| December 2023 NASDAQ: NRBO 1 May 2025 MetaVia Inc. NASDAQ: MTVA |
| 2 Forward-Looking Statements This presentation includes forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. Forward-looking statements include all statements that do not relate solely to historical or current facts and can be identified by the use of words such as “believes”, “expects”, “anticipates”, “may”, “will”, “should”, “seeks”, “approximately”, “intends”, “projects”, “plans”, “estimates” or the negative of these words or other comparable terminology (as well as other words or expressions referencing future events, conditions or circumstances). Forward-looking statements are predictions, projections and other statements about future events that are based on current expectations and assumptions and, as a result, are subject to risks and uncertainties. These forward-looking statements include, but are not limited to, statements regarding the market size and potential growth opportunities of our current product candidates; the safety, efficacy, tolerability and other potential benefits, such as weight loss, associated with our current product candidates; the competitive differentiators of our current product candidates; potential collaborations and/or licensing partners for our current product candidates; our planned clinical trial activities for our current product candidates; the expected timeline for topline data release dates; and our planned submissions of investigational new drug applications and meetings with the U.S. Food and Drug Administration. Many factors could cause actual future events to differ materially from the forward-looking statements in this presentation, including, without limitation, those risks associated with our ability to execute our commercial strategy; the sufficiency of our existing cash on hand to fund our operations; the timeline for regulatory submissions, regulatory steps and potential regulatory approval of our current and future product candidates; the ability to realize the benefits of the license agreement with Dong-A ST Co., Ltd., including the impact on our future financial and operating results; the ability to integrate our current and future product candidates into our business in a timely and cost-efficient manner; the cooperation of our contract manufacturers, clinical study partners and others involved in the development of our current and future product candidates; our ability to initiate clinical trials on a timely basis; our planned clinical trials and our ability to recruit subjects for our clinical trials; the costs related to the license agreement, known and unknown, including costs of any litigation or regulatory actions relating to the license agreement; the changes in applicable laws or regulations; and the effects of changes to our stock price on the terms of the license agreement and any future fundraising. These forward-looking statements are based on information currently available to us and our current plans or expectations and are subject to a number of known and unknown uncertainties, risks and other important factors that may cause our actual results, performance or achievements expressed or implied by the forward-looking statements. These and other important factors are described in detail in the “Risk Factors” section of our Annual Report on Form 10-K for the year ended December 31, 2024, and our other filings with the Securities and Exchange Commission. While we may elect to update such forward-looking statements at some point in the future, except as required by law, we disclaim any obligation to do so, even if subsequent events cause our views to change. Although we believe the expectations reflected in such forward-looking statements are reasonable, we