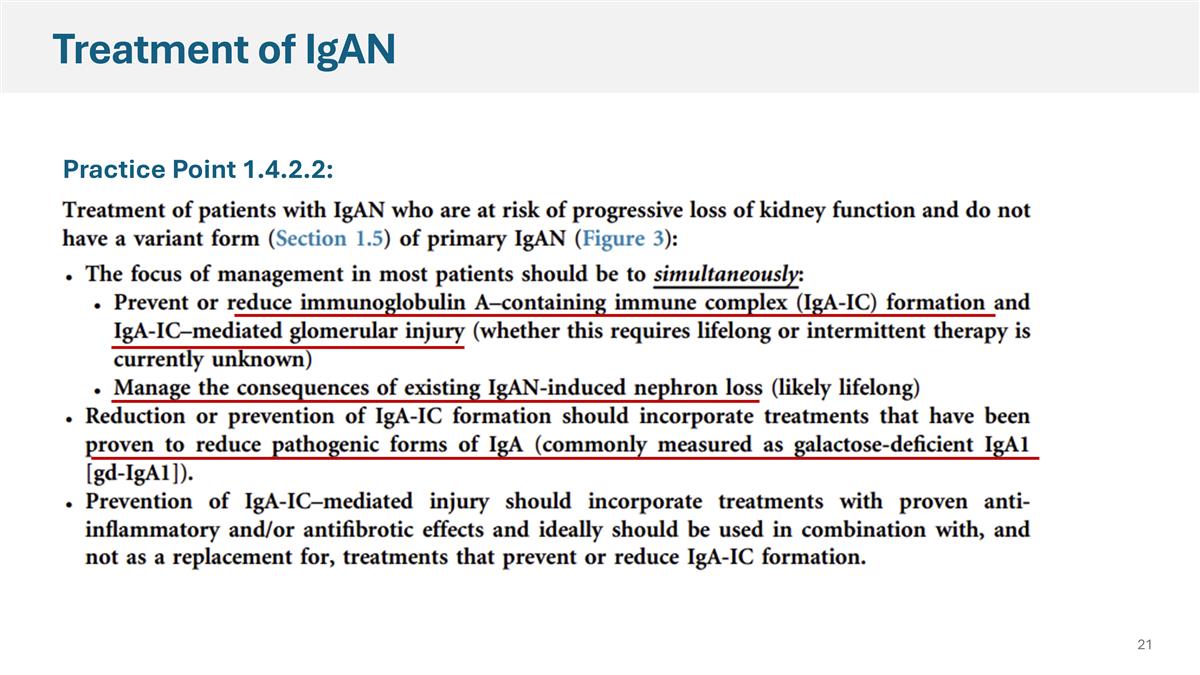
Treatment of IgAN Practice Point 1.4.2.2:
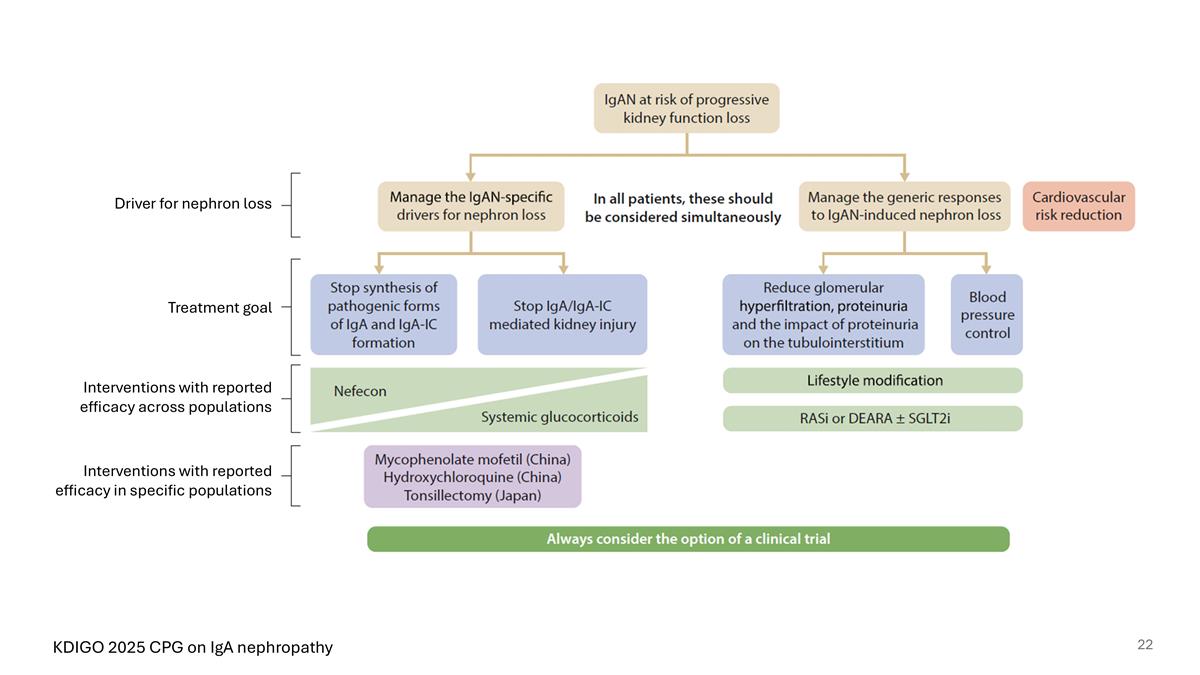
KDIGO 2025 CPG on IgA nephropathy Driver for nephron loss Treatment goal Interventions with reported efficacy across populations Interventions with reported efficacy in specific populations

Current understanding of BAFF/APRIL pathways
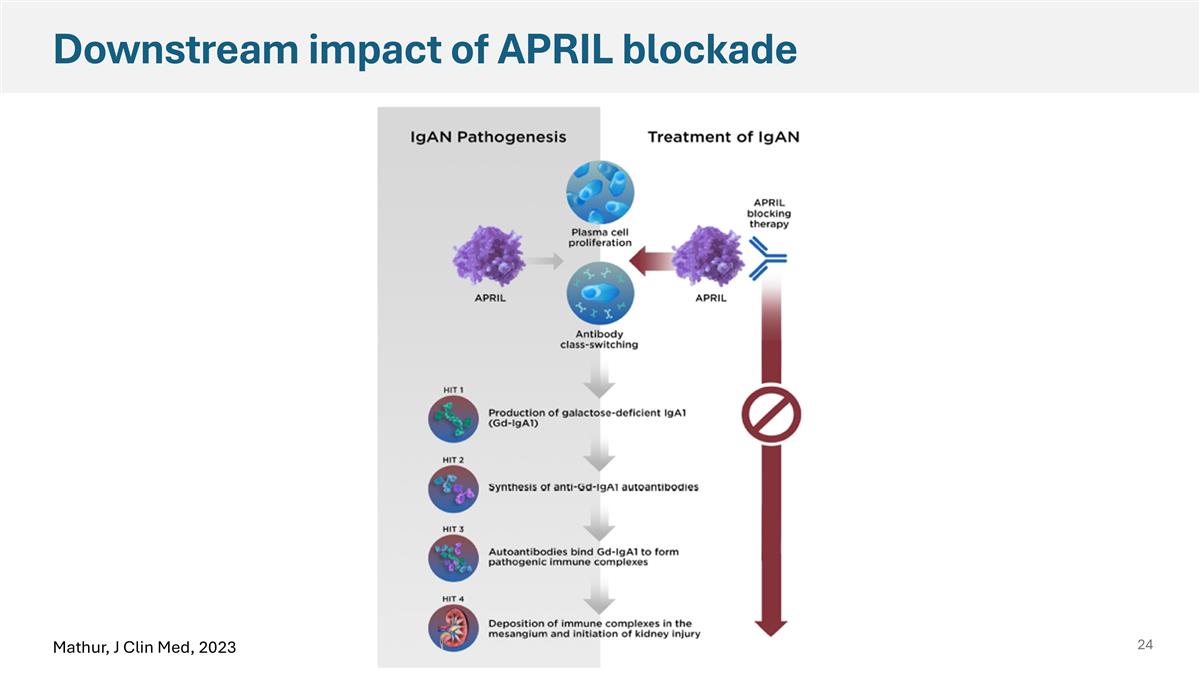
Downstream impact of APRIL blockade Mathur, J Clin Med, 2023
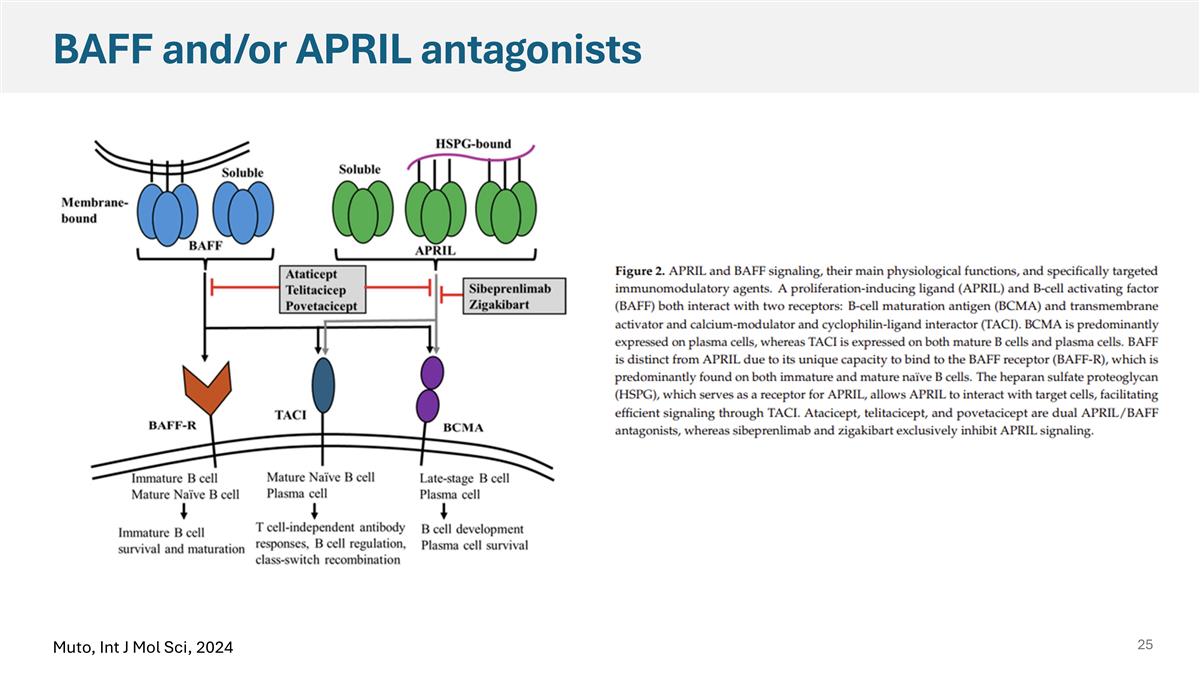
BAFF and/or APRIL antagonists Muto, Int J Mol Sci, 2024
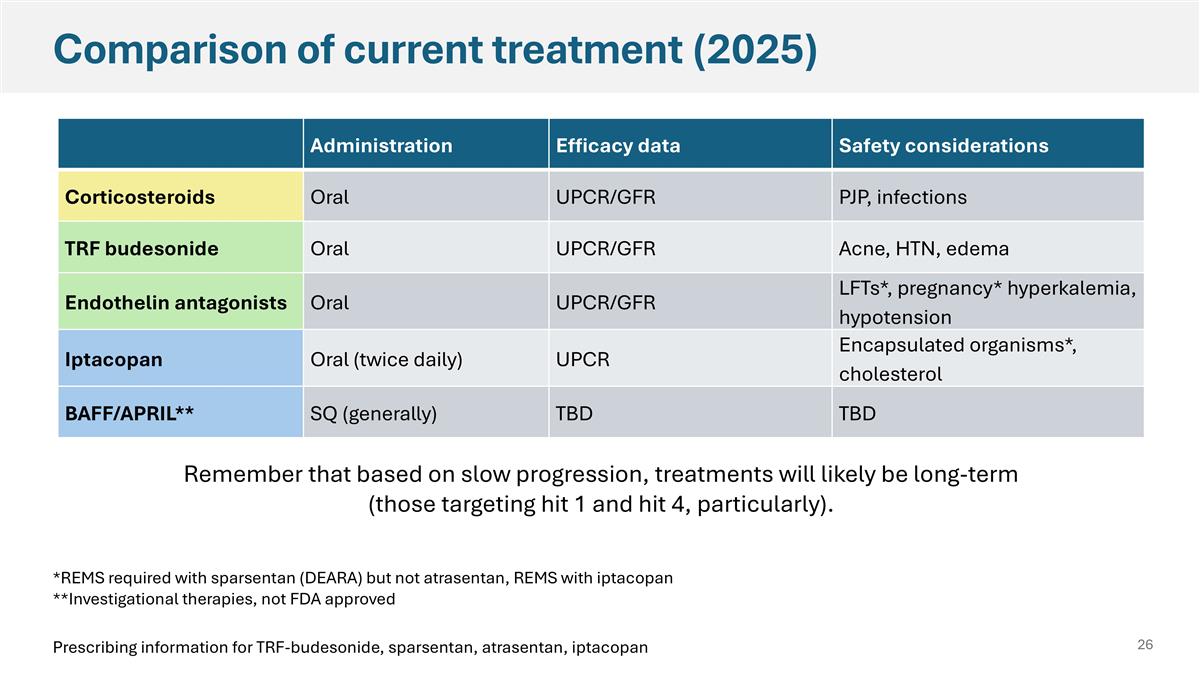
Comparison of current treatment (2025) Remember that based on slow progression, treatments will likely be long-term (those targeting hit 1 and hit 4, particularly). Administration Efficacy data Safety considerations Corticosteroids Oral UPCR/GFR PJP, infections TRF budesonide Oral UPCR/GFR Acne, HTN, edema Endothelin antagonists Oral UPCR/GFR LFTs*, pregnancy* hyperkalemia, hypotension Iptacopan Oral (twice daily) UPCR Encapsulated organisms*, cholesterol BAFF/APRIL** SQ (generally) TBD TBD *REMS required with sparsentan (DEARA) but not atrasentan, REMS with iptacopan **Investigational therapies, not FDA approved Prescribing information for TRF-budesonide, sparsentan, atrasentan, iptacopan

Where are we going in the future? KDIGO 2025 CPG on IgA nephropathy

Thanks!

