| Overview June 2025 |
| This presentation contains forward-looking statements that involve substantial risks and uncertainties. All statements, other than statements of historical facts, contained in this presentation, including statements about our expectations regarding the potential benefits, activity, effec tiveness, and safety of our product candidates, our expectations with regard to the design and results of our research and development programs, preclinical studies, and cli nical trials, including the timing and availability of data from such studies and trials, our preclinical, clinical, and regulatory development plans for our product candidates, including the timing or likelihood of regulatory filings and approvals for our product candidates, our expectations with regard to our ability to license, acquire, discover, and develop additional products candidates and advance such product candidates into, and successfully complete, preclinical studies and clinical trials, the potential market size and size of the potential patient populations for our product candidates and any future product candidates, our ability to maintain existing, and establish new, strategic collaborations, licensing, or other arrangements, the scope of protection we are able to establish and maintain for intellectual property rights covering our initial product candidates and any future product candidates, our business strategy, and our future results of operations and financial position, are forward-looking statements. The words “anticipate,” “believe,” “estimate,” “expect,” “intend,” “may,” “might,” “plan,” “predict,” “project,” “target,” “potential,” “will,” “would,” “could,” “should,” “continue,” and similar expressions are intended to identify forward-looking statements, although not all forward-looking statements contain these identifying words. These forward-looking statements are subject to a number of risks, uncertainties and assumptions. Risks regarding our business are described in detail in our Securities and Exchange Commission filings, including in our Quarterly Report on Form 10-Q for the quarter ended March 31, 2025, and our other filings with the SEC, which are available on the SEC’s website at www.sec.gov. We may not actually achieve the plans, intentions or expectations disclosed in our forward-looking statements, and you should not place undue reliance on our forward-looking statements. Actual results or events could differ materially from the plans, intentions and expectations disclosed in the forward-looking statements we make. The forward-looking statements contained in this presentation reflect our current views with respect to future events, and we assume no obligation to update any forward-looking statements except as required by applicable law. This presentation also contains estimates and other statistical data made by independent parties and by us relating to market size and growth and other data about our industry. This data involves a number of assumptions and limitations, and you are cautioned not to give undue weight to such estimates. Industry publications and third-party research, surveys and studies generally indicate that their information has been obtained from sources believed to be reliable, although they do not guarantee the accuracy or completeness of such information. In addition, projections, assumptions, and estimates of our future performance and the future performance of the markets in which we operate are necessarily subject to a high degree of uncertainty and risk. This presentation contains trademarks, service marks, trade names and copyrights of Tvardi and other companies which are the property of their respective owners. 2 Disclaimer and Forward-Looking Statements |
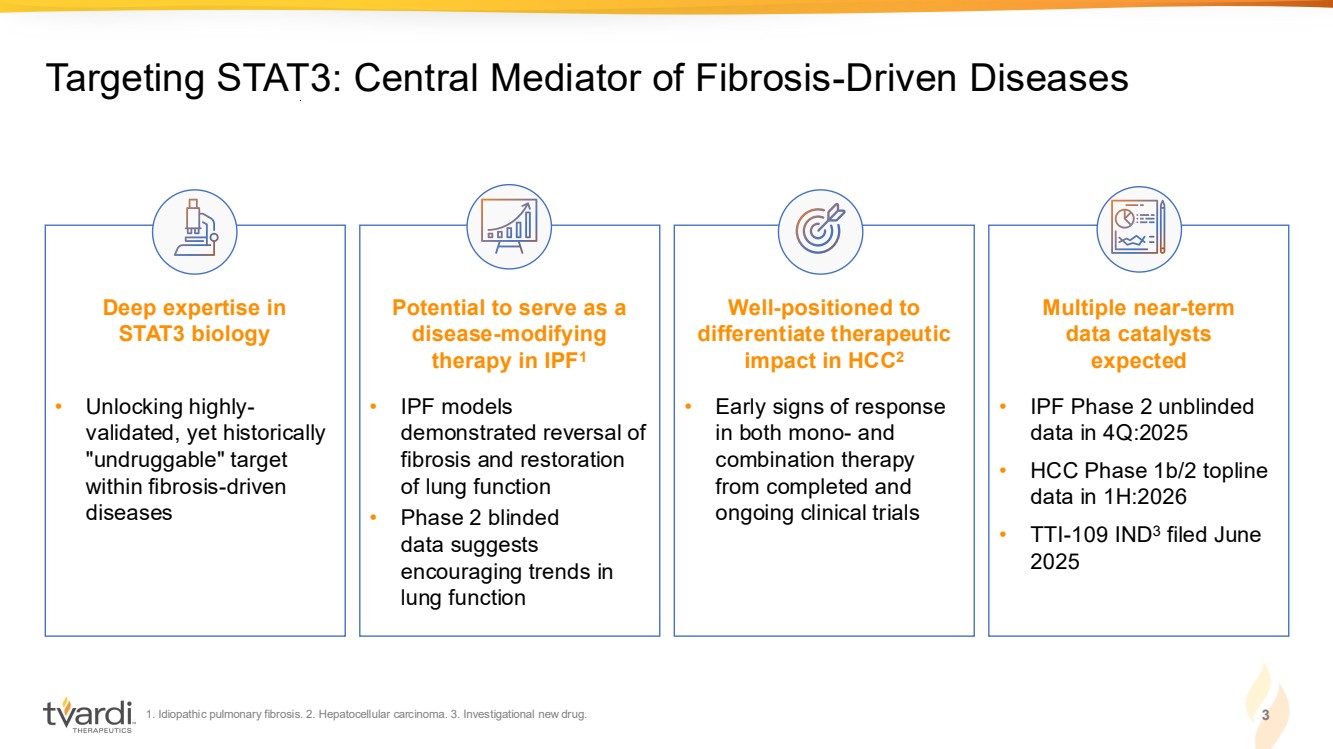
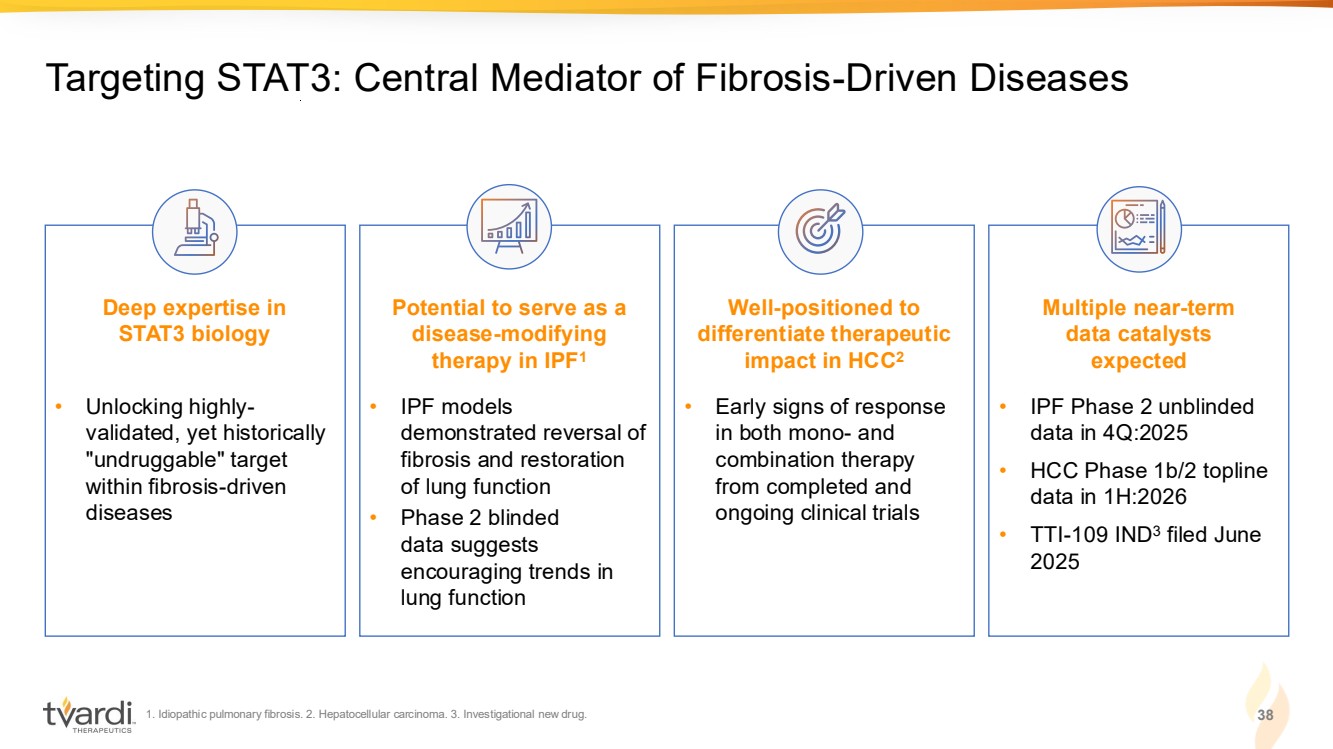
