

Breaking Boundaries. Igniting Change. IO Biotech Conference Call Pivotal Phase 3 Trial Results 11August2025 Exhibit 99.2

DISCLAIMER | Forward Looking Statements Certain information contained in this presentation includes “forward-looking statements” within the meaning of Section 27A of the Securities Act of 1933, as amended, and Section 21E of the Securities Exchange Act of 1934, as amended, related to our business plan, clinical trials and regulatory submissions. We may, in some cases, use terms such as “may,” “should,” “would,” “expects,” “plans,” “anticipates,” “could,” “intends,” “target,” “projects,” “contemplates,” “believes,” “estimates,” “predicts,” “potential” or “continue” or other words that convey uncertainty of the future events or outcomes to identify these forward-looking statements. Our forward-looking statements are based on current beliefs and expectations of our management team that involve risks, potential changes in circumstances, assumptions, and uncertainties. Any or all of the forward-looking statements may turn out to be wrong or be affected by inaccurate assumptions we might make or by known or unknown risks and uncertainties. These forward-looking statements are subject to risks and uncertainties including risks related to the execution of our business plan, success and timing of our clinical trials or other studies and the other risks set forth in our filings with the U.S. Securities and Exchange Commission. For all these reasons, actual results and developments could be materially different from those expressed in or implied by our forward-looking statements. You are cautioned not to place undue reliance on these forward-looking statements, which are made only as of the date of this presentation. We undertake no obligation to publicly update such forward-looking statements to reflect subsequent events or circumstances, except as required by law.

Agenda for Today’s Call Topic Speaker Introduction Mai-Britt Zocca, Ph.D., chief executive officer Cylembio Phase 3 Data Qasim Ahmad, M.D., chief medical officer First-line Advanced Melanoma Treatment Landscape Omid Hamid, MD, Director, Clinical Research and Immunotherapy at The Angeles Clinic and Research Institute, A Cedars Sinai Affiliate Company Milestones Amy Sullivan, chief financial officer

Cylembio is an investigational drug candidate that has not been approved for marketing by the US FDA or other regulatory authorities.
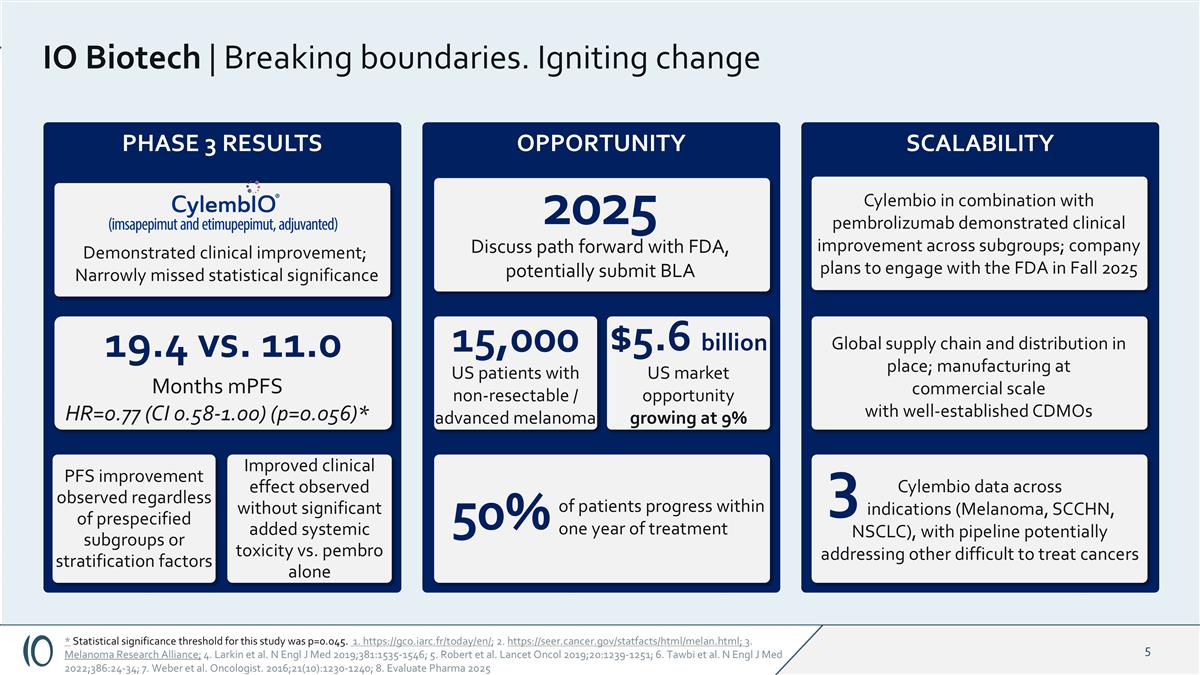
IO Biotech | Breaking boundaries. Igniting change of patients progress within one year of treatment 2025 Discuss path forward with FDA, potentially submit BLA PHASE 3 RESULTS * Statistical significance threshold for this study was p=0.045. 1. https://gco.iarc.fr/today/en/; 2. https://seer.cancer.gov/statfacts/html/melan.html; 3. Melanoma Research Alliance; 4. Larkin et al. N Engl J Med 2019;381:1535-1546; 5. Robert et al. Lancet Oncol 2019;20:1239-1251; 6. Tawbi et al. N Engl J Med 2022;386:24-34; 7. Weber et al. Oncologist. 2016;21(10):1230-1240; 8. Evaluate Pharma 2025 Cylembio in combination with pembrolizumab demonstrated clinical improvement across subgroups; company plans to engage with the FDA in Fall 2025 SCALABILITY OPPORTUNITY Improved clinical effect observed without significant added systemic toxicity vs. pembro alone 19.4 vs. 11.0 Months mPFS HR=0.77 (CI 0.58-1.00) (p=0.056)* PFS improvement observed regardless of prespecified subgroups or stratification factors 15,000 Demonstrated clinical improvement; Narrowly missed statistical significance Cylembio data across indications (Melanoma, SCCHN, NSCLC), with pipeline potentially addressing other difficult to treat cancers US patients with non-resectable / advanced melanoma 50% $5.6 billion US market opportunity growing at 9% Global supply chain and distribution in place; manufacturing at commercial scale with well-established CDMOs 3
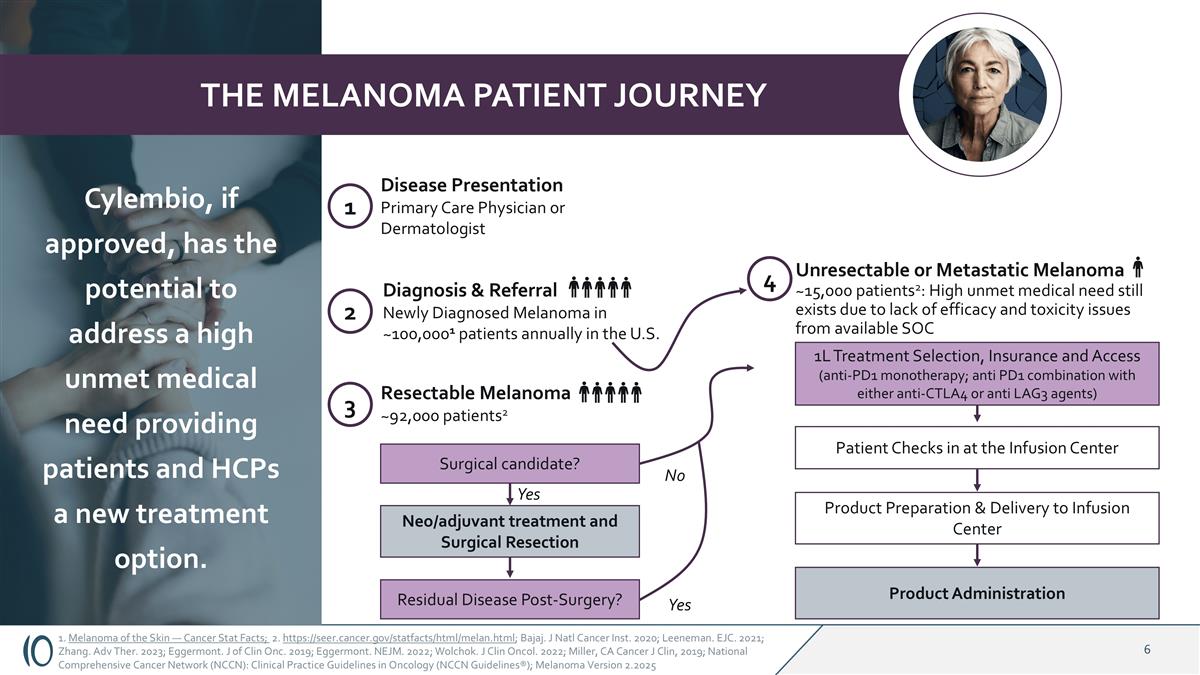
1. Melanoma of the Skin — Cancer Stat Facts; 2. https://seer.cancer.gov/statfacts/html/melan.html; Bajaj. J Natl Cancer Inst. 2020; Leeneman. EJC. 2021; Zhang. Adv Ther. 2023; Eggermont. J of Clin Onc. 2019; Eggermont. NEJM. 2022; Wolchok. J Clin Oncol. 2022; Miller, CA Cancer J Clin, 2019; National Comprehensive Cancer Network (NCCN): Clinical Practice Guidelines in Oncology (NCCN Guidelines®); Melanoma Version 2.2025 THE MELANOMA PATIENT JOURNEY 1 Disease Presentation Primary Care Physician or Dermatologist 2 Diagnosis & Referral Newly Diagnosed Melanoma in ~100,0001 patients annually in the U.S. 3 Resectable Melanoma ~92,000 patients2 Surgical candidate? Residual Disease Post-Surgery? Neo/adjuvant treatment and Surgical Resection 4 Unresectable or Metastatic Melanoma ~15,000 patients2: High unmet medical need still exists due to lack of efficacy and toxicity issues from available SOC 1L Treatment Selection, Insurance and Access (anti-PD1 monotherapy; anti PD1 combination with either anti-CTLA4 or anti LAG3 agents) Patient Checks in at the Infusion Center Product Preparation & Delivery to Infusion Center Product Administration Yes No Yes Cylembio, if approved, has the potential to address a high unmet medical need providing patients and HCPs a new treatment option.
