
GH Research R&D Update July 2025

This presentation has been prepared by GH Research PLC (“GH Research”).
Nothing contained in this presentation is, or should be construed as, a recommendation, promise or representation by the presenter or GH Research or any director, employee, agent, or adviser of GH Research. This presentation does not
purport to be all-inclusive or to contain all of the information you may desire. This presentation does not constitute an offer to sell or the solicitation of an offer to buy securities, nor shall there be any sale of securities in any
state or jurisdiction in which such offer, solicitation or sale would be unlawful prior to registration or qualification under the securities laws of any such state or jurisdiction. This presentation contains statements that are, or may
be deemed to be, forward-looking statements. All statements other than statements of historical fact included in this presentation, including statements regarding the clinical hold on GH001, including plans and expectations for
progressing any nonclinical programs and any other work needed to lift the continuing clinical hold and the timing required for the FDA to lift such clinical hold; our plans and expectations with respect to progressing development of
GH002 including with respect to the timing, scope and likelihood of IND submission and approval with the FDA; our targets regarding the initiation of our first global pivotal program; our future results of operations and financial
position, business strategy, product candidates, medical devices required to deliver these product candidates, research pipeline, ongoing and currently planned preclinical studies and clinical trials, regulatory submissions and approvals
and their effects on our business strategy, our expectations related to commencing trials in the US, research and development costs, cash runway, timing and likelihood of success, as well as plans and objectives of management for future
operations, are forward-looking statements. Forward-looking statements appear in a number of places in this presentation and include, but are not limited to, statements regarding our intent, believe or current expectations.
Forward-looking statements are based on our management’s beliefs and assumptions and on information currently available to our management. Such statements are subject to risks and uncertainties, and actual results may differ materially
from those expressed or implied in the forward-looking statements, due to various factors, including but not limited to, the risk that the FDA does not accept our responses to the clinical hold issues and that we will be unable to lift
the clinical hold on GH001; the risk that we may not be able to submit an IND for GH002, or to commence clinical trials in the United States on the timelines we are targeting; and other factors, risks and uncertainties described in our
filings with the U.S. Securities and Exchange Commission. No assurance can be given that such future results, plans, or expectations or targets will be achieved. Such forward-looking statements contained in this presentation speak only
as of the date of this presentation. We expressly disclaim any obligation or undertaking to update these forward-looking statements contained in this presentation to reflect any change in our expectations or any change in events,
conditions, or circumstances on which such statements are based unless required to do so by applicable law. No representations or warranties (expressed or implied) are made about the accuracy of any such forward-looking
statements. Disclaimer Regarding Forward-Looking Statements 2 2025© GH Research PLC

Summary of GH Research R&D Updates 1 2 3 On Track to Commence TRD Pivotal
Program in 2026 Strong and Consistent Final Data from GH Mebufotenin in TRD Best in Class, Best in Molecule, Best in Therapeutic Category GH001 & GH002 Formulations: Progress Update Note: To-date, no head-to-head comparisons of any
other products to any of our product candidates in any clinical trial have been completed; results have been obtained from different trials with different designs, endpoints and patient populations; results may not be comparable Abbreviations:
TRD = Treatment-Resistant Depression

GH001 Update IND Hold Response from the FDA received. Only one hold topic
remaining - the FDA requested that we either provide additional data or further justification related to the respiratory tract histology findings in rats. We strongly believe, based on scientific evidence, that the respiratory tract
histology findings are rat specific. There were no additional requests related to dog toxicology. There were no device related issues remaining. Next Steps Bolster rat specificity response with additional expert opinion, available
data and arguments. Engage with FDA on IND complete response. We are actively working to address the remaining issue. 4

Phase 2b Trial with an Open-Label Extension in Patients with Treatment-Resistant
DepressionFull Analysis Set // Efficacy Clinicaltrials.gov ID: NCT05800860 1 2 3 On Track to Commence TRD Pivotal Program in 2026 Strong and Consistent Final Data from GH Mebufotenin in TRD Best in Class, Best in Molecule, Best in
Therapeutic Category GH001 & GH002 Formulations: Progress Update GH001-TRD-201 Phase 2b TrialFull Analysis Set / New Data
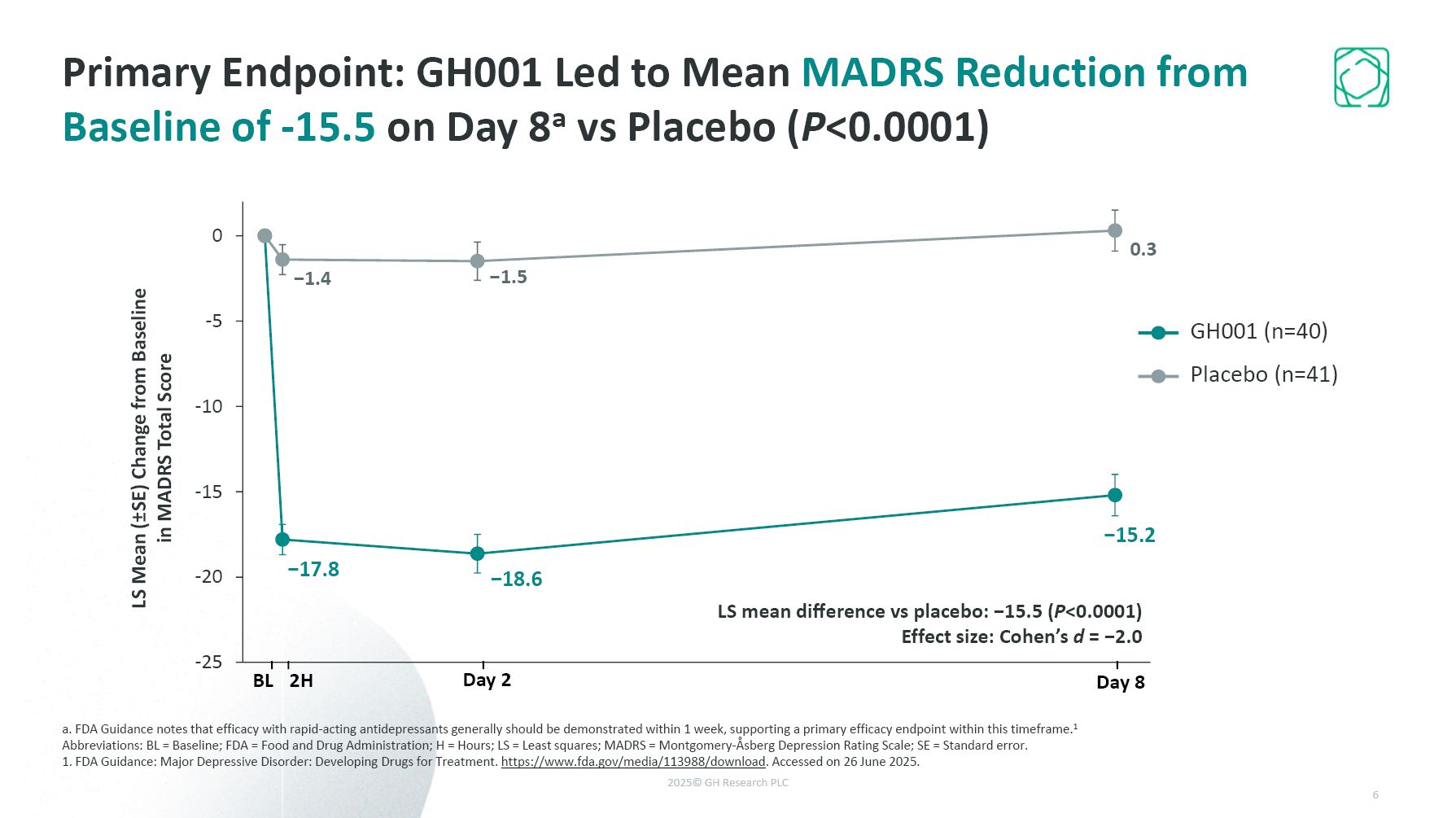
Primary Endpoint: GH001 Led to Mean MADRS Reduction from Baseline of -15.5 on Day
8a vs Placebo (P<0.0001) a. FDA Guidance notes that efficacy with rapid-acting antidepressants generally should be demonstrated within 1 week, supporting a primary efficacy endpoint within this timeframe.1 Abbreviations: BL = Baseline; FDA
= Food and Drug Administration; H = Hours; LS = Least squares; MADRS = Montgomery-Åsberg Depression Rating Scale; SE = Standard error. 1. FDA Guidance: Major Depressive Disorder: Developing Drugs for Treatment.
