

NASDAQ TIVC INVESTOR PRESENTATION 2022 SEPT The future of medicine is electronic. NASDAQ : TIVC INVESTOR PRESENTATION May 2024

Forward-Looking Statements This presentation contains forward-looking statements. All statements other than statements of historical facts contained in this presentation may be forward-looking statements. Statements regarding our future results of operations and financial position, economic performance, business strategy and plans and objectives of management for future operations, including, among others, statements regarding the consummation of the offering, our expected growth, investments, and future capital expenditures are all forward looking statements. Without limiting the generality of the foregoing, words such as “may,” “will,” “should,” “expect,” “believe,” “anticipate,” “intend,” “could,” “estimate,” “target,” “project,” “might,” “plan,” “predict” or “continue” or the negative or other variations thereof or comparable terminology are intended to identify forward-looking statements. We caution you that any such forward-looking statements are not guarantees of future performance, and are subject to risks, assumptions and uncertainties that are difficult to predict and beyond our ability and control. Although we believe that the expectations reflected in these forward-looking statements are reasonable as of the date made, actual results may prove to be materially different from the results expressed or implied by the forward-looking statements. Any differences could be caused by a number of factors, including but not limited to: our anticipated needs for working capital; our ability to secure additional financing; our ability to continue to satisfy the listing standards of Nasdaq; regulatory or legal developments in the United States and other countries; economic and market conditions; our expectation regarding timing, costs, conduct and development of our products and product candidates; and our efforts to expand our products and business. Many of the important factors that will determine these results are beyond our ability to control or predict. Accordingly, you should not place undue reliance on any such forward-looking statements. Any forward-looking statement speaks only as of the date on which it is made, and, except as otherwise required by law, we do not undertake any obligation to publicly update or review any forward-looking statement, whether as a result of new information, future developments or otherwise. New factors emerge from time to time, and it is not possible for us to predict which will arise. We cannot assess the impact of each factor on our business or the extent to which any factor, or combination of factors, may cause actual results to differ materially from those contained in any forward-looking statements. In addition, statements that “we believe” and similar statements reflect our beliefs and opinions on the relevant subject. These statements are based upon information available to us as of the date made, and while we believe such information forms a reasonable basis for such statements, such information may be limited or incomplete, and our statements should not be read to indicate that we have conducted an exhaustive inquiry into, or review of, all potentially available relevant information. These statements are inherently uncertain, and you are cautioned not to place undue reliance upon these statements. Industry Information Unless otherwise indicated, information contained in this presentation concerning our industry, competitive position and the markets in which we operate is based on information from independent industry and research organizations, other third-party sources, as well as data from our internal research, and are based on assumptions made by us upon reviewing such data, and our experience in, and knowledge of, such industry and markets, which we believe to be reasonable. In addition, projections, assumptions and estimates of the future performance of the industry in which we operate, our addressable market, and our future performance are necessarily subject to uncertainty and risk due to a variety of factors, which could cause results to differ materially from those expressed in the estimates made by the independent parties and by us. Additional Information This presentation shall not constitute an offer to sell or the solicitation of an offer to buy our securities, nor shall there be any sale of our securities in any state or jurisdiction in which such offer, solicitation, or sale would be unlawful prior to registration or qualification under the securities laws of any such state or jurisdiction. Potential investors should review our filings with the Securities and Exchange Commission (“SEC”), including our Annual Report on Form 10-K for the year ended December 31, 2023, filed with the SEC on March 25, 2024, and the Risk Factors included therein, as well as our subsequent filings with the SEC. These filings and other information about us are available on the SEC’s website at www.sec.gov. You may access these materials at our corporate website, www.tivichealth.com, free of charge as soon as reasonably practicable after they are electronically filed with, or furnished to, the SEC. Disclaimers / Safe Harbor Statement This presentation has been prepared to assist us in determining the potential level of investor interest in our company. This presentation and information contained in this presentation is confidential and proprietary to us and is being distributed to you on a confidential basis. No part of this presentation or the information contained herein may be reproduced, photocopied, redistributed or passed on, directly or indirectly, to any other person or published, in whole or in part, for any purpose.

References provided in Appendix . Investment Highlights TIVIC HEALTH Commercial-stage in Bioelectronic Medicine, field projected to grow at 35% CAGR 2019-20291 One of the most promising new fields of medicine. FDA-approved product in market targeting $9.1B addressable market2 First product (ClearUP) targets inflammation of sinus passages, proof source from which to build neuromodulation lines. New results on vagus nerve stimulation, which form basis for future growth Vagus nerve is a high-value target for medical applications. Tivic’s low-current, non-invasive approach has recently shown potential applications in cardiology, neurology, and psychiatry.3 Significant R&D inflection points within next 6 months Clinical read-outs on two research programs expected within next 6 months, including first published data from new area of Vagus Nerve Stimulation. Strong fundamental IP: 9 issued patents, 10 patents pending US, Europe, China, and others, issuance dates beginning in 2020. IP covers all factors that make trigeminal nerve stimulation effective, comfortable and easy to use. Recently filed VNS IP covers fundamental differentiation in stimulation circuitry and method. Experienced management team Backgrounds include growth through organic R&D, strategic partnerships, licensing, M&A and joint ventures.