can give no assurance that such expectations will prove to be correct. These forward-looking statements should not be relied upon as representing our views as of any date subsequent to this presentation. This presentation also may contain estimates and other statistical data made by independent parties and by us relating to market size and other data about our industry. This data involves a number of assumptions and limitations, and you are cautioned not to give undue weight to such estimates. In addition, projections, assumptions and estimates of our future performance and the future performance of the markets in which we operate are necessarily subject to a high degree of uncertainty and risk. |
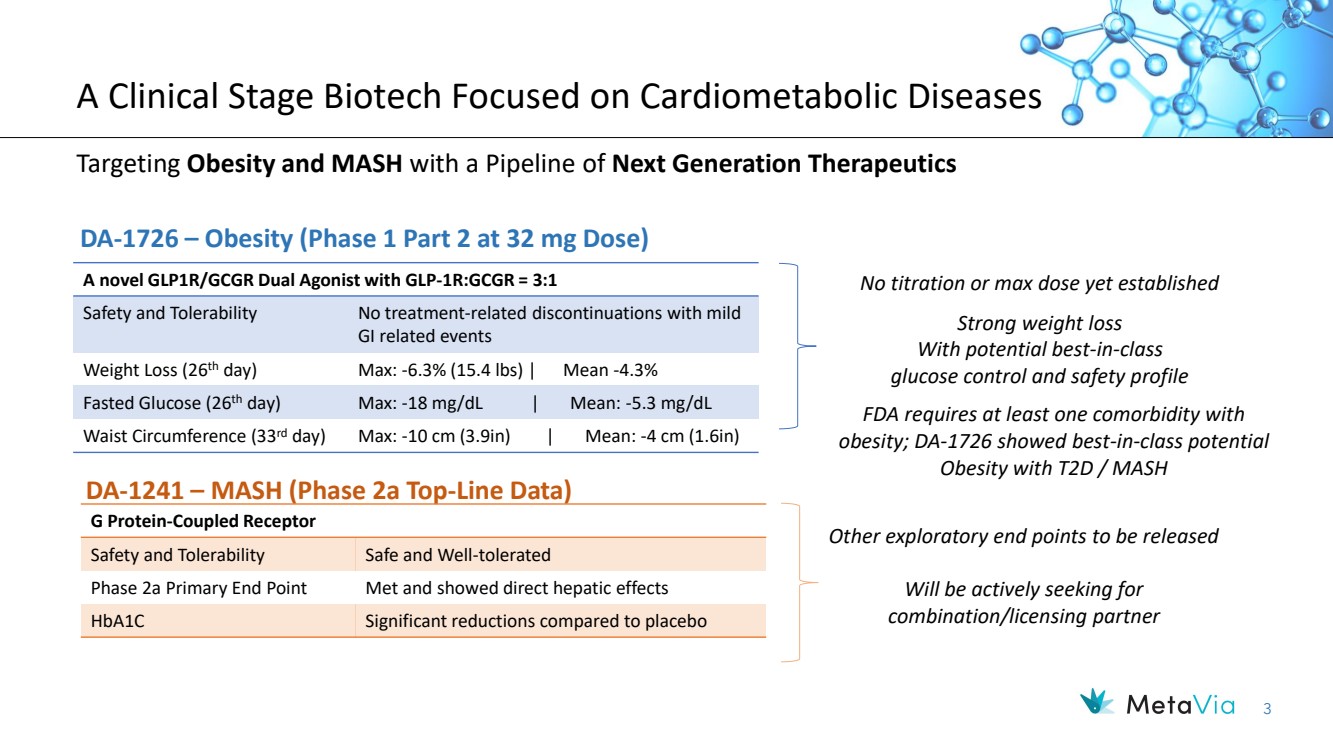
| 3 A Clinical Stage Biotech Focused on Cardiometabolic Diseases Targeting Obesity and MASH with a Pipeline of Next Generation Therapeutics A novel GLP1R/GCGR Dual Agonist with GLP-1R:GCGR = 3:1 Safety and Tolerability No treatment-related discontinuations with mild GI related events Weight Loss (26th day) Max: -6.3% (15.4 lbs) | Mean -4.3% Fasted Glucose (26th day) Max: -18 mg/dL | Mean: -5.3 mg/dL Waist Circumference (33rd day) Max: -10 cm (3.9in) | Mean: -4 cm (1.6in) No titration or max dose yet established DA-1726 – Obesity (Phase 1 Part 2 at 32 mg Dose) DA-1241 – MASH (Phase 2a Top-Line Data) G Protein-Coupled Receptor Safety and Tolerability Safe and Well-tolerated Phase 2a Primary End Point Met and showed direct hepatic effects HbA1C Significant reductions compared to placebo Other exploratory end points to be released Will be actively seeking for combination/licensing partner Strong weight loss With potential best-in-class glucose control and safety profile FDA requires at least one comorbidity with obesity; DA-1726 showed best-in-class potential Obesity with T2D / MASH |
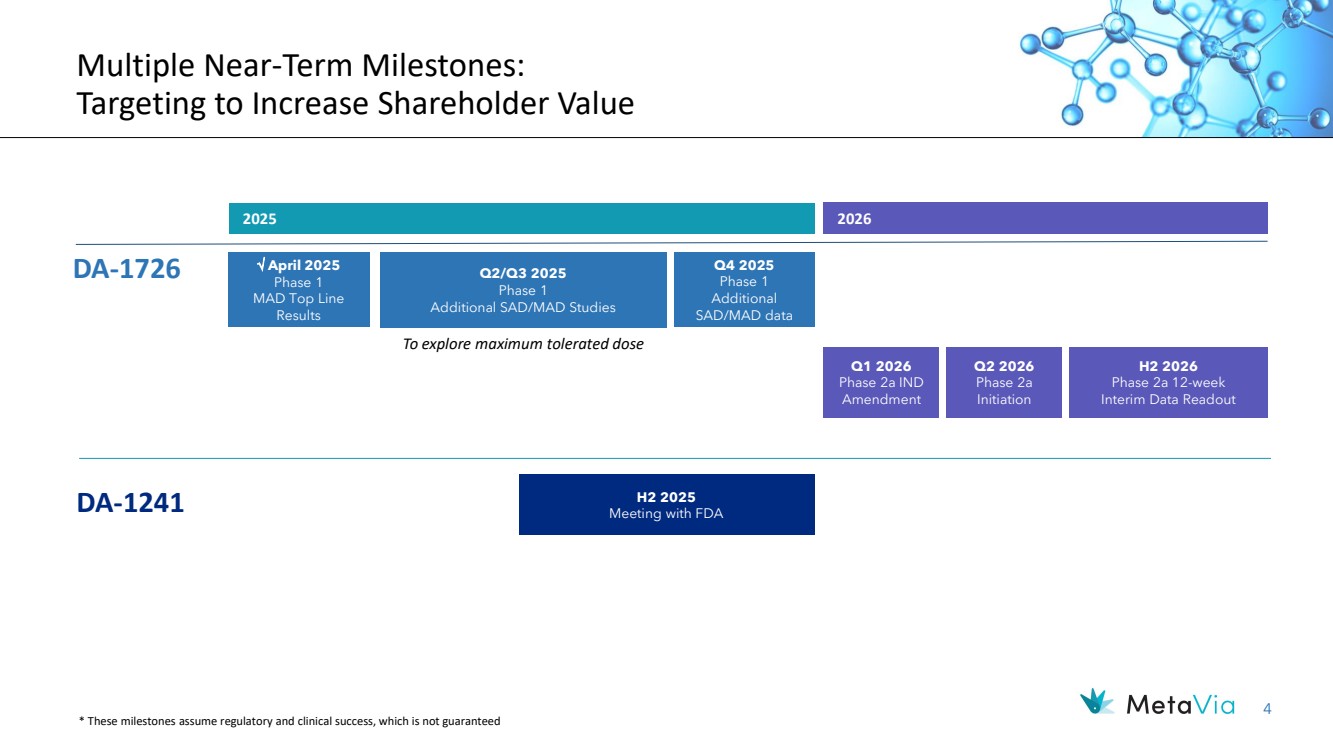
| 4 Multiple Near-Term Milestones: Targeting to Increase Shareholder Value 2026 DA-1241 H2 2025 Meeting with FDA April 2025 Phase 1 MAD Top Line Results DA-1726 Q1 2026 Phase 2a IND Amendment H2 2026 Phase 2a 12-week Interim Data Readout * These milestones assume regulatory and clinical success, which is not guaranteed Q2/Q3 2025 Phase 1 Additional SAD/MAD Studies Q4 2025 Phase 1 Additional SAD/MAD data To explore maximum tolerated dose 2025 Q2 2026 Phase 2a Initiation |
| December 2023 NASDAQ: NRBO 5 DA -1726 A Novel GLP1R/GCGR Dual Agonist for the Treatment of Obesity |
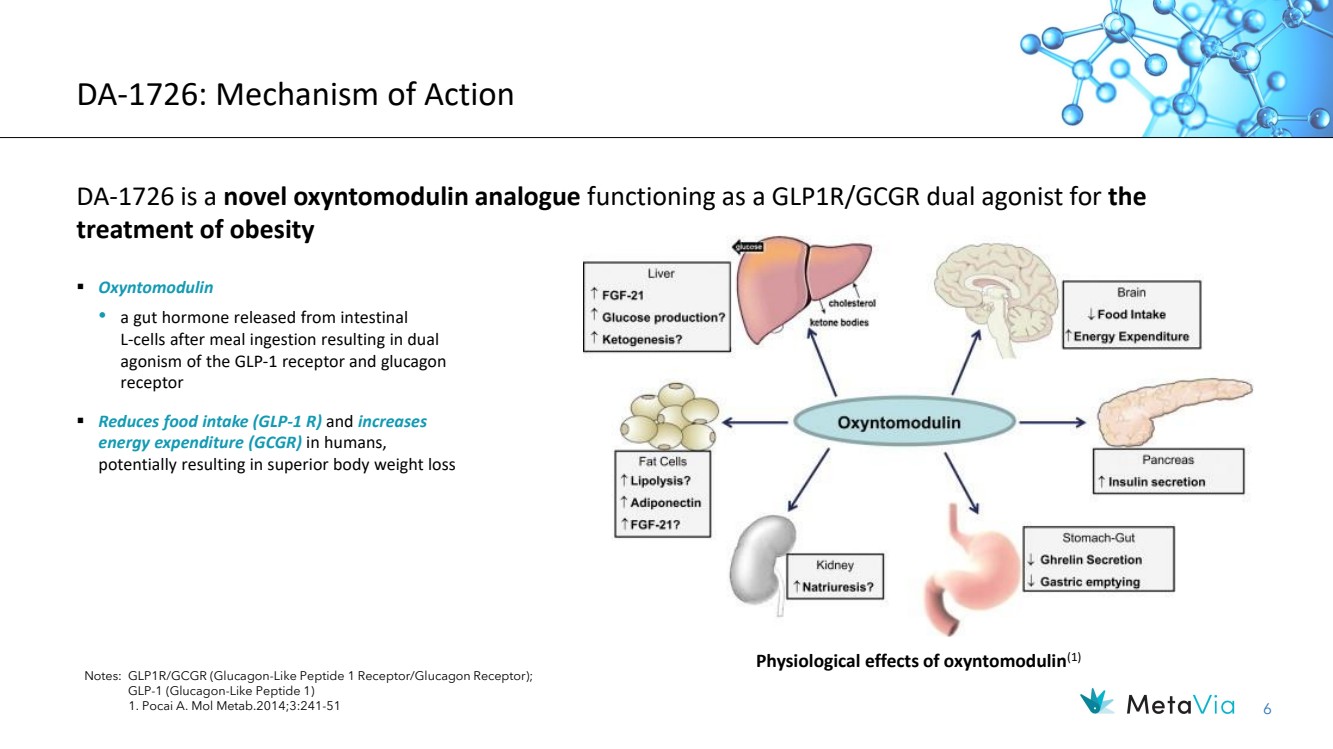
| 6 DA-1726: Mechanism of Action DA-1726 is a novel oxyntomodulin analogue functioning as a GLP1R/GCGR dual agonist for the treatment of obesity Notes: GLP1R/GCGR (Glucagon-Like Peptide 1 Receptor/Glucagon Receptor); GLP-1 (Glucagon-Like Peptide 1) 1. Pocai A. Mol Metab.2014;3:241-51 ▪ Oxyntomodulin • a gut hormone released from intestinal L-cells after meal ingestion resulting in dual agonism of the GLP-1 receptor and glucagon receptor ▪ Reduces food intake (GLP-1 R) and increases energy expenditure (GCGR) in humans, potentially resulting in superior body weight loss Physiological effects of oxyntomodulin(1) |
| 7 A Single Site, Randomized, Double-Blind, Placebo-Controlled, Parallel, Phase 1 Clinical Trial to Evaluate the Safety and Tolerability of DA-1726 in Obese Otherwise Healthy Subjects Primary Objective To evaluate the safety and tolerability of DA-1726 in obese but otherwise healthy patients NCT Number NCT06252220 Dosing Regimen DA-1726 4mg, 8mg, 16mg, 32mg or Matching Placebo, Sub Q, Once Weekly, No Titration Planned # of Subjects 9 subjects per cohort (6 active : 3 placebo) Future Plan Additional MAD cohorts to explore maximum tolerated dose DA-1726: Phase 1 MAD Clinical Study Subject Disposition Number of subjects (%) Pooled PBO 4 mg 8 mg 16 mg 32 mg Randomized 12 6 6 6 6 Completed the Study 10 (83.3%) 6 (100%) 5 (83.3%) 6 (100%) 6 (100%) Early Discontinuation from the Study 2 (16.7%) 0 1 (16.7%) 0 0 Reason for Study Discontinuation Treatment Related SAE 0 0 0 0 0 Non-Treatment Related SAE 0 0 1 (16.7%) * 0 0 Others 2 (16.7%) 0 0 0 0 * Hospitalization due to a car accident, subject was in a passenger seat. Not related to IP. |
