| Nasdaq: MRNS @MarinusPharma Photo Credit: Kelly Crews Photography Ryan (center) Living with CDKL5 deficiency disorder AES Investor Breakfast December 5, 2022 |
| ©2022 Marinus Pharmaceuticals. All Rights Reserved I To the extent that statements contained in this presentation are not descriptions of historical facts regarding Marinus, they ar e forward - looking statements reflecting the current beliefs and expectations of management made pursuant to the safe harbor provisions of the Private Securities Litigation Reform Act of 19 95. Words such as “may”, “will”, “expect”, “anticipate”, “estimate”, “intend”, “believe”, and similar expressions (as well as other words or expressions referencing future events, co ndi tions or circumstances) are intended to identify forward - looking statements. Examples of forward - looking statements contained in this presentation include, among others, statements rega rding our commercialization plans for ZTALMY® and clinical development plans for ganaxolone and the expected timing thereof ; expected dosing in our clinical trials; the clinical development schedule and milestones; our expected timing to begin and complete enrollment in our clinical trials; the expected trial design, target patient population and endpoints for our clinical trials; interpretation of scientific basis for ganaxolone use; timing for availability and release of data; the potential safety and efficacy and therapeutic potential of ganaxolone; tim ing and expectations regarding the potential benefits ZTALMY will provide for patients and physicians , our expectations regarding the ZTALMY One program; timing and expectations regarding regulatory communications and submissions; expectations regarding our agreement with BARDA; expectations regarding our collaborations; expectations regarding the potent ial market opportunities for our product candidates, including oral ganaxolone; potential commercial alliances; and our expectations regarding the effect of the COVID - 19 pandemic on our business and clinical development plans. Forward - looking statements in this presentation involve substantial risks and uncertainties that could cause our clinical development pr ograms, future results, performance or achievements to differ significantly from those expressed or implied by the forward - looking statements. Such risks and uncertainties include, am ong others, patient and physician acceptance of ZTALMY, our ability to obtain adequate market access for ZTALMY; our ability to comply with the FDA’s requirement for additional post - ma rket studies in the required timeframes; the timing of regulatory filings, including the timing of filing the ganaxolone MAA with the EMA; the potential that regulatory authorities , i ncluding the FDA and EMA, may not grant or may delay approval for our product candidates; uncertainties and delays relating to the design, enrollment, completion, and results of clinical tri als; unanticipated costs and expenses; early clinical trials may not be indicative of the results in later clinical trials; clinical trial results may not support regulatory approval or furt her development in a specified indication or at all; actions or advice of the FDA or EMA may affect the design, initiation, timing, continuation and/or progress of clinical trials or result in the ne ed for additional clinical trials; our ability to obtain and maintain regulatory approval for our product candidates; our ability to obtain, maintain, protect