
Exhibit 99.1

www.ClearmindMedicine.com Novel Psychedelics for Treating Addictions, Obesity & Mental Health Corporate and Science Presentation | July 2025 NASDAQ: CMND FSE: CWY0 Clearmind Medicine Inc.

Clearmind Medicine Inc. Page 2 All statements in this presentation, other than those relating to historical facts, are "forward-looking statements . ” Forward - looking statements contained in this presentation include, but are not limited to, statements regarding Clearmind Medicine Inc . (the “Company”) strategic and business plans, technology, relationships, objectives and expectations for its business, growth, the impact of trends on and interest in its business, intellectual property, products and its future results, operations and financial performance and condition and may be identified by the use of words such as “expects,” “intends,” “plans,” “believes,” “seeks,” “estimates,” and similar expressions or variations of such words are intended to identify forward - looking statements . For example, the Company is using forward - looking statements when it discusses financial plans, its project pipeline, its expected revenue models, the potential of its technology, its strategy, market potential for its technology and its future growth . Forward - looking statements are not historical facts, and are based upon management’s current expectations, beliefs and projections, many of which, by their nature, are inherently uncertain . Such expectations, beliefs and projections are expressed in good faith . However, there can be no assurance that management’s expectations, beliefs and projections will be achieved, and actual results may differ materially from what is expressed or indicated by the forward - looking statements . Forward - looking statements are subject to risks and uncertainties that could cause actual performance or results to differ materially from those expressed in the forward - looking statements . For a more detailed description of the risks and uncertainties affecting the Company, reference is made to the Company’s reports filed from time to time with the Securities and Exchange Commission (“SEC”), including, but not limited to, the risks detailed in the Company’s Annual Report on Form 20 - F for the fiscal year ended October 31 , 2024 filed with the SEC and in subsequent filings with the SEC . Forward - looking statements speak only as of the date the statements are made . The Company assumes no obligation to update forward - looking statements to reflect actual results, subsequent events or circumstances, changes in assumptions or changes in other factors affecting forward - looking information except to the extent required by applicable securities laws . If the Company does update one or more forward - looking statements, no inference should be drawn that the Company will make additional updates with respect thereto or with respect to other forward - looking statements . This presentation includes statistical, market and industry data and forecasts which we obtained from publicly available information and independent industry publications and reports that we believe to be reliable sources . These publicly available industry publications and reports generally state that they obtain their information from sources that they believe to be reliable, but they do not guarantee the accuracy or completeness of the information . Although we are responsible for all of the disclosures contained in this presentation, including such statistical, market and industry data, we have not independently verified any of the data from third - party sources, nor have we ascertained the underlying economic assumptions relied upon therein . In addition, while we believe the market opportunity information included in this presentation is generally reliable and is based on reasonable assumptions, the industry in which we operate is subject to a high degree of uncertainty and risk due to a variety of important factors that could cause results to differ materially from those expressed in the estimates made by third parties and by us . This presentation does not constitute an offer to sell or the solicitation of an offer to sell or the solicitation of an offer to buy any of our securities nor shall there be any sale of securities in any jurisdiction in which such offer, solicitation or sale would be unlawful prior to registration or qualification under the securities laws of any such jurisdiction. Any offering of securities can only be made in compliance with applicable securities laws. Trade names, trademarks and service marks of third parties in this presentation are the property of their respective holders. Disclaimer and Forward - Looking Statements Corporate Presentation | July 2025

