
Exhibit 99.1

©2024 60 Degrees Pharmaceuticals, Inc. CONFIDENTIAL All rights reserved. September 2025 H.C. Wainwright Presentation

Disclaimer and Forward - looking Statements ©2024 60 Degrees Pharmaceuticals, Inc. CONFIDENTIAL All rights reserved. DISCLAIMER. The information contained herein has been prepared to assist prospective investors in making their own evaluation of 60 Degrees Pharmaceuticals, Inc. (the “Company”) and does not purport to be all - inclusive or to contain all of the information a prospective or existing investor may desire. In all cases, interested parties will be expected to have conducted their own due diligence investigation regarding these and all other matters pertinent to investment in the Company. The Company makes no representation or warrant as to the accuracy or completeness of this information and shall not have any liability for any representations (expressed or implied) regarding information contained in, or for any omissions from, this information or any other written or oral communications transmitted to the recipient in the course of its evaluation of the Company. This presentation and contents herein are the exclusive property of the Company and may not be copied without the express prior written consent of the Company. FORWARD LOOKING STATEMENTS. This communication includes forward - looking statements based on the Company’s current expectations and projections about future events. All statements contained in this communication other than statements of historical fact, including any statements regarding our future operations, are forward - looking statements. The words “believe”, “may”, “will”, “estimate”, “continue”, “anticipate”, “intend”, “expect”, “could”, “would”, “project”, “plan”, “potentially”, “likely” and similar expressions are intended to identify forward - looking statements as defined in the Private securities Litigation Reform Act of 1995. The forward - looking statements contained in this communication are based on knowledge of the environment in which the Company currently operates and are subject to changed based on various important factors that may affect the Company’s operations, growth strategies, financial results and cash flows, and as well as other factors beyond the Company’s control as of the date of this presentation. Important factors that could cause our actual results and financial conditions to differ materially from those indicated in the forward - looking statements include, among others, the following: there is substantial doubt as to our ability to continue on a going - concern basis; we might not be eligible for Australian government research and development tax rebates; if we are not able to successfully develop, obtain FDA approval for, and otherwise provide for the commercialization of non - malaria prevention indications for Tafenoquine ( ARAKODA or other regimen) or Celgosivir/Australian Chestbut extracts in a timely manner, we may not be able to expand our business operations; we cannot guarantee our ability to conduct successful clinical trials; and we have no manufacturing capacity which poses the risk of lengthy and costly delays of bringing our products to market. More detailed information about the Company and the risk factors that may affect the realization of forward - looking statements is set forth in the Company’s filings with the Securities and Exchange Commission (SEC), including our Annual Report on Form 10 - K and our subsequent Quarterly Reports on Form 10 - Q. Investors and security holders are urged to read these documents free of charge on the SEC’s website at www.sec.gov. As a result of these matters, changes in fact, assumptions not being realized or other circumstances, the Company’s actual results may differ materially from the expected results discussed in the forward - looking statements contained in this presentation. In light of these risks, uncertainties and assumptions, you should not place undue reliance on these forward - looking statements, which speak only as of the date of this presentation. Although we believe our expectations are based on reasonable assumptions, we can give no assurance that our expectations will materialize. Unless required by law, we undertake no obligation to update or revise any forward - looking statements, whether as a result of new information, future events, or otherwise.

©2025 60 Degrees Pharmaceuticals, Inc. CONFIDENTIAL All rights reserved. Market Cap: $5.8 million
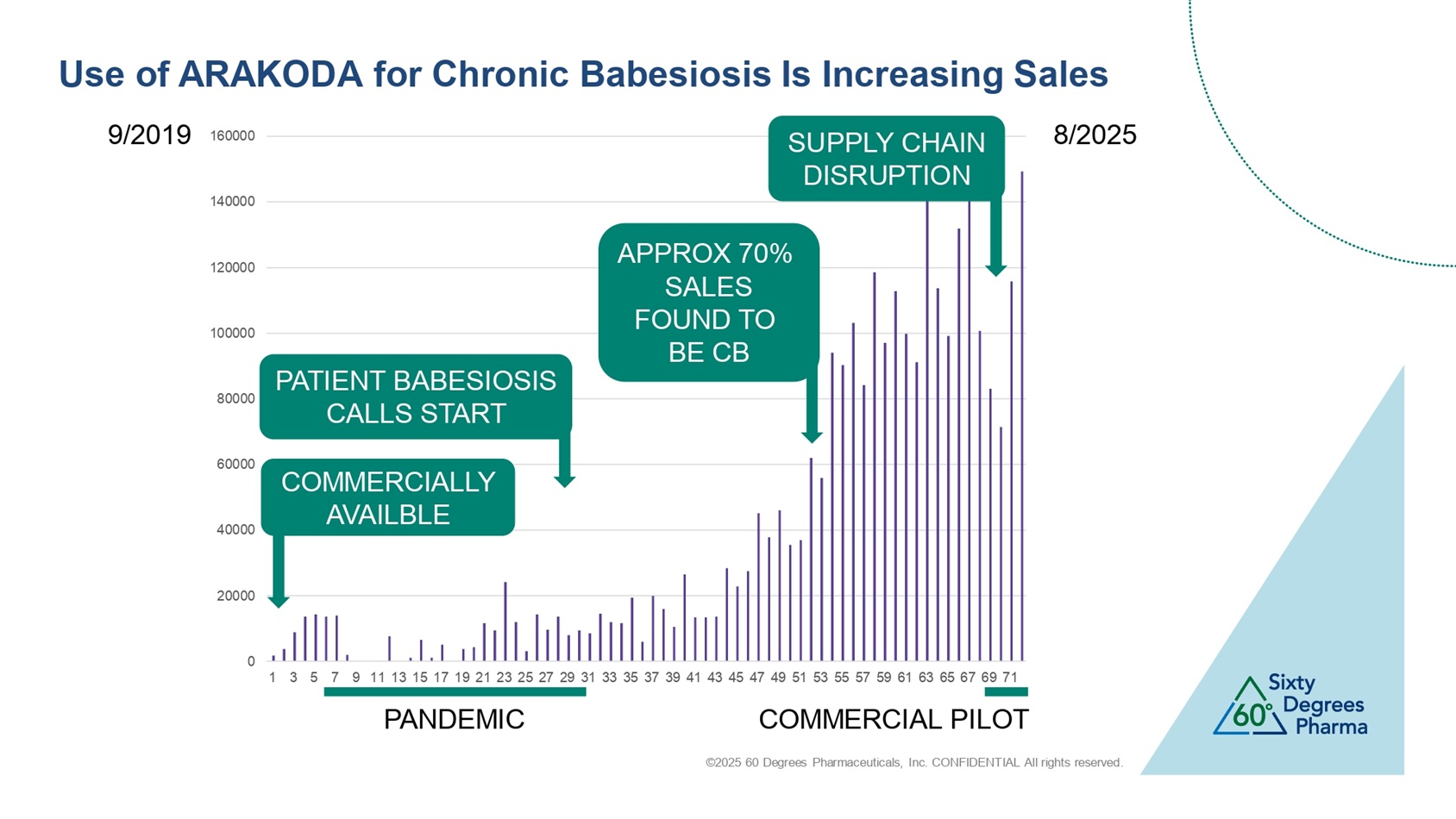
©2025 60 Degrees Pharmaceuticals, Inc. CONFIDENTIAL All rights reserved. 0 20000 40000 60000 80000 100000 120000 140000 160000 1 3 5 7 9 11 13 15 17 19 21 23 25 27 29 31 33 35 37 39 41 43 45 47 49 51 53 55 57 59 61 63 65 67 69 71 9/2019 8/2025 PANDEMIC SUPPLY CHAIN DISRUPTION PATIENT BABESIOSIS CALLS START APPROX 70% SALES FOUND TO BE CB COMMERCIAL PILOT Use of ARAKODA for Chronic Babesiosis Is Increasing Sales COMMERCIALLY AVAILBLE
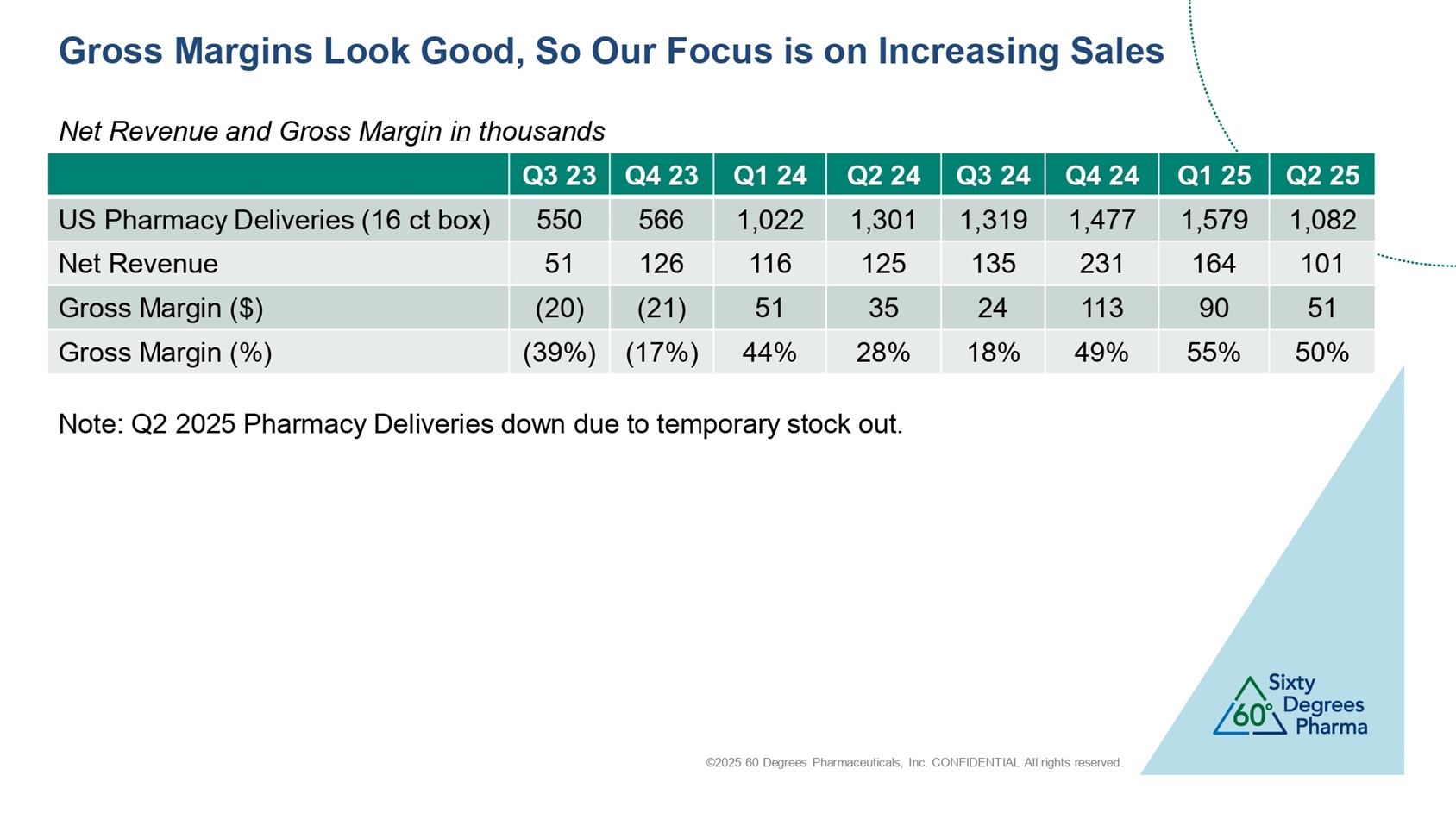
©2025 60 Degrees Pharmaceuticals, Inc. CONFIDENTIAL All rights reserved. Gross Margins Look Good, So Our Focus is on Increasing Sales Q2 25 Q1 25 Q4 24 Q3 24 Q2 24 Q1 24 Q4 23 Q3 23 1,082 1,579 1,477 1,319 1,301 1,022 566 550 US Pharmacy Deliveries (16 ct box) 101 164 231 135 125 116 126 51 Net Revenue 51 90 113 24 35 51 (21) (20) Gross Margin ($) 50% 55% 49% 18% 28% 44% (17%) (39%) Gross Margin (%) Net Revenue and Gross Margin in thousands Note: Q2 2025 Pharmacy Deliveries down due to temporary stock out.