THE BIG IDEA: BIOELECTRONIC MEDICINE The body is an electrochemical system. Restore healthy function by engaging the body’s electrical signals. Bioelectronic medicine represents a multi-billion-dollar opportunity and has the “potential to become a pillar of medical treatment.” Bioelectronics ‘jump-start’ the next wave of device therapeutics. – October 2019 4 4
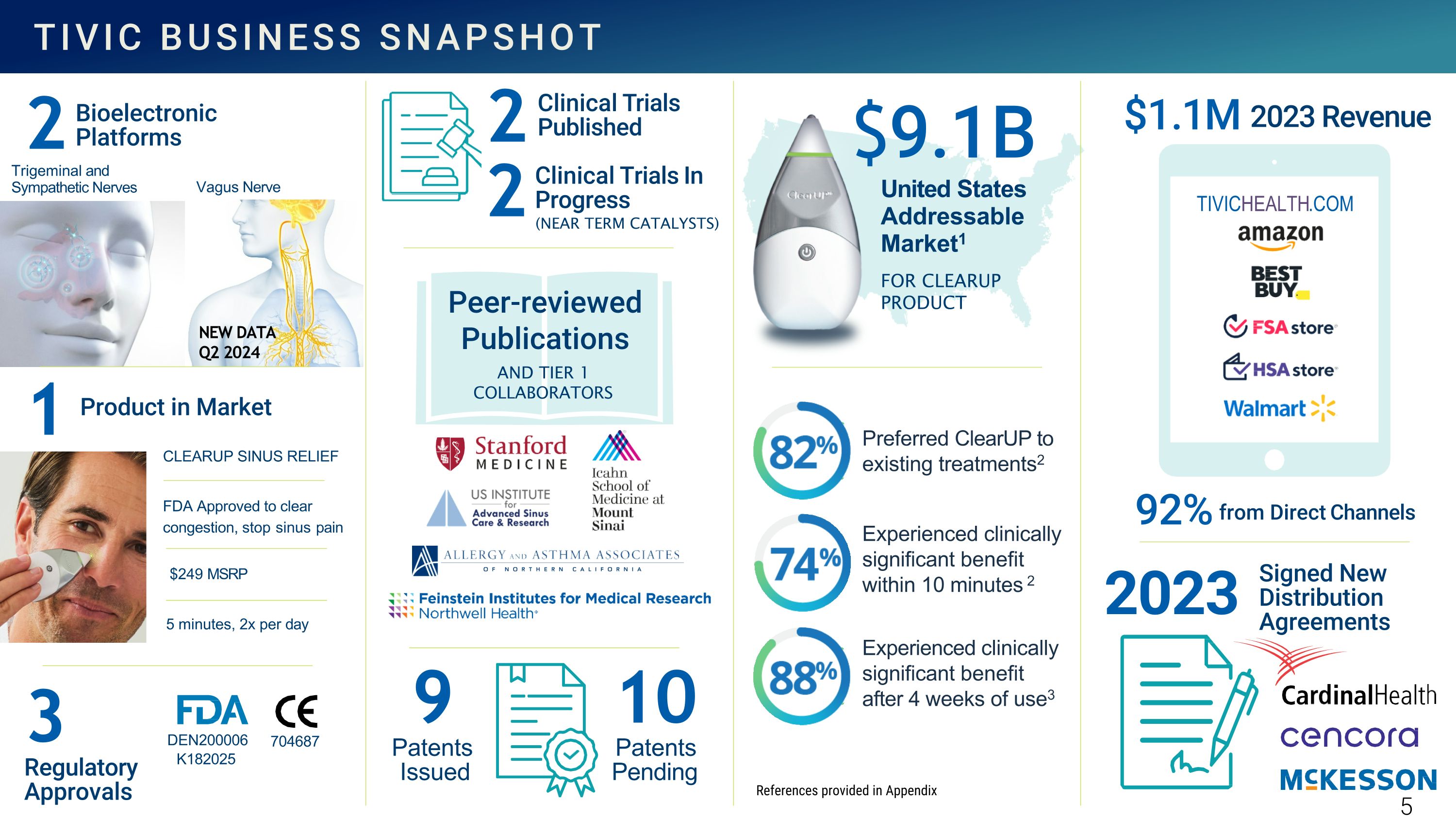
FDA Approved to clear congestion, stop sinus pain $249 MSRP 5 minutes, 2x per day Bioelectronic Platforms Regulatory Approvals Peer-reviewed Publications AND TIER 1 COLLABORATORS FOR CLEARUP PRODUCT 2 Product in Market 1 Trigeminal and Sympathetic Nerves Signed New Distribution Agreements 2023 Preferred ClearUP to existing treatments2 Experienced clinically significant benefit within 10 minutes 2 Experienced clinically significant benefit after 4 weeks of use3 3 Patents Pending Patents Issued 10 $9.1B United States Addressable Market1 704687 DEN200006 K182025 Vagus Nerve Clinical Trials In Progress 2 2 Clinical Trials Published TIVICHEALTH.COM 92% $1.1M 9 CLEARUP SINUS RELIEF 2023 Revenue from Direct Channels NEW DATA Q2 2024 (NEAR TERM CATALYSTS) References provided in Appendix TIVIC BUSINESS SNAPSHOT 5
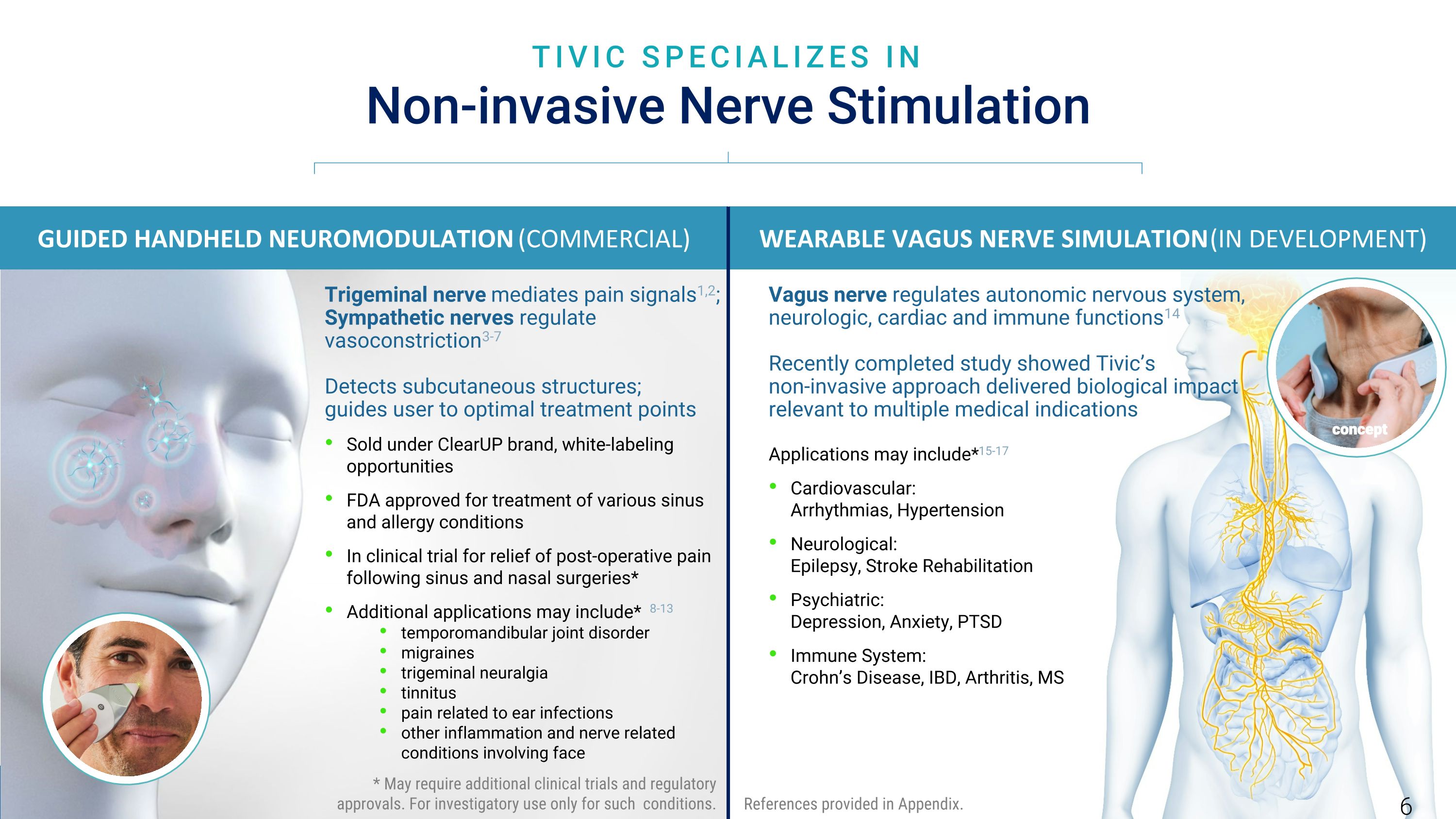
TIVIC SPECIALIZES IN Non-invasive Nerve Stimulation GUIDED HANDHELD NEUROMODULATION (COMMERCIAL) Trigeminal nerve mediates pain signals1,2; Sympathetic nerves regulate vasoconstriction3-7 Detects subcutaneous structures; guides user to optimal treatment points Sold under ClearUP brand, white-labeling opportunities FDA approved for treatment of various sinus and allergy conditions In clinical trial for relief of post-operative pain following sinus and nasal surgeries* Additional