| Advancing Dual-Pathway Biologic Candidates Addressing Unmet Needs in Autoimmune and Inflammatory Diseases Jefferies Global Healthcare Conference 2025 Wednesday, June 4, 2025 New York City #JefferiesHealthcare Nasdaq Ticker: ZURA |
| ©2025 Zura Bio Ltd. 2 Forward Looking Statements Disclaimer This communication includes “forward-looking statements” within the meaning of the “safe harbor” provisions of the Private Securities Litigation Reform Act of 1995. Words such as “expect,” “estimate,” “project,” “budget,” “forecast,” “anticipate,” “intend,” “plan,” “may,” “will,” “could,” “should,” “believe,” “predict,” “potential,” “continue,” “strategy,” “future,” “opportunity,” “would,” “seem,” “seek,” “outlook” and the negatives of such terms and similar expressions are intended to identify such forward-looking statements. Forward-looking statements are predictions, projections and other statements about future events that are based on current expectations and assumptions and, as a result, are subject to risks and uncertainties that could cause the actual results to differ materially from the expected results. These forward-looking statements may include, but are not limited to: expectations with respect to development and regulatory plans, trial designs, and the costs, timing and results thereof; expectations with respect to milestones and key events, including the timing of study initiation and completion; expectations with respect to Zura Bio’s development program, including clinical trials and the timing thereof, and expectations with respect to development programs, data readouts and product candidates of other parties; Zura Bio's cash resources and projected cash runway; the potential to raise additional capital to support the company's operations; the potential of pipeline assets to offer broader and improved clinical responses; expectations with respect to addressable markets, projected CAGRs and patient populations; and expectations with respect to the use of proceeds from any financing transactions. These statements are based on various assumptions, whether or not identified in this communication. These forward-looking statements are provided for illustrative purposes only and are not intended to serve as, and must not be relied on by an investor as, a guarantee, an assurance, a prediction or a definitive statement of fact or probability. Actual events are difficult or impossible to predict and could differ materially from those expressed or implied in such forward-looking statements, as a result of these risks and uncertainties, which include, but are not limited to: the potential of Zura Bio's product candidates and their related benefits, competing product candidates and products both in development and approved; Zura Bio's vision and strategy; the timing of key events and initiation of Zura Bio's studies and release of clinical data may take longer than anticipated or may not be achieved at all; the potential general acceptability and maintenance of Zura Bio's product candidates by regulatory authorities, payors, physicians, and patients may not be achieved; Zura Bio's ability to attract and retain key personnel; Zura Bio's future operating expenses, capital requirements and needs for additional financing may not be achieved; Zura Bio has not completed any clinical trials, and has no products approved for commercial sale; Zura Bio has incurred significant losses since inception, and expects to incur significant losses for