

RT-102 Phase I Study Part 2: Repeat-Dose Update December 2022

This presentation and the accompanying oral statements contain forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995 as contained in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act. Forward-looking statements are based on information available at the time those statements are made or on management's good faith beliefs and assumptions as of that time with respect to future events, and are subject to risks and uncertainties that could cause actual performance or results to differ materially from those expressed in, or suggested by, the forward-looking statements. In light of these risks and uncertainties, the forward-looking events and circumstances discussed in this presentation and the accompanying oral statements may not occur and actual results could differ materially from those anticipated or implied in the forward-looking statements. These risks and uncertainties include Rani Therapeutics Holdings, Inc.’s (“Rani,” “we,” “us,” or “our”) future financial performance, including our expectations regarding our revenues, cost of revenues, operating expenses, and our ability to achieve and maintain future profitability, those risks inherent in the preclinical and clinical development process and the regulatory approval process, the risks and uncertainties in commercialization and gaining market acceptance, the commercial potential of oral biologics, our ability to complete development of the RaniPill® HC or any redesign and conduct additional preclinical and clinical studies of the RaniPill HC or any future design of the RaniPill to accommodate higher target payloads, the risks associated with protecting and defending our patents or other proprietary rights, the risk that our proprietary rights may be insufficient to protect our product candidates, the risk that we will be unable to obtain necessary capital when needed on acceptable terms or at all, our ability to enter into strategic partnerships and to achieve the potential