

Pociredir Pioneer Study: 12 mg Cohort Data Release July 29, 2025

Disclaimer and Notice This presentation contains “forward-looking statements” of Fulcrum Therapeutics, Inc. (Fulcrum or Fulcrum Therapeutics) within the meaning of the Private Securities Litigation Reform Act of 1995 that involve substantial risks and uncertainties, including express or implied statements regarding Fulcrum’s goals for pociredir, pociredir’s best-in-class potential for the treatment of sickle cell disease, pociredir’s ability to induce fetal hemoglobin, vaso-occlusive crises during 12-week treatment period, enrollment in additional cohorts and timing of data releases, as well as timing and outcomes of meetings with the U.S. Food and Drug Administration, among others. All statements, other than statements of historical facts, contained in this presentation, including express or implied statements regarding Fulcrum's strategy, future operations, future financial position, prospects, plans and objectives of management, are forward-looking statements. The words “anticipate,” “believe,” “continue,” “could,” “estimate,” “expect,” “intend,” “may,” “plan,” “potential,” “predict,” “project,” “should,” “target,” “will,” “would” and similar expressions are intended to identify forward-looking statements, although not all forward-looking statements contain these identifying words. Any forward-looking statements are based on management’s current expectations of future events and are subject to a number of risks and uncertainties that could cause actual results to differ materially and adversely from those set forth in, or implied by, such forward-looking statements. These risks and uncertainties include, but are not limited to, risks associated with Fulcrum’s ability to continue to advance pociredir and its other product candidates in clinical trials, including enrollment and completion; estimating the potential patient population and/or market for Fulcrum's product candidates; replicating in clinical trials positive results found in preclinical studies and/or earlier-stage clinical trials pociredir and any other product candidates; obtaining, maintaining or protecting intellectual property rights related to Fulcrum’s product candidates; managing expenses; and raising the substantial additional capital needed to achieve its business objectives, among others. For a discussion of other risks and uncertainties, and other important factors, any of which could cause Fulcrum's actual results to differ from those contained in the forward-looking statements, see the “Risk Factors” section, as well as discussions of potential risks, uncertainties and other important factors, in Fulcrum's most recent filings with the Securities and Exchange Commission. In addition, the forward-looking statements included in this presentation represent Fulcrum's views as of the date hereof and should not be relied upon as representing Fulcrum's views as of any