

Corporate Presentation July 2025 NYSE AMERICAN: CATX

This presentation contains forward-looking statements within the meaning of the United States Private Securities Litigation Reform Act of 1995. Statements in this presentation that are not statements of historical fact are forward-looking statements. Words such as “may,” “will,” “should,” “expect,” “plan,” “anticipate,” “could,” “intend,” “target,” “project,” “estimate,” “believe,” “predict,” “potential” or “continue” or the negative of these terms or other similar expressions are intended to identify forward-looking statements, though not all forward-looking statements contain these identifying words. Forward-looking statements in this presentation include statements concerning, among other things, the Company's clinical development plans and the expected timing thereof; the expected timing for availability and release of data; the Company’s timing and expectations regarding regulatory communications, submissions and approvals; expectations regarding the potential market opportunities for the Company’s product candidates; the Company’s expected cash runway; the potential functionality, capabilities and benefits of the Company’s product candidates and the potential application of these product candidates for other disease indications; the potential size of the commercial market for the Company’s product candidates; the Company’s expectations, beliefs, intentions, and strategies regarding the future; the Company’s intentions to improve important aspects of care in cancer treatment; and other statements that are not historical fact. The Company may not actually achieve the plans, intentions or expectations disclosed in the forward-looking statements and you should not place undue reliance on the forward-looking statements. These forward-looking statements involve risks and uncertainties that could cause the Company’s actual results to differ materially from the results described in or implied by the forward-looking statements, including, without limitation, the potential that regulatory authorities may not grant or may delay approval for the Company’s product candidates; uncertainties and delays relating to the design, enrollment, completion and results of clinical trials; unanticipated costs and expenses; early clinical trials may not be indicative of the results in later clinical trials; clinical trial results may not support regulatory approval or further development in a specified indication or at all; actions or advice of regulatory authorities may affect the design, initiation, timing, continuation and/or progress of clinical trials or result in the need for additional clinical trials; the Company may not be able to maintain regulatory approval for the Company’s product candidates; delays, interruptions or failures in the manufacture and supply of the Company’s product candidates; the size and growth potential of the markets for the Company’s product candidates, and the Company’s ability to service those markets; the Company’s cash and cash equivalents may not be sufficient to support its operating plan for as long as anticipated; the Company’s expectations, projections and estimates regarding expenses, future revenue, capital requirements and the availability of and the need for additional financing; the Company’s ability to obtain additional funding to support its clinical development programs; the availability or potential availability of alternative products or treatments for conditions targeted by the Company that could affect the availability or commercial potential of its product candidates; the ability of the Company to manage growth; whether the Company can maintain its key employees; the ability of the Company to build out its manufacturing facilities and satisfy manufacturing-related regulatory requirements; whether there is sufficient training and use of the Company’s products and product candidates; the market acceptance and recognition of the Company’s products and product candidates; the Company’s ability to maintain and enforce its intellectual property rights; whether the Company can maintain its therapeutic isotope supply agreement with the DOE; the Company’s ability to maintain and increase its supply, manufacturing and distribution capabilities; whether the Company will continue to comply with the procedures and regulatory requirements mandated by the FDA for additional trials, Phase 1 and 2 approvals, and Fast Track approvals; and any changes in applicable laws and regulations. Certain factors that may cause the Company’s actual results to differ materially from those expressed or implied in the forward-looking statements in this presentation are described under the heading “Risk Factors” in the Company’s most recent Annual Report on Form 10-K filed with the Securities and Exchange Commission (the “SEC”), in the Company’s other filings with the SEC, and in the Company’s future reports to be filed with the SEC and available at www.sec.gov. Forward-looking statements contained in this presentation are made as of this date. Unless required to do so by law, the Company undertakes no obligation to publicly update or revise any forward-looking statements, whether as a result of new information, future events or otherwise. Legal Disclaimers
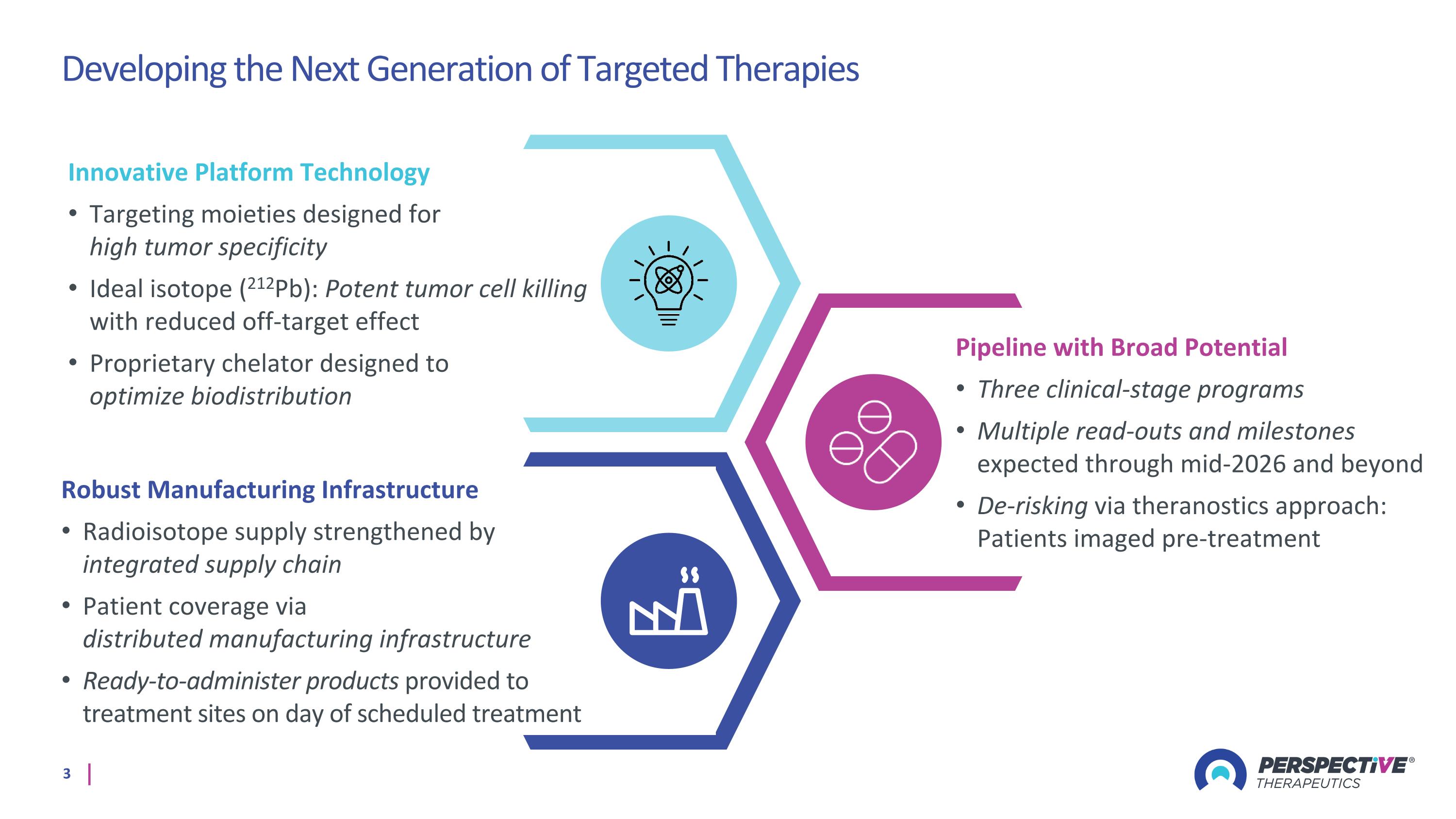
Developing the Next Generation of Targeted Therapies Robust Manufacturing Infrastructure Radioisotope supply strengthened by integrated supply chain Patient coverage via distributed manufacturing infrastructure Ready-to-administer products provided to treatment sites on day of scheduled treatment Innovative Platform Technology Targeting moieties designed for high tumor specificity Ideal isotope (212Pb): Potent tumor cell killing with reduced off-target effect Proprietary chelator designed to optimize biodistribution Pipeline with Broad Potential Three clinical-stage programs Multiple read-outs and milestones expected through mid-2026 and beyond De-risking via theranostics approach: Patients imaged pre-treatment
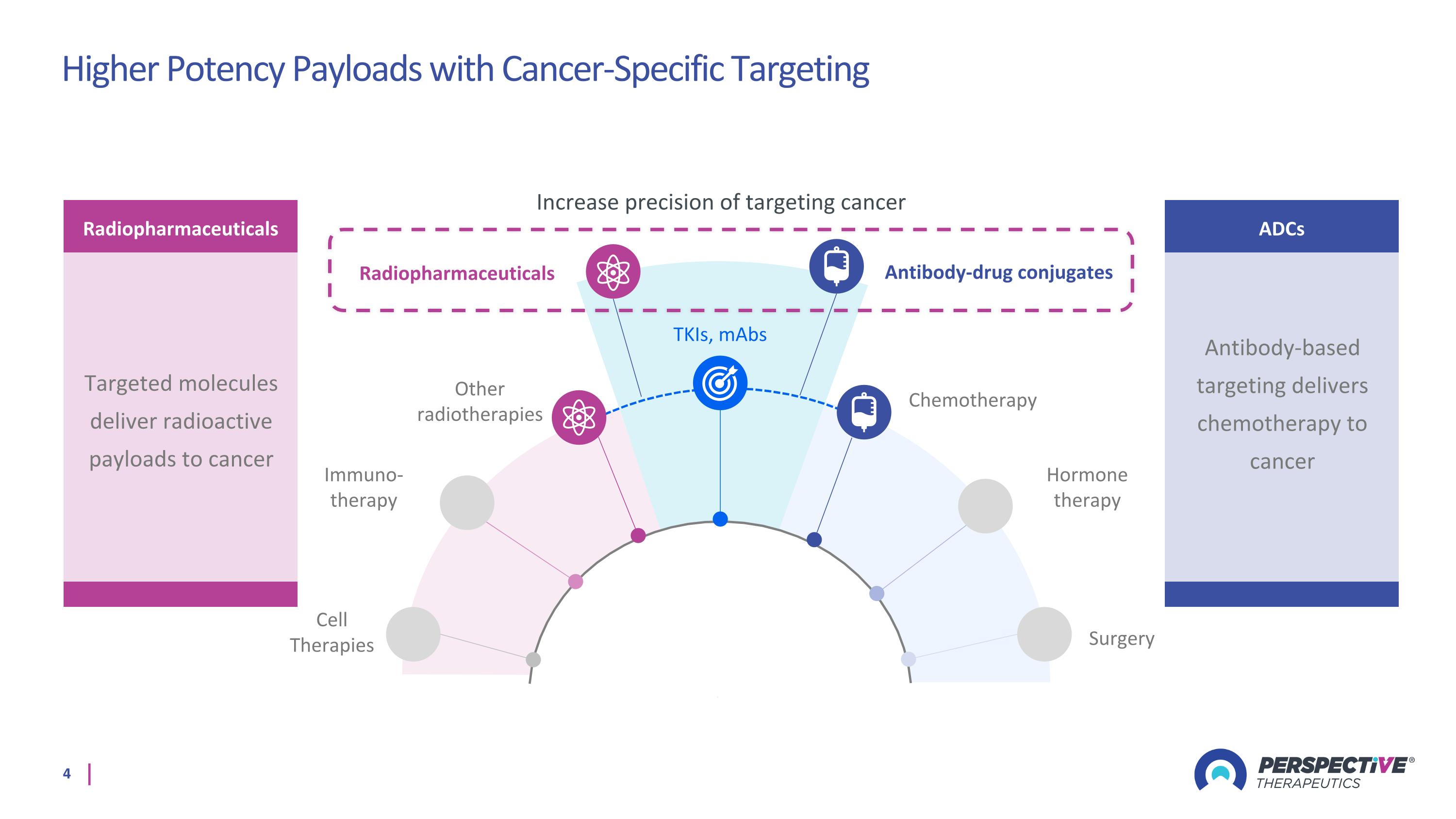
TKIs, mAbs Immuno-therapy Hormone therapy Other radiotherapies Chemotherapy Surgery Cell Therapies Antibody-drug conjugates Radiopharmaceuticals Increase precision of targeting cancer Radiopharmaceuticals Targeted molecules deliver radioactive payloads to cancer ADCs Antibody-based targeting delivers chemotherapy to cancer Higher Potency Payloads with Cancer-Specific Targeting
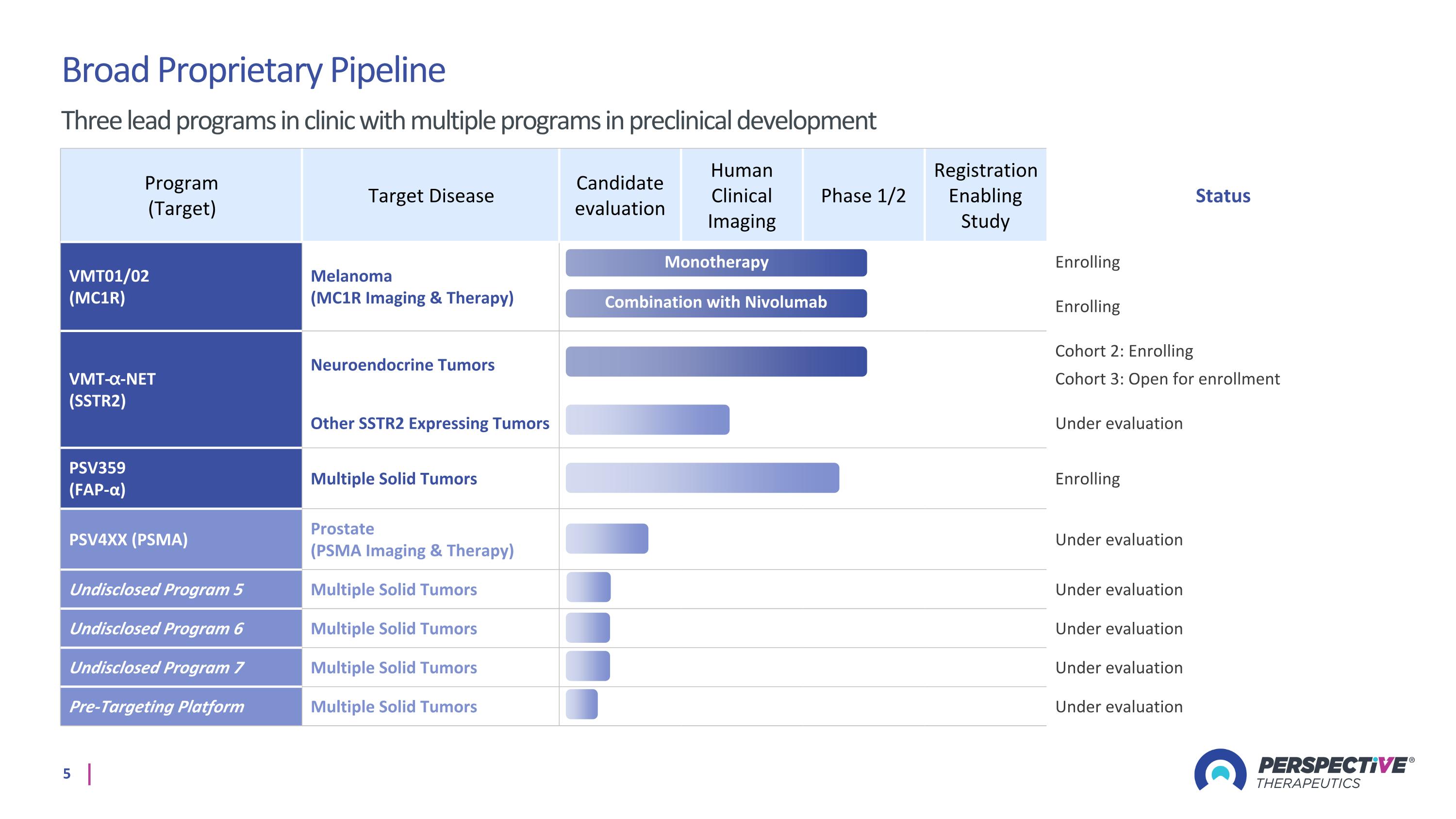
Program (Target) Target Disease Candidate evaluation Human Clinical Imaging Phase 1/2 Registration Enabling Study Status VMT01/02 (MC1R) Melanoma (MC1R Imaging & Therapy) Enrolling Enrolling VMT-⍺-NET (SSTR2) Neuroendocrine Tumors Cohort 2: Enrolling Cohort 3: Open for enrollment Other SSTR2 Expressing Tumors Under evaluation PSV359 (FAP-α) Multiple Solid Tumors Enrolling PSV4XX (PSMA) Prostate (PSMA Imaging & Therapy) Under evaluation Undisclosed Program 5 Multiple Solid Tumors Under evaluation Undisclosed Program 6 Multiple Solid Tumors Under evaluation Undisclosed Program 7 Multiple Solid Tumors Under evaluation Pre-Targeting Platform Multiple Solid Tumors Under evaluation Monotherapy Broad Proprietary Pipeline Three lead programs in clinic with multiple programs in preclinical development Combination with Nivolumab
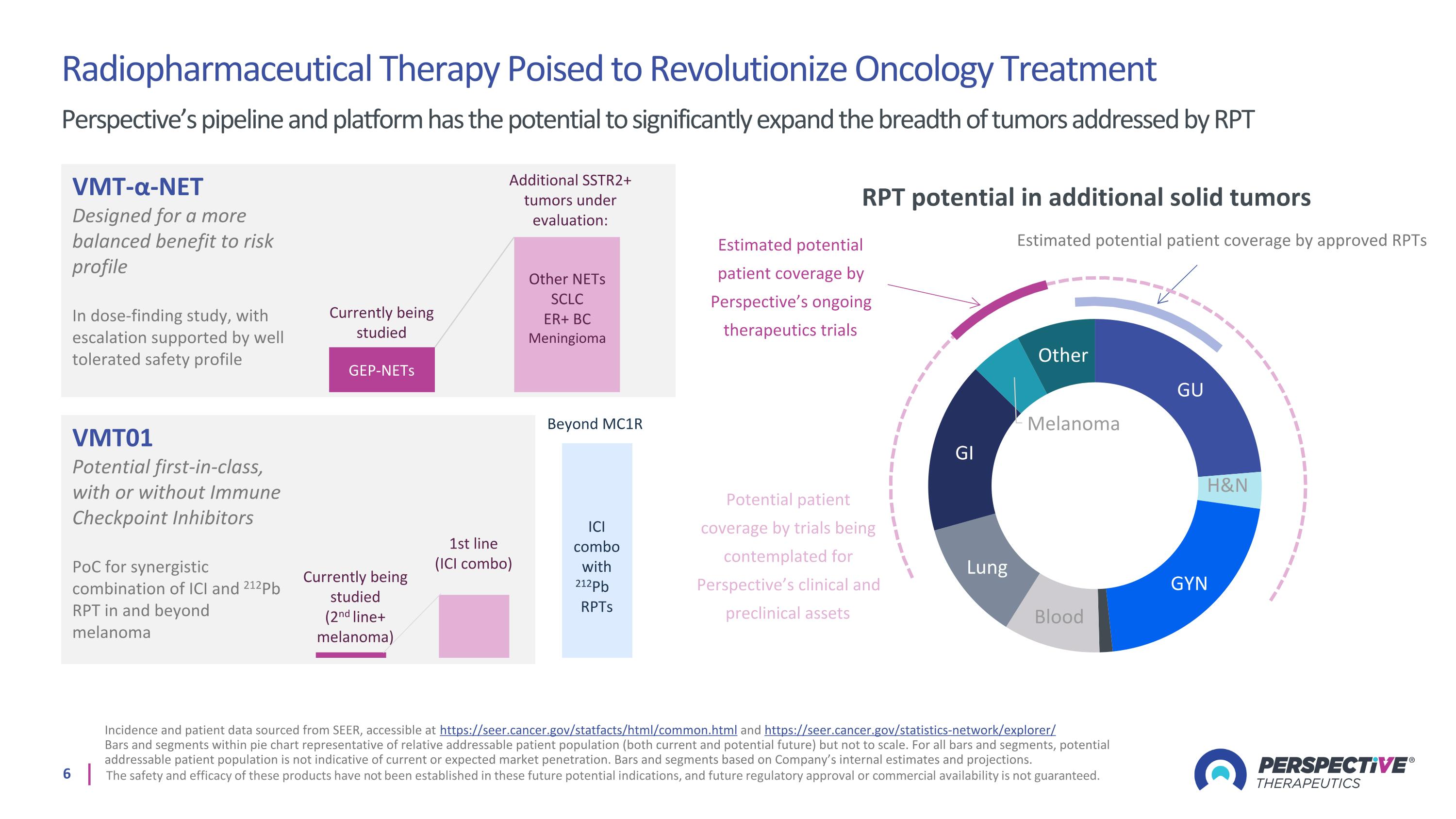
