| Dedicated to Dramatically Improving the Lives of Children and Adults with SMA Q2 2025 BUSINESS UPDATE August 6, 2025 © 2025 Scholar Rock, Inc. All rights reserved. Exhibit 99.2 |
| Q2 EARNINGS CALL © 2025 Scholar Rock, Inc. All rights reserved. 2 TOPIC SPEAKER SCHOLAR ROCK NEXT PHASE OF GROWTH David L. Hallal Chairman and Chief Executive Officer R&D PROGRESS Akshay Vaishnaw, M.D., Ph.D President of R&D COMMERCIAL READINESS Keith Woods Chief Operating Officer COMPANY FINANCIALS Vikas Sinha Chief Financial Officer Q&A SESSION |
| Forward-Looking Statements © 2025 Scholar Rock, Inc. All rights reserved. © 2025 Scholar Rock, Inc. All rights reserved. This presentation contains "forward-looking statements" within the meaning of the Private Securities Litigation Reform Act of 1995, including, but not limited to, statements regarding Scholar Rock’s future expectations, plans and prospects, including without limitation, Scholar Rock’s expectations regarding its growth, strategy, progress and timing of its clinical trials for apitegromab and its preclinical programs, including SRK- 439, and indication selection and development timing, including the timing of any regulatory submissions and anticipated approvals, the therapeutic potential, clinical benefits and safety of any product candidatesits cash runway, expectations regarding commercial launch timing in the US and in Europe, expectations regarding the achievement of important milestones, the ability of any product candidate to perform in humans in a manner consistent with earlier nonclinical, preclinical or clinical trial data, and the potential of its product candidates and proprietary platform. The use of words such as “may,” “might,” “could,” “will,” “should,” “expect,” “plan,” “anticipate,” “believe,” “estimate,” “project,” “intend,” “future,” “potential,” or “continue,” and other similar expressions are intended to identify such forward-looking statements. All such forward-looking statements are based on