date subsequent to the date hereof. Fulcrum anticipates that subsequent events and developments will cause Fulcrum's views to change. While Fulcrum may elect to update these forward-looking statements at some point in the future, Fulcrum specifically disclaims any obligation to do so. Unless otherwise indicated in this presentation, 12 mg data (n=16) discussed herein relates to cohort 3b. The incomplete prior 12 mg cohort (3a) conducted prior to study hold not included in this analysis.

Today’s Guest Speakers Drs. Alan and Smith are practicing physicians and paid Investigators in Fulcrum Therapeutics’ Pioneer Study. The views and opinions expressed by Drs. Alan and Smith are their own and do not necessarily reflect those of Fulcrum Therapeutics. Sheinei Alan, M.D. Director, Inova Adult Sickle Cell Program & Assistant Professor, UVA School of Medicine Inova Campus Wally Smith, M.D. Director, VCU Adult Sickle Cell Program & Florence Neal Cooper Smith Professor of Sickle Cell Disease at Virginia Commonwealth University

Agenda for Investor Call Introduction Alex Sapir, President & CEO Sickle Cell Disease (SCD) and the Potential of a Once Daily Oral HbF-Inducer Iain Fraser MBChB, D.Phil, SVP Early Clinical Development Pioneer Study Overview and 12 mg Pociredir Cohort Data Update Sheinei Alan, M.D., Director, Inova Adult Sickle Cell Program & Assistant Professor, UVA School of Medicine Expert Perspective on Pociredir and Its Potential as a Once Daily Oral HbF-Inducer for Treating SCD Wally Smith, M.D., Director, VCU Adult Sickle Cell Program and Professor, VCU School of Internal Medicine Q&A Fulcrum Management, Drs. Alan and Smith Closing Remarks Alex Sapir, President & CEO
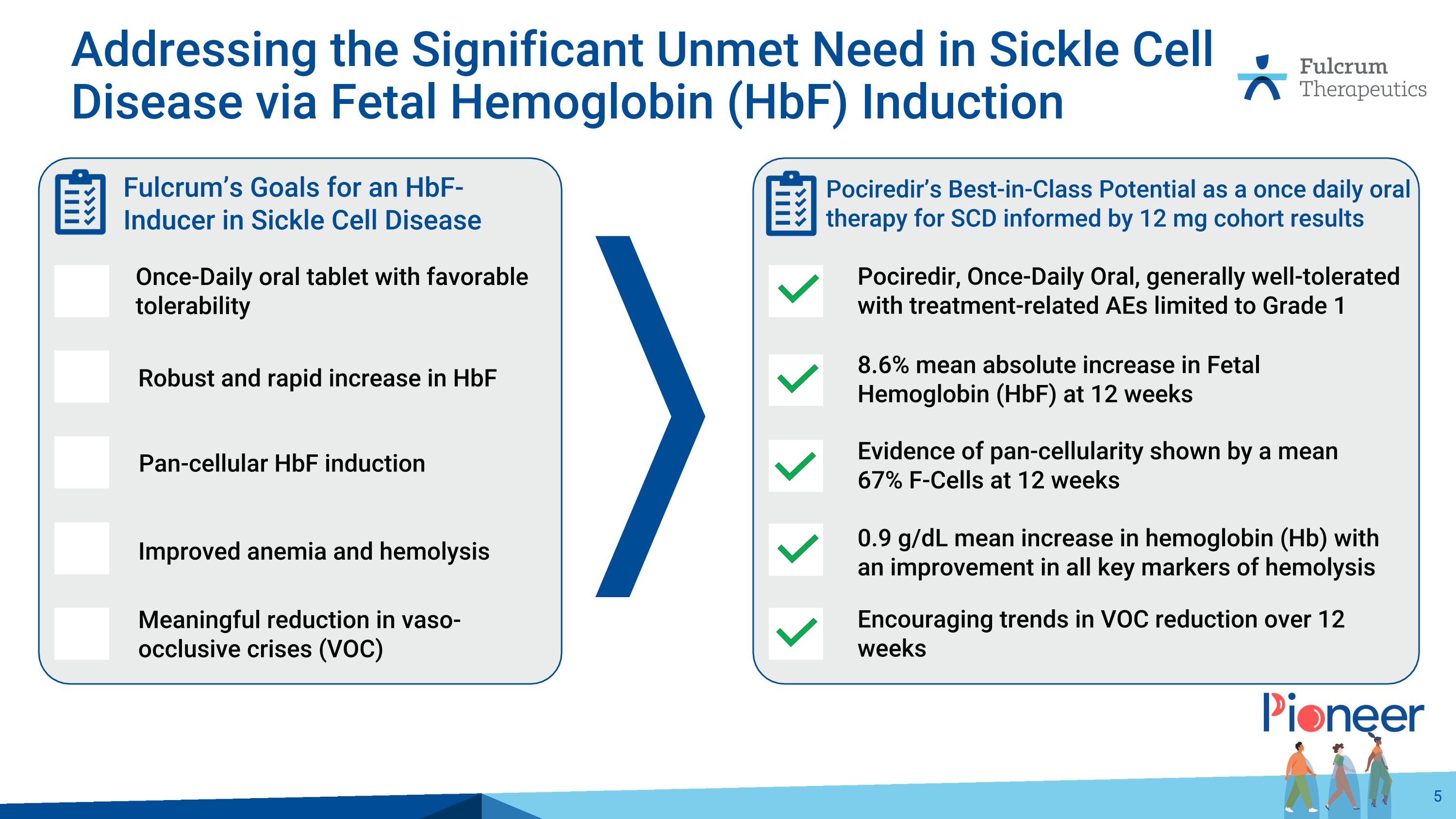
Evidence of pan-cellularity shown by a mean 67% F-Cells at 12 weeks 8.6% mean absolute increase in Fetal Hemoglobin (HbF) at 12 weeks 0.9 g/dL mean increase in hemoglobin (Hb) with an improvement in all key markers of hemolysis Encouraging trends in VOC reduction over 12 weeks Pociredir, Once-Daily Oral, generally well-tolerated with treatment-related AEs limited to Grade 1 Pociredir’s Best-in-Class Potential as a once daily oral therapy for SCD informed by 12 mg cohort results Addressing the Significant Unmet Need in Sickle Cell Disease via Fetal Hemoglobin (HbF) Induction Pan-cellular HbF induction Robust and rapid increase in HbF Improved anemia and hemolysis Meaningful reduction in vaso-occlusive crises (VOC) Once-Daily oral tablet with favorable tolerability Fulcrum’s Goals for an HbF-Inducer in Sickle Cell Disease

Agenda for Investor Call Introduction Alex Sapir, President & CEO Sickle Cell Disease (SCD) and the Potential of a Once Daily Oral HbF-Inducer Iain Fraser MBChB, D.Phil, SVP Early Clinical Development Pioneer Study Overview and 12mg Pociredir Cohort Data Update Sheinei Alan, M.D., Director, Inova Adult Sickle Cell Program & Assistant Professor, UVA School of Medicine Expert Perspective on Pociredir and Its Potential as a Once Daily Oral HbF-Inducer for Treating SCD Wally Smith, M.D., Director, VCU Adult Sickle Cell Program and Professor, VCU School of Internal Medicine Q&A Fulcrum Management, Dr. Alan and Dr. Smith Closing Remarks Alex Sapir, President & CEO
