| Deepening our Commitment to Patients Suffering from Chemotherapy Induced Peripheral Neuropathy NASDAQ: DWTX September 29th, 2025 |
| NASDAQ: DWTX 2 Agenda for Today ➢Great Progress on Ongoing Halneuron® Phase 2b Chemotherapy Induced Pain Study ➢Exciting New SP16 Cancer Related Pain Global License Overview ➢Q&A |
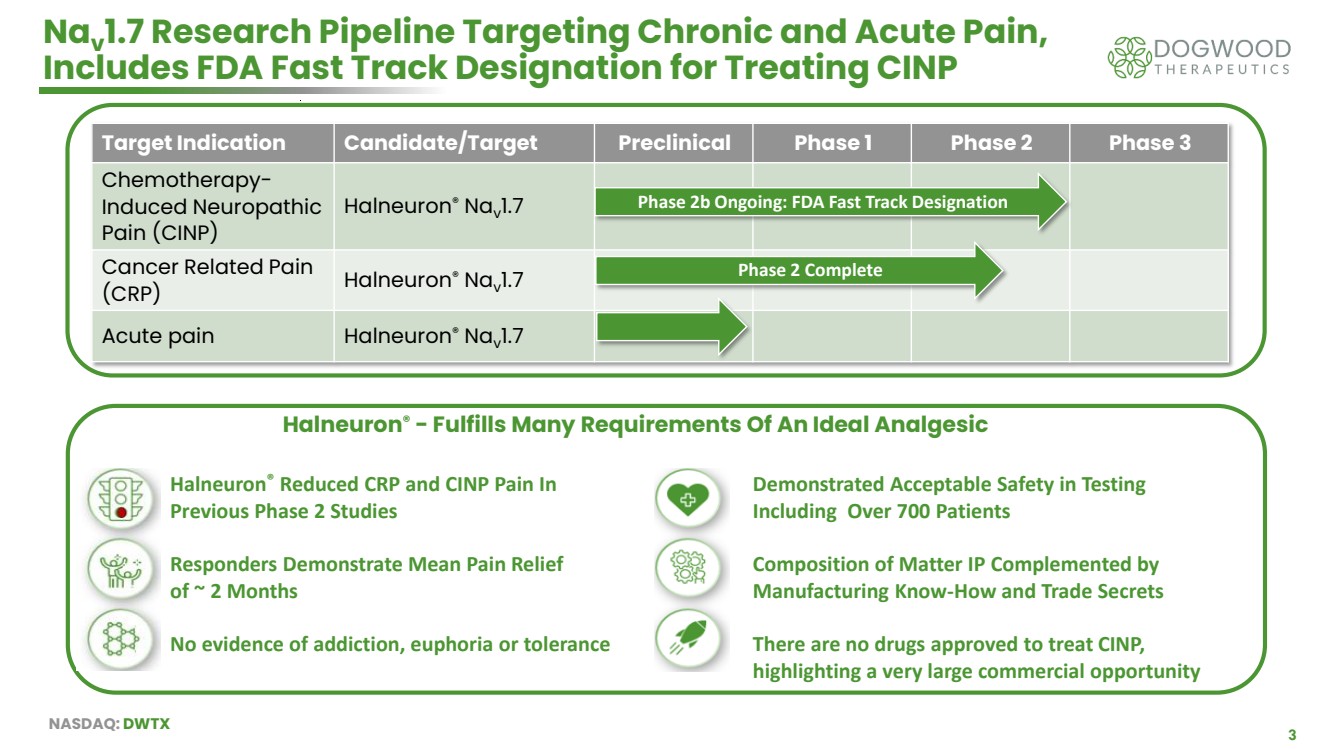
| NASDAQ: DWTX 3 Nav 1.7 Research Pipeline Targeting Chronic and Acute Pain, Includes FDA Fast Track Designation for Treating CINP Target Indication Candidate/Target Preclinical Phase 1 Phase 2 Phase 3 Chemotherapy-Induced Neuropathic Pain (CINP) Halneuron® Nav 1.7 Cancer Related Pain (CRP) Halneuron® Nav 1.7 Acute pain Halneuron® Nav 1.7 Halneuron® - Fulfills Many Requirements Of An Ideal Analgesic Phase 2b Ongoing: FDA Fast Track Designation Phase 2 Complete Halneuron® Reduced CRP and CINP Pain In Previous Phase 2 Studies Responders Demonstrate Mean Pain Relief of ~ 2 Months No evidence of addiction, euphoria or tolerance Demonstrated Acceptable Safety in Testing Including Over 700 Patients Composition of Matter IP Complemented by Manufacturing Know-How and Trade Secrets There are no drugs approved to treat CINP, highlighting a very large commercial opportunity |
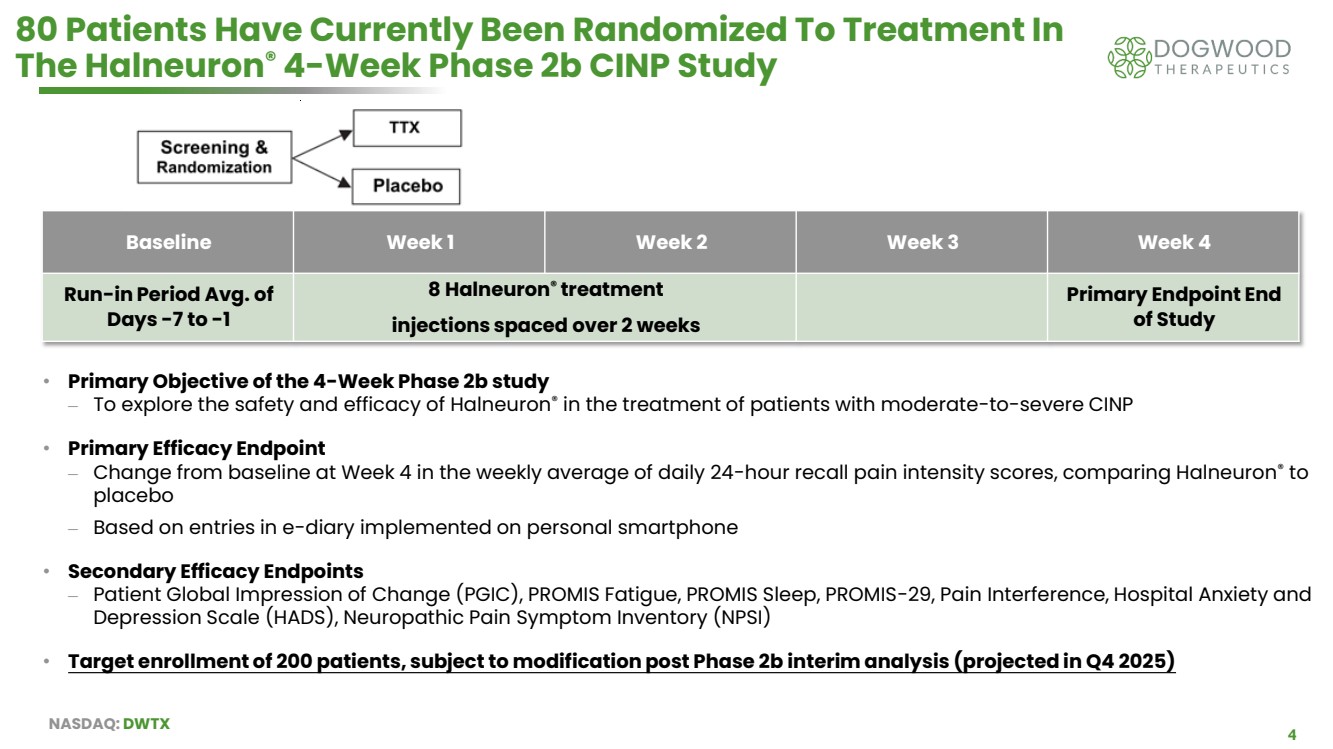