https://www.fda.gov/media/113988/download. Accessed on 26 June 2025. LS mean difference vs placebo: −15.5 (P<0.0001) Effect size: Cohen’s d = −2.0 LS Mean (±SE) Change from Baseline in MADRS Total Score BL 2H Day 2 Day 8 GH001
(n=40) Placebo (n=41) 6
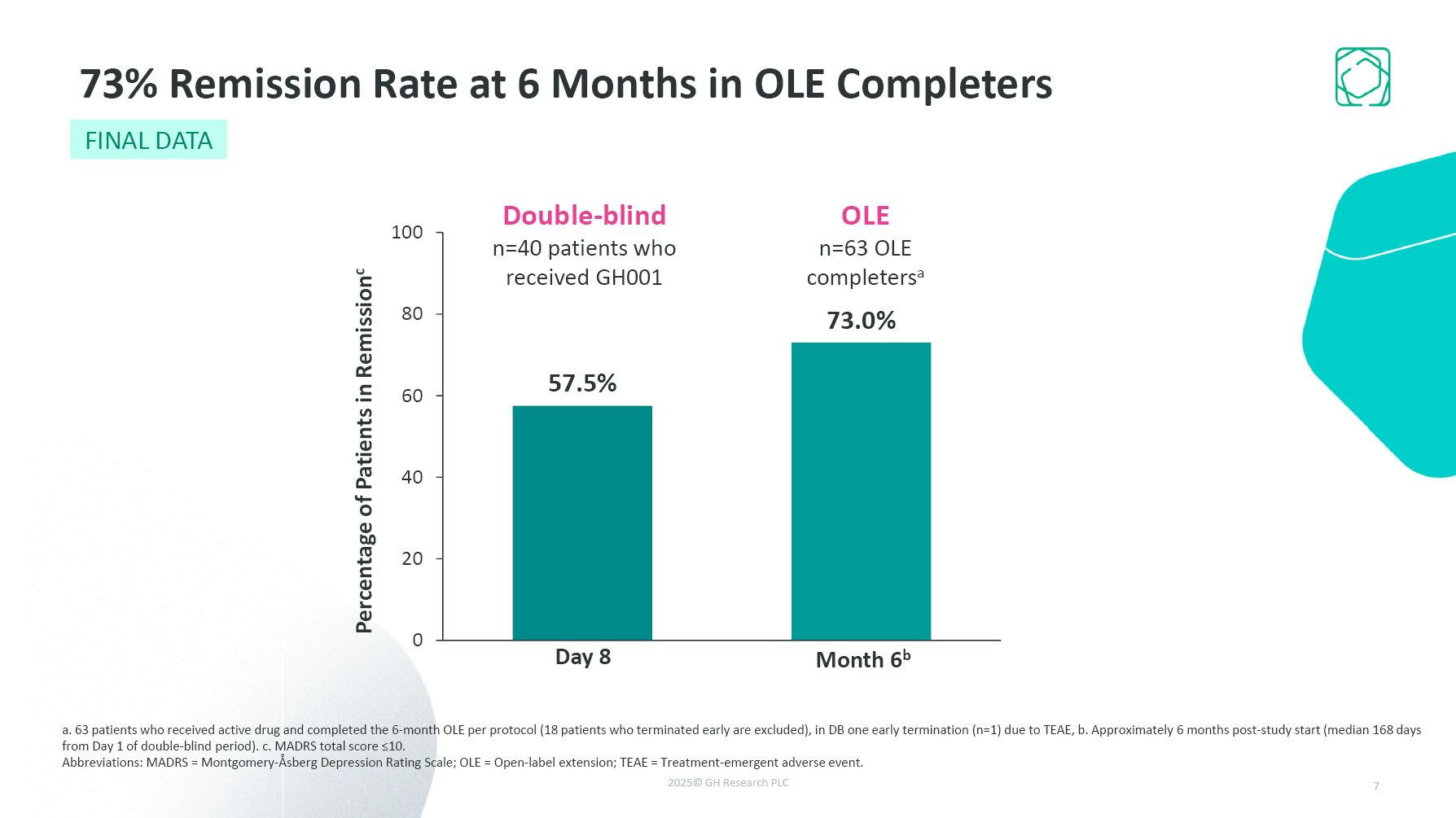
Double-blind n=40 patients who received GH001 OLE n=63 OLE completersa Day
8 Month 6b 73% Remission Rate at 6 Months in OLE Completers a. 63 patients who received active drug and completed the 6-month OLE per protocol (18 patients who terminated early are excluded), in DB one early termination (n=1) due to TEAE, b.
