

Corporate Presentation August 2025 Exhibit 99.2

Forward Looking Statements This presentation contains “forward-looking” statements within the meaning of the “safe harbor” provisions of the Private Securities Litigation Reform Act of 1995, including, but not limited to, anticipated discovery, preclinical and clinical development activities for Prelude’s product candidates, the potential safety, efficacy, benefits and addressable market for Prelude’s product candidates, the expected timeline for clinical trial results for Prelude’s product candidates including its SMARCA2 degrader molecules. Any statements contained herein or provided orally that are not statements of historical fact may be deemed to be forward-looking statements. In some cases, you can identify forward-looking statements by such terminology as ‘‘believe,’’ ‘‘may,’’ ‘‘will,’’ ‘‘potentially,’’ ‘‘estimate,’’ ‘‘continue,’’ ‘‘anticipate,’’ ‘‘intend,’’ ‘‘could,’’ ‘‘would,’’ ‘‘project,’’ ‘‘plan,’’ ‘‘expect’’ and similar expressions that convey uncertainty of future events or outcomes, although not all forward-looking statements contain these words. Statements, including forward-looking statements, speak only to the date they are provided (unless an earlier date is indicated). This presentation shall not constitute an offer to sell or the solicitation of an offer to buy, nor shall there be any sale of these securities in any state or other jurisdiction in which such offer, solicitation or sale would be unlawful prior to registration or qualification under the securities laws of any such state or other jurisdiction. These forward-looking statements are based on the beliefs of our management as well as assumptions made by and information currently available to us. Although we believe the expectations reflected in such forward-looking statements are reasonable, we can give no assurance that such expectations will prove to be correct. If such assumptions do not fully materialize or prove incorrect, the events or circumstances referred to in the forward-looking statements may not occur. We undertake no obligation to update publicly any forward-looking statements for any reason after the date of this presentation to conform these statements to actual results or to changes in our expectations, except as required by law. Accordingly, readers are cautioned not to place undue reliance on these forward-looking statements. Additional risks and uncertainties that could affect our business are included under the caption “Risk Factors” in our filings with the Securities and Exchange Commission, including our Annual Report on Form 10-K for the year ended December 31, 2023.

We are on a mission to extend the promise of precision medicine to every cancer patient in need Select the best modality to precisely target oncogenic mechanisms Strive for first-in-class science focused on areas of highest patient unmet need Draw on decades of experience and proven leadership to drive innovation

Kris Vaddi, PhD Chief Executive Officer Jane Huang M.D. President and Chief Medical Officer Andrew Combs, PhD Chief Chemistry Officer Sean Brusky, MBA Chief Business Officer Peggy Scherle, PhD Chief Scientific Officer Bryant Lim, J.D. Chief Financial Officer, Chief Legal Officer, and Corporate Secretary Experienced Leadership Team With Proven Track Records in Precision Oncology
