
Exhibit 99.3

Corporate Presentation Nasdaq: CMPX August 2025

This presentation has been prepared by Compass Therapeutics, Inc . ("we," "us," "our," or the “Company”) . Statements contained herein are made as of the date of this presentation unless stated otherwise, and this presentation shall not under any circumstances create an implication that the information contained herein is correct as of any time after such date or that information will be updated or revised to reflect information that subsequently becomes available or changes occurring after the date hereof . This presentation contains forward - looking statements. Statements in this presentation that are not purely historical are forwar d - looking statements. Such forward - looking statements include, among other things, references to Compass's financial position to continue advancing its product candidates, expecta tio ns about cash runway, business and development plans, and statements regarding Compass's product candidates, including their preclinical and clinical development, therapeutic pote nti al and tolerability profile, and clinical trial milestones such as the expected trial design, timing of enrollment, patient dosing and data readouts, regulatory plans with respect to Compas s's product candidates and the therapeutic potential thereof. Actual results could differ from those projected in any forward - looking statements due to numerous factors. Such factors include , among others, Compass's ability to raise the additional funding it will need to continue to pursue its business and product development plans, the inherent uncertainties associated wit h developing product candidates and operating as a development stage company, Compass's ability to identify additional product candidates for development, Compass's ability to dev elop, initiate and complete clinical trials for, obtain approvals for and commercialize any of its product candidates, competition in the industry in which Compass operates and mark et conditions. These forward - looking statements are made as of the date of this presentation, and Compass assumes no obligation to update the forward - looking statements, or to upda te the reasons why actual results could differ from those projected in the forward - looking statements, except as required by law. Investors should consult all of the information se t forth herein and should also refer to the risk factor disclosure set forth in the reports and other documents Compass files with the U.S. Securities and Exchange Commission (SEC) available at www.sec.gov, including without limitation Compass's latest Annual Report on Form 10 - K, Quarterly Report on Form 10 - Q and subsequent filings with the SEC. This presentation also contains estimates and other statistical data made by independent parties and by us relating to market size and growth and other data about our industry . This data involves a number of assumptions and limitations, and you are cautioned not to give undue weight to such estimates . In addition, projections, assumptions, and estimates of our future performance and the future performance of the markets in which we operate are necessarily subject to a high degree of uncertainty and risk . This presentation concerns drugs that are under clinical investigation, and which have not yet been approved for marketing by the U . S . Food and Drug Administration (FDA) . It is currently limited by Federal law to investigational use, and no representation is made as to its safety or effectiveness for the purposes for which it is being investigated . DISCLAIMER 2
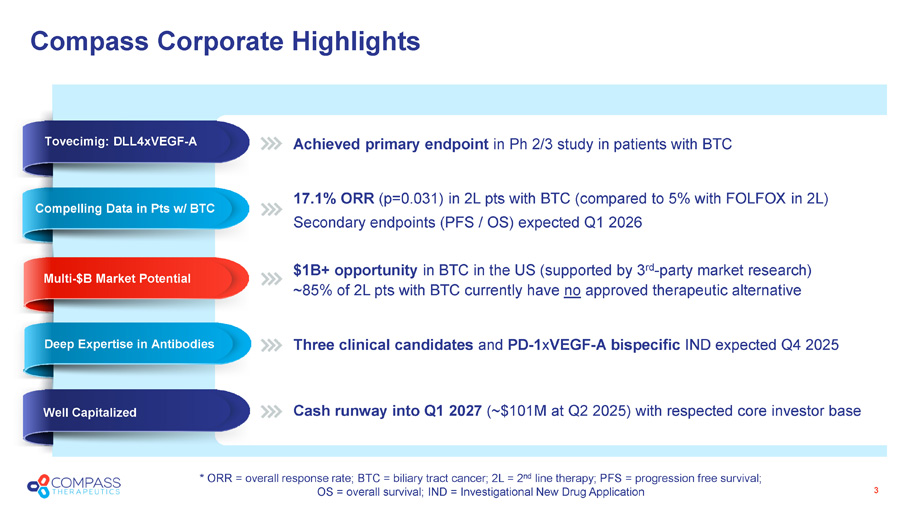
Compass Corporate Highlights 3 Achieved primary endpoint in Ph 2/3 study in patients with BTC 17.1% ORR (p=0.031) in 2L pts with BTC (compared to 5% with FOLFOX in 2L) Secondary endpoints (PFS / OS) expected Q1 2026 $ 1B+ opportunity in BTC in the US ( supported by 3 rd - party market research) ~85% of 2L pts with BTC currently have no approved therapeutic alternative Three clinical candidates and PD - 1 x VEGF - A bispecific IND expected Q4 2025 Cash runway into Q1 2027 (~$ 101 M at Q2 2025) with respected core investor base Well Capitalized Deep Expertise in Antibodies Multi - $B Market Potential Unprecedented Ph 2 Data * ORR = overall response rate; BTC = biliary tract cancer; 2L = 2 nd line therapy; PFS = progression free survival; OS = overall survival; IND = Investigational New Drug Application Compelling Data in Pts w/ BTC Tovecimig: DLL4xVEGF - A
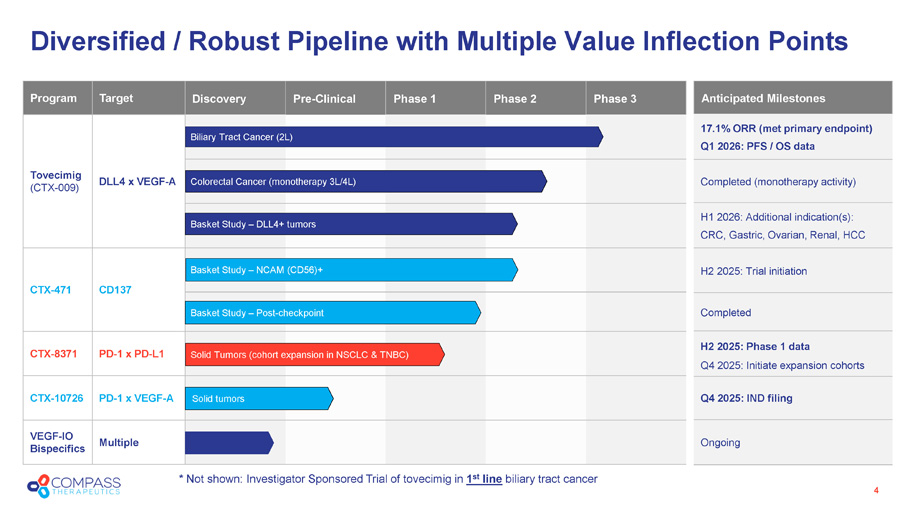
* Not shown: Investigator Sponsored Trial of tovecimig in 1 st line biliary tract cancer Diversified / Robust Pipeline with Multiple Value Inflection Points 4 Phase 3 Phase 2 Phase 1 Pre - Clinical Discovery Target Program DLL4 x VEGF - A Tovecimig (CTX - 009) CD137 CTX - 471 PD - 1 x PD - L1 CTX - 8371 PD - 1 x VEGF - A CTX - 10726 Multiple VEGF - IO Bispecifics Colorectal Cancer (monotherapy 3L/4L) Solid Tumors (cohort expansion in NSCLC & TNBC) Basket Study – DLL4+ tumors Biliary Tract Cancer (2L) Solid tumors Basket Study – Post - checkpoint Basket Study – NCAM (CD56)+ Anticipated Milestones 17.1% ORR (met primary endpoint) Q1 2026: PFS / OS data Completed (monotherapy activity) H1 2026: Additional indication(s): CRC, Gastric, Ovarian, Renal, HCC H2 2025: Trial initiation Completed H2 2025: Phase 1 data Q4 2025: Initiate expansion cohorts Q4 2025: IND filing Ongoing

Leadership Team Experienced in Drug Discovery and Development 5 Minori Rosales, MD, PhD SVP, Head of Clinical Development Bing Gong, PhD S VP, Discovery Research Jon Anderman , JD SVP, General Counsel & Corporate Secretary Thomas J. Schuetz, MD, PhD President , CEO, & Vice Chairman of the Board Neil Lerner, CPA, MIM S VP, CAO Ian Chia, PhD VP, Business Development Karin Herrera S VP, Clinical Operations James Kranz, PhD VP, CMC Kris Sachsenmeier , PhD VP, Translational Science Barry Shin , J D, MBA EVP, CFO

Tovecimig (CTX - 009) DLL4 X VEGF - A bispecific antibody
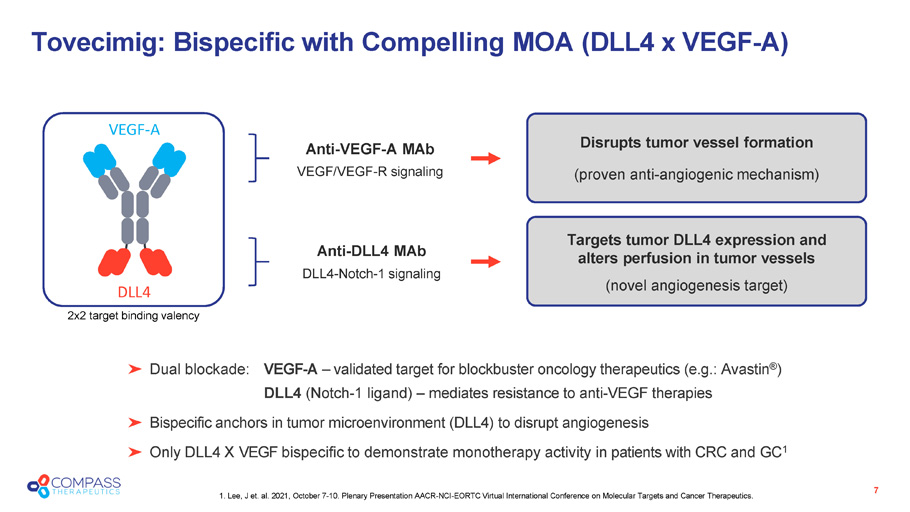
Anti - DLL4 MAb DLL4 - Notch - 1 signaling Anti - VEGF - A MAb VEGF/VEGF - R signaling Targets tumor DLL4 expression and alters perfusion in tumor vessels (novel angiogenesis target) Tovecimig: Bispecific with Compelling MOA ( DLL4 x VEGF - A ) 7 Dual blockade: VEGF - A – validated target for blockbuster oncology therapeutics (e.g.: Avastin ® ) DLL4 (Notch - 1 ligand) – mediates resistance to anti - VEGF therapies Bispecific anchors in tumor microenvironment (DLL4) to disrupt angiogenesis Only DLL4 X VEGF bispecific to demonstrate monotherapy activity in patients with CRC and GC 1 Disrupts tumor vessel formation (proven anti - angiogenic mechanism) 2x2 target binding valency VEGF - A DLL4 1. Lee, J et. al. 2021, October 7 - 10. Plenary Presentation AACR - NCI - EORTC Virtual International Conference on Molecular Targets and Cancer Therapeutics.