benefits of such partnerships, competition from other products or procedures, our reliance on third-parties to conduct our clinical and non-clinical trials, our reliance on single-source third-party suppliers to manufacture clinical, non-clinical and any future commercial supplies of our product candidates, our ability to continue to scale and optimize our manufacturing processes by expanding our use of automation, our expectations regarding the period during which we qualify as an emerging growth company under the JOBS Act, the extent and duration of the COVID-19 pandemic and the conflict between Ukraine and Russia, our expectations regarding customer demand for our product candidates, increased regulatory requirements and other factors that are set forth in our filings with the Securities and Exchange Commission (“SEC”), including under the caption “Risk Factors” in our Quarterly Report on Form 10-Q for the quarter ended March 31, 2022, which was filed with the SEC on May 11, 2022, and our other public filings made with the SEC and available at www.sec.gov. Trade names, trademarks and service marks of other companies appearing in this presentation are the property of their respective owners. Solely for convenience, the trademarks and trade names referred to in this presentation appear without the ® and ™ symbols, but those references are not intended to indicate, in any way, that we will not assert, to the fullest extent under applicable law, our rights, or the right of the applicable licensor to these trademarks and tradenames. Forward-looking statements are inherently subject to risks and uncertainties, some of which cannot be predicted or quantified. In some cases, you can identify forward-looking statements by terminology such as “anticipate,” “believe,” “contemplate,” “continue,” “could,” “estimate,” “expect,” “intend,” “may,” “plan,” “potential,” “predict,” “project,” “should,” “target,” “will” or “would,” or the negative of these terms or other comparable terminology. You should not put undue