CORPORATE OVERVIEW September 2025

Important Notice and Disclaimers This presentation contains forward-looking statements within the meaning of The Private Securities Litigation Reform Act of 1995. All statements other than statements of historical facts contained in this presentation, including express or implied statements regarding the Company’s beliefs and expectations related to: our preclinical and clinical development plans and timelines, the clinical and therapeutic potential of our product candidates, the strategy of our product candidates, our research and development activities, our future financial condition, our future operations and projected costs, prospects and plans of management are forward-looking statements. In some cases, you can identify forward-looking statements by terminology such as “anticipate,” “believe,” “continue,” “estimate,” “expect,” “intend,” “may,” “plan,” “potential,” “predict,” “seek,” “should,” “target,” “will” or the negative of these terms or other comparable terminology. Any forward-looking statements in this presentation are based on management's current expectations and beliefs of future events and are subject to known and unknown risks and uncertainties that may cause our actual results, performance or achievements to be materially different from any future results, performance or achievements expressed or implied by the forward-looking statements. These risks include, but are not limited to, the following: uncertainty regarding the timing and results of regulatory submissions; the risk that any INDs, NDAs or other global regulatory submissions we may file with the United States Food and Drug Administration or other global regulatory agencies are not cleared on our expected timelines, or at all; the success of our clinical trials and preclinical studies; the risks related to our ability to protect and maintain our intellectual property position; the risks related to manufacturing, supply, and distribution of our product candidates; the risk that any one or more of our product candidates, including those that are co-developed, will not be successfully developed and commercialized; the risk that the results of preclinical studies or clinical studies will not be predictive of future results in connection with future studies; the success of any collaboration, partnership, license or similar agreements; and other important risks and uncertainties discussed in our filings with the Securities and Exchange Commission, including under the caption “Risk Factors” in our most recent Annual Report on Form 10-K, Quarterly Report on Form 10-Q and other filings that we make with the SEC from time to time. These risks could cause actual results to differ materially from those indicated by the forward-looking statements made in this presentation. While we may elect to update such forward-looking statements at some point in the future, we disclaim any obligation to do so, even if subsequent events cause our views to change, except to the extent required by law. These forward-looking statements should not be relied upon as representing our views as of any date subsequent to the date of this presentation. Moreover, except as required by law, neither Cullinan nor any other person assumes responsibility for the accuracy and completeness of the forward-looking statements included in this presentation. Any forward-looking statement included in this presentation speaks only as of the date on which it was made. Certain information contained in this presentation relates to or is based on studies, publications, surveys and other data obtained from third-party sources and our own internal estimates and research. While we believe these third-party sources to be reliable as of the date of this presentation, we have not independently verified, and make no representation as to the adequacy, fairness, accuracy or completeness of, any information obtained from third-party sources. In addition, all of the market data included in this presentation involves a number of assumptions and limitations, and there can be no guarantee as to the accuracy or reliability of such assumptions. Finally, while we believe our own internal research is reliable, such research has not been verified by any independent source.

CULLINAN THERAPEUTICS Our Mission: Create new standards of care for patients We use a unique R&D model of identifying high-impact targets and then applying the best modality to address each target We are rigorously advancing only highly-differentiated molecules, yielding a robust portfolio of clinical-stage programs, including three T cell engagers, a core area of development expertise Cullinan has a leadership position in the development of T cell engagers for autoimmune diseases with CLN-978 (CD19xCD3) and velinotamig (BCMAxCD3), utilizing a comprehensive potential disease modifying approach through both B cell and plasma cell depletion. CLN-978 is being investigated in systemic lupus erythematosus (SLE), rheumatoid arthritis (RA) and Sjögren's disease (SjD). Genrix Bio is planning a Phase 1 study of velinotamig in China in patients with autoimmune diseases to begin later this year. We are simultaneously advancing a diversified pipeline of clinical-stage oncology programs, with multiple catalysts in 2025 We are well-positioned to execute on strategic goals and advance to a commercial-stage organization, with cash runway into 2028* © CULLINAN THERAPEUTICS, INC. ALL RIGHTS RESERVED. *As of June 30, 2025
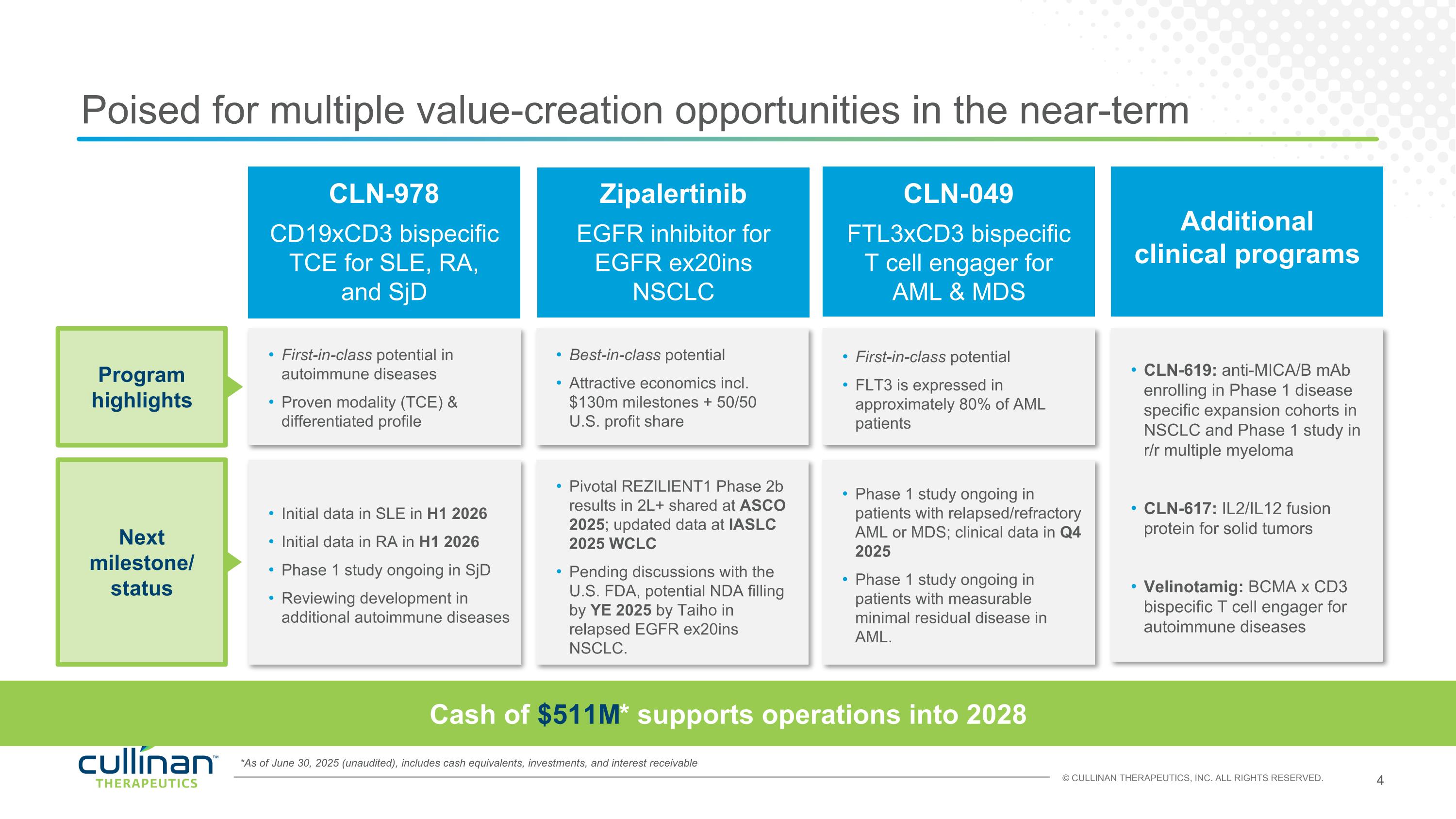
*As of June 30, 2025 (unaudited), includes cash equivalents, investments, and interest receivable Poised for multiple value-creation opportunities in the near-term Cash of $511M* supports operations into 2028 First-in-class potential in autoimmune diseases Proven modality (TCE) & differentiated profile First-in-class potential FLT3 is expressed in approximately 80% of AML patients Best-in-class potential Attractive economics incl. $130m milestones + 50/50 U.S. profit share CLN-619: anti-MICA/B mAb enrolling in Phase 1 disease specific expansion cohorts in NSCLC and Phase 1 study in r/r multiple myeloma CLN-617: IL2/IL12 fusion protein for solid tumors Velinotamig: BCMA x CD3 bispecific T cell engager for autoimmune diseases Initial data in SLE in H1 2026 Initial data in RA in H1 2026 Phase 1 study ongoing in SjD Reviewing development in additional autoimmune diseases Phase 1 study ongoing in patients with relapsed/refractory AML or MDS; clinical data in Q4 2025 Phase 1 study ongoing in patients with measurable minimal residual disease in AML. Pivotal REZILIENT1 Phase 2b results in 2L+ shared at ASCO 2025; updated data at IASLC 2025 WCLC Pending discussions with the U.S. FDA, potential NDA filling by YE 2025 by Taiho in relapsed EGFR ex20ins NSCLC. CLN-978 CD19xCD3 bispecific TCE for SLE, RA, and SjD CLN-049 FTL3xCD3 bispecific T cell engager for AML & MDS Additional clinical programs Zipalertinib EGFR inhibitor for EGFR ex20ins NSCLC Program highlights Next milestone/ status

T Cell Engagers in Autoimmune Diseases
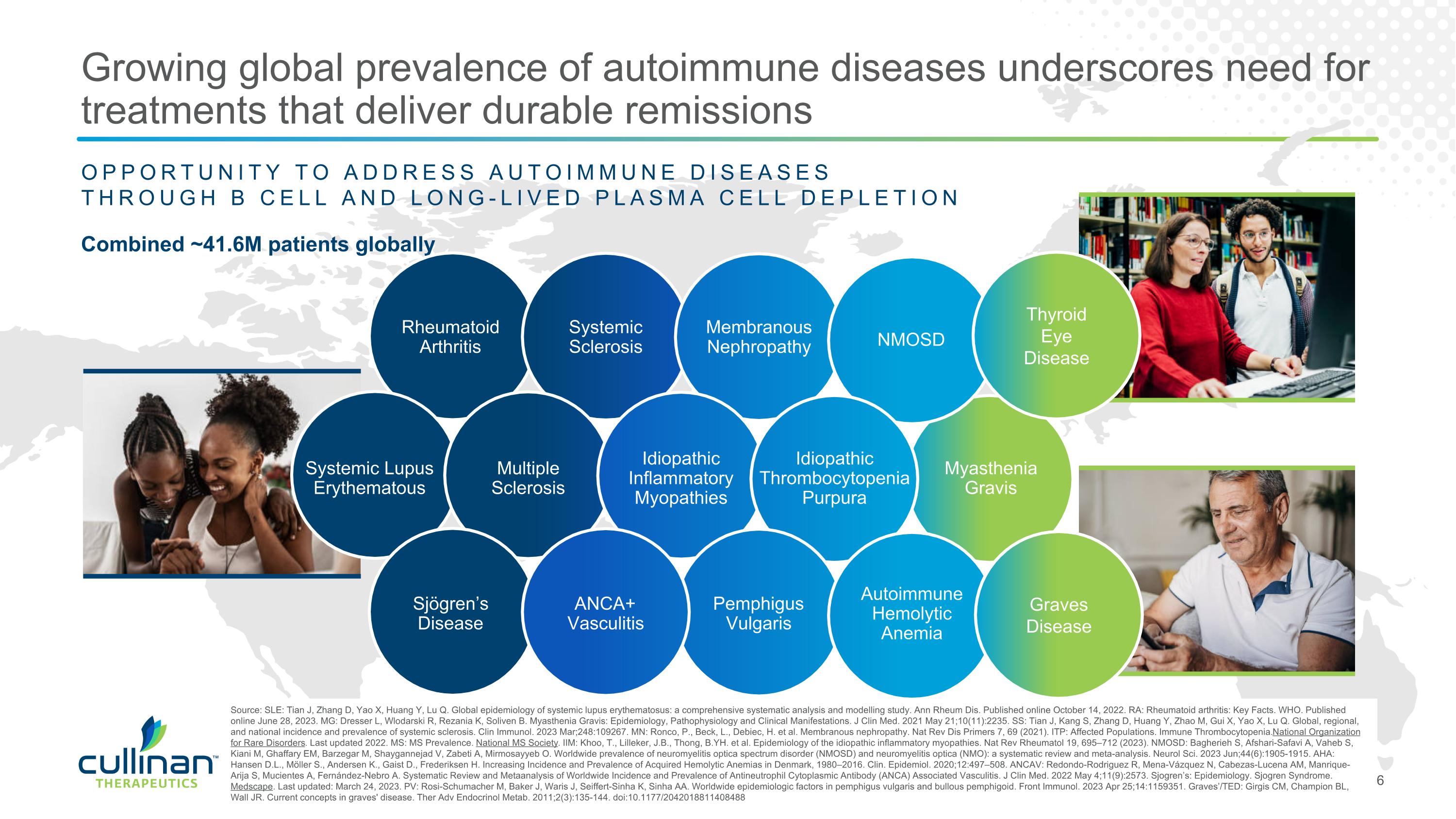
Combined ~41.6M patients globally Source: SLE: Tian J, Zhang D, Yao X, Huang Y, Lu Q. Global epidemiology of systemic lupus erythematosus: a comprehensive systematic analysis and modelling study. Ann Rheum Dis. Published online October 14, 2022. RA: Rheumatoid arthritis: Key Facts. WHO. Published online June 28, 2023. MG: Dresser L, Wlodarski R, Rezania K, Soliven B. Myasthenia Gravis: Epidemiology, Pathophysiology and Clinical Manifestations. J Clin Med. 2021 May 21;10(11):2235. SS: Tian J, Kang S, Zhang D, Huang Y, Zhao M, Gui X, Yao X, Lu Q. Global, regional, and national incidence and prevalence of systemic sclerosis. Clin Immunol. 2023 Mar;248:109267. MN: Ronco, P., Beck, L., Debiec, H. et al. Membranous nephropathy. Nat Rev Dis Primers 7, 69 (2021). ITP: Affected Populations. Immune Thrombocytopenia. National Organization for Rare Disorders. Last updated 2022. MS: MS Prevalence. National MS Society. IIM: Khoo, T., Lilleker, J.B., Thong, B.YH. et al. Epidemiology of the idiopathic inflammatory myopathies. Nat Rev Rheumatol 19, 695–712 (2023). NMOSD: Bagherieh S, Afshari-Safavi A, Vaheb S, Kiani M, Ghaffary EM, Barzegar M, Shaygannejad V, Zabeti A, Mirmosayyeb O. Worldwide prevalence of neuromyelitis optica spectrum disorder (NMOSD) and neuromyelitis optica (NMO): a systematic review and meta-analysis. Neurol Sci. 2023 Jun;44(6):1905-1915. AHA: Hansen D.L., Möller S., Andersen K., Gaist D., Frederiksen H. Increasing Incidence and Prevalence of Acquired Hemolytic Anemias in Denmark, 1980–2016. Clin. Epidemiol. 2020;12:497–508. ANCAV: Redondo-Rodriguez R, Mena-Vázquez N, Cabezas-Lucena AM, Manrique-Arija S, Mucientes A, Fernández-Nebro A. Systematic Review and Metaanalysis of Worldwide Incidence and Prevalence of Antineutrophil Cytoplasmic Antibody (ANCA) Associated Vasculitis. J Clin Med. 2022 May 4;11(9):2573. Sjogren’s: Epidemiology. Sjogren Syndrome. Medscape. Last updated: March 24, 2023. PV: Rosi-Schumacher M, Baker J, Waris J, Seiffert-Sinha K, Sinha AA. Worldwide epidemiologic factors in pemphigus vulgaris and bullous pemphigoid. Front Immunol. 2023 Apr 25;14:1159351. Graves’/TED: Girgis CM, Champion BL, Wall JR. Current concepts in graves' disease. Ther Adv Endocrinol Metab. 2011;2(3):135-144. doi:10.1177/2042018811408488 OPPORTUNITY TO ADDRESS AUTOIMMUNE DISEASES THROUGH B CELL AND LONG-LIVED PLASMA CELL DEPLETION Multiple Sclerosis Rheumatoid Arthritis Systemic Sclerosis Membranous Nephropathy Idiopathic Thrombocytopenia Purpura Sjögren’s Disease ANCA+ Vasculitis Pemphigus Vulgaris Autoimmune Hemolytic Anemia Idiopathic Inflammatory Myopathies Systemic Lupus Erythematous Myasthenia Gravis NMOSD Growing global prevalence of autoimmune diseases underscores need for treatments that deliver durable remissions Thyroid Eye Disease Graves Disease
