| US FDA Approval of PAPZIMEOS August 18, 2025 |
| 2 Forward-looking Statement This presentation contains “forward-looking” statements within the meaning of the safe harbor provisions of the US Private Securities Litigation Reform Act of 1995. Forward-looking statements can be identified by words such as: “anticipate,” “intend,” “plan,” “goal,” “seek,” “believe,” “project,” “estimate,” “expect,” “strategy,” “future,” “likely,” “may,” “should,” “will” and similar references to future periods. These statements are subject to numerous risks and uncertainties that could cause actual results to differ materially from what the Company expects. Examples of forward-looking statements include, among others, information relating to the Company’s business and business plans, the success of efforts to commercialize PAPZIMEOS (zopapogene imadenovec-drba) for the treatment of recurrent respiratory papillomatosis (RRP) in adults, the Company’s ability to successfully obtain foreign regulatory approvals for PAPZIMEOS, expectations about the safety and efficacy of PAPZIMEOS and the Company’s other product candidates, the timing of clinical trials and their results, the Company’s ability to commence clinical studies or complete ongoing clinical studies, and the ability of PAPZIMEOS to treat RRP. The Company has no obligation to provide any updates to these forward-looking statements even if its expectations change. All forward-looking statements are expressly qualified in their entirety by this cautionary statement. For further information on potential risks and uncertainties, and other important factors, any of which could cause the