

March 2024 July 2025 Corporate Presentation Exhibit 99.2

This presentation includes “forward-looking statements” within the meaning of the “safe harbor” provisions of the Private Securities Litigation Reform Act of 1995. ProKidney’s actual results may differ from its expectations, estimates and projections and consequently, you should not rely on these forward-looking statements as predictions of future events. Words such as “expect,” “estimate,” “project,” “budget,” “forecast,” “anticipate,” “intend,” “plan,” “may,” “will,” “could,” “should,” “believes,” “predicts,” “potential,” “continue,” and similar expressions (or the negative versions of such words or expressions) are intended to identify such forward-looking statements. These forward-looking statements include, without limitation, the Company’s beliefs that the FDA agrees that the Company’s Phase 3 REGEN-006 (PROACT 1) trial could be sufficient to support a potential BLA submission and full regulatory approval and that the Company could consider using eGFR slope as a surrogate endpoint on an accelerated approval pathway for rilparencel, expectations with respect to financial results and expected cash runway, including the Company’s expectation that current cash will support operating plans into mid-2027, future performance, development and commercialization of products, if approved, the potential benefits and impact of the Company’s products, if approved, potential regulatory approvals, the size and potential growth of current or future markets for the Company’s products, if approved, the advancement of the Company’s development programs into and through the clinic and the expected timing for reporting data, the making of regulatory filings or achieving other milestones related to the Company’s product candidates, and the advancement and funding of the Company’s developmental programs, generally. Most of these factors are outside of the Company’s control and are difficult to predict. Factors that may cause such differences include, but are not limited to: disruptions to our business that may otherwise materially harm our results of operations or financial condition as a result of our recent domestication to the United States; the inability to maintain the listing of the Company’s Class A common stock on Nasdaq; the inability of the Company’s Class A common stock to remain included in various indices and the potential negative impact on the trading price of the Class A common stock if excluded from such indices; the inability to implement business plans, forecasts, and other expectations or identify and realize additional opportunities, which may be affected by, among other things, competition and the ability of the Company to grow and manage growth profitably and retain its key employees; the risk of downturns and a changing regulatory landscape in the highly competitive biotechnology industry; the risk that results of the Company’s clinical trials may not support approval; the risk that the FDA could require additional studies before approving the Company’s drug candidates; the inability of the