the foreseeable future and may not be able to achieve or sustain profitability in the future; Zura Bio requires substantial additional capital to finance its operations, and if it is unable to raise such capital when needed or on acceptable terms, Zura Bio may be forced to delay, reduce, and/or eliminate one or more of its development programs or future commercialization efforts; Zura Bio may be unable to renew existing contracts or enter into new contracts; Zura Bio relies on third-party contract development manufacturing organizations for the manufacture of clinical materials; Zura Bio relies on contract research organizations, clinical trial sites, and other third parties to conduct of its preclinical studies and clinical trials; Zura Bio may be unable to obtain regulatory approval for its product candidates, and there may be related restrictions or limitations of any approved products; Zura Bio may be unable to successfully respond to general economic and geopolitical conditions; Zura Bio may be unable to effectively manage growth; Zura Bio faces competitive pressures from other companies worldwide; Zura Bio may be unable to adequately protect its intellectual property rights; and other factors set forth in documents filed, or to be filed by Zura Bio, with the SEC, including the risks and uncertainties described in the “Risk Factors” section of Zura Bio's Annual Report on Form 10-K for the year ended December 31, 2023 and other filings with the SEC. These risks and uncertainties may be amplified by health epidemics or other unanticipated global disruption events, which may continue to cause economic uncertainty. Zura Bio cautions that the foregoing list of factors is not exclusive or exhaustive and not to place undue reliance upon any forward-looking statements, including projections, which speak only as of the date made. Zura Bio gives no assurance that we will achieve our expectations. Zura Bio does not undertake or accept any obligation to publicly provide revisions or updates to any forward-looking statements, whether as a result of new information, future developments or otherwise, or should circumstances change, except as otherwise required by securities and other applicable laws. This presentation discusses product candidates that are under clinical study, and which have not yet been approved for marketing by the US Food and Drug Administration. No representation is made as to the safety or effectiveness of these product candidates for the use for which such product candidates are being studied. Caution should be exercised when comparing data across trials of different products and product candidates. Differences existing between trial designs and patient populations and characteristics. The results across such trials may not have interpretative value on Zura Bio’s existing or future results. Statements included herein concerning clinical trials for the product candidates have not been reviewed or endorsed by Eli Lilly ("Lilly") or Pfizer. This presentation shall not constitute an offer to sell or the solicitation of an offer to buy securities, nor shall there be any sale of securities in any state or jurisdiction in which such offer, solicitation or sale would be unlawful prior to registration or qualification under the securities laws of any such state orjurisdiction. |
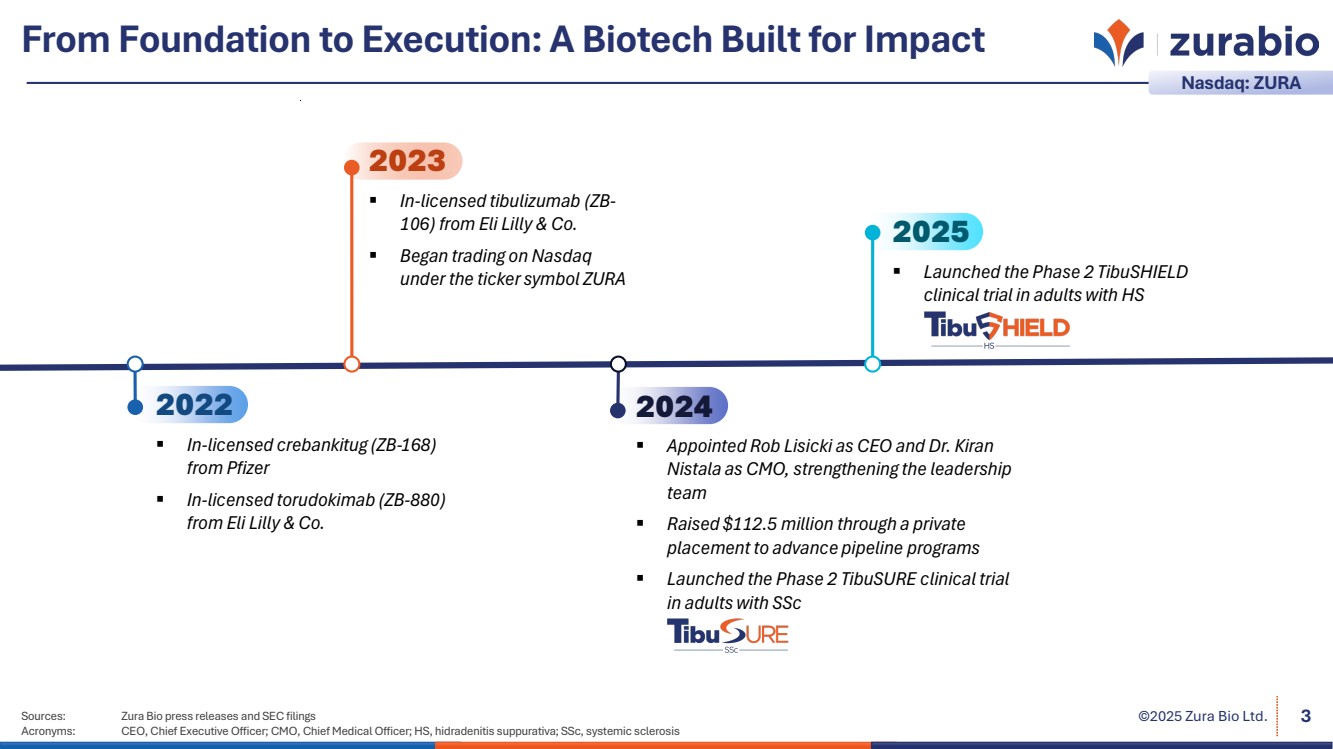