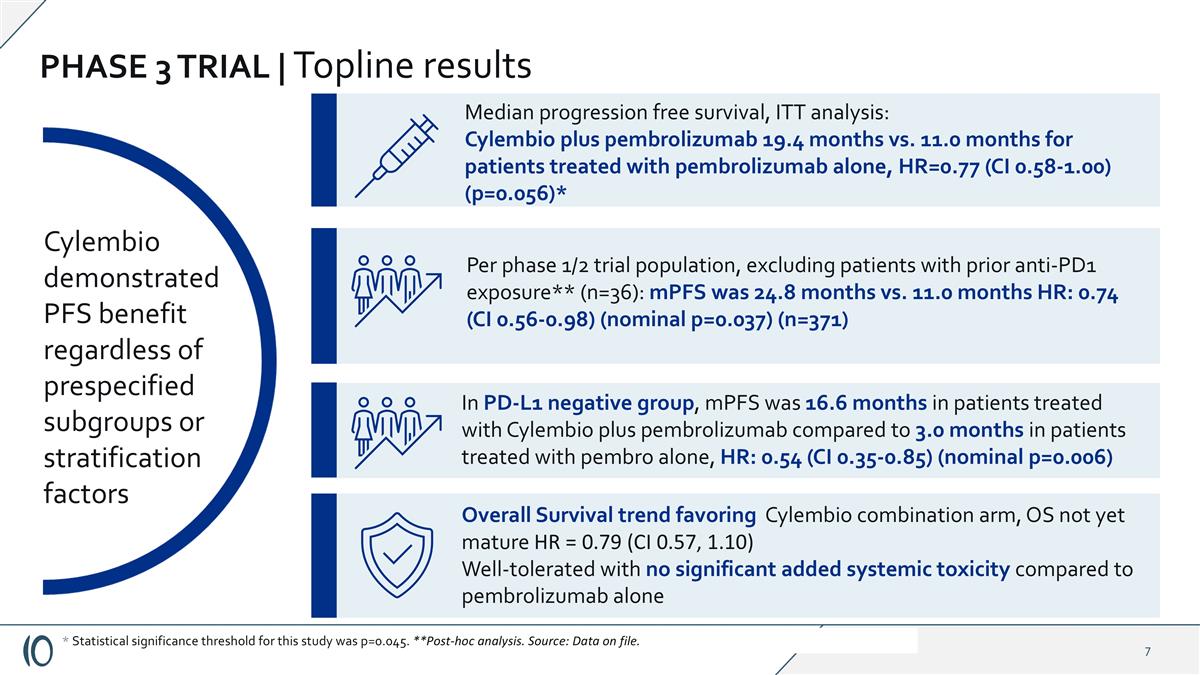
PHASE 3 TRIAL | Topline results Cylembio demonstrated PFS benefit regardless of prespecified subgroups or stratification factors Median progression free survival, ITT analysis: Cylembio plus pembrolizumab 19.4 months vs. 11.0 months for patients treated with pembrolizumab alone, HR=0.77 (CI 0.58-1.00) (p=0.056)* Overall Survival trend favoring Cylembio combination arm, OS not yet mature HR = 0.79 (CI 0.57, 1.10) Well-tolerated with no significant added systemic toxicity compared to pembrolizumab alone * Statistical significance threshold for this study was p=0.045. **Post-hoc analysis. Source: Data on file. In PD-L1 negative group, mPFS was 16.6 months in patients treated with Cylembio plus pembrolizumab compared to 3.0 months in patients treated with pembro alone, HR: 0.54 (CI 0.35-0.85) (nominal p=0.006) Per phase 1/2 trial population, excluding patients with prior anti-PD1 exposure** (n=36): mPFS was 24.8 months vs. 11.0 months HR: 0.74 (CI 0.56-0.98) (nominal p=0.037) (n=371)
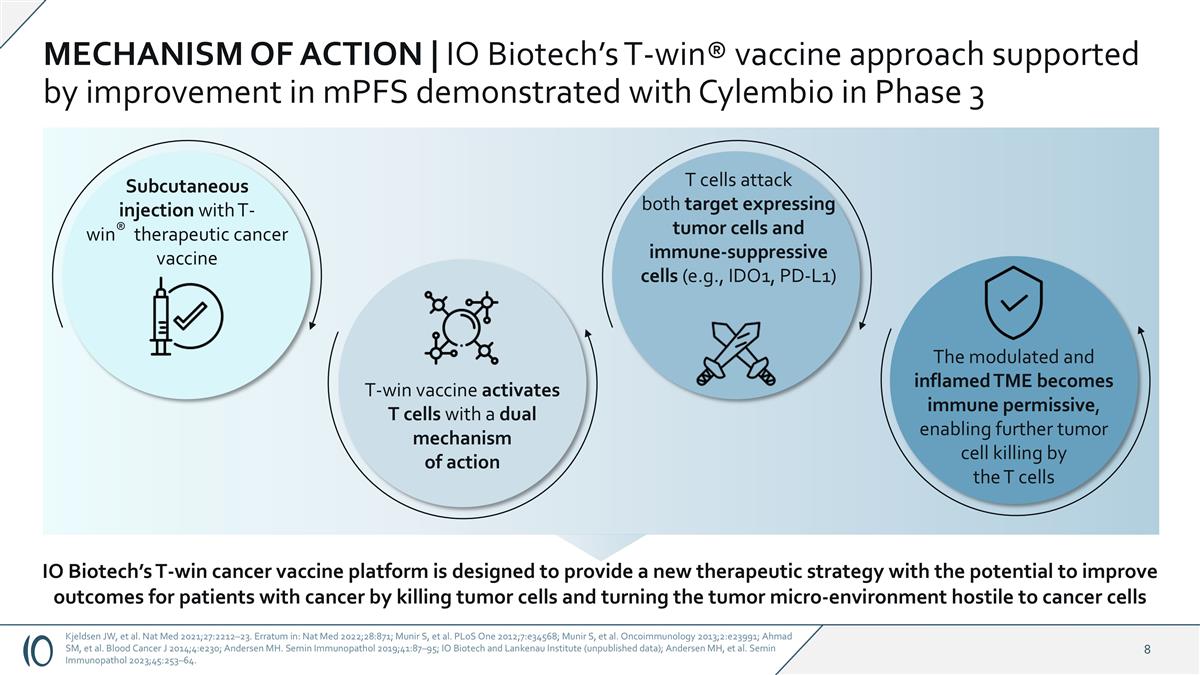
MECHANISM OF ACTION | IO Biotech’s T-win® vaccine approach supported by improvement in mPFS demonstrated with Cylembio in Phase 3 Subcutaneous injection with T-win® therapeutic cancer vaccine T-win vaccine activates T cells with a dual mechanism of action T cells attack both target expressing tumor cells and immune-suppressive cells (e.g., IDO1, PD-L1) The modulated and inflamed TME becomes immune permissive, enabling further tumor cell killing by the T cells IO Biotech’s T-win cancer vaccine platform is designed to provide a new therapeutic strategy with the potential to improve outcomes for patients with cancer by killing tumor cells and turning the tumor micro-environment hostile to cancer cells Kjeldsen JW, et al. Nat Med 2021;27:2212–23. Erratum in: Nat Med 2022;28:871; Munir S, et al. PLoS One 2012;7:e34568; Munir S, et al. Oncoimmunology 2013;2:e23991; Ahmad SM, et al. Blood Cancer J 2014;4:e230; Andersen MH. Semin Immunopathol 2019;41:87–95; IO Biotech and Lankenau Institute (unpublished data); Andersen MH, et al. Semin Immunopathol 2023;45:253–64.
