
June 10, 2025 Topline Results of Phase 2b Study of TPIP in PAH Patients TPIP:
Treprostinil Palmitil Inhalation Powder | PAH: pulmonary arterial hypertension

Forward Looking Statements This presentation contains forward-looking statements
that involve substantial risks and uncertainties. “Forward-looking statements,” as that term is defined in the Private Securities Litigation Reform Act of 1995, are statements that are not historical facts and involve a number of risks and
uncertainties. Words herein such as “may,” “will,” “should,” “could,” “would,” “expects,” “plans,” “anticipates,” “believes,” “estimates,” “projects,” “predicts,” “intends,” “potential,” “continues,” and similar expressions (as well as other
words or expressions referencing future events, conditions or circumstances) may identify forward-looking statements. The forward-looking statements in this presentation are based upon the Company’s current expectations and beliefs, and
involve known and unknown risks, uncertainties and other factors, which may cause the Company’s actual results, performance and achievements and the timing of certain events to differ materially from the results, performance, achievements or
timing discussed, projected, anticipated or indicated in any forward-looking statements. Such risks, uncertainties and other factors include, among others, the following: the risk that the full data set from the TPIP PAH study or data generated
in further clinical trials of TPIP will not be consistent with the topline results of the TPIP PAH study; failure to successfully conduct future clinical trials for TPIP, such as the Company’s planned Phase 3 program for TPIP, including due to
the Company's potential inability to enroll or retain sufficient patients to conduct and complete the trials or generate data necessary for regulatory approval, among other things; development of unexpected safety or efficacy concerns related
to TPIP; failure of third parties on which the Company is dependent to manufacture sufficient quantities of TPIP for clinical needs, to conduct the Company's clinical trials, or to comply with the Company's agreements or laws and regulations
