
Exhibit 99.1

Date: June 16, 2025 NYSEAmerican: IGC 1 NYSE AMERICAN: IGC - Service to humanity -

Safe Harbor The information presented in these slides includes forward - looking statements regarding the business prospects of IGC Pharma, Inc . ("IGC" or the "Company") . These forward - looking statements are based on management's current expectations as of the date of this presentation and are subject to a number of risks and uncertainties, many of which are beyond our control . Forward - looking statements can often be identified by words such as "believes," "expects," "anticipates," "plans," "will," "goal," "may," "intends," "assumes," or similar expressions . These forward - looking statements involve risks and uncertainties that could cause actual results to differ materially from those projected or implied . These risks and uncertainties include, but are not limited to : clinical trial outcomes, patient recruitment timelines, clinical site performance, management's ability to recruit sites, patients, and monitor clinical trials, regulatory approvals, competition, funding availability, intellectual property protection . The forward - looking statements are based on assumptions that the Company has made in light of its industry experience and its perceptions of historical trends, current conditions, expected future developments, and other factors believed to be appropriate under the circumstances . However, these assumptions are inherently subject to uncertainty and changes . IGC's actual results may differ materially from those anticipated in these forward - looking statements due to various factors . The forward - looking statements are not guarantees of future performance . Important factors that could cause actual results to differ materially from recent results or those projected in forward - looking statements are included in the Company's filings with the Securities and Exchange Commission (the "SEC"), including its most recent Annual Report on Form 10 - K and subsequent filings on Form 10 - Ǫ, which are accessible on the SEC's website ( www . sec . gov ) . The Company undertakes no obligation and expressly disclaims any obligation to update or revise any forward - looking statements, whether as a result of new information, future events, or otherwise, except as required by law . Potential investors are cautioned not to place undue reliance on these forward - looking statements, which speak only as of the date of this presentation . NYSEAmerican: IGC - Service to humanity 2

• Preclinical work done by Dr. Chuanhai Cao and his team at USF. • IGC - AD1’s technology is licensed from USF and protected by Patents. MoA Hypothesis: Dual - Agent Liquid Therapy for Alzheimer’s NYSEAmerican: IGC - Service to humanity 3
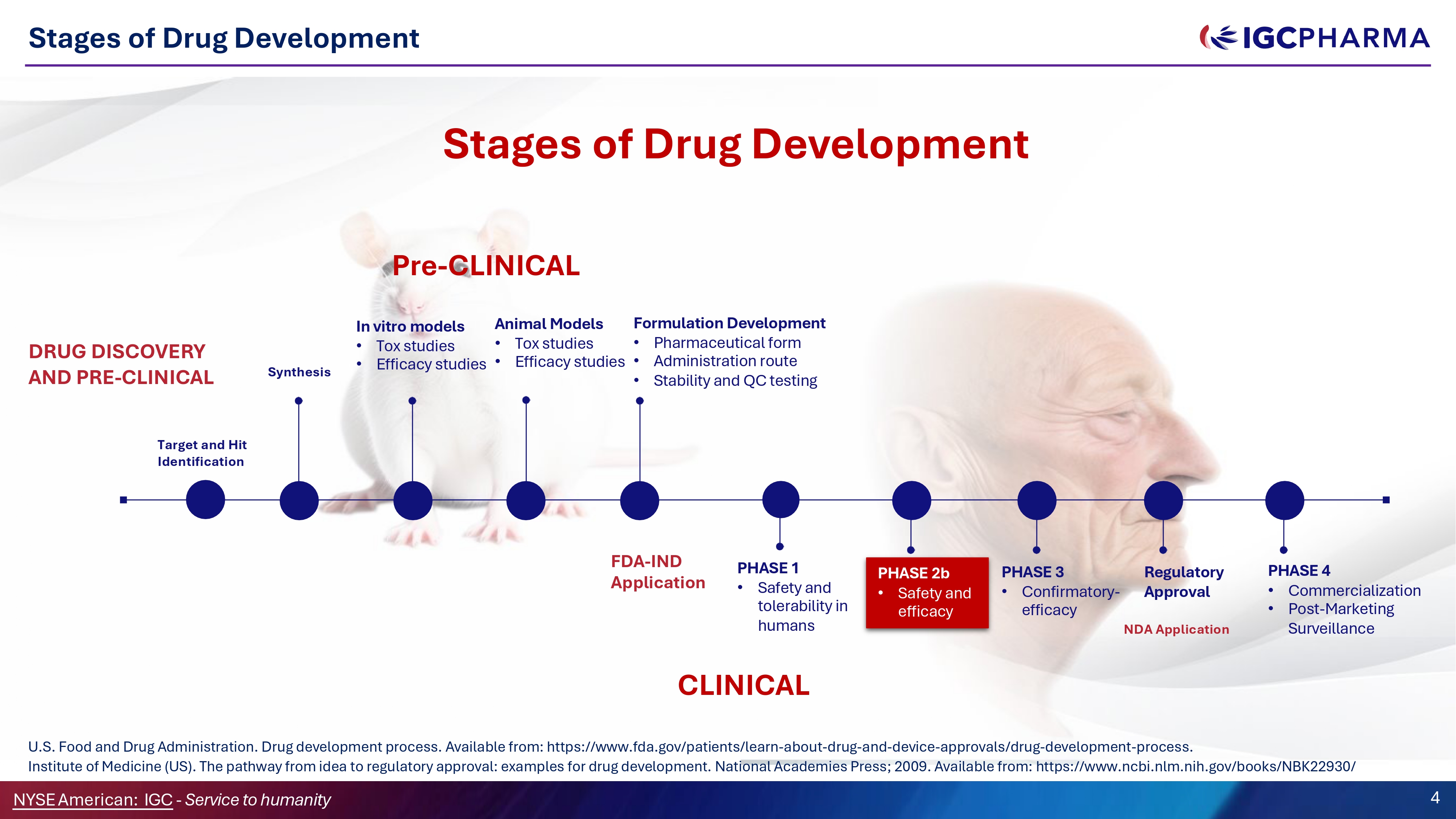
Stages of Drug Development Stages of Drug Development DRUG DISCOVERY AND PRE - CLINICAL CLINICAL Synthesis In vitro models • Tox studies • Efficacy studies Animal Models • Tox studies • Efficacy studies Formulation Development • Pharmaceutical form • Administration route • Stability and ǪC testing PHASE 1 • Safety and tolerability in humans PHASE 2b • Safety and efficacy PHASE 3 • Confirmatory - efficacy PHASE 4 • Commercialization • Post - Marketing Surveillance Regulatory Approval Target and Hit Identification NYSEAmerican: IGC - Service to humanity 4 FDA - IND Application NDA Application Pre - CLINICAL U.S. Food and Drug Administration. Drug development process. Available from: https:// www.fda.gov/patients/learn - about - drug - and - device - approvals/drug - development - process. Institute of Medicine (US). The pathway from idea to regulatory approval: examples for drug development. National Academies Press; 2009. Available from: https:// www.ncbi.nlm.nih.gov/books/NBK22930/
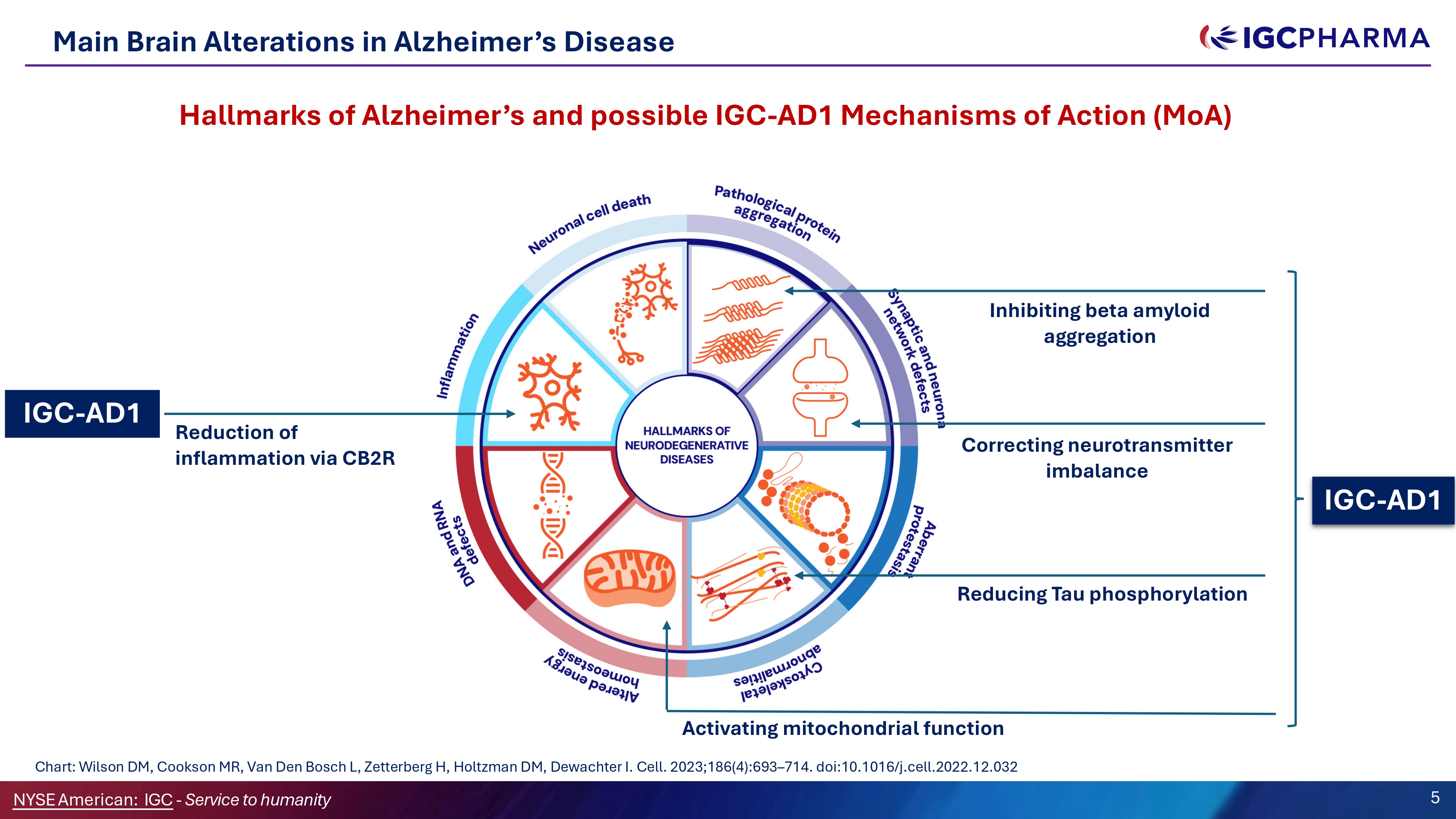
Main Brain Alterations in Alzheimer’s Disease IGC - AD1 IGC - AD1 Reduction of inflammation via CB2R Inhibiting beta amyloid aggregation Reducing Tau phosphorylation Activating mitochondrial function Chart: Wilson DM, Cookson MR, Van Den Bosch L, Zetterberg H, Holtzman DM, Dewachter I. Cell. 2023;186(4):693 – 714. doi:10.1016/j.cell.2022.12.032 Correcting neurotransmitter imbalance Hallmarks of Alzheimer’s and possible IGC - AD1 Mechanisms of Action (MoA) 5 NYSEAmerican: IGC - Service to humanity
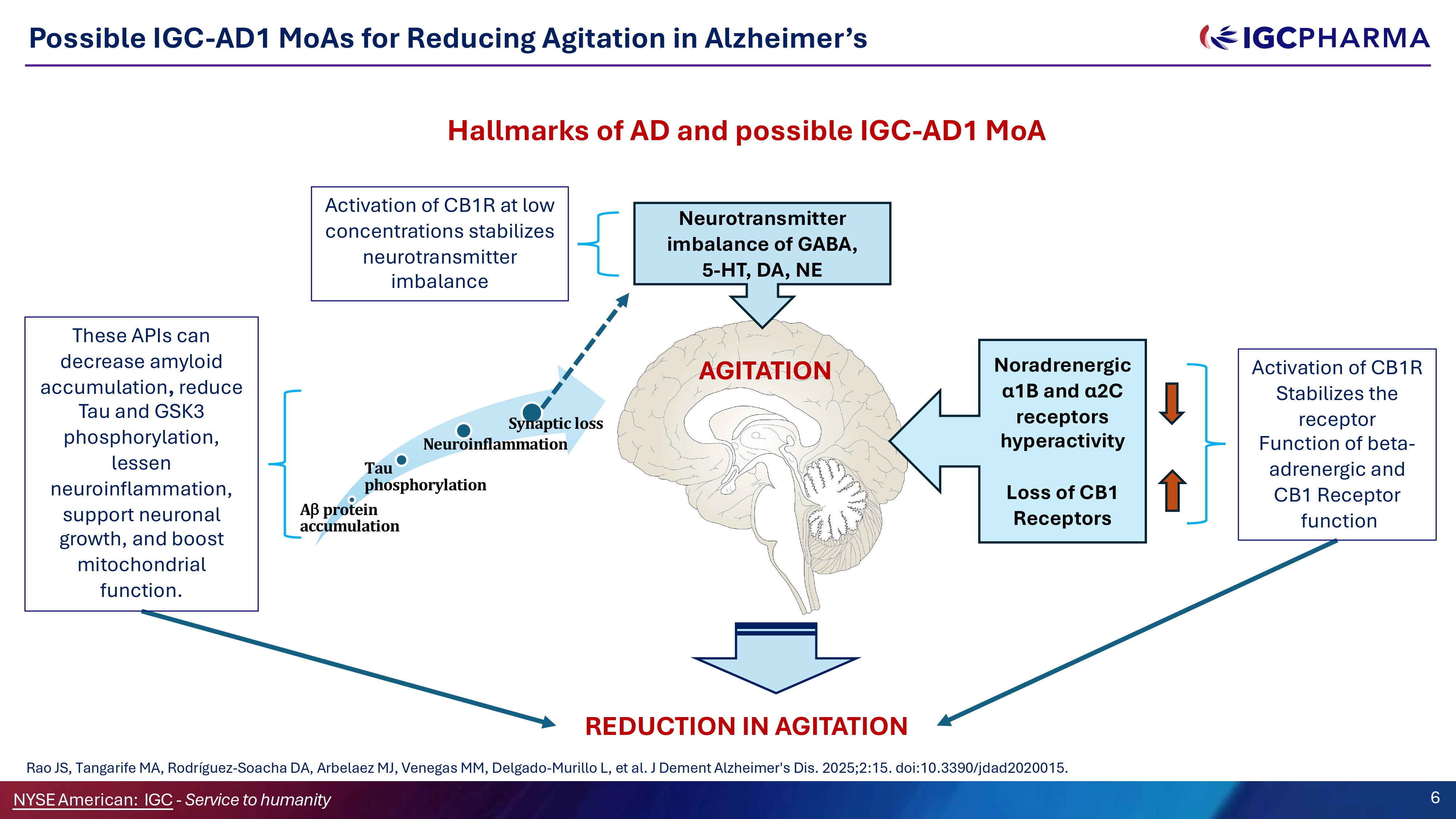
Possible IGC - AD1 MoAs for Reducing Agitation in Alzheimer’s Hallmarks of AD and possible IGC - AD1 MoA AGITATION Neurotransmitter imbalance of GABA, 5 - HT, DA, NE Tau phosphorylation A protein accumulation Synaptic loss Neuroinflammation Noradrenergic α1B and α2C receptors hyperactivity Loss of CB1 Receptors Activation of CB1R at low concentrations stabilizes neurotransmitter imbalance NYSEAmerican: IGC - Service to humanity 6 These APIs can decrease amyloid accumulation , reduce Tau and GSK3 phosphorylation, lessen neuroinflammation, support neuronal growth, and boost mitochondrial function. Activation of CB1R Stabilizes the receptor Function of beta - adrenergic and CB1 Receptor function REDUCTION IN AGITATION Rao JS, Tangarife MA, Rodríguez - Soacha DA, Arbelaez MJ, Venegas MM, Delgado - Murillo L, et al. J Dement Alzheimer's Dis. 2025;2:15. doi:10.3390/jdad2020015.