CLYM116 Anti-APRIL monoclonal antibody
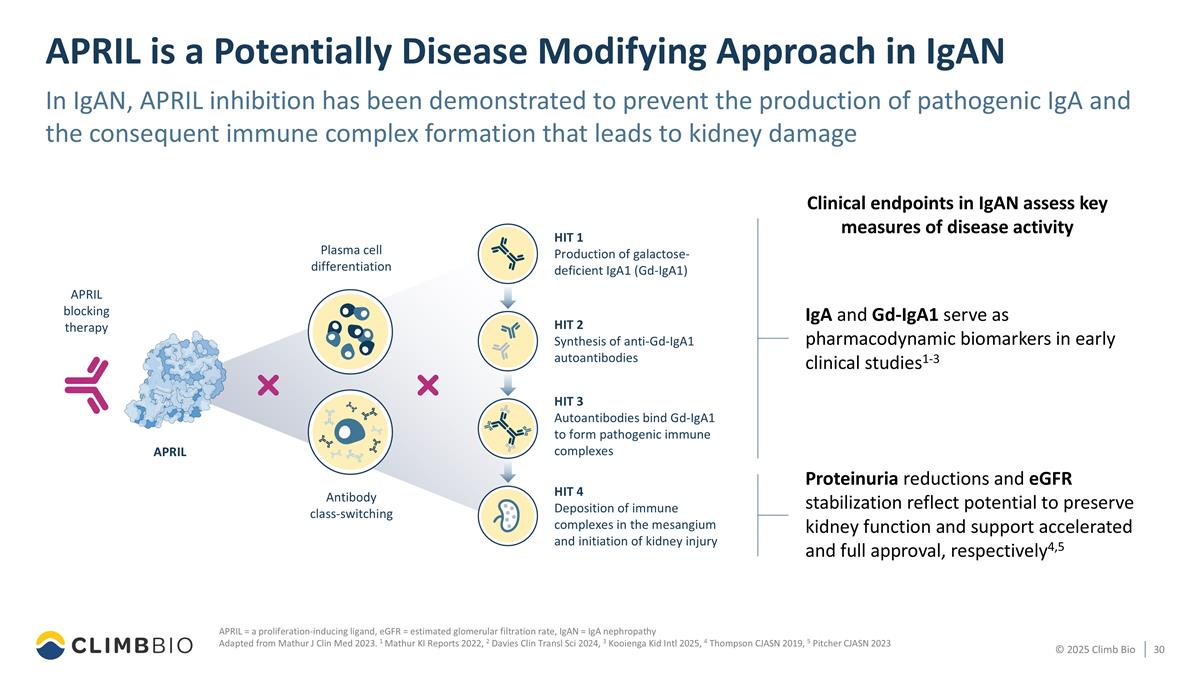
APRIL is a Potentially Disease Modifying Approach in IgAN In IgAN, APRIL inhibition has been demonstrated to prevent the production of pathogenic IgA and the consequent immune complex formation that leads to kidney damage APRIL = a proliferation-inducing ligand, eGFR = estimated glomerular filtration rate, IgAN = IgA nephropathy Adapted from Mathur J Clin Med 2023. 1 Mathur KI Reports 2022, 2 Davies Clin Transl Sci 2024, 3 Kooienga Kid Intl 2025, 4 Thompson CJASN 2019, 5 Pitcher CJASN 2023 IgA and Gd-IgA1 serve as pharmacodynamic biomarkers in early clinical studies1-3 Clinical endpoints in IgAN assess key measures of disease activity HIT 1 Production of galactose- deficient IgA1 (Gd-IgA1) HIT 2 Synthesis of anti-Gd-IgA1 autoantibodies HIT 3 Autoantibodies bind Gd-IgA1 to form pathogenic immune complexes HIT 4 Deposition of immune complexes in the mesangium and initiation of kidney injury Plasma cell differentiation Antibody class-switching APRIL APRIL blocking therapy Proteinuria reductions and eGFR stabilization reflect potential to preserve kidney function and support accelerated and full approval, respectively4,5
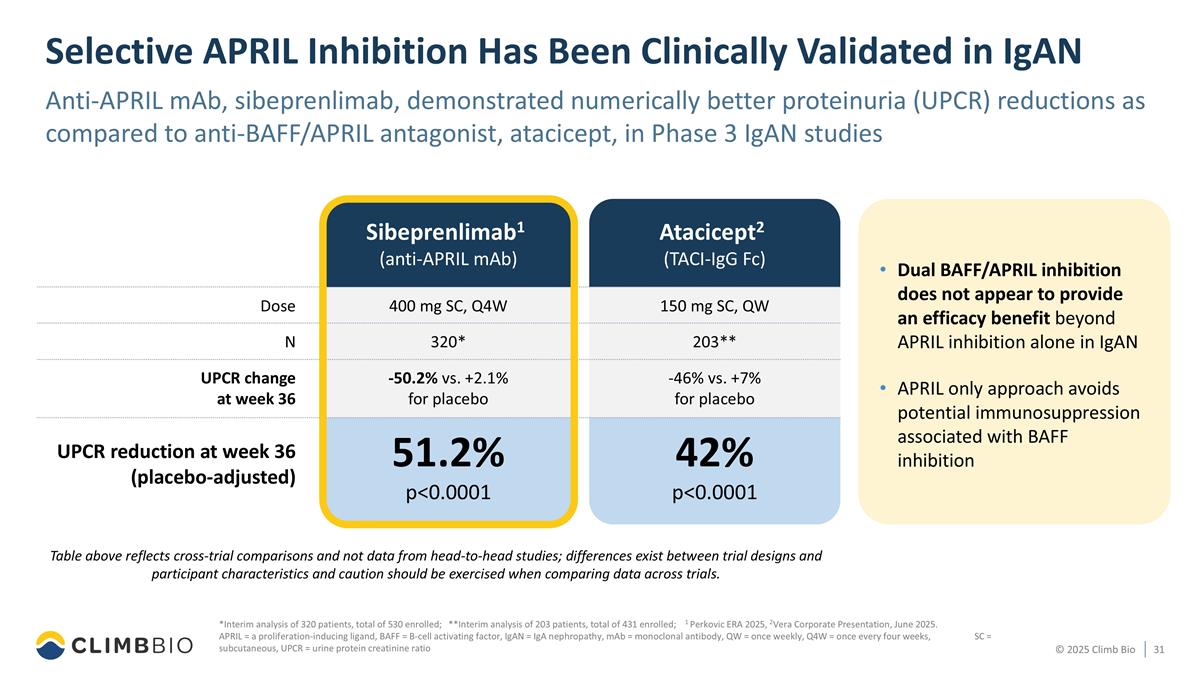
Selective APRIL Inhibition Has Been Clinically Validated in IgAN Anti-APRIL mAb, sibeprenlimab, demonstrated numerically better proteinuria (UPCR) reductions as compared to anti-BAFF/APRIL antagonist, atacicept, in Phase 3 IgAN studies Table above reflects cross-trial comparisons and not data from head-to-head studies; differences exist between trial designs and participant characteristics and caution should be exercised when comparing data across trials. Dual BAFF/APRIL inhibition does not appear to provide an efficacy benefit beyond APRIL inhibition alone in IgAN APRIL only approach avoids potential immunosuppression associated with BAFF inhibition 400 mg SC, Q4W 320* -50.2% vs. +2.1% for placebo 51.2% p<0.0001 Atacicept2 (TACI-IgG Fc) 150 mg SC, QW 203** -46% vs. +7% for placebo 42% p<0.0001 Dose N UPCR change at week 36 UPCR reduction at week 36 (placebo-adjusted) Sibeprenlimab1 (anti-APRIL mAb) *Interim analysis of 320 patients, total of 530 enrolled; **Interim analysis of 203 patients, total of 431 enrolled; 1 Perkovic ERA 2025, 2Vera Corporate Presentation, June 2025. APRIL = a proliferation-inducing ligand, BAFF = B-cell activating factor, IgAN = IgA nephropathy, mAb = monoclonal antibody, QW = once weekly, Q4W = once every four weeks, SC = subcutaneous, UPCR = urine protein creatinine ratio
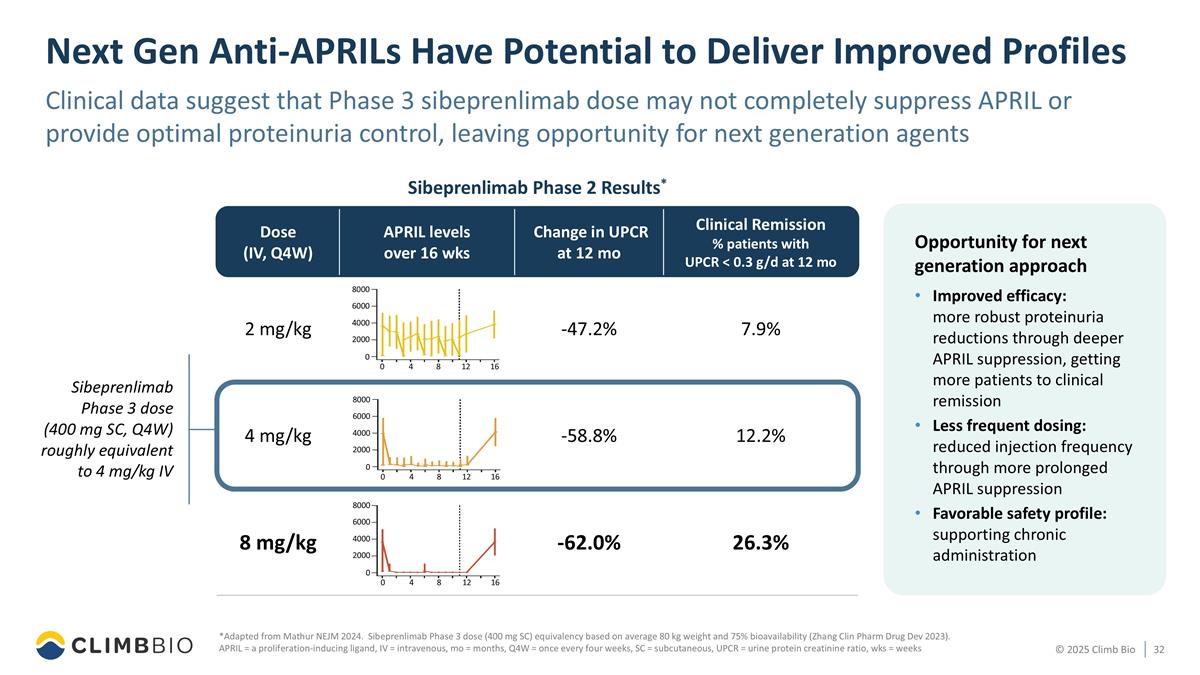