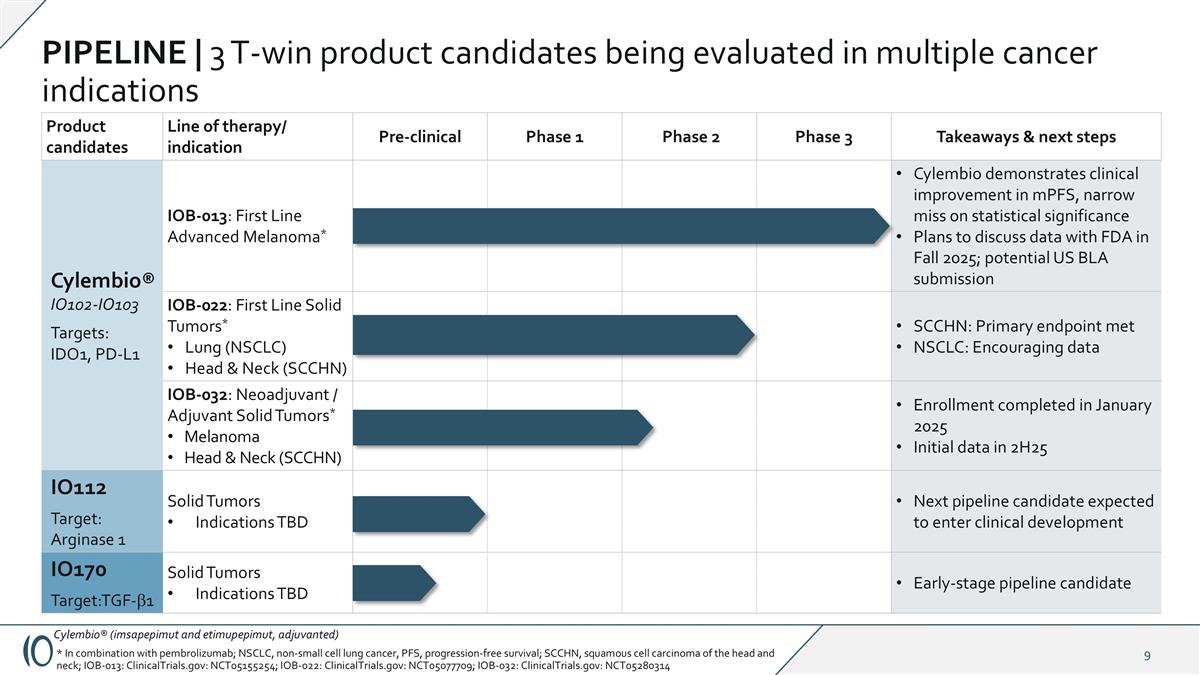
Product candidates Line of therapy/ indication Pre-clinical Phase 1 Phase 2 Phase 3 Takeaways & next steps Cylembio® IO102-IO103 Targets: IDO1, PD-L1 IOB-013: First Line Advanced Melanoma* Cylembio demonstrates clinical improvement in mPFS, narrow miss on statistical significance Plans to discuss data with FDA in Fall 2025; potential US BLA submission IO102–IO103 IOB-022: First Line Solid Tumors* Lung (NSCLC) Head & Neck (SCCHN) SCCHN: Primary endpoint met NSCLC: Encouraging data IOB-032: Neoadjuvant / Adjuvant Solid Tumors* Melanoma Head & Neck (SCCHN) Enrollment completed in January 2025 Initial data in 2H25 IO112 Target: Arginase 1 Solid Tumors Indications TBD Next pipeline candidate expected to enter clinical development IO170 Target:TGF-b1 Solid Tumors Indications TBD Early-stage pipeline candidate PIPELINE | 3 T-win product candidates being evaluated in multiple cancer indications Cylembio® (imsapepimut and etimupepimut, adjuvanted) * In combination with pembrolizumab; NSCLC, non-small cell lung cancer, PFS, progression-free survival; SCCHN, squamous cell carcinoma of the head and neck; IOB-013: ClinicalTrials.gov: NCT05155254; IOB-022: ClinicalTrials.gov: NCT05077709; IOB-032: ClinicalTrials.gov: NCT05280314

Cylembio Phase 3 Pivotal Trial Results
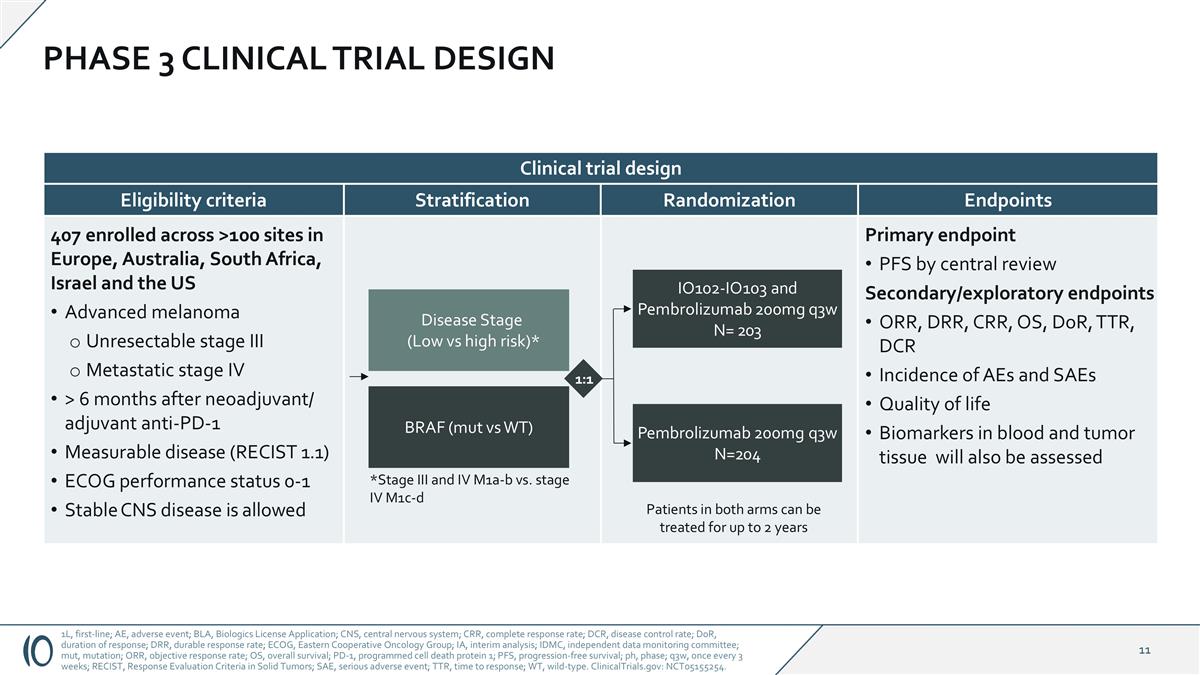
Primary endpoint PFS by central review Secondary/exploratory endpoints ORR, DRR, CRR, OS, DoR, TTR, DCR Incidence of AEs and SAEs Quality of life Biomarkers in blood and tumor tissue will also be assessed Stratification Endpoints Randomization 407 enrolled across >100 sites in Europe, Australia, South Africa, Israel and the US Advanced melanoma Unresectable stage III Metastatic stage IV > 6 months after neoadjuvant/ adjuvant anti-PD-1 Measurable disease (RECIST 1.1) ECOG performance status 0-1 Stable CNS disease is allowed Eligibility criteria PHASE 3 CLINICAL TRIAL DESIGN 1L, first-line; AE, adverse event; BLA, Biologics License Application; CNS, central nervous system; CRR, complete response rate; DCR, disease control rate; DoR, duration of response; DRR, durable response rate; ECOG, Eastern Cooperative Oncology Group; IA, interim analysis; IDMC, independent data monitoring committee; mut, mutation; ORR, objective response rate; OS, overall survival; PD-1, programmed cell death protein 1; PFS, progression-free survival; ph, phase; q3w, once every 3 weeks; RECIST, Response Evaluation Criteria in Solid Tumors; SAE, serious adverse event; TTR, time to response; WT, wild-type. ClinicalTrials.gov: NCT05155254. Disease Stage (Low vs high risk)* BRAF (mut vs WT) *Stage III and IV M1a-b vs. stage IV M1c-d IO102-IO103 and Pembrolizumab 200mg q3w N= 203 Pembrolizumab 200mg q3w N=204 1:1 Clinical trial design Patients in both arms can be treated for up to 2 years
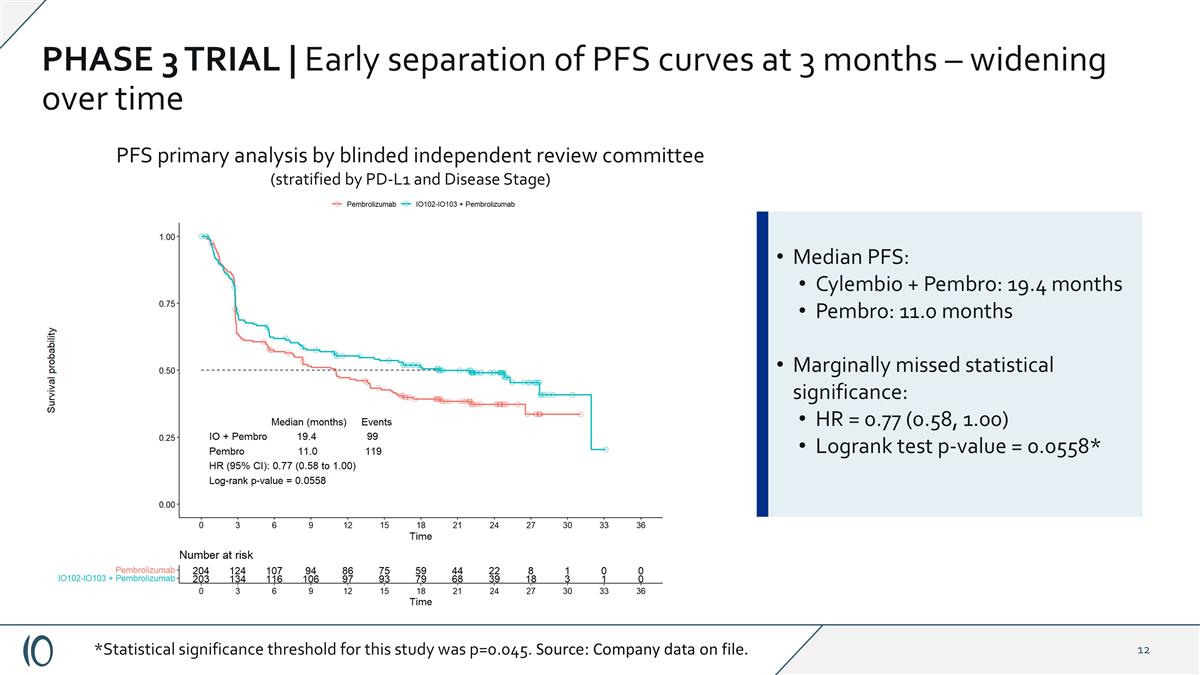
PHASE 3 TRIAL | Early separation of PFS curves at 3 months – widening over time PFS primary analysis by blinded independent review committee (stratified by PD-L1 and Disease Stage) Median PFS: Cylembio + Pembro: 19.4 months Pembro: 11.0 months Marginally missed statistical significance: HR = 0.77 (0.58, 1.00) Logrank test p-value = 0.0558* *Statistical significance threshold for this study was p=0.045. Source: Company data on file.