Company to raise financing in the future; the inability of the Company to obtain and maintain regulatory clearance or approval for its products, and any related restrictions and limitations of any cleared or approved product; the inability of the Company to identify, in-license or acquire additional technology; the inability of Company to compete with other companies currently marketing or engaged in the biologics market and in the area of treatment of kidney diseases; the size and growth potential of the markets for the Company’s products, if approved, and its ability to serve those markets, either alone or in partnership with others; the Company’s estimates regarding expenses, future revenue, capital requirements and needs for additional financing; the Company’s financial performance; the Company’s intellectual property rights; uncertainties inherent in cell therapy research and development, including the actual time it takes to initiate and complete clinical studies and the timing and content of decisions made by regulatory authorities; the fact that interim results from our clinical programs may not be indicative of future results; the impact of geo-political conflict on the Company’s business; and other risks and uncertainties included under the heading “Risk Factors” in the Company’s most recent Annual Report on Form 10-K, subsequent Quarterly Reports on Form 10-Q and other filings with the Securities and Exchange Commission. The Company cautions readers that the foregoing list of factors is not exclusive and cautions readers not to place undue reliance upon any forward-looking statements, which speak only as of the date made. The Company does not undertake or accept any obligation or undertaking to release publicly any updates or revisions to any forward-looking statements to reflect any change in its expectations or any change in events, conditions or circumstances on which any such statement is based. This presentation shall not constitute an offer to sell or the solicitation of an offer to buy these securities, nor shall there be any sale of these securities in any state or jurisdiction in which such offer, solicitation, or sale would be unlawful prior to registration or qualification under the securities laws of any such state or jurisdiction. Forward-looking Statements

Renal Autologous Cell Therapy: Rilparencel (REACT®) proprietary autologous cellular therapy being evaluated to preserve kidney function in patients with diabetes and advanced chronic kidney disease Disrupting the CKD Treatment Landscape

Recent Developments An Introduction to ProKidney CKD = chronic kidney disease RMAT = regenerative medicine advanced therapy Preserve kidney function in advanced CKD patients Preserve kidney function in patients with type 2 diabetes and advanced chronic kidney disease who are faced with limited options for care beyond transplantation or dialysis A proprietary autologous cellular therapy with RMAT designation Currently in pivotal Phase 3 clinical development with REGEN-006 (PROACT 1) Supported by three Phase 2 clinical trials in advanced CKD