Exhibit 99.1

Silencing Oncogenes at the Level of Gene Expression Nasdaq: SLXN Corporate
Presentation August 2025

The statements contained in this presentation that are not purely historical are
forward-looking statements. Our forward-looking statements include, but are not limited to, statements regarding our or our management team’s expectations, hopes, beliefs, intentions or strategies regarding the future. In addition, any
statements that refer to projections, forecasts or other characterizations of future events or circumstances, including any underlying assumptions, are forward-looking statements. The words “anticipate,” “believe,” “continue,” “could,”
“estimate,” “expect,” “intends,” “may,” “might,” “plan,” “possible,” “potential,” “predict,” “project,” “should,” “would” and similar expressions may identify forward-looking statements, but the absence of these words does not mean that a
statement is not forward-looking. Forward-looking statements in this presentation may include, for example, statements about: the future performance of the Company, including Silexion’s projected timeline for regulatory approvals of its
product candidates; and the Company’s future plans and opportunities. The forward-looking statements contained in this presentation are based on our current expectations and beliefs concerning future developments and their potential effects
on us. There can be no assurance that future developments affecting us will be those that we have anticipated. These forward-looking statements involve a number of risks, uncertainties (some of which are beyond our control) or other assumptions
that may cause actual results or performance to be materially different from those expressed or implied by these forward-looking statements. These risks and uncertainties include, but are not limited to, the items in the following
list: Silexion is a development-stage company and has a limited operating history on which to assess its business; Silexion has never generated any revenue from product sales and may never be profitable; The approach Silexion is taking to
discover and develop novel RNAi therapeutics is unproven for oncology and may never lead to marketable products; Silexion does not have experience producing its product candidates at commercial levels, currently has no marketing and sales
organization, has an uncertain market receptiveness to its product candidates, and is uncertain as to whether there will be insurance coverage and reimbursement for its potential products; Silexion may be unable to attract, develop and/or
retain its key personnel or additional employees required for its development and future success; Silexion may not succeed at maintaining its listing on Nasdaq; and Additional factors relating to the business, operations and financial
performance of Silexion. Should one or more of these risks or uncertainties materialize, or should any of our assumptions prove incorrect, actual results may vary in material respects from those projected in these forward-looking statements.
We undertake no obligation to update or revise any forward-looking statements, whether as a result of new information, future events or otherwise, except as may be required under applicable securities laws. 2 Forward-Looking Statements
Silexion: Silencing Cancer-Driving Proteins Before They Are Produced Mutated KRAS
proteins are the most prevalent cancer drivers: 92% pancreatic cancer 49% colorectal cancer 35% non-small-cell-non-squamous lung cancer They are well-validated targets, as KRAS inhibitor products represent a multi-billion dollar market
opportunity, despite limitations in side effects, resistance and efficacy Instead of targeting mutated KRAS proteins after they are formed (the approach of our competitors), Silexion’s siRNA technology silences their expression at the outset,
offering a potentially safer and more durable approach KRAS-Focused RNA Interference Platform 3

Silexion’s Phase 2 Proof-of-Concept (POC) Paves the Way for Late-Stage Clinical
Development First generation, KRAS mutation G12D/G12V “Loder” silencing RNA (siG12DLoder siRNA) Completed POC- Phase 2 clinical trial in locally advanced pancreatic cancer (LAPC), shows strong trend for 9.3 months improvement in overall
survival with siG12DLoder + SoC chemo vs. SoC chemo alone Second generation, pan-KRAS mutation silencing siRNA “SIL204” Significantly improved product stability and delivery while providing a broader gene silencing activity Successful
preclinical results support dual-route strategy to administer SIL204 both intratumorally targeting primary tumor and subcutaneously targeting metastases Key preclinical studies demonstrate SIL204’s pan-KRAS anti-tumor activity in pancreatic,
colorectal and lung cancers Oncogene silencing expected to overcome tumoral resistance observed with KRAS inhibitors Phase 2/3 clinical trial, planned Q2, 2026, using integrated approach administering systemic (subcutaneous) and
intratumorally Strong IP protection expected until 2043 plus extension up to ~2048 Promising Clinical Data in KRAS-Driven Locally Advanced Pancreatic Cancer Compelling Preclinical Results for Second Generation Product 4

Silexion is Positioned to Develop the Most Advanced Oncogene Silencing
Therapy NASDAQ listing – ticker SLXN Management team experienced with late-stage development Validated cancer target in mutated KRAS; KRAS-driven cancers represent large potential and need for oncogene silencing therapy in pancreatic, GI and
lung cancers Late-stage pan-KRAS silencing therapy, GMP-grade SIL204 manufactured with FDA/EU-acceptable partner, Q1, 2026 ready for Ph2/3 clinical trial Late-Stage Company with Advanced Stable siRNA Asset 5

Targeting Mutated KRAS by Silencing RNA 6
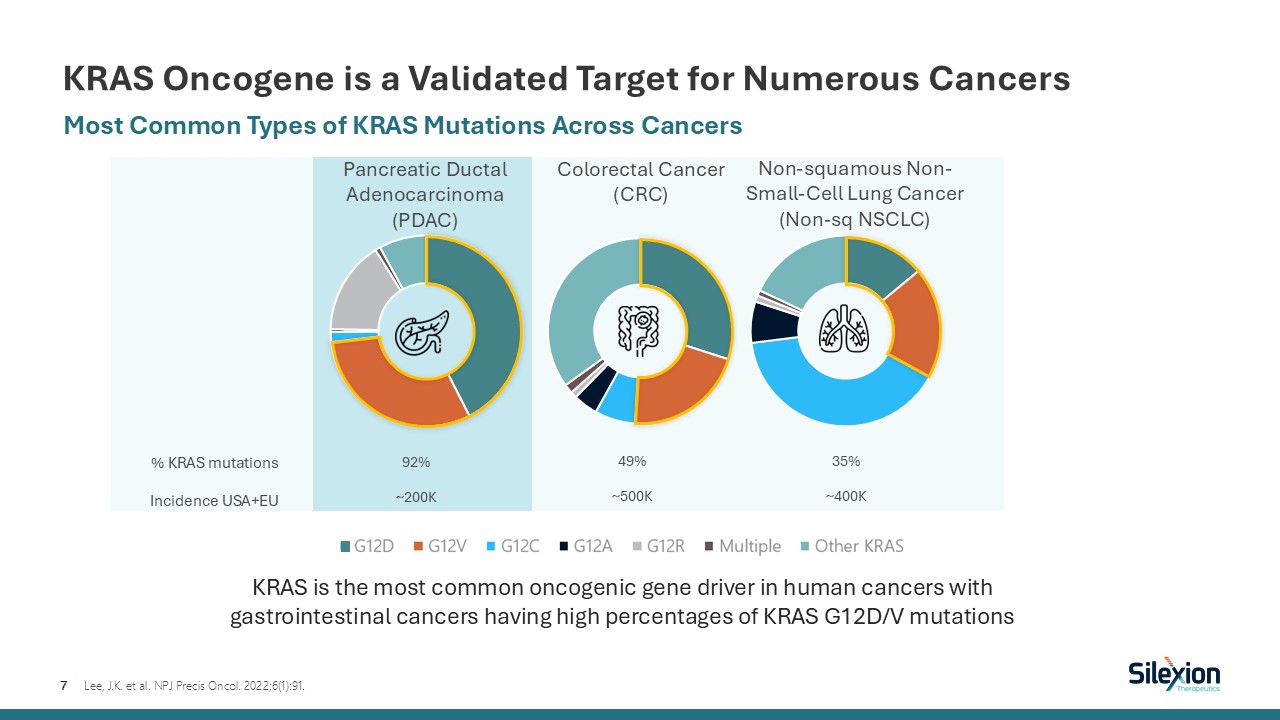
KRAS Oncogene is a Validated Target for Numerous Cancers 7 Most Common Types
of KRAS Mutations Across Cancers Lee, J.K. et al. NPJ Precis Oncol. 2022;6(1):91. Pancreatic Ductal Adenocarcinoma (PDAC) Colorectal Cancer (CRC) Non-squamous Non-Small-Cell Lung Cancer(Non-sq NSCLC) KRAS is the most common oncogenic
gene driver in human cancers with gastrointestinal cancers having high percentages of KRAS G12D/V mutations % KRAS mutations Incidence USA+EU 92% ~200K 49% ~500K 35% ~400K
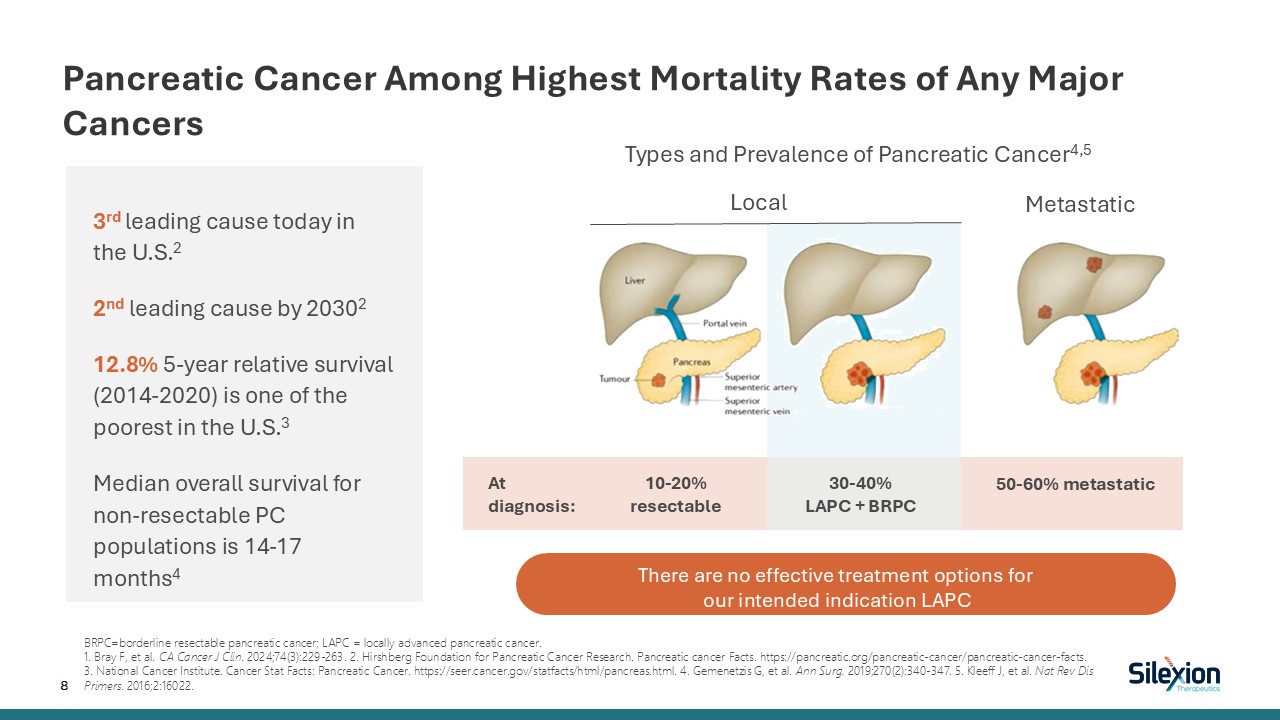
Pancreatic Cancer Among Highest Mortality Rates of Any Major
Cancers BRPC=borderline resectable pancreatic cancer; LAPC = locally advanced pancreatic cancer. 1. Bray F, et al. CA Cancer J Clin. 2024;74(3):229-263. 2. Hirshberg Foundation for Pancreatic Cancer Research. Pancreatic cancer Facts.
