
Exhibit 99.1

Immuron CEO, Steven Lydeamore presentation to Coffee Microcaps Conference
Melbourne, Australia, September 16, 2025: Immuron Limited (ASX: IMC; NASDAQ: IMRN) is pleased to advise our Chief Executive Officer, Steven Lydeamore will be presenting at the Coffee Microcaps VIP Conference on Tuesday 16th September 2025 (9.45am Australian Eastern time) in Sydney.
A copy of the presentation being made is included below.
This release has been authorised by the directors of Immuron Limited.
- - - END - - -
COMPANY CONTACT:
Steven Lydeamore
Chief Executive Officer
steve@immuron.com
About Immuron
Immuron Limited (ASX: IMC, NASDAQ: IMRN), is an Australian biopharmaceutical company focused on developing and commercializing orally delivered targeted polyclonal antibodies for the treatment of infectious diseases.
About Travelan®
Travelan® is an orally administered passive immunotherapy that prophylactically reduces the likelihood of contracting travelers’ diarrhea, a digestive tract disorder that is commonly caused by pathogenic bacteria and the toxins they produce. Travelan® is a highly purified tabletized preparation of hyper immune bovine antibodies and other factors, which when taken with meals bind to diarrhea-causing bacteria and prevent colonization and the pathology associated with travelers’ diarrhea. In Australia, Travelan® is a listed medicine on the Australian Register for Therapeutic Goods (AUST L 106709) and is indicated to reduce the risk of Travelers’ Diarrhea, reduce the risk of minor gastro-intestinal disorders and is antimicrobial. In Canada, Travelan® is a licensed natural health product (NPN 80046016) and is indicated to reduce the risk of Travelers’ Diarrhea. In the U.S., Travelan® is sold as a dietary supplement for digestive tract protection.
Travelers’ diarrhea (TD)
TD is generally defined as the passage of ≥ 3 unformed stools per 24 hours plus at least one additional symptom (such as nausea, vomiting, abdominal cramps, fever, blood/mucus in the stools, or fecal urgency) that develop while abroad or within 10 days of returning from any resource-limited destinations (Leung et al., 2006). Diarrhea continues to be the most frequent health problem among travelers to destinations in lower- and middle-income regions (Steffen, 2017). Deployed US military personnel, essentially representing a long-term traveller population, are particularly affected given their population dynamics and the context in which they seek care and treatment (Connor et al., 2012). Diarrhea is the leading infectious disease threat to the overall health and preparedness of deployed US armed forces, with diarrheagenic E. coli, Campylobacter spp., and Shigella spp. among the most commonly reported etiologies (Riddle et al., 2006).


Immuron Platform Technology
Immuron’s proprietary technology is based on polyclonal immunoglobulins (IgG) derived from engineered hyper-immune bovine colostrum. Immuron has the capability of producing highly specific immunoglobulins to any enteric pathogen and our products are orally active. Bovine IgG can withstand the acidic environment of the stomach and is resistant to proteolysis by the digestive enzymes found in the Gastrointestinal (GI) tract. Bovine IgG also possesses this unique ability to remain active in the human GI tract delivering its full benefits directly to the bacteria found there. The underlying nature of Immuron’s platform technology enables the development of medicines across a large range of infectious diseases. The platform can be used to block viruses or bacteria at mucosal surfaces such as the Gastrointestinal tract and neutralize the toxins they produce.
IMM-124E (Travelan®)
IMM-124E was developed using Immuron’s platform technology. IMM-124E is produced from the colostrum of birthing cattle that have been immunised during pregnancy with a vaccine containing the outer antigens of multiple human derived ETEC. A total of 13 ETEC strains are used in the vaccine to produce high levels of antibodies against selected surface antigens from the most common strains of ETEC. (Otto et al., 2011)
The resultant hyperimmune colostrum IMM-124E from ETEC vaccinated cows contains significant levels of polyclonal antibodies specific for ETEC antigens LPS, CFA-I and Flagellin (Sears et al., 2017).
The antibodies produced in IMM-124E have been found to have a stronger binding and neutralizing activity (than the antibodies of unvaccinated cattle) against a wide range of LPS antigens including both the variable O-polysaccharide region and the preserved oligosaccharide core ‘R’ region of LPS from the 13 serotypes used in the ETEC vaccine.
IMM-124E is manufactured into a tablet form referred to as Travelan®.
IMM-529
Immuron is developing IMM-529 as an adjunctive therapy in combination with standard of care antibiotics for the prevention and/or treatment of recurrent Clostridioides difficile infection (CDI). IMM-529 antibodies targeting Clostridioides difficile (C. diff) may help to clear CDI infection and promote a quicker re-establishment of normal gut flora, providing an attractive oral preventative for recurrent CDI.
Immuron is collaborating with Dr. Dena Lyras and her team at Monash University, Australia to develop vaccines to produce bovine colostrum-derived antibodies. Dairy cows were immunised to generate hyperimmune bovine colostrum (HBC) that contains antibodies targeting three essential C. diff virulence components. IMM-529 targets Toxin B (TcB), the spores and the surface layer proteins of the vegetative cells.
This unique 3-target approach has yielded promising results in pre-clinical infection and relapse models, including (1) Prevention of primary disease (80% P =0.0052); (2) Protection of disease recurrence (67%, P <0.01) and (3) Treatment of primary disease (78.6%, P<0.0001; TcB HBC). Importantly IMM-529 antibodies cross-react with whole cell lysates of many different human strains of C. diff including hypervirulent strains.
To our knowledge, IMM-529 is, to date, the only investigational drug that has shown therapeutic potential in all three phases of the disease (Hutton et al., 2017).
ProIBS®
Immuron has an exclusive distribution agreement with Calmino goup AB for the territories of Australia and New Zealand for ProIBS®. ProIBS® - to help patients treat IBS symptoms ProIBS® is a certified medical device for the treatment of IBS symptoms such as abdominal pain, bloating and unsettled bowel movements (diarrhoea and/or constipation). ProIBS® contains AVH200®, derived from the plant Aloe barbadensis. Mill. AVH200® has gel forming components which support the intestinal mucosal barrier. As IBS is known to affect individuals for a long period of time, it is essential to have a treatment appropriate for long- term use –as ProIBS® is. The product is safe, and no interactions with other medications are known. Science-driven innovative Calmino group AB, the developer of ProIBS®, conducted a usability study among 1,003 users. PROIBS® was helpful for 94% of them. 91% of the users experienced an improvement in daily life and 98% would recommend PROIBS® to someone else. To learn more please check: www.proibs.eu.


Irritable bowel syndrome (IBS) is a common condition where you experience symptoms related to your digestive system. This is sometimes linked to certain foods, lifestyle habits and stress levels or mood. IBS affects around 3 out of every 10 people.
Females are more likely than males to be affected. Some key symptoms of IBS include: abdominal pain or discomfort; stomach bloating and wind; chronic diarrhoea or constipation, or alternating between the two.(healthdirect.gov.au) According to available data, the IBS treatment market in Australia is estimated to be a part of the broader “Digestives & Intestinal Remedies” market, generating a revenue of around AU$221.14 million in 2025, with a projected annual growth rate of 3.28%.(Statista)
References
Connor P, Porter CK, Swierczewski B and Riddle MS. Diarrhea during military deployment: current concepts and future directions. Curr Opin Infect Dis. 25(5): 546-54; 2012.
