2022 ANNUAL INFORMATION FORM OF
ORGANIGRAM HOLDINGS INC.
For the Financial Year Ended August 31, 2022
Dated November 24, 2022
TABLE OF CONTENTS
ANNUAL INFORMATION FORM
In this annual information form (“Annual Information Form”), unless otherwise noted or the context indicates otherwise, the “Company”, “Organigram”, “we”, “us” and “our” refer to Organigram Holdings Inc. and its wholly-owned subsidiaries, including Organigram Inc. and the term “marijuana” has the meaning given to the term “cannabis” in the Cannabis Act (Canada) (the “Cannabis Act”). All financial information in this Annual Information Form is prepared in Canadian dollars and using International Financial Reporting Standards as issued by the International Accounting Standards Board. The information contained herein is dated as of August 31, 2022 unless otherwise stated.
FORWARD-LOOKING STATEMENTS
This Annual Information Form contains certain information that may constitute “forward-looking information” and “forward-looking statements” within the meaning of applicable securities laws (collectively, “forward-looking statements”) which are necessarily based upon the Company’s current internal expectations, estimates, forecasts, assumptions and beliefs regarding, among other things, the future performance and results of the Company’s business and operations, general economic conditions, global events and applicable regulatory regime. Such statements can be identified by the use of forward-looking terminology such as “expect”, “likely”, “may”, “will”, “should”, “intend”, “anticipate”, “potential”, “proposed”, “estimate” and other similar words, including negative and grammatical variations thereof, or statements that certain events or conditions “may”, “will” or “could” happen, or by discussions of strategy. Forward-looking statements include estimates, plans, expectations, opinions, forecasts, projections, targets, guidance, and all other statements that are not statements of fact. The forward-looking statements included in this Annual Information Form are made only as of the date of this Annual Information Form. Forward-looking statements in this Annual Information Form include, but are not limited to, statements with respect to:
•the expected performance of the Company’s business and operations;
•the intention to grow the business, operations and potential activities of the Company;
•the operation of the Moncton Campus (as defined below), the Winnipeg Facility (as defined below) and the Lac Supérieur Facility (as defined below), and receipt and continued maintenance of any required approvals from Health Canada with respect thereto;
•the expected growth in the number of customers and patients using the Company’s cannabis and extract based products;
•the expected growth in the Company’s growing and cannabis extraction capacity;
•the quantity of cannabis and cannabis related products expected to be used by each customer or patient;
•the expected prospects of the Company’s collaboration with BAT (as defined below) including pursuant to the PDC Agreement (as defined below);
•the successful integration and expected prospects for the Company’s recently acquired subsidiaries, EIC (as defined below) and LAU (as defined below);
•the methods expected to be used by the Company to deliver cannabis extract related products;
•the competitive conditions of the industry;
•the effect of government regulations (or changes thereto) with respect to the restrictions on production, sale, consumption, export controls, income taxes, expropriation of property, repatriation of profits, environmental legislation, land use, water use and receipt of necessary permits and approvals;
•the Company’s competitive and business strategies;
•the grant and impact of any license or supplemental license to conduct activities with hemp, cannabis and/or cannabis or hemp extracts or any amendments thereof;
•the anticipated future gross revenues and profit margins of the Company’s operations;
•the Company’s ability to raise additional funds including through the sale of equity or debt securities;
•future liquidity and financial capacity;
•expectations regarding revenues and expenses;
•the price of cannabis and derivative cannabis products;
•expectations with respect to the success of the Company’s research and development initiatives;
•the scope of protection the Company is able to establish and maintain, if any, for its intellectual property rights;
•the Company’s ability to enter into and maintain strategic arrangements with distributors and retailers, and the potential costs and benefits of such arrangements;
•the Company’s anticipated investments in community relations, cannabis health and safety education programming in the locations where the Company operates and the further development of its social responsibility programs;
•the targeting of international opportunities as the laws and regulations governing cannabis evolve internationally;
•the legalization of industrial hemp or cannabis for medical use or adult-use recreational cannabis in jurisdictions outside of Canada and the Company’s ability to participate in any such markets, if and when such use is legalized;
•the Company’s ability to import and export cannabis, hemp, and cannabis and hemp extracts to/from foreign jurisdictions it may identify from time to time;
•whether key personnel will continue their employment with the Company;
•the Company’s plan with respect to the payment of dividends;
•the Company’s ability to remain listed on the TSX (as defined below) and Nasdaq (as defined below) and the impact of any actions it may be required to take to remain listed;
•the Company’s possible exposure to liability, the perceived level of risk related thereto, and the anticipated results of any litigation or other similar disputes or proceedings involving the Company, including but not limited to the class action proceedings described herein; and
•the general economic conditions and global events including COVID-19 (as defined herein) retail store closures or reduced sales at retail stores or other impacts of COVID-19.
Certain of the forward-looking statements and other information contained herein are based on estimates prepared by the Company using data from publicly available governmental sources as well as from market research and industry analysis, and on assumptions based on data and knowledge of the medical cannabis industry, industrial hemp industry and the adult-use recreational cannabis industry which the Company believes to be reasonable. However, although generally indicative of relative market positions, market shares and performance characteristics, such data is inherently imprecise. While the Company is not aware of any misstatement regarding any industry or government data presented herein, the medical cannabis industry, industrial hemp industry, and the adult- use recreational cannabis industry involve risks and uncertainties that are subject to change based on various factors.
Although the Company believes that the expectations reflected in such forward-looking statements are reasonable, it can give no assurance that such expectations will prove to be correct. The Company’s forward-looking statements are expressly qualified in their entirety by this cautionary statement. A number of factors could cause actual events, performance or results to differ materially from what is projected in the forward-looking statements. These factors include, but are not limited to, risks related to competition, changes in the Canadian cannabis industry and market, governmental regulation, changes in laws, regulations and guidelines, reliance primarily on a single cultivation facility, volatility in the wholesale and retail prices of cannabis, the Company’s success in developing new products and finding a market for the sale of new products, license renewal risks, risk inherent in the agricultural business, rising energy costs, negative cash flows from operations, dividends, competition from illicit markets, acquisition and integration risk, volatility in the market for the Company’s securities, limited operating history and history of losses, product liability, sufficiency of insurance, TSX and Nasdaq requirements, management of growth, financing risks, risks relating to developing and maintaining effective internal controls for reliable financial reporting and for fraud prevention, reliance on key personnel, risks relating to the ongoing COVID-19 pandemic and catastrophic events, product recalls, risks relating to litigation and securities class actions, difficulties with forecasts, uninsured and uninsurable events risks, unknown health impacts of the use of cannabis and cannabis-derivatives, reliance on third-party transportation, ability to meet target production capacity, scale of operations, contracts or other arrangements with provincial governments are not guaranteed, continuing to meet listing standards for the TSX and the Nasdaq, risks relating to the Company’s designation as a “large accelerated filer”, differing shareholder protections across jurisdictions, increased volatility for dual-listed shares, market liquidity risks, investment risk, risks relating to the Company’s status as a foreign private issuer in the U.S., risks relating to expansion into new markets, foreign investment risk, risk of corruption and fraud in emerging markets and relating to ownership of real property, risks relating to the Company’s intellectual property, credit risk, liquidity risk, concentration risk, risks associated with significant shareholders, dividends, publicity
or consumer perception, cyber security and privacy, product security, environmental and employee health and safety regulations, reliance on key inputs, regulatory proceedings, investigations and audits, fraudulent or illegal activity by employees, restrictions on foreign investors, regulatory and operational risks associated with expansion into foreign jurisdictions, reliance on international advisers and consultants, anti-money laundering laws and regulation risks, anti-corruption and anti-bribery laws, global economic risks, future acquisitions, general business risks and liabilities, dilution, constraints on marketing products, provincial legislative controls, suppliers and skilled labour, conflicts of interest, risks associated with the Company’s status as a holding company and the other risks described in this Annual Information Form under the heading “Risk Factors”. Material factors and assumptions used in establishing forward-looking information include that construction and production activities will proceed as planned and regulatory conditions will advance in the manner expected by management. The purpose of forward-looking statements is only to provide the reader with a description of management’s expectations relating to future periods, and, as such, forward-looking statements are not appropriate for any other purpose. You should not place undue reliance on forward-looking statements contained in this Annual Information Form. We undertake no obligation to update or revise any forward-looking statements, whether as a result of new information, future events or otherwise, except as required by applicable law.