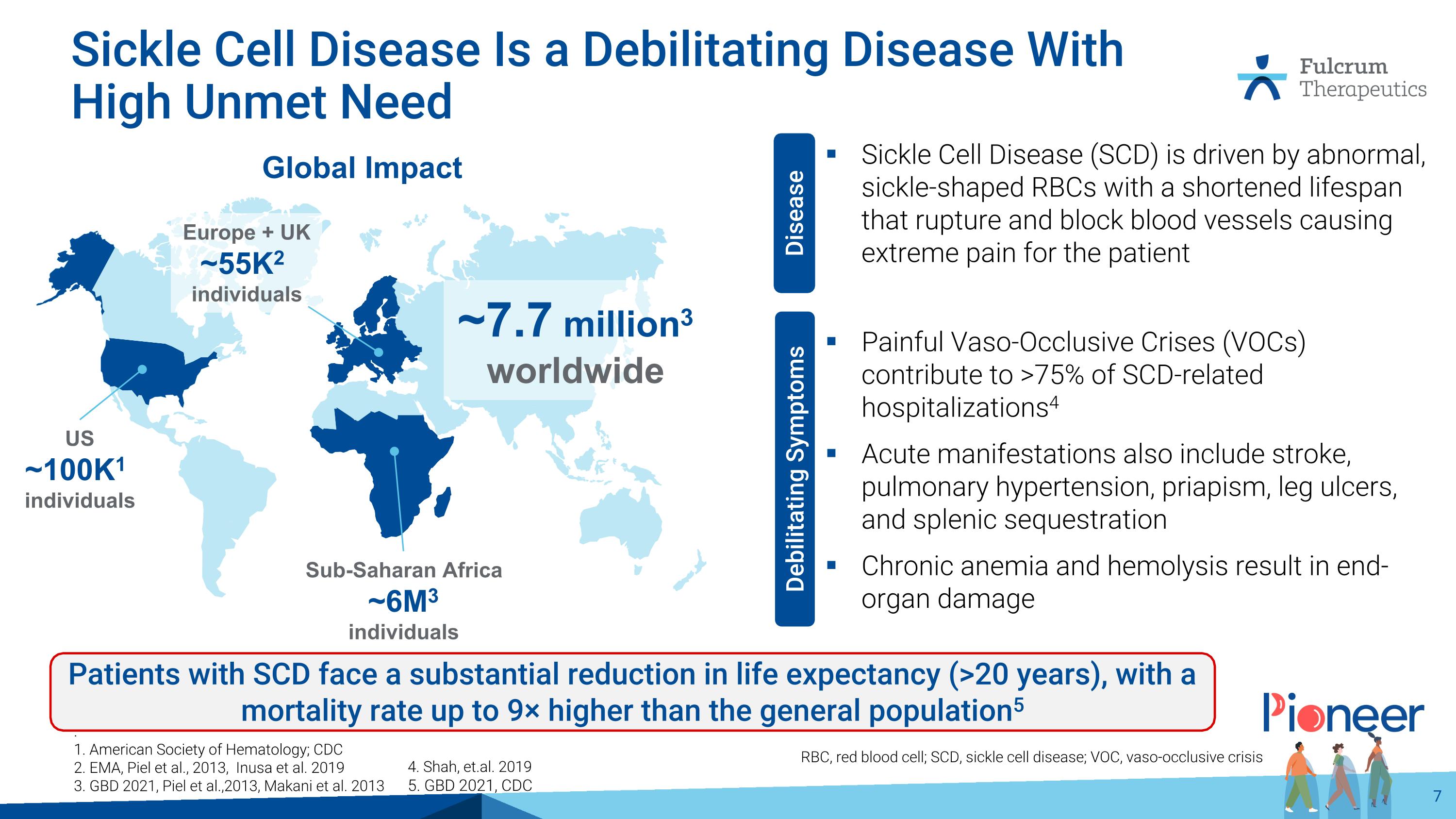
Sickle Cell Disease Is a Debilitating Disease With High Unmet Need Sickle Cell Disease (SCD) is driven by abnormal, sickle-shaped RBCs with a shortened lifespan that rupture and block blood vessels causing extreme pain for the patient Painful Vaso-Occlusive Crises (VOCs) contribute to >75% of SCD-related hospitalizations4 Acute manifestations also include stroke, pulmonary hypertension, priapism, leg ulcers, and splenic sequestration Chronic anemia and hemolysis result in end-organ damage . 1. American Society of Hematology; CDC 2. EMA, Piel et al., 2013, Inusa et al. 2019 3. GBD 2021, Piel et al.,2013, Makani et al. 2013 US ~100K1 individuals Sub-Saharan Africa ~6M3 individuals Global Impact ~7.7 million3 worldwide Europe + UK ~55K2 individuals Disease Debilitating Symptoms Patients with SCD face a substantial reduction in life expectancy (>20 years), with a mortality rate up to 9× higher than the general population5 RBC, red blood cell; SCD, sickle cell disease; VOC, vaso-occlusive crisis 4. Shah, et.al. 2019 5. GBD 2021, CDC
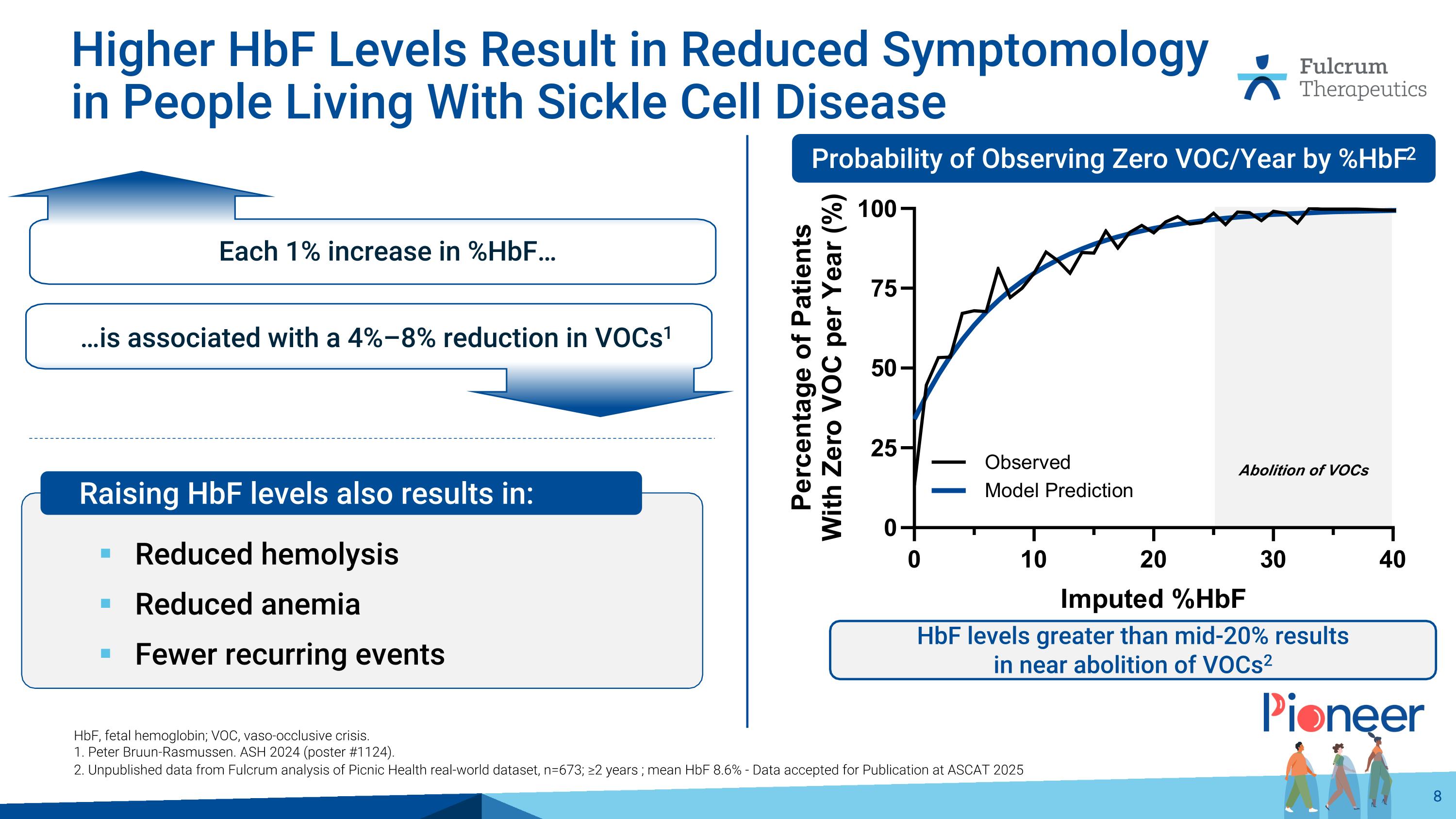
Abolition of VOCs Higher HbF Levels Result in Reduced Symptomology in People Living With Sickle Cell Disease Reduced hemolysis Reduced anemia Fewer recurring events HbF, fetal hemoglobin; VOC, vaso-occlusive crisis. 1. Peter Bruun-Rasmussen. ASH 2024 (poster #1124). 2. Unpublished data from Fulcrum analysis of Picnic Health real-world dataset, n=673; ≥2 years ; mean HbF 8.6% - Data accepted for Publication at ASCAT 2025 Probability of Observing Zero VOC/Year by %HbF2 HbF levels greater than mid-20% results in near abolition of VOCs2 Each 1% increase in %HbF… …is associated with a 4%–8% reduction in VOCs1 Raising HbF levels also results in:
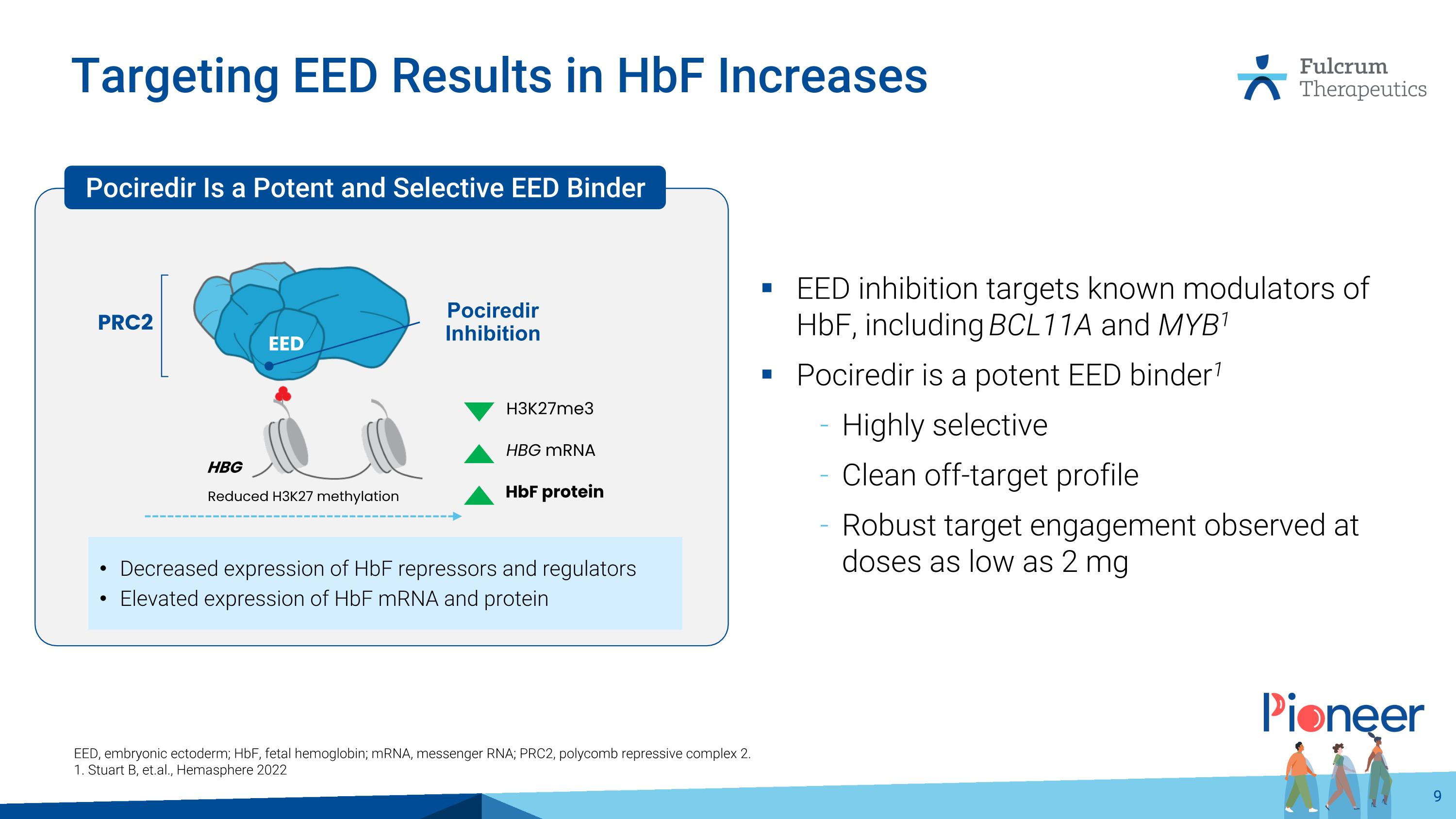
Targeting EED Results in HbF Increases EED inhibition targets known modulators of HbF, including BCL11A and MYB1 Pociredir is a potent EED binder1 Highly selective Clean off-target profile Robust target engagement observed at doses as low as 2 mg EED, embryonic ectoderm; HbF, fetal hemoglobin; mRNA, messenger RNA; PRC2, polycomb repressive complex 2. 1. Stuart B, et.al., Hemasphere 2022 Decreased expression of HbF repressors and regulators Elevated expression of HbF mRNA and protein Reduced H3K27 methylation EED H3K27me3 HBG mRNA HbF protein Pociredir Inhibition HBG Pociredir Is a Potent and Selective EED Binder PRC2
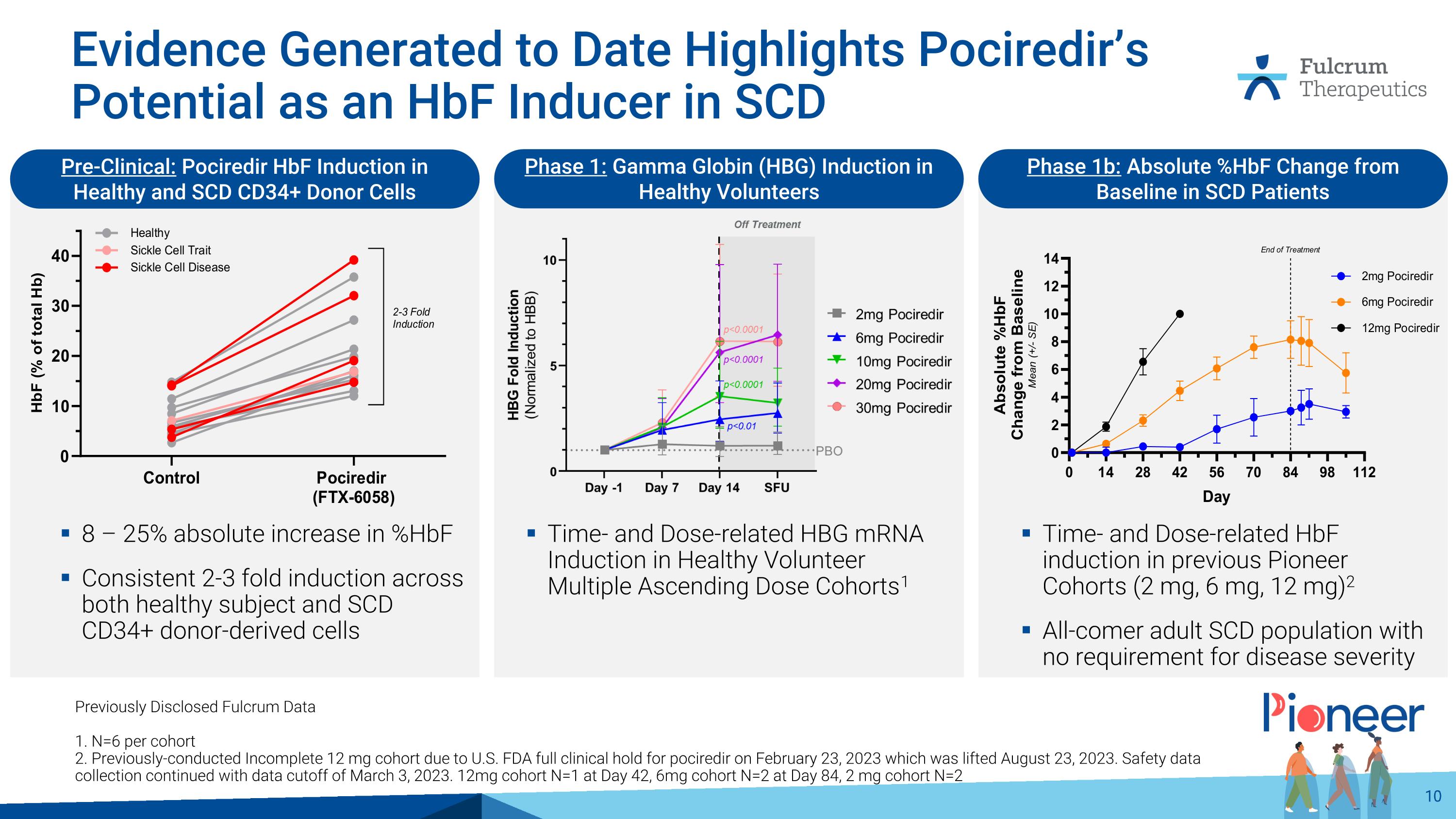