Company's actual results to differ from those contained in the forward-looking statements, see the section entitled “Risk Factors” in the Company’s most recent Annual Report on Form 10-K and subsequent reports filed with the Securities and Exchange Commission. |
| 3 Call Participants Helen Sabzevari, PhD President and CEO Phil Tennant Chief Commercial Officer Rutul Shah Chief Operating Officer Harry Thomasian Jr. Chief Financial Officer |
| 4 Agenda RRP Landscape PAPZIMEOS Prescribing Information and Label Details PAPZIMEOS Pivotal Clinical Data Commercial Strategy and Launch Plans Q&A |
| 5 First and Only FDA-Approved Therapy for the Treatment of Adults with Recurrent Respiratory Papillomatosis (RRP) www.PAPZIMEOS.com |
| 6 RRP Landscape |
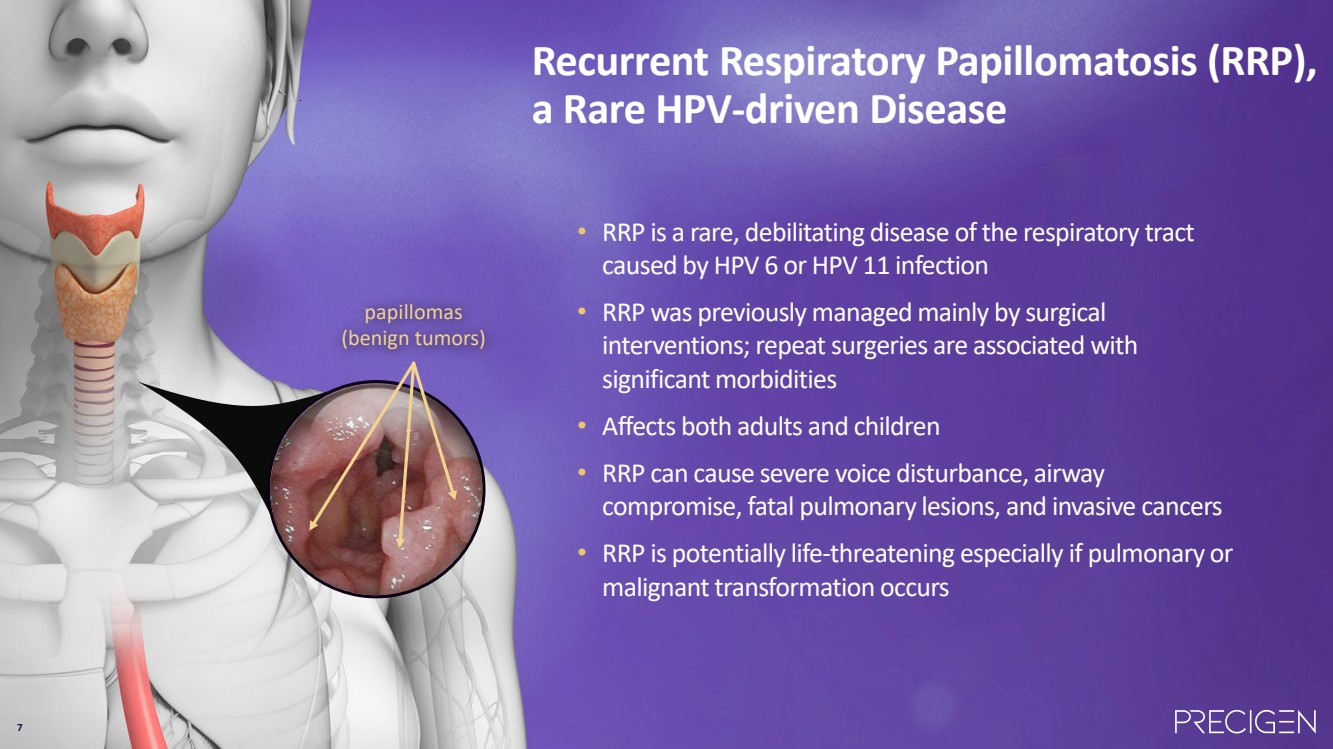
| 7 Recurrent Respiratory Papillomatosis (RRP), a Rare HPV-driven Disease papillomas (benign tumors) • RRP is a rare, debilitating disease of the respiratory tract caused by HPV 6 or HPV 11 infection • RRP was previously managed mainly by surgical interventions; repeat surgeries are associated with significant morbidities • Affects both adults and children • RRP can cause severe voice disturbance, airway compromise, fatal pulmonary lesions, and invasive cancers • RRP is potentially life-threatening especially if pulmonary or malignant transformation occurs 7 |
| 8 Prevalence of RRP in US1 PAPZIMEOS has Potential to Become the Standard-of-care for Adults with RRP 27,000 adult patients in US Approximately 1 Precigen-commissioned research; analysis derived from claims and electronic health record data PAPZIMEOS has the potential to define a new treatment paradigm for adults with RRP |
