

THE SCIENCE TO OVERCOME INFLAMMATION CORPORATE PRESENTATION JULY 2025 Exhibit 99.1

Disclaimer Statements in this Presentation that are not statements of historical fact are forward-looking statements. Such forward-looking statements include, without limitation, statements regarding the development of RPT904, including the expected timing of clinical trials and the availability of data therefrom and regulatory interactions; the therapeutic potential of RPT904; the potential commercial opportunity for RPT904; the ability to obtain necessary regulatory approvals; and pricing and reimbursement. Words such as "believe," "anticipate," "plan," "expect," "will," "may," “could,” "upcoming,“ “projected,” "milestone," "potential," "target" or the negative of these terms or similar expressions are intended to identify forward-looking statements, though not all forward-looking statements necessarily contain these identifying words. These forward-looking statements are based on the current beliefs of the Company's management with respect to future events and trends and are subject to known and unknown risks and uncertainties that may cause our actual performance or achievements to be materially different from any future performance or achievements expressed or implied by the forward-looking statements in this Presentation. Risks and uncertainties that may cause actual results to differ materially include: risks inherent in the initiation, progress and completion of clinical trials and clinical development of our product candidates; the risk that clinical trials may have unsatisfactory outcomes; risks associated with preclinical development of product candidates; regulatory authorities, including the U.S. Food and Drug Administration (FDA) may not agree with our interpretation of the data from clinical trials of our drug candidates; we may decide, or regulatory authorities may require us, to conduct additional clinical trials or to