About Clearmind Clearmind Medicine Inc. Corporate Presentation | July 2025 Why Psychedelics? Psychedelic compounds are increasingly being studied and many believe represent the future for treatment of a variety of mental health disorders. Clearmind’s solutions for addiction, especially alcoholism and obesity, hold the potential to better the lives of millions around the world. Nasdaq public company NASDAQ:CMND FSE:CWY0 Pharmaceutical company developing patent - protected psychedelic medicines to solve widespread mental health disorders Initially targeting addiction market, including alcoholism and obesity IND clearance from FDA Phase I/Iia Clinical trials commenced in 4 sites worldwide 19 patents families. 31 granted in the USA and other major jurisdictions Partnership with leading universities & KOLs Page 3
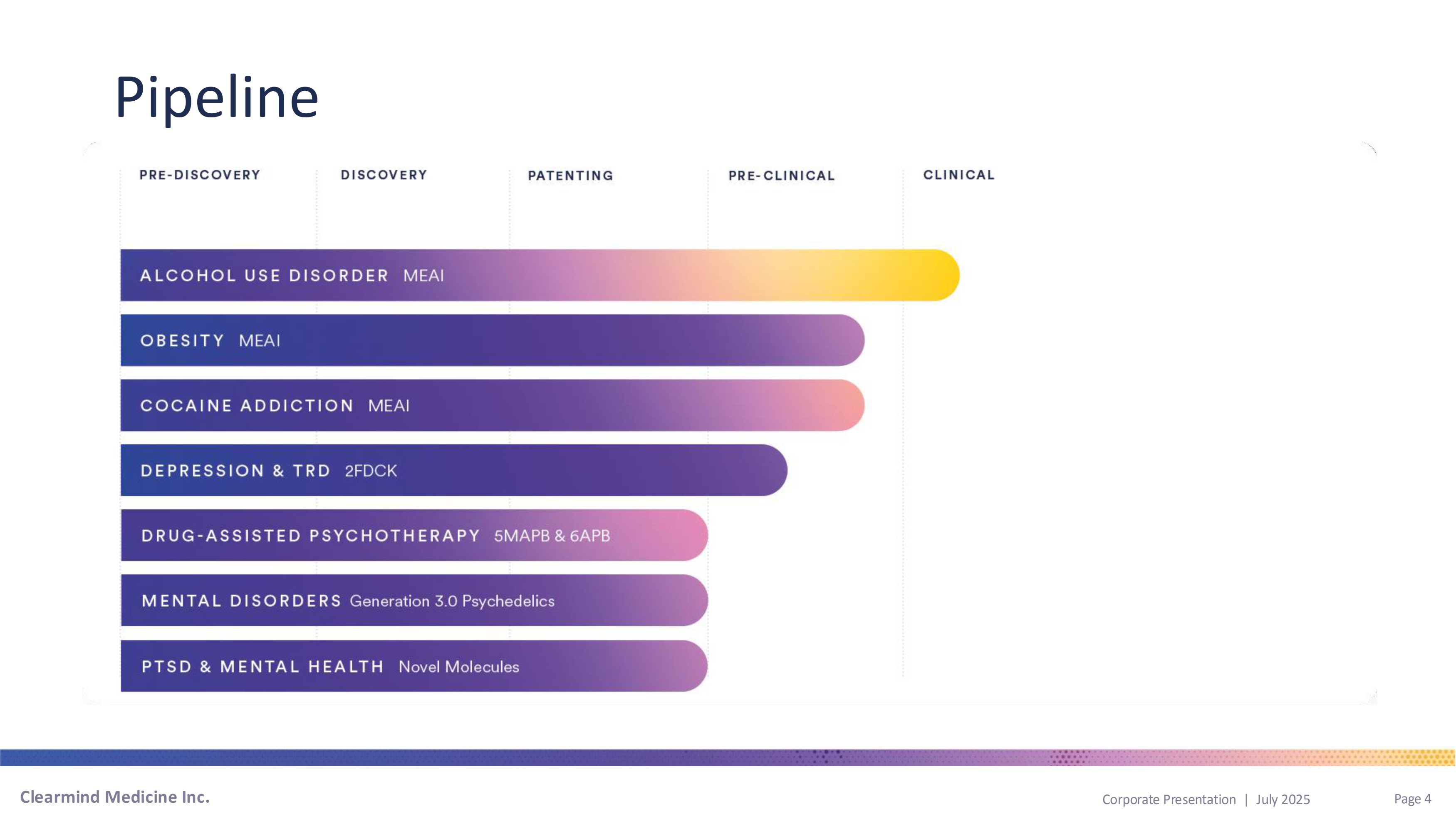
Pipeline Clearmind Medicine Inc. Page 4 Corporate Presentation | July 2025

Alcohol Use Disorder Only 4 FDA approved medicines for AUD Average $/Month Approved by FDA $42 1949 Antabuse Disulfiram $125 2004 Campral Acamprosate $205 1994 ReVia Naltrexone $1000 2006 Vivitrol Naltrexone Clearmind Medicine Inc. Page 5 Corporate Presentation | O c t o b e r 2024 Existing medicines are expensive, have low efficacy (less than 30 % ) 1 , 2 , and low patient compliance due to side effects . $400.4 million Vivitrol sales (2023) 1 Pharmacotherapy for Adults With Alcohol Use Disorders in Outpatient Settings A Systematic Review and Meta - analysis, 2014. 2 Win slow BT, Onysko M, Hebert M. Medications for Alcohol Use Disorder. Am Fam Physician. 2016 Mar 15;93(6):457 - 65. Clearmind’s flagship treatments are focused on Alcohol Use Disorder (AUD), which is extremely common. The yearly cost of excessive alcohol use in the US was almost $250 billion in 2010 https://pubmed.ncbi.nlm.nih.gov/26477807/ $28 billion of which has been accounted for as healthcare costs https:// www.cdc.gov/alcohol/images/real_cost_alcohol_use.jpg
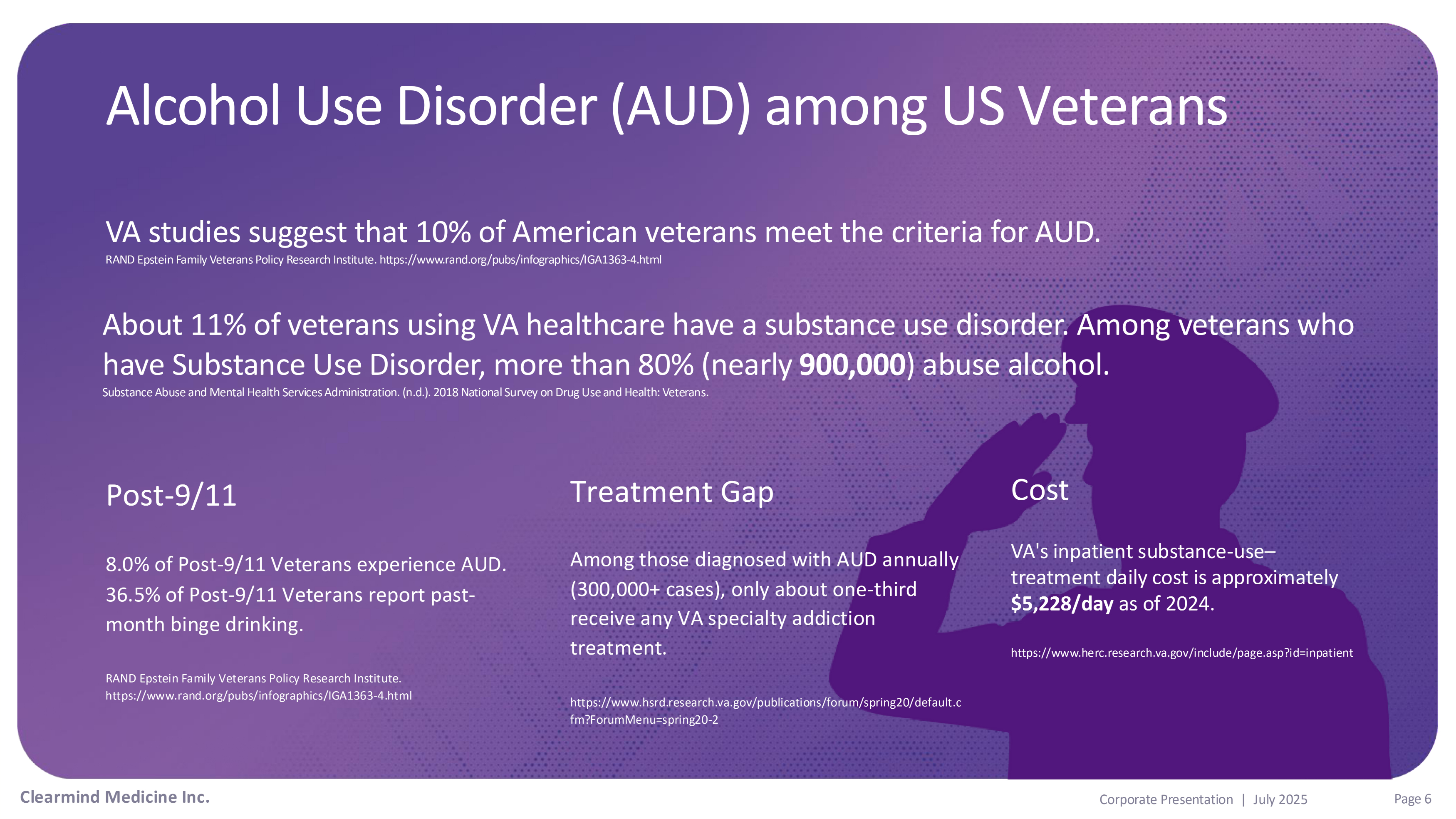
Alcohol Use Disorder (AUD) among US Veterans Treatment Gap Clearmind Medicine Inc. Page 6 Corporate Presentation | July 2025 Among those diagnosed with AUD annually (300,000+ cases), only about one - third receive any VA specialty addiction treatment. https:// www.hsrd.research.va.gov/publications/forum/spring20/default.c fm?ForumMenu=spring20 - 2 Cost VA's inpatient substance - use – treatment daily cost is approximately $5,228/day as of 2024. https:// www.herc.research.va.gov/include/page.asp?id=inpatient VA studies suggest that 10% of American veterans meet the criteria for AUD. RAND Epstein Family Veterans Policy Research Institute. https:// www.rand.org/pubs/infographics/IGA1363 - 4.html About 11% of veterans using VA healthcare have a substance use disorder. Among veterans who have Substance Use Disorder, more than 80% (nearly 900,000 ) abuse alcohol. Substance Abuse and Mental Health Services Administration. (n.d.). 2018 National Survey on Drug Use and Health: Veterans. Post - 9/11 8.0% of Post - 9/11 Veterans experience AUD. 36.5% of Post - 9/11 Veterans report past - month binge drinking. RAND Epstein Family Veterans Policy Research Institute. https:// www.rand.org/pubs/infographics/IGA1363 - 4.html
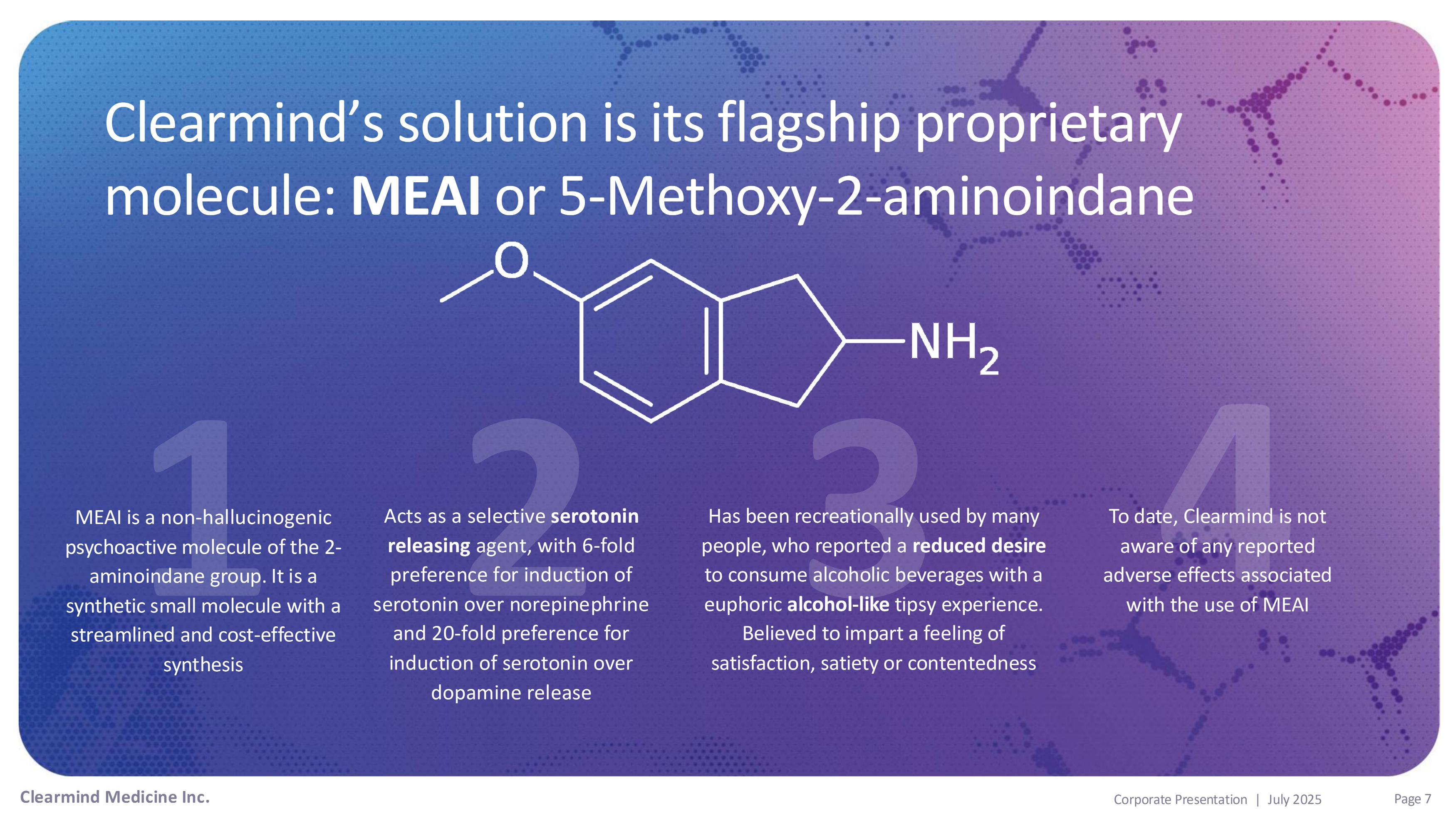
Clearmind’s solution is its flagship proprietary molecule: MEAI or 5 - Methoxy - 2 - aminoindane MEAI is a non - hallucinogenic psychoactive molecule of the 2 - aminoindane group. It is a synthetic small molecule with a streamlined and cost - effective synthesis Has been recreationally used by many people, who reported a reduced desire to consume alcoholic beverages with a euphoric alcohol - like tipsy experience. Believed to impart a feeling of satisfaction, satiety or contentedness To date, Clearmind is not aware of any reported adverse effects associated with the use of MEAI Acts as a selective serotonin releasing agent, with 6 - fold preference for induction of serotonin over norepinephrine and 20 - fold preference for induction of serotonin over dopamine release Clearmind Medicine Inc. Page 7 Corporate Presentation | July 2025
MEAI Mechanism of Action The efficacy and mechanism of action of MEAI has been investigated in vitro as well as in vivo Clearmind Medicine Inc. Page 8 Corporate Presentation | July 2025 In vitro binding studies established interactions mainly with the serotonergic system. MEAI was found to interact with the following receptors or enzymes: serotonin 5HT1A, 5HT2A, 5HT2B receptors; monoamine oxidase inhibitor A (MAO - A); and dopamine D2 and D3 receptors. Functionality has only been assessed at the 5HT2B receptor and was determined as non - agonist activity. MEAI was also shown to interact with plasma membrane monoamine transporters for dopamine (DAT), norepinephrine (NET) and serotonin (SERT) as well as α2 adrenergic receptors. The exact nature of these interactions as they relate to the mechanism of action has not been definitively clarified at this time.
