

PYNNACLE Phase 2 Clinical Data Update Investor Webinar September 10, 2025 Exhibit 99.1

Disclaimer Forward-Looking Statements This presentation contains forward looking statements. Such statements include, but are not limited to, statements regarding our research, preclinical and clinical development activities, plans and projected timelines for rezatapopt, including the timing of disclosures regarding clinical data updates of its current clinical trial for rezatapopt, expected therapeutic benefits of rezatapopt including potential efficacy and tolerability, plans regarding regulatory filings and approvals, including targeted dates for our NDA submission and initial FDA approval for platinum-resistant or refractory ovarian indication, ongoing safety and response rate of participants in our PYNNACLE study, as well as the overall timing and success of our current and future clinical trials for rezatapopt, and the adequacy of the data to support its regulatory approval, and our expectations regarding the therapeutic, addressable patient populations, timing for approval and commercial potential of rezatapopt, as well as our cash runway forecast. The words “believe,” “may,” “should,” “will,” “estimate,” “promise,” “plan”, “continue,” “anticipate,” “intend,” “expect,” “potential” and similar expressions (including the negative thereof), are intended to identify forward-looking statements. Because such statements are subject to risks and uncertainties, actual results may differ materially from those expressed or implied by such forward-looking statements. Risks that contribute to the uncertain nature of the forward-looking statements include: our preclinical studies and clinical trials may not be successful; the U.S. Food and Drug Administration (FDA) may not agree with our interpretation of the data from clinical trials of our product candidates; we may decide, or the FDA may require us, to conduct additional clinical trials or to modify our ongoing clinical trials, which could result in enrollment or other delays to our anticipated timelines; we may experience delays in the commencement, enrollment, completion or analysis of clinical testing for our product candidates, or significant issues regarding the adequacy of our clinical trial designs or the execution of our clinical trials may arise, which could result in increased costs and delays, or limit our ability to obtain regulatory approval; the commencement, enrollment and completion of clinical trials and the reporting of data; a global pandemic, other public health emergencies or geopolitical tensions or conflicts may disrupt our business and that of the third parties on which we depend, including delaying or otherwise disrupting our clinical trials and preclinical studies, manufacturing and supply chain, or impairing employee productivity; our product candidates may not receive regulatory approval or be successfully commercialized; unexpected adverse side effects or inadequate therapeutic efficacy of our product candidates could delay or prevent regulatory approval or commercialization; we may not be able to obtain additional financing on terms acceptable to us or at all; as well as those risks and uncertainties set forth in the sections entitled “Risk Factors” and "Management's Discussion and Analysis of Financial Condition and Results of Operations" in the Company’s Annual Report on Form 10-K, filed with the Securities and Exchange Commission (the “SEC”) on March 3, 2025, the Company’s Quarterly Report on Form 10-Q for the three months ended March 31, 2025, filed with the SEC on May 9, 2025, and the Company’s Quarterly Report on Form 10-Q for the three months ended June 30, 2025, filed with the SEC on August 7, 2025, and its other filings filed with the SEC.. Additional risks and uncertainties may emerge from time to time, and it is not possible for PMV Pharma’s management to predict all risk factors and uncertainties. All forward-looking statements contained in this presentation speak only as of the date on which they were made. We undertake no obligation to update such statements to reflect events that occur or circumstances that exist after the date on which they were made. This presentation does not constitute an offer to sell or a solicitation of an offer to buy securities, nor shall there be any sale of any securities in any state or jurisdiction in which such offer, solicitation or sale would be unlawful prior to registration or qualification under the securities laws of any such state or jurisdiction.

Today’s Objectives Rezatapopt background Ovarian cancer treatment landscape PYNNACLE Phase 2 interim data update Initial NDA strategy informed by FDA feedback Q&A 01 02 03 04 David Mack, PhD President and Chief Executive Officer Ramez N. Eskander, MD Professor, Department of Obstetrics, Gynecology, and Reproductive Sciences UC San Diego Deepika Jalota, PharmD Chief Development Officer Panel

Today’s Objectives Rezatapopt background Ovarian cancer treatment landscape PYNNACLE Phase 2 interim data update Initial NDA strategy informed by FDA feedback Q&A 01 02 03 04 David Mack, PhD President and Chief Executive Officer

PMV’s lead candidate is rezatapopt, a first-in-class, investigational p53 Y220C reactivator The p53 Y220C mutation, a previously undruggable target, is found in 2.9% of ovarian cancer and 1% of all solid tumors Phase 1 PYNNACLE study has achieved proof of concept data for rezatapopt Pivotal Phase 2 PYNNACLE study interim clinical data demonstrates favorable efficacy and safety across multiple tumor types Strong balance sheet with $148M as of June 30, 2025, with cash runway through 1Q2027 NDA submission planned in 1Q2027 in platinum-resistant/refractory ovarian cancer patients PMV Pharma is Harnessing the Power of p53 to Treat Cancer
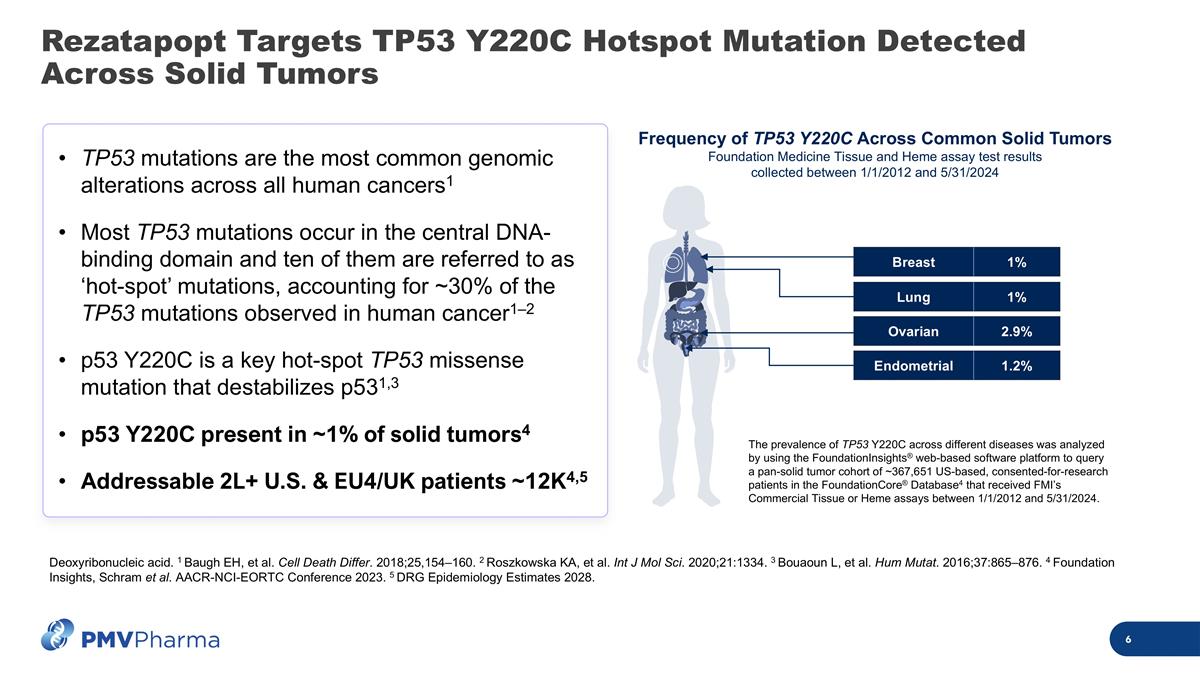
Rezatapopt Targets TP53 Y220C Hotspot Mutation Detected Across Solid Tumors TP53 mutations are the most common genomic alterations across all human cancers1 Most TP53 mutations occur in the central DNA-binding domain and ten of them are referred to as ‘hot-spot’ mutations, accounting for ~30% of the TP53 mutations observed in human cancer1–2 p53 Y220C is a key hot-spot TP53 missense mutation that destabilizes p531,3 p53 Y220C present in ~1% of solid tumors4 Addressable 2L+ U.S. & EU4/UK patients ~12K4,5 Deoxyribonucleic acid. 1 Baugh EH, et al. Cell Death Differ. 2018;25,154–160. 2 Roszkowska KA, et al. Int J Mol Sci. 2020;21:1334. 3 Bouaoun L, et al. Hum Mutat. 2016;37:865–876. 4 Foundation Insights, Schram et al. AACR-NCI-EORTC Conference 2023. 5 DRG Epidemiology Estimates 2028. Frequency of TP53 Y220C Across Common Solid Tumors Foundation Medicine Tissue and Heme assay test results collected between 1/1/2012 and 5/31/2024 The prevalence of TP53 Y220C across different diseases was analyzed by using the FoundationInsights® web-based software platform to query a pan-solid tumor cohort of ~367,651 US-based, consented-for-research patients in the FoundationCore® Database4 that received FMI’s Commercial Tissue or Heme assays between 1/1/2012 and 5/31/2024. Ovarian 2.9% Breast 1% Lung 1% Endometrial 1.2%
