
Exhibit 99.2

Unlocking Life Changing Therapies August 2025 C O R PO R AT E O VER VI E W

©2025 Relmada - All rights reserved Disclosures 2 The Private Securities Litigation Reform Act of 1995 provides a safe harbor for forward - looking statements made by us or on our behalf . This press release contains statements which constitute “forward - looking statements” within the meaning of Section 27 A of the Securities Act of 1933 and Section 21 E of the Securities Exchange Act of 1934 . Any statement that is not historical in nature is a forward - looking statement and may be identified by the use of words and phrases such as “if”, “may”, “expects”, “anticipates”, “believes”, “will”, “will likely result”, “will continue”, “plans to”, “potential”, “promising”, and similar expressions . These statements are based on management’s current expectations and beliefs and are subject to a number of risks, uncertainties and assumptions that could cause actual results to differ materially from those described in the forward - looking statements, including potential for Phase 2 NDV - 01 data to continue to deliver positive results supporting further development, potential for clinical trials to deliver statistically and/or clinically significant evidence of efficacy and/or safety, failure of top - line results to accurately reflect the complete results of the trial, failure of planned or ongoing preclinical and clinical studies to demonstrate expected results, potential failure to secure FDA agreement on the regulatory path for sepranolone, and NDV - 01 , or that future sepranolone, or NDV - 01 clinical results will be acceptable to the FDA, failure to secure adequate sepranolone, or NDV - 01 drug supply, and the other risk factors described under the heading “Risk Factors” set forth in the Company’s reports filed with the SEC from time to time . No forward - looking statement can be guaranteed, and actual results may differ materially from those projected . Relmada undertakes no obligation to publicly update any forward - looking statement, whether as a result of new information, future events, or otherwise . Readers are cautioned that it is not possible to predict or identify all the risks, uncertainties and other factors that may affect future results and that the risks described herein should not be a complete list .
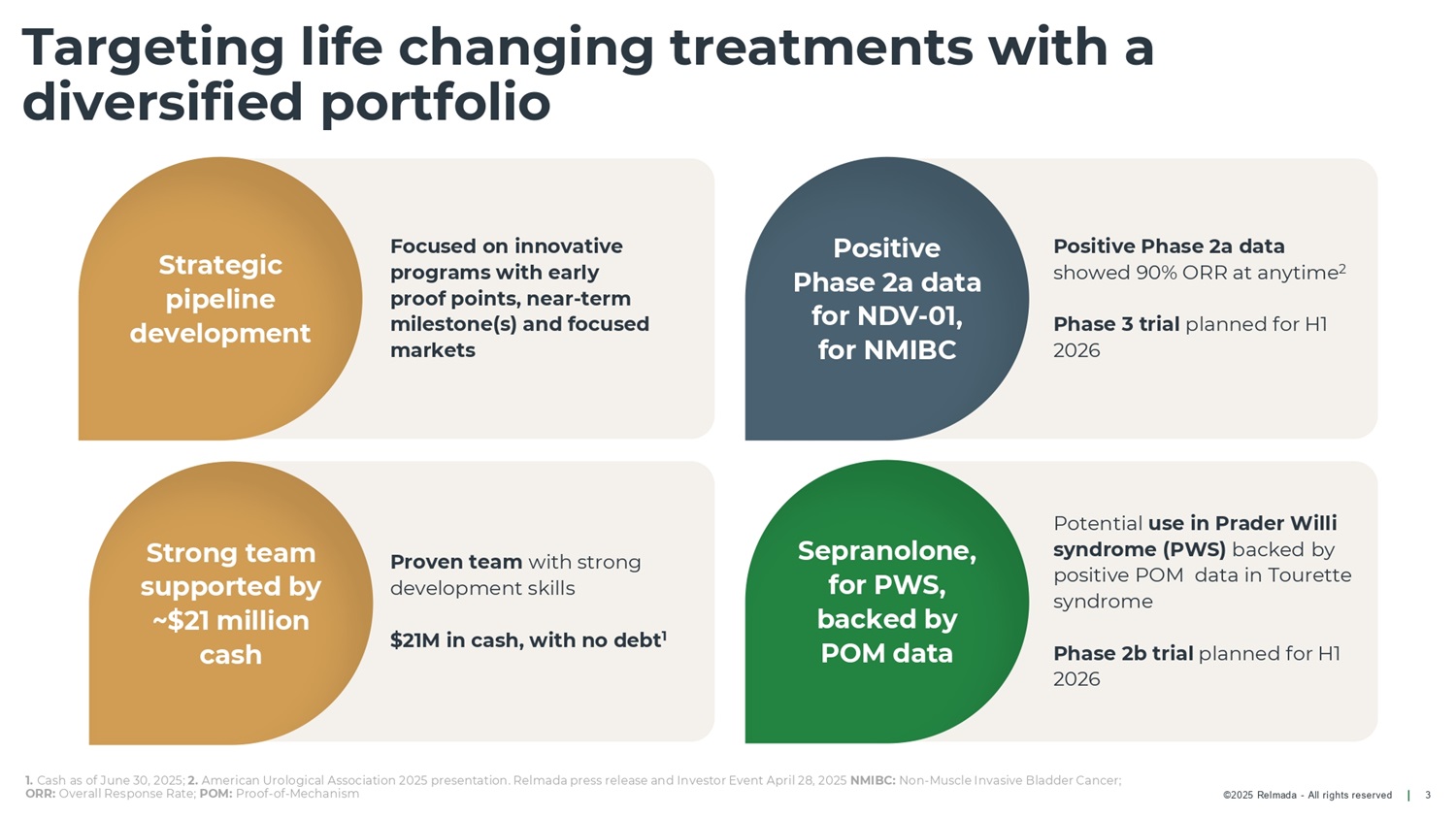
©2025 Relmada - All rights reserved Potential use in Prader Willi syndrome (PWS) backed by positive POM data in Tourette syndrome Phase 2b trial planned for H1 2026 Positive Phase 2a data showed 90% ORR at anytime 2 Phase 3 trial planned for H1 2026 Proven team with strong development skills $21M in cash, with no debt 1 Targeting life changing treatments with a diversified portfolio 3 Focused on innovative programs with early proof points, near - term milestone(s) and focused markets Strategic pipeline development Positive Phase 2a data for NDV - 01, for NMIBC Sepranolone, for PWS, backed by POM data Strong team supported by ~$21 million cash 1. Cash as of June 30, 2025; 2. American Urological Association 2025 presentation. Relmada press release and Investor Event April 28, 2025 NMIBC: Non - Muscle Invasive Bladder Cancer; ORR: Overall Response Rate; POM: Proof - of - Mechanism
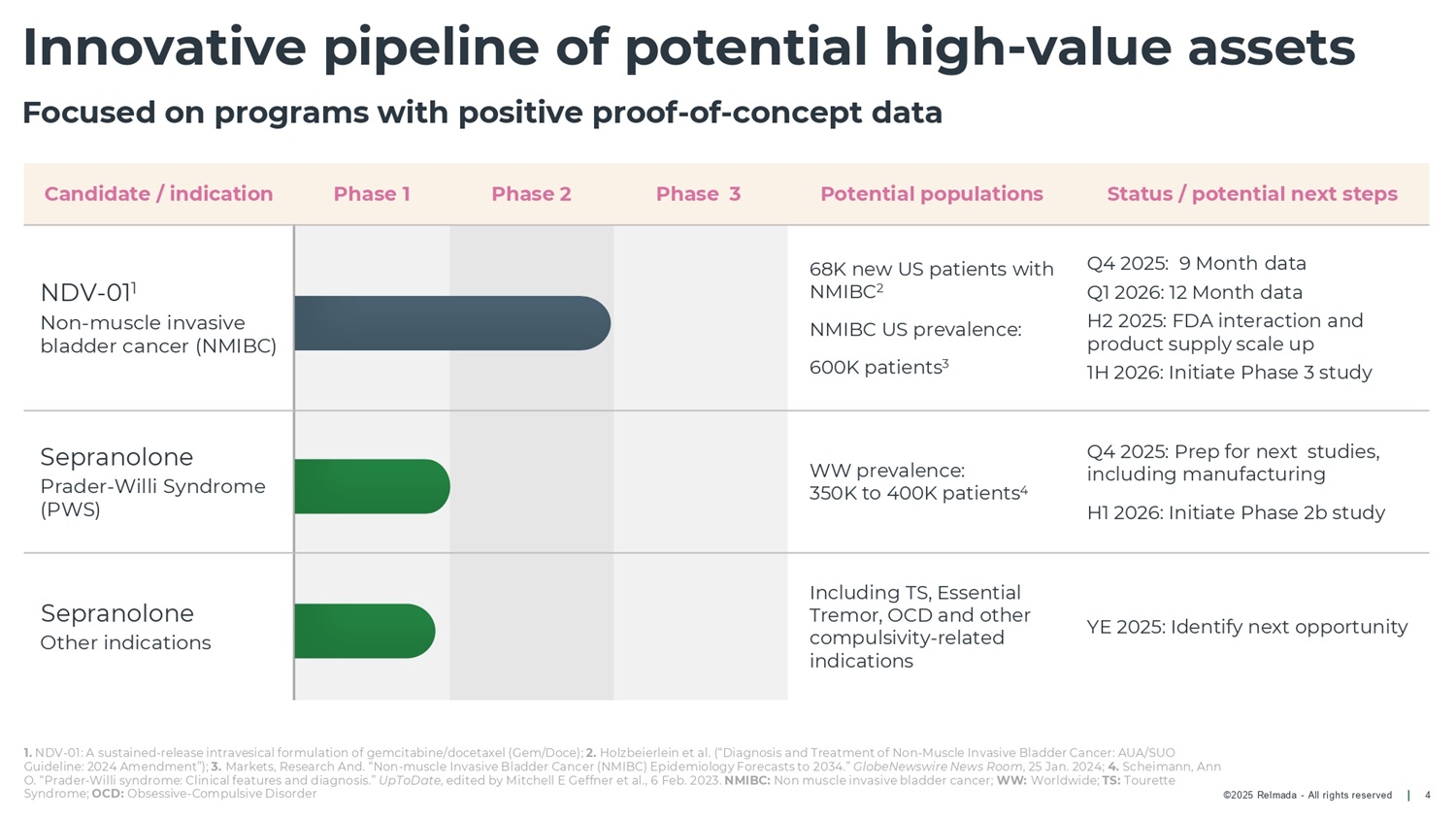