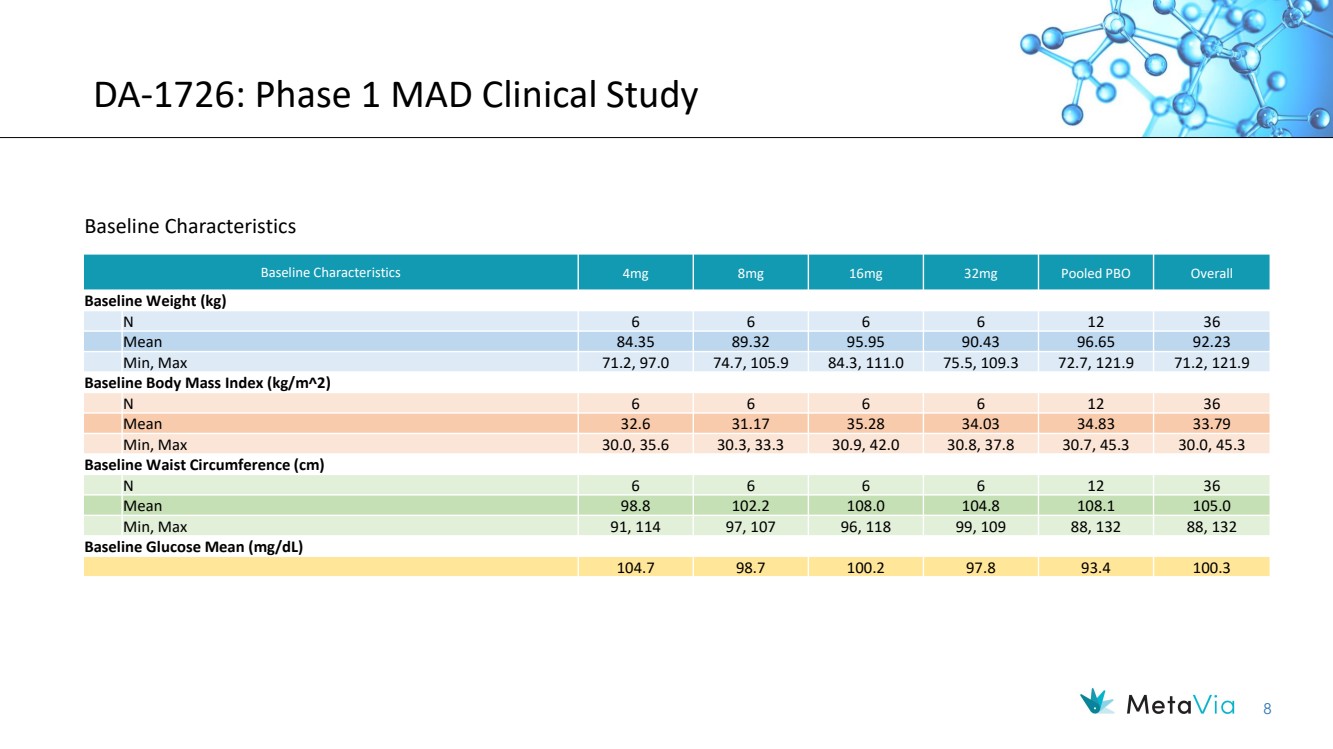
| 8 DA-1726: Phase 1 MAD Clinical Study Baseline Characteristics 4mg 8mg 16mg 32mg Pooled PBO Overall Baseline Weight (kg) N 6 6 6 6 12 36 Mean 84.35 89.32 95.95 90.43 96.65 92.23 Min, Max 71.2, 97.0 74.7, 105.9 84.3, 111.0 75.5, 109.3 72.7, 121.9 71.2, 121.9 Baseline Body Mass Index (kg/m2) N 6 6 6 6 12 36 Mean 32.6 31.17 35.28 34.03 34.83 33.79 Min, Max 30.0, 35.6 30.3, 33.3 30.9, 42.0 30.8, 37.8 30.7, 45.3 30.0, 45.3 Baseline Waist Circumference (cm) N 6 6 6 6 12 36 Mean 98.8 102.2 108.0 104.8 108.1 105.0 Min, Max 91, 114 97, 107 96, 118 99, 109 88, 132 88, 132 Baseline Glucose Mean (mg/dL) 104.7 98.7 100.2 97.8 93.4 100.3 Baseline Characteristics |
| 9 DA-1726: Phase 1 Safety Highlights (32 mg Dose) • Safety & Tolerability ✓ Most GI related AEs occurred after the 1st dose and resolved within 24 hours from occurrence ✓ No treatment-related discontinuations ✓ Mild vomiting occurred with 3 subjects but resolved within 9 hours, 2 subjects after 1st dose with no reoccurrence ✓ Mild nausea occurred with 2 subjects after the 1st dose but resolved within 12 hours, no nausea after 2nd to the last dose ✓ Constipation occurred with 2 subjects and resolved within 3 days ✓ Abdominal distension with 1 subject and resolved in 14 hours ✓ No diarrhea reported ✓ Mean heart rate showed slight decrease from baseline in all treatment groups other than the 16 mg cohort (baseline was significantly lower than other cohorts) ✓ No clinically significant increases in QTcF were noted and no drug-induced cardiovascular effects were observed at 4 weeks ❖ With no titration, 1st dose acted as the “titration” dose which will allow for predictive AE events resulting in increased tolerability • Notable AE ✓ Early satiety, suggesting efficacy of GLP-1R, was observed in five out of six subjects receiving the 32 mg dose ✓ Most subjects reported this AE after 3rd dosing, only one subject reporting after the 2nd dose ❖ These findings suggest greater weight loss may be seen in longer duration studies ❖ Further suggesting glucagon may have been the weight loss driver during the first 2 weeks |
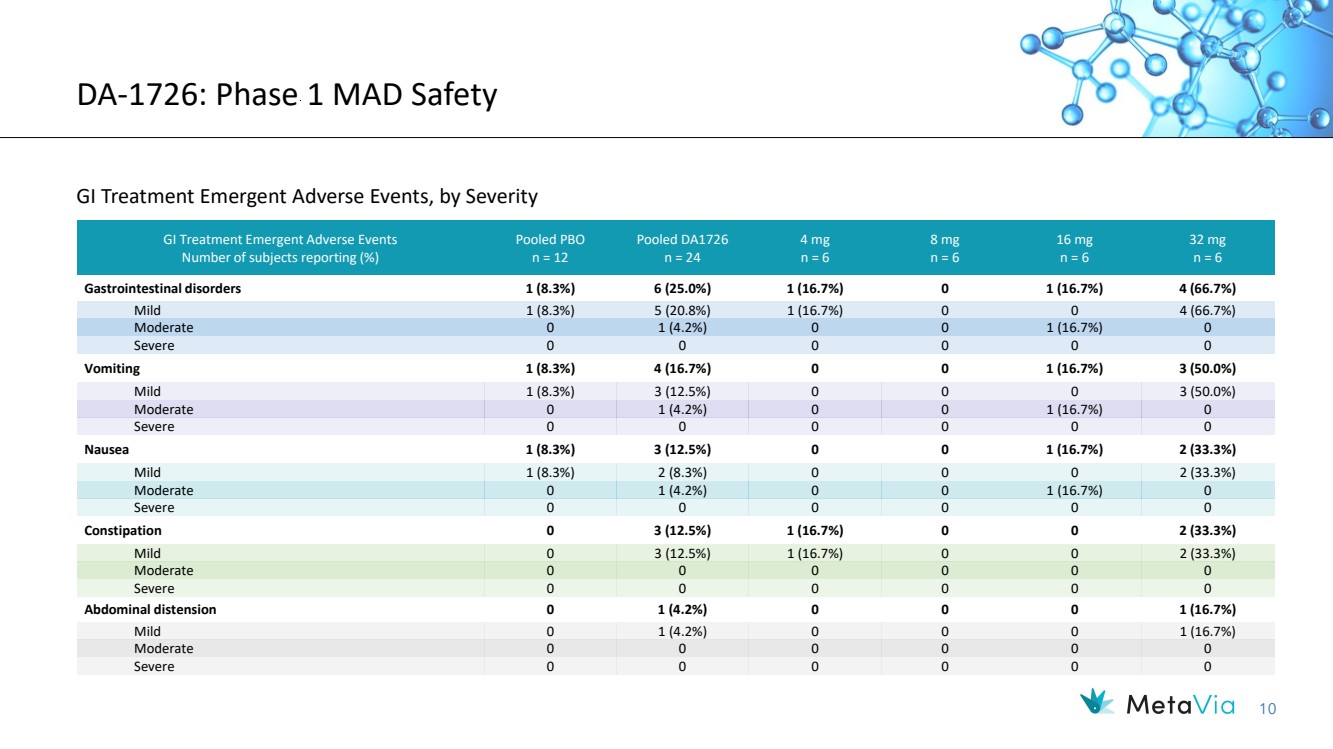
| 10 DA-1726: Phase 1 MAD Safety GI Treatment Emergent Adverse Events, by Severity GI Treatment Emergent Adverse Events Number of subjects reporting (%) Pooled PBO n = 12 Pooled DA1726 n = 24 4 mg n = 6 8 mg n = 6 16 mg n = 6 32 mg n = 6 Gastrointestinal disorders 1 (8.3%) 6 (25.0%) 1 (16.7%) 0 1 (16.7%) 4 (66.7%) Mild 1 (8.3%) 5 (20.8%) 1 (16.7%) 0 0 4 (66.7%) Moderate 0 1 (4.2%) 0 0 1 (16.7%) 0 Severe 0 0 0 0 0 0 Vomiting 1 (8.3%) 4 (16.7%) 0 0 1 (16.7%) 3 (50.0%) Mild 1 (8.3%) 3 (12.5%) 0 0 0 3 (50.0%) Moderate 0 1 (4.2%) 0 0 1 (16.7%) 0 Severe 0 0 0 0 0 0 Nausea 1 (8.3%) 3 (12.5%) 0 0 1 (16.7%) 2 (33.3%) Mild 1 (8.3%) 2 (8.3%) 0 0 0 2 (33.3%) Moderate 0 1 (4.2%) 0 0 1 (16.7%) 0 Severe 0 0 0 0 0 0 Constipation 0 3 (12.5%) 1 (16.7%) 0 0 2 (33.3%) Mild 0 3 (12.5%) 1 (16.7%) 0 0 2 (33.3%) Moderate 0 0 0 0 0 0 Severe 0 0 0 0 0 0 Abdominal distension 0 1 (4.2%) 0 0 0 1 (16.7%) Mild 0 1 (4.2%) 0 0 0 1 (16.7%) Moderate 0 0 0 0 0 0 Severe 0 0 0 0 0 0 |
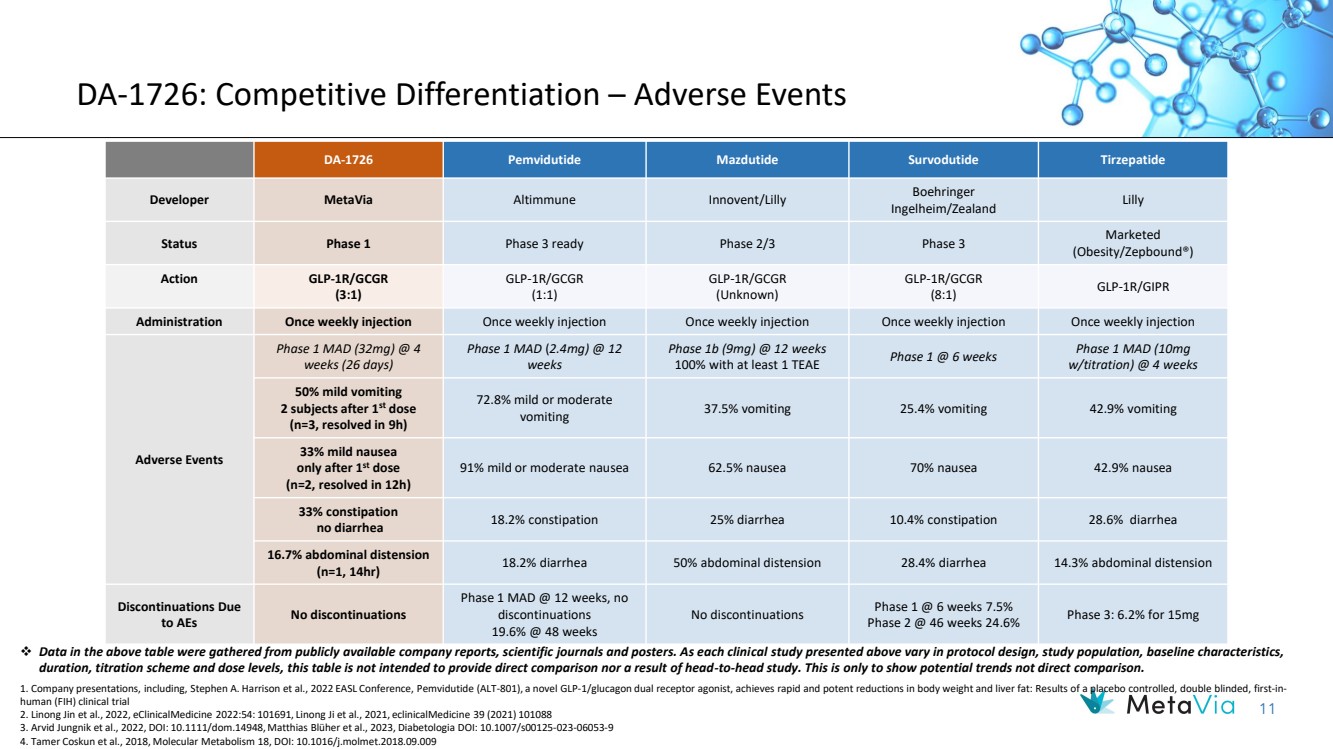