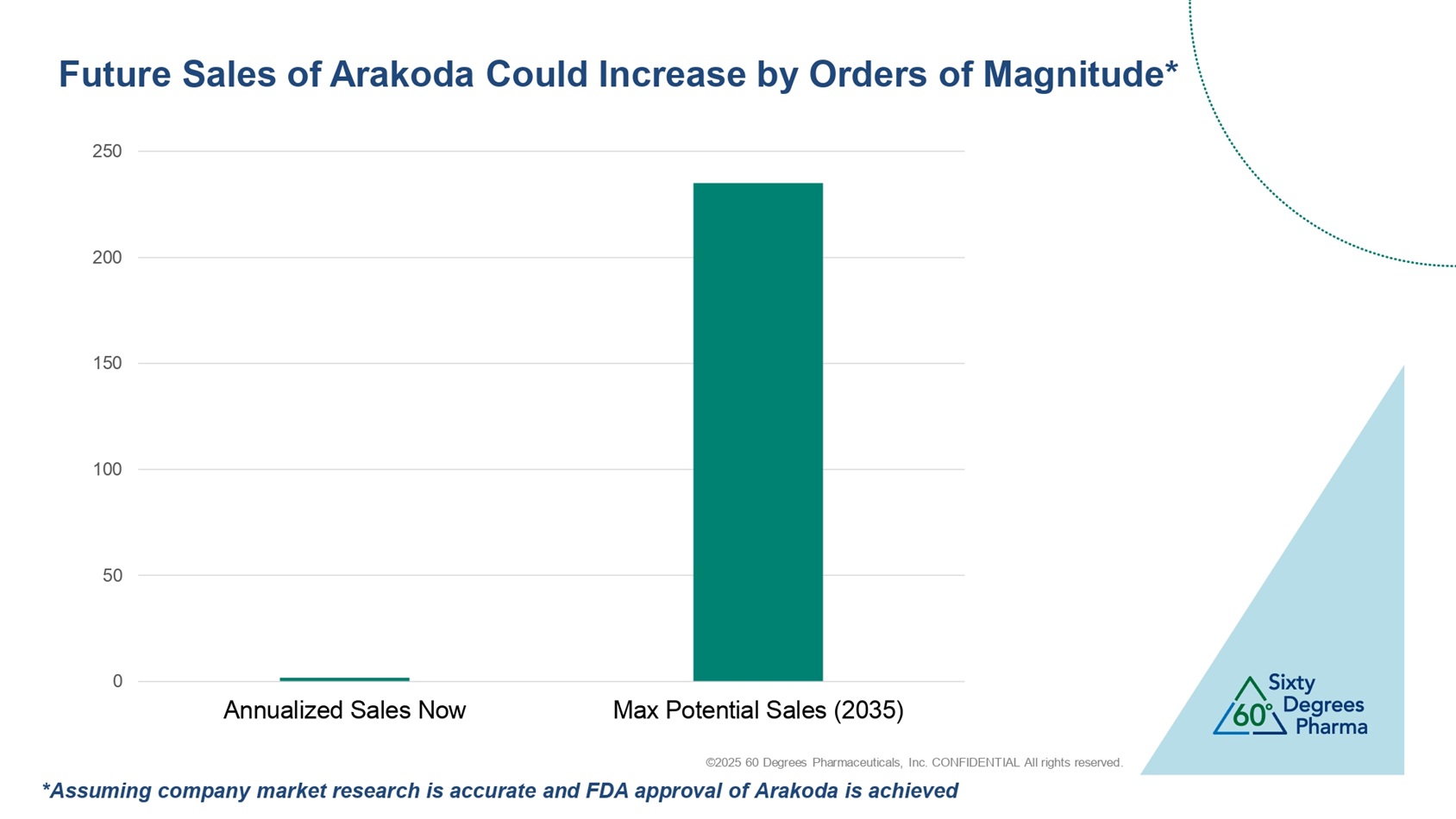
©2025 60 Degrees Pharmaceuticals, Inc. CONFIDENTIAL All rights reserved. 0 50 100 150 200 250 Annualized Sales Now Max Potential Sales (2035) Future Sales of Arakoda Could Increase by Orders of Magnitude* *Assuming company market research is accurate and FDA approval of Arakoda is achieved
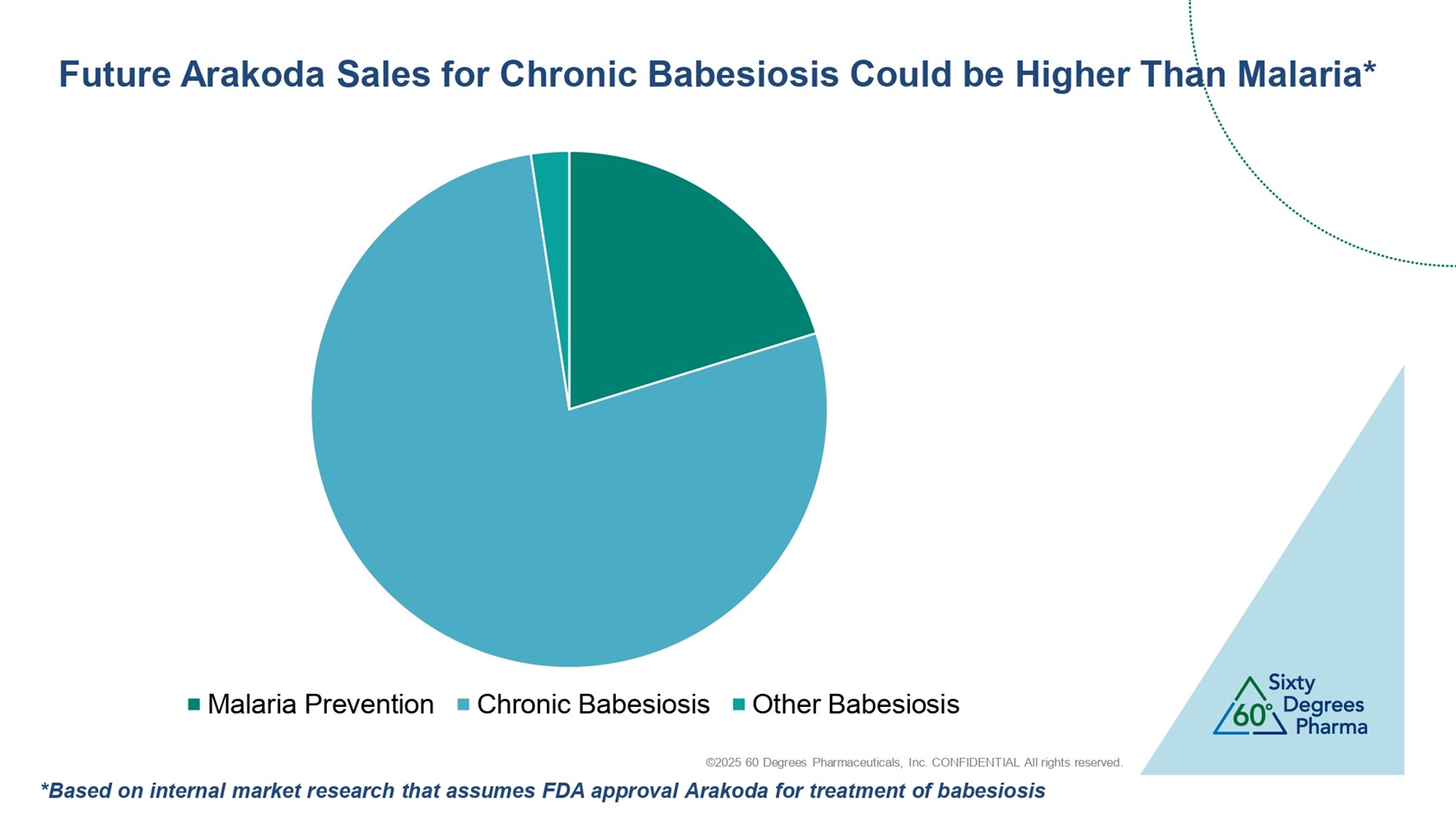
©2025 60 Degrees Pharmaceuticals, Inc. CONFIDENTIAL All rights reserved. Malaria Prevention Chronic Babesiosis Other Babesiosis Future Arakoda Sales for Chronic Babesiosis Could be Higher Than Malaria* *Based on internal market research that assumes FDA approval Arakoda for treatment of babesiosis

©2025 60 Degrees Pharmaceuticals, Inc. CONFIDENTIAL All rights reserved. Roberts wrote, “Malaria is an awful disease that needs to be attacked full - force when a diagnosis is made. In my time here, I’ve gone from being so sick that any dog would take pity on me to being ready to fire up the grill on Labor Day (well, maybe ready to watch the grill being fired up).”
