

On a Journey to Erase Cancer Erasca Investor Presentation June 5, 2023 Exhibit 99.1

We caution you that this presentation contains forward-looking statements. All statements other than statements of historical facts contained in this presentation, including statements regarding our future results of operations and financial position, business strategy, research and development plans, the anticipated timing (including the timing of initiation and the timing of data readouts), costs, design and conduct of our ongoing and planned preclinical studies and clinical trials for our product candidates, the potential benefits from our current or future arrangements with third parties, the timing and likelihood of success of our plans and objectives, the planned deprioritization of certain programs, and future results of anticipated product development efforts, are forward-looking statements. In some cases, you can identify forward-looking statements by terms such as “may,” “will,” “should,” “expect,” “plan,” “anticipate,” “could,” “intend,” “target,” “project,” “contemplates,” “believes,” “estimates,” “predicts,” “potential” or “continue” or the negative of these terms or other similar expressions. The inclusion of forward-looking statements should not be regarded as a representation by us that any of our plans will be achieved. Actual results may differ from those set forth in this presentation due to the risks and uncertainties inherent in our business, including, without limitation: our approach to the discovery and development of product candidates based on our singular focus on shutting down the RAS/MAPK pathway, a novel and unproven approach; we are early in our development efforts and have only four product candidates in clinical development and all of our other development efforts are in the preclinical or development stage; the retrospective analysis of pooled ERAS-007 data covers multiple clinical trials with different designs, inclusion criteria, and dosing regimens, which cannot be directly compared, and therefore may not be a reliable indicator of efficacy and safety data; we have not conducted any clinical trials of naporafenib and are reliant on data generated by Novartis in prior clinical trials conducted by it; our planned SEACRAFT trials may not support the registration of naporafenib; preliminary results of clinical trials are not necessarily indicative of final results and one or more of the clinical outcomes may materially change as patient enrollment continues, following more comprehensive reviews of the data and more patient data become available, including the risk that an uPR to treatment may not ultimately result in a cPR to treatment after follow-up evaluations; potential delays in the commencement, enrollment, and completion of clinical trials and preclinical studies; our dependence on third parties in connection with manufacturing, research, and preclinical and clinical testing; unexpected adverse side effects or inadequate efficacy of our product candidates that may limit their development, regulatory approval, and/or commercialization, or may result in recalls or product liability claims; unfavorable results from preclinical studies or clinical trials; results from preclinical studies or early clinical trials not necessarily being predictive of future results; the inability to realize any benefits from our current licenses, acquisitions, or collaborations, and any future licenses, acquisitions, or collaborations, and our ability to fulfill our obligations under such arrangements; our assumptions around which programs may have a higher probability of success may not be accurate, and we may expend our limited resources to pursue a particular product candidate and/or indication and fail to capitalize on product candidates or indications with greater development or commercial potential; regulatory developments in the United States and foreign countries; and other risks described in our prior filings with the Securities and Exchange Commission (SEC), including under the heading “Risk Factors” in our annual report on Form 10-K for the year ended December 31, 2022, and any subsequent filings with the SEC. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date hereof, and we undertake no obligation to update such statements to reflect events that occur or circumstances that exist after the date hereof. All forward-looking statements are qualified in their entirety by this cautionary statement, which is made under the safe harbor provisions of the Private Securities Litigation Reform Act of 1995. This presentation also contains estimates and other statistical data made by independent parties and by us relating to market size and growth and other data about our industry. This data involves a number of assumptions, and you are cautioned not to give undue weight to such estimates. In addition, projections, assumptions, and estimates of our future performance and the future performance of the markets in which we operate are necessarily subject to a high degree of uncertainty and risk. These and other factors could cause results to differ materially from those expressed in the estimates made by the independent parties and by us. Disclaimer: Forward Looking Statements & Market Data
Erasca Investor Presentation Agenda Portfolio prioritization Portfolio Update Q&A Session Development Update ERAS-007 monotherapy results HERKULES-3: ERAS-007 master protocol in GI cancers HERKULES-2: ERAS-007 master protocol in NSCLC
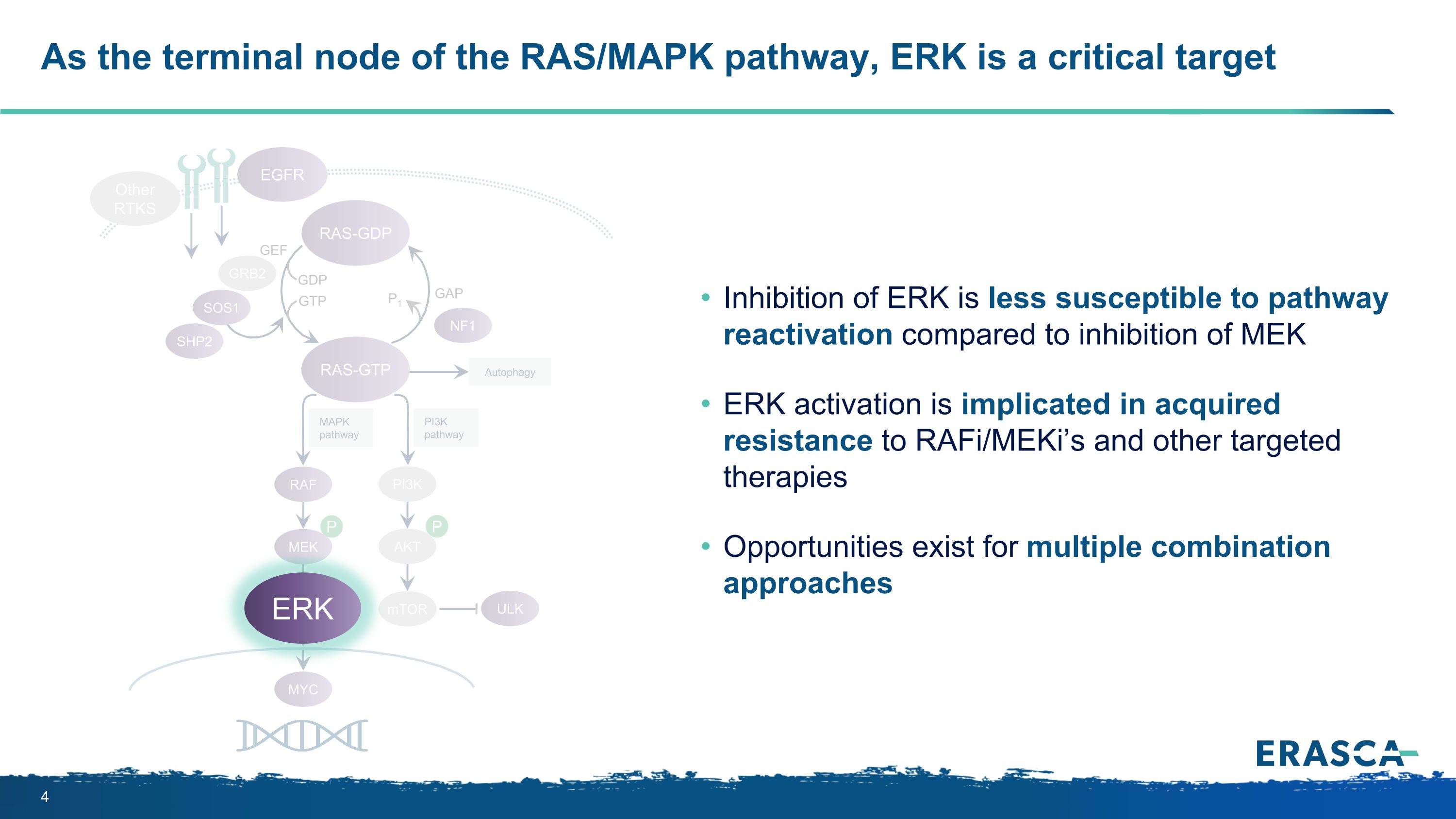
P P P Autophagy MAPK pathway ULK PI3K pathway RAS-GTP GRB2 SHP2 GAP GDP GTP P1 NF1 SOS1 RAF MEK PI3K AKT mTOR RAS-GDP EGFR Other RTKS MYC GEF As the terminal node of the RAS/MAPK pathway, ERK is a critical target ERK ERK Inhibition of ERK is less susceptible to pathway reactivation compared to inhibition of MEK ERK activation is implicated in acquired resistance to RAFi/MEKi’s and other targeted therapies Opportunities exist for multiple combination approaches
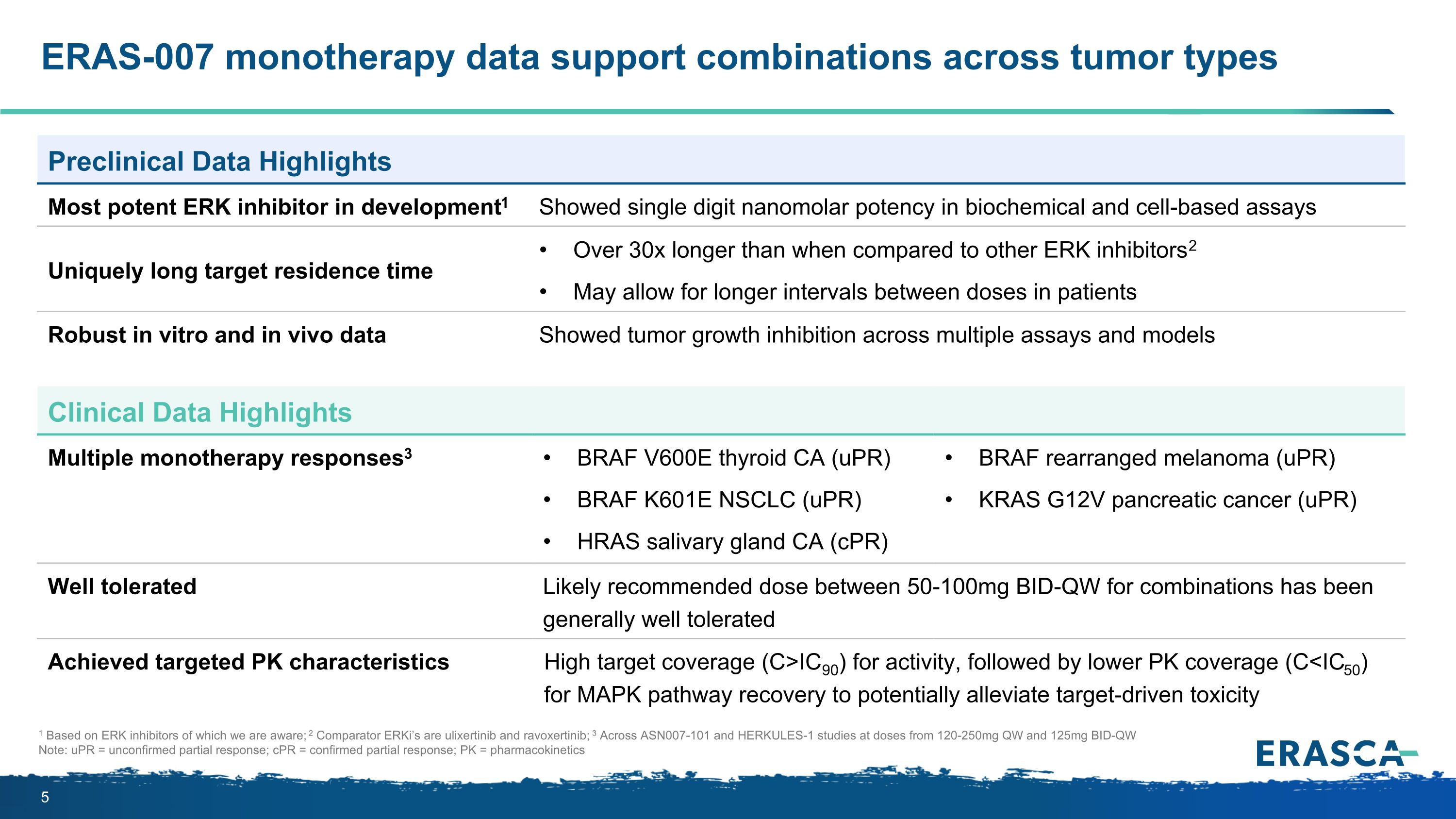
1 Based on ERK inhibitors of which we are aware; 2 Comparator ERKi’s are ulixertinib and ravoxertinib; 3 Across ASN007-101 and HERKULES-1 studies at doses from 120-250mg QW and 125mg BID-QW Note: uPR = unconfirmed partial response; cPR = confirmed partial response; PK = pharmacokinetics ERAS-007 monotherapy data support combinations across tumor types Preclinical Data Highlights Most potent ERK inhibitor in development1 Showed single digit nanomolar potency in biochemical and cell-based assays Uniquely long target residence time Over 30x longer than when compared to other ERK inhibitors2 May allow for longer intervals between doses in patients Robust in vitro and in vivo data Showed tumor growth inhibition across multiple assays and models Clinical Data Highlights Multiple monotherapy responses3 BRAF V600E thyroid CA (uPR) BRAF K601E NSCLC (uPR) HRAS salivary gland CA (cPR) BRAF rearranged melanoma (uPR) KRAS G12V pancreatic cancer (uPR) Well tolerated Likely recommended dose between 50-100mg BID-QW for combinations has been generally well tolerated Achieved targeted PK characteristics High target coverage (C>IC90) for activity, followed by lower PK coverage (C<IC50) for MAPK pathway recovery to potentially alleviate target-driven toxicity
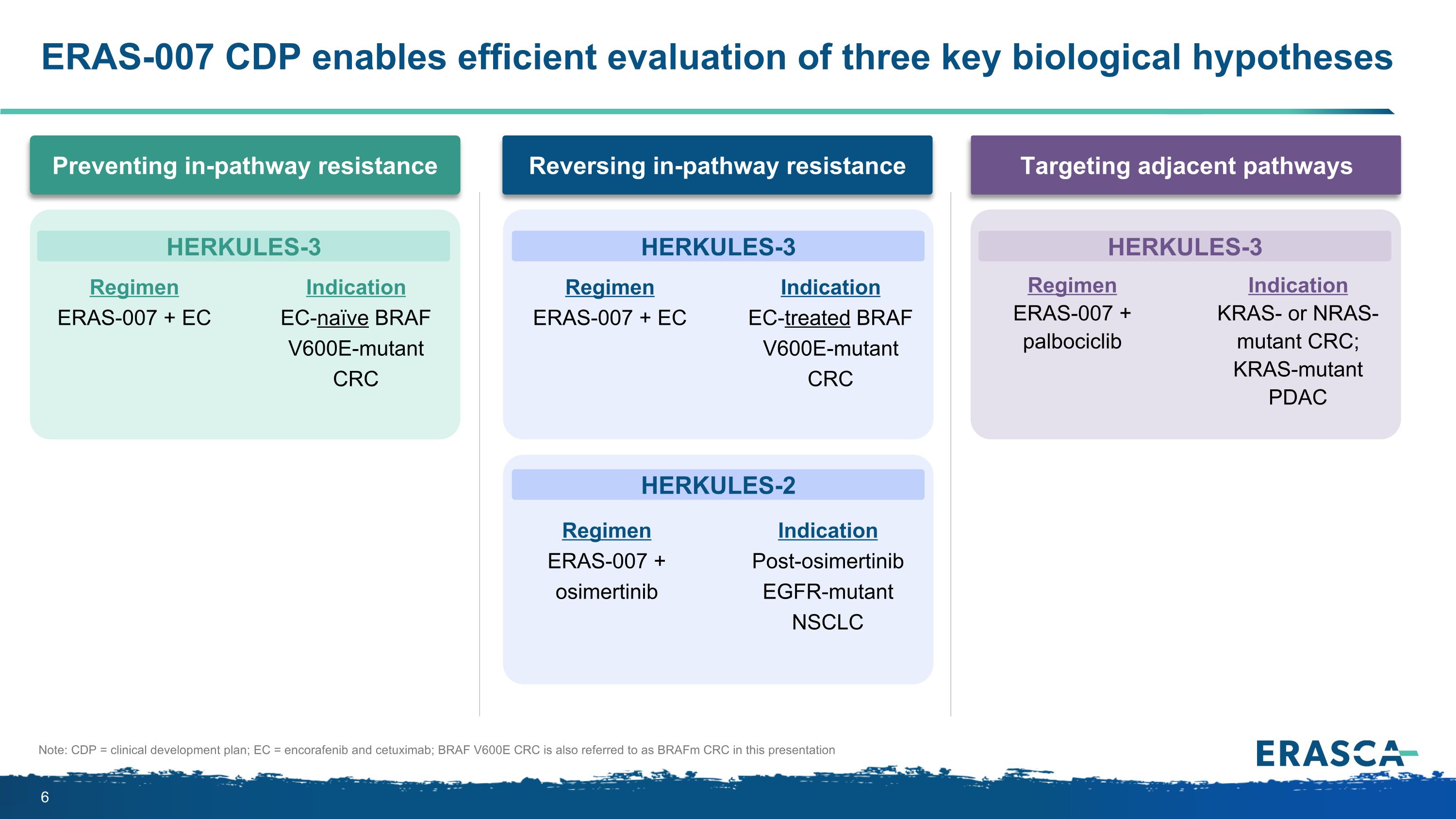
ERAS-007 CDP enables efficient evaluation of three key biological hypotheses Targeting adjacent pathways Preventing in-pathway resistance Reversing in-pathway resistance Regimen ERAS-007 + EC Indication EC-naïve BRAF V600E-mutant CRC Regimen ERAS-007 + EC Indication EC-treated BRAF V600E-mutant CRC Regimen ERAS-007 + osimertinib Indication Post-osimertinib EGFR-mutant NSCLC Regimen ERAS-007 + palbociclib Indication KRAS- or NRAS-mutant CRC; KRAS-mutant PDAC HERKULES-3 HERKULES-3 HERKULES-2 HERKULES-3 Note: CDP = clinical development plan; EC = encorafenib and cetuximab; BRAF V600E CRC is also referred to as BRAFm CRC in this presentation