| 9 PAPZIMEOS Prescribing Information and Label Details |
| 10 First FDA-approved Therapy for Adults with RRP PAPZIMEOS received full approval No requirement for the confirmatory clinical trial BROAD LABEL enables adults with RRP to be eligible for PAPZIMEOS Strong efficacy, durability of response, and favorable safety profile |
| 11 PAPZIMEOS US Prescribing Information Overview INDICATION PAPZIMEOS is a non-replicating adenoviral vector-based immunotherapy indicated for the treatment of adults with recurrent respiratory papillomatosis DOSAGE & ADMINISTRATION PAPZIMEOS is for subcutaneous injection only The recommended dose of PAPZIMEOS is 5×1011 particle units (PU) per injection administered by subcutaneous injection four (4) times over a 12-week interval Full prescribing information is available at www.PAPZIMEOS.com |
| 12 PAPZIMEOS US Prescribing Information Overview Warnings and Precautions Injection-site reactions: Injection-site reactions, have been observed. Monitor patients for local site reactions for at least 30 minutes after the initial treatment. Thrombotic events: Thrombotic events may occur following administration of adenoviral vector-based therapies. Monitor patients for signs and symptoms of thrombotic events and treat events according to clinical practice. Adverse Reactions The most common adverse reactions were injection site reactions, fatigue, chills, pyrexia, myalgia, and nausea. No REMS No Boxed Warnings No Contraindications Full prescribing information is available at www.PAPZIMEOS.com |
| 13 PAPZIMEOS Pivotal Clinical Data |
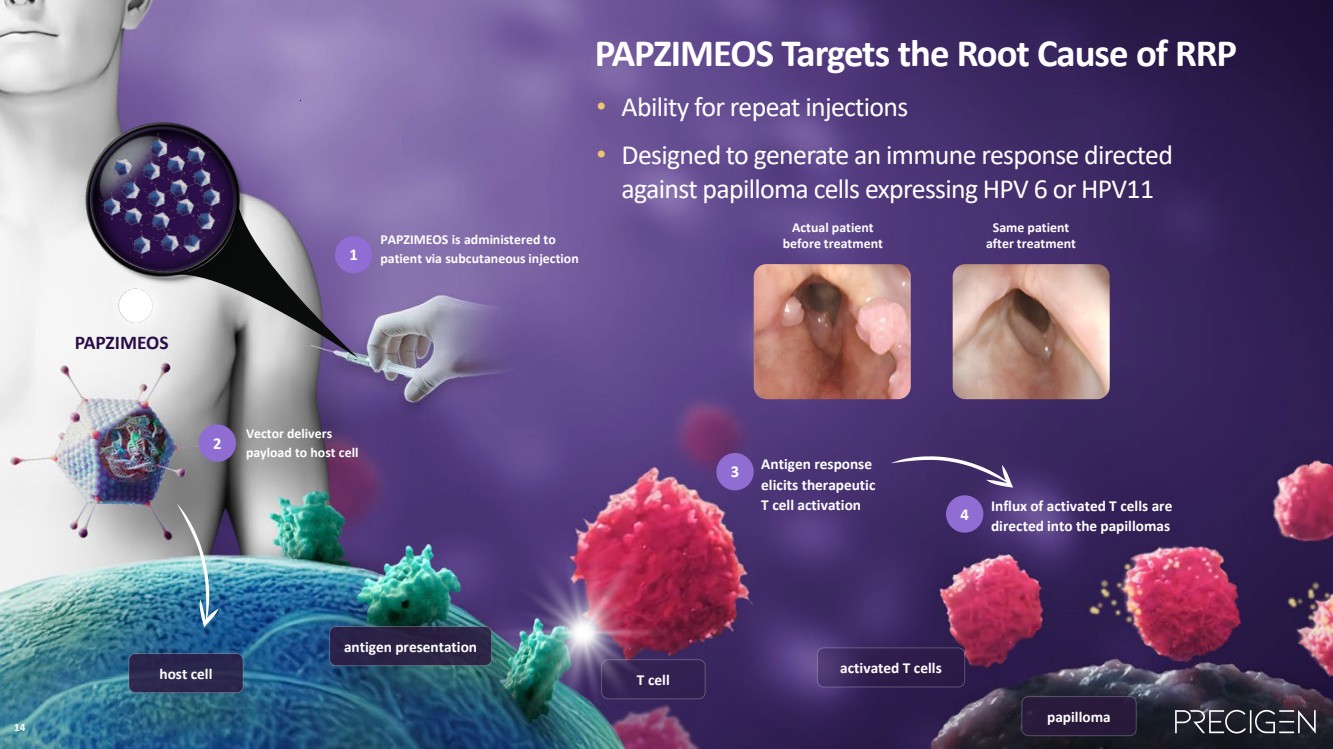