that impact the Company's business or agreements with the Company; failure to obtain regulatory approval for TPIP; inaccuracies in the Company's estimates of the size of the potential markets for TPIP or in data the Company has used to identify
physicians; expected rates of patient uptake, duration of expected treatment, or expected patient adherence or discontinuation rates, if TPIP is approved; inability of the Company or the Company's third-party manufacturers to comply with
regulatory requirements related to TPIP; the Company's inability to obtain adequate reimbursement from government or third-party payors for TPIP or acceptable prices for TPIP, if approved; restrictions or other obligations imposed on us by
agreements related to TPIP and failure to comply with our obligations under such agreements; risks that the Company's clinical studies will be delayed or that serious side effects will be identified during drug development; the strength and
enforceability of the Company’s intellectual property rights or the rights of third parties; and the cost and potential reputational damage resulting from litigation to which the Company may become a party, including product liability
claims. The Company may not actually achieve the results, plans, intentions or expectations indicated by the Company's forward-looking statements because, by their nature, forward-looking statements involve risks and uncertainties because
they relate to events and depend on circumstances that may or may not occur in the future. For additional information about the risks and uncertainties that may affect the Company's business, please see the factors discussed in Item 1A, "Risk
Factors," in the Company's Annual Report on Form 10-K for the year ended December 31, 2024, and any subsequent Company filings with the Securities and Exchange Commission (SEC). The Company cautions readers not to place undue reliance on any
such forward-looking statements, which speak only as of the date of this presentation. The Company disclaims any obligation, except as specifically required by law and the rules of the SEC, to publicly update or revise any such statements to
