

Obicetrapib in Alzheimer’s Disease (AD): First-in-Class Evidence from BROADWAY July 30, 2025

Disclaimer This presentation (together with oral statements made in connection herewith, this “Presentation”) is for informational purposes only. Forward Looking Statements Certain statements included in this presentation (together with oral statements made in connection herewith, this “Presentation”) that are not historical facts are forward-looking statements for purposes of the safe harbor provisions under the United States Private Securities Litigation Reform Act of 1995. Forward-looking statements generally are accompanied by words such as “believe,” “may,” “will,” “estimate,” “continue,” “anticipate,” “intend,” “expect,” “should,” “would,” “plan,” “predict,” “potential,” “seem,” “seek,” “future,” “outlook,” "aim" and similar expressions that predict or indicate future events or trends or that are not statements of historical matters. These forward-looking statements include, but are not limited to, statements by NewAmsterdam Pharma Company N.V. (“NewAmsterdam” or the “Company”) regarding estimates and forecasts of financial and performance metrics and projections of market opportunity; the Company's business and strategic plans; expectations and timing related to the success, cost and timing of product development activities, including timing of initiation, completion and data readouts for clinical trials and the potential approval of the Company’s product candidate; the timing for enrolling patients in clinical trials; the size and growth potential of the markets for the Company’s product candidate; the Company's ability to achieve the broad degree of physician adoption and use and market acceptance necessary for commercial success; the therapeutic potential of the Company’s product candidate including without limitation, its potential to favorably impact AD biomarkers and its potential to reduce neurodegenerative and heart disease risks; the Company’s plans to discuss the results of the AD analysis with regulatory authorities to determine next steps; financing and other business milestones; and the Company’s plans for commercialization. These statements are based on various assumptions, whether or not identified in this Presentation, and on the current expectations of the Company’s management and are not predictions of actual performance. These forward-looking statements are provided for illustrative purposes only and are not intended to serve as and must not be relied on as a guarantee, an assurance, a prediction, or a definitive statement of fact or probability. Actual events and circumstances are difficult or impossible to predict and may differ from assumptions. Many actual events and circumstances are beyond the control of the Company. These forward-looking statements are subject to a number of risks and uncertainties, including changes in domestic and foreign business, market, financial, political, and legal conditions; risks related to the approval of the Company’s product candidate and the timing of expected regulatory and business milestones; whether topline, initial or preliminary results from a particular clinical trial will be predictive of the final results of that trial and whether results of the AD analysis and other early clinical trials will be indicative of the results of later clinical trials; the potential for varying interpretations of the Alzheimer's disease analysis results and the results of other trials; uncertainty regarding outcomes of the Company’s ongoing clinical trials, particularly as they relate to regulatory review and potential approval of the Company’s product candidate; risks associated with the Company’s efforts to commercialize its product candidates; the impact of competing product candidates on the Company’s business and prospects; intellectual property-related claims; the Company’s ability to attract and retain qualified personnel; and the Company’s ability to continue to source the raw materials for its product candidates and manufacturer final product; and those risks, uncertainties and other factors discussed under the caption "Item 1A. Risk Factors" and elsewhere in the Company’s most recent Form 10-K, Form 10-Q and other public filings with the Securities and Exchange Commission - which are available at www.sec.gov. If any of these risks materialize or NewAmsterdam’s assumptions prove incorrect, actual results could differ materially from the results implied by these forward-looking statements. There may be additional risks that are presently unknown by the Company or that the Company currently believes are immaterial that could also cause actual results to differ from those contained in the forward-looking statements. In addition, forward-looking statements reflect NewAmsterdam’s expectations, plans, or forecasts of future events and views as of the date of this Presentation and are qualified in their entirety by reference to the cautionary statements herein. NewAmsterdam anticipates that subsequent events and developments will cause the Company’s assessments to change. These forward-looking statements should not be relied upon as representing NewAmsterdam’s assessments as of any date subsequent to the date of this Presentation. Accordingly, undue reliance should not be placed upon the forward-looking statements. Neither NewAmsterdam nor any of its affiliates undertakes any obligation to update these forward-looking statements, except as required by law. Market Data Certain information contained in this Presentation relates to or is based on third-party studies, publications, data, surveys and NewAmsterdam’s own internal estimates and research. In addition, all of the market data included in this Presentation involves a number of assumptions and limitations, and there can be no guarantee as to the accuracy or reliability of such assumptions. Finally, while NewAmsterdam believes its internal research is reliable, such research has not been verified by any independent source and NewAmsterdam cannot guarantee and makes no representation or warranty, express or implied, as to its accuracy and completeness. Trademarks This Presentation contains trademarks, service marks, trade names, and copyrights of NewAmsterdam and other companies, which are the property of their respective owners. The use or display of third parties’ trademarks, service marks, trade name or products in this Presentation is not intended to, and does not imply, a relationship with NewAmsterdam or an endorsement or sponsorship by or of NewAmsterdam. Solely for convenience, the trademarks, service marks and trade names referred to in this Presentation may appear with the TM or SM symbols, but such references are not intended to indicate, in any way, that NewAmsterdam will not assert, to the fullest extent permitted under applicable law, their rights or the right of the applicable licensor to these trademarks, service marks and trade names.
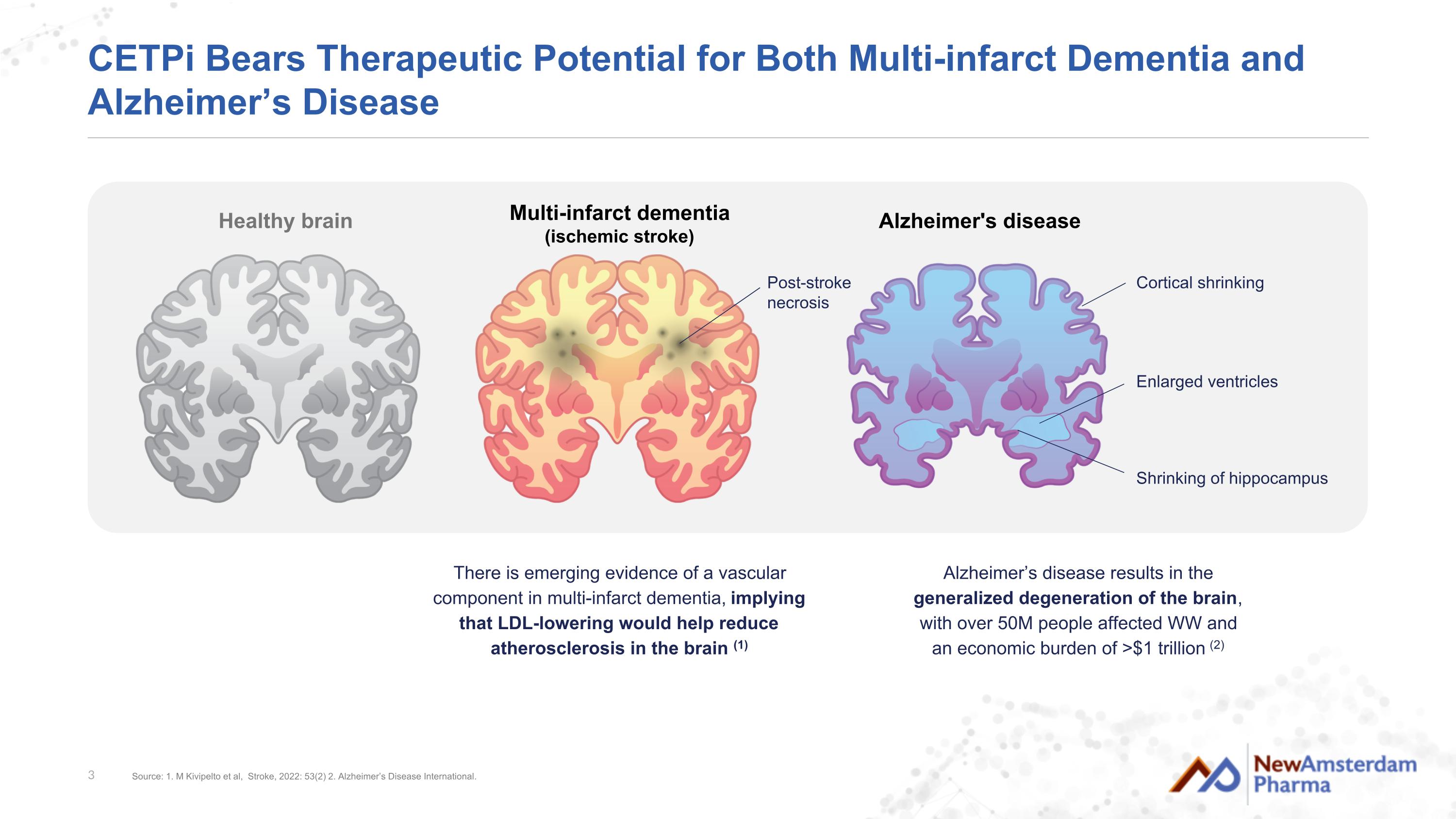
CETPi Bears Therapeutic Potential for Both Multi-infarct Dementia and Alzheimer’s Disease Alzheimer's disease Healthy brain Alzheimer’s disease results in the generalized degeneration of the brain, with over 50M people affected WW and an economic burden of >$1 trillion (2) There is emerging evidence of a vascular component in multi-infarct dementia, implying that LDL-lowering would help reduce atherosclerosis in the brain (1) Multi-infarct dementia (ischemic stroke) Cortical shrinking Enlarged ventricles Shrinking of hippocampus Post-stroke necrosis Source: 1. M Kivipelto et al, Stroke, 2022: 53(2) 2. Alzheimer’s Disease International.
