

Transforming Potential into Reality I-Mab Biopharma June 2025

Legal Disclaimer. This presentation has been prepared by I-Mab (the “Company”) solely for informational purposes. Certain of the information included herein was obtained from various sources, including certain third parties, and has not been independently verified by the Company. By viewing or accessing the information contained in this presentation, you hereby acknowledge and agree that no representations, warranties, or undertakings, express or implied, are made by the Company or any of its directors, shareholders, employees, agents, affiliates, advisors, or representatives as to, and no reliance should be placed on the truth, accuracy, fairness, completeness, or reasonableness of the information or opinions presented or contained in, and omission from, this presentation. Neither the Company nor any of its directors, employees, agents, affiliates, advisors, or representatives shall be responsible or liable whatsoever (in negligence or otherwise) for any loss, howsoever arising from any information presented or contained in this presentation or otherwise arising in connection with the presentation, except to the extent required by applicable law. The information presented or contained in this presentation speaks only as of the date hereof and is subject to change without notice. This presentation includes statistical and other industry and market data that we obtained from industry publications and research, surveys, and studies conducted by third parties, and our own estimates of potential market opportunities. All of the market data used in this presentation involves a number of assumptions and limitations, and you are cautioned not to give undue weight to such data. Industry publications and third-party research, surveys and studies generally indicate that their information has been obtained from sources believed to be reliable, although they do not guarantee the accuracy or completeness of such information. Our estimates of the potential market opportunities for our product candidates include several key assumptions based on our industry knowledge, industry publications, third-party research, and other surveys, which may be based on a small sample size and may fail to accurately reflect market opportunities. While we believe that our internal assumptions are reasonable, no independent source has verified such assumptions. Forward Looking Statements. This presentation contains forward-looking statements. These statements are made under the “safe harbor” provisions of the U.S. Private Securities Litigation Reform Act of 1995. These forward-looking statements can be identified by terminology such as “future”, “promising”, “may”, “plans”, “potential”, “will”, “could position”, “promise”, “advance”, “target”, “design”, “strategy”, “pipeline”, and “project”, and similar terms or the negative thereof. Statements that are not historical facts, including statements about I-Mab's beliefs and expectations, are forward-looking statements. The forward-looking statements in this presentation include, without limitation, statements regarding the following: the Company’s pipeline and capital strategy; the design and potential benefits, advantages, promise, attributes, and target usage of givastomig, ragistomig and uliledlimab; the projected development and advancement of the Company’s portfolio and anticipated milestones and related timing; the Company’s expectation regarding the potential market opportunity of gastric cancer, pancreatic ductal adenocarcinoma and cholangiocarcinoma; the market opportunity and I-Mab’s potential next steps (including the potential expansion, differentiation, or commercialization) for givastomig, ragistomig and uliledlimab; the Company’s expectations regarding the impact of data from past, ongoing and future studies and trials; the benefits of the Company’s collaboration with development partners; the timing and progress of studies (including with respect to patient enrollment and dosing); the availability of data and information from ongoing studies; the Company’s expectations regarding its cash runway and future use of its cash position. These forward-looking statements involve inherent risks and uncertainties that could cause