modify our ongoing clinical trials; we may experience delays in the commencement, enrollment, completion or analysis of clinical testing for our drug candidates, or significant issues regarding the adequacy of our clinical trial designs or the execution of our clinical trials may arise, which could result in increased costs and delays, or limit our ability to obtain regulatory approval; our drug candidates may not receive regulatory approval or be successfully commercialized; unexpected adverse side effects or inadequate therapeutic efficacy of our drug candidates could delay or prevent regulatory approval or commercialization; uncertainties inherent in the conduct of clinical trials, our reliance on third parties over which we may not always have full control; our ability to enter into strategic partnerships on commercially reasonable terms; our ability to obtain additional financing; the uncertainty regarding the macroeconomic environment and other risks and uncertainties that are described in the “Risk Factors” section of our most recent Form 10-Q filed with the Securities and Exchange Commission, and any current and periodic reports filed thereafter. These forward-looking statements should not be taken as forecasts or promises nor should they be taken as implying any indication, assurance or guarantee that any assumptions on which such forward-looking statements have been made are correct or exhaustive or, in the case of such assumptions, fully stated in the Presentation. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date this Presentation is given. Although we believe that the beliefs and assumptions reflected in the forward-looking statements are reasonable, we cannot guarantee future performance or achievements. Except as required by law, we undertake no obligation to update publicly any forward-looking statements for any reason after the date of this Presentation. This Presentation discusses drug candidates