IGC - AD1 Has Undergone Rigorous Preclinical and Regulatory Review and Is Approved for Clinical Investigation NYSEAmerican: IGC - Service to humanity 7
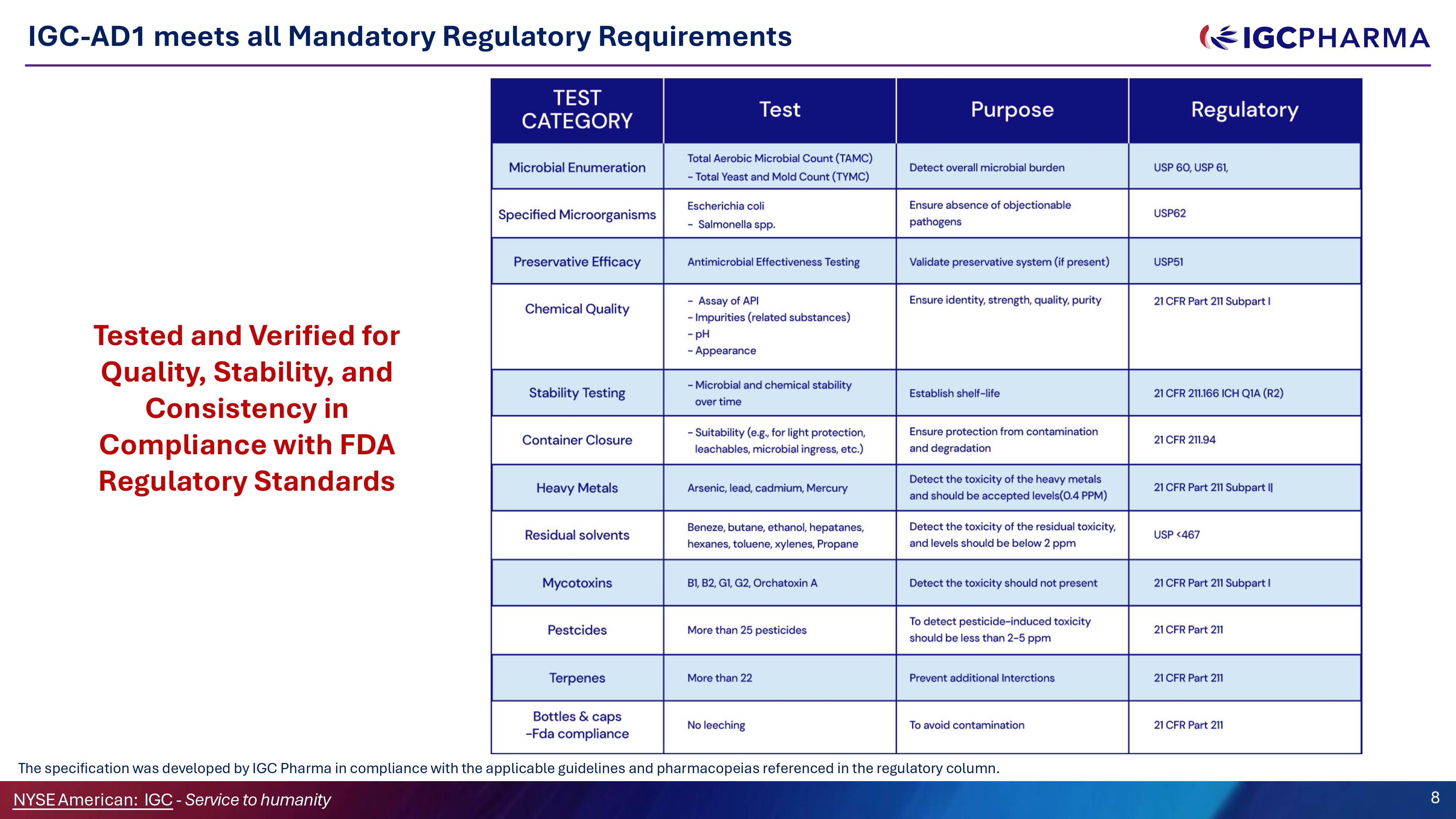
IGC - AD1 meets all Mandatory Regulatory Requirements Tested and Verified for Ǫuality, Stability, and Consistency in Compliance with FDA Regulatory Standards The specification was developed by IGC Pharma in compliance with the applicable guidelines and pharmacopeias referenced in the regulatory column. NYSEAmerican: IGC - Service to humanity 8
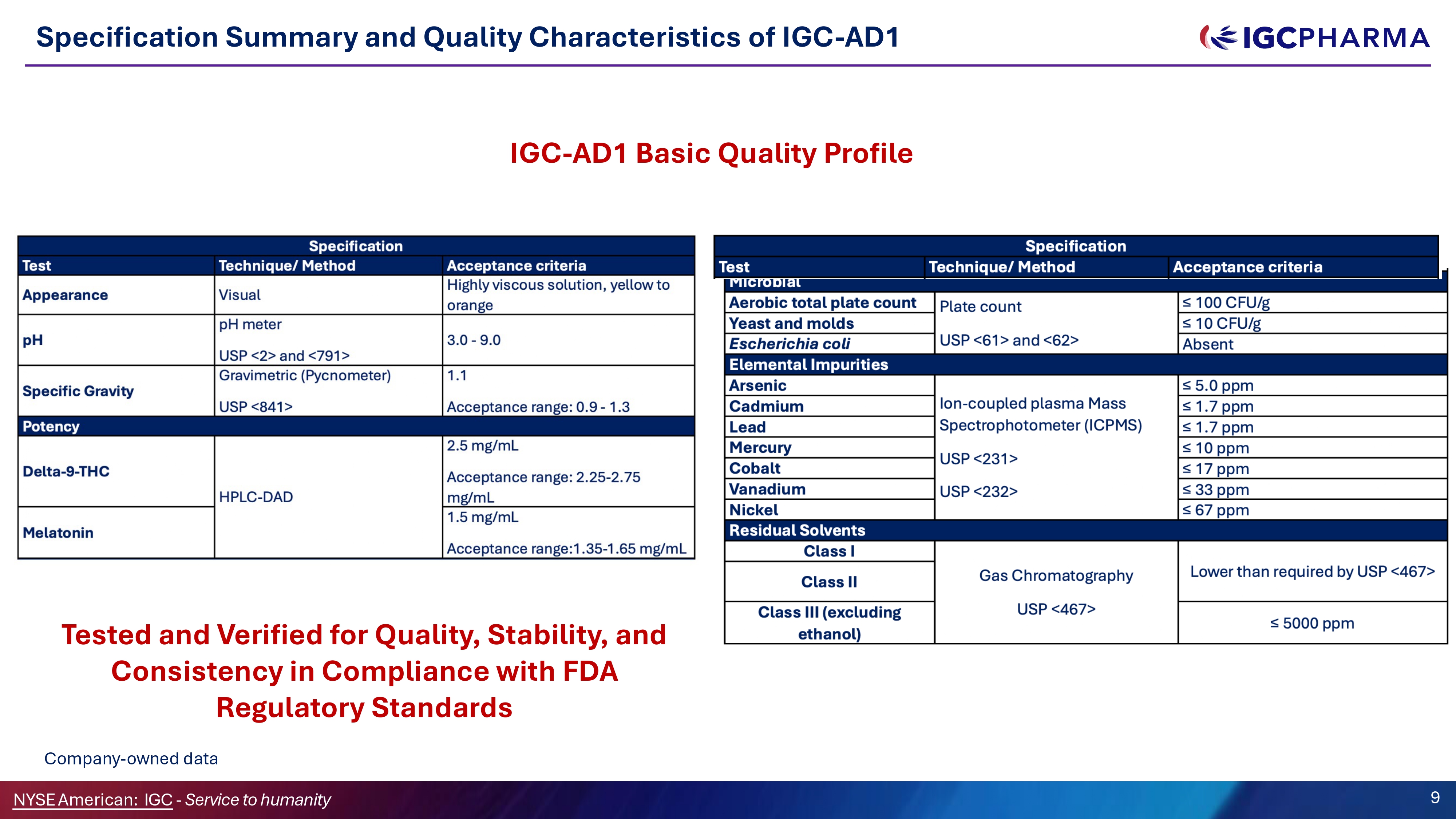
Specification Summary and Ǫuality Characteristics of IGC - AD1 IGC - AD1 Basic Ǫuality Profile Tested and Verified for Ǫuality, Stability, and Consistency in Compliance with FDA Regulatory Standards Company - owned data 9 NYSEAmerican: IGC - Service to humanity
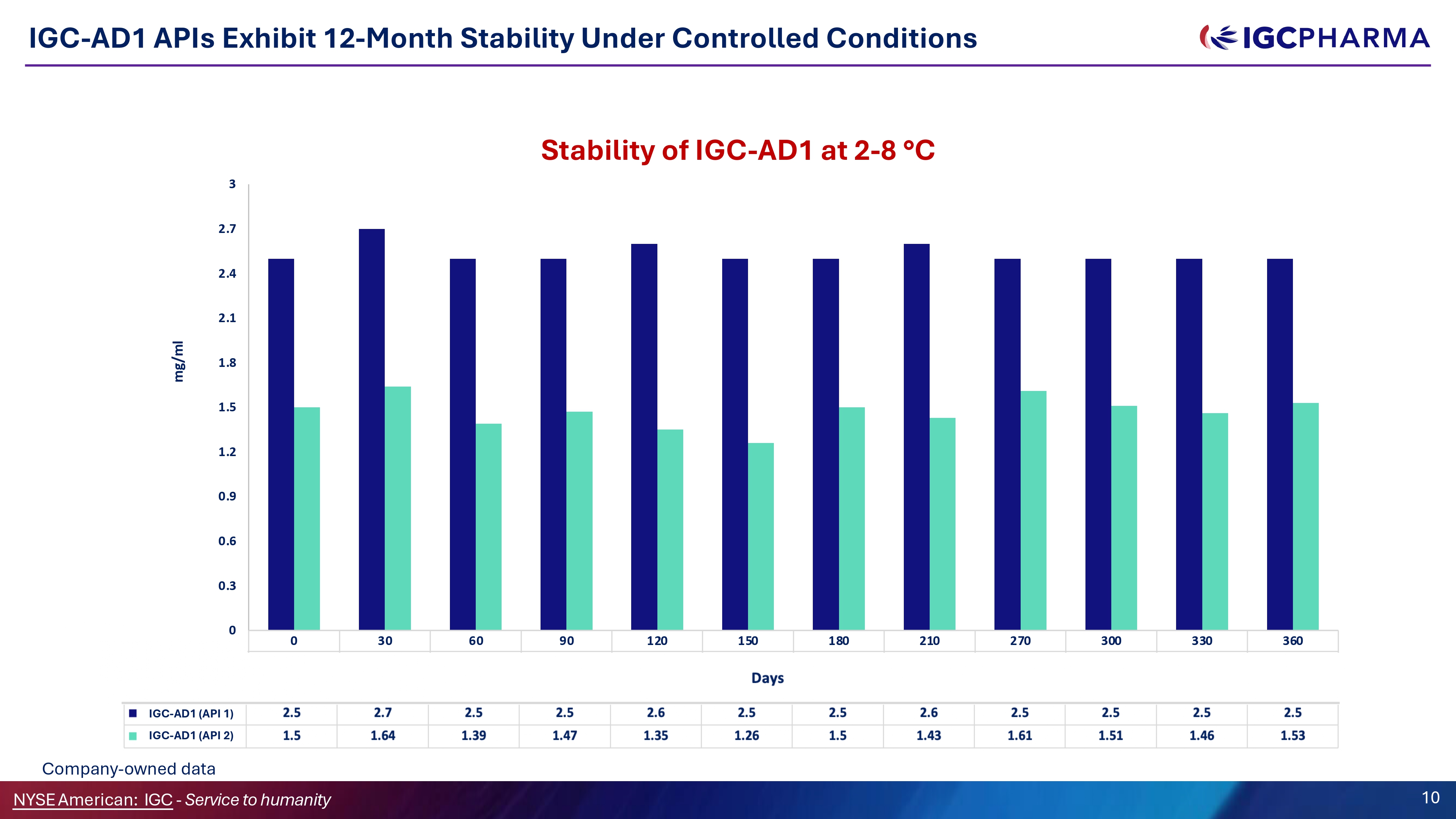
0 30 60 90 120 150 180 210 270 300 330 360 THC mg/ml 2.5 2.7 2.5 2.5 2.6 2.5 2.5 2.6 2.5 2.5 2.5 2.5 Melatonin mg/mL 1.5 1.64 1.39 1.47 1.35 1.26 1.5 1.43 1.61 1.51 1.46 1.53 0 3 2.7 2.4 2.1 1.8 1.5 1.2 0.9 0.6 0.3 mg/ml Days Stability of IGC - AD1 at 2 - 8 ƒ C IGC - AD1 APIs Exhibit 12 - Month Stability Under Controlled Conditions IGC - AD1 (API 1) NYSEAmerican: IGC - Service to humanity 10 IGC - AD1 (API 2) Company - owned data

Preclinical Toxicity Studies In vitro studies In vivo studies NYSEAmerican: IGC - Service to humanity 11
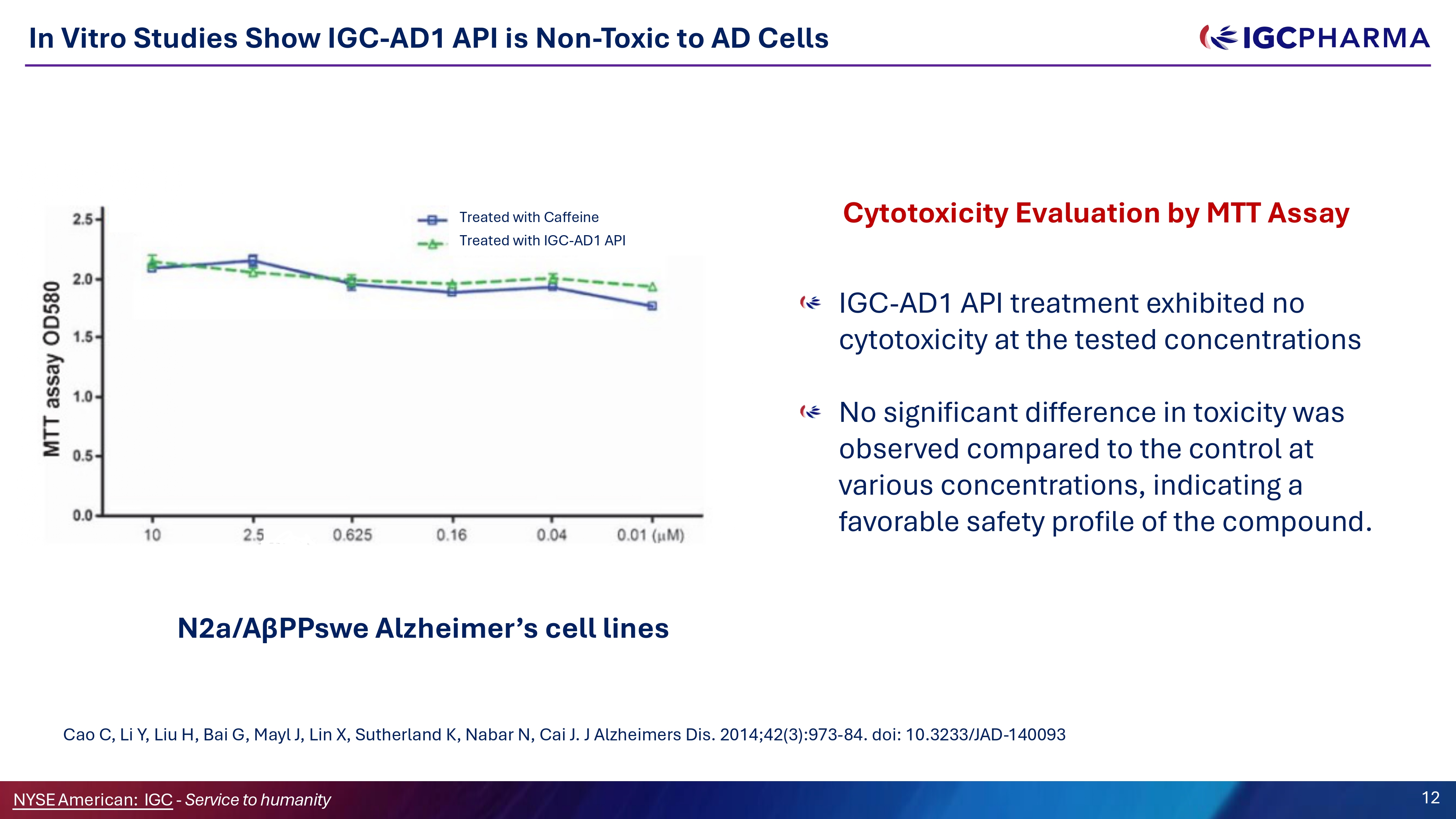
In Vitro Studies Show IGC - AD1 API is Non - Toxic to AD Cells Cao C, Li Y, Liu H, Bai G, Mayl J, Lin X, Sutherland K, Nabar N, Cai J. J Alzheimers Dis. 2014;42(3):973 - 84. doi: 10.3233/JAD - 140093 IGC - AD1 API treatment exhibited no cytotoxicity at the tested concentrations No significant difference in toxicity was observed compared to the control at various concentrations, indicating a favorable safety profile of the compound. N2a/AβPPswe Alzheimer’s cell lines Cytotoxicity Evaluation by MTT Assay Treated with Caffeine Treated with IGC - AD1 API Science Team 12 NYSEAmerican: IGC - Service to humanity
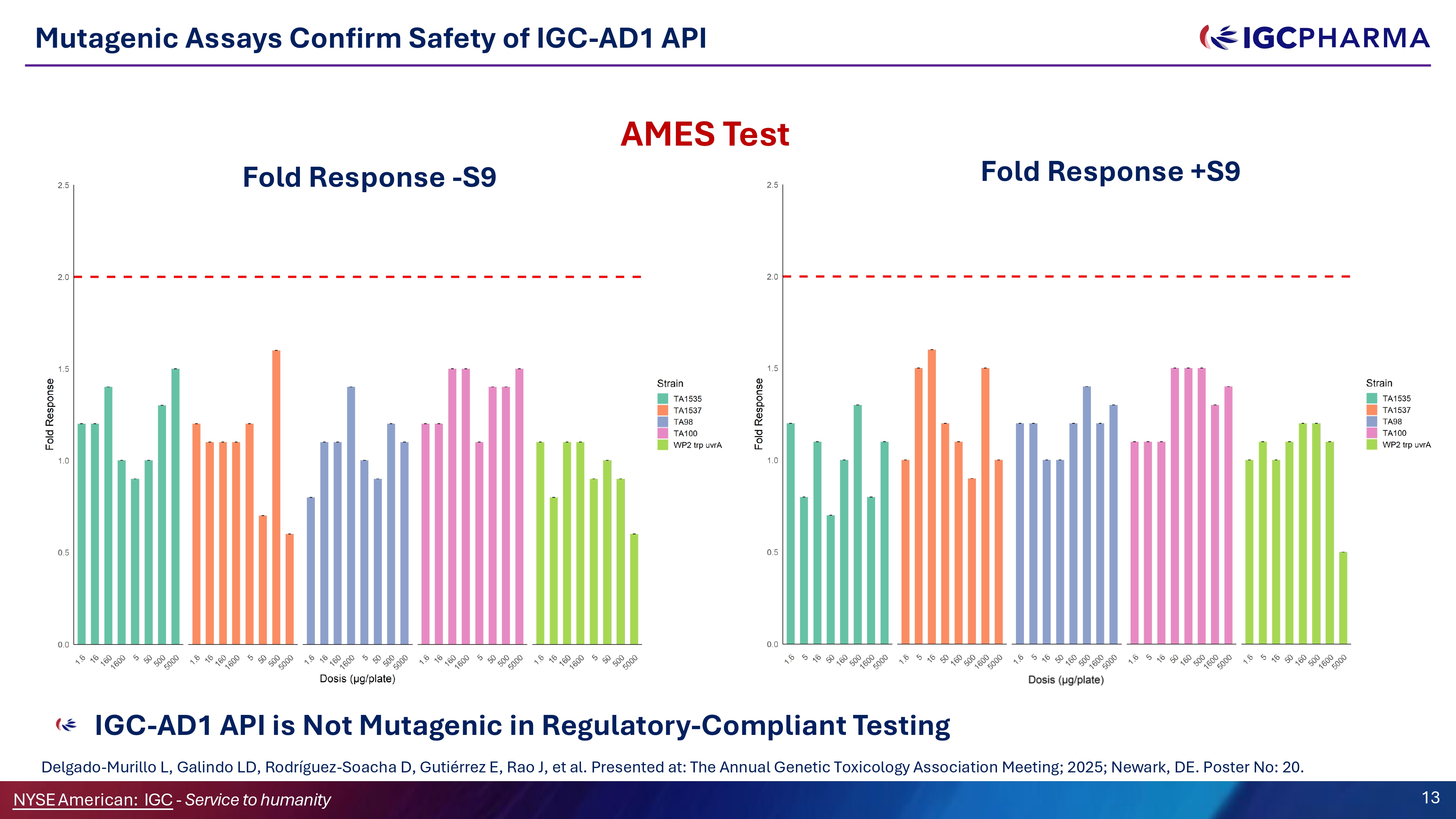
Mutagenic Assays Confirm Safety of IGC - AD1 API IGC - AD1 API is Not Mutagenic in Regulatory - Compliant Testing Delgado - Murillo L, Galindo LD, Rodríguez - Soacha D, Gutiérrez E, Rao J, et al. Presented at: The Annual Genetic Toxicology Association Meeting; 2025; Newark, DE. Poster No: 20. AMES Test Fold Response +SG Fold Response - SG Science Team 13 NYSEAmerican: IGC - Service to humanity

Preclinical Efficacy Studies In vitro studies In vivo studies NYSEAmerican: IGC - Service to humanity 14
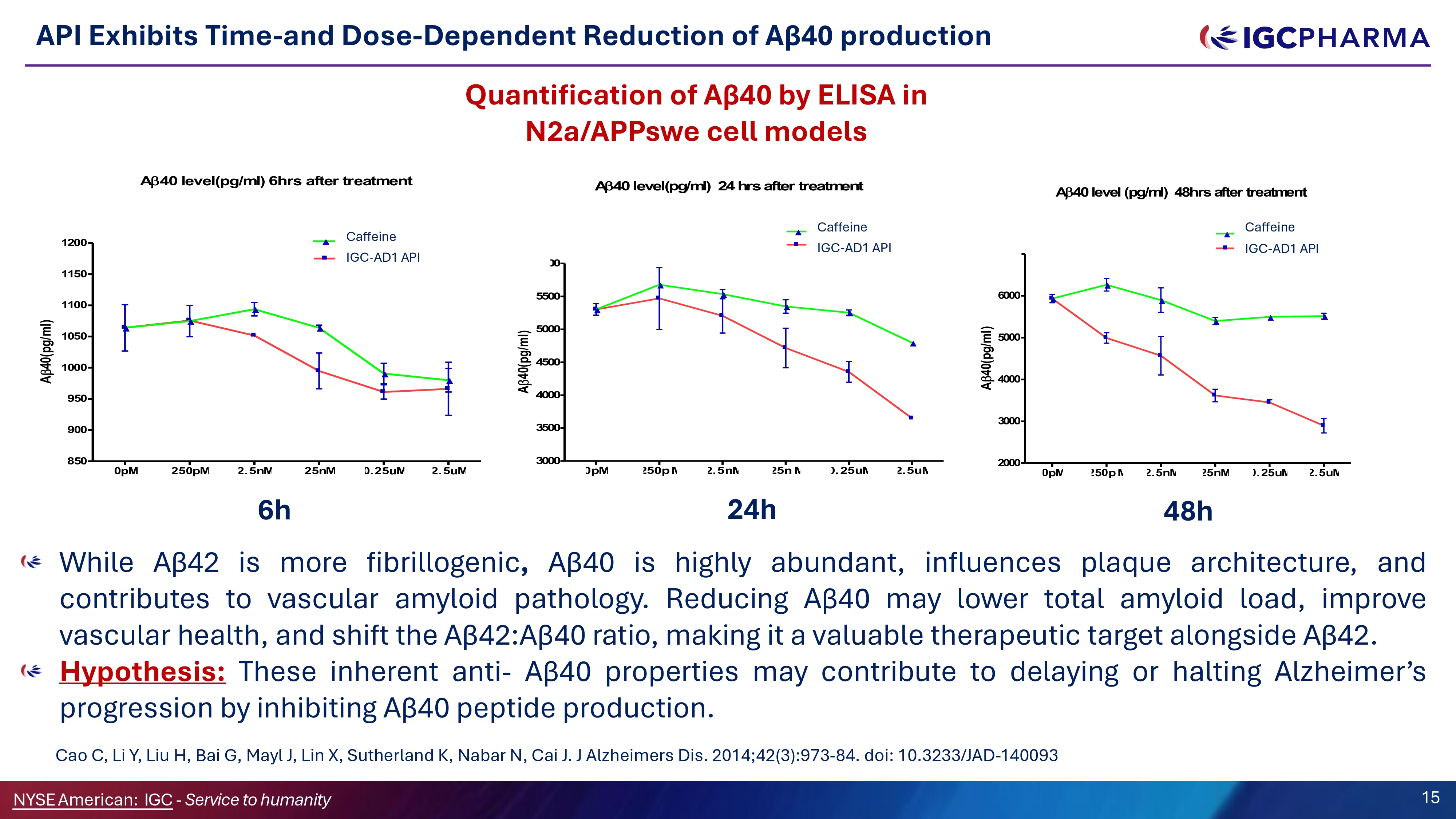
API Exhibits Time - and Dose - Dependent Reduction of Aβ40 production While Aβ 42 is more fibrillogenic , Aβ 40 is highly abundant, influences plaque architecture, and contributes to vascular amyloid pathology . Reducing Aβ 40 may lower total amyloid load, improve vascular health, and shift the Aβ 42 : Aβ 40 ratio, making it a valuable therapeutic target alongside Aβ 42 . Hypothesis : These inherent anti - Aβ 40 properties may contribute to delaying or halting Alzheimer’s progression by inhibiting Aβ 40 peptide production . Cao C, Li Y, Liu H, Bai G, Mayl J, Lin X, Sutherland K, Nabar N, Cai J . J Alzheimers Dis . 2014 ; 42 ( 3 ) : 973 - 84 . doi : 10 . 3233 /JAD - 140093 Ǫuantification of Aβ40 by ELISA in N2a/APPswe cell models 0pM 250pM 2. 5nM 25nM 0. 25uM 2. 5uM 850 900 950 1000 1050 1100 1150 1200 Caffien e A 40 level(pg/ml) 6hrs after treatment THC Concentration A 40(pg/ml) 0pM 50p M . 25uM 2. 5uM 3000 3500 4000 4500 5000 5500 00 THC 60 Caffein e A 40 level(pg/ml) 24 hrs after treatment THC Concentrations A 40(pg/ml) 0pM 2000 3000 4000 5000 6000 THC 7000 THC Caffeine A 40 level (pg/ml) 48hrs after treatment THC Concentration A 40(pg/ml ) Concentration IGC - AD1 API IGC - AD1 API IGC - AD1 API Caffeine Caffeine Caffeine 6h Science Team 15 NYSEAmerican: IGC - Service to humanity 2. 5nM 25n M 24h 50p M 2. 5nM 25nM 0. 25uM 2. 5uM 48h