CORPORATE STRUCTURE
Organigram Holdings Inc. was incorporated under the Business Corporations Act (British Columbia) on July 5, 2010 as “Inform Resources Corp.”. The Company changed its name to “0885160 B.C. Ltd.” on September 13, 2010, and subsequently to “Inform Exploration Corp.” (“Inform”) on February 16, 2011. On November 21, 2011, Inform completed its initial public offering and its common shares commenced trading on the TSX Venture Exchange (the “TSX-V”) on November 24, 2011.
On August 22, 2014, Inform and Organigram Inc. entered into an acquisition agreement (the “Acquisition Agreement”) pursuant to which Inform agreed, among other things, to change its name to “Organigram Holdings Inc.” and to effect a consolidation of the outstanding Inform common shares on a 0.883604747 to 1 basis. Under the Acquisition Agreement, Inform acquired all of the outstanding common shares of Organigram Inc. (the “RTO Transaction”). On April 6, 2016, the Company was continued under the Canada Business Corporations Act.
The Company graduated from the TSX-V to the Toronto Stock Exchange (the “TSX”) in August 2019. As a result of the graduation, the common shares of the Company (the “Common Shares”) commenced trading on the TSX under the symbol “OGI” effective August 22, 2019. The Common Shares have also been listed for trading on Nasdaq Global Select Market (the “Nasdaq”) under the symbol “OGI” effective May 21, 2019 and were delisted from the OTCQX® Best Market after market close on May 20, 2019.
The Company’s core operations are based in Moncton, New Brunswick with two other facilities in Winnipeg, Manitoba and Lac Supérieur, Québec. The Company’s head office is located at 333 Bay Street, Suite 1250, Toronto, Ontario and the registered office is located at 35 English Drive, Moncton, New Brunswick. The Company’s telephone number is 1 (844) 644-4726 and its corporate website is www.organigram.ca.
Subsidiaries
Organigram Inc. (“OGI”), a wholly-owned subsidiary of Organigram Holdings Inc., was incorporated under the Business Corporations Act (New Brunswick) on March 1, 2013.
10870277 Canada Inc., incorporated under the Canada Business Corporations Act on July 4, 2018, is a wholly owned subsidiary of Organigram Holdings Inc., and is used as a special purpose holding company for the Company for its investment in alpha-cannabis Pharma GmbH as further described herein.
The Edibles and Infusions Corporation (“EIC”), amalgamated under the Business Corporations Act (Ontario), was acquired and became a wholly owned subsidiary of the Company on April 6, 2021.
Laurentian Organic Inc. (“LAU”), incorporated under the Canada Business Corporations Act, was acquired and became a wholly owned subsidiary of the Company on December 21, 2021.
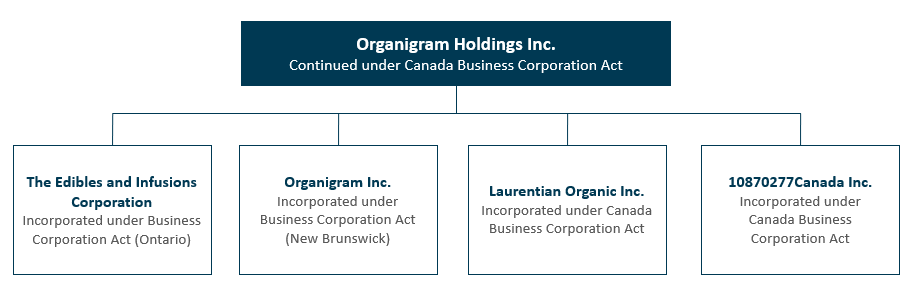
The following chart illustrates, as of August 31, 2022, the Company’s corporate structure:
Certain subsidiaries of the Company, each of which does not represent more than 10% of the consolidated assets of the Company and not more than 10% of the consolidated revenue of the Company, and all of which, in the aggregate, represent not more than 20% of the total consolidated assets and the total consolidated revenue of the Company as at the most recent financial year end of the Company, have been omitted from the chart above.
GENERAL DEVELOPMENT OF THE BUSINESS
Licenses
On March 26, 2014, Organigram Inc. was issued a producer’s license under the Marihuana for Medical Purposes Regulations (“MMPR”) promulgated under the Controlled Drugs and Substances Act (Canada) (the “CDSA”). Pursuant to this license, Organigram Inc. was permitted to produce, sell, possess and ship medical marijuana, in conformity with applicable regulations, and made its first shipment of medical marijuana to registered patients in September 2014.
On March 27, 2016, OGI’s license was amended by Health Canada to authorize the manufacturing of cannabis oil extracts under the class exemption available under Section 56 of the CDSA (the “Section 56 Exemption”). In June 2016, this license was further amended by Health Canada to also include the sale of cannabis oil extracts.
On August 24, 2016, the MMPR was replaced by the Access to Cannabis for Medical Purposes Regulations (“ACMPR”) promulgated under the CDSA. Under the transitional provisions of the ACMPR, a producer’s license issued under the MMPR was continued under the ACMPR and remained valid until that license expired or was revoked. Health Canada issued a license (the “OGI License”) to OGI under Section 35 of the ACMPR with an effective date of August 10, 2017. The License permitted OGI to produce, sell, possess and ship medical marijuana, in conformity with the ACMPR.
On May 15, 2018, OGI received a dealer’s license under the CDSA from Health Canada (the “Dealer’s License”). The Dealer’s License authorized the Company to develop, test and export and import for the purposes of reformulation an extensive range of products including, but not limited to, a range of cannabis oils as well as an extensive range of derivative based formulations. On June 27, 2018, the Dealer’s License was amended under the CDSA.
Effective October 17, 2018, cannabis is no longer regulated under the CDSA and is now regulated under the Cannabis Act as further described herein under “Canadian Regulatory Landscape”. As a result of the Cannabis Act coming into effect, every license issued under the ACMPR that was in force immediately before the day on which the Cannabis Act came into force was deemed to be a license under the Cannabis Act. Additionally, under the Cannabis Act export permits are applied for and received on a case by case basis.
On November 9, 2018, Health Canada reissued the License to OGI under the Cannabis Act as a license for standard cultivation, standard processing and sale for medical purposes while also authorizing the sale of dried cannabis and cannabis oil products to adult recreational use sales channels.
On October 21, 2019, after the amendment to the Cannabis Regulations, Health Canada amended the License to expand the classes of cannabis products that may be sold to adult-use recreational sales channels or sold for medical purposes, to include cannabis topicals, cannabis extracts and edible cannabis. Health Canada renewed the License effective as of March 20, 2020. The License has an expiry date of March 20, 2023. The Company intends to renew the License and it is anticipated that Health Canada will extend or renew the License at the end of its term. See Risk Factors “Reliance on License Renewal”.
OGI also holds a cannabis license under the Excise Act, 2001 (the “Excise Act”) most recently renewed on October 17, 2022 and expiring October 16, 2024. All holders of a license under the Cannabis Act who are authorized to cultivate, produce and package cannabis products are required to hold a cannabis license under the Excise Act. The Company intends to renew this license prior to expiry.
On October 23, 2019, Health Canada issued a license to OGI under the Cannabis Act for research (the “Research License”). The Research License allows the Company to test inhalable cannabis in the cannabis extracts class of cannabis, and ingestible cannabis in the cannabis extracts and edible cannabis classes of cannabis, on human research subjects for assessments of taste, sight, smell or touch. The activities authorized under the Research License support the Company’s commercialization of cannabis products in the cannabis extracts and edible cannabis classes of cannabis. The Research License has an expiry date of October 23, 2024. The Company intends to renew the Research License at the end of its term.
EIC, the Company’s wholly-owned subsidiary acquired on April 6, 2021, holds a research license having an effective date of November 22, 2019 and expiring on November 22, 2024 allowing EIC to develop confectionary cannabis product formulations. Further, on December 11, 2020, Health Canada issued EIC a standard processing and medical sales license under the Cannabis Act, expiring on December 11, 2023. On May 3, 2022, Health Canada issued EIC an amended license authorizing the sale of cannabis extract, edible and topical products to adult recreational sales channels (the “EIC License”). EIC also holds a cannabis license under the Excise Act most recently renewed on February 14, 2021 and expiring on February 13, 2023. The Company intends to renew these licenses at the end of their respective terms.
LAU, the Company’s wholly-owned subsidiary acquired on December 21, 20221, holds a standard cultivation and standard processing license, originally issued April 17, 2020, authorizing the cultivation of cannabis and manufacturing of dried cannabis, cannabis extract, edible and topical products. On December 3, 2020, Health Canada issued LAU an amended license authorizing the sale of dried cannabis, cannabis extract, edible and topical products to adult recreational sales channels, such license having an expiry date of April 17, 2023 (the “LAU License”). LAU also holds a cannabis license under the Excise Act most recently renewed on October 17, 2022 and expiring on October 16, 2024. The Company intends to renew these licenses at the end of their respective terms.