Approximately 6 months post-study start (median 168 days from Day 1 of double-blind period). c. MADRS total score ≤10. Abbreviations: MADRS = Montgomery-Åsberg Depression Rating Scale; OLE = Open-label extension; TEAE = Treatment-emergent
adverse event. 7 FINAL DATA
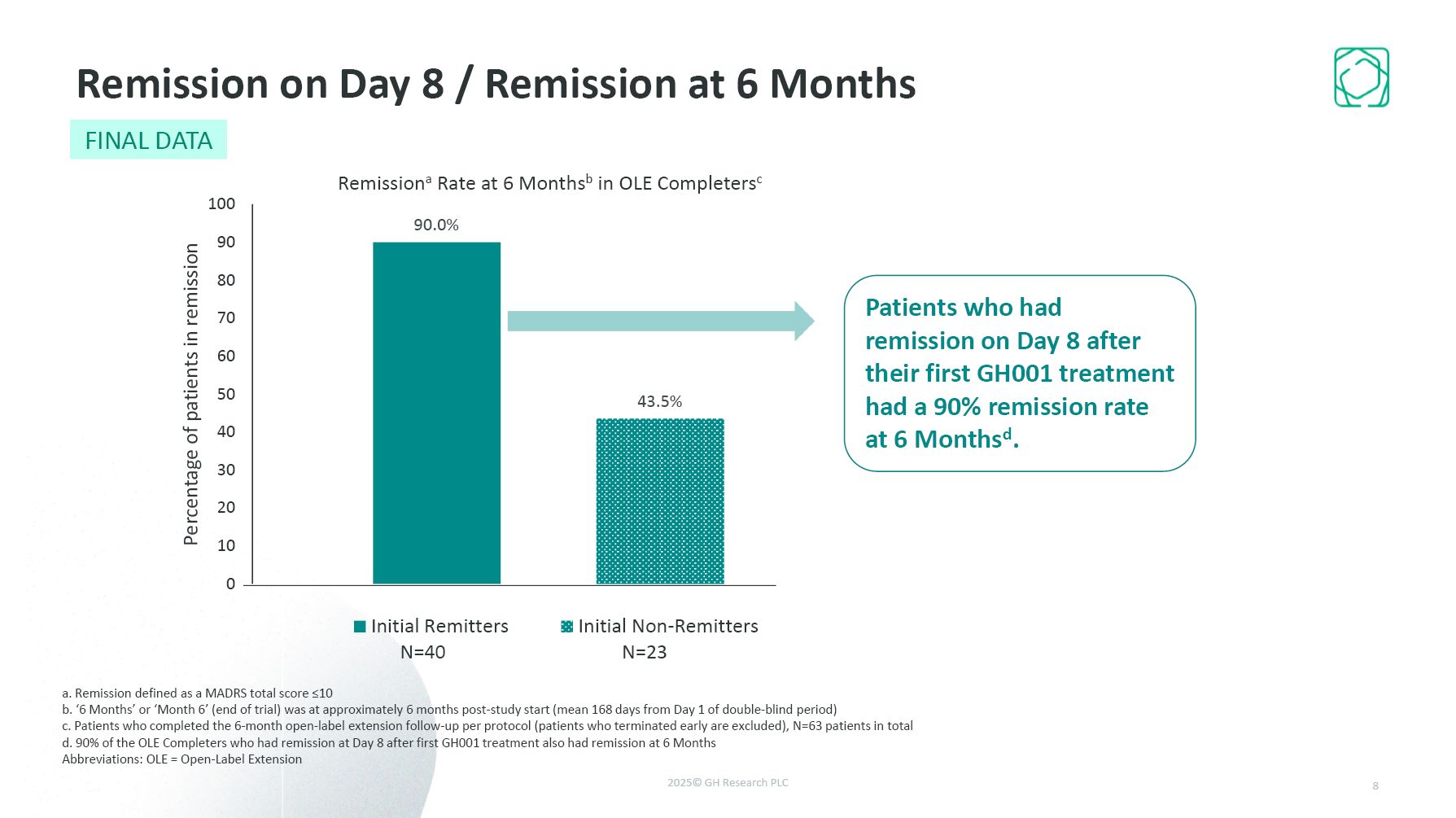
Remission on Day 8 / Remission at 6 Months a. Remission defined as a MADRS total
score ≤10 b. ‘6 Months’ or ‘Month 6’ (end of trial) was at approximately 6 months post-study start (mean 168 days from Day 1 of double-blind period) c. Patients who completed the 6-month open-label extension follow-up per protocol (patients
who terminated early are excluded), N=63 patients in total d. 90% of the OLE Completers who had remission at Day 8 after first GH001 treatment also had remission at 6 Months Abbreviations: OLE = Open-Label Extension Patients who had
remission on Day 8 after their first GH001 treatment had a 90% remission rate at 6 Monthsd. 8 FINAL DATA
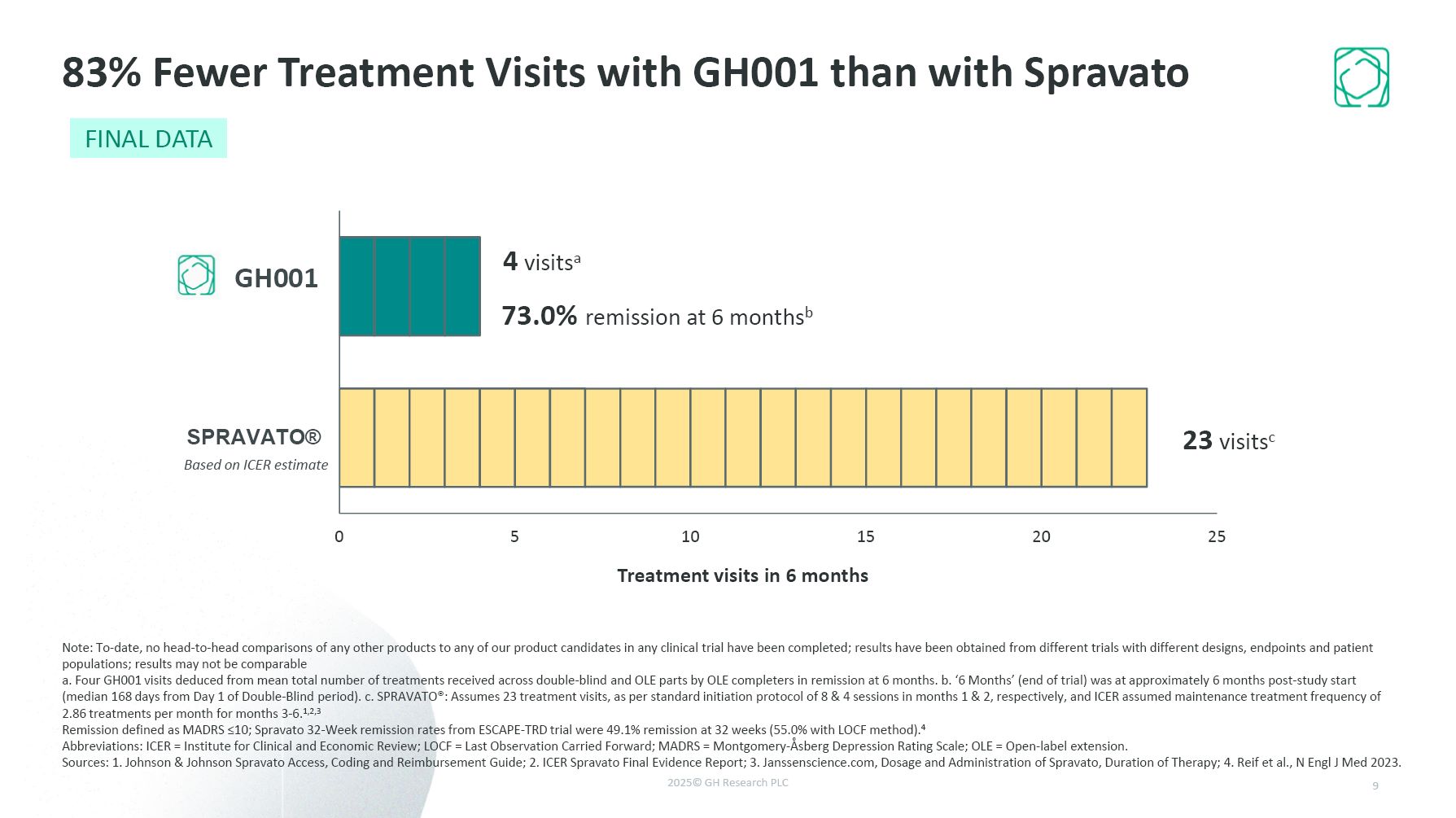
GH001 Based on ICER estimate Treatment visits in 6 months 4 visitsa 23
visitsc 73.0% remission at 6 monthsb 83% Fewer Treatment Visits with GH001 than with Spravato Note: To-date, no head-to-head comparisons of any other products to any of our product candidates in any clinical trial have been completed;
results have been obtained from different trials with different designs, endpoints and patient populations; results may not be comparable a. Four GH001 visits deduced from mean total number of treatments received across double-blind and OLE
parts by OLE completers in remission at 6 months. b. ‘6 Months’ (end of trial) was at approximately 6 months post-study start (median 168 days from Day 1 of Double-Blind period). c. SPRAVATO®: Assumes 23 treatment visits, as per standard
initiation protocol of 8 & 4 sessions in months 1 & 2, respectively, and ICER assumed maintenance treatment frequency of 2.86 treatments per month for months 3-6.1,2,3 Remission defined as MADRS ≤10; Spravato 32-Week remission rates
from ESCAPE-TRD trial were 49.1% remission at 32 weeks (55.0% with LOCF method).4 Abbreviations: ICER = Institute for Clinical and Economic Review; LOCF = Last Observation Carried Forward; MADRS = Montgomery-Åsberg Depression Rating Scale; OLE