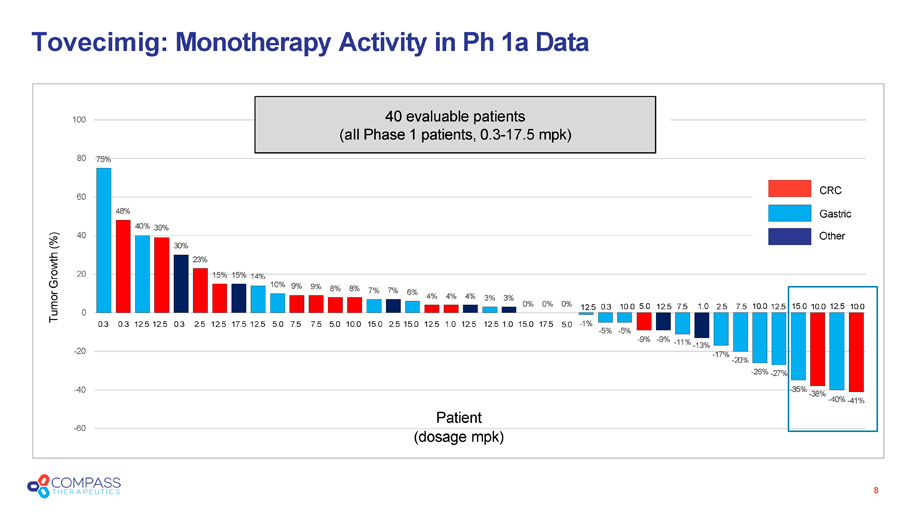
15% 15% 14% 10% 9% 9% 8% 8% 7% 7% 6% 4% 4% 4% 3% 3% 0% 0% 0% - 1% - 5% - 5% - 9% - 9% - 11% - 13% - 17% - 20% - 26% - 27% - 35% - 38% - 40% - 41% - 60 - 40 - 20 0 20 60 48% 40% 39% 40 30% 23% 80 75% 100 CRC Gastric Other 40 evaluable patients ( a ll Phase 1 p atients, 0.3 - 17.5 mpk ) Patient ( d osage mpk ) Tovecimig : Monotherapy Activity in Ph 1a Data 8 0.3 0.3 12.5 12.5 0.3 2.5 12.5 17.5 12.5 5.0 7.5 7.5 5.0 10.0 15.0 2.5 15.0 12.5 1.0 12.5 12.5 1.0 15.0 17.5 5.0 12.5 0.3 10.0 5.0 12.5 7.5 1.0 2.5 7.5 10.0 12.5 15.0 10.0 12.5 10.0 Tumor Growth (%)
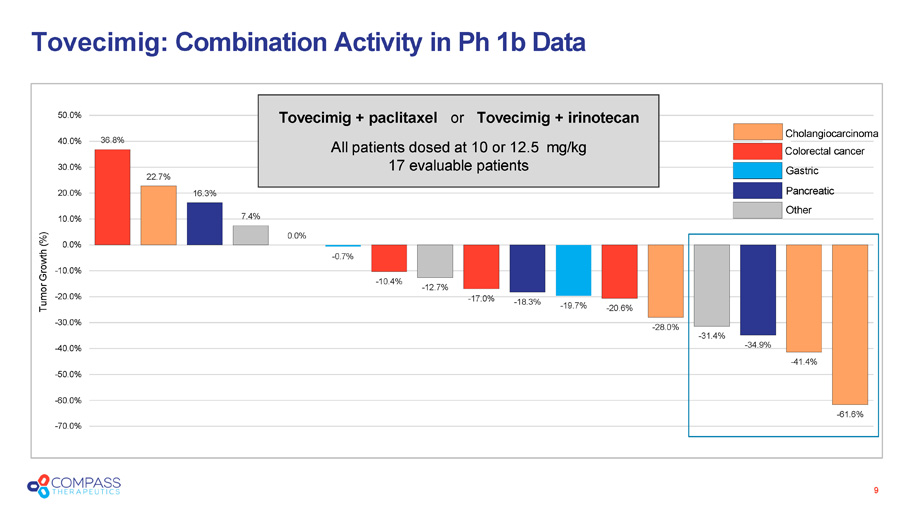
Tovecimig : Combination Activity in Ph 1b Data 9 36.8% 22.7% 16.3% 7.4% 0.0% - 0.7% - 10.4% - 12.7% - 17.0% - 18.3% - 19.7% - 20.6% - 28.0% - 31.4% - 34.9% - 41.4% - 61.6% - 70.0% - 50.0% - 60.0% - 40.0% - 30.0% - 20.0% - 10.0% 0.0% 10.0% 20.0% 30.0% 40.0% 50.0% Tumor Growth (%) Tovecimig + paclitaxel or Tovecimig + irinotecan All patients dosed at 10 or 12.5 mg/kg 17 evaluable patients Cholangiocarcinoma Colorectal cancer Gastric Pancreatic Other
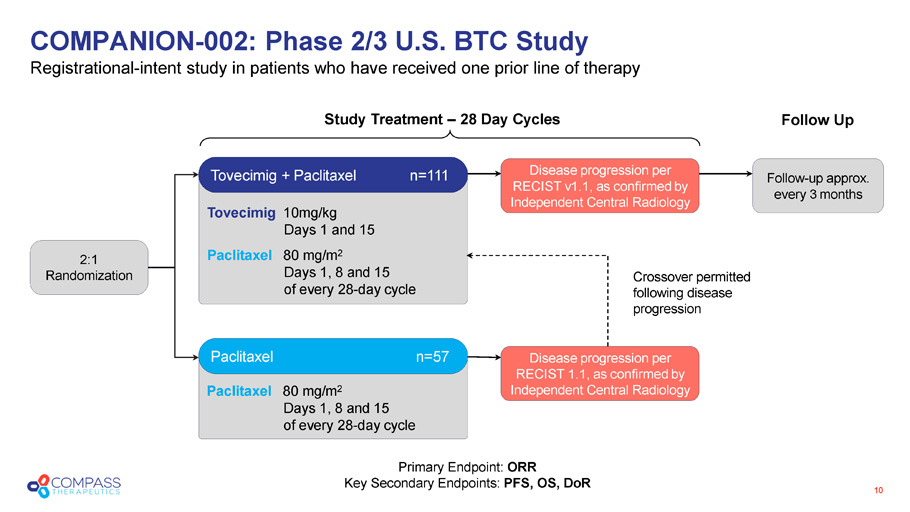
Paclitaxel 80 mg/m 2 Days 1, 8 and 15 of every 28 - day cycle Tovecimig 10mg/kg Days 1 and 15 Paclitaxel 80 mg/m 2 Days 1, 8 and 15 of every 28 - day cycle COMPANION - 002: Phase 2/3 U.S. BTC Study Registrational - intent s tudy in patients who have received one prior line of therapy 10 Crossover permitted following disease progression Study Treatment – 28 Day Cycles Follow Up Tovecimig + Paclitaxel n=111 Paclitaxel n= 57 Disease p rogression per RECIST v 1.1, as confirmed by Independent Central Radiology Disease p rogression per RECIST 1.1, as confirmed by Independent Central Radiology Follow - up approx. every 3 months 2:1 Randomization Primary Endpoint: ORR Key Secondary Endpoints: PFS, OS, DoR
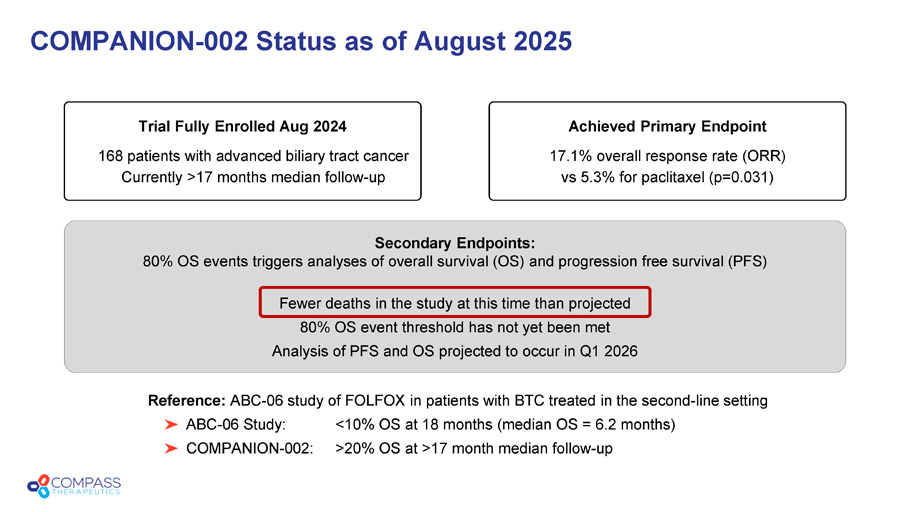
Secondary Endpoints: 80% OS events triggers analyses of overall survival (OS) and progression free survival (PFS) Fewer deaths in the study at this time than projected 80% OS event threshold has not yet been met Analysis of PFS and OS projected to occur in Q1 2026 COMPANION - 002 Status as of August 2025 Trial Fully Enrolled Aug 2024 168 patients with advanced biliary tract cancer Currently >17 months median follow - up Achieved Primary Endpoint 17.1% overall response rate (ORR) vs 5.3% for paclitaxel (p=0.031) Reference: ABC - 06 study of FOLFOX in patients with BTC treated in the second - line setting ABC - 06 Study: <10% OS at 18 months (median OS = 6.2 months) COMPANION - 002: >20% OS at >17 month median follow - up