Facilities Expansion
The Company acquired its Moncton campus (the “Moncton Campus”) located in Moncton, New Brunswick in March 2014, and undertook a series of expansions since its acquisition.
The Company continually assesses the critical facets of the lighting and environmental elements in its facilities in an effort to drive maximum quality and yield in the plants it produces. It is the Company’s intention to continually improve and refine its cultivation and post-harvesting practices in an effort to achieve a competitive advantage in the space. In July, 2022, the Company completed the expansion of 29 additional grow rooms. With the addition of environmental enhancements and the expansion, the Moncton Campus now has the capacity to produce approx. 85,000 kg of flower annually.
As part of the acquisition of EIC, the Company now has a purpose-built, highly-automated, 51,000-square-foot manufacturing facility in Winnipeg, Manitoba (the “Winnipeg Facility”). The facility design and the equipment specifications were designed under EU GMP standards and were also designed to handle both smaller-batch artisanal manufacturing as well as large-scale nutraceutical-grade high-
efficiency manufacturing, and to produce highly customizable, precise and scalable cannabis-infused products in various formats and dosages including pectin, gelatin, sugar-free soft chews (gummies), and toffee and caramel with novel capabilities such as infusions, striping and the possibility of using fruit purees. In May, 2022, a high speed packaging line for pouches was commissioned as part of the Company’s effort to optimize the Winnipeg Facility.
As part of the acquisition of LAU, the Company acquired a facility in Lac Supérieur having 6,800 square feet of cultivation area, (the “Lac Supérieur Facility”), which is currently being expanded to 33,000 square feet. The Lac-Supérieur Facility is currently equipped to produce approximately 600 kilograms of flower and 1 million packaged units of hash annually. The expansion program currently underway is expected to increase annual capacity to 2,400 kilograms of flower and 2 million packaged units of hash once completed
The forward-looking estimate of production capacity and budget amounts noted above are based on a number of material factors and assumptions including that: (a) the facility size will be as estimated with the same amount of cultivation space being used per grow room for cultivation as used to date; (b) the ratio of dried flower cultivated per canopy square foot of grow room will be consistent with historical output in the Company’s existing facilities; (c) all grow rooms designated as production rooms will be utilized for their intended purposes (from time to time rooms may be used for other purposes, such as for storage); and (d) a number of factors can cause actual costs to differ from estimates. See “Risk Factors”.
Three-Year History
Developments during the financial year ended August 31, 2020
Effective September 6, 2019, the Company received Health Canada’s approval for the licensing of 17 additional cultivation rooms.
On November 15, 2019, the Company amended the credit agreement entered into on May 31, 2019 with the Bank of Montreal and a syndicate including three other lenders (“Credit Agreement”): (i) extend the final draw deadline of the term loan from November 30, 2019 to March 31, 2020; (ii) postpone the commencement of principal repayments on the term loan to May 31, 2020; and (iii) realign the financial covenants structure, effective November 30, 2019, to be more consistent with industry norms up to and including May 31, 2020, which also provided the Company will greater flexibility around the timing and quantum of any incremental draws. Prior to the further amendment of the Credit Agreement in May 2020, which is described below, the financial covenants were to revert back to the original structure on August 31, 2020. On April 1, 2021, the Company repaid all outstanding balances (approximately C$58.5 million) under the facilities provided under the Credit Agreement.
On December 4, 2019, the Company launched an at-the-market equity program (the “December ATM Program”) that allowed the Company to issue and sell up to $55,000,000 (or its U.S. dollar equivalent) of Common Shares from treasury to the public, from time to time, at the Company’s discretion. All Common Shares sold through the December ATM Program were sold through the TSX, Nasdaq or another marketplace (as defined in National Instrument 21-101 – Marketplace Operations) upon which the Common Shares were then listed, quoted or otherwise traded, at the prevailing market price at the time of sale. Distributions of the Common Shares through the December ATM Program were made pursuant to an equity distribution agreement dated December 4, 2019 among the Company, BMO Nesbitt Burns Inc., as Canadian agent, and BMO Capital Markets Corp., as U.S. agent, and the volume and timing of distributions under the December ATM Program were determined in the Company’s sole discretion. All of the Common Shares issuable pursuant to the December ATM Program were issued as of February 13, 2020.
Effective December 12, 2019, the Company received Health Canada’s approval for the licensing of 16 additional cultivation rooms and additional drying and storage areas. The Company also received Health Canada’s approval for the expansion of the site perimeter for Phase 4C and Phase 5 and approval for the operations area that houses the Company’s state-of-the-art chocolate line.
On December 23, 2019, the Company announced that the first of its ‘Cannabis 2.0’ products had been released, which included Trailblazer Spark, Flicker and Glow 510-thread Torch vape cartridges.
On January 16, 2020, the Company announced that it had secured a supply agreement with Medical Cannabis by Shoppers (the “Shoppers Agreement”), the online medical cannabis platform by Shoppers Drug Mart Inc. (“Shoppers”). Under the terms of the Shoppers Agreement, the Company will provide Shoppers with a wide range of products including dried flower, oils and other future derivative products as they become available. The Shoppers Agreement is for a three-year term subject to automatic renewal for an additional two years.
On February 20, 2020, the Company announced the continued roll out of its innovative portfolio of adult-use recreational cannabis products including vape pens and cannabis-infused chocolate. The Company made its first shipments of the Edison vape pens powered by Feather technology to jurisdictions across Canada. The Company also announced the first shipment of cannabis-infused chocolate following its investment in a high-speed, high-capacity, fully automated production line. Edison Bytes, the Company’s premium cannabis-infused chocolate truffles were available in both milk and dark chocolate formulations and were the first of Organigram’s chocolate products to be available to Canadian adult consumers. See “Distribution and Sales – Cannabis Edibles”.
On March 4, 2020, Derrick West, then a member of the board of directors of the Company, was appointed as Chief Financial Officer.
On March 23, 2020, the Company announced that Health Canada approved the licensing of the remainder of the Company’s edibles and derivatives expansion area together with the renewal of its licenses for standard cultivation, standard processing and sale for medical purposes under the Cannabis Regulations. The terms of the licenses include approval of a two-floor production facility designed to support all processing activity as well as dedicated spaces for packaging of flower, pre-rolls and vape pens. The expansion also included a new extraction facility with increased capacity for CO2 extraction, and winterization as well as new capabilities designed for future product development. The amendments allowed for new purpose-built harvest and drying rooms and support areas for quality assurance, maintenance and sanitation. The licenses are valid for a three-year period until March 20, 2023 and are subject to customary terms and conditions.
On March 23, 2020, the Company announced that it was carefully monitoring and actively planning for the evolving situation related to COVID-19 and the potential impacts on the Company’s business and operations. The Company stated that it expected to materially reduce its workforce as a result of voluntary and company-imposed temporary lay-offs, which would result in corresponding production and packaging reductions.
On April 6, 2020, the Company announced the temporary layoff of approximately 45% of its workforce primarily in an effort to help contain COVID-19. The Company offered voluntary layoffs to certain staff and those who accepted made up the majority of the layoffs. In some cases, due to the impacts of COVID-19, some administrative, support and other functions were deemed non-essential to the short-term needs of the business and those employees were temporarily laid off. The temporary layoffs were initiated on March 24, 2020. Lump-sum payments (equating to approximately two weeks of work) were paid to the affected employees to help bridge the gap to available government programs. In addition, the Company absorbed the employee paid portion of health, dental and short-term disability premiums for all employees during this difficult time. The Company also put in place a number of health and safety measures during Q3 and Q4 2020. On July 3, 2020, in response to the continued impact of COVID-19 on the Company’s business and the continuing evolution of the Canadian cannabis industry, the Company further reduced its workforce by 25%. The Company workforce continues to fluctuate based on evolving needs and expansionary activities.
On April 22, 2020, the Company launched an at-the-market equity program (the “April 2020 ATM Program”) that allowed the Company to issue and sell up to $49,000,000 (or its U.S. dollar equivalent) of Common Shares from treasury to the public, from time to time, at the Company’s discretion. All Common Shares sold through the April 2020 ATM Program are sold through the TSX, Nasdaq or another marketplace (as defined in National Instrument 21-101 – Marketplace Operations) upon which the Common Shares were then listed, quoted or otherwise traded, at the prevailing market price at the time of sale. Distributions of the Common Shares through the April 2020 ATM Program are made pursuant to an equity distribution agreement dated April 22, 2020 among the Company, BMO Nesbitt Burns Inc., as Canadian agent, and BMO Capital Markets Corp., as U.S. agent, and the volume and timing of
distributions under the December ATM Program were determined in the Company’s sole discretion. All of the Common Shares issuable pursuant to the April 2020 ATM Program were issued as of June 23, 2020.