Next Gen Anti-APRILs Have Potential to Deliver Improved Profiles *Adapted from Mathur NEJM 2024. Sibeprenlimab Phase 3 dose (400 mg SC) equivalency based on average 80 kg weight and 75% bioavailability (Zhang Clin Pharm Drug Dev 2023). APRIL = a proliferation-inducing ligand, IV = intravenous, mo = months, Q4W = once every four weeks, SC = subcutaneous, UPCR = urine protein creatinine ratio, wks = weeks Sibeprenlimab Phase 2 Results* Opportunity for next generation approach Improved efficacy: more robust proteinuria reductions through deeper APRIL suppression, getting more patients to clinical remission Less frequent dosing: reduced injection frequency through more prolonged APRIL suppression Favorable safety profile: supporting chronic administration Sibeprenlimab Phase 3 dose (400 mg SC, Q4W) roughly equivalent to 4 mg/kg IV Dose (IV, Q4W) APRIL levels over 16 wks Change in UPCR at 12 mo Clinical Remission % patients with UPCR < 0.3 g/d at 12 mo 2 mg/kg -47.2% 7.9% 4 mg/kg -58.8% 12.2% 8 mg/kg -62.0% 26.3% 8000 6000 4000 2000 0 0 4 8 12 16 8000 6000 4000 2000 0 0 4 8 12 16 8000 6000 4000 2000 0 0 4 8 12 16 Clinical data suggest that Phase 3 sibeprenlimab dose may not completely suppress APRIL or provide optimal proteinuria control, leaving opportunity for next generation agents
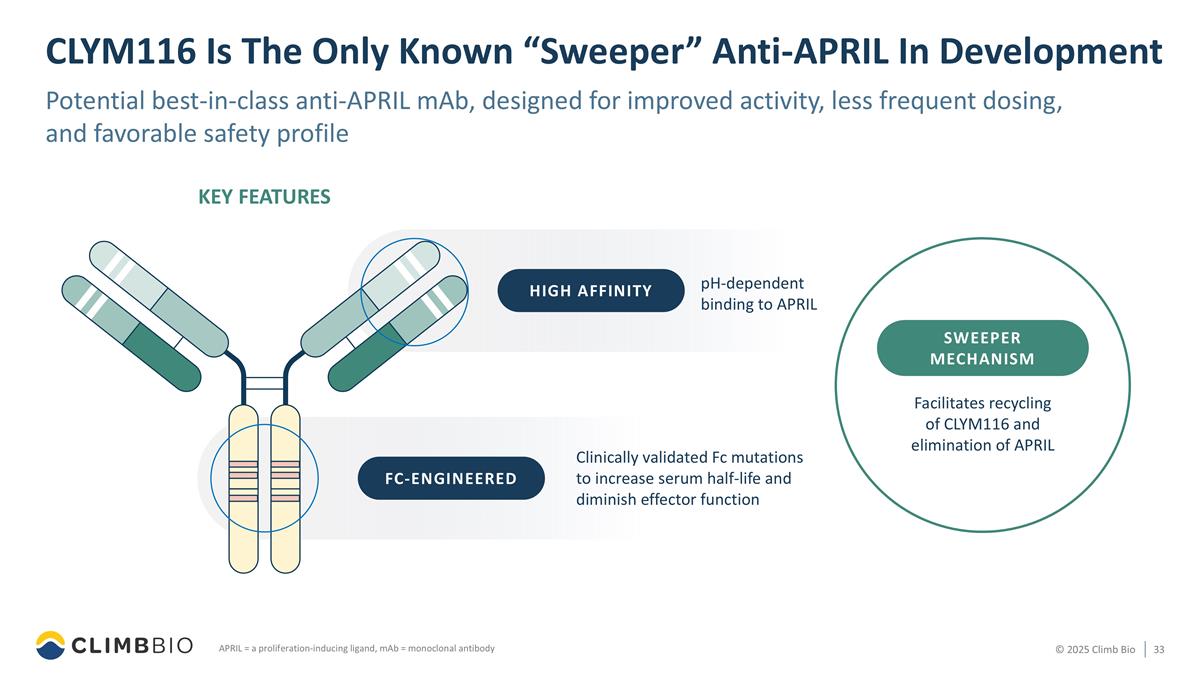
CLYM116 Is The Only Known “Sweeper” Anti-APRIL In Development Potential best-in-class anti-APRIL mAb, designed for improved activity, less frequent dosing, and favorable safety profile APRIL = a proliferation-inducing ligand, mAb = monoclonal antibody pH-dependent binding to APRIL Clinically validated Fc mutations to increase serum half-life and diminish effector function Facilitates recycling of CLYM116 and elimination of APRIL FC-ENGINEERED KEY FEATURES HIGH AFFINITY Sweeper mechanism
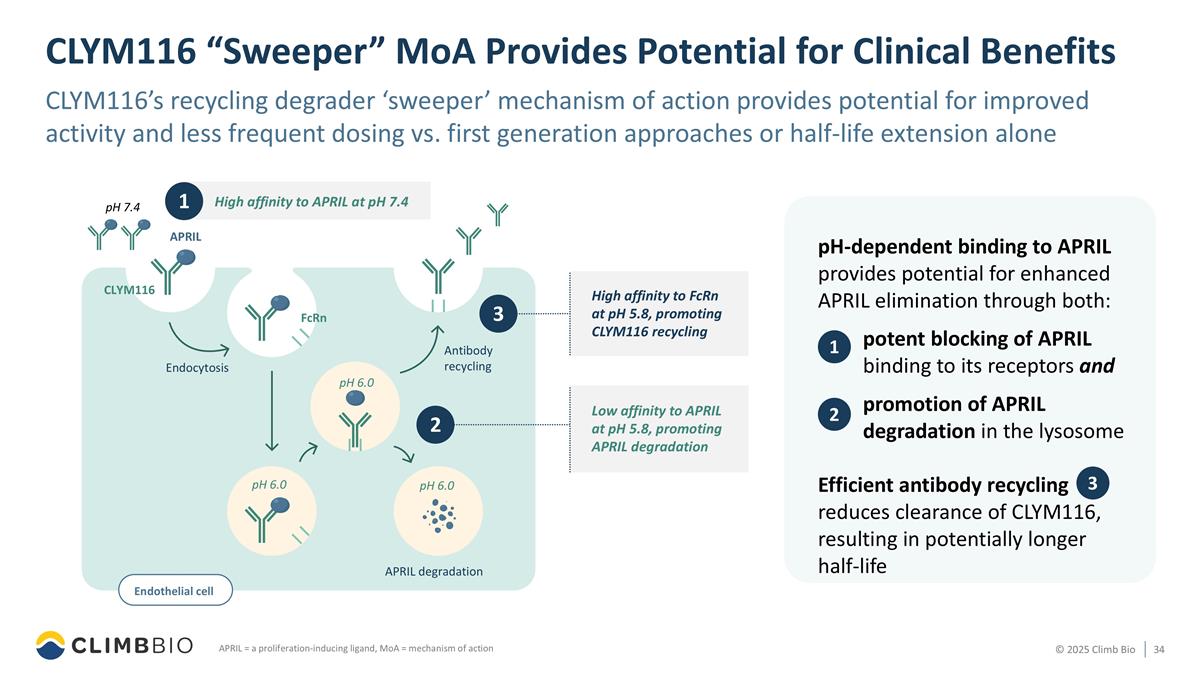
APRIL Endocytosis APRIL degradation pH 6.0 pH 6.0 pH 6.0 FcRn pH 7.4 CLYM116 Antibody recycling CLYM116 “Sweeper” MoA Provides Potential for Clinical Benefits CLYM116’s recycling degrader ‘sweeper’ mechanism of action provides potential for improved activity and less frequent dosing vs. first generation approaches or half-life extension alone APRIL = a proliferation-inducing ligand, MoA = mechanism of action pH-dependent binding to APRIL provides potential for enhanced APRIL elimination through both: potent blocking of APRIL binding to its receptors and promotion of APRIL degradation in the lysosome Efficient antibody recycling reduces clearance of CLYM116, resulting in potentially longer half-life Low affinity to APRIL at pH 5.8, promoting APRIL degradation 2 1 High affinity to APRIL at pH 7.4 Endothelial cell a High affinity to FcRn at pH 5.8, promoting CLYM116 recycling 3 1 2 3
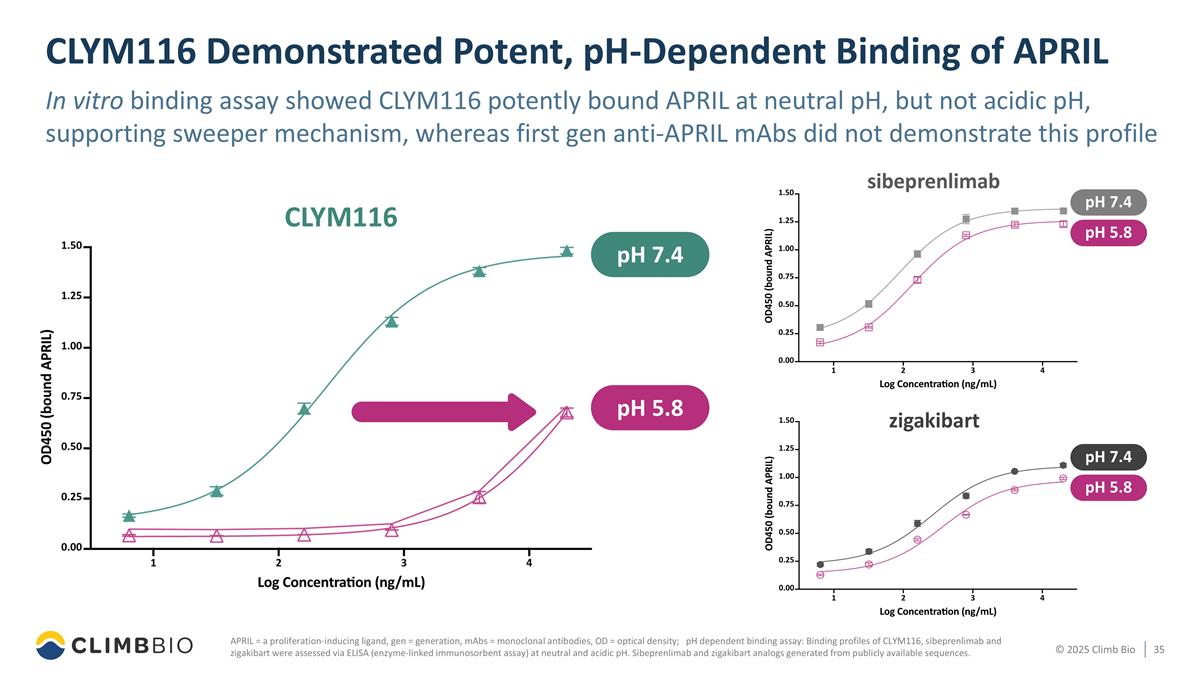
CLYM116 Demonstrated Potent, pH-Dependent Binding of APRIL In vitro binding assay showed CLYM116 potently bound APRIL at neutral pH, but not acidic pH, supporting sweeper mechanism, whereas first gen anti-APRIL mAbs did not demonstrate this profile APRIL = a proliferation-inducing ligand, gen = generation, mAbs = monoclonal antibodies, OD = optical density; pH dependent binding assay: Binding profiles of CLYM116, sibeprenlimab and zigakibart were assessed via ELISA (enzyme-linked immunosorbent assay) at neutral and acidic pH. Sibeprenlimab and zigakibart analogs generated from publicly available sequences. CLYM116 zigakibart pH 7.4 pH 5.8 pH 5.8 pH 7.4 sibeprenlimab pH 5.8 pH 7.4
