

A New Approach to Interstitial Lung Disease Topline Results for Phase 3 EFZO-FIT™ Study of Efzofitimod in Pulmonary Sarcoidosis September 15, 2025 Exhibit 99.1

Forward Looking Statements The following slides and any accompanying oral presentation contain forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995 and other federal securities laws. The use of words such as “may,” “might,” “will,” “should,” “expect,” “plan,” “anticipate,” “believe,” “estimate,” “project,” “intend,” “future,” “potential,” “opportunity,” or “continue,” and other similar expressions are intended to identify forward-looking statements. For example, all statements regarding: the potential therapeutic benefits of proteins derived from tRNA synthetase genes and our product candidates and development programs; the ability to successfully advance our product candidates and undertake certain development activities (such as the initiation of clinical trials, clinical trial enrollment, the conduct of clinical trials and announcement of clinical results) and accomplish certain development goals, and the timing of such events; the potential market opportunity for our product candidates; our ability to receive regulatory approvals for, and commercialize, our product candidates; our ability to identify and discover additional product candidates; potential activities and payments under collaboration agreements; and the ability of our intellectual property portfolio to provide protection are forward-looking statements. All forward-looking statements are based on estimates and assumptions by our management that, although we believe to be reasonable, are inherently uncertain. All forward-looking statements are subject to risks and uncertainties