Radiopharmaceutical Therapy Poised to Revolutionize Oncology Treatment Perspective’s pipeline and platform has the potential to significantly expand the breadth of tumors addressed by RPT Incidence and patient data sourced from SEER, accessible at https://seer.cancer.gov/statfacts/html/common.html and https://seer.cancer.gov/statistics-network/explorer/ Bars and segments within pie chart representative of relative addressable patient population (both current and potential future) but not to scale. For all bars and segments, potential addressable patient population is not indicative of current or expected market penetration. Bars and segments based on Company’s internal estimates and projections. The safety and efficacy of these products have not been established in these future potential indications, and future regulatory approval or commercial availability is not guaranteed. RPT potential in additional solid tumors Estimated potential patient coverage by approved RPTs Estimated potential patient coverage by Perspective’s ongoing therapeutics trials Potential patient coverage by trials being contemplated for Perspective’s clinical and preclinical assets VMT-α-NET Designed for a more balanced benefit to risk profile In dose-finding study, with escalation supported by well tolerated safety profile VMT01 Potential first-in-class, with or without Immune Checkpoint Inhibitors PoC for synergistic combination of ICI and 212Pb RPT in and beyond melanoma Other NETs SCLC ER+ BC Meningioma GEP-NETs Additional SSTR2+ tumors under evaluation: Currently being studied 1st line (ICI combo) Currently being studied (2nd line+ melanoma) Beyond MC1R ICI combo with 212Pb RPTs
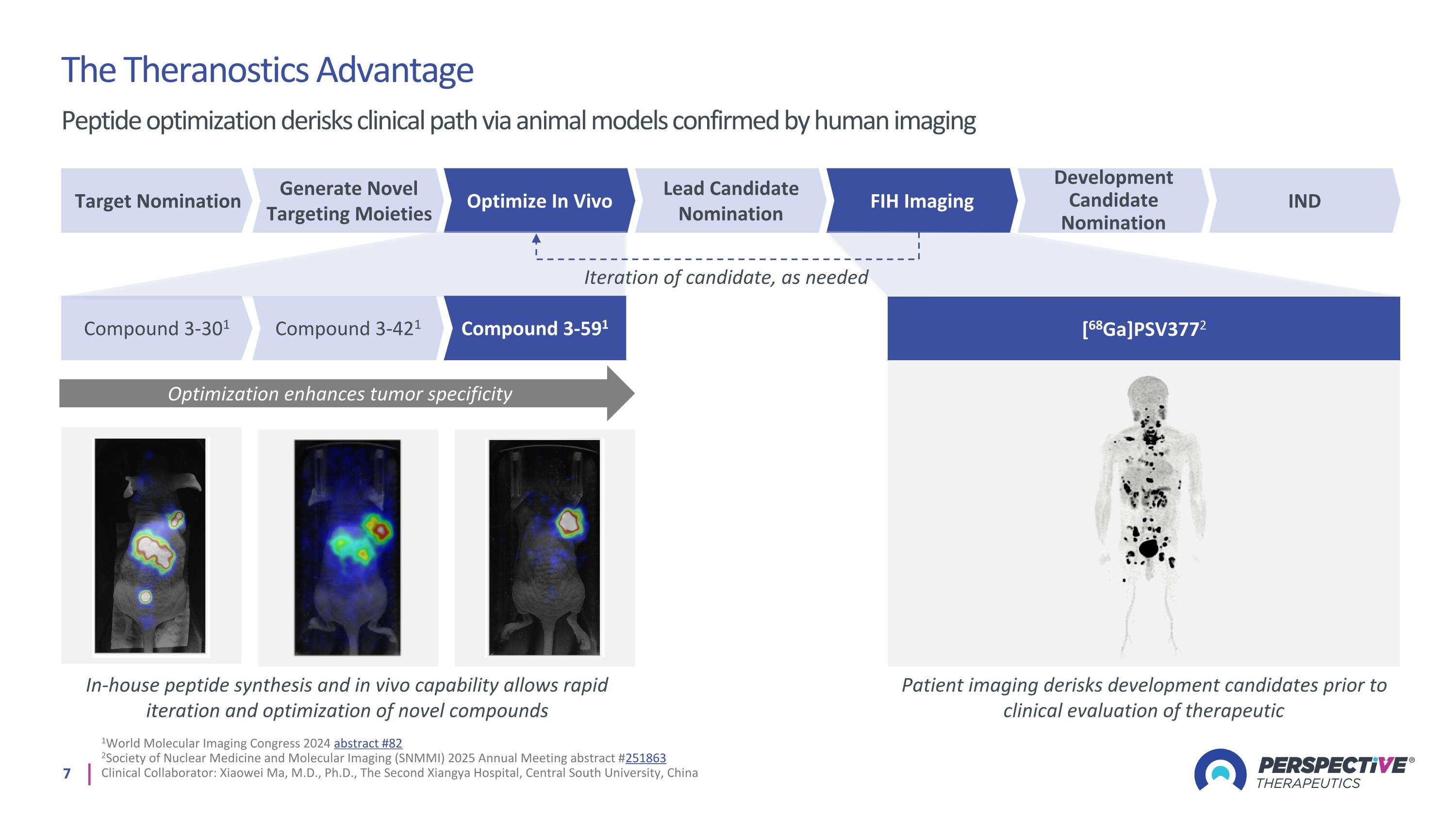
1World Molecular Imaging Congress 2024 abstract #82 2Society of Nuclear Medicine and Molecular Imaging (SNMMI) 2025 Annual Meeting abstract #251863 Clinical Collaborator: Xiaowei Ma, M.D., Ph.D., The Second Xiangya Hospital, Central South University, China The Theranostics Advantage Peptide optimization derisks clinical path via animal models confirmed by human imaging Optimization enhances tumor specificity In-house peptide synthesis and in vivo capability allows rapid iteration and optimization of novel compounds Patient imaging derisks development candidates prior to clinical evaluation of therapeutic Target Nomination Generate Novel Targeting Moieties Optimize In Vivo Lead Candidate Nomination FIH Imaging Development Candidate Nomination IND [68Ga]PSV3772 Compound 3-301 Compound 3-421 Compound 3-591 Iteration of candidate, as needed

Perspective’s Innovative Platform Enabling broad applications in the clinic
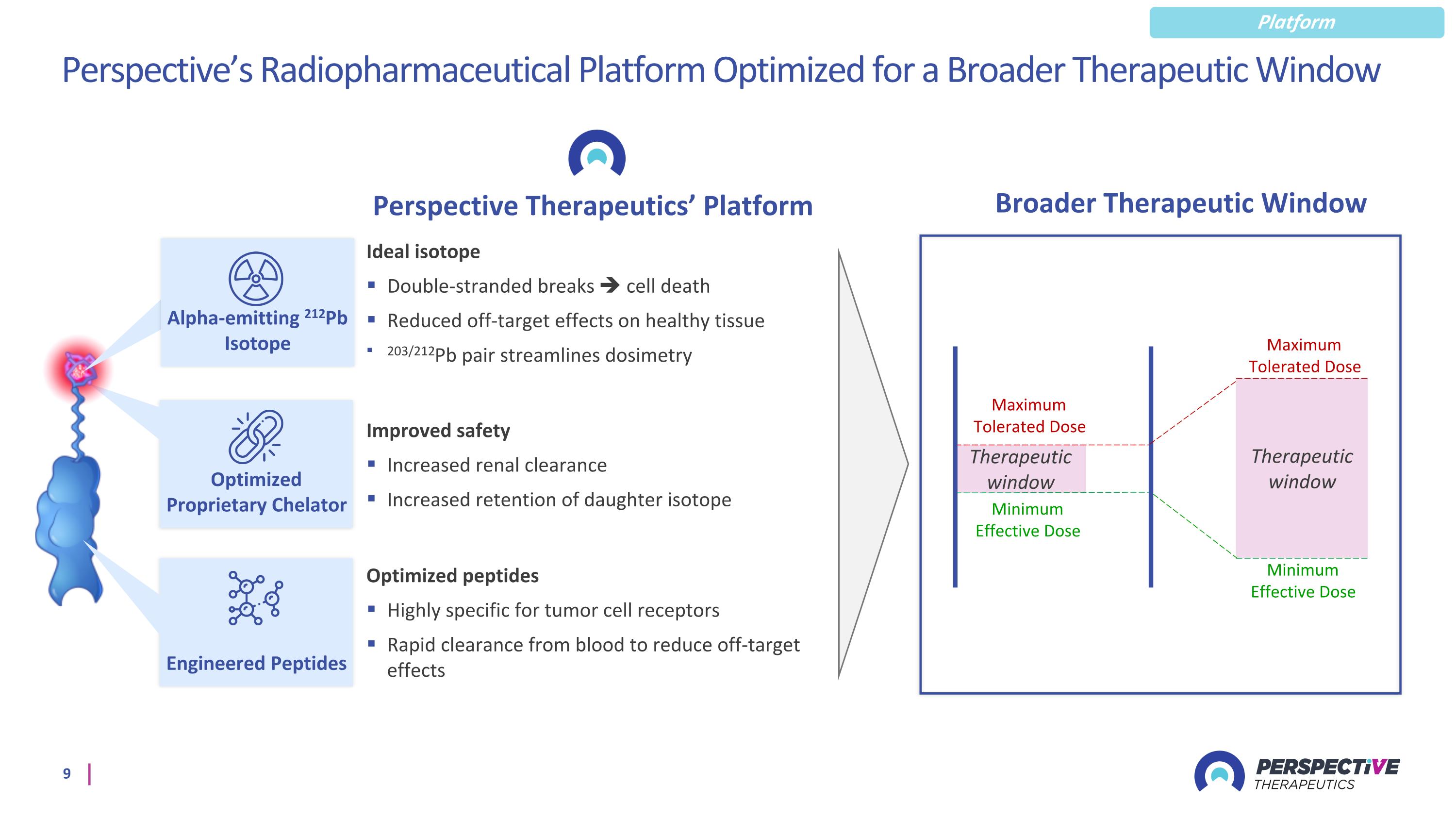
Perspective’s Radiopharmaceutical Platform Optimized for a Broader Therapeutic Window Perspective Therapeutics’ Platform Optimized peptides Highly specific for tumor cell receptors Rapid clearance from blood to reduce off-target effects Ideal isotope Double-stranded breaks cell death Reduced off-target effects on healthy tissue 203/212Pb pair streamlines dosimetry Improved safety Increased renal clearance Increased retention of daughter isotope Minimum Effective Dose Maximum Tolerated Dose Therapeutic window Minimum Effective Dose Maximum Tolerated Dose Therapeutic window Optimized Proprietary Chelator Engineered Peptides Broader Therapeutic Window Platform Alpha-emitting 212Pb Isotope
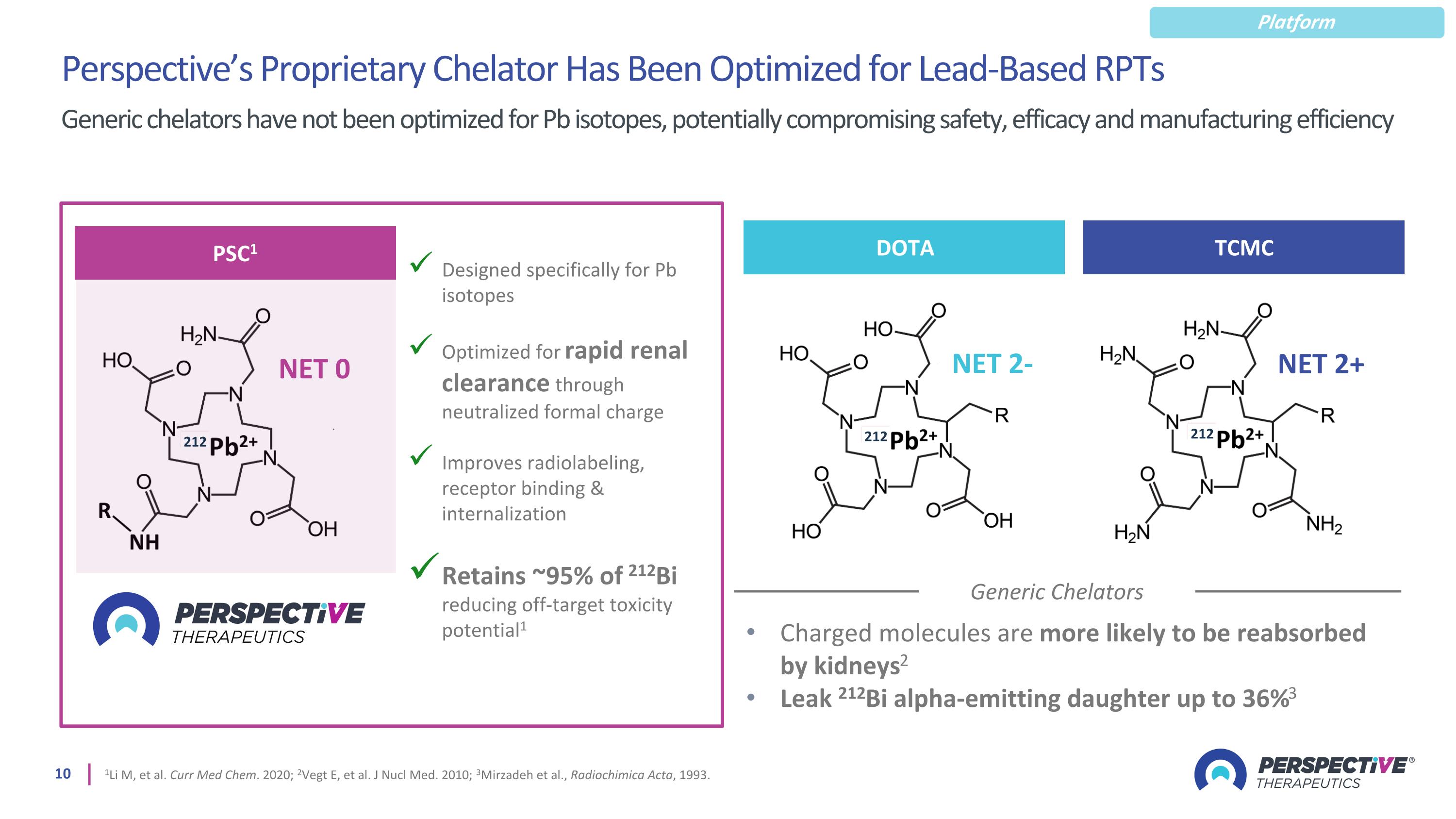
Perspective’s Proprietary Chelator Has Been Optimized for Lead-Based RPTs 1Li M, et al. Curr Med Chem. 2020; 2Vegt E, et al. J Nucl Med. 2010; 3Mirzadeh et al., Radiochimica Acta, 1993. Generic chelators have not been optimized for Pb isotopes, potentially compromising safety, efficacy and manufacturing efficiency Generic Chelators Charged molecules are more likely to be reabsorbed by kidneys2 Leak 212Bi alpha-emitting daughter up to 36%3 Designed specifically for Pb isotopes Optimized for rapid renal clearance through neutralized formal charge Improves radiolabeling, receptor binding & internalization Retains ~95% of 212Bi reducing off-target toxicity potential1 PSC1 NET 0 212 DOTA NET 2- 212 TCMC NET 2+ 212 Platform
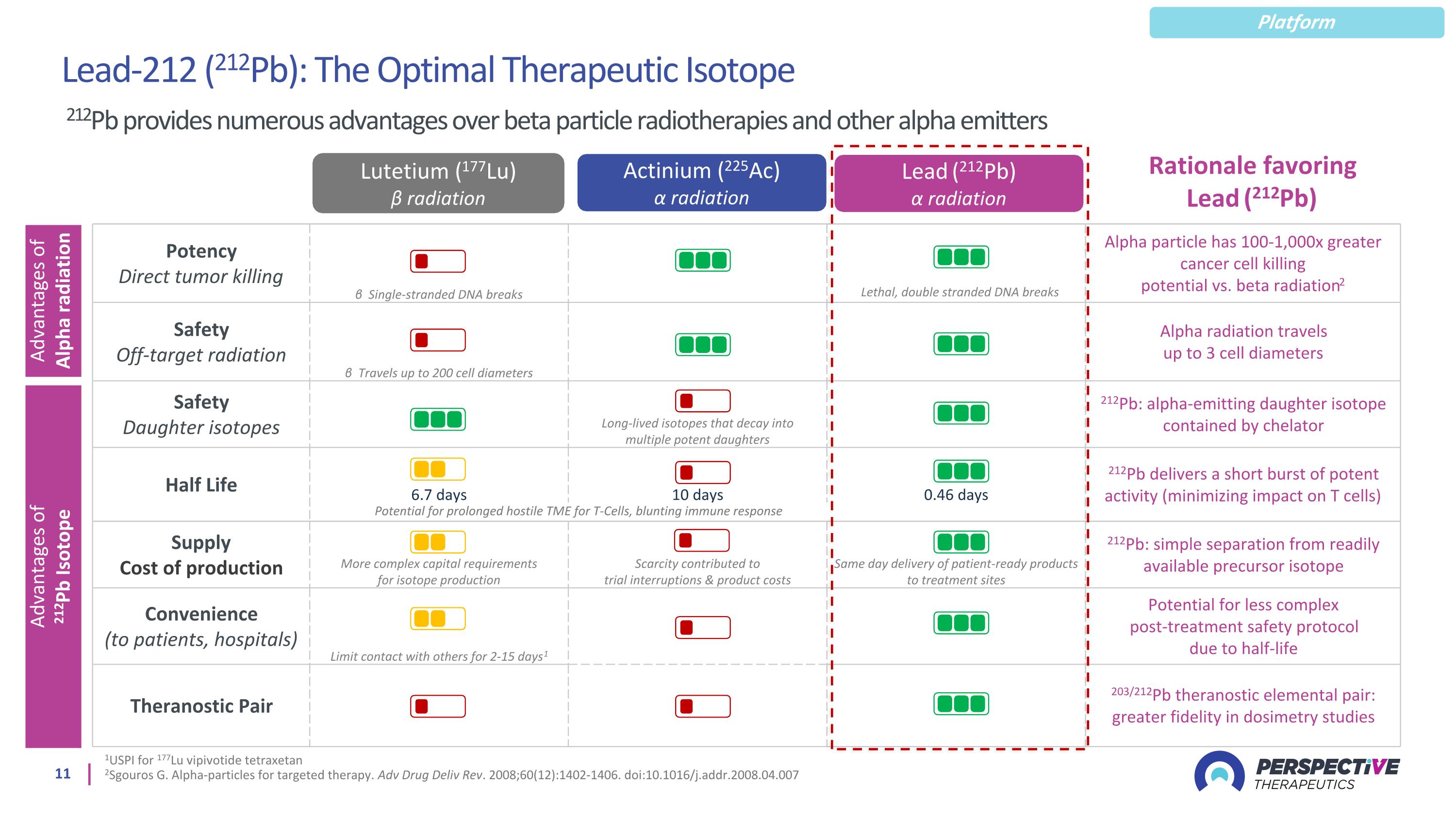
1USPI for 177Lu vipivotide tetraxetan 2Sgouros G. Alpha-particles for targeted therapy. Adv Drug Deliv Rev. 2008;60(12):1402-1406. doi:10.1016/j.addr.2008.04.007 Lead (212Pb) α radiation Actinium (225Ac) α radiation Lutetium (177Lu) ϐ radiation Potency Direct tumor killing β Single-stranded DNA breaks Alpha particle has 100-1,000x greater cancer cell killing potential vs. beta radiation2 Safety Off-target radiation β Travels up to 200 cell diameters Alpha radiation travels up to 3 cell diameters Safety Daughter isotopes Long-lived isotopes that decay into multiple potent daughters 212Pb: alpha-emitting daughter isotope contained by chelator Half Life 6.7 days 10 days 0.46 days 212Pb delivers a short burst of potent activity (minimizing impact on T cells) Supply Cost of production More complex capital requirements for isotope production Scarcity contributed to trial interruptions & product costs Same day delivery of patient-ready products to treatment sites 212Pb: simple separation from readily available precursor isotope Convenience (to patients, hospitals) Limit contact with others for 2-15 days1 Potential for less complex post-treatment safety protocol due to half-life Theranostic Pair 203/212Pb theranostic elemental pair: greater fidelity in dosimetry studies Advantages of Alpha radiation Advantages of 212Pb Isotope Potential for prolonged hostile TME for T-Cells, blunting immune response Rationale favoring Lead (212Pb) Lead-212 (212Pb): The Optimal Therapeutic Isotope 212Pb provides numerous advantages over beta particle radiotherapies and other alpha emitters Lethal, double stranded DNA breaks Platform
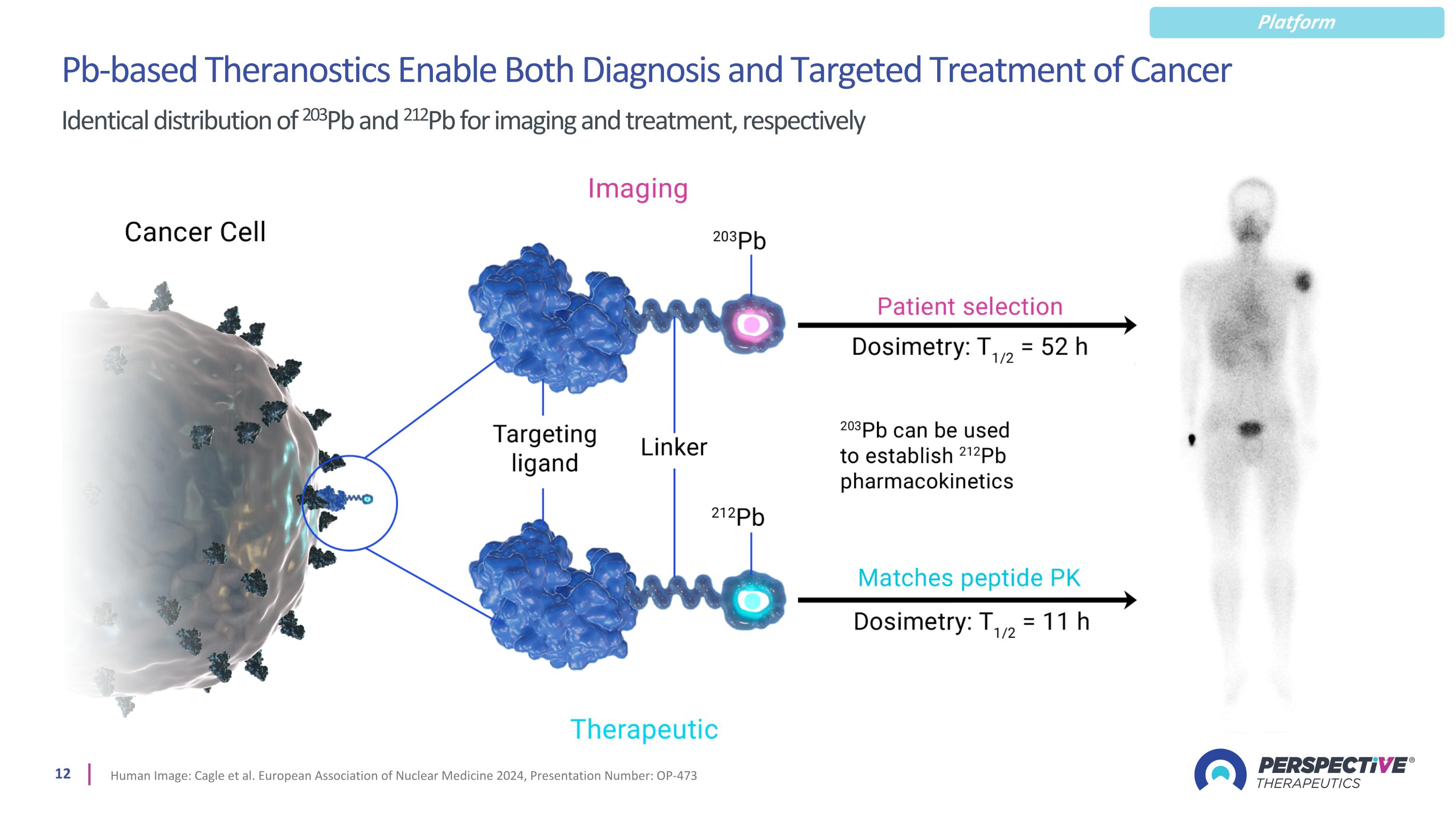
Pb-based Theranostics Enable Both Diagnosis and Targeted Treatment of Cancer Identical distribution of 203Pb and 212Pb for imaging and treatment, respectively Human Image: Cagle et al. European Association of Nuclear Medicine 2024, Presentation Number: OP-473 Platform
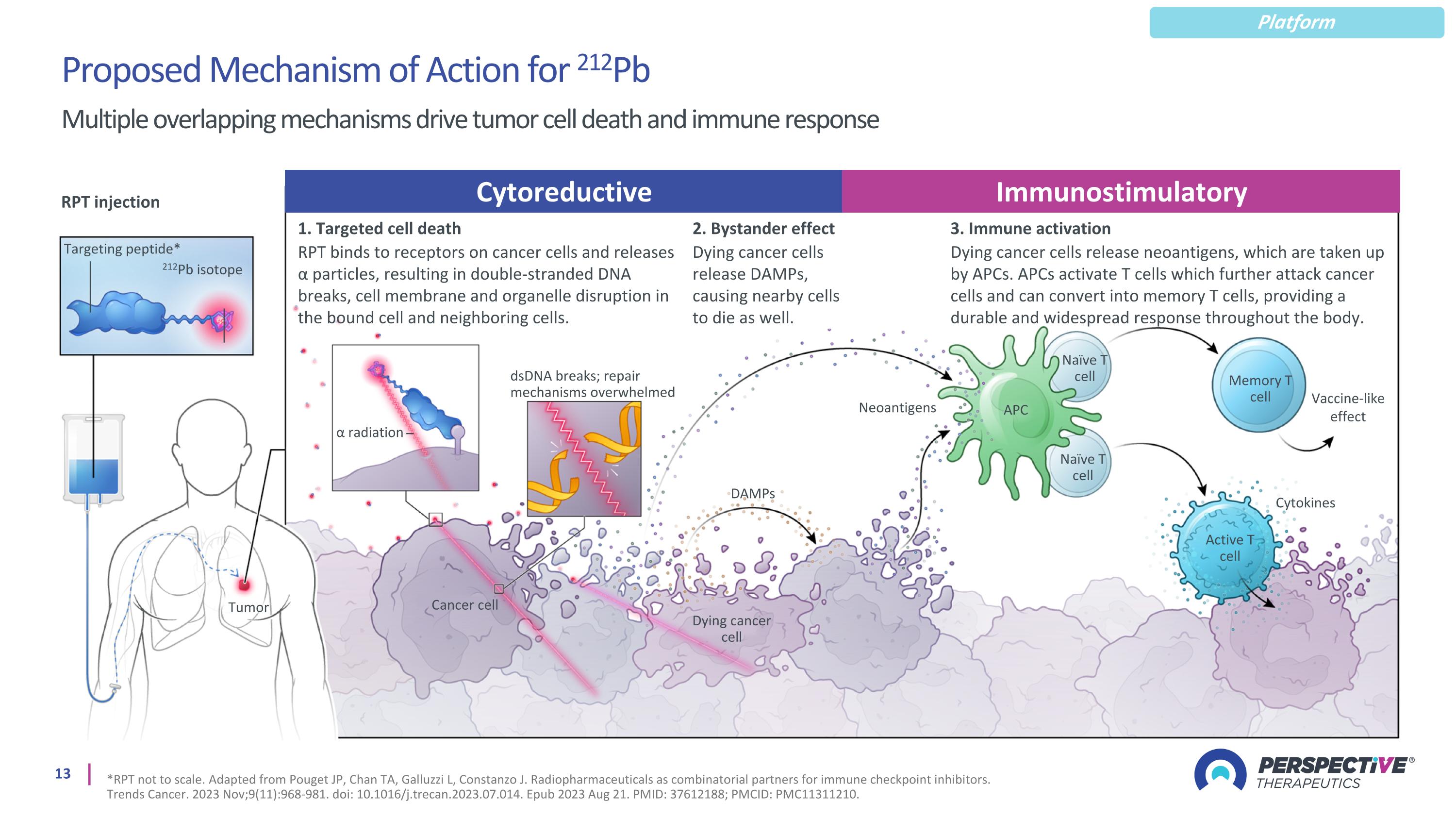
Multiple overlapping mechanisms drive tumor cell death and immune response Proposed Mechanism of Action for 212Pb Platform *RPT not to scale. Adapted from Pouget JP, Chan TA, Galluzzi L, Constanzo J. Radiopharmaceuticals as combinatorial partners for immune checkpoint inhibitors. Trends Cancer. 2023 Nov;9(11):968-981. doi: 10.1016/j.trecan.2023.07.014. Epub 2023 Aug 21. PMID: 37612188; PMCID: PMC11311210. RPT injection Targeting peptide* 212Pb isotope 1. Targeted cell death RPT binds to receptors on cancer cells and releases α particles, resulting in double-stranded DNA breaks, cell membrane and organelle disruption in the bound cell and neighboring cells. 3. Immune activation Dying cancer cells release neoantigens, which are taken up by APCs. APCs activate T cells which further attack cancer cells and can convert into memory T cells, providing a durable and widespread response throughout the body. α radiation dsDNA breaks; repair mechanisms overwhelmed Neoantigens Tumor Dying cancer cell DAMPs Cancer cell APC Naïve T cell Naïve T cell Memory T cell Active T cell Cytokines Vaccine-like effect Cytoreductive Immunostimulatory 2. Bystander effect Dying cancer cells release DAMPs, causing nearby cells to die as well.