= Open-label extension. Sources: 1. Johnson & Johnson Spravato Access, Coding and Reimbursement Guide; 2. ICER Spravato Final Evidence Report; 3. Janssenscience.com, Dosage and Administration of Spravato, Duration of Therapy; 4. Reif et
al., N Engl J Med 2023. 9 FINAL DATA
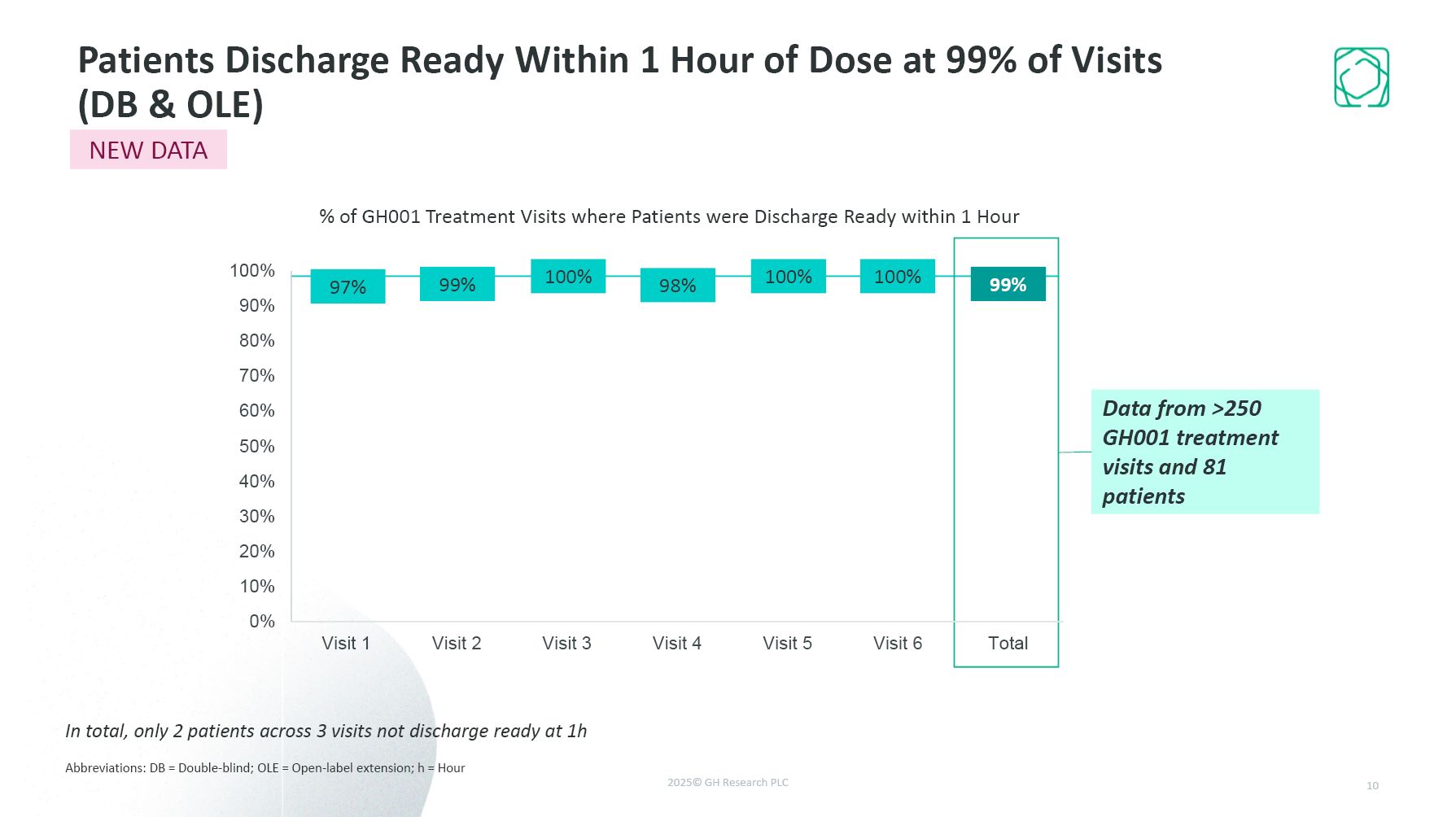
Patients Discharge Ready Within 1 Hour of Dose at 99% of Visits (DB &
OLE) Abbreviations: DB = Double-blind; OLE = Open-label extension; h = Hour 10 In total, only 2 patients across 3 visits not discharge ready at 1h Data from >250 GH001 treatment visits and 81 patients NEW DATA % of GH001 Treatment
Visits where Patients were Discharge Ready within 1 Hour 99% 97% 100% 98% 100% 100% 99%
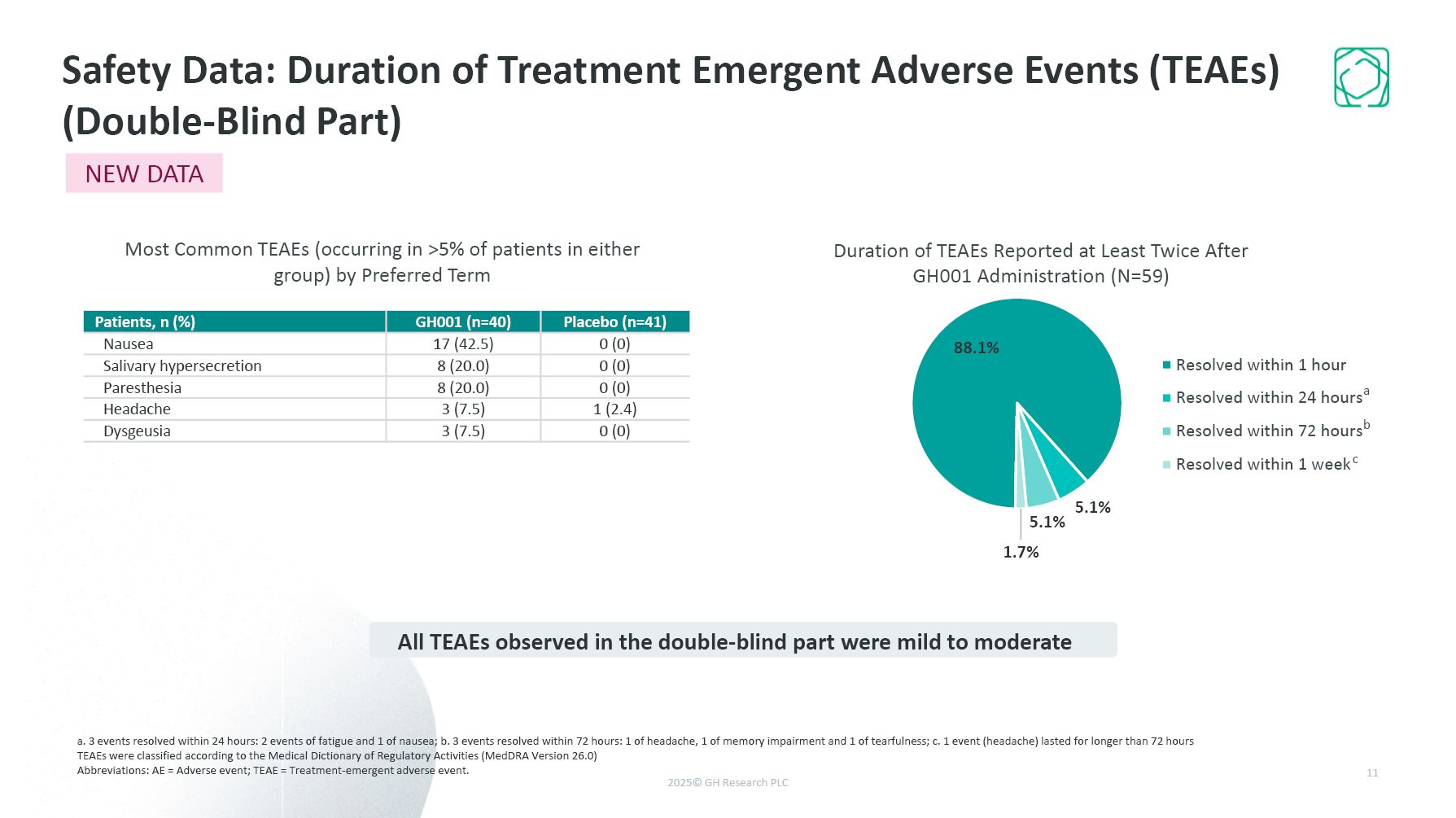
a. 3 events resolved within 24 hours: 2 events of fatigue and 1 of nausea; b. 3
events resolved within 72 hours: 1 of headache, 1 of memory impairment and 1 of tearfulness; c. 1 event (headache) lasted for longer than 72 hours TEAEs were classified according to the Medical Dictionary of Regulatory Activities (MedDRA
Version 26.0) Abbreviations: AE = Adverse event; TEAE = Treatment-emergent adverse event. Safety Data: Duration of Treatment Emergent Adverse Events (TEAEs) (Double-Blind Part) 11 Duration of TEAEs Reported at Least Twice After GH001
Administration (N=59) Patients, n (%) GH001 (n=40) Placebo (n=41) Nausea 17 (42.5) 0 (0) Salivary hypersecretion 8 (20.0) 0 (0) Paresthesia 8 (20.0) 0 (0) Headache 3 (7.5) 1 (2.4) Dysgeusia 3 (7.5) 0 (0) Most Common TEAEs
(occurring in >5% of patients in either group) by Preferred Term All TEAEs observed in the double-blind part were mild to moderate a b c NEW DATA
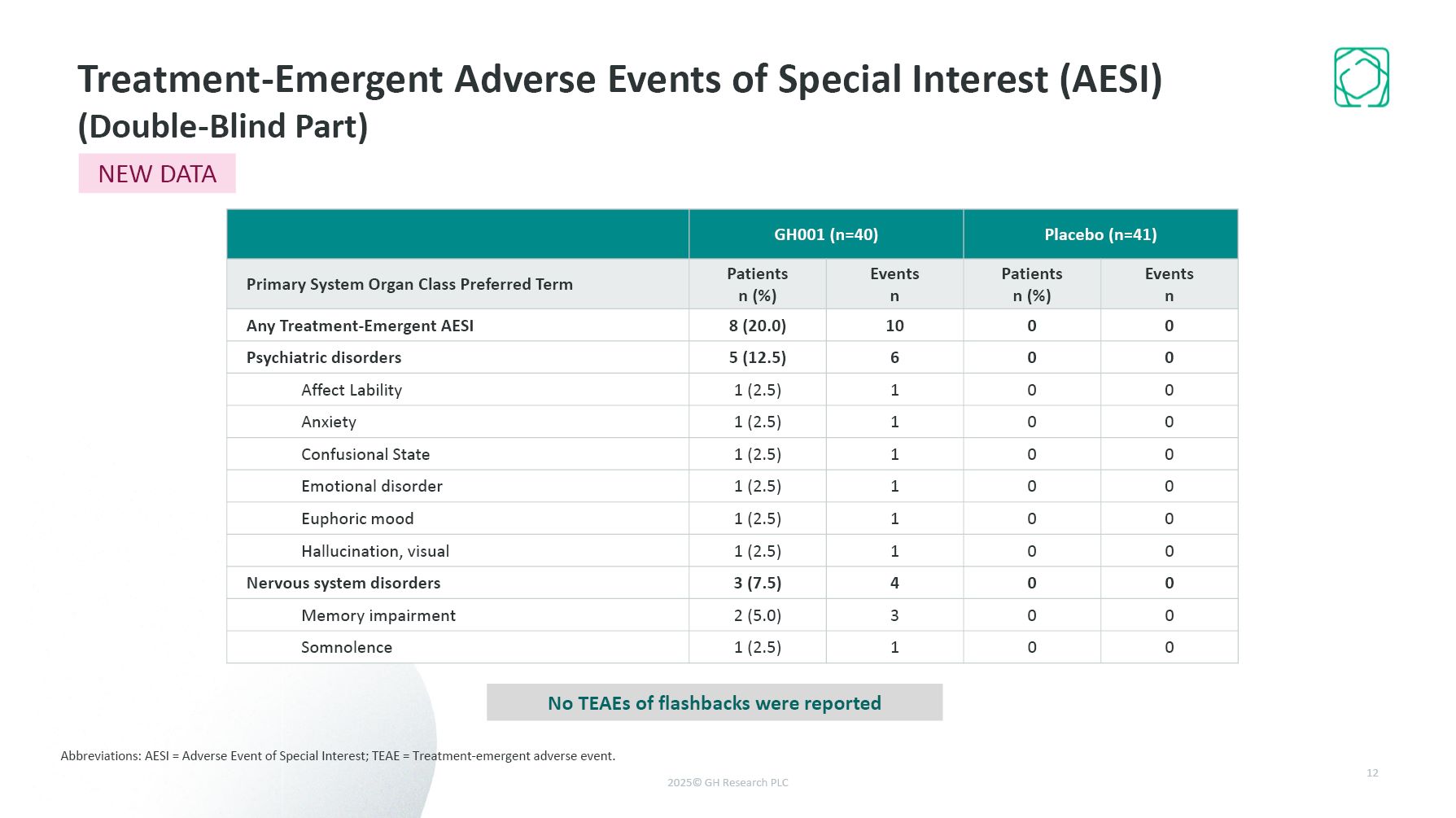
Treatment-Emergent Adverse Events of Special Interest (AESI) (Double-Blind Part)
Abbreviations: AESI = Adverse Event of Special Interest; TEAE = Treatment-emergent adverse event. 12 NEW DATA GH001 (n=40) Placebo (n=41) Primary System Organ Class Preferred Term Patients n (%) Events n Patients n (%) Events