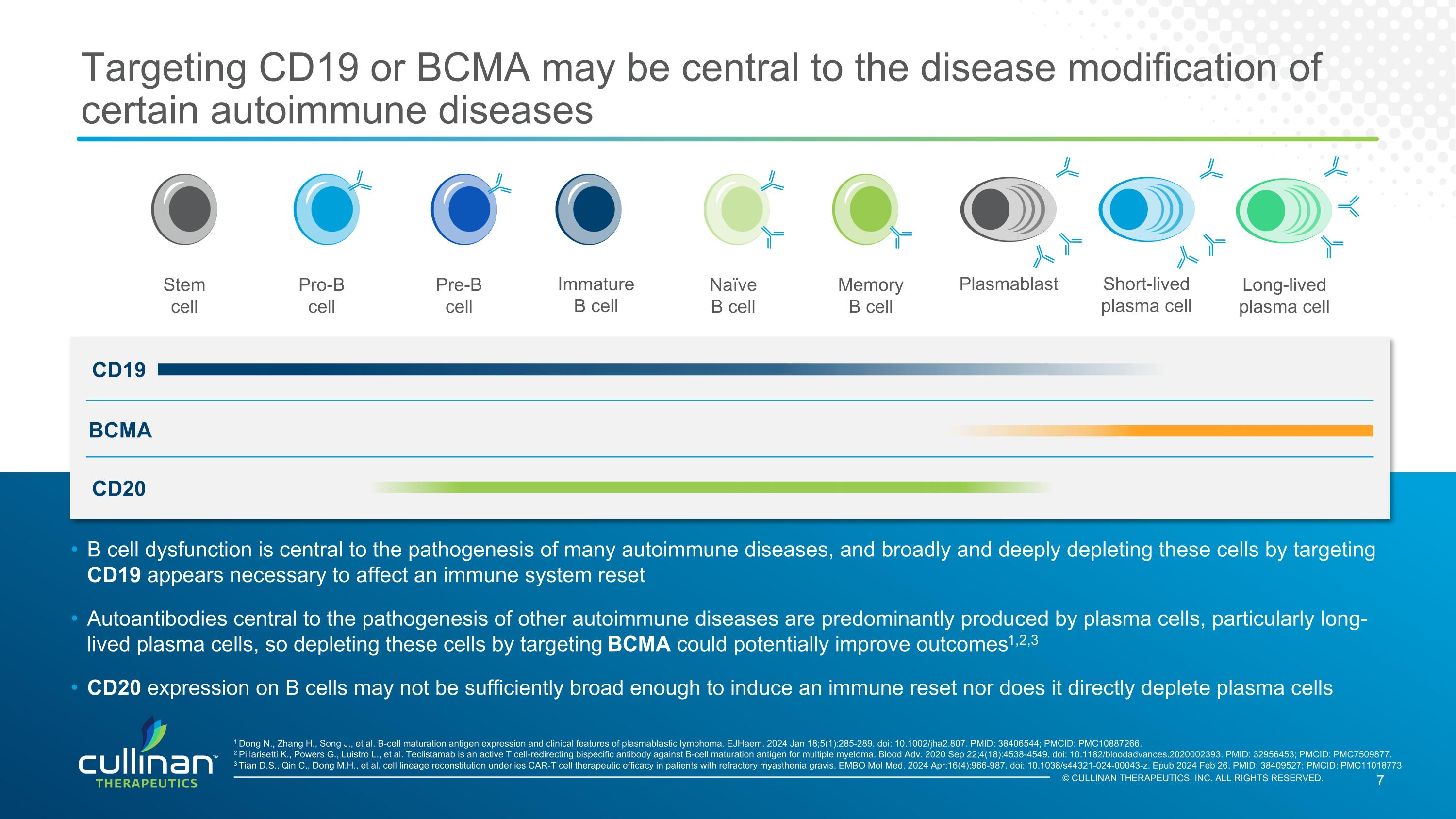
© CULLINAN THERAPEUTICS, INC. ALL RIGHTS RESERVED. Targeting CD19 or BCMA may be central to the disease modification of certain autoimmune diseases BCMA B cell dysfunction is central to the pathogenesis of many autoimmune diseases, and broadly and deeply depleting these cells by targeting CD19 appears necessary to affect an immune system reset Autoantibodies central to the pathogenesis of other autoimmune diseases are predominantly produced by plasma cells, particularly long-lived plasma cells, so depleting these cells by targeting BCMA could potentially improve outcomes1,2,3 CD20 expression on B cells may not be sufficiently broad enough to induce an immune reset nor does it directly deplete plasma cells Stem cell Pro-B cell Pre-B cell Immature B cell Naïve B cell Memory B cell Plasmablast Short-lived plasma cell Long-lived plasma cell 1 Dong N., Zhang H., Song J., et al. B-cell maturation antigen expression and clinical features of plasmablastic lymphoma. EJHaem. 2024 Jan 18;5(1):285-289. doi: 10.1002/jha2.807. PMID: 38406544; PMCID: PMC10887266. 2 Pillarisetti K., Powers G., Luistro L., et al. Teclistamab is an active T cell-redirecting bispecific antibody against B-cell maturation antigen for multiple myeloma. Blood Adv. 2020 Sep 22;4(18):4538-4549. doi: 10.1182/bloodadvances.2020002393. PMID: 32956453; PMCID: PMC7509877. 3 Tian D.S., Qin C., Dong M.H., et al. cell lineage reconstitution underlies CAR-T cell therapeutic efficacy in patients with refractory myasthenia gravis. EMBO Mol Med. 2024 Apr;16(4):966-987. doi: 10.1038/s44321-024-00043-z. Epub 2024 Feb 26. PMID: 38409527; PMCID: PMC11018773 CD19 CD20
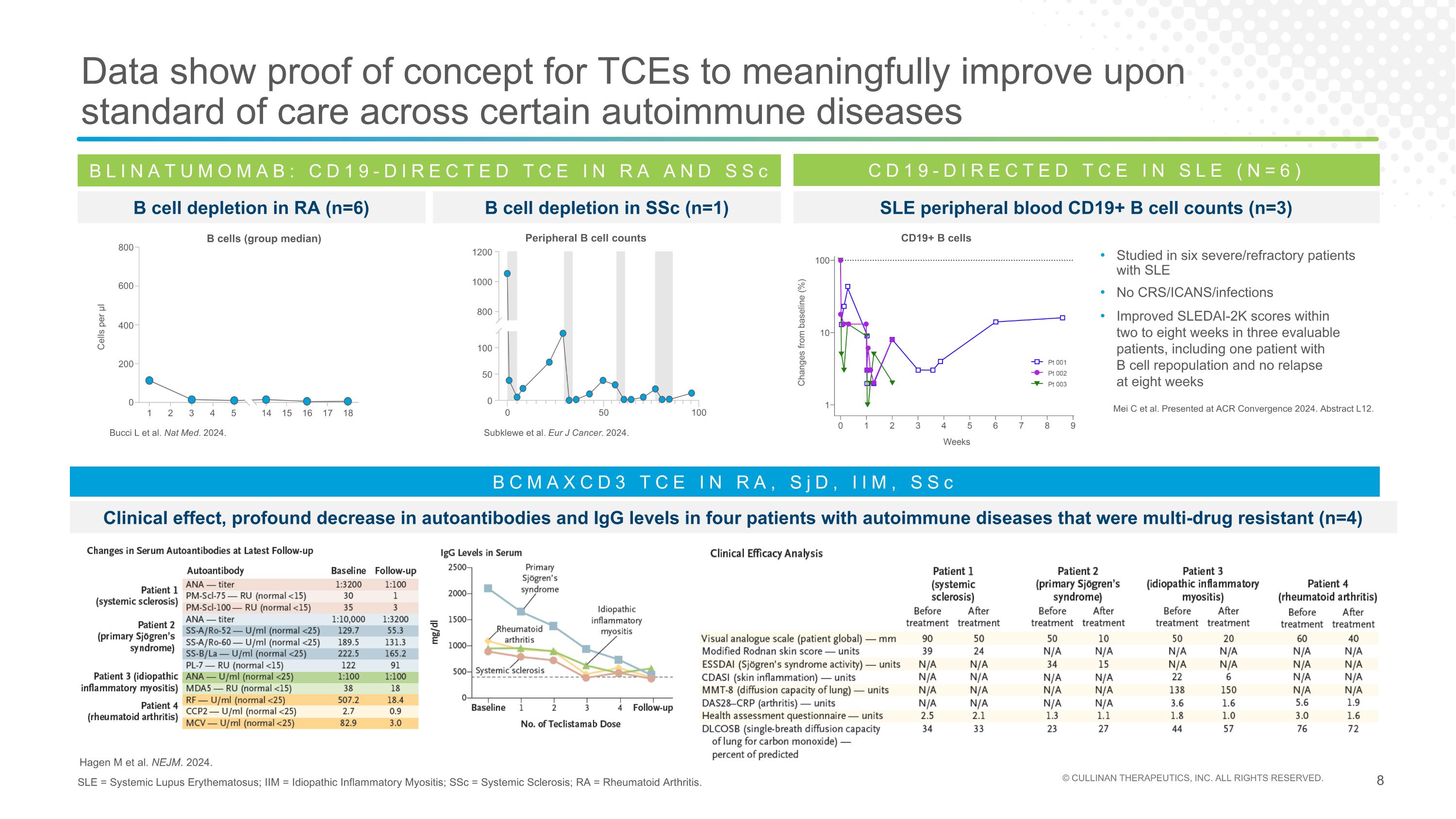
Data show proof of concept for TCEs to meaningfully improve upon standard of care across certain autoimmune diseases SLE = Systemic Lupus Erythematosus; IIM = Idiopathic Inflammatory Myositis; SSc = Systemic Sclerosis; RA = Rheumatoid Arthritis. BLINATUMOMAB: CD19-DIRECTED TCE IN RA AND SSc B cell depletion in RA (n=6) Bucci L et al. Nat Med. 2024. B Cells (Group median) Cells per µl 800 600 400 200 0 1 2 3 4 5 14 15 16 17 18 Subklewe et al. Eur J Cancer. 2024. Peripheral B cell counts 0 50 100 0 50 100 800 1000 1200 B cell depletion in SSc (n=1) CD19-DIRECTED TCE IN SLE (N=6) Mei C et al. Presented at ACR Convergence 2024. Abstract L12. SLE peripheral blood CD19+ B cell counts (n=3) Studied in six severe/refractory patients with SLE No CRS/ICANS/infections Improved SLEDAI-2K scores within two to eight weeks in three evaluable patients, including one patient with B cell repopulation and no relapse at eight weeks CD19+ B cells Hagen M et al. NEJM. 2024. BCMAXCD3 TCE IN RA, SjD, IIM, SSc Clinical effect, profound decrease in autoantibodies and IgG levels in four patients with autoimmune diseases that were multi-drug resistant (n=4) B cells (group median)
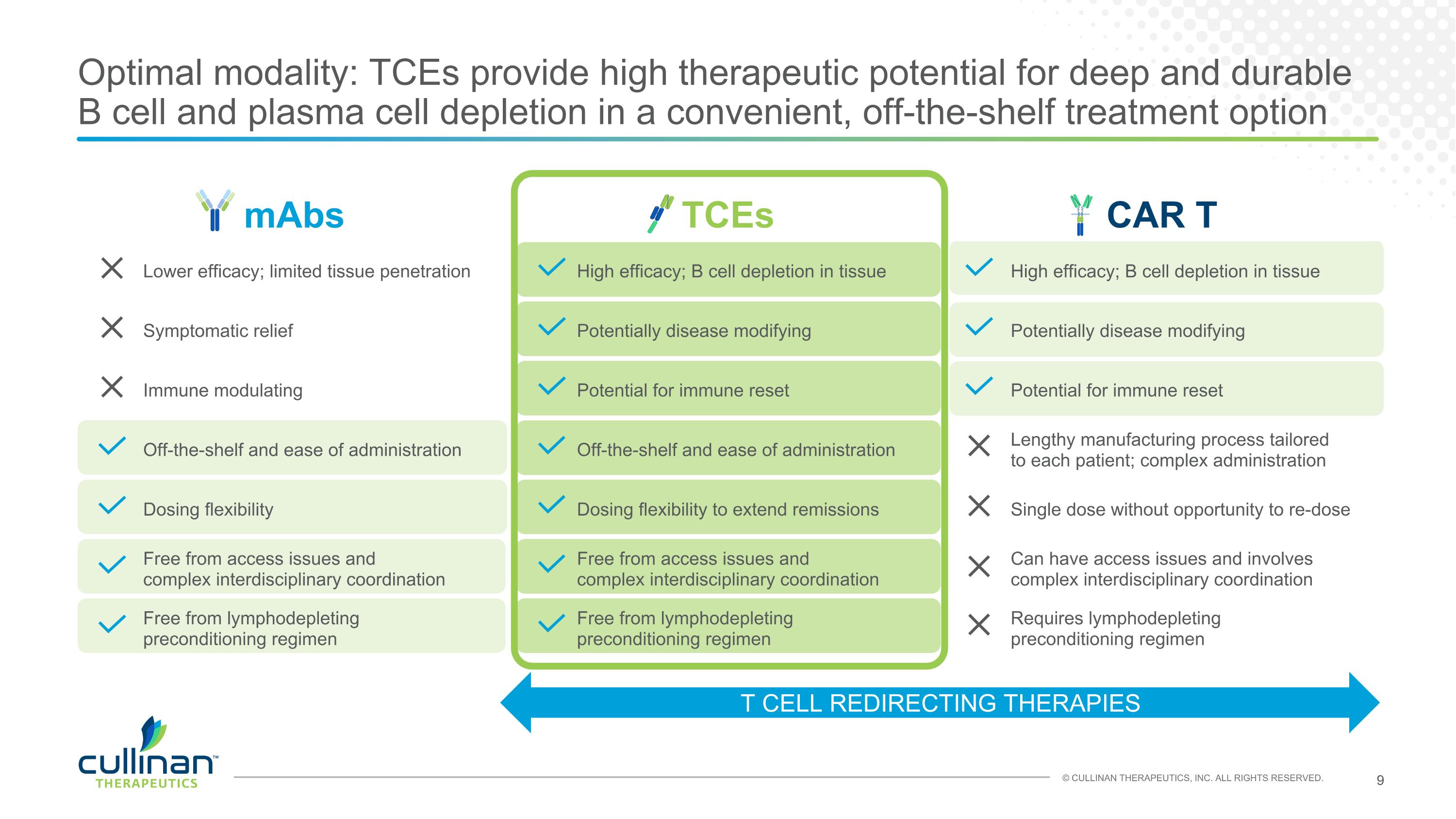
mAbs TCEs CAR T Lower efficacy; limited tissue penetration High efficacy; B cell depletion in tissue High efficacy; B cell depletion in tissue Symptomatic relief Potentially disease modifying Potentially disease modifying Immune modulating Potential for immune reset Potential for immune reset Off-the-shelf and ease of administration Off-the-shelf and ease of administration Lengthy manufacturing process tailored to each patient; complex administration Dosing flexibility Dosing flexibility to extend remissions Single dose without opportunity to re-dose Free from access issues and complex interdisciplinary coordination Free from access issues and complex interdisciplinary coordination Can have access issues and involves complex interdisciplinary coordination Free from lymphodepleting preconditioning regimen Free from lymphodepleting preconditioning regimen Requires lymphodepleting preconditioning regimen Optimal modality: TCEs provide high therapeutic potential for deep and durable B cell and plasma cell depletion in a convenient, off-the-shelf treatment option T CELL REDIRECTING THERAPIES
CLN-978 CD19xCD3 bispecific T cell engager
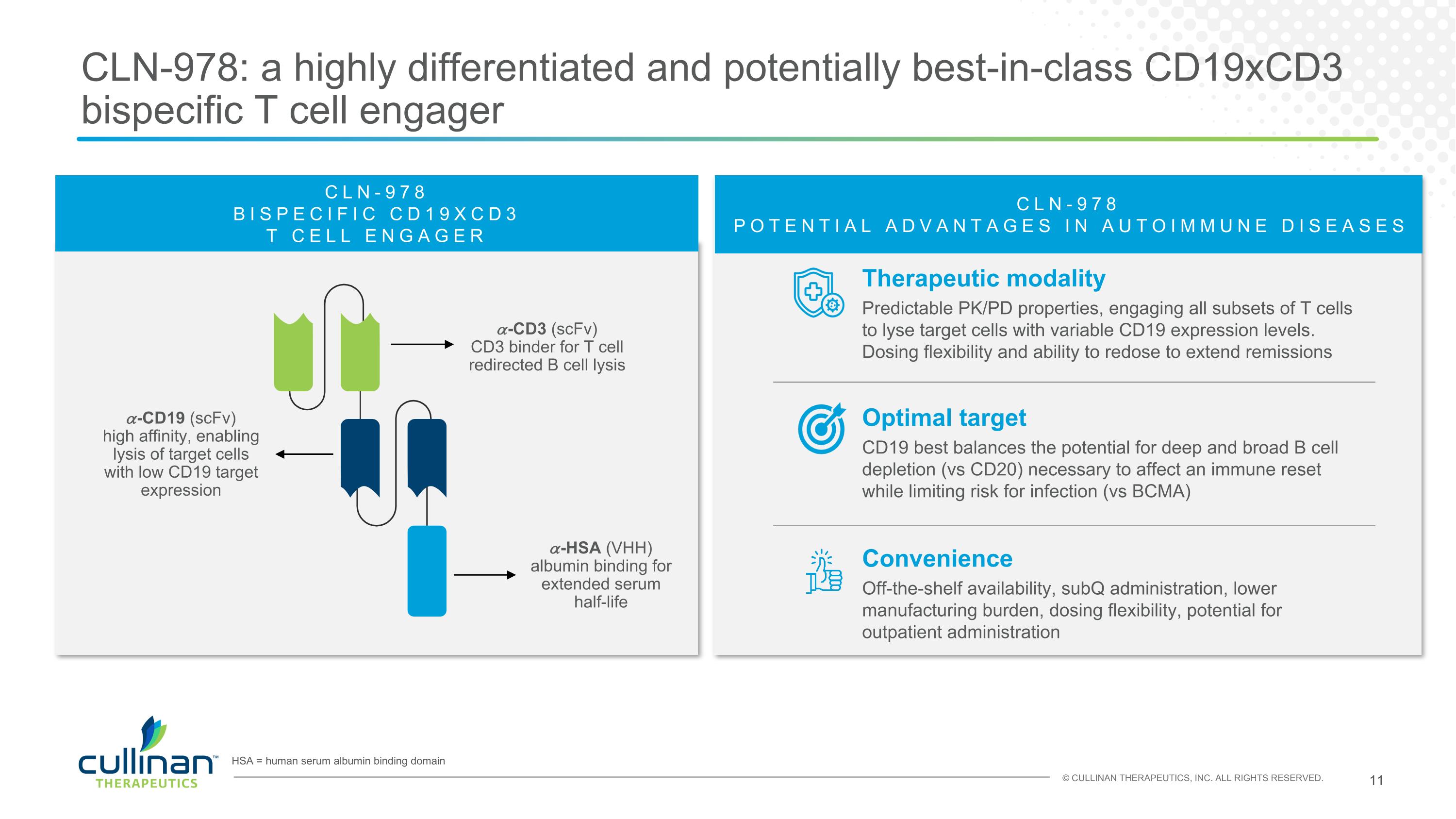
CLN-978: a highly differentiated and potentially best-in-class CD19xCD3 bispecific T cell engager HSA = human serum albumin binding domain Therapeutic modality Predictable PK/PD properties, engaging all subsets of T cells to lyse target cells with variable CD19 expression levels. Dosing flexibility and ability to redose to extend remissions CLN-978 POTENTIAL ADVANTAGES IN AUTOIMMUNE DISEASES Convenience Off-the-shelf availability, subQ administration, lower manufacturing burden, dosing flexibility, potential for outpatient administration Optimal target CD19 best balances the potential for deep and broad B cell depletion (vs CD20) necessary to affect an immune reset while limiting risk for infection (vs BCMA) 𝛼-CD19 (scFv) high affinity, enabling lysis of target cells with low CD19 target expression 𝛼-CD3 (scFv) CD3 binder for T cell redirected B cell lysis 𝛼-HSA (VHH) albumin binding for extended serum half-life CLN-978 BISPECIFIC CD19XCD3 T CELL ENGAGER
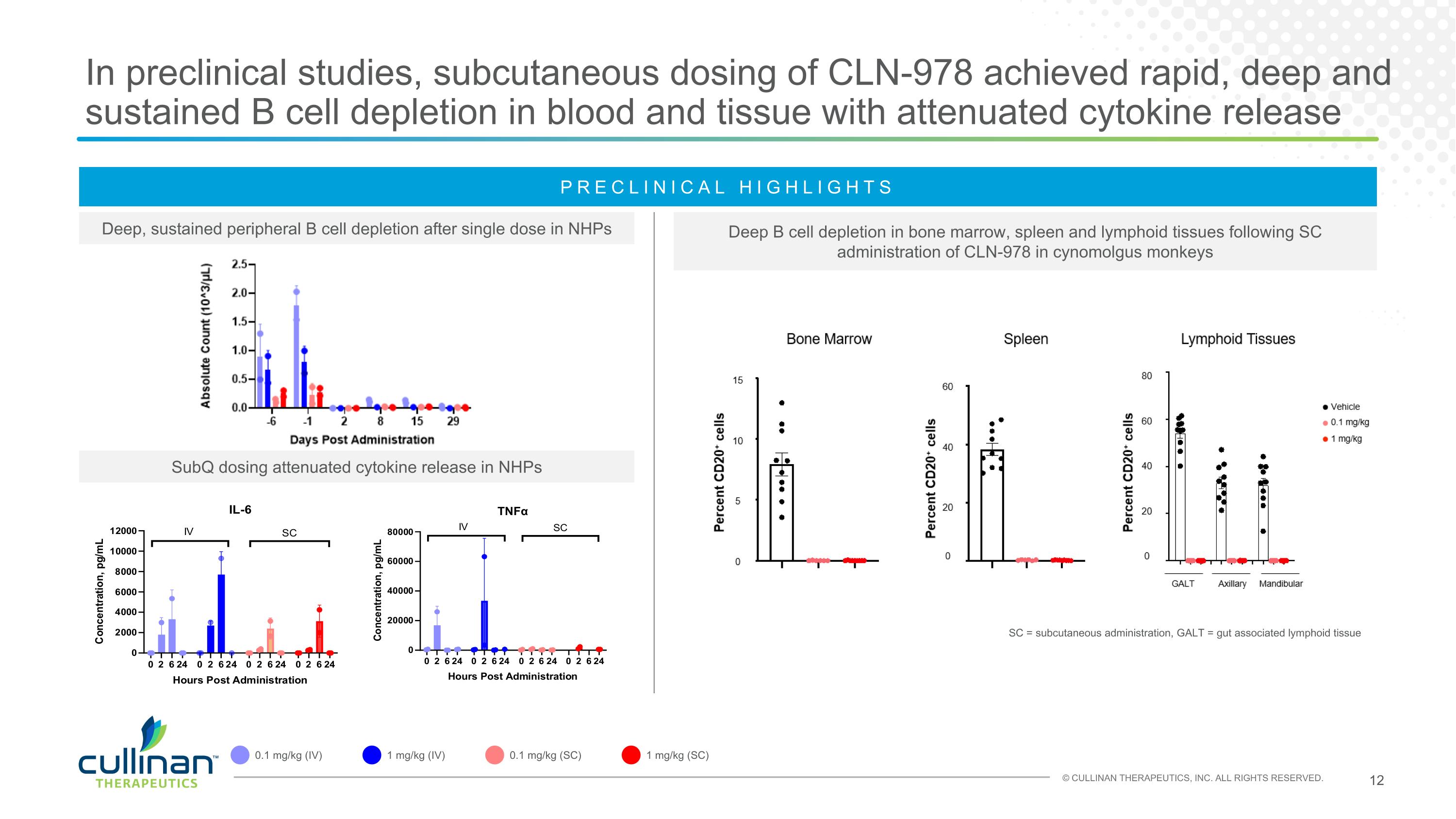
In preclinical studies, subcutaneous dosing of CLN-978 achieved rapid, deep and sustained B cell depletion in blood and tissue with attenuated cytokine release Deep, sustained peripheral B cell depletion after single dose in NHPs SubQ dosing attenuated cytokine release in NHPs Deep B cell depletion in bone marrow, spleen and lymphoid tissues following SC administration of CLN-978 in cynomolgus monkeys SC = subcutaneous administration, GALT = gut associated lymphoid tissue PRECLINICAL HIGHLIGHTS 0.1 mg/kg (IV) 1 mg/kg (IV) 0.1 mg/kg (SC) 1 mg/kg (SC)
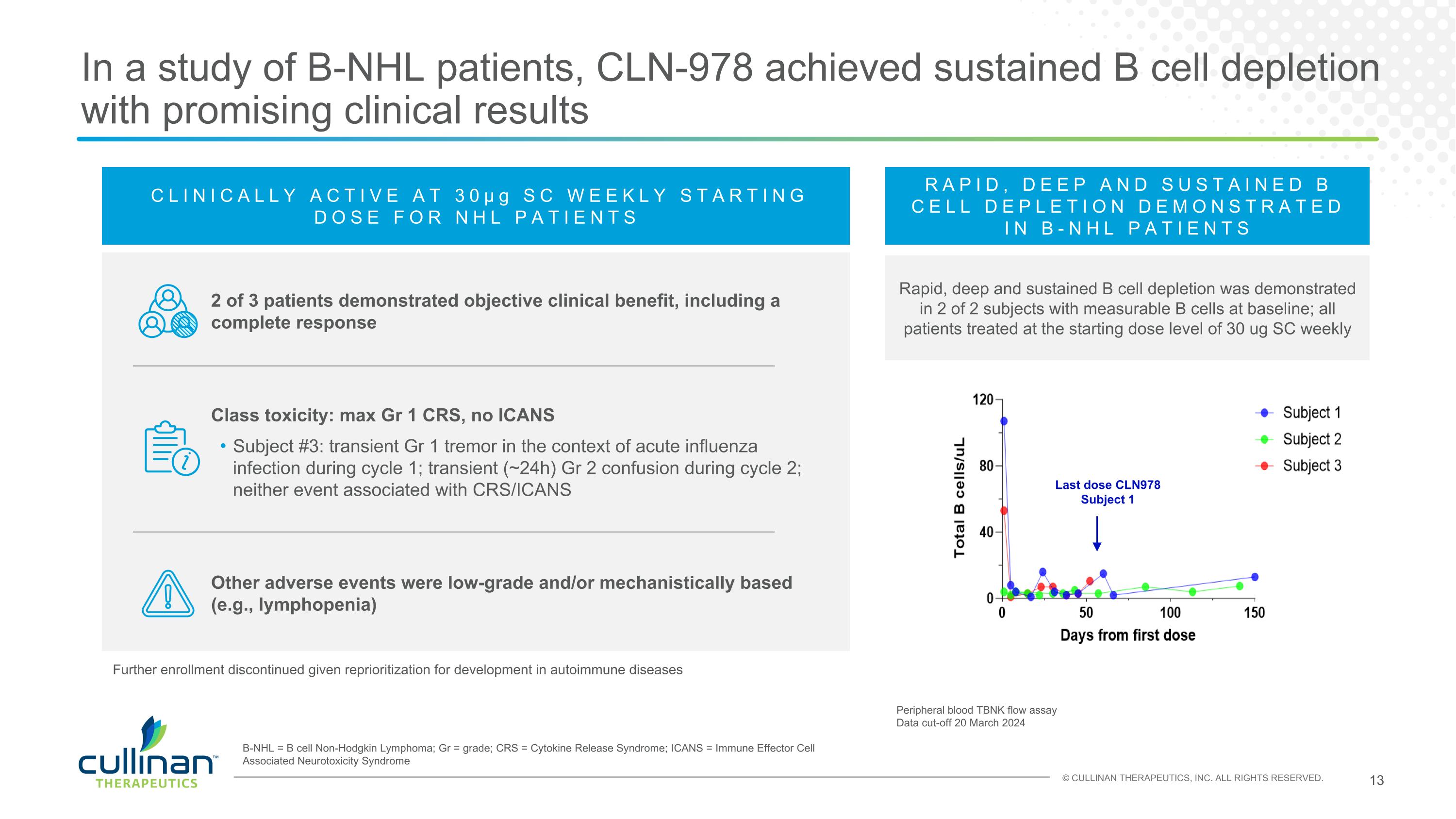
In a study of B-NHL patients, CLN-978 achieved sustained B cell depletion with promising clinical results CLINICALLY ACTIVE AT 30μg SC WEEKLY STARTING DOSE FOR NHL PATIENTS 2 of 3 patients demonstrated objective clinical benefit, including a complete response Class toxicity: max Gr 1 CRS, no ICANS Subject #3: transient Gr 1 tremor in the context of acute influenza infection during cycle 1; transient (~24h) Gr 2 confusion during cycle 2; neither event associated with CRS/ICANS Other adverse events were low-grade and/or mechanistically based (e.g., lymphopenia) Last dose CLN978 Subject 1 Peripheral blood TBNK flow assay Data cut-off 20 March 2024 RAPID, DEEP AND SUSTAINED B CELL DEPLETION DEMONSTRATED IN B-NHL PATIENTS Rapid, deep and sustained B cell depletion was demonstrated in 2 of 2 subjects with measurable B cells at baseline; all patients treated at the starting dose level of 30 ug SC weekly B-NHL = B cell Non-Hodgkin Lymphoma; Gr = grade; CRS = Cytokine Release Syndrome; ICANS = Immune Effector Cell Associated Neurotoxicity Syndrome Further enrollment discontinued given reprioritization for development in autoimmune diseases
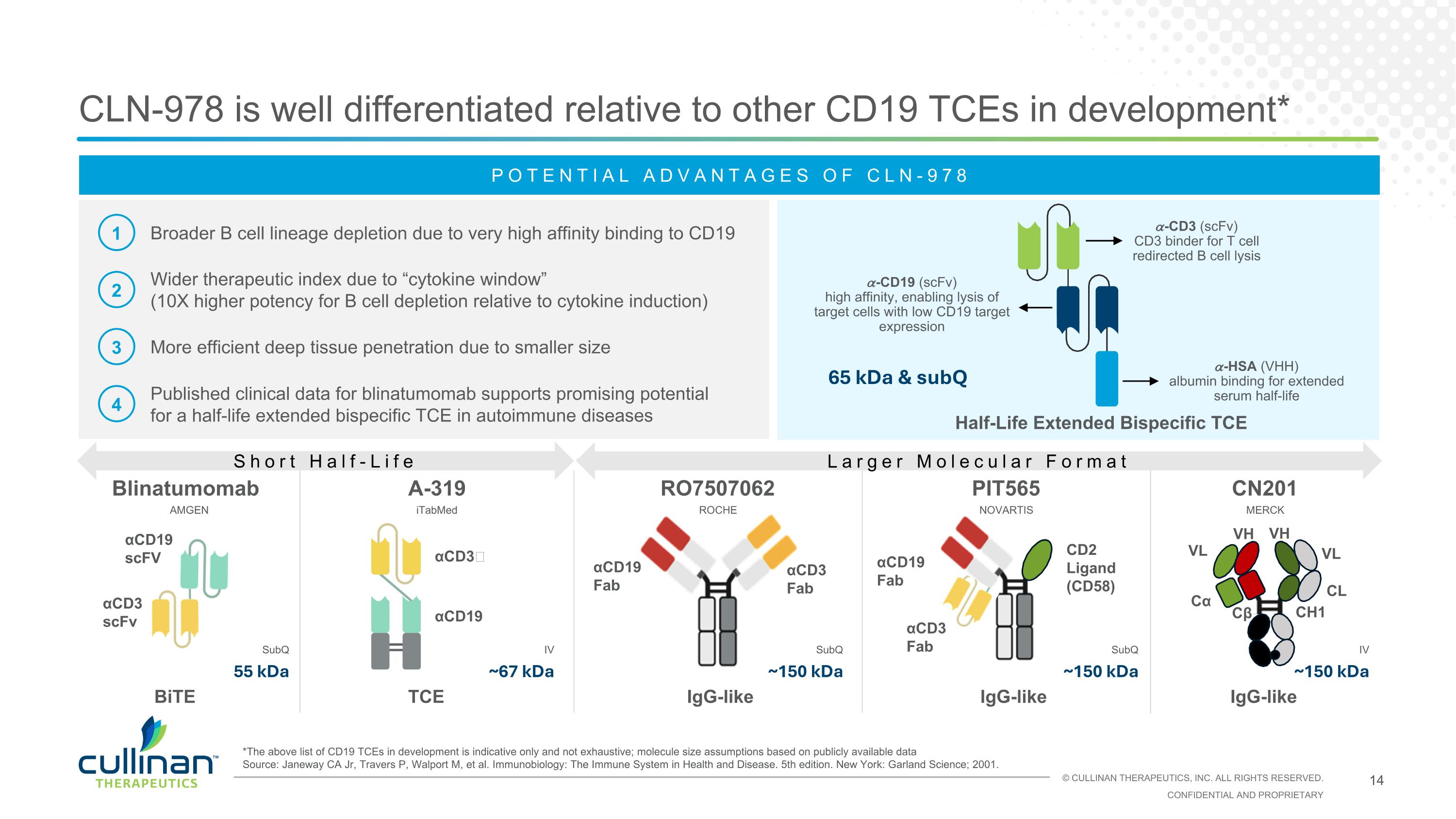
αCD3 scFv αCD19 scFV αCD3 Fab αCD19 Fab PIT565 RO7507062 CLN-978 is well differentiated relative to other CD19 TCEs in development* IgG-like Blinatumomab IgG-like αCD3 Fab αCD19 Fab CD2 Ligand (CD58) BiTE POTENTIAL ADVANTAGES OF CLN-978 1 2 3 4 Broader B cell lineage depletion due to very high affinity binding to CD19 Wider therapeutic index due to “cytokine window” (10X higher potency for B cell depletion relative to cytokine induction) More efficient deep tissue penetration due to smaller size Published clinical data for blinatumomab supports promising potential for a half-life extended bispecific TCE in autoimmune diseases CN201 *The above list of CD19 TCEs in development is indicative only and not exhaustive; molecule size assumptions based on publicly available data Source: Janeway CA Jr, Travers P, Walport M, et al. Immunobiology: The Immune System in Health and Disease. 5th edition. New York: Garland Science; 2001. AMGEN ROCHE NOVARTIS IgG-like Cα CL VL VH CH1 Cβ VL VH 55 kDa ~150 kDa SubQ MERCK SubQ ~150 kDa SubQ ~150 kDa IV A-319 TCE iTabMed ~67 kDa IV Half-Life Extended Bispecific TCE 65 kDa & subQ αCD3ɛ αCD19 Short Half-Life Larger Molecular Format 𝛼-CD19 (scFv) high affinity, enabling lysis of target cells with low CD19 target expression 𝛼-CD3 (scFv) CD3 binder for T cell redirected B cell lysis 𝛼-HSA (VHH) albumin binding for extended serum half-life