reflect any change in expectations or in events, conditions or circumstances on which any such statements may be based, or that may affect the likelihood that actual results will differ from those set forth in the forward-looking
statements. Additional Disclaimers: Please be aware that TPIP is an investigational product that has not been approved for sale or found safe or effective by the FDA or any regulatory authority. This presentation is not a promotion or
advertisement of TPIP. Insmed is a registered trademark of Insmed Incorporated. TPIP: Treprostinil Palmitil Inhalation Powder | PAH: pulmonary arterial hypertension

Opening Remarks Study Results Closing Remarks Q&A
Session 5 7-16 17 18 Slides Will Lewis Chair & CEO Agenda Speakers Gene Sullivan Chief Product Strategy Officer

Opening Remarks Will Lewis | Chair & CEO
Phase 2b Study of TPIP for PAH is a Clear Success TPIP: Treprostinil Palmitil
Inhalation Powder | PAH: pulmonary arterial hypertension | PVR: pulmonary vascular resistance 6MWD: six-minute walk distance | * Statistically significant at Week 16 35%* placebo-adjusted PVR reduction from baseline 35.5-meter
placebo-adjusted 6MWD improvement from baseline Efficacy endpoints measured ~24-hours after prior dose; included milder, heavily pre-treated patients Results illustrate a competitive efficacy and safety profile achieved with a once-daily
therapy

Study Results Gene Sullivan| Chief Product Strategy Officer
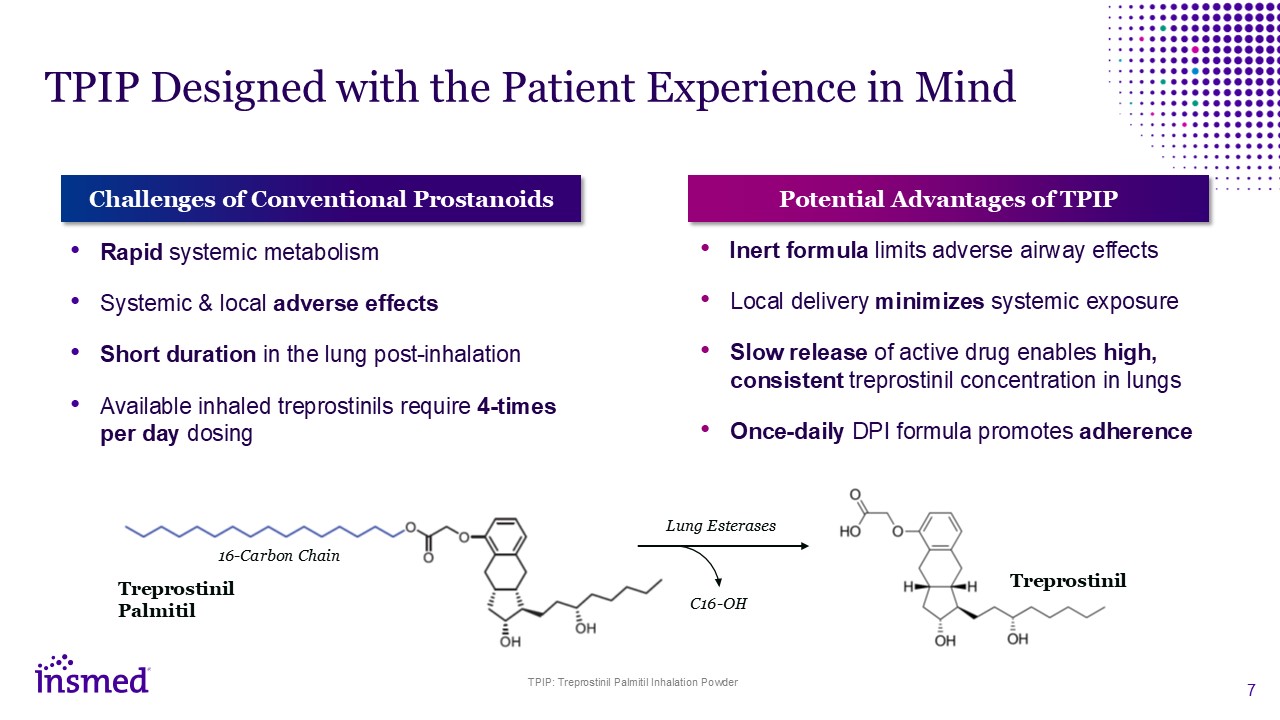
TPIP Designed with the Patient Experience in Mind Challenges of Conventional
Prostanoids Rapid systemic metabolism Systemic & local adverse effects Short duration in the lung post-inhalation Available inhaled treprostinils require 4-times per day dosing Potential Advantages of TPIP Inert formula limits
adverse airway effects Local delivery minimizes systemic exposure Slow release of active drug enables high, consistent treprostinil concentration in lungs Once-daily DPI formula promotes adherence Treprostinil Palmitil 16-Carbon
Chain Treprostinil C16-OH Lung Esterases TPIP: Treprostinil Palmitil Inhalation Powder
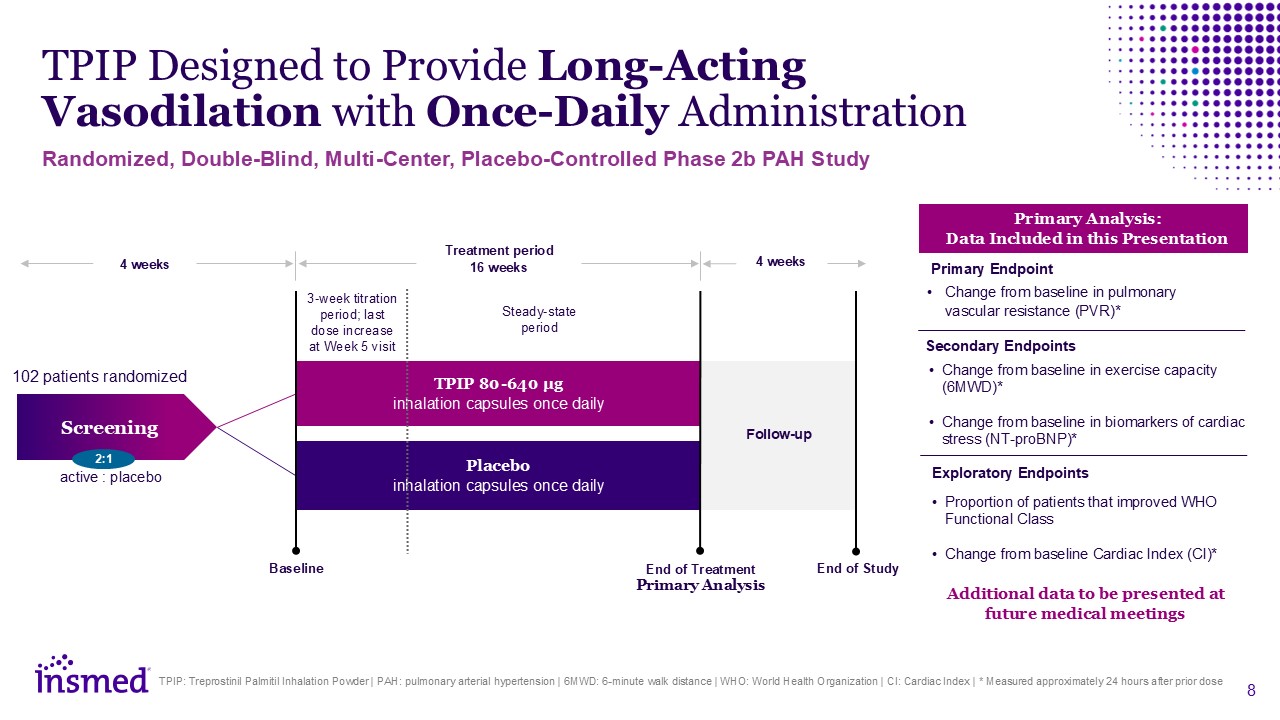