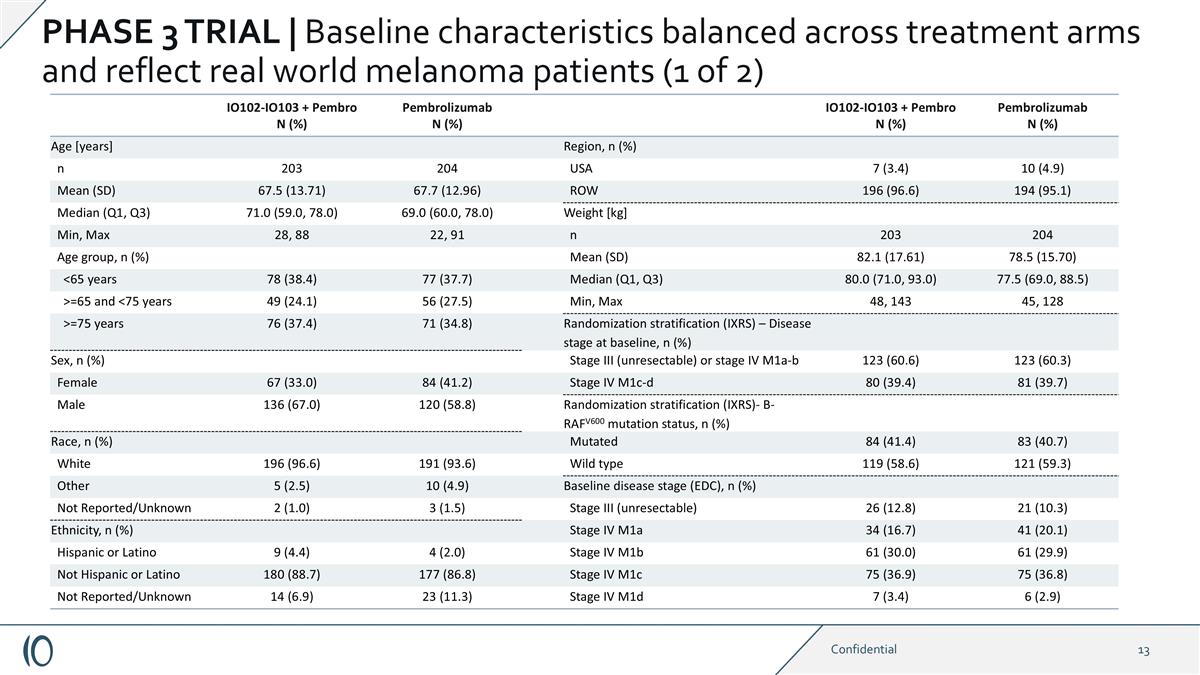
IO102-IO103 + Pembro N (%) Pembrolizumab N (%) IO102-IO103 + Pembro N (%) Pembrolizumab N (%) Age [years] Region, n (%) n 203 204 USA 7 (3.4) 10 (4.9) Mean (SD) 67.5 (13.71) 67.7 (12.96) ROW 196 (96.6) 194 (95.1) Median (Q1, Q3) 71.0 (59.0, 78.0) 69.0 (60.0, 78.0) Weight [kg] Min, Max 28, 88 22, 91 n 203 204 Age group, n (%) Mean (SD) 82.1 (17.61) 78.5 (15.70) <65 years 78 (38.4) 77 (37.7) Median (Q1, Q3) 80.0 (71.0, 93.0) 77.5 (69.0, 88.5) >=65 and <75 years 49 (24.1) 56 (27.5) Min, Max 48, 143 45, 128 >=75 years 76 (37.4) 71 (34.8) Randomization stratification (IXRS) – Disease stage at baseline, n (%) Sex, n (%) Stage III (unresectable) or stage IV M1a-b 123 (60.6) 123 (60.3) Female 67 (33.0) 84 (41.2) Stage IV M1c-d 80 (39.4) 81 (39.7) Male 136 (67.0) 120 (58.8) Randomization stratification (IXRS)- B-RAFV600 mutation status, n (%) Race, n (%) Mutated 84 (41.4) 83 (40.7) White 196 (96.6) 191 (93.6) Wild type 119 (58.6) 121 (59.3) Other 5 (2.5) 10 (4.9) Baseline disease stage (EDC), n (%) Not Reported/Unknown 2 (1.0) 3 (1.5) Stage III (unresectable) 26 (12.8) 21 (10.3) Ethnicity, n (%) Stage IV M1a 34 (16.7) 41 (20.1) Hispanic or Latino 9 (4.4) 4 (2.0) Stage IV M1b 61 (30.0) 61 (29.9) Not Hispanic or Latino 180 (88.7) 177 (86.8) Stage IV M1c 75 (36.9) 75 (36.8) Not Reported/Unknown 14 (6.9) 23 (11.3) Stage IV M1d 7 (3.4) 6 (2.9) Confidential PHASE 3 TRIAL | Baseline characteristics balanced across treatment arms and reflect real world melanoma patients (1 of 2)
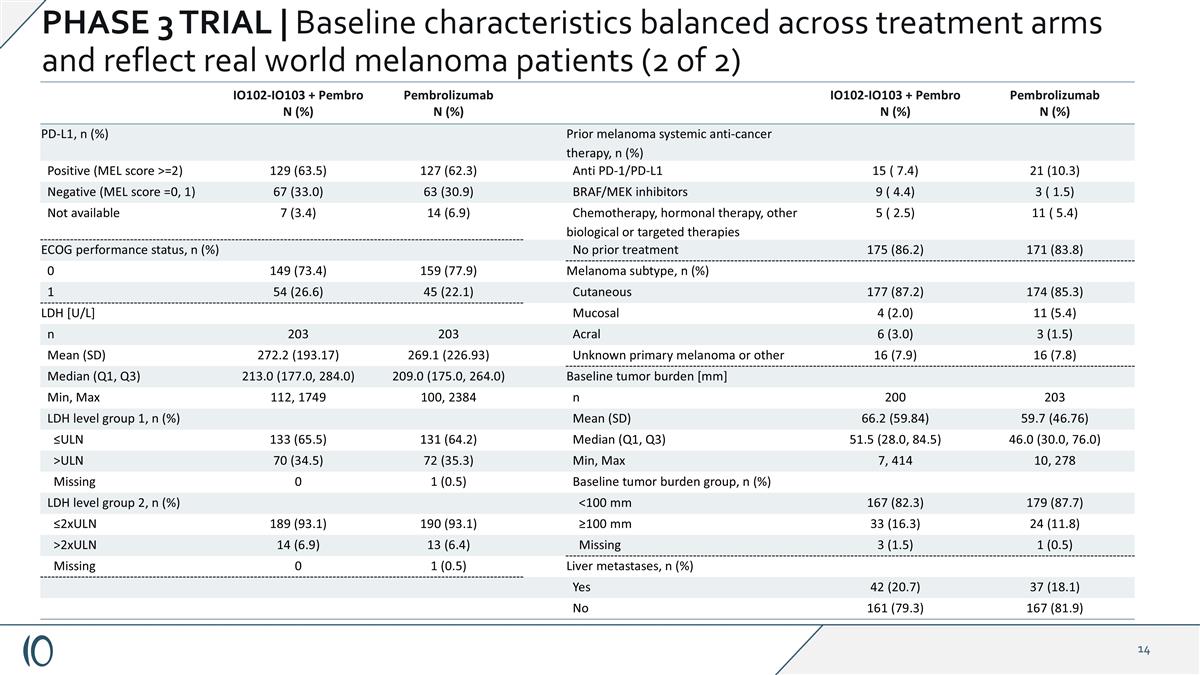
IO102-IO103 + Pembro N (%) Pembrolizumab N (%) IO102-IO103 + Pembro N (%) Pembrolizumab N (%) PD-L1, n (%) Prior melanoma systemic anti-cancer therapy, n (%) Positive (MEL score >=2) 129 (63.5) 127 (62.3) Anti PD-1/PD-L1 15 ( 7.4) 21 (10.3) Negative (MEL score =0, 1) 67 (33.0) 63 (30.9) BRAF/MEK inhibitors 9 ( 4.4) 3 ( 1.5) Not available 7 (3.4) 14 (6.9) Chemotherapy, hormonal therapy, other biological or targeted therapies 5 ( 2.5) 11 ( 5.4) ECOG performance status, n (%) No prior treatment 175 (86.2) 171 (83.8) 0 149 (73.4) 159 (77.9) Melanoma subtype, n (%) 1 54 (26.6) 45 (22.1) Cutaneous 177 (87.2) 174 (85.3) LDH [U/L] Mucosal 4 (2.0) 11 (5.4) n 203 203 Acral 6 (3.0) 3 (1.5) Mean (SD) 272.2 (193.17) 269.1 (226.93) Unknown primary melanoma or other 16 (7.9) 16 (7.8) Median (Q1, Q3) 213.0 (177.0, 284.0) 209.0 (175.0, 264.0) Baseline tumor burden [mm] Min, Max 112, 1749 100, 2384 n 200 203 LDH level group 1, n (%) Mean (SD) 66.2 (59.84) 59.7 (46.76) ≤ULN 133 (65.5) 131 (64.2) Median (Q1, Q3) 51.5 (28.0, 84.5) 46.0 (30.0, 76.0) >ULN 70 (34.5) 72 (35.3) Min, Max 7, 414 10, 278 Missing 0 1 (0.5) Baseline tumor burden group, n (%) LDH level group 2, n (%) <100 mm 167 (82.3) 179 (87.7) ≤2xULN 189 (93.1) 190 (93.1) ≥100 mm 33 (16.3) 24 (11.8) >2xULN 14 (6.9) 13 (6.4) Missing 3 (1.5) 1 (0.5) Missing 0 1 (0.5) Liver metastases, n (%) Yes 42 (20.7) 37 (18.1) No 161 (79.3) 167 (81.9) PHASE 3 TRIAL | Baseline characteristics balanced across treatment arms and reflect real world melanoma patients (2 of 2)
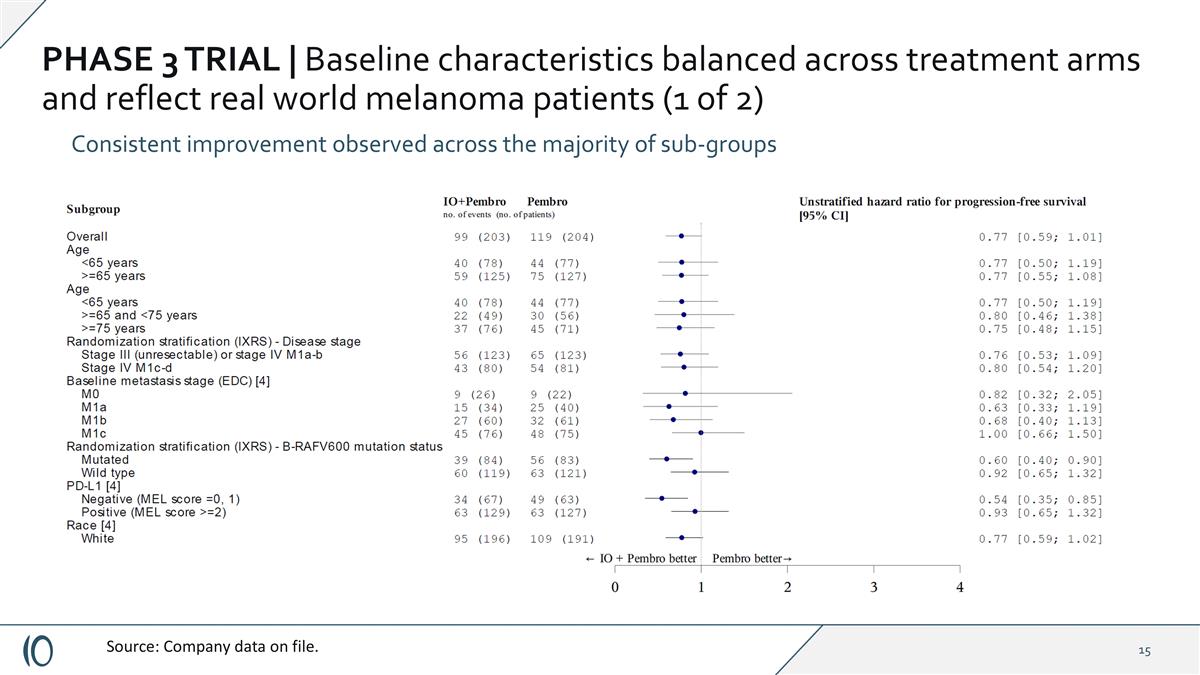
PHASE 3 TRIAL | Baseline characteristics balanced across treatment arms and reflect real world melanoma patients (1 of 2) Consistent improvement observed across the majority of sub-groups Source: Company data on file.
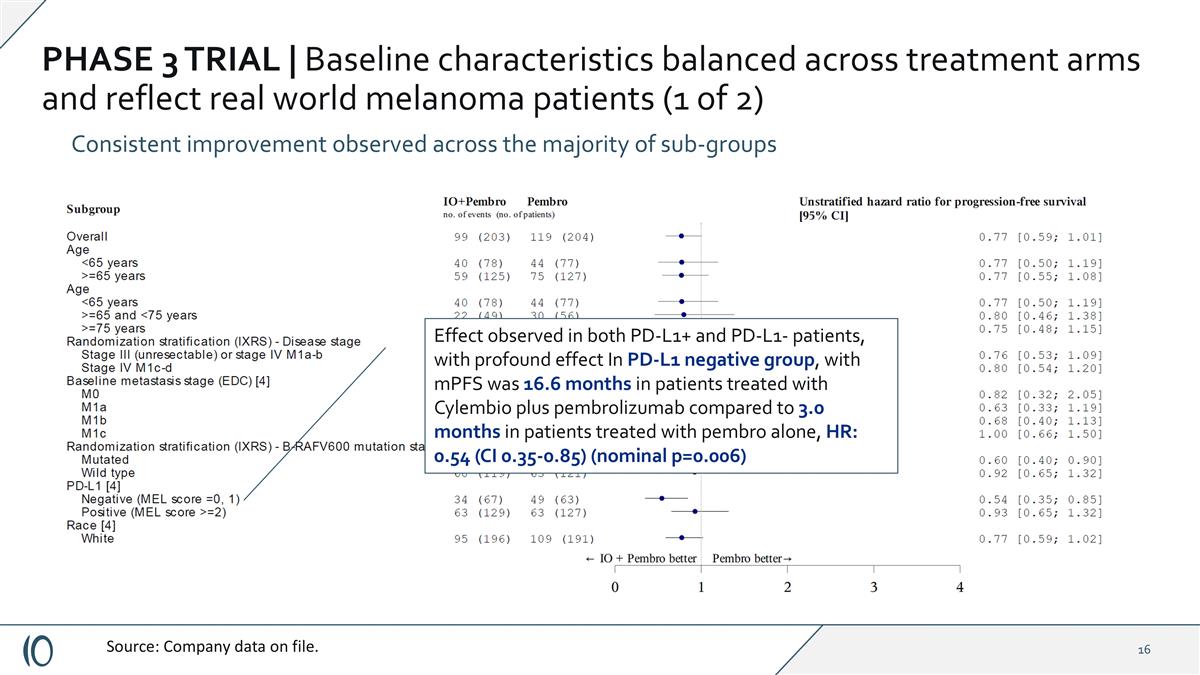
PHASE 3 TRIAL | Baseline characteristics balanced across treatment arms and reflect real world melanoma patients (1 of 2) Consistent improvement observed across the majority of sub-groups Source: Company data on file. Effect observed in both PD-L1+ and PD-L1- patients, with profound effect In PD-L1 negative group, with mPFS was 16.6 months in patients treated with Cylembio plus pembrolizumab compared to 3.0 months in patients treated with pembro alone, HR: 0.54 (CI 0.35-0.85) (nominal p=0.006)
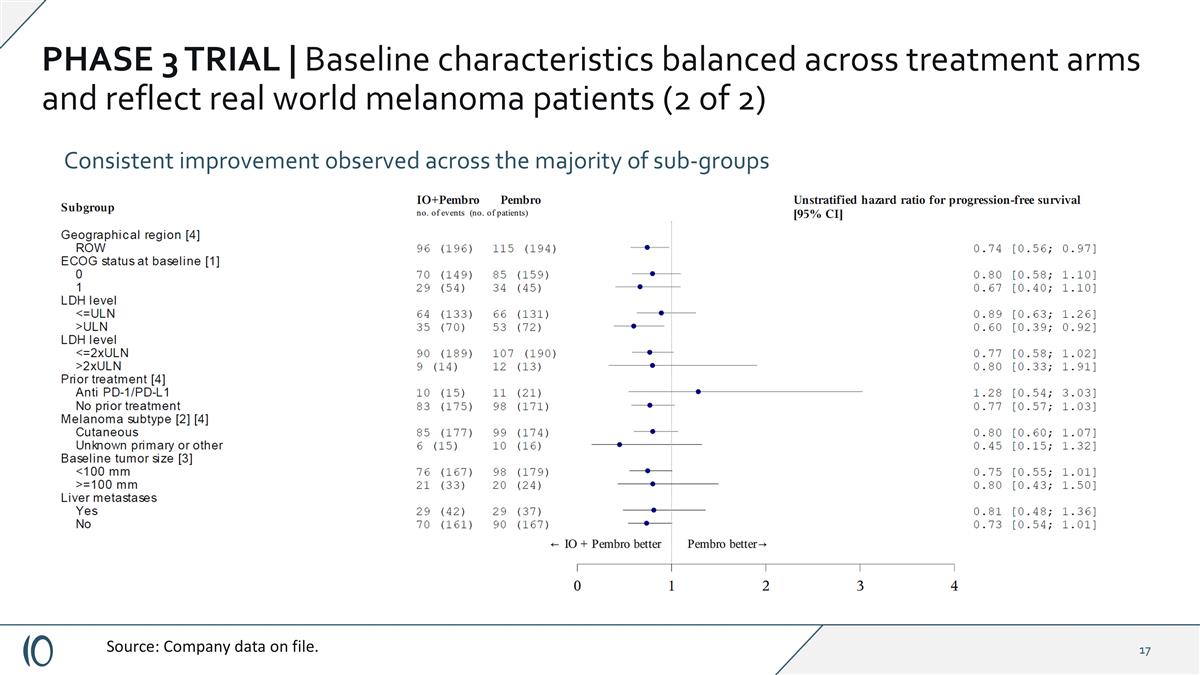
PHASE 3 TRIAL | Baseline characteristics balanced across treatment arms and reflect real world melanoma patients (2 of 2) Consistent improvement observed across the majority of sub-groups Source: Company data on file.