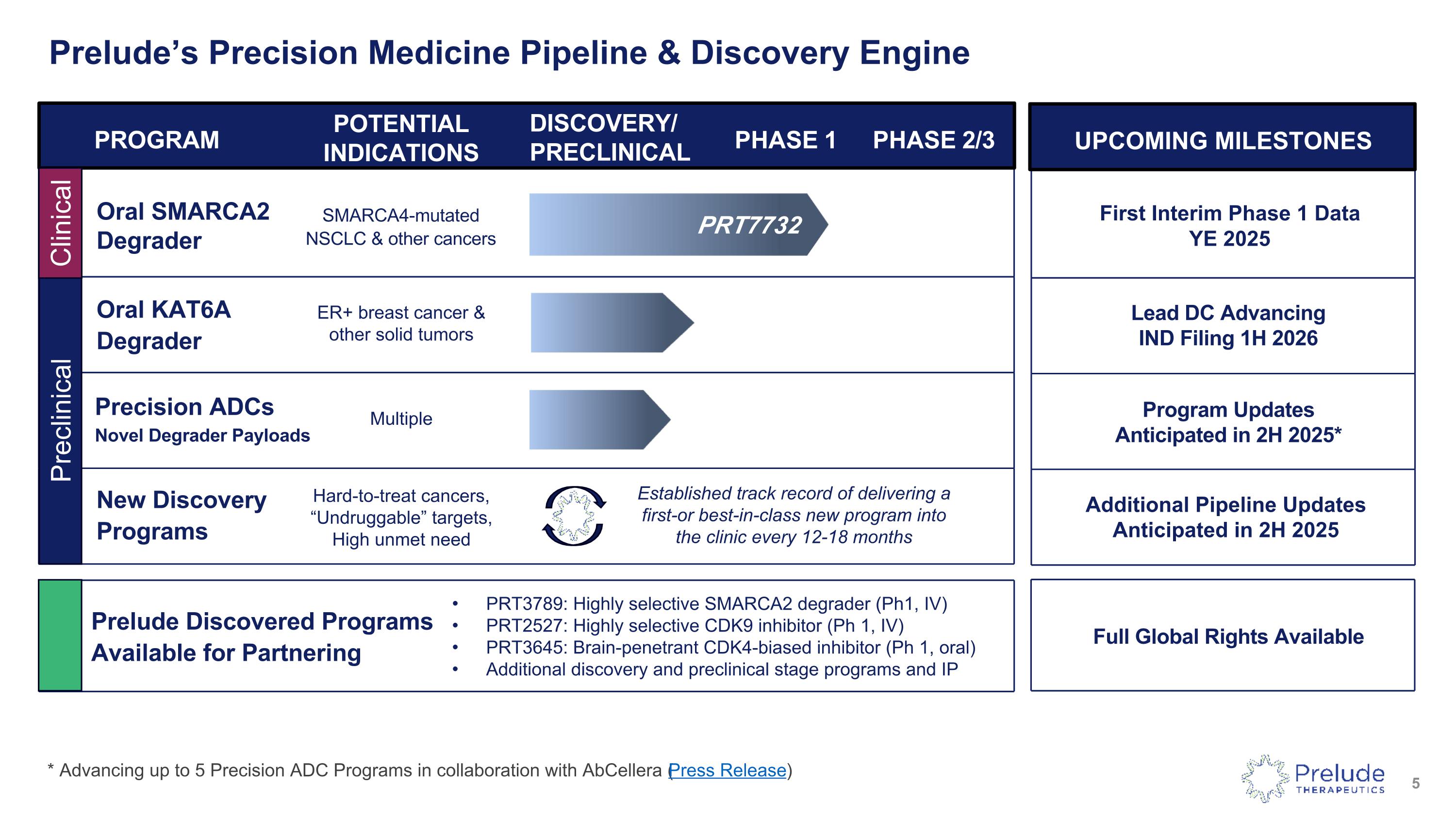
Prelude’s Precision Medicine Pipeline & Discovery Engine PROGRAM POTENTIAL INDICATIONS DISCOVERY/ PRECLINICAL PHASE 1 PHASE 2/3 Oral SMARCA2 Degrader SMARCA4-mutated NSCLC & other cancers Oral KAT6A Degrader UPCOMING MILESTONES Lead DC Advancing IND Filing 1H 2026 First Interim Phase 1 Data YE 2025 ER+ breast cancer & other solid tumors * Advancing up to 5 Precision ADC Programs in collaboration with AbCellera (Press Release) PRT7732 Full Global Rights Available Precision ADCs Novel Degrader Payloads Multiple Program Updates Anticipated in 2H 2025* Prelude Discovered Programs Available for Partnering PRT3789: Highly selective SMARCA2 degrader (Ph1, IV) PRT2527: Highly selective CDK9 inhibitor (Ph 1, IV) PRT3645: Brain-penetrant CDK4-biased inhibitor (Ph 1, oral) Additional discovery and preclinical stage programs and IP New Discovery Programs Additional Pipeline Updates Anticipated in 2H 2025 Hard-to-treat cancers, “Undruggable” targets, High unmet need Preclinical Established track record of delivering a first-or best-in-class new program into the clinic every 12-18 months Clinical
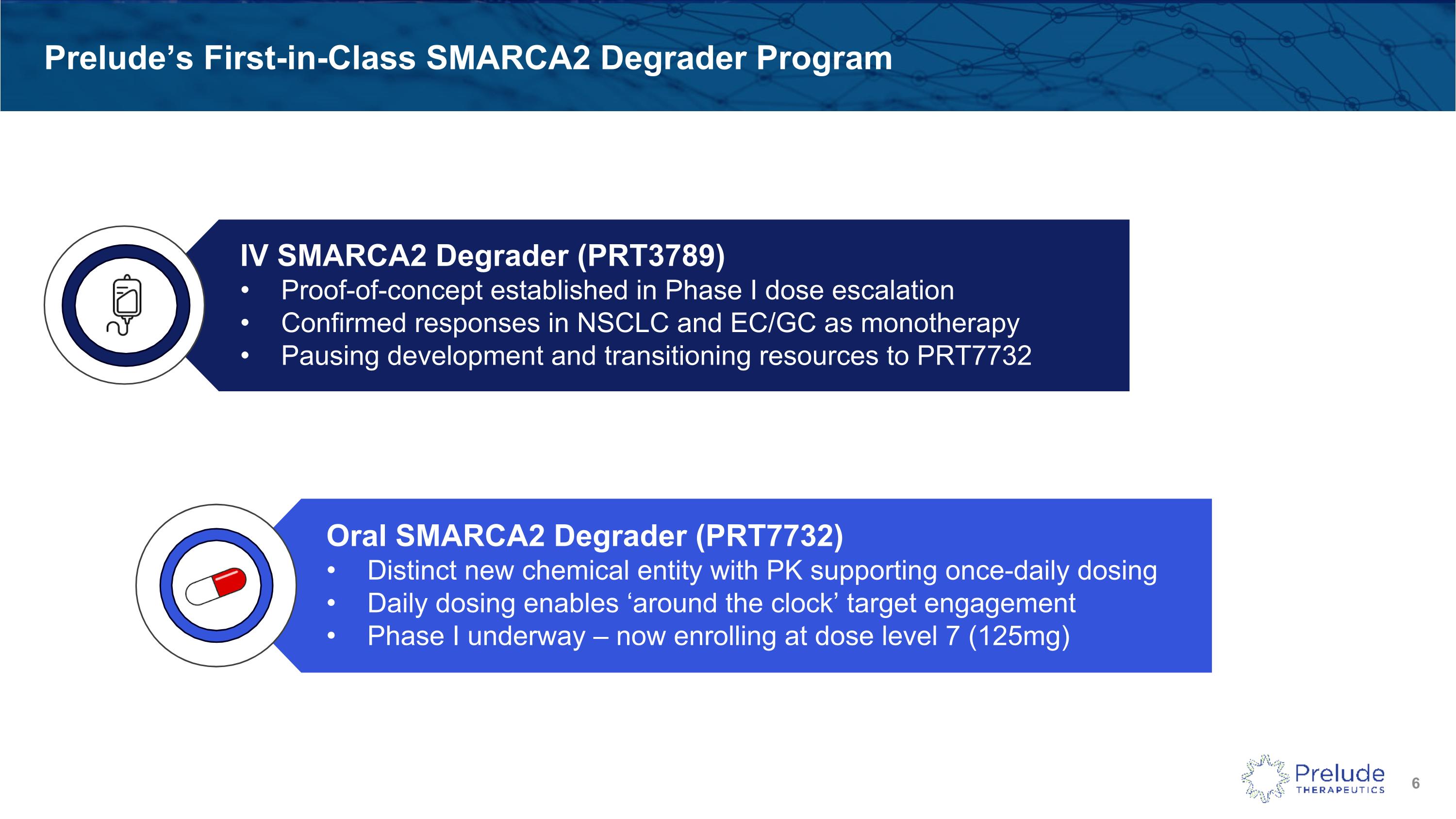
Prelude’s First-in-Class SMARCA2 Degrader Program Oral SMARCA2 Degrader (PRT7732) Distinct new chemical entity with PK supporting once-daily dosing Daily dosing enables ‘around the clock’ target engagement Phase I underway – now enrolling at dose level 7 (125mg) IV SMARCA2 Degrader (PRT3789) Proof-of-concept established in Phase I dose escalation Confirmed responses in NSCLC and EC/GC as monotherapy Pausing development and transitioning resources to PRT7732
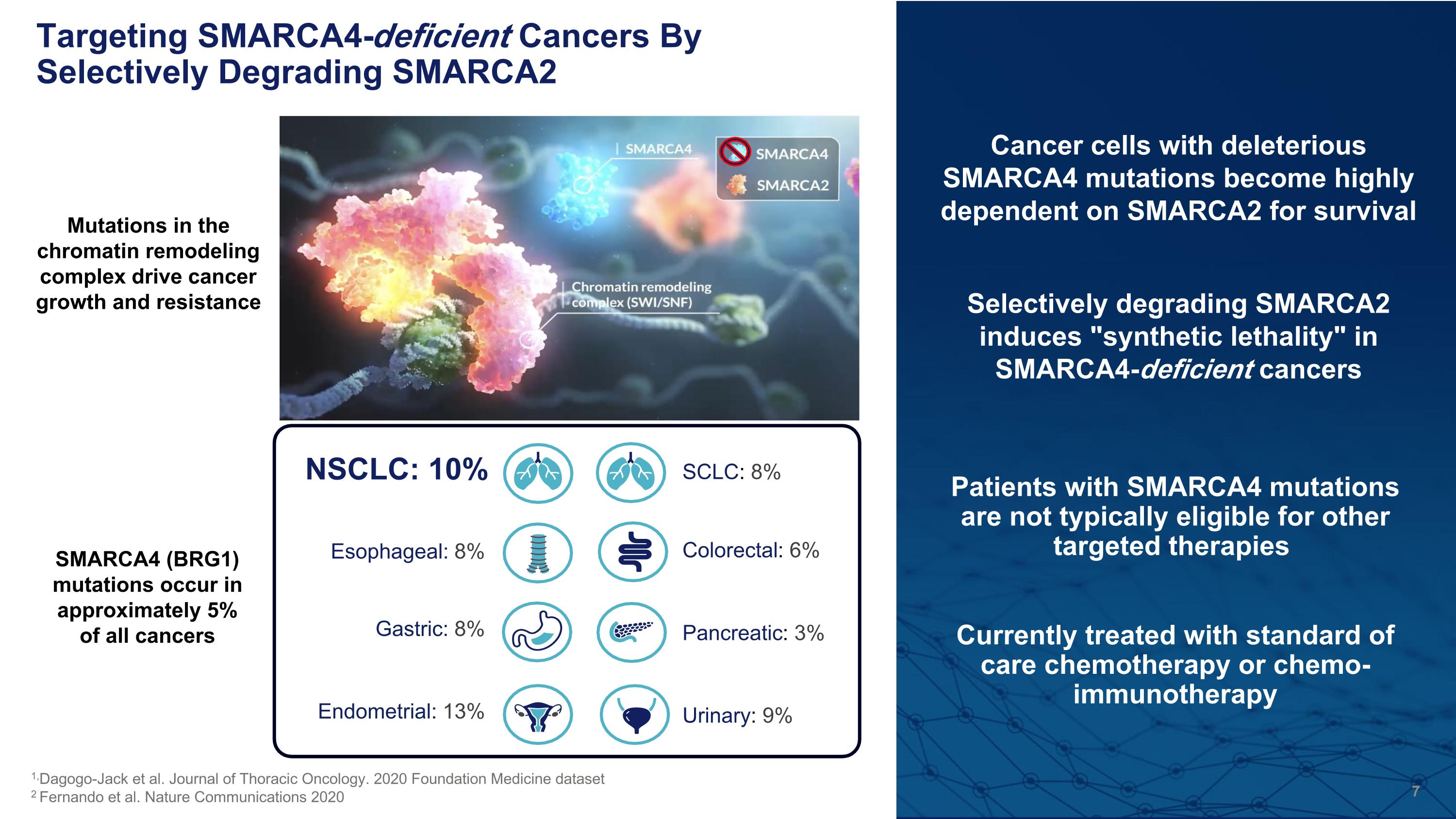
Patients with SMARCA4 mutations are not typically eligible for other targeted therapies Currently treated with standard of care chemotherapy or chemo-immunotherapy Targeting SMARCA4-deficient Cancers By Selectively Degrading SMARCA2 Pancreatic: 3% NSCLC: 10% Esophageal: 8% Gastric: 8% Endometrial: 13% SCLC: 8% Urinary: 9% Colorectal: 6% 1,Dagogo-Jack et al. Journal of Thoracic Oncology. 2020 Foundation Medicine dataset 2 Fernando et al. Nature Communications 2020 SMARCA4 (BRG1) mutations occur in approximately 5% of all cancers Mutations in the chromatin remodeling complex drive cancer growth and resistance Cancer cells with deleterious SMARCA4 mutations become highly dependent on SMARCA2 for survival Selectively degrading SMARCA2 induces "synthetic lethality" in SMARCA4-deficient cancers
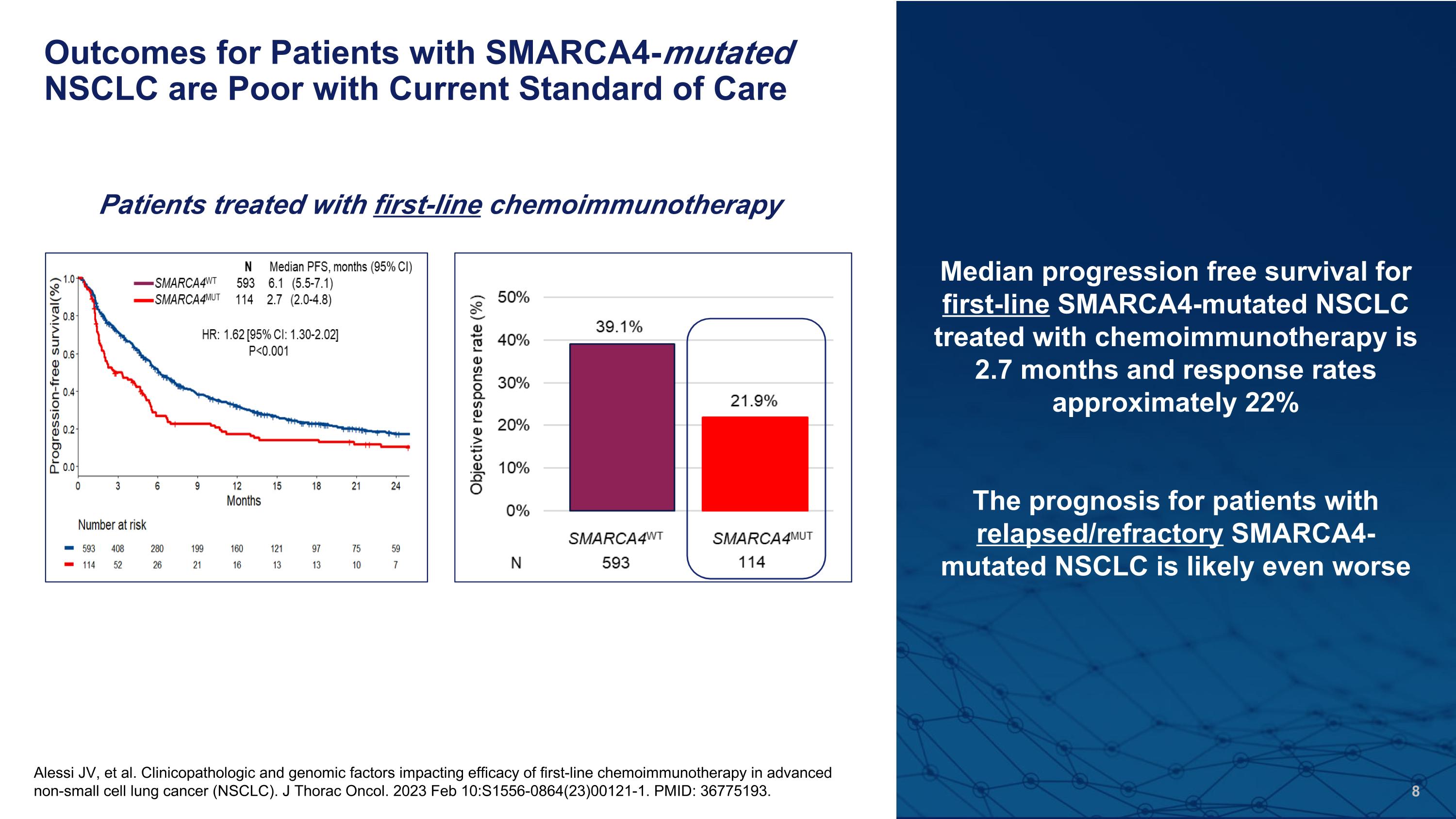
Outcomes for Patients with SMARCA4-mutated NSCLC are Poor with Current Standard of Care Alessi JV, et al. Clinicopathologic and genomic factors impacting efficacy of first-line chemoimmunotherapy in advanced non-small cell lung cancer (NSCLC). J Thorac Oncol. 2023 Feb 10:S1556-0864(23)00121-1. PMID: 36775193. Median progression free survival for first-line SMARCA4-mutated NSCLC treated with chemoimmunotherapy is 2.7 months and response rates approximately 22% The prognosis for patients with relapsed/refractory SMARCA4-mutated NSCLC is likely even worse Patients treated with first-line chemoimmunotherapy