| ©2025 Zura Bio Ltd. From Foundation to Execution: A Biotech Built for Impact Sources: Zura Bio press releases and SEC filings Acronyms: CEO, Chief Executive Officer; CMO, Chief Medical Officer; HS, hidradenitis suppurativa; SSc, systemic sclerosis 3 Nasdaq: ZURA 2022 In-licensed crebankitug (ZB-168) from Pfizer In-licensed torudokimab (ZB-880) from Eli Lilly & Co. 2023 In-licensed tibulizumab (ZB-106) from Eli Lilly & Co. Began trading on Nasdaq under the ticker symbol ZURA 2025 Launched the Phase 2 TibuSHIELD clinical trial in adults with HS 2024 Appointed Rob Lisicki as CEO and Dr. Kiran Nistala as CMO, strengthening the leadership team Raised $112.5 million through a private placement to advance pipeline programs Launched the Phase 2 TibuSURE clinical trial in adults with SSc |
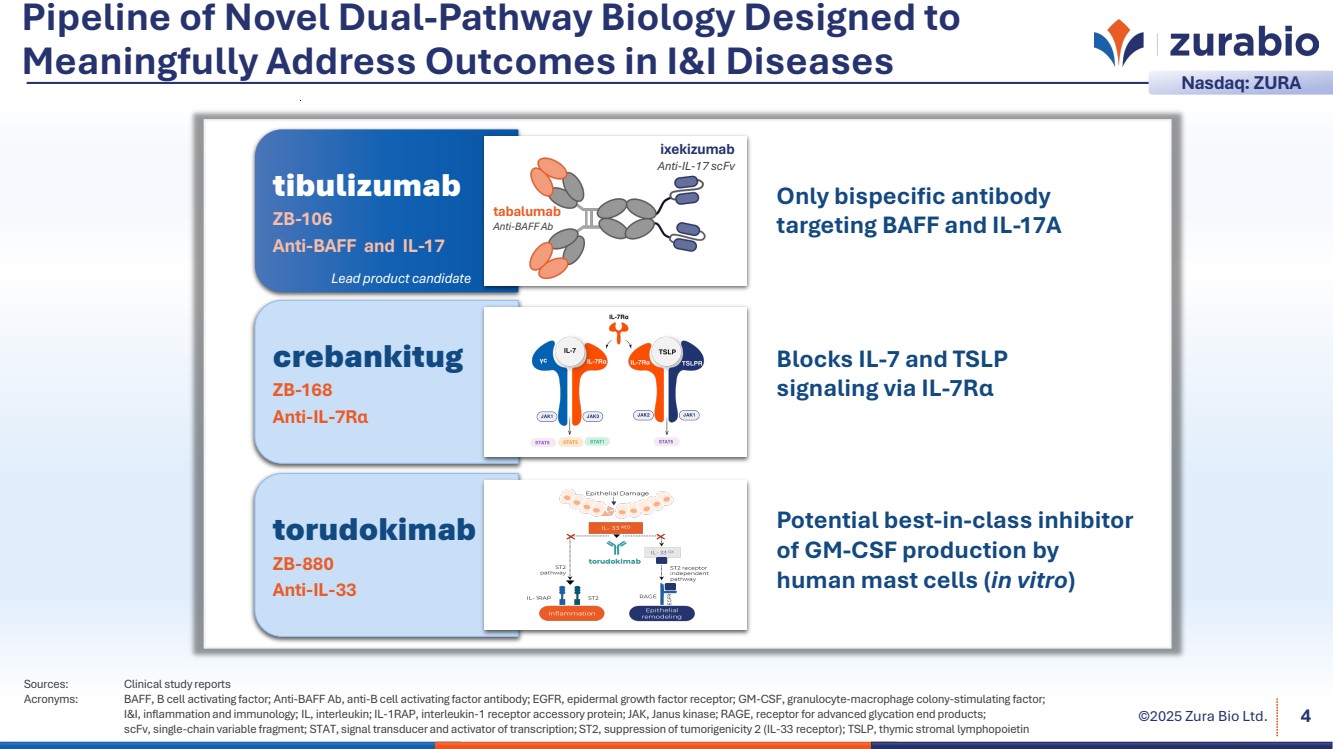
| ©2025 Zura Bio Ltd. Pipeline of Novel Dual-Pathway Biology Designed to Meaningfully Address Outcomes in I&I Diseases Sources: Clinical study reports Acronyms: BAFF, B cell activating factor; Anti-BAFF Ab, anti-B cell activating factor antibody; EGFR, epidermal growth factor receptor; GM-CSF, granulocyte-macrophage colony-stimulating factor; I&I, inflammation and immunology; IL, interleukin; IL-1RAP, interleukin-1 receptor accessory protein; JAK, Janus kinase; RAGE, receptor for advanced glycation end products; scFv, single-chain variable fragment; STAT, signal transducer and activator of transcription; ST2, suppression of tumorigenicity 2 (IL-33 receptor); TSLP, thymic stromal lymphopoietin 4 Nasdaq: ZURA crebankitug ZB-168 Anti-IL-7Rα torudokimab ZB-880 Anti-IL-33 Blocks IL-7 and TSLP signaling via IL-7Rα Potential best-in-class inhibitor of GM-CSF production by human mast cells (in vitro) Only bispecific antibody targeting BAFF and IL-17A tibulizumab ZB-106 Anti-BAFF and IL-17 Lead product candidate ixekizumab Anti-IL-17 scFv tabalumab Anti-BAFF Ab |
| Transformative Opportunity for Tibulizumab Tibulizumab | ZB-106 | Anti-BAFF and IL-17 Tibulizumab, is an investigational agent. Its efficacy and safety have not been established or approved by any regulatory agency worldwide. |
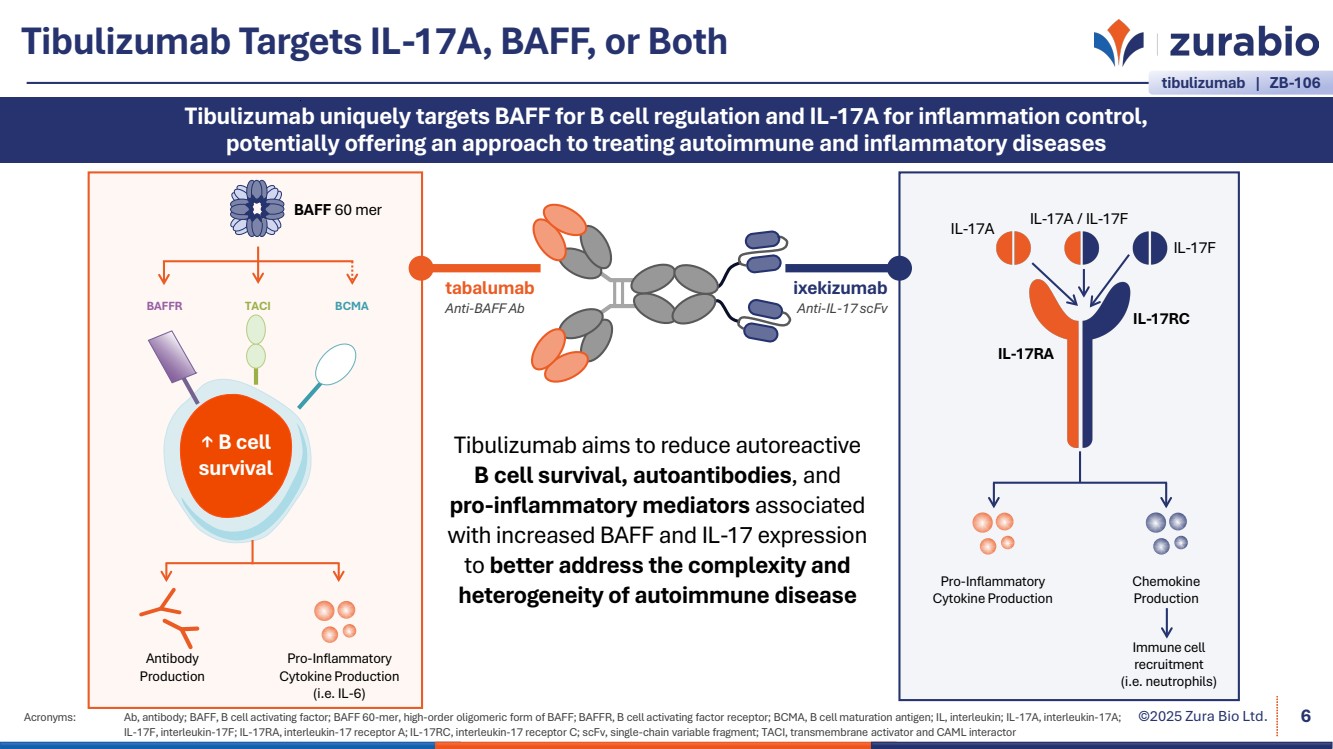
| ©2025 Zura Bio Ltd. Tibulizumab Targets IL-17A, BAFF, or Both Acronyms: Ab, antibody; BAFF, B cell activating factor; BAFF 60-mer, high-order oligomeric form of BAFF; BAFFR, B cell activating factor receptor; BCMA, B cell maturation antigen; IL, interleukin; IL-17A, interleukin-17A; IL-17F, interleukin-17F; IL-17RA, interleukin-17 receptor A; IL-17RC, interleukin-17 receptor C; scFv, single-chain variable fragment; TACI, transmembrane activator and CAML interactor 6 Tibulizumab uniquely targets BAFF for B cell regulation and IL-17A for inflammation control, potentially offering an approach to treating autoimmune and inflammatory diseases ixekizumab Anti-IL-17 scFv tabalumab Anti-BAFF Ab Tibulizumab aims to reduce autoreactive B cell survival, autoantibodies, and pro-inflammatory mediators associated with increased BAFF and IL-17 expression to better address the complexity and heterogeneity of autoimmune disease Pro-Inflammatory Cytokine Production Chemokine Production BAFF 60 mer ↑ B cell survival BAFFR TACI BCMA Antibody Production Pro-Inflammatory Cytokine Production (i.e. IL-6) IL-17RC IL-17RA IL-17A IL-17A / IL-17F IL-17F Immune cell recruitment (i.e. neutrophils) tibulizumab | ZB-106 |
| An Overview of Tibulizumab Tibulizumab | ZB-106 | Anti-BAFF and IL-17 Tibulizumab, is an investigational agent. Its efficacy and safety have not been established or approved by any regulatory agency worldwide. |