actual results to differ materially from those expressed or implied in such forward-looking statements. These risks and uncertainties include, but are not limited to, the following: I-Mab’s ability to demonstrate the safety and efficacy of its drug candidates; the clinical results for its drug candidates, which may or may not support further development or new drug application/biologics license application approval; the content and timing of decisions made by the relevant regulatory authorities regarding regulatory approval of I-Mab’s drug candidates; I-Mab’s ability to achieve commercial success for its drug candidates, if approved; I-Mab’s ability to obtain and maintain protection of intellectual property for its technology and drugs; I-Mab’s reliance on third parties to conduct drug development, manufacturing and other services; I-Mab’s limited operating history and I-Mab’s ability to obtain additional funding for operations and to complete the development and commercialization of its drug candidates; and discussions of potential risks, uncertainties, and other important factors in I-Mab’s most recent annual report on Form 20-F and I-Mab’s subsequent filings with the U.S. Securities and Exchange Commission (the “SEC”). I-Mab may also make written or oral forward-looking statements in its periodic reports to the SEC, in its annual report to shareholders, in press releases and other written materials, and in oral statements made by its officers, directors, or employees to third parties. All forward-looking statements are based on information currently available to I-Mab. I-Mab undertakes no obligation to publicly update or revise any forward-looking statements, whether as a result of new information, future events, or otherwise, except as may be required by law. Disclaimer
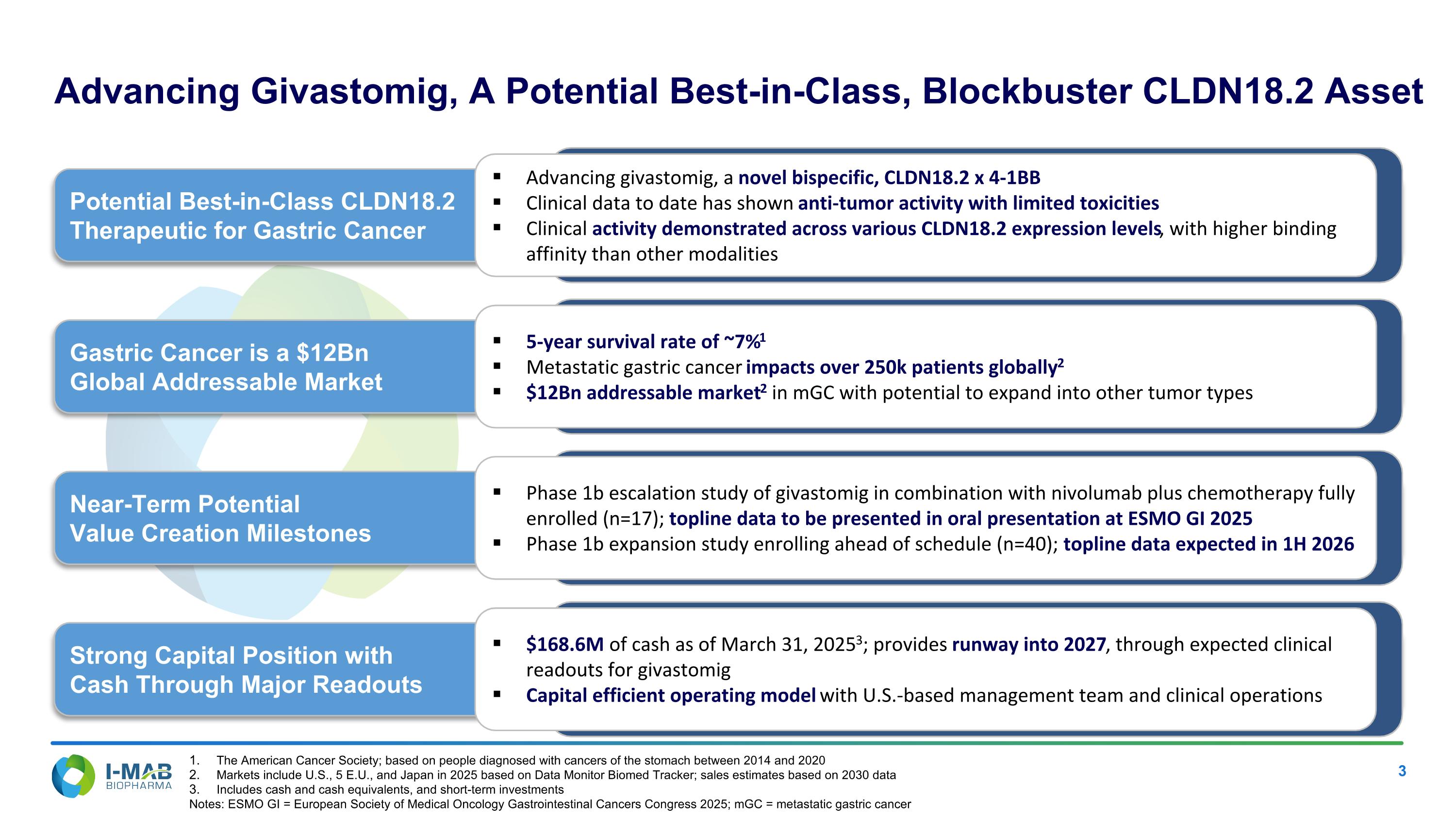
Advancing Givastomig, A Potential Best-in-Class, Blockbuster CLDN18.2 Asset Potential Best-in-Class CLDN18.2 Therapeutic for Gastric Cancer Advancing givastomig, a novel bispecific, CLDN18.2 x 4-1BB Clinical data to date has shown anti-tumor activity with limited toxicities Clinical activity demonstrated across various CLDN18.2 expression levels, with higher binding affinity than other modalities Gastric Cancer is a $12Bn Global Addressable Market 5-year survival rate of ~7%1 Metastatic gastric cancer impacts over 250k patients globally2 $12Bn addressable market2 in mGC with potential to expand into other tumor types Near-Term Potential Value Creation Milestones Phase 1b escalation study of givastomig in combination with nivolumab plus chemotherapy fully enrolled (n=17); topline data to be presented in oral presentation at ESMO GI 2025 Phase 1b expansion study enrolling ahead of schedule (n=40); topline data expected in 1H 2026 Strong Capital Position with Cash Through Major Readouts $168.6M of cash as of March 31, 20253; provides runway into 2027, through expected clinical readouts for givastomig Capital efficient operating model with U.S.-based management team and clinical operations The American Cancer Society; based on people diagnosed with cancers of the stomach between 2014 and 2020 Markets include U.S., 5 E.U., and Japan in 2025 based on Data Monitor Biomed Tracker; sales estimates based on 2030 data Includes cash and cash equivalents, and short-term investments Notes: ESMO GI = European Society of Medical Oncology Gastrointestinal Cancers Congress 2025; mGC = metastatic gastric cancer
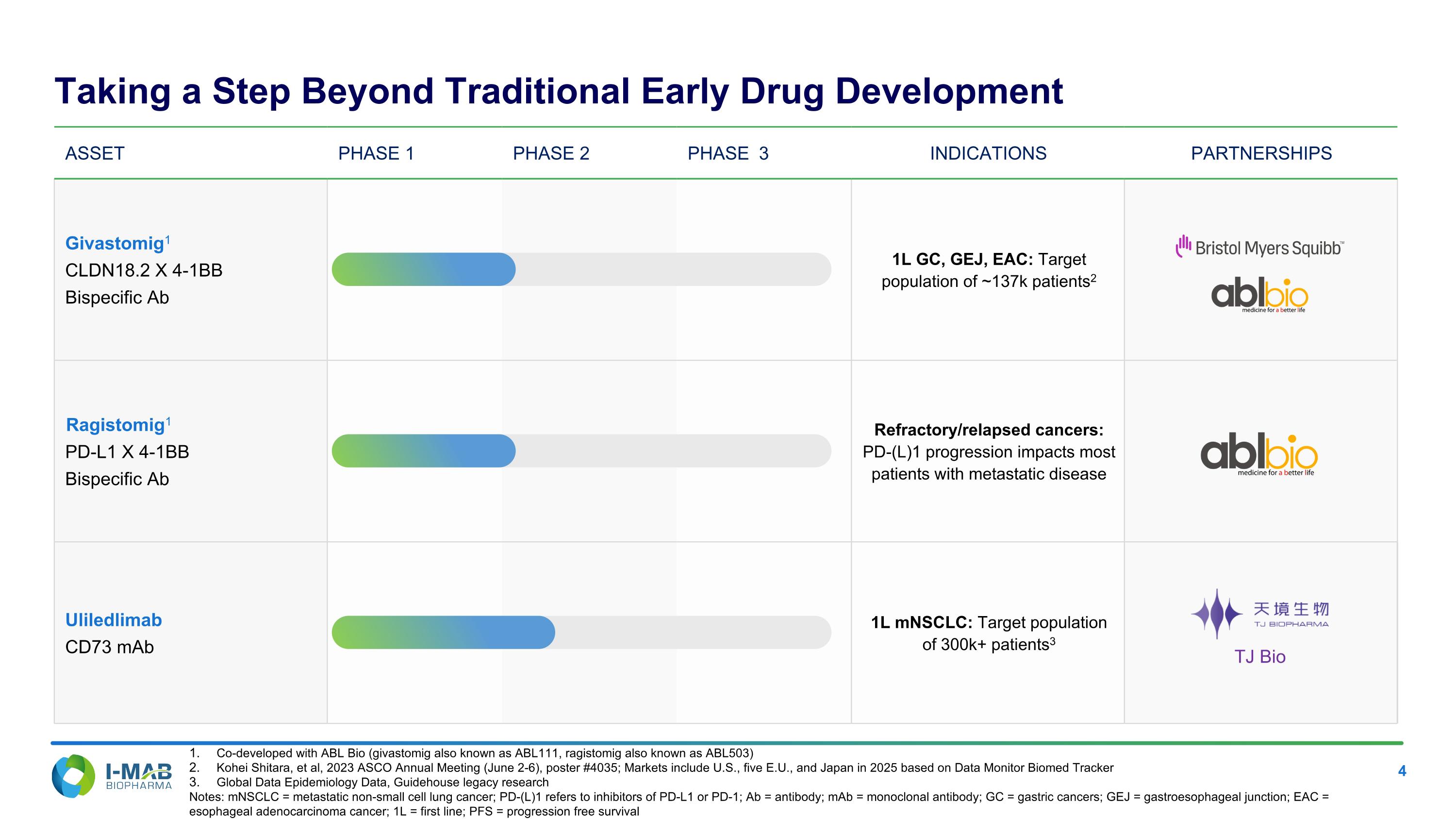
Asset PHASE 1 PHASE 2 PHASE 3 INDICATIONS PARTNERSHIPS Givastomig1 CLDN18.2 X 4-1BB Bispecific Ab 1L GC, GEJ, EAC: Target population of ~137k patients2 Ragistomig1 PD-L1 X 4-1BB Bispecific Ab Refractory/relapsed cancers: PD-(L)1 progression impacts most patients with metastatic disease Uliledlimab CD73 mAb 1L mNSCLC: Target population of 300k+ patients3 Co-developed with ABL Bio (givastomig also known as ABL111, ragistomig also known as ABL503) Kohei Shitara, et al, 2023 ASCO Annual Meeting (June 2-6), poster #4035; Markets include U.S., five E.U., and Japan in 2025 based on Data Monitor Biomed Tracker Global Data Epidemiology Data, Guidehouse legacy research Notes: mNSCLC = metastatic non-small cell lung cancer; PD-(L)1 refers to inhibitors of PD-L1 or PD-1; Ab = antibody; mAb = monoclonal antibody; GC = gastric cancers; GEJ = gastroesophageal junction; EAC = esophageal adenocarcinoma cancer; 1L = first line; PFS = progression free survival; TJ Bio Taking a Step Beyond Traditional Early Drug Development
