
Exhibit 99.2


This presentation has been prepared by Compass Therapeutics, Inc . ("we," "us," "our," or the “Company”) . Statements contained herein are made as of the date of this presentation unless stated otherwise, and this presentation shall not under any circumstances create an implication that the information contained herein is correct as of any time after such date or that information will be updated or revised to reflect information that subsequently becomes available or changes occurring after the date hereof . This presentation contains forward - looking statements. Statements in this presentation that are not purely historical are forwar d - looking statements. Such forward - looking statements include, among other things, references to Compass's financial position to continue advancing its product candidates, expecta tio ns about cash runway, business and development plans, and statements regarding Compass's product candidates, including their preclinical and clinical development, therapeutic pote nti al and tolerability profile, and clinical trial milestones such as the expected trial design, timing of enrollment, patient dosing and data readouts, regulatory plans with respect to Compas s's product candidates and the therapeutic potential thereof. Actual results could differ from those projected in any forward - looking statements due to numerous factors. Such factors include , among others, Compass's ability to raise the additional funding it will need to continue to pursue its business and product development plans, the inherent uncertainties associated wit h developing product candidates and operating as a development stage company, Compass's ability to identify additional product candidates for development, Compass's ability to dev elop, initiate and complete clinical trials for, obtain approvals for and commercialize any of its product candidates, competition in the industry in which Compass operates and mark et conditions. These forward - looking statements are made as of the date of this presentation, and Compass assumes no obligation to update the forward - looking statements, or to upda te the reasons why actual results could differ from those projected in the forward - looking statements, except as required by law. Investors should consult all of the information se t forth herein and should also refer to the risk factor disclosure set forth in the reports and other documents Compass files with the U.S. Securities and Exchange Commission (SEC) ava ilable at www.sec.gov, including without limitation Compass's latest Annual Report on Form 10 - K, Quarterly Report on Form 10 - Q and subsequent filings with the SEC. This presentation also contains estimates and other statistical data made by independent parties and by us relating to market size and growth and other data about our industry . This data involves a number of assumptions and limitations, and you are cautioned not to give undue weight to such estimates . In addition, projections, assumptions, and estimates of our future performance and the future performance of the markets in