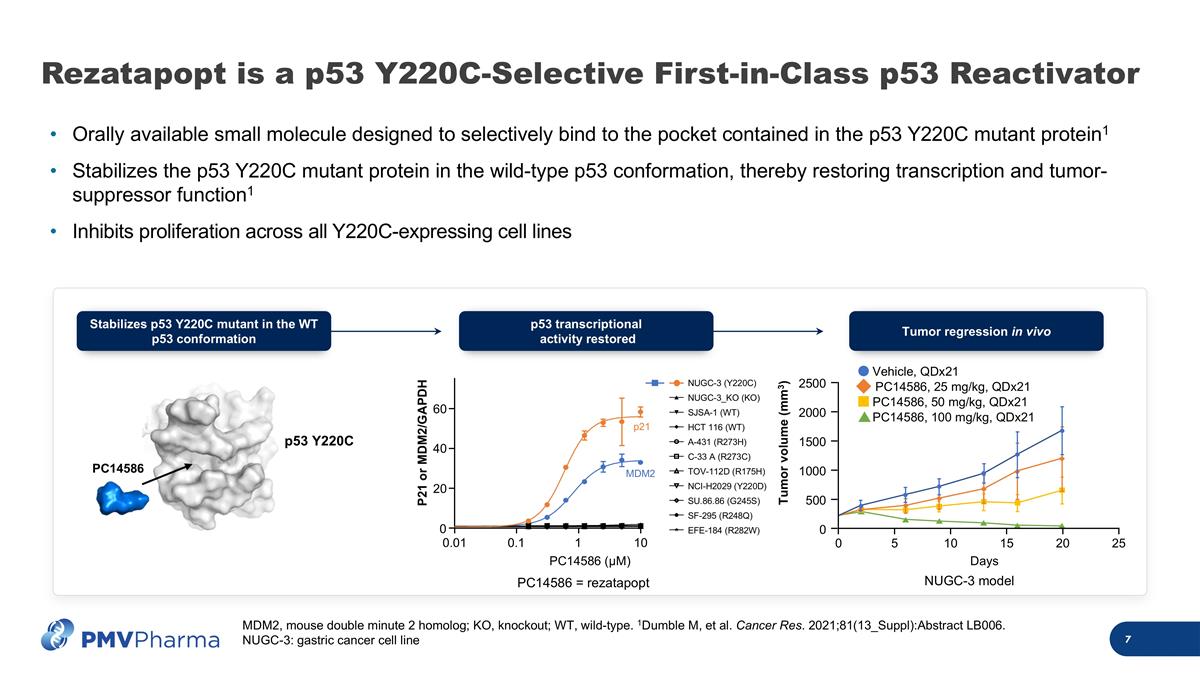
Rezatapopt is a p53 Y220C-Selective First-in-Class p53 Reactivator Orally available small molecule designed to selectively bind to the pocket contained in the p53 Y220C mutant protein1 Stabilizes the p53 Y220C mutant protein in the wild-type p53 conformation, thereby restoring transcription and tumor-suppressor function1 Inhibits proliferation across all Y220C-expressing cell lines p53 Y220C PC14586 Tumor regression in vivo 0 Days Tumor volume (mm3) 5 10 15 20 25 0 500 1000 1500 2000 2500 Vehicle, QDx21 PC14586, 25 mg/kg, QDx21 PC14586, 50 mg/kg, QDx21 PC14586, 100 mg/kg, QDx21 PC14586 (μM) P21 or MDM2/GAPDH 0.01 0.1 1 10 0 20 40 60 NUGC-3 (Y220C) NUGC-3_KO (KO) SJSA-1 (WT) HCT 116 (WT) A-431 (R273H) C-33 A (R273C) TOV-112D (R175H) NCI-H2029 (Y220D) SU.86.86 (G245S) SF-295 (R248Q) EFE-184 (R282W) p21 MDM2 p53 transcriptional activity restored Stabilizes p53 Y220C mutant in the WT p53 conformation PC14586 = rezatapopt NUGC-3 model MDM2, mouse double minute 2 homolog; KO, knockout; WT, wild-type. 1Dumble M, et al. Cancer Res. 2021;81(13_Suppl):Abstract LB006. NUGC-3: gastric cancer cell line
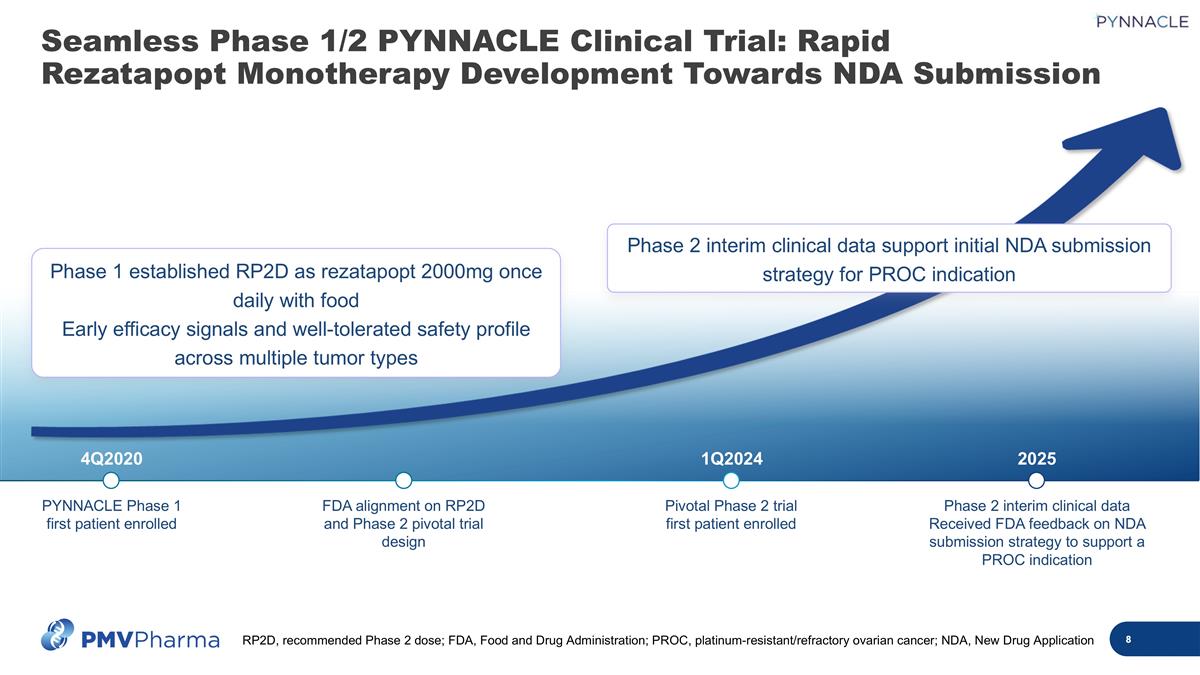
4Q2020 1Q2024 2025 PYNNACLE Phase 1 first patient enrolled FDA alignment on RP2D and Phase 2 pivotal trial design Pivotal Phase 2 trial first patient enrolled Phase 2 interim clinical data Received FDA feedback on NDA submission strategy to support a PROC indication Phase 2 interim clinical data support initial NDA submission strategy for PROC indication Phase 1 established RP2D as rezatapopt 2000mg once daily with food Early efficacy signals and well-tolerated safety profile across multiple tumor types Seamless Phase 1/2 PYNNACLE Clinical Trial: Rapid Rezatapopt Monotherapy Development Towards NDA Submission RP2D, recommended Phase 2 dose; FDA, Food and Drug Administration; PROC, platinum-resistant/refractory ovarian cancer; NDA, New Drug Application
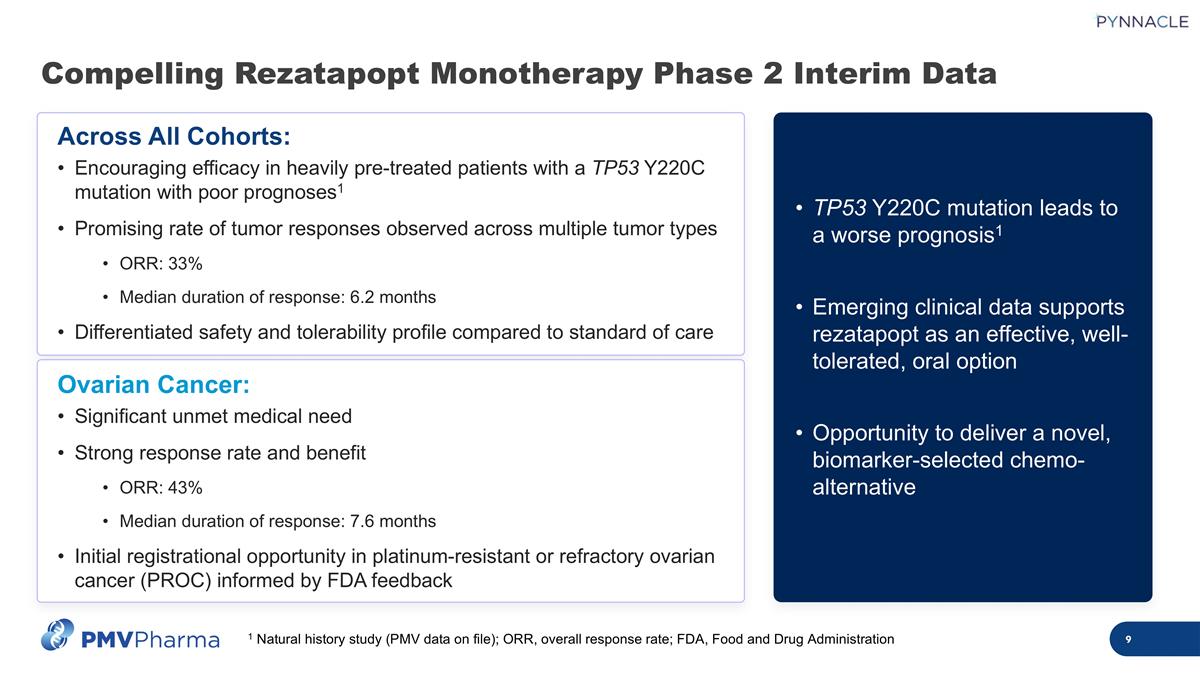
Compelling Rezatapopt Monotherapy Phase 2 Interim Data 1 Natural history study (PMV data on file); ORR, overall response rate; FDA, Food and Drug Administration Across All Cohorts: Encouraging efficacy in heavily pre-treated patients with a TP53 Y220C mutation with poor prognoses1 Promising rate of tumor responses observed across multiple tumor types ORR: 33% Median duration of response: 6.2 months Differentiated safety and tolerability profile compared to standard of care Ovarian Cancer: Significant unmet medical need Strong response rate and benefit ORR: 43% Median duration of response: 7.6 months Initial registrational opportunity in platinum-resistant or refractory ovarian cancer (PROC) informed by FDA feedback TP53 Y220C mutation leads to a worse prognosis1 Emerging clinical data supports rezatapopt as an effective, well-tolerated, oral option Opportunity to deliver a novel, biomarker-selected chemo-alternative

Today’s Objectives Rezatapopt background Ovarian cancer treatment landscape PYNNACLE Phase 2 interim data update Initial NDA strategy informed by FDA feedback Q&A 01 02 03 04 Ramez N. Eskander, MD Professor, Department of Obstetrics, Gynecology, and Reproductive Sciences UC San Diego

Disclosures Consultant/Advisory Board: AstraZeneca Clovis Oncology Daiichi Sankyo, Inc. Eisai Inc. Elevar Therapeutics GSK ImmunoGen, Inc. Mersana Therapeutics Myriad Genetics, Inc. Novocure GmbH Onconova Therapeutics Nuvectis PMV Pharmaceuticals Regeneron Lilly AbbVie Pfizer Other Financial or Material Support for GOG Associate Clinical Trial Advisor
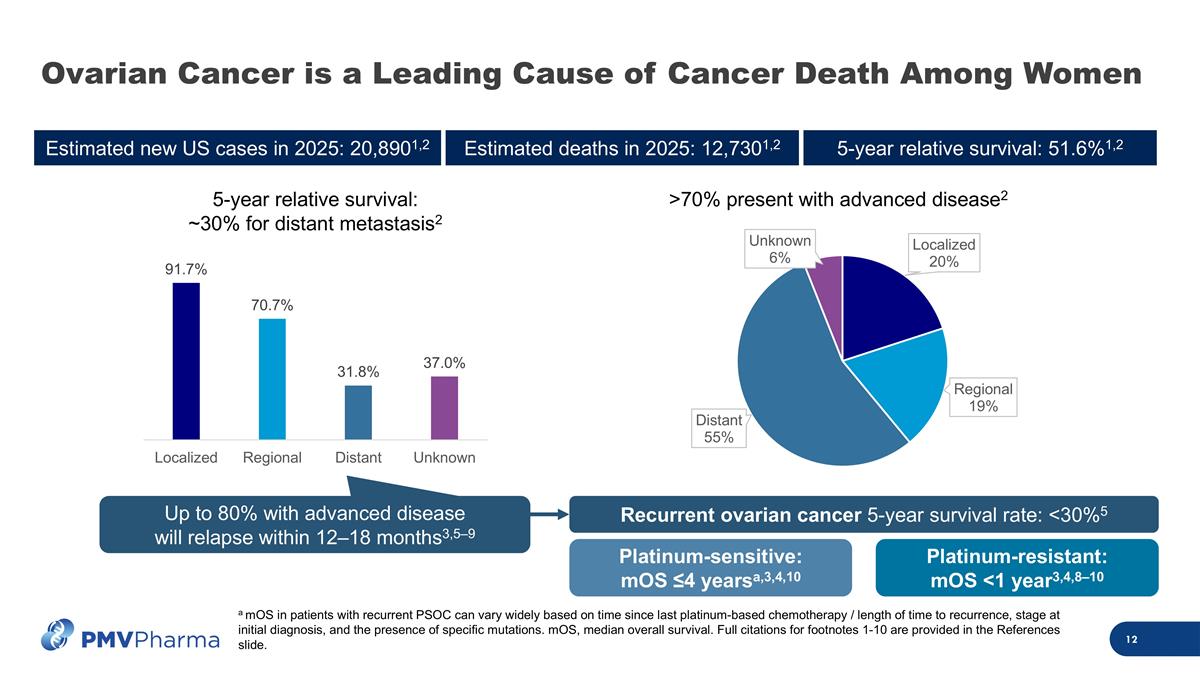
Ovarian Cancer is a Leading Cause of Cancer Death Among Women a mOS in patients with recurrent PSOC can vary widely based on time since last platinum-based chemotherapy / length of time to recurrence, stage at initial diagnosis, and the presence of specific mutations. mOS, median overall survival. Full citations for footnotes 1-10 are provided in the References slide. Estimated new US cases in 2025: 20,8901,2 Estimated deaths in 2025: 12,7301,2 5-year relative survival: 51.6%1,2 5-year relative survival: ~30% for distant metastasis2 Up to 80% with advanced disease will relapse within 12–18 months3,5–9 >70% present with advanced disease2 Platinum-sensitive: mOS ≤4 yearsa,3,4,10 Platinum-resistant: mOS <1 year3,4,8–10 Recurrent ovarian cancer 5-year survival rate: <30%5
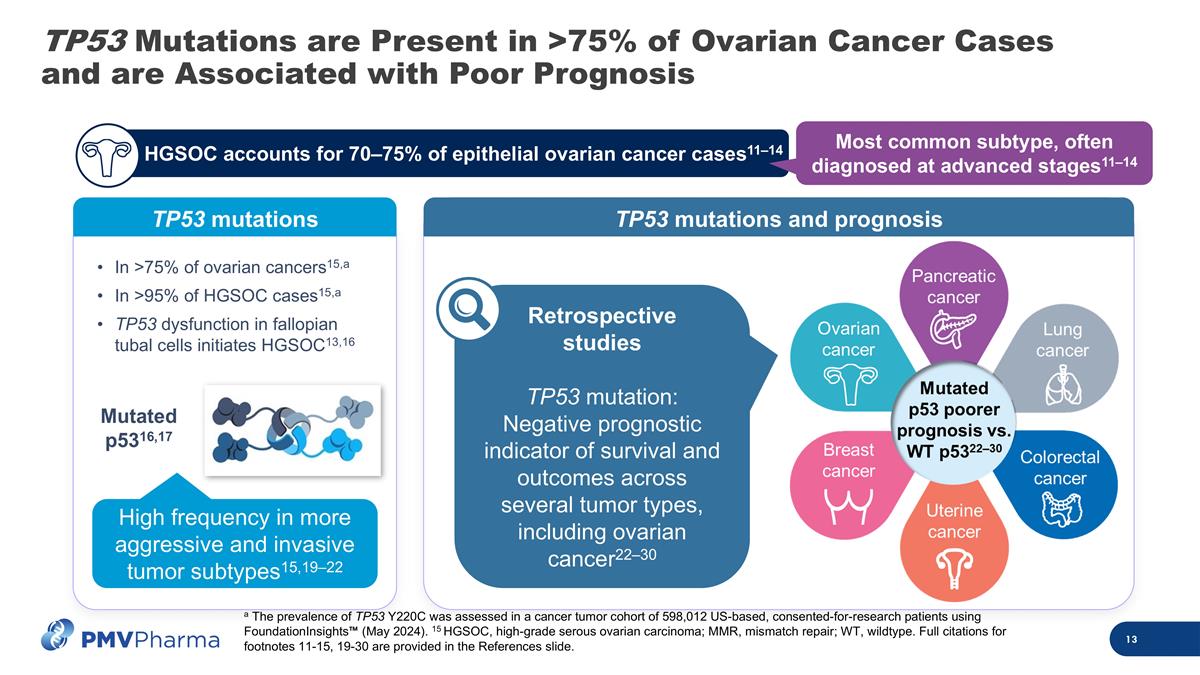
TP53 Mutations are Present in >75% of Ovarian Cancer Cases and are Associated with Poor Prognosis TP53 mutations Mutated p5316,17 High frequency in more aggressive and invasive tumor subtypes15,19–22 a The prevalence of TP53 Y220C was assessed in a cancer tumor cohort of 598,012 US-based, consented-for-research patients using FoundationInsights™ (May 2024). 15 HGSOC, high-grade serous ovarian carcinoma; MMR, mismatch repair; WT, wildtype. Full citations for footnotes 11-15, 19-30 are provided in the References slide. TP53 mutations and prognosis Retrospective studies TP53 mutation: Negative prognostic indicator of survival and outcomes across several tumor types, including ovarian cancer22–30 c HGSOC accounts for 70–75% of epithelial ovarian cancer cases11–14 Most common subtype, often diagnosed at advanced stages11–14 c In >75% of ovarian cancers15,a In >95% of HGSOC cases15,a TP53 dysfunction in fallopian tubal cells initiates HGSOC13,16
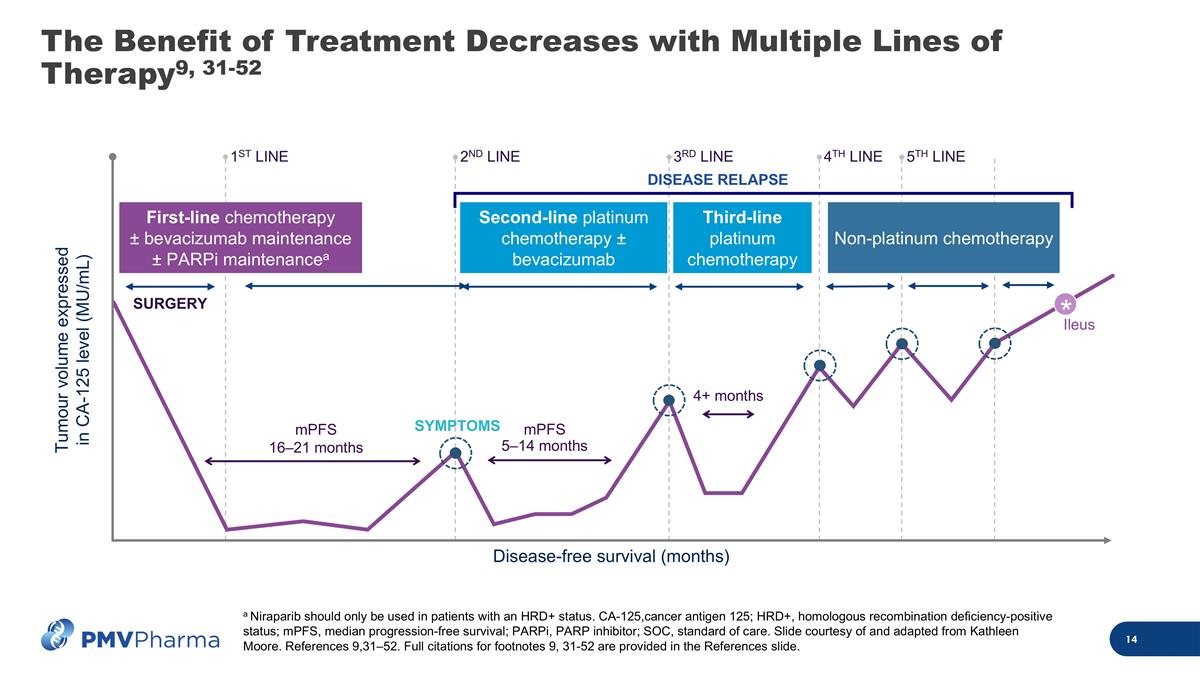
The Benefit of Treatment Decreases with Multiple Lines of Therapy9, 31-52 mPFS 16–21 months mPFS 5–14 months Tumour volume expressed in CA-125 level (MU/mL) Disease-free survival (months) Ileus SYMPTOMS 4+ months SURGERY DISEASE RELAPSE Second-line platinum chemotherapy ± bevacizumab Third-line platinum chemotherapy Non-platinum chemotherapy First-line chemotherapy ± bevacizumab maintenance ± PARPi maintenancea * 1ST LINE 5TH LINE 2ND LINE 3RD LINE 4TH LINE a Niraparib should only be used in patients with an HRD+ status. CA-125,cancer antigen 125; HRD+, homologous recombination deficiency-positive status; mPFS, median progression-free survival; PARPi, PARP inhibitor; SOC, standard of care. Slide courtesy of and adapted from Kathleen Moore. References 9,31–52. Full citations for footnotes 9, 31-52 are provided in the References slide.