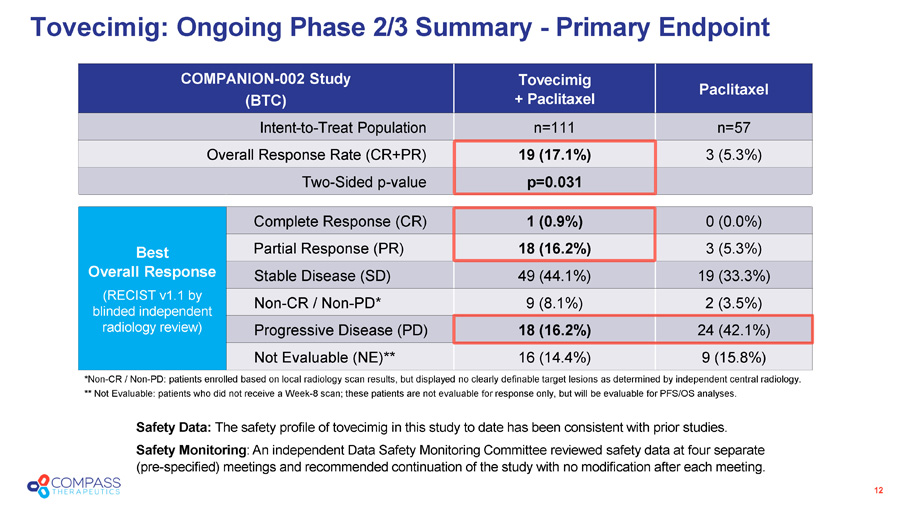
12 Paclitaxel Tovecimig + Paclitaxel COMPANION - 002 Study (BTC) n=57 n=111 Intent - to - Treat Population 3 (5.3%) 19 (17.1%) Overall Response Rate (CR+PR) p=0.031 Two - Sided p - value 0 (0.0%) 1 (0.9%) Complete Response (CR) Best Overall Response (RECIST v1.1 by blinded independent radiology review) 3 (5.3%) 18 (16.2%) Partial Response (PR) 19 (33.3%) 49 (44.1%) Stable Disease (SD) 2 (3.5%) 9 (8.1%) Non - CR / Non - PD* 24 (42.1%) 18 (16.2%) Progressive Disease (PD) 9 (15.8%) 16 (14.4%) Not Evaluable (NE)** Tovecimig: Ongoing Phase 2/3 Summary - Primary Endpoint Safety Data: The safety profile of tovecimig in this study to date has been consistent with prior studies. Safety Monitoring : An independent Data Safety Monitoring Committee reviewed safety data at four separate (pre - specified) meetings and recommended continuation of the study with no modification after each meeting. *Non - CR / Non - PD: patients enrolled based on local radiology scan results, but displayed no clearly definable target lesions as determined by independent central radiology. ** Not Evaluable: patients who did not receive a Week - 8 scan; these patients are not evaluable for response only, but will be evaluable for PFS/OS analyses.
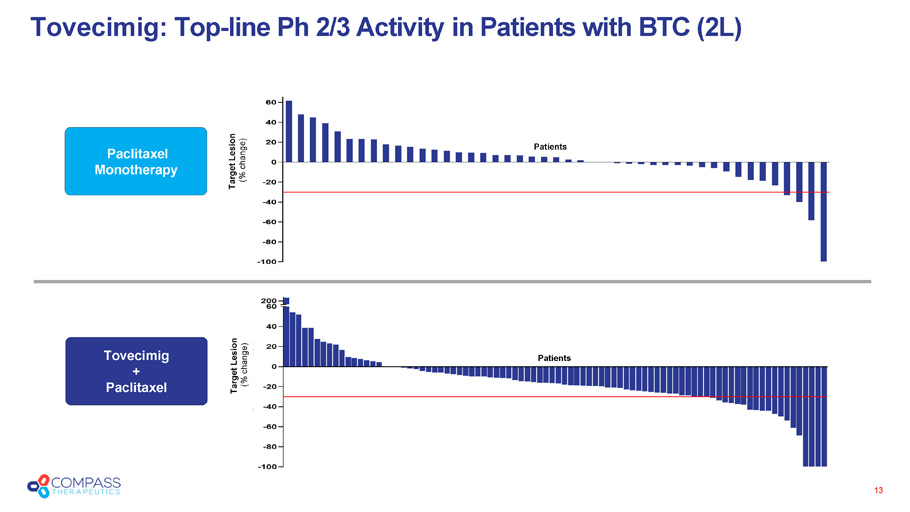
13 Tovecimig : Top - line Ph 2/3 Activity in Patients with BTC (2L) Target Lesion (% change) Paclitaxel Monotherapy Target Lesion (% change) Tovecimig + Paclitaxel Patients Patients
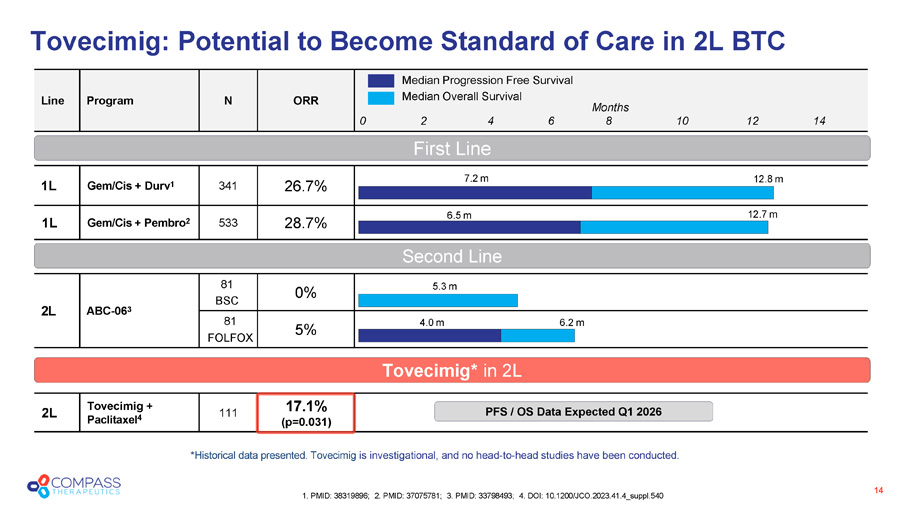
Tovecimig : Potential to Become Standard of Care in 2L BTC 14 1. PMID: 38319896; 2. PMID: 37075781; 3. PMID: 33798493; 4. DOI: 10.1200/JCO.2023.41.4_suppl.540 ORR N Program Line 26.7% 341 Gem/Cis + Durv 1 1L 28.7% 533 Gem/Cis + Pembro 2 1L 0% 81 BSC ABC - 06 3 2L 5% 81 FOLFOX 17.1% (p=0.031) 111 Tovecimig + Paclitaxel 4 2L Median Progression Free Survival Median Overall Survival 5.3 m 4.0 m 6.2 m 7.2 m 12.8 m 6.5 m 12.7 m First Line Second Line Months 0 2 4 6 8 10 12 14 * Historical d ata presented. Tovecimig is investigational, and no head - to - head studies have been conducted . Tovecimig* in 2L PFS / OS Data Expected Q1 2026