Hutton, M.L., Cunningham, B.A., Mackin, K.E. et al. Bovine antibodies targeting primary and recurrent Clostridium difficile disease are a potent antibiotic alternative. Sci Rep 7, 3665 (2017). https://doi.org/10.1038/s41598-017-03982-5
Leung AK, Robson WL, Davies HD. Travelers’ diarrhea. Adv Ther. Jul-Aug; 23(4): 519-27; 2006
Otto W, Najnigier B, Stelmasiak T and Robins-Browne RM. Randomized control trials using a tablet formulation of hyperimmune bovine colostrum to prevent diarrhea caused by enterotoxigenic Escherichia coli in volunteers Scandinavian Journal of Gastroenterology 46: 862– 868; 2011.
Riddle MS, Sanders JW, Putnam SD, and Tribble DR. Incidence, etiology, and impact of diarrhea among long-term travelers’ (US military and similar populations): A systematic review. American Journal of Tropical Medicine and Hygiene. 74(5): 891-900; 2006.
Sears KT, Tennant SM, Reymann MK, Simon R, Konstantopolos N, Blackwelder WC, Barry EM and Pasetti MF. Bioactive Immune Components of Anti-Diarrheagenic Enterotoxigenic Escherichia coli Hyperimmune Bovine Colostrum products. Clinical and Vaccine Immunology. 24 (8) 1-14; 2017.
Steffen R. Epidemiology of travelers’ diarrhea. J Travel Med. 24(suppl_1): S2-S5; 2017.
For more information visit: https://www.immuron.com.au/ and https://www.travelan.com
Subscribe to Immuron’s InvestorHub: Here
FORWARD-LOOKING STATEMENTS:
This press release may contain “forward-looking statements” within the meaning of Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, each as amended. Such statements include, but are not limited to, any statements relating to our growth strategy and product development programs and any other statements that are not historical facts. Forward-looking statements are based on management’s current expectations and are subject to risks and uncertainties that could negatively affect our business, operating results, financial condition, and stock value. Factors that could cause actual results to differ materially from those currently anticipated include: risks relating to our growth strategy; our ability to obtain, perform under and maintain financing and strategic agreements and relationships; risks relating to the results of research and development activities; risks relating to the timing of starting and completing clinical trials; uncertainties relating to preclinical and clinical testing; our dependence on third-party suppliers; our ability to attract, integrate and retain key personnel; the early stage of products under development; our need for substantial additional funds; government regulation; patent and intellectual property matters; competition; as well as other risks described in our SEC filings. We expressly disclaim any obligation or undertaking to release publicly any updates or revisions to any forward-looking statements contained herein to reflect any change in our expectations or any changes in events, conditions, or circumstances on which any such statement is based, except as required by law.


16 September 2025 Steven Lydeamore Chief Executive Officer Investor Presentation NASDAQ: IMRN ASX: IMC

Certain statements made in this presentation are forward - looking statements and are based on Immuron’s current expectations, estimates and projections. Words such as ” anticipates,” “expects,” “intends,” “plans,” “believes,” ” seeks,” “estimates,” “guidance” and s imilar expressions are intended to identify forward - looking statements. Although Immuron believes the forward - looking statements are based on reasonable assumptions, they are subject to certain r i sks and uncertainties, some of which are beyond Immuron’s control, including those r i sks or uncertainties inherent in the process of both developing and commercializing technology. As a result, actual results