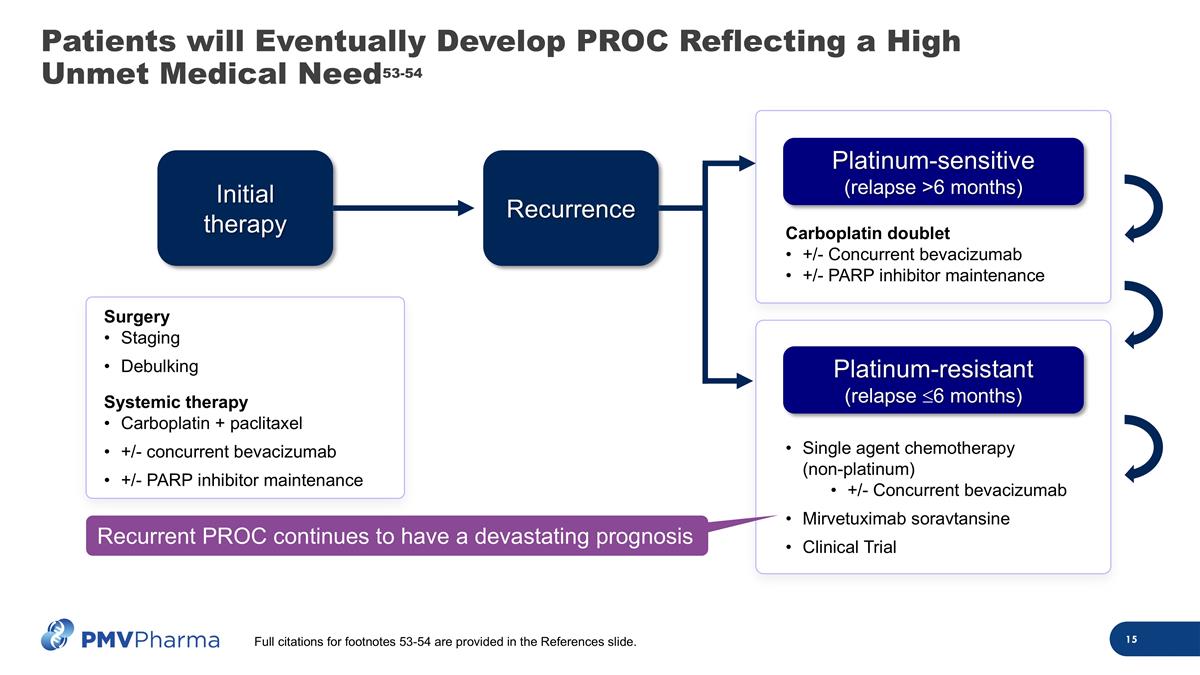
Single agent chemotherapy (non-platinum) +/- Concurrent bevacizumab Mirvetuximab soravtansine Clinical Trial Carboplatin doublet +/- Concurrent bevacizumab +/- PARP inhibitor maintenance Patients will Eventually Develop PROC Reflecting a High Unmet Medical Need53-54 Initial therapy Recurrence Platinum-sensitive (relapse >6 months) Platinum-resistant (relapse £6 months) Recurrent PROC continues to have a devastating prognosis Full citations for footnotes 53-54 are provided in the References slide. Surgery Staging Debulking Systemic therapy Carboplatin + paclitaxel +/- concurrent bevacizumab +/- PARP inhibitor maintenance
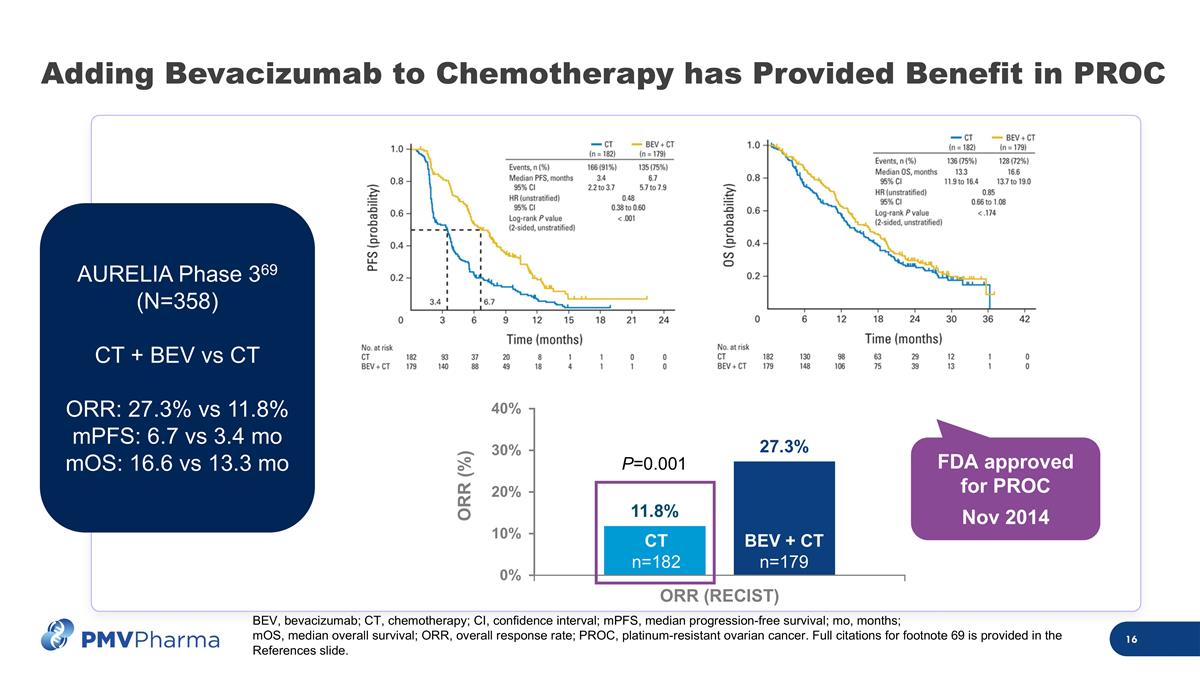
ORR (%) Adding Bevacizumab to Chemotherapy has Provided Benefit in PROC AURELIA Phase 369 (N=358) CT + BEV vs CT ORR: 27.3% vs 11.8% mPFS: 6.7 vs 3.4 mo mOS: 16.6 vs 13.3 mo P=0.001 FDA approved for PROC Nov 2014 CT n=182 BEV + CT n=179 BEV, bevacizumab; CT, chemotherapy; CI, confidence interval; mPFS, median progression-free survival; mo, months; mOS, median overall survival; ORR, overall response rate; PROC, platinum-resistant ovarian cancer. Full citations for footnote 69 is provided in the References slide.
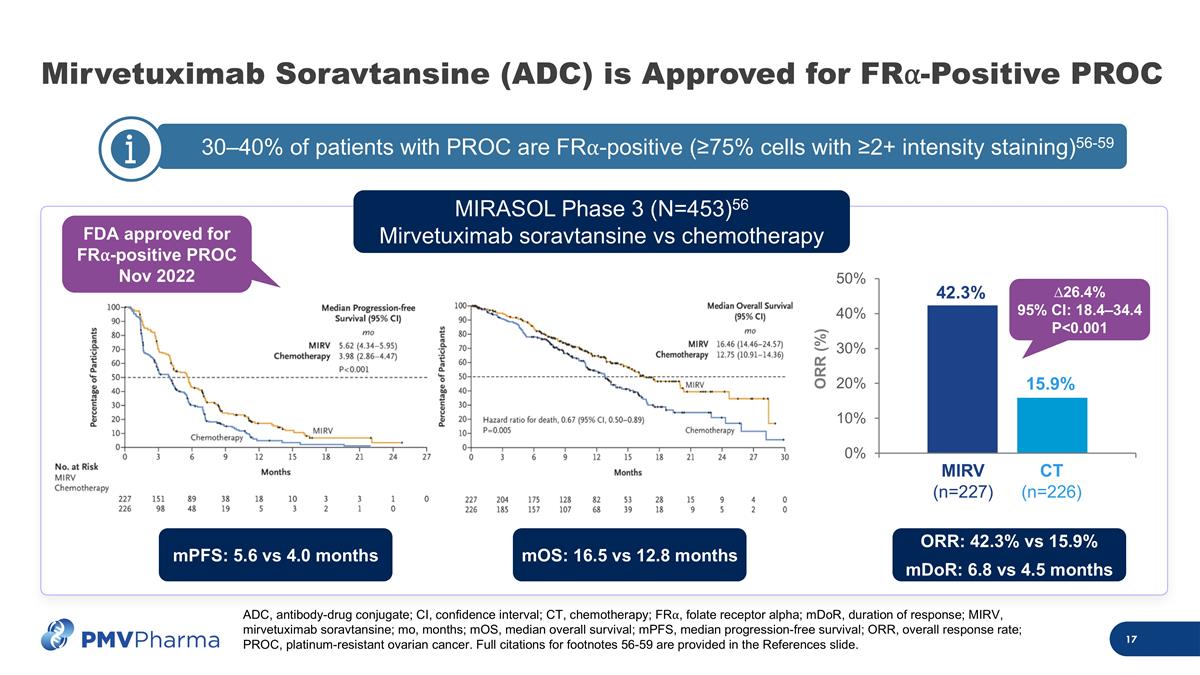
30–40% of patients with PROC are FR⍺-positive (≥75% cells with ≥2+ intensity staining)56-59 c Mirvetuximab Soravtansine (ADC) is Approved for FR⍺-Positive PROC MIRV (n=227) CT (n=226) 42.3% 15.9% ORR (%) ∆26.4% 95% CI: 18.4–34.4 P<0.001 MIRASOL Phase 3 (N=453)56 Mirvetuximab soravtansine vs chemotherapy FDA approved for FR⍺-positive PROC Nov 2022 ADC, antibody-drug conjugate; CI, confidence interval; CT, chemotherapy; FR⍺, folate receptor alpha; mDoR, duration of response; MIRV, mirvetuximab soravtansine; mo, months; mOS, median overall survival; mPFS, median progression-free survival; ORR, overall response rate; PROC, platinum-resistant ovarian cancer. Full citations for footnotes 56-59 are provided in the References slide. mPFS: 5.6 vs 4.0 months mOS: 16.5 vs 12.8 months ORR: 42.3% vs 15.9% mDoR: 6.8 vs 4.5 months
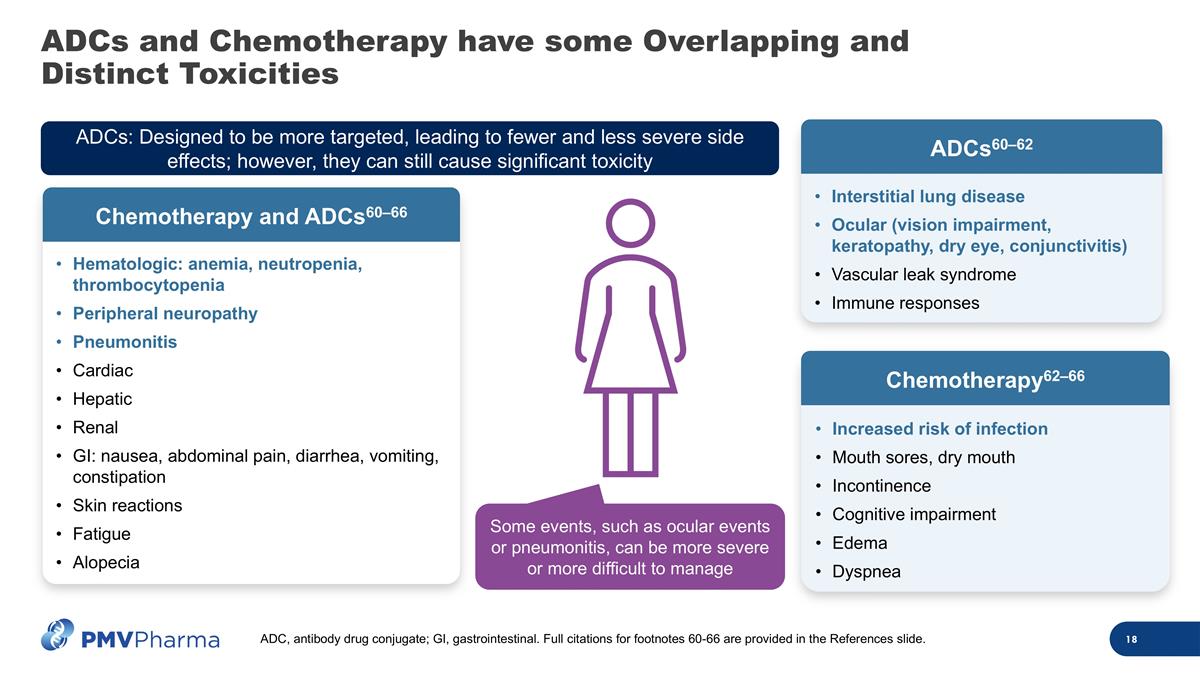
ADCs and Chemotherapy have some Overlapping and Distinct Toxicities Hematologic: anemia, neutropenia, thrombocytopenia Peripheral neuropathy Pneumonitis Cardiac Hepatic Renal GI: nausea, abdominal pain, diarrhea, vomiting, constipation Skin reactions Fatigue Alopecia Chemotherapy and ADCs60–66 ADCs: Designed to be more targeted, leading to fewer and less severe side effects; however, they can still cause significant toxicity Increased risk of infection Mouth sores, dry mouth Incontinence Cognitive impairment Edema Dyspnea Chemotherapy62–66 Interstitial lung disease Ocular (vision impairment, keratopathy, dry eye, conjunctivitis) Vascular leak syndrome Immune responses ADCs60–62 Some events, such as ocular events or pneumonitis, can be more severe or more difficult to manage ADC, antibody drug conjugate; GI, gastrointestinal. Full citations for footnotes 60-66 are provided in the References slide.