On March 3, 2017, the Claim (as defined herein) in connection with a proposed class action lawsuit was filed with the Nova Scotia Supreme Court. On April 30, 2020, the Company announced that the Nova Scotia Court of Appeal partially overturned the certification, thereby significantly reducing the scope of the Claim. On June 26, 2020, the plaintiff filed an application for leave to appeal the Nova Scotia Court of Appeal’s decision to the Supreme Court of Canada. On November 5, 2020, the Supreme Court of Canada dismissed the leave to appeal with costs. On June 23, 2022, the Company announced that it had reached a Settlement (as defined herein) with respect to the Claim. As part of the Settlement, the Company has agreed to pay an aggregate of $2,310,000. On August 31, 2022, the Court held a hearing and approved the Settlement. See “Legal Proceedings and Regulatory Actions”.
On May 28, 2020, the Company announced that as of May 19, 2020, the ready-to-use Edison Cannabis Company vape pens powered by Feather, Edison-branded pods specifically for PAX Era vaporizers, and Edison Bytes chocolates in dark and milk chocolate were made available to medical cannabis consumers.
On June 9, 2020, the Company announced that it entered into a multi-year agreement for the supply of dried flower to one of Israel’s largest and most established medical cannabis producers, Canndoc Ltd. (“Canndoc”), a subsidiary of InterCure Ltd. (TASE: INCR/INCR.TA) (the “2020 Canndoc Agreement”). Canndoc has EU GMP-approved medical cannabis products which are sold in pharmacies in Israel. Canndoc holds international cultivation and distribution agreements in the European Union and Canada. The 2020 Canndoc Agreement provided for a tiered pricing scheme and the exact value will vary depending on factors such as potency and product mix. The 2020 Canndoc Agreement also contemplated, among other things, an opportunity for the Company to launch branded medical products with Canndoc in Israeli and European Union markets, and granted exclusivity and related rights to Canndoc within the Israel market for a period of approximately 7.5 years. Activities under the 2020 Canndoc Agreement are subject to compliance with all applicable laws, including receipt of all requisite approvals from Health Canada, the Israeli Ministry of Health, and any other applicable regulatory authorities. As the supply commitments under the 2020 Canndoc Agreement have been fulfilled, the parties recently entered into a subsequent supply agreement. See update in “Developments during the financial year ended August 31, 2022” and “Israeli Regulatory Framework”.
On June 23, 2020, the Company announced a number of corporate updates including (i) the completion of its April 2020 ATM Program, issuing an aggregate of 21,080,229 Common Shares for gross proceeds of approximately $49 million, and (ii) the commencement of legal action in the Court of Queen’s Bench in Alberta, which action seeks damages against many of the largest Canadian cannabis companies, including the Company.
On July 20, 2020, the Company announced that Ray Gracewood, Senior Vice President of Marketing and Communications left the Company to pursue other interests. Julie Chamberlain was promoted to Vice President of Marketing. The Company’s senior marketing role is now occupied by Megan McCrae as described below.
On July 28, 2020, the Company announced the launch of its most recent cannabis 2.0 product Trailblazer Snax, a premium-quality, value-priced, cannabis infused chocolate bar. The Trailblazer Snax is a 42g bar with 10mg of THC and comes in both mint and mocha flavours.
On August 4, 2020, the Company announced the launch of three new strains of Edison Cannabis Co. dried flower products: (i) The General (Grapefruit GG4); (ii) Chemdog; and (iii) Samurai Spy (Ninja Fruit).
On August 14, 2020, the Company announced that it sent its first shipment of bulk dried flower to Canndoc. The shipment was part of the Canndoc Agreement and was valued at $2.47 million.
On August 18, 2020, the Company announced it partnered with Medical Cannabis by Shoppers and TruTrace in effort to track source and genetics of cannabis used by medical patients. The program is designed to genetically finger-print all participating cannabis products, tracking them throughout the
supply chain, from genome to patient, in order to provide real-time information about the composition of each cannabis product used by Medical Cannabis by Shoppers customers. The Company agreed to provide cannabis products to Shoppers for use in the tracking program.
Developments during the financial year ended August 31, 2021
On September 17, 2020, the Company announced the launch of SHRED, a high quality, high potency, affordable dried flower product pre-shredded for additional consumer convenience. SHRED offers three pre-milled varieties, all featuring THC of 18% or more and each contained in a two-way humidity system to preserve their unique flavour profiles.
On October 23, 2020, the Company announced the funding of an additional $2.5 million investment in Hyasynth Biologicals Inc. (“Hyasynth”) in accordance with the terms of the debenture purchase agreement dated September 12, 2020, between the Company and Hyasynth. This brought the Company’s total face value of convertible debentures investment in Hyasynth to $7.5 million, which provided the Company with a potential ownership interest of up to 46.7% on a fully diluted basis.
On November 10, 2020, the Company announced an underwritten public offering of 37,375,000 units of the Company (the “Units”) at a price of $1.85 per Unit (the “Offering”), including the full exercise of the over-allotment option, underwritten by a syndicate of underwriters led by Canaccord Genuity Corp. Each Unit was comprised of one common share of the Company and one half of one common share purchase warrant of the Company (each full common share purchase warrant, a “Warrant”). Each Warrant is exercisable to acquire one common share of the Company (a “Warrant Share”) for a period of 3 years following the closing date of the Offering at an exercise price of $2.50 per Warrant Share, subject to adjustment in certain events. The Offering closed on November 12, 2020.
On November 27, 2020, the Company further amended its credit facilities pursuant to an amended and restated credit agreement (the “Amended and Restated Credit Agreement”) with BMO to: (i) reduce the term loan amount from $115 million to $60 million (the “Term Loan”) based on a repayment of $55 million to be made on December 1, 2020 of the outstanding Term Loan balance of $115 million; (ii) have repayments on the balance of the Term Loan commence on February 28, 2021 in an amount equal to $1.5 million per quarter; (iii) reduce the revolver commitment to $2 million from up to $25 million; (iv) adjust the minimum quarterly EBITDA covenants to be maintained by the Company commencing on February 28, 2021 and continuing through to maturity, thereby removing this covenant for the fiscal period ended November 30, 2020 and eliminating the reversion of the financial covenants to that of the original structure on November 30, 2021; (v) modify the applicable margin pricing and standby fee terms to reflect current market conditions; and (vi) reduce the minimum unrestricted cash balance requirement to $20 million, which was already inclusive of the $8 million restricted investment then currently outstanding. The interest rate margin was fixed from November 27, 2020 through to maturity on May 31, 2021. The Company incurred an amendment fee of $217,000 plus legal expenses in connection with the amendment and restatement. On April 1, 2021, the Company repaid the $58.5 million outstanding balance under the facilities provided under the Amended and Restated Credit Agreement.
On December 22, 2020, the Company announced the launch of three new Edison Indica strains, namely, high potency Black Cherry Punch and Ice Cream Cake (I.C.C.) and full flavour Slurricane.
On January 11, 2021, the Company announced the appointment of Marni Wieshofer to the Company’s board of directors. Ms. Wieshofer is Organigram’s first U.S. domiciled director and assumed her board position effective January 12, 2021.
On February 2, 2021, the Company announced the departure of Matt Rogers, Senior Vice President, Operations who left the Company at the end of May 2021 to pursue other interests. Further, on the same date, the Company announced the appointment of Nathalie Batten as the Company’s plant manager. Effective June 1, 2021, Nathalie Batten was appointed as the Company’s Vice President, Operations.
On March 2, 2021, the Company announced the launch of two new additions to its adult-use recreational cannabis product portfolio which are SHRED Tropic Thunder Jar of Joints and Trailblazer SNAX Milk Chocolate Bars. SHRED’s Tropic Thunder is a combination of strains with citrus and tropical aromas featuring THC of 18% or more, and is available in a Jar of Joints. Trailblazer SNAX is the only chocolate
bar currently in market in which cannabis is infused into a rich, creamy cacao filling in the centre of the bar. See “Distribution and Sales – Cannabis Edibles”.
On March 11, 2021, the Company announced a $221 million strategic investment from a wholly-owned subsidiary of British American Tobacco (“BAT”), which subscribed for approximately 58.3 million Common Shares of the Company at $3.792 per Common Share, which represented a 19.9% equity interest in the Company on a post-transaction basis at the time of announcement. Concurrent with the investment, Organigram Inc. and BAT also entered into a product development collaboration agreement (the “PDC Agreement”) pursuant to which a “Center of Excellence” (a “CoE”) has been established at the Moncton Campus to focus on developing the next generation of cannabis products.