that are under clinical study and which have not yet been approved for marketing by the U.S. Food and Drug Administration. No representation is made as to the safety or effectiveness of any drug candidates for any use for which such drug candidates are being studied. This presentation also contains estimates and other statistical data made by independent parties and by us relating to market size and other data about our industry. These data involve a number of assumptions and limitations, and you are cautioned not to give undue weight to such estimates. In addition, projections, assumptions and estimates of our future performance and the future performance of the markets in which we operate are necessarily subject to a high degree of uncertainty and risk.
RAPT is Developing Transformative Therapies for High-Value Inflammatory Diseases RPT904 is a novel potential best-in-class half-life extended omalizumab (Xolair®) anti-IgE antibody designed for less frequent dosing and greater compliance Potential to transform treatment of Food Allergy (FA) and Chronic Spontaneous Urticaria (CSU), estimated >$40B and >$5B market opportunities in the US, respectively Multiple milestones anticipated over next two years Initiate Phase 2b trial in FA in 2H 2025; data expected 1H 2027 Partner Jemincare is in Phase 2 for CSU and Asthma; data expected in 2H 2025 Initiate Phase 2 or Phase 3 trial in CSU in 2026 Next generation oral CCR4 antagonist in preclinical development Preclinical candidate selected in Q2 with 20-fold increase in safety margins over previous generation Potential to address high unmet need for a safe oral option for many Th2-driven disorders Company well funded with cash runway projected through multiple clinical milestones including Phase 2b FA data XOLAIR® is a registered trademark of Novartis AG

RPT904 Potential to Transform the Treatment of Food Allergy and Urticaria
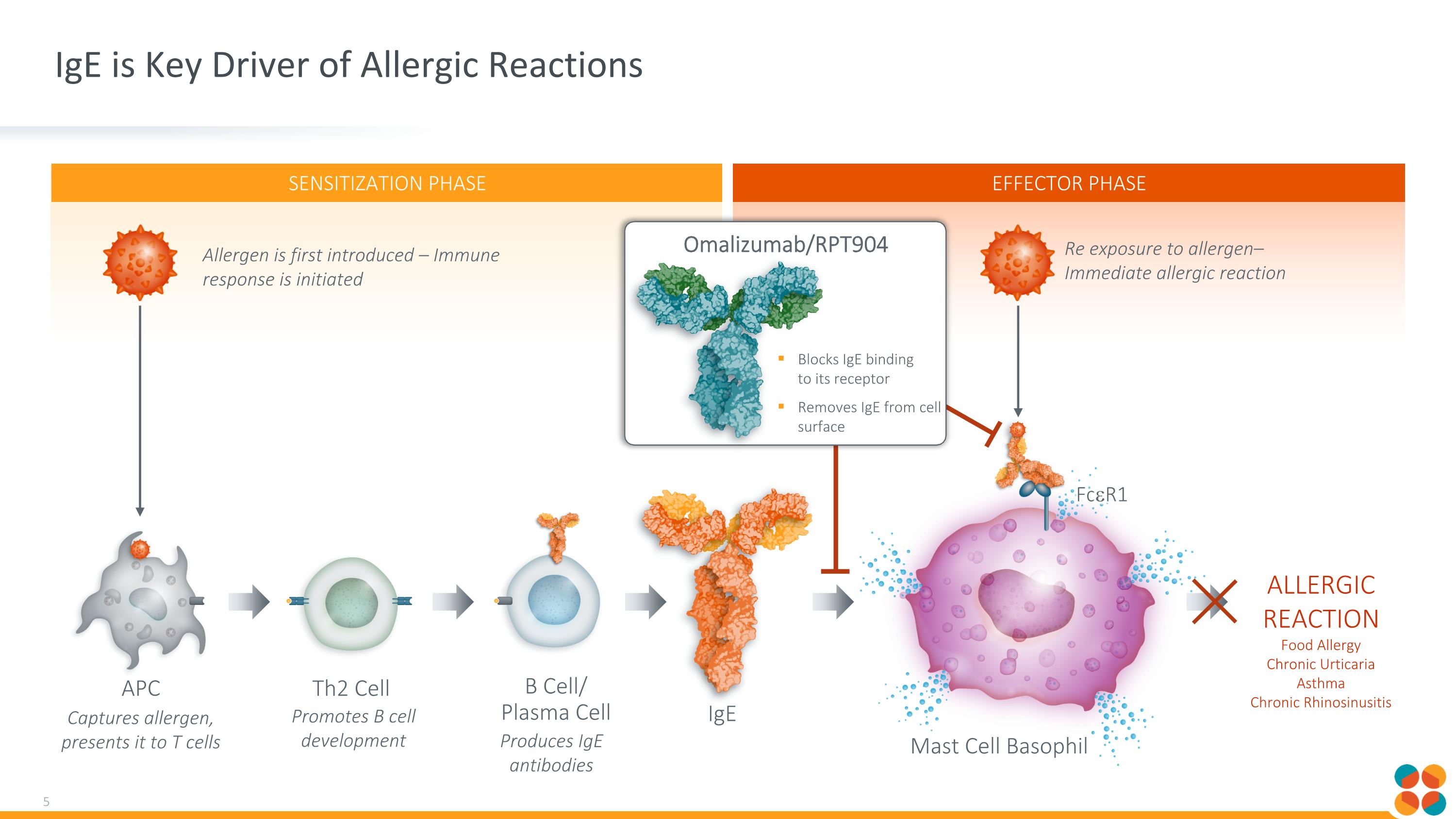
