
Exhibit 99.1

Genprex Issues Stockholder Letter and Provides 2025 Corporate Update
Company Achieves Multiple Clinical Development Milestones in 2025
Patient Treatment Continues in Two Lung Cancer Clinical Trials
AUSTIN, Texas — (Aug. 4, 2025) — Genprex, Inc. (“Genprex” or the “Company”) (NASDAQ: GNPX), a clinical-stage gene therapy company focused on developing life-changing therapies for patients with cancer and diabetes, today announced that it has issued a stockholder letter and corporate update summarizing the Company’s recent achievements across its oncology and diabetes programs and outlines key milestones for 2025 and beyond.
“Genprex has made significant progress across our technology programs this past year, and I am enthusiastic about Genprex’s future as we continue to advance our clinical development program while maintaining streamlined, focused strategies that are important to build value across the entire company,” said Ryan Confer, President and Chief Executive Officer. “We remain dedicated to transforming lives of patients battling cancer and diabetes through our novel gene therapies, and we look forward to executing on a number of upcoming milestones in the second half of 2025 and beyond.”
To read the stockholder letter in its entirety, a digital copy of the Company’s stockholder letter can be found on the Company’s website, www.genprex.com.
About Genprex, Inc.
Genprex, Inc. is a clinical-stage gene therapy company focused on developing life-changing therapies for patients with cancer and diabetes. Genprex’s technologies are designed to administer disease-fighting genes to provide new therapies for large patient populations with cancer and diabetes who currently have limited treatment options. Genprex works with world-class institutions and collaborators to develop drug candidates to further its pipeline of gene therapies in order to provide novel treatment approaches. Genprex’s oncology program utilizes its systemic, non-viral Oncoprex® Delivery System which encapsulates the gene-expressing plasmids using lipid-based nanoparticles in a lipoplex form. The resultant product is administered intravenously, where it is taken up by tumor cells that then express tumor suppressor proteins that were deficient in the tumor. The Company’s lead product candidate, Reqorsa® Gene Therapy (quaratusugene ozeplasmid), is being evaluated in two clinical trials as a treatment for NSCLC and SCLC. Each of Genprex’s lung cancer clinical programs has received a Fast Track Designation from the FDA for the treatment of that patient population, and Genprex’s SCLC program has received an FDA Orphan Drug Designation. Genprex’s diabetes gene therapy approach is comprised of a novel infusion process that uses an AAV vector to deliver Pdx1 and MafA genes directly to the pancreas. In models of Type 1 diabetes, GPX-002 transforms alpha cells in the pancreas into functional beta-like cells, which can produce insulin but may be distinct enough from beta cells to evade the body’s immune system. In a similar approach for Type 2 diabetes, where autoimmunity is not at play, GPX-002 is believed to rejuvenate and replenish exhausted beta cells.
Interested investors and shareholders are encouraged to sign up for press releases and industry updates by visiting the Company Website, registering for Email Alerts and by following Genprex on Twitter, Facebook and LinkedIn.
Cautionary Language Concerning Forward-Looking Statements
Statements contained in this press release regarding matters that are not historical facts are "forward-looking statements" within the meaning of the Private Securities Litigation Reform Act of 1995. These forward-looking statements are made on the basis of the current beliefs, expectations and assumptions of management, are not guarantees of performance and are subject to significant risks and uncertainty. These forward-looking statements should, therefore, be considered in light of various important factors, including those set forth in Genprex’s reports that it files from time to time with the Securities and Exchange Commission and which you should review, including those statements under “Item 1A – Risk Factors” in Genprex’s Annual Report on Form 10-K for the year ended December 31, 2024.
Because forward-looking statements are subject to risks and uncertainties, actual results may differ materially from those expressed or implied by such forward-looking statements. Such statements include, but are not limited to, statements regarding: Genprex’s ability to advance the clinical development, manufacturing and commercialization of its product candidates in accordance with projected timelines and specifications; the timing and success of Genprex’s clinical trials, its intended regulatory submissions and any resulting regulatory approvals; the effect of Genprex’s product candidates, alone and in combination with other therapies, on cancer and diabetes; Genprex’s future growth and financial status, including Genprex’s ability to maintain compliance with the continued listing requirements of The Nasdaq Capital Market and to continue as a going concern and to obtain capital to meet its long-term liquidity needs on acceptable terms, or at all; Genprex’s commercial and strategic partnerships, including those with its third party vendors, suppliers and manufacturers and their ability to successfully perform and scale up the manufacture of its product candidates; Genprex’s intellectual property and licenses; and Genprex’s current expectations, estimates, forecasts and projections about the industry and markets in which it operates.
These forward-looking statements should not be relied upon as predictions of future events and Genprex cannot assure you that the events or circumstances discussed or reflected in these statements will be achieved or will occur. If such forward-looking statements prove to be inaccurate, the inaccuracy may be material. You should not regard these statements as a representation or warranty by Genprex or any other person that Genprex will achieve its objectives and plans in any specified timeframe, or at all. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date of this press release. Genprex disclaims any obligation to publicly update or release any revisions to these forward-looking statements, whether as a result of new information, future events or otherwise, after the date of this press release or to reflect the occurrence of unanticipated events, except as required by law.
Genprex, Inc.
(877) 774-GNPX (4679)
GNPX Investor Relations
investors@genprex.com
GNPX Media Contact
Kalyn Dabbs
media@genprex.com