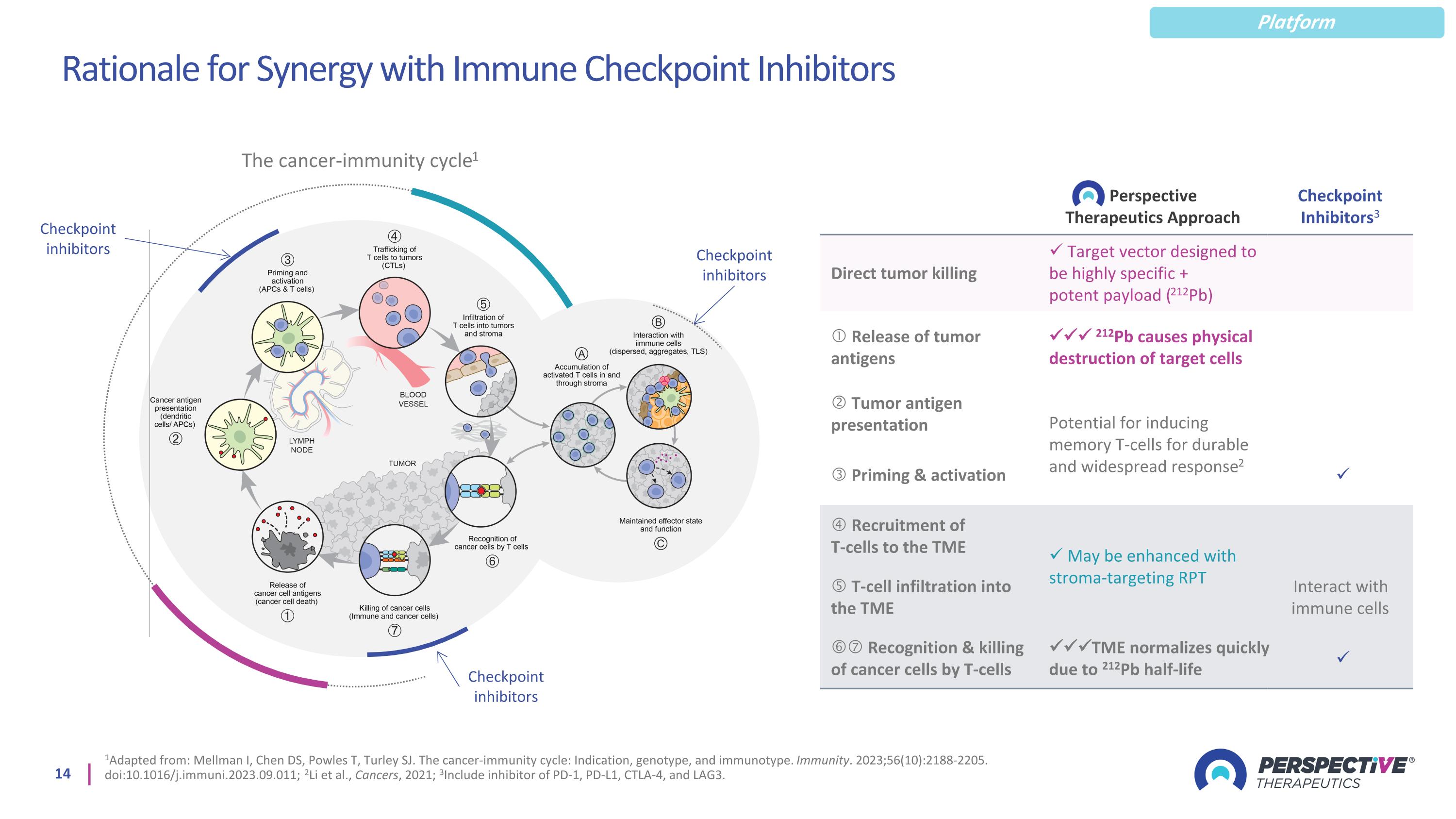
Rationale for Synergy with Immune Checkpoint Inhibitors 1Adapted from: Mellman I, Chen DS, Powles T, Turley SJ. The cancer-immunity cycle: Indication, genotype, and immunotype. Immunity. 2023;56(10):2188-2205. doi:10.1016/j.immuni.2023.09.011; 2Li et al., Cancers, 2021; 3Include inhibitor of PD-1, PD-L1, CTLA-4, and LAG3. Checkpoint inhibitors Perspective Therapeutics Approach Checkpoint Inhibitors3 Direct tumor killing Target vector designed to be highly specific + potent payload (212Pb) Release of tumor antigens 212Pb causes physical destruction of target cells Tumor antigen presentation Potential for inducing memory T-cells for durable and widespread response2 Priming & activation Recruitment of T-cells to the TME May be enhanced with stroma-targeting RPT T-cell infiltration into the TME Interact with immune cells Recognition & killing of cancer cells by T-cells TME normalizes quickly due to 212Pb half-life The cancer-immunity cycle1 Checkpoint inhibitors Checkpoint inhibitors Platform
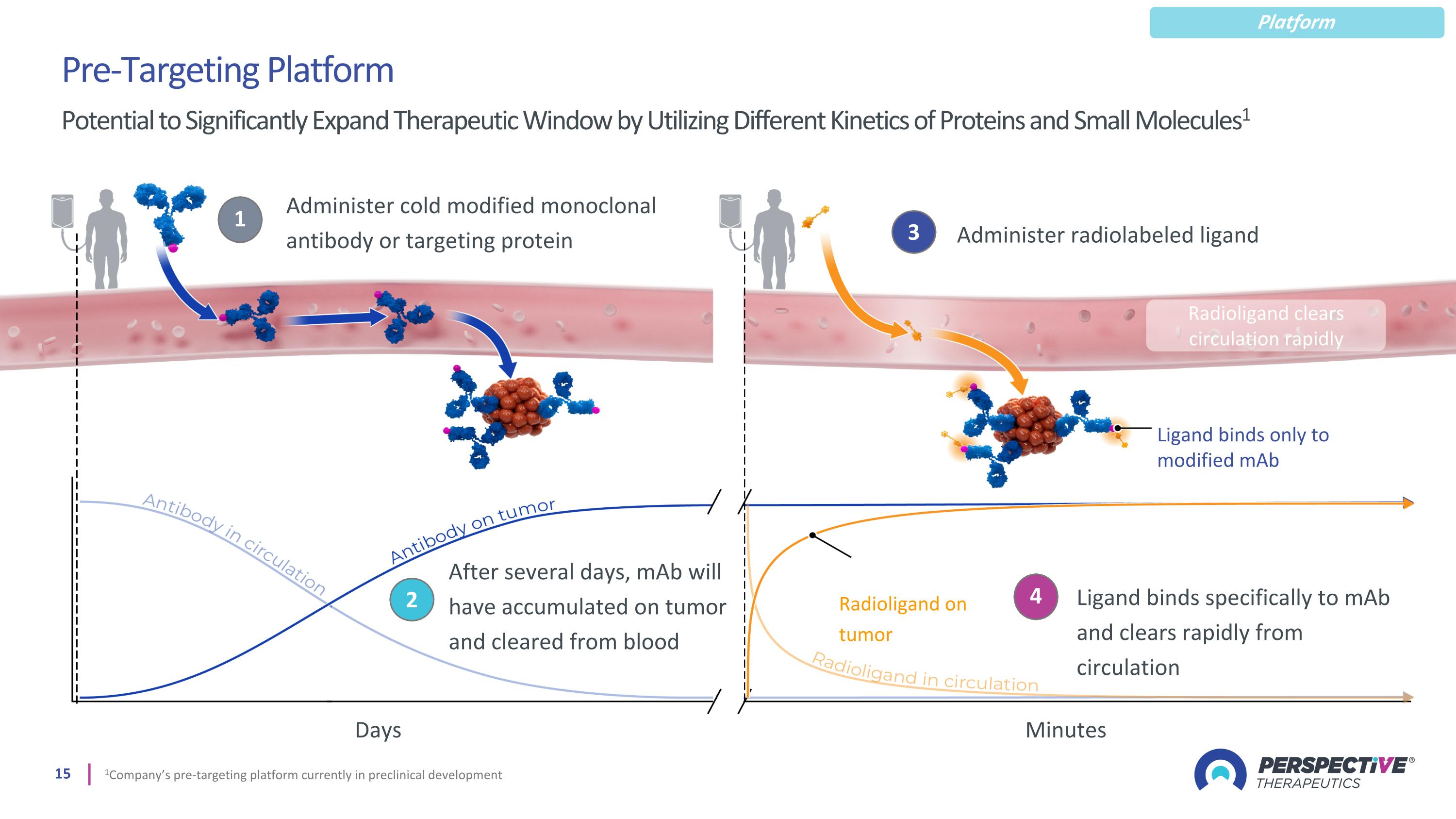
1 Administer cold modified monoclonal antibody or targeting protein 2 After several days, mAb will have accumulated on tumor and cleared from blood 3 Administer radiolabeled ligand 4 Ligand binds specifically to mAb and clears rapidly from circulation Radioligand on tumor Radioligand clears circulation rapidly Ligand binds only to modified mAb Pre-Targeting Platform Potential to Significantly Expand Therapeutic Window by Utilizing Different Kinetics of Proteins and Small Molecules1 Minutes Days 1Company’s pre-targeting platform currently in preclinical development Platform

Supply Chain and Manufacturing Infrastructure Ensuring radioisotope supply and ability to deliver ready-to-administer products to hospitals
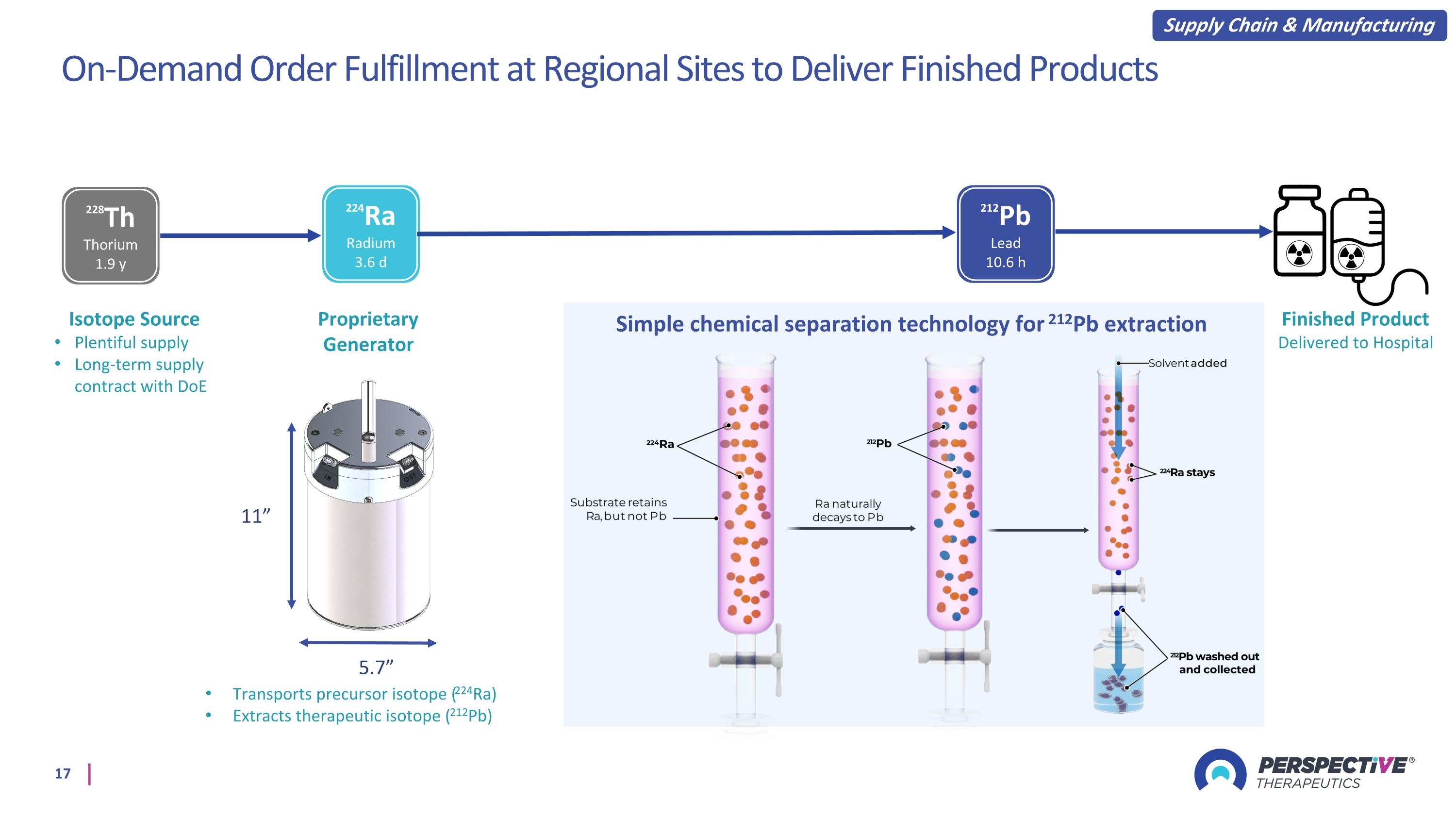
On-Demand Order Fulfillment at Regional Sites to Deliver Finished Products 5.7” 11” Proprietary Generator Simple chemical separation technology for 212Pb extraction 228Th Thorium 1.9 y 224Ra Radium 3.6 d 212Pb Lead 10.6 h Transports precursor isotope (224Ra) Extracts therapeutic isotope (212Pb) Isotope Source Plentiful supply Long-term supply contract with DoE Supply Chain & Manufacturing Finished Product Delivered to Hospital
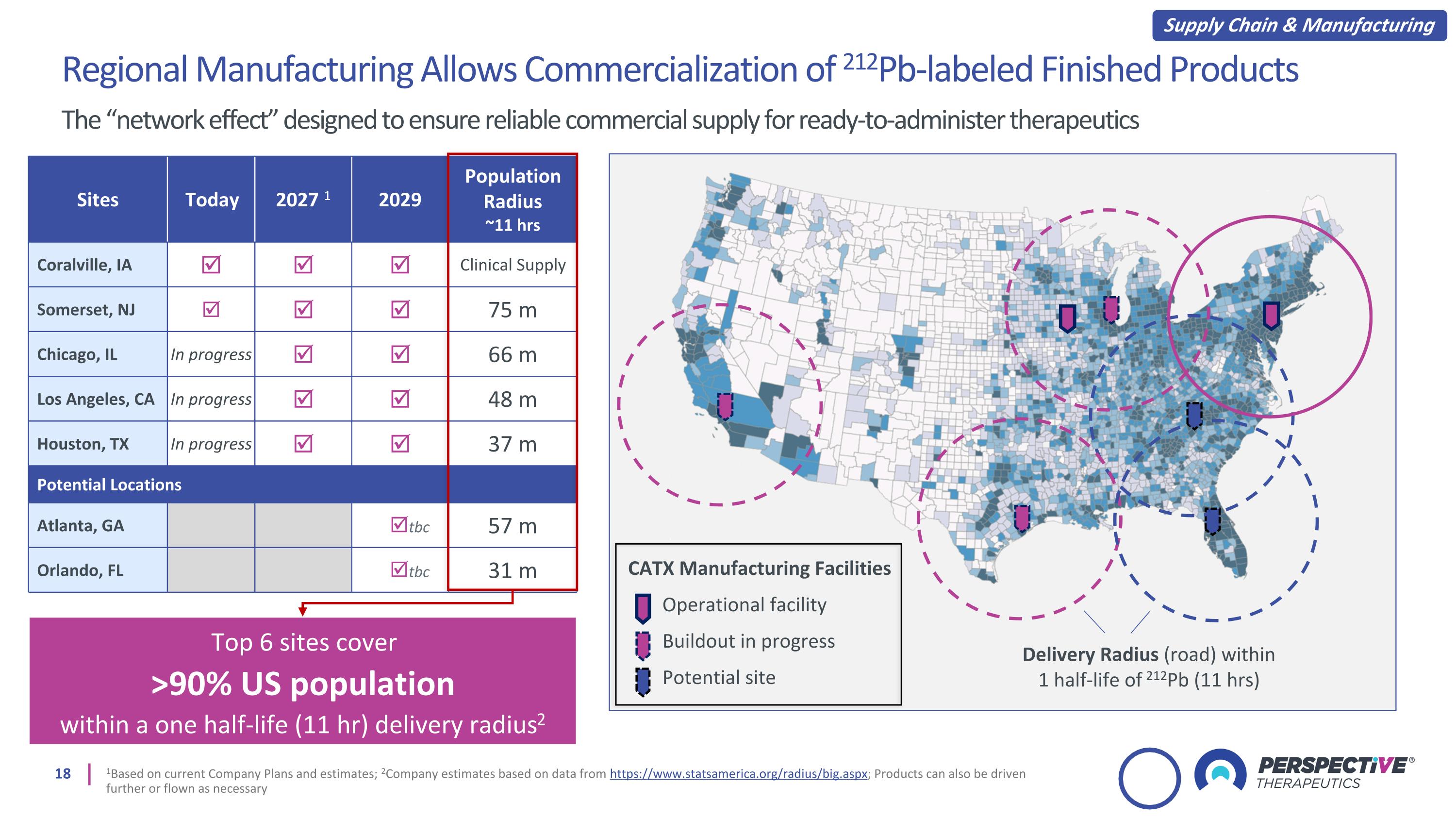
Sites Today 2027 1 2029 Population Radius ~11 hrs Coralville, IA Clinical Supply Somerset, NJ 75 m Chicago, IL In progress 66 m Los Angeles, CA In progress 48 m Houston, TX In progress 37 m Potential Locations Atlanta, GA tbc 57 m Orlando, FL tbc 31 m Top 6 sites cover >90% US population within a one half-life (11 hr) delivery radius2 Regional Manufacturing Allows Commercialization of 212Pb-labeled Finished Products The “network effect” designed to ensure reliable commercial supply for ready-to-administer therapeutics 1Based on current Company Plans and estimates; 2Company estimates based on data from https://www.statsamerica.org/radius/big.aspx; Products can also be driven further or flown as necessary CATX Manufacturing Facilities Operational facility Buildout in progress Potential site Delivery Radius (road) within 1 half-life of 212Pb (11 hrs) Supply Chain & Manufacturing

Neuroendocrine Tumors: VMT-⍺-NET Targeting the somatostatin receptor to treat rare neuroendocrine-type cancers
Whole body SPECT scan of NET patient imaged with [203Pb]VMT-𝛼-NET Neuroendocrine Tumors: VMT-⍺-NET Targeting the somatostatin receptor to treat neuroendocrine and other cancers Clinical Pipeline Unmet Need Disease Overview Program Overview 1Wu P, He D, Chang H, Zhang X. Epidemiologic trends of and factors associated with overall survival in patients with neuroendocrine tumors over the last two decades in the USA. Endocr Connect. 2023;12(12):e230331. Published 2023 Nov 23. doi:10.1530/EC-23-0331; 2LUTATHERA PI; 3Navalkissoor et al (2019) Neuroendocrine cells are specialized cells that secrete hormones and other bioactive substances Most neuroendocrine tumors highly express somatostatin receptor type 2 (SSTR2) Often grow in the pancreas or other areas of the gut; also widely expressed in a broad range of tumors including SCLC, breast cancer, meningioma, head & neck cancer ~12K new diagnoses annually in the US; ~170,000 people are living with this diagnosis in the US1 Existing radiopharmaceutical treatment LUTATHERA® (Novartis) has an overall response rate (ORR) of only 13–17%, and no overall survival (OS) benefit2 Broad acknowledgment that targeted alpha therapies are needed to improve care3 Fast Track Designation for SSTR2+ NETs regardless of prior treatment response Ongoing CATX-sponsored therapeutic dose-finding trial currently recruiting in PRRT-naïve setting throughout the US Investigator initiated clinical research in PRRT- refractory patients
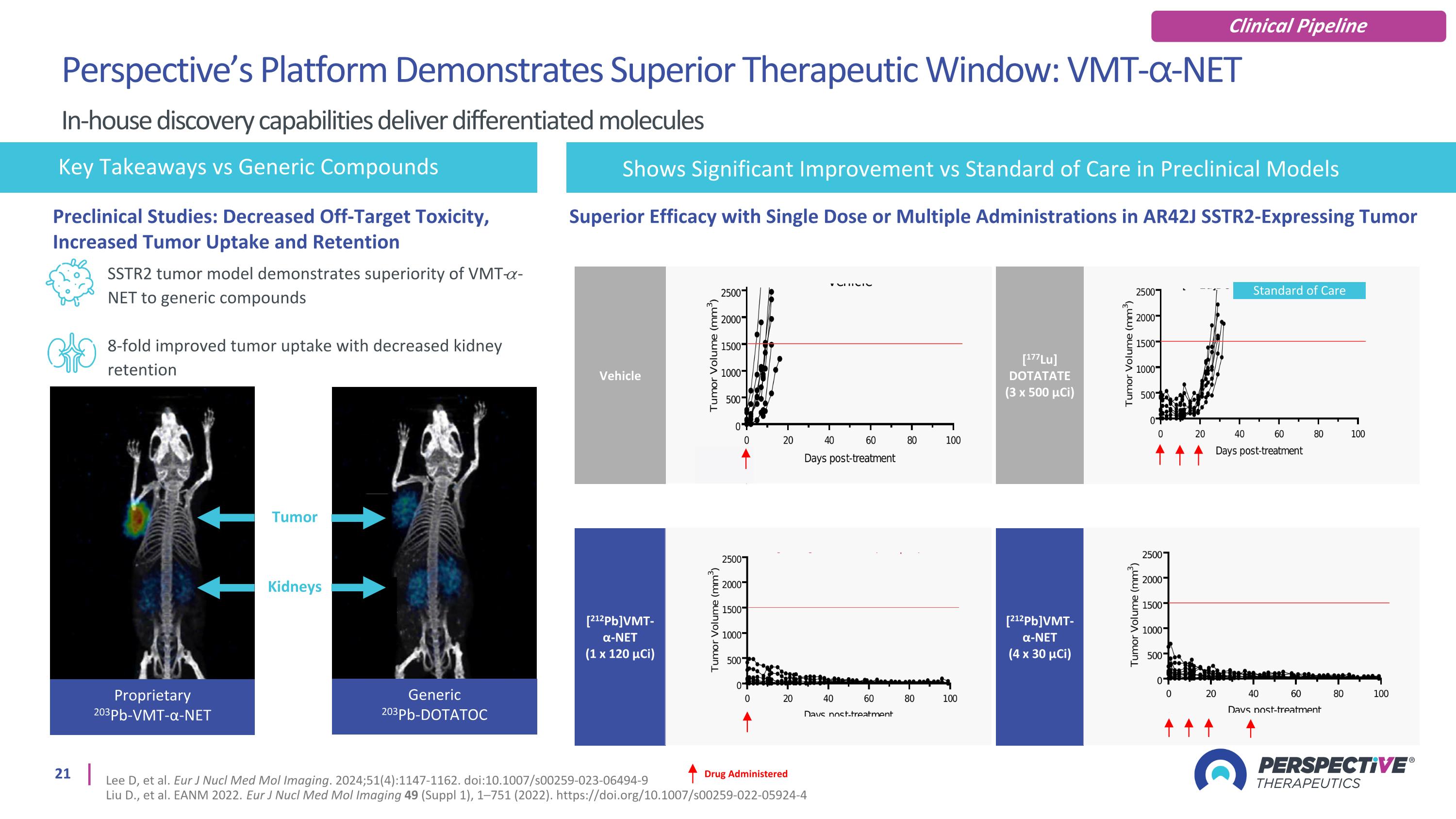
SSTR2 tumor model demonstrates superiority of VMT-𝛼-NET to generic compounds 8-fold improved tumor uptake with decreased kidney retention Key Takeaways vs Generic Compounds Generic 203Pb-DOTATOC Proprietary 203Pb-VMT-α-NET Tumor Kidneys Drug Administered [212Pb]VMT-α-NET (1 x 120 µCi) Vehicle [212Pb]VMT-α-NET (4 x 30 µCi) [177Lu] DOTATATE (3 x 500 µCi) Standard of Care Superior Efficacy with Single Dose or Multiple Administrations in AR42J SSTR2-Expressing Tumor Shows Significant Improvement vs Standard of Care in Preclinical Models Preclinical Studies: Decreased Off-Target Toxicity, Increased Tumor Uptake and Retention Perspective’s Platform Demonstrates Superior Therapeutic Window: VMT-⍺-NET In-house discovery capabilities deliver differentiated molecules Lee D, et al. Eur J Nucl Med Mol Imaging. 2024;51(4):1147-1162. doi:10.1007/s00259-023-06494-9 Liu D., et al. EANM 2022. Eur J Nucl Med Mol Imaging 49 (Suppl 1), 1–751 (2022). https://doi.org/10.1007/s00259-022-05924-4 Clinical Pipeline
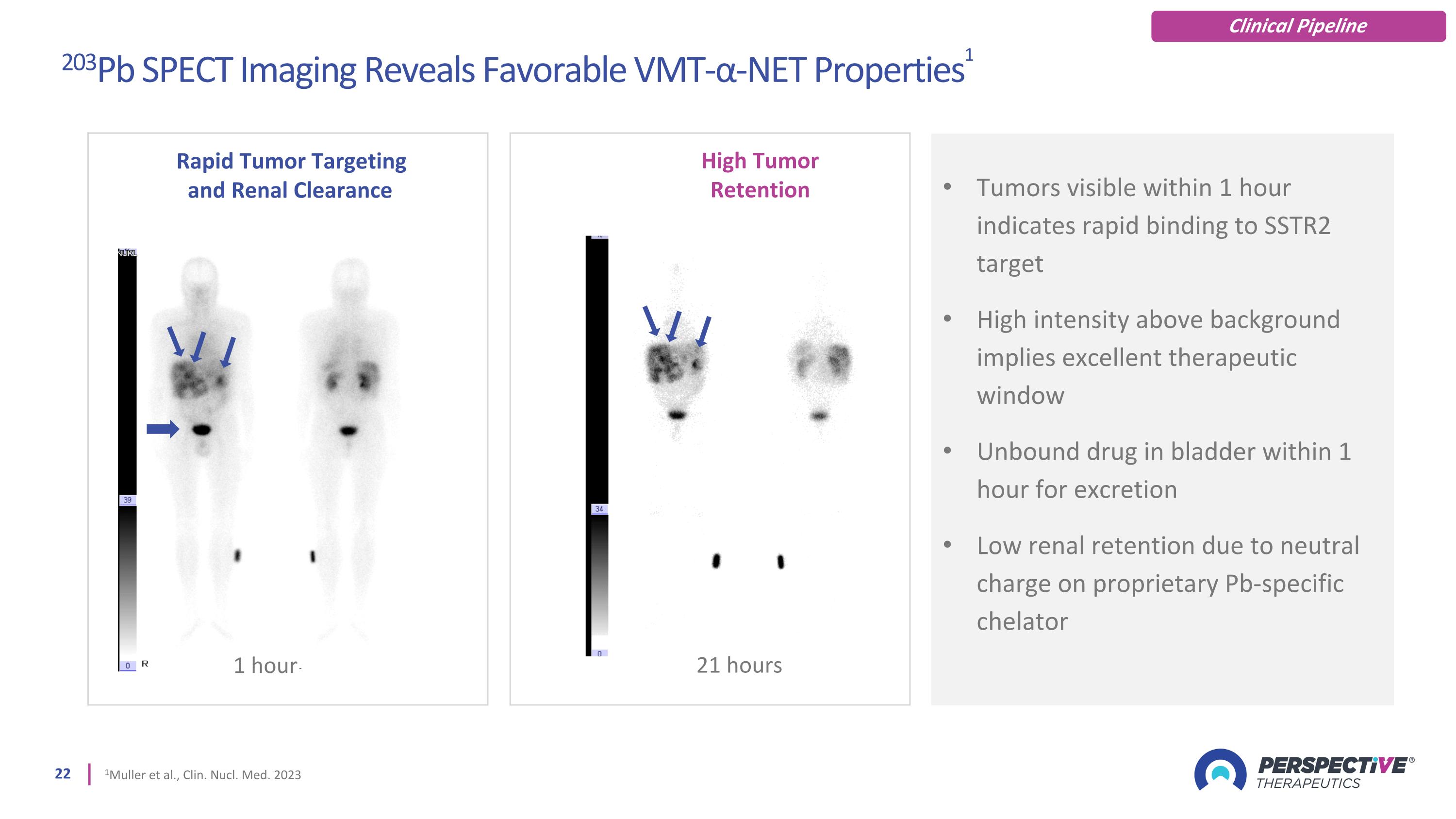
Tumors visible within 1 hour indicates rapid binding to SSTR2 target High intensity above background implies excellent therapeutic window Unbound drug in bladder within 1 hour for excretion Low renal retention due to neutral charge on proprietary Pb-specific chelator 21 hours Rapid Tumor Targeting and Renal Clearance 1 hour High Tumor Retention 203Pb SPECT Imaging Reveals Favorable VMT-α-NET Properties1 1Muller et al., Clin. Nucl. Med. 2023 Clinical Pipeline
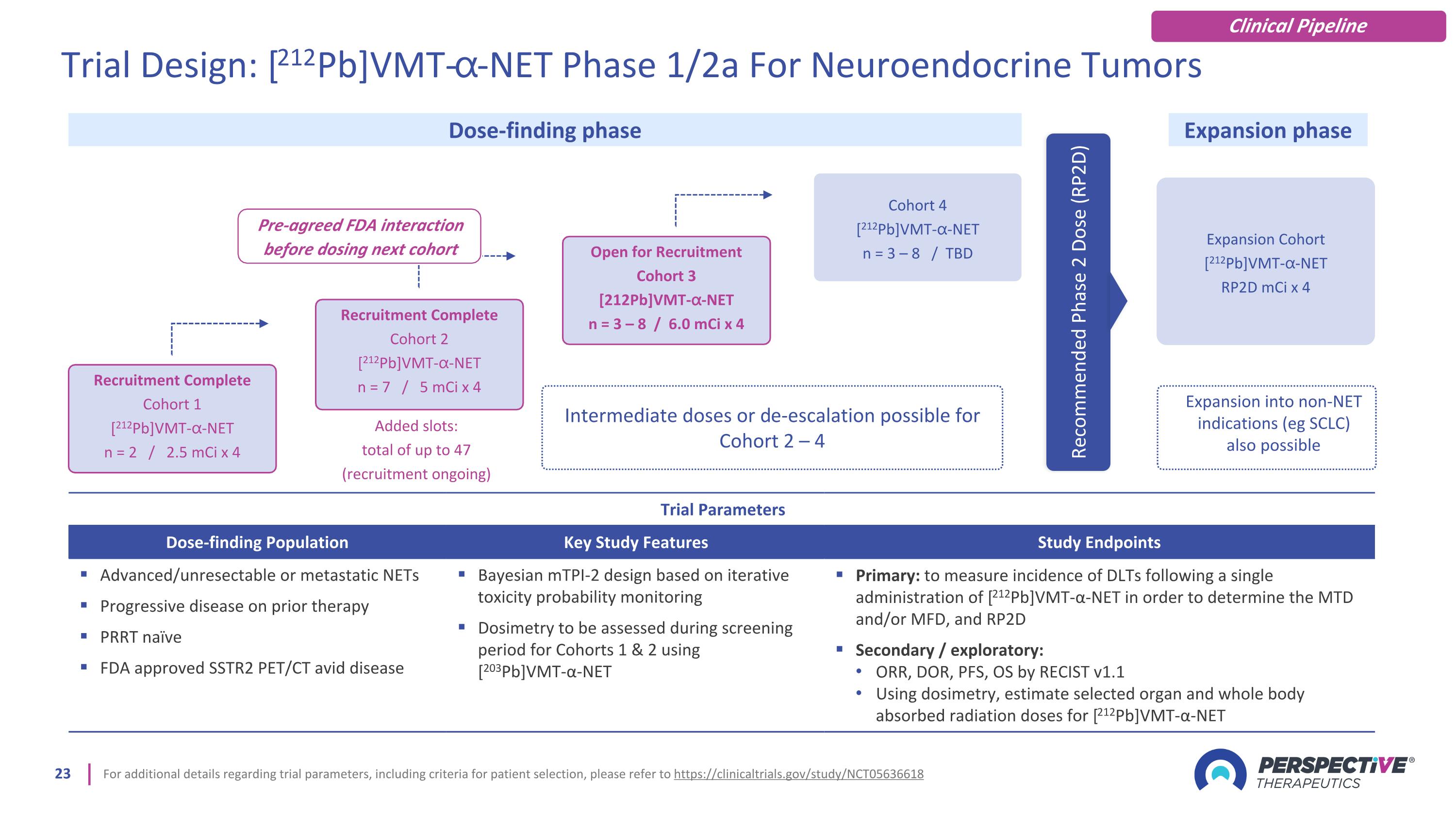