On March 11, 2021, the Company announced it had added Mr. Jeyan Heper to the board of directors, as one of the two nominees that BAT is entitled to nominate in connection with BAT’s strategic investment in the Company and the PDC Agreement. Mr. Jeyan Heper resigned from the Board effective October 31, 2021.
On April 6, 2021, the Company announced it had acquired all of the issued and outstanding shares of EIC (the “EIC Acquisition”) for share consideration of $22.0 million, plus up to an additional $13.0 million in shares payable upon the EIC business achieving certain earnout milestones. The EIC Acquisition further broadens the Company’s continuum of product offerings and provides an operational footprint in Western Canada. On September 8, 2021, the Company issued 1,039,192 Common Shares on EIC’s achievement of the first milestone earnout.
On April 22, 2021, the Company announced the launch of two new Edison dried flower strains – GMO Cookies and MAC1. Both strains contain a THC range of 20-26% feature a distinct phenotypic profile, flavour and aroma as a result of being grown in one of Organigram’s strain specific micro-climates and are available in 3.5g format or a package of three x 0.5g pre-rolls.
On May 3, 2021, the Company announced that Greg Engel had stepped away from his role as CEO and that, he would continue to act as a special advisor to the board of directors through a transition period until a new permanent CEO was appointed. Peter Amirault, chair of the board of directors, was appointed by the board of directors to serve as executive chair on an interim basis, and to oversee the day-to-day management of the Company until a permanent CEO is appointed.
On May 10, 2021, the Company announced the appointment of Borna Zlamalik as the Company’s Vice President of Innovation who is an accomplished consumer packaged goods marketer. Mr. Zlamalik most recently served as Vice President, Marketing & Communications, for The Valens Company, and has held senior roles in the past. Mr. Zlamalik oversees all R&D and product development and sits as one of Organigram’s representatives on the steering committee for the CoE. See “New Product Development”.
On May 18, 2021, the Company announced the launch of Big Bag o’ Buds, a lineup of dried flower products featuring a roster of well-known genetics and an exciting rotation of one-time offerings in a 28g format. Big Bag o’ Buds offers a minimum of 17% THC and a rich genetics assortment that includes Ultra Sour, a pungent, Sativa-leaning sour featuring the tartness of Meyer lemon and the diesel and pungent notes from the strain’s kush undertones.
On May 31, 2021, the Company announced the appointment of Megan McCrae as the Company’s Senior Vice President of Marketing and Communications.
On June 3, 2021, The Company announced the official launch of the CoE as outlined in the PDC Agreement. The Company also announced it was recruiting for as many as 75 positions, across most functional areas of the Company. Positions include those in operations, production, sanitation, cultivation, sales, marketing, research, and quality assurance, to build a world-class workforce to support the development of new, innovative and industry-leading products. See “New Product Development”.
On June 24, 2021, the Company announced the filing of a preliminary short form base shelf prospectus with the securities commissions in each of the provinces and territories of Canada, and the concurrent filing of a base shelf registration statement with the SEC on Form F-10 under the United States Securities Exchange Act of 1933, as amended, pursuant to the Multijurisdictional Disclosure System. The base shelf
allows the Company to qualify the distribution of up to $500 million of Common Shares, preferred shares, debt securities, subscription receipts, warrants, and units during the 25‐month period that the base shelf prospectus remains effective. The Company received a receipt for its final short form base shelf prospectus on August 31, 2021.
On July 15, 2021, the Company announced the launch of the Cannabis Innovators Panel, a cannabis consumer panel offering real-time insights into consumer preferences, usage occasions, and future development opportunities. This online panel engages with up to 2,500 participants across Canada on an ongoing basis, and contributes feedback on both existing product categories as well as guide areas of future research and development including flower, vapes, concentrates, edibles, flower and pre-rolls.
On August 4, 2021, the Company announced that Beena Goldenberg, formerly Chief Executive Officer of The Supreme Cannabis Company Inc. and previously Chief Executive Officer at Hain-Celestial Canada, ULC, was appointed as the Company’s new CEO. Ms. Goldenberg assumed the role on September 9, 2021.
On August 17, 2021, the Company announced the launch of Edison JOLTS, Canada’s first flavoured high potency THC lozenge. Edison JOLTS are available in a package of 10 mint flavoured lozenges with 10 mg of THC per lozenge for a total of 100mg per pack. Edison JOLTS are intended for sublingual or buccal absorption, which generally allows for faster absorption of active ingredients (in this case, THC) compared to a product that is chewed and swallowed. Edison JOLTS are also low-calorie and vegan friendly.
On August 25, 2021, the Company announced the launch of SHRED’ems, high-quality and bold flavoured cannabis-infused gummies. SHRED’ems is an extension of the Company’s value-priced SHRED product portfolio which includes SHRED milled flower and SHRED Jar of Joints. Reflecting the SHRED portfolio’s commitment to value, convenience, and bold flavour, SHRED’ems is competitively priced and is available in three bold, all-natural flavours. SHRED’ems gummies are also vegan friendly.
Developments during the financial year ended August 31, 2022
On November 1, 2021, the Company announced it made an international shipment to Canndoc in Q1 Fiscal 2022 under the Canndoc Agreement. The Company also announced it had signed a supply agreement with the Yukon Liquor Corporation adding territorial distribution to its existing 10 provincial distribution arrangements.
On November 18, 2021, the Company announced the launch of Monjour, the Company’s new wellness brand, offering high quality, CBD-forward products. Monjour’s first offerings included both vegan-friendly as well as sugar-free soft chews, both in assorted flavours.
On December 21, 2021, the Company announced the acquisition of LAU for $36 million, net of working capital adjustments, plus earnout share consideration, if any. The acquisition added more premium products to the Company’s portfolio and strengthened its presence in the province of Quebec.
On December 22, 2021, the Company announced that it had made an additional $2.5 million investment in Hyasynth. The Company has to date invested $10 million in Hyasynth.
On February 23, 2022, the Company announced that the BAT nominee, Mr. Simon Ashton, was appointed to the Company’s board of directors.
On March 1, 2022, the Company announced that BAT had invested $6.3 million to exercise its rights pursuant to the Investor Rights Agreement (as defined herein) to enhance its equity ownership position in the Company to 19.5% (as at December 31, 2021) from 18.8%.
On March 17, 2022, the Company launched its social impact strategy, Organigram Operating for Good, joining the Pledge 1% movement by donating up to 1% of employee time towards causes aimed at “Building Healthy Communities Where We Live and Work.”
On June 23, 2022, the Company announced that it had reached the Settlement with respect to the Claim. As part the Settlement, the Company has agreed to pay a settlement amount in the aggregate of $2,310,000 (the “Settlement Amount”). On August 31, 2022, the Court held a hearing and approved the Settlement. See “Legal Proceedings and Regulatory Actions”.
On November 17, 2022, the Company announced that it had entered into a new multi-year agreement for the supply of medical dried cannabis flower to Canndoc (the “2022 Canndoc Agreement”). The 2022 Canndoc Agreement provides for a commitment of 10,000kg of dried flower with an option for Canndoc to elect to order up to an additional 10,000kg during the three-year supply term. As of the date of the announcement, approximately 2,800kg had already been delivered to Canndoc and credited against the total volume commitment under the 2022 Canndoc Agreement. The Company’s has agreed to exclusively supply Canndoc in Israel during the three-year term with a right of first offer for an additional one year period following the end of the term to negotiate a further exclusive collaboration in Israel. Additionally, the Company granted exclusively on certain popular genetics for distribution into Canndoc’s international supply chain, subject to agreement between the parties and local regulations.
DESCRIPTION OF THE BUSINESS
Company Overview
The Company is a leading Canadian licensed producer of high quality cannabis and cannabis-derived products in Canada under the Cannabis Act. The Company is focused on producing high-quality cannabis and other cannabis derived products for adult-use recreational and medical consumers in Canada as well as developing international business partnerships to extend the Company’s global footprint.
A description of the regulatory framework is included below under the heading “Canadian Regulatory Framework”. For a summary of the Cannabis Act and Cannabis Regulations as well as the Company’s license issued under the Cannabis Act, see “Canadian Regulatory Framework – Licenses, Permits and Authorizations”.
Principal Products and Brands
The Company has been working on establishing strong brands for use in the adult-use recreational cannabis market place and is seeking to create a portfolio of diverse brands and products. The Company’s adult-use recreational cannabis brands strategy reflects the Company’s views about current and potential consumers, the industry, future product development and opportunities for growth.