| NASDAQ: DWTX 4 80 Patients Have Currently Been Randomized To Treatment In The Halneuron® 4-Week Phase 2b CINP Study Baseline Week 1 Week 2 Week 3 Week 4 Run-in Period Avg. of Days -7 to -1 8 Halneuron® treatment injections spaced over 2 weeks Primary Endpoint End of Study • Primary Objective of the 4-Week Phase 2b study ⎼ To explore the safety and efficacy of Halneuron® in the treatment of patients with moderate-to-severe CINP • Primary Efficacy Endpoint ⎼ Change from baseline at Week 4 in the weekly average of daily 24-hour recall pain intensity scores, comparing Halneuron® to placebo ⎼ Based on entries in e-diary implemented on personal smartphone • Secondary Efficacy Endpoints ⎼ Patient Global Impression of Change (PGIC), PROMIS Fatigue, PROMIS Sleep, PROMIS-29, Pain Interference, Hospital Anxiety and Depression Scale (HADS), Neuropathic Pain Symptom Inventory (NPSI) • Target enrollment of 200 patients, subject to modification post Phase 2b interim analysis (projected in Q4 2025) |
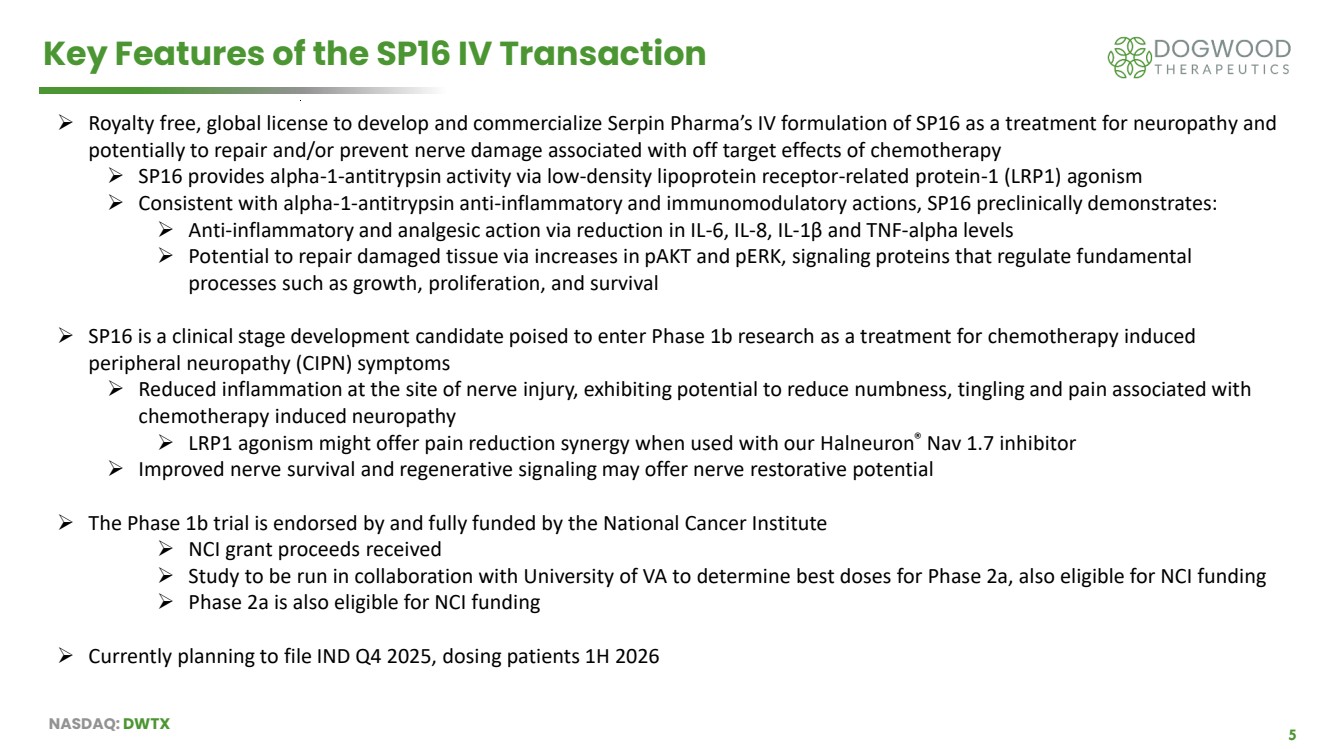
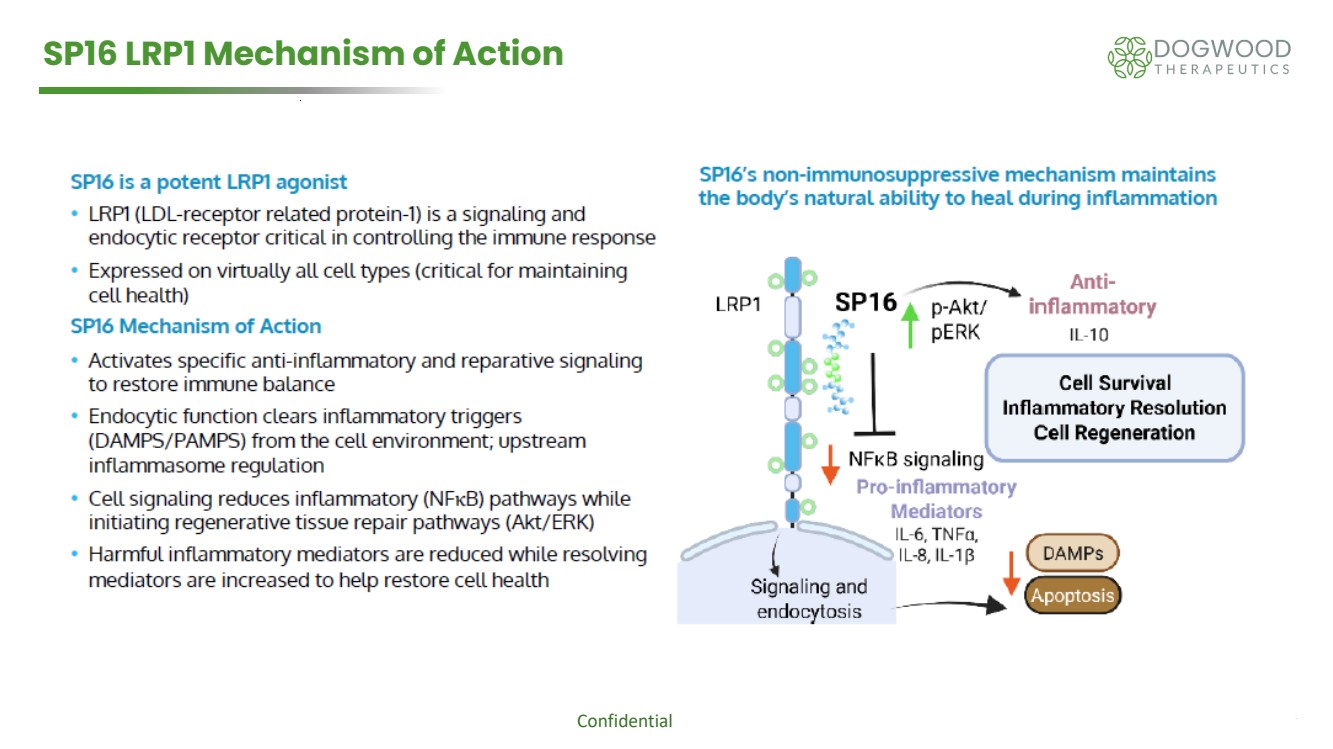
| NASDAQ: DWTX 5 Key Features of the SP16 IV Transaction ➢ Royalty free, global license to develop and commercialize Serpin Pharma’s IV formulation of SP16 as a treatment for neuropathy and potentially to repair and/or prevent nerve damage associated with off target effects of chemotherapy ➢ SP16 provides alpha-1-antitrypsin activity via low-density lipoprotein receptor-related