| 11 DA-1726: Competitive Differentiation – Adverse Events DA-1726 Pemvidutide Mazdutide Survodutide Tirzepatide Developer MetaVia Altimmune Innovent/Lilly Boehringer Ingelheim/Zealand Lilly Status Phase 1 Phase 3 ready Phase 2/3 Phase 3 Marketed (Obesity/Zepbound®) Action GLP-1R/GCGR (3:1) GLP-1R/GCGR (1:1) GLP-1R/GCGR (Unknown) GLP-1R/GCGR (8:1) GLP-1R/GIPR Administration Once weekly injection Once weekly injection Once weekly injection Once weekly injection Once weekly injection Adverse Events Phase 1 MAD (32mg) @ 4 weeks (26 days) Phase 1 MAD (2.4mg) @ 12 weeks Phase 1b (9mg) @ 12 weeks 100% with at least 1 TEAE Phase 1 @ 6 weeks Phase 1 MAD (10mg w/titration) @ 4 weeks 50% mild vomiting 2 subjects after 1st dose (n=3, resolved in 9h) 72.8% mild or moderate vomiting 37.5% vomiting 25.4% vomiting 42.9% vomiting 33% mild nausea only after 1st dose (n=2, resolved in 12h) 91% mild or moderate nausea 62.5% nausea 70% nausea 42.9% nausea 33% constipation no diarrhea 18.2% constipation 25% diarrhea 10.4% constipation 28.6% diarrhea 16.7% abdominal distension (n=1, 14hr) 18.2% diarrhea 50% abdominal distension 28.4% diarrhea 14.3% abdominal distension Discontinuations Due to AEs No discontinuations Phase 1 MAD @ 12 weeks, no discontinuations 19.6% @ 48 weeks No discontinuations Phase 1 @ 6 weeks 7.5% Phase 2 @ 46 weeks 24.6% Phase 3: 6.2% for 15mg ❖ Data in the above table were gathered from publicly available company reports, scientific journals and posters. As each clinical study presented above vary in protocol design, study population, baseline characteristics, duration, titration scheme and dose levels, this table is not intended to provide direct comparison nor a result of head-to-head study. This is only to show potential trends not direct comparison. 1. Company presentations, including, Stephen A. Harrison et al., 2022 EASL Conference, Pemvidutide (ALT-801), a novel GLP-1/glucagon dual receptor agonist, achieves rapid and potent reductions in body weight and liver fat: Results of a placebo controlled, double blinded, first-in-human (FIH) clinical trial 2. Linong Jin et al., 2022, eClinicalMedicine 2022:54: 101691, Linong Ji et al., 2021, eclinicalMedicine 39 (2021) 101088 3. Arvid Jungnik et al., 2022, DOI: 10.1111/dom.14948, Matthias Blüher et al., 2023, Diabetologia DOI: 10.1007/s00125-023-06053-9 4. Tamer Coskun et al., 2018, Molecular Metabolism 18, DOI: 10.1016/j.molmet.2018.09.009 |
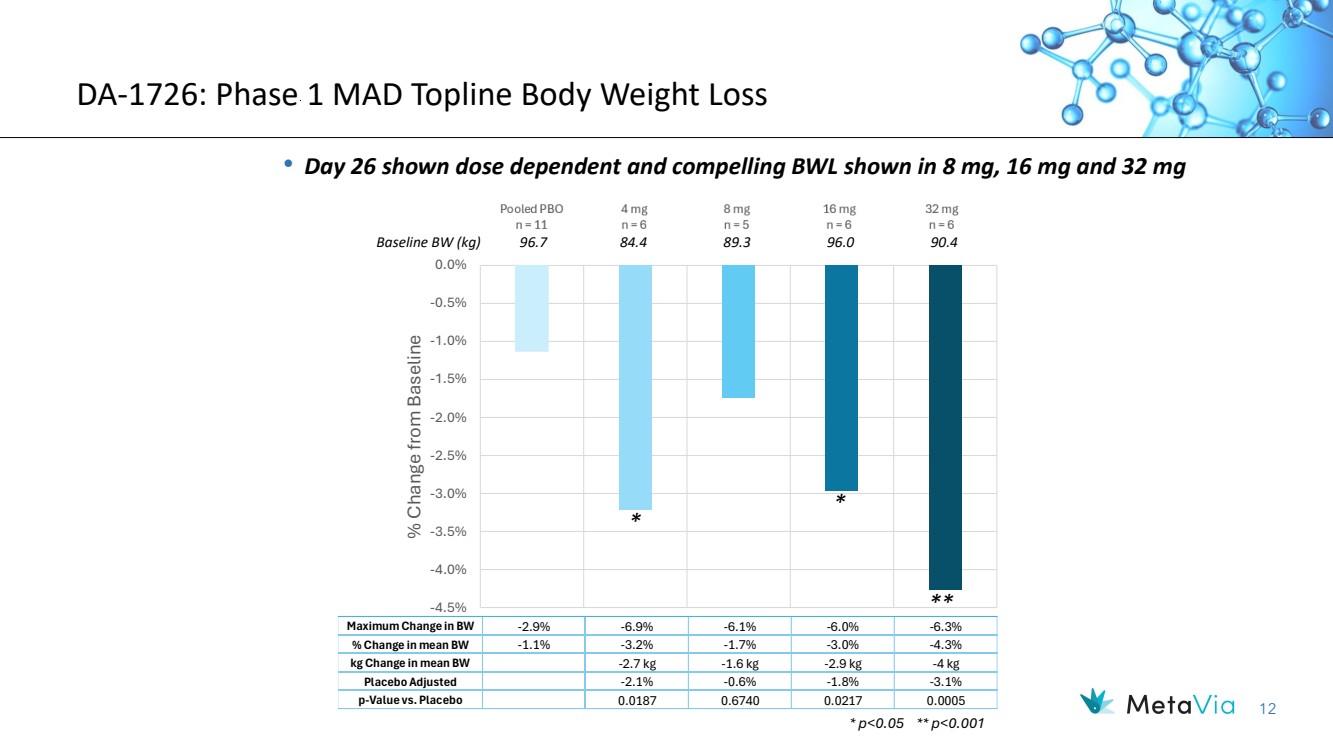
| 12 DA-1726: Phase 1 MAD Topline Body Weight Loss • Day 26 shown dose dependent and compelling BWL shown in 8 mg, 16 mg and 32 mg -4.5% -4.0% -3.5% -3.0% -2.5% -2.0% -1.5% -1.0% -0.5% 0.0% % Change from Baseline Maximum Change in BW -2.9% -6.9% -6.1% -6.0% -6.3% % Change in mean BW -1.1% -3.2% -1.7% -3.0% -4.3% kg Change in mean BW -2.7 kg -1.6 kg -2.9 kg -4 kg Placebo Adjusted -2.1% -0.6% -1.8% -3.1% p-Value vs. Placebo 0.0187 0.6740 0.0217 0.0005 * p<0.05 ** p<0.001 Baseline BW (kg) 96.7 84.4 89.3 96.0 90.4 * * ** |
| 13 DA-1726: MAD Cohort 1-4 - Changes in BMI |
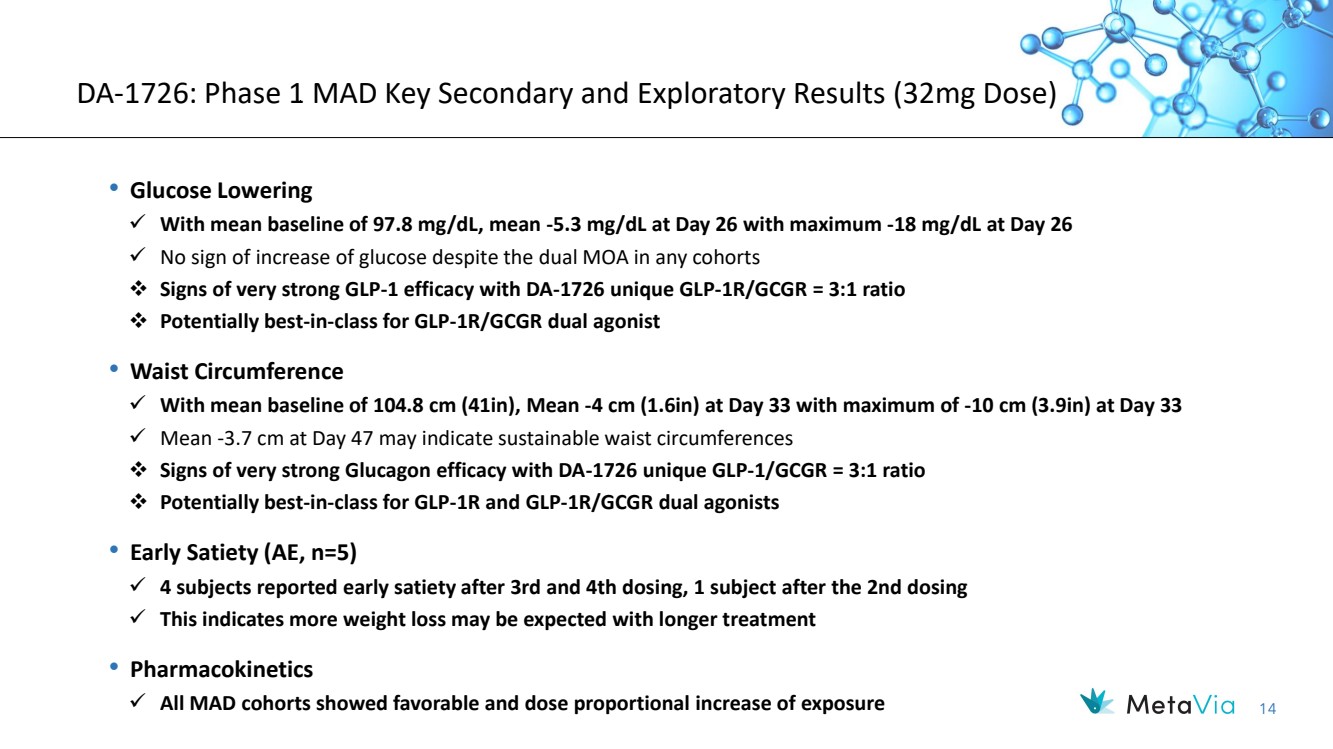
| 14 DA-1726: Phase 1 MAD Key Secondary and Exploratory Results (32mg Dose) • Glucose Lowering ✓ With mean baseline of 97.8 mg/dL, mean -5.3 mg/dL at Day 26 with maximum -18 mg/dL at Day 26 ✓ No sign of increase of glucose despite the dual MOA in any cohorts ❖ Signs of very strong GLP-1 efficacy with DA-1726 unique GLP-1R/GCGR = 3:1 ratio ❖ Potentially best-in-class for GLP-1R/GCGR dual agonist • Waist Circumference ✓ With mean baseline of 104.8 cm (41in), Mean -4 cm (1.6in) at Day 33 with maximum of -10 cm (3.9in) at Day 33 ✓ Mean -3.7 cm at Day 47 may indicate sustainable waist circumferences ❖ Signs of very strong Glucagon efficacy with DA-1726 unique GLP-1/GCGR = 3:1 ratio ❖ Potentially best-in-class for GLP-1R and GLP-1R/GCGR dual agonists • Early Satiety (AE, n=5) ✓ 4 subjects reported early satiety after 3rd and 4th dosing, 1 subject after the 2nd dosing ✓ This indicates more weight loss may be expected with longer treatment • Pharmacokinetics ✓ All MAD cohorts showed favorable and dose proportional increase of exposure |