reliance on any forward-looking statements. Forward-looking statements should not be read as a guarantee of future performance or results, and will not necessarily be accurate indications of the times at, or by, which such performance or results will be achieved, if at all. Except as required by law, Rani does not undertake any obligation to publicly update or revise any forward-looking statement, whether as a result of new information, future developments or otherwise. This presentation and the accompanying oral statements contain statistical data, estimates and forecasts that are based on independent industry publications or other publicly available information, as well as other information based on our internal sources. This information involves many assumptions and limitations, and you are cautioned not to give undue weight to such information. We have not independently verified the accuracy or completeness of the information contained in the industry publications and other publicly available information. Accordingly, we make no representations as to the accuracy or completeness of that information nor do we undertake to update such information after the date of this presentation. Forward-Looking Statements

Topline results from Part 2 of our RT-102 Phase 1 study Seven-day repeat-dose study in healthy volunteers First repeat-dose study of RaniPill capsule in humans collecting data on: Safety Tolerability Device Reliability Important data For the RT-102 program and Phase 2 plan for 2023 For RaniPill platform in general Presentation Overview
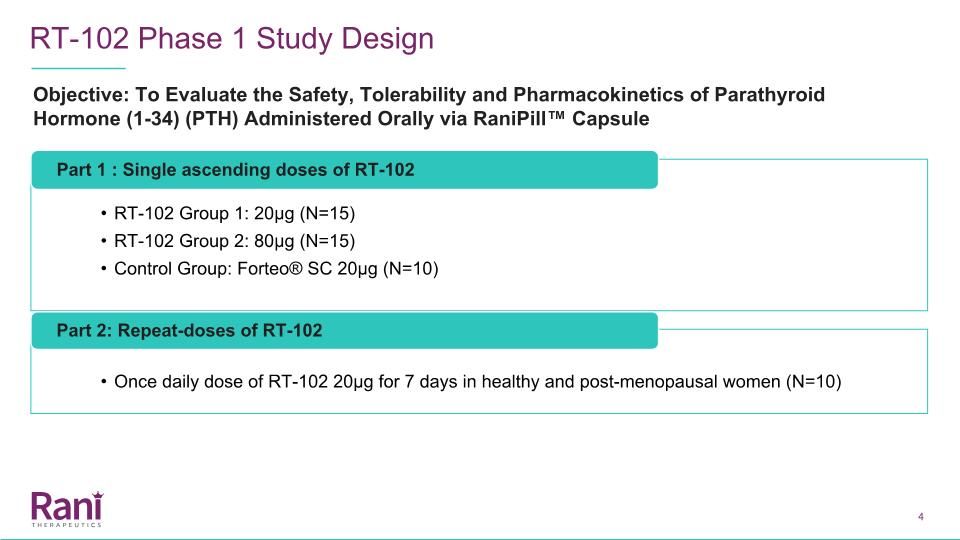
RT-102 Phase 1 Study Design Part 1 : Single ascending doses of RT-102 RT-102 Group 1: 20µg (N=15) RT-102 Group 2: 80µg (N=15) Control Group: Forteo® SC 20µg (N=10) Part 2: Repeat-doses of RT-102 Once daily dose of RT-102 20µg for 7 days in healthy and post-menopausal women (N=10) Objective: To Evaluate the Safety, Tolerability and Pharmacokinetics of Parathyroid Hormone (1-34) (PTH) Administered Orally via RaniPill™ Capsule