protein-1 (LRP1) agonism ➢ Consistent with alpha-1-antitrypsin anti-inflammatory and immunomodulatory actions, SP16 preclinically demonstrates: ➢ Anti-inflammatory and analgesic action via reduction in IL-6, IL-8, IL-1β and TNF-alpha levels ➢ Potential to repair damaged tissue via increases in pAKT and pERK, signaling proteins that regulate fundamental processes such as growth, proliferation, and survival ➢ SP16 is a clinical stage development candidate poised to enter Phase 1b research as a treatment for chemotherapy induced peripheral neuropathy (CIPN) symptoms ➢ Reduced inflammation at the site of nerve injury, exhibiting potential to reduce numbness, tingling and pain associated with chemotherapy induced neuropathy ➢ LRP1 agonism might offer pain reduction synergy when used with our Halneuron® Nav 1.7 inhibitor ➢ Improved nerve survival and regenerative signaling may offer nerve restorative potential ➢ The Phase 1b trial is endorsed by and fully funded by the National Cancer Institute ➢ NCI grant proceeds received ➢ Study to be run in collaboration with University of VA to determine best doses for Phase 2a, also eligible for NCI funding ➢ Phase 2a is also eligible for NCI funding ➢ Currently planning to file IND Q4 2025, dosing patients 1H 2026 |
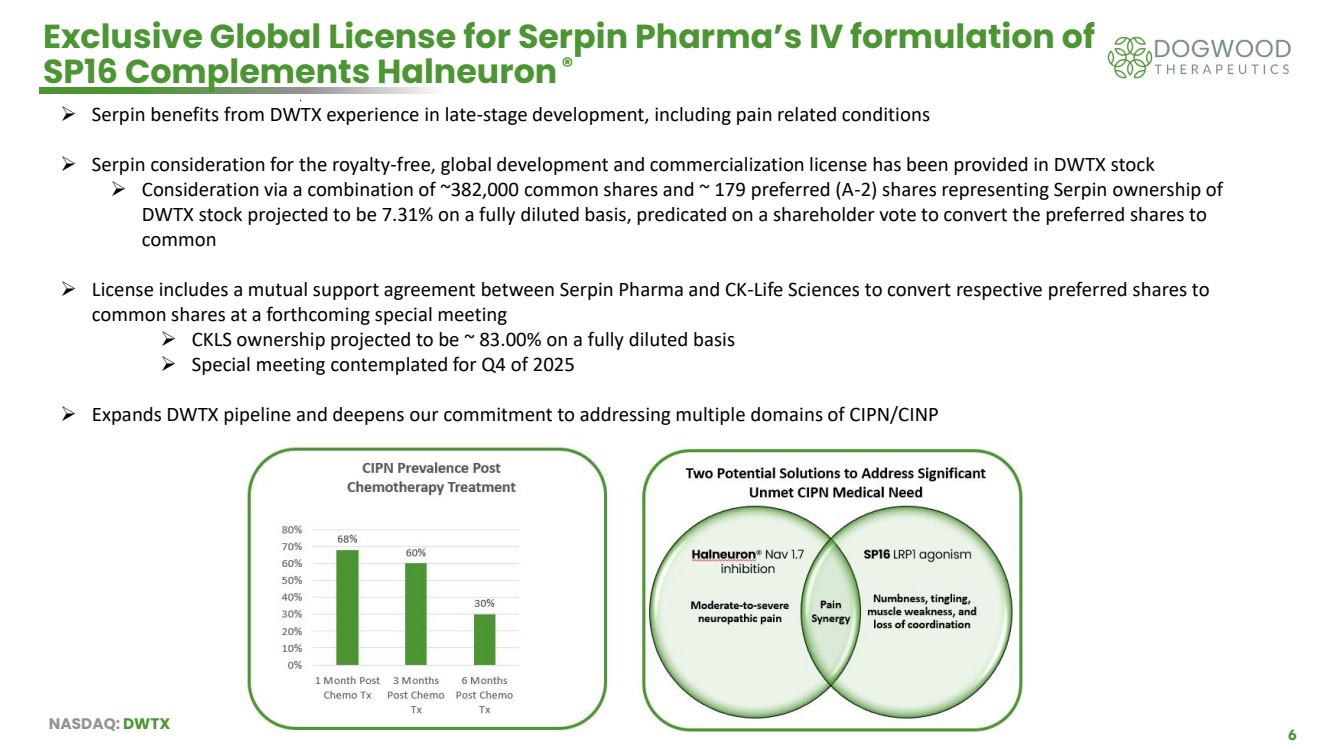
