| Corporate Overview June 2025 EMPOWERING PATIENTS THROUGH THERAPEUTIC INNOVATION |
| 2 Disclaimer and Cautionary Note Regarding Forward -Looking Statements Any statements contained in this presentation that do not describe historical facts may constitute forward-looking statements as that term is defined in the Private Securities Litigation Reform Act of 1995. These statements may be identified by words such as “anticipate,” “believe,” “expect,” “intend,” “may,” “plan,” “potential,” “will,” and similar expressions, and are based on Aclaris’ current beliefs and expectations. These forward-looking statements include expectations regarding the therapeutic potential of Aclaris' drug candidates, including bosakitug (ATI-045), ATI-052 and ATI-2138, to provide meaningful benefit to patients suffering from atopic dermatitis, COPD, asthma and/or other indications, the development of Aclaris’ drug candidates, including bosakitug, ATI-052, ATI-2138 and undisclosed next generation selective ITK inhibitors and bispecific antibodies, the timing of regulatory filings and initiation of clinical trials, the availability and timing of data from clinical trials, the potential of bosakitug to have extended dosing, and Aclaris’ cash runway, including the ability to fully fund preclinical and clinical development plans and the potential to extend the cash runway through non-dilutive opportunities. These statements involve risks and uncertainties that could cause actual results to differ materially from those reflected in such statements. Risks and uncertainties that may cause actual results to differ materially include uncertainties inherent in the conduct of clinical trials, Aclaris’ reliance on third parties over which it may not always have full control, Aclaris’ ability to enter into strategic partnerships on commercially reasonable terms, the uncertainty regarding the macroeconomic environment and other risks and uncertainties that are described in the “Risk Factors” section of Aclaris’ Annual Report on Form 10-K for the year ended December 31, 2024, and other filings Aclaris makes with the U.S. Securities and Exchange Commission from time to time. These documents are available under the “SEC Filings” page of the “Investors” section of Aclaris’ website at www.aclaristx.com. Any forward-looking statements speak only as of the date of this presentation and are based on information available to Aclaris as of the date of this presentation, and Aclaris assumes no obligation to, and does not intend to, update any forward-looking statements, whether as a result of new information, future events or otherwise. This presentation also contains estimates and other statistical data made by independent parties and by us relating to market size and other data about our industry. These data involve a