| 14 PAPZIMEOS Targets the Root Cause of RRP • Ability for repeat injections • Designed to generate an immune response directed against papilloma cells expressing HPV 6 or HPV11 antigen presentation Vector delivers payload to host cell host cell Antigen response elicits therapeutic T cell activation 2 3 Influx of activated T cells are directed into the papillomas 4 papilloma T cell activated T cells PAPZIMEOS PAPZIMEOS is administered to 1 patient via subcutaneous injection Actual patient before treatment Same patient after treatment 14 |
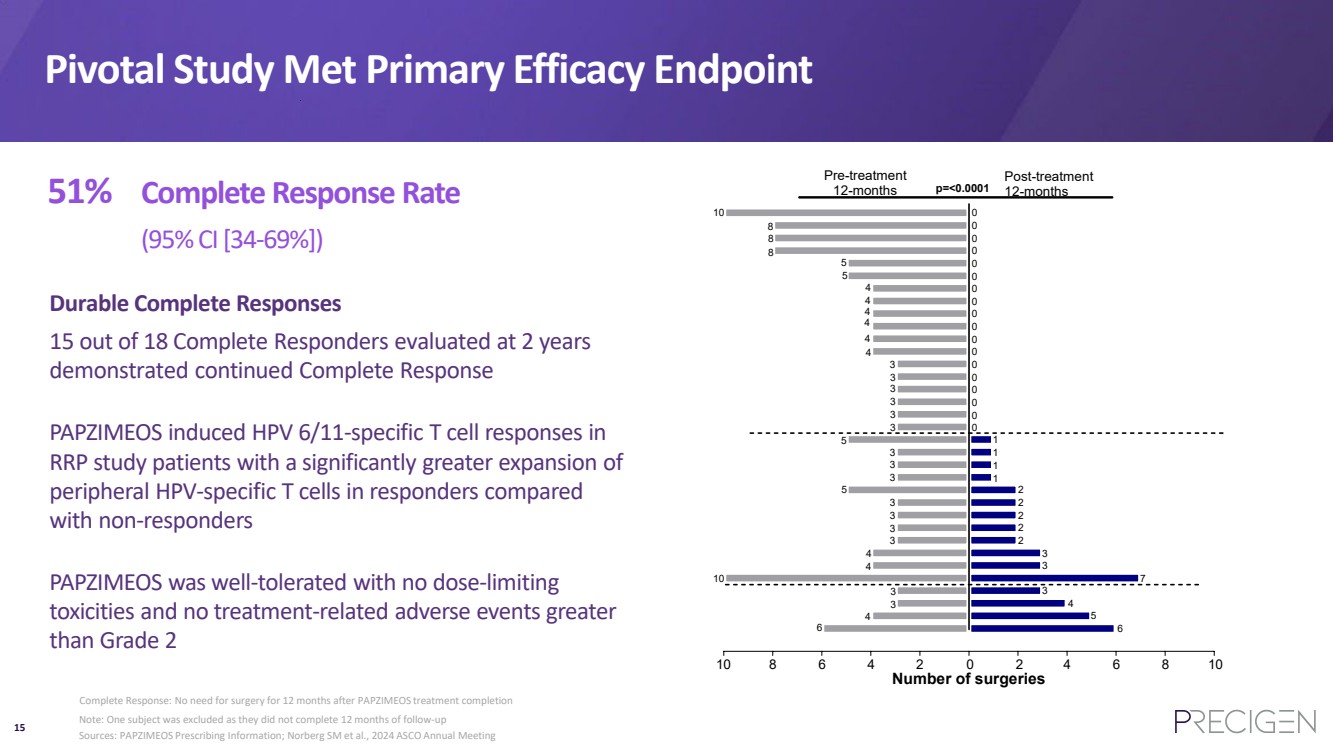
| 15 Pivotal Study Met Primary Efficacy Endpoint Number of surgeries 6 10 0 10 Pre-treatment 12-months Post-treatment 12-months 8 8 3 4 10 5 3 7 4 8 6 4 2 2 4 6 8 p=<0.0001 8 10 3 4 4 3 3 3 3 3 3 4 4 6 3 3 4 3 3 1 5 3 4 4 0 3 3 4 2 3 5 5 5 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 1 1 1 2 2 2 2 3 Note: One subject was excluded as they did not complete 12 months of follow-up Complete Response: No need for surgery for 12 months after PAPZIMEOS treatment completion 51% Complete Response Rate (95% CI [34-69%]) PAPZIMEOS was well-tolerated with no dose-limiting toxicities and no treatment-related adverse events greater than Grade 2 Durable Complete Responses 15 out of 18 Complete Responders evaluated at 2 years demonstrated continued Complete Response PAPZIMEOS induced HPV 6/11-specific T cell responses in RRP study patients with a significantly greater expansion of peripheral HPV-specific T cells in responders compared with non-responders Sources: PAPZIMEOS Prescribing Information; Norberg SM et al., 2024 ASCO Annual Meeting |