that may cause actual results to differ materially from those that we expected. These risks, uncertainties and other factors are more fully described in our filings with the U.S. Securities and Exchange Commission, including our Annual Report on Form 10-K, our subsequently filed Quarterly Reports on Form 10-Q, and in our other filings. The forward-looking statements in this presentation speak only as of the date of this presentation and neither we nor any other person assume responsibility for the accuracy and completeness of any forward-looking statement. We undertake no obligation to publicly update or revise any forward-looking statement, whether as a result of new information, future events or otherwise, except as required by law. We own various U.S. federal trademark applications and unregistered trademarks, including our company name. All other trademarks or trade names referred to in this presentation are the property of their respective owners. Solely for convenience, the trademarks and trade names in this presentation are referred to without the symbols ® and ™, but such references should not be construed as any indicator that their respective owners will not assert, to the fullest extent under applicable law, their rights thereto. This presentation discusses product candidates that are under clinical study and which have not yet been approved for marketing by the U.S. Food and Drug Administration. No representation is made as to the safety or effectiveness of these product candidates for the uses for which they are being studied. This presentation also contains estimates and other statistical data made by independent parties and by us relating to market size and growth and other data about our industry. This data involves a number of assumptions and limitations, and you are cautioned not to give undue weight to such estimates. In addition, projections, assumptions and estimates of our future performance and the future performance of the markets in which we operate are necessarily subject to a high degree of uncertainty and risk. These and other factors could cause results to differ materially from those expressed in the estimates made by the independent parties and us.
Summary of Key Findings Study did not meet primary endpoint in change from baseline in mean daily OCS dose at week 48 52.6% of patients treated with 5.0 mg/kg efzofitimod achieved complete steroid withdrawal at week 48 vs 40.2% on placebo (p=0.0919) Clinical improvement in KSQ-Lung score at week 48 observed in the 5.0 mg/kg efzofitimod treatment group vs placebo (p=0.0479). Greater proportion of patients achieved complete steroid withdrawal at week 48 with a KSQ-Lung score improvement in the 5.0 mg/kg efzofitimod treatment group (29.5%) vs placebo (14.4%) (p=0.0199) Lung function as measured by forced vital capacity (FVC) at week 48 was maintained Efzofitimod was generally well-tolerated at both the 3.0 mg/kg and 5.0 mg/kg doses, consistent with a previously observed safety profile in all trials conducted to date OCS = oral corticosteroids; KSQ = King’s Sarcoidosis Questionnaire; FDA = Food and Drug Administration As the primary endpoint did not achieve statistical significance, p-values for other endpoints should be interpreted as nominal p-values. Findings demonstrate drug activity for efzofitimod across multiple clinically relevant efficacy endpoints Company plans to engage with the U.S. FDA to determine the path forward for efzofitimod in pulmonary sarcoidosis