| 1. Idiopathic pulmonary fibrosis. 2. Hepatocellular carcinoma. 3. Investigational new drug. 3 Targeting STAT3: Central Mediator of Fibrosis-Driven Diseases Deep expertise in STAT3 biology Potential to serve as a disease-modifying therapy in IPF1 Well-positioned to differentiate therapeutic impact in HCC2 Multiple near-term data catalysts expected • Unlocking highly-validated, yet historically "undruggable" target within fibrosis-driven diseases • IPF models demonstrated reversal of fibrosis and restoration of lung function • Phase 2 blinded data suggests encouraging trends in lung function • Early signs of response in both mono- and combination therapy from completed and ongoing clinical trials • IPF Phase 2 unblinded data in 4Q:2025 • HCC Phase 1b/2 topline data in 1H:2026 • TTI-109 IND3 filed June 2025 |
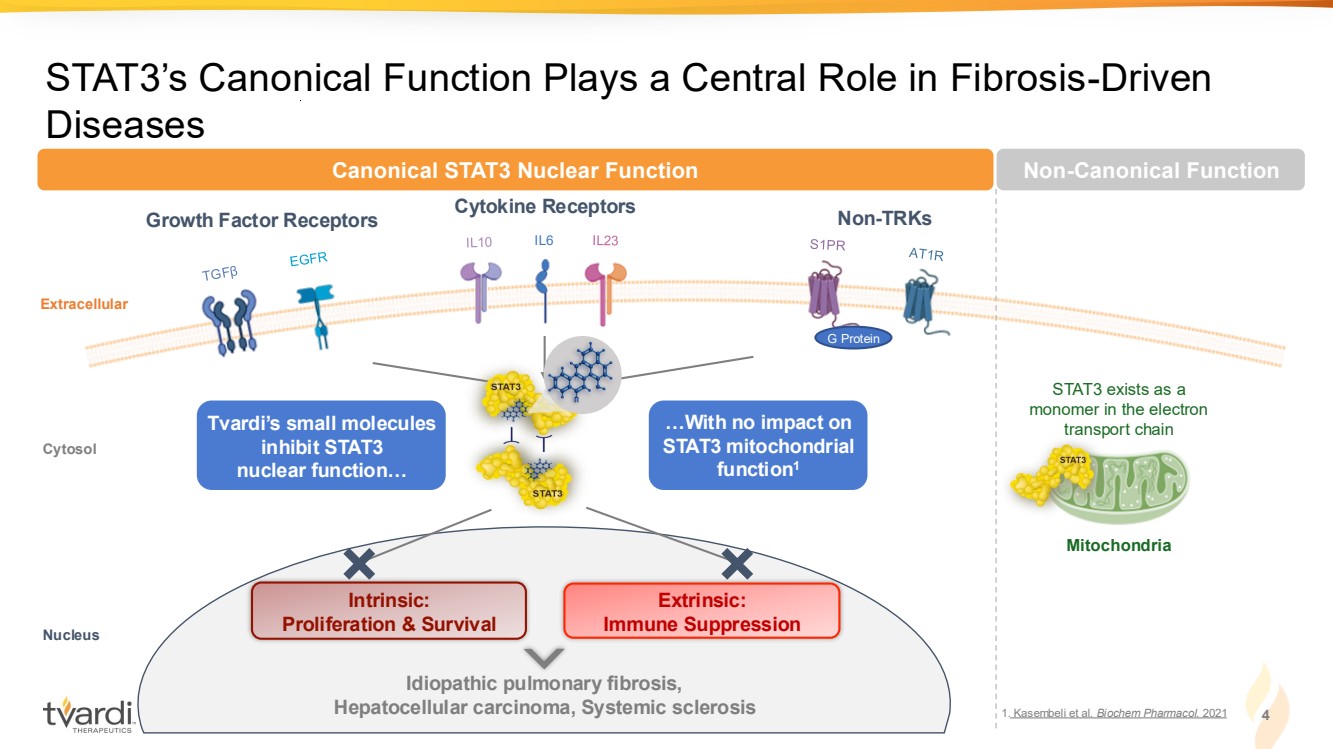
| 4 STAT3’s Canonical Function Plays a Central Role in Fibrosis-Driven Diseases IL6 Growth Factor Receptors Cytokine Receptors Non-TRKs Extracellular Intrinsic: Proliferation & Survival Extrinsic: Immune Suppression Idiopathic pulmonary fibrosis, Hepatocellular carcinoma, Systemic sclerosis Cytosol Nucleus G Protein Canonical STAT3 Nuclear Function Mitochondria STAT3 exists as a monomer in the electron Tvardi’s small molecules transport chain inhibit STAT3 nuclear function… …With no impact on STAT3 mitochondrial function1 Non-Canonical Function 1. Kasembeli et al. Biochem Pharmacol. 2021 |
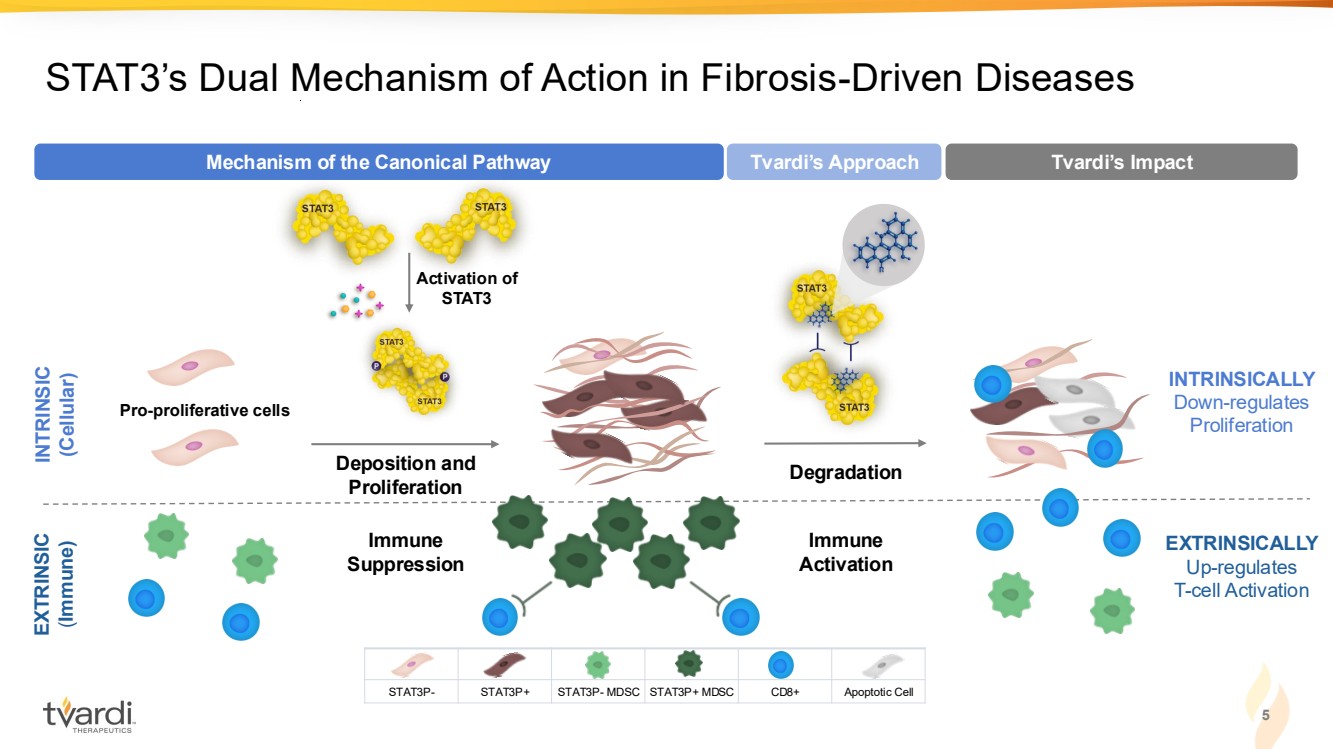
| 5 STAT3’s Dual Mechanism of Action in Fibrosis-Driven Diseases EXTRINSICALLY Up-regulates T-cell Activation Immune Activation Degradation Tvardi’s Approach Tvardi’s Impact INTRINSICALLY Down-regulates Proliferation INTRINSIC (Cellular) EXTRINSIC (Immune) Immune Suppression Pro-proliferative cells Activation of STAT3 Deposition and Proliferation Mechanism of the Canonical Pathway STAT3P- STAT3P+ STAT3P- MDSC STAT3P+ MDSC CD8+ Apoptotic Cell |
| 6 Seasoned Leadership: Deep R&D and Operational Expertise Sujal Shah Chairman Michael Wyzga Director Cynthia Smith Director Susan Shiff, PhD Director Shaheen Wirk, MD Director Wallace Hall Director Imran Alibhai, PhD CEO & Director Dan Conn, JD, MBA CFO John Kauh, MD CMO David Tweardy, MD Founder & Advisor Ron DePinho, MD Founder & Advisor Keith Flaherty, MD Advisor (Oncology) Jeff Swigris, DO Advisor (IPF) Management Team Scientific Advisory Board Board of Directors BioMatrix Partners |
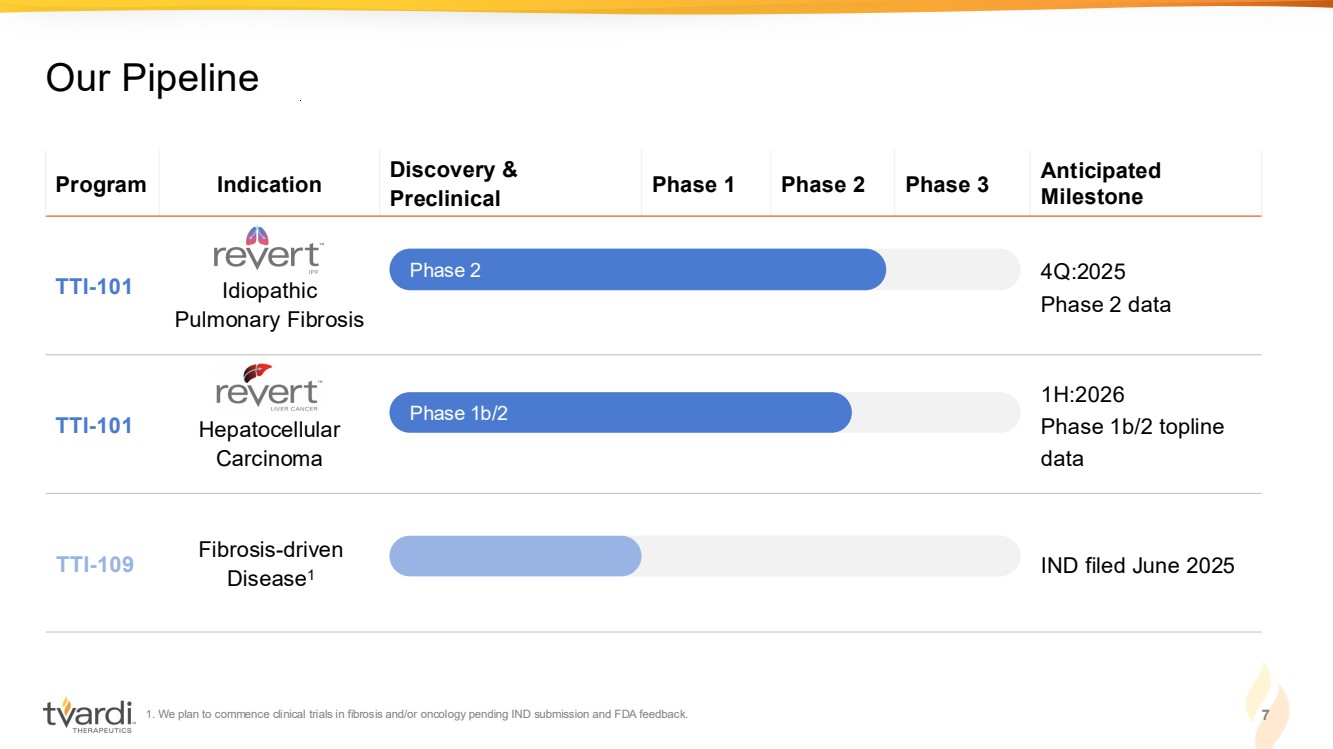
| 1. We plan to commence clinical trials in fibrosis and/or oncology pending IND submission and FDA feedback. 7 Our Pipeline Program Indication Discovery & Preclinical Phase 1 Phase 2 Phase 3 Anticipated Milestone TTI-101 Idiopathic Pulmonary Fibrosis 4Q:2025 Phase 2 data TTI-101 Hepatocellular Carcinoma 1H:2026 Phase 1b/2 topline data TTI-109 Fibrosis-driven Disease1 IND filed June 2025 Phase 2 Phase 1b/2 |
| TTI-101 in IPF |
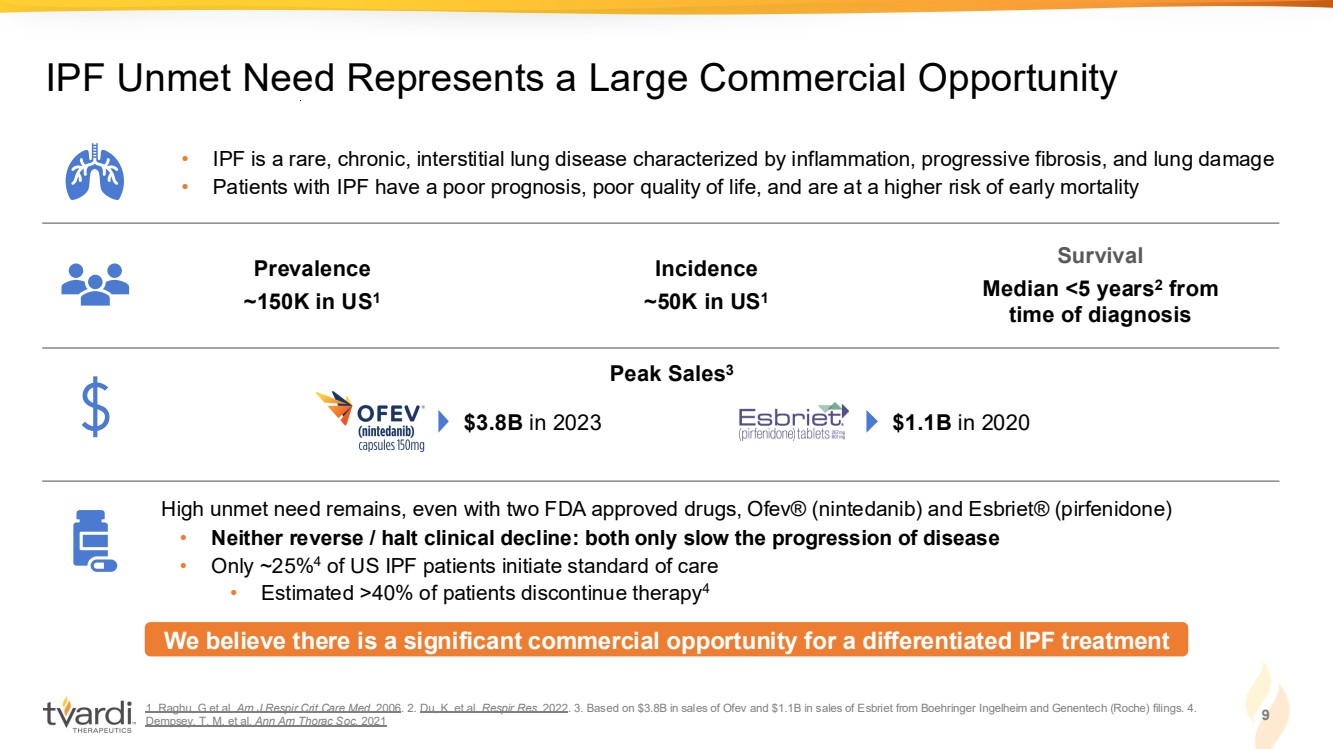
| 1. Raghu, G et al. Am J Respir Crit Care Med. 2006. 2. Du, K. et al. Respir Res. 2022. 3. Based on $3.8B in sales of Ofev and $1.1B in sales of Esbriet from Boehringer Ingelheim and Genentech (Roche) filings. 4. Dempsey, T. M. et al. Ann Am Thorac Soc. 2021 9 IPF Unmet Need Represents a Large Commercial Opportunity • IPF is a rare, chronic, interstitial lung disease characterized by inflammation, progressive fibrosis, and lung damage • Patients with IPF have a poor prognosis, poor quality of life, and are at a higher risk of early mortality We believe there is a significant commercial opportunity for a differentiated IPF treatment Prevalence ~150K in US1 Incidence ~50K in US1 Survival Median <5 years2 from time of diagnosis High unmet need remains, even with two FDA approved drugs, Ofev® (nintedanib) and Esbriet® (pirfenidone) • Neither reverse / halt clinical decline: both only slow the progression of disease • Only ~25%4 of US IPF patients initiate standard of care • Estimated >40% of patients discontinue therapy4 Peak Sales3 $3.8B in 2023 $1.1B in 2020 |
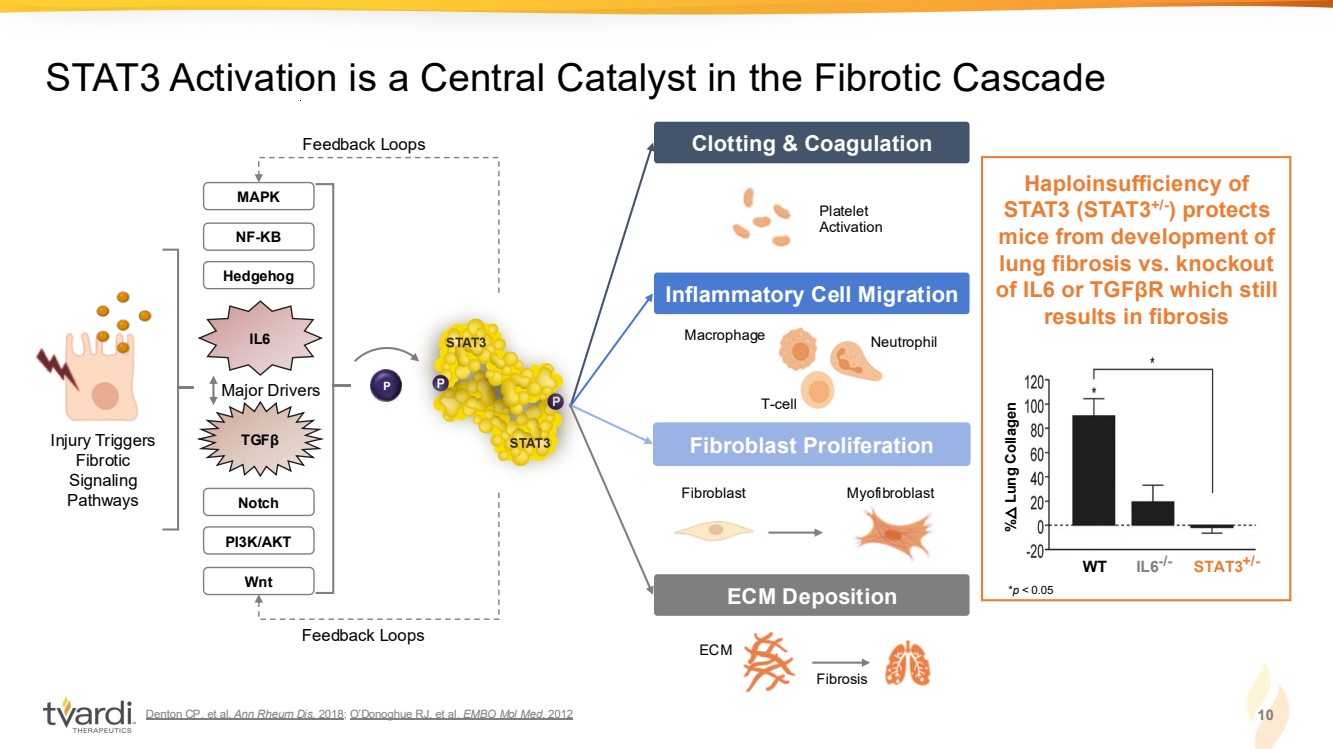
| Denton CP. et al. Ann Rheum Dis. 2018; O’Donoghue RJ. et al. EMBO Mol Med. 2012 10 STAT3 Activation is a Central Catalyst in the Fibrotic Cascade IL6-/- STAT3 WT +/- % △ Notch Lung Collagen IL6 TGFβ NF-KB MAPK Hedgehog PI3K/AKT Wnt Injury Triggers Fibrotic Signaling Pathways Haploinsufficiency of STAT3 (STAT3+/- ) protects mice from development of lung fibrosis vs. knockout of IL6 or TGFβR which still results in fibrosis Clotting & Coagulation Fibroblast Proliferation ECM Deposition Macrophage Neutrophil T-cell Platelet Activation ECM Fibrosis Fibroblast Myofibroblast Inflammatory Cell Migration Major Drivers P Feedback Loops Feedback Loops *p < 0.05 |