patient populations Leadership Team with Clinical Development & Regulatory Experience Together the team brings over 150 years cumulative experience in the discovery, development, manufacturing and commercialization of biotechnology, pharmaceutical, and device products Key Developments Across Clinical and Regulatory Objectives In July 2025, announced positive topline results for the Phase 2 REGEN-007 trial In a Q4 2024 Type B meeting, the FDA confirmed the accelerated approval pathway is available for rilparencel if an acceptable surrogate endpoint, such as eGFR slope, is used; additional details expected in mid-2025 Goal Rilparencel Leadership
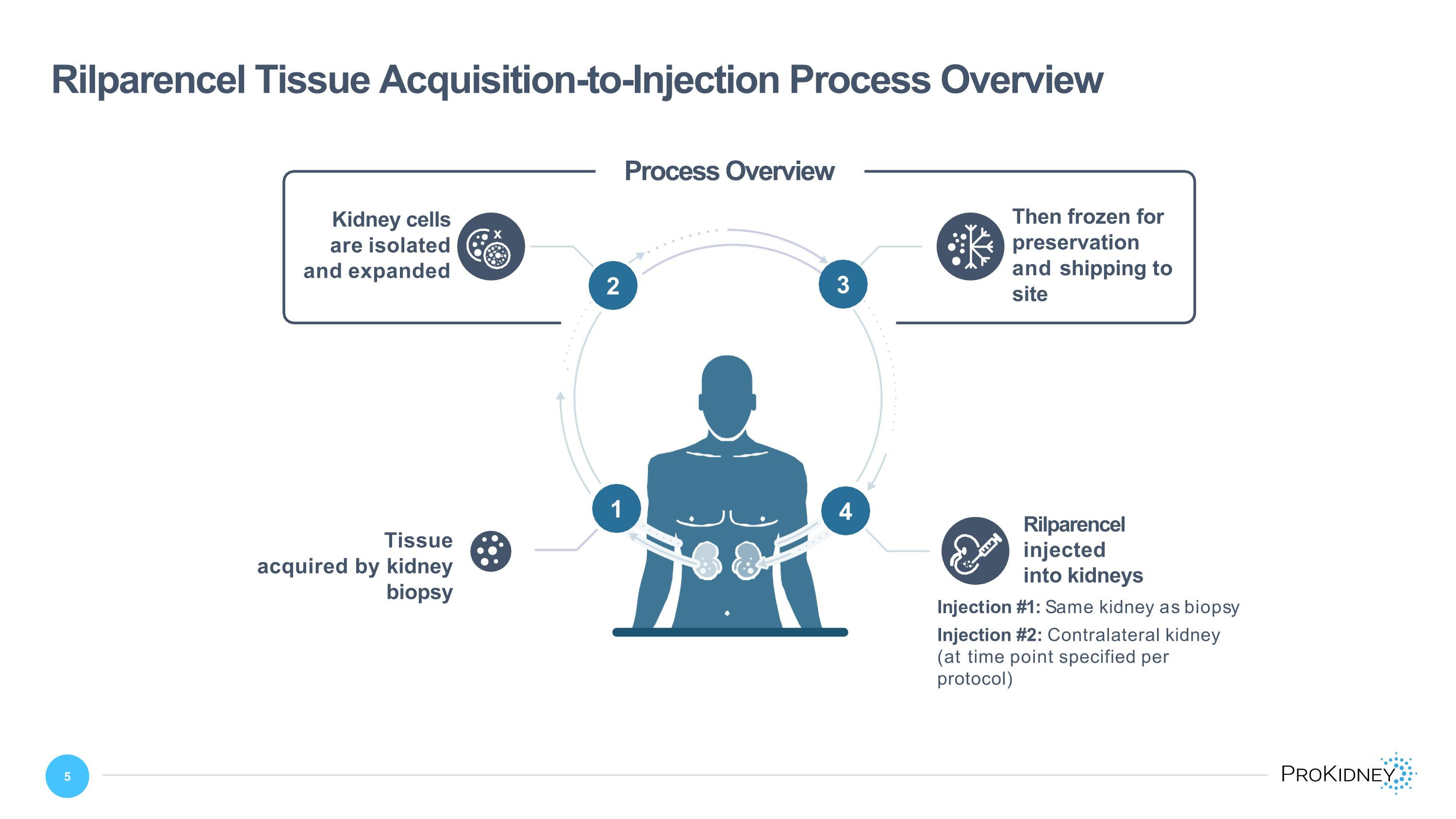
Rilparencel Tissue Acquisition-to-Injection Process Overview 4 2 3 x Then frozen for preservation and shipping to site Kidney cells are isolated and expanded Tissue acquired by kidney biopsy Rilparencel injected into kidneys 1 Process Overview Injection #1: Same kidney as biopsy Injection #2: Contralateral kidney (at time point specified per protocol) 13 2 3 4
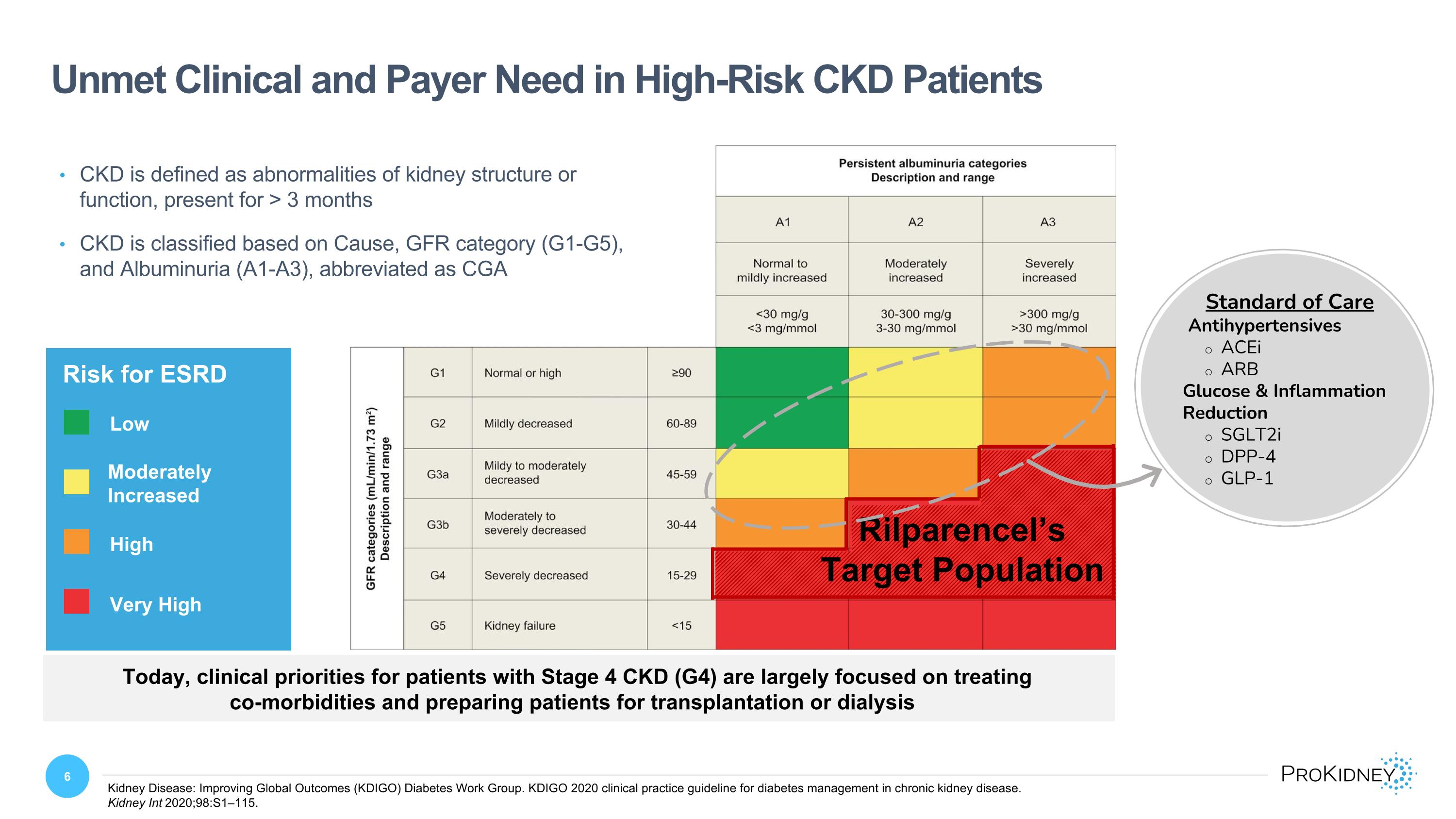
Unmet Clinical and Payer Need in High-Risk CKD Patients Kidney Disease: Improving Global Outcomes (KDIGO) Diabetes Work Group. KDIGO 2020 clinical practice guideline for diabetes management in chronic kidney disease. Kidney Int 2020;98:S1–115. Moderately Increased High Very High Rilparencel’s Target Population Risk for ESRD Low Moderately Increased High Very High CKD is defined as abnormalities of kidney structure or function, present for > 3 months CKD is classified based on Cause, GFR category (G1-G5), and Albuminuria (A1-A3), abbreviated as CGA Standard of Care Antihypertensives ACEi ARB Glucose & Inflammation Reduction SGLT2i DPP-4 GLP-1 Today, clinical priorities for patients with Stage 4 CKD (G4) are largely focused on treating co-morbidities and preparing patients for transplantation or dialysis Persistent albuminuria categories Description and range A1 Normal to mildly increased <30 mg/g <3 mg/mmol A2 Moderately increased 30-300 mg/g 3-30 mg/mmol A3 Severely increased >300 mg/g >30 mg/mmol GFR categories (mL/min/1.73m2) Description and range G1 Normal or high ≥90 G2 Mildly decreased 60-89 G3a Mildy to moderately decreased 45-59 G3b Moderately to severely decreased 30-44 G4 Severely decreased 15-29 G5 Kidney failure <15
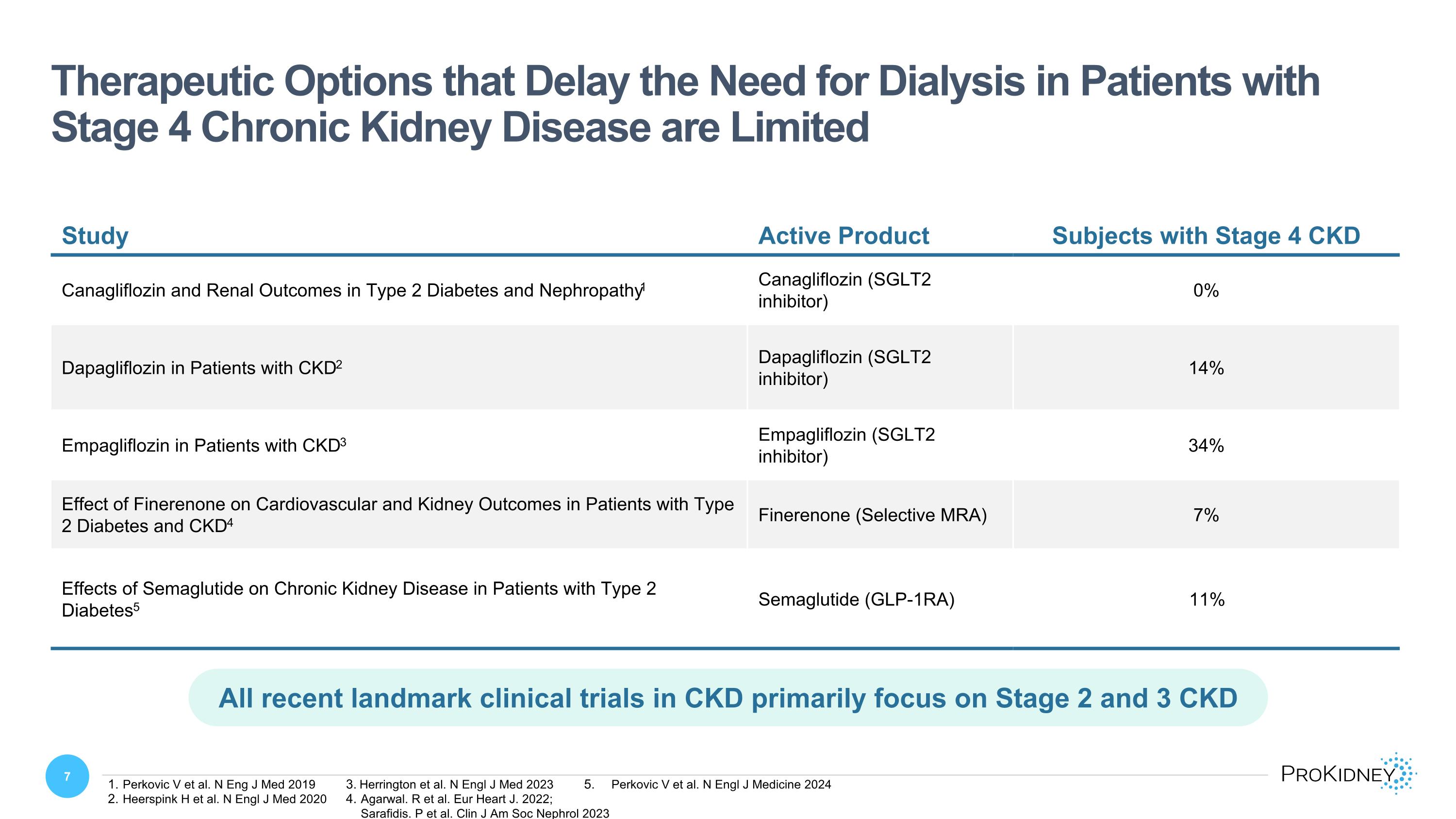
Study Active Product Subjects with Stage 4 CKD Canagliflozin and Renal Outcomes in Type 2 Diabetes and Nephropathy1 Canagliflozin (SGLT2 inhibitor) 0% Dapagliflozin in Patients with CKD2 Dapagliflozin (SGLT2 inhibitor) 14% Empagliflozin in Patients with CKD3 Empagliflozin (SGLT2 inhibitor) 34% Effect of Finerenone on Cardiovascular and Kidney Outcomes in Patients with Type 2 Diabetes and CKD4 Finerenone (Selective MRA) 7% Effects of Semaglutide on Chronic Kidney Disease in Patients with Type 2 Diabetes5 Semaglutide (GLP-1RA) 11% Therapeutic Options that Delay the Need for Dialysis in Patients with Stage 4 Chronic Kidney Disease are Limited All recent landmark clinical trials in CKD primarily focus on Stage 2 and 3 CKD Perkovic V et al. N Eng J Med 2019 Heerspink H et al. N Engl J Med 2020 Herrington et al. N Engl J Med 2023 Agarwal. R et al. Eur Heart J. 2022; Sarafidis. P et al. Clin J Am Soc Nephrol 2023 Perkovic V et al. N Engl J Medicine 2024