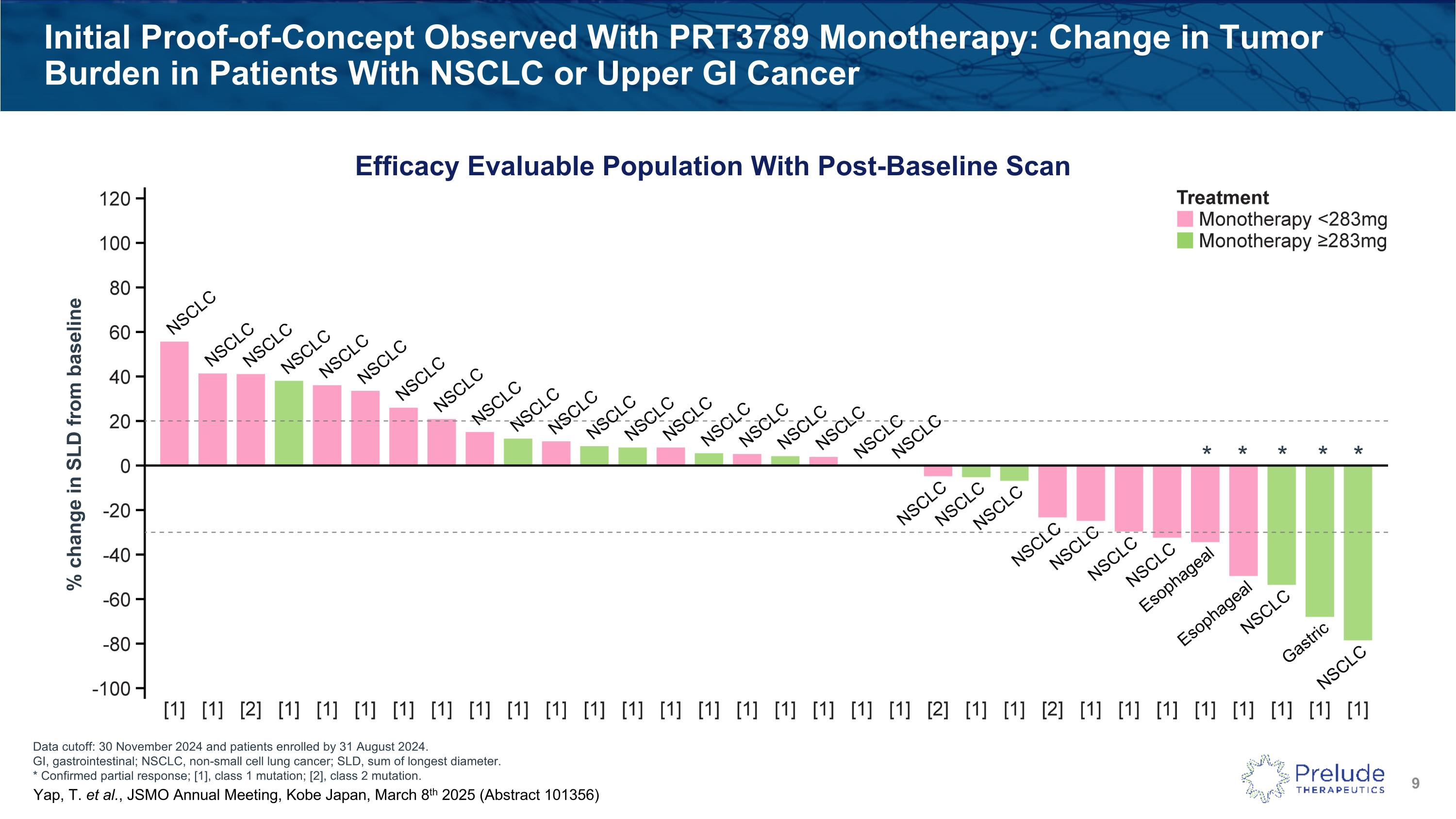
Data cutoff: 30 November 2024 and patients enrolled by 31 August 2024. GI, gastrointestinal; NSCLC, non-small cell lung cancer; SLD, sum of longest diameter. * Confirmed partial response; [1], class 1 mutation; [2], class 2 mutation. Initial Proof-of-Concept Observed With PRT3789 Monotherapy: Change in Tumor Burden in Patients With NSCLC or Upper GI Cancer Efficacy Evaluable Population With Post-Baseline Scan % change in SLD from baseline * * * * * Yap, T. et al., JSMO Annual Meeting, Kobe Japan, March 8th 2025 (Abstract 101356)
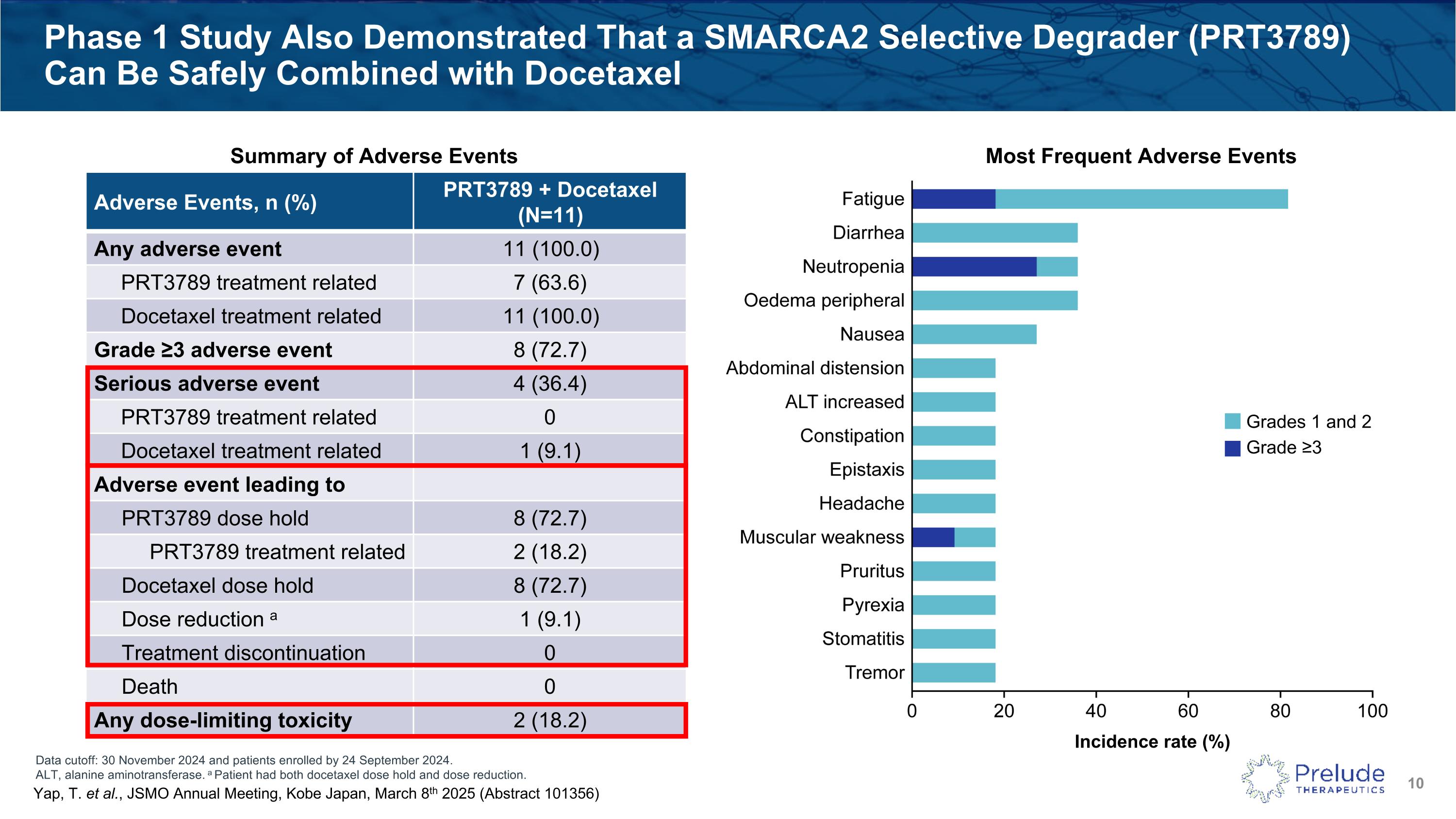
Data cutoff: 30 November 2024 and patients enrolled by 24 September 2024. ALT, alanine aminotransferase. a Patient had both docetaxel dose hold and dose reduction. Phase 1 Study Also Demonstrated That a SMARCA2 Selective Degrader (PRT3789) Can Be Safely Combined with Docetaxel Adverse Events, n (%) PRT3789 + Docetaxel (N=11) Any adverse event 11 (100.0) PRT3789 treatment related 7 (63.6) Docetaxel treatment related 11 (100.0) Grade ≥3 adverse event 8 (72.7) Serious adverse event 4 (36.4) PRT3789 treatment related 0 Docetaxel treatment related 1 (9.1) Adverse event leading to PRT3789 dose hold 8 (72.7) PRT3789 treatment related 2 (18.2) Docetaxel dose hold 8 (72.7) Dose reduction a 1 (9.1) Treatment discontinuation 0 Death 0 Any dose-limiting toxicity 2 (18.2) Summary of Adverse Events Most Frequent Adverse Events Incidence rate (%) Grades 1 and 2 Grade ≥3 Yap, T. et al., JSMO Annual Meeting, Kobe Japan, March 8th 2025 (Abstract 101356)
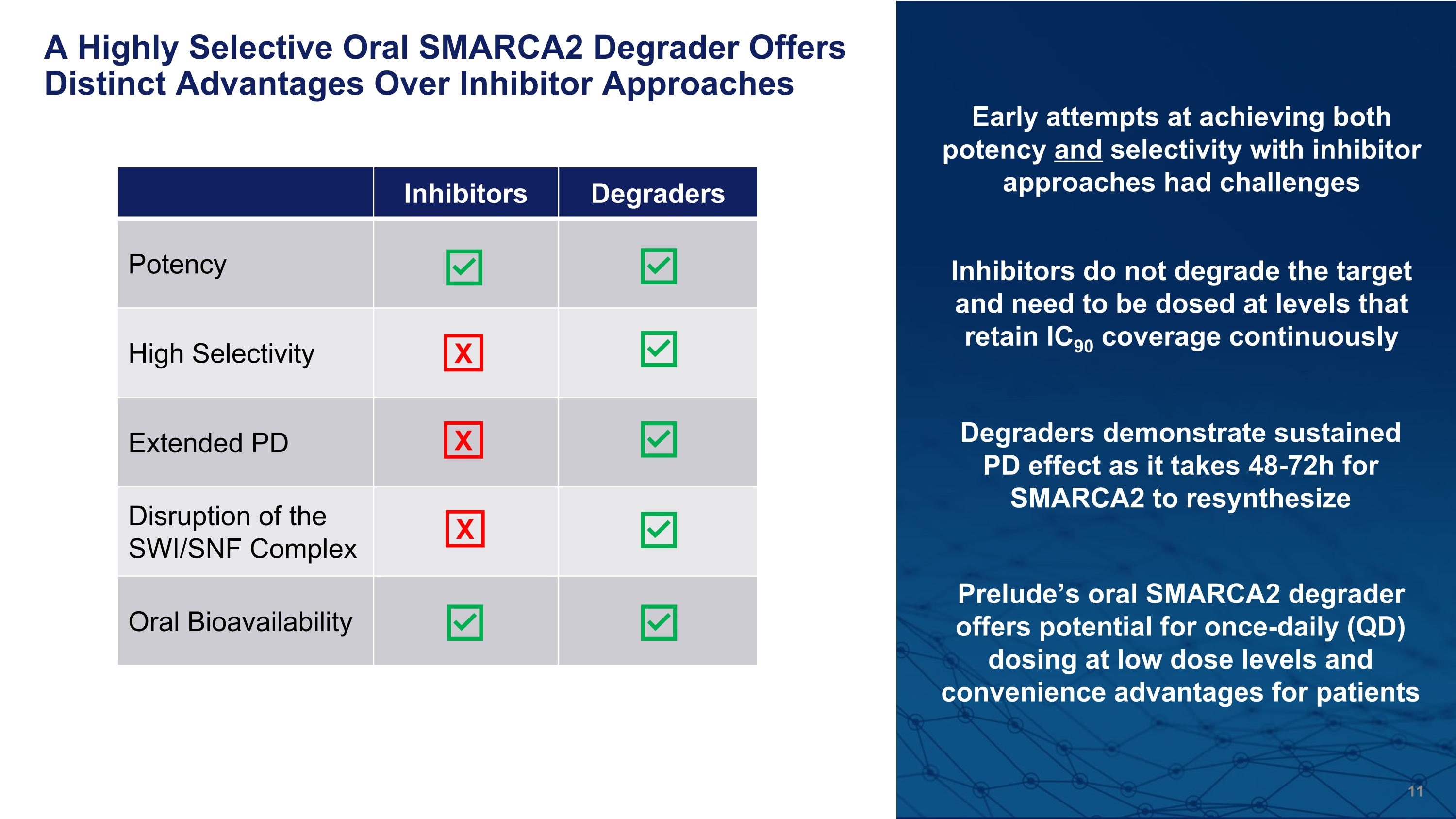
A Highly Selective Oral SMARCA2 Degrader Offers Distinct Advantages Over Inhibitor Approaches Inhibitors Degraders Potency High Selectivity Extended PD Disruption of the SWI/SNF Complex Oral Bioavailability X X Early attempts at achieving both potency and selectivity with inhibitor approaches had challenges Inhibitors do not degrade the target and need to be dosed at levels that retain IC90 coverage continuously Degraders demonstrate sustained PD effect as it takes 48-72h for SMARCA2 to resynthesize Prelude’s oral SMARCA2 degrader offers potential for once-daily (QD) dosing at low dose levels and convenience advantages for patients X
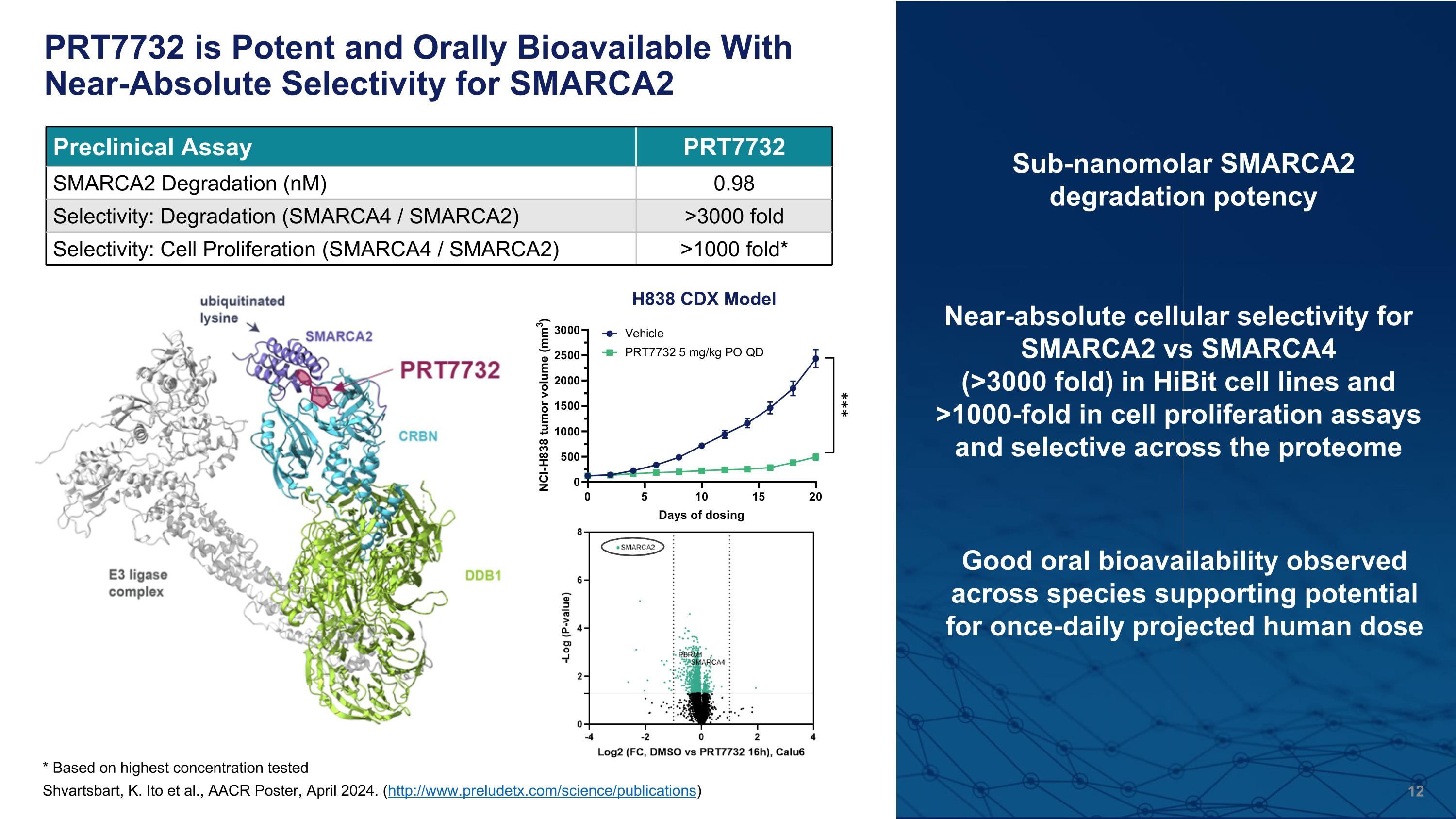
PRT7732 is Potent and Orally Bioavailable With Near-Absolute Selectivity for SMARCA2 Near-absolute cellular selectivity for SMARCA2 vs SMARCA4 (>3000 fold) in HiBit cell lines and >1000-fold in cell proliferation assays and selective across the proteome Sub-nanomolar SMARCA2 degradation potency * Based on highest concentration tested Good oral bioavailability observed across species supporting potential for once-daily projected human dose Preclinical Assay PRT7732 SMARCA2 Degradation (nM) 0.98 Selectivity: Degradation (SMARCA4 / SMARCA2) >3000 fold Selectivity: Cell Proliferation (SMARCA4 / SMARCA2) >1000 fold* Shvartsbart, K. Ito et al., AACR Poster, April 2024. (http://www.preludetx.com/science/publications) H838 CDX Model
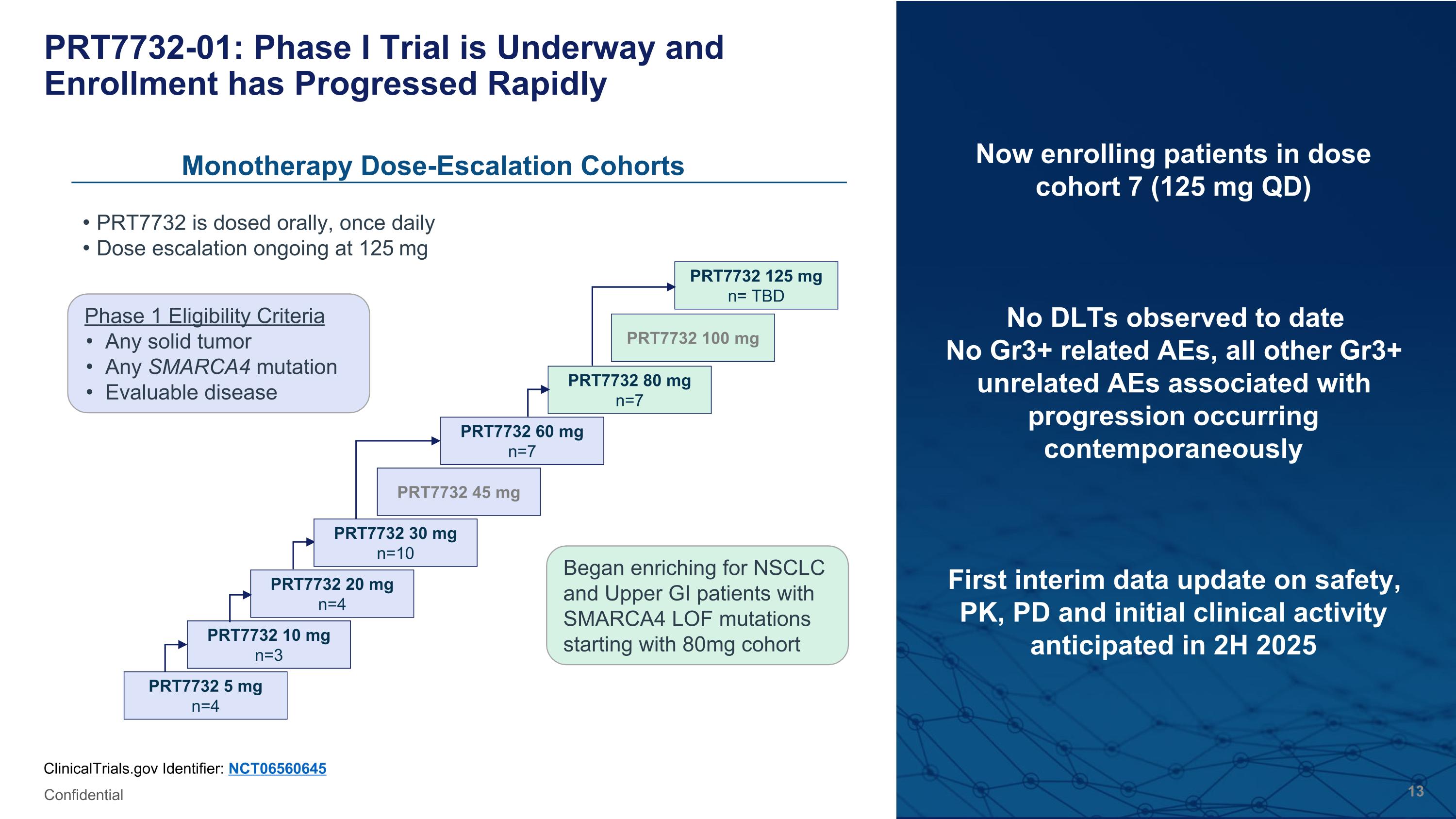
