
Targeted science, Tailored solutions for people with autoimmune
disease Batoclimab Graves’ Disease Proof-of-Concept Study Remission Data September 2025

Forward-looking statements This presentation contains forward-looking statements
for the purposes of the safe harbor provisions under The Private Securities Litigation Reform Act of 1995 and other federal securities laws. The use of words such as "can," “may,” “might,” “will,” “would,” “should,” “expect,” “believe,”
“estimate,” “design,” “plan,” "intend," "anticipate," and other similar expressions are intended to identify forward-looking statements. Such forward looking statements include Immunovant’s expectations regarding the clinical and therapeutic
benefits of its product candidates, including durability of response, efficacy, tolerability and the potential for disease modification and first-in-class potential; patient enrollment, timing, design, progress, scope and results of its
existing and planned clinical trials, including the potential registrational trials for IMVT-1402 in Graves’ disease and whether the trial designs will result in improved efficacy data; future development of IMVT-1402 and batoclimab, including
the timing and likelihood of expansion into additional indications; the evolution of the treatment paradigm for Graves’ disease in North America; the size and growth of the potential markets for Immunovant's product candidates and indication
selections, including any estimated market opportunities; expectations regarding the receipt of regulatory approval for its product candidates; and whether, if approved, IMVT-1402 or batcolimab will be successfully distributed, marketed or
commercialized. All forward-looking statements are based on estimates and assumptions by Immunovant’s management that, although Immunovant believes to be reasonable, are inherently uncertain. All forward-looking statements are subject to risks
and uncertainties that may cause actual results to differ materially from those that Immunovant expected. Such risks and uncertainties include, among others: initial results or other preliminary analyses or results of early clinical trials may
not be predictive of final trial results or of the results of later clinical trials; results of animal studies may not be predictive of results in humans; the timing and availability of data from clinical trials; the timing of discussions with
regulatory agencies, as well as regulatory submissions and potential approvals; the continued development of Immunovant’s product candidates, including the timing of the commencement of additional clinical trials; Immunovant’s scientific
approach, clinical trial design, indication selection, and general development progress; future clinical trials may not confirm any safety, potency, or other product characteristics described or assumed in this presentation; any product
candidate that Immunovant develops may not progress through clinical development or receive required regulatory approvals within expected timelines or at all; Immunovant’s product candidates may not be beneficial to patients, or even if
approved by regulatory authorities, successfully commercialized; the effect of global factors such as geopolitical tensions and adverse macroeconomic conditions on Immunovant’s business operations and supply chains, including its clinical
development plans and timelines; Immunovant’s business is heavily dependent on the successful development, regulatory approval and commercialization of batoclimab and IMVT-1402; Immunovant is in various stages of clinical development for
IMVT-1402 and batoclimab; and Immunovant will require additional capital to fund its operations and advance IMVT-1402 and batoclimab through clinical development. These and other risks and uncertainties are more fully described in Immunovant’s
periodic and other reports filed with the Securities and Exchange Commission (SEC), including in the section titled “Risk Factors” in Immunovant’s most recent Quarterly Report on Form 10-Q for the quarter ended June 30, 2025, filed with the SEC