and defend intellectual property for ou r product candidates; the potential negative impact of third party patents on our or our collaborators’ ability to commercialize ganaxolone; delays, interruptions or failures in the manu fac ture and supply of our product candidates; the size and growth potential of the markets for our product candidates, and our ability to service those markets; our cash and cash equiv ale nts may not be sufficient to support our operating plan for as long as anticipated; our expectations, projections and estimates regarding expenses, future revenue, capital requirements, an d the availability of and the need for additional financing; our ability to obtain additional funding to support our commercial and clinical development programs; the potential for our c oll aborators to breach our collaboration agreements or terminate the agreements in accordance with their respective terms; the effect of the COVID - 19 pandemic on our business, the med ical community, regulators and the global economy; and the availability or potential availability of alternative products or treatments for conditions targeted by us that could aff ect the availability or commercial potential of our product candidates. Marinus undertakes no obligation to update or revise any forward - looking statements. For a further description of th e risks and uncertainties that could cause actual results to differ from those expressed in these forward - looking statements, as well as risks relating to our business in general, see filin gs we have made with the Securities and Exchange Commission. You may access these documents for free by visiting EDGAR on the SEC web site at www.sec.gov. Safe Harbor Statement 2 |
| Agenda Welcome & Introduction Sasha Damouni Ellis, VP, Corporate Affairs & Investor Relations CDKL5 Deficiency Disorder: Phase 3 Open - Label Extension Alex Aimetti, Ph.D. VP, Scientific Affairs Tuberous Sclerosis Complex: DDI Study Results Ian Miller, M.D., VP, Clinical Development Second Generation Product Development Joseph Hulihan, M.D., Chief Medical Officer ZTALMY Launch Update Christy Shafer, Chief Commercial Officer ZTALMY Early Success Metrics & Key Field Insights Lisa Lejuwaan, VP, Sales ZTALMY Market Access and Patient Services Update Paul Voss, VP, Access & Policy ZTALMY Customer Engagement, Digital & AES footprint Catherine Galica, Sr. Director, Marketing Genetic Epilepsy IV Ganaxolone Commercial Strategy Kristin Rudisill, VP, Acute Care Q&A Scott Braunstein, M.D., Chairman & Chief Executive Officer 3 |
| CDKL5 Deficiency Disorder: Phase 3 Open - Label Extension Alex Aimetti, Ph.D. VP, Scientific Affairs |
| ©2022 Marinus Pharmaceuticals, Inc. All Rights Reserved I AES 2022 Marinus Abstract Highlights 5 • Dose - dependent pharmacokinetic and pharmacodynamic response following administration of IV ganaxolone in healthy volunteers • More pronounced BIS/ qEEG changes observed following IV bolus suggesting rapid brain penetration and clearance (i.e., rapid onset/offset) providing favorable properties as a potential treatment for SE • No clinically meaningful interactions between a single dose of ganaxolone and cannabidiol at steady state • Combination of ganaxolone and cannabidiol were generally well - tolerated in healthy subjects • Phase 3 TSC study will provide additional data in patients • Safety and efficacy data in patients with a minimum of 1 - year (June 2021 data cut) follow up • Ganaxolone was generally well - tolerated, with safety findings consistent with those previously reported • Data provide supportive evidence for maintenance of ganaxolone effectiveness in CDD |