applications may include* 8-13 temporomandibular joint disorder migraines trigeminal neuralgia tinnitus pain related to ear infections other inflammation and nerve related conditions involving face WEARABLE VAGUS NERVE SIMULATION (IN DEVELOPMENT) concept References provided in Appendix. Vagus nerve regulates autonomic nervous system, neurologic, cardiac and immune functions14 Recently completed study showed Tivic’s non-invasive approach delivered biological impact relevant to multiple medical indications Applications may include*15-17 Cardiovascular: Arrhythmias, Hypertension Neurological: Epilepsy, Stroke Rehabilitation Psychiatric: Depression, Anxiety, PTSD Immune System: Crohn’s Disease, IBD, Arthritis, MS * May require additional clinical trials and regulatory approvals. For investigatory use only for such conditions. 6
CORE TECHNOLOGY BIOELECTRONIC “PLATFORM” Programmable Signal Parameters Patented Electrical and Industrial Designs Proprietary Algorithms Tivic has unique expertise to optimize the delivery of effective, low-current, non-invasive neuromodulation 7
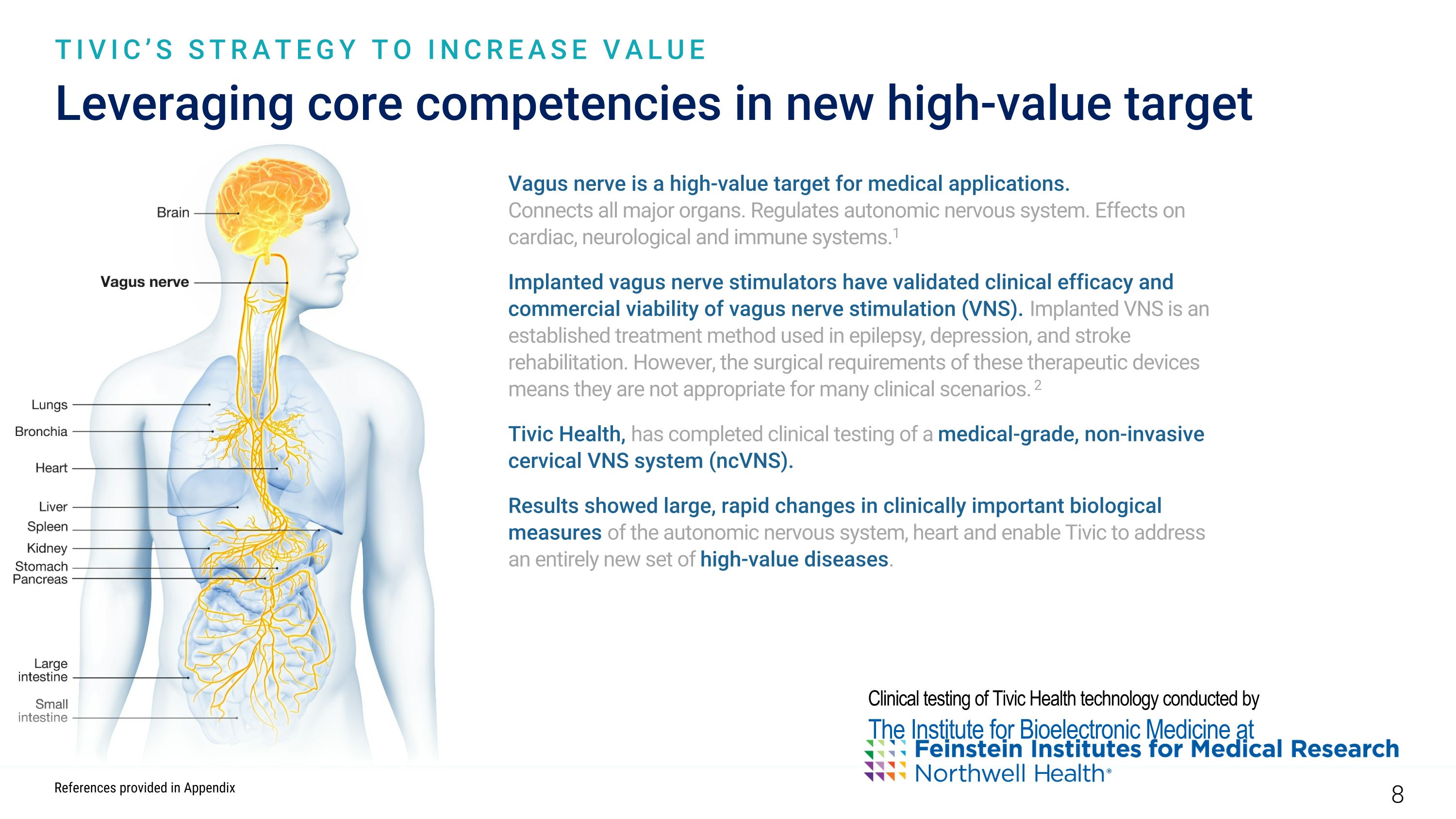
Leveraging core competencies in new high-value target TIVIC’S STRATEGY TO INCREASE VALUE Vagus nerve is a high-value target for medical applications. Connects all major organs. Regulates autonomic nervous system. Effects on cardiac, neurological and immune systems.1 Implanted vagus nerve stimulators have validated clinical efficacy and commercial viability of vagus nerve stimulation (VNS). Implanted VNS is an established treatment method used in epilepsy, depression, and stroke rehabilitation. However, the surgical requirements of these therapeutic devices means they are not appropriate for many clinical scenarios. 