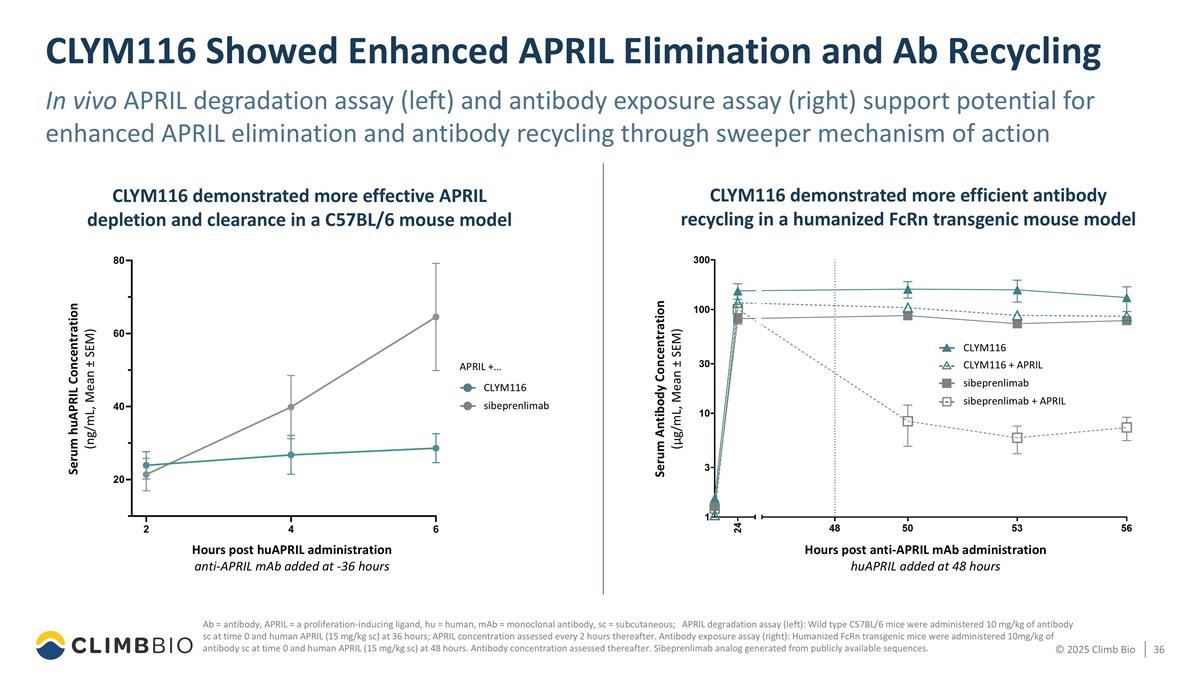
CLYM116 Showed Enhanced APRIL Elimination and Ab Recycling In vivo APRIL degradation assay (left) and antibody exposure assay (right) support potential for enhanced APRIL elimination and antibody recycling through sweeper mechanism of action Ab = antibody, APRIL = a proliferation-inducing ligand, hu = human, mAb = monoclonal antibody, sc = subcutaneous; APRIL degradation assay (left): Wild type C57BL/6 mice were administered 10 mg/kg of antibody sc at time 0 and human APRIL (15 mg/kg sc) at 36 hours; APRIL concentration assessed every 2 hours thereafter. Antibody exposure assay (right): Humanized FcRn transgenic mice were administered 10mg/kg of antibody sc at time 0 and human APRIL (15 mg/kg sc) at 48 hours. Antibody concentration assessed thereafter. Sibeprenlimab analog generated from publicly available sequences. CLYM116 demonstrated more effective APRIL depletion and clearance in a C57BL/6 mouse model CLYM116 demonstrated more efficient antibody recycling in a humanized FcRn transgenic mouse model Hours post anti-APRIL mAb administration huAPRIL added at 48 hours sibeprenlimab + APRIL sibeprenlimab CLYM116 + APRIL CLYM116 Serum Antibody Concentration (µg/mL, Mean ± SEM) Hours post huAPRIL administration anti-APRIL mAb added at -36 hours APRIL +… sibeprenlimab CLYM116 Serum huAPRIL Concentration (ng/mL, Mean ± SEM)
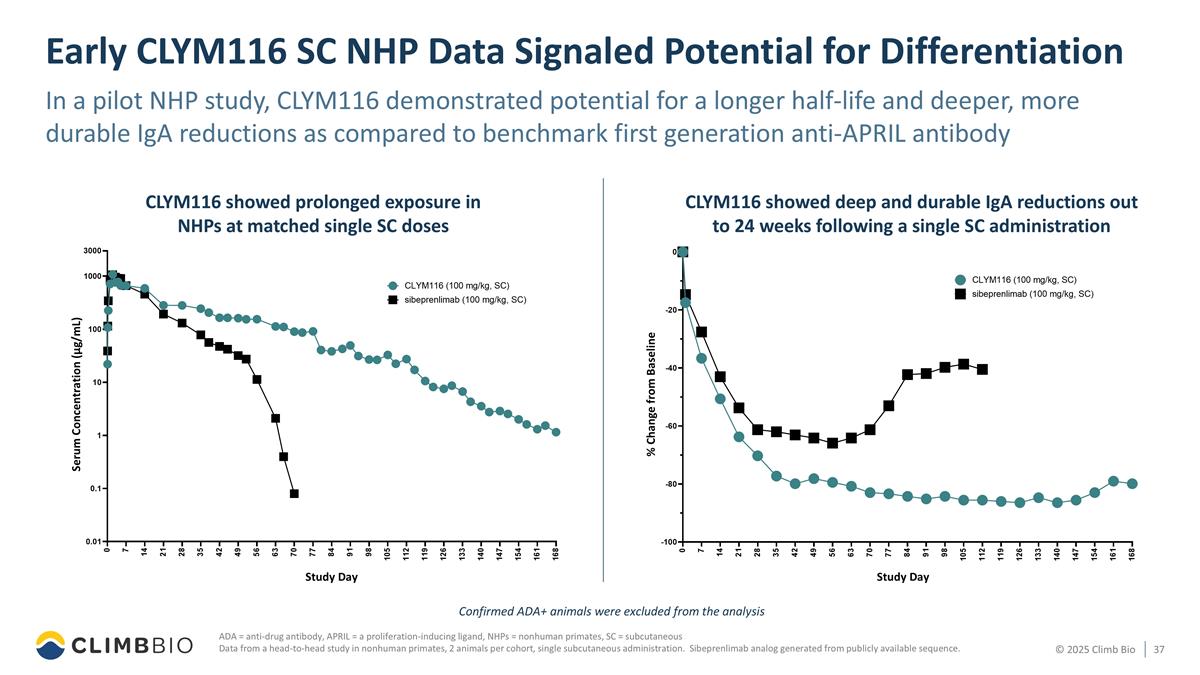
Early CLYM116 SC NHP Data Signaled Potential for Differentiation In a pilot NHP study, CLYM116 demonstrated potential for a longer half-life and deeper, more durable IgA reductions as compared to benchmark first generation anti-APRIL antibody ADA = anti-drug antibody, APRIL = a proliferation-inducing ligand, NHPs = nonhuman primates, SC = subcutaneous Data from a head-to-head study in nonhuman primates, 2 animals per cohort, single subcutaneous administration. Sibeprenlimab analog generated from publicly available sequence. Confirmed ADA+ animals were excluded from the analysis CLYM116 showed prolonged exposure in NHPs at matched single SC doses CLYM116 showed deep and durable IgA reductions out to 24 weeks following a single SC administration Study Day Serum Concentration (µg/mL) Study Day % Change from Baseline