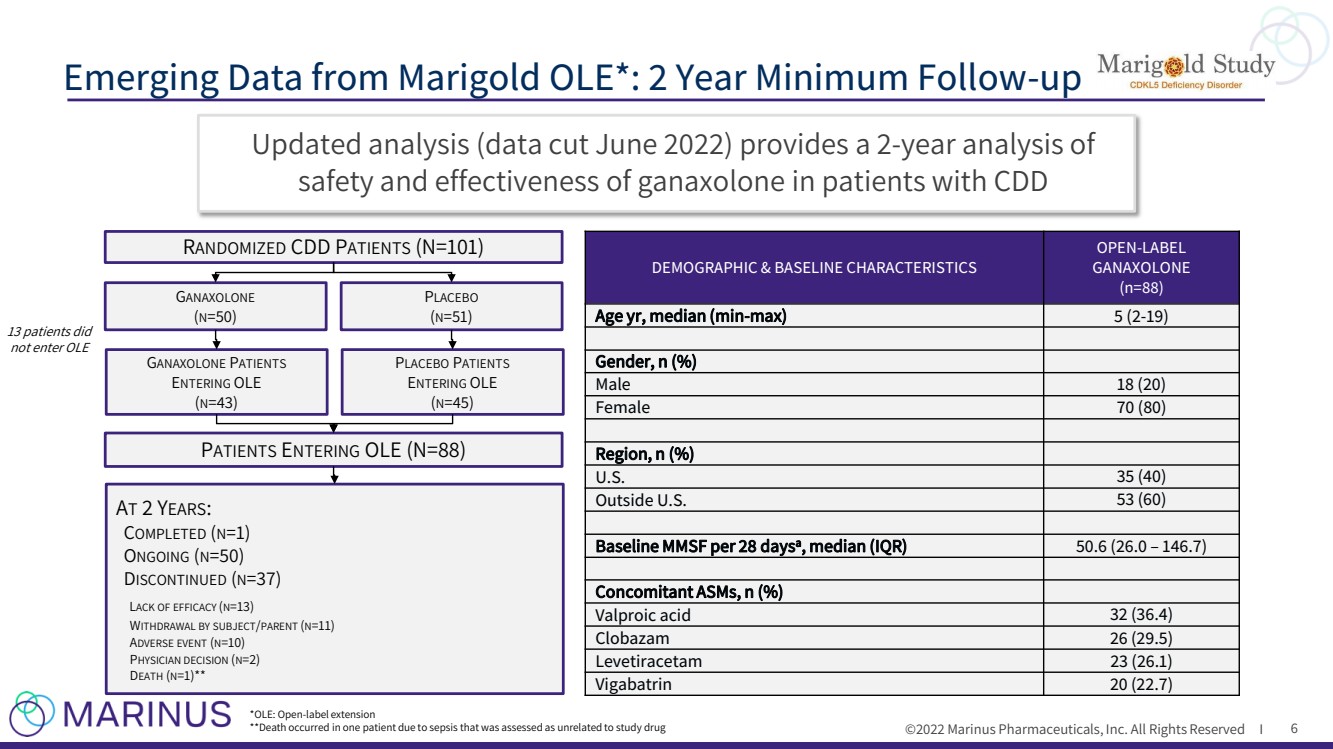
| ©2022 Marinus Pharmaceuticals, Inc. All Rights Reserved I Updated analysis (data cut June 2022) provides a 2 - year analysis of safety and effectiveness of ganaxolone in patients with CDD Emerging Data from Marigold OLE*: 2 Year Minimum Follow - up 6 DEMOGRAPHIC & BASELINE CHARACTERISTICS OPEN - LABEL GANAXOLONE (n= 88 ) Age yr , median ( min - max ) 5 ( 2 - 1 9 ) Gender, n (%) Male 1 8 ( 20 ) Female 70 ( 80 ) Region, n (%) U.S. 35 (40) Outside U.S. 53 (60) Baseline MMSF per 28 days a , median (IQR) 50.6 (26.0 – 146.7) Concomitant ASMs, n (%) Valproic acid 32 (36.4) Clobazam 26 (29.5) Levetiracetam 23 (26.1) Vigabatrin 20 (22.7) R ANDOMIZED CDD P ATIENTS (N=101) G ANAXOLONE ( N =50) P LACEBO ( N =51) P ATIENTS E NTERING OLE (N=88) G ANAXOLONE P ATIENTS E NTERING OLE ( N =43) P LACEBO P ATIENTS E NTERING OLE ( N =45) 13 patients did not enter OLE A T 2 Y EARS : C OMPLETED ( N =1) O NGOING ( N =50) D ISCONTINUED ( N =37) L ACK OF EFFICACY ( N =13) W ITHDRAWAL BY SUBJECT / PARENT ( N =11) A DVERSE EVENT ( N =10) P HYSICIAN DECISION ( N =2) D EATH ( N =1)** *OLE: Open - label extension **Death occurred in one patient due to sepsis that was assessed as unrelated to study drug |
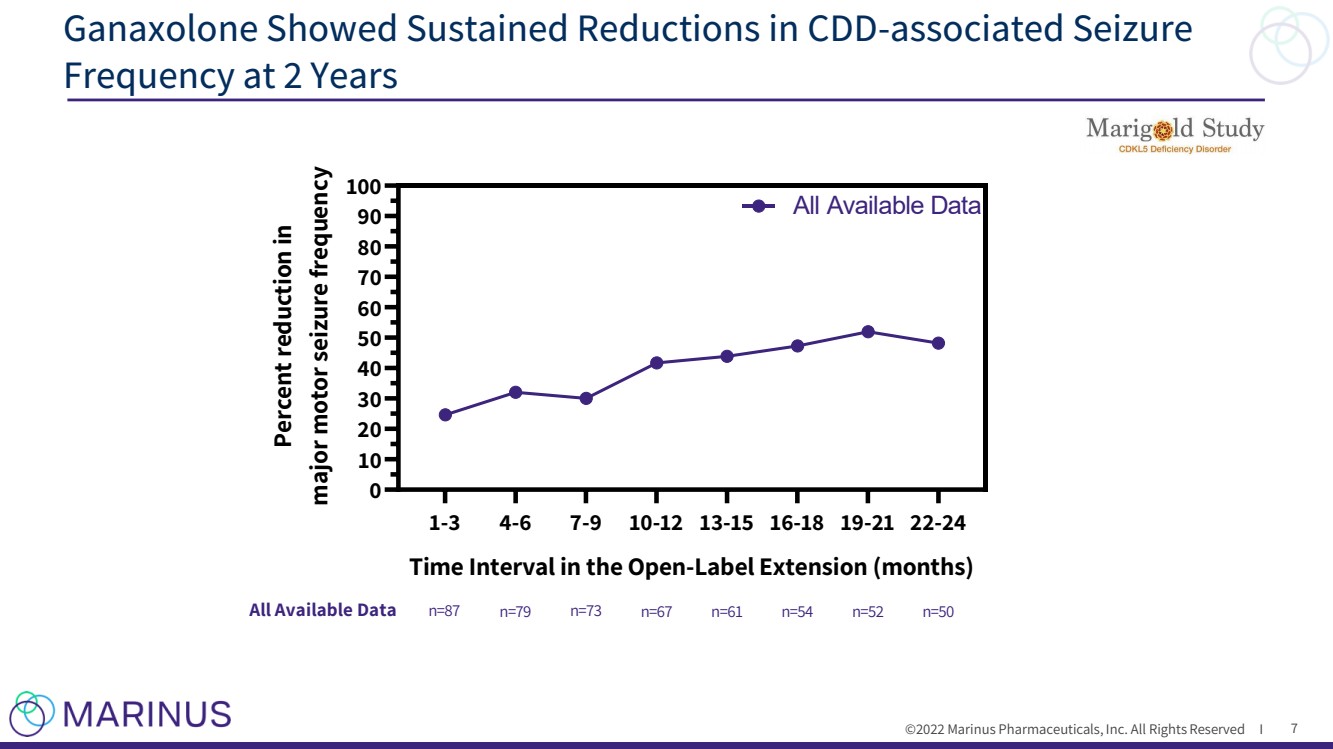
| ©2022 Marinus Pharmaceuticals, Inc. All Rights Reserved I Ganaxolone Showed Sustained Reductions in CDD - associated Seizure Frequency at 2 Years 7 1-3 4-6 7-9 10-12 13-15 16-18 19-21 22-24 0 10 20 30 40 50 60 70 80 90 100 Time Interval in the Open-Label Extension (months) Percent reduction in major motor seizure frequency All Available Data LOCF n=87 All Available Data n=79 n=73 n=67 LOCF n=61 n=54 n=52 n=50 n=87 n=87 n=87 n=87 n=87 n=87 n=87 n=87 |
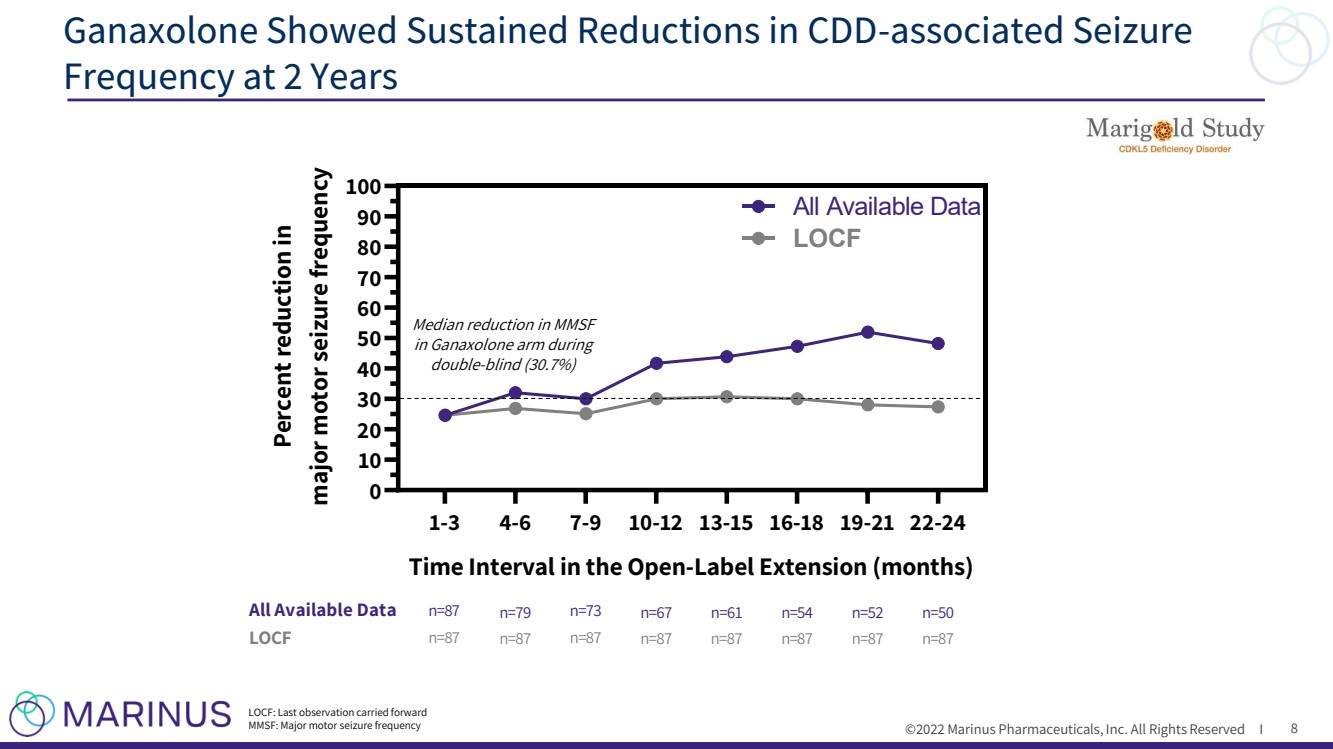
| ©2022 Marinus Pharmaceuticals, Inc. All Rights Reserved I 1-3 4-6 7-9 10-12 13-15 16-18 19-21 22-24 0 10 20 30 40 50 60 70 80 90 100 Time Interval in the Open-Label Extension (months) Percent reduction in major motor seizure frequency All Available Data LOCF n=87 All Available Data n=79 n=73 n=67 LOCF n=61 n=54 n=52 n=50 n=87 n=87 n=87 n=87 n=87 n=87 n=87 n=87 Ganaxolone Showed Sustained Reductions in CDD - associated Seizure Frequency at 2 Years 8 Median reduction in MMSF in Ganaxolone arm during double - blind (30.7%) LOCF: Last observation carried forward MMSF: Major motor seizure frequency |
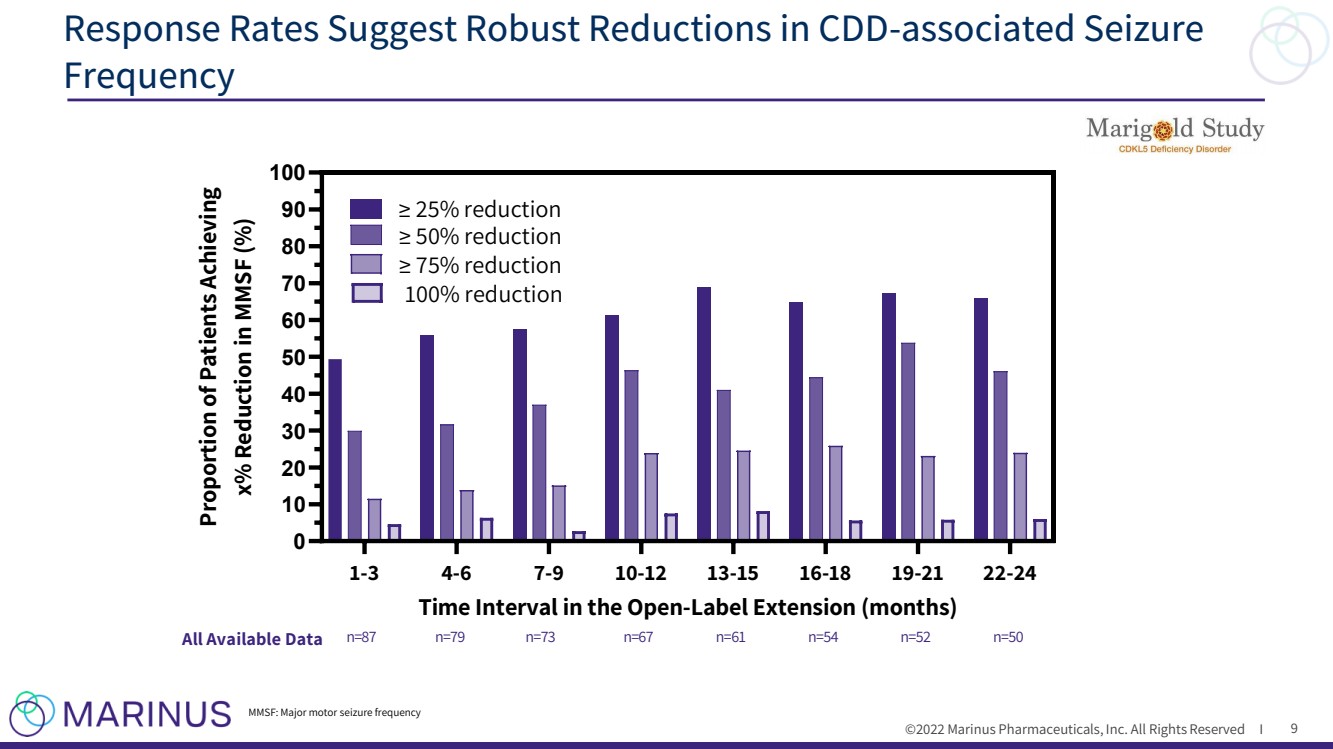
| ©2022 Marinus Pharmaceuticals, Inc. All Rights Reserved I Response Rates Suggest Robust Reductions in CDD - associated Seizure Frequency 9 1-3 4-6 7-9 10-12 13-15 16-18 19-21 22-24 0 10 20 30 40 50 60 70 80 90 100 Time Interval in the Open-Label Extension (months) Proportion of Patients Achieving x% Reduction in MMSF (%) ≥ 25% reduction ≥ 50% reduction ≥ 75% reduction n=87 n=79 n=73 n=67 n=61 n=54 n=52 n=50 100% reduction All Available Data MMSF: Major motor seizure frequency |
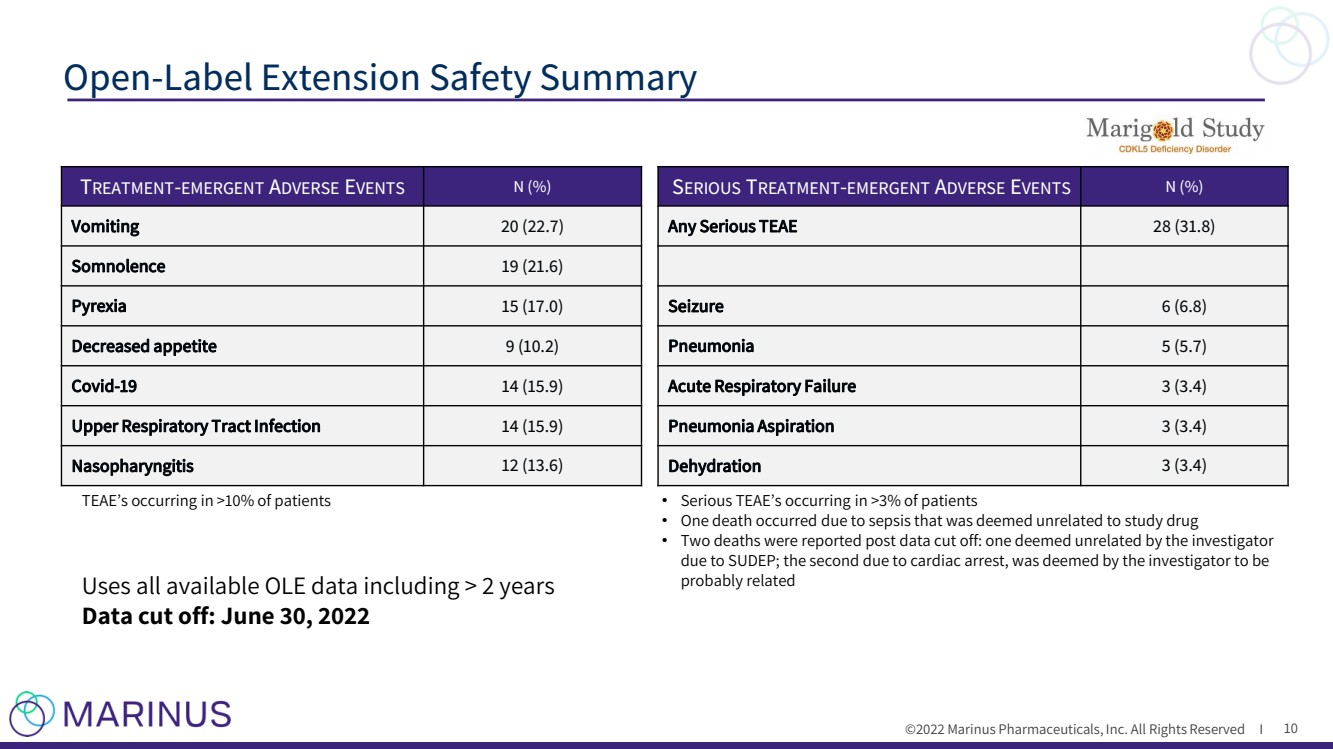
