

Innovating the future of cancer care to cure patients and preserve organ function August 2025 Exhibit 99.2

Legal disclosure This presentation contains forward-looking statements, all of which are qualified in their entirety by this cautionary statement. Many of the forward-looking statements contained herein can be identified by the use of forward-looking words such as "may", "anticipate", "believe", "could', "expect", "should", "plan", "intend", "estimate", "will", "potential" and "ongoing", among others, although not all forward-looking statements contain these identifying words. These forward-looking statements include statements about the initiation, timing, progress, results and cost of our research and development programs and our current and future nonclinical, preclinical studies and clinical trials, including statements regarding the timing of initiation and completion of studies or trials and related preparatory work, the period during which the results of the trials will become available and our research and development programs; our ability to efficiently develop our existing product candidates and discover new product candidates; our ability to successfully manufacture our drug substances and product candidates for preclinical use, for clinical trials and on a larger scale for commercial use, if approved; the ability and willingness of our third-party strategic collaborators to continue research and development activities relating to our development candidates and product candidates; our ability to commercialize our products, if approved; our ability to obtain funding for our operations necessary to complete further development and commercialization of our product candidates; our ability to obtain and maintain regulatory approval of our product candidates; statements regarding our beliefs and expectations for the high unmet medical need for an effective local treatment in ocular and urologic oncology to preserve organ function; the size and growth potential of the markets for our product candidates and our ability to serve those markets; our financial performance; our expected cash runway into the first half of 2027; and the implementation of our business model, including strategic plans for our business and product candidates. Except as otherwise noted, these forward-looking statements speak only as of the date of this presentation, and we undertake no obligation to update or revise any of such statements to reflect events or circumstances occurring after this presentation. Because forward-looking statements are inherently subject to risks and uncertainties, some of which cannot be predicted or quantified and some of which are beyond our control, you should not rely on these forward-looking statements as predictions of future events. For a discussion of these and other risks and uncertainties, and other important factors, any of which could cause our actual results to differ from those contained in the forward-looking statements, see the section entitled "Risk Factors" in our most recent Annual Report on Form 10-K and Quarterly Report on Form 10-Q filed with the Securities and Exchange Commission (SEC), as well as discussions of potential risks, uncertainties, and other important factors in our other subsequent filings with the SEC, which are available on the SEC's website at www.sec.gov. The events and circumstances reflected in our forward-looking statements may not be achieved or occur and actual results could differ materially from those projected in the forward-looking statements. We caution you not to place undue reliance on the forward-looking statements contained in this presentation. This presentation discusses product candidates that are under preclinical or clinical evaluation and that have not yet been approved for marketing by the U.S. Food and Drug Administration (FDA) or any other regulatory authority. Until finalized in a clinical study report, clinical trial data presented herein remain subject to adjustment as a result of clinical site audits and other review processes. No representation is made as to the safety or effectiveness of these product candidates for the use for which such product candidates are being studied. This presentation shall not constitute an offer to sell or the solicitation of an offer to buy, nor shall there be any sale of these securities in any state or other jurisdiction in which such offer, solicitation or sale would be unlawful prior to registration or qualification under the securities laws of any such state or other jurisdiction.
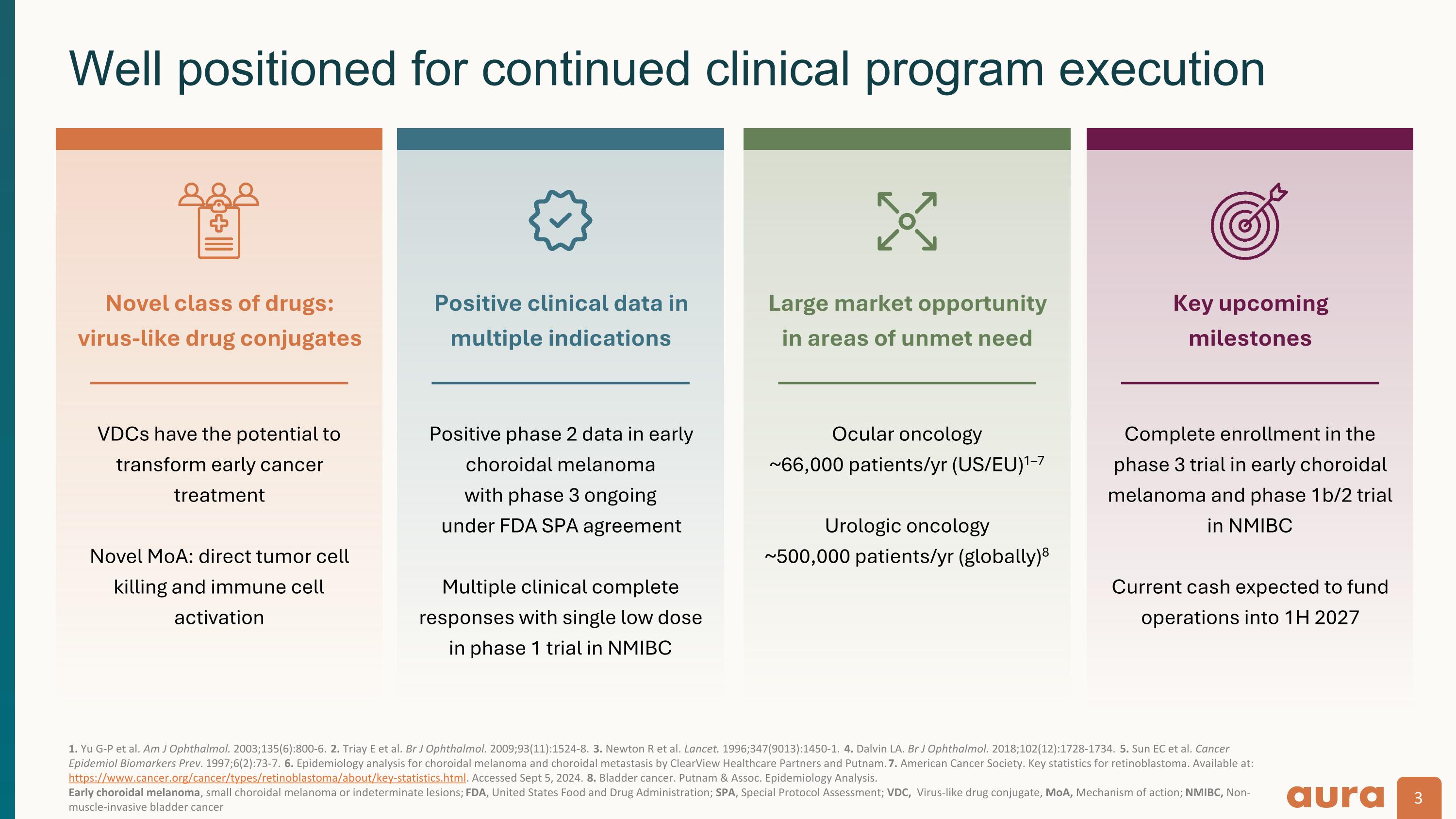
1. Yu G-P et al. Am J Ophthalmol. 2003;135(6):800-6. 2. Triay E et al. Br J Ophthalmol. 2009;93(11):1524-8. 3. Newton R et al. Lancet. 1996;347(9013):1450-1. 4. Dalvin LA. Br J Ophthalmol. 2018;102(12):1728-1734. 5. Sun EC et al. Cancer Epidemiol Biomarkers Prev. 1997;6(2):73-7. 6. Epidemiology analysis for choroidal melanoma and choroidal metastasis by ClearView Healthcare Partners and Putnam. 7. American Cancer Society. Key statistics for retinoblastoma. Available at: https://www.cancer.org/cancer/types/retinoblastoma/about/key-statistics.html. Accessed Sept 5, 2024. 8. Bladder cancer. Putnam & Assoc. Epidemiology Analysis. Early choroidal melanoma, small choroidal melanoma or indeterminate lesions; FDA, United States Food and Drug Administration; SPA, Special Protocol Assessment; VDC, Virus-like drug conjugate, MoA, Mechanism of action; NMIBC, Non-muscle-invasive bladder cancer Well positioned for continued clinical program execution VDCs have the potential to transform early cancer treatment Novel MoA: direct tumor cell killing and immune cell activation Novel class of drugs: virus-like drug conjugates Positive phase 2 data in early choroidal melanoma with phase 3 ongoing under FDA SPA agreement Multiple clinical complete responses with single low dose in phase 1 trial in NMIBC Positive clinical data in multiple indications Ocular oncology ~66,000 patients/yr (US/EU)1–7 Urologic oncology ~500,000 patients/yr (globally)8 Large market opportunity in areas of unmet need Complete enrollment in the phase 3 trial in early choroidal melanoma and phase 1b/2 trial in NMIBC Current cash expected to fund operations into 1H 2027 Key upcoming milestones
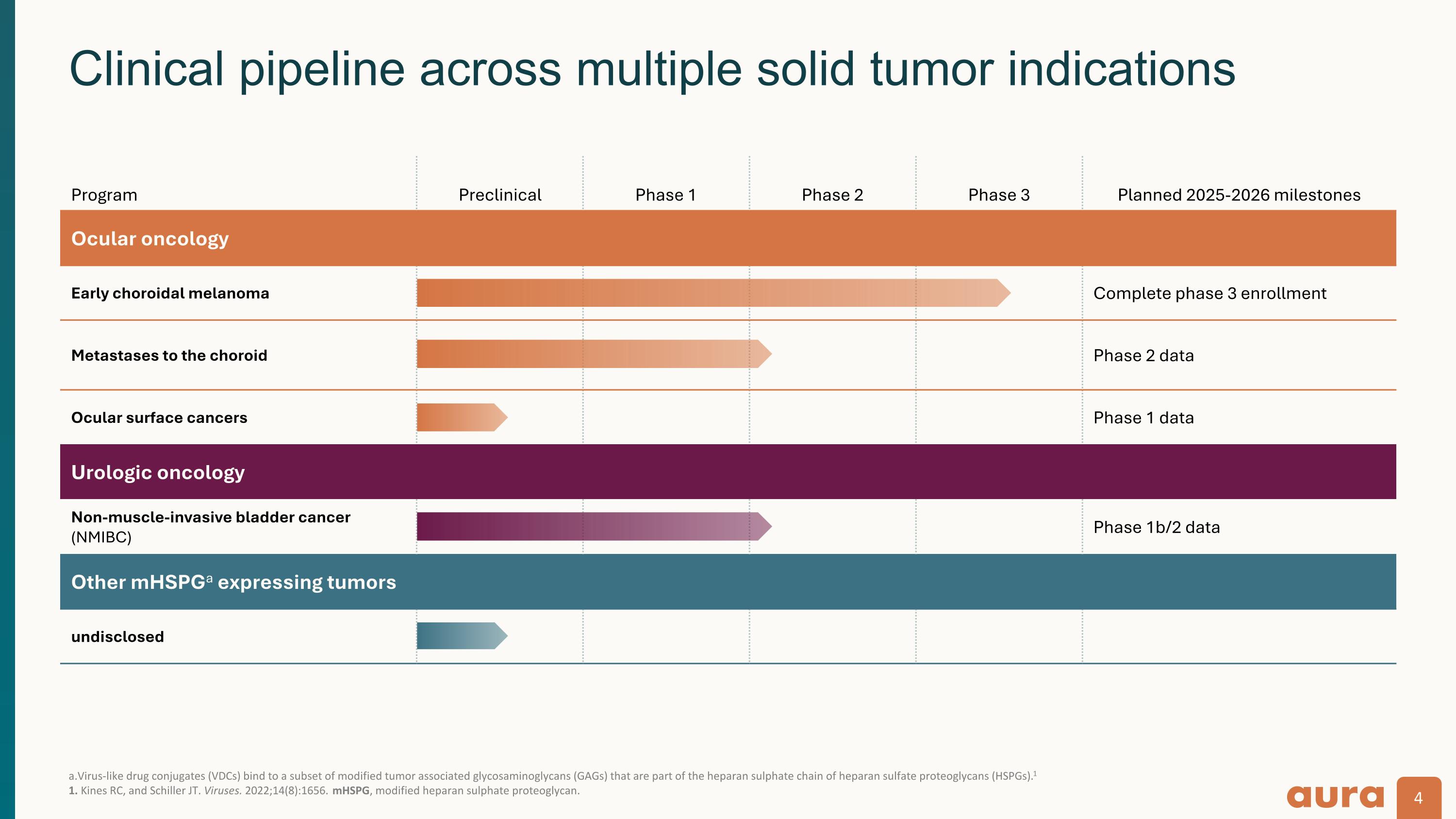