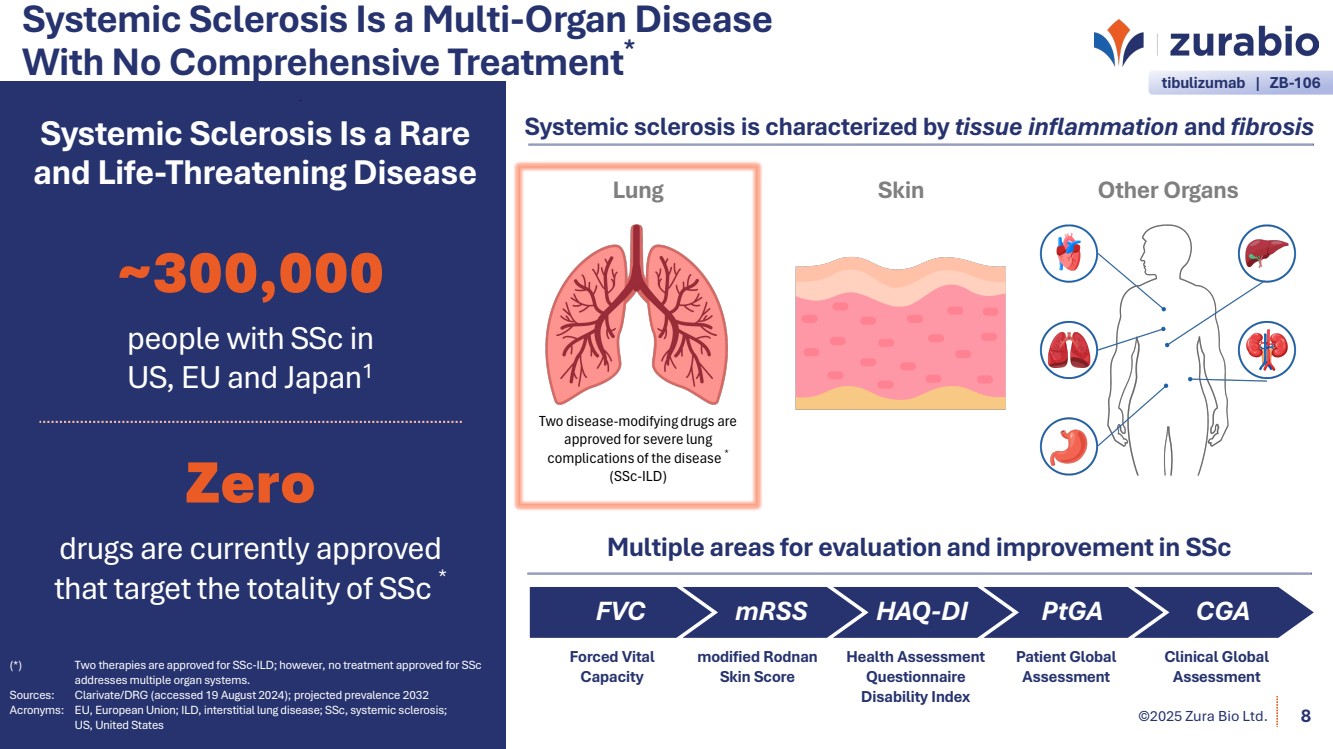
| ©2025 Zura Bio Ltd. Systemic Sclerosis Is a Multi-Organ Disease With No Comprehensive Treatment* 8 Systemic Sclerosis Is a Rare and Life-Threatening Disease ~300,000 people with SSc in US, EU and Japan1 Zero drugs are currently approved that target the totality of SSc * (*) Two therapies are approved for SSc-ILD; however, no treatment approved for SSc addresses multiple organ systems. Sources: Clarivate/DRG (accessed 19 August 2024); projected prevalence 2032 Acronyms: EU, European Union; ILD, interstitial lung disease; SSc, systemic sclerosis; US, United States Systemic sclerosis is characterized by tissue inflammation and fibrosis Multiple areas for evaluation and improvement in SSc FVC Forced Vital Capacity mRSS modified Rodnan Skin Score HAQ-DI Health Assessment Questionnaire Disability Index PtGA Patient Global Assessment CGA Clinical Global Assessment tibulizumab | ZB-106 Lung Skin Other Organs Two disease-modifying drugs are approved for severe lung complications of the disease * (SSc-ILD) |
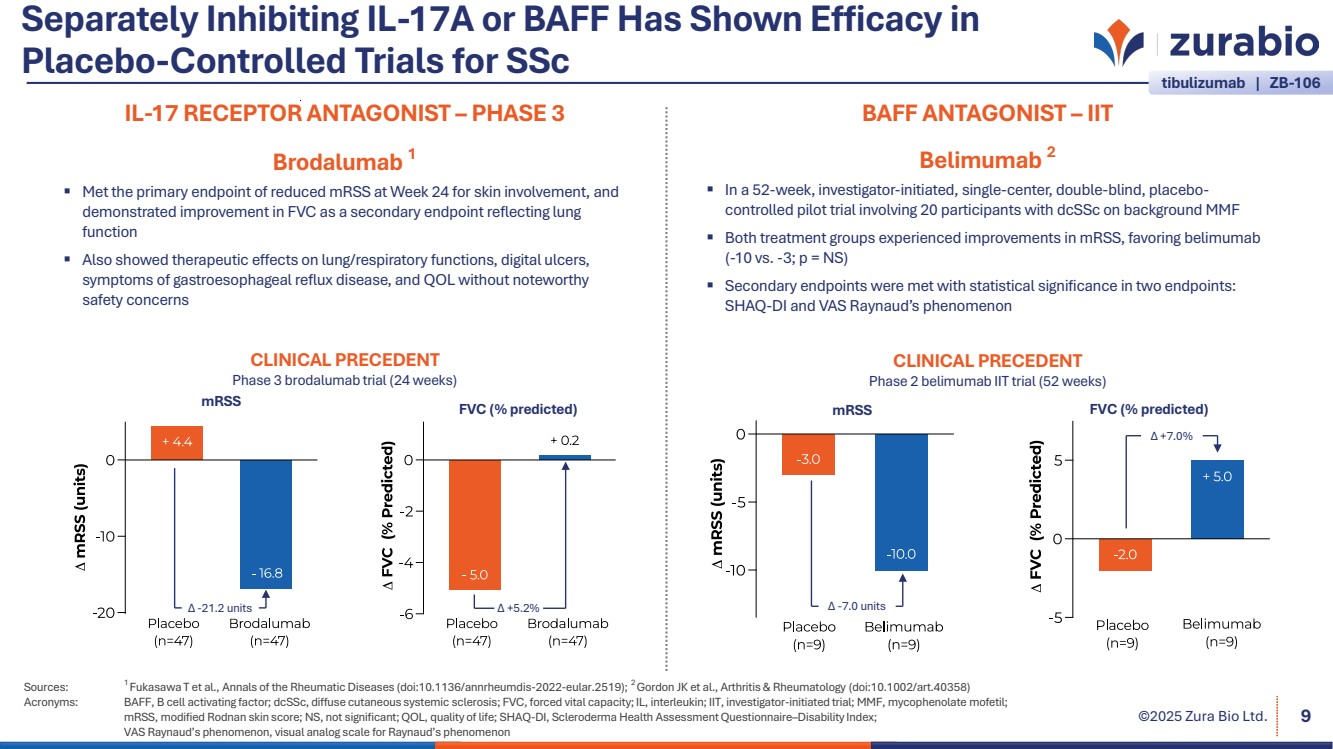
| ©2025 Zura Bio Ltd. Separately Inhibiting IL-17A or BAFF Has Shown Efficacy in Placebo-Controlled Trials for SSc Sources: 1 Fukasawa T et al., Annals of the Rheumatic Diseases (doi:10.1136/annrheumdis-2022-eular.2519); 2 Gordon JK et al., Arthritis & Rheumatology (doi:10.1002/art.40358) Acronyms: BAFF, B cell activating factor; dcSSc, diffuse cutaneous systemic sclerosis; FVC, forced vital capacity; IL, interleukin; IIT, investigator-initiated trial; MMF, mycophenolate mofetil; mRSS, modified Rodnan skin score; NS, not significant; QOL, quality of life; SHAQ-DI, Scleroderma Health Assessment Questionnaire–Disability Index; VAS Raynaud’s phenomenon, visual analog scale for Raynaud’s phenomenon 9 IL-17 RECEPTOR ANTAGONIST – PHASE 3 Brodalumab 1 Met the primary endpoint of reduced mRSS at Week 24 for skin involvement, and demonstrated improvement in FVC as a secondary endpoint reflecting lung function Also showed therapeutic effects on lung/respiratory functions, digital ulcers, symptoms of gastroesophageal reflux disease, and QOL without noteworthy safety concerns Δ -21.2 units Δ +5.2% CLINICAL PRECEDENT Phase 3 brodalumab trial (24 weeks) mRSS FVC (% predicted) BAFF ANTAGONIST – IIT Belimumab 2 In a 52-week, investigator-initiated, single-center, double-blind, placebo-controlled pilot trial involving 20 participants with dcSSc on background MMF Both treatment groups experienced improvements in mRSS, favoring belimumab (-10 vs. -3; p = NS) Secondary endpoints were met with statistical significance in two endpoints: SHAQ-DI and VAS Raynaud’s phenomenon CLINICAL PRECEDENT Phase 2 belimumab IIT trial (52 weeks) mRSS FVC (% predicted) Δ -7.0 units Δ +7.0% tibulizumab | ZB-106 |
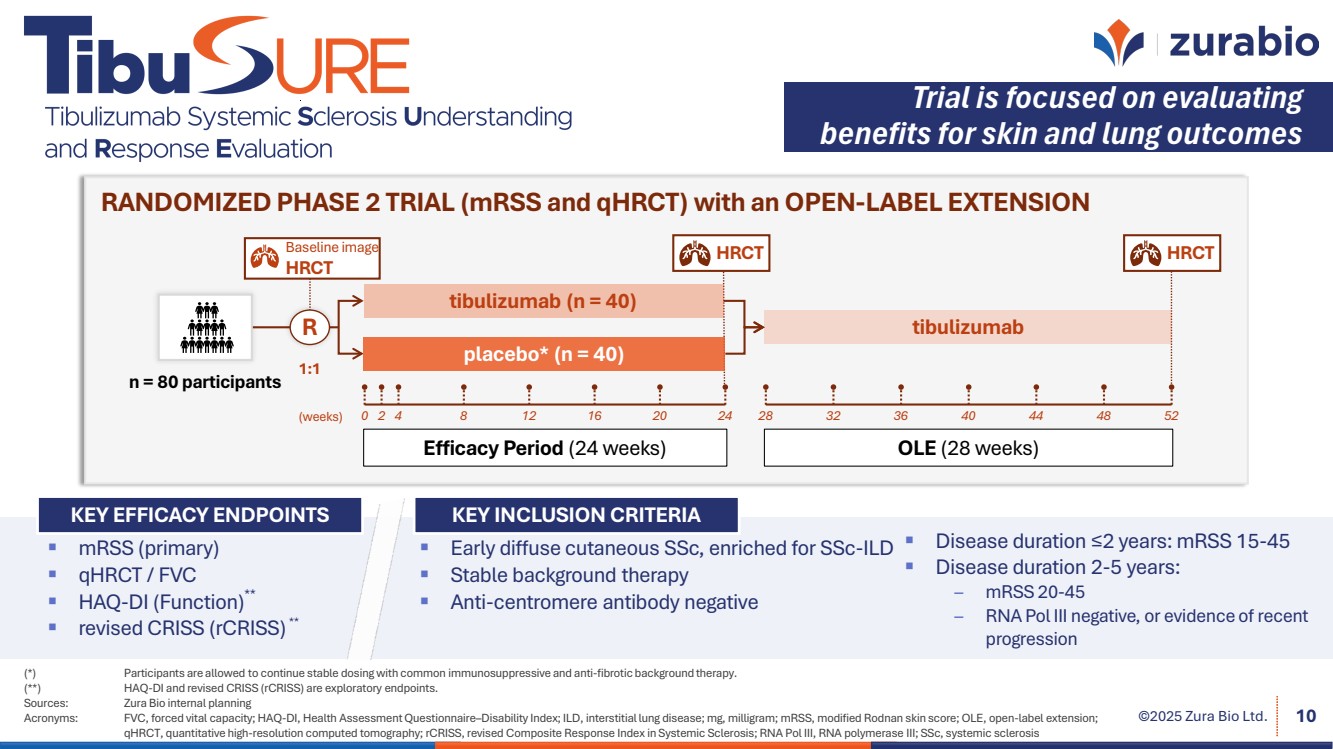
| ©2025 Zura Bio Ltd. 10 Efficacy Period (24 weeks) Baseline image HRCT HRCT OLE (28 weeks) 1:1 R tibulizumab tibulizumab (n = 40) placebo* (n = 40) n = 80 participants (weeks) 0 2 4 8 12 16 20 24 28 32 36 40 44 48 52 HRCT RANDOMIZED PHASE 2 TRIAL (mRSS and qHRCT) with an OPEN-LABEL EXTENSION KEY INCLUSION CRITERIA Early diffuse cutaneous SSc, enriched for SSc-ILD Stable background therapy Anti-centromere antibody negative Disease duration ≤2 years: mRSS 15-45 Disease duration 2-5 years: – mRSS 20-45 – RNA Pol III negative, or evidence of recent progression KEY EFFICACY ENDPOINTS mRSS (primary) qHRCT / FVC HAQ-DI (Function)** revised CRISS (rCRISS) ** Trial is focused on evaluating benefits for skin and lung outcomes (*) Participants are allowed to continue stable dosing with common immunosuppressive and anti-fibrotic background therapy. (**) HAQ-DI and revised CRISS (rCRISS) are exploratory endpoints. Sources: Zura Bio internal planning Acronyms: FVC, forced vital capacity; HAQ-DI, Health Assessment Questionnaire–Disability Index; ILD, interstitial lung disease; mg, milligram; mRSS, modified Rodnan skin score; OLE, open-label extension; qHRCT, quantitative high-resolution computed tomography; rCRISS, revised Composite Response Index in Systemic Sclerosis; RNA Pol III, RNA polymerase III; SSc, systemic sclerosis |
| Nasdaq: ZURA | www.zurabio.com An Overview of Hidradenitis Suppurativa ©2025 Zura Bio Ltd. 11 ~50% of patients report dissatisfaction with their treatment6-7 Average time to diagnosis is 7 years ~300K people living with HS in the U.S. DISEASE OVERVIEW CLINICAL OPPORTUNITY 6 Sources: 1 Moran B et al., Journal of Investigative Dermatology (doi:10.1016/j.jid.2017.05.033); 2 Banerjee A et al., Immunological Investigations (doi:10.1080/08820139.2016.1230867); 3 Sabat R et al., Journal of Allergy and Clinical Immunology (doi:10.1016/j.jaci.2022.10.034); 4 Garg A et al., JAMA Dermatology (doi:10.1001/jamadermatol.2017.0201); 5 Ingram JR, British Journal of Dermatology (doi:10.1111/bjd.19435); 6 Medical literature and MEDACorp KOL discussions; 7 Midgette B et al., British Journal of Dermatology (doi:10.1111/bjd.21798) Acronyms: HS, hidradenitis suppurativa; Th, T-helper cells; US, United States tibulizumab | ZB-106 Recurrent nodules and abscesses resulting in pus-like discharge Difficult-to-heal open wounds and scarring Hidradenitis suppurativa is an inflammatory follicular skin disease Increased Th1/Th17 and B cell mediated inflammation 1-3 Disproportionately affects women between adolescent age to 55 years of age 4-5 Skin lesions form in the armpits, groin, and under the breasts due to inflammation and infection of the sweat glands |
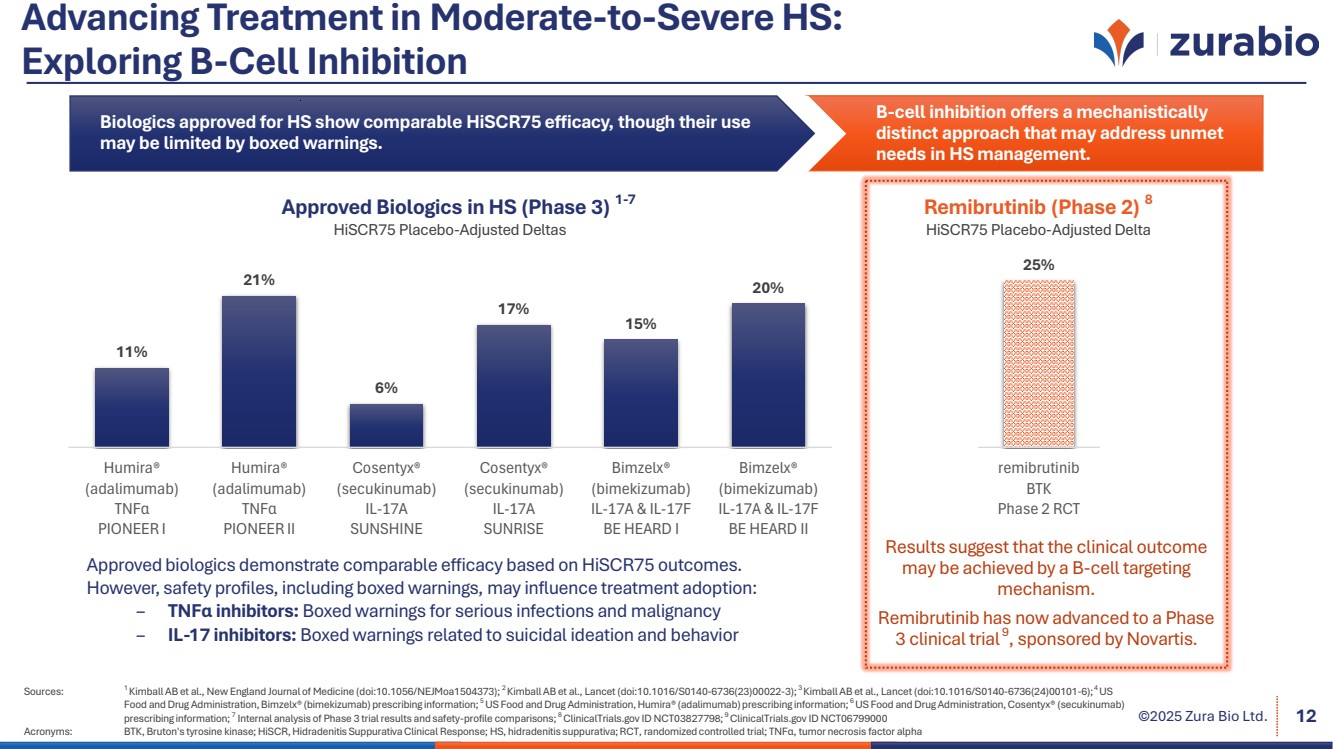
| ©2025 Zura Bio Ltd. Advancing Treatment in Moderate-to-Severe HS: Exploring B-Cell Inhibition B-cell inhibition offers a mechanistically distinct approach that may address unmet needs in HS management. Biologics approved for HS show comparable HiSCR75 efficacy, though their use may be limited by boxed warnings. Sources: 1 Kimball AB et al., New England Journal of Medicine (doi:10.1056/NEJMoa1504373); 2 Kimball AB et al., Lancet (doi:10.1016/S0140-6736(23)00022-3); 3 Kimball AB et al., Lancet (doi:10.1016/S0140-6736(24)00101-6);4 US Food and Drug Administration, Bimzelx® (bimekizumab) prescribing information; 5 US Food and Drug Administration, Humira® (adalimumab) prescribing information; 6 US Food and Drug Administration, Cosentyx® (secukinumab) prescribing information; 7 Internal analysis of Phase 3 trial results and safety-profile comparisons; 8 ClinicalTrials.gov ID NCT03827798; 9 ClinicalTrials.gov ID NCT06799000 Acronyms: BTK, Bruton's tyrosine kinase; HiSCR, Hidradenitis Suppurativa Clinical Response; HS, hidradenitis suppurativa; RCT, randomized controlled trial; TNFα, tumor necrosis factor alpha 12 11% 21% 6% 17% 15% 20% Humira® (adalimumab) TNFα PIONEER I Humira® (adalimumab) TNFα PIONEER II Cosentyx® (secukinumab) IL-17A SUNSHINE Cosentyx® (secukinumab) IL-17A SUNRISE Bimzelx® (bimekizumab) IL-17A & IL-17F BE HEARD I Bimzelx® (bimekizumab) IL-17A & IL-17F BE HEARD II Approved Biologics