| ©2022 Marinus Pharmaceuticals, Inc. All Rights Reserved I Open - Label Extension Safety Summary 10 T REATMENT - EMERGENT A DVERSE E VENTS N (%) Vomiting 20 (22.7) Somnolence 19 (21.6) Pyrexia 15 (17.0) Decreased appetite 9 (10.2) Covid - 19 14 (15.9) Upper Respiratory Tract Infection 14 (15.9) Nasopharyngitis 12 (13.6) TEAE’s occurring in >10% of patients S ERIOUS T REATMENT - EMERGENT A DVERSE E VENTS N (%) Any Serious TEAE 28 (31.8) Seizure 6 (6.8) Pneumonia 5 (5.7) Acute Respiratory Failure 3 (3.4) Pneumonia Aspiration 3 (3.4) Dehydration 3 (3.4) • Serious TEAE’s occurring in >3% of patients • One death occurred due to sepsis that was deemed unrelated to study drug • Two deaths were reported post data cut off: one deemed unrelated by the investigator due to SUDEP; the second due to cardiac arrest, was deemed by the investigator to be probably related Uses all available OLE data including > 2 years Data cut off: June 30, 2022 |
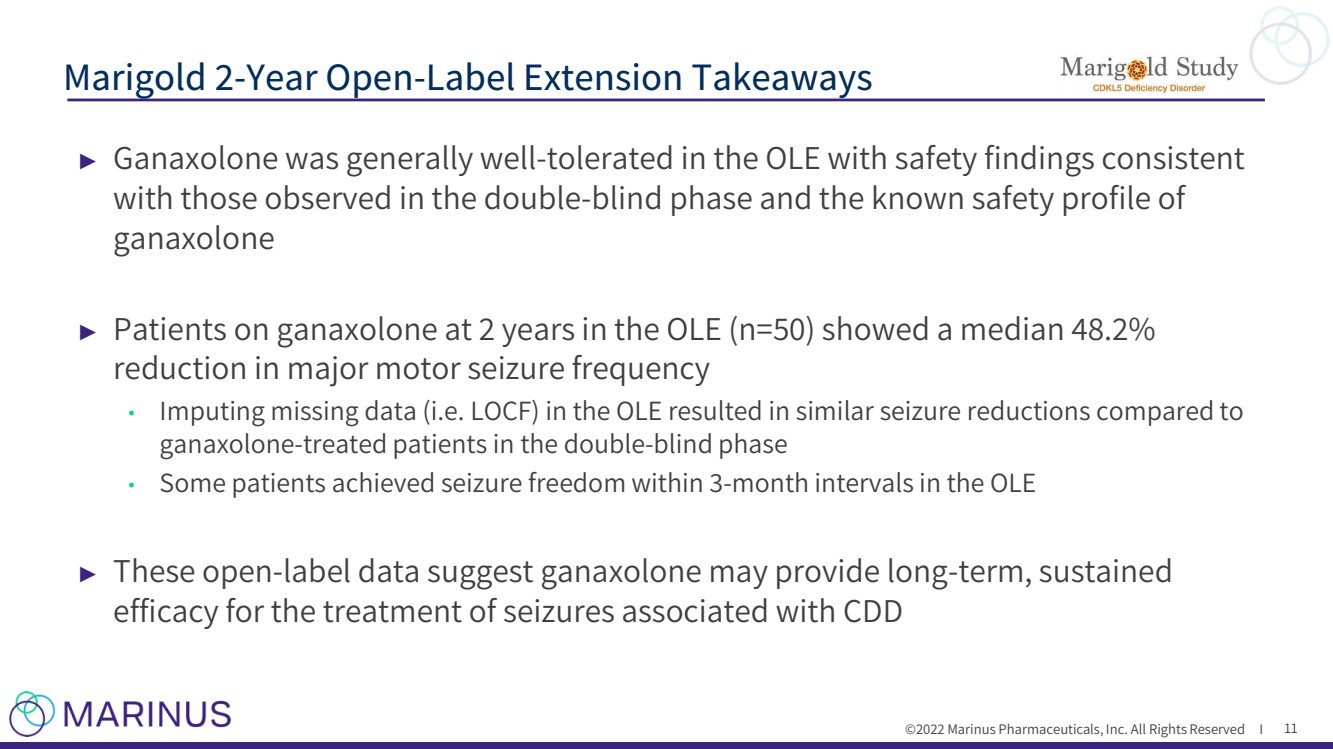
| ©2022 Marinus Pharmaceuticals, Inc. All Rights Reserved I ► Ganaxolone was generally well - tolerated in the OLE with safety findings consistent with those observed in the double - blind phase and the known safety profile of ganaxolone ► Patients on ganaxolone at 2 years in the OLE (n=50) showed a median 48.2% reduction in major motor seizure frequency • Imputing missing data ( i.e. LOCF) in the OLE resulted in similar seizure reductions compared to ganaxolone - treated patients in the double - blind phase • Some patients achieved seizure freedom within 3 - month intervals in the OLE ► These open - label data suggest ganaxolone may provide long - term, sustained efficacy for the treatment of seizures associated with CDD Marigold 2 - Year Open - Label Extension Takeaways 11 |
| Tuberous Sclerosis Complex DDI Study & Phase 3 TrustTSC Trial Ian Miller, M.D., VP, Clinical Development 12 |
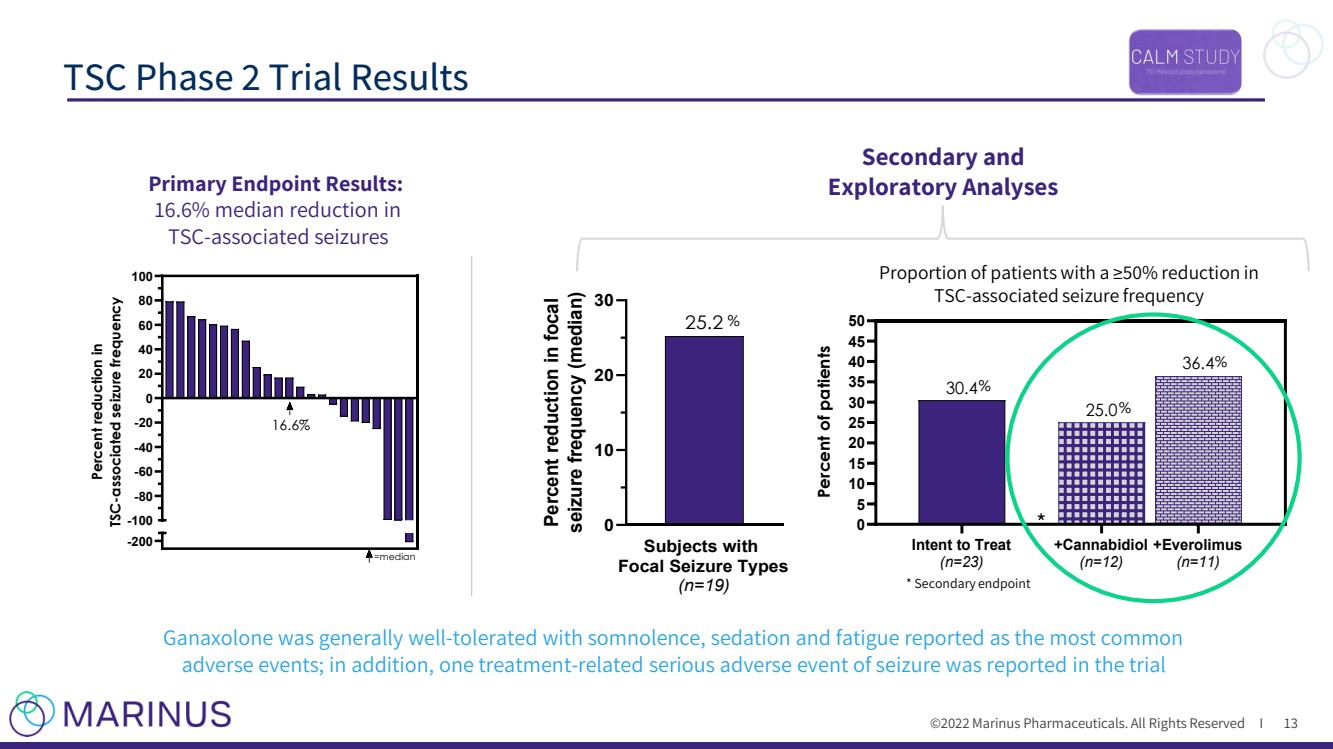
| ©2022 Marinus Pharmaceuticals. All Rights Reserved I TSC Phase 2 Trial Results 13 * -200 -100 -80 -60 -40 -20 0 20 40 60 80 100 Percent reduction in TSC-associated seizure frequency =median 16.6% Secondary and Exploratory Analyses Primary Endpoint Results: 16.6% median reduction in TSC - associated seizures Ganaxolone was generally well - tolerated with somnolence, sedation and fatigue reported as the most common adverse events; in addition, one treatment - related serious adverse event of seizure was reported in the trial * Secondary endpoint Proportion of patients with a ≥50% reduction in TSC - associated seizure frequency Intent to Treat (n=23) +Cannabidiol (n=12) +Everolimus (n=11) 0 5 10 15 20 25 30 35 40 45 50 36.4 25.0 30.4 Percent of patients % % % Subjects with Focal Seizure Types (n=19) 0 10 20 30 25.2 Percent reduction in focal seizure frequency (median) % |
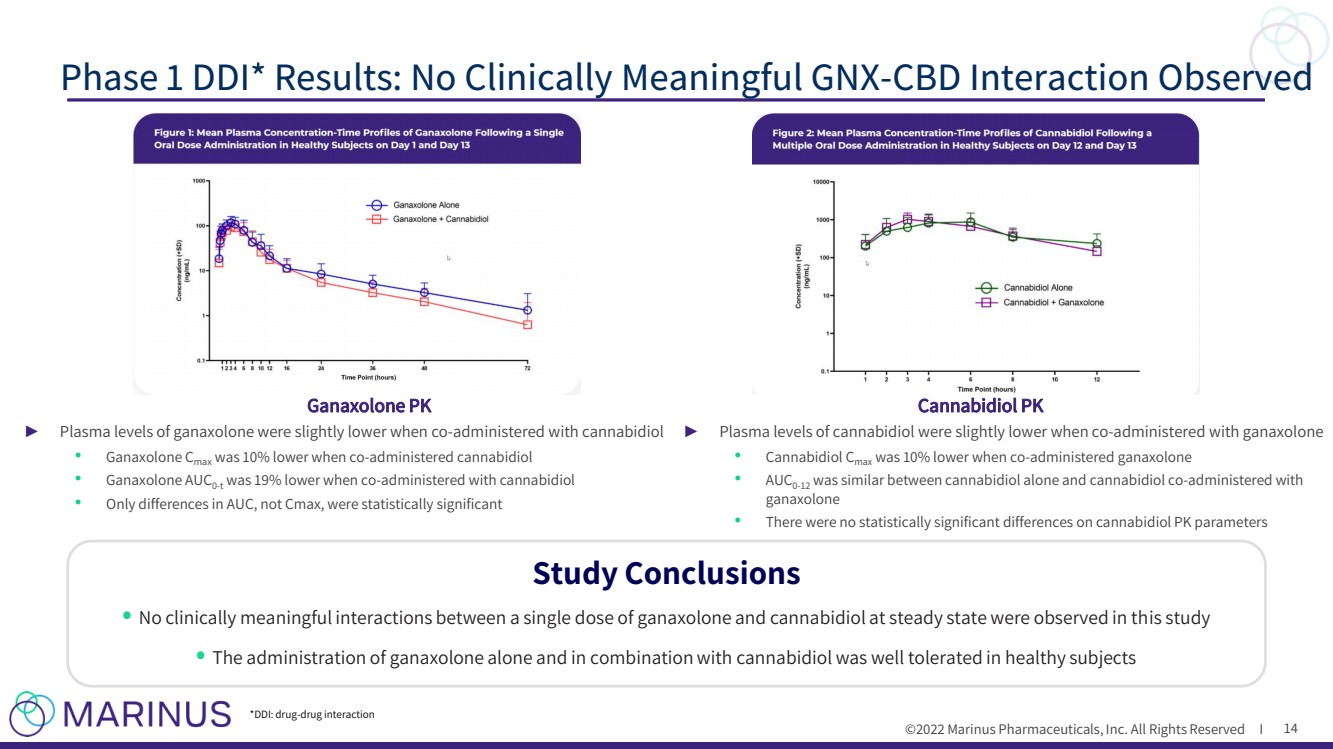
| ©2022 Marinus Pharmaceuticals, Inc. All Rights Reserved I Cannabidiol PK Ganaxolone PK ► Plasma levels of ganaxolone were slightly lower when co - administered with cannabidiol • Ganaxolone C max was 10% lower when co - administered cannabidiol • Ganaxolone AUC 0 - t was 19% lower when co - administered with cannabidiol • Only differences in AUC, not Cmax , were statistically significant ► Plasma levels of cannabidiol were slightly lower when co - administered with ganaxolone • Cannabidiol C max was 10% lower when co - administered ganaxolone • AUC 0 - 12 was similar between cannabidiol alone and cannabidiol co - administered with ganaxolone • There were no statistically significant differences on cannabidiol PK parameters Phase 1 DDI* Results: No Clinically Meaningful GNX - CBD Interaction Observed 14 Study Conclusions • No clinically meaningful interactions between a single dose of ganaxolone and cannabidiol at steady state were observed in th is study • The administration of ganaxolone alone and in combination with cannabidiol was well tolerated in healthy subjects *DDI: drug - drug interaction |