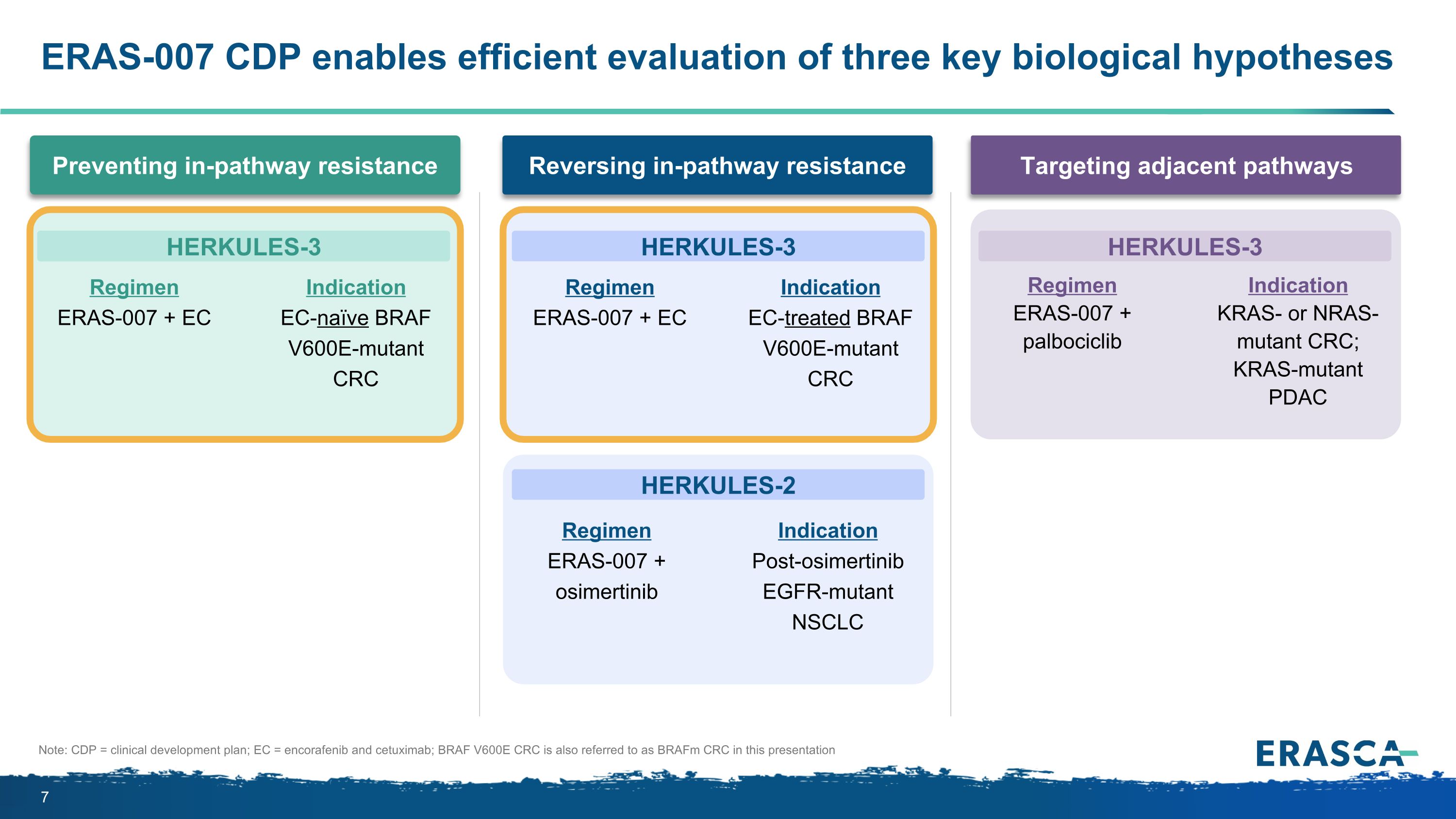
ERAS-007 CDP enables efficient evaluation of three key biological hypotheses Targeting adjacent pathways Preventing in-pathway resistance Reversing in-pathway resistance Regimen ERAS-007 + EC Indication EC-naïve BRAF V600E-mutant CRC Regimen ERAS-007 + EC Indication EC-treated BRAF V600E-mutant CRC Regimen ERAS-007 + osimertinib Indication Post-osimertinib EGFR-mutant NSCLC Regimen ERAS-007 + palbociclib Indication KRAS- or NRAS-mutant CRC; KRAS-mutant PDAC HERKULES-3 HERKULES-3 HERKULES-2 HERKULES-3 Note: CDP = clinical development plan; EC = encorafenib and cetuximab; BRAF V600E CRC is also referred to as BRAFm CRC in this presentation
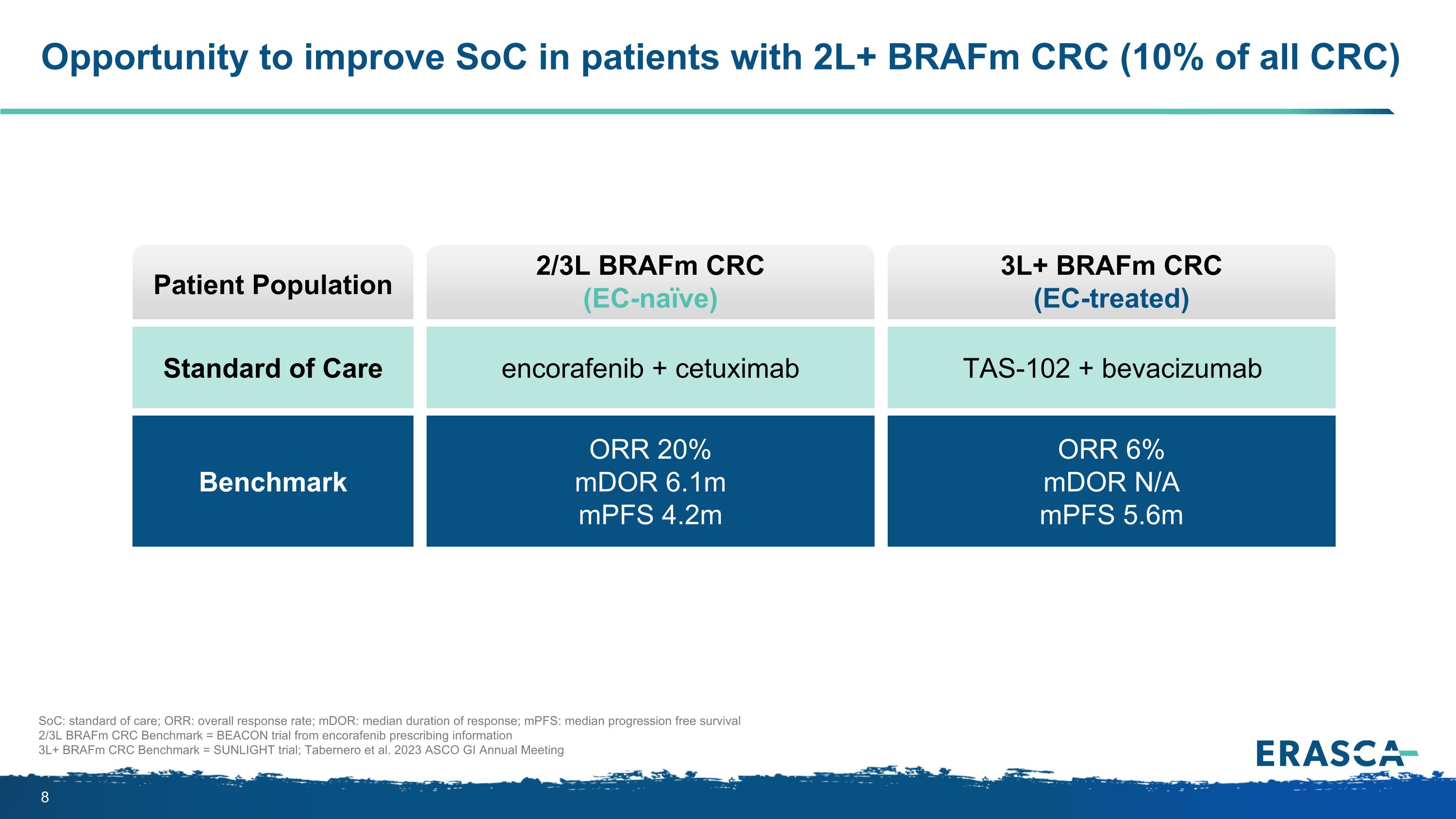
Opportunity to improve SoC in patients with 2L+ BRAFm CRC (10% of all CRC) Patient Population Benchmark Standard of Care 2/3L BRAFm CRC (EC-naïve) ORR 20% mDOR 6.1m mPFS 4.2m encorafenib + cetuximab 3L+ BRAFm CRC (EC-treated) ORR 6% mDOR N/A mPFS 5.6m TAS-102 + bevacizumab SoC: standard of care; ORR: overall response rate; mDOR: median duration of response; mPFS: median progression free survival 2/3L BRAFm CRC Benchmark = BEACON trial from encorafenib prescribing information 3L+ BRAFm CRC Benchmark = SUNLIGHT trial; Tabernero et al. 2023 ASCO GI Annual Meeting
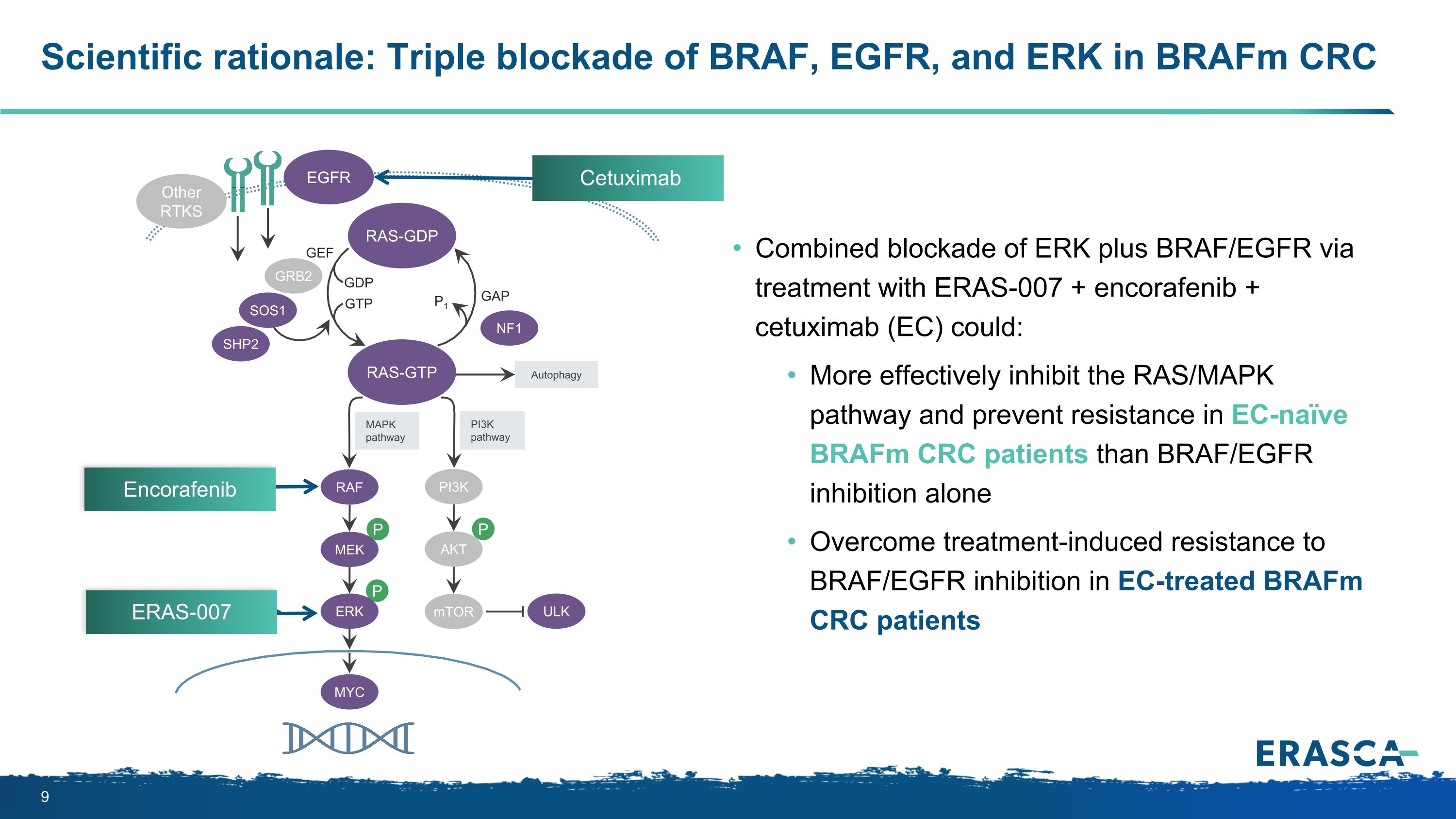
P P P Autophagy MAPK pathway ULK PI3K pathway RAS-GTP GRB2 GAP GDP GTP P1 NF1 SOS1 RAF MEK PI3K AKT mTOR RAS-GDP EGFR Other RTKS MYC GEF ERK Scientific rationale: Triple blockade of BRAF, EGFR, and ERK in BRAFm CRC SHP2 Combined blockade of ERK plus BRAF/EGFR via treatment with ERAS-007 + encorafenib + cetuximab (EC) could: More effectively inhibit the RAS/MAPK pathway and prevent resistance in EC-naïve BRAFm CRC patients than BRAF/EGFR inhibition alone Overcome treatment-induced resistance to BRAF/EGFR inhibition in EC-treated BRAFm CRC patients Cetuximab Encorafenib ERAS-007
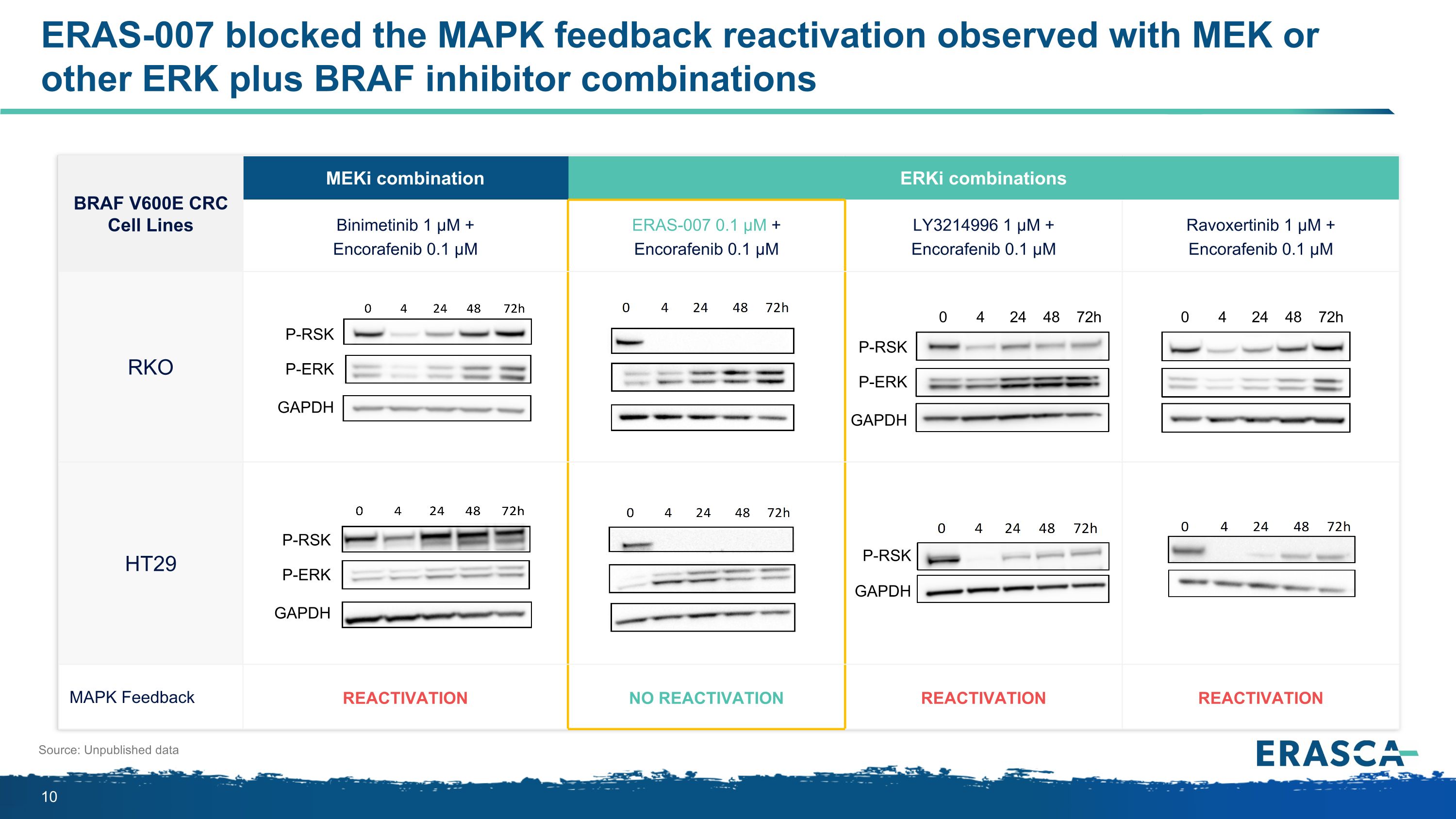
BRAF V600E CRC Cell Lines MEKi combination ERKi combinations Binimetinib 1 µM + Encorafenib 0.1 µM ERAS-007 0.1 µM + Encorafenib 0.1 µM LY3214996 1 µM + Encorafenib 0.1 µM Ravoxertinib 1 µM + Encorafenib 0.1 µM RKO HT29 MAPK Feedback Reactivation No Reactivation Reactivation Reactivation Source: Unpublished data P-RSK P-ERK GAPDH P-RSK P-ERK GAPDH P-RSK GAPDH P-RSK P-ERK GAPDH 0 4 24 48 72h 0 4 24 48 72h ERAS-007 blocked the MAPK feedback reactivation observed with MEK or other ERK plus BRAF inhibitor combinations
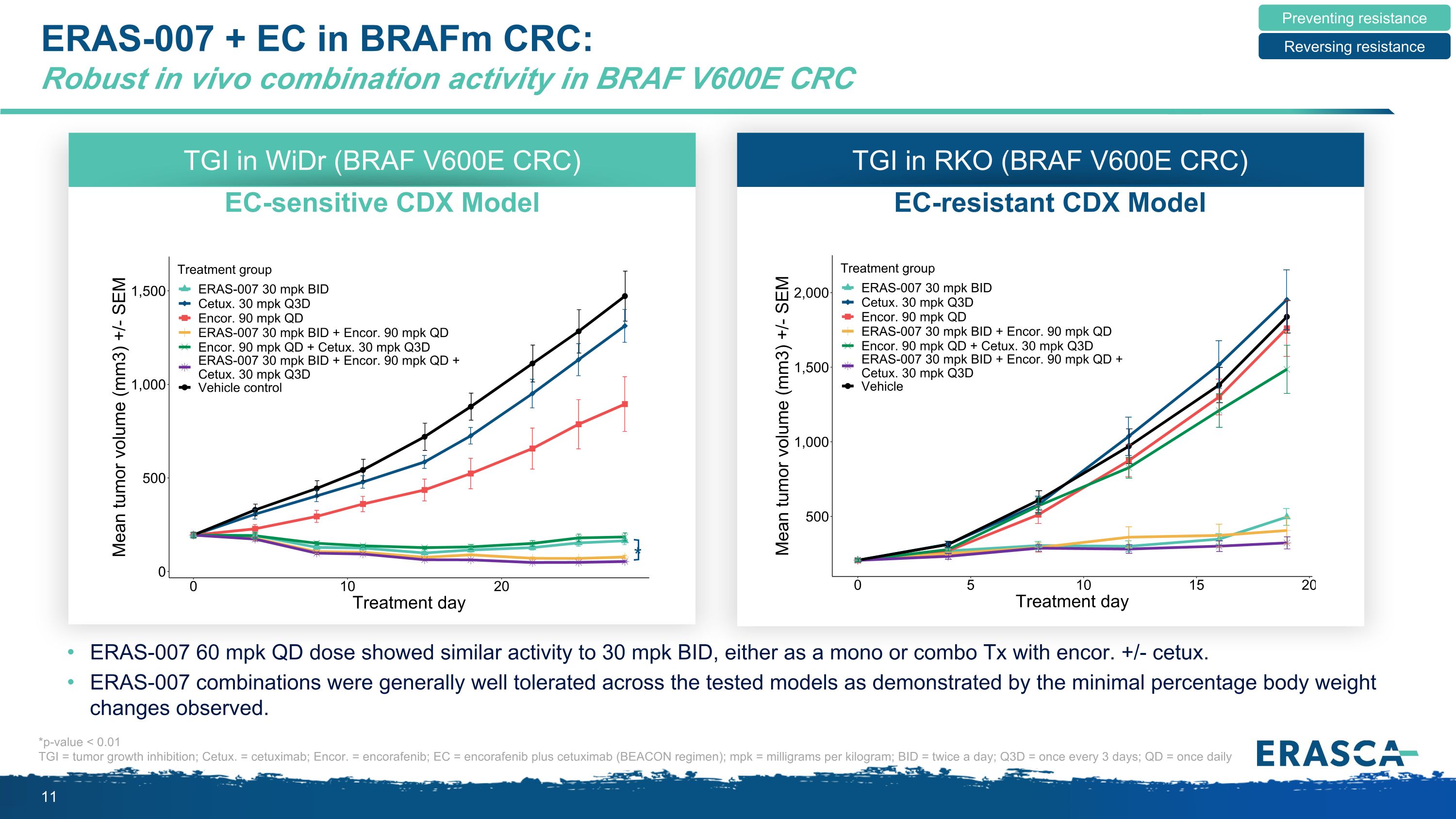
ERAS-007 + EC in BRAFm CRC: Robust in vivo combination activity in BRAF V600E CRC ERAS-007 60 mpk QD dose showed similar activity to 30 mpk BID, either as a mono or combo Tx with encor. +/- cetux. ERAS-007 combinations were generally well tolerated across the tested models as demonstrated by the minimal percentage body weight changes observed. TGI in WiDr (BRAF V600E CRC) EC-sensitive CDX Model * *p-value < 0.01 TGI = tumor growth inhibition; Cetux. = cetuximab; Encor. = encorafenib; EC = encorafenib plus cetuximab (BEACON regimen); mpk = milligrams per kilogram; BID = twice a day; Q3D = once every 3 days; QD = once daily Preventing resistance Reversing resistance TGI in RKO (BRAF V600E CRC) EC-resistant CDX Model
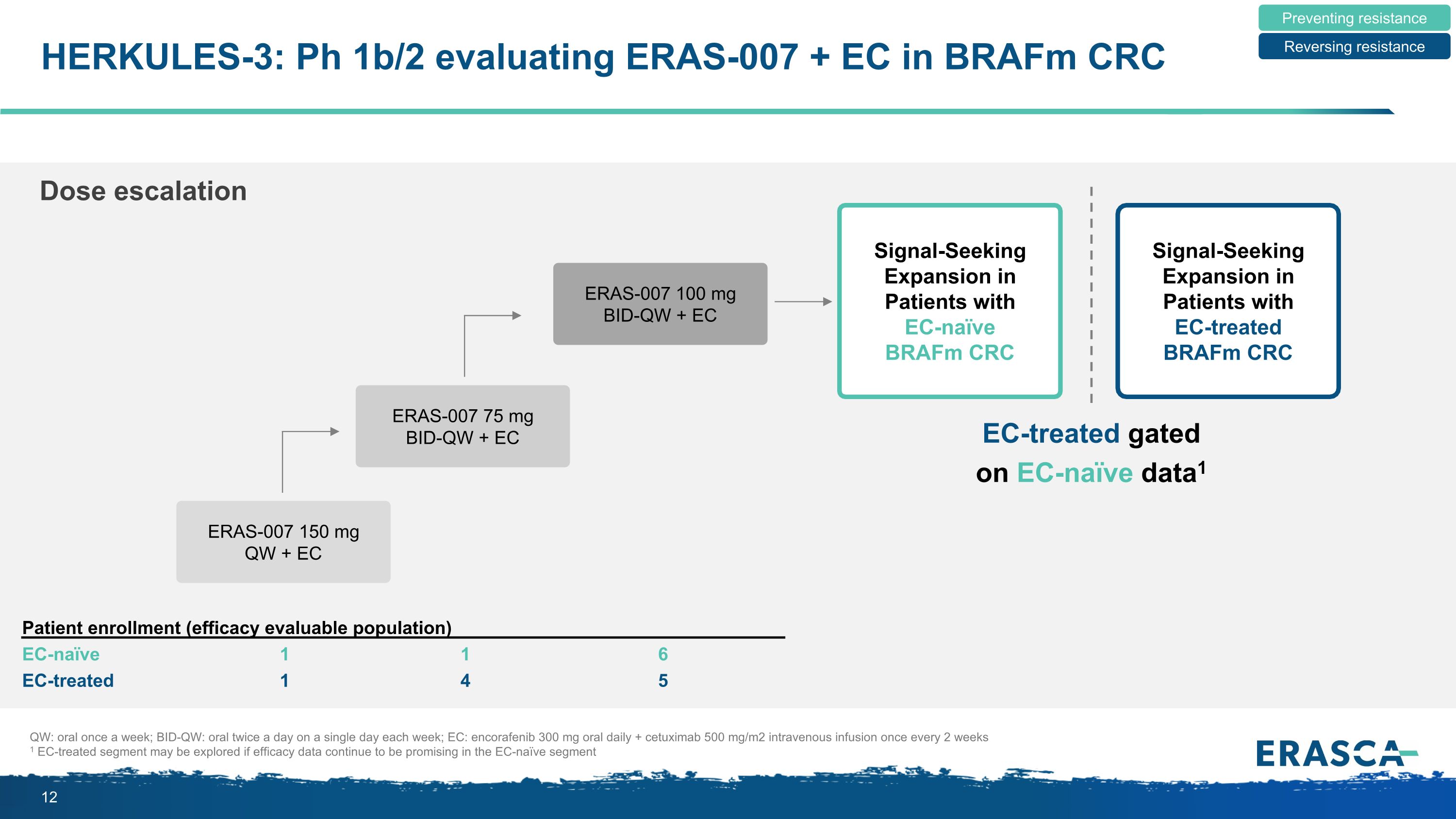
HERKULES-3: Ph 1b/2 evaluating ERAS-007 + EC in BRAFm CRC Preventing resistance Reversing resistance Signal-Seeking Expansion in Patients with EC-naïve BRAFm CRC Signal-Seeking Expansion in Patients with EC-treated BRAFm CRC ERAS-007 75 mg BID-QW + EC ERAS-007 100 mg BID-QW + EC ERAS-007 150 mg QW + EC Dose escalation QW: oral once a week; BID-QW: oral twice a day on a single day each week; EC: encorafenib 300 mg oral daily + cetuximab 500 mg/m2 intravenous infusion once every 2 weeks 1 EC-treated segment may be explored if efficacy data continue to be promising in the EC-naïve segment Patient enrollment (efficacy evaluable population) EC-naïve EC-treated 1 1 1 4 6 5 EC-treated gated on EC-naïve data1