PRT7732-01: Phase I Trial is Underway and Enrollment has Progressed Rapidly Now enrolling patients in dose cohort 7 (125 mg QD) No DLTs observed to date No Gr3+ related AEs, all other Gr3+ unrelated AEs associated with progression occurring contemporaneously First interim data update on safety, PK, PD and initial clinical activity anticipated in 2H 2025 ClinicalTrials.gov Identifier: NCT06560645 Confidential Monotherapy Dose-Escalation Cohorts Phase 1 Eligibility Criteria Any solid tumor Any SMARCA4 mutation Evaluable disease PRT7732 is dosed orally, once daily Dose escalation ongoing at 125 mg PRT7732 5 mg n=4 PRT7732 10 mg n=3 PRT7732 20 mg n=4 PRT7732 45 mg PRT7732 60 mg n=7 PRT7732 80 mg n=7 PRT7732 100 mg PRT7732 125 mg n= TBD PRT7732 30 mg n=10 Began enriching for NSCLC and Upper GI patients with SMARCA4 LOF mutations starting with 80mg cohort
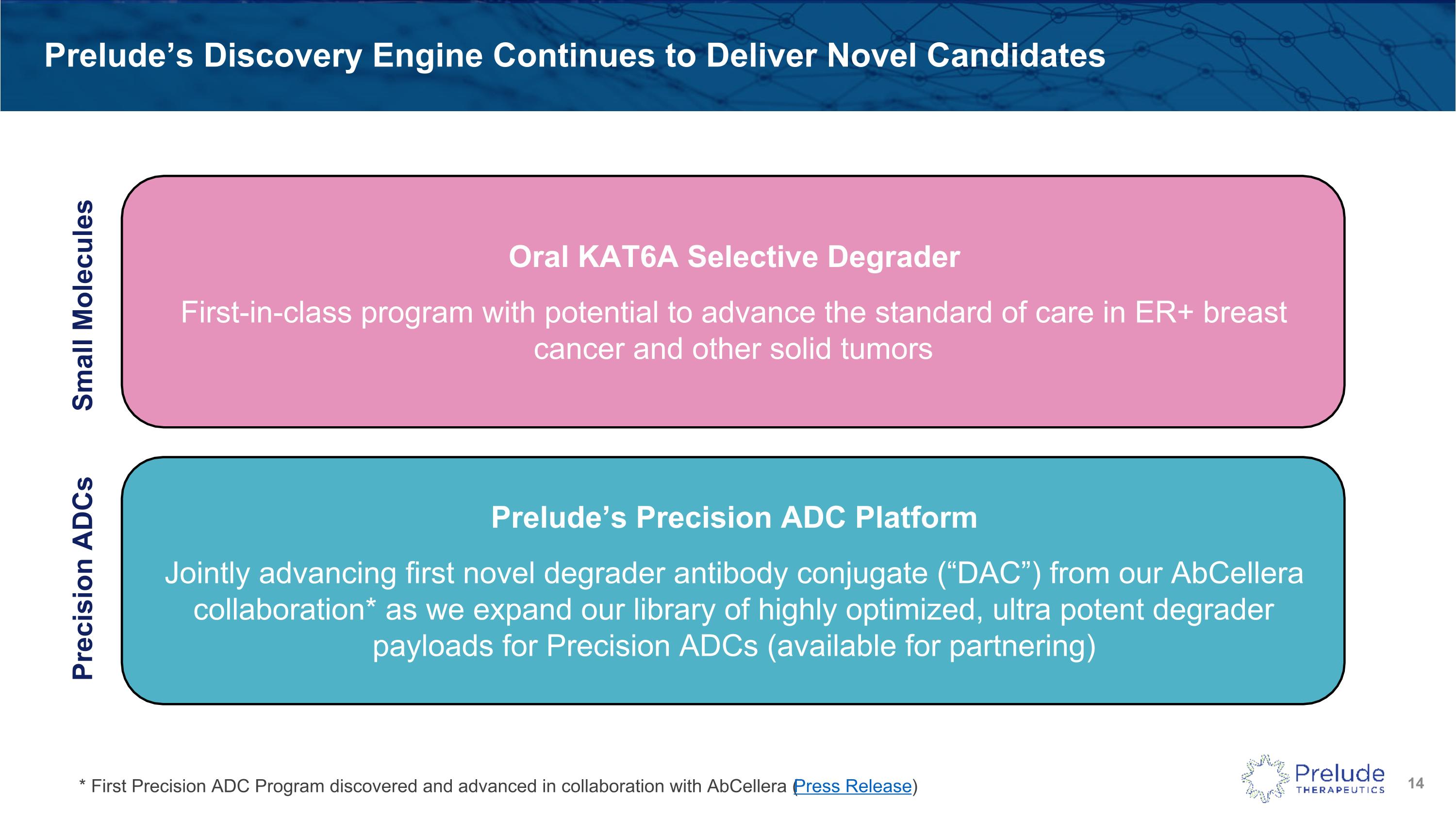
Prelude’s Discovery Engine Continues to Deliver Novel Candidates Oral KAT6A Selective Degrader First-in-class program with potential to advance the standard of care in ER+ breast cancer and other solid tumors Prelude’s Precision ADC Platform Jointly advancing first novel degrader antibody conjugate (“DAC”) from our AbCellera collaboration* as we expand our library of highly optimized, ultra potent degrader payloads for Precision ADCs (available for partnering) Small Molecules Precision ADCs * First Precision ADC Program discovered and advanced in collaboration with AbCellera (Press Release)
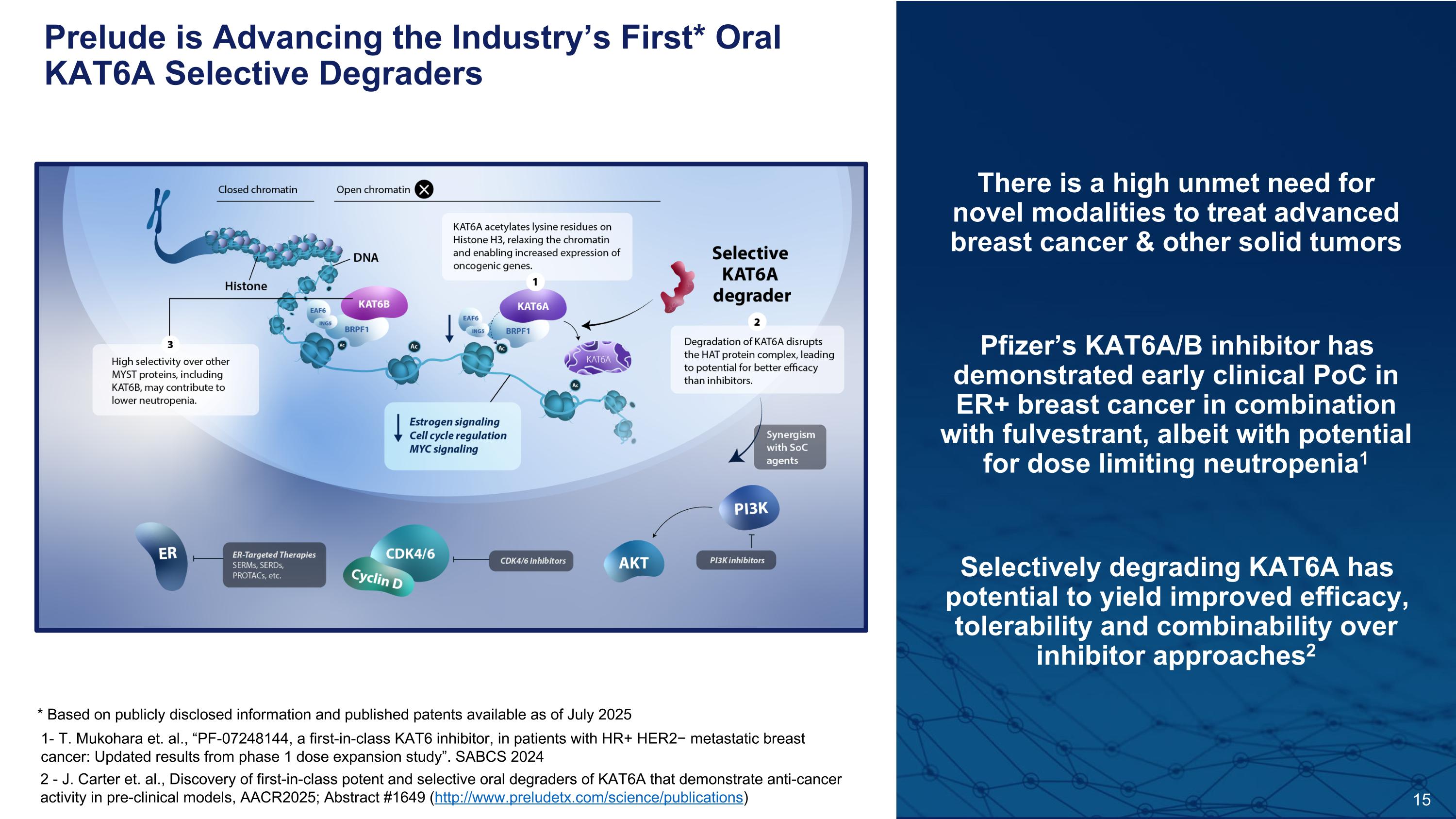
15 There is a high unmet need for novel modalities to treat advanced breast cancer & other solid tumors Pfizer’s KAT6A/B inhibitor has demonstrated early clinical PoC in ER+ breast cancer in combination with fulvestrant, albeit with potential for dose limiting neutropenia1 Selectively degrading KAT6A has potential to yield improved efficacy, tolerability and combinability over inhibitor approaches2 * Based on publicly disclosed information and published patents available as of July 2025 2 - J. Carter et. al., Discovery of first-in-class potent and selective oral degraders of KAT6A that demonstrate anti-cancer activity in pre-clinical models, AACR2025; Abstract #1649 (http://www.preludetx.com/science/publications) 1- T. Mukohara et. al., “PF-07248144, a first-in-class KAT6 inhibitor, in patients with HR+ HER2− metastatic breast cancer: Updated results from phase 1 dose expansion study”. SABCS 2024 Prelude is Advancing the Industry’s First* Oral KAT6A Selective Degraders
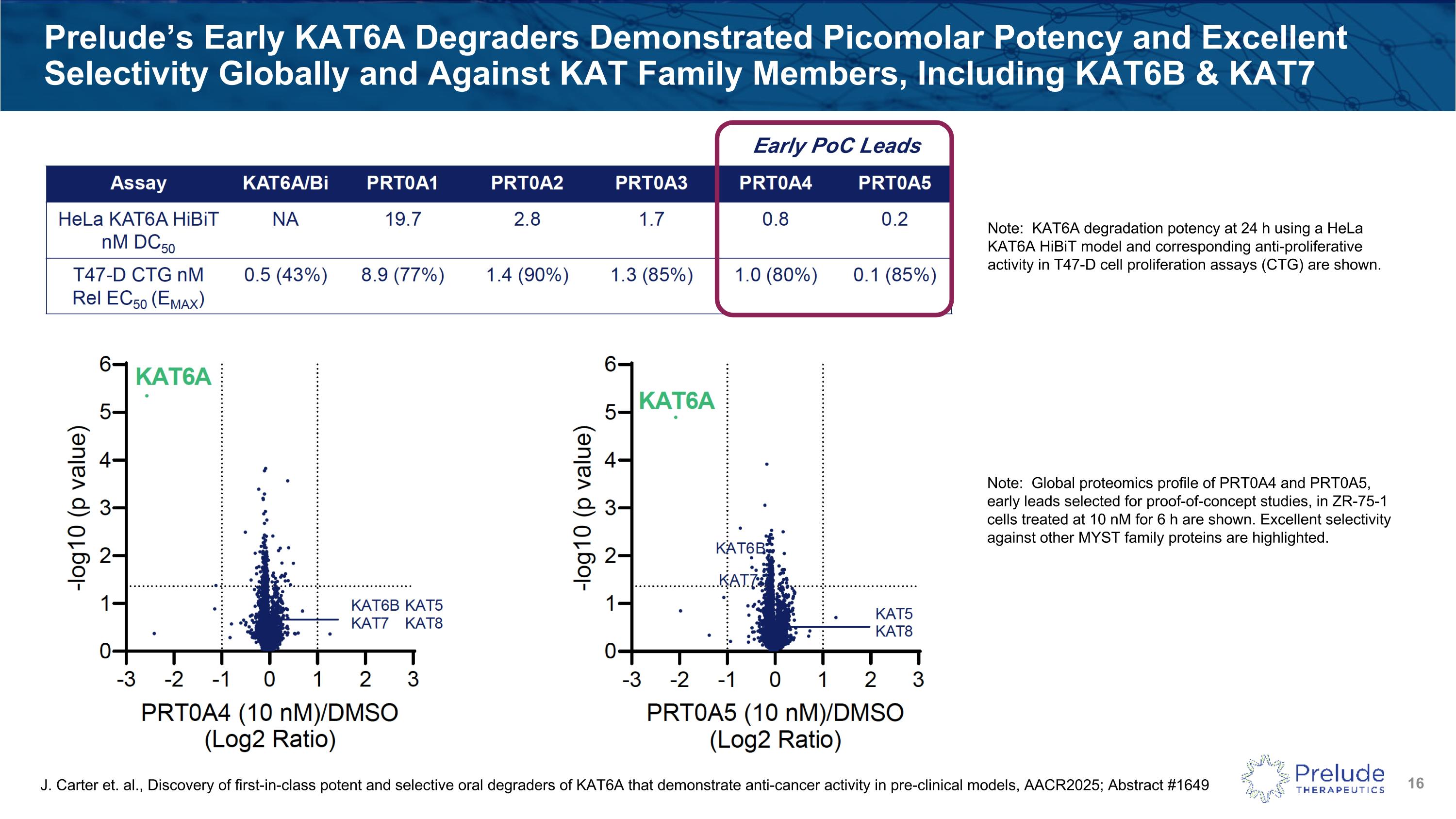
Prelude’s Early KAT6A Degraders Demonstrated Picomolar Potency and Excellent Selectivity Globally and Against KAT Family Members, Including KAT6B & KAT7 J. Carter et. al., Discovery of first-in-class potent and selective oral degraders of KAT6A that demonstrate anti-cancer activity in pre-clinical models, AACR2025; Abstract #1649 Note: KAT6A degradation potency at 24 h using a HeLa KAT6A HiBiT model and corresponding anti-proliferative activity in T47-D cell proliferation assays (CTG) are shown. Early PoC Leads Note: Global proteomics profile of PRT0A4 and PRT0A5, early leads selected for proof-of-concept studies, in ZR-75-1 cells treated at 10 nM for 6 h are shown. Excellent selectivity against other MYST family proteins are highlighted.