on August 11, 2025, and Immunovant’s subsequent filings with the SEC. Any forward-looking statement speaks only as of the date on which it was made. Immunovant undertakes no obligation to publicly update or revise any forward-looking statement,
whether as a result of new information, future events or otherwise. and IMMUNOVANT® are registered trademarks of Immunovant Sciences GmbH. All other trademarks, trade names, service marks, and copyrights appearing in this presentation are the
property of their respective owners. Dates used in this presentation refer to the applicable calendar year unless otherwise noted. 2
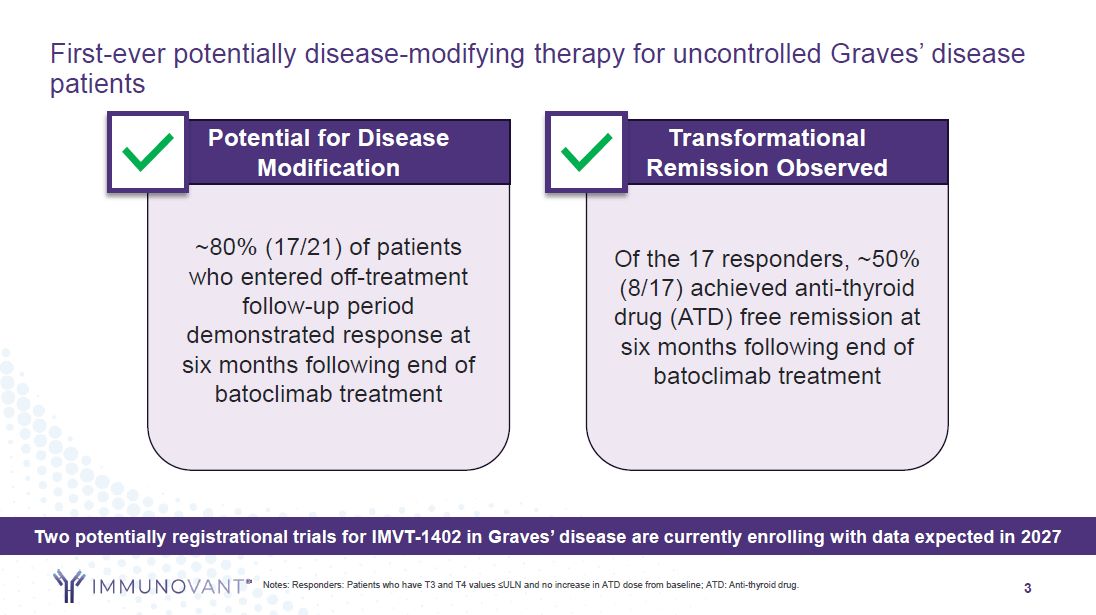
3 First-ever potentially disease-modifying therapy for uncontrolled Graves’
disease patients Notes: Responders: Patients who have T3 and T4 values ≤ULN and no increase in ATD dose from baseline; ATD: Anti-thyroid drug. ~80% (17/21) of patients who entered off-treatment follow-up period demonstrated response at six
months following end of batoclimab treatment Potential for Disease Modification Of the 17 responders, ~50% (8/17) achieved anti-thyroid drug (ATD) free remission at six months following end of batoclimab treatment Transformational Remission
Observed Two potentially registrational trials for IMVT-1402 in Graves’ disease are currently enrolling with data expected in 2027

Background: Graves’ Disease 4
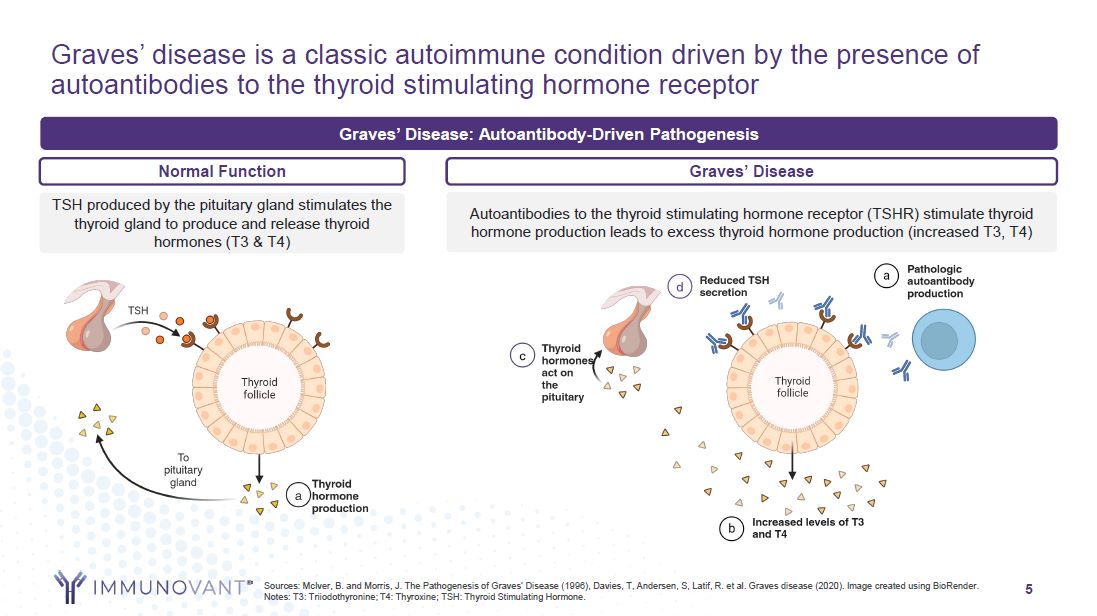
5 Graves’ disease is a classic autoimmune condition driven by the presence of
autoantibodies to the thyroid stimulating hormone receptor Sources: McIver, B. and Morris, J. The Pathogenesis of Graves’ Disease (1996), Davies, T, Andersen, S, Latif, R. et al. Graves disease (2020). Image created using BioRender. Notes: T3:
Triiodothyronine; T4: Thyroxine; TSH: Thyroid Stimulating Hormone. TSH produced by the pituitary gland stimulates the thyroid gland to produce and release thyroid hormones (T3 & T4) Autoantibodies to the thyroid stimulating hormone
receptor (TSHR) stimulate thyroid hormone production leads to excess thyroid hormone production (increased T3, T4) Graves’ Disease: Autoantibody-Driven Pathogenesis Normal Function Graves’ Disease
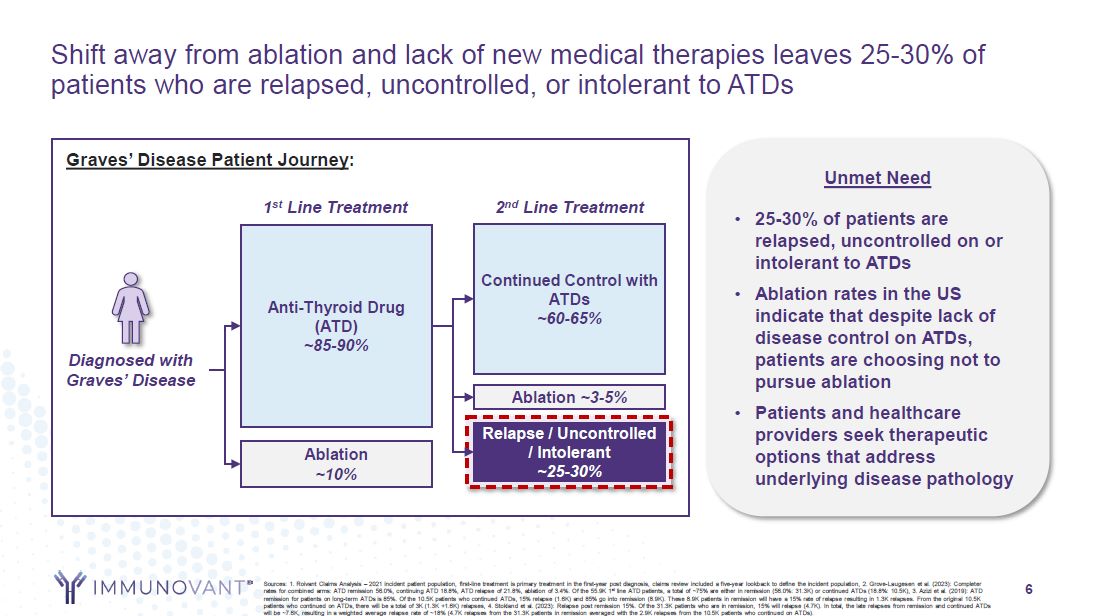
Shift away from ablation and lack of new medical therapies leaves 25-30% of
patients who are relapsed, uncontrolled, or intolerant to ATDs Diagnosed with Graves’ Disease Anti-Thyroid Drug (ATD) ~85-90% Ablation ~10% Continued Control with ATDs ~60-65% Ablation ~3-5% Relapse / Uncontrolled /
Intolerant ~25-30% 2nd Line Treatment Graves’ Disease Patient Journey: 1st Line Treatment Unmet Need 25-30% of patients are relapsed, uncontrolled on or intolerant to ATDs Ablation rates in the US indicate that despite lack of disease
control on ATDs, patients are choosing not to pursue ablation Patients and healthcare providers seek therapeutic options that address underlying disease pathology 6 Sources: 1. Roivant Claims Analysis – 2021 incident patient population,
first-line treatment is primary treatment in the first-year post diagnosis, claims review included a five-year lookback to define the incident population, 2. Grove-Laugesen et al. (2023): Completer rates for combined arms: ATD remission 56.0%,
continuing ATD 18.8%, ATD relapse of 21.8%, ablation of 3.4%. Of the 55.9K 1st line ATD patients, a total of ~75% are either in remission (56.0%: 31.3K) or continued ATDs (18.8%: 10.5K), 3. Azizi et al. (2019): ATD remission for patients on
long-term ATDs is 85%. Of the 10.5K patients who continued ATDs, 15% relapse (1.6K) and 85% go into remission (8.9K). These 8.9K patients in remission will have a 15% rate of relapse resulting in 1.3K relapses. From the original 10.5K patients
who continued on ATDs, there will be a total of 3K (1.3K +1.6K) relapses, 4. Stokland et al. (2023): Relapse post remission 15%. Of the 31.3K patients who are in remission, 15% will relapse (4.7K). In total, the late relapses from remission and
continued ATDs will be ~7.6K, resulting in a weighted average relapse rate of ~18% (4.7K relapses from the 31.3K patients in remission averaged with the 2.9K relapses from the 10.5K patients who continued on ATDs).