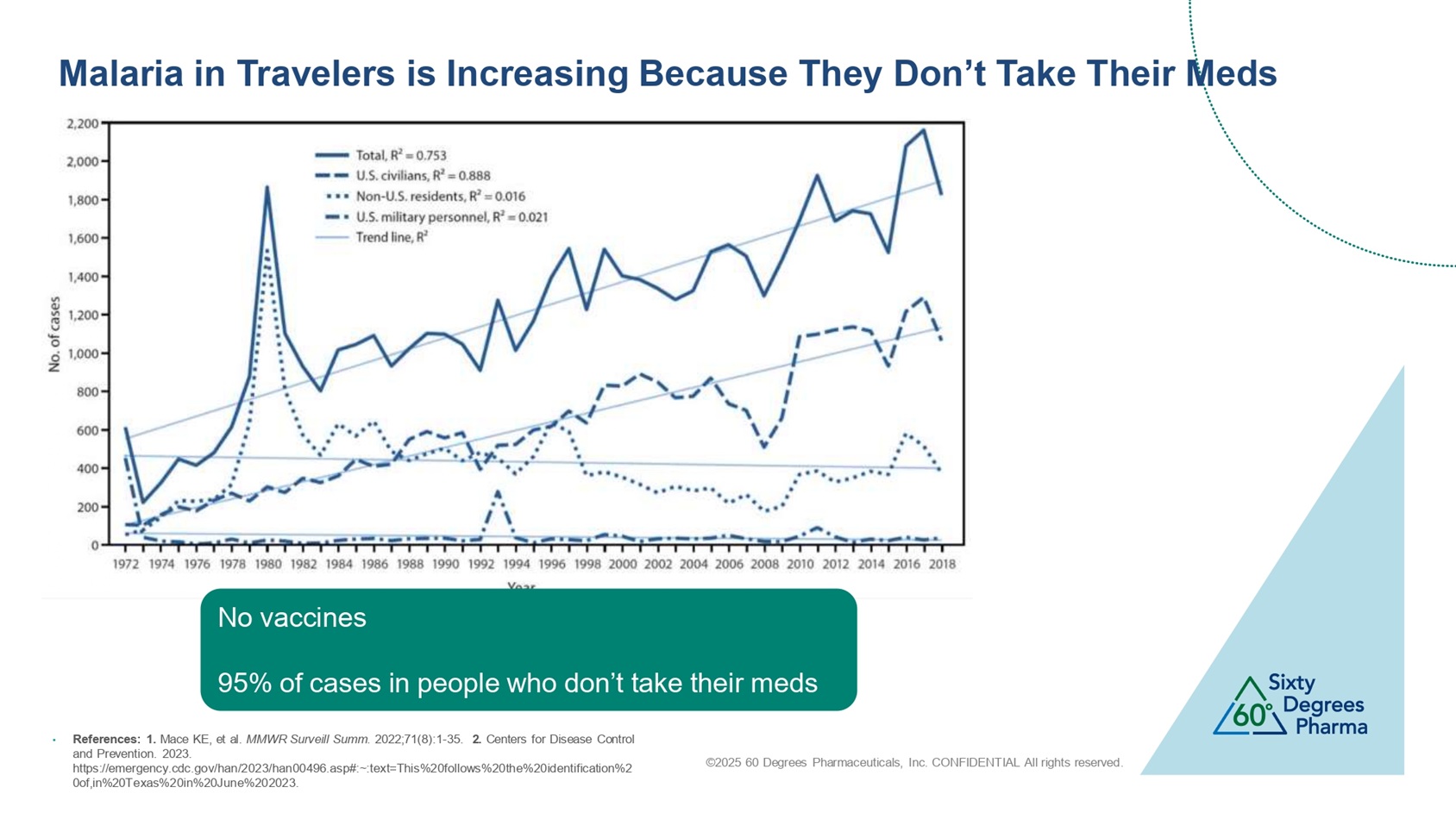
©2025 60 Degrees Pharmaceuticals, Inc. CONFIDENTIAL All rights reserved. Malaria in Travelers is Increasing Because They Don’t Take Their Meds No vaccines 95% of cases in people who don’t take their meds • References: 1. Mace KE, et al. MMWR Surveill Summ . 2022;71(8):1 - 35. 2. Centers for Disease Control and Prevention. 2023. https://emergency.cdc.gov/han/2023/han00496.asp#:~:text=This%20follows%20the%20identification%2 0of,in%20Texas%20in%20June%202023.
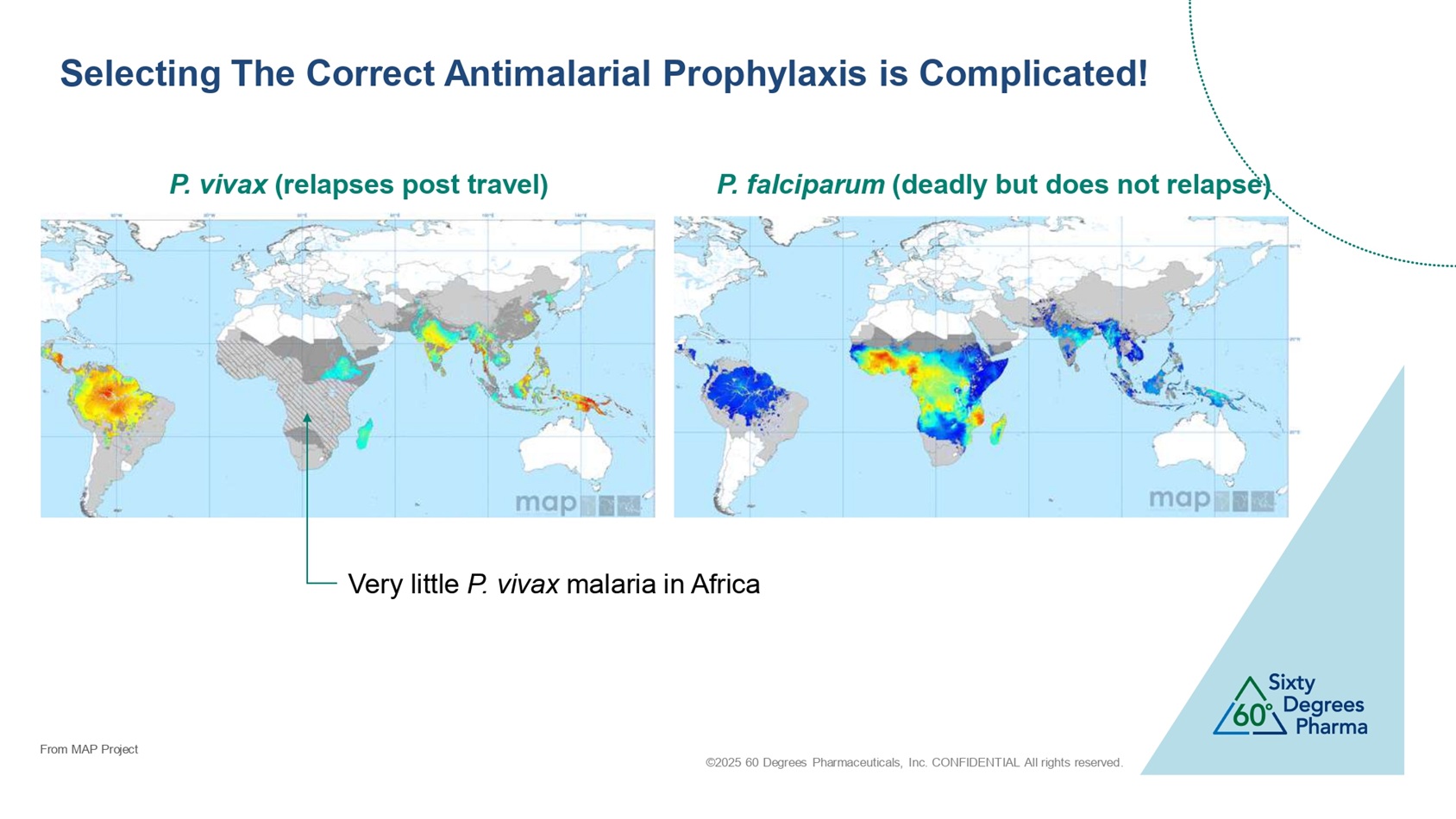
©2025 60 Degrees Pharmaceuticals, Inc. CONFIDENTIAL All rights reserved. Selecting The Correct Antimalarial Prophylaxis is Complicated! P. vivax (relapses post travel) P. falciparum (deadly but does not relapse) Very little P. vivax malaria in Africa From MAP Project
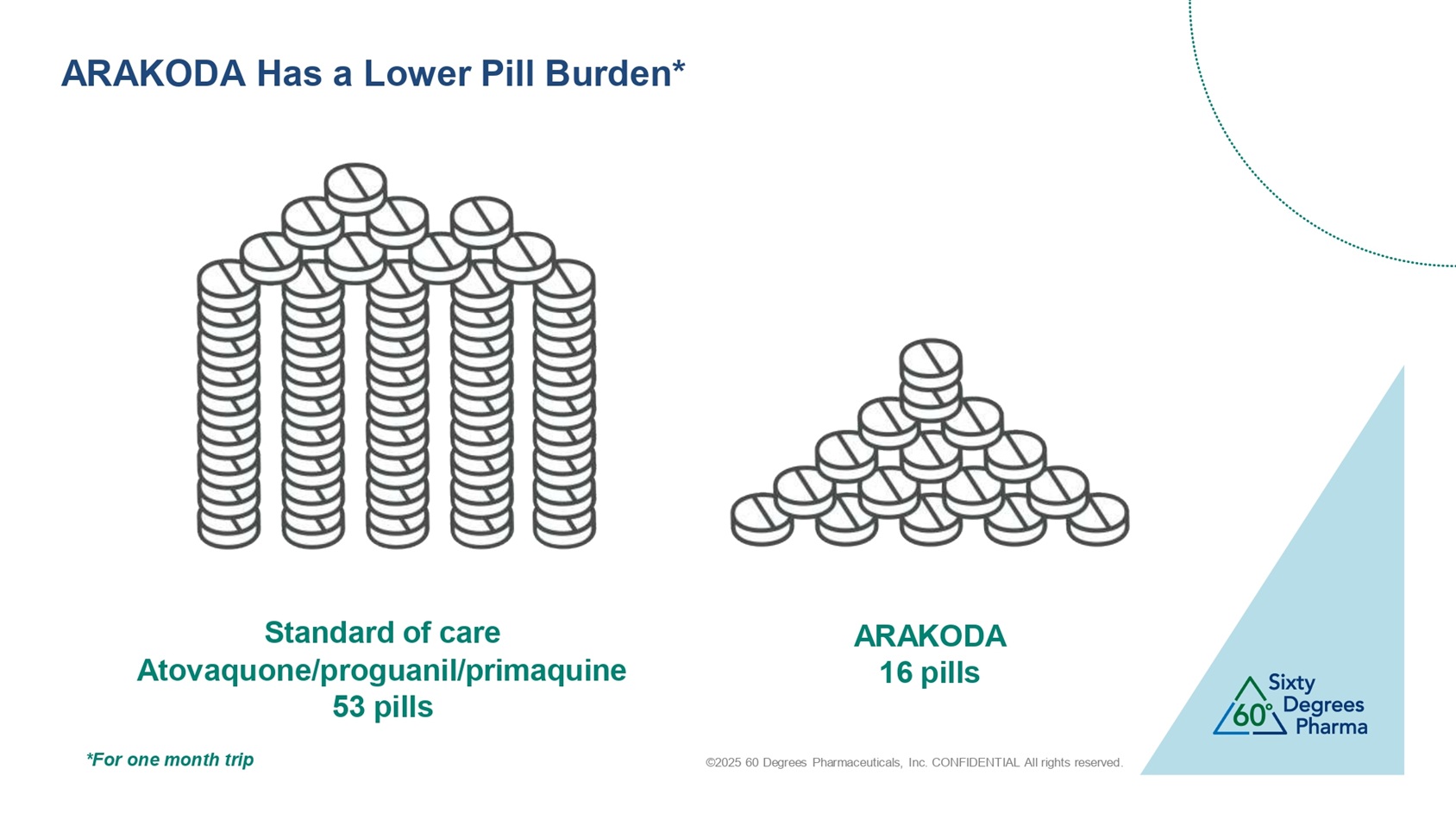
©2025 60 Degrees Pharmaceuticals, Inc. CONFIDENTIAL All rights reserved. ARAKODA Has a Lower Pill Burden* Standard of care Atovaquone/proguanil/primaquine 53 pills ARAKODA 16 pills *For one month trip