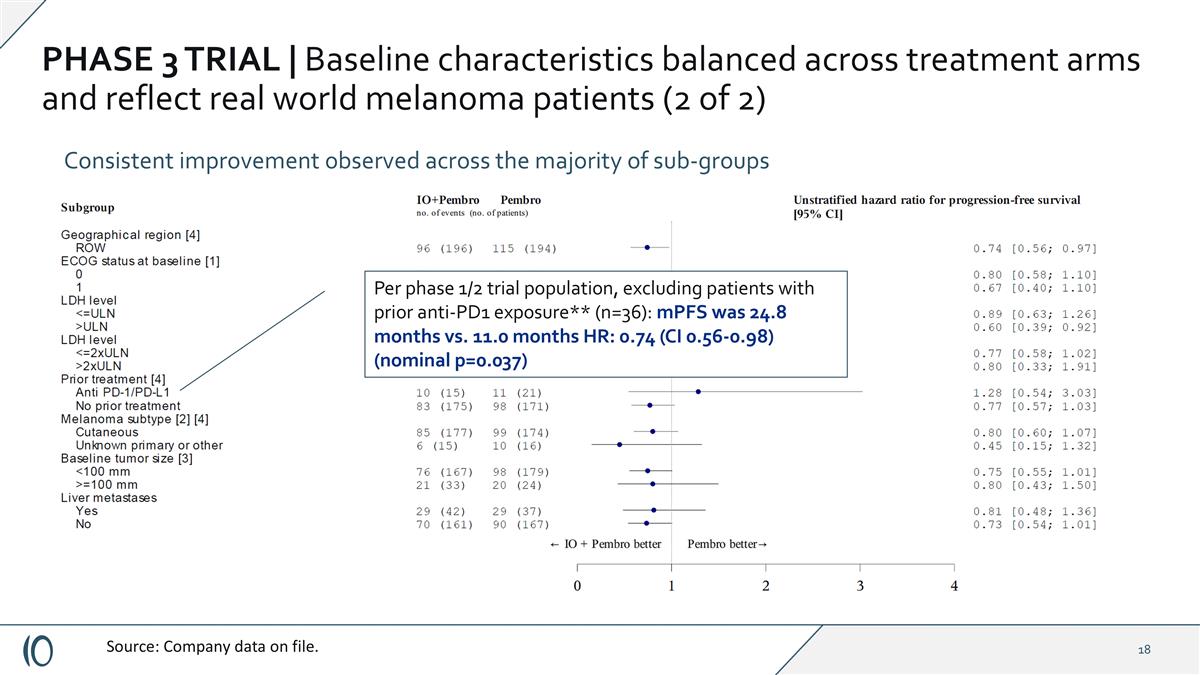
PHASE 3 TRIAL | Baseline characteristics balanced across treatment arms and reflect real world melanoma patients (2 of 2) Consistent improvement observed across the majority of sub-groups Source: Company data on file. Per phase 1/2 trial population, excluding patients with prior anti-PD1 exposure** (n=36): mPFS was 24.8 months vs. 11.0 months HR: 0.74 (CI 0.56-0.98) (nominal p=0.037)
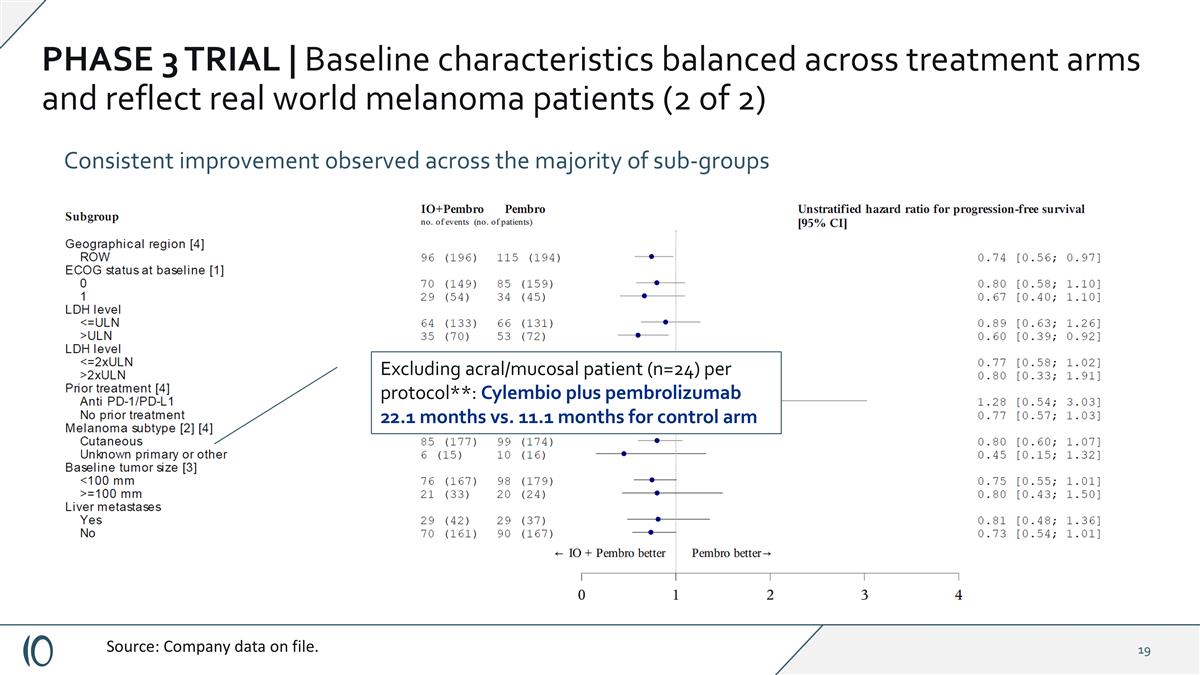
PHASE 3 TRIAL | Baseline characteristics balanced across treatment arms and reflect real world melanoma patients (2 of 2) Consistent improvement observed across the majority of sub-groups Source: Company data on file. Excluding acral/mucosal patient (n=24) per protocol**: Cylembio plus pembrolizumab 22.1 months vs. 11.1 months for control arm
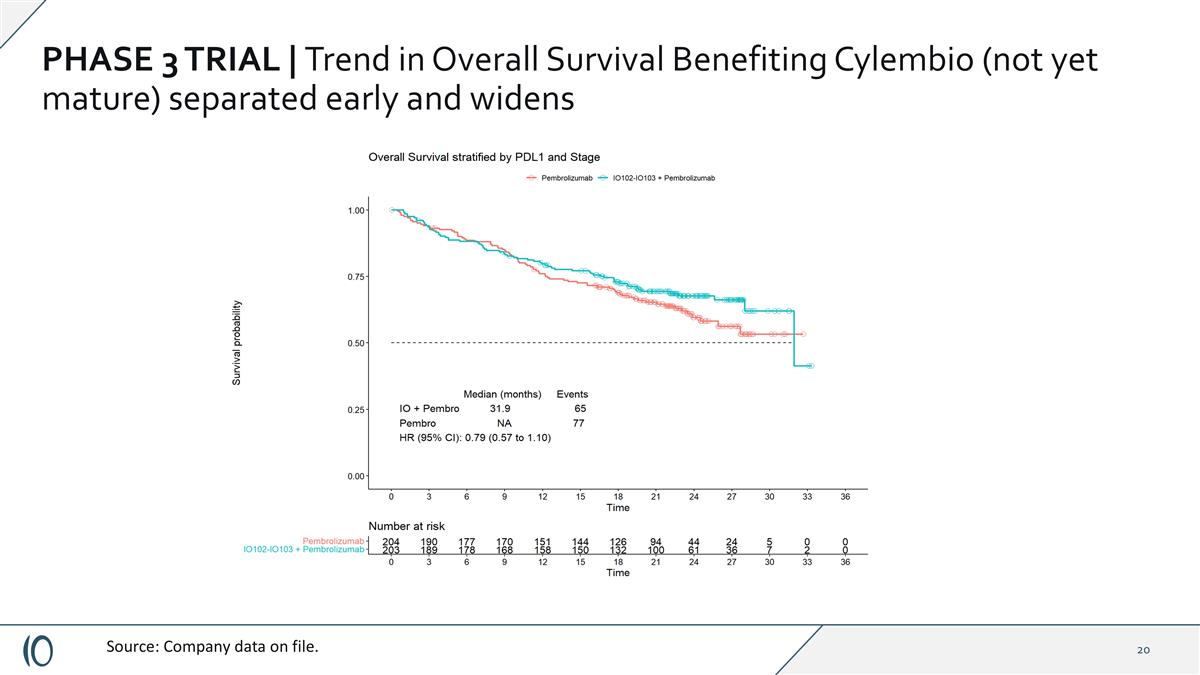
PHASE 3 TRIAL | Trend in Overall Survival Benefiting Cylembio (not yet mature) separated early and widens Source: Company data on file.