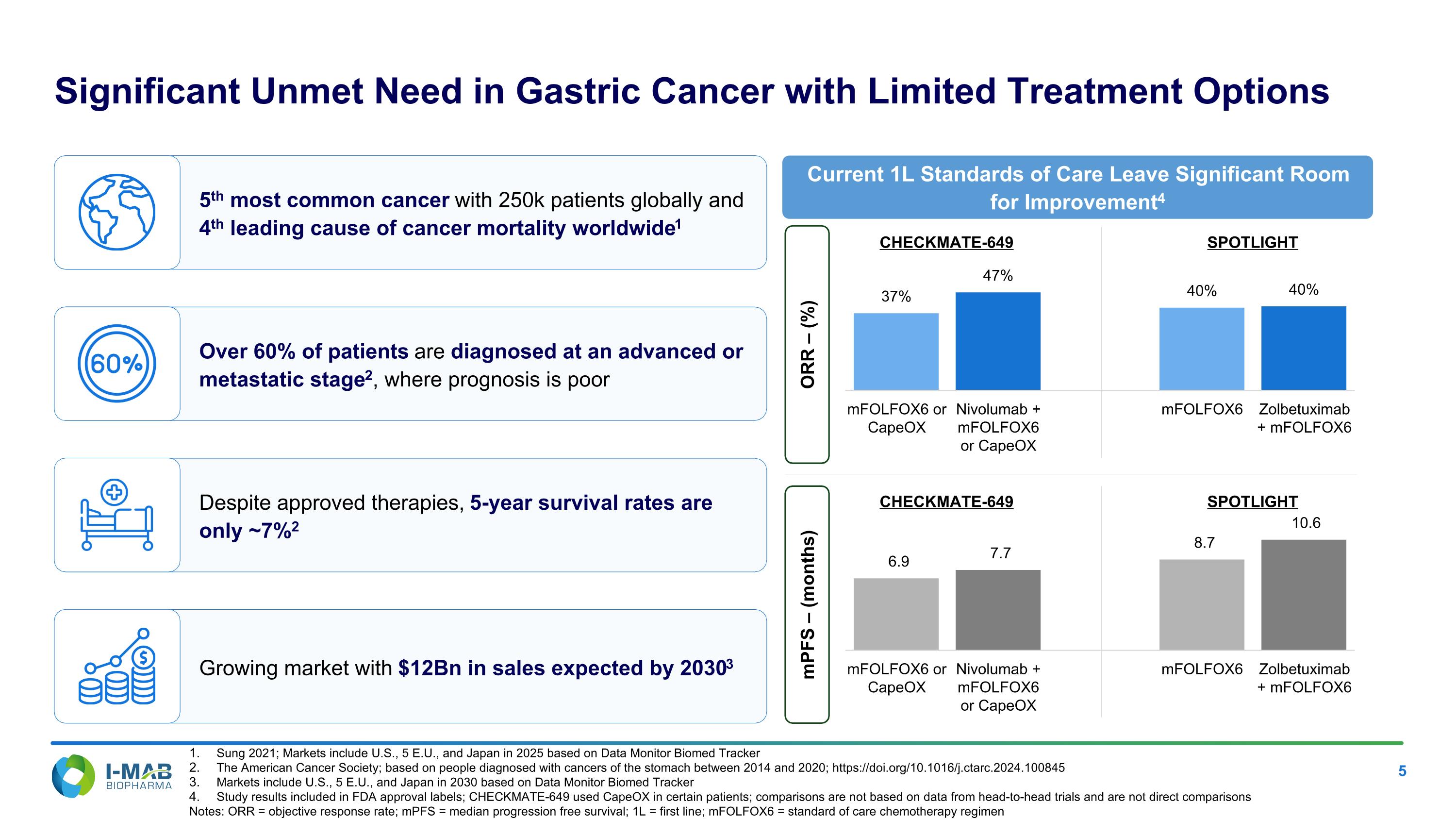
5th most common cancer with 250k patients globally and 4th leading cause of cancer mortality worldwide1 Over 60% of patients are diagnosed at an advanced or metastatic stage2, where prognosis is poor Despite approved therapies, 5-year survival rates are only ~7%2 Growing market with $12Bn in sales expected by 20303 Significant Unmet Need in Gastric Cancer with Limited Treatment Options Current 1L Standards of Care Leave Significant Room for Improvement4 ORR – (%) mPFS – (months) Sung 2021; Markets include U.S., 5 E.U., and Japan in 2025 based on Data Monitor Biomed Tracker The American Cancer Society; based on people diagnosed with cancers of the stomach between 2014 and 2020; https://doi.org/10.1016/j.ctarc.2024.100845 Markets include U.S., 5 E.U., and Japan in 2030 based on Data Monitor Biomed Tracker Study results included in FDA approval labels; CHECKMATE-649 used CapeOX in certain patients; comparisons are not based on data from head-to-head trials and are not direct comparisons Notes: ORR = objective response rate; mPFS = median progression free survival; 1L = first line; mFOLFOX6 = standard of care chemotherapy regimen CHECKMATE-649 SPOTLIGHT CHECKMATE-649 SPOTLIGHT
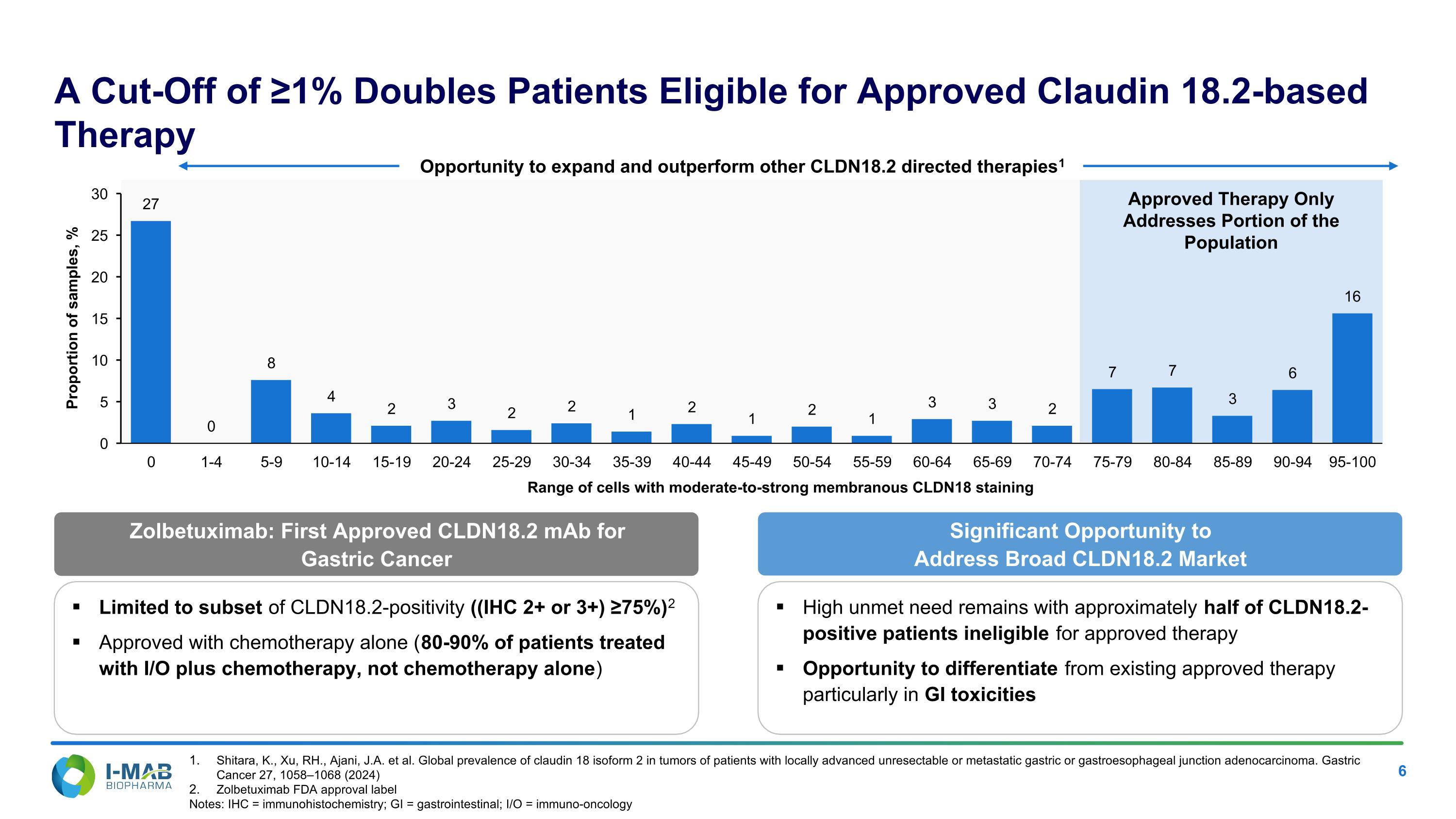
Zolbetuximab: First Approved CLDN18.2 mAb for Gastric Cancer A Cut-Off of ≥1% Doubles Patients Eligible for Approved Claudin 18.2-based Therapy Opportunity to expand and outperform other CLDN18.2 directed therapies1 Limited to subset of CLDN18.2-positivity ((IHC 2+ or 3+) ≥75%)2 Approved with chemotherapy alone (80-90% of patients treated with I/O plus chemotherapy, not chemotherapy alone) Significant Opportunity to Address Broad CLDN18.2 Market High unmet need remains with approximately half of CLDN18.2-positive patients ineligible for approved therapy Opportunity to differentiate from existing approved therapy particularly in GI toxicities Shitara, K., Xu, RH., Ajani, J.A. et al. Global prevalence of claudin 18 isoform 2 in tumors of patients with locally advanced unresectable or metastatic gastric or gastroesophageal junction adenocarcinoma. Gastric Cancer 27, 1058–1068 (2024) Zolbetuximab FDA approval label Notes: IHC = immunohistochemistry; GI = gastrointestinal; I/O = immuno-oncology Approved Therapy Only Addresses Portion of the Population
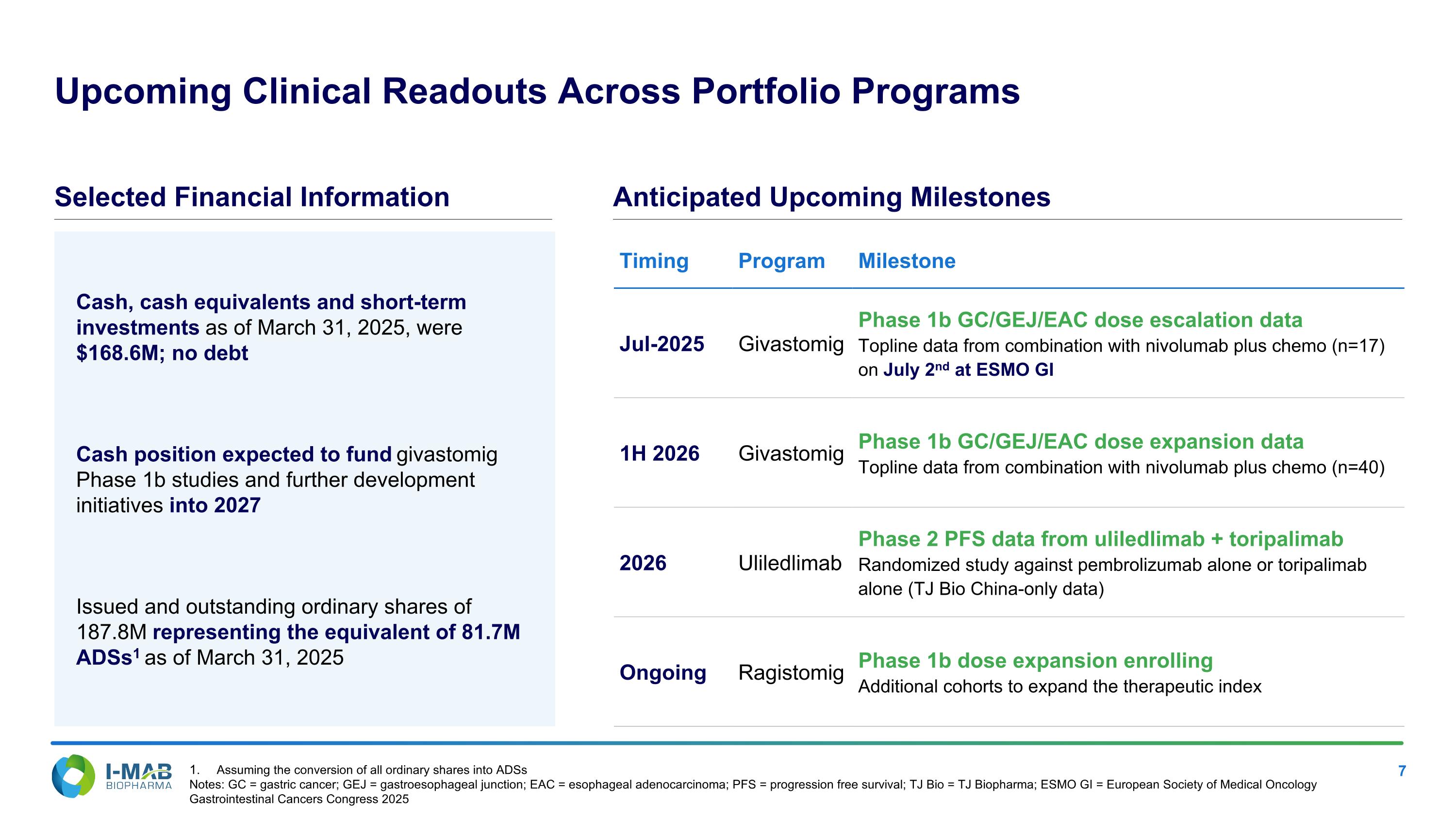
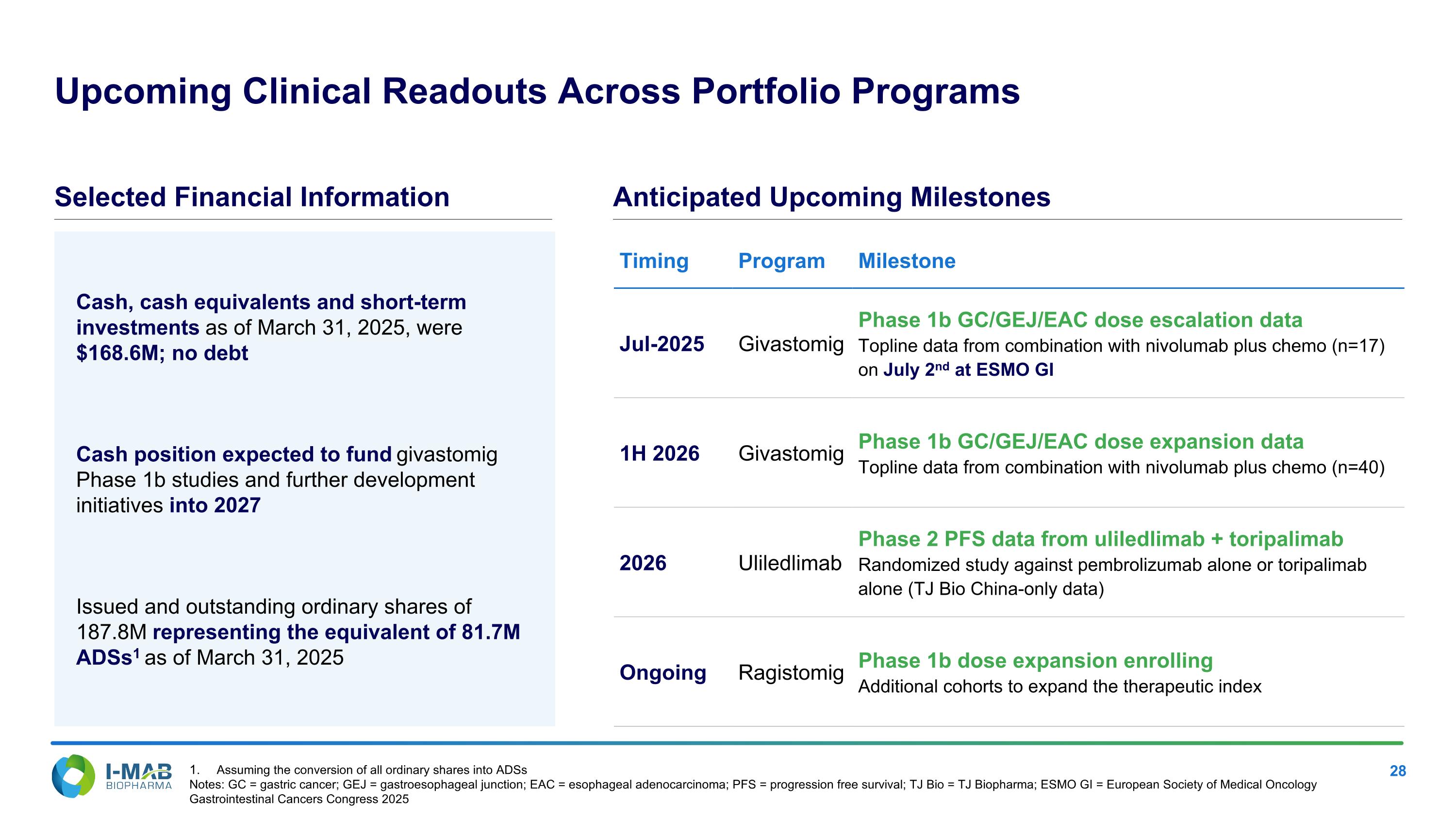
Cash, cash equivalents and short-term investments as of March 31, 2025, were $168.6M; no debt Cash position expected to fund givastomig Phase 1b studies and further development initiatives into 2027 Issued and outstanding ordinary shares of 187.8M representing the equivalent of 81.7M ADSs1 as of March 31, 2025 Upcoming Clinical Readouts Across Portfolio Programs Selected Financial Information Anticipated Upcoming Milestones Timing Program Milestone Jul-2025 Givastomig Phase 1b GC/GEJ/EAC dose escalation data Topline data from combination with nivolumab plus chemo (n=17) on July 2nd at ESMO GI 1H 2026 Givastomig Phase 1b GC/GEJ/EAC dose expansion data Topline data from combination with nivolumab plus chemo (n=40) 2026 Uliledlimab Phase 2 PFS data from uliledlimab + toripalimab Randomized study against pembrolizumab alone or toripalimab alone (TJ Bio China-only data) Ongoing Ragistomig Phase 1b dose expansion enrolling Additional cohorts to expand the therapeutic index Assuming the conversion of all ordinary shares into ADSs Notes: GC = gastric cancer; GEJ = gastroesophageal junction; EAC = esophageal adenocarcinoma; PFS = progression free survival; TJ Bio = TJ Biopharma; ESMO GI = European Society of Medical Oncology Gastrointestinal Cancers Congress 2025
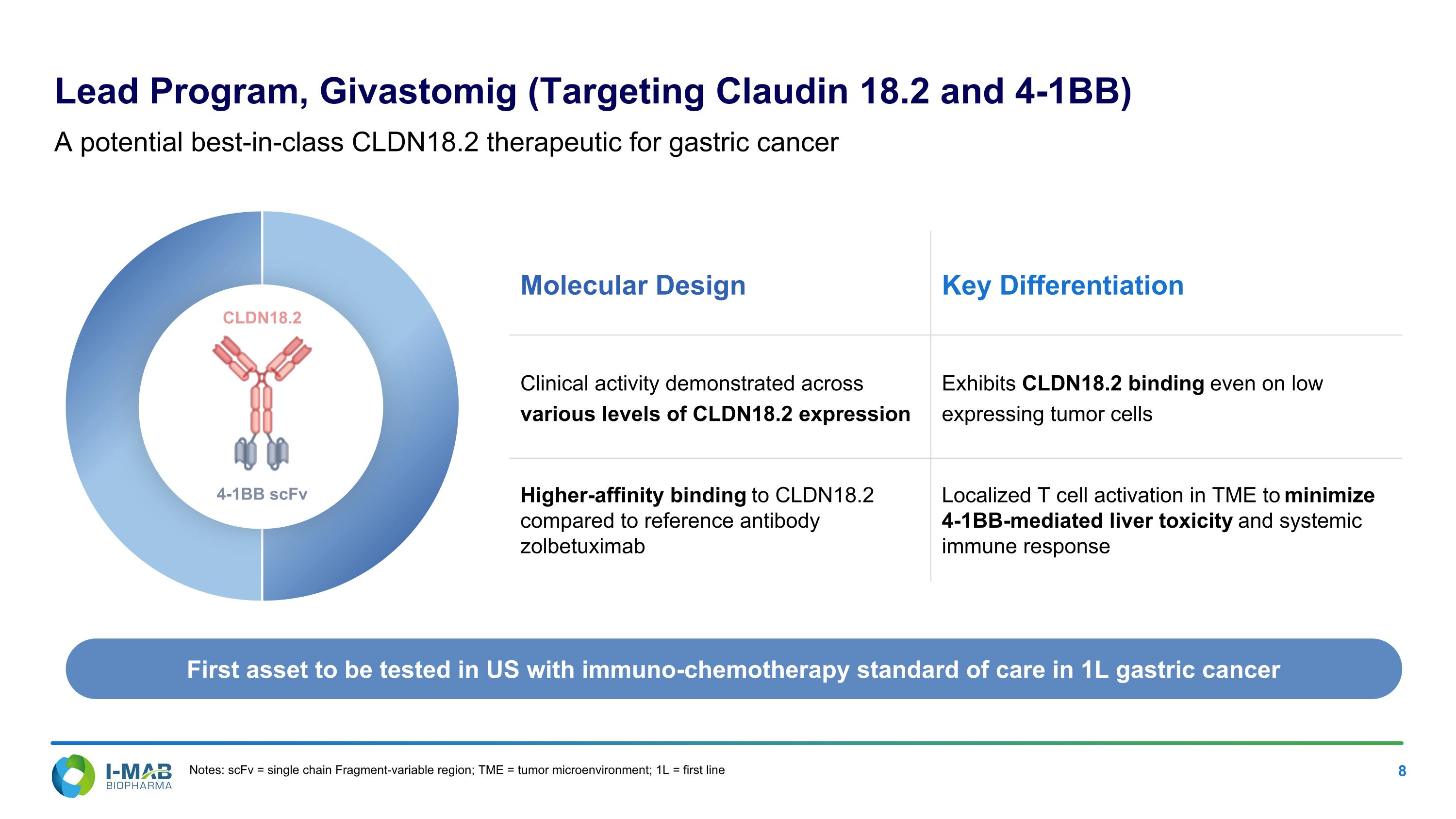