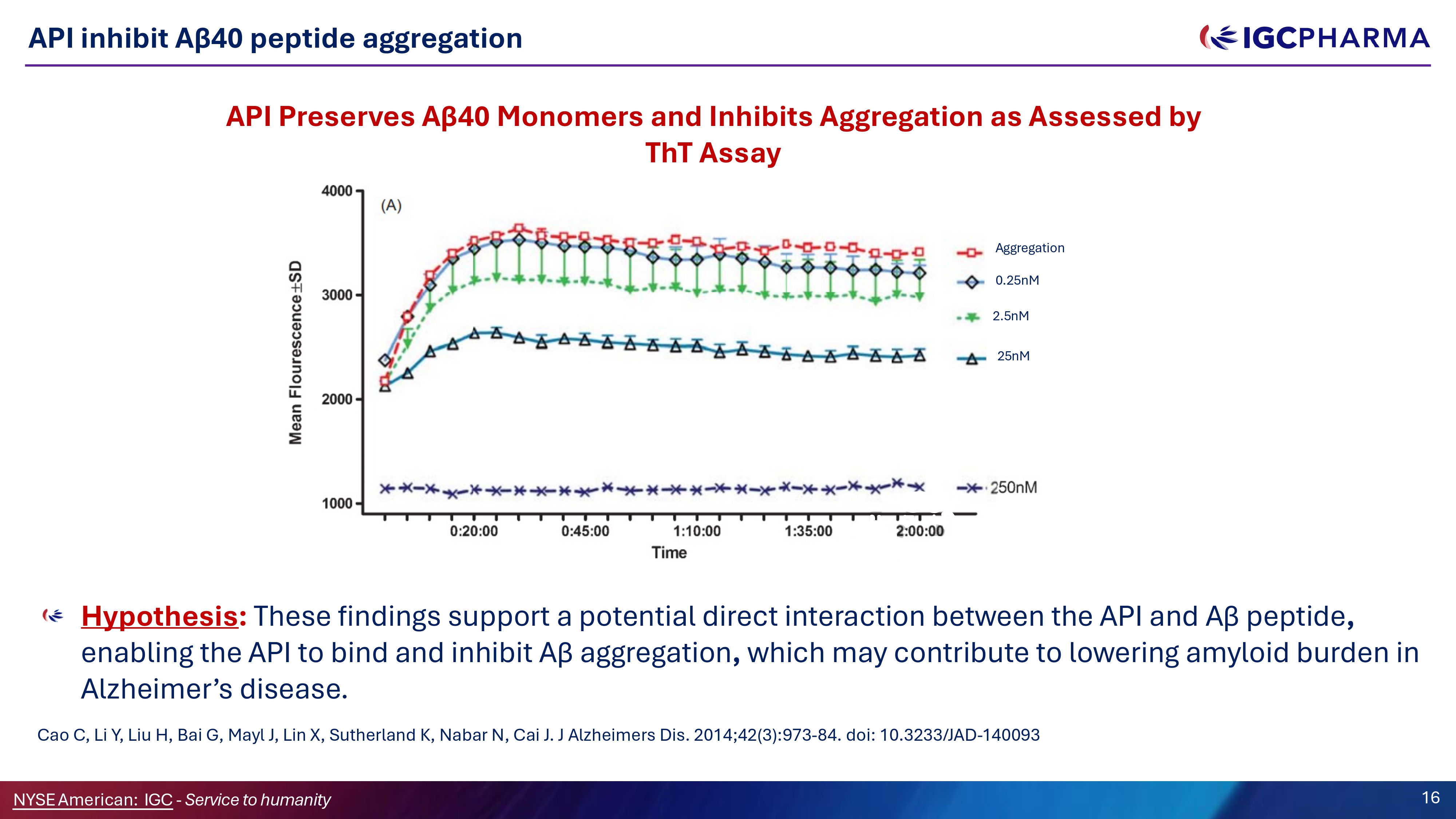
API inhibit Aβ40 peptide aggregation Hypothesis : These findings support a potential direct interaction between the API and Aβ peptide , enabling the API to bind and inhibit Aβ aggregation , which may contribute to lowering amyloid burden in Alzheimer’s disease. Cao C, Li Y, Liu H, Bai G, Mayl J, Lin X, Sutherland K, Nabar N, Cai J. J Alzheimers Dis. 2014;42(3):973 - 84. doi: 10.3233/JAD - 140093 API Preserves Aβ40 Monomers and Inhibits Aggregation as Assessed by ThT Assay Aggregation 0.25nM 2.5nM 25nM Science Team 16 NYSEAmerican: IGC - Service to humanity
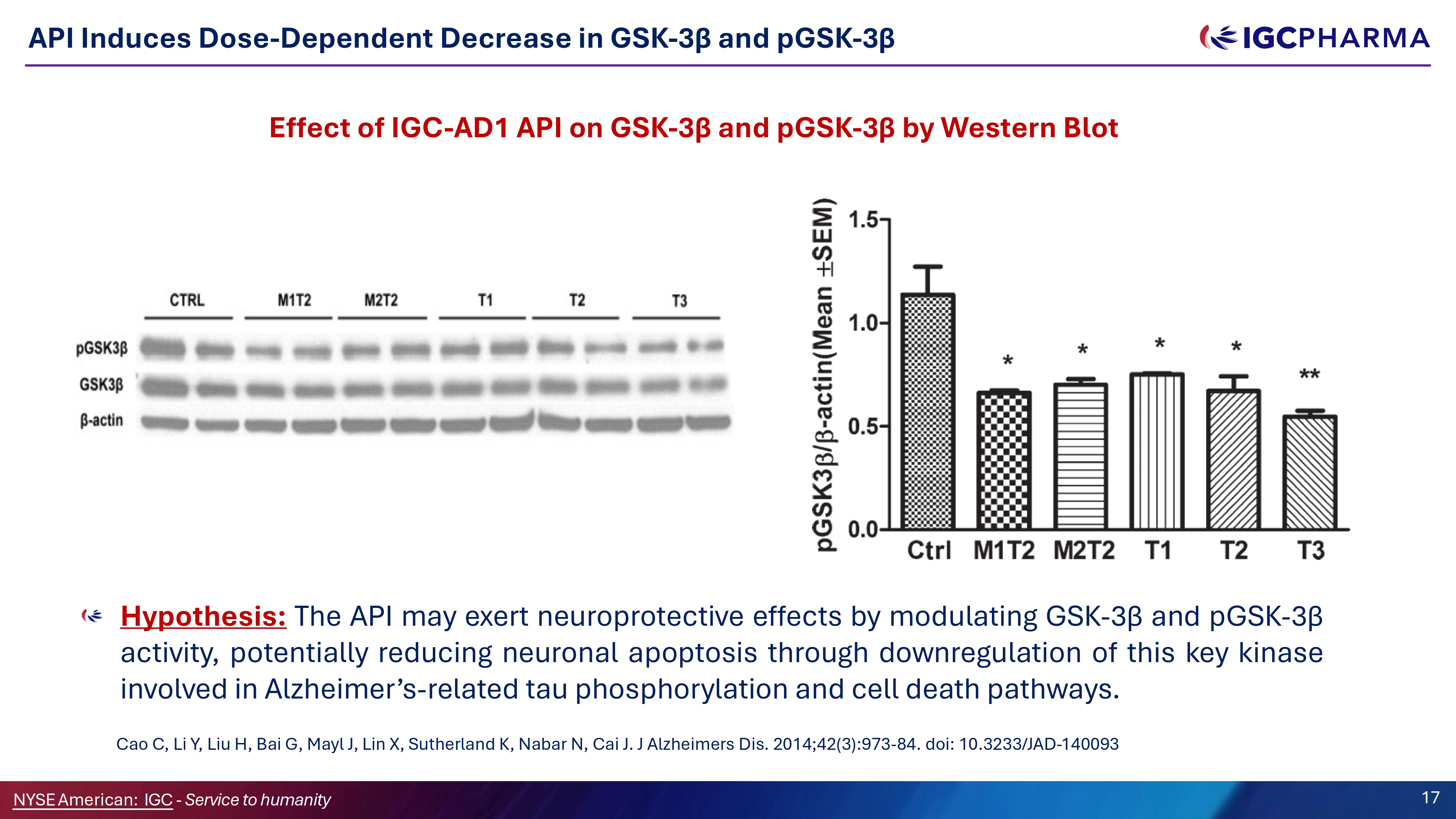
API Induces Dose - Dependent Decrease in GSK - 3β and pGSK - 3β Hypothesis : The API may exert neuroprotective effects by modulating GSK - 3 β and pGSK - 3 β activity, potentially reducing neuronal apoptosis through downregulation of this key kinase involved in Alzheimer’s - related tau phosphorylation and cell death pathways . Cao C, Li Y, Liu H, Bai G, Mayl J, Lin X, Sutherland K, Nabar N, Cai J . J Alzheimers Dis . 2014 ; 42 ( 3 ) : 973 - 84 . doi : 10 . 3233 /JAD - 140093 Effect of IGC - AD1 API on GSK - 3β and pGSK - 3β by Western Blot Science Team 17 NYSEAmerican: IGC - Service to humanity
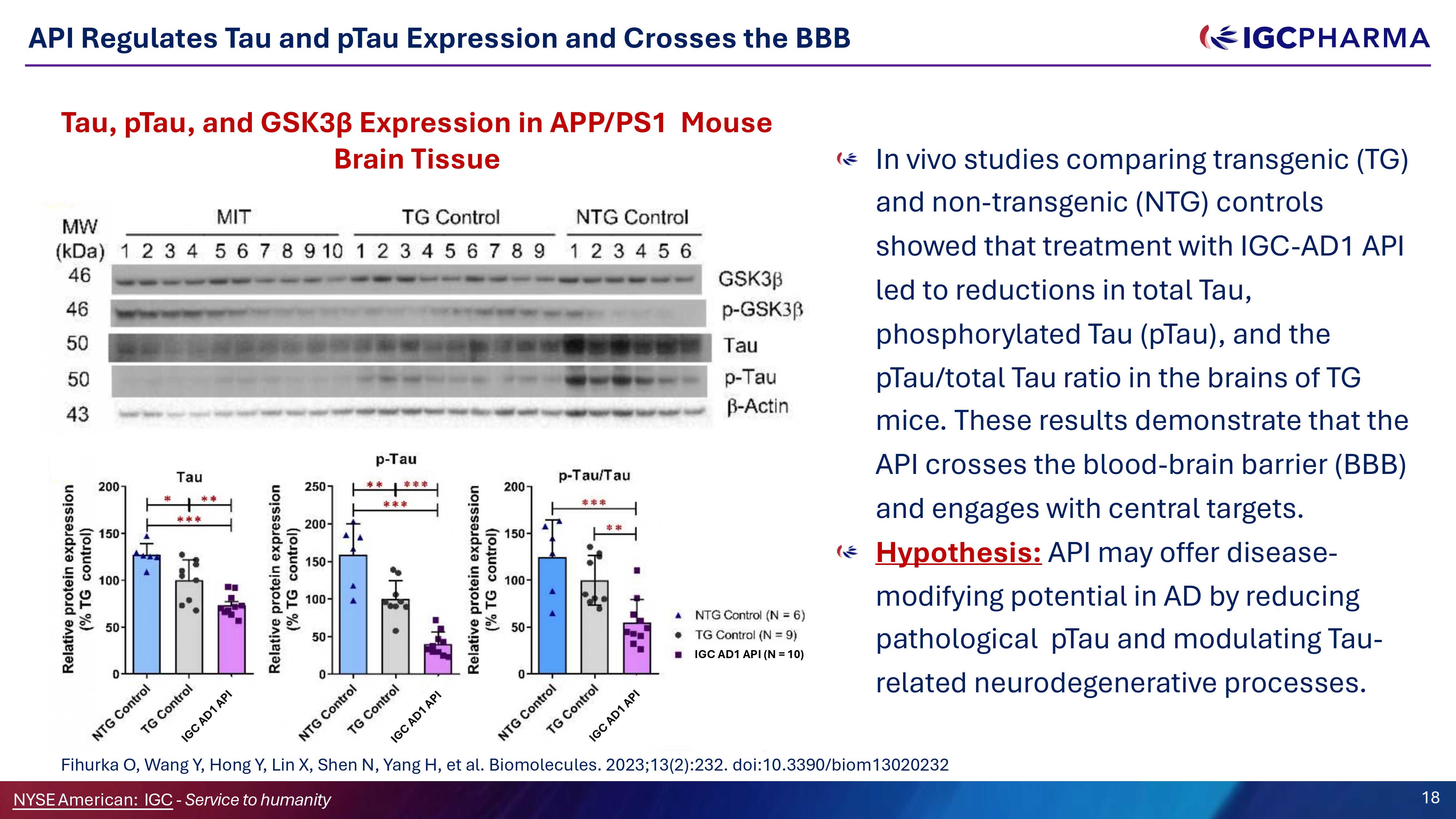
API Regulates Tau and pTau Expression and Crosses the BBB Tau, pTau, and GSK3β Expression in APP/PS1 Mouse Brain Tissue In vivo studies comparing transgenic (TG) and non - transgenic (NTG) controls showed that treatment with IGC - AD1 API led to reductions in total Tau, phosphorylated Tau (pTau), and the pTau/total Tau ratio in the brains of TG mice. These results demonstrate that the API crosses the blood - brain barrier (BBB) and engages with central targets. Hypothesis: API may offer disease - modifying potential in AD by reducing pathological pTau and modulating Tau - related neurodegenerative processes. Fihurka O, Wang Y, Hong Y, Lin X, Shen N, Yang H, et al. Biomolecules. 2023;13(2):232. doi:10.3390/biom13020232 IGC AD1 API (N = 10) Science Team 18 NYSEAmerican: IGC - Service to humanity
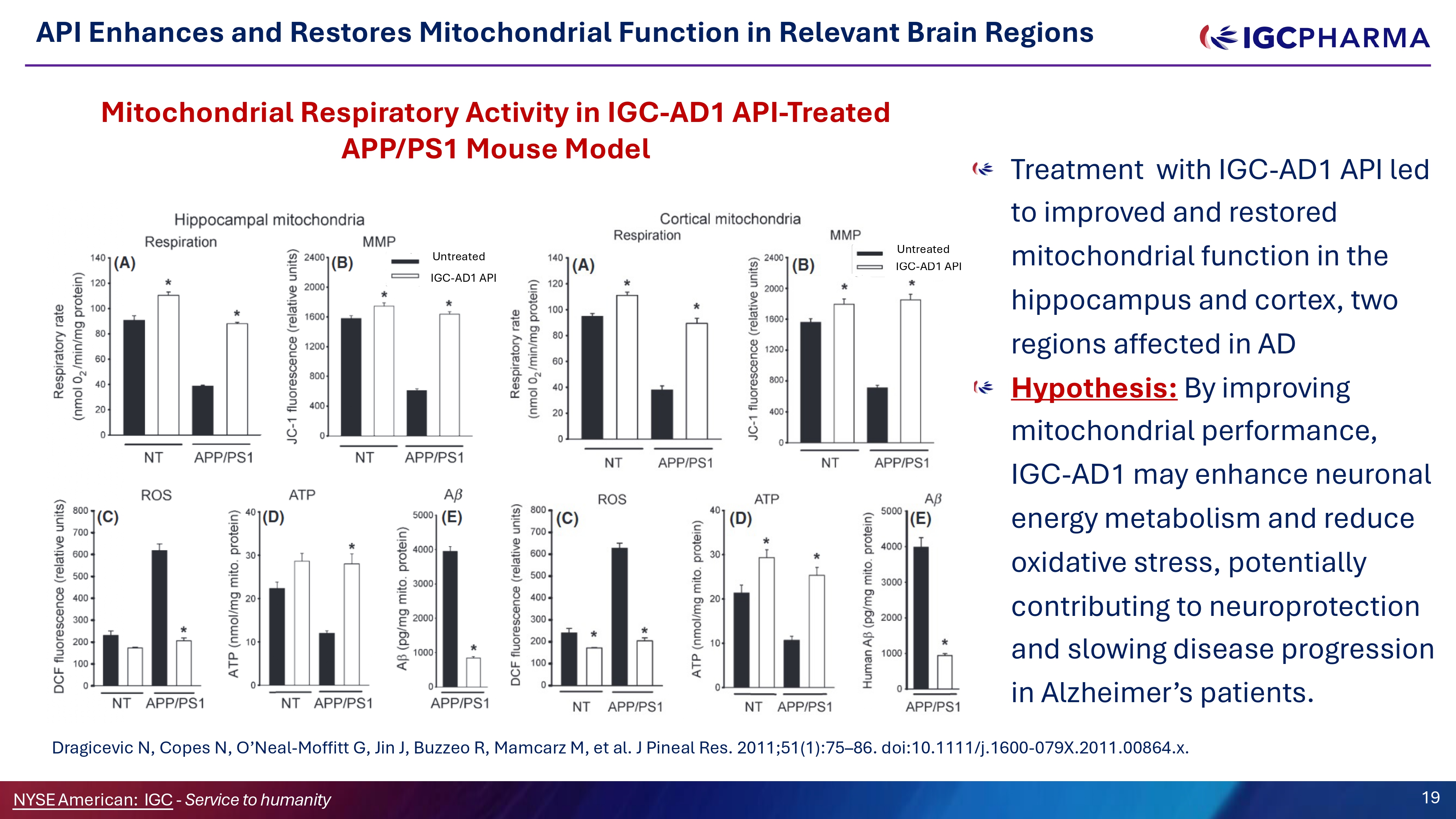
API Enhances and Restores Mitochondrial Function in Relevant Brain Regions Mitochondrial Respiratory Activity in IGC - AD1 API - Treated APP/PS1 Mouse Model Treatment with IGC - AD1 API led to improved and restored mitochondrial function in the hippocampus and cortex, two regions affected in AD Hypothesis: By improving mitochondrial performance, IGC - AD1 may enhance neuronal energy metabolism and reduce oxidative stress, potentially contributing to neuroprotection and slowing disease progression in Alzheimer’s patients. Dragicevic N, Copes N, O’Neal - Moffitt G, Jin J, Buzzeo R, Mamcarz M, et al. J Pineal Res. 2011;51(1):75 – 86. doi:10.1111/j.1600 - 079X.2011.00864.x. IGC - AD1 API Untreated IGC - AD1 API Untreated Science Team 19 NYSEAmerican: IGC - Service to humanity
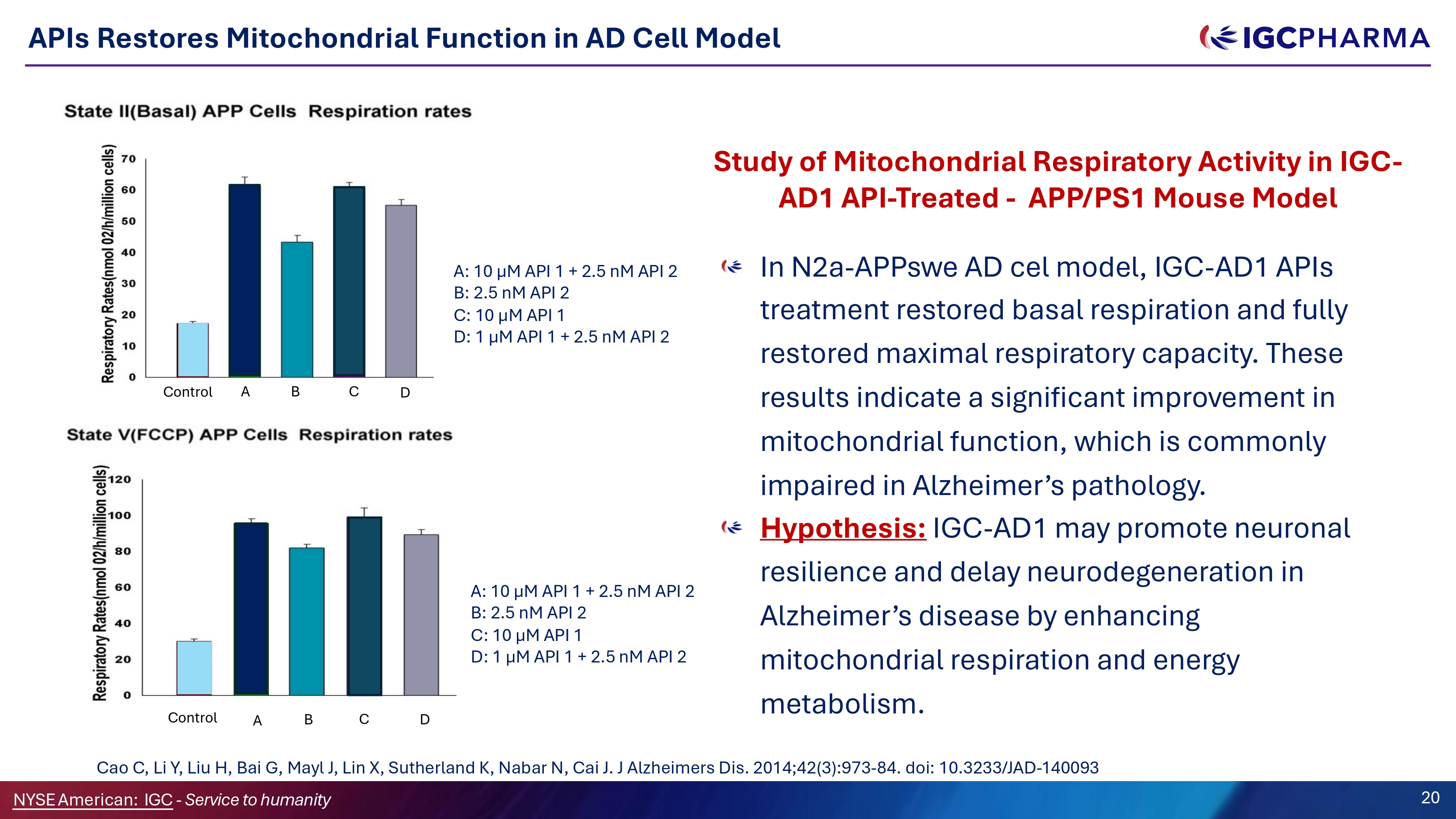
