

June 18, 2025 2025 EHA ANALYST AND INVESTOR EVENT Our goal is to develop transformative therapies to extend and improve the lives of patients with cancer Exhibit 99.1

FORWARD-LOOKING STATEMENTS This presentation contains forward-looking statements. Such statements include, but are not limited to, statements regarding our research, preclinical and clinical development activities, plans and projected timelines for ziftomenib, KO-2806 and tipifarnib, expectations regarding the relative benefits of our product candidates versus competitive therapies, expectations regarding the therapeutic and commercial potential of our product candidates, market opportunities and expectations regarding our collaboration with Kyowa Kirin. The words “believe,” “may,” “should,” “will,” “estimate,” “promise,” “plan”, “continue,” “anticipate,” “intend,” “expect,” “potential” and similar expressions (including the negative thereof) are intended to identify forward-looking statements. Because such statements are subject to risks and uncertainties, actual results may differ materially from those expressed or implied by such forward-looking statements. Risks that contribute to the uncertain nature of the forward-looking statements include: our preclinical studies and clinical trials may not be successful; the U.S. Food and Drug Administration (FDA) may not agree with our interpretation of the data from clinical trials of our product candidates; we may decide, or the FDA may require us, to conduct additional clinical trials or to modify our ongoing clinical trials; we may experience delays in the commencement, enrollment, completion or analysis of clinical testing for our product candidates, or in the reporting of data from such clinical testing, or significant issues regarding the adequacy of our clinical trial designs or the execution of our clinical trials may arise, which could result in increased costs and delays, or limit our ability to obtain regulatory approval; our product candidates may not receive regulatory approval or be successfully commercialized; unexpected adverse side effects or inadequate therapeutic efficacy of our product candidates could delay or prevent regulatory approval or commercialization; we may not be able to obtain additional financing; and our collaboration with Kyowa Kirin may not be successful. Additional risks and uncertainties may emerge from time to time, and it is not possible for Kura’s management to predict all risk factors and uncertainties. All forward-looking statements contained in this presentation speak only as of the date on which they were made. Other risks and uncertainties affecting us are described more fully in our filings with the Securities and Exchange Commission. We undertake no obligation to update such statements to reflect events that occur or circumstances that exist after the date on which they were made. This presentation may also contain statistical, preclinical and clinical data obtained from and prepared by third parties. The recipient is cautioned not to give undue weight to such disclosures. Neither the Company nor any other person makes any representation as to the accuracy or completeness of such data or undertakes any obligation to update such data after the date of this presentation.

ZIFTOMENIB Targeted investigational menin inhibitor for relapsed/refractory and newly diagnosed acute myeloid leukemia (AML) New Drug Application (NDA) based on positive results from the Phase 2 KOMET-001 trial NDA granted Priority Review and assigned Prescription Drug User Fee Act (PDUFA) target action date of November 30, 2025 Kyowa Kirin partnership funds expansive AML development program through 1L U.S. commercialization

AGENDA Ziftomenib Combined with Intensive Induction Chemotherapy (7+3) in Newly Diagnosed NPM1-m or KMT2A-r AML: Updated Phase 1a/b Results from KOMET-007 Unmet Need in Newly Diagnosed AML Ziftomenib Global Development Plan and KOMET-017 Phase 3 Clinical Trials Ziftomenib Market Opportunity in Newly Diagnosed NPM1-m and KMT2A-r AML

KEY OPINION LEADERS AND INVITED PARTICIPANTS Harry Erba, M.D., Ph.D. Director of Leukemia Program at the Duke Cancer Institute Ghayas C. Issa, M.D. Associate Professor of Leukemia at The University of Texas MD Anderson Cancer Center

UNMET NEED IN NEWLY DIAGNOSED AML Ghayas C. Issa, M.D.