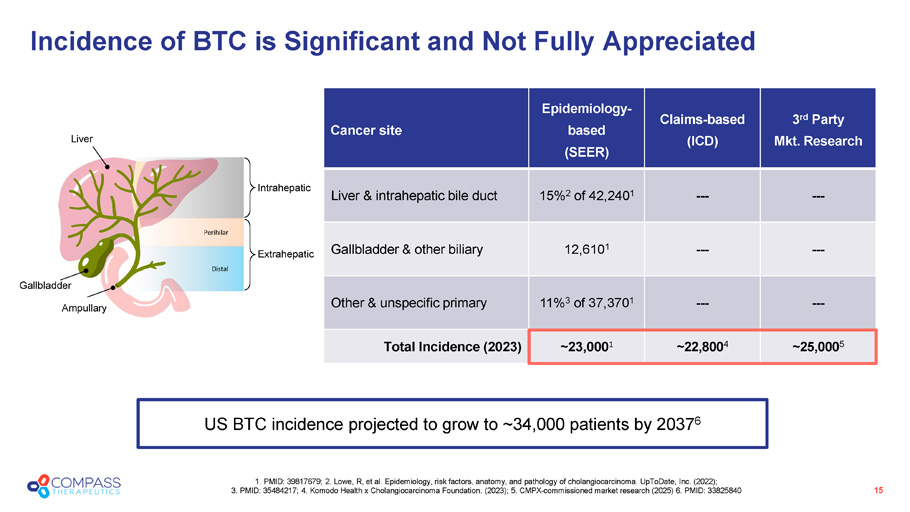
3 rd Party Mkt. Research Claims - based (ICD) Epidemiology - based (SEER) Cancer site --- --- 15% 2 of 42,240 1 Liver & intrahepatic bile duct --- --- 12,610 1 Gallbladder & other biliary --- --- 11% 3 of 37,370 1 Other & unspecific primary ~25,000 5 ~22,800 4 ~23,000 1 Total Incidence (2023) 15 1. PMID: 39817679; 2. Lowe, R, et al. Epidemiology, risk factors, anatomy, and pathology of cholangiocarcinoma. UpToDate, Inc . ( 2022); 3. PMID: 35484217; 4. Komodo Health x Cholangiocarcinoma Foundation. (2023); 5. CMPX - commissioned market research (2025) 6 . PMID: 33825840 Incidence of BTC is Significant and Not Fully Appreciated US BTC incidence projected to grow to ~34,000 patients by 2037 6 Gallbladder Ampullary Distal Intrahepatic Extrahepatic Perihilar Liver
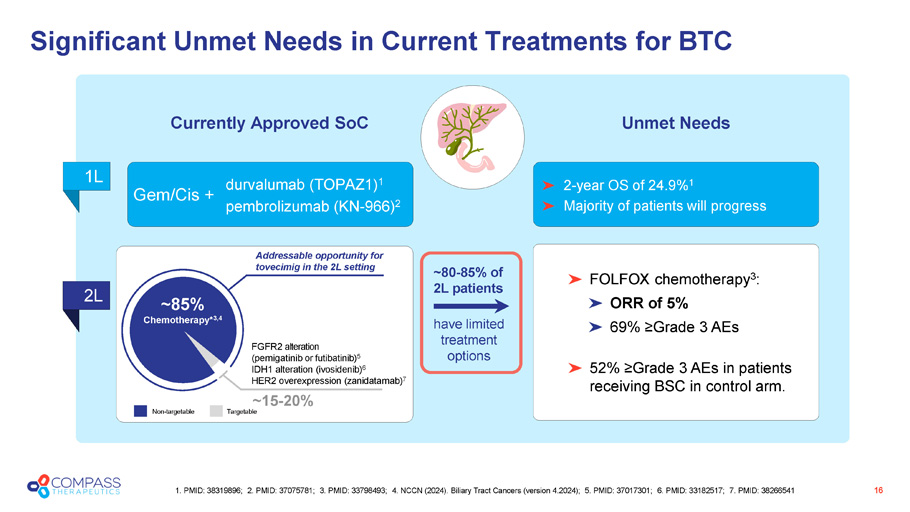
Significant Unmet Needs in Current Treatments for BTC 16 1. PMID: 38319896; 2. PMID: 37075781; 3. PMID: 33798493; 4 . NCCN (2024). Biliary Tract Cancers (version 4.2024); 5. PMID: 37017301; 6. PMID: 33182517; 7. PMID: 38266541 durvalumab (TOPAZ1) 1 pembrolizumab (KN - 966) 2 Gem/Cis + Currently Approved SoC Unmet Needs 1L 2L 2 - year OS of 24.9% 1 Majority of patients will progress FOLFOX chemotherapy 3 : ORR of 5% 69 % ≥Grade 3 AEs 52% ≥Grade 3 AEs in patients receiving BSC in control arm. Non - targetable Targetable FGFR2 alteration ( p emigatinib or futibatinib ) 5 IDH1 alteration ( ivosidenib ) 6 HER2 overexpression ( zanidatamab ) 7 Addressable opportunity for tovecimig in the 2L setting ~85% Chemotherapy* 3 ,4 ~15 - 20% have limited treatment options ~80 - 85% of 2L patients
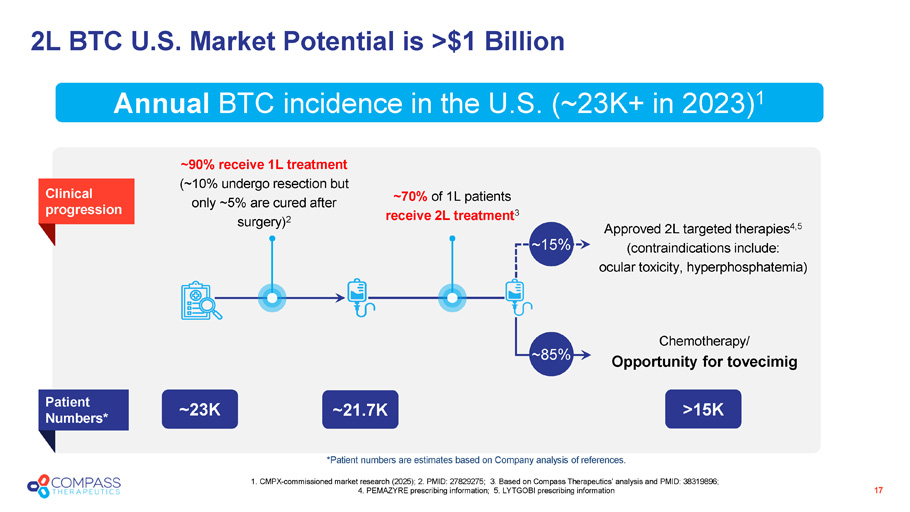
2L BTC U.S. Market Potential is >$1 Billion 17 1. CMPX - commissioned market research (2025); 2. PMID: 27829275; 3. Based on Compass Therapeutics’ analysis and PMID: 38319896; 4. PEMAZYRE prescribing information; 5. LYTGOBI prescribing information Annual BTC incidence in the U.S. (~23K+ in 2023) 1 ~90% receive 1L treatment (~10% undergo resection but only ~5% are cured after surgery) 2 ~70% of 1L patients receive 2L treatment 3 Approved 2L targeted therapies 4,5 (contraindications include: ocular toxicity, hyperphosphatemia) ~21.7K > 15K ~23K Patient Numbers* Chemotherapy/ Opportunity for t ovecimig Clinical progression ~ 15% ~ 85% * Patient numbers are estimates based on Company analysis of references .