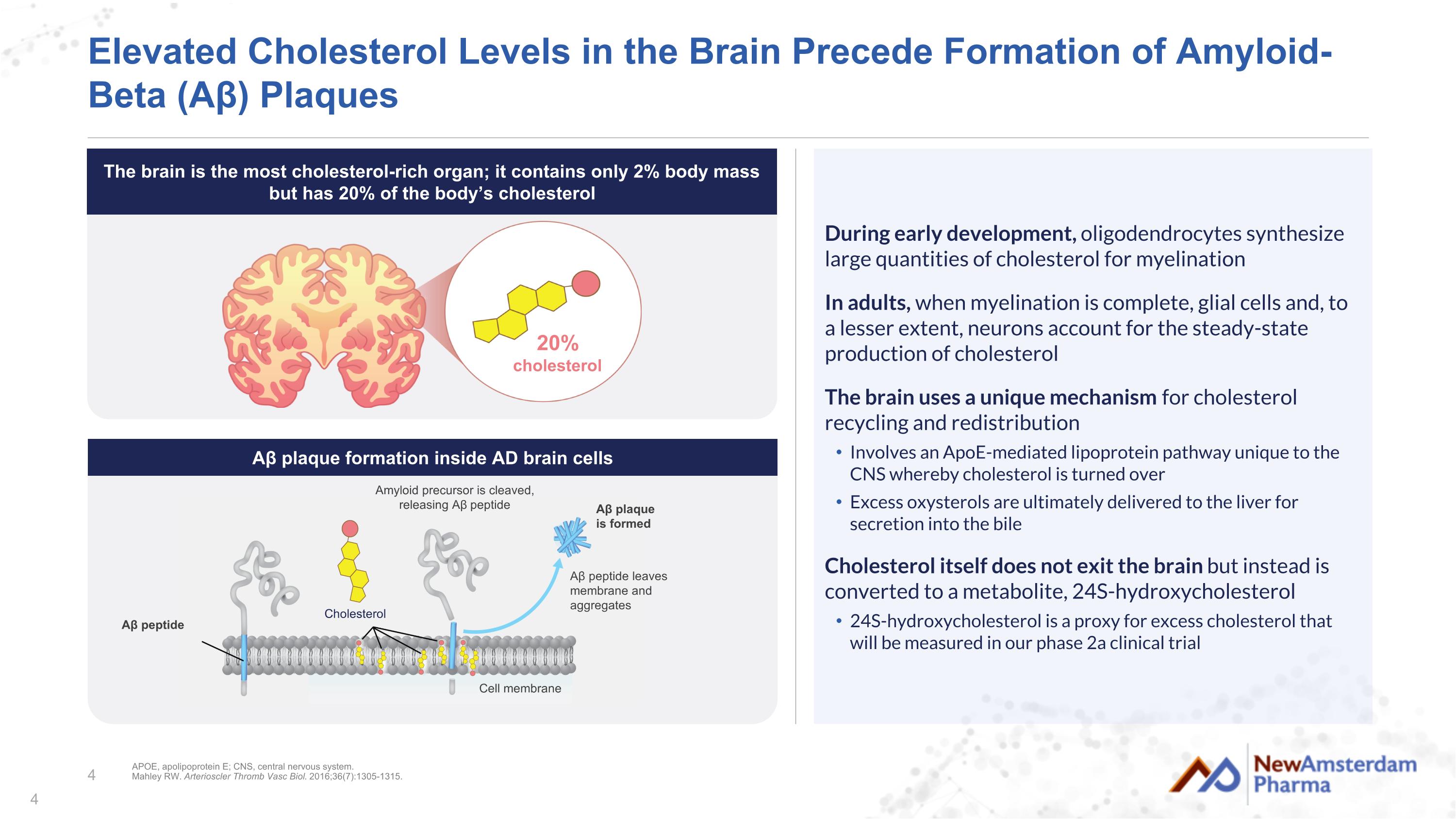
+ Elevated Cholesterol Levels in the Brain Precede Formation of Amyloid-Beta (Aβ) Plaques During early development, oligodendrocytes synthesize large quantities of cholesterol for myelination In adults, when myelination is complete, glial cells and, to a lesser extent, neurons account for the steady-state production of cholesterol The brain uses a unique mechanism for cholesterol recycling and redistribution Involves an ApoE-mediated lipoprotein pathway unique to the CNS whereby cholesterol is turned over Excess oxysterols are ultimately delivered to the liver for secretion into the bile Cholesterol itself does not exit the brain but instead is converted to a metabolite, 24S-hydroxycholesterol 24S-hydroxycholesterol is a proxy for excess cholesterol that will be measured in our phase 2a clinical trial 20% cholesterol APOE, apolipoprotein E; CNS, central nervous system. Mahley RW. Arterioscler Thromb Vasc Biol. 2016;36(7):1305-1315. Amyloid precursor is cleaved, releasing Aβ peptide Aβ peptide leaves membrane and aggregates Aβ plaque is formed Aβ peptide Cholesterol Cell membrane Aβ plaque formation inside AD brain cells The brain is the most cholesterol-rich organ; it contains only 2% body mass but has 20% of the body’s cholesterol
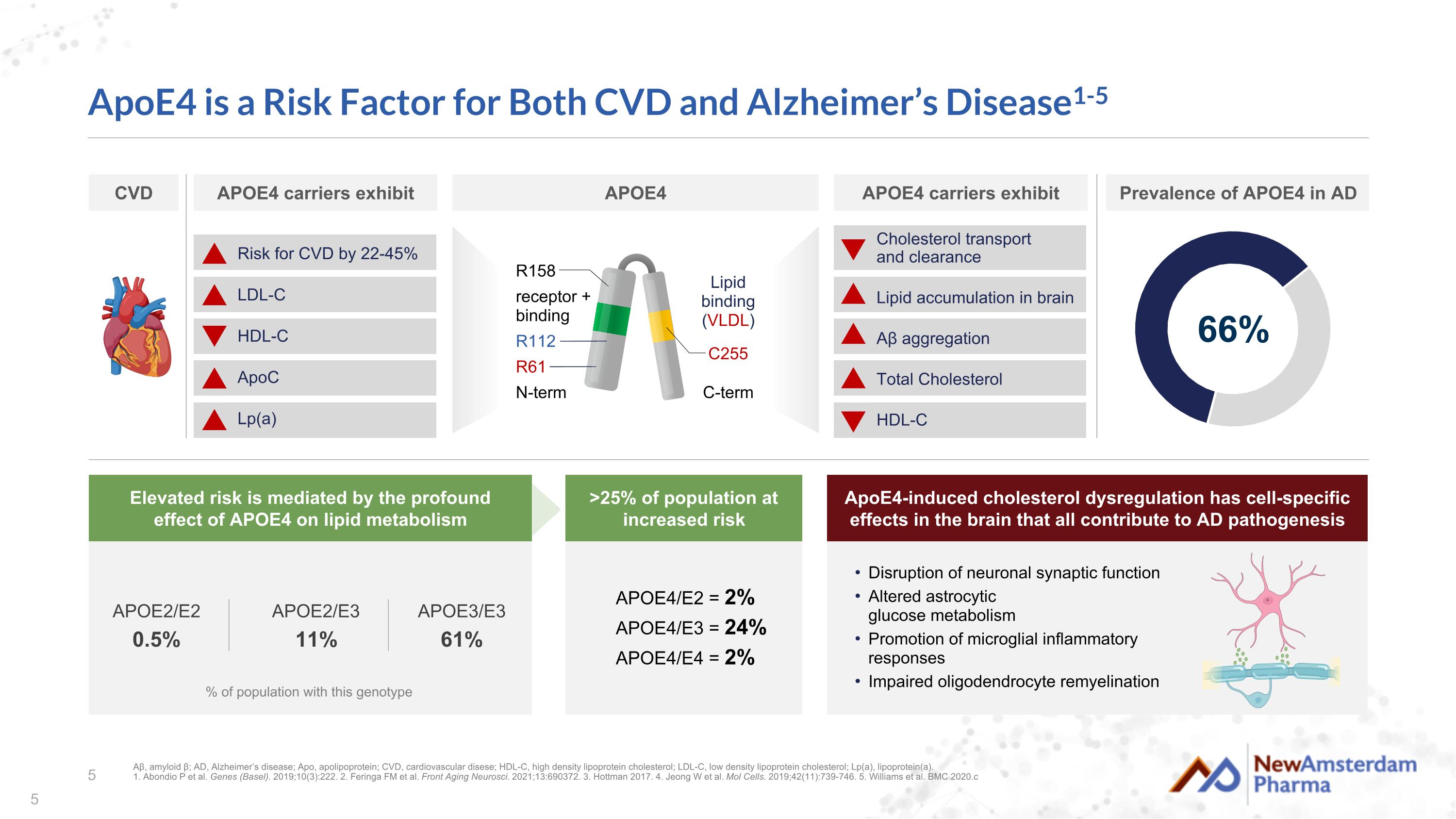
ApoE4 is a Risk Factor for Both CVD and Alzheimer’s Disease1-5 % of population with this genotype APOE2/E3 11% APOE3/E3 61% APOE2/E2 0.5% APOE4/E2 = 2% APOE4/E3 = 24% APOE4/E4 = 2% Cholesterol transport and clearance Lipid accumulation in brain Aβ aggregation Total Cholesterol HDL-C receptor + binding N-term C-term Lipid binding (VLDL) R158 R61 R112 C255 Disruption of neuronal synaptic function Altered astrocytic glucose metabolism Promotion of microglial inflammatory responses Impaired oligodendrocyte remyelination Aβ, amyloid β; AD, Alzheimer’s disease; Apo, apolipoprotein; CVD, cardiovascular disese; HDL-C, high density lipoprotein cholesterol; LDL-C, low density lipoprotein cholesterol; Lp(a), lipoprotein(a). 1. Abondio P et al. Genes (Basel). 2019;10(3):222. 2. Feringa FM et al. Front Aging Neurosci. 2021;13:690372. 3. Hottman 2017. 4. Jeong W et al. Mol Cells. 2019;42(11):739-746. 5. Williams et al. BMC 2020.c 66% APOE4 carriers exhibit Prevalence of APOE4 in AD ApoE4-induced cholesterol dysregulation has cell-specific effects in the brain that all contribute to AD pathogenesis >25% of population at increased risk Elevated risk is mediated by the profound effect of APOE4 on lipid metabolism CVD APOE4 carriers exhibit APOE4 Risk for CVD by 22-45% LDL-C HDL-C ApoC Lp(a)
BROADWAY AD Biomarker Analysis: Design and Baseline Characteristics AD, Alzheimer’s disease.