TPIP Designed to Provide Long-Acting Vasodilation with Once-Daily
Administration TPIP: Treprostinil Palmitil Inhalation Powder | PAH: pulmonary arterial hypertension | 6MWD: 6-minute walk distance | WHO: World Health Organization | CI: Cardiac Index | * Measured approximately 24 hours after prior dose 4
weeks TPIP 80-640 µg inhalation capsules once daily Placebo inhalation capsules once daily Screening Treatment period 16 weeks Baseline End of Study 4 weeks Follow-up 102 patients randomized Steady-state period 3-week titration
period; last dose increase at Week 5 visit active : placebo 2:1 End of Treatment Primary Analysis Randomized, Double-Blind, Multi-Center, Placebo-Controlled Phase 2b PAH Study Change from baseline in pulmonary vascular resistance
(PVR)* Change from baseline in exercise capacity (6MWD)* Change from baseline in biomarkers of cardiac stress (NT-proBNP)* Primary Analysis: Data Included in this Presentation Proportion of patients that improved WHO Functional
Class Change from baseline Cardiac Index (CI)* Primary Endpoint Secondary Endpoints Exploratory Endpoints Additional data to be presented at future medical meetings
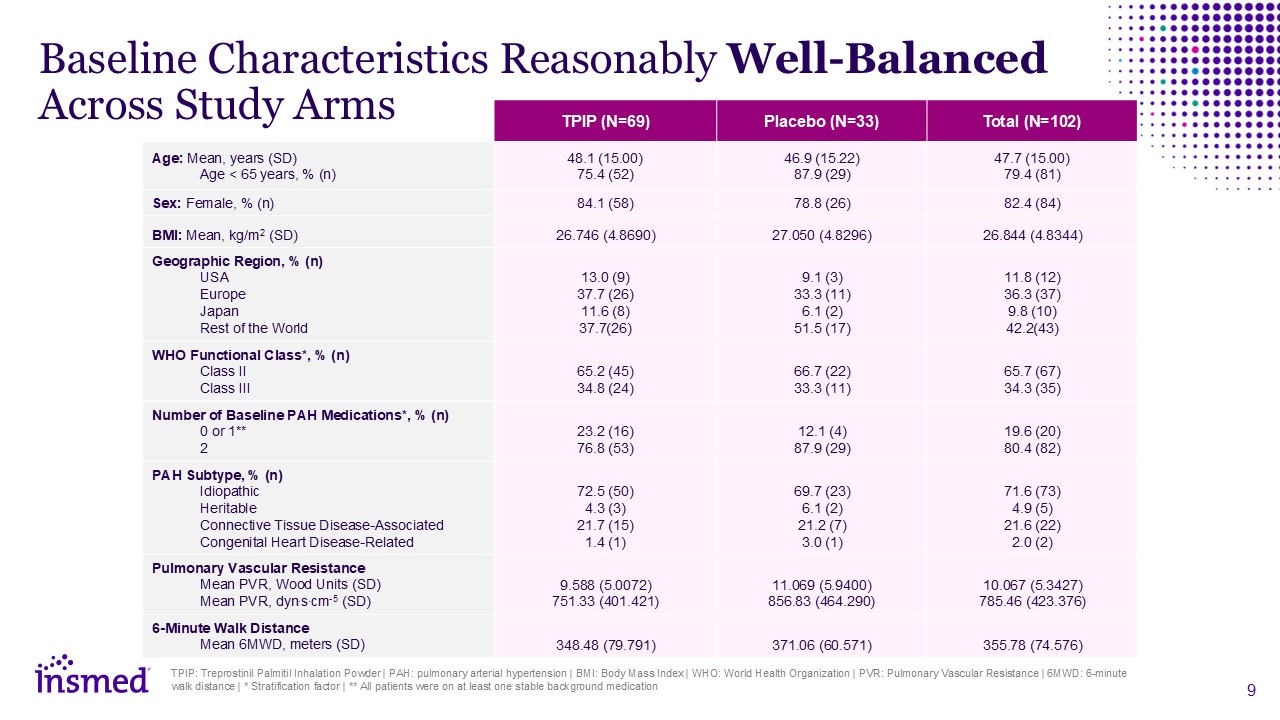
TPIP: Treprostinil Palmitil Inhalation Powder | PAH: pulmonary arterial
hypertension | BMI: Body Mass Index | WHO: World Health Organization | PVR: Pulmonary Vascular Resistance | 6MWD: 6-minute walk distance | * Stratification factor | ** All patients were on at least one stable background medication TPIP
(N=69) Placebo (N=33) Total (N=102) Age: Mean, years (SD) Age < 65 years, % (n) Adverse Events (%) 48.1 (15.00) 75.4 (52) 46.9 (15.22) 87.9 (29) 47.7 (15.00) 79.4 (81) Sex: Female, % (n) 84.1 (58) 78.8 (26) 82.4 (84) BMI:
Mean, kg/m2 (SD) 26.746 (4.8690) 27.050 (4.8296) 26.844 (4.8344) Geographic Region, % (n) USA Europe Japan Rest of the World Adverse Events (%) 13.0 (9) 37.7 (26) 11.6 (8) 37.7(26) 9.1 (3) 33.3 (11) 6.1 (2) 51.5 (17) 11.8
(12) 36.3 (37) 9.8 (10) 42.2(43) WHO Functional Class*, % (n) Class II Class III Serious Adverse Events (%) 65.2 (45) 34.8 (24) 66.7 (22) 33.3 (11) 65.7 (67) 34.3 (35) Number of Baseline PAH Medications*, % (n) 0 or