could materially differ f rom those expressed or forecasted in the forward - looking statements. The forward - looking statements made in this presentation relate only to events as of the date on which the statements are made. Immuron will not undertake any obligation to release publicly any revisions or updates to these forward - looking statements to reflect events, circumstances or unanticipated events occurring after the date of this presentation except as required by l aw or by any appropriate regulatory authority. FY 2025 results in this presentation are subject to audit review. SAFE HARBOR STATEMENT
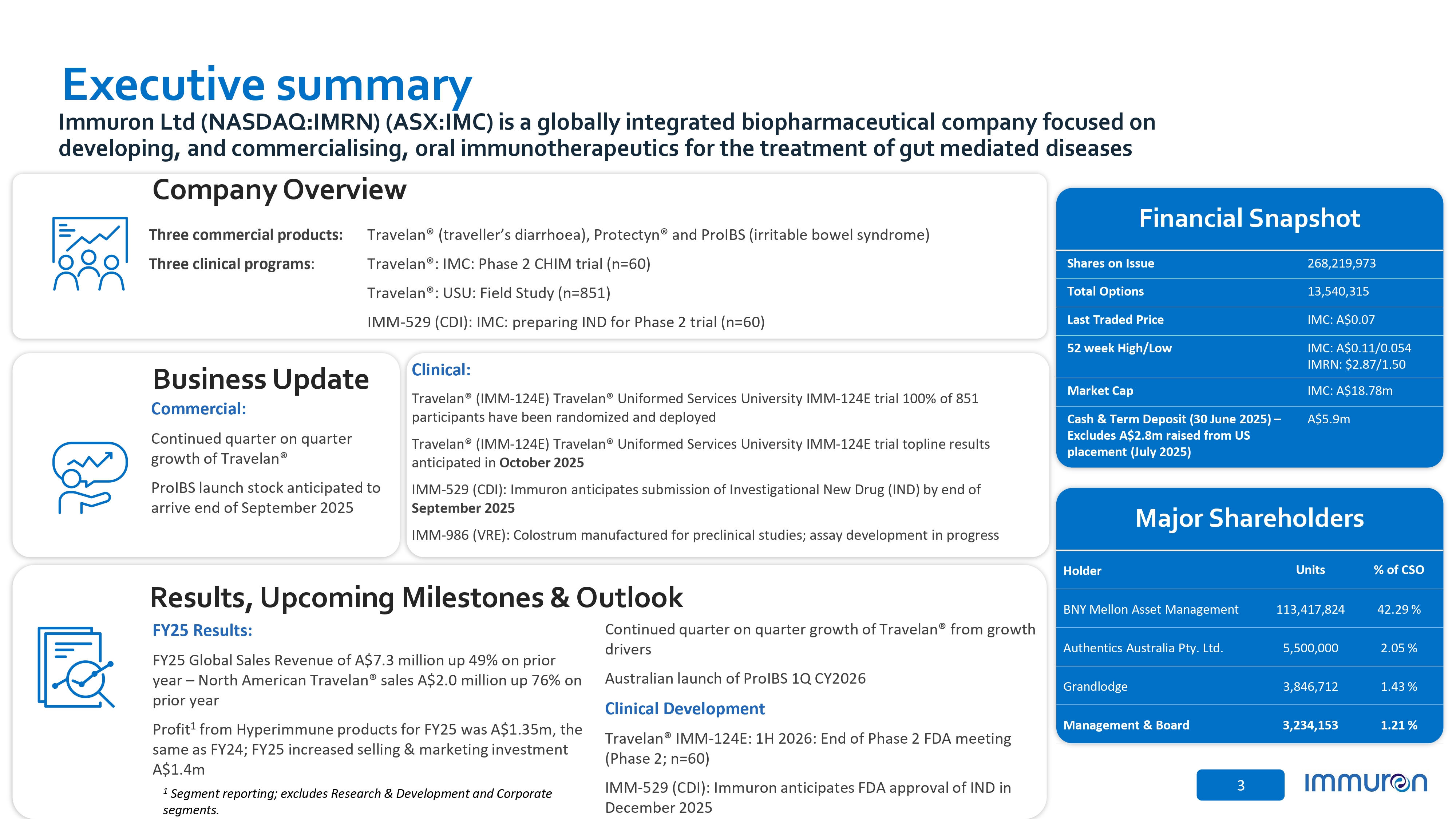
3 Continued quarter on quarter growth of Travelan® from growth drivers Australian launch of ProIBS 1Q CY2026 Clinical Development Travelan® IMM - 124E: 1H 2026: End of Phase 2 FDA meeting (Phase 2; n=60) IMM - 529 (CDI): Immuron anticipates FDA approval of IND in December 2025 Results, Upcoming Milestones & Outlook Executive summary Three commercial products: Three clinical programs : Travelan® (traveller’s diarrhoea), Protectyn® and ProIBS (irritable bowel syndrome) Travelan®: IMC: Phase 2 CHIM trial (n=60) Travelan®: USU: Field Study (n=851) IMM - 529 (CDI): IMC: preparing IND for Phase 2 trial (n=60) Major Shareholders Financial Snapshot 268,219,973 Shares on Issue 13,540,315 Total Options IMC: A$0.07 Last Traded Price IMC: A$0.11/0.054 IMRN: $2.87/1.50 52 week High/Low IMC: A$18.78m Market Cap A$5.9m Cash & Term Deposit (30 June 2025) – Excludes A$2.8m raised from US placement (July 2025) % of CSO Units Holder 42.29 % 113,417,824 BNY Mellon Asset Management 2.05 % 5,500,000 Authentics Australia Pty. Ltd. 1.43 % 3,846,712 Grandlodge 1.21 % 3,234,153 Management & Board Immuron Ltd (NASDAQ:IMRN) (ASX:IMC) is a globally integrated biopharmaceutical company focused on developing, and commercialising, oral immunotherapeutics for the treatment of gut mediated diseases Company Overview Clinical: Travelan® (IMM - 124E) Travelan® Uniformed Services University IMM - 124E trial 100% of 851 participants have been randomized and deployed Travelan® (IMM - 124E) Travelan® Uniformed Services University IMM - 124E trial topline results anticipated in October 2025 IMM - 529 (CDI): Immuron anticipates submission of Investigational New Drug (IND) by end of September 2025 IMM - 986 (VRE): Colostrum manufactured for preclinical studies; assay development in progress Business Update Commercial: Continued quarter on quarter growth of Travelan® ProIBS launch stock anticipated to arrive end of September 2025 FY25 Results: FY25 Global Sales Revenue of A$7.3 million up 49% on prior year – North American Travelan® sales A$2.0 million up 76% on prior year Profit 1 from Hyperimmune products for FY25 was A$1.35m, the same as FY24; FY25 increased selling & marketing investment A$1.4m 1 Segment reporting; excludes Research & Development and Corporate segments.

1. FINANCIAL & OPERATIONAL HIGHLIGHTS
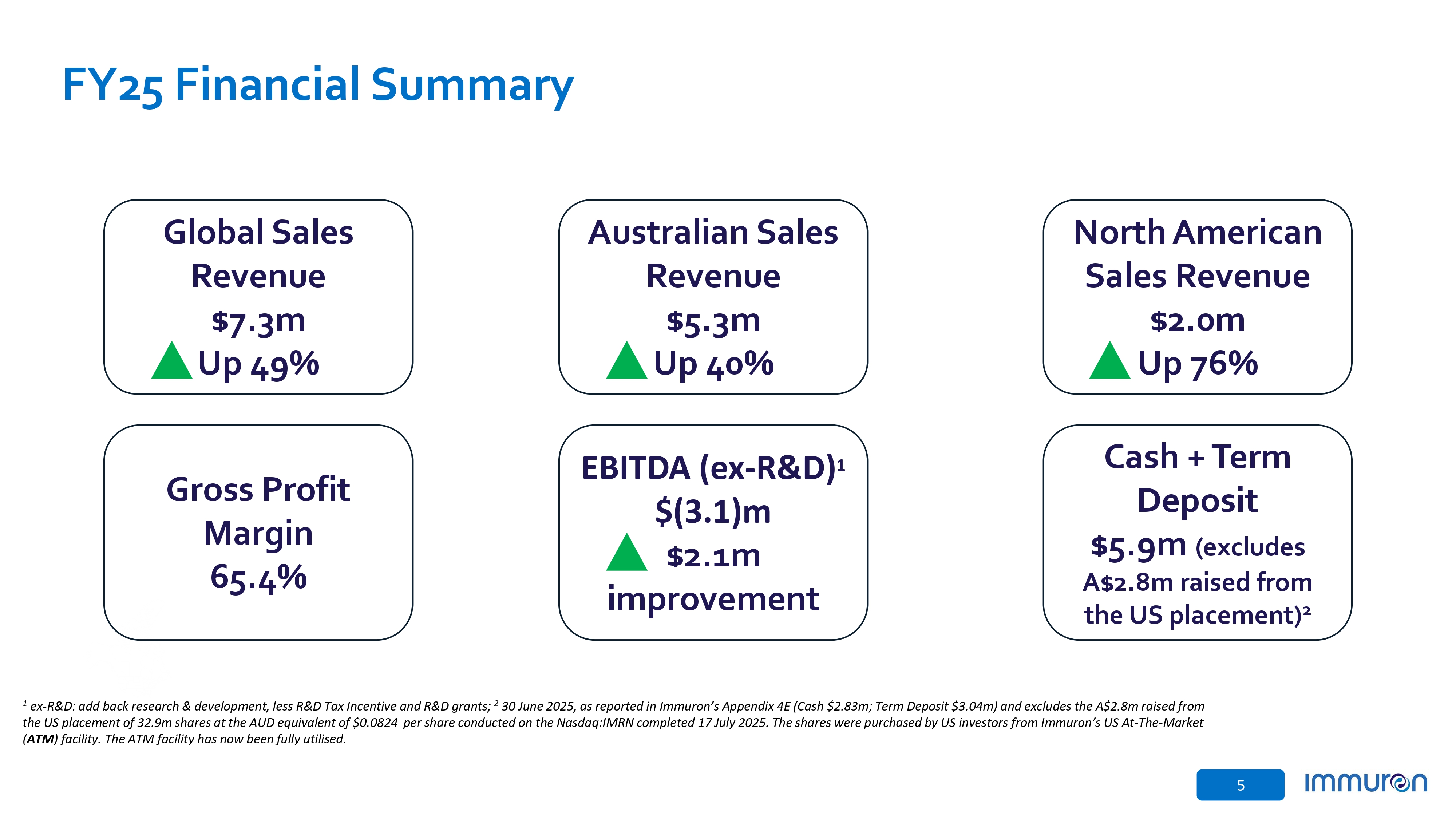
5 FY25 Financial Summary Global Sales Revenue $7.3m Up 49% Australian Sales Revenue $5.3m Up 40% North American Sales Revenue $2.0m Up 76% Gross Profit Margin 65.4% EBITDA (ex - R&D) 1 $(3.1)m $2.1m improvement Cash + Term Deposit $5.9m (excludes A$2.8m raised from the US placement) 2 1 ex - R&D : add back research & development, less R&D Tax Incentive and R&D grants ; 2 30 June 2025 , as reported in Immuron’s Appendix 4 E (Cash $ 2 . 83 m ; Term Deposit $ 3 . 04 m) and excludes the A $ 2 . 8 m raised from the US placement of 32 . 9 m shares at the AUD equivalent of $ 0 . 0824 per share conducted on the Nasdaq : IMRN completed 17 July 2025 . The shares were purchased by US investors from Immuron’s US At - The - Market ( ATM ) facility . The ATM facility has now been fully utilised .