Part 1 Summary Safety & Tolerability Both doses of RT-102 were well tolerated No serious adverse events (SAEs) reported in the study No adverse events (AEs) related to RaniPill reported Device Performance Drug delivery success rate of 95% Pharmacokinetics Bioavailability of PTH delivered via RT-102 was >300% higher than subcutaneous (SC) injection

Part 2: Arvinder Dhalla, PhD Vice President, Clinical Development

Part 2 Study Overview A Phase I Study to Evaluate the Pharmacokinetics of Parathyroid Hormone (1-34) (PTH) Administered Orally via RaniPill™ Capsule Objective To evaluate the safety and tolerability of repeat-doses of RT-102 Study Population Healthy women and Post-menopausal women (N=10) Study Site Single Site in Australia Study Group A single group receiving RT-102 20µg dose for 7 days End Points Safety and tolerability of repeat-doses of RT-102 Reliability of drug delivery Start Date August 1, 2022

Repeat-Dose: Study Procedures Fast >8 hrs. Participant Dosed 6 hr. 9 hr. Blood Sample Blood Sample Blood Sample Blood sample Fluoro image taken Participant Dosed Blood Sample Liquids provided Start PK sampling….. Serial fluoroscopic imaging done to determine T=0 Days 1-6 Day 7 Participation in the study was considered complete if a participant was able to complete all doses for 7 days and go through pharmacokinetic sampling on Day 7 Food provided 3 hr. Fast >8 hrs. 3 hr. Device Deployment confirmed

Topline Results
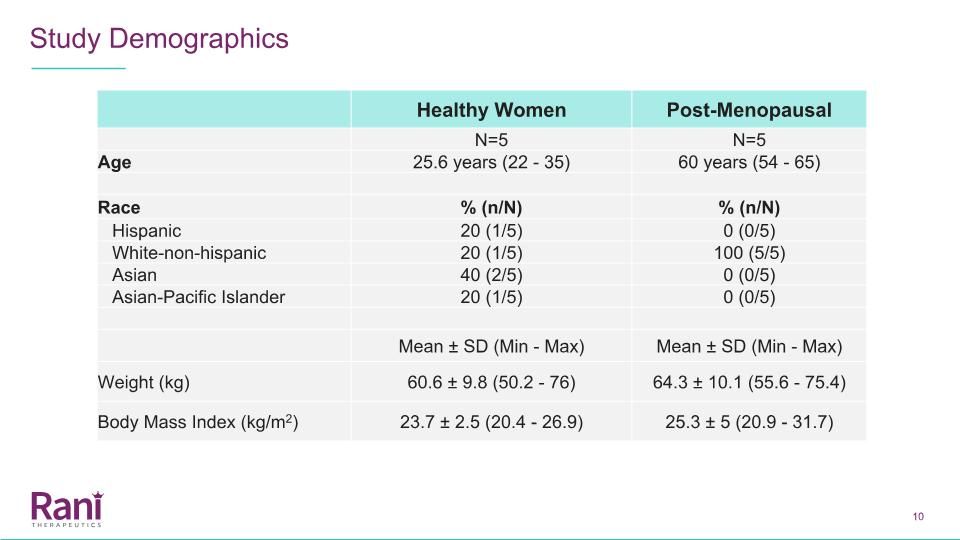
Healthy Women Post-Menopausal N=5 N=5 Age 25.6 years (22 - 35) 60 years (54 - 65) Race % (n/N) % (n/N) Hispanic 20 (1/5) 0 (0/5) White-non-hispanic 20 (1/5) 100 (5/5) Asian 40 (2/5) 0 (0/5) Asian-Pacific Islander 20 (1/5) 0 (0/5) Mean ± SD (Min - Max) Mean ± SD (Min - Max) Weight (kg) 60.6 ± 9.8 (50.2 - 76) 64.3 ± 10.1 (55.6 - 75.4) Body Mass Index (kg/m2) 23.7 ± 2.5 (20.4 - 26.9) 25.3 ± 5 (20.9 - 31.7) Study Demographics