©2025 60 Degrees Pharmaceuticals, Inc. CONFIDENTIAL All rights reserved. A rakoda Market Research Revealed Some Challenges to Solve Brand recognition is low HCPs and patients liked product profile Generic alternatives have lower out of pocket costs G6PD testing requirement a potential barrier for some travelers
Commercial Pilot for Malaria Indication Addresses Barriers to Use Increase Awareness and Differentiate ARAKODA Differentiate ARAKODA from the generic competition with a clear and compelling value story Drive ARAKODA Trial & Usage Encourage HCPS to prescribe ARAKODA for appropriate patients based on product attributes Facilitate Access & Affordability Manage high OOP costs with co - pay assistance program
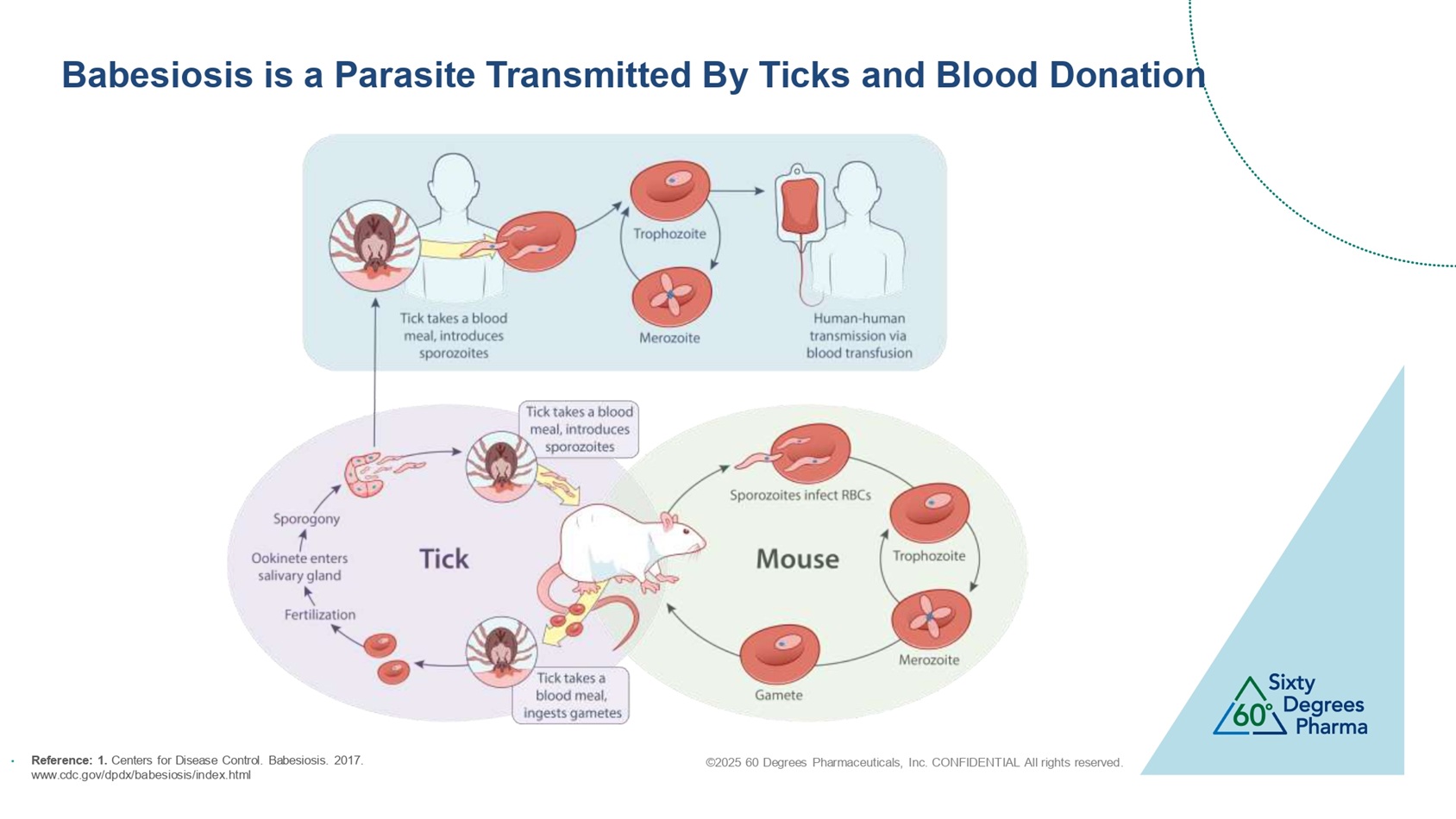
©2025 60 Degrees Pharmaceuticals, Inc. CONFIDENTIAL All rights reserved. Babesiosis is a Parasite Transmitted By Ticks and Blood Donation • Reference: 1. Centers for Disease Control. Babesiosis. 2017. www.cdc.gov/dpdx/babesiosis/index.html
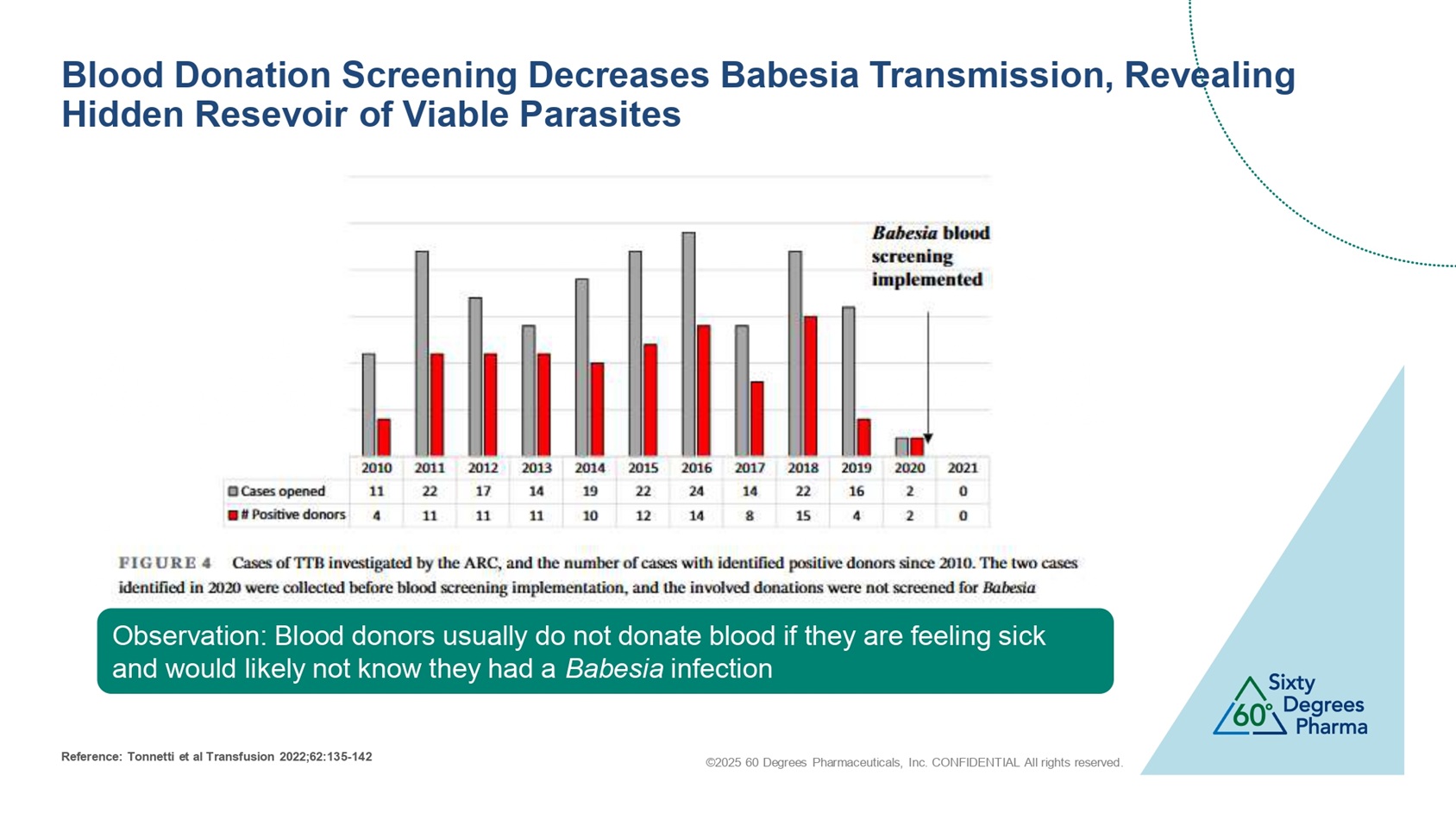
©2025 60 Degrees Pharmaceuticals, Inc. CONFIDENTIAL All rights reserved. Blood Donation Screening Decreases Babesia Transmission, Revealing Hidden Resevoir of Viable Parasites Observation: Blood donors usually do not donate blood if they are feeling sick and would likely not know they had a Babesia infection Reference: Tonnetti et al Transfusion 2022;62:135 - 142