n Any Treatment-Emergent AESI 8 (20.0) 10 0 0 Psychiatric disorders 5 (12.5) 6 0 0 Affect Lability 1 (2.5) 1 0 0 Anxiety 1 (2.5) 1 0 0 Confusional State 1 (2.5) 1 0 0 Emotional disorder 1 (2.5) 1 0 0 Euphoric
mood 1 (2.5) 1 0 0 Hallucination, visual 1 (2.5) 1 0 0 Nervous system disorders 3 (7.5) 4 0 0 Memory impairment 2 (5.0) 3 0 0 Somnolence 1 (2.5) 1 0 0 No TEAEs of flashbacks were reported
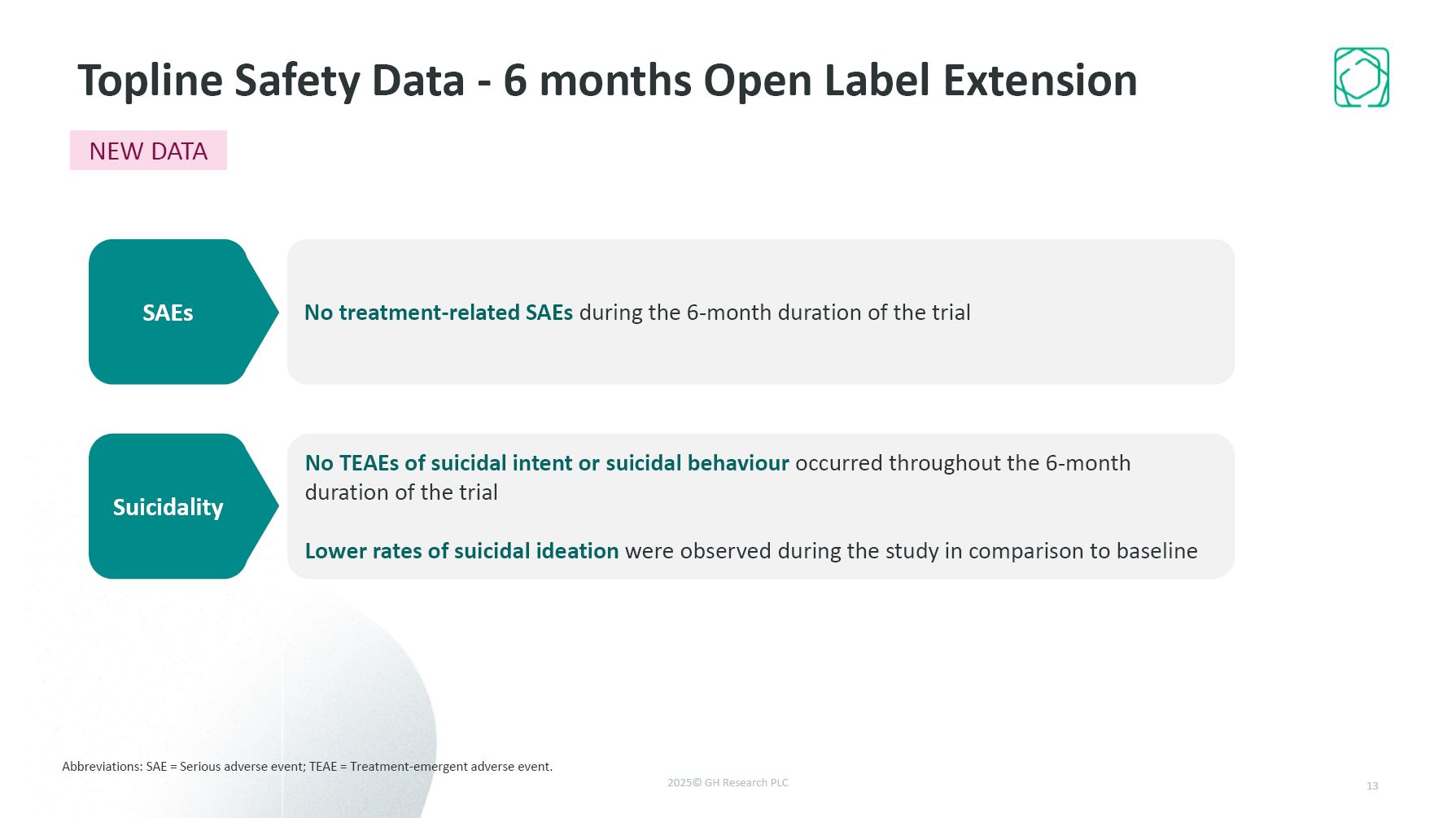
Abbreviations: SAE = Serious adverse event; TEAE = Treatment-emergent adverse
event. No treatment-related SAEs during the 6-month duration of the trial No TEAEs of suicidal intent or suicidal behaviour occurred throughout the 6-month duration of the trial Lower rates of suicidal ideation were observed during the study
in comparison to baseline 13 Topline Safety Data - 6 months Open Label Extension SAEs Suicidality NEW DATA
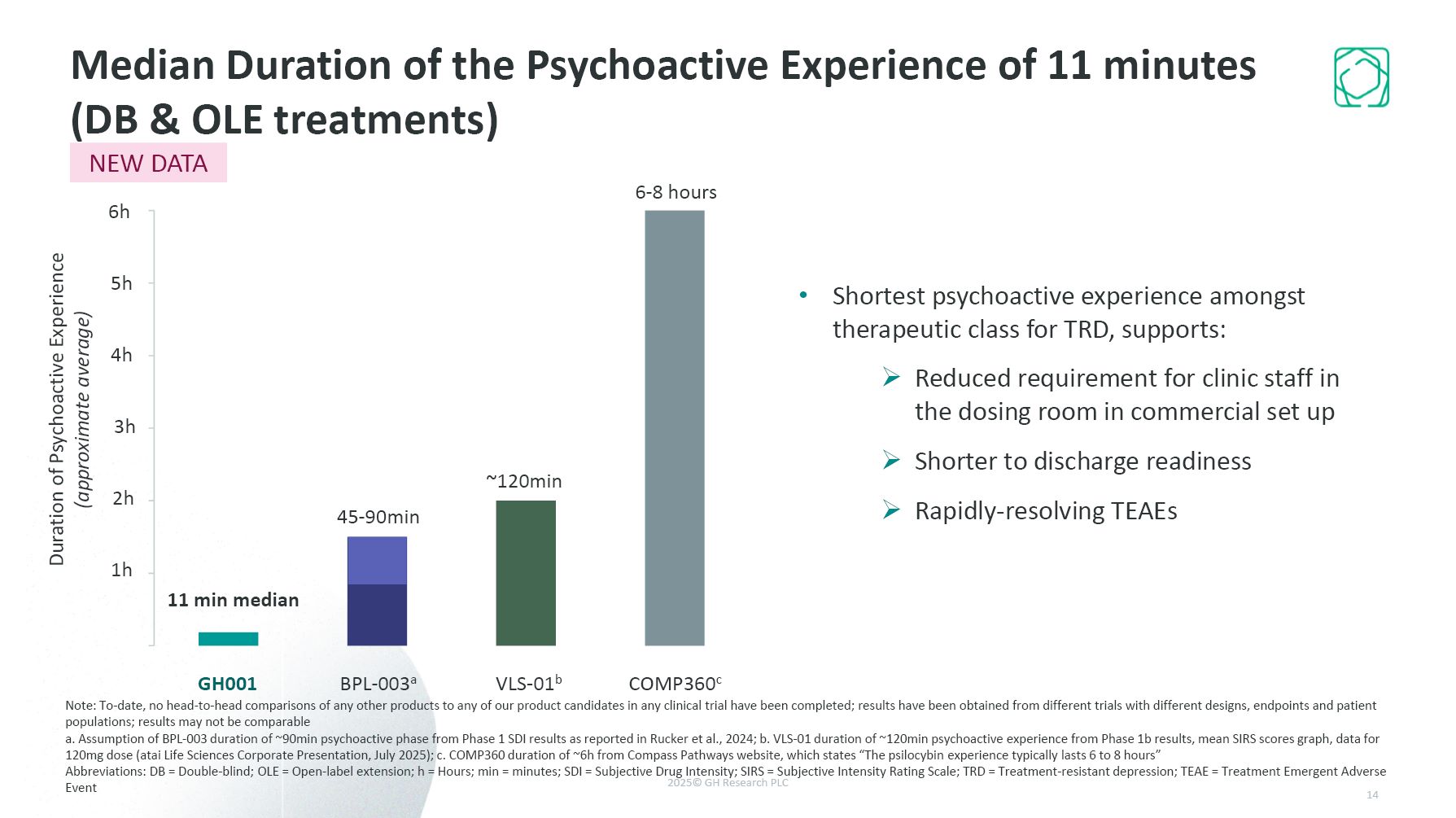
Shortest psychoactive experience amongst therapeutic class for TRD, supports:
Reduced requirement for clinic staff in the dosing room in commercial set up Shorter to discharge readiness Rapidly-resolving TEAEs GH001 2h 3h 4h 5h 6h Median Duration of the Psychoactive Experience of 11 minutes (DB & OLE
treatments) BPL-003a VLS-01b COMP360c 11 min median 1h Duration of Psychoactive Experience (approximate average) Note: To-date, no head-to-head comparisons of any other products to any of our product candidates in any clinical trial
have been completed; results have been obtained from different trials with different designs, endpoints and patient populations; results may not be comparable a. Assumption of BPL-003 duration of ~90min psychoactive phase from Phase 1 SDI
results as reported in Rucker et al., 2024; b. VLS-01 duration of ~120min psychoactive experience from Phase 1b results, mean SIRS scores graph, data for 120mg dose (atai Life Sciences Corporate Presentation, July 2025); c. COMP360 duration of
~6h from Compass Pathways website, which states “The psilocybin experience typically lasts 6 to 8 hours” Abbreviations: DB = Double-blind; OLE = Open-label extension; h = Hours; min = minutes; SDI = Subjective Drug Intensity; SIRS = Subjective
