
EXHIBIT 99.2

Test report for the VKIN 300 waste pre-treatment unit in accordance with NFX 30503-1 standard.
Hospital Hygiene Laboratory
Clermont-Ferrand University Hospital
May-June 2025
| EC-EFRES 2025 tests | Hospital Hygiene Laboratory CHU Clermont-Fd |
|
| 1 |

|
| ➢ | Airborne microbial contamination tests (NFX 30503-1 Annex A1) |
Methods
Sampling by Sampl'air biocollector (AES Laboratoires) calibrated on 20/10/2024.
Volume collected per sample: 100 liters by impaction on Trypcase Soy Agar (TSA) (Biomérieux, ref 43011).
Samples stored and transported at 4°C until incubation (< 24h).
TSA agar incubated 3 days at 30°C and 2 days at 25°C
Expression in colony-forming units / m3, conversion into log10.
Results
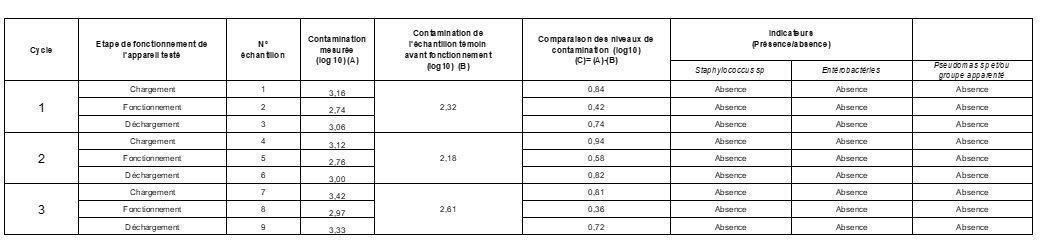
Absence of Staphylococcus spp, enterobacteria, yeasts, Pseudomonas spp and other non-fermentative Gram-negative bacilli
| EC-EFRES 2025 tests | Hospital Hygiene Laboratory CHU Clermont-Fd |
|
| 2 |

|
| ➢ | Treatment tests on biological indicators (NFX 30503-1 Annex A2) |
|
|
|
|
Methods
Description of germ carriers
Germ carrier consisting of square non-woven compresses (LCH ref 44564 Lot 4500005471)
|
| I. | Bacillus and Aspergillus |
Counting of bacterial and fungal suspensions
Dilute stock suspensions to 10.-8 for Bacillus and 10.-9 for Aspergillus in tryptone-salt broth.
- Spread 100 µl of dilutions 10.-5 to 10.-8 for Bacillus on TSA agar
- Spread 100 µl of dilutions 10.-6 to 10.-9 for Aspergillus on Sabouraud agar (Thermo Scientific ref PO5096A)
- Incubation 48-72 h at 30°C
Deposition of microorganisms on germ-bearing swabs
-Bacillus: deposit of 100 µl of bacterial suspension
-Aspergillus: deposition of 10 µl of bacterial suspension
Addition of sheep blood as an interfering substance (Labmedical ref. H04F-0817-P-0025) to the suspension
Inoculation of the suspension by depositing it on the entire germ carrier
After treatment (biological indicators and D curve)
The gauze is transferred to sterile Falcon tubes. Add 3 ml (minimum volume to ensure gauze is well impregnated) of Tryptone salt broth to each tube. Vortex for 5 min.
Suspension dilutions
- transport tube swabs: dilution down to 10-7
- biological indicator swabs and D curves: dilution to 10-4
- spread 100µl of dilutions on TSA agar (Sabouraud for Aspergillus). The remainder of the pure sample is inoculated onto additional Petri dishes in order to analyze the entire sample.
- Incubation 48-72h at 30°C
|
| II. | Adenovirus |
HeLa cell culture
The medium used for HeLa cell growth is MEM medium prepared as follows:
MEM (450 mL) + 10% decomplemented SVF filtered at 0.22 µm (50 mL) + ATB2x (1mL)
Virus: Human Adenovirus 5 (ATCC VR-5 TM) Lot 70053996 Validation date 26/09/2022
Virus culture methods
HeLa cells are seeded in 96-well microplates (Falcon, ref 353072) 48 h prior to infection.
Germ carriers are aseptically placed in a Falcon tube containing 3 mL of MEM medium.
Sonicate for 1 min (Branson 2510), then vortex for at least 2 min.
Series 10 dilution in MEM medium
Inoculation of microplates with pure sample and dilutions (100 µL per well, 8 wells per dilution). The remainder of the pure sample is inoculated into additional wells, so that the whole sample can be analyzed.
Incubate for 2 h at 37°C with gentle agitation, then add 100 µL of MEM medium.
Incubation at 37°C under 5% CO(2).
Read cytopathic effect at 2 and 3 days post-inoculation.
Determination of viral titer by Spearman Kärber method.
| EC-EFRES 2025 tests | Hospital Hygiene Laboratory CHU Clermont-Fd |
|
| 3 |