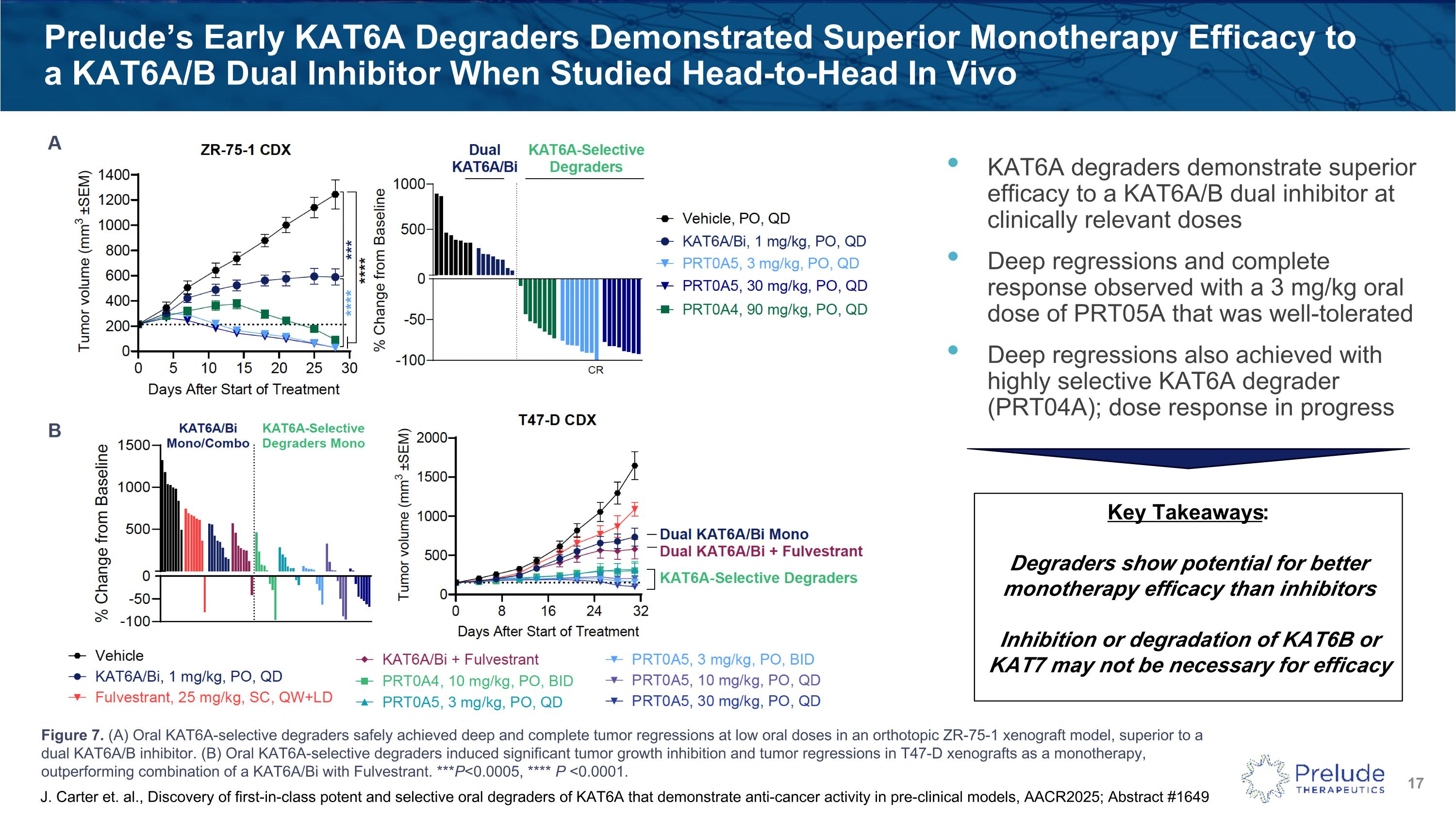
KAT6A degraders demonstrate superior efficacy to a KAT6A/B dual inhibitor at clinically relevant doses Deep regressions and complete response observed with a 3 mg/kg oral dose of PRT05A that was well-tolerated Deep regressions also achieved with highly selective KAT6A degrader (PRT04A); dose response in progress Prelude’s Early KAT6A Degraders Demonstrated Superior Monotherapy Efficacy to a KAT6A/B Dual Inhibitor When Studied Head-to-Head In Vivo Key Takeaways: Degraders show potential for better monotherapy efficacy than inhibitors Inhibition or degradation of KAT6B or KAT7 may not be necessary for efficacy J. Carter et. al., Discovery of first-in-class potent and selective oral degraders of KAT6A that demonstrate anti-cancer activity in pre-clinical models, AACR2025; Abstract #1649 Figure 7. (A) Oral KAT6A-selective degraders safely achieved deep and complete tumor regressions at low oral doses in an orthotopic ZR-75-1 xenograft model, superior to a dual KAT6A/B inhibitor. (B) Oral KAT6A-selective degraders induced significant tumor growth inhibition and tumor regressions in T47-D xenografts as a monotherapy, outperforming combination of a KAT6A/Bi with Fulvestrant. ***P<0.0005, **** P <0.0001.
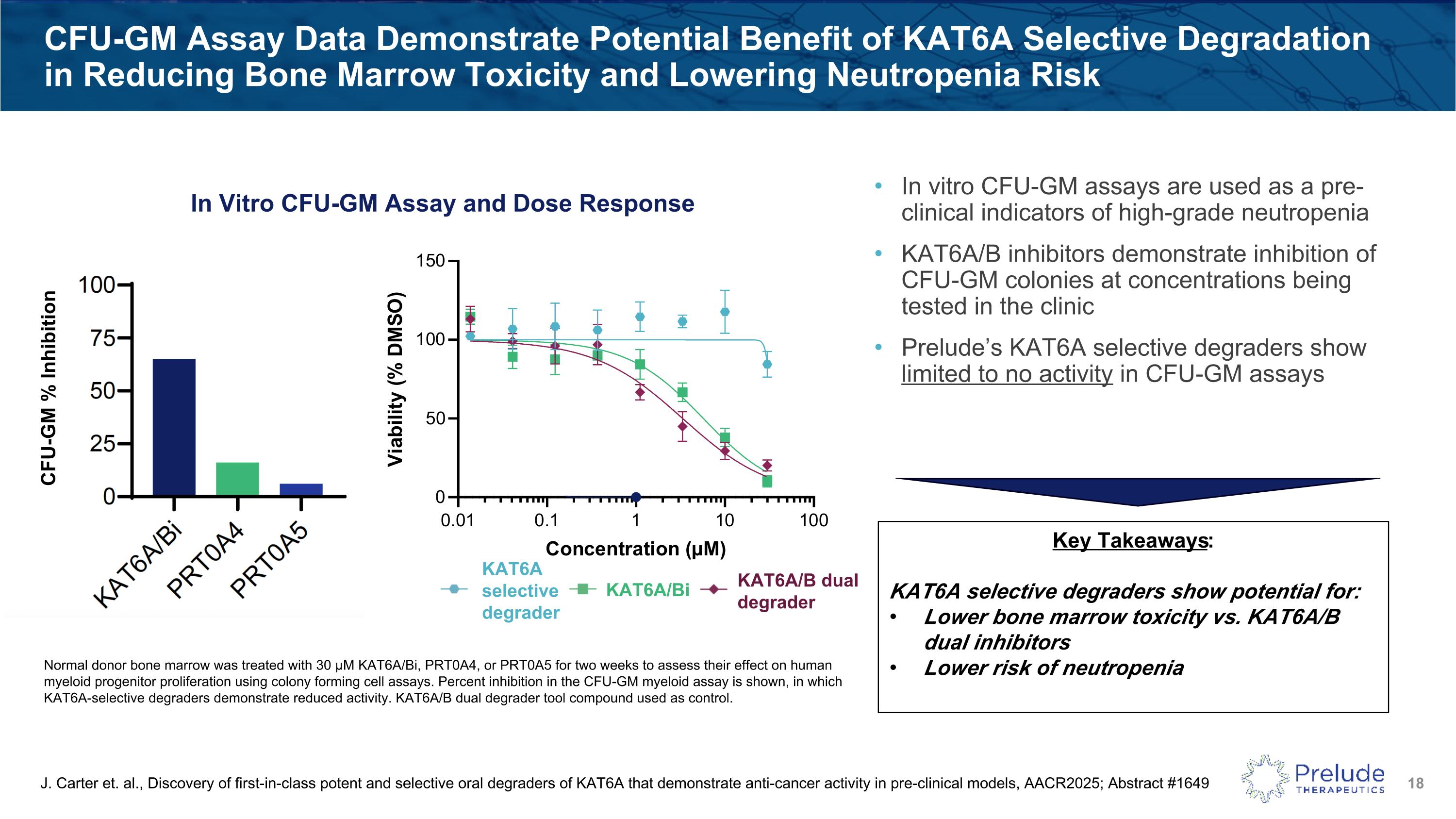
CFU-GM Assay Data Demonstrate Potential Benefit of KAT6A Selective Degradation in Reducing Bone Marrow Toxicity and Lowering Neutropenia Risk KAT6A selective degrader KAT6A/B dual degrader KAT6A/Bi Key Takeaways: KAT6A selective degraders show potential for: Lower bone marrow toxicity vs. KAT6A/B dual inhibitors Lower risk of neutropenia J. Carter et. al., Discovery of first-in-class potent and selective oral degraders of KAT6A that demonstrate anti-cancer activity in pre-clinical models, AACR2025; Abstract #1649 Normal donor bone marrow was treated with 30 μM KAT6A/Bi, PRT0A4, or PRT0A5 for two weeks to assess their effect on human myeloid progenitor proliferation using colony forming cell assays. Percent inhibition in the CFU-GM myeloid assay is shown, in which KAT6A-selective degraders demonstrate reduced activity. KAT6A/B dual degrader tool compound used as control. In vitro CFU-GM assays are used as a pre-clinical indicators of high-grade neutropenia KAT6A/B inhibitors demonstrate inhibition of CFU-GM colonies at concentrations being tested in the clinic Prelude’s KAT6A selective degraders show limited to no activity in CFU-GM assays In Vitro CFU-GM Assay and Dose Response CFU-GM % Inhibition
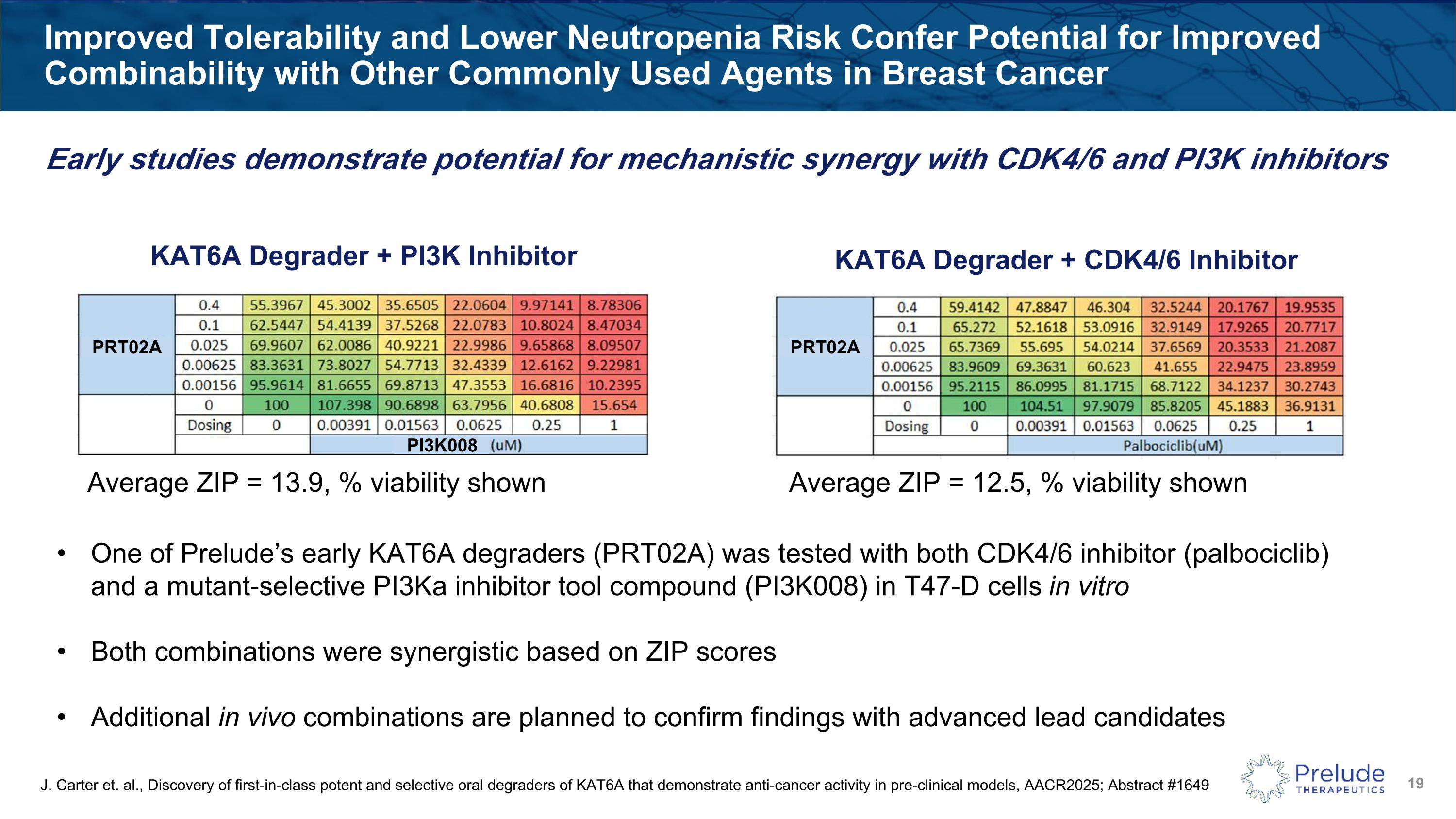
Improved Tolerability and Lower Neutropenia Risk Confer Potential for Improved Combinability with Other Commonly Used Agents in Breast Cancer Average ZIP = 13.9, % viability shown Average ZIP = 12.5, % viability shown KAT6A Degrader + PI3K Inhibitor KAT6A Degrader + CDK4/6 Inhibitor One of Prelude’s early KAT6A degraders (PRT02A) was tested with both CDK4/6 inhibitor (palbociclib) and a mutant-selective PI3Ka inhibitor tool compound (PI3K008) in T47-D cells in vitro Both combinations were synergistic based on ZIP scores Additional in vivo combinations are planned to confirm findings with advanced lead candidates PRT02A PRT02A PI3K008 J. Carter et. al., Discovery of first-in-class potent and selective oral degraders of KAT6A that demonstrate anti-cancer activity in pre-clinical models, AACR2025; Abstract #1649 Early studies demonstrate potential for mechanistic synergy with CDK4/6 and PI3K inhibitors