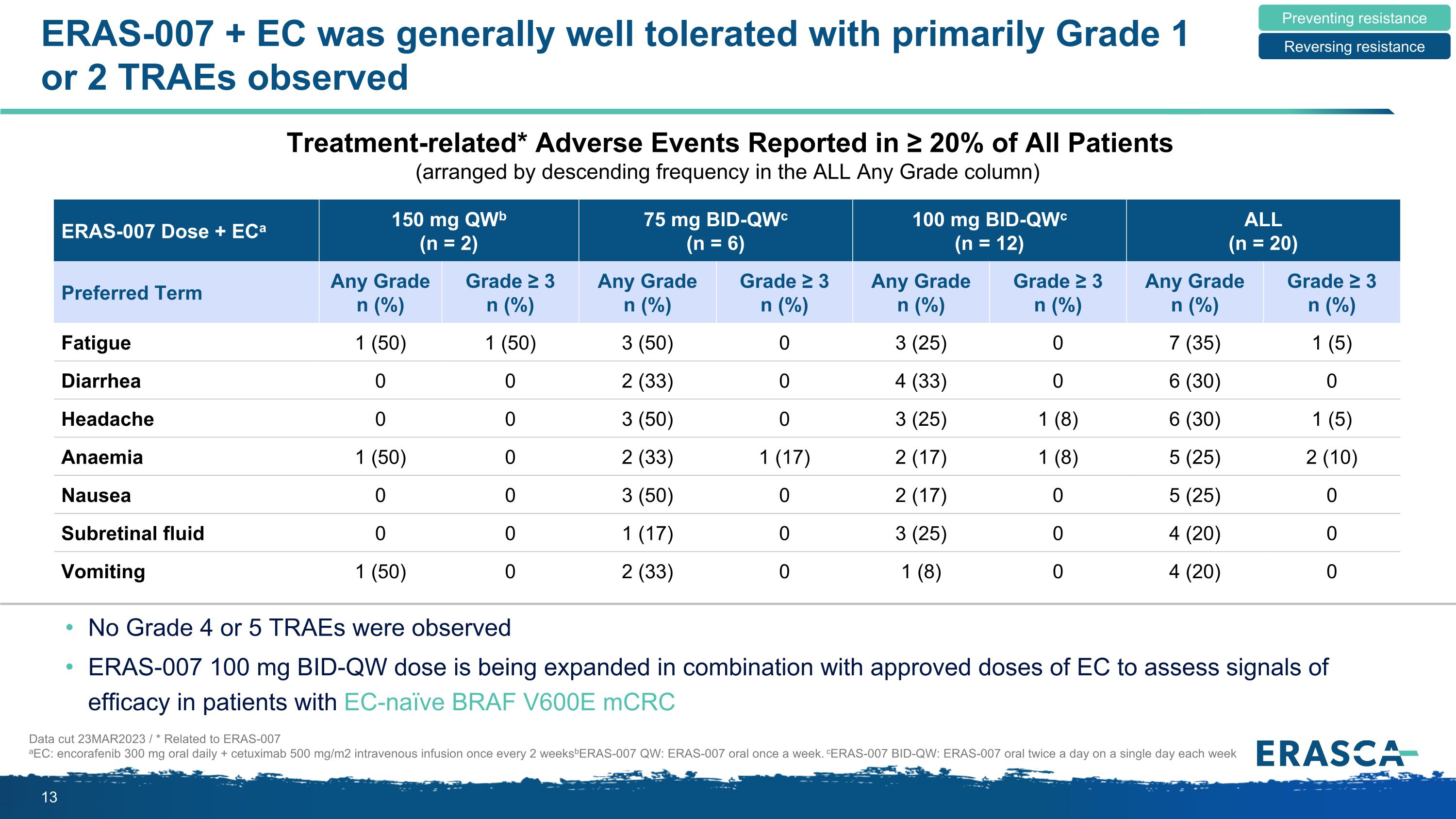
ERAS-007 + EC was generally well tolerated with primarily Grade 1 or 2 TRAEs observed Data cut 23MAR2023 / * Related to ERAS-007 aEC: encorafenib 300 mg oral daily + cetuximab 500 mg/m2 intravenous infusion once every 2 weeks bERAS-007 QW: ERAS-007 oral once a week. cERAS-007 BID-QW: ERAS-007 oral twice a day on a single day each week Preventing resistance Reversing resistance No Grade 4 or 5 TRAEs were observed ERAS-007 100 mg BID-QW dose is being expanded in combination with approved doses of EC to assess signals of efficacy in patients with EC-naïve BRAF V600E mCRC ERAS-007 Dose + ECa 150 mg QWb (n = 2) 75 mg BID-QWc (n = 6) 100 mg BID-QWc (n = 12) ALL (n = 20) Preferred Term Any Grade n (%) Grade ≥ 3 n (%) Any Grade n (%) Grade ≥ 3 n (%) Any Grade n (%) Grade ≥ 3 n (%) Any Grade n (%) Grade ≥ 3 n (%) Fatigue 1 (50) 1 (50) 3 (50) 0 3 (25) 0 7 (35) 1 (5) Diarrhea 0 0 2 (33) 0 4 (33) 0 6 (30) 0 Headache 0 0 3 (50) 0 3 (25) 1 (8) 6 (30) 1 (5) Anaemia 1 (50) 0 2 (33) 1 (17) 2 (17) 1 (8) 5 (25) 2 (10) Nausea 0 0 3 (50) 0 2 (17) 0 5 (25) 0 Subretinal fluid 0 0 1 (17) 0 3 (25) 0 4 (20) 0 Vomiting 1 (50) 0 2 (33) 0 1 (8) 0 4 (20) 0 Treatment-related* Adverse Events Reported in ≥ 20% of All Patients (arranged by descending frequency in the ALL Any Grade column)
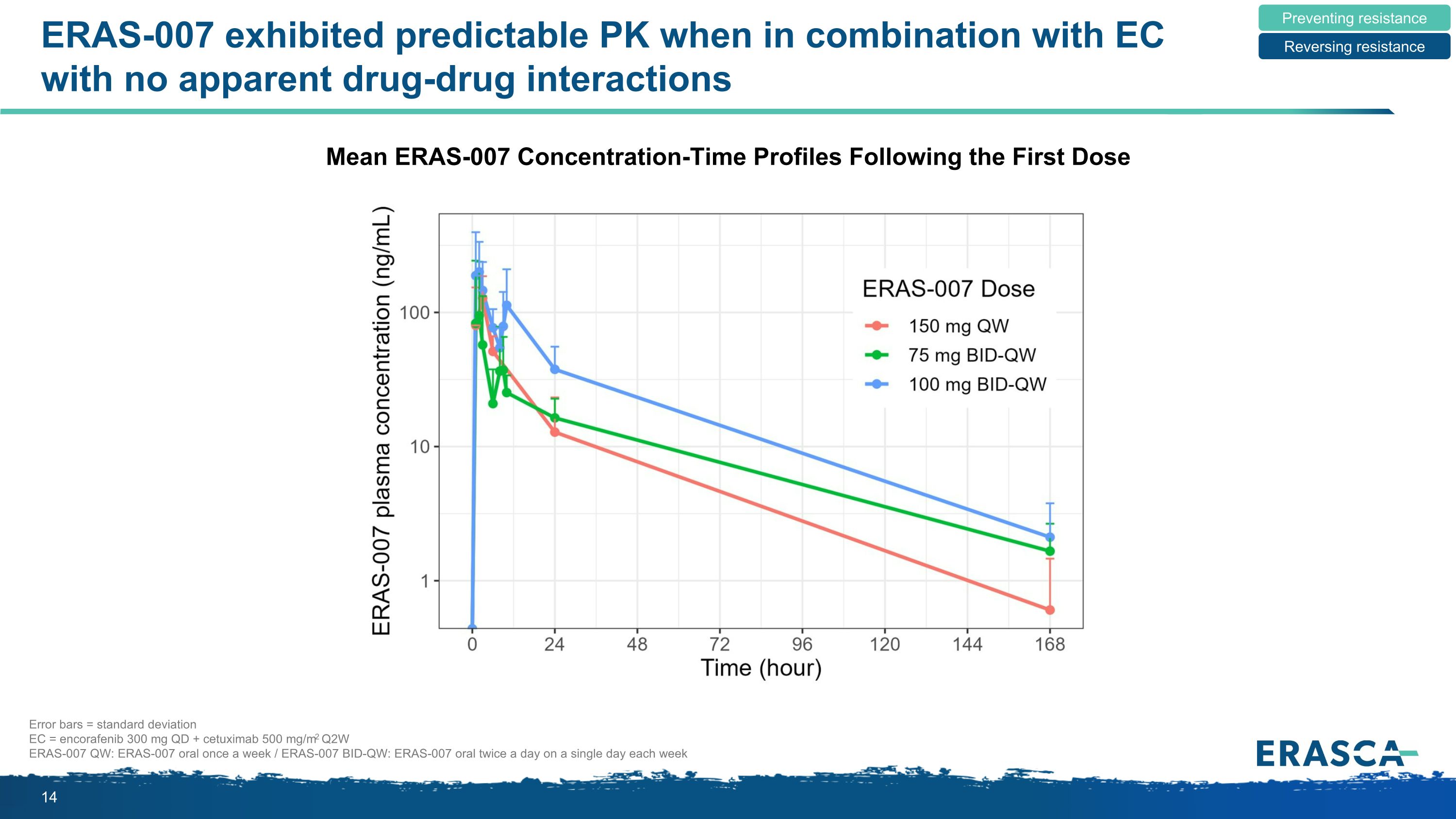
ERAS-007 exhibited predictable PK when in combination with EC with no apparent drug-drug interactions Mean ERAS-007 Concentration-Time Profiles Following the First Dose Preventing resistance Reversing resistance Error bars = standard deviation EC = encorafenib 300 mg QD + cetuximab 500 mg/m2 Q2W ERAS-007 QW: ERAS-007 oral once a week / ERAS-007 BID-QW: ERAS-007 oral twice a day on a single day each week
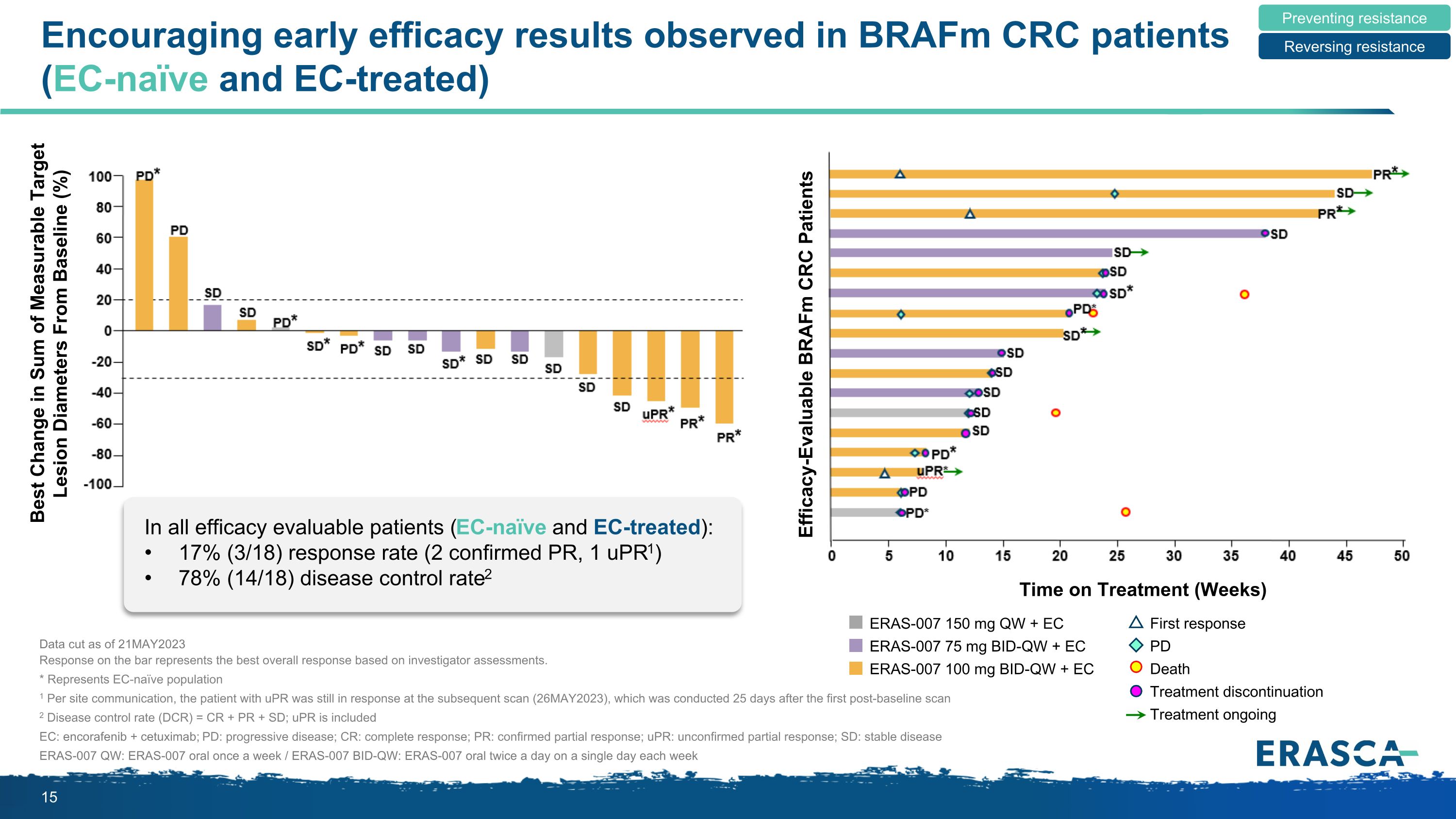
Encouraging early efficacy results observed in BRAFm CRC patients (EC-naïve and EC-treated) Data cut as of 21MAY2023 Response on the bar represents the best overall response based on investigator assessments. * Represents EC-naïve population 1 Per site communication, the patient with uPR was still in response at the subsequent scan (26MAY2023), which was conducted 25 days after the first post-baseline scan 2 Disease control rate (DCR) = CR + PR + SD; uPR is included EC: encorafenib + cetuximab; PD: progressive disease; CR: complete response; PR: confirmed partial response; uPR: unconfirmed partial response; SD: stable disease ERAS-007 QW: ERAS-007 oral once a week / ERAS-007 BID-QW: ERAS-007 oral twice a day on a single day each week Preventing resistance Reversing resistance Best Change in Sum of Measurable Target Lesion Diameters From Baseline (%) In all efficacy evaluable patients (EC-naïve and EC-treated): 17% (3/18) response rate (2 confirmed PR, 1 uPR1) 78% (14/18) disease control rate2 ERAS-007 150 mg QW + EC ERAS-007 75 mg BID-QW + EC ERAS-007 100 mg BID-QW + EC First response PD Death Treatment discontinuation Treatment ongoing Efficacy-Evaluable BRAFm CRC Patients Time on Treatment (Weeks)
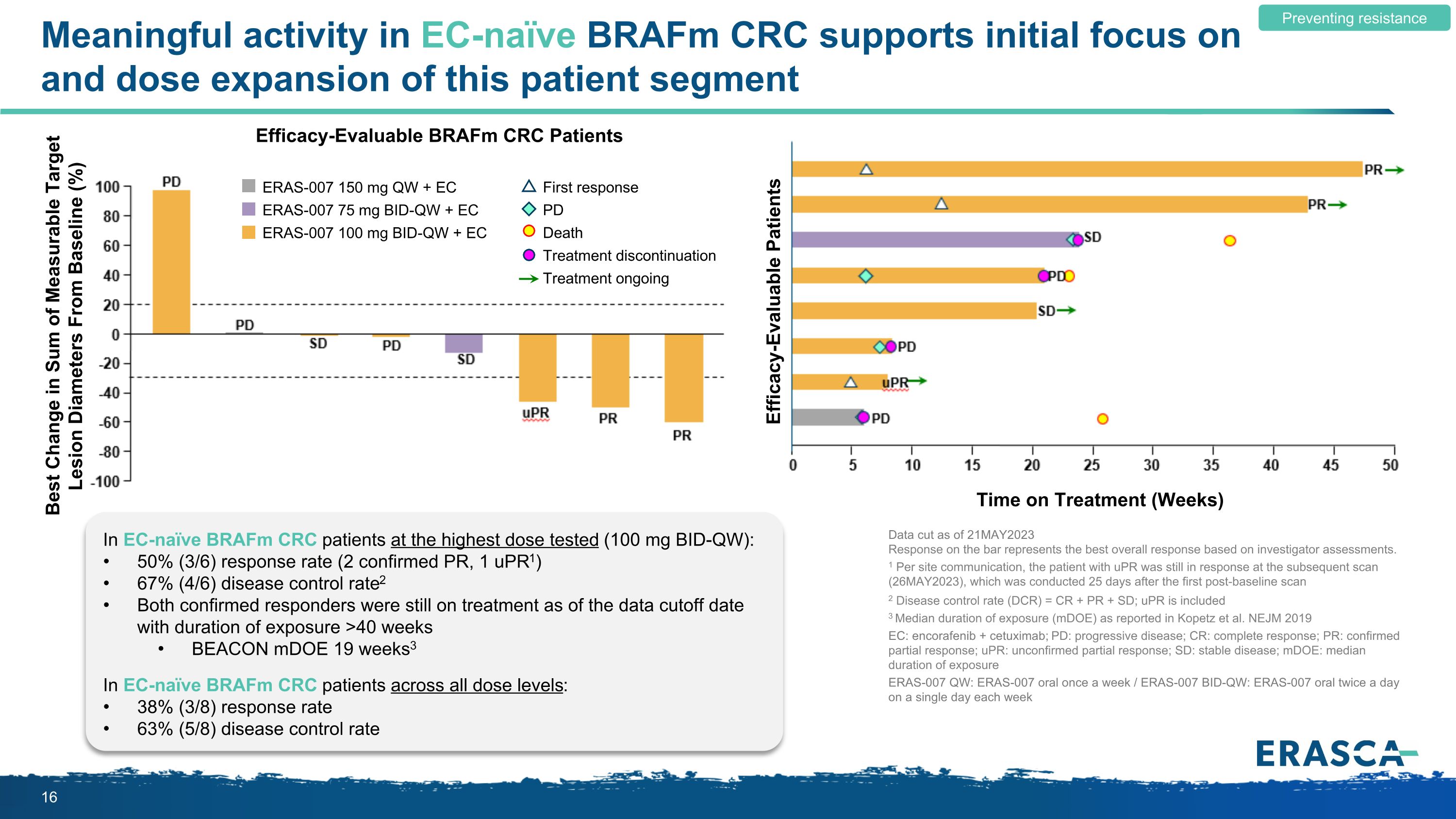