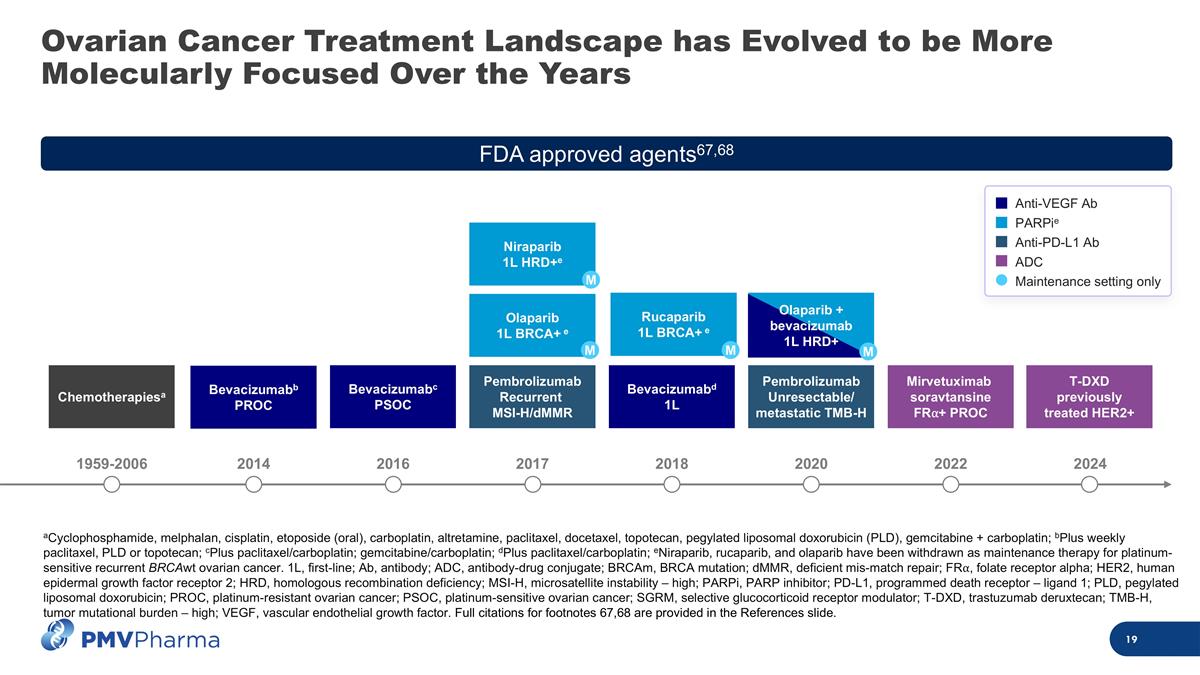
Ovarian Cancer Treatment Landscape has Evolved to be More Molecularly Focused Over the Years aCyclophosphamide, melphalan, cisplatin, etoposide (oral), carboplatin, altretamine, paclitaxel, docetaxel, topotecan, pegylated liposomal doxorubicin (PLD), gemcitabine + carboplatin; bPlus weekly paclitaxel, PLD or topotecan; cPlus paclitaxel/carboplatin; gemcitabine/carboplatin; dPlus paclitaxel/carboplatin; eNiraparib, rucaparib, and olaparib have been withdrawn as maintenance therapy for platinum-sensitive recurrent BRCAwt ovarian cancer. 1L, first-line; Ab, antibody; ADC, antibody-drug conjugate; BRCAm, BRCA mutation; dMMR, deficient mis-match repair; FR⍺, folate receptor alpha; HER2, human epidermal growth factor receptor 2; HRD, homologous recombination deficiency; MSI-H, microsatellite instability – high; PARPi, PARP inhibitor; PD-L1, programmed death receptor – ligand 1; PLD, pegylated liposomal doxorubicin; PROC, platinum-resistant ovarian cancer; PSOC, platinum-sensitive ovarian cancer; SGRM, selective glucocorticoid receptor modulator; T-DXD, trastuzumab deruxtecan; TMB-H, tumor mutational burden – high; VEGF, vascular endothelial growth factor. Full citations for footnotes 67,68 are provided in the References slide. 1959-2006 2016 2020 2024 2014 2017 2018 2022 Bevacizumabb PROC Chemotherapiesa Bevacizumabc PSOC Niraparib 1L HRD+e Rucaparib 1L BRCA+ e Mirvetuximab soravtansine FR⍺+ PROC T-DXD previously treated HER2+ Pembrolizumab Unresectable/ metastatic TMB-H Bevacizumabd 1L Olaparib 1L BRCA+ e Pembrolizumab Recurrent MSI-H/dMMR M M M Olaparib + bevacizumab 1L HRD+ M FDA approved agents67,68 Anti-VEGF Ab PARPie Anti-PD-L1 Ab ADC Maintenance setting only
The Unmet Medical Need is High for PROC Despite advances in therapeutic options there is still an unmet need in advanced ovarian cancer, particularly in PROC There is a lack of effective and durable treatment options, leading to poor prognosis and limited survival outcomes Patients with PROC often experience rapid progression of disease and have a median overall survival of <12 months Current treatments, primarily chemotherapy, are often ineffective, highlighting the urgent need for new tolerable therapies that can improve progression-free and overall survival PROC, platinum-resistant ovarian cancer.

Today’s Objectives Rezatapopt background Ovarian cancer treatment landscape PYNNACLE Phase 2 interim data update Initial NDA strategy informed by FDA feedback Q&A 01 02 03 04 Deepika Jalota, PharmD Chief Development Officer
Overview of PYNNACLE Phase 2 Interim Data Overall results across all cohorts Ovarian cohort results NDA submission strategy Looking ahead
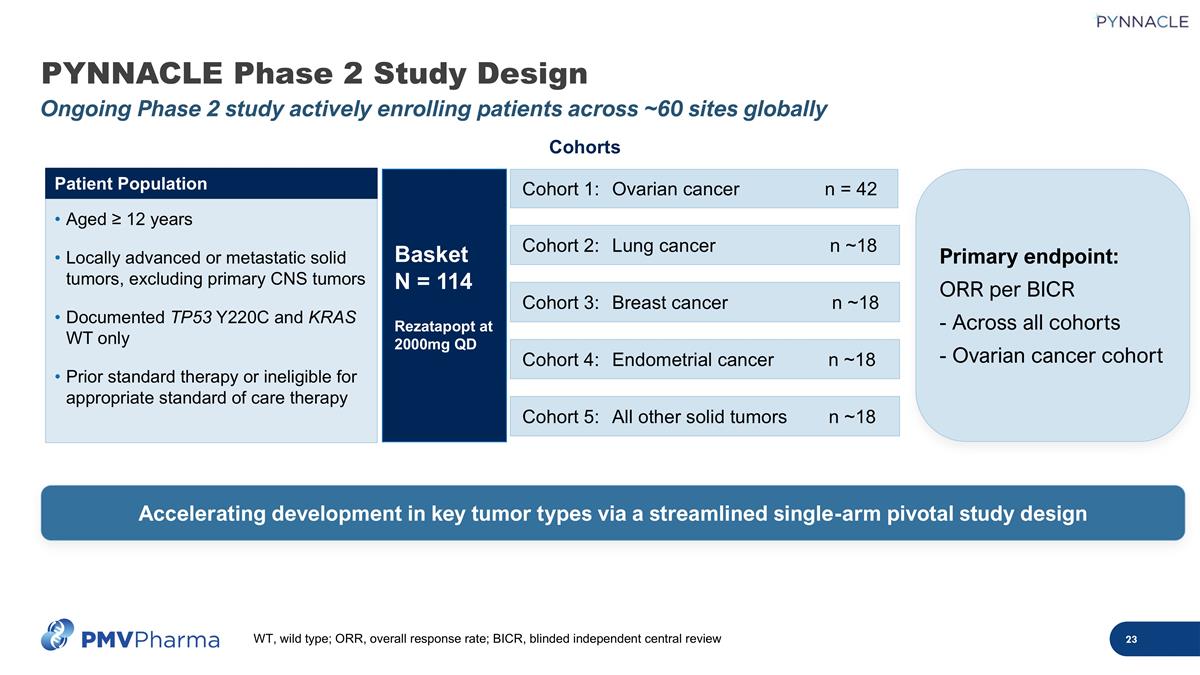
PYNNACLE Phase 2 Study Design Ongoing Phase 2 study actively enrolling patients across ~60 sites globally Cohort 2:Lung cancer n ~18 Cohort 1: Ovarian cancer n = 42 Cohorts Cohort 3:Breast cancer n ~18 Cohort 4:Endometrial cancer n ~18 Cohort 5:All other solid tumors n ~18 Basket N = 114 Rezatapopt at 2000mg QD Aged ≥ 12 years Locally advanced or metastatic solid tumors, excluding primary CNS tumors Documented TP53 Y220C and KRAS WT only Prior standard therapy or ineligible for appropriate standard of care therapy Patient Population Accelerating development in key tumor types via a streamlined single-arm pivotal study design Primary endpoint: ORR per BICR - Across all cohorts - Ovarian cancer cohort WT, wild type; ORR, overall response rate; BICR, blinded independent central review
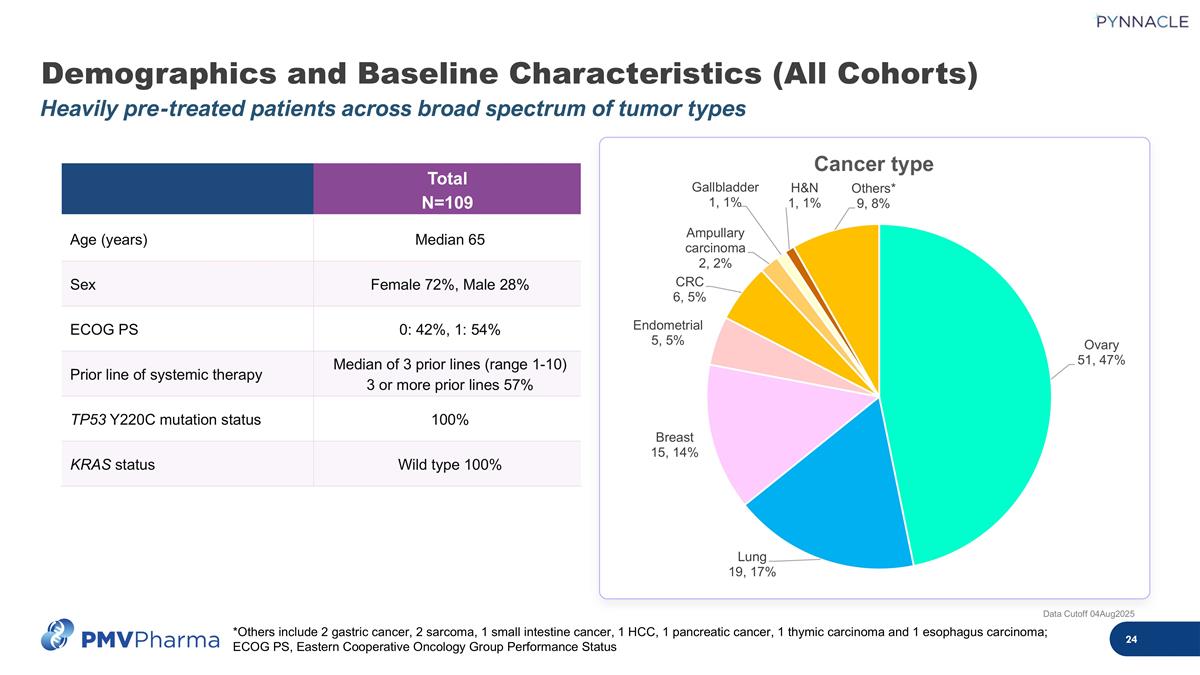
Demographics and Baseline Characteristics (All Cohorts) Heavily pre-treated patients across broad spectrum of tumor types Data Cutoff 04Aug2025 Total N=109 Age (years) Median 65 Sex Female 72%, Male 28% ECOG PS 0: 42%, 1: 54% Prior line of systemic therapy Median of 3 prior lines (range 1-10) 3 or more prior lines 57% TP53 Y220C mutation status 100% KRAS status Wild type 100% *Others include 2 gastric cancer, 2 sarcoma, 1 small intestine cancer, 1 HCC, 1 pancreatic cancer, 1 thymic carcinoma and 1 esophagus carcinoma; ECOG PS, Eastern Cooperative Oncology Group Performance Status