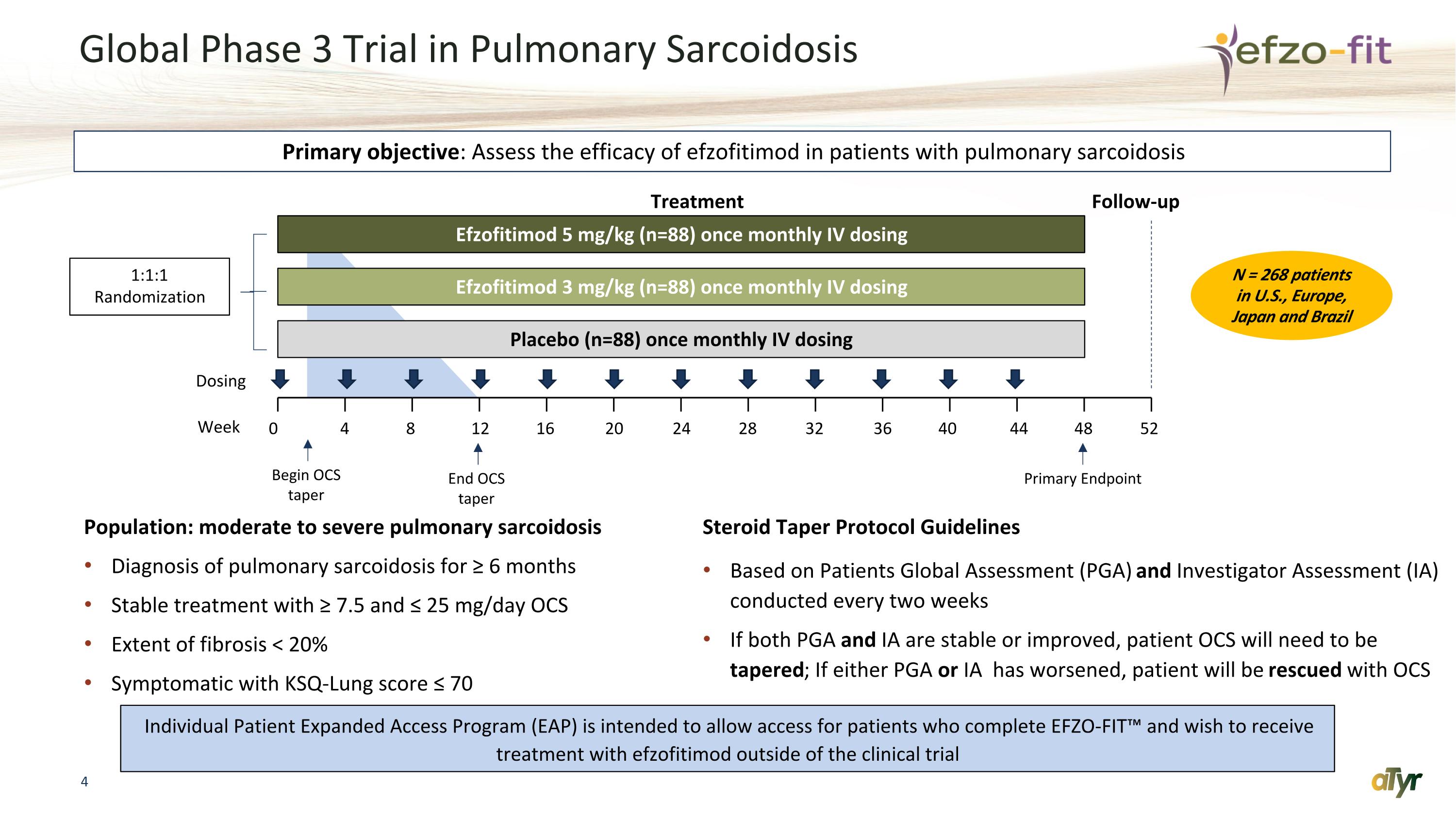
Global Phase 3 Trial in Pulmonary Sarcoidosis Population: moderate to severe pulmonary sarcoidosis Diagnosis of pulmonary sarcoidosis for ≥ 6 months Stable treatment with ≥ 7.5 and ≤ 25 mg/day OCS Extent of fibrosis < 20% Symptomatic with KSQ-Lung score ≤ 70 Steroid Taper Protocol Guidelines Based on Patients Global Assessment (PGA) and Investigator Assessment (IA) conducted every two weeks If both PGA and IA are stable or improved, patient OCS will need to be tapered; If either PGA or IA has worsened, patient will be rescued with OCS 0 4 8 12 16 20 24 28 Efzofitimod 5 mg/kg (n=88) once monthly IV dosing Efzofitimod 3 mg/kg (n=88) once monthly IV dosing Placebo (n=88) once monthly IV dosing Week Dosing 1:1:1 Randomization Treatment Follow-up Primary objective: Assess the efficacy of efzofitimod in patients with pulmonary sarcoidosis 32 36 40 44 48 52 Begin OCS taper End OCS taper Primary Endpoint N = 268 patients in U.S., Europe, Japan and Brazil Individual Patient Expanded Access Program (EAP) is intended to allow access for patients who complete EFZO-FIT™ and wish to receive treatment with efzofitimod outside of the clinical trial
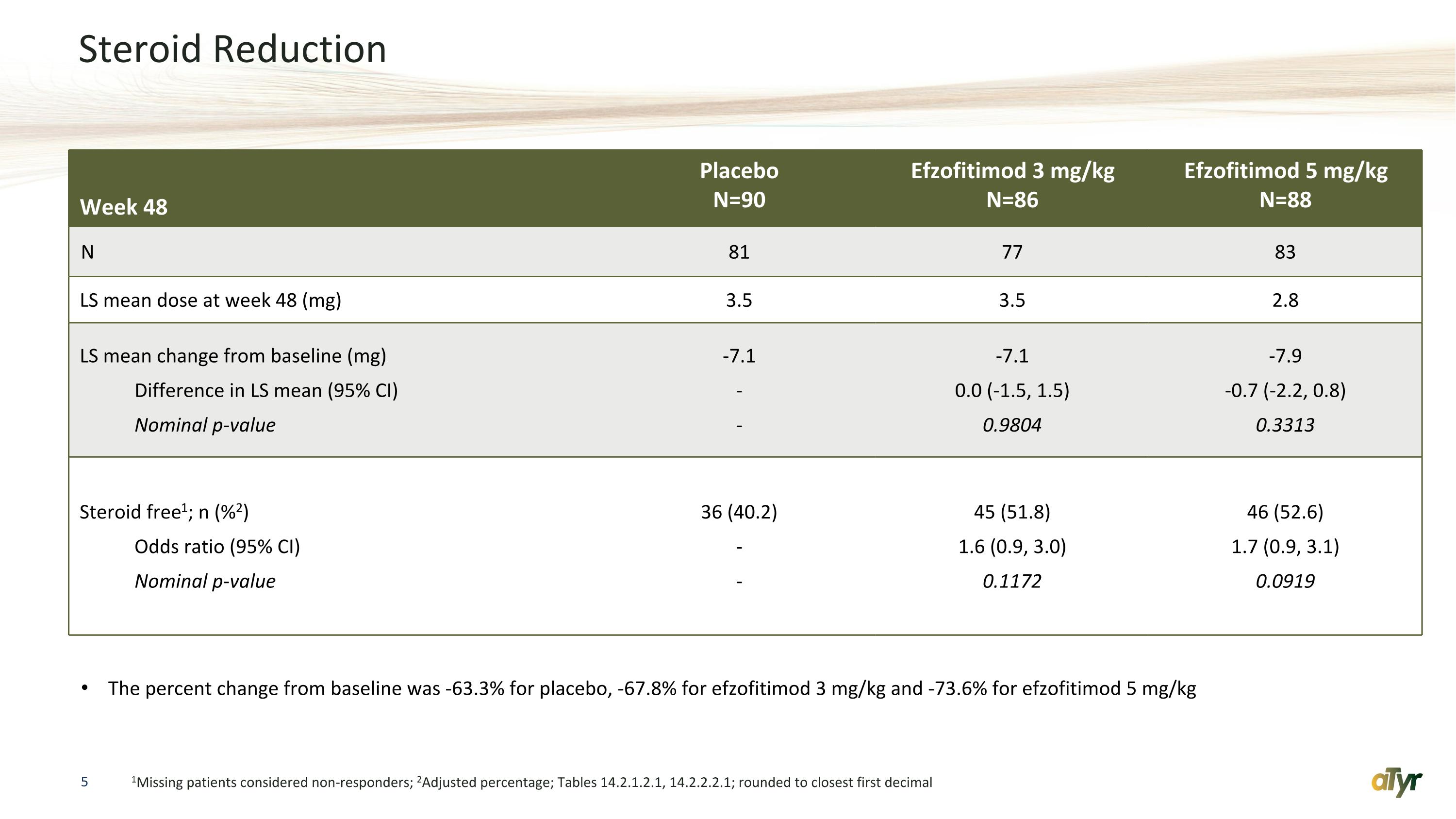
Steroid Reduction 1Missing patients considered non-responders; 2Adjusted percentage; Tables 14.2.1.2.1, 14.2.2.2.1; rounded to closest first decimal Week 48 Placebo N=90 Efzofitimod 3 mg/kg N=86 Efzofitimod 5 mg/kg N=88 N 81 77 83 LS mean dose at week 48 (mg) 3.5 3.5 2.8 LS mean change from baseline (mg) Difference in LS mean (95% CI) Nominal p-value -7.1 - - -7.1 0.0 (-1.5, 1.5) 0.9804 -7.9 -0.7 (-2.2, 0.8) 0.3313 Steroid free1; n (%2) Odds ratio (95% CI) Nominal p-value 36 (40.2) - - 45 (51.8) 1.6 (0.9, 3.0) 0.1172 46 (52.6) 1.7 (0.9, 3.1) 0.0919 The percent change from baseline was -63.3% for placebo, -67.8% for efzofitimod 3 mg/kg and -73.6% for efzofitimod 5 mg/kg