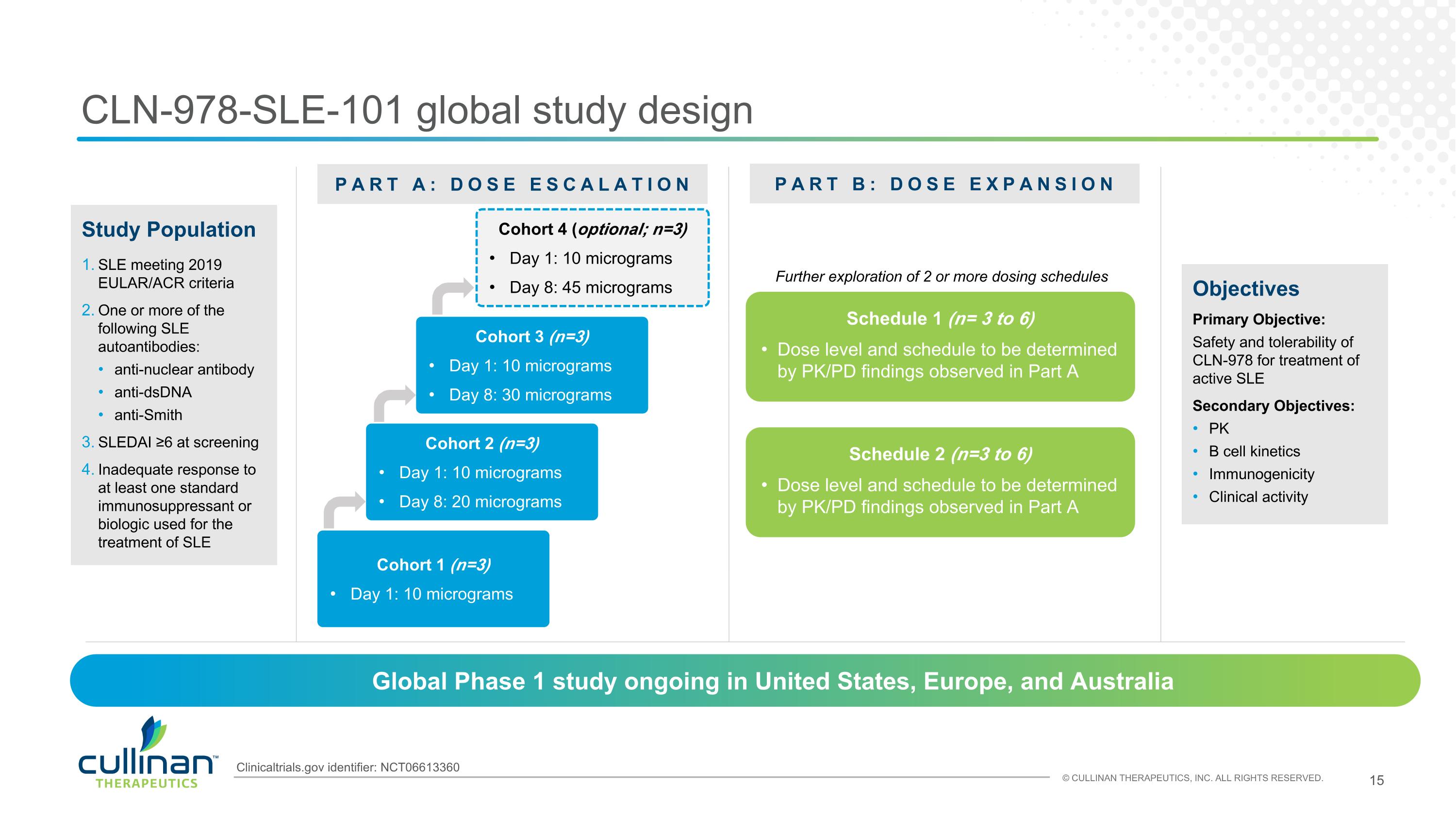
CLN-978-SLE-101 global study design Objectives Primary Objective: Safety and tolerability of CLN-978 for treatment of active SLE Secondary Objectives: PK B cell kinetics Immunogenicity Clinical activity PART A: DOSE ESCALATION PART B: DOSE EXPANSION Cohort 1 (n=3) Day 1: 10 micrograms Schedule 1 (n= 3 to 6) Dose level and schedule to be determined by PK/PD findings observed in Part A Study Population SLE meeting 2019 EULAR/ACR criteria One or more of the following SLE autoantibodies: anti-nuclear antibody anti-dsDNA anti-Smith SLEDAI ≥6 at screening Inadequate response to at least one standard immunosuppressant or biologic used for the treatment of SLE Global Phase 1 study ongoing in United States, Europe, and Australia Cohort 2 (n=3) Day 1: 10 micrograms Day 8: 20 micrograms Cohort 3 (n=3) Day 1: 10 micrograms Day 8: 30 micrograms Schedule 2 (n=3 to 6) Dose level and schedule to be determined by PK/PD findings observed in Part A Further exploration of 2 or more dosing schedules Cohort 4 (optional; n=3) Day 1: 10 micrograms Day 8: 45 micrograms Clinicaltrials.gov identifier: NCT06613360
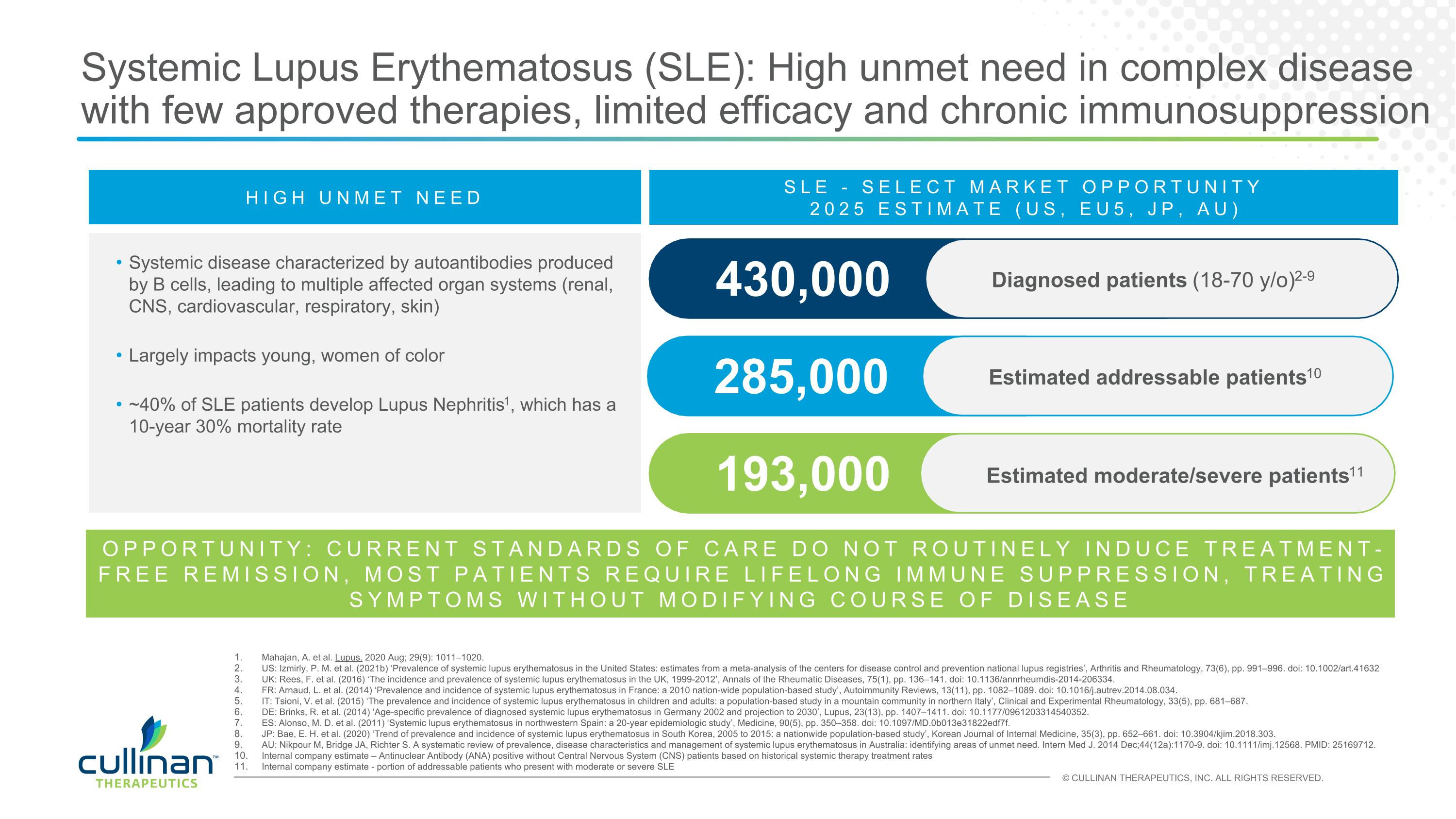
Systemic Lupus Erythematosus (SLE): High unmet need in complex disease with few approved therapies, limited efficacy and chronic immunosuppression HIGH UNMET NEED Systemic disease characterized by autoantibodies produced by B cells, leading to multiple affected organ systems (renal, CNS, cardiovascular, respiratory, skin) Largely impacts young, women of color ~40% of SLE patients develop Lupus Nephritis1, which has a 10-year 30% mortality rate 285,000 Estimated addressable patients10 430,000 Diagnosed patients (18-70 y/o)2-9 SLE - SELECT MARKET OPPORTUNITY 2025 ESTIMATE (US, EU5, JP, AU) 193,000 Estimated moderate/severe patients11 Mahajan, A. et al. Lupus. 2020 Aug; 29(9): 1011–1020. US: Izmirly, P. M. et al. (2021b) ‘Prevalence of systemic lupus erythematosus in the United States: estimates from a meta-analysis of the centers for disease control and prevention national lupus registries’, Arthritis and Rheumatology, 73(6), pp. 991–996. doi: 10.1002/art.41632 UK: Rees, F. et al. (2016) ‘The incidence and prevalence of systemic lupus erythematosus in the UK, 1999-2012’, Annals of the Rheumatic Diseases, 75(1), pp. 136–141. doi: 10.1136/annrheumdis-2014-206334. FR: Arnaud, L. et al. (2014) ‘Prevalence and incidence of systemic lupus erythematosus in France: a 2010 nation-wide population-based study’, Autoimmunity Reviews, 13(11), pp. 1082–1089. doi: 10.1016/j.autrev.2014.08.034. IT: Tsioni, V. et al. (2015) ‘The prevalence and incidence of systemic lupus erythematosus in children and adults: a population-based study in a mountain community in northern Italy’, Clinical and Experimental Rheumatology, 33(5), pp. 681–687. DE: Brinks, R. et al. (2014) ‘Age-specific prevalence of diagnosed systemic lupus erythematosus in Germany 2002 and projection to 2030’, Lupus, 23(13), pp. 1407–1411. doi: 10.1177/0961203314540352. ES: Alonso, M. D. et al. (2011) ‘Systemic lupus erythematosus in northwestern Spain: a 20-year epidemiologic study’, Medicine, 90(5), pp. 350–358. doi: 10.1097/MD.0b013e31822edf7f. JP: Bae, E. H. et al. (2020) ‘Trend of prevalence and incidence of systemic lupus erythematosus in South Korea, 2005 to 2015: a nationwide population-based study’, Korean Journal of Internal Medicine, 35(3), pp. 652–661. doi: 10.3904/kjim.2018.303. AU: Nikpour M, Bridge JA, Richter S. A systematic review of prevalence, disease characteristics and management of systemic lupus erythematosus in Australia: identifying areas of unmet need. Intern Med J. 2014 Dec;44(12a):1170-9. doi: 10.1111/imj.12568. PMID: 25169712. Internal company estimate – Antinuclear Antibody (ANA) positive without Central Nervous System (CNS) patients based on historical systemic therapy treatment rates Internal company estimate - portion of addressable patients who present with moderate or severe SLE OPPORTUNITY: CURRENT STANDARDS OF CARE DO NOT ROUTINELY INDUCE TREATMENT-FREE REMISSION, MOST PATIENTS REQUIRE LIFELONG IMMUNE SUPPRESSION, TREATING SYMPTOMS WITHOUT MODIFYING COURSE OF DISEASE
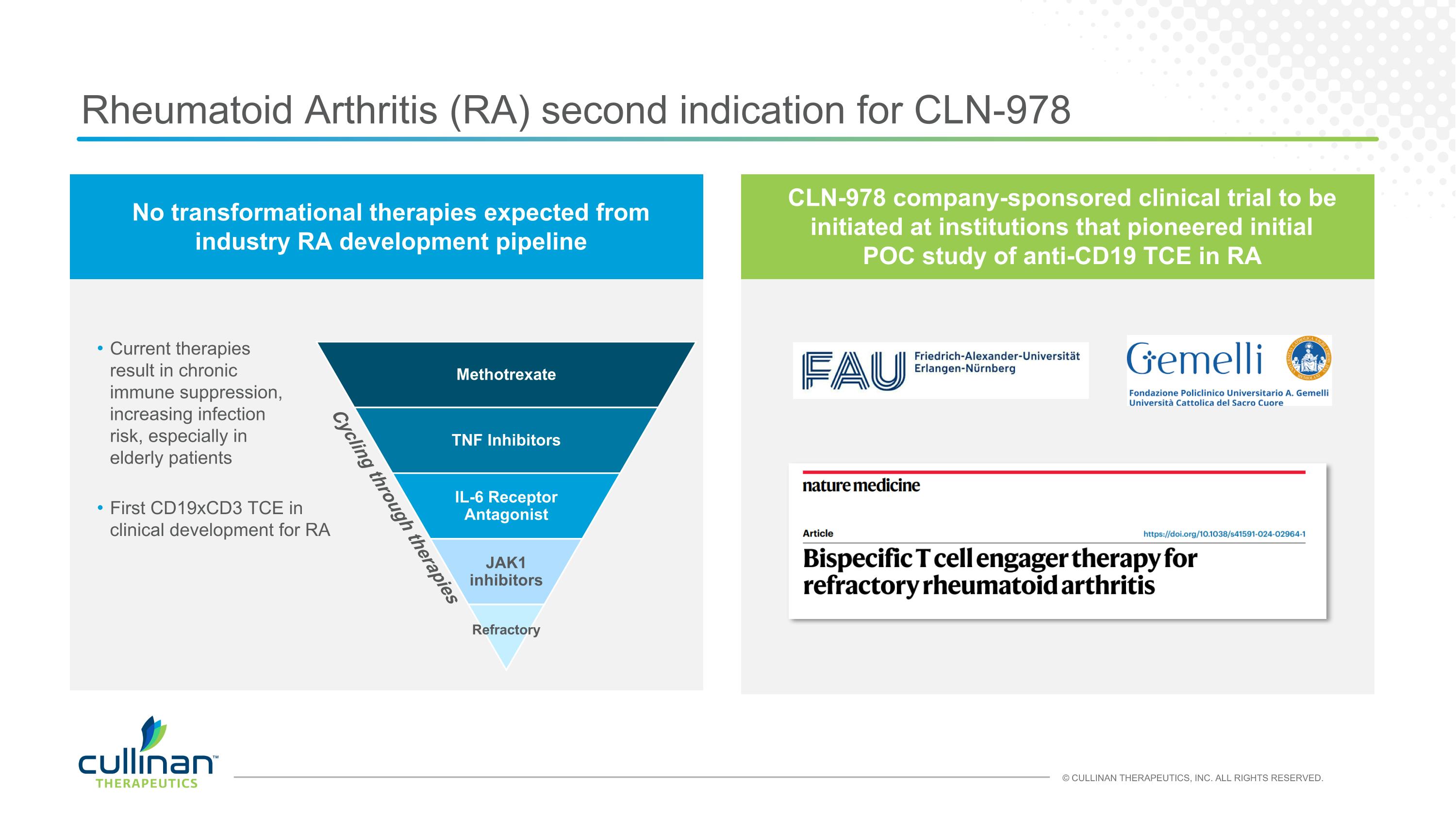
Rheumatoid Arthritis (RA) second indication for CLN-978 Methotrexate TNF Inhibitors IL-6 Receptor Antagonist JAK1 inhibitors Refractory Cycling through therapies Current therapies result in chronic immune suppression, increasing infection risk, especially in elderly patients No transformational therapies expected from industry RA development pipeline CLN-978 company-sponsored clinical trial to be initiated at institutions that pioneered initial POC study of anti-CD19 TCE in RA First CD19xCD3 TCE in clinical development for RA
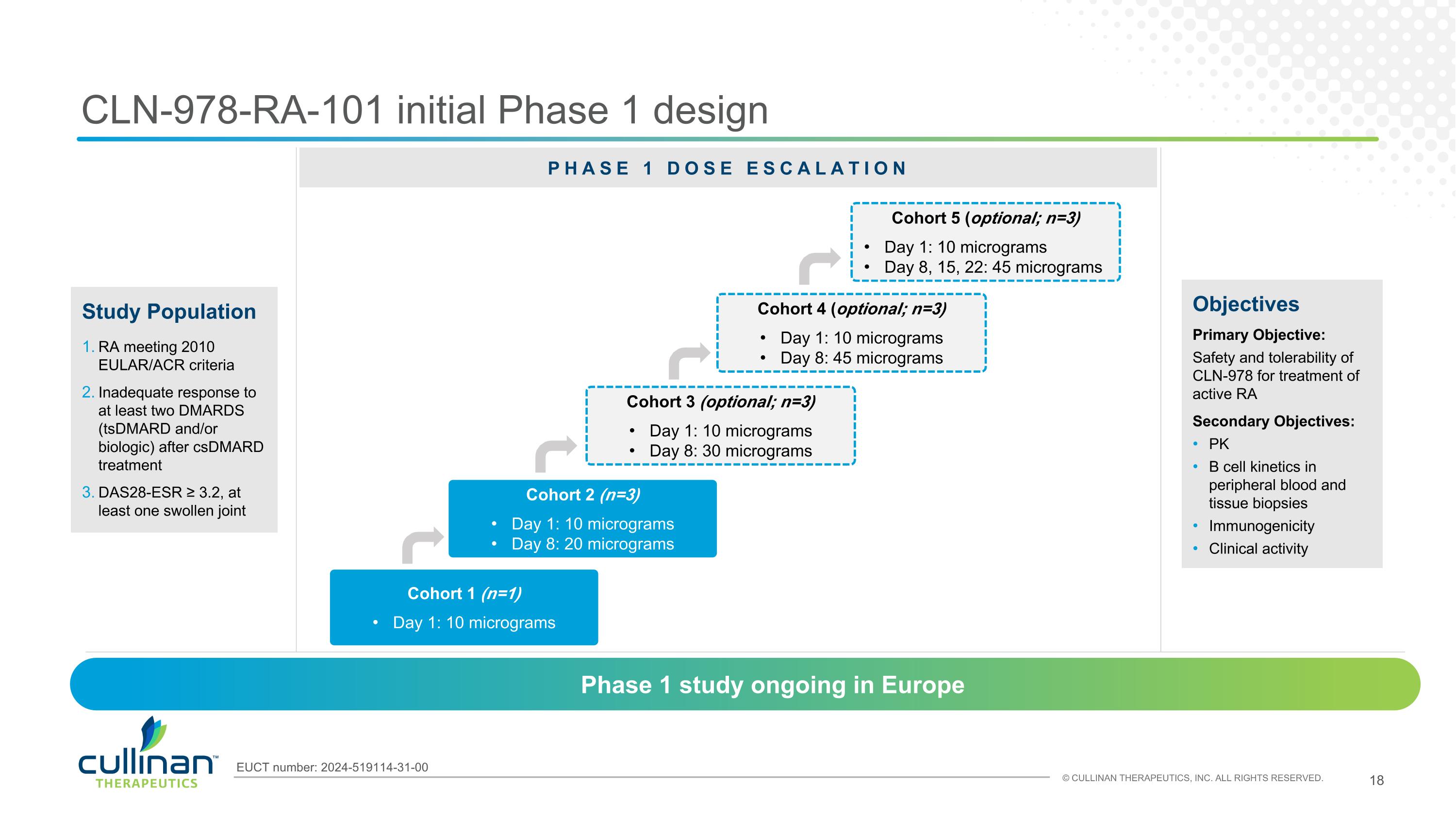
CLN-978-RA-101 initial Phase 1 design Objectives Primary Objective: Safety and tolerability of CLN-978 for treatment of active RA Secondary Objectives: PK B cell kinetics in peripheral blood and tissue biopsies Immunogenicity Clinical activity Cohort 1 (n=1) Day 1: 10 micrograms Study Population RA meeting 2010 EULAR/ACR criteria Inadequate response to at least two DMARDS (tsDMARD and/or biologic) after csDMARD treatment DAS28-ESR ≥ 3.2, at least one swollen joint Phase 1 study ongoing in Europe Cohort 2 (n=3) Day 1: 10 micrograms Day 8: 20 micrograms Cohort 3 (optional; n=3) Day 1: 10 micrograms Day 8: 30 micrograms Cohort 4 (optional; n=3) Day 1: 10 micrograms Day 8: 45 micrograms EUCT number: 2024-519114-31-00 Cohort 5 (optional; n=3) Day 1: 10 micrograms Day 8, 15, 22: 45 micrograms PHASE 1 DOSE ESCALATION
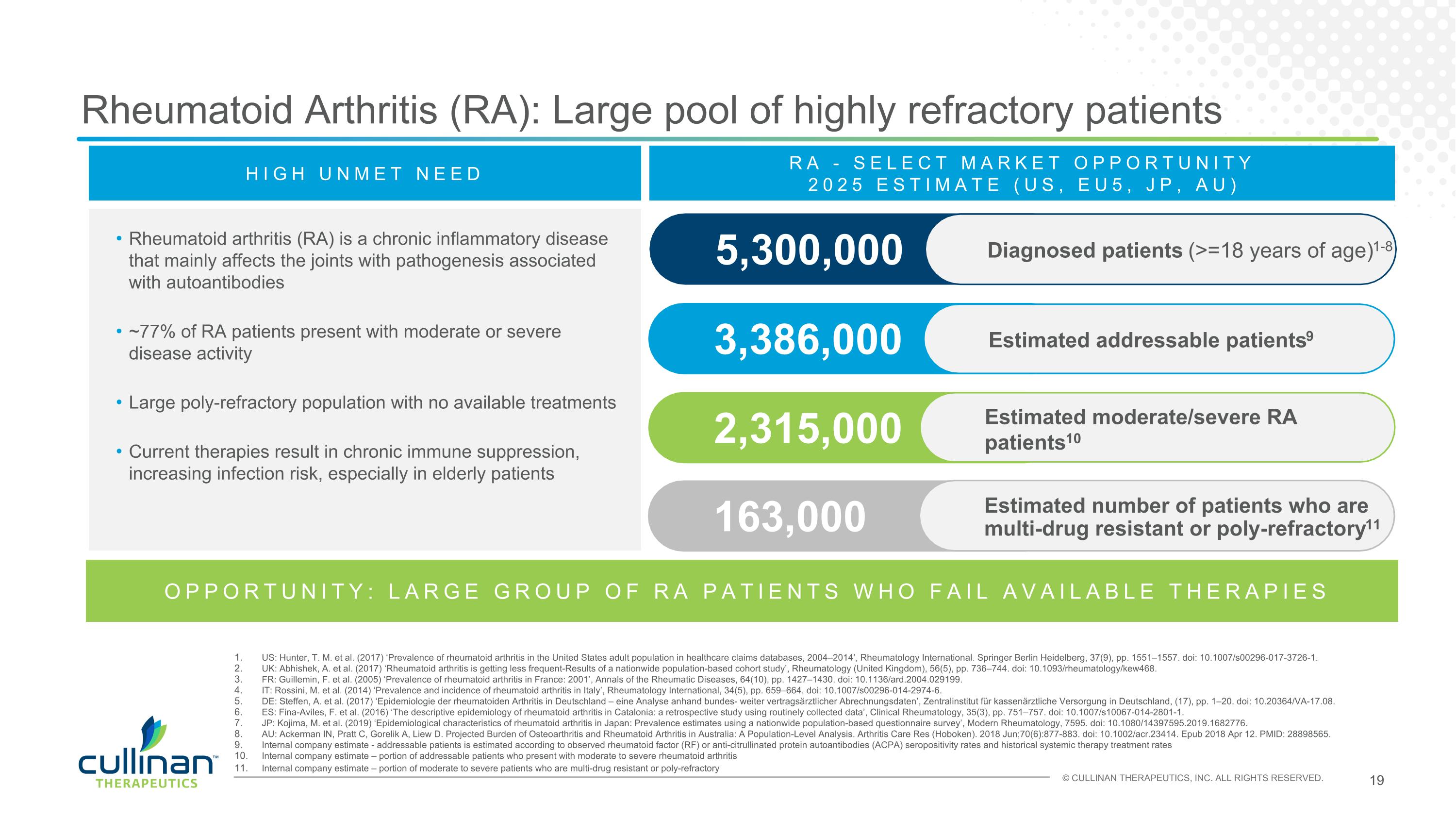
Rheumatoid arthritis (RA) is a chronic inflammatory disease that mainly affects the joints with pathogenesis associated with autoantibodies ~77% of RA patients present with moderate or severe disease activity Large poly-refractory population with no available treatments Current therapies result in chronic immune suppression, increasing infection risk, especially in elderly patients Rheumatoid Arthritis (RA): Large pool of highly refractory patients 3,386,000 Estimated addressable patients9 5,300,000 RA - SELECT MARKET OPPORTUNITY 2025 ESTIMATE (US, EU5, JP, AU) 2,315,000 Estimated moderate/severe RA patients10 163,000 Estimated number of patients who are multi-drug resistant or poly-refractory11 US: Hunter, T. M. et al. (2017) ‘Prevalence of rheumatoid arthritis in the United States adult population in healthcare claims databases, 2004–2014’, Rheumatology International. Springer Berlin Heidelberg, 37(9), pp. 1551–1557. doi: 10.1007/s00296-017-3726-1. UK: Abhishek, A. et al. (2017) ‘Rheumatoid arthritis is getting less frequent-Results of a nationwide population-based cohort study’, Rheumatology (United Kingdom), 56(5), pp. 736–744. doi: 10.1093/rheumatology/kew468. FR: Guillemin, F. et al. (2005) ‘Prevalence of rheumatoid arthritis in France: 2001’, Annals of the Rheumatic Diseases, 64(10), pp. 1427–1430. doi: 10.1136/ard.2004.029199. IT: Rossini, M. et al. (2014) ‘Prevalence and incidence of rheumatoid arthritis in Italy’, Rheumatology International, 34(5), pp. 659–664. doi: 10.1007/s00296-014-2974-6. DE: Steffen, A. et al. (2017) ‘Epidemiologie der rheumatoiden Arthritis in Deutschland – eine Analyse anhand bundes- weiter vertragsärztlicher Abrechnungsdaten’, Zentralinstitut für kassenärztliche Versorgung in Deutschland, (17), pp. 1–20. doi: 10.20364/VA-17.08. ES: Fina-Aviles, F. et al. (2016) ‘The descriptive epidemiology of rheumatoid arthritis in Catalonia: a retrospective study using routinely collected data’, Clinical Rheumatology, 35(3), pp. 751–757. doi: 10.1007/s10067-014-2801-1. JP: Kojima, M. et al. (2019) ‘Epidemiological characteristics of rheumatoid arthritis in Japan: Prevalence estimates using a nationwide population-based questionnaire survey’, Modern Rheumatology, 7595. doi: 10.1080/14397595.2019.1682776. AU: Ackerman IN, Pratt C, Gorelik A, Liew D. Projected Burden of Osteoarthritis and Rheumatoid Arthritis in Australia: A Population-Level Analysis. Arthritis Care Res (Hoboken). 2018 Jun;70(6):877-883. doi: 10.1002/acr.23414. Epub 2018 Apr 12. PMID: 28898565. Internal company estimate - addressable patients is estimated according to observed rheumatoid factor (RF) or anti-citrullinated protein autoantibodies (ACPA) seropositivity rates and historical systemic therapy treatment rates Internal company estimate – portion of addressable patients who present with moderate to severe rheumatoid arthritis Internal company estimate – portion of moderate to severe patients who are multi-drug resistant or poly-refractory OPPORTUNITY: LARGE GROUP OF RA PATIENTS WHO FAIL AVAILABLE THERAPIES HIGH UNMET NEED Diagnosed patients (>=18 years of age)1-8