Enrollment was done on a rolling basis to complete ten subjects, total number of participants enrolled were 17 Seven participants did not complete 7 days of dosing due to exclusions per protocol Two participants started menstruation on Day 4 One participant had cannulation issues on Day 7 One participant had elevated eosinophils on Day 4 due to an earring infection One participant had >7 hr. gastric residence time (GRT) on Day 7 Two participants had pill remnants exceeding the number (>3) allowed by protocol Exclusions after Enrollment None of the participants stopped the dosing due to any adverse events related to the RaniPill
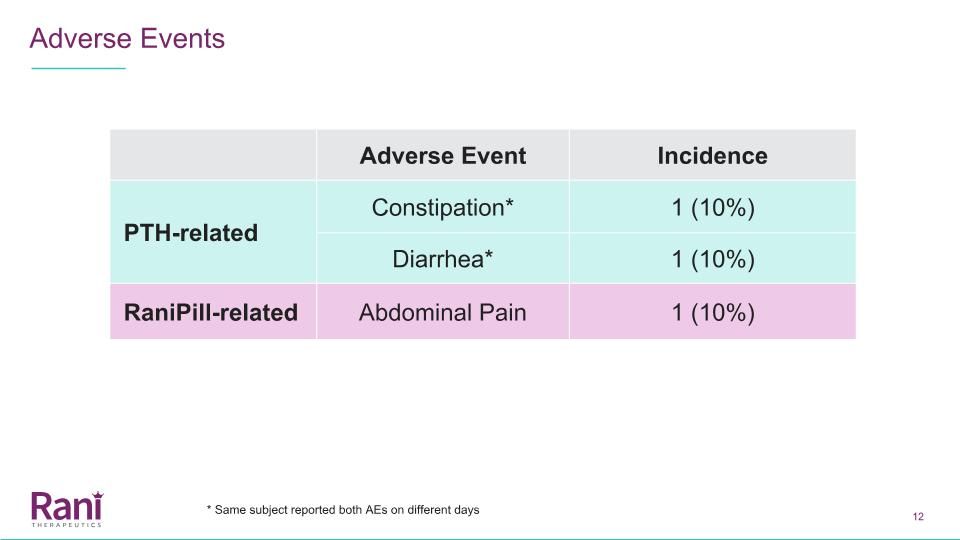
Adverse Events Adverse Event Incidence PTH-related Constipation* 1 (10%) Diarrhea* 1 (10%) RaniPill-related Abdominal Pain 1 (10%) * Same subject reported both AEs on different days
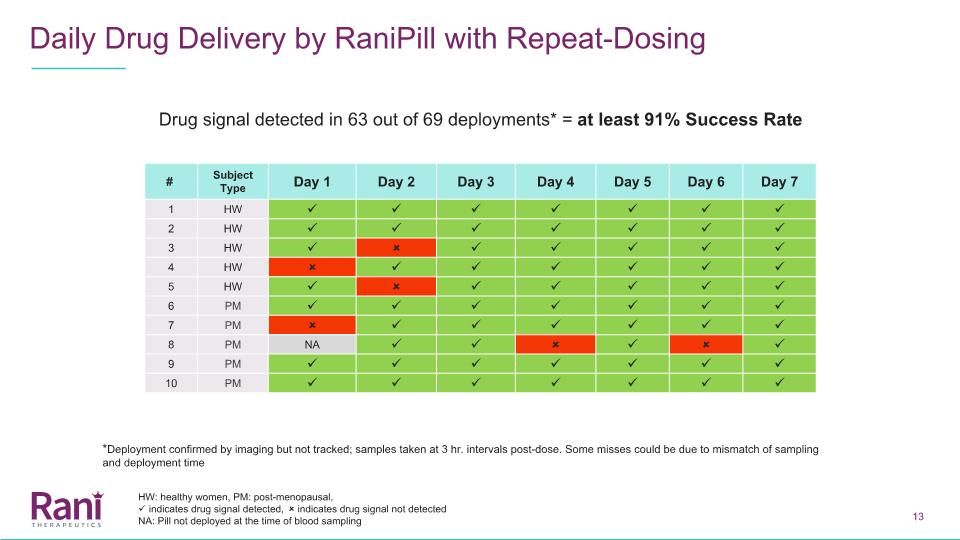
Daily Drug Delivery by RaniPill with Repeat-Dosing 25% 50% 80% Drug signal detected in 63 out of 69 deployments* = at least 91% Success Rate # Subject Type Day 1 Day 2 Day 3 Day 4 Day 5 Day 6 Day 7 1 HW 2 HW 3 HW 4 HW 5 HW 6 PM 7 PM 8 PM NA 9 PM 10 PM *Deployment confirmed by imaging but not tracked; samples taken at 3 hr. intervals post-dose. Some misses could be due to mismatch of sampling and deployment time HW: healthy women, PM: post-menopausal, indicates drug signal detected, indicates drug signal not detected NA: Pill not deployed at the time of blood sampling
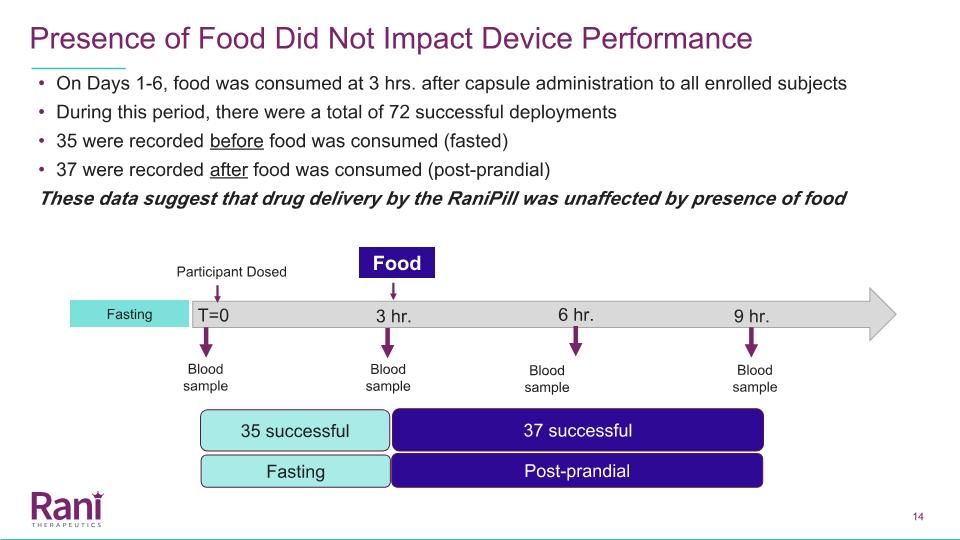
On Days 1-6, food was consumed at 3 hrs. after capsule administration to all enrolled subjects During this period, there were a total of 72 successful deployments 35 were recorded before food was consumed (fasted) 37 were recorded after food was consumed (post-prandial) These data suggest that drug delivery by the RaniPill was unaffected by presence of food Presence of Food Did Not Impact Device Performance Participant Dosed 6 hr. 9 hr. Blood sample Blood sample Blood sample Blood sample Food 3 hr. T=0 Fasting 35 successful 37 successful Fasting Post-prandial
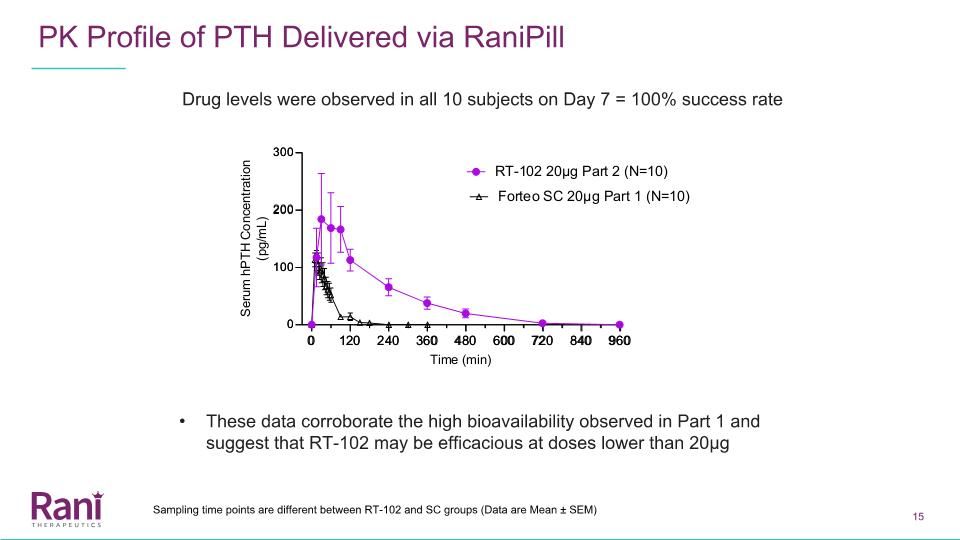