a.Virus-like drug conjugates (VDCs) bind to a subset of modified tumor associated glycosaminoglycans (GAGs) that are part of the heparan sulphate chain of heparan sulfate proteoglycans (HSPGs).1 1. Kines RC, and Schiller JT. Viruses. 2022;14(8):1656. mHSPG, modified heparan sulphate proteoglycan. Clinical pipeline across multiple solid tumor indications Program Preclinical Phase 1 Phase 2 Phase 3 Planned 2025-2026 milestones Ocular oncology Early choroidal melanoma Complete phase 3 enrollment Metastases to the choroid Phase 2 data Ocular surface cancers Phase 1 data Urologic oncology Non-muscle-invasive bladder cancer (NMIBC) Phase 1b/2 data Other mHSPGa expressing tumors undisclosed
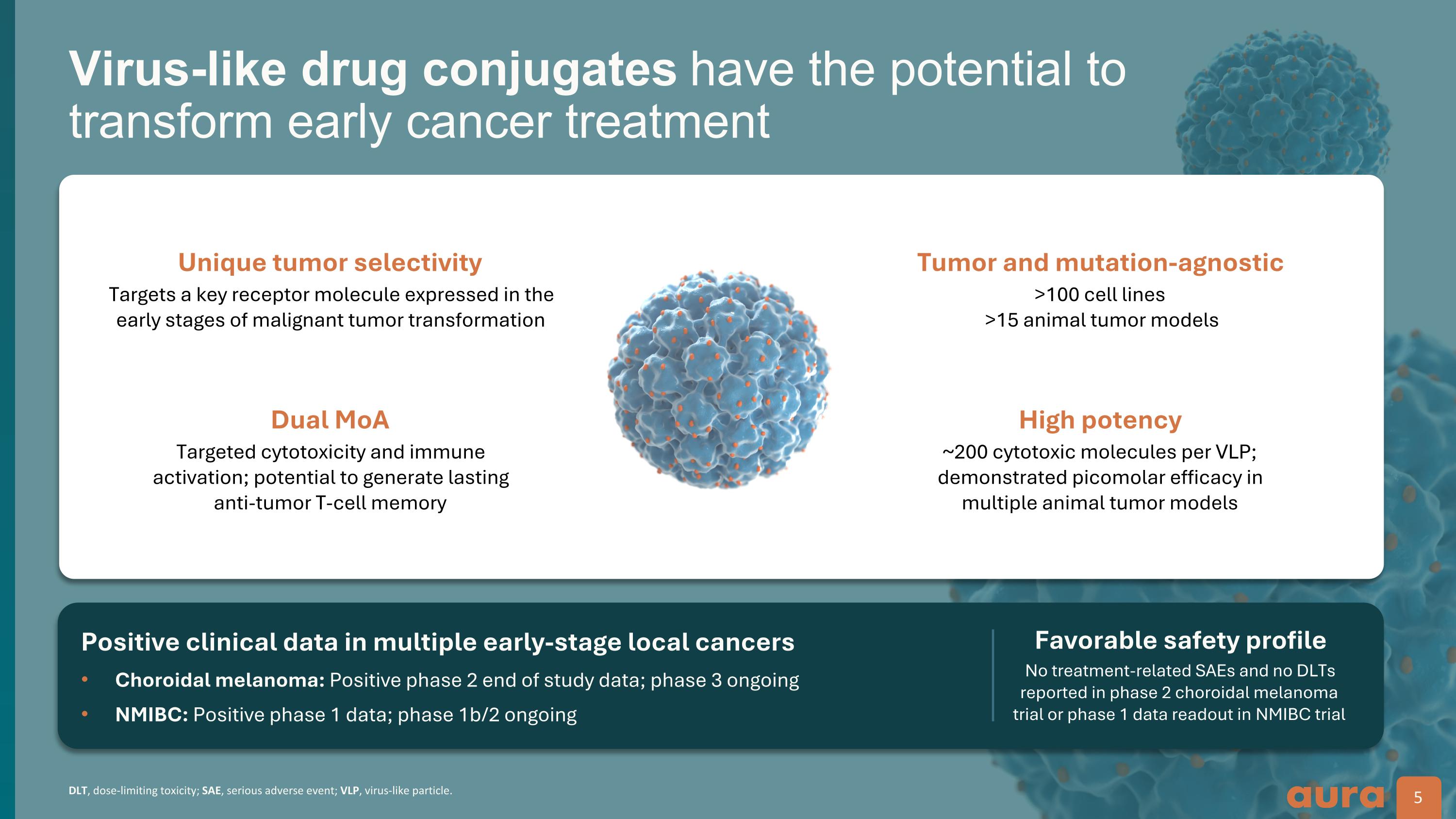
DLT, dose-limiting toxicity; SAE, serious adverse event; VLP, virus-like particle. Virus-like drug conjugates have the potential to transform early cancer treatment 5 Positive clinical data in multiple early-stage local cancers Choroidal melanoma: Positive phase 2 end of study data; phase 3 ongoing NMIBC: Positive phase 1 data; phase 1b/2 ongoing Favorable safety profile Unique tumor selectivity Dual MoA Targets a key receptor molecule expressed in the early stages of malignant tumor transformation Targeted cytotoxicity and immune activation; potential to generate lasting anti-tumor T-cell memory Tumor and mutation-agnostic High potency >100 cell lines >15 animal tumor models ~200 cytotoxic molecules per VLP; demonstrated picomolar efficacy in multiple animal tumor models No treatment-related SAEs and no DLTs reported in phase 2 choroidal melanoma trial or phase 1 data readout in NMIBC trial
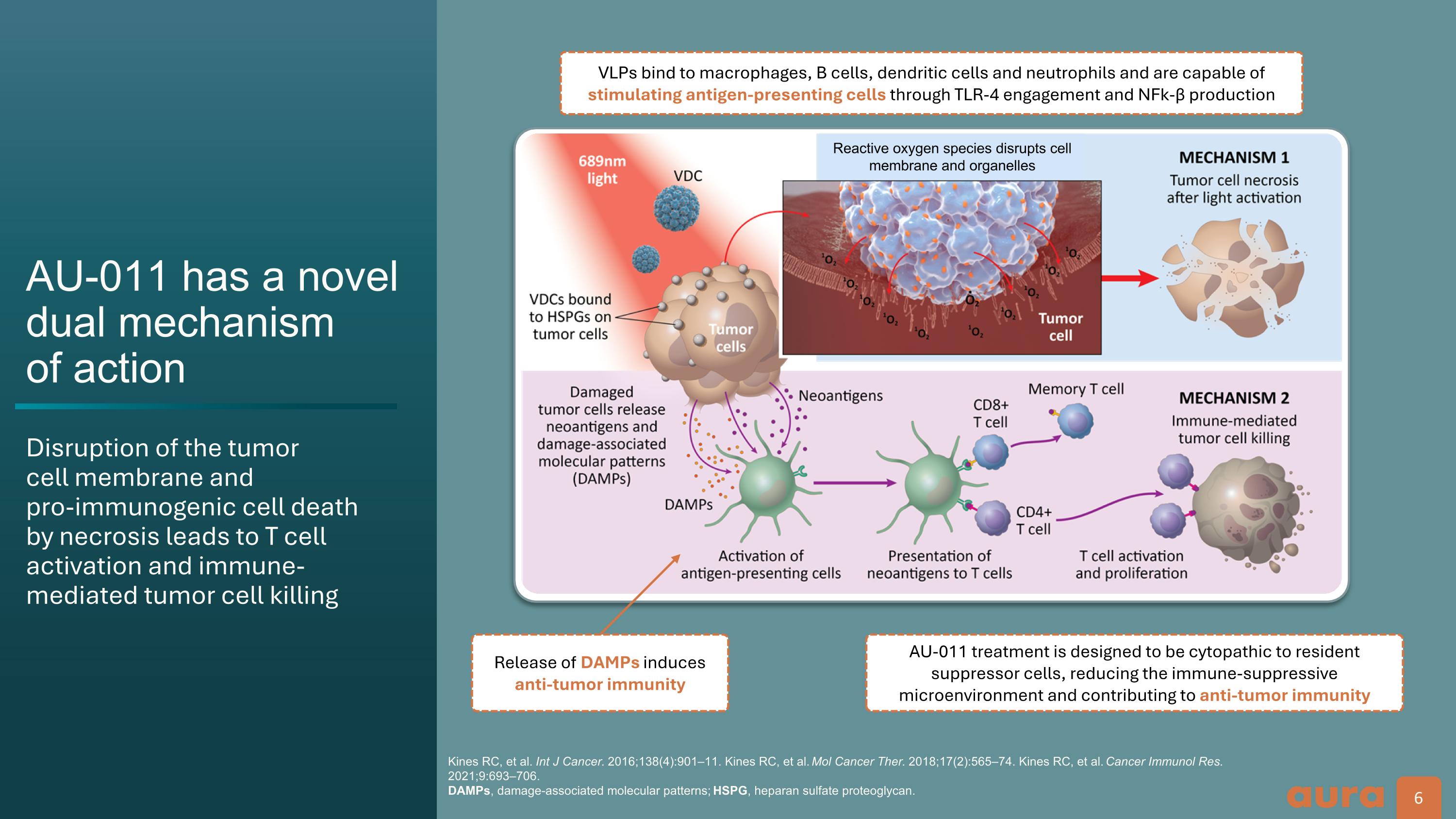
AU-011 has a novel dual mechanism of action Disruption of the tumor cell membrane and pro-immunogenic cell death by necrosis leads to T cell activation and immune-mediated tumor cell killing Kines RC, et al. Int J Cancer. 2016;138(4):901–11. Kines RC, et al. Mol Cancer Ther. 2018;17(2):565–74. Kines RC, et al. Cancer Immunol Res. 2021;9:693–706. DAMPs, damage-associated molecular patterns; HSPG, heparan sulfate proteoglycan. Release of DAMPs induces anti-tumor immunity AU-011 treatment is designed to be cytopathic to resident suppressor cells, reducing the immune-suppressive microenvironment and contributing to anti-tumor immunity VLPs bind to macrophages, B cells, dendritic cells and neutrophils and are capable of stimulating antigen-presenting cells through TLR-4 engagement and NFk-β production Reactive oxygen species disrupts cell membrane and organelles
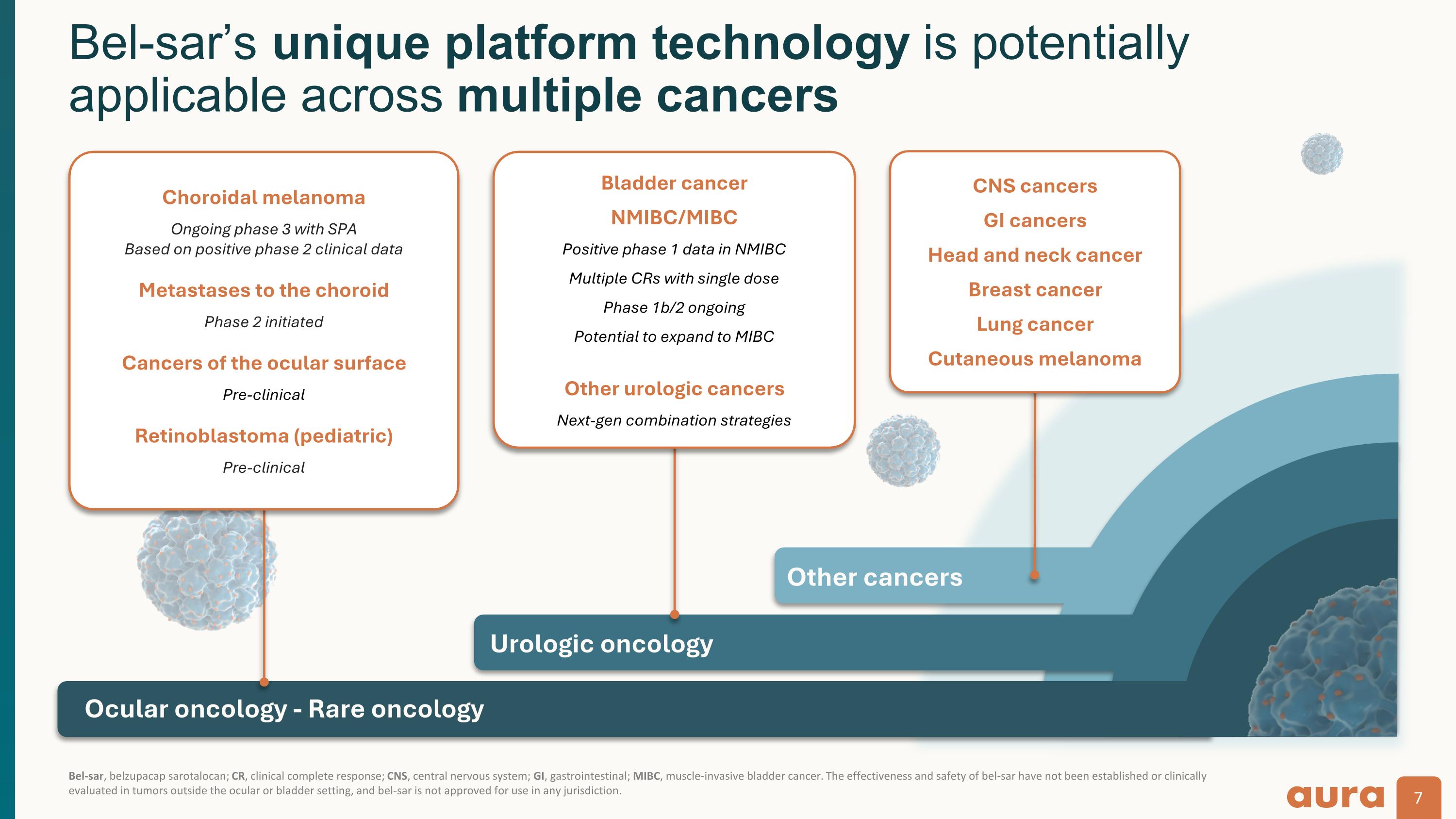
Bel-sar, belzupacap sarotalocan; CR, clinical complete response; CNS, central nervous system; GI, gastrointestinal; MIBC, muscle-invasive bladder cancer. The effectiveness and safety of bel-sar have not been established or clinically evaluated in tumors outside the ocular or bladder setting, and bel-sar is not approved for use in any jurisdiction. Bel-sar’s unique platform technology is potentially applicable across multiple cancers Urologic oncology Other cancers Bladder cancer NMIBC/MIBC Positive phase 1 data in NMIBC Multiple CRs with single dose Phase 1b/2 ongoing Potential to expand to MIBC Other urologic cancers Next-gen combination strategies CNS cancers GI cancers Head and neck cancer Breast cancer Lung cancer Cutaneous melanoma Ocular oncology - Rare oncology Choroidal melanoma Ongoing phase 3 with SPA Based on positive phase 2 clinical data Metastases to the choroid Phase 2 initiated Cancers of the ocular surface Pre-clinical Retinoblastoma (pediatric) Pre-clinical
Ocular Oncology Bel-sar target indications: Early choroidal melanoma | Metastases to the choroid | Ocular surface cancers
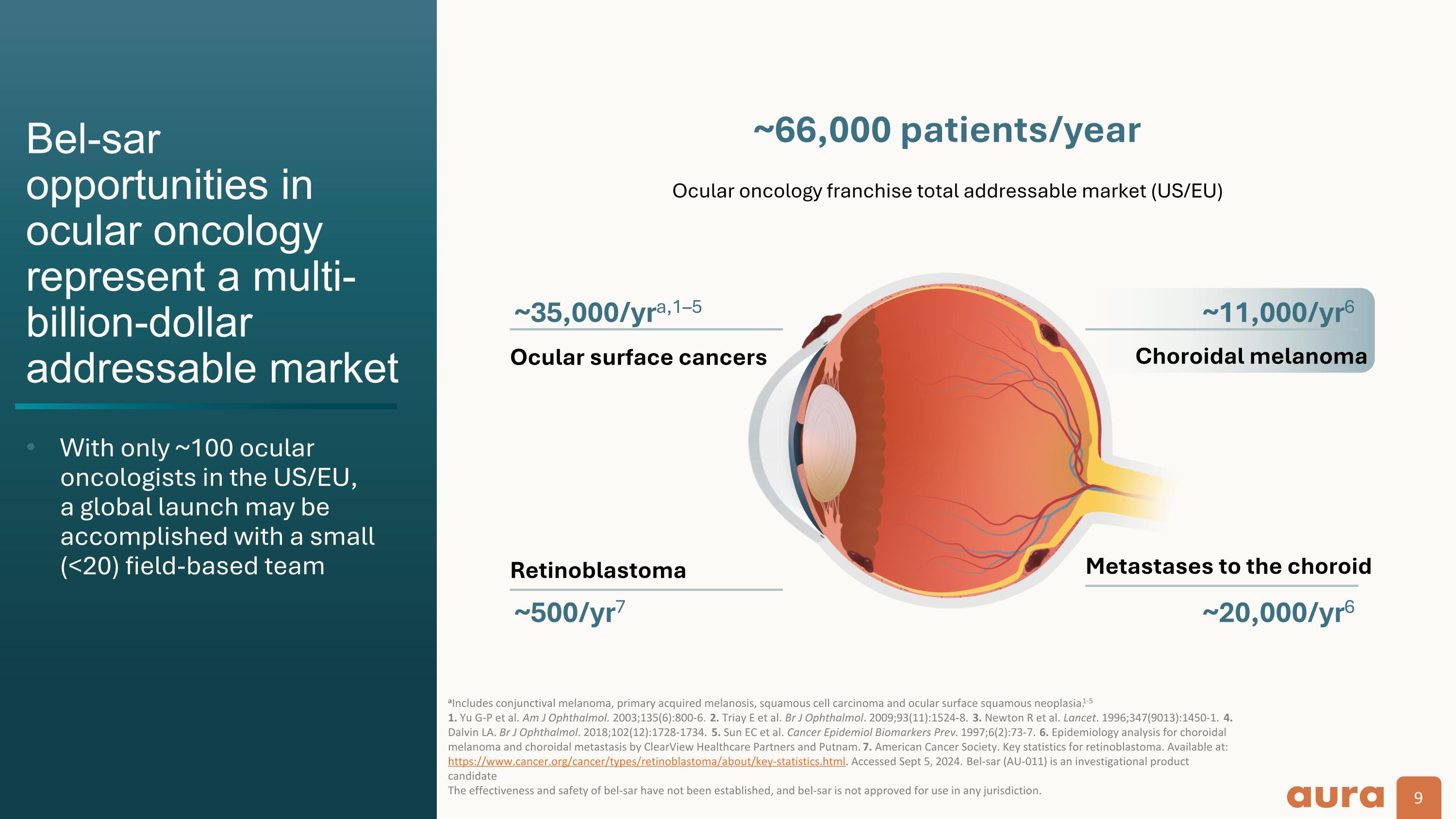
Bel-sar opportunities in ocular oncology represent a multi-billion-dollar addressable market With only ~100 ocular oncologists in the US/EU, a global launch may be accomplished with a small (<20) field-based team aIncludes conjunctival melanoma, primary acquired melanosis, squamous cell carcinoma and ocular surface squamous neoplasia.1-5 1. Yu G-P et al. Am J Ophthalmol. 2003;135(6):800-6. 2. Triay E et al. Br J Ophthalmol. 2009;93(11):1524-8. 3. Newton R et al. Lancet. 1996;347(9013):1450-1. 4. Dalvin LA. Br J Ophthalmol. 2018;102(12):1728-1734. 5. Sun EC et al. Cancer Epidemiol Biomarkers Prev. 1997;6(2):73-7. 6. Epidemiology analysis for choroidal melanoma and choroidal metastasis by ClearView Healthcare Partners and Putnam. 7. American Cancer Society. Key statistics for retinoblastoma. Available at: https://www.cancer.org/cancer/types/retinoblastoma/about/key-statistics.html. Accessed Sept 5, 2024. Bel-sar (AU-011) is an investigational product candidate The effectiveness and safety of bel-sar have not been established, and bel-sar is not approved for use in any jurisdiction. Ocular surface cancers ~66,000 patients/year ~35,000/yra,1–5 Choroidal melanoma ~11,000/yr6 Metastases to the choroid ~20,000/yr6 Retinoblastoma ~500/yr7 Ocular oncology franchise total addressable market (US/EU)
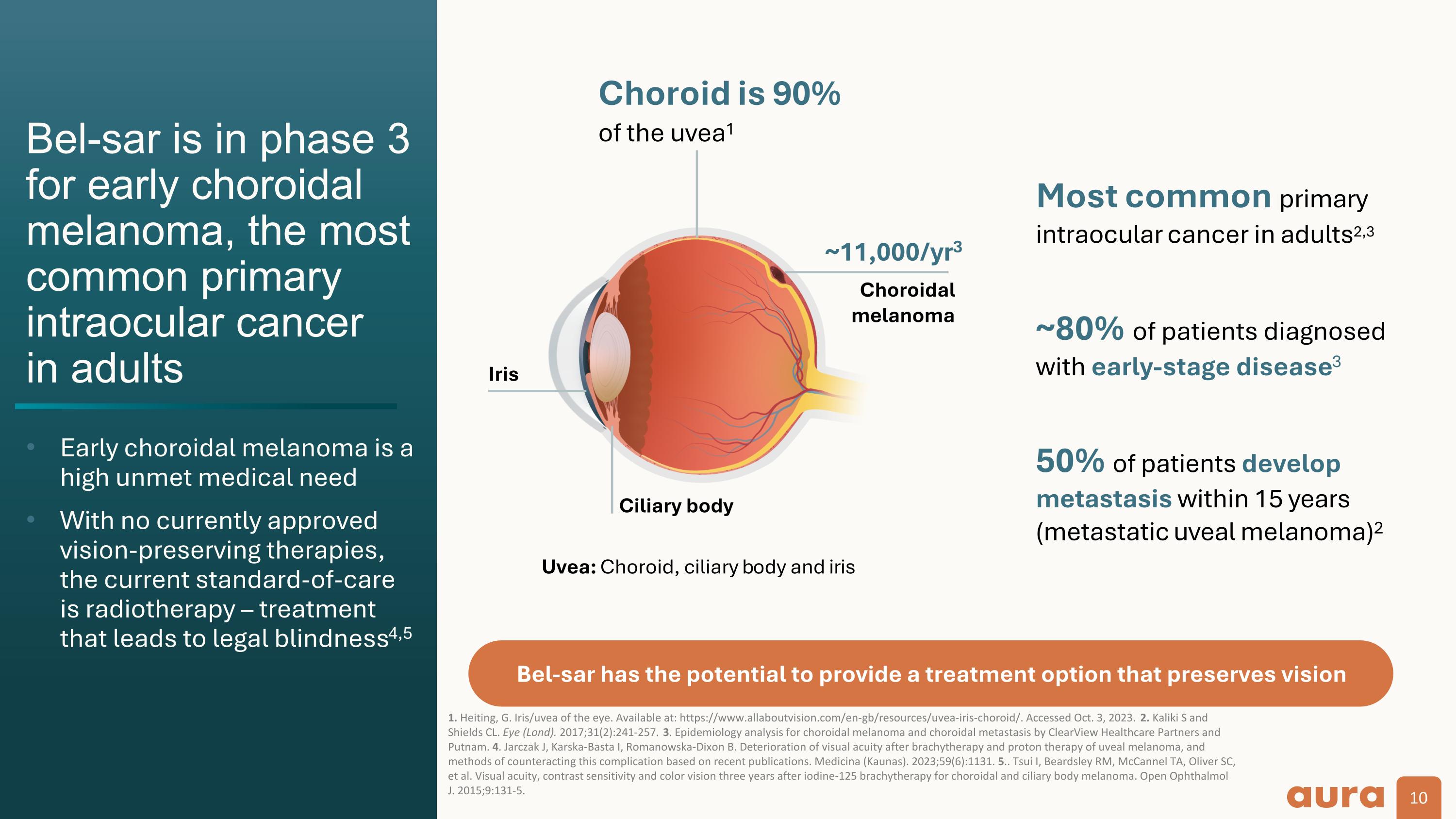
Bel-sar is in phase 3 for early choroidal melanoma, the most common primary intraocular cancer in adults Early choroidal melanoma is a high unmet medical need With no currently approved vision-preserving therapies, the current standard-of-care is radiotherapy – treatment that leads to legal blindness4,5 1. Heiting, G. Iris/uvea of the eye. Available at: https://www.allaboutvision.com/en-gb/resources/uvea-iris-choroid/. Accessed Oct. 3, 2023. 2. Kaliki S and Shields CL. Eye (Lond). 2017;31(2):241-257. 3. Epidemiology analysis for choroidal melanoma and choroidal metastasis by ClearView Healthcare Partners and Putnam. 4. Jarczak J, Karska-Basta I, Romanowska-Dixon B. Deterioration of visual acuity after brachytherapy and proton therapy of uveal melanoma, and methods of counteracting this complication based on recent publications. Medicina (Kaunas). 2023;59(6):1131. 5.. Tsui I, Beardsley RM, McCannel TA, Oliver SC, et al. Visual acuity, contrast sensitivity and color vision three years after iodine-125 brachytherapy for choroidal and ciliary body melanoma. Open Ophthalmol J. 2015;9:131-5. Choroid is 90% of the uvea1 Uvea: Choroid, ciliary body and iris Ciliary body Iris Most common primary intraocular cancer in adults2,3 50% of patients develop metastasis within 15 years (metastatic uveal melanoma)2 ~80% of patients diagnosed with early-stage disease3 Choroidal melanoma ~11,000/yr3 Bel-sar has the potential to provide a treatment option that preserves vision
