
Exhibit 99.2

Jasper Therapeutics SPOTLIGHT Data Update June 16, 2025

2 B RIQU ILIMAB IS AN INVES TIGATIVE DRU G AND IS NOT APPROVED FOR ANY INDICATION Safe Harbor Statements Forward - Looking Statements Certain statements in this Presentation include “forward - looking statements” within the meaning of the Private Securities Litigation Reform Act of 1995, as amended. All statements other than statements of historical fact contained in this Presentation, including statements regarding Jasper’s future opportunities and prospects, including milestones, potential regulatory filings and the anticipated timing thereof, patient enrollment, future timelines, business strategy, plans and objectives for future operations, Jasper’s ability to obtain additional funding for its operations in this or future offerings and its expectations related to the use of any net proceeds from this offering are forward - looking statements. Jasper has based these forward - looking statements on its estimates and assumptions and its current expectations and projections about future events. These forward - looking statements are subject to a number of risks, uncertainties and assumptions, including those contained in the "Risk Factors" section of the Company's Annual Report on Form 10 - K for the year ended December 31, 2024, Quarterly Reports on Form 10 - Q and Current Reports on Form 8 - K that the Company has subsequently filed or may subsequently file with the SEC. In light of these risks, uncertainties and assumptions, the forward - looking events and circumstances discussed in this