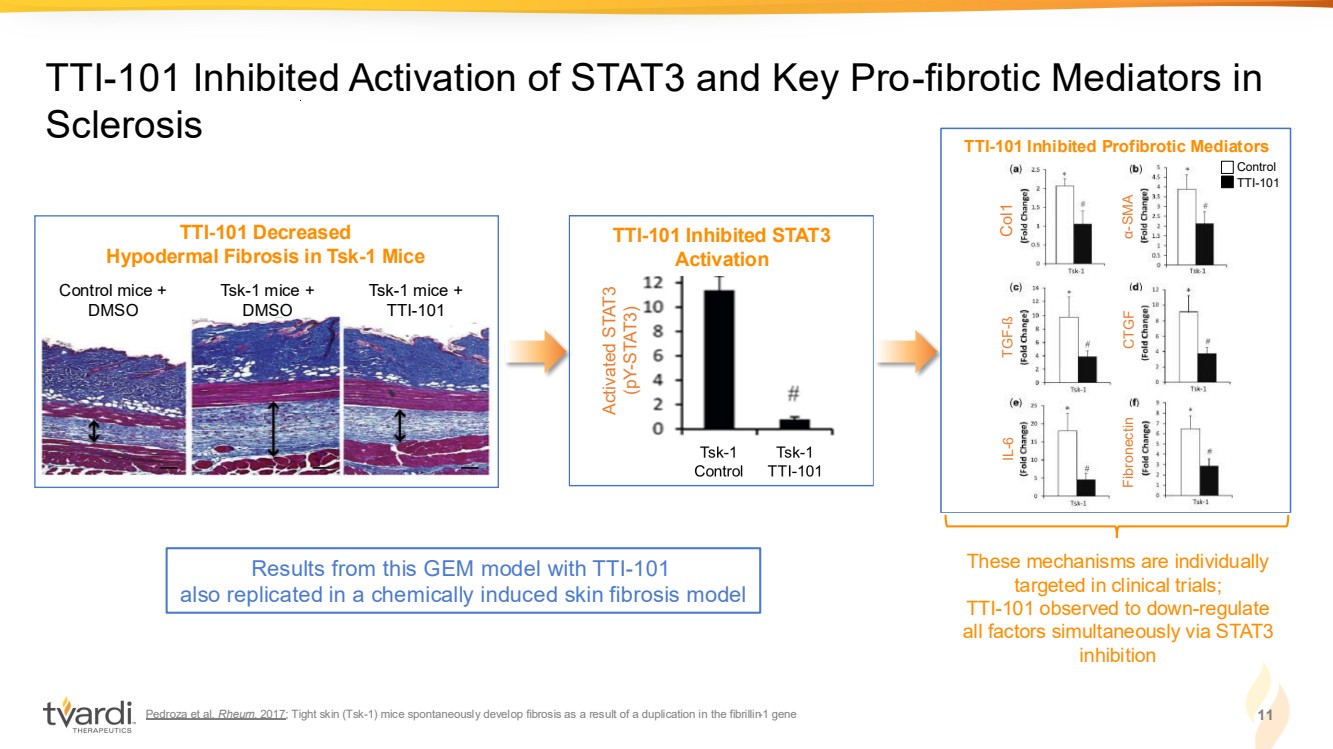
| Pedroza et al. Rheum. 2017; Tight skin (Tsk-1) mice spontaneously develop fibrosis as a result of a duplication in the fibrillin-1 gene 11 TTI-101 Inhibited Activation of STAT3 and Key Pro-fibrotic Mediators in Sclerosis TTI-101 Decreased Hypodermal Fibrosis in Tsk-1 Mice Control mice + DMSO Tsk-1 mice + DMSO Tsk-1 mice + TTI-101 These mechanisms are individually targeted in clinical trials; TTI-101 observed to down-regulate all factors simultaneously via STAT3 inhibition Results from this GEM model with TTI-101 also replicated in a chemically induced skin fibrosis model TTI-101 Inhibits STAT3 Activation Activated STAT3 (pY-STAT3) TTI-101 Inhibited STAT3 Activation TTI-101 Inhibits Pro-fibrotic Mediators Control TTI-101 Col1 TGF-ß IL-6 α-SMA CTGF Fibronectin TTI-101 Inhibited Profibrotic Mediators |
| Pedroza et al. FASEB J. 2016 12 STAT3 is Activated in Major Compartments of IPF-Affected Mouse and Human Lung Tissue Activated STAT3 is overexpressed in IPF human lung tissue Activated STAT3 is similarly overexpressed in lung tissue of murine IPF pY-STAT3 / GAPDH Fold Induction pY-STAT3 / Actin Fold Induction Alveolar Epithelial Cells Alveolar Fibroblasts Alveolar Macrophages Normal pY-STAT3 pY-STAT3 pY-STAT3 pY-STAT3 |
| Celada et al Sci Transl Med 2018 STAT3 Correlates with High Mortality in IPF Patients IPF Transplant-free survival over the course of 3.5 years post-diagnosis in a cohort of patients (n=55) based on STAT3 expression. Activated STAT3 (pSTAT3) induces the expression of STAT3 transcript. 13 |
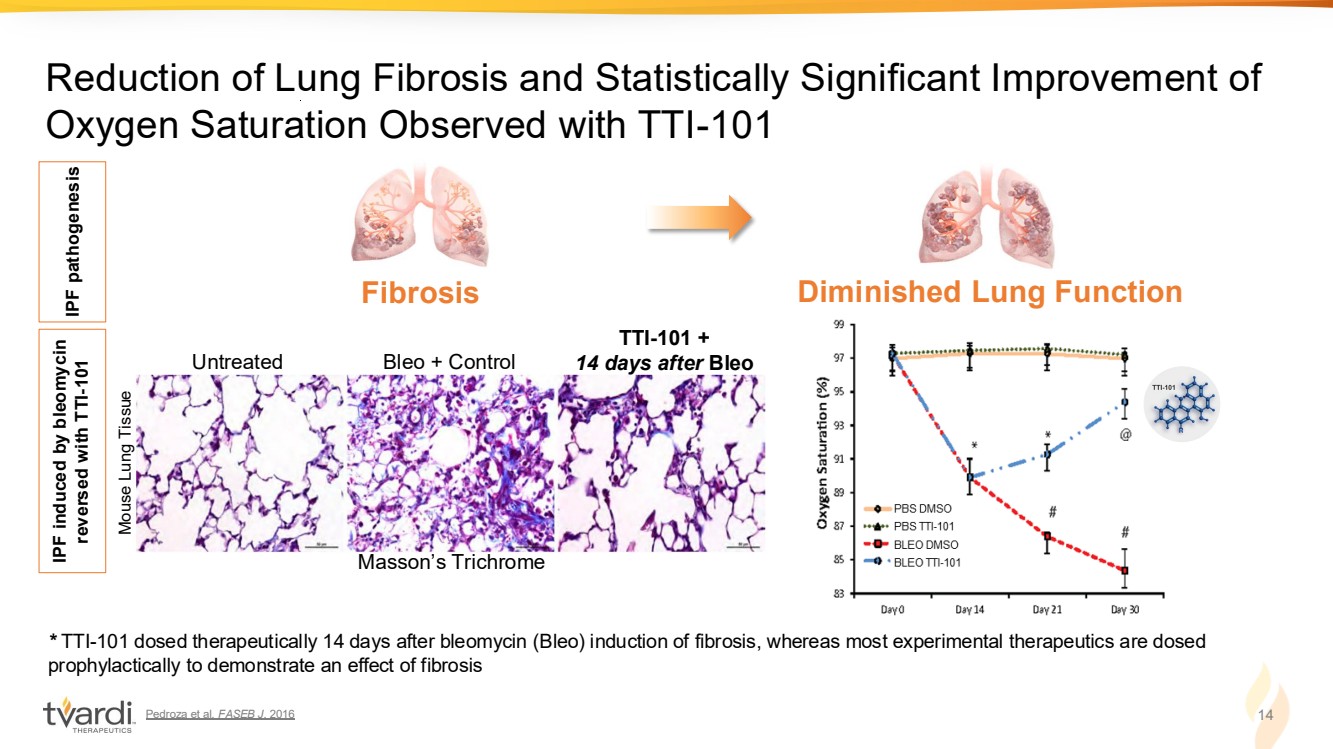
| Pedroza et al. FASEB J. 2016 14 Reduction of Lung Fibrosis and Statistically Significant Improvement of Oxygen Saturation Observed with TTI-101 * TTI-101 dosed therapeutically 14 days after bleomycin (Bleo) induction of fibrosis, whereas most experimental therapeutics are dosed prophylactically to demonstrate an effect of fibrosis Untreated Bleo + Control TTI-101 + 14 days after Bleo IPF pathogenesis IPF induced by bleomycin reversed with TTI-101 Fibrosis Diminished Lung Function Masson’s Trichrome Mouse Lung Tissue |
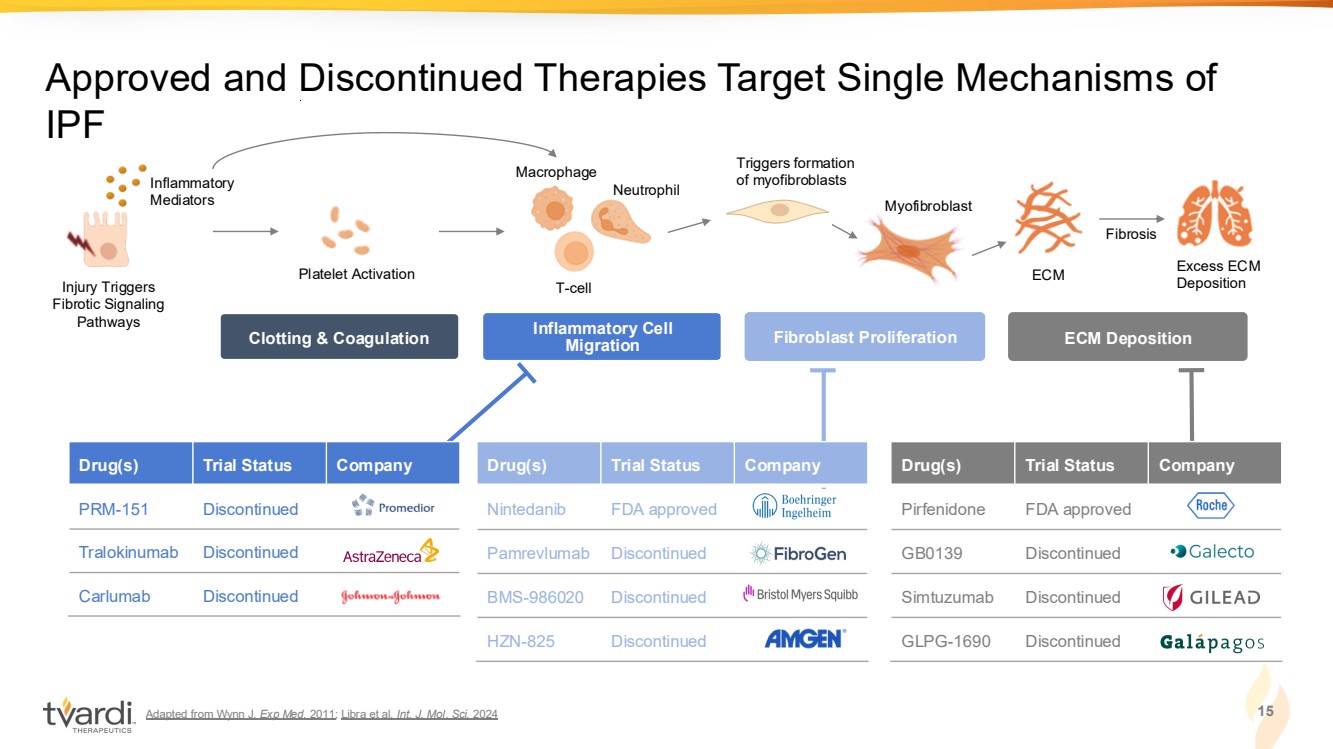
| Adapted from Wynn J. Exp Med. 2011; Libra et al. Int. J. Mol. Sci. 2024 15 Approved and Discontinued Therapies Target Single Mechanisms of IPF Injury Triggers Fibrotic Signaling Pathways Inflammatory Mediators ECM Fibrosis Excess ECM Deposition Macrophage Neutrophil T-cell Platelet Activation Myofibroblast Triggers formation of myofibroblasts Clotting & Coagulation Inflammatory Cell Migration Fibroblast Proliferation ECM Deposition Drug(s) Trial Status Company Pirfenidone FDA approved GB0139 Discontinued Simtuzumab Discontinued GLPG-1690 Discontinued Drug(s) Trial Status Company Nintedanib FDA approved Pamrevlumab Discontinued BMS-986020 Discontinued HZN-825 Discontinued Drug(s) Trial Status Company PRM-151 Discontinued Tralokinumab Discontinued Carlumab Discontinued |
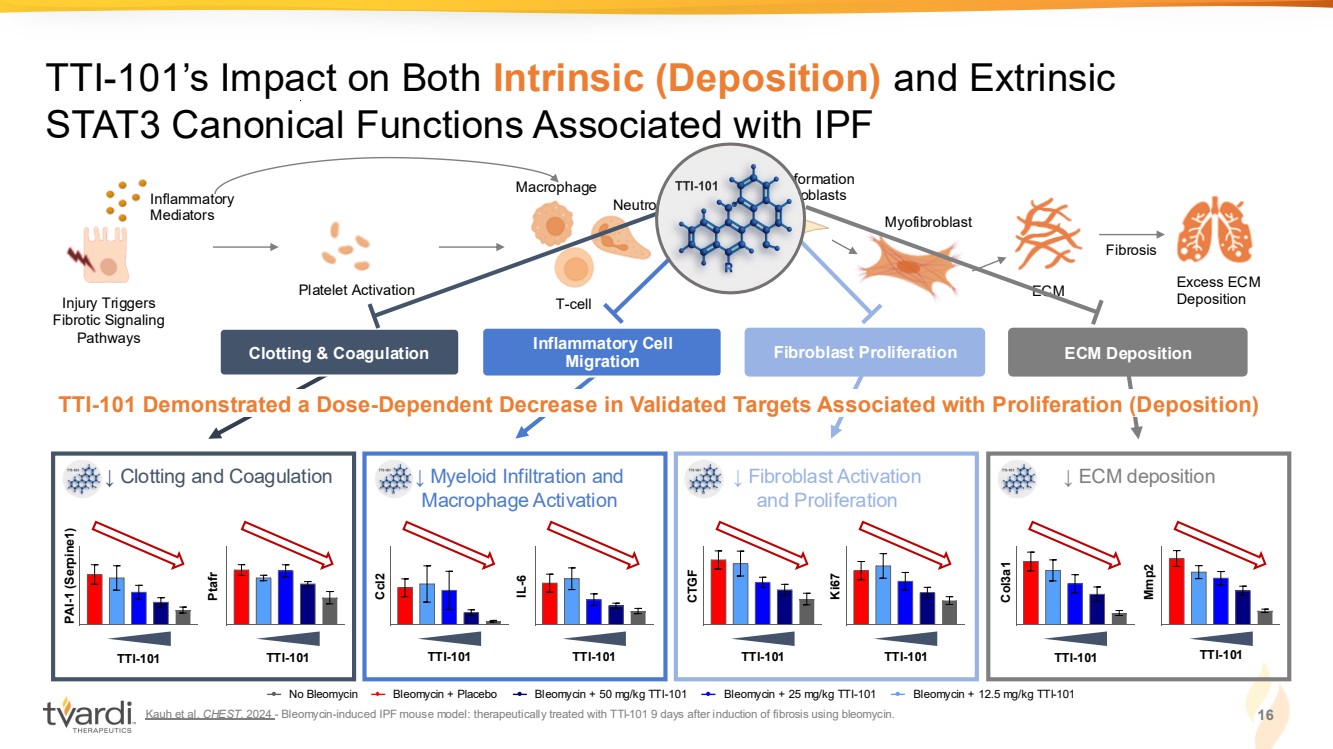
| Kauh et al. CHEST. 2024 - Bleomycin-induced IPF mouse model: therapeutically treated with TTI-101 9 days after induction of fibrosis using bleomycin. 16 TTI-101’s Impact on Both Intrinsic (Deposition) and Extrinsic STAT3 Canonical Functions Associated with IPF Injury Triggers Fibrotic Signaling Pathways Inflammatory Mediators ECM Fibrosis Excess ECM Deposition Macrophage Neutrophil T-cell Platelet Activation Myofibroblast Triggers formation of myofibroblasts Clotting & Coagulation Inflammatory Cell Migration Fibroblast Proliferation ECM Deposition ↓ Fibroblast Activation ↓ ECM deposition and Proliferation TTI-101 CTGF TTI-101 Ki67 Col3a1 Mmp2 TTI-101 TTI-101 TTI-101 Demonstrated a Dose-Dependent Decrease in Validated Targets Associated with Proliferation (Deposition) No Bleomycin Bleomycin + Placebo Bleomycin + 50 mg/kg TTI-101 Bleomycin + 25 mg/kg TTI-101 Bleomycin + 12.5 mg/kg TTI-101 ↓ Myeloid Infiltration and Macrophage Activation TTI-101 Ccl2 TTI-101 IL-6 TTI-101 ↓ Clotting and Coagulation PAI-1 (Serpine1) Ptafr TTI-101 |