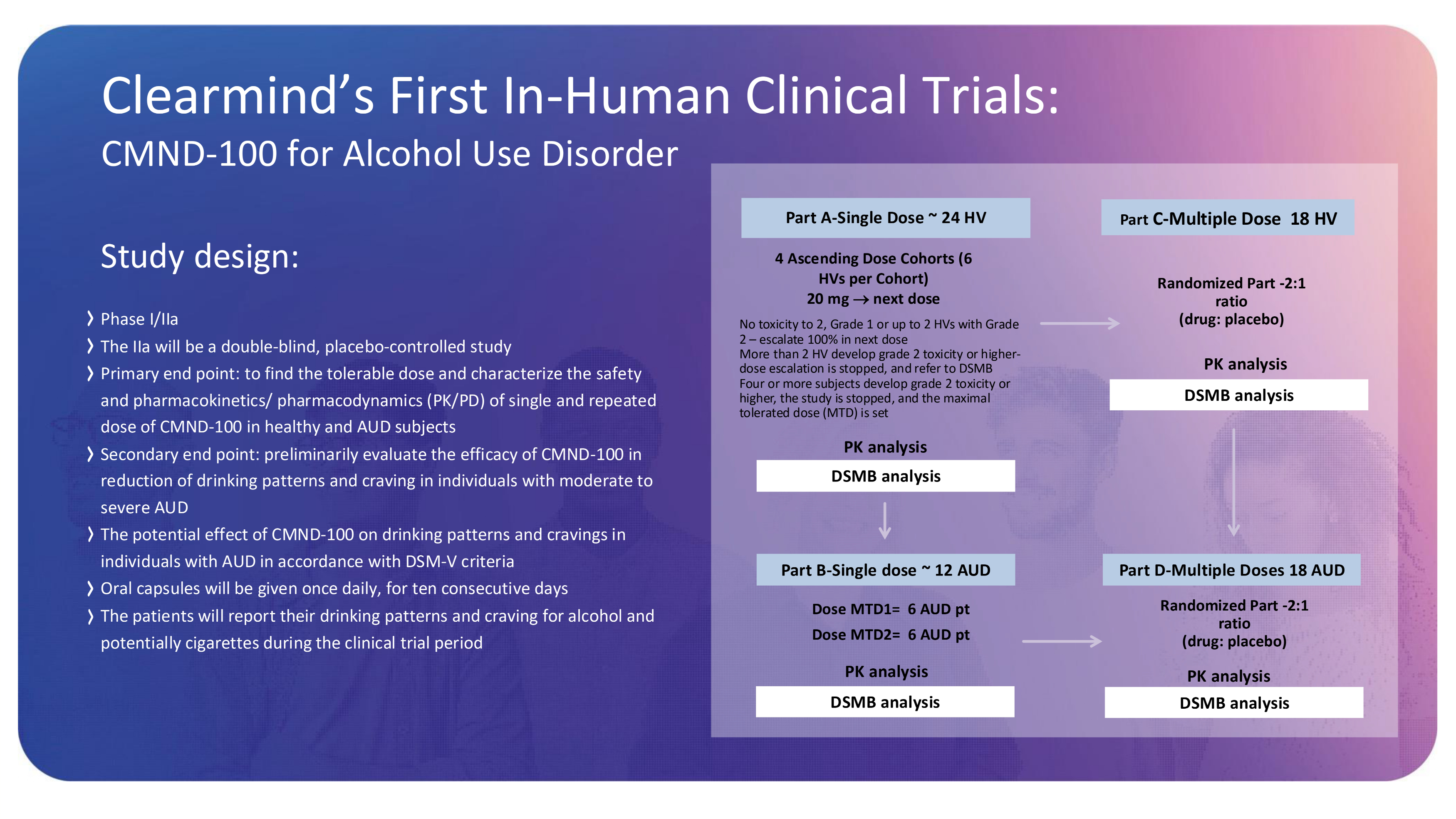
Clearmind’s First In - Human Clinical Trials: CMND - 100 for Alcohol Use Disorder Study design: Phase I/IIa The IIa will be a double - blind, placebo - controlled study Primary end point: to find the tolerable dose and characterize the safety and pharmacokinetics/ pharmacodynamics (PK/PD) of single and repeated dose of CMND - 100 in healthy and AUD subjects Secondary end point: preliminarily evaluate the efficacy of CMND - 100 in reduction of drinking patterns and craving in individuals with moderate to severe AUD The potential effect of CMND - 100 on drinking patterns and cravings in individuals with AUD in accordance with DSM - V criteria Oral capsules will be given once daily, for ten consecutive days The patients will report their drinking patterns and craving for alcohol and potentially cigarettes during the clinical trial period Part A - Single Dose ~ 24 HV PK analysis DSMB analysis Part B - Single dose ~ 12 AUD Dose MTD1= 6 AUD pt Dose MTD2= 6 AUD pt PK analysis DSMB analysis Part C - Multiple Dose 18 HV Randomized Part - 2:1 ratio (drug: placebo) PK analysis DSMB analysis Part D - Multiple Doses 18 AUD 4 Ascending Dose Cohorts (6 HVs per Cohort) 20 mg next dose No toxicity to 2, Grade 1 or up to 2 HVs with Grade 2 – escalate 100% in next dose More than 2 HV develop grade 2 toxicity or higher - dose escalation is stopped, and refer to DSMB Four or more subjects develop grade 2 toxicity or higher, the study is stopped, and the maximal tolerated dose (MTD) is set Randomized Part - 2:1 ratio (drug: placebo) PK analysis DSMB analysis

Ongoing clinical programs: Clinical sites Clearmind Medicine Inc. Page 10 Corporate Presentation | July 2025 CMND - 100 for Alcohol Use Disorder Johns Hopkins School of Medicine Baltimore, MD, USA. Principal investigators: Jennifer Ellis, PhD and Professor Eric Strain, MD. Yale School of Medicine, Department of Psychiatry New Haven, CT, USA. Principal investigator: Anahita Bassir Nia, MD. Christopher Pittenger, MD, PhD, Director, Clinical Neuroscience Research Unit, Psychiatry; Director, Yale Program for Psychedelic Science, Psychiatry. Hadassa - University Medical Center Jerusalem, Israel Principal Investigator: Prof. Joseph Karko , MD , Director of the Center for Clinical Research. Tel Aviv Sourasky Medical Center Tel - Aviv, Israel Principal Investigator: Prof. David Zeltser, Deputy Director, R&D and Innovation. Director, Internal Medicine Division
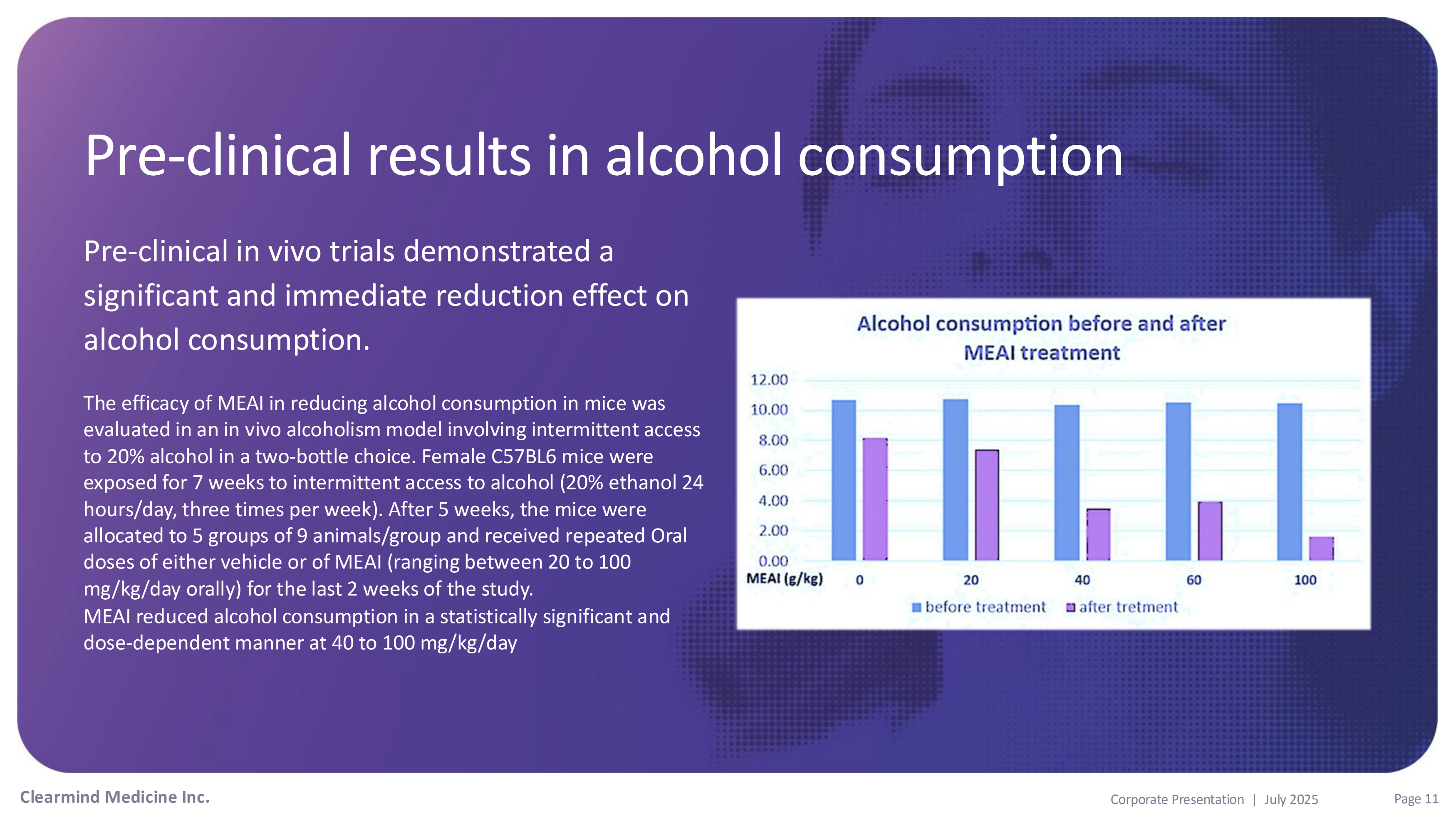
Pre - clinical results in alcohol consumption Pre - clinical in vivo trials demonstrated a significant and immediate reduction effect on alcohol consumption. The efficacy of MEAI in reducing alcohol consumption in mice was evaluated in an in vivo alcoholism model involving intermittent access to 20% alcohol in a two - bottle choice. Female C57BL6 mice were exposed for 7 weeks to intermittent access to alcohol (20% ethanol 24 hours/day, three times per week). After 5 weeks, the mice were allocated to 5 groups of 9 animals/group and received repeated Oral doses of either vehicle or of MEAI (ranging between 20 to 100 mg/kg/day orally) for the last 2 weeks of the study. MEAI reduced alcohol consumption in a statistically significant and dose - dependent manner at 40 to 100 mg/kg/day Clearmind Medicine Inc. Page 11 Corporate Presentation | July 2025
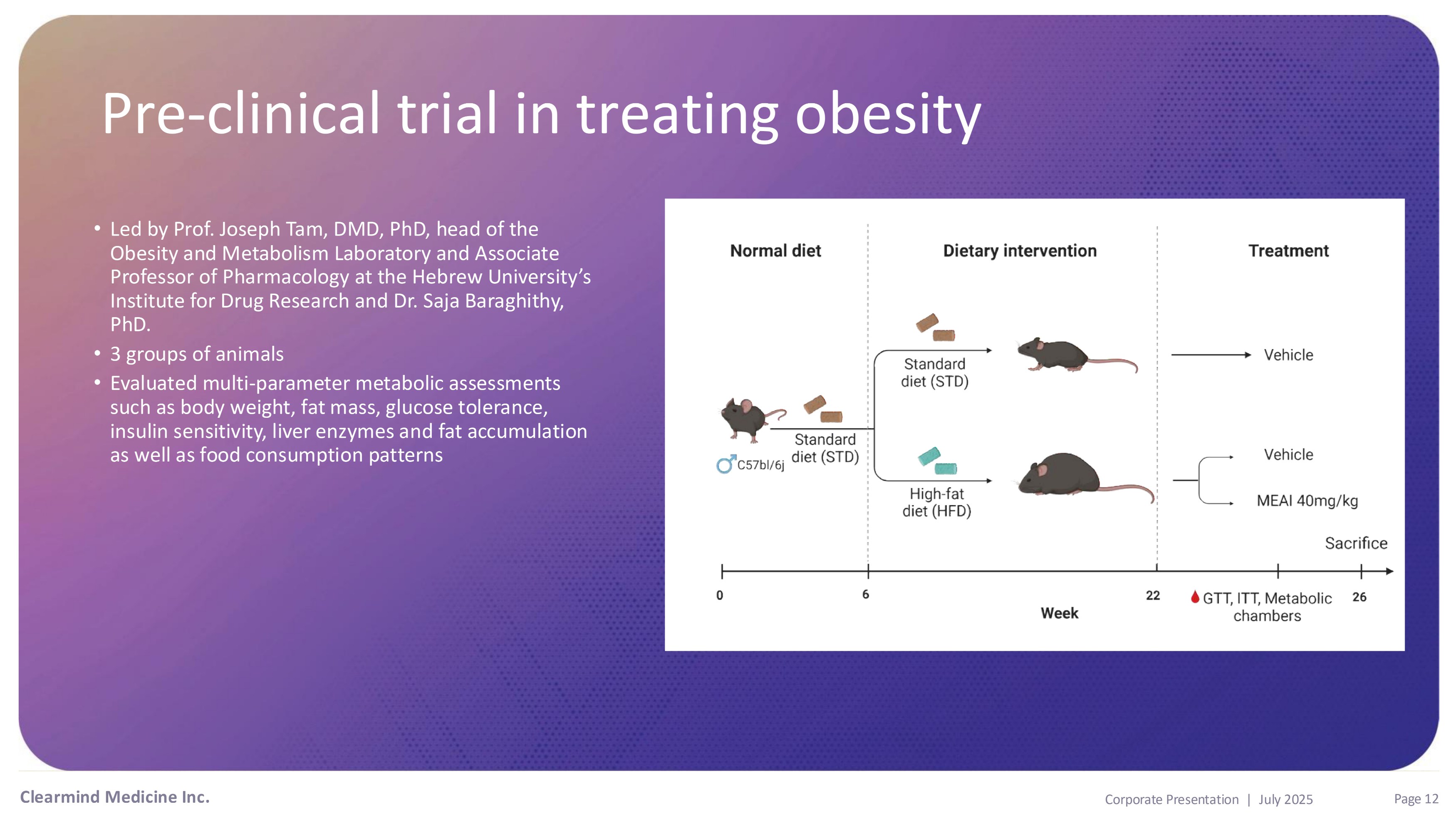
Pre - clinical results in treating Obesity • Led by Prof. Joseph Tam, DMD, PhD, head of the Obesity and Metabolism Laboratory and Associate Professor of Pharmacology at the Hebrew University’s Institute for Drug Research and Dr. Saja Baraghithy, PhD. • 3 groups of animals • Evaluated multi - parameter metabolic assessments such as body weight, fat mass, glucose tolerance, insulin sensitivity, liver enzymes and fat accumulation as well as food consumption patterns Pre - clinical trial in treating obesity Clearmind Medicine Inc. Page 12 Corporate Presentation | July 2025
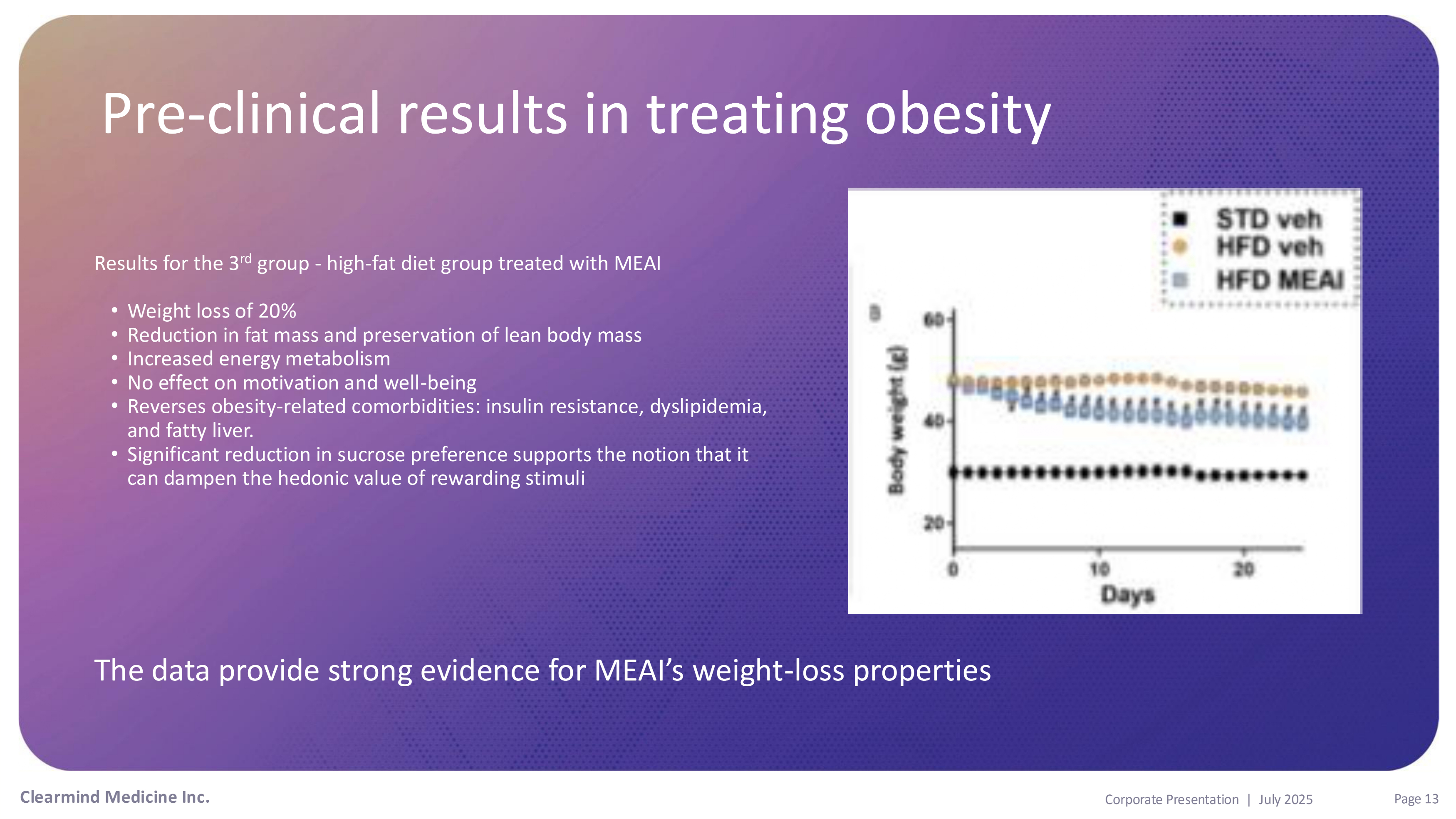
Pre - clinical results in treating Obesity Pre - clinical results in treating obesity Results for the 3 rd group - high - fat diet group treated with MEAI • Weight loss of 20% • Reduction in fat mass and preservation of lean body mass • Increased energy metabolism • No effect on motivation and well - being • Reverses obesity - related comorbidities: insulin resistance, dyslipidemia, and fatty liver. • Significant reduction in sucrose preference supports the notion that it can dampen the hedonic value of rewarding stimuli The data provide strong evidence for MEAI’s weight - loss properties Clearmind Medicine Inc. Page 13 Corporate Presentation | July 2025
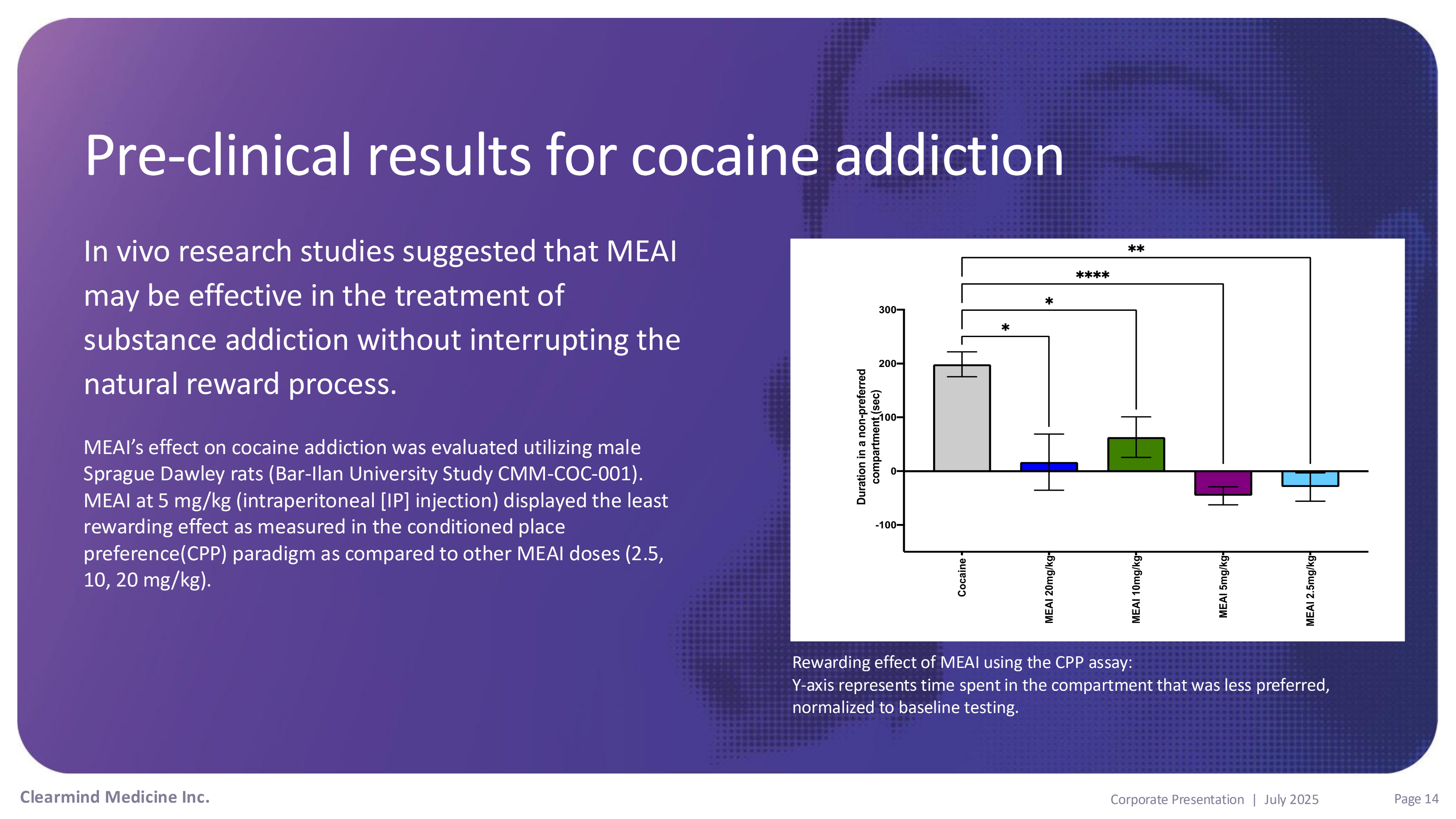
Pre - clinical results for cocaine addiction In vivo research studies suggested that MEAI may be effective in the treatment of substance addiction without interrupting the natural reward process. MEAI’s effect on cocaine addiction was evaluated utilizing male Sprague Dawley rats (Bar - Ilan University Study CMM - COC - 001). MEAI at 5 mg/kg (intraperitoneal [IP] injection) displayed the least rewarding effect as measured in the conditioned place preference(CPP) paradigm as compared to other MEAI doses (2.5, 10, 20 mg/kg). Rewarding effect of MEAI using the CPP assay: Y - axis represents time spent in the compartment that was less preferred, normalized to baseline testing. Clearmind Medicine Inc. Page 14 Corporate Presentation | July 2025
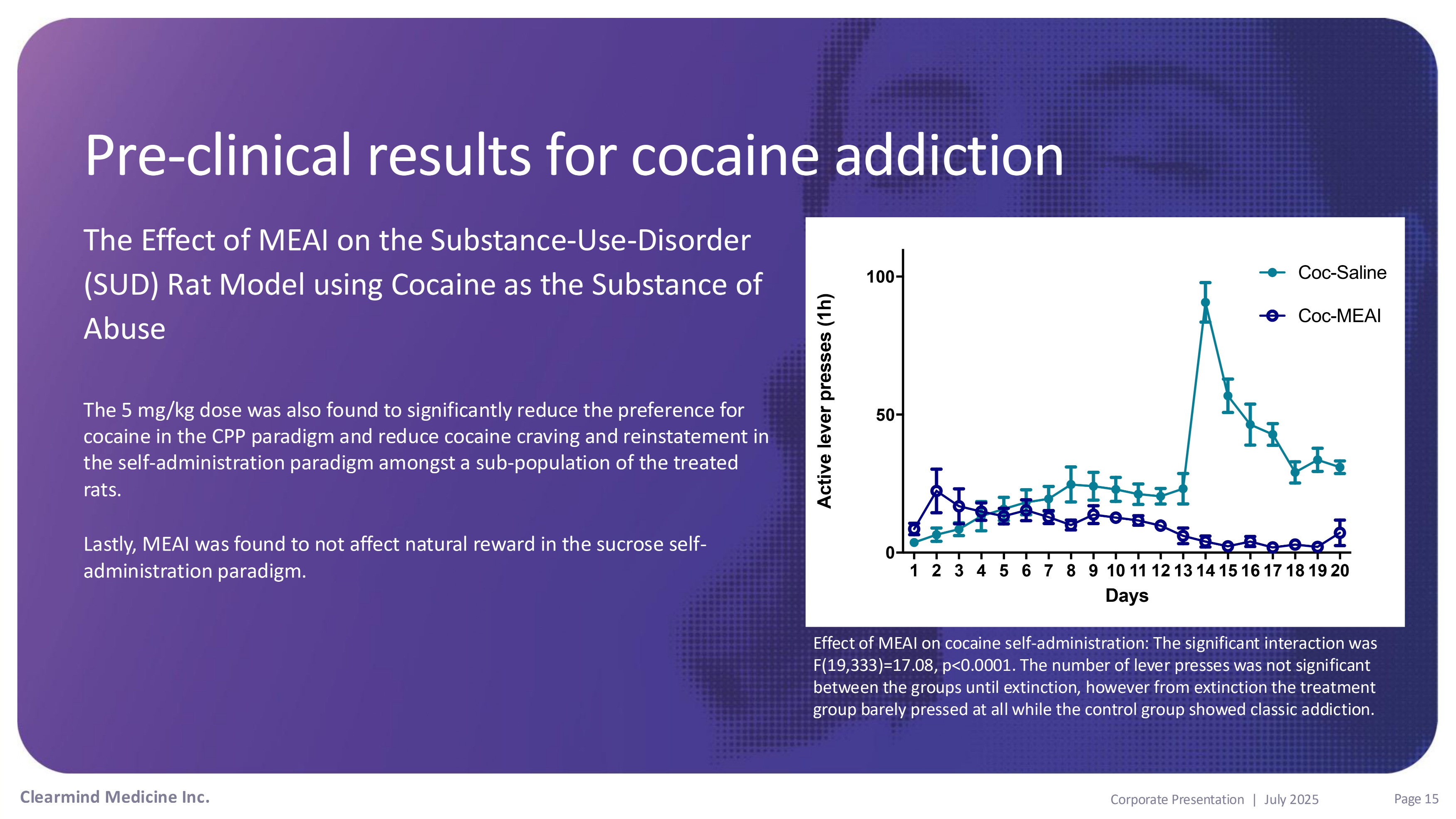
Pre - clinical results for cocaine addiction The Effect of MEAI on the Substance - Use - Disorder (SUD) Rat Model using Cocaine as the Substance of Abuse The 5 mg/kg dose was also found to significantly reduce the preference for cocaine in the CPP paradigm and reduce cocaine craving and reinstatement in the self - administration paradigm amongst a sub - population of the treated rats. Lastly, MEAI was found to not affect natural reward in the sucrose self - administration paradigm. 0 50 100 Coc - Saline Coc - MEAI Active lever presses (1h) Clearmind Medicine Inc. Page 15 Corporate Presentation | July 2025 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 Days Effect of MEAI on cocaine self - administration: The significant interaction was F(19,333)=17.08, p<0.0001. The number of lever presses was not significant between the groups until extinction, however from extinction the treatment group barely pressed at all while the control group showed classic addiction.
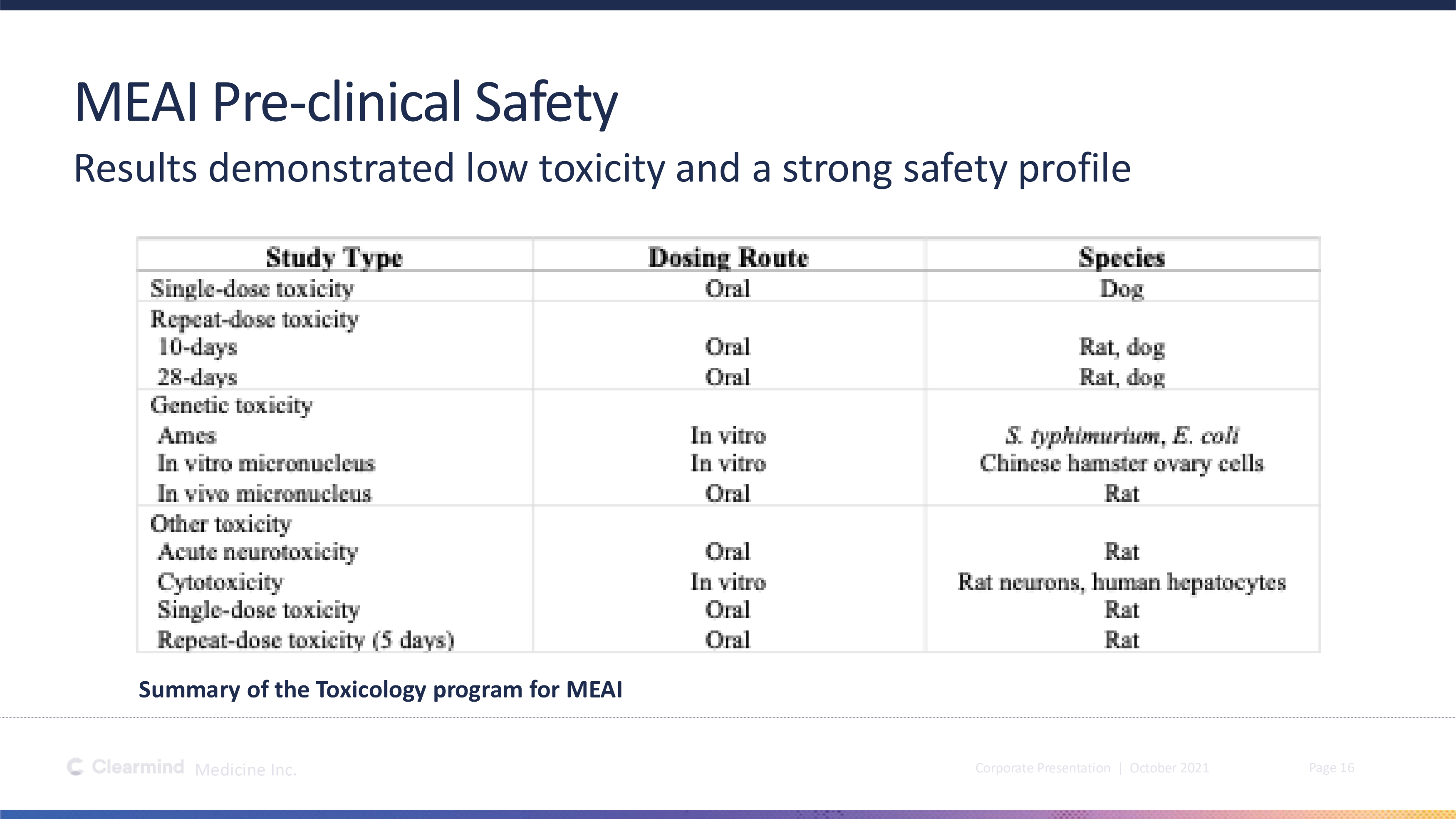
Corporate Presentation | October 2021 Medicine Inc. Page 16 MEAI Pre - clinical Safety Results demonstrated low toxicity and a strong safety profile Summary of the Toxicology program for MEAI

The Clearmind Advantage Next - gen and non - hallucinogenic MEAI is a next generation psychedelic, that does not induce hallucinations and is not addictive. MEAI is potentially effective for alcoholism, cocaine addiction and obesity. Accessible treatment Historical human data In 2018, an MEAI - based drink was sold in Canada by DACOA, an American company. DACOA organized consumer focus groups that consumed the drink and supplied feedback using a questionnaire. In this study, 96% of people who used MEAI recreationally described a reduction in alcohol consumption, 85% claimed that MEAI helped them refrain from overeating, and 78% claimed that MEAI helped them refrain from smoking. Thousands of MEAI - based drinks were sold and no adverse effects reported. Clearmind Medicine Inc. Corporate Presentation |J u l y 2 0 2 5 Page 17 CMND - 100 is designed to be self - administered, due to its high safety potential, and not in conjunction to psychotherapy. Sober lifestyle We are advancing a psychoactive, MEAI - derived beverage designed to replicate the social aspects of alcohol without its health consequences, targeting the fast - growing global no - alcohol market (valued at over $13 billion in 2023). Additionally, we have entered into an agreement to secure global manufacturers for our novel MEAI molecule. https://clearmindmedicine.com/news - release/clearmind - medicine - revolutionizes - the - 13 - billion - nonalcoholic - beverages - market - with - its - psychedelic - based - drink

Consists of 19 utility patent families owned by the company* Claims include composition of matter and method of use MEAI : 2 mature families for binge behaviour regulators and alcoholic beverage substitute MEAI + PEA : 4 families for combination treatment for binge behaviour , cocaine addiction, obesity and metabolic syndrome, and depression 6 families for combinations of PEA with: MDMA , ibogaine , ketamine , LSD , psilocybin and DMT 5MAPB, 6APB: as fail - safes for MDMA therapy 2FDCK: for treatment of depression and TRD 3 - MMC: for treatment of dyskinesia, and treatment of eating disorders Novel psychedelics, methods of preparation and uses Novel psychoactives, for treatment of mental disorders IP Portfolio: Clearmind Medicine Inc. Page 18 Corporate Presentation | July 2025 31 granted patents in major jurisdictions such as the US, the EU, China and India *Two of them jointly owned with Yissum and one jointly owned with BiIRAD

Strong and Experienced Management Team Adi Zuloff - Shani, PhD Chief Executive Officer Dr. Adi Zuloff - Shani is a Biomedical Research and Development Executive with vast experience and over 20 years of strategic and operational leadership in the healthcare industry, as well as a deep understanding of therapeutics development in heavily regulated environments. She has expertise in the pharmaceutical industry, leading cell and drug development through drug and product development, CMC, non - clinical, all stages of clinical development, as well as clinical development strategies and regulatory (FDA, EMEA, others) interactions, NDAs, leading INDs, as well as parallel EU activities. Dr. Zuloff - Shani holds a Ph.D. in human biology and immunology from Bar - Ilan University, Israel. Mark Haden, MSW VP Business Development Clearmind Medicine Inc. Page 19 Corporate Presentation | July 2025 Alan Rootenberg, CPA Chief Financial Officer Previously the Executive Director of MAPS (Multidisciplinary Association for Psychedelic Studies) Canada for 10 years, Mark is an adjunct professor at the University of British Columbia School of Population and Public Health, and has published on the issue of drug control policy and psychedelics. Mark also worked in the addictions field in counselling, supervisory and management positions for 28 years. Mark has provided public education on drugs and drug policy for over 30 years. Mark works with the Health Officers Council of British Columbia on their position papers on the issue of a regulated market for all currently illegal drugs. Has presented in conferences and training events in many countries. Awarded the Queen’s Diamond Jubilee Medal for drug policy reform work in 2013. Mr. Alan Rootenberg is a chartered professional accountant who has served as the Chief Financial Officer of a number of publicly traded companies, listed on the TSX, TSXV, CSE and OTCBB. Alan has a Bachelor of Commerce degree and received his CPA designation in Ontario, Canada. His extensive experience includes serving as Chief Financial Officer for mineral exploration, mining, technology and medical cannabis companies. Mr. Rootenberg holds a Certificate in the Theory of Accounting from University of Witwatersrand (1977), a bachelor’s degree in commerce from University of Witwatersrand (1975) and was registered as a CPA in 1980.

Team of International Experts Amit Shwartz, MSc R&D Consultant Shannon Smadella Community Development Yael Stav, PhD Program Management Sara Horn, PhD Clinical and Regulatory Affairs Nick Kadysh Special Advisor Alcohol Substitute Program Gadi Levin, MBA VP Finance Clearmind Medicine Inc. Page 20 Corporate Presentation | July 2025 Adi Varon Controller Mylene Touboul Accounting Manager

Award - Winning Advisory Board Prof. Alon Friedman MD, PhD, Dalhousie University, Halifax, Canada Prof. Christian Schütz MD, PhD, University of British Columbia, Canada Prof. John Krystal MD , Yale University, CT, US Prof. Henry R. Kranzler MD, PhD, University of Pennsylvania, PA, US Prof. Fatima Stanford MD, MPH, MPA, Harvard Medical School, MA, US Clearmind Medicine Inc. Page 21 Corporate Presentation | July 2025 Professor Kranzler is the Benjamin Rush Professor of Psychiatry, and Director of the Center for Studies of Addiction, at the University of Pennsylvania’s Perelman School of Medicine . His research focuses on the genetics and pharmacological treatment of substance dependence, with emphasis on precision addiction medicine. His research has been continuously supported since 1987 by grants from the National Institutes of Health . Professor Kranzler has authored or co - authored more than 600 journal articles, book chapters, and books, is a member of the editorial board of three peer - review journals as well as the editor of Alcohol : Clinical and Experimental Research . His work currently focuses on the molecular genetics of substance dependence and the personalized treatment of alcohol, opioid, and nicotine use disorders using a pharmacogenetic approach . Dr . Stanford is currently