Presentation are inherently uncertain and may not occur, and actual results could differ materially and adversely from those anticipated or implied in th e forward - looking statements. Accordingly, you should not rely upon forward - looking statements as predictions of future events. Jasper undertakes no obligation to update publicly or revise any forward - looking statements for any reason after the date of this Presentation or to conform these statements to actual results or to changes in Jasper's expectations. Industry and Market Data Certain data in this Presentation was obtained from various external sources, and neither the Company nor its affiliates, adv isers or representatives has verified such data with independent sources. Accordingly, neither the Company nor any of its affiliates, advisers or representatives makes any representations as to the accuracy or completeness of that data or undertakes any obligation to update such data after the date of this Presentation. Such data involves risks and uncertainties and is subject to change based on various factors. Trademarks The trademarks included herein are the property of the owners thereof and are used for reference purposes only. Such use should not be construed as an endorsement of the products or services of the Company.

B RIQU ILIMAB IS AN INVESTIGATIVE DRUG AND IS NOT APPROVED FOR ANY INDICATION 3 Today’s Agenda • Opening Remarks • SPOTLIGHT 180mg Results Summary • Upcoming Milestones and Closing Remarks • Question & Answer Session

SPOTLIGHT 180mg Results Summary
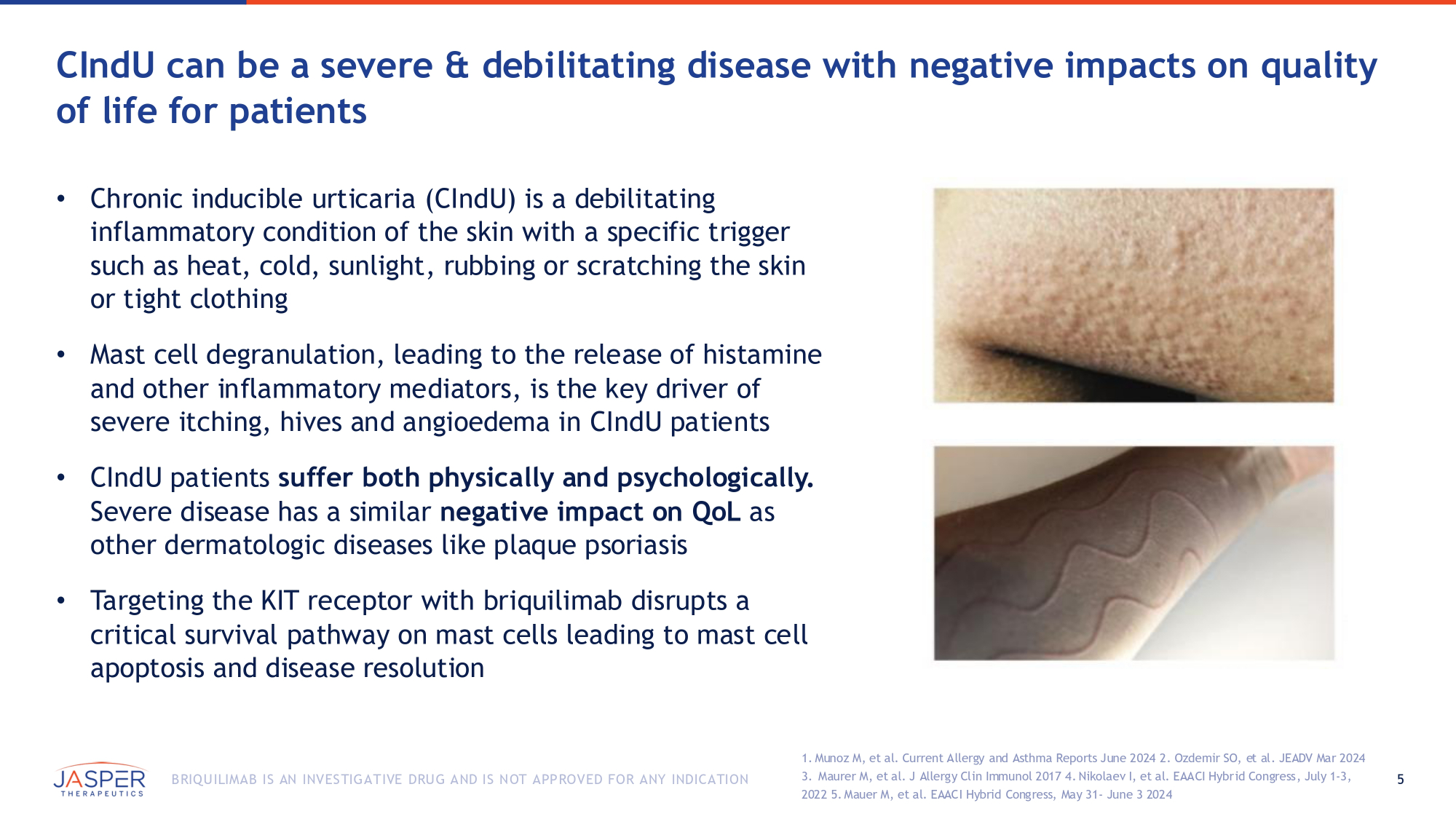