For additional details regarding trial parameters, including criteria for patient selection, please refer to https://clinicaltrials.gov/study/NCT05636618 Trial Parameters Dose-finding Population Key Study Features Study Endpoints Advanced/unresectable or metastatic NETs Progressive disease on prior therapy PRRT naïve FDA approved SSTR2 PET/CT avid disease Bayesian mTPI-2 design based on iterative toxicity probability monitoring Dosimetry to be assessed during screening period for Cohorts 1 & 2 using [203Pb]VMT-α-NET Primary: to measure incidence of DLTs following a single administration of [212Pb]VMT-α-NET in order to determine the MTD and/or MFD, and RP2D Secondary / exploratory: ORR, DOR, PFS, OS by RECIST v1.1 Using dosimetry, estimate selected organ and whole body absorbed radiation doses for [212Pb]VMT-α-NET Expansion Cohort [212Pb]VMT-⍺-NET RP2D mCi x 4 Dose-finding phase Expansion phase Expansion into non-NET indications (eg SCLC) also possible Recommended Phase 2 Dose (RP2D) Recruitment Complete Cohort 1 [212Pb]VMT-⍺-NET n = 2 / 2.5 mCi x 4 Recruitment Complete Cohort 2 [212Pb]VMT-⍺-NET n = 7 / 5 mCi x 4 Open for Recruitment Cohort 3 [212Pb]VMT-⍺-NET n = 3 – 8 / 6.0 mCi x 4 Cohort 4 [212Pb]VMT-⍺-NET n = 3 – 8 / TBD Intermediate doses or de-escalation possible for Cohort 2 – 4 Added slots: total of up to 47 (recruitment ongoing) Trial Design: [212Pb]VMT-⍺-NET Phase 1/2a For Neuroendocrine Tumors Pre-agreed FDA interaction before dosing next cohort Clinical Pipeline
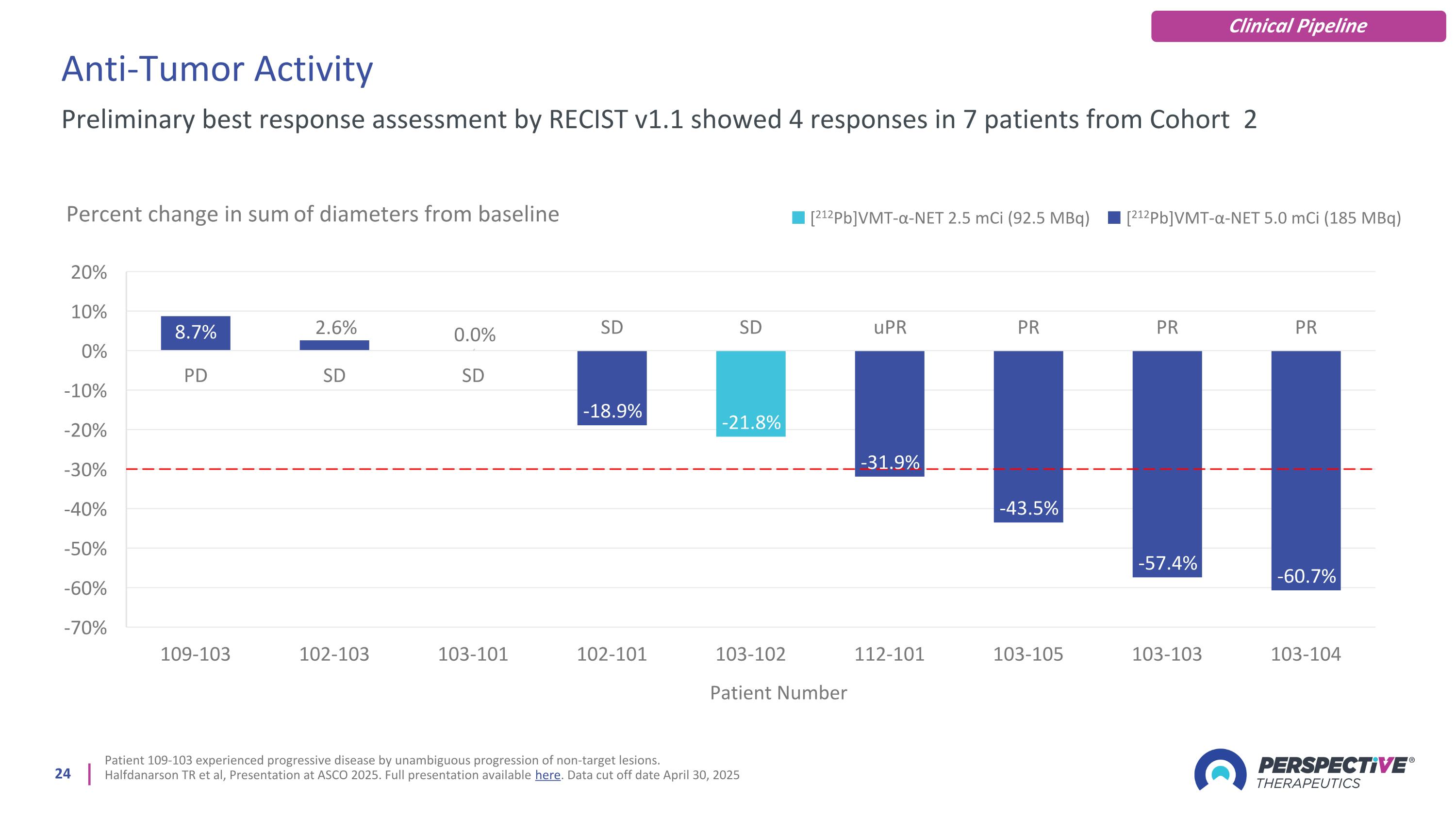
Anti-Tumor Activity Patient 109-103 experienced progressive disease by unambiguous progression of non-target lesions. Halfdanarson TR et al, Presentation at ASCO 2025. Full presentation available here. Data cut off date April 30, 2025 Preliminary best response assessment by RECIST v1.1 showed 4 responses in 7 patients from Cohort 2 [212Pb]VMT-α-NET 2.5 mCi (92.5 MBq) [212Pb]VMT-α-NET 5.0 mCi (185 MBq) Clinical Pipeline
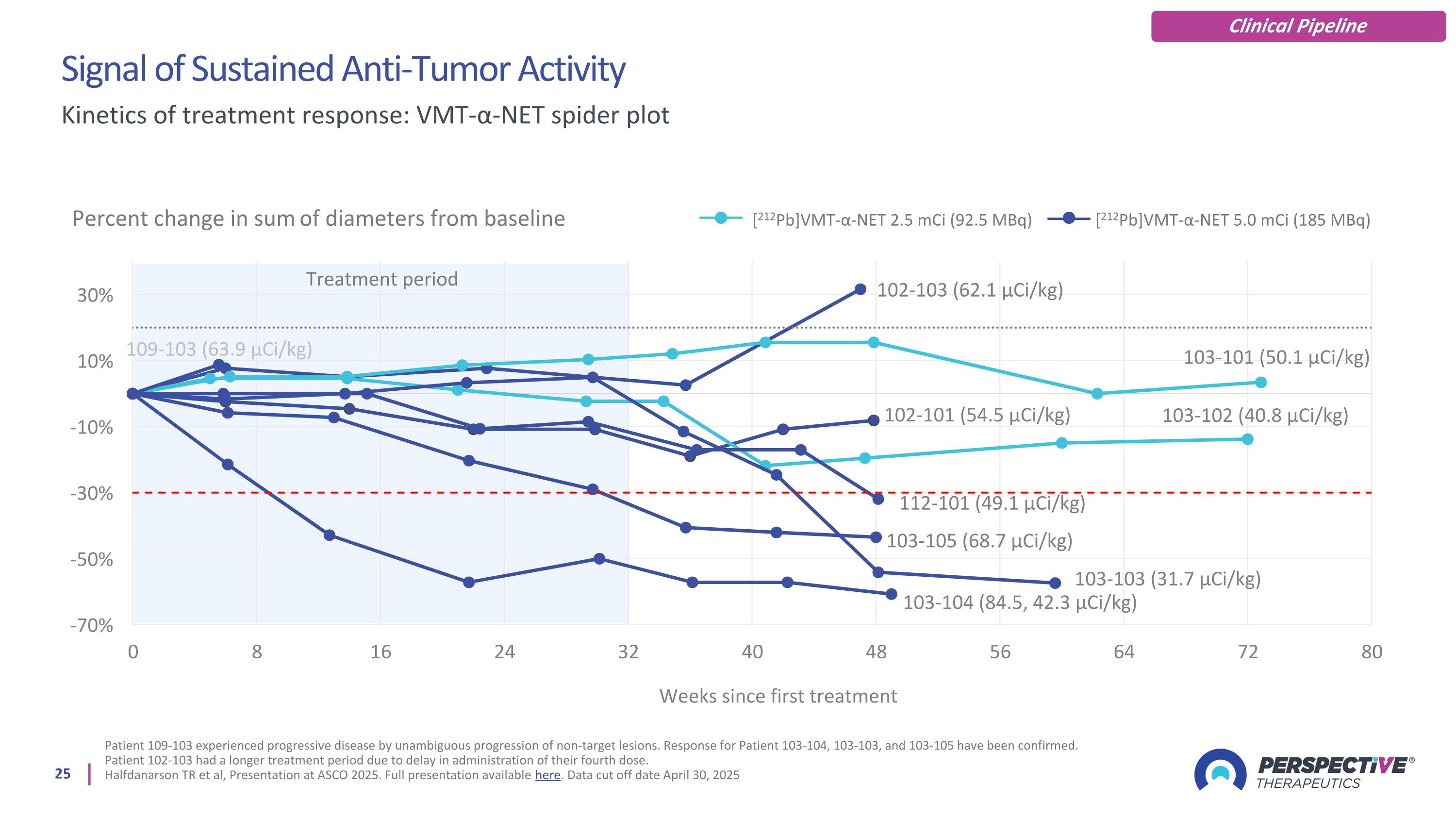
Signal of Sustained Anti-Tumor Activity Patient 109-103 experienced progressive disease by unambiguous progression of non-target lesions. Response for Patient 103-104, 103-103, and 103-105 have been confirmed. Patient 102-103 had a longer treatment period due to delay in administration of their fourth dose. Halfdanarson TR et al, Presentation at ASCO 2025. Full presentation available here. Data cut off date April 30, 2025 Kinetics of treatment response: VMT-α-NET spider plot Percent change in sum of diameters from baseline [212Pb]VMT-α-NET 2.5 mCi (92.5 MBq) [212Pb]VMT-α-NET 5.0 mCi (185 MBq) Treatment period Clinical Pipeline
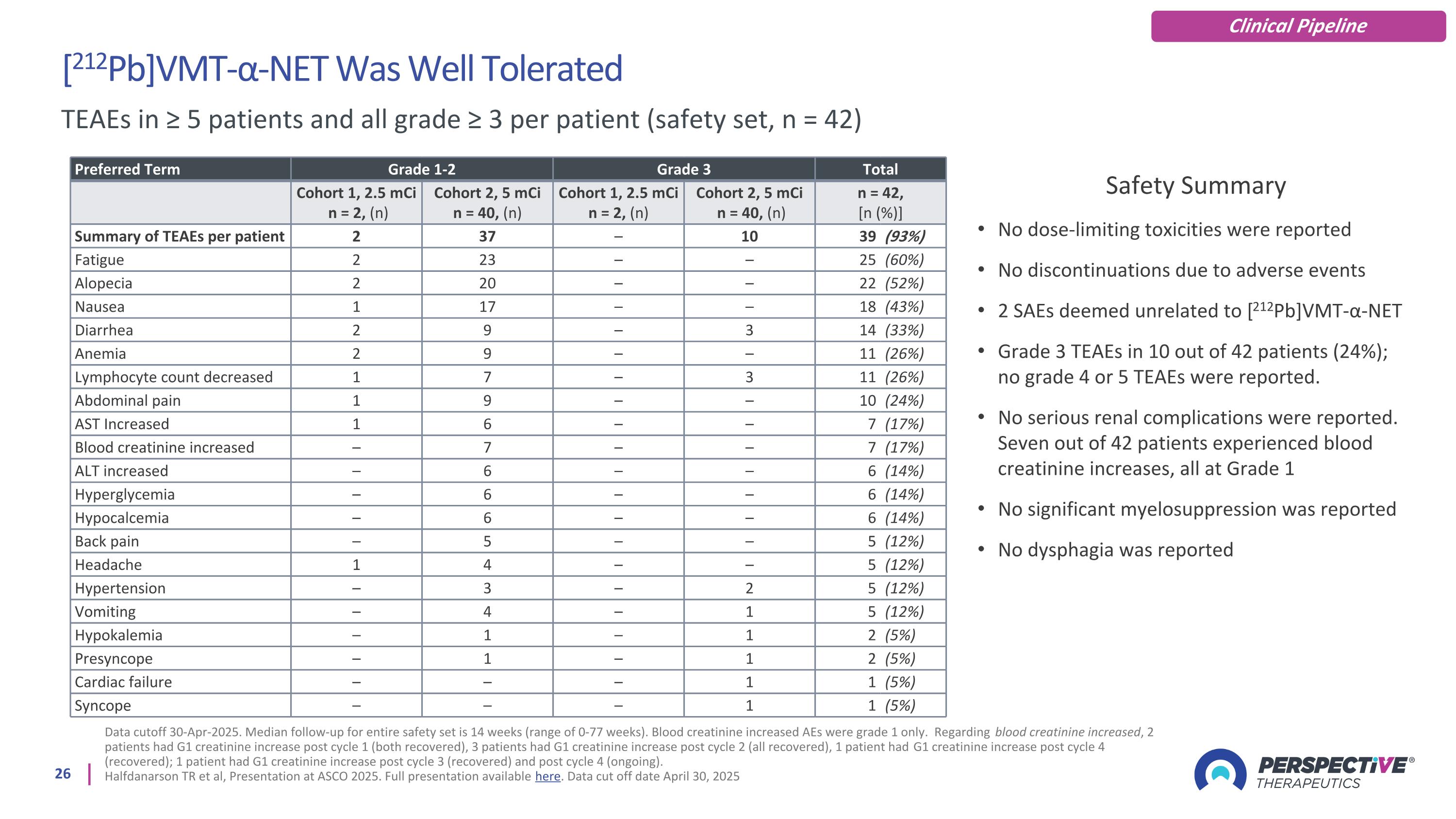
[212Pb]VMT-α-NET Was Well Tolerated Data cutoff 30-Apr-2025. Median follow-up for entire safety set is 14 weeks (range of 0-77 weeks). Blood creatinine increased AEs were grade 1 only. Regarding blood creatinine increased, 2 patients had G1 creatinine increase post cycle 1 (both recovered), 3 patients had G1 creatinine increase post cycle 2 (all recovered), 1 patient had G1 creatinine increase post cycle 4 (recovered); 1 patient had G1 creatinine increase post cycle 3 (recovered) and post cycle 4 (ongoing). Halfdanarson TR et al, Presentation at ASCO 2025. Full presentation available here. Data cut off date April 30, 2025 TEAEs in ≥ 5 patients and all grade ≥ 3 per patient (safety set, n = 42) Safety Summary No dose-limiting toxicities were reported No discontinuations due to adverse events 2 SAEs deemed unrelated to [212Pb]VMT-α-NET Grade 3 TEAEs in 10 out of 42 patients (24%); no grade 4 or 5 TEAEs were reported. No serious renal complications were reported. Seven out of 42 patients experienced blood creatinine increases, all at Grade 1 No significant myelosuppression was reported No dysphagia was reported Preferred Term Grade 1-2 Grade 3 Total Cohort 1, 2.5 mCi n = 2, (n) Cohort 2, 5 mCi n = 40, (n) Cohort 1, 2.5 mCi n = 2, (n) Cohort 2, 5 mCi n = 40, (n) n = 42, [n (%)] Summary of TEAEs per patient 2 37 – 10 39 (93%) Fatigue 2 23 – – 25 (60%) Alopecia 2 20 – – 22 (52%) Nausea 1 17 – – 18 (43%) Diarrhea 2 9 – 3 14 (33%) Anemia 2 9 – – 11 (26%) Lymphocyte count decreased 1 7 – 3 11 (26%) Abdominal pain 1 9 – – 10 (24%) AST Increased 1 6 – – 7 (17%) Blood creatinine increased – 7 – – 7 (17%) ALT increased – 6 – – 6 (14%) Hyperglycemia – 6 – – 6 (14%) Hypocalcemia – 6 – – 6 (14%) Back pain – 5 – – 5 (12%) Headache 1 4 – – 5 (12%) Hypertension – 3 – 2 5 (12%) Vomiting – 4 – 1 5 (12%) Hypokalemia – 1 – 1 2 (5%) Presyncope – 1 – 1 2 (5%) Cardiac failure – – – 1 1 (5%) Syncope – – – 1 1 (5%) Clinical Pipeline
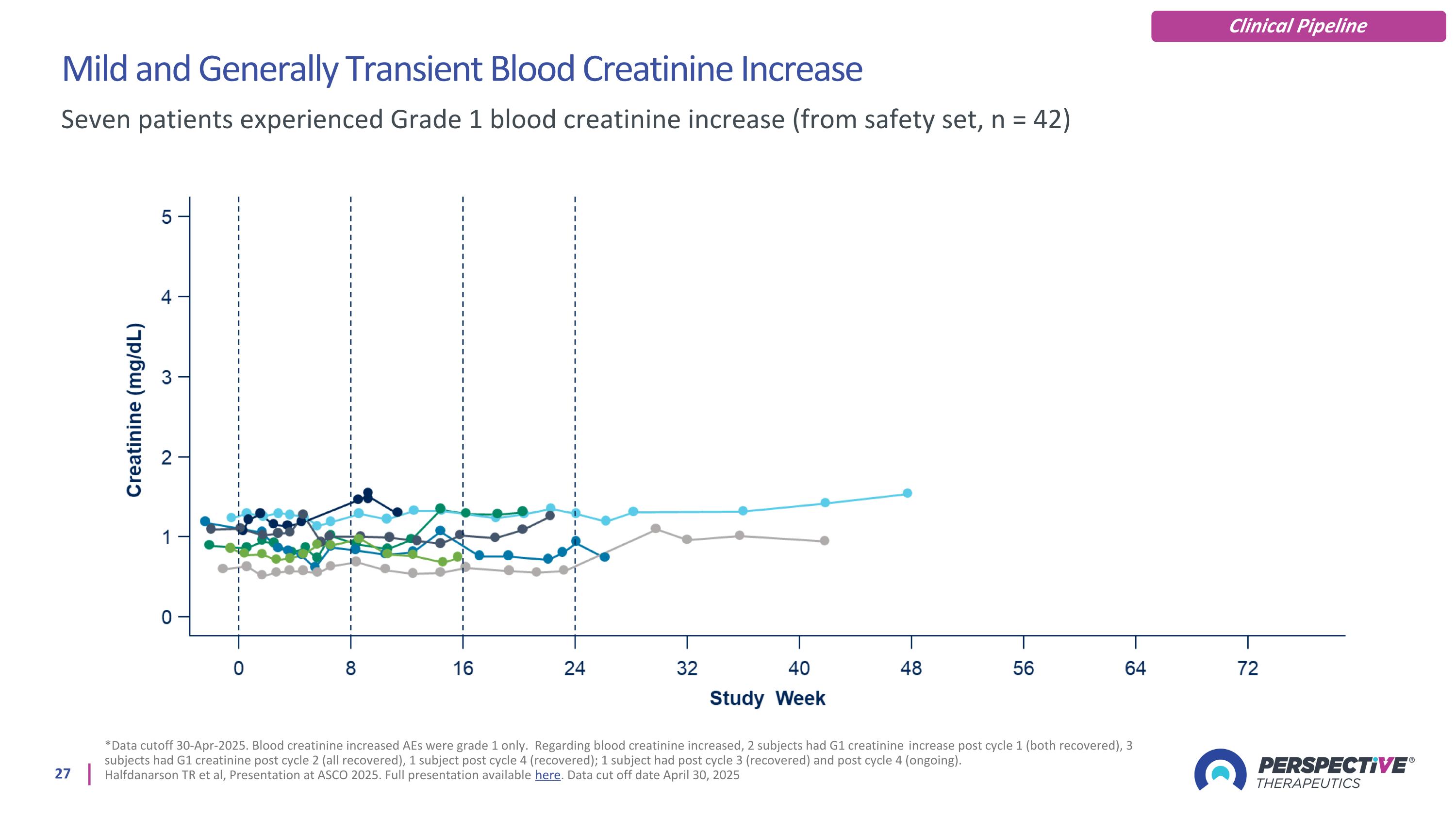
Mild and Generally Transient Blood Creatinine Increase *Data cutoff 30-Apr-2025. Blood creatinine increased AEs were grade 1 only. Regarding blood creatinine increased, 2 subjects had G1 creatinine increase post cycle 1 (both recovered), 3 subjects had G1 creatinine post cycle 2 (all recovered), 1 subject post cycle 4 (recovered); 1 subject had post cycle 3 (recovered) and post cycle 4 (ongoing). Halfdanarson TR et al, Presentation at ASCO 2025. Full presentation available here. Data cut off date April 30, 2025 Seven patients experienced Grade 1 blood creatinine increase (from safety set, n = 42) Clinical Pipeline
1Halfdanarson TR et al, Presentation at ASCO 2025. Full presentation available here. Data cut off date April 30, 2025 Conclusion & Next Steps [212Pb]VMT-α-NET was well tolerated, supporting rationale for potential dose escalation [212Pb]VMT-α-NET was well-tolerated1 No DLTs, no grade 4 or grade 5 AEs or deaths; 10 out of 42 (24%) patients experienced a Grade 3 TEAE No discontinuations due to AE have occurred No serious renal complications; blood creatinine increases in 7 out of 42 patients were all Grade 1 No significant myelosuppression was reported Appreciable activity was observed with treatment at this interim point in the study1 3 confirmed objective responses, 1 new response pending confirmation 7/9 (78%) patients remained free from disease progression after more than 1 year of follow-up The Safety Monitoring Committee has recommended dose escalation and re-opening of Cohort 2 FDA alignment to initiate next dosing cohort (Cohort 3) An additional 33 patients dosed in Cohort 2 since re-opening in August 2024, for a total of 40 in Cohort 2 as of April 30, 2025 Plan to submit data on patients who have had the opportunity to receive at least one scan after their full treatment for scientific congress presentation in 2H 2025 Ongoing evaluation of potential expansion into other SSTR2+ tumor types including breast, SCLC Clinical Pipeline
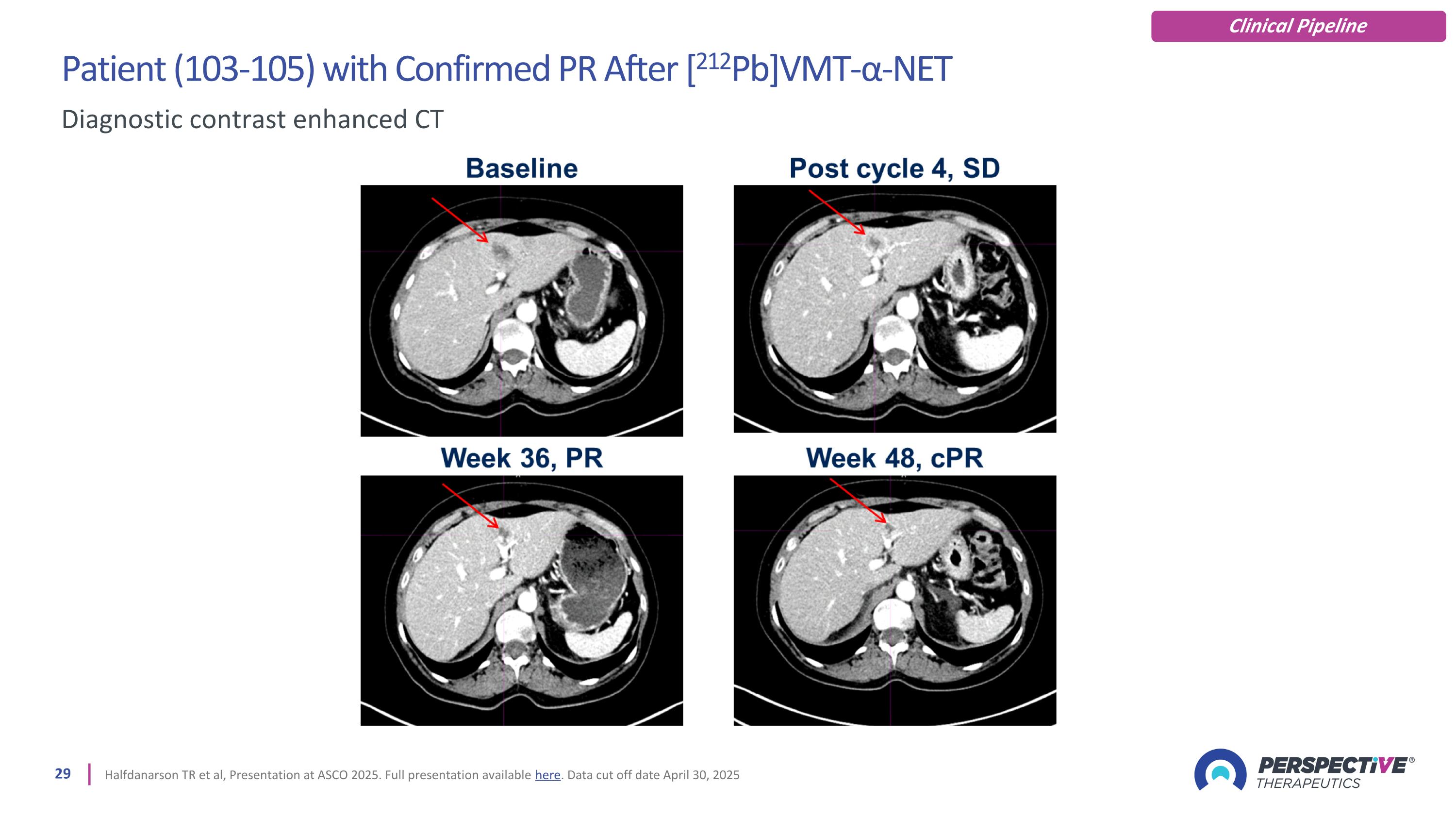
Patient (103-105) with Confirmed PR After [212Pb]VMT-α-NET Halfdanarson TR et al, Presentation at ASCO 2025. Full presentation available here. Data cut off date April 30, 2025 Diagnostic contrast enhanced CT Clinical Pipeline

Melanoma Program: VMT01/02 Using the melanocortin receptor (MC1R) to target melanoma for imaging and therapy
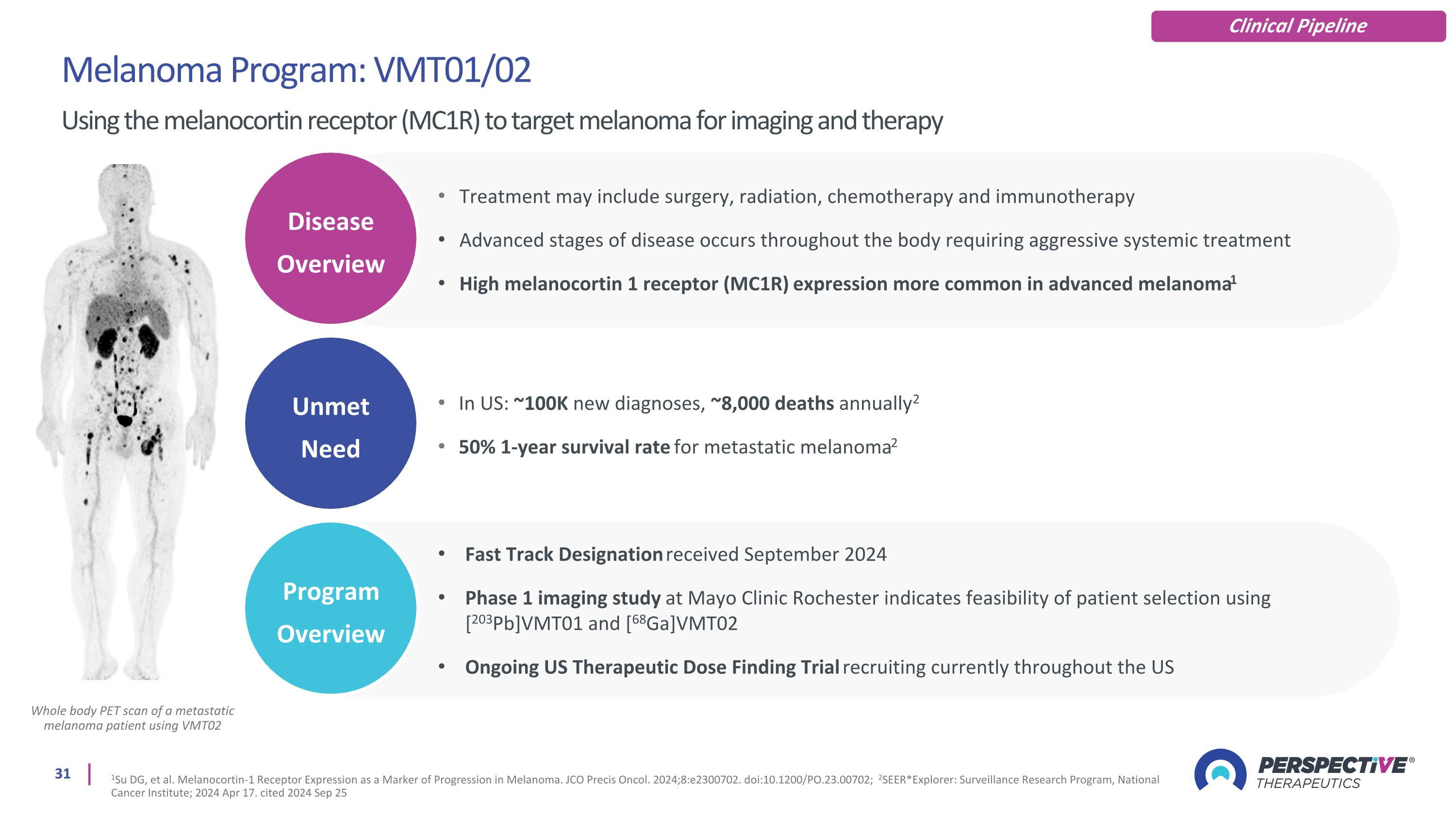
Whole body PET scan of a metastatic melanoma patient using VMT02 Melanoma Program: VMT01/02 Using the melanocortin receptor (MC1R) to target melanoma for imaging and therapy 1Su DG, et al. Melanocortin-1 Receptor Expression as a Marker of Progression in Melanoma. JCO Precis Oncol. 2024;8:e2300702. doi:10.1200/PO.23.00702; 2SEER*Explorer: Surveillance Research Program, National Cancer Institute; 2024 Apr 17. cited 2024 Sep 25 Clinical Pipeline Unmet Need Disease Overview Program Overview Treatment may include surgery, radiation, chemotherapy and immunotherapy Advanced stages of disease occurs throughout the body requiring aggressive systemic treatment High melanocortin 1 receptor (MC1R) expression more common in advanced melanoma1 In US: ~100K new diagnoses, ~8,000 deaths annually2 50% 1-year survival rate for metastatic melanoma2 Fast Track Designation received September 2024 Phase 1 imaging study at Mayo Clinic Rochester indicates feasibility of patient selection using [203Pb]VMT01 and [68Ga]VMT02 Ongoing US Therapeutic Dose Finding Trial recruiting currently throughout the US