Larger Study in NHPs Further Evaluated CLYM116 Profile 4-6 animals per arm per dose Single dose administration CLYM116 SC and IV at matched doses CLYM116 SC and sibeprenlimab SC at matched doses Pharmacokinetic and pharmacodynamic measures assessed daily through day 8 and weekly thereafter Additional head-to-head comparative study in NHPs conducted to further assess CLYM116 exposure, activity and tolerability profile NHP = nonhuman primate, , IV = intravenous, SC = subcutaneous, TMDD = target mediated drug disposition Confirm SC bioavailability and tolerability profile Assess pharmacokinetics, dose response, and potential effect of TMDD on exposure Replicate pilot NHP study results in a more robust sample Assess biomarkers in NHPs that have shown translatability to human clinical efficacy Support initiation of clinical development OBJECTIVES STUDY DESIGN
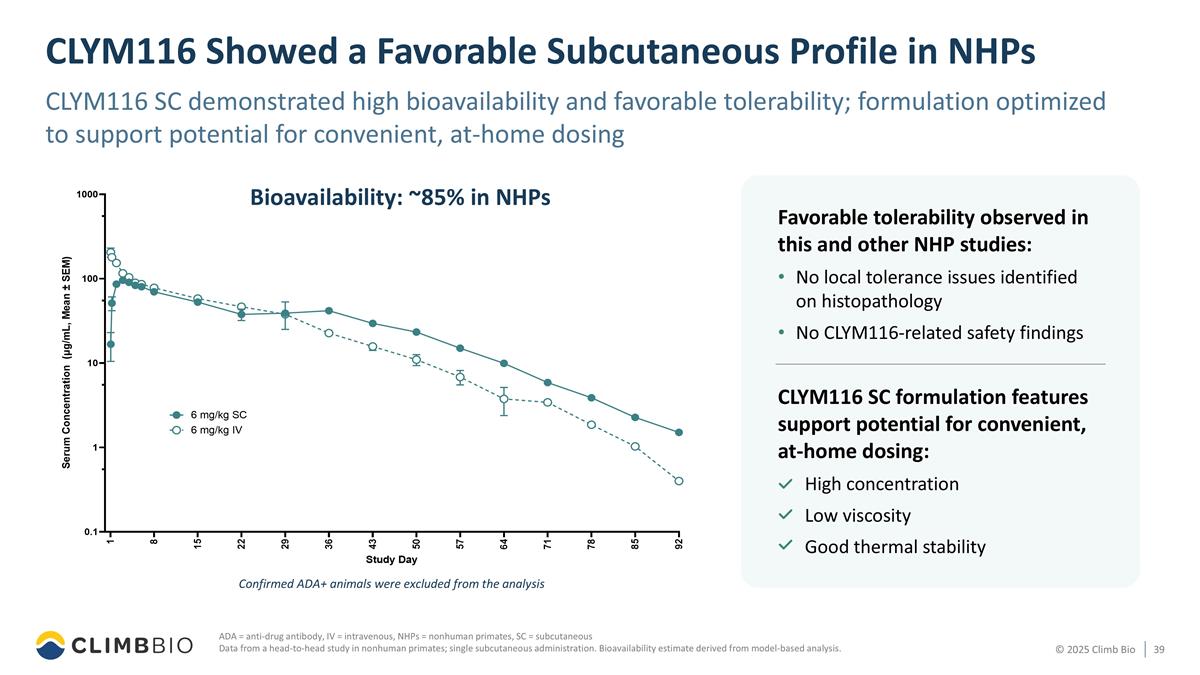
CLYM116 Showed a Favorable Subcutaneous Profile in NHPs CLYM116 SC demonstrated high bioavailability and favorable tolerability; formulation optimized to support potential for convenient, at-home dosing ADA = anti-drug antibody, IV = intravenous, NHPs = nonhuman primates, SC = subcutaneous Data from a head-to-head study in nonhuman primates; single subcutaneous administration. Bioavailability estimate derived from model-based analysis. CLYM116 SC formulation features support potential for convenient, at-home dosing: High concentration Low viscosity Good thermal stability Favorable tolerability observed in this and other NHP studies: No local tolerance issues identified on histopathology No CLYM116-related safety findings Bioavailability: ~85% in NHPs Confirmed ADA+ animals were excluded from the analysis
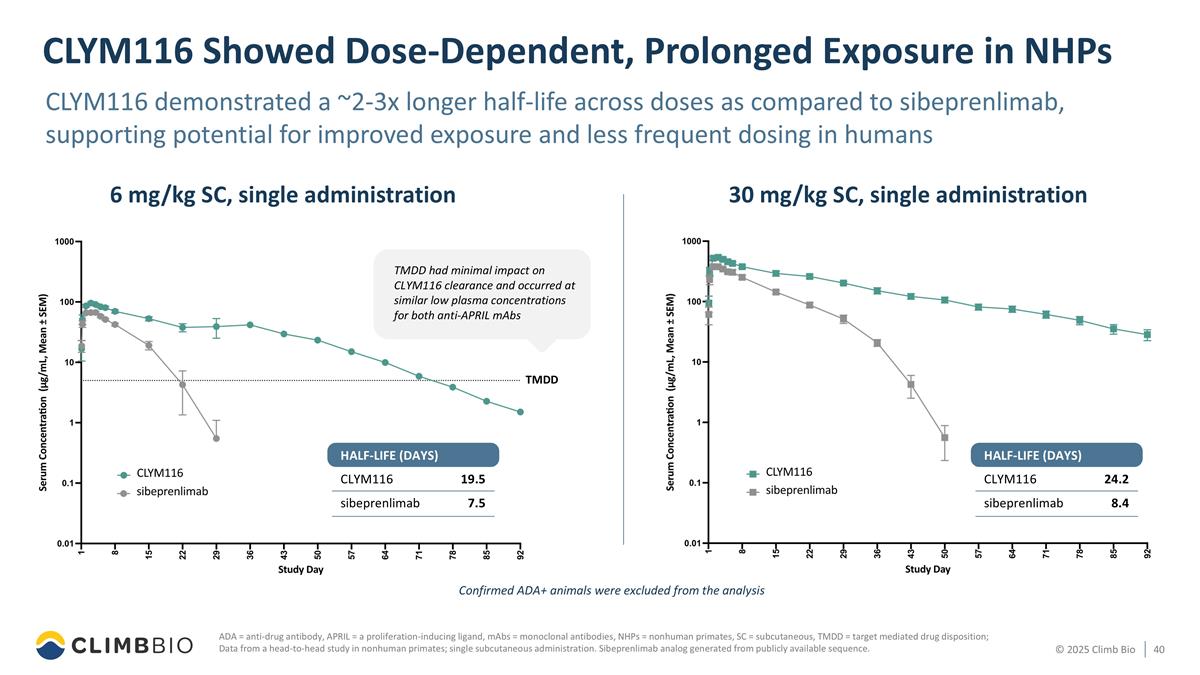
CLYM116 Showed Dose-Dependent, Prolonged Exposure in NHPs ADA = anti-drug antibody, APRIL = a proliferation-inducing ligand, mAbs = monoclonal antibodies, NHPs = nonhuman primates, SC = subcutaneous, TMDD = target mediated drug disposition; Data from a head-to-head study in nonhuman primates; single subcutaneous administration. Sibeprenlimab analog generated from publicly available sequence. CLYM116 demonstrated a ~2-3x longer half-life across doses as compared to sibeprenlimab, supporting potential for improved exposure and less frequent dosing in humans Confirmed ADA+ animals were excluded from the analysis 6 mg/kg SC, single administration 30 mg/kg SC, single administration TMDD CLYM116 sibeprenlimab 19.5 7.5 HALF-LIFE (DAYS) TMDD had minimal impact on CLYM116 clearance and occurred at similar low plasma concentrations for both anti-APRIL mAbs CLYM116 sibeprenlimab 24.2 8.4 HALF-LIFE (DAYS)
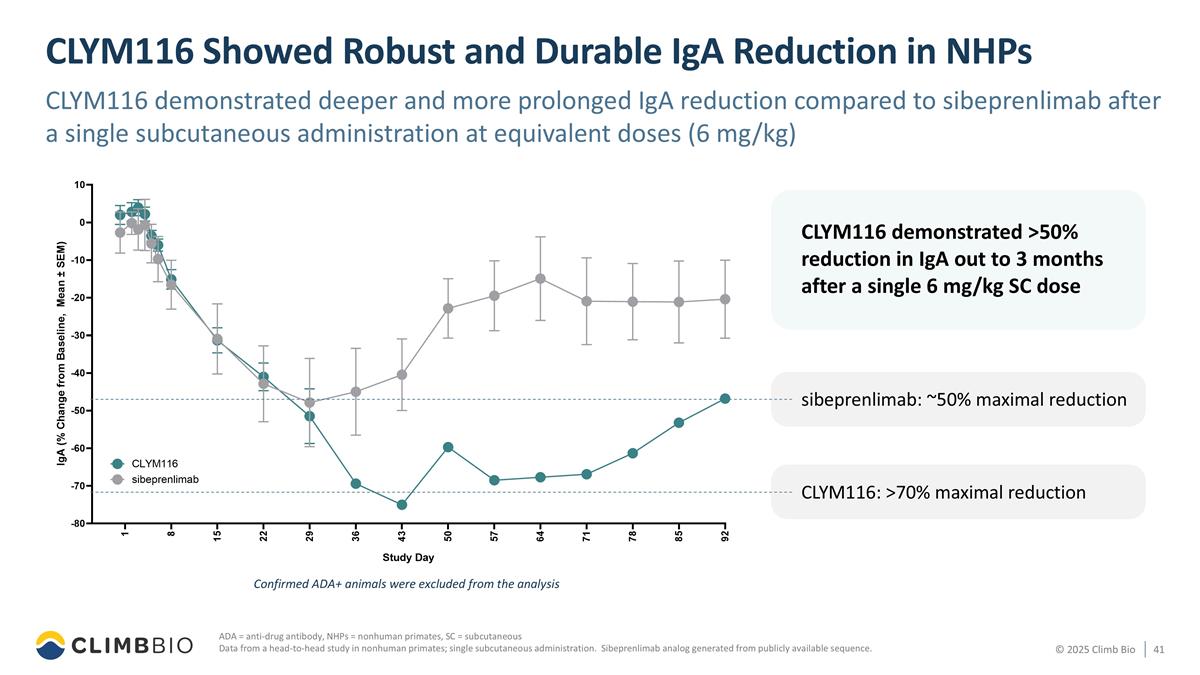
ADA = anti-drug antibody, NHPs = nonhuman primates, SC = subcutaneous Data from a head-to-head study in nonhuman primates; single subcutaneous administration. Sibeprenlimab analog generated from publicly available sequence. CLYM116 demonstrated >50% reduction in IgA out to 3 months after a single 6 mg/kg SC dose CLYM116 Showed Robust and Durable IgA Reduction in NHPs CLYM116 demonstrated deeper and more prolonged IgA reduction compared to sibeprenlimab after a single subcutaneous administration at equivalent doses (6 mg/kg) sibeprenlimab: ~50% maximal reduction CLYM116: >70% maximal reduction Confirmed ADA+ animals were excluded from the analysis