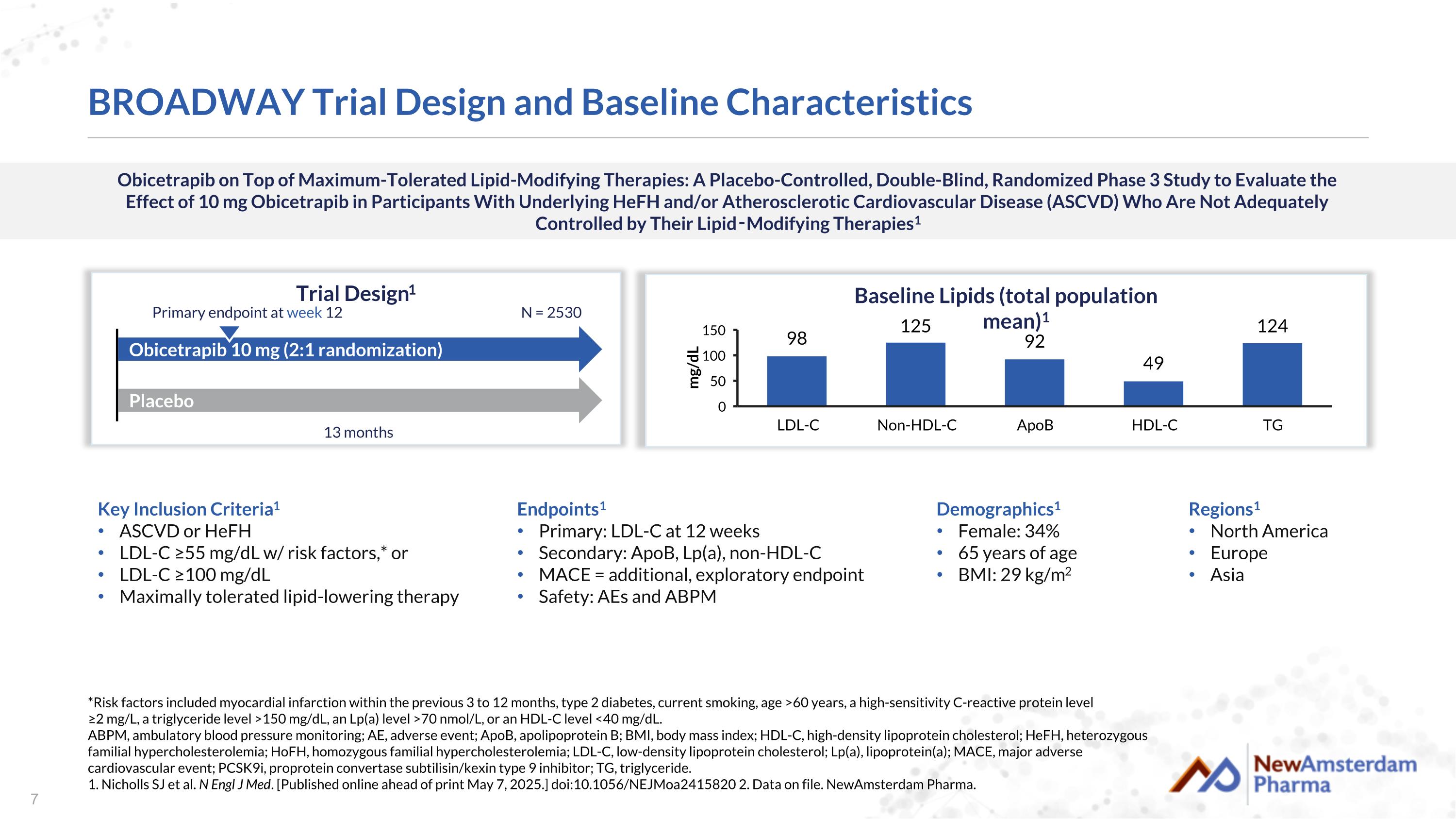
Obicetrapib on Top of Maximum-Tolerated Lipid-Modifying Therapies: A Placebo-Controlled, Double-Blind, Randomized Phase 3 Study to Evaluate the Effect of 10 mg Obicetrapib in Participants With Underlying HeFH and/or Atherosclerotic Cardiovascular Disease (ASCVD) Who Are Not Adequately Controlled by Their Lipid‑Modifying Therapies1 BROADWAY Trial Design and Baseline Characteristics Trial Design1 Key Inclusion Criteria1 ASCVD or HeFH LDL-C ≥55 mg/dL w/ risk factors,* or LDL-C ≥100 mg/dL Maximally tolerated lipid-lowering therapy Placebo Obicetrapib 10 mg (2:1 randomization) N = 2530 13 months Primary endpoint at week 12 Demographics1 Female: 34% 65 years of age BMI: 29 kg/m2 Baseline Lipids (total population mean)1 *Risk factors included myocardial infarction within the previous 3 to 12 months, type 2 diabetes, current smoking, age >60 years, a high-sensitivity C-reactive protein level ≥2 mg/L, a triglyceride level >150 mg/dL, an Lp(a) level >70 nmol/L, or an HDL-C level <40 mg/dL. ABPM, ambulatory blood pressure monitoring; AE, adverse event; ApoB, apolipoprotein B; BMI, body mass index; HDL-C, high-density lipoprotein cholesterol; HeFH, heterozygous familial hypercholesterolemia; HoFH, homozygous familial hypercholesterolemia; LDL-C, low-density lipoprotein cholesterol; Lp(a), lipoprotein(a); MACE, major adverse cardiovascular event; PCSK9i, proprotein convertase subtilisin/kexin type 9 inhibitor; TG, triglyceride. 1. Nicholls SJ et al. N Engl J Med. [Published online ahead of print May 7, 2025.] doi:10.1056/NEJMoa2415820 2. Data on file. NewAmsterdam Pharma. Endpoints1 Primary: LDL-C at 12 weeks Secondary: ApoB, Lp(a), non-HDL-C MACE = additional, exploratory endpoint Safety: AEs and ABPM Regions1 North America Europe Asia
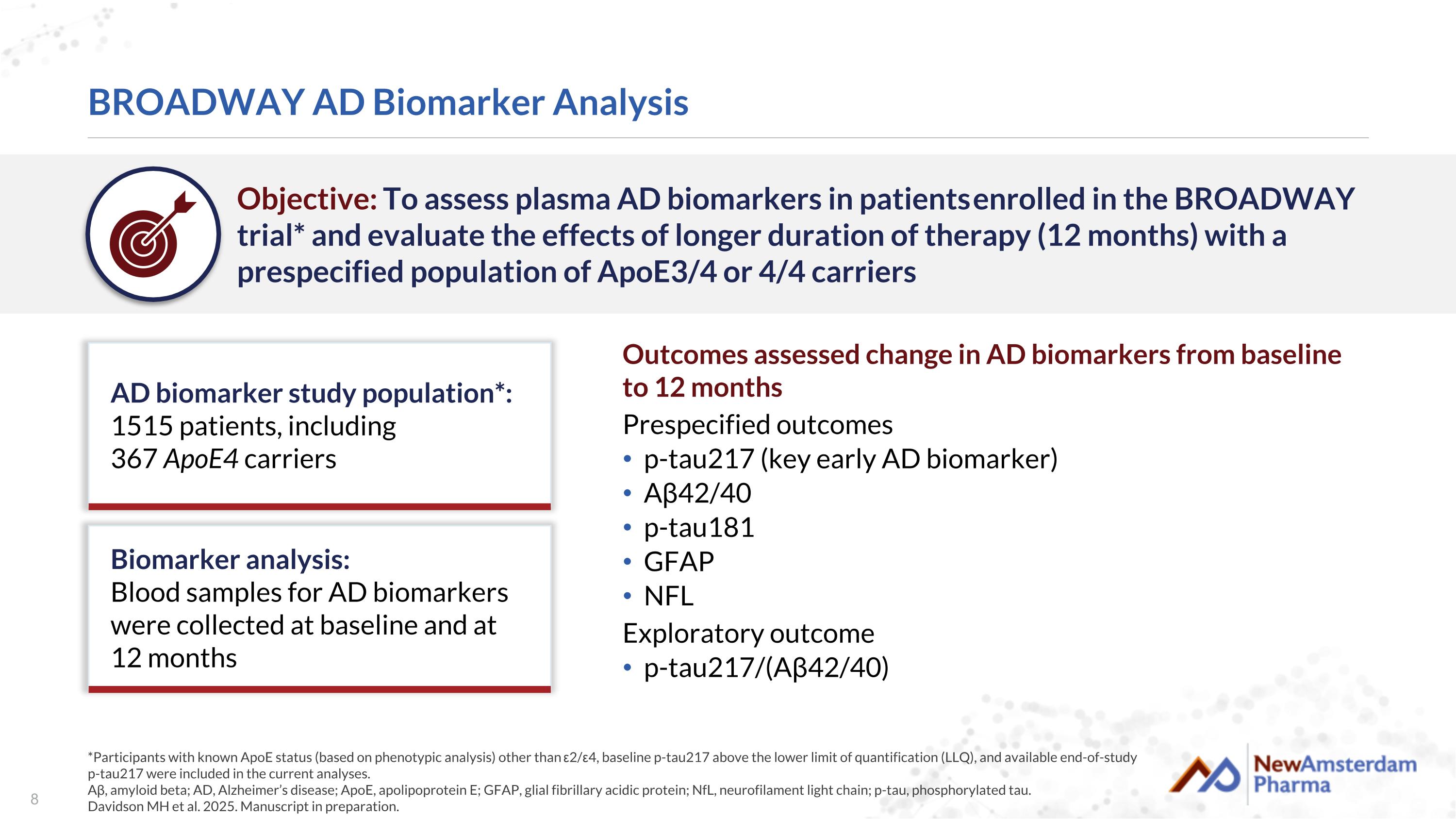