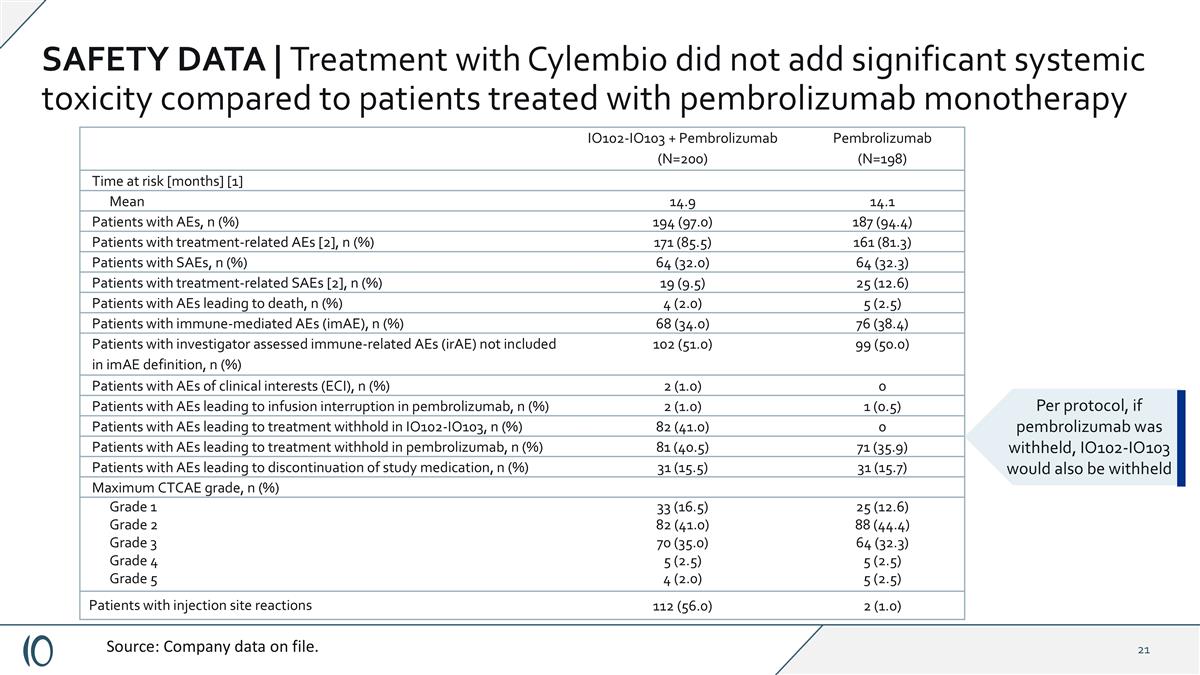
SAFETY DATA | Treatment with Cylembio did not add significant systemic toxicity compared to patients treated with pembrolizumab monotherapy IO102-IO103 + Pembrolizumab (N=200) Pembrolizumab (N=198) Time at risk [months] [1] Mean 14.9 14.1 Patients with AEs, n (%) 194 (97.0) 187 (94.4) Patients with treatment-related AEs [2], n (%) 171 (85.5) 161 (81.3) Patients with SAEs, n (%) 64 (32.0) 64 (32.3) Patients with treatment-related SAEs [2], n (%) 19 (9.5) 25 (12.6) Patients with AEs leading to death, n (%) 4 (2.0) 5 (2.5) Patients with immune-mediated AEs (imAE), n (%) 68 (34.0) 76 (38.4) Patients with investigator assessed immune-related AEs (irAE) not included in imAE definition, n (%) 102 (51.0) 99 (50.0) Patients with AEs of clinical interests (ECI), n (%) 2 (1.0) 0 Patients with AEs leading to infusion interruption in pembrolizumab, n (%) 2 (1.0) 1 (0.5) Patients with AEs leading to treatment withhold in IO102-IO103, n (%) 82 (41.0) 0 Patients with AEs leading to treatment withhold in pembrolizumab, n (%) 81 (40.5) 71 (35.9) Patients with AEs leading to discontinuation of study medication, n (%) 31 (15.5) 31 (15.7) Maximum CTCAE grade, n (%) Grade 1 Grade 2 Grade 3 Grade 4 Grade 5 33 (16.5) 82 (41.0) 70 (35.0) 5 (2.5) 4 (2.0) 25 (12.6) 88 (44.4) 64 (32.3) 5 (2.5) 5 (2.5) Patients with injection site reactions 112 (56.0) 2 (1.0) Source: Company data on file. Per protocol, if pembrolizumab was withheld, IO102-IO103 would also be withheld
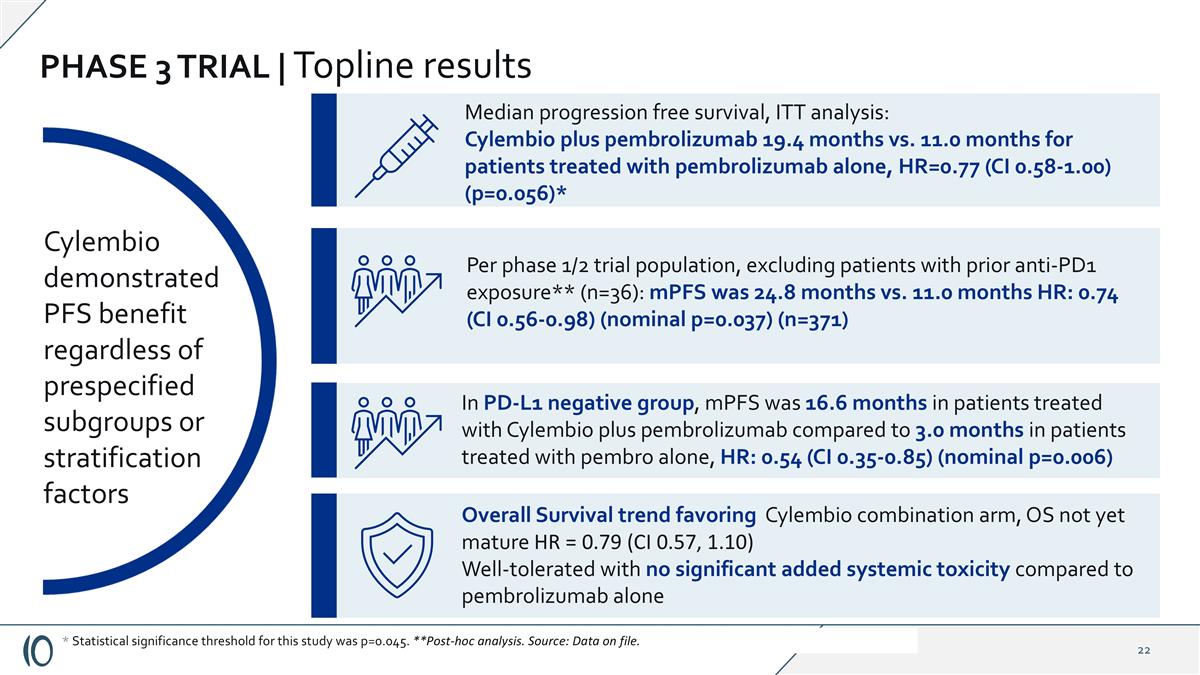
PHASE 3 TRIAL | Topline results Cylembio demonstrated PFS benefit regardless of prespecified subgroups or stratification factors Median progression free survival, ITT analysis: Cylembio plus pembrolizumab 19.4 months vs. 11.0 months for patients treated with pembrolizumab alone, HR=0.77 (CI 0.58-1.00) (p=0.056)* Overall Survival trend favoring Cylembio combination arm, OS not yet mature HR = 0.79 (CI 0.57, 1.10) Well-tolerated with no significant added systemic toxicity compared to pembrolizumab alone * Statistical significance threshold for this study was p=0.045. **Post-hoc analysis. Source: Data on file. In PD-L1 negative group, mPFS was 16.6 months in patients treated with Cylembio plus pembrolizumab compared to 3.0 months in patients treated with pembro alone, HR: 0.54 (CI 0.35-0.85) (nominal p=0.006) Per phase 1/2 trial population, excluding patients with prior anti-PD1 exposure** (n=36): mPFS was 24.8 months vs. 11.0 months HR: 0.74 (CI 0.56-0.98) (nominal p=0.037) (n=371)

1st Line Advanced Melanoma Treatment Landscape Omid Hamid, MD, Director, Clinical Research and Immunotherapy at The Angeles Clinic and Research Institute, A Cedars Sinai Affiliate
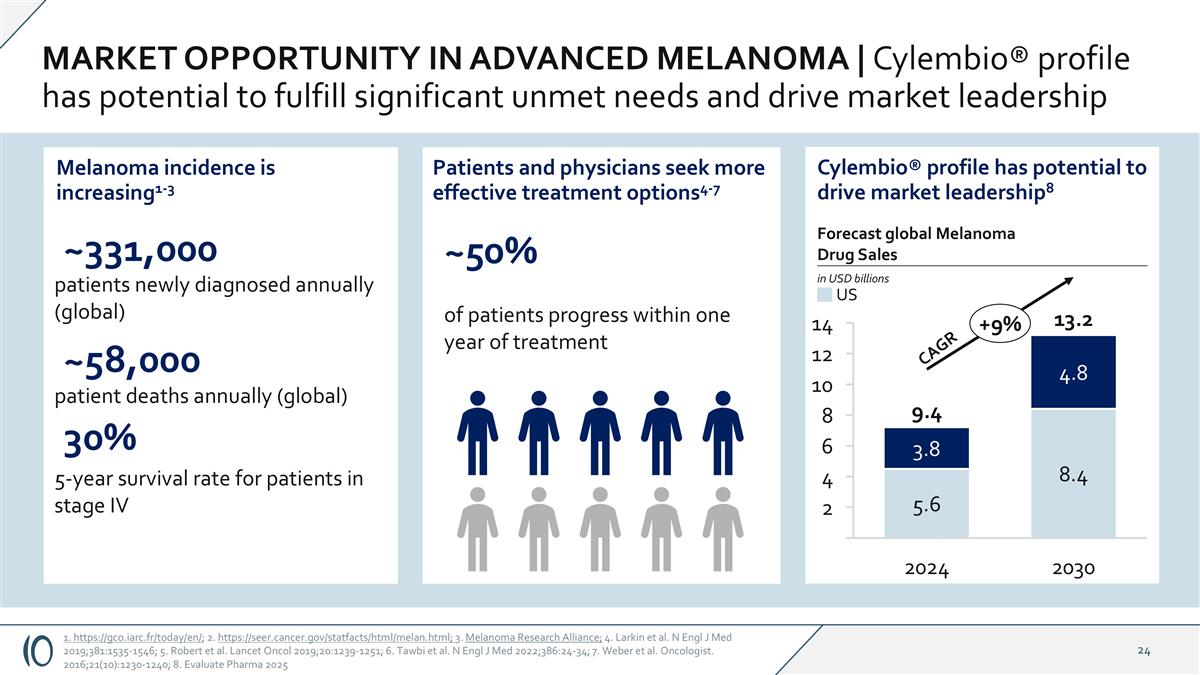