SENSITIZATION PHASE EFFECTOR PHASE IgE is Key Driver of Allergic Reactions Allergen is first introduced – Immune response is initiated Captures allergen, presents it to T cells Promotes B cell development Produces IgE antibodies Th2 Cell B Cell/ Plasma Cell APC ALLERGIC REACTION Food Allergy Chronic Urticaria Asthma Chronic Rhinosinusitis FceR1 Re exposure to allergen– Immediate allergic reaction Mast Cell Basophil IgE Blocks IgE binding to its receptor Removes IgE from cell surface Omalizumab/RPT904
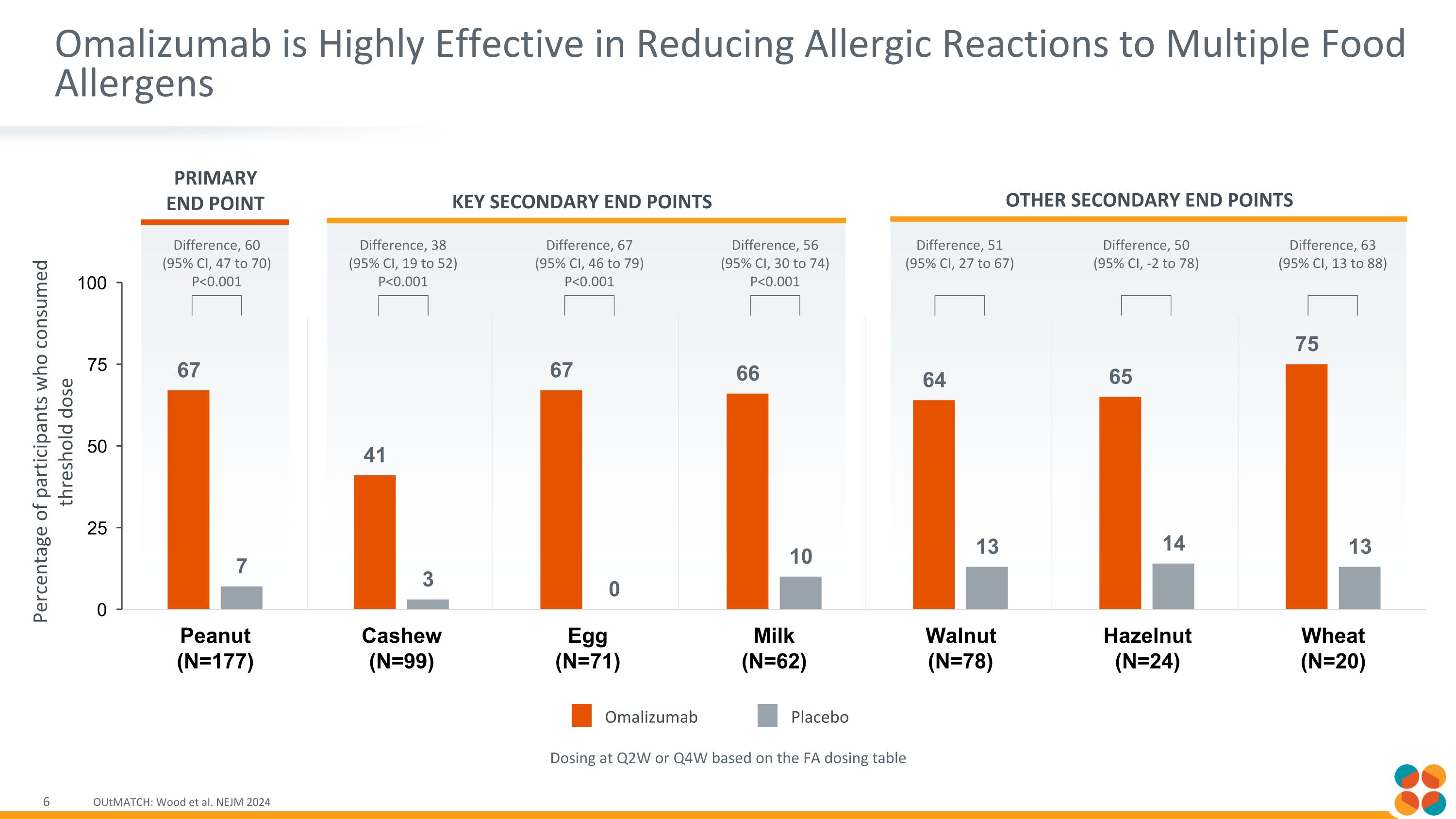
Omalizumab is Highly Effective in Reducing Allergic Reactions to Multiple Food Allergens OUtMATCH: Wood et al. NEJM 2024 Dosing at Q2W or Q4W based on the FA dosing table Primary End Point Key secondary End Points other secondary End Points Difference, 60 (95% CI, 47 to 70) P<0.001 Difference, 38 (95% CI, 19 to 52) P<0.001 Difference, 67 (95% CI, 46 to 79) P<0.001 Difference, 56 (95% CI, 30 to 74) P<0.001 Difference, 51 (95% CI, 27 to 67) Difference, 50 (95% CI, -2 to 78) Difference, 63 (95% CI, 13 to 88) Omalizumab Placebo Percentage of participants who consumed threshold dose
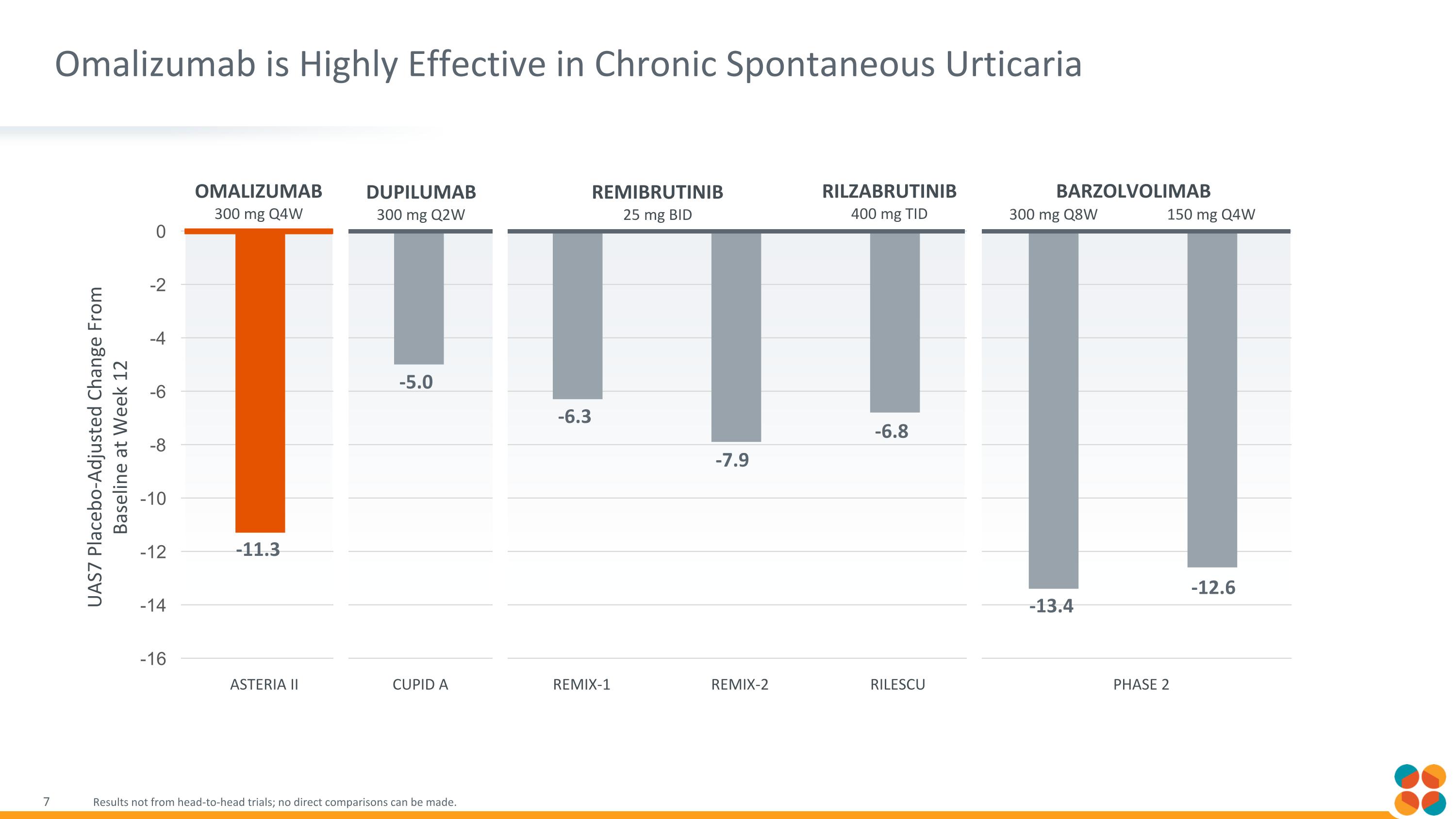
Omalizumab is Highly Effective in Chronic Spontaneous Urticaria Results not from head-to-head trials; no direct comparisons can be made. Omalizumab 300 mg Q4W REMIBRUTINIB 25 mg BID DUPILUMAB 300 mg Q2W -11.3 -5.0 -6.3 -7.9 -6.8 -13.4 -12.6 REMIX-1 REMIX-2 RILZABRUTINIB 400 mg TID BARZOLVOLIMAB 300 mg Q8W 150 mg Q4W ASTERIA II CUPID A RILESCU PHASE 2 UAS7 Placebo-Adjusted Change From Baseline at Week 12
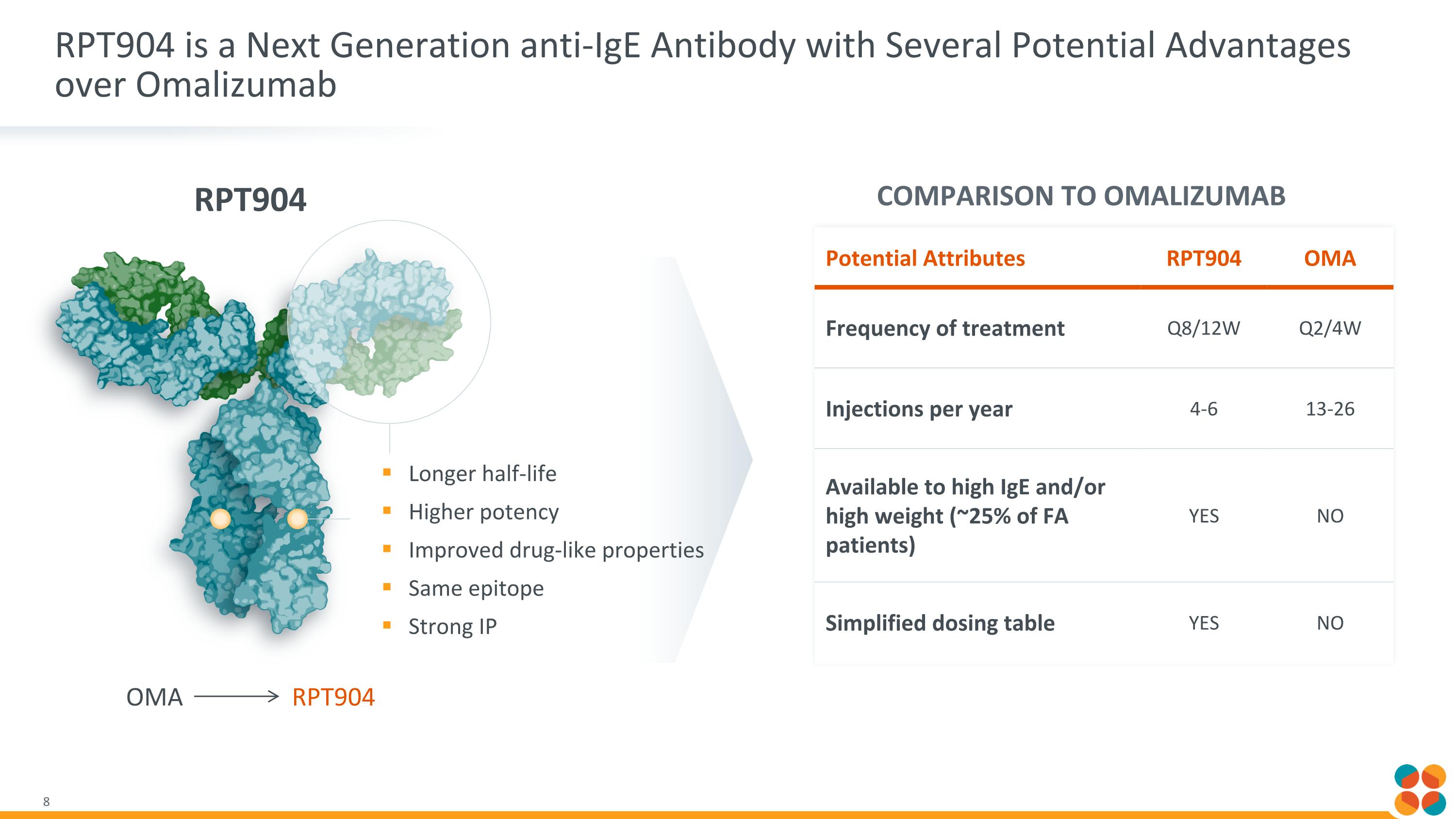
Potential Attributes RPT904 OMA Frequency of treatment Q8/12W Q2/4W Injections per year 4-6 13-26 Available to high IgE and/or high weight (~25% of FA patients) YES NO Simplified dosing table YES NO RPT904 is a Next Generation anti-IgE Antibody with Several Potential Advantages over Omalizumab Longer half-life Higher potency Improved drug-like properties Same epitope Strong IP RPT904 COMPARISON TO OMALIZUMAB OMA RPT904