SIGNIFICANT UNMET NEED REMAINS FOR AML PATIENTS AML, acute myeloid leukemia; CR, complete response. 1. National Cancer Institute. Accessed May 25, 2025. https://seer.cancer.gov/statfacts/html/amyl.html 2. Kumar CC. Genes Cancer. 2011;2(2):95-107. doi:10.1177/1947601911408076. ~ 70% Up to 70% of patients who achieve a first CR will see AML return within 3 years2 5-year survival rate for AML is 33% and as low as 8.6% for patients aged ≥ 65 years1 An estimated 22,000 new cases of AML diagnosed each year in the United States1 Median age at diagnosis is 69 years; majority of diagnoses made in patients aged 65 to 74 years.1 Current FDA approved therapies include combination chemotherapy regimens such as 7+3, venetoclax and hypomethylating agents (HMAs) and FLT3 inhibitors like midostaurin or quizartinib 33%
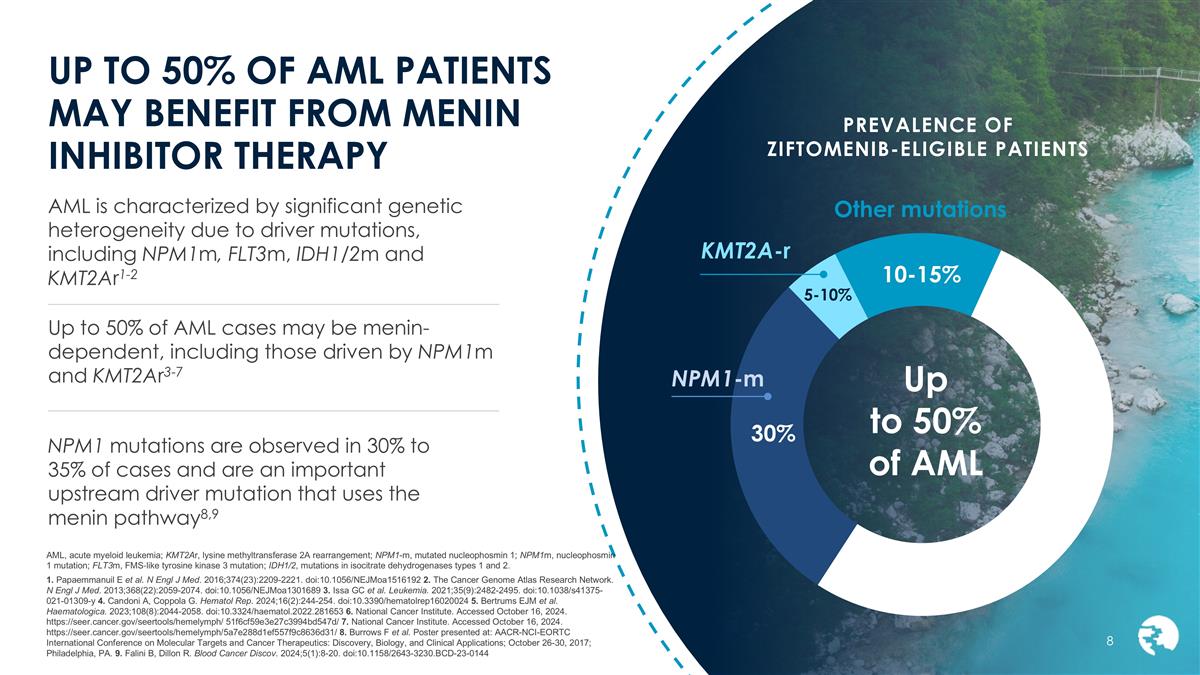
PREVALENCE OF ZIFTOMENIB-ELIGIBLE PATIENTS UP TO 50% OF AML PATIENTS MAY BENEFIT FROM MENIN INHIBITOR THERAPY AML, acute myeloid leukemia; KMT2Ar, lysine methyltransferase 2A rearrangement; NPM1-m, mutated nucleophosmin 1; NPM1m, nucleophosmin 1 mutation; FLT3m, FMS‐like tyrosine kinase 3 mutation; IDH1/2, mutations in isocitrate dehydrogenases types 1 and 2. 1. Papaemmanuil E et al. N Engl J Med. 2016;374(23):2209-2221. doi:10.1056/NEJMoa1516192 2. The Cancer Genome Atlas Research Network. N Engl J Med. 2013;368(22):2059-2074. doi:10.1056/NEJMoa1301689 3. Issa GC et al. Leukemia. 2021;35(9):2482-2495. doi:10.1038/s41375-021-01309-y 4. Candoni A, Coppola G. Hematol Rep. 2024;16(2):244-254. doi:10.3390/hematolrep16020024 5. Bertrums EJM et al. Haematologica. 2023;108(8):2044-2058. doi:10.3324/haematol.2022.281653 6. National Cancer Institute. Accessed October 16, 2024. https://seer.cancer.gov/seertools/hemelymph/ 51f6cf59e3e27c3994bd547d/ 7. National Cancer Institute. Accessed October 16, 2024. https://seer.cancer.gov/seertools/hemelymph/5a7e288d1ef557f9c8636d31/ 8. Burrows F et al. Poster presented at: AACR-NCI-EORTC International Conference on Molecular Targets and Cancer Therapeutics: Discovery, Biology, and Clinical Applications; October 26-30, 2017; Philadelphia, PA. 9. Falini B, Dillon R. Blood Cancer Discov. 2024;5(1):8-20. doi:10.1158/2643-3230.BCD-23-0144 AML is characterized by significant genetic heterogeneity due to driver mutations, including NPM1m, FLT3m, IDH1/2m and KMT2Ar1-2 Up to 50% of AML cases may be menin-dependent, including those driven by NPM1m and KMT2Ar3-7 NPM1 mutations are observed in 30% to 35% of cases and are an important upstream driver mutation that uses the menin pathway8,9 10-15% 5-10% Up to 50% of AML 30% NPM1-m KMT2A-r Other mutations
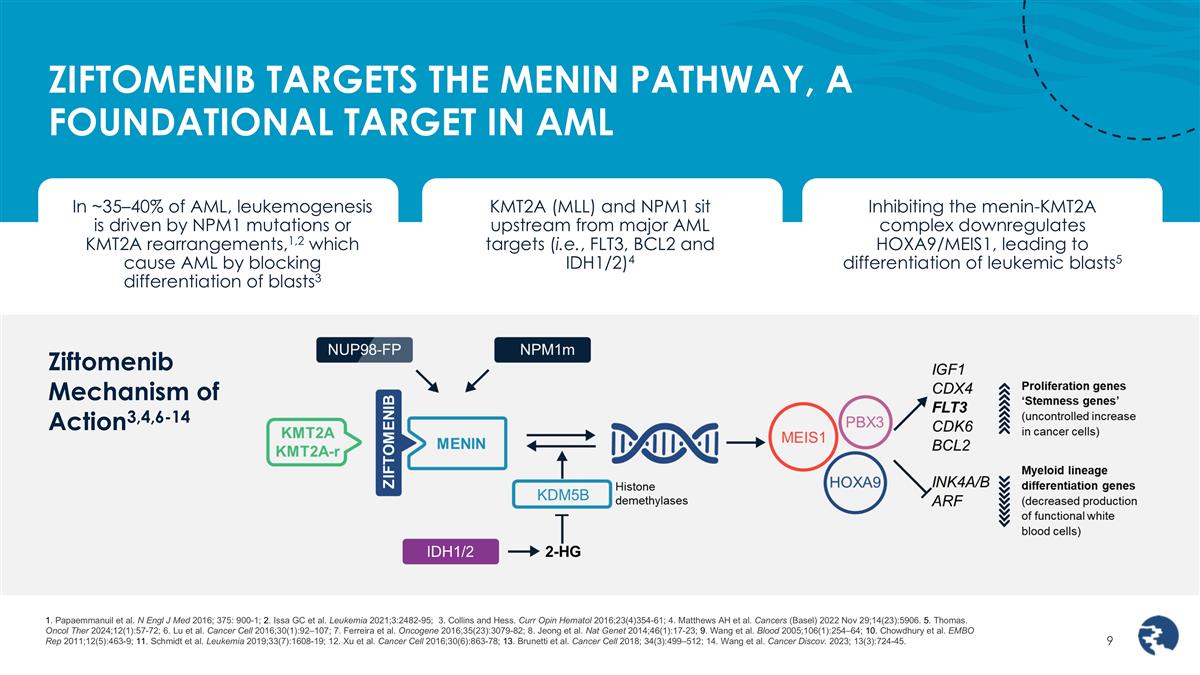
ZIFTOMENIB TARGETS THE MENIN PATHWAY, A FOUNDATIONAL TARGET IN AML Inhibiting the menin-KMT2A complex downregulates HOXA9/MEIS1, leading to differentiation of leukemic blasts5 KMT2A (MLL) and NPM1 sit upstream from major AML targets (i.e., FLT3, BCL2 and IDH1/2)4 In ~35–40% of AML, leukemogenesis is driven by NPM1 mutations or KMT2A rearrangements,1,2 which cause AML by blocking differentiation of blasts3 1. Papaemmanuil et al. N Engl J Med 2016; 375: 900-1; 2. Issa GC et al. Leukemia 2021;3:2482-95; 3. Collins and Hess. Curr Opin Hematol 2016;23(4)354-61; 4. Matthews AH et al. Cancers (Basel) 2022 Nov 29;14(23):5906. 5. Thomas. Oncol Ther 2024;12(1):57-72; 6. Lu et al. Cancer Cell 2016;30(1):92–107; 7. Ferreira et al. Oncogene 2016;35(23):3079-82; 8. Jeong et al. Nat Genet 2014;46(1):17-23; 9. Wang et al. Blood 2005;106(1):254–64; 10. Chowdhury et al. EMBO Rep 2011;12(5):463-9; 11. Schmidt et al. Leukemia 2019;33(7):1608-19; 12. Xu et al. Cancer Cell 2016;30(6):863-78; 13. Brunetti et al. Cancer Cell 2018; 34(3):499–512; 14. Wang et al. Cancer Discov. 2023; 13(3):724-45. Ziftomenib Mechanism of Action3,4,6-14
S136 1Duke Cancer Institute, Durham, NC, USA; 2Roswell Park Comprehensive Cancer Center, Buffalo, NY, USA; 3Massachusetts General Hospital, Harvard Medical School, Boston, MA, USA; 4Weill Cornell Medicine and The New York Presbyterian Hospital, New York, NY, USA; 5University of Texas Southwestern Medical Center, Dallas, TX, USA; 6SCRI at TriStar Centennial, Nashville, TN, USA; 7Karmanos Cancer Institute, Wayne State University, Detroit, MI, USA; 8Moores Cancer Center, University of California, San Diego, La Jolla, CA, USA; 9Abramson Cancer Center, University of Pennsylvania, Philadelphia, PA, USA; 10Cleveland Clinic, Cleveland, OH, USA; 11Sidney Kimmel Comprehensive Cancer Center, Johns Hopkins University School of Medicine, Baltimore, MD, USA; 12Robert H. Lurie Comprehensive Cancer Center, Northwestern University, Chicago, IL, USA; 13Colorado Blood Cancer Institute, Denver, CO, USA; 14Chao Family Comprehensive Cancer Center, University of California Irvine Health, Orange, CA, USA; 15Georgia Cancer Center, Augusta, GA, USA; 16Department of Hematology, University of Minnesota, Minneapolis, MN, USA; 17Ochsner MD Anderson Cancer Center, New Orleans, LA, USA; 18The Ohio State University Comprehensive Cancer Center, Columbus, OH, USA; 19Tisch Cancer Institute, Icahn School of Medicine at Mount Sinai, New York, NY, USA; 20Rutgers Cancer Institute of New Jersey, New Brunswick, NJ, USA; 21Carbone Cancer Center, University of Wisconsin-Madison, Madison, WI, USA; 22David Geffen School of Medicine at UCLA, Los Angeles, CA, USA; 23The University of Kansas Cancer Center, Kansas City, KS, USA; 24University of Oklahoma Health Sciences Center, Stephenson Cancer Center, Oklahoma, OK, USA; 25Stony Brook University Hospital Cancer Center, Stony Brook, NY, USA; 26Mayo Clinic, Jacksonville, FL, USA; 27Kura Oncology, Inc., San Diego, CA, USA; 28Yale University and Yale Cancer Center, New Haven, CT, USA Ziftomenib combined with intensive induction chemotherapy (7+3) in newly diagnosed NPM1-m or KMT2A-r acute myeloid leukemia: Updated phase 1a/b results from KOMET-007 Harry Erba1, Eunice S. Wang2, Amir T. Fathi3, Gail J. Roboz4, Yazan F. Madanat5, Stephen A. Strickland6, Suresh Balasubramanian7, James K. Mangan8, Keith Pratz9, Anjali Advani10, Ivana Gojo11, Jessica K. Altman12, Marcello Rotta13, Kiran Naqvi14, Jorge Cortes15, Mark Juckett16, Leonard C. Alsfeld17, James S. Blachly18, Marina Kremyanskaya19, Neil Palmisiano20, Kalyan V. Nadiminti21, Gary Schiller22, Tara L. Lin23, Mohamad Khawandanah24, Michael W. Schuster25, Talha Badar26, Julie Mackey Ahsan27, Tianle Chen27, Marcie Riches27, Daniel Corum27, Mollie Leoni27, and Amer M. Zeidan28
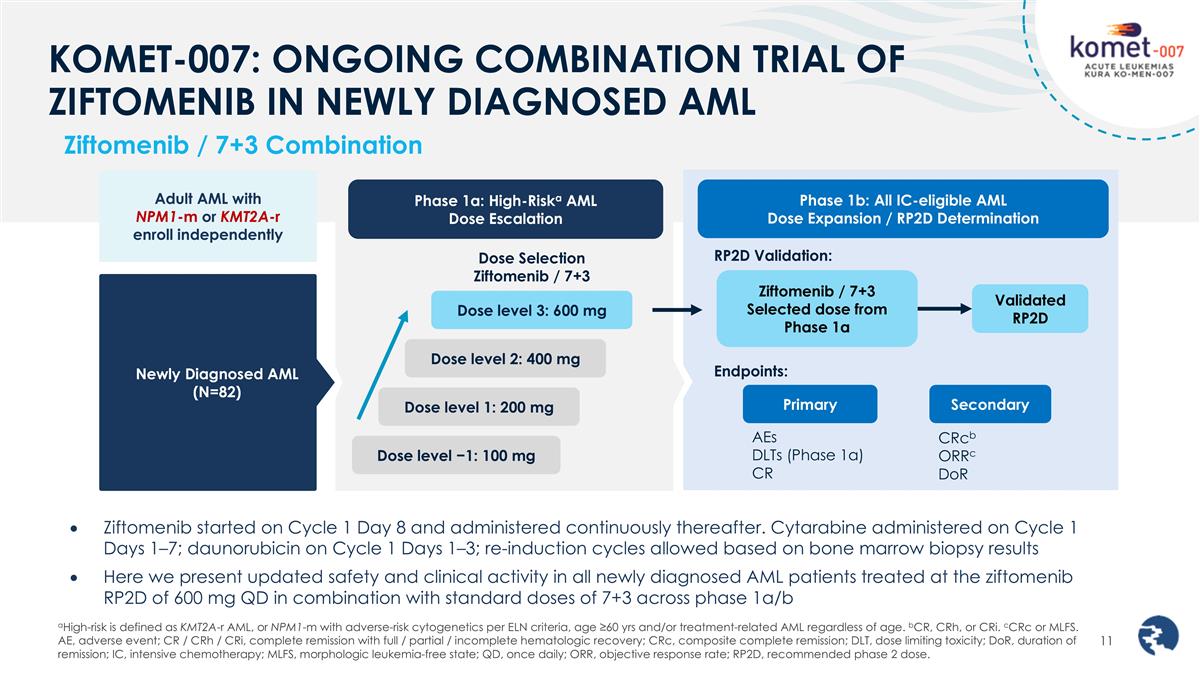
KOMET-007: ONGOING COMBINATION TRIAL OF ZIFTOMENIB IN NEWLY DIAGNOSED AML Ziftomenib started on Cycle 1 Day 8 and administered continuously thereafter. Cytarabine administered on Cycle 1 Days 1–7; daunorubicin on Cycle 1 Days 1–3; re-induction cycles allowed based on bone marrow biopsy results Here we present updated safety and clinical activity in all newly diagnosed AML patients treated at the ziftomenib RP2D of 600 mg QD in combination with standard doses of 7+3 across phase 1a/b Ziftomenib / 7+3 Combination Adult AML with NPM1-m or KMT2A-r enroll independently Newly Diagnosed AML (N=82) Phase 1a: High-Riska AML Dose Escalation Dose Selection Ziftomenib / 7+3 Dose level −1: 100 mg Dose level 3: 600 mg Dose level 2: 400 mg Dose level 1: 200 mg Phase 1b: All IC-eligible AML Dose Expansion / RP2D Determination Ziftomenib / 7+3 Selected dose from Phase 1a Validated RP2D RP2D Validation: Endpoints: Primary AEs DLTs (Phase 1a) CR Secondary CRcb ORRc DoR aHigh-risk is defined as KMT2A-r AML, or NPM1-m with adverse-risk cytogenetics per ELN criteria, age ≥60 yrs and/or treatment-related AML regardless of age. bCR, CRh, or CRi. cCRc or MLFS. AE, adverse event; CR / CRh / CRi, complete remission with full / partial / incomplete hematologic recovery; CRc, composite complete remission; DLT, dose limiting toxicity; DoR, duration of remission; IC, intensive chemotherapy; MLFS, morphologic leukemia-free state; QD, once daily; ORR, objective response rate; RP2D, recommended phase 2 dose.
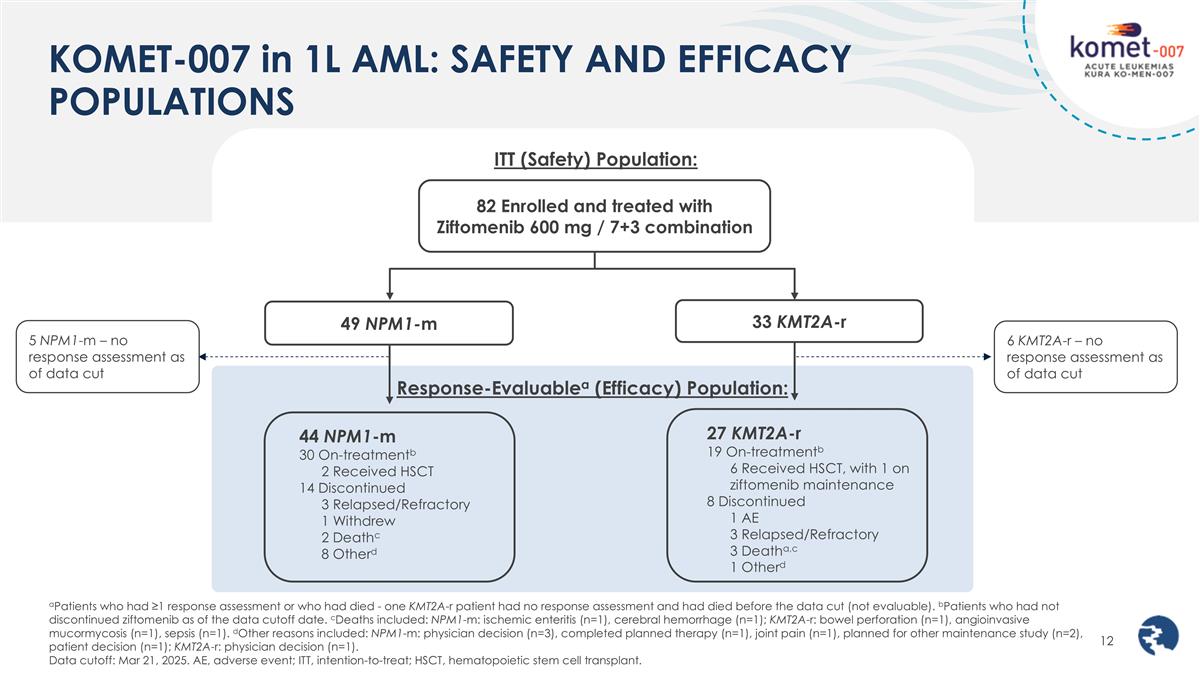
KOMET-007 in 1L AML: SAFETY AND EFFICACY POPULATIONS 82 Enrolled and treated with Ziftomenib 600 mg / 7+3 combination 49 NPM1-m 44 NPM1-m 30 On-treatmentb 2 Received HSCT 14 Discontinued 3 Relapsed/Refractory 1 Withdrew 2 Deathc 8 Otherd 33 KMT2A-r 27 KMT2A-r 19 On-treatmentb 6 Received HSCT, with 1 on ziftomenib maintenance 8 Discontinued 1 AE 3 Relapsed/Refractory 3 Deatha,c 1 Otherd ITT (Safety) Population: Response-Evaluablea (Efficacy) Population: aPatients who had ≥1 response assessment or who had died - one KMT2A-r patient had no response assessment and had died before the data cut (not evaluable). bPatients who had not discontinued ziftomenib as of the data cutoff date. cDeaths included: NPM1-m: ischemic enteritis (n=1), cerebral hemorrhage (n=1); KMT2A-r: bowel perforation (n=1), angioinvasive mucormycosis (n=1), sepsis (n=1). dOther reasons included: NPM1-m: physician decision (n=3), completed planned therapy (n=1), joint pain (n=1), planned for other maintenance study (n=2), patient decision (n=1); KMT2A-r: physician decision (n=1). Data cutoff: Mar 21, 2025. AE, adverse event; ITT, intention-to-treat; HSCT, hematopoietic stem cell transplant. 6 KMT2A-r – no response assessment as of data cut 5 NPM1-m – no response assessment as of data cut
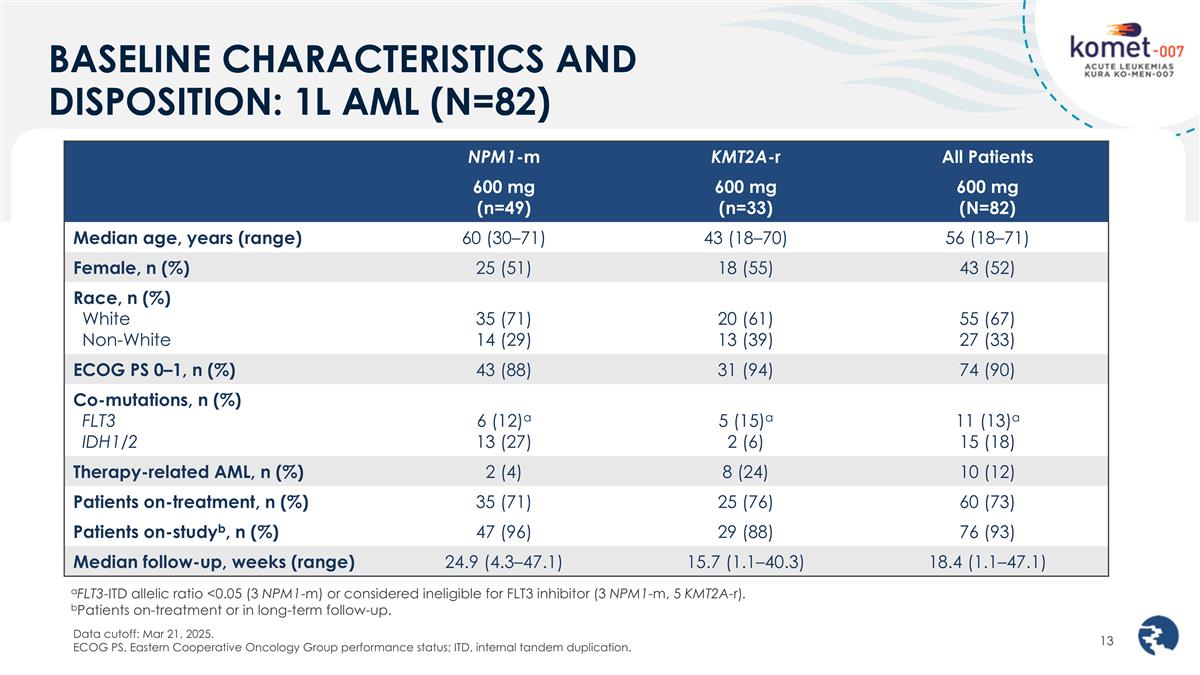
BASELINE CHARACTERISTICS AND DISPOSITION: 1L AML (N=82) Data cutoff: Mar 21, 2025. ECOG PS, Eastern Cooperative Oncology Group performance status; ITD, internal tandem duplication. NPM1-m KMT2A-r All Patients 600 mg (n=49) 600 mg (n=33) 600 mg (N=82) Median age, years (range) 60 (30–71) 43 (18–70) 56 (18–71) Female, n (%) 25 (51) 18 (55) 43 (52) Race, n (%) White Non-White 35 (71) 14 (29) 20 (61) 13 (39) 55 (67) 27 (33) ECOG PS 0–1, n (%) 43 (88) 31 (94) 74 (90) Co-mutations, n (%) FLT3 IDH1/2 6 (12)a 13 (27) 5 (15)a 2 (6) 11 (13)a 15 (18) Therapy-related AML, n (%) 2 (4) 8 (24) 10 (12) Patients on-treatment, n (%) 35 (71) 25 (76) 60 (73) Patients on-studyb, n (%) 47 (96) 29 (88) 76 (93) Median follow-up, weeks (range) 24.9 (4.3–47.1) 15.7 (1.1–40.3) 18.4 (1.1–47.1) aFLT3-ITD allelic ratio <0.05 (3 NPM1-m) or considered ineligible for FLT3 inhibitor (3 NPM1-m, 5 KMT2A-r). bPatients on-treatment or in long-term follow-up.
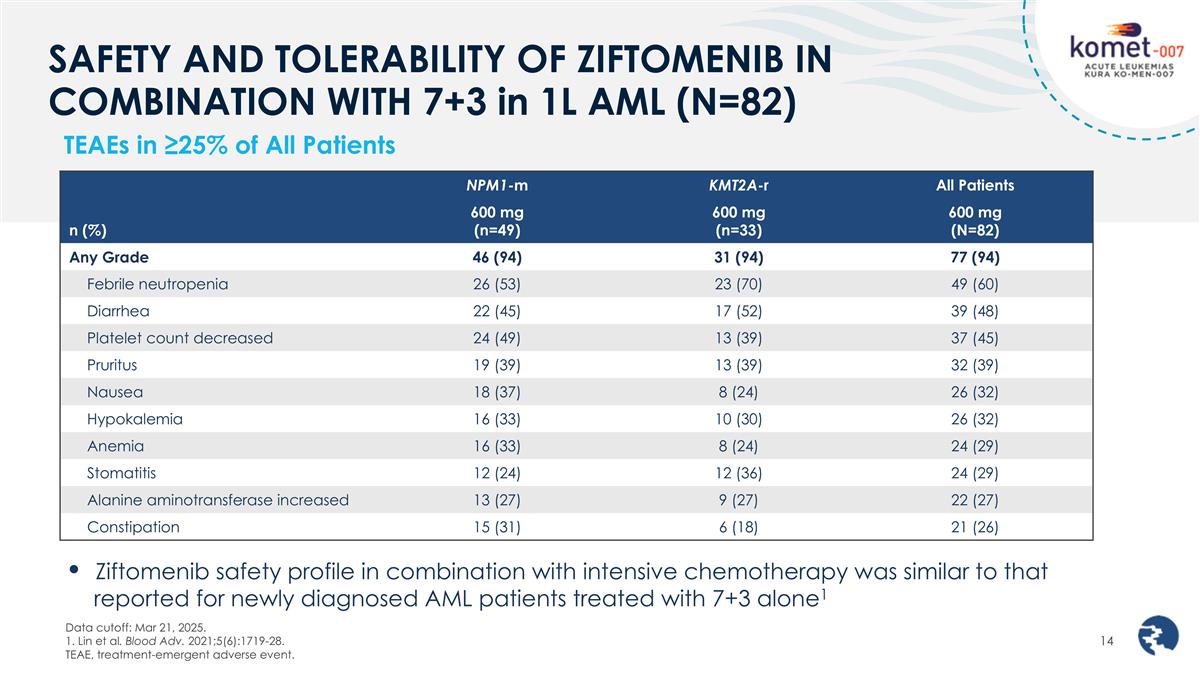
SAFETY AND TOLERABILITY OF ZIFTOMENIB IN COMBINATION WITH 7+3 in 1L AML (N=82) TEAEs in ≥25% of All Patients Data cutoff: Mar 21, 2025. 1. Lin et al. Blood Adv. 2021;5(6):1719-28. TEAE, treatment-emergent adverse event. Ziftomenib safety profile in combination with intensive chemotherapy was similar to that reported for newly diagnosed AML patients treated with 7+3 alone1 n (%) NPM1-m KMT2A-r All Patients 600 mg (n=49) 600 mg (n=33) 600 mg (N=82) Any Grade 46 (94) 31 (94) 77 (94) Febrile neutropenia 26 (53) 23 (70) 49 (60) Diarrhea 22 (45) 17 (52) 39 (48) Platelet count decreased 24 (49) 13 (39) 37 (45) Pruritus 19 (39) 13 (39) 32 (39) Nausea 18 (37) 8 (24) 26 (32) Hypokalemia 16 (33) 10 (30) 26 (32) Anemia 16 (33) 8 (24) 24 (29) Stomatitis 12 (24) 12 (36) 24 (29) Alanine aminotransferase increased 13 (27) 9 (27) 22 (27) Constipation 15 (31) 6 (18) 21 (26)
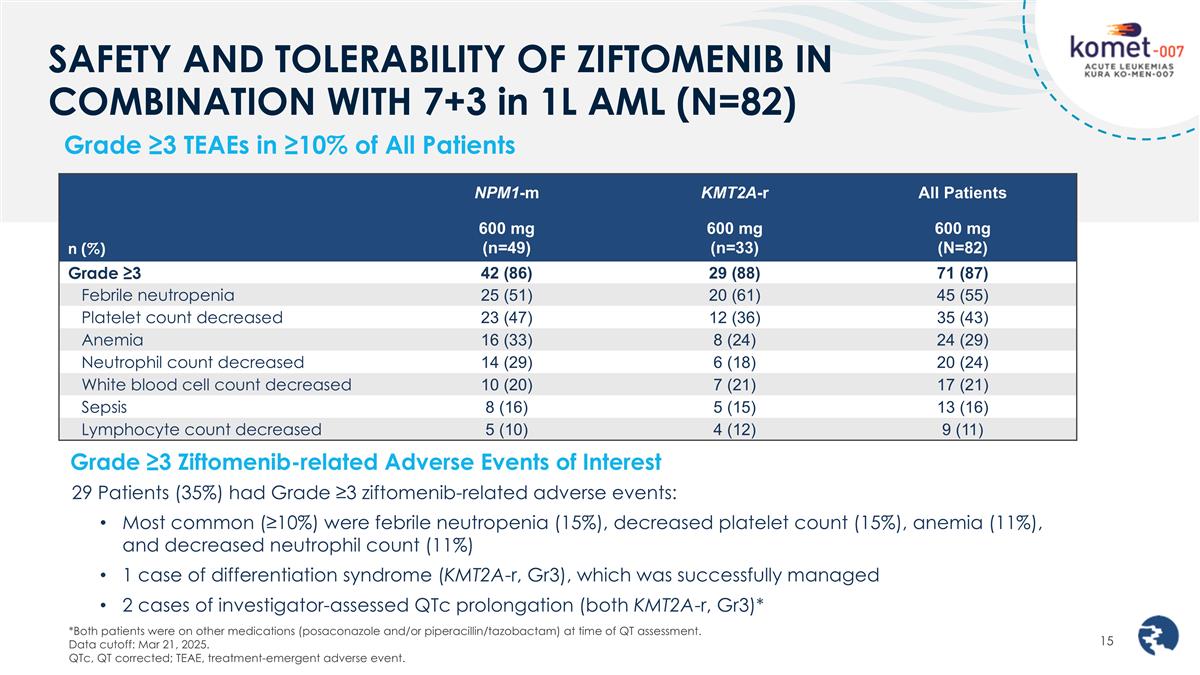
SAFETY AND TOLERABILITY OF ZIFTOMENIB IN COMBINATION WITH 7+3 in 1L AML (N=82) Grade ≥3 TEAEs in ≥10% of All Patients n (%) NPM1-m KMT2A-r All Patients 600 mg (n=49) 600 mg (n=33) 600 mg (N=82) Grade ≥3 42 (86) 29 (88) 71 (87) Febrile neutropenia 25 (51) 20 (61) 45 (55) Platelet count decreased 23 (47) 12 (36) 35 (43) Anemia 16 (33) 8 (24) 24 (29) Neutrophil count decreased 14 (29) 6 (18) 20 (24) White blood cell count decreased 10 (20) 7 (21) 17 (21) Sepsis 8 (16) 5 (15) 13 (16) Lymphocyte count decreased 5 (10) 4 (12) 9 (11) *Both patients were on other medications (posaconazole and/or piperacillin/tazobactam) at time of QT assessment. Data cutoff: Mar 21, 2025. QTc, QT corrected; TEAE, treatment-emergent adverse event. Grade ≥3 Ziftomenib-related Adverse Events of Interest 29 Patients (35%) had Grade ≥3 ziftomenib-related adverse events: Most common (≥10%) were febrile neutropenia (15%), decreased platelet count (15%), anemia (11%), and decreased neutrophil count (11%) 1 case of differentiation syndrome (KMT2A-r, Gr3), which was successfully managed 2 cases of investigator-assessed QTc prolongation (both KMT2A-r, Gr3)*
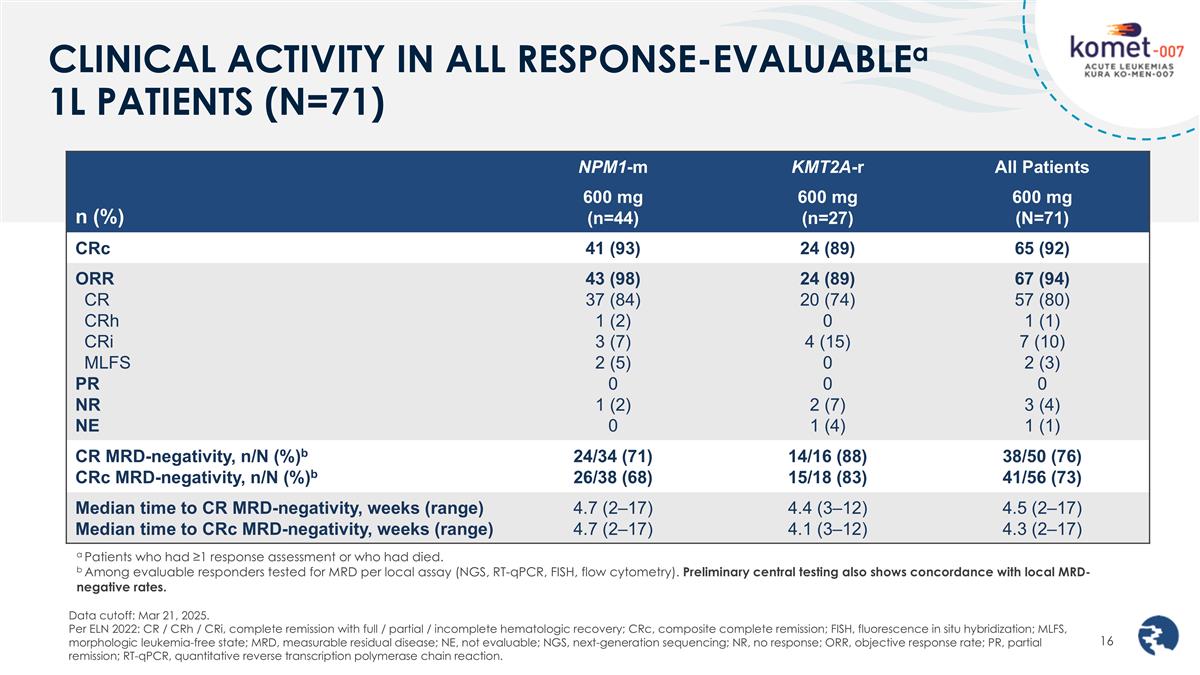
CLINICAL ACTIVITY IN ALL RESPONSE-EVALUABLEa 1L PATIENTS (N=71) a Patients who had ≥1 response assessment or who had died. b Among evaluable responders tested for MRD per local assay (NGS, RT-qPCR, FISH, flow cytometry). Preliminary central testing also shows concordance with local MRD-negative rates. Data cutoff: Mar 21, 2025. Per ELN 2022: CR / CRh / CRi, complete remission with full / partial / incomplete hematologic recovery; CRc, composite complete remission; FISH, fluorescence in situ hybridization; MLFS, morphologic leukemia-free state; MRD, measurable residual disease; NE, not evaluable; NGS, next-generation sequencing; NR, no response; ORR, objective response rate; PR, partial remission; RT-qPCR, quantitative reverse transcription polymerase chain reaction. n (%) NPM1-m KMT2A-r All Patients 600 mg (n=44) 600 mg (n=27) 600 mg (N=71) CRc 41 (93) 24 (89) 65 (92) ORR CR CRh CRi MLFS PR NR NE 43 (98) 37 (84) 1 (2) 3 (7) 2 (5) 0 1 (2) 0 24 (89) 20 (74) 0 4 (15) 0 0 2 (7) 1 (4) 67 (94) 57 (80) 1 (1) 7 (10) 2 (3) 0 3 (4) 1 (1) CR MRD-negativity, n/N (%)b CRc MRD-negativity, n/N (%)b 24/34 (71) 26/38 (68) 14/16 (88) 15/18 (83) 38/50 (76) 41/56 (73) Median time to CR MRD-negativity, weeks (range) Median time to CRc MRD-negativity, weeks (range) 4.7 (2–17) 4.7 (2–17) 4.4 (3–12) 4.1 (3–12) 4.5 (2–17) 4.3 (2–17)
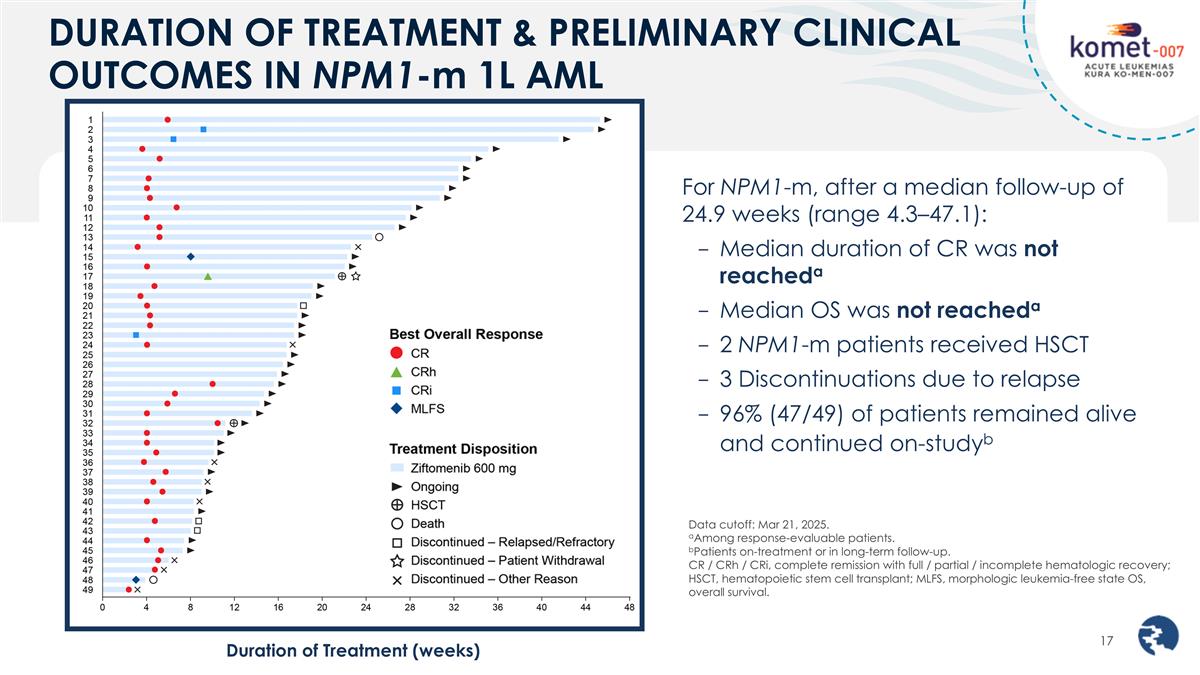
DURATION OF TREATMENT & PRELIMINARY CLINICAL OUTCOMES IN NPM1-m 1L AML Data cutoff: Mar 21, 2025. aAmong response-evaluable patients. bPatients on-treatment or in long-term follow-up. CR / CRh / CRi, complete remission with full / partial / incomplete hematologic recovery; HSCT, hematopoietic stem cell transplant; MLFS, morphologic leukemia-free state OS, overall survival. For NPM1-m, after a median follow-up of 24.9 weeks (range 4.3–47.1): Median duration of CR was not reacheda Median OS was not reacheda 2 NPM1-m patients received HSCT 3 Discontinuations due to relapse 96% (47/49) of patients remained alive and continued on-studyb Duration of Treatment (weeks)
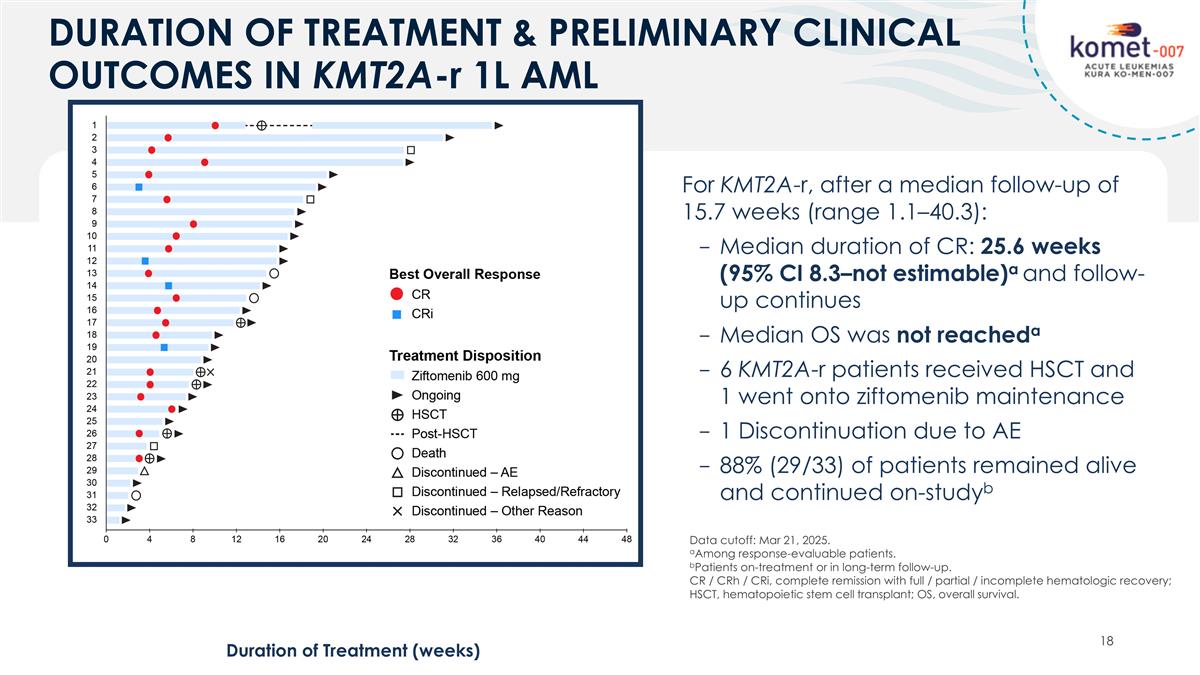
DURATION OF TREATMENT & PRELIMINARY CLINICAL OUTCOMES IN KMT2A-r 1L AML Duration of Treatment (weeks) For KMT2A-r, after a median follow-up of 15.7 weeks (range 1.1–40.3): Median duration of CR: 25.6 weeks (95% CI 8.3–not estimable)a and follow-up continues Median OS was not reacheda 6 KMT2A-r patients received HSCT and 1 went onto ziftomenib maintenance 1 Discontinuation due to AE 88% (29/33) of patients remained alive and continued on-studyb Data cutoff: Mar 21, 2025. aAmong response-evaluable patients. bPatients on-treatment or in long-term follow-up. CR / CRh / CRi, complete remission with full / partial / incomplete hematologic recovery; HSCT, hematopoietic stem cell transplant; OS, overall survival.
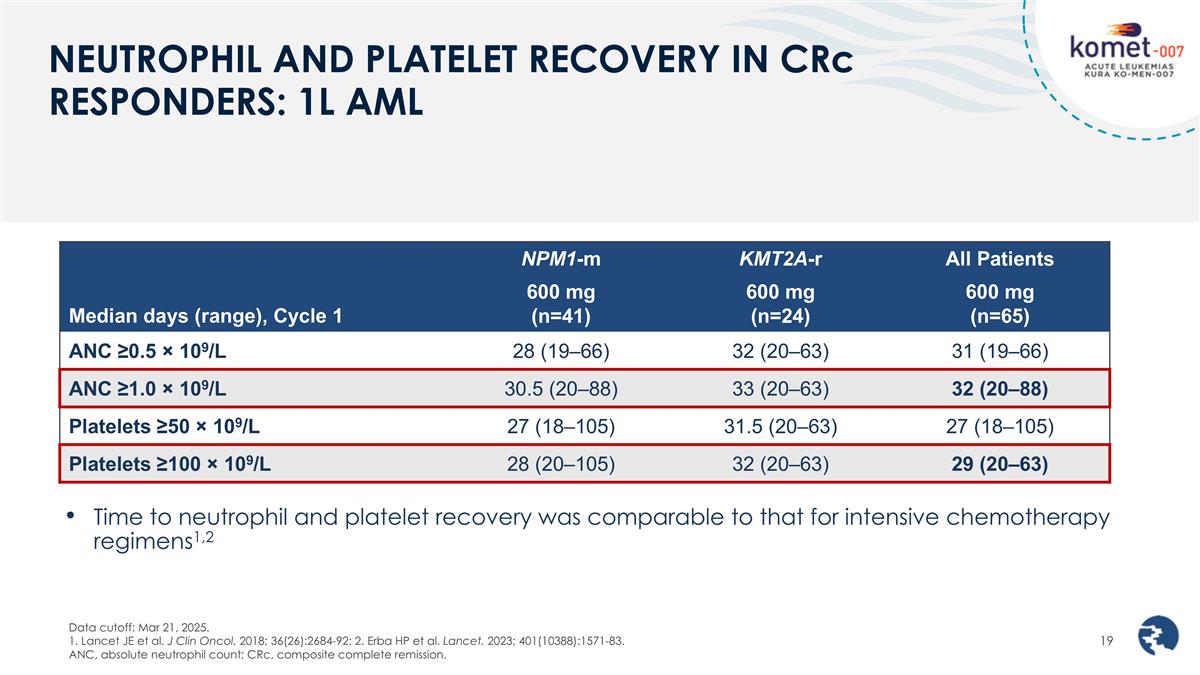
NEUTROPHIL AND PLATELET RECOVERY IN CRc RESPONDERS: 1L AML Data cutoff: Mar 21, 2025. 1. Lancet JE et al. J Clin Oncol. 2018; 36(26):2684-92; 2. Erba HP et al. Lancet. 2023; 401(10388):1571-83. ANC, absolute neutrophil count; CRc, composite complete remission. Median days (range), Cycle 1 NPM1-m KMT2A-r All Patients 600 mg (n=41) 600 mg (n=24) 600 mg (n=65) ANC ≥0.5 × 109/L 28 (19–66) 32 (20–63) 31 (19–66) ANC ≥1.0 × 109/L 30.5 (20–88) 33 (20–63) 32 (20–88) Platelets ≥50 × 109/L 27 (18–105) 31.5 (20–63) 27 (18–105) Platelets ≥100 × 109/L 28 (20–105) 32 (20–63) 29 (20–63) Time to neutrophil and platelet recovery was comparable to that for intensive chemotherapy regimens1,2
CONCLUSIONS In the ongoing KOMET-007 study, ziftomenib 600 mg QD combined with 7+3 was well tolerated, with a safety profile consistent with previous reports Low rates of ziftomenib-related cytopenia and no additional myelosuppression observed with the combination Ziftomenib 600 mg QD did not delay neutrophil and platelet count recovery 1 case of Gr3 differentiation syndrome (KMT2A-r), which was successfully managed Robust clinical activity with deep responses was demonstrated in newly diagnosed NPM1-m and KMT2A-r AML CRc: 93% for NPM1-m, 89% for KMT2A-r patients CRc MRD negativity: 68% for NPM1-m at median of 4.7 weeks, 83% for KMT2A-r at median of 4.1 weeks 96% (47/49) of NPM1-m and 88% (29/33) KMT2A-r patients remained alive and continued on-study (median follow-up of 25 and 16 weeks, respectively) Taken together, we believe these data support the Phase 3 advancement of ziftomenib combination in newly diagnosed NPM1-m and KMT2A-r AML (KOMET-017)