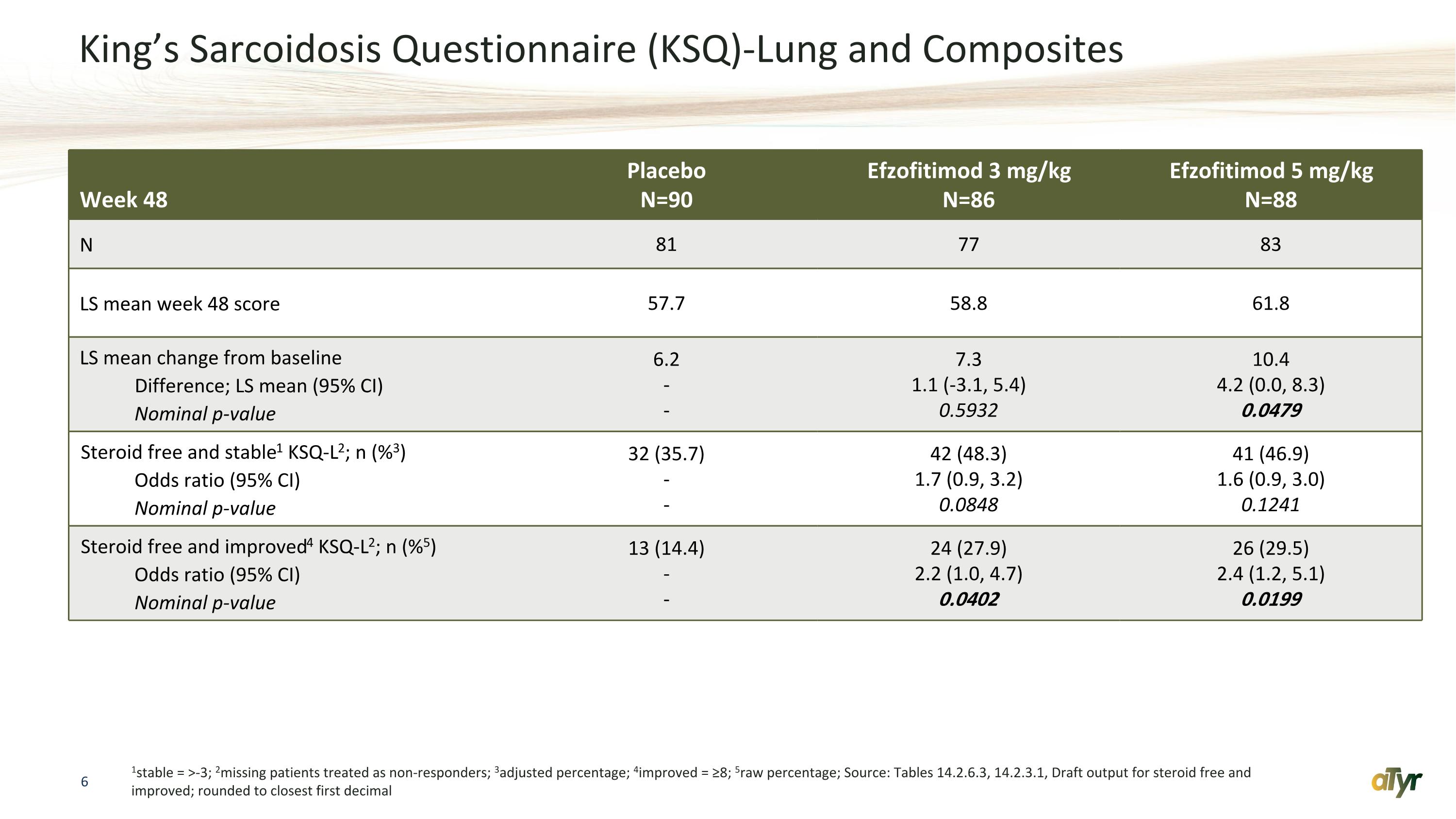
King’s Sarcoidosis Questionnaire (KSQ)-Lung and Composites 1stable = >-3; 2missing patients treated as non-responders; 3adjusted percentage; 4improved = ≥8; 5raw percentage; Source: Tables 14.2.6.3, 14.2.3.1, Draft output for steroid free and improved; rounded to closest first decimal Week 48 Placebo N=90 Efzofitimod 3 mg/kg N=86 Efzofitimod 5 mg/kg N=88 N 81 77 83 LS mean week 48 score 57.7 58.8 61.8 LS mean change from baseline Difference; LS mean (95% CI) Nominal p-value 6.2 - - 7.3 1.1 (-3.1, 5.4) 0.5932 10.4 4.2 (0.0, 8.3) 0.0479 Steroid free and stable1 KSQ-L2; n (%3) Odds ratio (95% CI) Nominal p-value 32 (35.7) - - 42 (48.3) 1.7 (0.9, 3.2) 0.0848 41 (46.9) 1.6 (0.9, 3.0) 0.1241 Steroid free and improved4 KSQ-L2; n (%5) Odds ratio (95% CI) Nominal p-value 13 (14.4) - - 24 (27.9) 2.2 (1.0, 4.7) 0.0402 26 (29.5) 2.4 (1.2, 5.1) 0.0199
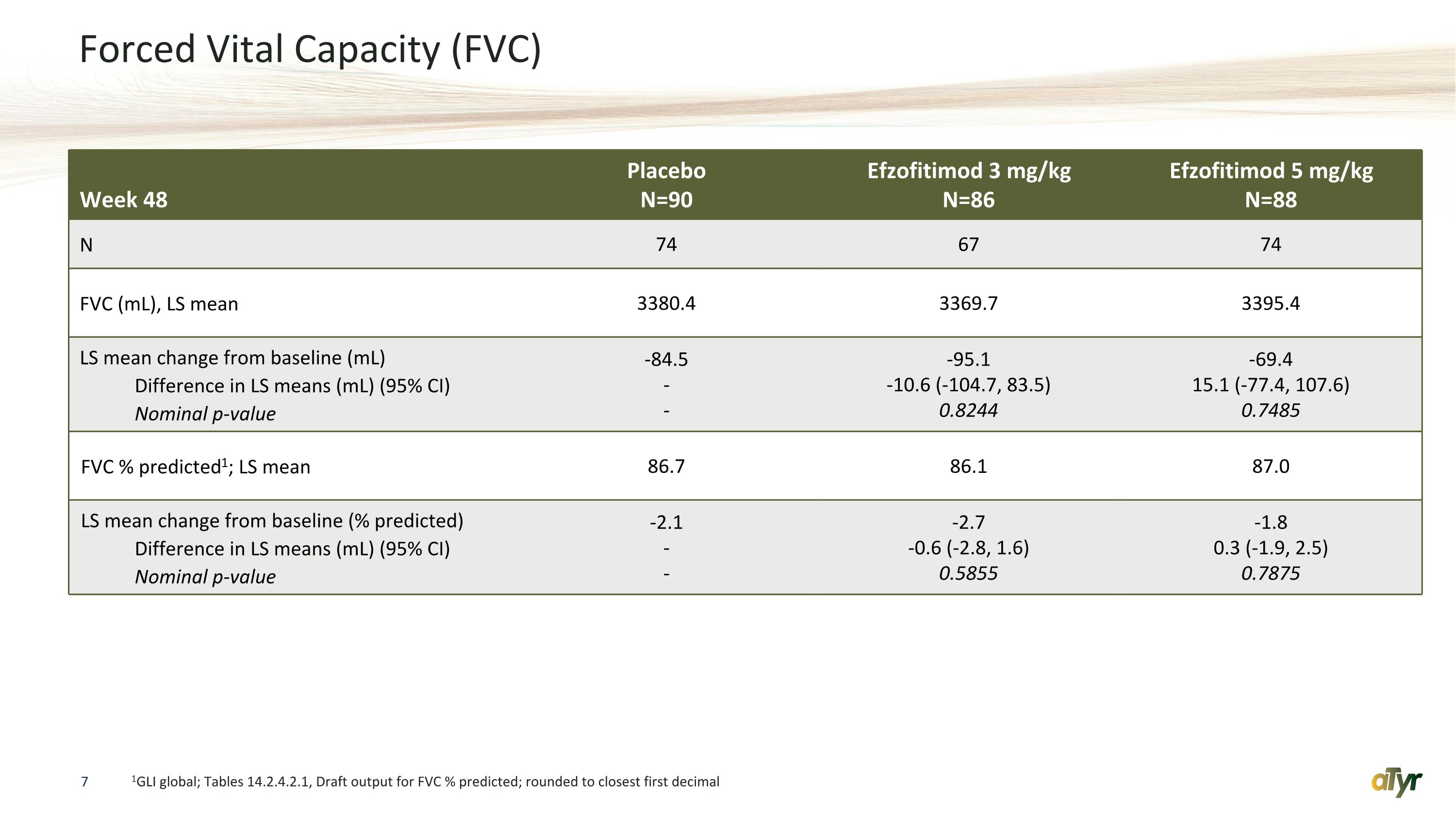
Forced Vital Capacity (FVC) 1GLI global; Tables 14.2.4.2.1, Draft output for FVC % predicted; rounded to closest first decimal Week 48 Placebo N=90 Efzofitimod 3 mg/kg N=86 Efzofitimod 5 mg/kg N=88 N 74 67 74 FVC (mL), LS mean 3380.4 3369.7 3395.4 LS mean change from baseline (mL) Difference in LS means (mL) (95% CI) Nominal p-value -84.5 - - -95.1 -10.6 (-104.7, 83.5) 0.8244 -69.4 15.1 (-77.4, 107.6) 0.7485 FVC % predicted1; LS mean 86.7 86.1 87.0 LS mean change from baseline (% predicted) Difference in LS means (mL) (95% CI) Nominal p-value -2.1 - - -2.7 -0.6 (-2.8, 1.6) 0.5855 -1.8 0.3 (-1.9, 2.5) 0.7875