BROADWAY AD Biomarker Analysis *Participants with known ApoE status (based on phenotypic analysis) other than ε2/ε4, baseline p-tau217 above the lower limit of quantification (LLQ), and available end-of-study p-tau217 were included in the current analyses. Aβ, amyloid beta; AD, Alzheimer’s disease; ApoE, apolipoprotein E; GFAP, glial fibrillary acidic protein; NfL, neurofilament light chain; p-tau, phosphorylated tau. Davidson MH et al. 2025. Manuscript in preparation. Objective: To assess plasma AD biomarkers in patients enrolled in the BROADWAY trial* and evaluate the effects of longer duration of therapy (12 months) with a prespecified population of ApoE3/4 or 4/4 carriers Outcomes assessed change in AD biomarkers from baseline to 12 months Prespecified outcomes p-tau217 (key early AD biomarker) Aβ42/40 p-tau181 GFAP NFL Exploratory outcome p-tau217/(Aβ42/40) AD biomarker study population*: 1515 patients, including 367 ApoE4 carriers Biomarker analysis: Blood samples for AD biomarkers were collected at baseline and at 12 months
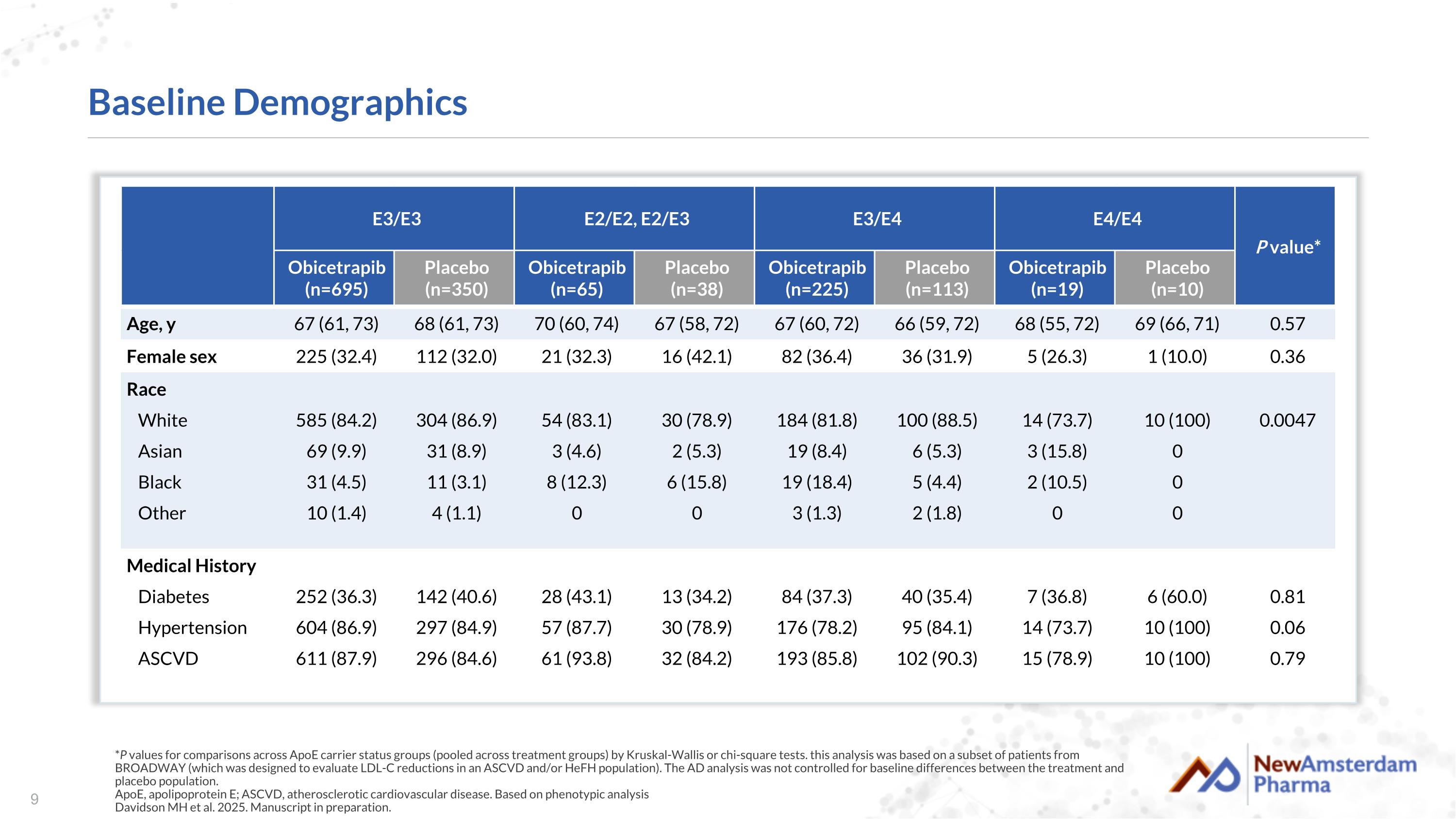
Baseline Demographics *P values for comparisons across ApoE carrier status groups (pooled across treatment groups) by Kruskal-Wallis or chi-square tests. this analysis was based on a subset of patients from BROADWAY (which was designed to evaluate LDL-C reductions in an ASCVD and/or HeFH population). The AD analysis was not controlled for baseline differences between the treatment and placebo population. ApoE, apolipoprotein E; ASCVD, atherosclerotic cardiovascular disease. Based on phenotypic analysis Davidson MH et al. 2025. Manuscript in preparation. E3/E3 E2/E2, E2/E3 E3/E4 E4/E4 P value* Obicetrapib (n=695) Placebo (n=350) Obicetrapib (n=65) Placebo (n=38) Obicetrapib (n=225) Placebo (n=113) Obicetrapib (n=19) Placebo (n=10) Age, y 67 (61, 73) 68 (61, 73) 70 (60, 74) 67 (58, 72) 67 (60, 72) 66 (59, 72) 68 (55, 72) 69 (66, 71) 0.57 Female sex 225 (32.4) 112 (32.0) 21 (32.3) 16 (42.1) 82 (36.4) 36 (31.9) 5 (26.3) 1 (10.0) 0.36 Race White Asian Black Other 585 (84.2) 69 (9.9) 31 (4.5) 10 (1.4) 304 (86.9) 31 (8.9) 11 (3.1) 4 (1.1) 54 (83.1) 3 (4.6) 8 (12.3) 0 30 (78.9) 2 (5.3) 6 (15.8) 0 184 (81.8) 19 (8.4) 19 (18.4) 3 (1.3) 100 (88.5) 6 (5.3) 5 (4.4) 2 (1.8) 14 (73.7) 3 (15.8) 2 (10.5) 0 10 (100) 0 0 0 0.0047 Medical History Diabetes Hypertension ASCVD 252 (36.3) 604 (86.9) 611 (87.9) 142 (40.6) 297 (84.9) 296 (84.6) 28 (43.1) 57 (87.7) 61 (93.8) 13 (34.2) 30 (78.9) 32 (84.2) 84 (37.3) 176 (78.2) 193 (85.8) 40 (35.4) 95 (84.1) 102 (90.3) 7 (36.8) 14 (73.7) 15 (78.9) 6 (60.0) 10 (100) 10 (100) 0.81 0.06 0.79