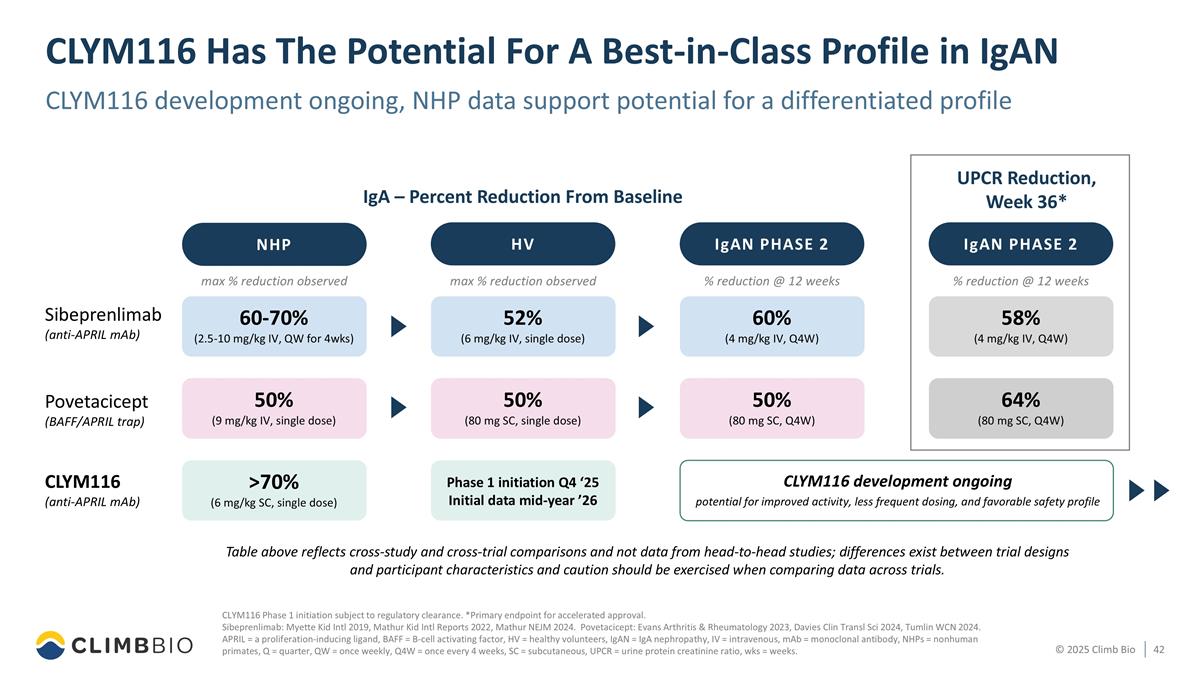
CLYM116 Has The Potential For A Best-in-Class Profile in IgAN CLYM116 development ongoing, NHP data support potential for a differentiated profile Sibeprenlimab (anti-APRIL mAb) Povetacicept (BAFF/APRIL trap) IgA – Percent Reduction From Baseline UPCR Reduction, Week 36* CLYM116 (anti-APRIL mAb) Table above reflects cross-study and cross-trial comparisons and not data from head-to-head studies; differences exist between trial designs and participant characteristics and caution should be exercised when comparing data across trials. CLYM116 Phase 1 initiation subject to regulatory clearance. *Primary endpoint for accelerated approval. Sibeprenlimab: Myette Kid Intl 2019, Mathur Kid Intl Reports 2022, Mathur NEJM 2024. Povetacicept: Evans Arthritis & Rheumatology 2023, Davies Clin Transl Sci 2024, Tumlin WCN 2024. APRIL = a proliferation-inducing ligand, BAFF = B-cell activating factor, HV = healthy volunteers, IgAN = IgA nephropathy, IV = intravenous, mAb = monoclonal antibody, NHPs = nonhuman primates, Q = quarter, QW = once weekly, Q4W = once every 4 weeks, SC = subcutaneous, UPCR = urine protein creatinine ratio, wks = weeks. NHP max % reduction observed 60-70% (2.5-10 mg/kg IV, QW for 4wks) 50% (9 mg/kg IV, single dose) >70% (6 mg/kg SC, single dose) HV 52% (6 mg/kg IV, single dose) 50% (80 mg SC, single dose) Phase 1 initiation Q4 ‘25 Initial data mid-year ’26 max % reduction observed IgAN PHASE 2 % reduction @ 12 weeks 60% (4 mg/kg IV, Q4W) 50% (80 mg SC, Q4W) IgAN PHASE 2 % reduction @ 12 weeks 58% (4 mg/kg IV, Q4W) 64% (80 mg SC, Q4W) CLYM116 development ongoing potential for improved activity, less frequent dosing, and favorable safety profile
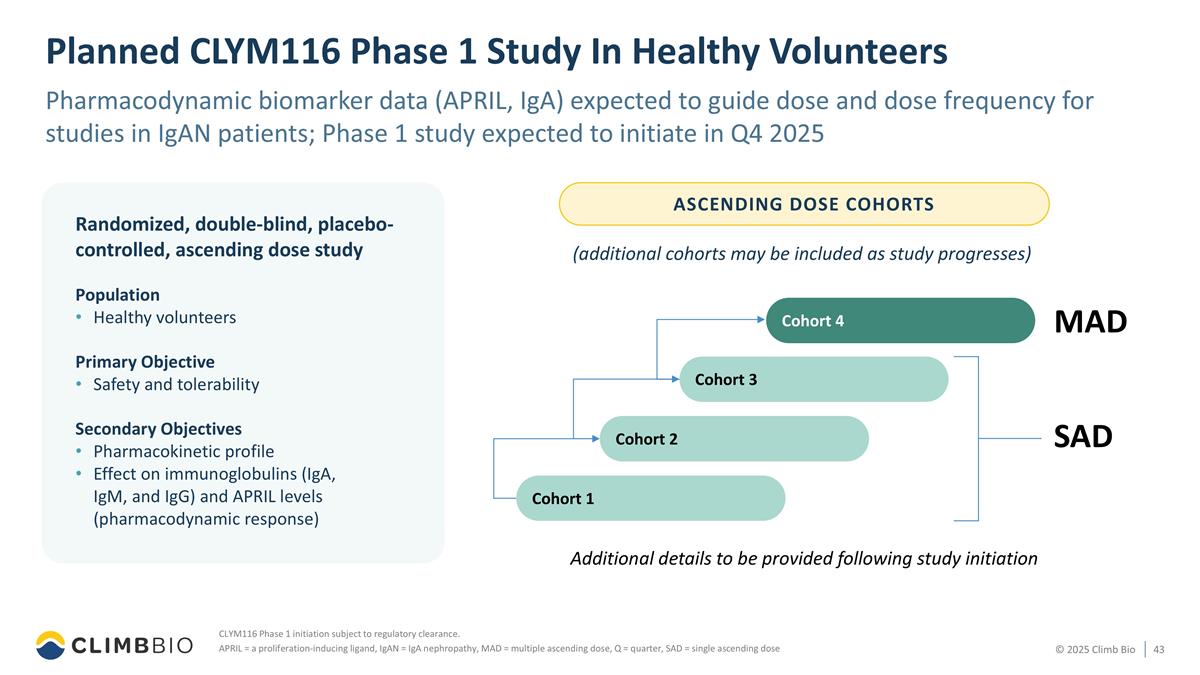
Planned CLYM116 Phase 1 Study In Healthy Volunteers Pharmacodynamic biomarker data (APRIL, IgA) expected to guide dose and dose frequency for studies in IgAN patients; Phase 1 study expected to initiate in Q4 2025 Population Healthy volunteers Primary Objective Safety and tolerability Secondary Objectives Pharmacokinetic profile Effect on immunoglobulins (IgA, IgM, and IgG) and APRIL levels (pharmacodynamic response) Randomized, double-blind, placebo-controlled, ascending dose study ASCENDING DOSE COHORTS CLYM116 Phase 1 initiation subject to regulatory clearance. APRIL = a proliferation-inducing ligand, IgAN = IgA nephropathy, MAD = multiple ascending dose, Q = quarter, SAD = single ascending dose Additional details to be provided following study initiation (additional cohorts may be included as study progresses) Cohort 3 Cohort 4 Cohort 2 Cohort 1 SAD MAD