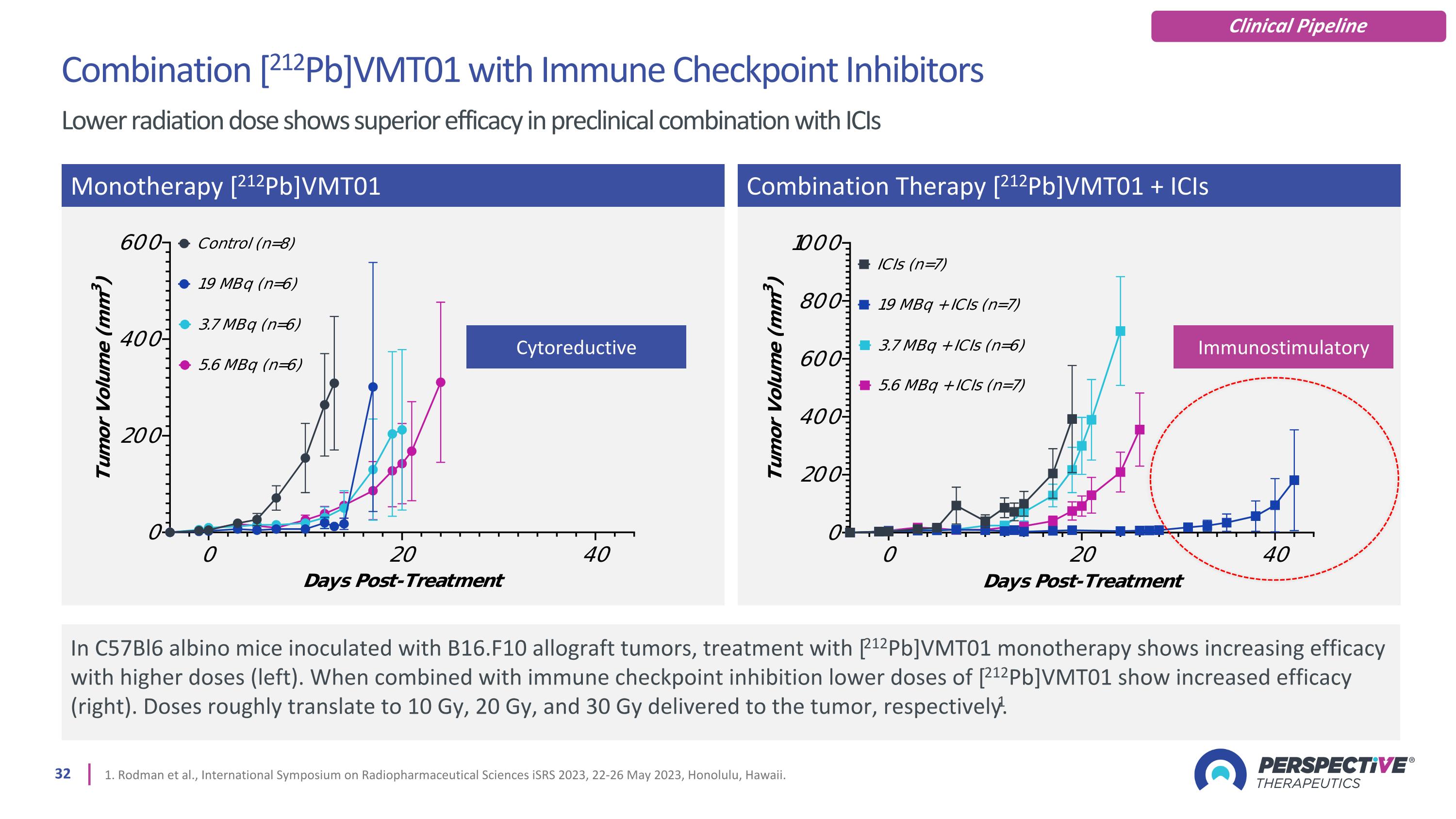
1. Rodman et al., International Symposium on Radiopharmaceutical Sciences iSRS 2023, 22-26 May 2023, Honolulu, Hawaii. In C57Bl6 albino mice inoculated with B16.F10 allograft tumors, treatment with [212Pb]VMT01 monotherapy shows increasing efficacy with higher doses (left). When combined with immune checkpoint inhibition lower doses of [212Pb]VMT01 show increased efficacy (right). Doses roughly translate to 10 Gy, 20 Gy, and 30 Gy delivered to the tumor, respectively.1 Monotherapy [212Pb]VMT01 Combination Therapy [212Pb]VMT01 + ICIs Combination [212Pb]VMT01 with Immune Checkpoint Inhibitors Lower radiation dose shows superior efficacy in preclinical combination with ICIs Cytoreductive Immunostimulatory Clinical Pipeline
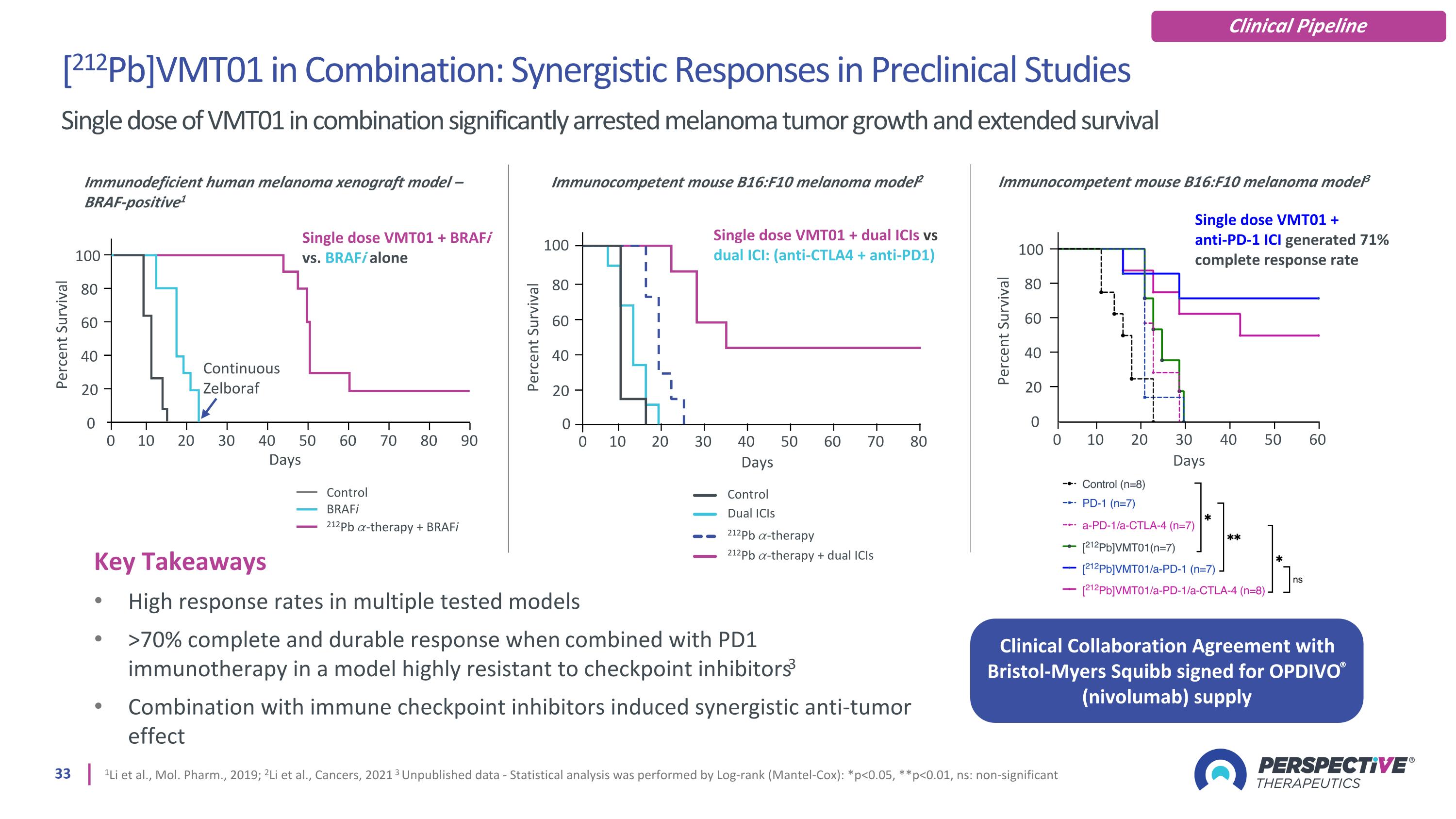
Key Takeaways High response rates in multiple tested models >70% complete and durable response when combined with PD1 immunotherapy in a model highly resistant to checkpoint inhibitors3 Combination with immune checkpoint inhibitors induced synergistic anti-tumor effect 1Li et al., Mol. Pharm., 2019; 2Li et al., Cancers, 2021 3 Unpublished data - Statistical analysis was performed by Log-rank (Mantel-Cox): *p<0.05, **p<0.01, ns: non-significant Immunodeficient human melanoma xenograft model – BRAF-positive1 Single dose VMT01 + BRAFi vs. BRAFi alone 0 0 Percent Survival Continuous Zelboraf Days 80 60 40 20 10 20 30 40 50 60 70 80 90 100 Control BRAFi 212Pb 𝛼-therapy + BRAFi Percent Survival Days 0 100 Immunocompetent mouse B16:F10 melanoma model2 Single dose VMT01 + dual ICIs vs dual ICI: (anti-CTLA4 + anti-PD1) Dual ICIs 212Pb 𝛼-therapy 212Pb 𝛼-therapy + dual ICIs Control 0 10 20 30 40 50 60 70 80 80 60 40 20 [212Pb]VMT01 in Combination: Synergistic Responses in Preclinical Studies Single dose of VMT01 in combination significantly arrested melanoma tumor growth and extended survival 0 0 Percent Survival Days 80 60 40 20 10 20 30 40 50 60 100 Immunocompetent mouse B16:F10 melanoma model3 Single dose VMT01 + anti-PD-1 ICI generated 71% complete response rate Clinical Collaboration Agreement with Bristol-Myers Squibb signed for OPDIVO® (nivolumab) supply Clinical Pipeline
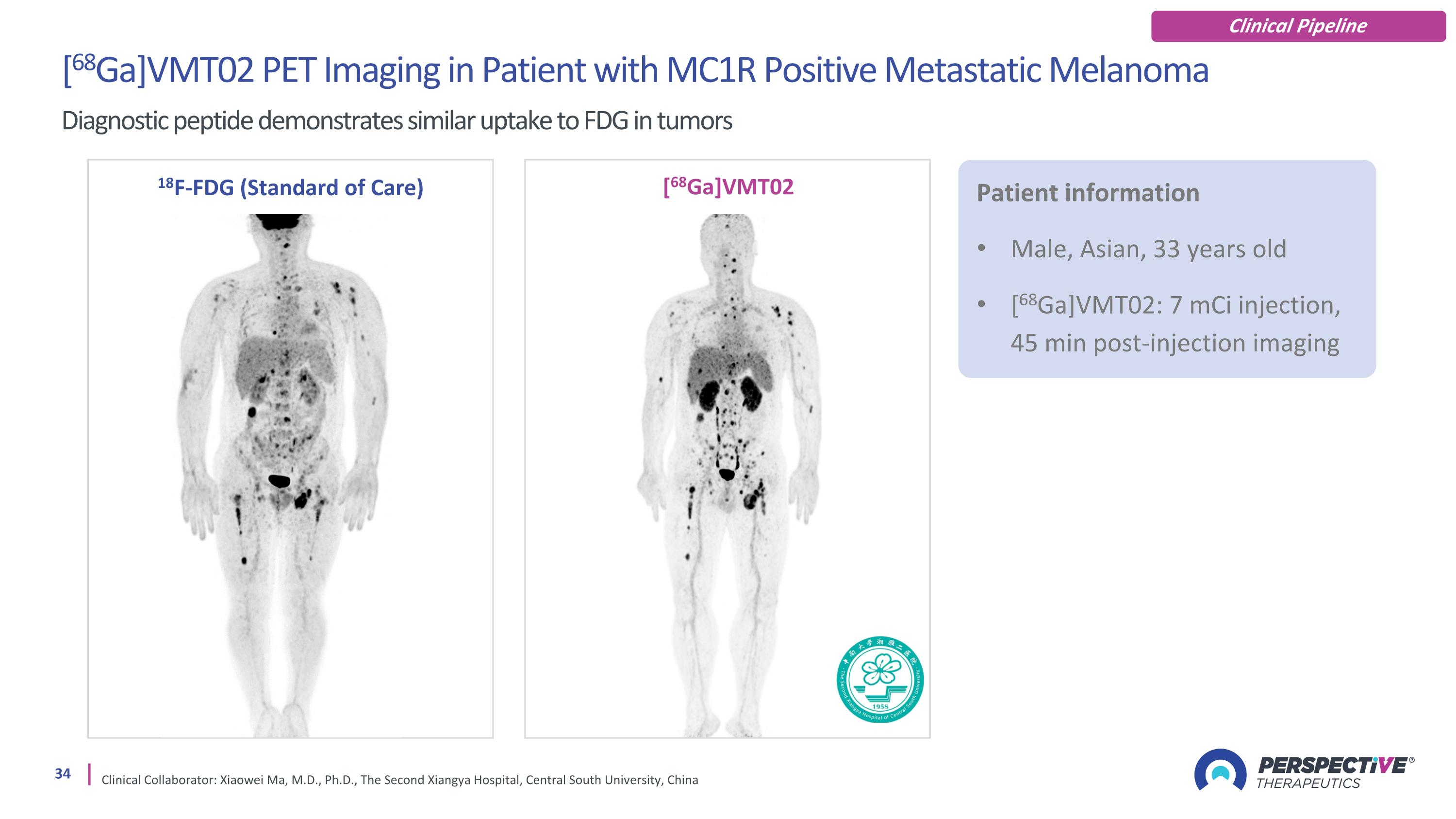
[68Ga]VMT02 PET Imaging in Patient with MC1R Positive Metastatic Melanoma Diagnostic peptide demonstrates similar uptake to FDG in tumors Clinical Collaborator: Xiaowei Ma, M.D., Ph.D., The Second Xiangya Hospital, Central South University, China 18F-FDG (Standard of Care) [68Ga]VMT02 Patient information Male, Asian, 33 years old [68Ga]VMT02: 7 mCi injection, 45 min post-injection imaging Clinical Pipeline
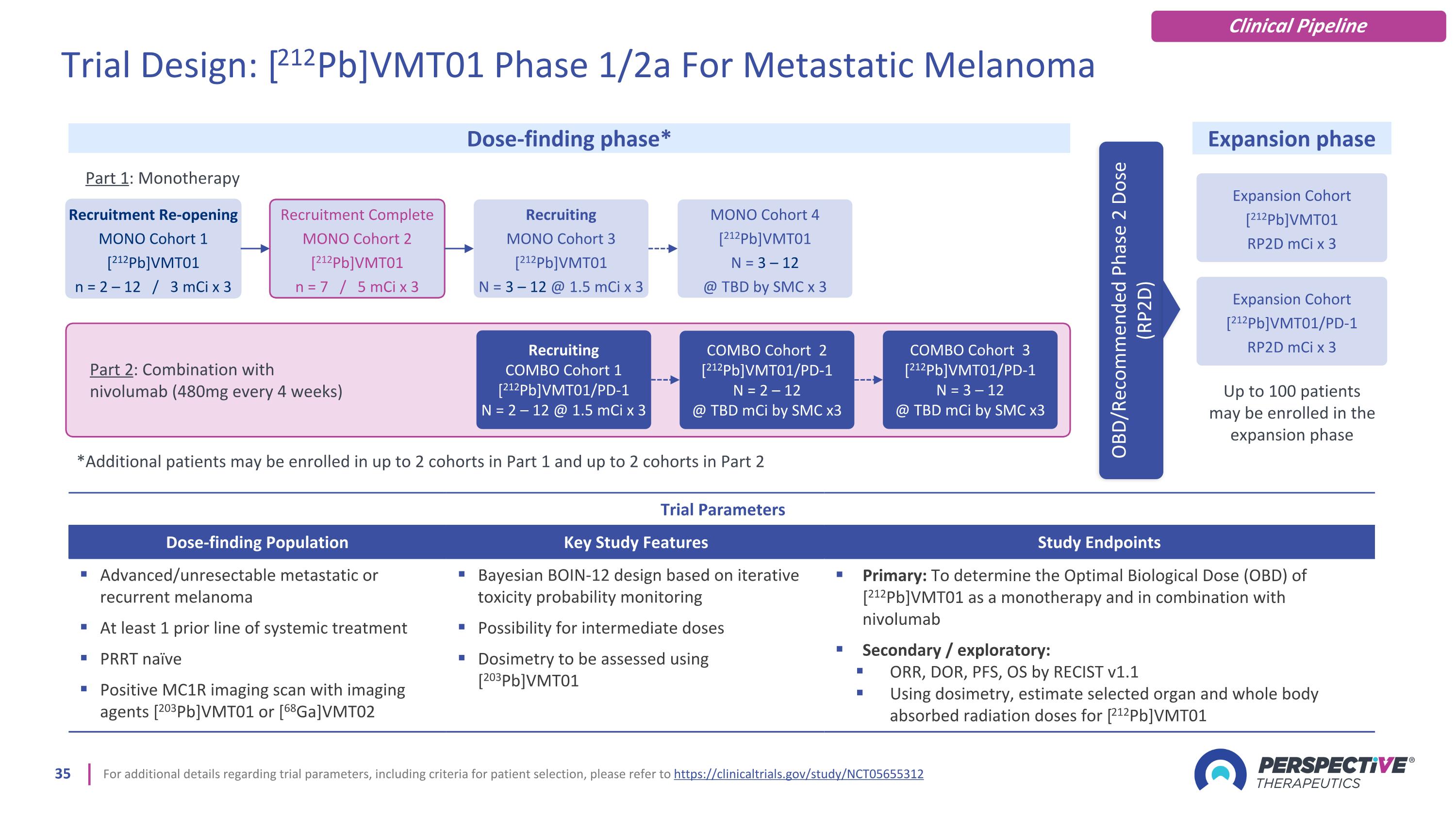
For additional details regarding trial parameters, including criteria for patient selection, please refer to https://clinicaltrials.gov/study/NCT05655312 Trial Parameters Dose-finding Population Key Study Features Study Endpoints Advanced/unresectable metastatic or recurrent melanoma At least 1 prior line of systemic treatment PRRT naïve Positive MC1R imaging scan with imaging agents [203Pb]VMT01 or [68Ga]VMT02 Bayesian BOIN-12 design based on iterative toxicity probability monitoring Possibility for intermediate doses Dosimetry to be assessed using [203Pb]VMT01 Primary: To determine the Optimal Biological Dose (OBD) of [212Pb]VMT01 as a monotherapy and in combination with nivolumab Secondary / exploratory: ORR, DOR, PFS, OS by RECIST v1.1 Using dosimetry, estimate selected organ and whole body absorbed radiation doses for [212Pb]VMT01 Dose-finding phase* Expansion phase OBD/Recommended Phase 2 Dose (RP2D) Trial Design: [212Pb]VMT01 Phase 1/2a For Metastatic Melanoma Clinical Pipeline Part 2: Combination with nivolumab (480mg every 4 weeks) Recruitment Re-opening MONO Cohort 1 [212Pb]VMT01 n = 2 – 12 / 3 mCi x 3 Recruitment Complete MONO Cohort 2 [212Pb]VMT01 n = 7 / 5 mCi x 3 Recruiting COMBO Cohort 1 [212Pb]VMT01/PD-1 N = 2 – 12 @ 1.5 mCi x 3 COMBO Cohort 2 [212Pb]VMT01/PD-1 N = 2 – 12 @ TBD mCi by SMC x3 Recruiting MONO Cohort 3 [212Pb]VMT01 N = 3 – 12 @ 1.5 mCi x 3 MONO Cohort 4 [212Pb]VMT01 N = 3 – 12 @ TBD by SMC x 3 Expansion Cohort [212Pb]VMT01 RP2D mCi x 3 COMBO Cohort 3 [212Pb]VMT01/PD-1 N = 3 – 12 @ TBD mCi by SMC x3 Part 1: Monotherapy *Additional patients may be enrolled in up to 2 cohorts in Part 1 and up to 2 cohorts in Part 2 Expansion Cohort [212Pb]VMT01/PD-1 RP2D mCi x 3 Up to 100 patients may be enrolled in the expansion phase
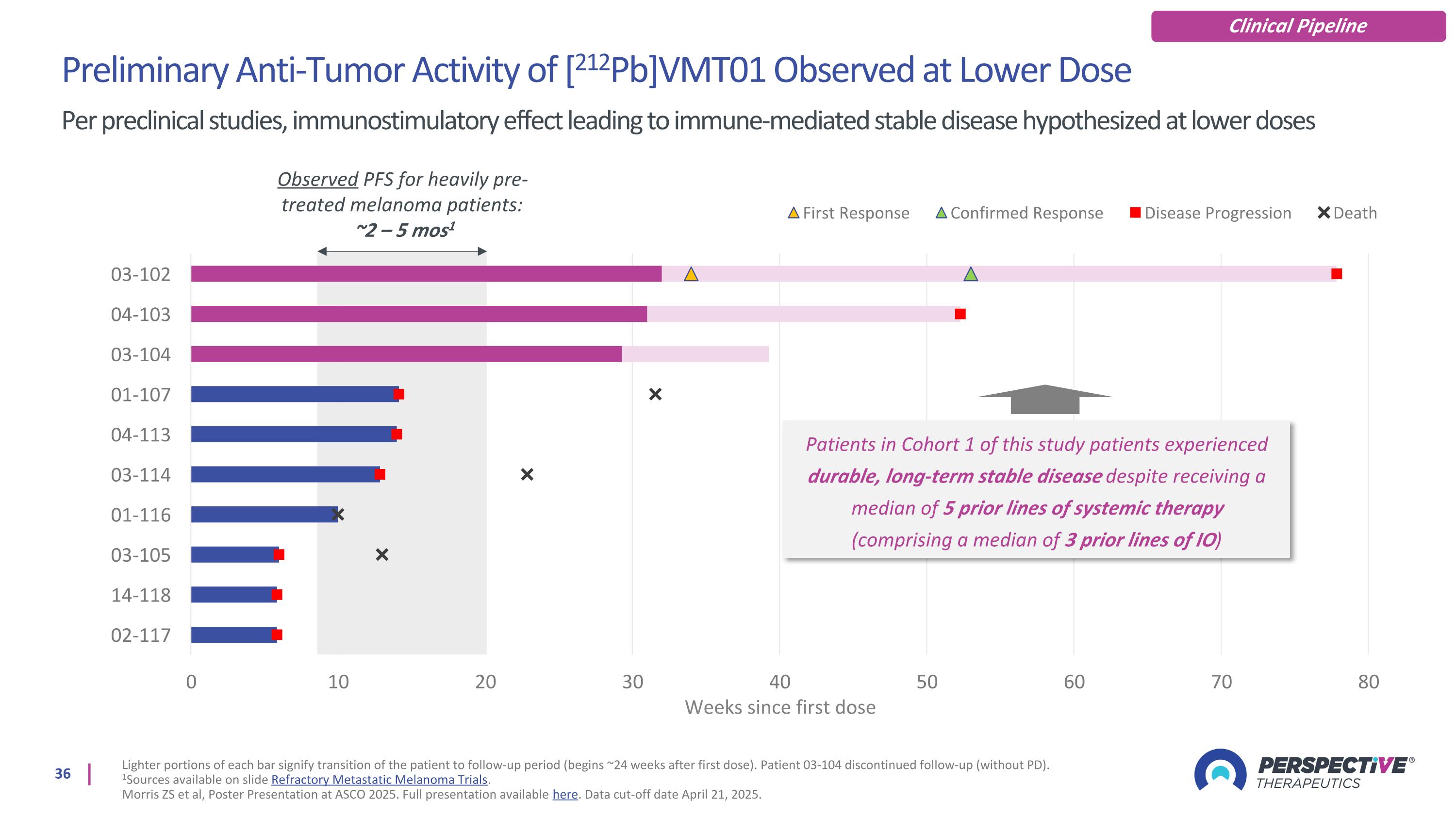
Preliminary Anti-Tumor Activity of [212Pb]VMT01 Observed at Lower Dose Per preclinical studies, immunostimulatory effect leading to immune-mediated stable disease hypothesized at lower doses Lighter portions of each bar signify transition of the patient to follow-up period (begins ~24 weeks after first dose). Patient 03-104 discontinued follow-up (without PD). 1Sources available on slide Refractory Metastatic Melanoma Trials. Morris ZS et al, Poster Presentation at ASCO 2025. Full presentation available here. Data cut-off date April 21, 2025. Clinical Pipeline Observed PFS for heavily pre-treated melanoma patients: ~2 – 5 mos1 Patients in Cohort 1 of this study patients experienced durable, long-term stable disease despite receiving a median of 5 prior lines of systemic therapy (comprising a median of 3 prior lines of IO)
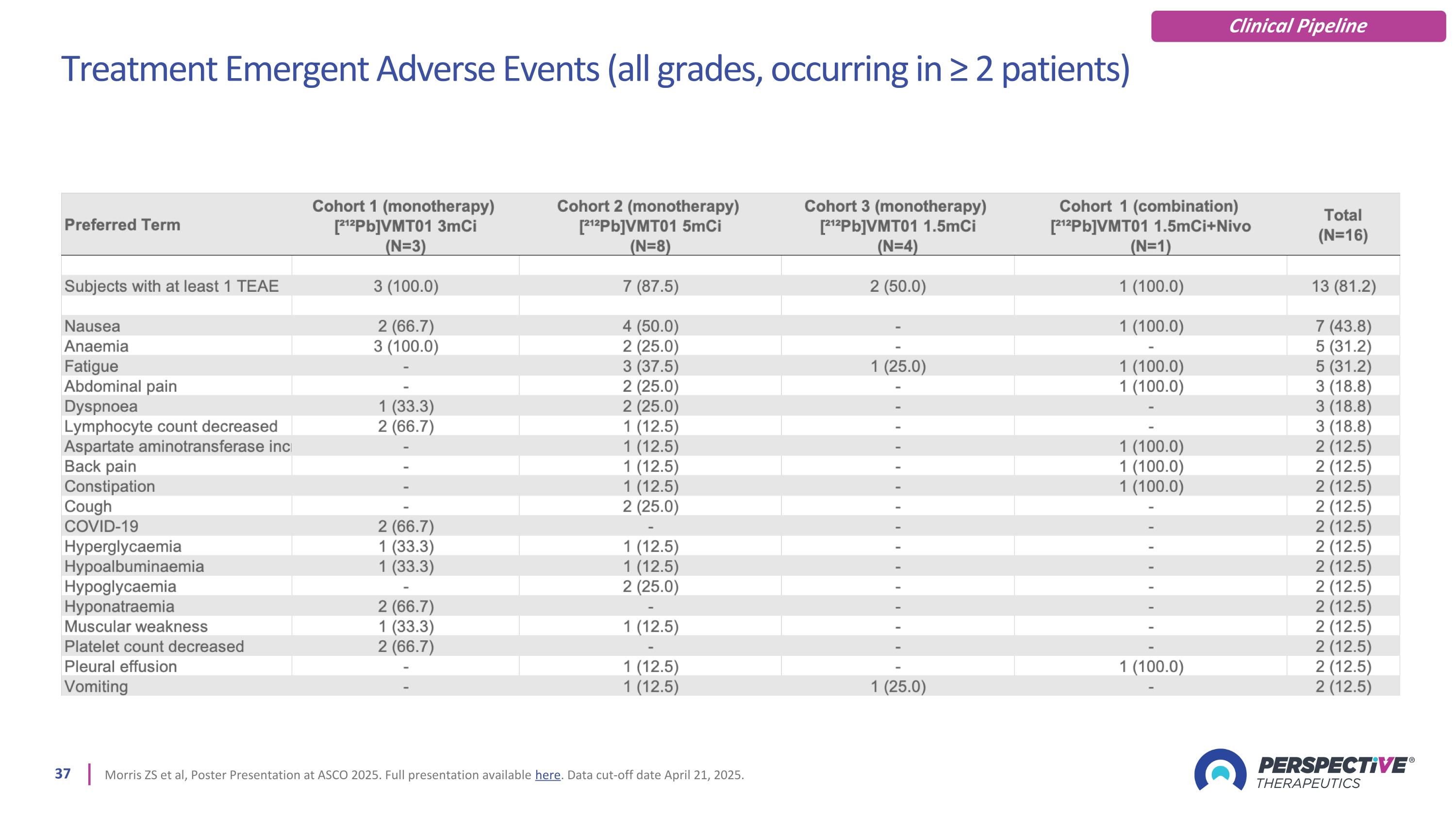
Treatment Emergent Adverse Events (all grades, occurring in ≥ 2 patients) Morris ZS et al, Poster Presentation at ASCO 2025. Full presentation available here. Data cut-off date April 21, 2025. Clinical Pipeline
1Morris ZS et al, Poster Presentation at ASCO 2025. Full presentation available here. Data cut-off date April 21, 2025. Conclusion & Next Steps Activity seen in 3 mCi dose cohort supports dose reduction and nivolumab combination Clinical Pipeline [212Pb]VMT01 was well-tolerated at doses studied1 At 3 mCi and 5 mCi activity levels, [212Pb]VMT01 was well-tolerated and no DLTs were observed Dosimetry demonstrates high kidney estimates may limit monotherapy escalation, although no renal toxicities were reported SMC recommendation included dose reduction to 1.5 mCi for both monotherapy and the combination cohort with nivolumab Objective response at lower dose cohort in line with preclinical data An objective response and prolonged PFS was observed at the 3 mCi activity level The observed preliminary anti-tumor activity in Cohort 1 may be a result of the immunostimulatory effect of low dose 212Pb-targeted alpha particle radiation on the TME, as observed in preclinical studies Lower dose and nivolumab combo cohorts enrolling First patient dosed in the combination cohort in March 2025 First patient dosed in the monotherapy cohort in April 2025