August 2025
To my fellow stockholders:
Genprex has made significant progress across our technology programs and administratively this past year, and we have had a strong start to the 2025 year. I am extremely proud of the entire Genprex team as we have worked tirelessly to advance our pipeline assets in oncology and diabetes. I am enthusiastic about our future as we continue to advance our clinical development program while maintaining streamlined, focused strategies that are important to build value across the entire company. We continue to evaluate ways to optimize our clinical and research programs and operational strategies, as part of our ongoing prioritization initiative. Additionally, we are considering various strategic alternatives and opportunities to enhance stockholder value. Importantly, we remain focused on our mission, which guides, inspires and fuels everything that we do.
A Strong Start to 2025:
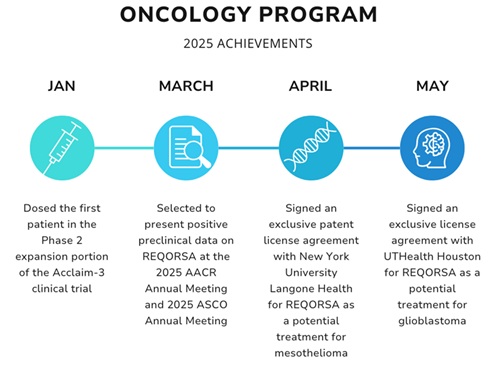
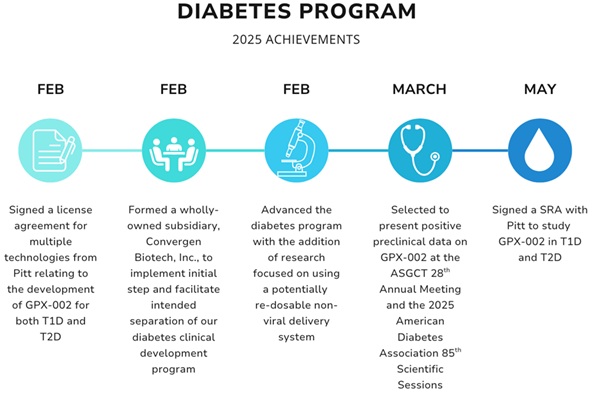
I am proud of the fact that our team is off to such a strong start in 2025 by achieving several key milestones so far this year, including:
3300 Bee Cave Road | Suite 650-227 | Austin, Texas 78746 | 1-877-774-GNPX | www.genprex.com

Oncology Program
In the second half of 2024, we announced plans to re-focus the oncology clinical development program in order to best support the most expeditious route to regulatory submissions for Reqorsa® Gene Therapy (quaratusugene ozeplasmid). This re-focus included the prioritization of resources to focus on the most promising aspects of the Acclaim-1 and Acclaim-3 lung cancer clinical trials. Both of the indications being studied in these Acclaim clinical trials have received U.S. Food and Drug Administration (FDA) Fast Track Designation, and REQORSA also has FDA Orphan Drug Designation for the treatment of small cell lung cancer (SCLC).
Acclaim-1
| We are currently enrolling and treating patients in the Phase 2a expansion portion of our Phase 1/2 Acclaim-1 clinical trial. The Acclaim-1 trial is evaluating the combination of REQORSA and AstraZeneca’s Tagrisso® (osimertinib) to treat patients with late-stage non-small cell lung cancer (NSCLC) who have activating EGFR mutations and disease progression after treatment with Tagrisso. Our Phase 2a expansion study follows the successful completion of the Phase 1 dose escalation portion of the study, which showed REQORSA was generally well tolerated with no dose limiting toxicities despite doubling the starting dose. Importantly, the results showed early signs of efficacy with some patients experiencing prolonged progression free survival and one patient having a partial response. |
 |
3300 Bee Cave Road | Suite 650-227 | Austin, Texas 78746 | 1-877-774-GNPX | www.genprex.com