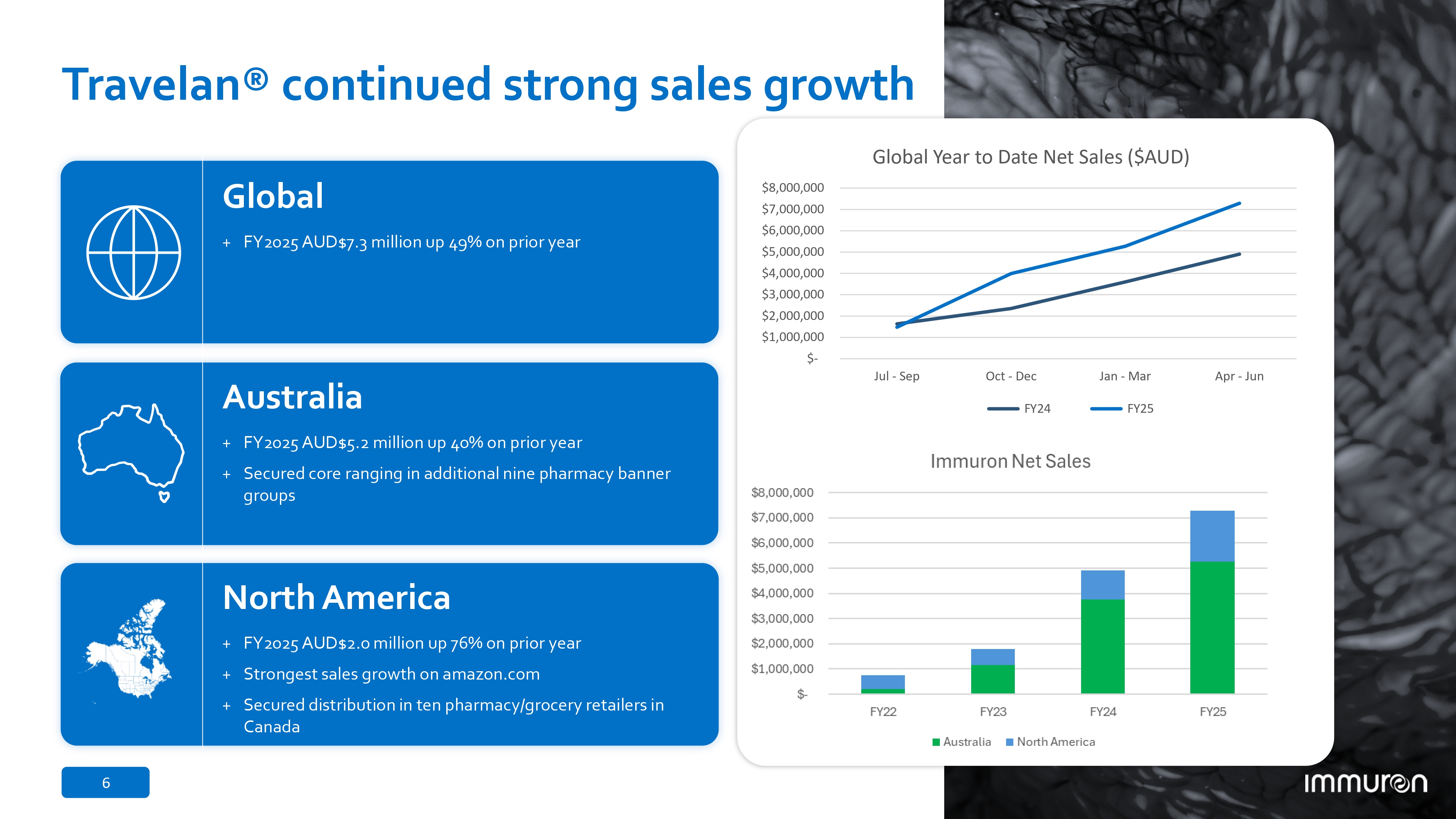
6 Travelan® continued strong sales growth Global + FY2025 AUD$7.3 million up 49% on prior year Australia + FY2025 AUD$5.2 million up 40% on prior year + Secured core ranging in additional nine pharmacy banner groups North America + FY2025 AUD$2.0 million up 76% on prior year + Strongest sales growth on amazon.com + Secured distribution in ten pharmacy/grocery retailers in Canada $8,000,000 $7,000,000 $6,000,000 $5,000,000 $4,000,000 $3,000,000 $2,000,000 $1,000,000 $ - Jul - Sep Oct - Dec Jan - Mar Apr - Jun Global Year to Date Net Sales ($AUD) FY24 FY25 6
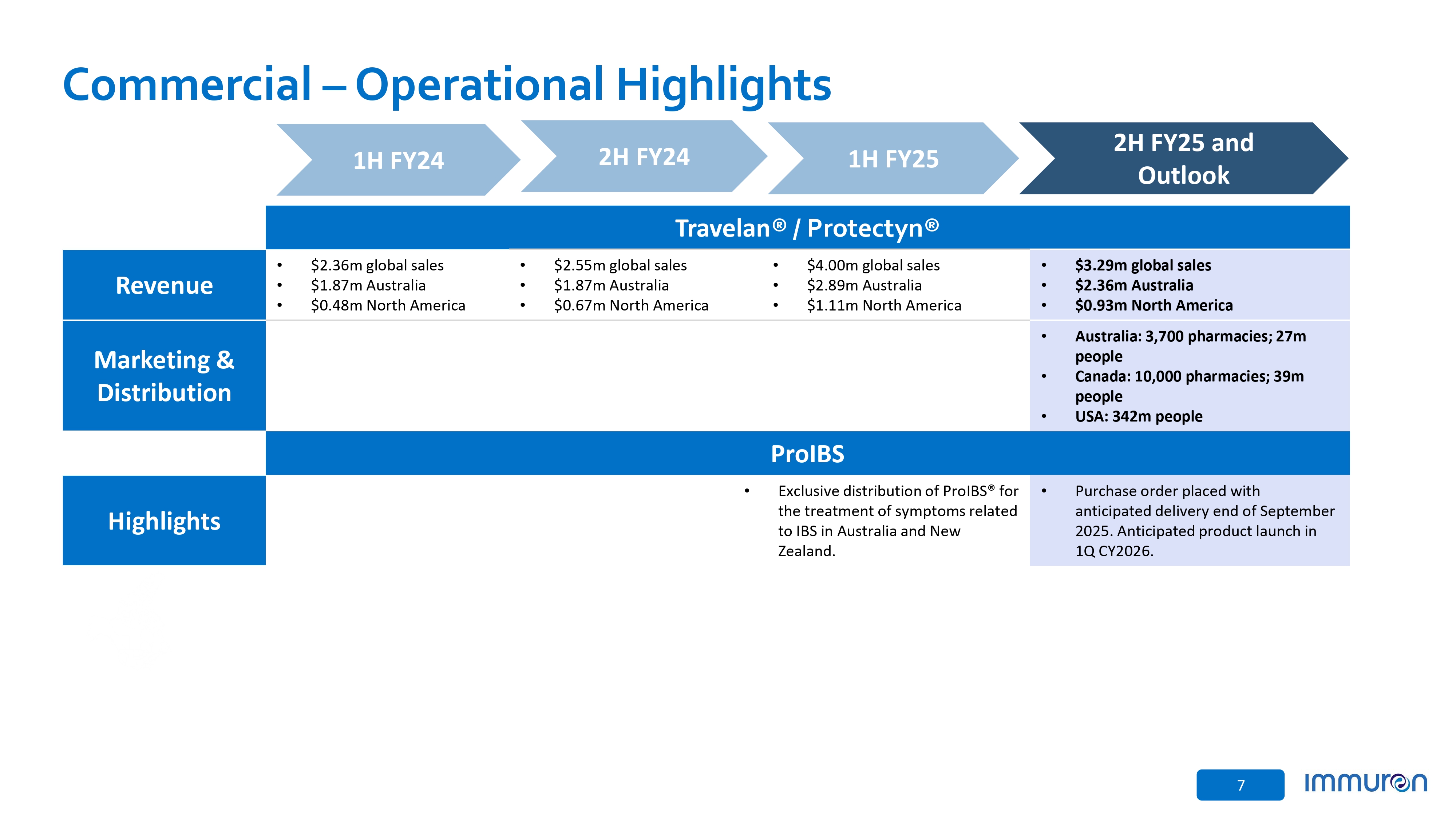
7 Commercial – Operational Highlights Travelan ® / Protectyn® • $3.29m global sales • $2.36m Australia • $0.93m North America • $4.00m global sales • $2.89m Australia • $1.11m North America • $2.55m global sales • $1.87m Australia • $0.67m North America • $2.36m global sales • $1.87m Australia • $0.48m North America Revenue • Australia: 3,700 pharmacies; 27m people • Canada: 10,000 pharmacies; 39m people • USA: 342m people Marketing & Distribution ProIBS • Purchase order placed with anticipated delivery end of September 2025. Anticipated product launch in 1Q CY2026. • Exclusive distribution of ProIBS® for the treatment of symptoms related to IBS in Australia and New Zealand. Highlights 1H FY24 2H FY24 1H FY25 2H FY25 and Outlook

8 Opportunity to Convert Billion Dollar Traveller’s Diarrhoea Market from Relief to Prevention by Travelan® Billion Dollar Market Traveller’s diarrhoea treatment market is large and growing at a CAGR of ~7% 1 Industry tailwinds International travel continues to grow Travel to high - risk destinations from Australia exceeds pre - pandemic levels and still growing Frequent Symptom 30% - 70% of travelers experience traveller’s diarrhoea 2 1 https://www.transparencymarketresearch.com/travelers - diarrhea - market.html ;Centers for Disease Control and Prevention CDC.gov 2024 Proprietary Vaccine Dairy cows inoculated with proprietary vaccines covering 13 strains of enterotoxigenic E.coli (ETEC) Product Differentiation • Colostrum has some antibacterial and immune modulatory properties. • However, Travelan® has in addition to the colostrum - derived compounds very high concentration of anti - E.coli antibodies. • Travelan® utilises