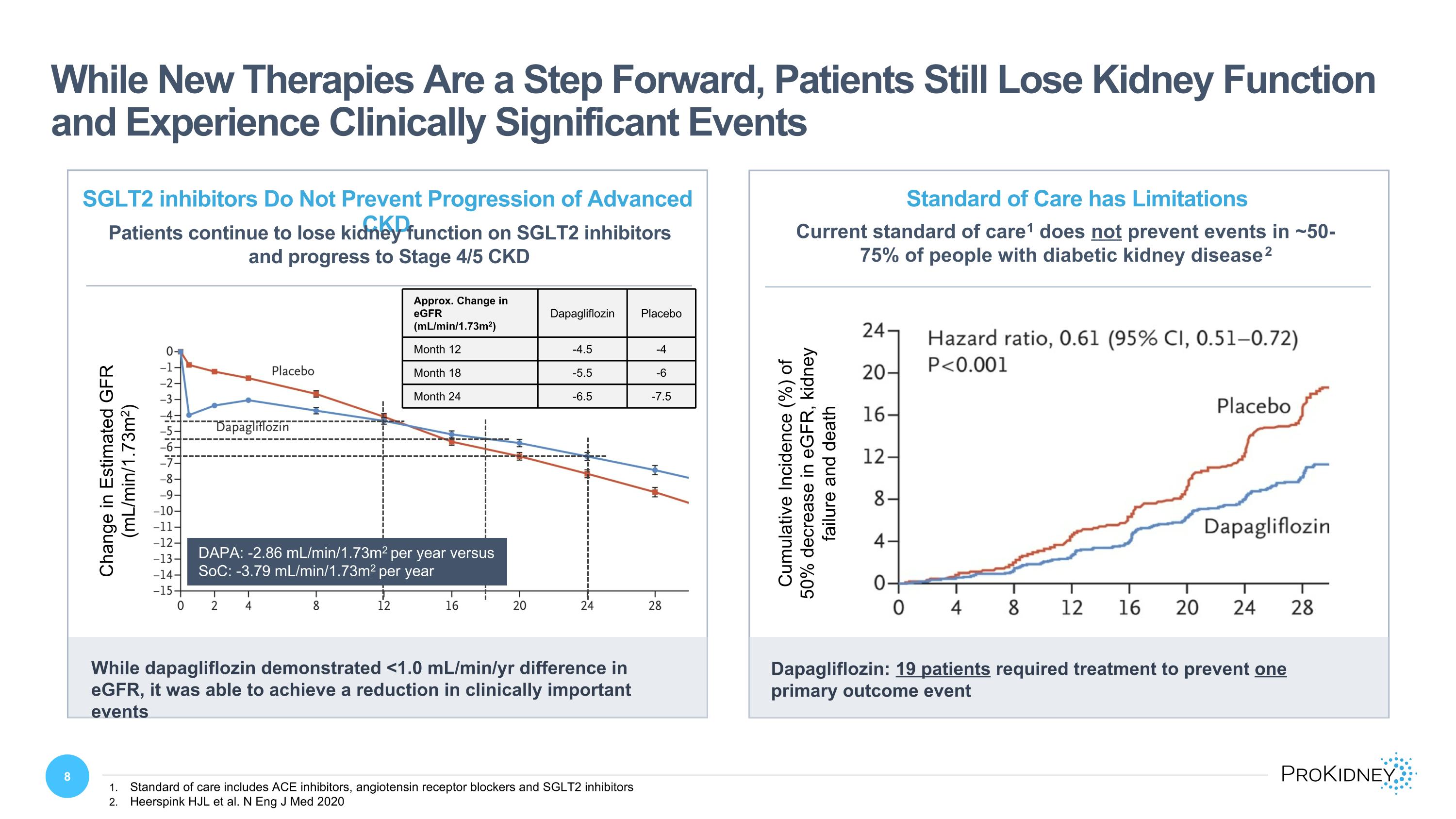
While New Therapies Are a Step Forward, Patients Still Lose Kidney Function and Experience Clinically Significant Events Standard of care includes ACE inhibitors, angiotensin receptor blockers and SGLT2 inhibitors Heerspink HJL et al. N Eng J Med 2020 Current standard of care1 does not prevent events in ~50-75% of people with diabetic kidney disease2 Standard of Care has Limitations Cumulative Incidence (%) of 50% decrease in eGFR, kidney failure and death Dapagliflozin: 19 patients required treatment to prevent one primary outcome event SGLT2 inhibitors Do Not Prevent Progression of Advanced CKD Patients continue to lose kidney function on SGLT2 inhibitors and progress to Stage 4/5 CKD Change in Estimated GFR (mL/min/1.73m2) While dapagliflozin demonstrated <1.0 mL/min/yr difference in eGFR, it was able to achieve a reduction in clinically important events Approx. Change in eGFR (mL/min/1.73m2) Dapagliflozin Placebo Month 12 -4.5 -4 Month 18 -5.5 -6 Month 24 -6.5 -7.5 DAPA: -2.86 mL/min/1.73m2 per year versus SoC: -3.79 mL/min/1.73m2 per year
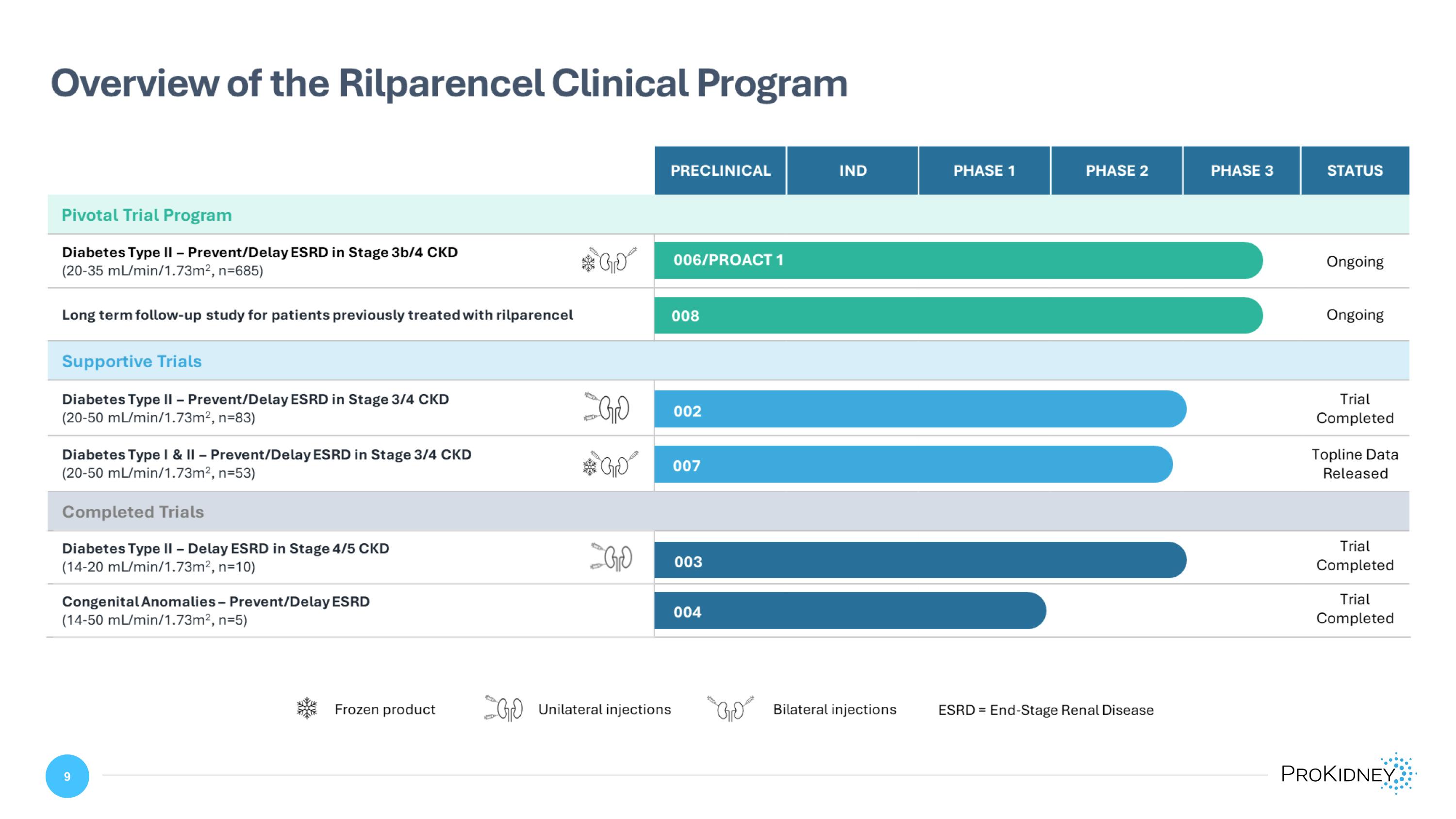
Overview of the Rilparencel Clinical Program PRECLINICAL IND PHASE 1 PHASE 2 PHASE 3 STATUS Pivotal Trial Program Diabetes Type II – Prevent/Delay ESRD in Stage 3b/4 CKD (20-35 mL/min/1.73m2, n=685) 006/PROACT 1 Ongoing Long term follow-up study for patients previously treated with rilparencel 008 Ongoing Supportive Trials Diabetes Type II – Prevent/Delay ESRD in Stage 3/4 CKD (20-50 mL/min/1.73m2, n=83) 002 Trial Completed Diabetes Type I & II – Prevent/Delay ESRD in Stage 3/4 CKD (20-50 mL/min/1.73m2, n=53) 007 Topline Data Released Completed Trials Diabetes Type II – Delay ESRD in Stage 4/5 CKD (14-20 mL/min/1.73m2, n=10) 003 Trial Completed Congenital Anomalies – Prevent/Delay ESRD (14-50 mL/min/1.73m2, n=5) 004 Trial Completed Frozen product Unilateral injections Bilateral injections ESRD = End-Stage Renal Disease

Advancing a Comprehensive Clinical Plan RMCL-002 Phase 2 Trial; full results published in May 2024 Open-label safety & efficacy trial of rilparencel in patients with Stage 3/4 CKD (eGFR 20-50) and type 2 diabetes Results indicated the potential to preserve kidney function for up to 30 months in several patient groups REGEN-006 (PROACT 1) Phase 3 Trial in patients with Stage 3b/4 CKD and type 2 diabetes In a Q4 2024 Type B meeting, FDA confirmed that PROACT 1 could be sufficient to support a full U.S. rilparencel approval FDA also confirmed that the accelerated approval pathway is available for rilparencel if an acceptable surrogate endpoint, such as eGFR slope, is used 2024 REGEN-007 Phase 2 Trial; positive topline results announced in July 2025 Open-label safety & efficacy trial of rilparencel in patients with Stage 3/4 CKD (eGFR 20-50) and diabetes Patients in Group 1 (n=24) met the primary