APIs Restores Mitochondrial Function in AD Cell Model Study of Mitochondrial Respiratory Activity in IGC - AD1 API - Treated - APP/PS1 Mouse Model In N2a - APPswe AD cel model, IGC - AD1 APIs treatment restored basal respiration and fully restored maximal respiratory capacity. These results indicate a significant improvement in mitochondrial function, which is commonly impaired in Alzheimer’s pathology. Hypothesis: IGC - AD1 may promote neuronal resilience and delay neurodegeneration in Alzheimer’s disease by enhancing mitochondrial respiration and energy metabolism. A: 10 µM API 1 + 2.5 nM API 2 B: 2.5 nM API 2 C: 10 µM API 1 D: 1 µM API 1 + 2.5 nM API 2 Control Science Team Cao C, Li Y, Liu H, Bai G, Mayl J, Lin X, Sutherland K, Nabar N, Cai J. J Alzheimers Dis. 2014;42(3):973 - 84. doi: 10.3233/JAD - 140093 NYSEAmerican: IGC - Service to humanity 20 Control A B C D A B C D A: 10 µM API 1 + 2.5 nM API 2 B: 2.5 nM API 2 C: 10 µM API 1 D: 1 µM API 1 + 2.5 nM API 2
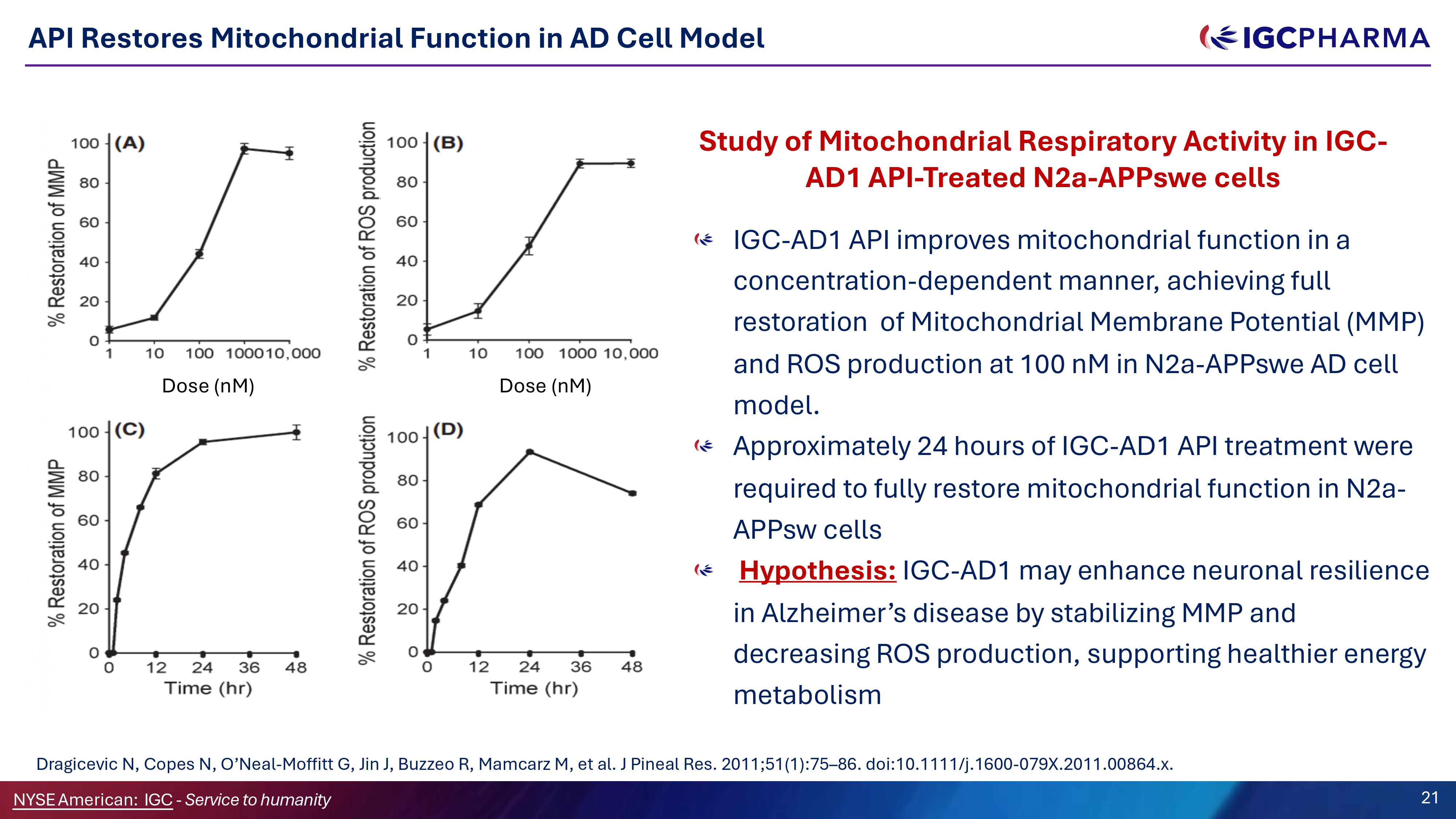
API Restores Mitochondrial Function in AD Cell Model Study of Mitochondrial Respiratory Activity in IGC - AD1 API - Treated N2a - APPswe cells IGC - AD1 API improves mitochondrial function in a concentration - dependent manner, achieving full restoration of Mitochondrial Membrane Potential (MMP) and ROS production at 100 nM in N2a - APPswe AD cell model. Approximately 24 hours of IGC - AD1 API treatment were required to fully restore mitochondrial function in N2a - APPsw cells Hypothesis: IGC - AD1 may enhance neuronal resilience in Alzheimer’s disease by stabilizing MMP and decreasing ROS production, supporting healthier energy metabolism Dose (nM) Science Team Dragicevic N, Copes N, O’Neal - Moffitt G, Jin J, Buzzeo R, Mamcarz M, et al. J Pineal Res. 2011;51(1):75 – 86. doi:10.1111/j.1600 - 079X.2011.00864.x. NYSEAmerican: IGC - Service to humanity 21 Dose (nM)
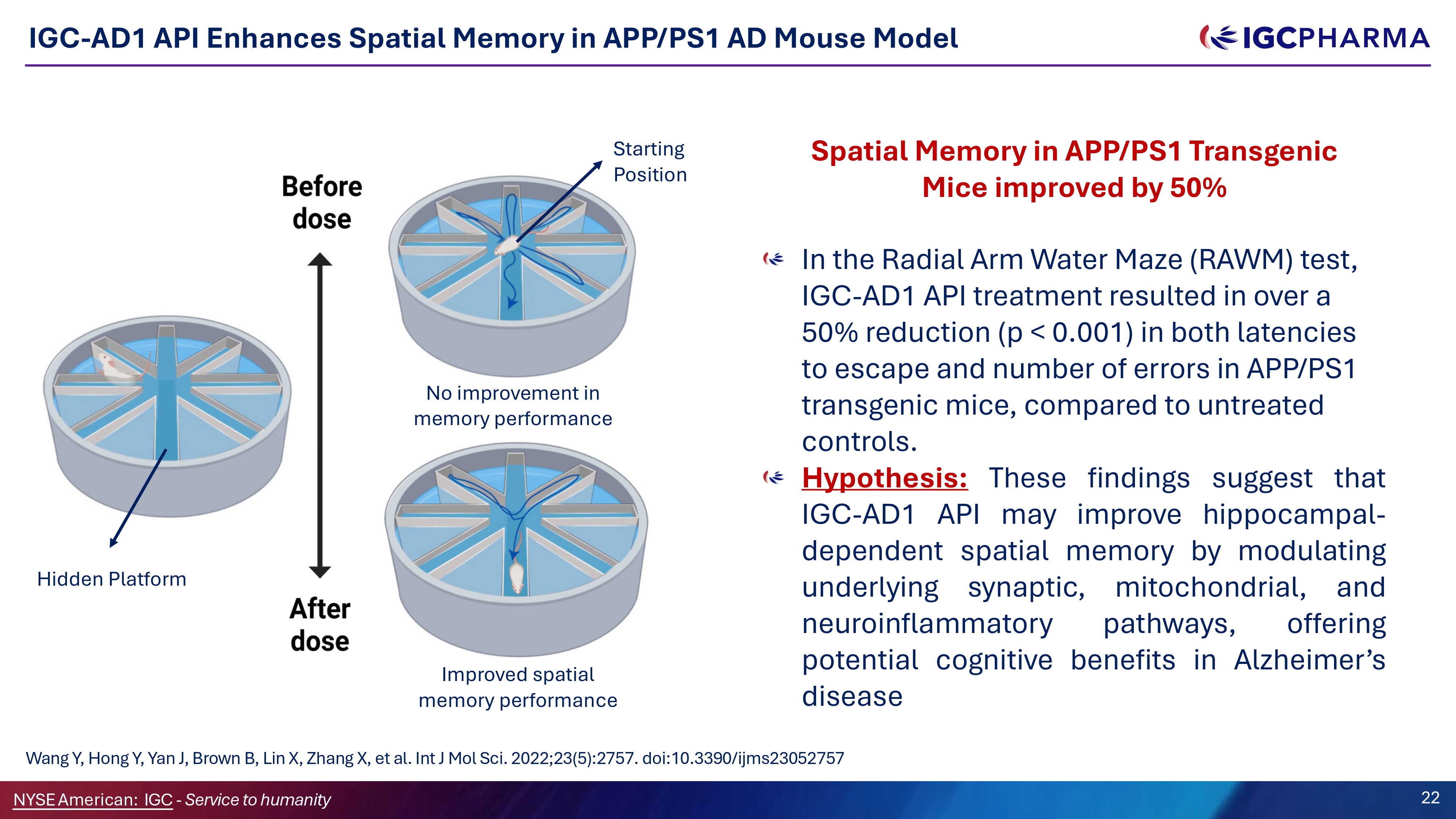
IGC - AD1 API Enhances Spatial Memory in APP/PS1 AD Mouse Model In the Radial Arm Water Maze (RAWM) test, IGC - AD1 API treatment resulted in over a 50% reduction (p < 0.001) in both latencies to escape and number of errors in APP/PS1 transgenic mice, compared to untreated controls. Hypothesis : These findings suggest that IGC - AD 1 API may improve hippocampal - dependent spatial memory by modulating underlying synaptic, mitochondrial, and neuroinflammatory pathways, offering potential cognitive benefits in Alzheimer’s disease Wang Y, Hong Y, Yan J, Brown B, Lin X, Zhang X, et al. Int J Mol Sci. 2022;23(5):2757. doi:10.3390/ijms23052757 Hidden Platform Science Team 22 NYSEAmerican: IGC - Service to humanity Starting Position No improvement in memory performance Improved spatial memory performance Spatial Memory in APP/PS1 Transgenic Mice improved by 50%
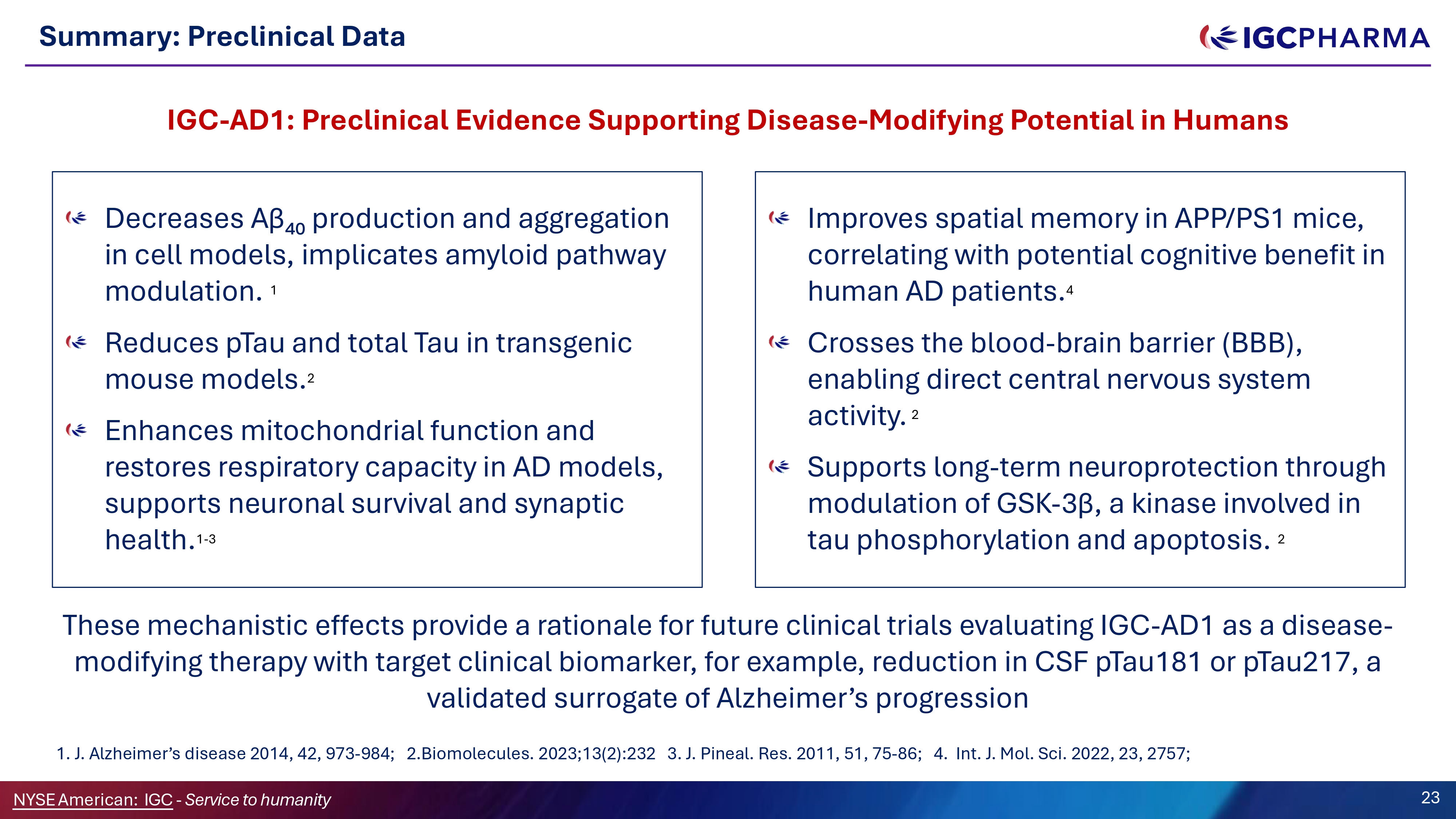
Summary: Preclinical Data Decreases Aβ₄₀ production and aggregation in cell models, implicates amyloid pathway modulation . 1 Reduces pTau and total Tau in transgenic mouse models. 2 Enhances mitochondrial function and restores respiratory capacity in AD models, supports neuronal survival and synaptic health. 1 - 3 IGC - AD1: Preclinical Evidence Supporting Disease - Modifying Potential in Humans Improves spatial memory in APP/PS1 mice, correlating with potential cognitive benefit in human AD patients. 4 Crosses the blood - brain barrier (BBB), enabling direct central nervous system activity . 2 Supports long - term neuroprotection through modulation of GSK - 3β, a kinase involved in tau phosphorylation and apoptosis. 2 23 NYSEAmerican: IGC - Service to humanity These mechanistic effects provide a rationale for future clinical trials evaluating IGC - AD1 as a disease - modifying therapy with target clinical biomarker, for example, reduction in CSF pTau181 or pTau217, a validated surrogate of Alzheimer’s progression 1. J. Alzheimer’s disease 2014, 42, 973 - 984; 2.Biomolecules. 2023;13(2):232 3. J. Pineal. Res. 2011, 51, 75 - 86; 4. Int. J. Mol. Sci. 2022, 23, 2757;

Phase I Randomized Placebo Controlled MAD Study to Evaluate Safety and Tolerability of IGC - AD1 in Subjects With Dementia Due to Alzheimer's Disease Phase 1 Trial Results 24 NYSEAmerican: IGC - Service to humanity