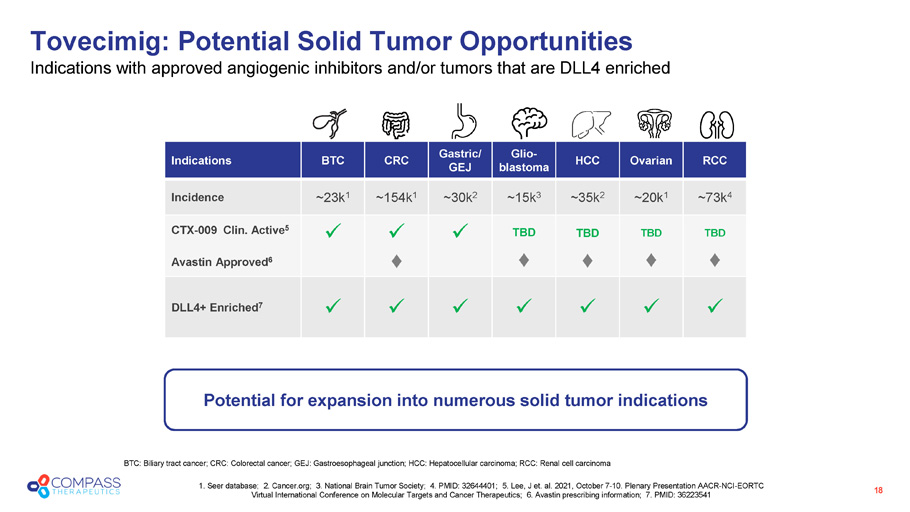
RCC Ovarian HCC Glio - blastoma Gastric/ GEJ CRC BTC Indications ~73k 4 ~20k 1 ~35k 2 ~15k 3 ~30k 2 ~154k 1 ~23k 1 Incidence TBD TBD TBD TBD x x x CTX - 009 Clin. Active 5 Avastin Approved 6 x x x x x x x DLL4+ Enriched 7 Tovecimig: Potential Solid Tumor Opportunities Indications with approved angiogenic inhibitors and/or tumors that are DLL4 enriched BTC: Biliary tract cancer; CRC: Colorectal cancer; GEJ: Gastroesophageal junction; HCC: Hepatocellular carcinoma; RCC: Renal cel l carcinoma 1. Seer database; 2. Cancer.org; 3. National Brain Tumor Society; 4. PMID: 32644401; 5. Lee, J et. al. 2021, October 7 - 10. P lenary Presentation AACR - NCI - EORTC Virtual International Conference on Molecular Targets and Cancer Therapeutics; 6. Avastin prescribing information; 7. PMID: 36 223541 Potential for expansion into numerous solid tumor indications 18
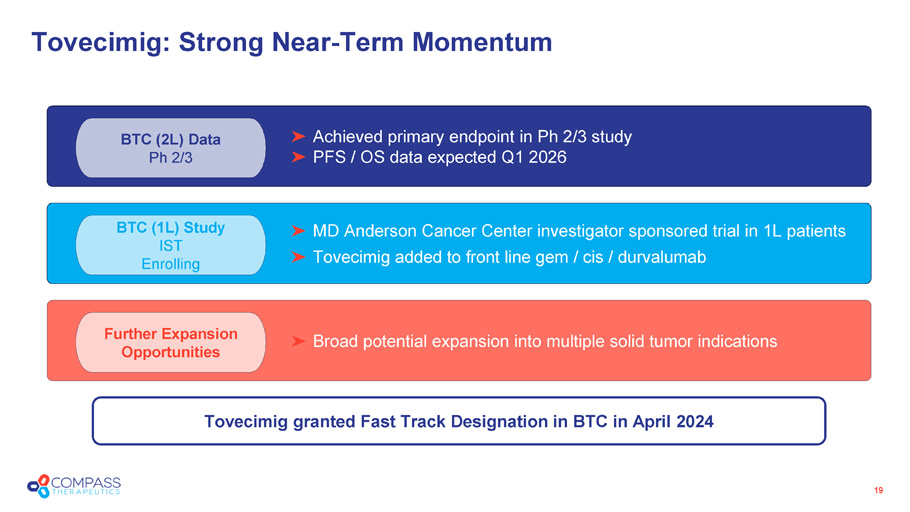
Further Expansion Opportunities Broad potential expansion into multiple solid tumor indications BTC (1L) Study IST Enrolling MD Anderson Cancer Center investigator sponsored trial in 1L patients Tovecimig added to front line gem / cis / durvalumab BTC (2L) Data Ph 2/3 Tovecimig: Strong Near - Term Momentum 19 Achieved primary endpoint in Ph 2/3 study PFS / OS data expected Q1 2026 Tovecimig granted Fast Track Designation in BTC in April 2024

CTX - 471 CD137 agonist
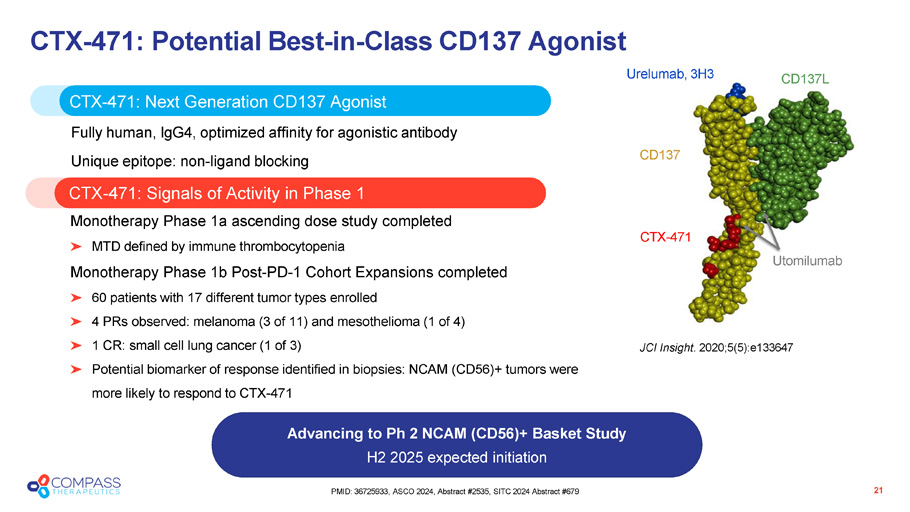
21 PMID: 36725933, ASCO 2024, Abstract #2535, SITC 2024 Abstract #679 CTX - 471: Potential Best - in - Class CD137 Agonist CTX - 471: Signals of Activity in Phase 1 Advancing to Ph 2 NCAM (CD56)+ Basket Study H2 2025 expected initiation CTX - 471: N ext G eneration CD137 A gonist Fully human, IgG4, optimized affinity for agonistic antibody Unique epitope: non - ligand blocking Monotherapy Phase 1a ascending dose study completed MTD defined by immune thrombocytopenia Monotherapy Phase 1b Post - PD - 1 Cohort Expansions completed 60 patients with 17 different tumor types enrolled 4 PRs observed: melanoma (3 of 11) and mesothelioma (1 of 4) 1 CR: small cell lung cancer (1 of 3) Potential biomarker of response identified in biopsies: NCAM (CD56)+ tumors were more likely to respond to CTX - 471 JCI Insight . 2020;5(5):e133647 CD137L CD137 Urelumab, 3H3 CTX - 471 Utomilumab JCI Insight . 2020;5(5):e133647
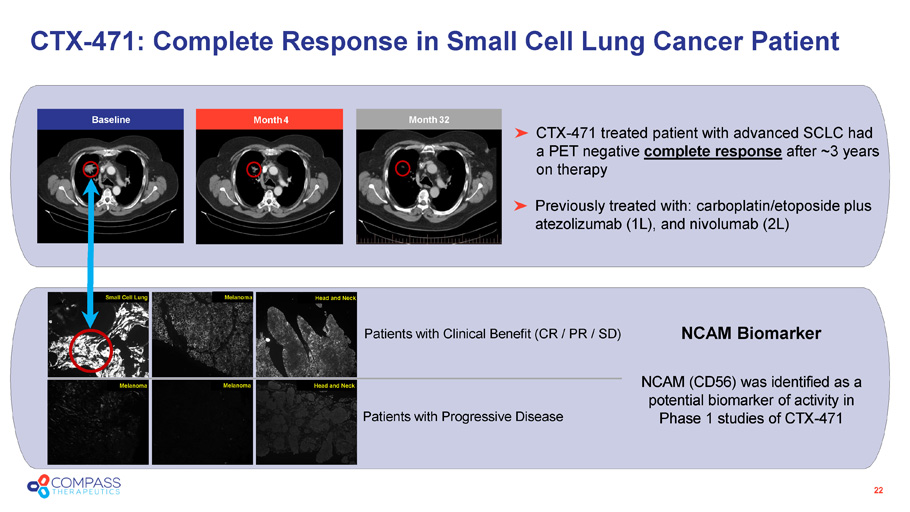