©2025 Relmada - All rights reserved Innovative pipeline of potential high - value assets 4 Focused on programs with positive proof - of - concept data Status / potential next steps Potential populations Phase 3 Phase 2 Phase 1 Candidate / indication Q4 2025: 9 Month data Q1 2026: 12 Month data H2 2025: FDA interaction and product supply scale up 1H 2026: Initiate Phase 3 study 68K new US patients with NMIBC 2 NMIBC US prevalence: 600K patients 3 NDV - 01 1 Non - muscle invasive bladder cancer (NMIBC) Q4 2025: Prep for next studies, including manufacturing H1 2026: Initiate Phase 2b study WW prevalence: 350K to 400K patients 4 Sepranolone Prader - Willi Syndrome (PWS) YE 2025: Identify next opportunity Including TS, Essential Tremor, OCD and other compulsivity - related indications Sepranolone Other indications 1. NDV - 01: A sustained - release intravesical formulation of gemcitabine/docetaxel (Gem/Doce); 2. Holzbeierlein et al. (“Diagnosis and Treatment of Non - Muscle Invasive Bladder Cancer: AUA/SUO Guideline: 2024 Amendment”); 3. Markets, Research And. “Non - muscle Invasive Bladder Cancer (NMIBC) Epidemiology Forecasts to 2034.” GlobeNewswire News Room , 25 Jan. 2024; 4. Scheimann, Ann O. “Prader - Willi syndrome: Clinical features and diagnosis.” UpToDate , edited by Mitchell E Geffner et al., 6 Feb. 2023. NMIBC: Non muscle invasive bladder cancer; WW: Worldwide; TS: Tourette Syndrome; OCD: Obsessive - Compulsive Disorder

©2025 Relmada - All rights reserved 5 NDV - 01 A sustained - release intravesical formulation of gemcitabine/docetaxel (Gem/Doce) for patients with NMIBC, with positive Phase 2a data 1 1. American Urological Association 2025 presentation. Relmada press release and Investor Event April 28, 2025 NMIBC: Non - Muscle Invasive Bladder. The graphic is for artistic purposes only, not a factual representation
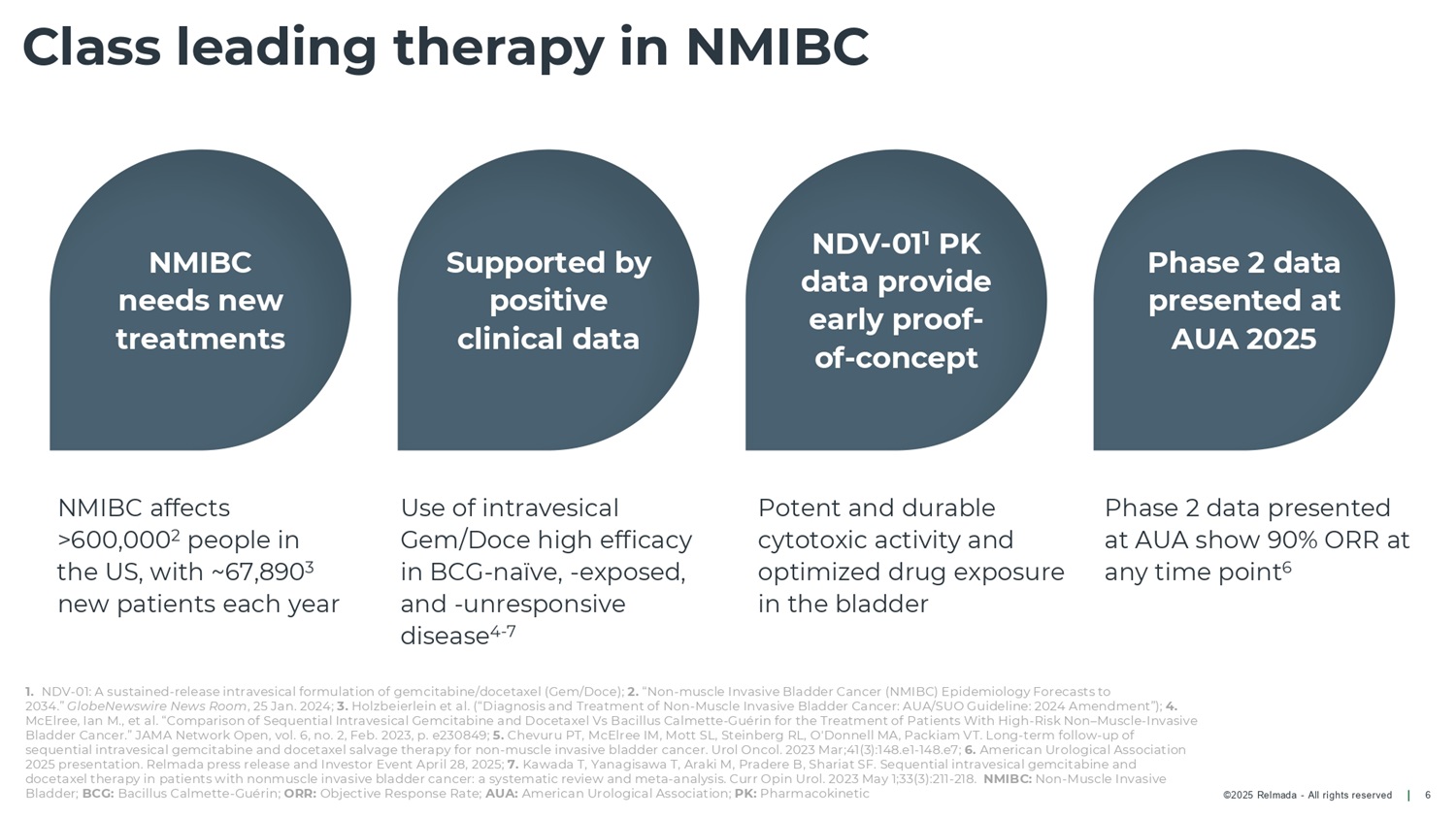
©2025 Relmada - All rights reserved Class leading therapy in NMIBC 6 Use of intravesical Gem/Doce high efficacy in BCG - naïve, - exposed, and - unresponsive disease 4 - 7 Phase 2 data presented at AUA show 90% ORR at any time point 6 Potent and durable cytotoxic activity and optimized drug exposure in the bladder NMIBC needs new treatments Supported by positive clinical data NDV - 01 1 PK data provide early proof - of - concept Phase 2 data presented at AUA 2025 1. NDV - 01: A sustained - release intravesical formulation of gemcitabine/docetaxel (Gem/Doce); 2. “Non - muscle Invasive Bladder Cancer (NMIBC) Epidemiology Forecasts to 2034.” GlobeNewswire News Room , 25 Jan. 2024; 3. Holzbeierlein et al. (“Diagnosis and Treatment of Non - Muscle Invasive Bladder Cancer: AUA/SUO Guideline: 2024 Amendment”); 4. McElree, Ian M., et al. “Comparison of Sequential Intravesical Gemcitabine and Docetaxel Vs Bacillus Calmette - Guérin for the Treatment of Patients With High - Risk Non – Muscle - Invasive Bladder Cancer.” JAMA Network Open, vol. 6, no. 2, Feb. 2023, p. e230849; 5. Chevuru PT, McElree IM, Mott SL, Steinberg RL, O'Donnell MA, Packiam VT. Long - term follow - up of sequential intravesical gemcitabine and docetaxel salvage therapy for non - muscle invasive bladder cancer. Urol Oncol. 2023 Mar;41(3):148.e1 - 148.e7; 6. American Urological Association 2025 presentation. Relmada press release and Investor Event April 28, 2025; 7. Kawada T, Yanagisawa T, Araki M, Pradere B, Shariat SF. Sequential intravesical gemcitabine and docetaxel therapy in patients with nonmuscle invasive bladder cancer: a systematic review and meta - analysis. Curr Opin Urol. 2023 May 1;33(3):211 - 218. NMIBC: Non - Muscle Invasive Bladder; BCG: Bacillus Calmette - Guérin; ORR: Objective Response Rate; AUA: American Urological Association; PK: Pharmacokinetic NMIBC affects >600,000 2 people in the US, with ~67,890 3 new patients each year