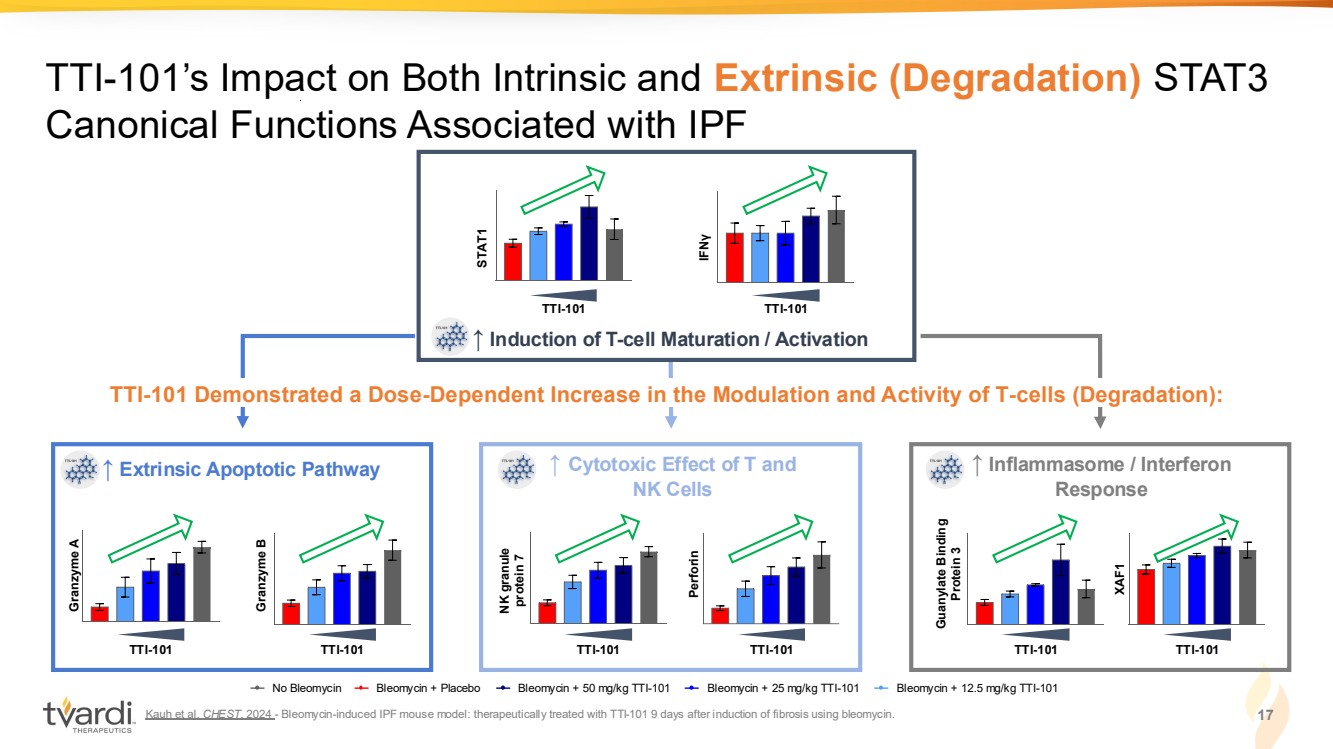
| Kauh et al. CHEST. 2024 - Bleomycin-induced IPF mouse model: therapeutically treated with TTI-101 9 days after induction of fibrosis using bleomycin. 17 TTI-101’s Impact on Both Intrinsic and Extrinsic (Degradation) STAT3 Canonical Functions Associated with IPF TTI-101 Demonstrated a Dose-Dependent Increase in the Modulation and Activity of T-cells (Degradation): No Bleomycin Bleomycin + Placebo Bleomycin + 50 mg/kg TTI-101 Bleomycin + 25 mg/kg TTI-101 Bleomycin + 12.5 mg/kg TTI-101 ↑ Induction of T-cell Maturation / Activation TTI-101 TTI-101 STAT1 IFN γ TTI-101 Perforin NK granule protein 7 TTI-101 ↑ Cytotoxic Effect of T and NK Cells ↑ Extrinsic Apoptotic Pathway Granzyme A Granzyme B TTI-101 TTI-101 Guanylate Binding Protein 3 XAF1 TTI-101 TTI-101 ↑ Inflammasome / Interferon Response |
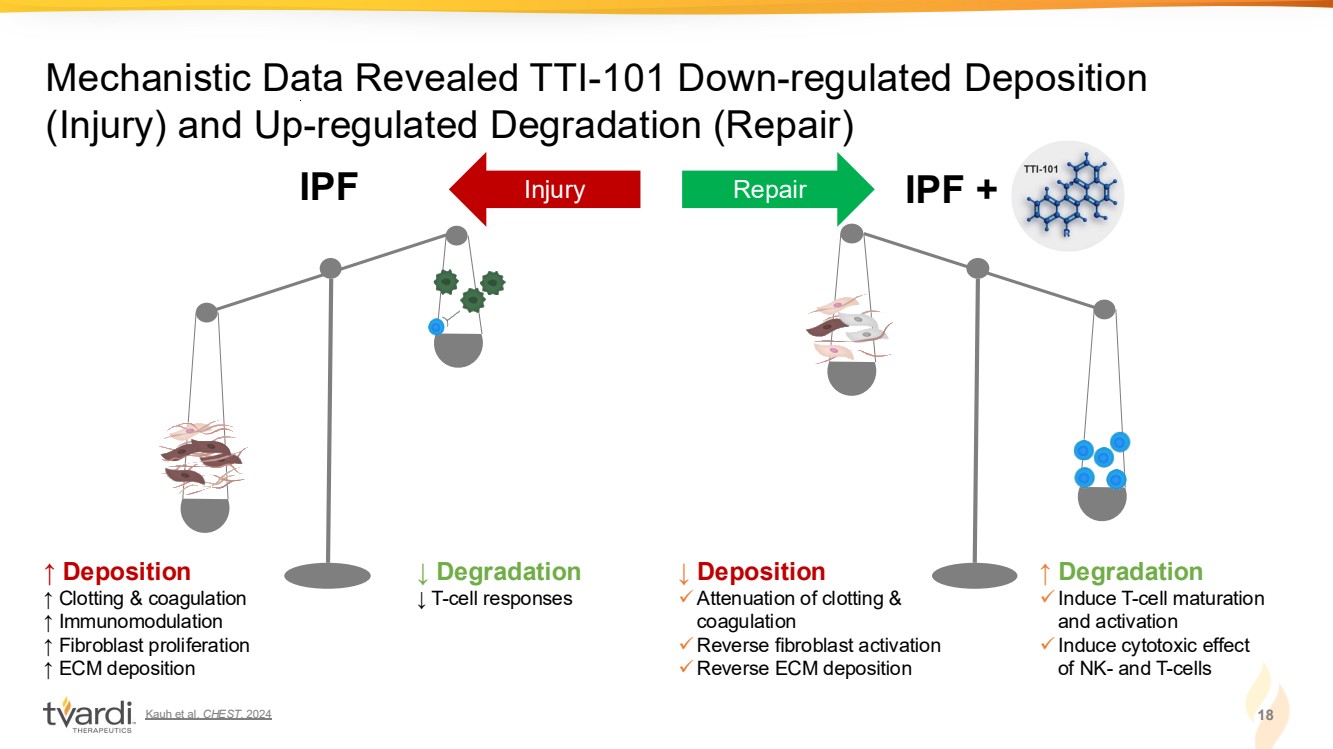
| Kauh et al. CHEST. 2024 18 Mechanistic Data Revealed TTI-101 Down-regulated Deposition (Injury) and Up-regulated Degradation (Repair) ↑ Deposition ↑ Clotting & coagulation ↑ Immunomodulation ↑ Fibroblast proliferation ↑ ECM deposition IPF ↓ Degradation ↓ T-cell responses ↓ Deposition ✓Attenuation of clotting & coagulation ✓Reverse fibroblast activation ✓Reverse ECM deposition IPF + ↑ Degradation ✓Induce T-cell maturation and activation ✓Induce cytotoxic effect of NK- and T-cells Injury Repair TUMORIGENESIS & DRUG RESISTANCE |
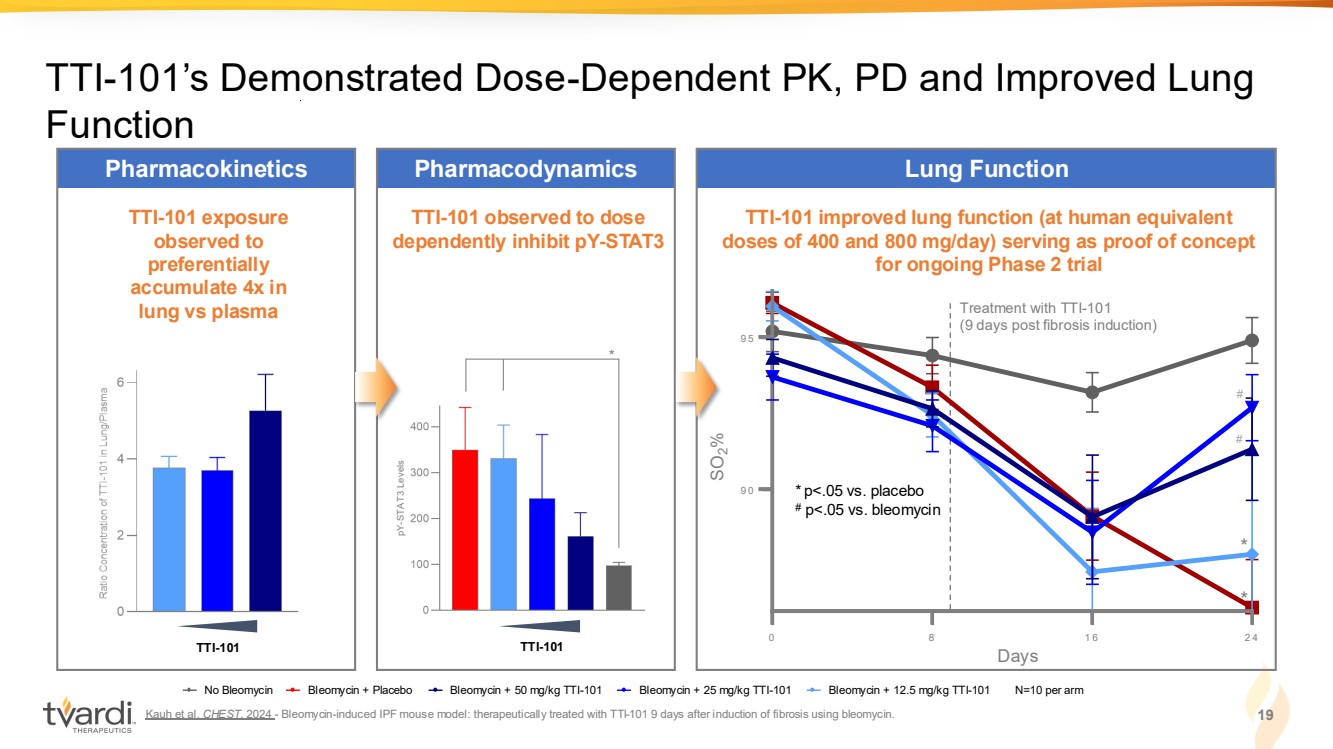
| Kauh et al. CHEST. 2024 - Bleomycin-induced IPF mouse model: therapeutically treated with TTI-101 9 days after induction of fibrosis using bleomycin. 19 TTI-101’s Demonstrated Dose-Dependent PK, PD and Improved Lung Function TTI-101 exposure observed to preferentially accumulate 4x in lung vs plasma Pharmacokinetics TTI-101 observed to dose dependently inhibit pY-STAT3 Pharmacodynamics TTI-101 improved lung function (at human equivalent doses of 400 and 800 mg/day) serving as proof of concept for ongoing Phase 2 trial Lung Function TTI-101 TTI-101 pY-STAT3 Levels * * # 0 8 1 6 2 4 9 0 9 5 Days SO %2 0 8 1 6 2 4 9 0 9 5 Days SO %2 0 8 1 6 2 4 9 0 9 5 Days SO %2 0 8 1 6 2 4 9 0 9 5 Days SO %2 * p<.05 vs. placebo # p<.05 vs. bleomycin No Bleomycin Bleomycin + Placebo Bleomycin + 50 mg/kg TTI-101 Bleomycin + 25 mg/kg TTI-101 Bleomycin + 12.5 mg/kg TTI-101 N=10 per arm Days Treatment with TTI-101 (9 days post fibrosis induction) # |
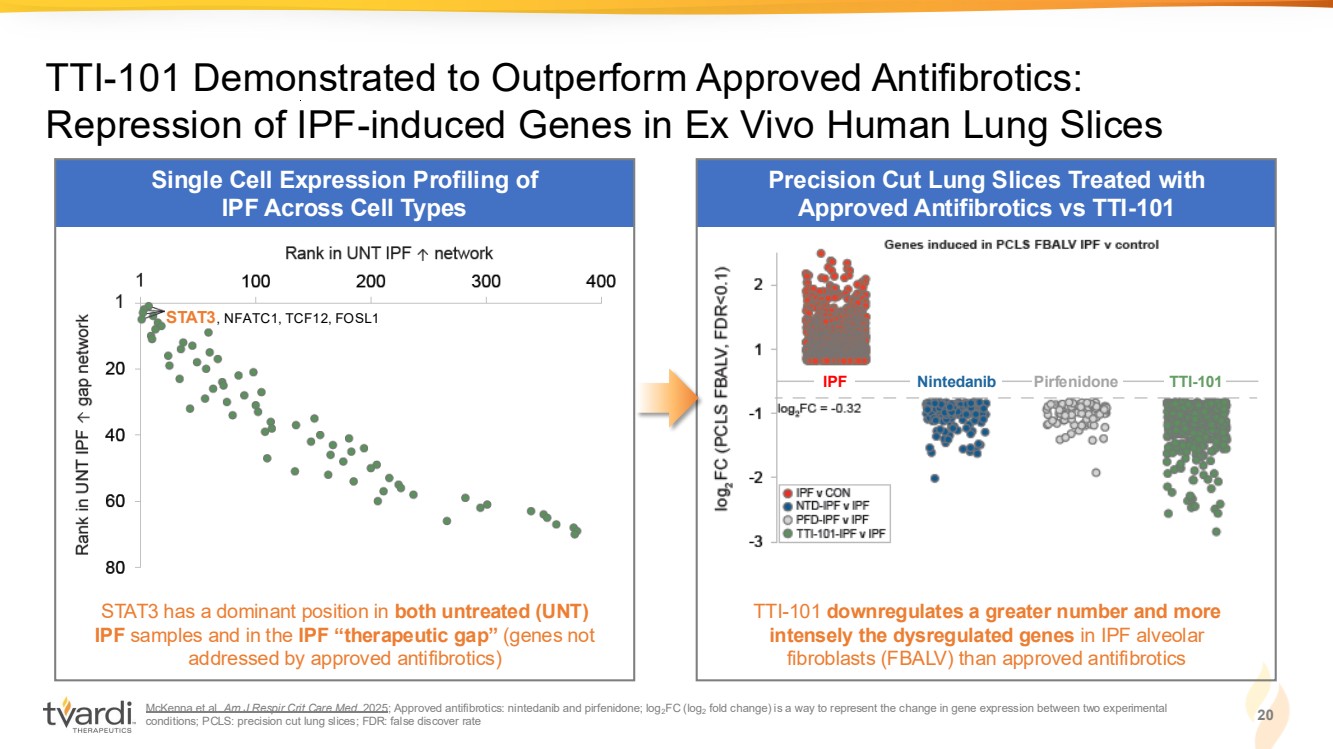
| Single Cell Expression Profiling of IPF Across Cell Types McKenna et al. Am J Respir Crit Care Med. 2025; Approved antifibrotics: nintedanib and pirfenidone; log2FC (log2 fold change) is a way to represent the change in gene expression between two experimental conditions; PCLS: precision cut lung slices; FDR: false discover rate 20 TTI-101 Demonstrated to Outperform Approved Antifibrotics: Repression of IPF-induced Genes in Ex Vivo Human Lung Slices Precision Cut Lung Slices Treated with Approved Antifibrotics vs TTI-101 STAT3 has a dominant position in both untreated (UNT) IPF samples and in the IPF “therapeutic gap” (genes not addressed by approved antifibrotics) TTI-101 downregulates a greater number and more intensely the dysregulated genes in IPF alveolar fibroblasts (FBALV) than approved antifibrotics IPF Nintedanib Pirfenidone TTI-101 STAT3, NFATC1, TCF12, FOSL1 |
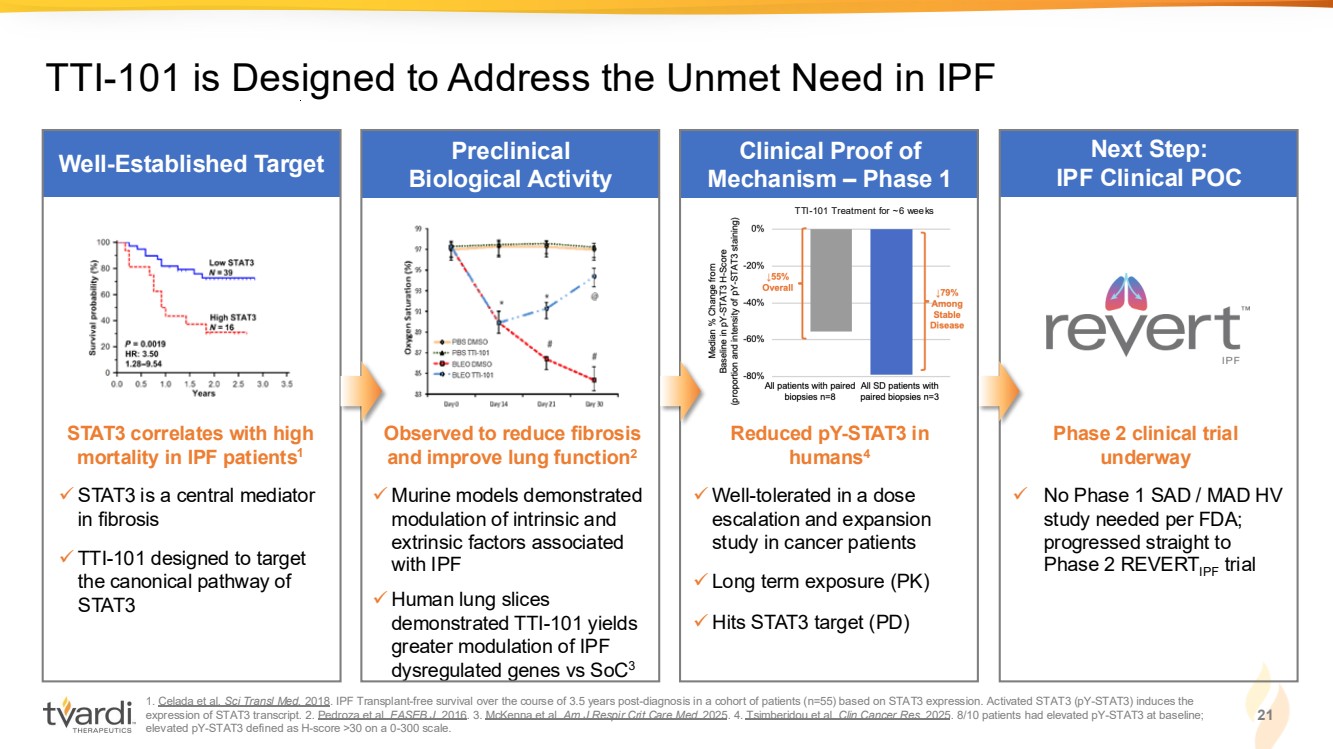
| 1. Celada et al. Sci Transl Med. 2018. IPF Transplant-free survival over the course of 3.5 years post-diagnosis in a cohort of patients (n=55) based on STAT3 expression. Activated STAT3 (pY-STAT3) induces the expression of STAT3 transcript. 2. Pedroza et al. FASEB J. 2016. 3. McKenna et al. Am J Respir Crit Care Med. 2025. 4. Tsimberidou et al. Clin Cancer Res. 2025. 8/10 patients had elevated pY-STAT3 at baseline; elevated pY-STAT3 defined as H-score >30 on a 0-300 scale. 21 Clinical Proof of Mechanism – Phase 1 Well-Established Target Preclinical Biological Activity Next Step: IPF Clinical POC ✓ STAT3 is a central mediator in fibrosis ✓ TTI-101 designed to target the canonical pathway of STAT3 STAT3 correlates with high mortality in IPF patients1 ✓ Well-tolerated in a dose escalation and expansion study in cancer patients ✓ Long term exposure (PK) ✓ Hits STAT3 target (PD) Reduced pY-STAT3 in humans4 ✓ No Phase 1 SAD / MAD HV study needed per FDA; progressed straight to Phase 2 REVERTIPF trial Phase 2 clinical trial underway ✓ Murine models demonstrated modulation of intrinsic and extrinsic factors associated with IPF ✓ Human lung slices demonstrated TTI-101 yields greater modulation of IPF dysregulated genes vs SoC3 Observed to reduce fibrosis and improve lung function2 TTI-101 is Designed to Address the Unmet Need in IPF TTI-101 Treatment for ~6 weeks |