specific antibodies to bind the bacteria and the toxins they produce effectively neutralising them and inhibiting their attachment to the gastrointestinal tract reducing LPS - related inflammation and bacterial. • These antibodies target the major bacteria which cause Traveller’s Diarrhoea. • Travelan® has a unique synergistic effect between the colostrum - derived products and the high concentration antibodies for suppressing the inflammation and targeting the bacteria which cause Traveller’s Diarrhoea in the gastrointestinal system. Bind and Neutralise to Prevent According to the Centers for Disease Control and Prevention Traveller’s Diarrhoea is a clinical syndrome resulting from microbial contamination of ingested food and water. Travelan® utilises specific antibodies to bind the bacteria and the toxins they produce effectively neutralising them and inhibiting their attachment to the gastrointestinal tract reducing LPS - related inflammation and bacterial colonisation.

EXPANSION OF TRAVELAN® DISTRIBUTION 9

2. CLINICAL DEVELOPMENT PROGRAM
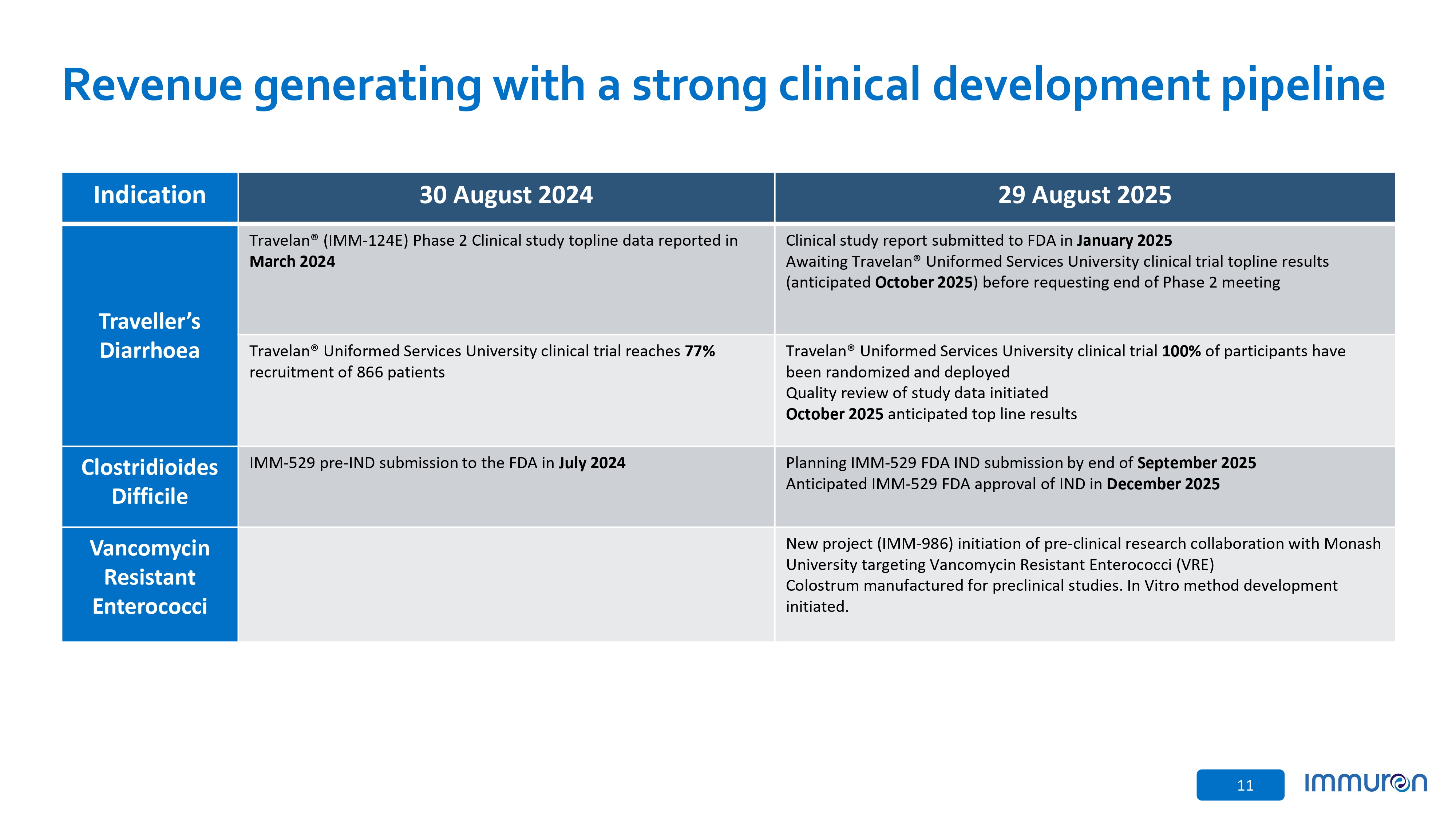
11 Revenue generating with a strong clinical development pipeline 29 August 2025 30 August 2024 Indication Clinical study report submitted to FDA in January 2025 Awaiting Travelan® Uniformed Services University clinical trial topline results (anticipated October 2025 ) before requesting end of Phase 2 meeting Travelan® (IMM - 124E) Phase 2 Clinical study topline data reported in March 2024 Traveller’s Diarrhoea Travelan® Uniformed Services University clinical trial 100% of participants have been randomized and deployed Quality review of study data initiated October 2025 anticipated top line results Travelan® Uniformed Services University clinical trial reaches 77% recruitment of 866 patients Planning IMM - 529 FDA IND submission by end of September 2025 Anticipated IMM - 529 FDA approval of IND in December 2025 IMM - 529 pre - IND submission to the FDA in July 2024 Clostridioides Difficile New project (IMM - 986) initiation of pre - clinical research collaboration with Monash University targeting Vancomycin Resistant Enterococci (VRE) Colostrum manufactured for preclinical studies. In Vitro method development initiated. Vancomycin Resistant Enterococci
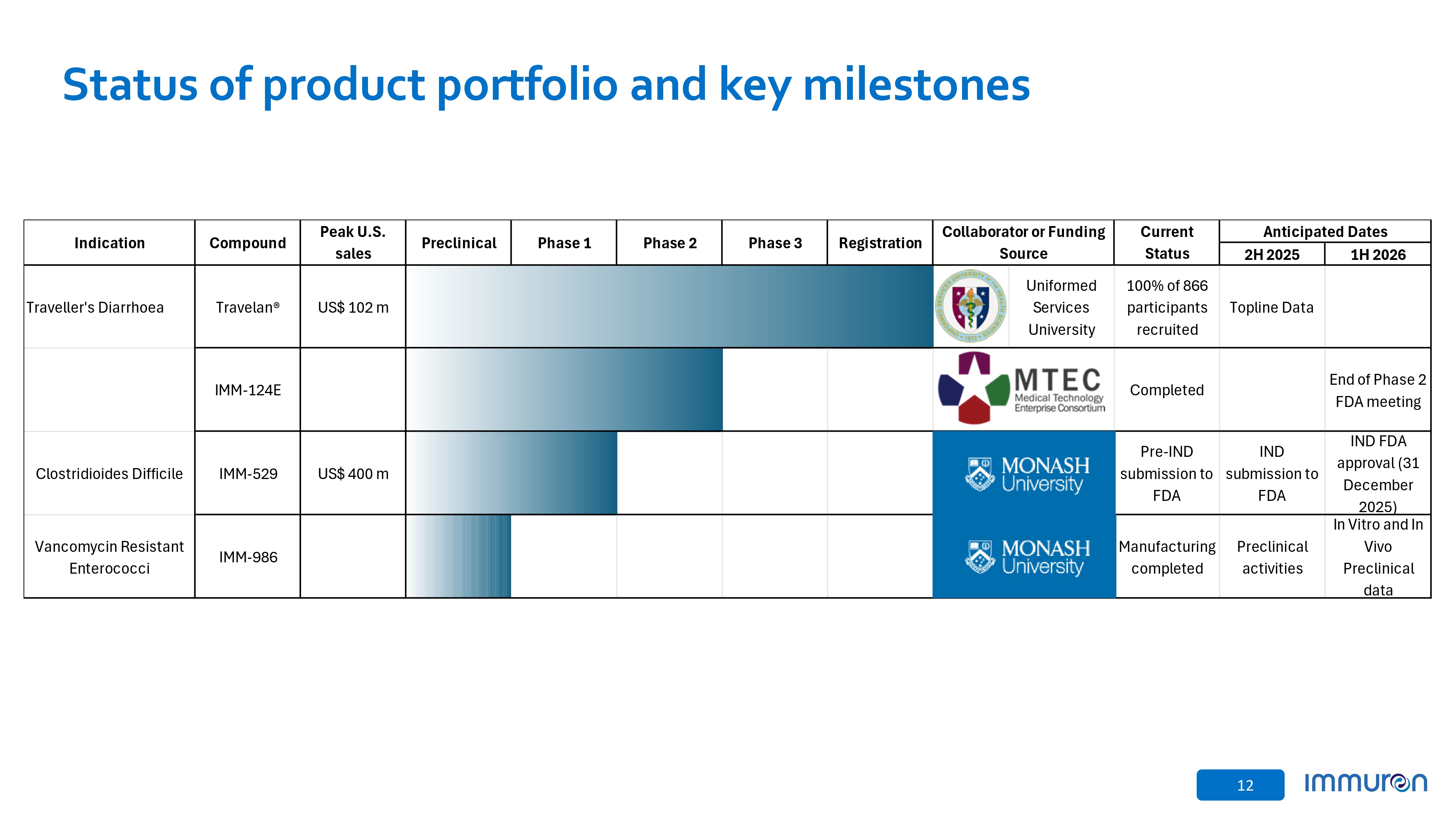