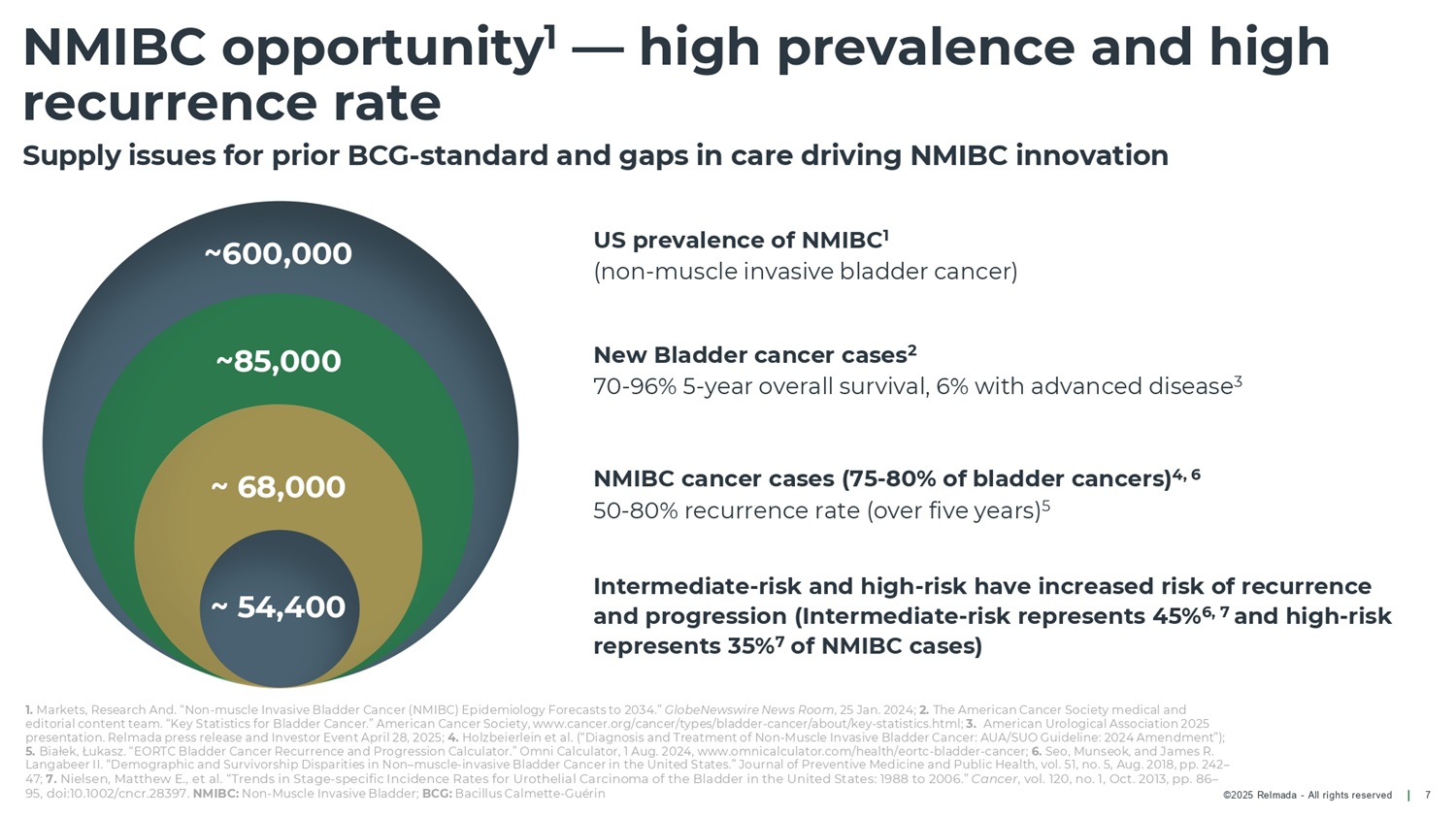
©2025 Relmada - All rights reserved NMIBC opportunity 1 — high prevalence and high recurrence rate Supply issues for prior BCG - standard and gaps in care driving NMIBC innovation 7 New Bladder cancer cases 2 70 - 96% 5 - year overall survival, 6% with advanced disease 3 NMIBC cancer cases (75 - 80% of bladder cancers) 4, 6 50 - 80% recurrence rate (over five years) 5 US prevalence of NMIBC 1 (non - muscle invasive bladder cancer) Intermediate - risk and high - risk have increased risk of recurrence and progression (Intermediate - risk represents 45% 6, 7 and high - risk represents 35% 7 of NMIBC cases) ~ 68,000 ~85,000 ~600,000 ~ 54,400 1. Markets, Research And. “Non - muscle Invasive Bladder Cancer (NMIBC) Epidemiology Forecasts to 2034.” GlobeNewswire News Room , 25 Jan. 2024; 2. The American Cancer Society medical and editorial content team. “Key Statistics for Bladder Cancer.” American Cancer Society, www.cancer.org/cancer/types/bladder - cancer/about/key - statistics.html; 3. American Urological Association 2025 presentation. Relmada press release and Investor Event April 28, 2025; 4. Holzbeierlein et al. (“Diagnosis and Treatment of Non - Muscle Invasive Bladder Cancer: AUA/SUO Guideline: 2024 Amendment”); 5. Białek, Łukasz. “EORTC Bladder Cancer Recurrence and Progression Calculator.” Omni Calculator, 1 Aug. 2024, www.omnicalculator.com/health/eortc - bladder - cancer; 6. Seo, Munseok, and James R. Langabeer II. “Demographic and Survivorship Disparities in Non – muscle - invasive Bladder Cancer in the United States.” Journal of Preventive Medicine and Public Health, vol. 51, no. 5, Aug. 2018, pp. 242 – 47; 7. Nielsen, Matthew E., et al. “Trends in Stage-specific Incidence Rates for Urothelial Carcinoma of the Bladder in the United States: 1988 to 2006.” Cancer , vol. 120, no. 1, Oct. 2013, pp. 86 – 95, doi:10.1002/cncr.28397. NMIBC: Non - Muscle Invasive Bladder; BCG: Bacillus Calmette - Guérin
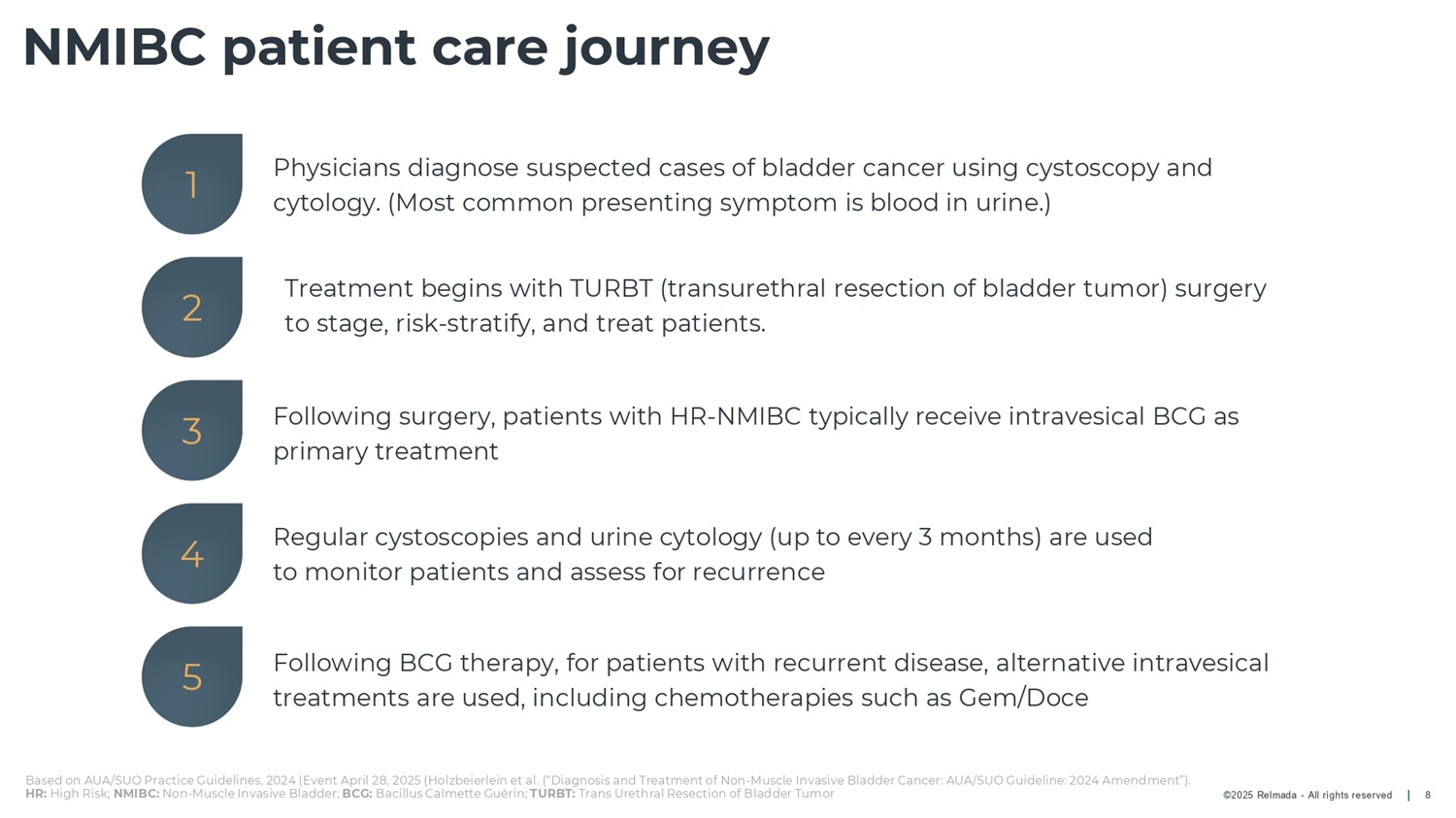
©2025 Relmada - All rights reserved NMIBC patient care journey 8 Treatment begins with TURBT (transurethral resection of bladder tumor) surgery to stage, risk - stratify, and treat patients. Following surgery, patients with HR - NMIBC typically receive intravesical BCG as primary treatment Physicians diagnose suspected cases of bladder cancer using cystoscopy and cytology. (Most common presenting symptom is blood in urine.) Following BCG therapy, for patients with recurrent disease, alternative intravesical treatments are used, including chemotherapies such as Gem/Doce Regular cystoscopies and urine cytology (up to every 3 months) are used to monitor patients and assess for recurrence 1 2 3 4 5 Based on AUA/SUO Practice Guidelines, 2024 (Event April 28, 2025 (Holzbeierlein et al. (“Diagnosis and Treatment of Non - Muscle Invasive Bladder Cancer: AUA/SUO Guideline: 2024 Amendment”). HR: High Risk; NMIBC: Non - Muscle Invasive Bladder; BCG: Bacillus Calmette Guérin; TURBT: Trans Urethral Resection of Bladder Tumor
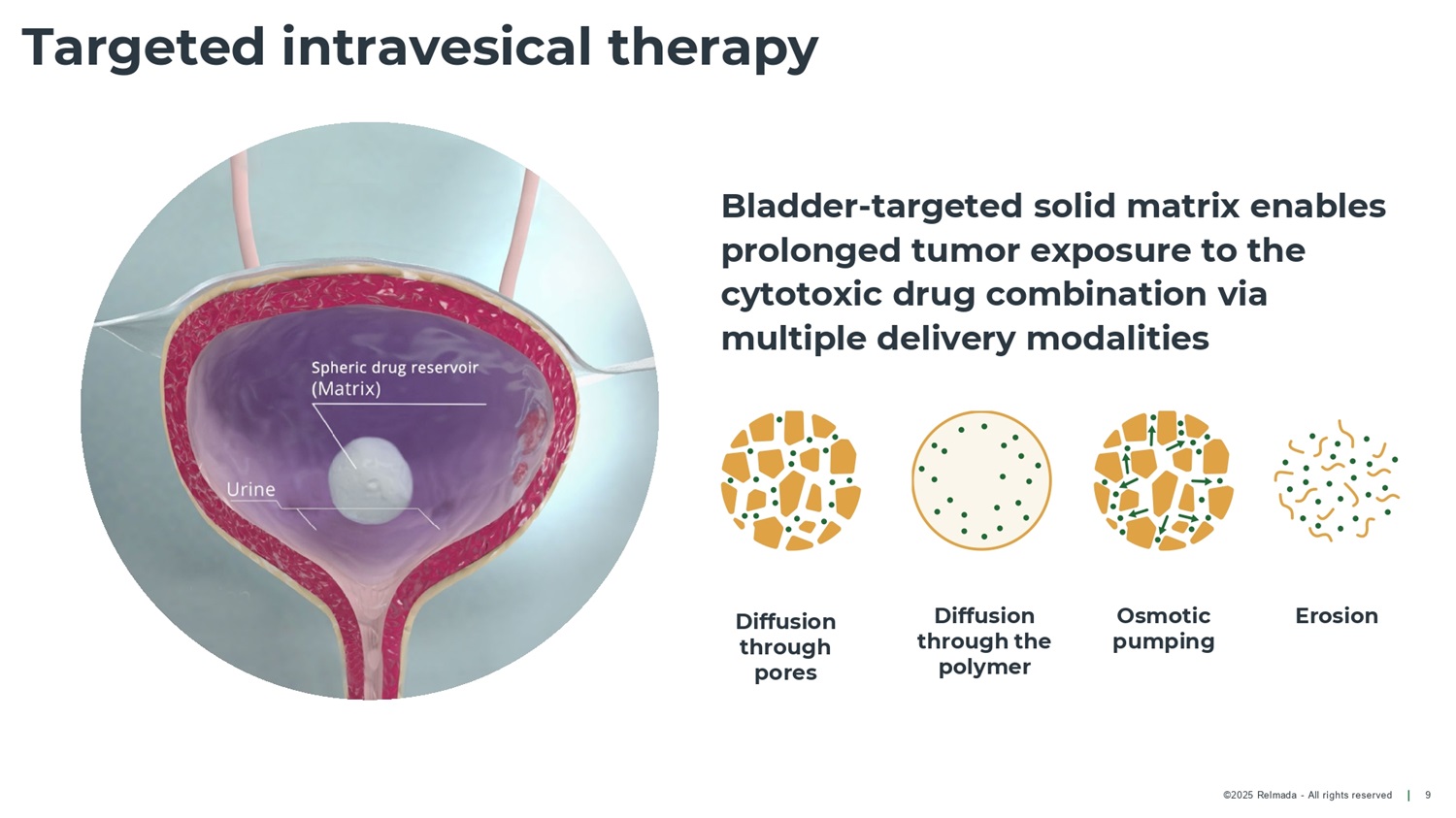
©2025 Relmada - All rights reserved Targeted intravesical therapy 9 Diffusion through pores Diffusion through the polymer Osmotic pumping Erosion Bladder - targeted solid matrix enables prolonged tumor exposure to the cytotoxic drug combination via multiple delivery modalities
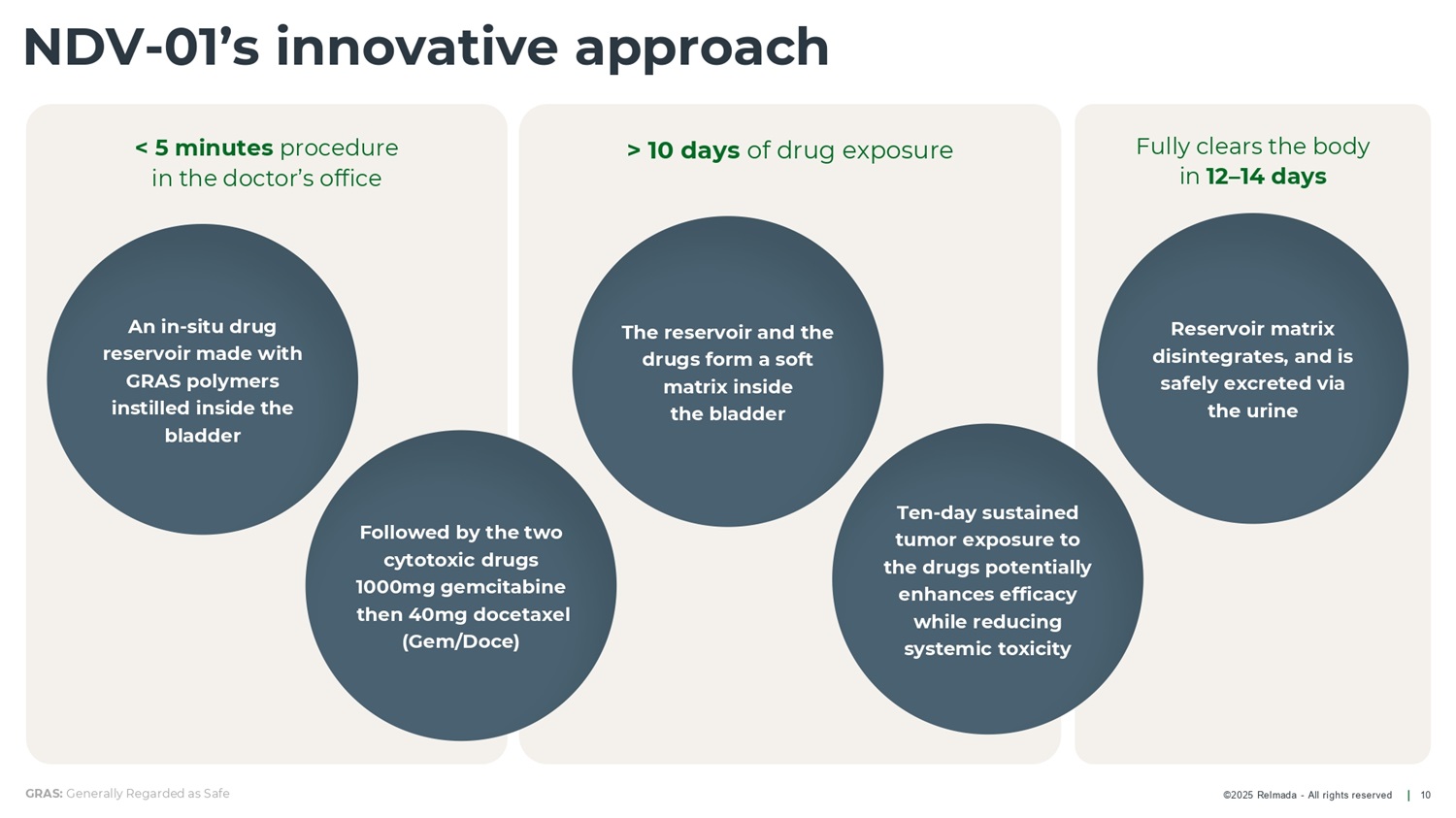