management's current expectations of future events and are subject to a number of risks and uncertainties that could cause actual results to differ materially and adversely from those set forth in or implied by such forward-looking statements. These risks and uncertainties include, without limitation, whether preclinical and clinical data, including the results from the Phase 3 SAPPHIRE trial will be sufficient to support regulatory approval, that the full results from the Phase 3 SAPPHIRE trial may differ from the topline data, that preclinical and clinical data, including the results from the Phase 2 or Phase 3 clinical trial of apitegromab, are not predictive of, may be inconsistent with, or more favorable than, data generated from future or ongoing clinical trials of the same product candidates; Scholar Rock’s ability to manage expenses or provide the financial support, resources and expertise necessary to identify and develop product candidates on the expected timeline; information provided or decisions made by regulatory authorities; competition from third parties that are developing products for similar uses; Scholar Rock’s ability to obtain, maintain and protect its intellectual property; and Scholar Rock’s dependence on third parties for development and manufacture of product candidates including, without limitation, to supply any clinical trials as well as those risks more fully discussed in the section entitled "Risk Factors" in Scholar Rock’s Quarterly Report on Form 10-Q for the quarter ended June 30, 2025, as well as discussions of potential risks, uncertainties, and other important factors in Scholar Rock’s subsequent filings with the Securities and Exchange Commission. Any forward-looking statements represent Scholar Rock’s views only as of today and should not be relied upon as representing its views as of any subsequent date. All information in this press release is as of the date of the release, and Scholar Rock undertakes no duty to update this information unless required by law. Apitegromab and SRK-439 have not been approved for any use by the FDA or any other regulatory agency and the safety and efficacy of apitegromab and SRK-439 have not been established. 3 |