Pan-Cancer Target: PSV359 Preclinical Activity and First in Human Images of Novel Peptide Targeting Fibroblast Activation Protein-alpha (FAP-ɑ)
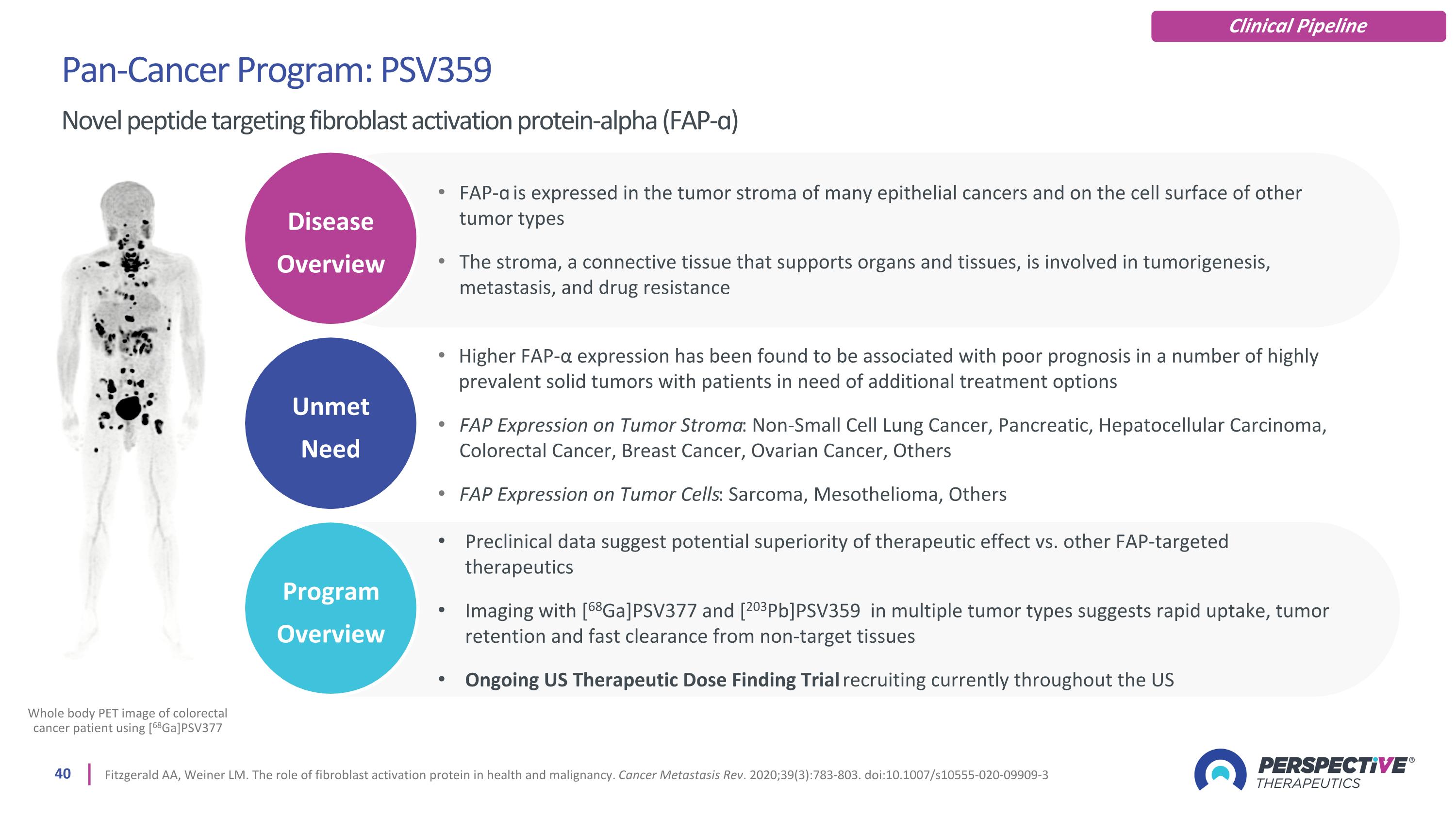
Pan-Cancer Program: PSV359 Novel peptide targeting fibroblast activation protein-alpha (FAP-ɑ) Fitzgerald AA, Weiner LM. The role of fibroblast activation protein in health and malignancy. Cancer Metastasis Rev. 2020;39(3):783-803. doi:10.1007/s10555-020-09909-3 Whole body PET image of colorectal cancer patient using [68Ga]PSV377 Clinical Pipeline Unmet Need Disease Overview Program Overview FAP-ɑ is expressed in the tumor stroma of many epithelial cancers and on the cell surface of other tumor types The stroma, a connective tissue that supports organs and tissues, is involved in tumorigenesis, metastasis, and drug resistance Higher FAP-α expression has been found to be associated with poor prognosis in a number of highly prevalent solid tumors with patients in need of additional treatment options FAP Expression on Tumor Stroma: Non-Small Cell Lung Cancer, Pancreatic, Hepatocellular Carcinoma, Colorectal Cancer, Breast Cancer, Ovarian Cancer, Others FAP Expression on Tumor Cells: Sarcoma, Mesothelioma, Others Preclinical data suggest potential superiority of therapeutic effect vs. other FAP-targeted therapeutics Imaging with [68Ga]PSV377 and [203Pb]PSV359 in multiple tumor types suggests rapid uptake, tumor retention and fast clearance from non-target tissues Ongoing US Therapeutic Dose Finding Trial recruiting currently throughout the US
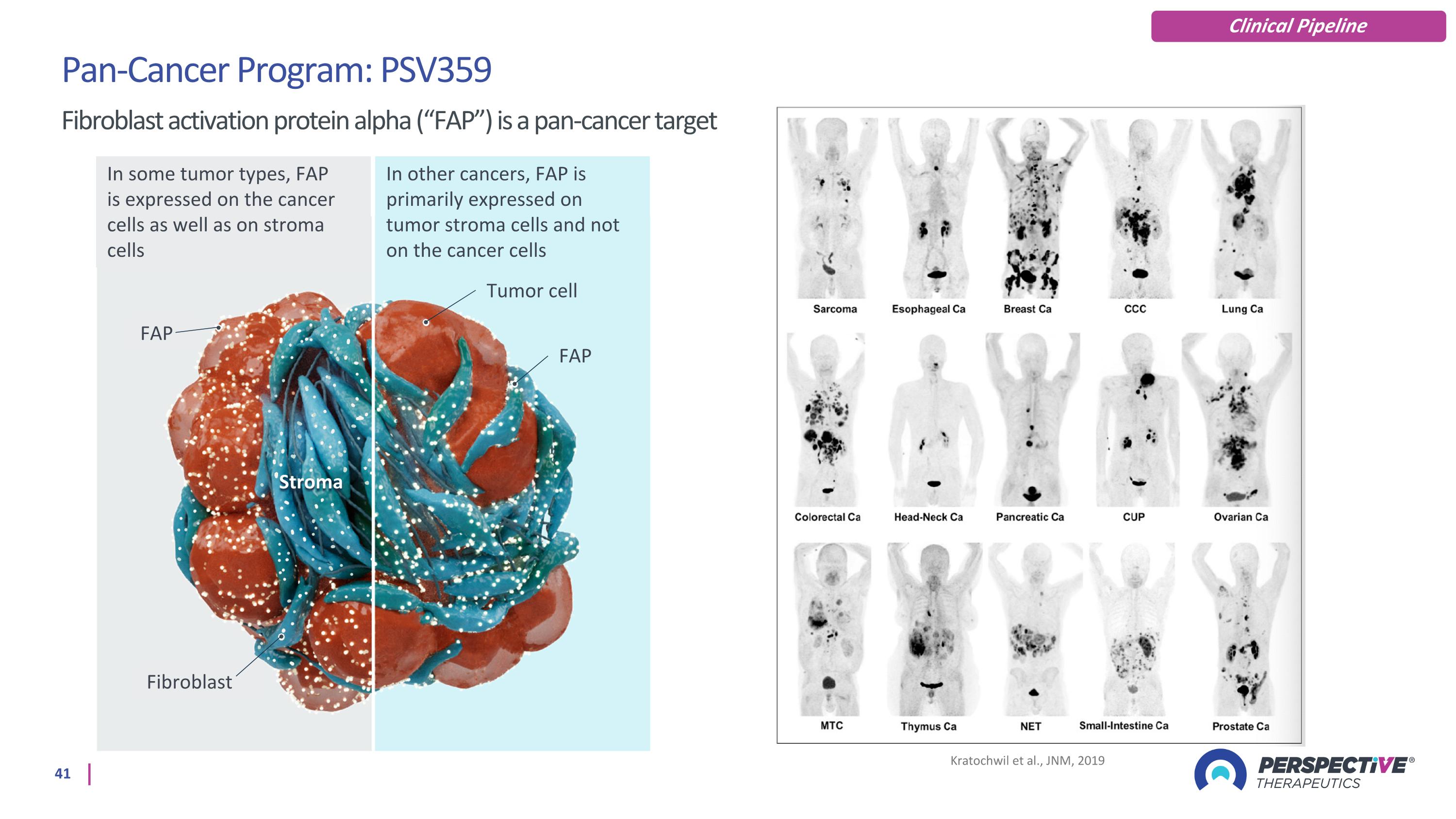
Kratochwil et al., JNM, 2019 Pan-Cancer Program: PSV359 Fibroblast activation protein alpha (“FAP”) is a pan-cancer target Clinical Pipeline In some tumor types, FAP is expressed on the cancer cells as well as on stroma cells In other cancers, FAP is primarily expressed on tumor stroma cells and not on the cancer cells FAP Tumor cell Fibroblast Stroma FAP
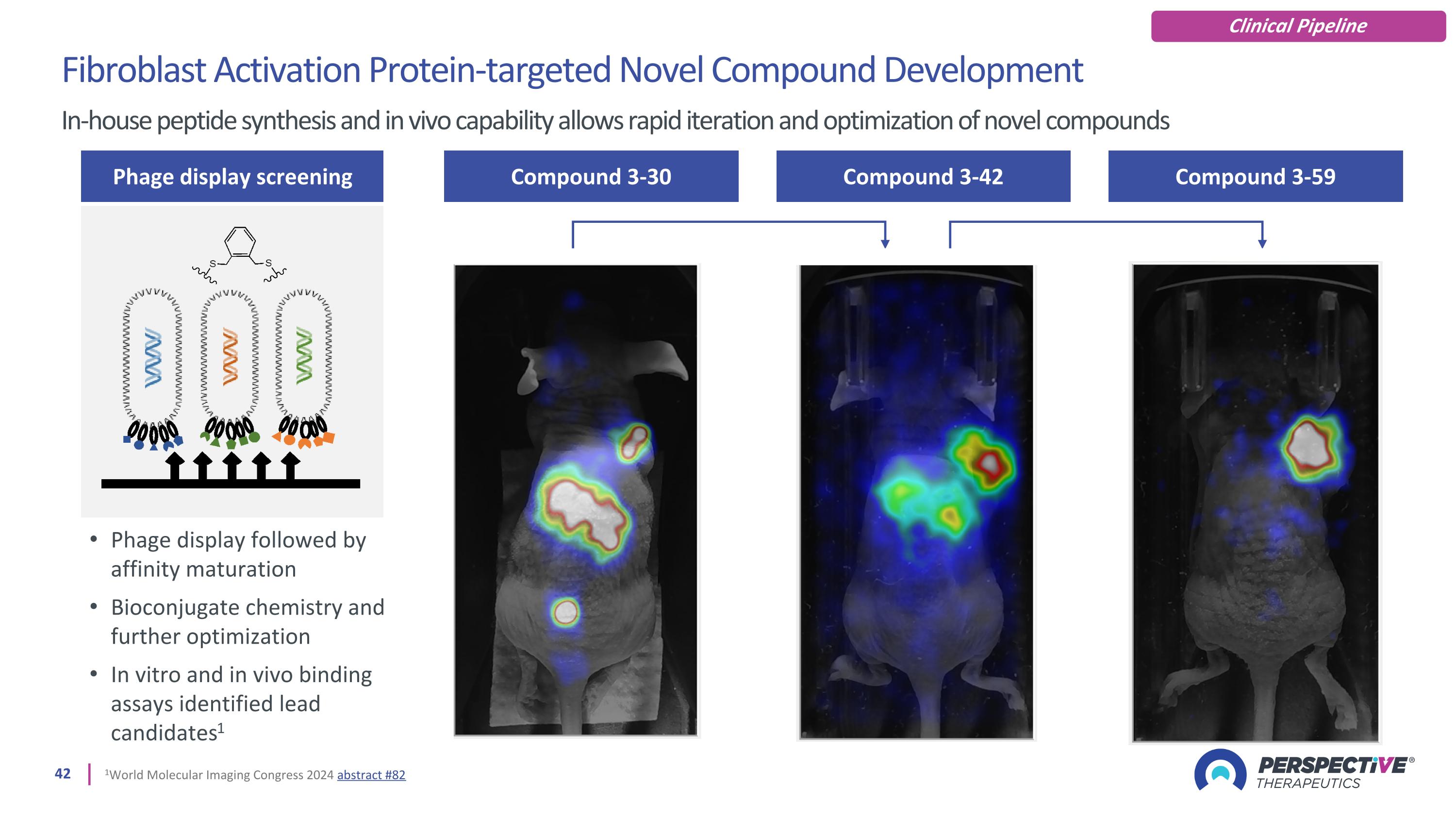
Compound 3-30 Compound 3-42 Compound 3-59 Fibroblast Activation Protein-targeted Novel Compound Development In-house peptide synthesis and in vivo capability allows rapid iteration and optimization of novel compounds Phage display screening Phage display followed by affinity maturation Bioconjugate chemistry and further optimization In vitro and in vivo binding assays identified lead candidates1 1World Molecular Imaging Congress 2024 abstract #82 Clinical Pipeline
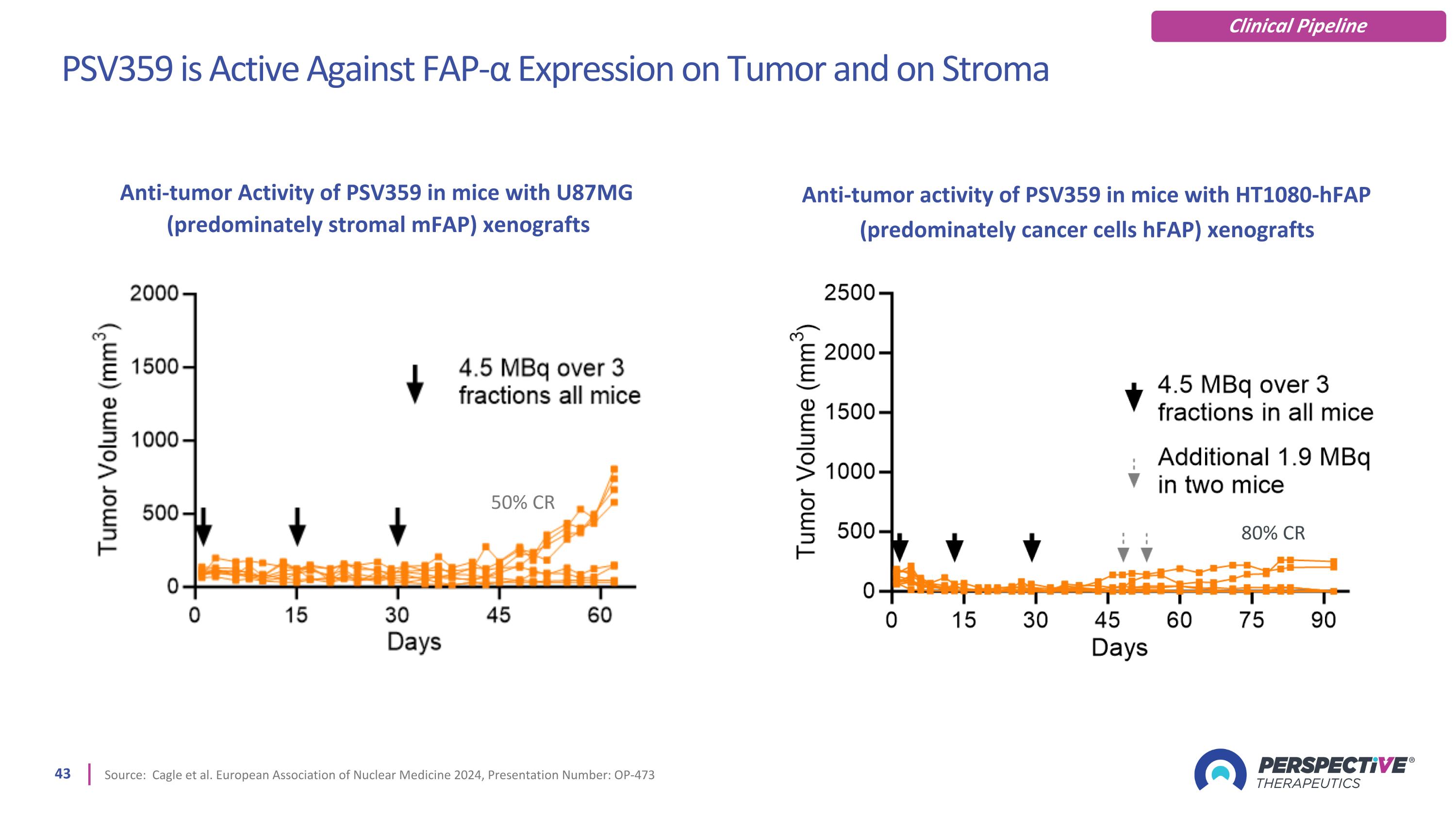
PSV359 is Active Against FAP-α Expression on Tumor and on Stroma Source: Cagle et al. European Association of Nuclear Medicine 2024, Presentation Number: OP-473 80% CR 50% CR Anti-tumor Activity of PSV359 in mice with U87MG (predominately stromal mFAP) xenografts Anti-tumor activity of PSV359 in mice with HT1080-hFAP (predominately cancer cells hFAP) xenografts Clinical Pipeline
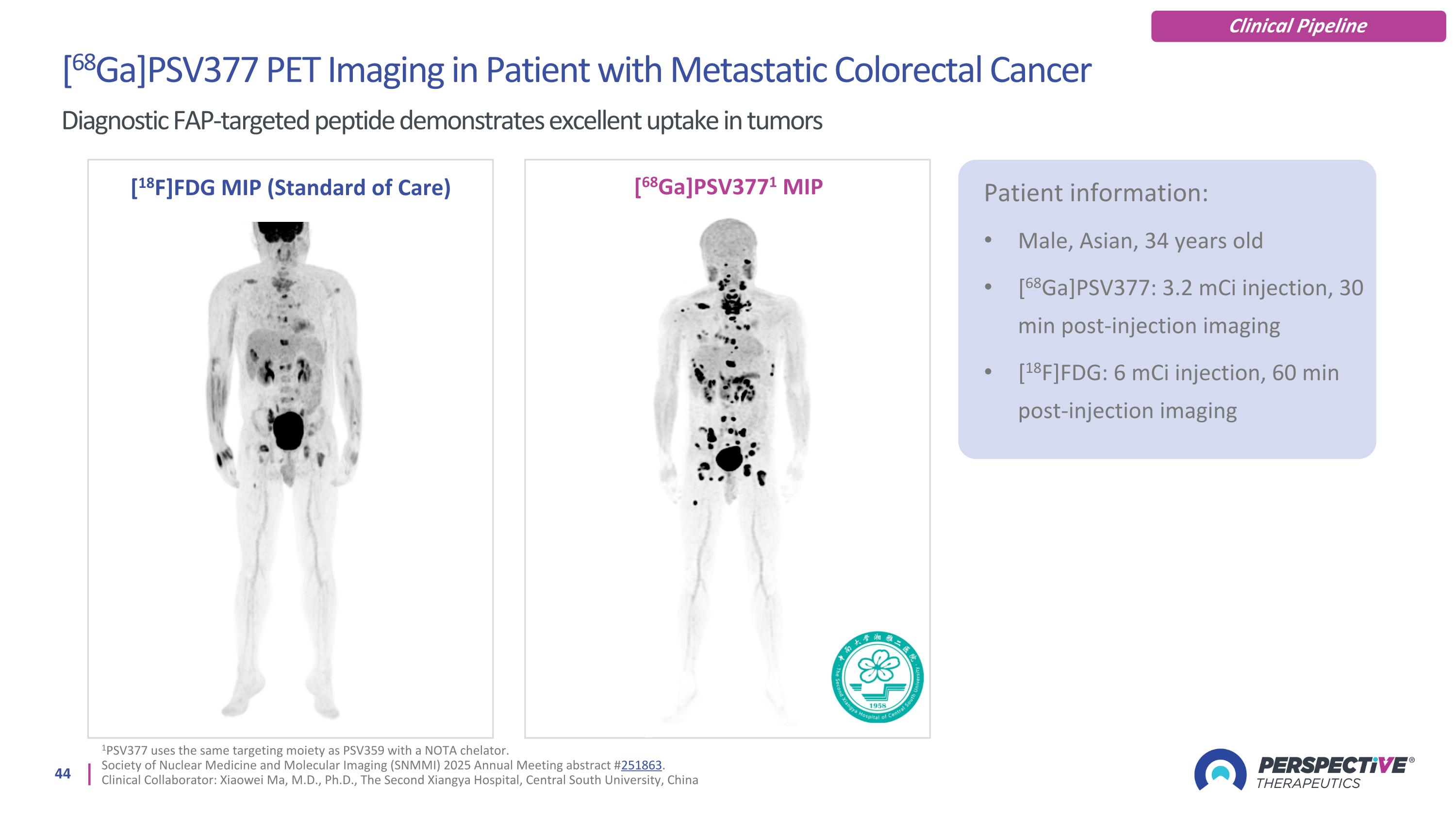
[68Ga]PSV377 PET Imaging in Patient with Metastatic Colorectal Cancer Diagnostic FAP-targeted peptide demonstrates excellent uptake in tumors [18F]FDG MIP (Standard of Care) [68Ga]PSV3771 MIP Patient information: Male, Asian, 34 years old [68Ga]PSV377: 3.2 mCi injection, 30 min post-injection imaging [18F]FDG: 6 mCi injection, 60 min post-injection imaging Clinical Pipeline 1PSV377 uses the same targeting moiety as PSV359 with a NOTA chelator. Society of Nuclear Medicine and Molecular Imaging (SNMMI) 2025 Annual Meeting abstract #251863. Clinical Collaborator: Xiaowei Ma, M.D., Ph.D., The Second Xiangya Hospital, Central South University, China
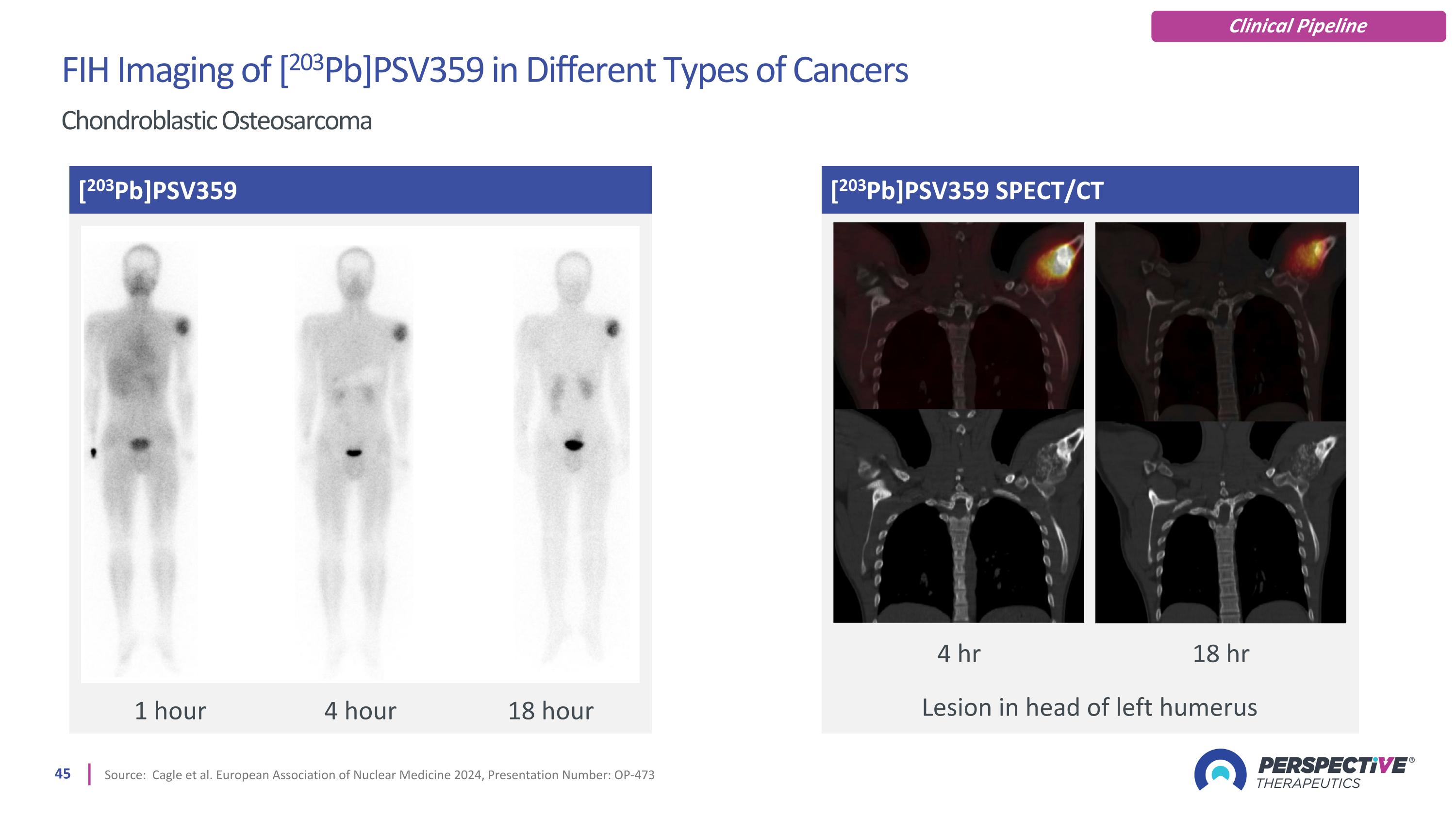
[203Pb]PSV359 SPECT/CT Source: Cagle et al. European Association of Nuclear Medicine 2024, Presentation Number: OP-473 [203Pb]PSV359 Lesion in head of left humerus 1 hour 4 hour 18 hour 4 hr 18 hr FIH Imaging of [203Pb]PSV359 in Different Types of Cancers Chondroblastic Osteosarcoma Clinical Pipeline
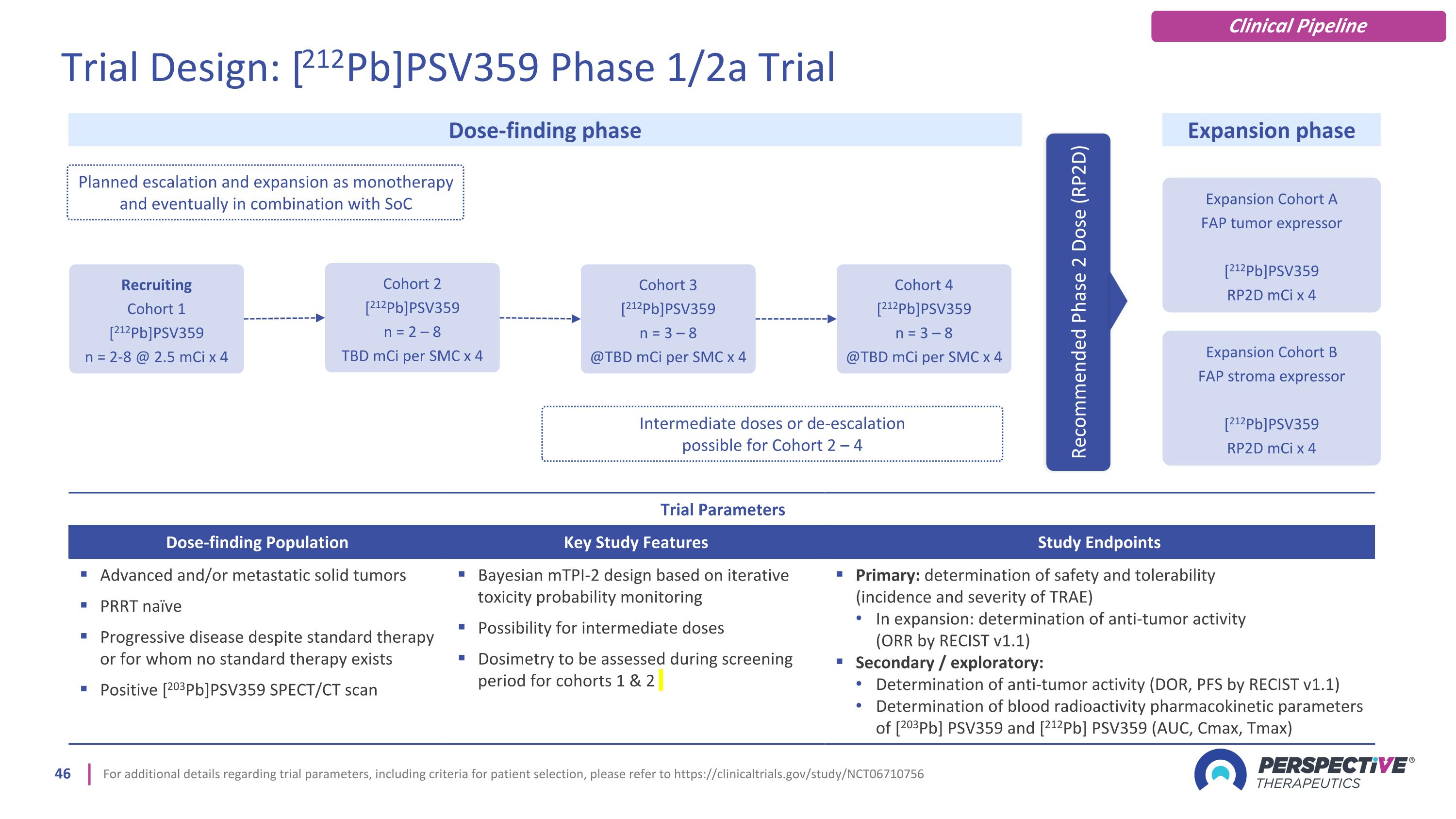
For additional details regarding trial parameters, including criteria for patient selection, please refer to https://clinicaltrials.gov/study/NCT06710756 Trial Parameters Dose-finding Population Key Study Features Study Endpoints Advanced and/or metastatic solid tumors PRRT naïve Progressive disease despite standard therapy or for whom no standard therapy exists Positive [203Pb]PSV359 SPECT/CT scan Bayesian mTPI-2 design based on iterative toxicity probability monitoring Possibility for intermediate doses Dosimetry to be assessed during screening period for cohorts 1 & 2 Primary: determination of safety and tolerability (incidence and severity of TRAE) In expansion: determination of anti-tumor activity (ORR by RECIST v1.1) Secondary / exploratory: Determination of anti-tumor activity (DOR, PFS by RECIST v1.1) Determination of blood radioactivity pharmacokinetic parameters of [203Pb] PSV359 and [212Pb] PSV359 (AUC, Cmax, Tmax) Expansion Cohort A FAP tumor expressor [212Pb]PSV359 RP2D mCi x 4 Dose-finding phase Expansion phase Planned escalation and expansion as monotherapy and eventually in combination with SoC Recommended Phase 2 Dose (RP2D) Recruiting Cohort 1 [212Pb]PSV359 n = 2-8 @ 2.5 mCi x 4 Cohort 2 [212Pb]PSV359 n = 2 – 8 TBD mCi per SMC x 4 Cohort 3 [212Pb]PSV359 n = 3 – 8 @TBD mCi per SMC x 4 Cohort 4 [212Pb]PSV359 n = 3 – 8 @TBD mCi per SMC x 4 Intermediate doses or de-escalation possible for Cohort 2 – 4 Trial Design: [212Pb]PSV359 Phase 1/2a Trial Clinical Pipeline Expansion Cohort B FAP stroma expressor [212Pb]PSV359 RP2D mCi x 4
Source: Cagle et al. European Association of Nuclear Medicine 2024, Presentation Number: OP-473 Conclusion & Next Steps Preclinical data and first-in-human imaging data supportive of FIH therapeutic study in 2025 Clinical Pipeline Strong scientific rationale FAP-ɑ is a pan-cancer target that is abundantly expressed in certain cancer cells as well as cancer-associated fibroblasts in tumor lesions and involved in promoting disease progression Perspective’s in-house discovery team has developed an optimized peptide with potential best-in-class characteristics as demonstrated in preclinical models Imaging agent has favorable characteristics First-in-human clinical SPECT/CT imaging revealed strong tumor uptake, fast clearance through the renal system, low accumulation in normal organs, and long tumor retention in three patients with FAP-expressing cancer Therapeutic study enrolling First patient dosed in the therapeutic study in April 2025 Activation activities are underway for additional sites

General Corporate Information