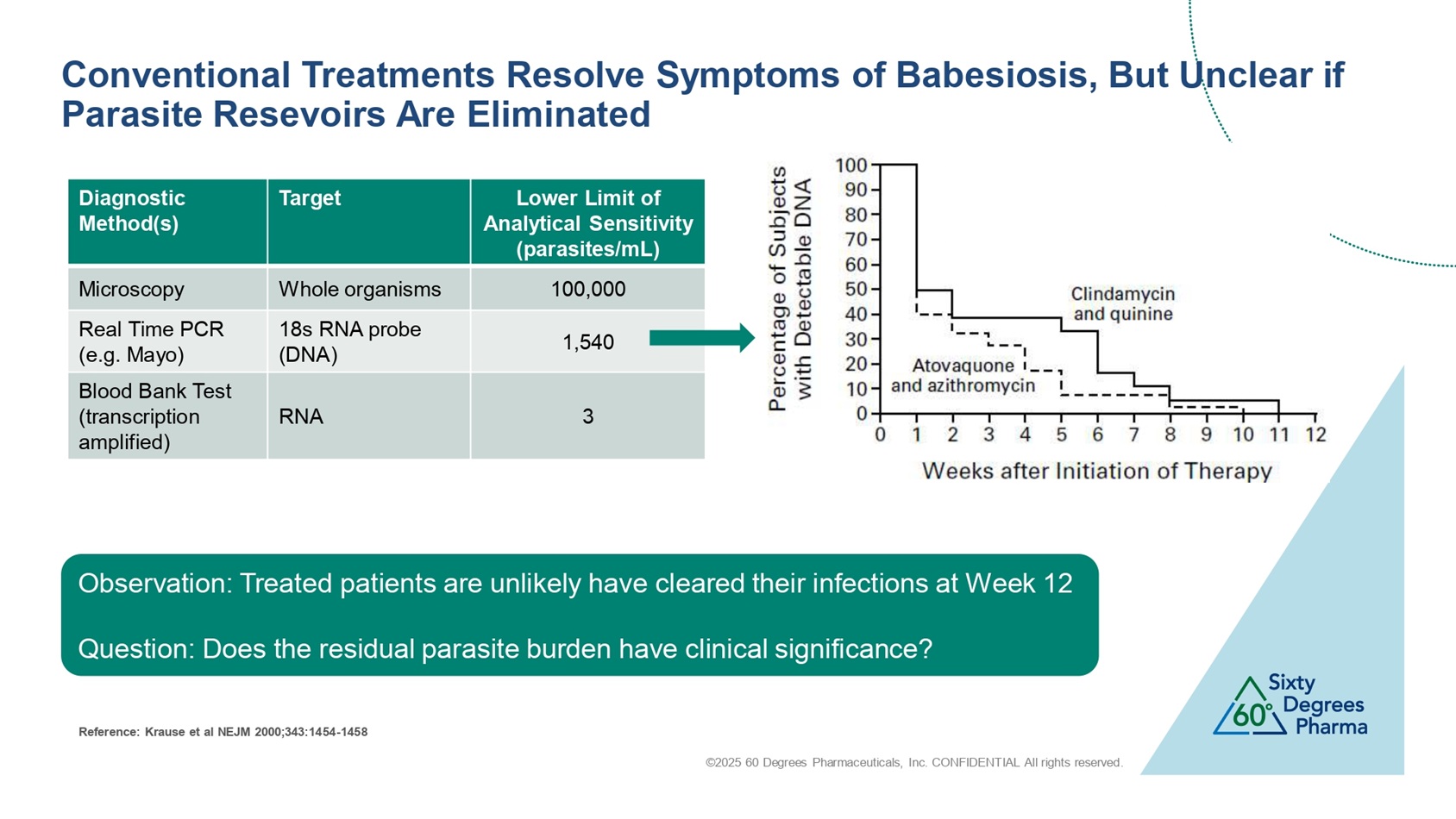
©2025 60 Degrees Pharmaceuticals, Inc. CONFIDENTIAL All rights reserved. Lower Limit of Analytical Sensitivity (parasites/mL) Target Diagnostic Method(s) 100,000 Whole organisms Microscopy 1,540 18s RNA probe (DNA) Real Time PCR (e.g. Mayo) 3 RNA Blood Bank Test (transcription amplified) Observation: Treated patients are unlikely have cleared their infections at Week 12 Question: Does the residual parasite burden have clinical significance? Conventional Treatments Resolve Symptoms of Babesiosis, But Unclear if Parasite Resevoirs Are Eliminated Reference: Krause et al NEJM 2000;343:1454 - 1458
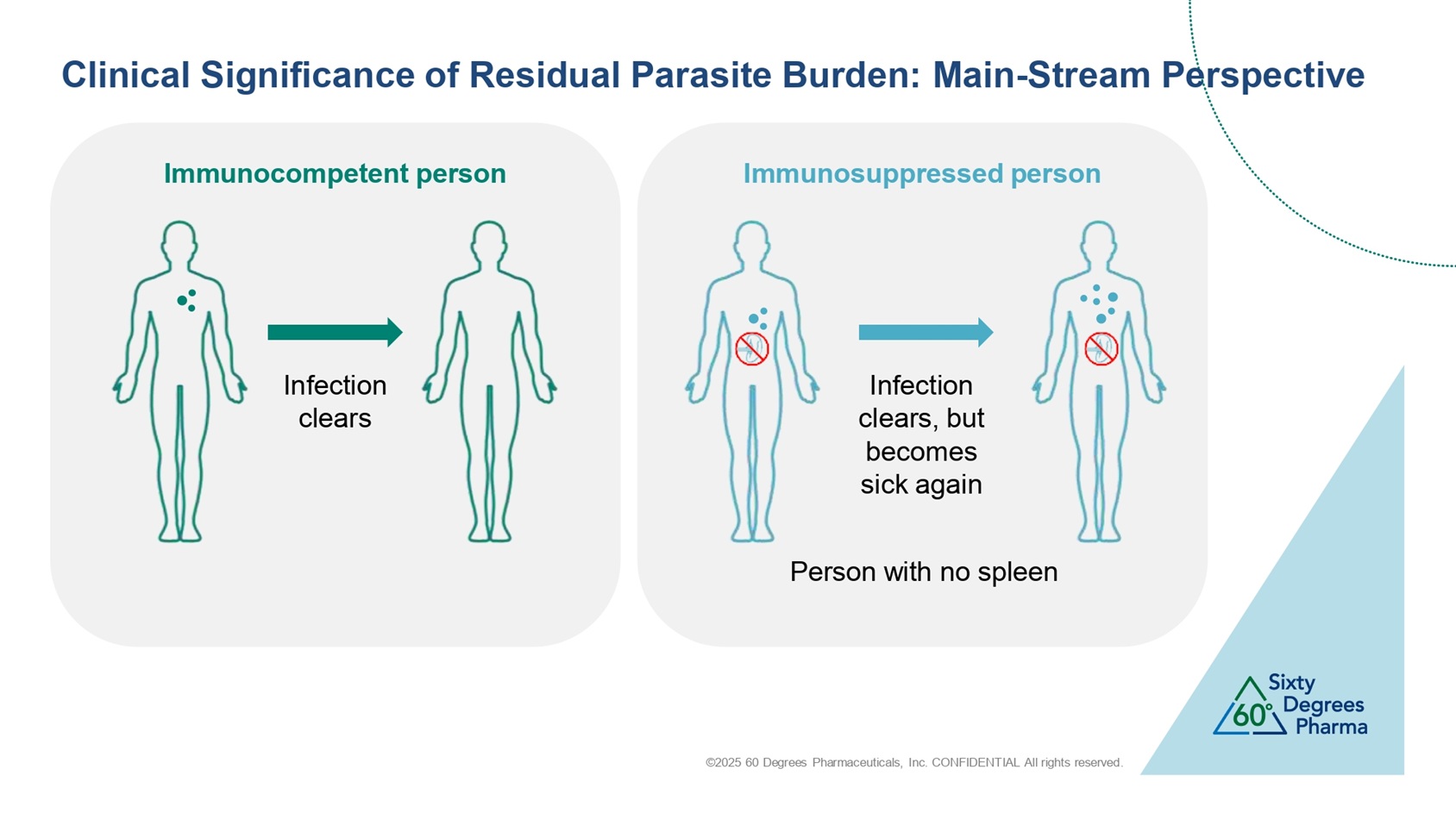
©2025 60 Degrees Pharmaceuticals, Inc. CONFIDENTIAL All rights reserved. Clinical Significance of Residual Parasite Burden: Main - Stream Perspective Immunocompetent person Immunosuppressed person Infection clears Infection clears, but becomes sick again Person with no spleen