BROADWAY AD Biomarker Study: Primary Results AD, Alzheimer’s disease.
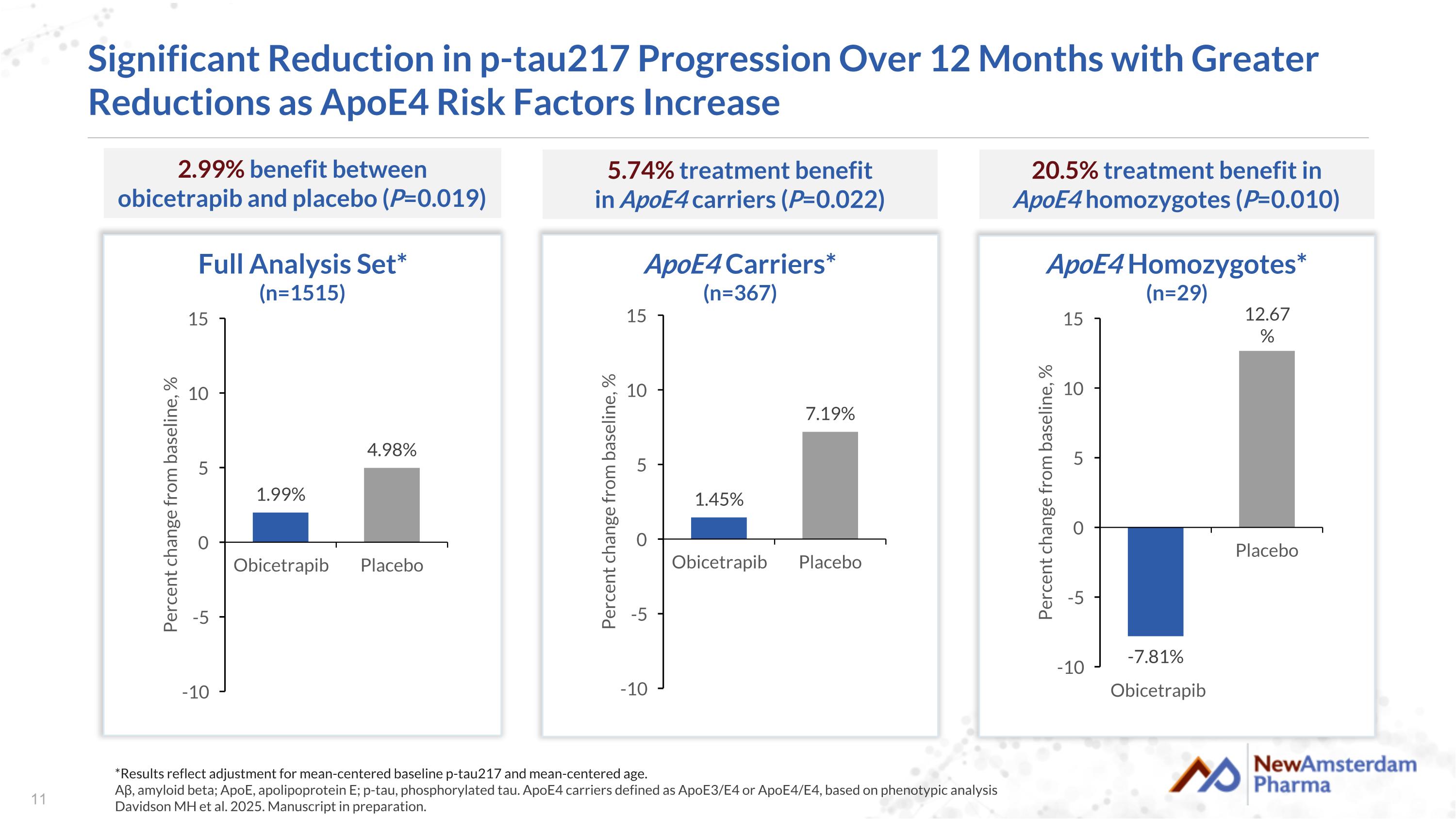
Significant Reduction in p-tau217 Progression Over 12 Months with Greater Reductions as ApoE4 Risk Factors Increase Full Analysis Set* (n=1515) 2.99% benefit between obicetrapib and placebo (P=0.019) ApoE4 Carriers* (n=367) 5.74% treatment benefit in ApoE4 carriers (P=0.022) *Results reflect adjustment for mean-centered baseline p-tau217 and mean-centered age. Aβ, amyloid beta; ApoE, apolipoprotein E; p-tau, phosphorylated tau. ApoE4 carriers defined as ApoE3/E4 or ApoE4/E4, based on phenotypic analysis Davidson MH et al. 2025. Manuscript in preparation. Obicetrapib ApoE4 Homozygotes* (n=29) 20.5% treatment benefit in ApoE4 homozygotes (P=0.010)
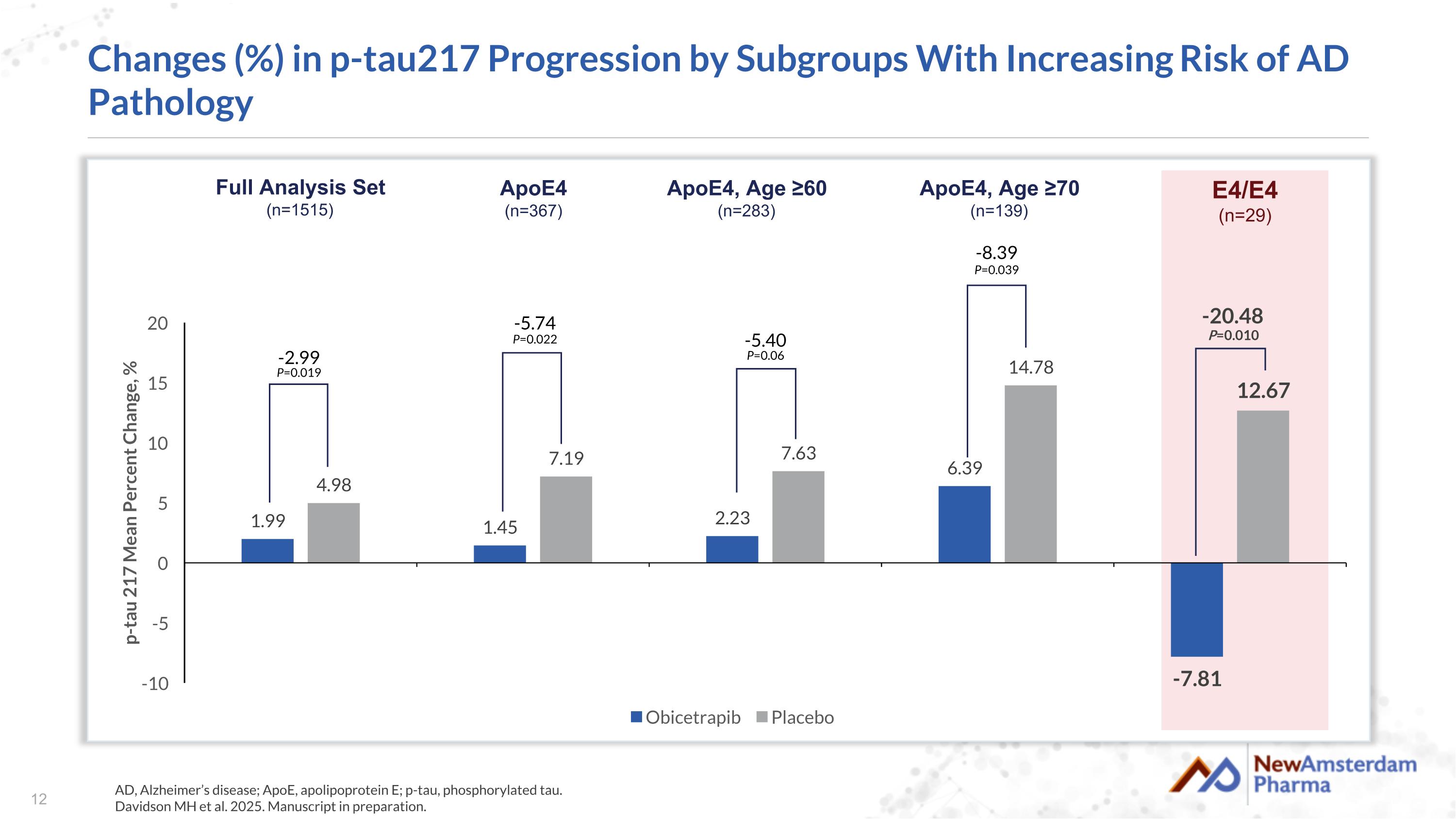
Changes (%) in p-tau217 Progression by Subgroups With Increasing Risk of AD Pathology AD, Alzheimer’s disease; ApoE, apolipoprotein E; p-tau, phosphorylated tau. Davidson MH et al. 2025. Manuscript in preparation. -8.39 P=0.039 -5.40 P=0.06 -5.74 P=0.022 -2.99 P=0.019 -20.48 P=0.010 Full Analysis Set (n=1515) ApoE4 (n=367) ApoE4, Age ≥60 (n=283) E4/E4 (n=29) ApoE4, Age ≥70 (n=139)
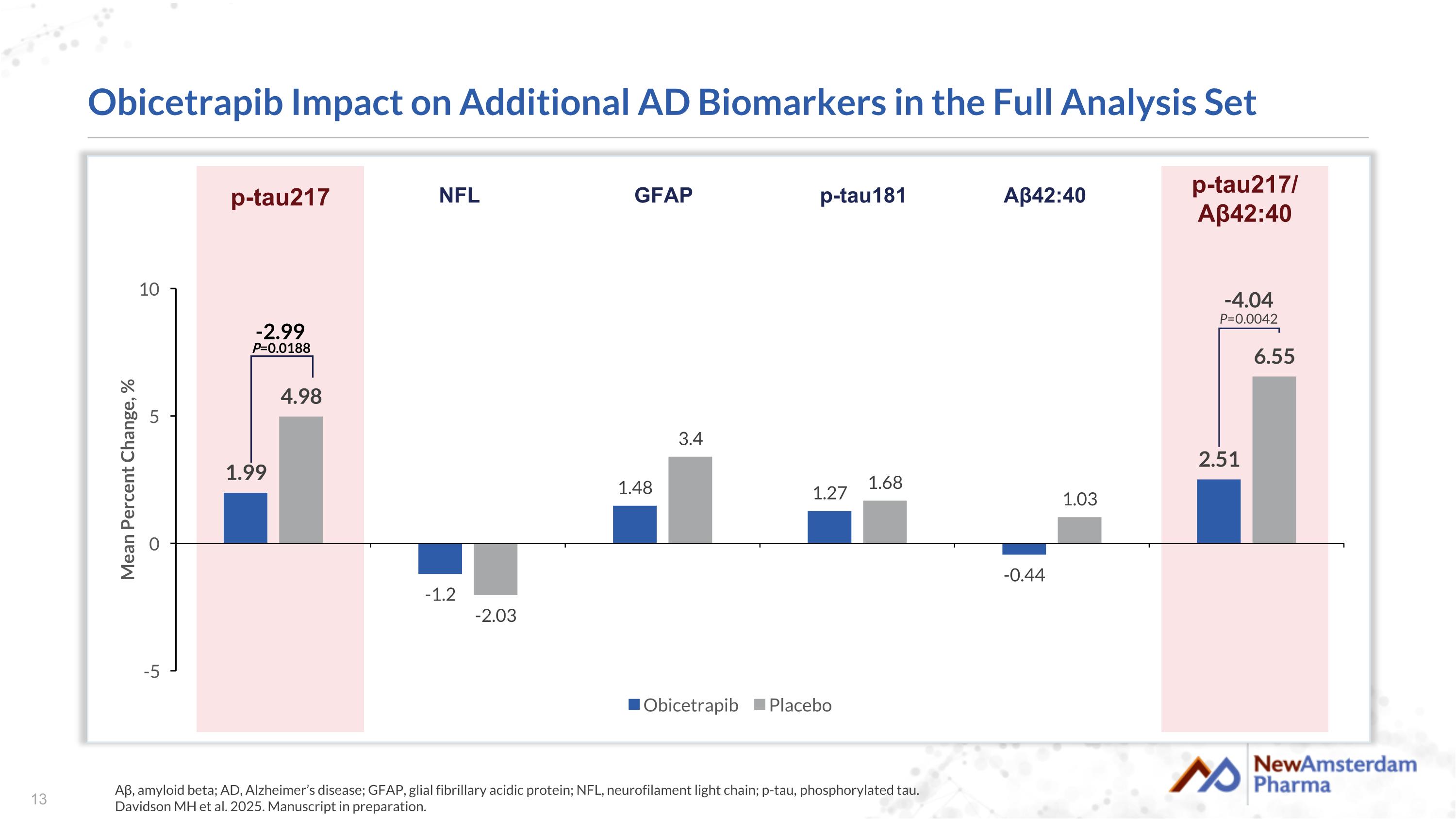
Obicetrapib Impact on Additional AD Biomarkers in the Full Analysis Set Aβ, amyloid beta; AD, Alzheimer’s disease; GFAP, glial fibrillary acidic protein; NFL, neurofilament light chain; p-tau, phosphorylated tau. Davidson MH et al. 2025. Manuscript in preparation. -2.99 P=0.0188 -4.04 P=0.0042 p-tau217 NFL GFAP p-tau181 Aβ42:40 p-tau217/ Aβ42:40
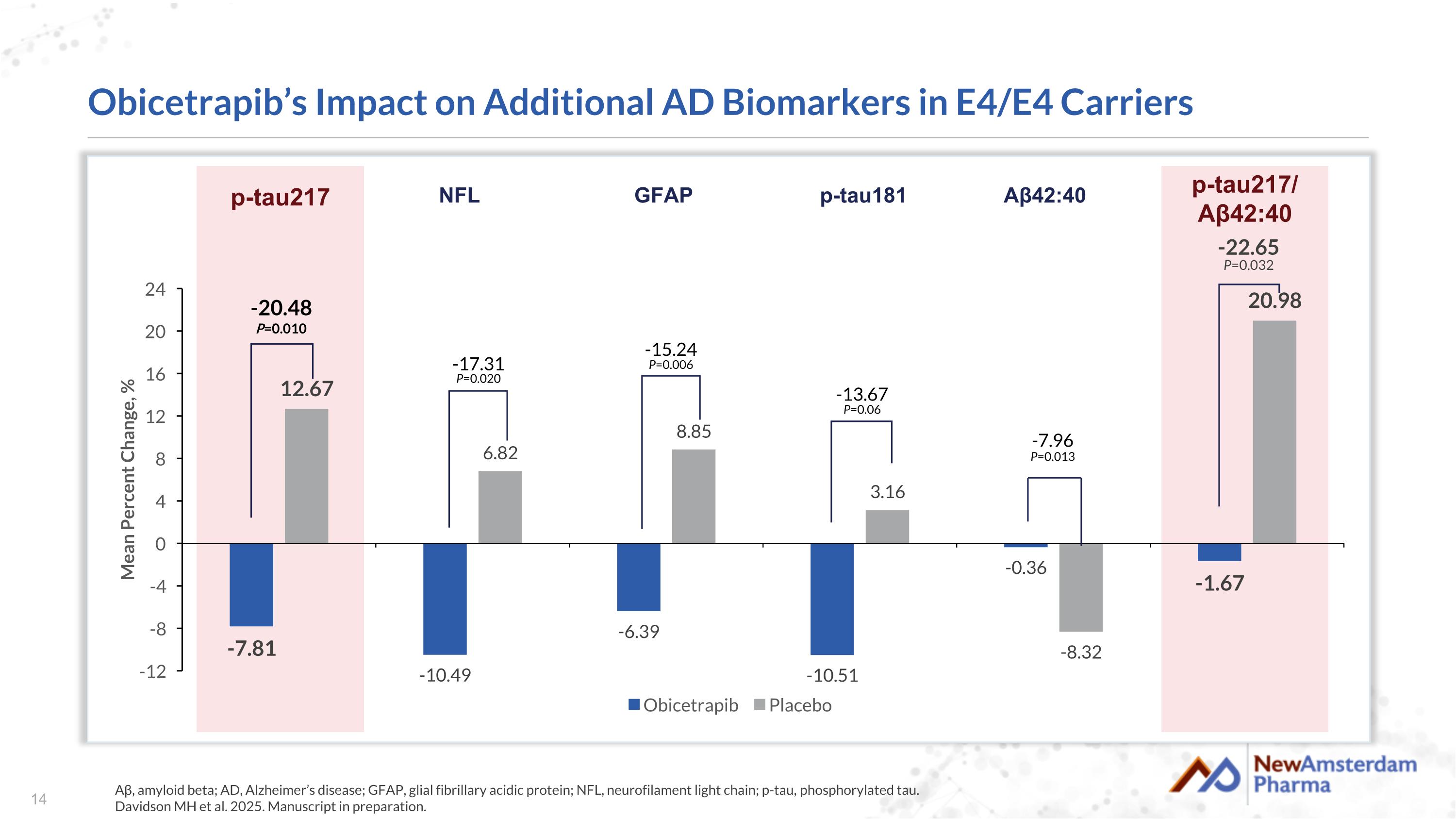
Obicetrapib’s Impact on Additional AD Biomarkers in E4/E4 Carriers Aβ, amyloid beta; AD, Alzheimer’s disease; GFAP, glial fibrillary acidic protein; NFL, neurofilament light chain; p-tau, phosphorylated tau. Davidson MH et al. 2025. Manuscript in preparation. -7.96 P=0.013 -13.67 P=0.06 -15.24 P=0.006 -17.31 P=0.020 -20.48 P=0.010 -22.65 P=0.032 p-tau217 NFL GFAP p-tau181 Aβ42:40 p-tau217/ Aβ42:40