endpoint demonstrating kidney function stabilization Safety profile was consistent with previously reported study results and comparable to a kidney biopsy Full results will be submitted as a late-breaking clinical trial to the American Society of Nephrology (ASN) Kidney Week REGEN-006 (PROACT 1) Phase 3 Trial in patients with Stage 3b/4 CKD and type 2 diabetes Upcoming FDA Type B meeting in summer 2025 to confirm the approach using eGFR slope as the surrogate endpoint for the accelerated approval path for rilparencel Additional details expected in mid-2025 Update on rilparencel mechanism of action in 2H 2025 2025 and beyond
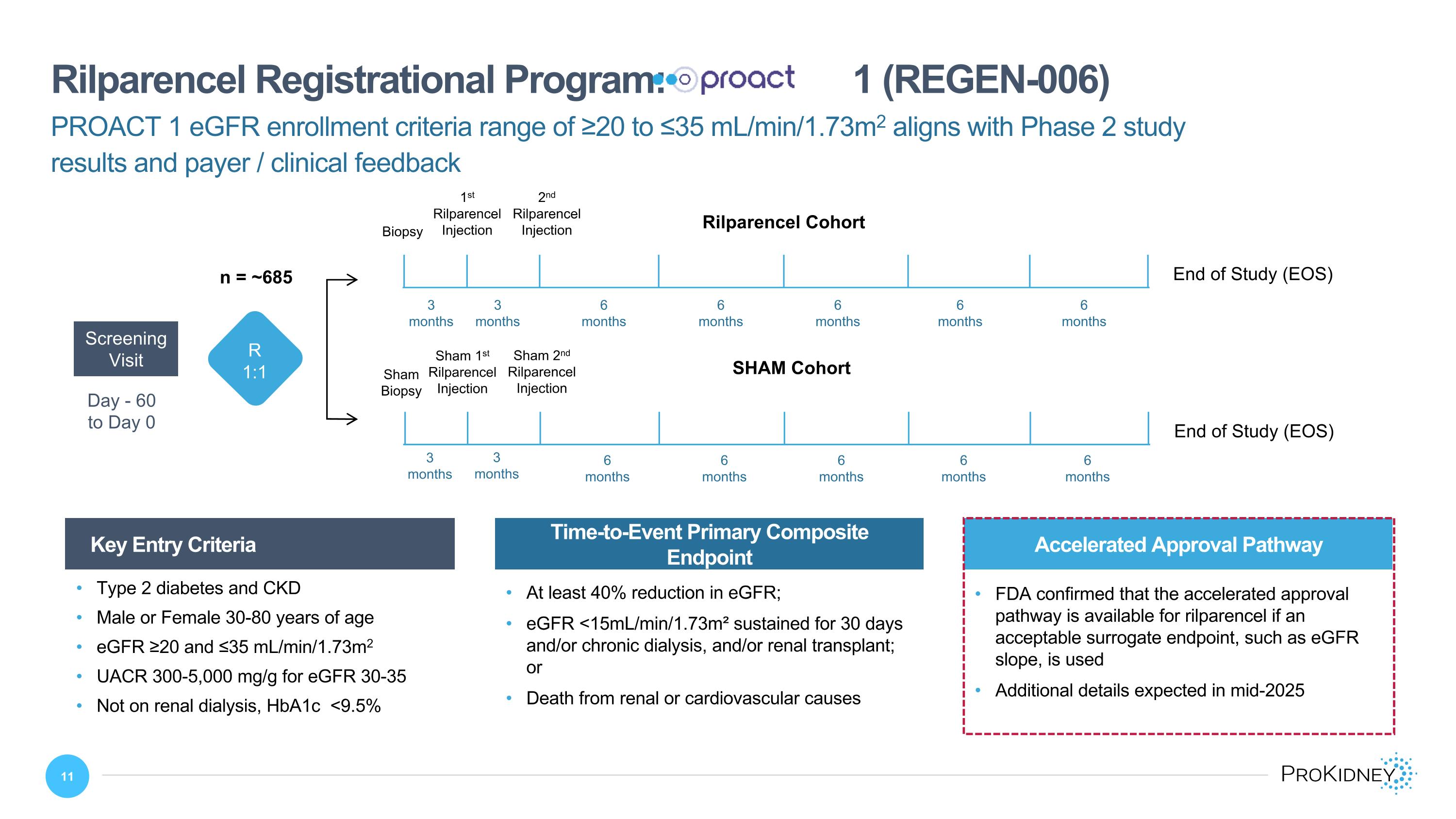
Day - 60 to Day 0 Screening Visit SHAM Cohort Sham 1st Rilparencel Injection Sham 2nd Rilparencel Injection Sham Biopsy End of Study (EOS) Rilparencel Cohort 1st Rilparencel Injection 2nd Rilparencel Injection Biopsy End of Study (EOS) PROACT 1 eGFR enrollment criteria range of ≥20 to ≤35 mL/min/1.73m2 aligns with Phase 2 study results and payer / clinical feedback Rilparencel Registrational Program: 1 (REGEN-006) Key Entry Criteria Time-to-Event Primary Composite Endpoint At least 40% reduction in eGFR; eGFR <15mL/min/1.73m² sustained for 30 days and/or chronic dialysis, and/or renal transplant; or Death from renal or cardiovascular causes Type 2 diabetes and CKD Male or Female 30-80 years of age eGFR ≥20 and ≤35 mL/min/1.73m2 UACR 300-5,000 mg/g for eGFR 30-35 Not on renal dialysis, HbA1c <9.5% 3 months 3 months 3 months 3 months 6 months 6 months 6 months 6 months 6 months 6 months 6 months 6 months 6 months 6 months R 1:1 n = ~685 Accelerated Approval Pathway FDA confirmed that the accelerated approval pathway is available for rilparencel if an acceptable surrogate endpoint, such as eGFR slope, is used Additional details expected in mid-2025

REGEN-007 Topline Results
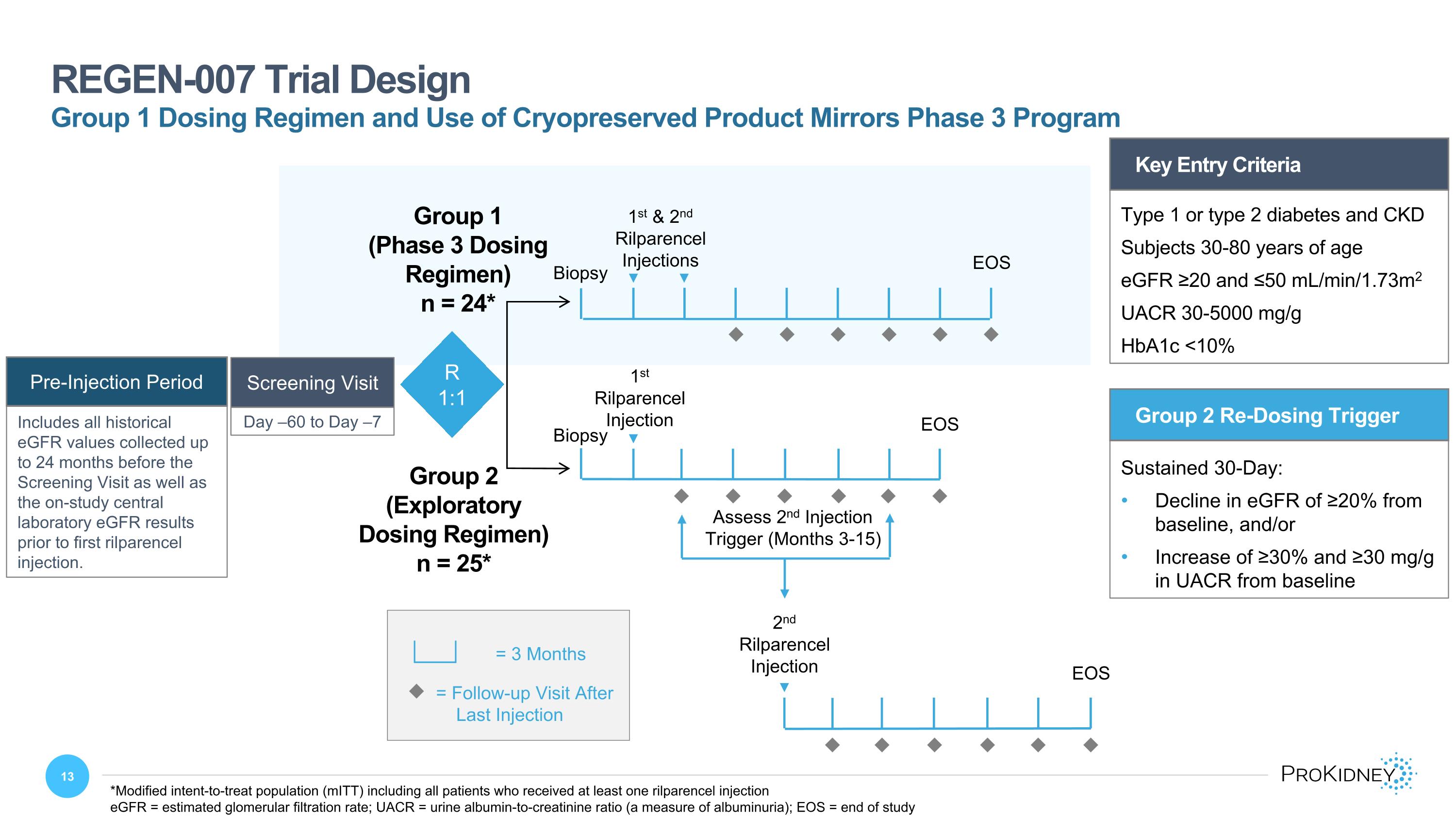
Key Entry Criteria Type 1 or type 2 diabetes and CKD Subjects 30-80 years of age eGFR ≥20 and ≤50 mL/min/1.73m2 UACR 30-5000 mg/g HbA1c <10% Day –60 to Day –7 Screening Visit REGEN-007 Trial Design u = Follow-up Visit After Last Injection = 3 Months Group 2 Re-Dosing Trigger Sustained 30-Day: Decline in eGFR of ≥20% from baseline, and/or Increase of ≥30% and ≥30 mg/g in UACR from baseline 2nd Rilparencel Injection Assess 2nd Injection Trigger (Months 3-15) Group 2 (Exploratory Dosing Regimen) n = 25* R 1:1 1st & 2nd Rilparencel Injections EOS Group 1 (Phase 3 Dosing Regimen) n = 24* EOS 1st Rilparencel Injection u u u u u u u u u u u u u u u u u u EOS *Modified intent-to-treat population (mITT) including all patients who received at least one rilparencel injection eGFR = estimated glomerular filtration rate; UACR = urine albumin-to-creatinine ratio (a measure of albuminuria); EOS = end of study Group 1 Dosing Regimen and Use of Cryopreserved Product Mirrors Phase 3 Program Biopsy Biopsy Pre-Injection Period Includes all historical eGFR values collected up to 24 months before the Screening Visit as well as the on-study central laboratory eGFR results prior to first rilparencel injection.