Our Goal: Quickly progress from Phase 1 study in healthy volunteers to studies in IgAN patients Decision to progress based upon PD (depth and duration of APRIL and IgA suppression) and safety readouts, which will inform: Dosing regimen/frequency Predicted efficacy Predicted safety/tolerability in IgAN patients CLYM116 Phase 1 initiation subject to regulatory clearance; APRIL = a proliferation-inducing ligand, eGFR = estimated glomerular filtration rate, HV = healthy volunteers, IgAN = IgA nephropathy, mAbs = monoclonal antibodies, PD = pharmacodynamic, UPCR = urine protein creatinine ratio Opportunities for development program efficiencies provided by: Internal and external biomarker data (correlations between APRIL suppression, IgA suppression and UPCR reduction, and link to eGFR stabilization) Observed safety/tolerability profile of anti-APRIL mAbs Parallel execution of early-stage studies by Mabworks (China), providing a complementary, independent dataset that will allow fulsome stage-gate decisioning Regulatory precedent CLYM116 Development Strategy and Path Forward Development strategy aimed at rapid progression into a registrational program, with streamlined phase transitions; initial data from Phase 1 trial in HVs anticipated mid-year 2026

CLYM116 is a promising investigational therapy for IgAN, with a unique ‘sweeper’ mechanism of action APRIL represents a validated target with potential to address underlying IgAN pathology CLYM116 is the only known ‘sweeper’ anti-APRIL mAb in development New CLYM116 NHP data demonstrated improvement versus sibeprenlimab (first-generation anti-APRIL mAb) Deeper and more durable IgA reductions – supporting potential for differentiated activity profile ~2-3x longer half-life – supporting potential for less frequent dosing Potential for CLYM116 to be a leading therapy in the large and growing IgAN market (est. $10-20B in US alone) IgAN typically diagnosed early in life and may require lifelong management New treatment guidelines recommend earlier treatment, indicating potential for further market expansion Derisked development opportunity, given established biology and regulatory path Established FDA pathway; endpoints of proteinuria and eGFR can potentially support product approval Biomarkers provide rapid assessment of clinical profile during early development Meaningful near-term CLYM116 anticipated milestones Initiation of Phase 1 HV study expected Q4 2025, with initial data (biomarkers, projected dosing interval) anticipated mid-year 2026 Parallel execution by Mabworks in China is expected to provide a complementary Phase 1 dataset CLYM116 is a Potential Best-in-Class anti-APRIL mAb for IgAN CLYM116 Phase 1 initiation subject to regulatory clearance; APRIL = a proliferation-inducing ligand, eGFR = estimated glomerular filtration rate, HV = healthy volunteers, IgAN = IgA nephropathy, mAb = monoclonal antibody, NHP = nonhuman primate, Q = quarter

Q&A Session Aoife Brennan, M.B., Ch.B. President and CEO, Climb Bio Craig Gordon, M.D., M.S. Professor, Tufts University School of Medicine Edgar Charles, M.D. Chief Medical Officer, Climb Bio Perrin Wilson, Ph.D. Chief Business Officer, Climb Bio