https://pancreatic.org/pancreatic-cancer/pancreatic-cancer-facts. 3. National Cancer Institute. Cancer Stat Facts: Pancreatic Cancer. https://seer.cancer.gov/statfacts/html/pancreas.html. 4. Gemenetzis G, et al. Ann Surg. 2019;270(2):340-347.
5. Kleeff J, et al. Nat Rev Dis Primers. 2016;2:16022. Local Metastatic At diagnosis: 10-20% resectable 30-40% LAPC + BRPC 50-60% metastatic Types and Prevalence of Pancreatic Cancer4,5 8 There are no effective treatment options for
our intended indication LAPC 3rd leading cause today in the U.S.2 2nd leading cause by 20302 12.8% 5-year relative survival (2014-2020) is one of the poorest in the U.S.3 Median overall survival for non-resectable PC populations is 14-17
months4
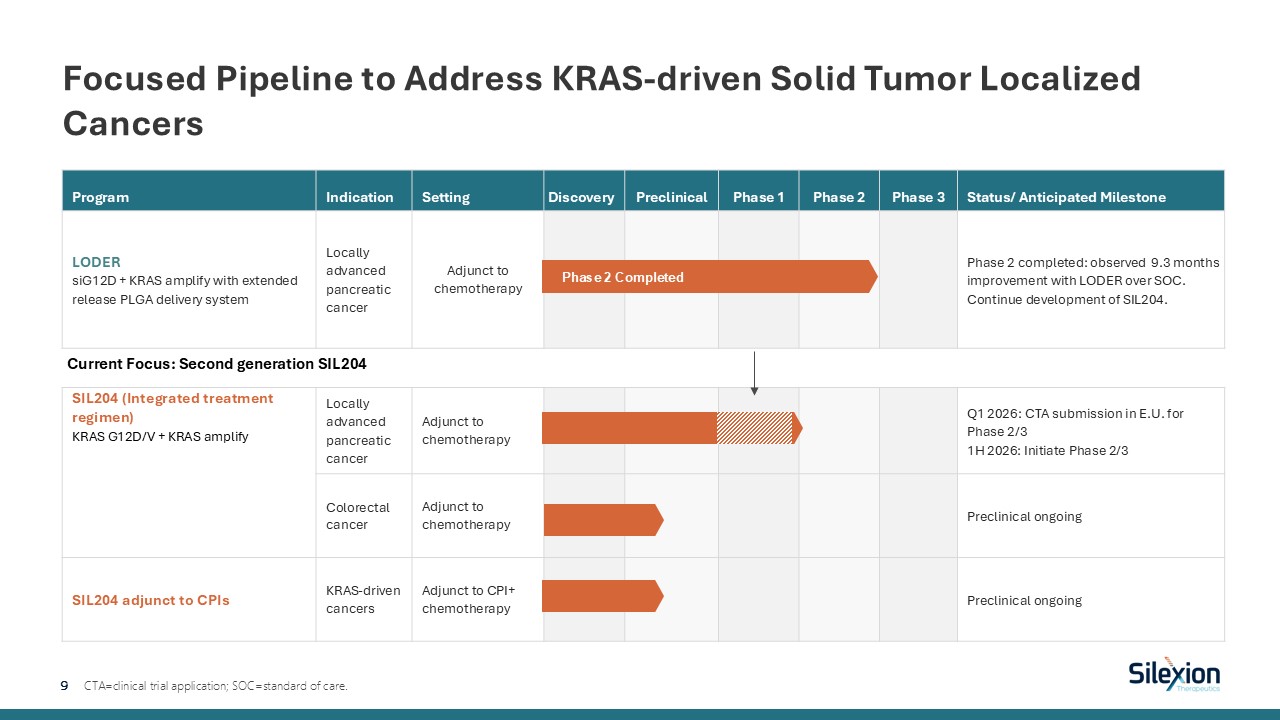
Program Indication Setting Discovery Preclinical Phase 1 Phase 2 Phase
3 Status/ Anticipated Milestone LODER siG12D + KRAS amplify with extended release PLGA delivery system Locally advanced pancreatic cancer Adjunct to chemotherapy Phase 2 completed: observed 9.3 months improvement with LODER over
SOC. Continue development of SIL204. SIL204 (Integrated treatment regimen) KRAS G12D/V + KRAS amplify Locally advanced pancreatic cancer Adjunct to chemotherapy Q1 2026: CTA submission in E.U. for Phase 2/31H 2026: Initiate Phase
2/3 Colorectal cancer Adjunct to chemotherapy Preclinical ongoing SIL204 adjunct to CPIs KRAS-driven cancers Adjunct to CPI+ chemotherapy Preclinical ongoing Focused Pipeline to Address KRAS-driven Solid Tumor Localized Cancers 9
CTA=clinical trial application; SOC=standard of care. Phase 2 Completed Current Focus: Second generation SIL204

LODER (First generation siRNA) Phase 2 Clinical Trial Data 10
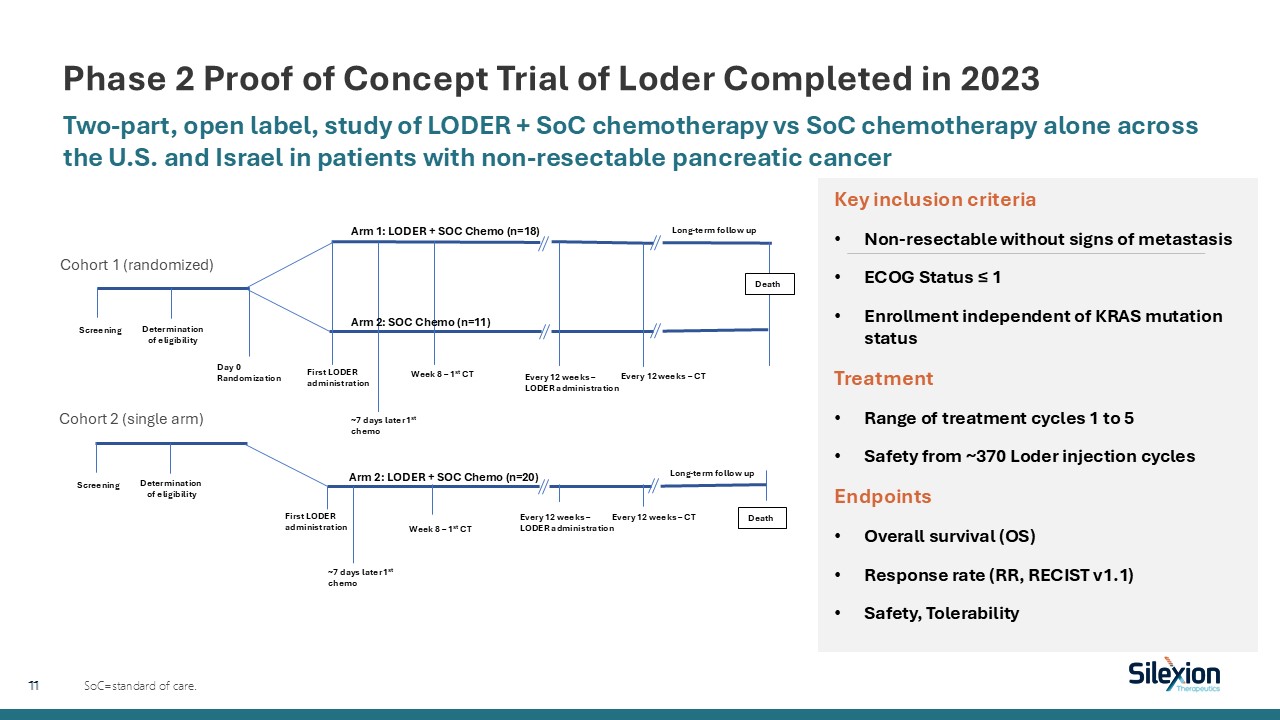
Phase 2 Proof of Concept Trial of Loder Completed in 2023 Two-part, open label,
study of LODER + SoC chemotherapy vs SoC chemotherapy alone across the U.S. and Israel in patients with non-resectable pancreatic cancer SoC=standard of care. Arm 1: LODER + SOC Chemo (n=18) Day 0 Randomization Long-term follow up