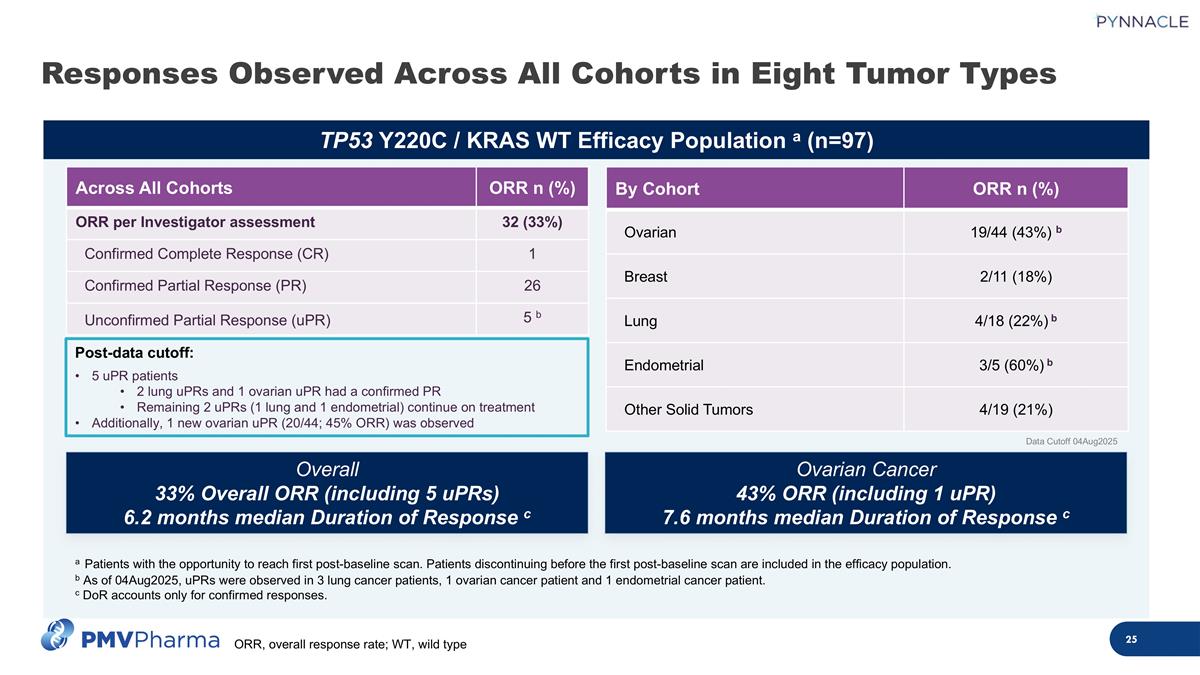
TP53 Y220C / KRAS WT Efficacy Population a (n=97) Responses Observed Across All Cohorts in Eight Tumor Types By Cohort ORR n (%) Ovarian 19/44 (43%) b Breast 2/11 (18%) Lung 4/18 (22%) b Endometrial 3/5 (60%) b Other Solid Tumors 4/19 (21%) Ovarian Cancer 43% ORR (including 1 uPR) 7.6 months median Duration of Response c Overall 33% Overall ORR (including 5 uPRs) 6.2 months median Duration of Response c Data Cutoff 04Aug2025 Across All Cohorts ORR n (%) ORR per Investigator assessment 32 (33%) Confirmed Complete Response (CR) 1 Confirmed Partial Response (PR) 26 Unconfirmed Partial Response (uPR) 5 b a Patients with the opportunity to reach first post-baseline scan. Patients discontinuing before the first post-baseline scan are included in the efficacy population. b As of 04Aug2025, uPRs were observed in 3 lung cancer patients, 1 ovarian cancer patient and 1 endometrial cancer patient. c DoR accounts only for confirmed responses. Post-data cutoff: 5 uPR patients 2 lung uPRs and 1 ovarian uPR had a confirmed PR Remaining 2 uPRs (1 lung and 1 endometrial) continue on treatment Additionally, 1 new ovarian uPR (20/44; 45% ORR) was observed ORR, overall response rate; WT, wild type
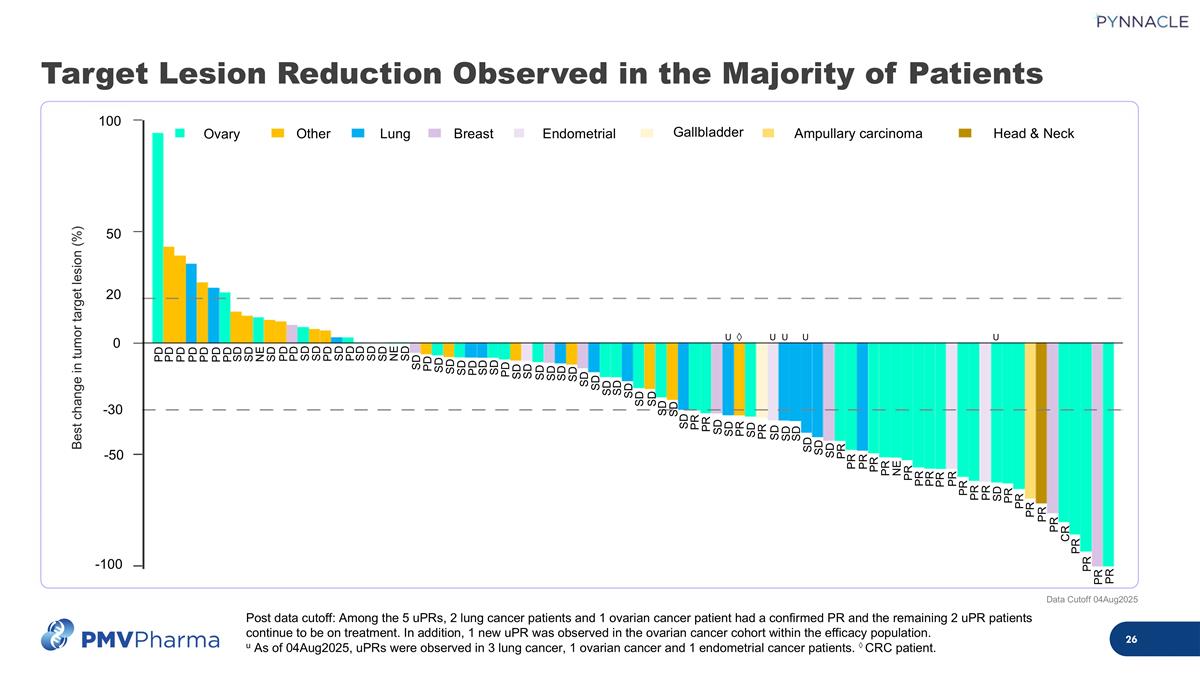
Target Lesion Reduction Observed in the Majority of Patients Post data cutoff: Among the 5 uPRs, 2 lung cancer patients and 1 ovarian cancer patient had a confirmed PR and the remaining 2 uPR patients continue to be on treatment. In addition, 1 new uPR was observed in the ovarian cancer cohort within the efficacy population. u As of 04Aug2025, uPRs were observed in 3 lung cancer, 1 ovarian cancer and 1 endometrial cancer patients. ◊ CRC patient. Data Cutoff 04Aug2025 U U U U U ◊ Best change in tumor target lesion (%) 100 -100 -50 50 0 20 -30 Ovary Other Lung Breast Endometrial Gallbladder Ampullary carcinoma Head & Neck PD PD PD PD PD PD PD SD SD NE SD PD PD SD SD PD SD PD SD SD SD NE SD PR PR PR PR CR PR PR PR PR PR SD PR PR PR PR PR PR PR PR NE PR PR PR PR PR SD SD SD SD SD SD PR SD SD PR PR PR SD SD SD SD SD SD SD SD SD SD SD SD SD SD SD SD SD PD SD SD PD SD SD SD PD SD
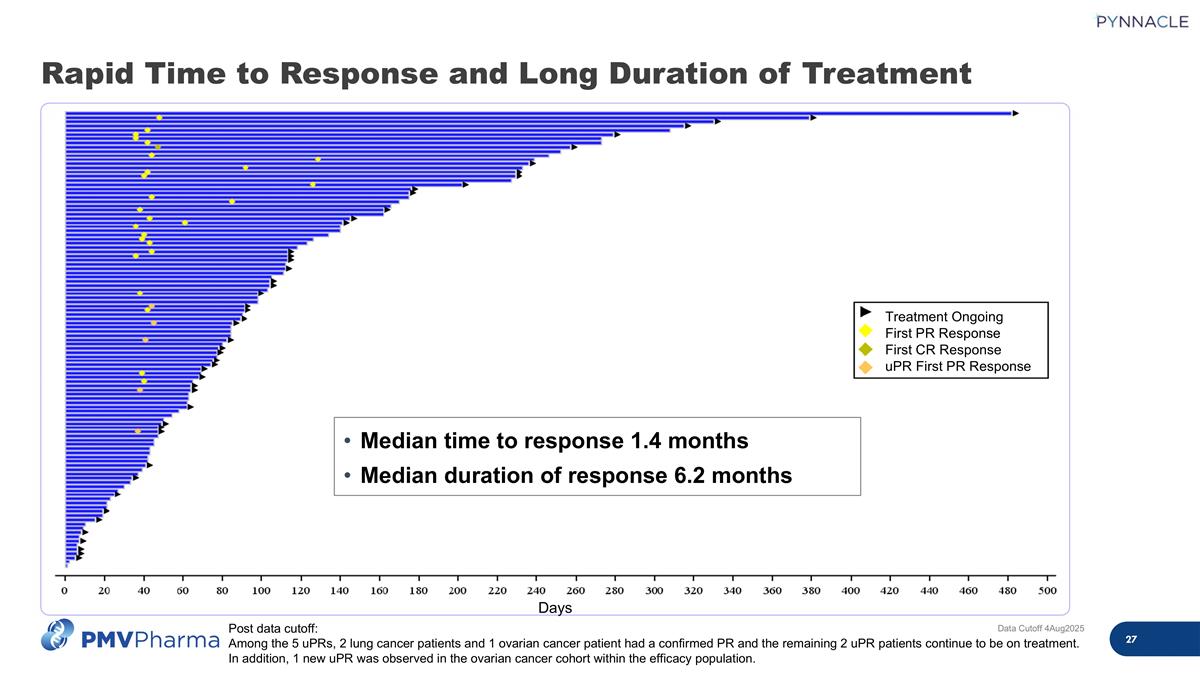
Data Cutoff 4Aug2025 Post data cutoff: Among the 5 uPRs, 2 lung cancer patients and 1 ovarian cancer patient had a confirmed PR and the remaining 2 uPR patients continue to be on treatment. In addition, 1 new uPR was observed in the ovarian cancer cohort within the efficacy population. Median time to response 1.4 months Median duration of response 6.2 months Days Treatment Ongoing First PR Response First CR Response uPR First PR Response Rapid Time to Response and Long Duration of Treatment
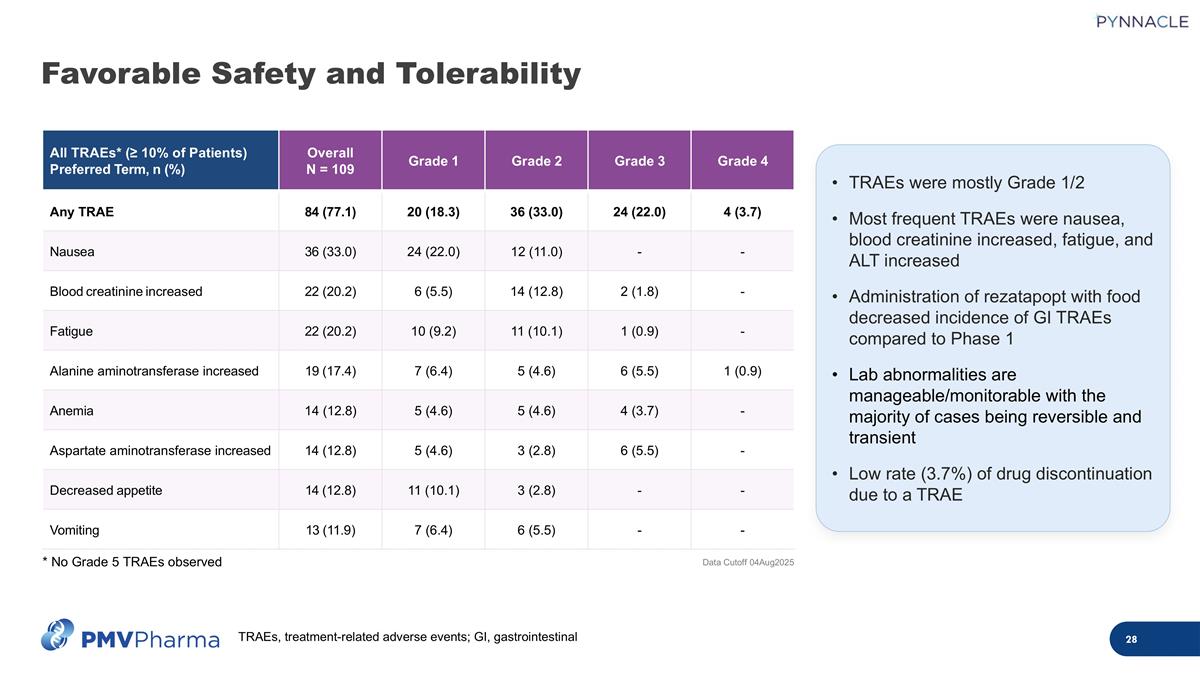
TRAEs were mostly Grade 1/2 Most frequent TRAEs were nausea, blood creatinine increased, fatigue, and ALT increased Administration of rezatapopt with food decreased incidence of GI TRAEs compared to Phase 1 Lab abnormalities are manageable/monitorable with the majority of cases being reversible and transient Low rate (3.7%) of drug discontinuation due to a TRAE All TRAEs* (≥ 10% of Patients) Preferred Term, n (%) Overall N = 109 Grade 1 Grade 2 Grade 3 Grade 4 Any TRAE 84 (77.1) 20 (18.3) 36 (33.0) 24 (22.0) 4 (3.7) Nausea 36 (33.0) 24 (22.0) 12 (11.0) - - Blood creatinine increased 22 (20.2) 6 (5.5) 14 (12.8) 2 (1.8) - Fatigue 22 (20.2) 10 (9.2) 11 (10.1) 1 (0.9) - Alanine aminotransferase increased 19 (17.4) 7 (6.4) 5 (4.6) 6 (5.5) 1 (0.9) Anemia 14 (12.8) 5 (4.6) 5 (4.6) 4 (3.7) - Aspartate aminotransferase increased 14 (12.8) 5 (4.6) 3 (2.8) 6 (5.5) - Decreased appetite 14 (12.8) 11 (10.1) 3 (2.8) - - Vomiting 13 (11.9) 7 (6.4) 6 (5.5) - - Data Cutoff 04Aug2025 * No Grade 5 TRAEs observed Favorable Safety and Tolerability TRAEs, treatment-related adverse events; GI, gastrointestinal