| David L. Hallal Chairman and Chief Executive Officer © 2025 Scholar Rock, Inc. All rights reserved. 4 SCHOLAR ROCK NEXT PHASE OF GROWTH |
| *Pending regulatory approval 2025 Priorities Support Long-Term Growth • Execute Successful US Commercial Launch* • Advance EU Launch Preparedness • Efficient Commercial Build • Phase Investments to Support Future High-value Commercial & Pipeline Initiatives Expand into Additional Rare, Severe & Debilitating Neuromuscular Diseases Apitegromab in SMA Regulatory Approvals & Commercialization Disciplined Capital Allocation • Apitegromab Development Program: Building a Pipeline in a Product • Leverage Highly Innovative Anti-myostatin Platform © 2025 Scholar Rock, Inc. All rights reserved. 5 |
| Progress at a Glance: Our Key Milestones Pending regulatory approval SPINAL MUSCULAR ATROPHY Positive SAPPHIRE Data Presented at Cure SMA Research Meeting FDA Target Action Date Sept 22, 2025 EMBRAZE Achieved Primary Endpoint Positive Ph 2 proof-of-concept trial in obesity File IND for SRK-439 in 2H 2025 PIPELINE Experienced Team Powering growth & accelerating transformation CORPORATE $295 Million Cash as of June 30, 2025 Commercial Buildout Customer-facing team deployed in the U.S. Initiate OPAL Trial in Q3 In infants & toddlers with SMA MAA Validated Anticipated 2026 approval © 2025 Scholar Rock, Inc. All rights reserved. 6 |
| © 2025 Scholar Rock, Inc. All rights reserved. 7 Fueling Scholar Rock’s long-term sustainable growth Apitegromab in SMA |
| Akshay Vaishnaw, M.D., Ph.D President of R&D R&D PROGRESS © 2025 Scholar Rock, Inc. All rights reserved. 8 |
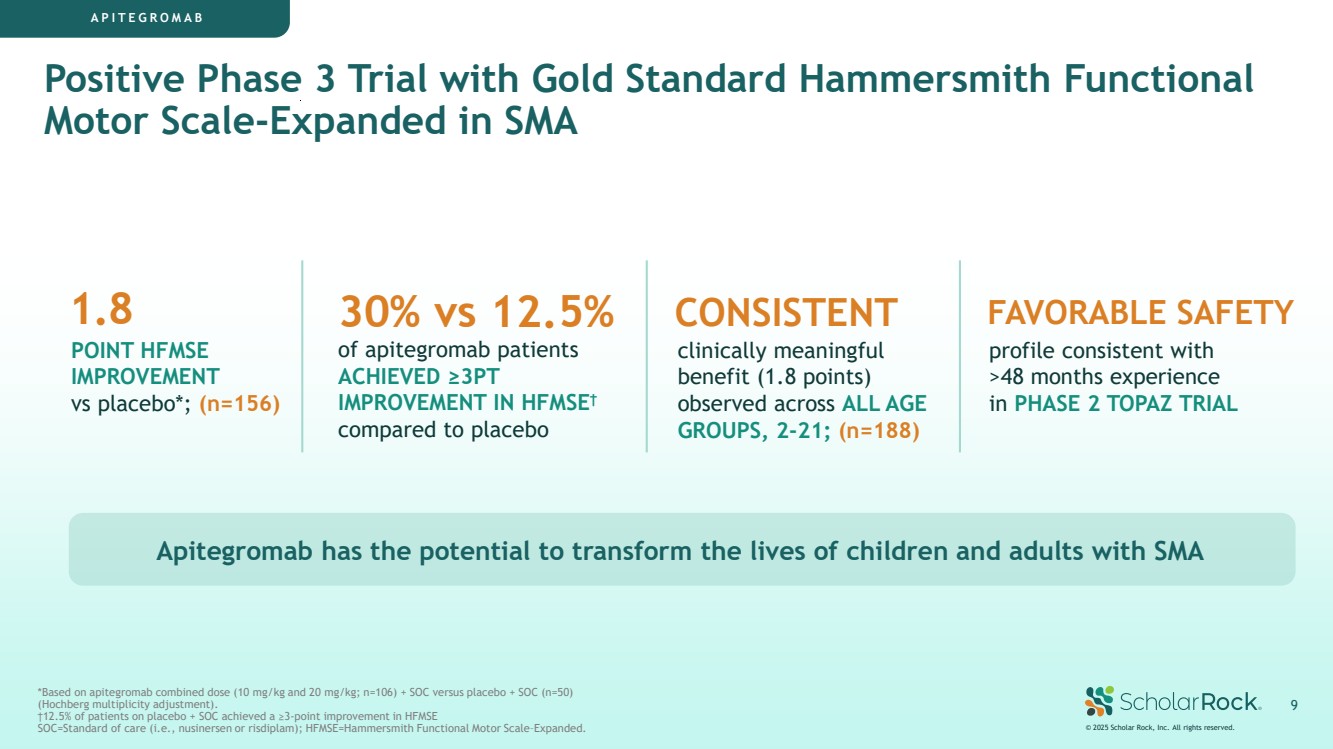