Meaningful activity in EC-naïve BRAFm CRC supports initial focus on and dose expansion of this patient segment Preventing resistance Efficacy-Evaluable BRAFm CRC Patients Time on Treatment (Weeks) ERAS-007 150 mg QW + EC ERAS-007 75 mg BID-QW + EC ERAS-007 100 mg BID-QW + EC First response PD Death Treatment discontinuation Treatment ongoing Best Change in Sum of Measurable Target Lesion Diameters From Baseline (%) Efficacy-Evaluable Patients In EC-naïve BRAFm CRC patients at the highest dose tested (100 mg BID-QW): 50% (3/6) response rate (2 confirmed PR, 1 uPR1) 67% (4/6) disease control rate2 Both confirmed responders were still on treatment as of the data cutoff date with duration of exposure >40 weeks BEACON mDOE 19 weeks3 In EC-naïve BRAFm CRC patients across all dose levels: 38% (3/8) response rate 63% (5/8) disease control rate Data cut as of 21MAY2023 Response on the bar represents the best overall response based on investigator assessments. 1 Per site communication, the patient with uPR was still in response at the subsequent scan (26MAY2023), which was conducted 25 days after the first post-baseline scan 2 Disease control rate (DCR) = CR + PR + SD; uPR is included 3 Median duration of exposure (mDOE) as reported in Kopetz et al. NEJM 2019 EC: encorafenib + cetuximab; PD: progressive disease; CR: complete response; PR: confirmed partial response; uPR: unconfirmed partial response; SD: stable disease; mDOE: median duration of exposure ERAS-007 QW: ERAS-007 oral once a week / ERAS-007 BID-QW: ERAS-007 oral twice a day on a single day each week

ERAS-007 CDP enables efficient evaluation of three key biological hypotheses Targeting adjacent pathways Preventing in-pathway resistance Reversing in-pathway resistance Regimen ERAS-007 + EC Indication EC-naïve BRAF V600E-mutant CRC Regimen ERAS-007 + EC Indication EC-treated BRAF V600E-mutant CRC Regimen ERAS-007 + osimertinib Indication Post-osimertinib EGFR-mutant NSCLC Regimen ERAS-007 + palbociclib Indication KRAS- or NRAS-mutant CRC; KRAS-mutant PDAC HERKULES-3 HERKULES-3 HERKULES-2 HERKULES-3 Note: CDP = clinical development plan; EC = encorafenib and cetuximab; BRAF V600E CRC is also referred to as BRAFm CRC in this presentation
HERKULES-2: ERAS-007 + osimertinib had acceptable and manageable AEs with no apparent DDI observed in patients with EGFRm NSCLC ERAS-007 150 mg QW + osimertinib 80mg QD identified as the MTD/RD Combination had acceptable and manageable AEs AEs similar to monotherapy ERAS-007 Most frequent (≥ 20%) TRAEs were diarrhea (50%), nausea (40%), rash (35%), vision blurred (35%) and vomiting (25%)1 No apparent drug-drug interactions (DDI) were observed ERAS-007 PK and osimertinib PK results were comparable to historical PK results observed in monotherapy trials 1 Data cut-off 23SEP22 MTD: maximum tolerated dose; RD: recommended dose Reversing resistance
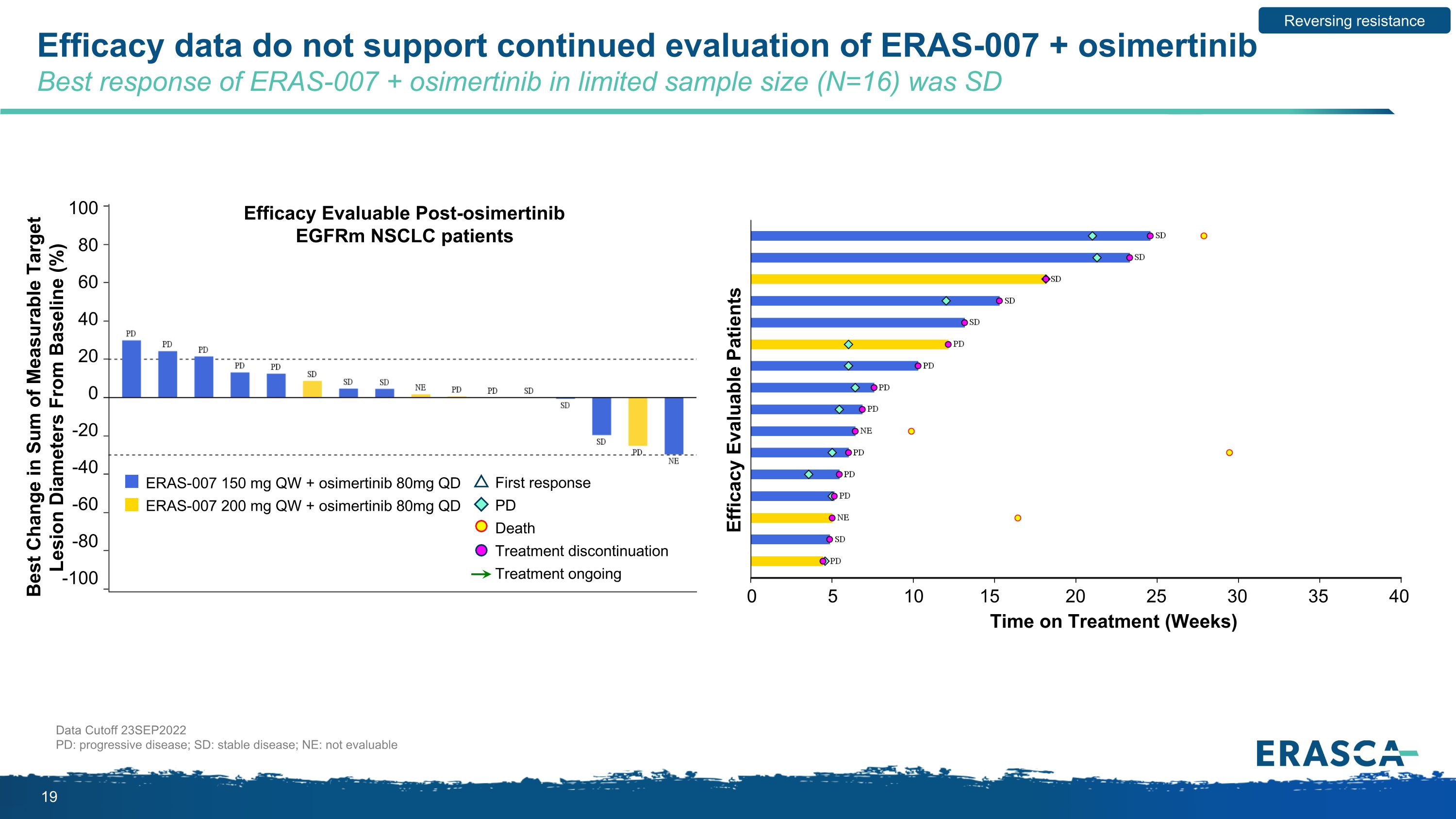
Efficacy data do not support continued evaluation of ERAS-007 + osimertinib Best response of ERAS-007 + osimertinib in limited sample size (N=16) was SD Data Cutoff 23SEP2022 PD: progressive disease; SD: stable disease; NE: not evaluable Reversing resistance 100 80 60 40 20 0 -20 -40 -60 -80 -100 0 5 10 15 20 25 30 35 40 Efficacy Evaluable Post-osimertinib EGFRm NSCLC patients Best Change in Sum of Measurable Target Lesion Diameters From Baseline (%) Efficacy Evaluable Patients Time on Treatment (Weeks) ERAS-007 150 mg QW + osimertinib 80mg QD ERAS-007 200 mg QW + osimertinib 80mg QD First response PD Death Treatment discontinuation Treatment ongoing
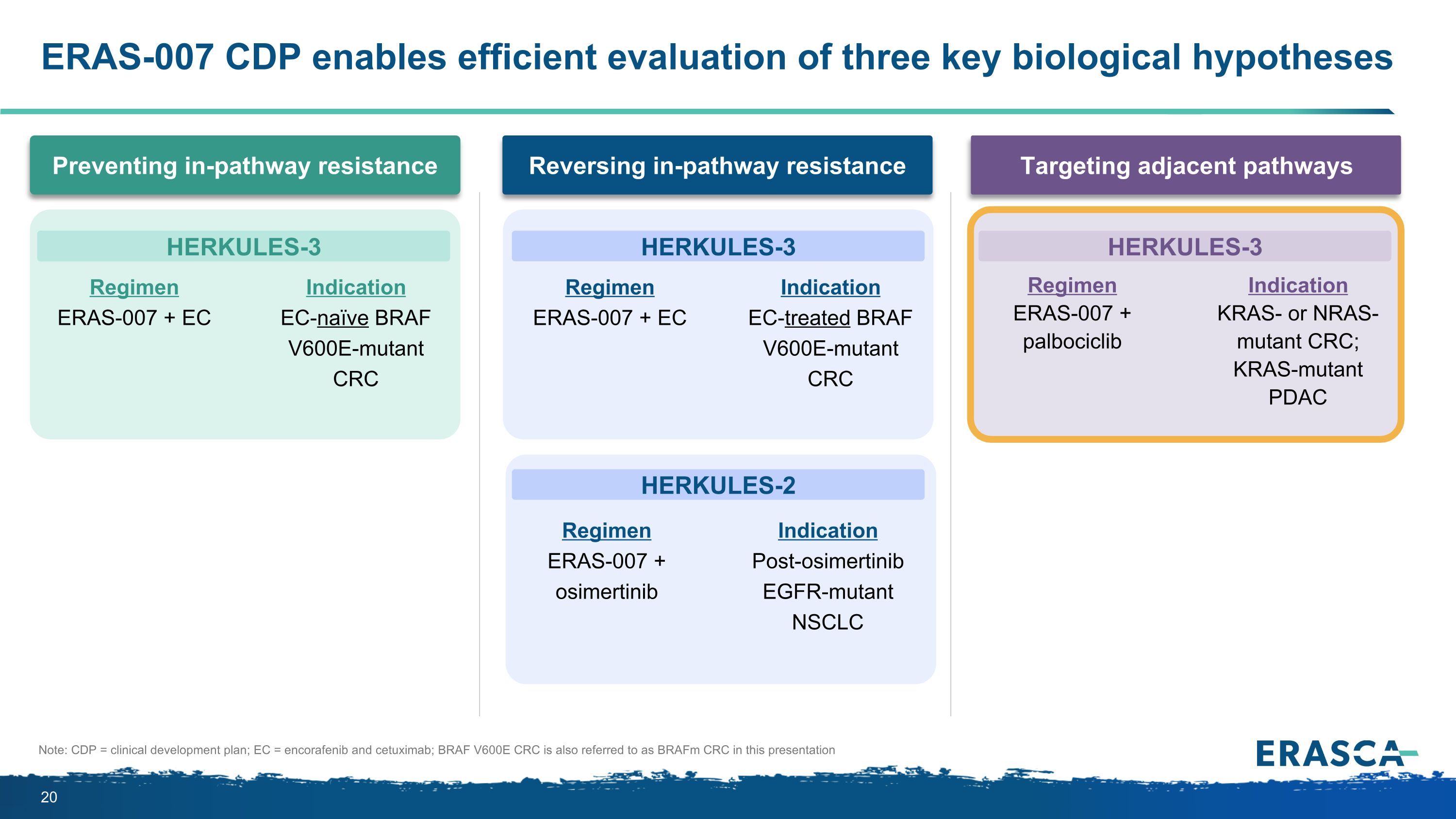
ERAS-007 CDP enables efficient evaluation of three key biological hypotheses Targeting adjacent pathways Preventing in-pathway resistance Reversing in-pathway resistance Regimen ERAS-007 + EC Indication EC-naïve BRAF V600E-mutant CRC Regimen ERAS-007 + EC Indication EC-treated BRAF V600E-mutant CRC Regimen ERAS-007 + osimertinib Indication Post-osimertinib EGFR-mutant NSCLC Regimen ERAS-007 + palbociclib Indication KRAS- or NRAS-mutant CRC; KRAS-mutant PDAC HERKULES-3 HERKULES-3 HERKULES-2 HERKULES-3 Note: CDP = clinical development plan; EC = encorafenib and cetuximab; BRAF V600E CRC is also referred to as BRAFm CRC in this presentation
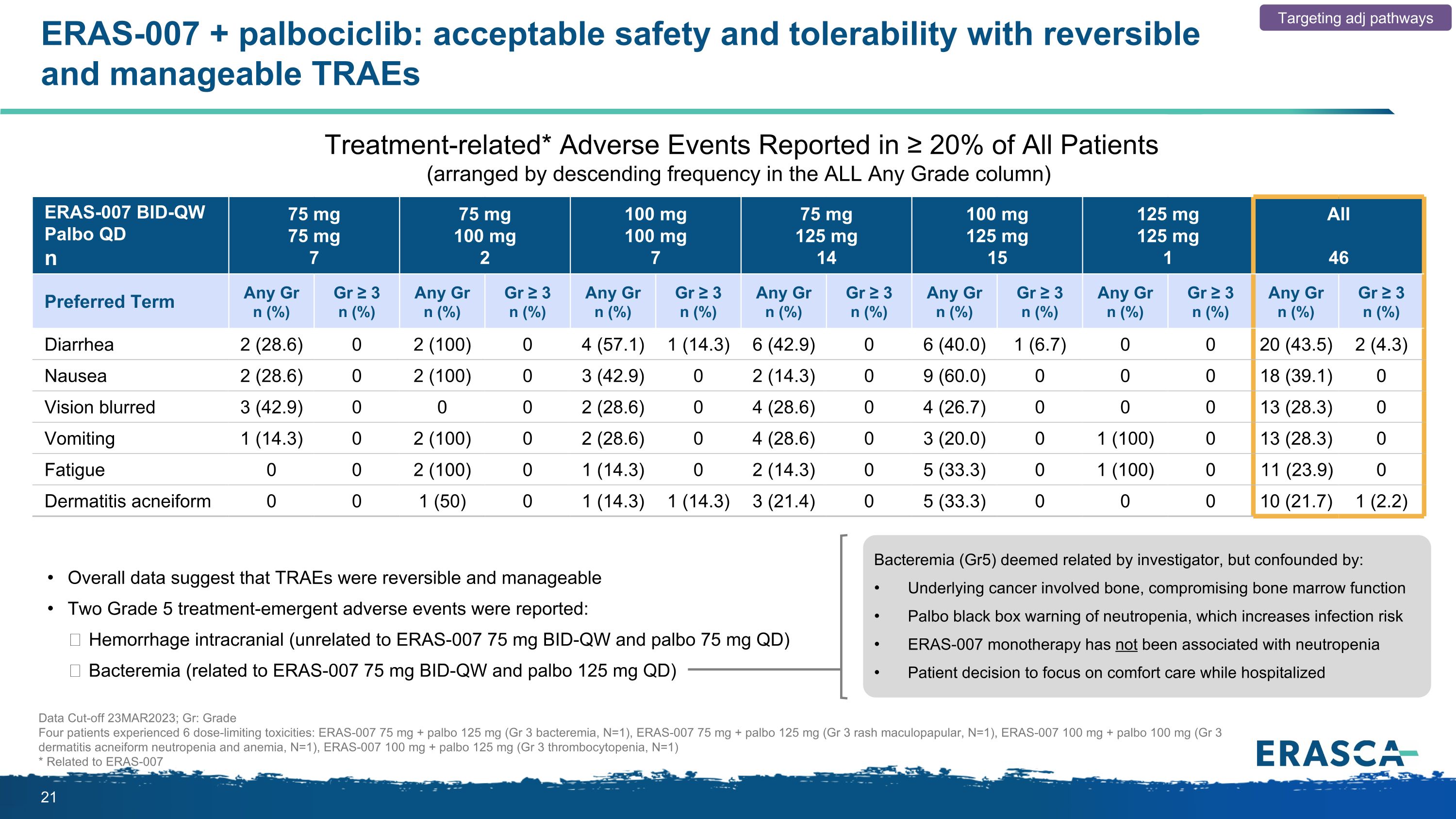