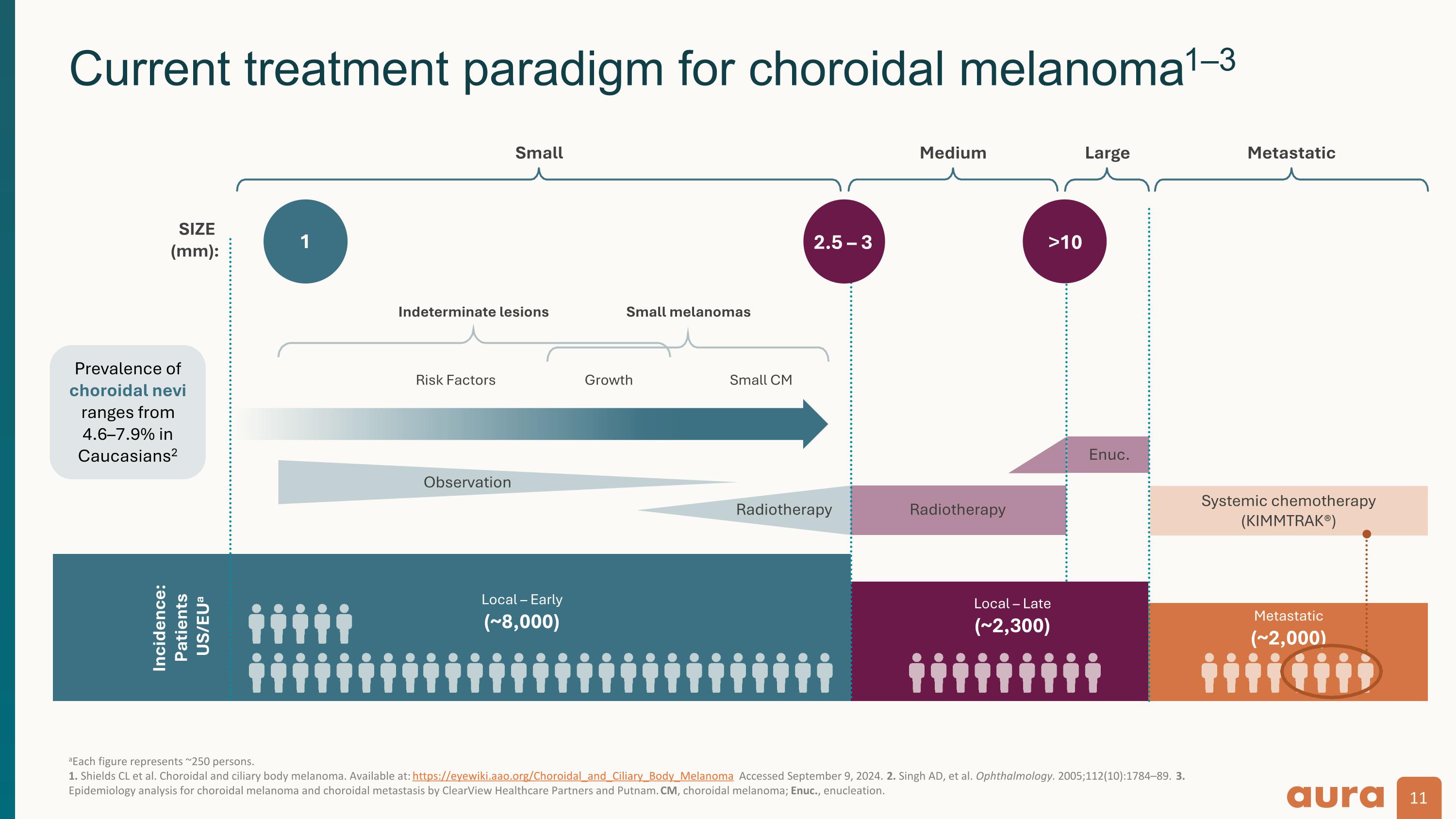
aEach figure represents ~250 persons. 1. Shields CL et al. Choroidal and ciliary body melanoma. Available at: https://eyewiki.aao.org/Choroidal_and_Ciliary_Body_Melanoma Accessed September 9, 2024. 2. Singh AD, et al. Ophthalmology. 2005;112(10):1784–89. 3. Epidemiology analysis for choroidal melanoma and choroidal metastasis by ClearView Healthcare Partners and Putnam. CM, choroidal melanoma; Enuc., enucleation. Current treatment paradigm for choroidal melanoma1–3 Indeterminate lesions Small melanomas Risk Factors Growth Small CM Observation Incidence: Patients US/EUa Local – Early (~8,000) Local – Late (~2,300) Metastatic (~2,000) SIZE (mm): Small Medium Large Metastatic Radiotherapy Radiotherapy 1 2.5 – 3 >10 Enuc. Systemic chemotherapy (KIMMTRAK®) Prevalence of choroidal nevi ranges from 4.6–7.9% in Caucasians2
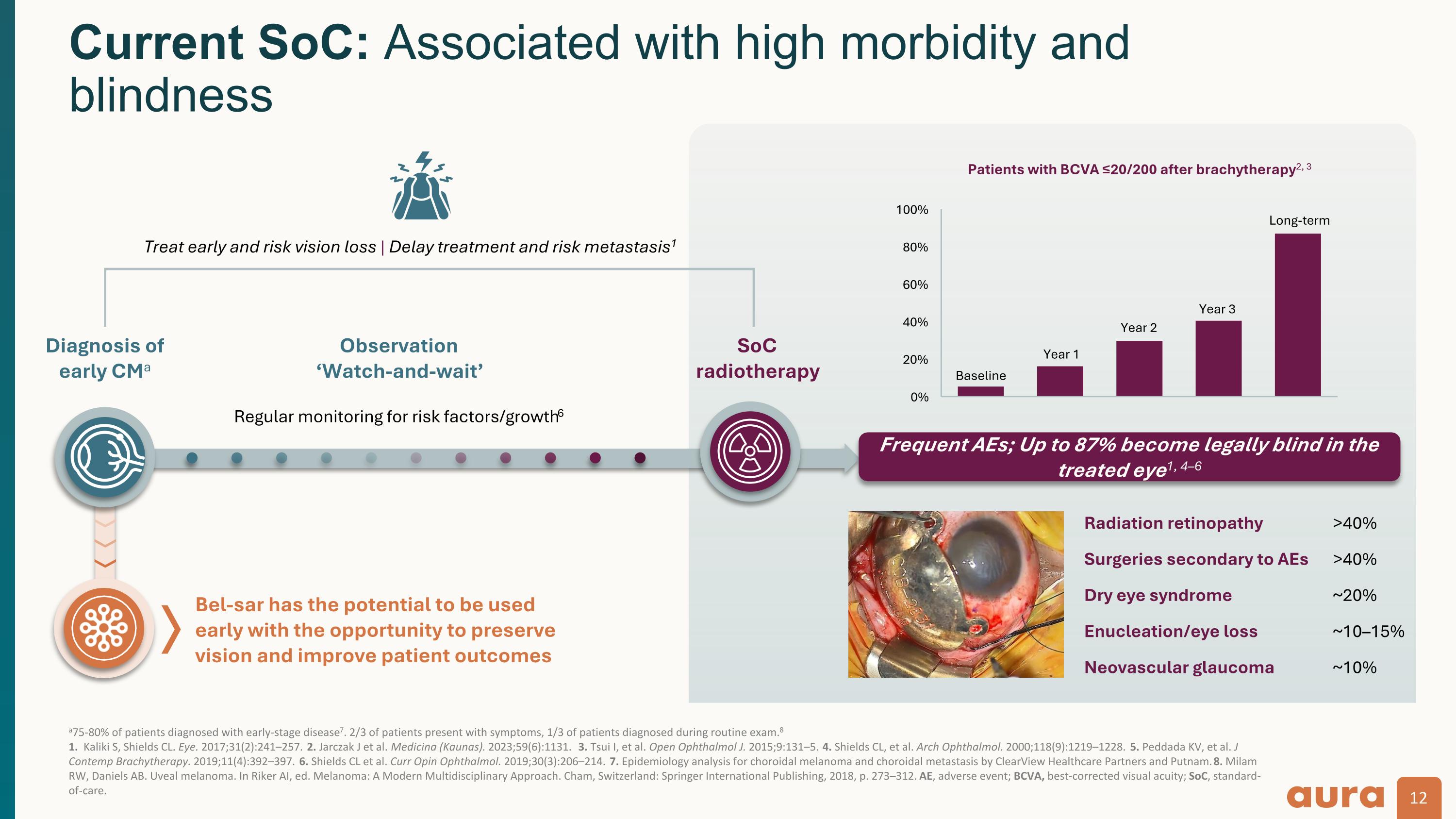
a75-80% of patients diagnosed with early-stage disease7. 2/3 of patients present with symptoms, 1/3 of patients diagnosed during routine exam.8 1. Kaliki S, Shields CL. Eye. 2017;31(2):241–257. 2. Jarczak J et al. Medicina (Kaunas). 2023;59(6):1131. 3. Tsui I, et al. Open Ophthalmol J. 2015;9:131–5. 4. Shields CL, et al. Arch Ophthalmol. 2000;118(9):1219–1228. 5. Peddada KV, et al. J Contemp Brachytherapy. 2019;11(4):392–397. 6. Shields CL et al. Curr Opin Ophthalmol. 2019;30(3):206–214. 7. Epidemiology analysis for choroidal melanoma and choroidal metastasis by ClearView Healthcare Partners and Putnam. 8. Milam RW, Daniels AB. Uveal melanoma. In Riker AI, ed. Melanoma: A Modern Multidisciplinary Approach. Cham, Switzerland: Springer International Publishing, 2018, p. 273–312. AE, adverse event; BCVA, best-corrected visual acuity; SoC, standard-of-care. Current SoC: Associated with high morbidity and blindness Observation ‘Watch-and-wait’ SoC radiotherapy Regular monitoring for risk factors/growth6 Treat early and risk vision loss | Delay treatment and risk metastasis1 Diagnosis of early CMa Frequent AEs; Up to 87% become legally blind in the treated eye1, 4–6 Radiation retinopathy >40% Surgeries secondary to AEs >40% Dry eye syndrome ~20% Enucleation/eye loss ~10–15% Neovascular glaucoma ~10% Year 1 Year 2 Year 3 Baseline Long-term Bel-sar has the potential to be used early with the opportunity to preserve vision and improve patient outcomes
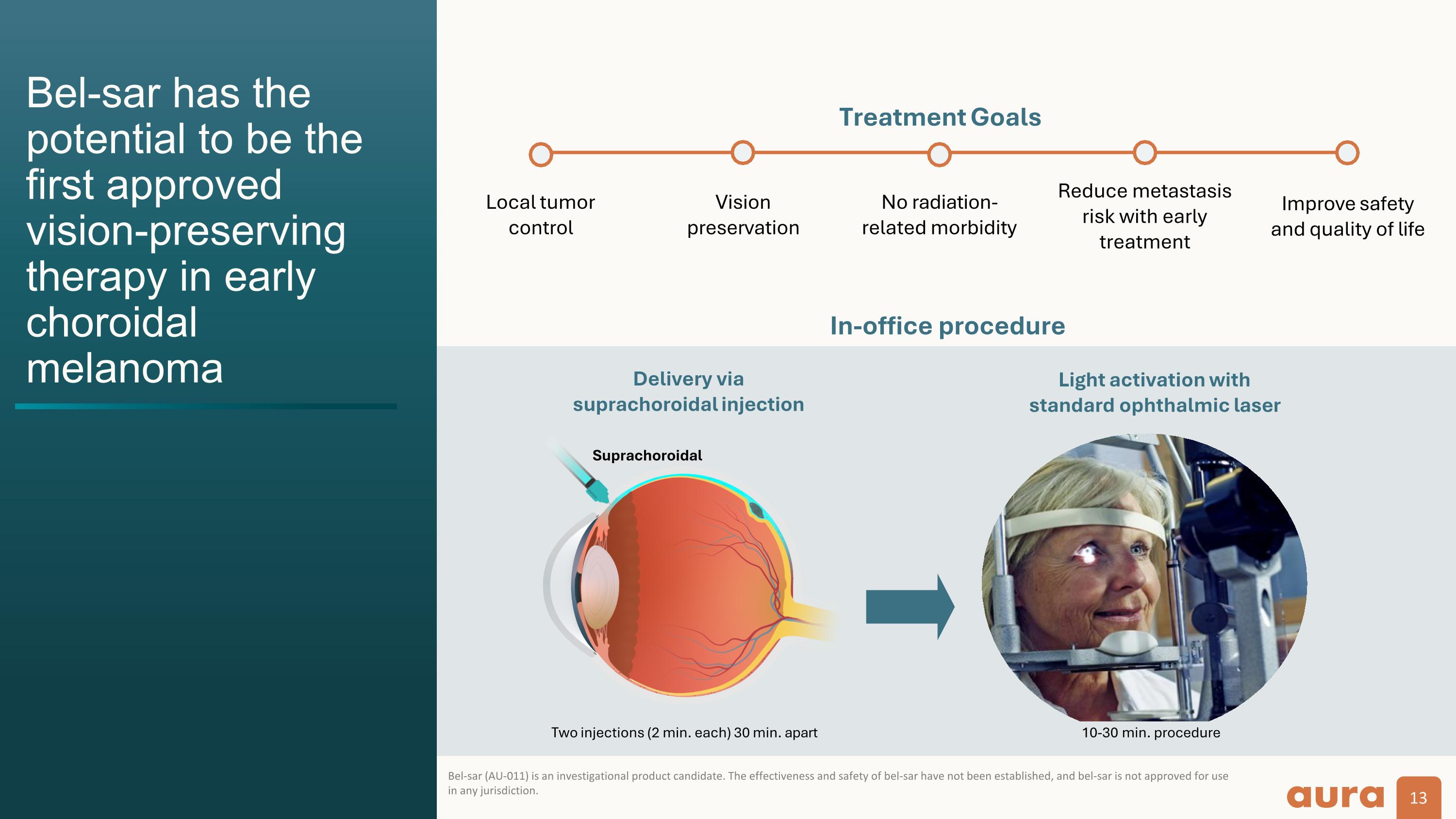
Bel-sar has the potential to be the first approved vision-preserving therapy in early choroidal melanoma Bel-sar (AU-011) is an investigational product candidate. The effectiveness and safety of bel-sar have not been established, and bel-sar is not approved for use in any jurisdiction. No radiation- related morbidity Vision preservation Local tumor control Reduce metastasis risk with early treatment Improve safety and quality of life Treatment Goals In-office procedure Two injections (2 min. each) 30 min. apart 10-30 min. procedure Delivery via suprachoroidal injection Light activation with standard ophthalmic laser Suprachoroidal
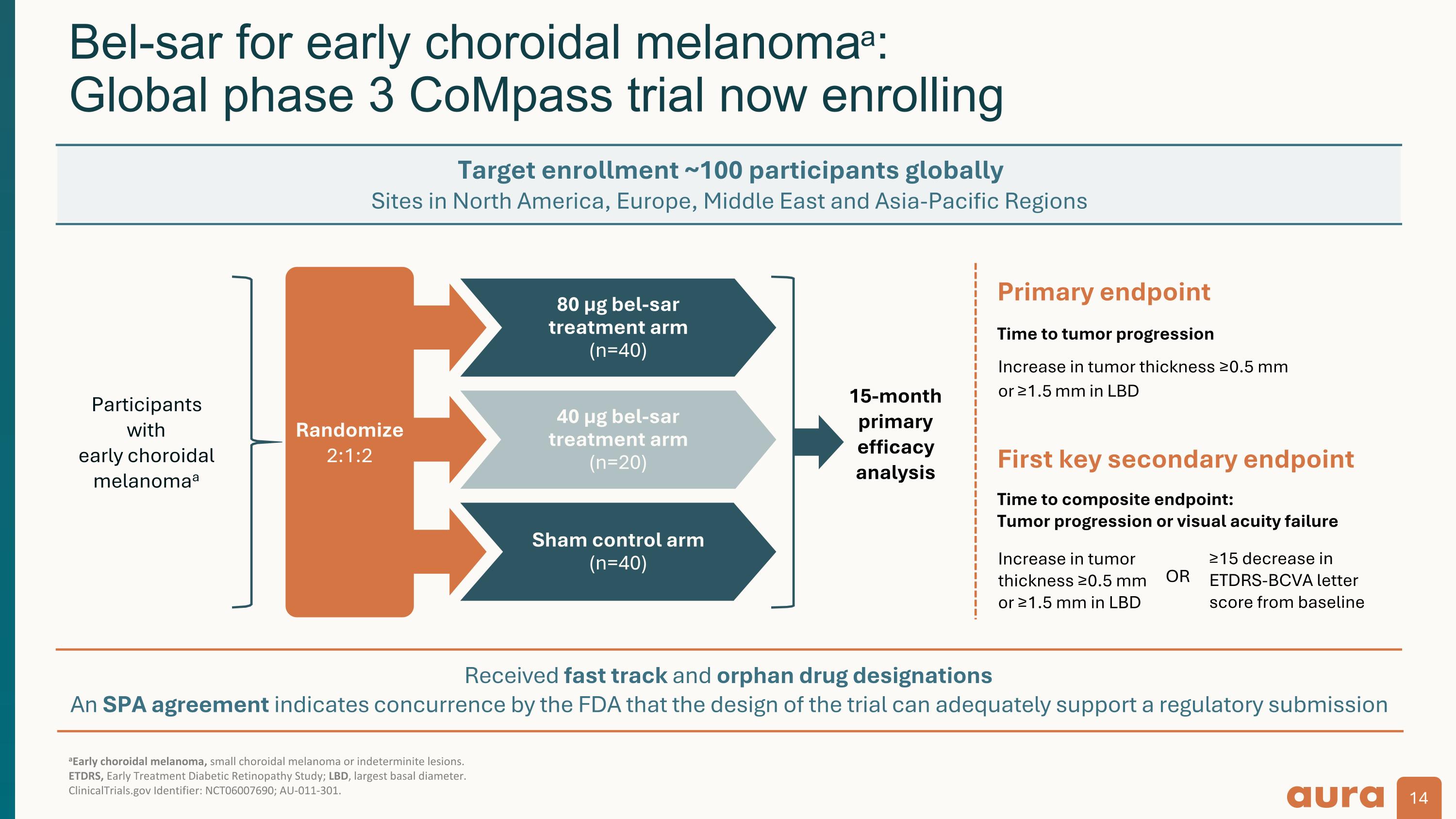
Received fast track and orphan drug designations An SPA agreement indicates concurrence by the FDA that the design of the trial can adequately support a regulatory submission aEarly choroidal melanoma, small choroidal melanoma or indeterminite lesions. ETDRS, Early Treatment Diabetic Retinopathy Study; LBD, largest basal diameter. ClinicalTrials.gov Identifier: NCT06007690; AU-011-301. Bel-sar for early choroidal melanomaa: Global phase 3 CoMpass trial now enrolling 15-month primary efficacy analysis 80 µg bel-sar treatment arm (n=40) 40 µg bel-sar treatment arm (n=20) Sham control arm (n=40) Participants with early choroidal melanomaa Randomize 2:1:2 First key secondary endpoint Primary endpoint Time to tumor progression Increase in tumor thickness ≥0.5 mm or ≥1.5 mm in LBD Time to composite endpoint: Tumor progression or visual acuity failure ≥15 decrease in ETDRS-BCVA letter score from baseline Increase in tumor thickness ≥0.5 mm or ≥1.5 mm in LBD OR Target enrollment ~100 participants globally Sites in North America, Europe, Middle East and Asia-Pacific Regions
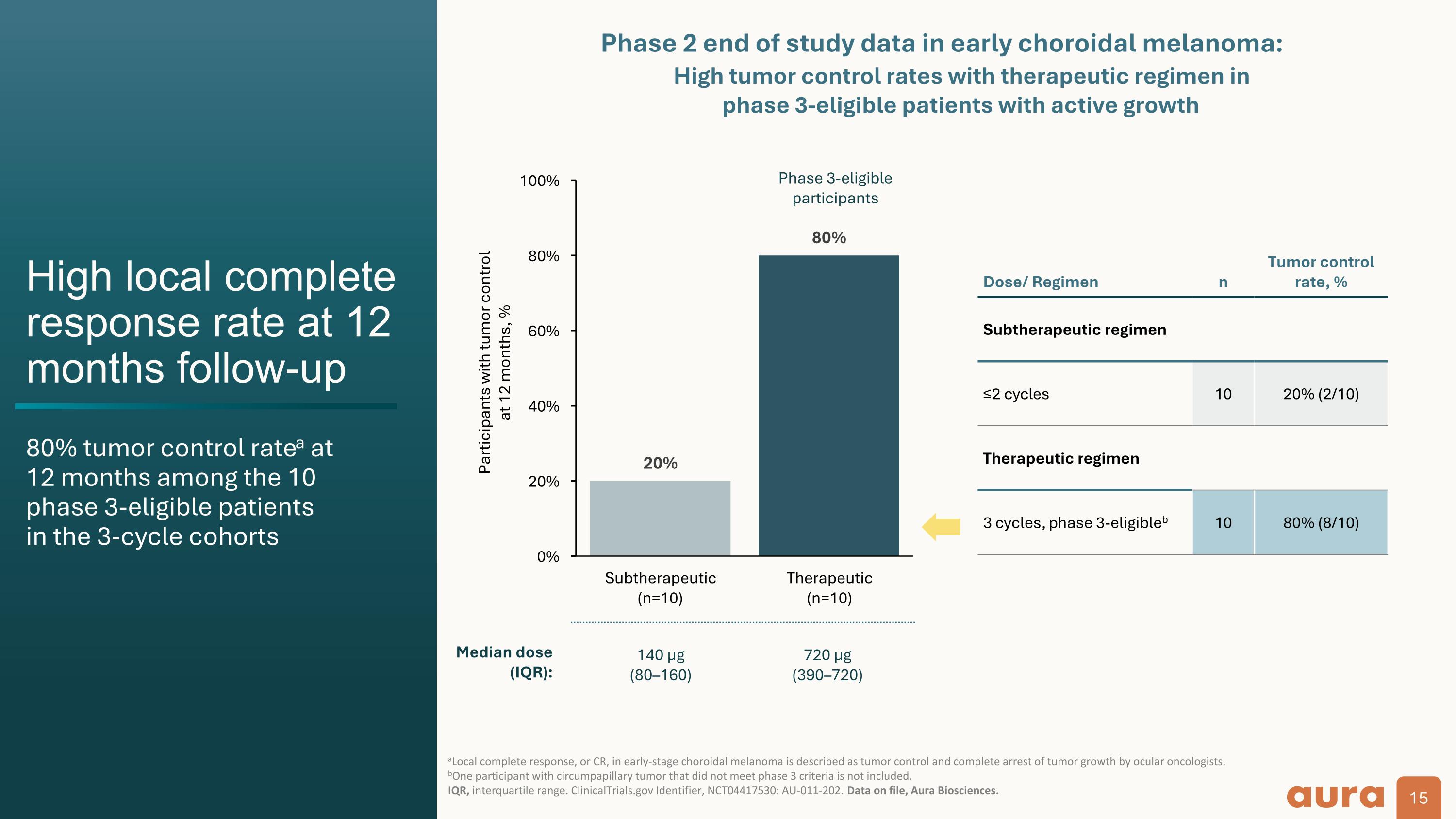
High local complete response rate at 12 months follow-up 80% tumor control ratea at 12 months among the 10 phase 3-eligible patients in the 3-cycle cohorts aLocal complete response, or CR, in early-stage choroidal melanoma is described as tumor control and complete arrest of tumor growth by ocular oncologists. bOne participant with circumpapillary tumor that did not meet phase 3 criteria is not included. IQR, interquartile range. ClinicalTrials.gov Identifier, NCT04417530: AU-011-202. Data on file, Aura Biosciences. Participants with tumor control at 12 months, % Dose/ Regimen n Tumor control rate, % Subtherapeutic regimen ≤2 cycles 10 20% (2/10) Therapeutic regimen 3 cycles, phase 3-eligibleb 10 80% (8/10) Phase 3-eligible participants High tumor control rates with therapeutic regimen in phase 3-eligible patients with active growth Median dose (IQR): 140 µg (80160) 720 µg (390–720) Phase 2 end of study data in early choroidal melanoma:
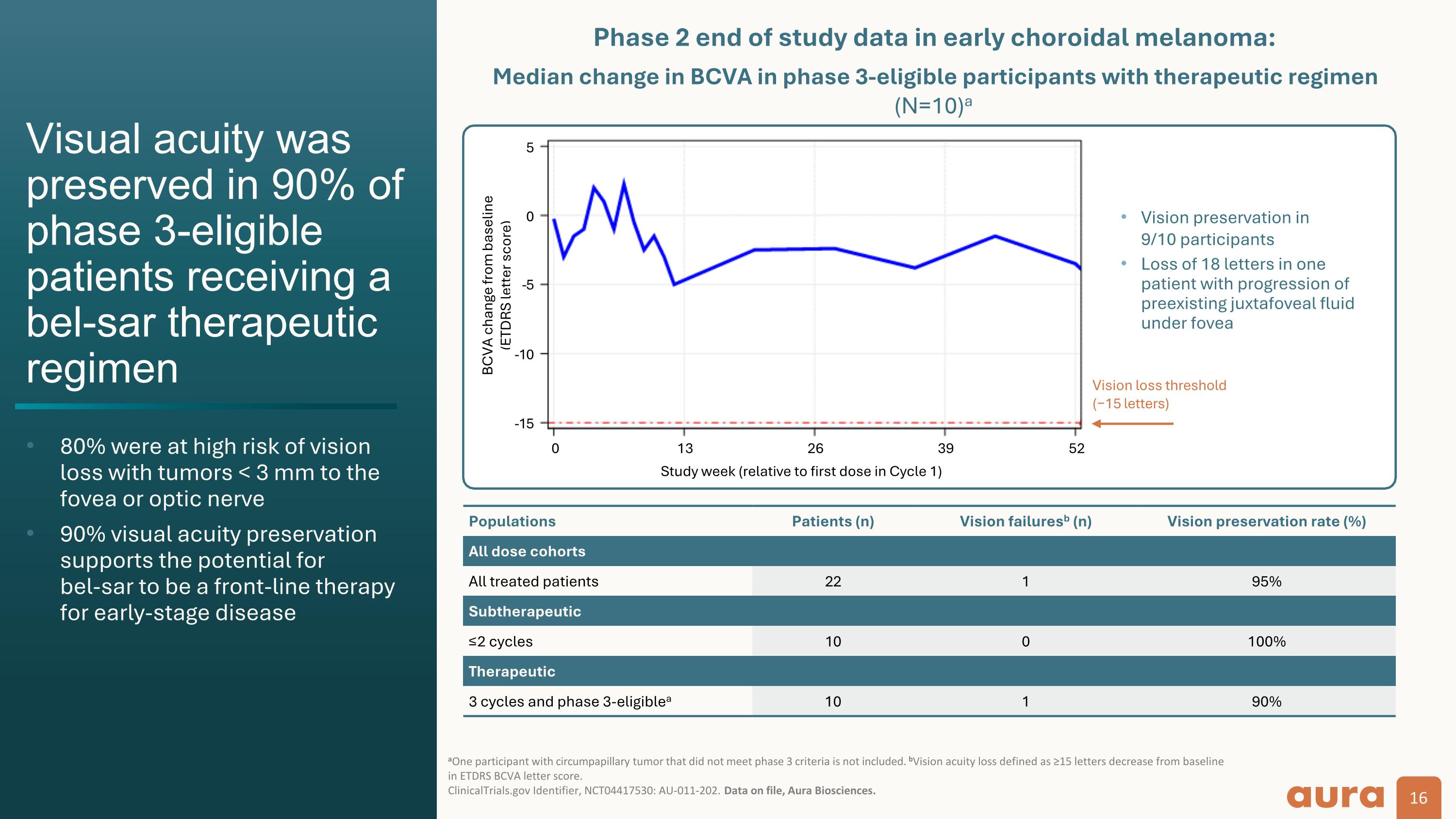
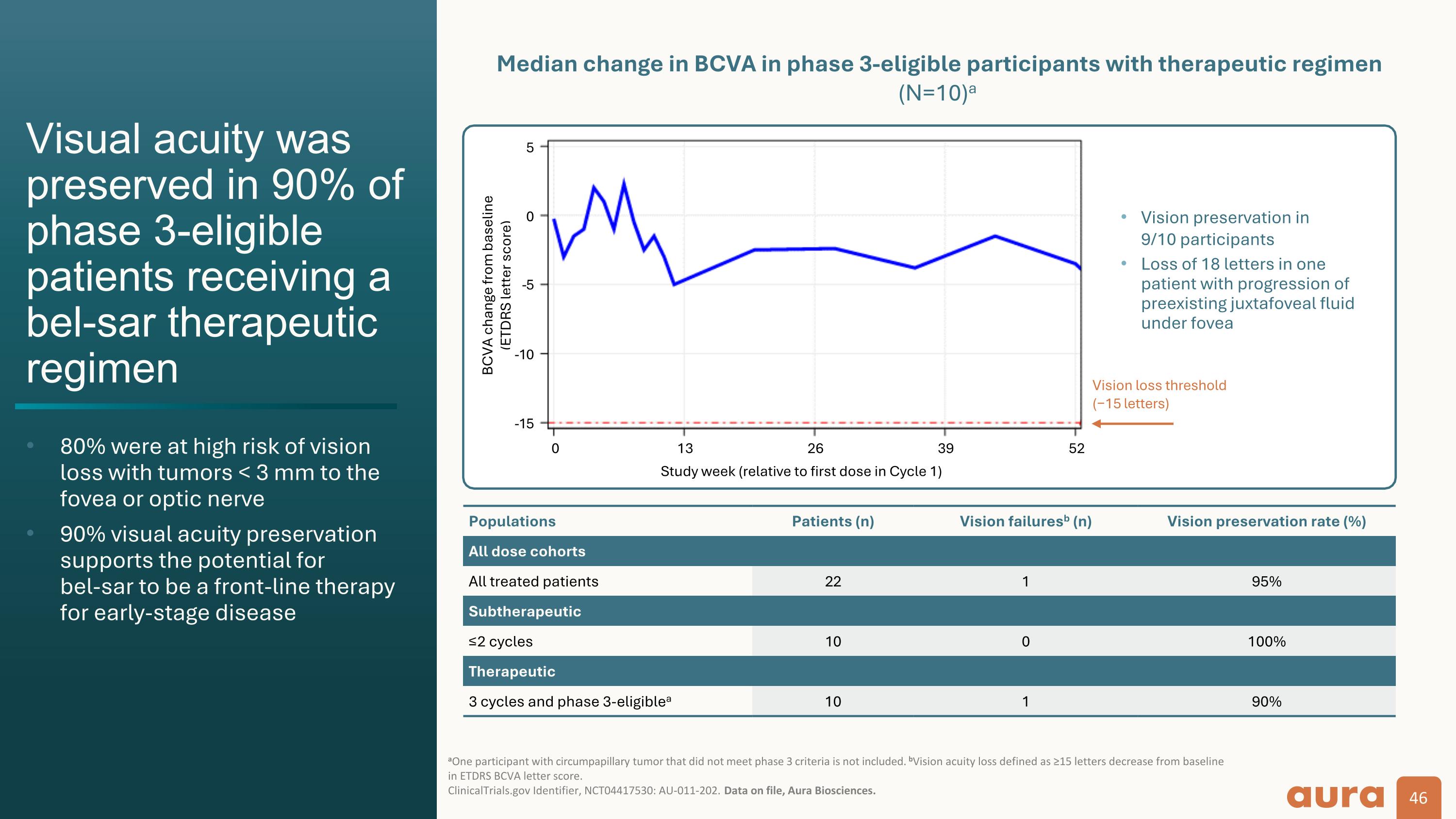
Vision loss threshold (−15 letters) Populations Patients (n) Vision failuresb (n) Vision preservation rate (%) All dose cohorts All treated patients 22 1 95% Subtherapeutic ≤2 cycles 10 0 100% Therapeutic 3 cycles and phase 3-eligiblea 10 1 90% BCVA change from baseline (ETDRS letter score) Median change in BCVA in phase 3-eligible participants with therapeutic regimen (N=10)a Visual acuity was preserved in 90% of phase 3-eligible patients receiving a bel-sar therapeutic regimen 80% were at high risk of vision loss with tumors < 3 mm to the fovea or optic nerve 90% visual acuity preservation supports the potential for bel-sar to be a front-line therapy for early-stage disease aOne participant with circumpapillary tumor that did not meet phase 3 criteria is not included. bVision acuity loss defined as ≥15 letters decrease from baseline in ETDRS BCVA letter score. ClinicalTrials.gov Identifier, NCT04417530: AU-011-202. Data on file, Aura Biosciences. Study week (relative to first dose in Cycle 1) Vision preservation in 9/10 participants Loss of 18 letters in one patient with progression of preexisting juxtafoveal fluid under fovea -5 0 5 -5 -10 -15 0 13 26 39 52 Phase 2 end of study data in early choroidal melanoma:
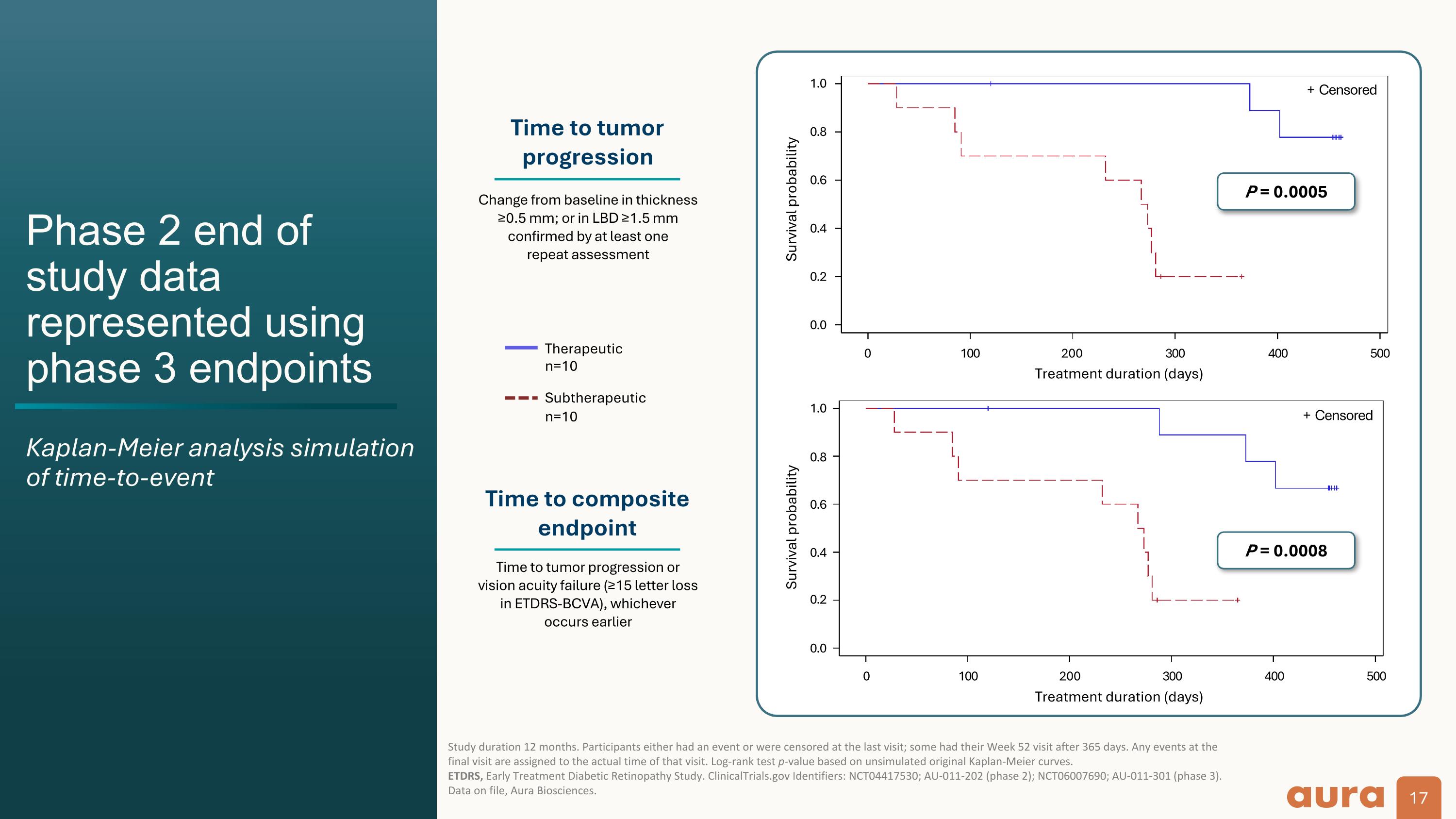
Phase 2 end of study data represented using phase 3 endpoints Kaplan-Meier analysis simulation of time-to-event Study duration 12 months. Participants either had an event or were censored at the last visit; some had their Week 52 visit after 365 days. Any events at the final visit are assigned to the actual time of that visit. Log-rank test p-value based on unsimulated original Kaplan-Meier curves. ETDRS, Early Treatment Diabetic Retinopathy Study. ClinicalTrials.gov Identifiers: NCT04417530; AU-011-202 (phase 2); NCT06007690; AU-011-301 (phase 3). Data on file, Aura Biosciences. Survival probability P = 0.0005 Time to tumor progression Time to composite endpoint Change from baseline in thickness ≥0.5 mm; or in LBD ≥1.5 mm confirmed by at least one repeat assessment Therapeutic n=10 Subtherapeutic n=10 Time to tumor progression or vision acuity failure (≥15 letter loss in ETDRS-BCVA), whichever occurs earlier 0.0 0.2 0.4 0.6 0.8 1.0 + Censored 0.0 0.2 0.4 0.6 0.8 1.0 0 100 200 300 400 500 P = 0.0008 Survival probability 0 100 200 300 400 500 + Censored Treatment duration (days) Treatment duration (days)
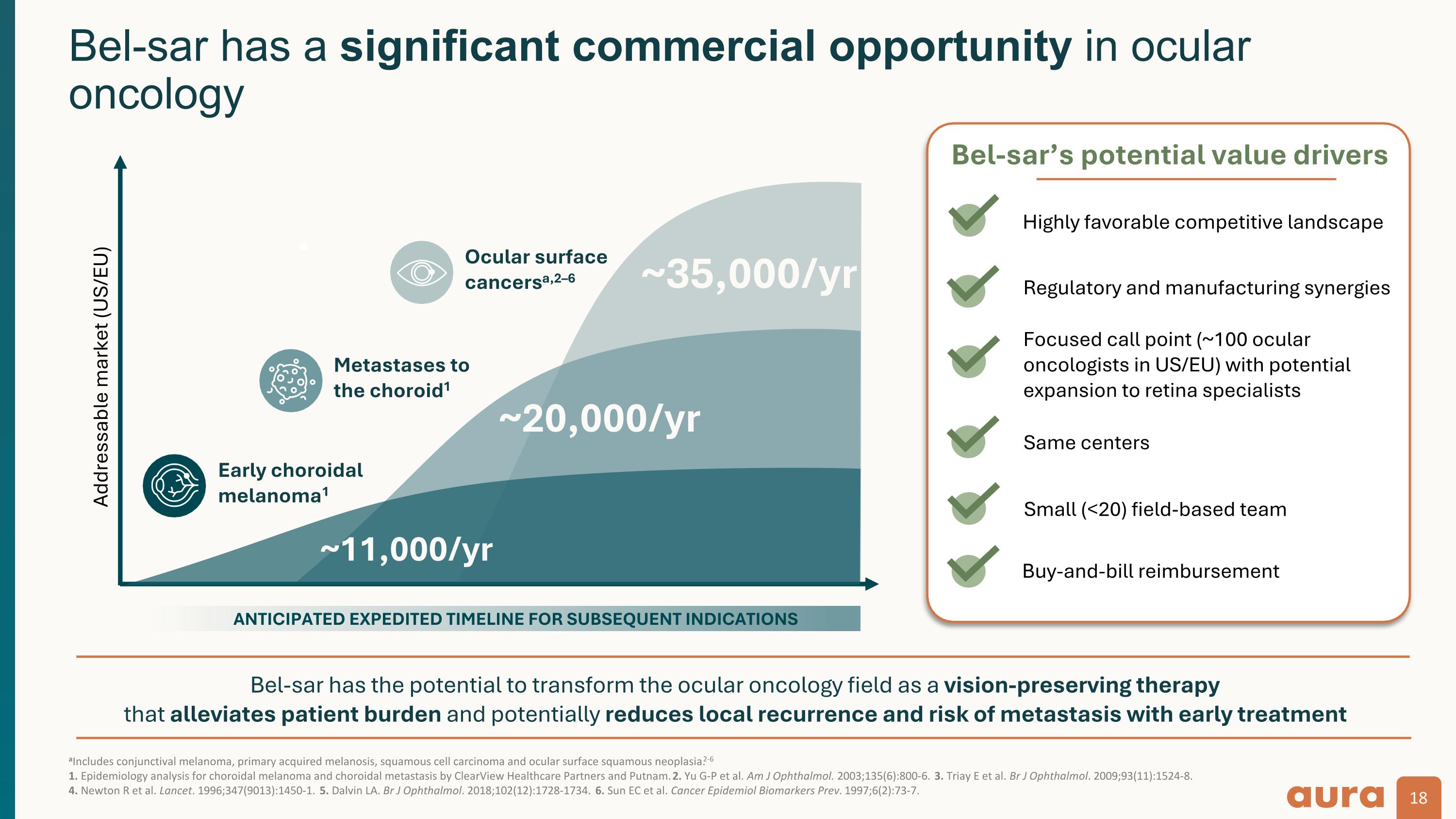
aIncludes conjunctival melanoma, primary acquired melanosis, squamous cell carcinoma and ocular surface squamous neoplasia.2-6 1. Epidemiology analysis for choroidal melanoma and choroidal metastasis by ClearView Healthcare Partners and Putnam. 2. Yu G-P et al. Am J Ophthalmol. 2003;135(6):800-6. 3. Triay E et al. Br J Ophthalmol. 2009;93(11):1524-8. 4. Newton R et al. Lancet. 1996;347(9013):1450-1. 5. Dalvin LA. Br J Ophthalmol. 2018;102(12):1728-1734. 6. Sun EC et al. Cancer Epidemiol Biomarkers Prev. 1997;6(2):73-7. Bel-sar has a significant commercial opportunity in ocular oncology ~35,000/yr Bel-sar has the potential to transform the ocular oncology field as a vision-preserving therapy that alleviates patient burden and potentially reduces local recurrence and risk of metastasis with early treatment Addressable market (US/EU) ~20,000/yr Early choroidal melanoma1 Metastases to the choroid1 ANTICIPATED EXPEDITED TIMELINE FOR SUBSEQUENT INDICATIONS ~11,000/yr Bel-sar’s potential value drivers Highly favorable competitive landscape Regulatory and manufacturing synergies Focused call point (~100 ocular oncologists in US/EU) with potential expansion to retina specialists Same centers Small (<20) field-based team Buy-and-bill reimbursement Ocular surface cancersa,2–6
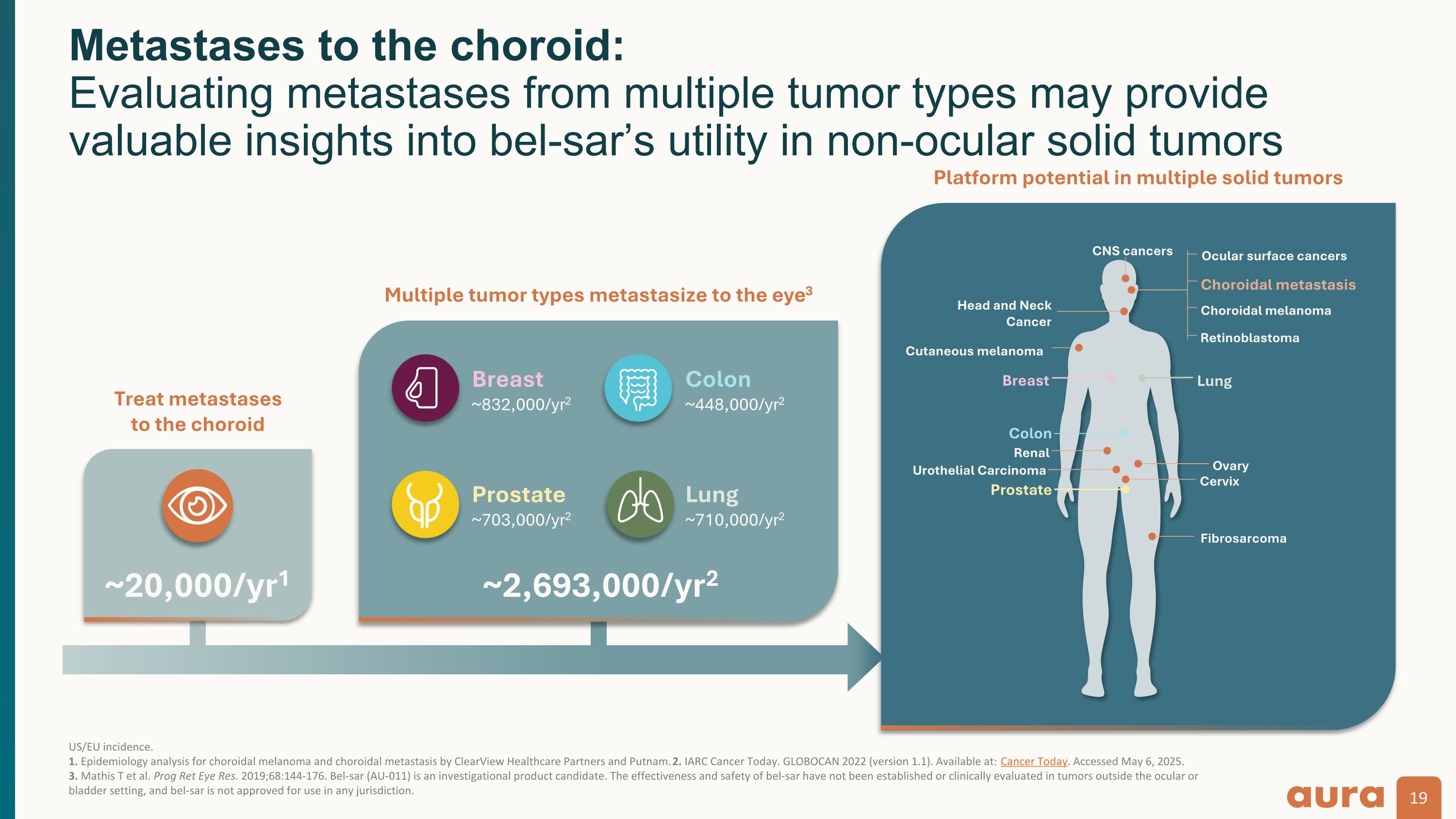
US/EU incidence. 1. Epidemiology analysis for choroidal melanoma and choroidal metastasis by ClearView Healthcare Partners and Putnam. 2. IARC Cancer Today. GLOBOCAN 2022 (version 1.1). Available at: Cancer Today. Accessed May 6, 2025. 3. Mathis T et al. Prog Ret Eye Res. 2019;68:144-176. Bel-sar (AU-011) is an investigational product candidate. The effectiveness and safety of bel-sar have not been established or clinically evaluated in tumors outside the ocular or bladder setting, and bel-sar is not approved for use in any jurisdiction. Metastases to the choroid: Evaluating metastases from multiple tumor types may provide valuable insights into bel-sar’s utility in non-ocular solid tumors Treat metastases to the choroid Multiple tumor types metastasize to the eye3 Platform potential in multiple solid tumors Choroidal melanoma Choroidal metastasis Ocular surface cancers Retinoblastoma CNS cancers Head and Neck Cancer Breast Lung Cutaneous melanoma Colon Renal Ovary Cervix Urothelial Carcinoma Prostate Fibrosarcoma ~20,000/yr1 Breast ~832,000/yr2 Prostate ~703,000/yr2 Colon ~448,000/yr2 Lung ~710,000/yr2 ~2,693,000/yr2
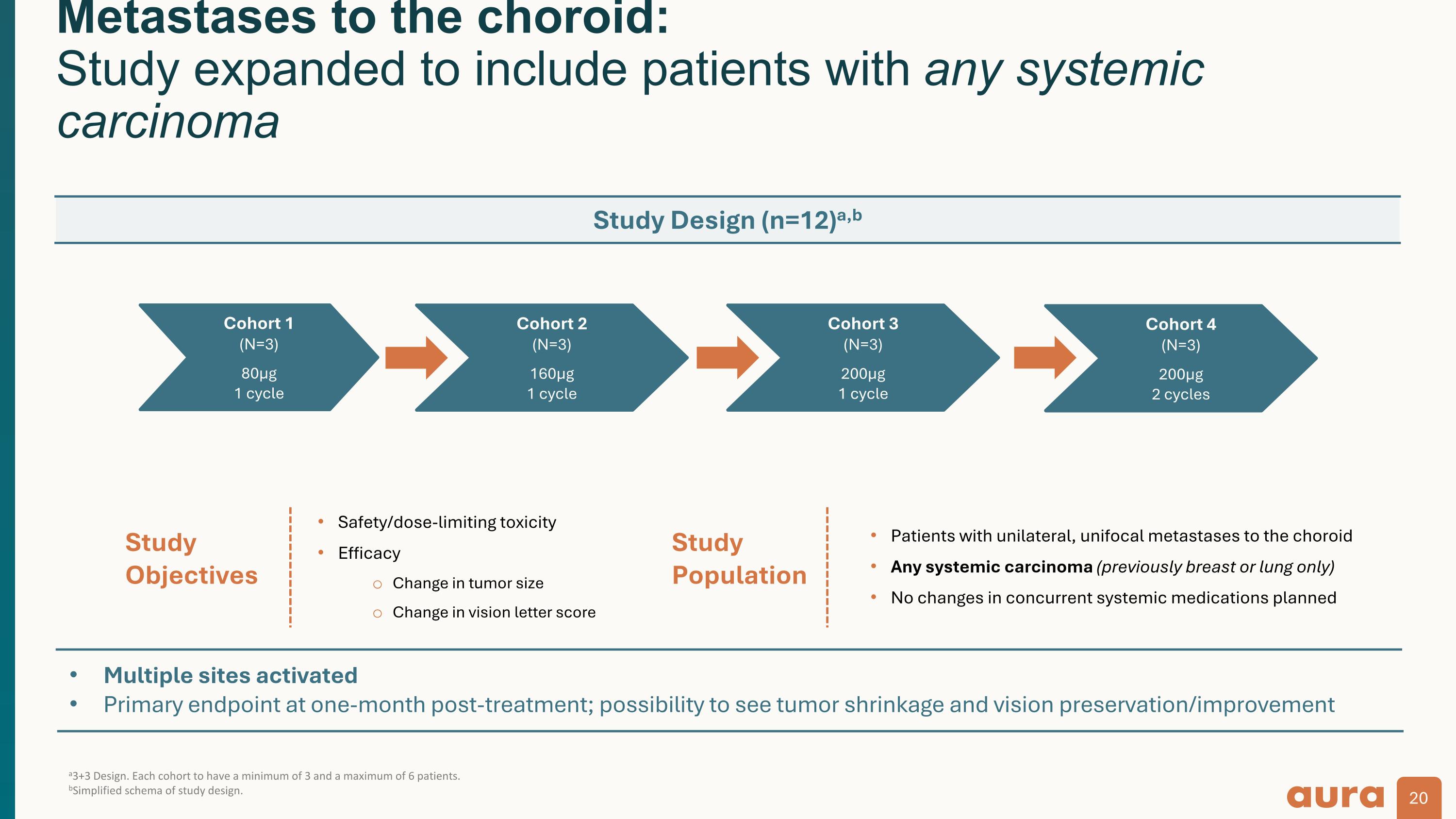
Multiple sites activated Primary endpoint at one-month post-treatment; possibility to see tumor shrinkage and vision preservation/improvement a3+3 Design. Each cohort to have a minimum of 3 and a maximum of 6 patients. bSimplified schema of study design. Metastases to the choroid: Study expanded to include patients with any systemic carcinoma Study Population Study Objectives Safety/dose-limiting toxicity Efficacy Change in tumor size Change in vision letter score Patients with unilateral, unifocal metastases to the choroid Any systemic carcinoma (previously breast or lung only) No changes in concurrent systemic medications planned Study Design (n=12)a,b Cohort 1 (N=3) 80µg 1 cycle Cohort 2 (N=3) 160µg 1 cycle Cohort 3 (N=3) 200µg 1 cycle Cohort 4 (N=3) 200µg 2 cycles
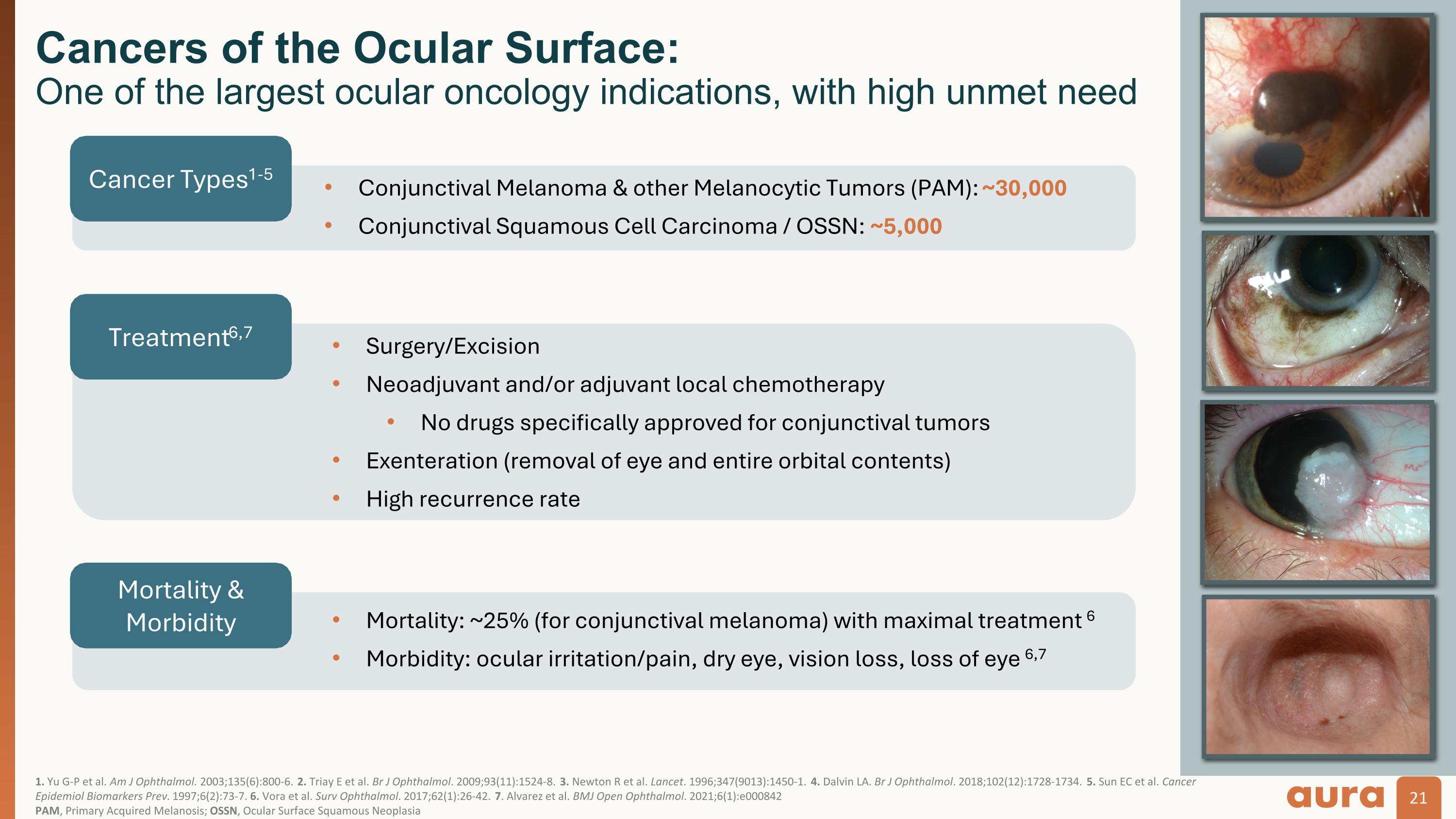
1. Yu G-P et al. Am J Ophthalmol. 2003;135(6):800-6. 2. Triay E et al. Br J Ophthalmol. 2009;93(11):1524-8. 3. Newton R et al. Lancet. 1996;347(9013):1450-1. 4. Dalvin LA. Br J Ophthalmol. 2018;102(12):1728-1734. 5. Sun EC et al. Cancer Epidemiol Biomarkers Prev. 1997;6(2):73-7. 6. Vora et al. Surv Ophthalmol. 2017;62(1):26-42. 7. Alvarez et al. BMJ Open Ophthalmol. 2021;6(1):e000842 PAM, Primary Acquired Melanosis; OSSN, Ocular Surface Squamous Neoplasia Cancers of the Ocular Surface: One of the largest ocular oncology indications, with high unmet need Cancer Types1-5 Conjunctival Melanoma & other Melanocytic Tumors (PAM): ~30,000 Conjunctival Squamous Cell Carcinoma / OSSN: ~5,000 Treatment6,7 Surgery/Excision Neoadjuvant and/or adjuvant local chemotherapy No drugs specifically approved for conjunctival tumors Exenteration (removal of eye and entire orbital contents) High recurrence rate Mortality & Morbidity Mortality: ~25% (for conjunctival melanoma) with maximal treatment 6 Morbidity: ocular irritation/pain, dry eye, vision loss, loss of eye 6,7
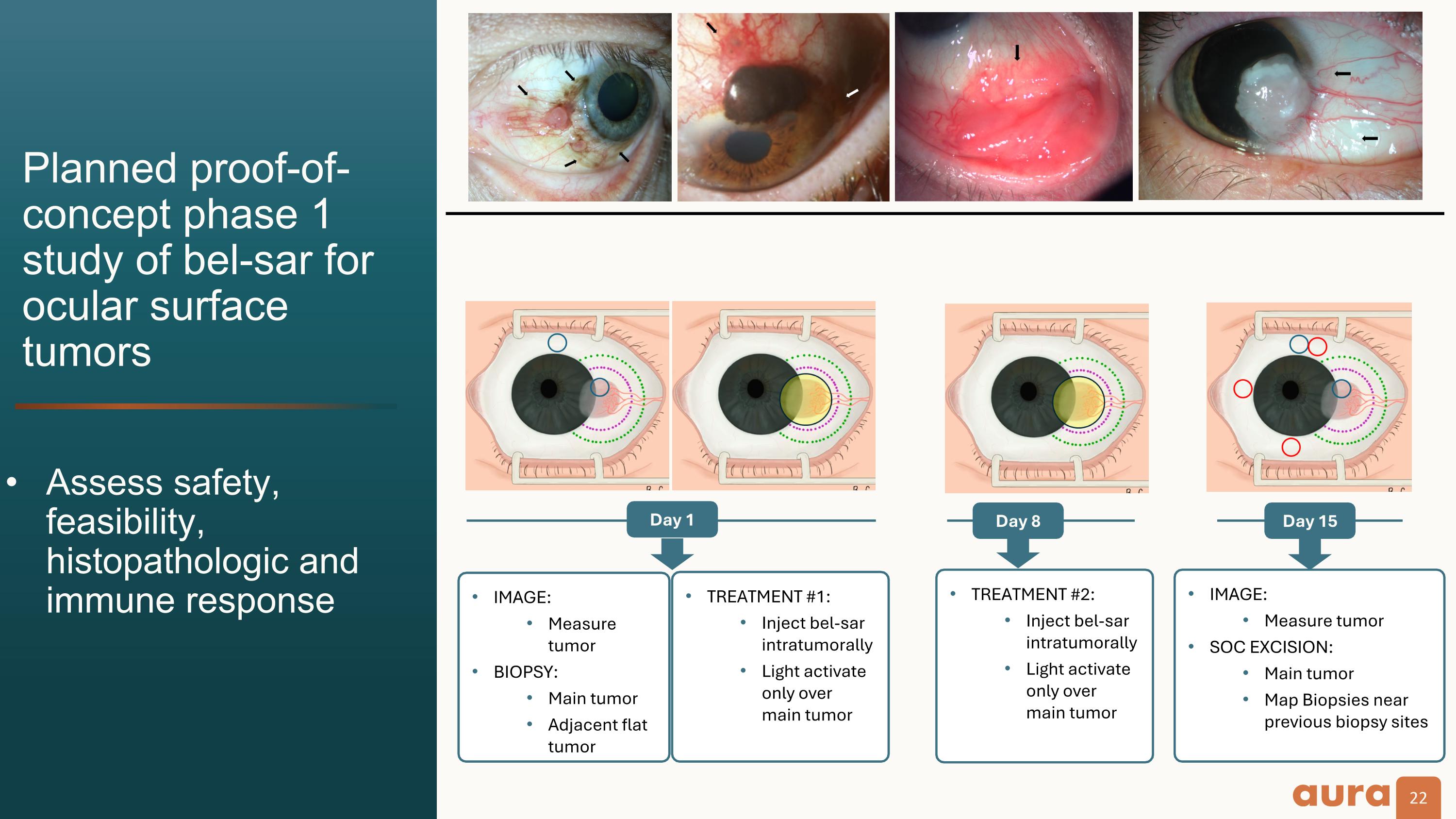
IMAGE: Measure tumor BIOPSY: Main tumor Adjacent flat tumor Planned proof-of-concept phase 1 study of bel-sar for ocular surface tumors Day 1 Day 8 TREATMENT #2: Inject bel-sar intratumorally Light activate only over main tumor Day 15 IMAGE: Measure tumor SOC EXCISION: Main tumor Map Biopsies near previous biopsy sites TREATMENT #1: Inject bel-sar intratumorally Light activate only over main tumor Assess safety, feasibility, histopathologic and immune response

Urologic Oncology Bel-sar target indications: Intermediate-risk NMIBC | High-risk NMIBC
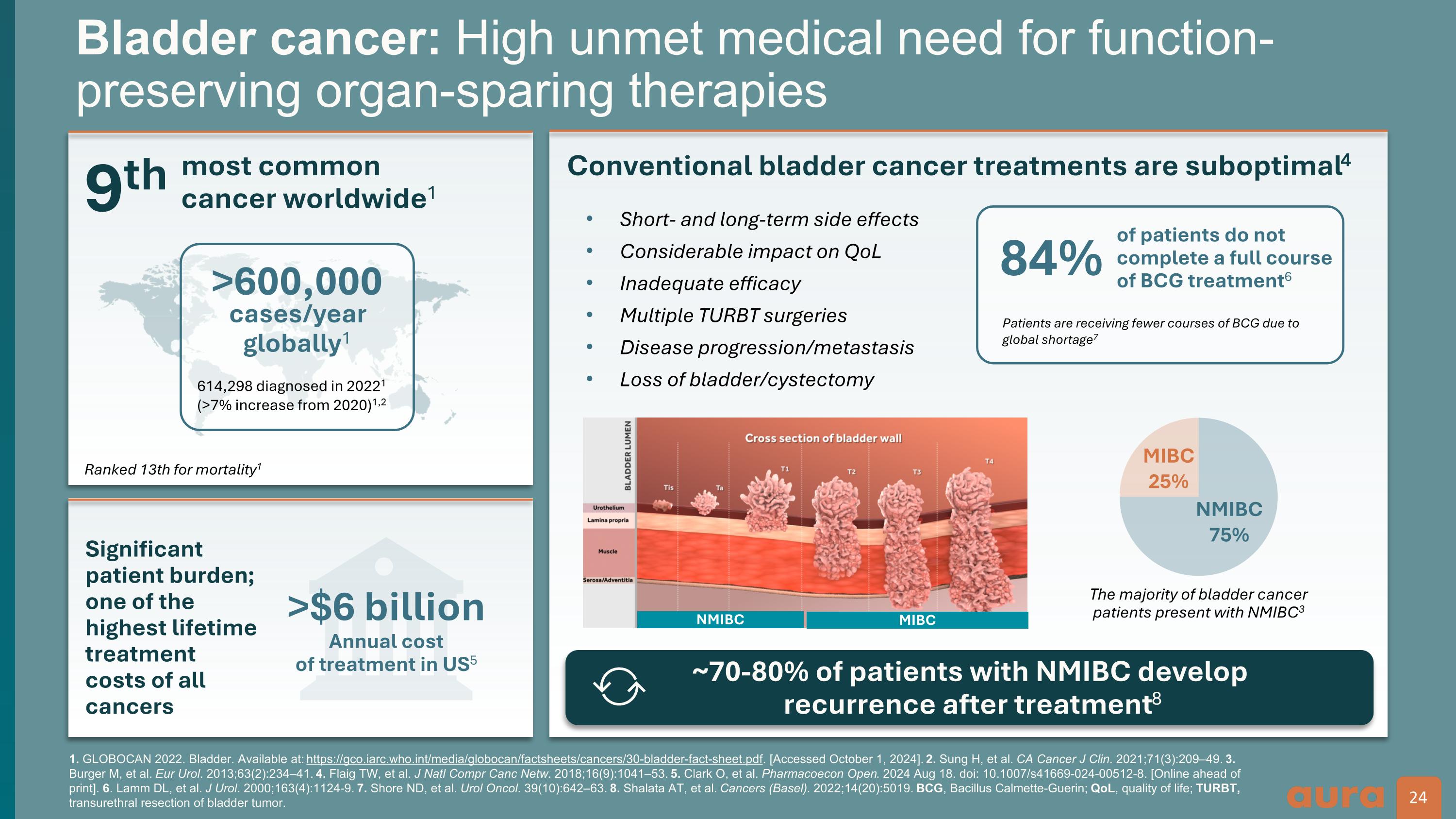