2 Tivic Health, has completed clinical testing of a medical-grade, non-invasive cervical VNS system (ncVNS). Results showed large, rapid changes in clinically important biological measures of the autonomic nervous system, heart and enable Tivic to address an entirely new set of high-value diseases. Clinical testing of Tivic Health technology conducted by The Institute for Bioelectronic Medicine at References provided in Appendix 8
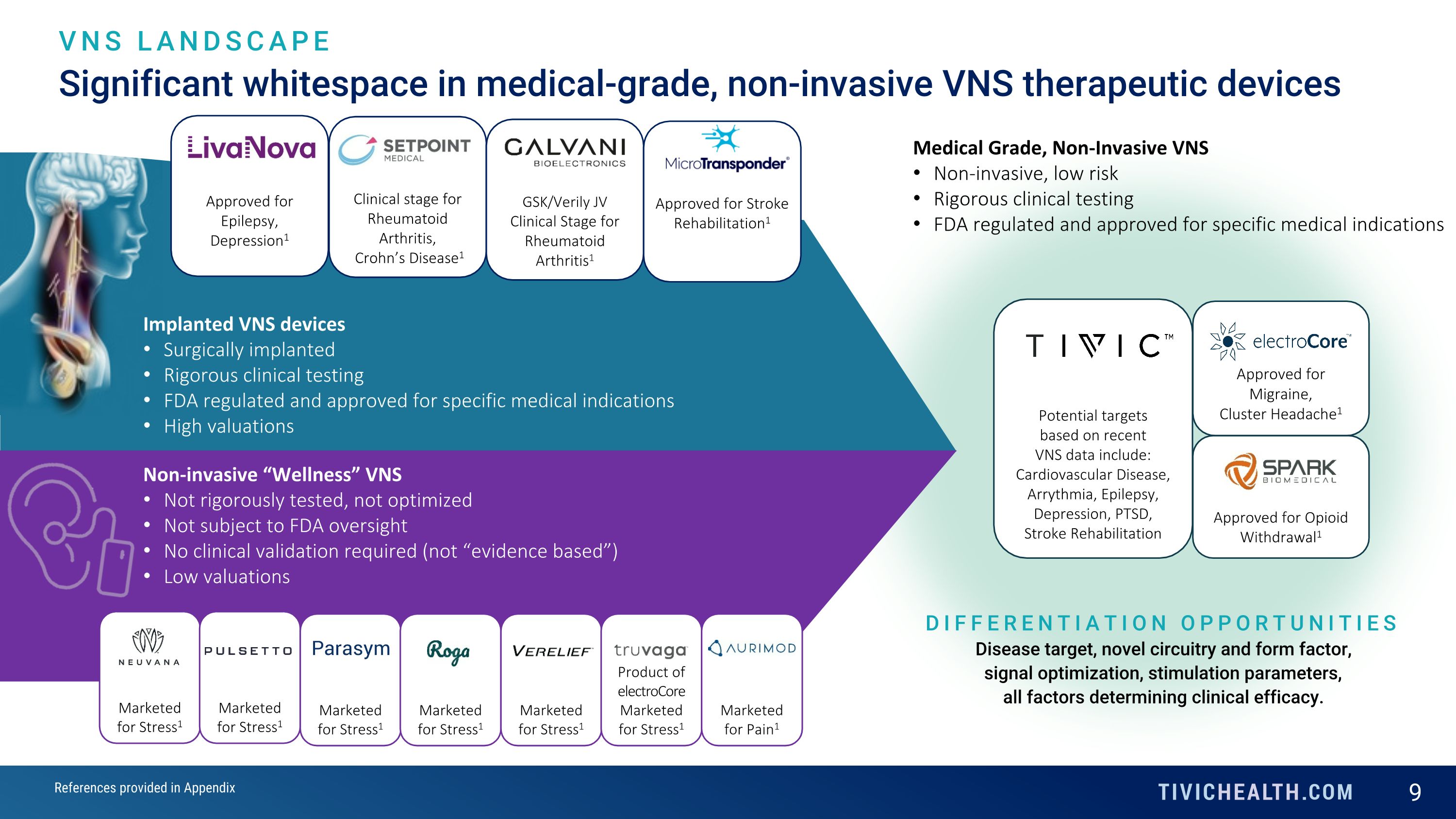
Significant whitespace in medical-grade, non-invasive VNS therapeutic devices VNS LANDSCAPE Non-invasive “Wellness” VNS Not rigorously tested, not optimized Not subject to FDA oversight No clinical validation required (not “evidence based”) Low valuations Marketed for Stress1 Marketed for Stress1 Marketed for Stress1 Marketed for Stress1 Marketed for Stress1 Product of electroCore Marketed for Stress1 Marketed for Pain1 Implanted VNS devices Surgically implanted Rigorous clinical testing FDA regulated and approved for specific medical indications High valuations Medical Grade, Non-Invasive VNS Non-invasive, low risk Rigorous clinical testing FDA regulated and approved for specific medical indications DIFFERENTIATION OPPORTUNITIES Disease target, novel circuitry and form factor, signal optimization, stimulation parameters, all factors determining clinical efficacy. Approved for Migraine, Cluster Headache1 Approved for Opioid Withdrawal1 Potential targets based on recent VNS data include: Cardiovascular Disease, Arrythmia, Epilepsy, Depression, PTSD, Stroke Rehabilitation References provided in Appendix Approved for Epilepsy, Depression1 Clinical stage for Rheumatoid Arthritis, Crohn’s Disease1 GSK/Verily JV Clinical Stage for Rheumatoid Arthritis1 Approved for Stroke Rehabilitation1