Deeply Experienced Management Team in Radiopharmaceuticals and Oncology Drug Development Michael Schultz, PHD Chief Science Officer Thijs Spoor Chief Executive Officer Jonathan Hunt Chief Accounting Officer Markus Puhlmann, MD MBA Chief Medical Officer Amos Hedt Chief Business Strategy Officer 20+ years of expertise in biotechnology companies; public and private companies; oncology and nuclear pharmacy 20+ years of oncology drug development across all phases, experience coordinating multiple regulatory filings 20+ years industry and research experience in radiopharmaceuticals; co-founder Viewpoint MT & inventor of Perspective products 20+ years of expertise in financial controls and public accounting for large and small companies across multiple industries 20+ years of expertise in early-stage pharmaceutical and biotech drug development; 10+ years in radiopharmaceuticals 20+ years of experience in life sciences globally, including as CFO of an emerging growth biopharmaceutical company after nearly two decades in Big Pharma Juan Graham Chief Financial Officer
8 Issued Patents (Expiry in 2037 and 2040, with potential PTE) Composition of matter for melanoma targeted radiopharmaceutical compounds; methods of combination therapies; methods of creating radiopharmaceutical compounds using Pb-specific chelators 3 Recently Allowed US and CA Patent Applications VMT-α-NET compounds, AlphaPRIME lead generation system and methods of creating radiopharmaceutical compounds using Pb-specific chelator 71 pending patent applications (including 9 PCT, 55 national, and 7 provisional) Composition of matter and method of use on: Radiometal separation technology Chelators SSTR2, MC1R, FAP, and PSMA targeting radiopharmaceuticals Pre-targeting technology platforms Methods of imaging and treatment Perspective-owned/Exclusively-licensed IP Strong Intellectual Property Portfolio As of May 8, 2025 IP Portfolio covers all aspects of radiopharmaceutical value chain Potential for Orphan Drug Designation
Funding into late 2026 Strong Financial Position Cash, cash equivalents and short-term investments as of March 31, 2025 ~$212 million Share count as of March 31, 2025 ~74.1 million Outstanding common stock warrants & options as of March 31, 2025 ~10.6 million Outstanding pre-funded warrants as of March 31, 2025 ~0.1 million Based on our current plans, which include advancing current clinical programs based on data readouts, progressing multiple pre-IND assets towards clinical trials, as well as building out regional manufacturing sites, we expect to have sufficient funding into late 2026.1 1As of filing date of Company’s Q1 2025 Form 10-Q, based on cash, cash equivalents and short-term investments as of March 31, 2025


Abbreviations AE: adverse event APC: antigen-presenting cell ASCO-GI: ASCO Gastrointestinal Cancers Symposium CTLA-4: human cytotoxic T-lymphocyte antigen 4 DAMPs: damage-associated molecular patterns DLT: dose limiting toxicities DOR: duration of response dsDNA: double-stranded DNA EANM: European Association of Nuclear Medicine eGFR: estimated glomerular filtration rate ER+ BC: estrogen receptor-positive breast cancer FDA: U.S. Food and Drug Administration GEP-NETs: gastroenteropancreatic-NETs GI: gastrointestinal GU: genitourinary GYN: gynecology H&N: head and neck HR: hazard ratio ICI: immune checkpoint inhibitor IND: investigational new drug LAG-3: lymphocyte activation gene-3 mAB: monoclonal antibodies MBq: megabecquerel MC1R: melanocortin 1 receptor mCi: millicurie MFD: maximum feasible radioactivity dose MIP: maximum intensity projection MTD: maximum tolerated dose mTPI-2: modified toxicity probability index NANETS: North American Neuroendocrine Tumor Society NETs: neuroendocrine tumor ORR: objective response rate OS: overall survival PD: progressive disease PD-1: programmed cell death receptor-1 PD-L1: programmed cell death 1 ligand PFS: progression free survival PoC: proof of concept PR: partial response PRRT: peptide receptor radionuclide therapy PSMA: prostate specific membrane antigen RP2D: recommended phase 2 dose RPT: radiopharmaceutical therapies SAE: serious adverse event SCLC: small cell lung cancer SD: stable disease SMC: safety monitoring committee SNMMI: Society of Nuclear Medicine and Molecular Imaging SOC: standard of care SSTR2: somatostatin receptor type 2 TEAE: treatment emergent adverse events TKI: tyrosine kinase inhibitors TME: tumor microenvironment TRAE: treatment related adverse events USPI: U.S. prescribing information
APPENDIX
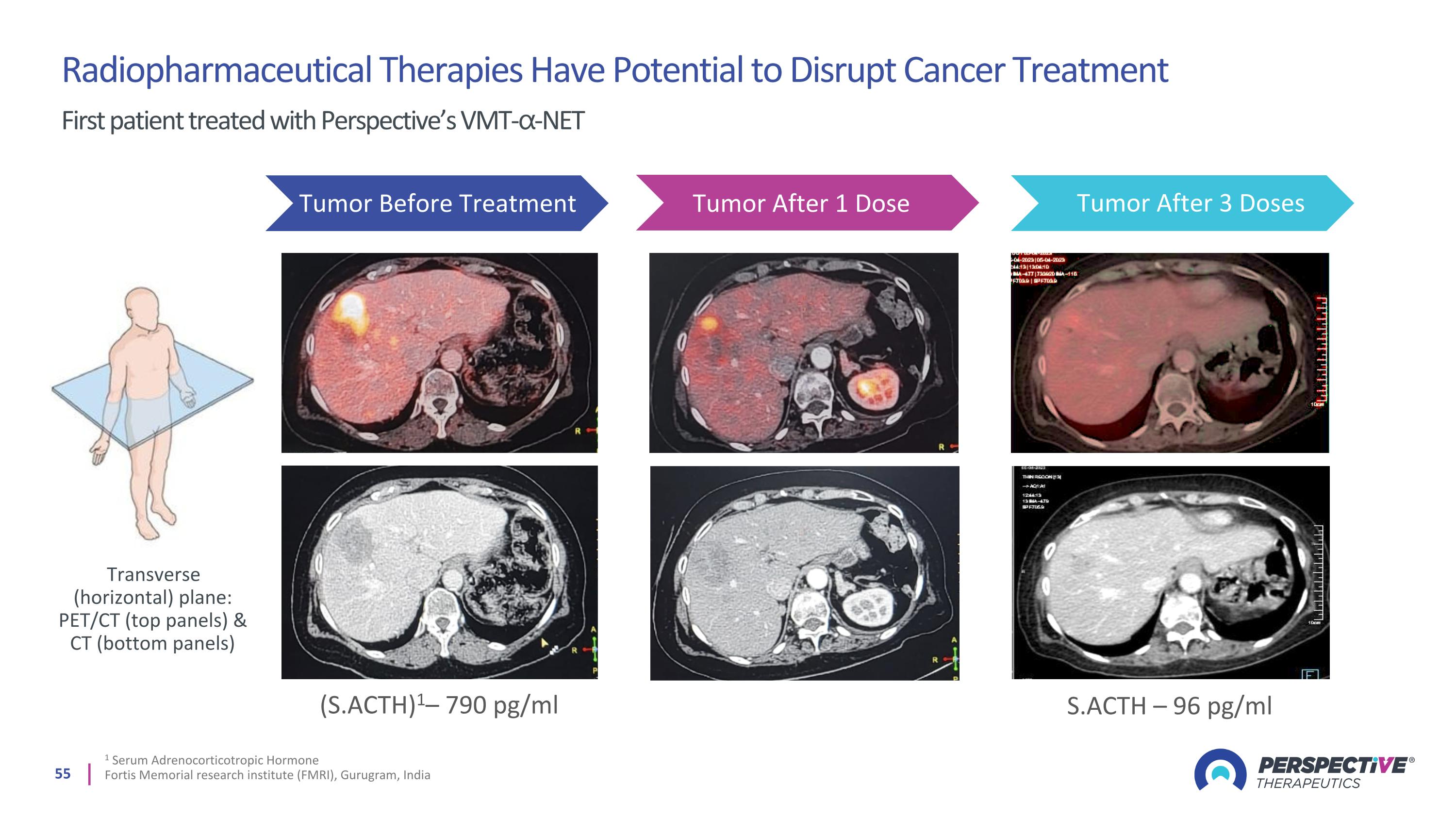
1 Serum Adrenocorticotropic Hormone Fortis Memorial research institute (FMRI), Gurugram, India (S.ACTH)1– 790 pg/ml S.ACTH – 96 pg/ml Tumor Before Treatment Tumor After 1 Dose Tumor After 3 Doses Radiopharmaceutical Therapies Have Potential to Disrupt Cancer Treatment First patient treated with Perspective’s VMT-⍺-NET Transverse (horizontal) plane: PET/CT (top panels) & CT (bottom panels)
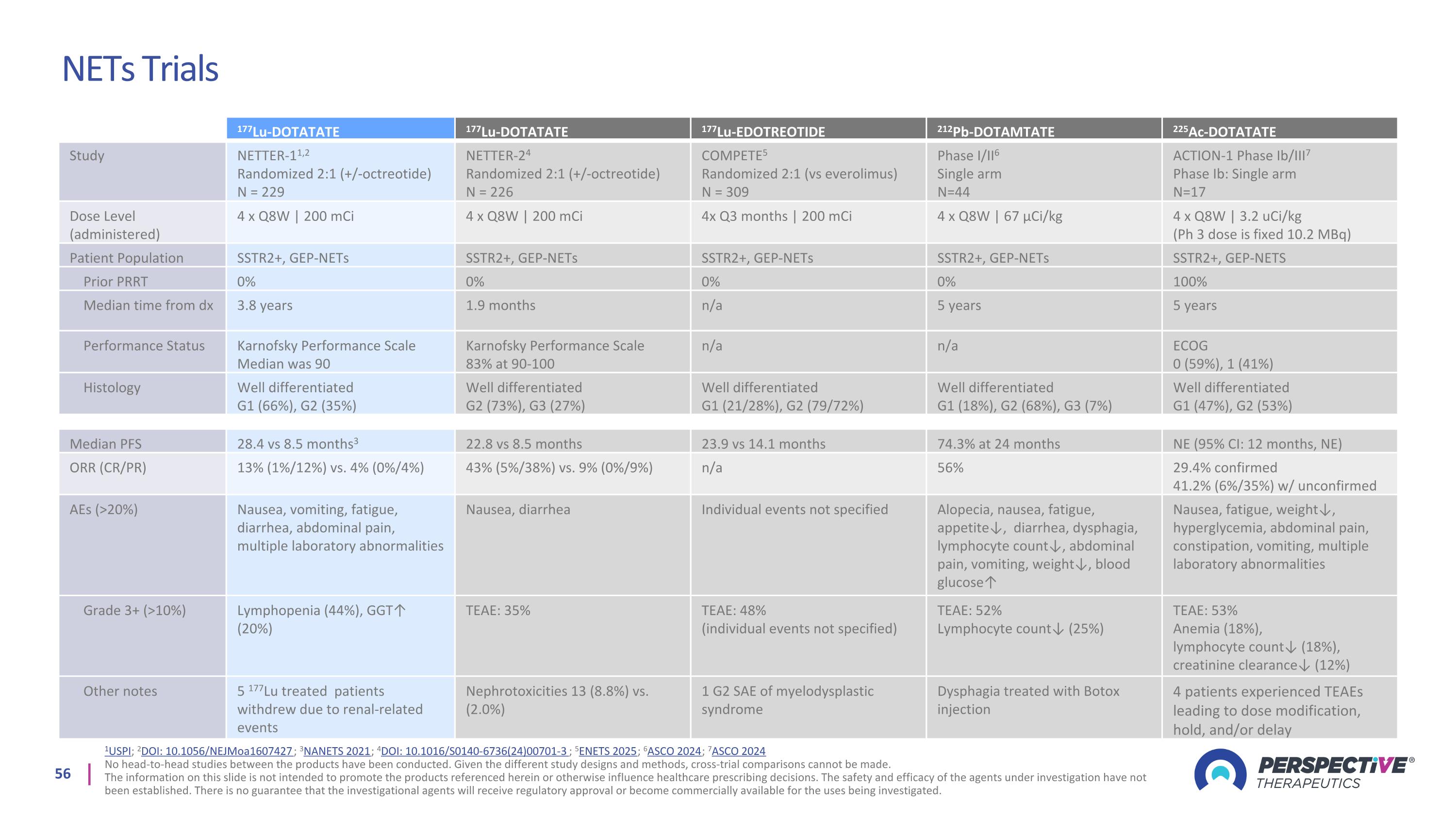
NETs Trials 1USPI; 2DOI: 10.1056/NEJMoa1607427; 3NANETS 2021; 4DOI: 10.1016/S0140-6736(24)00701-3; 5ENETS 2025; 6ASCO 2024; 7ASCO 2024 No head-to-head studies between the products have been conducted. Given the different study designs and methods, cross-trial comparisons cannot be made. The information on this slide is not intended to promote the products referenced herein or otherwise influence healthcare prescribing decisions. The safety and efficacy of the agents under investigation have not been established. There is no guarantee that the investigational agents will receive regulatory approval or become commercially available for the uses being investigated. 177Lu-DOTATATE 177Lu-DOTATATE 177Lu-EDOTREOTIDE 212Pb-DOTAMTATE 225Ac-DOTATATE Study NETTER-11,2 Randomized 2:1 (+/-octreotide) N = 229 NETTER-24 Randomized 2:1 (+/-octreotide) N = 226 COMPETE5 Randomized 2:1 (vs everolimus) N = 309 Phase I/II6 Single arm N=44 ACTION-1 Phase Ib/III7 Phase Ib: Single arm N=17 Dose Level (administered) 4 x Q8W | 200 mCi 4 x Q8W | 200 mCi 4x Q3 months | 200 mCi 4 x Q8W | 67 µCi/kg 4 x Q8W | 3.2 uCi/kg (Ph 3 dose is fixed 10.2 MBq) Patient Population SSTR2+, GEP-NETs SSTR2+, GEP-NETs SSTR2+, GEP-NETs SSTR2+, GEP-NETs SSTR2+, GEP-NETS Prior PRRT 0% 0% 0% 0% 100% Median time from dx 3.8 years 1.9 months n/a 5 years 5 years Performance Status Karnofsky Performance Scale Median was 90 Karnofsky Performance Scale 83% at 90-100 n/a n/a ECOG 0 (59%), 1 (41%) Histology Well differentiated G1 (66%), G2 (35%) Well differentiated G2 (73%), G3 (27%) Well differentiated G1 (21/28%), G2 (79/72%) Well differentiated G1 (18%), G2 (68%), G3 (7%) Well differentiated G1 (47%), G2 (53%) Median PFS 28.4 vs 8.5 months3 22.8 vs 8.5 months 23.9 vs 14.1 months 74.3% at 24 months NE (95% CI: 12 months, NE) ORR (CR/PR) 13% (1%/12%) vs. 4% (0%/4%) 43% (5%/38%) vs. 9% (0%/9%) n/a 56% 29.4% confirmed 41.2% (6%/35%) w/ unconfirmed AEs (>20%) Nausea, vomiting, fatigue, diarrhea, abdominal pain, multiple laboratory abnormalities Nausea, diarrhea Individual events not specified Alopecia, nausea, fatigue, appetite↓, diarrhea, dysphagia, lymphocyte count↓, abdominal pain, vomiting, weight↓, blood glucose↑ Nausea, fatigue, weight↓, hyperglycemia, abdominal pain, constipation, vomiting, multiple laboratory abnormalities Grade 3+ (>10%) Lymphopenia (44%), GGT↑ (20%) TEAE: 35% TEAE: 48% (individual events not specified) TEAE: 52% Lymphocyte count↓ (25%) TEAE: 53% Anemia (18%), lymphocyte count↓ (18%), creatinine clearance↓ (12%) Other notes 5 177Lu treated patients withdrew due to renal-related events Nephrotoxicities 13 (8.8%) vs. (2.0%) 1 G2 SAE of myelodysplastic syndrome Dysphagia treated with Botox injection 4 patients experienced TEAEs leading to dose modification, hold, and/or delay