©2025 Relmada - All rights reserved > 10 days of drug exposure Fully clears the body in 12 – 14 days < 5 minutes procedure in the doctor’s office NDV - 01’s innovative approach 10 GRAS: Generally Regarded as Safe Reservoir matrix disintegrates, and is safely excreted via the urine Followed by the two cytotoxic drugs 1000mg gemcitabine then 40mg docetaxel (Gem/Doce) An in - situ drug reservoir made with GRAS polymers instilled inside the bladder The reservoir and the drugs form a soft matrix inside the bladder Ten - day sustained tumor exposure to the drugs potentially enhances efficacy while reducing systemic toxicity

©2025 Relmada - All rights reserved Gem/Doce combination has been embraced by the urologic oncology community 11 Effective salvage treatment for patients who have failed or are intolerant to BCG with reported 2 - year RFS ~50% 1, 2, 3 Gem/Doce is an effective alternative first - line agent in high - risk BCG naïve patients with 2 - year RFS of 82% 4 Gem/Doce use expanding into intermediate - risk and low - grade tumors with reported 2 - year RFS of 70 - 80% 5, 6 Gem/Doce avoids/delays radical cystectomy 7, 8 Large ongoing cooperative “BRIDGE” study (n=870) evaluating Gem/Doce combination v. BCG (NCT05538663) 1. Steinberg RL, Thomas LJ, Brooks N, et al. Multi - Institution Evaluation of Sequential Gemcitabine/Docetaxel as Rescue Therapy for NMIBC. J Urol. 2020; 2. Garneau CA, Marcotte N, Lacombe L, et al. Salvage therapy for BCG failure with intravesical sequential Gem/Doce in patients with recurrent NMIBC. Can Urol Assoc J J Assoc Urol Can. 2024; 3. Yim K, Melnick K, Mott SL, et al. Sequential intravesical gemcitabine/docetaxel provides a durable remission in recurrent high - risk NMIBC following BCG therapy. Urol Oncol. 2023; 4. McElree IM, Steinberg RL, Martin AC, et al. Sequential Intravesical gemcitabine/docetaxel for BCG - Naïve High - Risk NMIBC. J Urol. 2022; 5. McElree IM, Orzel J, Stubbee R, et al. Sequential intravesical gemcitabine/docetaxel for treatment - naïve and previously treated intermediate - risk NMIBC. Urol Oncol. 2023; 6. Tan WS, McElree IM, Davaro F, et al. Sequential Intravesical Gemcitabine/Docetaxel is an Alternative to BCG for the Treatment of Intermediate - risk NMIBC. Eur Urol Oncol. 2023; 7. Chevuru PT, McElree IM, Mott SL, Steinberg RL, O’Donnell MA, Packiam VT. Long - term follow - up of sequential intravesical gemcitabine and docetaxel salvage therapy for NMIBC. Urol Oncol. 2023; 8. Narayan VM, Boorjian SA, Alemozaffar M, et al. Efficacy of Intravesical Nadofaragene Firadenovec for Patients With BCG - Unresponsive NMIBC: 5 - Year Follow - Up From a Phase 3 Trial. J Urol. 2024. RFS: Relapse Free Survival; BCG: Bacillus Calmette - Guérin; NMIBC: Non - muscle - Invasive Bladder Cancer
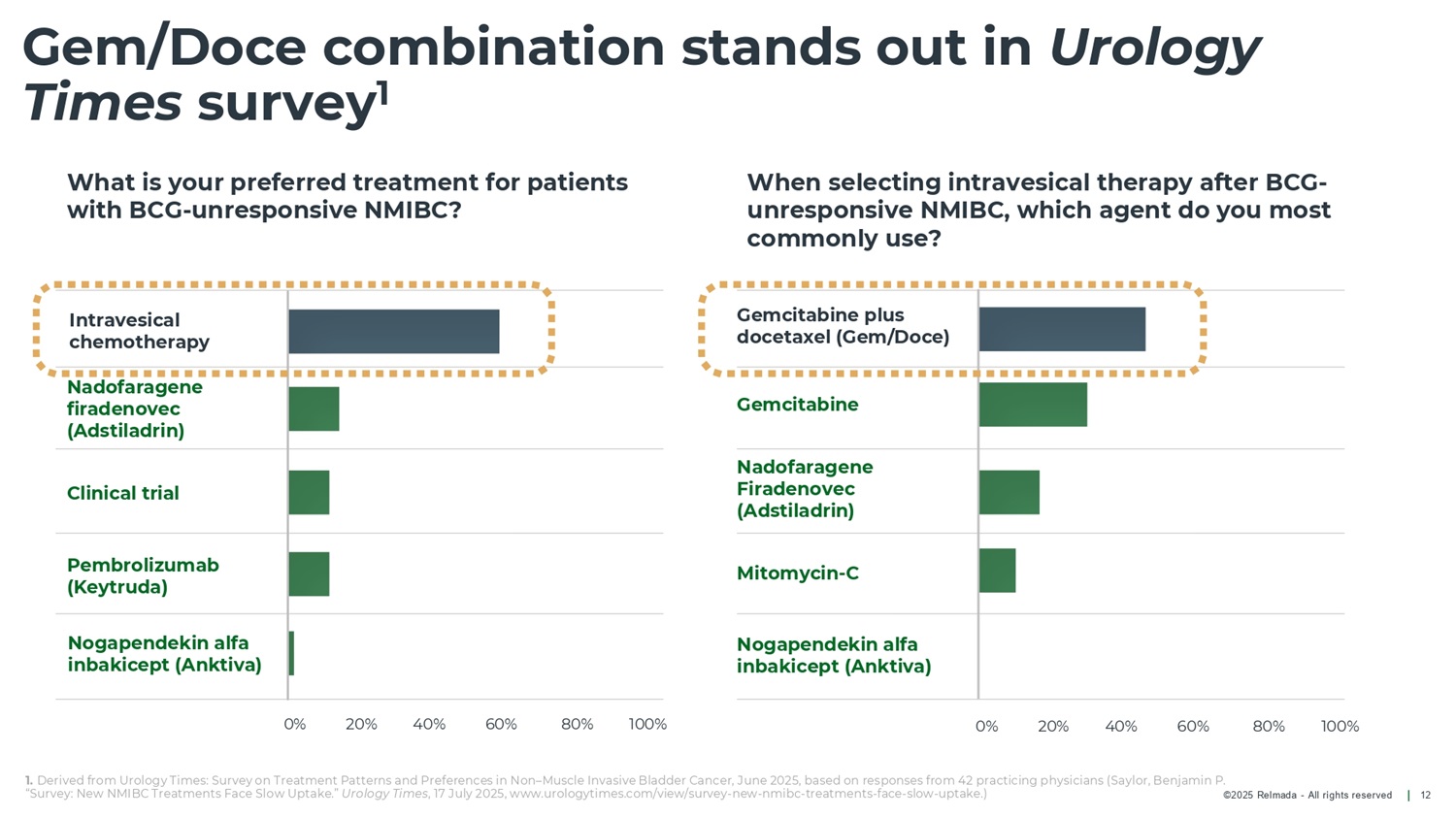
©2025 Relmada - All rights reserved Gem/Doce combination stands out in Urology Times survey 1 12 What is your preferred treatment for patients with BCG - unresponsive NMIBC? When selecting intravesical therapy after BCG - unresponsive NMIBC, which agent do you most commonly use? 1. Derived from Urology Times: Survey on Treatment Patterns and Preferences in Non – Muscle Invasive Bladder Cancer, June 2025, based on responses from 42 practicing physicians (Saylor, Benjamin P. “Survey: New NMIBC Treatments Face Slow Uptake.” Urology Times , 17 July 2025, www.urologytimes.com/view/survey - new - nmibc - treatments - face - slow - uptake.) Clinical trial Nogapendekin alfa inbakicept (Anktiva) Pembrolizumab (Keytruda) Intravesical chemotherapy Mitomycin - C Nogapendekin alfa inbakicept (Anktiva) Nadofaragene Firadenovec (Adstiladrin) Gemcitabine plus docetaxel (Gem/Doce) Gemcitabine Nadofaragene firadenovec (Adstiladrin) 0% 20% 40% 60% 80% 100% 0% 20% 40% 60% 80% 100%