1** 2 Serious Adverse Events (%) 23.2 (16) 76.8 (53) 12.1 (4) 87.9 (29) 19.6 (20) 80.4 (82) PAH Subtype, % (n) Idiopathic Heritable Connective Tissue Disease-Associated Congenital Heart Disease-Related 72.5 (50) 4.3 (3) 21.7
(15) 1.4 (1) 69.7 (23) 6.1 (2) 21.2 (7) 3.0 (1) 71.6 (73) 4.9 (5) 21.6 (22) 2.0 (2) Pulmonary Vascular Resistance Mean PVR, Wood Units (SD) Mean PVR, dyn.s.cm-5 (SD) 9.588 (5.0072) 751.33 (401.421) 11.069 (5.9400) 856.83
(464.290) 10.067 (5.3427) 785.46 (423.376) 6-Minute Walk Distance Mean 6MWD, meters (SD) 348.48 (79.791) 371.06 (60.571) 355.78 (74.576) Baseline Characteristics Reasonably Well-Balanced Across Study Arms
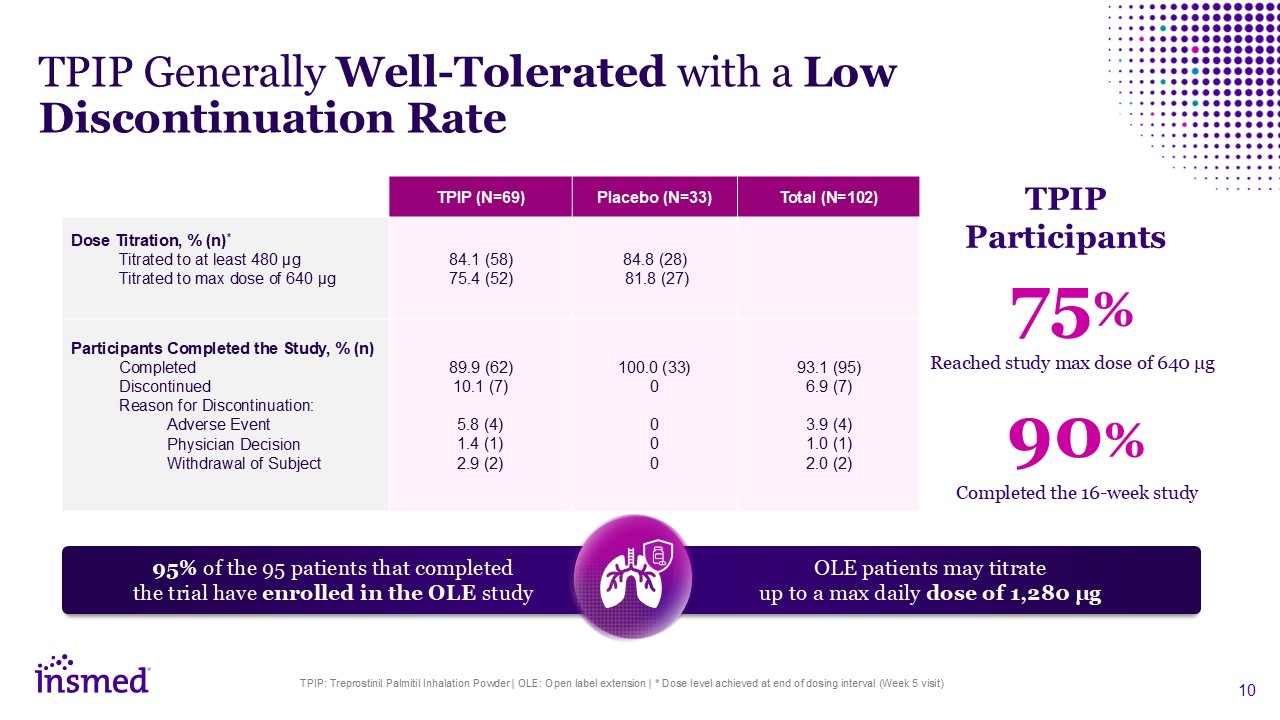
TPIP: Treprostinil Palmitil Inhalation Powder | OLE: Open label extension | * Dose
level achieved at end of dosing interval (Week 5 visit) TPIP (N=69) Placebo (N=33) Total (N=102) Dose Titration, % (n)* Titrated to at least 480 μg Titrated to max dose of 640 μg 84.1 (58) 75.4 (52) 84.8 (28) 81.8 (27) Participants
Completed the Study, % (n) Completed Discontinued Reason for Discontinuation: Adverse Event Physician Decision Withdrawal of Subject Adverse Events (%) 89.9 (62) 10.1 (7) 5.8 (4) 1.4 (1) 2.9 (2) 100.0 (33) 0 0 0 0 93.1
(95) 6.9 (7) 3.9 (4) 1.0 (1) 2.0 (2) TPIP Generally Well-Tolerated with a Low Discontinuation Rate 75% 90% Reached study max dose of 640 μg Completed the 16-week study TPIP Participants 95% of the 95 patients that completed the
trial have enrolled in the OLE study OLE patients may titrate up to a max daily dose of 1,280 μg
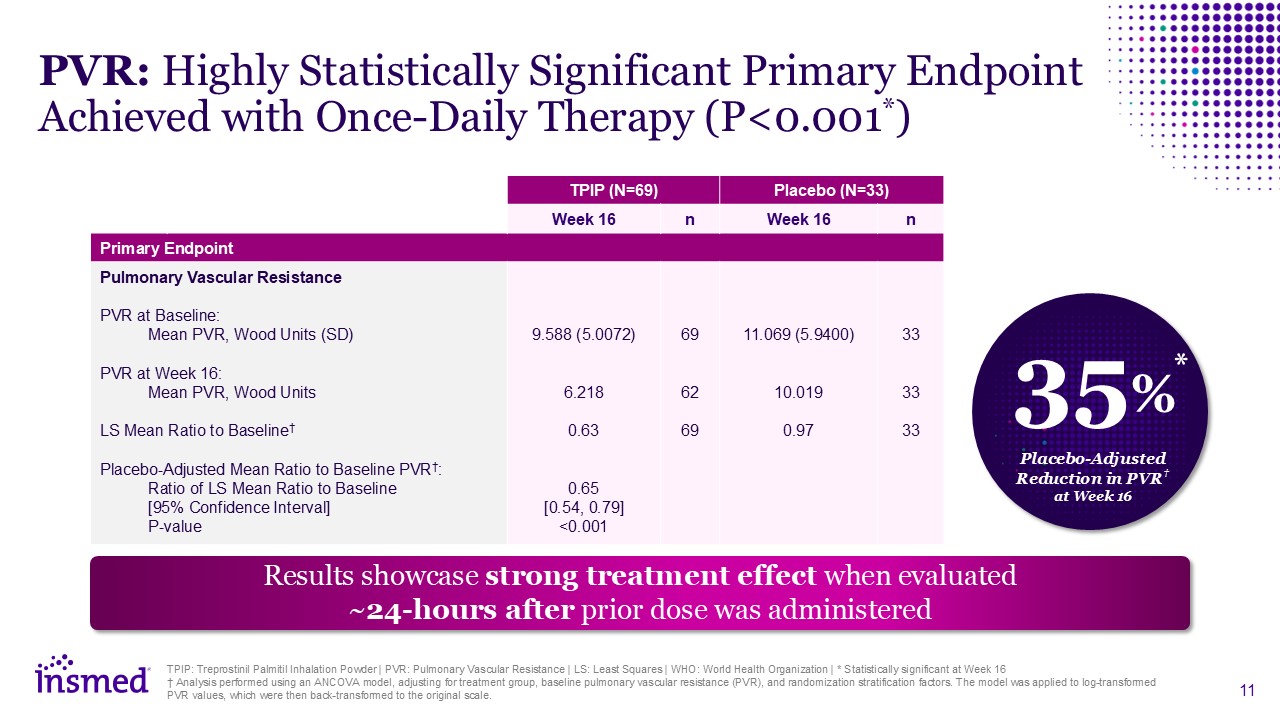
TPIP: Treprostinil Palmitil Inhalation Powder | PVR: Pulmonary Vascular Resistance
| LS: Least Squares | WHO: World Health Organization | * Statistically significant at Week 16 † Analysis performed using an ANCOVA model, adjusting for treatment group, baseline pulmonary vascular resistance (PVR), and randomization
stratification factors. The model was applied to log-transformed PVR values, which were then back-transformed to the original scale. TPIP (N=69) Placebo (N=33) Week 16 n Week 16 n Primary Endpoint Pulmonary Vascular Resistance PVR at