in HS (Phase 3) 1-7 HiSCR75 Placebo-Adjusted Deltas Remibrutinib (Phase 2) 8 HiSCR75 Placebo-Adjusted Delta 25% remibrutinib BTK Phase 2 RCT Results suggest that the clinical outcome may be achieved by a B-cell targeting mechanism. Remibrutinib has now advanced to a Phase 3 clinical trial 9 , sponsored by Novartis. Approved biologics demonstrate comparable efficacy based on HiSCR75 outcomes. However, safety profiles, including boxed warnings, may influence treatment adoption: – TNFα inhibitors: Boxed warnings for serious infections and malignancy – IL-17 inhibitors: Boxed warnings related to suicidal ideation and behavior |
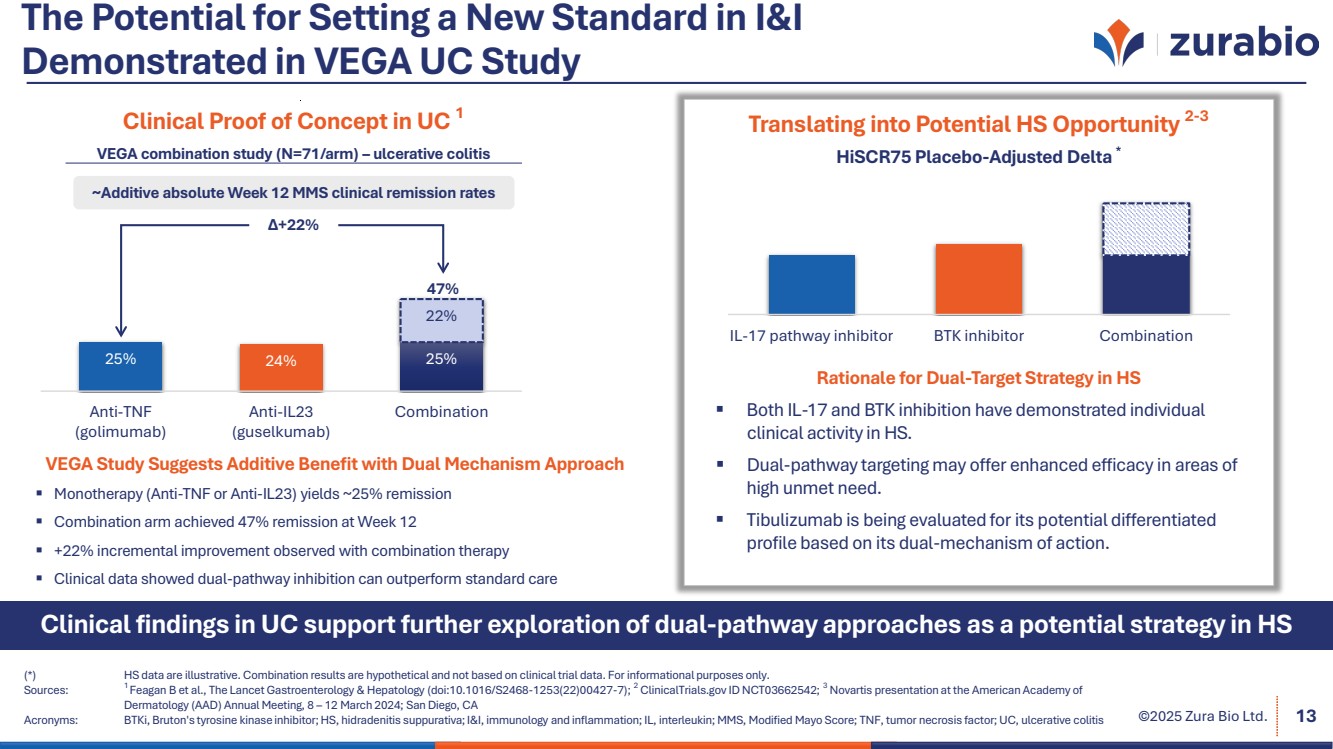
| ©2025 Zura Bio Ltd. The Potential for Setting a New Standard in I&I Demonstrated in VEGA UC Study (*) HS data are illustrative. Combination results are hypothetical and not based on clinical trial data. For informational purposes only. Sources: 1 Feagan B et al., The Lancet Gastroenterology & Hepatology (doi:10.1016/S2468-1253(22)00427-7); 2 ClinicalTrials.gov ID NCT03662542; 3 Novartis presentation at the American Academy of Dermatology (AAD) Annual Meeting, 8 – 12 March 2024; San Diego, CA Acronyms: BTKi, Bruton's tyrosine kinase inhibitor; HS, hidradenitis suppurativa; I&I, immunology and inflammation; IL, interleukin; MMS, Modified Mayo Score; TNF, tumor necrosis factor; UC, ulcerative colitis 13 Clinical findings in UC support further exploration of dual-pathway approaches as a potential strategy in HS VEGA Study Suggests Additive Benefit with Dual Mechanism Approach Monotherapy (Anti-TNF or Anti-IL23) yields ~25% remission Combination arm achieved 47% remission at Week 12 +22% incremental improvement observed with combination therapy Clinical data showed dual-pathway inhibition can outperform standard care Clinical Proof of Concept in UC 1 25% 24% 25% 22% Anti-TNF (golimumab) Anti-IL23 (guselkumab) Combination 47% Δ+22% ~Additive absolute Week 12 MMS clinical remission rates VEGA combination study (N=71/arm) – ulcerative colitis Translating into Potential HS Opportunity 2-3 IL-17 pathway inhibitor BTK inhibitor Combination HiSCR75 Placebo-Adjusted Delta * Rationale for Dual-Target Strategy in HS Both IL-17 and BTK inhibition have demonstrated individual clinical activity in HS. Dual-pathway targeting may offer enhanced efficacy in areas of high unmet need. Tibulizumab is being evaluated for its potential differentiated profile based on its dual-mechanism of action. |
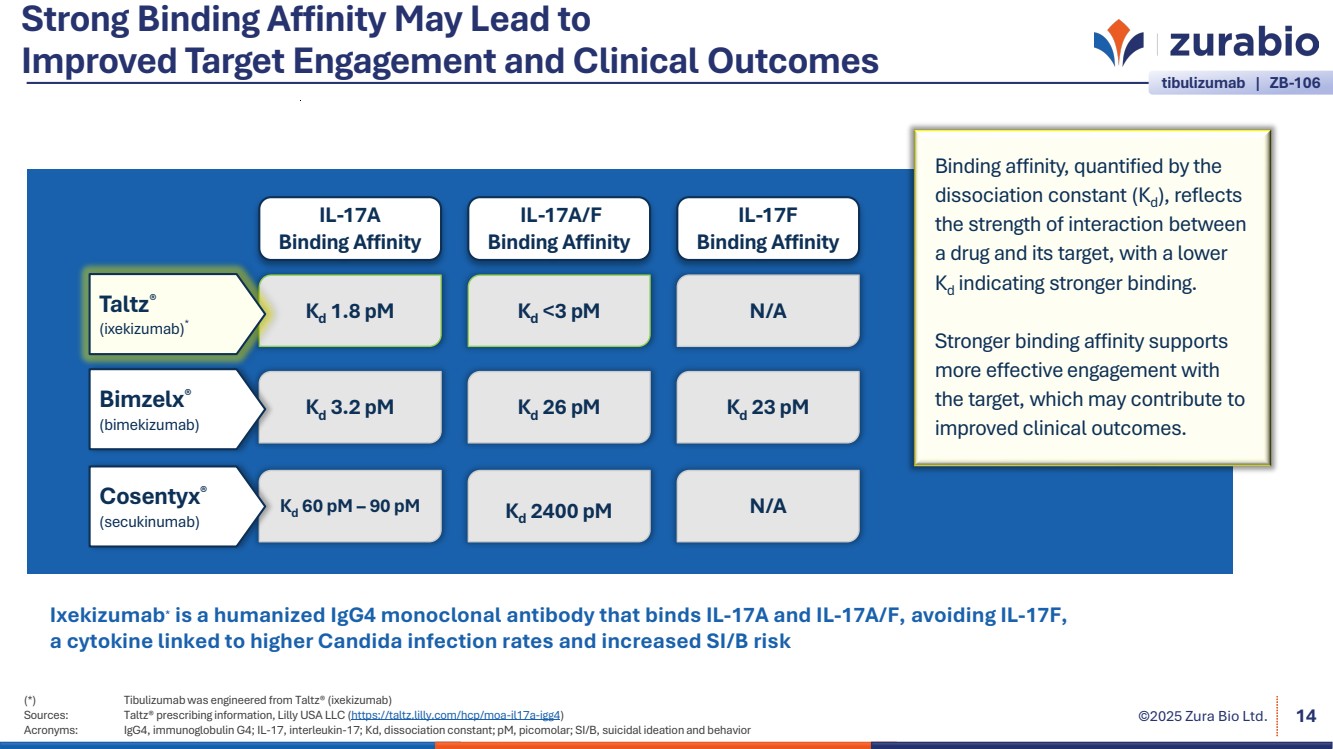
| ©2025 Zura Bio Ltd. Strong Binding Affinity May Lead to Improved Target Engagement and Clinical Outcomes (*) Tibulizumab was engineered from Taltz® (ixekizumab) Sources: Taltz® prescribing information, Lilly USA LLC (https://taltz.lilly.com/hcp/moa-il17a-igg4) Acronyms: IgG4, immunoglobulin G4; IL-17, interleukin-17; Kd, dissociation constant; pM, picomolar; SI/B, suicidal ideation and behavior 14 IL-17F Binding Affinity N/A Kd 23 pM N/A Kd <3 pM Kd 26 pM IL-17A/F Binding Affinity Kd 1.8 pM Kd 3.2 pM Kd 60 pM – 90 pM IL-17A Binding Affinity Taltz® (ixekizumab)* Bimzelx® (bimekizumab) Cosentyx® (secukinumab) Binding affinity, quantified by the dissociation constant (Kd), reflects the strength of interaction between a drug and its target, with a lower Kd indicating stronger binding. Stronger binding affinity supports more effective engagement with the target, which may contribute to improved clinical outcomes. Kd 2400 pM tibulizumab | ZB-106 Ixekizumab* is a humanized IgG4 monoclonal antibody that binds IL-17A and IL-17A/F, avoiding IL-17F, a cytokine linked to higher Candida infection rates and increased SI/B risk |