As a part of our re-focused strategy, we updated the Phase 2a clinical trial design to combine treatment of ES-SCLC patients progressing on Tagrisso alone and those progressing on Tagrisso-containing regimens into one Phase 2a group.
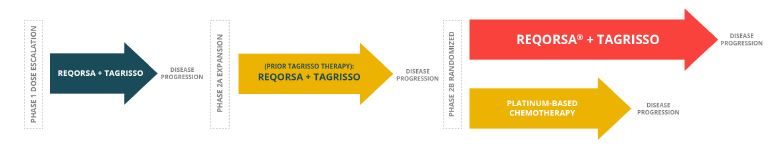
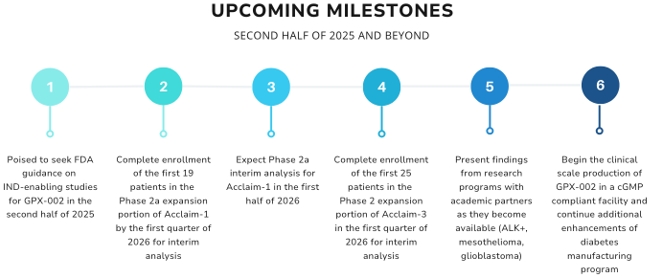
The Phase 2a expansion portion of the trial, consisting of one patient cohort, is expected to enroll approximately 33 patients who have previously received Tagrisso treatment and will determine the safety profile of patients with different eligibility criteria, and it will evaluate efficacy and other endpoints. Our team will conduct an interim analysis following the treatment of 19 patients. We expect to complete the Phase 2 expansion enrollment needed for interim analysis (19 patients) by the end of the first quarter of 2026, and thus we expect to report on our interim analysis in the first half of 2026.
We believe the expansion portion of this study will provide early insight into drug effectiveness and increase the likelihood of a successful randomized Phase 2b trial, which will follow the expansion portion study.
Acclaim-3
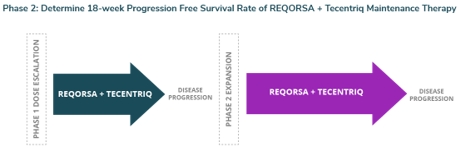
| Similarly, we are currently enrolling and treating patients in the Phase 2 expansion portion of our Phase 1/2 Acclaim-3 clinical trial. Our Acclaim-3 clinical trial uses a combination of REQORSA and Genentech’s Tecentriq® (atezolizumab) as maintenance therapy in patients with extensive stage small cell lung cancer (ES-SCLC) who are candidates for maintenance therapy after receiving Tecentriq and chemotherapy as standard of care initial treatment. In our study, patients will be treated with REQORSA and Tecentriq until disease progression or unacceptable toxicity is experienced. Our Phase 2 expansion study follows the successful completion of the Phase 1 dose escalation portion of the study, which showed REQORSA was generally well tolerated. There were no dose limiting toxicities, and in Acclaim-3, the Phase 2 patients are receiving the same dose of REQORSA as patients in the Phase 2 portion of Acclaim-1. |
 |
3300 Bee Cave Road | Suite 650-227 | Austin, Texas 78746 | 1-877-774-GNPX | www.genprex.com

The Phase 2 expansion portion is expected to enroll approximately 50 patients at approximately 10-15 U.S. sites. Patients will be treated with REQORSA and Tecentriq until disease progression or unacceptable toxicity is experienced. The primary endpoint of the Phase 2 portion is to determine the 18-week progression-free survival rate from the time of the start of maintenance therapy with REQORSA and Tecentriq in patients with ES-SCLC. Patients will also be followed for survival. A Phase 2 interim analysis will be performed after the 25th patient enrolled and treated reaches 18 weeks of follow up. We expect to complete enrollment of the first 25 patients for interim analysis in the Phase 2 expansion portion of the study in the first quarter of 2026.
Establishing Biomarkers to Enrich Clinical Trial Patient Populations
Continuing with our efforts to most expeditiously route REQORSA to regulatory submission, we are collaborating with researchers at The University of Texas MD Anderson Cancer Center (MD Anderson) to discover, develop and utilize biomarkers to:
|
● |
select the patient population most likely to respond to REQORSA; and |
|
|
● |
enable decisions on progression of REQORSA to the next phase of development. |
MD Anderson researchers currently are analyzing biomarkers that may indicate a strong positive or negative response to REQORSA in lung cancer that could be used to enrich the population of responders in our clinical trials.
3300 Bee Cave Road | Suite 650-227 | Austin, Texas 78746 | 1-877-774-GNPX | www.genprex.com