| NASDAQ: DWTX 6 Exclusive Global License for Serpin Pharma’s IV formulation of SP16 Complements Halneuron® ➢ Serpin benefits from DWTX experience in late-stage development, including pain related conditions ➢ Serpin consideration for the royalty-free, global development and commercialization license has been provided in DWTX stock ➢ Consideration via a combination of ~382,000 common shares and ~ 179 preferred (A-2) shares representing Serpin ownership of DWTX stock projected to be 7.31% on a fully diluted basis, predicated on a shareholder vote to convert the preferred shares to common ➢ License includes a mutual support agreement between Serpin Pharma and CK-Life Sciences to convert respective preferred shares to common shares at a forthcoming special meeting ➢ CKLS ownership projected to be ~ 83.00% on a fully diluted basis ➢ Special meeting contemplated for Q4 of 2025 ➢ Expands DWTX pipeline and deepens our commitment to addressing multiple domains of CIPN/CINP |
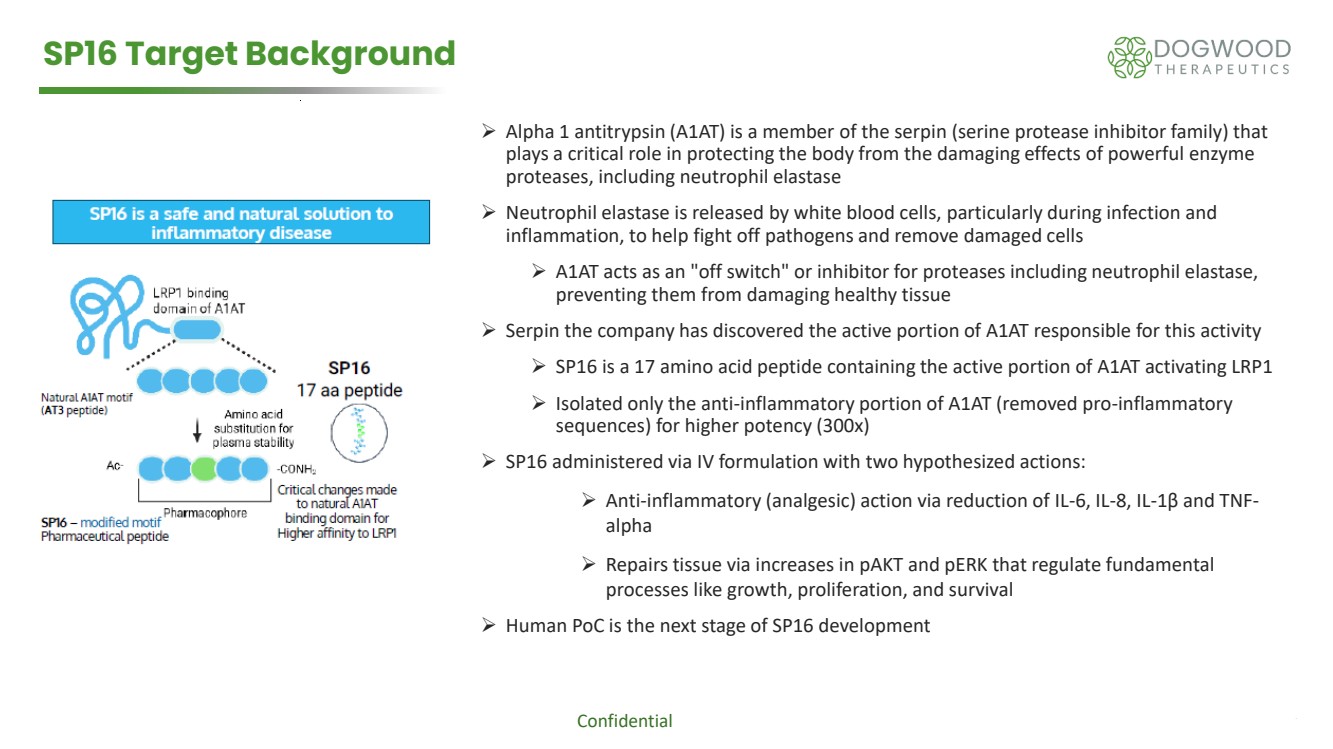
| Confidential 7 SP16 Target Background ➢ Alpha 1 antitrypsin (A1AT) is a member of the serpin (serine protease inhibitor family) that plays a critical role in protecting the body from the damaging effects of powerful enzyme proteases, including neutrophil elastase ➢ Neutrophil elastase is released by white blood cells, particularly during infection and inflammation, to help fight off pathogens and remove