ERAS-007 + palbociclib: acceptable safety and tolerability with reversible and manageable TRAEs ERAS-007 BID-QW Palbo QD n 75 mg 75 mg 7 75 mg 100 mg 2 100 mg 100 mg 7 75 mg 125 mg 14 100 mg 125 mg 15 125 mg 125 mg 1 All 46 Preferred Term Any Gr n (%) Gr ≥ 3 n (%) Any Gr n (%) Gr ≥ 3 n (%) Any Gr n (%) Gr ≥ 3 n (%) Any Gr n (%) Gr ≥ 3 n (%) Any Gr n (%) Gr ≥ 3 n (%) Any Gr n (%) Gr ≥ 3 n (%) Any Gr n (%) Gr ≥ 3 n (%) Diarrhea 2 (28.6) 0 2 (100) 0 4 (57.1) 1 (14.3) 6 (42.9) 0 6 (40.0) 1 (6.7) 0 0 20 (43.5) 2 (4.3) Nausea 2 (28.6) 0 2 (100) 0 3 (42.9) 0 2 (14.3) 0 9 (60.0) 0 0 0 18 (39.1) 0 Vision blurred 3 (42.9) 0 0 0 2 (28.6) 0 4 (28.6) 0 4 (26.7) 0 0 0 13 (28.3) 0 Vomiting 1 (14.3) 0 2 (100) 0 2 (28.6) 0 4 (28.6) 0 3 (20.0) 0 1 (100) 0 13 (28.3) 0 Fatigue 0 0 2 (100) 0 1 (14.3) 0 2 (14.3) 0 5 (33.3) 0 1 (100) 0 11 (23.9) 0 Dermatitis acneiform 0 0 1 (50) 0 1 (14.3) 1 (14.3) 3 (21.4) 0 5 (33.3) 0 0 0 10 (21.7) 1 (2.2) Treatment-related* Adverse Events Reported in ≥ 20% of All Patients (arranged by descending frequency in the ALL Any Grade column) Data Cut-off 23MAR2023; Gr: Grade Four patients experienced 6 dose-limiting toxicities: ERAS-007 75 mg + palbo 125 mg (Gr 3 bacteremia, N=1), ERAS-007 75 mg + palbo 125 mg (Gr 3 rash maculopapular, N=1), ERAS-007 100 mg + palbo 100 mg (Gr 3 dermatitis acneiform neutropenia and anemia, N=1), ERAS-007 100 mg + palbo 125 mg (Gr 3 thrombocytopenia, N=1) * Related to ERAS-007 Targeting adj pathways Overall data suggest that TRAEs were reversible and manageable Two Grade 5 treatment-emergent adverse events were reported: Hemorrhage intracranial (unrelated to ERAS-007 75 mg BID-QW and palbo 75 mg QD) Bacteremia (related to ERAS-007 75 mg BID-QW and palbo 125 mg QD) Bacteremia (Gr5) deemed related by investigator, but confounded by: Underlying cancer involved bone, compromising bone marrow function Palbo black box warning of neutropenia, which increases infection risk ERAS-007 monotherapy has not been associated with neutropenia Patient decision to focus on comfort care while hospitalized
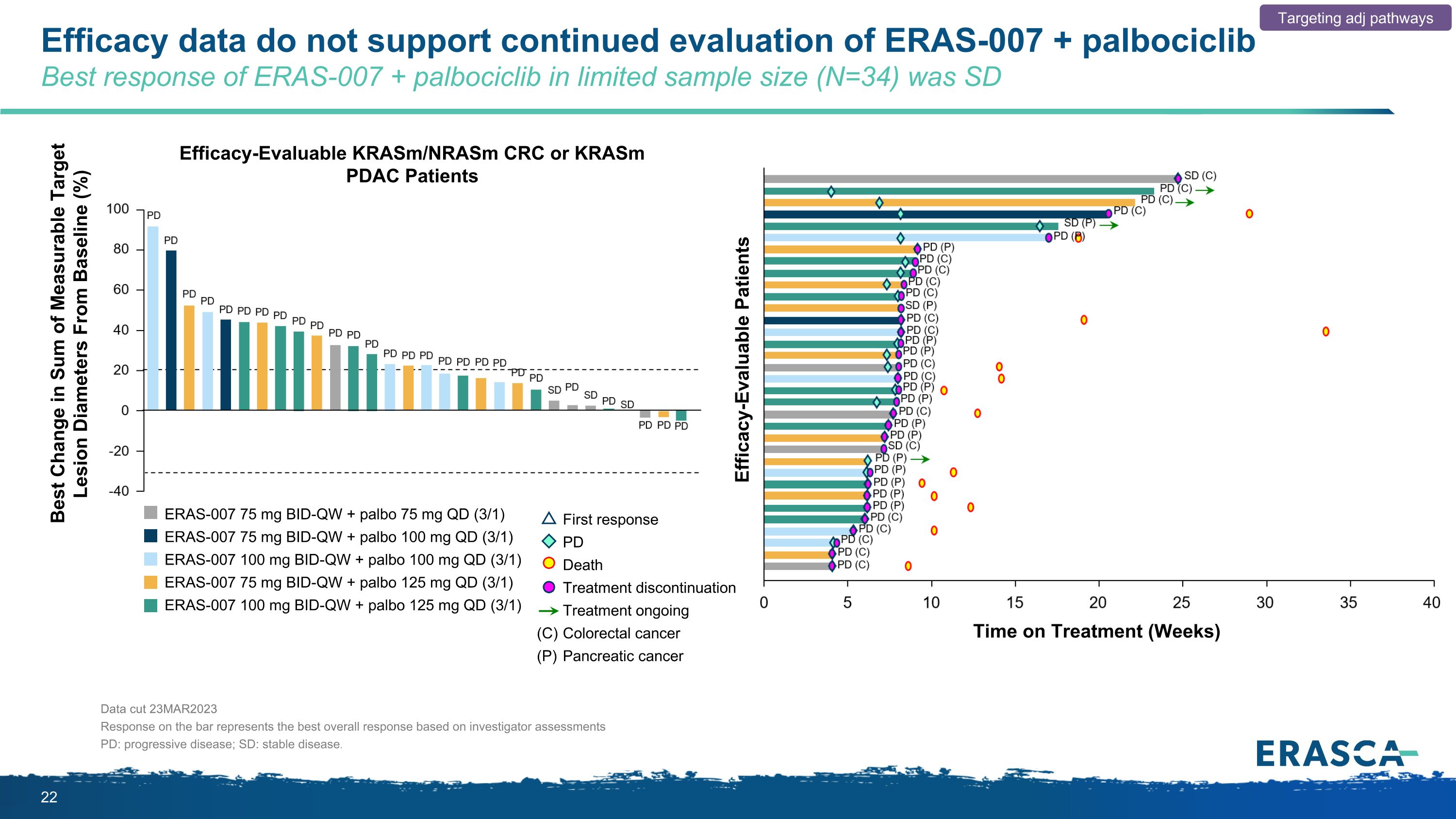
Efficacy data do not support continued evaluation of ERAS-007 + palbociclib Best response of ERAS-007 + palbociclib in limited sample size (N=34) was SD Targeting adj pathways Best Change in Sum of Measurable Target Lesion Diameters From Baseline (%) Efficacy-Evaluable KRASm/NRASm CRC or KRASm PDAC Patients Efficacy-Evaluable Patients Time on Treatment (Weeks) Data cut 23MAR2023 Response on the bar represents the best overall response based on investigator assessments PD: progressive disease; SD: stable disease. First response PD Death Treatment discontinuation Treatment ongoing Colorectal cancer Pancreatic cancer (C) (P) ERAS-007 75 mg BID-QW + palbo 75 mg QD (3/1) ERAS-007 75 mg BID-QW + palbo 100 mg QD (3/1) ERAS-007 100 mg BID-QW + palbo 100 mg QD (3/1) ERAS-007 75 mg BID-QW + palbo 125 mg QD (3/1) ERAS-007 100 mg BID-QW + palbo 125 mg QD (3/1)

Other ERAS-007 Clinical Update / Wrap-up

HERKULES-1: ERAS-601 + ERAS-007 combination safety data do not support continued evaluation of regimen tested Dosing Regimen Tested: ERAS-601 40 mg BID (3 weeks on/1 week off) + ERAS-007 50 mg BID-QW Rationale: ERAS-601 was positioned at its MTD as backbone therapy with dose escalation of ERAS-007 2 DLTs were reported in 6 DLT-evaluable patients enrolled in the first dosing cohort Grade 3 elevated AST Grade 3 neutropenia Data as reported by investigators on 25MAY2023 investigator call and subsequent follow up BID: twice a day; BID-QW: oral twice a day on a single day each week; MTD: maximum tolerated dose; DLT: dose limiting toxicity
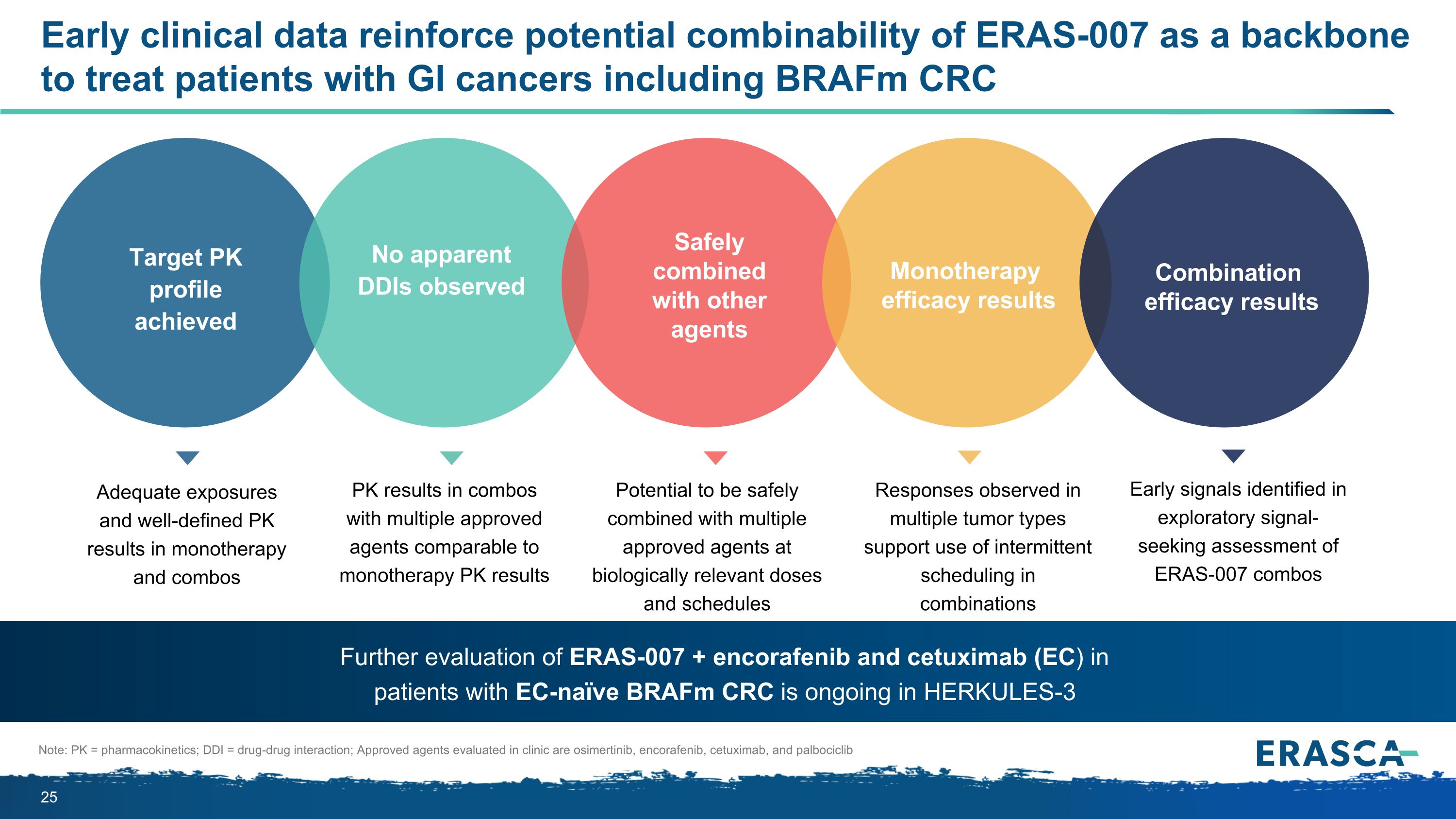