Phase 1 · Objectives and End Points Primary: Safety and tolerability of IGC - AD 1 , in a double - blind, multiple ascending dose (MAD) study, measured by solicited and non - solicited Adverse Events (AEs) in participants with mild to severe AD . Secondary: Compare (NPS) pre and post intervention neuropsychiatric symptoms measured by the neuropsychiatric inventory (NPI - 12 ) . Exploratory: Pharmacokinetics (PK) of IGC - AD1, and the impact of polymorphisms of CYP2C9 on PK. Trial on www.clinicaltrials.gov Science Team 25 NYSEAmerican: IGC - Service to humanity
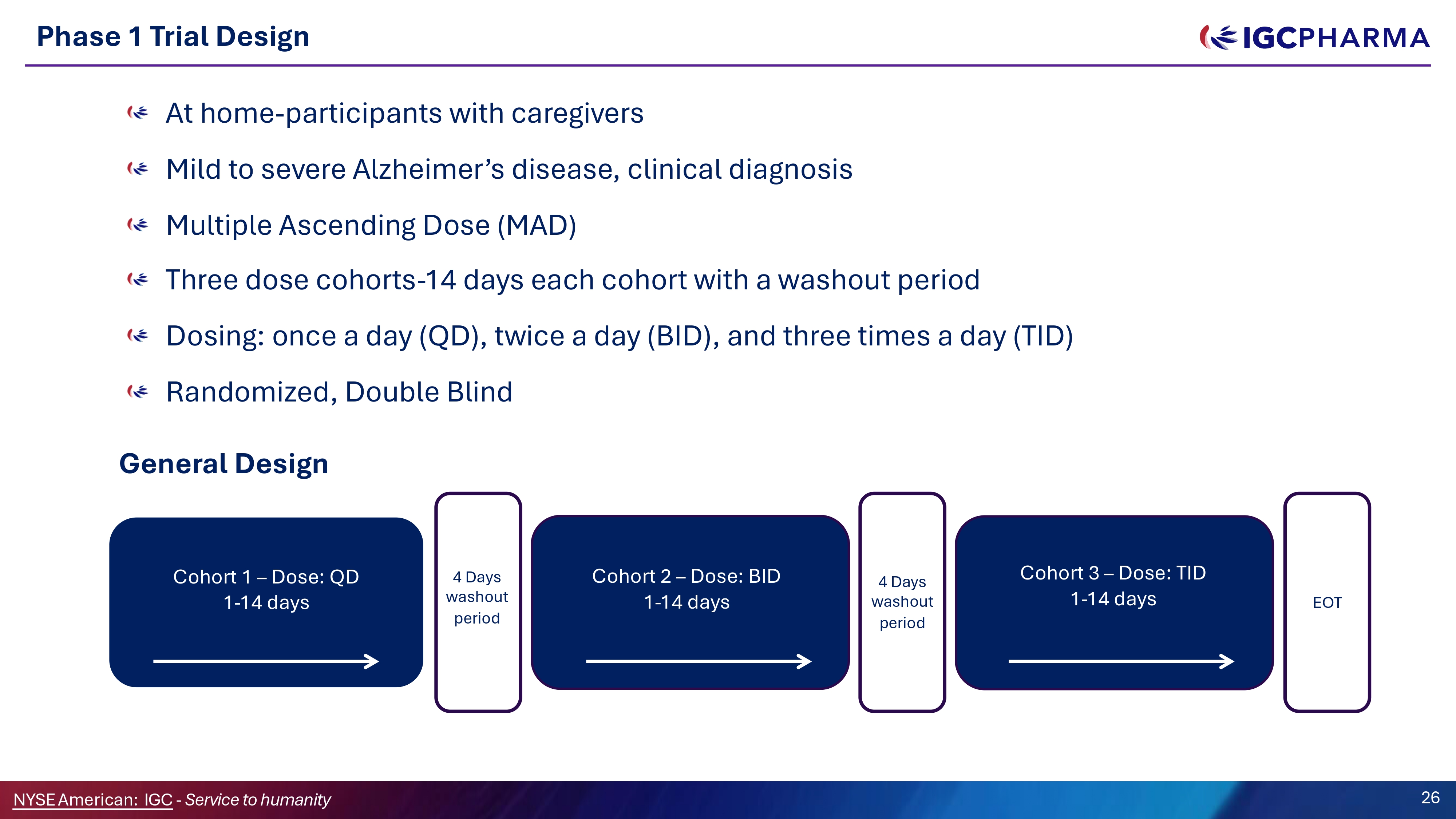
Phase 1 Trial Design At home - participants with caregivers Mild to severe Alzheimer’s disease, clinical diagnosis Multiple Ascending Dose (MAD) Three dose cohorts - 14 days each cohort with a washout period Dosing: once a day (ǪD), twice a day (BID), and three times a day (TID) Randomized, Double Blind General Design Cohort 1 – Dose: ǪD 1 - 14 days Cohort 2 – Dose: BID 1 - 14 days Cohort 3 – Dose: TID 1 - 14 days 4 Days washout period EOT 4 Days washout period 26 NYSEAmerican: IGC - Service to humanity
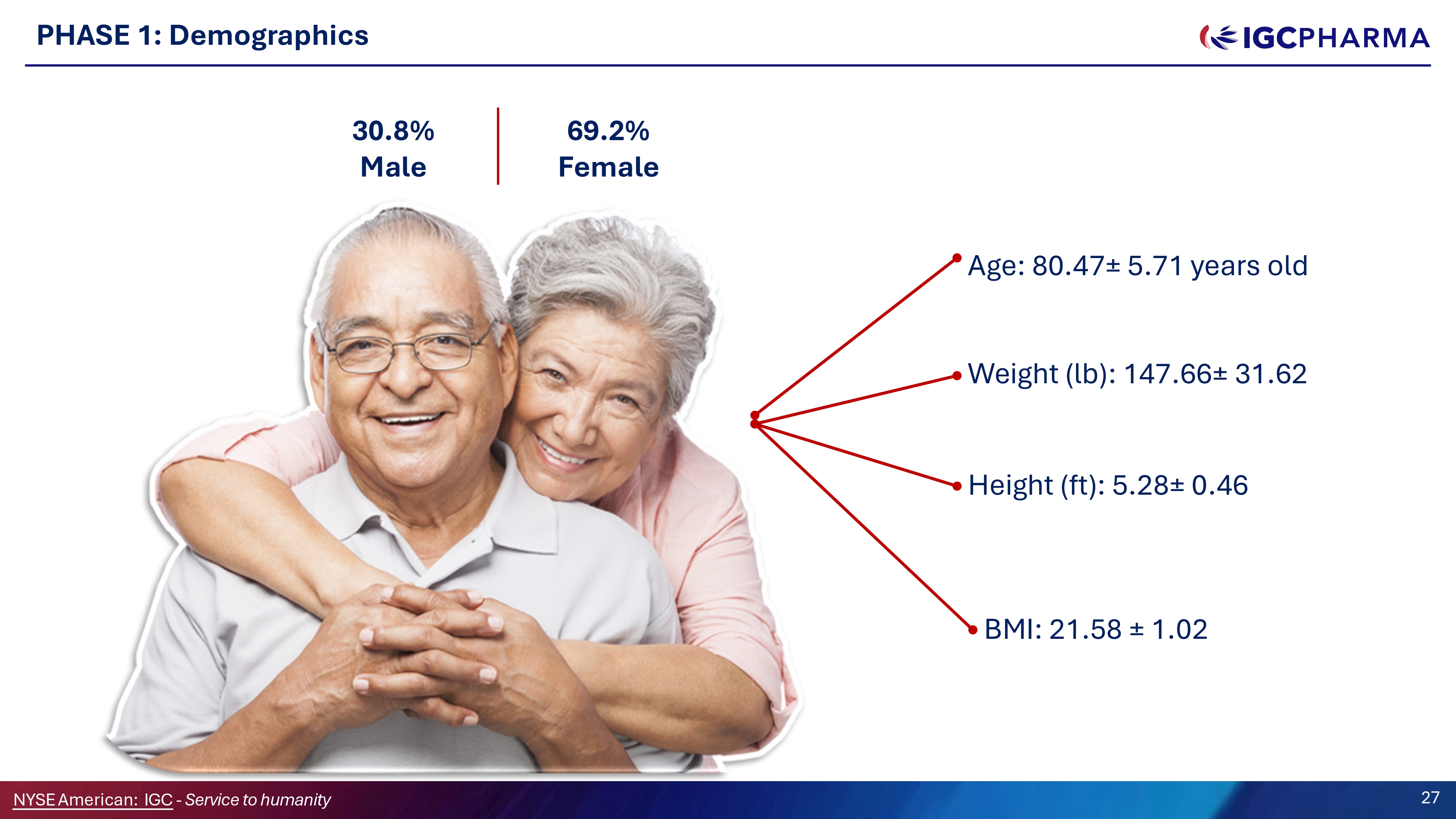
PHASE 1: Demographics 6G.2% Female 30.8% Male Age: 80.47 “ 5.71 years old Weight (lb): 147.66 “ 31.62 Height (ft): 5.28 “ 0.46 BMI: 21.58 “ 1.02 Science Team 27 NYSEAmerican: IGC - Service to humanity
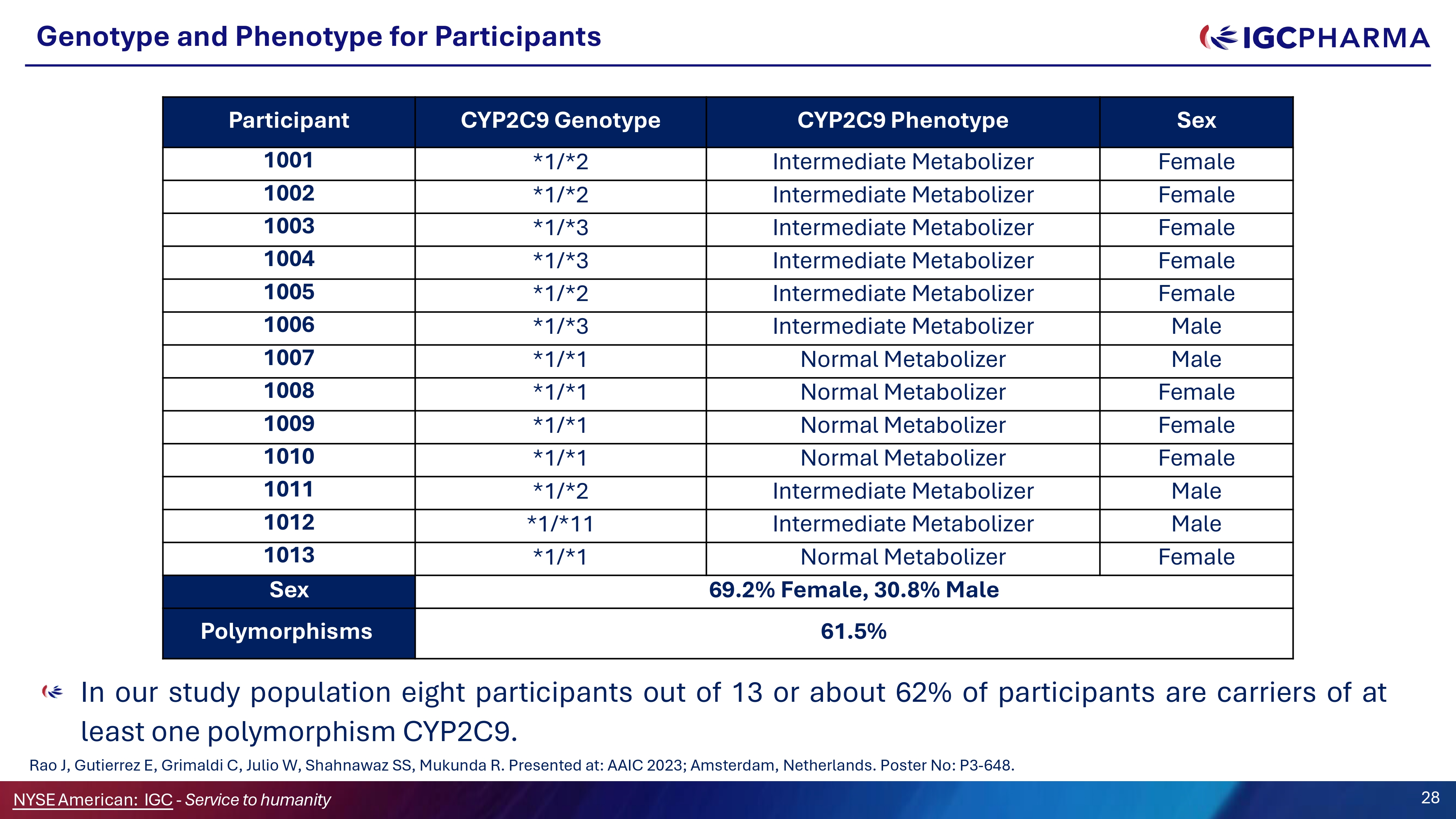
Genotype and Phenotype for Participants In our study population eight participants out of 13 or about 62% of participants are carriers of at least one polymorphism CYP2C9. Rao J, Gutierrez E, Grimaldi C, Julio W, Shahnawaz SS, Mukunda R. Presented at: AAIC 2023; Amsterdam, Netherlands. Poster No: P3 - 648. Sex CYP2CG Phenotype CYP2CG Genotype Participant Female Intermediate Metabolizer *1/*2 1001 Female Intermediate Metabolizer *1/*2 1002 Female Intermediate Metabolizer *1/*3 1003 Female Intermediate Metabolizer *1/*3 1004 Female Intermediate Metabolizer *1/*2 1005 Male Intermediate Metabolizer *1/*3 1006 Male Normal Metabolizer *1/*1 1007 Female Normal Metabolizer *1/*1 1008 Female Normal Metabolizer *1/*1 100G Female Normal Metabolizer *1/*1 1010 Male Intermediate Metabolizer *1/*2 1011 Male Intermediate Metabolizer *1/*11 1012 Female Normal Metabolizer *1/*1 1013 6G.2% Female, 30.8% Male Sex 61.5% Polymorphisms 28 NYSEAmerican: IGC - Service to humanity

Phase 1 Trial Safety Results IGC - AD1 was well - tolerated at all three dose levels There were no: Serious Adverse Events (SAEs) Life - threatening AEs Deaths reported Science Team 29 NYSEAmerican: IGC - Service to humanity
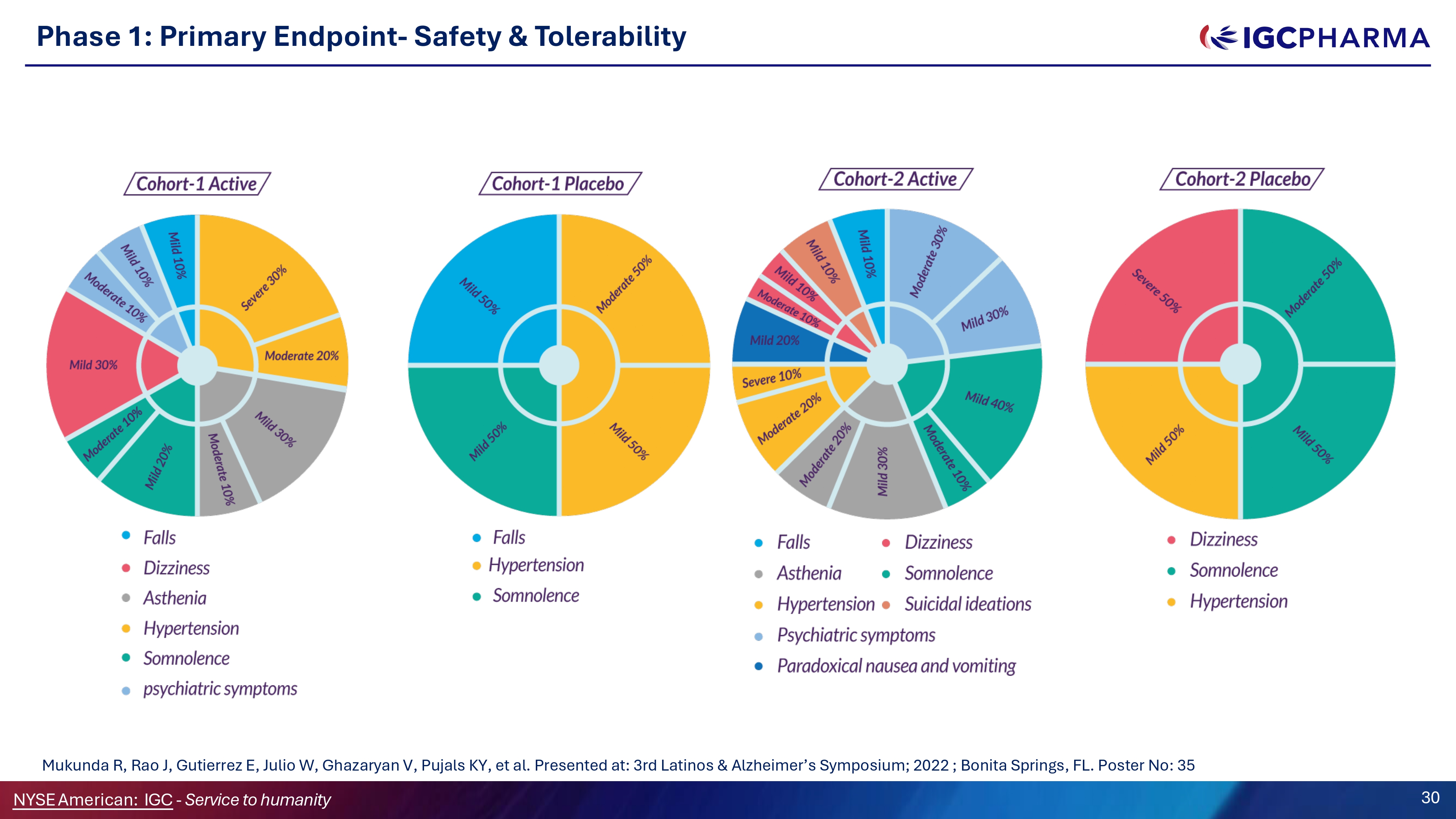
Phase 1: Primary Endpoint - Safety G Tolerability Science Team Mukunda R, Rao J, Gutierrez E, Julio W, Ghazaryan V, Pujals KY, et al. Presented at: 3rd Latinos C Alzheimer’s Symposium; 2022 ; Bonita Springs, FL. Poster No: 35 NYSEAmerican: IGC - Service to humanity 30
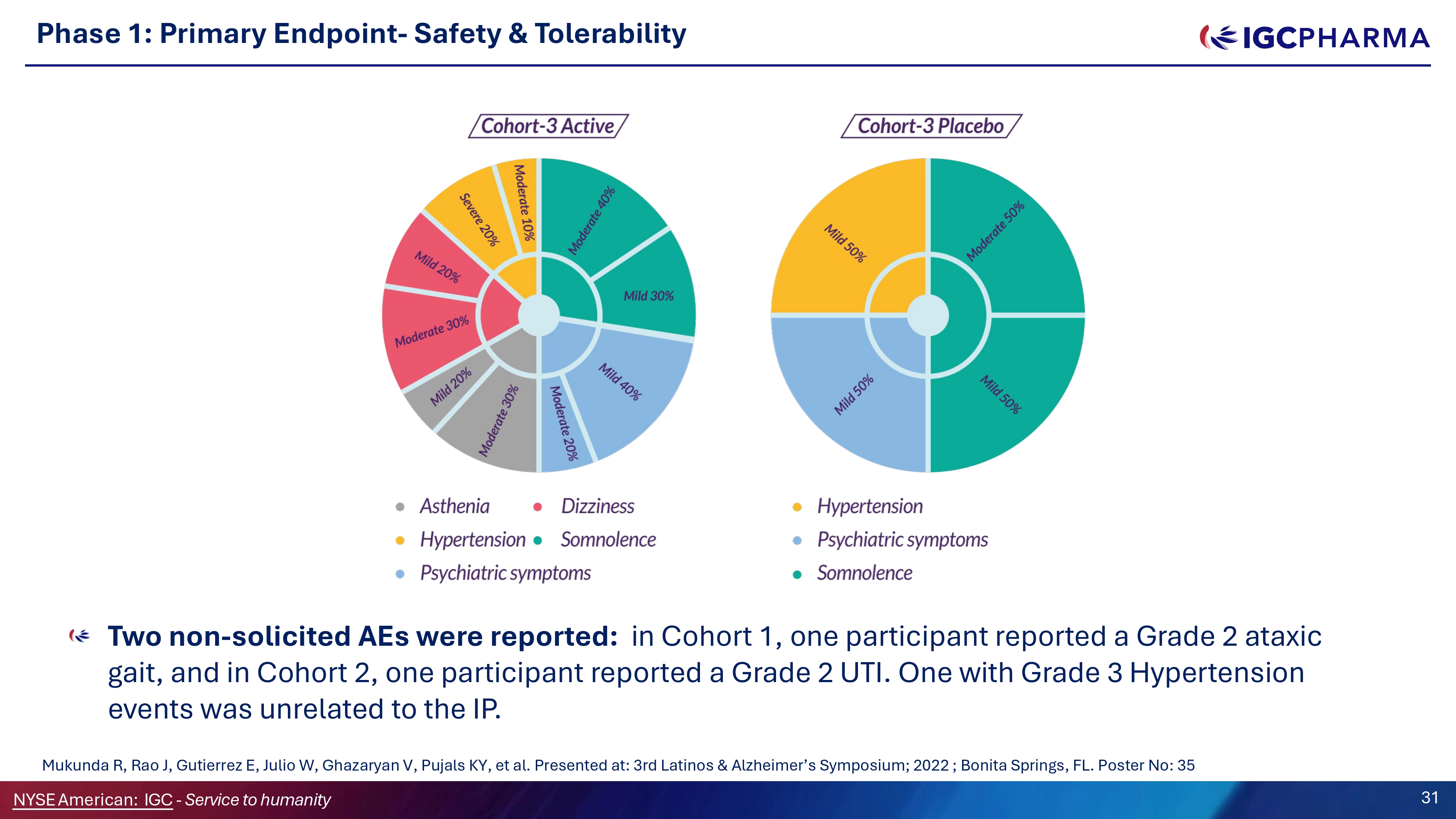
Two non - solicited AEs were reported: in Cohort 1, one participant reported a Grade 2 ataxic gait, and in Cohort 2, one participant reported a Grade 2 UTI. One with Grade 3 Hypertension events was unrelated to the IP. Phase 1: Primary Endpoint - Safety G Tolerability Mukunda R, Rao J, Gutierrez E, Julio W, Ghazaryan V, Pujals KY, et al. Presented at: 3rd Latinos C Alzheimer’s Symposium; 2022 ; Bonita Springs, FL. Poster No: 35 NYSEAmerican: IGC - Service to humanity 31
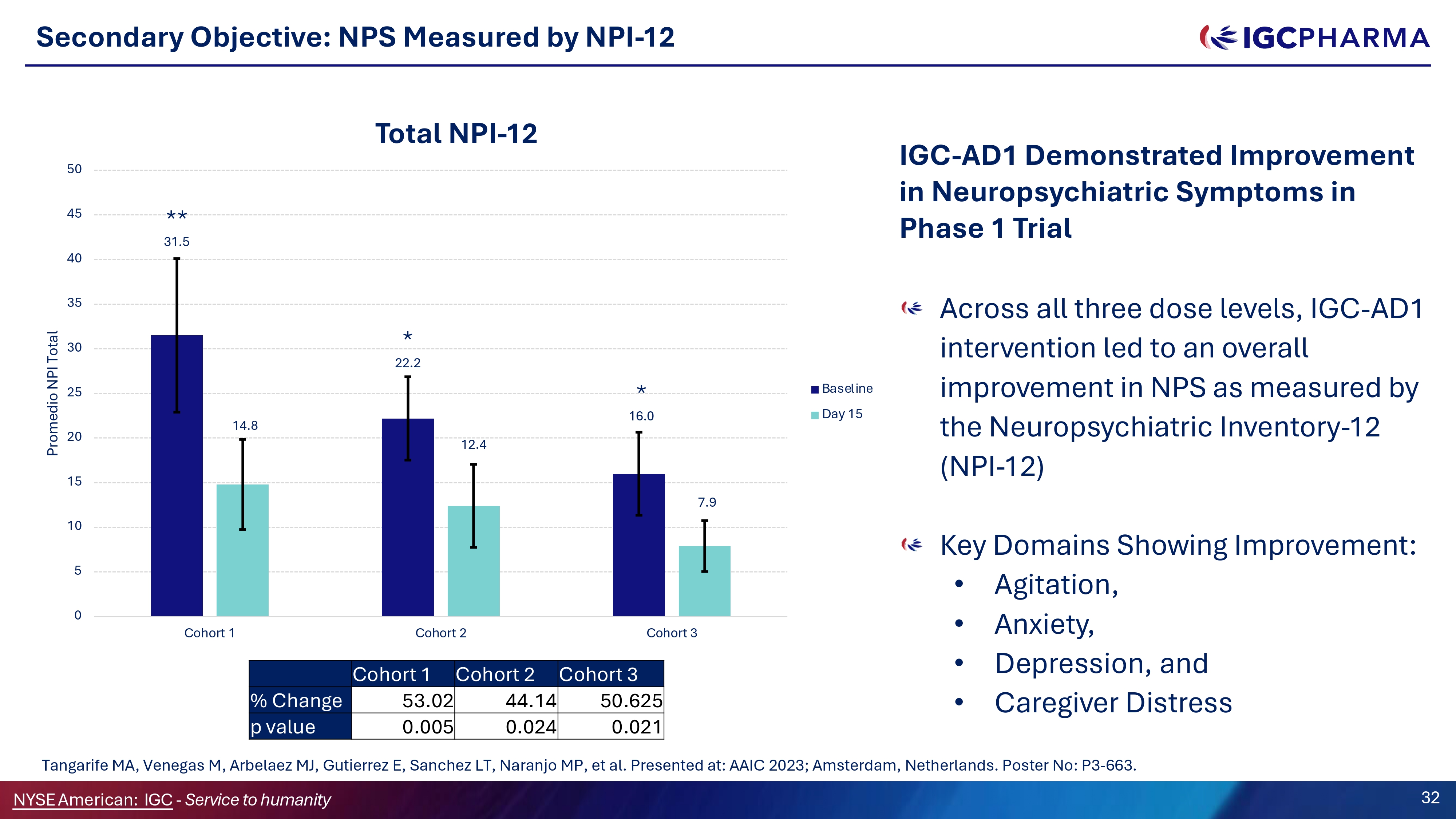