damaged cells ➢ A1AT acts as an "off switch" or inhibitor for proteases including neutrophil elastase, preventing them from damaging healthy tissue ➢ Serpin the company has discovered the active portion of A1AT responsible for this activity ➢ SP16 is a 17 amino acid peptide containing the active portion of A1AT activating LRP1 ➢ Isolated only the anti-inflammatory portion of A1AT (removed pro-inflammatory sequences) for higher potency (300x) ➢ SP16 administered via IV formulation with two hypothesized actions: ➢ Anti-inflammatory (analgesic) action via reduction of IL-6, IL-8, IL-1β and TNF-alpha ➢ Repairs tissue via increases in pAKT and pERK that regulate fundamental processes like growth, proliferation, and survival ➢ Human PoC is the next stage of SP16 development |
| Confidential 8 SP16 LRP1 Mechanism of Action |
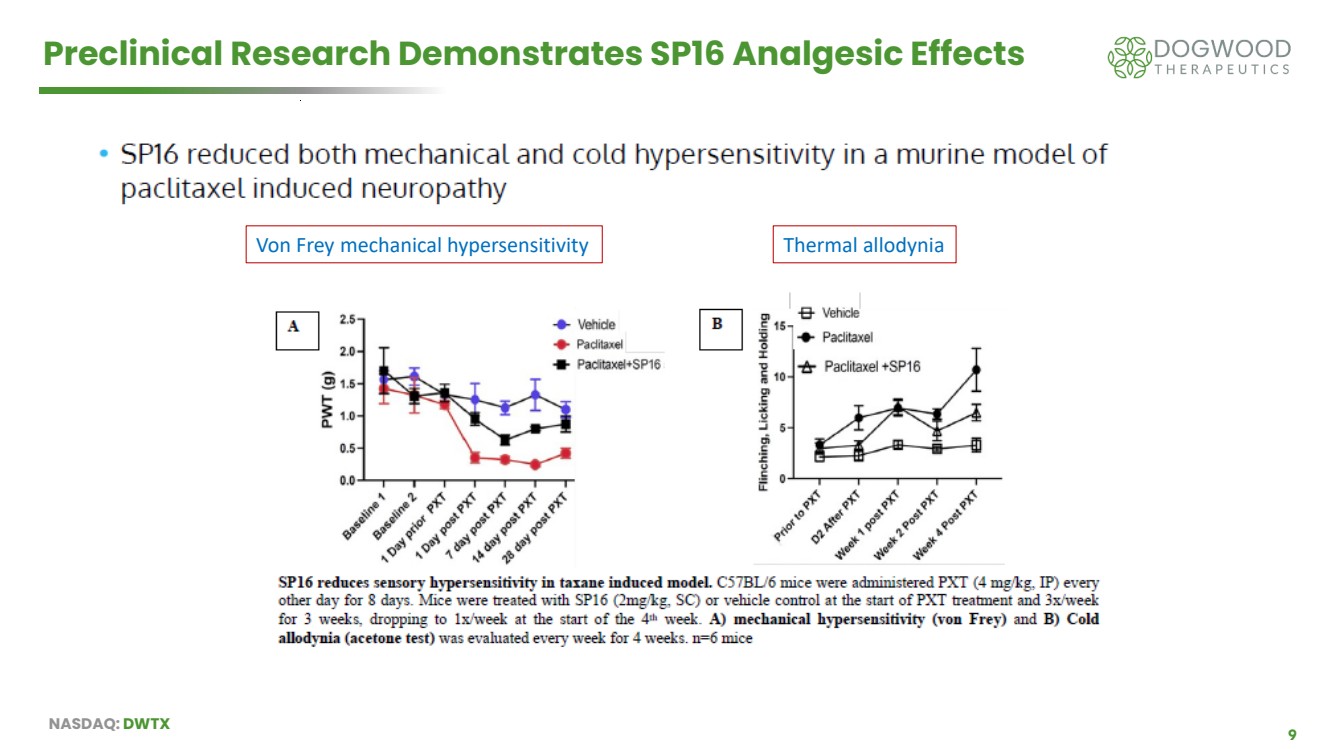
| NASDAQ: DWTX 9 Preclinical Research Demonstrates SP16 Analgesic Effects Von Frey mechanical hypersensitivity Thermal allodynia |
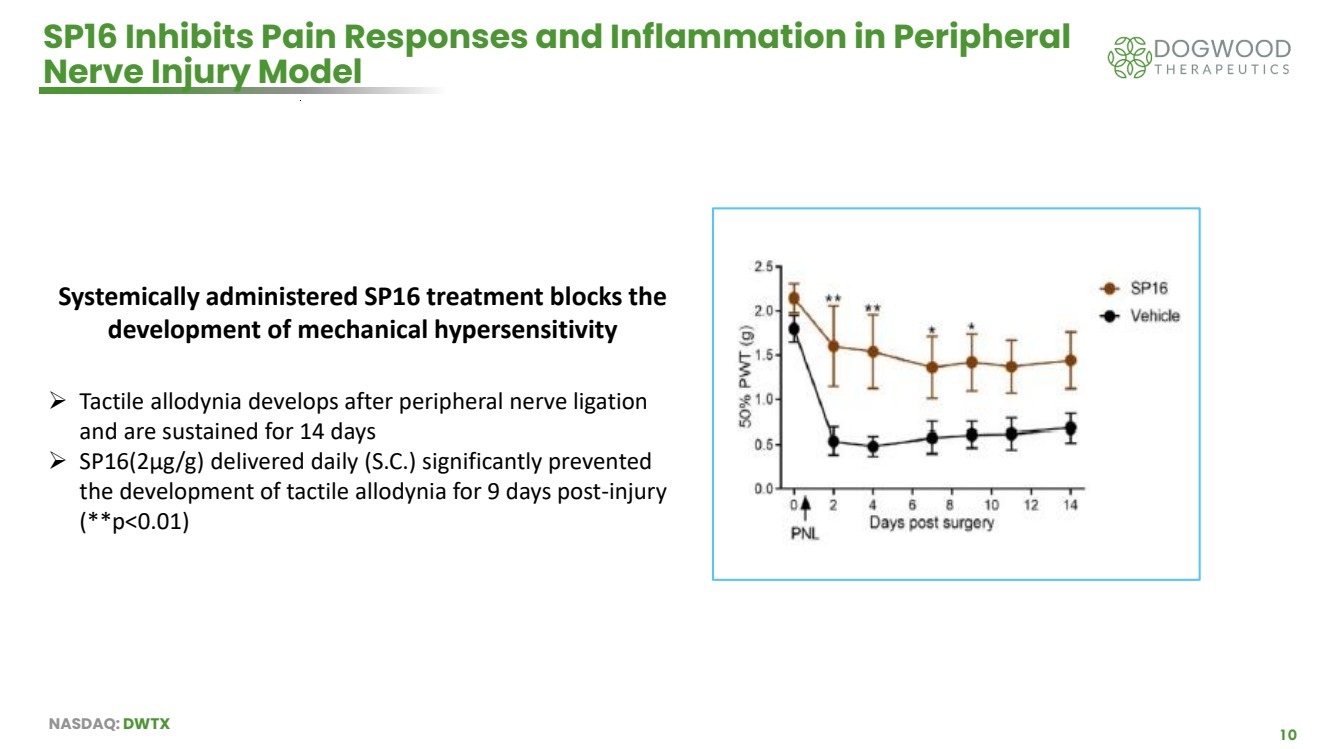
| NASDAQ: DWTX 10 SP16 Inhibits Pain Responses and Inflammation in Peripheral Nerve Injury Model Systemically administered SP16 treatment blocks the development of mechanical hypersensitivity ➢ Tactile allodynia develops after peripheral nerve ligation and are sustained for 14 days ➢ SP16(2μg/g) delivered daily (S.C.) significantly prevented the development of tactile allodynia for 9 days post-injury (**p<0.01) |