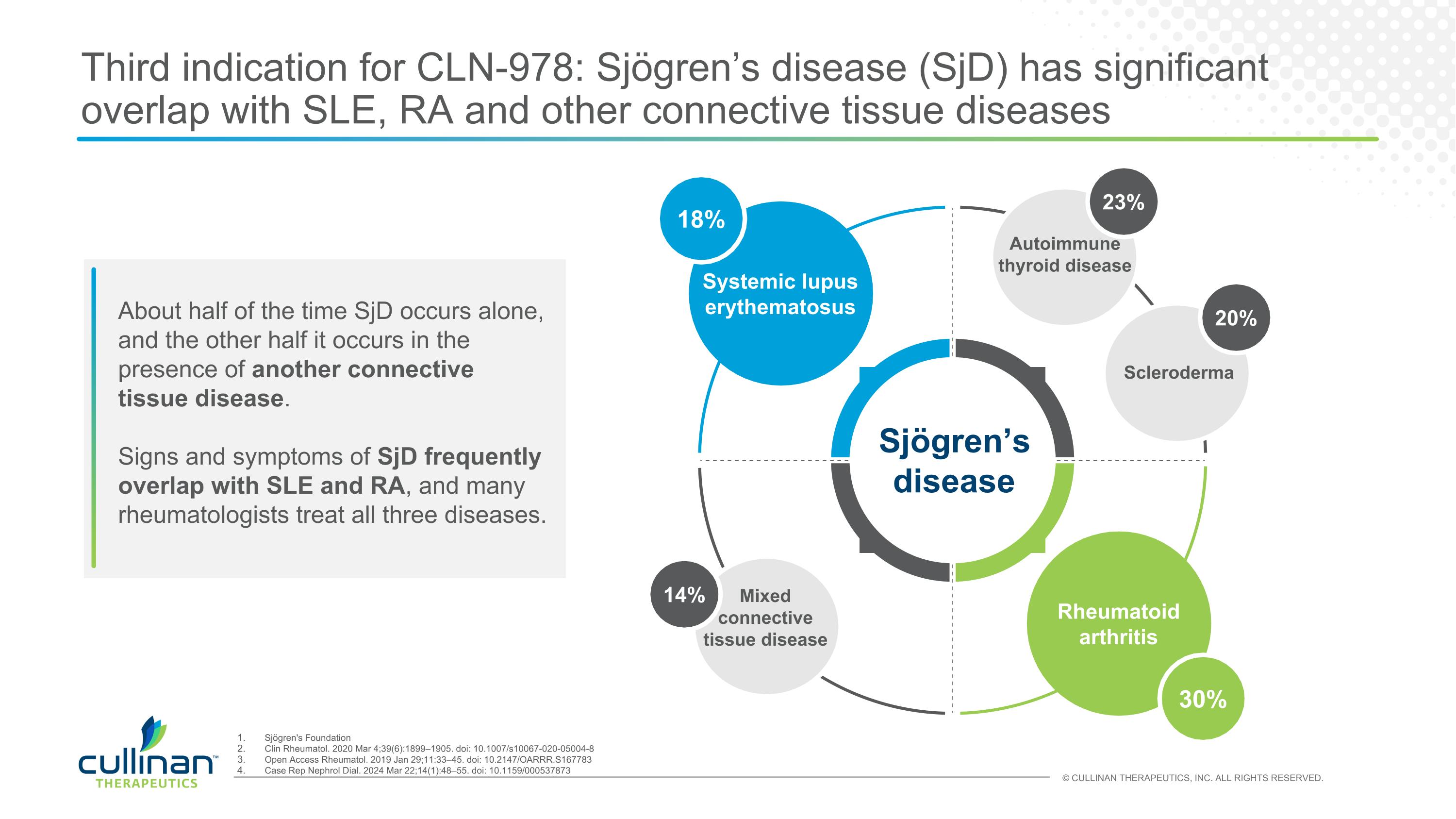
Third indication for CLN-978: Sjögren’s disease (SjD) has significant overlap with SLE, RA and other connective tissue diseases About half of the time SjD occurs alone, and the other half it occurs in the presence of another connective tissue disease. Signs and symptoms of SjD frequently overlap with SLE and RA, and many rheumatologists treat all three diseases. 18% 30% Systemic lupus erythematosus Rheumatoid arthritis Sjögren’s disease 20% Scleroderma 23% Autoimmune thyroid disease 14% Mixed connective tissue disease Sjögren's Foundation Clin Rheumatol. 2020 Mar 4;39(6):1899–1905. doi: 10.1007/s10067-020-05004-8 Open Access Rheumatol. 2019 Jan 29;11:33–45. doi: 10.2147/OARRR.S167783 Case Rep Nephrol Dial. 2024 Mar 22;14(1):48–55. doi: 10.1159/000537873
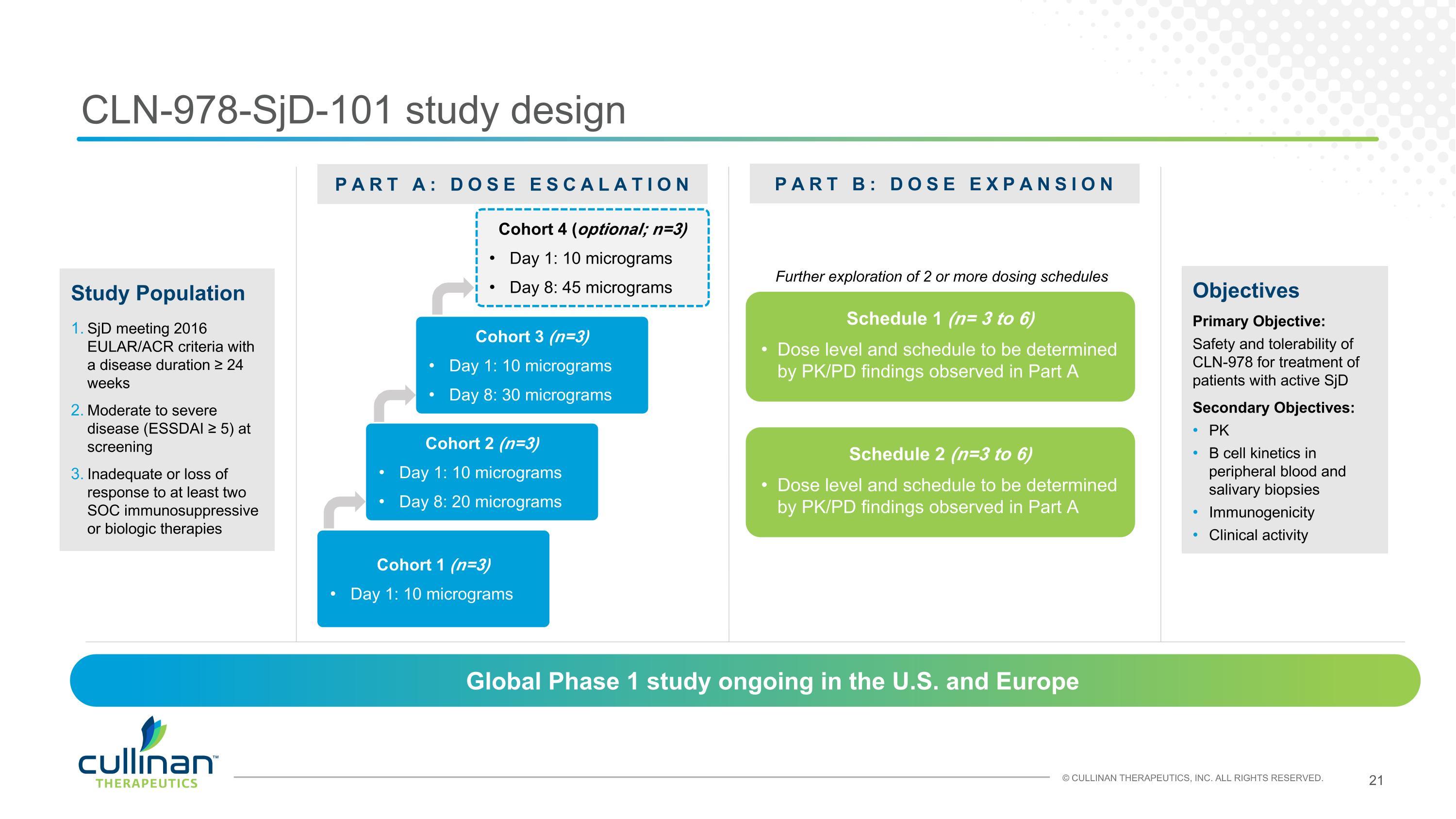
CLN-978-SjD-101 study design Objectives Primary Objective: Safety and tolerability of CLN-978 for treatment of patients with active SjD Secondary Objectives: PK B cell kinetics in peripheral blood and salivary biopsies Immunogenicity Clinical activity PART A: DOSE ESCALATION PART B: DOSE EXPANSION Cohort 1 (n=3) Day 1: 10 micrograms Schedule 1 (n= 3 to 6) Dose level and schedule to be determined by PK/PD findings observed in Part A Study Population SjD meeting 2016 EULAR/ACR criteria with a disease duration ≥ 24 weeks Moderate to severe disease (ESSDAI ≥ 5) at screening Inadequate or loss of response to at least two SOC immunosuppressive or biologic therapies Global Phase 1 study ongoing in the U.S. and Europe Cohort 2 (n=3) Day 1: 10 micrograms Day 8: 20 micrograms Cohort 3 (n=3) Day 1: 10 micrograms Day 8: 30 micrograms Schedule 2 (n=3 to 6) Dose level and schedule to be determined by PK/PD findings observed in Part A Further exploration of 2 or more dosing schedules Cohort 4 (optional; n=3) Day 1: 10 micrograms Day 8: 45 micrograms
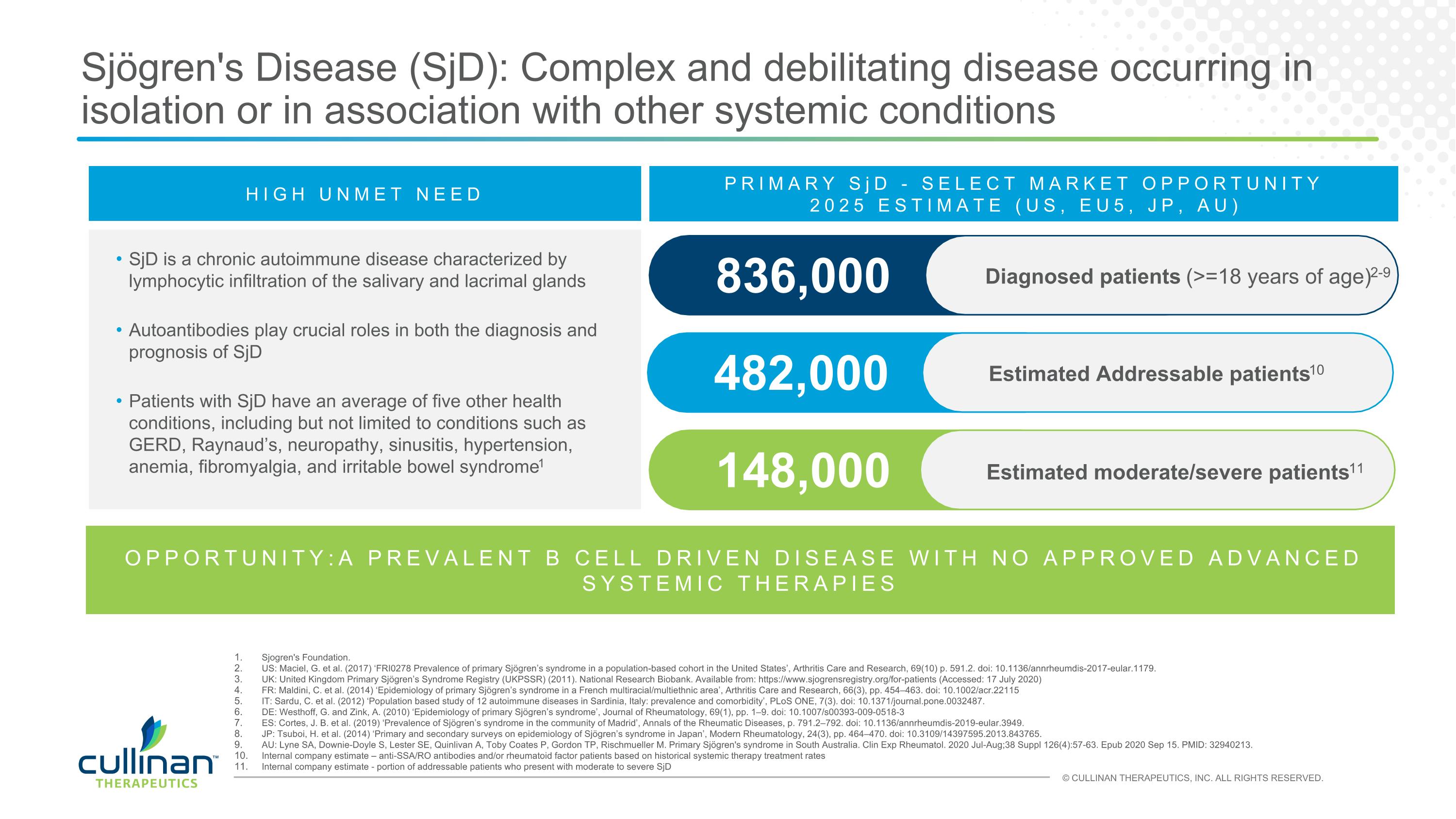
Sjögren's Disease (SjD): Complex and debilitating disease occurring in isolation or in association with other systemic conditions HIGH UNMET NEED SjD is a chronic autoimmune disease characterized by lymphocytic infiltration of the salivary and lacrimal glands Autoantibodies play crucial roles in both the diagnosis and prognosis of SjD Patients with SjD have an average of five other health conditions, including but not limited to conditions such as GERD, Raynaud’s, neuropathy, sinusitis, hypertension, anemia, fibromyalgia, and irritable bowel syndrome1 482,000 Estimated Addressable patients10 836,000 PRIMARY SjD - SELECT MARKET OPPORTUNITY 2025 ESTIMATE (US, EU5, JP, AU) 148,000 Estimated moderate/severe patients11 Sjogren's Foundation. US: Maciel, G. et al. (2017) ‘FRI0278 Prevalence of primary Sjögren’s syndrome in a population-based cohort in the United States’, Arthritis Care and Research, 69(10) p. 591.2. doi: 10.1136/annrheumdis-2017-eular.1179. UK: United Kingdom Primary Sjögren’s Syndrome Registry (UKPSSR) (2011). National Research Biobank. Available from: https://www.sjogrensregistry.org/for-patients (Accessed: 17 July 2020) FR: Maldini, C. et al. (2014) ‘Epidemiology of primary Sjögren’s syndrome in a French multiracial/multiethnic area’, Arthritis Care and Research, 66(3), pp. 454–463. doi: 10.1002/acr.22115 IT: Sardu, C. et al. (2012) ‘Population based study of 12 autoimmune diseases in Sardinia, Italy: prevalence and comorbidity’, PLoS ONE, 7(3). doi: 10.1371/journal.pone.0032487. DE: Westhoff, G. and Zink, A. (2010) ‘Epidemiology of primary Sjögren’s syndrome’, Journal of Rheumatology, 69(1), pp. 1–9. doi: 10.1007/s00393-009-0518-3 ES: Cortes, J. B. et al. (2019) ‘Prevalence of Sjögren’s syndrome in the community of Madrid’, Annals of the Rheumatic Diseases, p. 791.2–792. doi: 10.1136/annrheumdis-2019-eular.3949. JP: Tsuboi, H. et al. (2014) ‘Primary and secondary surveys on epidemiology of Sjögren’s syndrome in Japan’, Modern Rheumatology, 24(3), pp. 464–470. doi: 10.3109/14397595.2013.843765. AU: Lyne SA, Downie-Doyle S, Lester SE, Quinlivan A, Toby Coates P, Gordon TP, Rischmueller M. Primary Sjögren's syndrome in South Australia. Clin Exp Rheumatol. 2020 Jul-Aug;38 Suppl 126(4):57-63. Epub 2020 Sep 15. PMID: 32940213. Internal company estimate – anti-SSA/RO antibodies and/or rheumatoid factor patients based on historical systemic therapy treatment rates Internal company estimate - portion of addressable patients who present with moderate to severe SjD OPPORTUNITY:A PREVALENT B CELL DRIVEN DISEASE WITH NO APPROVED ADVANCED SYSTEMIC THERAPIES Diagnosed patients (>=18 years of age)2-9

Velinotamig BCMAxCD3 bispecific T cell engager
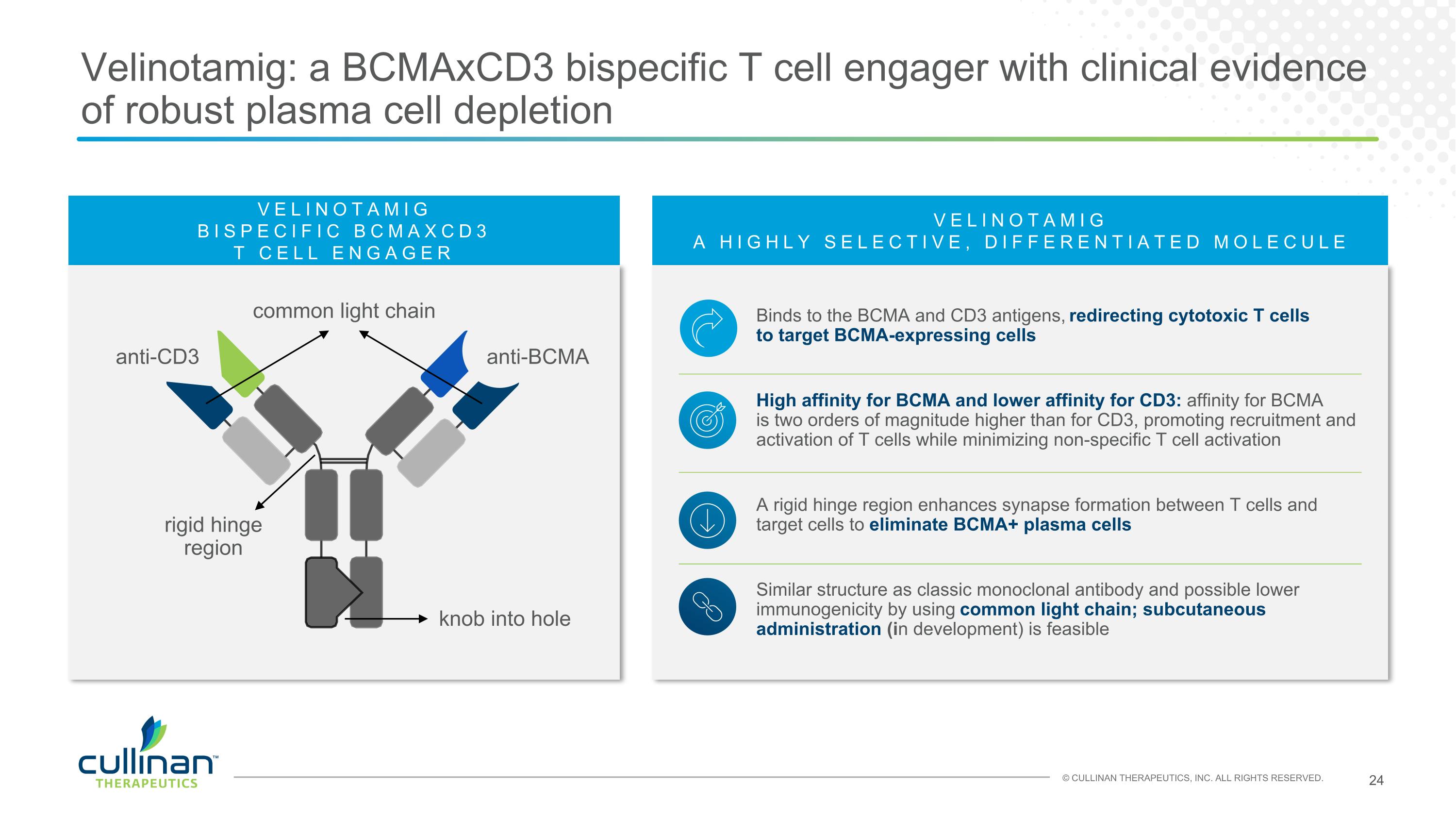
VELINOTAMIG A HIGHLY SELECTIVE, DIFFERENTIATED MOLECULE Binds to the BCMA and CD3 antigens, redirecting cytotoxic T cells to target BCMA-expressing cells High affinity for BCMA and lower affinity for CD3: affinity for BCMA is two orders of magnitude higher than for CD3, promoting recruitment and activation of T cells while minimizing non-specific T cell activation A rigid hinge region enhances synapse formation between T cells and target cells to eliminate BCMA+ plasma cells Similar structure as classic monoclonal antibody and possible lower immunogenicity by using common light chain; subcutaneous administration (in development) is feasible Velinotamig: a BCMAxCD3 bispecific T cell engager with clinical evidence of robust plasma cell depletion VELINOTAMIG BISPECIFIC BCMAXCD3 T CELL ENGAGER common light chain anti-BCMA anti-CD3 rigid hinge region knob into hole

Velinotamig current clinical development status in China GR1803-001: A (follow-up) completed Phase 1 study in patients with relapsed/refractory multiple myeloma GR1803-002: A Phase 2 pivotal study in patients with relapsed/refractory multiple myeloma GR1803-003: A Phase 2 pivotal study in patients with relapsed/refractory multiple myeloma with extramedullary lesions Velinotamig received Breakthrough Therapy Designation by the Center for Drug Evaluation (CDE) for the treatment of relapsed/refractory multiple myeloma PLANNED CLINICAL TRIAL IN AUTOIMMUNE DISEASES Genrix Bio plans to conduct a Phase 1 study in China in patients with autoimmune diseases beginning later this year Cullinan expects the data generated to accelerate the global clinical development of velinotamig in autoimmune diseases Following the completion of the Genrix Bio Phase 1 study, Cullinan will conduct all further development of velinotamig in autoimmune diseases COMPLETED/ ONGOING CLINICAL TRIALS IN MULTIPLE MYELOMA
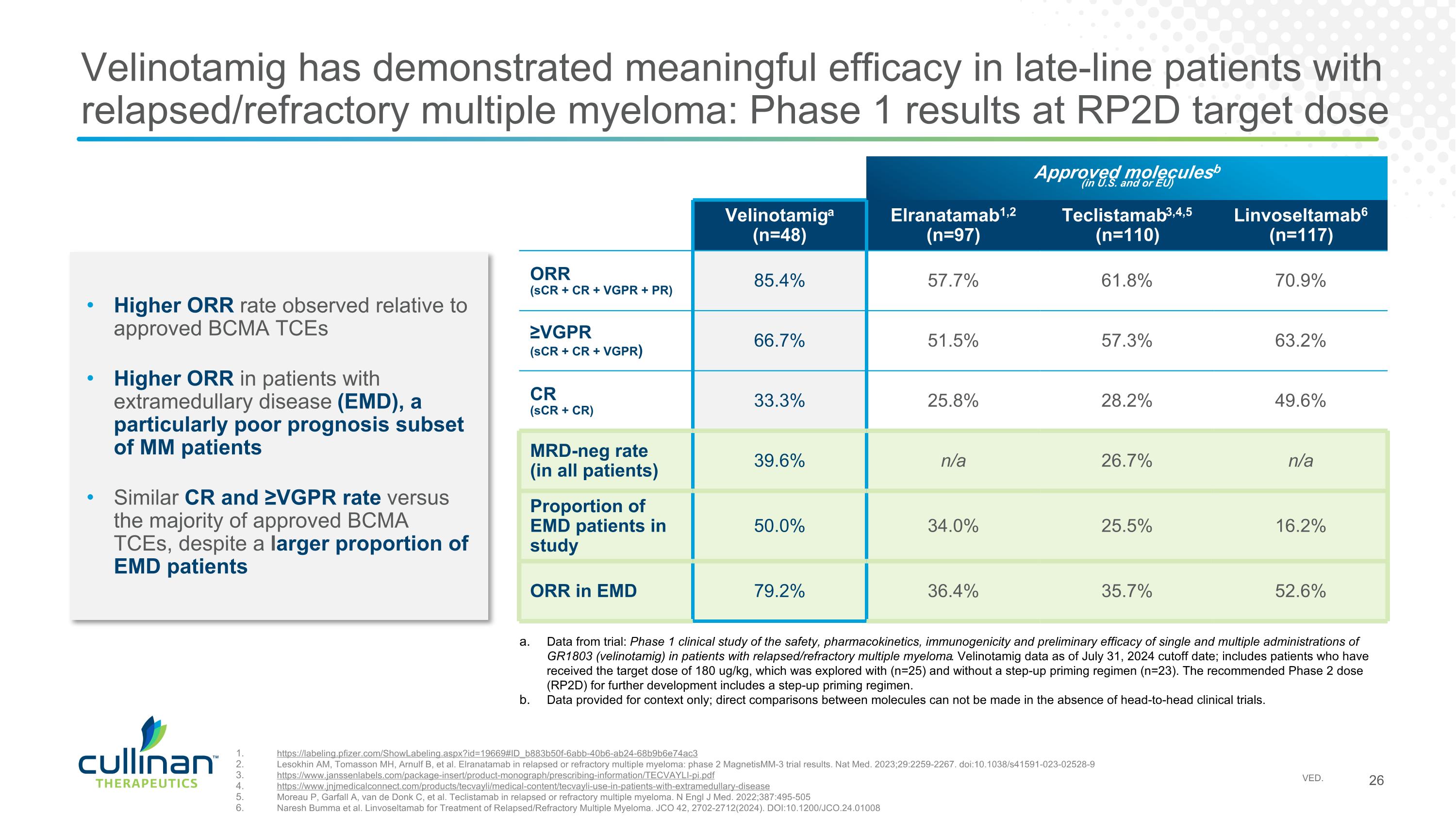
Velinotamig has demonstrated meaningful efficacy in late-line patients with relapsed/refractory multiple myeloma: Phase 1 results at RP2D target dose Approved moleculesb (in U.S. and or EU) Velinotamiga (n=48) Elranatamab1,2 (n=97) Teclistamab3,4,5 (n=110) Linvoseltamab6 (n=117) ORR (sCR + CR + VGPR + PR) 85.4% 57.7% 61.8% 70.9% ≥VGPR (sCR + CR + VGPR) 66.7% 51.5% 57.3% 63.2% CR (sCR + CR) 33.3% 25.8% 28.2% 49.6% MRD-neg rate (in all patients) 39.6% n/a 26.7% n/a Proportion of EMD patients in study 50.0% 34.0% 25.5% 16.2% ORR in EMD 79.2% 36.4% 35.7% 52.6% Higher ORR rate observed relative to approved BCMA TCEs Higher ORR in patients with extramedullary disease (EMD), a particularly poor prognosis subset of MM patients Similar CR and ≥VGPR rate versus the majority of approved BCMA TCEs, despite a larger proportion of EMD patients https://labeling.pfizer.com/ShowLabeling.aspx?id=19669#ID_b883b50f-6abb-40b6-ab24-68b9b6e74ac3 Lesokhin AM, Tomasson MH, Arnulf B, et al. Elranatamab in relapsed or refractory multiple myeloma: phase 2 MagnetisMM-3 trial results. Nat Med. 2023;29:2259-2267. doi:10.1038/s41591-023-02528-9 https://www.janssenlabels.com/package-insert/product-monograph/prescribing-information/TECVAYLI-pi.pdf https://www.jnjmedicalconnect.com/products/tecvayli/medical-content/tecvayli-use-in-patients-with-extramedullary-disease Moreau P, Garfall A, van de Donk C, et al. Teclistamab in relapsed or refractory multiple myeloma. N Engl J Med. 2022;387:495-505 Naresh Bumma et al. Linvoseltamab for Treatment of Relapsed/Refractory Multiple Myeloma. JCO 42, 2702-2712(2024). DOI:10.1200/JCO.24.01008 Data from trial: Phase 1 clinical study of the safety, pharmacokinetics, immunogenicity and preliminary efficacy of single and multiple administrations of GR1803 (velinotamig) in patients with relapsed/refractory multiple myeloma. Velinotamig data as of July 31, 2024 cutoff date; includes patients who have received the target dose of 180 ug/kg, which was explored with (n=25) and without a step-up priming regimen (n=23). The recommended Phase 2 dose (RP2D) for further development includes a step-up priming regimen. Data provided for context only; direct comparisons between molecules can not be made in the absence of head-to-head clinical trials.