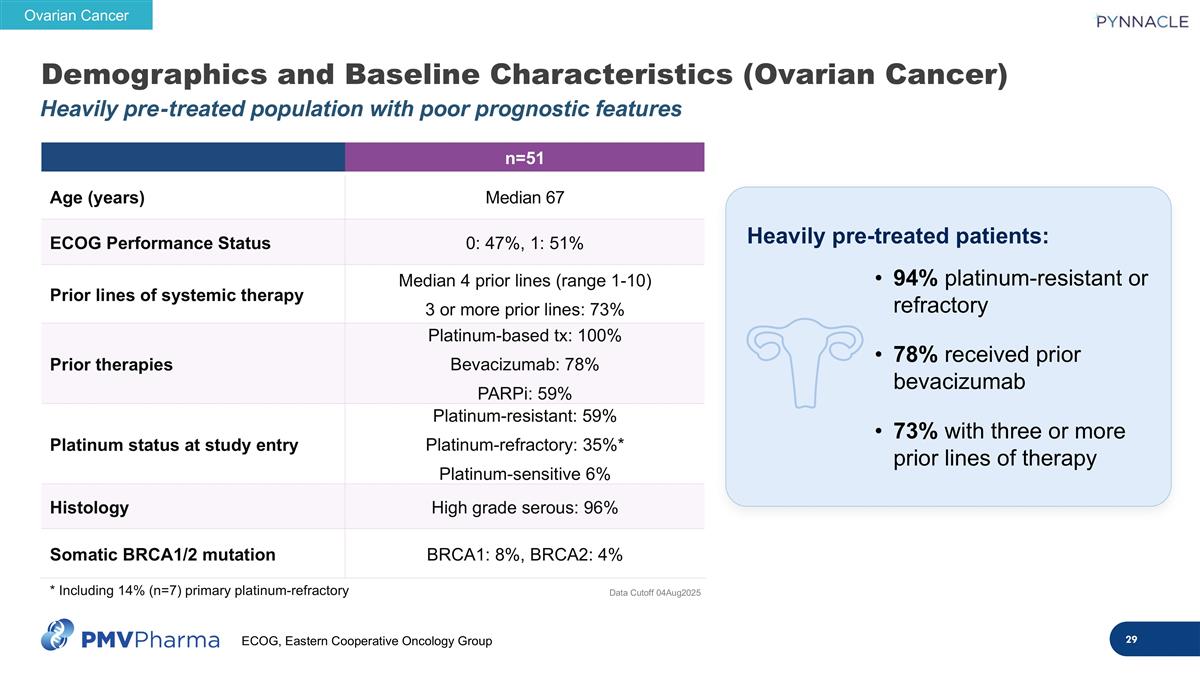
Demographics and Baseline Characteristics (Ovarian Cancer) Heavily pre-treated population with poor prognostic features n=51 Age (years) Median 67 ECOG Performance Status 0: 47%, 1: 51% Prior lines of systemic therapy Median 4 prior lines (range 1-10) 3 or more prior lines: 73% Prior therapies Platinum-based tx: 100% Bevacizumab: 78% PARPi: 59% Platinum status at study entry Platinum-resistant: 59% Platinum-refractory: 35%* Platinum-sensitive 6% Histology High grade serous: 96% Somatic BRCA1/2 mutation BRCA1: 8%, BRCA2: 4% Heavily pre-treated patients: 94% platinum-resistant or refractory 78% received prior bevacizumab 73% with three or more prior lines of therapy Data Cutoff 04Aug2025 Ovarian Cancer * Including 14% (n=7) primary platinum-refractory ECOG, Eastern Cooperative Oncology Group
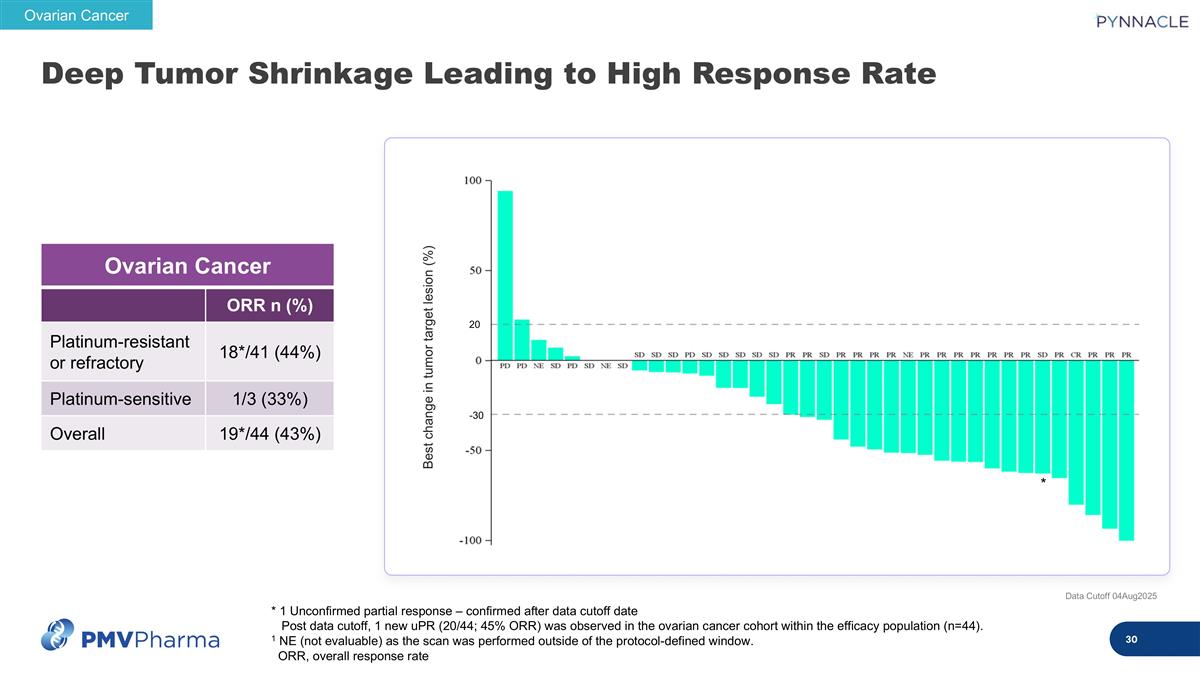
Deep Tumor Shrinkage Leading to High Response Rate Data Cutoff 04Aug2025 * 1 Unconfirmed partial response – confirmed after data cutoff date Post data cutoff, 1 new uPR (20/44; 45% ORR) was observed in the ovarian cancer cohort within the efficacy population (n=44). 1 NE (not evaluable) as the scan was performed outside of the protocol-defined window. ORR, overall response rate Ovarian Cancer ORR n (%) Platinum-resistant or refractory 18*/41 (44%) Platinum-sensitive 1/3 (33%) Overall 19*/44 (43%) Ovarian Cancer Best change in tumor target lesion (%) * 20 -30
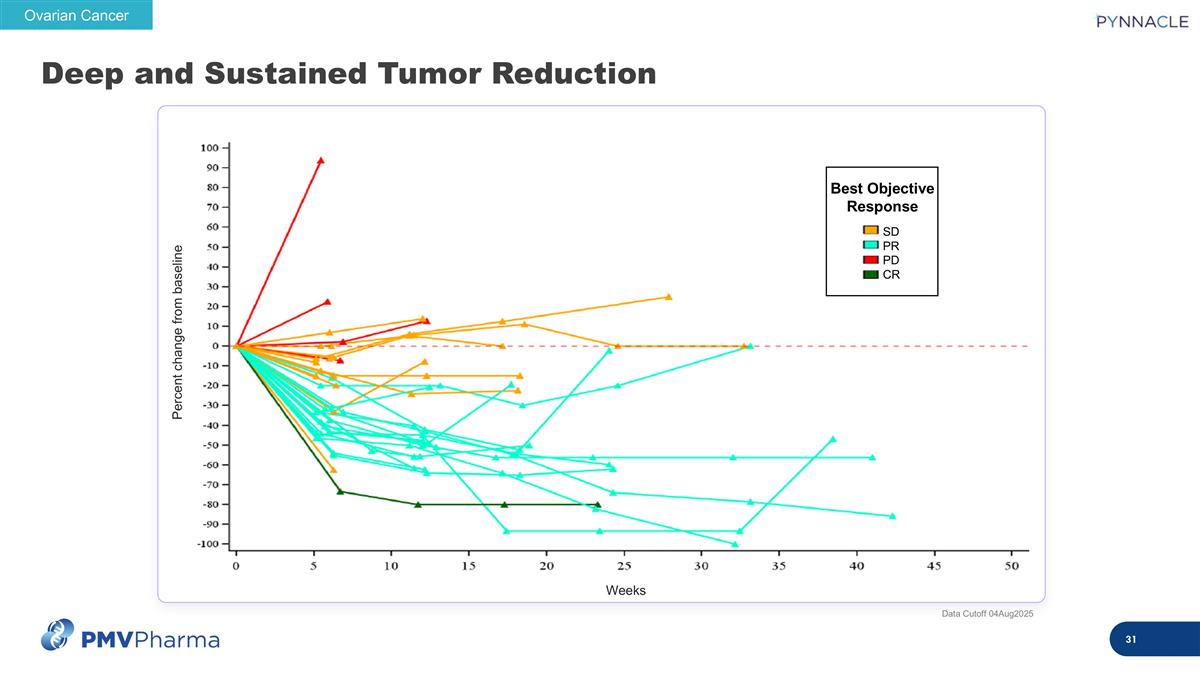
Deep and Sustained Tumor Reduction Weeks Percent change from baseline Ovarian Cancer SD PR PD CR Best Objective Response Data Cutoff 04Aug2025
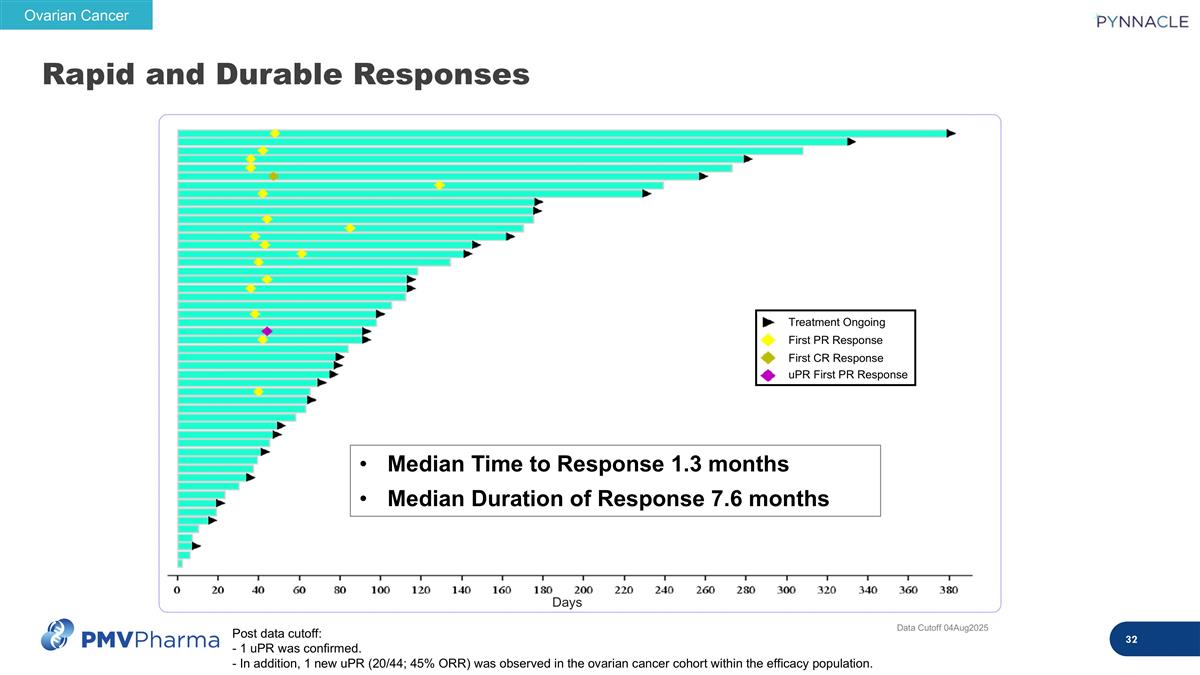
Rapid and Durable Responses Post data cutoff: - 1 uPR was confirmed. - In addition, 1 new uPR (20/44; 45% ORR) was observed in the ovarian cancer cohort within the efficacy population. Ovarian Cancer Days Median Time to Response 1.3 months Median Duration of Response 7.6 months Treatment Ongoing First PR Response First CR Response uPR First PR Response Data Cutoff 04Aug2025
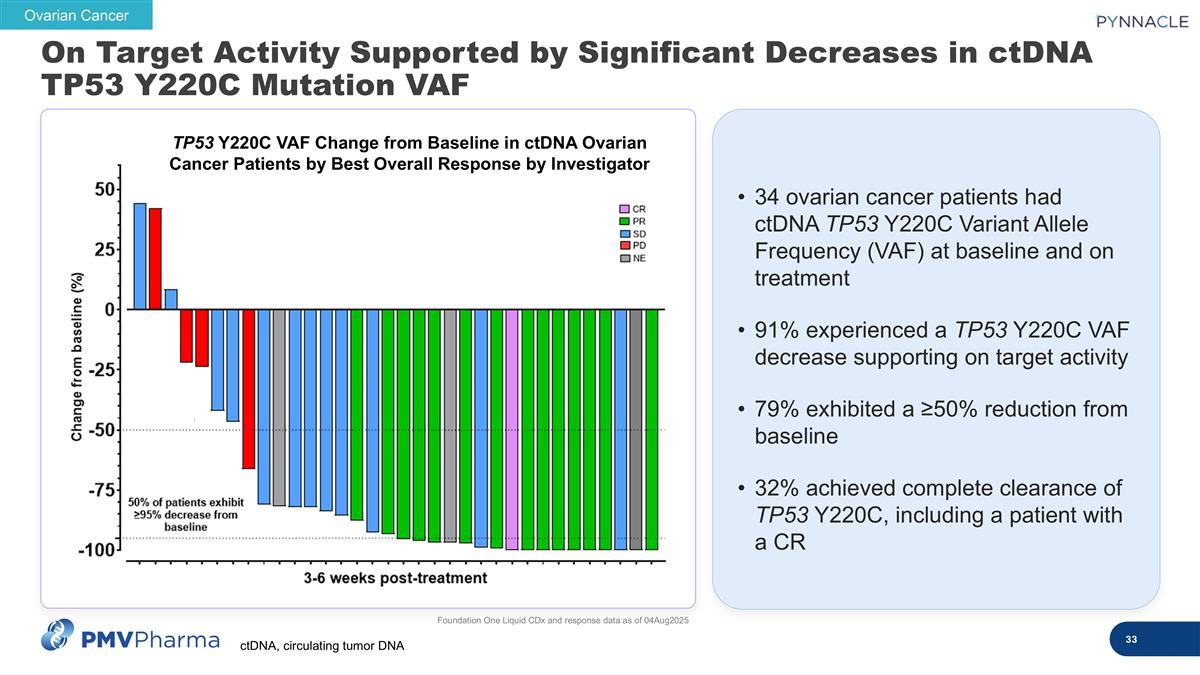
34 ovarian cancer patients had ctDNA TP53 Y220C Variant Allele Frequency (VAF) at baseline and on treatment 91% experienced a TP53 Y220C VAF decrease supporting on target activity 79% exhibited a ≥50% reduction from baseline 32% achieved complete clearance of TP53 Y220C, including a patient with a CR Foundation One Liquid CDx and response data as of 04Aug2025 Ovarian Cancer TP53 Y220C VAF Change from Baseline in ctDNA Ovarian Cancer Patients by Best Overall Response by Investigator On Target Activity Supported by Significant Decreases in ctDNA TP53 Y220C Mutation VAF ctDNA, circulating tumor DNA
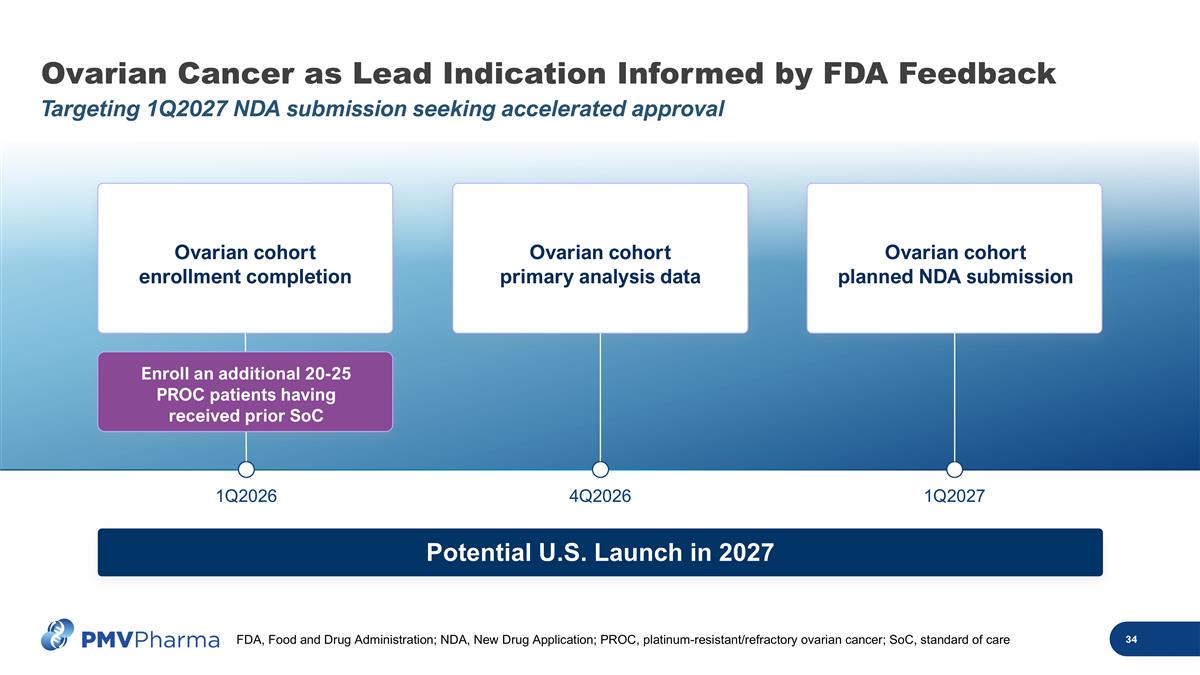
Ovarian Cancer as Lead Indication Informed by FDA Feedback Targeting 1Q2027 NDA submission seeking accelerated approval 1Q2026 4Q2026 1Q2027 Potential U.S. Launch in 2027 Ovarian cohort enrollment completion Ovarian cohort primary analysis data Ovarian cohort planned NDA submission Enroll an additional 20-25 PROC patients having received prior SoC FDA, Food and Drug Administration; NDA, New Drug Application; PROC, platinum-resistant/refractory ovarian cancer; SoC, standard of care

Market Research Feedback I would think this is going to be the go-to agent [for ovarian cancer]. With this current data, this beats everything in the market—if the patient does carry the mutation.” Community Oncologist, TX It’s meaningful if a patient doesn’t have to come in for an infusion all the time. PO [oral] is attractive...” Community Gynecologic Oncologist, GA Commercial Opportunity: De-risked opportunity in PROC as lead indication with projected 2027 launch Potential to expand label beyond PROC Clear value proposition for rezatapopt relative to existing and emerging treatments TP53 Y220C mutation broadly identifiable Rezatapopt Offers Compelling Commercial Opportunity “ PROC, platinum-resistant/refractory ovarian cancer