Baseline: Mean PVR, Wood Units (SD) PVR at Week 16: Mean PVR, Wood Units LS Mean Ratio to Baseline† Placebo-Adjusted Mean Ratio to Baseline PVR†: Ratio of LS Mean Ratio to Baseline [95% Confidence Interval] P-value 9.588
(5.0072) 6.218 0.63 0.65 [0.54, 0.79] <0.001 69 62 69 11.069 (5.9400) 10.019 0.97 33 33 33 PVR: Highly Statistically Significant Primary Endpoint Achieved with Once-Daily Therapy (P<0.001*) Results showcase strong
treatment effect when evaluated ~24-hours after prior dose was administered 35% Placebo-Adjusted Reduction in PVR† at Week 16 *
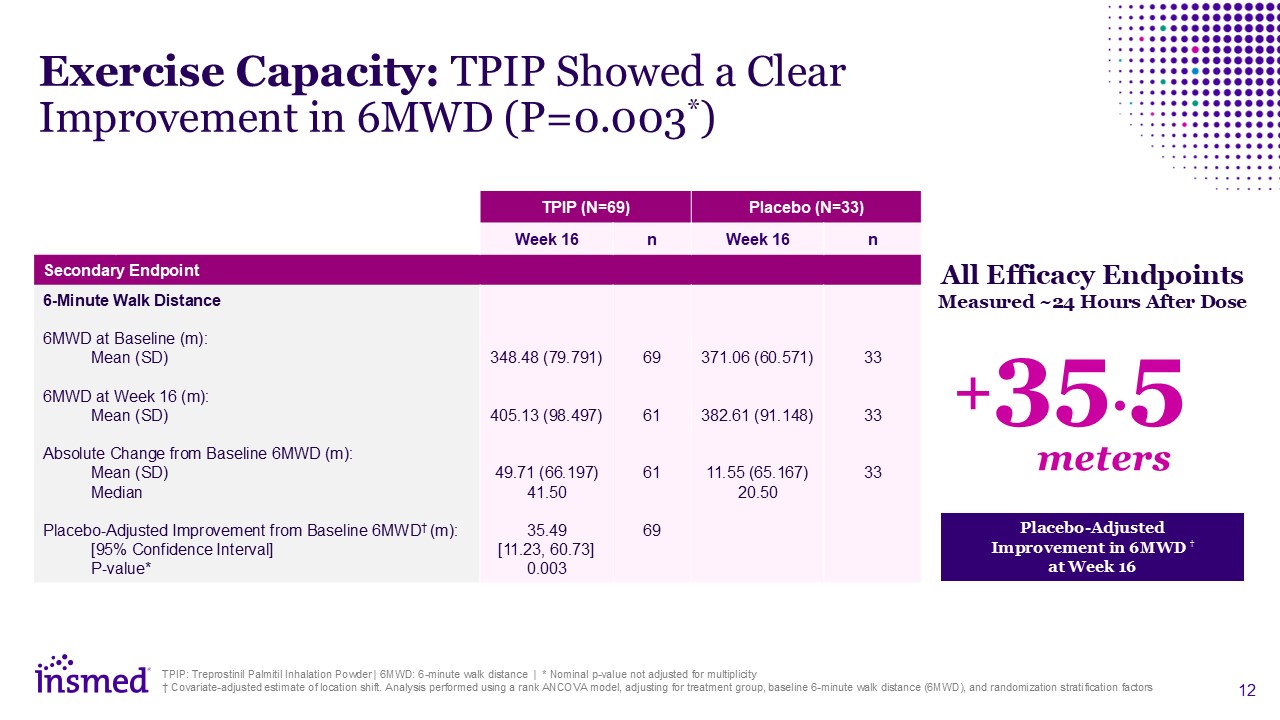
TPIP (N=69) Placebo (N=33) Week 16 n Week 16 n Secondary Endpoint 6-Minute
Walk Distance 6MWD at Baseline (m): Mean (SD) 6MWD at Week 16 (m): Mean (SD) Absolute Change from Baseline 6MWD (m): Mean (SD) Median Placebo-Adjusted Improvement from Baseline 6MWD† (m): [95% Confidence Interval] P-value* Adverse
Events (%) 348.48 (79.791) 405.13 (98.497) 49.71 (66.197) 41.50 35.49 [11.23, 60.73] 0.003 69 61 61 69 371.06 (60.571) 382.61 (91.148) 11.55 (65.167) 20.50 33 33 33 Exercise Capacity: TPIP Showed a Clear Improvement in 6MWD
(P=0.003*) TPIP: Treprostinil Palmitil Inhalation Powder | 6MWD: 6-minute walk distance | * Nominal p-value not adjusted for multiplicity † Covariate-adjusted estimate of location shift. Analysis performed using a rank ANCOVA model, adjusting
for treatment group, baseline 6-minute walk distance (6MWD), and randomization stratification factors Placebo-Adjusted Improvement in 6MWD † at Week 16 All Efficacy Endpoints Measured ~24 Hours After Dose +35.5 meters
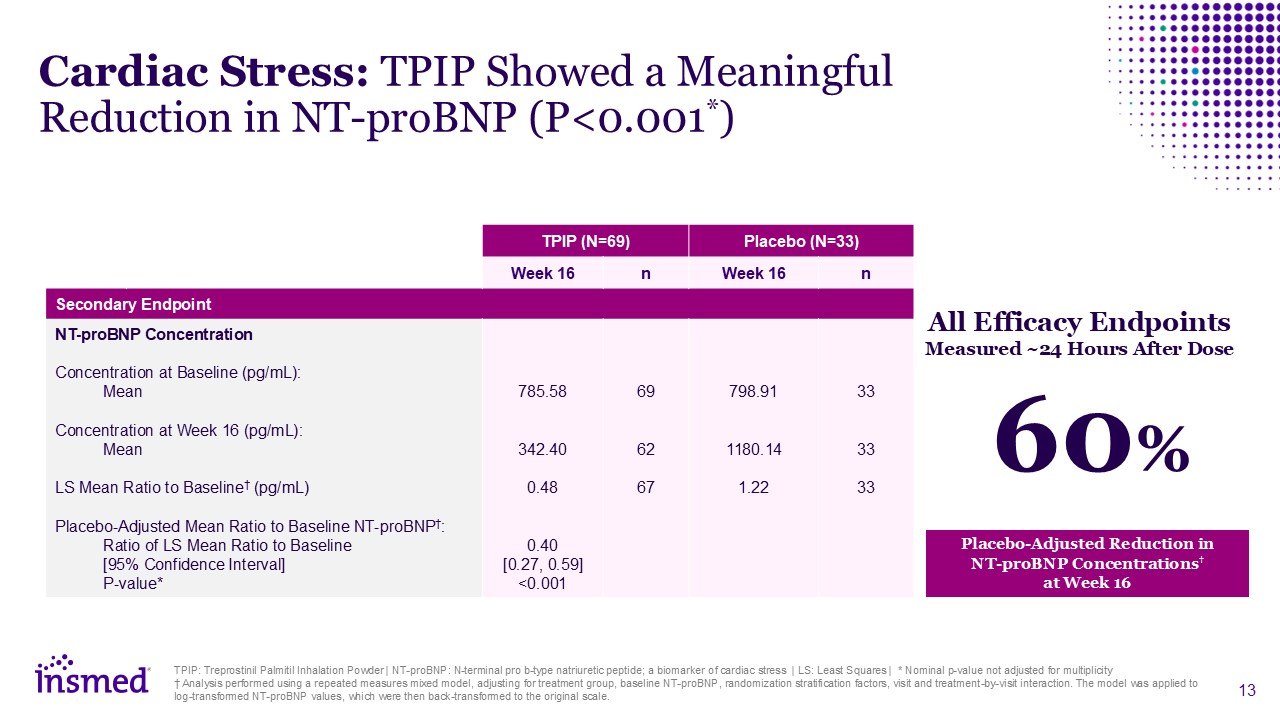
TPIP: Treprostinil Palmitil Inhalation Powder | NT-proBNP: N-terminal pro b-type
natriuretic peptide; a biomarker of cardiac stress | LS: Least Squares | * Nominal p-value not adjusted for multiplicity † Analysis performed using a repeated measures mixed model, adjusting for treatment group, baseline NT-proBNP,
randomization stratification factors, visit and treatment-by-visit interaction. The model was applied to log-transformed NT-proBNP values, which were then back-transformed to the original scale. TPIP (N=69) Placebo (N=33) Week 16 n Week