First asset to be tested in US with immuno-chemotherapy standard of care in 1L gastric cancer Molecular Design Key Differentiation Clinical activity demonstrated across various levels of CLDN18.2 expression Exhibits CLDN18.2 binding even on low expressing tumor cells Higher-affinity binding to CLDN18.2 compared to reference antibody zolbetuximab Localized T cell activation in TME to minimize 4-1BB-mediated liver toxicity and systemic immune response Lead Program, Givastomig (Targeting Claudin 18.2 and 4-1BB) A potential best-in-class CLDN18.2 therapeutic for gastric cancer Notes: scFv = single chain Fragment-variable region; TME = tumor microenvironment; 1L = first line 4-1BB scFv CLDN18.2
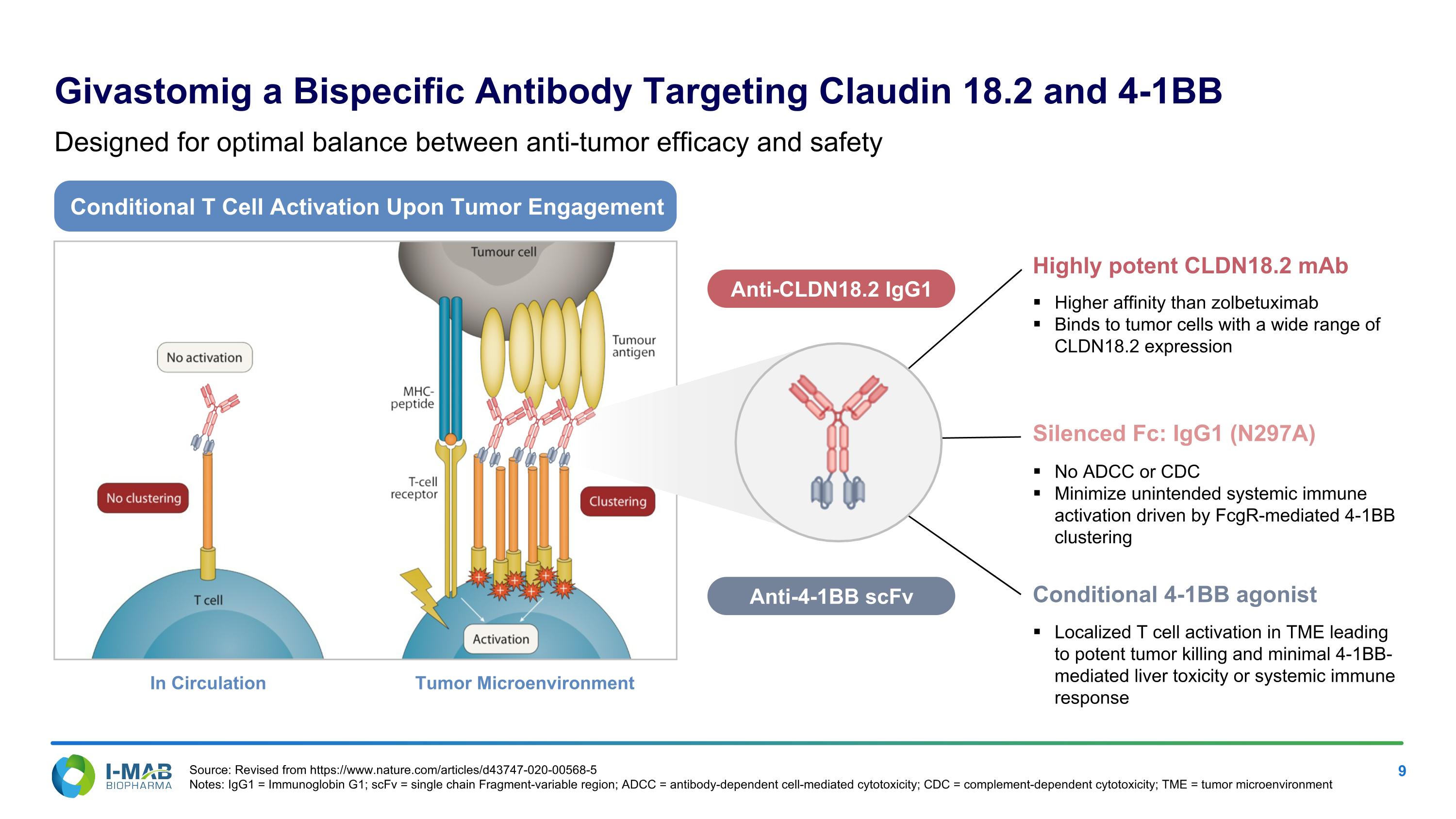
Conditional T Cell Activation Upon Tumor Engagement In Circulation Tumor Microenvironment Anti-CLDN18.2 IgG1 Anti-4-1BB scFv Highly potent CLDN18.2 mAb Higher affinity than zolbetuximab Binds to tumor cells with a wide range of CLDN18.2 expression Silenced Fc: IgG1 (N297A) No ADCC or CDC Minimize unintended systemic immune activation driven by FcgR-mediated 4-1BB clustering Conditional 4-1BB agonist Localized T cell activation in TME leading to potent tumor killing and minimal 4-1BB-mediated liver toxicity or systemic immune response Givastomig a Bispecific Antibody Targeting Claudin 18.2 and 4-1BB Designed for optimal balance between anti-tumor efficacy and safety Source: Revised from https://www.nature.com/articles/d43747-020-00568-5 Notes: IgG1 = Immunoglobin G1; scFv = single chain Fragment-variable region; ADCC = antibody-dependent cell-mediated cytotoxicity; CDC = complement-dependent cytotoxicity; TME = tumor microenvironment
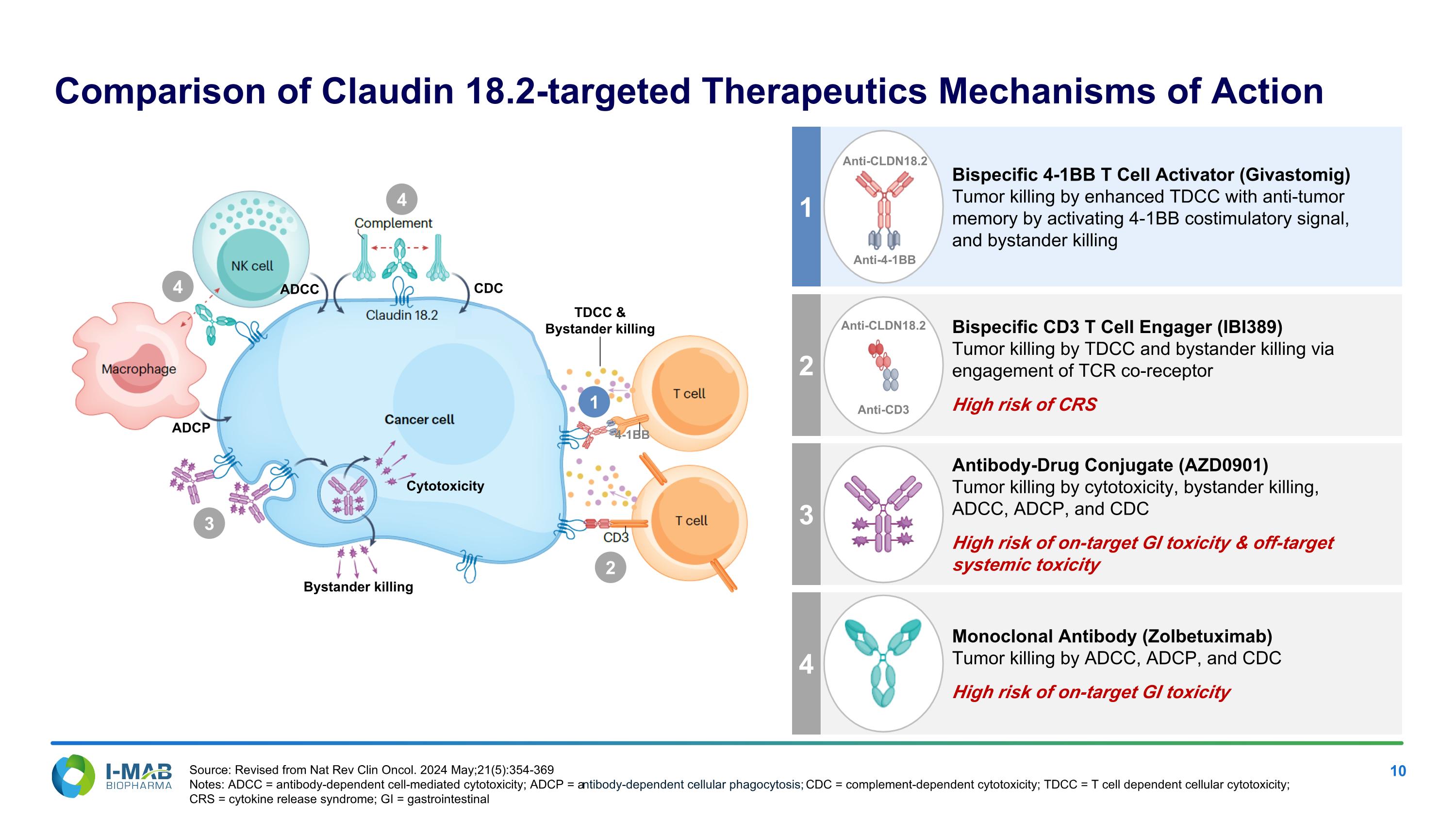
1 4 3 2 Antibody-Drug Conjugate (AZD0901) Tumor killing by cytotoxicity, bystander killing, ADCC, ADCP, and CDC High risk of on-target GI toxicity & off-target systemic toxicity Bispecific CD3 T Cell Engager (IBI389) Tumor killing by TDCC and bystander killing via engagement of TCR co-receptor High risk of CRS Monoclonal Antibody (Zolbetuximab) Tumor killing by ADCC, ADCP, and CDC High risk of on-target GI toxicity Comparison of Claudin 18.2-targeted Therapeutics Mechanisms of Action Anti-CD3 Anti-CLDN18.2 Bispecific 4-1BB T Cell Activator (Givastomig) Tumor killing by enhanced TDCC with anti-tumor memory by activating 4-1BB costimulatory signal, and bystander killing Anti-CLDN18.2 Anti-4-1BB CDC ADCC TDCC & Bystander killing Cytotoxicity Bystander killing 4-1BB ADCP 4 4 3 2 1 Source: Revised from Nat Rev Clin Oncol. 2024 May;21(5):354-369 Notes: ADCC = antibody-dependent cell-mediated cytotoxicity; ADCP = antibody-dependent cellular phagocytosis; CDC = complement-dependent cytotoxicity; TDCC = T cell dependent cellular cytotoxicity; CRS = cytokine release syndrome; GI = gastrointestinal
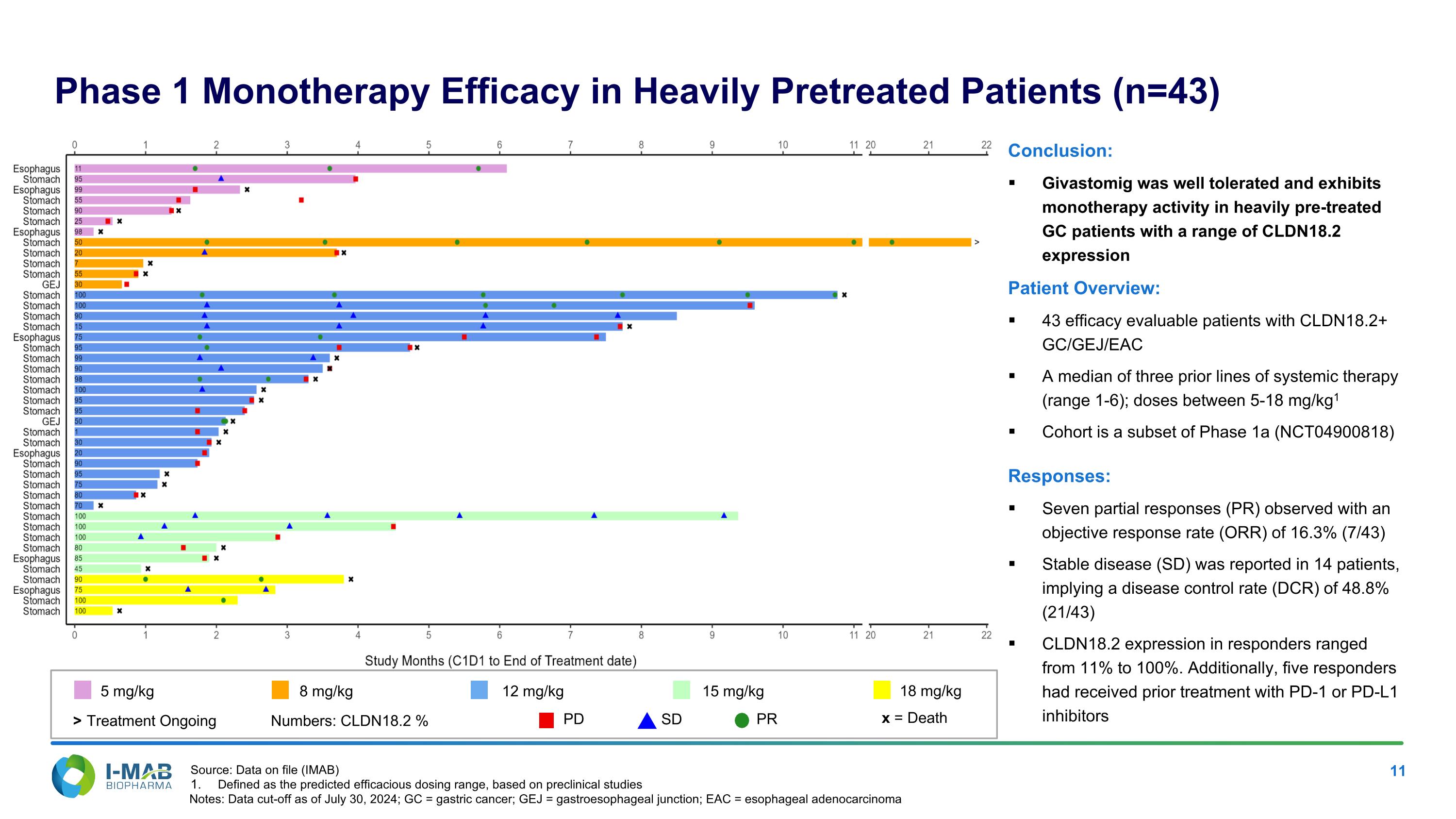
Phase 1 Monotherapy Efficacy in Heavily Pretreated Patients (n=43) Conclusion: Givastomig was well tolerated and exhibits monotherapy activity in heavily pre-treated GC patients with a range of CLDN18.2 expression Patient Overview: 43 efficacy evaluable patients with CLDN18.2+ GC/GEJ/EAC A median of three prior lines of systemic therapy (range 1-6); doses between 5-18 mg/kg1 Cohort is a subset of Phase 1a (NCT04900818) Responses: Seven partial responses (PR) observed with an objective response rate (ORR) of 16.3% (7/43) Stable disease (SD) was reported in 14 patients, implying a disease control rate (DCR) of 48.8% (21/43) CLDN18.2 expression in responders ranged from 11% to 100%. Additionally, five responders had received prior treatment with PD-1 or PD-L1 inhibitors Source: Data on file (IMAB) Defined as the predicted efficacious dosing range, based on preclinical studies Notes: Data cut-off as of July 30, 2024; GC = gastric cancer; GEJ = gastroesophageal junction; EAC = esophageal adenocarcinoma 5 mg/kg Numbers: CLDN18.2 % PD SD PR x = Death 8 mg/kg 12 mg/kg 15 mg/kg 18 mg/kg Treatment Ongoing >
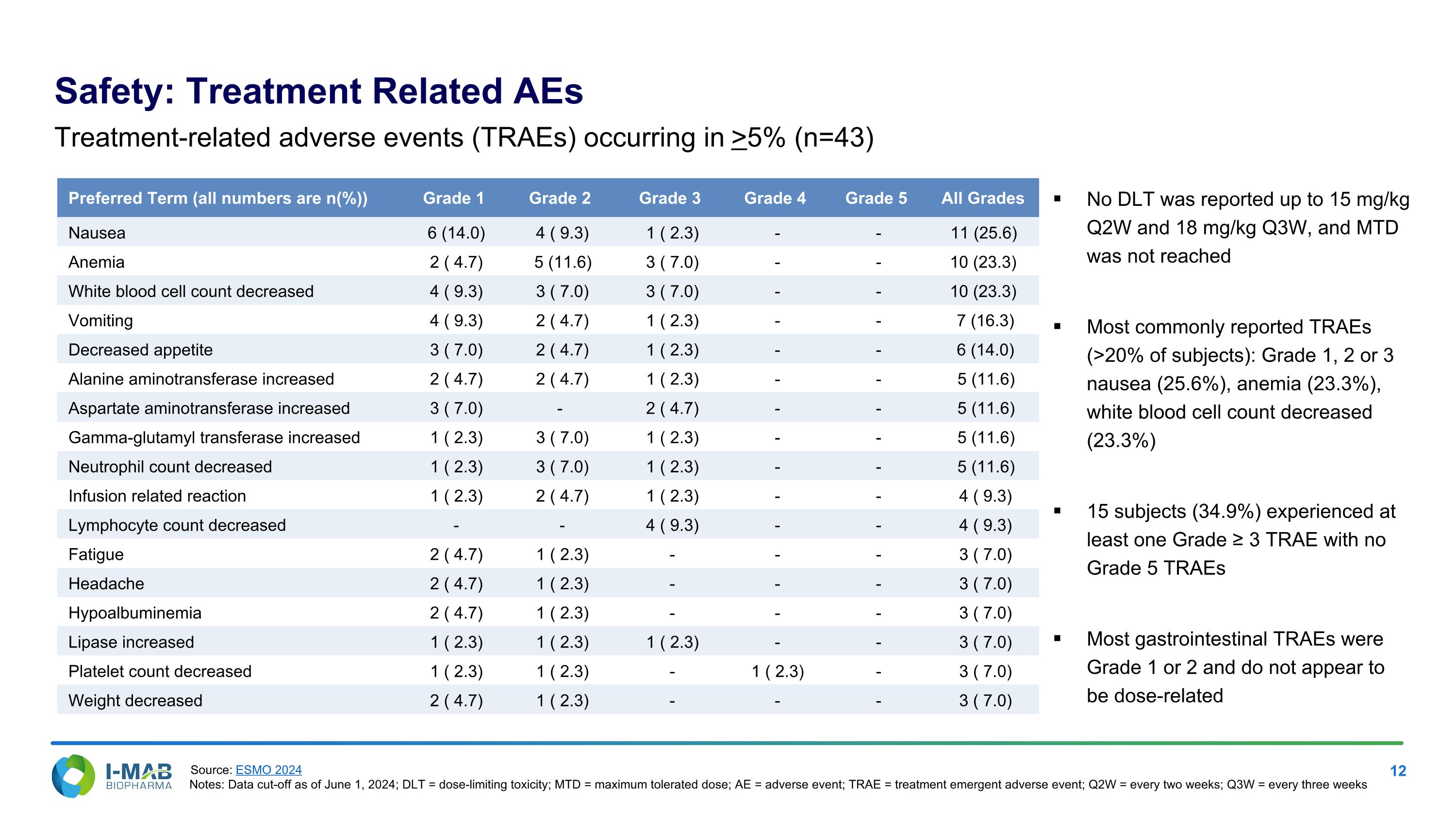