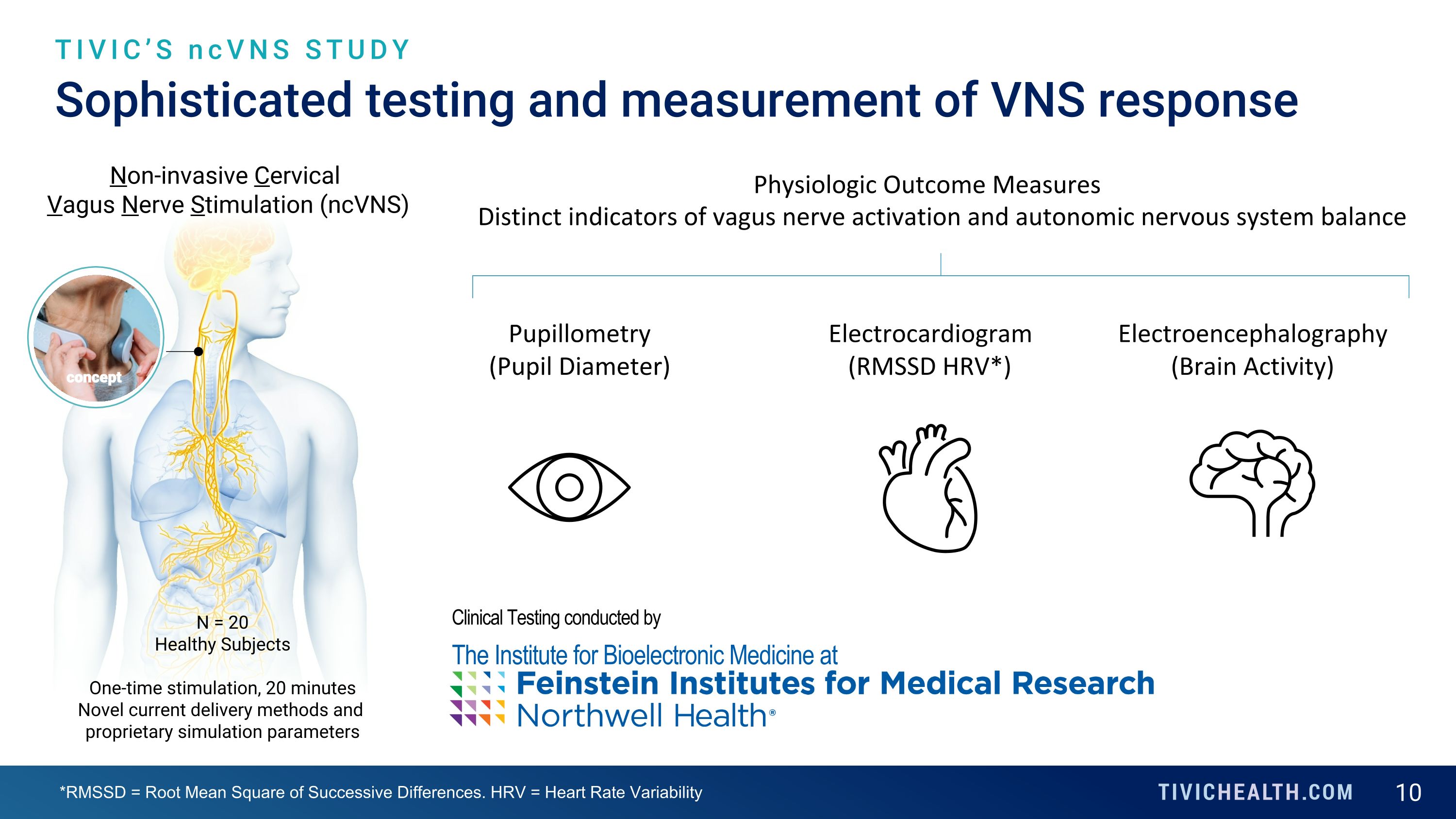
Sophisticated testing and measurement of VNS response TIVIC’S ncVNS STUDY Non-invasive Cervical Vagus Nerve Stimulation (ncVNS) N = 20 Healthy Subjects One-time stimulation, 20 minutes Novel current delivery methods and proprietary simulation parameters Physiologic Outcome Measures Pupillometry (Pupil Diameter) Electrocardiogram (RMSSD HRV*) Electroencephalography (Brain Activity) Distinct indicators of vagus nerve activation and autonomic nervous system balance Clinical Testing conducted by The Institute for Bioelectronic Medicine at *RMSSD = Root Mean Square of Successive Differences. HRV = Heart Rate Variability concept
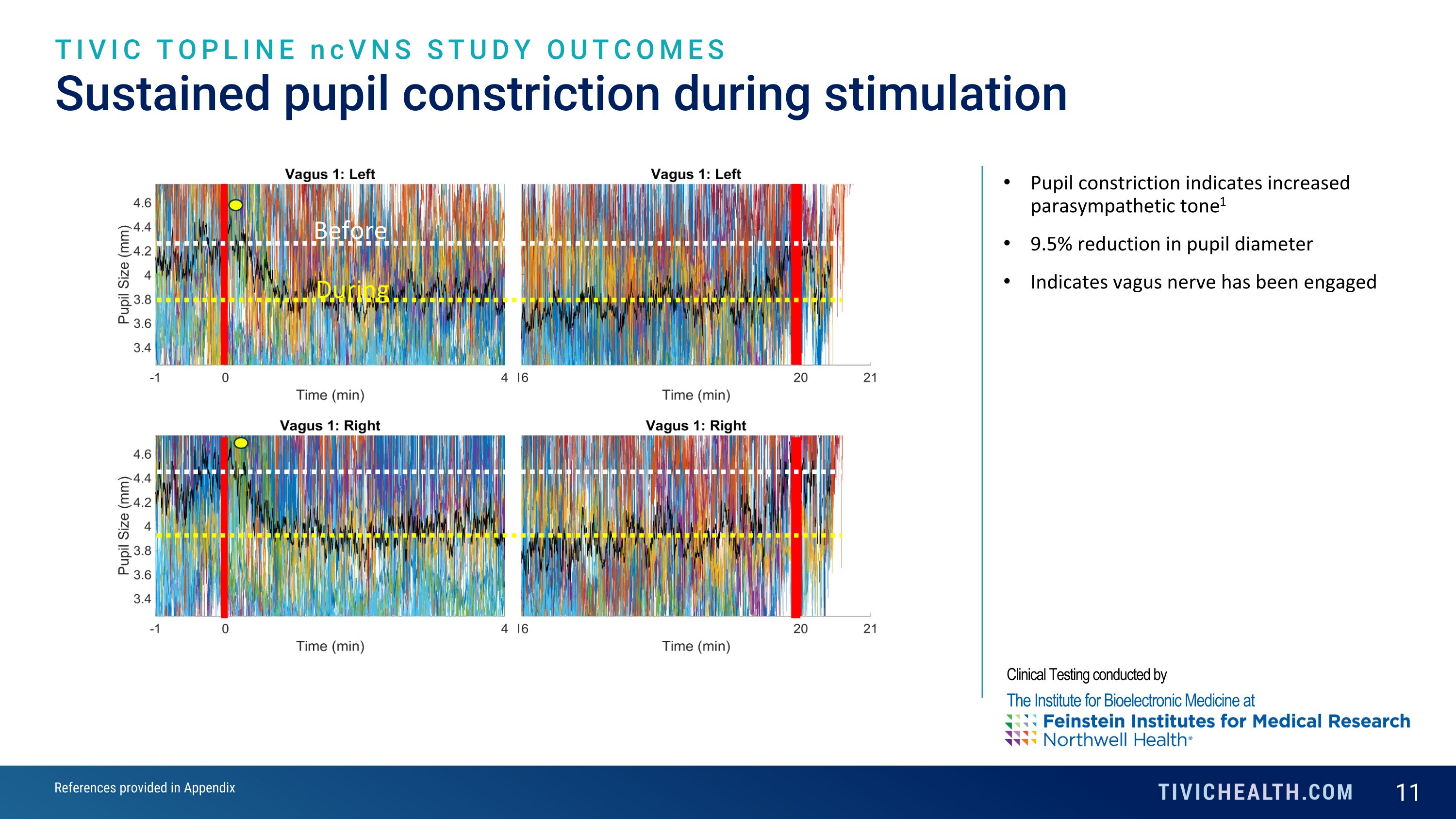
Sustained pupil constriction during stimulation TIVIC TOPLINE ncVNS STUDY OUTCOMES Pupil constriction indicates increased parasympathetic tone1 9.5% reduction in pupil diameter Indicates vagus nerve has been engaged Before During Clinical Testing conducted by The Institute for Bioelectronic Medicine at References provided in Appendix

Large, clinically meaningful increase in RMSSD – a measure of heart rate variability, vagal tone and parasympathetic activity TIVIC TOPLINE ncVNS STUDY OUTCOMES Importance of RMSSD RMSSD is an accepted proxy for vagal tone and parasympathetic activity1 Increasing parasympathetic activity may treat or prevent cardiac arrythmia2 Higher RMSSD is associated with lower morbidity and mortality in cardiovascular disease3 Tivic Topline Study Results 97% increase in RMSSD (2x) 170% increase in RMSSD in responders (2.7x) 60% Responder Rate (comparable to implants)4 Implantable devices have shown similar results4 Non-invasive devices have