Evidence Generated to Date Highlights Pociredir’s Potential as an HbF Inducer in SCD Previously Disclosed Fulcrum Data 1. N=6 per cohort 2. Previously-conducted Incomplete 12 mg cohort due to U.S. FDA full clinical hold for pociredir on February 23, 2023 which was lifted August 23, 2023. Safety data collection continued with data cutoff of March 3, 2023. 12mg cohort N=1 at Day 42, 6mg cohort N=2 at Day 84, 2 mg cohort N=2 Pre-Clinical: Pociredir HbF Induction in Healthy and SCD CD34+ Donor Cells Phase 1: Gamma Globin (HBG) Induction in Healthy Volunteers Phase 1b: Absolute %HbF Change from Baseline in SCD Patients 8 – 25% absolute increase in %HbF Consistent 2-3 fold induction across both healthy subject and SCD CD34+ donor-derived cells Time- and Dose-related HBG mRNA Induction in Healthy Volunteer Multiple Ascending Dose Cohorts1 Time- and Dose-related HbF induction in previous Pioneer Cohorts (2 mg, 6 mg, 12 mg)2 All-comer adult SCD population with no requirement for disease severity

Agenda for Investor Call Introduction Alex Sapir, President & CEO Sickle Cell Disease (SCD) and the Potential of a Once Daily Oral HbF-Inducer Iain Fraser MBChB, D.Phil, SVP Early Clinical Development Pioneer Study Overview and 12 mg Pociredir Cohort Data Update Sheinei Alan, M.D., Director, Inova Adult Sickle Cell Program & Assistant Professor, UVA School of Medicine Expert Perspective on Pociredir and Its Potential as a Once Daily Oral HbF-Inducer for Treating SCD Wally Smith, M.D., Director, VCU Adult Sickle Cell Program and Professor, VCU School of Internal Medicine Q&A Fulcrum Management, Dr. Alan and Dr. Smith Closing Remarks Alex Sapir, President & CEO
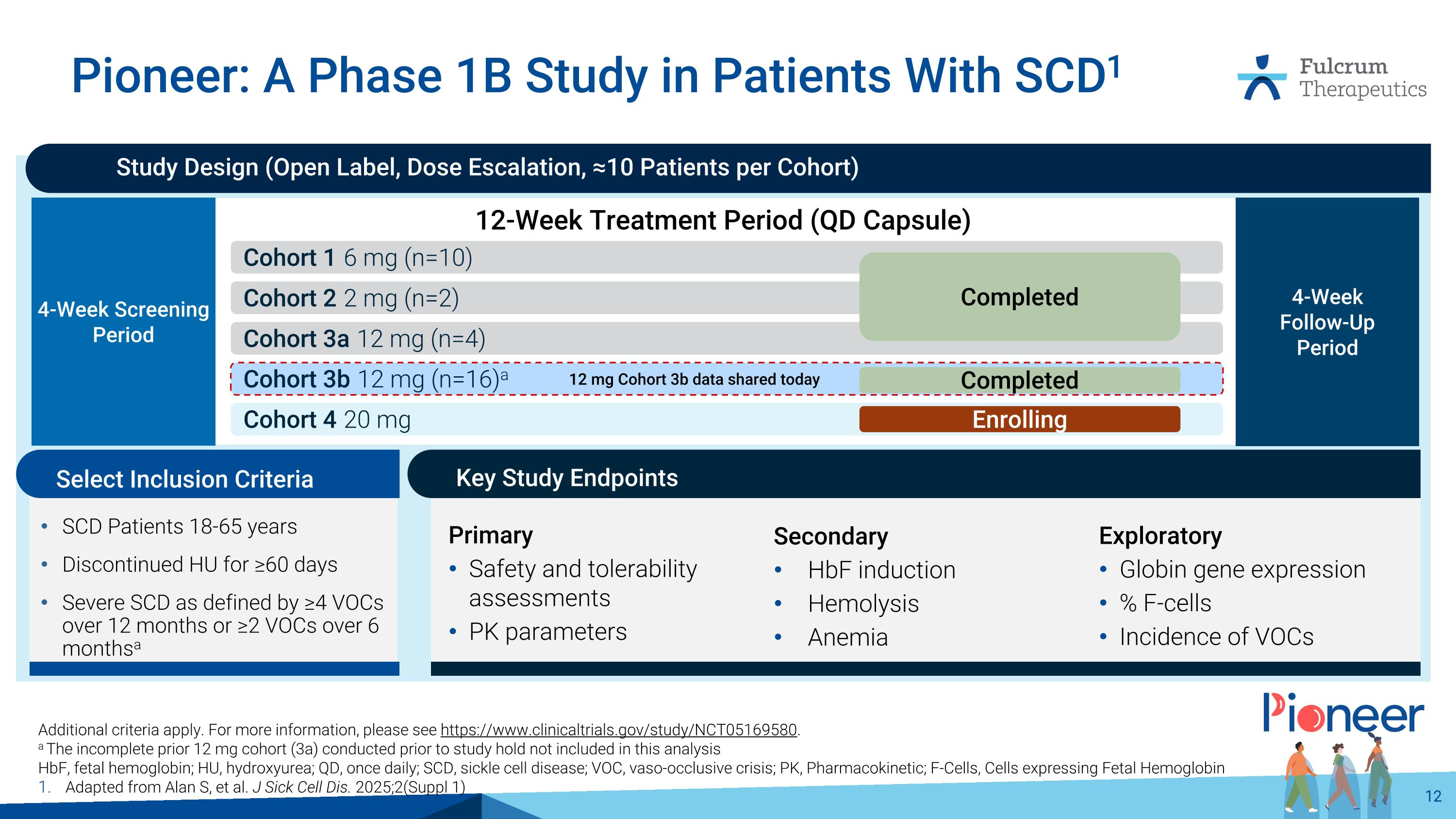
Pioneer: A Phase 1B Study in Patients With SCD1 Additional criteria apply. For more information, please see https://www.clinicaltrials.gov/study/NCT05169580. a The incomplete prior 12 mg cohort (3a) conducted prior to study hold not included in this analysis HbF, fetal hemoglobin; HU, hydroxyurea; QD, once daily; SCD, sickle cell disease; VOC, vaso-occlusive crisis; PK, Pharmacokinetic; F-Cells, Cells expressing Fetal Hemoglobin Adapted from Alan S, et al. J Sick Cell Dis. 2025;2(Suppl 1) Study Design (Open Label, Dose Escalation, ≈10 Patients per Cohort) 12-Week Treatment Period (QD Capsule) 4-Week Screening Period 4-Week Follow-Up Period Cohort 1 6 mg (n=10) Cohort 2 2 mg (n=2) Cohort 3b 12 mg (n=16)a Cohort 4 20 mg Enrolling Completed 12 mg Cohort 3b data shared today Cohort 3a 12 mg (n=4) Completed Key Study Endpoints SCD Patients 18-65 years Discontinued HU for ≥60 days Severe SCD as defined by ≥4 VOCs over 12 months or ≥2 VOCs over 6 monthsa Select Inclusion Criteria Secondary HbF induction Hemolysis Anemia Primary Safety and tolerability assessments PK parameters Exploratory Globin gene expression % F-cells Incidence of VOCs
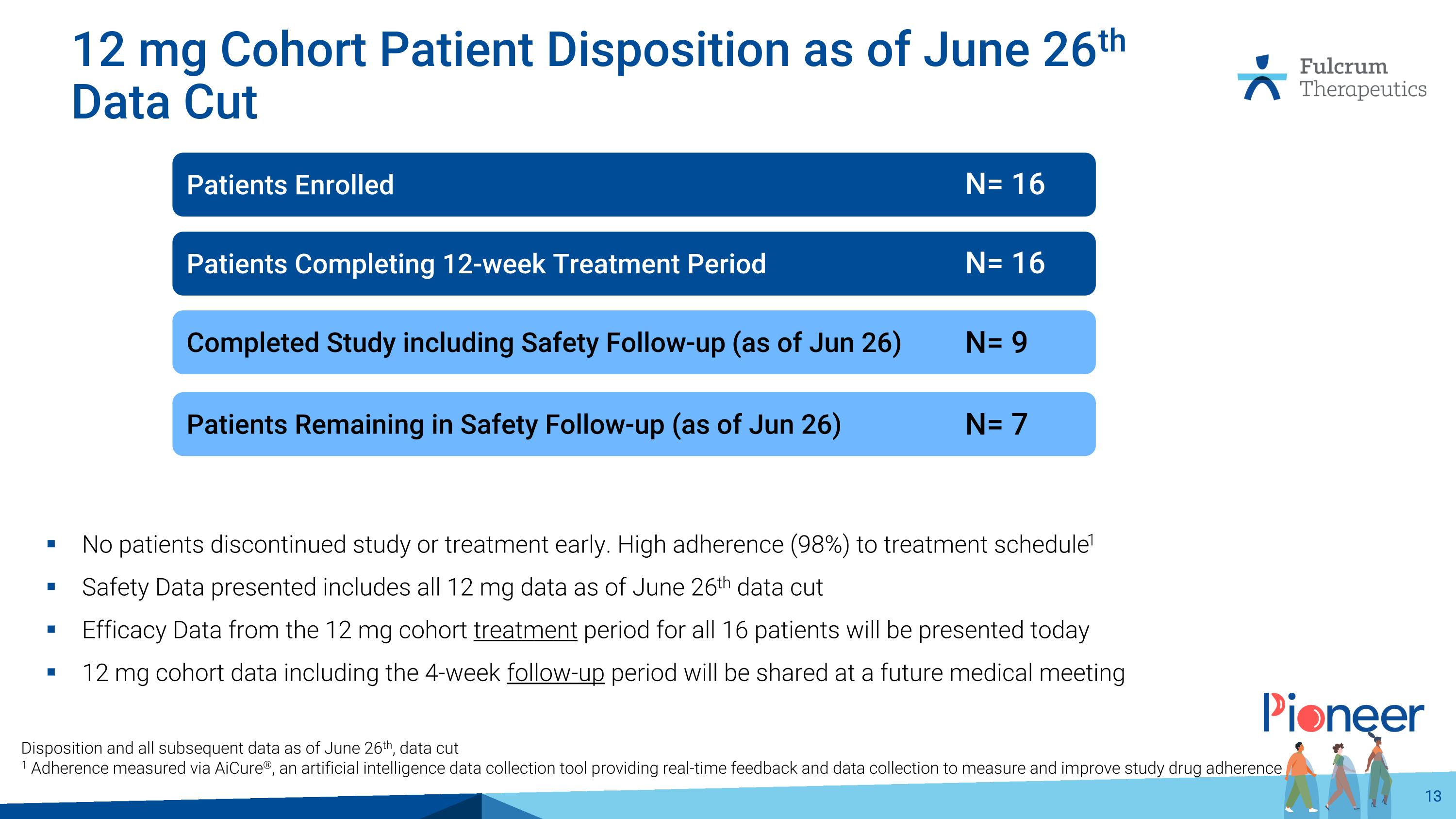
12 mg Cohort Patient Disposition as of June 26th Data Cut No patients discontinued study or treatment early. High adherence (98%) to treatment schedule1 Safety Data presented includes all 12 mg data as of June 26th data cut Efficacy Data from the 12 mg cohort treatment period for all 16 patients will be presented today 12 mg cohort data including the 4-week follow-up period will be shared at a future medical meeting Disposition and all subsequent data as of June 26th, data cut 1 Adherence measured via AiCure®, an artificial intelligence data collection tool providing real-time feedback and data collection to measure and improve study drug adherence Patients Enrolled Patients Completing 12-week Treatment Period Patients Remaining in Safety Follow-up (as of Jun 26) Completed Study including Safety Follow-up (as of Jun 26) N= 16 N= 16 N= 7 N= 9
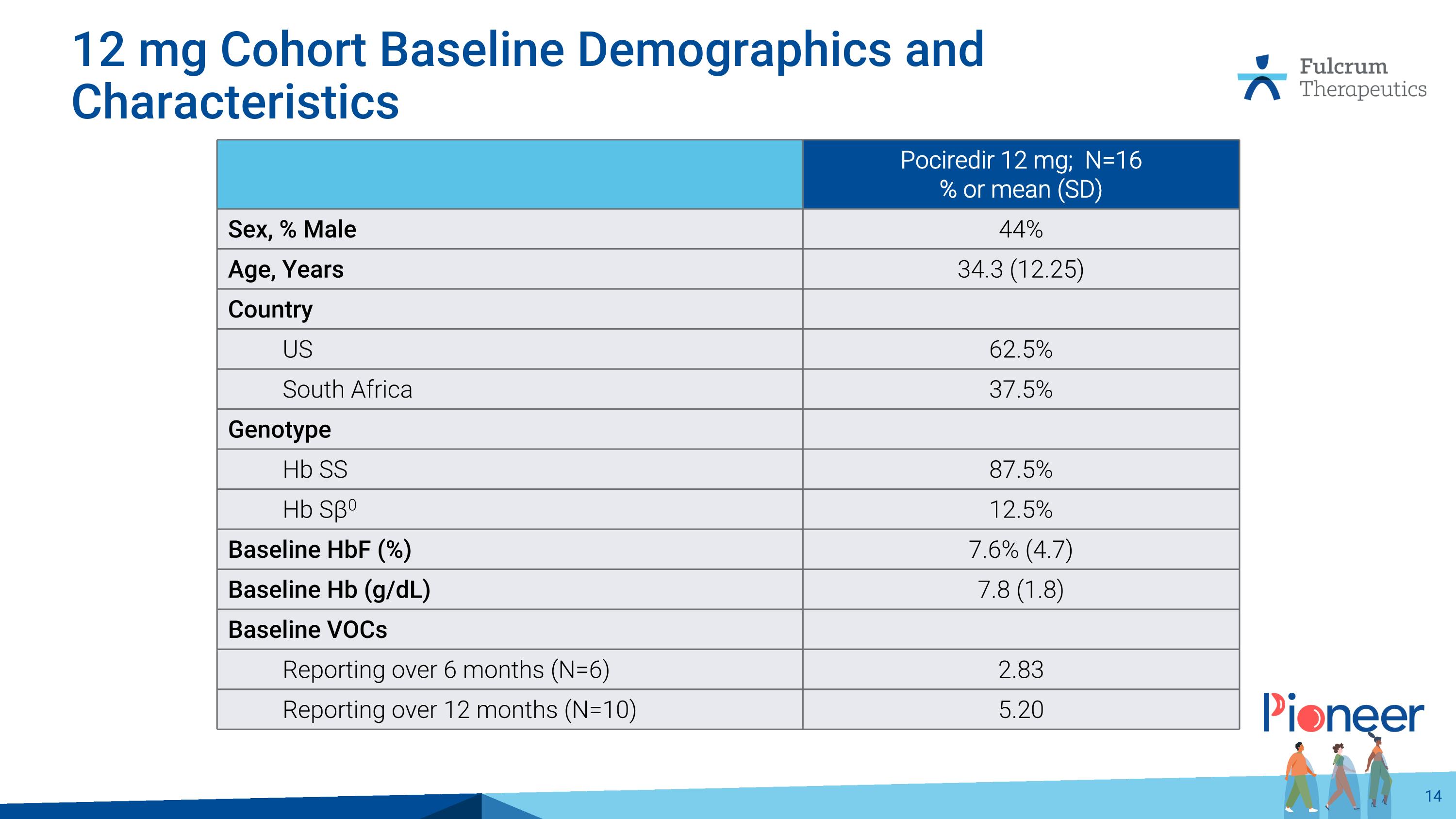
12 mg Cohort Baseline Demographics and Characteristics Pociredir 12 mg; N=16 % or mean (SD) Sex, % Male 44% Age, Years 34.3 (12.25) Country US 62.5% South Africa 37.5% Genotype Hb SS 87.5% Hb Sβ0 12.5% Baseline HbF (%) 7.6% (4.7) Baseline Hb (g/dL) 7.8 (1.8) Baseline VOCs Reporting over 6 months (N=6) 2.83 Reporting over 12 months (N=10) 5.20
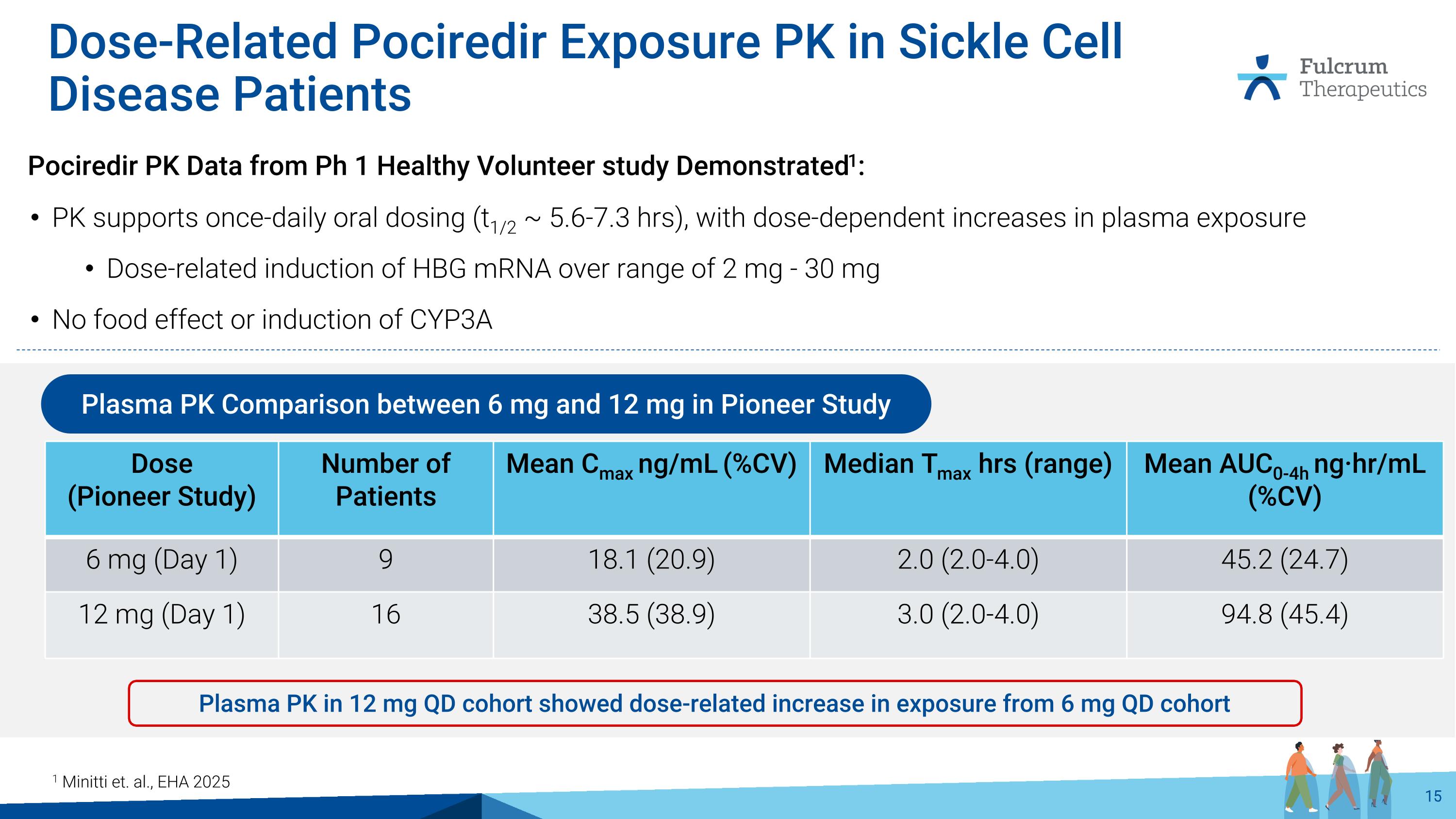
Dose-Related Pociredir Exposure PK in Sickle Cell Disease Patients Dose (Pioneer Study) Number of Patients Mean Cmax ng/mL (%CV) Median Tmax hrs (range) Mean AUC0-4h ng·hr/mL (%CV) 6 mg (Day 1) 9 18.1 (20.9) 2.0 (2.0-4.0) 45.2 (24.7) 12 mg (Day 1) 16 38.5 (38.9) 3.0 (2.0-4.0) 94.8 (45.4) Plasma PK Comparison between 6 mg and 12 mg in Pioneer Study Plasma PK in 12 mg QD cohort showed dose-related increase in exposure from 6 mg QD cohort 1 Minitti et. al., EHA 2025 Pociredir PK Data from Ph 1 Healthy Volunteer study Demonstrated1: PK supports once-daily oral dosing (t1/2 ~ 5.6-7.3 hrs), with dose-dependent increases in plasma exposure Dose-related induction of HBG mRNA over range of 2 mg - 30 mg No food effect or induction of CYP3A
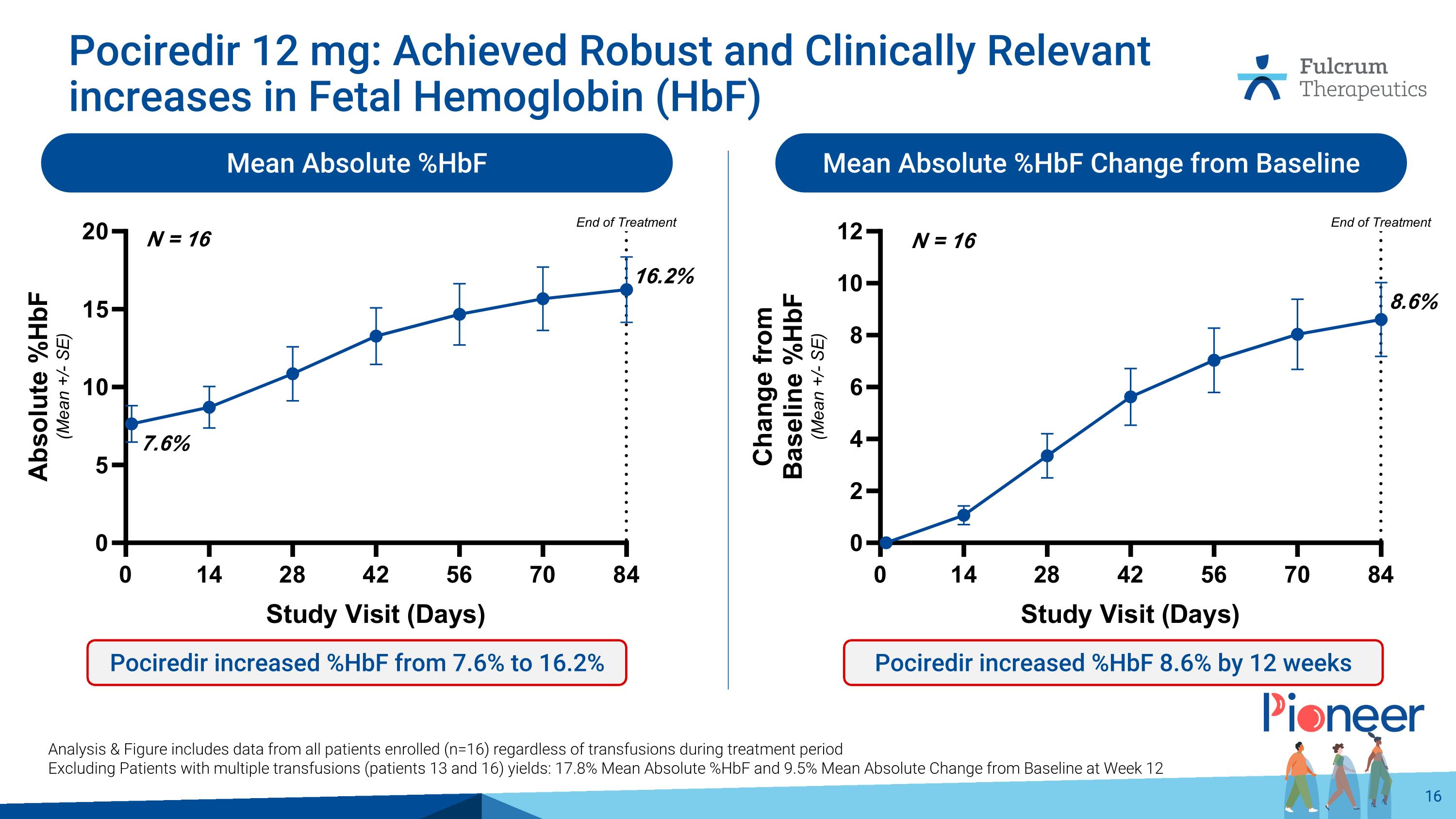
Pociredir 12 mg: Achieved Robust and Clinically Relevant increases in Fetal Hemoglobin (HbF) Mean Absolute %HbF Mean Absolute %HbF Change from Baseline Pociredir increased %HbF from 7.6% to 16.2% Pociredir increased %HbF 8.6% by 12 weeks Analysis & Figure includes data from all patients enrolled (n=16) regardless of transfusions during treatment period Excluding Patients with multiple transfusions (patients 13 and 16) yields: 17.8% Mean Absolute %HbF and 9.5% Mean Absolute Change from Baseline at Week 12 8.6% 16.2% 7.6% N = 16 N = 16
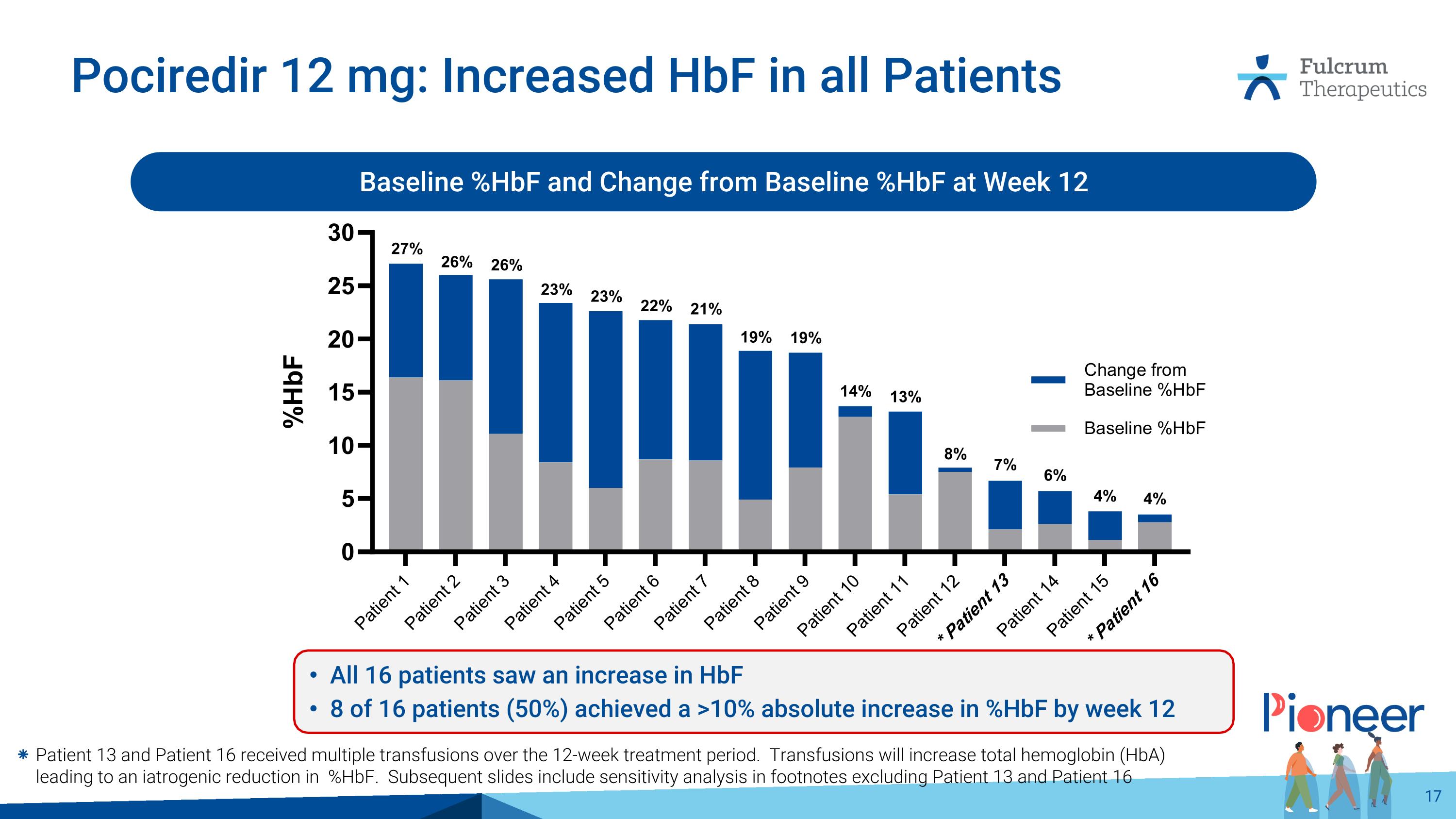
Pociredir 12 mg: Increased HbF in all Patients Baseline %HbF and Change from Baseline %HbF at Week 12 Patient 13 and Patient 16 received multiple transfusions over the 12-week treatment period. Transfusions will increase total hemoglobin (HbA) leading to an iatrogenic reduction in %HbF. Subsequent slides include sensitivity analysis in footnotes excluding Patient 13 and Patient 16 All 16 patients saw an increase in HbF 8 of 16 patients (50%) achieved a >10% absolute increase in %HbF by week 12 27% 26% 26% 23% 23% 22% 21% 19% 19% 14% 13% 8% 7% 6% 4% 4%
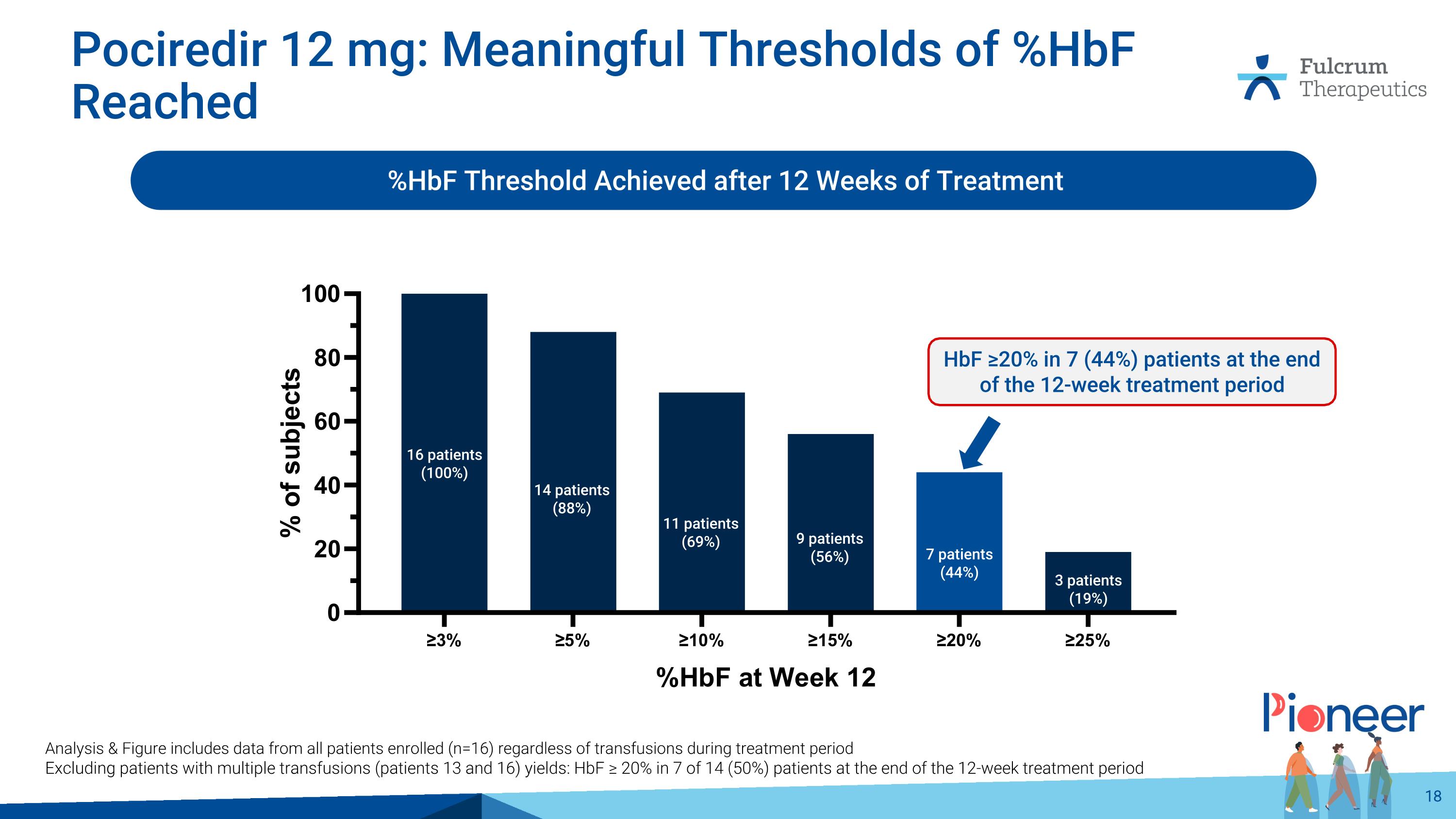
Pociredir 12 mg: Meaningful Thresholds of %HbF Reached %HbF Threshold Achieved after 12 Weeks of Treatment Analysis & Figure includes data from all patients enrolled (n=16) regardless of transfusions during treatment period Excluding patients with multiple transfusions (patients 13 and 16) yields: HbF ≥ 20% in 7 of 14 (50%) patients at the end of the 12-week treatment period 16 patients (100%) 7 patients (44%) 3 patients (19%) 14 patients (88%) 11 patients (69%) 9 patients (56%) HbF ≥20% in 7 (44%) patients at the end of the 12-week treatment period
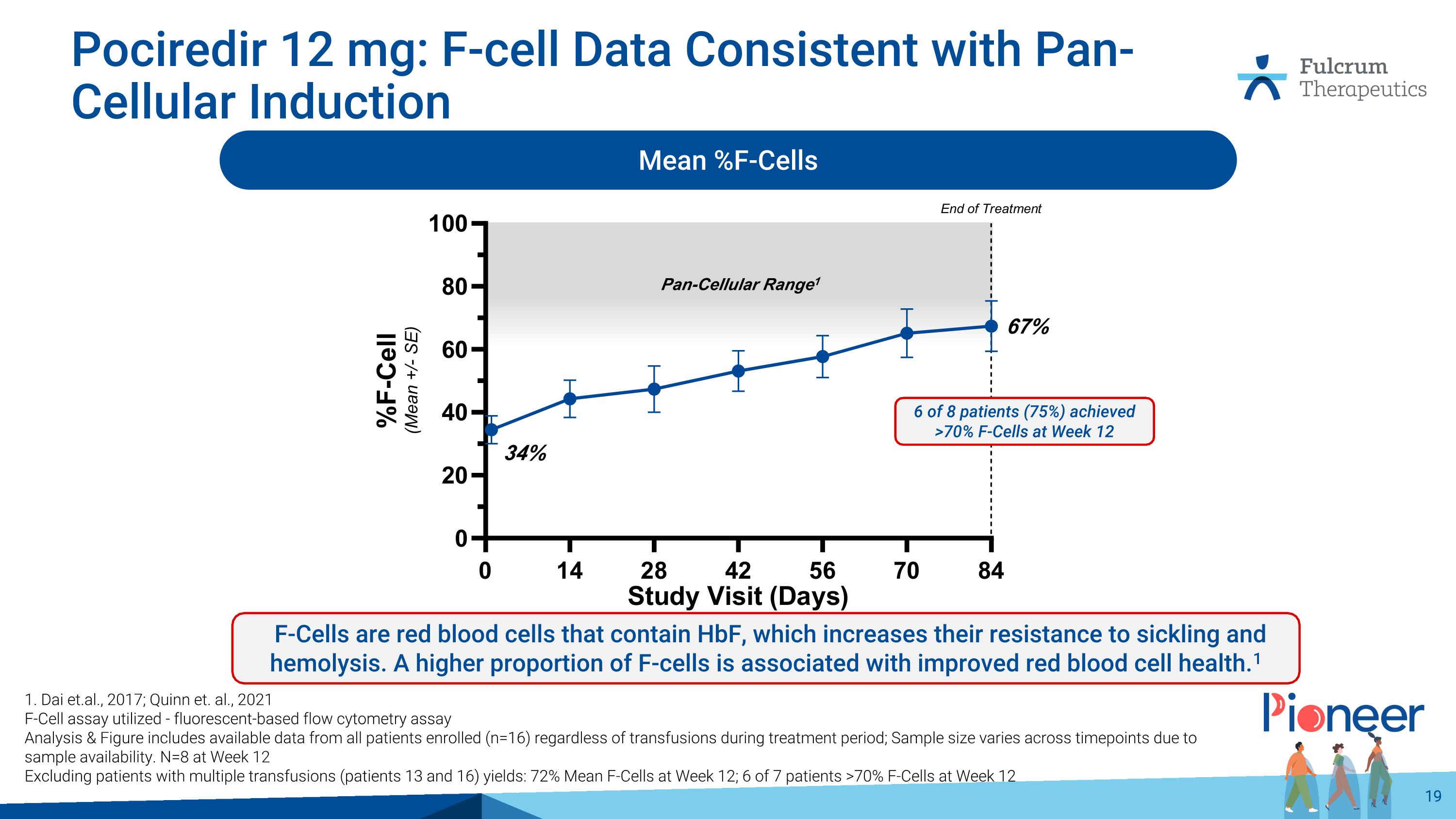
Pan-Cellular Range1 Pociredir 12 mg: F-cell Data Consistent with Pan-Cellular Induction 1. Dai et.al., 2017; Quinn et. al., 2021 F-Cell assay utilized - fluorescent-based flow cytometry assay Analysis & Figure includes available data from all patients enrolled (n=16) regardless of transfusions during treatment period; Sample size varies across timepoints due to sample availability. N=8 at Week 12 Excluding patients with multiple transfusions (patients 13 and 16) yields: 72% Mean F-Cells at Week 12; 6 of 7 patients >70% F-Cells at Week 12 Mean %F-Cells 6 of 8 patients (75%) achieved >70% F-Cells at Week 12 67% 34% F-Cells are red blood cells that contain HbF, which increases their resistance to sickling and hemolysis. A higher proportion of F-cells is associated with improved red blood cell health.1
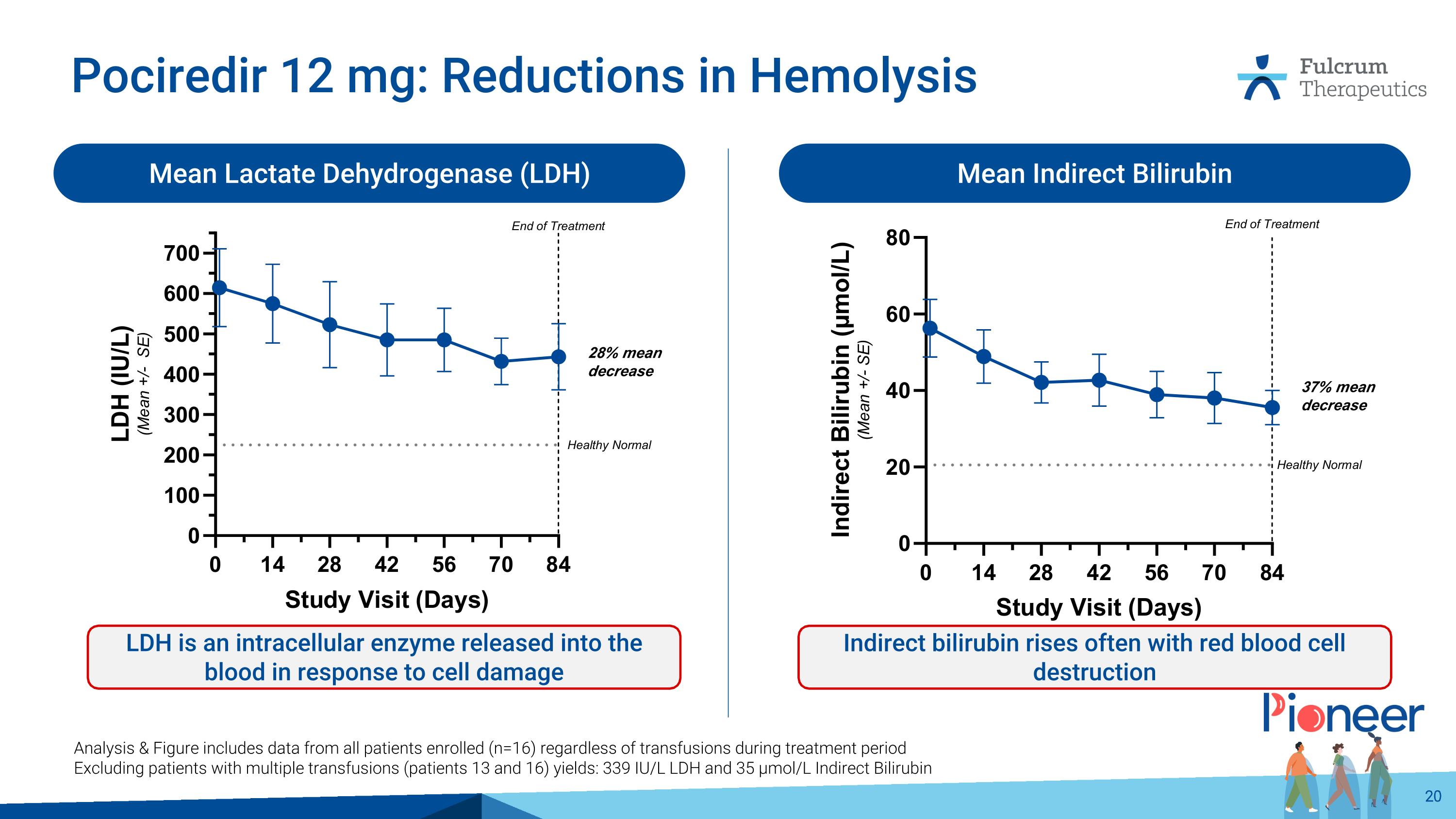
Pociredir 12 mg: Reductions in Hemolysis Mean Indirect Bilirubin Mean Lactate Dehydrogenase (LDH) LDH is an intracellular enzyme released into the blood in response to cell damage Indirect bilirubin rises often with red blood cell destruction Analysis & Figure includes data from all patients enrolled (n=16) regardless of transfusions during treatment period Excluding patients with multiple transfusions (patients 13 and 16) yields: 339 IU/L LDH and 35 µmol/L Indirect Bilirubin 28% mean decrease 37% mean decrease
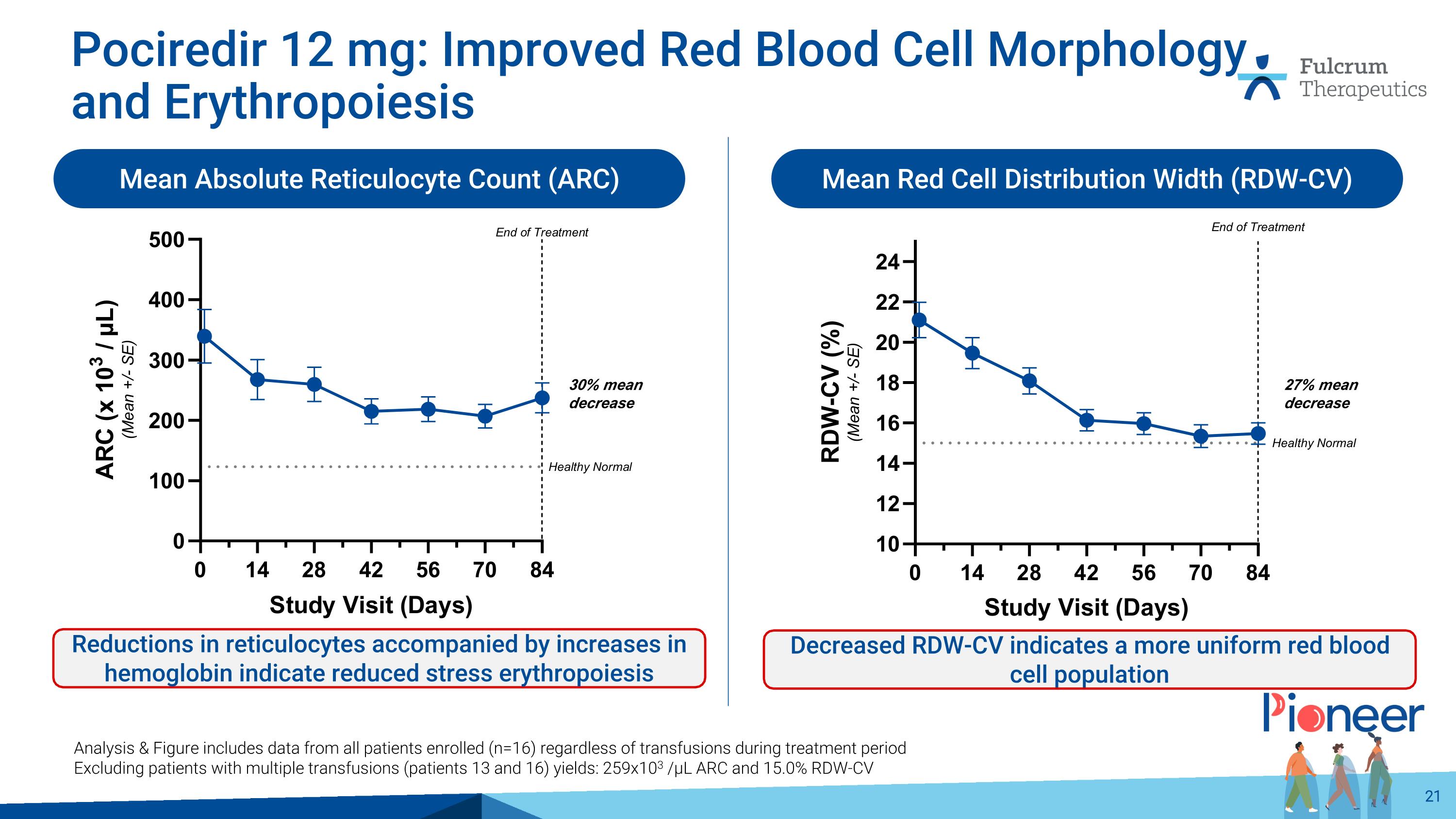
Pociredir 12 mg: Improved Red Blood Cell Morphology and Erythropoiesis Mean Absolute Reticulocyte Count (ARC) Mean Red Cell Distribution Width (RDW-CV) Reductions in reticulocytes accompanied by increases in hemoglobin indicate reduced stress erythropoiesis Decreased RDW-CV indicates a more uniform red blood cell population Analysis & Figure includes data from all patients enrolled (n=16) regardless of transfusions during treatment period Excluding patients with multiple transfusions (patients 13 and 16) yields: 259x103 /µL ARC and 15.0% RDW-CV 30% mean decrease 27% mean decrease
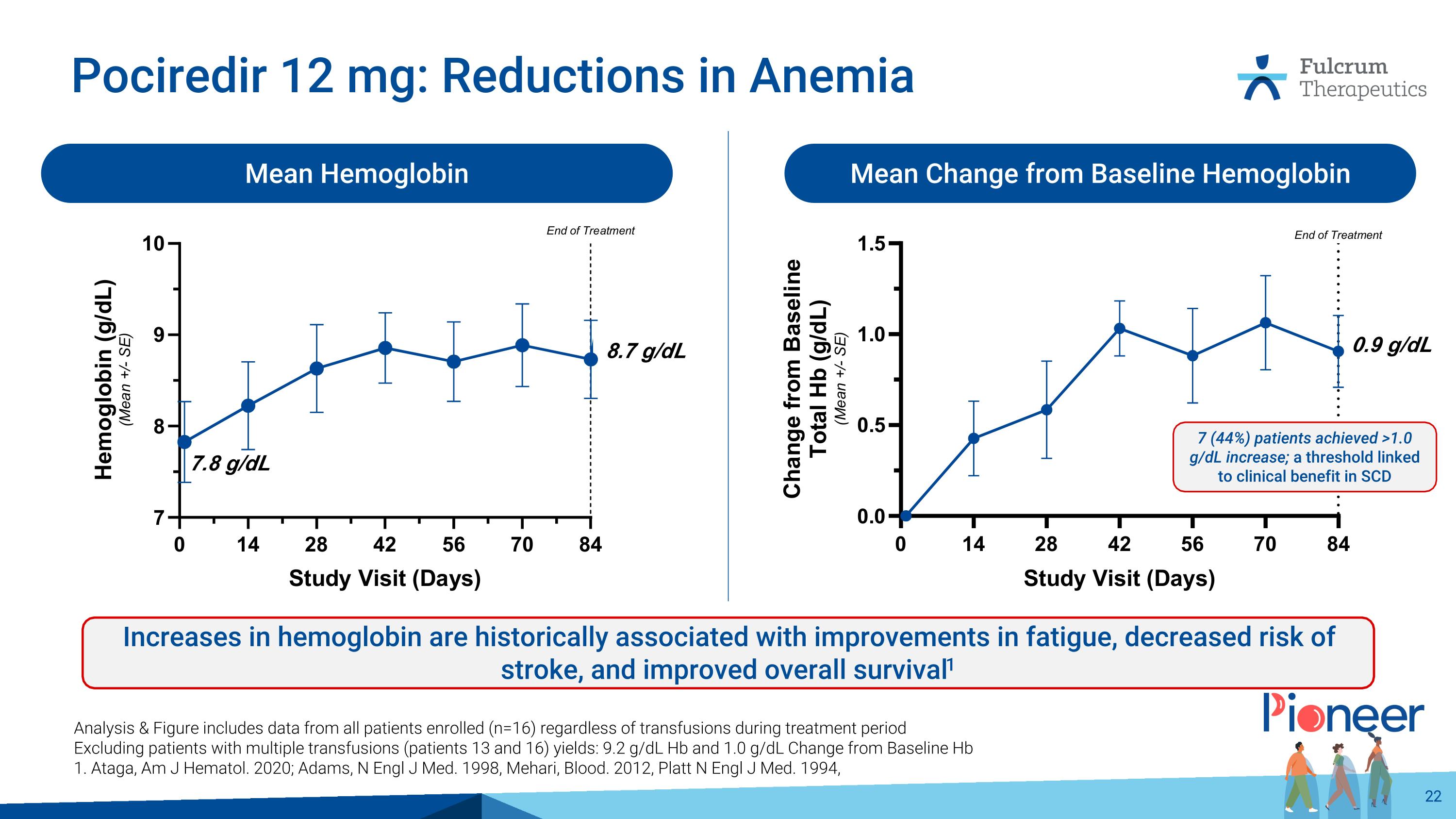
Pociredir 12 mg: Reductions in Anemia Mean Hemoglobin Mean Change from Baseline Hemoglobin Increases in hemoglobin are historically associated with improvements in fatigue, decreased risk of stroke, and improved overall survival1 Analysis & Figure includes data from all patients enrolled (n=16) regardless of transfusions during treatment period Excluding patients with multiple transfusions (patients 13 and 16) yields: 9.2 g/dL Hb and 1.0 g/dL Change from Baseline Hb 1. Ataga, Am J Hematol. 2020; Adams, N Engl J Med. 1998, Mehari, Blood. 2012, Platt N Engl J Med. 1994, 8.7 g/dL 0.9 g/dL 7 (44%) patients achieved >1.0 g/dL increase; a threshold linked to clinical benefit in SCD 7.8 g/dL
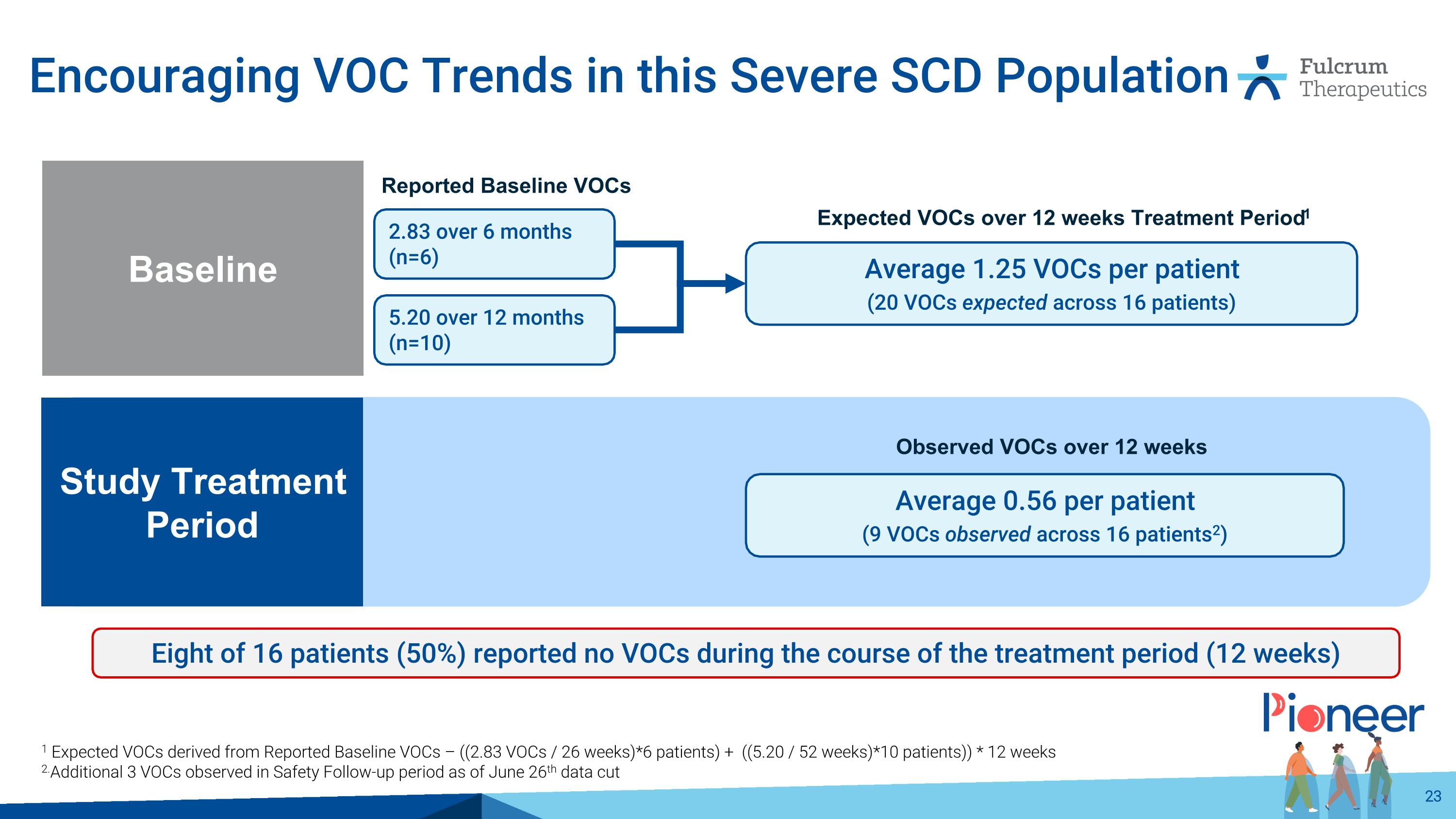
Encouraging VOC Trends in this Severe SCD Population 1 Expected VOCs derived from Reported Baseline VOCs – ((2.83 VOCs / 26 weeks)*6 patients) + ((5.20 / 52 weeks)*10 patients)) * 12 weeks 2.Additional 3 VOCs observed in Safety Follow-up period as of June 26th data cut Eight of 16 patients (50%) reported no VOCs during the course of the treatment period (12 weeks) Reported Baseline VOCs 2.83 over 6 months (n=6) 5.20 over 12 months (n=10) Average 1.25 VOCs per patient (20 VOCs expected across 16 patients) Expected VOCs over 12 weeks Treatment Period1 Study Treatment Period Baseline Average 0.56 per patient (9 VOCs observed across 16 patients2) Observed VOCs over 12 weeks
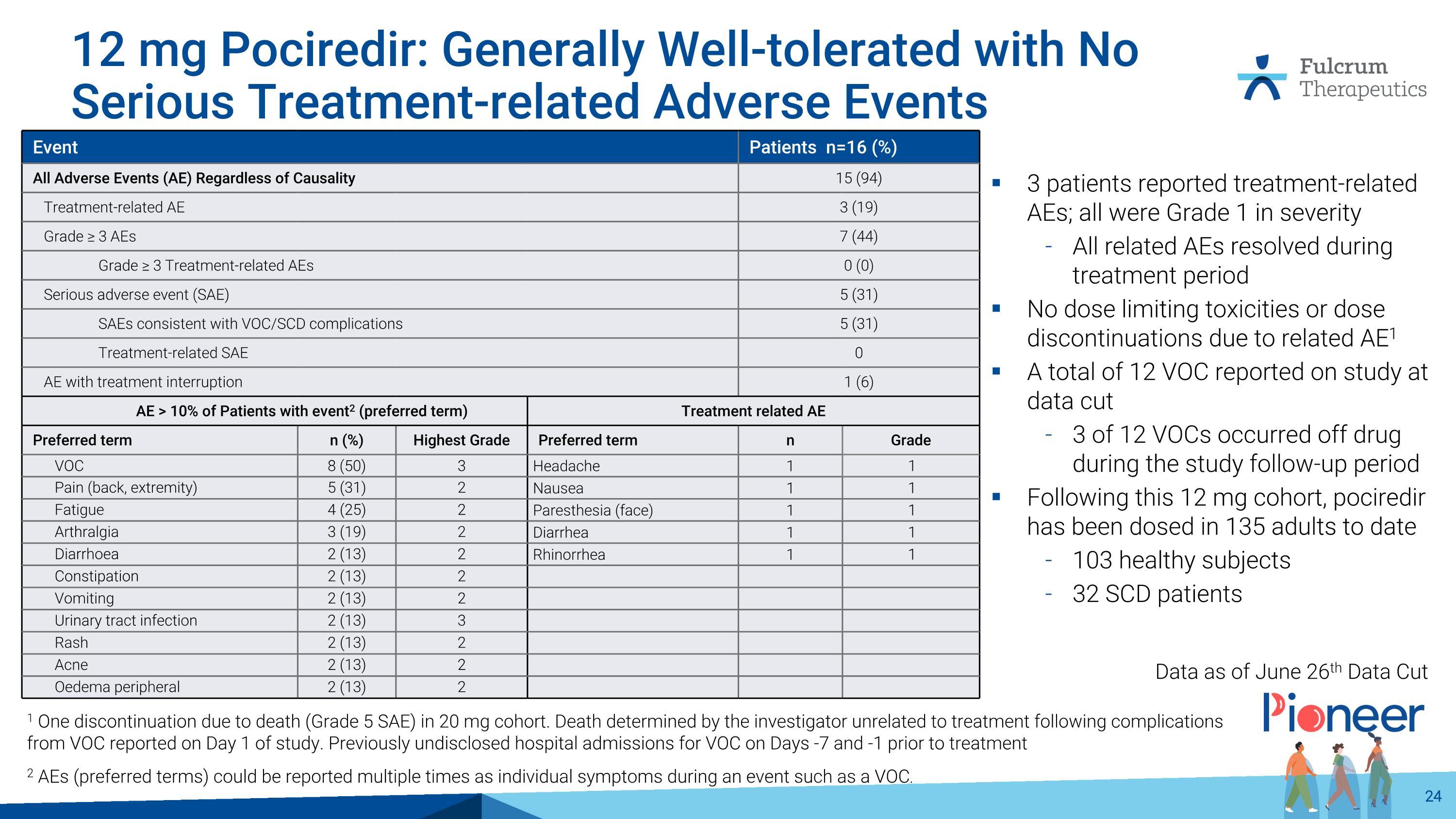
12 mg Pociredir: Generally Well-tolerated with No Serious Treatment-related Adverse Events 2 AEs (preferred terms) could be reported multiple times as individual symptoms during an event such as a VOC. Event Patients n=16 (%) All Adverse Events (AE) Regardless of Causality 15 (94) Treatment-related AE 3 (19) Grade ≥ 3 AEs 7 (44) Grade ≥ 3 Treatment-related AEs 0 (0) Serious adverse event (SAE) 5 (31) SAEs consistent with VOC/SCD complications 5 (31) Treatment-related SAE 0 AE with treatment interruption 1 (6) AE > 10% of Patients with event2 (preferred term) Treatment related AE Preferred term n (%) Highest Grade Preferred term n Grade VOC 8 (50) 3 Headache 1 1 Pain (back, extremity) 5 (31) 2 Nausea 1 1 Fatigue 4 (25) 2 Paresthesia (face) 1 1 Arthralgia 3 (19) 2 Diarrhea 1 1 Diarrhoea 2 (13) 2 Rhinorrhea 1 1 Constipation 2 (13) 2 Vomiting 2 (13) 2 Urinary tract infection 2 (13) 3 Rash 2 (13) 2 Acne 2 (13) 2 Oedema peripheral 2 (13) 2 1 One discontinuation due to death (Grade 5 SAE) in 20 mg cohort. Death determined by the investigator unrelated to treatment following complications from VOC reported on Day 1 of study. Previously undisclosed hospital admissions for VOC on Days -7 and -1 prior to treatment 3 patients reported treatment-related AEs; all were Grade 1 in severity All related AEs resolved during treatment period No dose limiting toxicities or dose discontinuations due to related AE1 A total of 12 VOC reported on study at data cut 3 of 12 VOCs occurred off drug during the study follow-up period Following this 12 mg cohort, pociredir has been dosed in 135 adults to date 103 healthy subjects 32 SCD patients Data as of June 26th Data Cut