Adult Use Recreational Cannabis
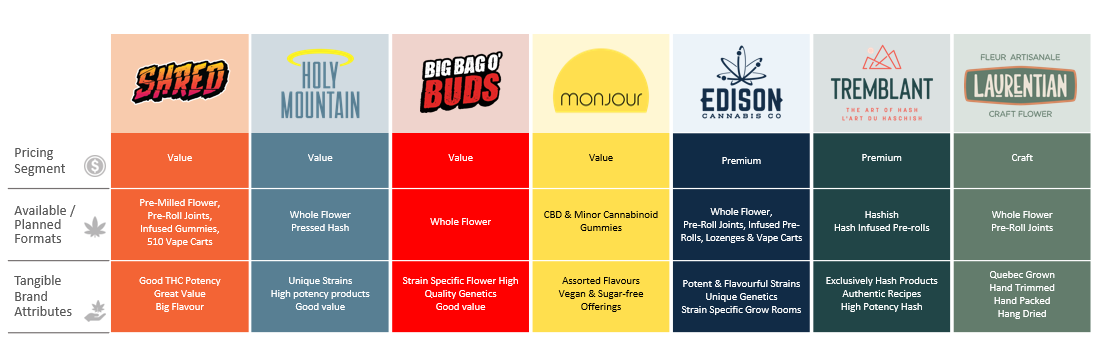
The Company developed its initial portfolio of adult-use recreational cannabis brands to specifically meet the evolving needs of Canada’s adult-use recreational cannabis market. The suite of brands created by the Company for Canada’s adult-use market include SHRED, Big Bag O’ Buds, Monjour, Edison Cannabis Co., Tremblant and Laurentian. Each brand is unique to a specific customer demographic with a product offering designed to meet the needs of its target audience, as described below, including as to potency and price point.
Medical Cannabis
The Company offers a broad offering of medical cannabis products, including cannabis flowers, cannabis oils and vaporizers to suit a variety of preferences.
New Product Development and Innovation
Research & Development
The Company continues to focus on consumer driven innovation, research and product development. It has made significant changes to its R&D process to ensure focus, development agility and increase effectiveness of both derivative product development and utilization of all cannabis material for innovation. In June 2022, the Company’s bio laboratory was commissioned and several plant science discoveries have already been made which should benefit the Company’s current plant portfolio and long term cultivation strategy. The sensory testing program has been expanded to over 200 expert panel members, segmented and trained to be able to assess the qualities and attributes of different product categories. Commercialization has been enhanced and automated using new, advanced project management software to allow for more efficient and agile planning and deployment of products into market.
As part of waste stream innovation efforts, the Company has optimized its CO2 and solventless extraction capabilities. The CO2 optimization has results in significant increase to distillate capacity reducing reliance on purchases from third parties. The solventless extraction stream manufactures kief in various grades used in the concentrates and hash making process. With these new processes, the Company is now utilizing a significant amount of cannabis trim which otherwise would’ve been wasted.
CoE and other Investments
The PDC Agreement entered into with BAT in March 2021 and the strategic investment of approximately $221 million in the Company by BAT is another example of the Company’s hallmark dedication to consumer driven product innovation. The strategic collaboration with BAT strengthens Organigram’s ability to create innovative, differentiated products that appeal to adult consumers. In addition, the Company continues to support efforts in cannabinoid biosynthesis via its investment in Hysaysnth. No assurance can be given that the Company will be successful in bringing these products to the market. See Risk Factors “The Company May Not be Able to Successfully Develop New Products or Find a Market for Their Sale”.
The Company and BAT entered into the PDC Agreement pursuant to which the CoE has been established to focus on research and product development activities for the next generation of cannabis products with an initial focus on CBD. The CoE is located at the Moncton Campus, which holds the Health Canada licenses required to conduct R&D activities with cannabis products. Both companies are contributing scientists, researchers, and product developers to the CoE which is governed and supervised by a steering committee consisting of an equal number of senior members from both companies. Under the terms of the PDC Agreement, both Organigram and BAT have access to certain of each other’s intellectual property
(“IP”) and, subject to certain limitations, have the right to independently, globally commercialize the products, technologies and IP created by the CoE pursuant to the PDC Agreement.
Per the PDC Agreement, Organigram and BAT have agreed to jointly develop cannabis vapour products, cannabis oral products and any other products, IP or technologies the parties mutually agree to develop. BAT will own all IP developed under this collaboration and will grant to Organigram a royalty-free, perpetual, global licence to all such IP. Each party has also agreed to grant to the other a non-exclusive, perpetual and irrevocable license to certain existing IP of such party and its affiliates for purposes of conducting the development activities and exploiting the products, technologies and IP created by the CoE per the PDC Agreement, subject to certain restrictions.
All key working spaces of the CoE have now been completed including the R&D laboratories, enhanced analytics space, quality assurance and control laboratory, GPP production space, sensory testing laboratory and a state of the art biolab for advanced plant science research. The CoE has undertaken initial stage development and safety studies on first generation edibles and novel beverages as part of its scope of work. As part of the development, the CoE has created and assessed numerous delivery systems and created over 60 unique formulations to develop differentiated products in the future. In addition, since September 2021, the CoE has completed early-stage work on first generation vapour innovation, with a focus to developing an enhanced consumer sensorial experience and addressing current product category challenges.
Distribution and Sales
Adult-Use Recreational Cannabis
The Cannabis Act provides provincial, territorial and municipal governments with the authority to prescribe regulations regarding retail and distribution of adult-use recreational cannabis. As such the distribution model for adult-use recreational cannabis is prescribed by provincial regulations and differs from province to province. Some provinces have government-run retailers, while others have government-licensed private retailers, and some have a combination of the two. The Company is authorized by its License for wholesale distribution of adult-use recreational cannabis and certain derivative products in all provinces of Canada and in Yukon, and has supply arrangements in different forms with the responsible government agency or equivalent in each province and in Yukon.
The Cannabis Act introduced restrictions on the promotion of cannabis products, cannabis accessories and services related to cannabis. These include restrictions on the content of promotions as well as locations where promotions may take place. With this in mind, the Company has created a portfolio of brands that address unique customer needs, including potency, yield, flavours, occasions, price points, volume discounts or promotional pricing. As the industry matures, certain seasonal sales trends are starting to emerge such as an increased popularity for pre-rolls during the summer months and an increased popularity for vape pens during the winter months. See “Description of the Business - Canadian Regulatory Framework” for additional information on current distribution channels under the Cannabis Act.
Medical Cannabis
The Company recently changed its distribution model for medical cannabis and as of November 1, 2022, exclusively distributes medical cannabis through Medical Cannabis by Shoppers, the online medical cannabis platform by Shoppers. The online medical cannabis platform by Shoppers offers a wide range of medical products and dedicated customer service. The Company’s prices for cannabis and derivative products vary based on growth time, strain yield and market prices. The Company may from time to time offer volume discount or promotional pricing. The Company is also authorized for wholesale shipping of medical cannabis plant cuttings and dried flower to other licensed producers. The Company is also able to ship wholesale medical cannabis products to certain international jurisdictions by obtaining the required approvals and permits from Health Canada and the applicable regulatory authority of the purchaser. See “Description of the Business - Canadian Regulatory Framework” for additional information on current distribution channels under the Cannabis Act.
Cannabis Edibles
Effective October 17, 2019, the Cannabis Regulations permit the sale to the public of edible cannabis products and concentrates through medical and adult-use consumer channels. Following the investment in a high-speed, high-capacity, fully automated production line, the Company began shipping their cannabis-infused chocolates to retailers across Canada in February 2020. The Company subsequently began evaluating future prospects for its chocolate production line based on its declining utilization and declining revenues from its chocolate output and ultimately made the strategic decision to cease manufacturing chocolate products. Following the EIC acquisition, the Company launched SHRED’ems cannabis-infused gummies and Monjour, a brand focusing on CBD-forward products.
Cannabis Extracts and Concentrates
The Company continues to expand its vape pen offering by having introduced a live resin vape cartridge as well as 3 ShredX vapes featuring distillate and proprietary botanical terpene blends. The Company has added three new flavours to its existing high potency THC lozenge, Edison JOLTS. As part of the LAU acquisition, the Company has also established a robust hash innovation pipeline, has expanded the Tremblant hash brand nationally via new higher potency hash temple balls, and has launched 2 new hash products into the market.
Operations
The Company has assembled a capable management team with significant experience in the management and growth of successful enterprises.