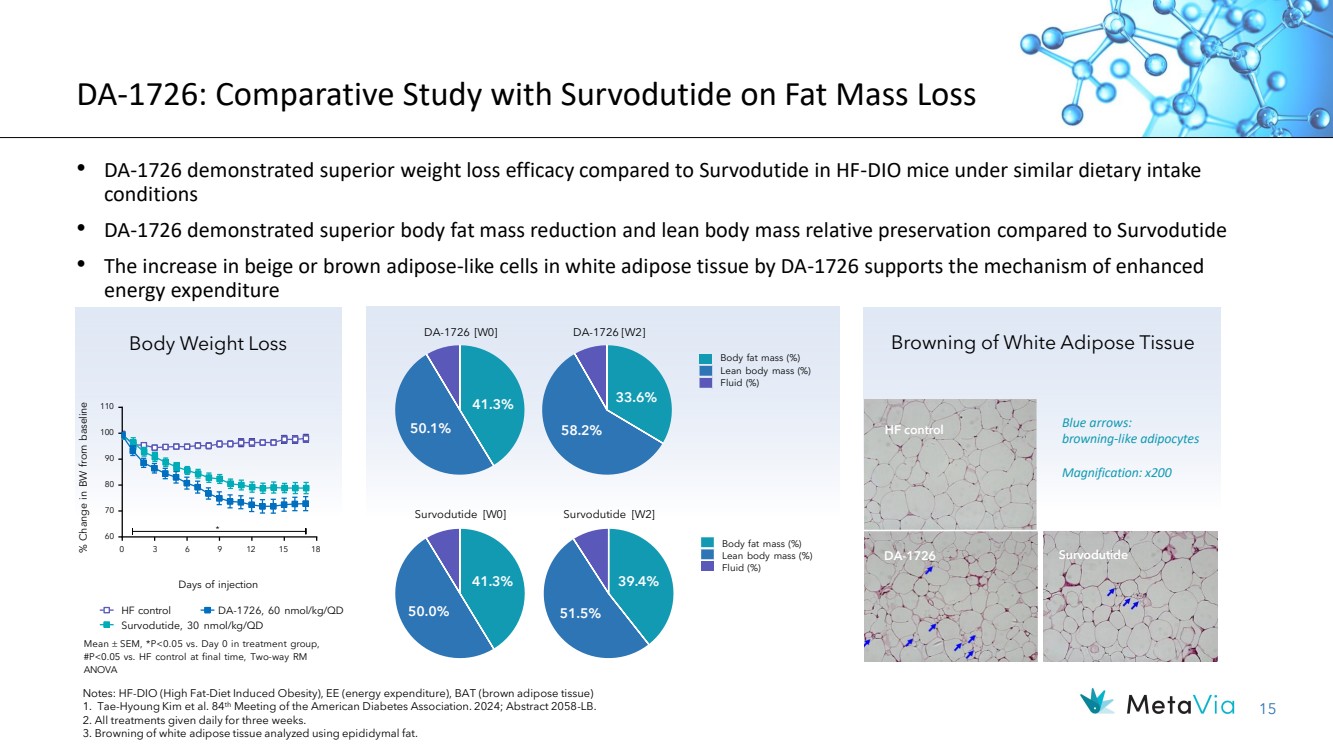
| 15 DA-1726: Comparative Study with Survodutide on Fat Mass Loss • DA-1726 demonstrated superior weight loss efficacy compared to Survodutide in HF-DIO mice under similar dietary intake conditions • DA-1726 demonstrated superior body fat mass reduction and lean body mass relative preservation compared to Survodutide • The increase in beige or brown adipose-like cells in white adipose tissue by DA-1726 supports the mechanism of enhanced energy expenditure Browning of White Adipose Tissue 41.3% 50.1% Survodutide [W0] 41.3% 50.0% 33.6% 58.2% Body fat mass (%) Lean body mass (%) Fluid (%) Survodutide [W2] 39.4% 51.5% Body fat mass (%) Lean body mass (%) Fluid (%) DA-1726 Survodutide HF control Blue arrows: browning-like adipocytes Magnification: x200 HF control Survodutide, 30 nmol/kg/QD DA-1726, 60 nmol/kg/QD Days of injection 0 60 70 80 90 100 110 * % 3 6 9 12 15 18 Change in BW from baseline Body Weight Loss DA-1726 [W0] DA-1726 [W2] Notes: HF-DIO (High Fat-Diet Induced Obesity), EE (energy expenditure), BAT (brown adipose tissue) 1. Tae-Hyoung Kim et al. 84th Meeting of the American Diabetes Association. 2024; Abstract 2058-LB. 2. All treatments given daily for three weeks. 3. Browning of white adipose tissue analyzed using epididymal fat. Mean SEM, *P<0.05 vs. Day 0 in treatment group, #P<0.05 vs. HF control at final time, Two-way RM ANOVA |
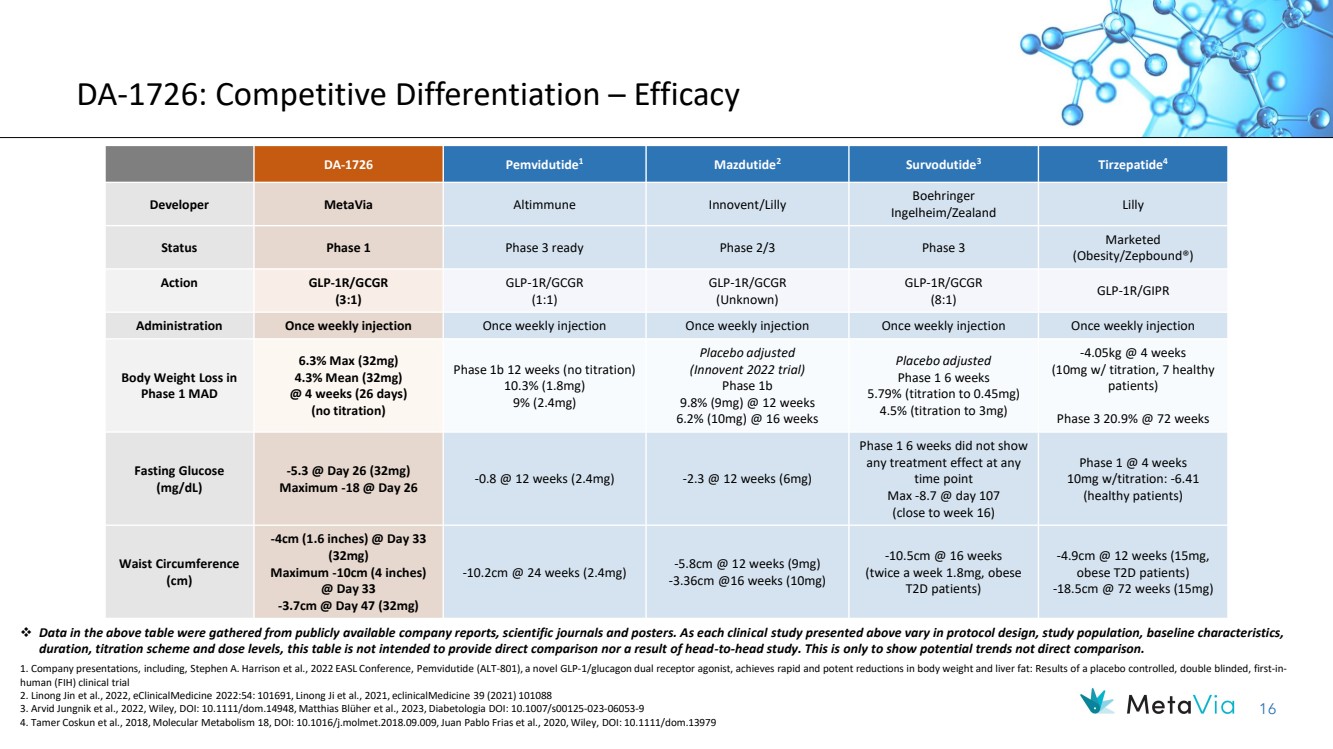
| 16 DA-1726: Competitive Differentiation – Efficacy DA-1726 Pemvidutide1 Mazdutide2 Survodutide3 Tirzepatide4 Developer MetaVia Altimmune Innovent/Lilly Boehringer Ingelheim/Zealand Lilly Status Phase 1 Phase 3 ready Phase 2/3 Phase 3 Marketed (Obesity/Zepbound®) Action GLP-1R/GCGR (3:1) GLP-1R/GCGR (1:1) GLP-1R/GCGR (Unknown) GLP-1R/GCGR (8:1) GLP-1R/GIPR Administration Once weekly injection Once weekly injection Once weekly injection Once weekly injection Once weekly injection Body Weight Loss in Phase 1 MAD 6.3% Max (32mg) 4.3% Mean (32mg) @ 4 weeks (26 days) (no titration) Phase 1b 12 weeks (no titration) 10.3% (1.8mg) 9% (2.4mg) Placebo adjusted (Innovent 2022 trial) Phase 1b 9.8% (9mg) @ 12 weeks 6.2% (10mg) @ 16 weeks Placebo adjusted Phase 1 6 weeks 5.79% (titration to 0.45mg) 4.5% (titration to 3mg) -4.05kg @ 4 weeks (10mg w/ titration, 7 healthy patients) Phase 3 20.9% @ 72 weeks Fasting Glucose (mg/dL) -5.3 @ Day 26 (32mg) Maximum -18 @ Day 26 -0.8 @ 12 weeks (2.4mg) -2.3 @ 12 weeks (6mg) Phase 1 6 weeks did not show any treatment effect at any time point Max -8.7 @ day 107 (close to week 16) Phase 1 @ 4 weeks 10mg w/titration: -6.41 (healthy patients) Waist Circumference (cm) -4cm (1.6 inches) @ Day 33 (32mg) Maximum -10cm (4 inches) @ Day 33 -3.7cm @ Day 47 (32mg) -10.2cm @ 24 weeks (2.4mg) -5.8cm @ 12 weeks (9mg) -3.36cm @16 weeks (10mg) -10.5cm @ 16 weeks (twice a week 1.8mg, obese T2D patients) -4.9cm @ 12 weeks (15mg, obese T2D patients) -18.5cm @ 72 weeks (15mg) ❖ Data in the above table were gathered from publicly available company reports, scientific journals and posters. As each clinical study presented above vary in protocol design, study population, baseline characteristics, duration, titration scheme and dose levels, this table is not intended to provide direct comparison nor a result of head-to-head study. This is only to show potential trends not direct comparison. 1. Company presentations, including, Stephen A. Harrison et al., 2022 EASL Conference, Pemvidutide (ALT-801), a novel GLP-1/glucagon dual receptor agonist, achieves rapid and potent reductions in body weight and liver fat: Results of a placebo controlled, double blinded, first-in-human (FIH) clinical trial 2. Linong Jin et al., 2022, eClinicalMedicine 2022:54: 101691, Linong Ji et al., 2021, eclinicalMedicine 39 (2021) 101088 3. Arvid Jungnik et al., 2022, Wiley, DOI: 10.1111/dom.14948, Matthias Blüher et al., 2023, Diabetologia DOI: 10.1007/s00125-023-06053-9 4. Tamer Coskun et al., 2018, Molecular Metabolism 18, DOI: 10.1016/j.molmet.2018.09.009, Juan Pablo Frias et al., 2020, Wiley, DOI: 10.1111/dom.13979 |