2025 American Association of Cancer Research (AACR)
We continue to explore how REQORSA may be beneficial for patients with specific types of lung cancer, other cancers and other diseases through our strong partnerships with academic institutions and our preclinical programs.
|
At the recent 2025 AACR annual meeting in April, our research collaborators at MD Anderson presented positive preclinical data for REQORSA for the treatment of KRASG12C mutant NSCLC. In the poster presentation, entitled “Overcoming sotorasib acquired resistance in KRASG12C mutant NSCLC by TUSC2 gene therapy,” researchers demonstrated that TUSC2 gene therapy (REQORSA) effectively overcomes sotorasib Acquired Resistance (AR) in KRASG12C mutant NSCLC mouse xenografts. |
 |
|
● |
REQORSA, alone or in combination with sotorasib, induced apoptosis, inhibited colony formation, and showed significant antitumor efficacy in KRASG12C mutant sotorasib-AR xenograft and PDX tumors. |
|
|
● |
Treatment with REQORSA alone was highly effective in controlling tumor growth both in xenografts from a cell line with acquired resistance to sotorasib and in xenografts derived from a patient’s lung cancer. |
|
|
● |
For treatment of xenografts with acquired resistance to sotorasib, REQORSA alone was just as effective in controlling tumor growth as the combination of REQORSA and sotorasib. |
A copy of the 2025 AACR poster can be found on Genprex’s website here.
2025 American Society of Clinical Oncology (ASCO) Annual Meeting
| Additionally, Genprex was selected to present the trial design of the Acclaim-3 clinical trial at the 2025 ASCO Annual Meeting for the Trials in Progress portion of the conference. The poster, entitled, “A phase 1/2 clinical trial of quaratusugene ozeplasmid gene therapy and atezolizumab maintenance therapy in patients with extensive stage small cell lung cancer (ES-SCLC)” provides an overview of Acclaim-3. Bo Wang, MD, at Oncology Associates of Oregon, presented the poster before an audience of organizations and industry leaders committed to shaping the future of cancer care. A copy of the 2025 ASCO Trials in Progress poster can be found on Genprex’s website here. |
 |
3300 Bee Cave Road | Suite 650-227 | Austin, Texas 78746 | 1-877-774-GNPX | www.genprex.com

Expansion of Intellectual Property Portfolio to Support Preclinical Programs
We are very excited and proud of the research being done in our preclinical programs with many academic partners. In 2025 to date, we have signed two exclusive license agreements related to REQORSA, one with New York University Langone Health for REQORSA as a potential treatment for mesothelioma and another with UTHealth Houston for REQORSA as a potential treatment for glioblastoma.
These license agreements strengthen our intellectual property portfolio for REQORSA, enabling us to expand our research programs to continue exploring how REQORSA may serve as a therapeutic treatment for some of the most difficult to treat cancers and diseases. These agreements position Genprex to expand our clinical development pipeline with future clinical studies within the scope of our licensed patents.
Studying REQORSA as a Treatment for Mesothelioma
Expression of TUSC2, which is the tumor suppressor gene used in REQORSA, is downregulated in 84% of mesotheliomas. Research collaborators at NYU presented positive preclinical data from a study of REQORSA for the treatment of mesothelioma at the 2024 EORTC-NCI-AACR Symposium on Molecular Targets and Cancer Therapeutics.
|
● |
In this study, the researchers demonstrated that REQORSA treatment resulted in a significant decrease in cell proliferation, cell invasion, and a significant increase in cell apoptosis in four Malignant Pleural Mesothelioma (MPM) cell lines. |
|
|
● |
Data also demonstrated potent tumor suppressive activity of the TUSC2 gene delivered by REQORSA, and thus, its re-expression could serve as a potential therapeutic strategy for the treatment of MPM. |
A copy of the poster presented at the 2024 EORTC-NCI-AACR Symposium on Molecular Targets and Cancer Therapeutics conference can be found on Genprex’s website here.
3300 Bee Cave Road | Suite 650-227 | Austin, Texas 78746 | 1-877-774-GNPX | www.genprex.com