©2025 60 Degrees Pharmaceuticals, Inc. CONFIDENTIAL All rights reserved. Chronic Babesiosis is a Problem: Prescribing Physician Perspective Dr. Richard Horowitz: “Chronic Babesiosis is seen in the vast majority of my chronically ill patients with Lyme disease. Present regimens with Mepron and Zithromax or clindamycin and quinine are insufficient in many cases to clear the infection. We need more studies on drugs like tafenoquine, for Chronic Babesiosis, as this persistent parasite is one of the reasons my patients do not get better” Dr. Shannon Delaney: “Every week I see kids and adults who have manifestations of Chronic Babesiosis, often presenting as severe neuropsychiatric symptoms”
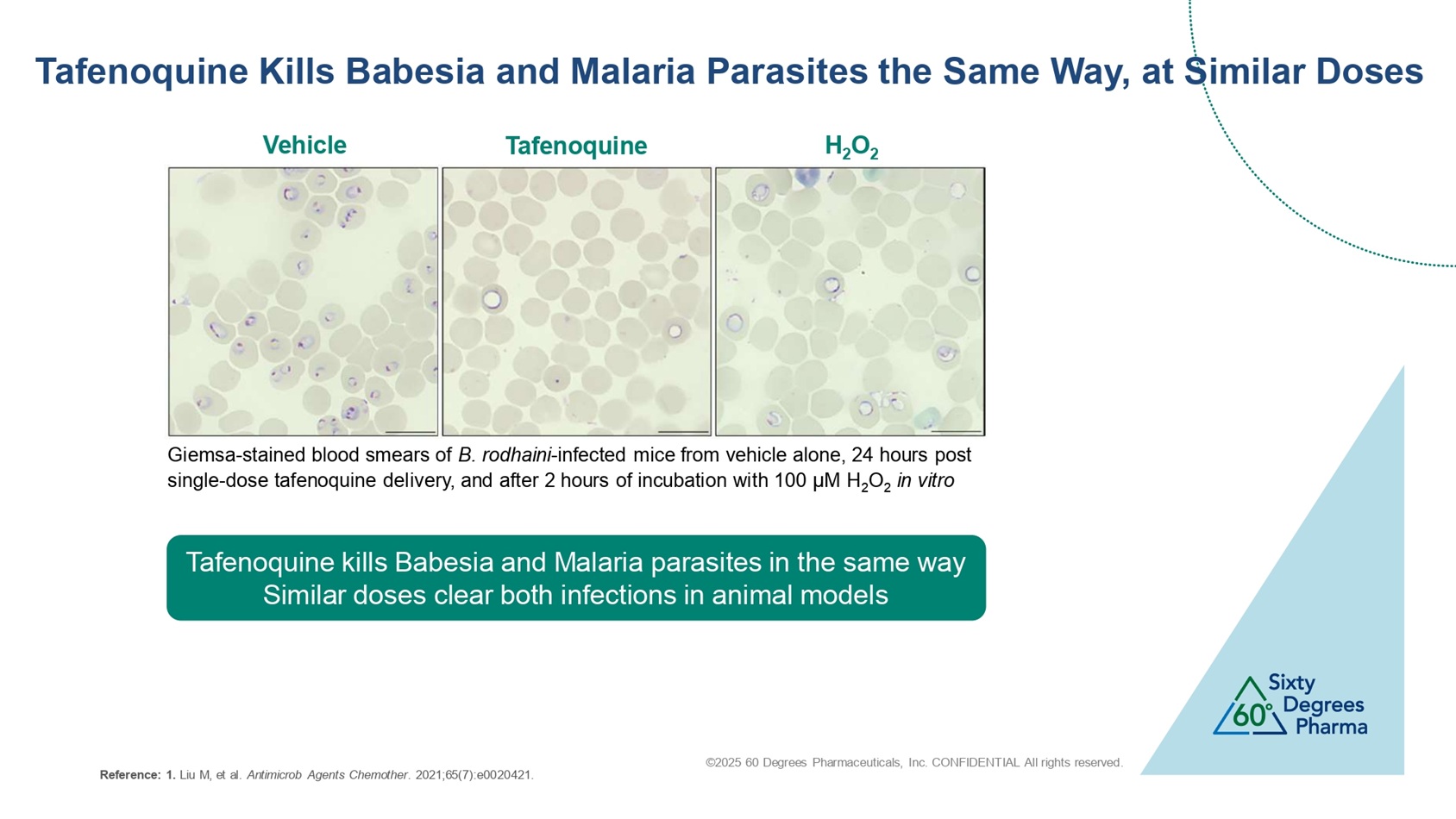
©2025 60 Degrees Pharmaceuticals, Inc. CONFIDENTIAL All rights reserved. Tafenoquine Kills Babesia and Malaria Parasites the Same Way, at Similar Doses Tafenoquine kills Babesia and Malaria parasites in the same way Similar doses clear both infections in animal models Giemsa - stained blood smears of B. rodhaini - infected mice from vehicle alone, 24 hours post single - dose tafenoquine delivery, and after 2 hours of incubation with 100 µ M H 2 O 2 in vitro Vehicle Tafenoquine H 2 O 2 Reference: 1. Liu M, et al. Antimicrob Agents Chemother . 2021;65(7):e0020421.
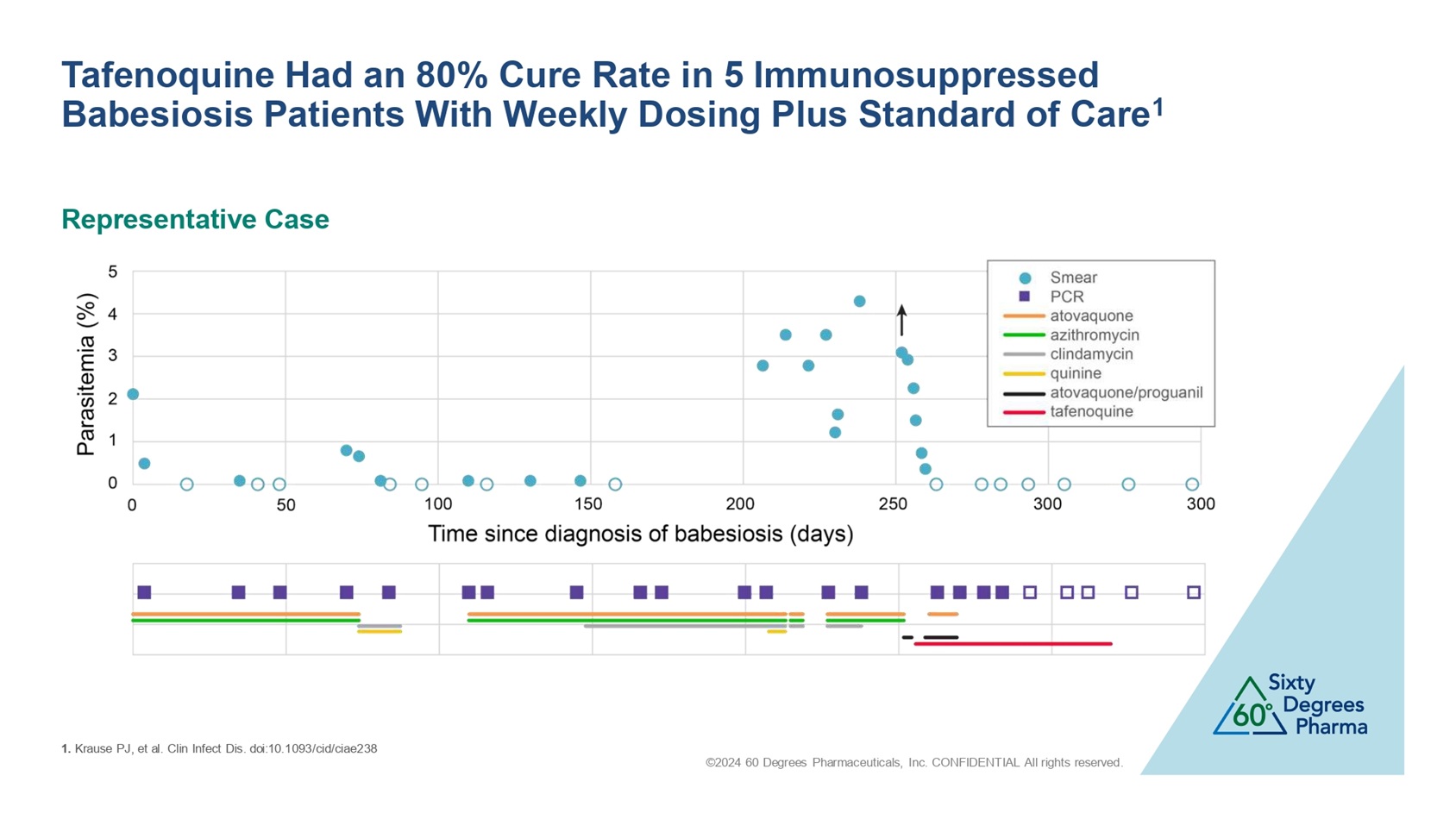
©2024 60 Degrees Pharmaceuticals, Inc. CONFIDENTIAL All rights reserved. Tafenoquine Had an 80% Cure Rate in 5 Immunosuppressed Babesiosis Patients With Weekly Dosing Plus Standard of Care 1 Representative Case 1. Krause PJ, et al. Clin Infect Dis. doi:10.1093/ cid /ciae238
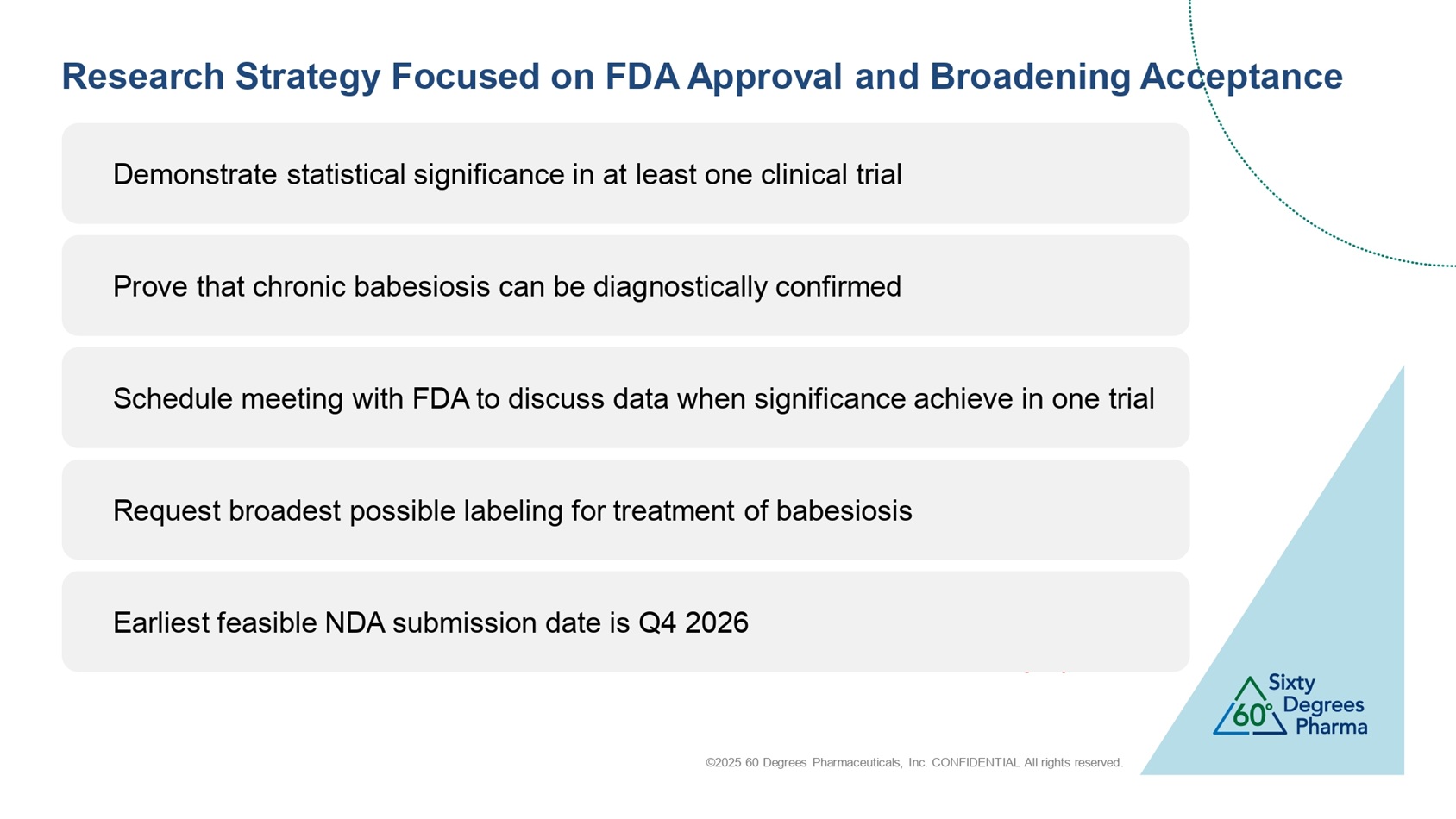
©2025 60 Degrees Pharmaceuticals, Inc. CONFIDENTIAL All rights reserved. Demonstrate statistical significance in at least one clinical trial Research Strategy Focused on FDA Approval and Broadening Acceptance Tidy up Prove that chronic babesiosis can be diagnostically confirmed Schedule meeting with FDA to discuss data when significance achieve in one trial Request broadest possible labeling for treatment of babesiosis Earliest feasible NDA submission date is Q4 2026
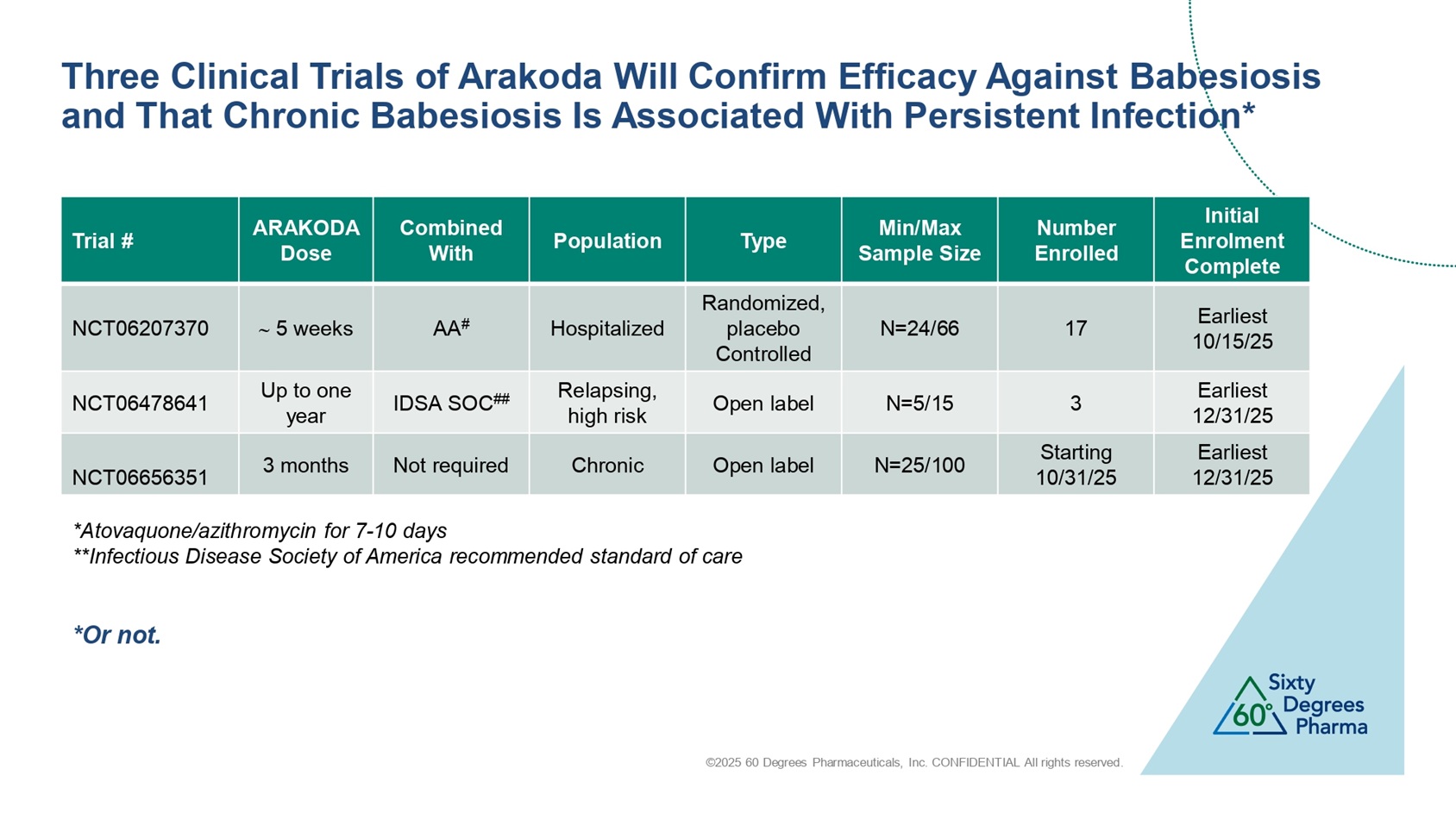
©2025 60 Degrees Pharmaceuticals, Inc. CONFIDENTIAL All rights reserved. Three Clinical Trials of Arakoda Will Confirm Efficacy Against Babesiosis and That Chronic Babesiosis Is Associated With Persistent Infection* Initial Enrolment Complete Number Enrolled Min/Max Sample Size Type Population Combined With ARAKODA Dose Trial # Earliest 10/15/25 17 N=24/66 Randomized, placebo Controlled Hospitalized AA # 5 weeks NCT06207370 Earliest 12/31/25 3 N=5/15 Open label Relapsing, high risk IDSA SOC ## Up to one year NCT06478641 Earliest 12/31/25 Starting 10/31/25 N=25/100 Open label Chronic Not required 3 months NCT06656351 *Atovaquone/azithromycin for 7 - 10 days **Infectious Disease Society of America recommended standard of care * Or not.