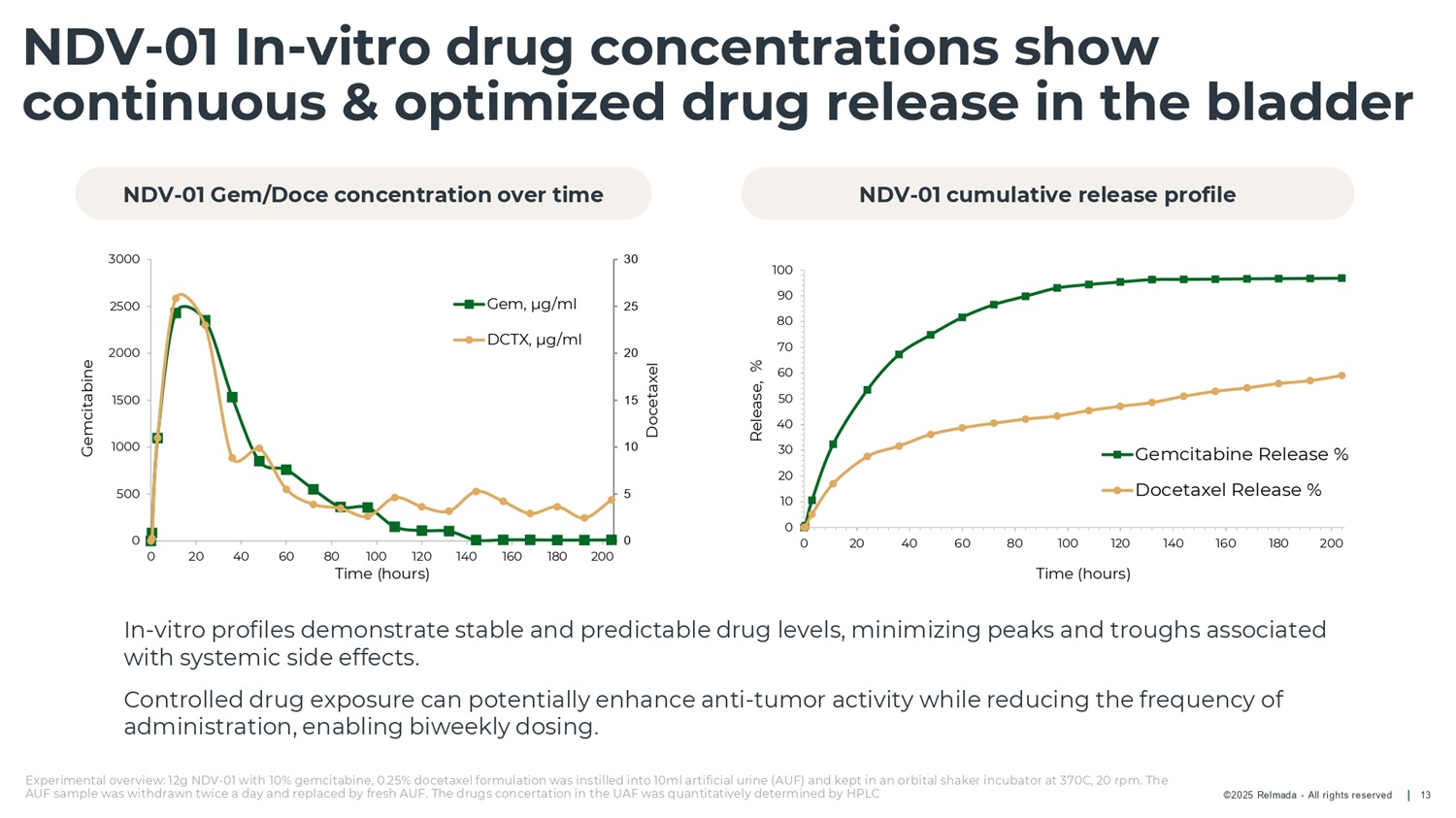
©2025 Relmada - All rights reserved NDV - 01 In - vitro drug concentrations show continuous & optimized drug release in the bladder 13 In - vitro profiles demonstrate stable and predictable drug levels, minimizing peaks and troughs associated with systemic side effects. Controlled drug exposure can potentially enhance anti - tumor activity while reducing the frequency of administration, enabling biweekly dosing. 100 90 80 70 60 50 40 30 20 10 0 0 20 40 60 80 100 120 Time (hours) 140 160 180 200 Release, % Gemcitabine Release % Docetaxel Release % 0 5 10 15 20 25 30 0 500 1000 1500 2000 2500 3000 0 20 40 60 80 100 120 140 160 180 200 Time (hours) Docetaxel Gemcitabine Gem, µg/ml DCTX, µg/ml NDV - 01 Gem/Doce concentration over time NDV - 01 cumulative release profile Experimental overview: 12g NDV - 01 with 10% gemcitabine, 0.25% docetaxel formulation was instilled into 10ml artificial urine (AUF) and kept in an orbital shaker incubator at 370C, 20 rpm. The AUF sample was withdrawn twice a day and replaced by fresh AUF. The drugs concertation in the UAF was quantitatively determined by HPLC
©2025 Relmada - All rights reserved 14 Study TRCG - 011 high - risk NMIBC patients An open - label, single - arm, single - center study to evaluate safety and efficacy of NDV - 01 in HR NMIBC patients (NCT06663137) American Urological Association 2025 presentation. Relmada press release and Investor Event April 28, 2025. HR: High Risk; NMIBC: Non muscle invasive bladder cancer
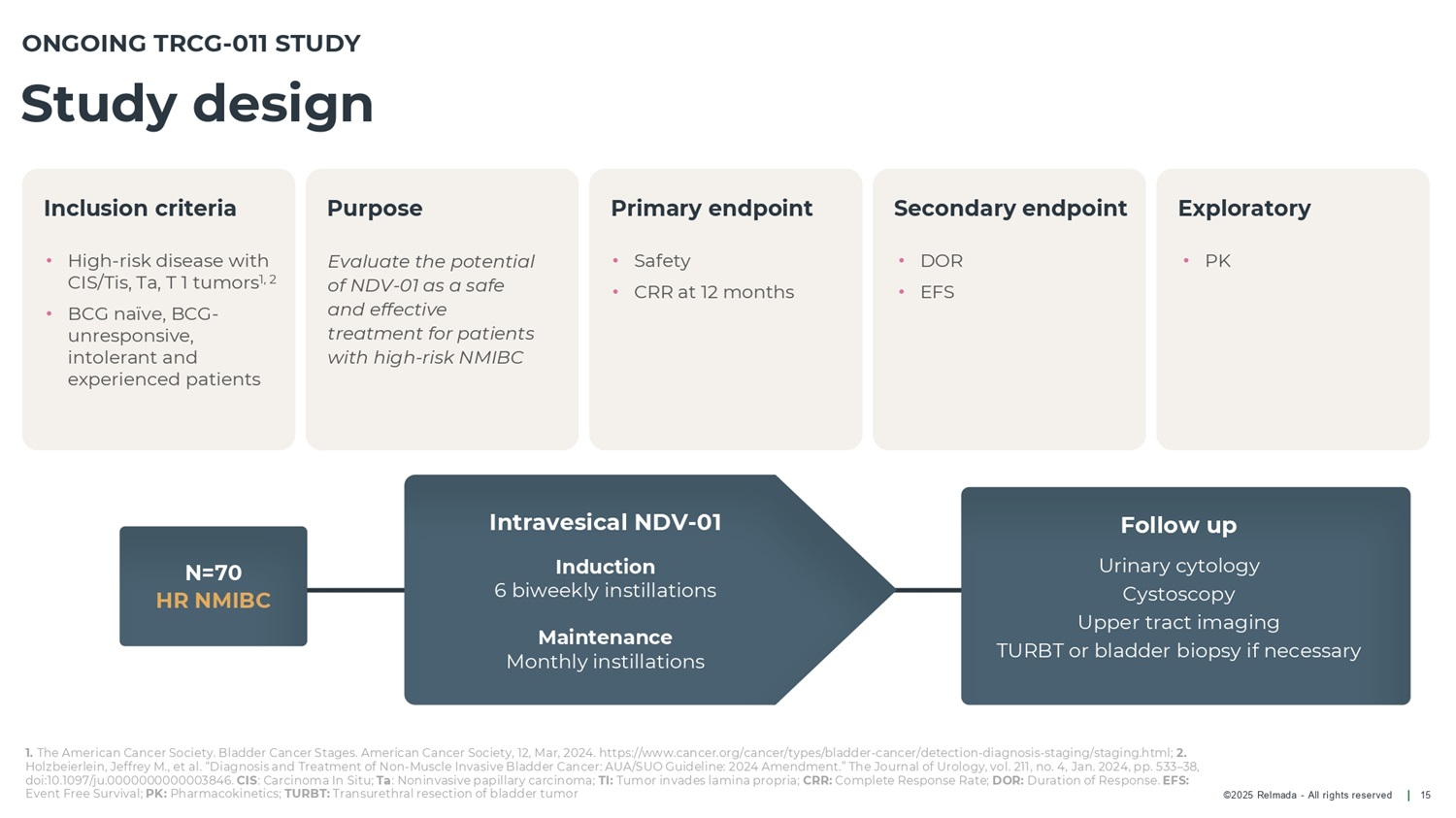
©2025 Relmada - All rights reserved Inclusion criteria Purpose Primary endpoint Secondary endpoint Exploratory Study design 15 N=70 HR NMIBC • High - risk disease with CIS/Tis, Ta, T 1 tumors 1, 2 • BCG naïve, BCG - unresponsive, intolerant and experienced patients Evaluate the potential of NDV - 01 as a safe and effective treatment for patients with high - risk NMIBC • Safety • CRR at 12 months • DOR • EFS • PK ONGOING TRCG - 011 STUDY Follow up Urinary cytology Cystoscopy Upper tract imaging TURBT or bladder biopsy if necessary Intravesical NDV - 01 Induction 6 biweekly instillations Maintenance Monthly instillations 1. The American Cancer Society. Bladder Cancer Stages. American Cancer Society, 12, Mar, 2024. https:// www.cancer.org/cancer/types/bladder - cancer/detection - diagnosis - staging/staging.html; 2. Holzbeierlein, Jeffrey M., et al. “Diagnosis and Treatment of Non - Muscle Invasive Bladder Cancer: AUA/SUO Guideline: 2024 Amendment.” The Journal of Urology, vol. 211, no. 4, Jan. 2024, pp. 533 – 38, doi:10.1097/ju.0000000000003846. CIS : Carcinoma In Situ; Ta : Noninvasive papillary carcinoma; TI: Tumor invades lamina propria; CRR: Complete Response Rate; DOR: Duration of Response. EFS: Event Free Survival; PK: Pharmacokinetics; TURBT: Transurethral resection of bladder tumor
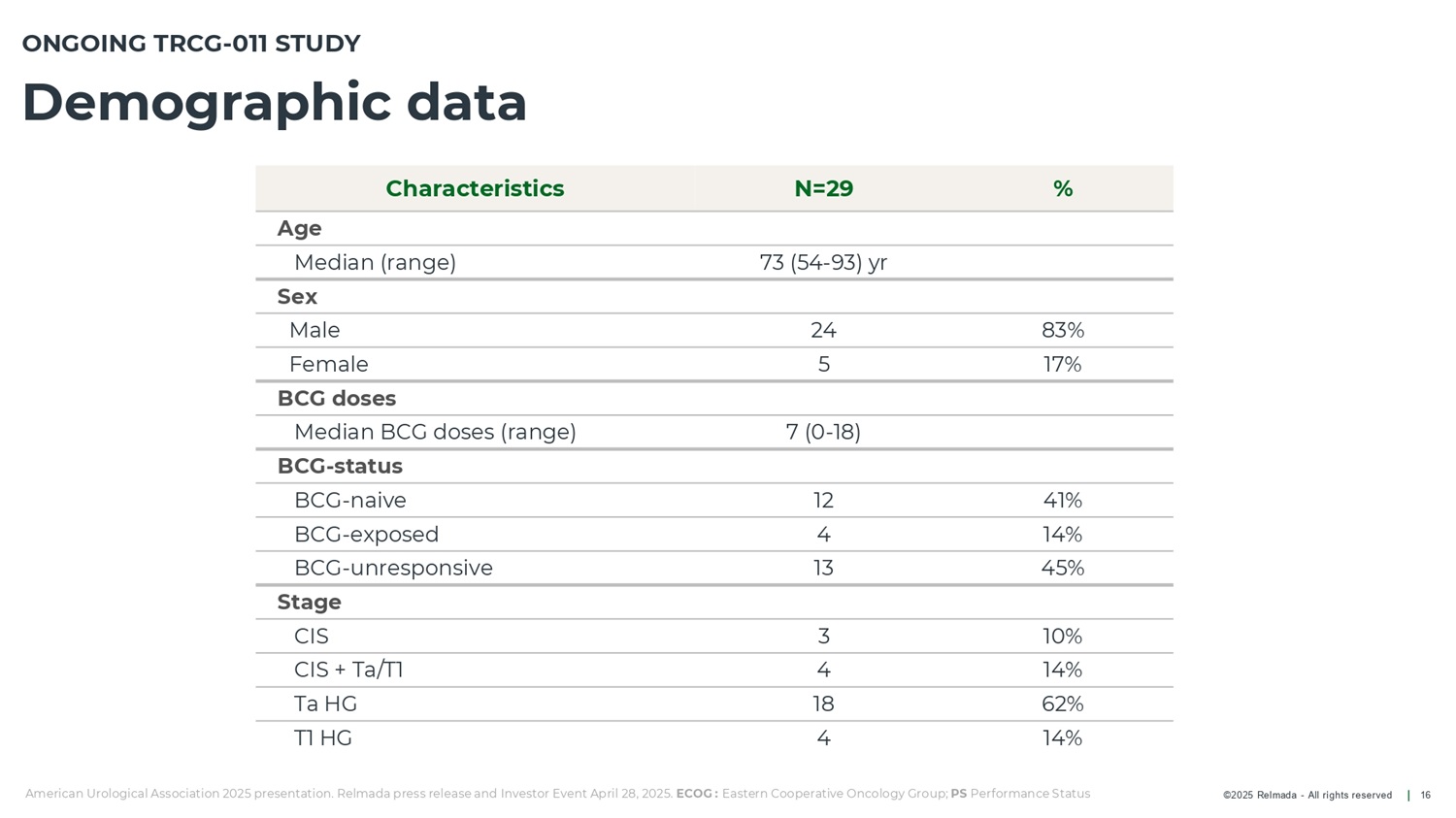
©2025 Relmada - All rights reserved ONGOING TRCG - 011 STUDY Demographic data 16 American Urological Association 2025 presentation. Relmada press release and Investor Event April 28, 2025. ECOG : Eastern Cooperative Oncology Group; PS Performance Status % N=29 Characteristics Age 73 (54 - 93) yr Median (range) Sex 83% 24 Male 17% 5 Female BCG doses 7 (0 - 18) Median BCG doses (range) BCG - status 41% 12 BCG - naive 14% 4 BCG - exposed 45% 13 BCG - unresponsive Stage 10% 3 CIS 14% 4 CIS + Ta/T1 62% 18 Ta HG 14% 4 T1 HG
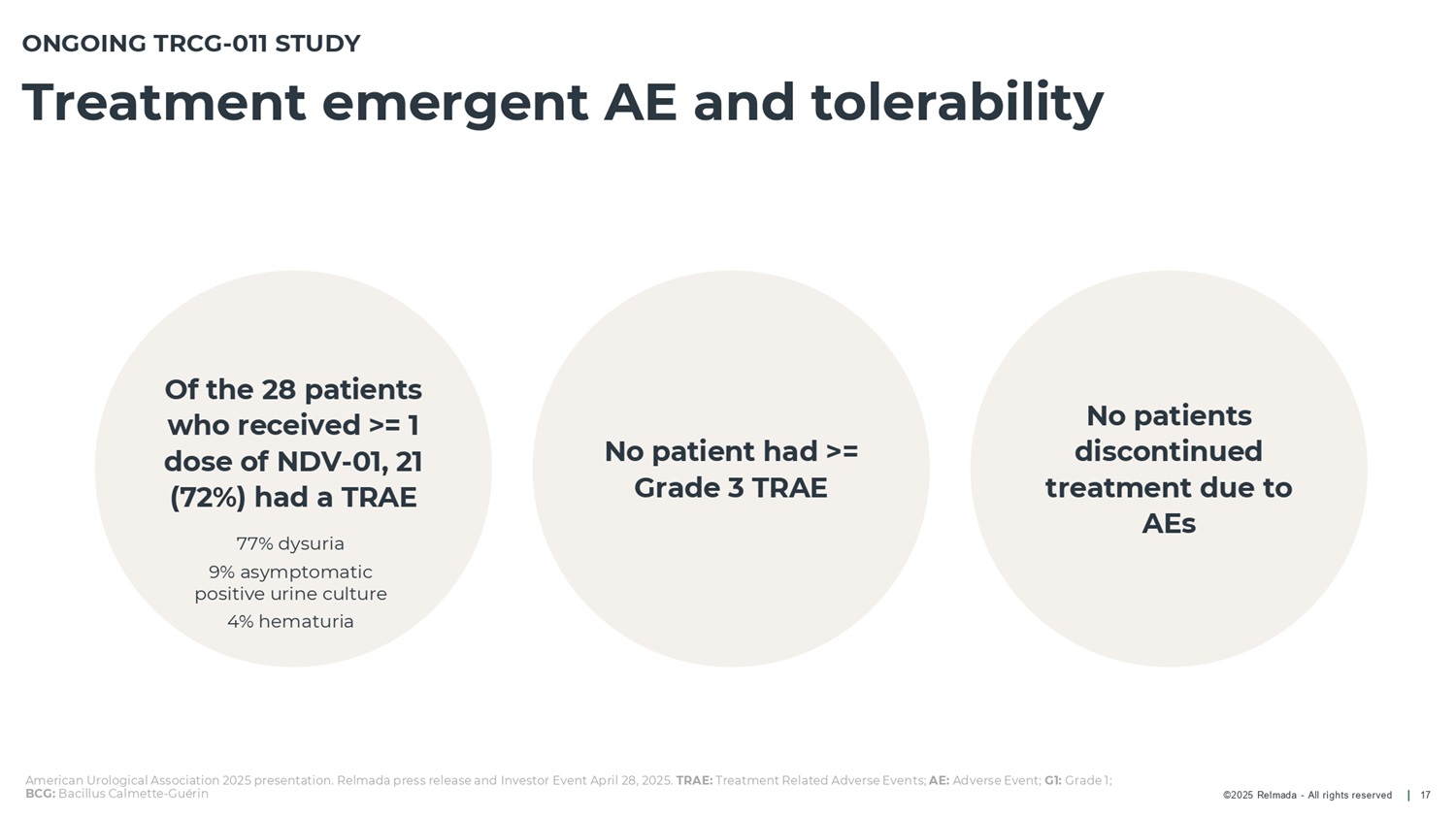