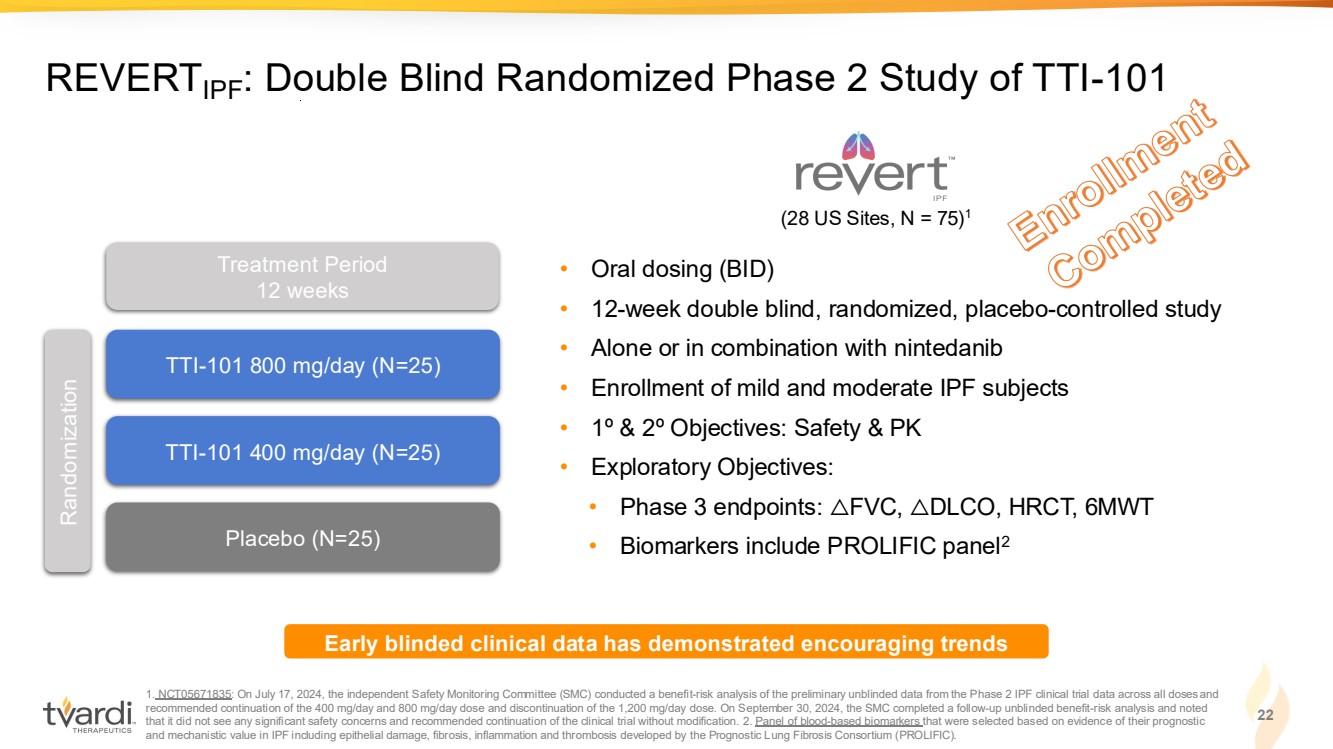
| Enrollment Completed 1. NCT05671835: On July 17, 2024, the independent Safety Monitoring Committee (SMC) conducted a benefit-risk analysis of the preliminary unblinded data from the Phase 2 IPF clinical trial data across all doses and recommended continuation of the 400 mg/day and 800 mg/day dose and discontinuation of the 1,200 mg/day dose. On September 30, 2024, the SMC completed a follow-up unblinded benefit-risk analysis and noted that it did not see any significant safety concerns and recommended continuation of the clinical trial without modification. 2. Panel of blood-based biomarkers that were selected based on evidence of their prognostic and mechanistic value in IPF including epithelial damage, fibrosis, inflammation and thrombosis developed by the Prognostic Lung Fibrosis Consortium (PROLIFIC). 22 REVERTIPF: Double Blind Randomized Phase 2 Study of TTI-101 • Oral dosing (BID) • 12-week double blind, randomized, placebo-controlled study • Alone or in combination with nintedanib • Enrollment of mild and moderate IPF subjects • 1º & 2º Objectives: Safety & PK • Exploratory Objectives: • Phase 3 endpoints: △FVC, △DLCO, HRCT, 6MWT • Biomarkers include PROLIFIC panel2 TTI-101 800 mg/day (N=25) TTI-101 400 mg/day (N=25) Placebo (N=25) Randomization Treatment Period 12 weeks Early blinded clinical data has demonstrated encouraging trends (28 US Sites, N = 75)1 |
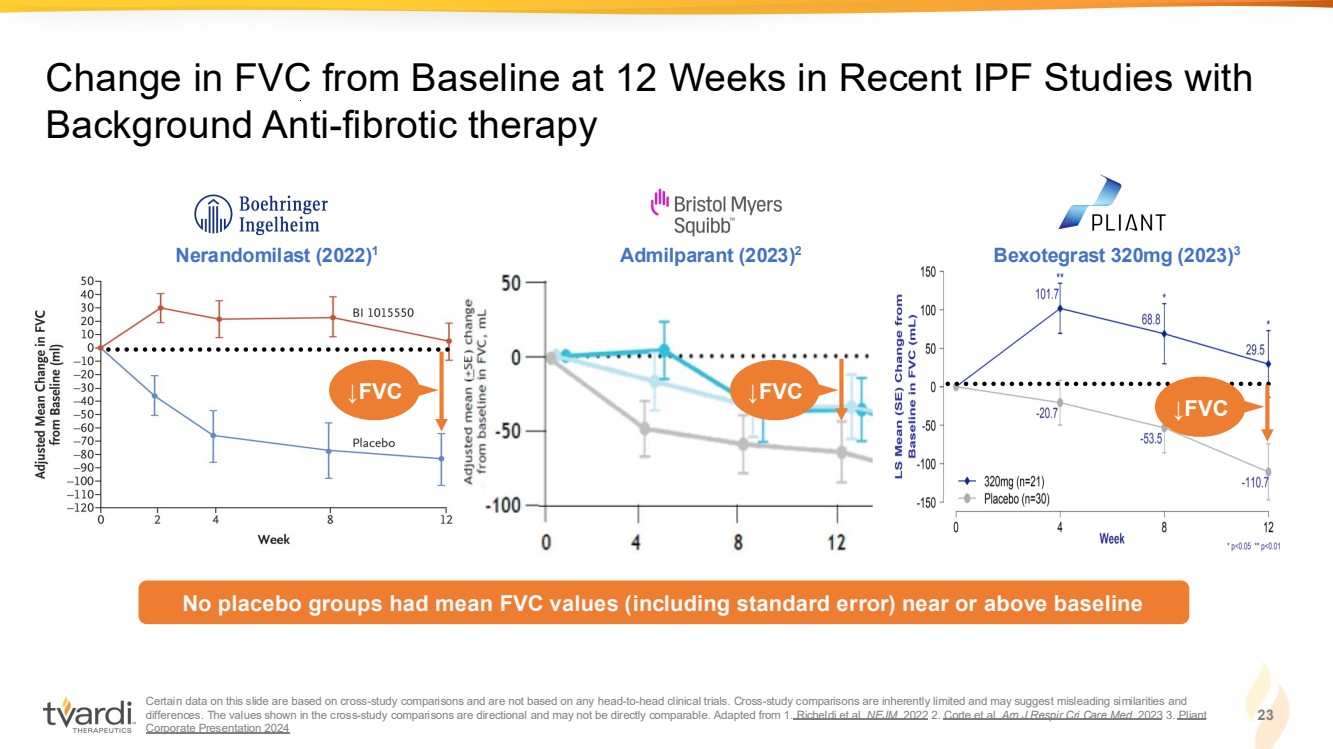
| Certain data on this slide are based on cross-study comparisons and are not based on any head-to-head clinical trials. Cross-study comparisons are inherently limited and may suggest misleading similarities and differences. The values shown in the cross-study comparisons are directional and may not be directly comparable. Adapted from 1. Richeldi et al. NEJM. 2022 2. Corte et al. Am J Respir Cri Care Med. 2023 3. Pliant Corporate Presentation 2024 23 Change in FVC from Baseline at 12 Weeks in Recent IPF Studies with Background Anti-fibrotic therapy Bexotegrast 320mg (2023) A 3 dmilparant (2023) Nerandomilast 2 (2022)1 No placebo groups had mean FVC values (including standard error) near or above baseline ↓FVC ↓FVC ↓FVC |
| *Trial is blinded to Sponsor, investigators and patients; unknown exposure to 400mg/day of TTI-101, 800mg/day of TTI-101 or placebo. Data as of September 2024. Preliminary data for efficacy-evaluable patients defined as: patients with acceptable baseline and at least one on-treatment pulmonary function test (PFT). At the time of analysis, the absolute FVCs comparing percent change from baseline to last visit on treatment were available for the following timepoints: 12 weeks (n=19); 8 weeks (n=9); 4 weeks (n=10). Increase FVC levels from baseline defined as >1% change from baseline FVC; stable FVC levels from baseline defined as −1% to 1% change from baseline FVC; decrease in FVC levels from baseline defined as <−1% change from baseline FVC. Due to the preliminary and blinded nature of the data, this interim data set was not subject to the standard quality control measures typically associated with final clinical trial results. 24 REVERTIPF: Preliminary Blinded Percent Change in FVC from Baseline (N=38) Stable −1% to 1% ~ −35 to +35ml Decrease <−1% Increase >1% 34% Increase 26% Stable 39% Decrease ↓ in FVC from baseline ↑ in FVC from baseline Trial Design: 1:1:1 Randomization TTI-101 800 mg/day (N=25) TTI-101 400 mg/day (N=25) Placebo (N=25) Natural course of IPF: decline in FVC Blinded data suggests >50% of subjects have FVC values near or above baseline 0 Near or above baseline is clinically significant |