Early clinical data reinforce potential combinability of ERAS-007 as a backbone to treat patients with GI cancers including BRAFm CRC Note: PK = pharmacokinetics; DDI = drug-drug interaction; Approved agents evaluated in clinic are osimertinib, encorafenib, cetuximab, and palbociclib Adequate exposures and well-defined PK results in monotherapy and combos PK results in combos with multiple approved agents comparable to monotherapy PK results Early signals identified in exploratory signal-seeking assessment of ERAS-007 combos Further evaluation of ERAS-007 + encorafenib and cetuximab (EC) in patients with EC-naïve BRAFm CRC is ongoing in HERKULES-3 Responses observed in multiple tumor types support use of intermittent scheduling in combinations Target PK profile achieved No apparent DDIs observed Combination efficacy results Monotherapy efficacy results Safely combined with other agents Potential to be safely combined with multiple approved agents at biologically relevant doses and schedules

Portfolio Update
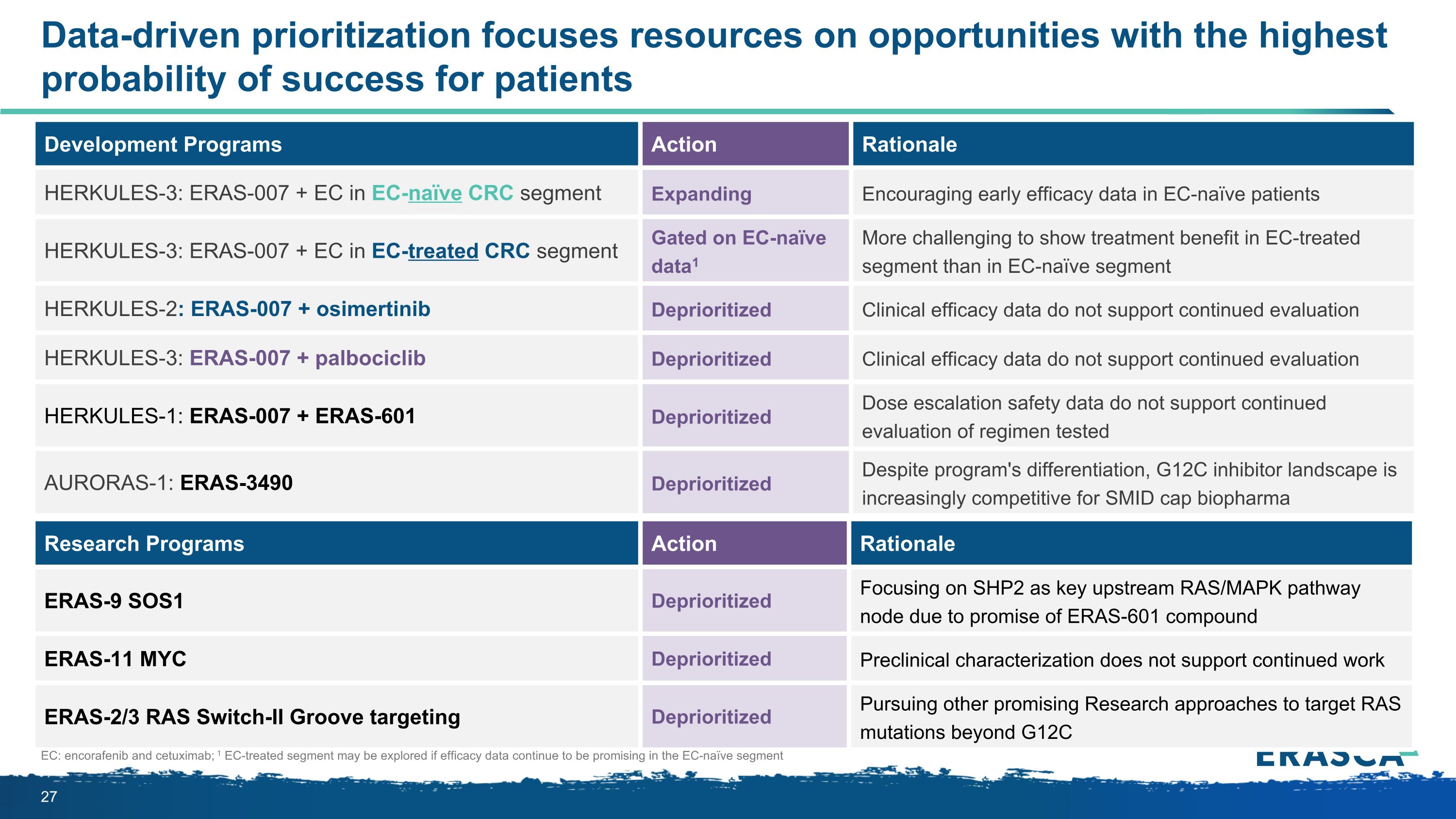
Research Programs Action Rationale ERAS-9 SOS1 Deprioritized Focusing on SHP2 as key upstream RAS/MAPK pathway node due to promise of ERAS-601 compound ERAS-11 MYC Deprioritized Preclinical characterization does not support continued work ERAS-2/3 RAS Switch-II Groove targeting Deprioritized Pursuing other promising Research approaches to target RAS mutations beyond G12C Data-driven prioritization focuses resources on opportunities with the highest probability of success for patients EC: encorafenib and cetuximab; 1 EC-treated segment may be explored if efficacy data continue to be promising in the EC-naïve segment Development Programs Action Rationale HERKULES-3: ERAS-007 + EC in EC-naïve CRC segment Expanding Encouraging early efficacy data in EC-naïve patients HERKULES-3: ERAS-007 + EC in EC-treated CRC segment Gated on EC-naïve data1 More challenging to show treatment benefit in EC-treated segment than in EC-naïve segment HERKULES-2: ERAS-007 + osimertinib Deprioritized Clinical efficacy data do not support continued evaluation HERKULES-3: ERAS-007 + palbociclib Deprioritized Clinical efficacy data do not support continued evaluation HERKULES-1: ERAS-007 + ERAS-601 Deprioritized Dose escalation safety data do not support continued evaluation of regimen tested AURORAS-1: ERAS-3490 Deprioritized Despite program's differentiation, G12C inhibitor landscape is increasingly competitive for SMID cap biopharma
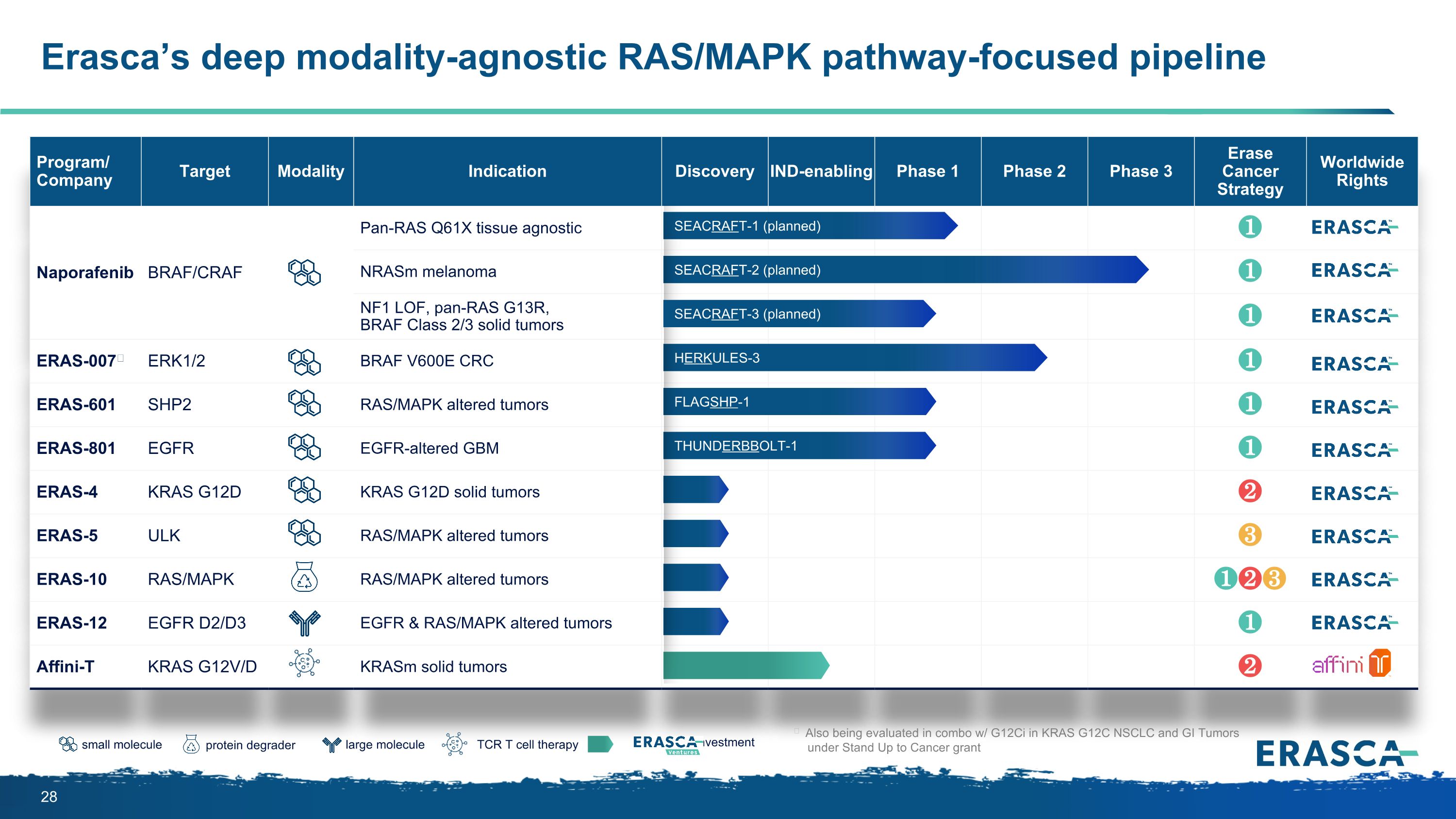
Program/ Company Target Modality Indication Discovery IND-enabling Phase 1 Phase 2 Phase 3 Erase Cancer Strategy Worldwide Rights Naporafenib BRAF/CRAF Pan-RAS Q61X tissue agnostic ❶ NRASm melanoma ❶ NF1 LOF, pan-RAS G13R, BRAF Class 2/3 solid tumors ❶ ERAS-007ǂ ERK1/2 BRAF V600E CRC ❶ ERAS-601 SHP2 RAS/MAPK altered tumors ❶ ERAS-801 EGFR EGFR-altered GBM ❶ ERAS-4 KRAS G12D KRAS G12D solid tumors ❷ ERAS-5 ULK RAS/MAPK altered tumors ❸ ERAS-10 RAS/MAPK RAS/MAPK altered tumors ❶❷❸ ERAS-12 EGFR D2/D3 EGFR & RAS/MAPK altered tumors ❶ Affini-T KRAS G12V/D KRASm solid tumors ❷ ǂ Also being evaluated in combo w/ G12Ci in KRAS G12C NSCLC and GI Tumors under Stand Up to Cancer grant large molecule protein degrader small molecule TCR T cell therapy investment SEACRAFT-2 (planned) HERKULES-3 SEACRAFT-1 (planned) THUNDERBBOLT-1 FLAGSHP-1 Erasca’s deep modality-agnostic RAS/MAPK pathway-focused pipeline SEACRAFT-3 (planned)
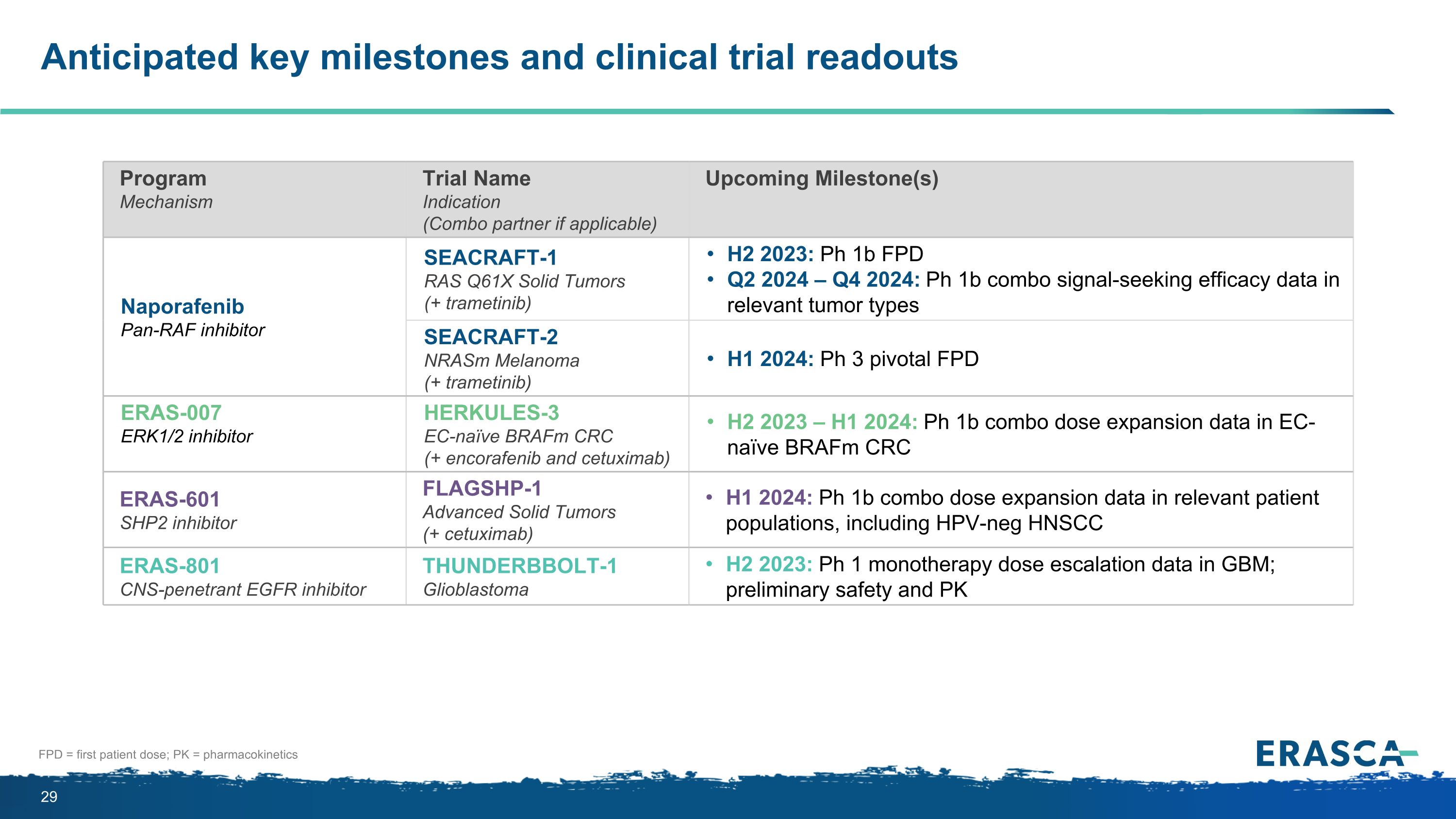
Anticipated key milestones and clinical trial readouts FPD = first patient dose; PK = pharmacokinetics Program Mechanism Trial Name Indication (Combo partner if applicable) Upcoming Milestone(s) Naporafenib Pan-RAF inhibitor SEACRAFT-1 RAS Q61X Solid Tumors (+ trametinib) H2 2023: Ph 1b FPD Q2 2024 – Q4 2024: Ph 1b combo signal-seeking efficacy data in relevant tumor types SEACRAFT-2 NRASm Melanoma (+ trametinib) H1 2024: Ph 3 pivotal FPD ERAS-007 ERK1/2 inhibitor HERKULES-3 EC-naïve BRAFm CRC (+ encorafenib and cetuximab) H2 2023 – H1 2024: Ph 1b combo dose expansion data in EC-naïve BRAFm CRC ERAS-601 SHP2 inhibitor FLAGSHP-1 Advanced Solid Tumors (+ cetuximab) H1 2024: Ph 1b combo dose expansion data in relevant patient populations, including HPV-neg HNSCC ERAS-801 CNS-penetrant EGFR inhibitor THUNDERBBOLT-1 Glioblastoma H2 2023: Ph 1 monotherapy dose escalation data in GBM; preliminary safety and PK

