
Addressing the Growing Incidence of HPV16-Related Cancers NASDAQ: PDSB June
2025 Precision Designed Science For Immunotherapy

Forward-Looking Statements This communication contains forward-looking statements
(including within the meaning of Section 21E of the United States Securities Exchange Act of 1934, as amended, and Section 27A of the United States Securities Act of 1933, as amended) concerning PDS Biotechnology Corporation (the “Company”) and
other matters. These statements may discuss goals, intentions and expectations as to future plans, trends, events, results of operations or financial condition, or otherwise, based on current beliefs of the Company’s management, as well as
assumptions made by, and information currently available to, management. Forward-looking statements generally include statements that are predictive in nature and depend upon or refer to future events or conditions, and include words such as
“may,” “will,” “should,” “would,” “expect,” “anticipate,” “plan,” “likely,” “believe,” “estimate,” “project,” “intend,” “forecast,” “guidance”, “outlook” and other similar expressions among others. Forward-looking statements are based on
current beliefs and assumptions that are subject to risks and uncertainties and are not guarantees of future performance. Actual results could differ materially from those contained in any forward-looking statement as a result of various
factors, including, without limitation: the Company’s ability to protect its intellectual property rights; the Company’s anticipated capital requirements, including the Company’s anticipated cash runway and the Company’s current expectations
regarding its plans for future equity financings; the Company’s dependence on additional financing to fund its operations and complete the development and commercialization of its product candidates, and the risks that raising such additional
capital may restrict the Company’s operations or require the Company to relinquish rights to the Company’s technologies or product candidates; the Company’s limited operating history in the Company’s current line of business, which makes it
difficult to evaluate the Company’s prospects, the Company’s business plan or the likelihood of the Company’s successful implementation of such business plan; the timing for the Company or its partners to initiate the planned clinical trials
for Versamune® HPV, PDS01ADC and other Versamune® based product candidates; the future success of such trials; the successful implementation of the Company’s research and development programs and collaborations, including any collaboration
studies concerning Versamune® HPV, PDS01ADC and other Versamune® based product candidates and the Company’s interpretation of the results and findings of such programs and collaborations and whether such results are sufficient to support the
future success of the Company’s product candidates; the success, timing and cost of the Company’s ongoing clinical trials and anticipated clinical trials for the Company’s current product candidates, including statements regarding the timing of
initiation, pace of enrollment and completion of the trials (including the Company’s ability to fully fund its disclosed clinical trials, which assumes no material changes to the Company’s currently projected expenses), futility analyses,
presentations at conferences and data reported in an abstract, and receipt of interim or preliminary results (including, without limitation, any preclinical results or data), which are not necessarily indicative of the final results of the
Company’s ongoing clinical trials; any Company statements about its understanding of product candidates mechanisms of action and interpretation of preclinical and early clinical results from its clinical development programs and any
collaboration studies; the Company’s ability to continue as a going concern; and other factors, including legislative, regulatory, political and economic developments not within the Company’s control. The foregoing review of important factors
that could cause actual events to differ from expectations should not be construed as exhaustive and should be read in conjunction with statements that are included herein and elsewhere, including the other risks, uncertainties, and other
factors described under “Risk Factors,” “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and elsewhere in the documents we file with the U.S. Securities and Exchange Commission. The forward-looking
statements are made only as of the date of this press release and, except as required by applicable law, the Company undertakes no obligation to revise or update any forward-looking statement, or to make any other forward-looking statements,
whether as a result of new information, future events or otherwise. Versamune® is a registered trademark of PDS Biotechnology Corporation. KEYTRUDA® is a registered trademark of Merck Sharp and Dohme LLC, a subsidiary of Merck & Co.,
Inc., Rahway, NJ, USA.
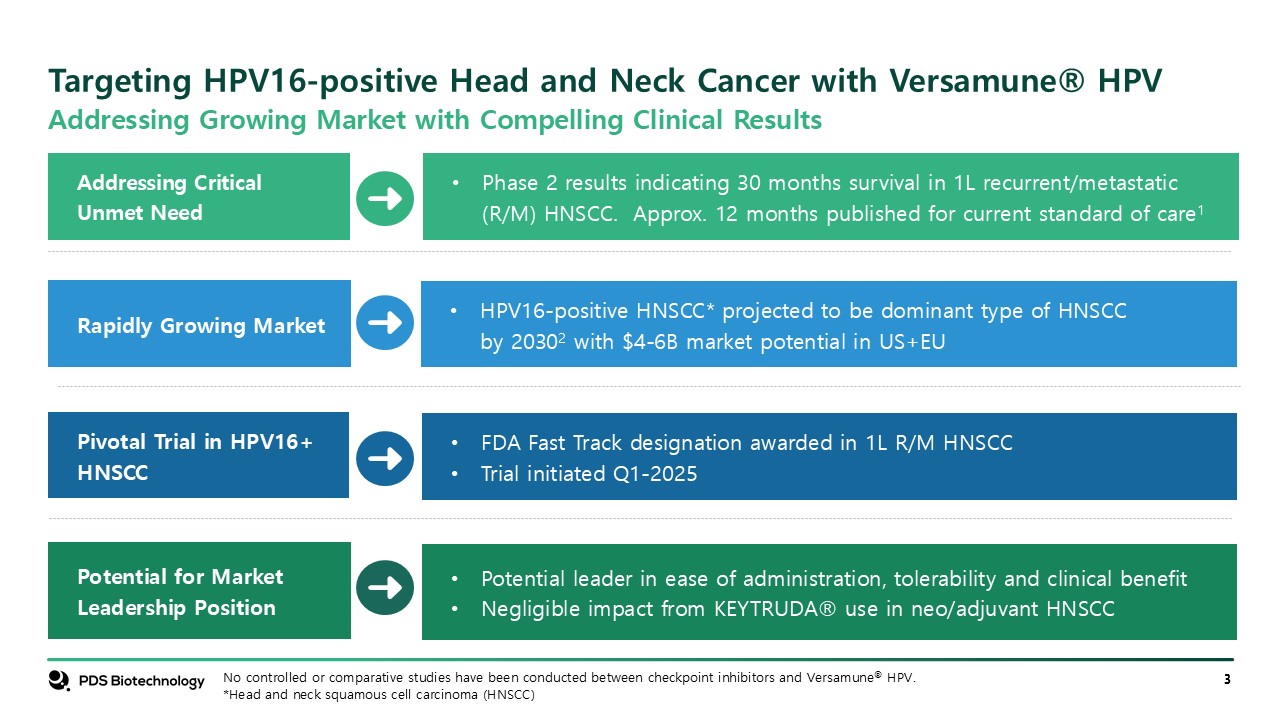
Targeting HPV16-positive Head and Neck Cancer with Versamune® HPV Potential for
Market Leadership Position Addressing Critical Unmet Need Rapidly Growing Market Pivotal Trial in HPV16+ HNSCC HPV16-positive HNSCC* projected to be dominant type of HNSCC by 20302 with $4-6B market potential in US+EU Phase 2 results
indicating 30 months survival in 1L recurrent/metastatic (R/M) HNSCC. Approx. 12 months published for current standard of care1 FDA Fast Track designation awarded in 1L R/M HNSCC Trial initiated Q1-2025 Potential leader in ease of
administration, tolerability and clinical benefit Negligible impact from KEYTRUDA® use in neo/adjuvant HNSCC No controlled or comparative studies have been conducted between checkpoint inhibitors and Versamune® HPV. *Head and neck squamous
cell carcinoma (HNSCC) Addressing Growing Market with Compelling Clinical Results
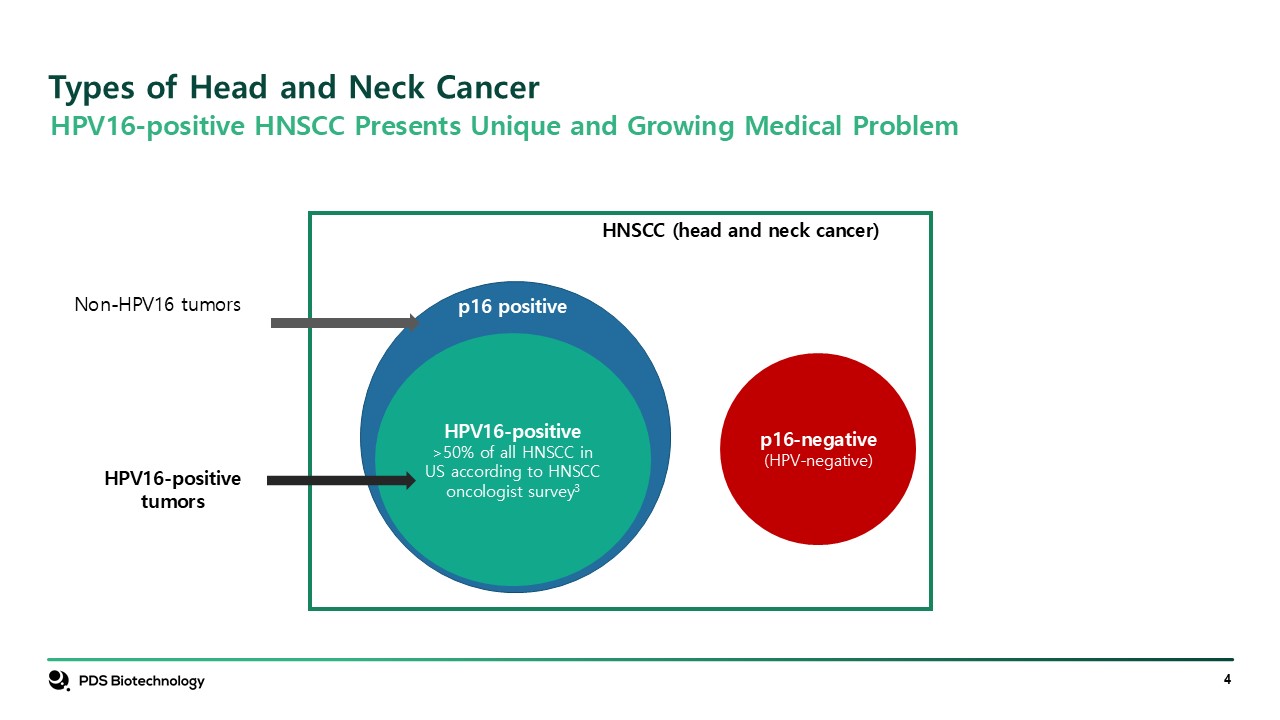
Types of Head and Neck Cancer HPV16-positive >50% of all HNSCC in US
according to HNSCC oncologist survey3 p16 positive p16-negative (HPV-negative) HPV16-positive tumors HNSCC (head and neck cancer) Non-HPV16 tumors HPV16-positive HNSCC Presents Unique and Growing Medical Problem
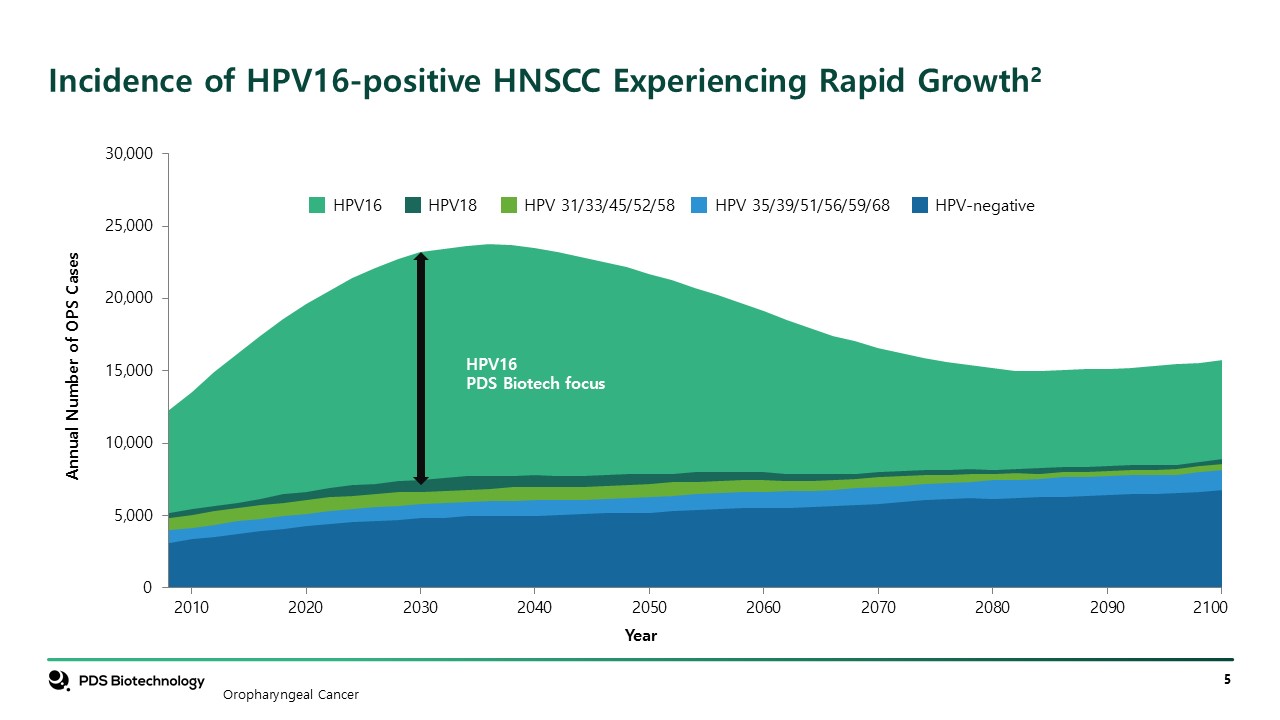
Incidence of HPV16-positive HNSCC Experiencing Rapid Growth2 HPV16 HPV18 HPV
31/33/45/52/58 HPV 35/39/51/56/59/68 HPV-negative Oropharyngeal Cancer
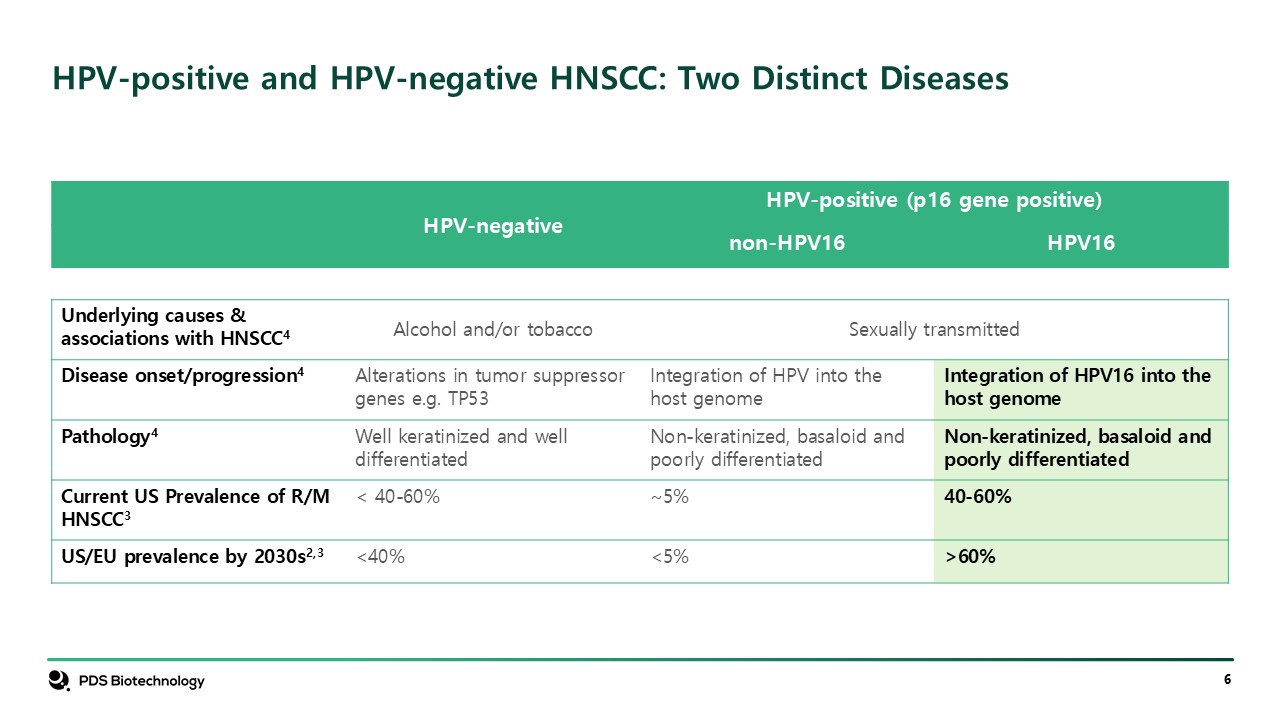
HPV-negative HPV-positive (p16 gene positive) non-HPV16 HPV16 Underlying
causes & associations with HNSCC4 Alcohol and/or tobacco Sexually transmitted Disease onset/progression4 Alterations in tumor suppressor genes e.g. TP53 Integration of HPV into the host genome Integration of HPV16 into the host
genome Pathology4 Well keratinized and well differentiated Non-keratinized, basaloid and poorly differentiated Non-keratinized, basaloid and poorly differentiated Current US Prevalence of R/M HNSCC3 < 40-60% ~5% 40-60% US/EU
prevalence by 2030s2,3 <40% <5% >60% HPV-positive and HPV-negative HNSCC: Two Distinct Diseases
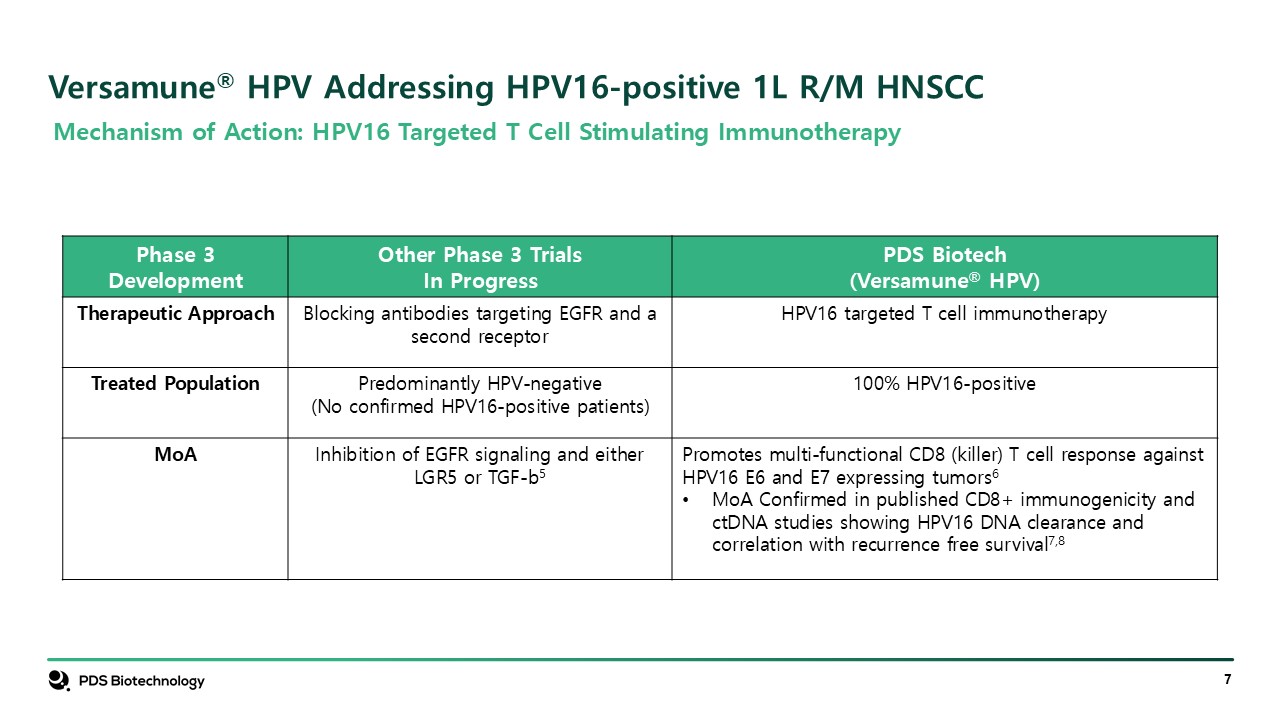
Versamune® HPV Addressing HPV16-positive 1L R/M HNSCC Phase 3 Development Other
Phase 3 Trials In Progress PDS Biotech (Versamune® HPV) Therapeutic Approach Blocking antibodies targeting EGFR and a second receptor HPV16 targeted T cell immunotherapy Treated Population Predominantly HPV-negative (No confirmed
HPV16-positive patients) 100% HPV16-positive MoA Inhibition of EGFR signaling and either LGR5 or TGF-b5 Promotes multi-functional CD8 (killer) T cell response against HPV16 E6 and E7 expressing tumors6 MoA Confirmed in published CD8+
immunogenicity and ctDNA studies showing HPV16 DNA clearance and correlation with recurrence free survival7,8 Mechanism of Action: HPV16 Targeted T Cell Stimulating Immunotherapy
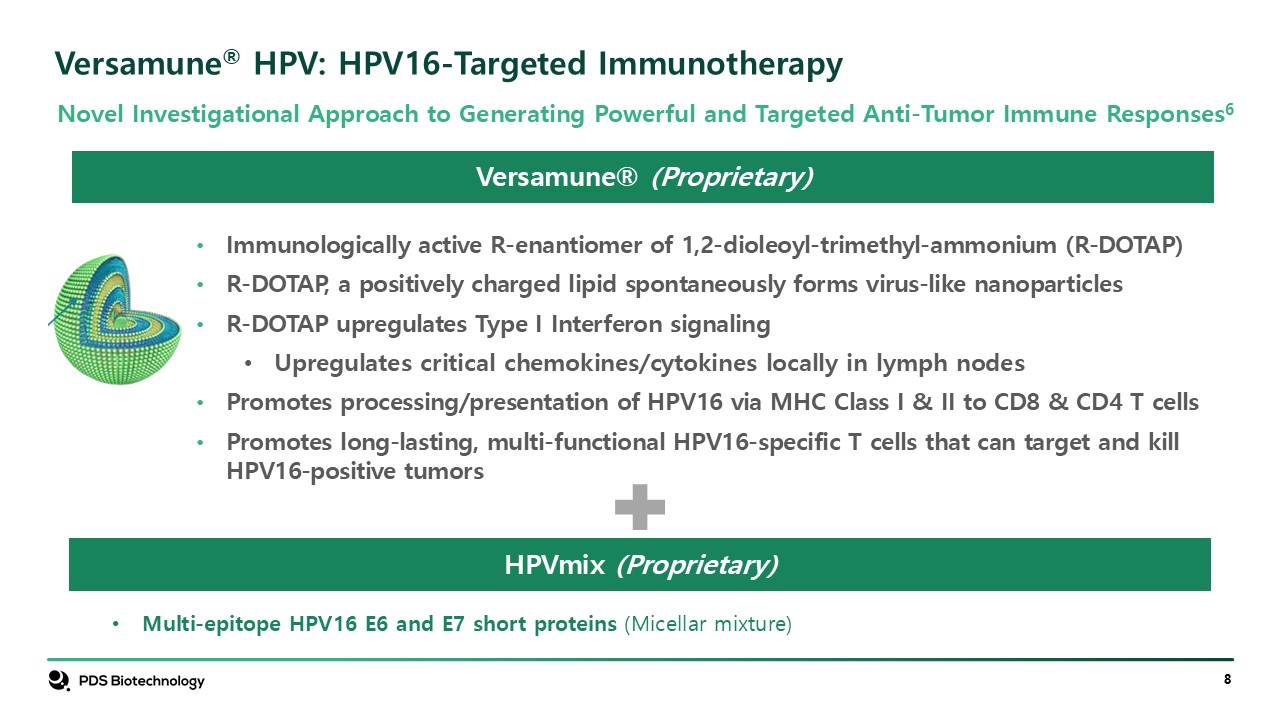
Versamune® HPV: HPV16-Targeted Immunotherapy Versamune®
(Proprietary) Immunologically active R-enantiomer of 1,2-dioleoyl-trimethyl-ammonium (R-DOTAP) R-DOTAP, a positively charged lipid spontaneously forms virus-like nanoparticles R-DOTAP upregulates Type I Interferon signaling Upregulates
critical chemokines/cytokines locally in lymph nodes Promotes processing/presentation of HPV16 via MHC Class I & II to CD8 & CD4 T cells Promotes long-lasting, multi-functional HPV16-specific T cells that can target and kill
HPV16-positive tumors Multi-epitope HPV16 E6 and E7 short proteins (Micellar mixture) HPVmix (Proprietary) Novel Investigational Approach to Generating Powerful and Targeted Anti-Tumor Immune Responses6
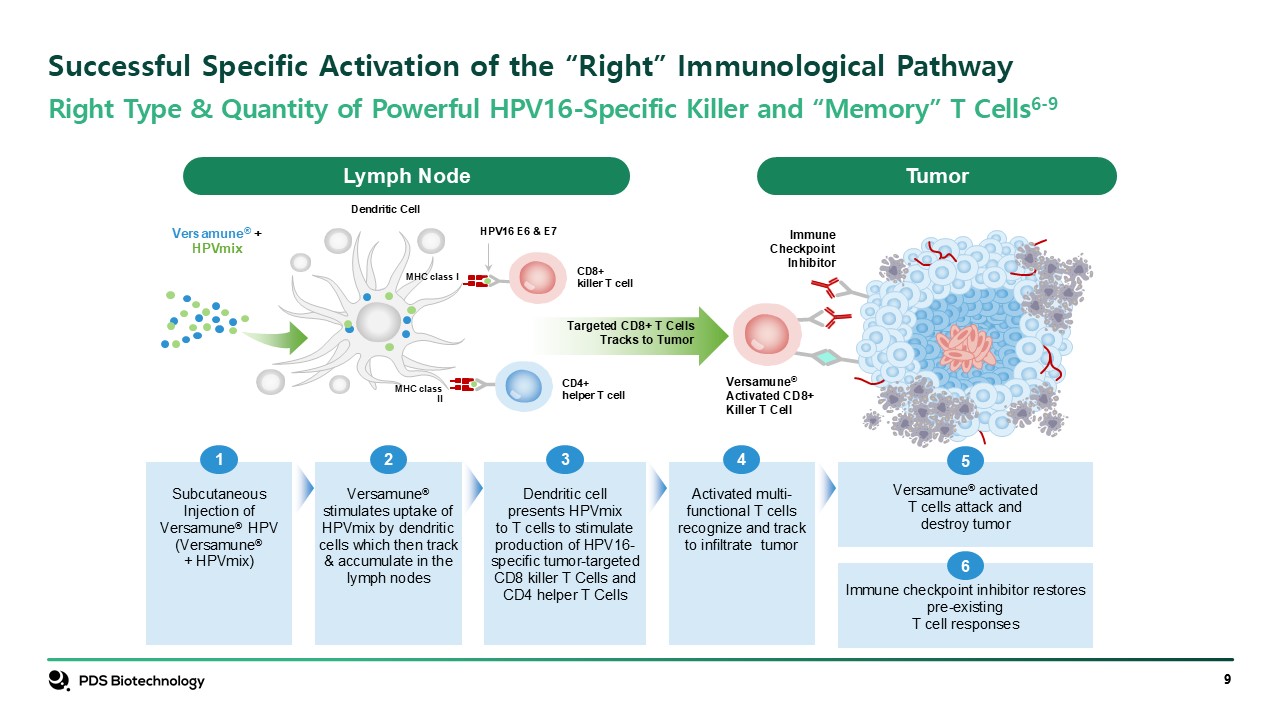
Right Type & Quantity of Powerful HPV16-Specific Killer and “Memory” T
Cells6-9 Successful Specific Activation of the “Right” Immunological Pathway Lymph Node Tumor CD4+helper T cell CD8+killer T cell HPV16 E6 & E7 Versamune®Activated CD8+Killer T Cell ImmuneCheckpointInhibitor Versamune®
+HPVmix Targeted CD8+ T CellsTracks to Tumor Dendritic Cell MHC class I MHC class II Subcutaneous Injection of Versamune® HPV (Versamune® + HPVmix) 1 Versamune® stimulates uptake of HPVmix by dendritic cells which then track &
accumulate in the lymph nodes 2 Dendritic cellpresents HPVmixto T cells to stimulateproduction of HPV16- specific tumor-targetedCD8 killer T Cells and CD4 helper T Cells 3 Activated multi-functional T cells recognize and trackto infiltrate
tumor 4 Versamune® activated T cells attack anddestroy tumor 5 Immune checkpoint inhibitor restores pre-existing T cell responses 6
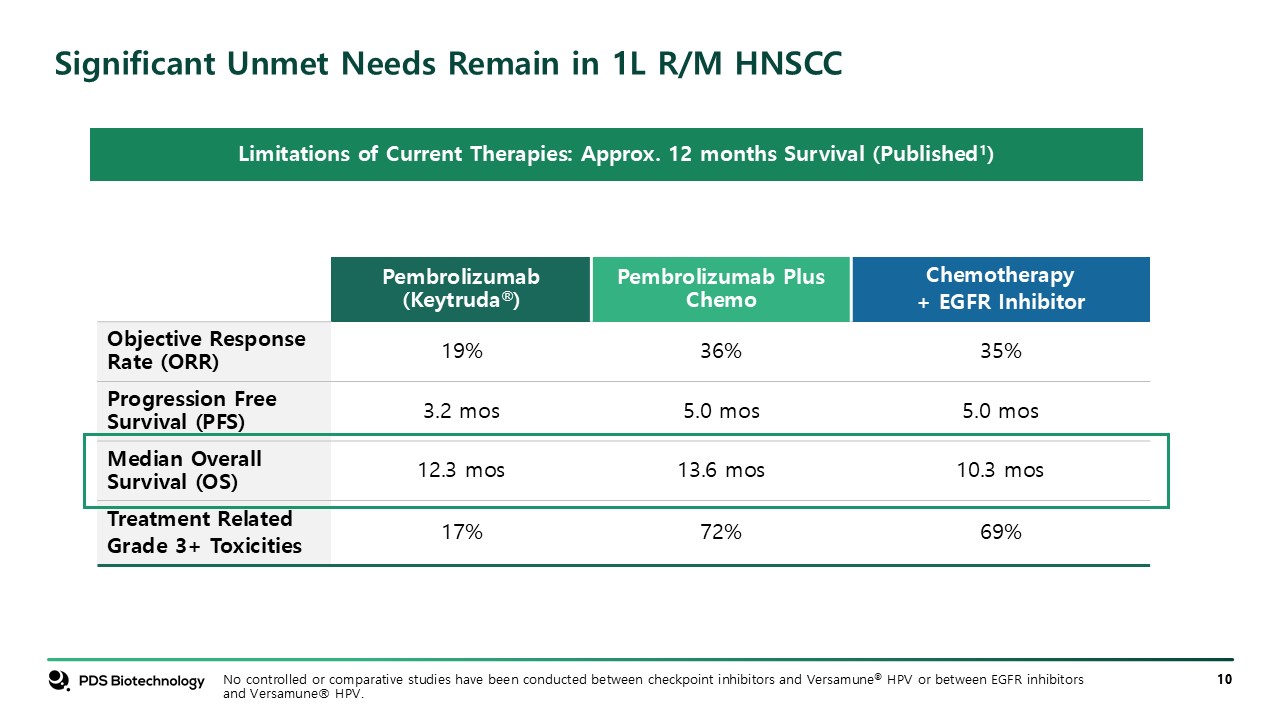
Significant Unmet Needs Remain in 1L R/M HNSCC Pembrolizumab
(Keytruda®) Pembrolizumab Plus Chemo Chemotherapy + EGFR Inhibitor Objective Response Rate (ORR) 19% 36% 35% Progression Free Survival (PFS) 3.2 mos 5.0 mos 5.0 mos Median Overall Survival (OS) 12.3 mos 13.6 mos 10.3
mos Treatment Related Grade 3+ Toxicities 17% 72% 69% Limitations of Current Therapies: Approx. 12 months Survival (Published1) No controlled or comparative studies have been conducted between checkpoint inhibitors and Versamune® HPV or
between EGFR inhibitors and Versamune® HPV.
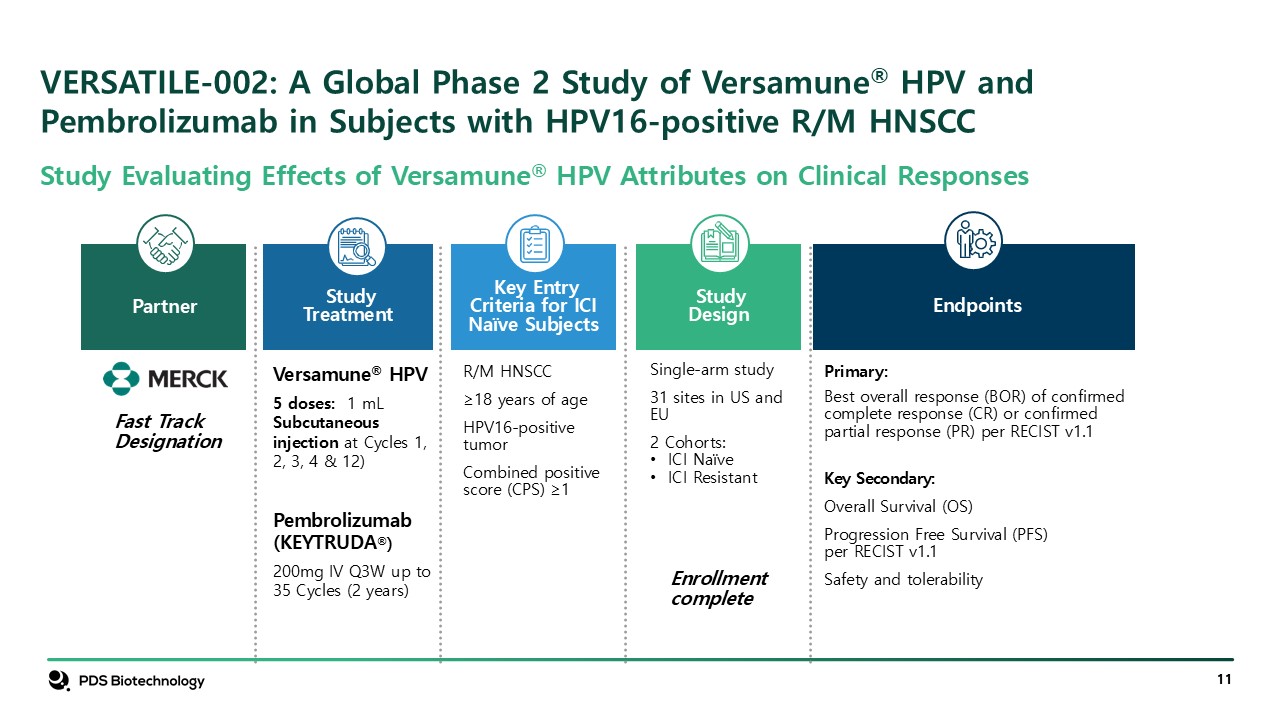
VERSATILE-002: A Global Phase 2 Study of Versamune® HPV and Pembrolizumab in
Subjects with HPV16-positive R/M HNSCC Partner StudyDesign Single-arm study 31 sites in US and EU 2 Cohorts: ICI Naïve ICI Resistant Enrollment complete Key Entry Criteria for ICI Naïve Subjects R/M HNSCC ≥18 years of
age HPV16-positive tumor Combined positive score (CPS) ≥1 Versamune® HPV 5 doses: 1 mL Subcutaneous injection at Cycles 1, 2, 3, 4 & 12) Pembrolizumab (KEYTRUDA®) 200mg IV Q3W up to 35 Cycles (2 years) Study
Treatment Primary: Best overall response (BOR) of confirmed complete response (CR) or confirmed partial response (PR) per RECIST v1.1 Key Secondary: Overall Survival (OS) Progression Free Survival (PFS) per RECIST v1.1 Safety and
tolerability Endpoints Fast Track Designation Study Evaluating Effects of Versamune® HPV Attributes on Clinical Responses
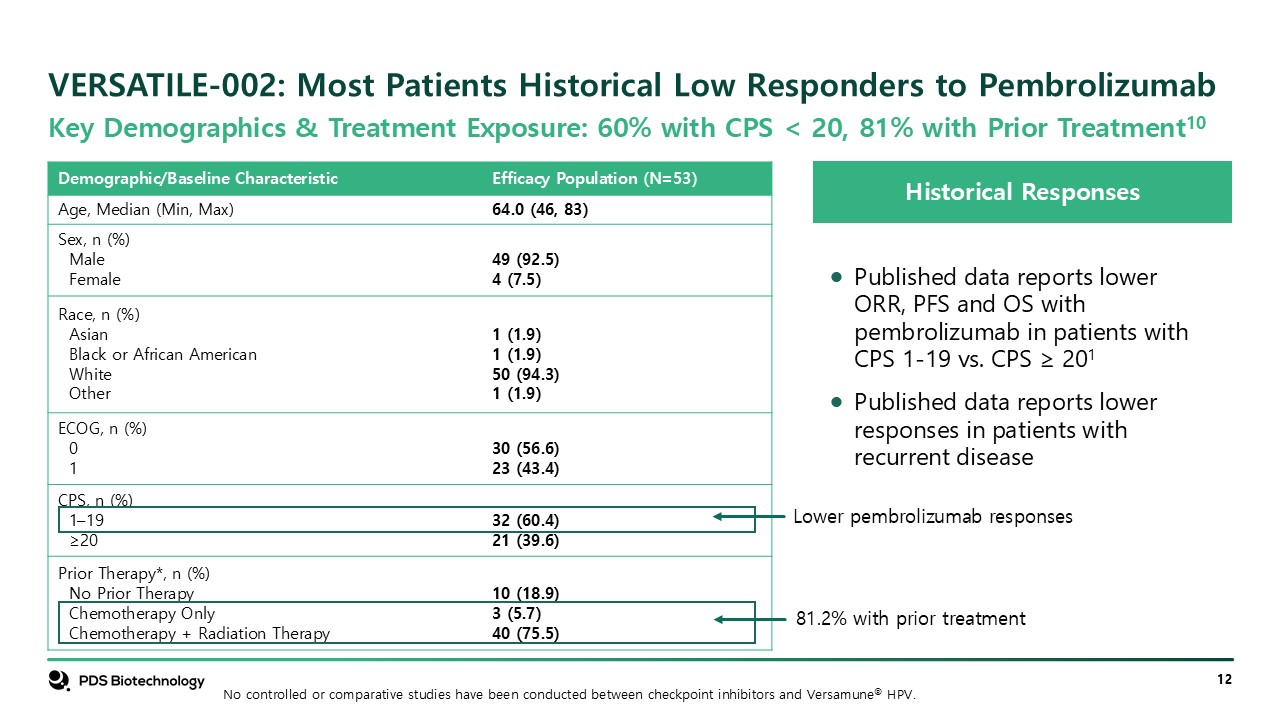
VERSATILE-002: Most Patients Historical Low Responders to
Pembrolizumab Historical Responses Published data reports lower ORR, PFS and OS with pembrolizumab in patients with CPS 1-19 vs. CPS ≥ 201 Published data reports lower responses in patients with recurrent disease Key Demographics &
Treatment Exposure: 60% with CPS < 20, 81% with Prior Treatment10 Demographic/Baseline Characteristic Efficacy Population (N=53) Age, Median (Min, Max) 64.0 (46, 83) Sex, n (%) Male Female 49 (92.5) 4 (7.5) Race, n
(%) Asian Black or African American White Other 1 (1.9) 1 (1.9) 50 (94.3) 1 (1.9) ECOG, n (%) 0 1 30 (56.6) 23 (43.4) CPS, n (%) 1–19 ≥20 32 (60.4) 21 (39.6) Prior Therapy*, n (%) No Prior Therapy Chemotherapy
Only Chemotherapy + Radiation Therapy 10 (18.9) 3 (5.7) 40 (75.5) Lower pembrolizumab responses 81.2% with prior treatment No controlled or comparative studies have been conducted between checkpoint inhibitors and Versamune® HPV.
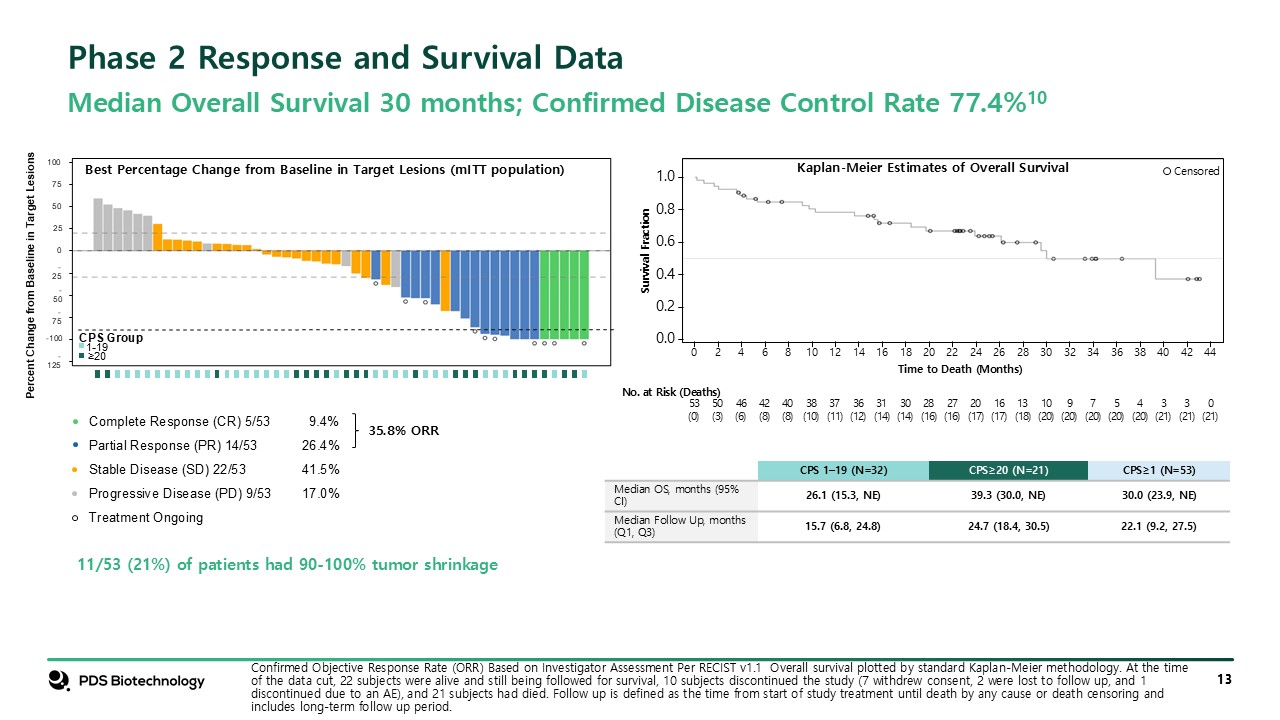
Confirmed Objective Response Rate (ORR) Based on Investigator Assessment Per
RECIST v1.1 Overall survival plotted by standard Kaplan-Meier methodology. At the time of the data cut, 22 subjects were alive and still being followed for survival, 10 subjects discontinued the study (7 withdrew consent, 2 were lost to follow
up, and 1 discontinued due to an AE), and 21 subjects had died. Follow up is defined as the time from start of study treatment until death by any cause or death censoring and includes long-term follow up period. Phase 2 Response and Survival
Data Median Overall Survival 30 months; Confirmed Disease Control Rate 77.4%10 Percent Change from Baseline in Target Lesions -50 -25 25 50 75 100 0 -75 -100 -125 CPS Group 1-19 ≥20 Complete Response (CR) 5/53 9.4% Partial
Response (PR) 14/53 26.4% Stable Disease (SD) 22/53 41.5% Progressive Disease (PD) 9/53 17.0% Treatment Ongoing Best Percentage Change from Baseline in Target Lesions (mITT population) 11/53 (21%) of patients had 90-100% tumor shrinkage
35.8% ORR 0.0 0.2 0.4 0.6 0.8 1.0 0 2 4 6 8 10 12 14 16 18 20 22 24 26 28 30 32 34 44 36 38 40 42 Time to Death (Months) No. at Risk (Deaths) 53 (0) 50 (3) 46 (6) 42 (8) 40 (8) 38 (10) 37 (11) 36
(12) 31 (14) 30 (14) 28 (16) 27 (16) 20 (17) 16 (17) 13 (18) 10 (20) 9 (20) 7 (20) 5 (20) 4 (20) 3 (21) 3 (21) 0 (21) Survival Fraction Censored CPS 1–19 (N=32) CPS≥20 (N=21) CPS≥1 (N=53) Median OS, months (95% CI) 26.1
(15.3, NE) 39.3 (30.0, NE) 30.0 (23.9, NE) Median Follow Up, months (Q1, Q3) 15.7 (6.8, 24.8) 24.7 (18.4, 30.5) 22.1 (9.2, 27.5) Kaplan-Meier Estimates of Overall Survival
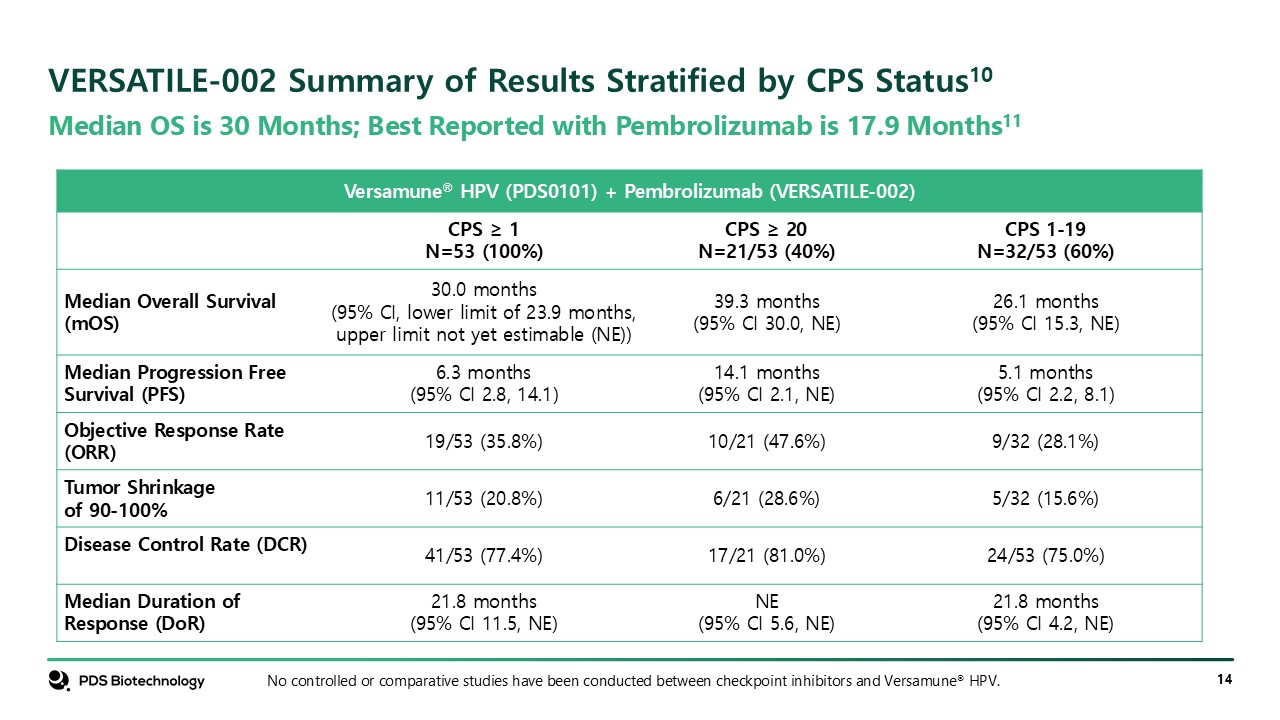
Median OS is 30 Months; Best Reported with Pembrolizumab is 17.9
Months11 VERSATILE-002 Summary of Results Stratified by CPS Status10 No controlled or comparative studies have been conducted between checkpoint inhibitors and Versamune® HPV. Versamune® HPV (PDS0101) + Pembrolizumab (VERSATILE-002) CPS
≥ 1 N=53 (100%) CPS ≥ 20 N=21/53 (40%) CPS 1-19 N=32/53 (60%) Median Overall Survival (mOS) 30.0 months (95% CI, lower limit of 23.9 months, upper limit not yet estimable (NE)) 39.3 months (95% CI 30.0, NE) 26.1 months (95% CI 15.3,
NE) Median Progression Free Survival (PFS) 6.3 months (95% CI 2.8, 14.1) 14.1 months (95% CI 2.1, NE) 5.1 months (95% CI 2.2, 8.1) Objective Response Rate (ORR) 19/53 (35.8%) 10/21 (47.6%) 9/32 (28.1%) Tumor Shrinkage of
90-100% 11/53 (20.8%) 6/21 (28.6%) 5/32 (15.6%) Disease Control Rate (DCR) 41/53 (77.4%) 17/21 (81.0%) 24/53 (75.0%) Median Duration of Response (DoR) 21.8 months (95% CI 11.5, NE) NE (95% CI 5.6, NE) 21.8 months (95% CI 4.2, NE)
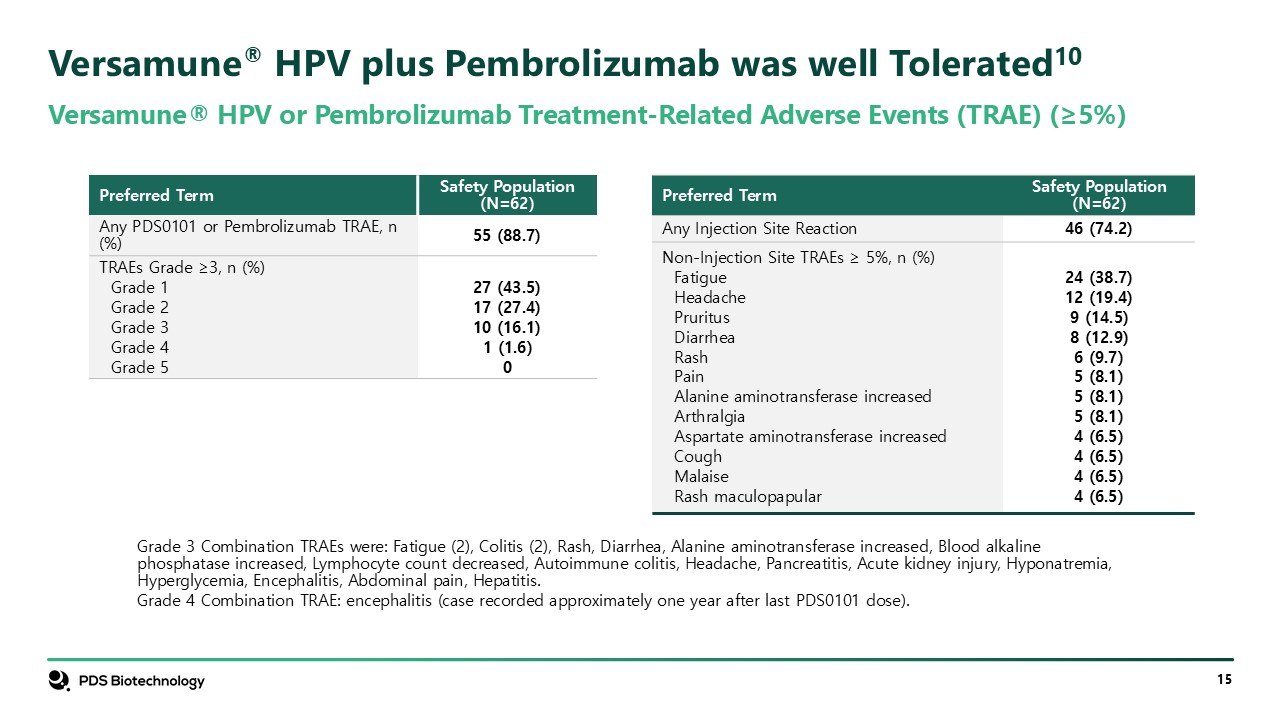
Versamune® HPV plus Pembrolizumab was well Tolerated10 Versamune® HPV or
Pembrolizumab Treatment-Related Adverse Events (TRAE) (≥5%) Grade 3 Combination TRAEs were: Fatigue (2), Colitis (2), Rash, Diarrhea, Alanine aminotransferase increased, Blood alkaline phosphatase increased, Lymphocyte count decreased,
Autoimmune colitis, Headache, Pancreatitis, Acute kidney injury, Hyponatremia, Hyperglycemia, Encephalitis, Abdominal pain, Hepatitis. Grade 4 Combination TRAE: encephalitis (case recorded approximately one year after last PDS0101
dose). Preferred Term Safety Population (N=62) Any PDS0101 or Pembrolizumab TRAE, n (%) 55 (88.7) TRAEs Grade ≥3, n (%) Grade 1 Grade 2 Grade 3 Grade 4 Grade 5 27 (43.5) 17 (27.4) 10 (16.1) 1 (1.6) 0 Preferred Term Safety
Population (N=62) Any Injection Site Reaction 46 (74.2) Non-Injection Site TRAEs ≥ 5%, n (%) Fatigue Headache Pruritus Diarrhea Rash Pain Alanine aminotransferase increased Arthralgia Aspartate aminotransferase
increased Cough Malaise Rash maculopapular 24 (38.7) 12 (19.4) 9 (14.5) 8 (12.9) 6 (9.7) 5 (8.1) 5 (8.1) 5 (8.1) 4 (6.5) 4 (6.5) 4 (6.5) 4 (6.5)
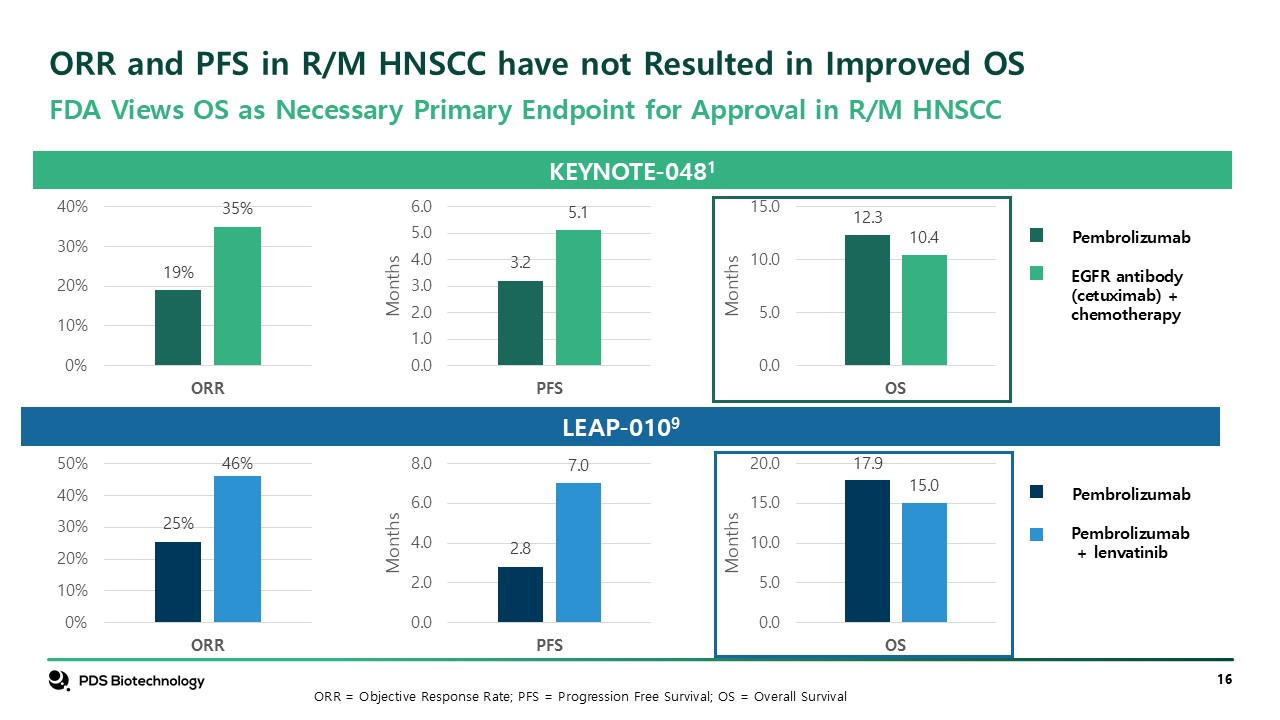
ORR and PFS in R/M HNSCC have not Resulted in Improved
OS KEYNOTE-0481 Pembrolizumab EGFR antibody (cetuximab) + chemotherapy LEAP-0109 Pembrolizumab Pembrolizumab + lenvatinib FDA Views OS as Necessary Primary Endpoint for Approval in R/M HNSCC ORR = Objective Response Rate; PFS =
Progression Free Survival; OS = Overall Survival
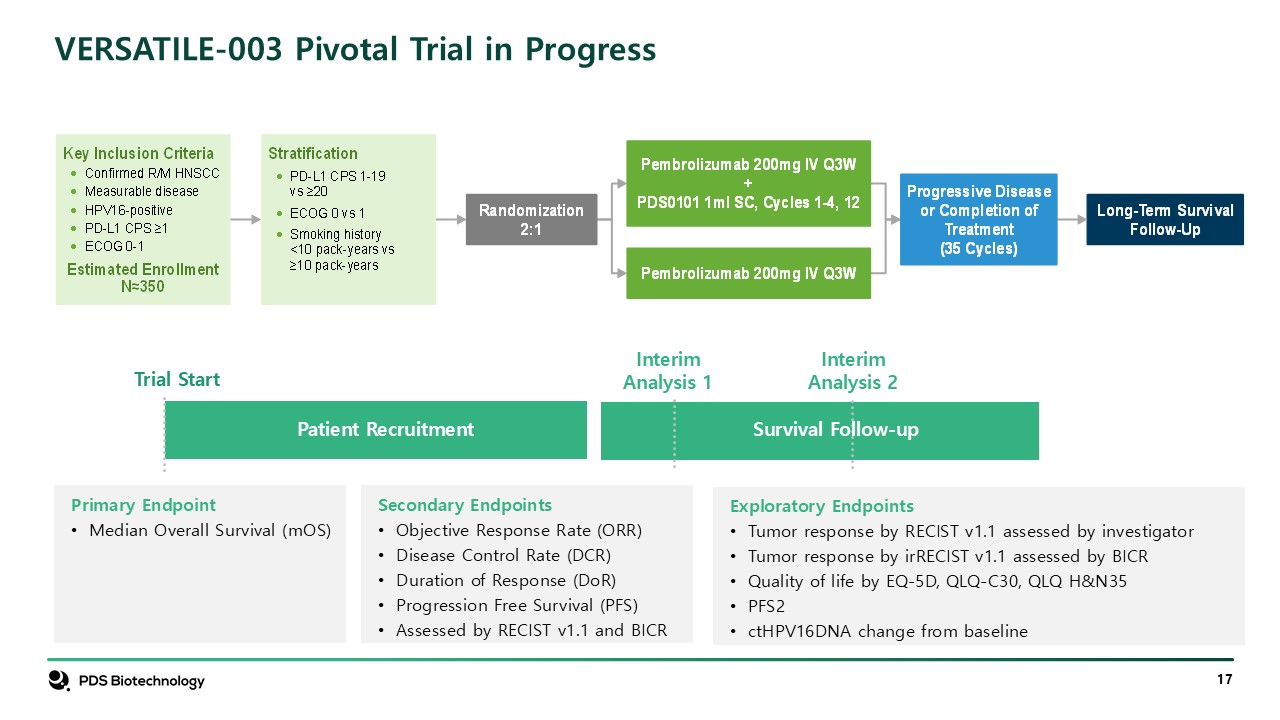
VERSATILE-003 Pivotal Trial in Progress Interim Analysis 1 Trial
Start Patient Recruitment Survival Follow-up Interim Analysis 2 Secondary Endpoints Objective Response Rate (ORR) Disease Control Rate (DCR) Duration of Response (DoR) Progression Free Survival (PFS) Assessed by RECIST v1.1 and
BICR Primary Endpoint Median Overall Survival (mOS) Exploratory Endpoints Tumor response by RECIST v1.1 assessed by investigator Tumor response by irRECIST v1.1 assessed by BICR Quality of life by EQ-5D, QLQ-C30, QLQ
H&N35 PFS2 ctHPV16DNA change from baseline
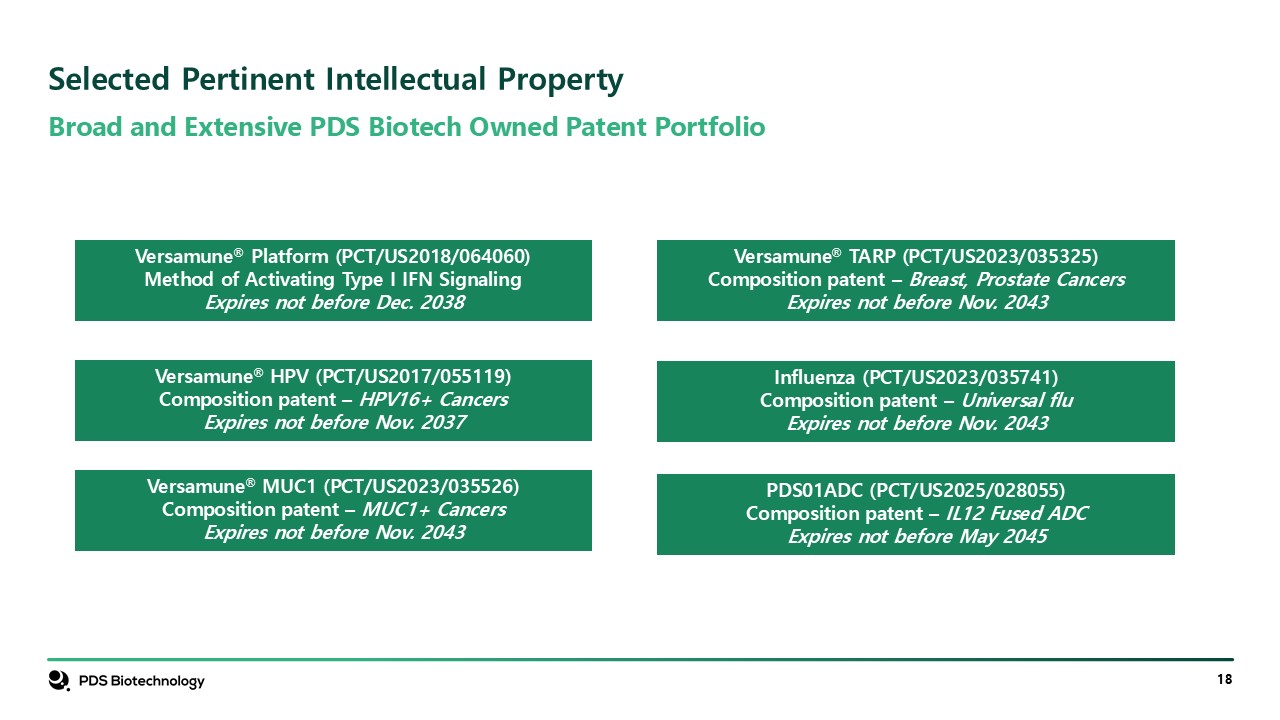
Broad and Extensive PDS Biotech Owned Patent Portfolio Selected Pertinent
Intellectual Property Versamune® Platform (PCT/US2018/064060) Method of Activating Type I IFN Signaling Expires not before Dec. 2038 Confidential Versamune® HPV (PCT/US2017/055119) Composition patent – HPV16+ Cancers Expires not before
Nov. 2037 Versamune® MUC1 (PCT/US2023/035526) Composition patent – MUC1+ Cancers Expires not before Nov. 2043 Versamune® TARP (PCT/US2023/035325) Composition patent – Breast, Prostate Cancers Expires not before Nov. 2043 Influenza
(PCT/US2023/035741) Composition patent – Universal flu Expires not before Nov. 2043 PDS01ADC (PCT/US2025/028055) Composition patent – IL12 Fused ADC Expires not before May 2045
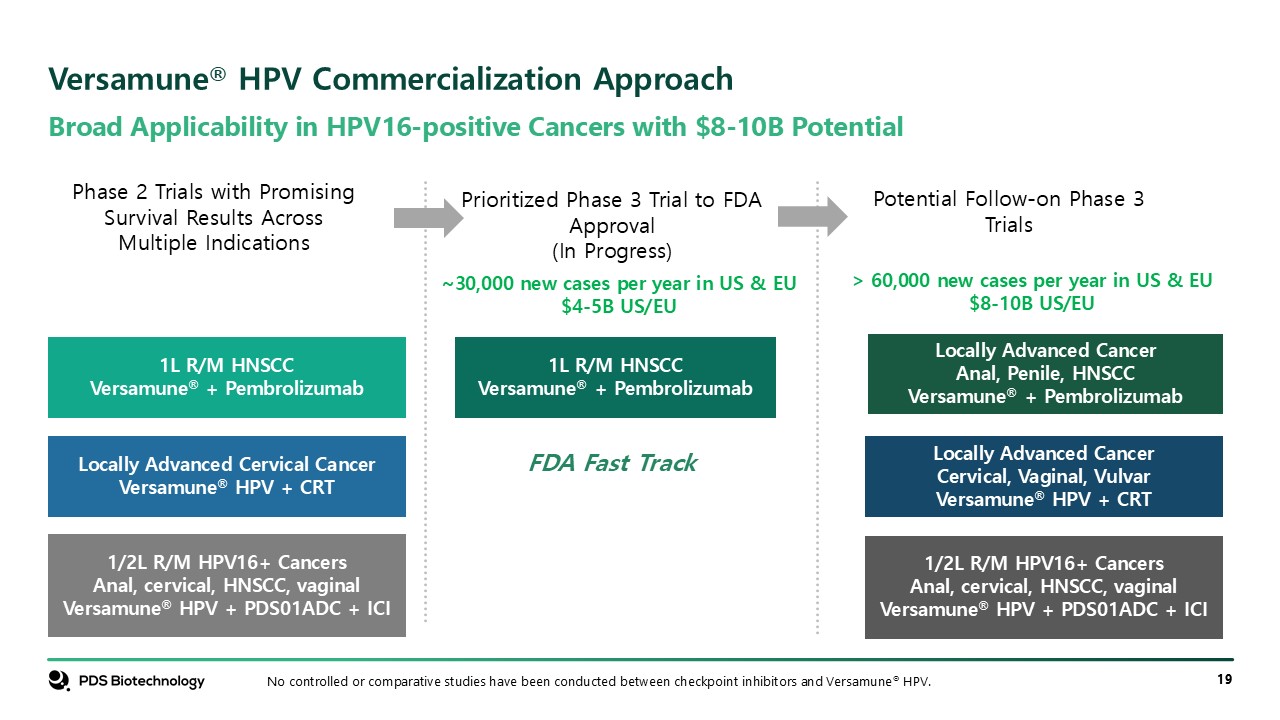
Broad Applicability in HPV16-positive Cancers with $8-10B Potential Versamune®
HPV Commercialization Approach 1L R/M HNSCC Versamune® + Pembrolizumab Confidential No controlled or comparative studies have been conducted between checkpoint inhibitors and Versamune® HPV. Locally Advanced Cervical Cancer Versamune® HPV
+ CRT 1/2L R/M HPV16+ Cancers Anal, cervical, HNSCC, vaginal Versamune® HPV + PDS01ADC + ICI 1L R/M HNSCC Versamune® + Pembrolizumab Phase 2 Trials with Promising Survival Results Across Multiple Indications Prioritized Phase 3 Trial to
FDA Approval (In Progress) Potential Follow-on Phase 3 Trials Locally Advanced Cancer Anal, Penile, HNSCC Versamune® + Pembrolizumab Locally Advanced Cancer Cervical, Vaginal, Vulvar Versamune® HPV + CRT 1/2L R/M HPV16+ Cancers Anal,
cervical, HNSCC, vaginal Versamune® HPV + PDS01ADC + ICI ~30,000 new cases per year in US & EU $4-5B US/EU > 60,000 new cases per year in US & EU $8-10B US/EU FDA Fast Track
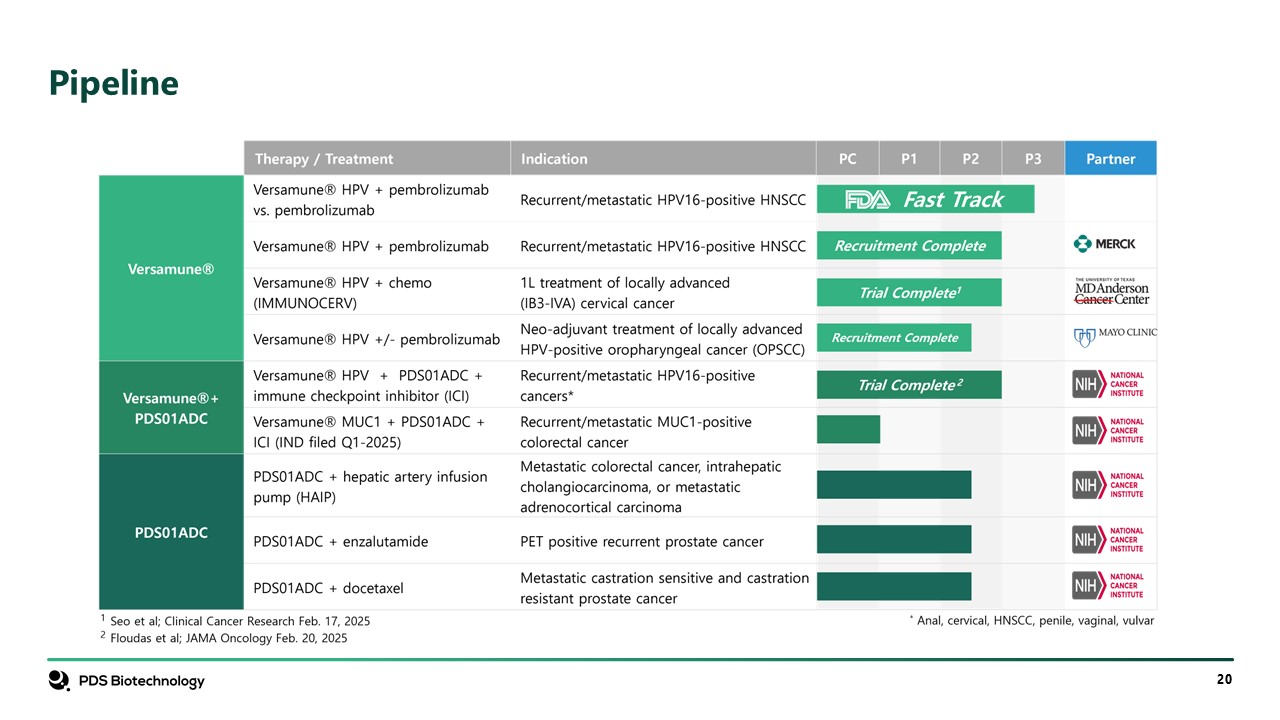
Pipeline
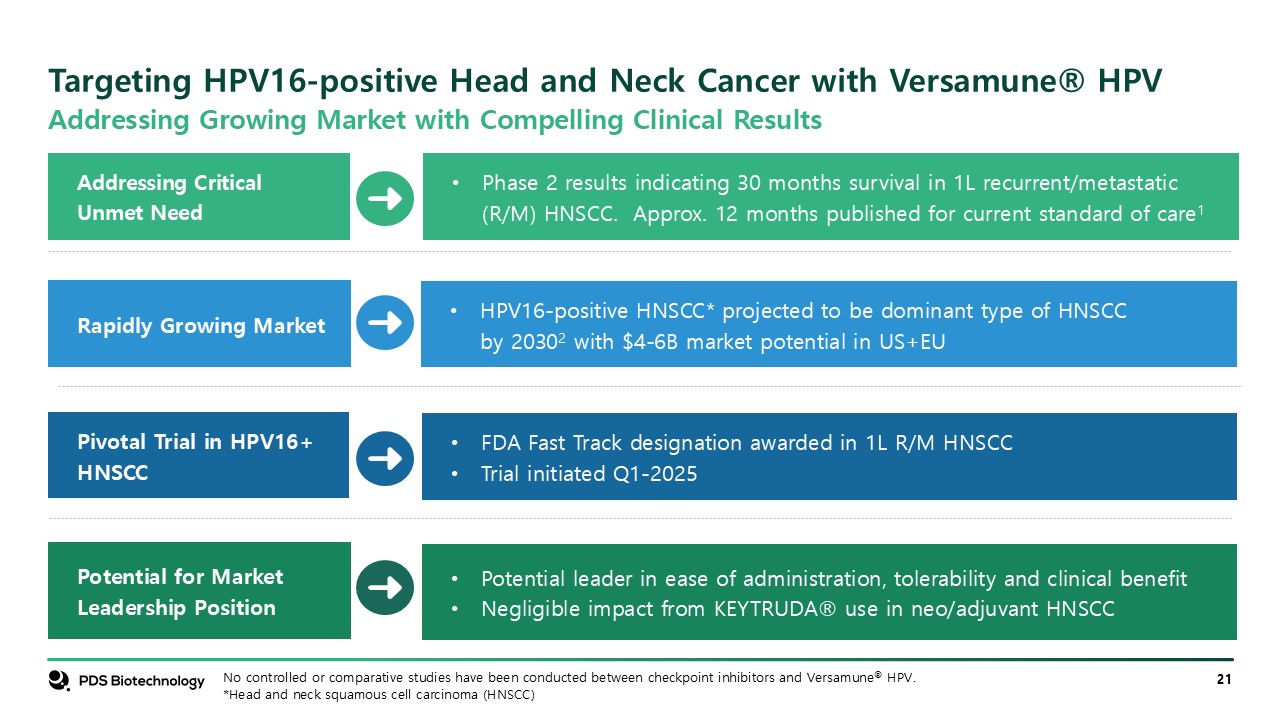
Targeting HPV16-positive Head and Neck Cancer with Versamune® HPV Potential for
Market Leadership Position Addressing Critical Unmet Need Rapidly Growing Market Pivotal Trial in HPV16+ HNSCC HPV16-positive HNSCC* projected to be dominant type of HNSCC by 20302 with $4-6B market potential in US+EU Phase 2 results
indicating 30 months survival in 1L recurrent/metastatic (R/M) HNSCC. Approx. 12 months published for current standard of care1 FDA Fast Track designation awarded in 1L R/M HNSCC Trial initiated Q1-2025 Potential leader in ease of
administration, tolerability and clinical benefit Negligible impact from KEYTRUDA® use in neo/adjuvant HNSCC No controlled or comparative studies have been conducted between checkpoint inhibitors and Versamune® HPV. *Head and neck squamous
cell carcinoma (HNSCC) Addressing Growing Market with Compelling Clinical Results
References

References Burtness B, Harrington KJ, Greil R, et al. Pembrolizumab alone or with
chemotherapy versus cetuximab with chemotherapy for recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-048): a randomized, open-label, phase 3 study. Lancet. 2019;394:1915-28. Damgacioglu H, Sonawane K, Chhatwal J,
et al. Long-term impact of HPV vaccination and COVID-19 pandemic on oropharyngeal cancer incidence and burden among men in the USA: A modeling Study. The Lancet Regional Health – Americas. 2022;8:100143. PDS Biotech sponsored third-party
market research, November 2024. Wang H, Wang S, Tang YJ, et al. The Double-Edged Sword—How Human Papillomaviruses Interact With Immunity in Head and Neck Cancer. Front. Immunol. 2019;10:doi:10.3389/fimmu.2019.00653. Lundberg AS, Geuijen CAW,
Hill S, et al. Petosemtamab, a Bispecific Antibody Targeting Epidermal Growth Factor Receptor (EGFR) and Leucine-Rich G Repeat-Containing Protein-Coupled Receptor (LGR5) Designed for Broad Clinical Applications. Cancers.
2025;17(10):https://doi.org/10.3390/cancers17101665 Gandhapudi SK, Ward M, Bush JPC, Bedu-Addo F, Conn G, Woodward JG. Antigen Priming with Enantiospecific Cationic Lipid Nanoparticles Induces Potent Antitumor CTL Responses through Novel
Induction of a Type I IFN Response. J Immunol. 2019;202:3524-3536. Seo et al, Human Papilloma Virus Circulating Cell-Free DNA Kinetics in Patients with Cervical Cancer Undergoing Definitive Chemoradiation, Clinical Cancer Research, 2025, 31
(4): 697–706. Yoshida-Court K, Gjyshi O, Lin L, et al. IMMUNOCERV, an ongoing Phase II trial combining PDS0101, an HPV-specific T cell immunotherapy with chemotherapy and radiation for treatment of locally advanced cervical cancers
(NCT04580771). Poster Presented at: SITC; November 8-12, 2022; Boston MA. Wood L, et al. A Novel Enantio-Specific Cationic Lipid R-DOTAP + HPV16 E6 & E7 Antigens Induces Potent Antigen-Specific CD8+ T Cell Responses In-Vivo in Subjects
with CIN and High-Risk Human Papillomavirus Infection. Nov 8, 2019. SITC. Presentation O17. Weiss J, et al. Overall Survival of HPV16-Positive Recurrent/Metastatic Head and Neck Squamous Cell Carcinoma Patients Treated with T Cell Stimulating
Immunotherapy PDS0101 and Pembrolizumab; Poster Presented at: ASCO Annual Meeting; June 2, 2025; Chicago, IL. Licitra L, Tahara M, Harrington K, et al. Pembrolizumab With or Without Lenvatinib As First-Line Therapy for Recurrent or Metastatic
Head and Neck Squamous Cell Carcinoma (R/M HNSCC): Phase 3 LEAP-10 Study. Poster or Paper presented at: Multidisciplinary Head and Neck Cancers Symposium; February 29-March 2, 2024; Phoenix, AZ.