varianble results5 Responses in clinical populations with medical issues expected to be larger All Subjects 2x Mean Increase 2.7x Mean Increase Responders (60% responder rate) Heart rate variability (RMSSD; higher is better) Before ncVNS After ncVNS Before ncVNS After ncVNS References provided in Appendix *RMSSD = Root Mean Square of Successive Differences. HRV = Heart Rate Variability
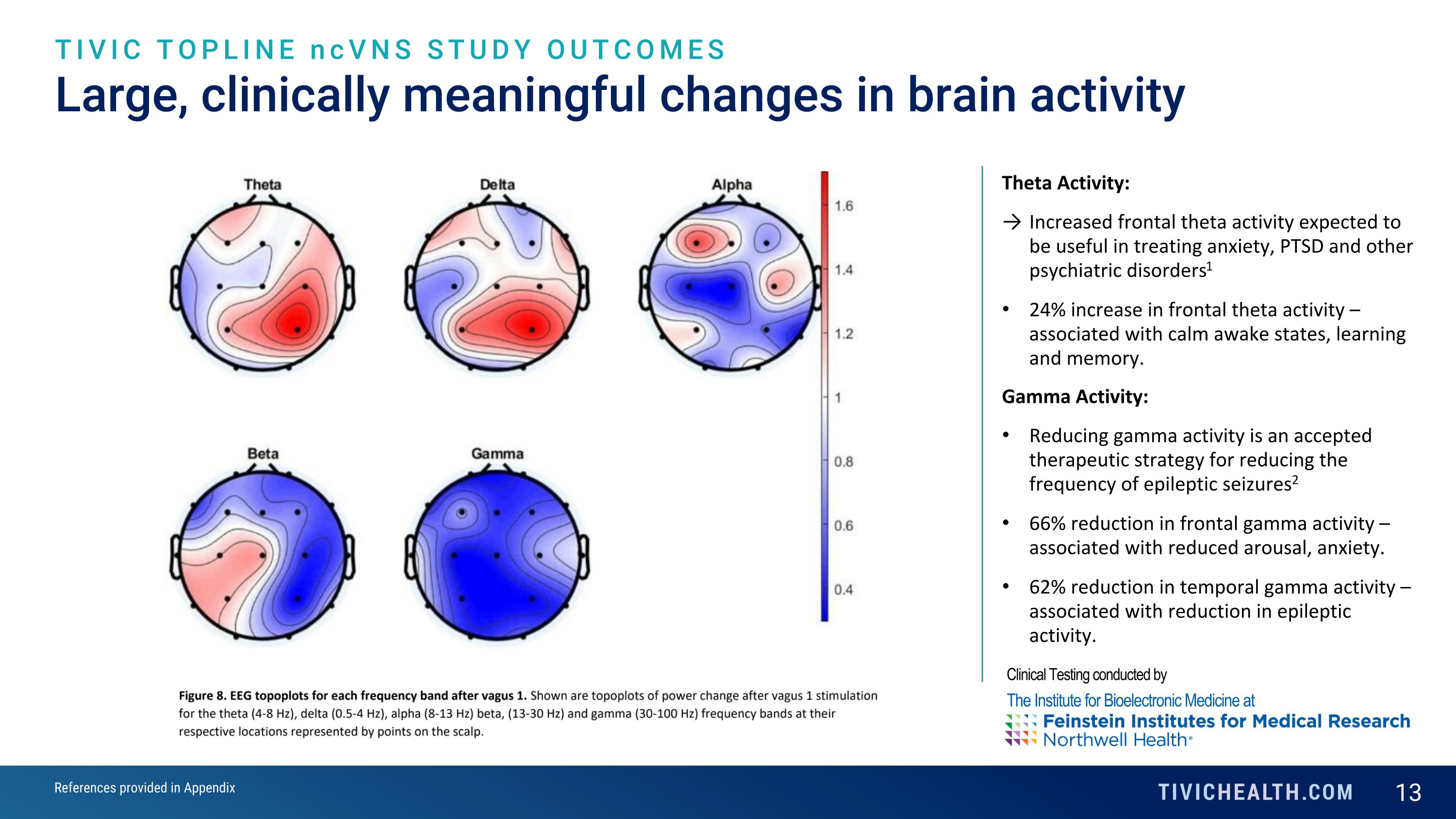
Large, clinically meaningful changes in brain activity TIVIC TOPLINE ncVNS STUDY OUTCOMES Theta Activity: Increased frontal theta activity expected to be useful in treating anxiety, PTSD and other psychiatric disorders1 24% increase in frontal theta activity – associated with calm awake states, learning and memory. Gamma Activity: Reducing gamma activity is an accepted therapeutic strategy for reducing the frequency of epileptic seizures2 66% reduction in frontal gamma activity – associated with reduced arousal, anxiety. 62% reduction in temporal gamma activity – associated with reduction in epileptic activity. Clinical Testing conducted by The Institute for Bioelectronic Medicine at References provided in Appendix
Why this matters Our topline ncVNS data indicate large rapid effects in clinically important biological measures (cardiac, brain, autonomic). Implanted technologies have validated clinical efficacy and commercial viability of VNS but are not always appropriate for the clinical scenario.1 Non-invasive VNS approaches to date have had not been adequately optimized resulting in variable responses and modest efficacy.2 References provided in Appendix There is a sizeable technical and commercial gap in medical-grade, non-invasive VNS. Tivic Health is poised to capitalize on that gap.