Presently, a significant portion of the Company’s revenue is derived from the sale of cannabis, cannabis product and cannabis plant material produced, cultivated and/or processed by the Company at its Moncton Campus. The Company grows cannabis at its Moncton Campus for the purposes of sale and distribution of finished products in accordance with the Cannabis Act. The Moncton Campus has 115 grow rooms with staggered cultivation cycles averaging 14 weeks per cycle. As the grow rooms are all indoor, seasonality has negligible impact on cultivation cycles.
In July 2022, the Company acquired the Winnipeg Facility which it had been previously leasing. EIC currently holds a research license, standard processing license and sales license issued under the Cannabis Act. The Company is currently producing cannabis-infused gummies and cannabis-infused lozenges at the Winnipeg Facility.
Storage and Security
The Cannabis Act prescribes physical security requirements that are necessary to secure sites where licensed producers conduct activities with cannabis. All facilities currently in production operate in accordance with the Cannabis Act requirements, including in relation to the security requirements. Health Canada conducts ad hoc, unscheduled site inspections of licensed producers under the Cannabis Act. The Company has been subject to these inspections numerous times. The Company has responded to and complied with all requests from Health Canada within the time frames indicated in such requests. As of the date hereof, there are no outstanding inspection issues with Health Canada beyond day-to-day adjustments that may occur in order to ensure ongoing compliance. For a summary of the requirements. See “Risk Factors”.
Specialized Skill and Knowledge
The nature of growing cannabis is not substantially different from the nature of growing other agricultural products. Variables such as temperature, humidity, and lighting, air flow, watering and feeding cycles are meticulously defined and controlled to produce consistent product and to avoid contamination. The product is cut, sorted and dried under defined conditions that are established to protect the activity and purity of the product. Once processing is complete, each and every processed batch is subjected to full testing against stringent quality specifications set for activity and purity. The Company has recruited a
production team with specialized skill sets unique to indoor agricultural cultivation and processing of cannabis plants and products at industrial scale.
In addition, in order to ensure compliance with the Cannabis Act and any directives issued by Health Canada, which includes strict security measures, equipment required to manage production, HVAC systems, odour control systems and laboratory equipment to monitor and test product quality, the Company must employ a number of regulatory personnel to assist the Company to remain compliant with the complex and rapidly evolving regulations applicable to the industry. The Company has successfully recruited the necessary personnel with this skill set.
The Company’s management includes individuals who have extensive expertise in the cannabis industry. In addition, the Company’s board of directors is constituted by experienced professionals from various relevant industries. See “Directors and Executive Officers” for additional information.
Competitive Conditions
As of the date hereof, Health Canada has issued cultivation, processing or cannabis sales licences to a total of 926 licence holders. There are also a number of unlicensed growers of cannabis which compete with the legal market. The Cannabis Act allows for adults to legally grow up to four cannabis plants for personal use, however, the Company believes that competition from homegrown cannabis is limited and does not currently have a significant impact on market demand for high quality cannabis flower.
On May 8, 2019, Health Canada introduced changes to the cannabis licensing process. Under the new system, new applicants for licenses will be required by Health Canada to have a fully built site that meets all the requirements under the Cannabis Regulations at the time of their application. The Company believes that this requirement in addition to the extensive regulatory restrictions and large amounts of financing required for operations, reduces the number of large-scale licensed producers that can compete nationally and internationally, at least in the short term.
However, as the demand for cannabis and cannabis products increases both nationally and internationally, the Company believes new competitors will enter the market. The principal aspects of competition between the Company and its competitors will be the price and quality of the products offered and client service provided to patients, government entities and private retailers.
As edible cannabis products have now been legalized in Canada, they have become a large market for licensed producers. Edibles are an attractive alternative that appeals to a broader audience, particularly to those who are not interested in smoking. The Company continues to invest in new product development through research and development, the acquisition of new technologies and the EIC and LAU acquisitions. The Company deployed a strategy aimed at product depth as opposed to breadth to maintain its strong track record of delivering on supply commitments, which is critical to building brand equity. The Company has launched vaporizer pen products, hash products, cannabis-infused gummies, and first-to-market ingestible extracts.
In respect of hemp and hemp-derived CBD, with the increased interest in CBD internationally, the Company believes that the industrial hemp market will likely continue to expand. Market entrants in Canada face regulatory hurdles which may impede or delay access to the market.
There is potential that the Company will face intense competition from other cannabis producers, some of which having longer operating histories and more financial resources and manufacturing and marketing experience than the Company. Increased competition by larger and better financed competitors could materially and adversely affect the business, financial condition and results of operations of the Company.
Employees
As of August 31, 2022, the Company employed approximately 882 employees, with approximately 722 working at the Moncton Campus, and as of October 31, 2022, the Company employed approximately 931 employees.
Canadian Regulatory Framework
On October 17, 2018, the Cannabis Act and the Cannabis Regulations came into force, legalizing the sale of cannabis for adult recreational use in Canada. Prior to the Cannabis Act and the Cannabis Regulations coming into force, only the sale of medical cannabis was legal. Such sales of medical cannabis were regulated under the ACMPR.
The Cannabis Act and Cannabis Regulations establish a licensing and permitting scheme for the production, importation, exportation, testing, packaging, labelling, sending, delivery, transportation, sale, possession and disposal of cannabis both for medical and non-medical use (i.e. adult recreational use).
The Cannabis Act and the Cannabis Regulations replaced the CDSA and the ACMPR regarding rules for the production, sale and distribution of medical cannabis and related CANNABIS oil extract. The Cannabis Act maintains separate access to cannabis for medical purposes, including providing that import and export licenses and permits will be issued only in respect of cannabis for medical or scientific purposes or in respect of industrial hemp.
Transitional provisions of the Cannabis Act provided that every license, permit and security clearance issued under the ACMPR that was in force immediately before the day on which the Cannabis Act came into force (being October 17, 2018) is deemed to be a license or permit issued under the Cannabis Act and that such license or permit will continue in force until it is revoked or expires.
On May 8, 2019, Health Canada changed its licensing criteria for new applicants for licenses to cultivate, process and sell cannabis for medical purposes. These categories of license applicants are now required to have a fully built site that meets all the requirements of the Cannabis Regulations at the time of their application, as well as satisfying any other applicable application criteria.
On October 17, 2019, the Regulations Amending the Cannabis Regulations (the “Amended Cannabis Regulations”) came into force. The Amended Cannabis Regulations amend the Cannabis Act and Cannabis Regulations to, among other things, allow for the production, distribution and sale of cannabis extracts, cannabis topicals and cannabis edibles in addition to the other previously permitted product categories. The Amended Cannabis Regulations set out certain requirements for the sale of cannabis products, including limiting the THC content and serving size of certain product forms.
Certain provinces have imposed restrictions on the launch and sale of edible and vaporizable products in their markets, including Quebec and Newfoundland and Labrador. Additionally, in February 2021 Health Canada announced its intent to restrict the use of flavours in vaporizable products in the near future and in June 2021 Health Canada proposed amendments that would restrict inhalable cannabis extracts from having a flavour other than the flavour of cannabis. These amendments were expected to come into force in 2022, however they are not yet in force as of the date hereof. As the market and regulations continue to develop the impact of these announcements is not readily determinable at this time. Given that the Cannabis Act and the Cannabis Regulations only recently came into force, the full impact of such regulatory changes on the Company’s business is unknown. See Risk Factors “Changes in Laws, Regulations and Guidelines”.
Licenses, Permits and Authorizations
The Cannabis Regulations establish six classes of licenses under the Cannabis Act: cultivation licenses; processing licenses; analytical testing licenses; licenses for sale; research licenses; and cannabis drug licenses. The Cannabis Regulations also create subclasses for cultivation licenses (standard cultivation, micro-cultivation and nursery) and processing licenses (standard processing and micro-processing). Different licenses and each subclass therein carry differing rules and requirements that are intended to be proportional to the public health and safety risks posed by each license category and subclass. The Cannabis Regulations provide that all licenses issued under the Cannabis Act must include both the effective date and expiry date of the license, and may be renewed on or before the expiry date.
The Cannabis Regulations permit license holders to conduct activities only at the site and building set out in the license (except for destruction, antimicrobial treatment and distribution) and no licensed activities
can take place in a “dwelling-house”. The holder of a license must not produce, test, store, package or label cannabis outdoors, except for obtaining cannabis by cultivating, propagating or harvesting it.
The Industrial Hemp Regulations (“IHR”) promulgated under the Cannabis Act came into force on October 17, 2018. The regulatory scheme for industrial hemp remained largely the same; however, the IHR permit the sale of hemp plants to licensed cannabis producers, the use of additional parts of the hemp plant and licensing requirements have been eased in accordance with the low risk posed by industrial hemp. The IHR define “industrial hemp” as cannabis plants whose leaves and flowering heads do not contain more than 0.3% THC.