ZIFTOMENIB GLOBAL DEVELOPMENT PLAN AND KOMET-017 PHASE 3 CLINICAL TRIALS Mollie Leoni, M.D. – Chief Medical Officer, Kura Oncology Ghayas C. Issa, M.D.
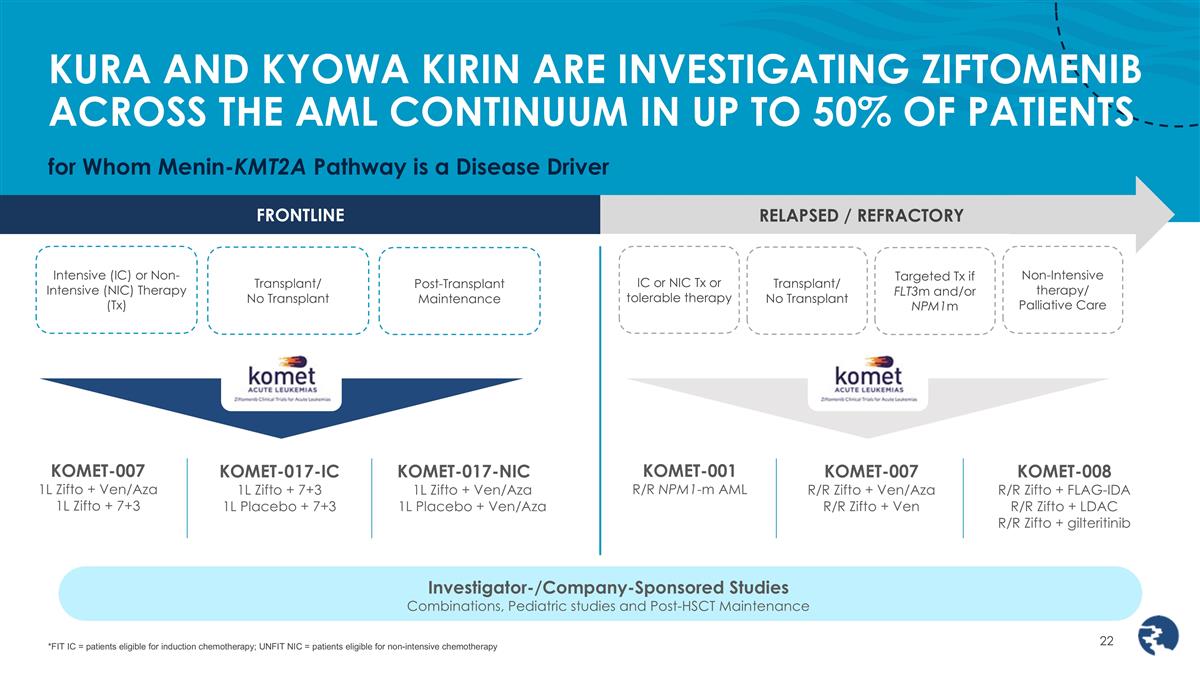
for Whom Menin-KMT2A Pathway is a Disease Driver FRONTLINE IC or NIC Tx or tolerable therapy Transplant/ No Transplant Targeted Tx if FLT3m and/or NPM1m Non-Intensive therapy/ Palliative Care KOMET-001 R/R NPM1-m AML KOMET-007 R/R Zifto + Ven/Aza R/R Zifto + Ven Investigator-/Company-Sponsored Studies Combinations, Pediatric studies and Post-HSCT Maintenance RELAPSED / REFRACTORY KOMET-008 R/R Zifto + FLAG-IDA R/R Zifto + LDAC R/R Zifto + gilteritinib Intensive (IC) or Non-Intensive (NIC) Therapy (Tx) Transplant/ No Transplant Post-Transplant Maintenance KOMET-007 1L Zifto + Ven/Aza 1L Zifto + 7+3 KOMET-017-IC 1L Zifto + 7+3 1L Placebo + 7+3 KOMET-017-NIC 1L Zifto + Ven/Aza 1L Placebo + Ven/Aza *FIT IC = patients eligible for induction chemotherapy; UNFIT NIC = patients eligible for non-intensive chemotherapy KURA AND KYOWA KIRIN ARE INVESTIGATING ZIFTOMENIB ACROSS THE AML CONTINUUM IN UP TO 50% OF PATIENTS

Expected to start in 2H 2025 (see Zeidan AM et al. EHA 2025 Abstract #PB2573) KOMET-017-NIC: Non-intensive therapy – Ziftomenib + ven/aza combo Patients NPM1-m AML Age ≥75 yrs, or 18 to <75 yrs with comorbidities Arm B Arm A Primary endpoints CR OS Ziftomenib Venetoclax + Azacitidine Placebo Venetoclax + Azacitidine R 1:1 KOMET-017-IC: Intensive therapy – Ziftomenib and 7+3 combo Patients NPM1-m or KMT2A-r AMLa FLT3 wildtype or ITD ratio of <0.05b Age ≥18 yrs ECOG PS 0–2 Primary endpoints CR MRD-negativity (NPM1-m only) EFS Arm C Arm A Arm B Ziftomenib 7+3 Induction(s) Consolidation Placebo 7+3 Induction(s) Consolidation Ziftomenib 7+3 Induction(s) Consolidation Placebo R 1:1:1 aExcluding partial tandem duplication. bUnless ineligible for FLT3-targeted therapy. CR, complete remission; ECOG PS, Eastern Cooperative Oncology Group performance status; EFS, event-free survival; ITD, internal tandem duplication; MRD, measurable residual disease; OS, overall survival. Two independently powered, registration-enabling, randomized Phase 3 studies in fit and unfit newly diagnosed AML KOMET-017: PHASE 3 ZIFTOMENIB PIVOTAL 1L COMBINATION STUDIES
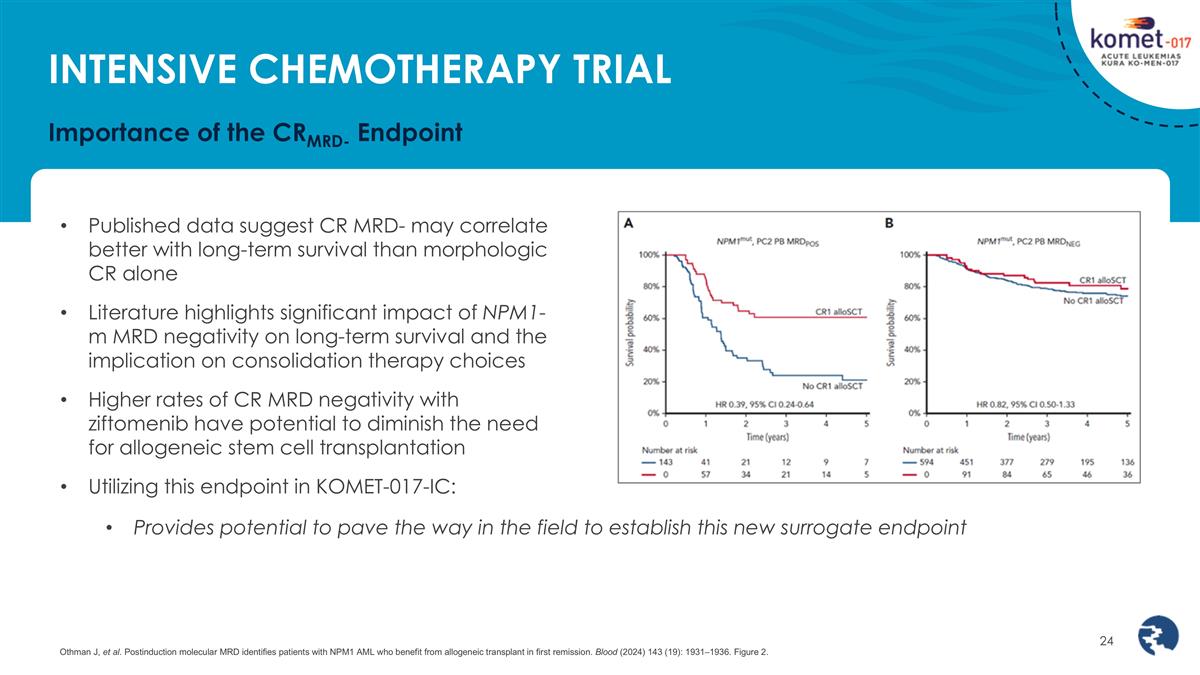
Importance of the CRMRD- Endpoint Published data suggest CR MRD- may correlate better with long-term survival than morphologic CR alone Literature highlights significant impact of NPM1-m MRD negativity on long-term survival and the implication on consolidation therapy choices Higher rates of CR MRD negativity with ziftomenib have potential to diminish the need for allogeneic stem cell transplantation Utilizing this endpoint in KOMET-017-IC: Provides potential to pave the way in the field to establish this new surrogate endpoint Othman J, et al. Postinduction molecular MRD identifies patients with NPM1 AML who benefit from allogeneic transplant in first remission. Blood (2024) 143 (19): 1931–1936. Figure 2. INTENSIVE CHEMOTHERAPY TRIAL

ZIFTOMENIB MARKET OPPORTUNITY IN NEWLY DIAGNOSED NPM1-m AND KMT2A-r AML Brian Powl – Chief Commercial Officer, Kura Oncology
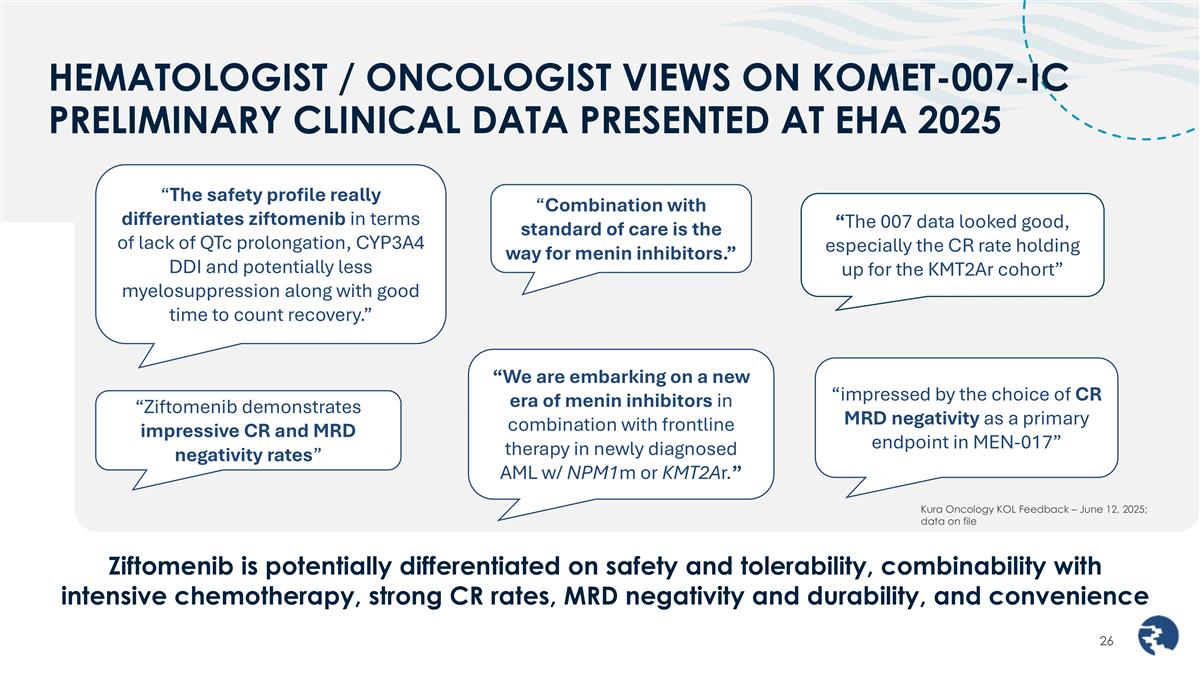
HEMATOLOGIST / ONCOLOGIST VIEWS ON KOMET-007-IC PRELIMINARY CLINICAL DATA PRESENTED AT EHA 2025 “The 007 data looked good, especially the CR rate holding up for the KMT2Ar cohort” “We are embarking on a new era of menin inhibitors in combination with frontline therapy in newly diagnosed AML w/ NPM1m or KMT2Ar.” “impressed by the choice of CR MRD negativity as a primary endpoint in MEN-017” “Combination with standard of care is the way for menin inhibitors.” Ziftomenib is potentially differentiated on safety and tolerability, combinability with intensive chemotherapy, strong CR rates, MRD negativity and durability, and convenience Kura Oncology KOL Feedback – June 12, 2025; data on file “The safety profile really differentiates ziftomenib in terms of lack of QTc prolongation, CYP3A4 DDI and potentially less myelosuppression along with good time to count recovery.” “Ziftomenib demonstrates impressive CR and MRD negativity rates”