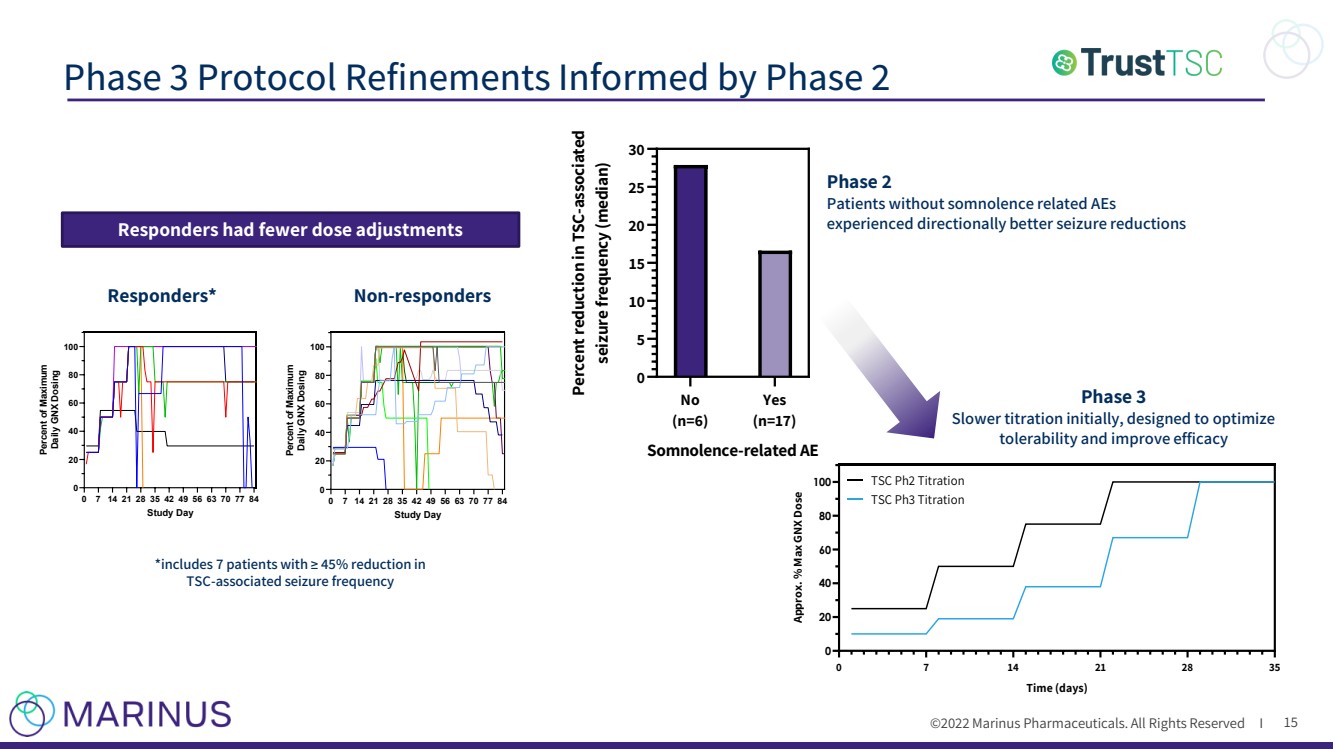
| ©2022 Marinus Pharmaceuticals. All Rights Reserved I Phase 3 Protocol Refinements Informed by Phase 2 15 Phase 3 Slower titration initially, designed to optimize tolerability and improve efficacy 0 7 14 21 28 35 0 20 40 60 80 100 Time (days) Approx. % Max GNX Dose TSC Ph2 Titration TSC Ph3 Titration No (n=6) Yes (n=17) 0 5 10 15 20 25 30 Somnolence-related AE Percent reduction in TSC-associated seizure frequency (median) Phase 2 Patients without somnolence related AEs experienced directionally better seizure reductions Responders had fewer dose adjustments Responders* Non - responders *includes 7 patients with ≥ 45% reduction in TSC - associated seizure frequency 0 7 14 21 28 35 42 49 56 63 70 77 84 0 20 40 60 80 100 Study Day Percent of Maximum Daily GNX Dosing 001001 001002 001004 001005 004002 004003 005003 008002 0 7 14 21 28 35 42 49 56 63 70 77 84 0 20 40 60 80 100 Study Day Percent of Maximum Daily GNX Dosing 008001 007001 006002 006001 005004 005002 005001 004005 004004 002004 002003 002002 002001 001006 001003 |
| ©2022 Marinus Pharmaceuticals, Inc. All Rights Reserved I Second Generation Product Development Joseph Hulihan, M.D., Chief Medical Officer |
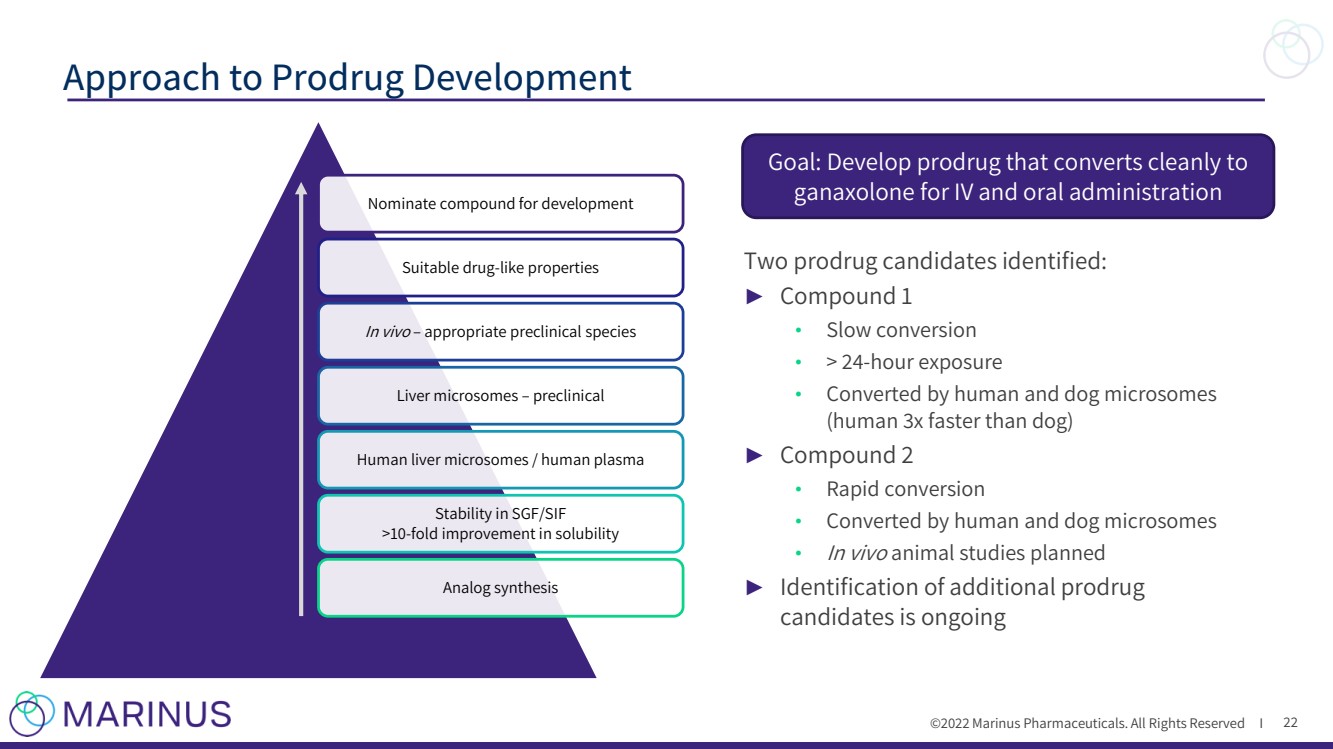
| ©2022 Marinus Pharmaceuticals, Inc. All Rights Reserved I ► Reformulation • Goals – Consistent dose - exposure relationship – Ability to individualize dosing – Dose once - or twice - daily – Reduce peak/trough variability • Two candidates selected for clinical assessment ► Prodrug • Goal is to develop oral and IV prodrug formulations – Oral prodrug: improve bioavailability and provide sustained delivery of ganaxolone (slow conversion) – IV prodrug: improve solubility and allow delivery of higher doses (rapid conversion) • A lead candidate has been identified for each of the oral and IV prodrug development programs Approaches to Second Generation Ganaxolone Development 17 |
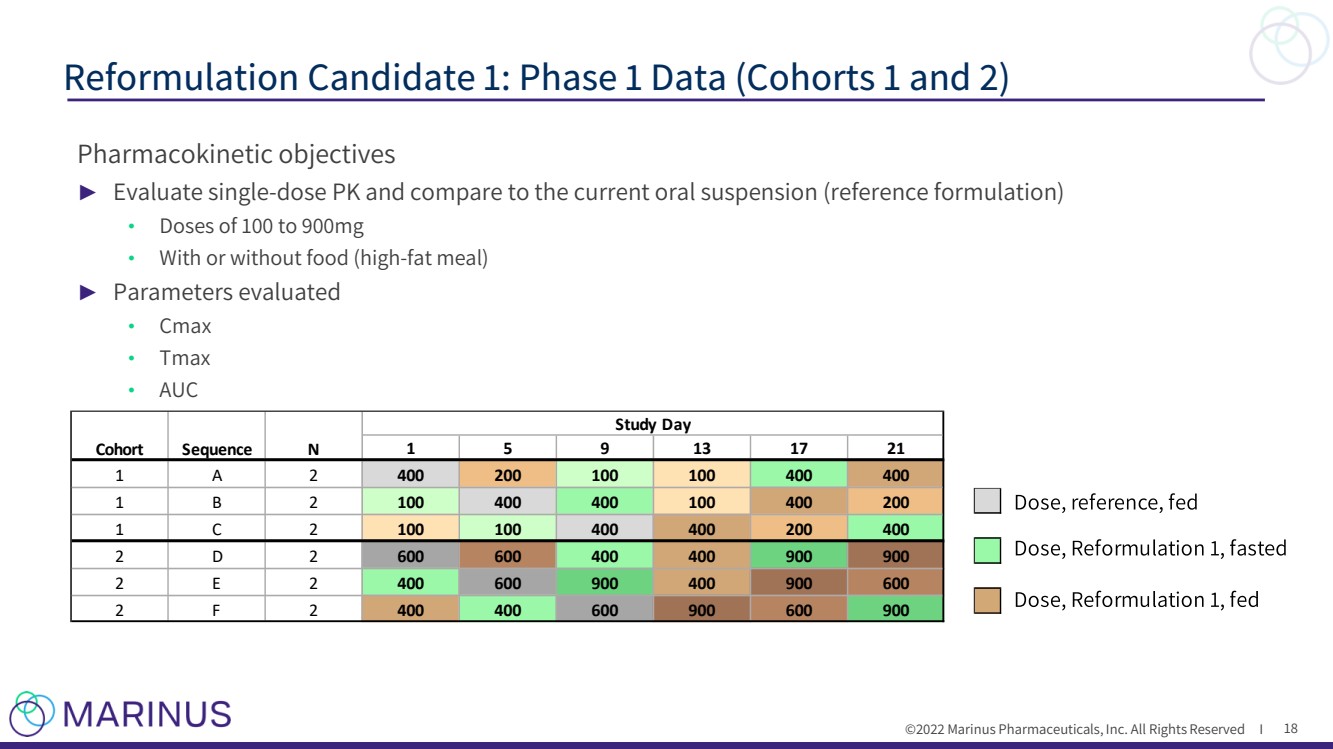
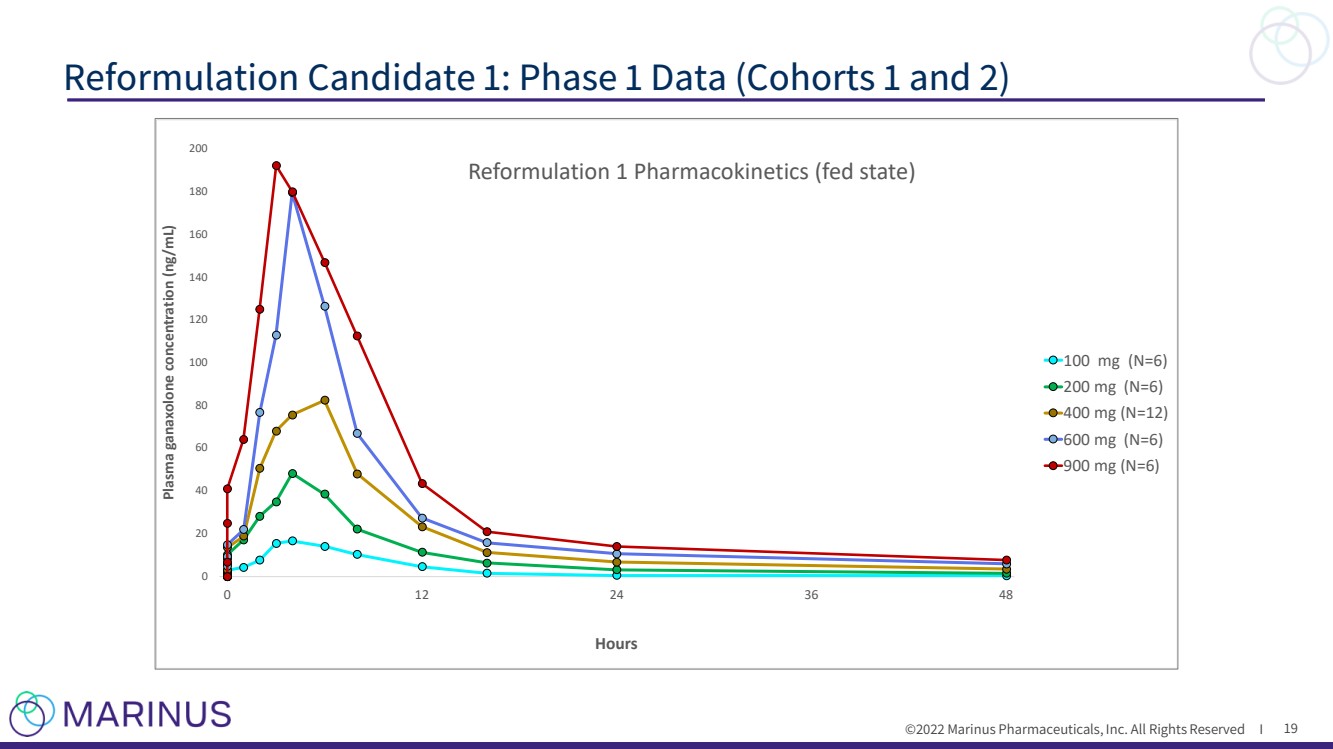
| ©2022 Marinus Pharmaceuticals, Inc. All Rights Reserved I Pharmacokinetic objectives ► Evaluate single - dose PK and compare to the current oral suspension (reference formulation) • Doses of 100 to 900mg • With or without food (high - fat meal) ► Parameters evaluated • Cmax • Tmax • AUC Reformulation Candidate 1: Phase 1 Data (Cohorts 1 and 2) 18 1 5 9 1 3 1 7 2 1 1 A 2 4 0 0 2 0 0 1 0 0 1 0 0 4 0 0 4 0 0 1 B 2 1 0 0 4 0 0 4 0 0 1 0 0 4 0 0 2 0 0 1 C 2 1 0 0 1 0 0 4 0 0 4 0 0 2 0 0 4 0 0 2 D 2 6 0 0 6 0 0 4 0 0 4 0 0 9 0 0 9 0 0 2 E 2 4 0 0 6 0 0 9 0 0 4 0 0 9 0 0 6 0 0 2 F 2 4 0 0 4 0 0 6 0 0 9 0 0 6 0 0 9 0 0 S t u d y D a y S e q u e n c e C o h o r t N |
| ©2022 Marinus Pharmaceuticals, Inc. All Rights Reserved I Reformulation Candidate 1: Phase 1 Data (Cohorts 1 and 2) 19 0 2 0 4 0 6 0 8 0 1 0 0 1 2 0 1 4 0 1 6 0 1 8 0 2 0 0 0 1 2 2 4 3 6 4 8 P l a s m a g a n a x o l o n e c o n c e n t r a t i o n ( n g / m L ) H o u r s R e f o r m u l a t i o n 1 P h a r m a c o k i n e t i c s ( f e d s t a t e ) 1 0 0 m g ( N = 6 ) 2 0 0 m g ( N = 6 ) 4 0 0 m g ( N = 1 2 ) 6 0 0 m g ( N = 6 ) 9 0 0 m g ( N = 6 ) |
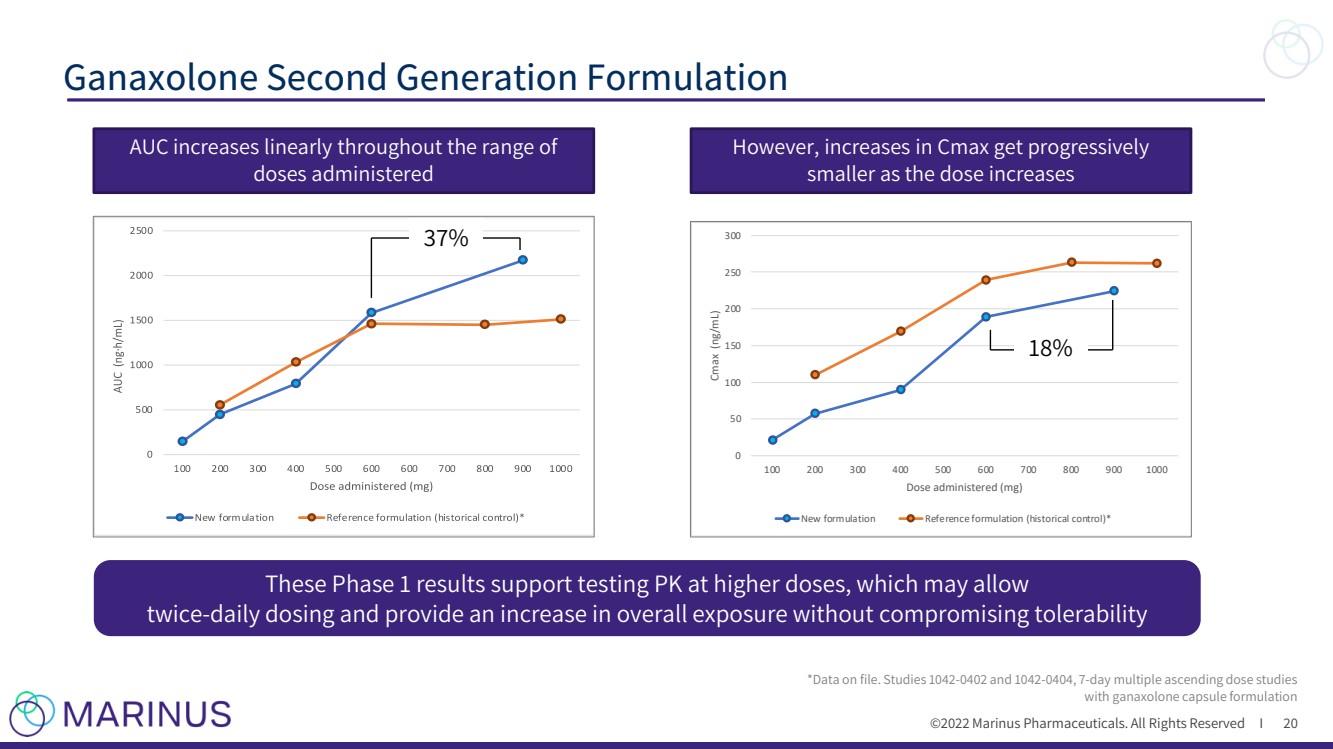