5 BRIQUILIMAB IS AN INVESTIGATIVE DRUG AND IS NOT APPROVED FOR ANY INDICATION CIndU can be a severe & debilitating disease with negative impacts on quality of life for patients • Chronic inducible urticaria (CIndU) is a debilitating inflammatory condition of the skin with a specific trigger such as heat, cold, sunlight, rubbing or scratching the skin or tight clothing • Mast cell degranulation, leading to the release of histamine and other inflammatory mediators, is the key driver of severe itching, hives and angioedema in CIndU patients • CIndU patients suffer both physically and psychologically. Severe disease has a similar negative impact on QoL as other dermatologic diseases like plaque psoriasis • Targeting the KIT receptor with briquilimab disrupts a critical survival pathway on mast cells leading to mast cell apoptosis and disease resolution 1. Munoz M, et al. Current Allergy and Asthma Reports June 2024 2. Ozdemir SO, et al. JEADV Mar 2024 3. Maurer M, et al. J Allergy Clin Immunol 2017 4. Nikolaev I, et al. EAACI Hybrid Congress, July 1 - 3, 2022 5. Mauer M, et al. EAACI Hybrid Congress, May 31 - June 3 2024
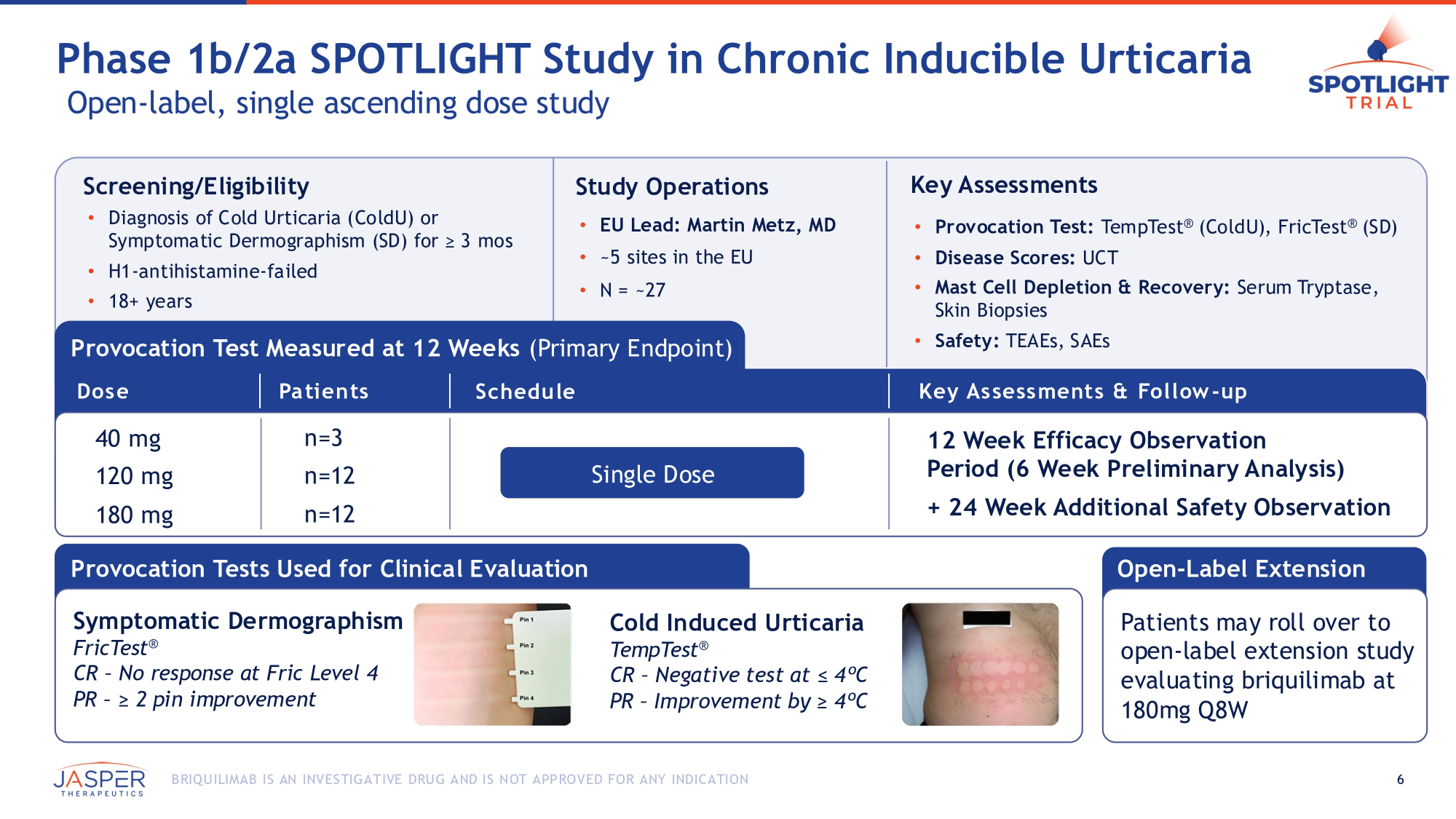
Screening/Eligibility • Diagnosis of Cold Urticaria (ColdU) or Symptomatic Dermographism (SD) for ≥ 3 mos • H1 - antihistamine - failed • 18+ years • 18 - 65 years of age Study Operations • EU Lead: Martin Metz, MD • ~5 sites in the EU • N = ~27 Key Assessments • Provocation Test: TempTest ® (ColdU), FricTest ® (SD) • Disease Scores: UCT • Mast Cell Depletion & Recovery: Serum Tryptase, Skin Biopsies • Safety: TEAEs, SAEs Provocation Test Measured at 12 Weeks (Primary Endpoint) Provocation Tests Used for Clinical Evaluation 40 mg 120 mg 180 mg n=3 n=12 n=12 Symptomatic Dermographism FricTest ® CR – No response at Fric Level 4 PR – ≥ 2 pin improvement Cold Induced Urticaria TempTest ® CR – Negative test at ≤ 4ºC PR – Improvement by ≥ 4ºC Single Dose Dose Patients Schedule Key Assessments & Follow - up 12 Week Efficacy Observation Period (6 Week Preliminary Analysis) + 24 Week Additional Safety Observation Phase 1b/2a SPOTLIGHT Study in Chronic Inducible Urticaria Open - label, single ascending dose study Open - Label Extension 6 B RIQU ILIMAB IS AN INVES TIGATIVE DRU G AND IS NOT APPROVED FOR ANY INDICATION Patients may roll over to open - label extension study evaluating briquilimab at 180mg Q8W
Rapid Onset of Deep and Durable Efficacy Observed at 180mg Dose 7 B RIQU ILIMAB IS AN INVES TIGATIVE DRU G AND IS NOT APPROVED FOR ANY INDICATION Depth of Response • 12 of 12 patients (100%) at 180mg achieved clinical response following single - dose of briquilimab • 11 of 12 patients (92%) at 180mg achieved complete response (CR) • 10 of 12 patients (83%) at 180mg had tryptase measurements below lower limit of quantification Rapid Onset of Effect • 67% of 180mg patients with CR or partial response (PR) at week 2 assessment Durability of Effect • 5 CRs and 2 PRs maintained at week 8 (58%, 7/12), durability assessment ongoing
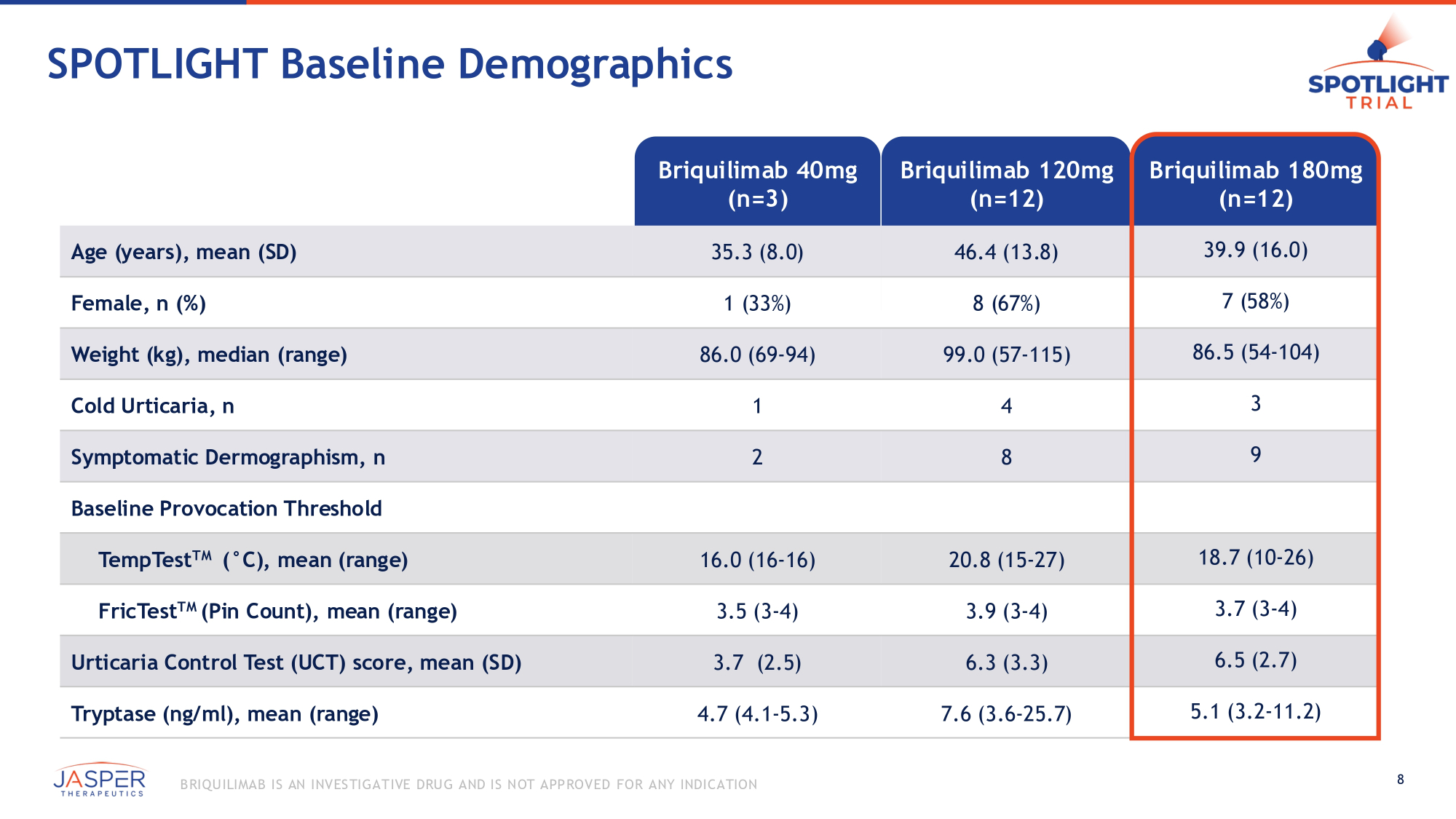