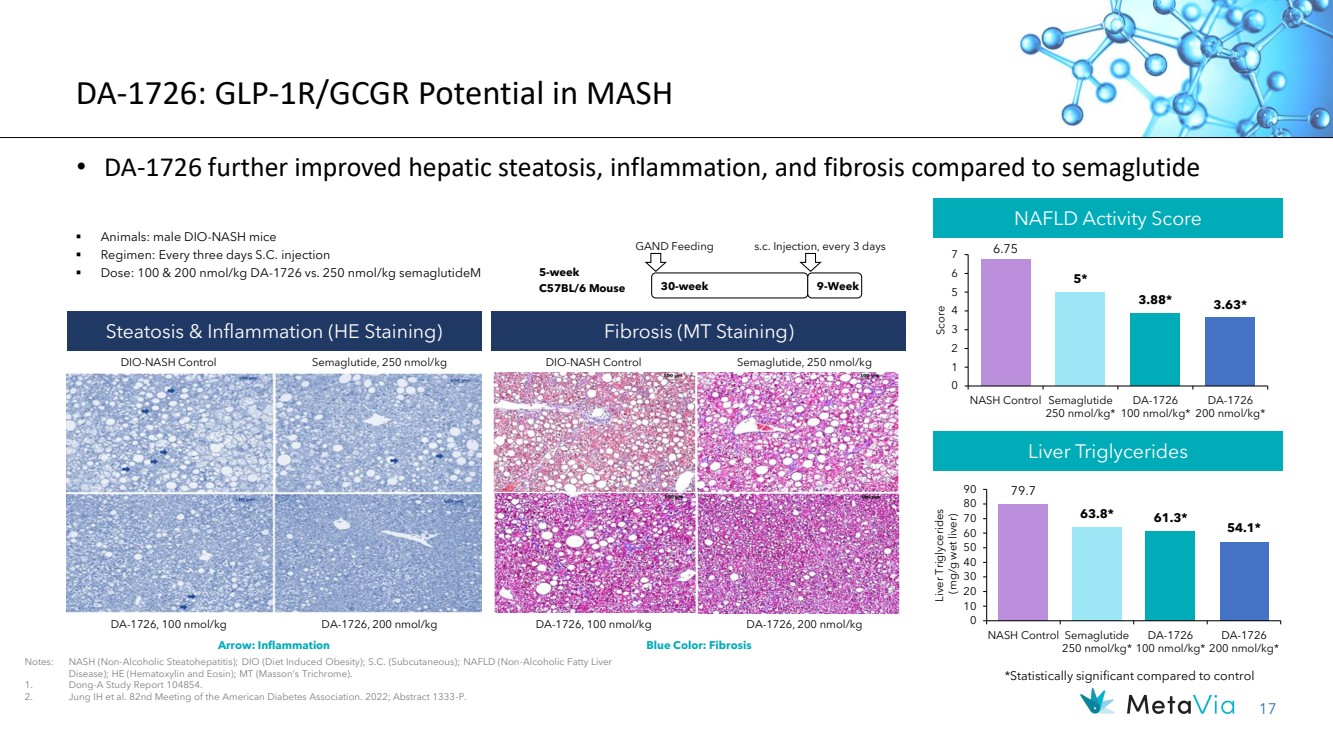
| 17 6.75 5* 3.88* 3.63* 0 1 2 3 4 5 6 7 NASH Control Semaglutide 250 nmol/kg* DA-1726 100 nmol/kg* DA-1726 200 nmol/kg* Score • DA-1726 further improved hepatic steatosis, inflammation, and fibrosis compared to semaglutide ▪ Animals: male DIO-NASH mice ▪ Regimen: Every three days S.C. injection ▪ Dose: 100 & 200 nmol/kg DA-1726 vs. 250 nmol/kg semaglutideM Steatosis & Inflammation (HE Staining) DA-1726, 100 nmol/kg Notes: NASH (Non-Alcoholic Steatohepatitis); DIO (Diet Induced Obesity); S.C. (Subcutaneous); NAFLD (Non-Alcoholic Fatty Liver Disease); HE (Hematoxylin and Eosin); MT (Masson’s Trichrome). 1. Dong-A Study Report 104854. 2. Jung IH et al. 82nd Meeting of the American Diabetes Association. 2022; Abstract 1333-P. 30-week GAND Feeding s.c. Injection, every 3 days 5-week C57BL/6 Mouse 9-Week NAFLD Activity Score Fibrosis (MT Staining) DA-1726, 200 nmol/kg DIO-NASH Control Semaglutide, 250 nmol/kg DIO-NASH Control Semaglutide, 250 nmol/kg DA-1726, 100 nmol/kg DA-1726, 200 nmol/kg Liver Triglycerides Arrow: Inflammation Blue Color: Fibrosis 79.7 63.8* 61.3* 54.1* 0 10 20 30 40 50 60 70 80 90 NASH Control Semaglutide 250 nmol/kg* DA-1726 100 nmol/kg* DA-1726 200 nmol/kg* Liver Triglycerides (mg/g wet liver) *Statistically significant compared to control DA-1726: GLP-1R/GCGR Potential in MASH |
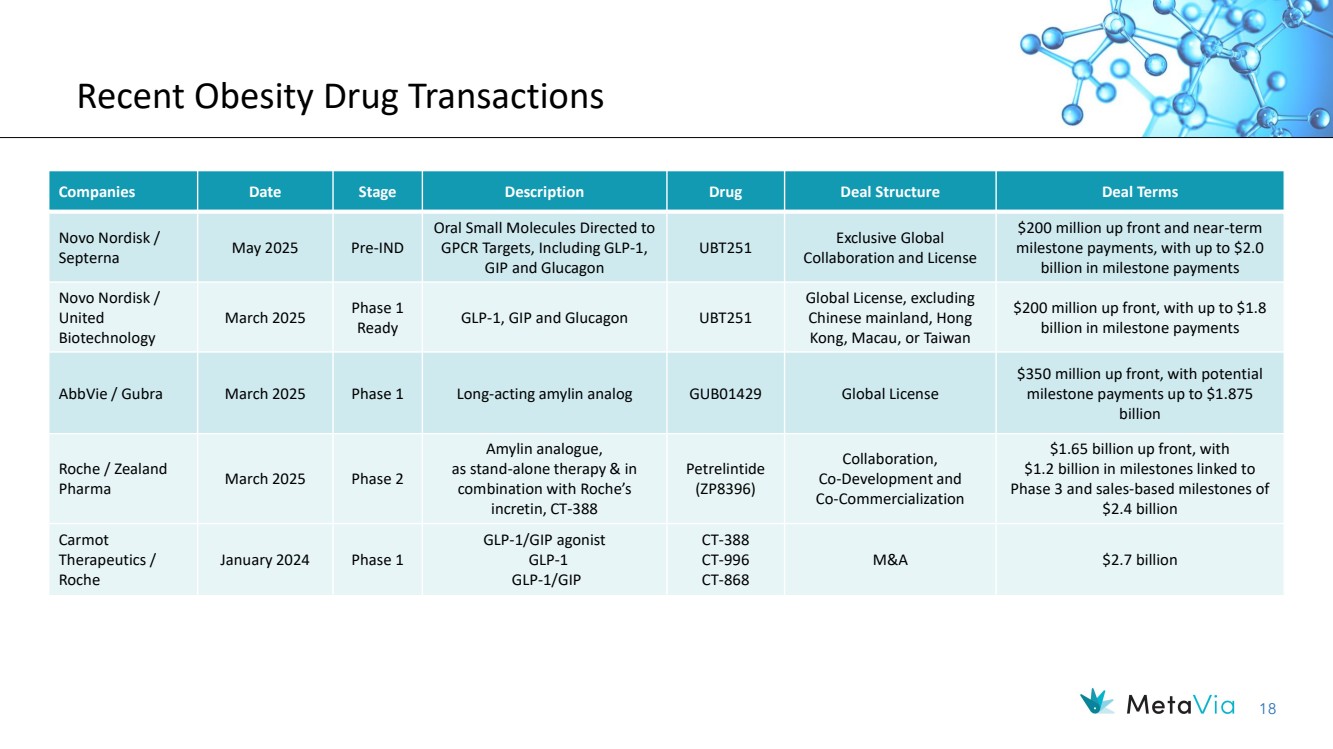
| 18 Recent Obesity Drug Transactions Companies Date Stage Description Drug Deal Structure Deal Terms Novo Nordisk / Septerna May 2025 Pre-IND Oral Small Molecules Directed to GPCR Targets, Including GLP-1, GIP and Glucagon UBT251 Exclusive Global Collaboration and License $200 million up front and near-term milestone payments, with up to $2.0 billion in milestone payments Novo Nordisk / United Biotechnology March 2025 Phase 1 Ready GLP-1, GIP and Glucagon UBT251 Global License, excluding Chinese mainland, Hong Kong, Macau, or Taiwan $200 million up front, with up to $1.8 billion in milestone payments AbbVie / Gubra March 2025 Phase 1 Long-acting amylin analog GUB01429 Global License $350 million up front, with potential milestone payments up to $1.875 billion Roche / Zealand Pharma March 2025 Phase 2 Amylin analogue, as stand-alone therapy & in combination with Roche’s incretin, CT-388 Petrelintide (ZP8396) Collaboration, Co-Development and Co-Commercialization $1.65 billion up front, with $1.2 billion in milestones linked to Phase 3 and sales-based milestones of $2.4 billion Carmot Therapeutics / Roche January 2024 Phase 1 GLP-1/GIP agonist GLP-1 GLP-1/GIP CT-388 CT-996 CT-868 M&A $2.7 billion |
| December 2023 NASDAQ: NRBO 19 DA-1241 Orally Available, Potential First-in-Class GPR119 Agonist for the Treatment of MASH |
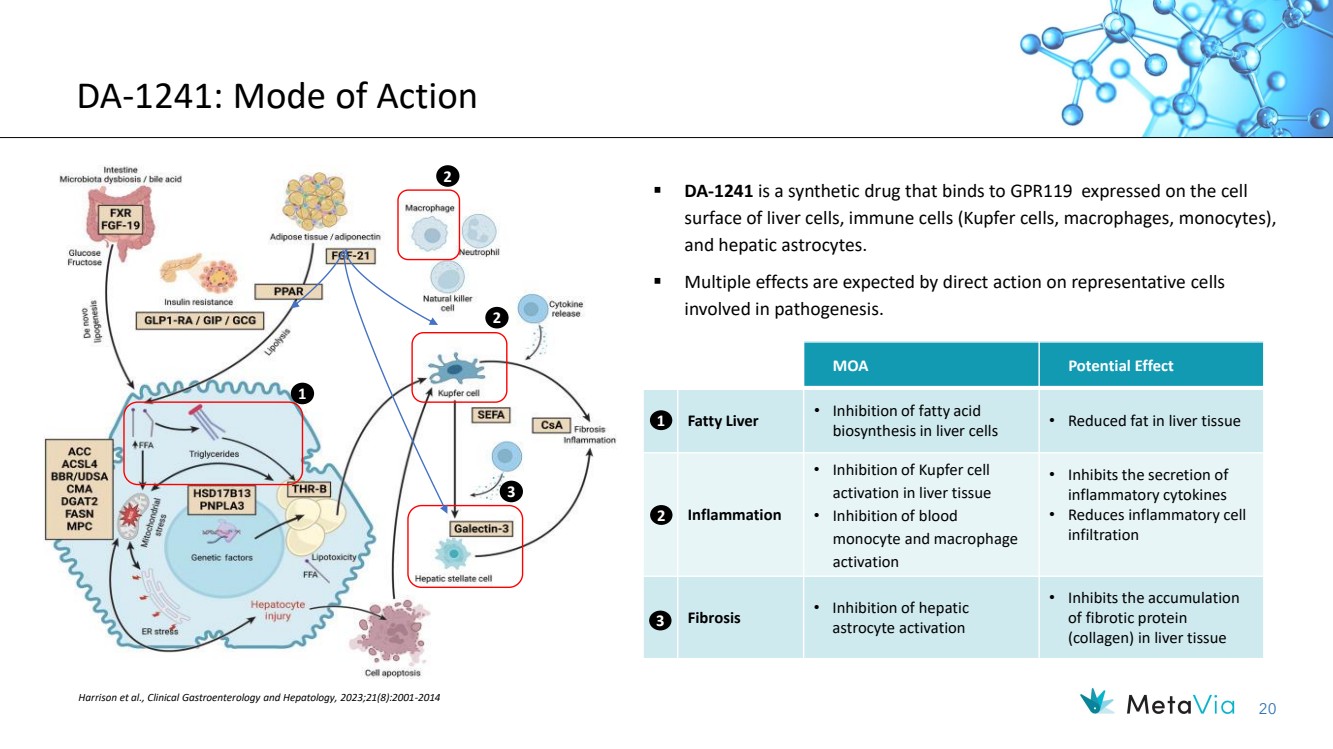
| 20 ▪ DA-1241 is a synthetic drug that binds to GPR119 expressed on the cell surface of liver cells, immune cells (Kupfer cells, macrophages, monocytes), and hepatic astrocytes. ▪ Multiple effects are expected by direct action on representative cells involved in pathogenesis. Harrison et al., Clinical Gastroenterology and Hepatology, 2023;21(8):2001-2014 DA-1241: Mode of Action MOA Potential Effect Fatty Liver • Inhibition of fatty acid biosynthesis in liver cells • Reduced fat in liver tissue Inflammation • Inhibition of Kupfer cell activation in liver tissue • Inhibition of blood monocyte and macrophage activation • Inhibits the secretion of inflammatory cytokines • Reduces inflammatory cell infiltration Fibrosis • Inhibition of hepatic astrocyte activation • Inhibits the accumulation of fibrotic protein (collagen) in liver tissue 1 2 3 1 2 2 3 |