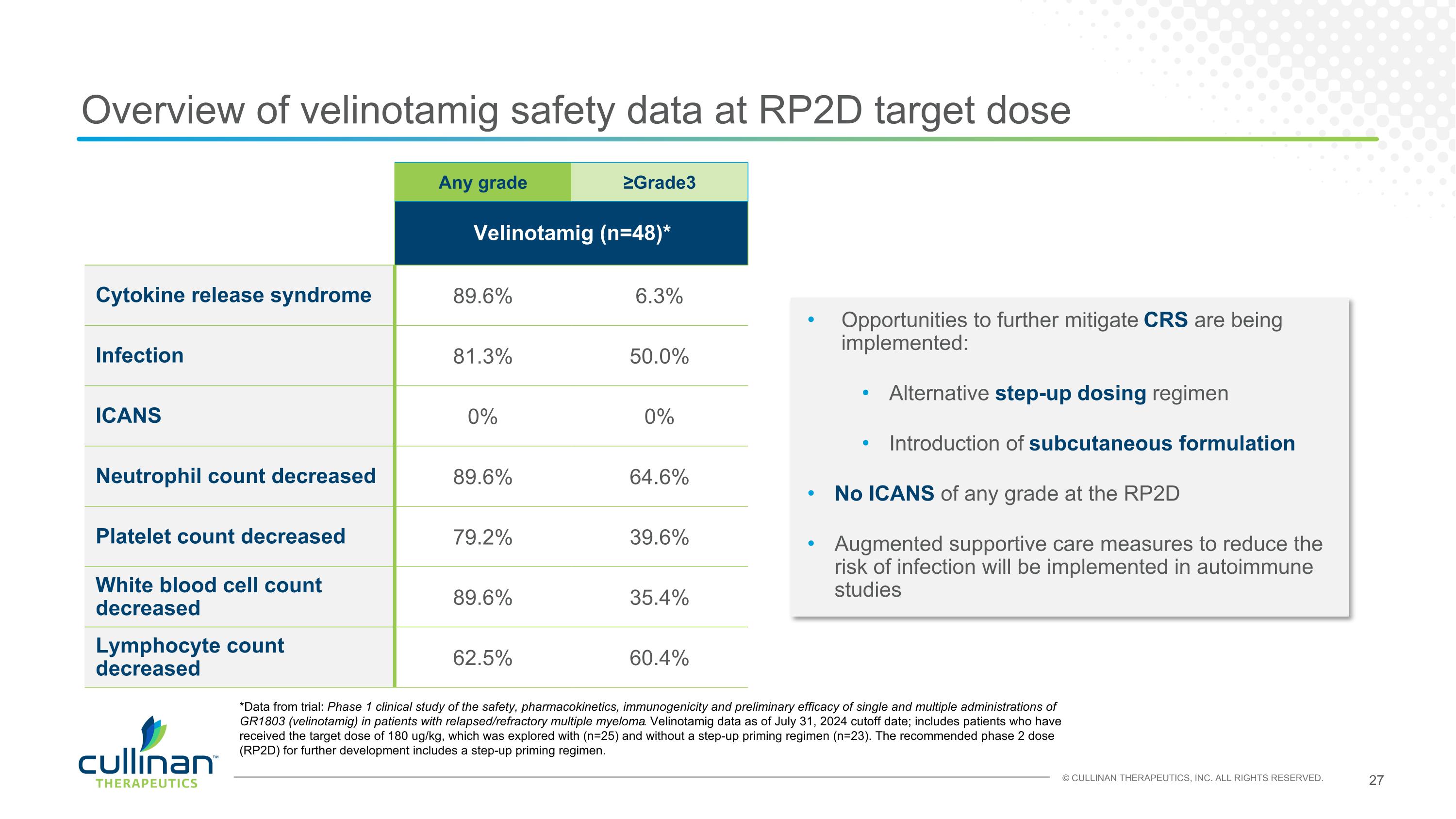
Overview of velinotamig safety data at RP2D target dose Any grade ≥Grade3 Velinotamig (n=48)* Cytokine release syndrome 89.6% 6.3% Infection 81.3% 50.0% ICANS 0% 0% Neutrophil count decreased 89.6% 64.6% Platelet count decreased 79.2% 39.6% White blood cell count decreased 89.6% 35.4% Lymphocyte count decreased 62.5% 60.4% Opportunities to further mitigate CRS are being implemented: Alternative step-up dosing regimen Introduction of subcutaneous formulation No ICANS of any grade at the RP2D Augmented supportive care measures to reduce the risk of infection will be implemented in autoimmune studies *Data from trial: Phase 1 clinical study of the safety, pharmacokinetics, immunogenicity and preliminary efficacy of single and multiple administrations of GR1803 (velinotamig) in patients with relapsed/refractory multiple myeloma. Velinotamig data as of July 31, 2024 cutoff date; includes patients who have received the target dose of 180 ug/kg, which was explored with (n=25) and without a step-up priming regimen (n=23). The recommended phase 2 dose (RP2D) for further development includes a step-up priming regimen.

ZIPALERTINIB (CLN-081/TAS6417) EGFRex20ins inhibitor
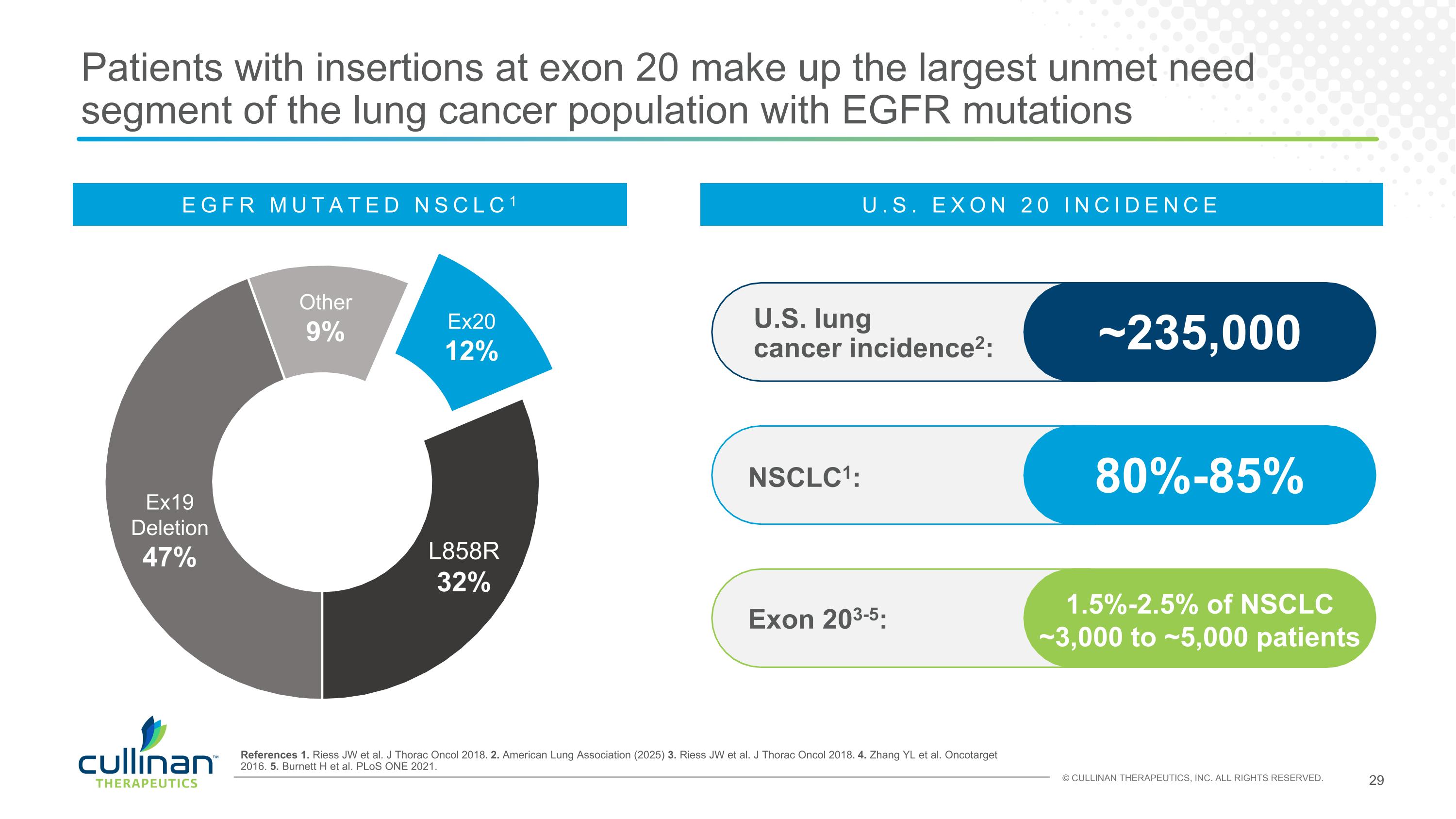
Patients with insertions at exon 20 make up the largest unmet need segment of the lung cancer population with EGFR mutations References 1. Riess JW et al. J Thorac Oncol 2018. 2. American Lung Association (2025) 3. Riess JW et al. J Thorac Oncol 2018. 4. Zhang YL et al. Oncotarget 2016. 5. Burnett H et al. PLoS ONE 2021. NSCLC1: 80%-85% U.S. lung cancer incidence2: ~235,000 Exon 203-5: 1.5%-2.5% of NSCLC ~3,000 to ~5,000 patients Other 9% Ex19 Deletion 47% L858R 32% Ex20 12% EGFR MUTATED NSCLC1 U.S. EXON 20 INCIDENCE
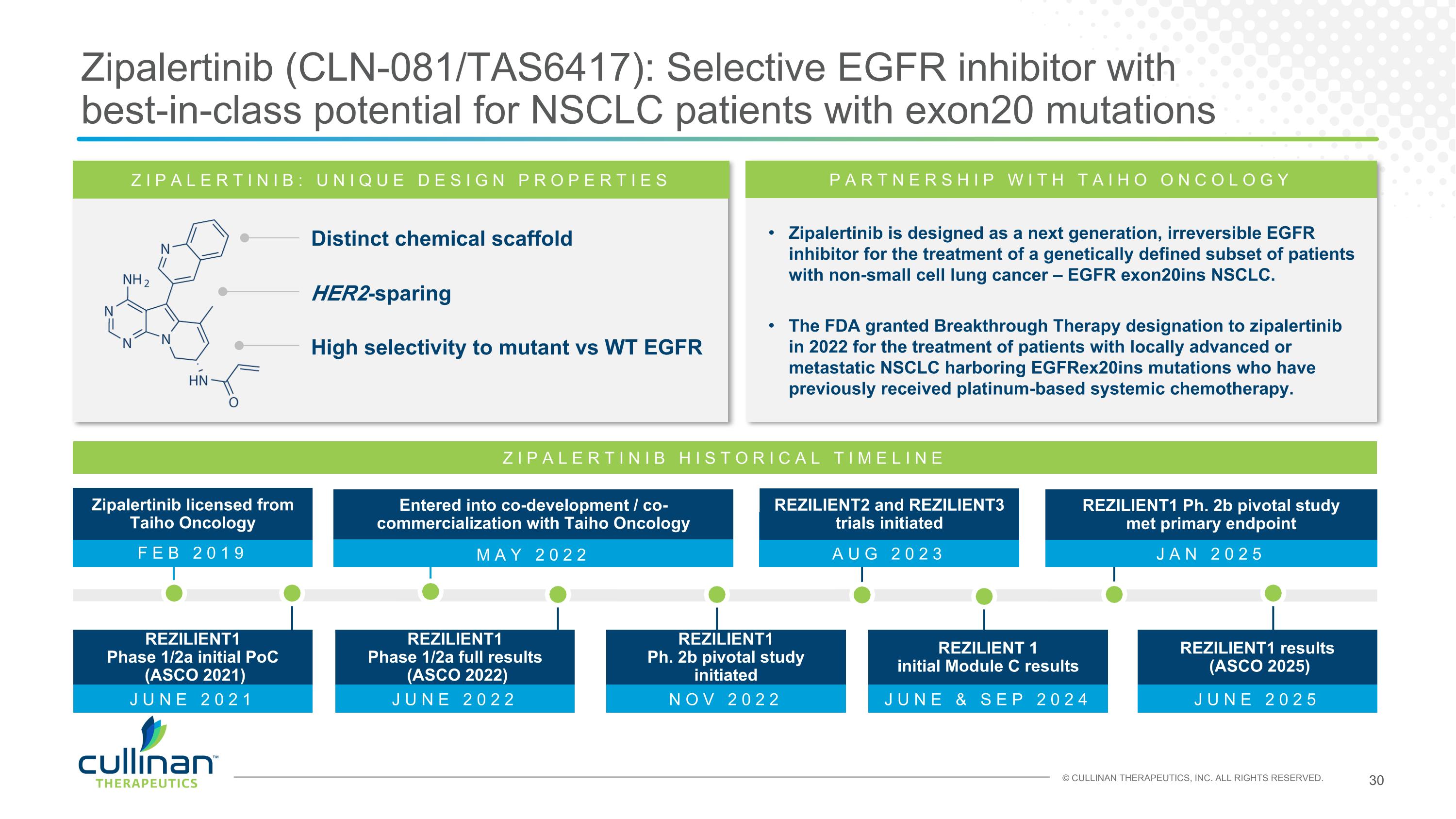
MAY 2022 Zipalertinib (CLN-081/TAS6417): Selective EGFR inhibitor with best-in-class potential for NSCLC patients with exon20 mutations ZIPALERTINIB: UNIQUE DESIGN PROPERTIES Distinct chemical scaffold HER2-sparing High selectivity to mutant vs WT EGFR Zipalertinib is designed as a next generation, irreversible EGFR inhibitor for the treatment of a genetically defined subset of patients with non-small cell lung cancer – EGFR exon20ins NSCLC. The FDA granted Breakthrough Therapy designation to zipalertinib in 2022 for the treatment of patients with locally advanced or metastatic NSCLC harboring EGFRex20ins mutations who have previously received platinum-based systemic chemotherapy. PARTNERSHIP WITH TAIHO ONCOLOGY ZIPALERTINIB HISTORICAL TIMELINE Entered into co-development / co-commercialization with Taiho Oncology FEB 2019 Zipalertinib licensed from Taiho Oncology JAN 2025 REZILIENT1 Ph. 2b pivotal study met primary endpoint AUG 2023 REZILIENT2 and REZILIENT3 trials initiated JUNE 2021 REZILIENT1 Phase 1/2a initial PoC (ASCO 2021) JUNE 2022 REZILIENT1 Phase 1/2a full results (ASCO 2022) JUNE 2025 REZILIENT1 results (ASCO 2025) JUNE & SEP 2024 REZILIENT 1 initial Module C results NOV 2022 REZILIENT1 Ph. 2b pivotal study initiated
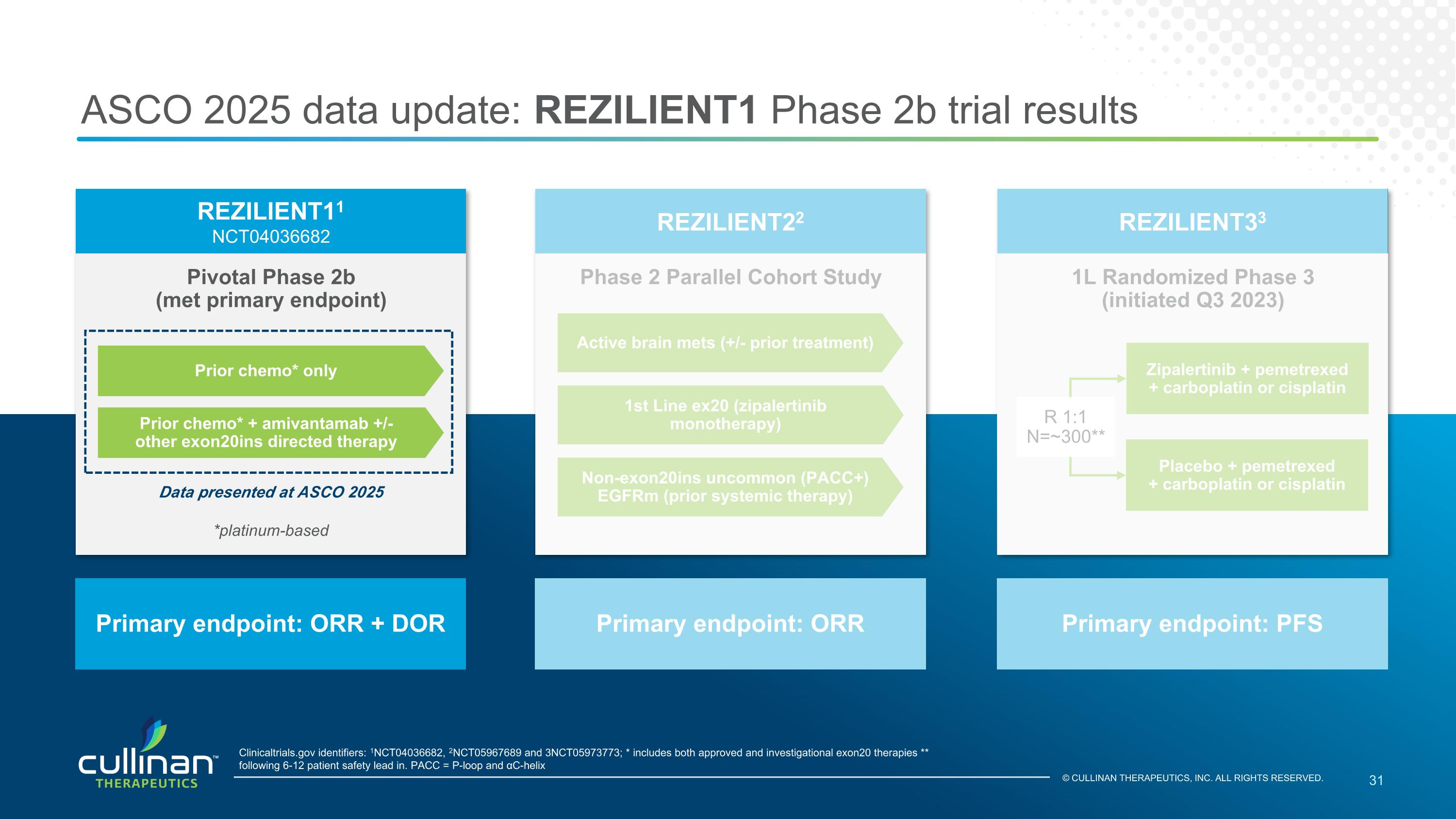
ASCO 2025 data update: REZILIENT1 Phase 2b trial results Pivotal Phase 2b (met primary endpoint) REZILIENT11 NCT04036682 Primary endpoint: ORR + DOR Clinicaltrials.gov identifiers: 1NCT04036682, 2NCT05967689 and 3NCT05973773; * includes both approved and investigational exon20 therapies ** following 6-12 patient safety lead in. PACC = P-loop and αC-helix 1L Randomized Phase 3 (initiated Q3 2023) REZILIENT33 Primary endpoint: PFS Phase 2 Parallel Cohort Study REZILIENT22 Primary endpoint: ORR Prior chemo* only Prior chemo* + amivantamab +/- other exon20ins directed therapy Active brain mets (+/- prior treatment) 1st Line ex20 (zipalertinib monotherapy) Non-exon20ins uncommon (PACC+) EGFRm (prior systemic therapy) Data presented at ASCO 2025 *platinum-based Zipalertinib + pemetrexed + carboplatin or cisplatin Placebo + pemetrexed + carboplatin or cisplatin R 1:1 N=~300** © CULLINAN THERAPEUTICS, INC. ALL RIGHTS RESERVED.