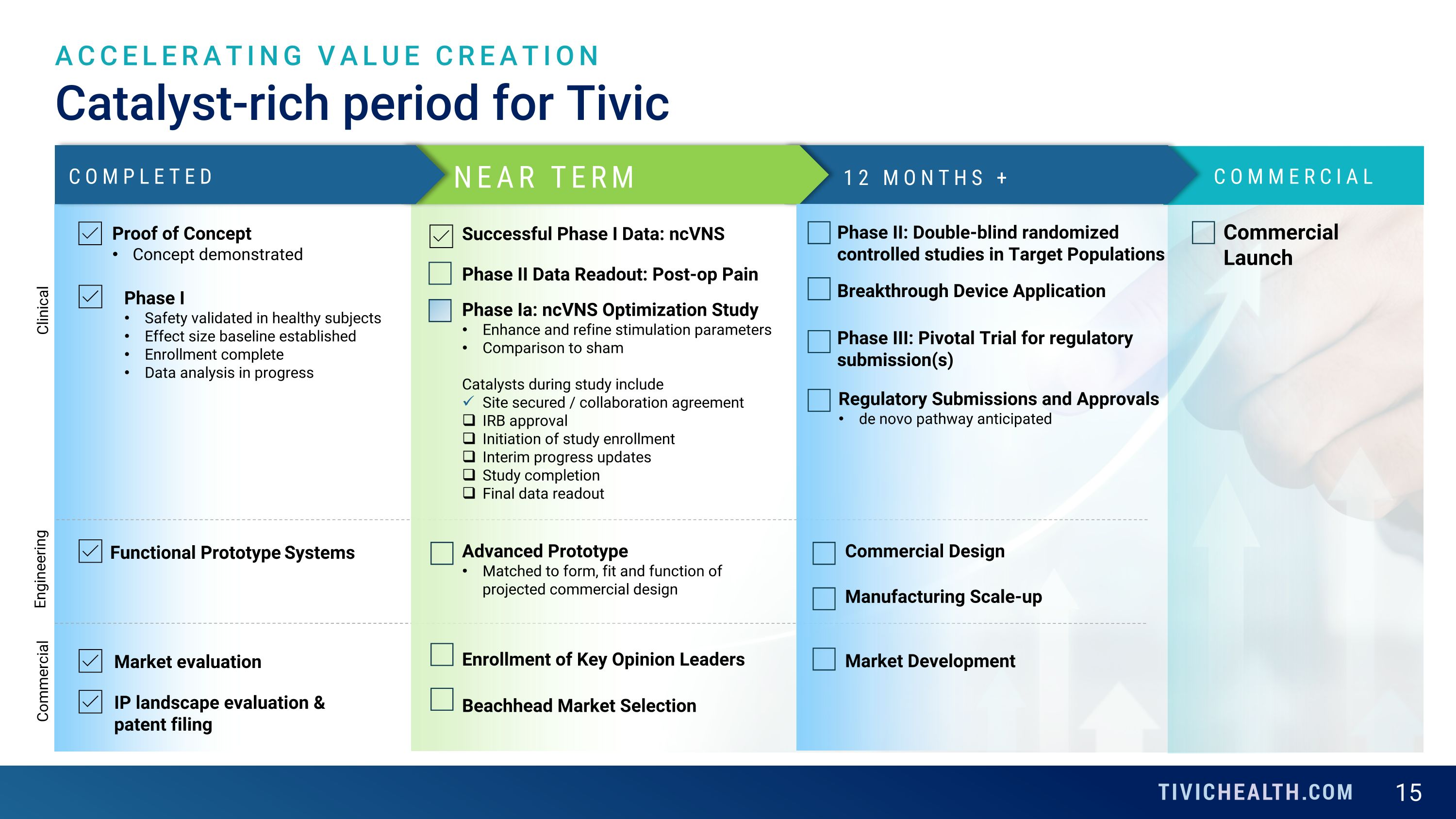
cv Catalyst-rich period for Tivic ACCELERATING VALUE CREATION NEAR TERM 12 MONTHS + COMPLETED Clinical Engineering Commercial Proof of Concept Concept demonstrated Phase I Safety validated in healthy subjects Effect size baseline established Enrollment complete Data analysis in progress Successful Phase I Data: ncVNS Phase Ia: ncVNS Optimization Study Enhance and refine stimulation parameters Comparison to sham Catalysts during study include Site secured / collaboration agreement IRB approval Initiation of study enrollment Interim progress updates Study completion Final data readout Functional Prototype Systems Advanced Prototype Matched to form, fit and function of projected commercial design Enrollment of Key Opinion Leaders Beachhead Market Selection COMMERCIAL Market evaluation Phase II: Double-blind randomized controlled studies in Target Populations Phase III: Pivotal Trial for regulatory submission(s) Commercial Design Manufacturing Scale-up Market Development Regulatory Submissions and Approvals de novo pathway anticipated Phase II Data Readout: Post-op Pain Commercial Launch Breakthrough Device Application IP landscape evaluation & patent filing

Experienced, Execution-oriented Team LEADERSHIP TEAM Jennifer Ernst Chief Executive Officer, MBA Founded company in 2016, built Tivic Health from founding to IPO in 5 years; first medical product in market in 3 years. Took prior company (Thin Film Electronics ASA) from 8-person R&D company to $480MM market cap as CEO of US subsidiary. International business development acumen. Blake Gurfein, Ph.D. Chief Scientific Officer Joined 2017. UCSF Neurosurgery Faculty. Neurotechnology thought leader with 5 previous products. Named 40 under 40 by Silicon Valley Business Journal. Journal publications cited more than 1500 times. Expertise in clinical, regulatory, commercialization, and intellectual property strategy. Kimberly Bambach Chief Financial Officer (Interim) Joined 2023. 30 years of financial leadership experience in public and private companies. Recently served as Chief Financial Officer of Jushi Holdings Inc., driving over a dozen acquisitions in 18 months. Experience in consumer, medical, ecommerce, retail and media.

. Investment Highlights TIVIC HEALTH Commercial-stage in Bioelectronic Medicine, field projected to grow at 35% CAGR 2019-20291 One of the most promising new fields of medicine. FDA-approved product in market targeting $9.1B addressable market2 First product (ClearUP) targets inflammation of sinus passages, proof source from which to build neuromodulation lines. New results on vagus nerve stimulation, which form basis for future growth Vagus nerve is a high-value target for medical applications. Tivic’s low-current, non-invasive approach has recently shown potential applications in cardiology, neurology, and psychiatry.3 Significant R&D inflection points within next 6 months Clinical read-outs on two research programs expected within next 6 months, including first published data from new area of Vagus Nerve Stimulation. Strong fundamental IP: 9 issued patents, 10 patents pending US, Europe, China, and others, issuance dates beginning in 2020. IP covers all factors that make trigeminal nerve stimulation effective, comfortable and easy to use. Recently filed VNS IP covers fundamental differentiation in stimulation circuitry and method. Experienced management team Backgrounds include growth through organic R&D, strategic partnerships, licensing, M&A and joint ventures. References provided in Appendix

Jennifer Ernst CEO, Tivic Health jennifer.ernst@tivichealth.com

APPENDIX: FOOTNOTES NON-INVASIVE NERVE STIMULATION Wilson-Pauwels, L., Akesson, E. J., Stewart, P. A. Cranial Nerves: Anatomy and Clinical Comments. B. C. Decker, 1998. Maul, Ximena A., et al. "Microcurrent technology for rapid relief of sinus pain: a randomized, placebo‐controlled, double‐blinded clinical trial." International forum of allergy & rhinology. Vol. 9. No. 4. 2019. Fischer, Laurent, et al. "Adrenergic and non-adrenergic vasoconstrictor mechanisms in the human nasal mucosa." Rhinology 31.1 (1993): 11-15. Mandel, Yossi, et al. "Vasoconstriction by electrical stimulation: new approach to control of non-compressible hemorrhage." Scientific reports 3.1 (2013): 1-7. Franco, O.S., et al. “Effects of different frequencies of transcutaneous electrical nerve stimulation on venous vascular reactivity.” Brazilian Journal of Medical and Biological Research 47.5 (2014): 411-418. Malm, L. “Stimulation of sympathetic nerve fibres to the nose in cats.” Acta otolaryngologica 75.2-6 (1973); 519-526. Fischer, Laurent, et al. “Adrenergic and non-adrenergic vasoconstrictor mechanisms in the human nasal mucosa.” Rhinology 31.1 (1993): 11-15. https://americanmigrainefoundation.org/resource-library/what-is-migraine/; Amiri P, Kazeminasab S, Nejadghaderi SA, Mohammadinasab R, Pourfathi H, Araj-Khodaei M, Sullman MJM, Kolahi AA, Safiri S. Migraine: A Review on Its History, Global Epidemiology, Risk Factors, and Comorbidities. Front Neurol. 2022 Feb 23 Bhattacharyya N. Ambulatory sinus and nasal surgery in the United States: demographics and perioperative outcomes. Laryngoscope. 