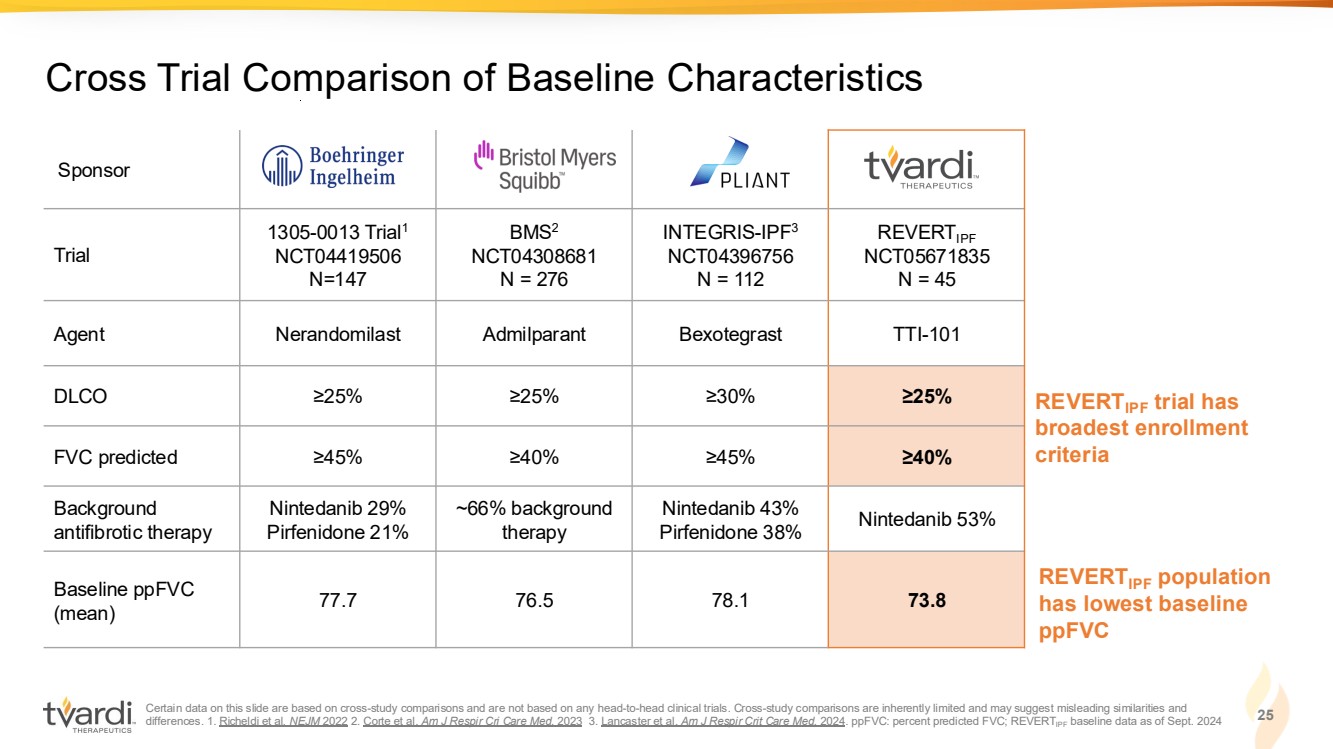
| Certain data on this slide are based on cross-study comparisons and are not based on any head-to-head clinical trials. Cross-study comparisons are inherently limited and may suggest misleading similarities and differences. 1. Richeldi et al. NEJM 2022 2. Corte et al. Am J Respir Cri Care Med. 2023 3. Lancaster et al. Am J Respir Crit Care Med. 2024. ppFVC: percent predicted FVC; REVERTIPF baseline data as of Sept. 2024 25 Cross Trial Comparison of Baseline Characteristics Sponsor Trial 1305-0013 Trial1 NCT04419506 N=147 BMS2 NCT04308681 N = 276 INTEGRIS-IPF3 NCT04396756 N = 112 REVERTIPF NCT05671835 N = 45 Agent Nerandomilast Admilparant Bexotegrast TTI-101 DLCO ≥25% ≥25% ≥30% ≥25% FVC predicted ≥45% ≥40% ≥45% ≥40% Background antifibrotic therapy Nintedanib 29% Pirfenidone 21% ~66% background therapy Nintedanib 43% Pirfenidone 38% Nintedanib 53% Baseline ppFVC (mean) 77.7 76.5 78.1 73.8 REVERTIPF trial has broadest enrollment criteria REVERTIPF population has lowest baseline ppFVC |
| 26 Key Takeaways: TTI-101 in IPF Driving inhibition of STAT3 activation to address both IPF disease pathologies (downregulating deposition and upregulating degradation) Ongoing Phase 2 trial → preliminary blinded data demonstrated ~50% of participants near or above baseline FVC Results from ongoing Phase 2 REVERTIPF trial expected in 4Q:2025 Compelling and validated target → central mediator in fibrosis STAT3: Well-Established Biology Differentiated Approach Encouraging Clinical Activity Near-Term Clinical Milestone |
| TTI-101 in HCC |
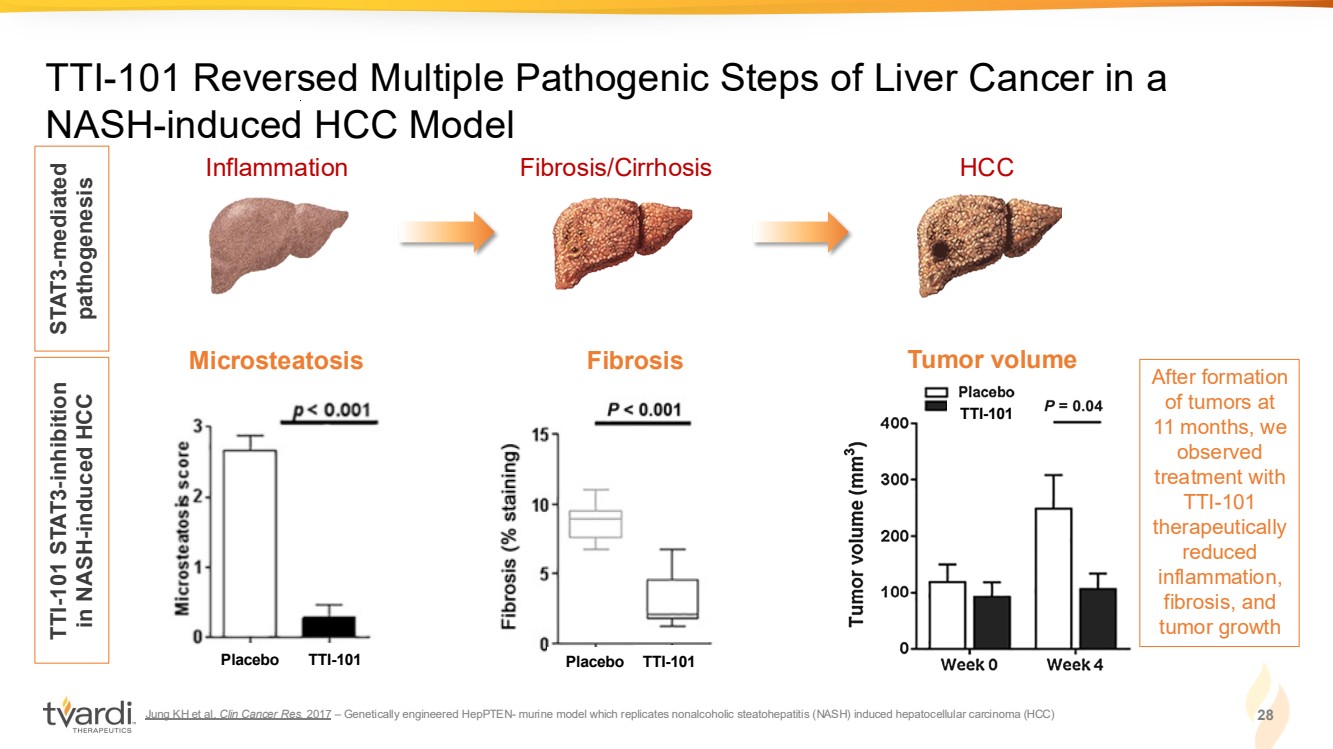
| Jung KH et al. Clin Cancer Res. 2017 – Genetically engineered HepPTEN- murine model which replicates nonalcoholic steatohepatitis (NASH) induced hepatocellular carcinoma (HCC) 28 TTI-101 Reversed Multiple Pathogenic Steps of Liver Cancer in a NASH-induced HCC Model Inflammation Fibrosis/Cirrhosis HCC STAT3-mediated pathogenesis TTI-101 STAT3-inhibition in NASH-induced HCC TTI-101 Microsteatosis Tumor volume Placebo TTI-101 TTI-101 Fibrosis Placebo TTI-101 After formation of tumors at 11 months, we observed treatment with TTI-101 therapeutically reduced inflammation, fibrosis, and tumor growth |
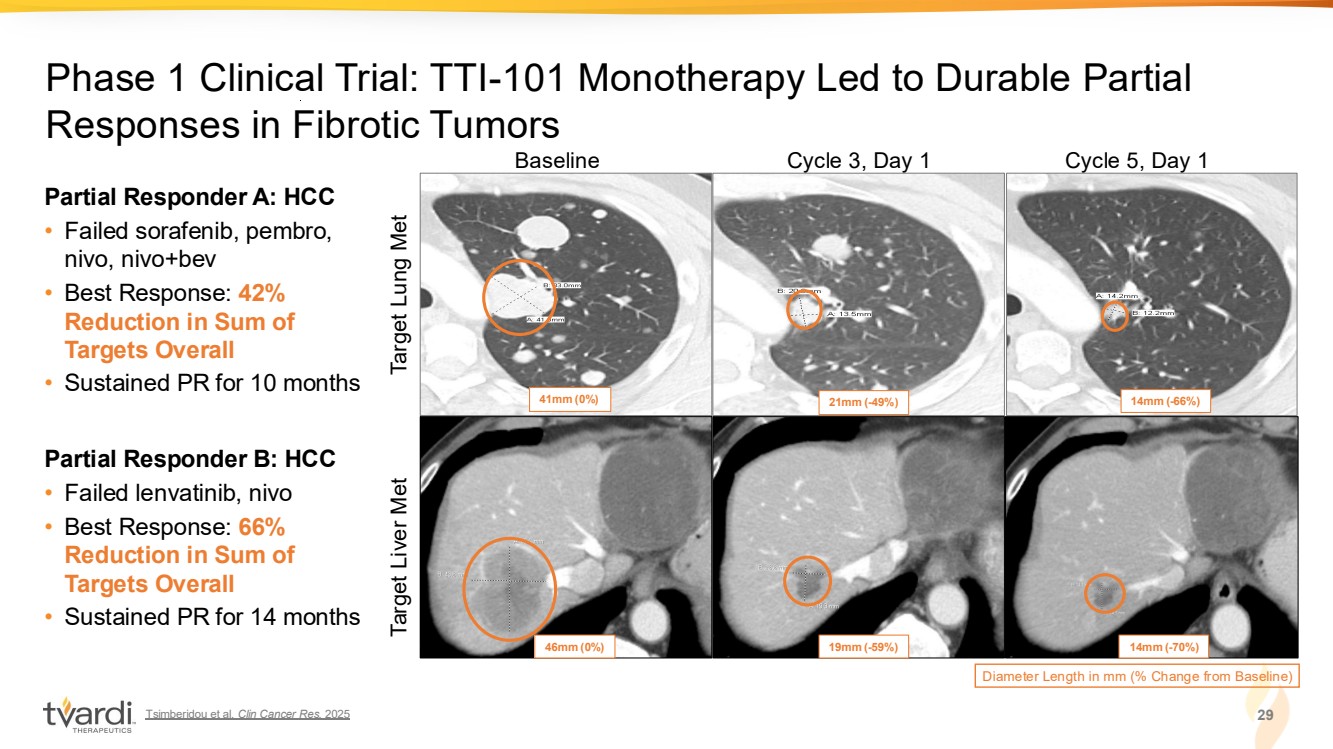
| Tsimberidou et al. Clin Cancer Res. 2025 29 Phase 1 Clinical Trial: TTI-101 Monotherapy Led to Durable Partial Responses in Fibrotic Tumors Baseline Cycle 3, Day 1 Cycle 5, Day 1 Diameter Length in mm (% Change from Baseline) 41mm (0%) 21mm (-49%) 14mm (-66%) 46mm (0%) 19mm (-59%) 14mm (-70%) Target Lung Met Target Liver Met Partial Responder A: HCC • Failed sorafenib, pembro, nivo, nivo+bev • Best Response: 42% Reduction in Sum of Targets Overall • Sustained PR for 10 months Partial Responder B: HCC • Failed lenvatinib, nivo • Best Response: 66% Reduction in Sum of Targets Overall • Sustained PR for 14 months |
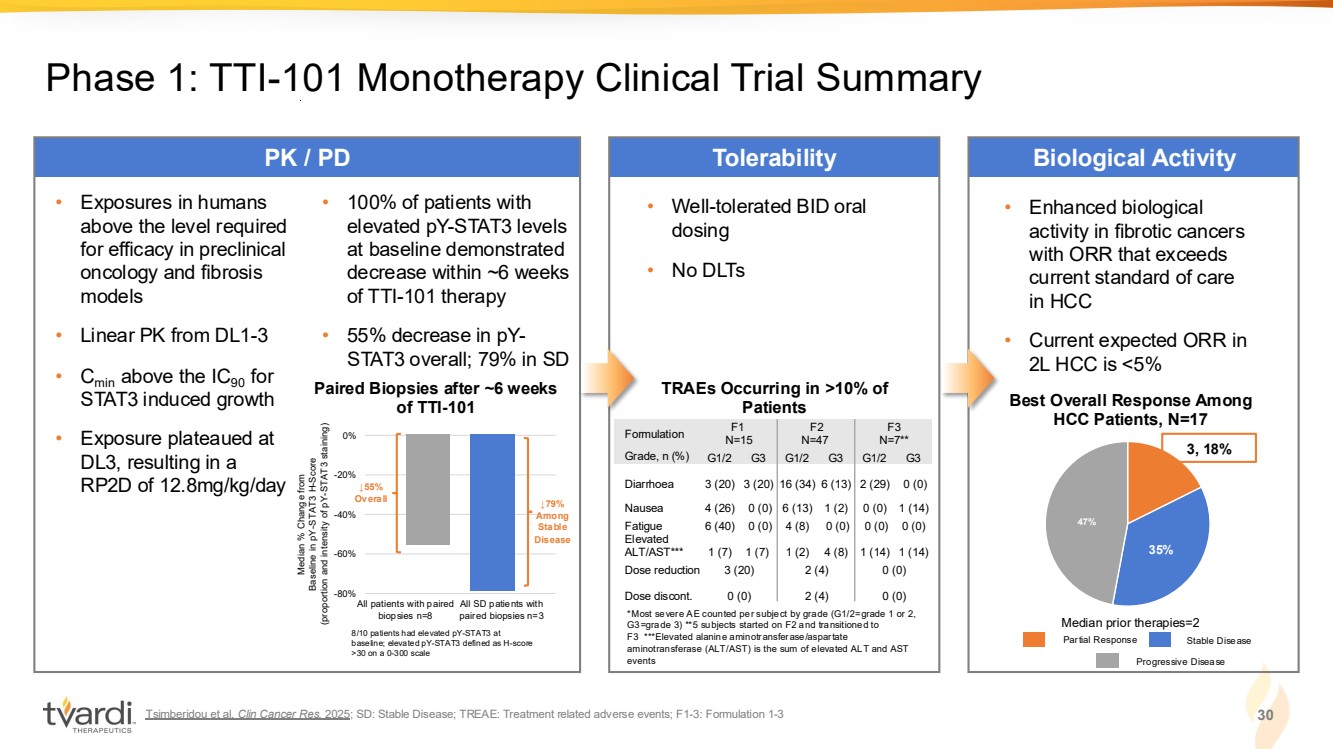
| Tsimberidou et al. Clin Cancer Res. 2025; SD: Stable Disease; TREAE: Treatment related adverse events; F1-3: Formulation 1-3 30 Phase 1: TTI-101 Monotherapy Clinical Trial Summary PK / PD Tolerability Biological Activity • Well-tolerated BID oral dosing • No DLTs *Most severe AE counted per subject by grade (G1/2=grade 1 or 2, G3=grade 3) **5 subjects started on F2 and transitioned to F3 ***Elevated alanine aminotransferase/aspartate aminotransferase (ALT/AST) is the sum of elevated ALT and AST events TRAEs Occurring in >10% of Patients • Exposures in humans above the level required for efficacy in preclinical oncology and fibrosis models • Linear PK from DL1-3 • Cmin above the IC90 for STAT3 induced growth • Exposure plateaued at DL3, resulting in a RP2D of 12.8mg/kg/day Median % Change from Baseline in pY-STAT3 H-Score (proportion and intensity of pY-STAT3 staining) All patients with paired biopsies n=8 All SD patients with paired biopsies n=3 -80% -60% -40% -20% 0% ↓79% Among Stable Disease 8/10 patients had elevated pY-STAT3 at baseline; elevated pY-STAT3 defined as H-score >30 on a 0-300 scale ↓55% Overall • 100% of patients with elevated pY-STAT3 levels at baseline demonstrated decrease within ~6 weeks of TTI-101 