©2025 Relmada - All rights reserved Treatment emergent AE and tolerability 17 Of the 28 patients who received >= 1 dose of NDV - 01 , 21 ( 72 % ) had a TRAE 77% dysuria 9% asymptomatic positive urine culture 4% hematuria ONGOING TRCG - 011 STUDY No patient had >= Grade 3 TRAE American Urological Association 2025 presentation. Relmada press release and Investor Event April 28, 2025. TRAE: Treatment Related Adverse Events; AE: Adverse Event; G1: Grade 1; BCG: Bacillus Calmette - Guérin No patients discontinued treatment due to AEs
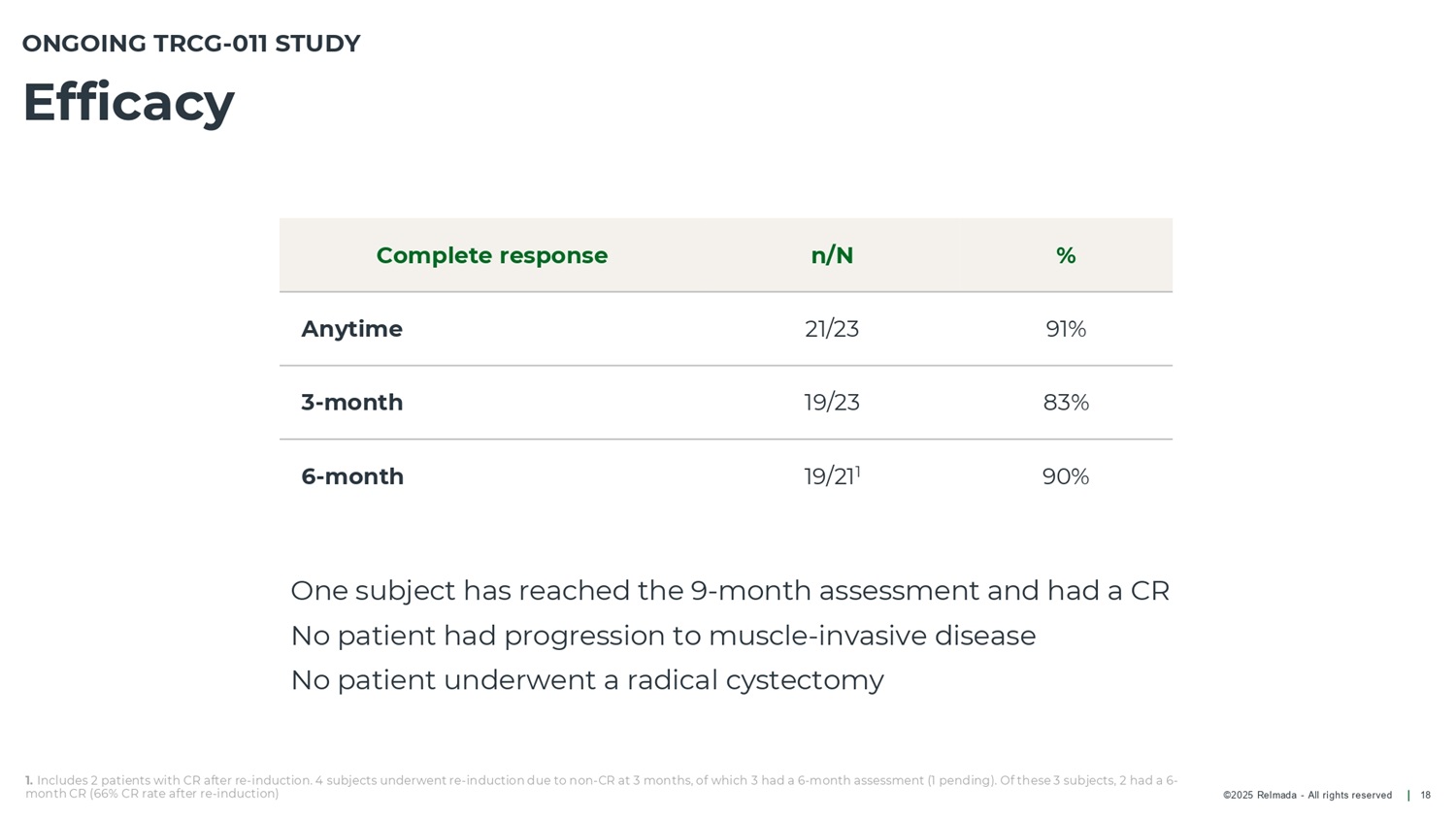
©2025 Relmada - All rights reserved Efficacy 18 ONGOING TRCG - 011 STUDY 1. Includes 2 patients with CR after re - induction. 4 subjects underwent re - induction due to non - CR at 3 months, of which 3 had a 6 - month assessment (1 pending). Of these 3 subjects, 2 had a 6 - month CR (66% CR rate after re - induction) % n/N Complete response 91% 21/23 Anytime 83% 19/23 3 - month 90% 19/21 1 6 - month One subject has reached the 9 - month assessment and had a CR No patient had progression to muscle - invasive disease No patient underwent a radical cystectomy
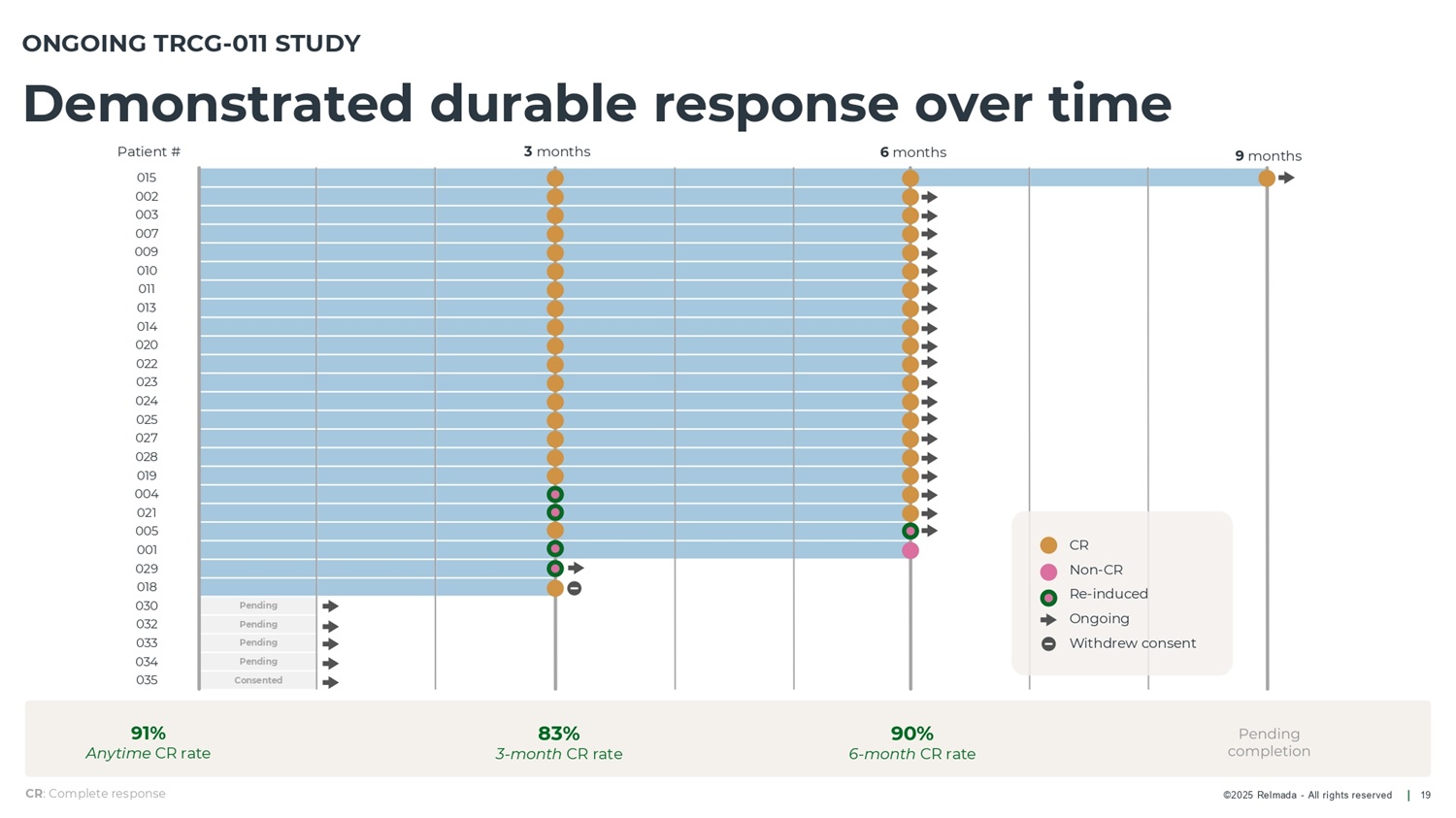
©2025 Relmada - All rights reserved Demonstrated durable response over time 19 ONGOING TRCG - 011 STUDY Pending Pending Pending Pending Consented 3 months 6 months 9 months CR : Complete response 91% Anytime CR rate 83% 3 - month CR rate 90% 6 - month CR rate CR Non - CR Re - induced Ongoing Withdrew consent Patient # 015 002 003 007 009 010 011 013 014 020 022 023 024 025 027 028 019 004 021 005 001 029 018 030 032 033 034 035
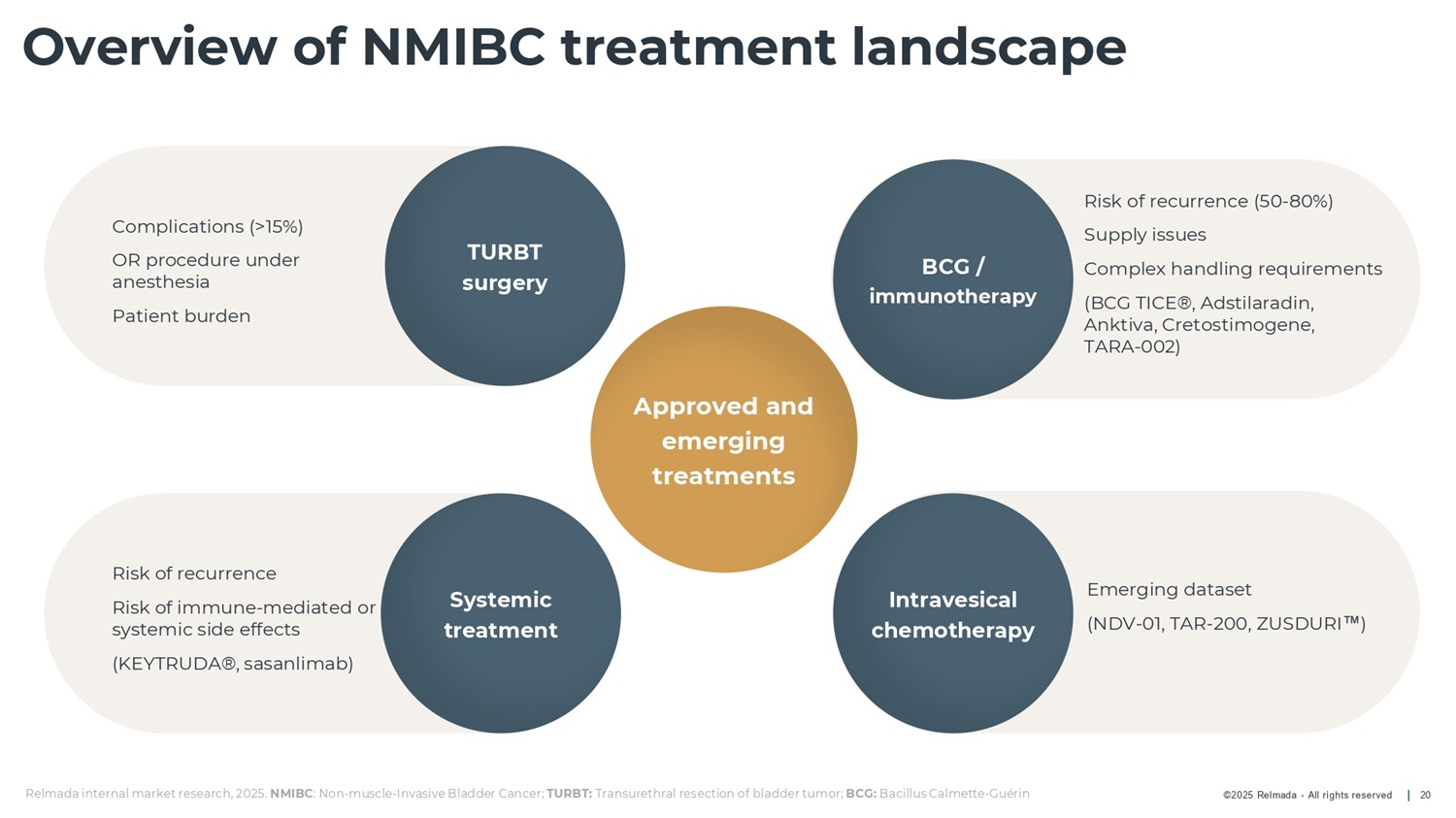
©2025 Relmada - All rights reserved Risk of recurrence (50 - 80%) Supply issues Complex handling requirements (BCG TICE®, Adstilaradin, Anktiva, Cretostimogene, TARA - 002 ) Emerging dataset (NDV - 01, TAR - 200, ZUSDURI ) Complications (>15%) OR procedure under anesthesia Patient burden Risk of recurrence Risk of immune - mediated or systemic side effects (KEYTRUDA®, sasanlimab) Intravesical chemotherapy Overview of NMIBC treatment landscape 20 Systemic treatment Approved and emerging treatments BCG / immunotherapy TURBT surgery Relmada internal market research, 2025. NMIBC : Non - muscle - Invasive Bladder Cancer; TURBT: Transurethral resection of bladder tumor; BCG: Bacillus Calmette - Guérin
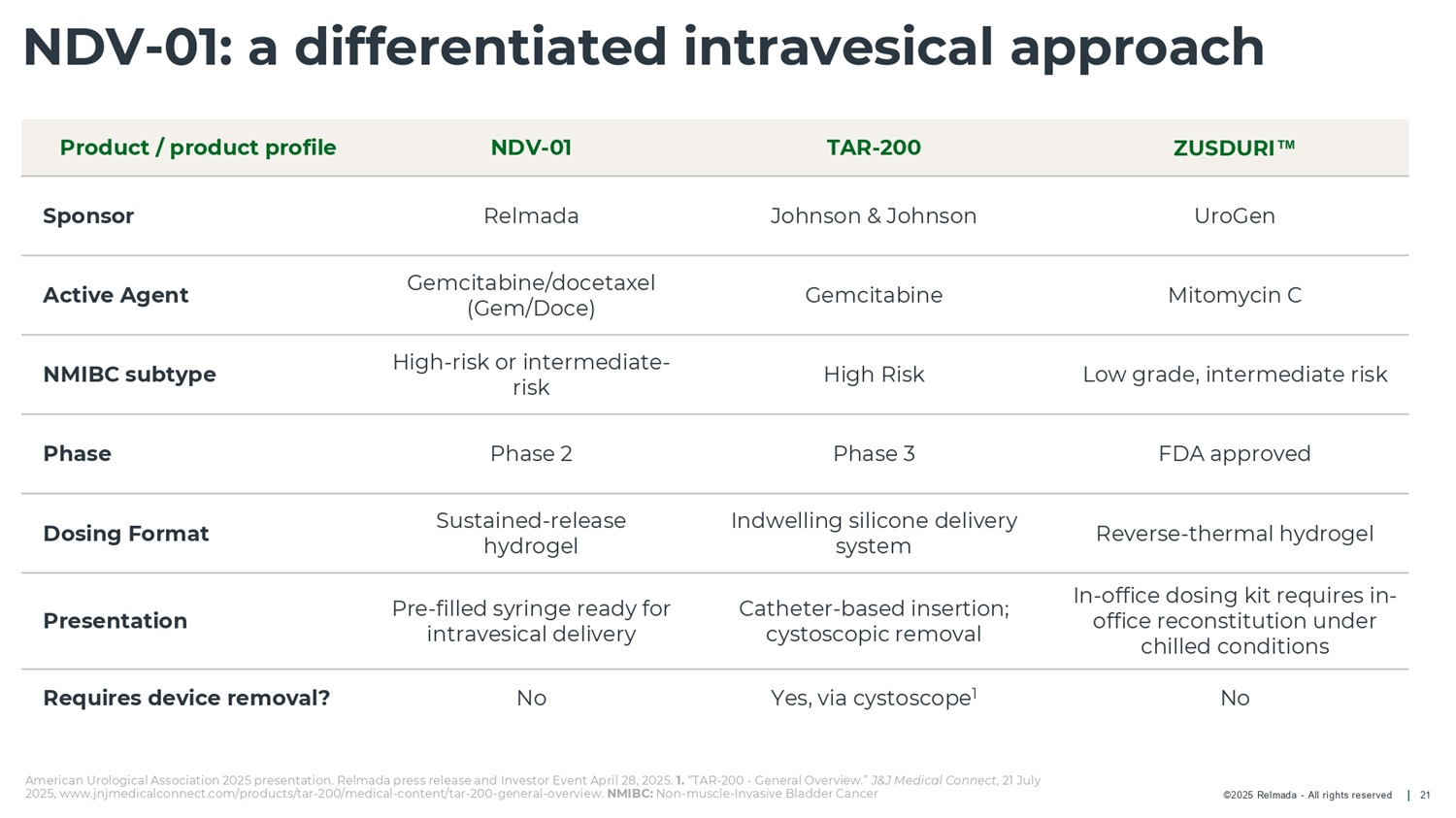
©2025 Relmada - All rights reserved NDV - 01: a differentiated intravesical approach 21 ZUSDURI TAR - 200 NDV - 01 Product / product profile UroGen Johnson & Johnson Relmada Sponsor Mitomycin C Gemcitabine Gemcitabine/docetaxel (Gem/Doce) Active Agent Low grade, intermediate risk High Risk High - risk or intermediate - risk NMIBC subtype FDA approved Phase 3 Phase 2 Phase Reverse - thermal hydrogel Indwelling silicone delivery system Sustained - release hydrogel Dosing Format In - office dosing kit requires in - office reconstitution under chilled conditions Catheter - based insertion; cystoscopic removal Pre - filled syringe ready for intravesical delivery Presentation No Yes, via cystoscope 1 No Requires device removal? American Urological Association 2025 presentation. Relmada press release and Investor Event April 28, 2025. 1. “TAR - 200 - General Overview.” J&J Medical Connect , 21 July 2025, www.jnjmedicalconnect.com/products/tar - 200/medical - content/tar - 200 - general - overview. NMIBC: Non - muscle - Invasive Bladder Cancer
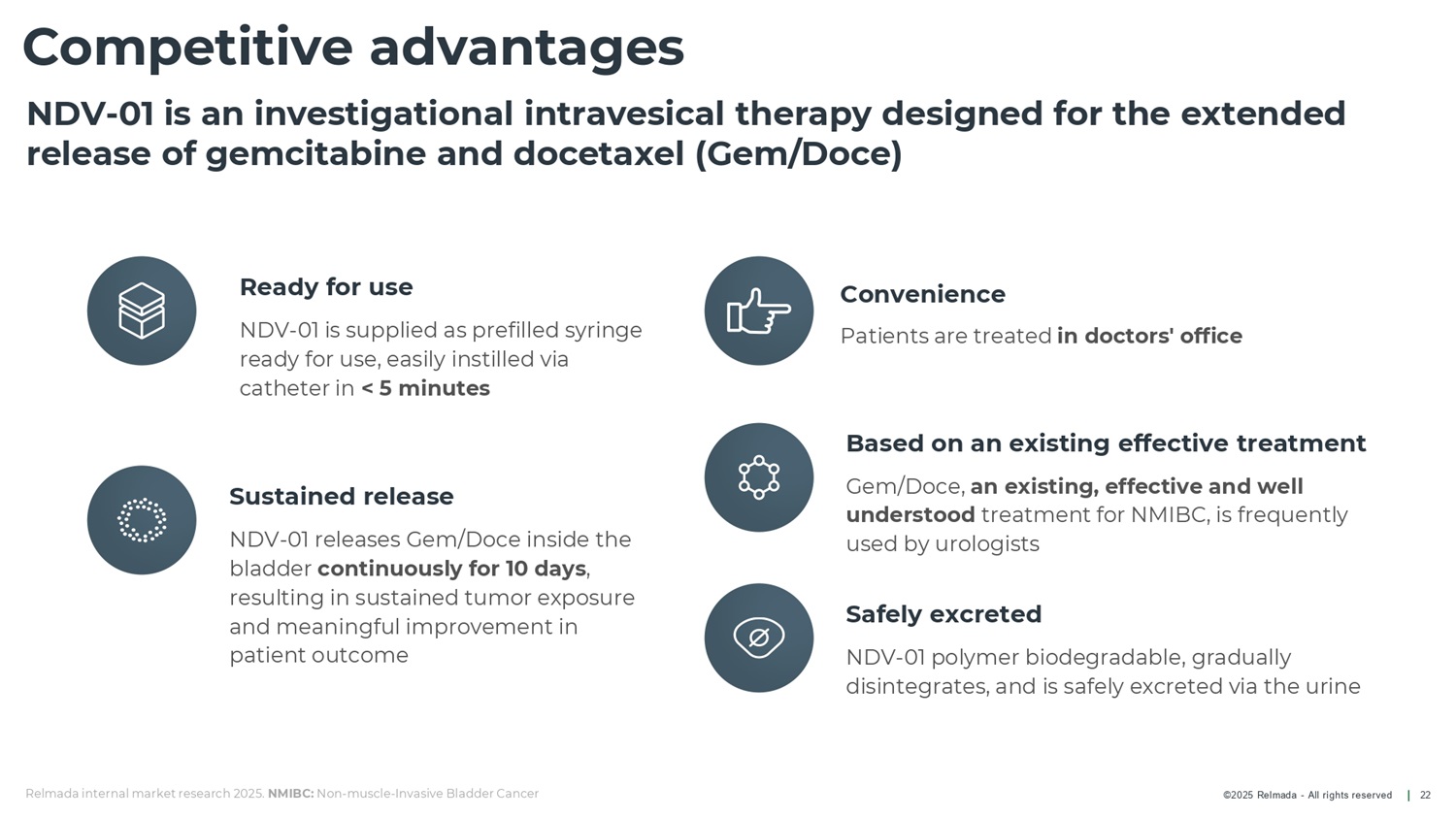