| 16 Commercial Strategy and Launch Plans |
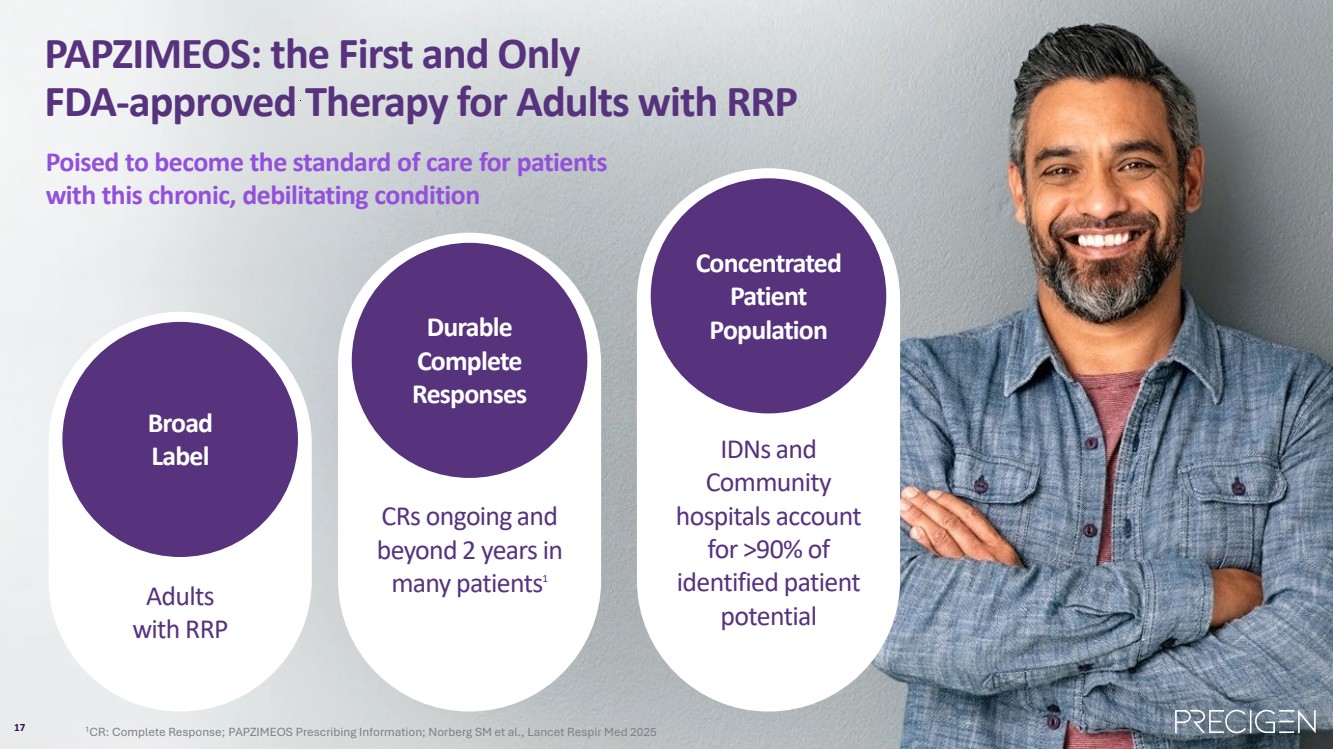
| 17 PAPZIMEOS: the First and Only FDA-approved Therapy for Adults with RRP Poised to become the standard of care for patients with this chronic, debilitating condition Broad Label Adults with RRP Durable Complete Responses CRs ongoing and beyond 2 years in many patients1 Concentrated Patient Population IDNs and Community hospitals account for >90% of identified patient potential 17 1CR: Complete Response; PAPZIMEOS Prescribing Information; Norberg SM et al., Lancet Respir Med 2025 |
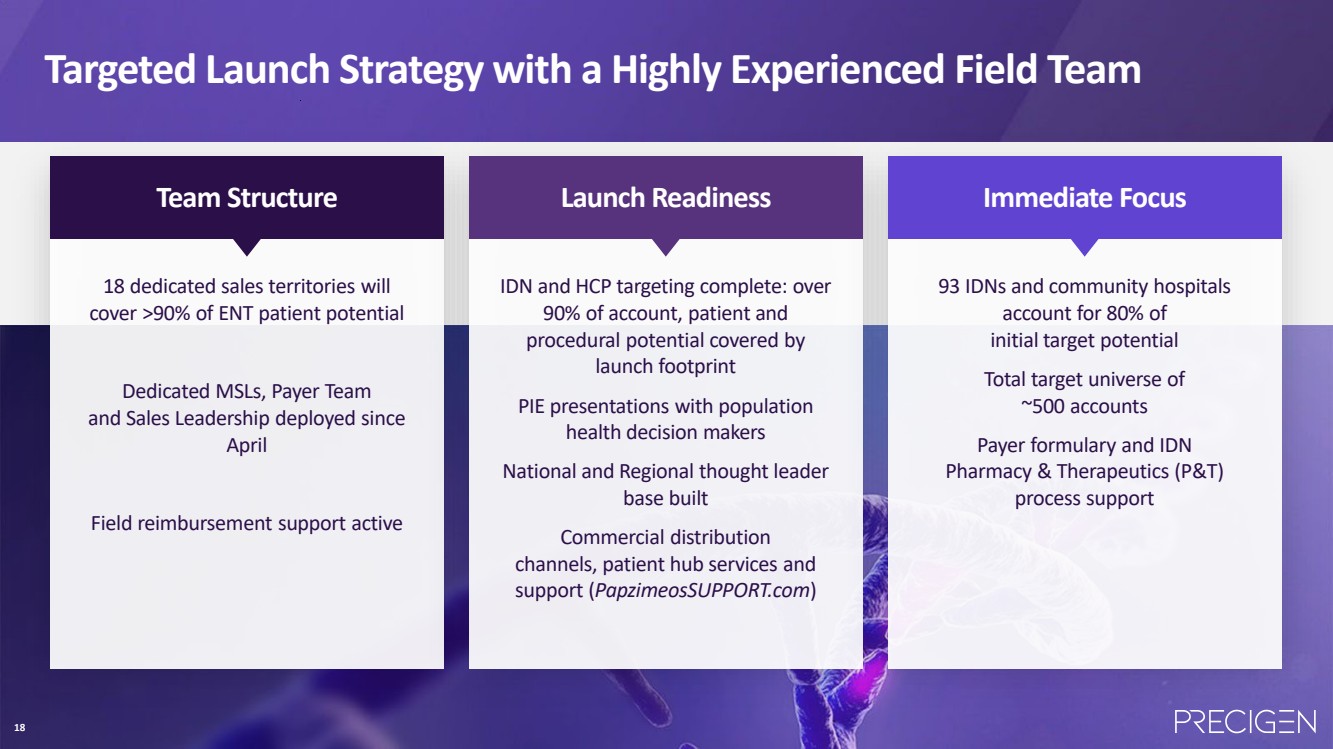
| 18 18 dedicated sales territories will cover >90% of ENT patient potential Dedicated MSLs, Payer Team and Sales Leadership deployed since April Field reimbursement support active IDN and HCP targeting complete: over 90% of account, patient and procedural potential covered by launch footprint PIE presentations with population health decision makers National and Regional thought leader base built Commercial distribution channels, patient hub services and support (PapzimeosSUPPORT.com) 93 IDNs and community hospitals account for 80% of initial target potential Total target universe of ~500 accounts Payer formulary and IDN Pharmacy & Therapeutics (P&T) process support Team Structure Launch Readiness Immediate Focus Targeted Launch Strategy with a Highly Experienced Field Team 18 |
| 19 Key Indicators of Launch Success KOL asset advocacy Prescription dynamics at HCP and Institutional level Formulary status / covered lives with favorable access Patient Hub dynamics |