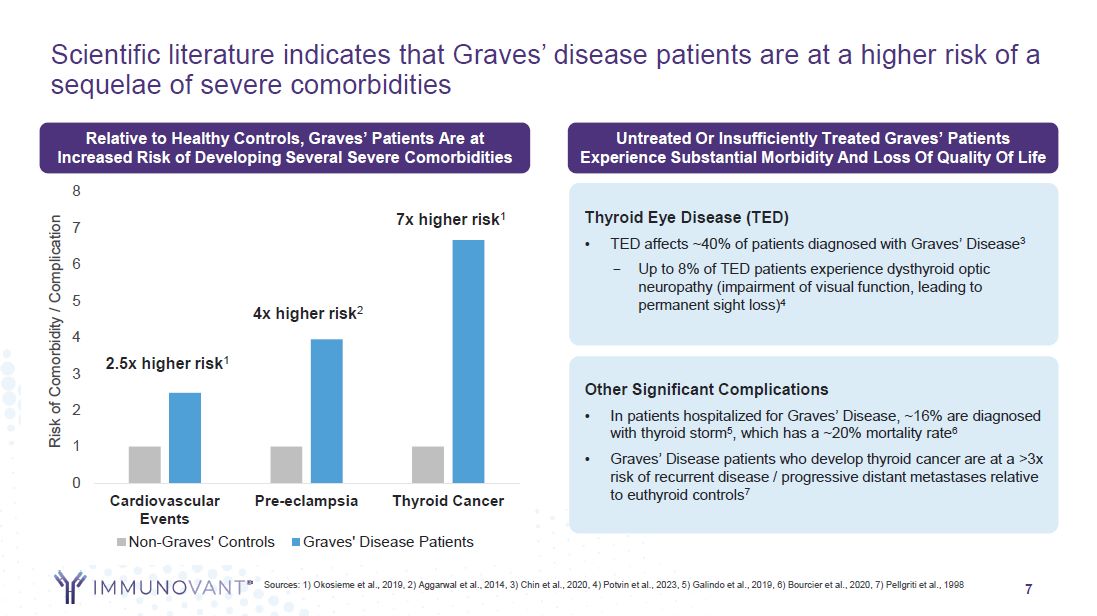
7 Scientific literature indicates that Graves’ disease patients are at a higher
risk of a sequelae of severe comorbidities Sources: 1) Okosieme et al., 2019, 2) Aggarwal et al., 2014, 3) Chin et al., 2020, 4) Potvin et al., 2023, 5) Galindo et al., 2019, 6) Bourcier et al., 2020, 7) Pellgriti et al.,
1998 0 1 2 3 4 5 6 7 Cardiovascular Events Pre-eclampsia Thyroid Cancer Risk of Comorbidity / Complication Non-Graves' Controls Graves' Disease Patients 7x higher risk1 4x higher risk2 2.5x higher risk1 Relative to Healthy
Controls, Graves’ Patients Are at Increased Risk of Developing Several Severe Comorbidities 8 Untreated Or Insufficiently Treated Graves’ Patients Experience Substantial Morbidity And Loss Of Quality Of Life Thyroid Eye Disease (TED) TED
affects ~40% of patients diagnosed with Graves’ Disease3 – Up to 8% of TED patients experience dysthyroid optic neuropathy (impairment of visual function, leading to permanent sight loss)4 Other Significant Complications In patients
hospitalized for Graves’ Disease, ~16% are diagnosed with thyroid storm5, which has a ~20% mortality rate6 Graves’ Disease patients who develop thyroid cancer are at a >3x risk of recurrent disease / progressive distant metastases relative
to euthyroid controls7
Classic autoimmune condition where disease pathology is driven by autoantibodies
to thyroid stimulating hormone receptor High unmet need with 25-30% of ATD treatment patients either uncontrolled, relapsed, or intolerant to ATDs No existing disease modifying therapy; ablative options continue to be used less frequently
with physicians and patients Patients with uncontrolled Graves’ disease experience greater risk of a sequelae of severe comorbidities (e.g., CV events, TED, thyroid storm) Significant unmet need with 65K incident population and upside from an
untapped prevalent pool of patients who remain uncontrolled but choose not to undergo ablation 8 Graves’ disease represents a high unmet need, underserved patient population with meaningful opportunity for innovation in ATD-uncontrolled
patients ATD: Anti-thyroid drug; CV: Cardiovascular; TED: Thyroid eye disease

Batoclimab Phase 2 Remission Data 9
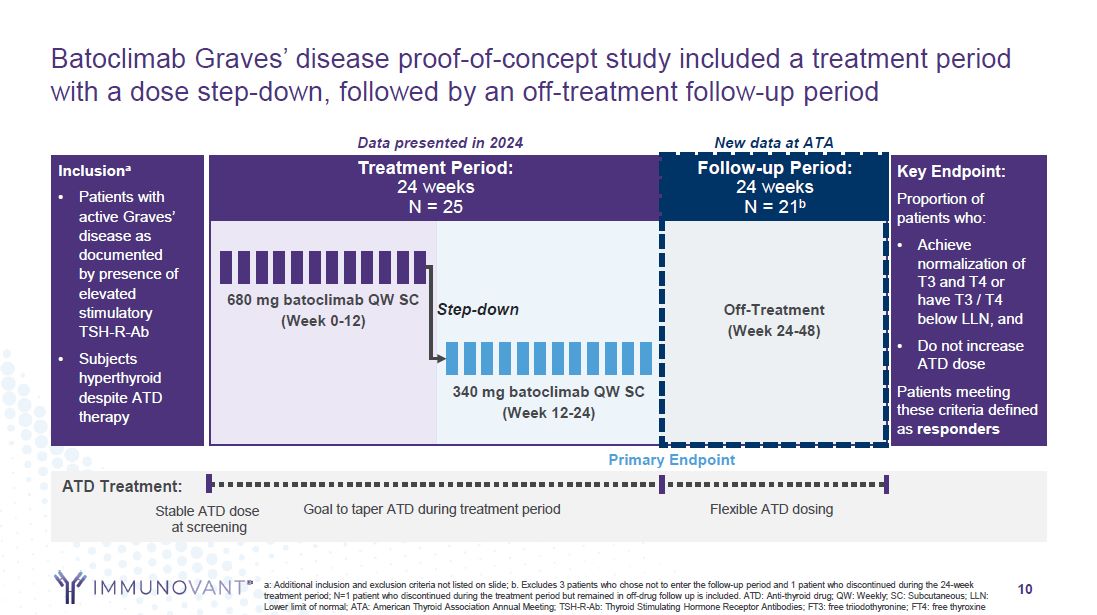
10 Batoclimab Graves’ disease proof-of-concept study included a treatment period
with a dose step-down, followed by an off-treatment follow-up period a: Additional inclusion and exclusion criteria not listed on slide; b. Excludes 3 patients who chose not to enter the follow-up period and 1 patient who discontinued during
the 24-week treatment period; N=1 patient who discontinued during the treatment period but remained in off-drug follow up is included. ATD: Anti-thyroid drug; QW: Weekly; SC: Subcutaneous; LLN: Lower limit of normal; ATA: American Thyroid