1. GLOBOCAN 2022. Bladder. Available at: https://gco.iarc.who.int/media/globocan/factsheets/cancers/30-bladder-fact-sheet.pdf. [Accessed October 1, 2024]. 2. Sung H, et al. CA Cancer J Clin. 2021;71(3):209–49. 3. Burger M, et al. Eur Urol. 2013;63(2):234–41. 4. Flaig TW, et al. J Natl Compr Canc Netw. 2018;16(9):1041–53. 5. Clark O, et al. Pharmacoecon Open. 2024 Aug 18. doi: 10.1007/s41669-024-00512-8. [Online ahead of print]. 6. Lamm DL, et al. J Urol. 2000;163(4):1124-9. 7. Shore ND, et al. Urol Oncol. 39(10):642–63. 8. Shalata AT, et al. Cancers (Basel). 2022;14(20):5019. BCG, Bacillus Calmette-Guerin; QoL, quality of life; TURBT, transurethral resection of bladder tumor. Bladder cancer: High unmet medical need for function-preserving organ-sparing therapies 9th most common cancer worldwide1 >$6 billion Annual cost of treatment in US5 Significant patient burden; one of the highest lifetime treatment costs of all cancers Conventional bladder cancer treatments are suboptimal4 Short- and long-term side effects Considerable impact on QoL Inadequate efficacy Multiple TURBT surgeries Disease progression/metastasis Loss of bladder/cystectomy MIBC 25% NMIBC 75% Ranked 13th for mortality1 24 24 The majority of bladder cancer patients present with NMIBC3 ~70-80% of patients with NMIBC develop recurrence after treatment8 NMIBC MIBC >600,000 614,298 diagnosed in 20221 (>7% increase from 2020)1,2 cases/year globally1 84% Patients are receiving fewer courses of BCG due to global shortage7 of patients do not complete a full course of BCG treatment6
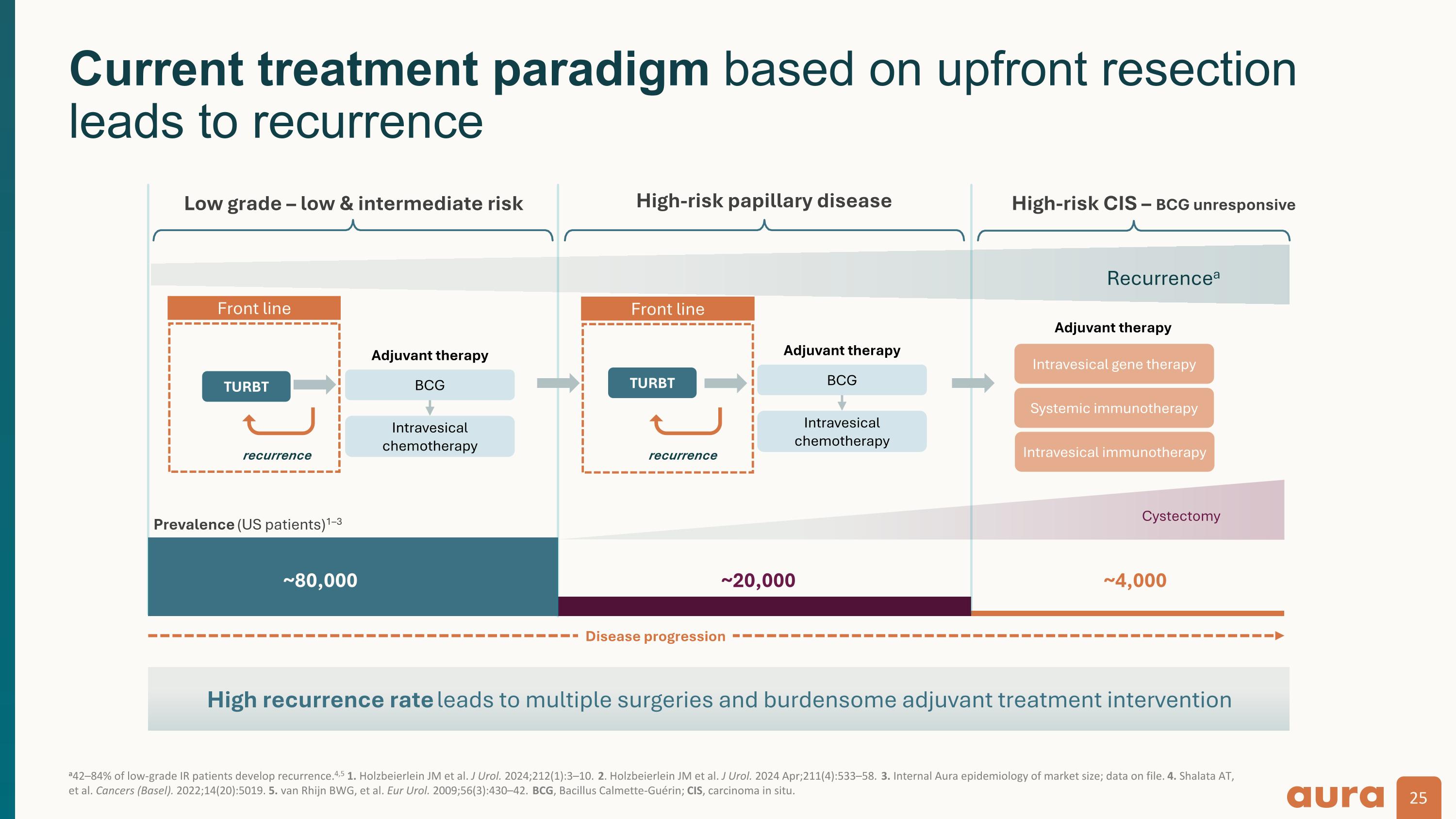
~80,000 ~20,000 Low grade – low & intermediate risk High-risk papillary disease High-risk CIS – BCG unresponsive BCG Intravesical chemotherapy ~4,000 TURBT recurrence Intravesical gene therapy Systemic immunotherapy TURBT recurrence Adjuvant therapy Adjuvant therapy Intravesical immunotherapy BCG Intravesical chemotherapy Adjuvant therapy Cystectomy Disease progression Recurrencea Prevalence (US patients)1–3 a42–84% of low-grade IR patients develop recurrence.4,5 1. Holzbeierlein JM et al. J Urol. 2024;212(1):3–10. 2. Holzbeierlein JM et al. J Urol. 2024 Apr;211(4):533–58. 3. Internal Aura epidemiology of market size; data on file. 4. Shalata AT, et al. Cancers (Basel). 2022;14(20):5019. 5. van Rhijn BWG, et al. Eur Urol. 2009;56(3):430–42. BCG, Bacillus Calmette-Guérin; CIS, carcinoma in situ. Current treatment paradigm based on upfront resection leads to recurrence Front line Front line High recurrence rate leads to multiple surgeries and burdensome adjuvant treatment intervention
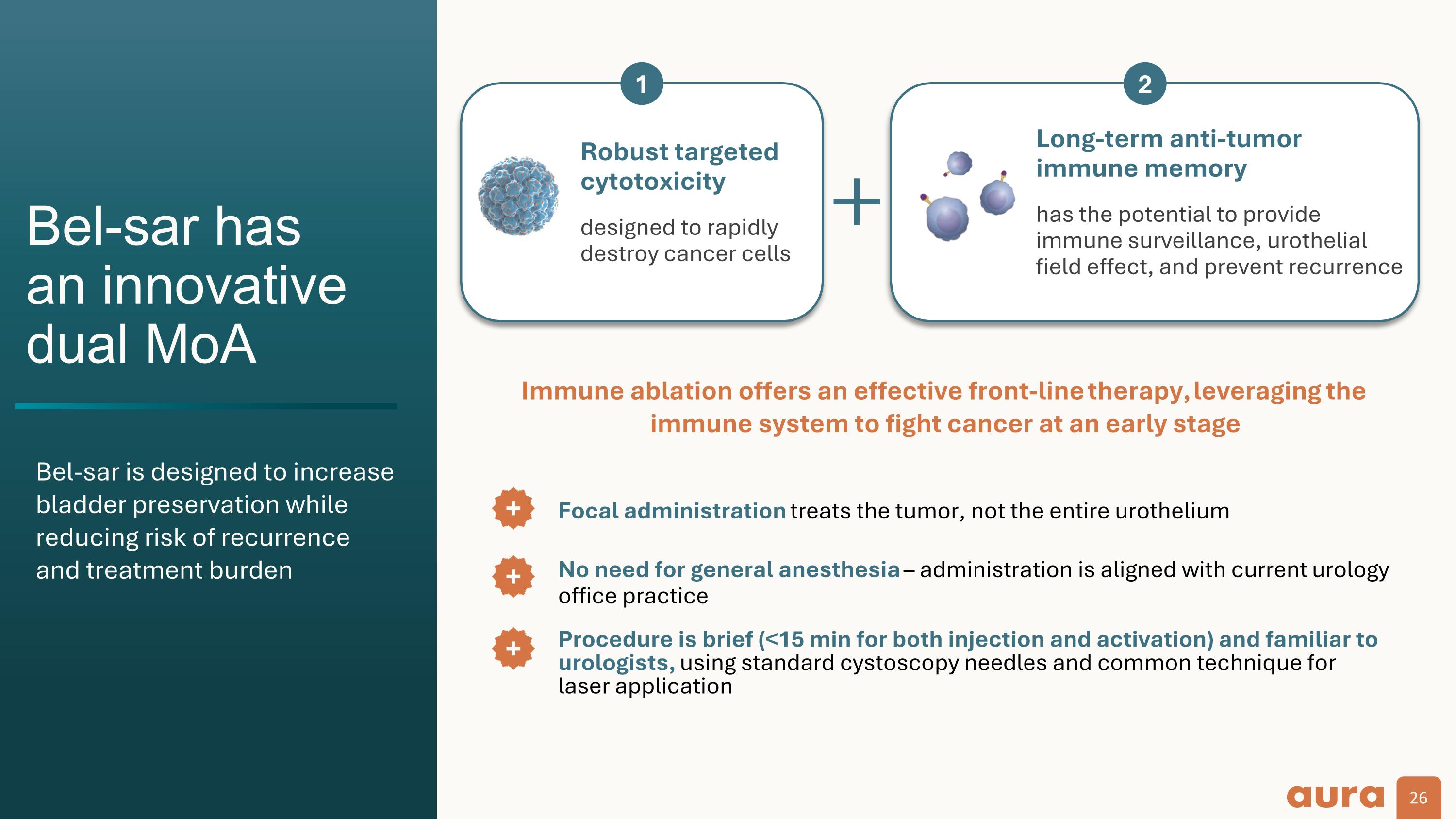
Bel-sar has an innovative dual MoA Long-term anti-tumor immune memory has the potential to provide immune surveillance, urothelial field effect, and prevent recurrence Robust targeted cytotoxicity designed to rapidly destroy cancer cells 1 2 Focal administration treats the tumor, not the entire urothelium Immune ablation offers an effective front-line therapy, leveraging the immune system to fight cancer at an early stage No need for general anesthesia – administration is aligned with current urology office practice Procedure is brief (<15 min for both injection and activation) and familiar to urologists, using standard cystoscopy needles and common technique for laser application Bel-sar is designed to increase bladder preservation while reducing risk of recurrence and treatment burden
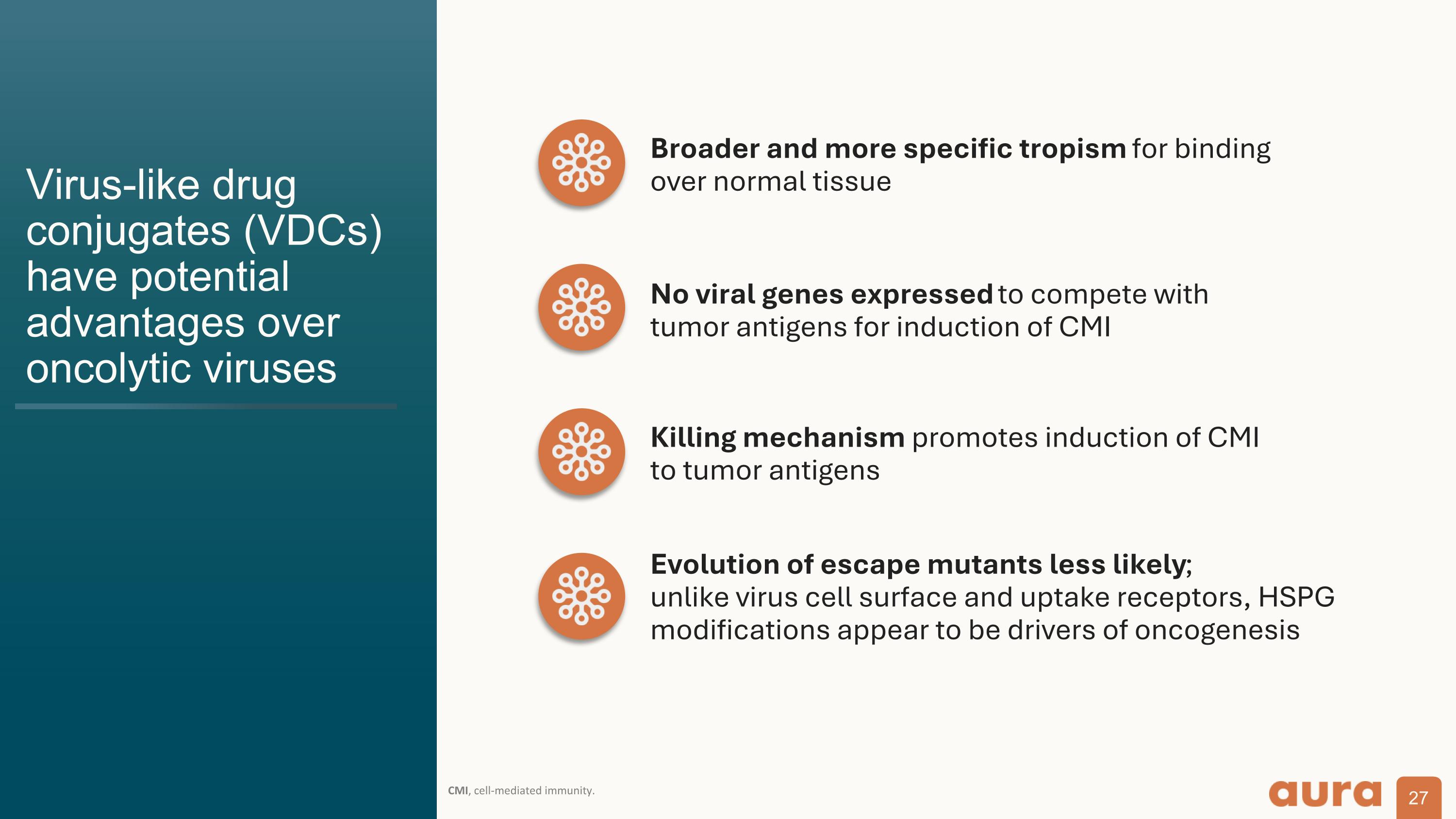
Virus-like drug conjugates (VDCs) have potential advantages over oncolytic viruses CMI, cell-mediated immunity. Broader and more specific tropism for binding over normal tissue No viral genes expressed to compete with tumor antigens for induction of CMI Killing mechanism promotes induction of CMI to tumor antigens Evolution of escape mutants less likely; unlike virus cell surface and uptake receptors, HSPG modifications appear to be drivers of oncogenesis
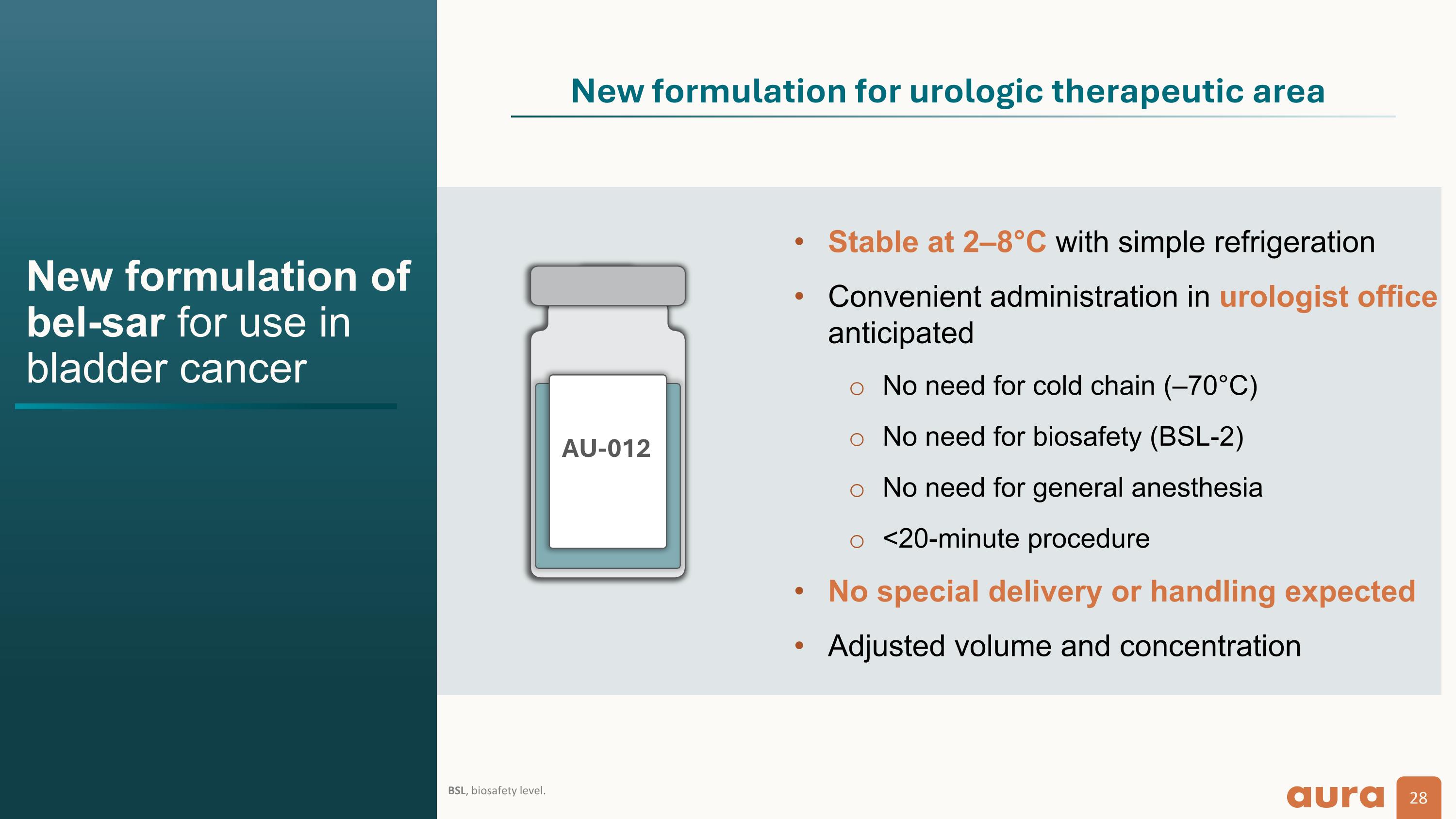
New formulation of bel-sar for use in bladder cancer BSL, biosafety level. AU-012 Stable at 2–8°C with simple refrigeration Convenient administration in urologist office anticipated No need for cold chain (–70°C) No need for biosafety (BSL-2) No need for general anesthesia <20-minute procedure No special delivery or handling expected Adjusted volume and concentration New formulation for urologic therapeutic area
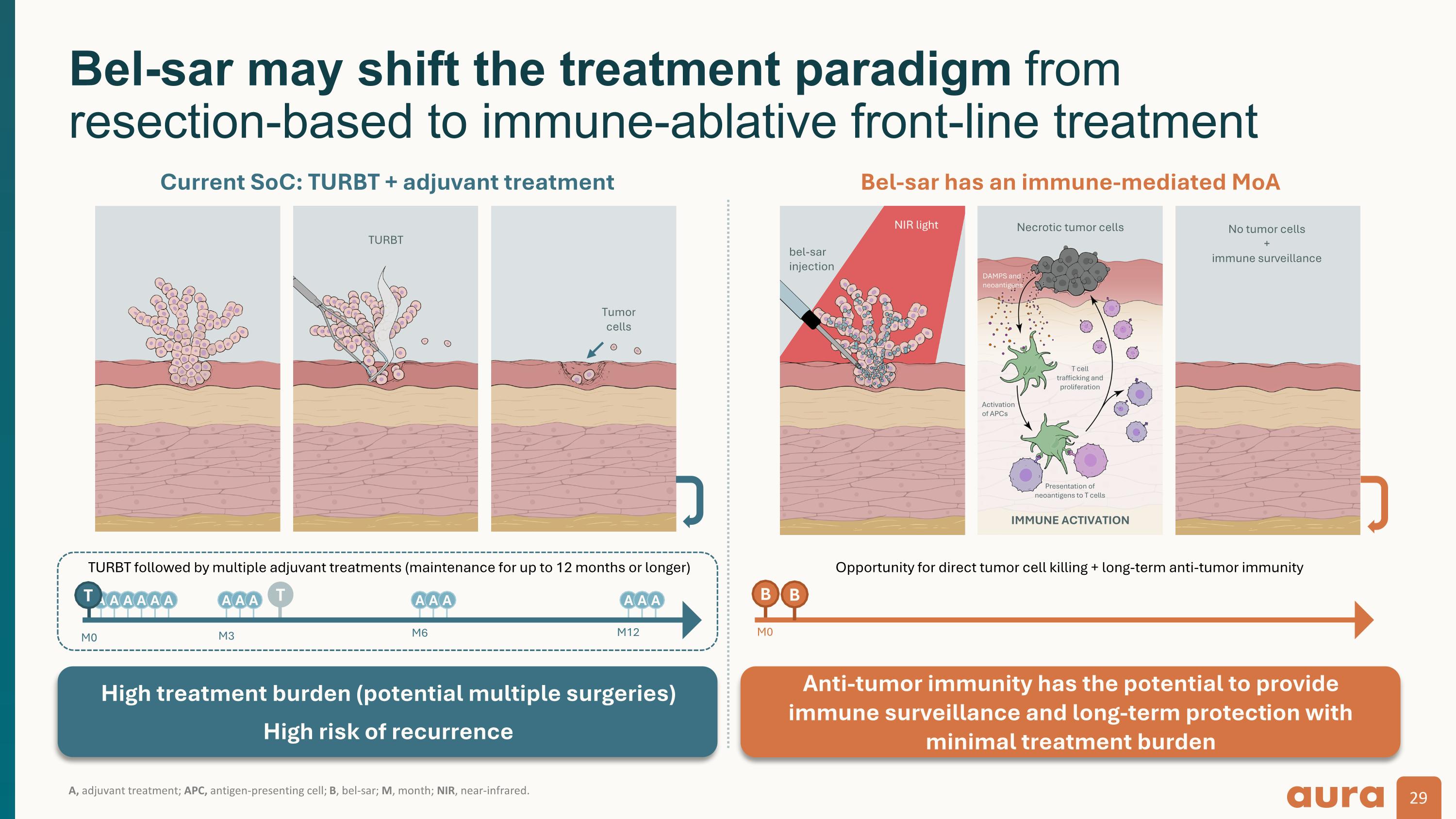
A, adjuvant treatment; APC, antigen-presenting cell; B, bel-sar; M, month; NIR, near-infrared. Bel-sar may shift the treatment paradigm from resection-based to immune-ablative front-line treatment Opportunity for direct tumor cell killing + long-term anti-tumor immunity B A A A T A A A A A A A A A A A A M0 M3 M6 M12 M0 T High treatment burden (potential multiple surgeries) High risk of recurrence Anti-tumor immunity has the potential to provide immune surveillance and long-term protection with minimal treatment burden TURBT followed by multiple adjuvant treatments (maintenance for up to 12 months or longer) B Current SoC: TURBT + adjuvant treatment Bel-sar has an immune-mediated MoA NIR light bel-sar injection Necrotic tumor cells IMMUNE ACTIVATION DAMPS and neoantigens Activation of APCs Presentation of neoantigens to T cells T cell trafficking and proliferation TURBT Tumor cells No tumor cells + immune surveillance
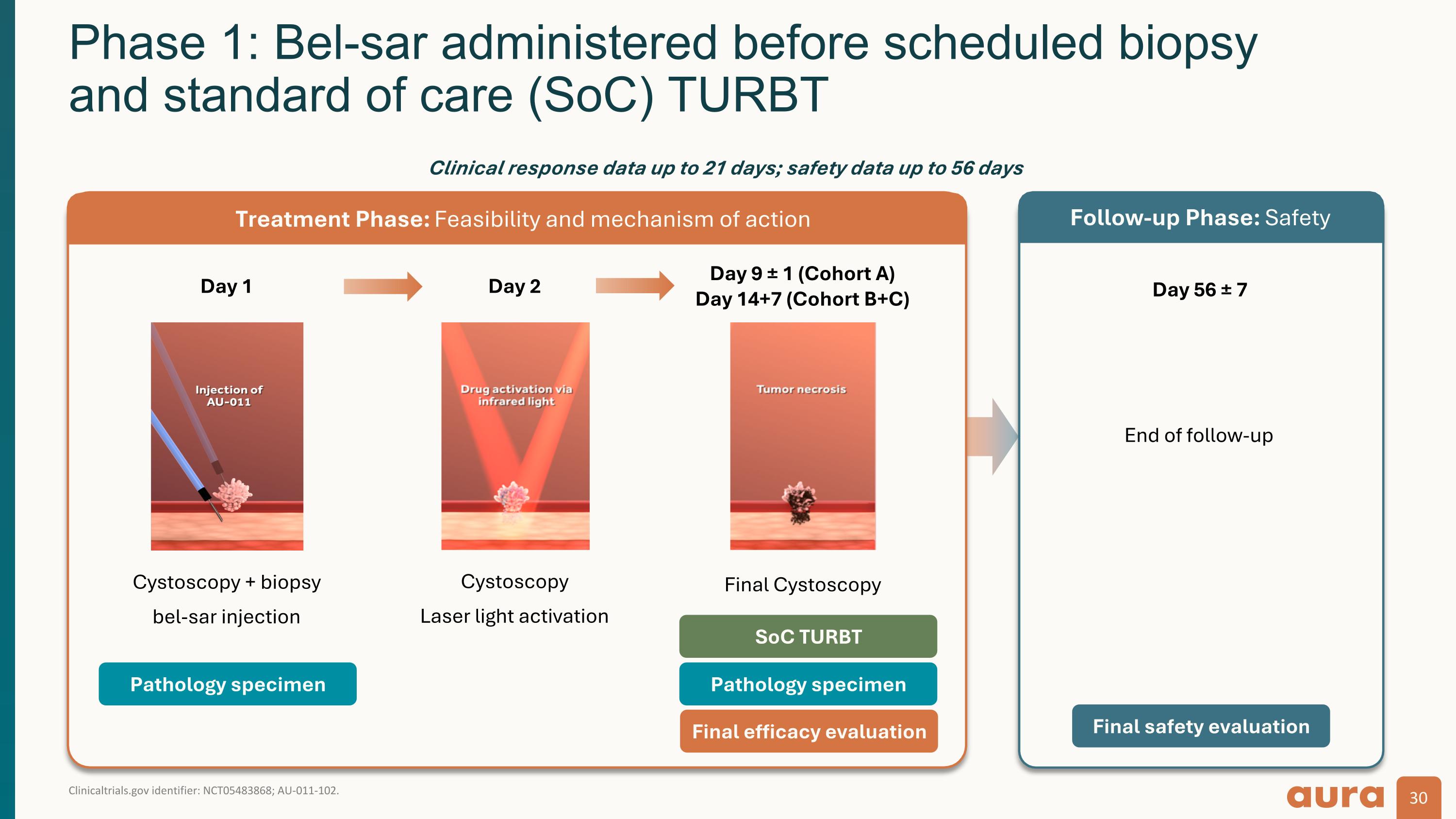
Clinicaltrials.gov identifier: NCT05483868; AU-011-102. Phase 1: Bel-sar administered before scheduled biopsy and standard of care (SoC) TURBT Day 1 Cystoscopy + biopsy bel-sar injection Day 2 Cystoscopy Laser light activation Day 9 ± 1 (Cohort A) Day 14+7 (Cohort B+C) Day 56 ± 7 End of follow-up Pathology specimen Pathology specimen Final efficacy evaluation Final safety evaluation Treatment Phase: Feasibility and mechanism of action Follow-up Phase: Safety Clinical response data up to 21 days; safety data up to 56 days SoC TURBT Final Cystoscopy
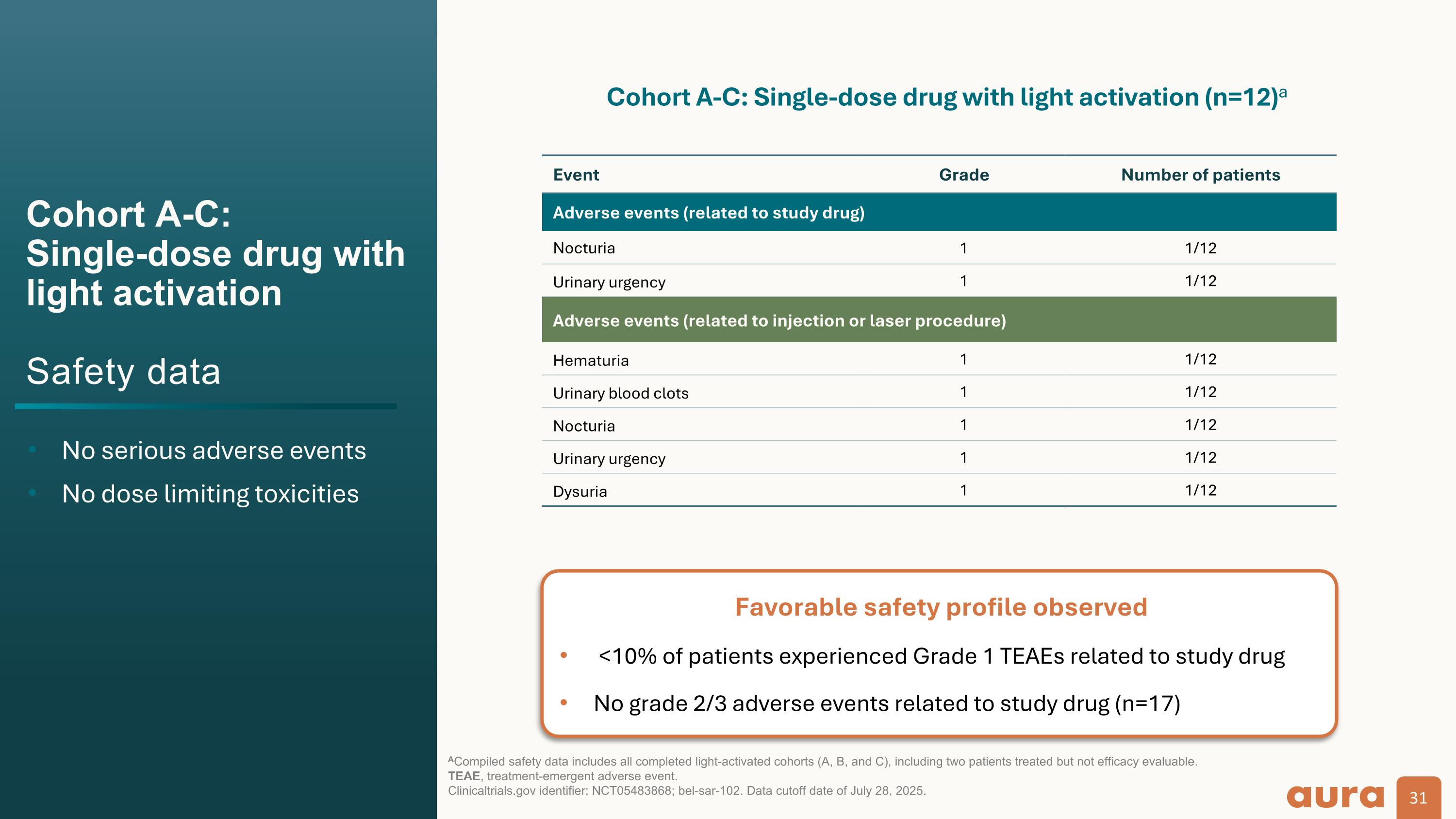