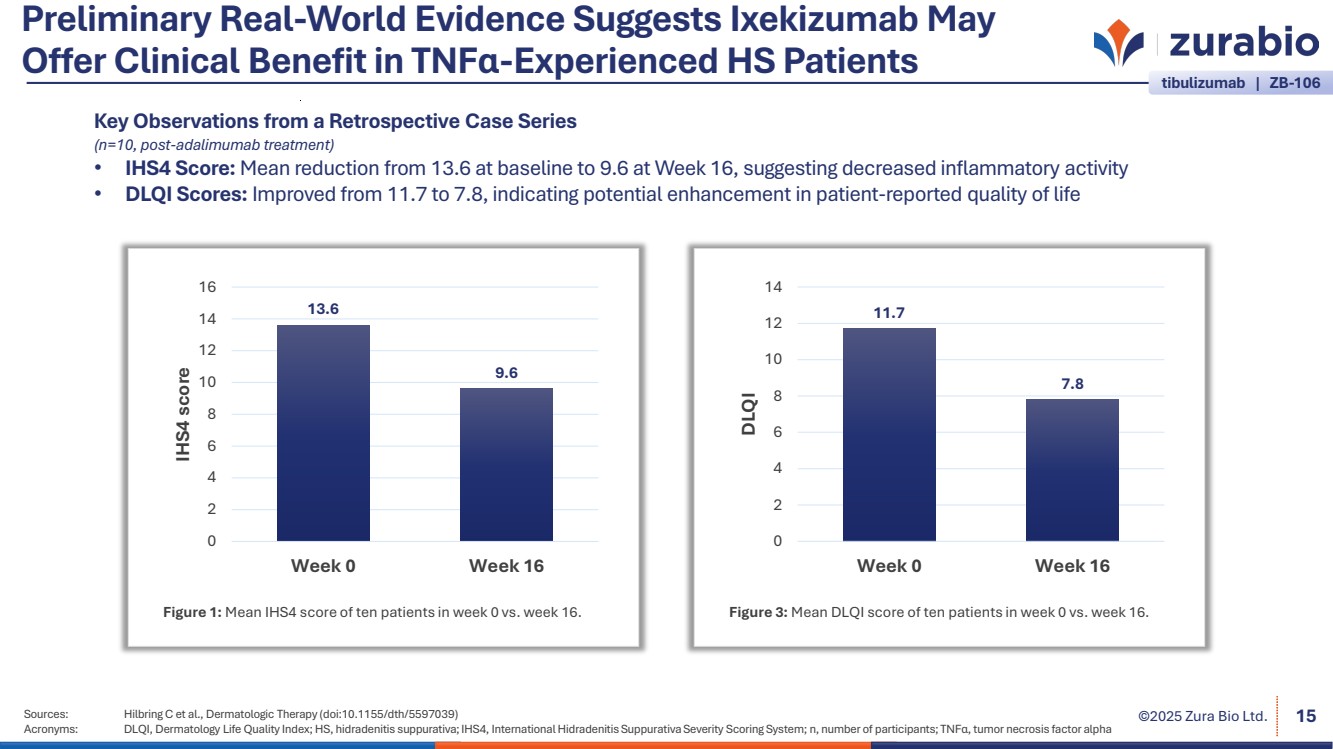
| ©2025 Zura Bio Ltd. Preliminary Real-World Evidence Suggests Ixekizumab May Offer Clinical Benefit in TNFα-Experienced HS Patients Sources: Hilbring C et al., Dermatologic Therapy (doi:10.1155/dth/5597039) Acronyms: DLQI, Dermatology Life Quality Index; HS, hidradenitis suppurativa; IHS4, International Hidradenitis Suppurativa Severity Scoring System; n, number of participants; TNFα, tumor necrosis factor alpha 15 13.6 9.6 0 2 4 6 8 10 12 14 16 Week 0 Week 16 IHS4 score Figure 1: Mean IHS4 score of ten patients in week 0 vs. week 16. 11.7 7.8 0 2 4 6 8 10 12 14 Week 0 Week 16 DLQI Figure 3: Mean DLQI score of ten patients in week 0 vs. week 16. Key Observations from a Retrospective Case Series (n=10, post-adalimumab treatment) • IHS4 Score: Mean reduction from 13.6 at baseline to 9.6 at Week 16, suggesting decreased inflammatory activity • DLQI Scores: Improved from 11.7 to 7.8, indicating potential enhancement in patient-reported quality of life tibulizumab | ZB-106 |
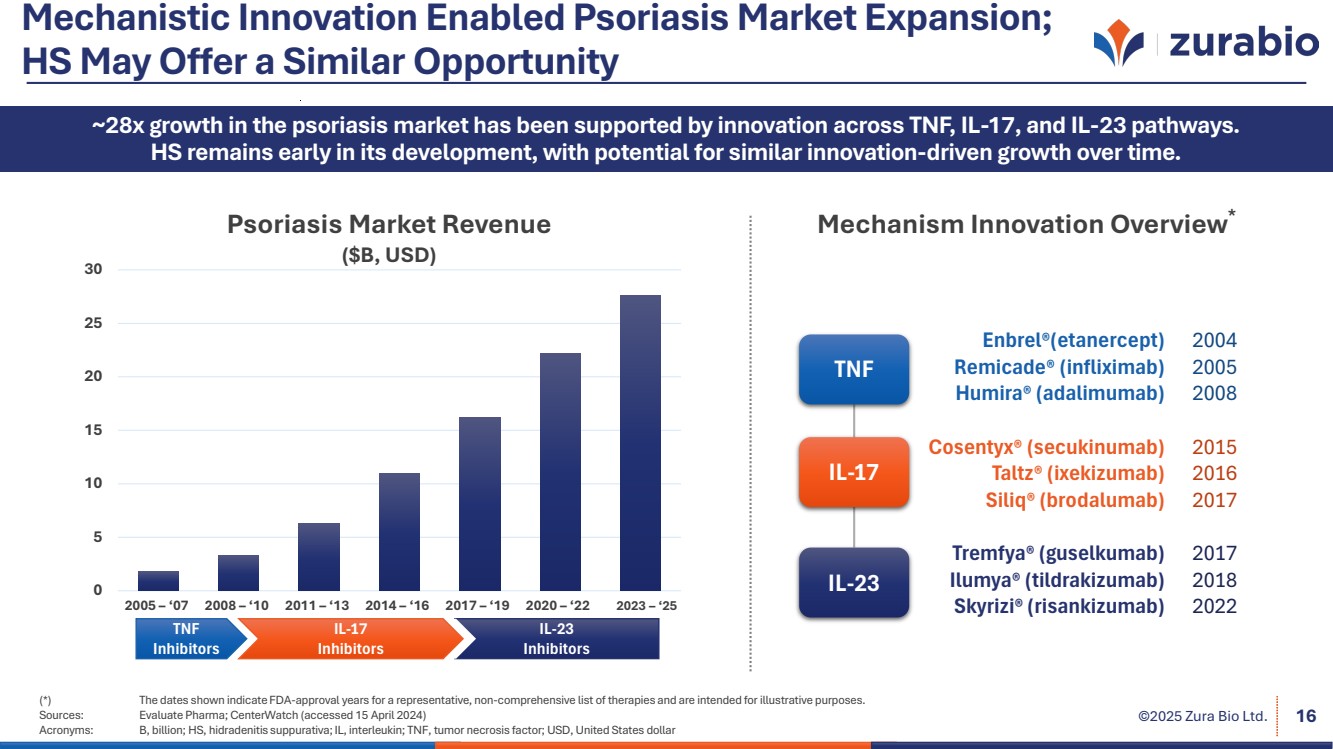
| ©2025 Zura Bio Ltd. Mechanistic Innovation Enabled Psoriasis Market Expansion; HS May Offer a Similar Opportunity (*) The dates shown indicate FDA-approval years for a representative, non-comprehensive list of therapies and are intended for illustrative purposes. Sources: Evaluate Pharma; CenterWatch (accessed 15 April 2024) Acronyms: B, billion; HS, hidradenitis suppurativa; IL, interleukin; TNF, tumor necrosis factor; USD, United States dollar 16 ~28x growth in the psoriasis market has been supported by innovation across TNF, IL-17, and IL-23 pathways. HS remains early in its development, with potential for similar innovation-driven growth over time. Enbrel®(etanercept) 2004 Remicade® (infliximab) 2005 Humira® (adalimumab) 2008 Cosentyx® (secukinumab) 2015 Taltz® (ixekizumab) 2016 Siliq® (brodalumab) 2017 Tremfya® (guselkumab) 2017 Ilumya® (tildrakizumab) 2018 Skyrizi® (risankizumab) 2022 Mechanism Innovation Overview* TNF IL-17 0 IL-23 5 10 15 20 25 30 Psoriasis Market Revenue ($B, USD) 2005 – ‘07 2008 – ‘10 2011 – ‘13 2014 – ‘16 2017 – ‘19 2020 – ‘22 2023 – ‘25 IL-23 Inhibitors IL-23 Surge TNF Inhibitors IL-17 Inhibitors |
| ©2025 Zura Bio Ltd. Tibulizumab Offers Encouraging Combination of Attributes for HS Sources: 1 van Straalen KR et al., Journal of Investigative Dermatology (doi:10.1016/j.jaci.2022.02.003); 2 Malvaso D et al., Pharmaceutics (doi:10.3390/pharmaceutics15102450); 3 US Food and Drug Administration, Bimzelx® (bimekizumab) prescribing information; 4 Novartis presentation at the American Academy of Dermatology (AAD) Annual Meeting, 8 – 12 March 2024; San Diego, CA; 5 US Food and Drug Administration, Taltz® (ixekizumab) prescribing information; 6 Internal market research Acronyms: BAFF, B-cell activating factor; HS, hidradenitis suppurativa; IL-17, interleukin-17; Kd, dissociation constant 17 tibulizumab | ZB-106 Clinically Relevant Pathways in HS IL-17 and B cell pathways are supported by clinical evidence as relevant targets in HS.1-4 High Affinity for Key IL-17 Isoforms The IL-17A-targeting component of tibulizumab shows strong binding affinity for IL-17A/A and IL-17A/F, which are thought to play key roles in inflammation. Selective Approach: No IL-17F/F Inhibition The benefit of targeting IL-17F/F remains unclear and may carry safety concerns.3 Favorable Safety and Tolerability Profile Tibulizumab combines ixekizumab and tabalumab, both of which have established safety and tolerability profiles as individual monoclonal antibodies.5 Kd Qualitative Research Suggests Attractive Potential for Commercial Opportunity 6 |