16 n Secondary Endpoint NT-proBNP Concentration Concentration at Baseline (pg/mL): Mean Concentration at Week 16 (pg/mL): Mean LS Mean Ratio to Baseline† (pg/mL) Placebo-Adjusted Mean Ratio to Baseline NT-proBNP†: Ratio of LS Mean
Ratio to Baseline [95% Confidence Interval] P-value* 785.58 342.40 0.48 0.40 [0.27, 0.59] <0.001 69 62 67 798.91 1180.14 1.22 33 33 33 Cardiac Stress: TPIP Showed a Meaningful Reduction in NT-proBNP
(P<0.001*) Placebo-Adjusted Reduction in NT-proBNP Concentrations† at Week 16 All Efficacy Endpoints Measured ~24 Hours After Dose 60%
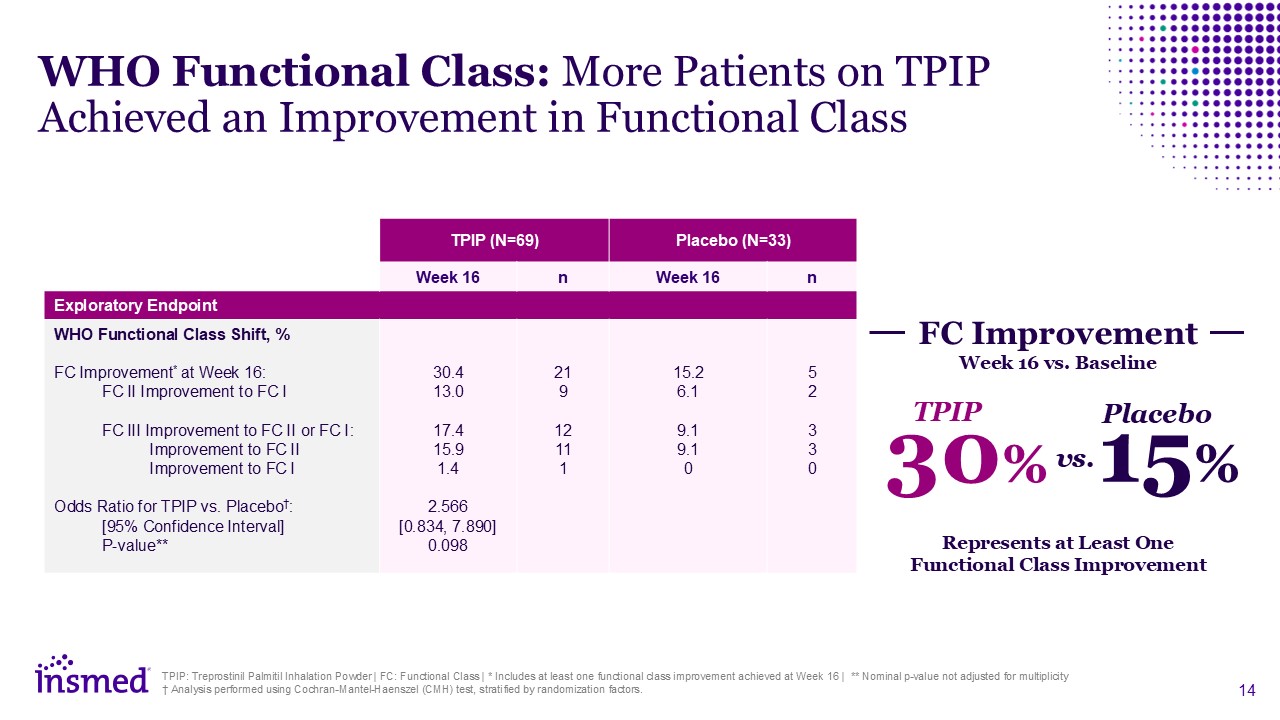
Represents at Least One Functional Class Improvement
30% TPIP 15% Placebo vs. FC Improvement Week 16 vs. Baseline TPIP: Treprostinil Palmitil Inhalation Powder | FC: Functional Class | * Includes at least one functional class improvement achieved at Week 16 | ** Nominal p-value not
adjusted for multiplicity † Analysis performed using Cochran-Mantel-Haenszel (CMH) test, stratified by randomization factors. TPIP (N=69) Placebo (N=33) Week 16 n Week 16 n Exploratory Endpoint WHO Functional Class Shift, % FC
Improvement* at Week 16: FC II Improvement to FC I FC III Improvement to FC II or FC I: Improvement to FC II Improvement to FC I Odds Ratio for TPIP vs. Placebo†: [95% Confidence Interval] P-value** Adverse Events (%) 30.4
13.0 17.4 15.9 1.4 2.566 [0.834, 7.890] 0.098 21 9 12 11 1 15.2 6.1 9.1 9.1 0 5 2 3 3 0 WHO Functional Class: More Patients on TPIP Achieved an Improvement in Functional Class
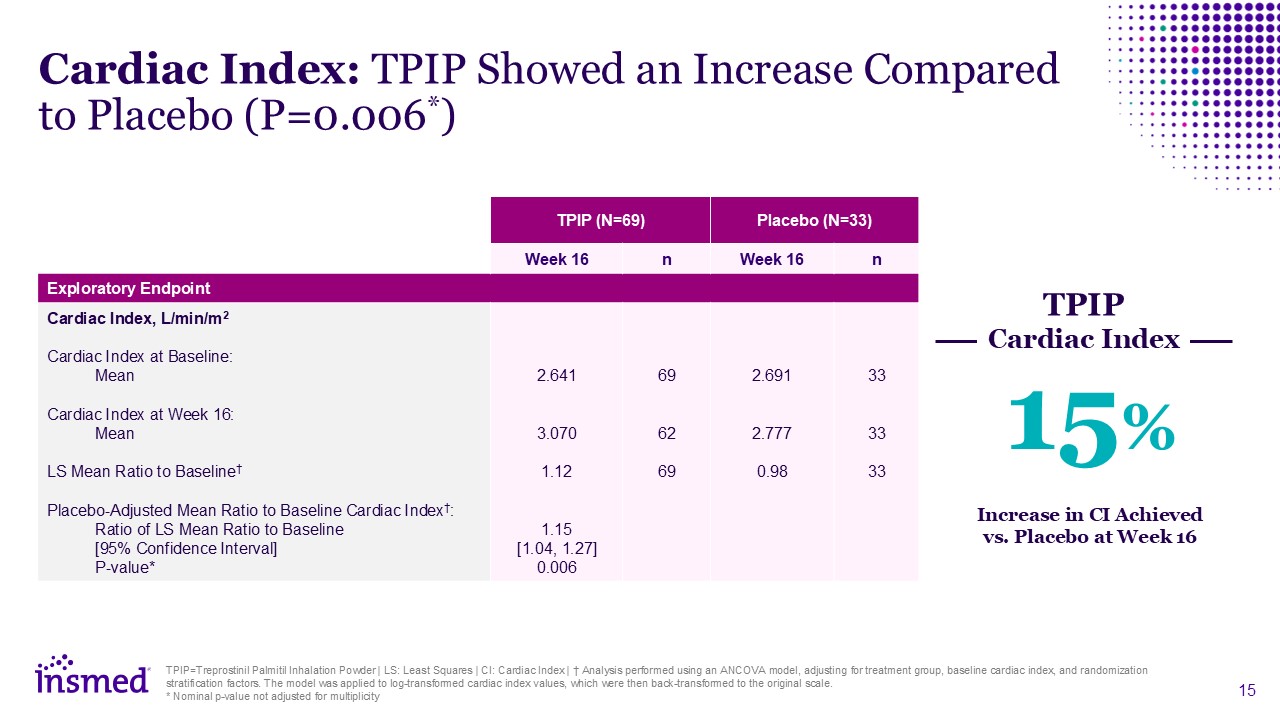
TPIP=Treprostinil Palmitil Inhalation Powder | LS: Least Squares | CI: Cardiac
Index | † Analysis performed using an ANCOVA model, adjusting for treatment group, baseline cardiac index, and randomization stratification factors. The model was applied to log-transformed cardiac index values, which were then back-transformed
to the original scale. * Nominal p-value not adjusted for multiplicity Cardiac Index: TPIP Showed an Increase Compared to Placebo (P=0.006*) TPIP (N=69) Placebo (N=33) Week 16 n Week 16 n Exploratory Endpoint Cardiac Index,