Cohort A-C: Single-dose drug with light activation Safety data No serious adverse events No dose limiting toxicities ACompiled safety data includes all completed light-activated cohorts (A, B, and C), including two patients treated but not efficacy evaluable. TEAE, treatment-emergent adverse event. Clinicaltrials.gov identifier: NCT05483868; bel-sar-102. Data cutoff date of July 28, 2025. Event Grade Number of patients Adverse events (related to study drug) Nocturia 1 1/12 Urinary urgency 1 1/12 Adverse events (related to injection or laser procedure) Hematuria 1 1/12 Urinary blood clots 1 1/12 Nocturia 1 1/12 Urinary urgency 1 1/12 Dysuria 1 1/12 Cohort A-C: Single-dose drug with light activation (n=12)a Favorable safety profile observed <10% of patients experienced Grade 1 TEAEs related to study drug No grade 2/3 adverse events related to study drug (n=17)
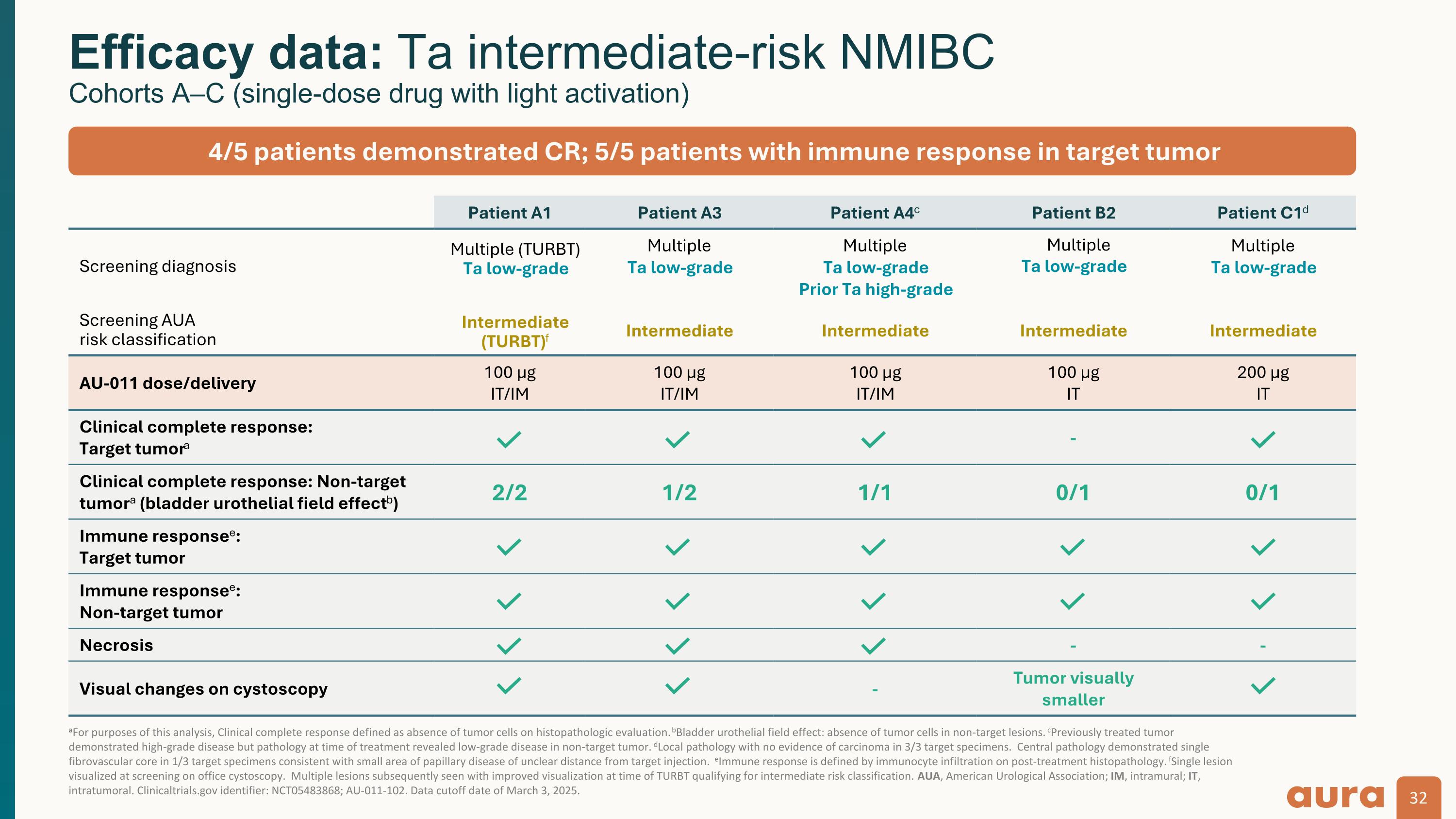
aFor purposes of this analysis, Clinical complete response defined as absence of tumor cells on histopathologic evaluation. bBladder urothelial field effect: absence of tumor cells in non-target lesions. cPreviously treated tumor demonstrated high-grade disease but pathology at time of treatment revealed low-grade disease in non-target tumor. dLocal pathology with no evidence of carcinoma in 3/3 target specimens. Central pathology demonstrated single fibrovascular core in 1/3 target specimens consistent with small area of papillary disease of unclear distance from target injection. eImmune response is defined by immunocyte infiltration on post-treatment histopathology. fSingle lesion visualized at screening on office cystoscopy. Multiple lesions subsequently seen with improved visualization at time of TURBT qualifying for intermediate risk classification. AUA, American Urological Association; IM, intramural; IT, intratumoral. Clinicaltrials.gov identifier: NCT05483868; AU-011-102. Data cutoff date of March 3, 2025. Efficacy data: Ta intermediate-risk NMIBC Cohorts A–C (single-dose drug with light activation) Patient A1 Patient A3 Patient A4c Patient B2 Patient C1d Screening diagnosis Multiple (TURBT) Ta low-grade Multiple Ta low-grade Multiple Ta low-grade Prior Ta high-grade Multiple Ta low-grade Multiple Ta low-grade Screening AUA risk classification Intermediate (TURBT)f Intermediate Intermediate Intermediate Intermediate AU-011 dose/delivery 100 µg IT/IM 100 µg IT/IM 100 µg IT/IM 100 µg IT 200 µg IT Clinical complete response: Target tumora - Clinical complete response: Non-target tumora (bladder urothelial field effectb) 2/2 1/2 1/1 0/1 0/1 Immune responsee: Target tumor Immune responsee: Non-target tumor Necrosis - - Visual changes on cystoscopy - Tumor visually smaller 4/5 patients demonstrated CR; 5/5 patients with immune response in target tumor
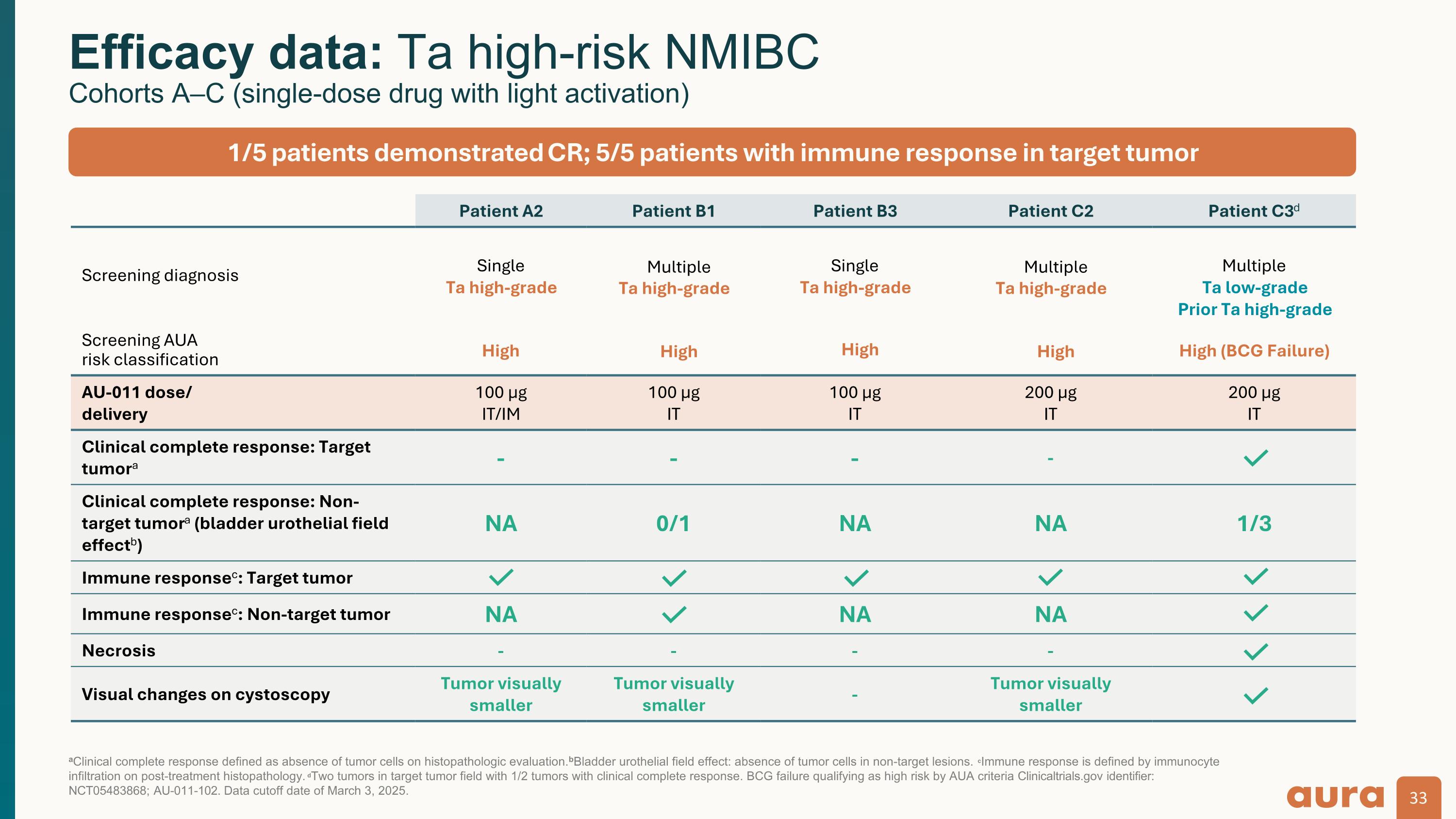
aClinical complete response defined as absence of tumor cells on histopathologic evaluation. bBladder urothelial field effect: absence of tumor cells in non-target lesions. cImmune response is defined by immunocyte infiltration on post-treatment histopathology. dTwo tumors in target tumor field with 1/2 tumors with clinical complete response. BCG failure qualifying as high risk by AUA criteria. Clinicaltrials.gov identifier: NCT05483868; AU-011-102. Data cutoff date of March 3, 2025. Efficacy data: Ta high-risk NMIBC Cohorts A–C (single-dose drug with light activation) Patient A2 Patient B1 Patient B3 Patient C2 Patient C3d Screening diagnosis Single Ta high-grade Multiple Ta high-grade Single Ta high-grade Multiple Ta high-grade Multiple Ta low-grade Prior Ta high-grade Screening AUA risk classification High High High High High (BCG Failure) AU-011 dose/ delivery 100 µg IT/IM 100 µg IT 100 µg IT 200 µg IT 200 µg IT Clinical complete response: Target tumora - - - - Clinical complete response: Non-target tumora (bladder urothelial field effectb) NA 0/1 NA NA 1/3 Immune responsec: Target tumor Immune responsec: Non-target tumor NA NA NA Necrosis - - - - Visual changes on cystoscopy Tumor visually smaller Tumor visually smaller - Tumor visually smaller 1/5 patients demonstrated CR; 5/5 patients with immune response in target tumor
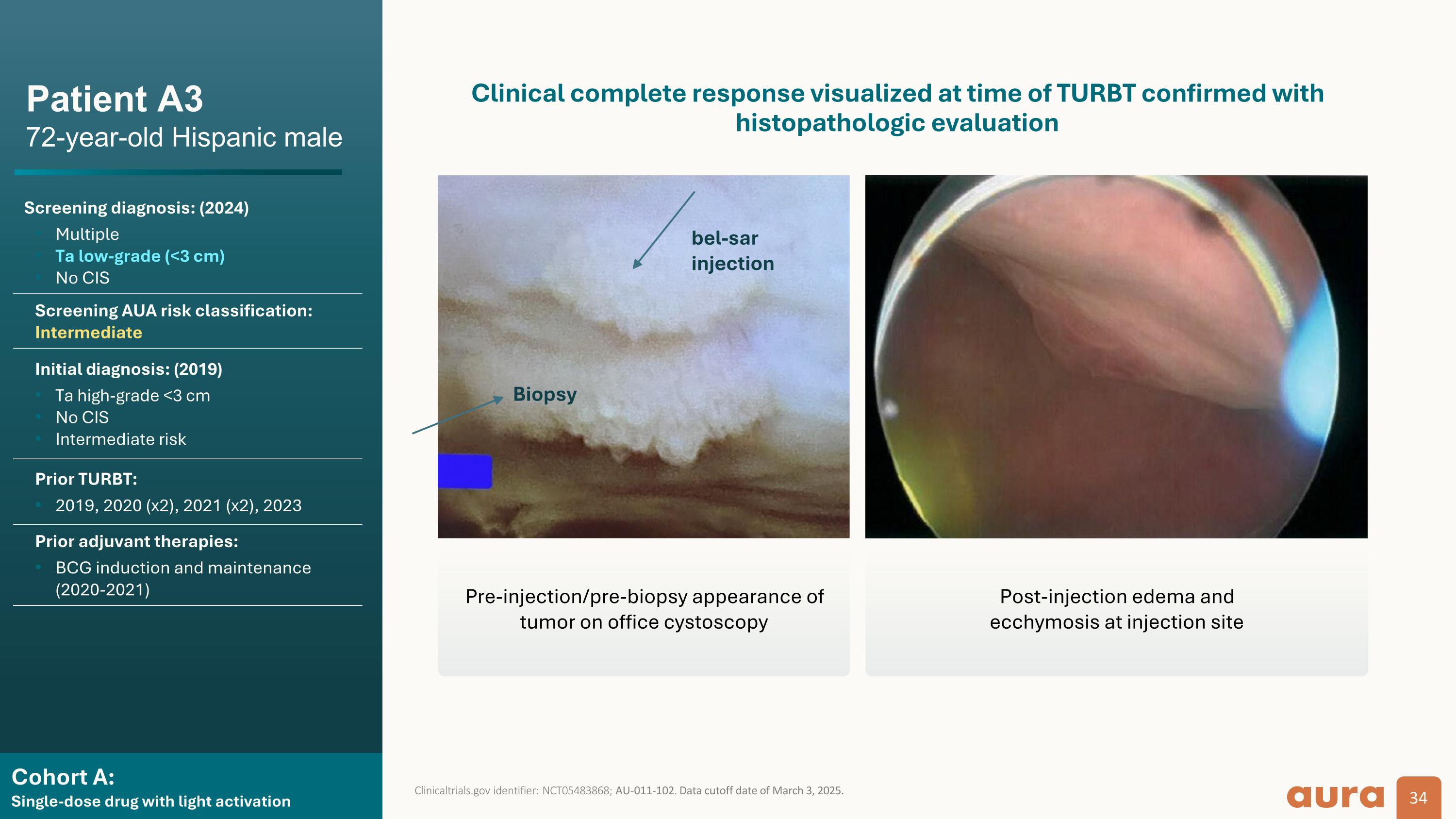
Cohort A: Single-dose drug with light activation Patient A3 72-year-old Hispanic male Screening diagnosis: (2024) Multiple Ta low-grade (<3 cm) No CIS Screening AUA risk classification: Intermediate Initial diagnosis: (2019) Ta high-grade <3 cm No CIS Intermediate risk Prior TURBT: 2019, 2020 (x2), 2021 (x2), 2023 Prior adjuvant therapies: BCG induction and maintenance (2020-2021) Clinicaltrials.gov identifier: NCT05483868; AU-011-102. Data cutoff date of March 3, 2025. Clinical complete response visualized at time of TURBT confirmed with histopathologic evaluation Biopsy bel-sar injection Pre-injection/pre-biopsy appearance of tumor on office cystoscopy Post-injection edema and ecchymosis at injection site
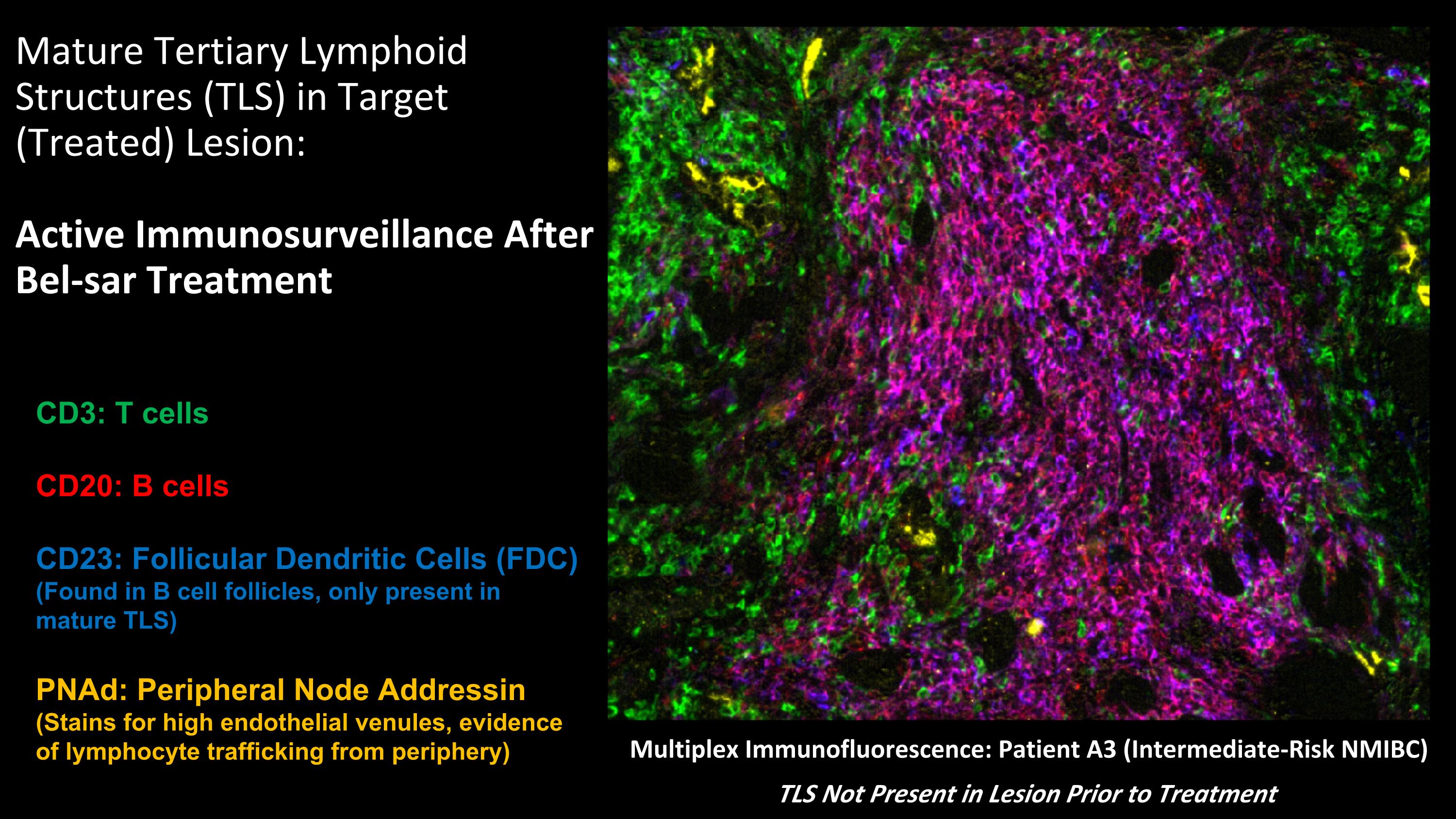
Multiplex Immunofluorescence: Patient A3 (Intermediate-Risk NMIBC) TLS Not Present in Lesion Prior to Treatment Mature Tertiary Lymphoid Structures (TLS) in Target (Treated) Lesion: Active Immunosurveillance After Bel-sar Treatment CD3: T cells CD20: B cells CD23: Follicular Dendritic Cells (FDC) (Found in B cell follicles, only present in mature TLS) PNAd: Peripheral Node Addressin (Stains for high endothelial venules, evidence of lymphocyte trafficking from periphery)
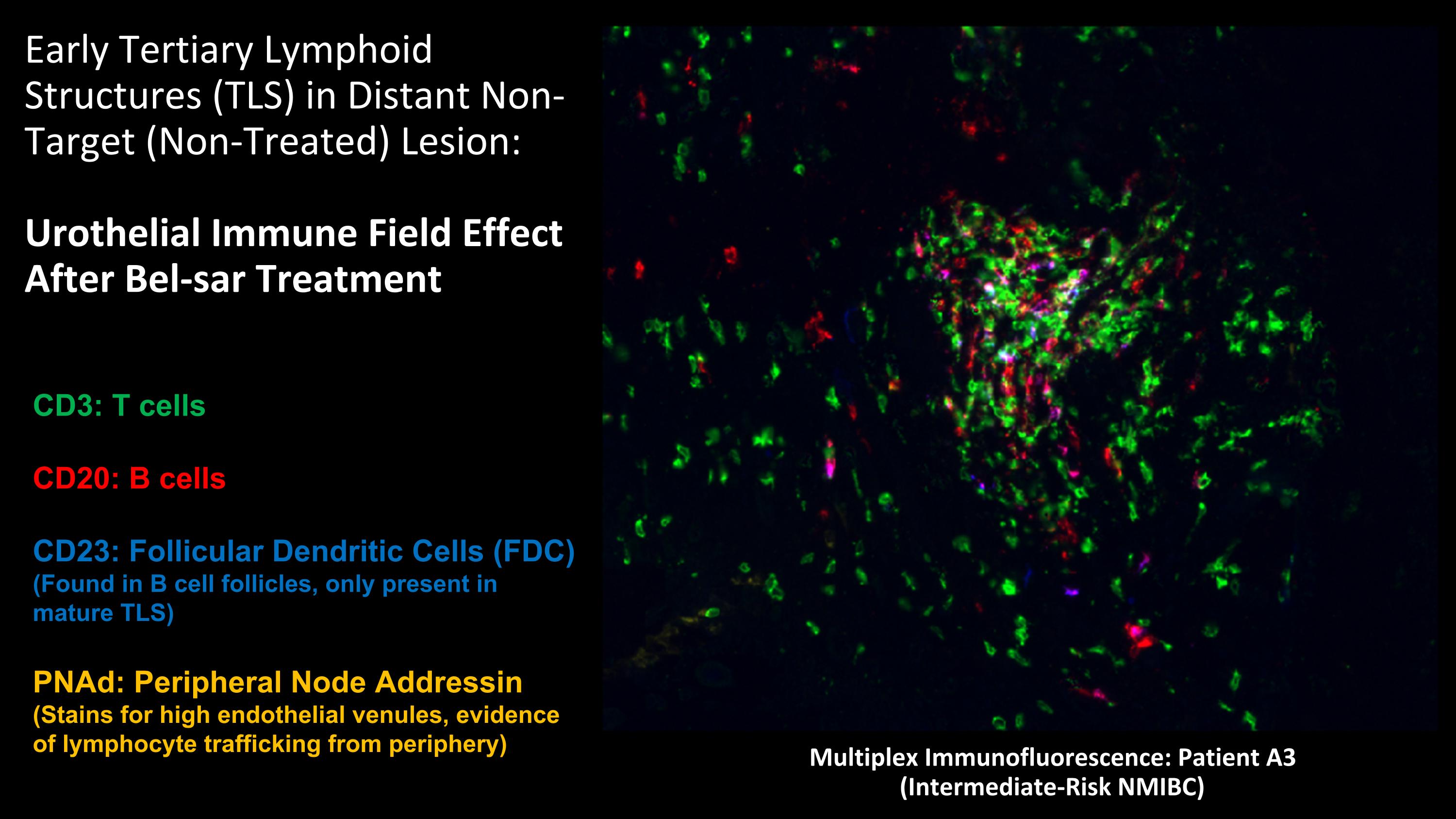
Early Tertiary Lymphoid Structures (TLS) in Distant Non-Target (Non-Treated) Lesion: Urothelial Immune Field Effect After Bel-sar Treatment Multiplex Immunofluorescence: Patient A3 (Intermediate-Risk NMIBC) CD3: T cells CD20: B cells CD23: Follicular Dendritic Cells (FDC) (Found in B cell follicles, only present in mature TLS) PNAd: Peripheral Node Addressin (Stains for high endothelial venules, evidence of lymphocyte trafficking from periphery)
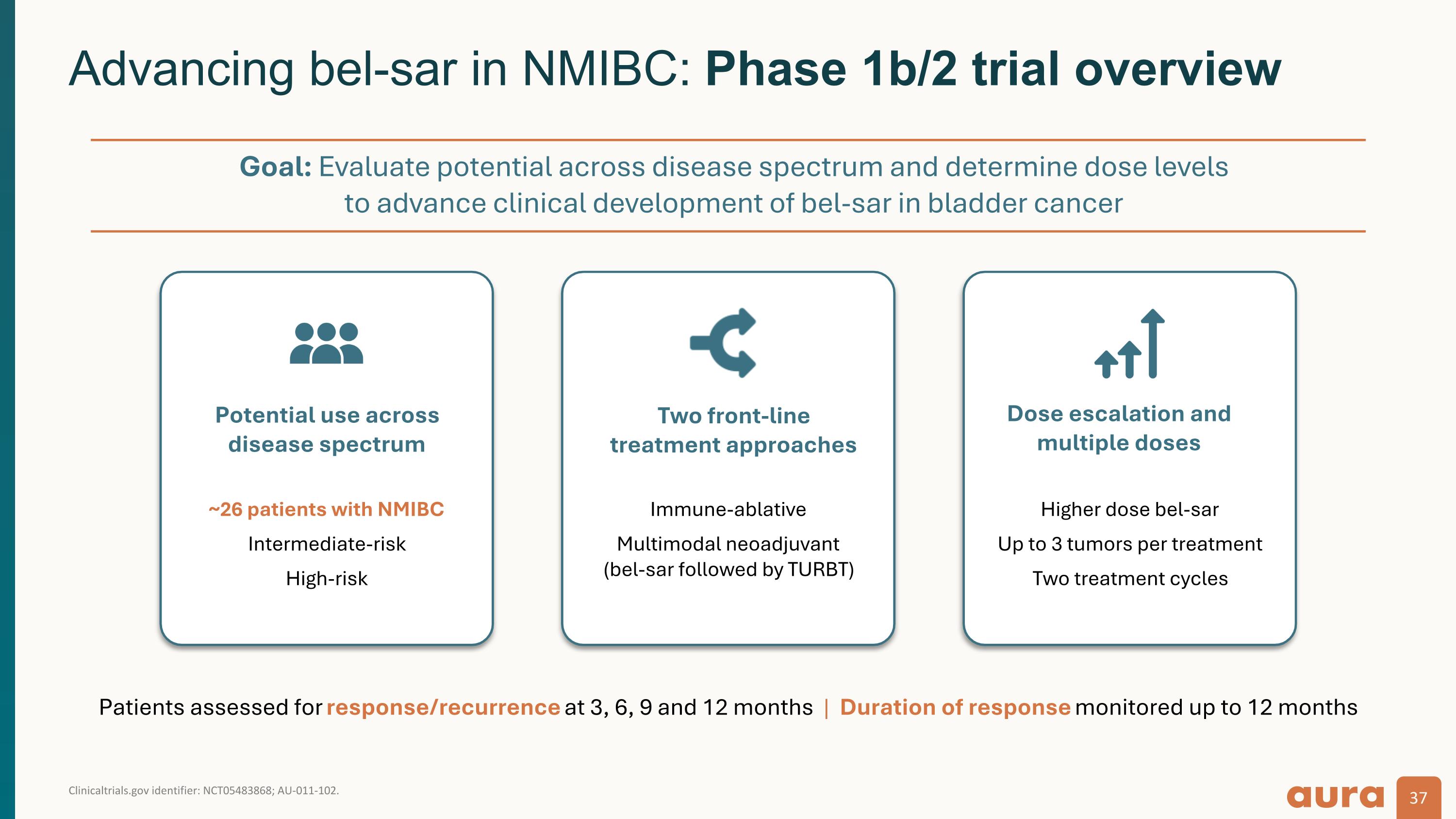
Clinicaltrials.gov identifier: NCT05483868; AU-011-102. Advancing bel-sar in NMIBC: Phase 1b/2 trial overview ~26 patients with NMIBC Intermediate-risk High-risk Potential use across disease spectrum Immune-ablative Multimodal neoadjuvant (bel-sar followed by TURBT) Two front-line treatment approaches Higher dose bel-sar Up to 3 tumors per treatment Two treatment cycles Dose escalation and multiple doses Patients assessed for response/recurrence at 3, 6, 9 and 12 months | Duration of response monitored up to 12 months Goal: Evaluate potential across disease spectrum and determine dose levels to advance clinical development of bel-sar in bladder cancer
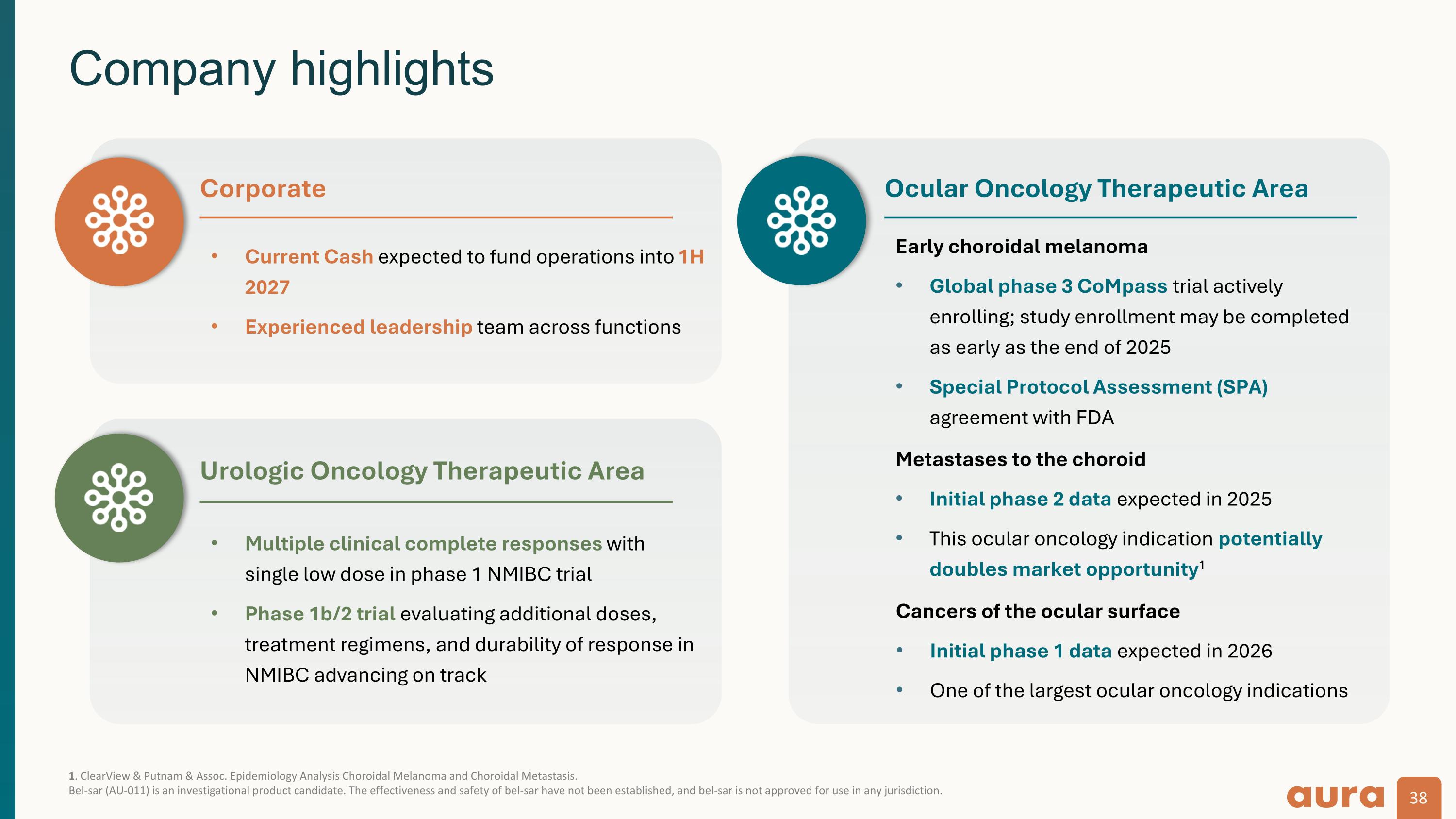