©2025 Relmada - All rights reserved Competitive advantages 22 Based on an existing effective treatment Gem/Doce, an existing, effective and well understood treatment for NMIBC, is frequently used by urologists Ready for use NDV - 01 is supplied as prefilled syringe ready for use, easily instilled via catheter in < 5 minutes Sustained release NDV - 01 releases Gem/Doce inside the bladder continuously for 10 days , resulting in sustained tumor exposure and meaningful improvement in patient outcome Safely excreted NDV - 01 polymer biodegradable, gradually disintegrates, and is safely excreted via the urine NDV - 01 is an investigational intravesical therapy designed for the extended release of gemcitabine and docetaxel (Gem/Doce) Convenience Patients are treated in doctors' office Relmada internal market research 2025. NMIBC: Non - muscle - Invasive Bladder Cancer

©2025 Relmada - All rights reserved Expecting to advance NDV - 01 towards registration - track studies in H1 2026 23 Phase 2 data update Results from 9 - month follow - up Q1 2026 FDA Engagement Including planned FDA interactions and manufacturing H1 2026 Initiate Phase 3 study Target population to be confirmed through FDA discussions Q4 2025 Phase 2 data update Results from 12 - month follow - up

©2025 Relmada - All rights reserved Sepranolone A novel candidate, with potential to overcome the challenges of current therapies for compulsivity disorders The graphic is for artistic purposes only, not a factual representation
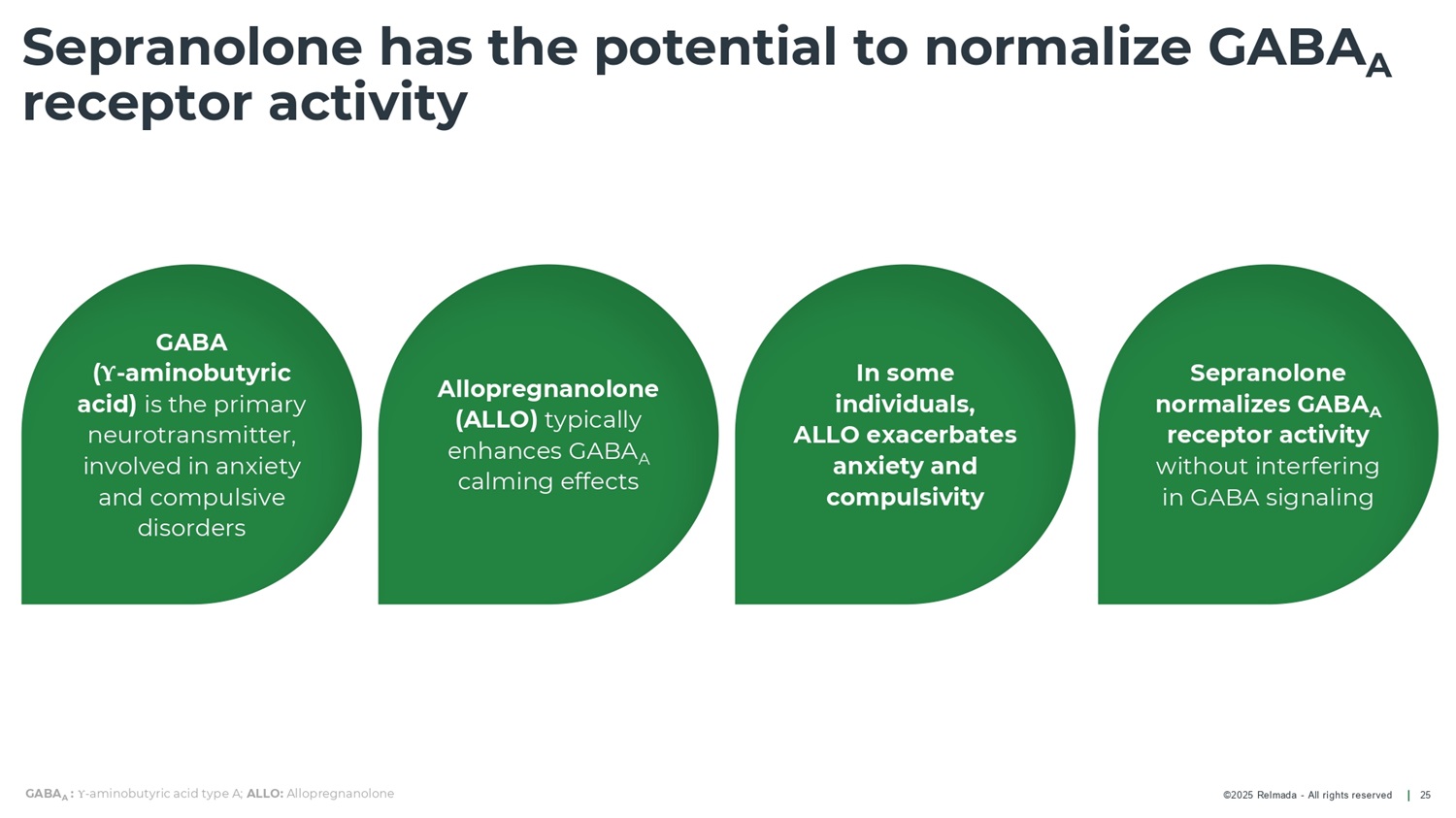
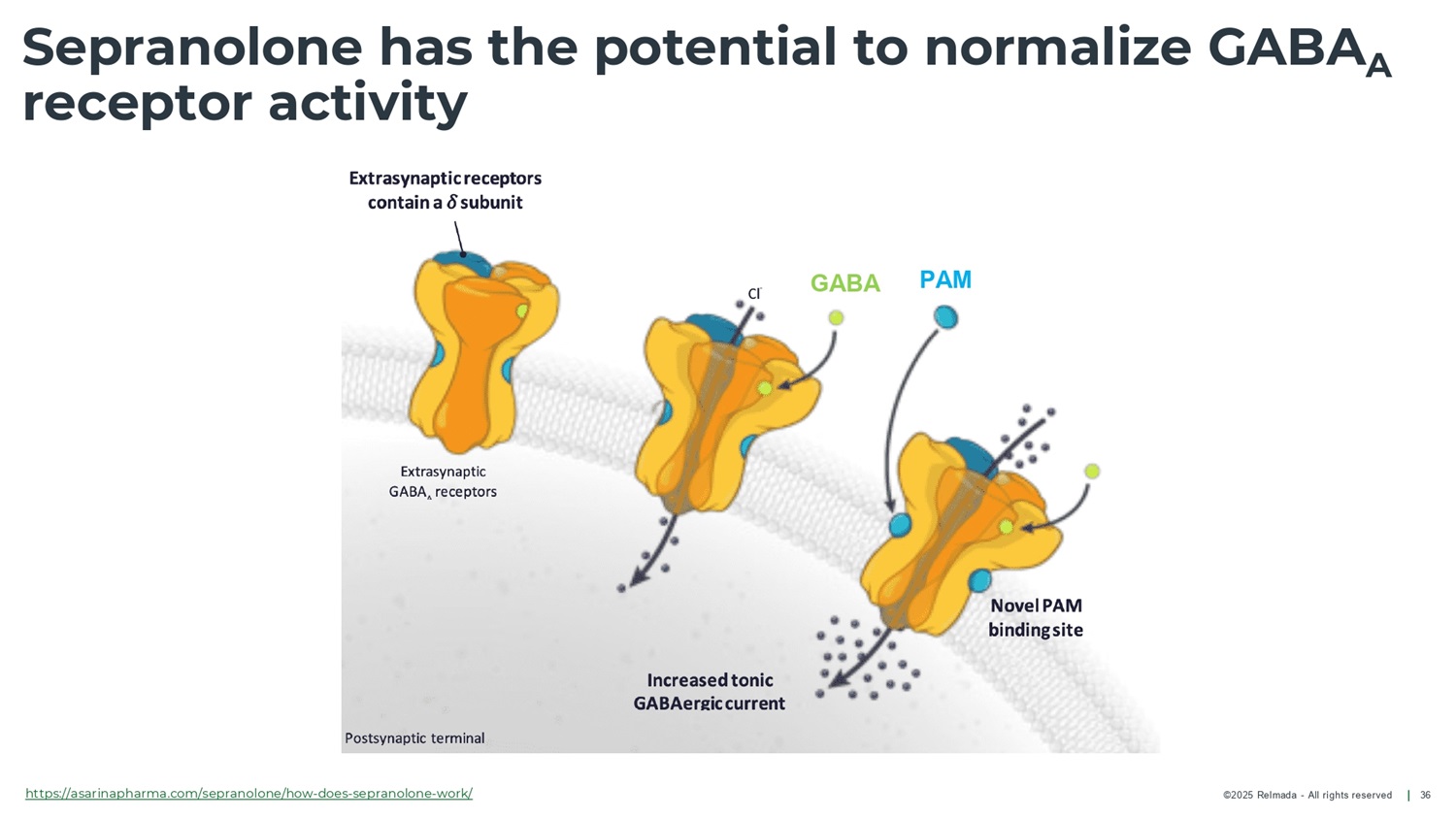
©2025 Relmada - All rights reserved Sepranolone has the potential to normalize GABA A receptor activity 25 GABA ( Υ - aminobutyric acid) is the primary neurotransmitter, involved in anxiety and compulsive disorders Allopregnanolone (ALLO) typically enhances GABA A calming effects In some individuals, ALLO exacerbates anxiety and compulsivity Sepranolone normalizes GABA A receptor activity without interfering in GABA signaling GABA A : Υ - aminobutyric acid type A; ALLO: Allopregnanolone
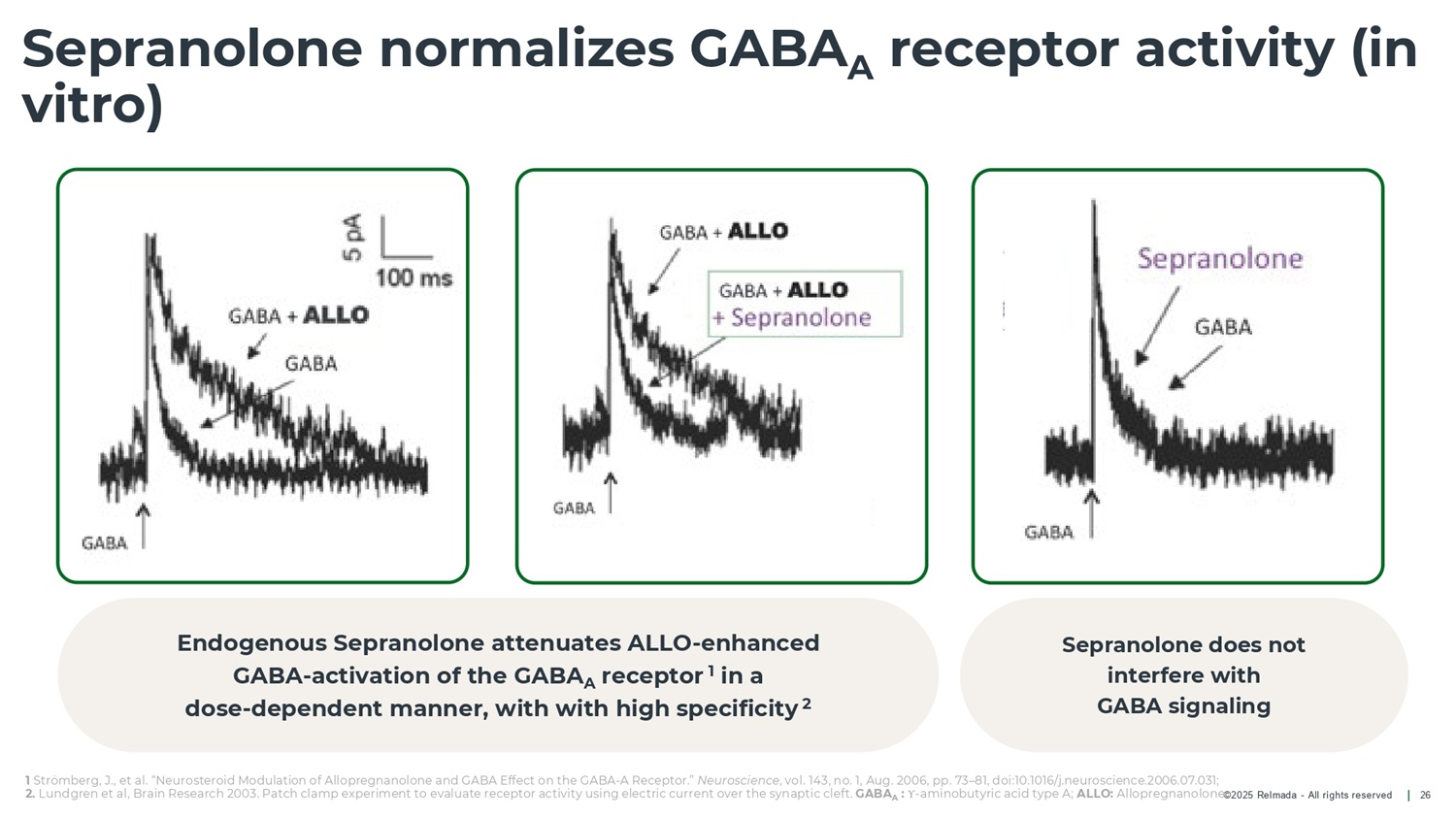
1 Strömberg, J., et al. “Neurosteroid Modulation of Allopregnanolone and GABA Effect on the GABA - A Receptor.” Neuroscience , vol. 143, no. 1, Aug. 2006, pp. 73 – 81, doi:10.1016/j.neuroscience.2006.07.031; 2. Lundgren et al, Brain Research 2003. Patch clamp experiment to evaluate receptor activity using electric current over the synaptic cleft. GABA A : Υ - aminobutyric acid type A; ALLO: Allopregnanolone ©2025 Relmada - All rights reserved Sepranolone normalizes GABA A receptor activity (in vitro) 26 Endogenous Sepranolone attenuates ALLO - enhanced GABA - activation of the GABA A receptor 1 in a dose - dependent manner, with with high specificity 2 Sepranolone does not interfere with GABA signaling

©2025 Relmada - All rights reserved Topline sepranolone safety and efficacy data 1 27 Sepranolone treatment produced a 28% drop in tic severity (p=0.051), with consistent positive impact on secondary endpoints Positive results across secondary Quality of Life measures , using widely accept scoring systems including the Gilles de la Tourette Syndrome Quality of Life total score (69% increase) No CNS off - target or systemic side effects were observed 1. Sepranolone Phase 2 Tourette data, Relmada, Feb 06, 2025 press release. CNS: Central Nervous System
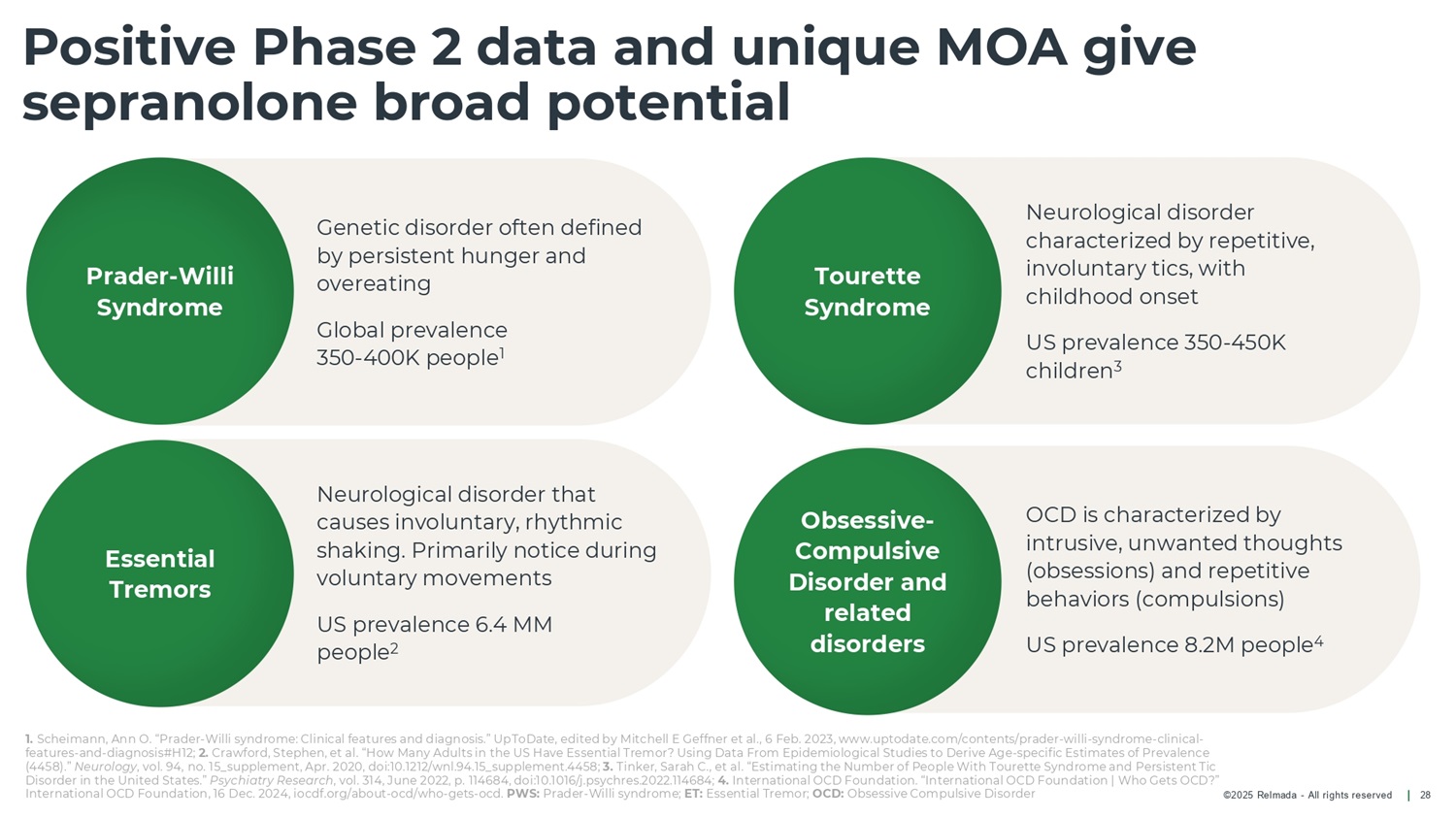