RPT904 Phase 1 Healthy Volunteer Data and Dose Estimations
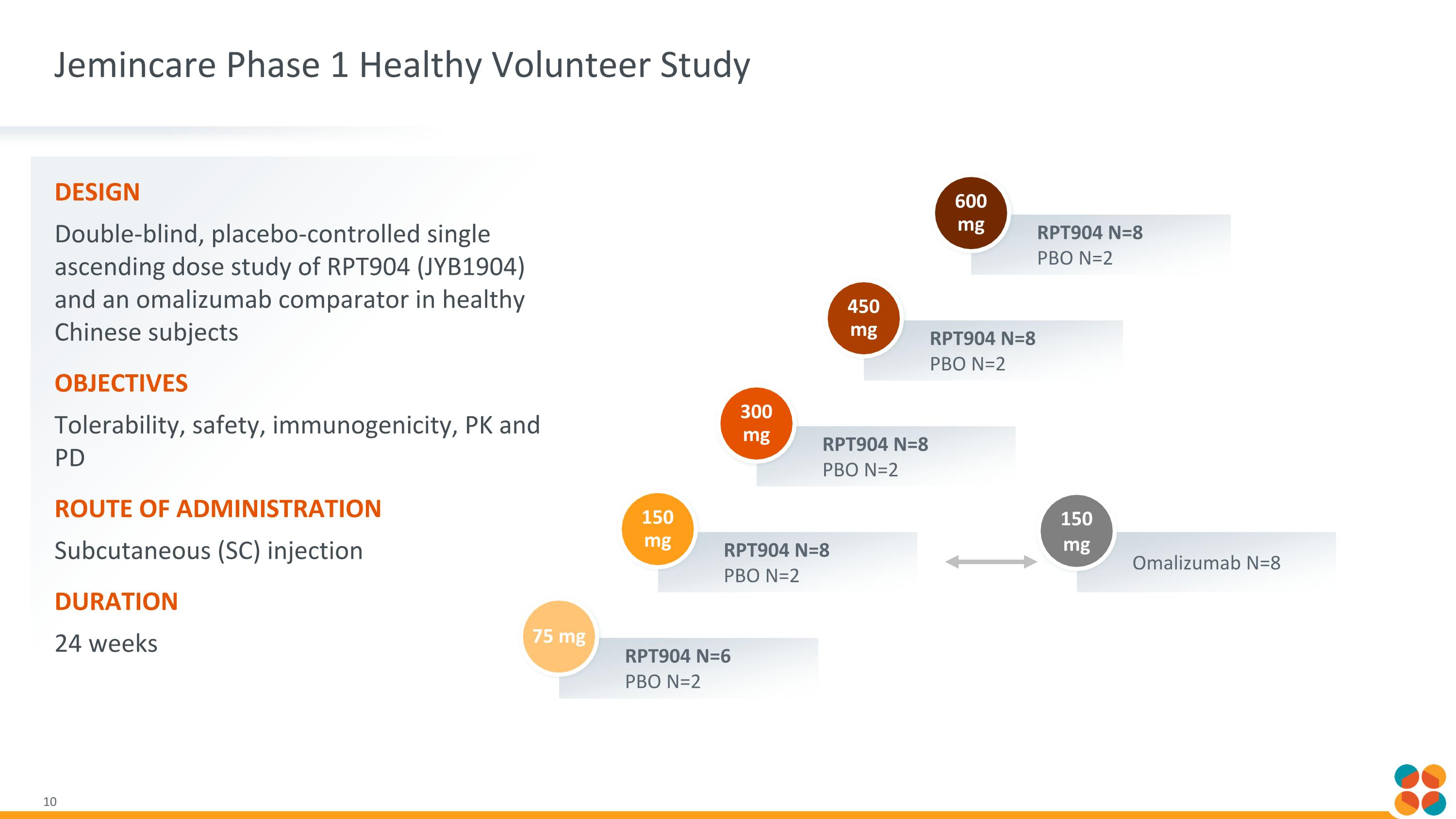
Jemincare Phase 1 Healthy Volunteer Study Design Double-blind, placebo-controlled single ascending dose study of RPT904 (JYB1904) and an omalizumab comparator in healthy Chinese subjects Objectives Tolerability, safety, immunogenicity, PK and PD Route of administration Subcutaneous (SC) injection Duration 24 weeks RPT904 N=6 PBO N=2 75 mg RPT904 N=8 PBO N=2 150 mg RPT904 N=8 PBO N=2 300 mg RPT904 N=8 PBO N=2 450 mg RPT904 N=8 PBO N=2 600 mg Omalizumab N=8 150 mg
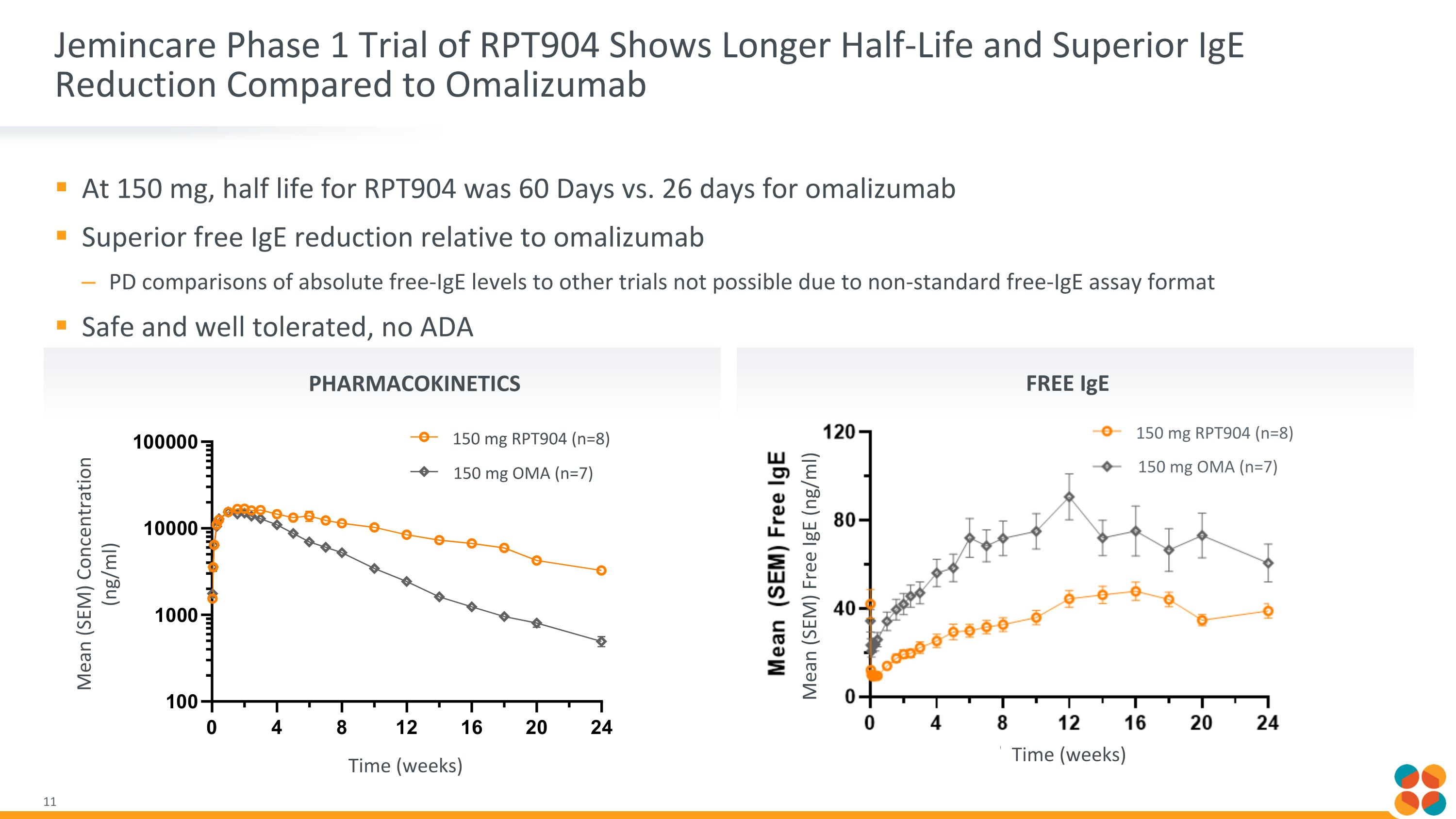
At 150 mg, half life for RPT904 was 60 Days vs. 26 days for omalizumab Superior free IgE reduction relative to omalizumab PD comparisons of absolute free-IgE levels to other trials not possible due to non-standard free-IgE assay format Safe and well tolerated, no ADA Jemincare Phase 1 Trial of RPT904 Shows Longer Half-Life and Superior IgE Reduction Compared to Omalizumab Pharmacokinetics Free IgE Time (weeks) Mean (SEM) Free IgE (ng/ml) 150 mg RPT904 (n=8) 150 mg OMA (n=7) Mean (SEM) Concentration (ng/ml) Time (weeks) 150 mg RPT904 (n=8) 150 mg OMA (n=7)
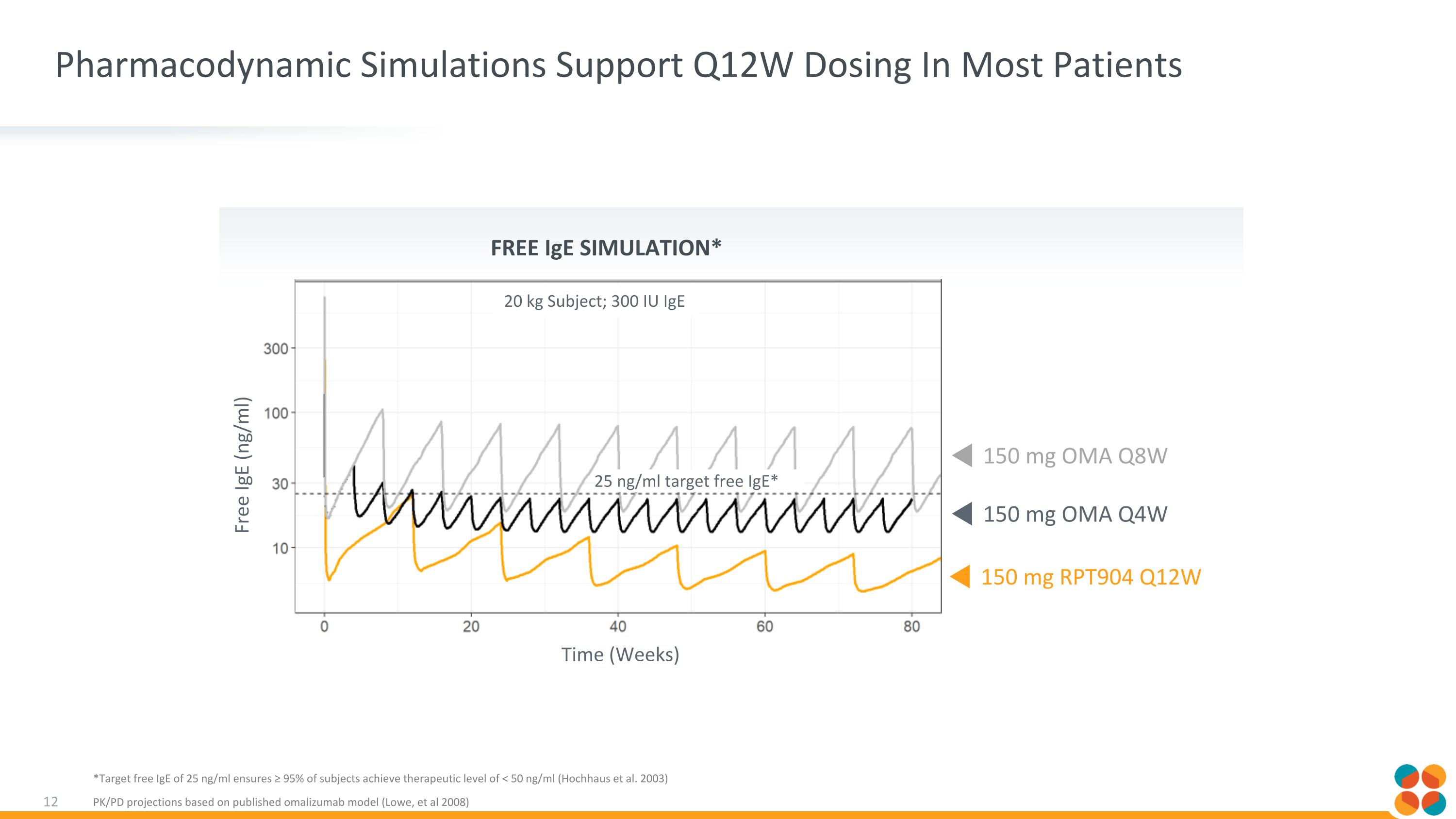
Pharmacodynamic Simulations Support Q12W Dosing In Most Patients *Target free IgE of 25 ng/ml ensures ≥ 95% of subjects achieve therapeutic level of < 50 ng/ml (Hochhaus et al. 2003) PK/PD projections based on published omalizumab model (Lowe, et al 2008) 25 ng/ml target free IgE* Free IgE (ng/ml) Time (Weeks) 20 kg Subject; 300 IU IgE Free IgE Simulation* 150 mg RPT904 Q12W 150 mg OMA Q4W 150 mg OMA Q8W
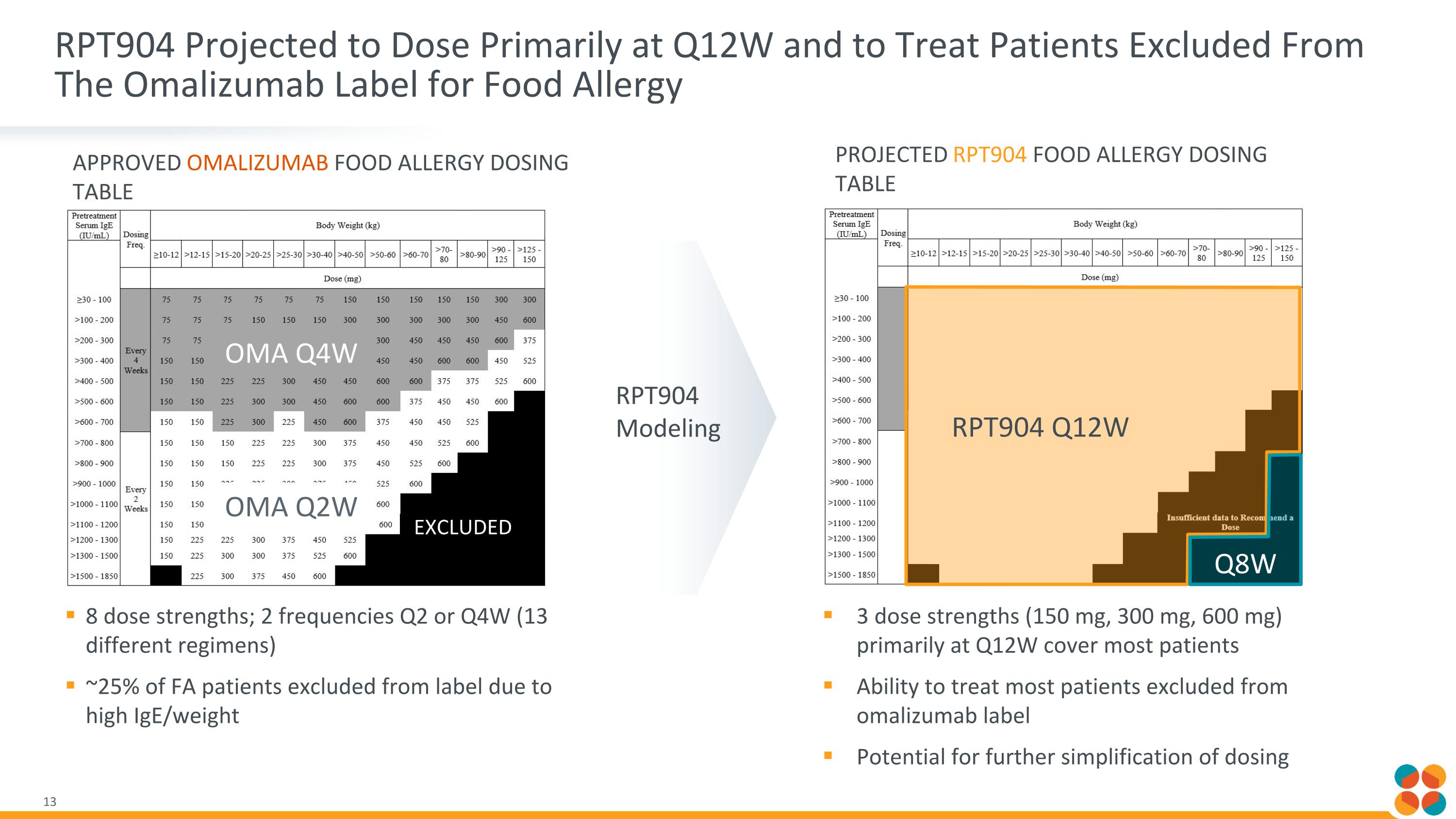