which we operate are necessarily subject to a high degree of uncertainty and risk . This presentation concerns drugs that are under clinical investigation, and which have not yet been approved for marketing by the U . S . Food and Drug Administration (FDA) . It is currently limited by Federal law to investigational use, and no representation is made as to its safety or effectiveness for the purposes for which it is being investigated . DISCLAIMER 2

Thomas Schuetz, MD, PhD CEO and Co - founder
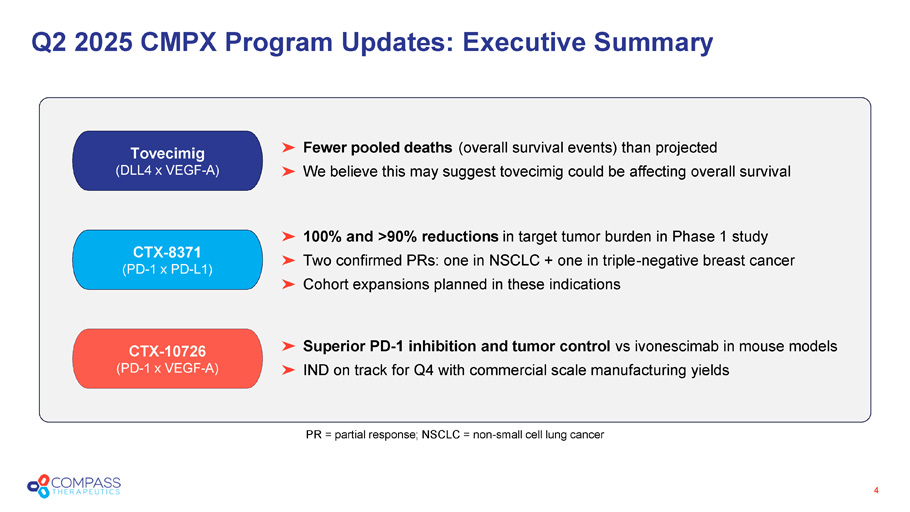
Q2 2025 CMPX Program Updates: Executive Summary 4 CTX - 10726 (PD - 1 x VEGF - A) Superior PD - 1 inhibition and tumor control vs ivonescimab in mouse models IND on track for Q4 with commercial scale manufacturing yields CTX - 8371 (PD - 1 x PD - L1) 100% and >90% reductions in target tumor burden in Phase 1 study Two confirmed PRs: one in NSCLC + one in triple - negative breast cancer Cohort expansions planned in these indications Tovecimig (DLL4 x VEGF - A) Fewer pooled deaths (overall survival events) than projected We believe this may suggest tovecimig could be affecting overall survival PR = partial response; NSCLC = non - small cell lung cancer
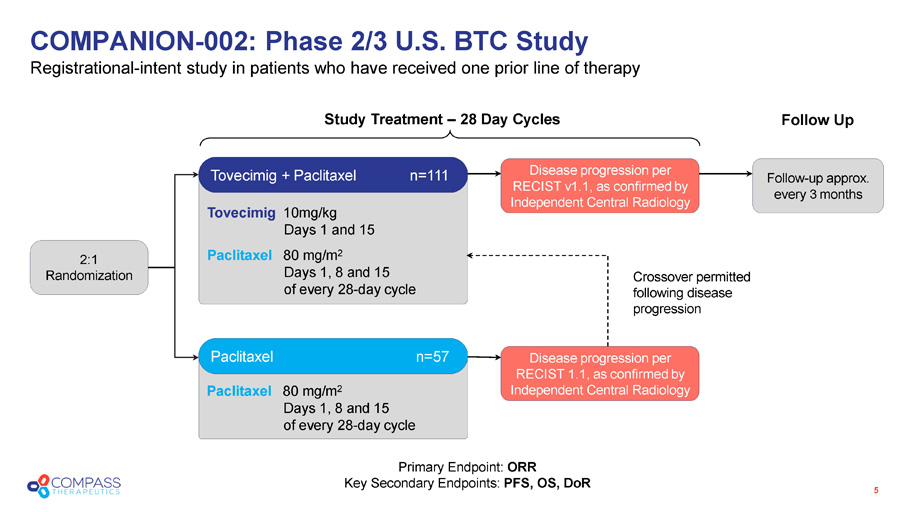
Paclitaxel 80 mg/m 2 Days 1, 8 and 15 of every 28 - day cycle Tovecimig 10mg/kg Days 1 and 15 Paclitaxel 80 mg/m 2 Days 1, 8 and 15 of every 28 - day cycle COMPANION - 002: Phase 2/3 U.S. BTC Study Registrational - intent s tudy in patients who have received one prior line of therapy 5 Crossover permitted following disease progression Study Treatment – 28 Day Cycles Follow Up Tovecimig + Paclitaxel n=111 Paclitaxel n= 57 Disease p rogression per RECIST v 1.1, as confirmed by Independent Central Radiology Disease p rogression per RECIST 1.1, as confirmed by Independent Central Radiology Follow - up approx. every 3 months 2:1 Randomization Primary Endpoint: ORR Key Secondary Endpoints: PFS, OS, DoR
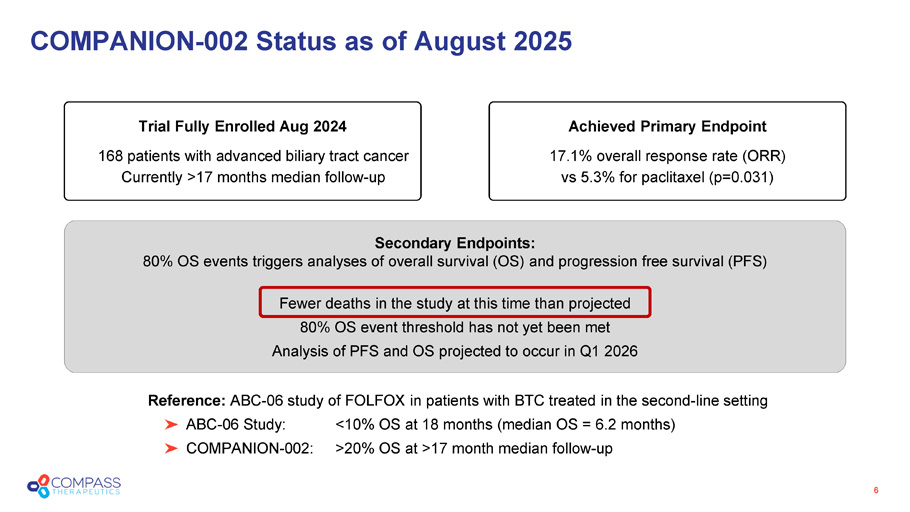
Secondary Endpoints: 80% OS events triggers analyses of overall survival (OS) and progression free survival (PFS) Fewer deaths in the study at this time than projected 80% OS event threshold has not yet been met Analysis of PFS and OS projected to occur in Q1 2026 COMPANION - 002 Status as of August 2025 Trial Fully Enrolled Aug 2024 168 patients with advanced biliary tract cancer Currently >17 months median follow - up Achieved Primary Endpoint 17.1% overall response rate (ORR) vs 5.3% for paclitaxel (p=0.031) Reference: ABC - 06 study of FOLFOX in patients with BTC treated in the second - line setting ABC - 06 Study: <10% OS at 18 months (median OS = 6.2 months) COMPANION - 002: >20% OS at >17 month median follow - up 6

CTX - 8371 PD - 1 x PD - L1 bispecific antibody
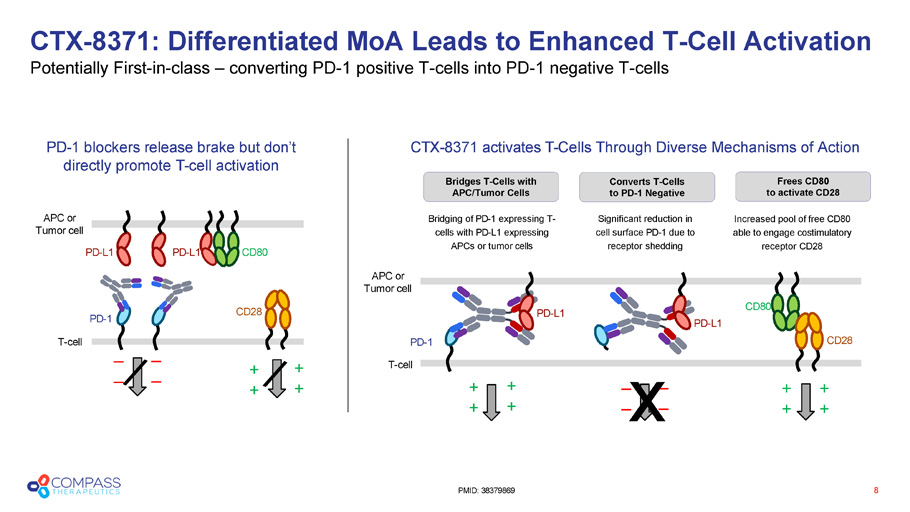
Bridges T - Cells with APC/Tumor Cells PD - L1 PD - L1 CD28 PD - 1 T - cell CD80 APC or Tumor cell PD - 1 blockers release brake but don’t directly promote T - cell activation _ _ _ _ PD - L1 CD28 PD - 1 CD80 PD - L1 Bridging of PD - 1 expressing T - cells with PD - L1 expressing APCs or tumor cells Significant reduction in cell surface PD - 1 due to receptor shedding APC or Tumor cell T - cell Increased pool of free CD80 able to engage costimulatory receptor CD28 + + _ _ CTX - 8371 activates T - Cells Through Diverse Mechanisms of Action + + + + + + _ _ x + + + + CTX - 8371: Differentiated MoA Leads to Enhanced T - Cell Activation Potentially First - in - class – converting PD - 1 positive T - cells into PD - 1 negative T - cells 8 PMID: 38379869 Converts T - Cells to PD - 1 Negative Frees CD80 to activate CD28
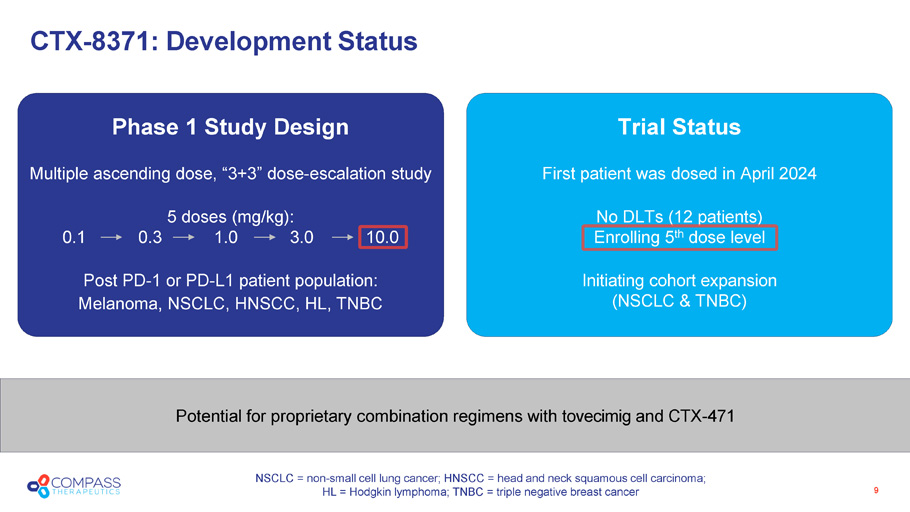
Trial Status First patient was dosed in April 2024 No DLTs (12 patients) Enrolling 5 th dose level Initiating cohort expansion (NSCLC & TNBC) Phase 1 Study Design Multiple ascending dose, “3+3” dose - escalation study 5 doses (mg/kg): 0.1 0.3 1.0 3.0 10.0 Post PD - 1 or PD - L1 patient population: Melanoma, NSCLC, HNSCC, HL, TNBC Potential for proprietary combination regimens with tovecimig and CTX - 471 9 CTX - 8371: Development Status NSCLC = non - small cell lung cancer; HNSCC = head and neck squamous cell carcinoma; HL = Hodgkin lymphoma; TNBC = triple negative breast cancer
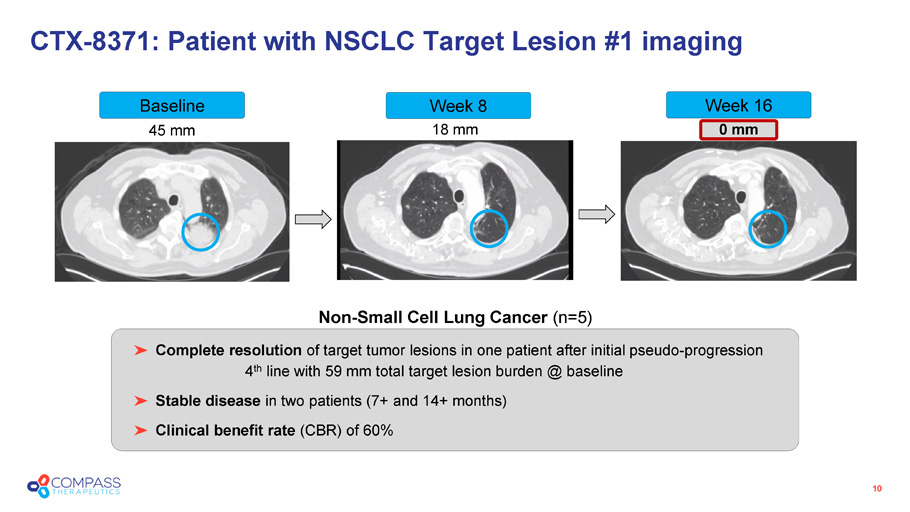
45 mm 18 mm 0 mm CTX - 8371: Patient with NSCLC Target Lesion #1 imaging 10 Non - Small Cell Lung Cancer (n=5) Complete resolution of target tumor lesions in one patient after initial pseudo - progression 4 th line with 59 mm total target lesion burden @ baseline Stable disease in two patients (7+ and 14+ months) Clinical benefit rate (CBR) of 60% Baseline Week 8 Week 16
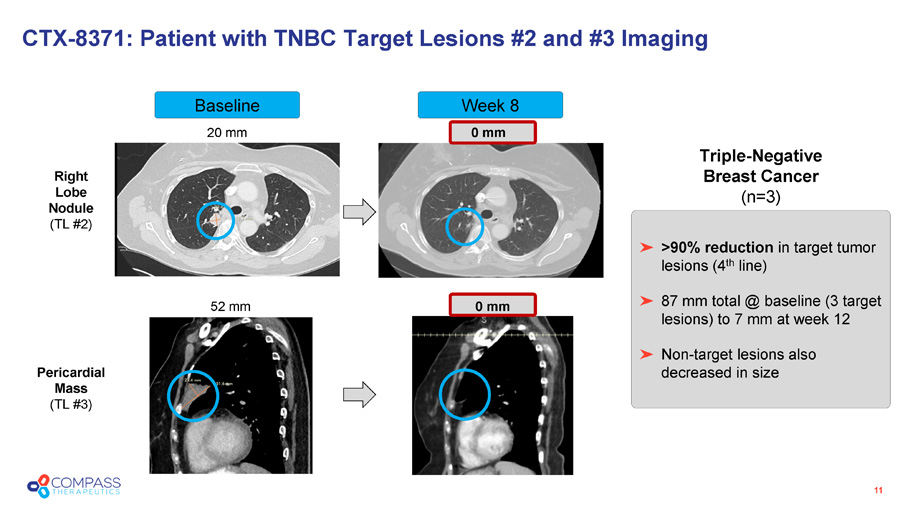
Week 8 Baseline CTX - 8371: Patient with TNBC Target Lesions #2 and #3 Imaging 11 20 mm 0 mm 52 mm 0 mm >90% reduction in target tumor lesions (4 th line) 87 mm total @ baseline (3 target lesions) to 7 mm at week 12 Non - target lesions also decreased in size Triple - Negative Breast Cancer (n=3) Right Lobe Nodule (TL #2) Pericardial Mass (TL #3)

CTX - 10726 PD - 1 x VEGF - A bispecific antibody
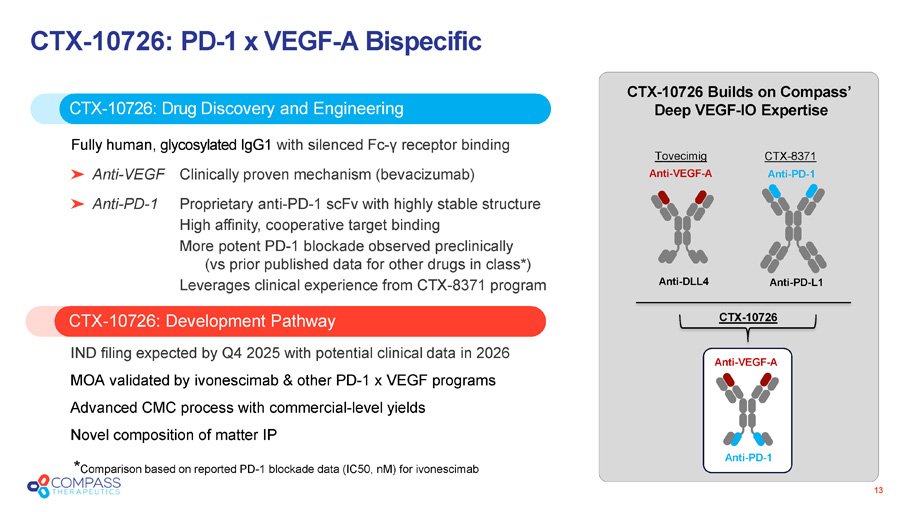
13 CTX - 10726 : PD - 1 x VEGF - A Bispecific CTX - 10726: Development Pathway CTX - 10726 : Drug Discovery and Engineering Fully human, glycosylated IgG1 with silenced Fc - γ receptor binding Anti - VEGF Clinically proven mechanism (bevacizumab) Anti - PD - 1 Proprietary anti - PD - 1 scFv with highly stable structure High affinity, cooperative target binding More potent PD - 1 blockade observed preclinically (vs prior published data for other drugs in class*) Leverages clinical experience from CTX - 8371 program IND filing expected by Q4 2025 with potential clinical data in 2026 MOA validated by ivonescimab & other PD - 1 x VEGF programs Advanced CMC process with commercial - level yields Novel composition of matter IP CTX - 10726 Builds on Compass’ Deep VEGF - IO Expertise Anti - VEGF - A Anti - DLL4 Tovecimig CTX - 8371 Anti - PD - 1 Anti - PD - L1 Anti - PD - 1 Anti - VEGF - A CTX - 10726 * Comparison based on reported PD - 1 blockade data (IC50, nM ) for ivonescimab
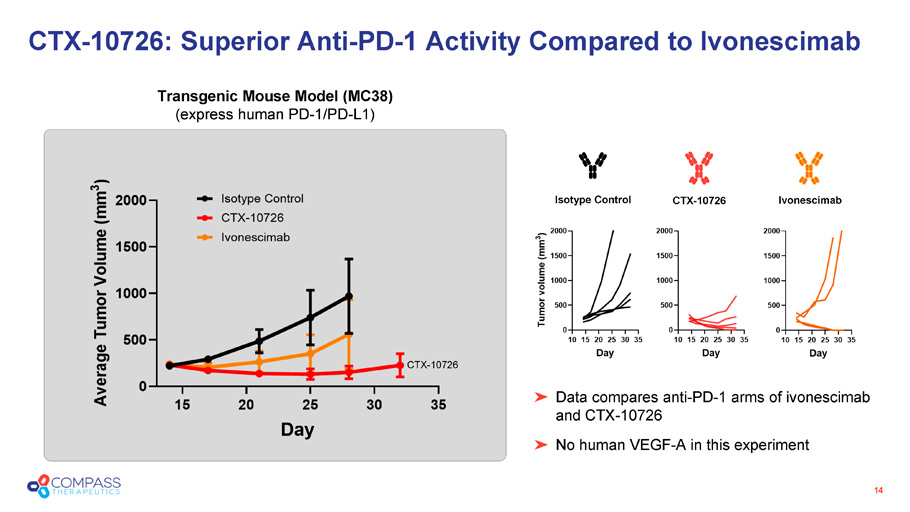