Now Advancing a First-in-Class Oral KAT6A Selective Degrader Development Candidate Highly potent (picomolar) and highly selective for KAT6A Excellent oral bioavailability across species Complete regressions observed with in vivo efficacy models as monotherapy at low doses No activity in CFU-GM assay (model of bone marrow toxicity) Studies in Progress Additional profiling, including in vivo PK/PD and efficacy studies Dose range finding toxicology studies On Track for IND Filing in 1H 2026 KAT6A Selective Degrader Program Summary & Next Steps Evidence for Improved Safety Profile (BM Tox) Excellent Oral PK Picomolar Potency Milestones Achieved Evidence Supporting Potential for Synergy with Other Modalities In Vivo Efficacy – Regressions as Monotherapy at Low Oral Doses Selective KAT6A degraders have the potential for better monotherapy efficacy, improved tolerability (neutropenia) and combinability, and synergy with other approved agents
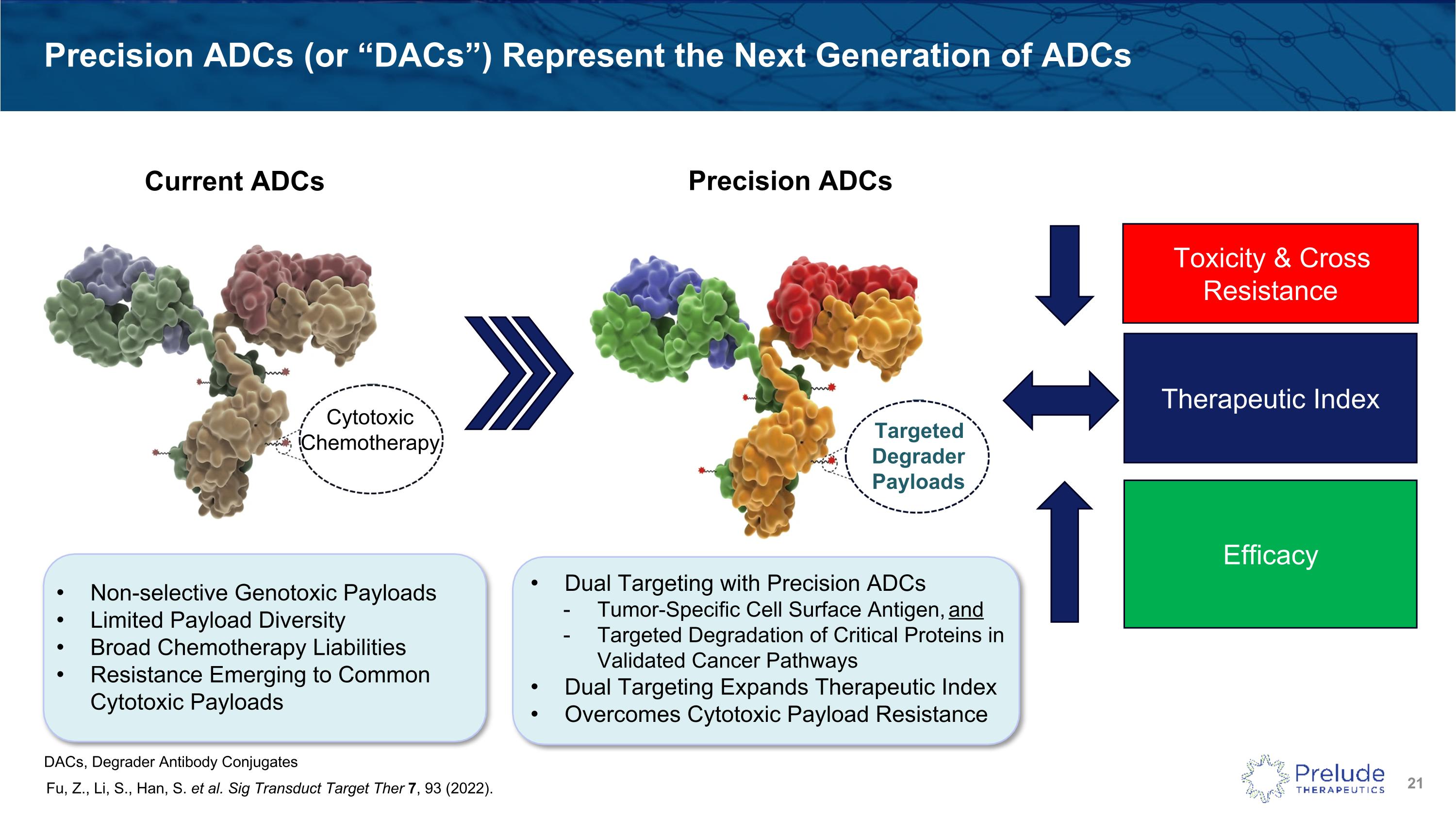
Precision ADCs (or “DACs”) Represent the Next Generation of ADCs Fu, Z., Li, S., Han, S. et al. Sig Transduct Target Ther 7, 93 (2022). Toxicity & Cross Resistance Efficacy Therapeutic Index Non-selective Genotoxic Payloads Limited Payload Diversity Broad Chemotherapy Liabilities Resistance Emerging to Common Cytotoxic Payloads Dual Targeting with Precision ADCs Tumor-Specific Cell Surface Antigen, and Targeted Degradation of Critical Proteins in Validated Cancer Pathways Dual Targeting Expands Therapeutic Index Overcomes Cytotoxic Payload Resistance Targeted Degrader Payloads Current ADCs Precision ADCs Cytotoxic Chemotherapy DACs, Degrader Antibody Conjugates
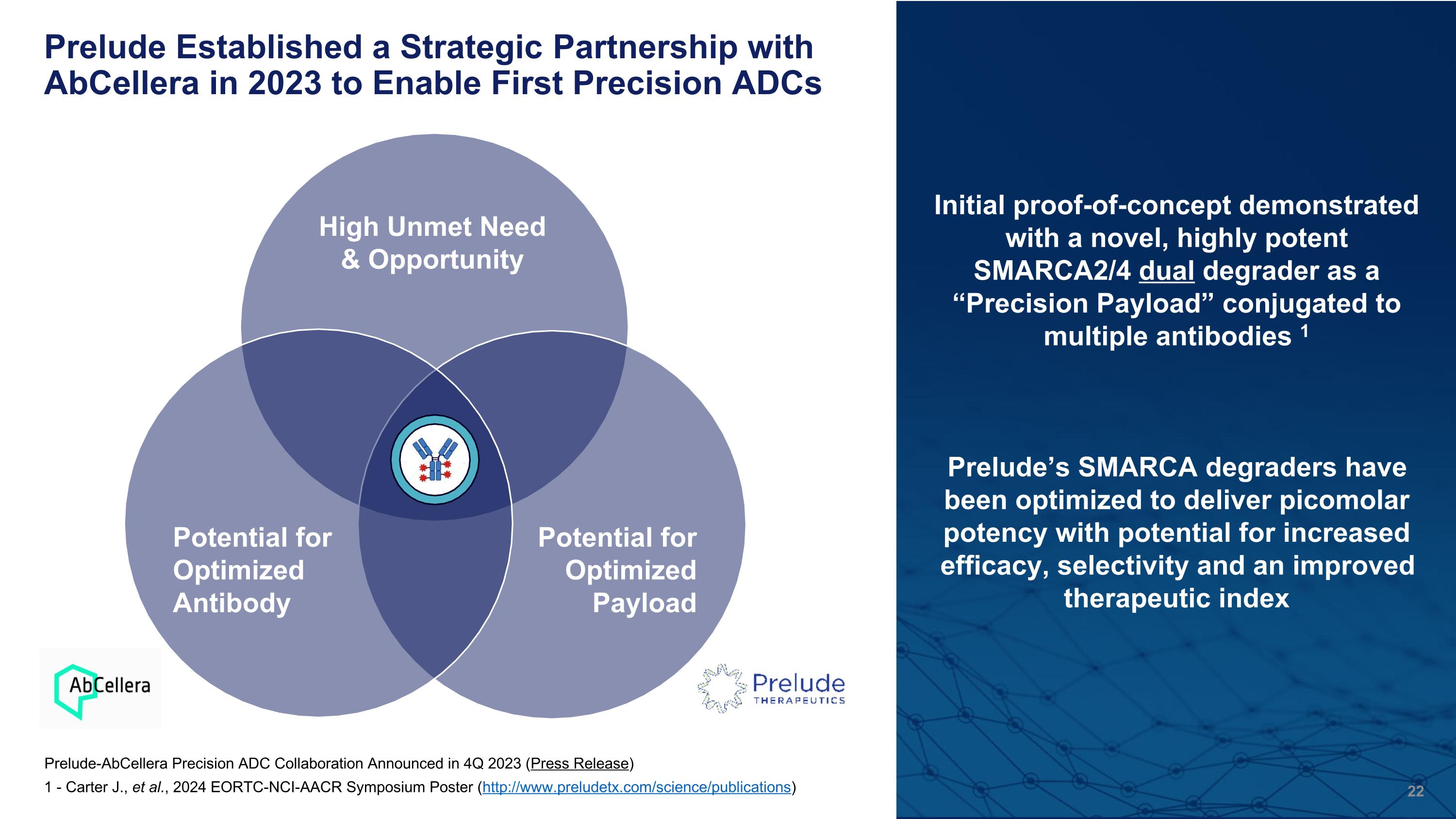
Prelude Established a Strategic Partnership with AbCellera in 2023 to Enable First Precision ADCs High Unmet Need & Opportunity Potential for Optimized Payload Potential for Optimized Antibody Initial proof-of-concept demonstrated with a novel, highly potent SMARCA2/4 dual degrader as a “Precision Payload” conjugated to multiple antibodies 1 Prelude’s SMARCA degraders have been optimized to deliver picomolar potency with potential for increased efficacy, selectivity and an improved therapeutic index Prelude-AbCellera Precision ADC Collaboration Announced in 4Q 2023 (Press Release) 1 - Carter J., et al., 2024 EORTC-NCI-AACR Symposium Poster (http://www.preludetx.com/science/publications)
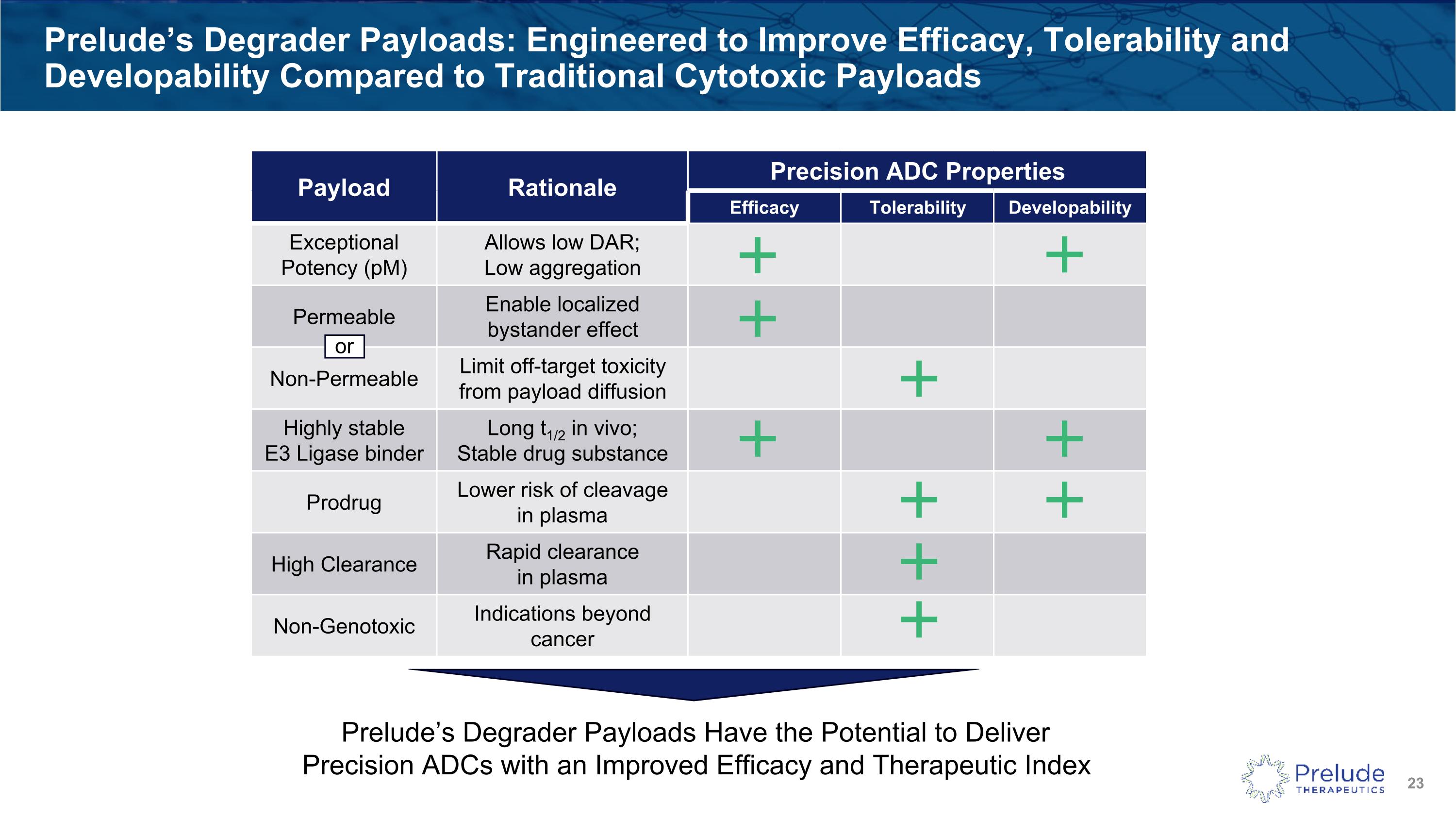
Prelude’s Degrader Payloads: Engineered to Improve Efficacy, Tolerability and Developability Compared to Traditional Cytotoxic Payloads Payload Rationale Precision ADC Properties Efficacy Tolerability Developability Exceptional Potency (pM) Allows low DAR; Low aggregation Permeable Enable localized bystander effect Non-Permeable Limit off-target toxicity from payload diffusion Highly stable E3 Ligase binder Long t1/2 in vivo; Stable drug substance Prodrug Lower risk of cleavage in plasma High Clearance Rapid clearance in plasma Non-Genotoxic Indications beyond cancer Prelude’s Degrader Payloads Have the Potential to Deliver Precision ADCs with an Improved Efficacy and Therapeutic Index or
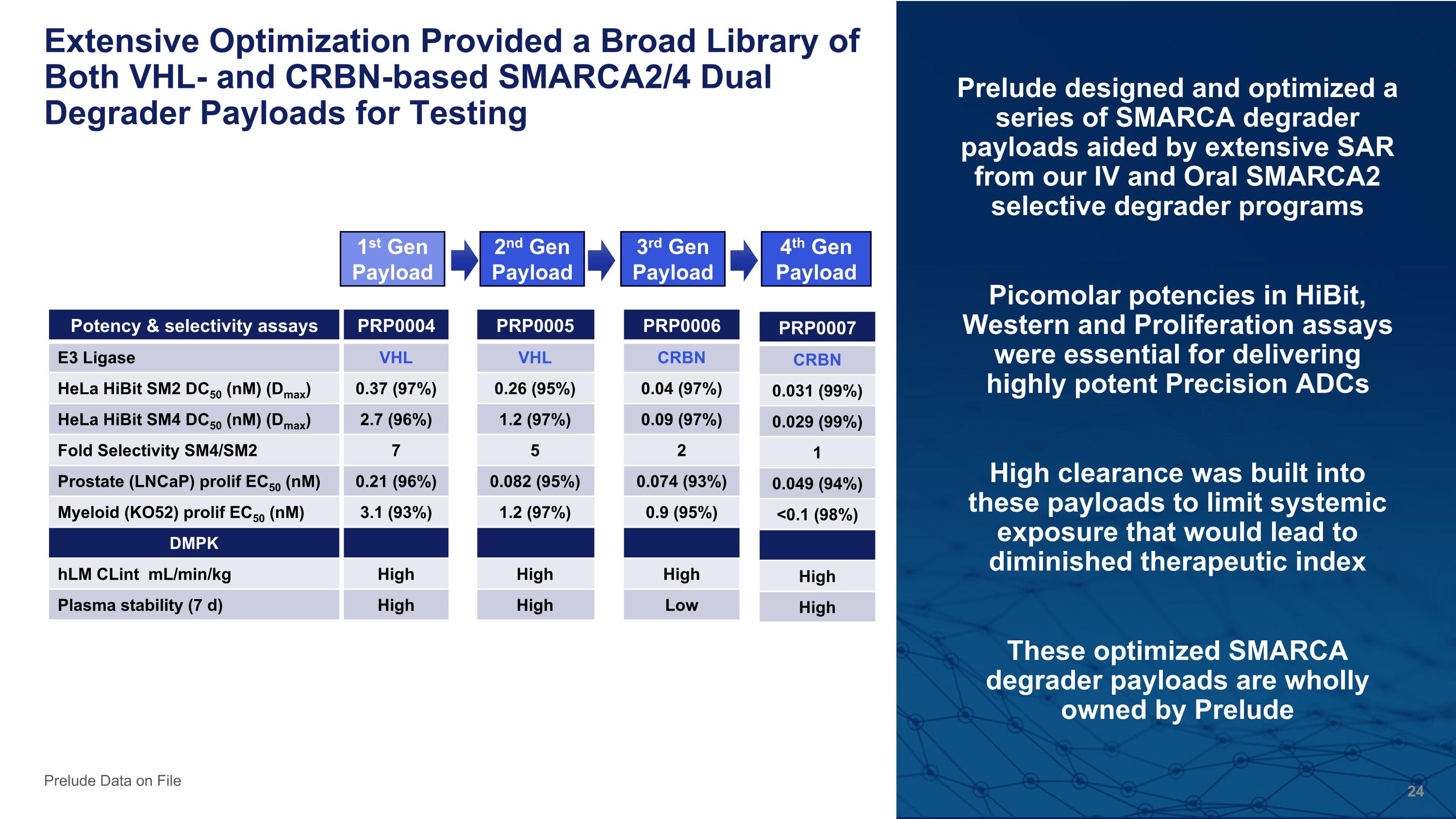
Extensive Optimization Provided a Broad Library of Both VHL- and CRBN-based SMARCA2/4 Dual Degrader Payloads for Testing Prelude designed and optimized a series of SMARCA degrader payloads aided by extensive SAR from our IV and Oral SMARCA2 selective degrader programs Picomolar potencies in HiBit, Western and Proliferation assays were essential for delivering highly potent Precision ADCs High clearance was built into these payloads to limit systemic exposure that would lead to diminished therapeutic index These optimized SMARCA degrader payloads are wholly owned by Prelude Prelude Data on File Potency & selectivity assays PRP0004 E3 Ligase VHL HeLa HiBit SM2 DC50 (nM) (Dmax) 0.37 (97%) HeLa HiBit SM4 DC50 (nM) (Dmax) 2.7 (96%) Fold Selectivity SM4/SM2 7 Prostate (LNCaP) prolif EC50 (nM) 0.21 (96%) Myeloid (KO52) prolif EC50 (nM) 3.1 (93%) DMPK hLM CLint mL/min/kg High Plasma stability (7 d) High PRP0006 CRBN 0.04 (97%) 0.09 (97%) 2 0.074 (93%) 0.9 (95%) High Low PRP0005 VHL 0.26 (95%) 1.2 (97%) 5 0.082 (95%) 1.2 (97%) High High 1st Gen Payload PRP0007 CRBN 0.031 (99%) 0.029 (99%) 1 0.049 (94%) <0.1 (98%) High High 2nd Gen Payload 3rd Gen Payload 4th Gen Payload
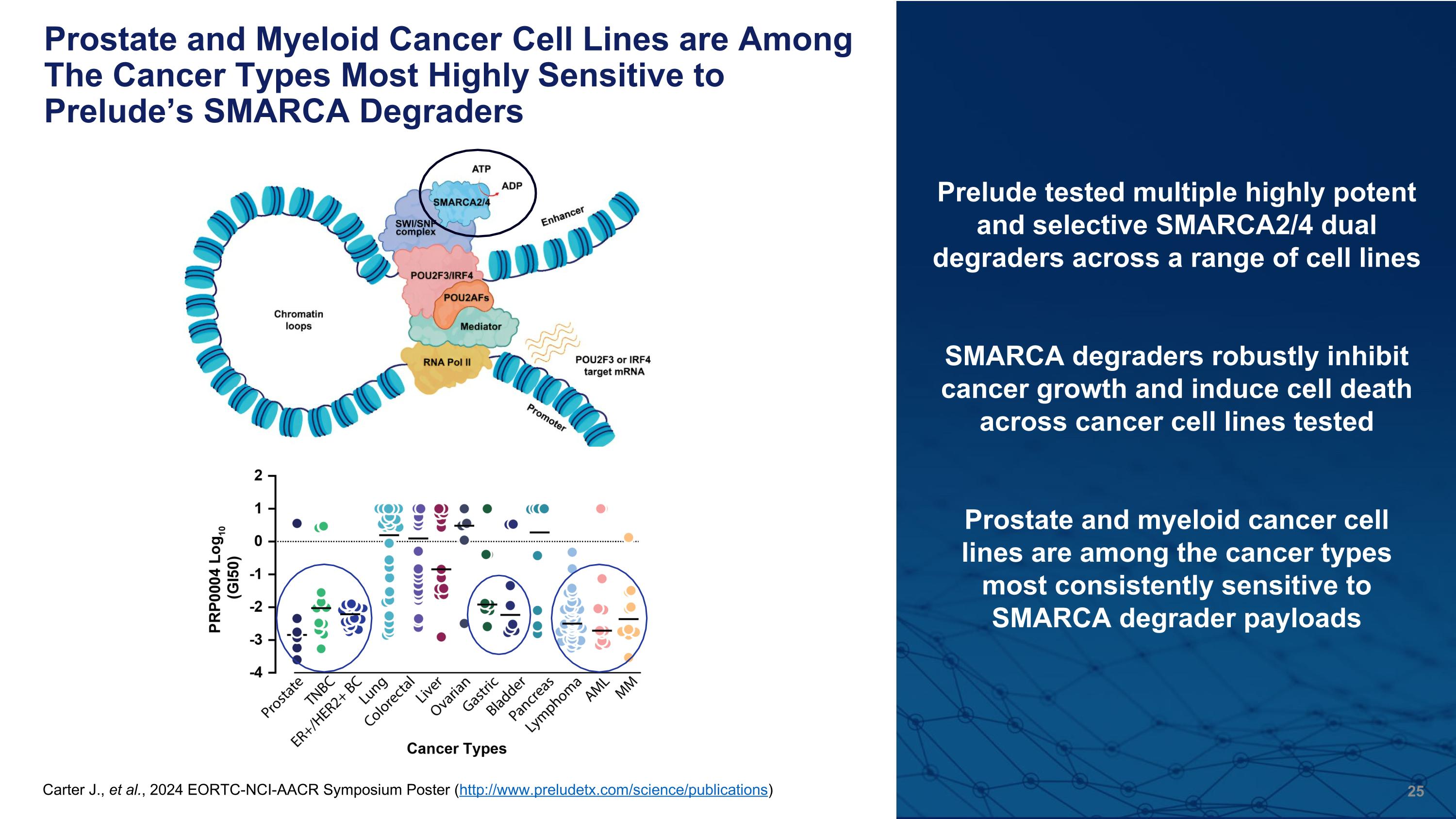
Prostate and Myeloid Cancer Cell Lines are Among The Cancer Types Most Highly Sensitive to Prelude’s SMARCA Degraders Carter J., et al., 2024 EORTC-NCI-AACR Symposium Poster (http://www.preludetx.com/science/publications) Prelude tested multiple highly potent and selective SMARCA2/4 dual degraders across a range of cell lines SMARCA degraders robustly inhibit cancer growth and induce cell death across cancer cell lines tested Prostate and myeloid cancer cell lines are among the cancer types most consistently sensitive to SMARCA degrader payloads
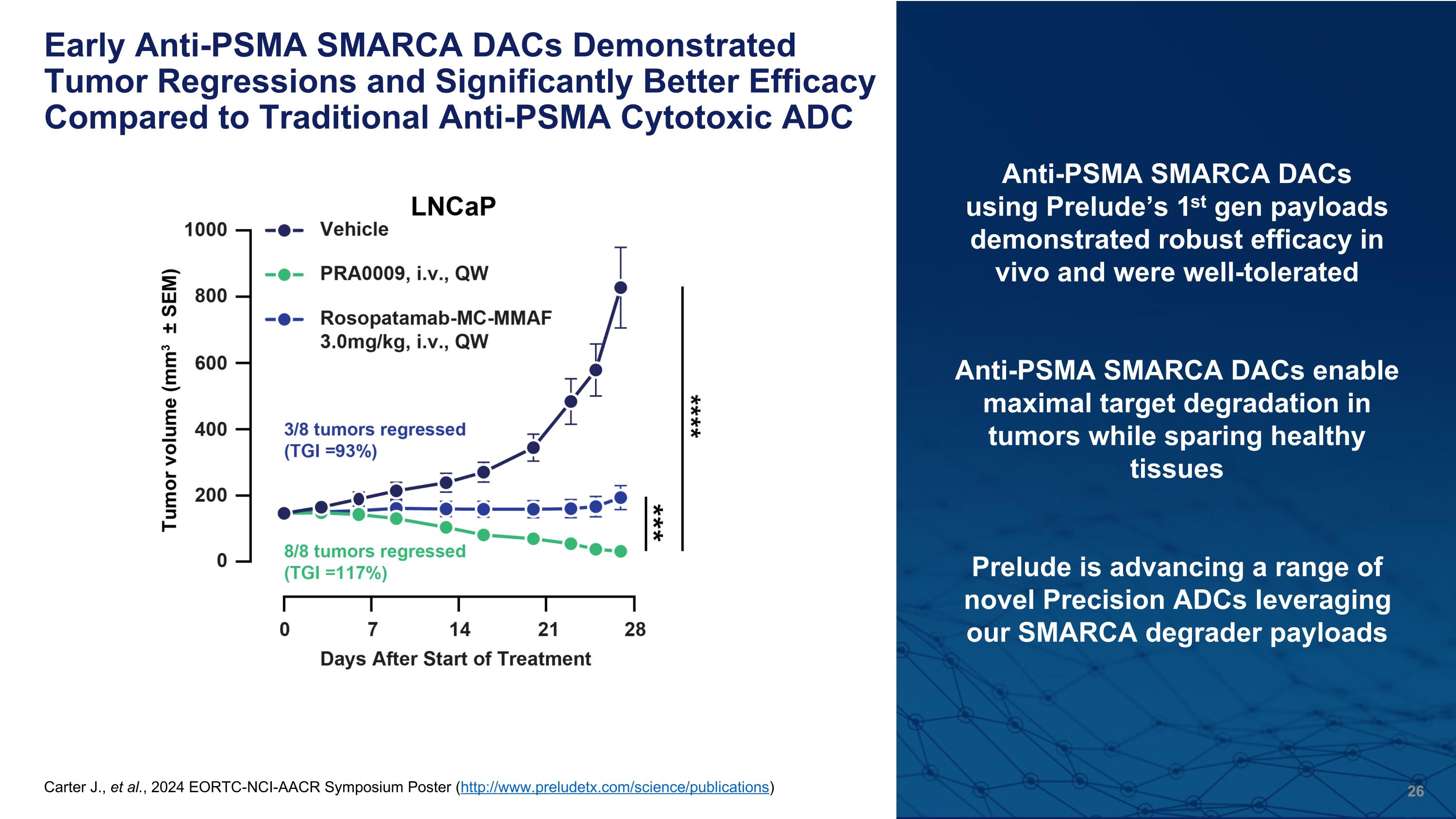
Early Anti-PSMA SMARCA DACs Demonstrated Tumor Regressions and Significantly Better Efficacy Compared to Traditional Anti-PSMA Cytotoxic ADC Anti-PSMA SMARCA DACs using Prelude’s 1st gen payloads demonstrated robust efficacy in vivo and were well-tolerated Anti-PSMA SMARCA DACs enable maximal target degradation in tumors while sparing healthy tissues Prelude is advancing a range of novel Precision ADCs leveraging our SMARCA degrader payloads (A) SMARCA2/4 protein expression was analyzed in DAC PRA0002 and payload PRP0004-treated LNCaP tumors at the indicated time points following a single dose. Graphs are quantitation of western blots. (B) Weekly i.v. administration of PRA0002 was well-tolerated and demonstrated significant tumor growth inhibition (89%) of PSMA+ LNCaP tumors. (C) Weekly i.v. administration of PRA0002 did not induce significant tumor growth inhibition in PSMA- PC3 tumors, in comparison to PRP0004 which was efficacious, but caused mouse body weight loss and death (D) Weekly i.v. administration of PRA0009 demonstrated tumor regression and significantly better efficacy compared to a PSMA cytotoxic ADC (Rosopatamab-MC-MMAF, DAR2) in LNCaP tumors. Carter J., et al., 2024 EORTC-NCI-AACR Symposium Poster (http://www.preludetx.com/science/publications)