number of assumptions and limitations, and you are cautioned not to give undue weight to such estimates. In addition, projections, assumptions and estimates of our future performance and the future performance of the markets in which we operate are necessarily subject to a high degree of uncertainty and risk. Tradenames, trademarks and service marks of other companies appearing in this presentation are the property of their respective owners. |
| 3 Strong balance sheet expected to fund operations through the first half of 2028 Current cash runway expected to fully fund preclinical and clinical development plans Potential opportunities for additional non-dilutive financing and development partners Bosakitug (ATI-045): Uniquely potent monoclonal antibody targeting TSLP ATI-052: Bispecific antibody (BsAb) targeting both TSLP and IL-4R ATI-2138: Potent and selective oral inhibitor of ITK/JAK3 Discovery/preclinical evaluation ongoing for novel ITK inhibitors and BsAbs Bosakitug: Two-arm pbo-controlled Phase 2 trial initiated in 2Q 2025 ATI-052: Pbo-controlled Phase 1a/1b SAD MAD program initiated in 2Q 2025 ATI-2138: Top line results from Phase 2a OL trial expected in July 2025; awaiting PD results New INDs starting in 2026 expected from discovery engine Aclaris Therapeutics: Opportunities for Significant Growth Small & large molecule discovery and development expertise: >12 biologics approved, >30 small molecules advanced to clinical development, six small molecules approved Proprietary kinase small molecule discovery engine complemented by in-house multidisciplinary scientific team Potential Best -in-Class Clinical Assets Continued Business Execution Well Positioned for Future Growth TSLP=Thymic Stromal Lymphopoietin; ITK=IL-2-Inducible Tyrosine Kinase; SAD/MAD=single/multiple ascending dose; OL=open label; IND=Investigational New Drug application World Class Expertise/Capability |
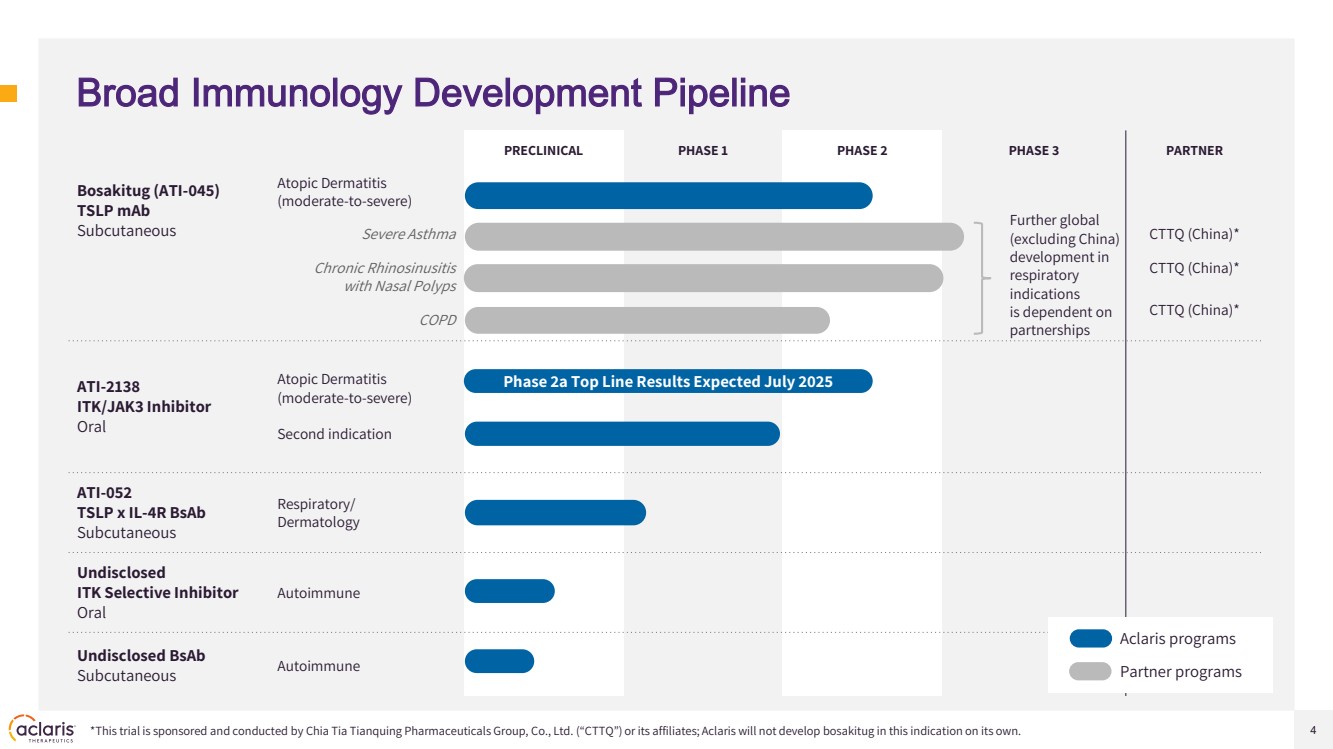
| 4 PRECLINICAL PHASE 1 PHASE 2 PHASE 3 PARTNER Bosakitug (ATI-045) TSLP mAb Subcutaneous Atopic Dermatitis (moderate-to-severe) Severe Asthma CTTQ (China)* Chronic Rhinosinusitis with Nasal Polyps CTTQ (China)* COPD CTTQ (China)* ATI-2138 ITK/JAK3 Inhibitor Oral Atopic Dermatitis (moderate-to-severe) Second indication ATI-052 TSLP x IL-4R BsAb Subcutaneous Respiratory/ Dermatology Undisclosed ITK Selective Inhibitor Oral Autoimmune Undisclosed BsAb Subcutaneous Autoimmune Broad Immunology Development Pipeline Phase 2a Top Line Results Expected July 2025 Further global (excluding China) development in respiratory indications is dependent on partnerships *This trial is sponsored and conducted by Chia Tia Tianquing Pharmaceuticals Group, Co., Ltd. (“CTTQ”) or its affiliates; Aclaris will not develop bosakitug in this indication on its own. Aclaris programs Partner programs |