22 CTX - 471 treated patient with advanced SCLC had a PET negative complete response after ~3 years on therapy Previously treated with: carboplatin/etoposide plus atezolizumab (1L), and nivolumab (2L) CTX - 471: Complete Response in Small Cell Lung Cancer Patient NCAM (CD56) was identified as a potential biomarker of activity in Phase 1 studies of CTX - 471 Patients with Clinical Benefit (CR / PR / SD) NCAM Biomarker Patients with Progressive Disease Small Cell Lung Small Cell Lung Melanoma Melanoma Melanoma Head and Neck Head and Neck Month 4 Month 32 Baseline
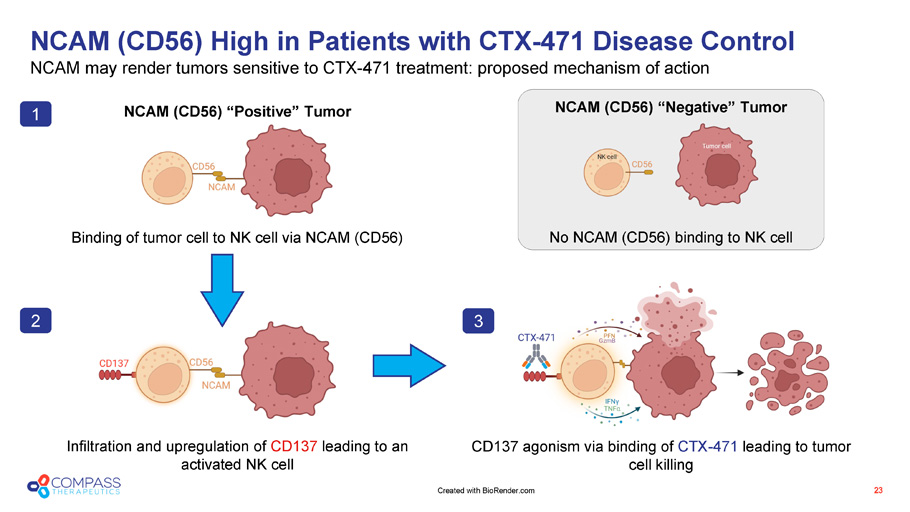
NCAM (CD56) High in Patients with CTX - 471 Disease Control NCAM may render tumors sensitive to CTX - 471 treatment: proposed mechanism of action 23 NCAM (CD56) “Positive” Tumor Binding of tumor cell to NK cell via NCAM (CD56) NCAM (CD56) “Negative” Tumor No NCAM (CD56) binding to NK cell Infiltration and upregulation of CD137 leading to an activated NK cell CD137 agonism via binding of CTX - 471 leading to tumor cell killing 1 2 3 Created with BioRender.com
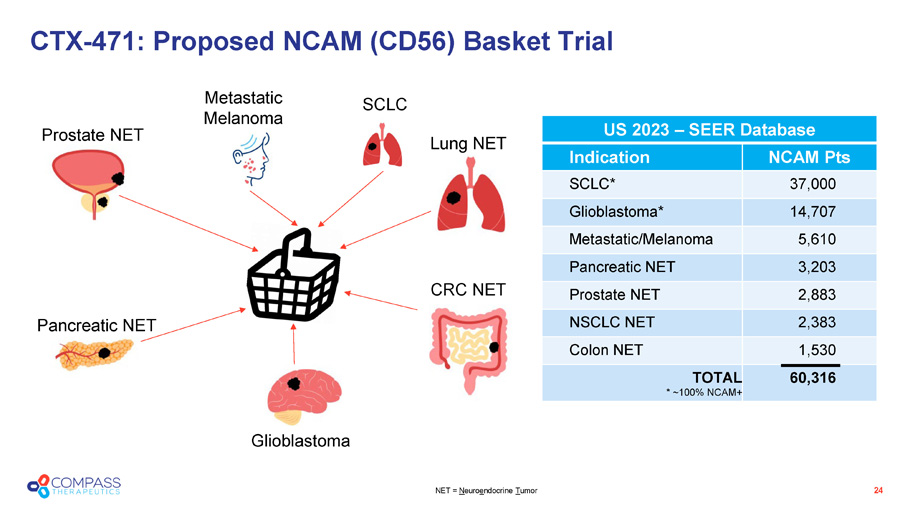
CTX - 471: Proposed NCAM (CD56) Basket Trial NET = N euro e ndocrine T umor SCLC Lung NET CRC NET Metastatic Melanoma Glioblastoma Pancreatic NET Prostate NET US 2023 – SEER Database US 2023 – SEER Database NCAM Pts Indication 37,000 SCLC* 14,707 Glioblastoma* 5,610 Metastatic/Melanoma 3,203 Pancreatic NET 2,883 Prostate NET 2,383 NSCLC NET 1,530 Colon NET 60,316 TOTAL * ~100% NCAM+ 24

CTX - 8371 PD - 1 x PD - L1 bispecific antibody
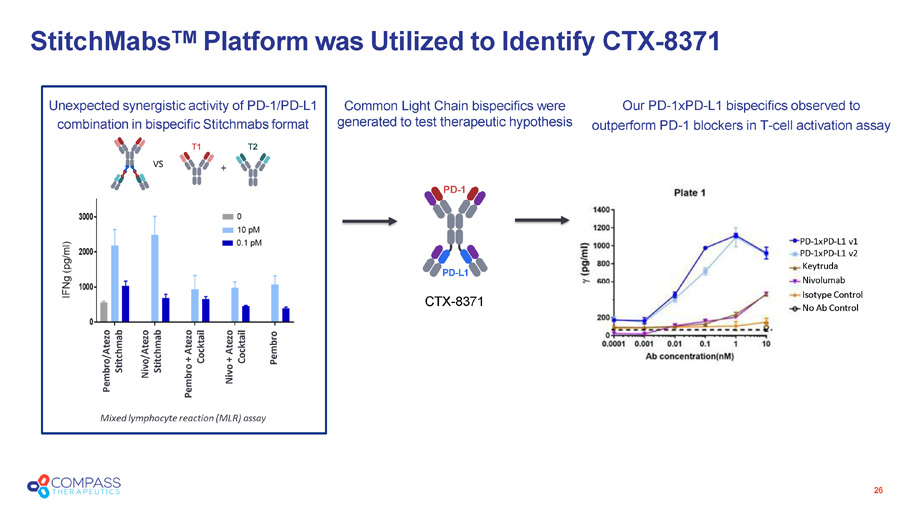
26 Mixed lymphocyte reaction (MLR) assay Pembro/Atezo Stitchmab Nivo/Atezo Stitchmab Pembro + Atezo Cocktail Nivo + Atezo Cocktail Pembro vs PD - 1 PD - L1 + Unexpected synergistic activity of PD - 1/PD - L1 combination in bispecific Stitchmab s format T1 T2 PD - 1xPD - L1 v1 PD - 1xPD - L1 v2 Keytruda Nivolumab Isotype Control No Ab Control CTX - 8371 StitchMabs TM Platform was Utilized to Identify CTX - 8371 Common Light Chain bispecifics were generated to test therapeutic hypothesis Our PD - 1xPD - L1 bispecifics observed to outperform PD - 1 blockers in T - cell activation assay
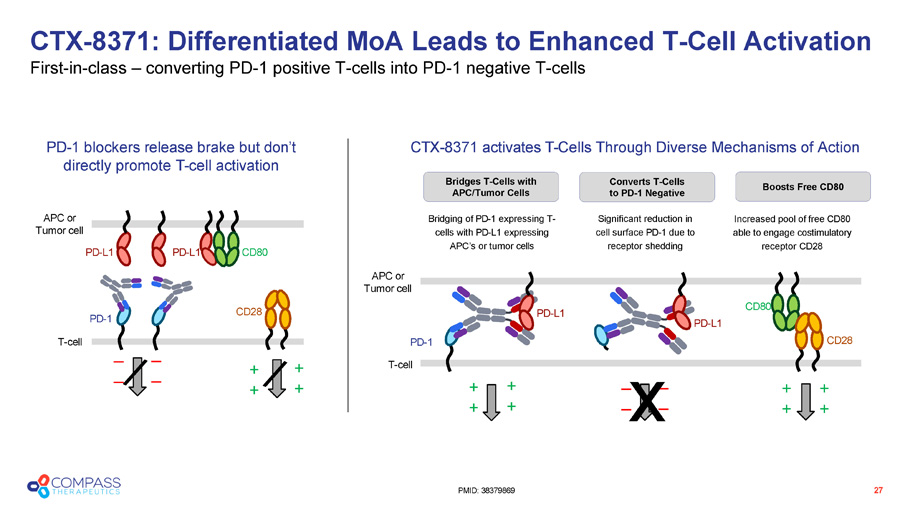
Bridges T - Cells with APC/Tumor Cells PD - L1 PD - L1 CD28 PD - 1 T - cell CD80 APC or Tumor cell PD - 1 blockers release brake but don’t directly promote T - cell activation _ _ _ _ PD - L1 CD28 PD - 1 CD80 PD - L1 Bridging of PD - 1 expressing T - cells with PD - L1 expressing APC’s or tumor cells Significant reduction in cell surface PD - 1 due to receptor shedding APC or Tumor cell T - cell Increased pool of free CD80 able to engage costimulatory receptor CD28 + + _ _ CTX - 8371 activates T - Cells Through Diverse Mechanisms of Action + + + + + + _ _ x + + + + CTX - 8371: Differentiated MoA Leads to Enhanced T - Cell Activation First - in - class – converting PD - 1 positive T - cells into PD - 1 negative T - cells 27 PMID: 38379869 Converts T - Cells to PD - 1 Negative Boosts Free CD80