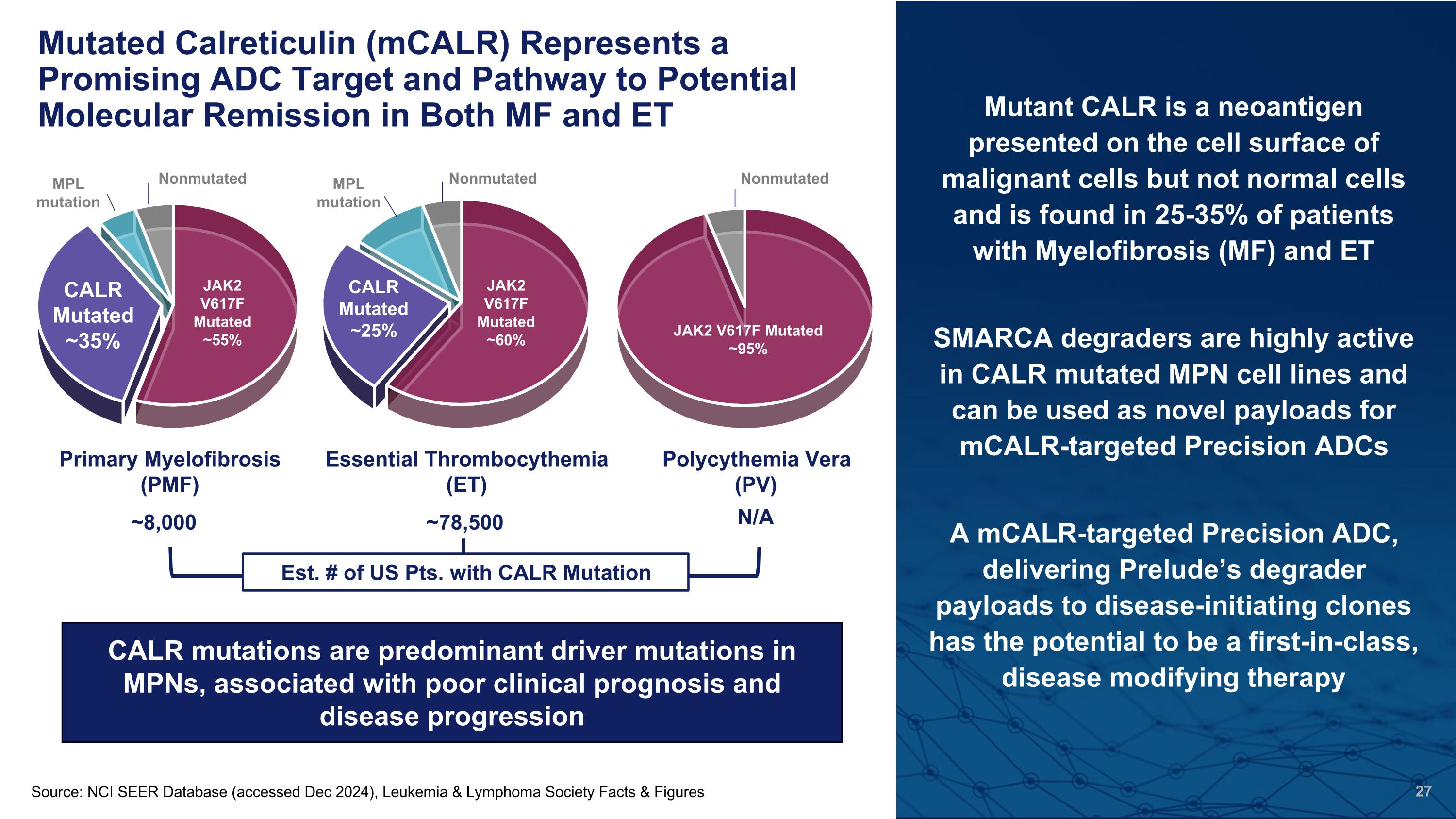
Mutated Calreticulin (mCALR) Represents a Promising ADC Target and Pathway to Potential Molecular Remission in Both MF and ET Mutant CALR is a neoantigen presented on the cell surface of malignant cells but not normal cells and is found in 25-35% of patients with Myelofibrosis (MF) and ET SMARCA degraders are highly active in CALR mutated MPN cell lines and can be used as novel payloads for mCALR-targeted Precision ADCs A mCALR-targeted Precision ADC, delivering Prelude’s degrader payloads to disease-initiating clones has the potential to be a first-in-class, disease modifying therapy Primary Myelofibrosis (PMF) Essential Thrombocythemia (ET) Polycythemia Vera (PV) JAK2 V617F Mutated ~95% MPL mutation MPL mutation Nonmutated Nonmutated CALR mutations are predominant driver mutations in MPNs, associated with poor clinical prognosis and disease progression Nonmutated ~8,000 ~78,500 N/A Est. # of US Pts. with CALR Mutation Source: NCI SEER Database (accessed Dec 2024), Leukemia & Lymphoma Society Facts & Figures JAK2 V617F Mutated ~95% JAK2 V617F Mutated ~60% JAK2 V617F Mutated ~55% CALR Mutated ~35% CALR Mutated ~25%
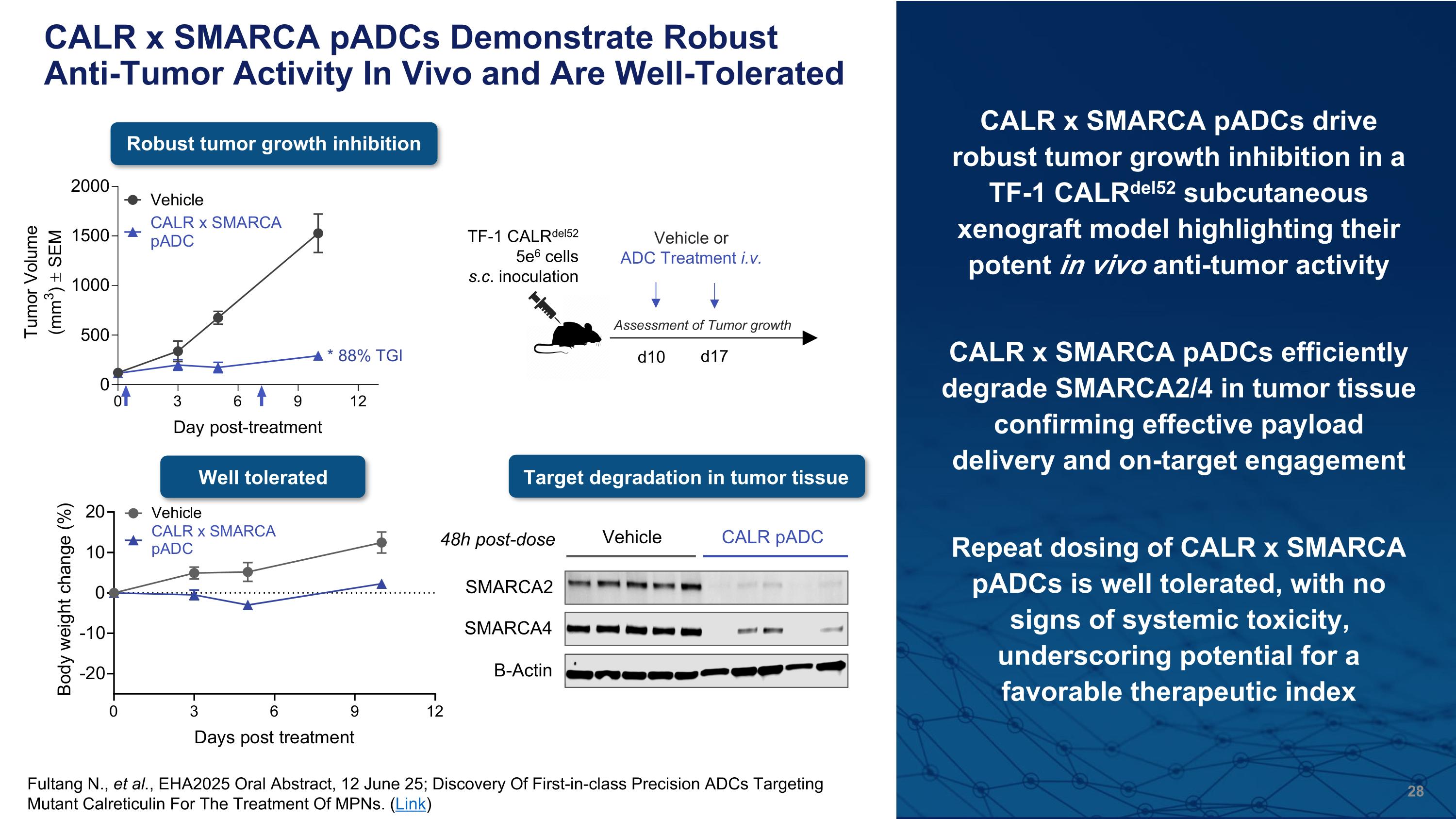
CALR x SMARCA pADCs Demonstrate Robust Anti-Tumor Activity In Vivo and Are Well-Tolerated CALR x SMARCA pADCs drive robust tumor growth inhibition in a TF-1 CALRdel52 subcutaneous xenograft model highlighting their potent in vivo anti-tumor activity CALR x SMARCA pADCs efficiently degrade SMARCA2/4 in tumor tissue confirming effective payload delivery and on-target engagement Repeat dosing of CALR x SMARCA pADCs is well tolerated, with no signs of systemic toxicity, underscoring potential for a favorable therapeutic index Robust tumor growth inhibition Well tolerated SMARCA2 SMARCA4 Β-Actin Vehicle CALR pADC 48h post-dose TF-1 CALRdel52 5e6 cells s.c. inoculation d10 Vehicle or ADC Treatment i.v. d17 Assessment of Tumor growth Target degradation in tumor tissue Fultang N., et al., EHA2025 Oral Abstract, 12 June 25; Discovery Of First-in-class Precision ADCs Targeting Mutant Calreticulin For The Treatment Of MPNs. (Link)

Our Focus is Continued Execution Across Strategic Priorities PRT2527 CDK9 PROGRAM Selective CDK Inhibitor PRT2527 PRT7732 SMARCA2 Degrader Program Discovery Engine KAT6A & Precision ADCs IND filing for lead KAT6A degrader development candidate(s) Advance first Precision ADC candidate in partnership w/ AbCellera EXPECTED DELIVERABLE MILESTONE BY 1H 2026 2H 2025 Cash, cash equivalents, restricted cash and marketable securities of $77.3 Million as of 6/30/2025 Report Phase 1 data Report first Phase 1 interim data (Safety, PK/PD, Clinical Activity) YE2025 YE2025 PRT3789

Thank You Contact Us: Robert Doody SVP, Investor Relations rdoody@preludetx.com