| ©2022 Marinus Pharmaceuticals. All Rights Reserved I Ganaxolone Second Generation Formulation 20 However, increases in Cmax get progressively smaller as the dose increases AUC increases linearly throughout the range of doses administered These Phase 1 results support testing PK at higher doses, which may allow twice - daily dosing and provide an increase in overall exposure without compromising tolerability 0 5 0 1 0 0 1 5 0 2 0 0 2 5 0 3 0 0 1 0 0 2 0 0 3 0 0 4 0 0 5 0 0 6 0 0 7 0 0 8 0 0 9 0 0 1 0 0 0 C m a x ( n g / m L ) D o s e a d m i n i s t e r e d ( m g ) N e w f o r m u l a t i o n R e f e r e n c e f o r m u l a t i o n ( h i s t o r i c a l c o n t r o l ) * *Data on file. Studies 1042 - 0402 and 1042 - 0404, 7 - day multiple ascending dose studies with ganaxolone capsule formulation 37% 18% |
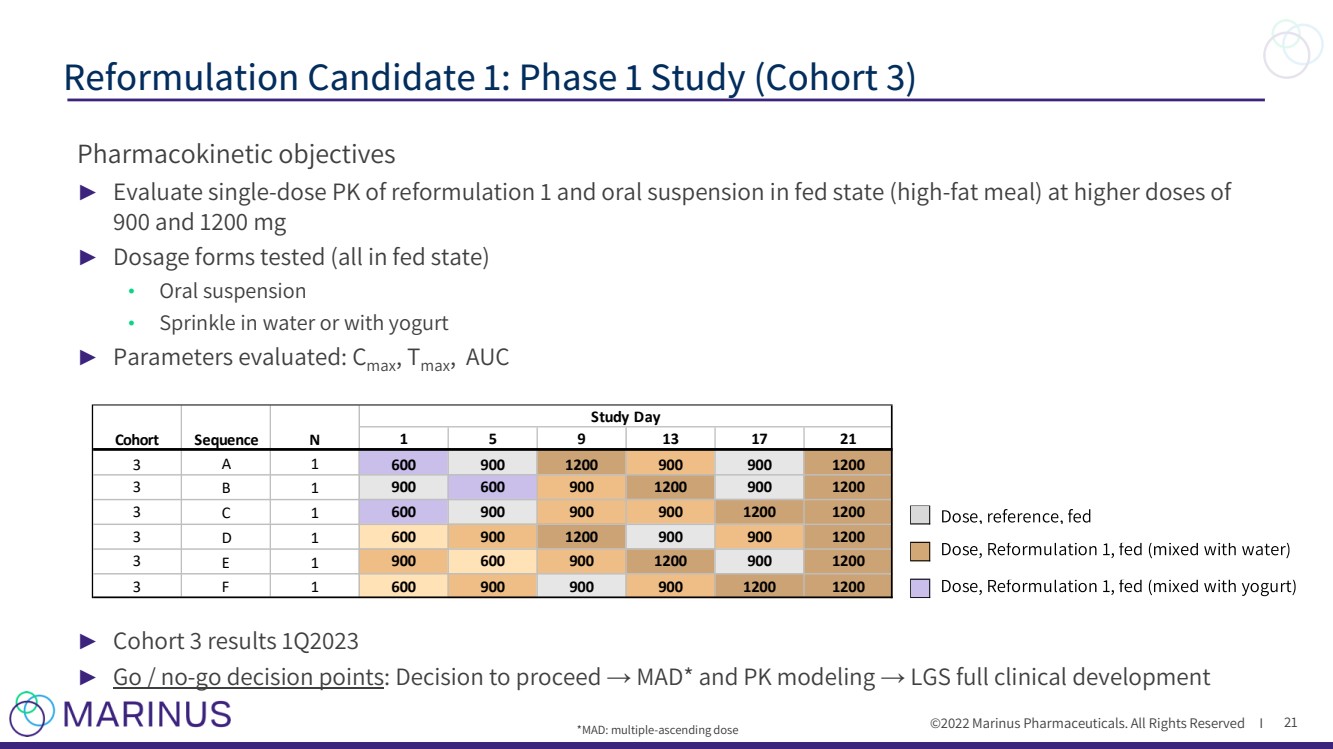
| ©2022 Marinus Pharmaceuticals. All Rights Reserved I Pharmacokinetic objectives ► Evaluate single - dose PK of reformulation 1 and oral suspension in fed state (high - fat meal) at higher doses of 900 and 1200 mg ► Dosage forms tested (all in fed state) • Oral suspension • Sprinkle in water or with yogurt ► Parameters evaluated: C max , T max , AUC ► Cohort 3 results 1Q2023 ► Go / no - go decision points : Decision to proceed → MAD* and PK modeling → LGS full clinical development Reformulation Candidate 1: Phase 1 Study (Cohort 3) 21 1 5 9 1 3 1 7 2 1 3 A 1 6 0 0 9 0 0 1 2 0 0 9 0 0 9 0 0 1 2 0 0 3 B 1 9 0 0 6 0 0 9 0 0 1 2 0 0 9 0 0 1 2 0 0 3 C 1 6 0 0 9 0 0 9 0 0 9 0 0 1 2 0 0 1 2 0 0 3 D 1 6 0 0 9 0 0 1 2 0 0 9 0 0 9 0 0 1 2 0 0 3 E 1 9 0 0 6 0 0 9 0 0 1 2 0 0 9 0 0 1 2 0 0 3 F 1 6 0 0 9 0 0 9 0 0 9 0 0 1 2 0 0 1 2 0 0 C o h o r t N S e q u e n c e S t u d y D a y *MAD: multiple - ascending dose |
| ©2022 Marinus Pharmaceuticals. All Rights Reserved I Two prodrug candidates identified: ► Compound 1 • Slow conversion • > 24 - hour exposure • Converted by human and dog microsomes (human 3x faster than dog) ► Compound 2 • Rapid conversion • Converted by human and dog microsomes • In vivo animal studies planned ► Identification of additional prodrug candidates is ongoing Approach to Prodrug Development 22 Goal: Develop prodrug that converts cleanly to ganaxolone for IV and oral administration Nominate compound for development Suitable drug - like properties In vivo – appropriate preclinical species Liver microsomes – preclinical Human liver microsomes / human plasma Stability in SGF/SIF >10 - fold improvement in solubility Analog synthesis |
| Not for promotional use ZTALMY ® (ganaxolone) oral suspension CV Launch Update Christy Shafer, Chief Commercial Officer |
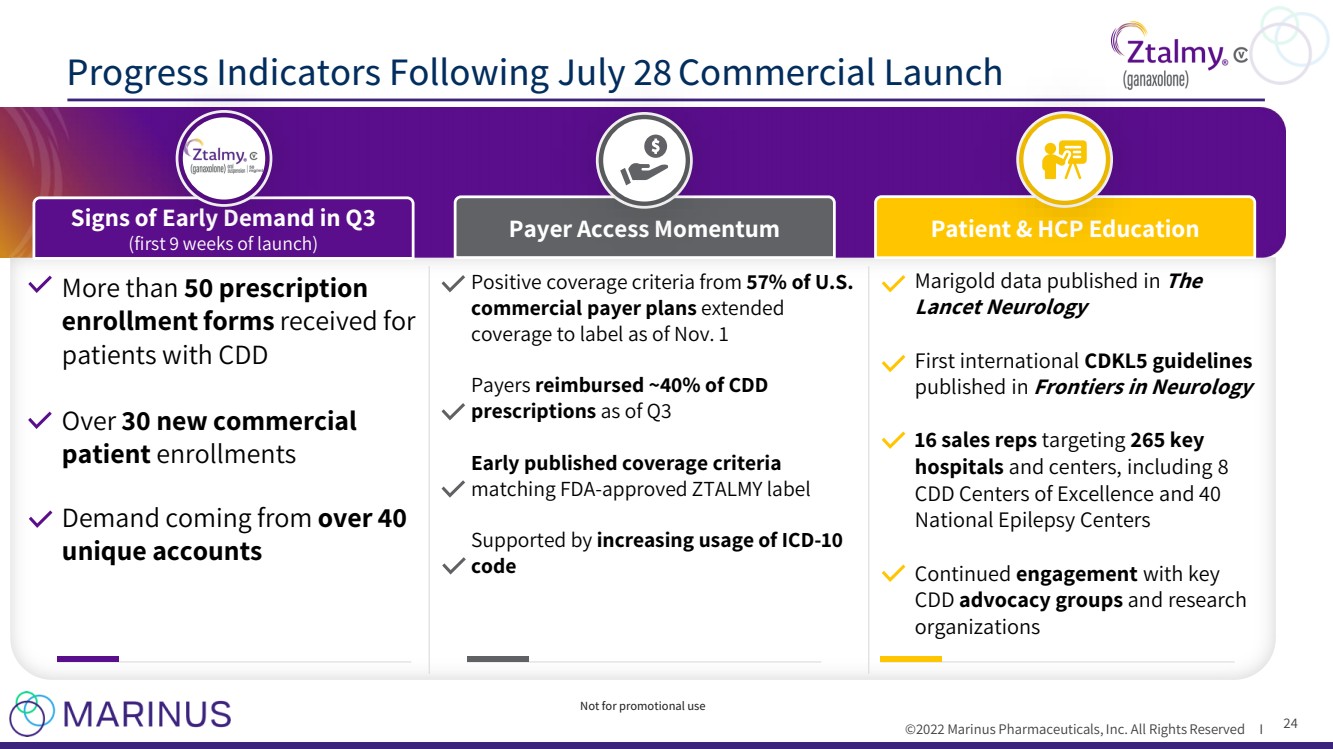
| ©2022 Marinus Pharmaceuticals, Inc. All Rights Reserved I Progress Indicators Following July 28 Commercial Launch 24 More than 50 prescription enrollment forms received for patients with CDD Over 30 new commercial patient enrollments Demand coming from over 40 unique accounts Positive coverage criteria from 57% of U.S. commercial payer plans extended coverage to label as of Nov. 1 Payers reimbursed ~40% of CDD prescriptions as of Q3 Early published coverage criteria matching FDA - approved ZTALMY label Supported by increasing usage of ICD - 10 code Marigold data published in The Lancet Neurology First international CDKL5 guidelines published in Frontiers in Neurology 16 sales reps targeting 265 key hospitals and centers, including 8 CDD Centers of Excellence and 40 National Epilepsy Centers Continued engagement with key CDD advocacy groups and research organizations Signs of Early Demand in Q3 (first 9 weeks of launch) Payer Access Momentum Patient & HCP Education Not for promotional use |