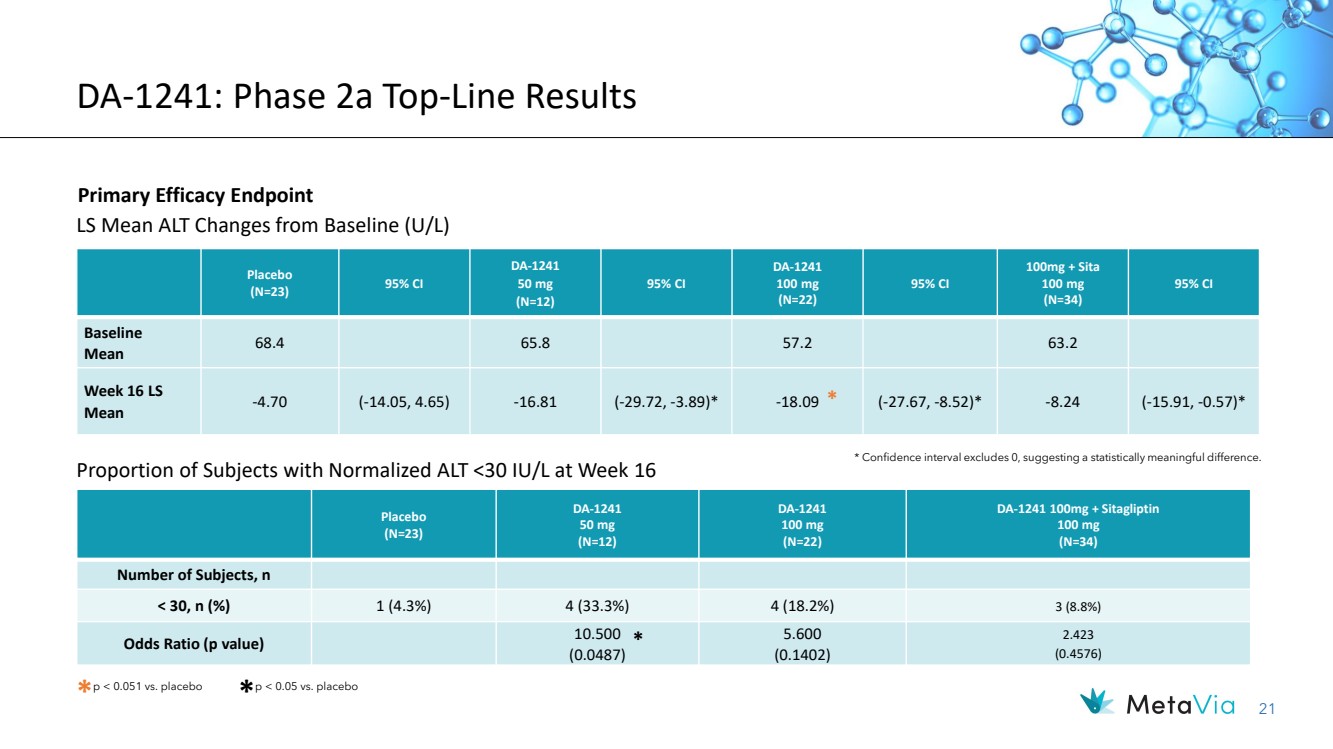
| 21 Placebo (N=23) DA-1241 50 mg (N=12) DA-1241 100 mg (N=22) DA-1241 100mg + Sitagliptin 100 mg (N=34) Number of Subjects, n < 30, n (%) 1 (4.3%) 4 (33.3%) 4 (18.2%) 3 (8.8%) Odds Ratio (p value) 10.500 (0.0487) 5.600 (0.1402) 2.423 (0.4576) DA-1241: Phase 2a Top-Line Results Primary Efficacy Endpoint * Confidence interval excludes 0, suggesting a statistically meaningful difference. LS Mean ALT Changes from Baseline (U/L) * p < 0.051 vs. placebo * p < 0.05 vs. placebo * Placebo (N=23) 95% CI DA-1241 50 mg (N=12) 95% CI DA-1241 100 mg (N=22) 95% CI 100mg + Sita 100 mg (N=34) 95% CI Baseline Mean 68.4 65.8 57.2 63.2 Week 16 LS Mean -4.70 (-14.05, 4.65) -16.81 (-29.72, -3.89)* -18.09 (-27.67, -8.52)* -8.24 (-15.91, -0.57)* Proportion of Subjects with Normalized ALT <30 IU/L at Week 16 * |
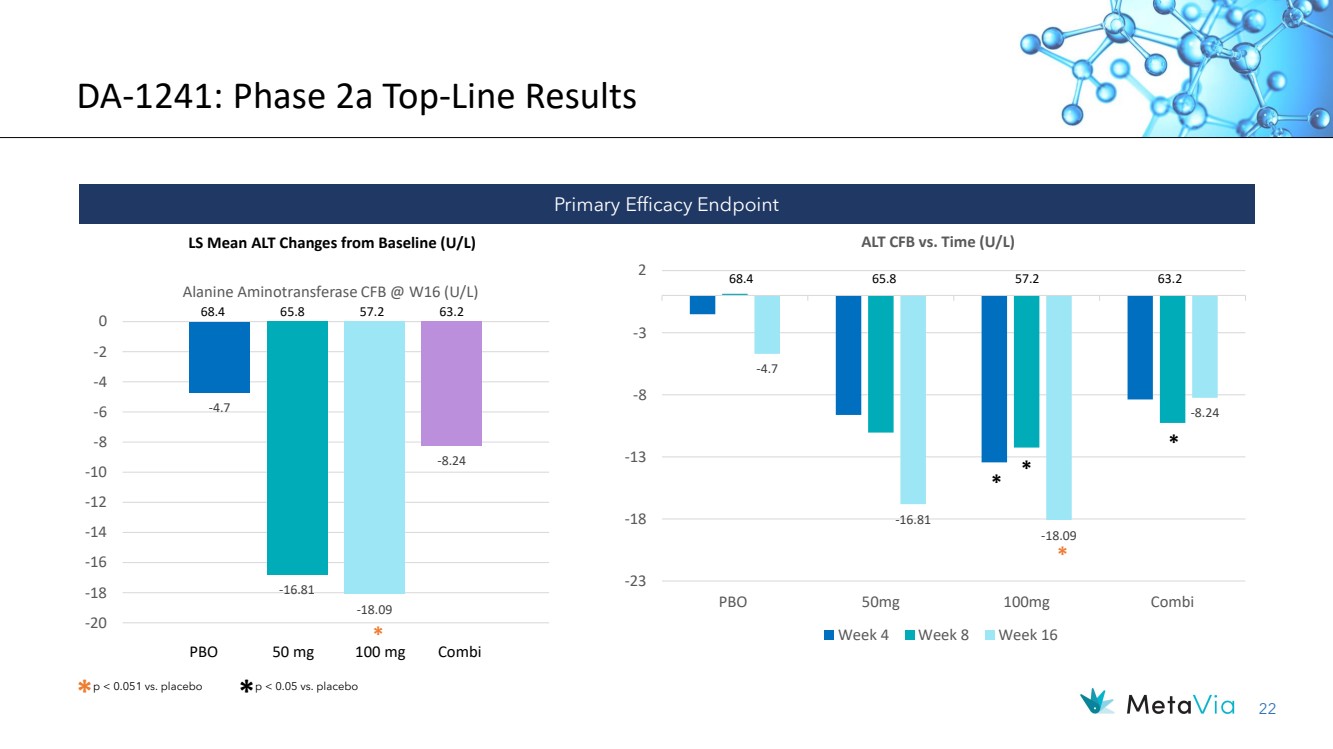
| 22 DA-1241: Phase 2a Top-Line Results LS Mean ALT Changes from Baseline (U/L) -4.7 -16.81 -18.09 -8.24 -20 -18 -16 -14 -12 -10 -8 -6 -4 -2 0 Alanine Aminotransferase CFB @ W16 (U/L) 68.4 65.8 57.2 63.2 -4.7 -16.81 -18.09 -8.24 -23 -18 -13 -8 -3 2 PBO 50mg 100mg Combi ALT CFB vs. Time (U/L) Week 4 Week 8 Week 16 68.4 65.8 57.2 63.2 PBO 50 mg 100 mg Combi Primary Efficacy Endpoint * p < 0.051 vs. placebo * p < 0.05 vs. placebo * * * * * |
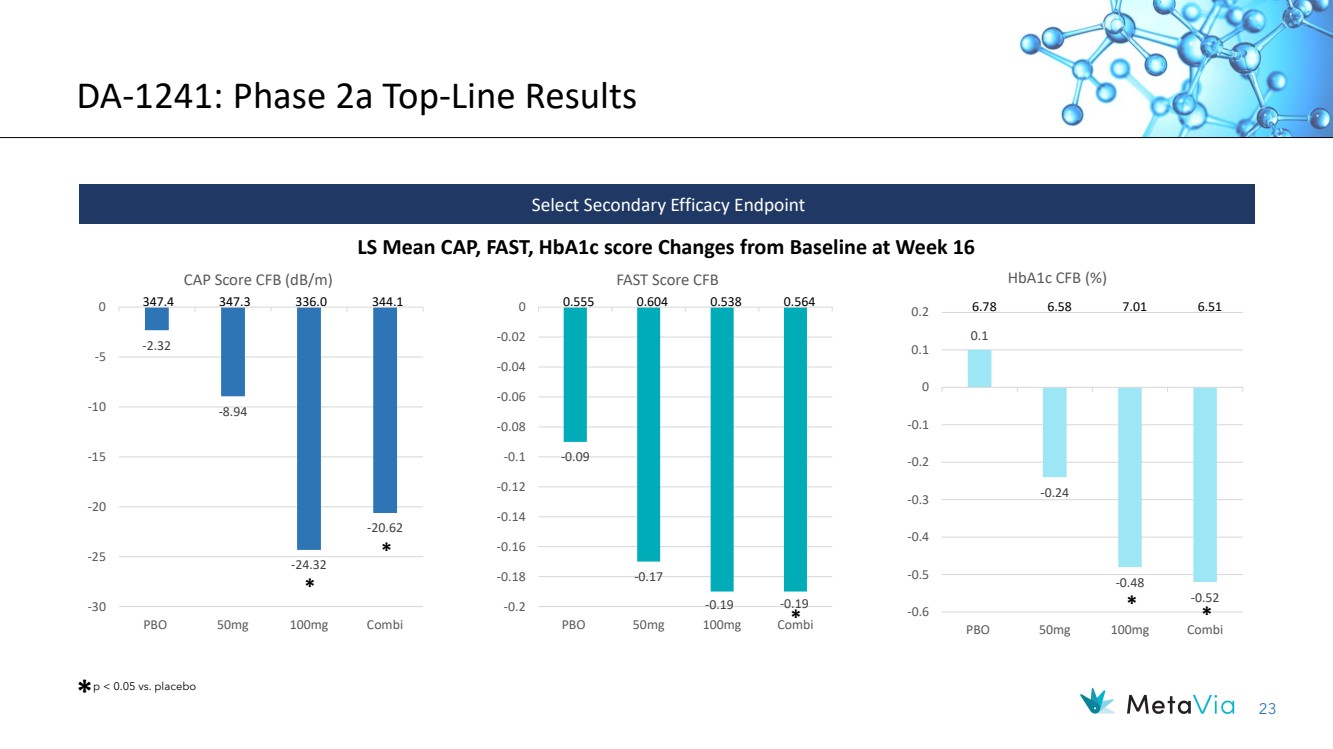
| 23 DA-1241: Phase 2a Top-Line Results LS Mean CAP, FAST, HbA1c score Changes from Baseline at Week 16 Select Secondary Efficacy Endpoint * p < 0.05 vs. placebo * -0.09 -0.17 -0.2 -0.19 -0.19 -0.18 -0.16 -0.14 -0.12 -0.1 -0.08 -0.06 -0.04 -0.02 0 PBO 50mg 100mg Combi FAST Score CFB 347.4 347.3 336.0 344.1 0.555 0.604 0.538 0.564 0.1 -0.24 -0.48 -0.52 -0.6 -0.5 -0.4 -0.3 -0.2 -0.1 0 0.1 0.2 PBO 50mg 100mg Combi HbA1c CFB (%) 6.78 6.58 7.01 6.51 * -2.32 -8.94 -24.32 -20.62 -30 -25 -20 -15 -10 -5 0 PBO 50mg 100mg Combi CAP Score CFB (dB/m) * * * |
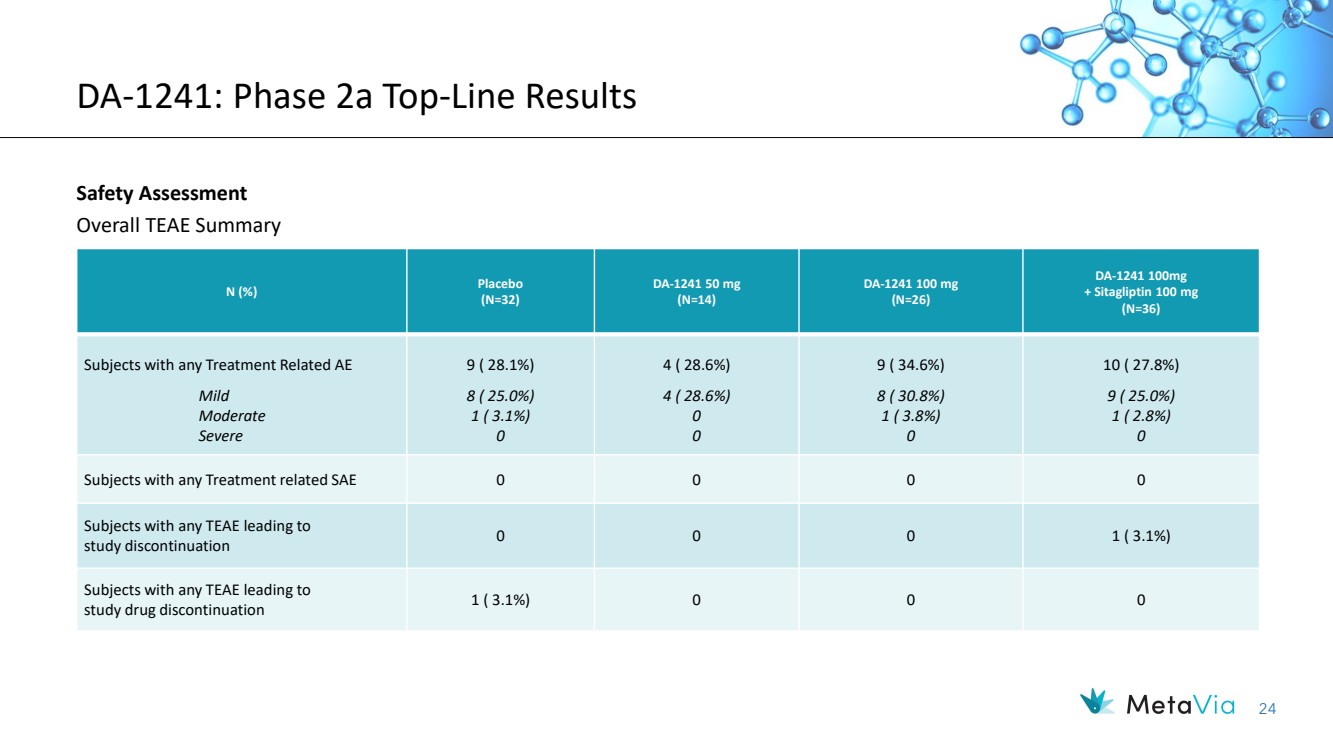
| 24 DA-1241: Phase 2a Top-Line Results Safety Assessment Overall TEAE Summary N (%) Placebo (N=32) DA-1241 50 mg (N=14) DA-1241 100 mg (N=26) DA-1241 100mg + Sitagliptin 100 mg (N=36) Subjects with any Treatment Related AE Mild Moderate Severe 9 ( 28.1%) 8 ( 25.0%) 1 ( 3.1%) 0 4 ( 28.6%) 4 ( 28.6%) 0 0 9 ( 34.6%) 8 ( 30.8%) 1 ( 3.8%) 0 10 ( 27.8%) 9 ( 25.0%) 1 ( 2.8%) 0 Subjects with any Treatment related SAE 0 0 0 0 Subjects with any TEAE leading to study discontinuation 0 0 0 1 ( 3.1%) Subjects with any TEAE leading to study drug discontinuation 1 ( 3.1%) 0 0 0 |
| December 2023 NASDAQ: NRBO 25 Financials and Capitalization |
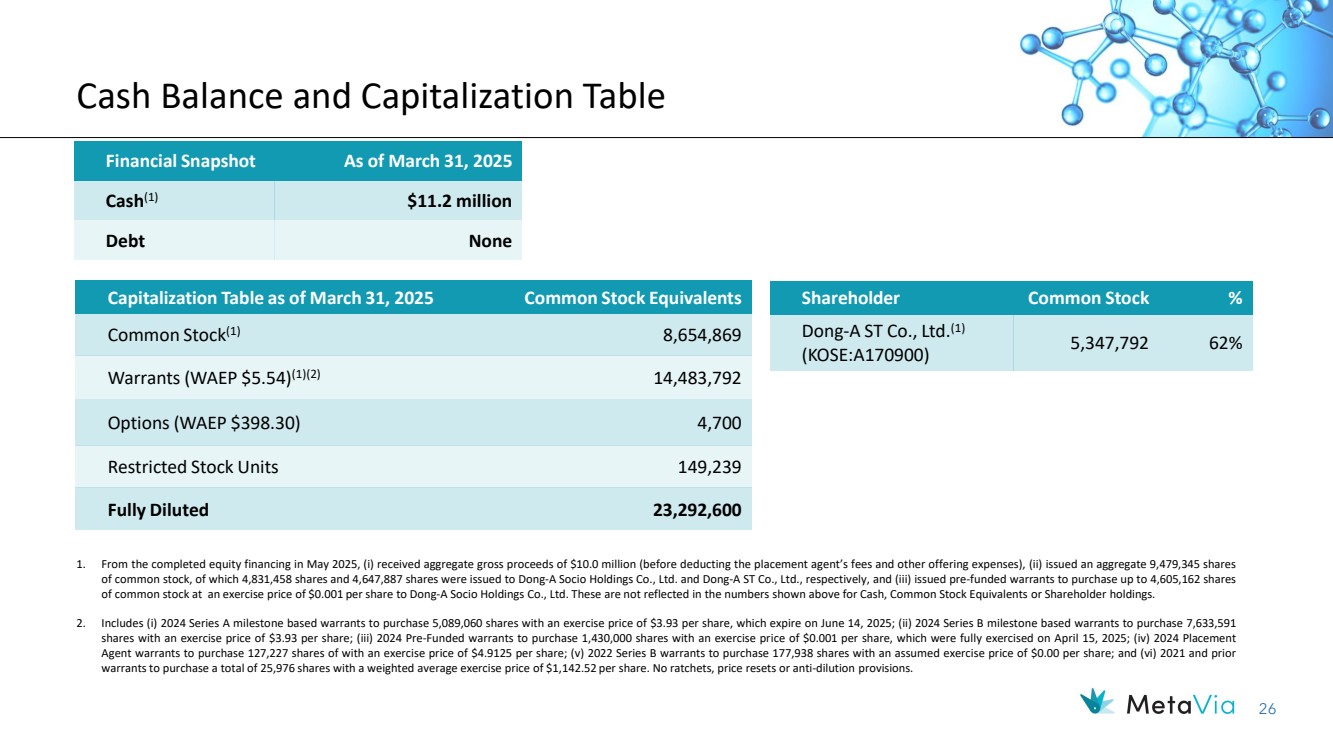
| 26 Cash Balance and Capitalization Table Financial Snapshot As of March 31, 2025 Cash(1) $11.2 million Debt None Capitalization Table as of March 31, 2025 Common Stock Equivalents Common Stock(1) 8,654,869 Warrants (WAEP $5.54)(1)(2) 14,483,792 Options (WAEP $398.30) 4,700 Restricted Stock Units 149,239 Fully Diluted 23,292,600 1. From the completed equity financing in May 2025, (i) received aggregate gross proceeds of $10.0 million (before deducting the placement agent’s fees and other offering expenses), (ii) issued an aggregate 9,479,345 shares of common stock, of which 4,831,458 shares and 4,647,887 shares were issued to Dong-A Socio Holdings Co., Ltd. and Dong-A ST Co., Ltd., respectively, and (iii) issued pre-funded warrants to purchase up to 4,605,162 shares of common stock at an exercise price of $0.001 per share to Dong-A Socio Holdings Co., Ltd. These are not reflected in the numbers shown above for Cash, Common Stock Equivalents or Shareholder holdings. 