SPOTLIGHT Baseline Demographics Briquilimab 180mg Briquilimab 120mg Briquilimab 40mg (n=12) (n=12) (n=3) 39.9 (16.0) 46.4 (13.8) 35.3 (8.0) Age (years), mean (SD) 7 (58%) 8 (67%) 1 (33%) Female, n (%) 86.5 (54 - 104) 99.0 (57 - 115) 86.0 (69 - 94) Weight (kg), median (range) 3 4 1 Cold Urticaria, n 9 8 2 Symptomatic Dermographism, n Baseline Provocation Threshold 18.7 (10 - 26) 20.8 (15 - 27) 16.0 (16 - 16) TempTest TM ( ƒ C), mean (range) 3.7 (3 - 4) 3.9 (3 - 4) 3.5 (3 - 4) FricTest TM (Pin Count), mean (range) 6.5 (2.7) 6.3 (3.3) 3.7 (2.5) Urticaria Control Test (UCT) score, mean (SD) 5.1 (3.2 - 11.2) 7.6 (3.6 - 25.7) 4.7 (4.1 - 5.3) Tryptase (ng/ml), mean (range) 8 B RIQU ILIMAB IS AN INVES TIGATIVE DRU G AND IS NOT APPROVED FOR ANY INDICATION
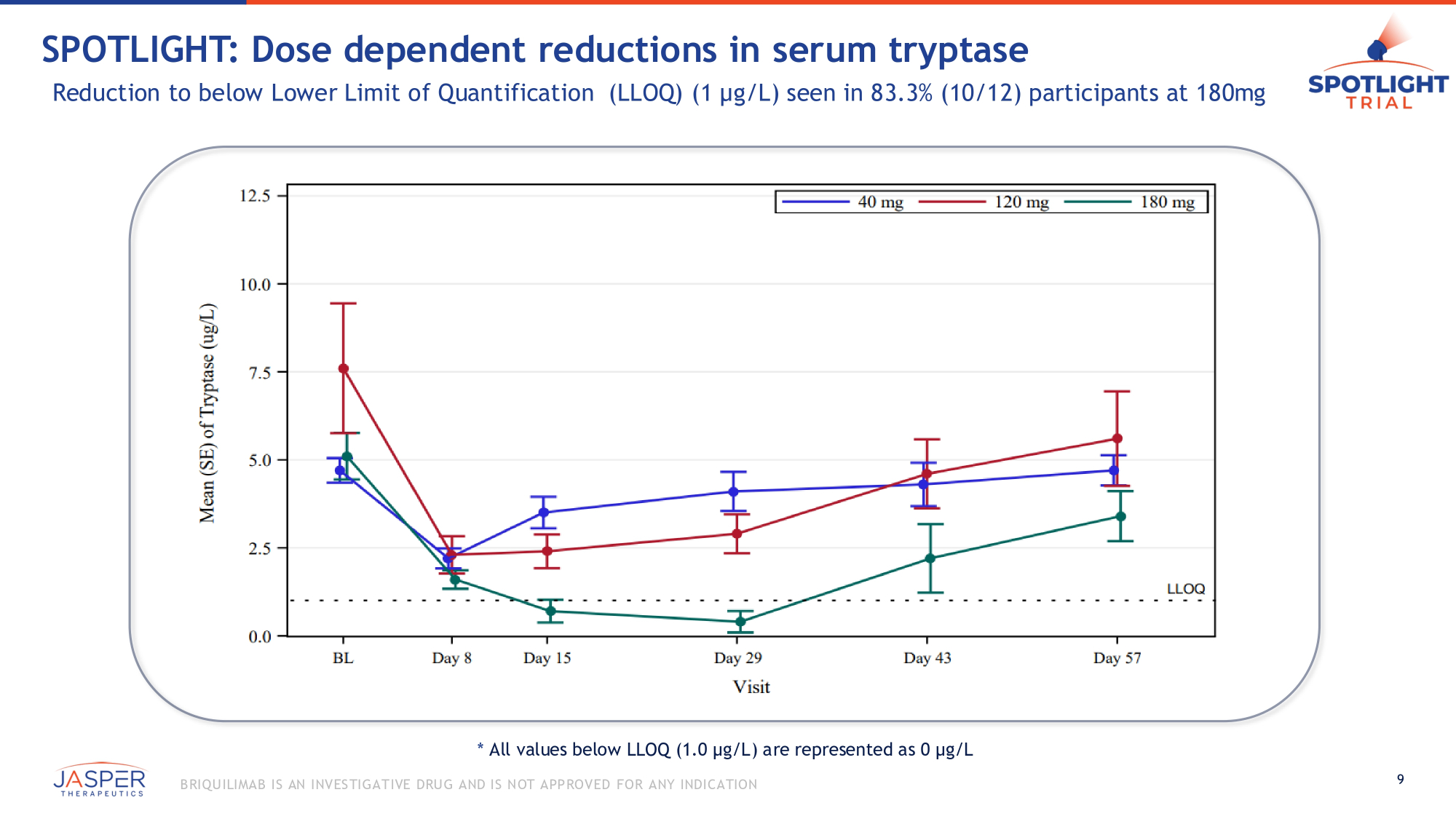
* All values below LLOQ (1.0 µg/L) are represented as 0 µg/L 9 B RIQU ILIMAB IS AN INVES TIGATIVE DRU G AND IS NOT APPROVED FOR ANY INDICATION SPOTLIGHT: Dose dependent reductions in serum tryptase Reduction to below Lower Limit of Quantification (LLOQ) (1 µg/L) seen in 83.3% (10/12) participants at 180mg
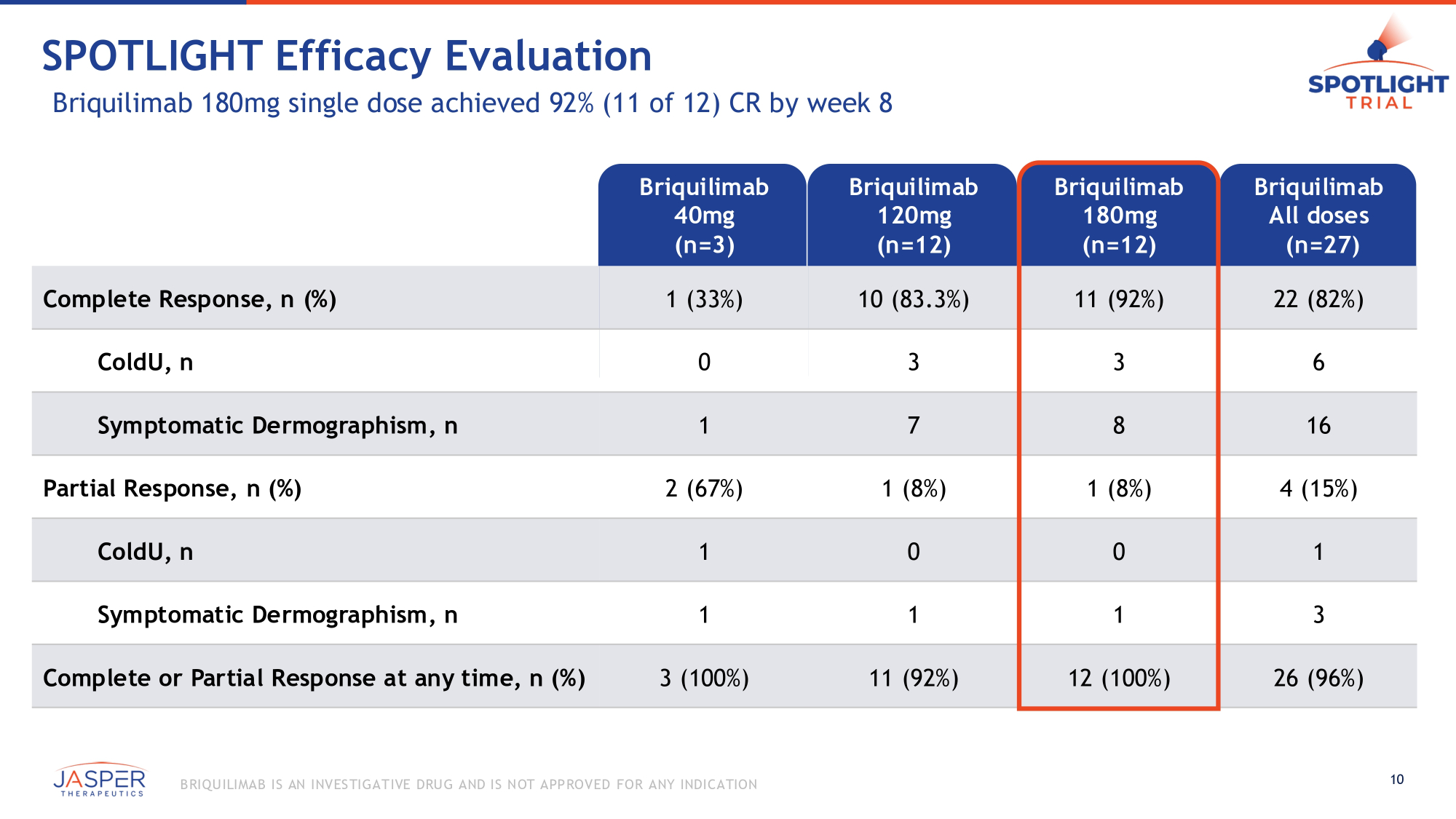
SPOTLIGHT Efficacy Evaluation Briquilimab 180mg single dose achieved 92% (11 of 12) CR by week 8 Briquilimab 40mg (n=3) Briquilimab 120mg (n=12) Briquilimab 180mg (n=12) Briquilimab All doses (n=27) Complete Response, n (%) 1 (33%) 10 (83.3%) 11 (92%) 22 (82%) ColdU, n 0 3 3 6 Symptomatic Dermographism, n 1 7 8 16 Partial Response, n (%) 2 (67%) 1 (8%) 1 (8%) 4 (15%) ColdU, n 1 0 0 1 Symptomatic Dermographism, n 1 1 1 3 Complete or Partial Response at any time, n (%) 3 (100%) 11 (92%) 12 (100%) 26 (96%) 10 B RIQU ILIMAB IS AN INVES TIGATIVE DRU G AND IS NOT APPROVED FOR ANY INDICATION