| Not for promotional use ZTALMY Early Success Metrics & Key Field Insights Lisa Lejuwaan, VP, Sales |
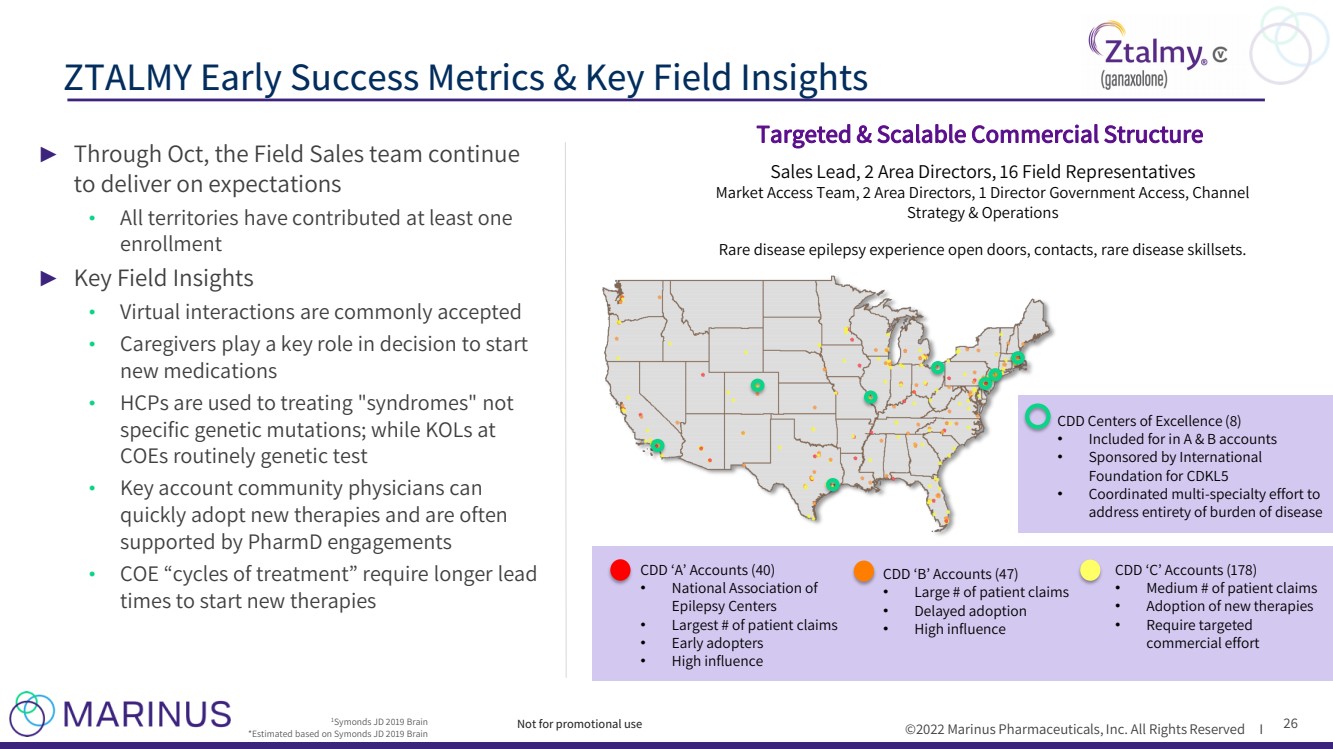
| ©2022 Marinus Pharmaceuticals, Inc. All Rights Reserved I ZTALMY Early Success Metrics & Key Field Insights 26 1 Symonds JD 2019 Brain *Estimated based on Symonds JD 2019 Brain CDD ‘A’ Accounts (40) • National Association of Epilepsy Centers • Largest # of patient claims • Early adopters • High influence CDD ‘B’ Accounts (47) • Large # of patient claims • Delayed adoption • High influence CDD ‘C’ Accounts (178) • Medium # of patient claims • Adoption of new therapies • Require targeted commercial effort CDD Centers of Excellence (8) • Included for in A & B accounts • Sponsored by International Foundation for CDKL5 • Coordinated multi - specialty effort to address entirety of burden of disease Sales Lead, 2 Area Directors, 16 Field Representatives Market Access Team, 2 Area Directors, 1 Director Government Access, Channel Strategy & Operations Rare disease epilepsy experience open doors, contacts, rare disease skillsets. Targeted & Scalable Commercial Structure Not for promotional use ► Through Oct, the Field Sales team continue to deliver on expectations • All territories have contributed at least one enrollment ► Key Field Insights • Virtual interactions are commonly accepted • Caregivers play a key role in decision to start new medications • HCPs are used to treating "syndromes" not specific genetic mutations; while KOLs at COEs routinely genetic test • Key account community physicians can quickly adopt new therapies and are often supported by PharmD engagements • COE “cycles of treatment” require longer lead times to start new therapies |
| Not for promotional use ZTALMY Market Access Patient Services Update Paul Voss, VP, Access & Policy |
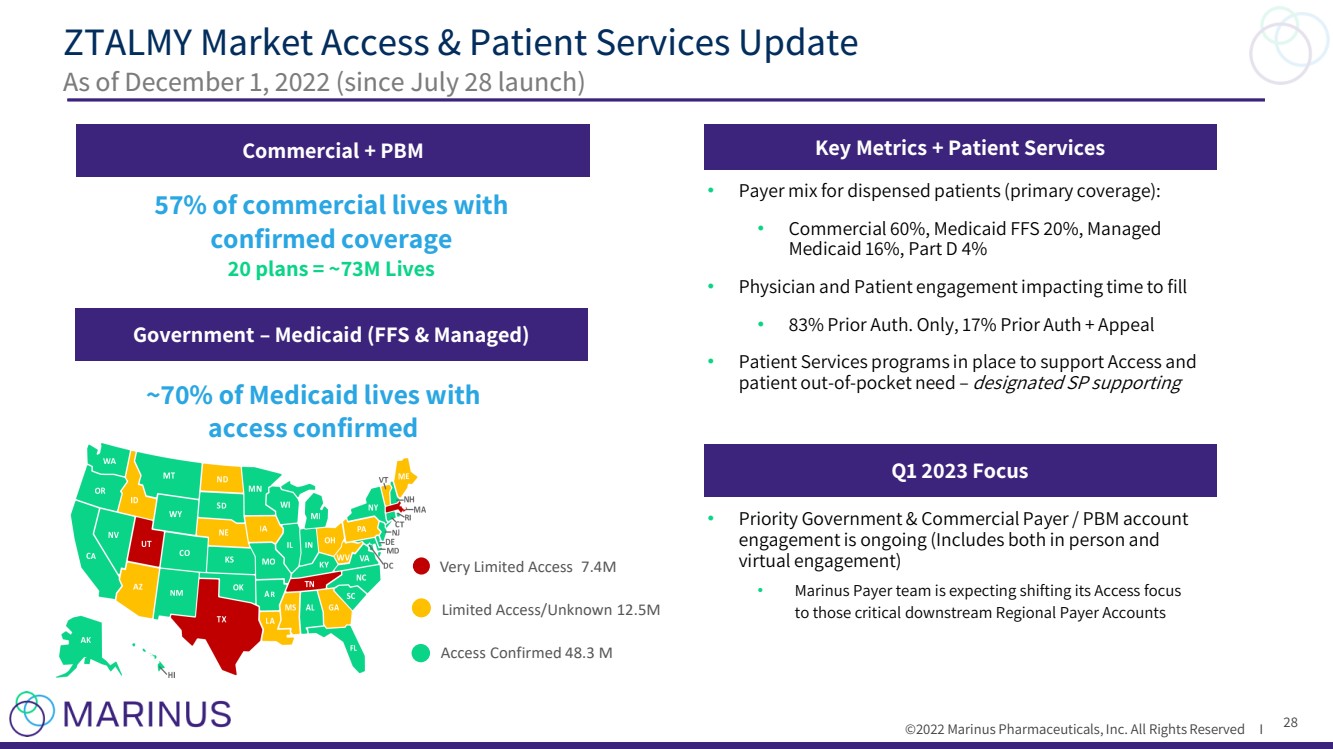
| ©2022 Marinus Pharmaceuticals, Inc. All Rights Reserved I ZTALMY Market Access & Patient Services Update As of December 1, 2022 (since July 28 launch) Commercial + PBM Government – Medicaid (FFS & Managed) 57% of commercial lives with confirmed coverage 20 plans = ~73M Lives Very Limited Access 7.4M Limited Access/Unknown 12.5M Access Confirmed 48.3 M Key Metrics + Patient Services • Payer mix for dispensed patients (primary coverage): • Commercial 60%, Medicaid FFS 20%, Managed Medicaid 16%, Part D 4% • Physician and Patient engagement impacting time to fill • 83% Prior Auth. Only, 17% Prior Auth + Appeal • Patient Services programs in place to support Access and patient out - of - pocket need – designated SP supporting Q1 2023 Focus • Priority Government & Commercial Payer / PBM account engagement is ongoing (Includes both in person and virtual engagement) • Marinus Payer team is expecting shifting its Access focus to those critical downstream Regional Payer Accounts ~70% of Medicaid lives with access confirmed 28 |
| Not for promotional use ZTALMY Customer Engagement, Digital & AES Footprint Catherine Galica, Sr. Director, Marketing Genetic Epilepsy |
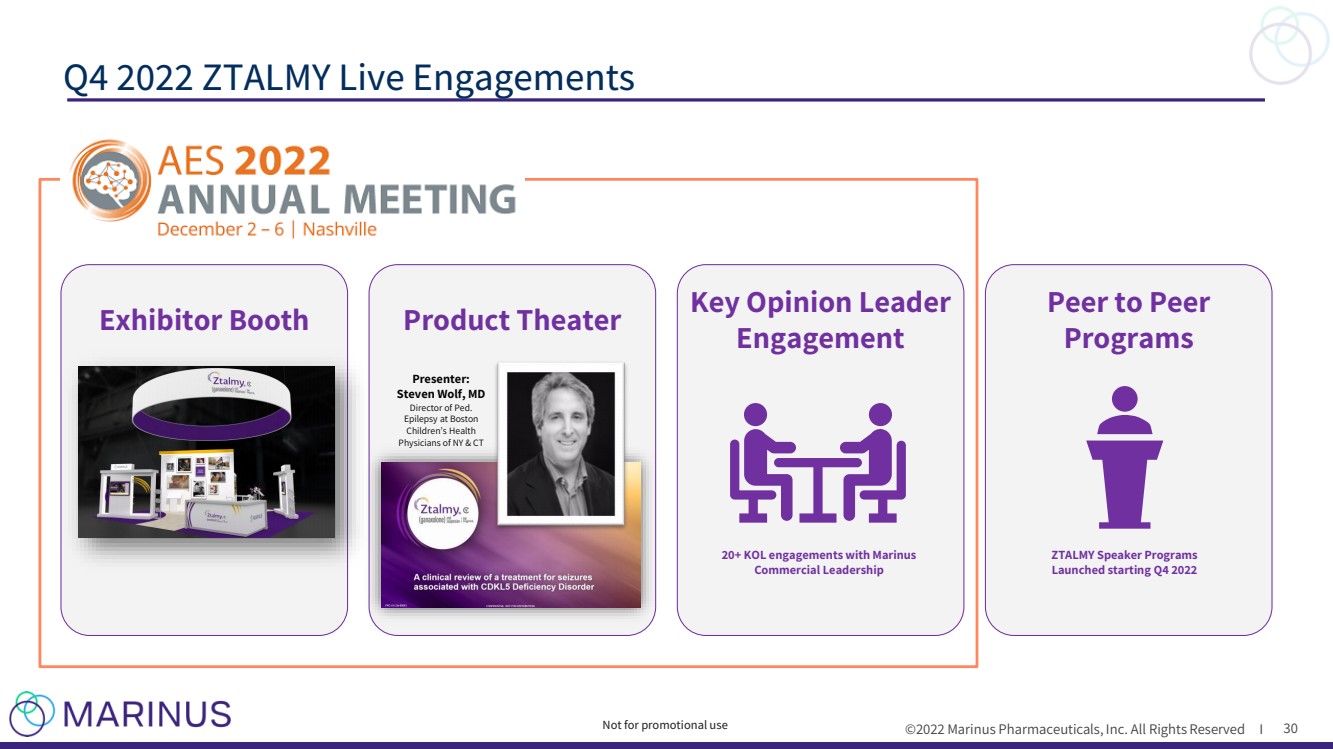
| ©2022 Marinus Pharmaceuticals, Inc. All Rights Reserved I Exhibitor Booth Product Theater Key Opinion Leader Engagement Peer to Peer Programs Q4 2022 ZTALMY Live Engagements 30 Presenter: Steven Wol f, MD Director of Ped. Epilepsy at Boston Children’s Health Physicians of NY & CT 20+ KOL engagements with Marinus Commercial Leadership ZTALMY Speaker Programs Launched starting Q4 2022 Not for promotional use |
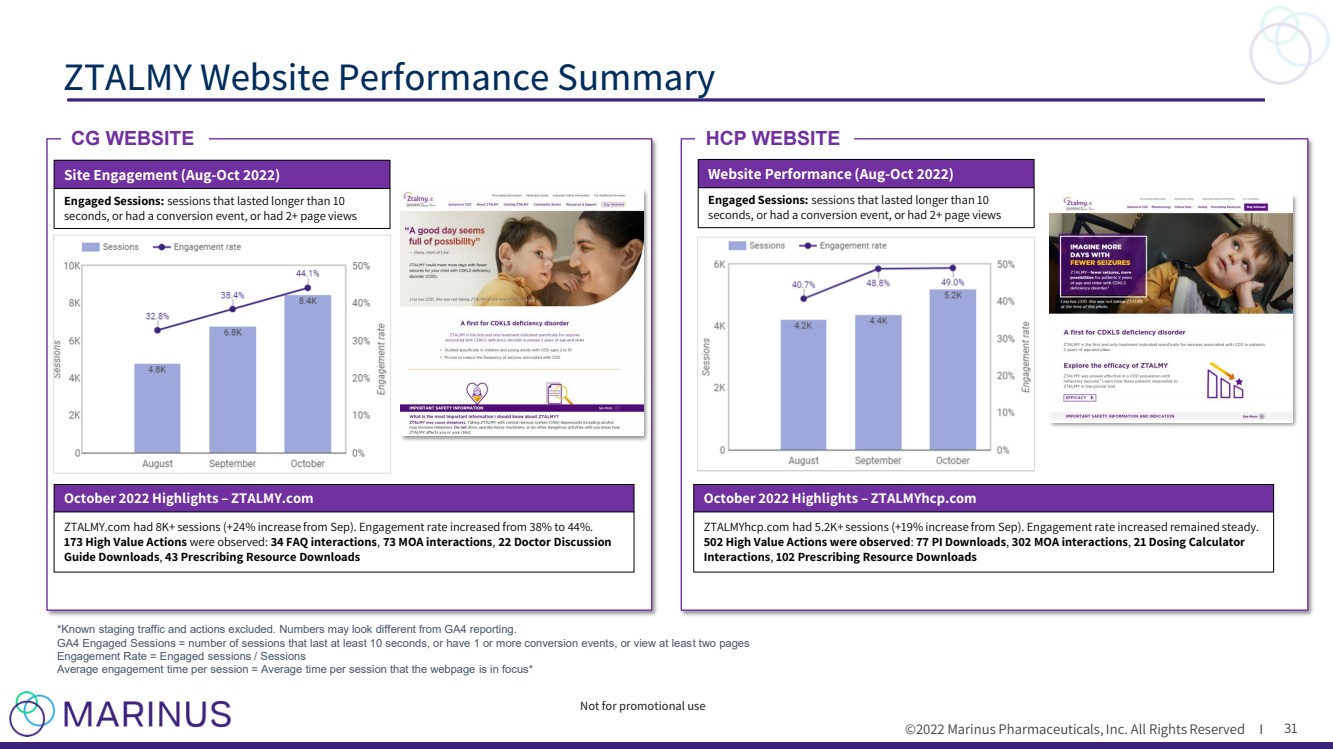