1. ClearView & Putnam & Assoc. Epidemiology Analysis Choroidal Melanoma and Choroidal Metastasis. Bel-sar (AU-011) is an investigational product candidate. The effectiveness and safety of bel-sar have not been established, and bel-sar is not approved for use in any jurisdiction. Company highlights Current Cash expected to fund operations into 1H 2027 Experienced leadership team across functions Corporate Ocular Oncology Therapeutic Area Early choroidal melanoma Global phase 3 CoMpass trial actively enrolling; study enrollment may be completed as early as the end of 2025 Special Protocol Assessment (SPA) agreement with FDA Metastases to the choroid Initial phase 2 data expected in 2025 This ocular oncology indication potentially doubles market opportunity1 Urologic Oncology Therapeutic Area Multiple clinical complete responses with single low dose in phase 1 NMIBC trial Phase 1b/2 trial evaluating additional doses, treatment regimens, and durability of response in NMIBC advancing on track Cancers of the ocular surface Initial phase 1 data expected in 2026 One of the largest ocular oncology indications

Appendix VDC Platform
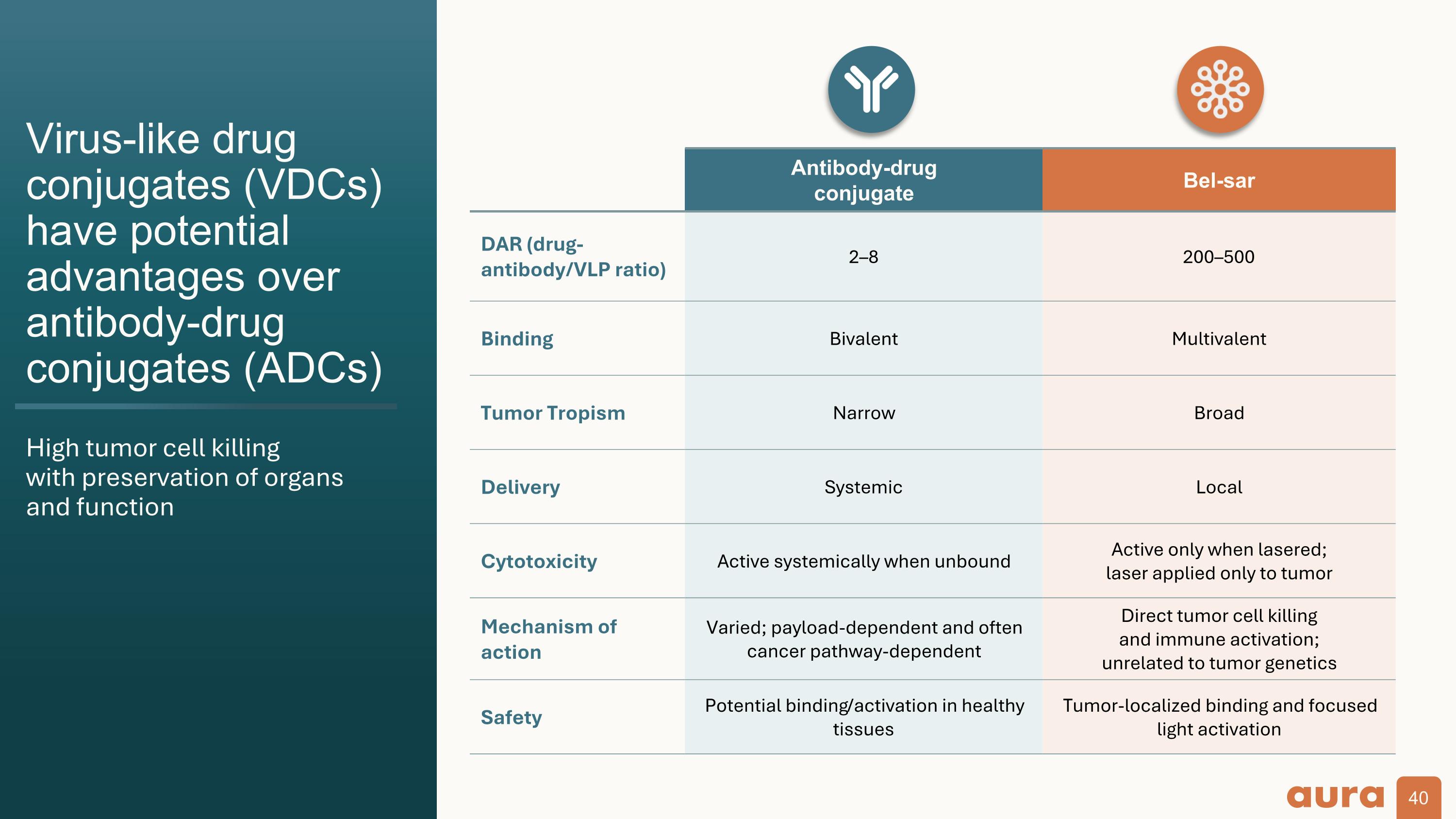
Virus-like drug conjugates (VDCs) have potential advantages over antibody-drug conjugates (ADCs) High tumor cell killing with preservation of organs and function Antibody-drug conjugate Bel-sar DAR (drug-antibody/VLP ratio) 2–8 200–500 Binding Bivalent Multivalent Tumor Tropism Narrow Broad Delivery Systemic Local Cytotoxicity Active systemically when unbound Active only when lasered; laser applied only to tumor Mechanism of action Varied; payload-dependent and often cancer pathway-dependent Direct tumor cell killing and immune activation; unrelated to tumor genetics Safety Potential binding/activation in healthy tissues Tumor-localized binding and focused light activation

Appendix Ocular Oncology
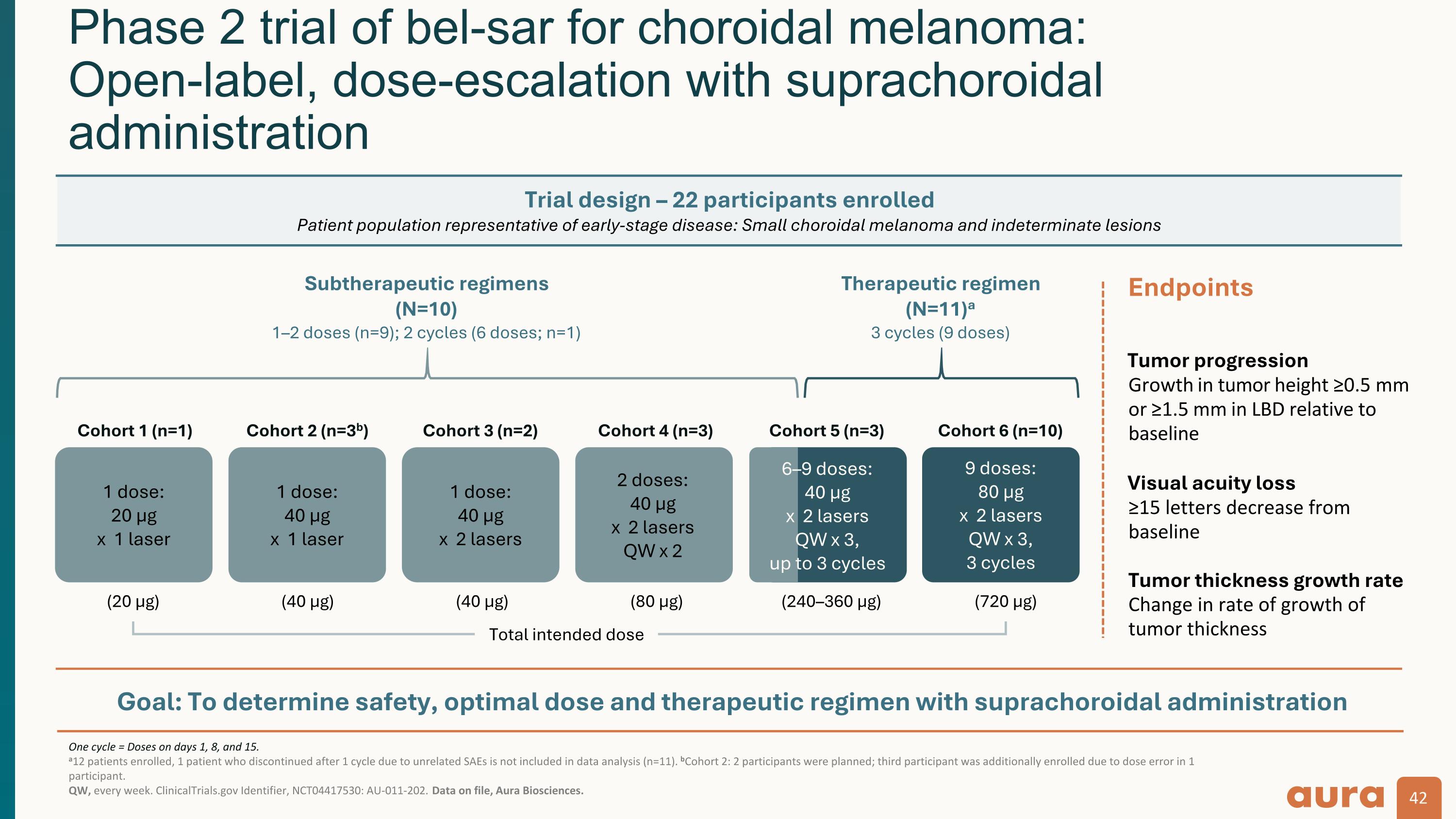
Goal: To determine safety, optimal dose and therapeutic regimen with suprachoroidal administration One cycle = Doses on days 1, 8, and 15. a12 patients enrolled, 1 patient who discontinued after 1 cycle due to unrelated SAEs is not included in data analysis (n=11). bCohort 2: 2 participants were planned; third participant was additionally enrolled due to dose error in 1 participant. QW, every week. ClinicalTrials.gov Identifier, NCT04417530: AU-011-202. Data on file, Aura Biosciences. Phase 2 trial of bel-sar for choroidal melanoma: Open-label, dose-escalation with suprachoroidal administration Trial design – 22 participants enrolled Patient population representative of early-stage disease: Small choroidal melanoma and indeterminate lesions Endpoints Tumor progression Growth in tumor height ≥0.5 mm or ≥1.5 mm in LBD relative to baseline Visual acuity loss ≥15 letters decrease from baseline Tumor thickness growth rate Change in rate of growth of tumor thickness 1 dose: 20 μg x 1 laser 1 dose: 40 μg x 1 laser 1 dose: 40 μg x 2 lasers 2 doses: 40 μg x 2 lasers QW x 2 9 doses: 80 μg x 2 lasers QW x 3, 3 cycles Subtherapeutic regimens (N=10) 1–2 doses (n=9); 2 cycles (6 doses; n=1) Therapeutic regimen (N=11)a 3 cycles (9 doses) Cohort 1 (n=1) Cohort 2 (n=3b) Cohort 3 (n=2) Cohort 4 (n=3) Cohort 5 (n=3) Cohort 6 (n=10) 6–9 doses: 40 μg x 2 lasers QW x 3, up to 3 cycles (20 µg) (40 µg) (40 µg) (80 µg) (240–360 µg) (720 µg) Total intended dose
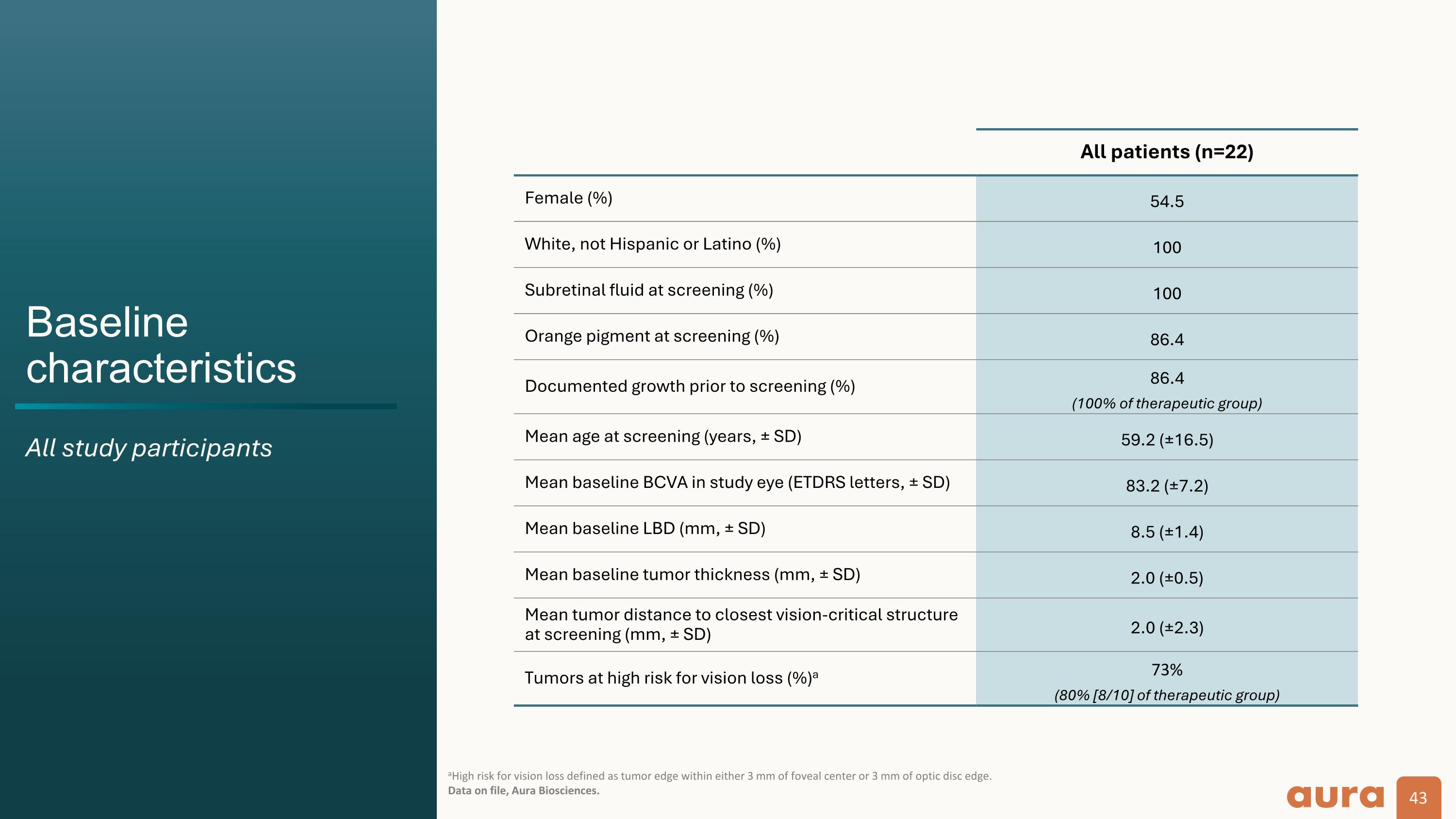
Baseline characteristics All study participants aHigh risk for vision loss defined as tumor edge within either 3 mm of foveal center or 3 mm of optic disc edge. Data on file, Aura Biosciences. All patients (n=22) Female (%) 54.5 White, not Hispanic or Latino (%) 100 Subretinal fluid at screening (%) 100 Orange pigment at screening (%) 86.4 Documented growth prior to screening (%) 86.4 (100% of therapeutic group) Mean age at screening (years, ± SD) 59.2 (±16.5) Mean baseline BCVA in study eye (ETDRS letters, ± SD) 83.2 (±7.2) Mean baseline LBD (mm, ± SD) 8.5 (±1.4) Mean baseline tumor thickness (mm, ± SD) 2.0 (±0.5) Mean tumor distance to closest vision-critical structure at screening (mm, ± SD) 2.0 (±2.3) Tumors at high risk for vision loss (%)a 73% (80% [8/10] of therapeutic group)
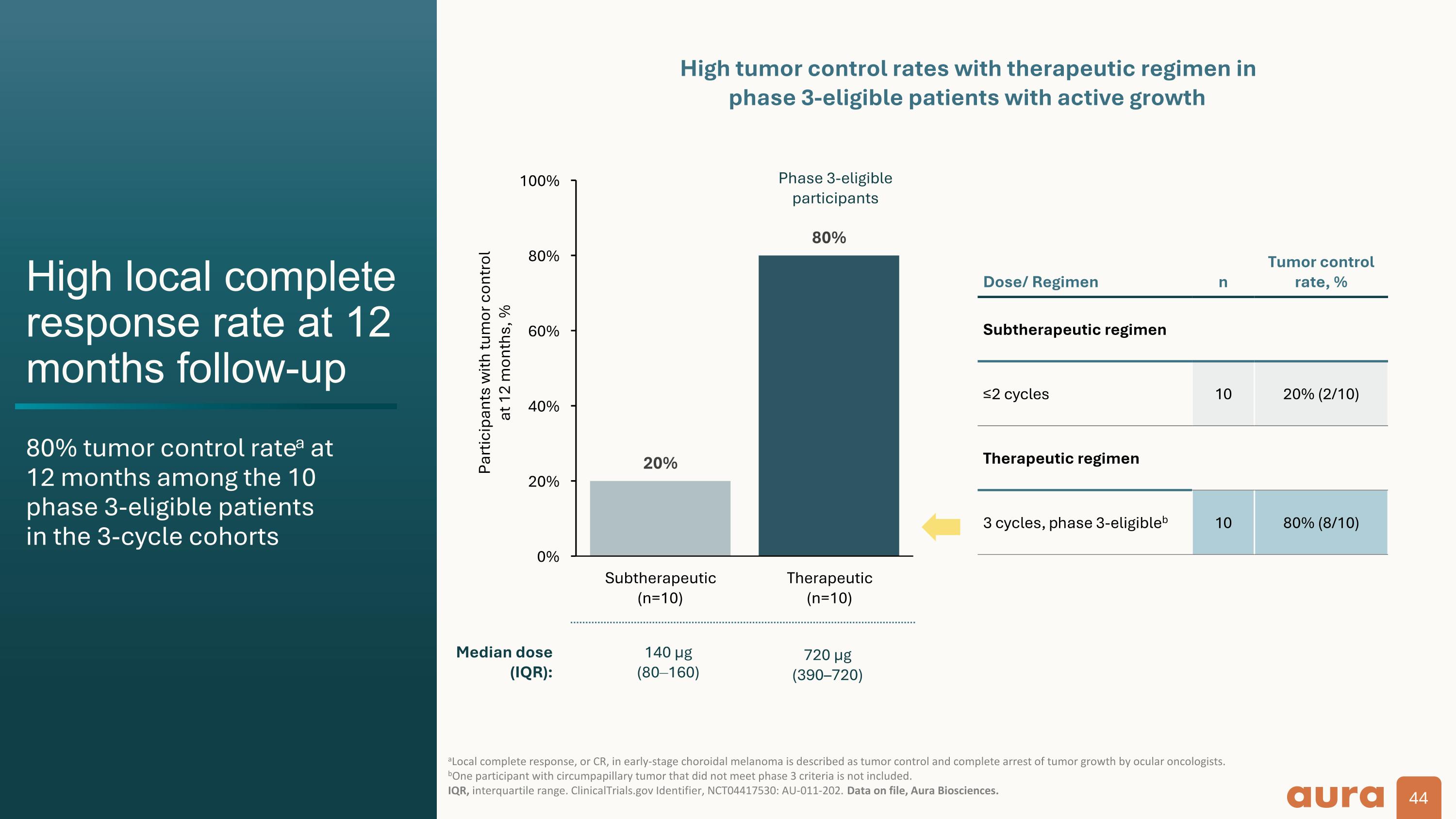
High local complete response rate at 12 months follow-up 80% tumor control ratea at 12 months among the 10 phase 3-eligible patients in the 3-cycle cohorts aLocal complete response, or CR, in early-stage choroidal melanoma is described as tumor control and complete arrest of tumor growth by ocular oncologists. bOne participant with circumpapillary tumor that did not meet phase 3 criteria is not included. IQR, interquartile range. ClinicalTrials.gov Identifier, NCT04417530: AU-011-202. Data on file, Aura Biosciences. Participants with tumor control at 12 months, % Dose/ Regimen n Tumor control rate, % Subtherapeutic regimen ≤2 cycles 10 20% (2/10) Therapeutic regimen 3 cycles, phase 3-eligibleb 10 80% (8/10) Phase 3-eligible participants High tumor control rates with therapeutic regimen in phase 3-eligible patients with active growth Median dose (IQR): 140 µg (80160) 720 µg (390–720)
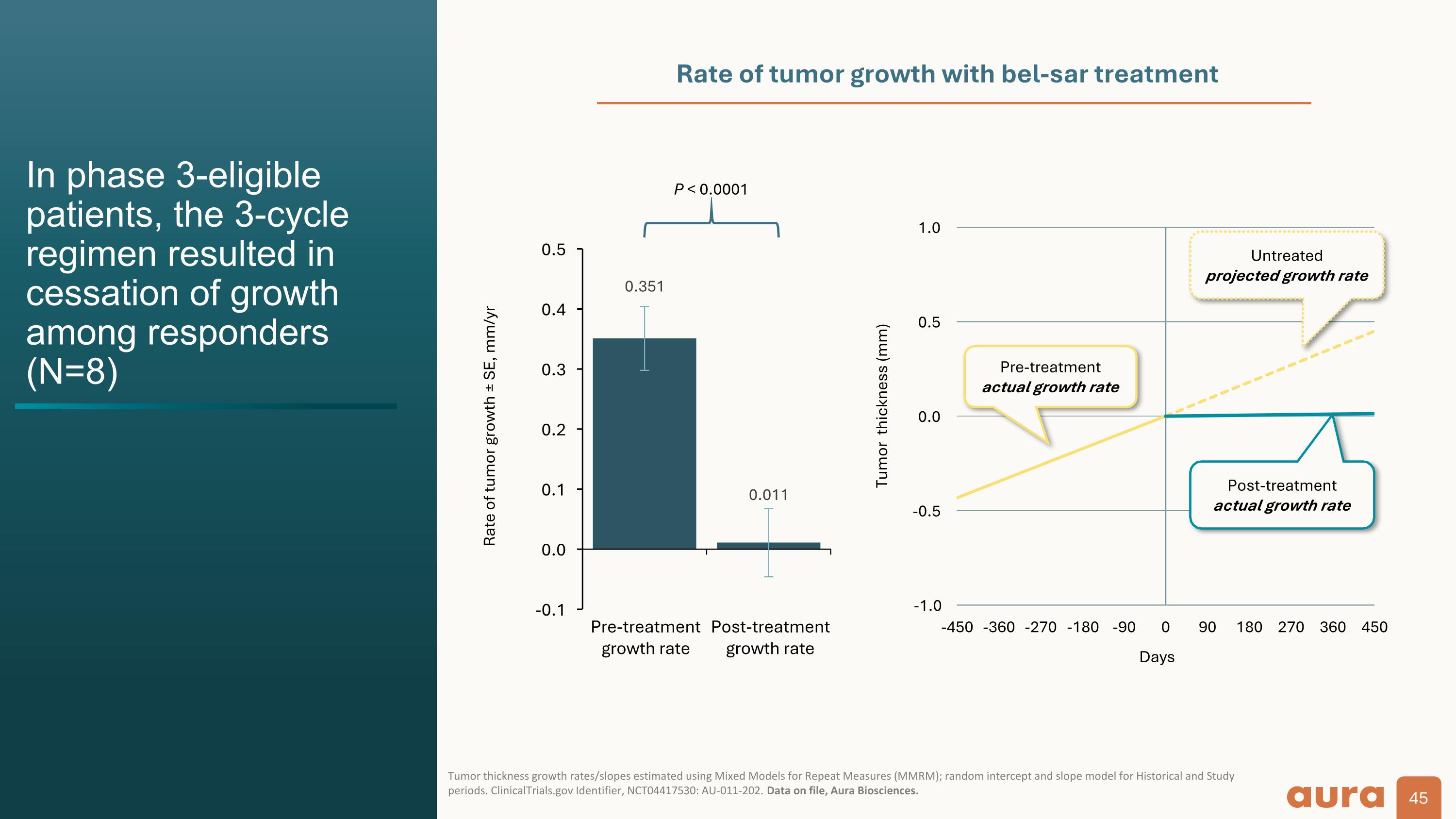
Rate of tumor growth ± SE, mm/yr P < 0.0001 Rate of tumor growth with bel-sar treatment In phase 3-eligible patients, the 3-cycle regimen resulted in cessation of growth among responders (N=8) Tumor thickness growth rates/slopes estimated using Mixed Models for Repeat Measures (MMRM); random intercept and slope model for Historical and Study periods. ClinicalTrials.gov Identifier, NCT04417530: AU-011-202. Data on file, Aura Biosciences. Post-treatment actual growth rate Untreated projected growth rate Pre-treatment actual growth rate
Vision loss threshold (−15 letters) Populations Patients (n) Vision failuresb (n) Vision preservation rate (%) All dose cohorts All treated patients 22 1 95% Subtherapeutic ≤2 cycles 10 0 100% Therapeutic 3 cycles and phase 3-eligiblea 10 1 90% BCVA change from baseline (ETDRS letter score) Median change in BCVA in phase 3-eligible participants with therapeutic regimen (N=10)a Visual acuity was preserved in 90% of phase 3-eligible patients receiving a bel-sar therapeutic regimen 80% were at high risk of vision loss with tumors < 3 mm to the fovea or optic nerve 90% visual acuity preservation supports the potential for bel-sar to be a front-line therapy for early-stage disease aOne participant with circumpapillary tumor that did not meet phase 3 criteria is not included. bVision acuity loss defined as ≥15 letters decrease from baseline in ETDRS BCVA letter score. ClinicalTrials.gov Identifier, NCT04417530: AU-011-202. Data on file, Aura Biosciences. Study week (relative to first dose in Cycle 1) Vision preservation in 9/10 participants Loss of 18 letters in one patient with progression of preexisting juxtafoveal fluid under fovea -5 0 5 -5 -10 -15 0 13 26 39 52