Screening Death Determination of eligibility First LODER administration ~7 days later 1st chemo Week 8 – 1st CT Arm 2: SOC Chemo (n=11) Every 12 weeks – LODER administration Every 12 weeks – CT Screening Death Determination of
eligibility First LODER administration ~7 days later 1st chemo Week 8 – 1st CT Arm 2: LODER + SOC Chemo (n=20) Every 12 weeks – LODER administration Every 12 weeks – CT Long-term follow up Cohort 1 (randomized) Cohort 2 (single
arm) Key inclusion criteria Non-resectable without signs of metastasis ECOG Status ≤ 1 Enrollment independent of KRAS mutation status Treatment Range of treatment cycles 1 to 5 Safety from ~370 Loder injection cycles Endpoints Overall
survival (OS) Response rate (RR, RECIST v1.1) Safety, Tolerability 11
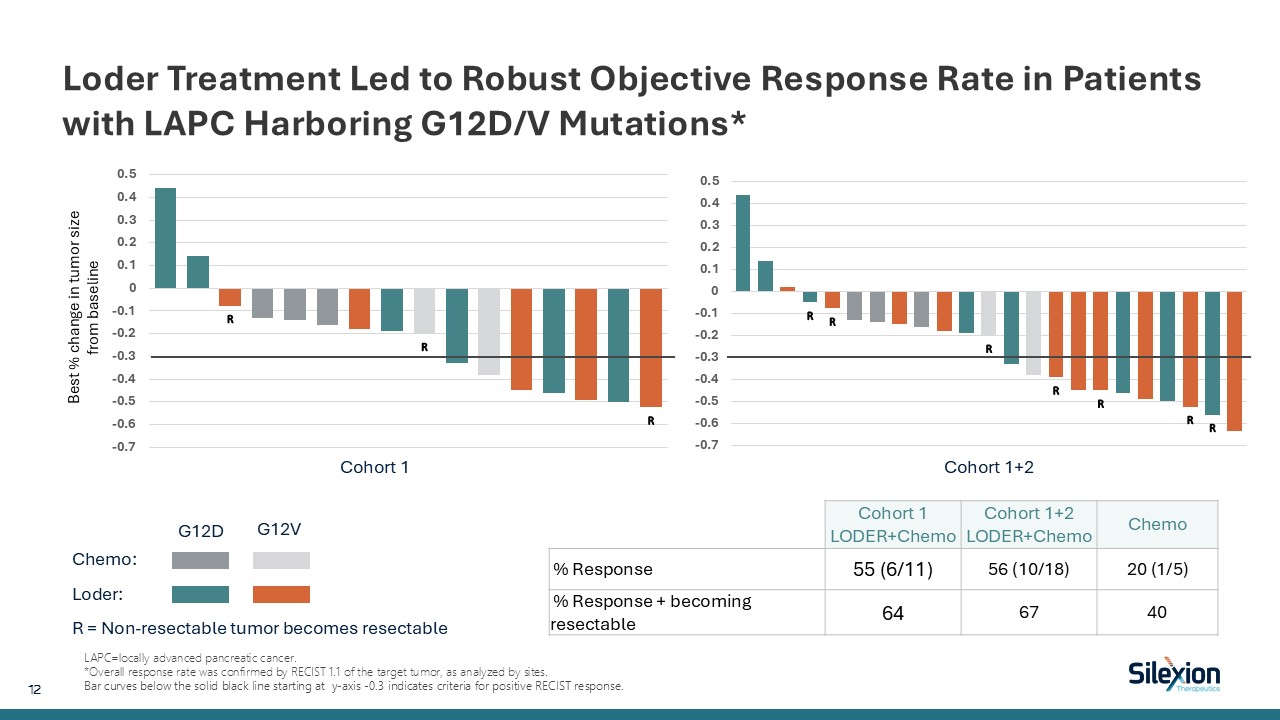
Loder Treatment Led to Robust Objective Response Rate in Patients with LAPC
Harboring G12D/V Mutations* LAPC=locally advanced pancreatic cancer. *Overall response rate was confirmed by RECIST 1.1 of the target tumor, as analyzed by sites. Bar curves below the solid black line starting at y-axis -0.3 indicates
criteria for positive RECIST response. Chemo: Loder: R = Non-resectable tumor becomes resectable G12D G12V Cohort 1 Cohort 1+2 Best % change in tumor size from baseline Cohort 1 LODER+Chemo Cohort 1+2 LODER+Chemo Chemo %
Response 55 (6/11) 56 (10/18) 20 (1/5) % Response + becoming resectable 64 67 40 12
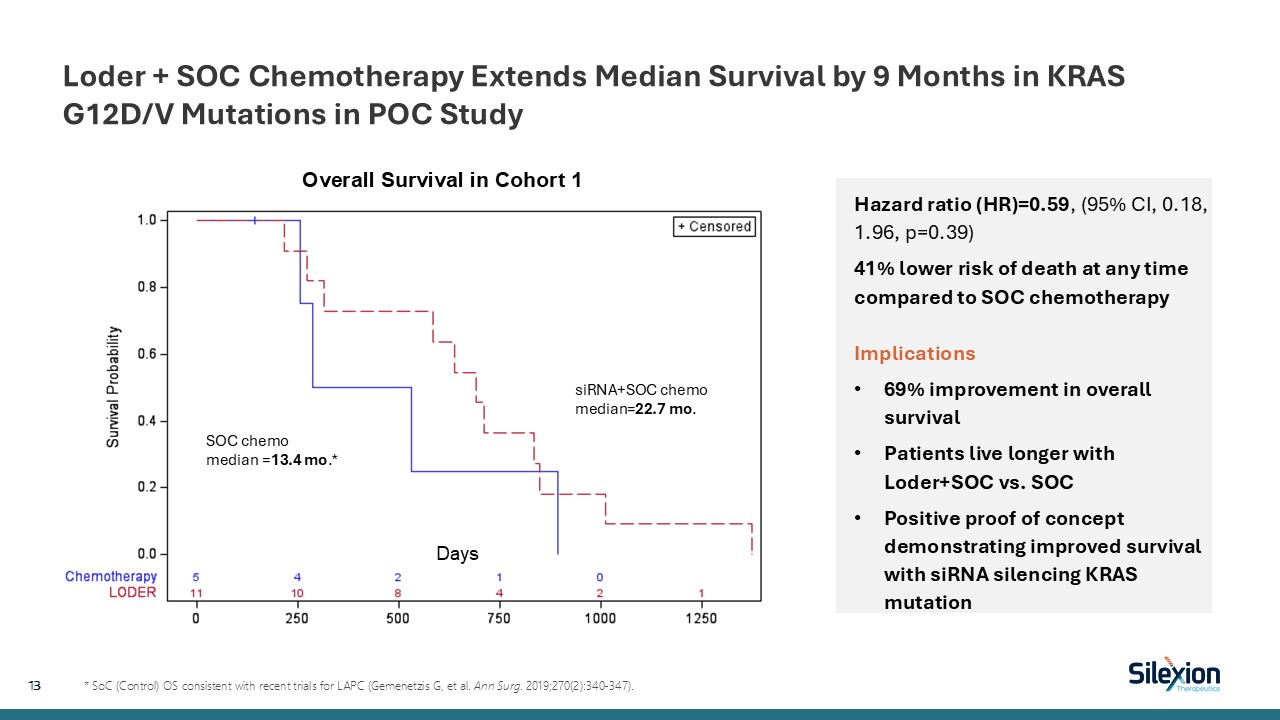
Loder + SOC Chemotherapy Extends Median Survival by 9 Months in KRAS G12D/V
Mutations in POC Study 13 * SoC (Control) OS consistent with recent trials for LAPC (Gemenetzis G, et al. Ann Surg. 2019;270(2):340-347). Hazard ratio (HR)=0.59, (95% CI, 0.18, 1.96, p=0.39) 41% lower risk of death at any time compared to
SOC chemotherapyImplications 69% improvement in overall survival Patients live longer with Loder+SOC vs. SOC Positive proof of concept demonstrating improved survival with siRNA silencing KRAS mutation Days Overall Survival in Cohort