Secondary Objective: NPS Measured by NPI - 12 ** 31.5 * 22.2 * 16.0 14.8 12.4 7.9 0 5 10 15 20 25 30 35 40 45 50 Cohort 1 Cohort 2 Cohort 3 Promedio NPI Total Total NPI - 12 Baseline Day 15 Across all three dose levels, IGC - AD1 intervention led to an overall improvement in NPS as measured by the Neuropsychiatric Inventory - 12 (NPI - 12) Key Domains Showing Improvement: • Agitation, • Anxiety, • Depression, and • Caregiver Distress Cohort 3 Cohort 2 Cohort 1 50.625 44.14 53.02 % Change 0.021 0.024 0.005 p value Science Team Tangarife MA, Venegas M, Arbelaez MJ, Gutierrez E, Sanchez LT, Naranjo MP, et al. Presented at: AAIC 2023; Amsterdam, Netherlands. Poster No: P3 - 663. NYSEAmerican: IGC - Service to humanity 32 IGC - AD1 Demonstrated Improvement in Neuropsychiatric Symptoms in Phase 1 Trial
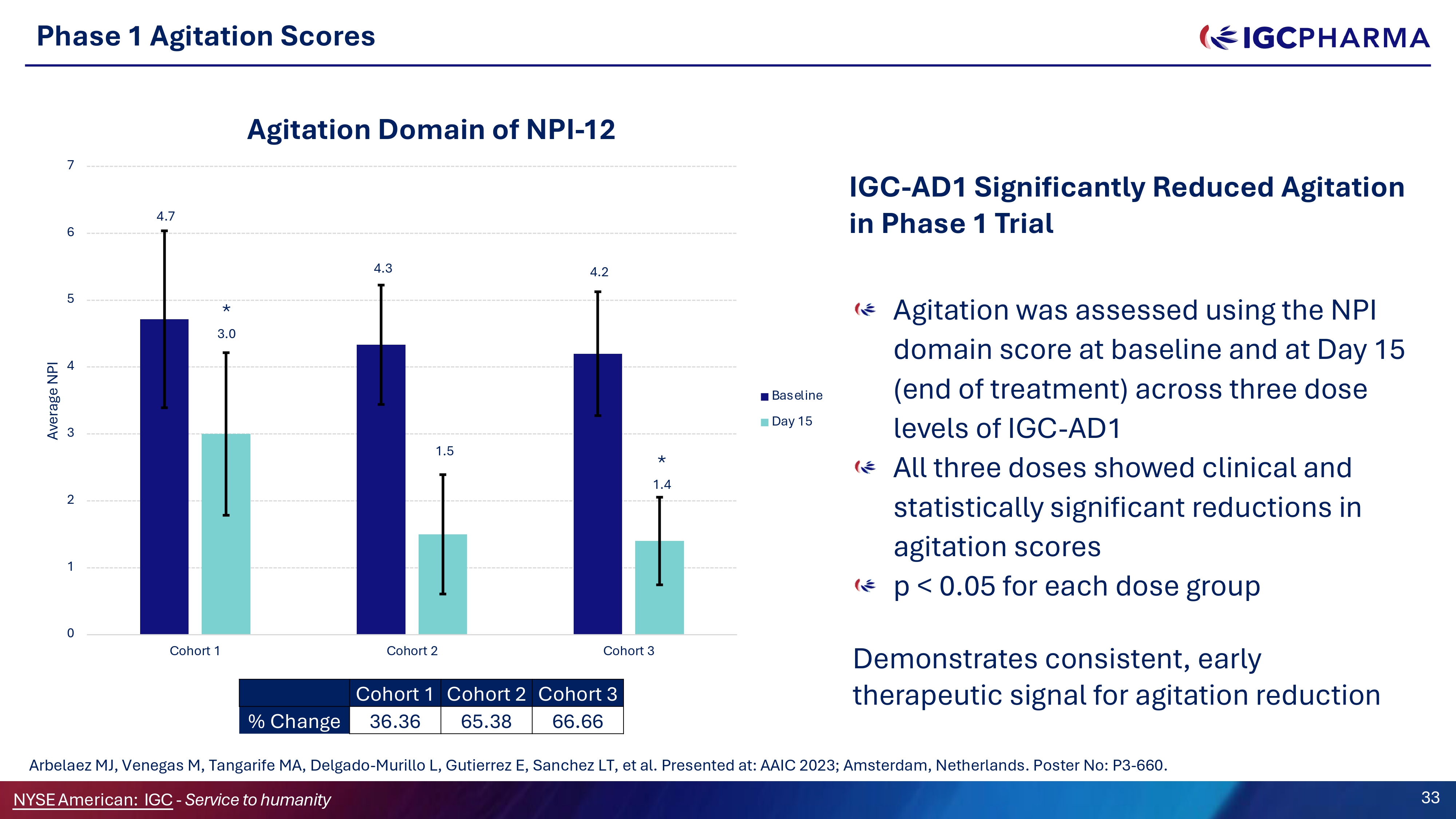
Phase 1 Agitation Scores Agitation was assessed using the NPI domain score at baseline and at Day 15 (end of treatment) across three dose levels of IGC - AD1 All three doses showed clinical and statistically significant reductions in agitation scores p < 0.05 for each dose group Demonstrates consistent, early therapeutic signal for agitation reduction 4.7 4.3 4.2 * 3.0 1.5 * 1.4 0 1 2 3 4 5 6 7 Cohort 1 Cohort 2 Cohort 3 Average NPI Agitation Domain of NPI - 12 Baseline Day 15 Cohort 3 Cohort 2 Cohort 1 66.66 65.38 36.36 % Change Science Team Arbelaez MJ, Venegas M, Tangarife MA, Delgado - Murillo L, Gutierrez E, Sanchez LT, et al. Presented at: AAIC 2023; Amsterdam, Netherlands. Poster No: P3 - 660. NYSEAmerican: IGC - Service to humanity 33 IGC - AD1 Significantly Reduced Agitation in Phase 1 Trial
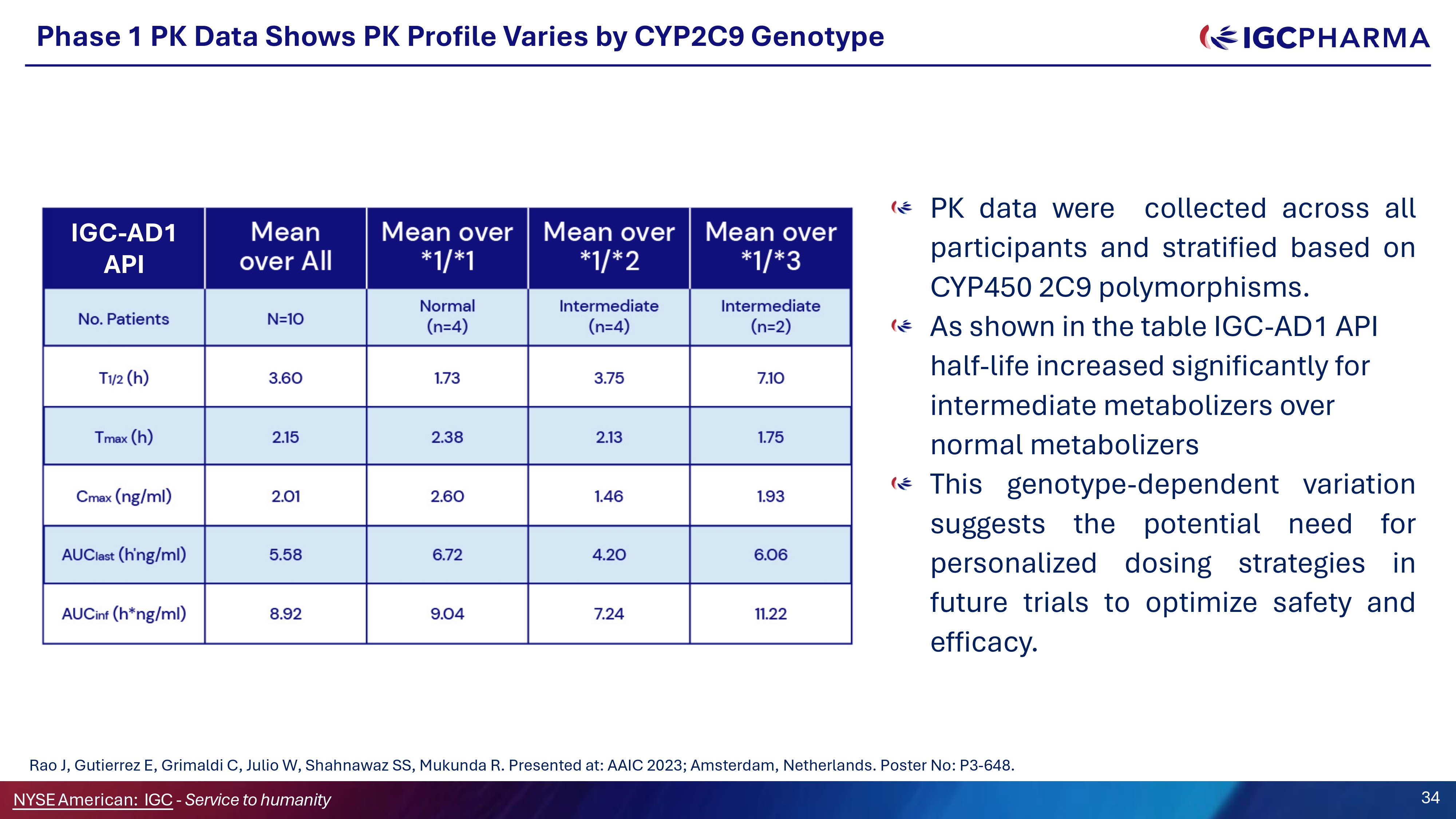
Phase 1 PK Data Shows PK Profile Varies by CYP2CG Genotype PK data were collected across all participants and stratified based on CYP 450 2 C 9 polymorphisms . As shown in the table IGC - AD1 API half - life increased significantly for intermediate metabolizers over normal metabolizers This genotype - dependent variation suggests the potential need for personalized dosing strategies in future trials to optimize safety and efficacy . IGC - AD1 API Rao J, Gutierrez E, Grimaldi C, Julio W, Shahnawaz SS, Mukunda R. Presented at: AAIC 2023; Amsterdam, Netherlands. Poster No: P3 - 648. NYSEAmerican: IGC - Service to humanity 34

IGC Pharma Poster Presentations at the AAIC 2023 in Netherlands Company - owned data NYSEAmerican: IGC - Service to humanity 35
IGC Pharma Poster Presentations at the AAIC 2023 in Netherlands Company - owned data NYSEAmerican: IGC - Service to humanity 36

IGC Pharma Poster Presentations at the AAIC 2024 in Philadelphia Company - owned data NYSEAmerican: IGC - Service to humanity 37

IGC Pharma Poster Presentations at the AAIC 2024 in Philadelphia Company - owned data NYSEAmerican: IGC - Service to humanity 38
IGC Pharma Poster Presentations at the AAIC 2024 in Philadelphia Company - owned data NYSEAmerican: IGC - Service to humanity 39

IGC - AD1 Phase 2 Strategy: Agitation in AD Preclinical studies demonstrated disease modifying potential, including reduction in pTau, Aβ aggregation, and mitochondrial dysfunction. Phase 1 clinical data showed clinical and statistically significant improvements in results agitation symptoms in patients with AD Based on the strength of agitation signal and urgent unmet clinical need, IGC strategically prioritized a Phase 2 trial targeting Agitation in AD dementia. Disease modification remains a long - term objective 40 NYSEAmerican: IGC - Service to humanity

Phase 2, Multi - Center, Double - Blind, Randomized, Placebo - Controlled, Trial of the Safety and Efficacy of IGC - AD1 on Agitation in Participants with Dementia due to Alzheimer’s Disease. Phase 2 in Progress 41 NYSEAmerican: IGC - Service to humanity