| APITEGROMAB Positive Phase 3 Trial with Gold Standard Hammersmith Functional Motor Scale-Expanded in SMA 1.8 POINT HFMSE IMPROVEMENT vs placebo*; (n=156) Apitegromab has the potential to transform the lives of children and adults with SMA © 2025 Scholar Rock, Inc. All rights reserved. 9 FAVORABLE SAFETY profile consistent with >48 months experience in PHASE 2 TOPAZ TRIAL CONSISTENT clinically meaningful benefit (1.8 points) observed across ALL AGE GROUPS, 2-21; (n=188) 30% vs 12.5% of apitegromab patients ACHIEVED ≥3PT IMPROVEMENT IN HFMSE† compared to placebo *Based on apitegromab combined dose (10 mg/kg and 20 mg/kg; n=106) + SOC versus placebo + SOC (n=50) (Hochberg multiplicity adjustment). †12.5% of patients on placebo + SOC achieved a ≥3-point improvement in HFMSE SOC=Standard of care (i.e., nusinersen or risdiplam); HFMSE=Hammersmith Functional Motor Scale–Expanded. |
| FDA Accepted BLA Under Priority Review – Target Action Date September 22 APITEGROMAB Potential clinical benefits of apitegromab, as demonstrated by our Phase 3 trial, are underscored by the FDA’s priority review designation © 2025 Scholar Rock, Inc. All rights reserved. 1 0 Providing a significant improvement in safety or effectiveness over existing treatments. By definition, a priority review designation by the FDA conveys the capacity of apitegromab to potentially impact unmet need in SMA by: Being a treatment for a serious or life-threatening condition. |
| Expanding Our Impact: Initiating Phase 2 OPAL Trial in Q3 Including evaluation of apitegromab in patients who received gene therapy EXPANDING OUR IMPACT Potential to alter the course of SMA in children under 2 ADDRESSING THE NEEDS OF INFANTS & TODDLERS WITH SMA Reaching patients earlier TIME IS MUSCLE APITEGROMAB Studying apitegromab in patients under 2 years old © 2025 Scholar Rock, Inc. All rights reserved. 1 1 |
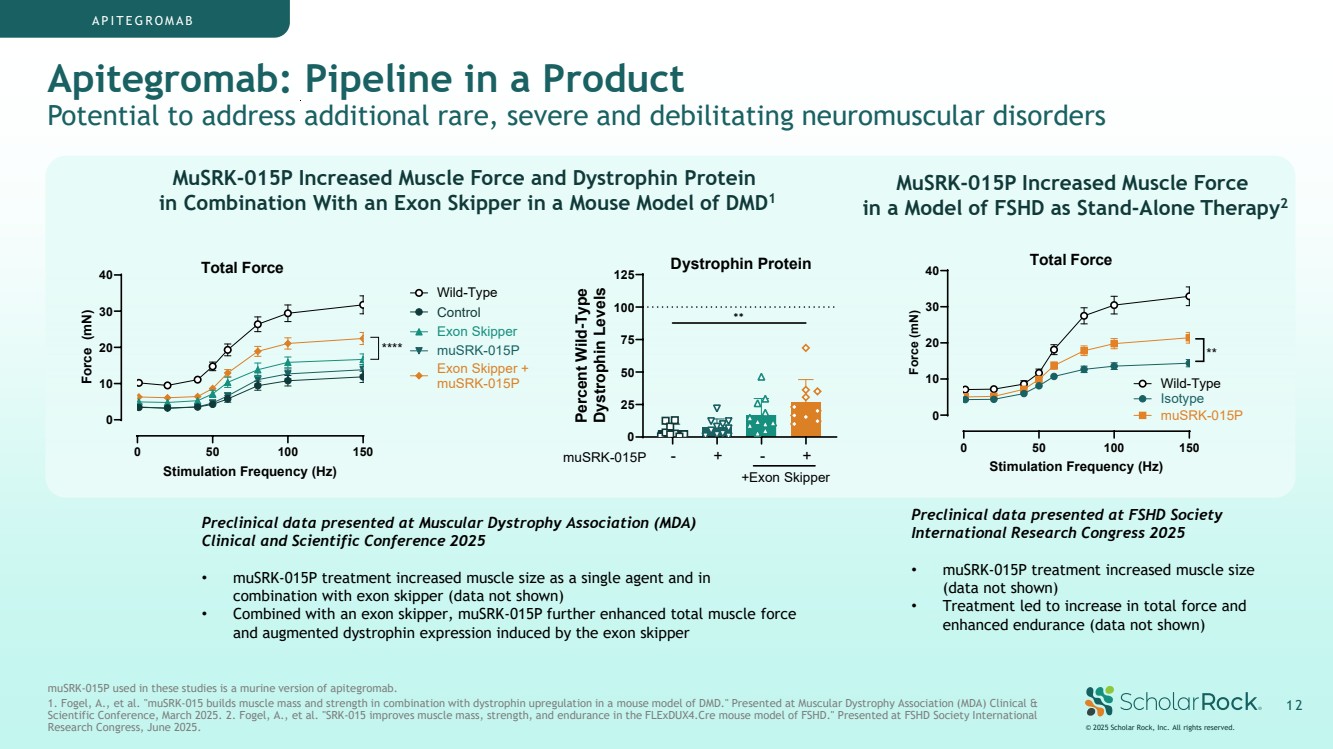