Expansive Market Opportunity ZIFTOMENIB MARKET POTENTIAL IN NEWLY DIAGNOSED AML AML, acute myeloid leukemia; CR, complete response. 1. Kumar CC. Genes Cancer. 2011;2(2):95-107. doi:10.1177/1947601911408076 2. National Cancer Institute. Accessed May 25, 2025. https://seer.cancer.gov/statfacts/html/amyl.html. Potential for benefit / risk to support sustained treatment 12-24 months Analog pricing, including for recently approved product $36-40k months Potential peak sales for menin inhibitors in 1L AML >$7B/yr Combination of encouraging clinical activity and safety in a once-daily oral medication could unlock a large market opportunity Potential for benefit / risk to support sustained treatment 12-24 months Newly diagnosed cases of AML each year in the U.S.2 ~22,000 Analog pricing, including for recently approved product $36-40k /month Large Population & Potential for Sustained Benefit High Unmet Medical Need of patients who achieve a first CR will relapse within 3 years1 ~70% 5-year survival rate is 33% for all ages; as low as 8.6% for patients aged ≥ 65 years2 33% >$7B/yr Annual U.S. market opportunity in 1L AML

CORPORATE OVERVIEW Troy Wilson, Ph.D., J.D. – Chief Executive Officer, Kura Oncology
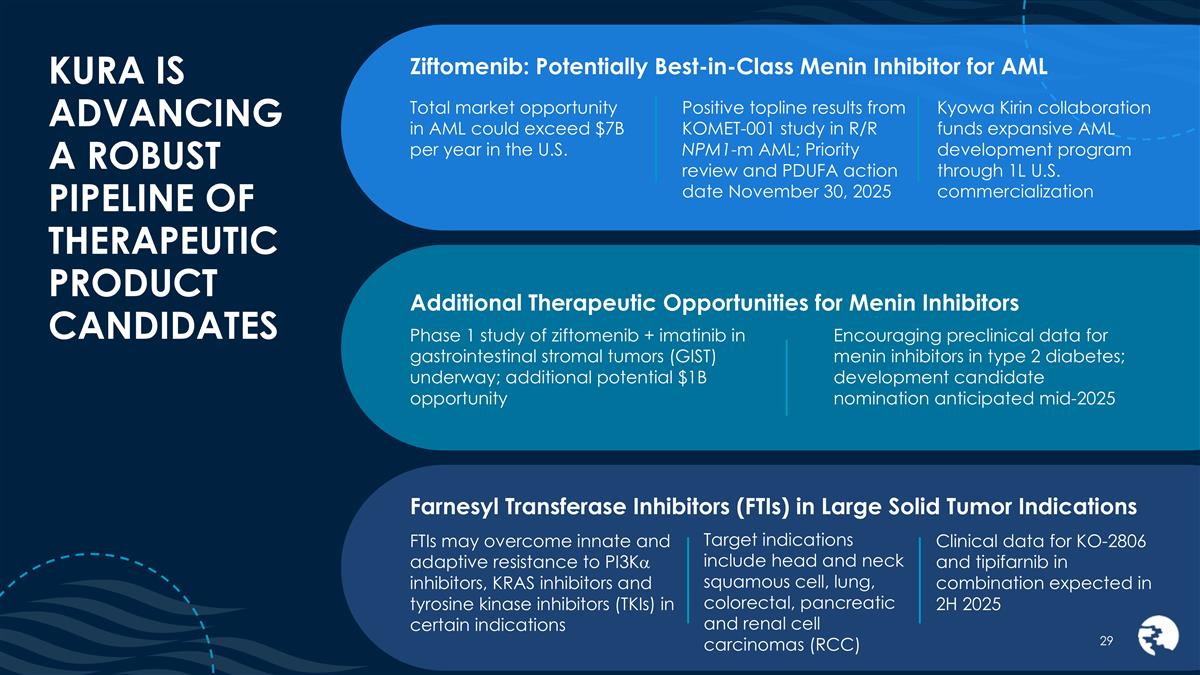
KURA IS ADVANCING A ROBUST PIPELINE OF THERAPEUTIC PRODUCT CANDIDATES Ziftomenib: Potentially Best-in-Class Menin Inhibitor for AML Total market opportunity in AML could exceed $7B per year in the U.S. Positive topline results from KOMET-001 study in R/R NPM1-m AML; Priority review and PDUFA action date November 30, 2025 Kyowa Kirin collaboration funds expansive AML development program through 1L U.S. commercialization Target indications include head and neck squamous cell, lung, colorectal, pancreatic and renal cell carcinomas (RCC) Farnesyl Transferase Inhibitors (FTIs) in Large Solid Tumor Indications FTIs may overcome innate and adaptive resistance to PI3Ka inhibitors, KRAS inhibitors and tyrosine kinase inhibitors (TKIs) in certain indications Clinical data for KO-2806 and tipifarnib in combination expected in 2H 2025 Additional Therapeutic Opportunities for Menin Inhibitors Encouraging preclinical data for menin inhibitors in type 2 diabetes; development candidate nomination anticipated mid-2025 Phase 1 study of ziftomenib + imatinib in gastrointestinal stromal tumors (GIST) underway; additional potential $1B opportunity