References of products used for cell and viral cultures:
MEM with glutamine (Dutscher ref. L0416 - 500)
PBS without calcium or magnesium (ref Dutscher L0615 - 500)
Trypsin with phenol red, calcium- and magnesium-free (Dutscher ref. L0930 - 100)
Fetal calf serum
Antibiotics Penicillin Streptomycin Neomycin (ref Sigma P4083)
Results
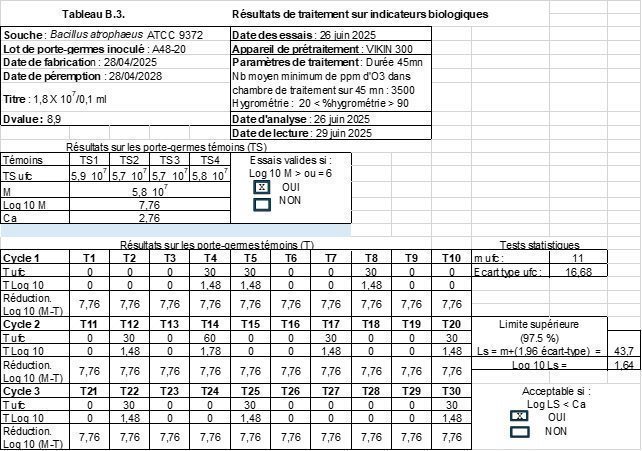
| EC-EFRES 2025 tests | Hospital Hygiene Laboratory CHU Clermont-Fd |
|
| 4 |

|
| ➢ | Treatment tests on infectious waste and reviviscence tests (NFX 30503-1 Annex A3) |
|
|
|
|
Methods
Waste before treatment
- Weigh 5 g of HIW
- Add 45 ml Tryptone salt broth (Dutscher ref 693424)
- Vortex
- Leave in contact at room temperature for 1 hour, vortexing regularly
- Make dilutions down to 10-7 in tryptone-salt broth
- Spread 100 µl of dilutions 10.-4 to 10.-7 on TSA agar plates
-Incubate 48-72h at 30°C
- Count the revivifiable aerobic bacterial flora and test for the presence of Staphylococcus spp, Enterobacteriaceae, yeasts, Pseudomonas spp and other non-fermentative Gram-negative bacilli.
Waste after treatment
- Weigh out 5 g of HIW
- Add 45 ml tryptone-salt broth
- Leave in contact at room temperature for 1 hour, vortexing regularly
- Make dilutions from 10.-1 to 10.-5 in tryptone-salt broth
- Spread 100 µl of the 5 dilutions on TSA agar plates
- Incubate 48-72 h at 30°C
- Count the revivifiable aerobic bacterial flora and test for the presence of Staphylococcus spp, Enterobacteriaceae, yeasts, Pseudomonas spp and other non-fermentative Gram-negative bacilli.
Results (May 7, 8 and 9, 2025)
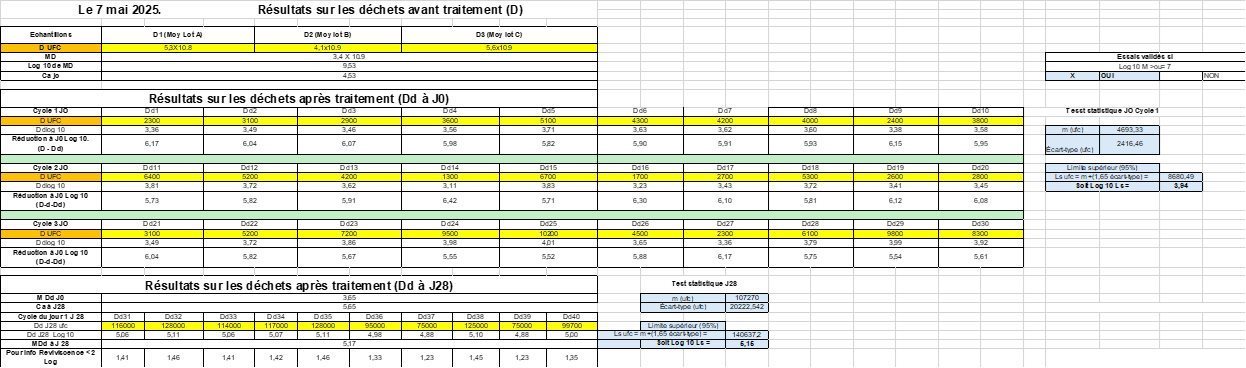
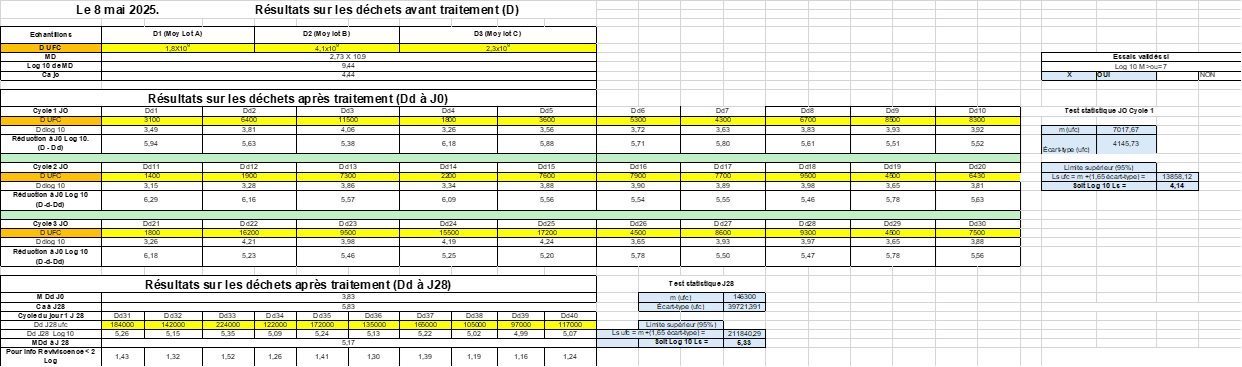
| EC-EFRES 2025 tests | Hospital Hygiene Laboratory CHU Clermont-Fd |
|
| 5 |