1 SOC chemo median =13.4 mo.* siRNA+SOC chemo median=22.7 mo.

LODER Was Overall Well Tolerated with an Acceptable Safety Profile Combination
siG12D-LODER and SoC chemotherapy generally well-tolerated All serious TEAEs resolved: 9 (pyrexia x2, abdominal pain, hyperbilirubinemia, pancreas infection x2, sepsis, procedural hemorrhage/presyncope, gastric hemorrhage) Two Grade 5
events reported in the siG12D-LODER population, both assessed unrelated to treatment: acute intestinal ischemia and gram negative sepsis. No Treatment Emergent Adverse Events (TEAEs) leading to study discontinuation related to LODER treatment
No meaningful observations in any vital sign parameter nor any physical examination findings in the study Independent Drug Safety Monitoring Board (DSMB) Reviews had no safety concerns nor safety restrictions In a subset analysis, no
measurable amount of siG12D siRNA was detected (<BLQ, 0.25ng/mL) in any plasma samples suggesting low systemic levels 14

SIL204 (Second Generation siRNA) 15
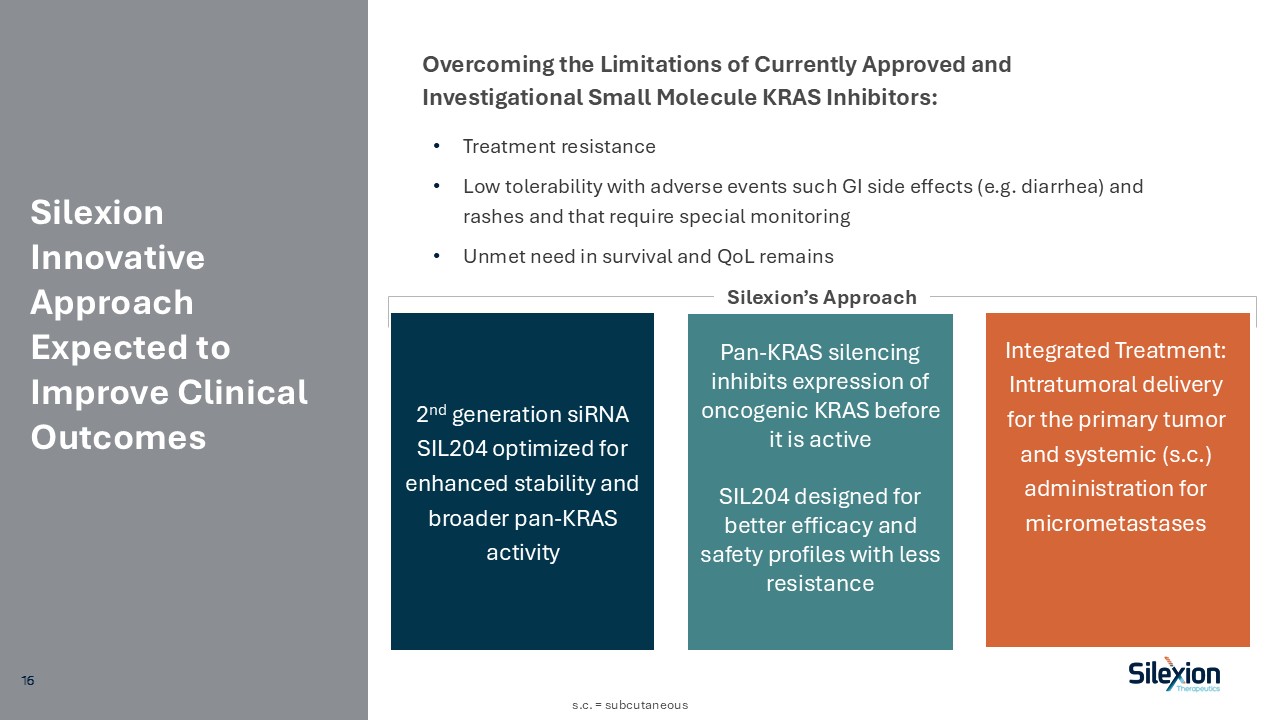
Silexion Innovative Approach Expected to Improve Clinical Outcomes Treatment
resistance Low tolerability with adverse events such GI side effects (e.g. diarrhea) and rashes and that require special monitoring Unmet need in survival and QoL remains Overcoming the Limitations of Currently Approved and Investigational
Small Molecule KRAS Inhibitors: 2nd generation siRNA SIL204 optimized for enhanced stability and broader pan-KRAS activity Integrated Treatment: Intratumoral delivery for the primary tumor and systemic (s.c.) administration for
micrometastases Pan-KRAS silencing inhibits expression of oncogenic KRAS before it is active SIL204 designed for better efficacy and safety profiles with less resistance Silexion’s Approach s.c. = subcutaneous 16
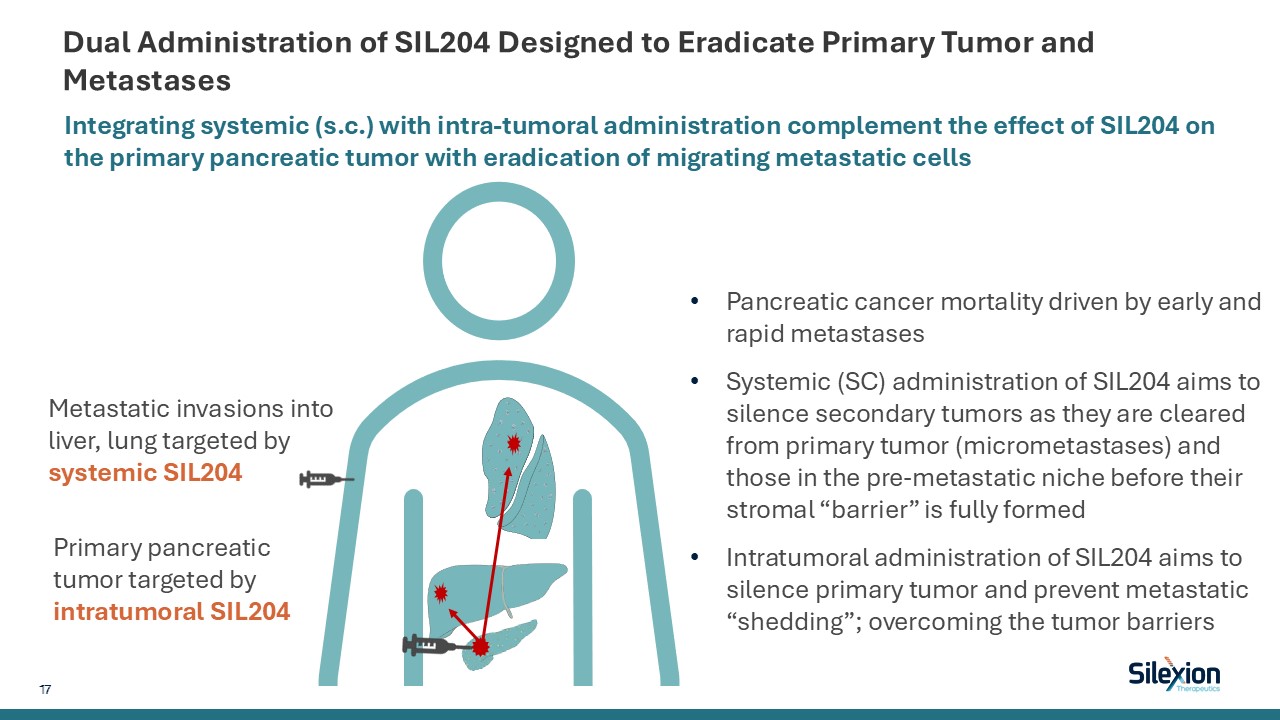
Dual Administration of SIL204 Designed to Eradicate Primary Tumor and
Metastases Integrating systemic (s.c.) with intra-tumoral administration complement the effect of SIL204 on the primary pancreatic tumor with eradication of migrating metastatic cells Primary pancreatic tumor targeted by intratumoral
SIL204 Metastatic invasions into liver, lung targeted by systemic SIL204 Pancreatic cancer mortality driven by early and rapid metastases Systemic (SC) administration of SIL204 aims to silence secondary tumors as they are cleared from
primary tumor (micrometastases) and those in the pre-metastatic niche before their stromal “barrier” is fully formed Intratumoral administration of SIL204 aims to silence primary tumor and prevent metastatic “shedding”; overcoming the tumor
barriers 17
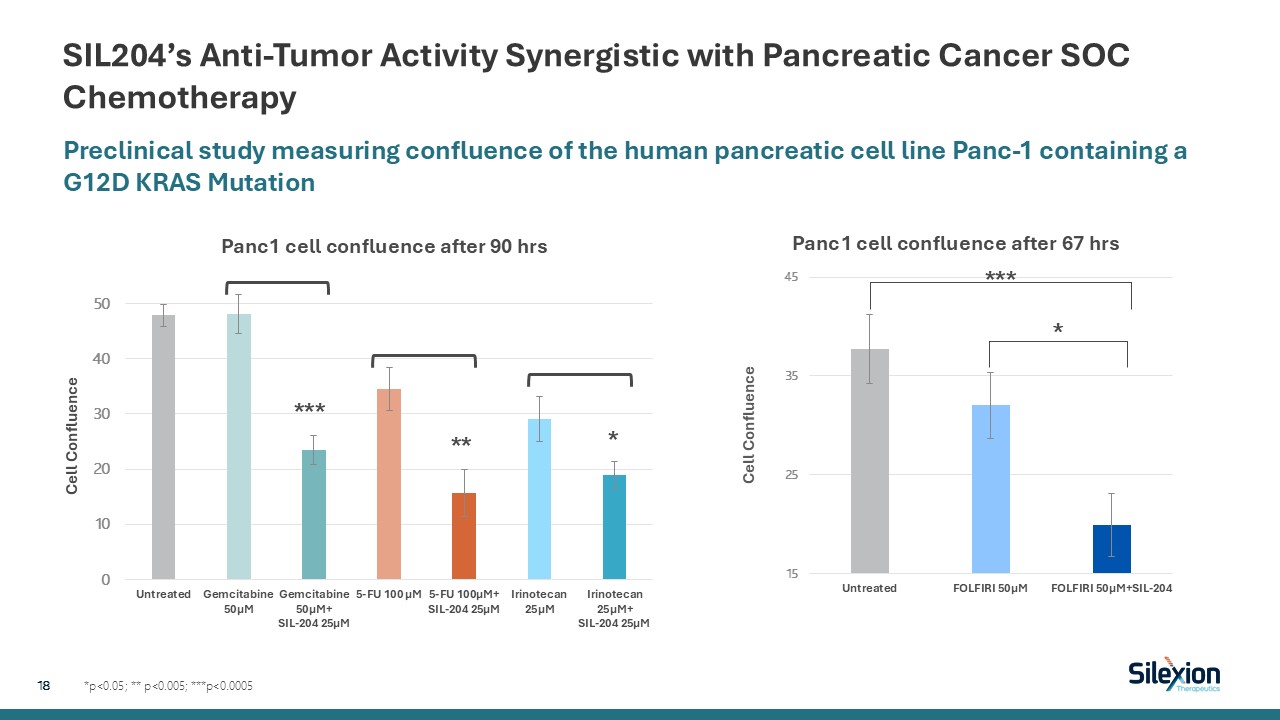
SIL204’s Anti-Tumor Activity Synergistic with Pancreatic Cancer SOC
Chemotherapy *p<0.05; ** p<0.005; ***p<0.0005 Preclinical study measuring confluence of the human pancreatic cell line Panc-1 containing a G12D KRAS Mutation 18 *** ** * *** *
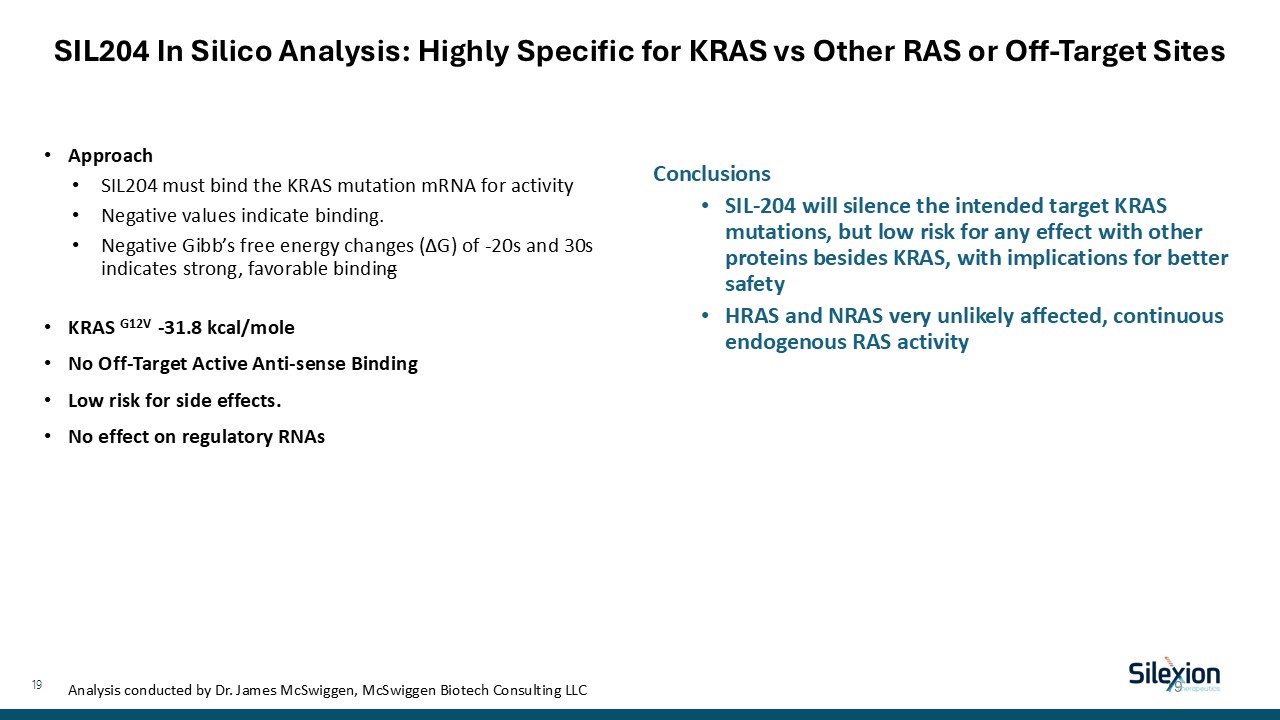
SIL204 In Silico Analysis: Highly Specific for KRAS vs Other RAS or Off-Target