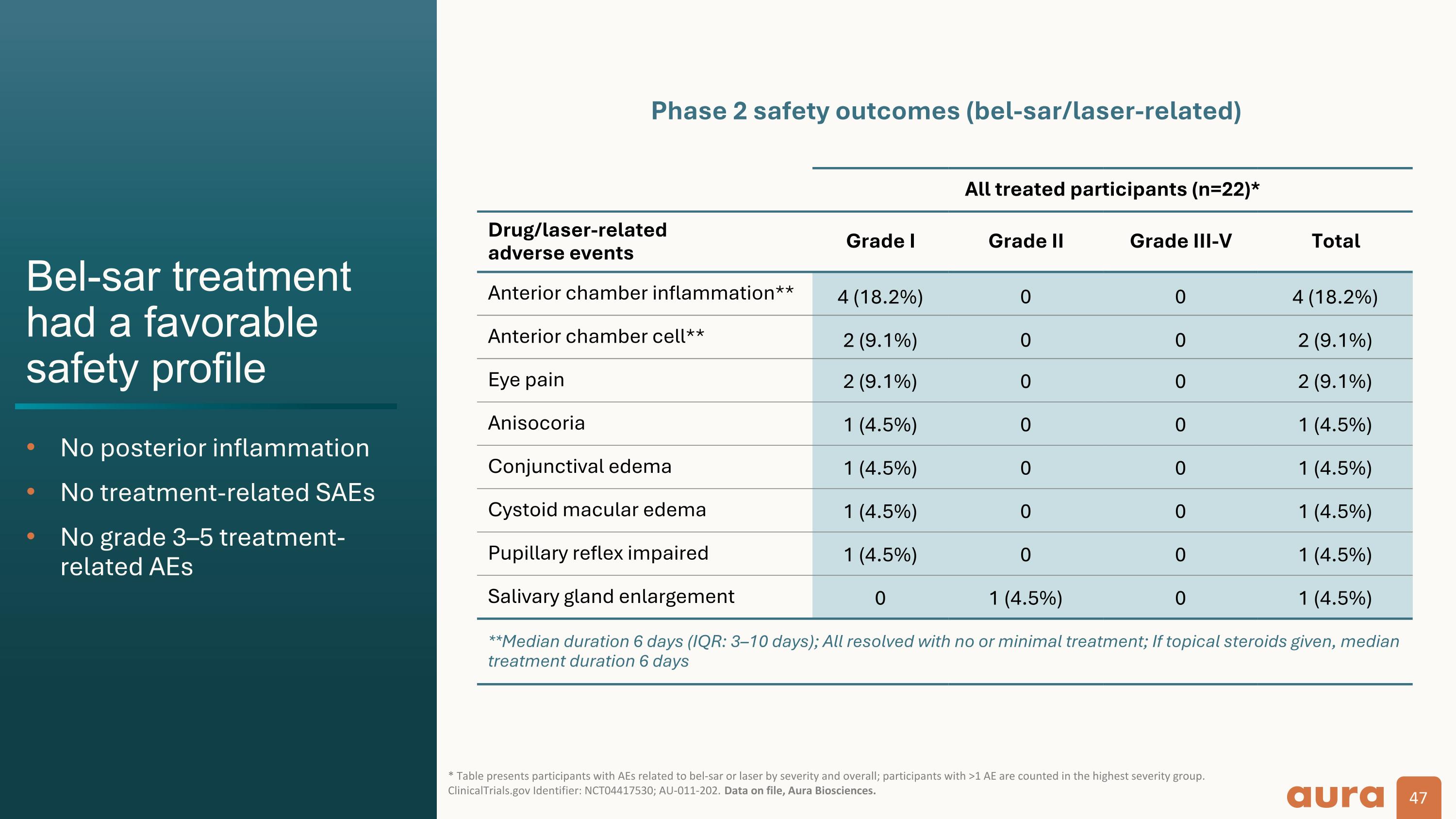
Bel-sar treatment had a favorable safety profile No posterior inflammation No treatment-related SAEs No grade 3–5 treatment-related AEs * Table presents participants with AEs related to bel-sar or laser by severity and overall; participants with >1 AE are counted in the highest severity group. ClinicalTrials.gov Identifier: NCT04417530; AU-011-202. Data on file, Aura Biosciences. All treated participants (n=22)* Drug/laser-related adverse events Grade I Grade II Grade III-V Total Anterior chamber inflammation** 4 (18.2%) 0 0 4 (18.2%) Anterior chamber cell** 2 (9.1%) 0 0 2 (9.1%) Eye pain 2 (9.1%) 0 0 2 (9.1%) Anisocoria 1 (4.5%) 0 0 1 (4.5%) Conjunctival edema 1 (4.5%) 0 0 1 (4.5%) Cystoid macular edema 1 (4.5%) 0 0 1 (4.5%) Pupillary reflex impaired 1 (4.5%) 0 0 1 (4.5%) Salivary gland enlargement 0 1 (4.5%) 0 1 (4.5%) **Median duration 6 days (IQR: 3–10 days); All resolved with no or minimal treatment; If topical steroids given, median treatment duration 6 days Phase 2 safety outcomes (bel-sar/laser-related)
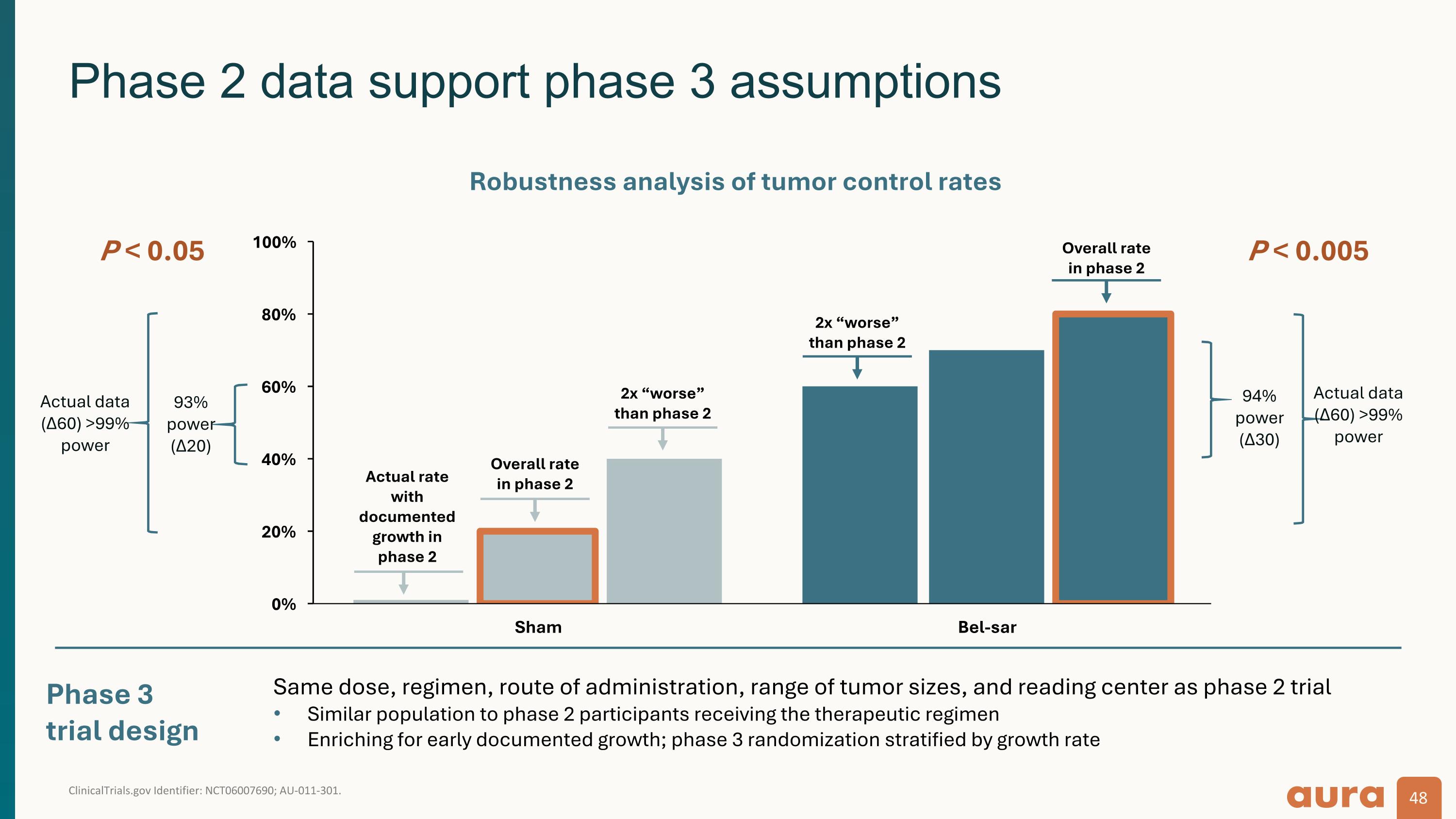
ClinicalTrials.gov Identifier: NCT06007690; AU-011-301. Phase 2 data support phase 3 assumptions Phase 3 trial design P < 0.005 93% power (Δ20) Actual data (Δ60) >99% power P < 0.05 Robustness analysis of tumor control rates Overall rate in phase 2 2x “worse” than phase 2 2x “worse” than phase 2 Actual rate with documented growth in phase 2 Overall rate in phase 2 94% power (Δ30) Actual data (Δ60) >99% power Same dose, regimen, route of administration, range of tumor sizes, and reading center as phase 2 trial Similar population to phase 2 participants receiving the therapeutic regimen Enriching for early documented growth; phase 3 randomization stratified by growth rate

Appendix Urologic Oncology
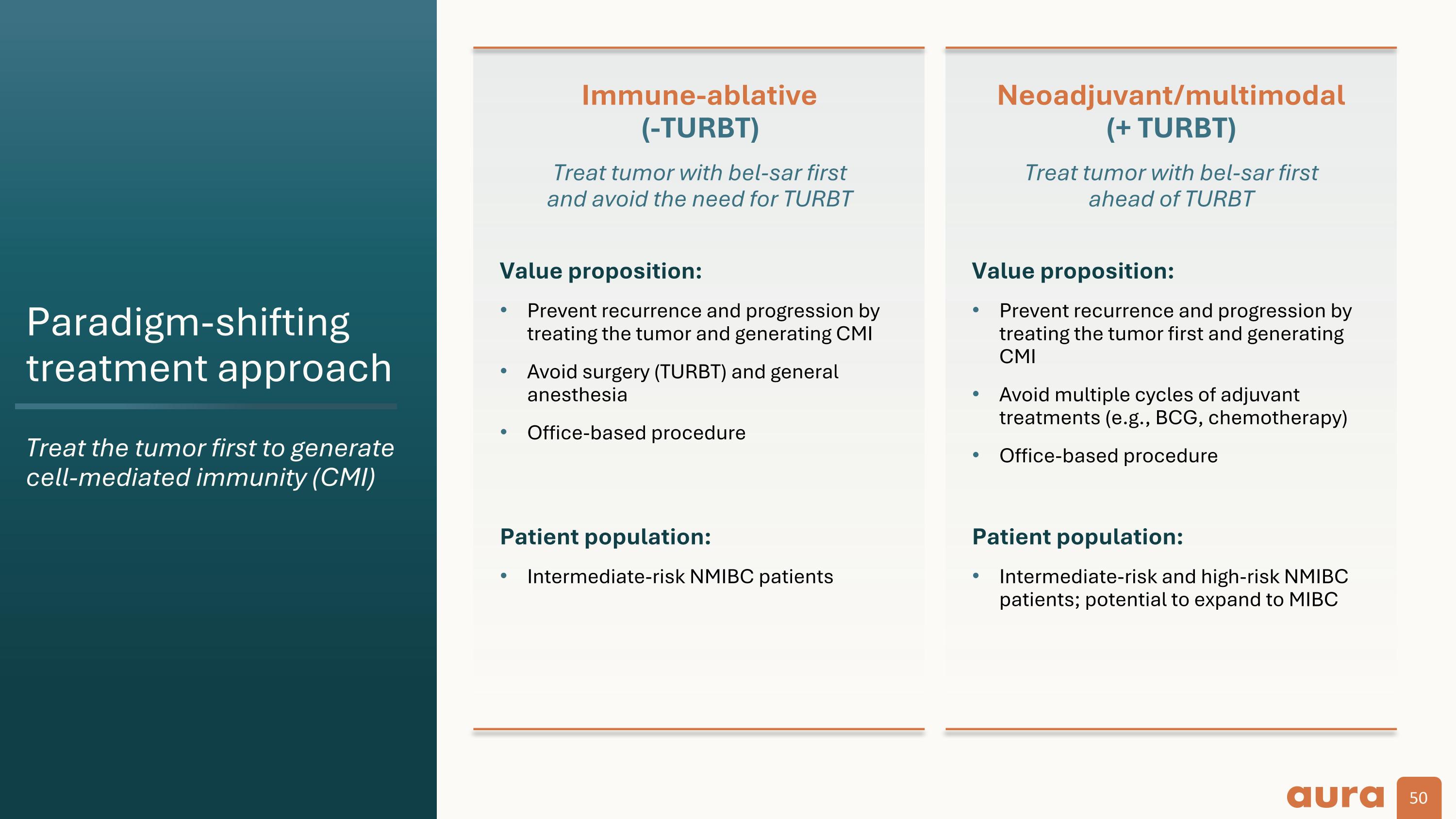
Paradigm-shifting treatment approach Treat the tumor first to generate cell-mediated immunity (CMI) Neoadjuvant/multimodal (+ TURBT) Treat tumor with bel-sar first ahead of TURBT Immune-ablative (-TURBT) Treat tumor with bel-sar first and avoid the need for TURBT Value proposition: Prevent recurrence and progression by treating the tumor first and generating CMI Avoid multiple cycles of adjuvant treatments (e.g., BCG, chemotherapy) Office-based procedure Patient population: Intermediate-risk and high-risk NMIBC patients; potential to expand to MIBC Value proposition: Prevent recurrence and progression by treating the tumor and generating CMI Avoid surgery (TURBT) and general anesthesia Office-based procedure Patient population: Intermediate-risk NMIBC patients

Bel-sar has potential as a standalone immune-ablative treatment or as a neoadjuvant to TURBT Immune-ablative approach could eliminate the need for TURBT, or be used prior to resection to improve treatment outcomes HR, high-risk; IR, intermediate risk; LR, low-risk. Immune-ablative treatment without TURBT (LR/IR NMIBC) 1 Neoadjuvant/ multimodal therapy followed by TURBT (IR/HR NMIBC) 2 NIR light bel-sar injection Necrotic tumor cells IMMUNE ACTIVATION DAMPS and neoantigens Activation of APCs Presentation of neoantigens to T cells T cell trafficking and proliferation NIR light bel-sar injection Necrotic tumor cells IMMUNE ACTIVATION DAMPS and neoantigens Activation of APCs Presentation of neoantigens to T cells T cell trafficking and proliferation Necrotic tissue TURBT No tumor cells + immune surveillance No tumor cells + immune surveillance
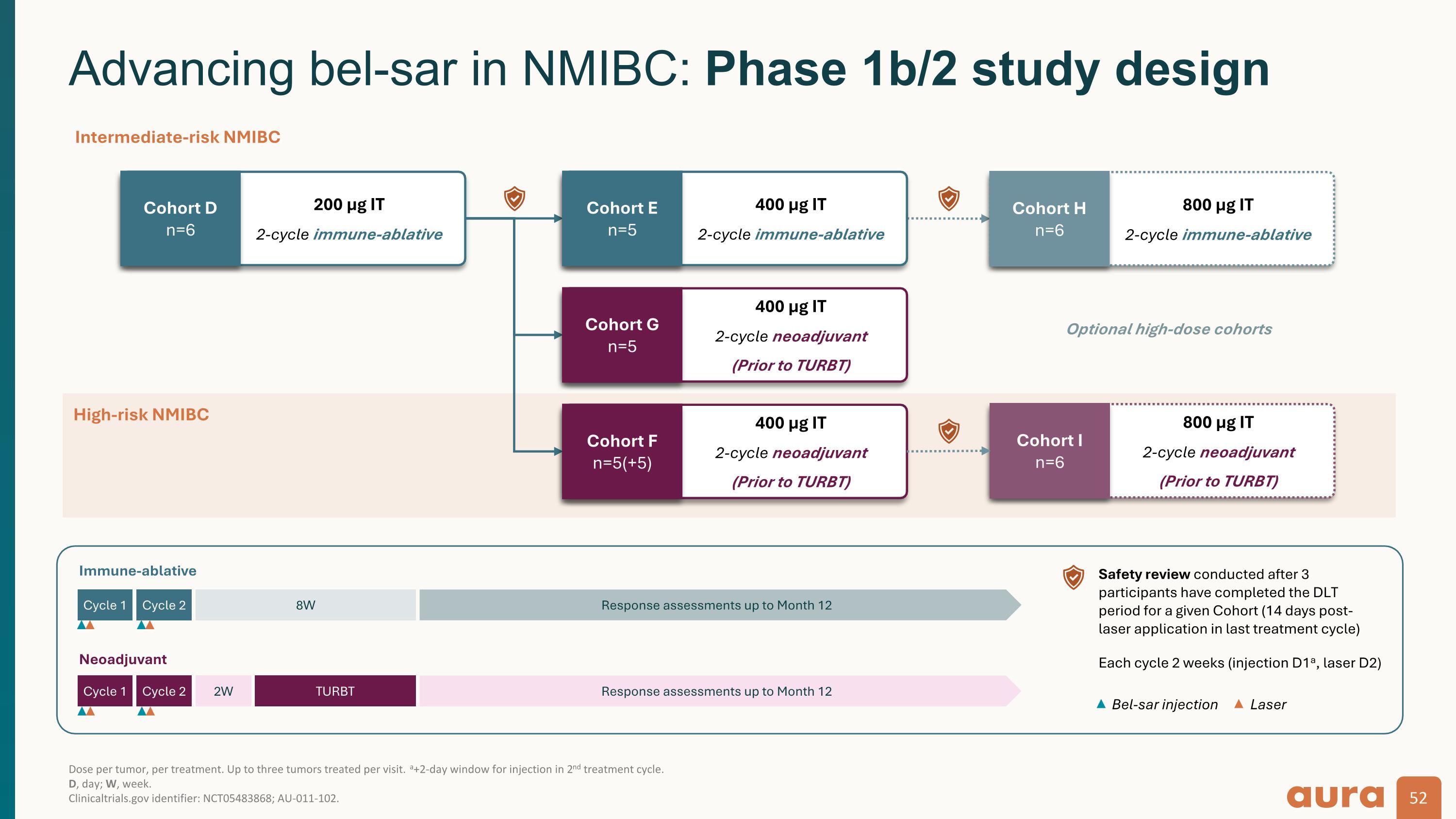
Dose per tumor, per treatment. Up to three tumors treated per visit. a+2-day window for injection in 2nd treatment cycle. D, day; W, week. Clinicaltrials.gov identifier: NCT05483868; AU-011-102. Advancing bel-sar in NMIBC: Phase 1b/2 study design 52 Immune-ablative Cycle 1 Cycle 2 8W Response assessments up to Month 12 Neoadjuvant Cycle 1 Cycle 2 2W Response assessments up to Month 12 TURBT Each cycle 2 weeks (injection D1a, laser D2) Bel-sar injection Laser Safety review conducted after 3 participants have completed the DLT period for a given Cohort (14 days post-laser application in last treatment cycle) Optional high-dose cohorts 200 µg IT 2-cycle immune-ablative Cohort D n=6 400 µg IT 2-cycle immune-ablative Cohort E n=5 400 µg IT 2-cycle neoadjuvant (Prior to TURBT) Cohort G n=5 400 µg IT 2-cycle neoadjuvant (Prior to TURBT) Cohort F n=5(+5) Intermediate-risk NMIBC High-risk NMIBC 800 µg IT 2-cycle immune-ablative Cohort H n=6 800 µg IT 2-cycle neoadjuvant (Prior to TURBT) Cohort I n=6
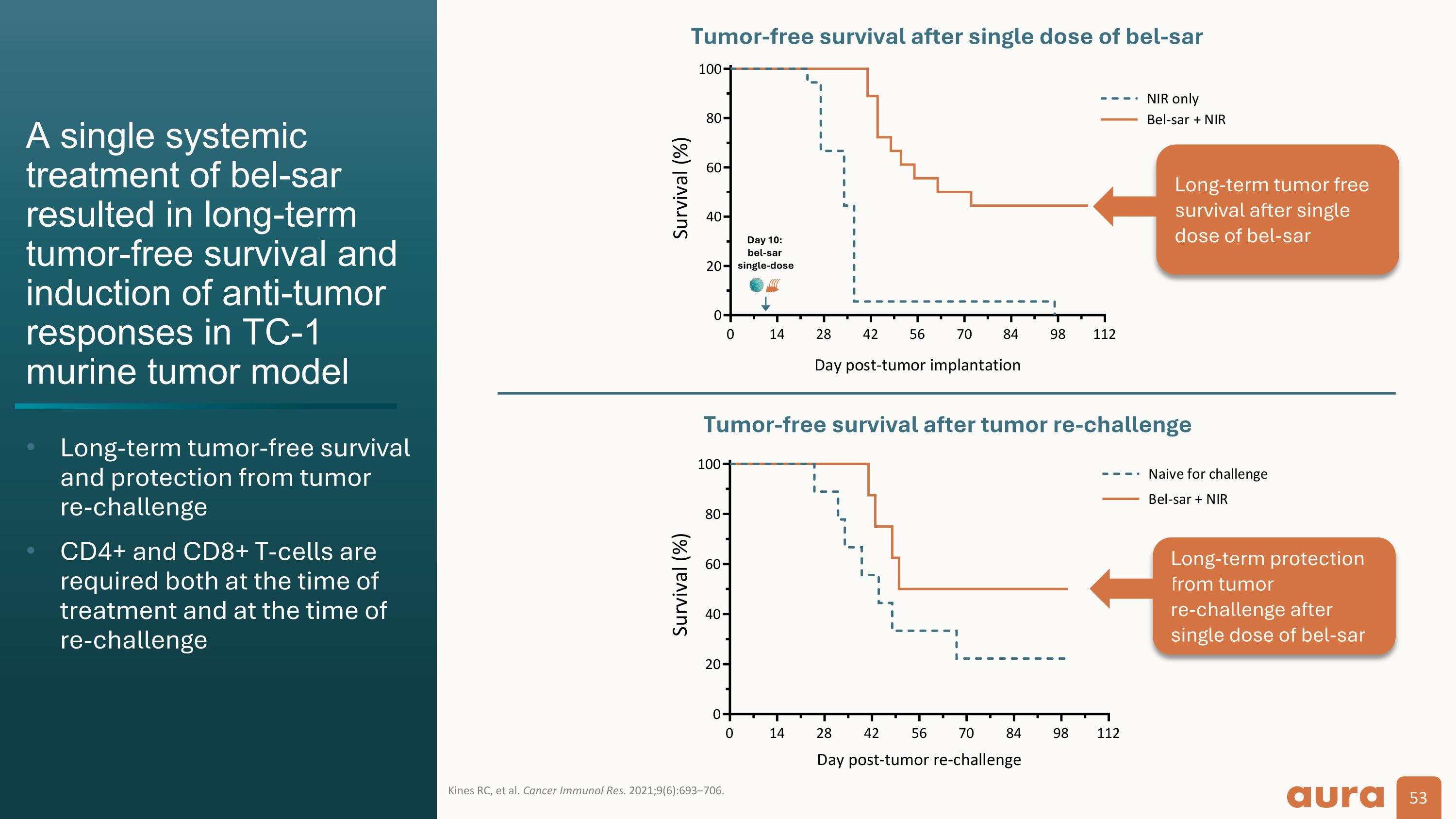
A single systemic treatment of bel-sar resulted in long-term tumor-free survival and induction of anti-tumor responses in TC-1 murine tumor model Long-term tumor-free survival and protection from tumor re-challenge CD4+ and CD8+ T-cells are required both at the time of treatment and at the time of re-challenge Kines RC, et al. Cancer Immunol Res. 2021;9(6):693–706. Tumor-free survival after tumor re-challenge Day 10: bel-sar single-dose Long-term protection from tumor re-challenge after single dose of bel-sar Long-term tumor free survival after single dose of bel-sar Tumor-free survival after single dose of bel-sar
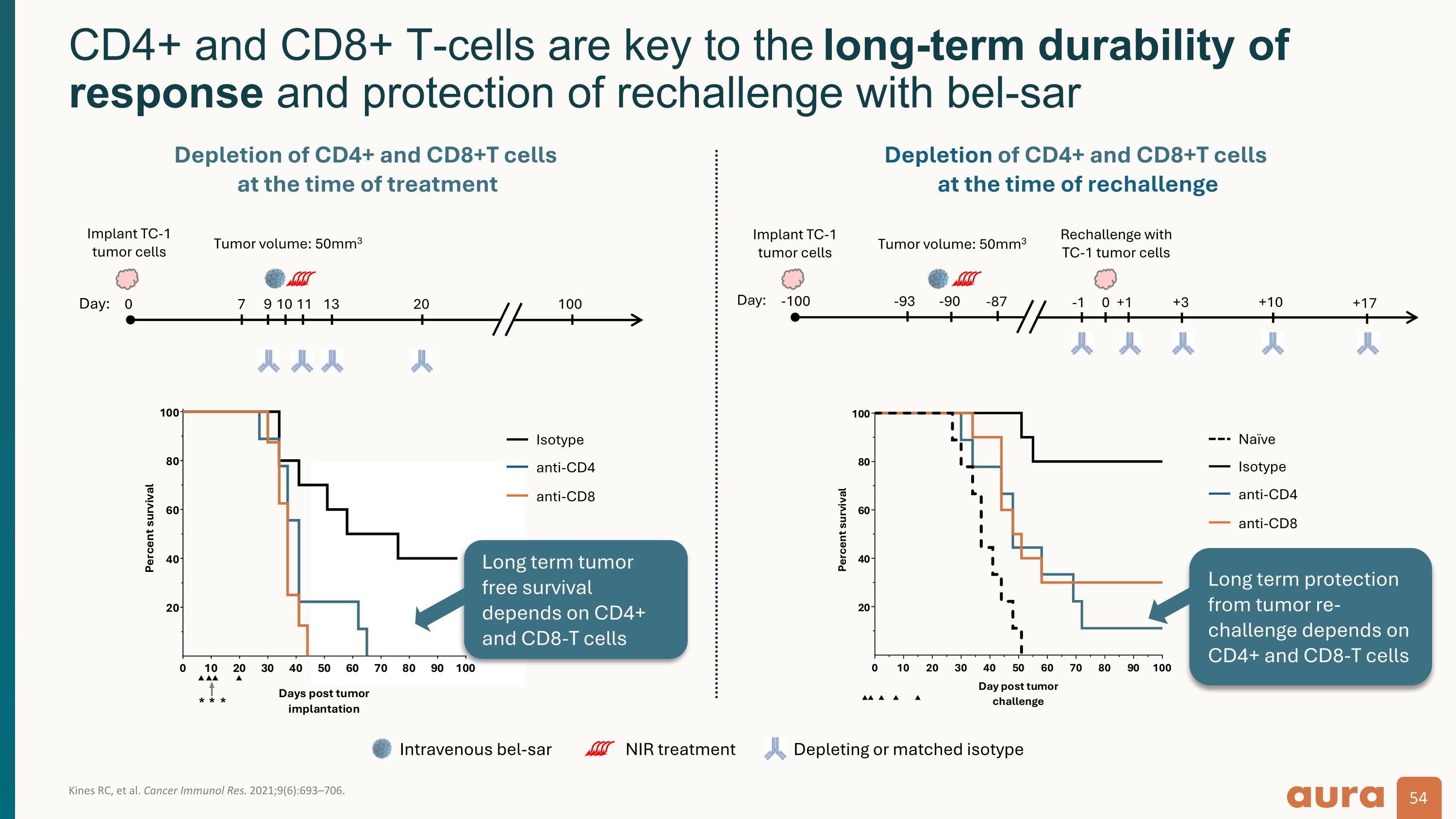
Kines RC, et al. Cancer Immunol Res. 2021;9(6):693–706. CD4+ and CD8+ T-cells are key to the long-term durability of response and protection of rechallenge with bel-sar +1 9 11 Day: 0 100 Implant TC-1 tumor cells Tumor volume: 50mm3 7 10 13 20 Day: -100 0 Implant TC-1 tumor cells Rechallenge with TC-1 tumor cells Tumor volume: 50mm3 -93 -90 -87 +10 -1 +17 +3 Depletion of CD4+ and CD8+T cells at the time of treatment Depletion of CD4+ and CD8+T cells at the time of rechallenge Depleting or matched isotype Intravenous bel-sar NIR treatment Isotype anti-CD4 anti-CD8 Isotype anti-CD4 anti-CD8 Naïve Long term protection from tumor re-challenge depends on CD4+ and CD8-T cells Long term tumor free survival depends on CD4+ and CD8-T cells