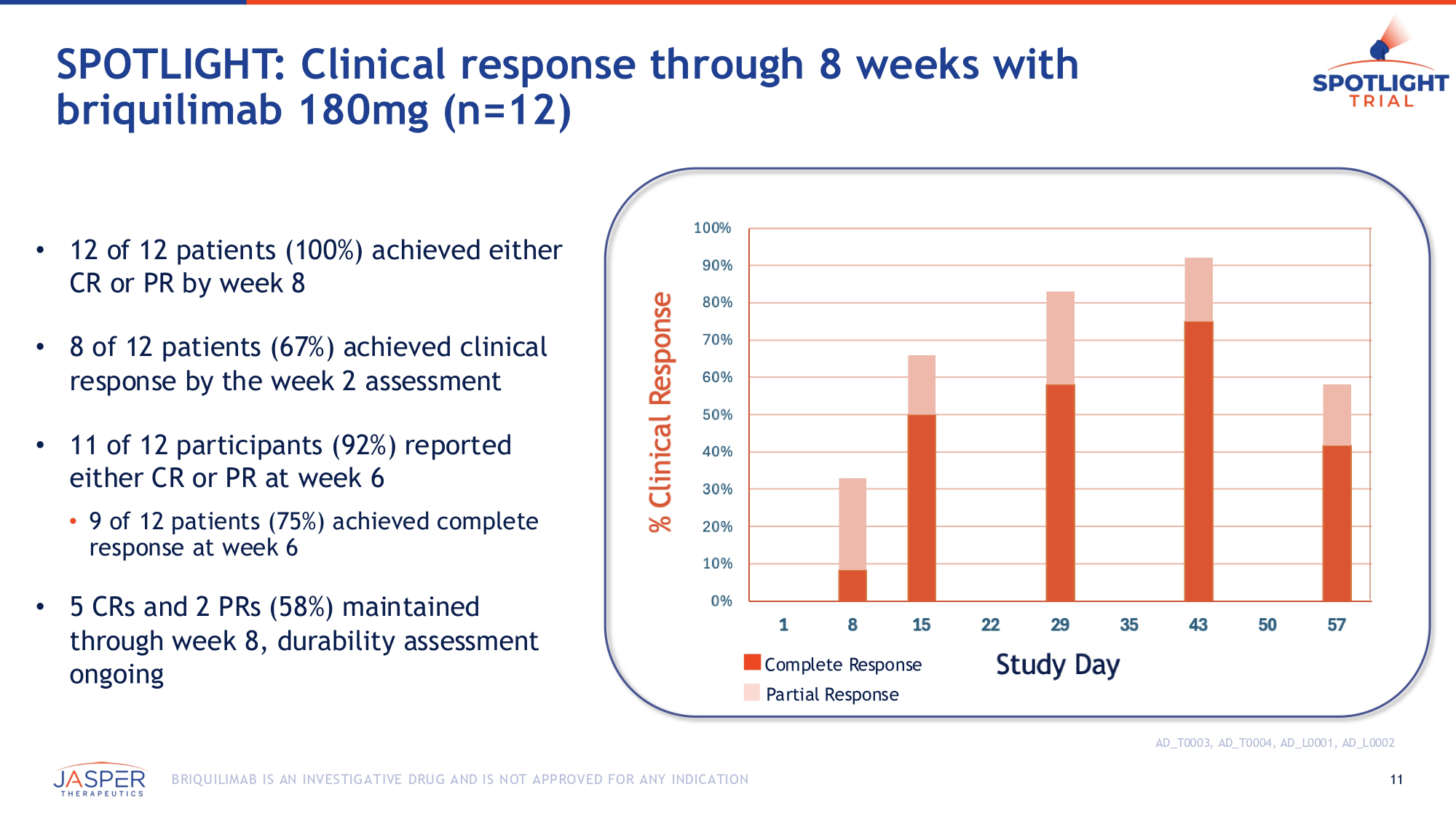
SPOTLIGHT: Clinical response through 8 weeks with briquilimab 180mg (n=12) AD_T0003, AD_T0004, AD_L0001, AD_L0002 • 12 of 12 patients (100%) achieved either CR or PR by week 8 • 8 of 12 patients (67%) achieved clinical response by the week 2 assessment • 11 of 12 participants (92%) reported either CR or PR at week 6 • 9 of 12 patients (75%) achieved complete response at week 6 • 5 CRs and 2 PRs (58%) maintained through week 8, durability assessment ongoing Complete Response Partial Response 11 B RIQU ILIMAB IS AN INVES TIGATIVE DRU G AND IS NOT APPROVED FOR ANY INDICATION
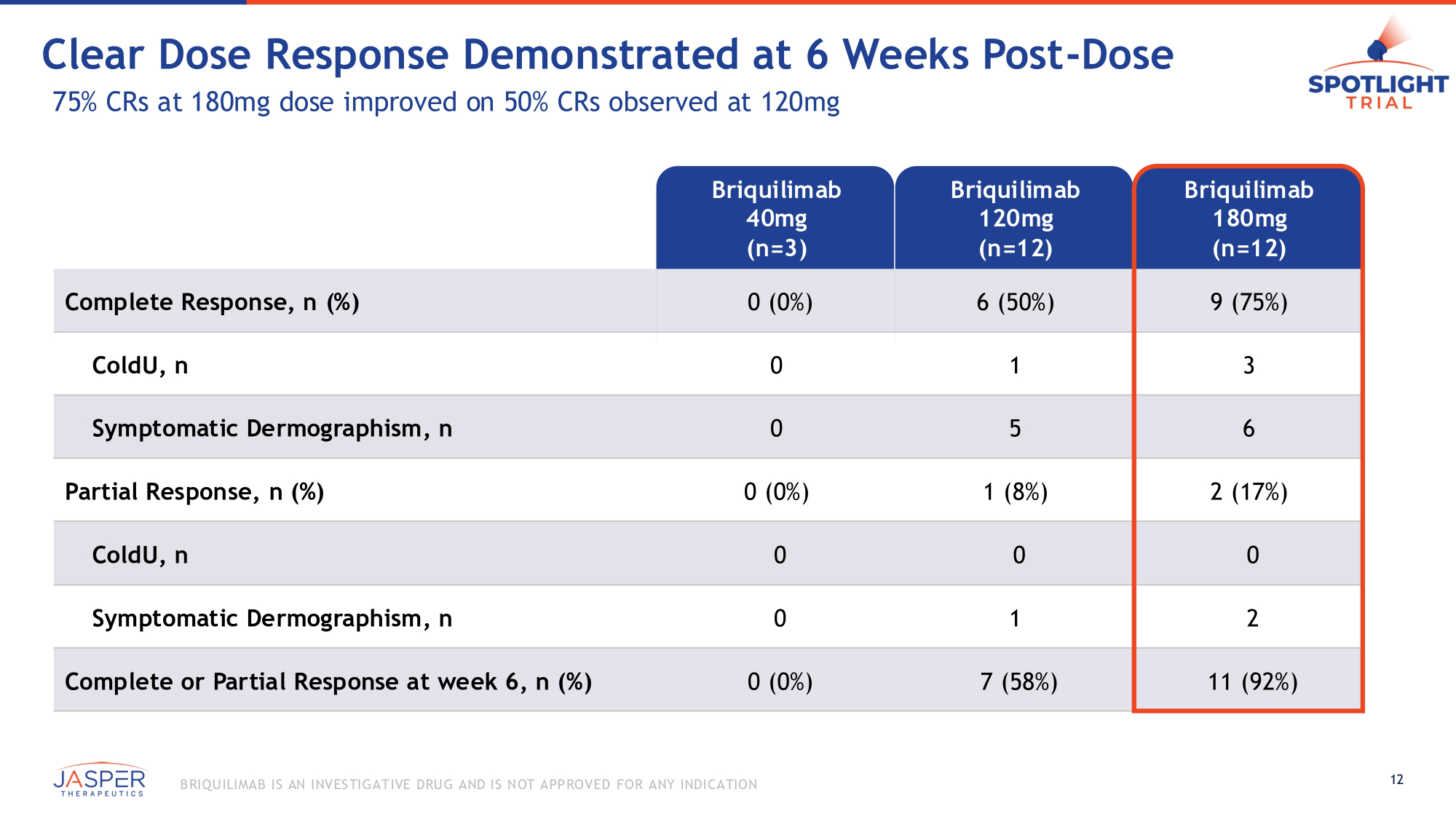
Clear Dose Response Demonstrated at 6 Weeks Post - Dose 75% CRs at 180mg dose improved on 50% CRs observed at 120mg Briquilimab 40mg (n=3) Briquilimab 120mg (n=12) Briquilimab 180mg (n=12) Complete Response, n (%) 0 (0%) 6 (50%) 9 (75%) ColdU, n 0 1 3 Symptomatic Dermographism, n 0 5 6 Partial Response, n (%) 0 (0%) 1 (8%) 2 (17%) ColdU, n 0 0 0 Symptomatic Dermographism, n 0 1 2 Complete or Partial Response at week 6, n (%) 0 (0%) 7 (58%) 11 (92%) 12 B RIQU ILIMAB IS AN INVES TIGATIVE DRU G AND IS NOT APPROVED FOR ANY INDICATION
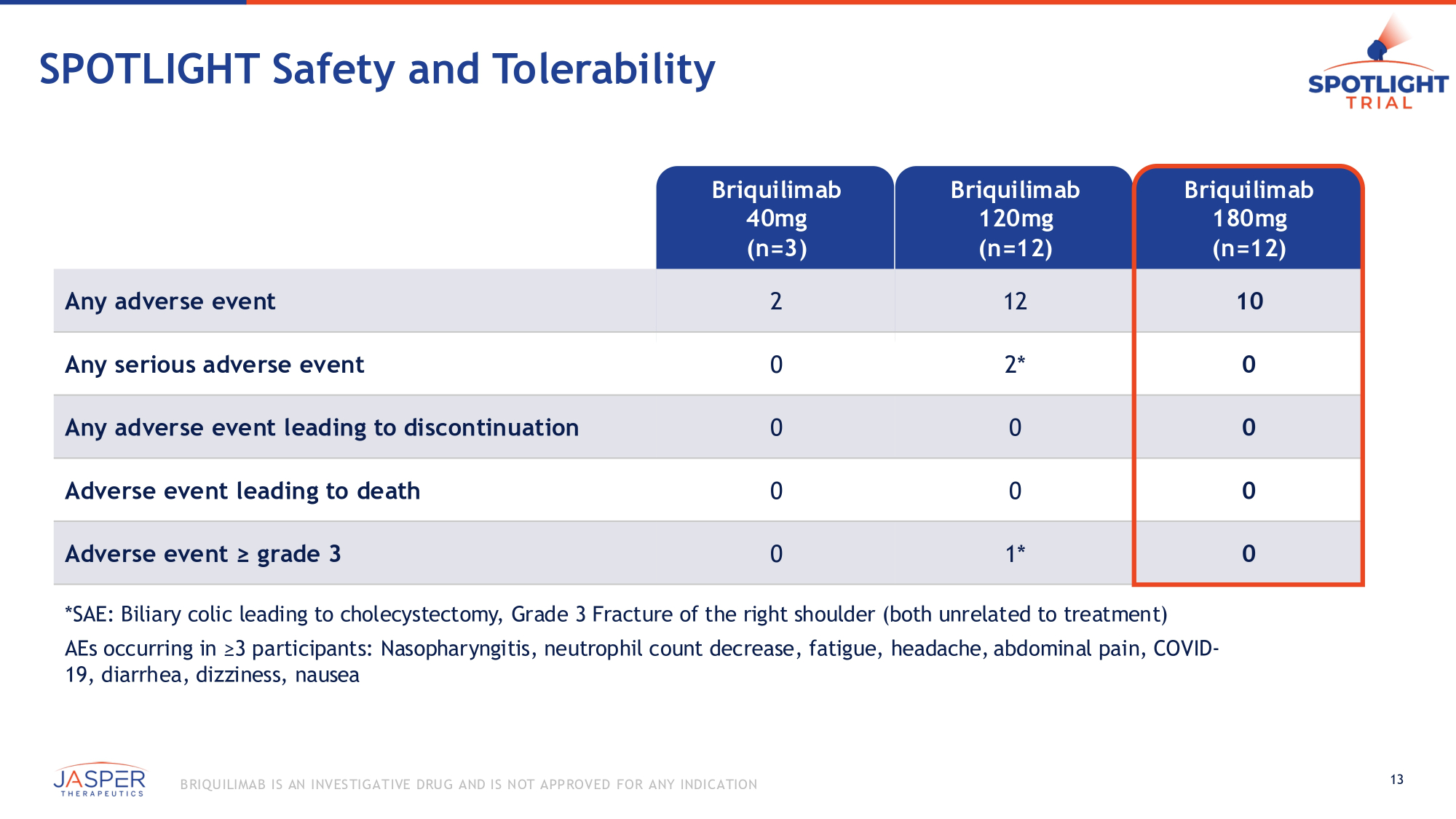
SPOTLIGHT Safety and Tolerability Briquilimab 40mg (n=3) Briquilimab 120mg (n=12) Briquilimab 180mg (n=12) Any adverse event 2 12 10 Any serious adverse event 0 2* 0 Any adverse event leading to discontinuation 0 0 0 Adverse event leading to death 0 0 0 0 Adverse event ≥ grade 3 0 1* *SAE: Biliary colic leading