Sites Approach SIL204 must bind the KRAS mutation mRNA for activity Negative values indicate binding. Negative Gibb’s free energy changes (∆G) of -20s and 30s indicates strong, favorable binding KRAS G12V -31.8 kcal/mole No Off-Target
Active Anti-sense Binding Low risk for side effects. No effect on regulatory RNAs Conclusions SIL-204 will silence the intended target KRAS mutations, but low risk for any effect with other proteins besides KRAS, with implications for
better safety HRAS and NRAS very unlikely affected, continuous endogenous RAS activity Analysis conducted by Dr. James McSwiggen, McSwiggen Biotech Consulting LLC 19 19
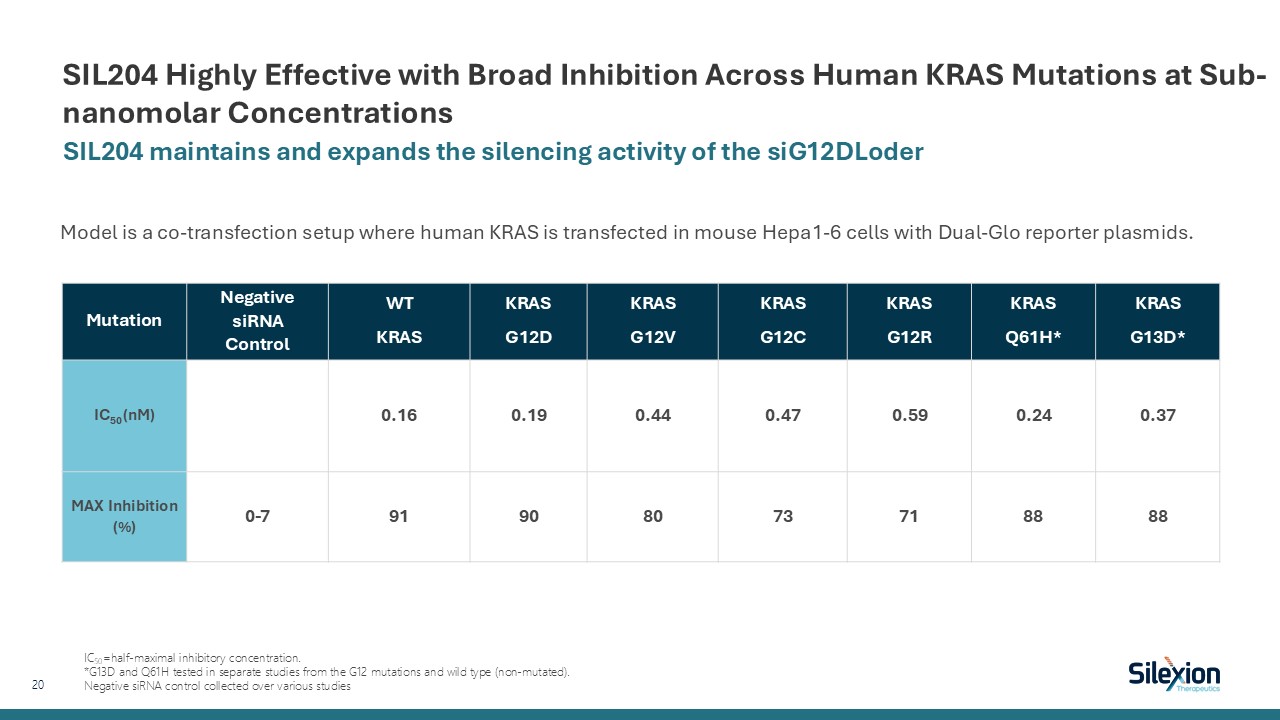
SIL204 Highly Effective with Broad Inhibition Across Human KRAS Mutations at
Sub-nanomolar Concentrations SIL204 maintains and expands the silencing activity of the siG12DLoder 20 Model is a co-transfection setup where human KRAS is transfected in mouse Hepa1-6 cells with Dual-Glo reporter
plasmids. Mutation NegativesiRNAControl WT KRAS KRAS G12D KRAS G12V KRAS G12C KRAS G12R KRAS Q61H* KRAS G13D* IC50 (nM) 0.16 0.19 0.44 0.47 0.59 0.24 0.37 MAX Inhibition
(%) 0-7 91 90 80 73 71 88 88 IC50=half-maximal inhibitory concentration. *G13D and Q61H tested in separate studies from the G12 mutations and wild type (non-mutated). Negative siRNA control collected over various studies
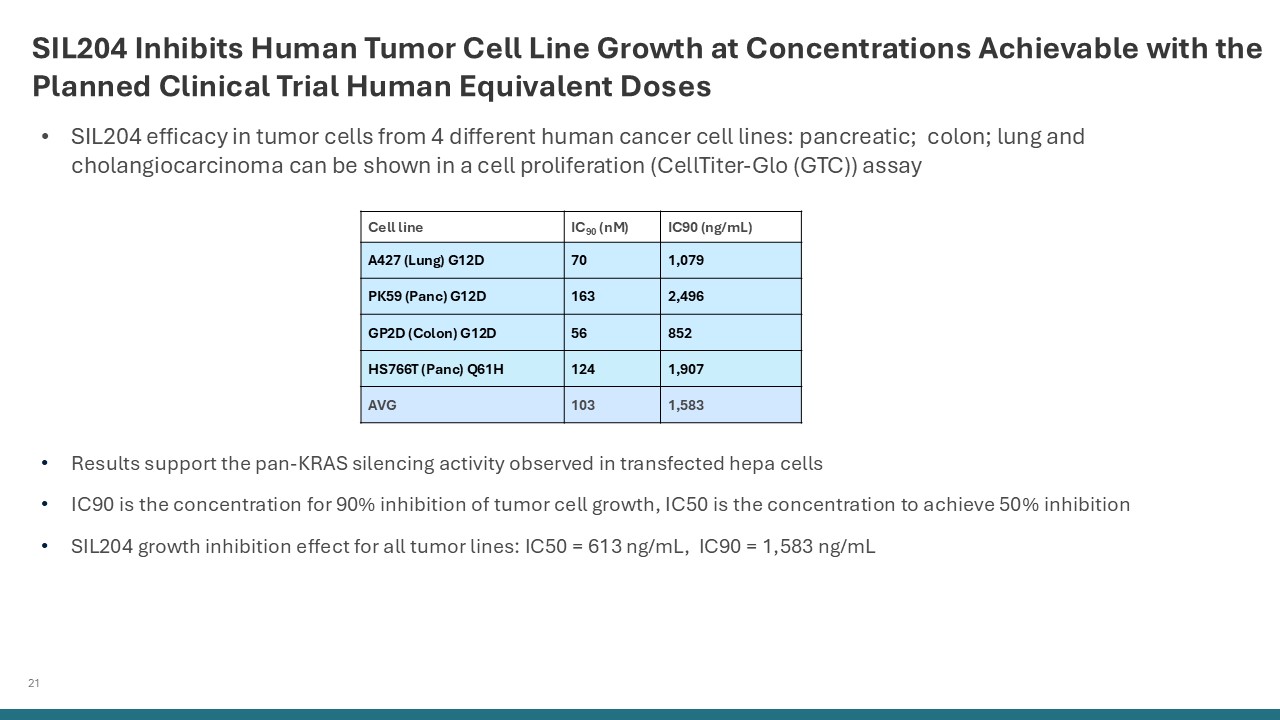
21 SIL204 Inhibits Human Tumor Cell Line Growth at Concentrations Achievable
with the Planned Clinical Trial Human Equivalent Doses Results support the pan-KRAS silencing activity observed in transfected hepa cells IC90 is the concentration for 90% inhibition of tumor cell growth, IC50 is the concentration to achieve
50% inhibition SIL204 growth inhibition effect for all tumor lines: IC50 = 613 ng/mL, IC90 = 1,583 ng/mL Cell line IC90 (nM) IC90 (ng/mL) A427 (Lung) G12D 70 1,079 PK59 (Panc) G12D 163 2,496 GP2D (Colon) G12D 56 852 HS766T (Panc)
Q61H 124 1,907 AVG 103 1,583 SIL204 efficacy in tumor cells from 4 different human cancer cell lines: pancreatic; colon; lung and cholangiocarcinoma can be shown in a cell proliferation (CellTiter-Glo (GTC)) assay
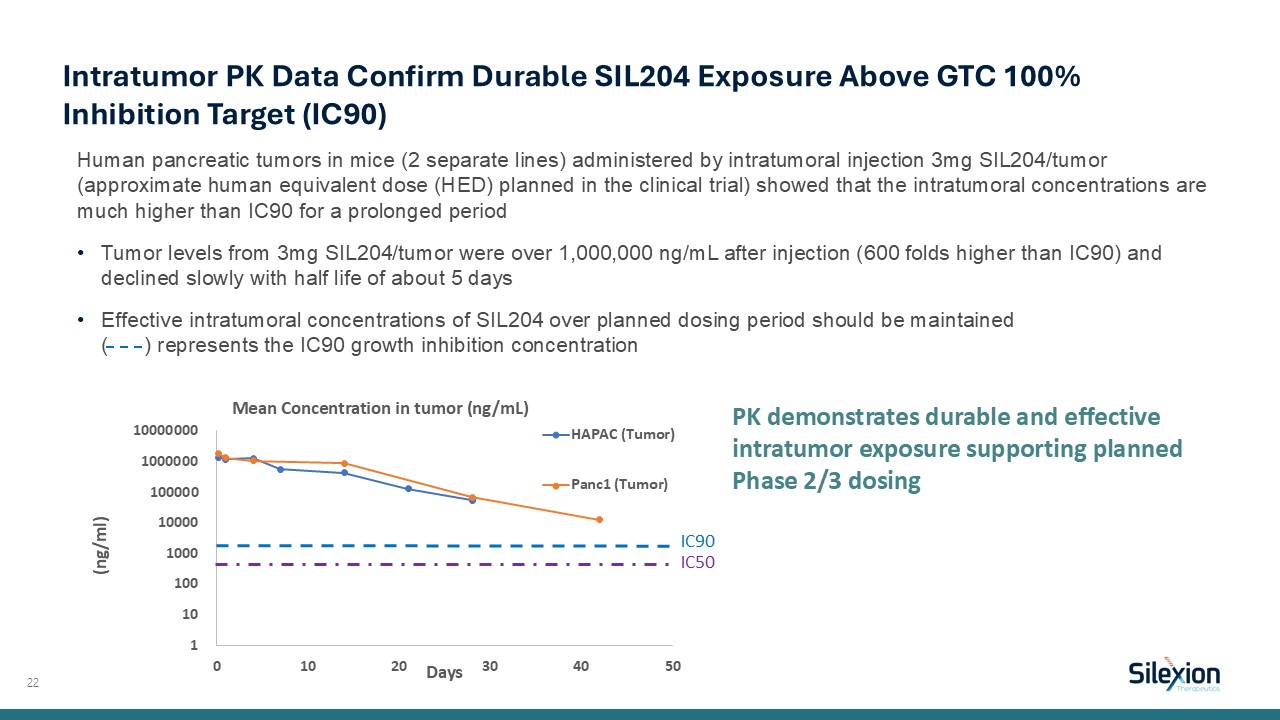
Human pancreatic tumors in mice (2 separate lines) administered by intratumoral
injection 3mg SIL204/tumor (approximate human equivalent dose (HED) planned in the clinical trial) showed that the intratumoral concentrations are much higher than IC90 for a prolonged period Tumor levels from 3mg SIL204/tumor were over
1,000,000 ng/mL after injection (600 folds higher than IC90) and declined slowly with half life of about 5 days Effective intratumoral concentrations of SIL204 over planned dosing period should be maintained ( ) represents the IC90 growth
inhibition concentration 22 Intratumor PK Data Confirm Durable SIL204 Exposure Above GTC 100% Inhibition Target (IC90) PK demonstrates durable and effective intratumor exposure supporting planned Phase 2/3 dosing IC90 IC50
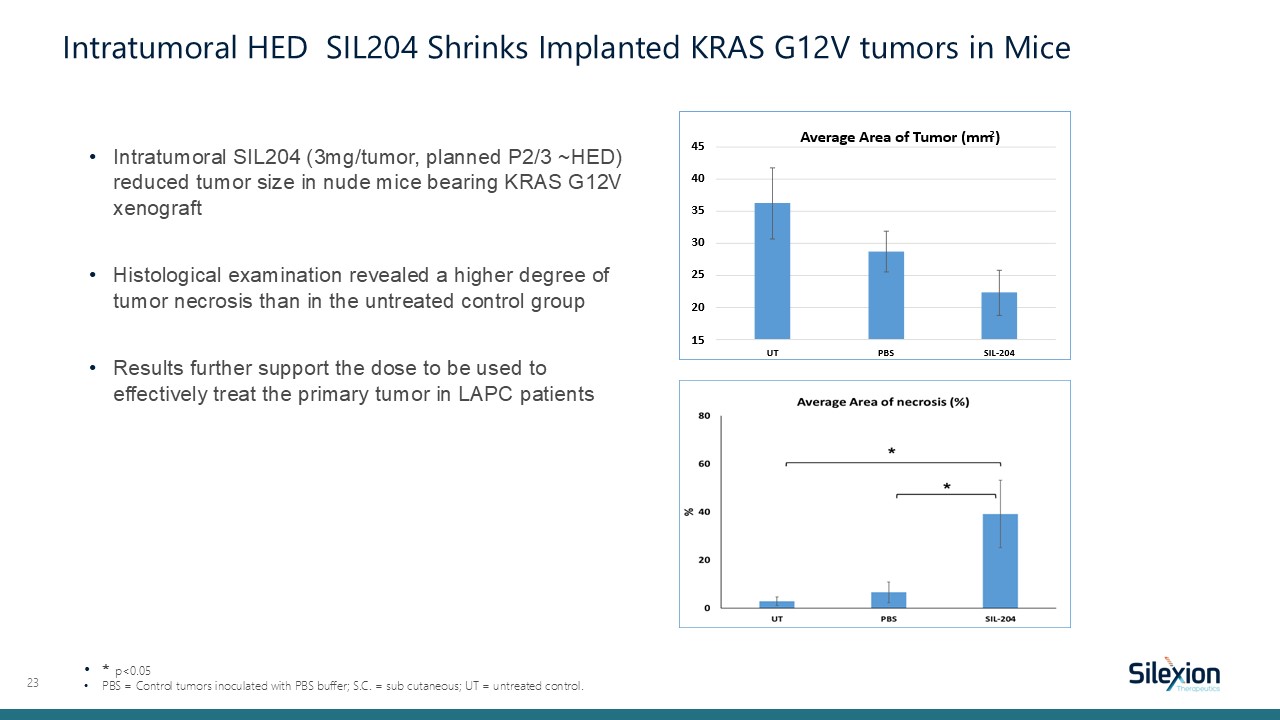
Intratumoral SIL204 (3mg/tumor, planned P2/3 ~HED) reduced tumor size in nude mice