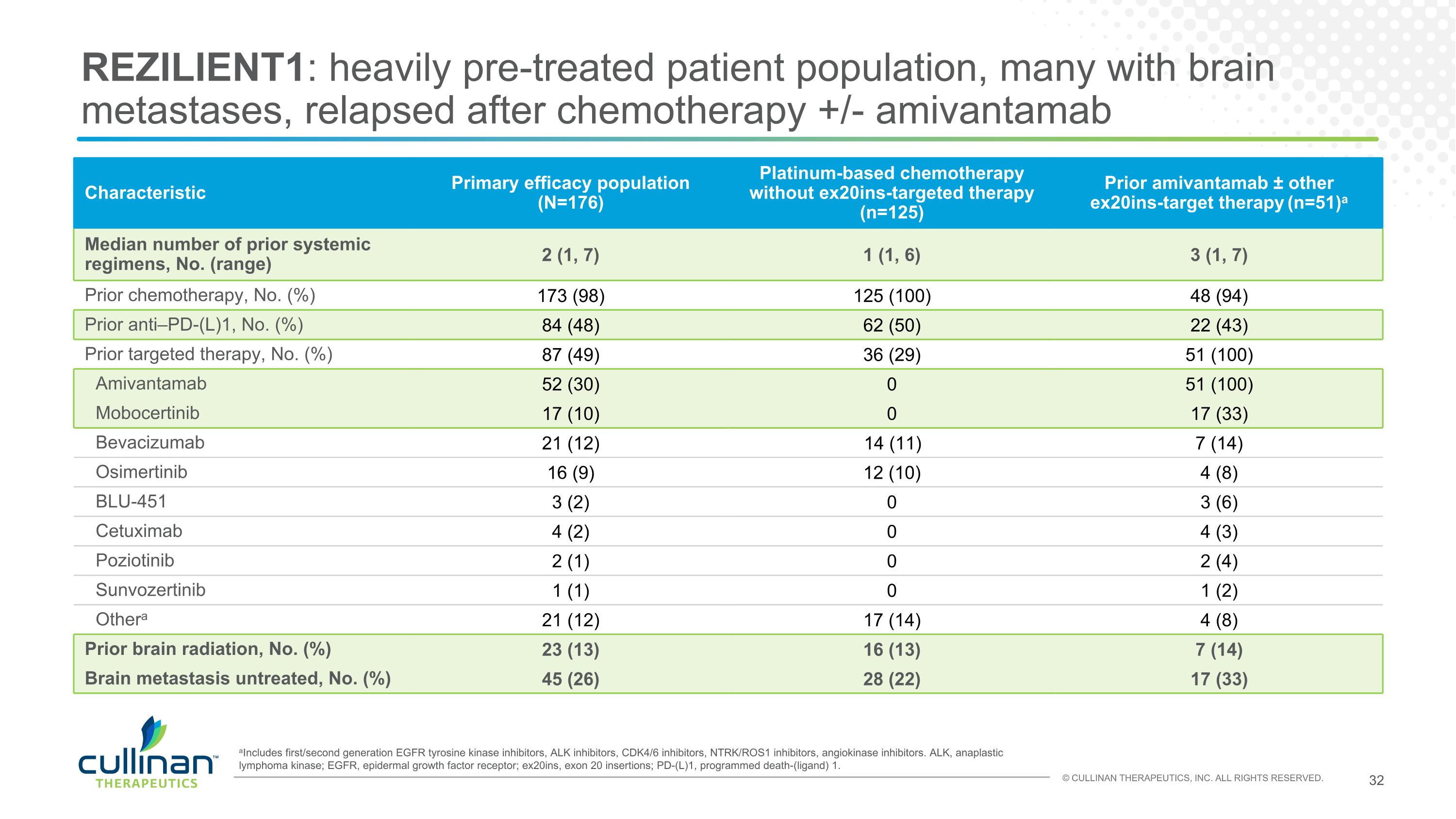
REZILIENT1: heavily pre-treated patient population, many with brain metastases, relapsed after chemotherapy +/- amivantamab aIncludes first/second generation EGFR tyrosine kinase inhibitors, ALK inhibitors, CDK4/6 inhibitors, NTRK/ROS1 inhibitors, angiokinase inhibitors. ALK, anaplastic lymphoma kinase; EGFR, epidermal growth factor receptor; ex20ins, exon 20 insertions; PD-(L)1, programmed death-(ligand) 1. Characteristic Primary efficacy population (N=176) Platinum-based chemotherapy without ex20ins-targeted therapy (n=125) Prior amivantamab ± other ex20ins-target therapy (n=51)a Median number of prior systemic regimens, No. (range) 2 (1, 7) 1 (1, 6) 3 (1, 7) Prior chemotherapy, No. (%) 173 (98) 125 (100) 48 (94) Prior anti–PD-(L)1, No. (%) 84 (48) 62 (50) 22 (43) Prior targeted therapy, No. (%) 87 (49) 36 (29) 51 (100) Amivantamab 52 (30) 0 51 (100) Mobocertinib 17 (10) 0 17 (33) Bevacizumab 21 (12) 14 (11) 7 (14) Osimertinib 16 (9) 12 (10) 4 (8) BLU-451 3 (2) 0 3 (6) Cetuximab 4 (2) 0 4 (3) Poziotinib 2 (1) 0 2 (4) Sunvozertinib 1 (1) 0 1 (2) Othera 21 (12) 17 (14) 4 (8) Prior brain radiation, No. (%) 23 (13) 16 (13) 7 (14) Brain metastasis untreated, No. (%) 45 (26) 28 (22) 17 (33)
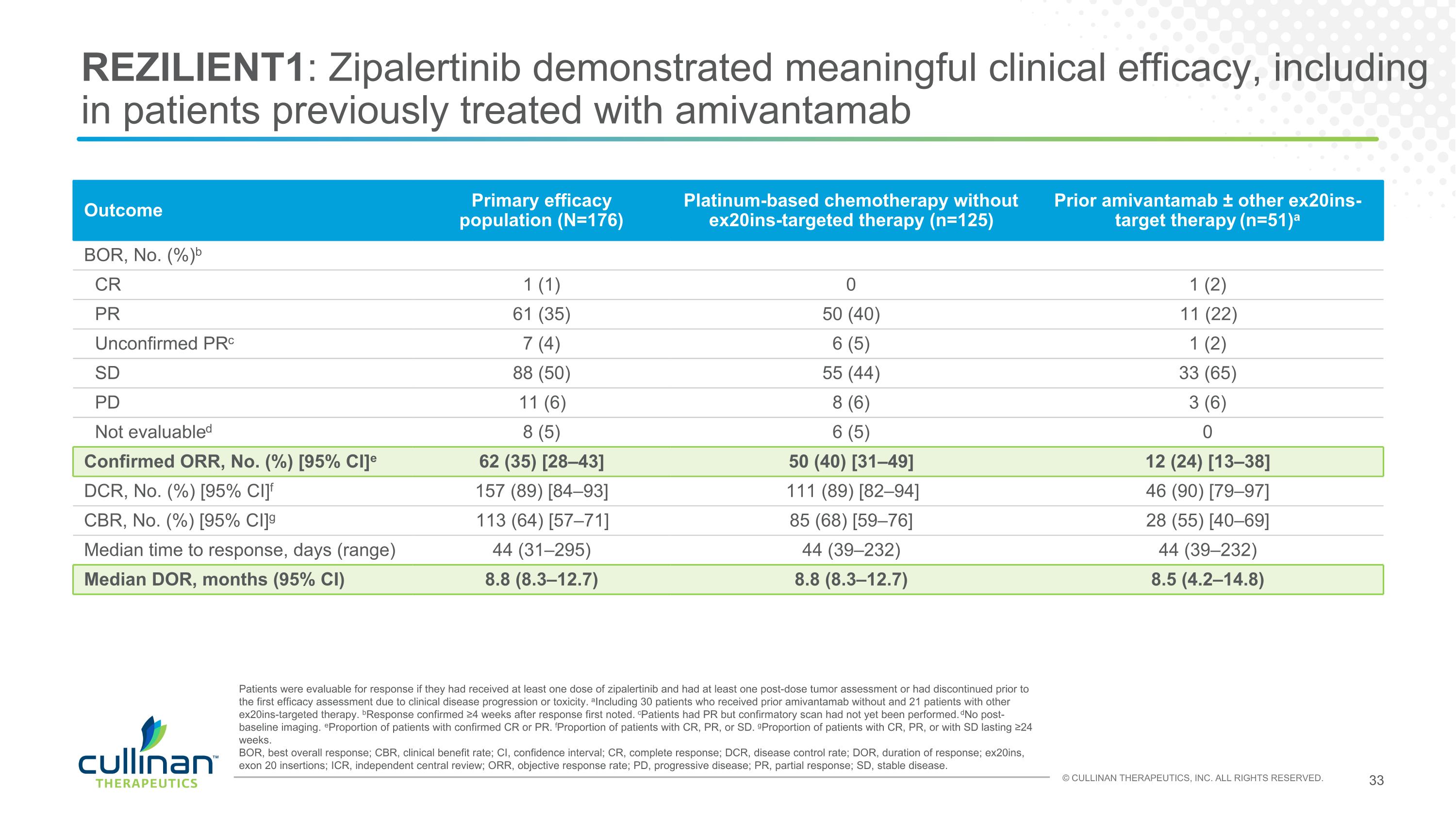
REZILIENT1: Zipalertinib demonstrated meaningful clinical efficacy, including in patients previously treated with amivantamab Outcome Primary efficacy population (N=176) Platinum-based chemotherapy without ex20ins-targeted therapy (n=125) Prior amivantamab ± other ex20ins-target therapy (n=51)a BOR, No. (%)b CR 1 (1) 0 1 (2) PR 61 (35) 50 (40) 11 (22) Unconfirmed PRc 7 (4) 6 (5) 1 (2) SD 88 (50) 55 (44) 33 (65) PD 11 (6) 8 (6) 3 (6) Not evaluabled 8 (5) 6 (5) 0 Confirmed ORR, No. (%) [95% CI]e 62 (35) [28–43] 50 (40) [31–49] 12 (24) [13–38] DCR, No. (%) [95% CI]f 157 (89) [84–93] 111 (89) [82–94] 46 (90) [79–97] CBR, No. (%) [95% CI]g 113 (64) [57–71] 85 (68) [59–76] 28 (55) [40–69] Median time to response, days (range) 44 (31–295) 44 (39–232) 44 (39–232) Median DOR, months (95% CI) 8.8 (8.3–12.7) 8.8 (8.3–12.7) 8.5 (4.2–14.8) Patients were evaluable for response if they had received at least one dose of zipalertinib and had at least one post-dose tumor assessment or had discontinued prior to the first efficacy assessment due to clinical disease progression or toxicity. aIncluding 30 patients who received prior amivantamab without and 21 patients with other ex20ins-targeted therapy. bResponse confirmed ≥4 weeks after response first noted. cPatients had PR but confirmatory scan had not yet been performed. dNo post-baseline imaging. eProportion of patients with confirmed CR or PR. fProportion of patients with CR, PR, or SD. gProportion of patients with CR, PR, or with SD lasting ≥24 weeks. BOR, best overall response; CBR, clinical benefit rate; CI, confidence interval; CR, complete response; DCR, disease control rate; DOR, duration of response; ex20ins, exon 20 insertions; ICR, independent central review; ORR, objective response rate; PD, progressive disease; PR, partial response; SD, stable disease.
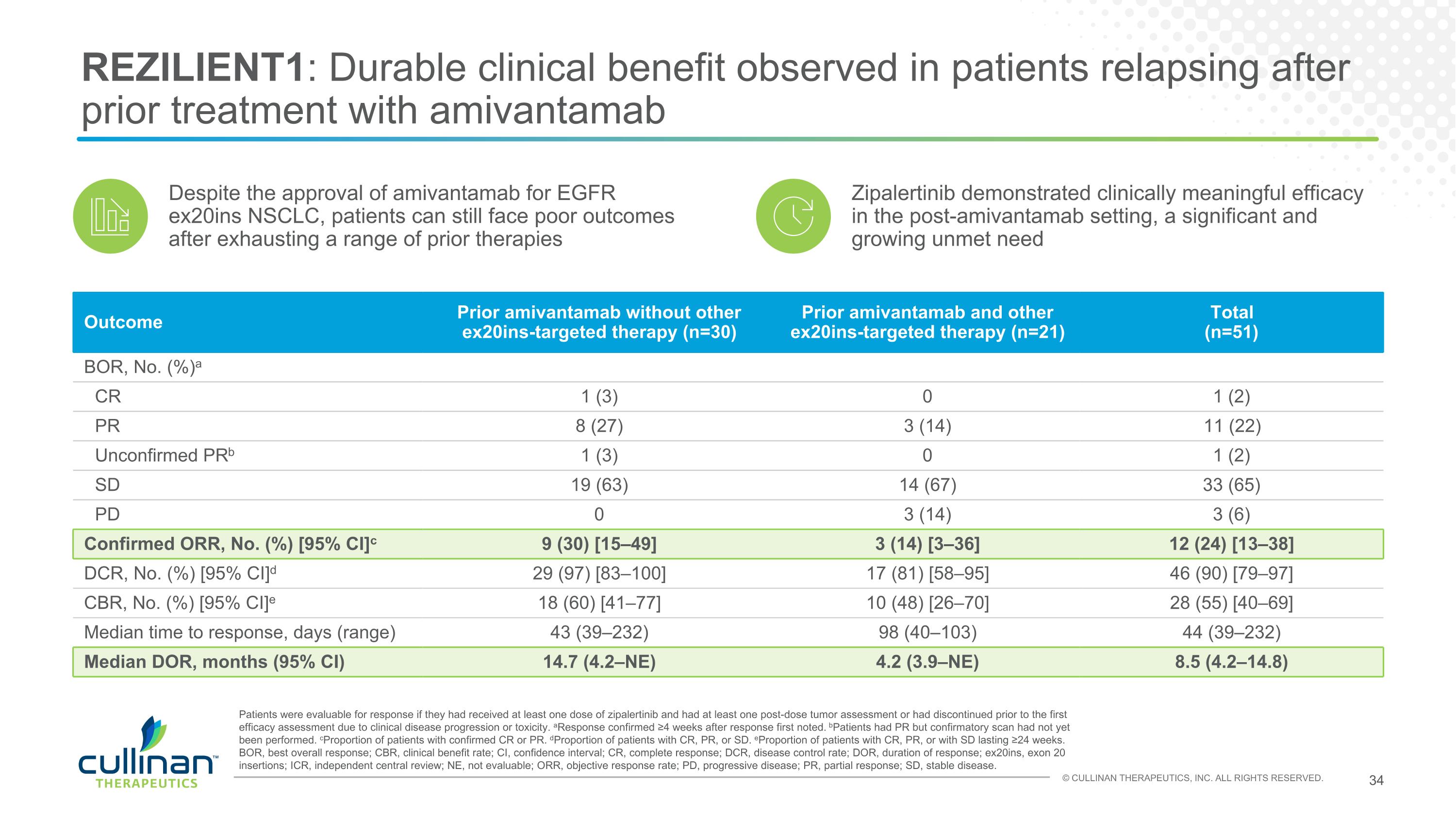
REZILIENT1: Durable clinical benefit observed in patients relapsing after prior treatment with amivantamab Despite the approval of amivantamab for EGFR ex20ins NSCLC, patients can still face poor outcomes after exhausting a range of prior therapies Outcome Prior amivantamab without other ex20ins-targeted therapy (n=30) Prior amivantamab and other ex20ins-targeted therapy (n=21) Total (n=51) BOR, No. (%)a CR 1 (3) 0 1 (2) PR 8 (27) 3 (14) 11 (22) Unconfirmed PRb 1 (3) 0 1 (2) SD 19 (63) 14 (67) 33 (65) PD 0 3 (14) 3 (6) Confirmed ORR, No. (%) [95% CI]c 9 (30) [15–49] 3 (14) [3–36] 12 (24) [13–38] DCR, No. (%) [95% CI]d 29 (97) [83–100] 17 (81) [58–95] 46 (90) [79–97] CBR, No. (%) [95% CI]e 18 (60) [41–77] 10 (48) [26–70] 28 (55) [40–69] Median time to response, days (range) 43 (39–232) 98 (40–103) 44 (39–232) Median DOR, months (95% CI) 14.7 (4.2–NE) 4.2 (3.9–NE) 8.5 (4.2–14.8) Patients were evaluable for response if they had received at least one dose of zipalertinib and had at least one post-dose tumor assessment or had discontinued prior to the first efficacy assessment due to clinical disease progression or toxicity. aResponse confirmed ≥4 weeks after response first noted. bPatients had PR but confirmatory scan had not yet been performed. cProportion of patients with confirmed CR or PR. dProportion of patients with CR, PR, or SD. eProportion of patients with CR, PR, or with SD lasting ≥24 weeks. BOR, best overall response; CBR, clinical benefit rate; CI, confidence interval; CR, complete response; DCR, disease control rate; DOR, duration of response; ex20ins, exon 20 insertions; ICR, independent central review; NE, not evaluable; ORR, objective response rate; PD, progressive disease; PR, partial response; SD, stable disease. Zipalertinib demonstrated clinically meaningful efficacy in the post-amivantamab setting, a significant and growing unmet need
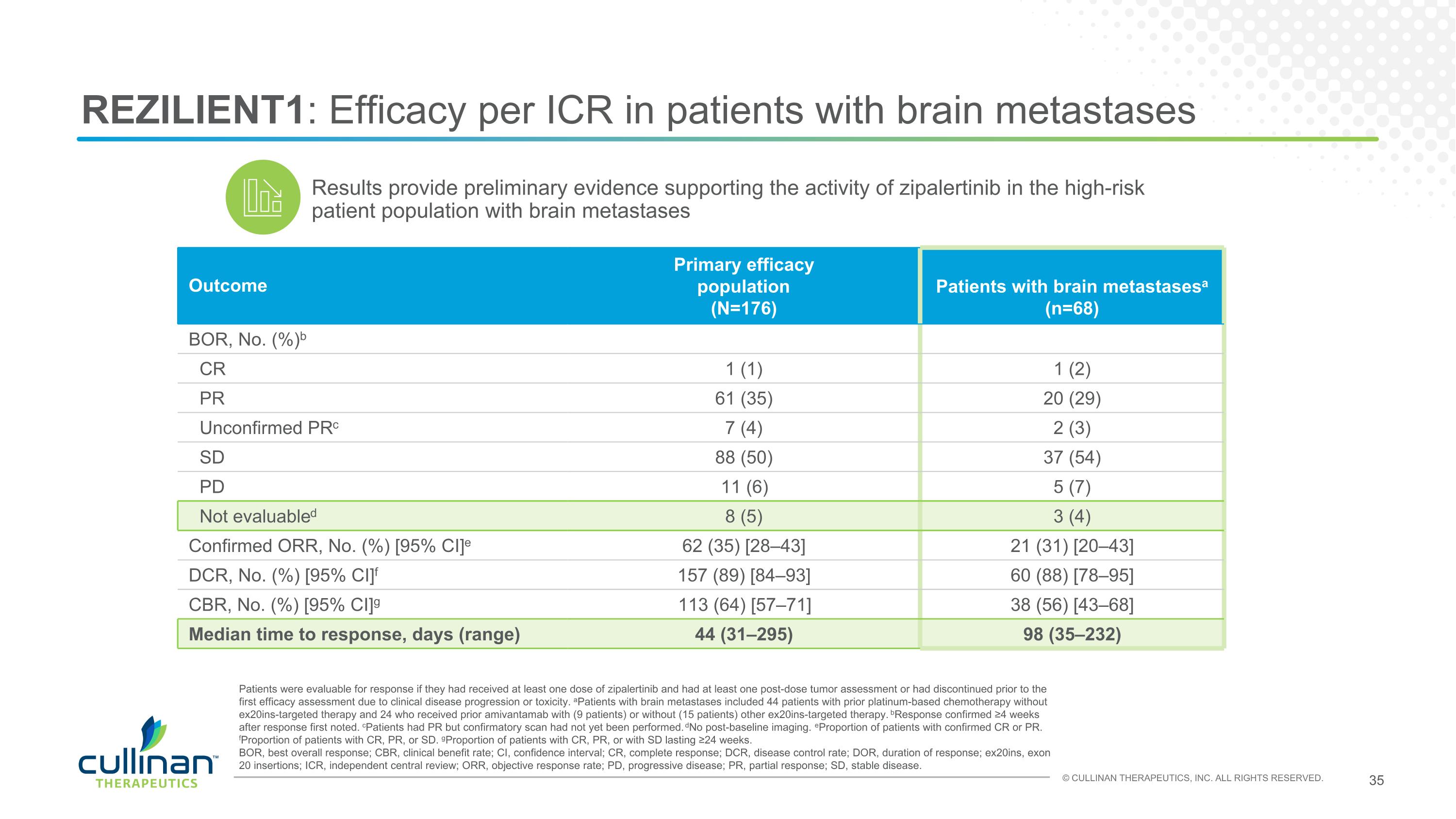
REZILIENT1: Efficacy per ICR in patients with brain metastases Outcome Primary efficacy population (N=176) Patients with brain metastasesa (n=68) BOR, No. (%)b CR 1 (1) 1 (2) PR 61 (35) 20 (29) Unconfirmed PRc 7 (4) 2 (3) SD 88 (50) 37 (54) PD 11 (6) 5 (7) Not evaluabled 8 (5) 3 (4) Confirmed ORR, No. (%) [95% CI]e 62 (35) [28–43] 21 (31) [20–43] DCR, No. (%) [95% CI]f 157 (89) [84–93] 60 (88) [78–95] CBR, No. (%) [95% CI]g 113 (64) [57–71] 38 (56) [43–68] Median time to response, days (range) 44 (31–295) 98 (35–232) Patients were evaluable for response if they had received at least one dose of zipalertinib and had at least one post-dose tumor assessment or had discontinued prior to the first efficacy assessment due to clinical disease progression or toxicity. aPatients with brain metastases included 44 patients with prior platinum-based chemotherapy without ex20ins-targeted therapy and 24 who received prior amivantamab with (9 patients) or without (15 patients) other ex20ins-targeted therapy. bResponse confirmed ≥4 weeks after response first noted. cPatients had PR but confirmatory scan had not yet been performed. dNo post-baseline imaging. eProportion of patients with confirmed CR or PR. fProportion of patients with CR, PR, or SD. gProportion of patients with CR, PR, or with SD lasting ≥24 weeks. BOR, best overall response; CBR, clinical benefit rate; CI, confidence interval; CR, complete response; DCR, disease control rate; DOR, duration of response; ex20ins, exon 20 insertions; ICR, independent central review; ORR, objective response rate; PD, progressive disease; PR, partial response; SD, stable disease. Results provide preliminary evidence supporting the activity of zipalertinib in the high-risk patient population with brain metastases