Preferred Term (all numbers are n(%)) Grade 1 Grade 2 Grade 3 Grade 4 Grade 5 All Grades Nausea 6 (14.0) 4 ( 9.3) 1 ( 2.3) - - 11 (25.6) Anemia 2 ( 4.7) 5 (11.6) 3 ( 7.0) - - 10 (23.3) White blood cell count decreased 4 ( 9.3) 3 ( 7.0) 3 ( 7.0) - - 10 (23.3) Vomiting 4 ( 9.3) 2 ( 4.7) 1 ( 2.3) - - 7 (16.3) Decreased appetite 3 ( 7.0) 2 ( 4.7) 1 ( 2.3) - - 6 (14.0) Alanine aminotransferase increased 2 ( 4.7) 2 ( 4.7) 1 ( 2.3) - - 5 (11.6) Aspartate aminotransferase increased 3 ( 7.0) - 2 ( 4.7) - - 5 (11.6) Gamma-glutamyl transferase increased 1 ( 2.3) 3 ( 7.0) 1 ( 2.3) - - 5 (11.6) Neutrophil count decreased 1 ( 2.3) 3 ( 7.0) 1 ( 2.3) - - 5 (11.6) Infusion related reaction 1 ( 2.3) 2 ( 4.7) 1 ( 2.3) - - 4 ( 9.3) Lymphocyte count decreased - - 4 ( 9.3) - - 4 ( 9.3) Fatigue 2 ( 4.7) 1 ( 2.3) - - - 3 ( 7.0) Headache 2 ( 4.7) 1 ( 2.3) - - - 3 ( 7.0) Hypoalbuminemia 2 ( 4.7) 1 ( 2.3) - - - 3 ( 7.0) Lipase increased 1 ( 2.3) 1 ( 2.3) 1 ( 2.3) - - 3 ( 7.0) Platelet count decreased 1 ( 2.3) 1 ( 2.3) - 1 ( 2.3) - 3 ( 7.0) Weight decreased 2 ( 4.7) 1 ( 2.3) - - - 3 ( 7.0) Treatment-related adverse events (TRAEs) occurring in >5% (n=43) No DLT was reported up to 15 mg/kg Q2W and 18 mg/kg Q3W, and MTD was not reached Most commonly reported TRAEs (>20% of subjects): Grade 1, 2 or 3 nausea (25.6%), anemia (23.3%), white blood cell count decreased (23.3%) 15 subjects (34.9%) experienced at least one Grade ≥ 3 TRAE with no Grade 5 TRAEs Most gastrointestinal TRAEs were Grade 1 or 2 and do not appear to be dose-related Safety: Treatment Related AEs Source: ESMO 2024 Notes: Data cut-off as of June 1, 2024; DLT = dose-limiting toxicity; MTD = maximum tolerated dose; AE = adverse event; TRAE = treatment emergent adverse event; Q2W = every two weeks; Q3W = every three weeks
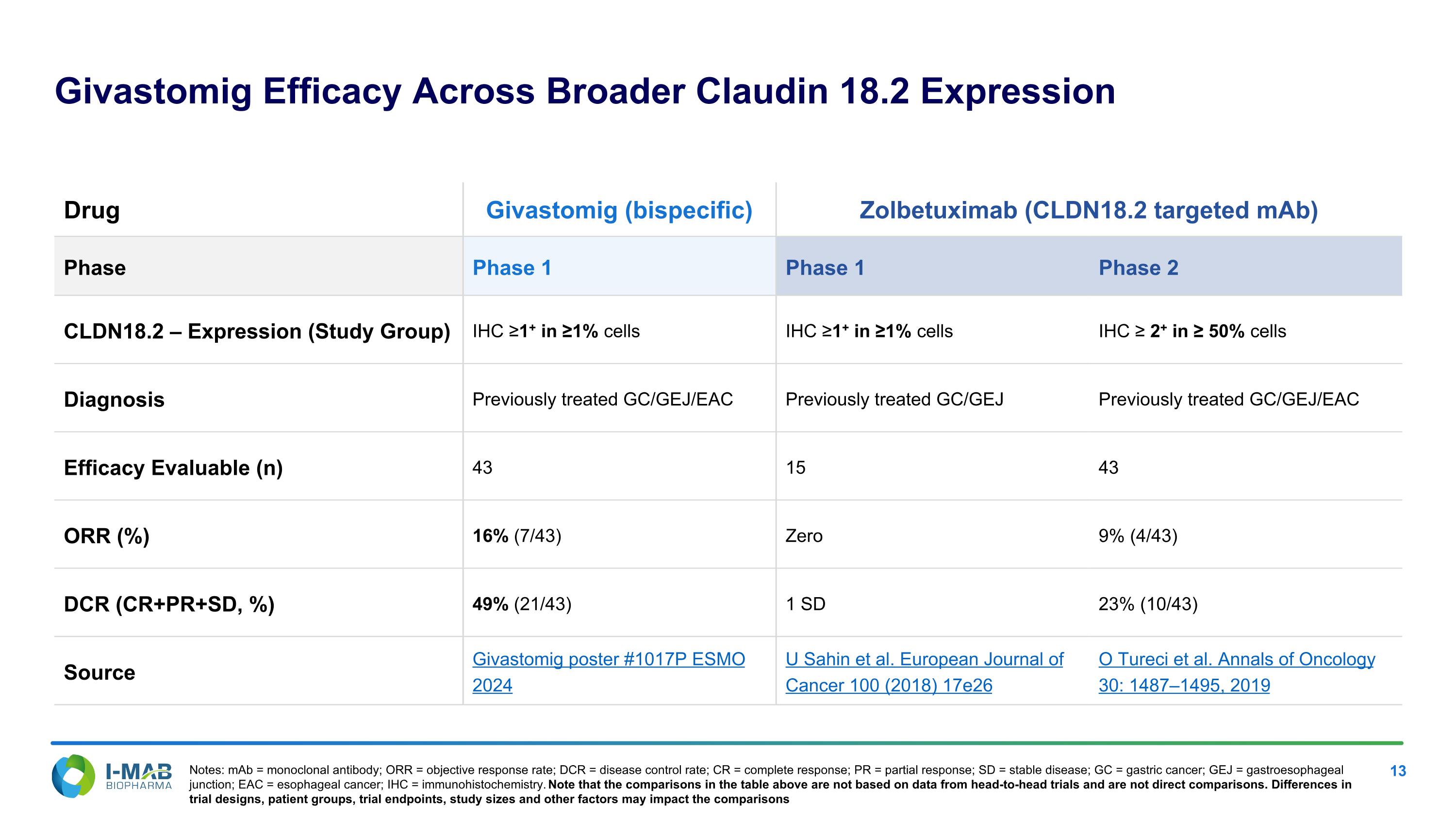
Givastomig Efficacy Across Broader Claudin 18.2 Expression Drug Givastomig (bispecific) Zolbetuximab (CLDN18.2 targeted mAb) Phase Phase 1 Phase 1 Phase 2 CLDN18.2 – Expression (Study Group) IHC ≥1+ in ≥1% cells IHC ≥1+ in ≥1% cells IHC ≥ 2+ in ≥ 50% cells Diagnosis Previously treated GC/GEJ/EAC Previously treated GC/GEJ Previously treated GC/GEJ/EAC Efficacy Evaluable (n) 43 15 43 ORR (%) 16% (7/43) Zero 9% (4/43) DCR (CR+PR+SD, %) 49% (21/43) 1 SD 23% (10/43) Source Givastomig poster #1017P ESMO 2024 U Sahin et al. European Journal of Cancer 100 (2018) 17e26 O Tureci et al. Annals of Oncology 30: 1487–1495, 2019 Notes: mAb = monoclonal antibody; ORR = objective response rate; DCR = disease control rate; CR = complete response; PR = partial response; SD = stable disease; GC = gastric cancer; GEJ = gastroesophageal junction; EAC = esophageal cancer; IHC = immunohistochemistry. Note that the comparisons in the table above are not based on data from head-to-head trials and are not direct comparisons. Differences in trial designs, patient groups, trial endpoints, study sizes and other factors may impact the comparisons
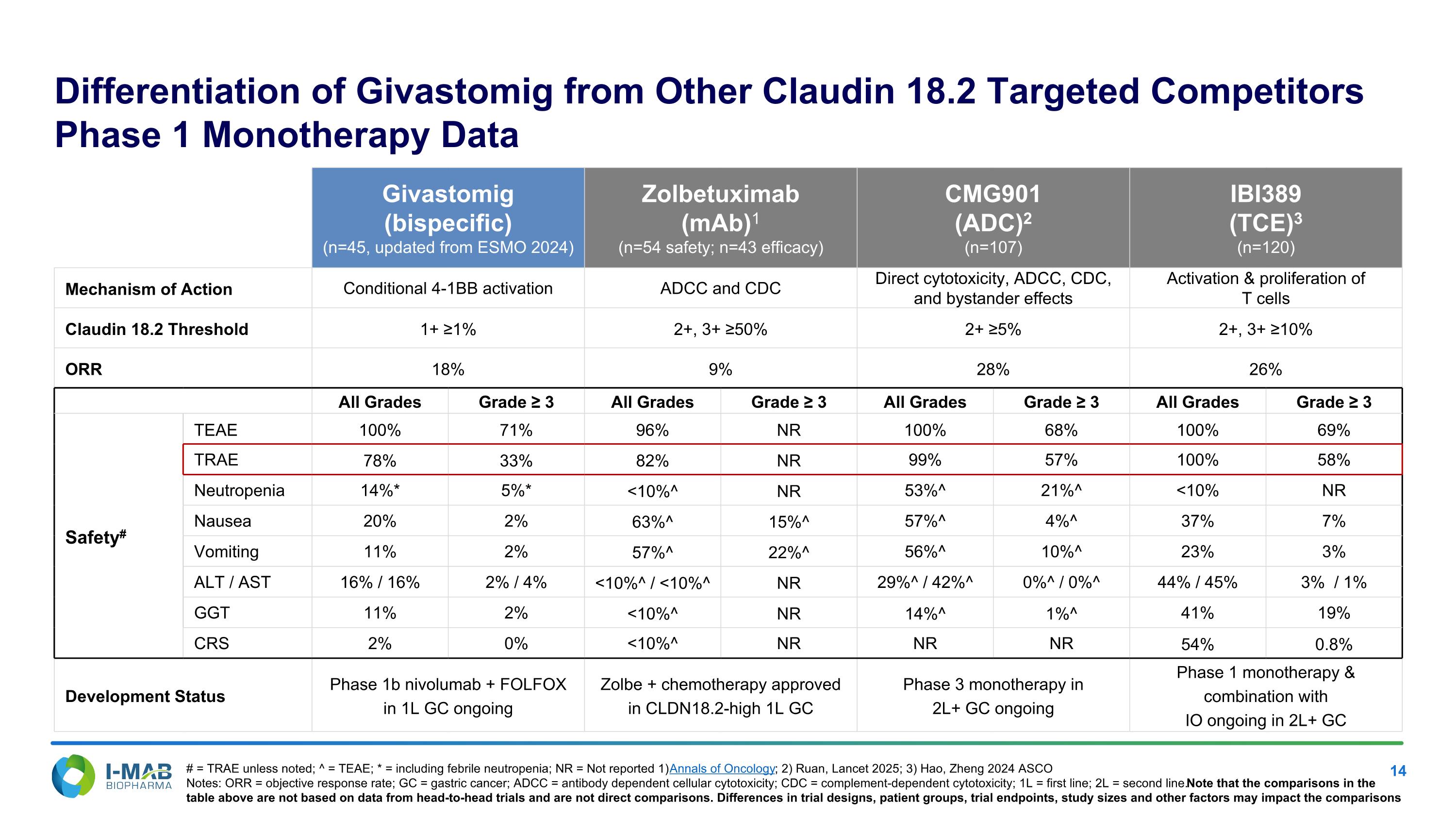
Differentiation of Givastomig from Other Claudin 18.2 Targeted Competitors Phase 1 Monotherapy Data Givastomig (bispecific) (n=45, updated from ESMO 2024) Zolbetuximab (mAb)1 (n=54 safety; n=43 efficacy) CMG901 (ADC)2 (n=107) IBI389 (TCE)3 (n=120) Mechanism of Action Conditional 4-1BB activation ADCC and CDC Direct cytotoxicity, ADCC, CDC, and bystander effects Activation & proliferation of T cells Claudin 18.2 Threshold 1+ ≥1% 2+, 3+ ≥50% 2+ ≥5% 2+, 3+ ≥10% ORR 18% 9% 28% 26% All Grades Grade ≥ 3 All Grades Grade ≥ 3 All Grades Grade ≥ 3 All Grades Grade ≥ 3 Safety# TEAE 100% 71% 96% NR 100% 68% 100% 69% TRAE 78% 33% 82% NR 99% 57% 100% 58% Neutropenia 14%* 5%* <10%^ NR 53%^ 21%^ <10% NR Nausea 20% 2% 63%^ 15%^ 57%^ 4%^ 37% 7% Vomiting 11% 2% 57%^ 22%^ 56%^ 10%^ 23% 3% ALT / AST 16% / 16% 2% / 4% <10%^ / <10%^ NR 29%^ / 42%^ 0%^ / 0%^ 44% / 45% 3% / 1% GGT 11% 2% <10%^ NR 14%^ 1%^ 41% 19% CRS 2% 0% <10%^ NR NR NR 54% 0.8% Development Status Phase 1b nivolumab + FOLFOX in 1L GC ongoing Zolbe + chemotherapy approved in CLDN18.2-high 1L GC Phase 3 monotherapy in 2L+ GC ongoing Phase 1 monotherapy & combination with IO ongoing in 2L+ GC # = TRAE unless noted; ^ = TEAE; * = including febrile neutropenia; NR = Not reported 1) Annals of Oncology; 2) Ruan, Lancet 2025; 3) Hao, Zheng 2024 ASCO Notes: ORR = objective response rate; GC = gastric cancer; ADCC = antibody dependent cellular cytotoxicity; CDC = complement-dependent cytotoxicity; 1L = first line; 2L = second line. Note that the comparisons in the table above are not based on data from head-to-head trials and are not direct comparisons. Differences in trial designs, patient groups, trial endpoints, study sizes and other factors may impact the comparisons
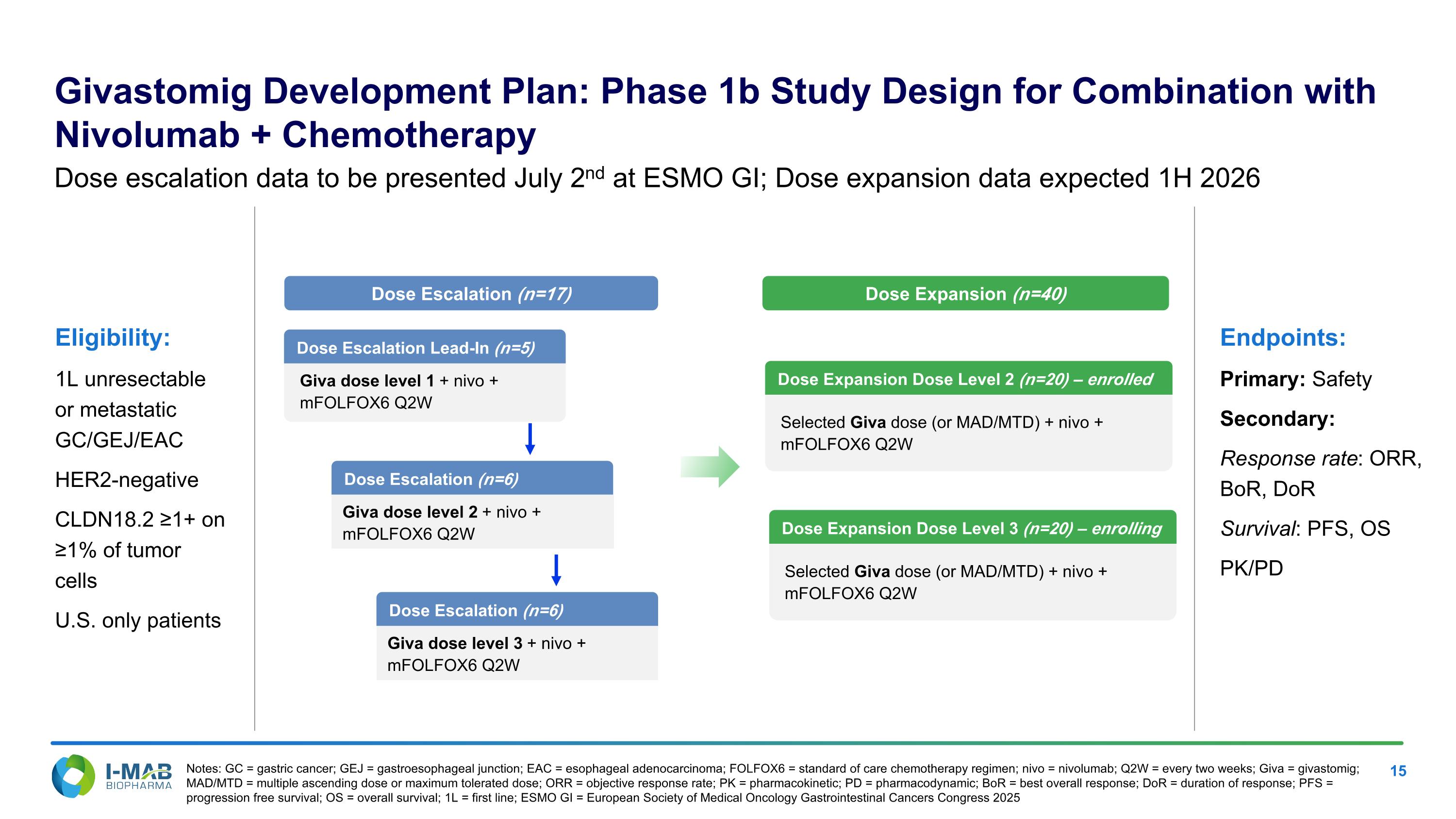