to cholecystectomy, Grade 3 Fracture of the right shoulder (both unrelated to treatment) AEs occurring in ≥3 participants: Nasopharyngitis, neutrophil count decrease, fatigue, headache, abdominal pain, COVID - 19, diarrhea, dizziness, nausea 13 B RIQU ILIMAB IS AN INVES TIGATIVE DRU G AND IS NOT APPROVED FOR ANY INDICATION
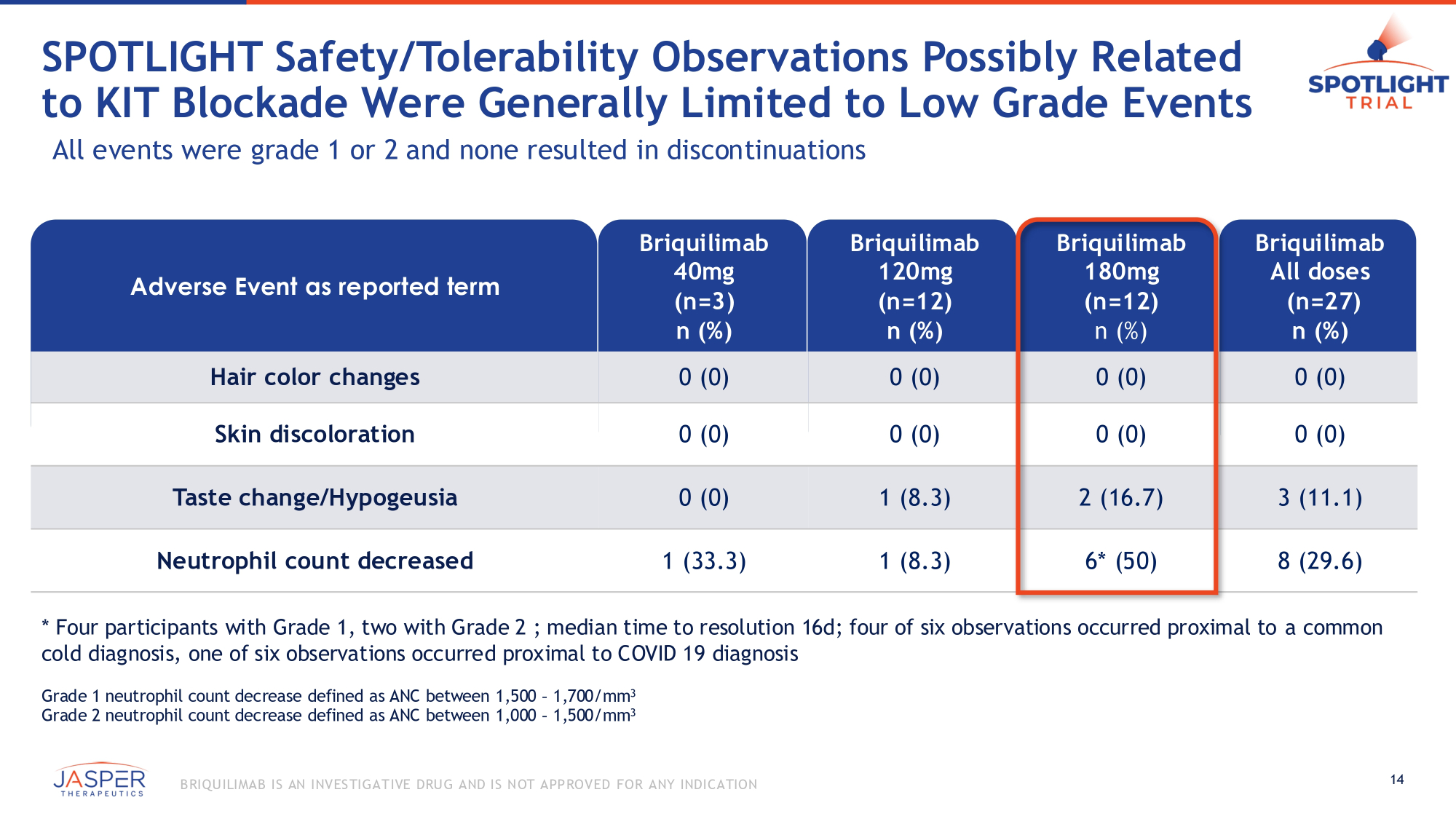
SPOTLIGHT Safety/Tolerability Observations Possibly Related to KIT Blockade Were Generally Limited to Low Grade Events All events were grade 1 or 2 and none resulted in discontinuations Adverse Event as reported term Briquilimab 40mg (n=3) n (%) Briquilimab 120mg (n=12) n (%) Briquilimab 180mg (n=12) n (%) Briquilimab All doses (n=27) n (%) Hair color changes 0 (0) 0 (0) 0 (0) 0 (0) Skin discoloration 0 (0) 0 (0) 0 (0) 0 (0) Taste change/Hypogeusia 0 (0) 1 (8.3) 2 (16.7) 3 (11.1) Neutrophil count decreased 1 (33.3) 1 (8.3) 6* (50) 8 (29.6) * Four participants with Grade 1, two with Grade 2 ; median time to resolution 16d; four of six observations occurred proximal to a common cold diagnosis, one of six observations occurred proximal to COVID 19 diagnosis Grade 1 neutrophil count decrease defined as ANC between 1,500 – 1,700/mm 3 Grade 2 neutrophil count decrease defined as ANC between 1,000 – 1,500/mm 3 14 B RIQU ILIMAB IS AN INVES TIGATIVE DRU G AND IS NOT APPROVED FOR ANY INDICATION
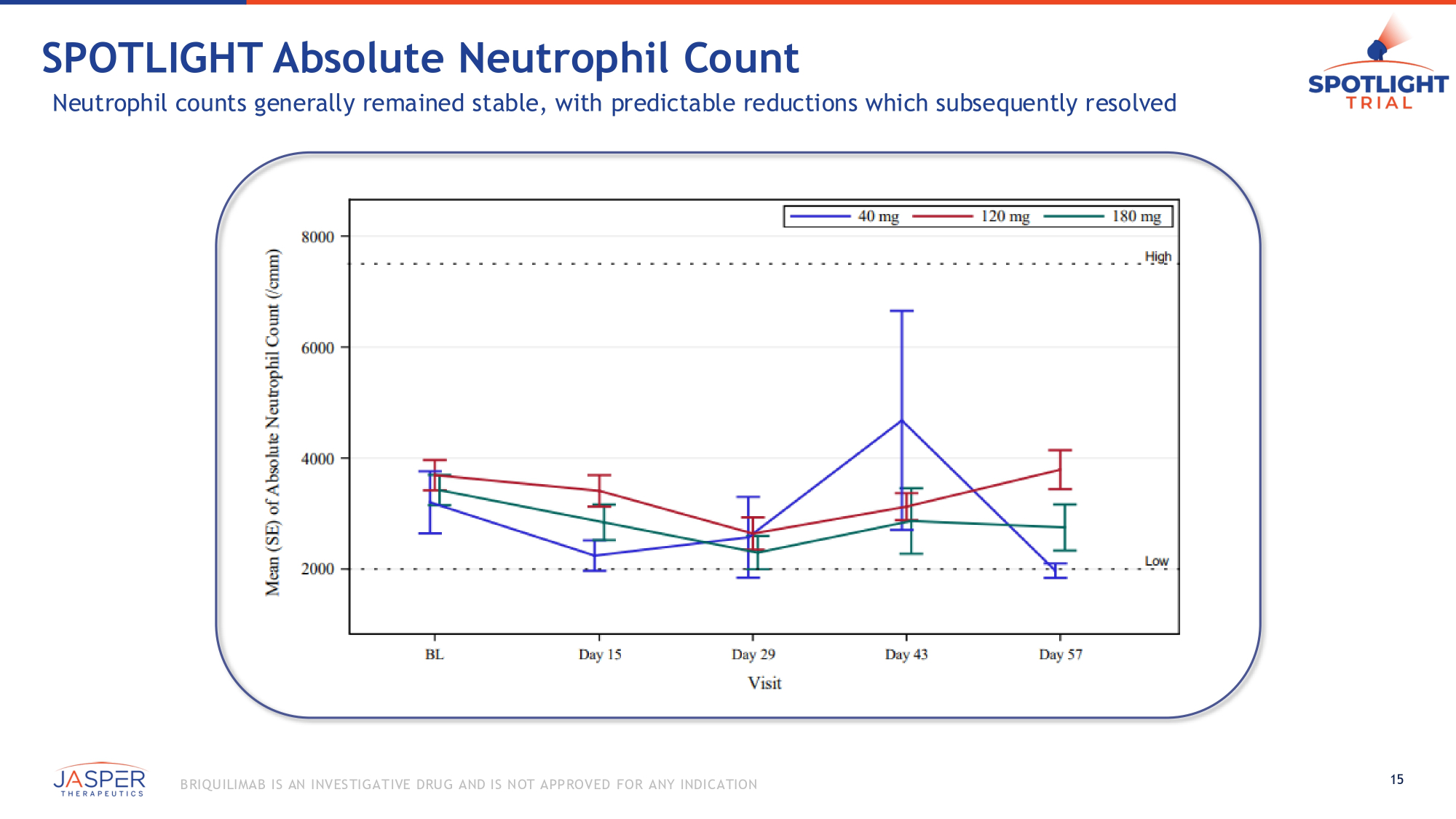
SPOTLIGHT Absolute Neutrophil Count Neutrophil counts generally remained stable, with predictable reductions which subsequently resolved 15 B RIQU ILIMAB IS AN INVES TIGATIVE DRU G AND IS NOT APPROVED FOR ANY INDICATION