| Apitegromab: Pipeline in a Product Potential to address additional rare, severe and debilitating neuromuscular disorders © 2025 Scholar Rock, Inc. All rights reserved. 1 2 MuSRK-015P Increased Muscle Force and Dystrophin Protein in Combination With an Exon Skipper in a Mouse Model of DMD1 MuSRK-015P Increased Muscle Force in a Model of FSHD as Stand-Alone Therapy2 APITEGROMAB Preclinical data presented at Muscular Dystrophy Association (MDA) Clinical and Scientific Conference 2025 • muSRK-015P treatment increased muscle size as a single agent and in combination with exon skipper (data not shown) • Combined with an exon skipper, muSRK-015P further enhanced total muscle force and augmented dystrophin expression induced by the exon skipper Preclinical data presented at FSHD Society International Research Congress 2025 • muSRK-015P treatment increased muscle size (data not shown) • Treatment led to increase in total force and enhanced endurance (data not shown) muSRK-015P used in these studies is a murine version of apitegromab. 1. Fogel, A., et al. "muSRK-015 builds muscle mass and strength in combination with dystrophin upregulation in a mouse model of DMD." Presented at Muscular Dystrophy Association (MDA) Clinical & Scientific Conference, March 2025. 2. Fogel, A., et al. "SRK-015 improves muscle mass, strength, and endurance in the FLExDUX4.Cre mouse model of FSHD." Presented at FSHD Society International Research Congress, June 2025. 0 50 100 150 0 10 20 30 40 Total Force Stimulation Frequency (Hz) Force (mN) Wild-Type Control Exon Skipper muSRK-015P Exon Skipper + muSRK-015P **** 0 25 50 75 100 125 Percent Wild-Type Dystrophin Levels ✱✱ muSRK-015P - + - + +Exon Skipper Dystrophin Protein 0 50 100 150 0 10 20 30 40 Stimulation Frequency (Hz) Force (mN) Isotype muSRK-015P Wild-Type ** Total Force |
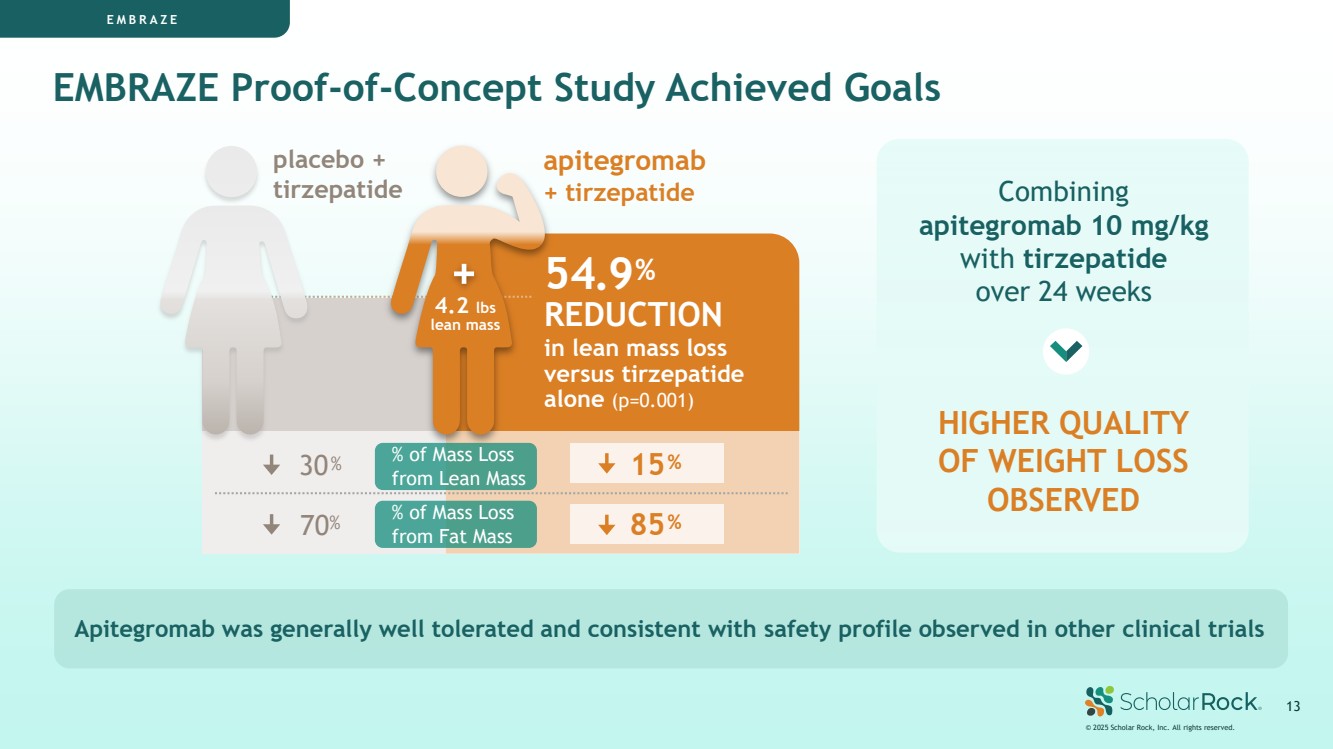
| EMBRAZE Apitegromab was generally well tolerated and consistent with safety profile observed in other clinical trials © 2025 Scholar Rock, Inc. All rights reserved. 13 apitegromab + tirzepatide 70% 30% 85% 15% placebo + tirzepatide 54.9% REDUCTION in lean mass loss versus tirzepatide alone (p=0.001) Combining apitegromab 10 mg/kg with tirzepatide over 24 weeks HIGHER QUALITY OF WEIGHT LOSS OBSERVED EMBRAZE Proof-of-Concept Study Achieved Goals 4.2 lbs lean mass % of Mass Loss from Fat Mass % of Mass Loss from Lean Mass |