2. Includes (i) 2024 Series A milestone based warrants to purchase 5,089,060 shares with an exercise price of $3.93 per share, which expire on June 14, 2025; (ii) 2024 Series B milestone based warrants to purchase 7,633,591 shares with an exercise price of $3.93 per share; (iii) 2024 Pre-Funded warrants to purchase 1,430,000 shares with an exercise price of $0.001 per share, which were fully exercised on April 15, 2025; (iv) 2024 Placement Agent warrants to purchase 127,227 shares of with an exercise price of $4.9125 per share; (v) 2022 Series B warrants to purchase 177,938 shares with an assumed exercise price of $0.00 per share; and (vi) 2021 and prior warrants to purchase a total of 25,976 shares with a weighted average exercise price of $1,142.52 per share. No ratchets, price resets or anti-dilution provisions. Shareholder Common Stock % Dong-A ST Co., Ltd.(1) (KOSE:A170900) 5,347,792 62% |
| 27 Compelling Investment Opportunity Targeting Obesity and MASH with a Pipeline of Next Generation Therapeutics ▪ Aiming to increase Shareholder Value through Multiple, Near-Term, Value Creating Milestones • DA-1726 ✓ With strong weight loss at 4 weeks, potentially best-in-class tolerability and compelling safety with potential pathway to not only obese otherwise healthy patients but also obese T2D and obese MASH patients ✓ 32 mg (not the max dose) with no titration showed max weight loss of -6.3% (15.4 lbs.) and mean weight loss of -4.3% at Day 26 ✓ Potentially best-in-class lowering of fasted glucose of -5.3 mg/dL and maximum of -18 mg/dL at Day 26 at 32 mg dose with strong signals of GLP-1R efficacy ✓ Potentially best-in-class waist circumference reduction with mean of 1.6 inches and maximum of 3.9 inches at Day 33 at 32 mg dose with strong signals of GCGR efficacy ✓ No treatment-related discontinuations, with mild GI related AEs at 32 mg dose o Additional cohort(s) are being added to Part 1 (SAD) and Part 2 (MAD) to explore maximum tolerable dose • DA-1241 ✓ Phase 2a top-line data in subjects with presumed MASH met primary end point and showed direct hepatic effects ✓ With added significant reductions in HbA1C from baseline at 100 mg dosing at Week 16 compared to the placebo group o Other exploratory end points including MRI-PDFF to be released at major medical conferences o Will be actively searching for a combination/licensing partner |
| December 2023 NASDAQ: NRBO 28 Thank You! Investor Contacts: Rx Communications Group Michael Miller +1 917.633.6086 mmiller@rxir.com MetaVia Marshall Woodworth +1 919.749.8748 marshall.woodworth@metaviatx.com |
| December 2023 NASDAQ: NRBO 29 Appendix |
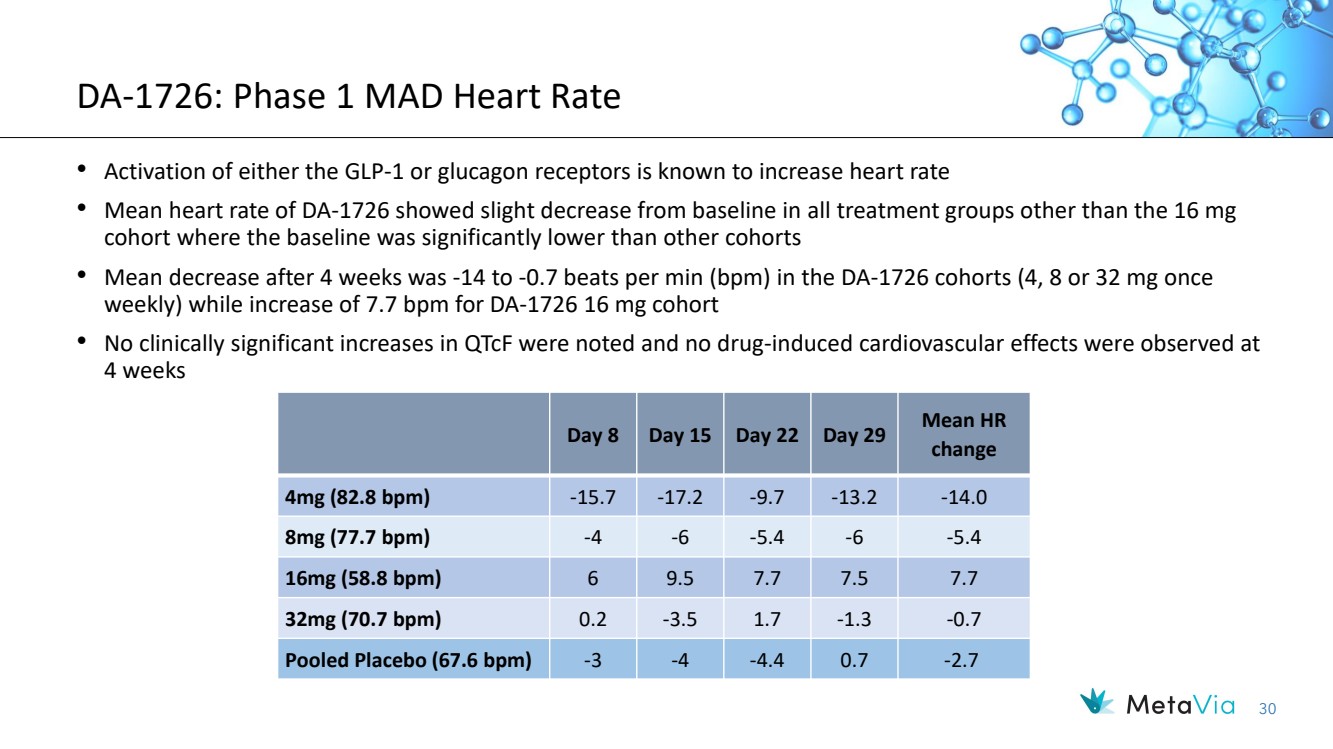
| 30 DA-1726: Phase 1 MAD Heart Rate Day 8 Day 15 Day 22 Day 29 Mean HR change 4mg (82.8 bpm) -15.7 -17.2 -9.7 -13.2 -14.0 8mg (77.7 bpm) -4 -6 -5.4 -6 -5.4 16mg (58.8 bpm) 6 9.5 7.7 7.5 7.7 32mg (70.7 bpm) 0.2 -3.5 1.7 -1.3 -0.7 Pooled Placebo (67.6 bpm) -3 -4 -4.4 0.7 -2.7 • Activation of either the GLP-1 or glucagon receptors is known to increase heart rate • Mean heart rate of DA-1726 showed slight decrease from baseline in all treatment groups other than the 16 mg cohort where the baseline was significantly lower than other cohorts • Mean decrease after 4 weeks was -14 to -0.7 beats per min (bpm) in the DA-1726 cohorts (4, 8 or 32 mg once weekly) while increase of 7.7 bpm for DA-1726 16 mg cohort • No clinically significant increases in QTcF were noted and no drug-induced cardiovascular effects were observed at 4 weeks |