Association Annual Meeting; TSH-R-Ab: Thyroid Stimulating Hormone Receptor Antibodies; FT3: free triiodothyronine; FT4: free thyroxine Inclusiona Patients with active Graves’ disease as documented by presence of elevated stimulatory
TSH-R-Ab Subjects hyperthyroid despite ATD therapy ATD Treatment: Stable ATD dose at screening Goal to taper ATD during treatment period Flexible ATD dosing Treatment Period: 24 weeks N = 25 Follow-up Period: 24 weeks N = 21b Key
Endpoint: Proportion of patients who: Achieve normalization of T3 and T4 or have T3 / T4 below LLN, and Do not increase ATD dose Patients meeting these criteria defined as responders 680 mg batoclimab QW SC (Week
0-12) Step-down Off-Treatment (Week 24-48) 340 mg batoclimab QW SC (Week 12-24) Data presented in 2024 New data at ATA Primary Endpoint
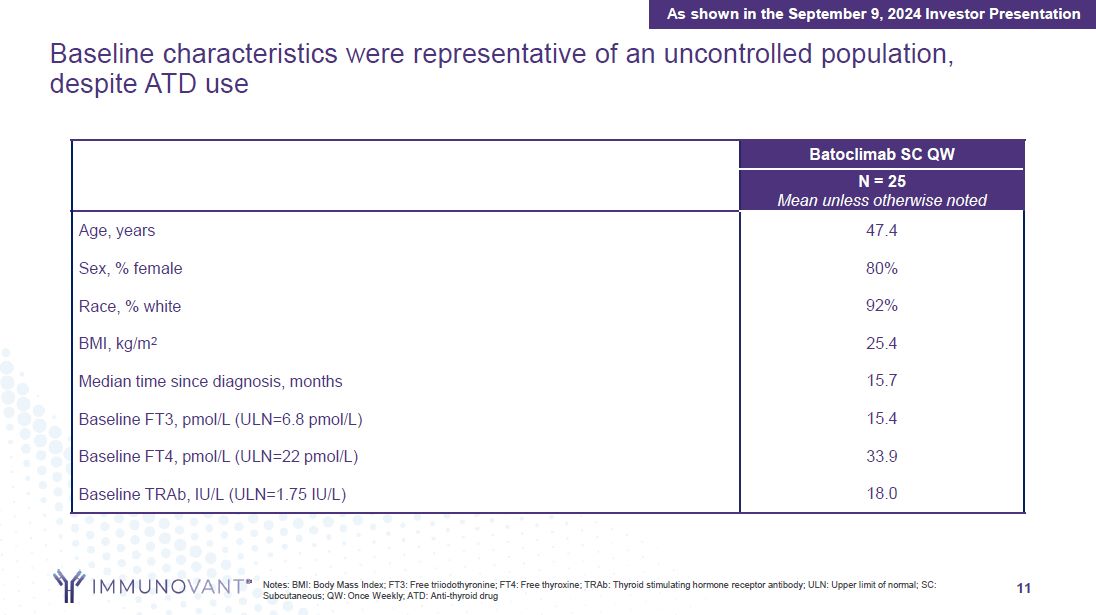
11 Baseline characteristics were representative of an uncontrolled population,
despite ATD use Batoclimab SC QW N = 25 Mean unless otherwise noted Age, years 47.4 Sex, % female 80% Race, % white 92% BMI, kg/m2 25.4 Median time since diagnosis, months 15.7 Baseline FT3, pmol/L (ULN=6.8 pmol/L) 15.4 Baseline
FT4, pmol/L (ULN=22 pmol/L) 33.9 Baseline TRAb, IU/L (ULN=1.75 IU/L) 18.0 As shown in the September 9, 2024 Investor Presentation Notes: BMI: Body Mass Index; FT3: Free triiodothyronine; FT4: Free thyroxine; TRAb: Thyroid stimulating
hormone receptor antibody; ULN: Upper limit of normal; SC: Subcutaneous; QW: Once Weekly; ATD: Anti-thyroid drug
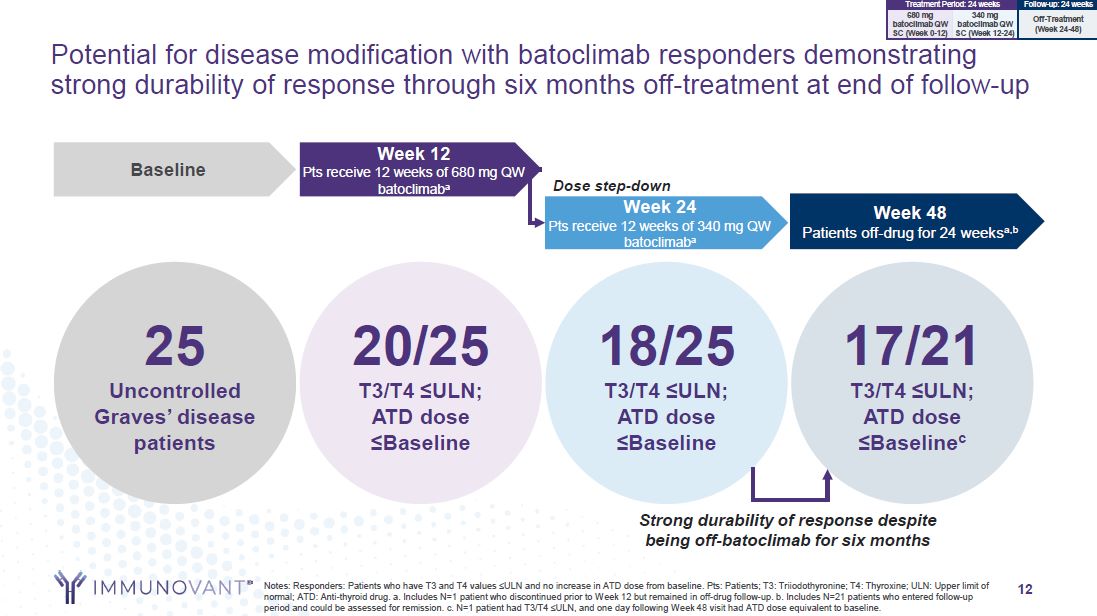
12 Potential for disease modification with batoclimab responders demonstrating
strong durability of response through six months off-treatment at end of follow-up 25 Uncontrolled Graves’ disease patients Baseline Week 48 Patients off-drug for 24 weeksa,b Week 12 Pts receive 12 weeks of 680 mg QW batoclimaba Dose
step-down Week 24 Pts receive 12 weeks of 340 mg QW batoclimaba 20/25 T3/T4 ≤ULN; ATD dose ≤Baseline 18/25 T3/T4 ≤ULN; ATD dose ≤Baseline 17/21 T3/T4 ≤ULN; ATD dose ≤Baselinec Notes: Responders: Patients who have T3 and T4 values
≤ULN and no increase in ATD dose from baseline. Pts: Patients; T3: Triiodothyronine; T4: Thyroxine; ULN: Upper limit of normal; ATD: Anti-thyroid drug. a. Includes N=1 patient who discontinued prior to Week 12 but remained in off-drug
follow-up. b. Includes N=21 patients who entered follow-up period and could be assessed for remission. c. N=1 patient had T3/T4 ≤ULN, and one day following Week 48 visit had ATD dose equivalent to baseline. Strong durability of response
despite being off-batoclimab for six months 340 mg batoclimab QW SC (Week 12-24) 680 mg batoclimab QW SC (Week 0-12) Treatment Period: 24 weeks Follow-up: 24 weeks Off-Treatment (Week 24-48)
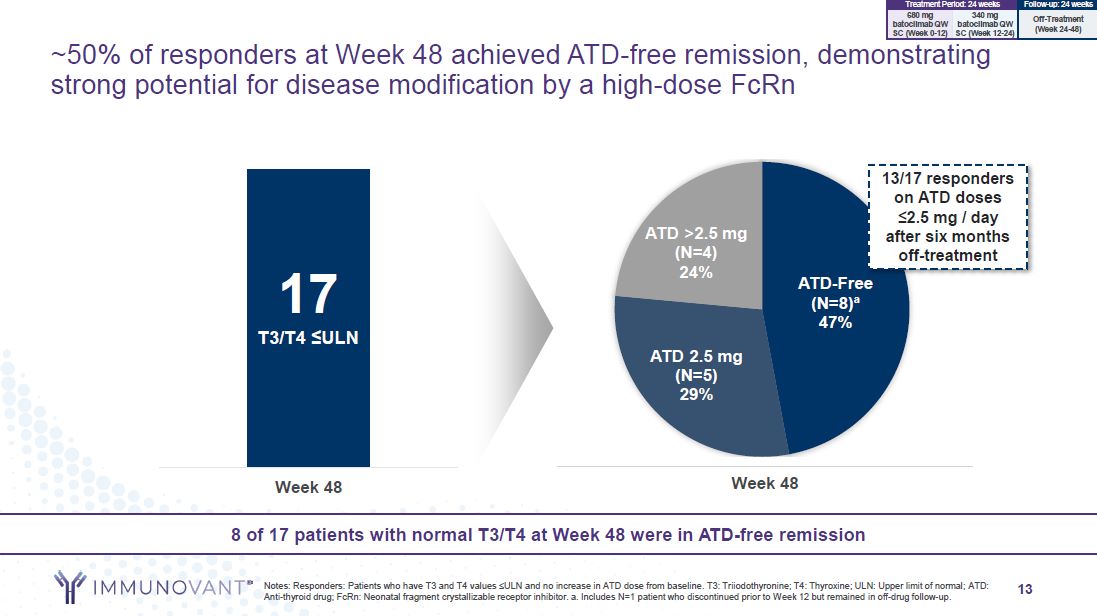
13 ~50% of responders at Week 48 achieved ATD-free remission, demonstrating
strong potential for disease modification by a high-dose FcRn 8 of 17 patients with normal T3/T4 at Week 48 were in ATD-free remission Notes: Responders: Patients who have T3 and T4 values ≤ULN and no increase in ATD dose from baseline. T3:
Triiodothyronine; T4: Thyroxine; ULN: Upper limit of normal; ATD: Anti-thyroid drug; FcRn: Neonatal fragment crystallizable receptor inhibitor. a. Includes N=1 patient who discontinued prior to Week 12 but remained in off-drug follow-up. Week
48 17 T3/T4 ≤ULN ATD-Free (N=8)a 47% ATD 2.5 mg (N=5) 29% ATD >2.5 mg (N=4) 24% Week 48 13/17 responders on ATD doses ≤2.5 mg / day after six months off-treatment 680 mg 340 mg batoclimab QW batoclimab QW SC (Week 0-12) SC (Week
12-24) Treatment Period: 24 weeks Follow-up: 24 weeks Off-Treatment (Week 24-48)
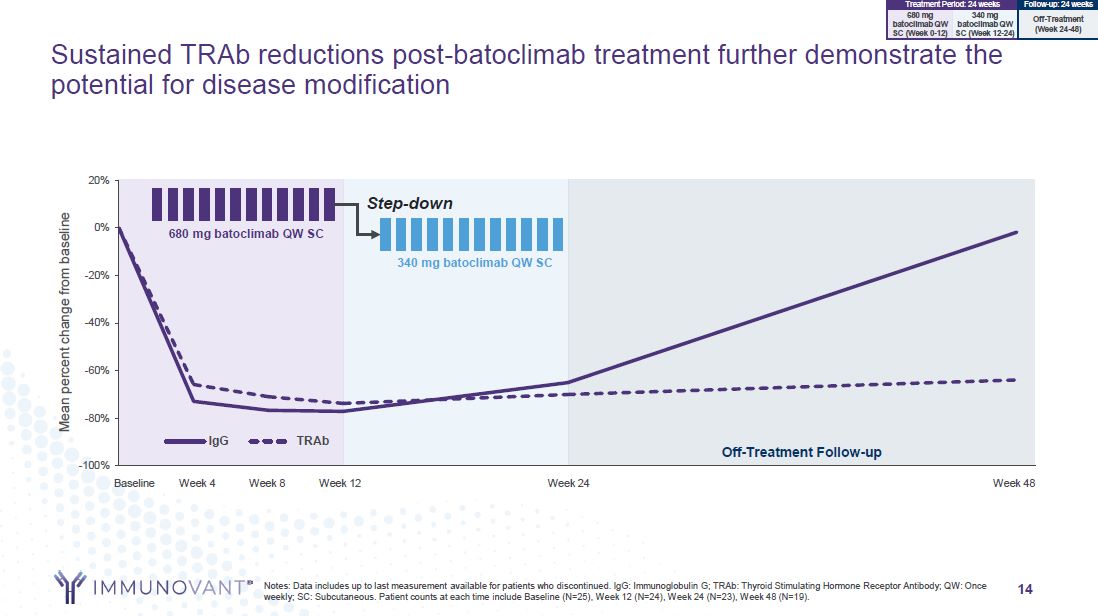
Off-Treatment Follow-up -100% -80% -60% -40% -20% 0% 20% IgG TRAb Mean
percent change from baseline Baseline Week 4 Week 8 Week 12 Week 24 Week 48 340 mg batoclimab QW SC 680 mg batoclimab QW SC Step-down Sustained TRAb reductions post-batoclimab treatment further demonstrate the potential for disease
modification 340 mg batoclimab QW SC (Week 12-24) 680 mg batoclimab QW SC (Week 0-12) Treatment Period: 24 weeks Follow-up: 24 weeks Off-Treatment (Week 24-48) Notes: Data includes up to last measurement available for patients who
discontinued. IgG: Immunoglobulin G; TRAb: Thyroid Stimulating Hormone Receptor Antibody; QW: Once weekly; SC: Subcutaneous. Patient counts at each time include Baseline (N=25), Week 12 (N=24), Week 24 (N=23), Week 48 (N=19). 14
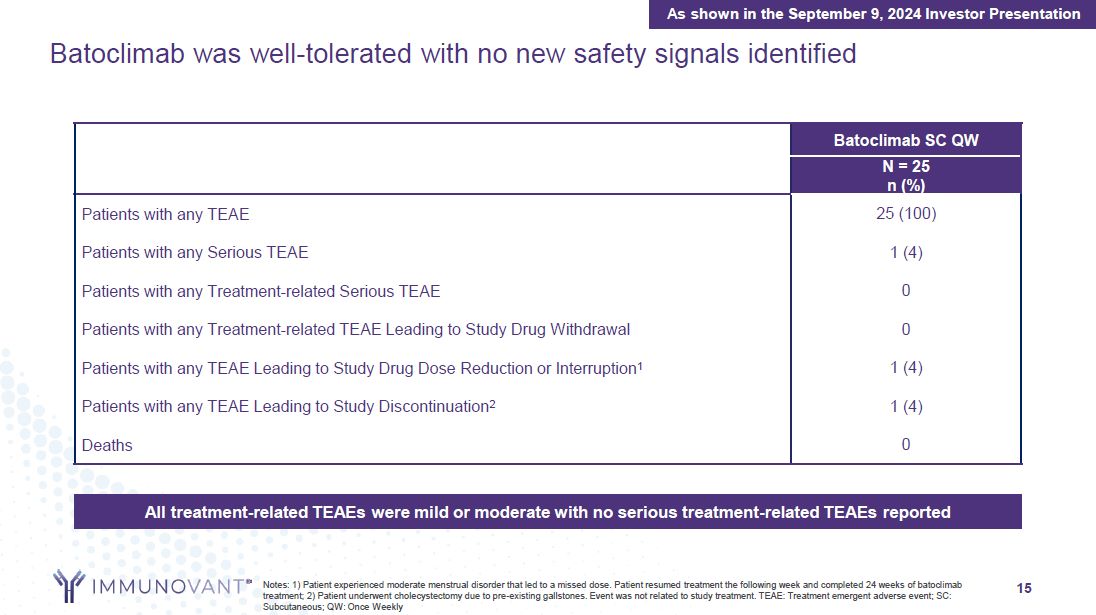
15 Batoclimab was well-tolerated with no new safety signals
identified Batoclimab SC QW N = 25 n (%) Patients with any TEAE 25 (100) Patients with any Serious TEAE 1 (4) Patients with any Treatment-related Serious TEAE 0 Patients with any Treatment-related TEAE Leading to Study Drug
Withdrawal 0 Patients with any TEAE Leading to Study Drug Dose Reduction or Interruption1 1 (4) Patients with any TEAE Leading to Study Discontinuation2 1 (4) Deaths 0 All treatment-related TEAEs were mild or moderate with no serious
treatment-related TEAEs reported Notes: 1) Patient experienced moderate menstrual disorder that led to a missed dose. Patient resumed treatment the following week and completed 24 weeks of batoclimab treatment; 2) Patient underwent
cholecystectomy due to pre-existing gallstones. Event was not related to study treatment. TEAE: Treatment emergent adverse event; SC: Subcutaneous; QW: Once Weekly As shown in the September 9, 2024 Investor Presentation
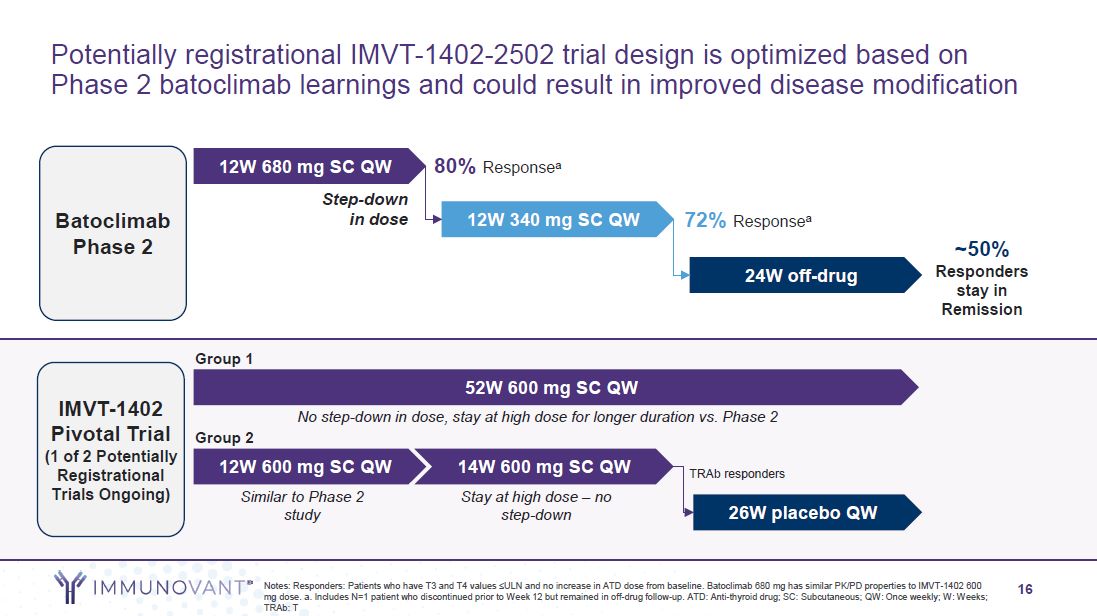
16 Potentially registrational IMVT-1402-2502 trial design is optimized based on
Phase 2 batoclimab learnings and could result in improved disease modification Batoclimab Phase 2 IMVT-1402 Pivotal Trial (1 of 2 Potentially Registrational Trials Ongoing) 12W 340 mg SC QW 80% Responsea 72% Responsea 12W 680 mg SC