therapy • 55% decrease in pY-STAT3 overall; 79% in SD • Enhanced biological activity in fibrotic cancers with ORR that exceeds current standard of care in HCC • Current expected ORR in 2L HCC is <5% 35% 47% 3, 18% Best Overall Response Among HCC Patients, N=17 Median prior therapies=2 Paired Biopsies after ~6 weeks of TTI-101 Partial Response Stable Disease Progressive Disease Formulation F1 N=15 F2 N=47 F3 N=7** Grade, n (%) G1/2 G3 G1/2 G3 G1/2 G3 Diarrhoea 3 (20) 3 (20) 16 (34) 6 (13) 2 (29) 0 (0) Nausea 4 (26) 0 (0) 6 (13) 1 (2) 0 (0) 1 (14) Fatigue 6 (40) 0 (0) 4 (8) 0 (0) 0 (0) 0 (0) Elevated ALT/AST*** 1 (7) 1 (7) 1 (2) 4 (8) 1 (14) 1 (14) Dose reduction 3 (20) 2 (4) 0 (0) Dose discont. 0 (0) 2 (4) 0 (0) |
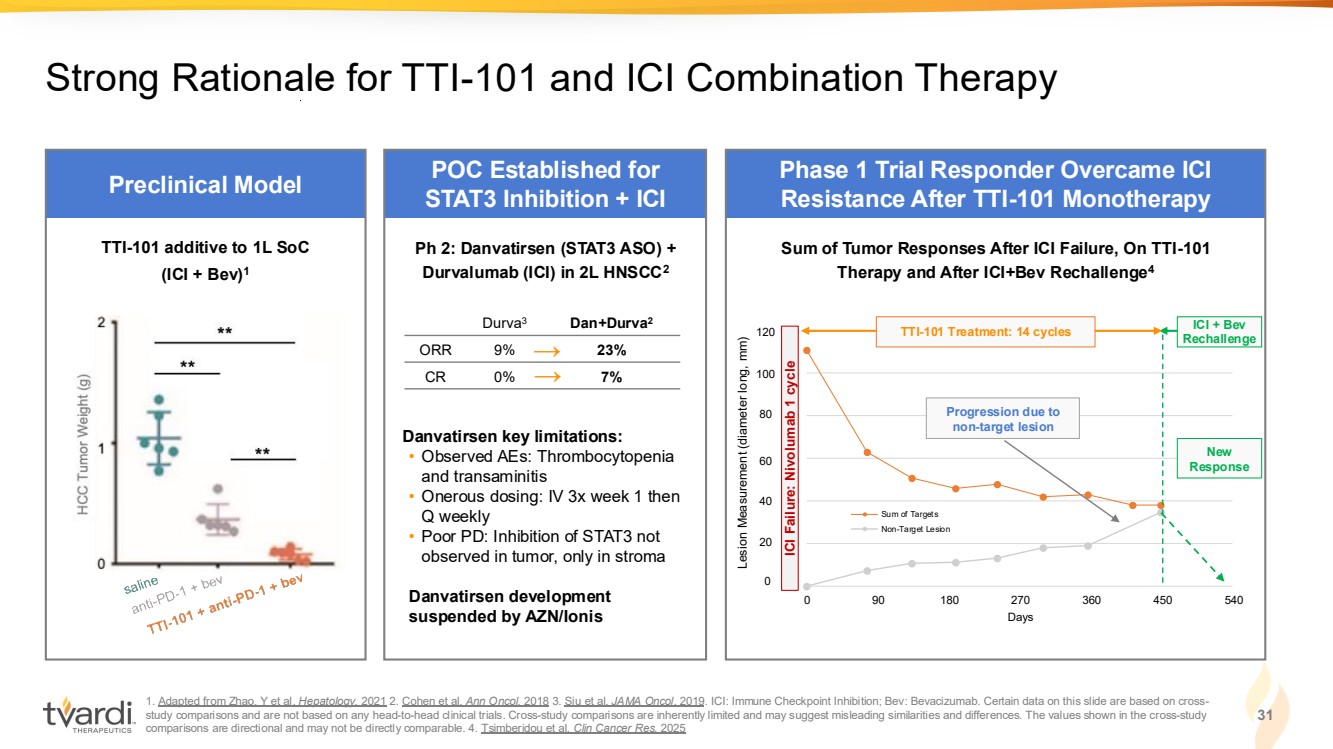
| 1. Adapted from Zhao, Y et al. Hepatology. 2021 2. Cohen et al. Ann Oncol. 2018 3. Siu et al. JAMA Oncol. 2019. ICI: Immune Checkpoint Inhibition; Bev: Bevacizumab. Certain data on this slide are based on cross-study comparisons and are not based on any head-to-head clinical trials. Cross-study comparisons are inherently limited and may suggest misleading similarities and differences. The values shown in the cross-study comparisons are directional and may not be directly comparable. 4. Tsimberidou et al. Clin Cancer Res. 2025 31 Strong Rationale for TTI-101 and ICI Combination Therapy 0 20 40 60 80 100 120 0 90 180 270 360 450 540 Days Sum of Targets Non-Target Lesion Preclinical Model POC Established for STAT3 Inhibition + ICI Phase 1 Trial Responder Overcame ICI Resistance After TTI-101 Monotherapy → → Ph 2: Danvatirsen (STAT3 ASO) + Durvalumab (ICI) in 2L HNSCC2 Danvatirsen key limitations: • Observed AEs: Thrombocytopenia and transaminitis • Onerous dosing: IV 3x week 1 then Q weekly • Poor PD: Inhibition of STAT3 not observed in tumor, only in stroma Danvatirsen development suspended by AZN/Ionis Durva3 Dan+Durva2 ORR 9% 23% CR 0% 7% TTI-101 additive to 1L SoC (ICI + Bev)1 Progression due to non-target lesion New Response ICI + Bev Rechallenge Sum of Tumor Responses After ICI Failure, On TTI-101 Therapy and After ICI+Bev Rechallenge4 0 20 40 60 80 100 120 ICI Failure: Nivolumab 1 cycle Lesion Measurement (diameter long, mm) TTI-101 Treatment: 14 cycles |
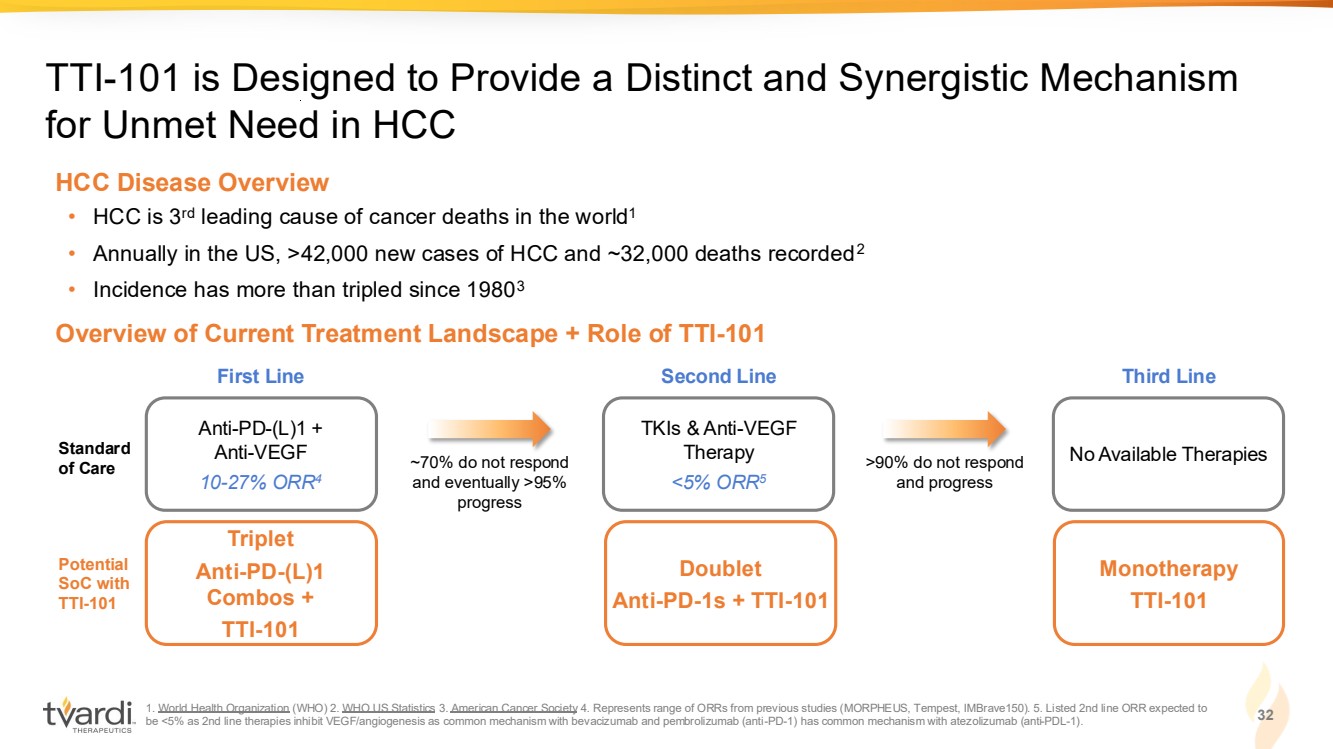
| 1. World Health Organization (WHO) 2. WHO US Statistics 3. American Cancer Society 4. Represents range of ORRs from previous studies (MORPHEUS, Tempest, IMBrave150). 5. Listed 2nd line ORR expected to be <5% as 2nd line therapies inhibit VEGF/angiogenesis as common mechanism with bevacizumab and pembrolizumab (anti-PD-1) has common mechanism with atezolizumab (anti-PDL-1). 32 TTI-101 is Designed to Provide a Distinct and Synergistic Mechanism for Unmet Need in HCC Overview of Current Treatment Landscape + Role of TTI-101 • HCC is 3rd leading cause of cancer deaths in the world1 • Annually in the US, >42,000 new cases of HCC and ~32,000 deaths recorded2 • Incidence has more than tripled since 19803 HCC Disease Overview Triplet Anti-PD-(L)1 Combos + TTI-101 Potential SoC with TTI-101 Doublet Anti-PD-1s + TTI-101 Monotherapy TTI-101 Anti-PD-(L)1 + Anti-VEGF 10-27% ORR4 Standard of Care First Line TKIs & Anti-VEGF Therapy <5% ORR5 Second Line No Available Therapies Third Line ~70% do not respond and eventually >95% progress >90% do not respond and progress |
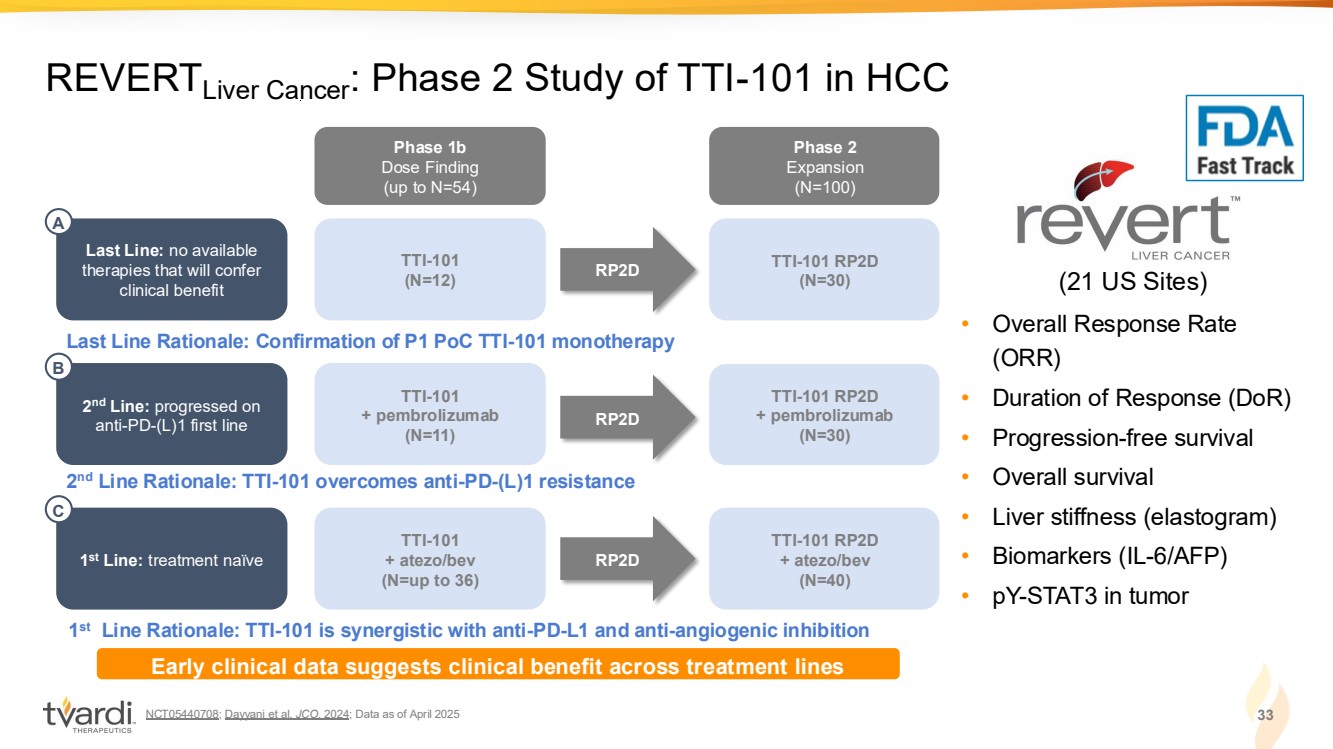
| NCT05440708; Dayyani et al. JCO. 2024; Data as of April 2025 33 REVERTLiver Cancer: Phase 2 Study of TTI-101 in HCC • Overall Response Rate (ORR) • Duration of Response (DoR) • Progression-free survival • Overall survival • Liver stiffness (elastogram) • Biomarkers (IL-6/AFP) • pY-STAT3 in tumor (21 US Sites) Phase 1b Dose Finding (up to N=54) Phase 2 Expansion (N=100) TTI-101 RP2D (N=30) TTI-101 (N=12) Last Line: no available therapies that will confer clinical benefit RP2D Last Line Rationale: Confirmation of P1 PoC TTI-101 monotherapy A TTI-101 RP2D + pembrolizumab (N=30) TTI-101 + pembrolizumab (N=11) RP2D 2 nd Line Rationale: TTI-101 overcomes anti-PD-(L)1 resistance 2 nd Line: progressed on anti-PD-(L)1 first line B TTI-101 RP2D + atezo/bev (N=40) TTI-101 + atezo/bev (N=up to 36) RP2D 1 st Line Rationale: TTI-101 is synergistic with anti-PD-L1 and anti-angiogenic inhibition 1 st Line: treatment naïve C Early clinical data suggests clinical benefit across treatment lines |