| ©2022 Marinus Pharmaceuticals, Inc. All Rights Reserved I ZTALMY Website Performance Summary 31 *Known staging traffic and actions excluded. Numbers may look different from GA4 reporting. GA4 Engaged Sessions = number of sessions that last at least 10 seconds, or have 1 or more conversion events, or view at leas t t wo pages Engagement Rate = Engaged sessions / Sessions Average engagement time per session = Average time per session that the webpage is in focus* CG WEBSITE HCP WEBSITE Website Performance (Aug - Oct 2022) Engaged Sessions: sessions that lasted longer than 10 seconds, or had a conversion event, or had 2+ page views Not for promotional use Site Engagement (Aug - Oct 2022) Engaged Sessions: sessions that lasted longer than 10 seconds, or had a conversion event, or had 2+ page views October 2022 Highlights – ZTALMY.com ZTALMY.com had 8K+ sessions (+24% increase from Sep). Engagement rate increased from 38% to 44%. 173 High Value Actions were observed: 34 FAQ interactions , 73 MOA interactions , 22 Doctor Discussion Guide Downloads , 43 Prescribing Resource Downloads October 2022 Highlights – ZTALMYhcp.com ZTALMYhcp.com had 5.2K+ sessions (+19% increase from Sep). Engagement rate increased remained steady. 502 High Value Actions were observed : 77 PI Downloads , 302 MOA interactions , 21 Dosing Calculator Interactions , 102 Prescribing Resource Downloads |
| Building Commercial Infrastructure for Future IV Launches Kristin Rudisill, VP, Acute Care |
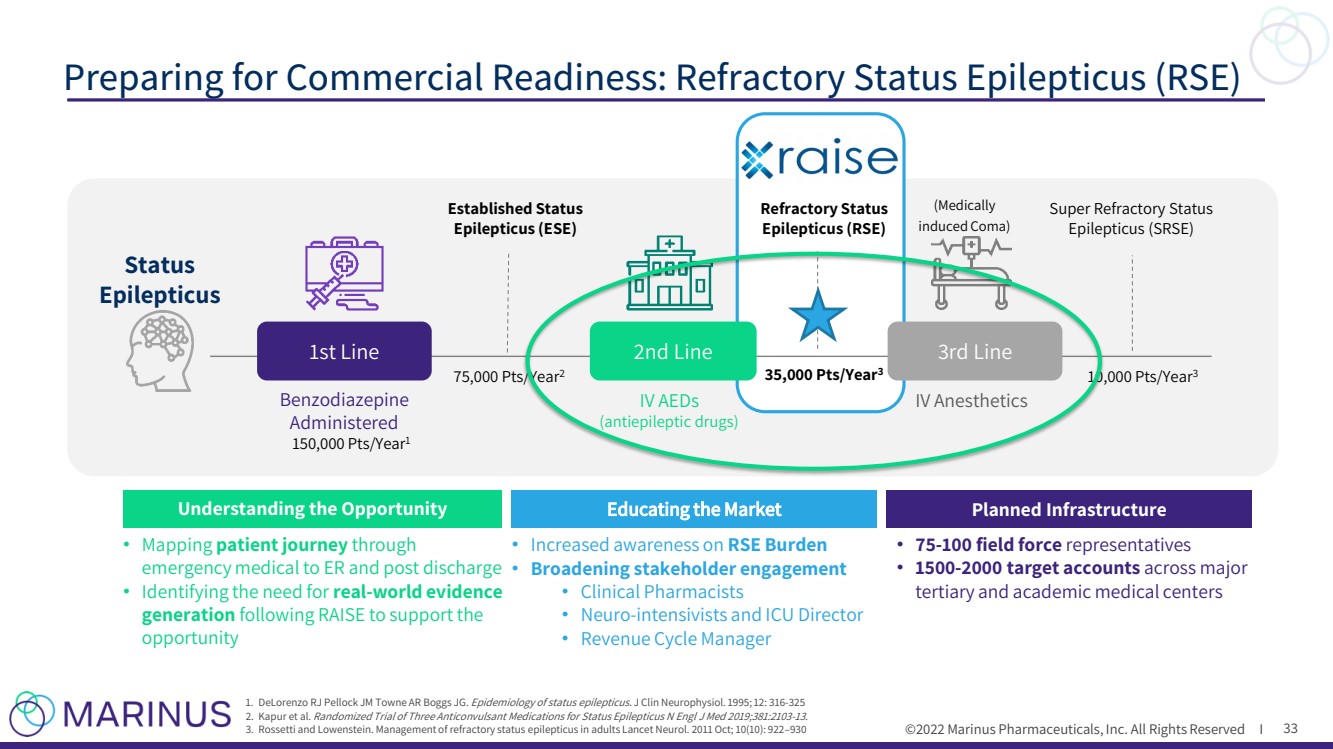
| ©2022 Marinus Pharmaceuticals, Inc. All Rights Reserved I Preparing for Co mmercial Readiness: Refractory Status Epilepticus (RSE) 33 1. DeLorenzo RJ Pellock JM Towne AR Boggs JG. Epidemiology of status epilepticus. J Clin Neurophysiol .. 1995; 12: 316 - 325 2. Kapur et al. Randomized Trial of Three Anticonvulsant Medications for Status Epilepticus N Engl J Med 2019;381:2103 - 13. 3. Rossetti and Lowenstein. Management of refractory status epilepticus in adults Lancet Neurol. 2011 Oct; 10(10): 922 – 930 Benzodiazepine Administered (Medically induced Coma) Established Status Epilepticus (ESE) IV AEDs (antiepileptic drugs) IV Anesthetics Super Refractory Status Epilepticus (SRSE) Refractory Status Epilepticus (RSE) 1st Line Status Epilepticus 3rd Line 2nd Line 150,000 Pts/Year 1 75,000 Pts/Year 2 35,000 Pts/Year 3 10,000 Pts/Year 3 Planned Infrastructure • 75 - 100 field force representatives • 1500 - 2000 target accounts across major tertiary and academic medical centers Educating the Market • Increased awareness on RSE Burden • Broadening stakeholder engagement • Clinical Pharmacists • Neuro - intensivists and ICU Director • Revenue Cycle Manager Understanding the Opportunity • Mapping patient journey through emergency medical to ER and post discharge • Identifying the need for real - world evidence generation following RAISE to support the opportunity |
| ©2022 Marinus Pharmaceuticals, Inc. All Rights Reserved I Key Considerations to Support Commercial Readiness for IV Franchise 34 Defining the Value Proposition Clinical Differentiation Economic Benefits Total Value Proposition Pricing Considerations ► Conducting ongoing pricing research to support our total value & alignment • Assess cost burden before and after hospital admission ► Cost avoidance from consequences related to initiating IV anesthetics/mechanical ventilation • Subsequent reductions in healthcare utilization: LOS, ICU bed/staffing, supportive care |
| Q&A |