bearing KRAS G12V xenograft Histological examination revealed a higher degree of tumor necrosis than in the untreated control group Results further support the dose to be used to effectively treat the primary tumor in LAPC patients 23 *
p<0.05 PBS = Control tumors inoculated with PBS buffer; S.C. = sub cutaneous; UT = untreated control. Intratumoral HED SIL204 Shrinks Implanted KRAS G12V tumors in Mice
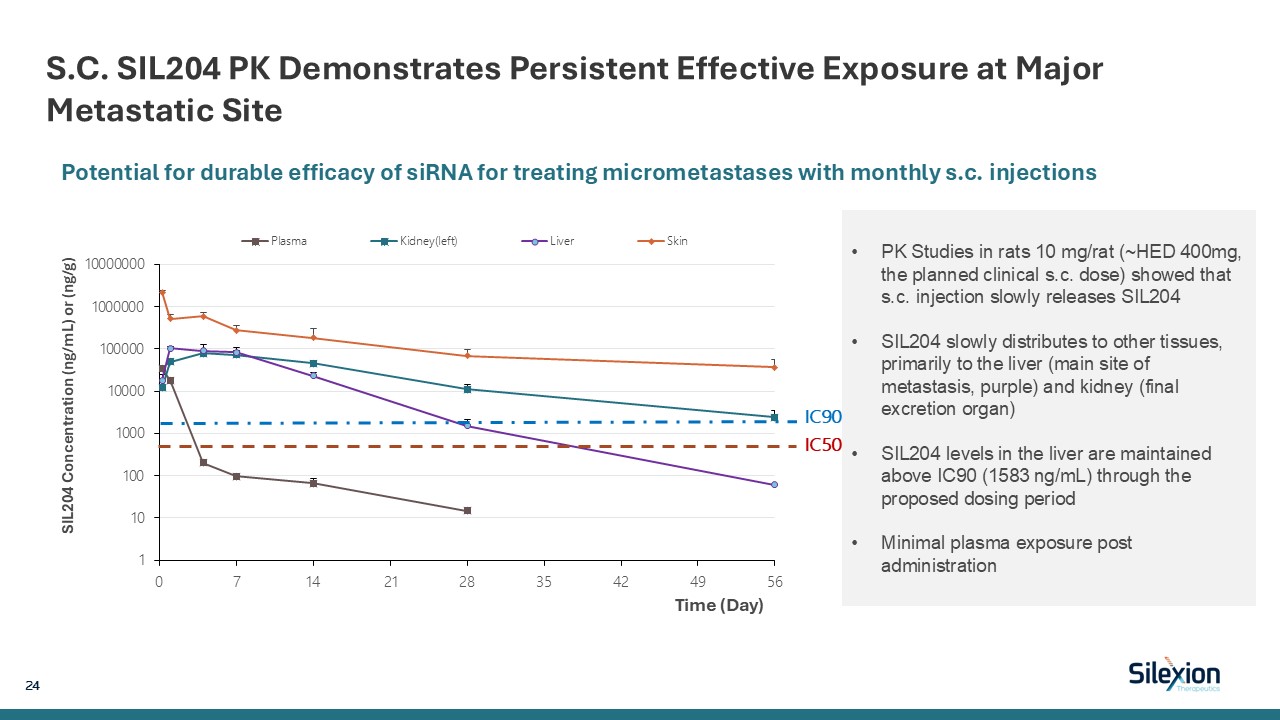
S.C. SIL204 PK Demonstrates Persistent Effective Exposure at Major Metastatic Site
24 PK Studies in rats 10 mg/rat (~HED 400mg, the planned clinical s.c. dose) showed that s.c. injection slowly releases SIL204 SIL204 slowly distributes to other tissues, primarily to the liver (main site of metastasis, purple) and kidney
(final excretion organ) SIL204 levels in the liver are maintained above IC90 (1583 ng/mL) through the proposed dosing period Minimal plasma exposure post administration Potential for durable efficacy of siRNA for treating micrometastases
with monthly s.c. injections IC90IC50
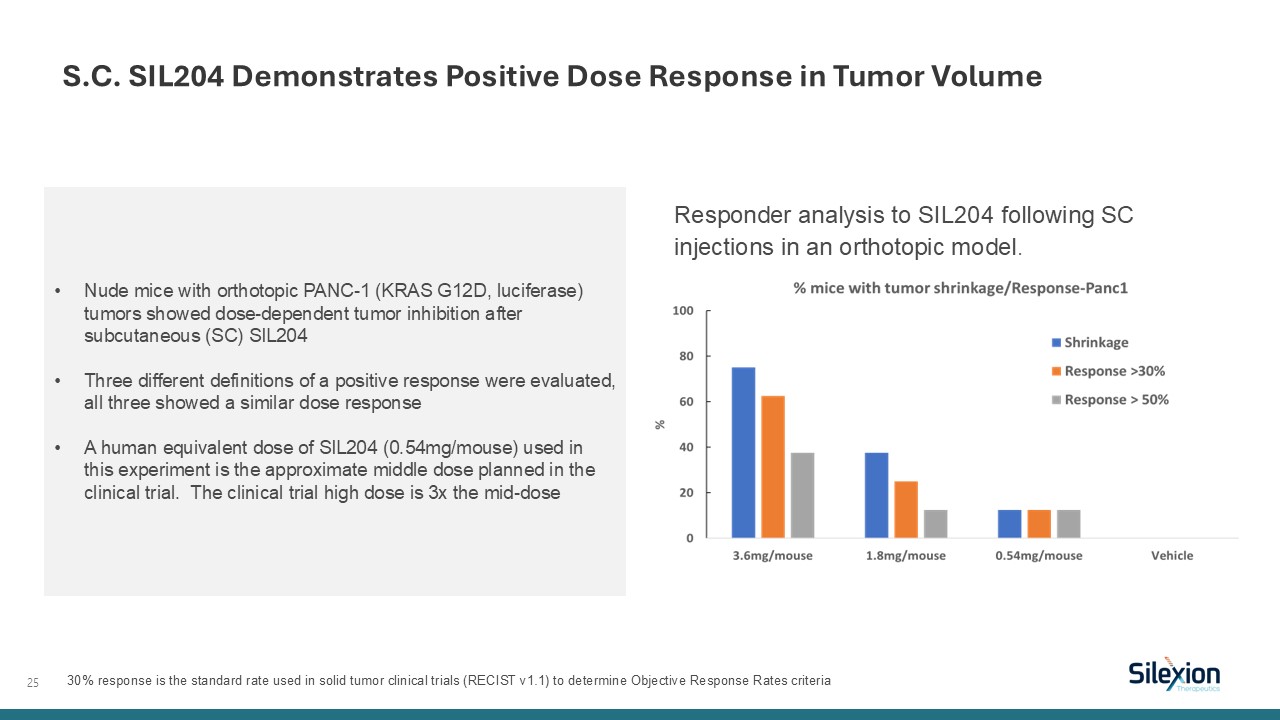
25 Responder analysis to SIL204 following SC injections in an orthotopic
model. S.C. SIL204 Demonstrates Positive Dose Response in Tumor Volume Nude mice with orthotopic PANC-1 (KRAS G12D, luciferase) tumors showed dose-dependent tumor inhibition after subcutaneous (SC) SIL204 Three different definitions of a
positive response were evaluated, all three showed a similar dose response A human equivalent dose of SIL204 (0.54mg/mouse) used in this experiment is the approximate middle dose planned in the clinical trial. The clinical trial high dose is
3x the mid-dose 30% response is the standard rate used in solid tumor clinical trials (RECIST v1.1) to determine Objective Response Rates criteria
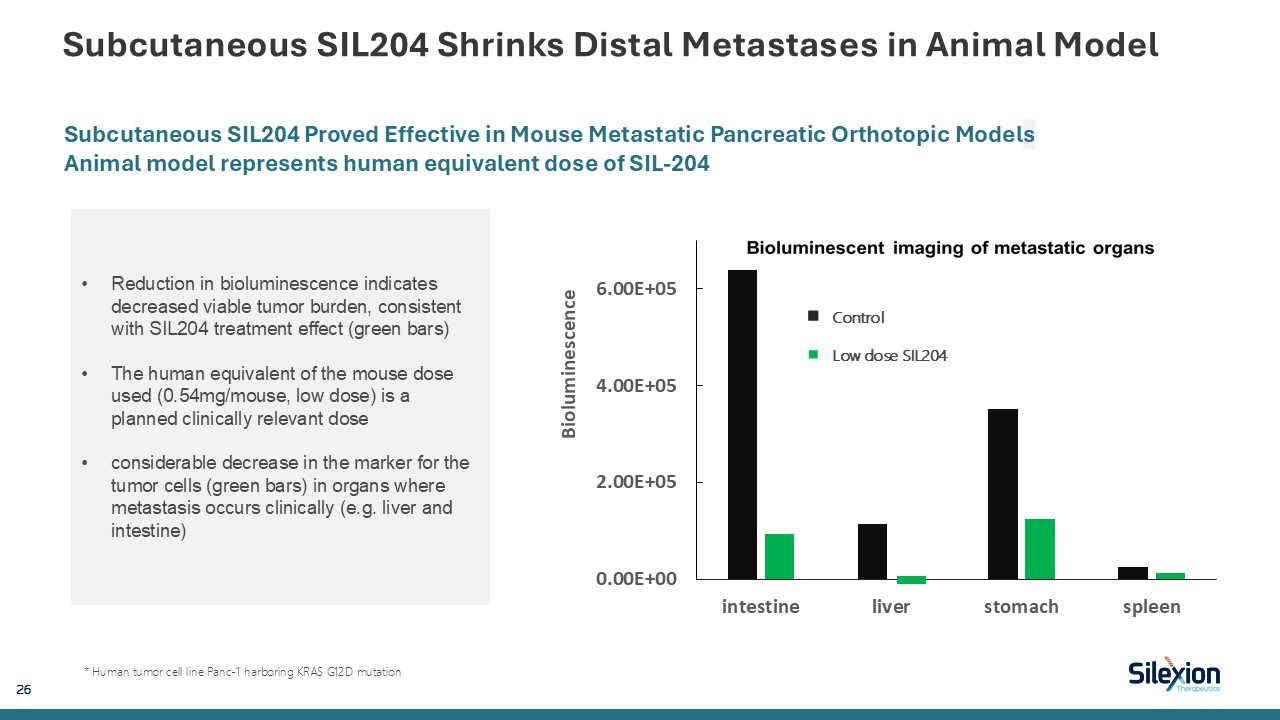
Subcutaneous SIL204 Shrinks Distal Metastases in Animal Model 26 Subcutaneous
SIL204 Proved Effective in Mouse Metastatic Pancreatic Orthotopic Models Animal model represents human equivalent dose of SIL-204 * Human tumor cell line Panc-1 harboring KRAS G12D mutation Control Low dose SIL204 Reduction in
bioluminescence indicates decreased viable tumor burden, consistent with SIL204 treatment effect (green bars) The human equivalent of the mouse dose used (0.54mg/mouse, low dose) is a planned clinically relevant dose considerable decrease in
the marker for the tumor cells (green bars) in organs where metastasis occurs clinically (e.g. liver and intestine)
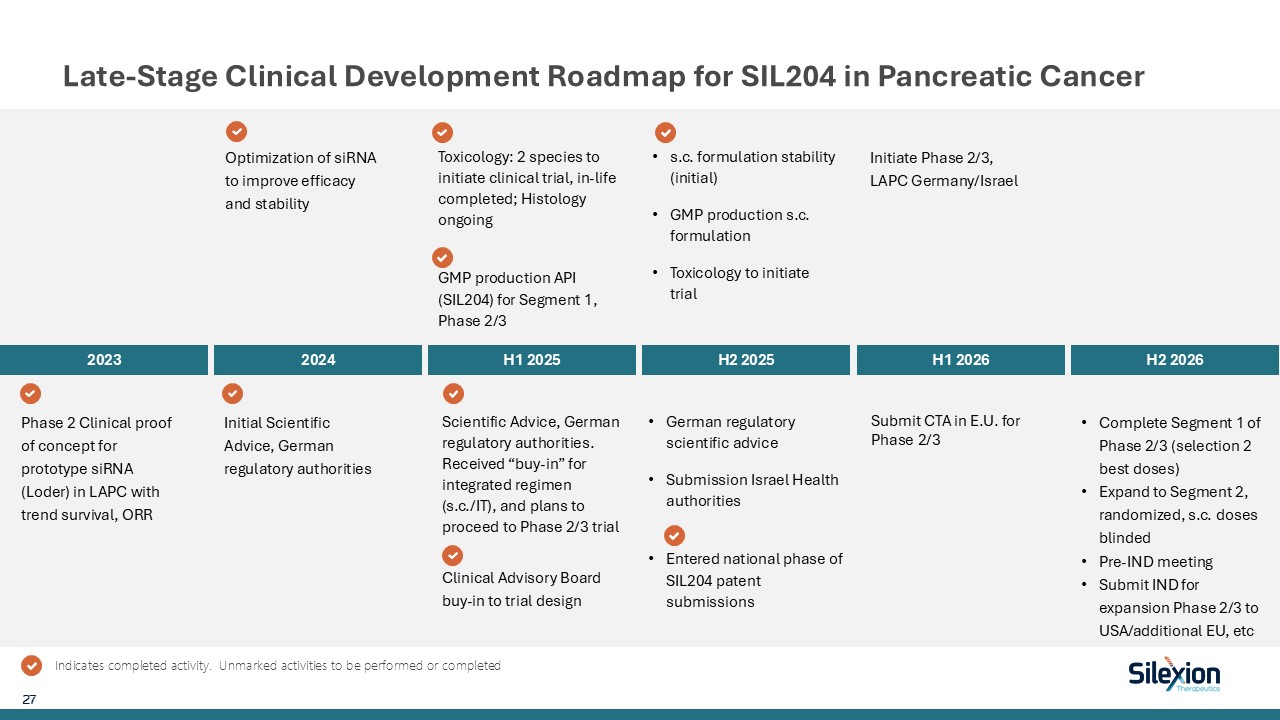
Late-Stage Clinical Development Roadmap for SIL204 in Pancreatic
Cancer 2023 2024 H1 2025 H2 2025 H1 2026 H2 2026 Phase 2 Clinical proof of concept for prototype siRNA (Loder) in LAPC with trend survival, ORR Optimization of siRNA to improve efficacy and stability Initial Scientific Advice, German
regulatory authorities Toxicology: 2 species to initiate clinical trial, in-life completed; Histology ongoing GMP production API (SIL204) for Segment 1, Phase 2/3 Initiate Phase 2/3, LAPC Germany/Israel Complete Segment 1 of Phase 2/3
(selection 2 best doses) Expand to Segment 2, randomized, s.c. doses blinded Pre-IND meeting Submit IND for expansion Phase 2/3 to USA/additional EU, etc 27 Indicates completed activity. Unmarked activities to be performed or