Ziftomenib ANTICIPATED UPCOMING MILESTONES: ADDITIONAL 2025 DATA READ-OUTS ACROSS MULTIPLE PROGRAMS Report topline results from KOMET-001 Phase 2 registration-directed trial in R/R NPM1-m AML ü FDA feedback on KOMET-017 registration-enabling protocol in 1L NPM1-m and KMT2A-r intensive and non-intensive AML ü NDA submission for ziftomenib in R/R NPM1-m AML ü Present topline data from KOMET-001 Phase 2 registration-directed trial in R/R NPM1-m AML ü Initiate KOMET-015 Phase 1 trial of ziftomenib in combination with imatinib in patients with advanced GIST ü Present preliminary clinical data from KOMET-007 Phase 1b trial in 1L intensive AML ü Initiate KOMET-017 Phase 3 registration-enabling trials in 1L NPM1-m and KMT2A-r intensive and non-intensive AML 2H 2025 Present preliminary clinical data from Phase 1b expansion of KOMET-007 in 1L non-intensive AML 2H 2025 KO-2806 / tipifarnib Initiate one or more expansion cohorts in combination with cabozantinib in RCC 2H 2025 Present preliminary clinical data from FIT-001 trial for KO-2806 as monotherapy and combo with cabozantinib in RCC 2H 2025 Present clinical data from the KURRENT-HN trial of tipifarnib in combo with alpelisib in PIK3CA-dependent HNSCC 2H 2025 Next-gen Menin Nominate a development candidate for next-generation menin inhibitor program for diabetes Mid-2025
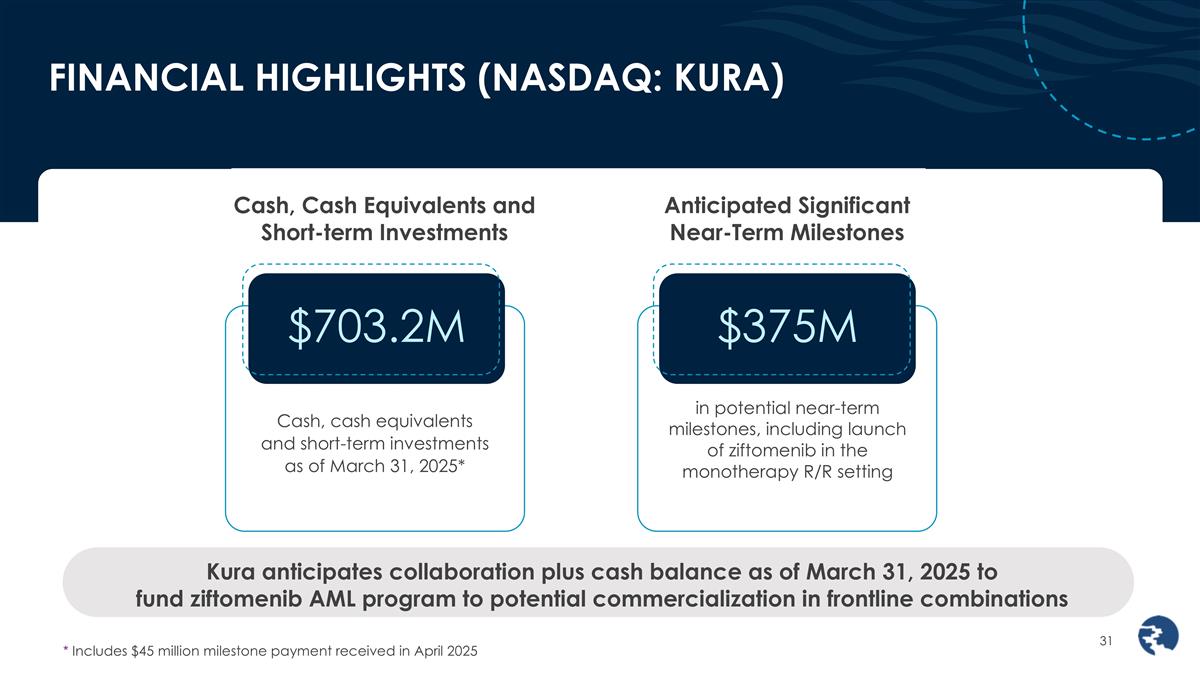
FINANCIAL HIGHLIGHTS (NASDAQ: KURA) Cash, Cash Equivalents and Short-term Investments Anticipated Significant Near-Term Milestones Cash, cash equivalents and short-term investments as of March 31, 2025* Kura anticipates collaboration plus cash balance as of March 31, 2025 to fund ziftomenib AML program to potential commercialization in frontline combinations in potential near-term milestones, including launch of ziftomenib in the monotherapy R/R setting $703.2M $375M * Includes $45 million milestone payment received in April 2025

QUESTIONS & ANSWERS

THANK YOU Our goal is to develop transformative therapies to extend and improve the lives of patients with cancer