RPT904 Projected to Dose Primarily at Q12W and to Treat Patients Excluded From The Omalizumab Label for Food Allergy 8 dose strengths; 2 frequencies Q2 or Q4W (13 different regimens) ~25% of FA patients excluded from label due to high IgE/weight APPROVED OMALIZUMAB FOOD ALLERGY DOSING TABLE 3 dose strengths (150 mg, 300 mg, 600 mg) primarily at Q12W cover most patients Ability to treat most patients excluded from omalizumab label Potential for further simplification of dosing RPT904 Modeling PROJECTED RPT904 FOOD ALLERGY DOSING TABLE RPT904 Q12W Q8W OMA Q4W OMA Q2W EXCLUDED

RPT904 Commercial Opportunity and Positioning in FA and CSU
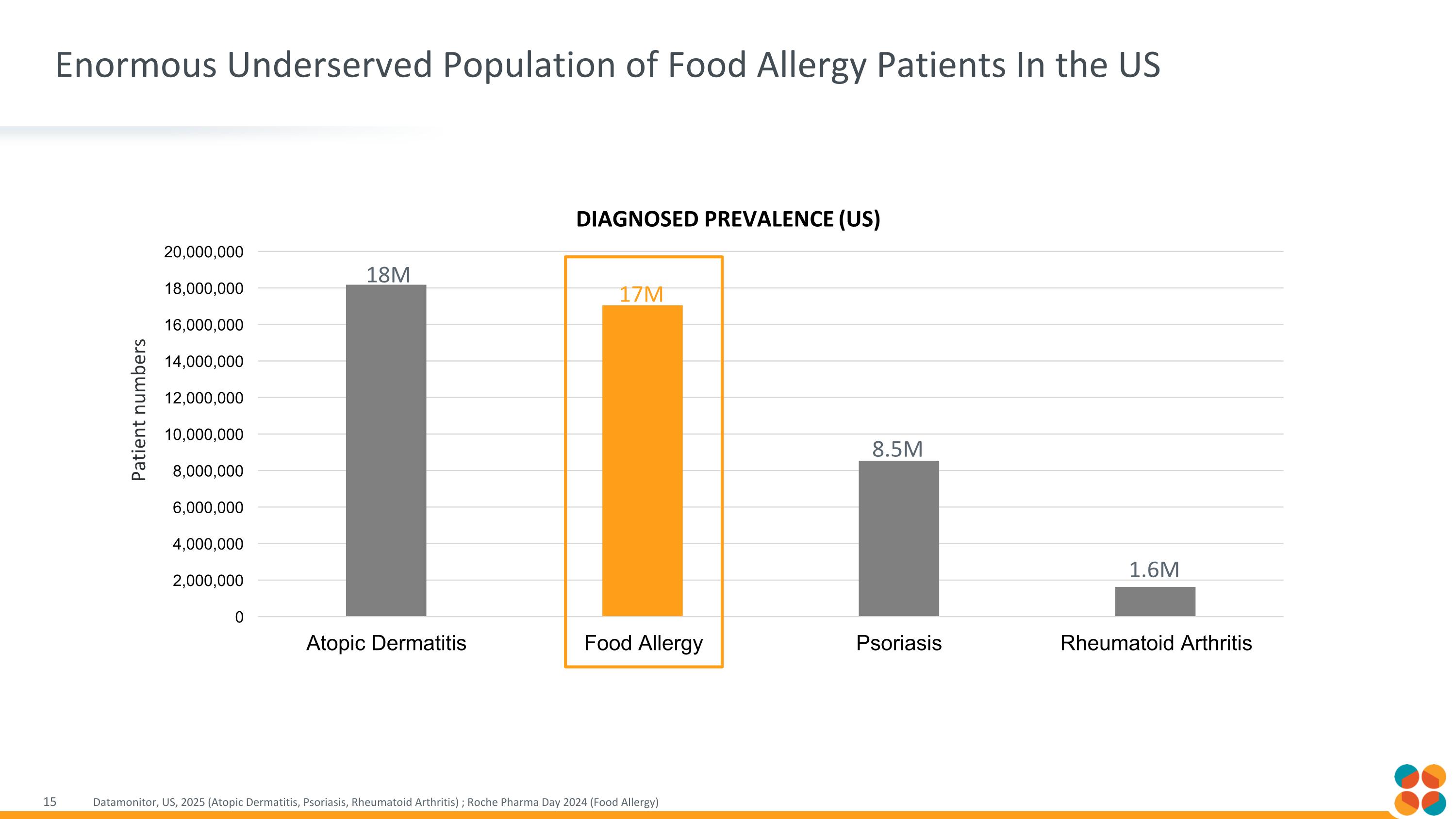
Enormous Underserved Population of Food Allergy Patients In the US Datamonitor, US, 2025 (Atopic Dermatitis, Psoriasis, Rheumatoid Arthritis) ; Roche Pharma Day 2024 (Food Allergy) Patient numbers 18M 17M 8.5M 1.6M
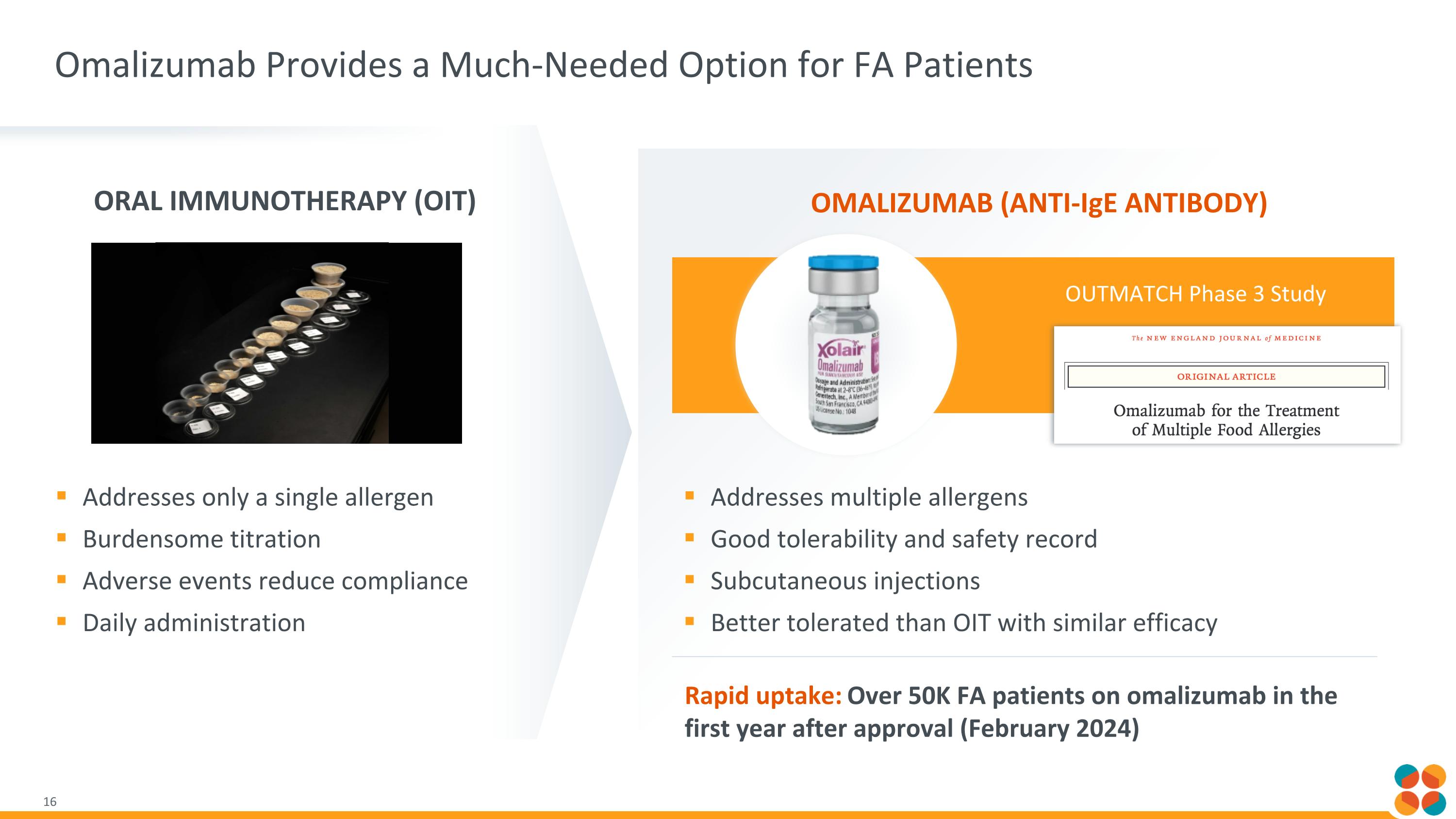
OUTMATCH Phase 3 Study Omalizumab Provides a Much-Needed Option for FA Patients Addresses multiple allergens Good tolerability and safety record Subcutaneous injections Better tolerated than OIT with similar efficacy Addresses only a single allergen Burdensome titration Adverse events reduce compliance Daily administration Rapid uptake: Over 50K FA patients on omalizumab in the first year after approval (February 2024) Oral Immunotherapy (OIT) Omalizumab (Anti-IgE antibody)
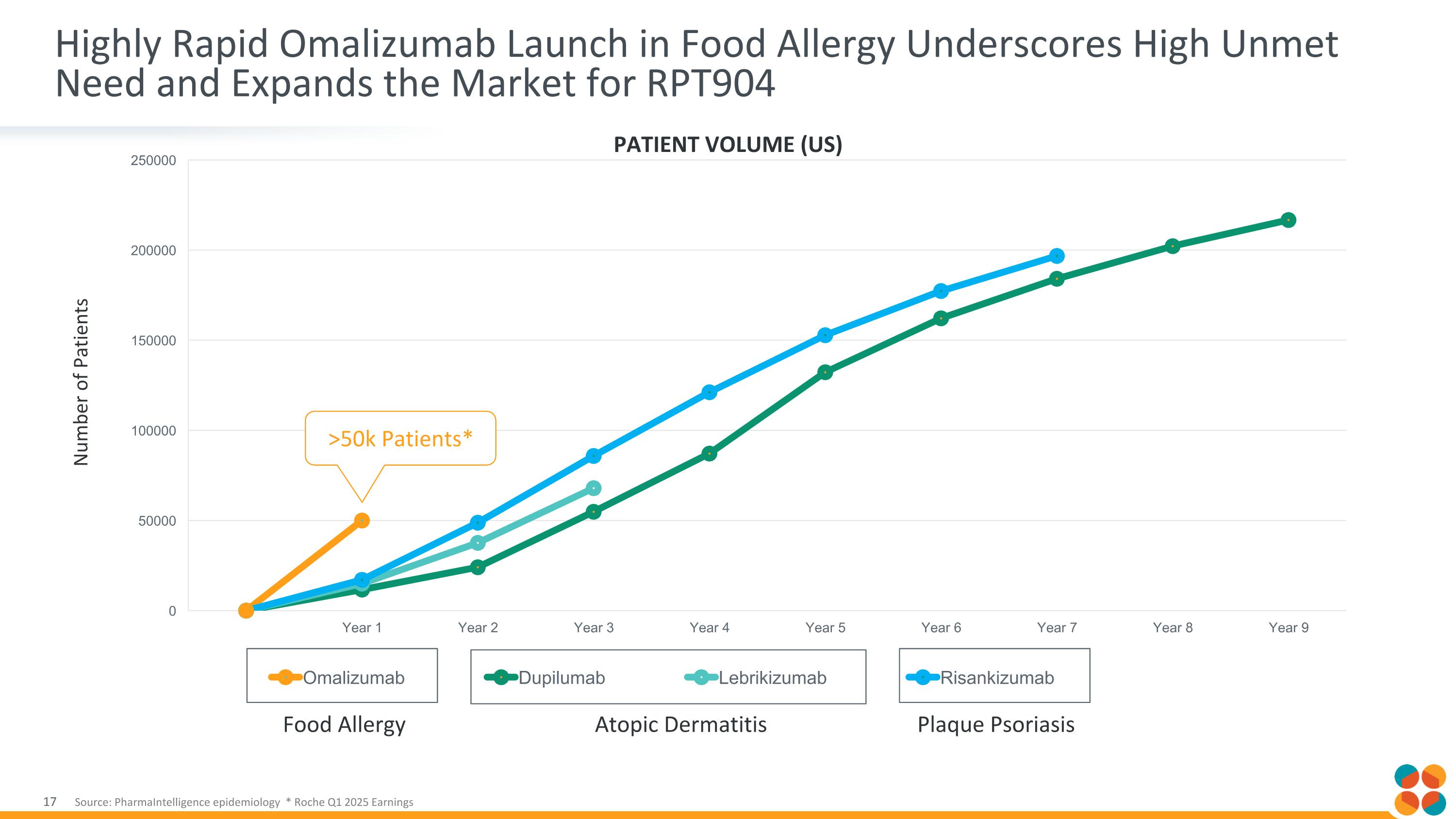
Highly Rapid Omalizumab Launch in Food Allergy Underscores High Unmet Need and Expands the Market for RPT904 Atopic Dermatitis Plaque Psoriasis Food Allergy Number of Patients PATIENT VOLUME (US) Source: PharmaIntelligence epidemiology * Roche Q1 2025 Earnings >50k Patients*
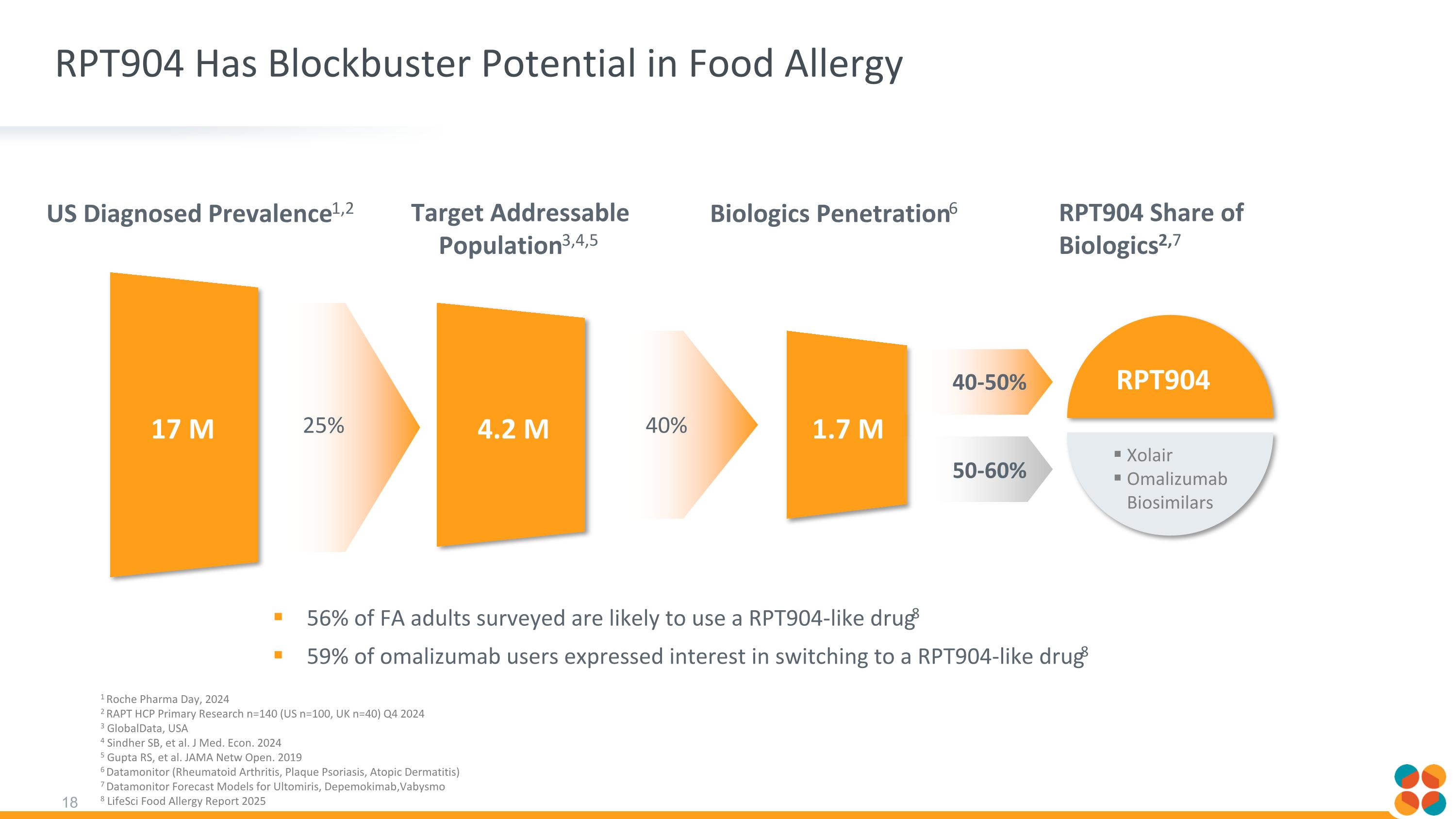
RPT904 Has Blockbuster Potential in Food Allergy US Diagnosed Prevalence1,2 17 M 4.2 M RPT904 Share of Biologics2,7 Target Addressable Population3,4,5 Biologics Penetration6 1.7 M 1 Roche Pharma Day, 2024 2 RAPT HCP Primary Research n=140 (US n=100, UK n=40) Q4 2024 3 GlobalData, USA 4 Sindher SB, et al. J Med. Econ. 2024 5 Gupta RS, et al. JAMA Netw Open. 2019 6 Datamonitor (Rheumatoid