QW Step-down in dose 24W off-drug ~50% Responders stay in Remission 26W placebo QW Notes: Responders: Patients who have T3 and T4 values ≤ULN and no increase in ATD dose from baseline. Batoclimab 680 mg has similar PK/PD properties to
IMVT-1402 600 mg dose. a. Includes N=1 patient who discontinued prior to Week 12 but remained in off-drug follow-up. ATD: Anti-thyroid drug; SC: Subcutaneous; QW: Once weekly; W: Weeks; TRAb: T 14W 600 mg SC QW Stay at high dose – no
step-down Group 2 12W 600 mg SC QW Similar to Phase 2 study Group 1 52W 600 mg SC QW No step-down in dose, stay at high dose for longer duration vs. Phase 2 TRAb responders
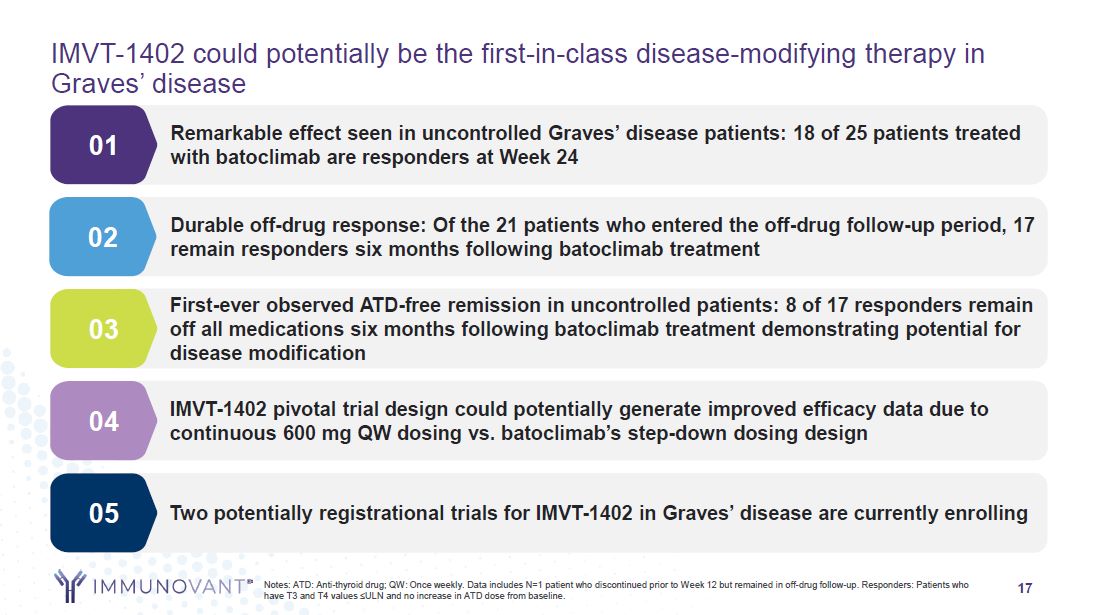
17 IMVT-1402 could potentially be the first-in-class disease-modifying therapy in
Graves’ disease Remarkable effect seen in uncontrolled Graves’ disease patients: 18 of 25 patients treated with batoclimab are responders at Week 24 01 Durable off-drug response: Of the 21 patients who entered the off-drug follow-up period,
17 remain responders six months following batoclimab treatment 02 IMVT-1402 pivotal trial design could potentially generate improved efficacy data due to continuous 600 mg QW dosing vs. batoclimab’s step-down dosing design 04 Two
potentially registrational trials for IMVT-1402 in Graves’ disease are currently enrolling 05 Notes: ATD: Anti-thyroid drug; QW: Once weekly. Data includes N=1 patient who discontinued prior to Week 12 but remained in off-drug follow-up.
Responders: Patients who have T3 and T4 values ≤ULN and no increase in ATD dose from baseline. First-ever observed ATD-free remission in uncontrolled patients: 8 of 17 responders remain off all medications six months following batoclimab
treatment demonstrating potential for disease modification 03

Appendix 18
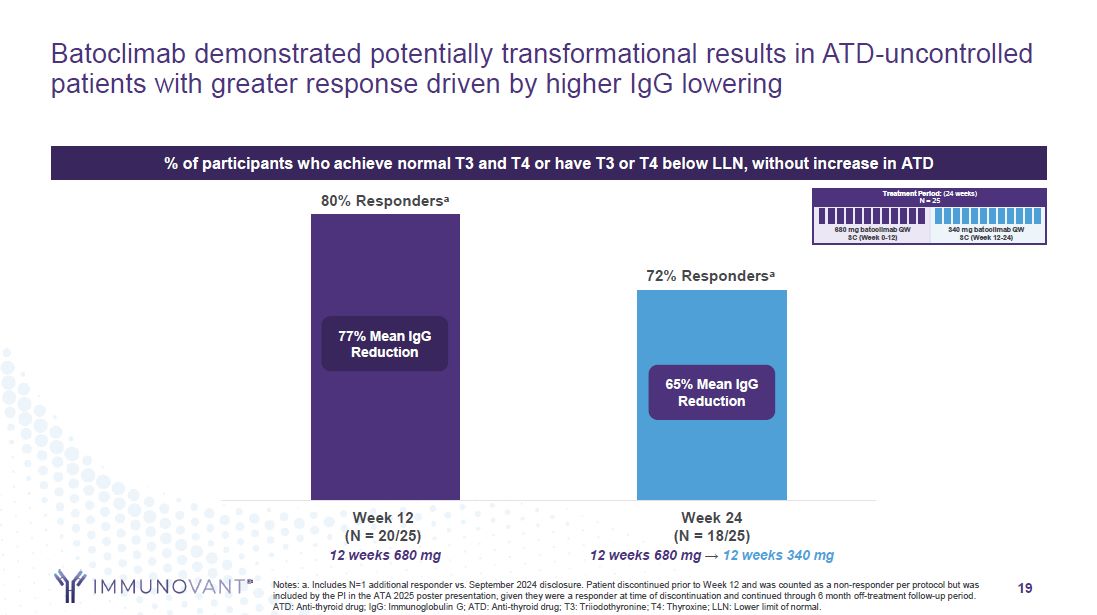
19 Batoclimab demonstrated potentially transformational results in
ATD-uncontrolled patients with greater response driven by higher IgG lowering Notes: a. Includes N=1 additional responder vs. September 2024 disclosure. Patient discontinued prior to Week 12 and was counted as a non-responder per protocol but
was included by the PI in the ATA 2025 poster presentation, given they were a responder at time of discontinuation and continued through 6 month off-treatment follow-up period. ATD: Anti-thyroid drug; IgG: Immunoglobulin G; ATD: Anti-thyroid
drug; T3: Triiodothyronine; T4: Thyroxine; LLN: Lower limit of normal. % of participants who achieve normal T3 and T4 or have T3 or T4 below LLN, without increase in ATD 80% Respondersa 72% Respondersa 77% Mean IgG Reduction 65% Mean IgG
Reduction Week 24 (N = 18/25) 12 weeks 680 mg → 12 weeks 340 mg Week 12 (N = 20/25) 12 weeks 680 mg Treatment Period: (24 weeks) N = 25 680 mg batoclimab QW SC (Week 0-12) 340 mg batoclimab QW SC (Week 12-24)
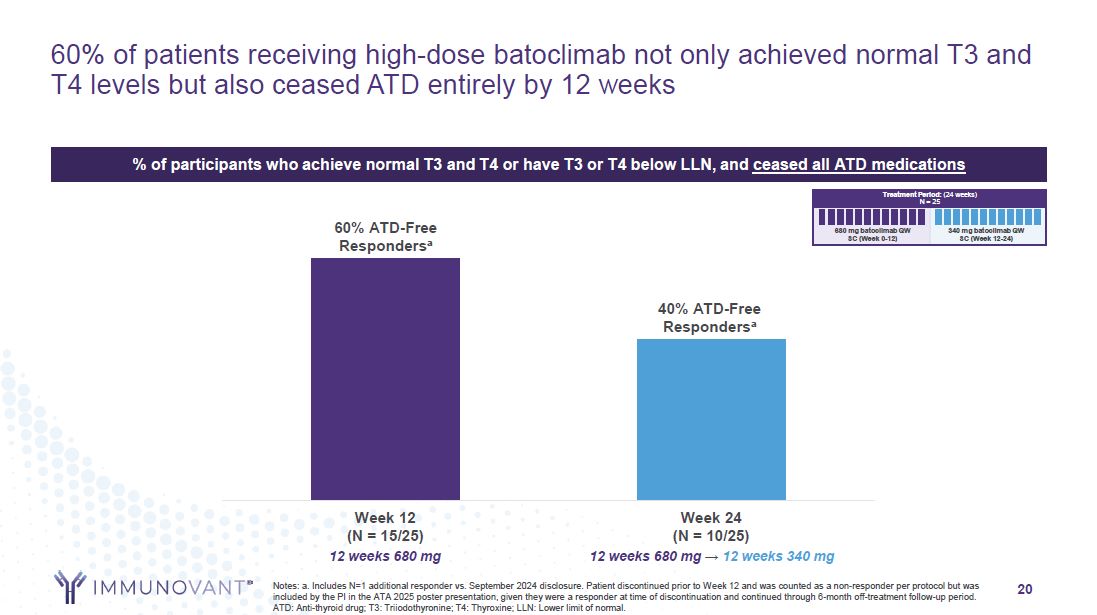
60% ATD-Free Respondersa 40% ATD-Free Respondersa 20 60% of patients