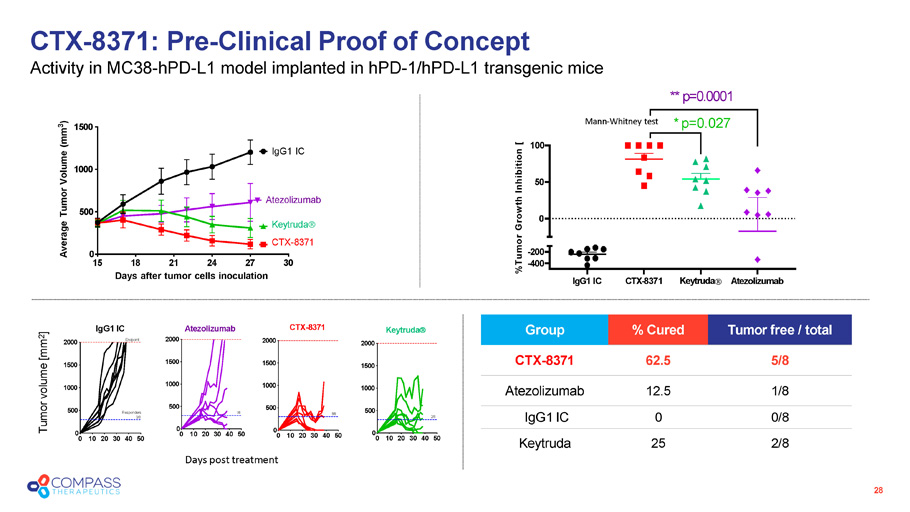
CTX - 8371: Pre - Clinical Proof of Concept Activity in MC38 - hPD - L1 model implanted in hPD - 1/hPD - L1 transgenic mice 28 15 30 0 500 1000 1500 18 21 24 27 Days after tumor cells inoculation Average Tumor Volume (mm 3 ) IgG1 IC Keytruda CTX - 8371 Atezolizumab Days post treatment IgG1 IC CTX - 8371 Keytruda Atezolizumab - 200 - 400 0 50 100 %Tumor Growth Inhibition [ ** p=0.0001 * p=0.027 Tumor free / total % Cured Group 5/8 62.5 CTX - 8371 1/8 12.5 Atezolizumab 0/8 0 IgG1 IC 2/8 25 Keytruda Mann - Whitney test 0 10 20 30 40 50 0 500 1000 1500 2000 IgG1 IC Endpoint Responders 0/8 0 10 20 30 40 50 0 500 1000 1500 2000 CTX - 8371 5/8 0 10 20 30 40 50 0 500 1000 1500 2000 Keytruda 2/8 0 10 20 30 40 50 0 500 1000 1500 Atezolizumab 2000 1/8 Tumor volume [mm 2 ]
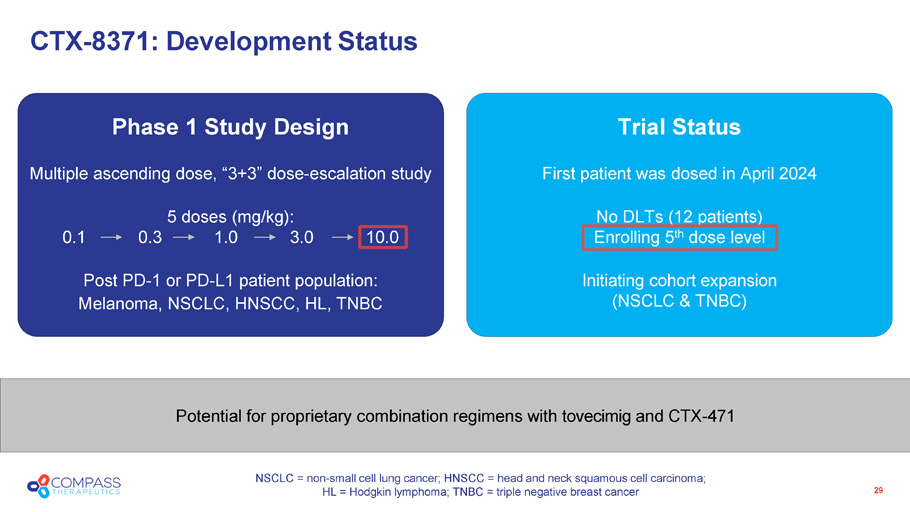
Trial Status First patient was dosed in April 2024 No DLTs (12 patients) Enrolling 5 th dose level Initiating cohort expansion (NSCLC & TNBC) Phase 1 Study Design Multiple ascending dose, “3+3” dose - escalation study 5 doses (mg/kg): 0.1 0.3 1.0 3.0 10.0 Post PD - 1 or PD - L1 patient population: Melanoma, NSCLC, HNSCC, HL, TNBC Potential for proprietary combination regimens with tovecimig and CTX - 471 29 CTX - 8371: Development Status NSCLC = non - small cell lung cancer; HNSCC = head and neck squamous cell carcinoma; HL = Hodgkin lymphoma; TNBC = triple negative breast cancer
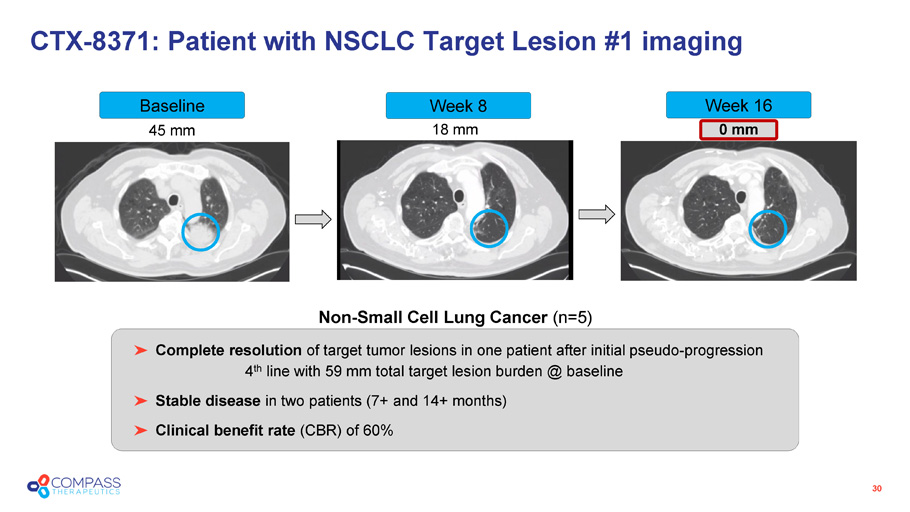
45 mm 18 mm 0 mm CTX - 8371: Patient with NSCLC Target Lesion #1 imaging 30 Non - Small Cell Lung Cancer (n=5) Baseline Week 8 Week 16 Complete resolution of target tumor lesions in one patient after initial pseudo - progression 4 th line with 59 mm total target lesion burden @ baseline Stable disease in two patients (7+ and 14+ months) Clinical benefit rate (CBR) of 60%
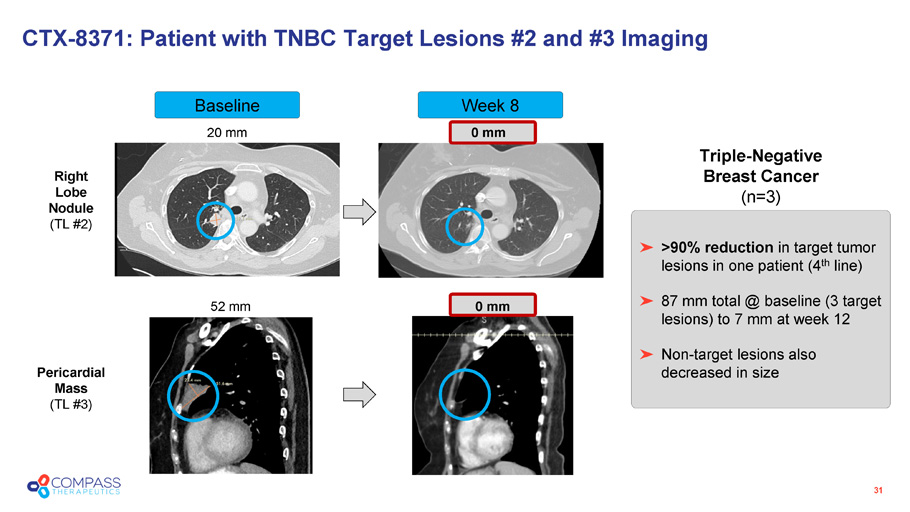
Week 8 Baseline CTX - 8371: Patient with TNBC Target Lesions #2 and #3 Imaging 31 20 mm 0 mm 52 mm 0 mm >90% reduction in target tumor lesions in one patient (4 th line) 87 mm total @ baseline (3 target lesions) to 7 mm at week 12 Non - target lesions also decreased in size Triple - Negative Breast Cancer (n=3) Right Lobe Nodule (TL #2) Pericardial Mass (TL #3)

CTX - 10726 PD - 1 x VEGF - A bispecific antibody
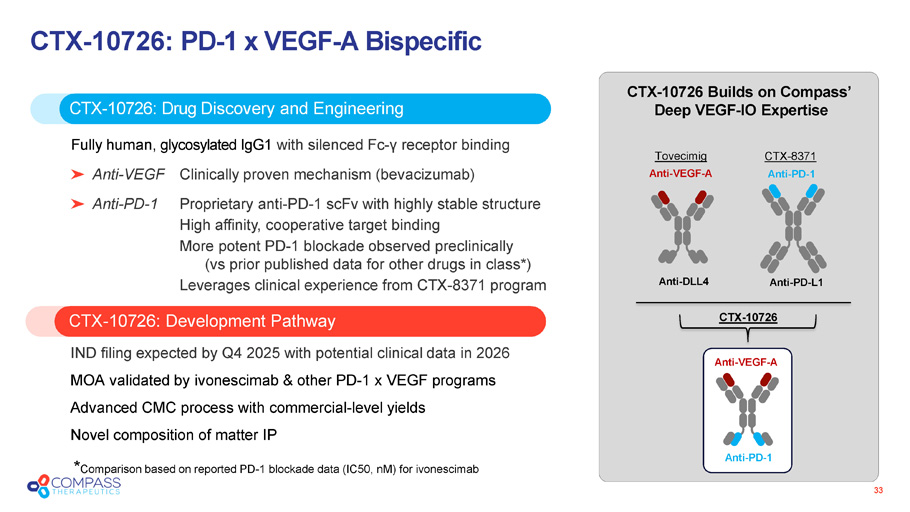