L/min/m2 Cardiac Index at Baseline: Mean Cardiac Index at Week 16: Mean LS Mean Ratio to Baseline† Placebo-Adjusted Mean Ratio to Baseline Cardiac Index†: Ratio of LS Mean Ratio to Baseline [95% Confidence Interval] P-value* Adverse
Events (%) 2.641 3.070 1.12 1.15 [1.04, 1.27] 0.006 69 62 69 2.691 2.777 0.98 33 33 33 Increase in CI Achieved vs. Placebo at Week 16 15% TPIP Cardiac Index
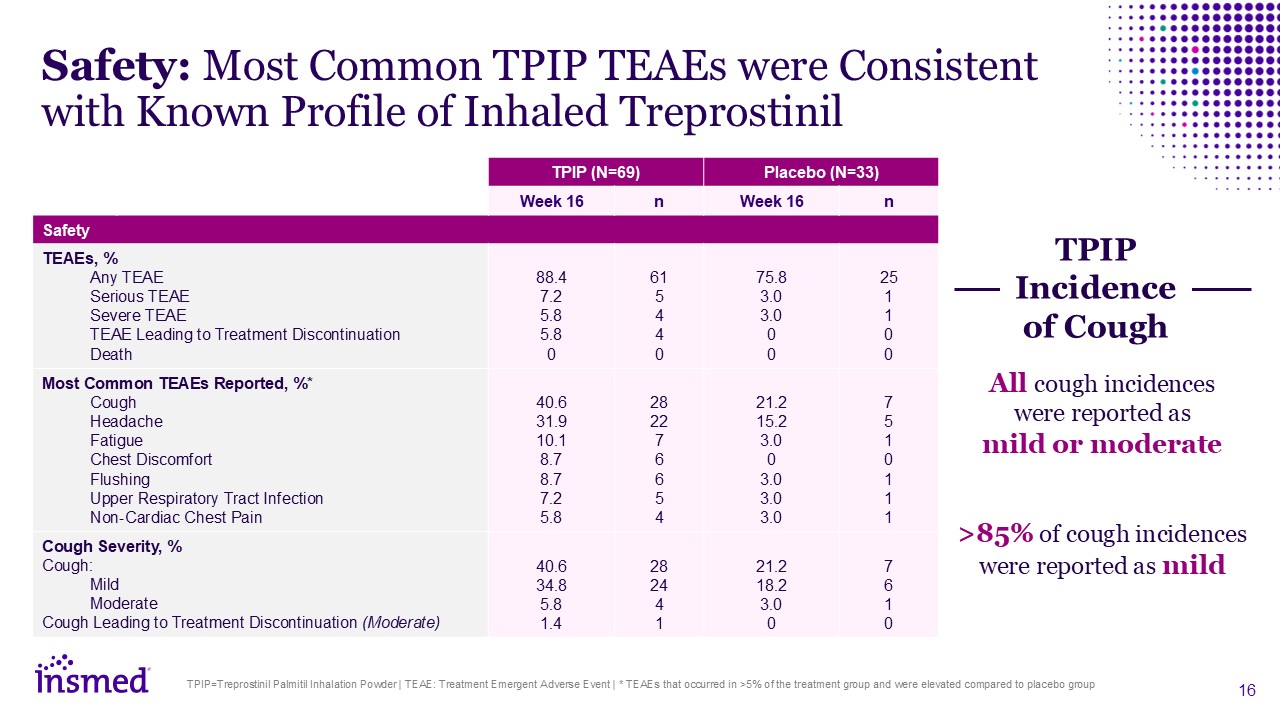
TPIP=Treprostinil Palmitil Inhalation Powder | TEAE: Treatment Emergent Adverse
Event | * TEAEs that occurred in >5% of the treatment group and were elevated compared to placebo group Safety: Most Common TPIP TEAEs were Consistent with Known Profile of Inhaled Treprostinil TPIP (N=69) Placebo (N=33) Week 16 n Week
16 n Safety TEAEs, % Any TEAE Serious TEAE Severe TEAE TEAE Leading to Treatment Discontinuation Death 88.4 7.2 5.8 5.8 0 61 5 4 4 0 75.8 3.0 3.0 0 0 25 1 1 0 0 Most Common TEAEs Reported,
%* Cough Headache Fatigue Chest Discomfort Flushing Upper Respiratory Tract Infection Non-Cardiac Chest Pain Adverse Events
(%) 40.6 31.9 10.1 8.7 8.7 7.2 5.8 28 22 7 6 6 5 4 21.2 15.2 3.0 0 3.0 3.0 3.0 7 5 1 0 1 1 1 Cough Severity, % Cough: Mild Moderate Cough Leading to Treatment Discontinuation
(Moderate) 40.6 34.8 5.8 1.4 28 24 4 1 21.2 18.2 3.0 0 7 6 1 0 All cough incidences were reported as mild or moderate >85% of cough incidences were reported as mild TPIP Incidence of Cough
Closing Remarks Goal of TPIP: once-daily therapy that combines the continuity of
parenteral treatment with the localization and convenience of inhaled therapy Results reinforce the promise of TPIP as a potential prostanoid of choice Plan to advance TPIP to Phase 3 before the end of 2025 for PH-ILD and in early 2026 for
PAH Potential to realize stronger efficacy with a longer titration window and a higher maximum dose in future Phase 3 studies TPIP=Treprostinil Palmitil Inhalation Powder | PAH: Pulmonary Arterial Hypertension | PH-ILD: Pulmonary Hypertension
due to Interstitial Lung Disease

Q&A Session Will Lewis Chair & CEO Sara Bonstein Chief Financial
Officer Martina Flammer Chief Medical Officer Gene Sullivan Chief Product Strategy Officer Thank you to the patients and investigators who participated in this study!