MARKET OPPORTUNITY IN ADVANCED MELANOMA | Cylembio® profile has potential to fulfill significant unmet needs and drive market leadership 1. https://gco.iarc.fr/today/en/; 2. https://seer.cancer.gov/statfacts/html/melan.html; 3. Melanoma Research Alliance; 4. Larkin et al. N Engl J Med 2019;381:1535-1546; 5. Robert et al. Lancet Oncol 2019;20:1239-1251; 6. Tawbi et al. N Engl J Med 2022;386:24-34; 7. Weber et al. Oncologist. 2016;21(10):1230-1240; 8. Evaluate Pharma 2025 Melanoma incidence is increasing1-3 ~331,000 patients newly diagnosed annually (global) ~58,000 patient deaths annually (global) 30% 5-year survival rate for patients in stage IV Cylembio® profile has potential to drive market leadership8 2030 2024 14 12 10 8 6 4 2 +9% 13.2 9.4 Forecast global Melanoma Drug Sales in USD billions US CAGR Patients and physicians seek more effective treatment options4-7 ~50% of patients progress within one year of treatment
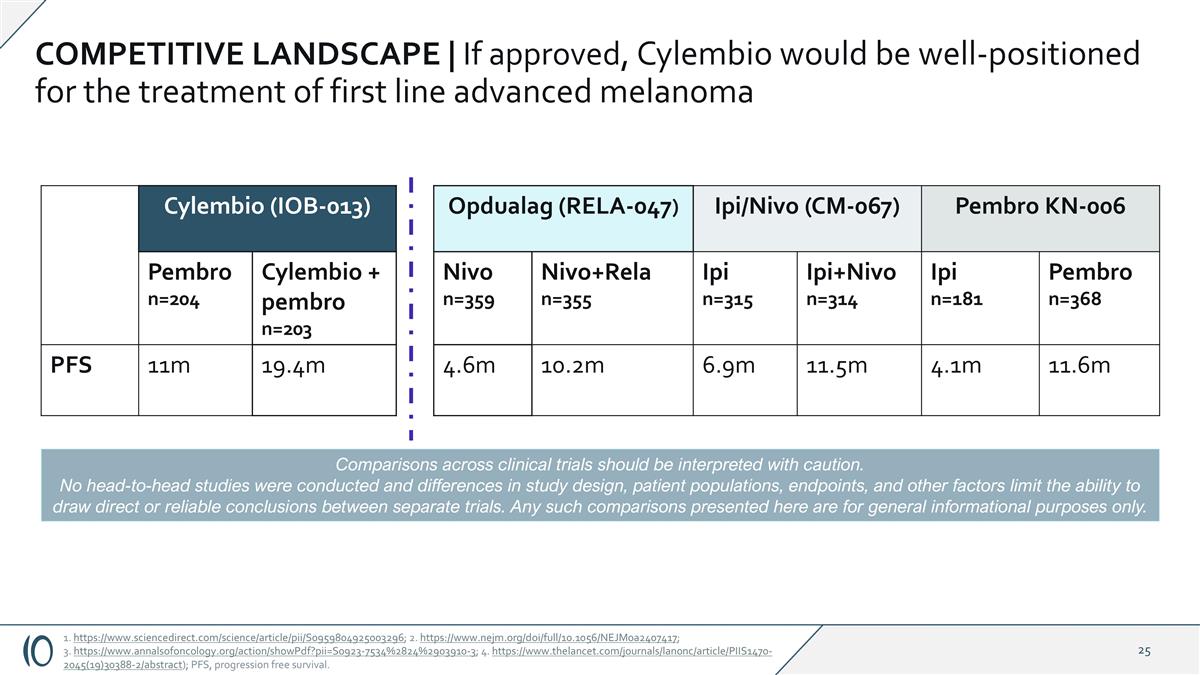
COMPETITIVE LANDSCAPE | If approved, Cylembio would be well-positioned for the treatment of first line advanced melanoma Cylembio (IOB-013) Opdualag (RELA-047) Ipi/Nivo (CM-067) Pembro KN-006 Pembro n=204 Cylembio + pembro n=203 Nivo n=359 Nivo+Rela n=355 Ipi n=315 Ipi+Nivo n=314 Ipi n=181 Pembro n=368 PFS 11m 19.4m 4.6m 10.2m 6.9m 11.5m 4.1m 11.6m Comparisons across clinical trials should be interpreted with caution. No head-to-head studies were conducted and differences in study design, patient populations, endpoints, and other factors limit the ability to draw direct or reliable conclusions between separate trials. Any such comparisons presented here are for general informational purposes only. 1. https://www.sciencedirect.com/science/article/pii/S0959804925003296; 2. https://www.nejm.org/doi/full/10.1056/NEJMoa2407417; 3. https://www.annalsofoncology.org/action/showPdf?pii=S0923-7534%2824%2903910-3; 4. https://www.thelancet.com/journals/lanonc/article/PIIS1470-2045(19)30388-2/abstract); PFS, progression free survival.

Company Milestones
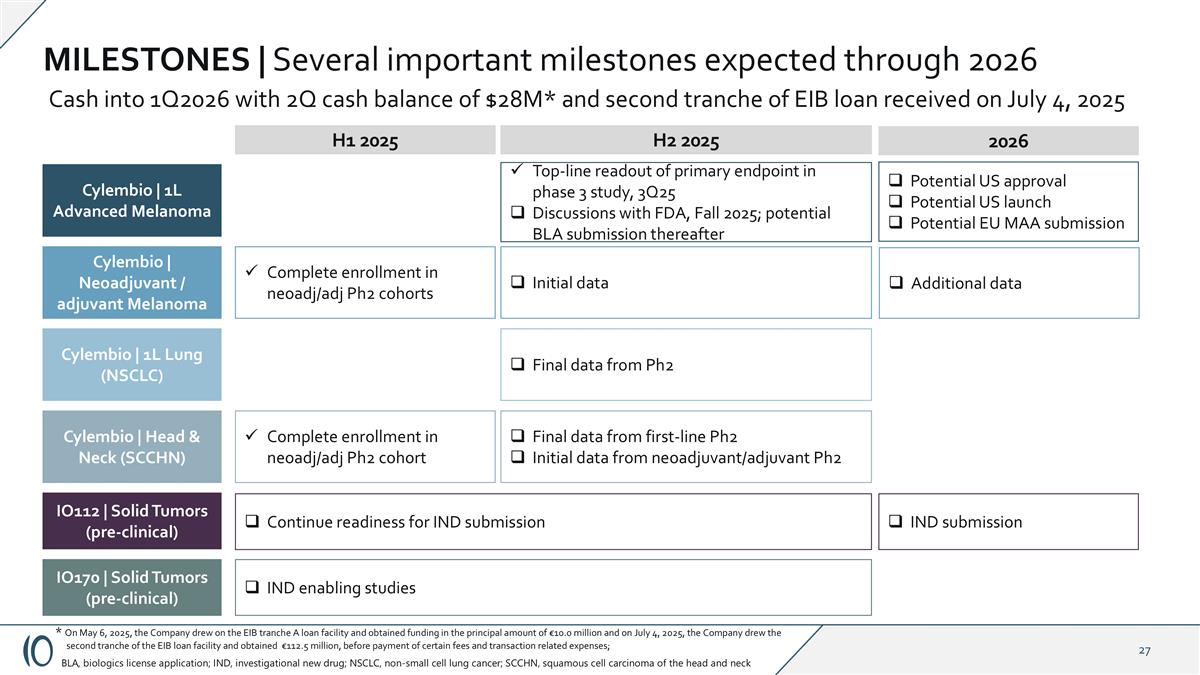
MILESTONES | Several important milestones expected through 2026 Cylembio | 1L Advanced Melanoma Top-line readout of primary endpoint in phase 3 study, 3Q25 Discussions with FDA, Fall 2025; potential BLA submission thereafter Initial data Complete enrollment in neoadj/adj Ph2 cohorts Final data from first-line Ph2 Initial data from neoadjuvant/adjuvant Ph2 Complete enrollment in neoadj/adj Ph2 cohort IND enabling studies H1 2025 H2 2025 Continue readiness for IND submission Cylembio | Neoadjuvant / adjuvant Melanoma Cylembio | 1L Lung (NSCLC) Cylembio | Head & Neck (SCCHN) IO112 | Solid Tumors (pre-clinical) IO170 | Solid Tumors (pre-clinical) Final data from Ph2 BLA, biologics license application; IND, investigational new drug; NSCLC, non-small cell lung cancer; SCCHN, squamous cell carcinoma of the head and neck Potential US approval Potential US launch Potential EU MAA submission 2026 IND submission Additional data Cash into 1Q2026 with 2Q cash balance of $28M* and second tranche of EIB loan received on July 4, 2025 * On May 6, 2025, the Company drew on the EIB tranche A loan facility and obtained funding in the principal amount of €10.0 million and on July 4, 2025, the Company drew the second tranche of the EIB loan facility and obtained €112.5 million, before payment of certain fees and transaction related expenses;

Breaking Boundaries. Igniting Change. IO Biotech Corporate Presentation Pivotal Phase 3 Trial Results Conference Call 11August2025