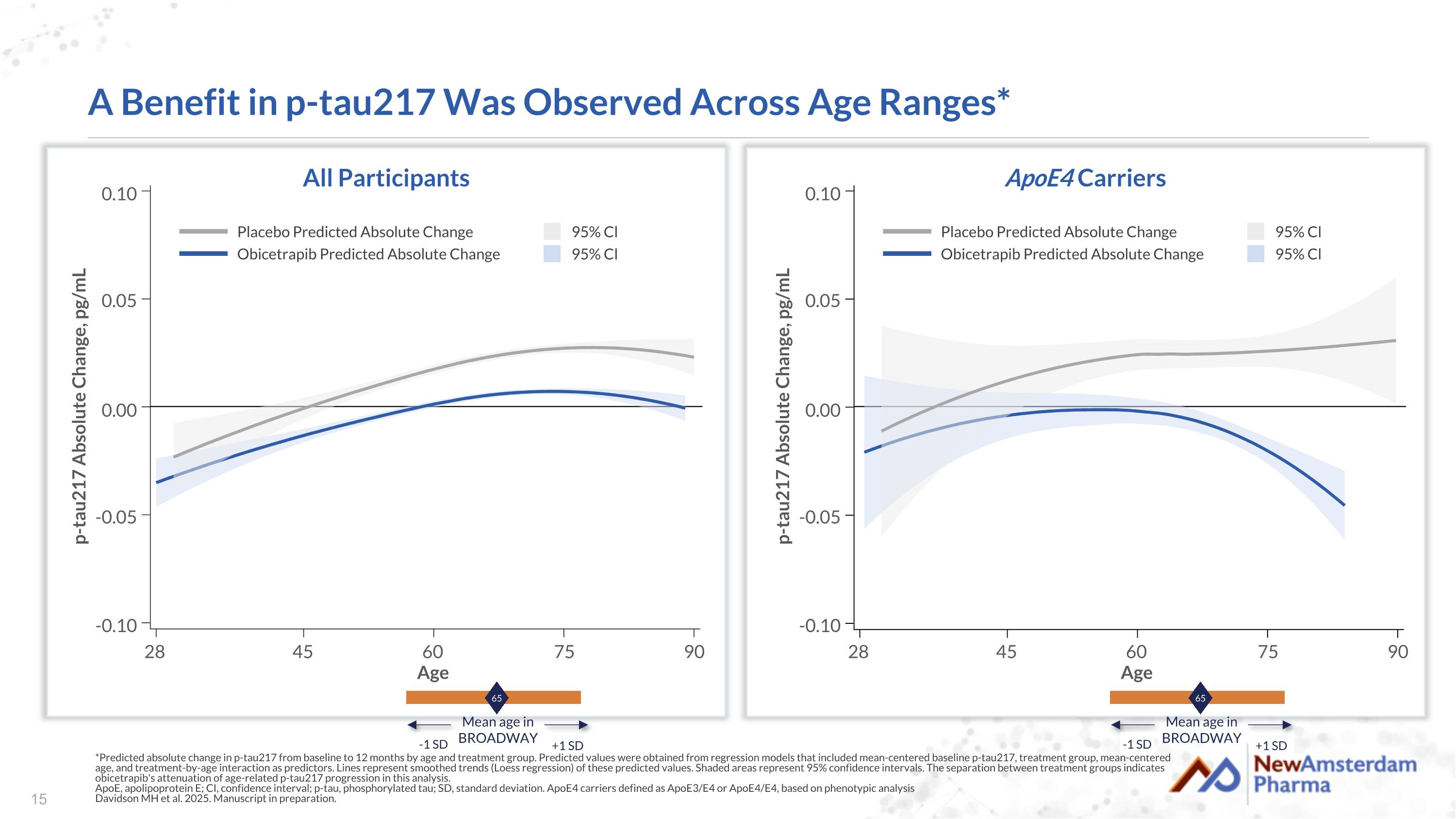
A Benefit in p-tau217 Was Observed Across Age Ranges* *Predicted absolute change in p-tau217 from baseline to 12 months by age and treatment group. Predicted values were obtained from regression models that included mean-centered baseline p-tau217, treatment group, mean-centered age, and treatment-by-age interaction as predictors. Lines represent smoothed trends (Loess regression) of these predicted values. Shaded areas represent 95% confidence intervals. The separation between treatment groups indicates obicetrapib's attenuation of age-related p-tau217 progression in this analysis. ApoE, apolipoprotein E; CI, confidence interval; p-tau, phosphorylated tau; SD, standard deviation. ApoE4 carriers defined as ApoE3/E4 or ApoE4/E4, based on phenotypic analysis Davidson MH et al. 2025. Manuscript in preparation. All Participants ApoE4 Carriers Placebo Predicted Absolute Change Obicetrapib Predicted Absolute Change 95% Cl 95% Cl 65 Mean age in BROADWAY -1 SD +1 SD p-tau217 Absolute Change, pg/mL Age 90 75 60 45 28 -0.10 -0.05 0.00 0.05 0.10 Placebo Predicted Absolute Change Obicetrapib Predicted Absolute Change 95% Cl 95% Cl 65 Mean age in BROADWAY -1 SD +1 SD p-tau217 Absolute Change, pg/mL Age 90 75 60 45 28 -0.10 -0.05 0.00 0.05 0.10
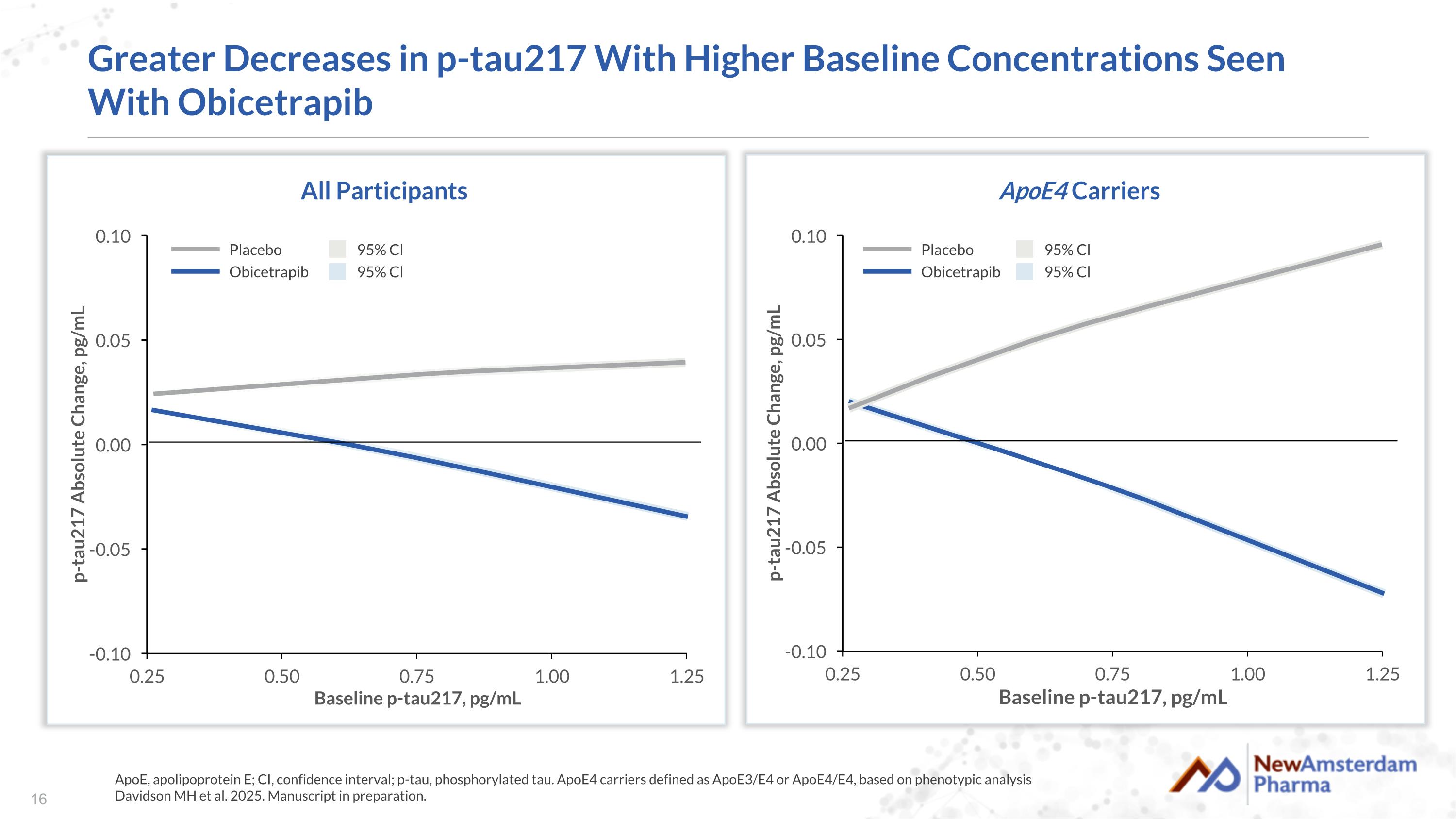
Greater Decreases in p-tau217 With Higher Baseline Concentrations Seen With Obicetrapib ApoE, apolipoprotein E; CI, confidence interval; p-tau, phosphorylated tau. ApoE4 carriers defined as ApoE3/E4 or ApoE4/E4, based on phenotypic analysis Davidson MH et al. 2025. Manuscript in preparation. All Participants Placebo Obicetrapib 95% Cl 95% Cl ApoE4 Carriers Placebo Obicetrapib 95% Cl 95% Cl
APOE4 and p-tau217
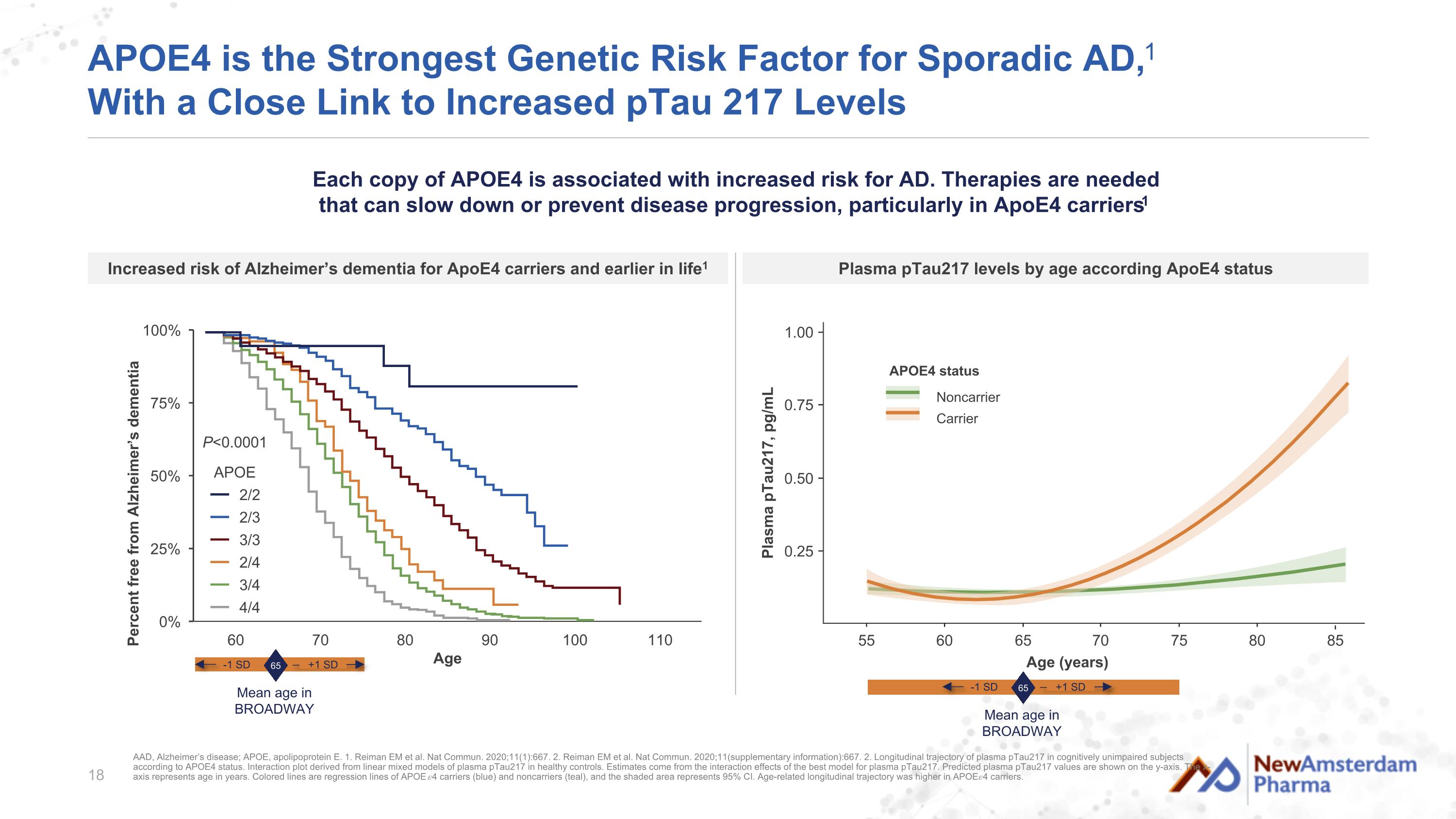
APOE4 is the Strongest Genetic Risk Factor for Sporadic AD,1 With a Close Link to Increased pTau 217 Levels P<0.0001 APOE 2/2 2/3 3/3 2/4 3/4 4/4 Age (years) 0.25 0.50 1.00 Plasma pTau217, pg/mL 0.75 80 55 60 65 70 75 85 Noncarrier Carrier APOE4 status AAD, Alzheimer’s disease; APOE, apolipoprotein E. 1. Reiman EM et al. Nat Commun. 