Arthritis, Plaque Psoriasis, Atopic Dermatitis) 7 Datamonitor Forecast Models for Ultomiris, Depemokimab,Vabysmo 8 LifeSci Food Allergy Report 2025 40% 25% 40-50% 50-60% RPT904 Xolair Omalizumab Biosimilars 56% of FA adults surveyed are likely to use a RPT904-like drug8 59% of omalizumab users expressed interest in switching to a RPT904-like drug8
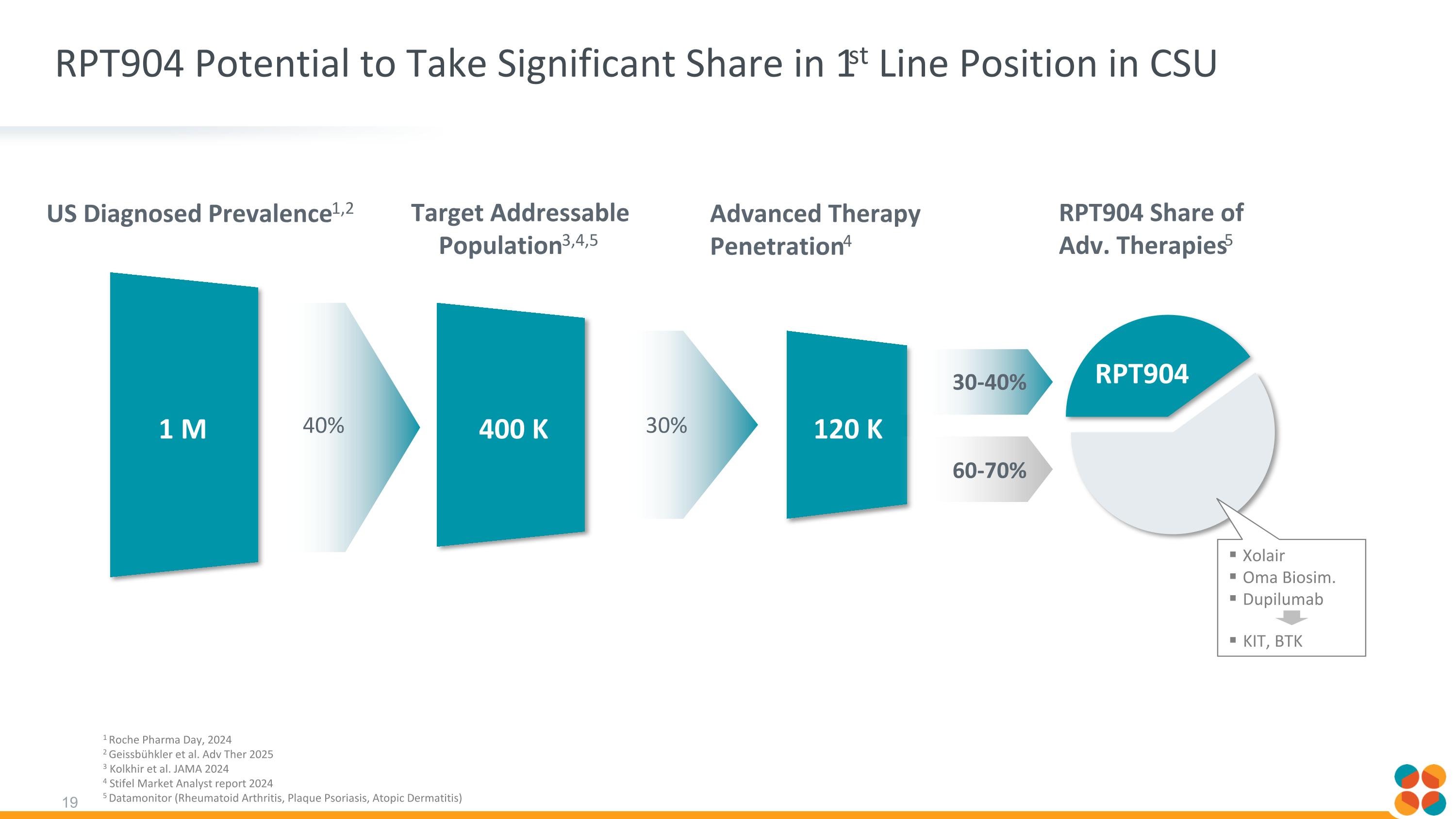
RPT904 Potential to Take Significant Share in 1st Line Position in CSU US Diagnosed Prevalence1,2 1 M 400 K RPT904 Share of Adv. Therapies5 Target Addressable Population3,4,5 Advanced Therapy Penetration4 120 K 30% 40% 30-40% 60-70% RPT904 1 Roche Pharma Day, 2024 2 Geissbühkler et al. Adv Ther 2025 3 Kolkhir et al. JAMA 2024 4 Stifel Market Analyst report 2024 5 Datamonitor (Rheumatoid Arthritis, Plaque Psoriasis, Atopic Dermatitis) Xolair Oma Biosim. Dupilumab KIT, BTK
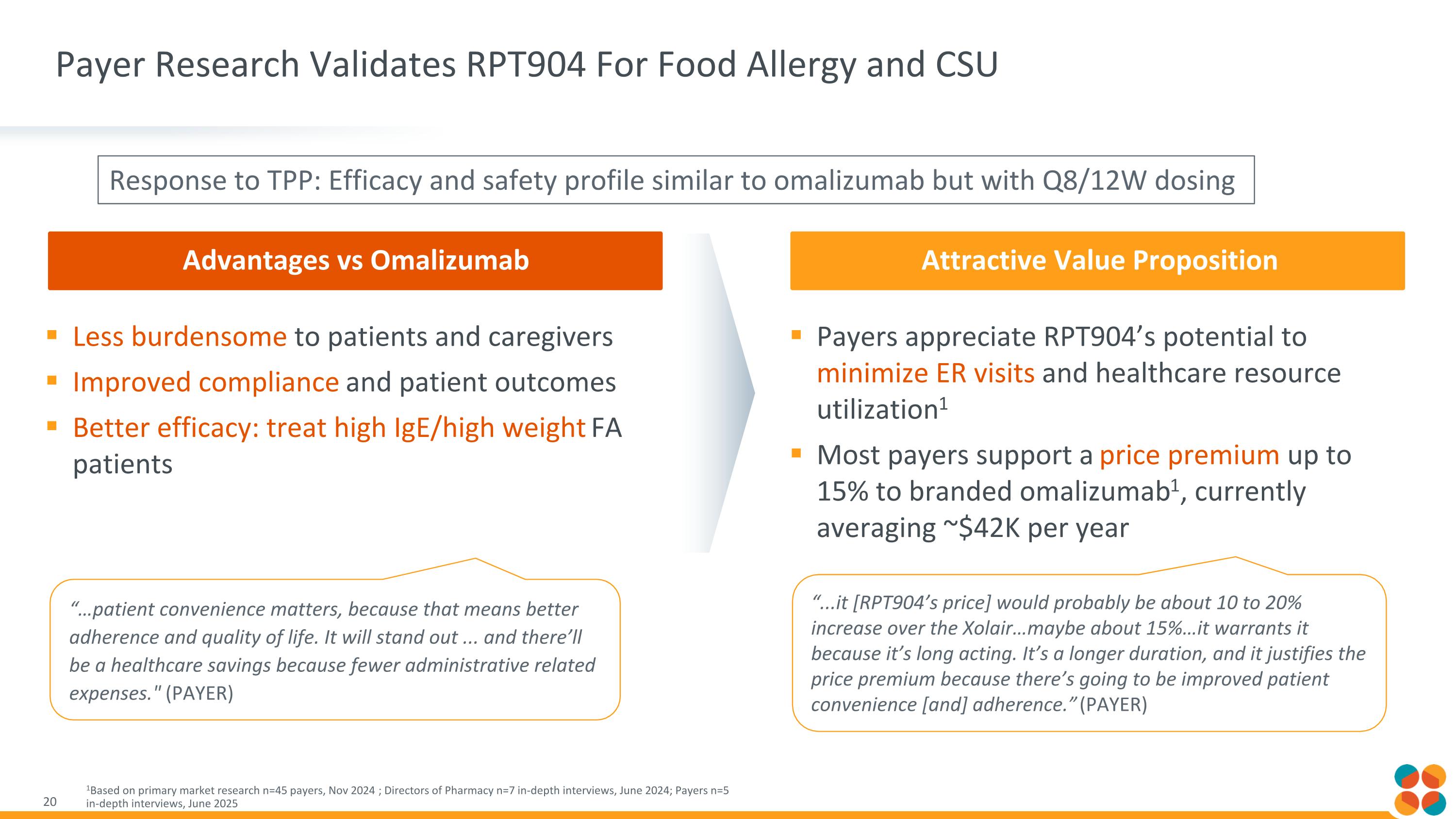
Advantages vs Omalizumab Payer Research Validates RPT904 For Food Allergy and CSU 1Based on primary market research n=45 payers, Nov 2024 ; Directors of Pharmacy n=7 in-depth interviews, June 2024; Payers n=5 in-depth interviews, June 2025 Well Positioned to Be First Choice Attractive Value Proposition Less burdensome to patients and caregivers Improved compliance and patient outcomes Better efficacy: treat high IgE/high weight FA patients Payers appreciate RPT904’s potential to minimize ER visits and healthcare resource utilization1 Most payers support a price premium up to 15% to branded omalizumab1, currently averaging ~$42K per year “...it [RPT904’s price] would probably be about 10 to 20% increase over the Xolair…maybe about 15%…it warrants it because it’s long acting. It’s a longer duration, and it justifies the price premium because there’s going to be improved patient convenience [and] adherence.” (PAYER) “…patient convenience matters, because that means better adherence and quality of life. It will stand out ... and there’ll be a healthcare savings because fewer administrative related expenses." (PAYER) Response to TPP: Efficacy and safety profile similar to omalizumab but with Q8/12W dosing Attractive Value Proposition