Studying REQORSA as a Treatment for Glioblastoma
Genprex also reported positive preclinical data on the efficacy of REQORSA in glioblastoma at the 2024 EORTC-NCI-AACR Symposium on Molecular Targets and Cancer Therapeutics. Research collaborators from UTHealth Houston reported TUSC2 as a novel tumor suppressor for glioblastoma, the most common and deadliest primary brain tumor in adults. In their latest study, UTHealth Houston researchers used patient-derived glioblastoma (GBM) cell lines and patient-derived glioma stem cell (PD-GSC) lines. REQORSA was used to restore TUSC2 expression.
|
● |
Researchers at UTHealth Houston observed that REQORSA significantly reduced GBM cell viability, and the results of a migration assay demonstrated that REQORSA suppressed GBM cell migration independent of its ability to suppress cell viability. |
|
|
● |
REQORSA demonstrated promising in vitro efficacy in GBM and PD-GSC cell lines, and these results support further evaluation of its in vivo anti-tumor efficacy in malignant gliomas using mouse models. |
A copy of the poster presented at the 2024 EORTC-NCI-AACR Symposium on Molecular Targets and Cancer Therapeutics conference can be found on Genprex’s website here.
Studying REQORSA as a Treatment for ALK-Positive Lung Cancer
In October 2024, we signed a Sponsored Research Agreement (SRA) with the University of Michigan Rogel Cancer Center to study TUSC2, the tumor suppressor gene used in REQORSA, in combination with ALK-inhibitors in ALK-EML4 positive (ALK+) translocated lung cancer. Through this SRA, we also announced our collaboration with ALK Positive, a non-profit patient-driven research organization dedicated to improving the life expectancy and quality of life for ALK+ lung cancer patients.
In November 2024, we signed an exclusive license agreement with the University of Michigan for REQORSA as a potential treatment for ALK-EML4 positive (ALK+) translocated lung cancer. This license, too, strengthens our intellectual property portfolio and provides us with protection for the drug combination of REQORSA with ALK inhibitors, widening our exclusivity of drug combinations for REQORSA.
REQORSA in combination with ALK inhibitors could be a potential therapeutic treatment for ALK+ lung cancer. Research collaborators at the University of Michigan Rogel Cancer Center’s Judith Tam ALK Lung Cancer Research Initiative presented positive preclinical data at the April 2024 AACR Annual Meeting, reporting that REQORSA induced apoptosis in alectinib resistant EML4-ALK positive non-small cell lung cancer (NSCLC) cell lines.
3300 Bee Cave Road | Suite 650-227 | Austin, Texas 78746 | 1-877-774-GNPX | www.genprex.com

|
● |
The study found that the use of REQORSA or a TUSC2-containing plasmid to overexpress TUSC2 in ALK+ NSCLC cell lines was effective in decreasing cell growth and proliferation through the activation of apoptotic pathways. |
|
|
● |
Researchers believe the results of this preclinical work support further clinical study of REQORSA as an anti-ALK NSCLC treatment strategy. |
|
|
● |
Genprex believes this research suggests that REQORSA may be an effective treatment in patients progressing on ALK inhibitors. |
To review the poster presented at the 2024 AACR Annual meeting, visit Genprex’s website.
Studying REQORSA as a Single Agent Drug
As a further bolster to our preclinical program, we are also researching REQORSA as a single agent drug; this means we are studying REQORSA as a stand-alone treatment. While data to date has demonstrated that REQORSA is synergistic with other cancer drugs, we believe REQORSA has potential to fight cancer and other diseases as a therapeutic treatment on its own. Not only are we researching REQORSA as a single agent as a potential treatment for mesothelioma and glioblastoma with NYU and UTHealth Houston, but we have also partnered with Meharry Medical College to study how REQORSA alone modulates the immune response against cancer.
Research collaborators at Meharry Medical College presented positive preclinical data on REQORSA as a single agent at the 39th Annual Society for Immunotherapy of Cancer (SITC) Meeting in November 2024.
The poster, entitled, “TUSC2 Modulates Cancer Immune Responses” demonstrated REQORSA treatment leads to increased immune response against tumors in TUSC2 Knock Out (KO) mice.
|
● |
Researchers used comparative flow cytometry analysis of splenocytes, lymph nodes cells, and tumor infiltrating leucocytes (TILs) from TUSC2 KO and TUSC2 Wild Type (WT) mice. FACS analysis was focused on the differences in T reg, Cytotoxic T cells and NK cells between the two mouse groups. |
|
|
● |
For analysis of immune responses to tumors, researchers challenged two groups of TUSC2 KO mice with syngeneic 129 Sv background metastatic lung cancer 344SQ cells and injected one group with REQORSA. |
|
|
● |
In the REQORSA supplemented group, the tumor growth was diminished. A significant reduction in T reg and significant increase in Cytotoxic T cells and NK cells were observed within TILs. A significant increase in Granzyme B expression within Cytotoxic T cells and NK cells was shown in REQORSA supplemented mice compared to the control group. |
3300 Bee Cave Road | Suite 650-227 | Austin, Texas 78746 | 1-877-774-GNPX | www.genprex.com