33 CTX - 10726 : PD - 1 x VEGF - A Bispecific CTX - 10726: Development Pathway CTX - 10726 : Drug Discovery and Engineering Fully human, glycosylated IgG1 with silenced Fc - γ receptor binding Anti - VEGF Clinically proven mechanism (bevacizumab) Anti - PD - 1 Proprietary anti - PD - 1 scFv with highly stable structure High affinity, cooperative target binding More potent PD - 1 blockade observed preclinically (vs prior published data for other drugs in class*) Leverages clinical experience from CTX - 8371 program IND filing expected by Q4 2025 with potential clinical data in 2026 MOA validated by ivonescimab & other PD - 1 x VEGF programs Advanced CMC process with commercial - level yields Novel composition of matter IP CTX - 10726 Builds on Compass’ Deep VEGF - IO Expertise Anti - VEGF - A Anti - DLL4 Tovecimig CTX - 8371 Anti - PD - 1 Anti - PD - L1 Anti - PD - 1 Anti - VEGF - A CTX - 10726 * Comparison based on reported PD - 1 blockade data (IC50, nM ) for ivonescimab
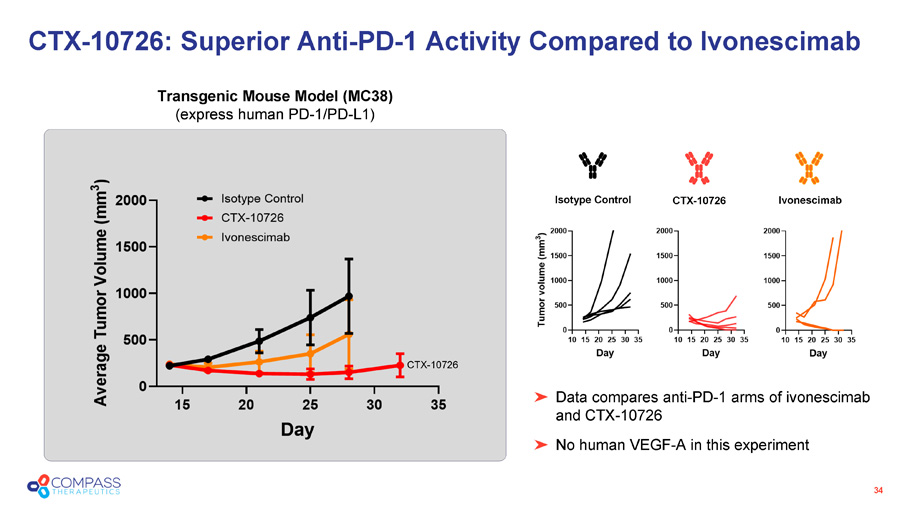
34 Data compares anti - PD - 1 arms of ivonescimab and CTX - 10726 No human VEGF - A in this experiment CTX - 10726: Superior Anti - PD - 1 Activity Compared to Ivonescimab Transgenic Mouse Model (MC38) (express human PD - 1/PD - L1) CTX - 10726 Isotype Control Ivonescimab CTX - 10726
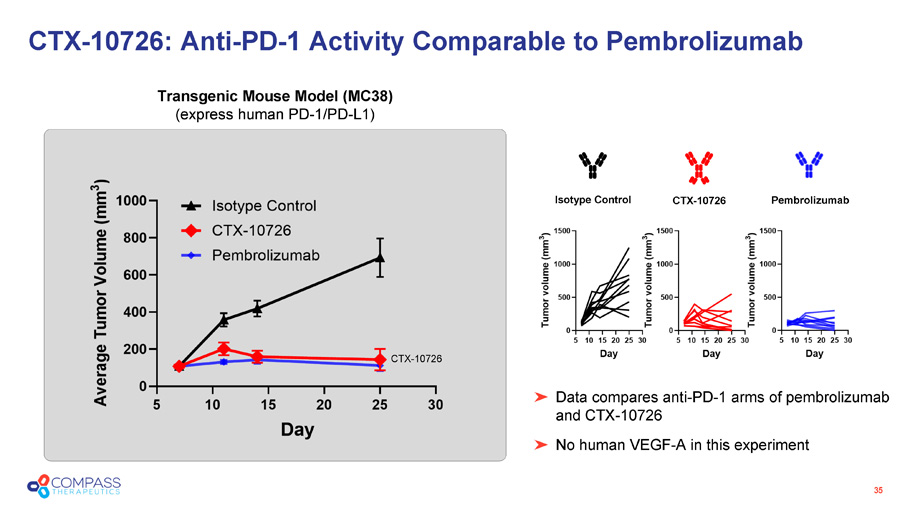
35 CTX - 10726: Anti - PD - 1 Activity Comparable to Pembrolizumab Transgenic Mouse Model (MC38) (express human PD - 1/PD - L1) CTX - 10726 Data compares anti - PD - 1 arms of pembrolizumab and CTX - 10726 No human VEGF - A in this experiment Isotype Control Pembrolizumab CTX - 10726
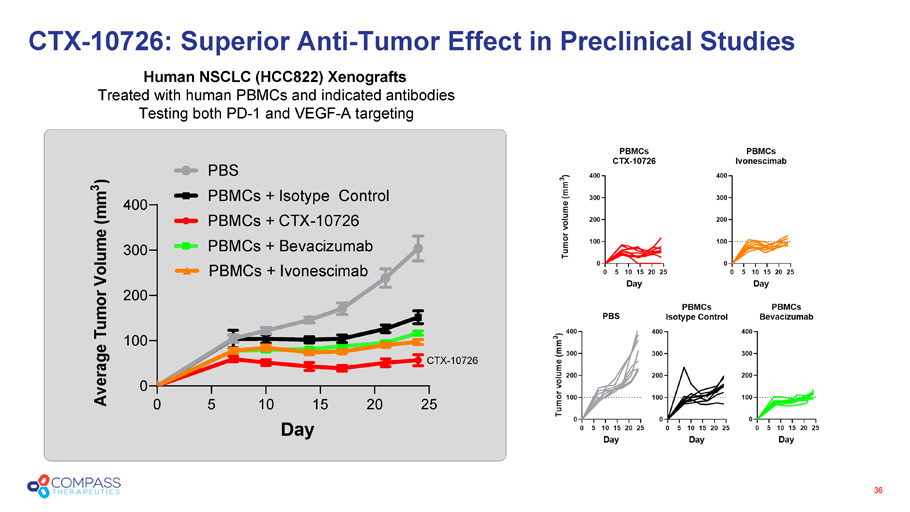
36 CTX - 10726 0 5 10 15 20 25 0 100 200 300 400 Day A v e r a g e T u m o r V o l u m e ( m m 3 ) PBS PBMCs + Isotype Control PBMCs + CTX-10726 PBMCs + Bevacizumab PBMCs + Ivonescimab Human NSCLC (HCC822) Xenografts Treated with human PBMCs and indicated antibodies Testing both PD - 1 and VEGF - A targeting CTX - 10726: Superior Anti - Tumor Effect in Preclinical Studies
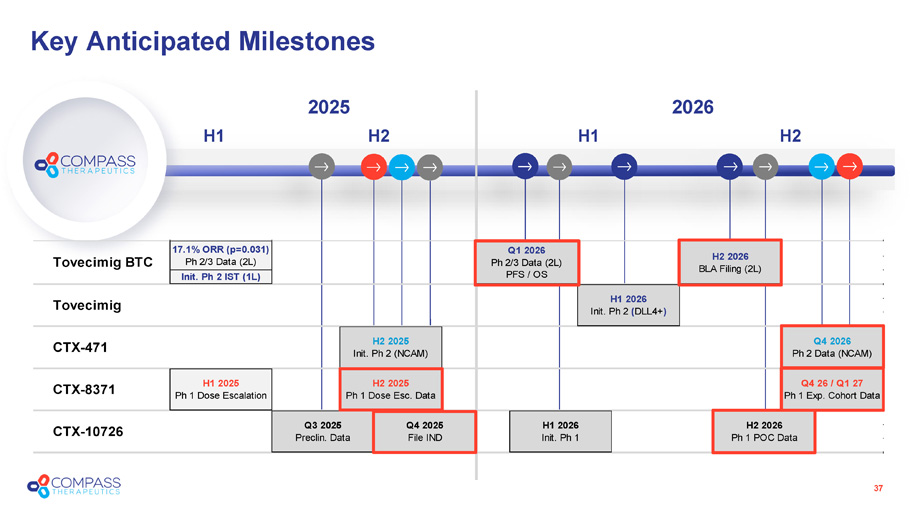
Key Anticipated Milestones 2025 H2 H1 37 2026 H2 H1 Tovecimig BTC 17.1% ORR (p=0.031) Ph 2/3 Data (2L) Q1 2026 Ph 2/3 Data (2L) PFS / OS Init. Ph 2 IST (1L) Q4 2026 Ph 2 Data (NCAM) CTX-8371 H1 2025 Ph 1 Dose Escalation H2 2025 Ph 1 Dose Esc. Data Q4 26 / Q1 27 Ph 1 Exp. Cohort Data H2 2026 BLA Filing (2L) Tovecimig H1 2026 Init. Ph 2 (DLL4+) CTX-471 H2 2025 Init. Ph 2 (NCAM) CTX-10726 Q3 2025 Preclin. Data Q4 2025 File IND H1 2026 Init. Ph 1 H2 2026 Ph 1 POC Data

Website: compasstherapeutics.com Nasdaq: CMPX Compass Therapeutics