2010 Mar https://tmj.org/living-with-tmj/basics/ https://my.clevelandclinic.org/health/diseases/14164-tinnitus https://www.aans.org/Patients/Neurosurgical-Conditions-and-Treatments/Trigeminal-Neuralgia https://jamanetwork.com/journals/jamapediatrics/fullarticle/2759422 Johnson, Rhaya L., and Christopher G. Wilson. "A review of vagus nerve stimulation as a therapeutic intervention." Journal of inflammation research (2018): 203-213. IBID Toffa, Dènahin Hinnoutondji, et al. "Learnings from 30 years of reported efficacy and safety of vagus nerve stimulation (VNS) for epilepsy treatment: a critical review." Seizure 83 (2020): 104-123. Badran, Bashar W., and Christopher W. Austelle. "The future is noninvasive: a brief review of the evolution and clinical utility of vagus nerve stimulation." Focus 20.1 (2022): 3-7. SIGNIFICANT WHITESPACE IN MEDICAL GRADE, NON-INVASIVE VNS THERAPEUTIC DEVICES Information on clinical stage, approvals, and target indications from individual company websites. LARGE, CLINICALLY MEANINGFUL CHANGES IN BRAIN ACTIVITY McLoughlin, Gráinne, et al. "Midfrontal theta activity in psychiatric illness: an index of cognitive vulnerabilities across disorders." Biological psychiatry 91.2 (2022): 173-182. 4. Hughes, John R. "Gamma, fast, and ultrafast waves of the brain: their relationships with epilepsy and behavior." Epilepsy & Behavior 13.1 (2008): 25-31. TIVIC BUSINESS SNAPSHOT See Investment Highlights: Footnote 2 Maul, Ximena A., et al. "Microcurrent technology for rapid relief of sinus pain: a randomized, placebo‐controlled, double‐blinded clinical trial." International forum of allergy & rhinology. Vol. 9. No. 4. 2019. Mandel, Yossi, et al. "Vasoconstriction by electrical stimulation: new approach to control of non-compressible hemorrhage." Scientific reports 3.1 (2013): 1-7. INVESTMENT HIGHLIGHTS IDTechEx Bioelectronic Medicine, 2019-2029. https://markets.businessinsider.com/news/stocks/growth-of-non-invasive-peripheral-nerve-stimulation-technologies-supported-by-positive-results-idtechex-finds-1029798202 Multiple billion-dollar segments: Data from publicly available census information (that has not been independently verified by the company) combined with company sponsored market research study of 2000+ people with recurring sinus conditions (conducted by consumer research firm, Intellego Insights). Intellego Intelligence conducted a 2000-person market segmentation study that identified the following addressable markets in the United States, based on the Company’s current pricing structure for ClearUP Individuals with severe sinus conditions representing a $0.9 billion addressable market, of which the Company has achieved less than 0.002% penetration Allergy sufferers seeking to avoid pharmaceutical side effects, representing $1.2 billion addressable market, of which the Company has achieved less than 0.002 penetration. High-performance athletes seeking improved breathing to enhance performance, representing a $2.4 billion addressable market that the Company has not yet targeted. CPAP and Sleep Apnea sufferers for whom congestion is a significant contributing factor to lack of quality sleep and/or CPAP compliance, representing a $0.6 billion addressable market. Individuals for whom sinus conditions cascade into severe headaches and migraine, representing a $4.0 billion addressable market. Claims for migraine would require separate FDA submissions beyond the Company’s currently approved indications. Unpublished data from clinical research program at The Feinstein Institutes for Medical Research at Northwell Health LEVERAGING CORE COMPETENCIES IN NEW HIGH-VALUE TARGET Johnson, Rhaya L., and Christopher G. Wilson. "A review of vagus nerve stimulation as a therapeutic intervention." Journal of inflammation research (2018): 203-213. Toffa, Dènahin Hinnoutondji, et al. "Learnings from 30 years of reported efficacy and safety of vagus nerve stimulation (VNS) for epilepsy treatment: a critical review." Seizure 83 (2020): 104-123. Badran, Bashar W., and Christopher W. Austelle. "The future is noninvasive: a brief review of the evolution and clinical utility of vagus nerve stimulation." Focus 20.1 (2022): 3-7. LARGE, CLINICALLY MEANINGFUL INCREASE IN RMSSD Laborde, Sylvain, Emma Mosley, and Julian F. Thayer. "Heart rate variability and cardiac vagal tone in psychophysiological research–recommendations for experiment planning, data analysis, and data reporting." Frontiers in psychology 8 (2017): 238557. Kharbanda, Rohit K., et al. "Vagus nerve stimulation and atrial fibrillation: revealing the paradox." Neuromodulation: Technology at the Neural Interface 25.3 (2022): 356-365. Jarczok, Marc N., et al. "Heart rate variability in the prediction of mortality: A systematic review and meta-analysis of healthy and patient populations." Neuroscience & Biobehavioral Reviews 143 (2022): 104907. Toffa, Dènahin Hinnoutondji, et al. "Learnings from 30 years of reported efficacy and safety of vagus nerve stimulation (VNS) for epilepsy treatment: a critical review." Seizure 83 (2020): 104-123. Badran, Bashar W., and Christopher W. Austelle. "The future is noninvasive: a brief review of the evolution and clinical utility of vagus nerve stimulation." Focus 20.1 (2022): 3-7. WHY THIS MATTERS Toffa, Dènahin Hinnoutondji, et al. "Learnings from 30 years of reported efficacy and safety of vagus nerve stimulation (VNS) for epilepsy treatment: a critical review." Seizure 83 (2020): 104-123. Badran, Bashar W., and Christopher W. Austelle. "The future is noninvasive: a brief review of the evolution and clinical utility of vagus nerve stimulation." Focus 20.1 (2022): 3-7. SUSTAINED PUPIL CONSTRICTION DURING STIMULATION McDougal, David H., and Paul D. Gamlin. "Autonomic control of the eye." Comprehensive physiology 5.1 (2015): 439. THE BIG IDEA: BIOELECTRONIC MEDICINE https://www.mckinsey.com/industries/life-sciences/our-insights/bioelectronics-jump-start-the-next-wave-of-device-therapeutics