receiving high-dose batoclimab not only achieved normal T3 and T4 levels but also ceased ATD entirely by 12 weeks Notes: a. Includes N=1 additional responder vs. September 2024 disclosure. Patient discontinued prior to Week 12 and was counted
as a non-responder per protocol but was included by the PI in the ATA 2025 poster presentation, given they were a responder at time of discontinuation and continued through 6-month off-treatment follow-up period. ATD: Anti-thyroid drug; T3:
Triiodothyronine; T4: Thyroxine; LLN: Lower limit of normal. Week 24 (N = 10/25) 12 weeks 680 mg → 12 weeks 340 mg Week 12 (N = 15/25) 12 weeks 680 mg Treatment Period: (24 weeks) N = 25 680 mg batoclimab QW SC (Week 0-12) 340 mg
batoclimab QW SC (Week 12-24) % of participants who achieve normal T3 and T4 or have T3 or T4 below LLN, and ceased all ATD medications
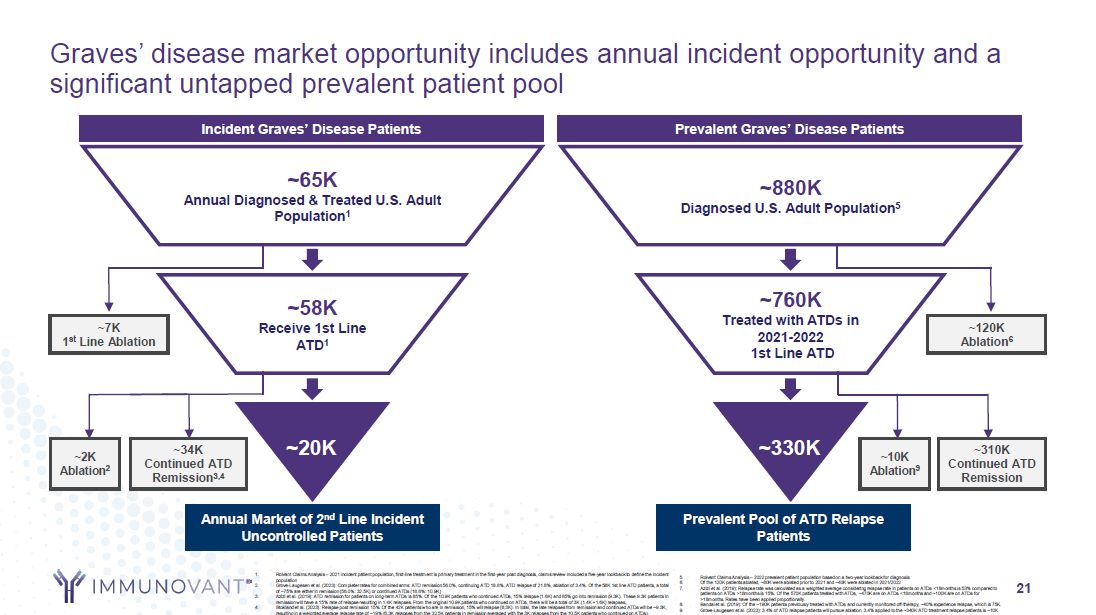
21 Graves’ disease market opportunity includes annual incident opportunity and a
significant untapped prevalent patient pool 5. 6. 8. 9. Roivant Claims Analysis – 2022 prevalent patient population based on a two-year lookback for diagnosis Of the 120K patients ablated, ~80K were ablated prior to 2021 and ~40K were
ablated in 2021/2022 Azizi et al. (2019): Relapse rate was calculated as a weighted average considering relapse rate in patients on ATDs <18months is 53% compared to patients on ATDs >18months is 15%. Of the 570K patients treated with
ATDs, ~470K are on ATDs <18months and ~100K are on ATDs for >18months. Rates have been applied proportionally. Bandai et al. (2019): Of the ~190K patients previously treated with ATDs and currently monitored off-therapy, ~40% experience
relapse, which is 75K. Grove-Laugesen et al. (2023): 3.4% of ATD relapse patients will pursue ablation. 3.4% applied to the ~340K ATD treatment relapse patients is ~10K 1. Roivant Claims Analysis – 2021 incident patient population, first-line
treatment is primary treatment in the first-year post diagnosis, claims review included a five-year lookback to define the incident population 2. Grove-Laugesen et al. (2023): Completer rates for combined arms: ATD remission 56.0%, continuing
ATD 18.8%, ATD relapse of 21.8%, ablation of 3.4%. Of the 58K 1st line ATD patients, a total 7. 3. 4. of ~75% are either in remission (56.0%: 32.5K) or continued ATDs (18.8%: 10.9K) Azizi et al. (2019): ATD remission for patients on
long-term ATDs is 85%. Of the 10.9K patients who continued ATDs, 15% relapse (1.6K) and 85% go into remission (9.3K). These 9.3K patients in remission will have a 15% rate of relapse resulting in 1.4K relapses. From the original 10.9K patients
who continued on ATDs, there will be a total of 3K (1.4K +1.6K) relapses, Stokland et al. (2023): Relapse post remission 15%. Of the 42K patients who are in remission, 15% will relapse (6.3K). In total, the late relapses from remission and