14 Data compares anti - PD - 1 arms of ivonescimab and CTX - 10726 No human VEGF - A in this experiment CTX - 10726: Superior Anti - PD - 1 Activity Compared to Ivonescimab Transgenic Mouse Model (MC38) (express human PD - 1/PD - L1) CTX - 10726 Isotype Control Ivonescimab CTX - 10726
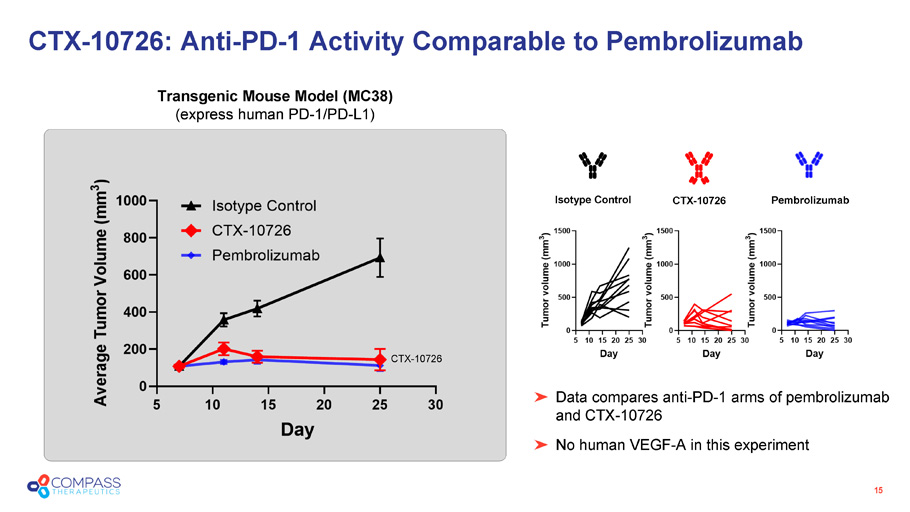
15 CTX - 10726: Anti - PD - 1 Activity Comparable to Pembrolizumab Transgenic Mouse Model (MC38) (express human PD - 1/PD - L1) CTX - 10726 Data compares anti - PD - 1 arms of pembrolizumab and CTX - 10726 No human VEGF - A in this experiment Isotype Control Pembrolizumab CTX - 10726
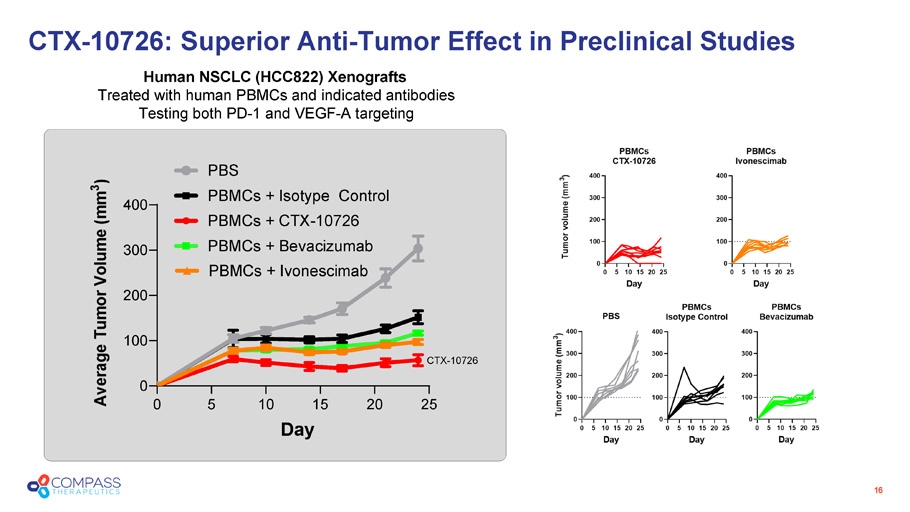
16 CTX - 10726 0 5 10 15 20 25 0 100 200 300 400 Day A v e r a g e T u m o r V o l u m e ( m m 3 ) PBS PBMCs + Isotype Control PBMCs + CTX-10726 PBMCs + Bevacizumab PBMCs + Ivonescimab Human NSCLC (HCC822) Xenografts Treated with human PBMCs and indicated antibodies Testing both PD - 1 and VEGF - A targeting CTX - 10726: Superior Anti - Tumor Effect in Preclinical Studies
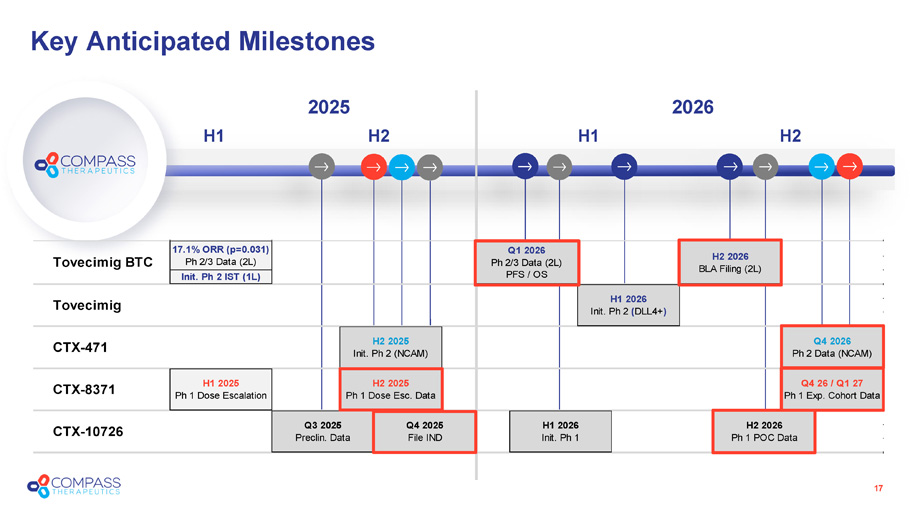
Key Anticipated Milestones 2025 H2 H1 17 2026 H2 H1 Tovecimig BTC 17.1% ORR (p=0.031) Ph 2/3 Data (2L) Q1 2026 Ph 2/3 Data (2L) PFS / OS Init. Ph 2 IST (1L) Q4 2026 Ph 2 Data (NCAM) CTX-8371 H1 2025 Ph 1 Dose Escalation H2 2025 Ph 1 Dose Esc. Data Q4 26 / Q1 27 Ph 1 Exp. Cohort Data H2 2026 BLA Filing (2L) Tovecimig H1 2026 Init. Ph 2 (DLL4+) CTX-471 H2 2025 Init. Ph 2 (NCAM) CTX-10726 Q3 2025 Preclin. Data Q4 2025 File IND H1 2026 Init. Ph 1 H2 2026 Ph 1 POC Data

Website: compasstherapeutics.com Nasdaq: CMPX Compass Therapeutics