Upcoming Milestones and Closing Remarks

Takeaways 17 B RIQU ILIMAB IS AN INVES TIGATIVE DRU G AND IS NOT APPROVED FOR ANY INDICATION • Single 180mg dose of briquilimab demonstrated 100% clinical response in participants with CIndU • Deep reduction of tryptase observed with 83% of participants below LLOQ in 180 mg cohort • Rapid onset of symptom control with 67% achieving clinical response by week 2 • 91.6% of participants in 180 mg cohort achieved CR vs 83% in 120 mg cohort • Durability of 180mg dose shown with 58% clinical response maintained at 8 wks (5 CRs and 2 PRs) • Briquilimab was well tolerated in participants with CIndU • All possibly KIT related adverse events observed were low - grade and transient in nature • Briquilimab’s robust efficacy and safety, supports continued evaluation in chronic urticaria • Full SPOTLIGHT study results expected at medical conference in 2nd half of 2025
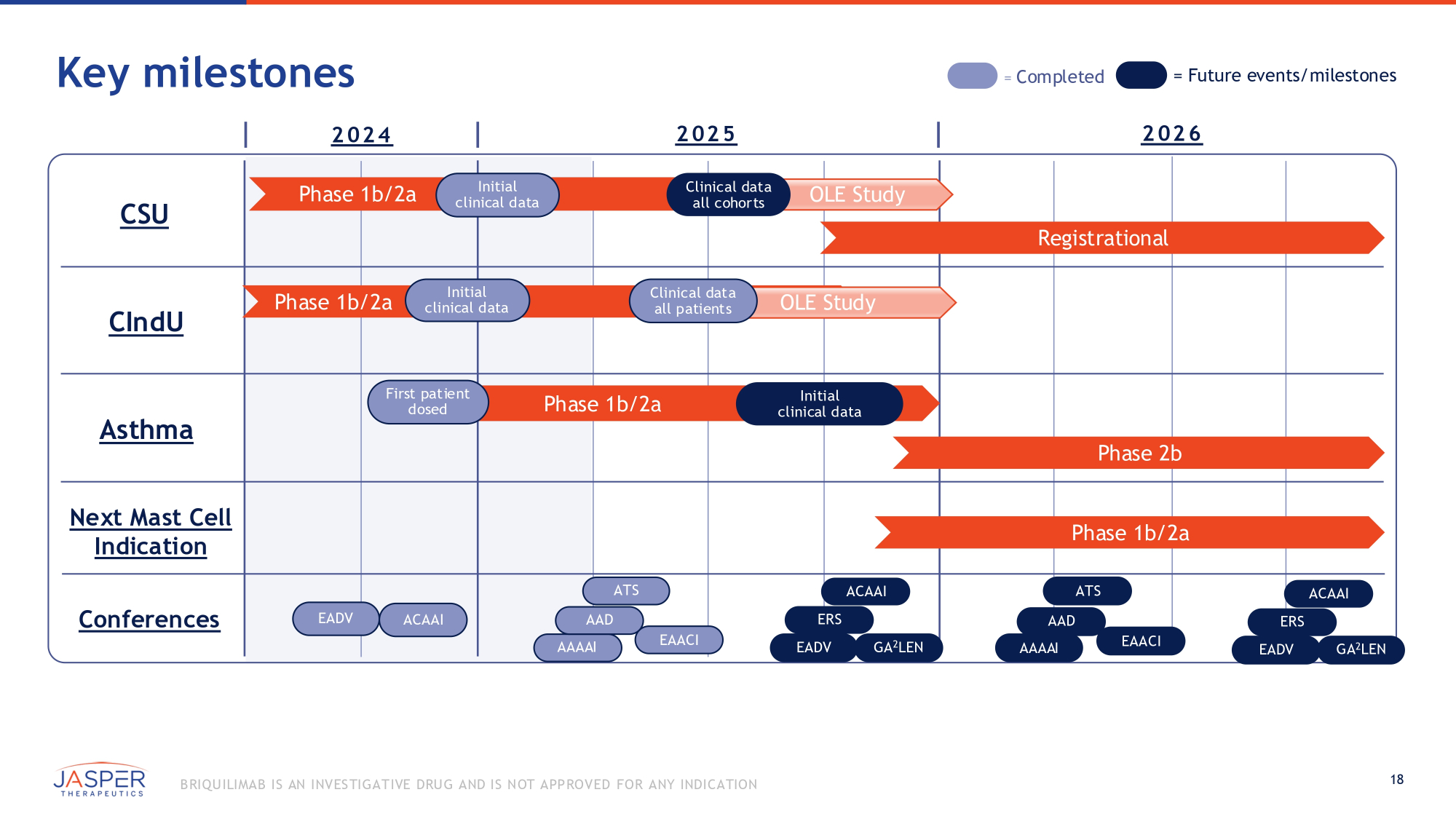
Key milestones = Completed = Future events/milestones 2 02 4 CSU CIndU Asthma Conferences 2 02 5 2 02 6 Registrational Phase 1b/2a Initial clinical data OLE Study Phase 1b/2a OLE E Study Clinical data all patients Next Mast Cell Indication Initial clinical data Phase 1b/2a First patient dosed Initial clinical data Phase 2b Phase 1b/2a EADV ACAAI AAAAI EAACI GA 2 LEN ATS ACAAI AAD EAACI ATS AAD AAAAI ERS EADV GA 2 LEN ACAAI ERS EADV Clinical data all cohorts 18 B RIQU ILIMAB IS AN INVES TIGATIVE DRU G AND IS NOT APPROVED FOR ANY INDICATION

Jasper Therapeutics SPOTLIGHT Data Update June 16, 2025