Evidence of pan-cellularity as shown by a mean 67% F-Cells at 12 weeks 8.6% mean absolute increase in Fetal Hemoglobin (HbF) at 12 weeks 0.9 g/dL mean increase in hemoglobin (Hb) with an improvement in all key markers of hemolysis Encouraging trends in VOC reduction over 12 weeks Pociredir, once-daily oral, generally well-tolerated with treatment-related AEs limited to Grade 1 Pociredir’s Best-in-Class Potential as a once daily oral therapy for SCD informed by 12 mg cohort results Addressing the Significant Unmet Need in Sickle Cell Disease via Fetal Hemoglobin (HbF) Induction

Agenda for Investor Call Introduction Alex Sapir, President & CEO Sickle Cell Disease (SCD) and the Potential of a Once Daily Oral HbF-Inducer Iain Fraser MBChB, D.Phil, SVP Early Clinical Development Pioneer Study Overview and 12 mg Pociredir Cohort Data Update Sheinei Alan, M.D., Director, Inova Adult Sickle Cell Program & Assistant Professor, UVA School of Medicine Expert Perspective on Pociredir and Its Potential as a Once Daily Oral HbF-Inducer for Treating SCD Wally Smith, M.D., Director, VCU Adult Sickle Cell Program and Professor, VCU School of Internal Medicine Q&A Fulcrum Management, Dr. Alan and Dr. Smith Closing Remarks Alex Sapir, President & CEO

Expert Perspective on HbF Induction and Clinical Benefit in SCD Patients Wally Smith, M.D. Director, VCU Adult Sickle Cell Program & Professor School of Medicine at Virginia Commonwealth University

Q&A
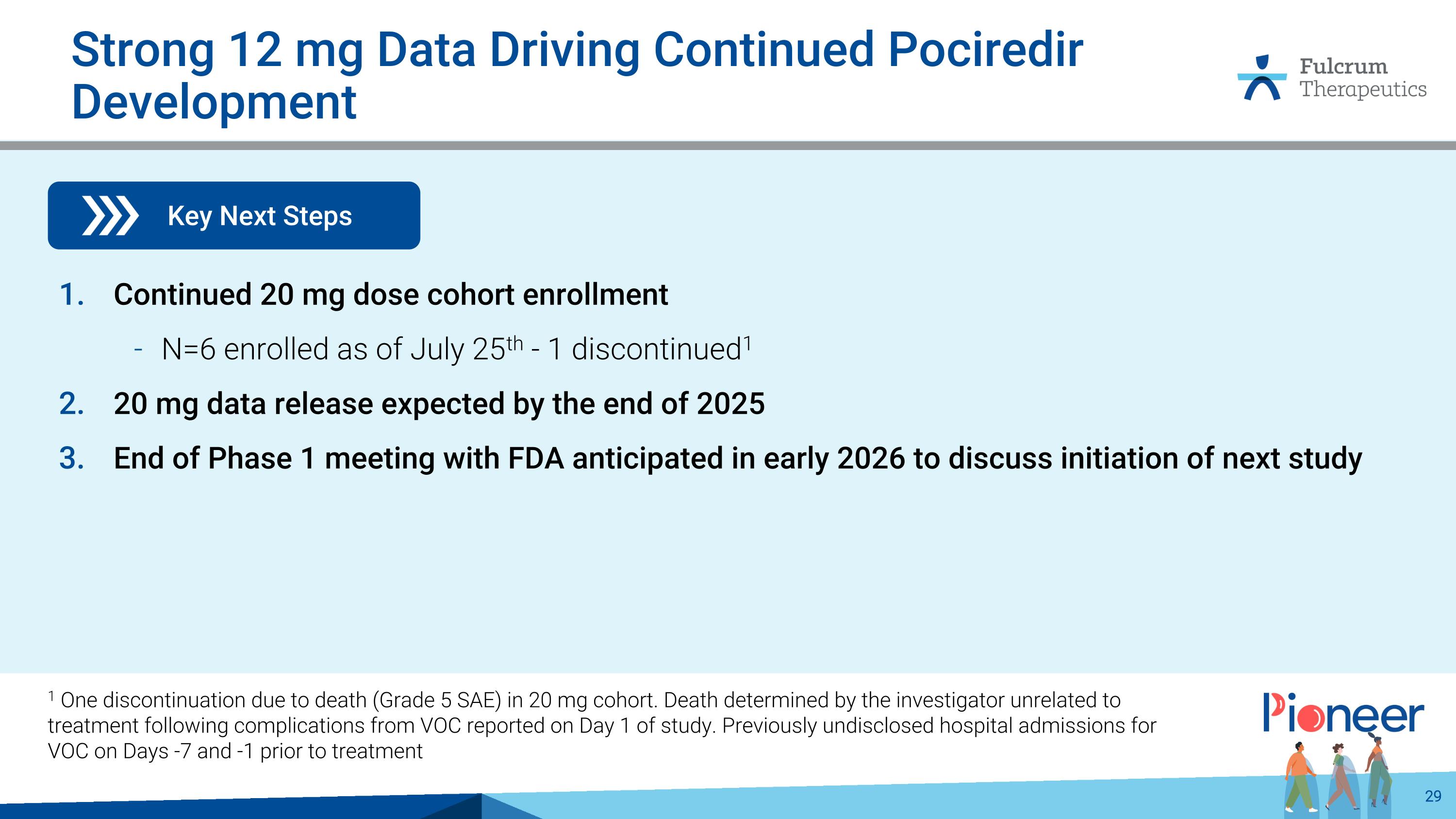
Strong 12 mg Data Driving Continued Pociredir Development Key Next Steps Continued 20 mg dose cohort enrollment N=6 enrolled as of July 25th - 1 discontinued1 20 mg data release expected by the end of 2025 End of Phase 1 meeting with FDA anticipated in early 2026 to discuss initiation of next study 1 One discontinuation due to death (Grade 5 SAE) in 20 mg cohort. Death determined by the investigator unrelated to treatment following complications from VOC reported on Day 1 of study. Previously undisclosed hospital admissions for VOC on Days -7 and -1 prior to treatment
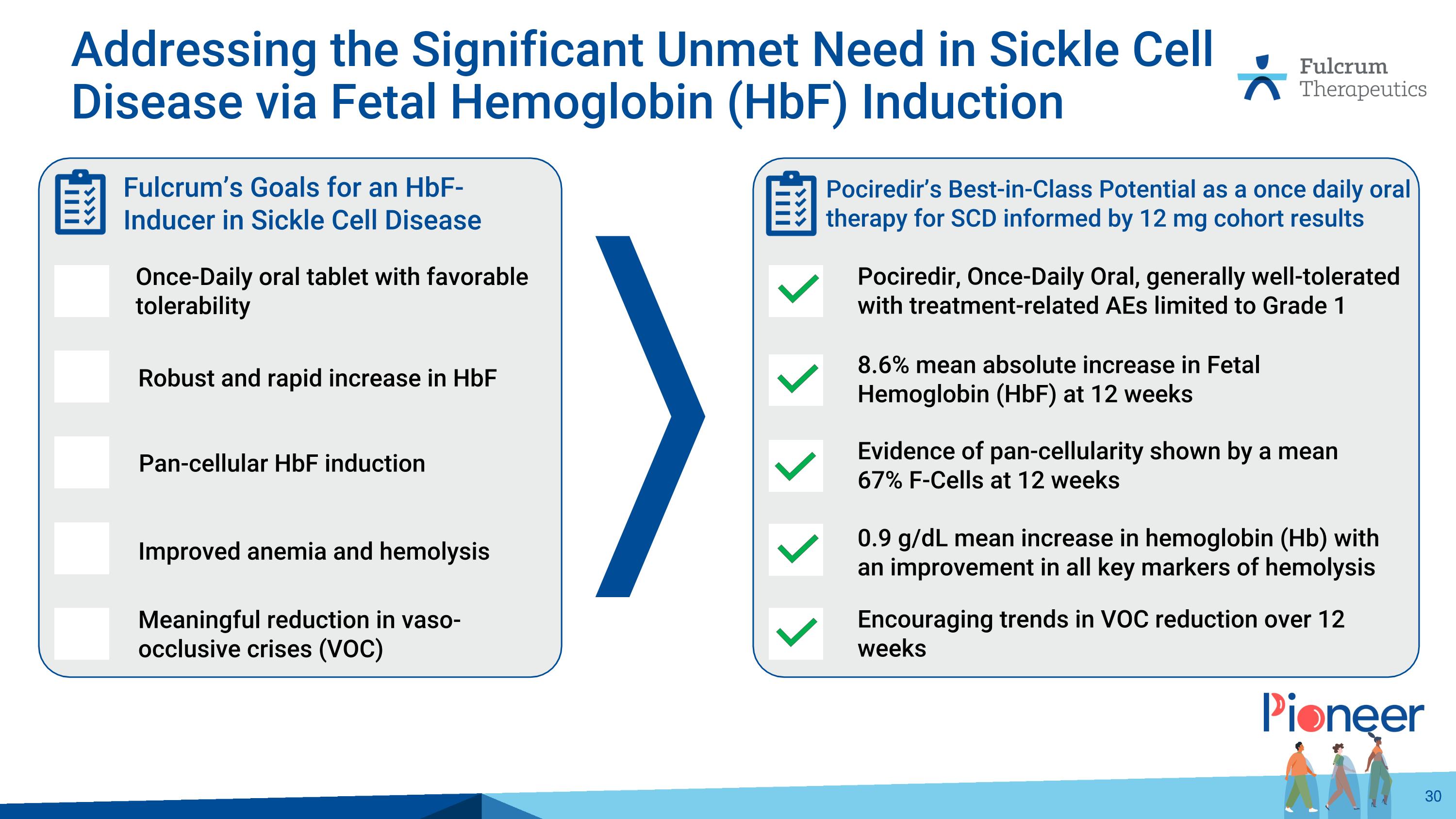
Evidence of pan-cellularity shown by a mean 67% F-Cells at 12 weeks 8.6% mean absolute increase in Fetal Hemoglobin (HbF) at 12 weeks 0.9 g/dL mean increase in hemoglobin (Hb) with an improvement in all key markers of hemolysis Encouraging trends in VOC reduction over 12 weeks Pociredir, Once-Daily Oral, generally well-tolerated with treatment-related AEs limited to Grade 1 Pociredir’s Best-in-Class Potential as a once daily oral therapy for SCD informed by 12 mg cohort results Addressing the Significant Unmet Need in Sickle Cell Disease via Fetal Hemoglobin (HbF) Induction Pan-cellular HbF induction Robust and rapid increase in HbF Improved anemia and hemolysis Meaningful reduction in vaso-occlusive crises (VOC) Once-Daily oral tablet with favorable tolerability Fulcrum’s Goals for an HbF-Inducer in Sickle Cell Disease

We thank the patients and their caregivers who participated in Pioneer, and our investigators and their teams