12 Status of product portfolio and key milestones Anticipated Dates Current Status Collaborator or Funding Source Registration Phase 3 Phase 2 Phase 1 Preclinical Peak U.S. sales Compound Indication 1H 2026 2H 2025 Topline Data 100% of 866 participants recruited Uniformed Services University US$ 102 m Travelan® Traveller’s Diarrhoea End of Phase 2 FDA meeting Completed IMM - 124E IND FDA approval (31 December 2025) IND submission to FDA Pre - IND submission to FDA US$ 400 m IMM - 529 Clostridioides Difficile In Vitro and In Vivo Preclinical data Preclinical activities Manufacturing completed IMM - 986 Vancomycin Resistant Enterococci
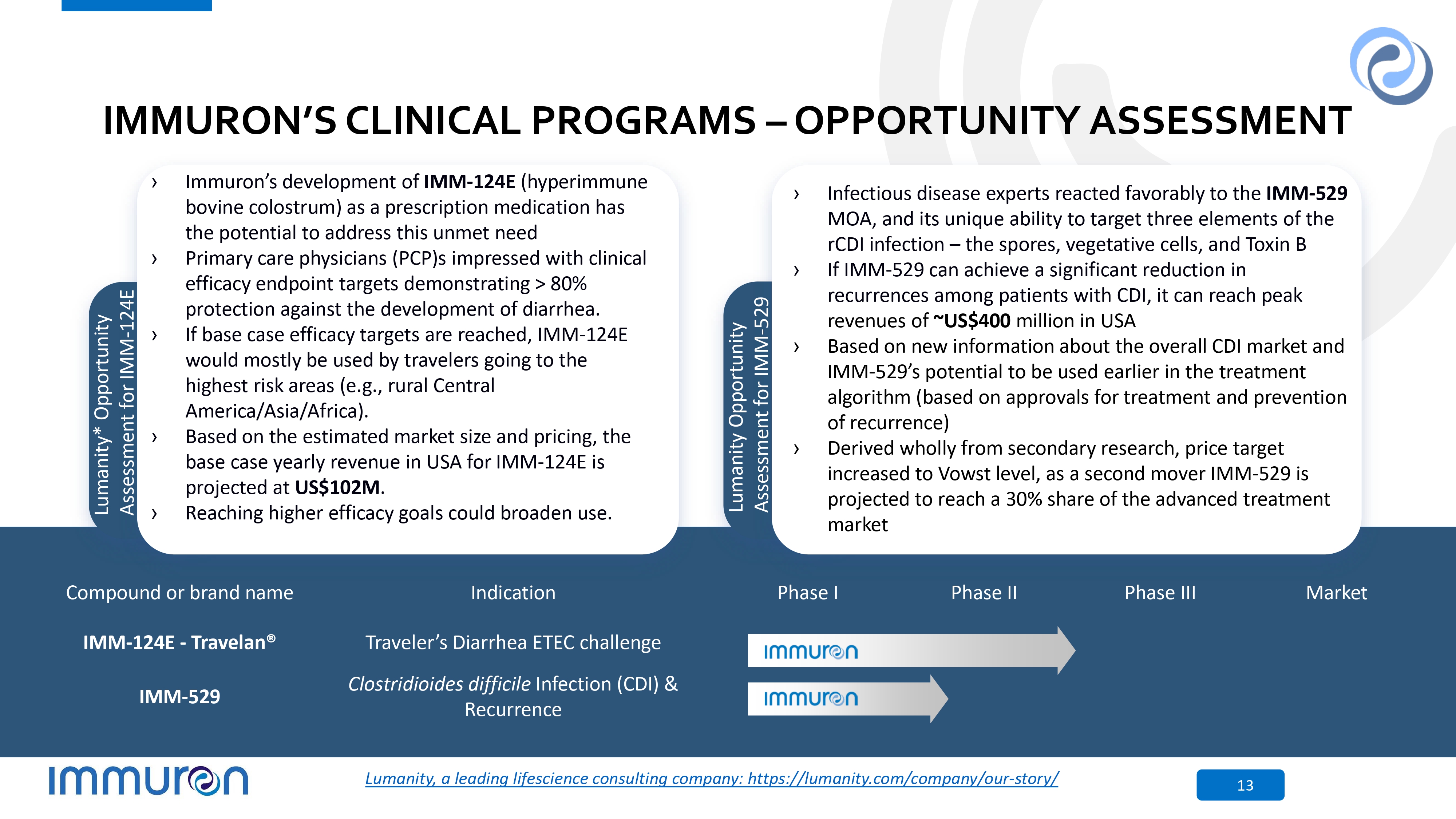
IMMURON’S CLINICAL PROGRAMS – OPPORTUNITY ASSESSMENT › Immuron’s development of IMM - 124E (hyperimmune bovine colostrum) as a prescription medication has the potential to address this unmet need › Primary care physicians (PCP)s impressed with clinical efficacy endpoint targets demonstrating > 80% protection against the development of diarrhea. › If base case efficacy targets are reached, IMM - 124E would mostly be used by travelers going to the highest risk areas (e.g., rural Central America/Asia/Africa). › Based on the estimated market size and pricing, the base case yearly revenue in USA for IMM - 124E is projected at US$102M . › Reaching higher efficacy goals could broaden use. L u m C a o n r i t p y o * r a O t p e p or t un i t y Assessment for IMM - 124E › Infectious disease experts reacted favorably to the IMM - 529 MOA, and its unique ability to target three elements of the rCDI infection – the spores, vegetative cells, and Toxin B › If IMM - 529 can achieve a significant reduction in recurrences among patients with CDI, it can reach peak revenues of ~US$400 million in USA › Based on new information about the overall CDI market and IMM - 529’s potential to be used earlier in the treatment algorithm (based on approvals for treatment and prevention of recurrence) › Derived wholly from secondary research, price target increased to Vowst level, as a second mover IMM - 529 is projected to reach a 30% share of the advanced treatment market L u m C a o n r i p t y o r O a p t e p or t un i t y Assessment for IMM - 529 Market Phase III Phase II Phase I Indication Compound or brand name Traveler’s Diarrhea ETEC challenge IMM - 124E - Travelan® Clostridioides difficile Infection (CDI) & Recurrence IMM - 529 Lumanity, a leading lifescience consulting company: https://lumanity.com/company/our - story/ 13