Intensity Rating Scale; TRD = Treatment-resistant depression; TEAE = Treatment Emergent Adverse Event 45-90min ~120min 6-8 hours NEW DATA 14
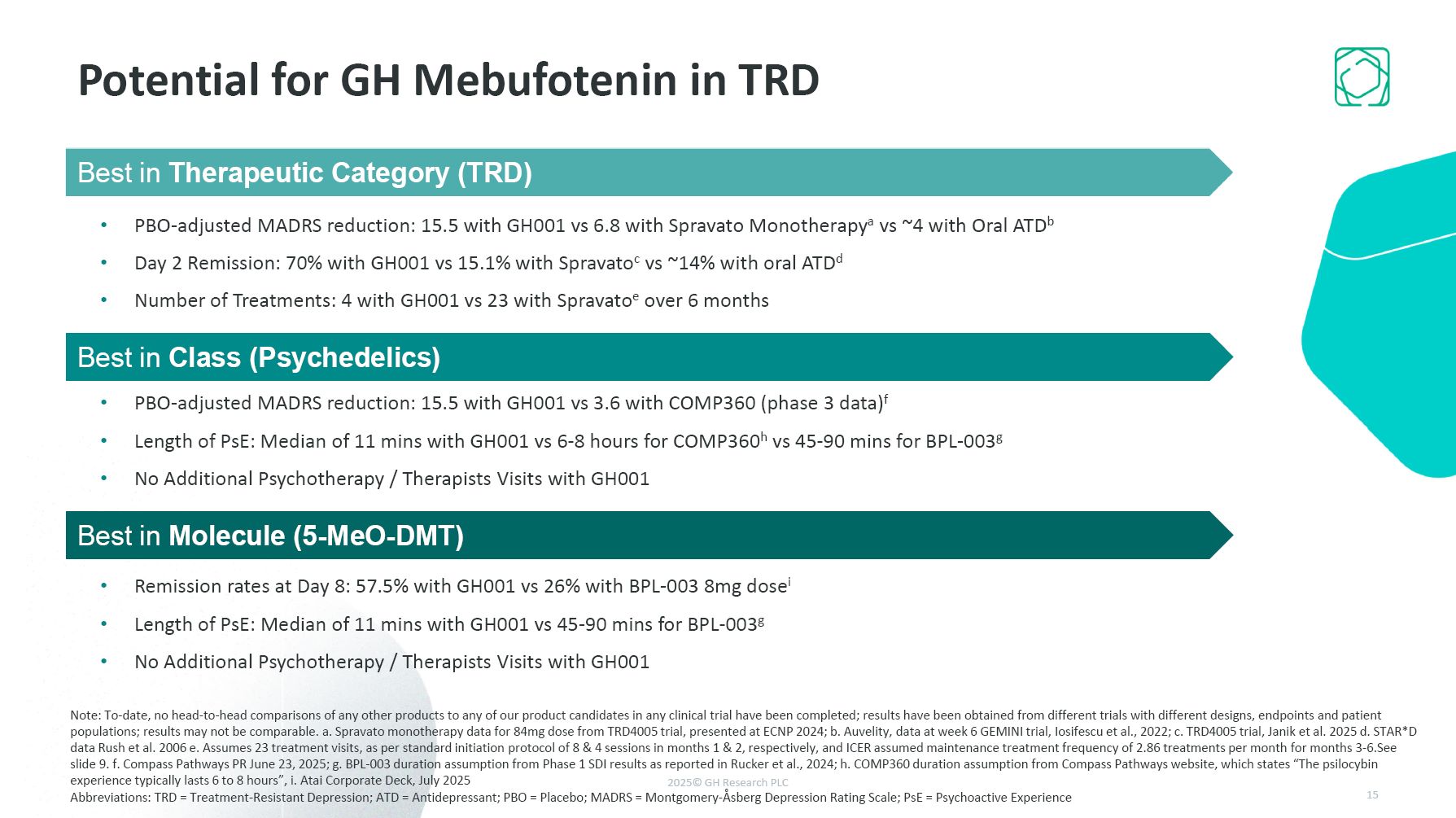
Potential for GH Mebufotenin in TRD Best in Molecule Fast in Fast out (FIFO)
formulations shown to be better than slower release formulations (intranasal) 15,5 vs 5 pbo corrected No psychotherapy needed Minimal Aes , minimal psychoactive phase Best in Class GH formulations shown far better efficacy than any other
psychedelic and at the same time the shortest psychoactive period Best in Therapeutic Category Pbo adjusted effects of 15.5 MADRS points is best in therapeutic Category, and achieved intraday. Repeat dosing yields same effect, and on-demand
dosing feasible with long effect window, and no SAEs. Best in Therapeutic Category (TRD) PBO-adjusted MADRS reduction: 15.5 with GH001 vs 6.8 with Spravato Monotherapya vs ~4 with Oral ATDb Day 2 Remission: 70% with GH001 vs 15.1% with
Spravatoc vs ~14% with oral ATDd Number of Treatments: 4 with GH001 vs 23 with Spravatoe over 6 months Best in Class (Psychedelics) PBO-adjusted MADRS reduction: 15.5 with GH001 vs 3.6 with COMP360 (phase 3 data)f Length of PsE: Median of
11 mins with GH001 vs 6-8 hours for COMP360h vs 45-90 mins for BPL-003g No Additional Psychotherapy / Therapists Visits with GH001 Best in Molecule (5-MeO-DMT) Remission rates at Day 8: 57.5% with GH001 vs 26% with BPL-003 8mg dosei Length
of PsE: Median of 11 mins with GH001 vs 45-90 mins for BPL-003g No Additional Psychotherapy / Therapists Visits with GH001 Note: To-date, no head-to-head comparisons of any other products to any of our product candidates in any clinical trial
have been completed; results have been obtained from different trials with different designs, endpoints and patient populations; results may not be comparable. a. Spravato monotherapy data for 84mg dose from TRD4005 trial, presented at ECNP
2024; b. Auvelity, data at week 6 GEMINI trial, Iosifescu et al., 2022; c. TRD4005 trial, Janik et al. 2025 d. STAR*D data Rush et al. 2006 e. Assumes 23 treatment visits, as per standard initiation protocol of 8 & 4 sessions in months 1