PK Profile of PTH Delivered via RaniPill Drug levels were observed in all 10 subjects on Day 7 = 100% success rate These data corroborate the high bioavailability observed in Part 1 and suggest that RT-102 may be efficacious at doses lower than 20µg Sampling time points are different between RT-102 and SC groups (Data are Mean ± SEM)
Repeat Doses of RT-102 Key Takeaways RT-102 was well tolerated with repeat dosing No SAEs were reported in the study Device remnants were eliminated without sequelae in all subjects RT-102 RaniPill delivered PTH with reliability of >90% Device performance was unaffected by presence of food Cmax comparable to SC Forteo No accumulation of PTH observed

Next Steps Talat Imran, CEO

Pre-IND meeting request Conduct 28-day GLP study Early 2023 Initiate Phase 2 Study 2H 2023 Plan to publish RT-102 Phase 1 Study results Additional 2023 prospective milestones: Initiation of a Phase 1 study of RT-111 containing an ustekinumab biosimilar Initiation of a Phase 1 study of RT-105 containing an adalimumab biosimilar Initiation of a Phase 1 study of RT-110 containing PTH for hypo-parathyroidism Next Steps and Upcoming Potential Milestones

Appendix
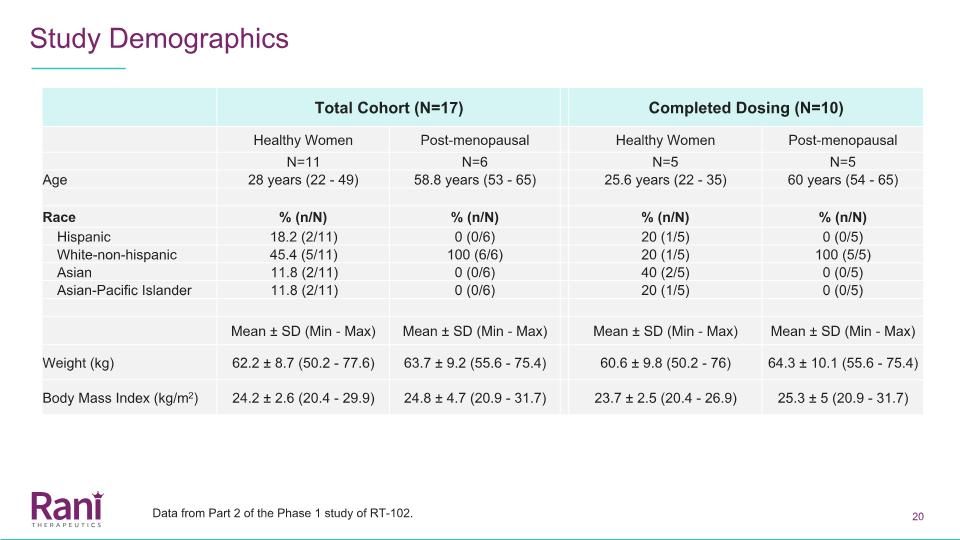
Total Cohort (N=17) Completed Dosing (N=10) Healthy Women Post-menopausal Healthy Women Post-menopausal N=11 N=6 N=5 N=5 Age 28 years (22 - 49) 58.8 years (53 - 65) 25.6 years (22 - 35) 60 years (54 - 65) Race % (n/N) % (n/N) % (n/N) % (n/N) Hispanic 18.2 (2/11) 0 (0/6) 20 (1/5) 0 (0/5) White-non-hispanic 45.4 (5/11) 100 (6/6) 20 (1/5) 100 (5/5) Asian 11.8 (2/11) 0 (0/6) 40 (2/5) 0 (0/5) Asian-Pacific Islander 11.8 (2/11) 0 (0/6) 20 (1/5) 0 (0/5) Mean ± SD (Min - Max) Mean ± SD (Min - Max) Mean ± SD (Min - Max) Mean ± SD (Min - Max) Weight (kg) 62.2 ± 8.7 (50.2 - 77.6) 63.7 ± 9.2 (55.6 - 75.4) 60.6 ± 9.8 (50.2 - 76) 64.3 ± 10.1 (55.6 - 75.4) Body Mass Index (kg/m2) 24.2 ± 2.6 (20.4 - 29.9) 24.8 ± 4.7 (20.9 - 31.7) 23.7 ± 2.5 (20.4 - 26.9) 25.3 ± 5 (20.9 - 31.7) Study Demographics Data from Part 2 of the Phase 1 study of RT-102.