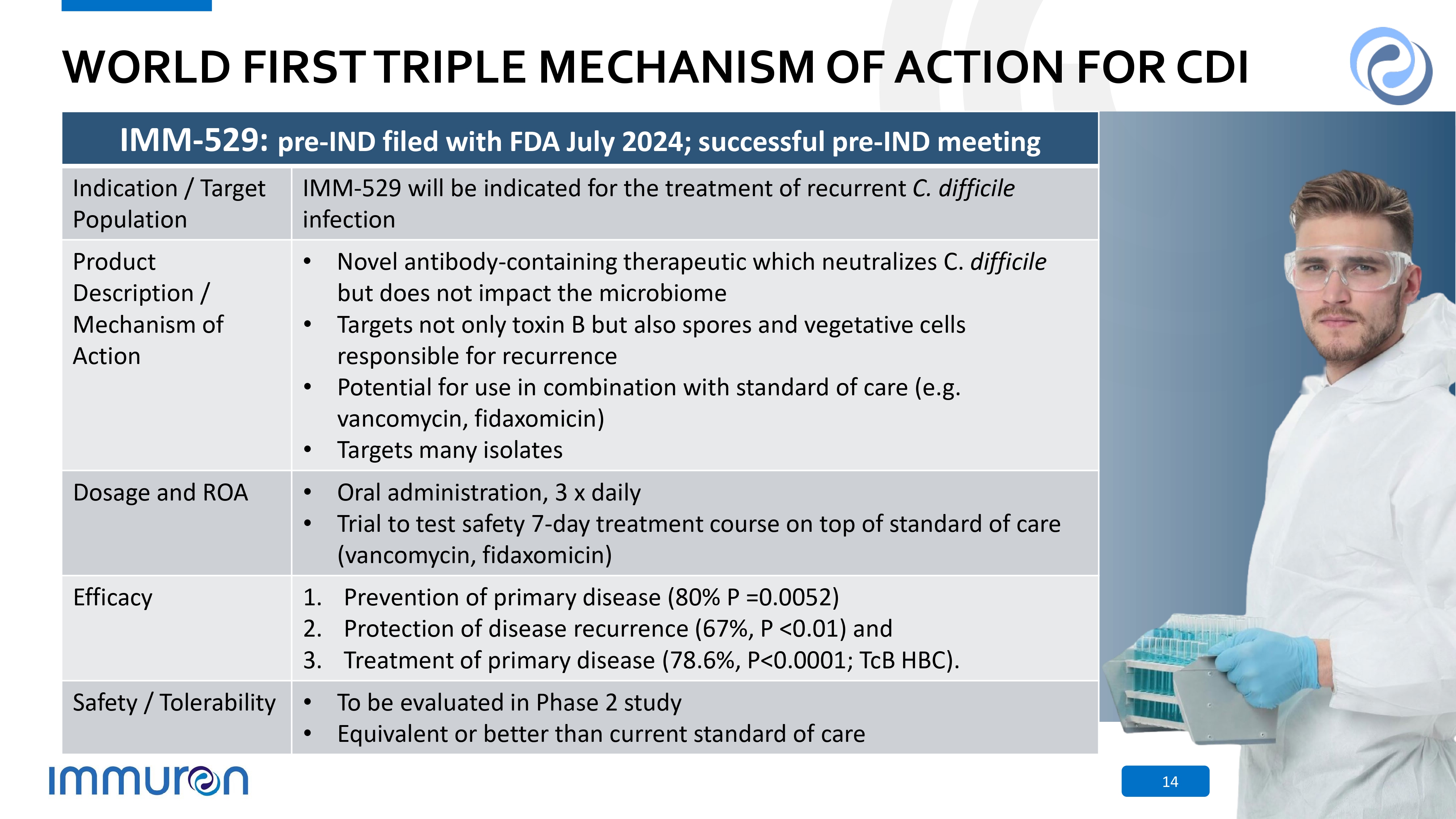
WORLD FIRST TRIPLE MECHANISM OF ACTION FOR CDI IMM - 529: pre - IND filed with FDA July 2024; successful pre - IND meeting IMM - 529 will be indicated for the treatment of recurrent C. difficile infection Indication / Target Population • Novel antibody - containing therapeutic which neutralizes C. difficile but does not impact the microbiome • Targets not only toxin B but also spores and vegetative cells responsible for recurrence • Potential for use in combination with standard of care (e.g. vancomycin, fidaxomicin) • Targets many isolates Product Description / Mechanism of Action • Oral administration, 3 x daily • Trial to test safety 7 - day treatment course on top of standard of care (vancomycin, fidaxomicin) Dosage and ROA 1. Prevention of primary disease (80% P =0.0052) 2. Protection of disease recurrence (67%, P <0.01) and 3. Treatment of primary disease (78.6%, P<0.0001; TcB HBC). Efficacy • To be evaluated in Phase 2 study • Equivalent or better than current standard of care Safety / Tolerability 14

3. UPCOMING MILESTONES & OUTLOOK
16 Upcoming Milestones & Outlook Commercial + Continued quarter on quarter growth of Travelan® from growth drivers + Australian launch of ProIBS in Q1 CY2026 + Planned growth in profitability 1 of Hyperimmune products net of planned increased investment in selling & marketing Clinical IMM - 124E (Travelan®): Traveller’s Diarrhoea + IMM - 124E: completed 100% recruitment, randomization and deployment (Phase 4; n=866) + IMM - 124E: October 2025: Topline data (Phase 4; n=866) + IMM - 124E: 1H 2026: End of Phase 2 FDA meeting (Phase 2; n=60) IMM - 529: Clostridioides difficile infection (C.diff, CDI) + IMM - 529: 30 September: FDA IND Submission + IMM - 529: 31 December: FDA IND Approval 1 Segment reporting; excludes Research & Development and Corporate segments. 16