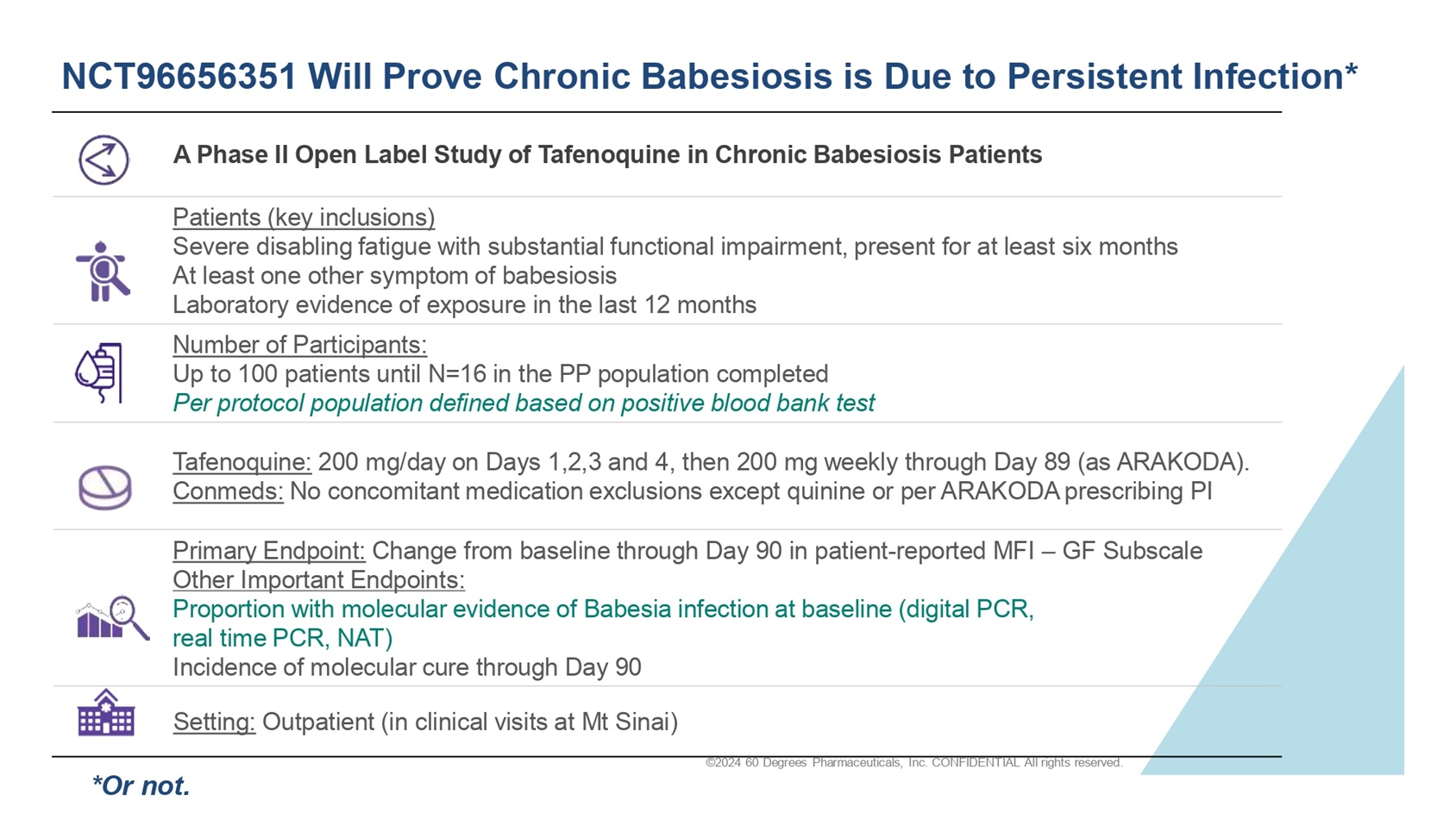
©2024 60 Degrees Pharmaceuticals, Inc. CONFIDENTIAL All rights reserved. NCT96656351 Will Prove Chronic Babesiosis is Due to Persistent Infection* A Phase II Open Label Study of Tafenoquine in Chronic Babesiosis Patients Patients (key inclusions) Severe disabling fatigue with substantial functional impairment, present for at least six months At least one other symptom of babesiosis Laboratory evidence of exposure in the last 12 months Number of Participants: Up to 100 patients until N=16 in the PP population completed Per protocol population defined based on positive blood bank test Tafenoquine: 200 mg/day on Days 1,2,3 and 4, then 200 mg weekly through Day 89 (as ARAKODA). Conmeds : No concomitant medication exclusions except quinine or per ARAKODA prescribing PI Primary Endpoint: Change from baseline through Day 90 in patient - reported MFI – GF Subscale Other Important Endpoints : Proportion with molecular evidence of Babesia infection at baseline (digital PCR, real time PCR, NAT) Incidence of molecular cure through Day 90 Setting: Outpatient (in clinical visits at Mt Sinai) *Or not.
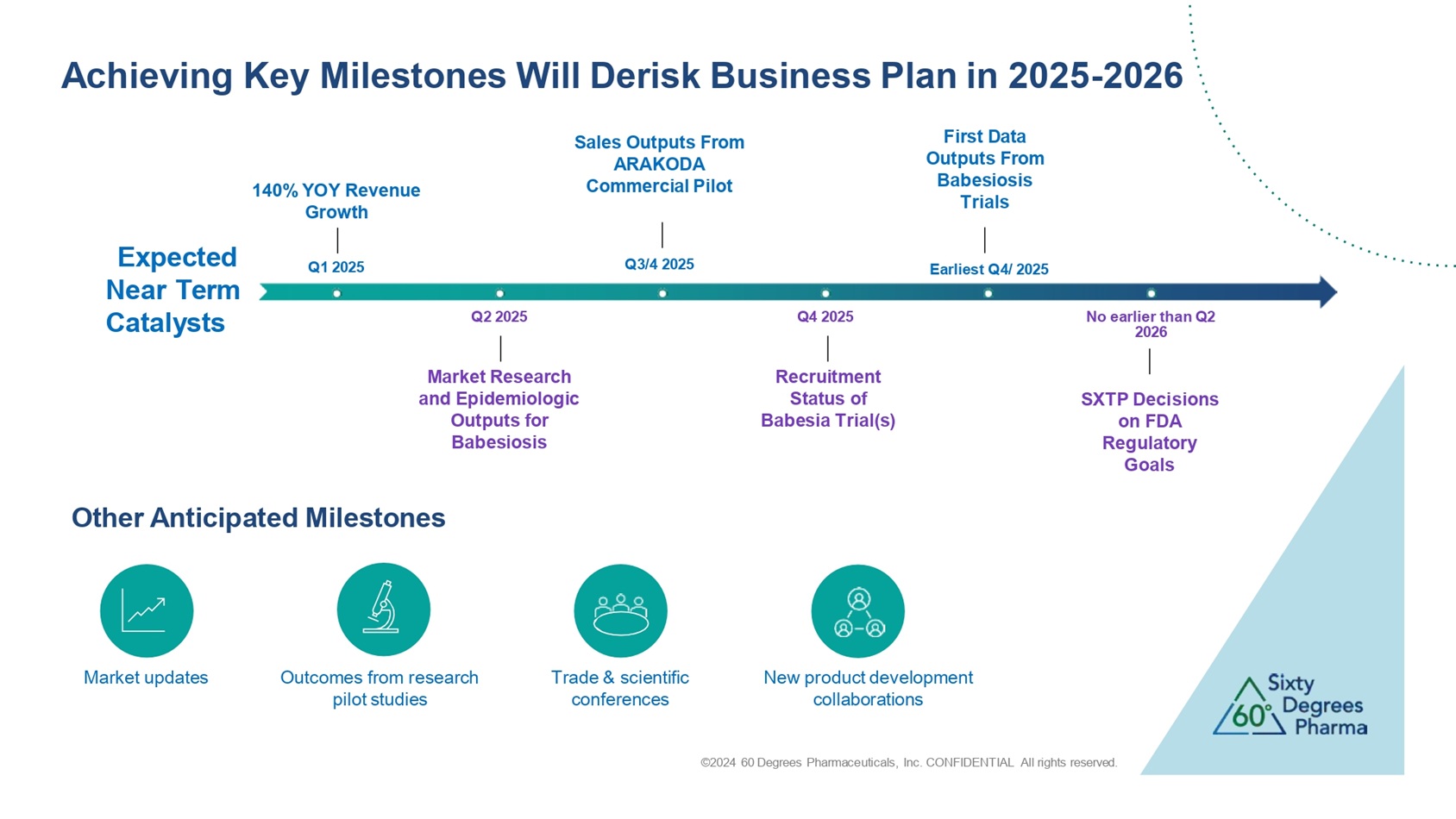
Achieving Key Milestones Will Derisk Business Plan in 2025 - 2026 Q1 2025 Q2 2025 Q3 /4 2025 Q4 2025 Earliest Q 4 / 202 5 No earlier than Q2 2026 140% YOY Revenue Growth Recruitment Status of Babesia Trial(s) Market Research and Epidemiologic Outputs for Babesiosis Sales Outputs From ARAKODA Commercial Pilot First Data Outputs From Babesiosis Trials SXTP Decisions on FDA Regulatory Goals Expected Near Term Catalysts Other Anticipated Milestones Outcomes from research pilot studies ©2024 60 Degrees Pharmaceuticals, Inc. CONFIDENTIAL All rights reserved. New product development collaborations Market updates Trade & scientific conferences