2020;11(1):667. 2. Reiman EM et al. Nat Commun. 2020;11(supplementary information):667. 2. Longitudinal trajectory of plasma pTau217 in cognitively unimpaired subjects according to APOE4 status. Interaction plot derived from linear mixed models of plasma pTau217 in healthy controls. Estimates come from the interaction effects of the best model for plasma pTau217. Predicted plasma pTau217 values are shown on the y-axis. The x-axis represents age in years. Colored lines are regression lines of APOE 𝜀4 carriers (blue) and noncarriers (teal), and the shaded area represents 95% CI. Age-related longitudinal trajectory was higher in APOE 𝜀4 carriers. Each copy of APOE4 is associated with increased risk for AD. Therapies are needed that can slow down or prevent disease progression, particularly in ApoE4 carriers1 Plasma pTau217 levels by age according ApoE4 status Increased risk of Alzheimer’s dementia for ApoE4 carriers and earlier in life1 65 Mean age in BROADWAY -1 SD +1 SD 65 Mean age in BROADWAY -1 SD +1 SD
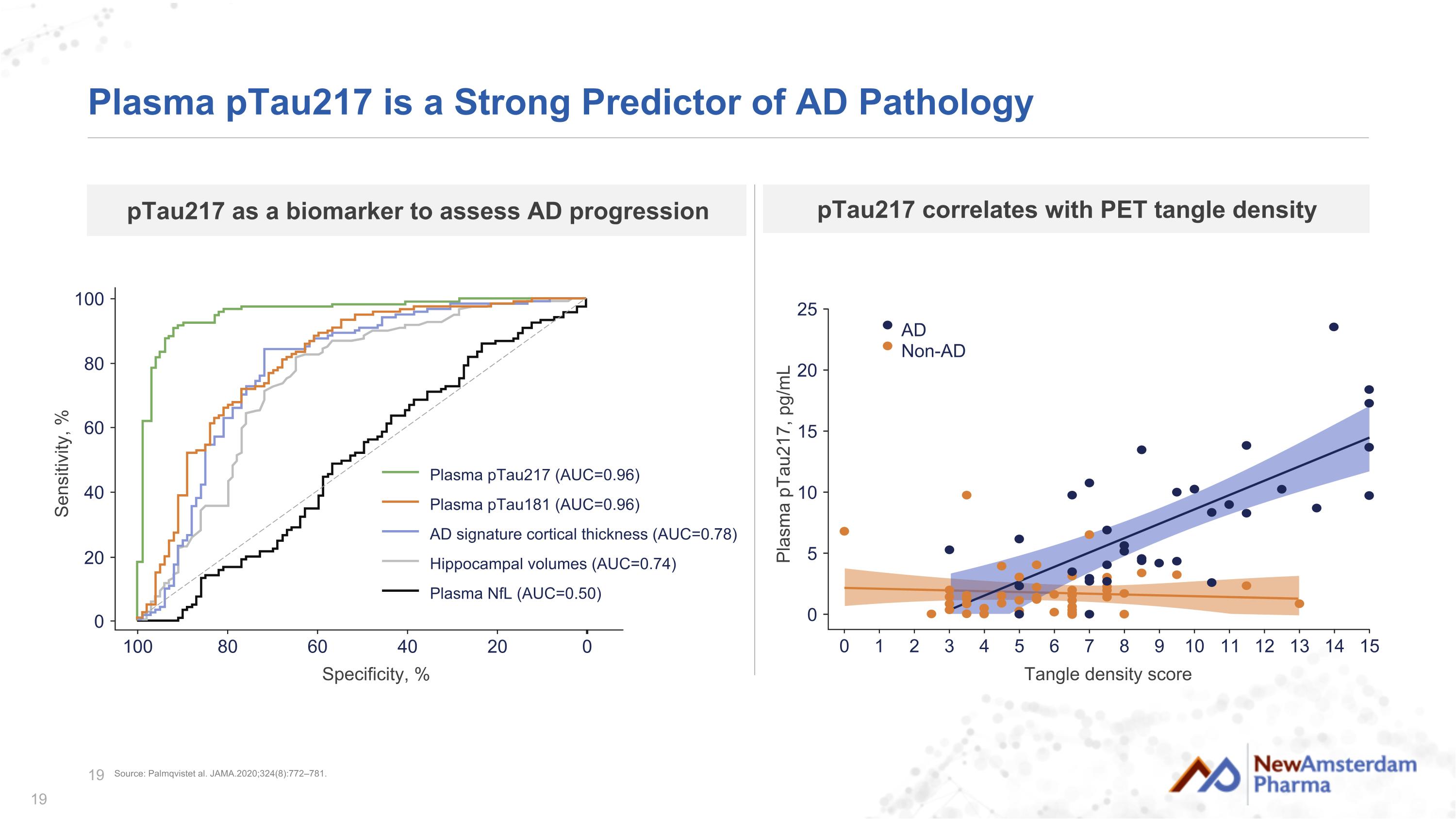
Plasma pTau217 is a Strong Predictor of AD Pathology Specificity, % 0 20 100 Sensitivity, % 80 20 100 80 60 40 0 60 40 Plasma pTau217 (AUC=0.96) Plasma pTau181 (AUC=0.96) AD signature cortical thickness (AUC=0.78) Hippocampal volumes (AUC=0.74) Plasma NfL (AUC=0.50) pTau217 as a biomarker to assess AD progression Tangle density score 0 1 2 3 4 0 5 10 15 25 Plasma pTau217, pg/mL 20 15 5 6 7 8 9 10 11 12 13 14 AD Non-AD pTau217 correlates with PET tangle density Source: Palmqvistet al. JAMA.2020;324(8):772–781.
Conclusions
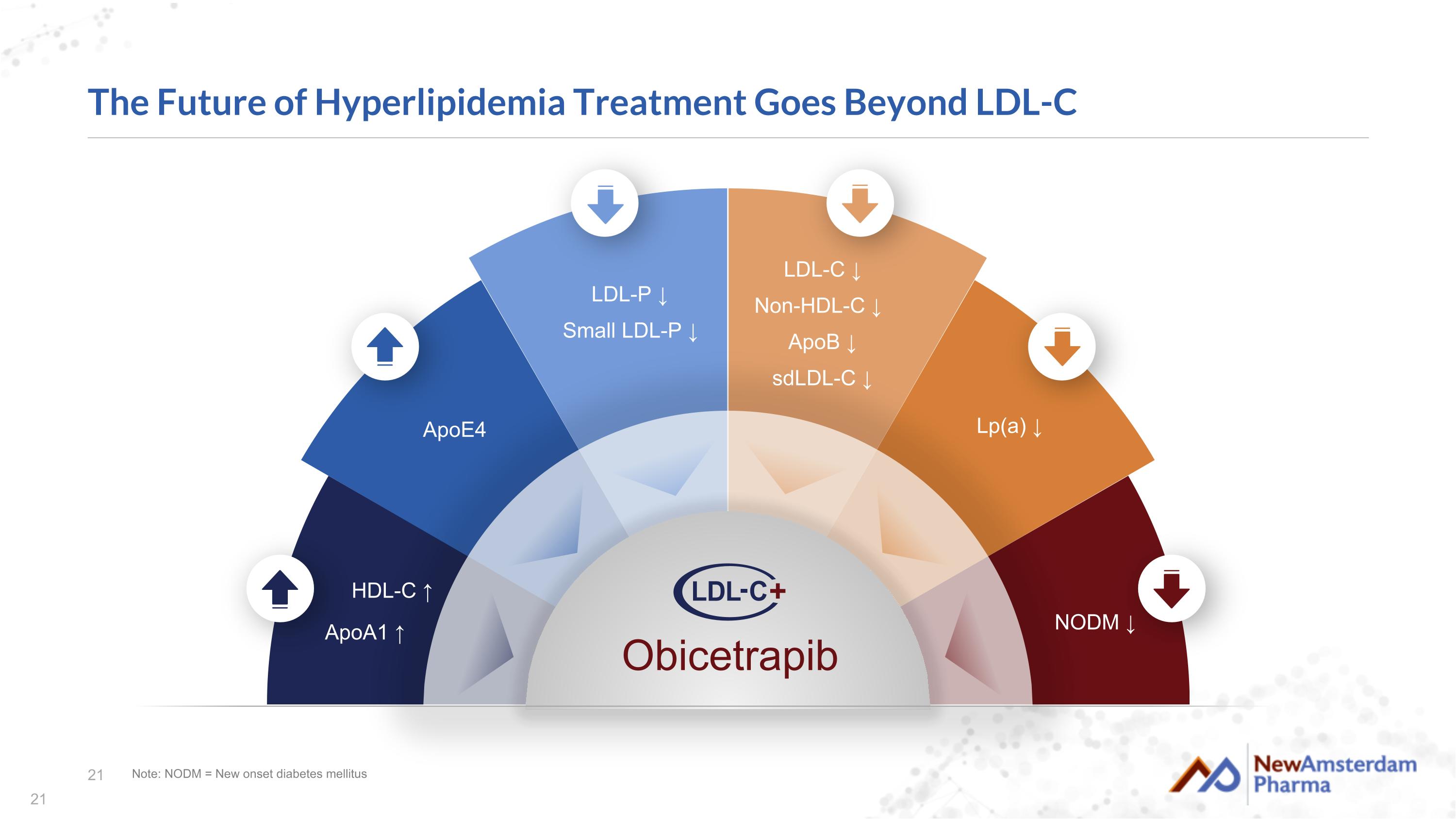
The Future of Hyperlipidemia Treatment Goes Beyond LDL-C Note: NODM = New onset diabetes mellitus ApoE4 LDL-P ↓ Small LDL-P ↓ NODM ↓ HDL-C ↑ ApoA1 ↑ LDL-C ↓ Non-HDL-C ↓ ApoB ↓ sdLDL-C ↓ Lp(a) ↓ Obicetrapib