17 Scientific references Travelan® (IMM - 124E) Scandinavian Journal of Gastroenterology, 46:7 - 8, 862 - 868, DOI: 10.3109/00365521.2011.574726 Travelan® has been shown to reduce both the incidence and severity of ETEC - induced diarrhea in up to 90% of volunteers Military Health System Research Symposium 14 - 17 Aug 2023_Abstract 1 Clinical Evaluation of Travelan® an Oral Prophylactic for Prevention of Travelers’ Diarrhea in Active Duty Military Service Assigned Abroad. US Department of Defense, Armed Forces Research Institute of Medical Sciences (AFRIM), 4 September, 2019 Travelan® demonstrates broad reactivity to Vibrio cholera strains from Southeast Asia indicating broad potential for prevention of traveler’s diarrhea US Department of Defense, Armed Forces Research Institute of Medical Sciences (AFRIM), 5 September, 2018 Travelan® prevented clinical shigellosis (bacillary dysentery) in 75% of Travelan® treated animals compared to placebo and demonstrated a significant clinical benefit US Department of Defense, Armed Forces Research Institute of Medical Sciences (AFRIM), 30 January, 2017 Travelan® able to bind and was reactive to 60 clinical isolates of each bacteria, Campylobacter, ETEC, and Shigella Islam D, Ruamsap N, Imerbsin R, Khanijou P, Gonwong S, Wegner MD, et al. (2023) Bioactivity and efficacy of a hyperimmune bovine colostrum product - Travelan, against shigellosis in a non - Human primate model (Macaca mulatta). PLoS ONE 18(12): e0294021. Bioactivity and efficacy of a hyperimmune bovine colostrum product - Travelan, against shigellosis in a non - Human primate model (Macaca mulatta) Clin Vaccine Immunol 24:e00186 - 16 . https://doi.org/10.1128/CVI.00186 - 16 Bioactive Immune Components of Travelan® I nfect Immun. 2023 Nov; 91(11): e00097 - 23. Hyperimmune bovine colostrum containing lipopolysaccharide antibodies (IMM - 124E) has a non - detrimental effect on gut microbial communities in unchallenged mice Journal of Crohn’s and Colitis, Volume 13, Issue 6, June 2019, Pages 785 – 797, https://doi.org/10.1093/ecco - jcc/jjy213 Administration of the Hyper - immune Bovine Colostrum Extract IMM - 124E Ameliorates Experimental Murine Colitis IMM - 529 Sci Rep 7, 3665 (2017). https://doi.org/10.1038/s41598 - 017 - 03982 - 5 Bovine antibodies targeting primary and recurrent Clostridium difficile disease are a potent antibiotic alternative 17

EMAIL: STEVE@IMMURON.COM PHONE: AUSTRALIA: +61 438 027 172 STEVEN LYDEAMORE CHIEF EXECUTIVE OFFICER IMMURON LIMITED CONTACT INFORMATION: