Exhibit 99.2

August 7, 2025 Second-Quarter 2025 Earnings Presentation

Forward Looking Statements This presentation contains forward-looking
statements that involve substantial risks and uncertainties. “Forward-looking statements,” as that term is defined in the Private Securities Litigation Reform Act of 1995, are statements that are not historical facts and involve a number of
risks and uncertainties. Words herein such as “may,” “will,” “should,” “could,” “would,” “expects,” “plans,” “anticipates,” “believes,” “estimates,” “projects,” “predicts,” “intends,” “potential,” “continues,” and similar expressions (as well
as other words or expressions referencing future events, conditions or circumstances) may identify forward-looking statements. The forward-looking statements in this presentation are based upon the Company’s current expectations and beliefs,
and involve known and unknown risks, uncertainties and other factors, which may cause the Company’s actual results, performance and achievements and the timing of certain events to differ materially from the results, performance, achievements
or timing discussed, projected, anticipated or indicated in any forward-looking statements. Such risks, uncertainties and other factors include, among others, the following: failure to continue to successfully commercialize ARIKAYCE, our only
approved product, in the U.S., Europe or Japan (amikacin liposome inhalation suspension, Liposomal 590 mg Nebuliser Dispersion, and amikacin sulfate inhalation drug product, respectively), or to maintain US, European or Japanese approval for
ARIKAYCE; our inability to obtain full approval of ARIKAYCE from the FDA, including the risk that we will not successfully or in a timely manner complete the confirmatory post-marketing clinical trial required for full approval of ARIKAYCE,
or our failure to obtain regulatory approval to expand ARIKAYCE’s indication to a broader patient population; failure to obtain, or delays in obtaining, regulatory approvals for brensocatib, TPIP or our other product candidates in the US,
Europe or Japan or for ARIKAYCE outside the US, Europe or Japan, including separate regulatory approval for Lamira® in each market and for each usage; failure to successfully commercialize brensocatib, TPIP or our other product candidates, if
approved by applicable regulatory authorities, or to maintain applicable regulatory approvals for brensocatib, TPIP or our other product candidates, if approved; uncertainties or changes in the degree of market acceptance of ARIKAYCE or, if
approved, brensocatib, TPIP or our other product candidates by physicians, patients, third-party payors and others in the healthcare community; our inability to obtain and maintain adequate reimbursement from government or third-party payors
for ARIKAYCE or, if approved, brensocatib, TPIP or our other product candidates, or acceptable prices for ARIKAYCE or, if approved, brensocatib, TPIP or our other product candidates; inaccuracies in our estimates of the size of the potential
markets for ARIKAYCE, brensocatib, TPIP or our other product candidates or in data we have used to identify physicians, expected rates of patient uptake, duration of expected treatment, or expected patient adherence or discontinuation rates;
failure of third parties on which the Company is dependent to manufacture sufficient quantities of ARIKAYCE, brensocatib, or TPIP for commercial or clinical needs, to conduct the Company's clinical trials, or to comply with the Company's
agreements or laws and regulations that impact the Company's business; the risks and uncertainties associated with, and the perceived benefits of, our senior secured loan with certain funds managed by Pharmakon Advisors LP and our royalty
financing with OrbiMed Royalty & Credit Opportunities IV, LP, including our ability to maintain compliance with the covenants in the agreements for the senior secured loan and royalty financing and the impact of the restrictions on our
operations under these agreements; our inability to create or maintain an effective direct sales and marketing infrastructure or to partner with third parties that offer such an infrastructure for distribution of ARIKAYCE or any of our
product candidates that are approved in the future; failure to successfully conduct future clinical trials for ARIKAYCE, brensocatib, TPIP or our other product candidates and our potential inability to enroll or retain sufficient patients to
conduct and complete the trials or generate data necessary for regulatory approval of our product candidates or to permit the use of ARIKAYCE in the broader population of patients with MAC lung disease, among other things; development of
unexpected safety or efficacy concerns related to ARIKAYCE, brensocatib, TPIP or our other product candidates; risks that our clinical studies will be delayed, that serious side effects will be identified during drug development, or that any
protocol amendments submitted will be rejected; failure to successfully predict the time and cost of development, regulatory approval and commercialization for novel gene therapy products; the risk that interim, topline or preliminary data
from our clinical trials that we announce or publish from time to time may change as more patient data become available or may be interpreted differently if additional data are disclosed, or that blinded data will not be predictive of
unblinded data; risk that our competitors may obtain orphan drug exclusivity for a product that is essentially the same as a product we are developing for a particular indication; our inability to attract and retain key personnel or to
effectively manage our growth; our inability to successfully integrate our recent acquisitions and appropriately manage the amount of management’s time and attention devoted to integration activities; risks that our acquired technologies,
products and product candidates will not be commercially successful; inability to adapt to our highly competitive and changing environment; inability to access, upgrade or expand our technology systems or difficulties in updating our existing
technology or developing or implementing new technology; risk that we are unable to maintain our significant customers; risk that government healthcare reform materially increases our costs and damages our financial condition; business or
economic disruptions due to catastrophes or other events, including natural disasters or public health crises; risk that our current and potential future use of artificial intelligence and machine learning may not be successful; deterioration
in general economic conditions in the US, Europe, Japan and globally, including the effect of prolonged periods of inflation, affecting us, our suppliers, third-party service providers and potential partners; the risk that we could become
involved in costly intellectual property disputes, be unable to adequately protect our intellectual property rights or prevent disclosure of our trade secrets and other proprietary information, and incur costs associated with litigation or
other proceedings related to such matters; restrictions or other obligations imposed on us by agreements related to ARIKAYCE, brensocatib or our other product candidates, including our license agreements with PARI and AstraZeneca AB , and
failure to comply with our obligations under such agreements; the cost and potential reputational damage resulting from litigation to which we are or may become a party, including product liability claims; risk that our operations are subject
to a material disruption in the event of a cybersecurity attack or issue; our limited experience operating internationally; changes in laws and regulations applicable to our business, including any pricing reform and laws that impact our
ability to utilize certain third parties in the research, development or manufacture of our product candidates, and failure to comply with such laws and regulations; our history of operating losses, and the possibility that we never achieve
or maintain profitability; goodwill impairment charges affecting our results of operations and financial condition; inability to repay our existing indebtedness and uncertainties with respect to our ability to access future capital; and
delays in the execution of plans to build out an additional third-party manufacturing facility approved by the appropriate regulatory authorities and unexpected expenses associated with those plans. The Company may not actually achieve the
results, plans, intentions or expectations indicated by the Company's forward-looking statements because, by their nature, forward-looking statements involve risks and uncertainties because they relate to events and depend on circumstances
that may or may not occur in the future. For additional information about the risks and uncertainties that may affect the Company's business, please see the factors discussed in Item 1A, "Risk Factors," in the Company's Annual Report on Form
10-K for the year ended December 31, 2024 and any subsequent Company filings with the Securities and Exchange Commission (SEC). The Company cautions readers not to place undue reliance on any such forward-looking statements, which speak only
as of the date of this presentation. The Company disclaims any obligation, except as specifically required by law and the rules of the SEC, to publicly update or revise any such statements to reflect any change in expectations or in events,
conditions or circumstances on which any such statements may be based, or that may affect the likelihood that actual results will differ from those set forth in the forward-looking statements. Additional Disclaimers: Please be aware that
brensocatib and TPIP are investigational products that have not been approved for sale or found safe or effective by the FDA or any regulatory authority. In addition, ARIKAYCE has not been approved for the treatment of all patients with MAC
lung disease. This presentation is not promotion or advertisement of ARIKAYCE, brensocatib, or TPIP. Insmed and ARIKAYCE are registered trademarks of Insmed Incorporated. All other trademarks are property of their respective owner(s). TPIP:
Treprostinil Palmitil Inhalation Powder | MAC / MAC LD: Mycobacterium avium complex lung disease | FDA: Food & Drug Administration
Opening Remarks Brensocatib Updates TPIP Updates Financial Results Q&A
Session 5-8 10-11 13 16-18 20 Slides Agenda Speakers Will Lewis Chair & CEO Sara Bonstein Chief Financial Officer Roger Adsett Chief Operating Officer

Opening Remarks Will Lewis | Chair & CEO

First-Half 2025 Highlights * Late-stage assets refers to assets that have
demonstrated clinical success in either Phase 2 or Phase 3 of clinical development for at least one indication, including: ARIKAYCE® (Phase 3 ARISE data), brensocatib (Phase 3 ASPEN data), TPIP (Phase 2 data in PAH & PH-ILD) Three for
Three: All three late-stage assets have now demonstrated clinical success* Recent successes reflect the hard work invested by teams across the organization over the last 18+ months Position of strength reinforced by consistent ARIKAYCE®
performance and capital raise

NCFB: non-cystic fibrosis bronchiectasis | MAC / MAC LD: Mycobacterium avium
complex lung disease | CRSsNP: chronic rhinosinusitis without nasal polyps HS: hidradenitis suppurativa | TPIP: Treprostinil Palmitil Inhalation Powder | PH-ILD: pulmonary hypertension due to interstitial lung disease | PAH: pulmonary
arterial hypertension | Late-stage assets refers to assets that have completed at least one Phase 2 or Phase 3 trial in at least one indication | * pending regulatory approval Shaping a Portfolio with Winning Potential Over the Next 12+
Months Brensocatib 2 3Q:25 U.S. launch in NCFB* YE:25 Phase 2 BiRCh data in CRSsNP 1Q:26 Phase 2 CEDAR futility analysis in HS 2026 Ex-U.S. launches in NCFB* TPIP 3 2H:25 Initiate Phase 3 study in PH-ILD Early 2026 Initiate Phase 3
study in PAH ARIKAYCE® 1 FY:25 On track to achieve sales guidance in Refractory MAC 1H:26 Phase 3 ENCORE readout in all MAC LD Three Late-Stage Assets 2 Million Patients in the coming years with the potential to serve more than
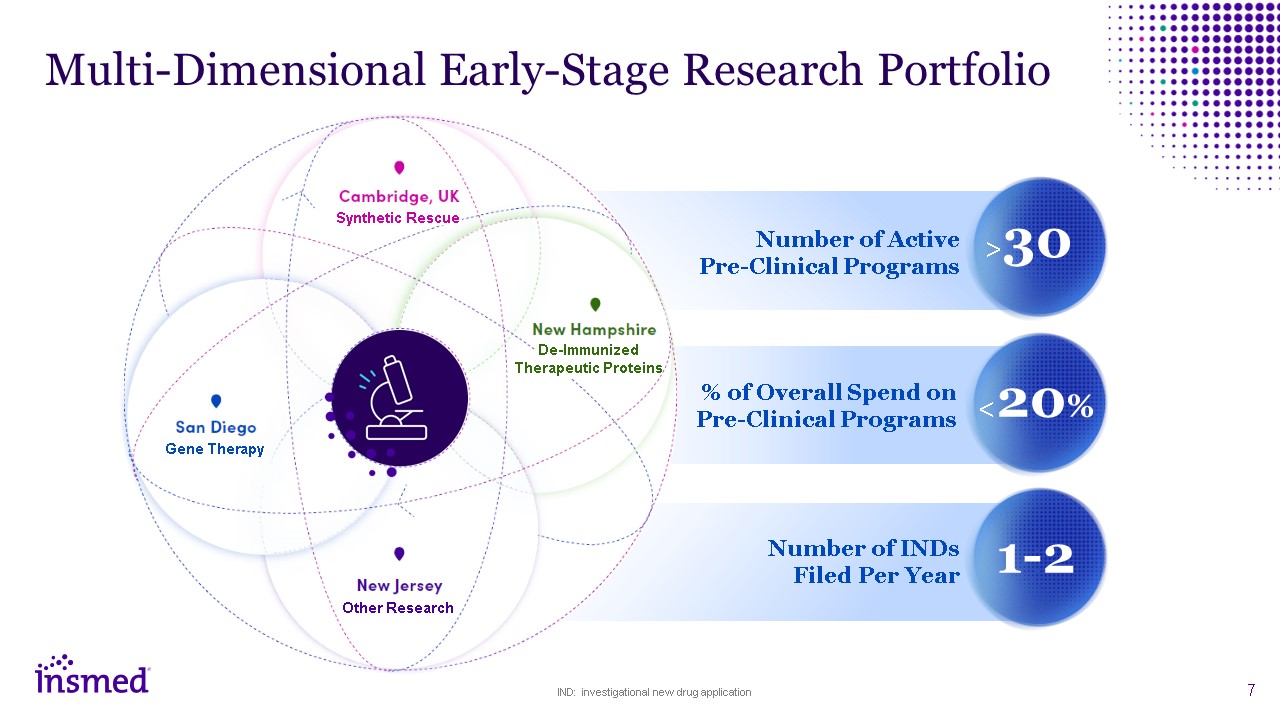
Multi-Dimensional Early-Stage Research Portfolio Number of Active
Pre-Clinical Programs % of Overall Spend on Pre-Clinical Programs Number of INDs Filed Per Year >30 <20% 1-2 IND: investigational new drug application Gene Therapy Other Research De-Immunized Therapeutic
Proteins Synthetic Rescue
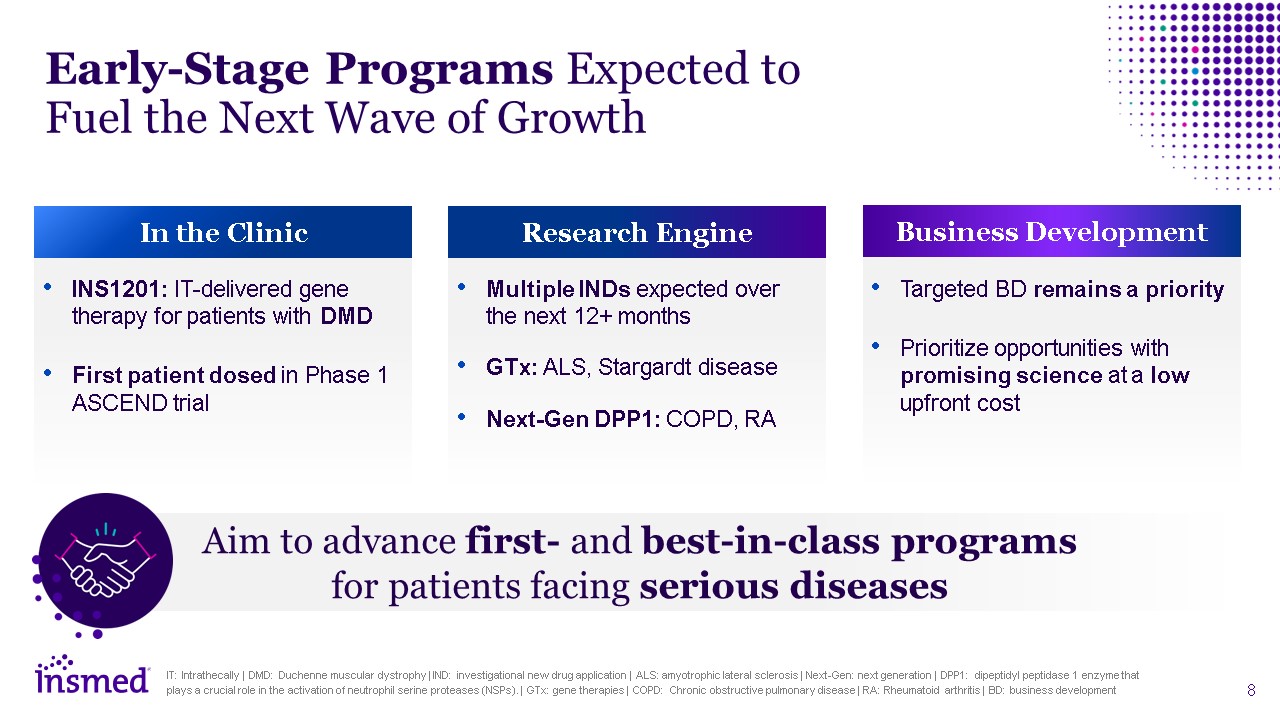
Early-Stage Programs Expected to Fuel the Next Wave of Growth In the
Clinic Research Engine Business Development INS1201: IT-delivered gene therapy for patients with DMD First patient dosed in Phase 1 ASCEND trial Multiple INDs expected over the next 12+ months GTx: ALS, Stargardt disease Next-Gen DPP1:
COPD, RA Targeted BD remains a priority Prioritize opportunities with promising science at a low upfront cost Aim to advance first- and best-in-class programs for patients facing serious diseases IT: Intrathecally | DMD: Duchenne
muscular dystrophy | IND: investigational new drug application | ALS: amyotrophic lateral sclerosis | Next-Gen: next generation | DPP1: dipeptidyl peptidase 1 enzyme that plays a crucial role in the activation of neutrophil serine proteases
(NSPs). | GTx: gene therapies | COPD: Chronic obstructive pulmonary disease | RA: Rheumatoid arthritis | BD: business development

Brensocatib Updates Will Lewis | Chair & CEO Roger Adsett | Chief
Operating Officer

COO Reflections: Brensocatib’s U.S. Launch is Uniquely Positioned for
Success Launch Readiness Trained & deployed our expanded salesforce >10 months pre-approval ~90% of surveyed physicians intend to prescribe to patients with ≥2 PEs* Speak up culture that promotes agility Brensocatib has
the potential to have one of the best launches in the specialty respiratory space COO: Chief Operating Officer | PE: pulmonary exacerbations | Insmed’s inLighten patient support program launched March 2025 | * Based on U.S. Demand
Study (fieldwork: June/July 2024)
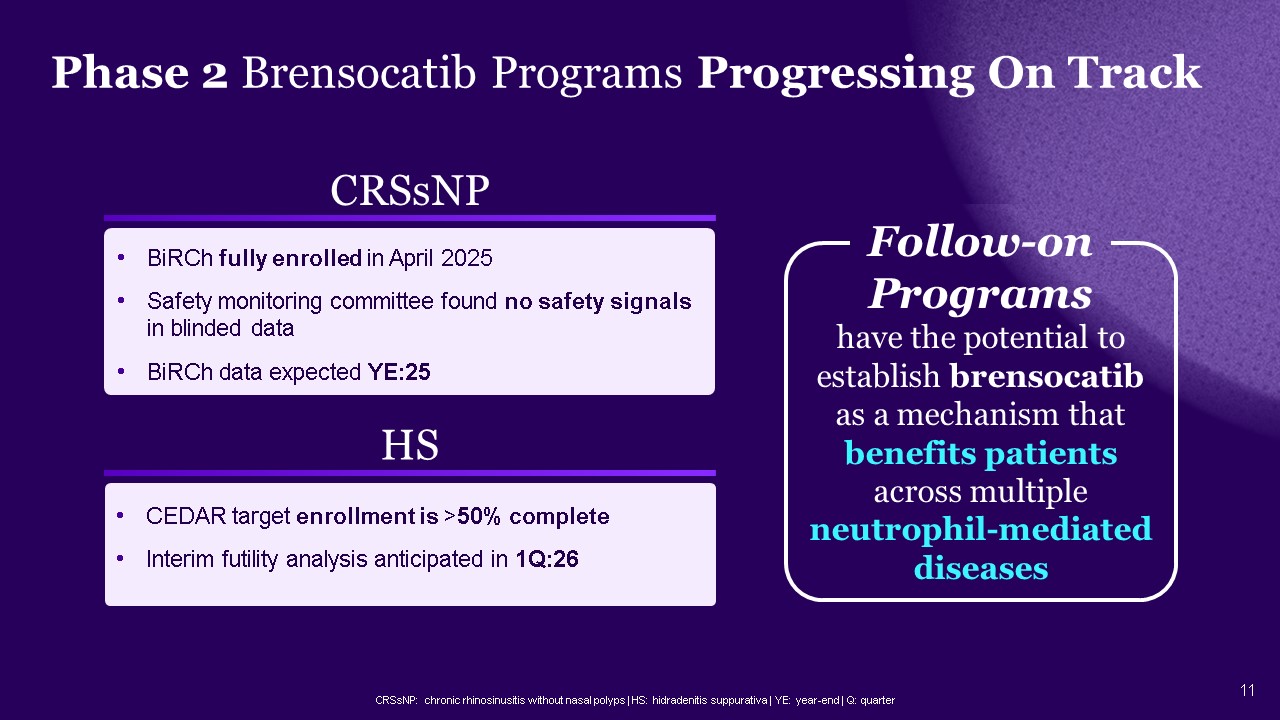
CRSsNP: chronic rhinosinusitis without nasal polyps | HS: hidradenitis
suppurativa | YE: year-end | Q: quarter CRSsNP BiRCh fully enrolled in April 2025 Safety monitoring committee found no safety signals in blinded data BiRCh data expected YE:25 HS CEDAR target enrollment is >50% complete Interim
futility analysis anticipated in 1Q:26 Follow-on Programs have the potential to establish brensocatib as a mechanism that benefits patients across multiple neutrophil-mediated diseases Phase 2 Brensocatib Programs Progressing On
Track

TPIP Updates TPIP: Treprostinil Palmitil Inhalation Powder Will Lewis | Chair
& CEO
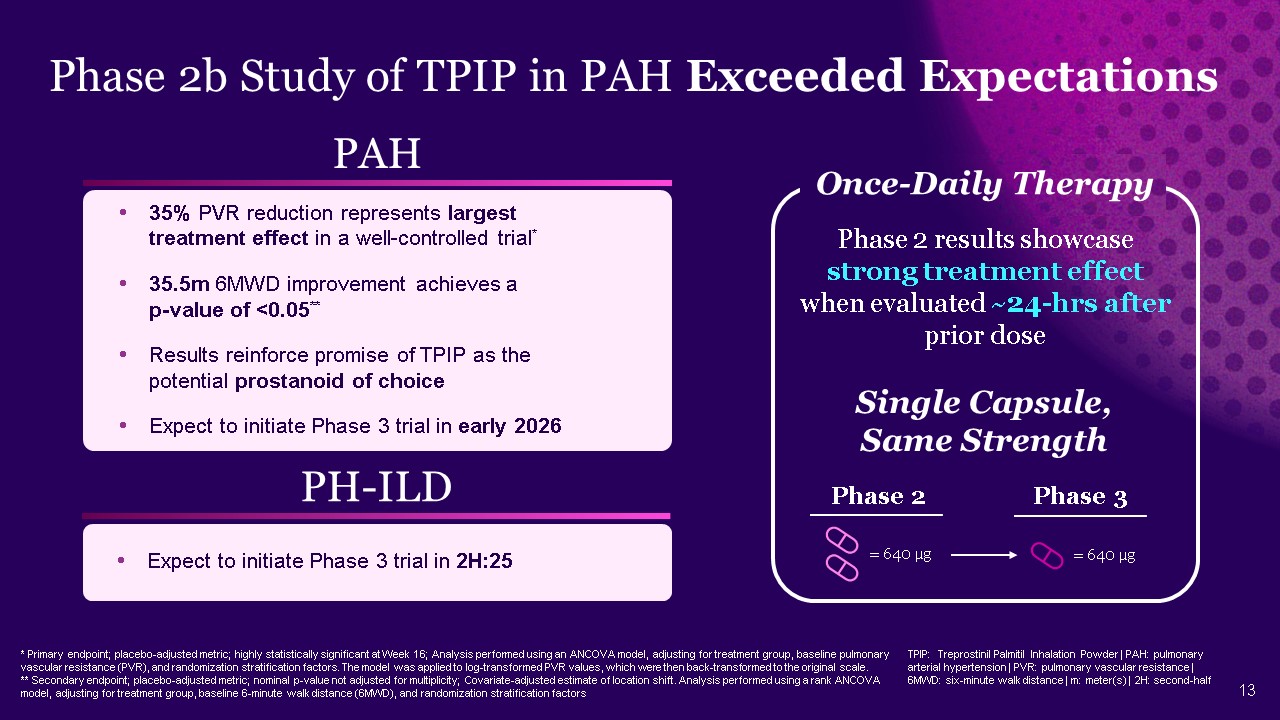
Phase 2b Study of TPIP in PAH Exceeded Expectations * Primary endpoint;
placebo-adjusted metric; highly statistically significant at Week 16; Analysis performed using an ANCOVA model, adjusting for treatment group, baseline pulmonary vascular resistance (PVR), and randomization stratification factors. The model
was applied to log-transformed PVR values, which were then back-transformed to the original scale. ** Secondary endpoint; placebo-adjusted metric; nominal p-value not adjusted for multiplicity; Covariate-adjusted estimate of location shift.
Analysis performed using a rank ANCOVA model, adjusting for treatment group, baseline 6-minute walk distance (6MWD), and randomization stratification factors PAH 35% PVR reduction represents largest treatment effect in a well-controlled
trial* 35.5m 6MWD improvement achieves a p-value of <0.05** Results reinforce promise of TPIP as the potential prostanoid of choice Expect to initiate Phase 3 trial in early 2026 PH-ILD Expect to initiate Phase 3 trial in 2H:25 Phase
2 = 640 μg Phase 3 = 640 μg Single Capsule, Same Strength Phase 2 results showcase strong treatment effect when evaluated ~24-hrs after prior dose Once-Daily Therapy TPIP: Treprostinil Palmitil Inhalation Powder | PAH: pulmonary
arterial hypertension | PVR: pulmonary vascular resistance | 6MWD: six-minute walk distance | m: meter(s) | 2H: second-half

Let’s Recap Execution on near-term catalysts has the potential to create a
profound difference in patient’s lives Achievement of goals enabled by a corporate culture that supports and empowers people to do their best work Well-positioned and motivated to deliver on clinical and commercial opportunities
ahead Certified Great Place to Work for Five Years in a Row!

Financial Results Sara Bonstein | Chief Financial Officer
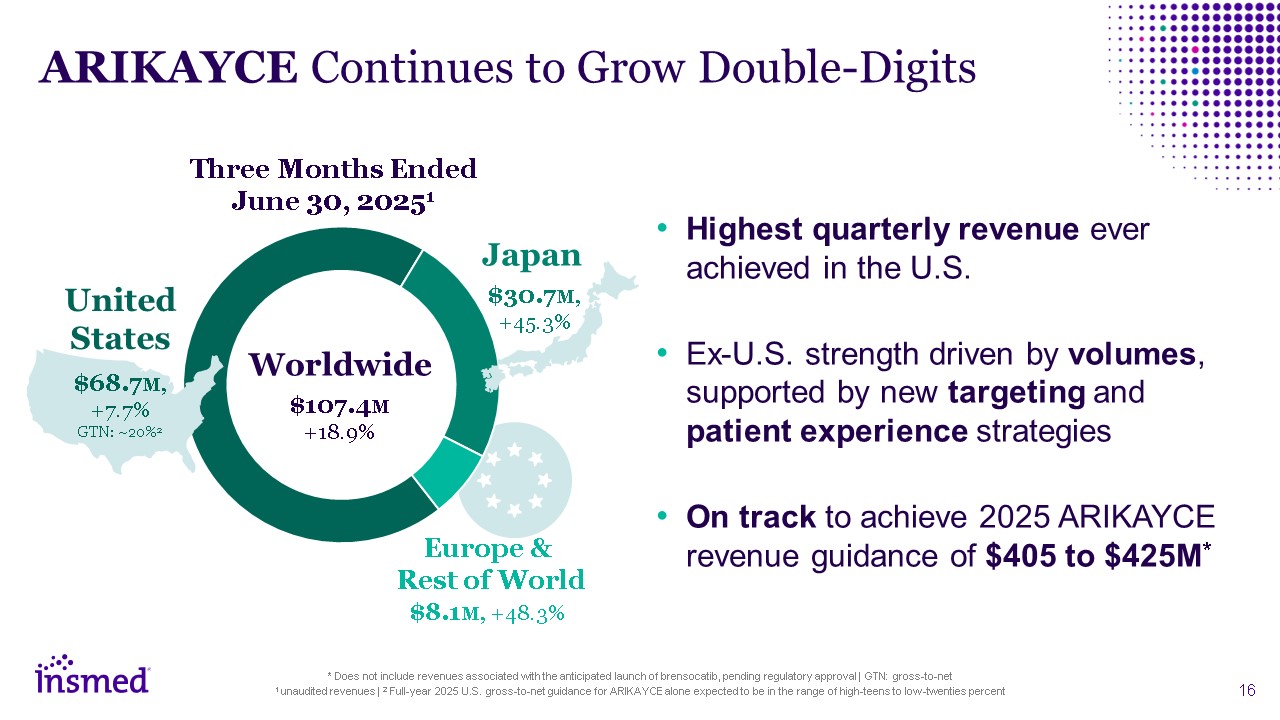
ARIKAYCE Continues to Grow Double-Digits Highest quarterly revenue ever
achieved in the U.S. Ex-U.S. strength driven by volumes, supported by new targeting and patient experience strategies On track to achieve 2025 ARIKAYCE revenue guidance of $405 to $425M* United States $68.7M, +7.7% GTN:
~20%2 Japan $30.7M, +45.3% Europe & Rest of World $8.1M, +48.3% Worldwide $107.4M +18.9% Three Months Ended June 30, 20251 * Does not include revenues associated with the anticipated launch of brensocatib, pending regulatory
approval | GTN: gross-to-net 1 unaudited revenues | 2 Full-year 2025 U.S. gross-to-net guidance for ARIKAYCE alone expected to be in the range of high-teens to low-twenties percent
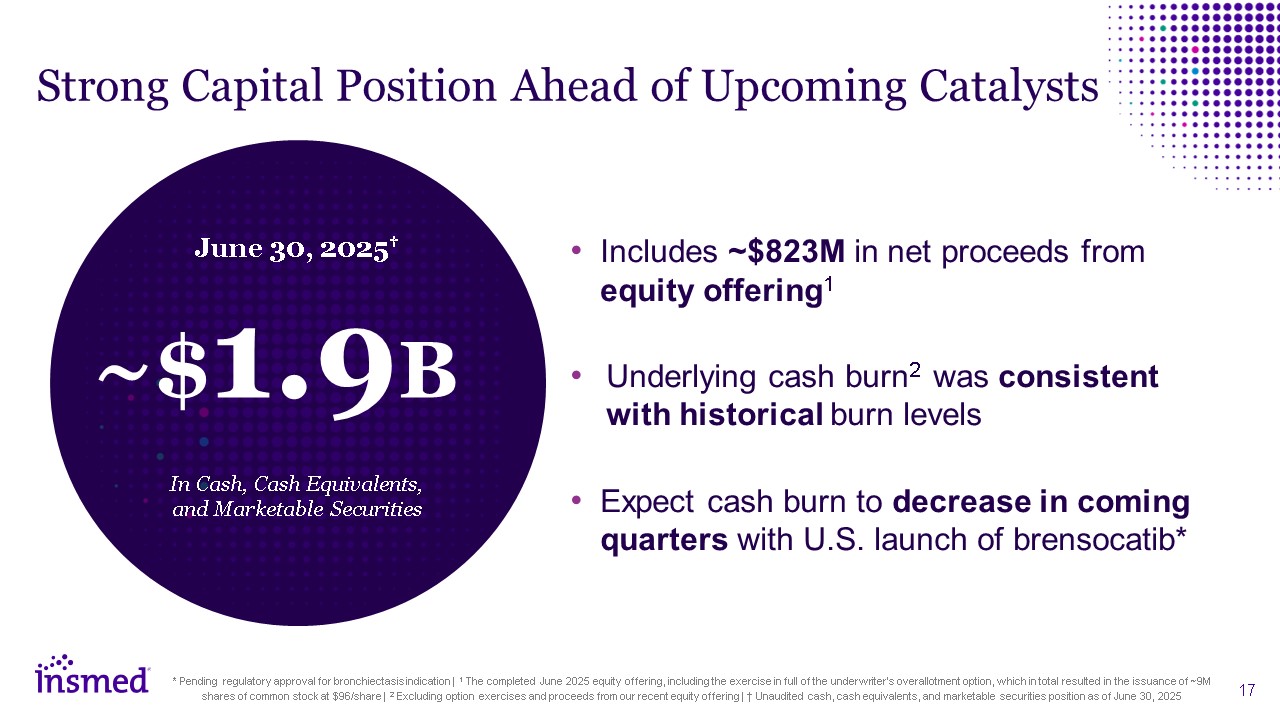
Strong Capital Position Ahead of Upcoming Catalysts Includes ~$823M in net
proceeds from equity offering1 Underlying cash burn2 was consistent with historical burn levels Expect cash burn to decrease in coming quarters with U.S. launch of brensocatib* * Pending regulatory approval for bronchiectasis indication |
1 The completed June 2025 equity offering, including the exercise in full of the underwriter’s overallotment option, which in total resulted in the issuance of ~9M shares of common stock at $96/share | 2 Excluding option exercises and
proceeds from our recent equity offering | † Unaudited cash, cash equivalents, and marketable securities position as of June 30, 2025 ~$1.9B June 30, 2025† In Cash, Cash Equivalents, and Marketable Securities
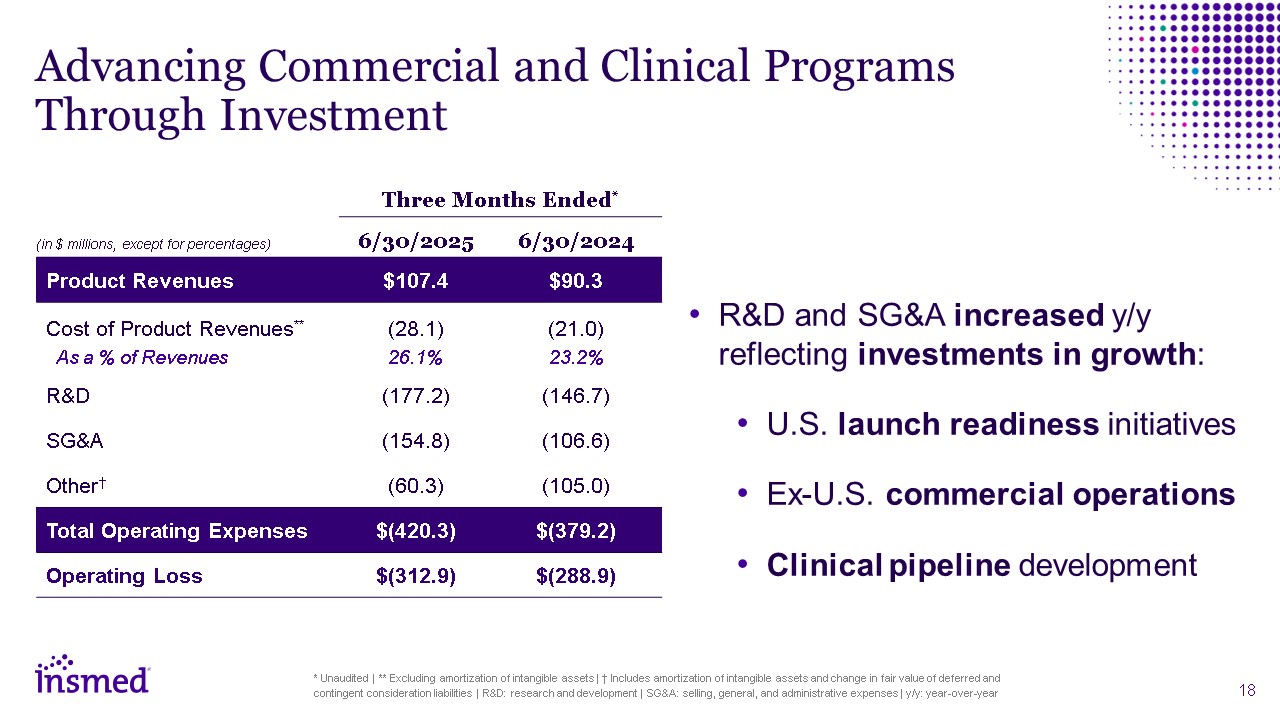
Advancing Commercial and Clinical Programs Through Investment Three Months
Ended* 6/30/2025 6/30/2024 Product Revenues $107.4 $90.3 Cost of Product Revenues** As a % of Revenues (28.1) 26.1% (21.0) 23.2% R&D (177.2) (146.7) SG&A (154.8) (106.6) Other† (60.3) (105.0) Total Operating
Expenses $(420.3) $(379.2) Operating Loss $(312.9) $(288.9) R&D and SG&A increased y/y reflecting investments in growth: U.S. launch readiness initiatives Ex-U.S. commercial operations Clinical pipeline development *
Unaudited | ** Excluding amortization of intangible assets | † Includes amortization of intangible assets and change in fair value of deferred and contingent consideration liabilities | R&D: research and development | SG&A: selling,
general, and administrative expenses | y/y: year-over-year (in $ millions, except for percentages)

Closing Remarks Next 12+ Months: Anticipate multiple commercial, clinical, and
regulatory milestones with the potential to drive value Strong cash position to execute on upcoming catalysts and maintain line-of-sight to profitability Remain committed to thoughtfully deploying capital to maximize opportunities for
patients

Q&A Session Will Lewis Chair & CEO Sara Bonstein Chief Financial
Officer Dr. Martina Flammer Chief Medical Officer Roger Adsett Chief Operating Officer