& 2, respectively, and ICER assumed maintenance treatment frequency of 2.86 treatments per month for months 3-6.See slide 9. f. Compass Pathways PR June 23, 2025; g. BPL-003 duration assumption from Phase 1 SDI results as reported in Rucker
et al., 2024; h. COMP360 duration assumption from Compass Pathways website, which states “The psilocybin experience typically lasts 6 to 8 hours”, i. Atai Corporate Deck, July 2025 Abbreviations: TRD = Treatment-Resistant Depression; ATD =
Antidepressant; PBO = Placebo; MADRS = Montgomery-Åsberg Depression Rating Scale; PsE = Psychoactive Experience 15

Summary of GH Research R&D Updates 1 2 3 On Track to Commence TRD Pivotal
Program in 2026 Strong and Consistent Final Data from GH Mebufotenin in TRD Best in Class, Best in Molecule, Best in Therapeutic Category GH001 & GH002 Formulations: Progress Update

Two ‘Fast-in-Fast-Out’ Mebufotenin Formulations GH001 (Inhaled Mebufotenin) T1/2
=y cmax = z, psychoactive period = 10-15m Regulatory status: UK MHRA approval and cross over study completed with new device US: IND resubmitted. Received response letter clearing 3 of 4 issues. Call for more data and arguments, both which
are in house available and will be submitted imminently. Resolution expected q3/q4 2025. next steps prior to pivotal are XYZ GH002 (I.V. Mebufotenin) T1/2=x cmax = y, psychoactive period = 10-15m Healthy Volunteer study completed More on
Status here. Next Steps prior to pivotal program MAKE CLEAR WHY YOU WOULD BE IN POSITION TO CHOSE Final PIVOTAL FORMULATION IN Q2 2026 or whatever date is best. i.e. make sure the individual steps for the 2 FIFO formulations align with
this GH001 (Inhaled Mebufotenin, Free Base) Expected Pivotal Program Start: 2026 Ongoing Pivotal Program preparation activities currently synergistic across GH001 and GH002 programs Status: Phase 1 dose ranging study in HVs
completed Comparative PK equivalence with inhaled formulation - achieved Doses for next phase of development selected Patent protected Next steps for Pivotal Program Readiness: IND submission expected Q4 2025 Status: Phase 2b study in