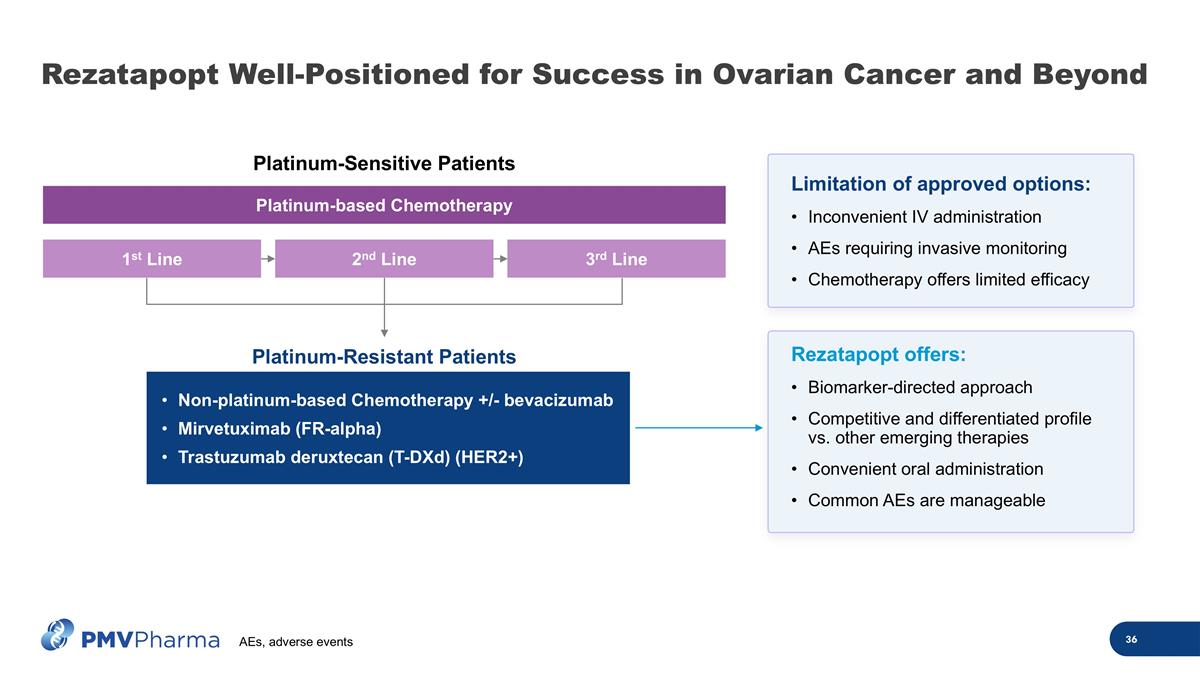
Non-platinum-based Chemotherapy +/- bevacizumab Mirvetuximab (FR-alpha) Trastuzumab deruxtecan (T-DXd) (HER2+) Platinum-Resistant Patients Platinum-Sensitive Patients 1st Line 2nd Line 3rd Line Platinum-based Chemotherapy Limitation of approved options: Inconvenient IV administration AEs requiring invasive monitoring Chemotherapy offers limited efficacy Rezatapopt offers: Biomarker-directed approach Competitive and differentiated profile vs. other emerging therapies Convenient oral administration Common AEs are manageable Rezatapopt Well-Positioned for Success in Ovarian Cancer and Beyond AEs, adverse events

TP53 Y220C Mutation is Broadly Identifiable on Existing NGS Panels Molecular testing is now recommended by NCCN and ESMO across many cancer types including ovarian cancer, breast cancer, NSCLC, endometrial and others Reimbursement of NGS testing is widely covered by Medicare and private insurance for qualifying patients NGS, next-generation sequencing; NCCN, National Comprehensive Cancer Network; ESMO, European Society for Medical Oncology; NSCLC, non-small cell lung cancer
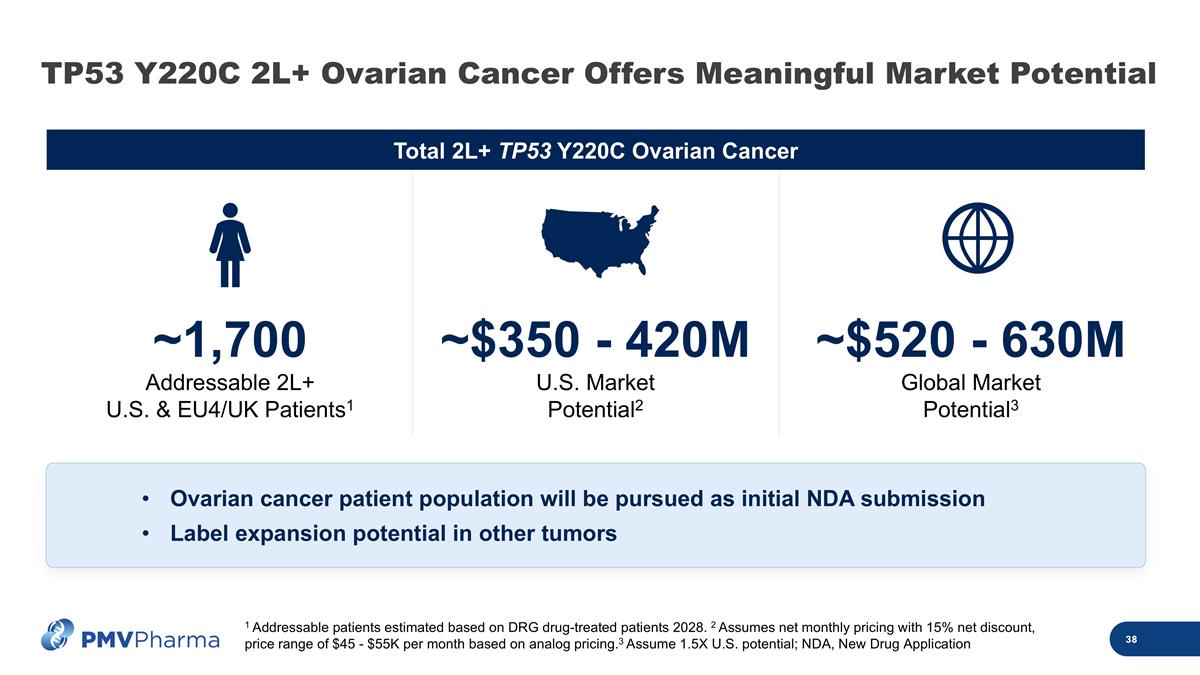
Total 2L+ TP53 Y220C Ovarian Cancer 1 Addressable patients estimated based on DRG drug-treated patients 2028. 2 Assumes net monthly pricing with 15% net discount, price range of $45 - $55K per month based on analog pricing.3 Assume 1.5X U.S. potential; NDA, New Drug Application ~1,700 Addressable 2L+ U.S. & EU4/UK Patients1 ~$350 - 420M U.S. Market Potential2 ~$520 - 630M Global Market Potential3 Ovarian cancer patient population will be pursued as initial NDA submission Label expansion potential in other tumors TP53 Y220C 2L+ Ovarian Cancer Offers Meaningful Market Potential
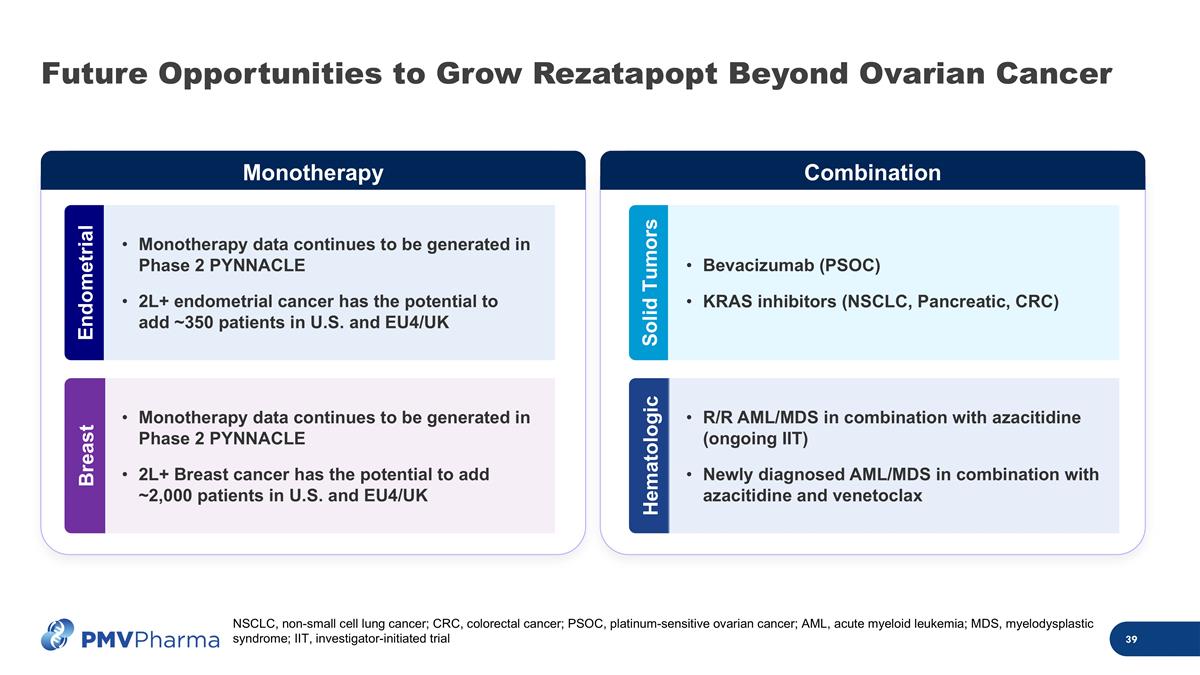
Future Opportunities to Grow Rezatapopt Beyond Ovarian Cancer Monotherapy data continues to be generated in Phase 2 PYNNACLE 2L+ endometrial cancer has the potential to add ~350 patients in U.S. and EU4/UK Endometrial Monotherapy data continues to be generated in Phase 2 PYNNACLE 2L+ Breast cancer has the potential to add ~2,000 patients in U.S. and EU4/UK Breast Monotherapy Combination Solid Tumors Hematologic Bevacizumab (PSOC) KRAS inhibitors (NSCLC, Pancreatic, CRC) R/R AML/MDS in combination with azacitidine (ongoing IIT) Newly diagnosed AML/MDS in combination with azacitidine and venetoclax NSCLC, non-small cell lung cancer; CRC, colorectal cancer; PSOC, platinum-sensitive ovarian cancer; AML, acute myeloid leukemia; MDS, myelodysplastic syndrome; IIT, investigator-initiated trial
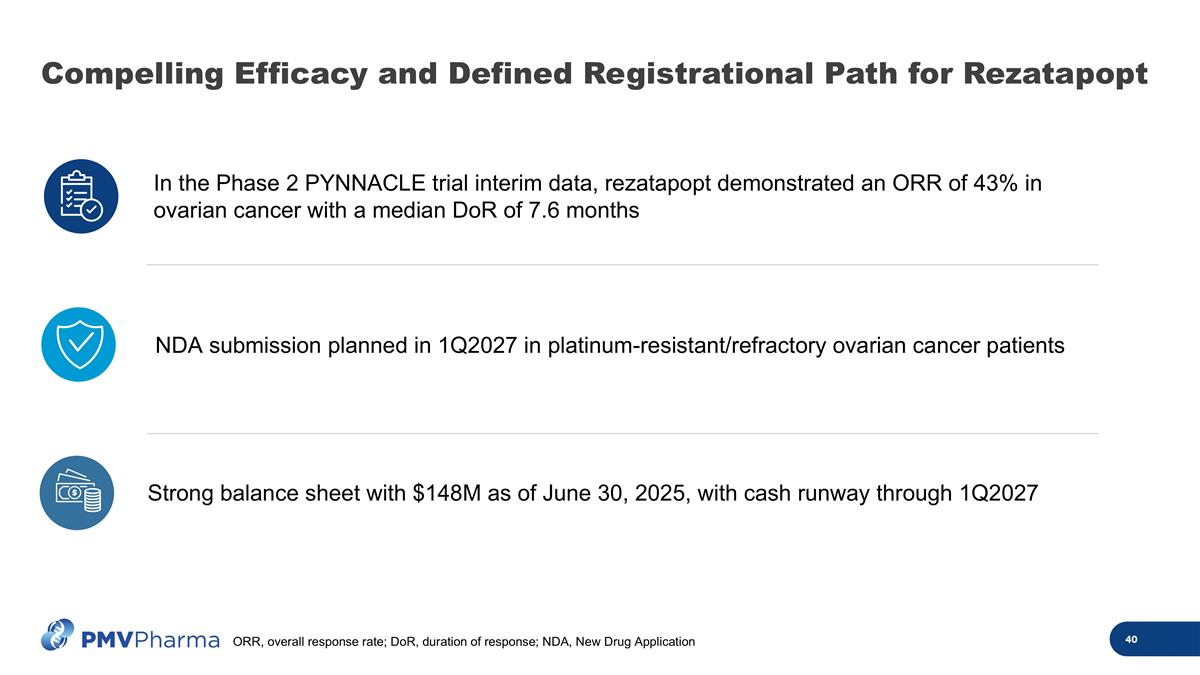
Compelling Efficacy and Defined Registrational Path for Rezatapopt In the Phase 2 PYNNACLE trial interim data, rezatapopt demonstrated an ORR of 43% in ovarian cancer with a median DoR of 7.6 months Strong balance sheet with $148M as of June 30, 2025, with cash runway through 1Q2027 NDA submission planned in 1Q2027 in platinum-resistant/refractory ovarian cancer patients ORR, overall response rate; DoR, duration of response; NDA, New Drug Application

Today’s Objectives Rezatapopt background Ovarian cancer treatment landscape PYNNACLE Phase 2 interim data update Initial NDA strategy informed by FDA feedback Q&A 01 02 03 04 David Mack, PhD President and Chief Executive Officer Ramez N. Eskander, MD Professor, Department of Obstetrics, Gynecology, and Reproductive Sciences UC San Diego Deepika Jalota, PharmD Chief Development Officer Panel

Thank You
American Cancer Society. Key statistics for ovarian cancer. https://www.cancer.org/cancer/types/ovarian-cancer/about/key-statistics.html. Accessed June 2025. National Cancer Institute. SEER Cancer Stat Facts: Ovarian Cancer. Bethesda, MD. https://seer.cancer.gov/statfacts/html/ovary.html. Accessed June 2025. Elyashiv O, et al. Cancers. 2024;16(3):641. Davies A, et al. Gynecol Oncol. 2014;133:624–31. Colombo N, et al. Int J Gynecol Cancer. 2017;27:1134–40. Luvero D, et al. Crit Rev Oncol Hematol. 2019:140:28–38. Garzon S, et al. Gland Surg. 2020;9:1118–29. Hanker LC, et al. Ann Oncol. 2012;23:2605–12. Kinney RE, et al. Oncologist. 2023;28:e478–86. Rose PG, et al. Obstet Gynecol. 2019;133:245–54. Momenimovahed Z, et al. Int J Womens Health. 2019;11:287–299. Kim J, et al. Cancers (Basel). 2018 Nov 12;10(11):433. Hatano Y, et al. Adv Anat Pathol. 2019;26:329–339. Ovarian Research Cancer Reliance. https://ocrahope.org/news/high-grade-serous-carcinoma/. Accessed July 2025. FoundationInsights™. A proprietary database used under license with review and approval from Foundation Medicine®. Available at: https://www.foundationmedicine.com/service/genomic-data-solutions. Accessed July 2024. Kuhn E, et al. Mod Pathol. 2016;29:1254–61. Vu B, et al. ACS Med Chem Lett. 2024;16:34–39. Dixit U, et al. J Virol. 2015;89(15):7905–7921. Olivier M, et al. Cold Spring Harb Perspect Biol. 2010;2:a001008. Berke T, et al. Onco Targets Ther. 2022;15:23–30. Nakamura M, et al. Int J Mol Sci. 2019;20:5482. Donehower LA, et al. Cell Rep. 2019;28(5):1370–84. Sadighi S, et al. PLoS One. 2017;12(8):e0182444. Li VD, et al. J Cancer Res Clin Oncol. 2019;145(3):625–36. Tuna M, et al. Br J Cancer. 2020;122(3):405–12. Li C, et al. Cell Physiol Biochem. 2018;51(6):2829–42. Li H, et al. Int J Mol Sci. 2019;20:5999. Bischof K, et al. Sci Rep. 2019;9:5244. Deacu M, et al. Rom J Morphol Embryol. 2021;62:63–71. Chen X, et al. Cell Death Dis. 2022;13:974. Gupta S, et al. J Ovarian Res. 2019;12:103. du Bois A, et al. J Natl Cancer Inst. 2003;95:1320–1329. Neijt JP, et al. J Clin Oncol. 2000;18:3084–3092. Ozols RF, et al. J Clin Oncol. 2003;21:3194–3200. Perren TJ, et al. N Engl J Med. 2011;365:2484–2496. Coleman RL, et al. Lancet Oncol. 2017;18:779–791. Aghajanian C, et al. J Clin Oncol. 2012;30:2039–2045. Wagner U, et al. Br J Can. 2012;107:588–591. Lord R, et al. Int J Gynecol Cancer. 2020;30:1026–1033 Moore K, et al. N Engl J Med. 2018;379:2495–505. Moore K, et al. N Engl J Med. 2018;379:Supplementary Appendix. Moore K, et al. N Engl J Med. 2018;379:Clinical Study Protocol. Gonzalez Martin A et al. N Engl J Med. 2019;381:2391–2402. Coleman RL, et al. N Engl J Med. 2019;381:2403–2415. Penson RT, et al. Presented at ESMO 2017. Poster #932PD. Pujade-Lauraine E, et al. Lancet Oncol. 2017;18:1274–1284 Friedlander M, et al. Lancet Oncol. 2018;19:1126–1134. Mirza MR, et al. N Engl J Med. 2016;375:2154–2164. Mirza MR, et al. N Engl J Med.2016;375:Suppl appendix. Coleman RL, et al. Lancet. 2017;390:1949–1961. Ledermann J, et al. N Engl J Med. 2012;366:1382–1392. Giornelli GH. Springerplus. 2016;5:1197. NCCN Guidelines®. Ovarian Cancer. v3.2024. Gonzalez-Martin A, et al. Ann Oncol. 2023;34:833–848. Pujade-Lauraine E, et al. J Clin Oncol. 2014;32:1302–8. Moore KN, et al. N Engl J Med 2023;389:2162–74. Matulonis UA, et al. J Clin Oncol. 2023;41:2436–45. Moore KN. https://www.targetedonc.com/view/mirvetuximab-soravtansine-results-from-mirasol-deemed-practice-changing-for-fr-platinum-resistant-ovarian-cancer. Accessed June 2025. Themeles M, et al. Cancer Res. 2025; 85: 8_Supplement_1:5910. Sato S, et al. Cancers (Basel). 2024;16:2545. Cheng X, et al. J Ovarian Res. 2024;17:196. Rached L, et al. Crit Rev Oncol Hematol. 2024:193:104212. American Cancer Society. https://www.cancer.org/cancer/managing-cancer/treatment-types/chemotherapy/chemotherapy-side-effects.html. Accessed June 2025. National Cancer Institute. https://www.cancer.gov/about-cancer/treatment/side-effects. Accessed June 2025. Lustberg M, et al. Nat Rev Clin Oncol. 2023;20:527–42. Kuderer NM, et al. Nature Nat Rev Clin Oncol. 2022;19:681–97. FDA. US prescribing information. Accessed June 2025. WAGO 2025 Highlights. References for Section 2: Current State: Ovarian Cancer