RPT904 Clinical Development Plans
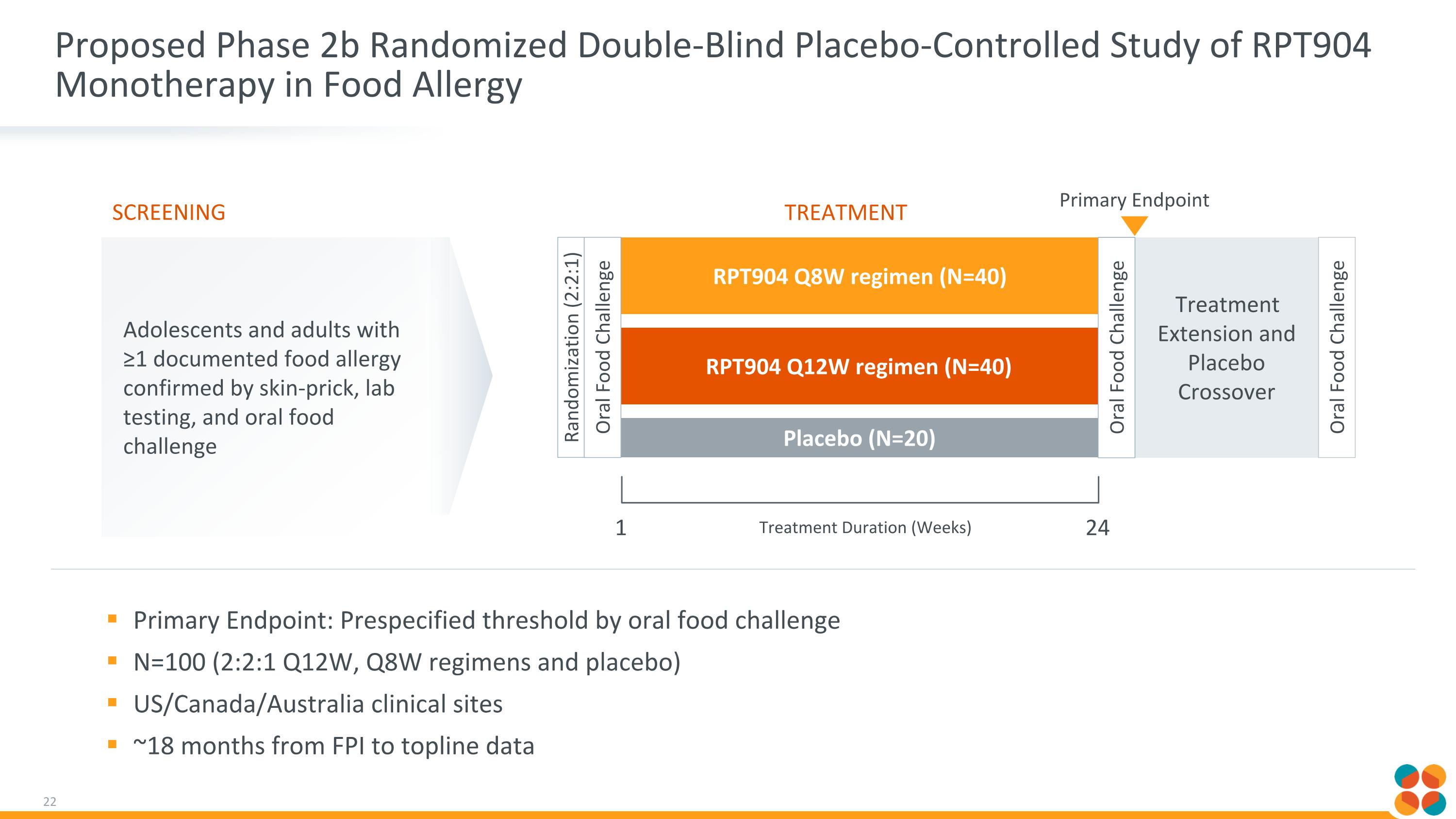
Proposed Phase 2b Randomized Double-Blind Placebo-Controlled Study of RPT904 Monotherapy in Food Allergy Primary Endpoint: Prespecified threshold by oral food challenge N=100 (2:2:1 Q12W, Q8W regimens and placebo) US/Canada/Australia clinical sites ~18 months from FPI to topline data Treatment Extension and Placebo Crossover RPT904 Q12W regimen (N=40) Adolescents and adults with ≥1 documented food allergy confirmed by skin-prick, lab testing, and oral food challenge Screening RPT904 Q8W regimen (N=40) Placebo (N=20) Randomization (2:2:1) Treatment 1 Treatment Duration (Weeks) Oral Food Challenge 24 Oral Food Challenge Oral Food Challenge Primary Endpoint
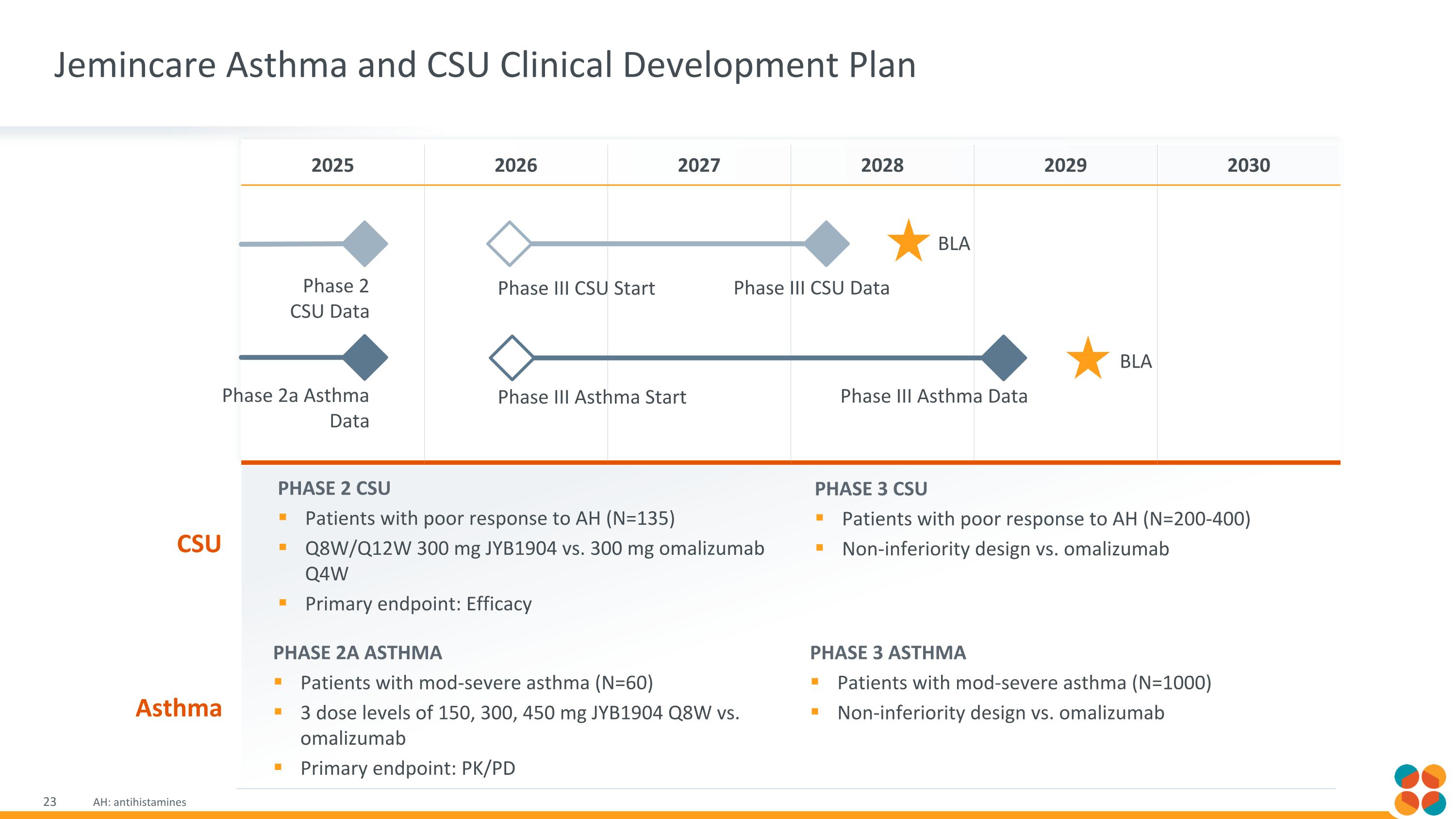
Jemincare Asthma and CSU Clinical Development Plan AH: antihistamines 2025 2026 2027 2028 2029 2030 Phase 2 CSU Data Phase III CSU Start Phase III CSU Data BLA Phase 2a Asthma Data Phase III Asthma Start Phase III Asthma Data BLA Phase 2a Asthma Patients with mod-severe asthma (N=60) 3 dose levels of 150, 300, 450 mg JYB1904 Q8W vs. omalizumab Primary endpoint: PK/PD Phase 3 Asthma Patients with mod-severe asthma (N=1000) Non-inferiority design vs. omalizumab Asthma Phase 2 CSU Patients with poor response to AH (N=135) Q8W/Q12W 300 mg JYB1904 vs. 300 mg omalizumab Q4W Primary endpoint: Efficacy Phase 3 CSU Patients with poor response to AH (N=200-400) Non-inferiority design vs. omalizumab CSU
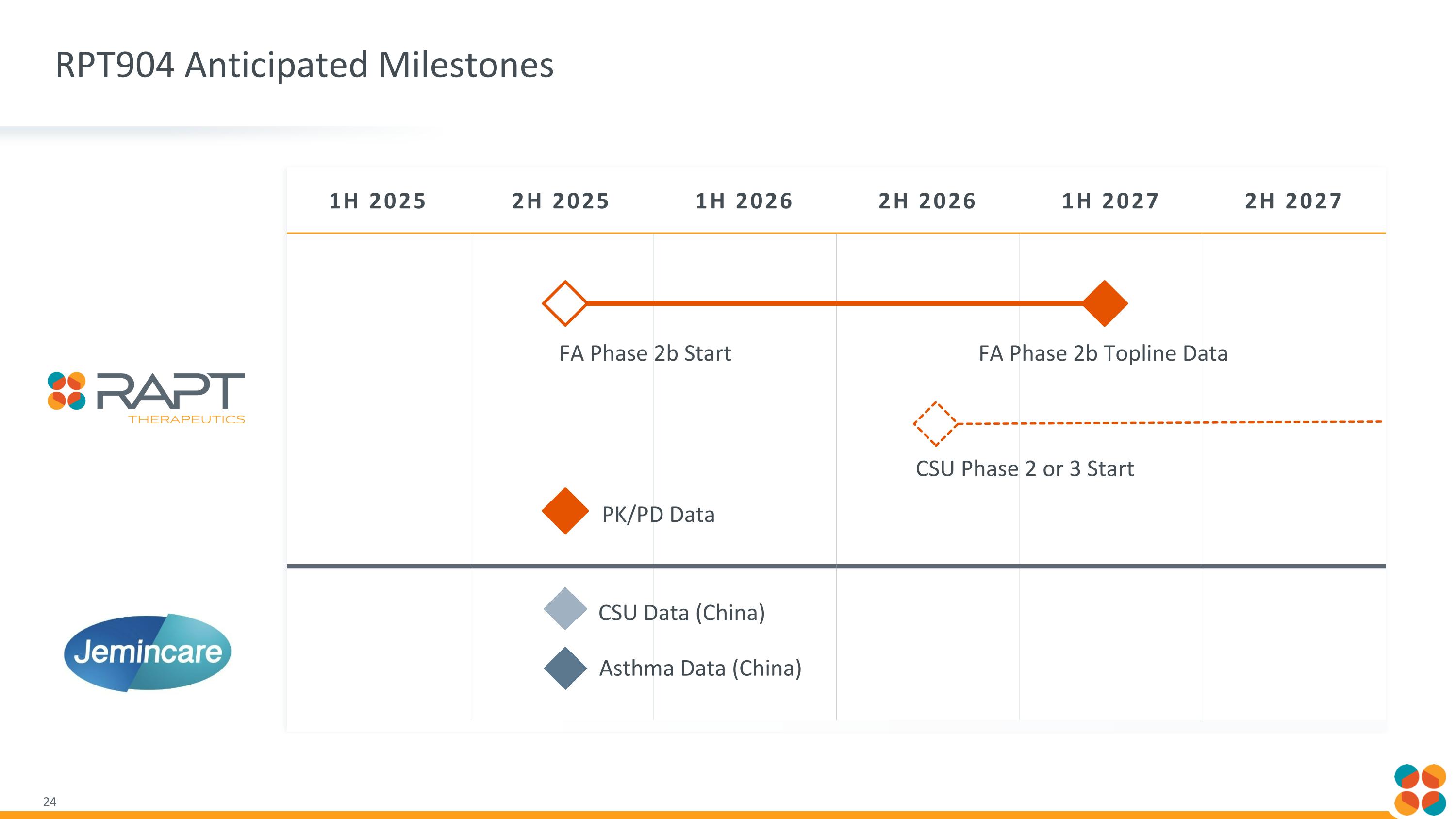
1H 2025 2H 2025 1H 2026 2H 2026 1H 2027 2H 2027 FA Phase 2b Start FA Phase 2b Topline Data CSU Phase 2 or 3 Start CSU Data (China) PK/PD Data RPT904 Anticipated Milestones Asthma Data (China)

Thank You Please visit www.rapt.com