Safety and Tolerability Efzofitimod was generally well-tolerated at both the 3.0 mg/kg and 5.0 mg/kg doses, consistent with a previously observed safety profile in all trials conducted to date Adverse events (AEs) were mostly mild or moderate in severity and generally assessed as unrelated to the study drug Serious adverse events (SAEs) were limited and balanced between treatment groups Proportion of patients with treatment-related SAEs and events leading to discontinuation was small and balanced between treatment groups. Proportion of patients who developed antidrug antibodies was small and balanced between treatment groups

Key Takeaways and Next Steps Evidence of drug activity observed for 5.0 mg/kg efzofitimod across multiple clinically relevant efficacy endpoints Clinical improvement in quality of life as measured by the KSQ-Lung for 5.0 mg/kg efzofitimod vs placebo Preservation of lung function with efzofitimod 5.0 mg/kg Generally well-tolerated at both the 3.0 mg/kg and 5.0 mg/kg doses, consistent with a previously observed safety profile in all trials conducted to date Planned Next Steps Present EFZO-FIT™ topline results at the European Respiratory Society Congress on September 30, 2025, at 8:44am CEST in Amsterdam, Netherlands Engage with the U.S. FDA to determine the path forward for efzofitimod in pulmonary sarcoidosis

KOL Commentary Robert P. Baughman, M.D. Emeritus Professor of Medicine, University of Cincinnati Editor, Sarcoidosis, Vasculitis, and Diffuse Lung Disease Editor, Current Opinion in Pulmonary Medicine Past President, World Association of Sarcoidosis and Other Granulomatous Disorders (WASOG) Member, Trial Steering Committee for the EFZO-FIT™ study

Q&A