Eligibility: 1L unresectable or metastatic GC/GEJ/EAC HER2-negative CLDN18.2 ≥1+ on ≥1% of tumor cells U.S. only patients Endpoints: Primary: Safety Secondary: Response rate: ORR, BoR, DoR Survival: PFS, OS PK/PD Dose Escalation Lead-In (n=5) Giva dose level 1 + nivo + mFOLFOX6 Q2W Dose Escalation (n=17) Dose Expansion (n=40) Dose Expansion Dose Level 2 (n=20) – enrolled Selected Giva dose (or MAD/MTD) + nivo + mFOLFOX6 Q2W Dose Escalation (n=6) Giva dose level 2 + nivo + mFOLFOX6 Q2W Dose Escalation (n=6) Giva dose level 3 + nivo + mFOLFOX6 Q2W Dose Expansion Dose Level 3 (n=20) – enrolling Selected Giva dose (or MAD/MTD) + nivo + mFOLFOX6 Q2W Dose escalation data to be presented July 2nd at ESMO GI; Dose expansion data expected 1H 2026 Notes: GC = gastric cancer; GEJ = gastroesophageal junction; EAC = esophageal adenocarcinoma; FOLFOX6 = standard of care chemotherapy regimen; nivo = nivolumab; Q2W = every two weeks; Giva = givastomig; MAD/MTD = multiple ascending dose or maximum tolerated dose; ORR = objective response rate; PK = pharmacokinetic; PD = pharmacodynamic; BoR = best overall response; DoR = duration of response; PFS = progression free survival; OS = overall survival; 1L = first line; ESMO GI = European Society of Medical Oncology Gastrointestinal Cancers Congress 2025 Givastomig Development Plan: Phase 1b Study Design for Combination with Nivolumab + Chemotherapy
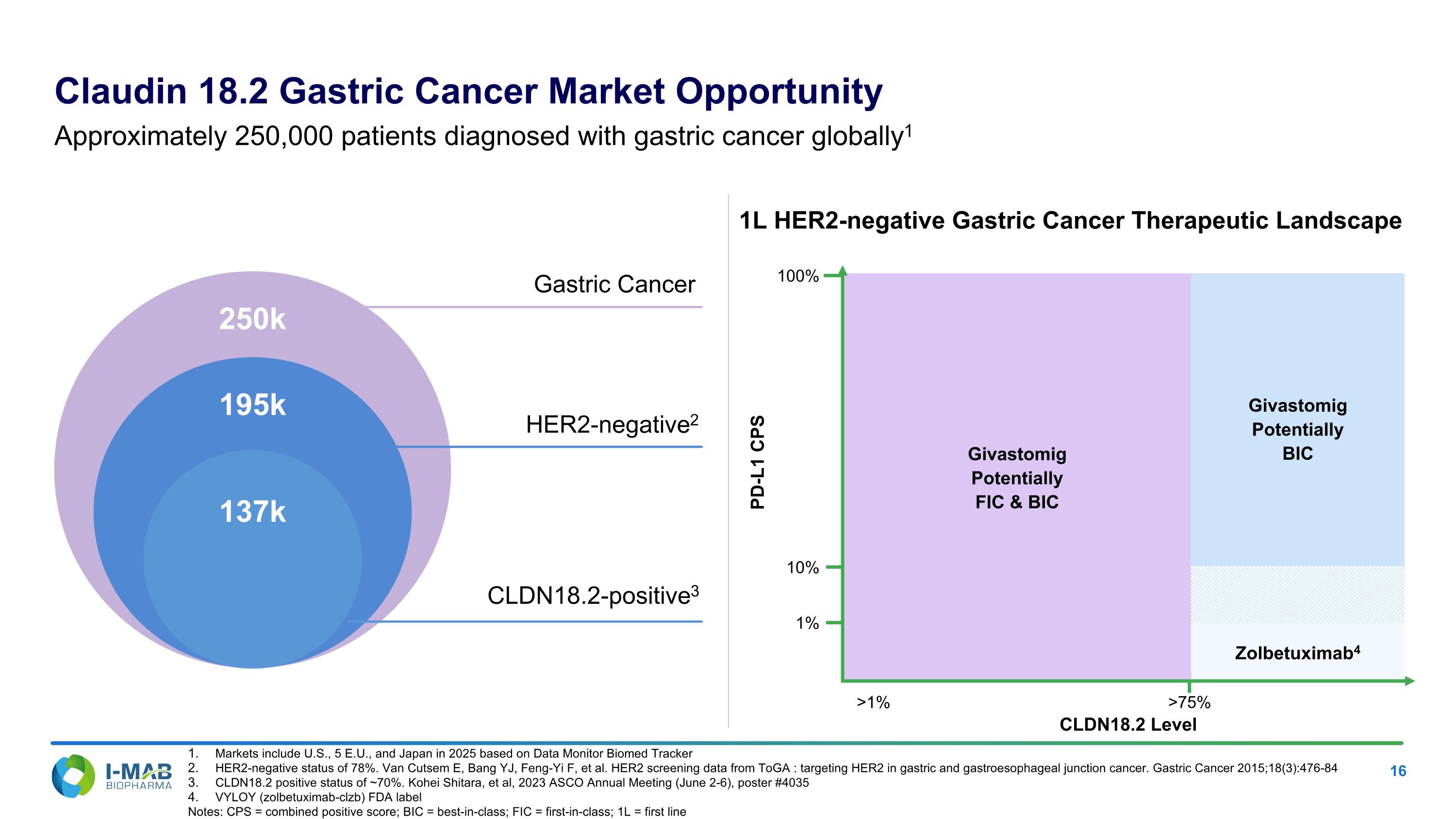
Givastomig Potentially FIC & BIC Givastomig Potentially BIC Zolbetuximab4 Gastric Cancer HER2-negative2 CLDN18.2-positive3 250k 195k 137k PD-L1 CPS CLDN18.2 Level 100% >75% >1% 1L HER2-negative Gastric Cancer Therapeutic Landscape Claudin 18.2 Gastric Cancer Market Opportunity Approximately 250,000 patients diagnosed with gastric cancer globally1 Markets include U.S., 5 E.U., and Japan in 2025 based on Data Monitor Biomed Tracker HER2-negative status of 78%. Van Cutsem E, Bang YJ, Feng-Yi F, et al. HER2 screening data from ToGA : targeting HER2 in gastric and gastroesophageal junction cancer. Gastric Cancer 2015;18(3):476-84 CLDN18.2 positive status of ~70%. Kohei Shitara, et al, 2023 ASCO Annual Meeting (June 2-6), poster #4035 VYLOY (zolbetuximab-clzb) FDA label Notes: CPS = combined positive score; BIC = best-in-class; FIC = first-in-class; 1L = first line 10% 1%
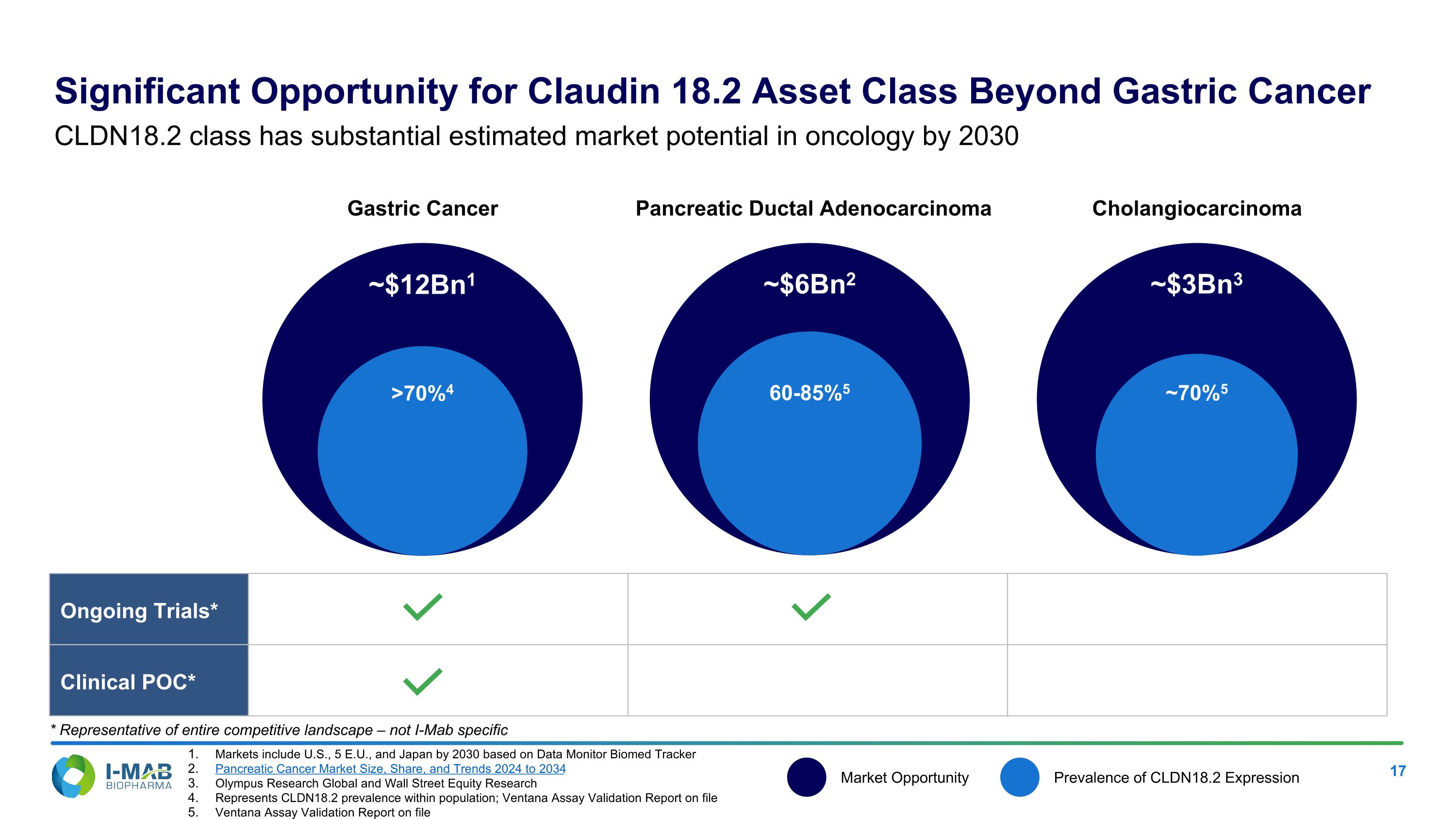
Markets include U.S., 5 E.U., and Japan by 2030 based on Data Monitor Biomed Tracker Pancreatic Cancer Market Size, Share, and Trends 2024 to 2034 Olympus Research Global and Wall Street Equity Research Represents CLDN18.2 prevalence within population; Ventana Assay Validation Report on file Ventana Assay Validation Report on file >70%4 Gastric Cancer ~$12Bn1 60-85%5 Pancreatic Ductal Adenocarcinoma ~$6Bn2 ~70%5 Cholangiocarcinoma ~$3Bn3 Ongoing Trials* Clinical POC* Market Opportunity Prevalence of CLDN18.2 Expression Significant Opportunity for Claudin 18.2 Asset Class Beyond Gastric Cancer CLDN18.2 class has substantial estimated market potential in oncology by 2030 * Representative of entire competitive landscape – not I-Mab specific
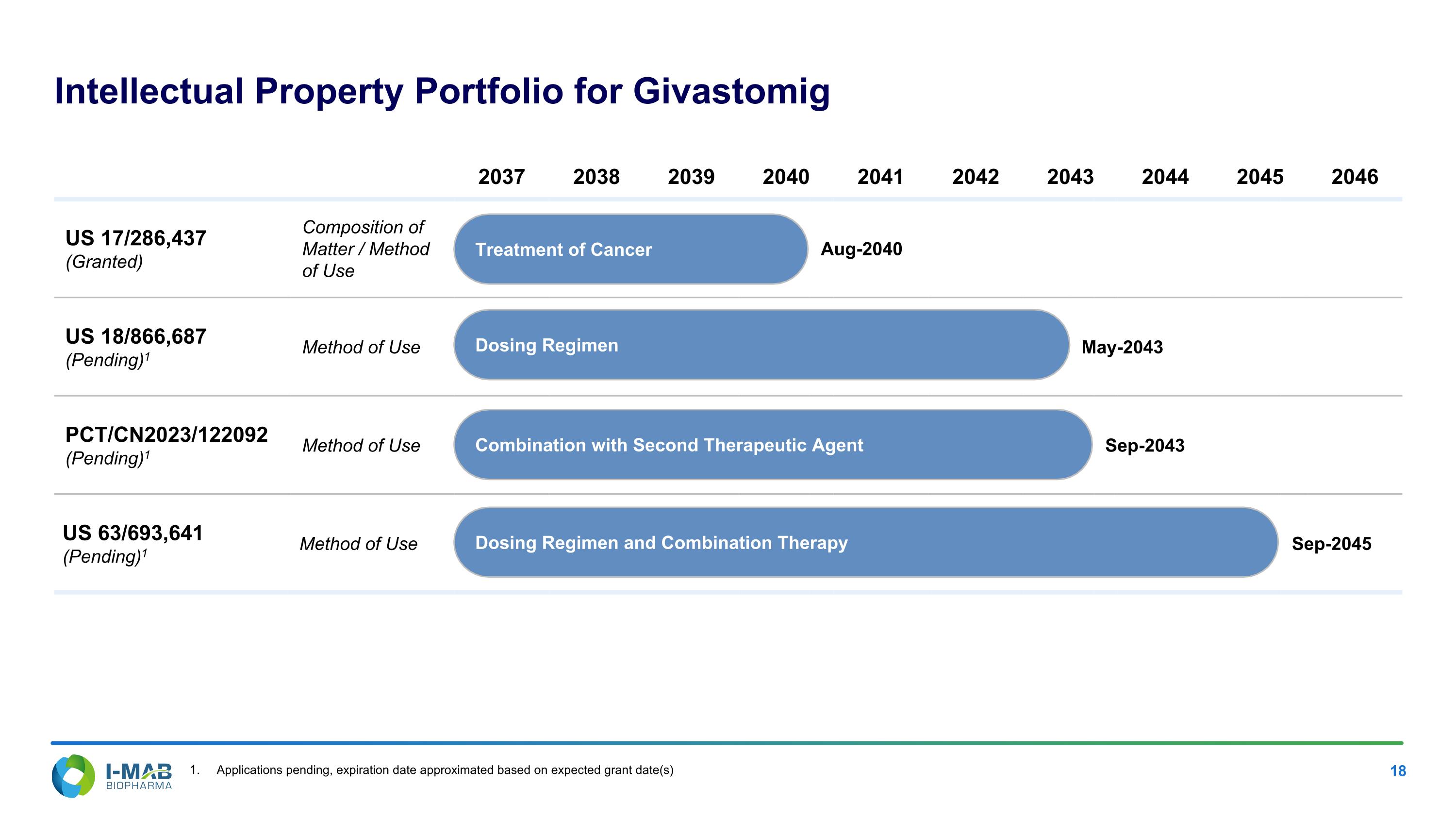
Intellectual Property Portfolio for Givastomig 2037 2038 2039 2040 2041 2042 2043 2044 2045 2046 US 17/286,437 (Granted) Composition of Matter / Method of Use Treatment of Cancer Aug-2040 US 18/866,687 (Pending)1 Method of Use Dosing Regimen May-2043 PCT/CN2023/122092 (Pending)1 Method of Use Combination with Second Therapeutic Agent Sep-2043 US 63/693,641 (Pending)1 Method of Use Dosing Regimen and Combination Therapy Sep-2045 Applications pending, expiration date approximated based on expected grant date(s) Treatment of Cancer Dosing Regimen Combination with Second Therapeutic Agent Dosing Regimen and Combination Therapy
Givastomig, a Potential Best-in-Class Claudin 18.2 Therapeutic First CLDN18.2 asset tested in U.S. with immuno-chemotherapy standard of care in 1L gastric cancer Notes: scFv = single chain Fragment-variable region; 1L = first line High CLDN18.2 affinity with conditional 4-1BB activation Designed for lasting immune responses across wide range of CLDN expression Response rate and tolerability stand out versus benchmarks Phase 1b dose escalation in Jul-2025; dose expansion data in 1H 2026 4-1BB scFv CLDN18.2