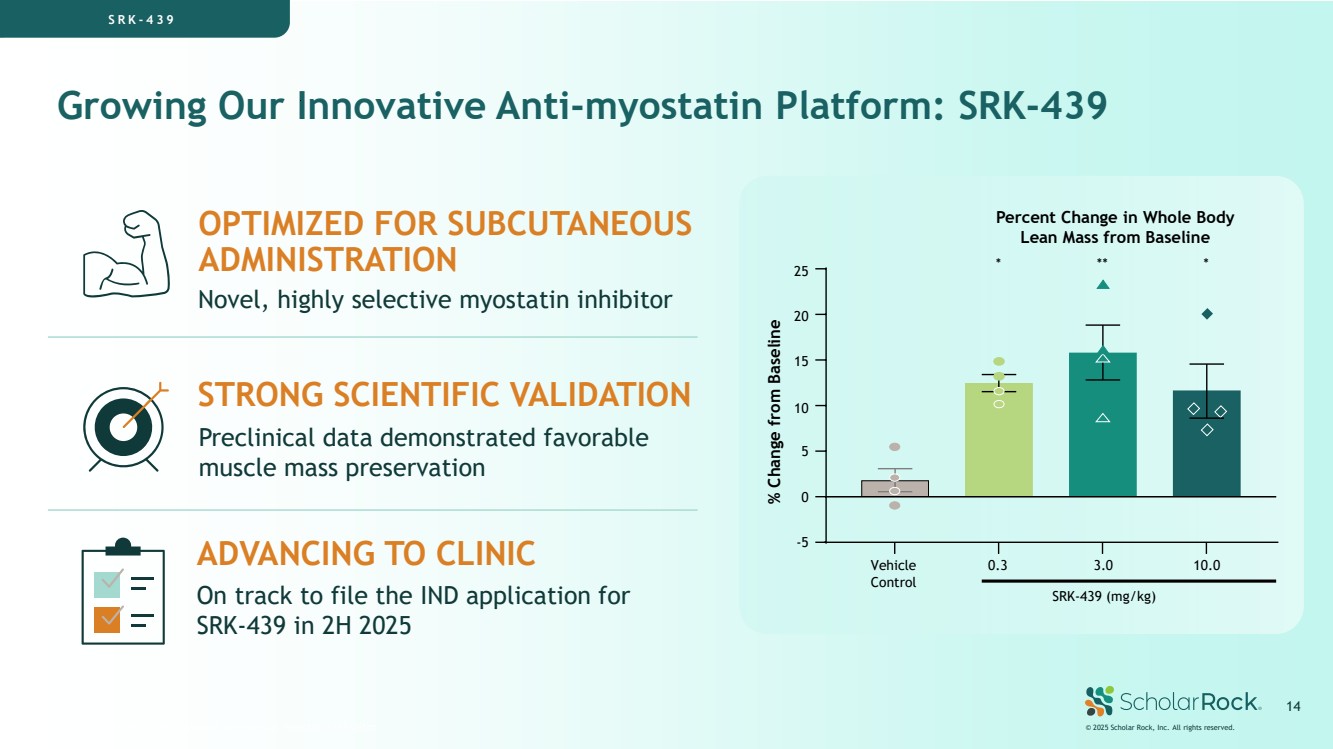
| Growing Our Innovative Anti-myostatin Platform: SRK-439 SRK-439 is a novel, investigational, preclinical myostatin inhibitor On track to file the IND application for SRK-439 in 2H 2025 Preclinical data demonstrated favorable muscle mass preservation Novel, highly selective myostatin inhibitor ADVANCING TO CLINIC STRONG SCIENTIFIC VALIDATION OPTIMIZED FOR SUBCUTANEOUS ADMINISTRATION SRK - 439 * ** * Vehicle Control 0.3 3.0 10.0 SRK-439 (mg/kg) -5 0 5 10 15 20 25 % Change from Baseline Percent Change in Whole Body Lean Mass from Baseline © 2025 Scholar Rock, Inc. All rights reserved. 14 |
| Remain Focused On Delivering R&D Priorities Advance toward anticipated US approval of apitegromab in Q3 2025; anticipated EU approval in 2026 Initiate Ph 2 OPAL Trial in Q3 2025 File IND for SRK-439 in 2H 2025 Complete clinical development plans: apitegromab in additional rare, severe, and debilitating neuromuscular disorders 1 2 3 4 © 2025 Scholar Rock, Inc. All rights reserved. 1 5 |
| Keith Woods Chief Operating Officer COMMERCIAL READINESS © 2025 Scholar Rock, Inc. All rights reserved. 1 6 |
| Ushering in a New Treatment Era for SMA *Pending regulatory approvals 1. Cure SMA State of SMA 2024 Report. https://www.curesma.org/wp-content/uploads/2025/04/State-of-SMA-Report2024_vWeb.pdf Apitegromab is an investigational drug candidate under evaluation and has not been approved by any regulatory agency. Apitegromab is the first and only muscle-targeted treatment to show clinically meaningful and statistically significant motor function improvement in SMA. Preparing for a global launch of apitegromab in SMA* SMA is a progressive and devastating disease that leads to loss of mobility, limited activities of daily living, and lack of independence 1 1 7 © 2025 Scholar Rock, Inc. All rights reserved. |