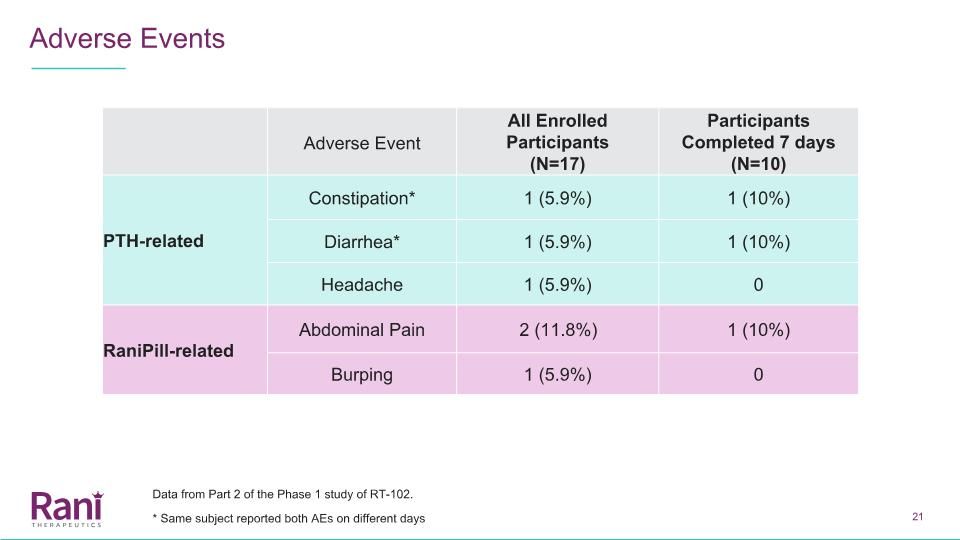
Adverse Events Adverse Event All Enrolled Participants (N=17) Participants Completed 7 days (N=10) PTH-related Constipation* 1 (5.9%) 1 (10%) Diarrhea* 1 (5.9%) 1 (10%) Headache 1 (5.9%) 0 RaniPill-related Abdominal Pain 2 (11.8%) 1 (10%) Burping 1 (5.9%) 0 * Same subject reported both AEs on different days Data from Part 2 of the Phase 1 study of RT-102.
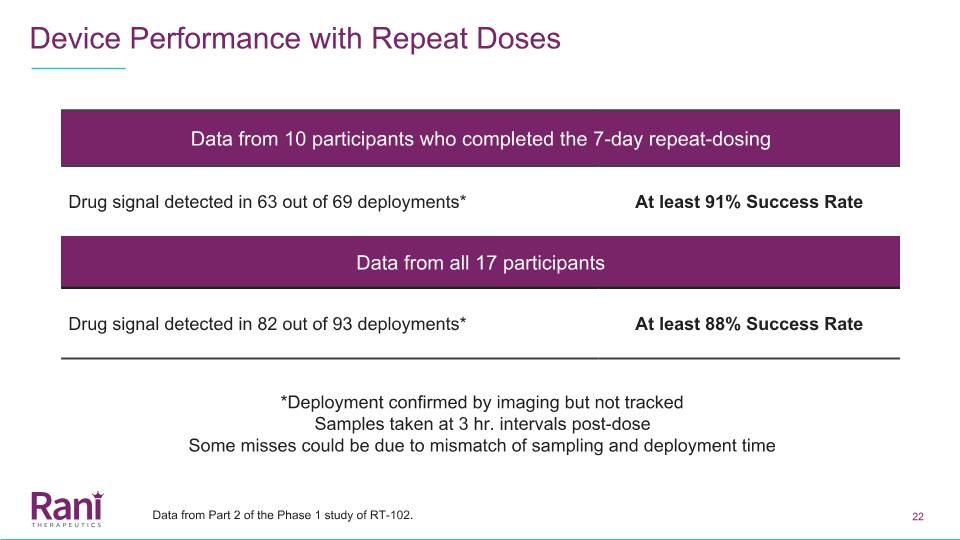
Device Performance with Repeat Doses Data from 10 participants who completed the 7-day repeat-dosing Drug signal detected in 63 out of 69 deployments* At least 91% Success Rate Data from all 17 participants Drug signal detected in 82 out of 93 deployments* At least 88% Success Rate *Deployment confirmed by imaging but not tracked Samples taken at 3 hr. intervals post-dose Some misses could be due to mismatch of sampling and deployment time Data from Part 2 of the Phase 1 study of RT-102.