completed Scientific Advice, German regulatory authorities. Received “buy-in” for integrated regimen (s.c./IT), and plans to proceed to Phase 2/3 trial s.c. formulation stability (initial) GMP production s.c. formulation Toxicology to
initiate trial German regulatory scientific advice Submission Israel Health authorities Entered national phase of SIL204 patent submissions Clinical Advisory Board buy-in to trial design Submit CTA in E.U. for Phase 2/3
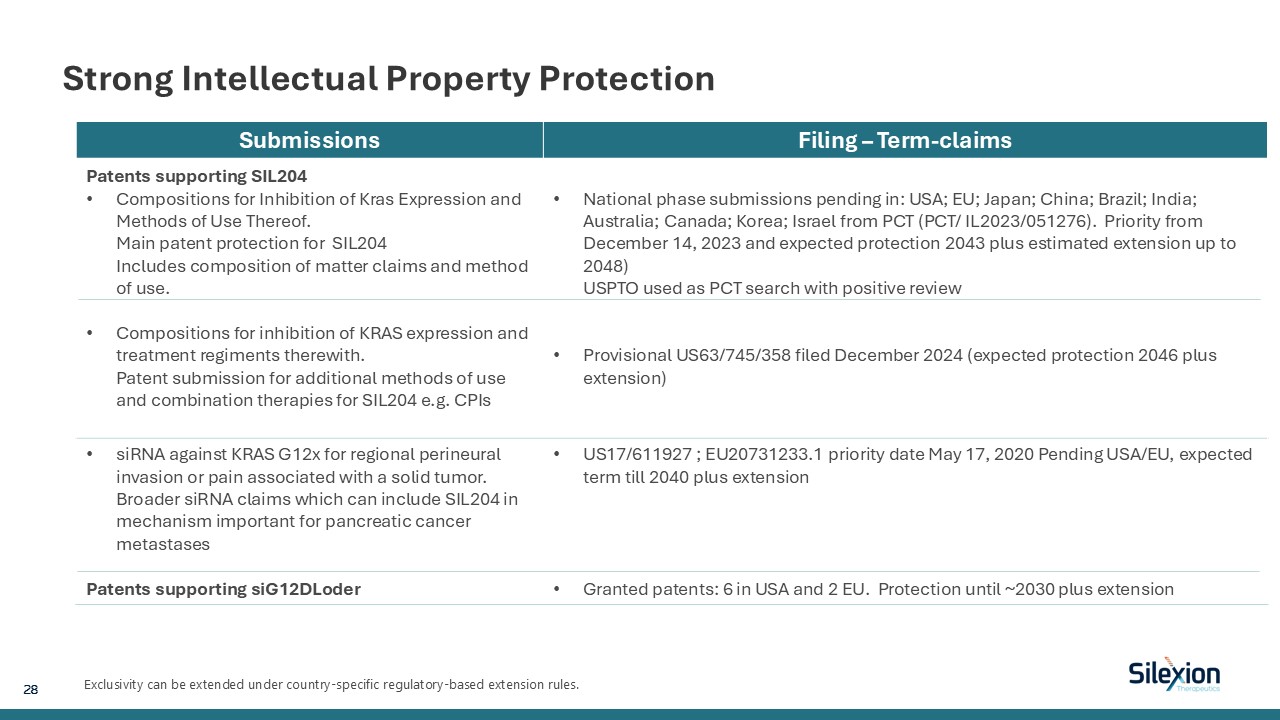
Strong Intellectual Property Protection Exclusivity can be extended under
country-specific regulatory-based extension rules. Submissions Filing – Term-claims Patents supporting SIL204 Compositions for Inhibition of Kras Expression and Methods of Use Thereof.Main patent protection for SIL204Includes composition of
matter claims and method of use. Compositions for inhibition of KRAS expression and treatment regiments therewith.Patent submission for additional methods of use and combination therapies for SIL204 e.g. CPIs National phase submissions
pending in: USA; EU; Japan; China; Brazil; India; Australia; Canada; Korea; Israel from PCT (PCT/ IL2023/051276). Priority from December 14, 2023 and expected protection 2043 plus estimated extension up to 2048) USPTO used as PCT search with
positive review Provisional US63/745/358 filed December 2024 (expected protection 2046 plus extension) siRNA against KRAS G12x for regional perineural invasion or pain associated with a solid tumor.Broader siRNA claims which can include
SIL204 in mechanism important for pancreatic cancer metastases Patents supporting siG12DLoder US17/611927 ; EU20731233.1 priority date May 17, 2020 Pending USA/EU, expected term till 2040 plus extension Granted patents: 6 in USA and 2 EU.
Protection until ~2030 plus extension 28

Silexion Positioned to Develop the Most Advanced Oncogene Silencing
Therapy Late-Stage Ready Asset with Regulatory Path Forward Strong Partnerships with Solid IP Portfolio Instead of targeting mutated KRAS proteins after they are formed (as competing technologies do) Silexion’s siRNA technology silences
their expression at the outset, offering a potentially safer and more durable approach KRAS-Focused RNA Interference Platform First generation siG12DLoder siRNA Completed POC- Phase 2 clinical trial in LAPC Demonstrated improvement in
overall survival. Expected that siRNA silencing mechanism of the oncogene will overcome the tumoral resistance mechanism observed with KRAS inhibitors Second generation, silencing siRNA “SIL204,” significantly improved product stability and
delivery while providing a broader gene silencing activity. Preclinical results demonstrate SIL204’s pan-KRAS anti-tumor activity in pancreatic, colorectal and lung; supports dual-route strategy to administer SIL204 both intratumorally and s.c.
to target primary tumor and metastases. Phase 2/3 clinical trial planned Q2, 2026; using integrated administration approach, s.c. plus intratumor Promising Clinical Data in KRAS-Driven Locally Advanced Pancreatic Cancer Compelling