continued ATDs will be ~9.3K, resulting in a weighted average relapse rate of ~19% (6.3K relapses from the 32.5K patients in remission averaged with the 3K relapses from the 10.5K patients who continued on ATDs). Annual Market of 2nd Line
Incident Uncontrolled Patients ~7K 1st Line Ablation ~34K Continued ATD Remission3,4 ~65K Annual Diagnosed & Treated U.S. Adult Population1 ~58K Receive 1st Line ATD1 ~20K ~2K Ablation2 Prevalent Pool of ATD Relapse
Patients ~120K Ablation6 ~310K Continued ATD Remission ~880K Diagnosed U.S. Adult Population5 ~760K Treated with ATDs in 2021-2022 1st Line ATD ~330K ~10K Ablation9 Incident Graves’ Disease Patients Prevalent Graves’ Disease
Patients
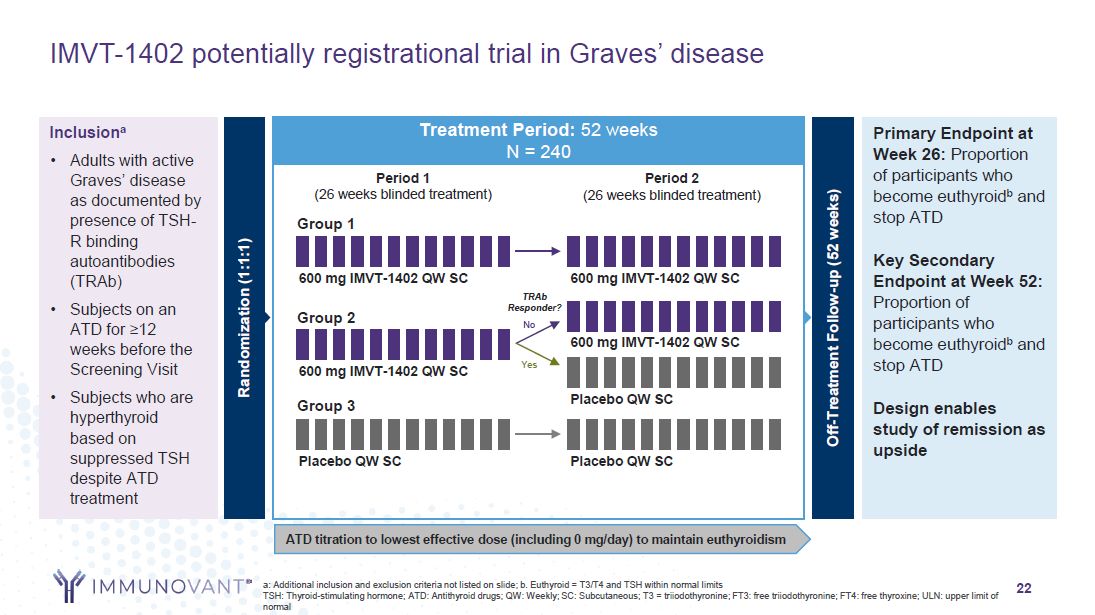
22 IMVT-1402 potentially registrational trial in Graves’ disease Treatment
Period: 52 weeks N = 240 Randomization (1:1:1) Primary Endpoint at Week 26: Proportion of participants who become euthyroidb and stop ATD Key Secondary Endpoint at Week 52: Proportion of participants who become euthyroidb and stop
ATD Design enables study of remission as upside Inclusiona Adults with active Graves’ disease as documented by presence of TSH- R binding autoantibodies (TRAb) Subjects on an ATD for ≥12 weeks before the Screening Visit Subjects who are
hyperthyroid based on suppressed TSH despite ATD treatment Group 3 ATD titration to lowest effective dose (including 0 mg/day) to maintain euthyroidism Period 2 (26 weeks blinded treatment) Period 1 (26 weeks blinded treatment) Group
1 600 mg IMVT-1402 QW SC 600 mg IMVT-1402 QW SC Group 2 600 mg IMVT-1402 QW SC Placebo QW SC Placebo QW SC Placebo QW SC 600 mg IMVT-1402 QW SC Off-Treatment Follow-up (52 weeks) TRAb Responder? No Yes a: Additional inclusion and
exclusion criteria not listed on slide; b. Euthyroid = T3/T4 and TSH within normal limits TSH: Thyroid-stimulating hormone; ATD: Antithyroid drugs; QW: Weekly; SC: Subcutaneous; T3 = triiodothyronine; FT3: free triiodothyronine; FT4: free
thyroxine; ULN: upper limit of normal
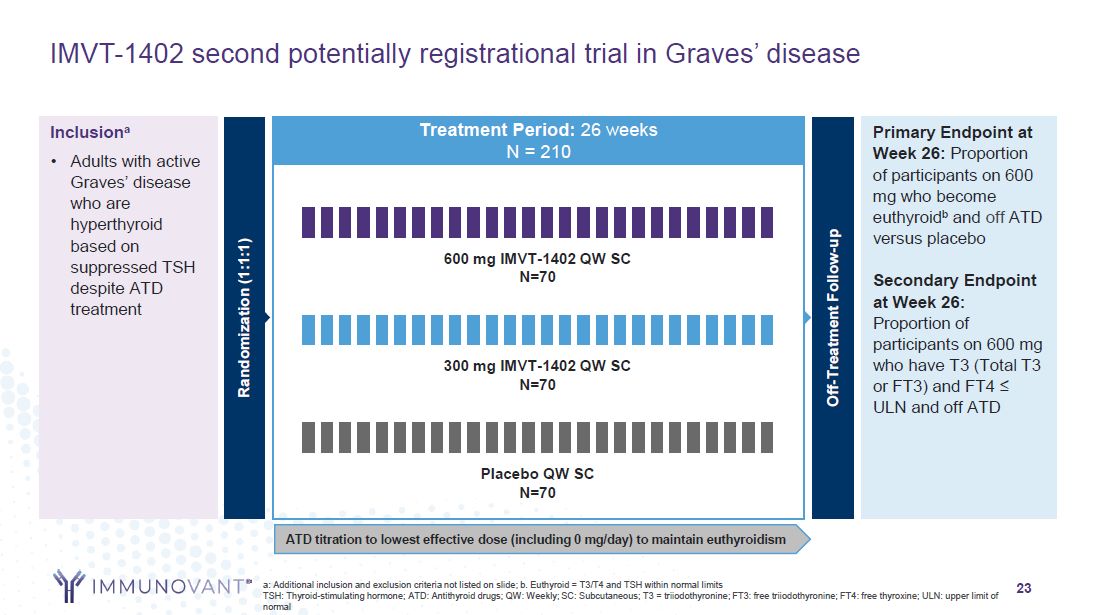
Primary Endpoint at Week 26: Proportion of participants on 600 mg who become
euthyroidb and off ATD versus placebo Secondary Endpoint at Week 26: Proportion of participants on 600 mg who have T3 (Total T3 or FT3) and FT4 ≤ ULN and off ATD Inclusiona Adults with active Graves’ disease who are hyperthyroid based on
suppressed TSH despite ATD treatment a: Additional inclusion and exclusion criteria not listed on slide; b. Euthyroid = T3/T4 and TSH within normal limits TSH: Thyroid-stimulating hormone; ATD: Antithyroid drugs; QW: Weekly; SC: Subcutaneous;
T3 = triiodothyronine; FT3: free triiodothyronine; FT4: free thyroxine; ULN: upper limit of normal Placebo QW SC N=70 600 mg IMVT-1402 QW SC N=70 300 mg IMVT-1402 QW SC N=70 23 IMVT-1402 second potentially registrational trial in Graves’
disease Randomization (1:1:1) Treatment Period: 26 weeks N = 210 Off-Treatment Follow-up ATD titration to lowest effective dose (including 0 mg/day) to maintain euthyroidism