©2025 Relmada - All rights reserved OCD is characterized by intrusive, unwanted thoughts (obsessions) and repetitive behaviors (compulsions) US prevalence 8.2M people 4 Neurological disorder characterized by repetitive, involuntary tics, with childhood onset US prevalence 350 - 450K children 3 Neurological disorder that causes involuntary, rhythmic shaking. Primarily notice during voluntary movements US prevalence 6.4 MM people 2 Genetic disorder often defined by persistent hunger and overeating Global prevalence 350 - 400K people 1 Positive Phase 2 data and unique MOA give sepranolone broad potential 28 Prader - Willi Syndrome Essential Tremors Tourette Syndrome Obsessive - Compulsive Disorder and related disorders 1. Scheimann, Ann O. “Prader - Willi syndrome: Clinical features and diagnosis.” UpToDate, edited by Mitchell E Geffner et al., 6 Feb. 2023, www.uptodate.com/contents/prader - willi - syndrome - clinical - features - and - diagnosis#H12; 2. Crawford, Stephen, et al. “How Many Adults in the US Have Essential Tremor? Using Data From Epidemiological Studies to Derive Age - specific Estimates of Prevalence (4458).” Neurology , vol. 94, no. 15_supplement, Apr. 2020, doi:10.1212/wnl.94.15_supplement.4458; 3. Tinker, Sarah C., et al. “Estimating the Number of People With Tourette Syndrome and Persistent Tic Disorder in the United States.” Psychiatry Research , vol. 314, June 2022, p. 114684, doi:10.1016/j.psychres.2022.114684; 4. International OCD Foundation. “International OCD Foundation | Who Gets OCD?” International OCD Foundation, 16 Dec. 2024, iocdf.org/about - ocd/who - gets - ocd. PWS: Prader - Willi syndrome; ET: Essential Tremor; OCD: Obsessive Compulsive Disorder
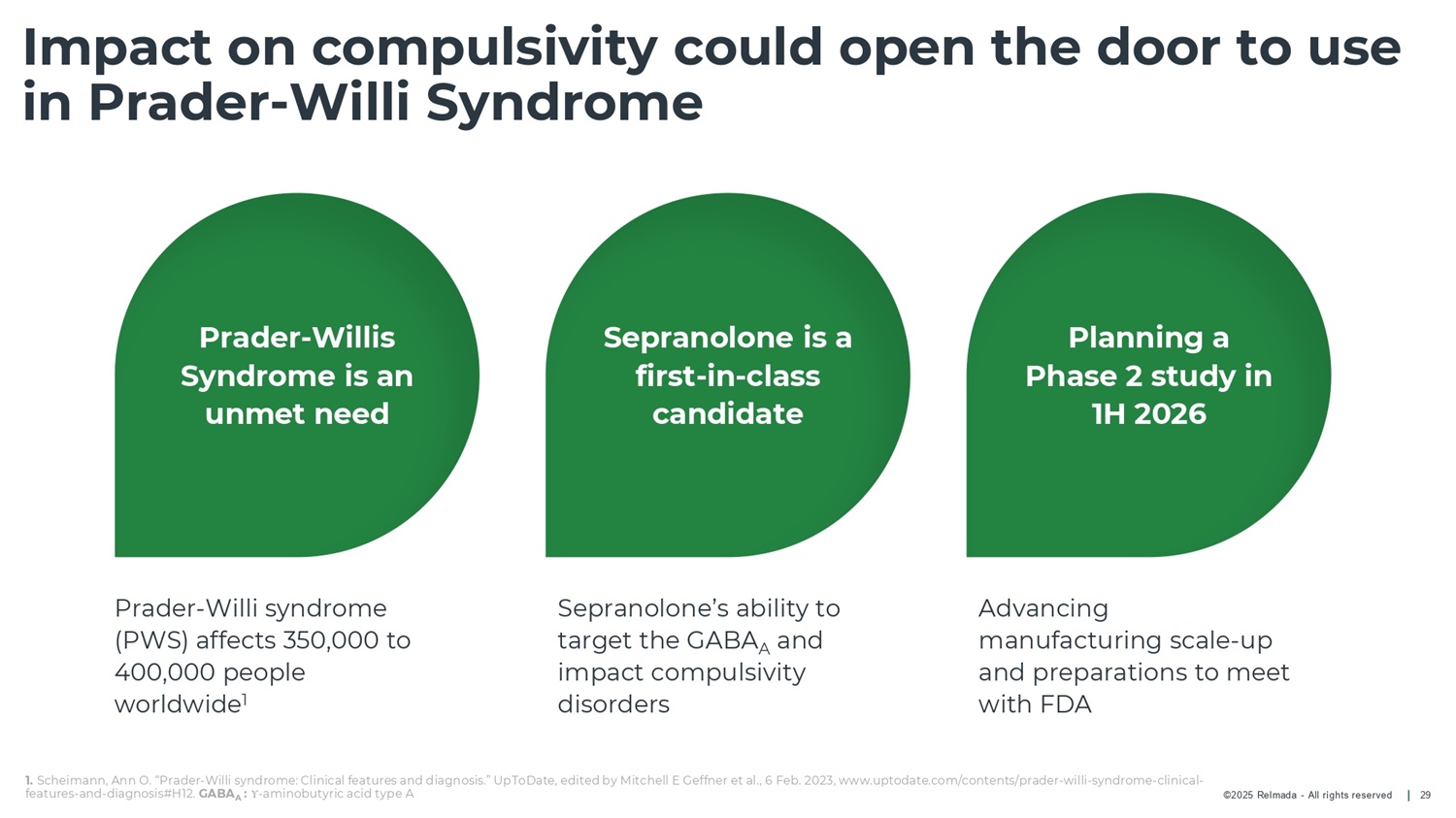
©2025 Relmada - All rights reserved Impact on compulsivity could open the door to use in Prader - Willi Syndrome 29 Prader - Willis Syndrome is an unmet need Sepranolone is a first - in - class candidate Planning a Phase 2 study in 1H 2026 Prader - Willi syndrome (PWS) affects 350,000 to 400,000 people worldwide 1 Advancing manufacturing scale - up and preparations to meet with FDA Sepranolone’s ability to target the GABA A and impact compulsivity disorders 1. Scheimann, Ann O. “Prader - Willi syndrome: Clinical features and diagnosis.” UpToDate, edited by Mitchell E Geffner et al., 6 Feb. 2023, www.uptodate.com/contents/prader - willi - syndrome - clinical - features - and - diagnosis#H12. GABA A : Υ - aminobutyric acid type A

©2025 Relmada - All rights reserved Expecting to advance sepranolone towards Phase 2 studies in Prader - Willi Syndrome in H1 2026 30 Q4 2025 Phase 2 PWS preparations Including planned FDA interactions and further development of product supply H1 2026 Initiation of Pilot Phase 2 study in Prader - Willi Syndrome Focus on evaluating early proof - of - concept PWS: Prader - Willi syndrome

©2025 Relmada - All rights reserved 31 Corporate summary
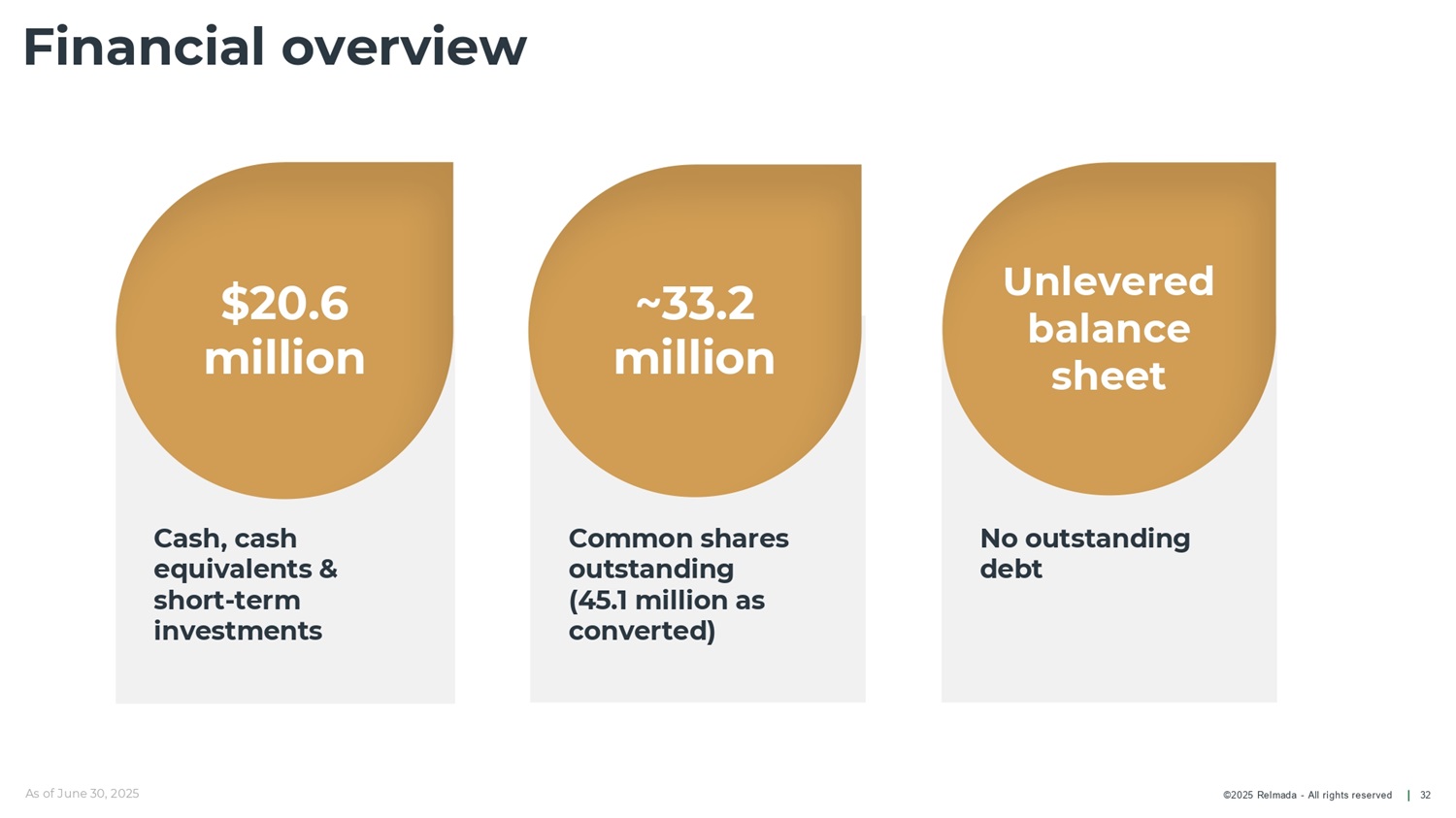
©2025 Relmada - All rights reserved Financial overview 32 Common shares outstanding (45.1 million as converted) Cash, cash equivalents & short - term investments As of June 30, 2025 No outstanding debt $20.6 million ~33.2 million Unlevered balance sheet

©2025 Relmada - All rights reserved NDV - 01 and sepranolone poised to make important progress in 2025 - 2026 33 Q4 2025 Q4 2025 H1 2026 H1 2026 NDV - 01 NDV - 01 Sepranolone Sepranolone Planned FDA interactions, manufacturing build - out Planned FDA interactions, product supply expansion Initiate registration - track study Initiate pilot PWS study PWS: Prader - Willi syndrome

Thank you!

Appendix
©2025 Relmada - All rights reserved Sepranolone has the potential to normalize GABA A receptor activity 36 GABA PAM https://asarinapharma.com/sepranolone/how - does - sepranolone - work/