associated with Massachusetts General Hospital and is an Associate Professor of Medicine and Pediatrics at Harvard Medical School, where she serves as an educator, researcher, and policy maker . Her extensive academic background includes a Bachelor of Science and Masters of Public Health from Emory University, a MD from the Medical College of Georgia School of Medicine, a Masters of Public Administration from the Harvard Kennedy School of Government and MBA from the Quantic School of Business and Technology . Dr . Stanford completed her Obesity Medicine & Nutrition Fellowship at Massachusetts General Hospital and Harvard Medical School, further enhancing her expertise in the field . With a career dedicated to bridging the gaps between medicine, public health, and policy, Dr . Stanford has become a sought - after expert in obesity medicine, both nationally and internationally. Professor Alon Friedman is Professor of Neuroscience at both Ben - Gurion University of the Negev (BGU) in Beersheba, Israel, and Dalhousie University, Halifax, Canada . He is best known for his discoveries of the link between blood – brain barrier dysfunction (BBBd) and neuropathologies, and the mechanisms underlying BBBd - related diseases . His team developed novel imaging modalities for the diagnosis of BBBd in neuro - psychiatric disorders . Professor Schütz, an Associate Professor at the Department of Psychiatry in University of British Columbia's Faculty of Medicine, focuses on clinical interventions and health service in substance use disorders and dual diagnoses (mental and substance use disorders), as well as neurobiological and neurocognitive aspects of impulsive decision - making . His main research focuses on understanding mechanisms of relapse and impulsive decision - making to improve treatment of addiction and concurrent disorders . He has published over 100 research articles and a dozen chapters . Dr . Krystal is the Robert L . McNeil, Jr . , Professor of Translational Research ; Professor of Psychiatry, Neuroscience, and Psychology ; and Chair of the Department of Psychiatry at the Yale University . He is also Chief of Psychiatry and Behavioral Health at Yale - New Haven Hospital . He is a graduate of the University of Chicago, Yale University School of Medicine, and the Yale Psychiatry Residency Training Program . He has published extensively on the neurobiology and treatment of schizophrenia, alcoholism, PTSD, and depression . Notably, his laboratory discovered the rapid antidepressant effects of ketamine in humans . He is the Director of the NIAAA Center for the Translational Neuroscience of Alcoholism and the Clinical Neuroscience Division of the VA National Center for PTSD . Dr . Krystal is a member of the U . S . National Academy of Medicine and a Fellow of the American Association for the Advancement of Science . Currently, he is co - director of the Neuroscience Forum of the U . S . National Academies of Sciences, Engineering, and Medicine ; and editor of Biological Psychiatry (IF= 12 . 1 ) . He has chaired the NIMH Board of Scientific Counselors and served on the national advisory councils for both NIMH and NIAAA . Also, he is past president of the American College of Neuropsychopharmacology (ACNP) and International College of Neuropsychopharmacology (CINP) .