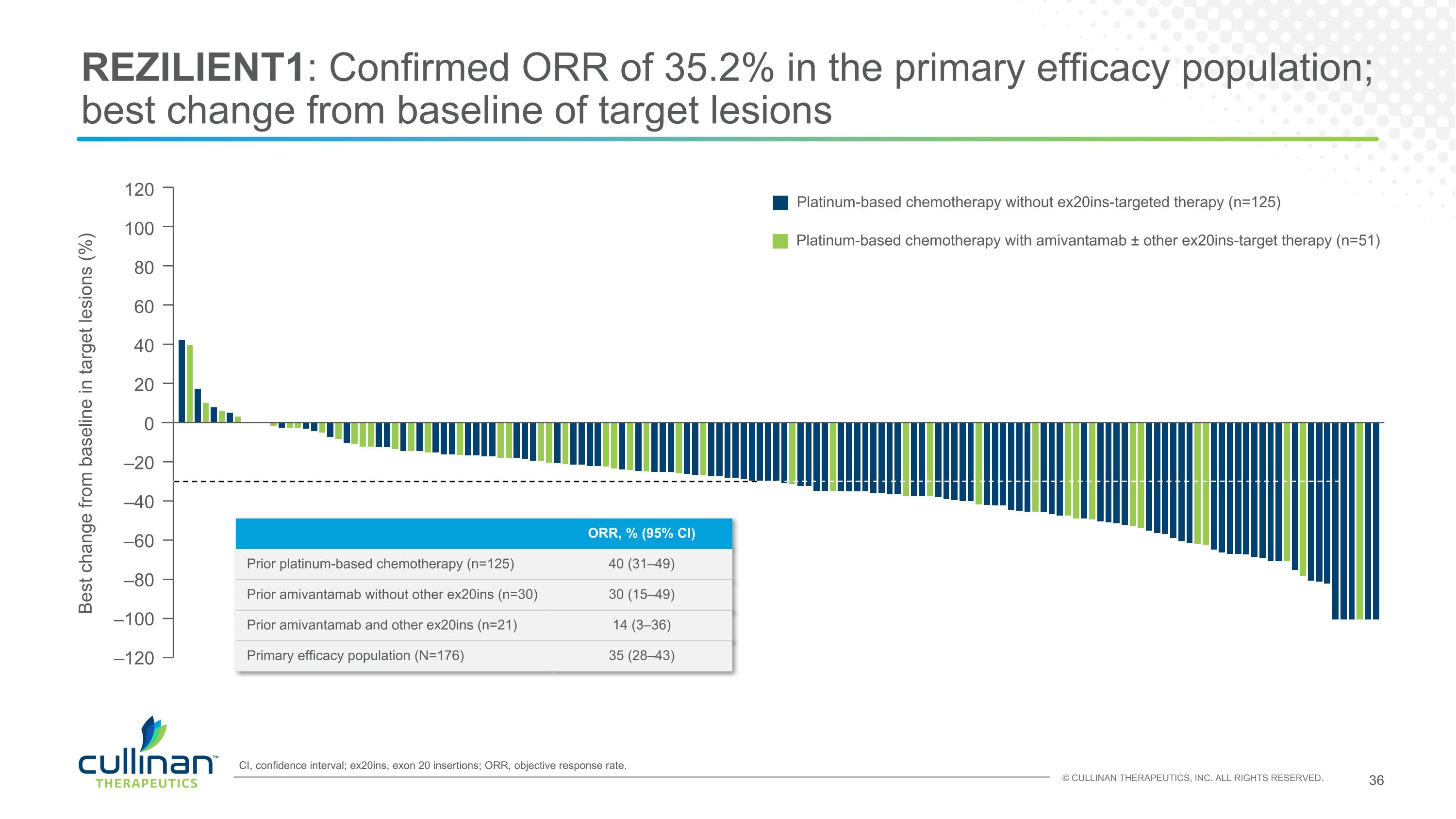
REZILIENT1: Confirmed ORR of 35.2% in the primary efficacy population; best change from baseline of target lesions Platinum-based chemotherapy without ex20ins-targeted therapy (n=125) Platinum-based chemotherapy with amivantamab ± other ex20ins-target therapy (n=51) 120 100 80 60 40 20 0 Best change from baseline in target lesions (%) –20 –40 –60 –80 –100 –120 CI, confidence interval; ex20ins, exon 20 insertions; ORR, objective response rate. ORR, % (95% CI) Prior platinum-based chemotherapy (n=125) 40 (31–49) Prior amivantamab without other ex20ins (n=30) 30 (15–49) Prior amivantamab and other ex20ins (n=21) 14 (3–36) Primary efficacy population (N=176) 35 (28–43)
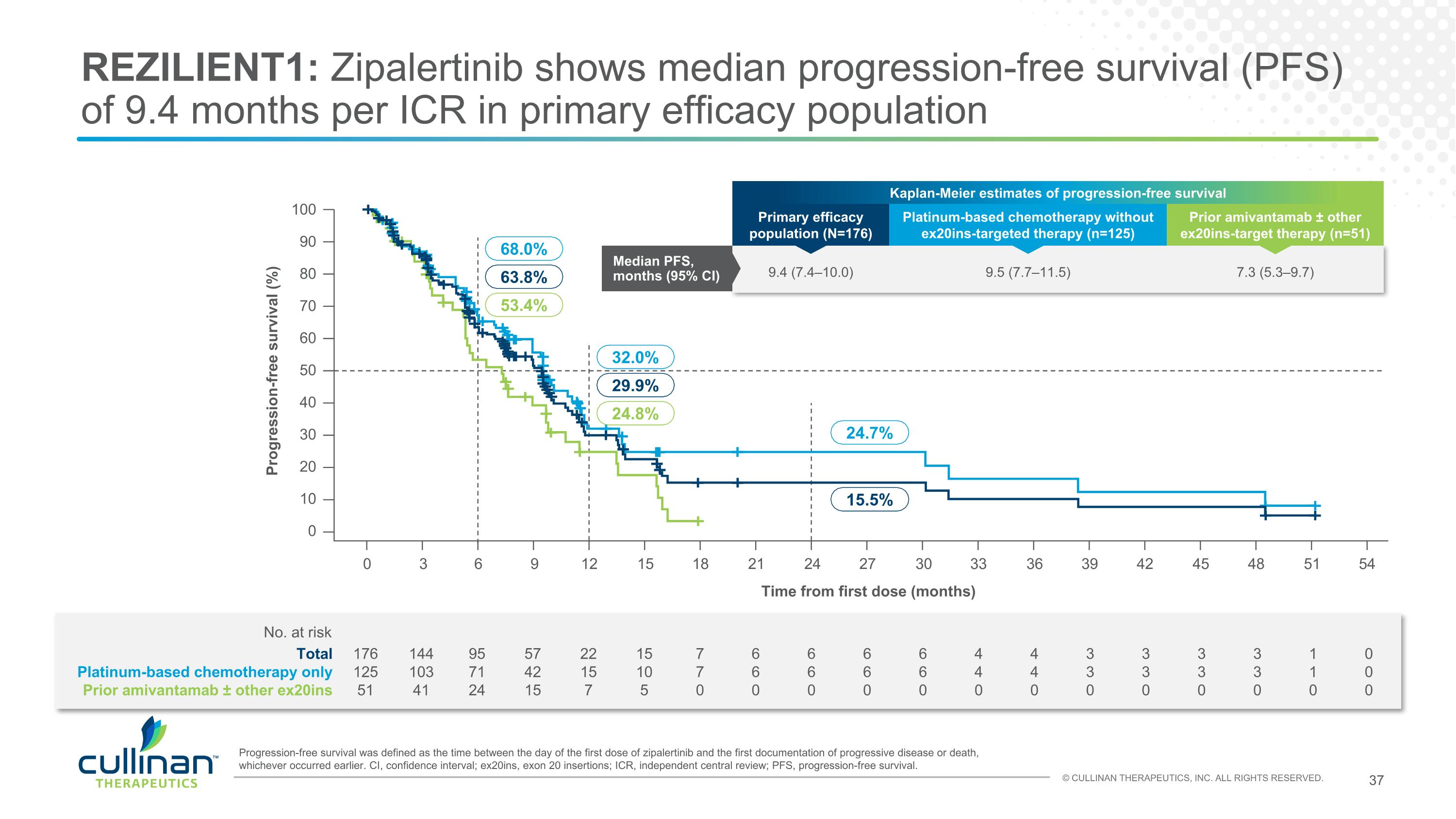
REZILIENT1: Zipalertinib shows median progression-free survival (PFS) of 9.4 months per ICR in primary efficacy population 176 125 51 144 103 41 95 71 24 57 42 15 22 15 7 15 10 5 7 7 0 6 6 0 6 6 0 6 6 0 6 6 0 4 4 0 4 4 0 3 3 0 3 3 0 3 3 0 3 3 0 1 1 0 0 0 0 Total Platinum-based chemotherapy only Prior amivantamab ± other ex20ins No. at risk Progression-free survival was defined as the time between the day of the first dose of zipalertinib and the first documentation of progressive disease or death, whichever occurred earlier. CI, confidence interval; ex20ins, exon 20 insertions; ICR, independent central review; PFS, progression-free survival. 100 90 80 70 60 50 40 30 20 10 0 Progression-free survival (%) Time from first dose (months) 24.7% 15.5% 0 3 6 9 12 15 18 21 24 27 30 33 36 39 42 45 48 51 54 32.0% 29.9% 24.8% 68.0% 63.8% 53.4% Kaplan-Meier estimates of progression-free survival Primary efficacy population (N=176) Platinum-based chemotherapy without ex20ins-targeted therapy (n=125) Prior amivantamab ± other ex20ins-target therapy (n=51) Median PFS, months (95% CI) 9.4 (7.4–10.0) 9.5 (7.7–11.5) 7.3 (5.3–9.7)
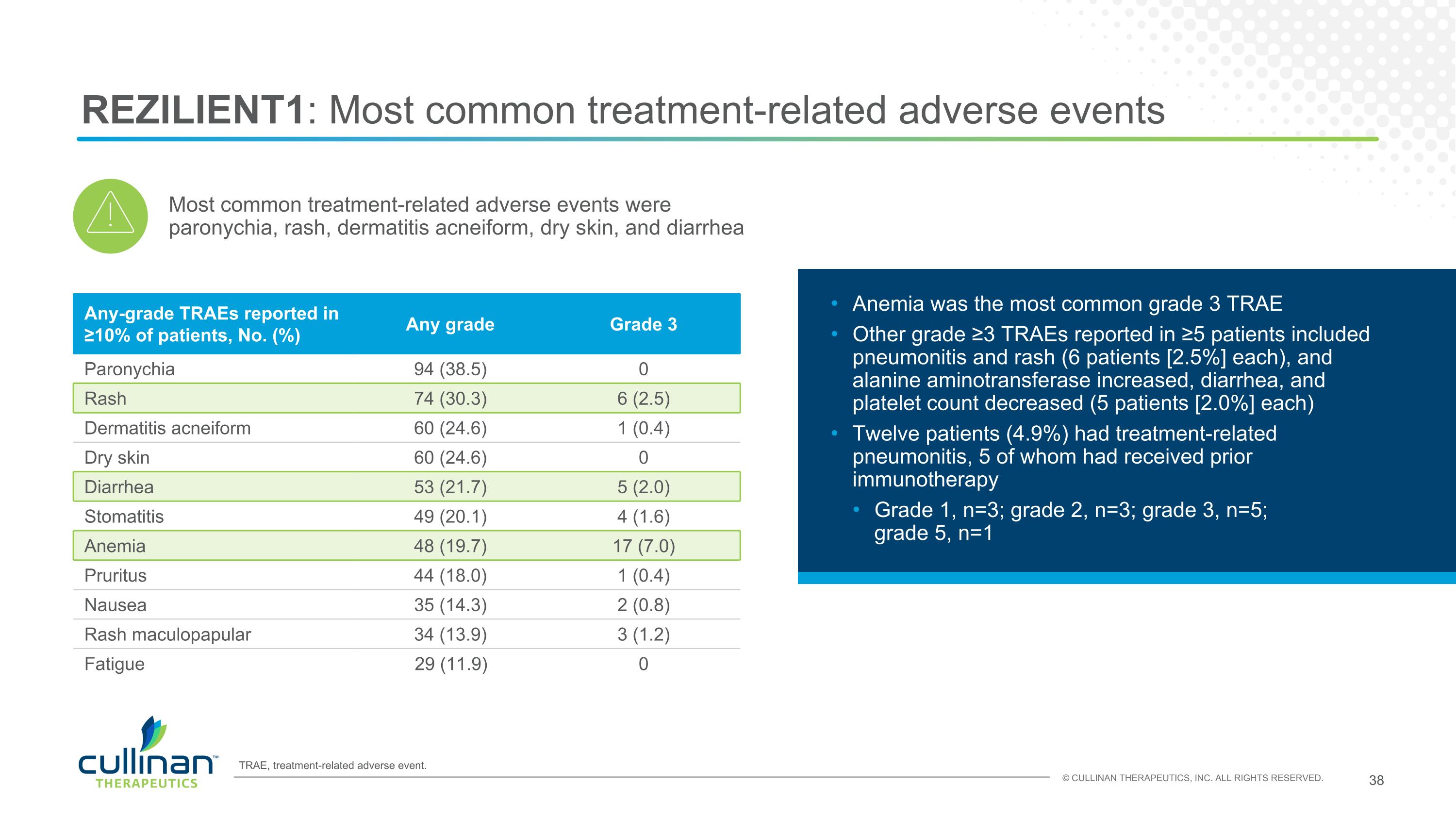
REZILIENT1: Most common treatment-related adverse events Any-grade TRAEs reported in ≥10% of patients, No. (%) Any grade Grade 3 Paronychia 94 (38.5) 0 Rash 74 (30.3) 6 (2.5) Dermatitis acneiform 60 (24.6) 1 (0.4) Dry skin 60 (24.6) 0 Diarrhea 53 (21.7) 5 (2.0) Stomatitis 49 (20.1) 4 (1.6) Anemia 48 (19.7) 17 (7.0) Pruritus 44 (18.0) 1 (0.4) Nausea 35 (14.3) 2 (0.8) Rash maculopapular 34 (13.9) 3 (1.2) Fatigue 29 (11.9) 0 Anemia was the most common grade 3 TRAE Other grade ≥3 TRAEs reported in ≥5 patients included pneumonitis and rash (6 patients [2.5%] each), and alanine aminotransferase increased, diarrhea, and platelet count decreased (5 patients [2.0%] each) Twelve patients (4.9%) had treatment-related pneumonitis, 5 of whom had received prior immunotherapy Grade 1, n=3; grade 2, n=3; grade 3, n=5; grade 5, n=1 Most common treatment-related adverse events were paronychia, rash, dermatitis acneiform, dry skin, and diarrhea TRAE, treatment-related adverse event.
REZILIENT1: Pivotal Phase 2b study in 2L+ met the primary endpoint of ORR and DOR, demonstrated a manageable safety profile for zipalertinib Pivotal Phase 2b (met primary endpoint) REZILIENT11 Primary endpoint: ORR and DOR Clinicaltrials.gov identifiers: 1NCT04036682, 2NCT05967689 and 3NCT05973773; * includes both approved and investigational exon20 therapies ** following 6-12 patient safety lead in. PACC = P-loop and αC-helix Prior platinum-based chemo only Prior platinum-based chemo + amivantamab +/- other exon20ins directed therapy These findings support zipalertinib as a potential treatment option for patients with EGFR ex20ins-mutant NSCLC after progression on platinum-based chemotherapy, including in the post-amivantamab setting, a significant and growing unmet need Updated efficacy and safety data in patients previously treated with amivantamab to be shared at the IASLC 2025 WCLC in September 2025 Pending discussions with the U.S. Food and Drug Administration, potential NDA filling in by YE 2025 by Taiho in relapsed EGFR ex20ins NSCLC. Randomized REZILIENT3 Phase 3 frontline study ongoing; complete enrollment expected in H1 2026 Next steps REZILIENT1 pivotal Phase 2b study in 2L+ met its primary endpoint of overall response rate and duration of response Zipalertinib demonstrated clinically meaningful efficacy Zipalertinib demonstrated a manageable safety profile, consistent with previously reported data Data
REZILIENT program: broad development of zipalertinib across multiple studies and indications in collaboration with Taiho Oncology 40 Pivotal Phase 2b (met primary endpoint) REZILIENT11 Primary endpoint: ORR + DOR Clinicaltrials.gov identifiers: 1NCT04036682, 2NCT05967689 and 3NCT05973773; * includes both approved and investigational exon20 therapies ** following 6-12 patient safety lead in. PACC = P-loop and αC-helix 1L Randomized Phase 3 (initiated Q3 2023) REZILIENT33 Primary endpoint: PFS Phase 2 Parallel Cohort Study REZILIENT22 Primary endpoint: ORR Prior chemo* only Prior chemo* + amivantamab +/- other exon20ins directed therapy Active brain mets (+/- prior treatment) 1st Line ex20 (zipalertinib monotherapy) Non-exon20ins uncommon (PACC+) EGFRm (prior systemic therapy) Data presented at ASCO 2025 *platinum-based Zipalertinib + pemetrexed + carboplatin or cisplatin Placebo + pemetrexed + carboplatin or cisplatin R 1:1 N=~300** © CULLINAN THERAPEUTICS, INC. ALL RIGHTS RESERVED.
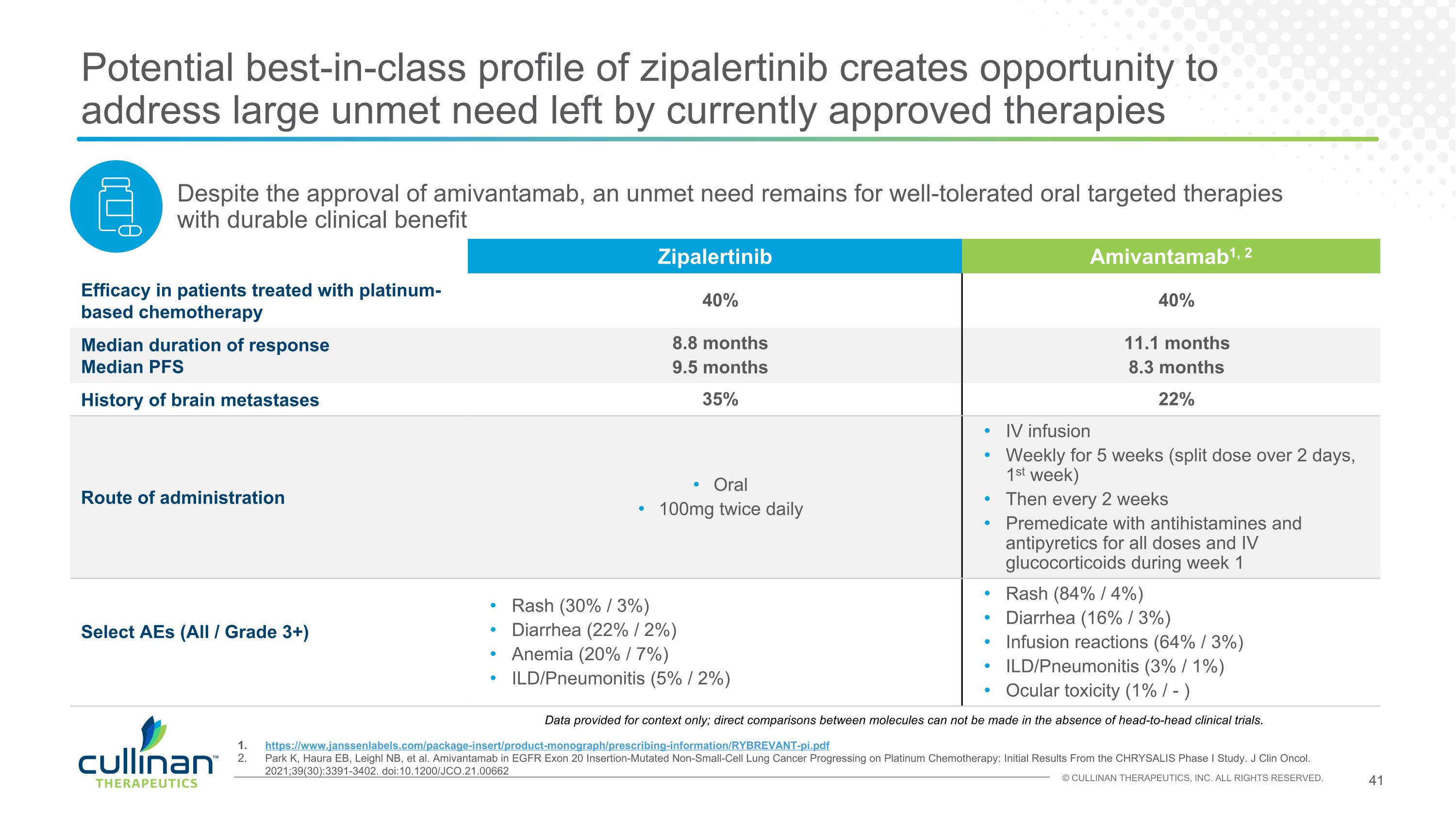
Potential best-in-class profile of zipalertinib creates opportunity to address large unmet need left by currently approved therapies KEY DATA https://www.janssenlabels.com/package-insert/product-monograph/prescribing-information/RYBREVANT-pi.pdf Park K, Haura EB, Leighl NB, et al. Amivantamab in EGFR Exon 20 Insertion-Mutated Non-Small-Cell Lung Cancer Progressing on Platinum Chemotherapy: Initial Results From the CHRYSALIS Phase I Study. J Clin Oncol. 2021;39(30):3391-3402. doi:10.1200/JCO.21.00662 Zipalertinib Amivantamab1, 2 Efficacy in patients treated with platinum-based chemotherapy 40% 40% Median duration of response Median PFS 8.8 months 9.5 months 11.1 months 8.3 months History of brain metastases 35% 22% Route of administration Oral 100mg twice daily IV infusion Weekly for 5 weeks (split dose over 2 days, 1st week) Then every 2 weeks Premedicate with antihistamines and antipyretics for all doses and IV glucocorticoids during week 1 Select AEs (All / Grade 3+) Rash (30% / 3%) Diarrhea (22% / 2%) Anemia (20% / 7%) ILD/Pneumonitis (5% / 2%) Rash (84% / 4%) Diarrhea (16% / 3%) Infusion reactions (64% / 3%) ILD/Pneumonitis (3% / 1%) Ocular toxicity (1% / - ) Despite the approval of amivantamab, an unmet need remains for well-tolerated oral targeted therapies with durable clinical benefit Data provided for context only; direct comparisons between molecules can not be made in the absence of head-to-head clinical trials.
Taiho zipalertinib collaboration provides Cullinan with financial and strategic benefits 1. Excludes rights to Japan, which were already held by Taiho, and rights to Greater China, which were previously licensed to Zai Labs Upfront Payment $275 million to Cullinan received in 2022 in exchange for providing 50% of U.S. and 100% of ex-U.S. rights to Taiho1 Milestone Payments Cullinan is eligible to receive up to $130 million in payments for EGFR exon 20 NSCLC U.S. regulatory milestones Collaboration Taiho and Cullinan entered into a U.S. co-development and co-commercialization agreement, providing Cullinan with a co-promote option Profit Sharing Parties will share 50/50 U.S. development costs and potential profits
REZILIENT program: upcoming milestones and next steps Initial results from REZILIENT2 Cohort C to be shared at ESMO Congress 2025 in October 2025 Initial results from Cohort C of REZILIENT2, which is exploring zipalertinib monotherapy in patients with active brain metastases who may or may not have had prior treatment for advanced disease Initial results from REZILIENT2 Cohort D to be shared at IASLC 2025 WCLC in September 2025 Initial results from Cohort D of REZILIENT3, which is exploring zipalertinib monotherapy in patients with uncommon EGFR mutations, such as PACC, who may or may not have had prior systemic therapy NDA Submission in 2L+ Pending discussions with the U.S. Food and Drug Administration, potential NDA filling by YE 2025 by Taiho in relapsed EGFR ex20ins NSCLC REZILIENT3 status update Expected to complete enrollment in H1 2026 REZILIENT1 Update Updated efficacy and safety data in patients previously treated with amivantamab to be shared at the IASLC 2025 WCLC in September 2025
CLN-049 FLT3xCD3 bispecific T cell Engager
Significant unmet need in adult AML References 1. American Cancer Society (2025) 2. DeWolf and Tallman. Blood 2020. The only curative therapy is intensive chemotherapy +/- stem cell transplantation Curative therapy remains out of reach for most AML patients: 85% patients >60 years old are ineligible for intensive chemotherapy Recent treatment advancements have not significantly improved the likelihood of cure for the majority of AML patients A significant unmet need remains for – a broadly applicable therapy that can produce high rates of durable response eradication of measurable residual disease (MRD) that portends relapse even when patients meet clinical criteria for complete remission 5-year survival 10% or less in relapsed setting2 Average age at diagnosis 681 US AML Incidence 22,0101
FLT3: An optimal target for AML immunotherapy 1 2 3 4 Promising therapeutic potential: FLT3 is expressed on leukemic stem cells as well as blast cells, which may increase response durability. Since FLT3 is an oncogenic driver, target loss is unlikely Validation: FLT3 plays a key role in promoting leukemic cell proliferation and survival. Tyrosine kinase inhibitors (XOSPATA®, RYDAPT®) treat 20-30% of AML by targeting only mutant FLT3 Potential for reduced toxicity risk: FLT3 expression is very low on most mature normal myeloid cells compared to other frequently used targets for T cell engagers in AML like CD123 and CD33. FLT3 expression is very low on normal pluripotent stem cells Potential for treatment of broad AML population: Targeting the extracellular domain of FLT3 could address ~80% of AML patients that express FLT3 on the cell surface, either wildtype or mutant forms
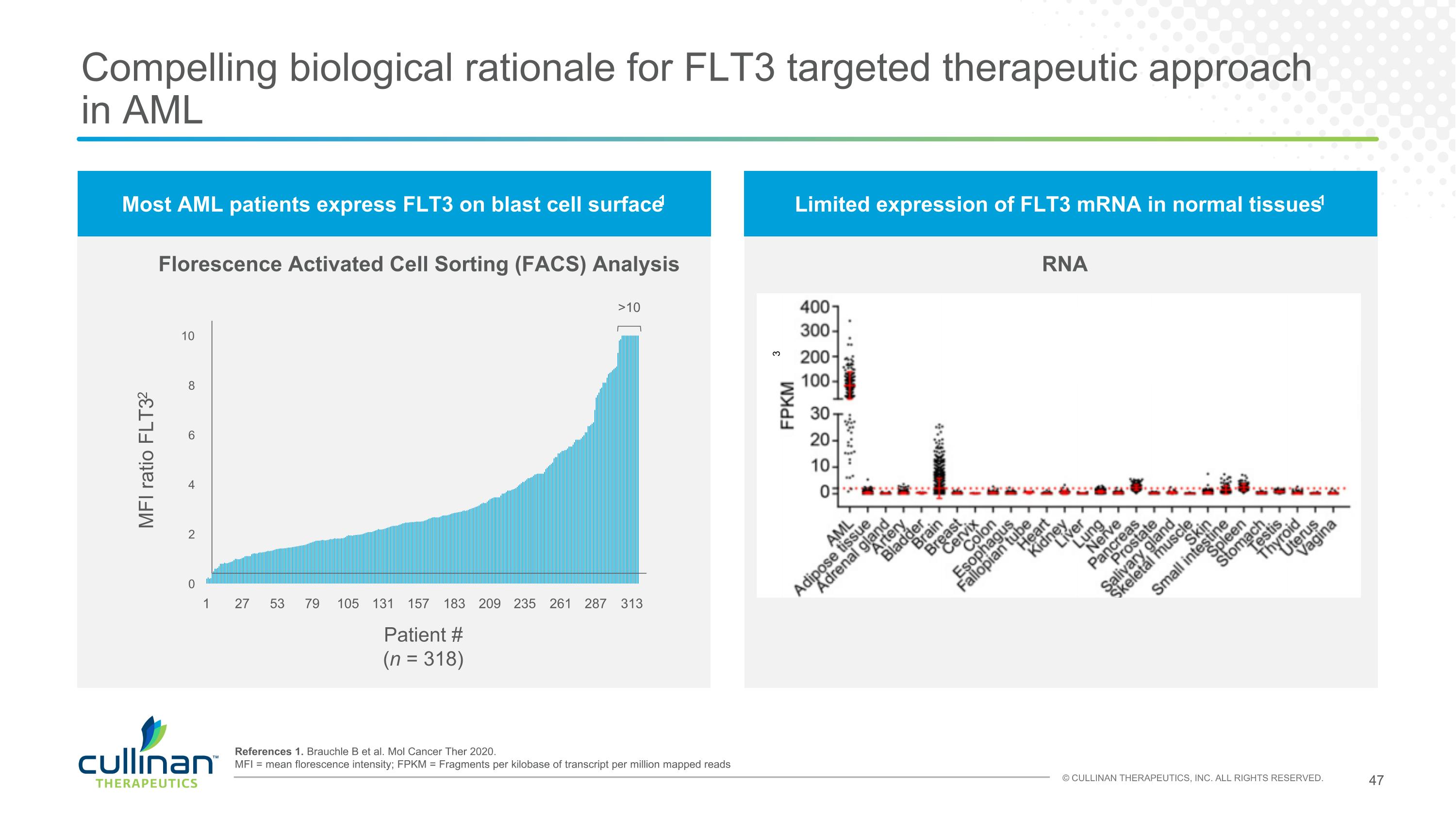
Most AML patients express FLT3 on blast cell surface1 Limited expression of FLT3 mRNA in normal tissues1 Compelling biological rationale for FLT3 targeted therapeutic approach in AML Florescence Activated Cell Sorting (FACS) Analysis RNA 1 27 53 79 105 131 157 183 209 235 261 287 313 >10 MFI ratio FLT32 Patient # (n = 318) 3 References 1. Brauchle B et al. Mol Cancer Ther 2020. MFI = mean florescence intensity; FPKM = Fragments per kilobase of transcript per million mapped reads
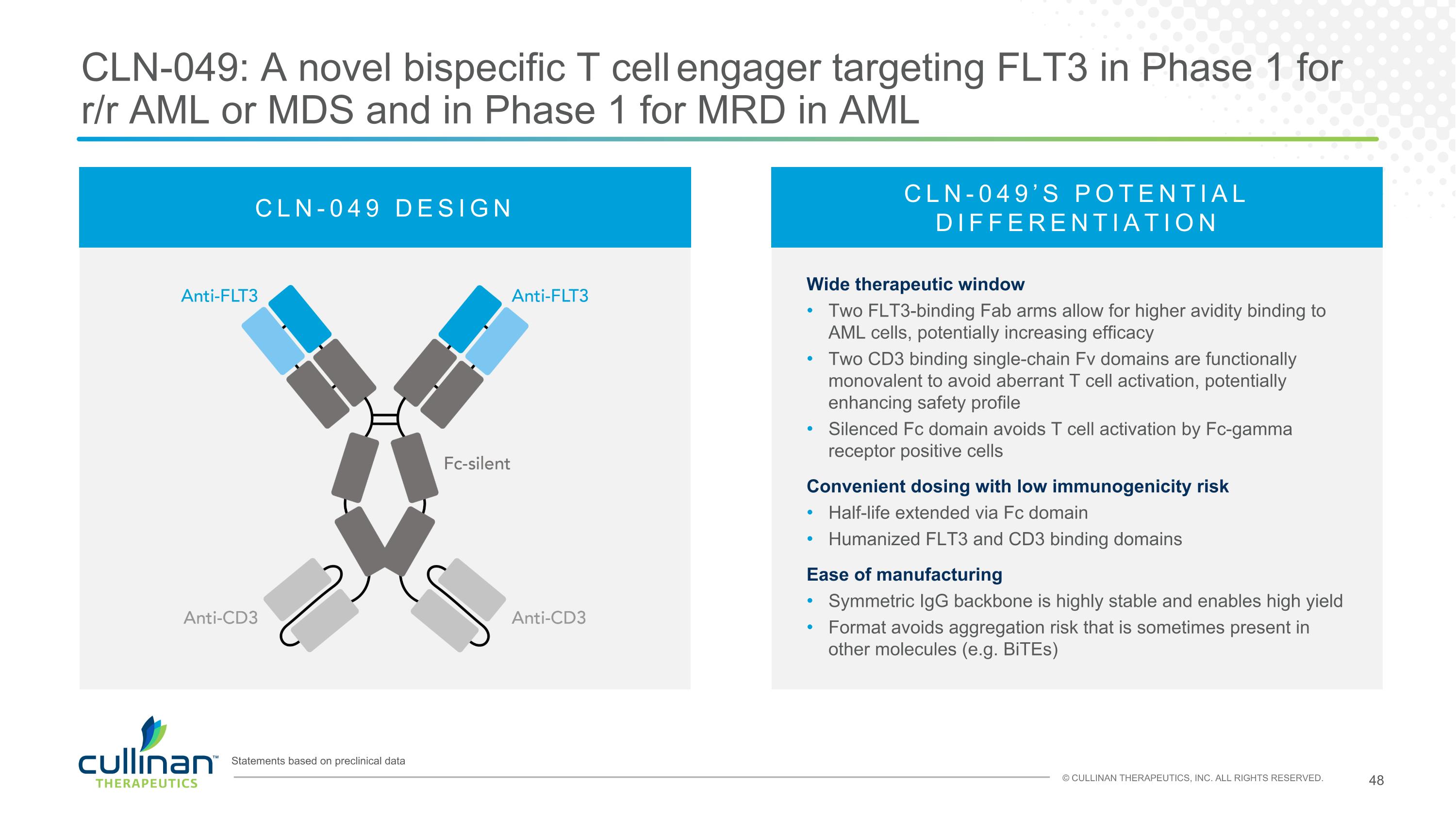
CLN-049: A novel bispecific T cell engager targeting FLT3 in Phase 1 for r/r AML or MDS and in Phase 1 for MRD in AML Statements based on preclinical data CLN-049 DESIGN CLN-049’S POTENTIAL DIFFERENTIATION Wide therapeutic window Two FLT3-binding Fab arms allow for higher avidity binding to AML cells, potentially increasing efficacy Two CD3 binding single-chain Fv domains are functionally monovalent to avoid aberrant T cell activation, potentially enhancing safety profile Silenced Fc domain avoids T cell activation by Fc-gamma receptor positive cells Convenient dosing with low immunogenicity risk Half-life extended via Fc domain Humanized FLT3 and CD3 binding domains Ease of manufacturing Symmetric IgG backbone is highly stable and enables high yield Format avoids aggregation risk that is sometimes present in other molecules (e.g. BiTEs)
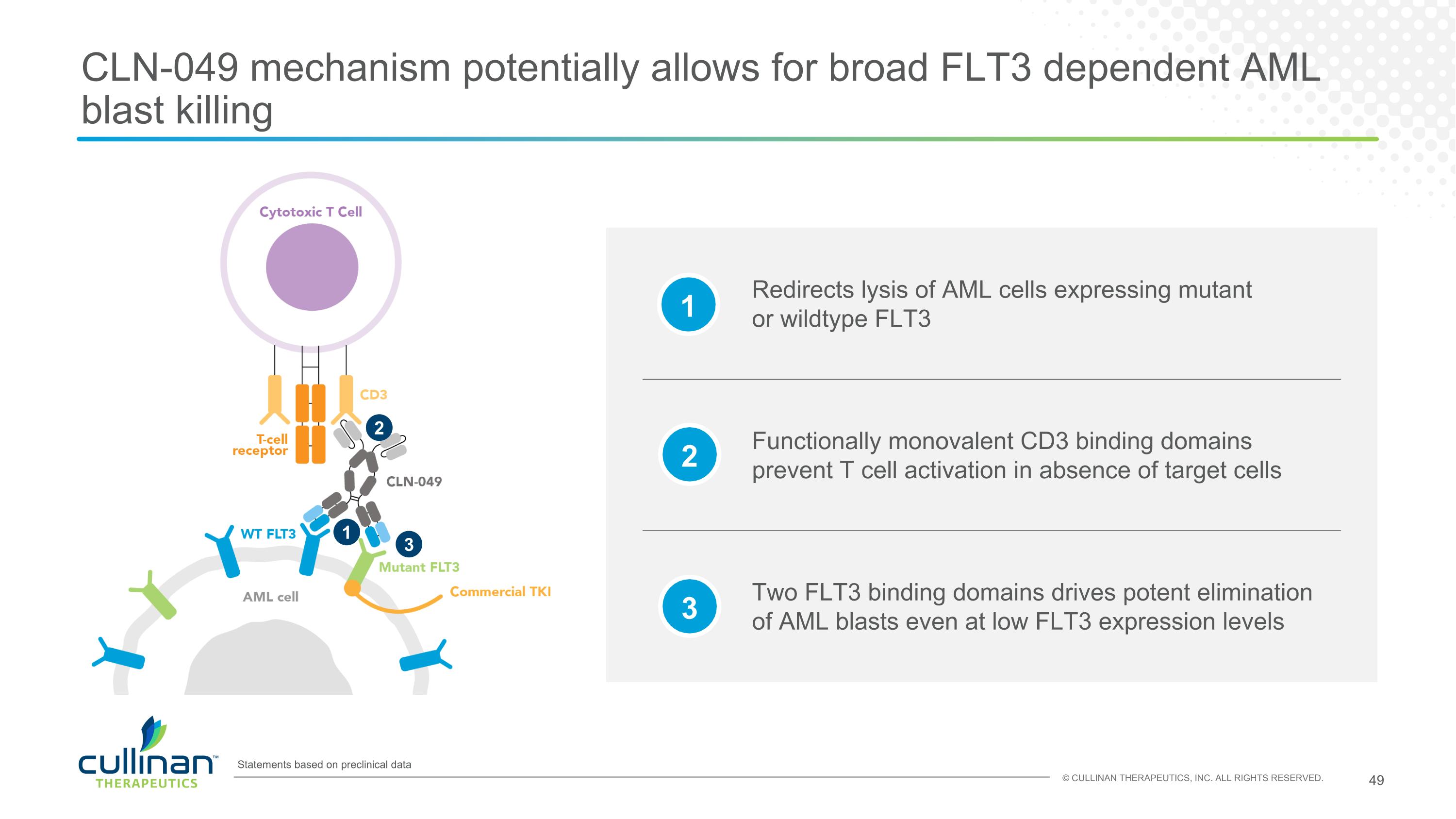
CLN-049 mechanism potentially allows for broad FLT3 dependent AML blast killing 1 2 3 Statements based on preclinical data Redirects lysis of AML cells expressing mutant or wildtype FLT3 Two FLT3 binding domains drives potent elimination of AML blasts even at low FLT3 expression levels Functionally monovalent CD3 binding domains prevent T cell activation in absence of target cells 1 2 3
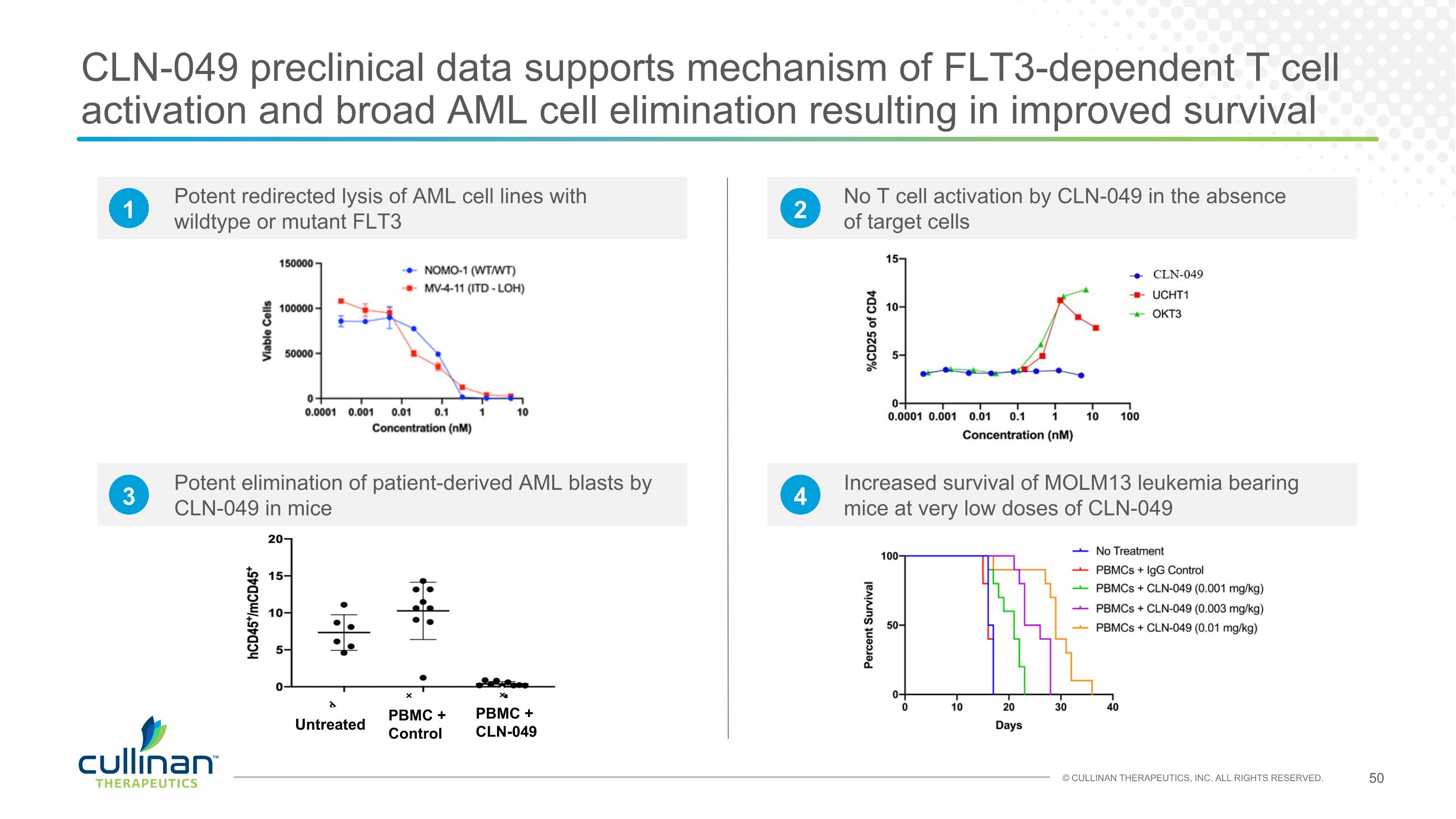
CLN-049 preclinical data supports mechanism of FLT3-dependent T cell activation and broad AML cell elimination resulting in improved survival Potent redirected lysis of AML cell lines with wildtype or mutant FLT3 No T cell activation by CLN-049 in the absence of target cells Potent elimination of patient-derived AML blasts by CLN-049 in mice Increased survival of MOLM13 leukemia bearing mice at very low doses of CLN-049 Untreated PBMC + Control PBMC + CLN-049 1 2 3 4
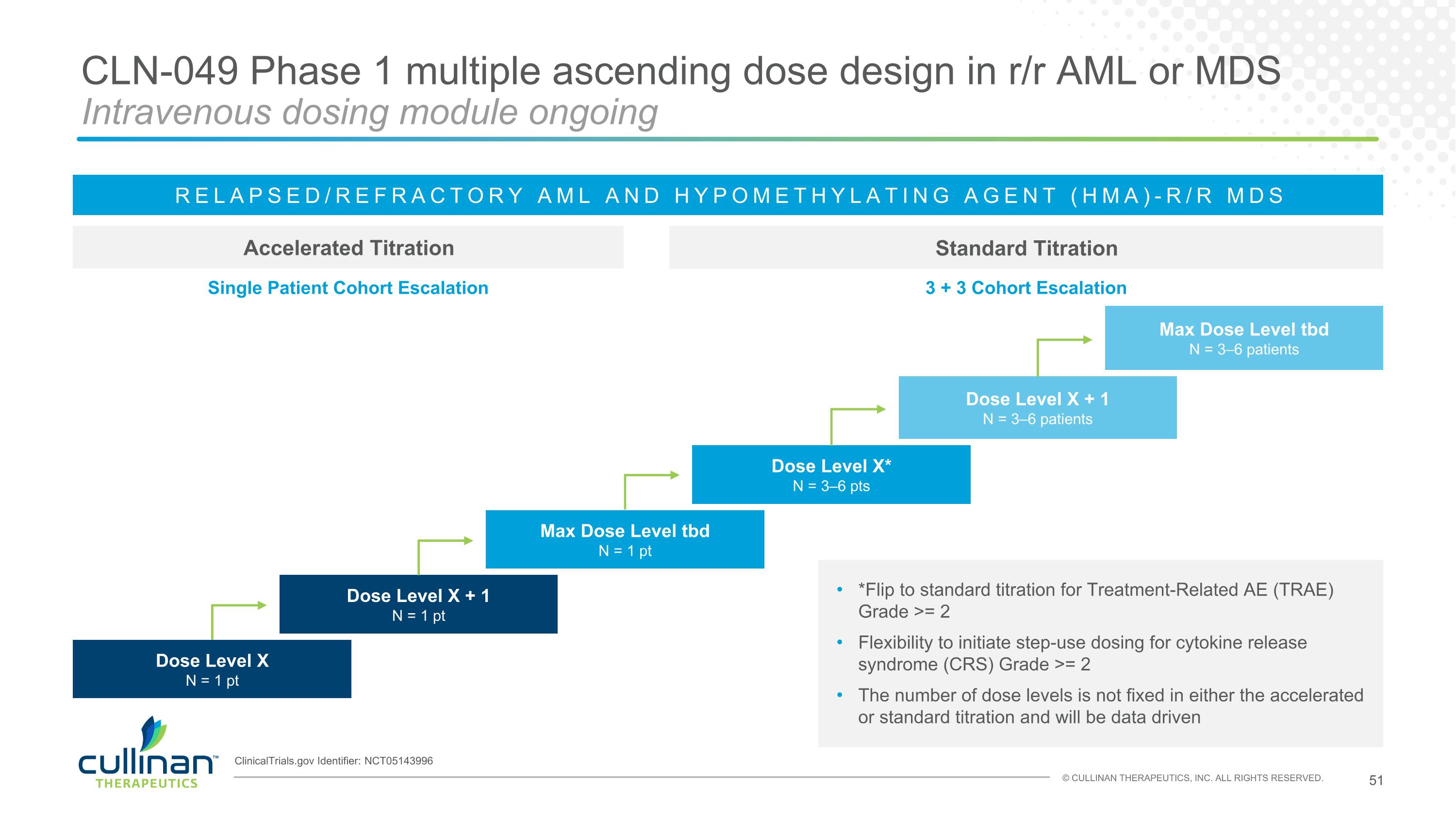
CLN-049 Phase 1 multiple ascending dose design in r/r AML or MDS Intravenous dosing module ongoing Accelerated Titration Standard Titration RELAPSED/REFRACTORY AML AND HYPOMETHYLATING AGENT (HMA)-R/R MDS Dose Level X* N = 3–6 pts Max Dose Level tbd N = 3–6 patients Dose Level X + 1 N = 3–6 patients Single Patient Cohort Escalation 3 + 3 Cohort Escalation Dose Level X N = 1 pt Dose Level X + 1 N = 1 pt Max Dose Level tbd N = 1 pt *Flip to standard titration for Treatment-Related AE (TRAE) Grade >= 2 Flexibility to initiate step-use dosing for cytokine release syndrome (CRS) Grade >= 2 The number of dose levels is not fixed in either the accelerated or standard titration and will be data driven ClinicalTrials.gov Identifier: NCT05143996
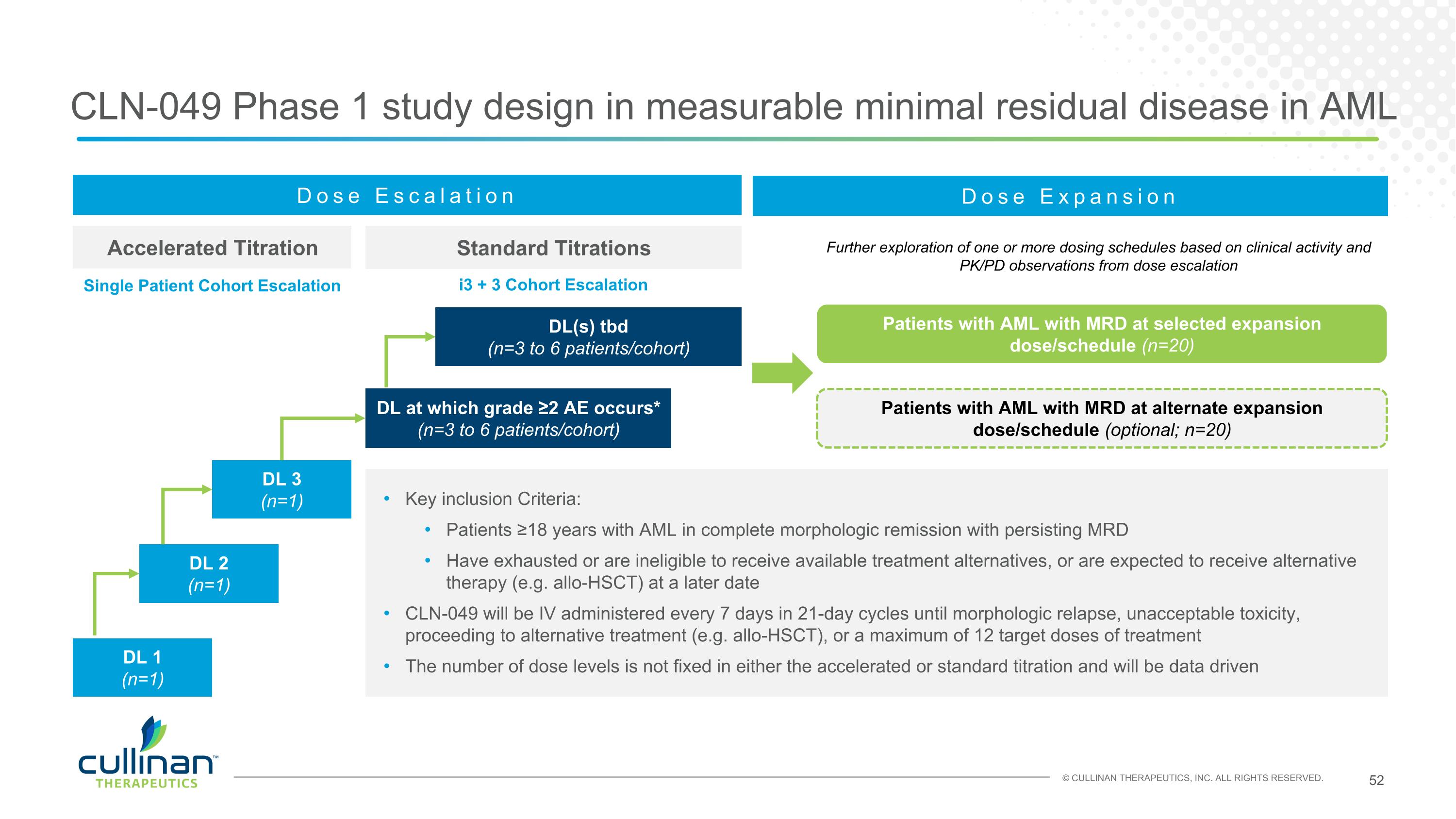
CLN-049 Phase 1 study design in measurable minimal residual disease in AML Accelerated Titration Standard Titrations Dose Escalation DL at which grade ≥2 AE occurs* (n=3 to 6 patients/cohort) DL 1 (n=1) Key inclusion Criteria: Patients ≥18 years with AML in complete morphologic remission with persisting MRD Have exhausted or are ineligible to receive available treatment alternatives, or are expected to receive alternative therapy (e.g. allo-HSCT) at a later date CLN-049 will be IV administered every 7 days in 21-day cycles until morphologic relapse, unacceptable toxicity, proceeding to alternative treatment (e.g. allo-HSCT), or a maximum of 12 target doses of treatment The number of dose levels is not fixed in either the accelerated or standard titration and will be data driven DL 2 (n=1) DL 3 (n=1) DL(s) tbd (n=3 to 6 patients/cohort) Dose Expansion Patients with AML with MRD at selected expansion dose/schedule (n=20) Patients with AML with MRD at alternate expansion dose/schedule (optional; n=20) Further exploration of one or more dosing schedules based on clinical activity and PK/PD observations from dose escalation Single Patient Cohort Escalation i3 + 3 Cohort Escalation