TRD completed Bridging HV study with GH proprietary device in progress (UK) Engagement with FDA on IND complete response ongoing: Only one hold topic remaining - related to the respiratory tract histology findings in rats. No additional
requests related to dog toxicology. No device related issues remaining. Patent protected Next steps for Pivotal Program Readiness: EoP2 meeting with FDA/EMA Scientific Advice GH002 (IV Mebufotenin, HBr Salt) Abbreviations: IV =
intravenous; HBr = Hydrobromide; HV = Healthy Volunteer; TRD = Treatment-Resistant Depression; IND = Investigational New Drug; FDA = U.S. Food and Drug Administration; EMA = European Medicines Agency; EoP2 = End of Phase 2; PK =
Pharmacokinetics

Summary of GH Research R&D Updates 1 2 3 On Track to Commence TRD Pivotal
Program in 2026 Strong and Consistent Final Data from GH Mebufotenin in TRD Best in Class, Best in Molecule, Best in Therapeutic Category GH001 & GH002 Formulations: Progress Update
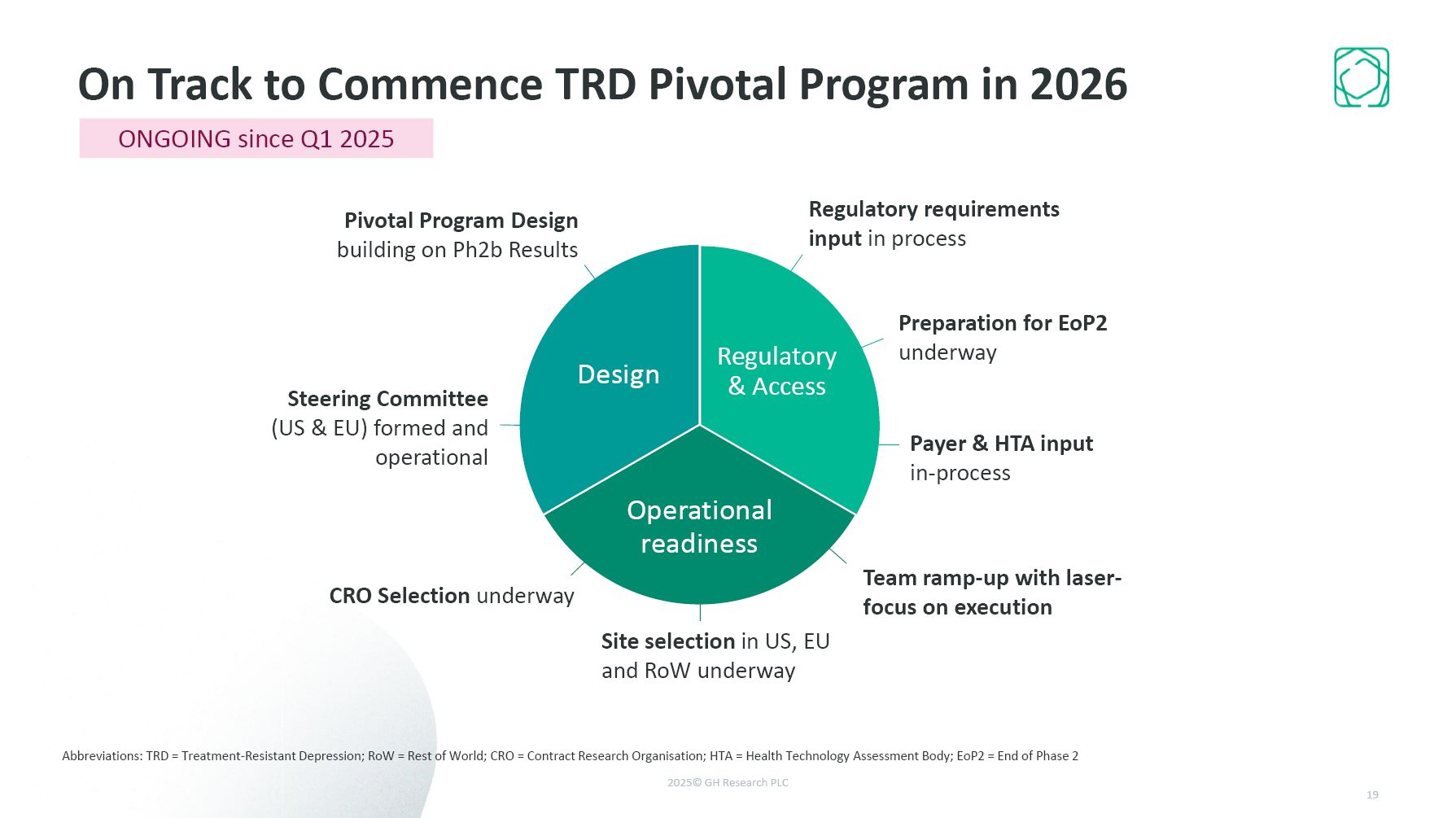
On Track to Commence TRD Pivotal Program in 2026 Abbreviations: TRD =
Treatment-Resistant Depression; RoW = Rest of World; CRO = Contract Research Organisation; HTA = Health Technology Assessment Body; EoP2 = End of Phase 2 Pivotal Program Design building on Ph2b Results Site selection in US, EU and RoW
underway CRO Selection underway Steering Committee (US & EU) formed and operational Payer & HTA input in-process Regulatory requirements input in process Team ramp-up with laser-focus on execution Preparation for EoP2
underway ONGOING since Q1 2025
Summary of GH Research R&D Updates 1 2 3 On Track to Commence TRD Pivotal
Program in 2026 Strong and Consistent Final Data from GH Mebufotenin in TRD Best in Class, Best in Molecule, Best in Therapeutic Category GH001 & GH002 Formulations: 2 PK Equivalent Solutions 4 $315.3 million cash and investments as
of March 31, 2025 to execute Note: To-date, no head-to-head comparisons of any other products to any of our product candidates in any clinical trial have been completed; results have been obtained from different trials with different designs,
endpoints and patient populations; results may not be comparable Abbreviations: TRD = Treatment-Resistant Depression