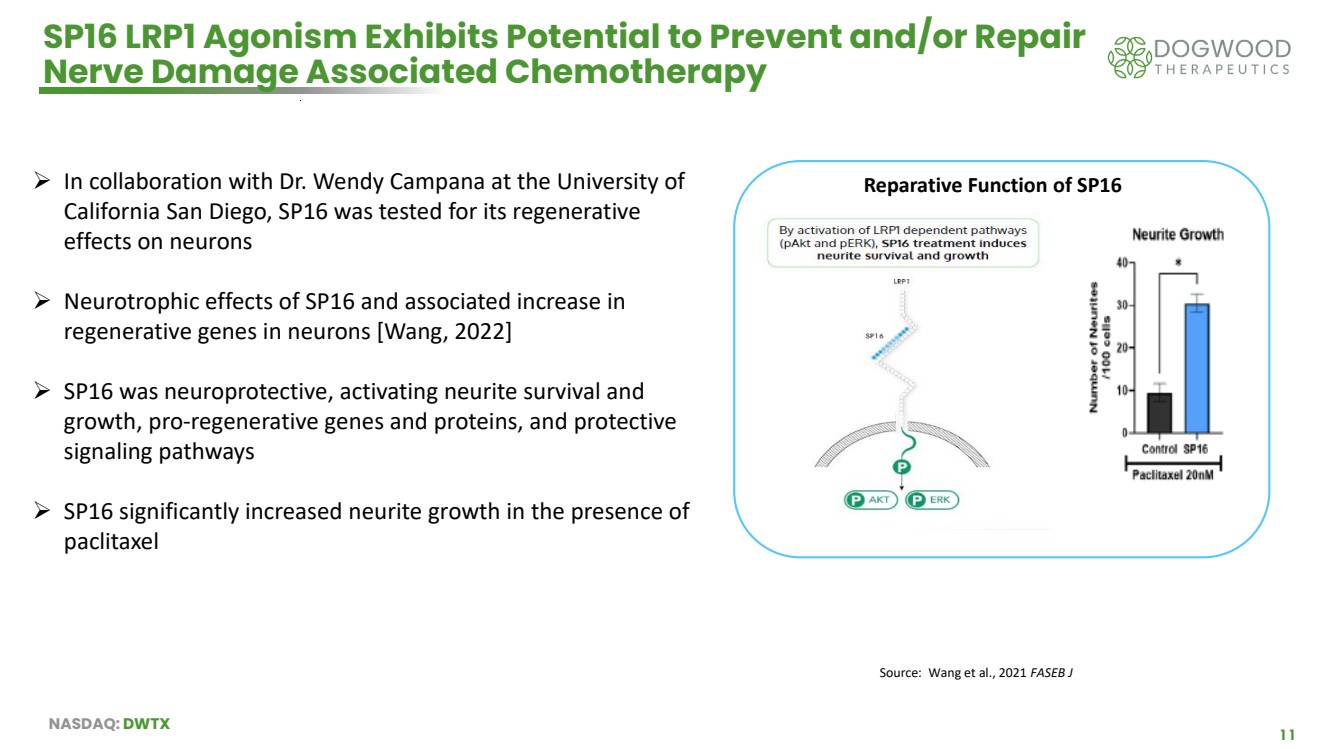
| NASDAQ: DWTX 11 SP16 LRP1 Agonism Exhibits Potential to Prevent and/or Repair Nerve Damage Associated Chemotherapy ➢ In collaboration with Dr. Wendy Campana at the University of California San Diego, SP16 was tested for its regenerative effects on neurons ➢ Neurotrophic effects of SP16 and associated increase in regenerative genes in neurons [Wang, 2022] ➢ SP16 was neuroprotective, activating neurite survival and growth, pro-regenerative genes and proteins, and protective signaling pathways ➢ SP16 significantly increased neurite growth in the presence of paclitaxel Source: Wang et al., 2021 FASEB J Reparative Function of SP16 |
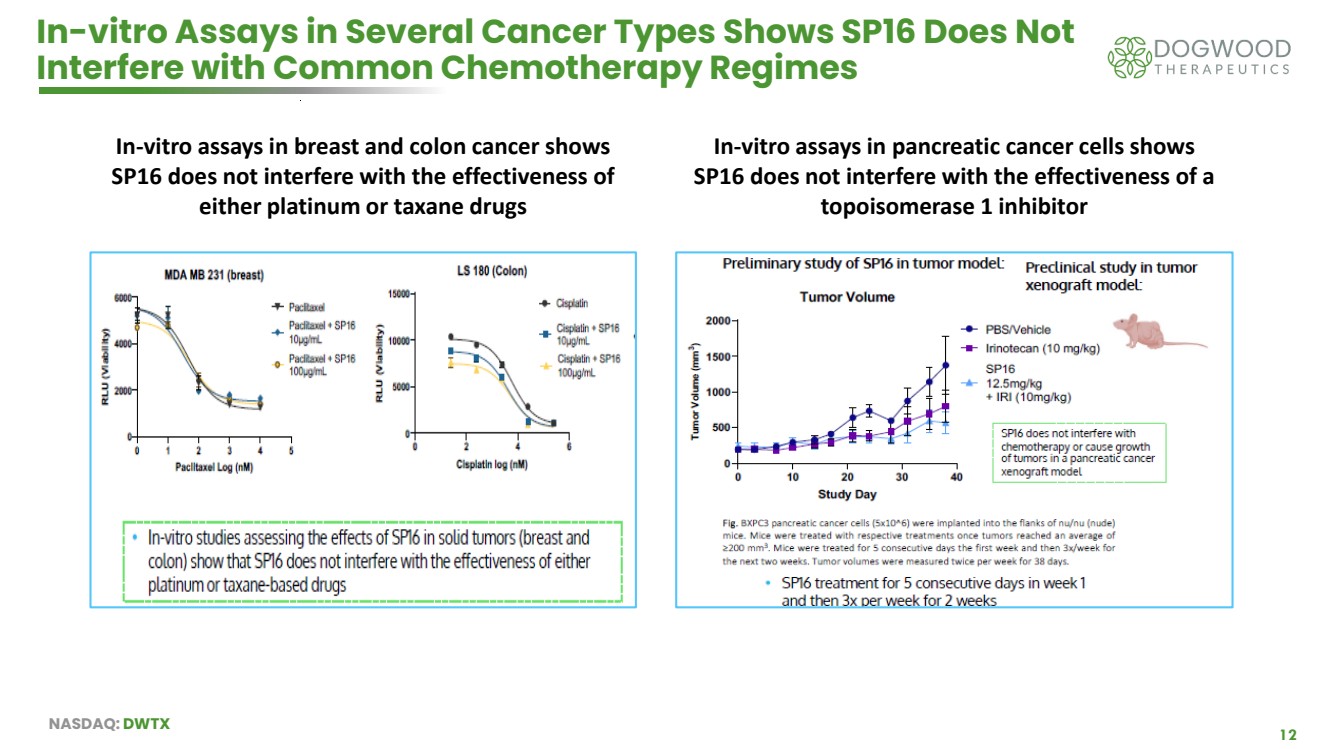
| NASDAQ: DWTX 12 In-vitro Assays in Several Cancer Types Shows SP16 Does Not Interfere with Common Chemotherapy Regimes In-vitro assays in breast and colon cancer shows SP16 does not interfere with the effectiveness of either platinum or taxane drugs In-vitro assays in pancreatic cancer cells shows SP16 does not interfere with the effectiveness of a topoisomerase 1 inhibitor |
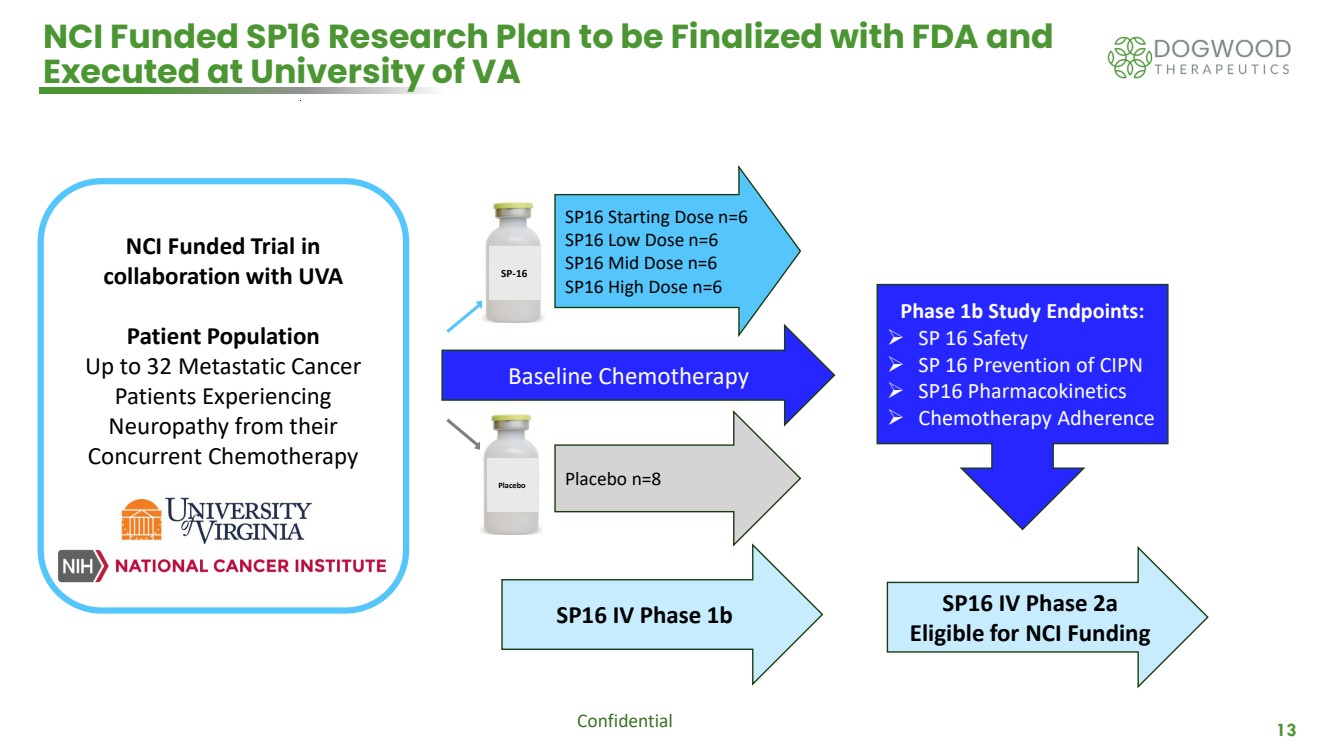