Molecular Design: Molecule binds to PD-L1 for activation of 4-1BB in the TME Implications: Mitigation of liver toxicity and systemic immune response Enhancement of anti-tumor immunity and re-invigoration of exhausted T cells1 Development: Co-development with ABL Bio Combinations will require maximizing the therapeutic index Implications: Further testing of additional doses and interval administration to maximize the therapeutic index Ragistomig (ABL503) targeting PD-L1 and 4-1BB A novel bispecific integrates PD-L1 as a tumor engager and 4-1BB as a conditional T cell activator 4-1BB scFv PD-L1 IgG JITC 2021 Notes: scFv = single chain Fragment-variable region; TME = tumor microenvironment

Phase 1 Data Support Further Development as a Monotherapy and in Combination with Other Agents Overview: 44 efficacy evaluable patients (53 enrolled) with advanced or relapsed/refractory solid tumors (NCT04762641) 64.2% (34/53) of patients enrolled had at least three prior lines of systemic anti-cancer treatment Efficacy Results at 3 and 5 mg/kg Q2W: Objective Response Rate (ORR) of 26.9% (7/26), Clinical Benefit Ratio (CBR) of 69.2% (18/26) One CR, six PRs, eleven SDs 71.4% of responders had received prior anti-PD-(L)-1 inhibitors The CR was observed in a heavily pretreated ovarian cancer patient dosed at 3 mg/kg (seven lines of prior therapy) Conclusion: Compelling clinical data in checkpoint inhibitor relapsed/refractory and IO naïve patients Treatment Duration (Days) CR start PR start On-going PD start 0.7 mg 2 mg/kg 2 mg 3 mg/kg 7 mg 5 mg/kg 0.3 mg/kg 7 mg/kg 1 mg/kg 10 mg/kg Source: ASCO 2024 Notes: Data cut-off as of April 19, 2024. CR = complete response; PR = partial response; PD = progressive disease; SD = stable disease; IO = Immuno-oncology; Q2W = every two weeks
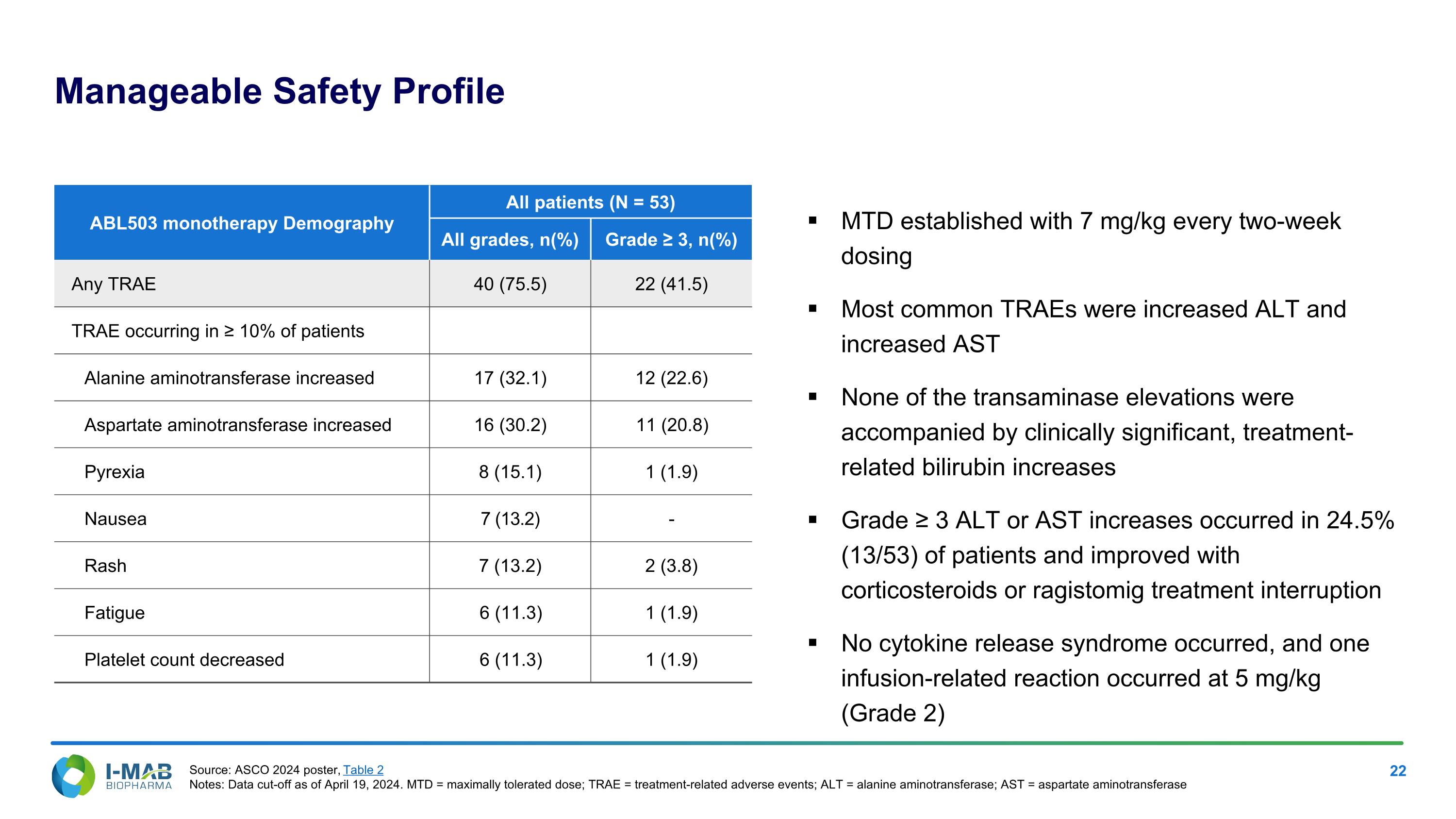
Manageable Safety Profile MTD established with 7 mg/kg every two-week dosing Most common TRAEs were increased ALT and increased AST None of the transaminase elevations were accompanied by clinically significant, treatment-related bilirubin increases Grade ≥ 3 ALT or AST increases occurred in 24.5% (13/53) of patients and improved with corticosteroids or ragistomig treatment interruption No cytokine release syndrome occurred, and one infusion-related reaction occurred at 5 mg/kg (Grade 2) ABL503 monotherapy Demography All patients (N = 53) All grades, n(%) Grade ≥ 3, n(%) Any TRAE 40 (75.5) 22 (41.5) TRAE occurring in ≥ 10% of patients Alanine aminotransferase increased 17 (32.1) 12 (22.6) Aspartate aminotransferase increased 16 (30.2) 11 (20.8) Pyrexia 8 (15.1) 1 (1.9) Nausea 7 (13.2) - Rash 7 (13.2) 2 (3.8) Fatigue 6 (11.3) 1 (1.9) Platelet count decreased 6 (11.3) 1 (1.9) Source: ASCO 2024 poster, Table 2 Notes: Data cut-off as of April 19, 2024. MTD = maximally tolerated dose; TRAE = treatment-related adverse events; ALT = alanine aminotransferase; AST = aspartate aminotransferase
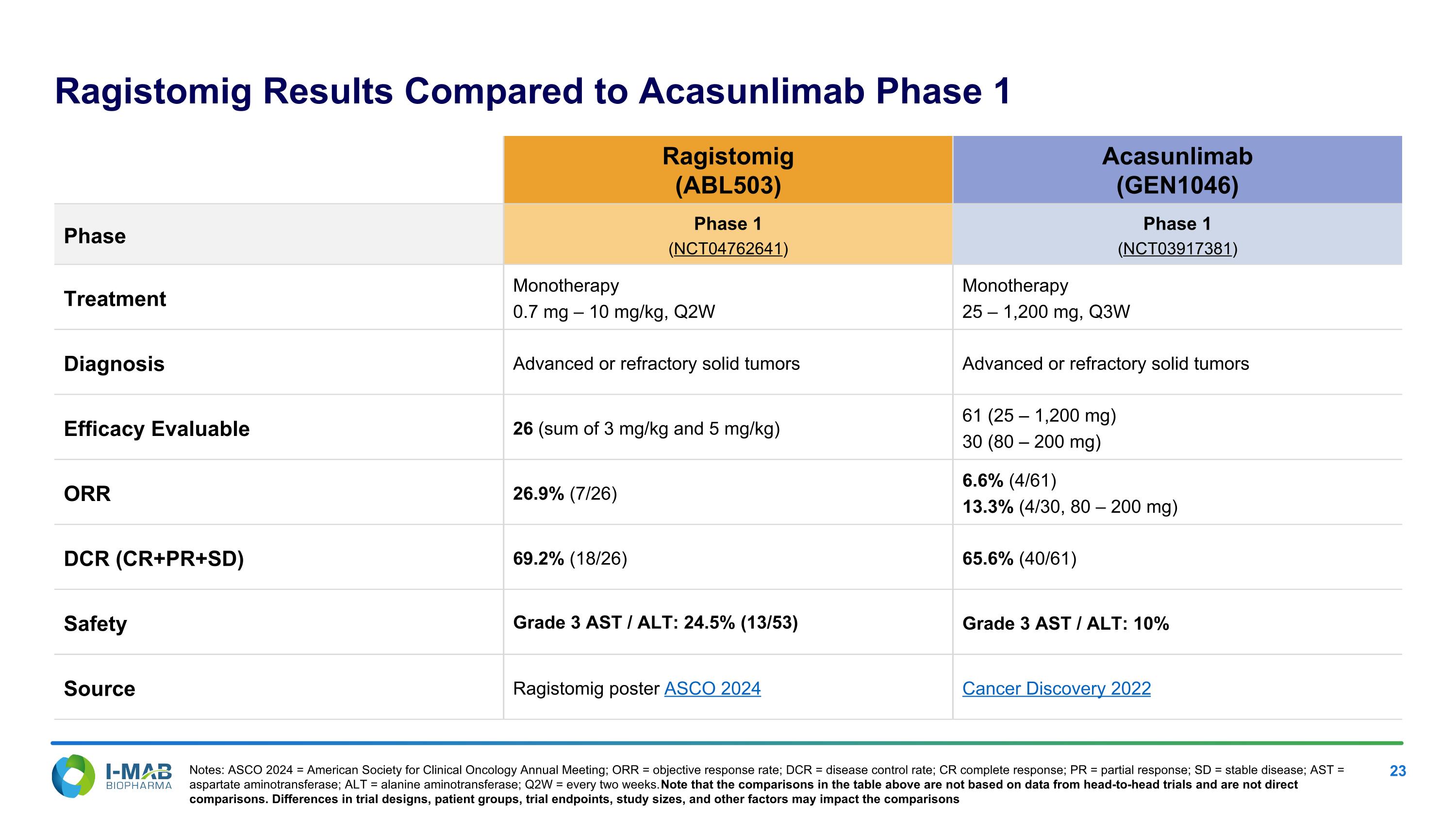
Ragistomig Results Compared to Acasunlimab Phase 1 Ragistomig (ABL503) Acasunlimab (GEN1046) Phase Phase 1 (NCT04762641) Phase 1 (NCT03917381) Treatment Monotherapy 0.7 mg – 10 mg/kg, Q2W Monotherapy 25 – 1,200 mg, Q3W Diagnosis Advanced or refractory solid tumors Advanced or refractory solid tumors Efficacy Evaluable 26 (sum of 3 mg/kg and 5 mg/kg) 61 (25 – 1,200 mg) 30 (80 – 200 mg) ORR 26.9% (7/26) 6.6% (4/61) 13.3% (4/30, 80 – 200 mg) DCR (CR+PR+SD) 69.2% (18/26) 65.6% (40/61) Safety Grade 3 AST / ALT: 24.5% (13/53) Grade 3 AST / ALT: 10% Source Ragistomig poster ASCO 2024 Cancer Discovery 2022 Notes: ASCO 2024 = American Society for Clinical Oncology Annual Meeting; ORR = objective response rate; DCR = disease control rate; CR complete response; PR = partial response; SD = stable disease; AST = aspartate aminotransferase; ALT = alanine aminotransferase; Q2W = every two weeks. Note that the comparisons in the table above are not based on data from head-to-head trials and are not direct comparisons. Differences in trial designs, patient groups, trial endpoints, study sizes, and other factors may impact the comparisons