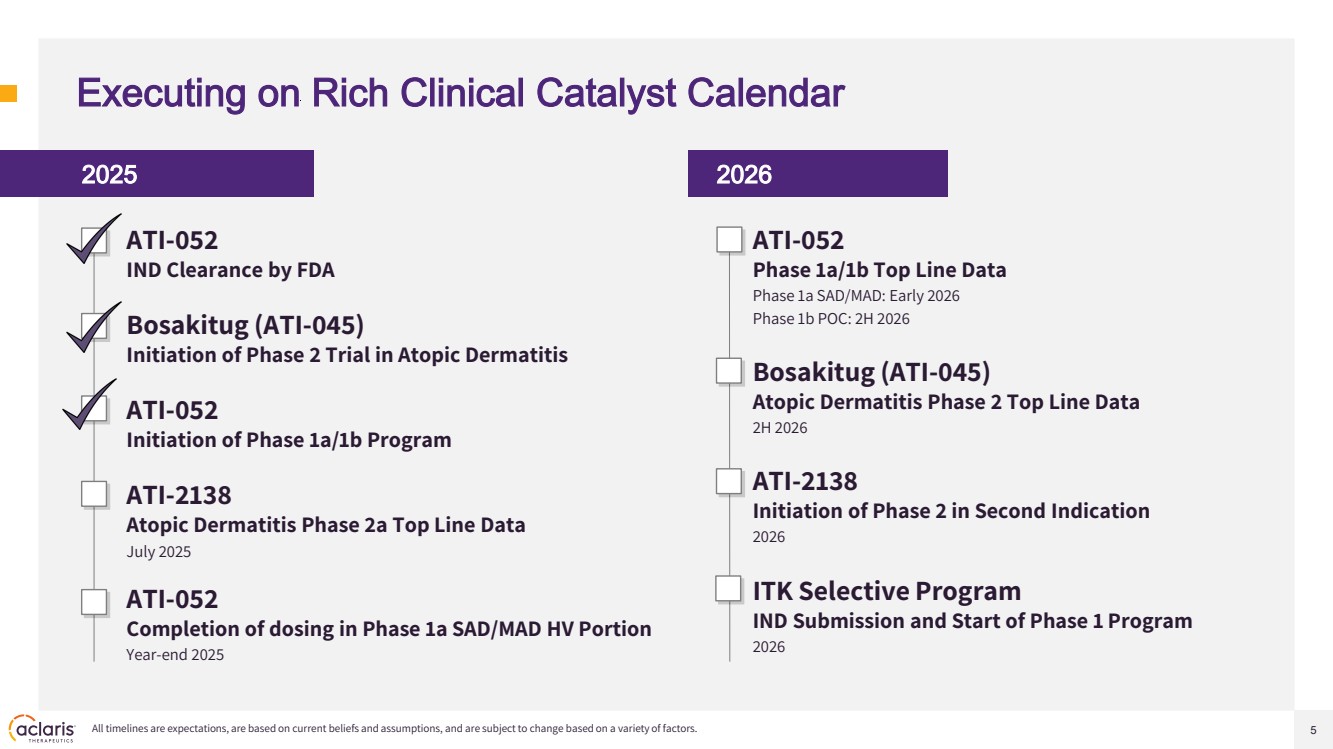
| 5 Executing on Rich Clinical Catalyst Calendar 2025 ATI-052 IND Clearance by FDA Bosakitug (ATI-045) Initiation of Phase 2 Trial in Atopic Dermatitis ATI-052 Initiation of Phase 1a/1b Program ATI-2138 Atopic Dermatitis Phase 2a Top Line Data July 2025 ATI-052 Completion of dosing in Phase 1a SAD/MAD HV Portion Year-end 2025 2026 All timelines are expectations, are based on current beliefs and assumptions, and are subject to change based on a variety of factors. ATI-052 Phase 1a/1b Top Line Data Phase 1a SAD/MAD: Early 2026 Phase 1b POC: 2H 2026 Bosakitug (ATI-045) Atopic Dermatitis Phase 2 Top Line Data 2H 2026 ATI-2138 Initiation of Phase 2 in Second Indication 2026 ITK Selective Program IND Submission and Start of Phase 1 Program 2026 |
| 6 Bosakitug (ATI -045) Anti -TSLP Monoclonal Antibody Program Differentiated Investigational Drug Candidate with Best-In-Class Potential |
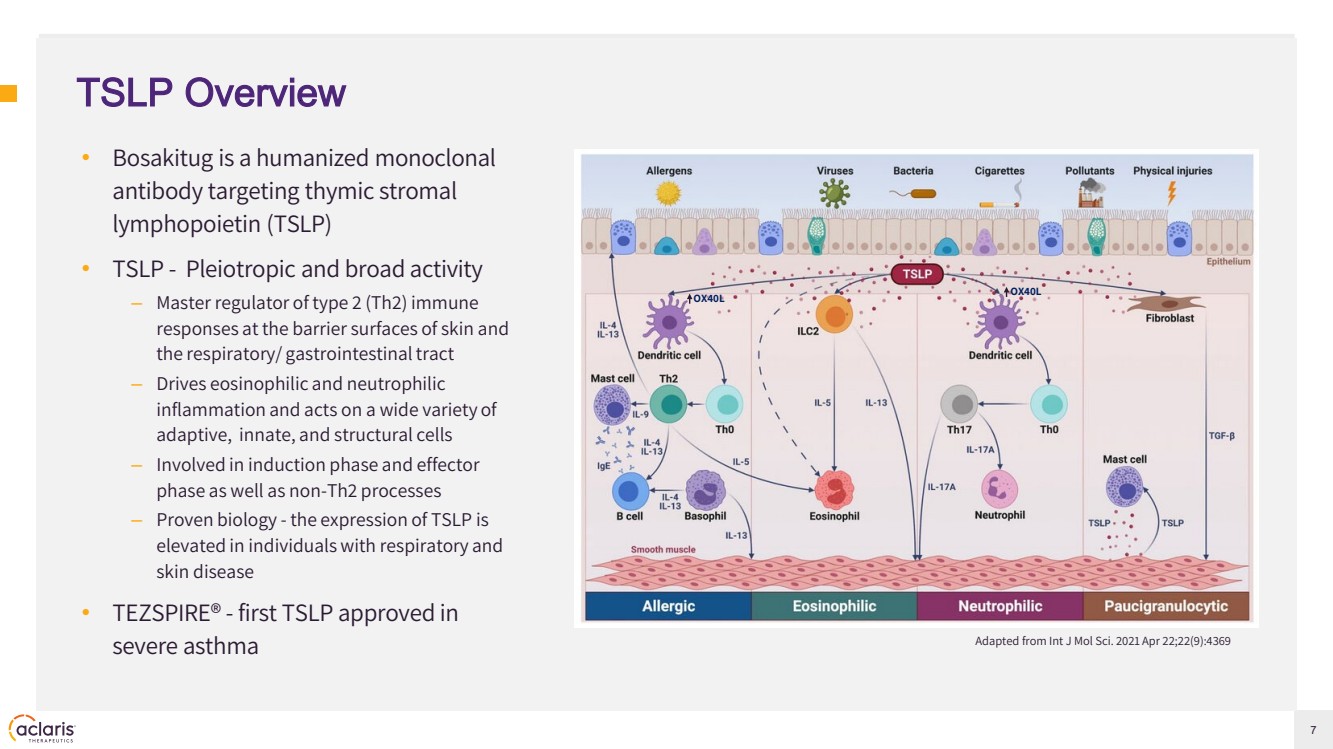
| 7 TSLP Overview • Bosakitug is a humanized monoclonal antibody targeting thymic stromal lymphopoietin (TSLP) • TSLP - Pleiotropic and broad activity – Master regulator of type 2 (Th2) immune responses at the barrier surfaces of skin and the respiratory/ gastrointestinal tract – Drives eosinophilic and neutrophilic inflammation and acts on a wide variety of adaptive, innate, and structural cells – Involved in induction phase and effector phase as well as non-Th2 processes – Proven biology - the expression of TSLP is elevated in individuals with respiratory and skin disease • TEZSPIRE® - first TSLP approved in severe asthma Adapted from Int J Mol Sci. 2021 Apr 22;22(9):4369 OX40L OX40L |
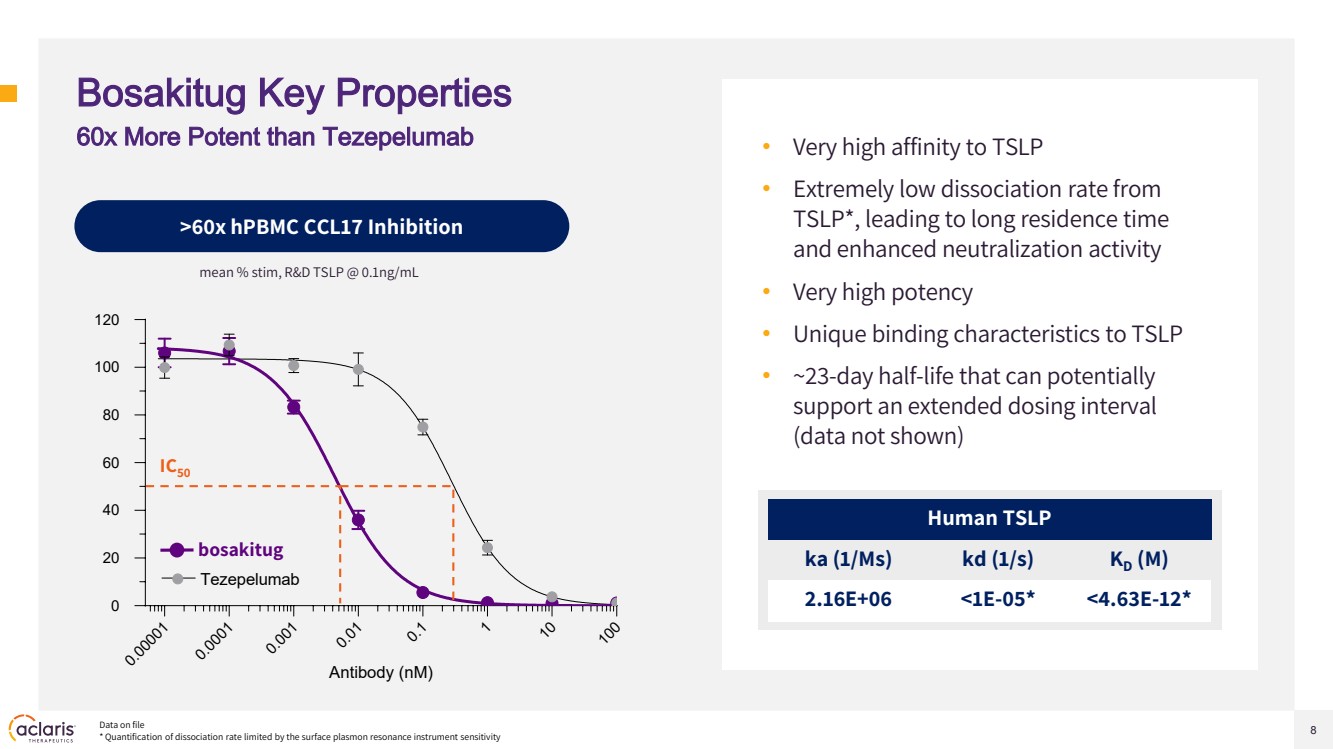
| 8 Bosakitug Key Properties • Very high affinity to TSLP • Extremely low dissociation rate from TSLP*, leading to long residence time and enhanced neutralization activity • Very high potency • Unique binding characteristics to TSLP • ~23-day half-life that can potentially support an extended dosing interval (data not shown) Human TSLP ka (1/Ms) kd (1/s) KD (M) 2.16E+06 <1E-05* <4.63E-12* >60x hPBMC CCL17 Inhibition Data on file * Quantification of dissociation rate limited by the surface plasmon resonance instrument sensitivity 60x More Potent than Tezepelumab mean % stim, R&D TSLP @ 0.1ng/mL 0.00001 0.0001 0.001 0.01 0.1 1 10 100 0 20 40 60 80 100 120 Antibody (nM) ATI-045 Tezepelumab IC50 bosakitug |
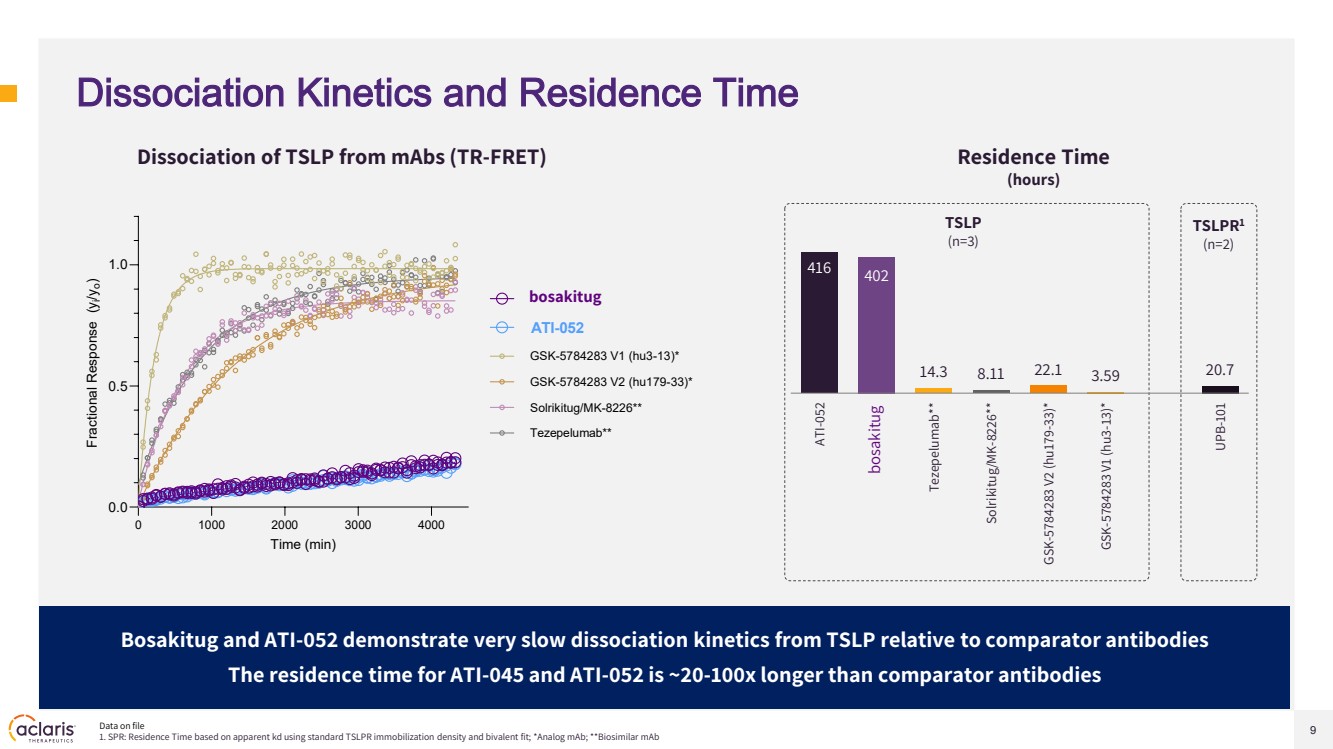
| 9 Dissociation Kinetics and Residence Time Residence Time (hours) Dissociation of TSLP from mAbs (TR-FRET) 416 402 14.3 8.11 22.1 3.59 20.7 ATI-052 ATI-045 Tezepelumab** Solrikitug/MK-8226** GSK-5784283 V2 (hu179-33)* GSK-5784283 V1 (hu3-13)* UPB-101 Bosakitug and ATI-052 demonstrate very slow dissociation kinetics from TSLP relative to comparator antibodies The residence time for ATI-045 and ATI-052 is ~20-100x longer than comparator antibodies Data on file 1. SPR: Residence Time based on apparent kd using standard TSLPR immobilization density and bivalent fit; *Analog mAb; **Biosimilar mAb TSLPR1 (n=2) TSLP (n=3) 0 1000 2000 3000 4000 0.0 0.5 1.0 Time (min) Fractional Response (yi/yo) ATI-045 ATI-052 GSK-5784283 V1 (hu3-13)* GSK-5784283 V2 (hu179-33)* Solrikitug/MK-8226** Tezepelumab** bosakitug bosakitug |
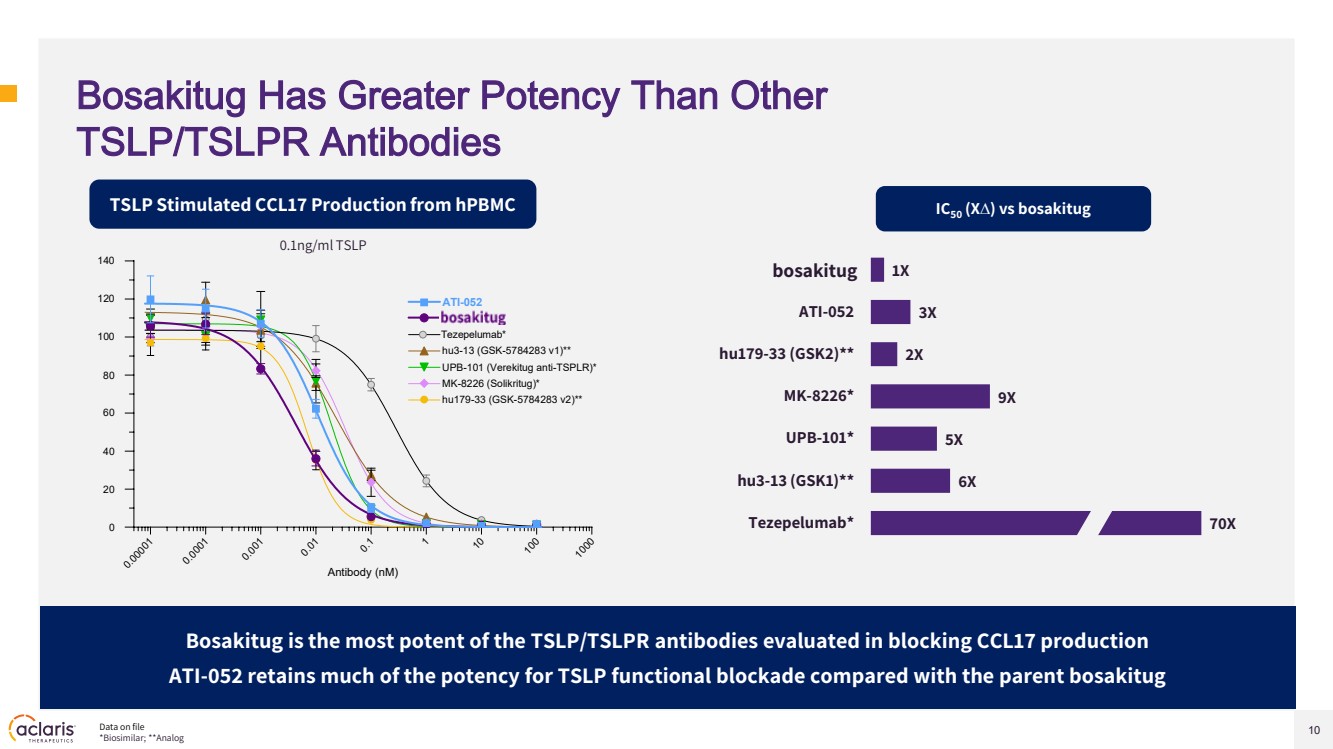
| 10 Bosakitug Has Greater Potency Than Other TSLP/TSLPR Antibodies TSLP Stimulated CCL17 Production from hPBMC Bosakitug is the most potent of the TSLP/TSLPR antibodies evaluated in blocking CCL17 production ATI-052 retains much of the potency for TSLP functional blockade compared with the parent bosakitug 70X 6X 5X 9X 2X 3X 1X Tezepelumab* hu3-13 (GSK1)** UPB-101* MK-8226* hu179-33 (GSK2)** ATI-052 ATI-045 IC50 (X∆) vs bosakitug 0.1ng/ml TSLP 0.00001 0.0001 0.001 0.01 0.1 1 10 100 1000 0 20 40 60 80 100 120 140 Antibody (nM) UPB-101 (Verekitug anti-TSPLR)* ATI-045 Tezepelumab* hu3-13 (GSK-5784283 v1)** MK-8226 (Solikritug)* hu179-33 (GSK-5784283 v2)** ATI-052 Data on file *Biosimilar; **Analog bosakitug |
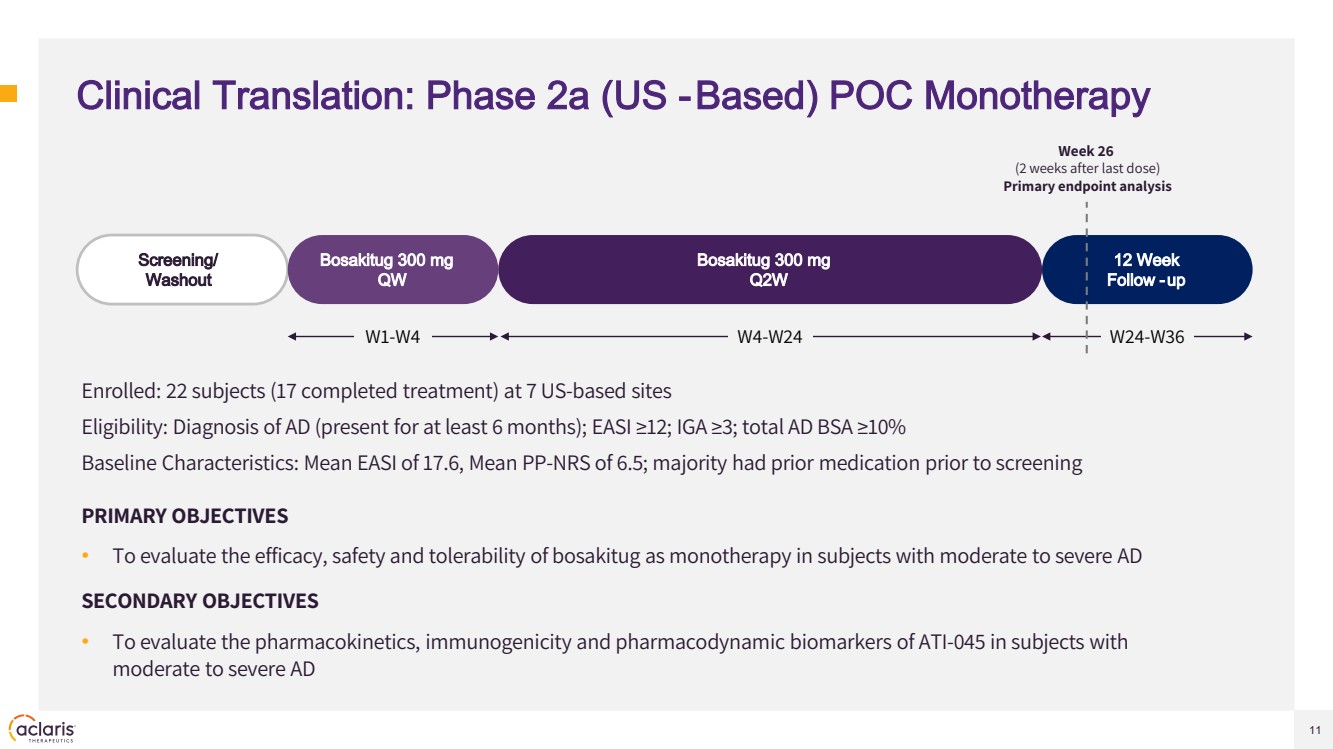
| 11 Clinical Translation: Phase 2a (US -Based) POC Monotherapy Enrolled: 22 subjects (17 completed treatment) at 7 US-based sites Eligibility: Diagnosis of AD (present for at least 6 months); EASI ≥12; IGA ≥3; total AD BSA ≥10% Baseline Characteristics: Mean EASI of 17.6, Mean PP-NRS of 6.5; majority had prior medication prior to screening PRIMARY OBJECTIVES • To evaluate the efficacy, safety and tolerability of bosakitug as monotherapy in subjects with moderate to severe AD SECONDARY OBJECTIVES • To evaluate the pharmacokinetics, immunogenicity and pharmacodynamic biomarkers of ATI-045 in subjects with moderate to severe AD Screening/ Washout Bosakitug 300 mg QW 12 Week Follow -up Bosakitug 300 mg Q2W Week 26 (2 weeks after last dose) Primary endpoint analysis W1-W4 W4-W24 W24-W36 |
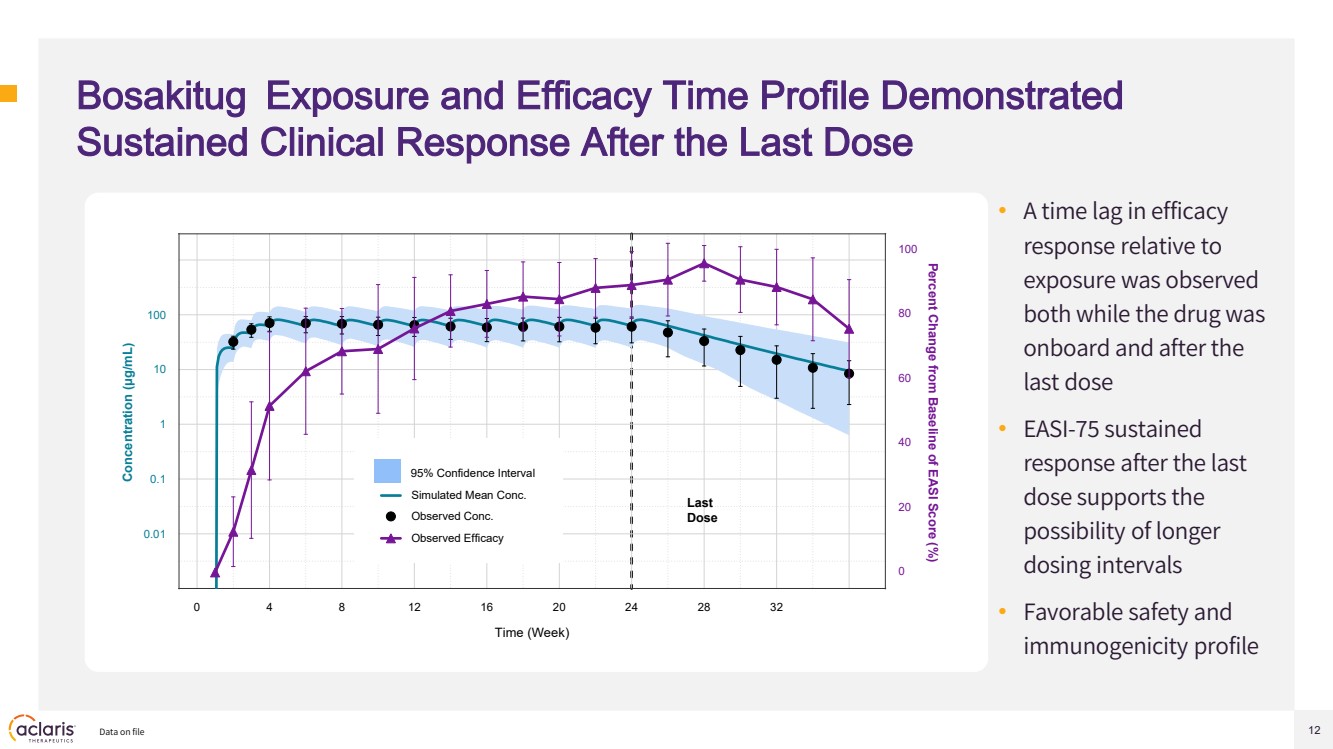
| 12 Bosakitug Exposure and Efficacy Time Profile Demonstrated Sustained Clinical Response After the Last Dose • A time lag in efficacy response relative to exposure was observed both while the drug was onboard and after the last dose • EASI-75 sustained response after the last dose supports the possibility of longer dosing intervals • Favorable safety and immunogenicity profile Data on file 0 4 8 12 16 20 24 28 32 0.01 0.1 1 10 100 0 20 40 60 80 100 Time (Week) Concentration (μg/mL) Percent Change from Baseline of EASI Score (%) Last Dose Simulated Mean Conc. Observed Conc. Observed Efficacy 95% Confidence Interval |
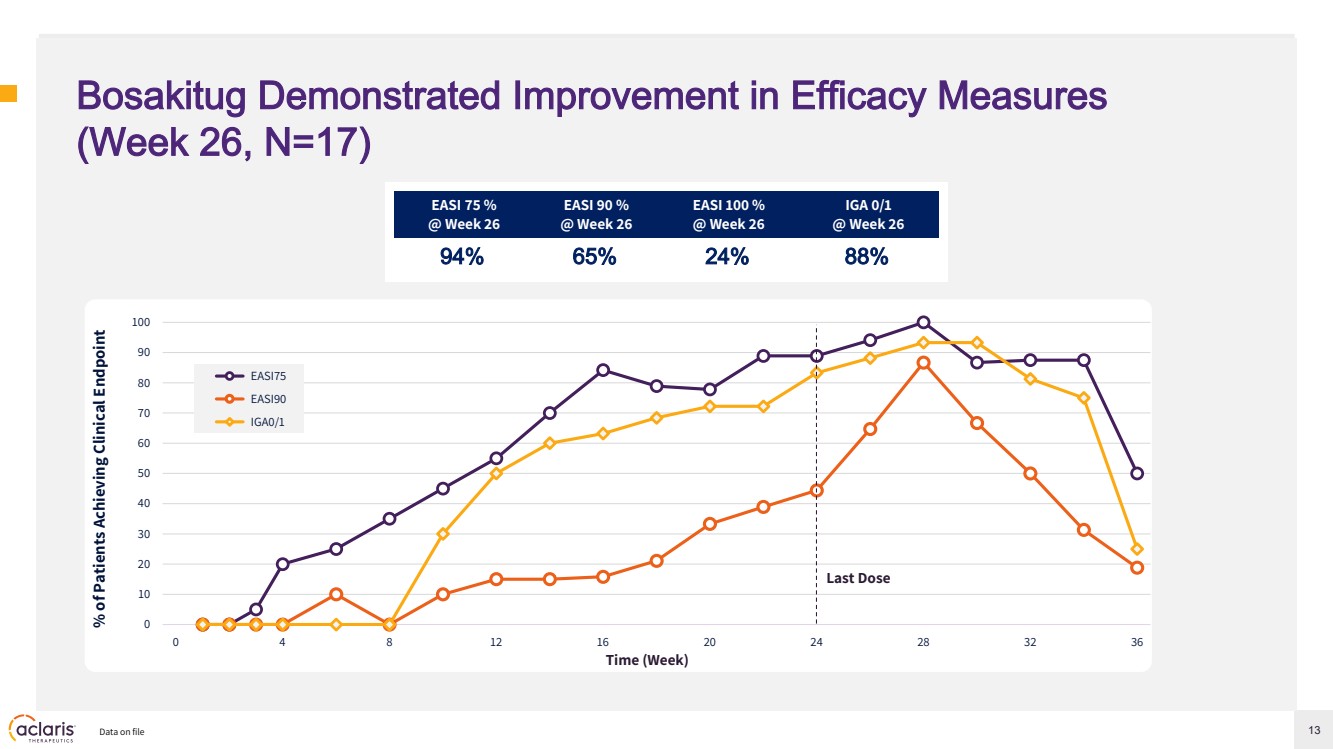
| 13 Bosakitug Demonstrated Improvement in Efficacy Measures (Week 26, N=17) EASI 75 % @ Week 26 EASI 90 % @ Week 26 EASI 100 % @ Week 26 IGA 0/1 @ Week 26 94% 65% 24% 88% Data on file % of Patients Achieving Clinical Endpoint Time (Week) 0 10 20 30 40 50 60 70 80 90 100 0 4 8 12 16 20 24 28 32 36 EASI75 EASI90 IGA0/1 Last Dose |
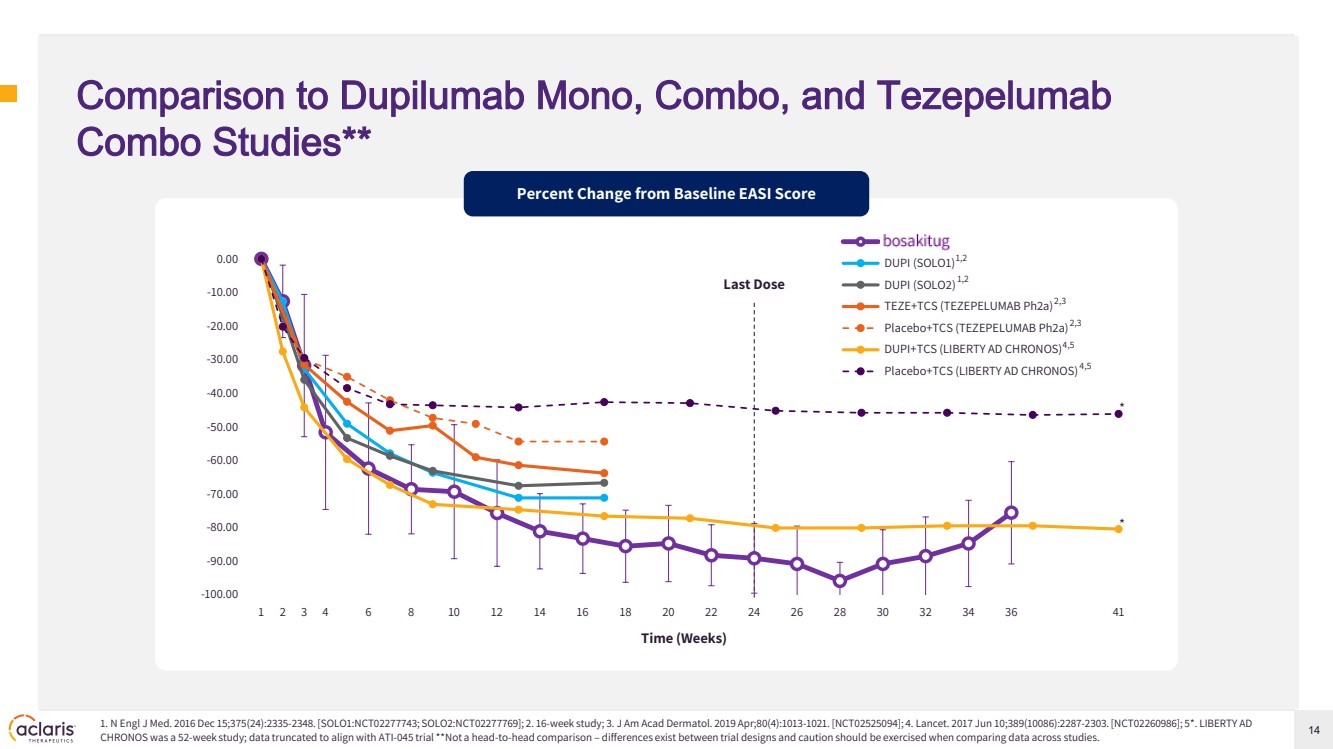
| 14 Comparison to Dupilumab Mono, Combo, and Tezepelumab Combo Studies** 1. N Engl J Med. 2016 Dec 15;375(24):2335-2348. [SOLO1:NCT02277743; SOLO2:NCT02277769]; 2. 16-week study; 3. J Am Acad Dermatol. 2019 Apr;80(4):1013-1021. [NCT02525094]; 4. Lancet. 2017 Jun 10;389(10086):2287-2303. [NCT02260986]; 5*. LIBERTY AD CHRONOS was a 52-week study; data truncated to align with ATI-045 trial **Not a head-to-head comparison – differences exist between trial designs and caution should be exercised when comparing data across studies. Percent Change from Baseline EASI Score -100.00 -90.00 -80.00 -70.00 -60.00 -50.00 -40.00 -30.00 -20.00 -10.00 0.00 1 2 3 4 6 8 10 12 14 16 18 20 22 24 26 28 30 32 34 36 41 ATI-045 DUPI (SOLO1) DUPI (SOLO2) TEZE+TCS (TEZEPELUMAB Ph2a) Placebo+TCS (TEZEPELUMAB Ph2a) DUPI+TCS (LIBERTY AD CHRONOS) Placebo+TCS (LIBERTY AD CHRONOS) 1,2 1,2 2,3 2,3 4,5 4,5 Last Dose Time (Weeks) * * |
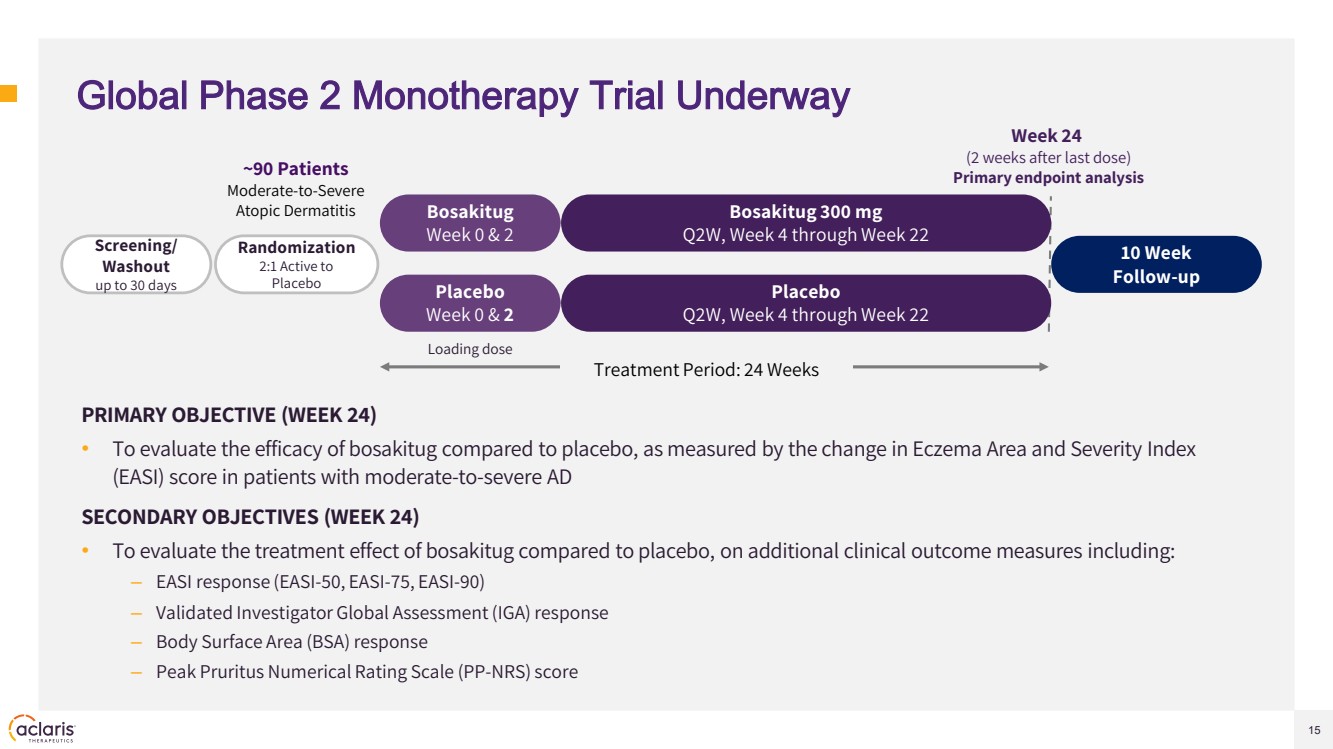
| 15 Global Phase 2 Monotherapy Trial Underway PRIMARY OBJECTIVE (WEEK 24) • To evaluate the efficacy of bosakitug compared to placebo, as measured by the change in Eczema Area and Severity Index (EASI) score in patients with moderate-to-severe AD SECONDARY OBJECTIVES (WEEK 24) • To evaluate the treatment effect of bosakitug compared to placebo, on additional clinical outcome measures including: – EASI response (EASI-50, EASI-75, EASI-90) – Validated Investigator Global Assessment (IGA) response – Body Surface Area (BSA) response – Peak Pruritus Numerical Rating Scale (PP-NRS) score Screening/ Washout up to 30 days Placebo Week 0 & 2 10 Week Follow-up Placebo Q2W, Week 4 through Week 22 Week 24 (2 weeks after last dose) Primary endpoint analysis Treatment Period: 24 Weeks Bosakitug Week 0 & 2 Bosakitug 300 mg Q2W, Week 4 through Week 22 Randomization 2:1 Active to Placebo ~90 Patients Moderate-to-Severe Atopic Dermatitis Loading dose |
| 16 Next Steps with Bosakitug • Competitively positioned as potential best-in-class TSLP mAb. Preclinical and clinical data generated to date reinforce the enhanced potency of bosakitug and support further development in dermatological conditions • Two-arm placebo-controlled Phase 2 trial of bosakitug in moderate-to-severe AD ongoing (initiated 2Q 2025) – Results expected in 2H 2026 • Aclaris is seeking partners to develop bosakitug in respiratory indications; further global (excluding China) development in these indications is dependent on entering into potential partnerships |
| 17 ATI-052: Anti -TSLP x IL-4R Bispecific Antibody Program Highly Potent and Bioactive Investigational Drug Candidate |
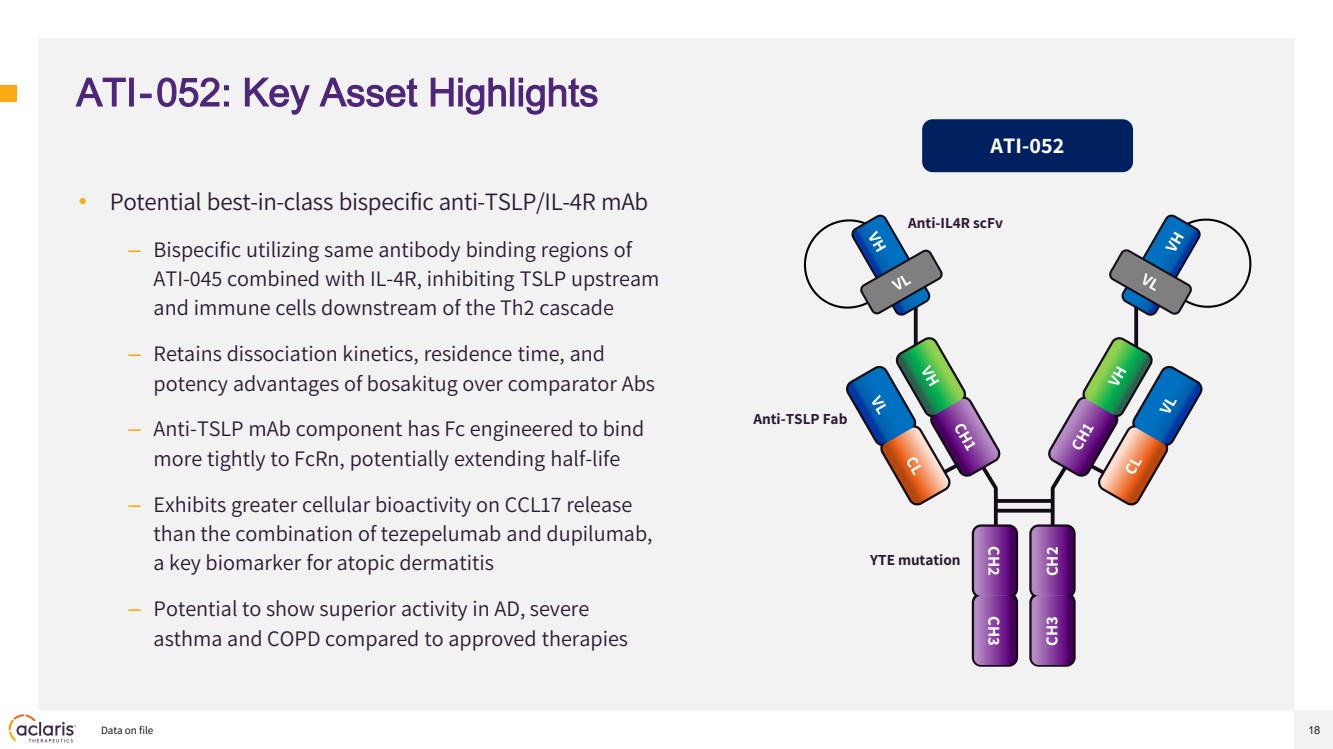
| 18 ATI-052: Key Asset Highlights • Potential best-in-class bispecific anti-TSLP/IL-4R mAb – Bispecific utilizing same antibody binding regions of ATI-045 combined with IL-4R, inhibiting TSLP upstream and immune cells downstream of the Th2 cascade – Retains dissociation kinetics, residence time, and potency advantages of bosakitug over comparator Abs – Anti-TSLP mAb component has Fc engineered to bind more tightly to FcRn, potentially extending half-life – Exhibits greater cellular bioactivity on CCL17 release than the combination of tezepelumab and dupilumab, a key biomarker for atopic dermatitis – Potential to show superior activity in AD, severe asthma and COPD compared to approved therapies Data on file ATI-052 CH2 CH3 CH2 CH3 Anti-IL4R scFv Anti-TSLP Fab YTE mutation |
| 19 ATI-052 Demonstrates Greater Potency than the Combination of Tezepelumab and Dupilumab on CCL17 Release • ATI-052 is 3-5x more potent than the combination of Tezepelumab + Dupilumab Effect on CCL17 Release Induced by 0.1 ng/mL IL4 plus 0.1 ng/mL TSLP Ab Concentration (nM) 140 120 100 80 60 40 20 0 CCL17 Level Antibody IC50 (nM) ATI-052 0.016 Tezepelumab + Dupilumab 0.069 Fold change 4.3 Tezepelumab + Dupilumab ATI-052 Data on file |
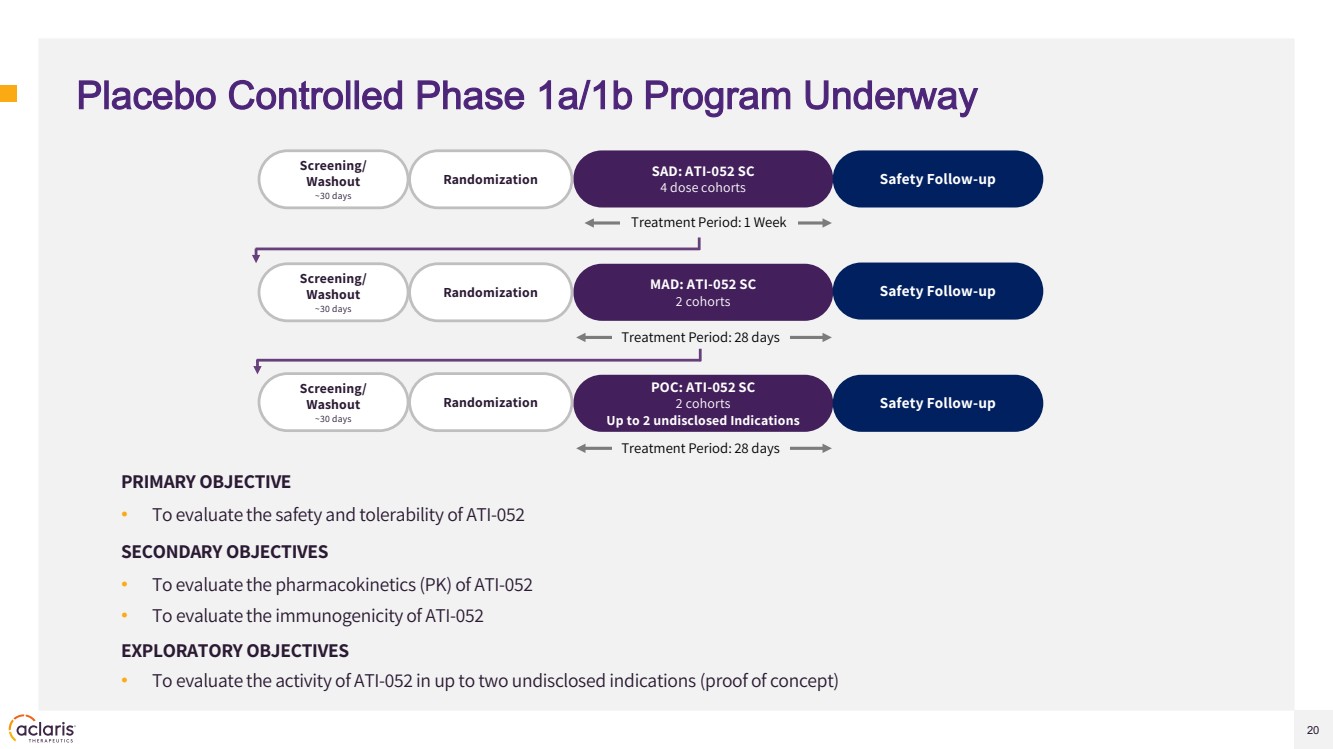
| 20 Placebo Controlled Phase 1a/1b Program Underway PRIMARY OBJECTIVE • To evaluate the safety and tolerability of ATI-052 SECONDARY OBJECTIVES • To evaluate the pharmacokinetics (PK) of ATI-052 • To evaluate the immunogenicity of ATI-052 EXPLORATORY OBJECTIVES • To evaluate the activity of ATI-052 in up to two undisclosed indications (proof of concept) Screening/ Washout ~30 days Treatment Period: 1 Week SAD: ATI-052 SC 4 dose cohorts Randomization MAD: ATI-052 SC 2 cohorts POC: ATI-052 SC 2 cohorts Up to 2 undisclosed Indications Screening/ Washout ~30 days Randomization Screening/ Washout ~30 days Randomization Safety Follow-up Treatment Period: 28 days Treatment Period: 28 days Safety Follow-up Safety Follow-up |
| 21 Next Steps with ATI -052 • IND cleared by US Food and Drug Administration (April 2025) • Phase 1a/1b program underway – Dosing in Phase 1a SAD/MAD portion expected to be complete by year-end 2025 followed by top line results in early 2026 – Top line results from expected Phase 1b POC portion anticipated in the second half of 2026 |
| 22 ATI-2138: A First-Generation Novel ITK/JAK3 Inhibitor for T Cell -Mediated Diseases Potent and Selective Investigational Drug Candidate with Strong Tolerability Profile |
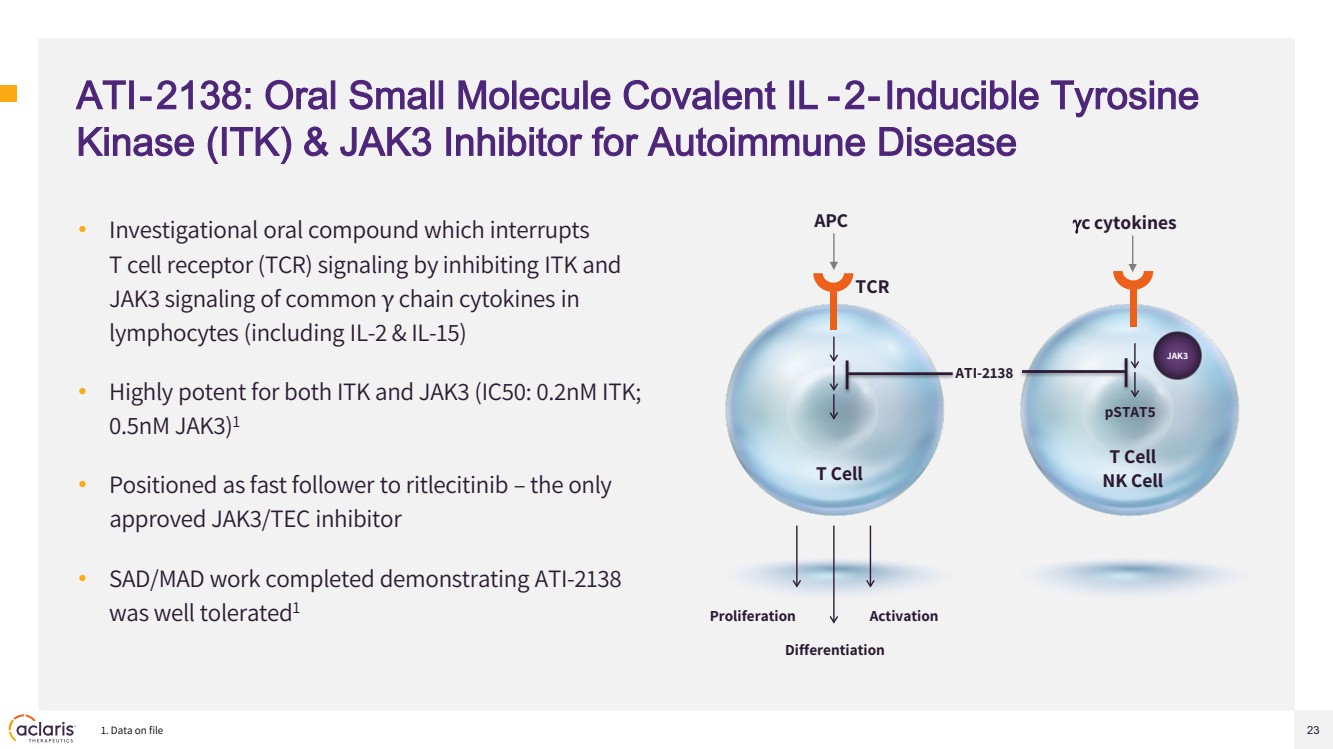
| 23 ATI-2138: Oral Small Molecule Covalent IL -2-Inducible Tyrosine Kinase (ITK) & JAK3 Inhibitor for Autoimmune Disease • Investigational oral compound which interrupts T cell receptor (TCR) signaling by inhibiting ITK and JAK3 signaling of common γ chain cytokines in lymphocytes (including IL-2 & IL-15) • Highly potent for both ITK and JAK3 (IC50: 0.2nM ITK; 0.5nM JAK3)1 • Positioned as fast follower to ritlecitinib – the only approved JAK3/TEC inhibitor • SAD/MAD work completed demonstrating ATI-2138 was well tolerated1 1. Data on file TCR APC T Cell Proliferation Differentiation Activation γc cytokines JAK3 pSTAT5 ATI-2138 T Cell NK Cell |
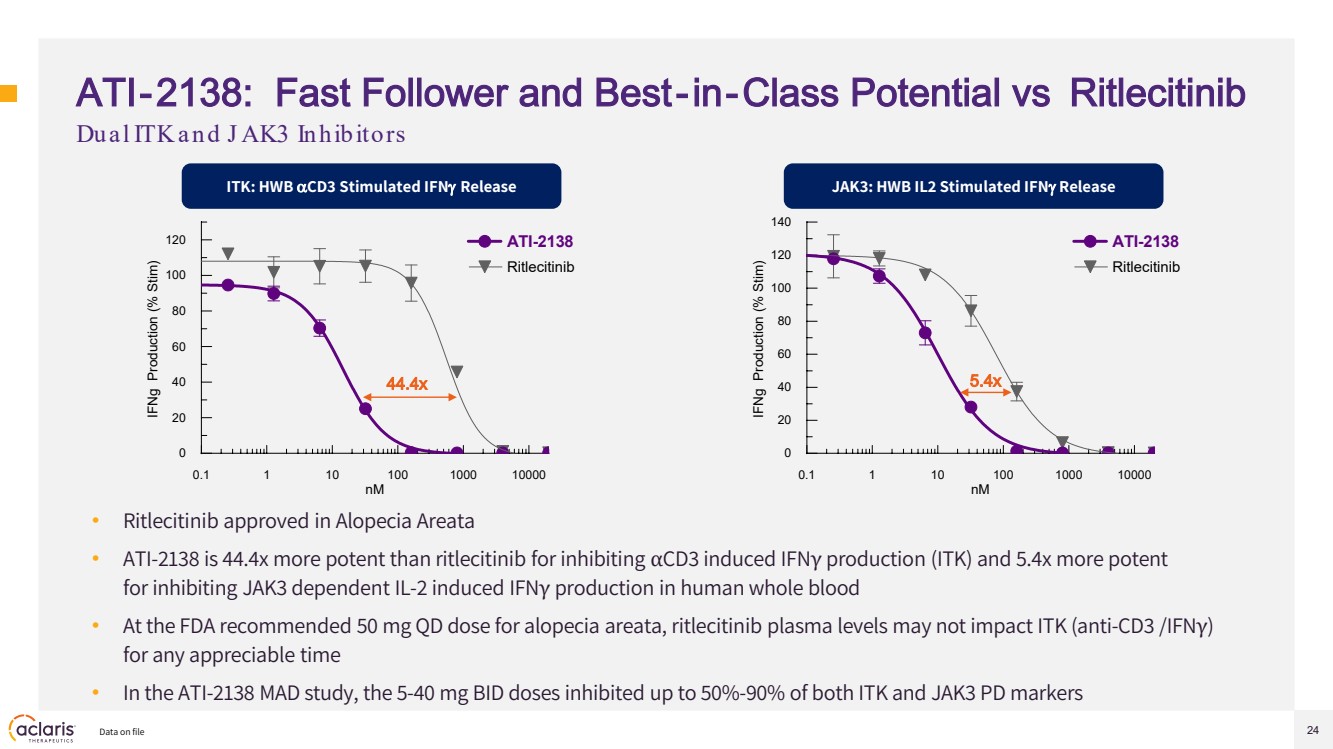
| 24 ATI-2138: Fast Follower and Best-in-Class Potential vs Ritlecitinib • Ritlecitinib approved in Alopecia Areata • ATI-2138 is 44.4x more potent than ritlecitinib for inhibiting αCD3 induced IFNγ production (ITK) and 5.4x more potent for inhibiting JAK3 dependent IL-2 induced IFNγ production in human whole blood • At the FDA recommended 50 mg QD dose for alopecia areata, ritlecitinib plasma levels may not impact ITK (anti-CD3 /IFNγ) for any appreciable time • In the ATI-2138 MAD study, the 5-40 mg BID doses inhibited up to 50%-90% of both ITK and JAK3 PD markers Data on file Dual ITK and J AK3 Inhibitors ITK: HWB αCD3 Stimulated IFNγ Release JAK3: HWB IL2 Stimulated IFNγ Release 0.1 1 10 100 1000 10000 0 20 40 60 80 100 120 140 nM IFNg Production (% Stim) ATI-2138 Ritlecitinib 0.1 1 10 100 1000 10000 0 20 40 60 80 100 120 nM IFNg Production (% Stim) ATI-2138 Ritlecitinib 44.4x 5.4x |
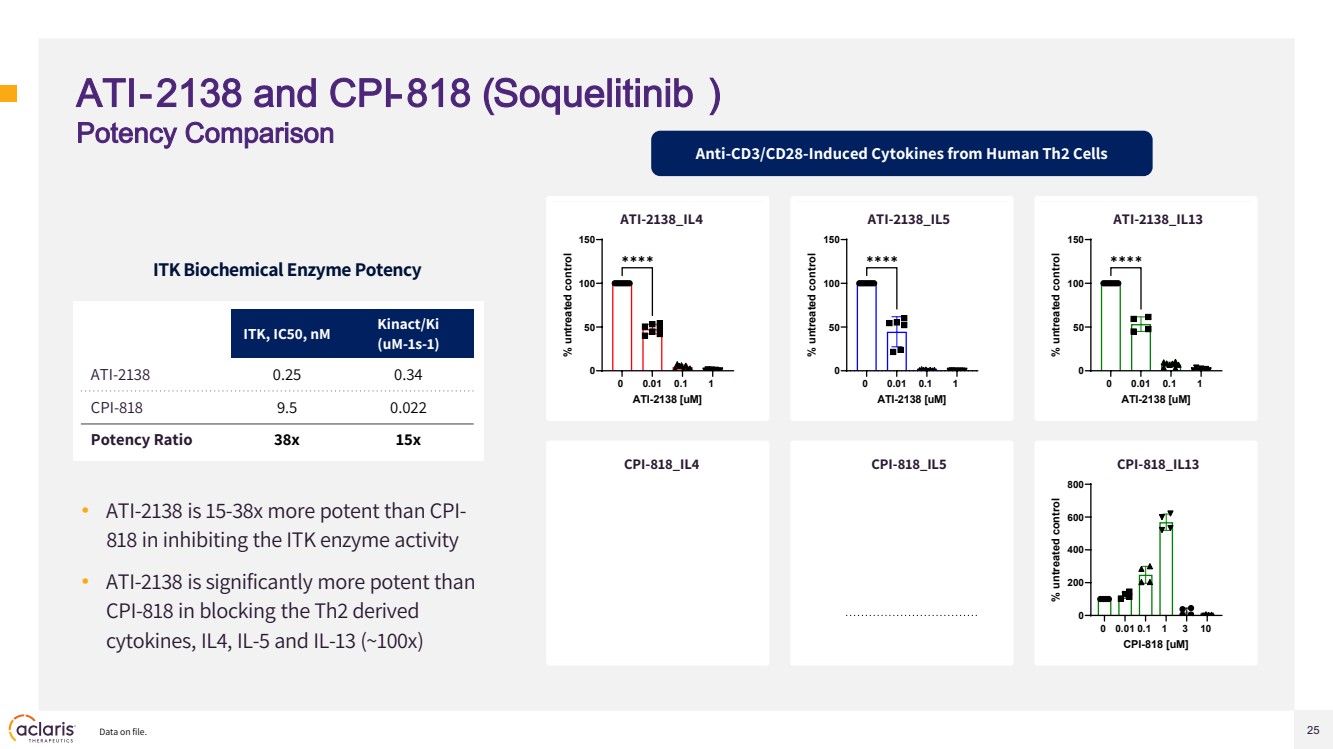
| 25 ATI-2138 and CPI-818 (Soquelitinib ) Potency Comparison • ATI-2138 is 15-38x more potent than CPI-818 in inhibiting the ITK enzyme activity • ATI-2138 is significantly more potent than CPI-818 in blocking the Th2 derived cytokines, IL4, IL-5 and IL-13 (~100x) 0 0.01 0.1 1 3 10 0 200 400 600 800 CPI-818_IL13 CPI-818 [uM] % untreated control 0 0.01 0.1 1 0 50 100 150 ATI-2138_IL4 ATI-2138 [uM] % untreated control ✱✱✱✱ 0 0.01 0.1 1 0 50 100 150 ATI-2138_IL5 ATI-2138 [uM] % untreated control ✱✱✱✱ 0 0.01 0.1 1 0 50 100 150 ATI-2138_IL13 ATI-2138 [uM] % untreated control ✱✱✱✱ Data on file. ITK, IC50, nM Kinact/Ki (uM-1s-1) ATI-2138 0.25 0.34 CPI-818 9.5 0.022 Potency Ratio 38x 15x ITK Biochemical Enzyme Potency Anti-CD3/CD28-Induced Cytokines from Human Th2 Cells ATI-2138_IL4 ATI-2138_IL5 ATI-2138_IL13 CPI-818_IL4 CPI-818_IL5 CPI-818_IL13 |
| 26 ATI-2138 Phase 2a Trial Design in Atopic Dermatitis Single Arm Open -Label Trial: Complete; Awaiting PD Results Screening ~30 days Follow-up 2 weeks ATI-2138 (10mg BID) Treatment Period: 12 Weeks Week 12 Efficacy endpoint analysis 14 enrolled patients Moderate-to-Severe Atopic Dermatitis PRIMARY OBJECTIVE • Safety SECONDARY/EXPLORATORY OBJECTIVES • Pharmacokinetics, pharmacodynamics (RNA analysis, proteomics, IHC to analyze specific pathway inhibition) • Efficacy measurements at week 12 Single arm Phase 2a conducted to assess tolerability and ITK contribution (PD), and identify efficacy signals to be explored in future clinical trials |
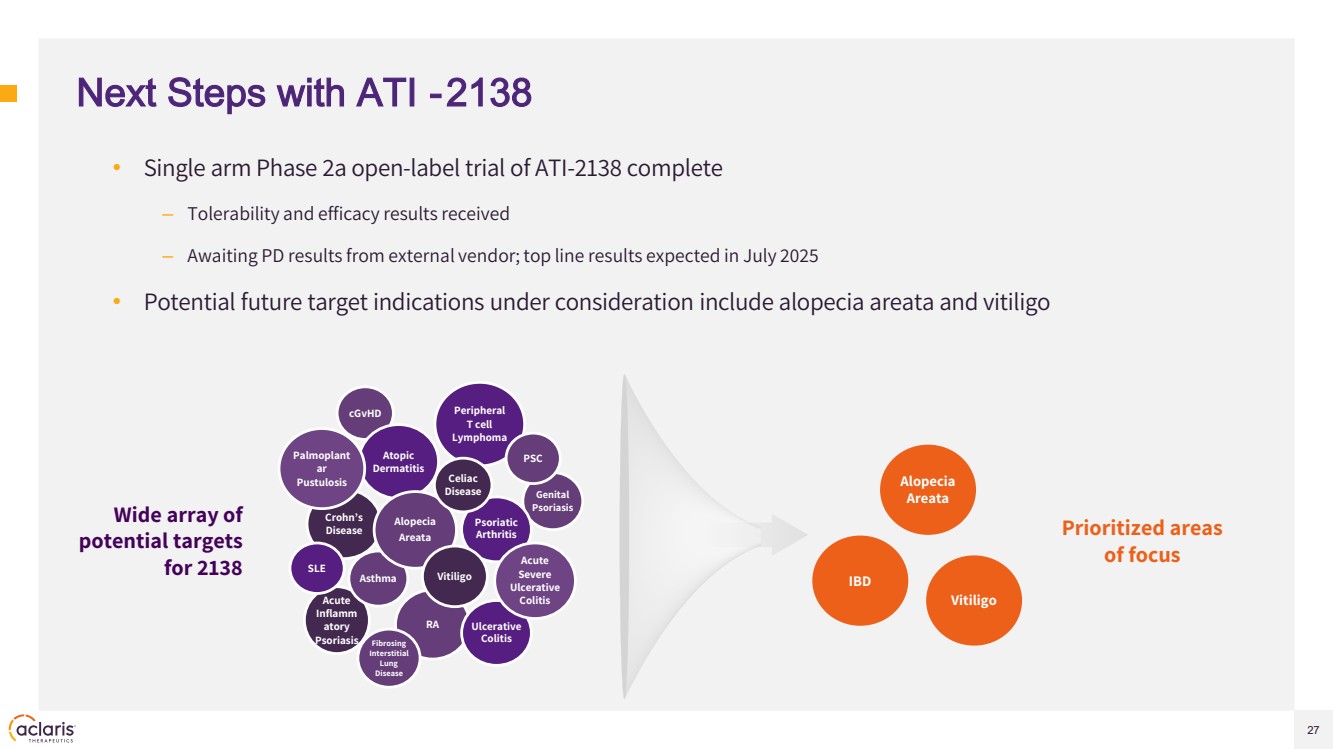
| 27 Next Steps with ATI -2138 • Single arm Phase 2a open-label trial of ATI-2138 complete – Tolerability and efficacy results received – Awaiting PD results from external vendor; top line results expected in July 2025 • Potential future target indications under consideration include alopecia areata and vitiligo Alopecia Areata IBD Vitiligo cGvHD Genital Psoriasis Acute Inflamm atory Psoriasis Asthma RA Crohn’s Disease Ulcerative Colitis Psoriatic Arthritis Atopic Dermatitis Palmoplant ar Pustulosis Alopecia Areata Peripheral T cell Lymphoma Celiac Disease Fibrosing Interstitial Lung Disease Acute Severe Ulcerative Colitis Vitiligo PSC SLE Prioritized areas of focus Wide array of potential targets for 2138 |
| 28 Next-Generation Selective ITK Inhibitor |
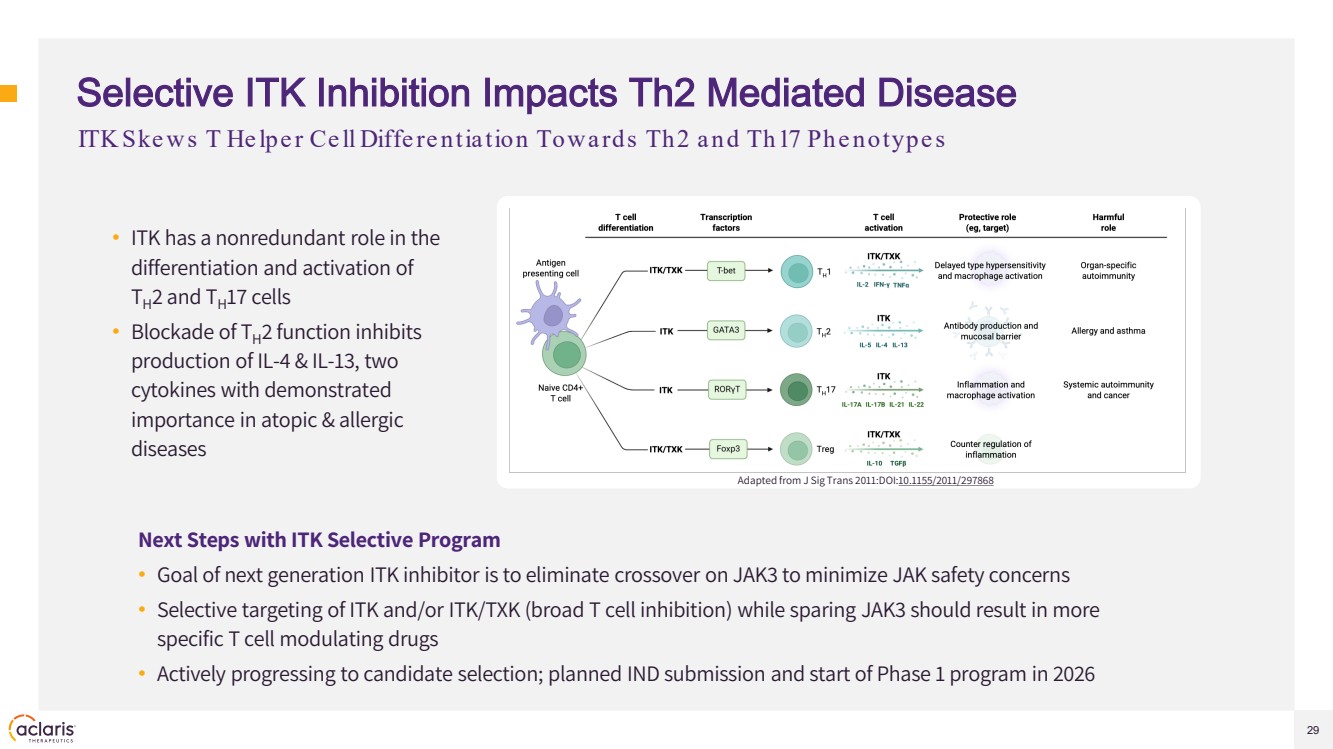
| 29 Selective ITK Inhibition Impacts Th2 Mediated Disease • ITK has a nonredundant role in the differentiation and activation of TH2 and TH17 cells • Blockade of TH2 function inhibits production of IL-4 & IL-13, two cytokines with demonstrated importance in atopic & allergic diseases Adapted from J Sig Trans 2011:DOI:10.1155/2011/297868 ITK Ske ws T He lpe r Ce ll Diffe re ntiation Towards Th2 and Th17 Phe notype s Next Steps with ITK Selective Program • Goal of next generation ITK inhibitor is to eliminate crossover on JAK3 to minimize JAK safety concerns • Selective targeting of ITK and/or ITK/TXK (broad T cell inhibition) while sparing JAK3 should result in more specific T cell modulating drugs • Actively progressing to candidate selection; planned IND submission and start of Phase 1 program in 2026 |
| 30 Company Summary * Without giving effect to additional business development transactions or financing activities. Proven track record of R&D, business development and scientific leadership in immuno-inflammatory diseases Proprietary discovery engine enables targeted design of novel drug candidates Multiple therapeutic programs ranging from discovery to clinical development Multiple milestones expected in 2025 and 2026 Executive Team KINect Technology Platform Pipeline Intellectual Property Financial Strength Global IP estate Cash, cash equivalents and marketable securities as of 1Q25 of $190.5M Cash runway expected through the first half of 2028* Potential to extend runway further through non-dilutive opportunities Focus on addressing the needs of patients with immuno-inflammatory diseases who lack satisfactory treatment options Commitment to Patients |
| June 2025 Corporate Overview EMPOWERING PATIENTS THROUGH THERAPEUTIC INNOVATION |