| Confidential 13 NCI Funded SP16 Research Plan to be Finalized with FDA and Executed at University of VA Baseline Chemotherapy SP16 Starting Dose n=6 SP16 Low Dose n=6 SP16 Mid Dose n=6 SP16 High Dose n=6 Placebo n=8 SP16 IV Phase 1b SP16 IV Phase 2a Eligible for NCI Funding Phase 1b Study Endpoints: ➢ SP 16 Safety ➢ SP 16 Prevention of CIPN ➢ SP16 Pharmacokinetics ➢ Chemotherapy Adherence NCI Funded Trial in collaboration with UVA Patient Population Up to 32 Metastatic Cancer Patients Experiencing Neuropathy from their Concurrent Chemotherapy SP-16 Placebo |
| NASDAQ: DWTX 14 Halneuron® Fit with SP16 for CINP and More Broadly for Cancer Related Pain Deal Rationale: ➢ Halneuron® (TTX) = Nav1.7 channel blocker, analgesic → best for treating established CINP pain ➢ Halneuron® is in later-stage development for CINP ➢ SP16 = LRP1-agonist, anti-inflammatory, neuroprotection → best for attenuation of CIPN during chemo; ➢ SP16 is in early clinical stage development and may enable neuroprotection, may preserve full chemo regimen and potential to synergistically complement Halneuron® in treating pain post chemotherapy ➢ Common commercial call Points (oncology and pain Centers) and potential partners ➢ Bundled protocols/formularies with major cancer centers/providers ➢ Co-promotion targeting infusion suites and pain clinics ➢ Together deeper penetration into the global CINP treatment opportunity ~$1.5B market ➢ Ability to expand into larger Cancer Related Pain market ➢ Increased “shots on