Uliledlimab (Targeting CD73) A potential best-in-class CD73 therapeutic Anti-CD73 CD73 Biology: CD73 is the rate-limiting enzyme and best target in the adenosine immunosuppressive pathway Key Advantages: Uliledlimab completely inhibits CD73 activity and the production of adenosine without the “hook effect”1 Development: Coordinated global development with TJ Bio Status: I-Mab development paused pending positive data from TJ Bio’s ongoing doublet study in 1L CD73+ NSCLC AACR 2021 Note: mNSCLC = metastatic non-small cell lung cancer; AMP = adenosine monophosphate; TJ Bio = TJ Biopharma
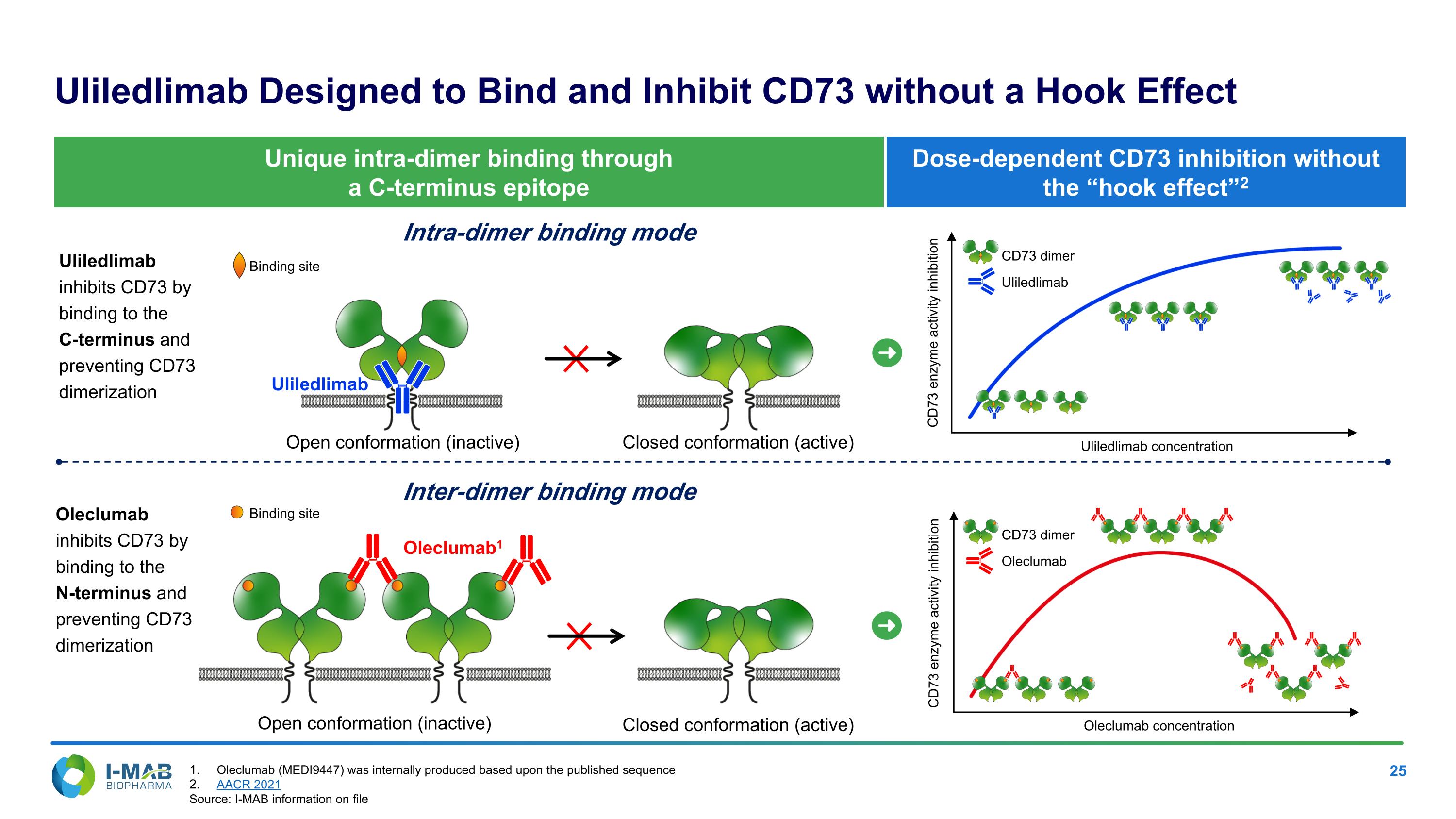
CD73 enzyme activity inhibition Dose-dependent CD73 inhibition without the “hook effect”2 Uliledlimab Designed to Bind and Inhibit CD73 without a Hook Effect Open conformation (inactive) Closed conformation (active) Oleclumab1 Intra-dimer binding mode Inter-dimer binding mode Open conformation (inactive) Closed conformation (active) Unique intra-dimer binding through a C-terminus epitope Uliledlimab inhibits CD73 by binding to the C-terminus and preventing CD73 dimerization Oleclumab inhibits CD73 by binding to the N-terminus and preventing CD73 dimerization Uliledlimab CD73 enzyme activity inhibition Uliledlimab concentration Oleclumab concentration Uliledlimab CD73 dimer Oleclumab CD73 dimer Binding site Binding site Oleclumab (MEDI9447) was internally produced based upon the published sequence AACR 2021 Source: I-MAB information on file
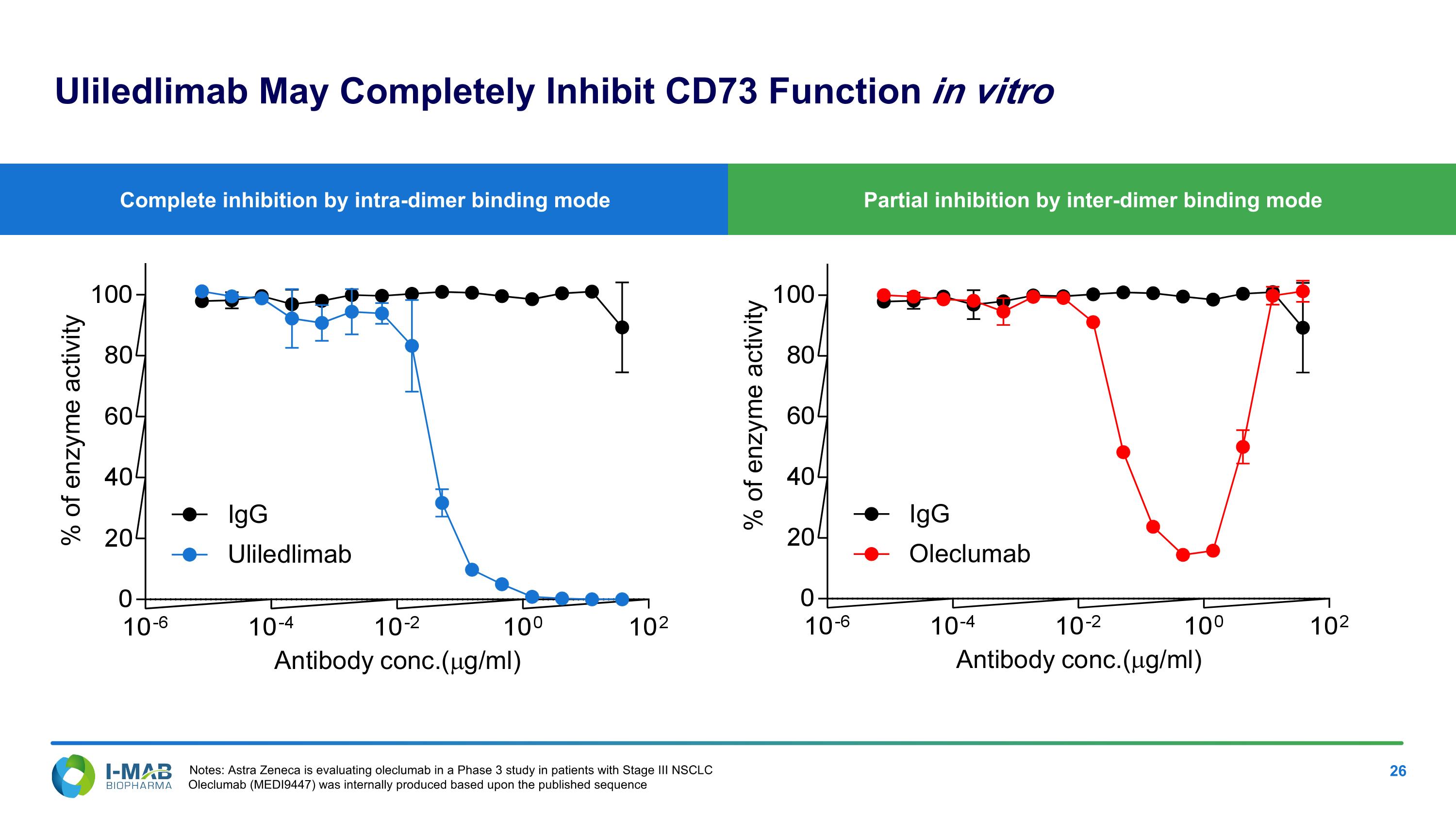
Partial inhibition by inter-dimer binding mode Complete inhibition by intra-dimer binding mode Uliledlimab May Completely Inhibit CD73 Function in vitro Notes: Astra Zeneca is evaluating oleclumab in a Phase 3 study in patients with Stage III NSCLC Oleclumab (MEDI9447) was internally produced based upon the published sequence
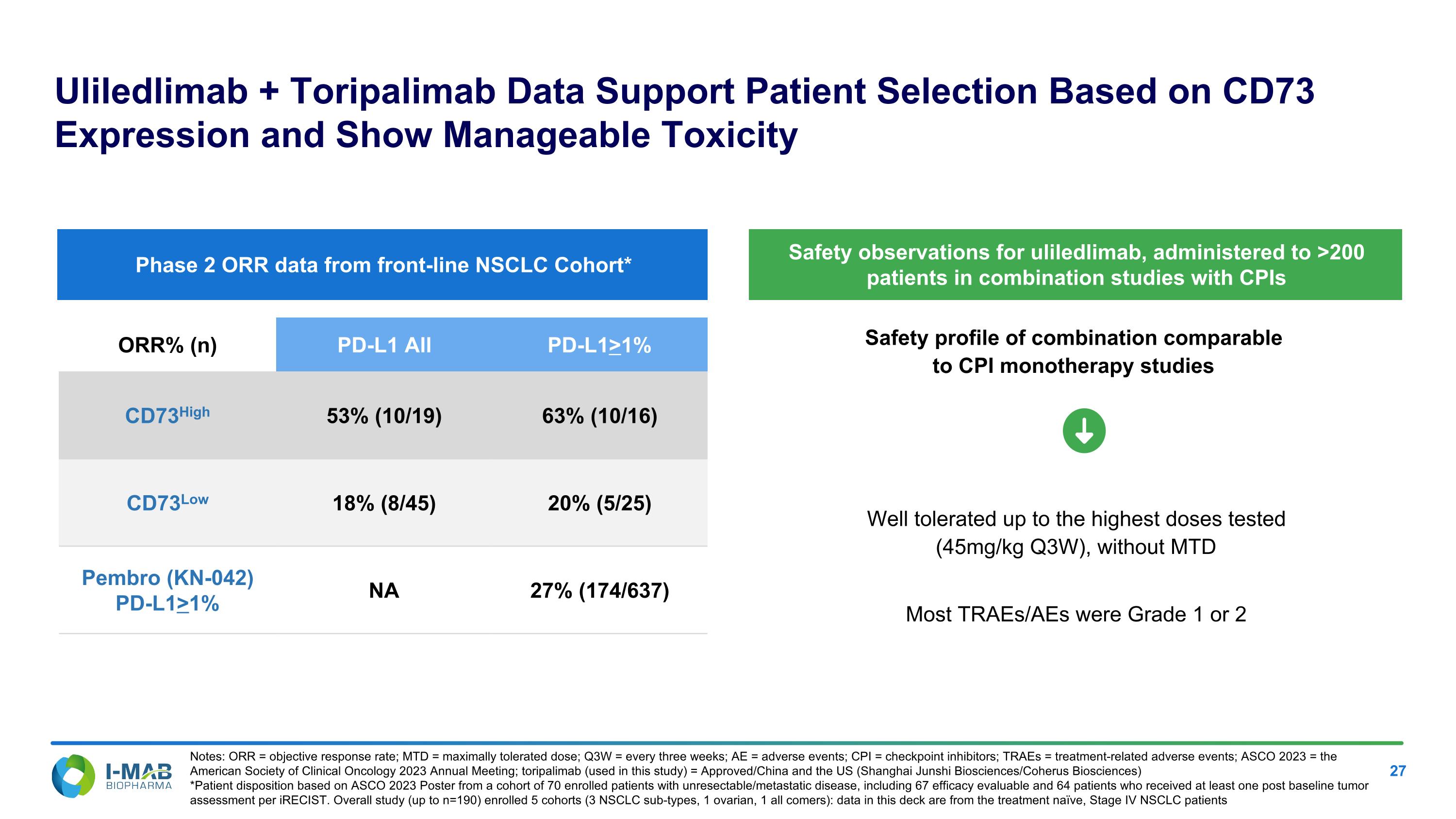
Safety profile of combination comparable to CPI monotherapy studies Uliledlimab + Toripalimab Data Support Patient Selection Based on CD73 Expression and Show Manageable Toxicity Well tolerated up to the highest doses tested (45mg/kg Q3W), without MTD Most TRAEs/AEs were Grade 1 or 2 ORR% (n) PD-L1 All PD-L1>1% CD73High 53% (10/19) 63% (10/16) CD73Low 18% (8/45) 20% (5/25) Pembro (KN-042) PD-L1>1% NA 27% (174/637) Phase 2 ORR data from front-line NSCLC Cohort* Safety observations for uliledlimab, administered to >200 patients in combination studies with CPIs Notes: ORR = objective response rate; MTD = maximally tolerated dose; Q3W = every three weeks; AE = adverse events; CPI = checkpoint inhibitors; TRAEs = treatment-related adverse events; ASCO 2023 = the American Society of Clinical Oncology 2023 Annual Meeting; toripalimab (used in this study) = Approved/China and the US (Shanghai Junshi Biosciences/Coherus Biosciences) *Patient disposition based on ASCO 2023 Poster from a cohort of 70 enrolled patients with unresectable/metastatic disease, including 67 efficacy evaluable and 64 patients who received at least one post baseline tumor assessment per iRECIST. Overall study (up to n=190) enrolled 5 cohorts (3 NSCLC sub-types, 1 ovarian, 1 all comers): data in this deck are from the treatment naïve, Stage IV NSCLC patients
Cash, cash equivalents and short-term investments as of March 31, 2025, were $168.6M; no debt Cash position expected to fund givastomig Phase 1b studies and further development initiatives into 2027 Issued and outstanding ordinary shares of 187.8M representing the equivalent of 81.7M ADSs1 as of March 31, 2025 Upcoming Clinical Readouts Across Portfolio Programs Selected Financial Information Anticipated Upcoming Milestones Timing Program Milestone Jul-2025 Givastomig Phase 1b GC/GEJ/EAC dose escalation data Topline data from combination with nivolumab plus chemo (n=17) on July 2nd at ESMO GI 1H 2026 Givastomig Phase 1b GC/GEJ/EAC dose expansion data Topline data from combination with nivolumab plus chemo (n=40) 2026 Uliledlimab Phase 2 PFS data from uliledlimab + toripalimab Randomized study against pembrolizumab alone or toripalimab alone (TJ Bio China-only data) Ongoing Ragistomig Phase 1b dose expansion enrolling Additional cohorts to expand the therapeutic index Assuming the conversion of all ordinary shares into ADSs Notes: GC = gastric cancer; GEJ = gastroesophageal junction; EAC = esophageal adenocarcinoma; PFS = progression free survival; TJ Bio = TJ Biopharma; ESMO GI = European Society of Medical Oncology Gastrointestinal Cancers Congress 2025

I-Mab Biopharma IR Contact IR@imabbio.com Stay connected