Award - Winning Advisory Board Prof. Gabriele Fischer MD, PhD, Medical University Vienna, Austria Prof. Michael Davidson MD, Israeli Medical Centre for Alzheimer, Israel Prof. Gal Yadid PhD, Bar Ilan University, Israel Prof. Wim van den Brink MD, PhD, University of Amsterdam, The Netherlands Prof. Joseph Tam DMD, PhD, Hebrew University, Israel Professor Tam is Professor of Pharmacology at the Hebrew University’s Institute for Drug Research . He is the Head of the Obesity and Metabolism Laboratory and the Director of the Multidisciplinary Center for Cannabinoid Research at the Hebrew University of Jerusalem . He is also a member of the Harvey M . Krueger Family Center for Nanoscience and Nanotechnology, served as president of the International Cannabinoid Research Society, and was a postdoctoral fellow at the National Institute on Alcohol Abuse and Alcoholism (NIAAA) of the NIH . Tam led the pre - clinical studies for MEAI at his lab at the Hebrew University . Clearmind Medicine Inc. Page 22 Corporate Presentation | July 2025 Professor Davidson has published over 350 articles in peer reviewed prestigious journals in the area of Psychiatry and of Alzheimer’s disease, including on development of novel treatments . Previously he was Department Chairman at the Tel Aviv University and Chief Editor of the European Neuropsy - chopharmacology journal . He is currently Chairman of the department of psychiatry at the Nicosia University medical school, the President of the Alzheimer Center in Israel, and actively involved in drug development . Professor Gabriele Fischer did her residency in psychiatry at Washington University, USA and at the Medical University Vienna, where she serves at head of the Addiction clinic . During her long research career she published > 150 scientific papers with > 400 presentations on different topics related to psychopharmacology . For many decades she works as a consultant for UNODC, WHO and other international organizations, in addition to her duty as member of the scientific board of EMCDDA (European monitoring center for drug & drug addiction); next to research studies in neuropsychopharmacology, her special science topics include human rights issues & gender related aspects. Wim van den Brink received his medical degree from the Free University in Amsterdam . He received his PhD degree form the State University of Groningen . Since 1992 he is full professor of Addiction Psychiatry at the Academic Medical Center of the University of Amsterdam . In 2014 he received the lifetime achievement award for science from the Netherlands Association of Psychiatry and in 2015 he was granted the status of honorable member of the Spanish Society for Dual disorders . In 2017 he received the European Addiction Research Award from the European Federation of Addiction Societies (EUFAS) and in 2020 he became a Doctor et Professor Honoris Causa at the Eötvös Loránd University in Budapest, Hungary . Until recently, he was an associate editor of Drug and Alcohol Dependence and chief editor of European Addiction Research . He has been the chair of the Workgroups that developed the Dutch Treatment Guidelines on Alcohol Use Disorders, Opiate Addiction and Drugs other than opioids . He is one of the founders and president of the International Collaboration of ADHD and Substance Abuse (ICASA) . Professor Gal Yadid is an expert in neuropsy - chopharmacology, and is a Lab Chief in the Brain center of the Bar Ilan University . His field research and treatment development for reward - related psychiatric disorders, specifically Addiction, PTSD and depression . His research moved from preclinical (using unique animal models) to clinical applying various methods (behavioral, cognitive, histochemical, neurochemical, genetic/epigenetic, microbiome .

Highly Accomplished Board of Directors Asaf Itzhaik Director Amitay Weiss Chairman of the Board Oz Adler Director Yehonatan Shachar Director Mr . Asaf Itzhaik has served as our Director since November 2022 . Mr . Itzhaik is a seasoned international businessman in retail, BTC, BTB and real estate . He is serving as a director in Tzmiha Ltd (Israel), GIX Internet (Israel), Jeff Brands (Nasdaq), and Rani Zim (Israel) . Mr . Itzhaik has 28 years of experience running an optic brand specializing in athletes . Mr . Yehonatan Shachar has served as our Director since April 15 , 2020 . Mr . Shachar has served as the Chief Executive Officer of Heroic Media Ltd . , a digital marketing agency that works with top Israeli e - commerce brands since February 2020 . Before this role, from June 2019 until February 2021 , Mr . Shachar served as the CEO of Chiron Refineries, where he led a merger with Upsellon brands . Mr . Shachar has an LLB in Law and MBA from the IDC International University in Herzliya, Israel . Mr . Oz Adler has served as our Director since September 2021 . Mr . Adler currently serves as the Chief Executive Officer and Chief Financial Officer of SciSparc Ltd . where he has served since April 2018 and between September 2017 and March 2018 , he served as VP Finance of SciSparc Ltd . From December 2020 to May 2021 , Mr . Adler served as the Chief Financial Officer of Medigus Ltd . Mr . Adler also worked in the audit department of Kost Forer Gabbay & Kasierer, a member of Ernst & Young Global between December 2012 and August 2017 . Mr . Adler currently serves on the board of directors of numerous private and publicly traded companies, including Elbit Imaging Ltd . (TASE : EMITF), Rail Vision Ltd . (NASDAQ : RVSN), Jeffs’ Brands Ltd . , Polyrizon Ltd . and Charging Robotics Ltd . Mr . Adler is a certified public accountant in Israel and holds a BA degree in Accounting and Business Management from The College of Management, Israel . Mr . Amitay Weiss has served Chairman of our Board of Directors since August 19 , 2019 . Mr . Weiss served as the Chief Executive Officer of SciSparc Ltd . from August 2020 until January 2022 , and since January 2022 , Mr . Weiss has served as the Chairman of SciScparc’s Board of Directors . In addition, Mr . Weiss currently serves as Chairman of the Board of Directors of both Automax Ltd . (TASE : AMX) and Save Foods, Inc . In 2016 , Mr . Weiss founded Amity Weiss Management Ltd . and now serves as its chief executive officer . From 2001 until 2015 , Mr . Weiss served as vice president of business marketing & development and in various other positions at Bank Poalei Agudat Israel Ltd . of the First International Bank of Israel group . Mr . Weiss holds a BA in economics from New England College, as well as MBA and LLB from Ono Academic College in Israel, an Israeli branch of University of Manchester . Hila Kiron - Revach Director Ms . Hila Kiron - Revach has served as our director since September 2024 . Ms . Kiron - Revach has served on the board of directors of Rail Vision Ltd . (Nasdaq : RVSN) since January 2024 . Ms . Kiron - Revach has served as a member of the board of directors of Geffen Biomed Ltd . since 2014 and has been a member of the board of directors of Zmiha Investment House Ltd . since 2021 . In 2021 , Ms . Kiron - Revach served as a professional advisor to the chairman of the board of directors and acting secretary of Eilat Ashkelon Pipeline Company . From 2015 to 2021 , Ms . Kiron - Revach served as a senior professional advisor to ministers in the Israeli government, including the minister of foreign affairs and minister of transportation . From 2012 until 2015 , Ms . Kiron - Revach served as CEO of Hamil 38 – the Israeli Center for National Master Plan to Strengthen Existing Building in the Face of Earthquakes, Tama 38 Ltd . and as an attorney at Tabakman & Co . Law Firm . Ms . Kiron - Revach holds an LLB from the Netanya Academic College and is a licensed attorney in Israel . Clearmind Medicine Inc. Page 23 Corporate Presentation | July 2025

Let's make history t o g e t h e r . Clearmind Medicine Inc. Page 24 Corporate Presentation | July 2025 Join our journey Investor Relations US: CMND@crescendo - IR.com invest@clearmindmedicine.com clearmindmedicine.com