goal” by doubling down on the channel into CIPN/ CINP ➢ Independent endpoints (prevention/regeneration vs treatment) allow parallel development ➢ Positive readouts in either arm create unique revenue pathways, while combination studies are designed |
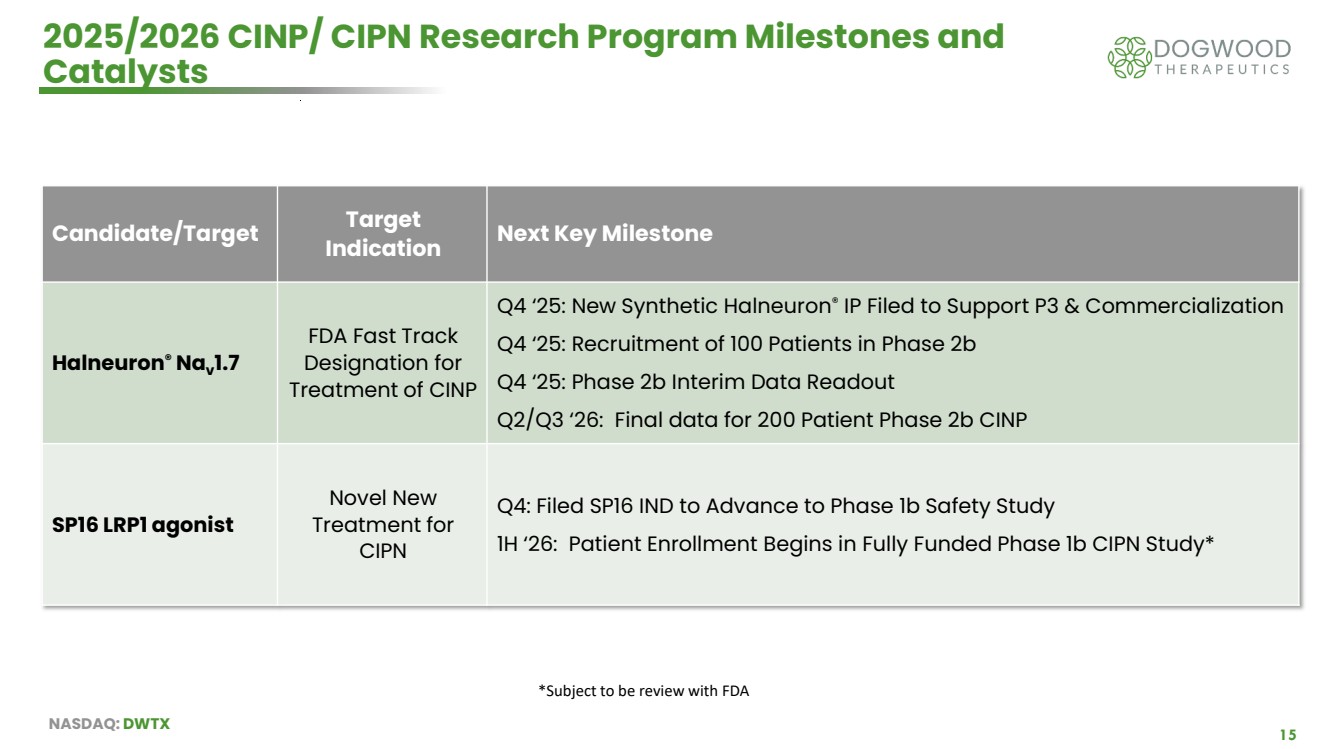
| NASDAQ: DWTX 15 2025/2026 CINP/ CIPN Research Program Milestones and Catalysts Candidate/Target Target Indication Next Key Milestone Halneuron® Nav 1.7 FDA Fast Track Designation for Treatment of CINP Q4 ‘25: New Synthetic Halneuron® IP Filed to Support P3 & Commercialization Q4 ‘25: Recruitment of 100 Patients in Phase 2b Q4 ‘25: Phase 2b Interim Data Readout Q2/Q3 ‘26: Final data for 200 Patient Phase 2b CINP SP16 LRP1 agonist Novel New Treatment for CIPN Q4: Filed SP16 IND to Advance to Phase 1b Safety Study 1H ‘26: Patient Enrollment Begins in Fully Funded Phase 1b CIPN Study* *Subject to be review with FDA |
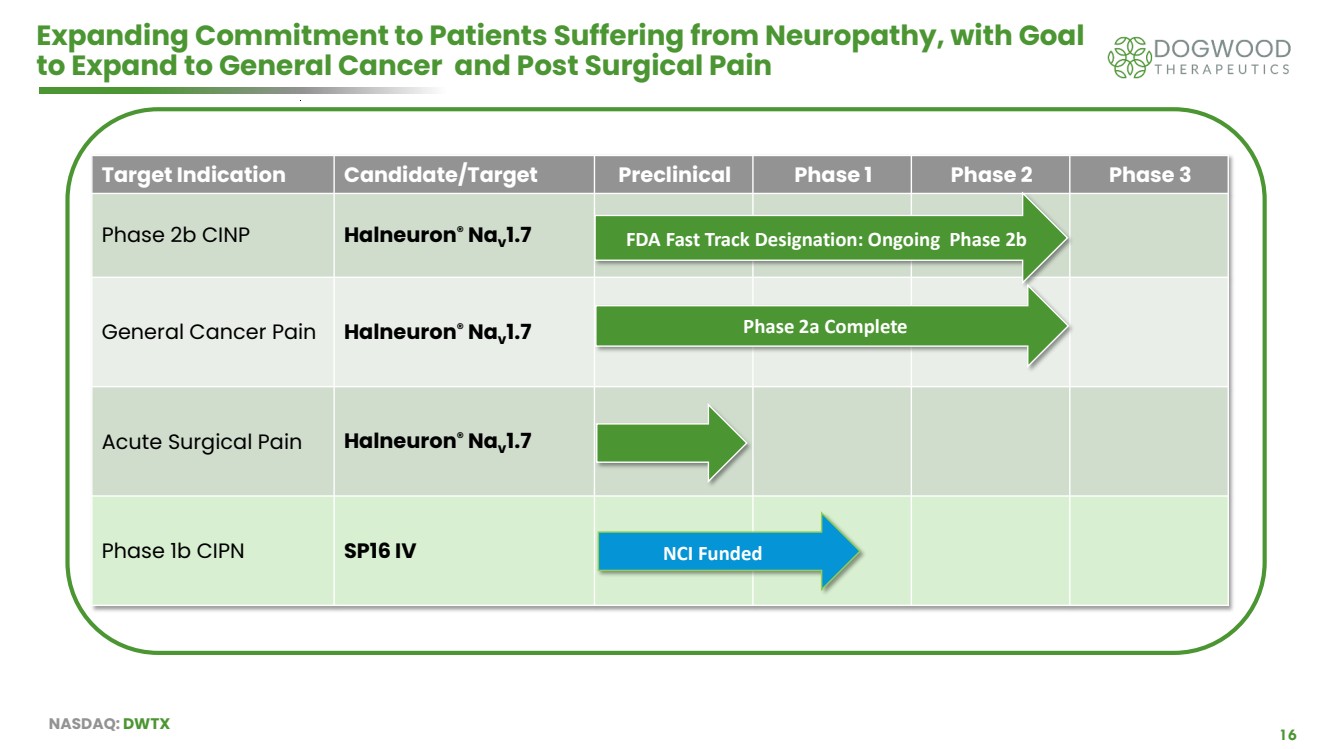
| NASDAQ: DWTX 16 Expanding Commitment to Patients Suffering from Neuropathy, with Goal to Expand to General Cancer and Post Surgical Pain Target Indication Candidate/Target Preclinical Phase 1 Phase 2 Phase 3 Phase 2b CINP Halneuron® Nav 1.7 General Cancer Pain Halneuron® Nav 1.7 Acute Surgical Pain Halneuron® Nav 1.7 Phase 1b CIPN SP16 IV FDA Fast Track Designation: Ongoing Phase 2b NCI Funded Phase 2a Complete |