Preclinical Results for Second Generation Product NASDAQ listed with experienced management team Validated cancer target in mutated KRAS Late-stage pan-KRAS silencing therapy. SIL204 API already manufactured by under GMP and ready for Phase
2/3 clinical trial Late-Stage Company with a Stable Advanced siRNA Asset 29

World-Renowned Expert Scientific Advisory Board Eileen M. O'Reilly, MD Memorial
Sloan Kettering, NY, NY Winthrop Rockefeller Endowed Chair of Medical Oncology; Co-Director, Medical Initiatives, David M. Rubenstein Center for Pancreatic Cancer Research; Section Head, Hepatopancreatobi Hana Algul, MD Technical University
of Munich, Germany chair for tumor metabolism; Director of the Comprehensive Cancer Center Munich, Germany at the Klinikum rechts der Isar, and Mildred-Scheel-professor and Milind Javle, MD The University of Texas & MD Anderson Cancer
Center, Houston, TX Professor, Department of Gastrointestinal (GI) Medical Oncology, Division of Cancer Medicine Philip A. Philip, MD Henry Ford Health, Detroit, MI Director, Gastrointestinal Oncology; Co-Director, Pancreatic Cancer
Center; Medical Director, Research and Clinical Care Integration, Henry Ford Cancer Institute Talia Golan, MD Sheba Tel Hashomer Hospital,, Israel Head, Sheba Pancreatic Cancer Center - SPCC Matthew Katz, MD The University of Texas &
MD Anderson Cancer Center, Houston, TX Department Chair, Department of Surgical Oncology, Division of Surgery and Professor. Andrew M. Lowy, MD UC San Diego, San Diego, CA Chief, Division of Surgical Oncology; Professor of Surgery Mark A.
Schattner, MD Memorial Sloan Kettering, NY, NY Chief, Gastroenterology, Hepatology and Nutrition Service 30 Thomas Seufferlein, MD University Hospital Ulm, German Director of Internal Medicine University Hospital Ulm, President German
Cancer Society

Highly Experienced Leadership Team Ilan Hadar, MBA Chairman and Chief Executive
Officer Over 25 years of multinational executive managerial and corporate experience with pharmaceutical and high-tech companies. CEO PainReform (Nasdaq: “PRFX”), CFO and Country Manager Foamix Pharmaceuticals Inc. (Currently Nasdaq:
“VYNE”) Mitchell Shirvan, PhD, MBA Chief Scientific and Development Officer Over 30 years of experience in R&D, innovation and discovery in biotech companies. CEO Macrocure Ltd., Sr. V.P. R&D Foamix Pharmaceuticals Inc. Currently
Nasdaq “VYNE”), Sr. Director Strategic Business Planning Teva Pharmaceuticals Industries Inc. Mirit Horenshtein Hadar, CPA Chief Financial Officer Over 15 years of corporate finance experience in senior financial positions of public companies
and privately held companies, in the pharmaceutical and high-tech industries. CFO Gouzy Israel (Nasdaq: “GAUZ”). V.P. Finance Foamix Pharmaceuticals Inc. (currently Nasdaq“VYNE”) 31

Thank You Nasdaq: SLXN Ilan Hadar Chairman & Chief Executive
Officer email: ihadar@silexion.com Dr. Mitchell Shirvan Chief Scientific and Development Officer email: mshirvan@silexion.com Mirit Horenshtein-Hadar Chief Financial Officer email: mirit@silexion.com 32