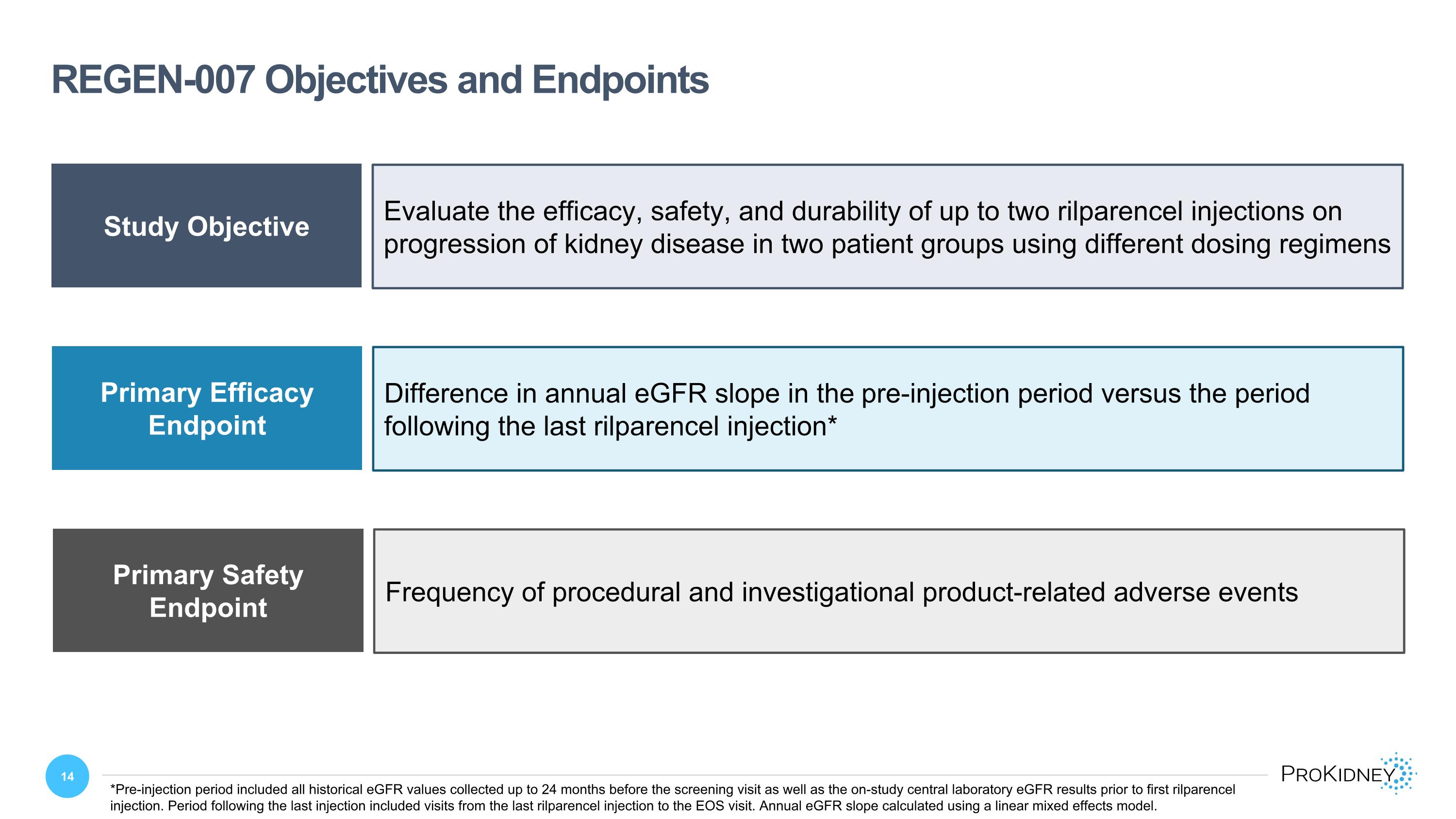
REGEN-007 Objectives and Endpoints Study Objective Endpoints Evaluate the efficacy, safety, and durability of up to two rilparencel injections on progression of kidney disease in two patient groups using different dosing regimens Primary Efficacy Endpoint Difference in annual eGFR slope in the pre-injection period versus the period following the last rilparencel injection* Primary Safety Endpoint Frequency of procedural and investigational product-related adverse events *Pre-injection period included all historical eGFR values collected up to 24 months before the screening visit as well as the on-study central laboratory eGFR results prior to first rilparencel injection. Period following the last injection included visits from the last rilparencel injection to the EOS visit. Annual eGFR slope calculated using a linear mixed effects model.