* Source: Toth, Peter P. et al. Atherosclerosis. Volume 235, Issue 2, 585 - 591. ^Hoogeveen RC, Gaubatz JW, Sun W, Dodge RC, Crosby JR, Jiang J, Couper D, Virani SS, Kathiresan S, Boerwinkle E, Ballantyne CM. Small dense low-density lipoprotein-cholesterol concentrations predict risk for coronary heart disease: the Atherosclerosis Risk In Communities (ARIC) study. Arterioscler Thromb Vasc Biol. 2014 May;34(5):1069-77. doi: 10.1161/ATVBAHA.114.303284. Epub 2014 Feb 20. PMID: 24558110; PMCID: PMC3999643. * Ray K, et al. European Journal of Preventive Cardiology (2021) 28, 1279–1289. ^ Footnote: Prediabetes was defined as fasting plasma glucose values of 100 to 125 mg/dL or A1C values of 5.7% to 6.4%. Reference: National Diabetes Statistics Report | Diabetes | CDC** Tsimikas et al. J Am Coll Cardiol. 2022; 80: 934-946 ^^ Shapiro, M et al. Journal of Clinical Lipidology, Volume 19, Issue 1, 28 – 38 Aβ, amyloid β; AD, Alzheimer’s disease; Apo, apolipoprotein; CVD, cardiovascular disese; HDL-C, high density lipoprotein cholesterol; LDL-C, low density lipoprotein cholesterol; Lp(a), lipoprotein(a). 1. Abondio P et al. Genes (Basel). 2019;10(3):222. 2. Feringa FM et al. Front Aging Neurosci. 2021;13:690372. 3. Hottman 2017. 4. Jeong W et al. Mol Cells. 2019;42(11):739-746. 5. Williams et al. BMC 2020.c Approximately 43 million patients with hyperlipidemia exhibit elevated levels of LDL-P of > 1300 nmol/L* Prevalence is even higher in individuals with type 2 diabetes^ Presence of LDL-P is a significant independent risk factor for coronary heart disease (CHD), even when traditional lipid measures like LDL-C and triglycerides are within normal ranges^ The Future of Hyperlipidemia Treatment Goes Beyond LDL-C In the US, close to 75 million patients have an elevated Lp(a) score of > 100-125 nmol/L** ^^ An estimated 14.3 million of these patients also have hypercholesterolemia 27.9 million hyperlipidemia patients are estimated to have concomitant diabetes* In addition, there are an estimated 27.2 million^ individuals with hyperlipidemia that are pre-diabetic ApoE4 is the strongest genetic risk factor for late-onset AD1-5 Approximately 20 million patients with hyperlipidemia are at increased risk for AD Prevalence of ApoE4 in AD is up to 66%1-5 LDL Particles Lp(a) Diabetes ApoE4 Obicetrapib was observed to impact multiple risk factors, potentially leading to more comprehensive risk management across additional ASCVD patient subpopulations