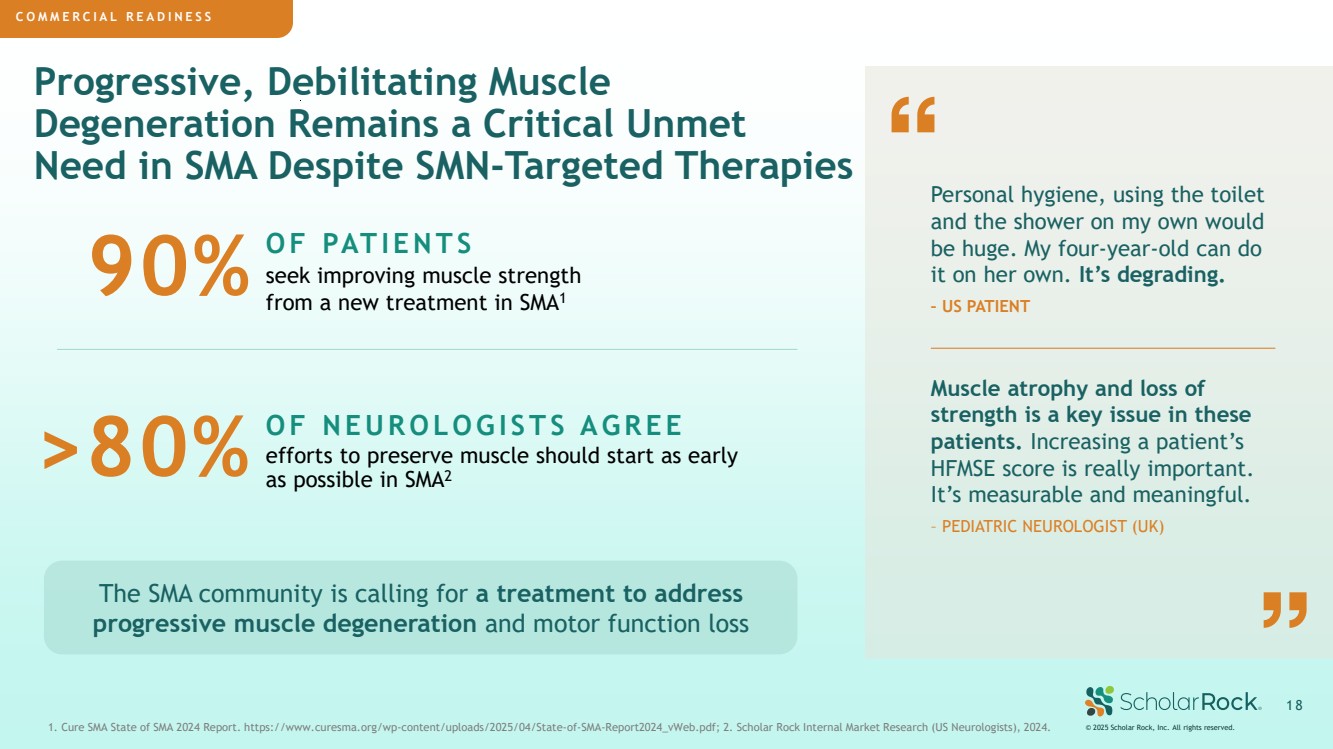
| COMMERCIAL PREPAREDNESS Progressive, Debilitating Muscle Degeneration Remains a Critical Unmet Need in SMA Despite SMN-Targeted Therapies © 2025 Scholar Rock, Inc. All rights reserved. 1 8 1. Cure SMA State of SMA 2024 Report. https://www.curesma.org/wp-content/uploads/2025/04/State-of-SMA-Report2024_vWeb.pdf; 2. Scholar Rock Internal Market Research (US Neurologists), 2024. OF NEUROLOGISTS AGREE efforts to preserve muscle should start as early as possible in SMA2 OF PATIENTS seek improving muscle strength from a new treatment in SMA1 Muscle atrophy and loss of strength is a key issue in these patients. Increasing a patient’s HFMSE score is really important. It’s measurable and meaningful. – PEDIATRIC NEUROLOGIST (UK) Personal hygiene, using the toilet and the shower on my own would be huge. My four-year-old can do it on her own. It’s degrading. – US PATIENT The SMA community is calling for a treatment to address progressive muscle degeneration and motor function loss COMMERCIAL READINESS COMMERCIAL PREPAREDNESS 90% >80% |
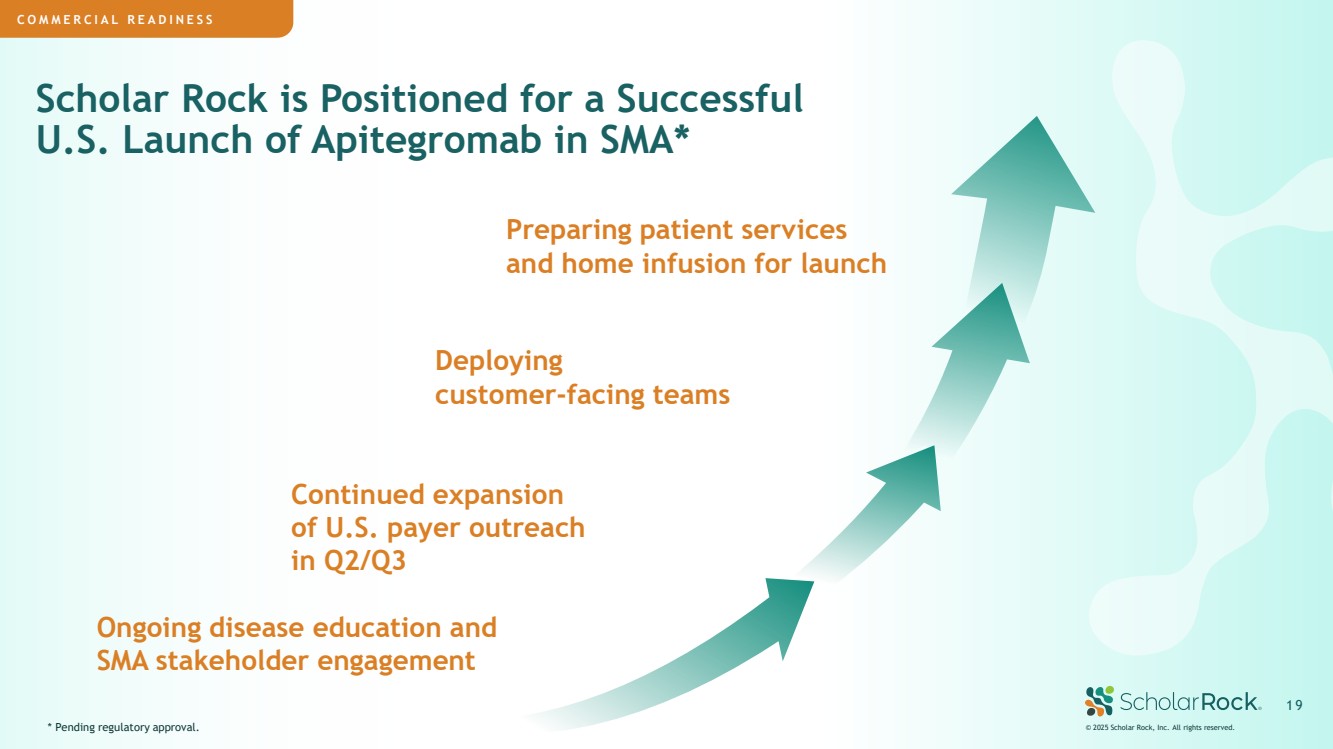
| Scholar Rock is Positioned for a Successful U.S. Launch of Apitegromab in SMA* COMMERCIAL READINESS * Pending regulatory approval. Continued expansion of U.S. payer outreach in Q2/Q3 Ongoing disease education and SMA stakeholder engagement Deploying customer-facing teams Preparing patient services and home infusion for launch © 2025 Scholar Rock, Inc. All rights reserved. 1 9 |
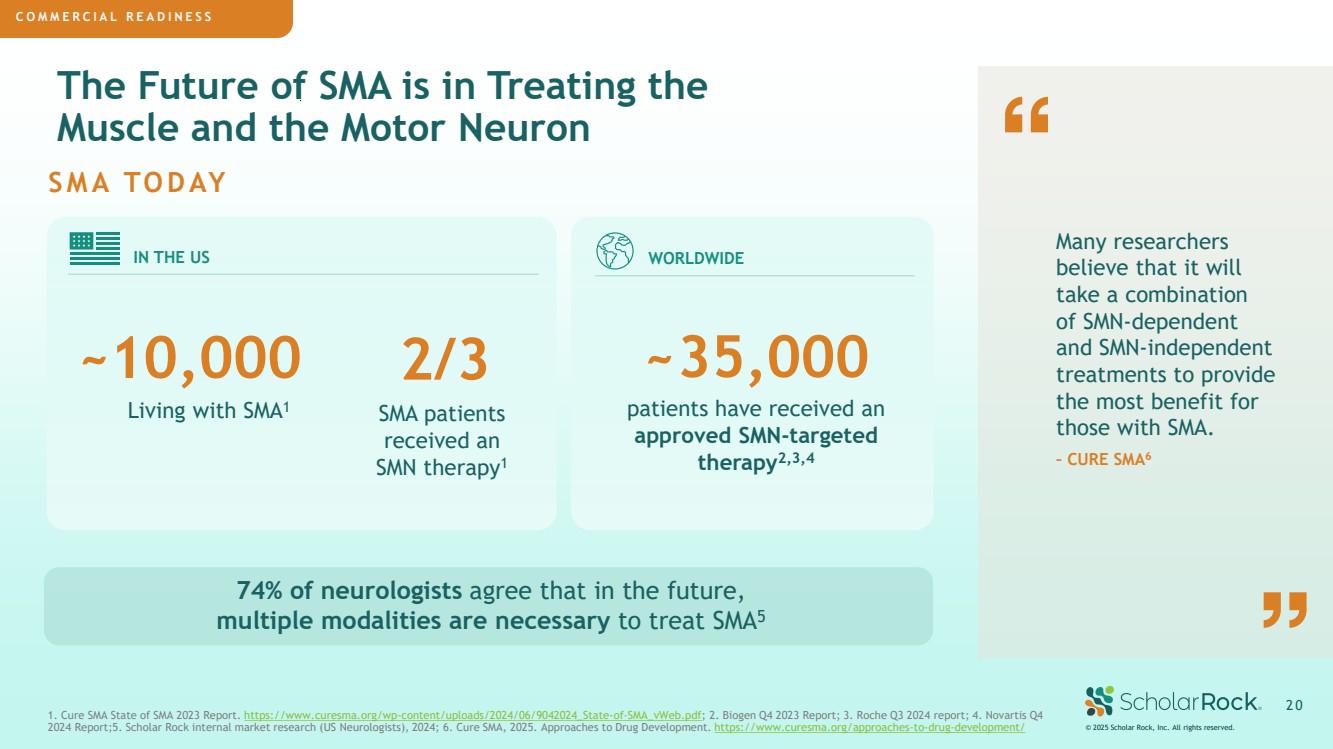
| COMMERCIAL PREPAREDNESS © 2025 Scholar Rock, Inc. All rights reserved. 2 0 1. Cure SMA State of SMA 2023 Report. https://www.curesma.org/wp-content/uploads/2024/06/9042024_State-of-SMA_vWeb.pdf; 2. Biogen Q4 2023 Report; 3. Roche Q3 2024 report; 4. Novartis Q4 2024 Report;5. Scholar Rock internal market research (US Neurologists), 2024; 6. Cure SMA, 2025. Approaches to Drug