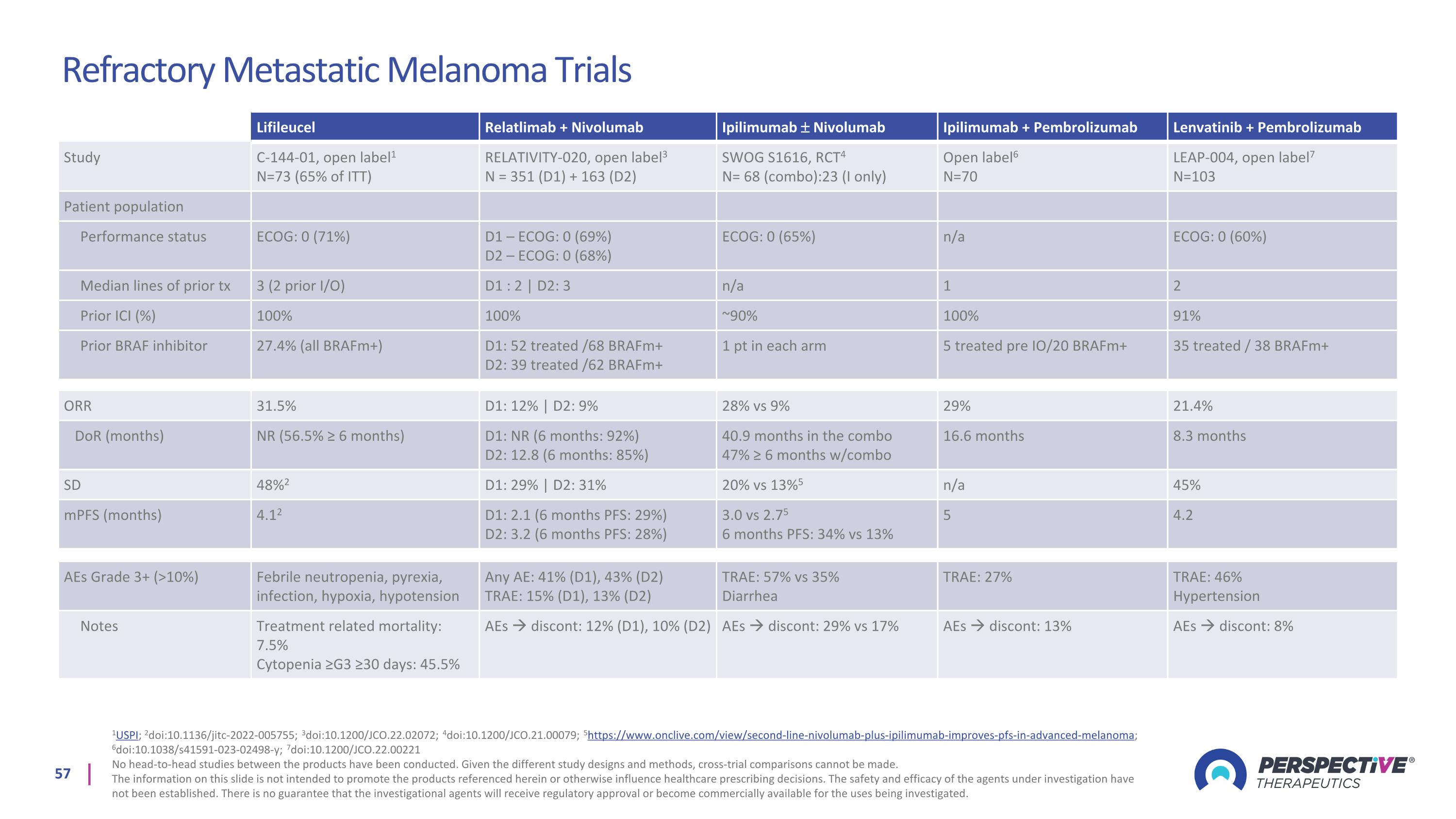
Refractory Metastatic Melanoma Trials 1USPI; 2doi:10.1136/jitc-2022-005755; 3doi:10.1200/JCO.22.02072; 4doi:10.1200/JCO.21.00079; 5https://www.onclive.com/view/second-line-nivolumab-plus-ipilimumab-improves-pfs-in-advanced-melanoma; 6doi:10.1038/s41591-023-02498-y; 7doi:10.1200/JCO.22.00221 No head-to-head studies between the products have been conducted. Given the different study designs and methods, cross-trial comparisons cannot be made. The information on this slide is not intended to promote the products referenced herein or otherwise influence healthcare prescribing decisions. The safety and efficacy of the agents under investigation have not been established. There is no guarantee that the investigational agents will receive regulatory approval or become commercially available for the uses being investigated. Lifileucel Relatlimab + Nivolumab Ipilimumab Nivolumab Ipilimumab + Pembrolizumab Lenvatinib + Pembrolizumab Study C-144-01, open label1 N=73 (65% of ITT) RELATIVITY-020, open label3 N = 351 (D1) + 163 (D2) SWOG S1616, RCT4 N= 68 (combo):23 (I only) Open label6 N=70 LEAP-004, open label7 N=103 Patient population Performance status ECOG: 0 (71%) D1 – ECOG: 0 (69%) D2 – ECOG: 0 (68%) ECOG: 0 (65%) n/a ECOG: 0 (60%) Median lines of prior tx 3 (2 prior I/O) D1 : 2 | D2: 3 n/a 1 2 Prior ICI (%) 100% 100% ~90% 100% 91% Prior BRAF inhibitor 27.4% (all BRAFm+) D1: 52 treated /68 BRAFm+ D2: 39 treated /62 BRAFm+ 1 pt in each arm 5 treated pre IO/20 BRAFm+ 35 treated / 38 BRAFm+ ORR 31.5% D1: 12% | D2: 9% 28% vs 9% 29% 21.4% DoR (months) NR (56.5% ≥ 6 months) D1: NR (6 months: 92%) D2: 12.8 (6 months: 85%) 40.9 months in the combo 47% ≥ 6 months w/combo 16.6 months 8.3 months SD 48%2 D1: 29% | D2: 31% 20% vs 13%5 n/a 45% mPFS (months) 4.12 D1: 2.1 (6 months PFS: 29%) D2: 3.2 (6 months PFS: 28%) 3.0 vs 2.75 6 months PFS: 34% vs 13% 5 4.2 AEs Grade 3+ (>10%) Febrile neutropenia, pyrexia, infection, hypoxia, hypotension Any AE: 41% (D1), 43% (D2) TRAE: 15% (D1), 13% (D2) TRAE: 57% vs 35% Diarrhea TRAE: 27% TRAE: 46% Hypertension Notes Treatment related mortality: 7.5% Cytopenia ≥G3 ≥30 days: 45.5% AEs discont: 12% (D1), 10% (D2) AEs discont: 29% vs 17% AEs discont: 13% AEs discont: 8%
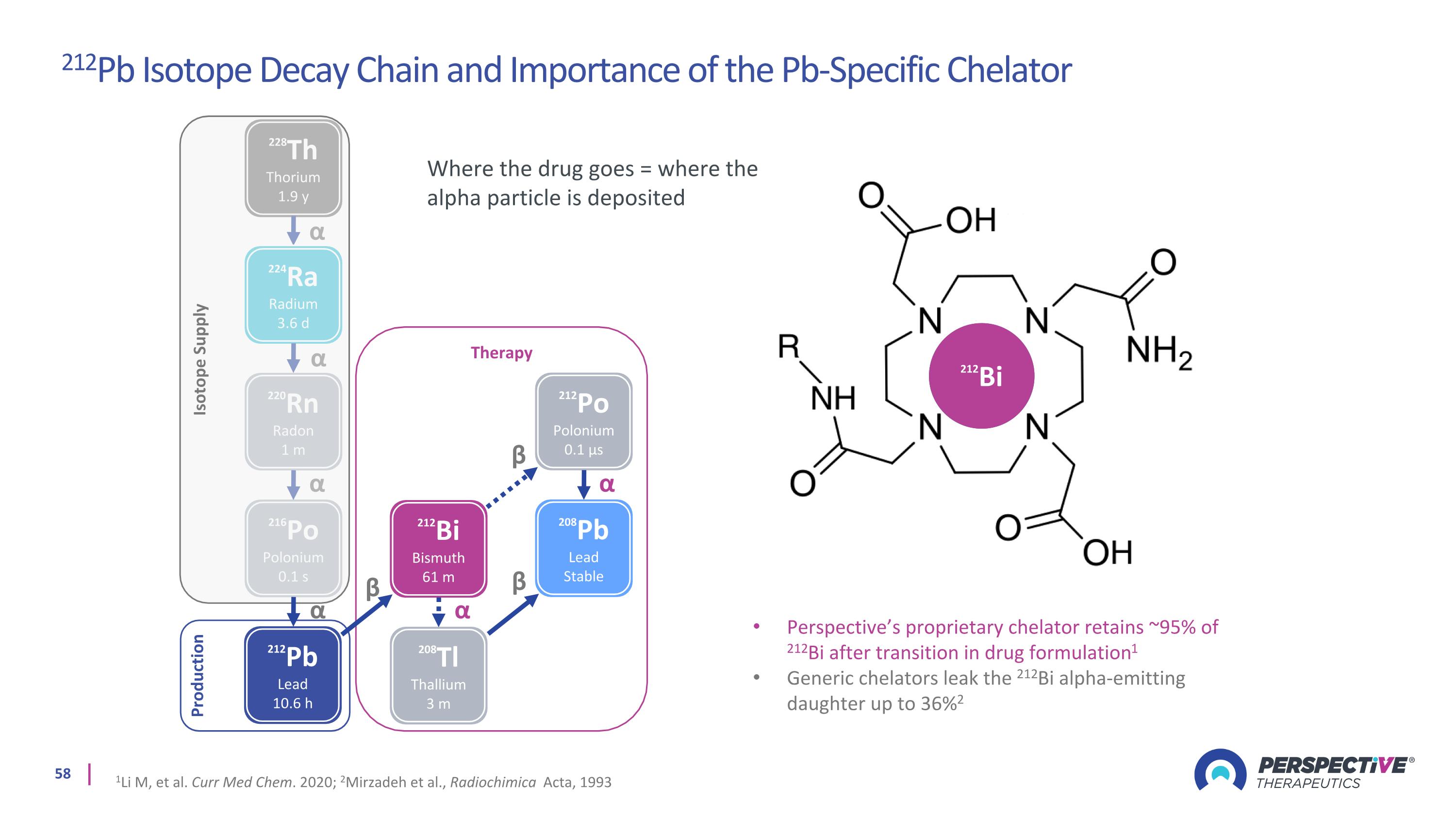
Therapy 228Th Thorium 1.9 y 224Ra Radium 3.6 d 220Rn Radon 1 m 216Po Polonium 0.1 s 212Bi Bismuth 61 m 208Tl Thallium 3 m 212Po Polonium 0.1 µs 208Pb Lead Stable 212Pb Lead 10.6 h α α α α α α β β β Isotope Supply Production α β 212Pb 212Bi Perspective’s proprietary chelator retains ~95% of 212Bi after transition in drug formulation1 Generic chelators leak the 212Bi alpha-emitting daughter up to 36%2 1Li M, et al. Curr Med Chem. 2020; 2Mirzadeh et al., Radiochimica Acta, 1993 Where the drug goes = where the alpha particle is deposited 212Pb Isotope Decay Chain and Importance of the Pb-Specific Chelator
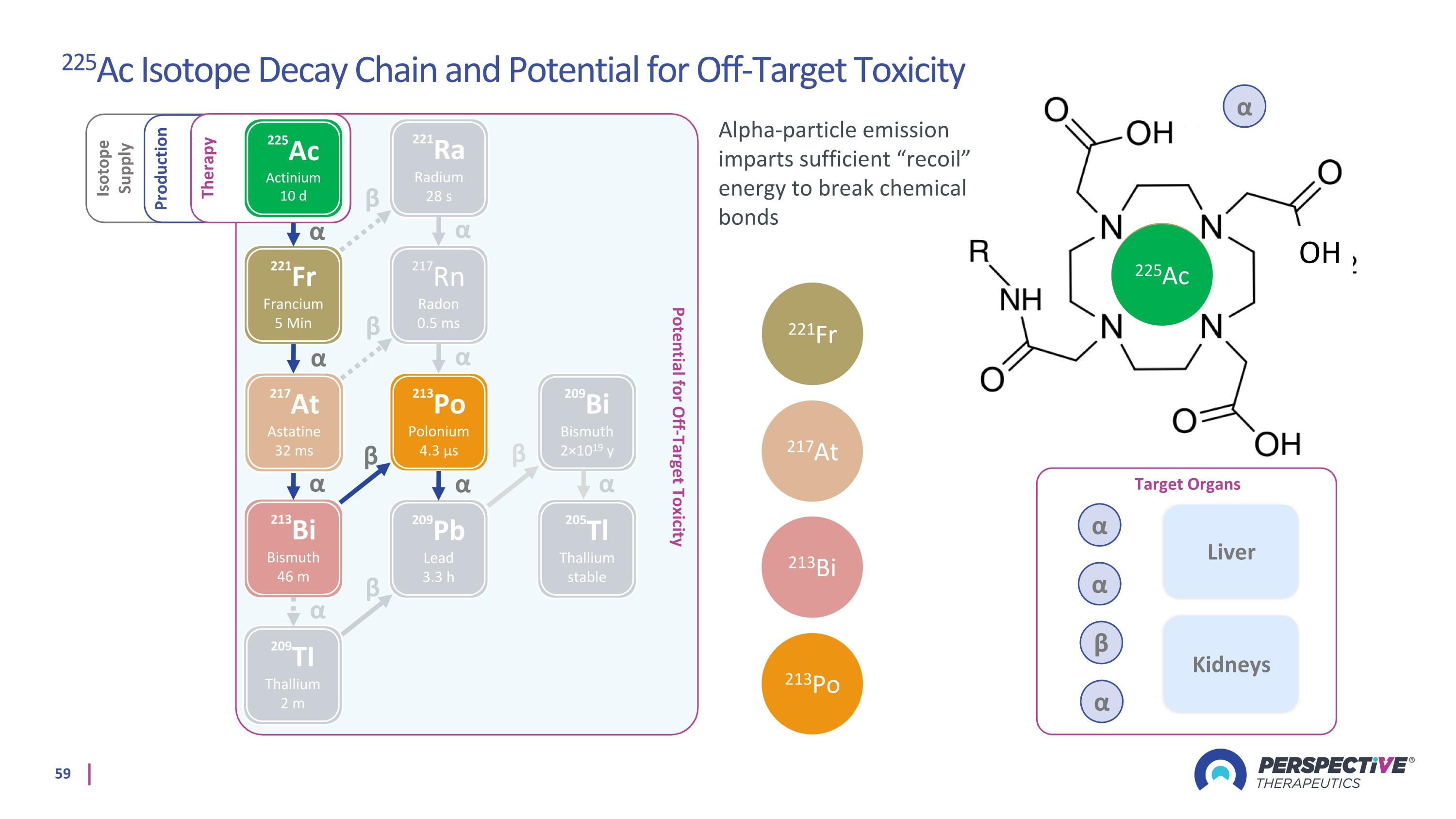
α α ꞵ α OH Potential for Off-Target Toxicity 221Fr Francium 5 Min 217Rn Radon 0.5 ms 213Bi Bismuth 46 m 209Pb Lead 3.3 h 213Po Polonium 4.3 µs 209TI Thallium 2 m α α α α α β β Isotope Supply Production 221Ra Radium 28 s 217At Astatine 32 ms 205Tl Thallium stable 209Bi Bismuth 2×1019 y β β α α α β Therapy 225Ac Actinium 10 d α 221Fr 217At 213Bi 213Po 225Ac Target Organs Liver Kidneys Alpha-particle emission imparts sufficient “recoil” energy to break chemical bonds 225Ac Isotope Decay Chain and Potential for Off-Target Toxicity α α β α 221Fr α
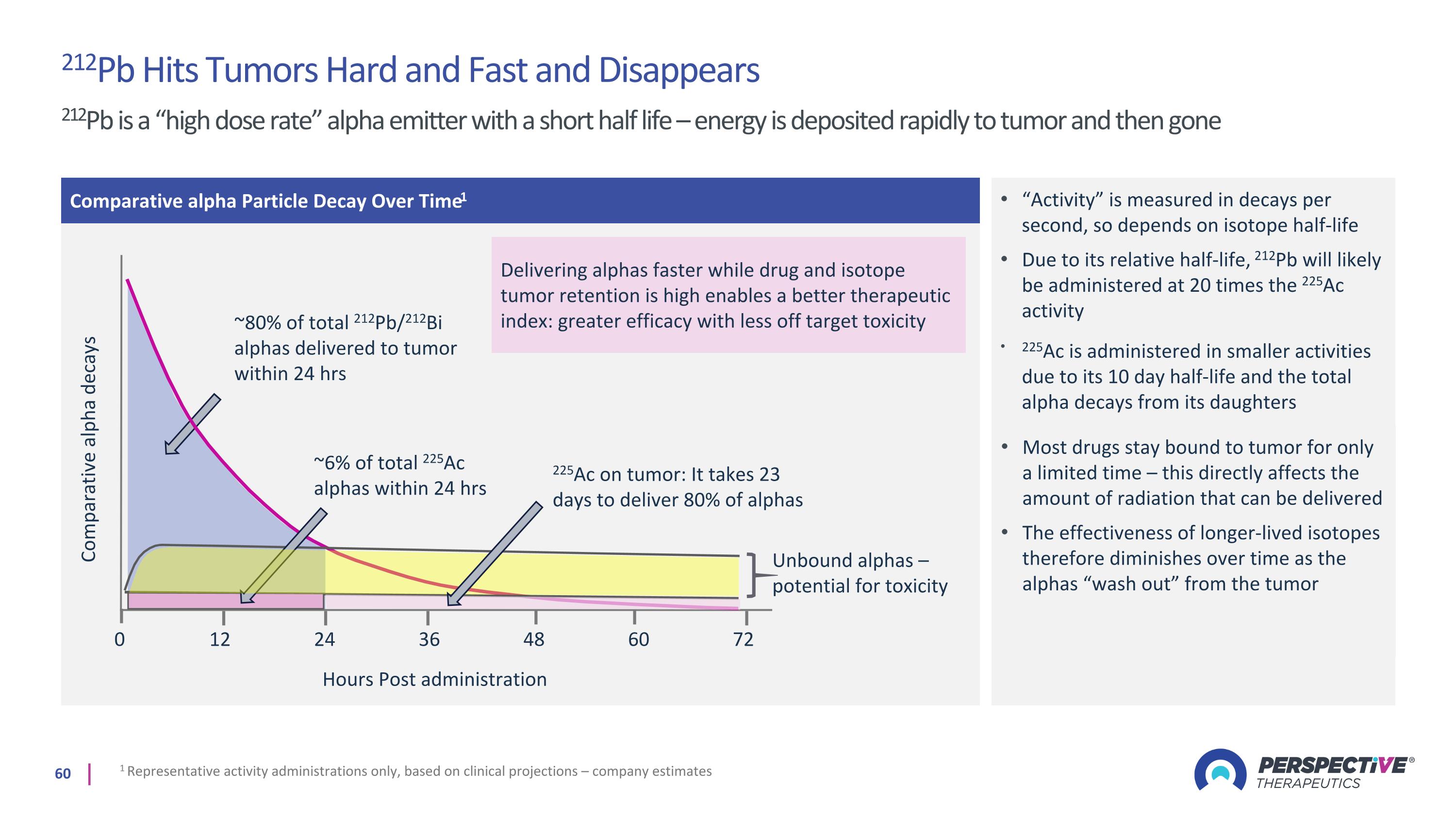
212Pb Hits Tumors Hard and Fast and Disappears 212Pb is a “high dose rate” alpha emitter with a short half life – energy is deposited rapidly to tumor and then gone Comparative alpha Particle Decay Over Time1 Comparative alpha decays 0 ~80% of total 212Pb/212Bi alphas delivered to tumor within 24 hrs Hours Post administration 12 24 36 48 60 72 “Activity” is measured in decays per second, so depends on isotope half-life Due to its relative half-life, 212Pb will likely be administered at 20 times the 225Ac activity 1 Representative activity administrations only, based on clinical projections – company estimates ~6% of total 225Ac alphas within 24 hrs 225Ac is administered in smaller activities due to its 10 day half-life and the total alpha decays from its daughters 225Ac on tumor: It takes 23 days to deliver 80% of alphas Unbound alphas – potential for toxicity Most drugs stay bound to tumor for only a limited time – this directly affects the amount of radiation that can be delivered The effectiveness of longer-lived isotopes therefore diminishes over time as the alphas “wash out” from the tumor Delivering alphas faster while drug and isotope tumor retention is high enables a better therapeutic index: greater efficacy with less off target toxicity
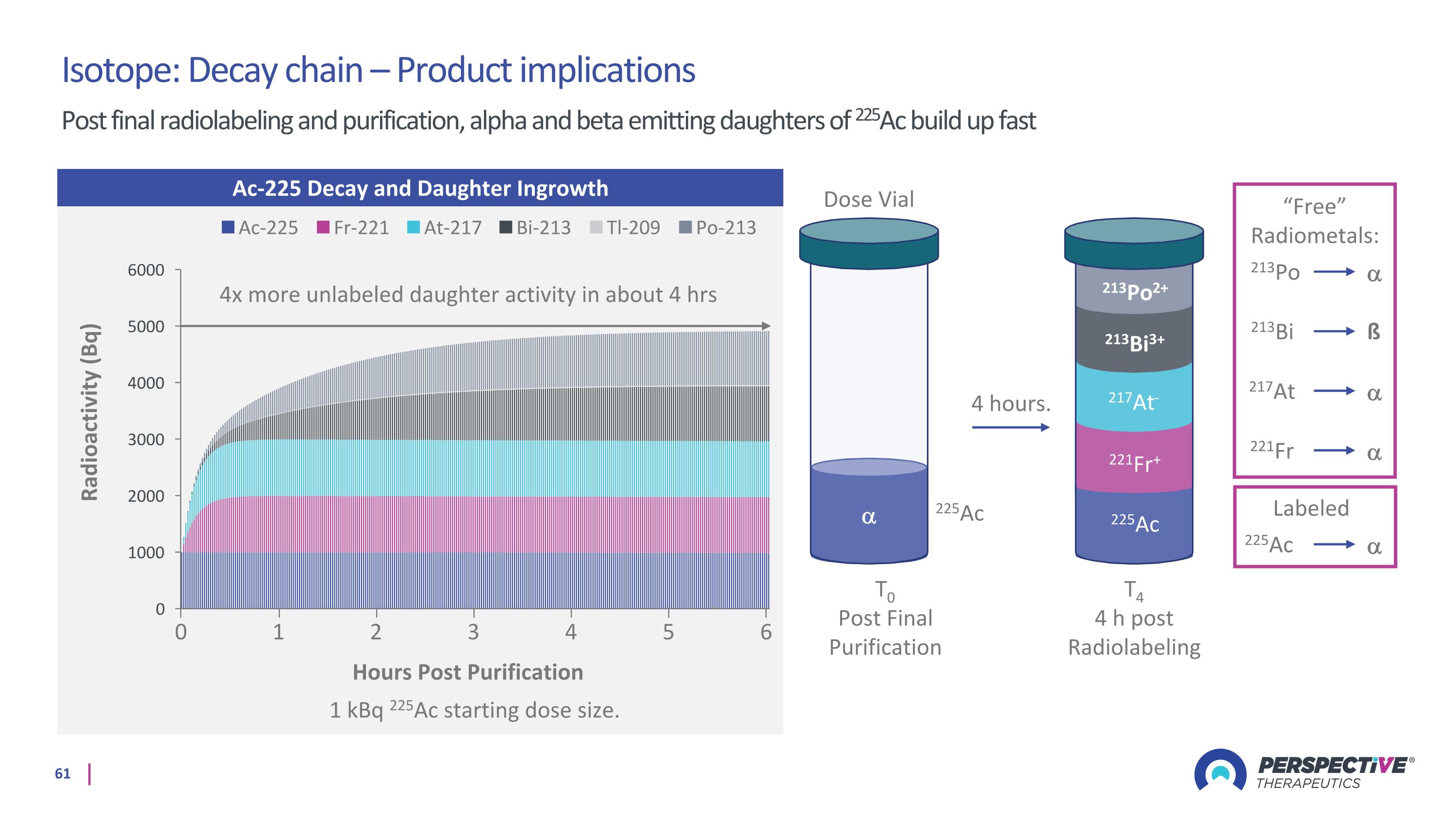
T0 Post Final Purification Dose Vial 225Ac 225Ac 221Fr 217At 213Po 213Bi ß “Free” Radiometals: Labeled 4 hours. T4 4 h post Radiolabeling 221Fr+ 217At- 213Bi3+ 213Po2+ 225Ac Ac-225 Decay and Daughter Ingrowth 1 kBq 225Ac starting dose size. Hours Post Purification 0 2 3 4 5 6 1 4x more unlabeled daughter activity in about 4 hrs Isotope: Decay chain – Product implications Post final radiolabeling and purification, alpha and beta emitting daughters of 225Ac build up fast
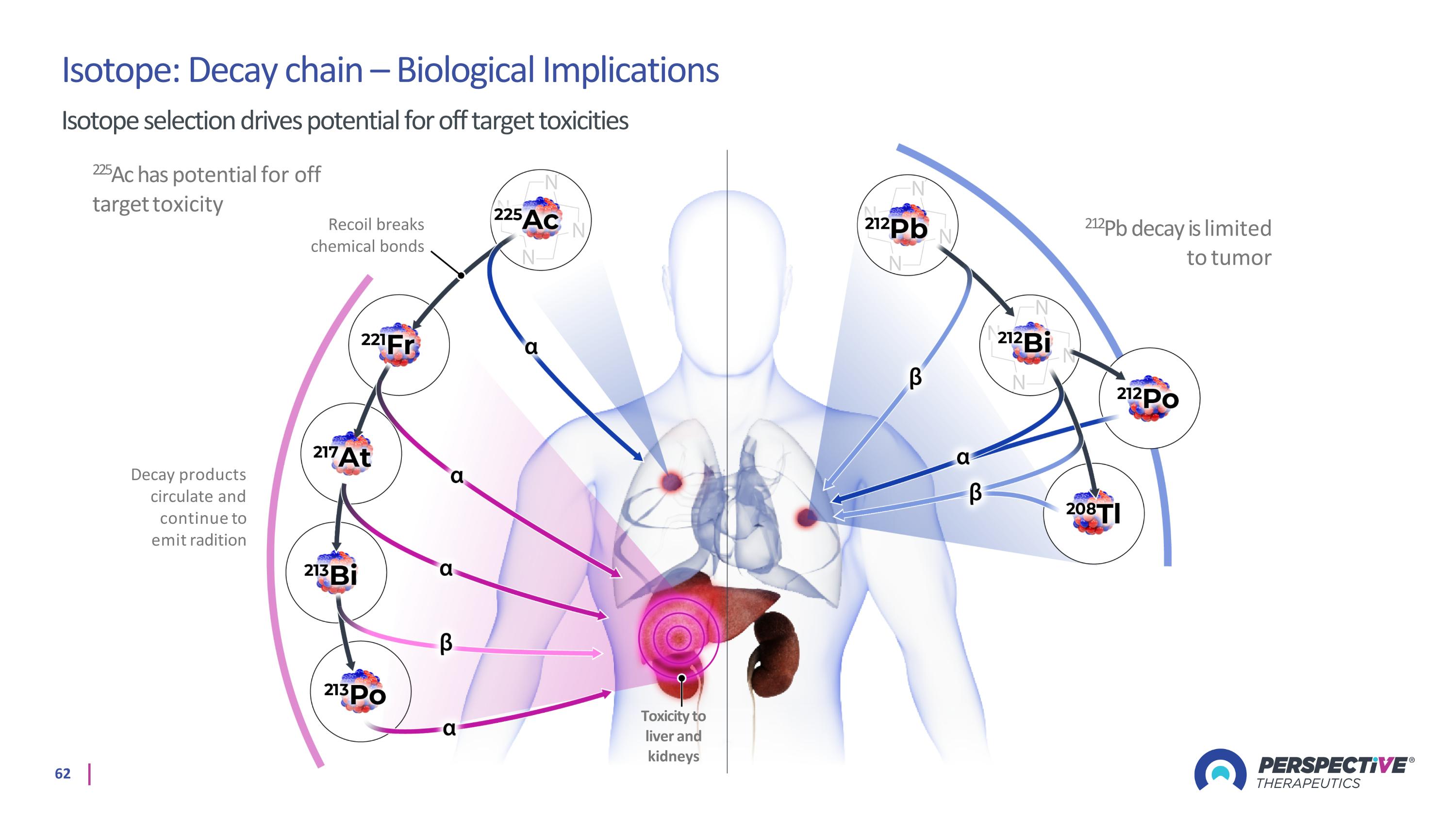
Isotope: Decay chain – Biological Implications Isotope selection drives potential for off target toxicities 225Ac has potential for off target toxicity Decay products circulate and continue to emit radition 212Pb decay is limited to tumor Toxicity to liver and kidneys Recoil breaks chemical bonds
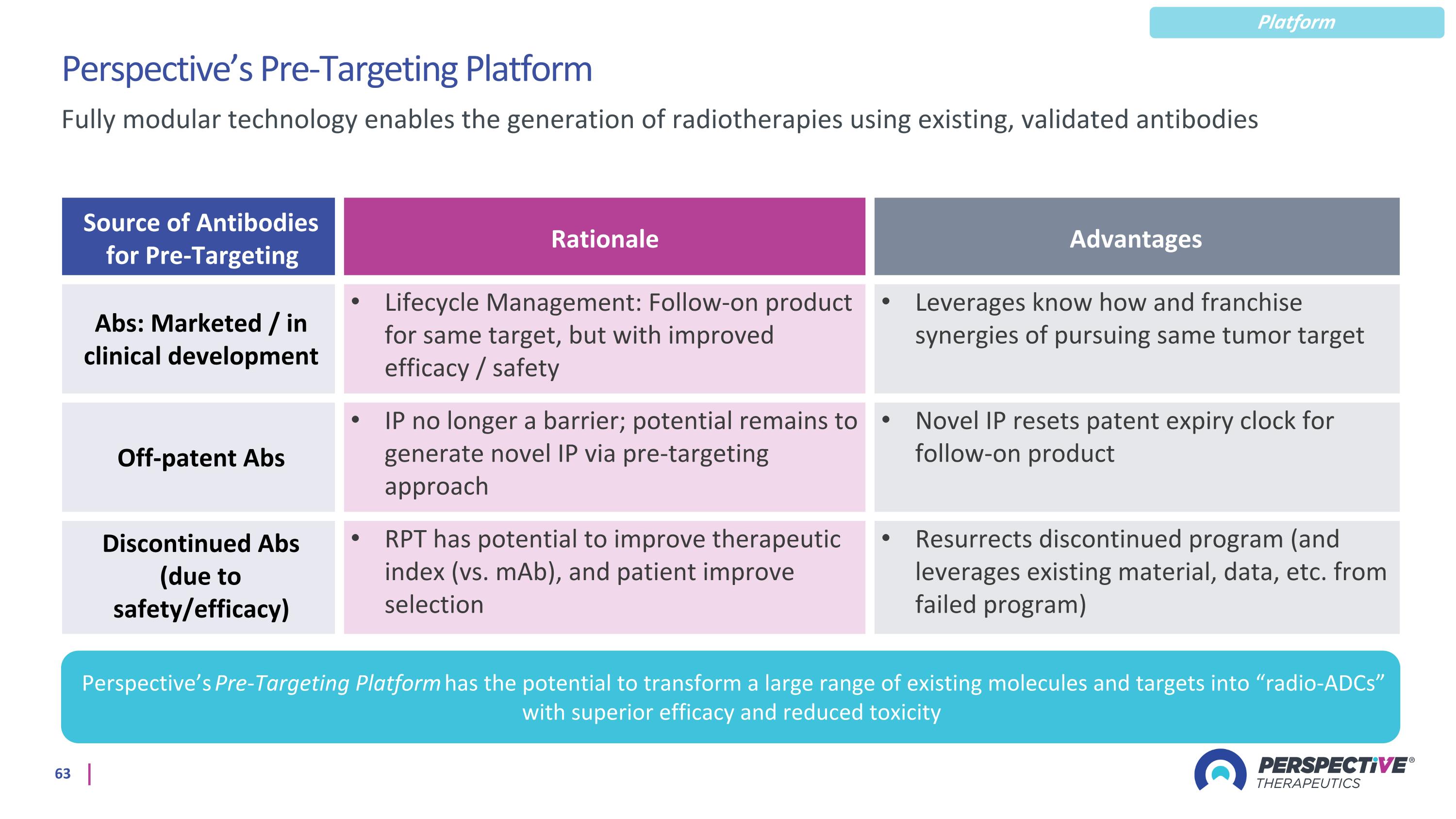
Perspective’s Pre-Targeting Platform Source of Antibodies for Pre-Targeting Rationale Advantages Abs: Marketed / in clinical development Lifecycle Management: Follow-on product for same target, but with improved efficacy / safety Leverages know how and franchise synergies of pursuing same tumor target Off-patent Abs IP no longer a barrier; potential remains to generate novel IP via pre-targeting approach Novel IP resets patent expiry clock for follow-on product Discontinued Abs (due to safety/efficacy) RPT has potential to improve therapeutic index (vs. mAb), and patient improve selection Resurrects discontinued program (and leverages existing material, data, etc. from failed program) Fully modular technology enables the generation of radiotherapies using existing, validated antibodies Perspective’s Pre-Targeting Platform has the potential to transform a large range of existing molecules and targets into “radio-ADCs” with superior efficacy and reduced toxicity Platform