A copy of the 2024 SITC poster can be found on Genprex’s website here.
Diabetes Program
I am very excited with the progress of our diabetes program this past year, and I am passionate about our aim of bringing our drug candidate, GPX-002, to market for diabetic patients.
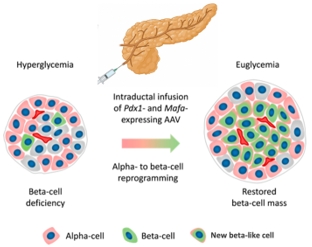
GPX-002 is currently being developed using the same construct for the treatment of both Type 1 diabetes (T1D) and Type 2 diabetes (T2D). The same general novel approach is used in each of T1D and T2D whereby an adeno-associated virus (AAV) vector containing the Pdx1 and MafA genes is administered directly into the pancreatic duct. In humans, this can be done with a routine endoscopy procedure.
|
In T1D, GPX-002 is designed to work by transforming alpha cells in the pancreas into functional beta-like cells, which can produce insulin but may be distinct enough from beta cells to evade the body’s immune system. In vivo, preclinical studies show that GPX-002 restored normal blood glucose levels for an extended period of time in T1D mouse models. In T2D, where autoimmunity is not at play, GPX-002 is believed to rejuvenate and replenish exhausted beta cells.
We have finalized components of the diabetes construct, and these technologies are currently being evaluated in preclinical studies at the University of Pittsburgh (Pitt). We plan to request FDA’s guidance for the preclinical studies needed to file an Investigational New Drug (IND) application and initiate first-in-human studies, and we believe we are poised to seek further FDA guidance on IND-enabling studies in the second half of 2025.
Researching a Non-Viral Delivery System |
 |
Another way that we are advancing the diabetes program is through our strategic collaboration with a contract development and manufacturing organization (CDMO) to research an alternative second-generation approach for GPX-002 using a non-viral lipid nanoparticle delivery system. This collaboration with our CDMO partner is separate from our existing preclinical research programs and seeks to evaluate potential next generation construct optimization.
3300 Bee Cave Road | Suite 650-227 | Austin, Texas 78746 | 1-877-774-GNPX | www.genprex.com

Many gene therapies rely on viral based delivery systems. The benefit of the viral system is that viruses are skilled at penetrating cells. However, a viral capsid cannot be readministered, due to the development of an immune response against this delivery vehicle.
This exploratory research represents our forward-thinking approach within our diabetes program to determine if our novel diabetes gene therapy could be delivered using a non-viral delivery system, similar to the lipid nanoparticle approach used in our oncology program. While AAV delivery is a well understood delivery mechanism, there are many benefits to a non-viral delivery system, including the potential for re-dosing patients to optimize treatment. The benefit of re-dosing could be significant for those patients with T1D, where the patient’s immune system attacks insulin-producing beta cells.
We believe this research and collaboration position us as a leader in gene therapy and within the diabetes market.
License Agreement with the University of Pittsburgh
Earlier this year, we signed a new license agreement with Pitt, which updates and consolidates most of our licensed technologies from Pitt into a single updated agreement, replacing the prior license agreements with Pitt. The latest exclusive license agreement strengthens our intellectual property portfolio for the diabetes gene therapy program and provides us with valuable rights over multiple diabetes gene therapy combinations that we believe have the potential to disrupt the diabetes market.
Sponsored Research Agreement with the University of Pittsburgh
We recently signed a new SRA with Pitt to study GPX-002 in T1D and T2D in animal models. After completing our first SRA with Pitt, which produced data in mouse and non-human primate (NHP) models of T1D, we have now expanded the SRA to study both T1D and T2D in NHPs. We believe these studies set us on a path toward human clinical trials.
3300 Bee Cave Road | Suite 650-227 | Austin, Texas 78746 | 1-877-774-GNPX | www.genprex.com

