

pro June 2025 Larimar Therapeutics Nomlabofusp Regulatory Update Exhibit 99.2

This presentation contains forward-looking statements that are based on the beliefs and assumptions of Larimar Therapeutics, Inc. ( “Company”) and on information currently available to management. All statements contained in this presentation other than statements of historical fact are forward-looking statements, including but not limited to Larimar’s ability to develop and commercialize nomlabofusp (CTI-1601) and other planned product candidates, Larimar’s planned research and development efforts, including the timing of its nomlabofusp clinical trials and non-clinical investigations and overall development plan expectations with respect to the FDA START pilot program, interactions with FDA, expectations regarding potential for accelerated approval or accelerated access and time to market and other matters regarding Larimar’s business strategies, ability to raise capital, use of capital, results of operations and financial position, and plans and objectives for future operations. In some cases, you can identify forward-looking statements by the words “may,” “will,” “could,” “would,” “should,” “expect,” “intend,” “plan,” “anticipate,” “believe,” “estimate,” “predict,” “project,” “potential,” “continue,” “ongoing” or the negative of these terms or other comparable terminology, although not all forward-looking statements contain these words. These statements involve risks, uncertainties and other factors that may cause actual results, performance, or achievements to be materially different from the information expressed or implied by these forward-looking statements. These risks, uncertainties and other factors include, among others, the success, cost and timing of Larimar’s product development activities, nonclinical studies and clinical trials, including nomlabofusp clinical milestones and continued interactions with the FDA; that preliminary clinical trial results may differ from final clinical trial results, that earlier non-clinical and clinical data and testing of nomlabofusp may not be predictive of the results or success of later non-clinical or clinical trials, and assessments; that the FDA may not ultimately agree with Larimar’s nomlabofusp development strategy; the potential impact of public health crises on Larimar’s future clinical trials, manufacturing, regulatory, nonclinical study timelines and operations, and general economic conditions; Larimar’s ability and the ability of third-party manufacturers Larimar engages, to optimize and scale nomlabofusp’s manufacturing process; Larimar’s ability to obtain regulatory approvals for nomlabofusp and future product candidates; Larimar’s ability to develop sales and marketing capabilities, whether alone or with potential future collaborators, and to successfully commercialize any approved product candidates; Larimar’s ability to raise the necessary capital to conduct its product development activities; and other risks described in the filings made by Larimar with the Securities and Exchange Commission (SEC), including but not limited to Larimar’s periodic reports, including the annual report on Form 10-K, quarterly reports on Form 10-Q and current reports on Form 8-K, filed with or furnished to the SEC and available at www.sec.gov. These forward-looking statements are based on a combination of facts and factors currently known by Larimar and its projections of the future, about which it cannot be certain. As a result, the forward-looking statements may not prove to be accurate. The forward-looking statements in this presentation represent Larimar’s management’s views only as of the date hereof. Larimar undertakes no obligation to update any forward-looking statements for any reason, except as required by law. Forward-Looking Statements
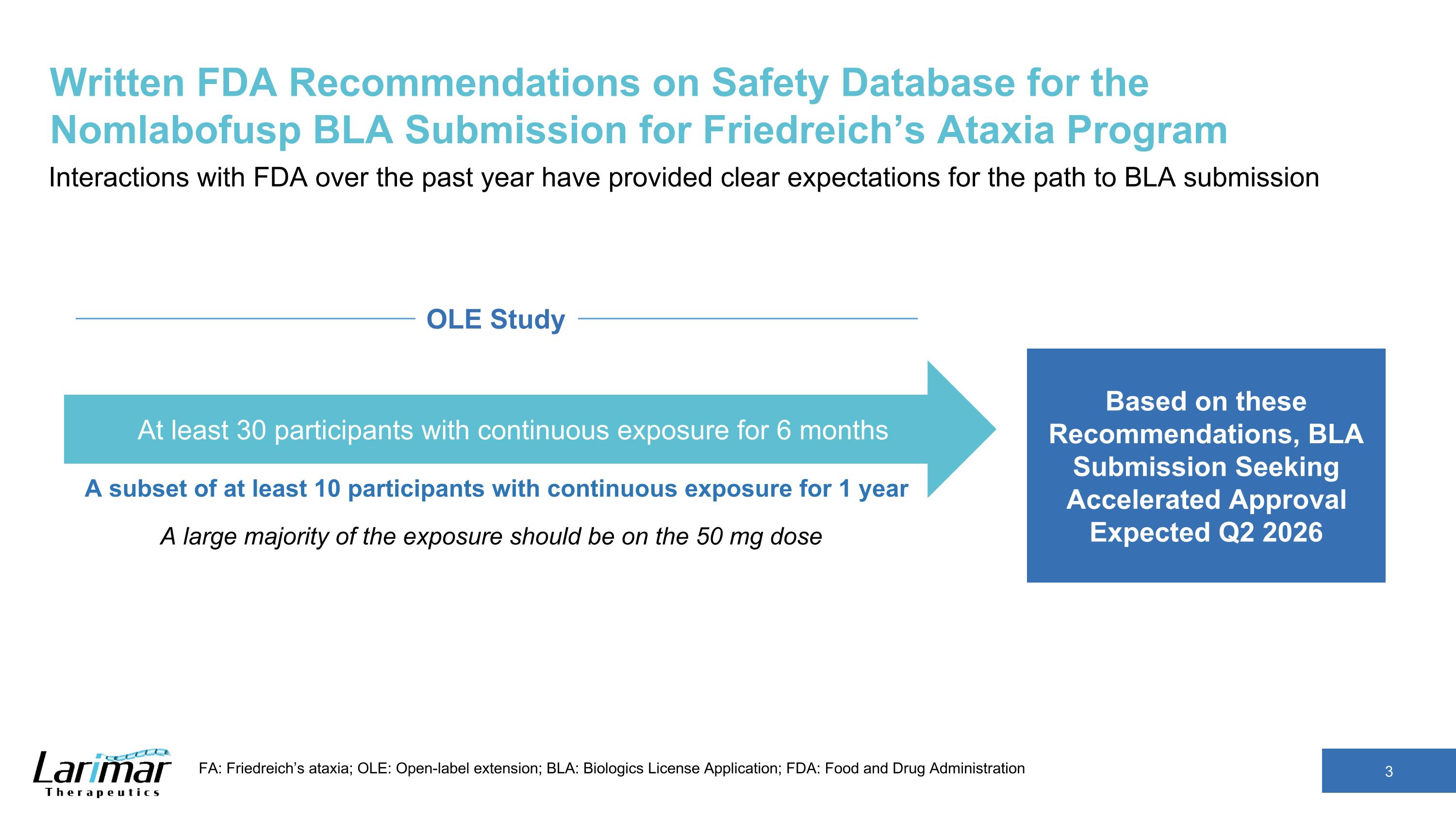
Written FDA Recommendations on Safety Database for the Nomlabofusp BLA Submission for Friedreich’s Ataxia Program Interactions with FDA over the past year have provided clear expectations for the path to BLA submission FA: Friedreich’s ataxia; OLE: Open-label extension; BLA: Biologics License Application; FDA: Food and Drug Administration Based on these Recommendations, BLA Submission Seeking Accelerated Approval Expected Q2 2026 A subset of at least 10 participants with continuous exposure for 1 year At least 30 participants with continuous exposure for 6 months A large majority of the exposure should be on the 50 mg dose OLE Study

Clear FDA Expectations for Path to BLA Submission Seeking Accelerated Approval Planned in Q2 2026 Elements of BLA Submission FDA Recommendations Use of Skin FXN Concentrations as a Surrogate Endpoint Open to use of increases in skin FXN concentrations as a RLSE Acknowledged submitted data appears to support a relationship between increased skin FXN and relevant tissues such as the heart, dorsal root ganglion, and skeletal muscle Acceptability of increases in skin FXN for accelerated approval will be decided during future BLA review Safety Database At least 30 participants with continuous exposure for 6-months A subset of at least 10 participants with continuous exposure for 1-year A large majority of the exposure should be on the 50 mg dose Clinical Data Package and Global Phase 3 Study SAD and MAD Phase 1 studies established safety and tolerability Phase 2 dose exploration study data and PK/PD data from ongoing OLE study supported 50 mg as the recommended dose Long-term data from ongoing OLE study including increases in skin FXN concentrations and safety data Global Phase 3 study, intended as the confirmatory study, to evaluate clinical outcomes including upright stability and mFARS expected to be underway at the time of BLA submission Pharmacology and Toxicology Nonclinical data supporting the use of FXN as a novel surrogate endpoint Complete toxicology package including juvenile toxicology study Clinical data includes trends towards normalization of patient lipid profiles and gene expression Chemistry Manufacturing and Controls Data supporting the lyophilized drug product with stability at room temperature Data on batches manufactured at a commercial scale Analytical methods and proposed specifications FXN: Frataxin; OLE: Open-label extension; BLA: Biologics License Application; FDA: Food and Drug Administration; RLSE: reasonably likely surrogate endpoint; SAD: Single ascending-dose ; MAD: Multiple ascending dose
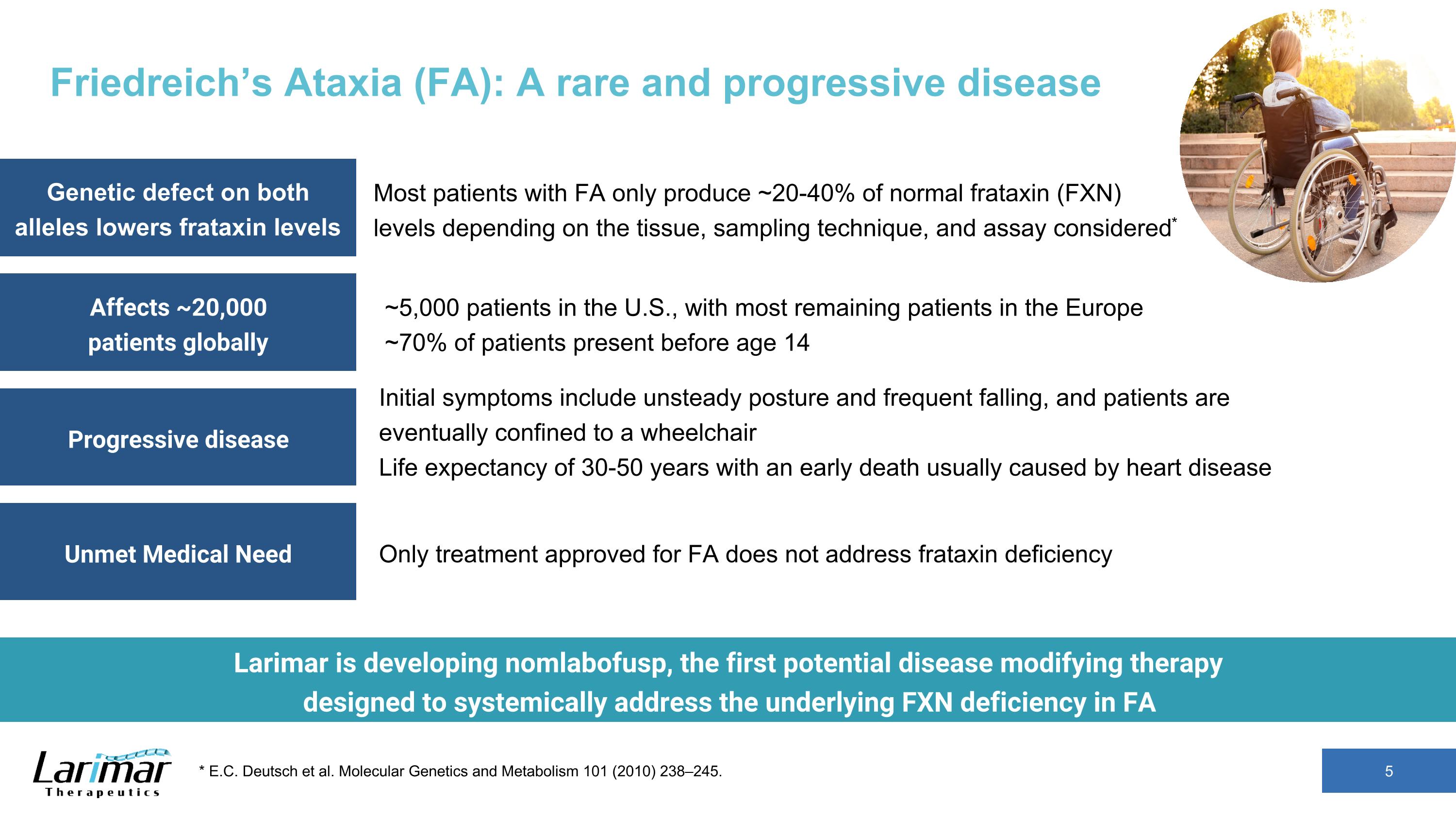
Friedreich’s Ataxia (FA): A rare and progressive disease 5 * E.C. Deutsch et al. Molecular Genetics and Metabolism 101 (2010) 238–245. Most patients with FA only produce ~20-40% of normal frataxin (FXN) levels depending on the tissue, sampling technique, and assay considered* Initial symptoms include unsteady posture and frequent falling, and patients are eventually confined to a wheelchair Life expectancy of 30-50 years with an early death usually caused by heart disease ~5,000 patients in the U.S., with most remaining patients in the Europe ~70% of patients present before age 14 Only treatment approved for FA does not address frataxin deficiency Genetic defect on both alleles lowers frataxin levels Affects ~20,000 patients globally Progressive disease Unmet Medical Need Larimar is developing nomlabofusp, the first potential disease modifying therapy designed to systemically address the underlying FXN deficiency in FA
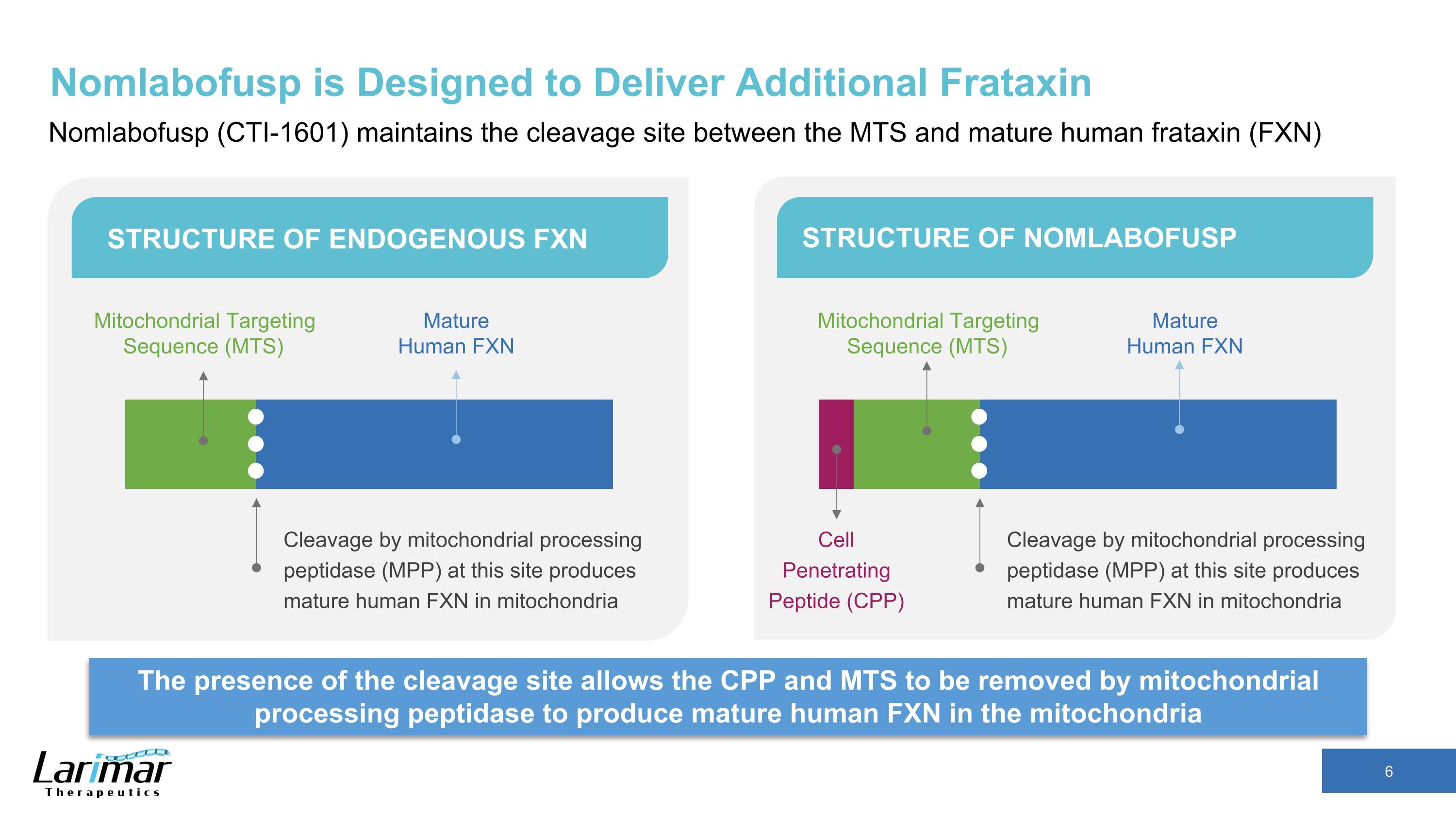
Nomlabofusp is Designed to Deliver Additional Frataxin Nomlabofusp (CTI-1601) maintains the cleavage site between the MTS and mature human frataxin (FXN) The presence of the cleavage site allows the CPP and MTS to be removed by mitochondrial processing peptidase to produce mature human FXN in the mitochondria STRUCTURE OF ENDOGENOUS FXN STRUCTURE OF NOMLABOFUSP Cleavage by mitochondrial processing peptidase (MPP) at this site produces mature human FXN in mitochondria Mitochondrial Targeting Sequence (MTS) Mature Human FXN Cleavage by mitochondrial processing peptidase (MPP) at this site produces mature human FXN in mitochondria Mature Human FXN Cell Penetrating Peptide (CPP) Mitochondrial Targeting Sequence (MTS)
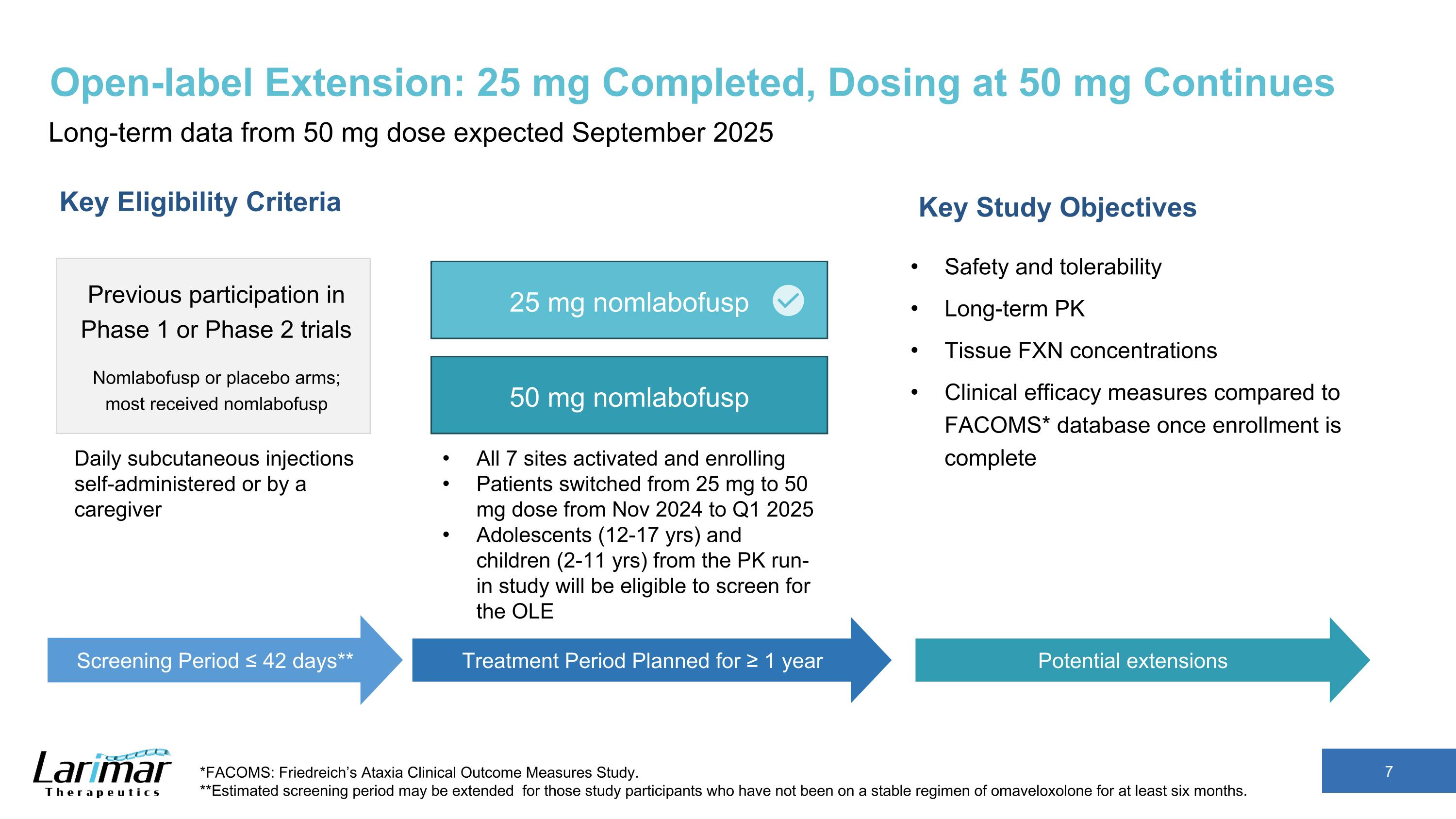
Open-label Extension: 25 mg Completed, Dosing at 50 mg Continues Long-term data from 50 mg dose expected September 2025 Key Eligibility Criteria Previous participation in Phase 1 or Phase 2 trials Nomlabofusp or placebo arms; most received nomlabofusp Key Study Objectives Safety and tolerability Long-term PK Tissue FXN concentrations Clinical efficacy measures compared to FACOMS* database once enrollment is complete *FACOMS: Friedreich’s Ataxia Clinical Outcome Measures Study. **Estimated screening period may be extended for those study participants who have not been on a stable regimen of omaveloxolone for at least six months. Screening Period ≤ 42 days** Treatment Period Planned for ≥ 1 year All 7 sites activated and enrolling Patients switched from 25 mg to 50 mg dose from Nov 2024 to Q1 2025 Adolescents (12-17 yrs) and children (2-11 yrs) from the PK run-in study will be eligible to screen for the OLE Potential extensions Daily subcutaneous injections self-administered or by a caregiver 25 mg nomlabofusp 50 mg nomlabofusp
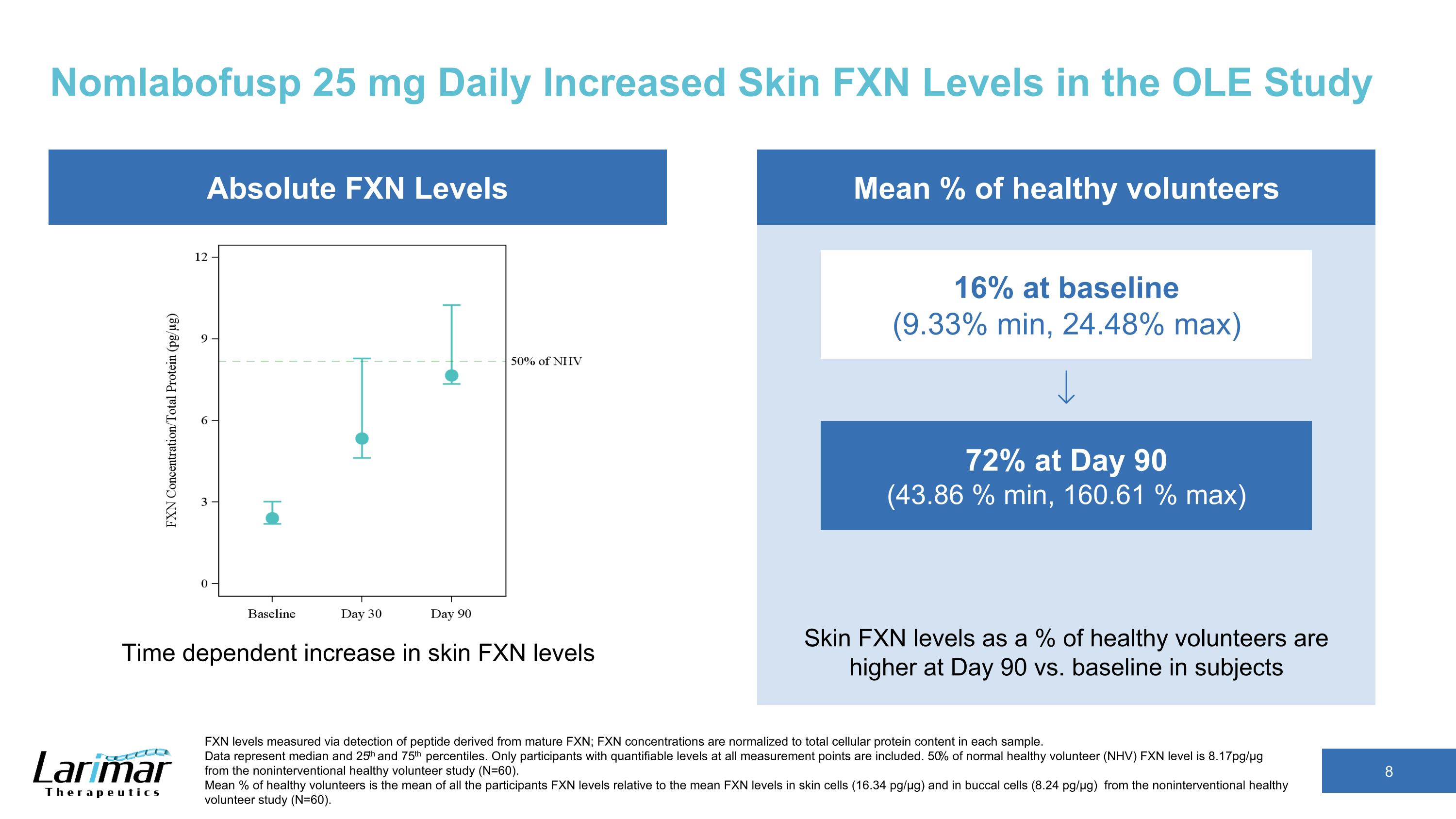
Nomlabofusp 25 mg Daily Increased Skin FXN Levels in the OLE Study 72% at Day 90 (43.86 % min, 160.61 % max) 16% at baseline (9.33% min, 24.48% max) Mean % of healthy volunteers FXN levels measured via detection of peptide derived from mature FXN; FXN concentrations are normalized to total cellular protein content in each sample. Data represent median and 25th and 75th percentiles. Only participants with quantifiable levels at all measurement points are included. 50% of normal healthy volunteer (NHV) FXN level is 8.17pg/µg from the noninterventional healthy volunteer study (N=60). Mean % of healthy volunteers is the mean of all the participants FXN levels relative to the mean FXN levels in skin cells (16.34 pg/µg) and in buccal cells (8.24 pg/µg) from the noninterventional healthy volunteer study (N=60). Skin FXN levels as a % of healthy volunteers are higher at Day 90 vs. baseline in subjects Absolute FXN Levels Time dependent increase in skin FXN levels
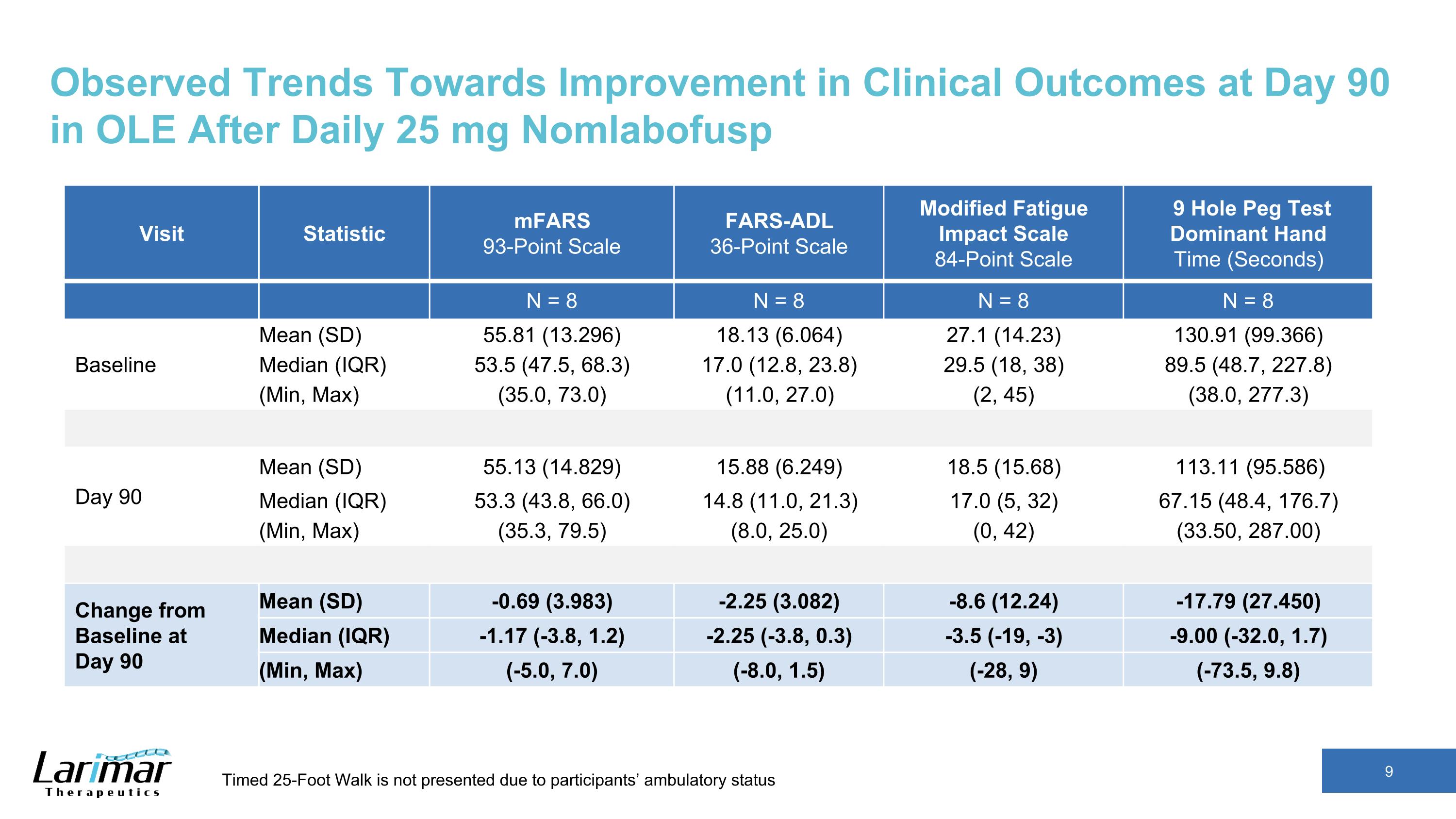
Observed Trends Towards Improvement in Clinical Outcomes at Day 90 in OLE After Daily 25 mg Nomlabofusp Visit Statistic mFARS 93-Point Scale FARS-ADL 36-Point Scale Modified Fatigue Impact Scale 84-Point Scale 9 Hole Peg Test Dominant Hand Time (Seconds) N = 8 N = 8 N = 8 N = 8 Baseline Mean (SD) 55.81 (13.296) 18.13 (6.064) 27.1 (14.23) 130.91 (99.366) Median (IQR) 53.5 (47.5, 68.3) 17.0 (12.8, 23.8) 29.5 (18, 38) 89.5 (48.7, 227.8) (Min, Max) (35.0, 73.0) (11.0, 27.0) (2, 45) (38.0, 277.3) Day 90 Mean (SD) 55.13 (14.829) 15.88 (6.249) 18.5 (15.68) 113.11 (95.586) Median (IQR) 53.3 (43.8, 66.0) 14.8 (11.0, 21.3) 17.0 (5, 32) 67.15 (48.4, 176.7) (Min, Max) (35.3, 79.5) (8.0, 25.0) (0, 42) (33.50, 287.00) Change from Baseline at Day 90 Mean (SD) -0.69 (3.983) -2.25 (3.082) -8.6 (12.24) -17.79 (27.450) Median (IQR) -1.17 (-3.8, 1.2) -2.25 (-3.8, 0.3) -3.5 (-19, -3) -9.00 (-32.0, 1.7) (Min, Max) (-5.0, 7.0) (-8.0, 1.5) (-28, 9) (-73.5, 9.8) Timed 25-Foot Walk is not presented due to participants’ ambulatory status
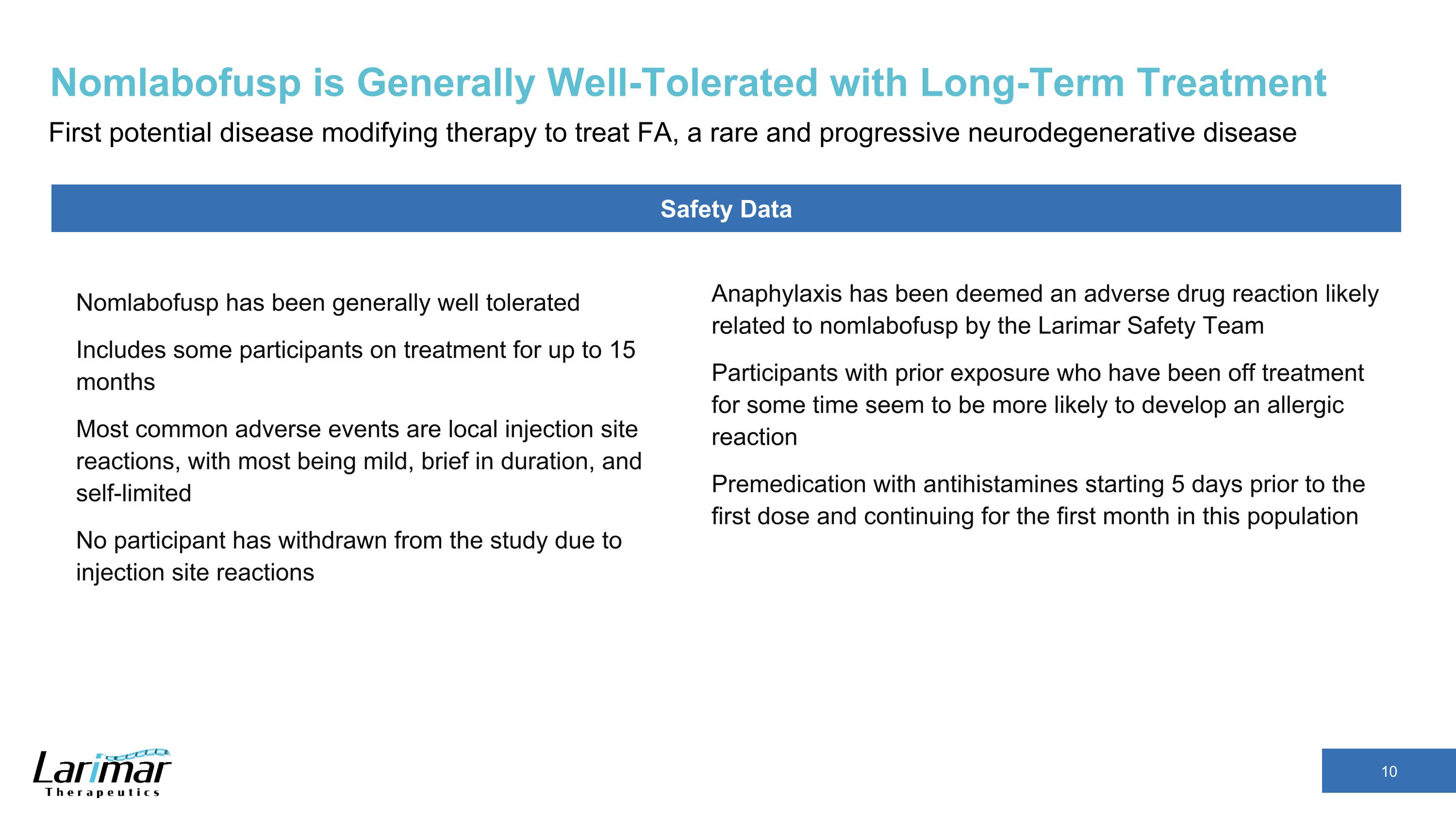
Nomlabofusp is Generally Well-Tolerated with Long-Term Treatment First potential disease modifying therapy to treat FA, a rare and progressive neurodegenerative disease Anaphylaxis has been deemed an adverse drug reaction likely related to nomlabofusp by the Larimar Safety Team Participants with prior exposure who have been off treatment for some time seem to be more likely to develop an allergic reaction Premedication with antihistamines starting 5 days prior to the first dose and continuing for the first month in this population Safety Data Nomlabofusp has been generally well tolerated Includes some participants on treatment for up to 15 months Most common adverse events are local injection site reactions, with most being mild, brief in duration, and self-limited No participant has withdrawn from the study due to injection site reactions
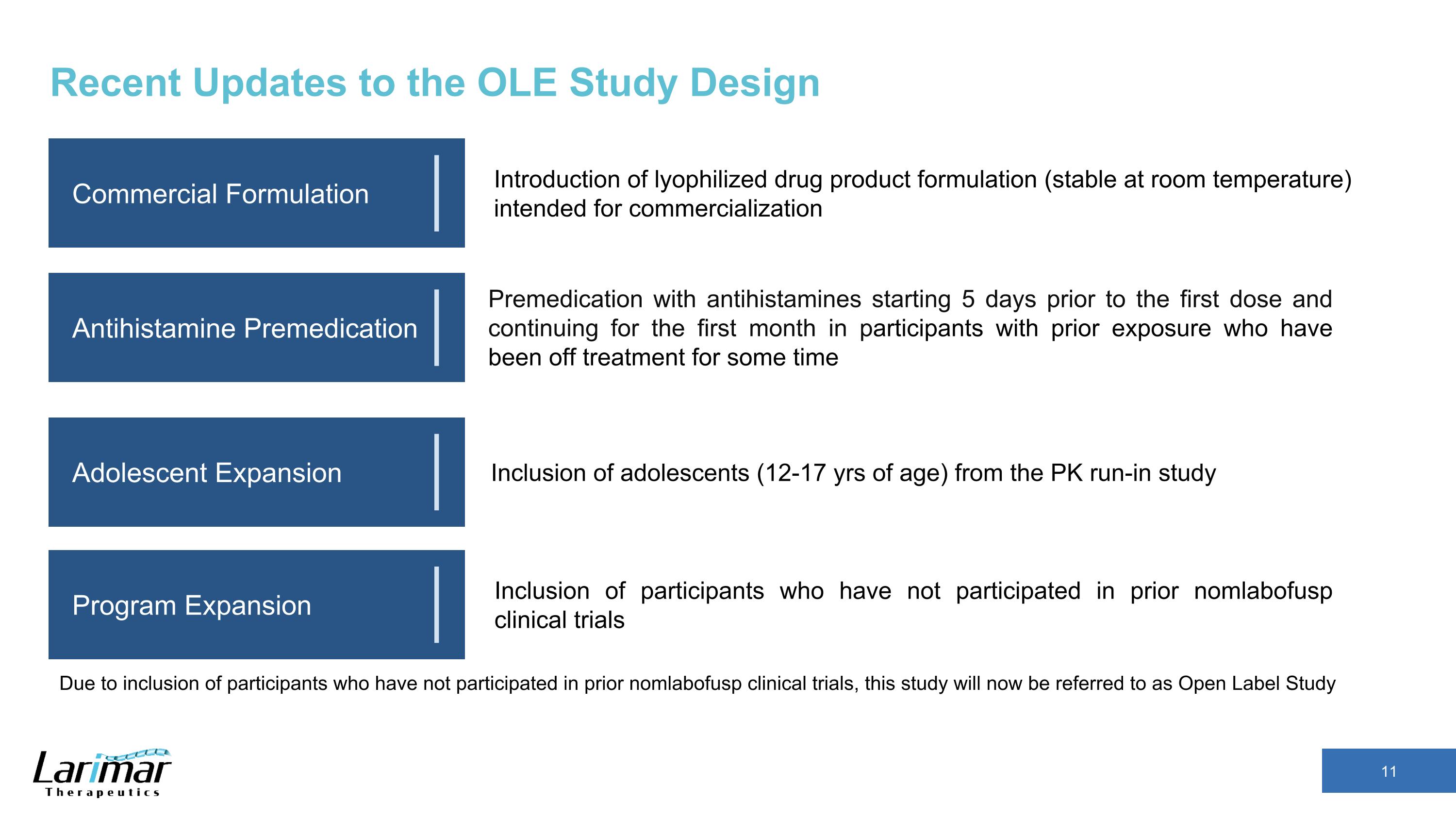
Recent Updates to the OLE Study Design Commercial Formulation Antihistamine Premedication Adolescent Expansion Program Expansion Premedication with antihistamines starting 5 days prior to the first dose and continuing for the first month in participants with prior exposure who have been off treatment for some time Introduction of lyophilized drug product formulation (stable at room temperature) intended for commercialization Inclusion of adolescents (12-17 yrs of age) from the PK run-in study Inclusion of participants who have not participated in prior nomlabofusp clinical trials Due to inclusion of participants who have not participated in prior nomlabofusp clinical trials, this study will now be referred to as Open Label Study
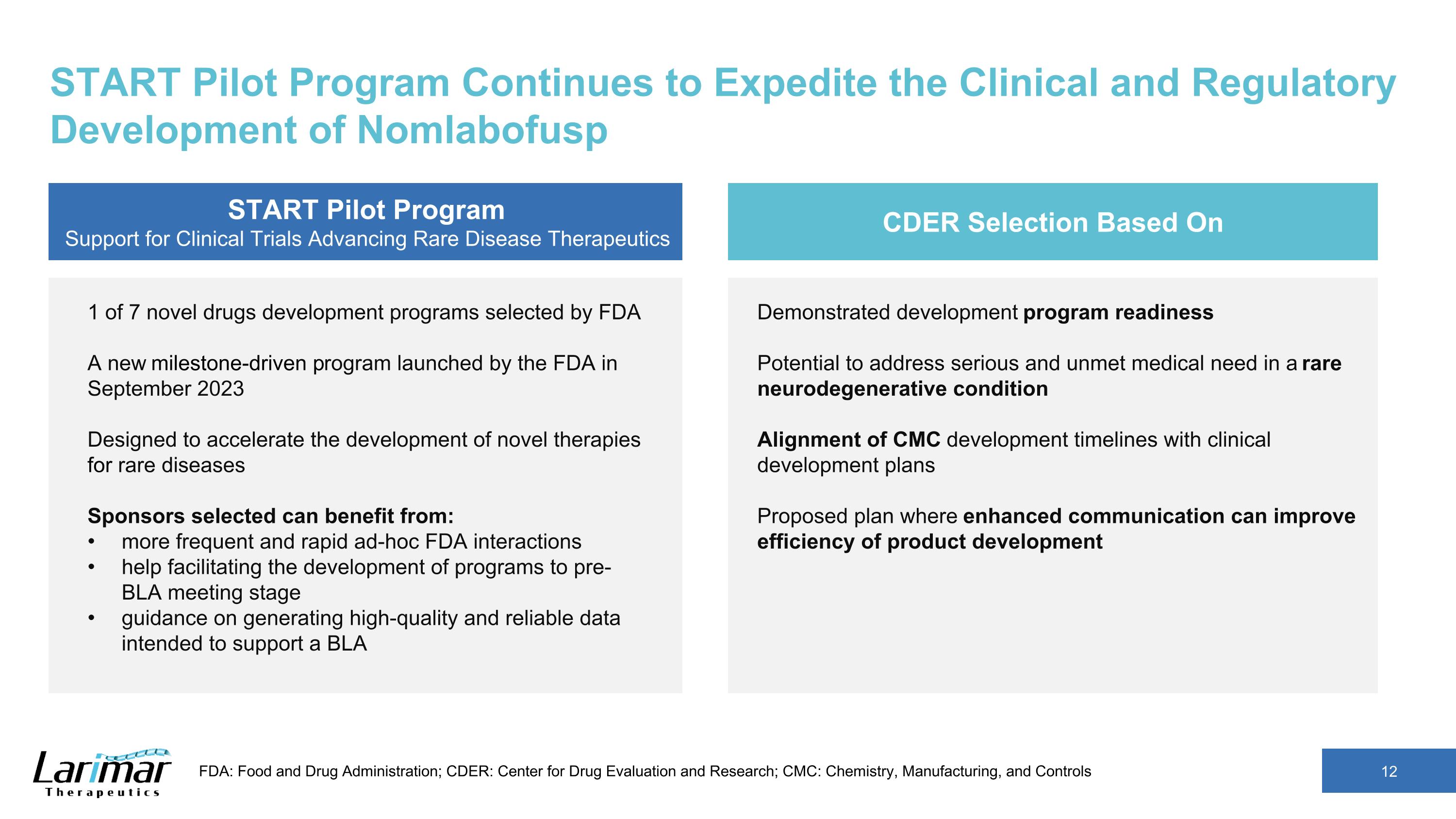
START Pilot Program Continues to Expedite the Clinical and Regulatory Development of Nomlabofusp START Pilot Program Support for Clinical Trials Advancing Rare Disease Therapeutics 1 of 7 novel drugs development programs selected by FDA A new milestone-driven program launched by the FDA in September 2023 Designed to accelerate the development of novel therapies for rare diseases Sponsors selected can benefit from: more frequent and rapid ad-hoc FDA interactions help facilitating the development of programs to pre-BLA meeting stage guidance on generating high-quality and reliable data intended to support a BLA CDER Selection Based On Demonstrated development program readiness Potential to address serious and unmet medical need in a rare neurodegenerative condition Alignment of CMC development timelines with clinical development plans Proposed plan where enhanced communication can improve efficiency of product development FDA: Food and Drug Administration; CDER: Center for Drug Evaluation and Research; CMC: Chemistry, Manufacturing, and Controls
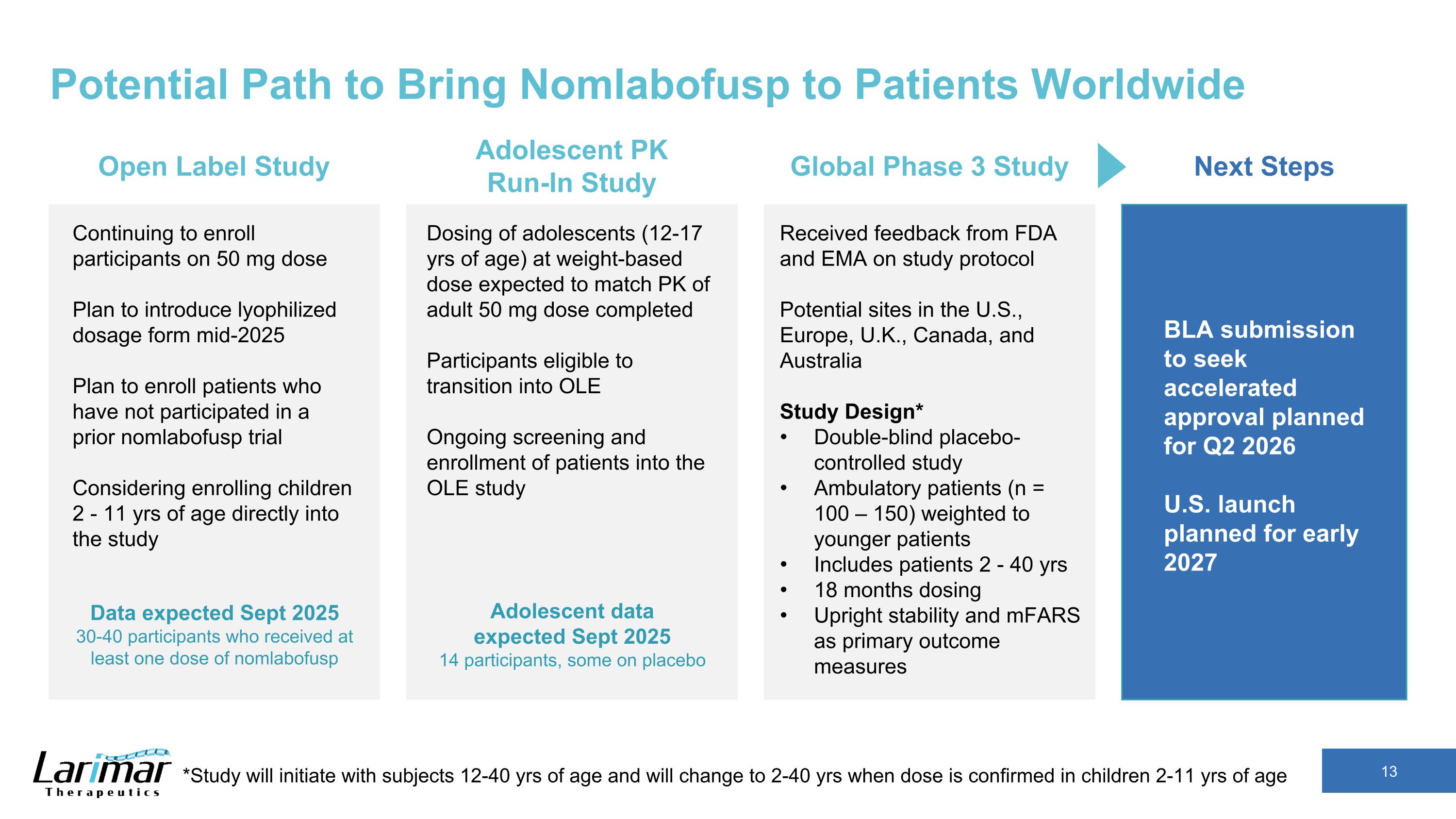
Potential Path to Bring Nomlabofusp to Patients Worldwide Received feedback from FDA and EMA on study protocol Potential sites in the U.S., Europe, U.K., Canada, and Australia Study Design* Double-blind placebo-controlled study Ambulatory patients (n = 100 – 150) weighted to younger patients Includes patients 2 - 40 yrs 18 months dosing Upright stability and mFARS as primary outcome measures Global Phase 3 Study BLA submission to seek accelerated approval planned for Q2 2026 U.S. launch planned for early 2027 Next Steps Continuing to enroll participants on 50 mg dose Plan to introduce lyophilized dosage form mid-2025 Plan to enroll patients who have not participated in a prior nomlabofusp trial Considering enrolling children 2 - 11 yrs of age directly into the study Open Label Study Data expected Sept 2025 30-40 participants who received at least one dose of nomlabofusp Dosing of adolescents (12-17 yrs of age) at weight-based dose expected to match PK of adult 50 mg dose completed Participants eligible to transition into OLE Ongoing screening and enrollment of patients into the OLE study Adolescent PK Run-In Study Adolescent data expected Sept 2025 14 participants, some on placebo *Study will initiate with subjects 12-40 yrs of age and will change to 2-40 yrs when dose is confirmed in children 2-11 yrs of age
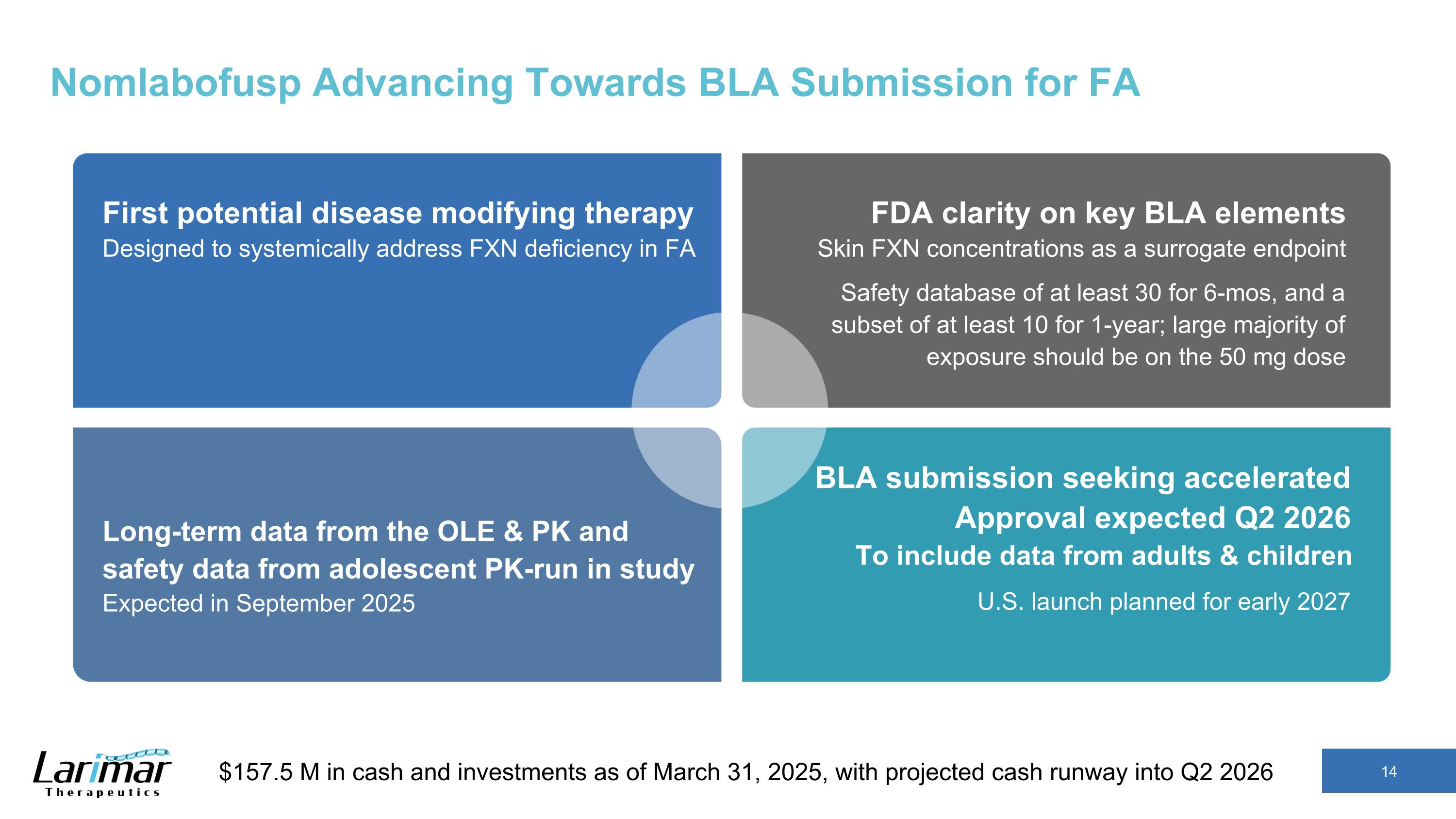
Nomlabofusp Advancing Towards BLA Submission for FA First potential disease modifying therapy Designed to systemically address FXN deficiency in FA FDA clarity on key BLA elements Skin FXN concentrations as a surrogate endpoint Safety database of at least 30 for 6-mos, and a subset of at least 10 for 1-year; large majority of exposure should be on the 50 mg dose Long-term data from the OLE & PK and safety data from adolescent PK-run in study Expected in September 2025 BLA submission seeking accelerated Approval expected Q2 2026 To include data from adults & children U.S. launch planned for early 2027 $157.5 M in cash and investments as of March 31, 2025, with projected cash runway into Q2 2026