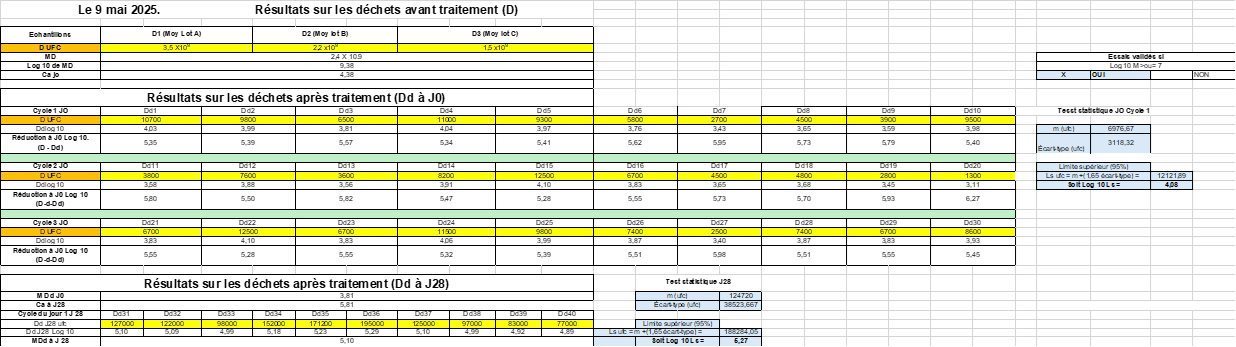
|
| ➢ | Granulometry tests |
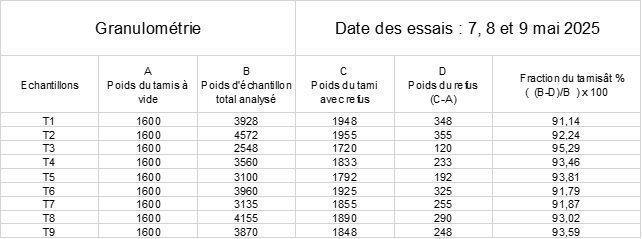
NB: the formula in table B5 of appendix A of the standard is incorrect in column D (reject weight): (C-A)/B should be replaced by (C-A).
Conclusion:
The tests carried out in accordance with standard NFX 30503-1 by the laboratory demonstrate that the VKIN 300 waste pre-treatment unit meets the specifications of this standard.
| EC-EFRES 2025 tests | Hospital Hygiene Laboratory CHU Clermont-Fd |
|
| 6 |

Pr O Traoré
| EC-EFRES 2025 tests | Hospital Hygiene Laboratory CHU Clermont-Fd |
|
| 7 |