Formation of Convergen Biotech, Inc., a Wholly-Owned Subsidiary
This year we formed a wholly-owned subsidiary, Convergen Biotech, Inc., to implement the initial step and facilitate the intended separation of our diabetes program from our oncology program. We believe this intended separation once complete will allow for the enhancement of each program by focusing on the needs of their respective markets and patients. We also believe this separation could expedite clinical development and enable greater opportunity for direct investment and strategic collaboration into the diabetes program.
Technology Transfer of GPX-002
A significant milestone for us is the successful technology transfer of the manufacturing process for the production of GPX-002 from our academic collaborators at Pitt, where it was previously manufactured, to our experienced, integrated network of commercial CDMOs and other vendors. Novel advanced technologies were incorporated in these processes to optimize the plasmid construct to increase stability of expression and modify the backbone to align with other plasmids used for AAV products. The new plasmid was cloned, purified, and manufactured and is currently being used in the manufacture of AAV. This marks significant achievement for us because we can begin the clinical scale production of GPX-002 in a current Good Manufacturing Practices (cGMP) compliant facility. This technology transfer also allows us to accelerate manufacturing processes.
2025 American Society of Gene and Cell Therapy (ASGCT) 28th Annual Meeting
| Our research collaborators at Pitt were selected to present positive preclinical data and research from studies of GPX-002 at the 2025 ASGCT Annual Meeting. In an oral presentation, entitled, “Immune Modulation Sustains Alpha Cell Reprogramming and Mitigates Immune Responses to AAV in a Diabetic Non-Human Primate Model,” researchers discussed a study that evaluated the immune response to direct infusion of recombinant adeno-associated virus (rAAV) into the pancreatic duct of NHPs with streptozotocin-induced diabetes and they evaluated how to best manage immune responses. |
 |
|
● |
One-month post-infusion, NHPs showed improved glucose tolerance and reduced insulin requirements. |
|
● |
Additionally, rAAV gene therapy in these animals remained effective and glucose tolerance continued improving in the presence of immunosuppression (IS). |
3300 Bee Cave Road | Suite 650-227 | Austin, Texas 78746 | 1-877-774-GNPX | www.genprex.com

|
● |
In conclusion, researchers developed a novel rAAV gene therapy approach and demonstrated that infusion of rAAV directly into the pancreatic duct of NHPs induces an anti-viral immune response. The anti-viral immune response in NHPs can be largely prevented by administration of a multi-agent IS and can allow for sustained therapeutic effects. |
2025 American Diabetes Association’s (ADA) 85th Scientific Sessions
| Both our collaborators, including the CDMO working with us on non-viral delivery and our collaborators at Pitt, were selected to present positive preclinical data on GPX-002 at the 2025 American Diabetes Association’s 85th Scientific Sessions. We are thrilled that our collaborators were selected to present new research on our novel gene therapy for diabetes at the largest worldwide scientific meeting for clinicians and researchers in diabetes. |
 |
The first poster presentation, entitled “Selecting Lipid Nanoparticles for Transfection of Islets of Langerhans Cells” provided an overview of an alternative second-generation approach with a non-viral lipid nanoparticle (LNP) delivery system. This research used a patented LNP and a luciferase mRNA payload, resulting in 100 times more luciferase activity in the pancreas than in other organs, demonstrating the specificity of transfection using infusion into the biliary duct/pancreatic duct. Researchers also demonstrated the ability of this LNP to cross the basement membrane in the pancreatic duct and to penetrate into the islets of Langerhans in the pancreas and transfect cells there. We believe we are the first and only company to be doing this type of research in the pancreatic duct using an LNP in place of a viral AAV construct. This research provides the proof of concept needed to move forward with additional studies, and it opens the door for a future second generation approach that would allow multiple dosing of patients.
A copy of this poster can be found on Genprex’s website here.
In an oral presentation, entitled “Recombinant AAV-mediated Gene Therapy For The Treatment Of Streptozotocin-Induced Diabetes in Non-Human Primates” researchers provided an overview of studies that demonstrated infusion of rAAV directly into the pancreatic duct of NHPs leads to transdifferentiation of alpha cells to beta-like cells with restoration of glucose homeostasis. Additionally, the beta-like cells were still providing improved control of glucose levels after three months. Immunosuppression (IS), including steroids, is necessary for a number of months to prevent anti-viral immunity in NHPs.
3300 Bee Cave Road | Suite 650-227 | Austin, Texas 78746 | 1-877-774-GNPX | www.genprex.com