Agitation refers to a range of behaviors, excessive motor activity, verbal aggression, or physical aggression that is severe enough to impair personal relationships, social functioning, and/or daily activities ( 1 ) . Agitation has a significant impact on both the individuals with Alzheimer's and their caregivers . Agitated behavior is always socially inappropriate . It may be abusive or aggressive towards self or others . It may be appropriate behavior performed with inappropriate frequency, such as constantly asking questions . References: (1) Cummings J et al., 2014 (2) Front Neurol , 2021, 12: 644317 What is Agitation in Dementia Due to Alzheimer’s Disease? April 2024 42 NYSEAmerican: IGC - Service to humanity
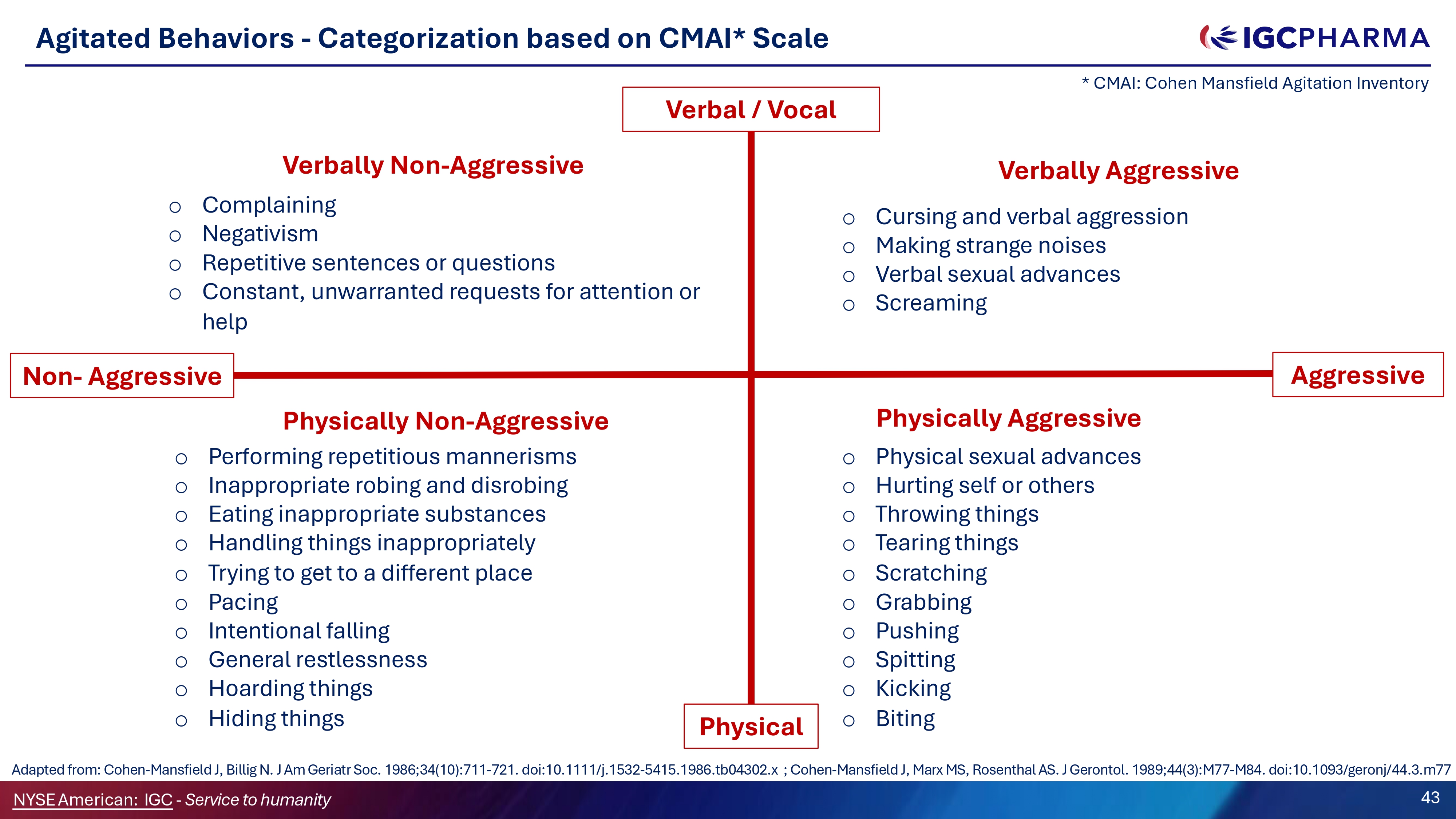
Agitated Behaviors - Categorization based on CMAI* Scale Verbal / Vocal Physical Aggressive Verbally Non - Aggressive o Complaining o Negativism o Repetitive sentences or questions o Constant, unwarranted requests for attention or help Verbally Aggressive o Cursing and verbal aggression o Making strange noises o Verbal sexual advances o Screaming Physically Non - Aggressive o Performing repetitious mannerisms o Inappropriate robing and disrobing o Eating inappropriate substances o Handling things inappropriately o Trying to get to a different place o Pacing o Intentional falling o General restlessness o Hoarding things o Hiding things Physically Aggressive o Physical sexual advances o Hurting self or others o Throwing things o Tearing things o Scratching o Grabbing o Pushing o Spitting o Kicking o Biting Non - Aggressive * CMAI: Cohen Mansfield Agitation Inventory 43 NYSEAmerican: IGC - Service to humanity Adapted from: Cohen - Mansfield J, Billig N. J Am Geriatr Soc. 1986;34(10):711 - 721. doi:10.1111/j.1532 - 5415.1986.tb04302.x ; Cohen - Mansfield J, Marx MS, Rosenthal AS. J Gerontol. 1989;44(3):M77 - M84. doi:10.1093/geronj/44.3.m77
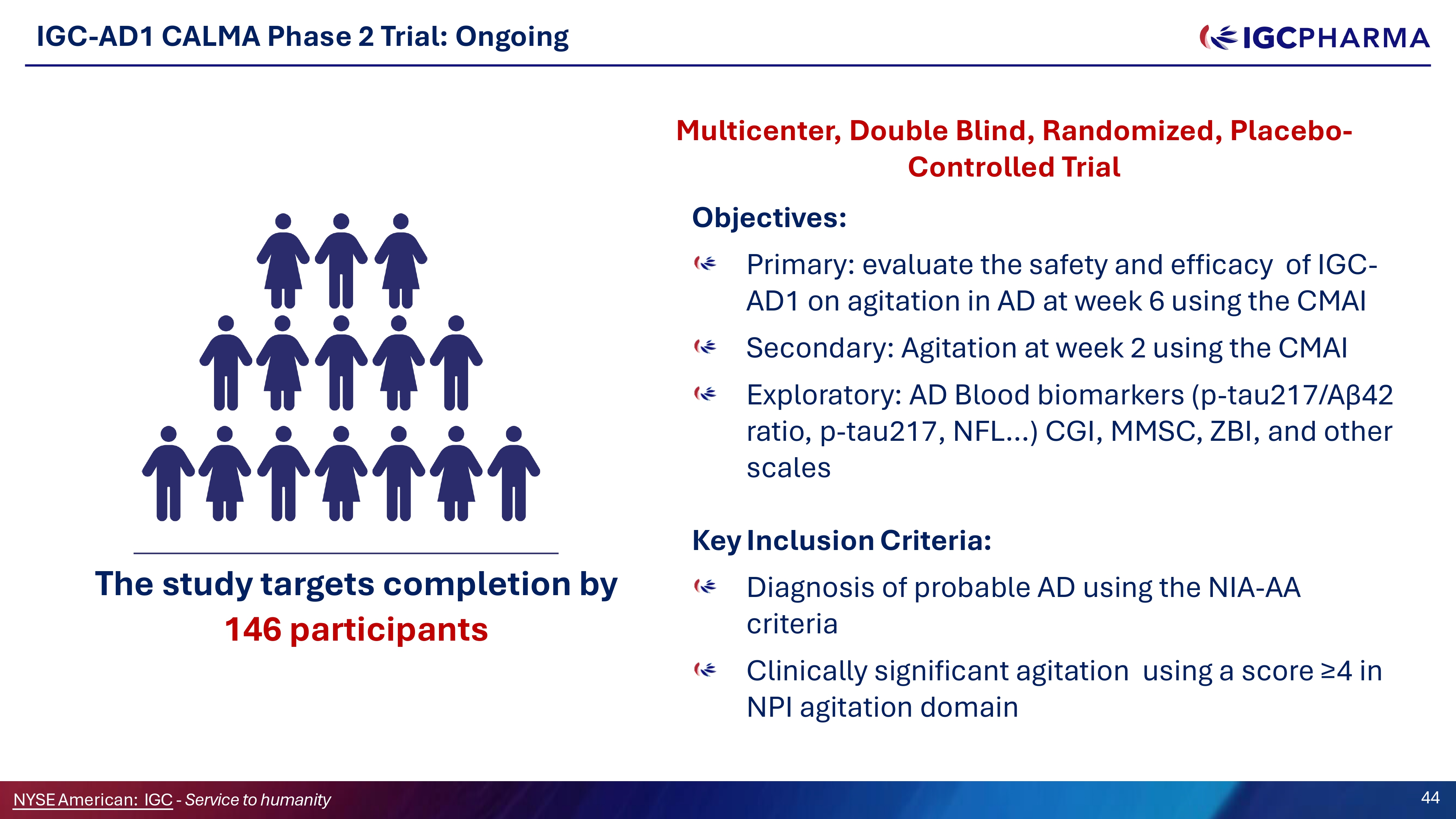
IGC - AD1 CALMA Phase 2 Trial: Ongoing Multicenter, Double Blind, Randomized, Placebo - Controlled Trial Objectives: Primary : evaluate the safety and efficacy of IGC - AD 1 on agitation in AD at week 6 using the CMAI Secondary : Agitation at week 2 using the CMAI Exploratory : AD Blood biomarkers (p - tau 217 /Aβ 42 ratio, p - tau 217 , NFL … ) CGI, MMSC, ZBI, and other scales Key Inclusion Criteria: Diagnosis of probable AD using the NIA - AA criteria Clinically significant agitation using a score ≥4 in NPI agitation domain The study targets completion by 146 participants Science Team 44 NYSEAmerican: IGC - Service to humanity
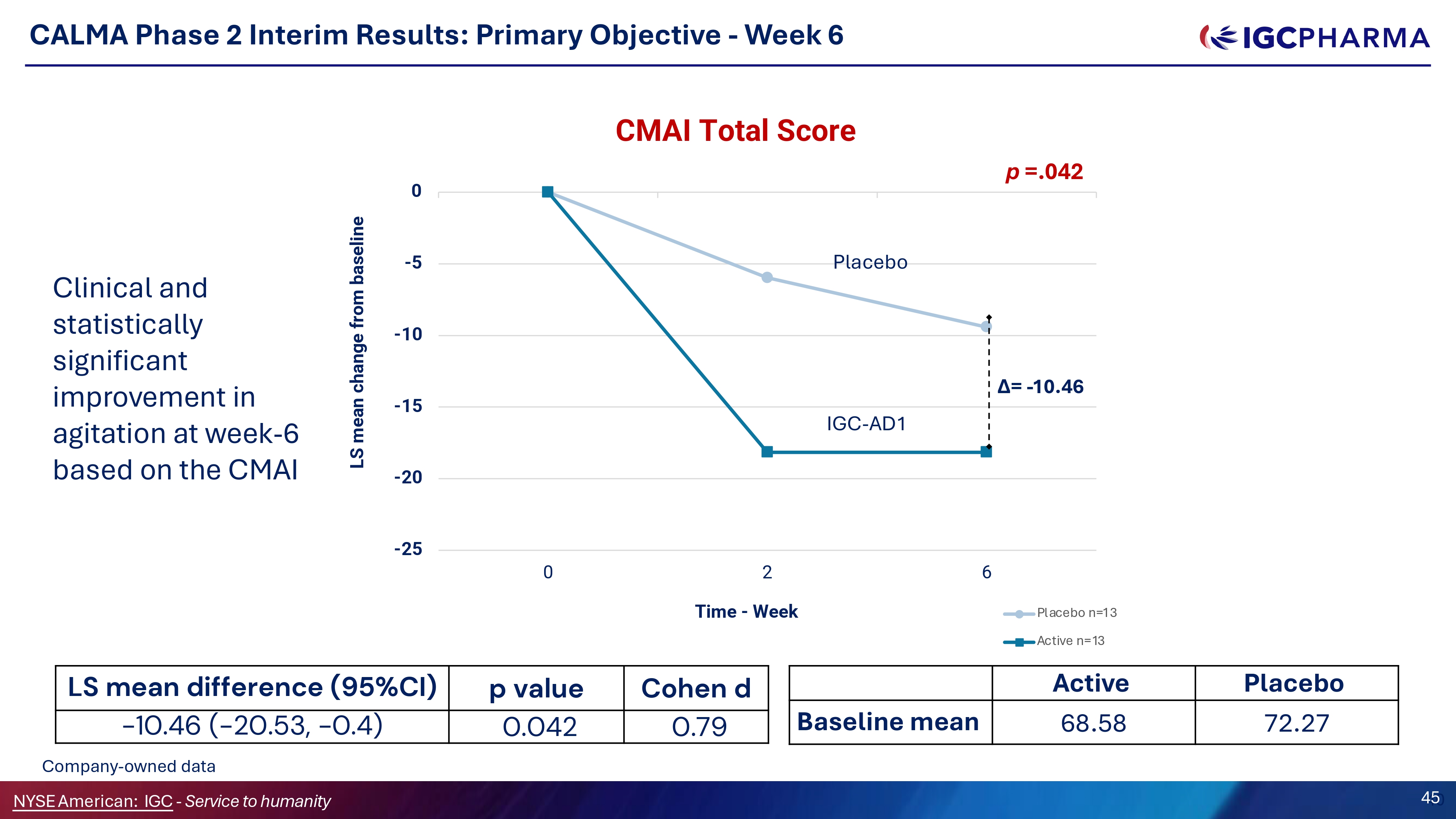
CALMA Phase 2 Interim Results: Primary Objective - Week 6 Cohen d p value LS mean difference (95%CI) 0.79 0.042 - 10.46 ( - 20.53, - 0.4) - 25 - 20 - 15 - 10 - 5 0 0 2 Time - Week 6 LS mean change from baseline CMAI Total Score Placebo n=13 Active n=13 Placebo Active 72.27 68.58 Baseline mean Placebo IGC - AD1 Δ= - 10.46 p =.042 Clinical and statistically significant improvement in agitation at week - 6 based on the CMAI Company - owned data NYSEAmerican: IGC - Service to humanity 4 4 5 0
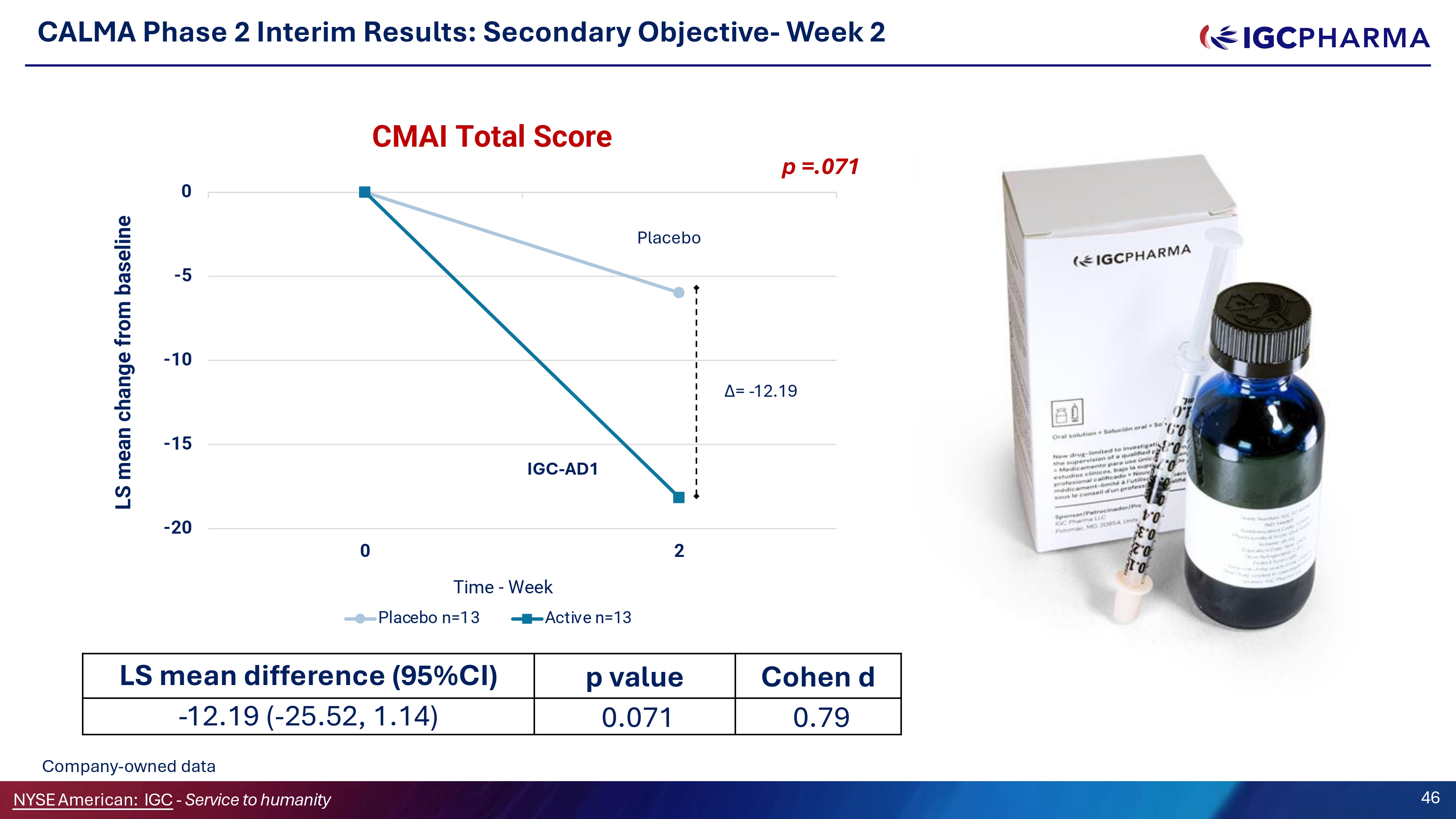
CALMA Phase 2 Interim Results: Secondary Objective - Week 2 - 20 - 15 - 10 - 5 0 0 2 LS mean change from baseline CMAI Total Score Time - Week Placebo n=13 Active n=13 Placebo IGC - AD1 Δ= - 12.19 p =.071 Cohen d p value LS mean difference (G5%CI) 0.79 0.071 - 12.19 ( - 25.52, 1.14) Company - owned data NYSEAmerican: IGC - Service to humanity 46
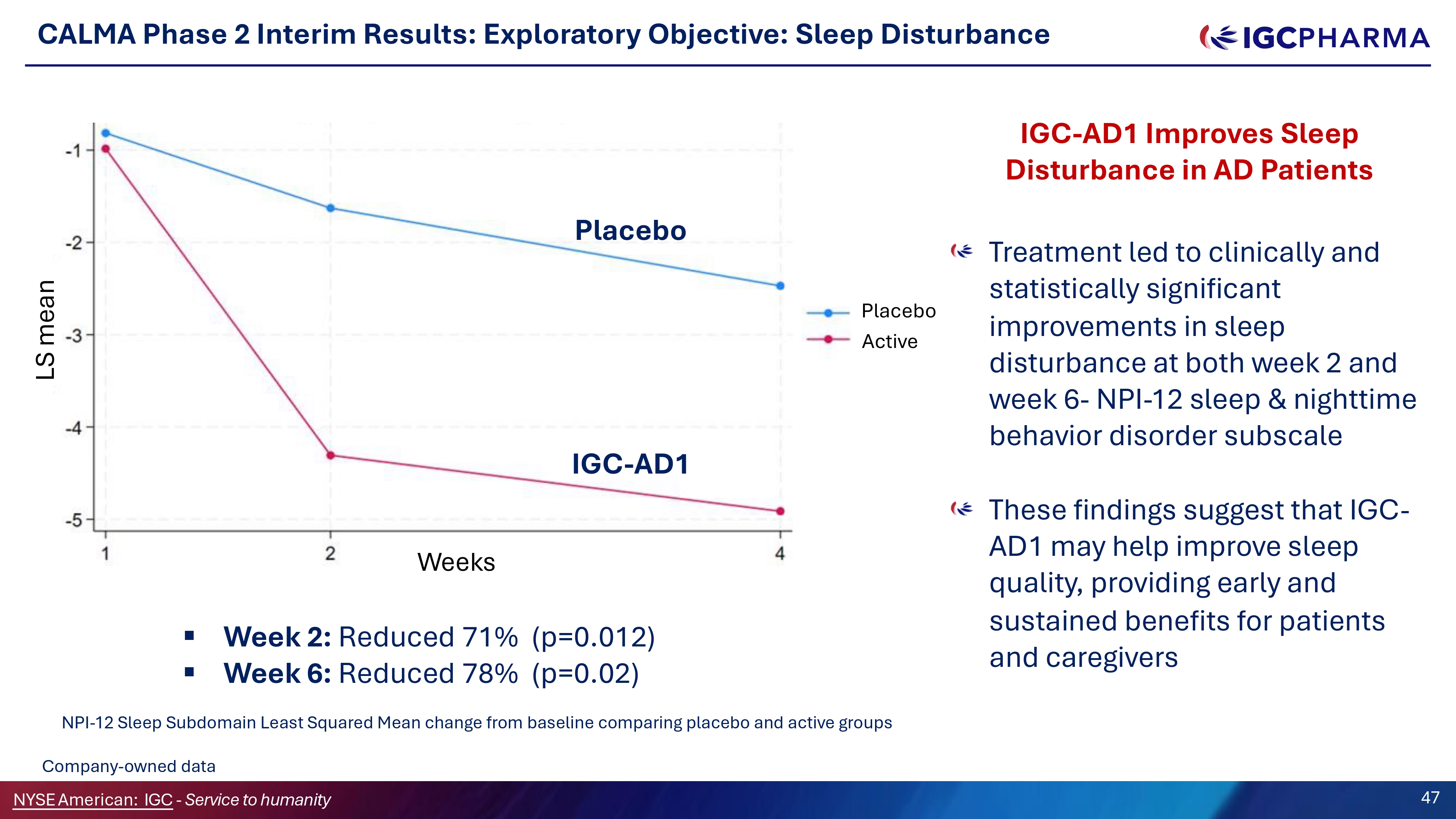