Thank You!
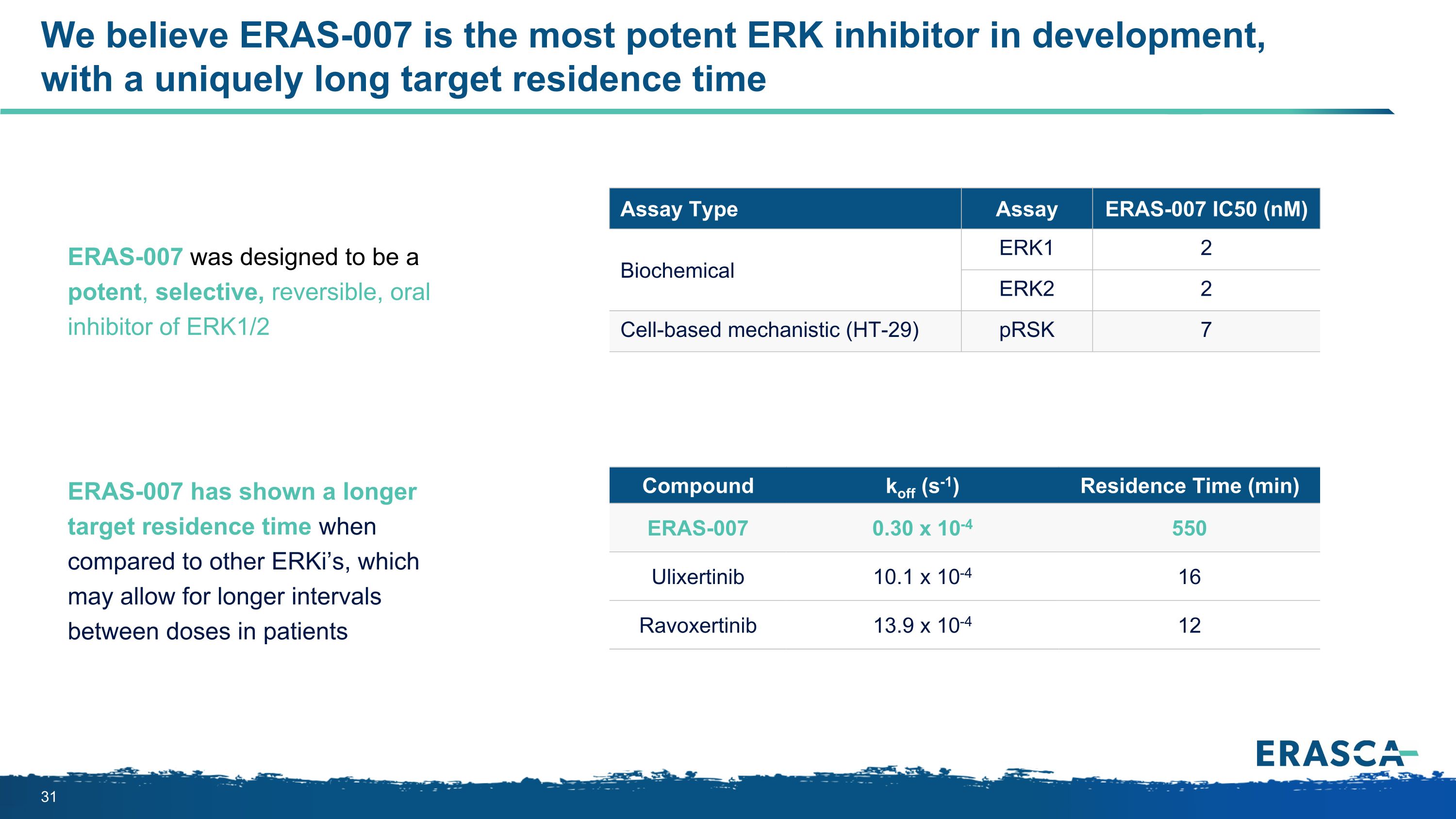
We believe ERAS-007 is the most potent ERK inhibitor in development, with a uniquely long target residence time Assay Type Assay ERAS-007 IC50 (nM) Biochemical ERK1 2 ERK2 2 Cell-based mechanistic (HT-29) pRSK 7 ERAS-007 was designed to be a potent, selective, reversible, oral inhibitor of ERK1/2 Compound koff (s-1) Residence Time (min) ERAS-007 0.30 x 10-4 550 Ulixertinib 10.1 x 10-4 16 Ravoxertinib 13.9 x 10-4 12 ERAS-007 has shown a longer target residence time when compared to other ERKi’s, which may allow for longer intervals between doses in patients
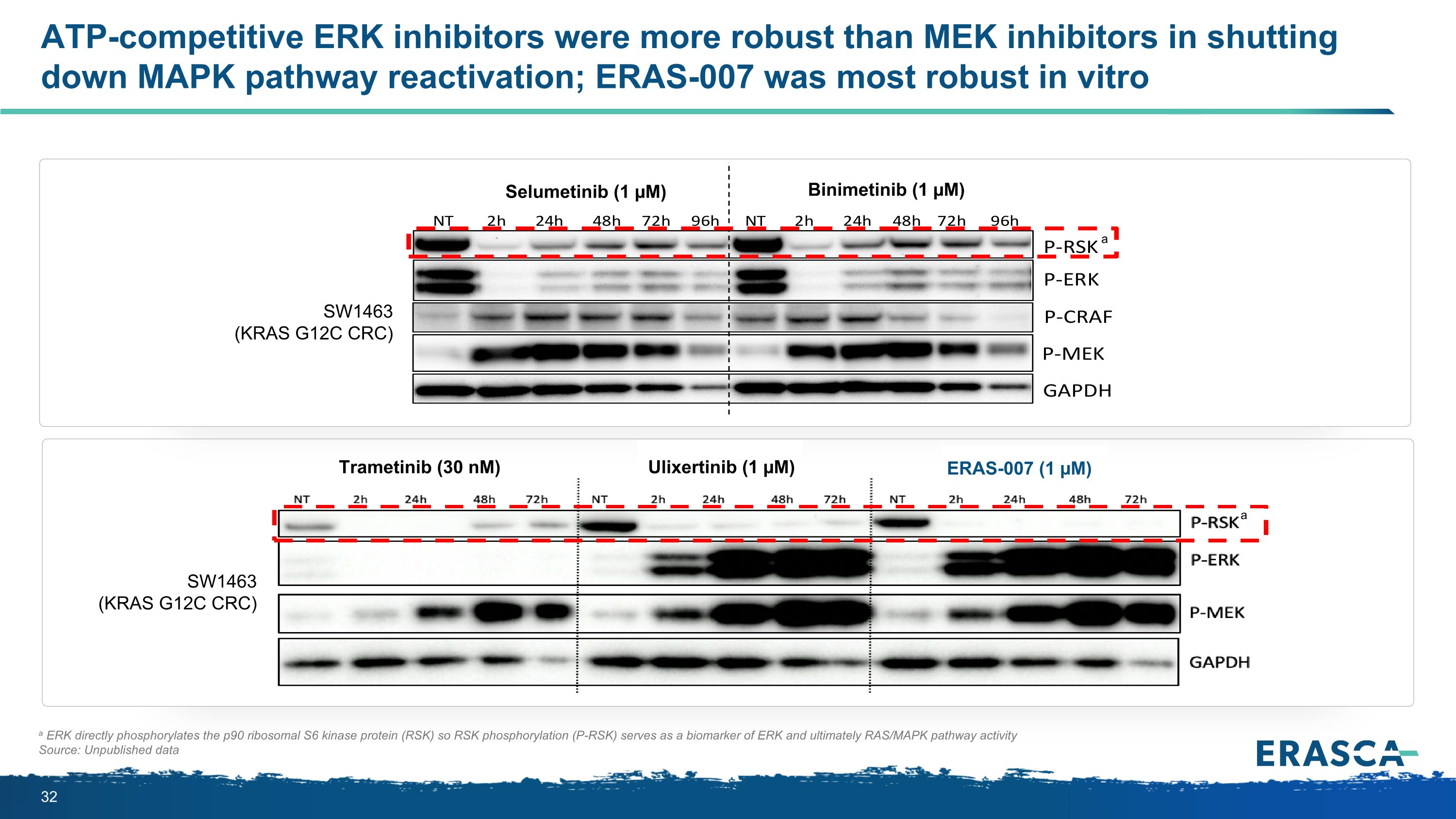
ATP-competitive ERK inhibitors were more robust than MEK inhibitors in shutting down MAPK pathway reactivation; ERAS-007 was most robust in vitro a ERK directly phosphorylates the p90 ribosomal S6 kinase protein (RSK) so RSK phosphorylation (P-RSK) serves as a biomarker of ERK and ultimately RAS/MAPK pathway activity Source: Unpublished data SW1463 (KRAS G12C CRC) Selumetinib (1 µM) Binimetinib (1 µM) SW1463 (KRAS G12C CRC) Trametinib (30 nM) Ulixertinib (1 µM) ERAS-007 (1 µM) a a
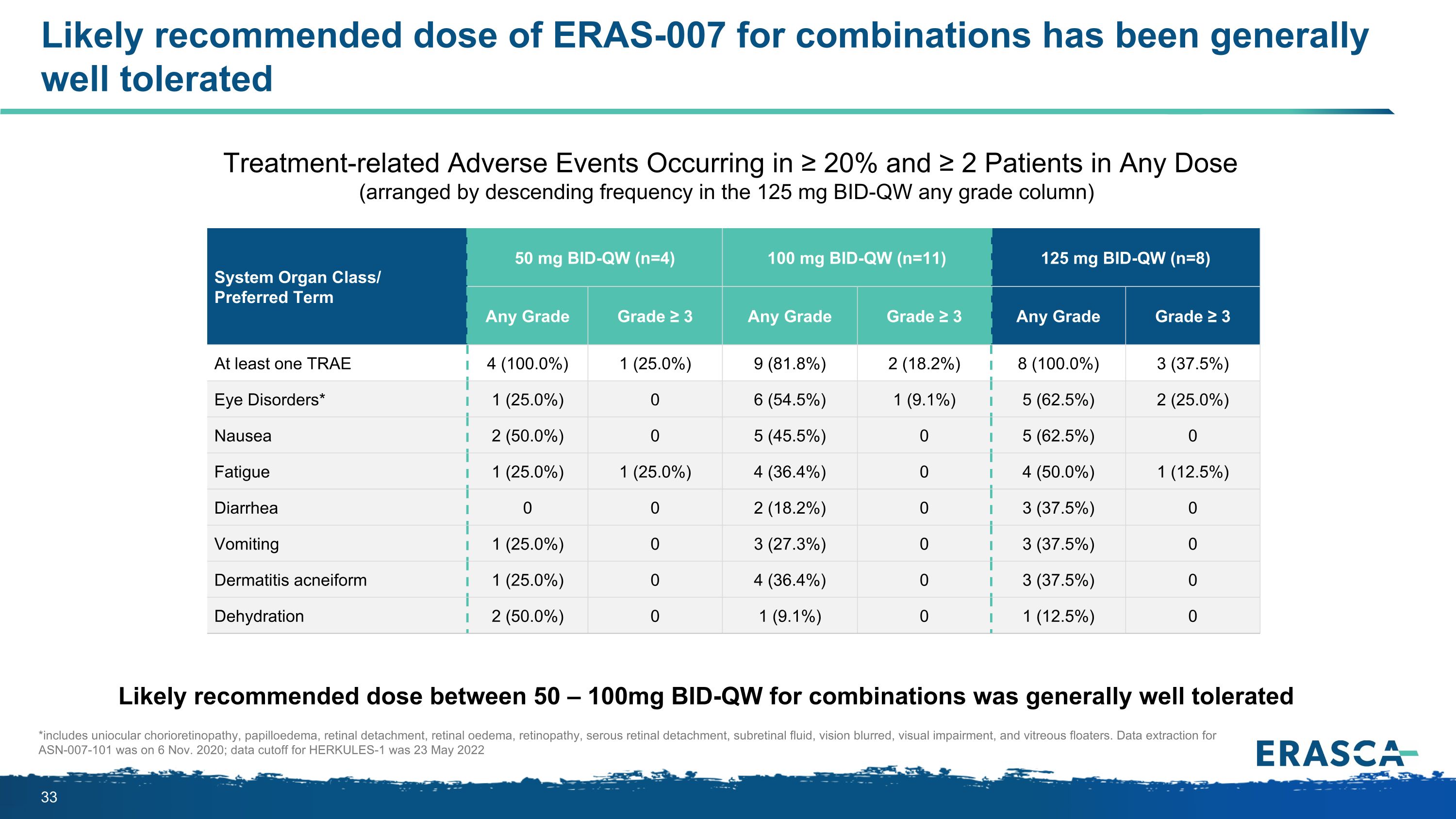
Likely recommended dose of ERAS-007 for combinations has been generally well tolerated *includes uniocular chorioretinopathy, papilloedema, retinal detachment, retinal oedema, retinopathy, serous retinal detachment, subretinal fluid, vision blurred, visual impairment, and vitreous floaters. Data extraction for ASN-007-101 was on 6 Nov. 2020; data cutoff for HERKULES-1 was 23 May 2022 System Organ Class/ Preferred Term 50 mg BID-QW (n=4) 100 mg BID-QW (n=11) 125 mg BID-QW (n=8) Any Grade Grade ≥ 3 Any Grade Grade ≥ 3 Any Grade Grade ≥ 3 At least one TRAE 4 (100.0%) 1 (25.0%) 9 (81.8%) 2 (18.2%) 8 (100.0%) 3 (37.5%) Eye Disorders* 1 (25.0%) 0 6 (54.5%) 1 (9.1%) 5 (62.5%) 2 (25.0%) Nausea 2 (50.0%) 0 5 (45.5%) 0 5 (62.5%) 0 Fatigue 1 (25.0%) 1 (25.0%) 4 (36.4%) 0 4 (50.0%) 1 (12.5%) Diarrhea 0 0 2 (18.2%) 0 3 (37.5%) 0 Vomiting 1 (25.0%) 0 3 (27.3%) 0 3 (37.5%) 0 Dermatitis acneiform 1 (25.0%) 0 4 (36.4%) 0 3 (37.5%) 0 Dehydration 2 (50.0%) 0 1 (9.1%) 0 1 (12.5%) 0 Treatment-related Adverse Events Occurring in ≥ 20% and ≥ 2 Patients in Any Dose (arranged by descending frequency in the 125 mg BID-QW any grade column) Likely recommended dose between 50 – 100mg BID-QW for combinations was generally well tolerated
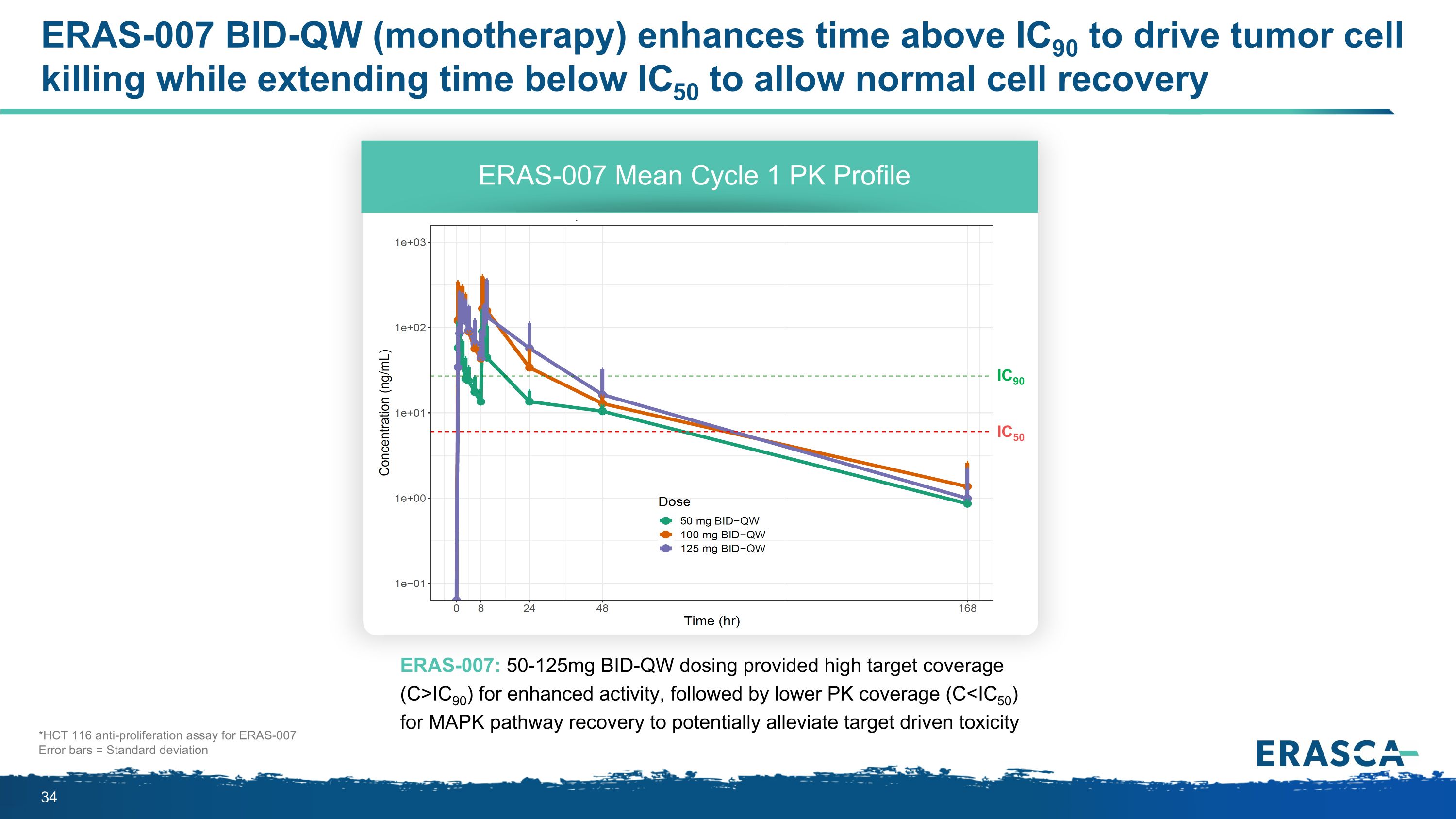
ERAS-007 BID-QW (monotherapy) enhances time above IC90 to drive tumor cell killing while extending time below IC50 to allow normal cell recovery ERAS-007: 50-125mg BID-QW dosing provided high target coverage (C>IC90) for enhanced activity, followed by lower PK coverage (C<IC50) for MAPK pathway recovery to potentially alleviate target driven toxicity ERAS-007 Mean Cycle 1 PK Profile *HCT 116 anti-proliferation assay for ERAS-007 Error bars = Standard deviation IC90 IC50
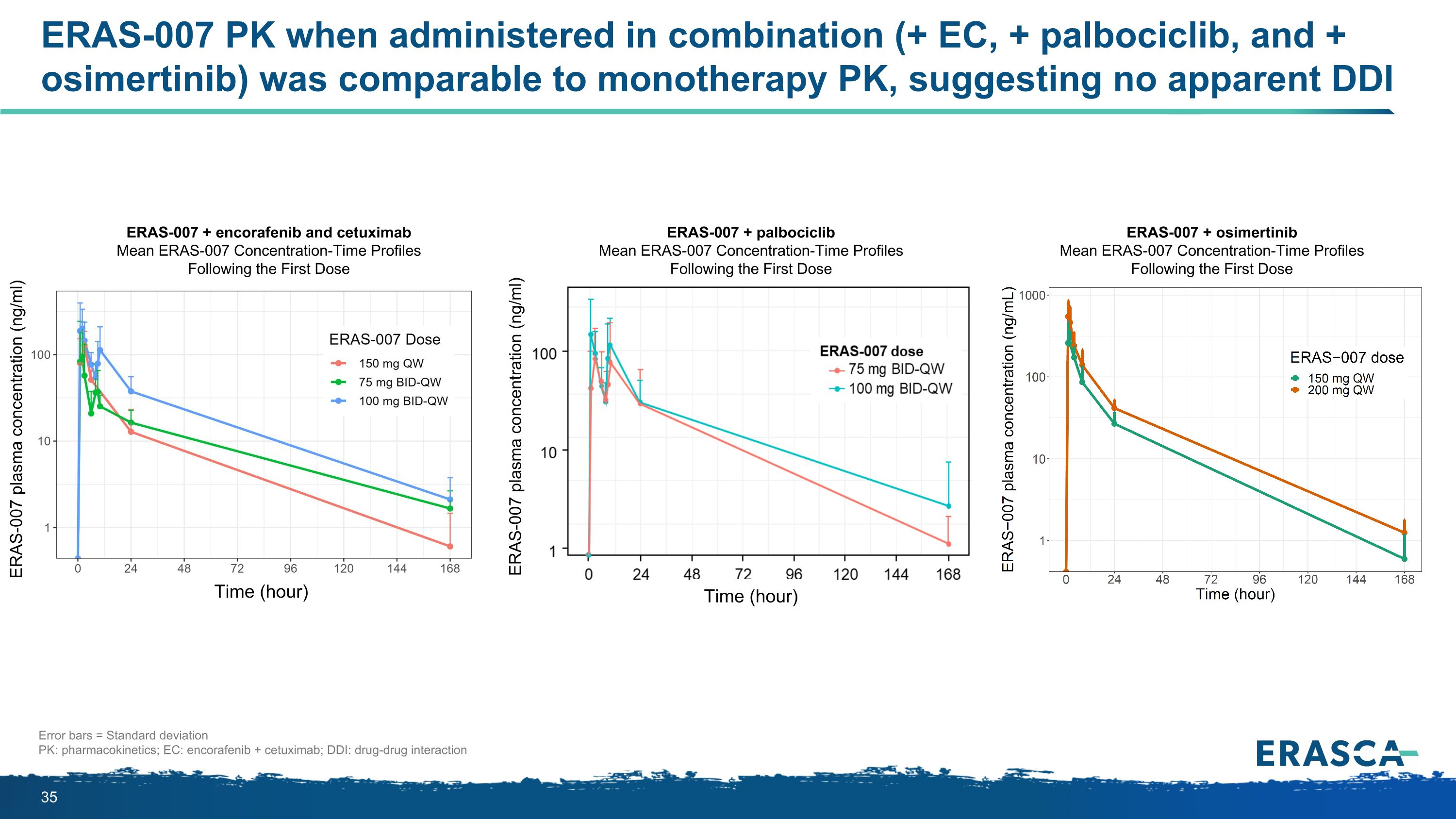
ERAS-007 PK when administered in combination (+ EC, + palbociclib, and + osimertinib) was comparable to monotherapy PK, suggesting no apparent DDI Error bars = Standard deviation PK: pharmacokinetics; EC: encorafenib + cetuximab; DDI: drug-drug interaction ERAS-007 + encorafenib and cetuximab Mean ERAS-007 Concentration-Time Profiles Following the First Dose ERAS-007 plasma concentration (ng/ml) ERAS-007 plasma concentration (ng/ml) Time (hour) Time (hour) ERAS-007 + palbociclib Mean ERAS-007 Concentration-Time Profiles Following the First Dose ERAS-007 + osimertinib Mean ERAS-007 Concentration-Time Profiles Following the First Dose