Researchers are continuing preclinical studies of GPX-002 in NHP models of both T1D and T2D to generate additional data, and current studies are evaluating viral efficacy after six months of IS.
What’s Ahead in 2025 and Beyond
We have been working relentlessly to advance our oncology and diabetes clinical programs. We are dedicated to transforming the lives of patients battling cancer and diabetes through our pioneering gene therapies. I believe in our innovative approaches: reintroducing tumor suppressor genes to combat cancer and revitalizing insulin-producing cells to stabilize glucose levels in diabetes. With multiple FDA Fast Track Designations and promising clinical trial results, Genprex is at the forefront of medical advancements, striving to bring hope and improved outcomes to patients worldwide.
As we look ahead to the second-half of 2025 and beyond, we have several key milestones ahead of us, including:
We have never had a time in our Company history where we have had such a robust pipeline. I am confident in the technology achievement we’ve made to date, and I believe we are at a momentous inflection point for Genprex as we execute our strategic plans for the company and achieve potentially value-creating milestones ahead of us. We thank you, our loyal stockholders, for your continued interest and support in our programs. We recognize that you have come along-side us in our mission to bring potentially life-changing gene therapies to patients with cancer and diabetes.
3300 Bee Cave Road | Suite 650-227 | Austin, Texas 78746 | 1-877-774-GNPX | www.genprex.com

With numerous important milestones ahead of us, I am excited for Genprex’s future. I look forward to sharing our future success together.
Sincerely,
Ryan Confer
President and Chief Executive Officer at Genprex, Inc.
Cautionary Language Concerning Forward-Looking Statements
Statements contained in this stockholder letter regarding matters that are not historical facts are "forward-looking statements" within the meaning of the Private Securities Litigation Reform Act of 1995. These forward-looking statements are made on the basis of the current beliefs, expectations and assumptions of management, are not guarantees of performance and are subject to significant risks and uncertainty. These forward-looking statements should, therefore, be considered in light of various important factors, including those set forth in Genprex’s reports that it files from time to time with the Securities and Exchange Commission and which you should review, including those statements under “Item 1A – Risk Factors” in Genprex’s Annual Report on Form 10-K for the year ended December 31, 2024. Because forward-looking statements are subject to risks and uncertainties, actual results may differ materially from those expressed or implied by such forward-looking statements. Such statements include, but are not limited to, statements regarding: Genprex’s assumptions, expectations and beliefs, and Genprex’s plans, intentions and strategies, and its ability to execute the foregoing in accordance with the expected and/or desired results; Genprex’s ability to advance the clinical development, manufacturing and commercialization of its product candidates in accordance with projected timelines and specifications; the timing and success of Genprex’s clinical trials, its intended regulatory submissions and any resulting regulatory approvals; the effect of Genprex’s product candidates, alone and in combination with other therapies, on cancer and diabetes; Genprex’s future growth and financial status, including Genprex’s ability to maintain compliance with the continued listing requirements of The Nasdaq Capital Market and to continue as a going concern and to obtain capital to meet its long-term liquidity needs on acceptable terms, or at all; Genprex’s commercial and strategic partnerships, including those with its third party vendors, suppliers and manufacturers and their ability to successfully perform and scale up the manufacture of its product candidates; Genprex’s intellectual property and licenses; and Genprex’s current expectations, estimates, forecasts and projections about the industry and markets in which it operates. These forward-looking statements should not be relied upon as predictions of future events and Genprex cannot assure you that the events or circumstances discussed or reflected in these statements will be achieved or will occur. If such forward-looking statements prove to be inaccurate, the inaccuracy may be material. You should not regard these statements as a representation or warranty by Genprex or any other person that Genprex will achieve its objectives and plans in any specified timeframe, or at all. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date of this press release. Genprex disclaims any obligation to publicly update or release any revisions to these forward-looking statements, whether as a result of new information, future events or otherwise, after the date of this press release or to reflect the occurrence of unanticipated events, except as required by law.
3300 Bee Cave Road | Suite 650-227 | Austin, Texas 78746 | 1-877-774-GNPX | www.genprex.com