| 20 Comprehensive PAPZIMEOS Support Program for HCPs and Patients Treatment Education Support through the treatment process, including education about PAPZIMEOS and help locating and coordinating treatment Insurance Navigation Support during the process of determining a patient’s insurance benefits for PAPZIMEOS and eligibility for affordability programs Order Support Help coordinating ordering and delivery of PAPZIMEOS via specialty pharmacy services Understanding Coverage Help understanding the insurance process and what information their health plan may need Financial Assistance Information about available resources that may help to reduce or eliminate their out-of-pocket costs Support for HCPs Support for Patients 20 Papzimeos SUPPORT can help support your patients and care team at any site of care throughout the access process. Download the enrollment form at www.PapzimeosSUPPORT.com. For questions or support, call (866) 827-8180, Monday to Friday, 8 am to 8 pm ET. |
| 21 PAPZIMEOS Offers a Significant Value Proposition First and Only FDA-approved RRP Treatment Transformative Clinical Benefit Chronic, Debilitating Rare Disease Committed to Serving RRP Community Our goal is to ensure every eligible RRP patient has access to PAPZIMEOS |
| 22 Q&A |
| 23 “This long -awaited FDA approval represents a momentous milestone for the RRP community. For the first time, adult patients with RRP have access to an FDA -approved therapy that offers the potential to reduce — or even eliminate —endless repeated surgeries. This breakthrough brings long -overdue hope to patients and families who have endured so much.” Kim McClellan President, RRP Foundation 23 |
| 24 |