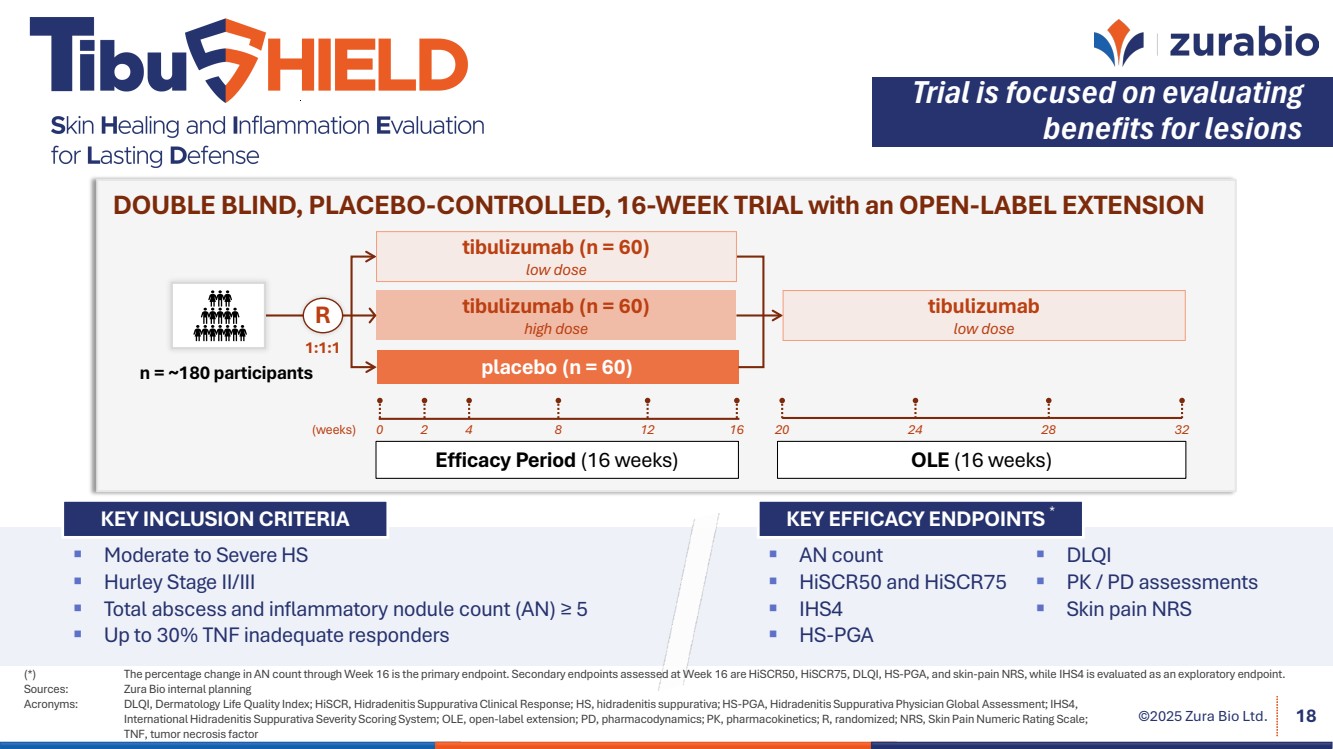
| ©2025 Zura Bio Ltd. 18 Efficacy Period (16 weeks) OLE (16 weeks) 1:1:1 R tibulizumab (n = 60) high dose n = ~180 participants placebo (n = 60) (weeks) 20 24 28 32 DOUBLE BLIND, PLACEBO-CONTROLLED, 16-WEEK TRIAL with an OPEN-LABEL EXTENSION tibulizumab (n = 60) low dose tibulizumab low dose 0 2 4 8 12 16 AN count HiSCR50 and HiSCR75 IHS4 HS-PGA DLQI PK / PD assessments Skin pain NRS KEY EFFICACY ENDPOINTS * KEY INCLUSION CRITERIA Moderate to Severe HS Hurley Stage II/III Total abscess and inflammatory nodule count (AN) ≥ 5 Up to 30% TNF inadequate responders Trial is focused on evaluating benefits for lesions (*) The percentage change in AN count through Week 16 is the primary endpoint. Secondary endpoints assessed at Week 16 are HiSCR50, HiSCR75, DLQI, HS-PGA, and skin-pain NRS, while IHS4 is evaluated as an exploratory endpoint. Sources: Zura Bio internal planning Acronyms: DLQI, Dermatology Life Quality Index; HiSCR, Hidradenitis Suppurativa Clinical Response; HS, hidradenitis suppurativa; HS-PGA, Hidradenitis Suppurativa Physician Global Assessment; IHS4, International Hidradenitis Suppurativa Severity Scoring System; OLE, open-label extension; PD, pharmacodynamics; PK, pharmacokinetics; R, randomized; NRS, Skin Pain Numeric Rating Scale; TNF, tumor necrosis factor |
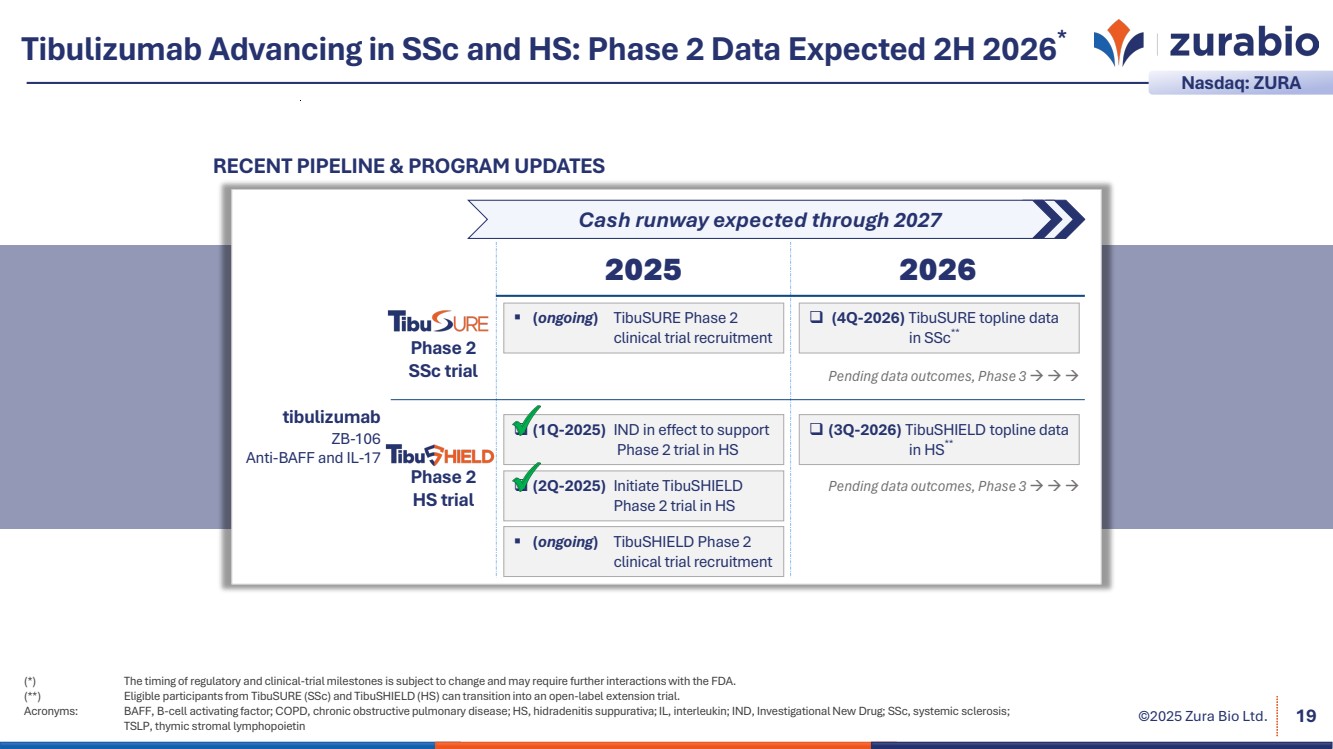
| ©2025 Zura Bio Ltd. Tibulizumab Advancing in SSc and HS: Phase 2 Data Expected 2H 2026* (*) The timing of regulatory and clinical-trial milestones is subject to change and may require further interactions with the FDA. (**) Eligible participants from TibuSURE (SSc) and TibuSHIELD(HS) can transition into an open-label extension trial. Acronyms: BAFF, B-cell activating factor; COPD, chronic obstructive pulmonary disease; HS, hidradenitis suppurativa; IL, interleukin; IND, Investigational New Drug; SSc, systemic sclerosis; TSLP, thymic stromal lymphopoietin 19 2025 2026 tibulizumab ZB-106 Anti-BAFF and IL-17 Phase 2 SSc trial Phase 2 HS trial RECENT PIPELINE & PROGRAM UPDATES Nasdaq: ZURA (ongoing) TibuSURE Phase 2 clinical trial recruitment (4Q-2026) TibuSURE topline data in SSc** (2Q-2025) Initiate TibuSHIELD Phase 2 trial in HS (1Q-2025) IND in effect to support Phase 2 trial in HS (3Q-2026) TibuSHIELD topline data in HS** Pending data outcomes, Phase 3 Pending data outcomes, Phase 3 Cash runway expected through 2027 (ongoing) TibuSHIELD Phase 2 clinical trial recruitment |
| ©2025 Zura Bio Ltd. Tibulizumab is currently being evaluated in two ongoing Phase 2 clinical trials: TibuSURE for adults with systemic sclerosis and TibuSHIELD for adults with hidradenitis suppurativa The strategy follows validated pathways with the potential for improved outcomes, supported by related mechanism-of-action data from IL-17 and BAFF/B-cell depletion therapies We believe tibulizumab is positioned as a potential first-in-class dual-pathway biologic The next 12 to 18 months offer growth opportunities, with multiple key milestones anticipated Tibulizumab Summary Acronyms: BAFF, B cell-activating factor; IL, interleukin ©2025 Zura Bio Ltd. 20 |
| Nasdaq: ZURA | www.zurabio.com Thank You |