CALMA Phase 2 Interim Results: Exploratory Objective: Sleep Disturbance Treatment led to clinically and statistically significant improvements in sleep disturbance at both week 2 and week 6 - NPI - 12 sleep C nighttime behavior disorder subscale These findings suggest that IGC - AD1 may help improve sleep quality, providing early and sustained benefits for patients and caregivers ▪ Week 2: Reduced 71% (p=0.012) ▪ Week 6: Reduced 78% (p=0.02) NPI - 12 Sleep Subdomain Least Squared Mean change from baseline comparing placebo and active groups Placebo IGC - AD1 Placebo Active Science Team Company - owned data NYSEAmerican: IGC - Service to humanity 47 LS mean Weeks IGC - AD1 Improves Sleep Disturbance in AD Patients
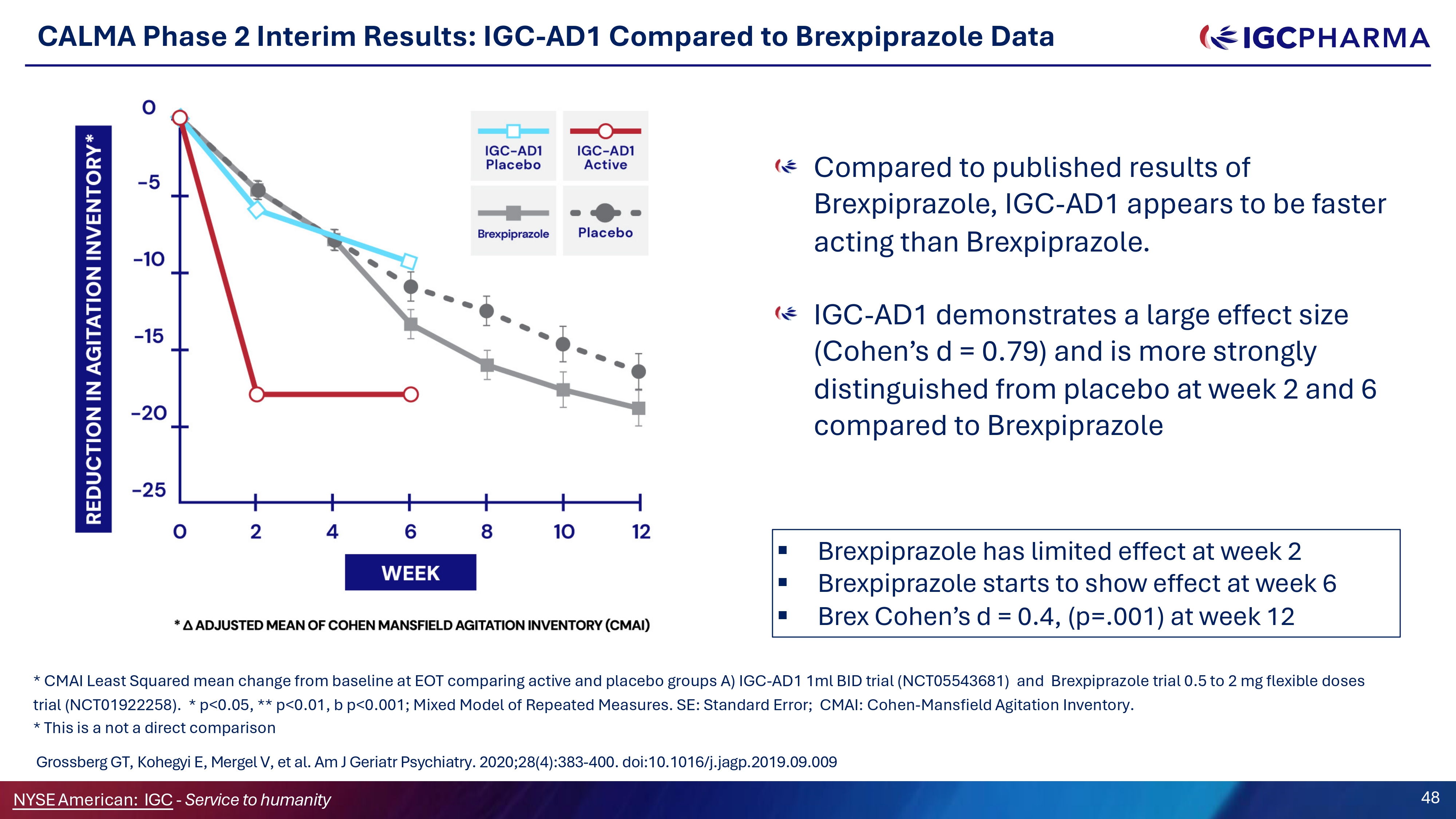
CALMA Phase 2 Interim Results: IGC - AD1 Compared to Brexpiprazole Data Compared to published results of Brexpiprazole, IGC - AD1 appears to be faster acting than Brexpiprazole. IGC - AD1 demonstrates a large effect size (Cohen’s d = 0.79) and is more strongly distinguished from placebo at week 2 and 6 compared to Brexpiprazole ▪ Brexpiprazole has limited effect at week 2 ▪ Brexpiprazole starts to show effect at week 6 ▪ Brex Cohen’s d = 0.4, (p=.001) at week 12 * CMAI Least Squared mean change from baseline at EOT comparing active and placebo groups A) IGC - AD1 1ml BID trial (NCT05543681) and Brexpiprazole trial 0.5 to 2 mg flexible doses trial (NCT01922258). * p<0.05, ** p<0.01, b p<0.001; Mixed Model of Repeated Measures. SE: Standard Error; CMAI: Cohen - Mansfield Agitation Inventory. * This is a not a direct comparison Grossberg GT, Kohegyi E, Mergel V, et al. Am J Geriatr Psychiatry. 2020;28(4):383 - 400. doi:10.1016/j.jagp.2019.09.009 Science Team 48 NYSEAmerican: IGC - Service to humanity
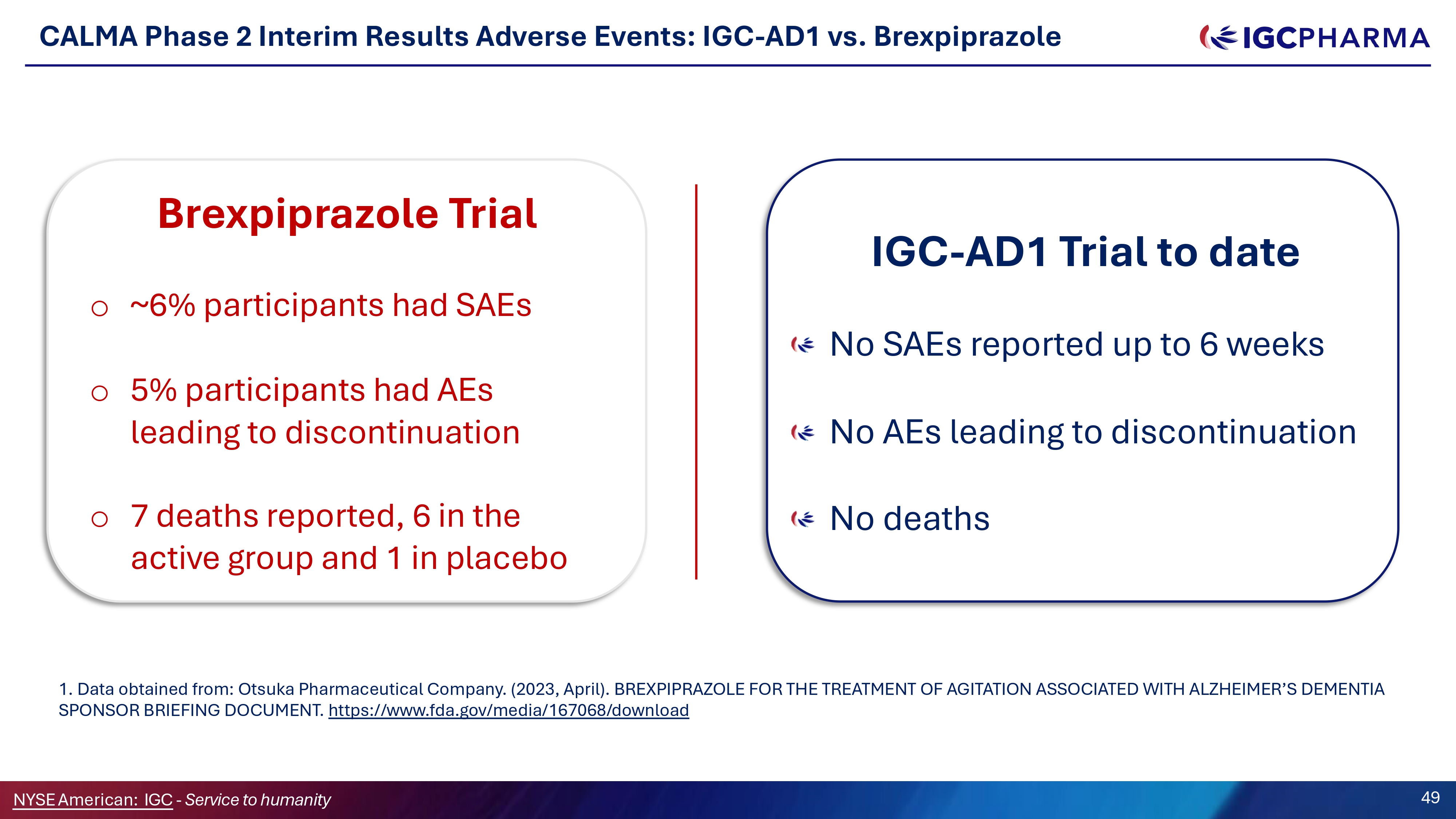
CALMA Phase 2 Interim Results Adverse Events: IGC - AD1 vs. Brexpiprazole t Brexpiprazole Trial o ~6% participants had SAEs o 5% participants had AEs leading to discontinuation o 7 deaths reported, 6 in the active group and 1 in placebo IGC - AD1 Trial to date No SAEs reported up to 6 weeks No AEs leading to discontinuation No deaths 1. Data obtained from: Otsuka Pharmaceutical Company. (2023, April). BREXPIPRAZOLE FOR THE TREATMENT OF AGITATION ASSOCIATED WITH ALZHEIMER’S DEMENTIA SPONSOR BRIEFING DOCUMENT. https:// www.fda.gov/media/167068/download NYSEAmerican: IGC - Service to humanity 49
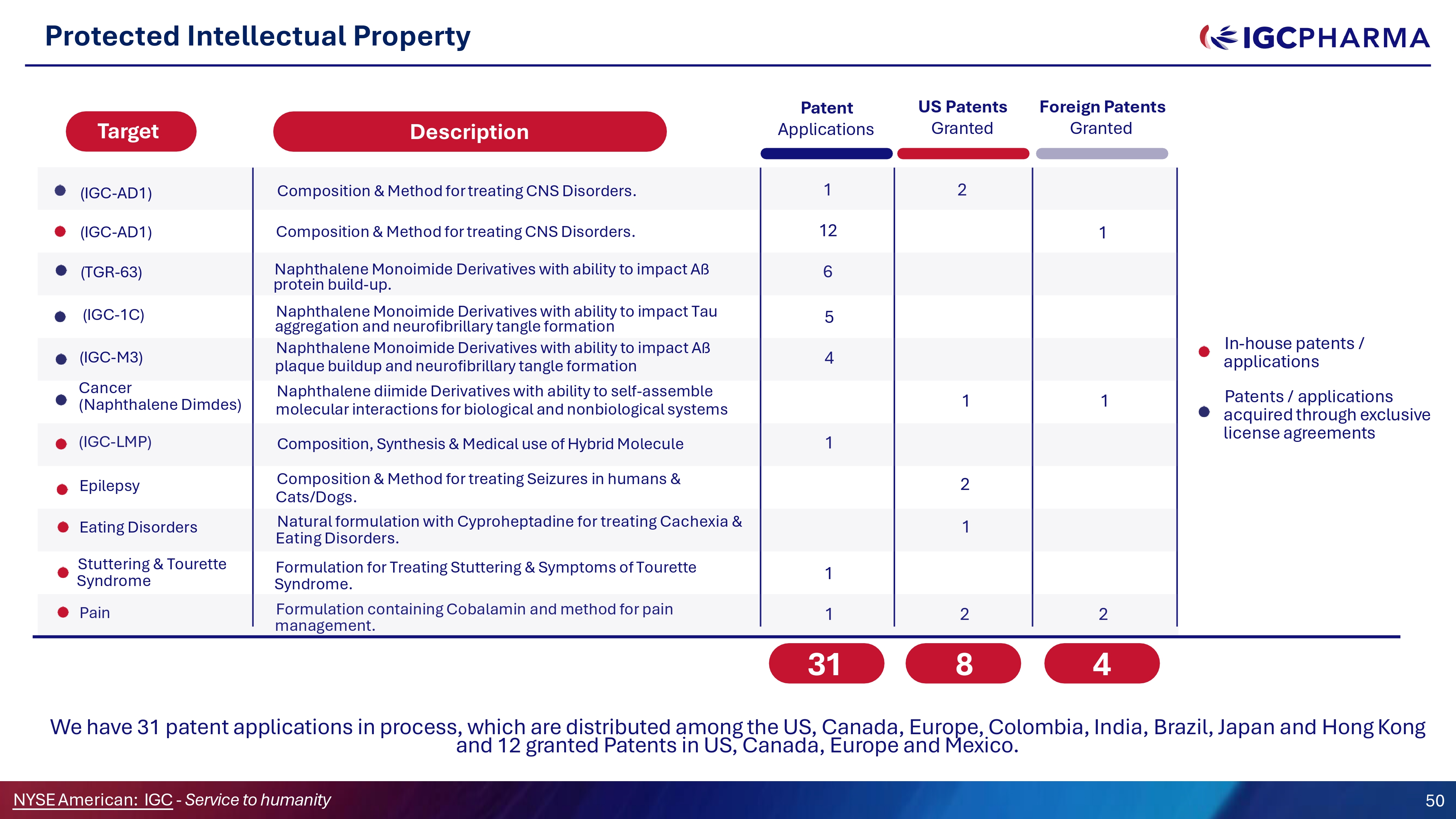
We have 31 patent applications in process, which are distributed among the US, Canada, Europe, Colombia, India, Brazil, Japan and Hong Kong and 12 granted Patents in US, Canada, Europe and Mexico. 6 5 4 1 1 1 2 (IGC - LMP) Formulation containing Cobalamin and method for pain management. Pain 1 2 1 4 Composition, Synthesis C Medical use of Hybrid Molecule Composition C Method for treating Seizures in humans C Cats/Dogs. Naphthalene Monoimide Derivatives with ability to impact Aß protein build - up. (TGR - 63) Naphthalene Monoimide Derivatives with ability to impact Tau aggregation and neurofibrillary tangle formation (IGC - 1C) Epilepsy (IGC - M3) Cancer (Naphthalene Dimdes) Naphthalene Monoimide Derivatives with ability to impact Aß plaque buildup and neurofibrillary tangle formation Naphthalene diimide Derivatives with ability to self - assemble molecular interactions for biological and nonbiological systems Natural formulation with Cyproheptadine for treating Cachexia C Eating Disorders. In - house patents / applications Patents / applications acquired through exclusive license agreements Formulation for Treating Stuttering C Symptoms of Tourette Syndrome. Eating Disorders Stuttering C Tourette Syndrome 2 1 1 2 1 8 31 Patent Applications US Patents Granted Foreign Patents Granted Composition C Method for treating CNS Disorders. (IGC - AD1) (IGC - AD1) Composition C Method for treating CNS Disorders. 12 Protected Intellectual Property Target Description NYSEAmerican: IGC - Service to humanity 50