Development. https://www.curesma.org/approaches-to-drug-development/ IN THE US WORLDWIDE ~10,000 The Future of SMA is in Treating the Muscle and the Motor Neuron Many researchers believe that it will take a combination of SMN-dependent and SMN-independent treatments to provide the most benefit for those with SMA. – CURE SMA6 74% of neurologists agree that in the future, multiple modalities are necessary to treat SMA5 SMA TODAY COMMERCIAL READINESS 2/3 patients have received an approved SMN-targeted therapy2,3,4 Living with SMA1 SMA patients received an SMN therapy1 ~35,000 |
| © 2025 Scholar Rock, Inc. All rights reserved. 2 1 Scholar Rock FINANCIALS Vikas Sinha Chief Financial Officer © 2025 Scholar Rock, Inc. All rights reserved. |
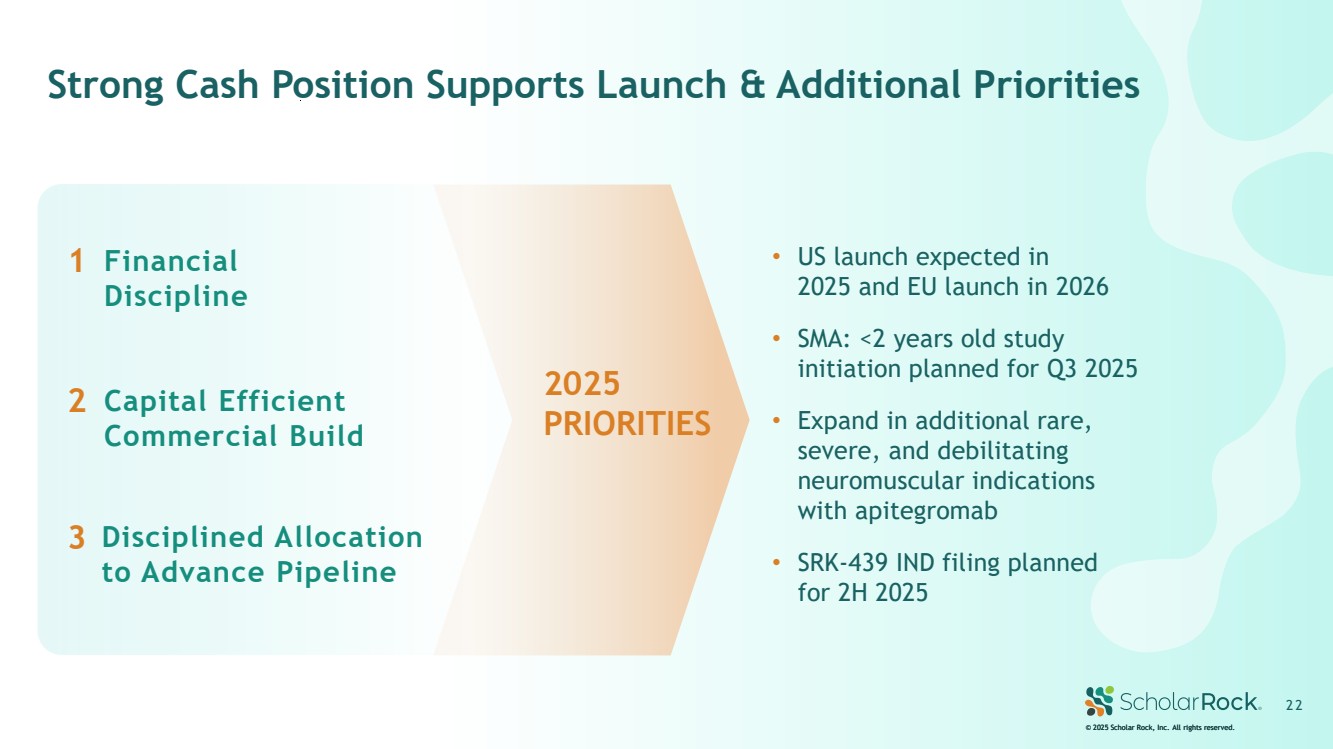
| SRK-439 is a novel, investigational, preclinical myostatin inhibitor © 2025 Scholar Rock, Inc. All rights reserved. 2 2 Strong Cash Position Supports Launch & Additional Priorities Capital Efficient Commercial Build Financial Discipline Disciplined Allocation to Advance Pipeline 1 2 3 • US launch expected in 2025 and EU launch in 2026 • SMA: <2 years old study initiation planned for Q3 2025 • Expand in additional rare, severe, and debilitating neuromuscular indications with apitegromab • SRK-439 IND filing planned for 2H 2025 2025 PRIORITIES |
| *Pending regulatory approval 2025 Priorities Support Long-Term Growth • Execute Successful US Commercial Launch* • Advance EU Launch Preparedness • Efficient Commercial Build • Phase Investments to Support Future High-value Commercial & Pipeline Initiatives Expand into Additional Rare, Severe & Debilitating Neuromuscular Diseases Apitegromab in SMA Regulatory Approvals & Commercialization Disciplined Capital Allocation • Apitegromab Development Program: Building a Pipeline in a Product • Leverage Highly Innovative Anti-myostatin Platform © 2025 Scholar Rock, Inc. All rights reserved. 2 3 |
| Q&A © 2025 Scholar Rock, Inc. All rights reserved. 2 4 |