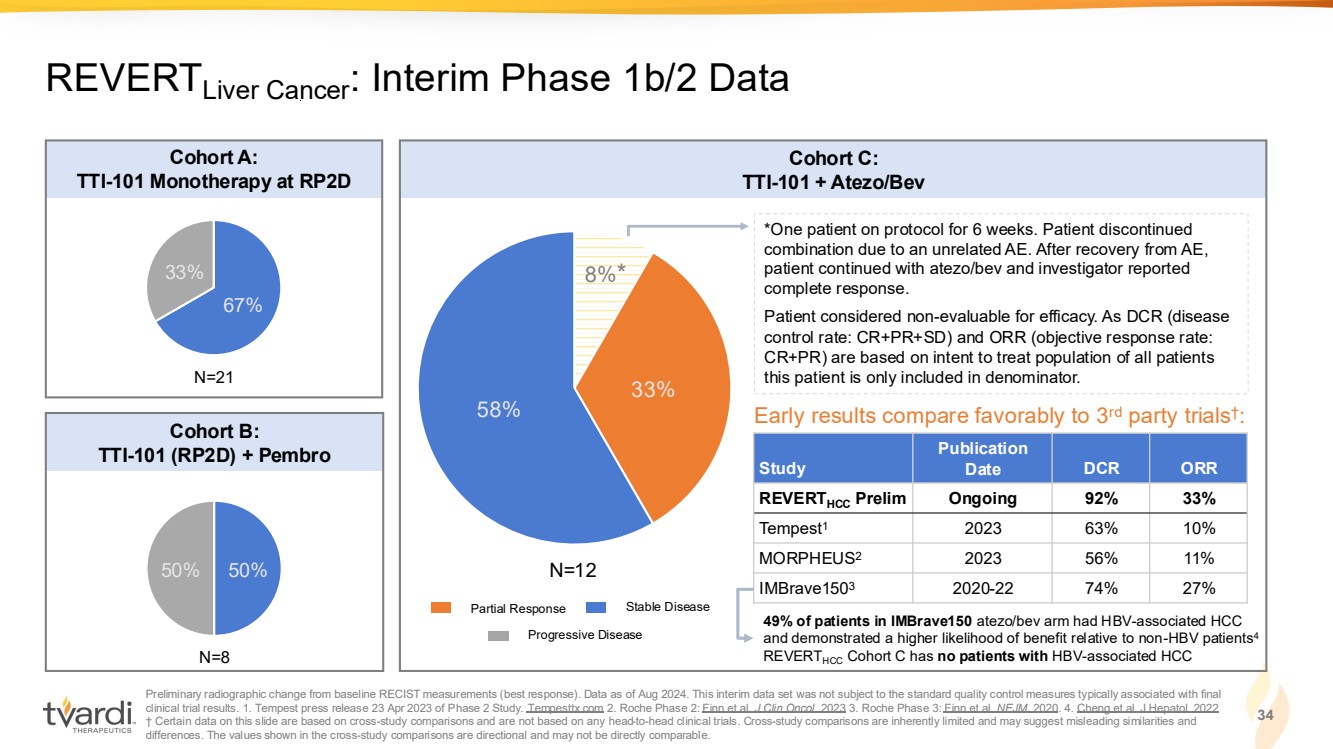
| Preliminary radiographic change from baseline RECIST measurements (best response). Data as of Aug 2024. This interim data set was not subject to the standard quality control measures typically associated with final clinical trial results. 1. Tempest press release 23 Apr 2023 of Phase 2 Study. Tempesttx.com 2. Roche Phase 2: Finn et al. J Clin Oncol. 2023 3. Roche Phase 3: Finn et al. NEJM. 2020. 4. Cheng et al. J Hepatol. 2022 † Certain data on this slide are based on cross-study comparisons and are not based on any head-to-head clinical trials. Cross-study comparisons are inherently limited and may suggest misleading similarities and differences. The values shown in the cross-study comparisons are directional and may not be directly comparable. 34 REVERTLiver Cancer: Interim Phase 1b/2 Data 67% 33% 50% 50% 8% 33% 58% Cohort A: TTI-101 Monotherapy at RP2D Cohort B: TTI-101 (RP2D) + Pembro Cohort C: TTI-101 + Atezo/Bev *One patient on protocol for 6 weeks. Patient discontinued combination due to an unrelated AE. After recovery from AE, patient continued with atezo/bev and investigator reported complete response. Patient considered non-evaluable for efficacy. As DCR (disease control rate: CR+PR+SD) and ORR (objective response rate: CR+PR) are based on intent to treat population of all patients N=21 this patient is only included in denominator. N=8 N=12 Study Publication Date DCR ORR REVERTHCC Prelim Ongoing 92% 33% Tempest1 2023 63% 10% MORPHEUS2 2023 56% 11% IMBrave1503 2020-22 74% 27% Early results compare favorably to 3rd party trials† : Partial Response Stable Disease Progressive Disease * 49% of patients in IMBrave150 atezo/bev arm had HBV-associated HCC and demonstrated a higher likelihood of benefit relative to non-HBV patients4 REVERTHCC Cohort C has no patients with HBV-associated HCC |
| 35 Key Takeaways: TTI-101 in HCC Inhibition of STAT3 activation to have dual therapeutic effect on cancer cells – overcoming tumorigenesis and immune suppression Clinically meaningful activity in both monotherapy and combination therapy in areas of unmet need Topline results from ongoing Phase 2 REVERTLIVER CANCER trial expected in 1H:2026 STAT3 long recognized as prime target in oncology; >95% of patients with HCC have activated STAT3 in their tumors STAT3: Well-Established Biology Differentiated Approach Encouraging Clinical Activity Near-Term Clinical Milestone |
| TTI-109 |
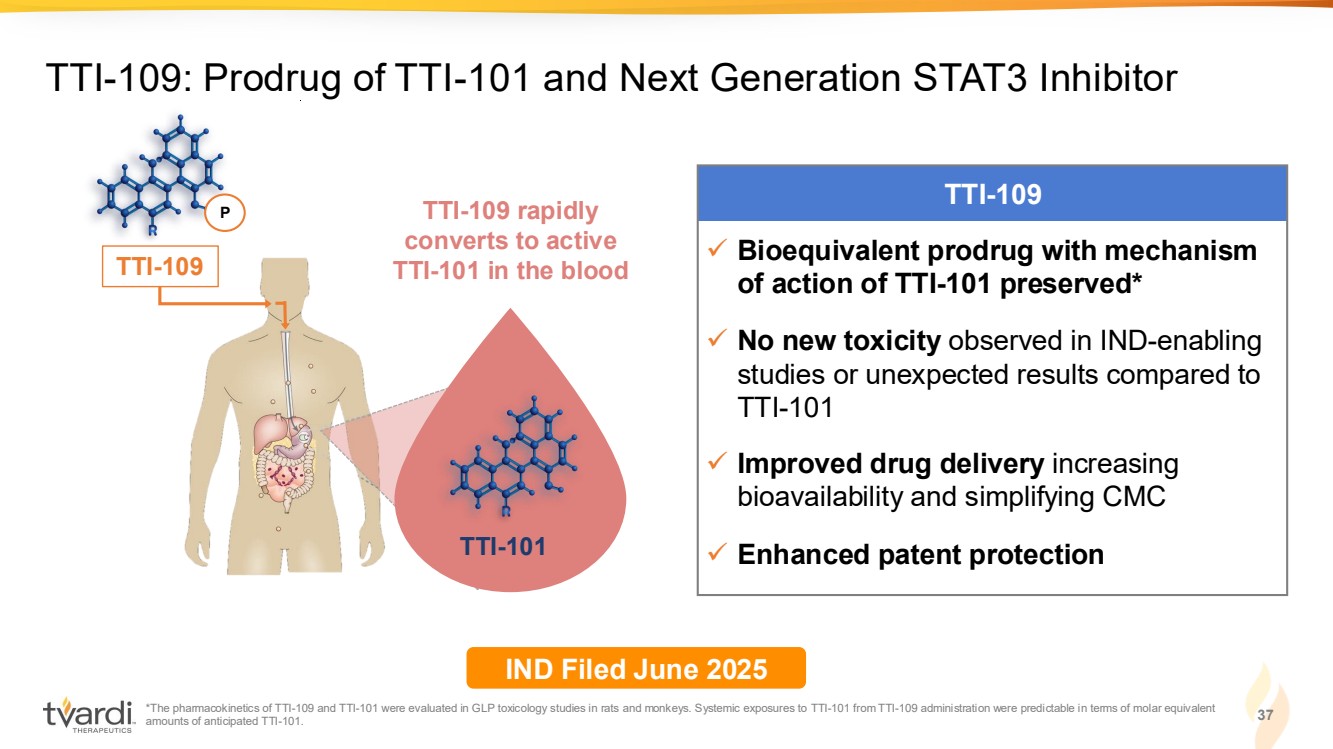
| 37 TTI-109: Prodrug of TTI-101 and Next Generation STAT3 Inhibitor TTI-109 ✓ Bioequivalent prodrug with mechanism of action of TTI-101 preserved* ✓ No new toxicity observed in IND-enabling studies or unexpected results compared to TTI-101 ✓ Improved drug delivery increasing bioavailability and simplifying CMC ✓ Enhanced patent protection TTI-101 TTI-109 rapidly converts to active TTI-101 in the blood P TTI-109 IND Filed June 2025 *The pharmacokinetics of TTI-109 and TTI-101 were evaluated in GLP toxicology studies in rats and monkeys. Systemic exposures to TTI-101 from TTI-109 administration were predictable in terms of molar equivalent amounts of anticipated TTI-101. |
| 1. Idiopathic pulmonary fibrosis. 2. Hepatocellular carcinoma. 3. Investigational new drug. 38 Targeting STAT3: Central Mediator of Fibrosis-Driven Diseases Deep expertise in STAT3 biology Potential to serve as a disease-modifying therapy in IPF1 Well-positioned to differentiate therapeutic impact in HCC2 Multiple near-term data catalysts expected • Unlocking highly-validated, yet historically "undruggable" target within fibrosis-driven diseases • IPF models demonstrated reversal of fibrosis and restoration of lung function • Phase 2 blinded data suggests encouraging trends in lung function • Early signs of response in both mono- and combination therapy from completed and ongoing clinical trials • IPF Phase 2 unblinded data in 4Q:2025 • HCC Phase 1b/2 topline data in 1H:2026 • TTI-109 IND3 filed June 2025 |