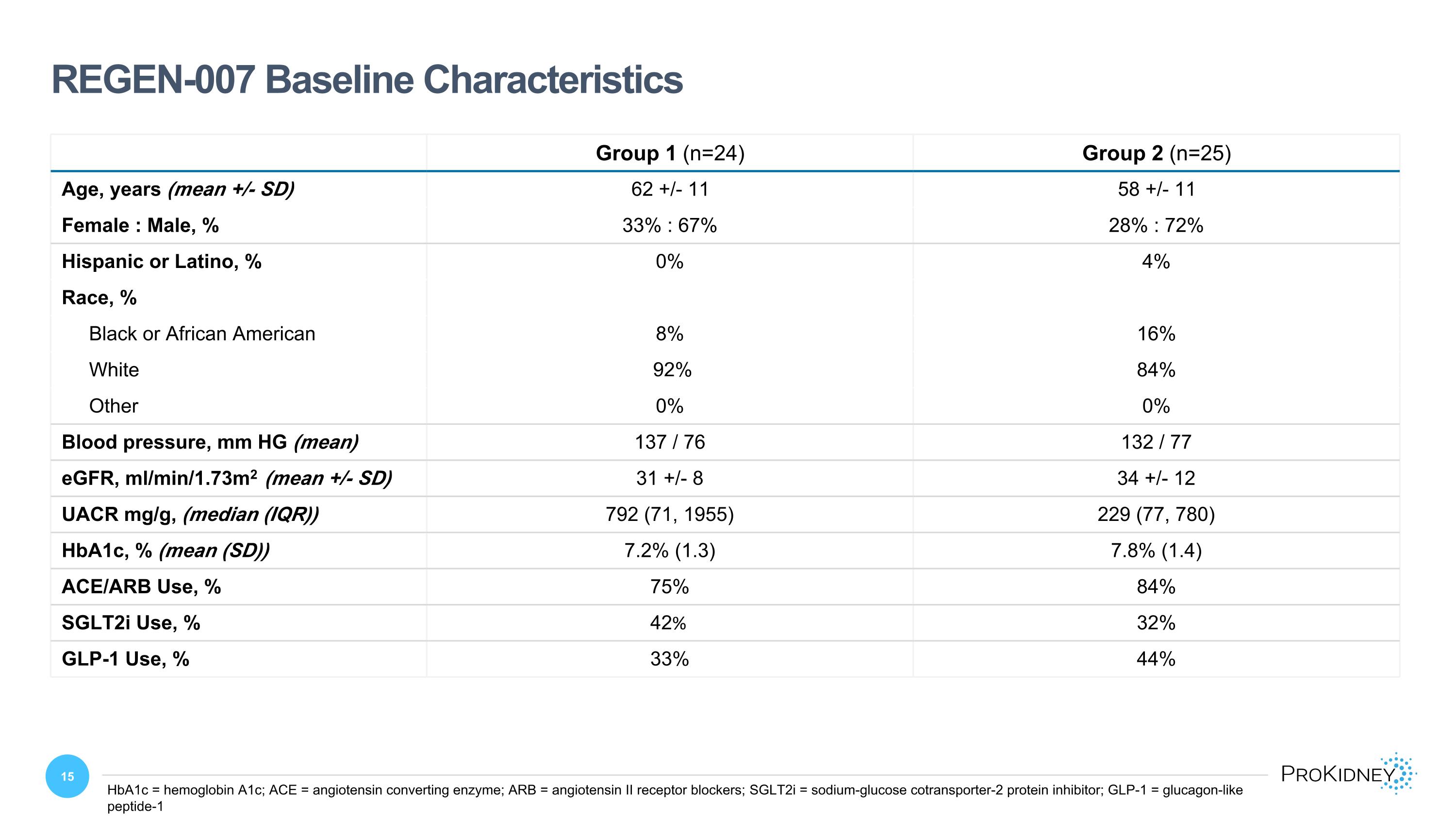
Group 1 (n=24) Group 2 (n=25) Age, years (mean +/- SD) 62 +/- 11 58 +/- 11 Female : Male, % 33% : 67% 28% : 72% Hispanic or Latino, % 0% 4% Race, % Black or African American 8% 16% White 92% 84% Other 0% 0% Blood pressure, mm HG (mean) 137 / 76 132 / 77 eGFR, ml/min/1.73m2 (mean +/- SD) 31 +/- 8 34 +/- 12 UACR mg/g, (median (IQR)) 792 (71, 1955) 229 (77, 780) HbA1c, % (mean (SD)) 7.2% (1.3) 7.8% (1.4) ACE/ARB Use, % 75% 84% SGLT2i Use, % 42% 32% GLP-1 Use, % 33% 44% REGEN-007 Baseline Characteristics HbA1c = hemoglobin A1c; ACE = angiotensin converting enzyme; ARB = angiotensin II receptor blockers; SGLT2i = sodium-glucose cotransporter-2 protein inhibitor; GLP-1 = glucagon-like peptide-1
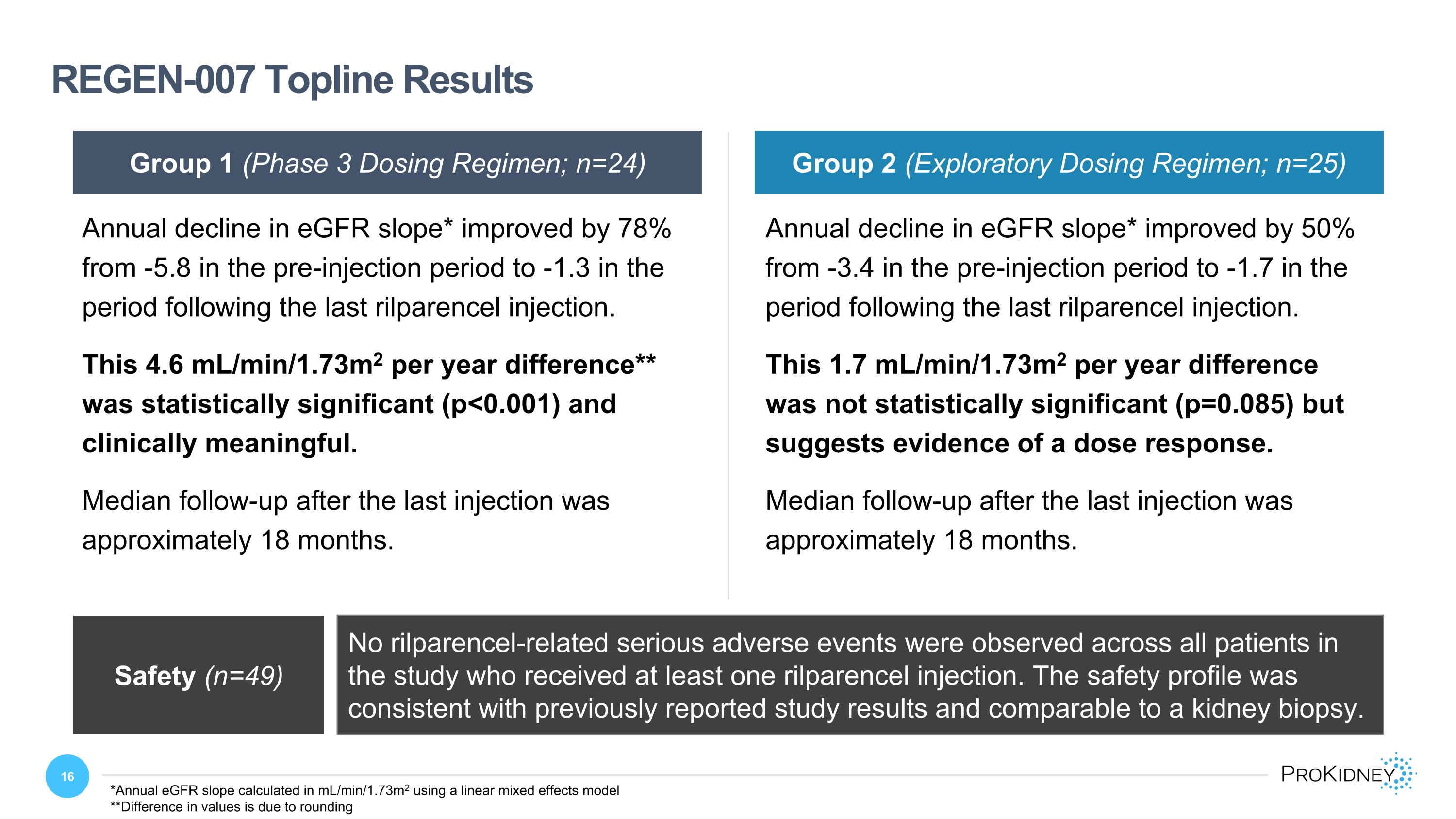
REGEN-007 Topline Results Group 1 (Phase 3 Dosing Regimen; n=24) Safety (n=49) No rilparencel-related serious adverse events were observed across all patients in the study who received at least one rilparencel injection. The safety profile was consistent with previously reported study results and comparable to a kidney biopsy. Group 2 (Exploratory Dosing Regimen; n=25) Annual decline in eGFR slope* improved by 78% from -5.8 in the pre-injection period to -1.3 in the period following the last rilparencel injection. This 4.6 mL/min/1.73m2 per year difference** was statistically significant (p<0.001) and clinically meaningful. Median follow-up after the last injection was approximately 18 months. Annual decline in eGFR slope* improved by 50% from -3.4 in the pre-injection period to -1.7 in the period following the last rilparencel injection. This 1.7 mL/min/1.73m2 per year difference was not statistically significant (p=0.085) but suggests evidence of a dose response. Median follow-up after the last injection was approximately 18 months. *Annual eGFR slope calculated in mL/min/1.73m2 using a linear mixed effects model **Difference in values is due to rounding
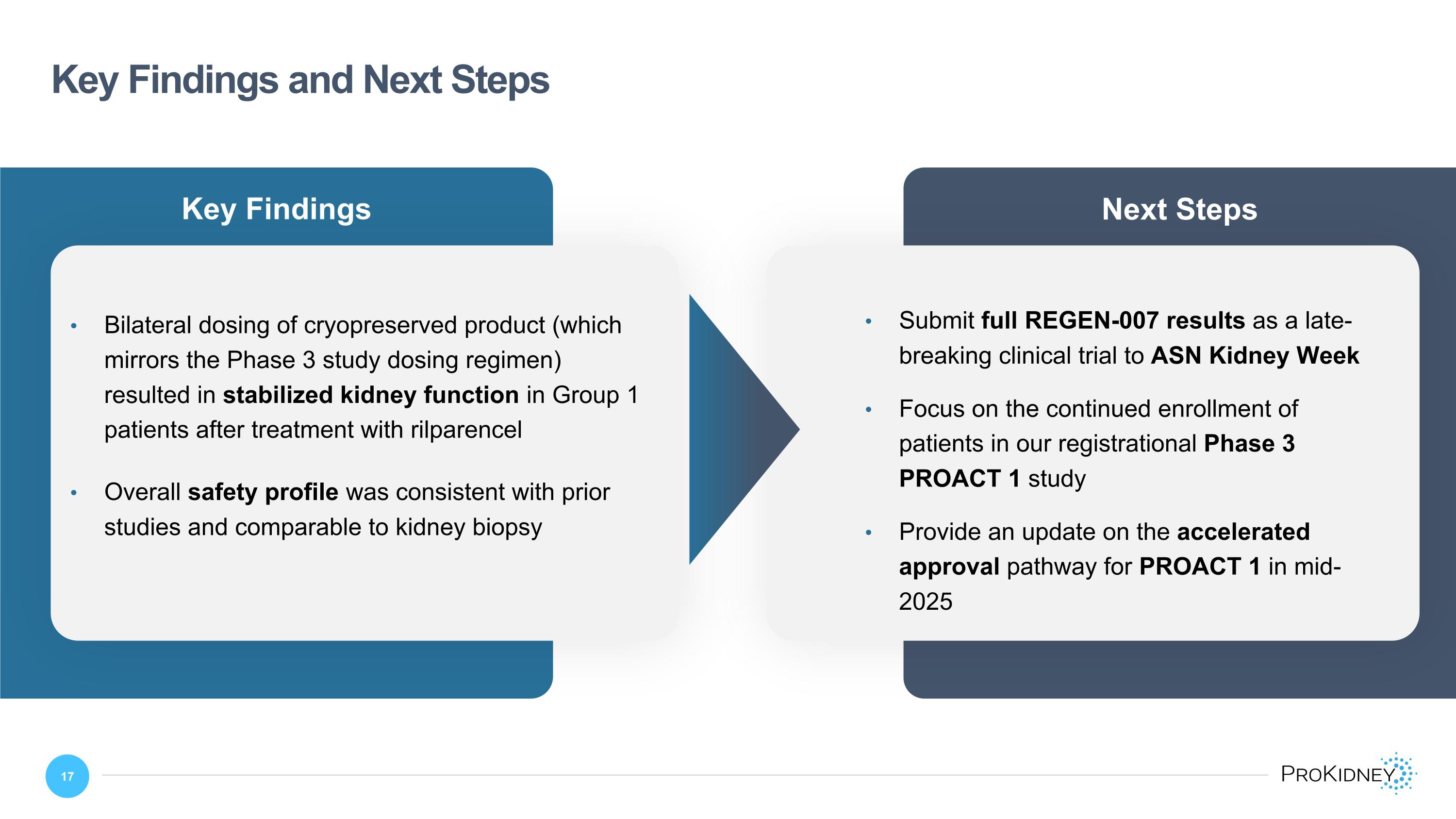
Key Findings Key Findings and Next Steps Bilateral dosing of cryopreserved product (which mirrors the Phase 3 study dosing regimen) resulted in stabilized kidney function in Group 1 patients after treatment with rilparencel Overall safety profile was consistent with prior studies and comparable to kidney biopsy Next Steps Submit full REGEN-007 results as a late-breaking clinical trial to ASN Kidney Week Focus on the continued enrollment of patients in our registrational Phase 3 PROACT 1 study Provide an update on the accelerated approval pathway for PROACT 1 in mid-2025
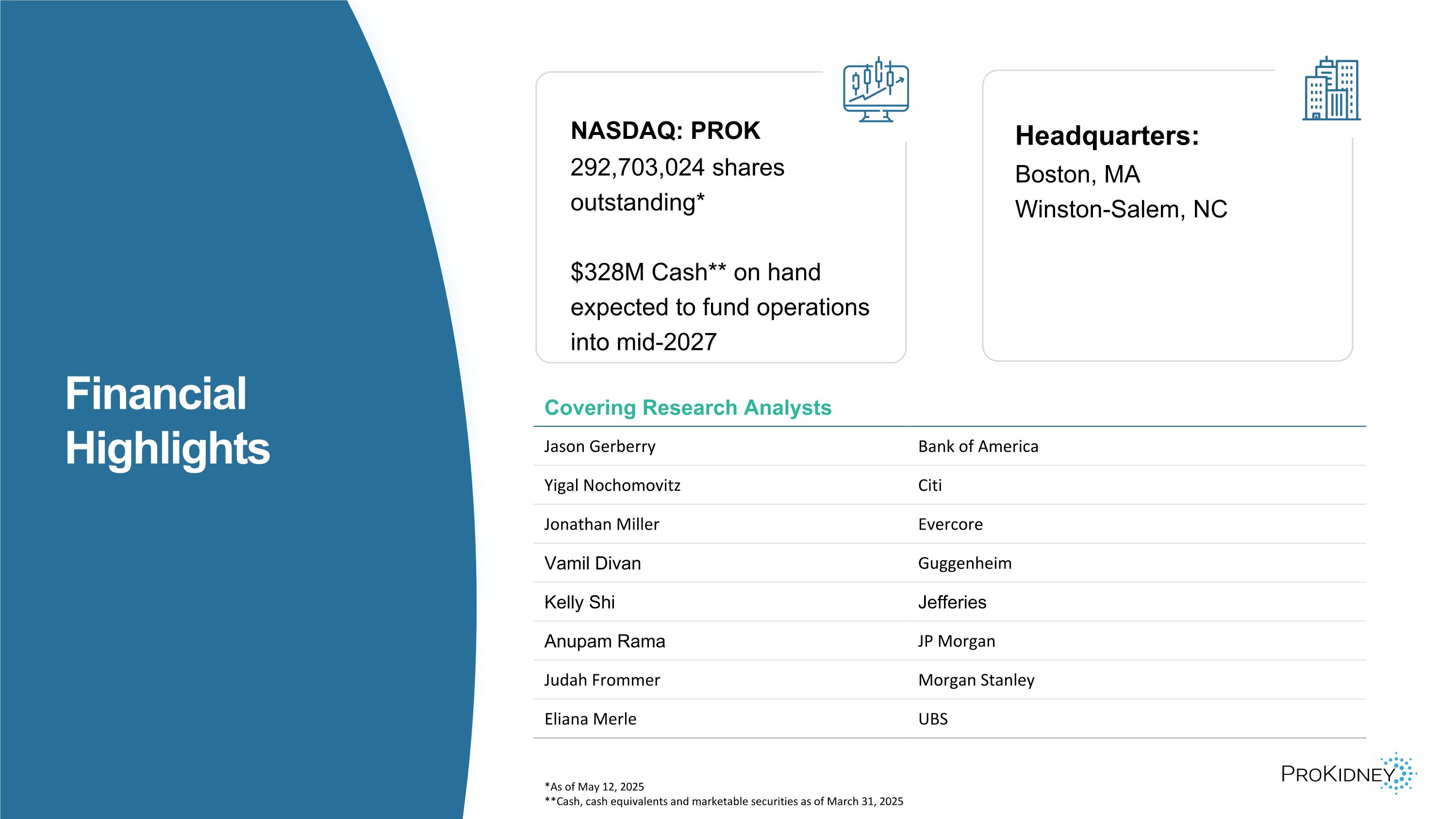
*As of May 12, 2025 **Cash, cash equivalents and marketable securities as of March 31, 2025 Covering Research Analysts Jason Gerberry Bank of America Yigal Nochomovitz Citi Jonathan Miller Evercore Vamil Divan Guggenheim Kelly Shi Jefferies Anupam Rama JP Morgan Judah Frommer Morgan Stanley Eliana Merle UBS NASDAQ: PROK 292,703,024 shares outstanding* $328M Cash** on hand expected to fund operations into mid-2027 Headquarters: Boston, MA Winston-Salem, NC Financial Highlights