Security Clearances
Certain people associated with cannabis licensees, including individuals occupying a “key position” such as directors, officers, significant shareholders and individuals identified by Canada’s Minister of Health (the “Minister”), must hold a valid security clearance issued by the Minister. Under the Cannabis Regulations, the Minister may refuse to grant security clearances to individuals with associations to organized crime or with past convictions for, or in association with, drug trafficking, corruption or violent offences. This is largely the approach that has been in place under the ACMPR and other related regulations governing the licensed production of cannabis for medical purposes. Individuals who have histories of nonviolent, lower-risk criminal activity (e.g. simple possession of cannabis, or small-scale cultivation of cannabis plants) are not precluded by legislation from participating in the legal cannabis industry, and the grant of security clearance to such individuals is at the discretion of the Minister and such applications are reviewed on a case-by-case basis. Security clearances issued under the ACMPR are considered to be security clearances for the purposes of the Cannabis Act and the Cannabis Regulations.
Cannabis Tracking and Licensing System
Under the Cannabis Act, the Minister is authorized to establish and maintain a national cannabis tracking system. The Cannabis Regulations set out a national cannabis tracking system to track cannabis throughout the supply chain to help prevent diversion of cannabis into, and out of, the legal market. The Cannabis Act also provides the Minister with the authority to make a ministerial order requiring certain persons named in such order to report specific information about their authorized activities with cannabis, in the form and manner specified by the Minister. Accordingly, the Minister has introduced the Cannabis Tracking and Licensing System, and license holders are required to use this system to submit monthly reports to the Minister, among other things, pursuant to the Cannabis Tracking System Order.
Cannabis Products
As of October 17, 2018, the Cannabis Act and Cannabis Regulations permitted the sale to the public of dried cannabis, cannabis oil, fresh cannabis, cannabis plants, and cannabis seeds by authorized license holders. On October 17, 2019, the Amended Cannabis Regulations came into force adding edibles containing cannabis, cannabis extracts and cannabis topicals as new classes of cannabis permitted to be sold through medical and adult-use consumer channels. The THC content and serving size of cannabis products is limited by the Cannabis Regulations. Effective October 17, 2020 cannabis oil was deleted from the Cannabis Act and products must be sold as cannabis extracts, subject to compliance with other prohibitions and requirements of the Cannabis Regulations. See “Changes in Laws, Regulations and Guidelines”.
Packaging and Labelling
The Cannabis Regulations set out requirements pertaining to the packaging and labelling of cannabis products, which requirements are intended to promote informed consumer choice and allow for the safe handling and transportation of cannabis, while also reducing the appeal of cannabis to youth and promoting safe consumption. These requirements include plain packaging for cannabis products, strict requirements for logos, colours and branding as well as packaging that is tamper-proof and child-resistant. The Cannabis Regulations further require mandatory health warnings, product source information, including the class of cannabis and the name, phone number and email of the processor, the standardized cannabis symbol and specific product information including the THC and CBD content.
Advertising
The Cannabis Act introduces strict restrictions on the promotion of cannabis products to, among other things, prevent promotion that could be appealing to young persons or evoke a positive or negative emotion about or image of, a way of life. Specifically, the Cannabis Act prohibits the promotion of cannabis, cannabis accessories or any services related to cannabis, unless such promotion is authorized under the Cannabis Act. Therefore, the Company may only advertise or promote its products in compliance with the provisions of the Cannabis Act.
Cannabis for Medical Purposes
The Cannabis Regulations set out the regime governing access to medical cannabis which largely reflects the rules under the ACMPR. Patients who have the authorization of their healthcare provider continue to have access to medical cannabis, either purchased directly from a federally licensed producer, or by registering to produce a limited amount of cannabis for their own medical purposes, or designating someone to produce cannabis for them.
Provincial Regulatory Framework
While the Cannabis Act provides for the regulation of the commercial production of cannabis for adult-use recreational purposes and related matters by the Government of Canada, the Cannabis Act enables the provinces and territories of Canada to regulate other aspects of adult-use recreational cannabis (similar to what is currently the case for liquor and tobacco products), such as sale and distribution, minimum age requirements, places where cannabis can be consumed, and a range of other matters.
As at the date hereof, the Company has entered into arrangements with distributors in all the provinces of Canada and the Yukon. The nature of these arrangements vary by jurisdiction.
The governments of every Canadian province and territory have, to varying degrees, enacted regulatory regimes for the distribution and sale of cannabis for adult-use recreational purposes within those jurisdictions. Most of these Canadian jurisdictions have a minimum age of 19 years old, except for Québec and Alberta, where the minimum age is 21 and 18, respectively.
There are three general frameworks enabled by provincial and territorial governments: (i) private cannabis retailers licensed by the provincial government; (ii) government run retail stores; and (iii) a combination of both frameworks. Regardless of the framework, the adult-use recreational cannabis market is ultimately supplied by federally licensed cultivators and processors. In most instances, provinces and territories have a government run wholesaler that is the exclusive source of cannabis products for retailers. The wholesalers, in turn, acquire cannabis products from the federally licensed cultivators and processors. The following chart outlines the current basic regime for adult-use recreational cannabis sales in each province and territory of Canada.
| | | | | | | | |
| Activity | Privately Operated | Publicly Operated |
| Storefront Adult Use Sale | British Columbia
Alberta
Saskatchewan
Manitoba
Ontario
Newfoundland and Labrador Nunavut Yukon Northwest Territories | British Columbia
Quebec
New Brunswick
Nova Scotia
Prince Edward Island |
| Online Adult Use Sale | Alberta Saskatchewan
Manitoba Nunavut Yukon | British Columbia
Ontario
Quebec
New Brunswick
Nova Scotia
Prince Edward Island
Newfoundland and Labrador Northwest Territories |
Israeli Regulatory Framework
Cannabis, including all parts of the plant and the roots (but excluding oil extracted from its seeds) is defined, under the Israeli Dangerous Drugs Ordinance (New Version), 5737-1973, as a “dangerous drug” such that the sale and use of cannabis are prohibited unless a permit has been granted by the Israeli Ministry of Health (the “MOH”).
In recent years, the MOH has made a significant progress in regulating cannabis for medical use – with the end goal of treating medical cannabis as any other pharmaceutical drug. As part of such “medicalization” of cannabis progress, the Israeli Medical Cannabis Agency (the “IMCA”), was established (acting as a “Government Agency” pursuant to the United Nations Single Convention on Narcotic Drugs of 1961), and has been granted the authority to issue licenses for the use of cannabis for medical purposes and for the following aspects related to medical cannabis supply chain: cultivation, manufacturing, storage, sale and for medical cannabis related research. Under such medicalization reform, the IMCA has issued several directives, which set clear and detailed standards and requirements for obtaining licenses for all such aspects of the medical cannabis.
The MOH issued, in December 2016 (last updated in November 2021) a procedure titled “Guidelines for Approval of Applications for Importation of Dangerous Drug of Cannabis Type for Medical Use and for Research” (“Procedure 109”). Procedure 109 provides guidelines for import applications and respective approval process, for research and medical use of cannabis. According to Procedure 109, the following permits and licenses are required to be obtained in order to be granted cannabis import license (for medical and research uses): (i) IMCA granted license to possess and operate in the medical cannabis field; (ii) a license to import plant material (to the extent that the imported cannabis is in a form of a plant, such as seeds, tissue culture), granted by the applicable department within the Israeli Ministry of Agriculture; (iii) a permit to import narcotic drugs, pursuant to the Dangerous Drugs Ordinance; and (iv) a license to import a dangerous drug, granted by the Department of Pharmaceutical Imports and Narcotics within the MOH. The IMCA has the discretion to grant or refuse to grant an import license, as well as to revoke a license previously granted. According to Procedure 109, importation of raw materials (cannabis inflorescence) will only be permitted if such plant materials were grown and cultivated in a post-harvest IMC-GAP certified facility (or an equivalent certification, such as CUMCS), or alternatively if the farm is GACP certificated (or equivalent), and the post-harvest facility if EU-GMP certified. Finished medical cannabis products are only allowed to be imported if they were manufactured in accordance with IMC-GMP or equivalent standard (e.g. EU-GMP).
On January 31, 2022, the Economic Affairs Committee of the Israeli Parliament held a discussion regarding the adverse effect of substantial import of medical cannabis on the local industry. The discussion was summoned with a request that the MOH would study the matter and consider halt of importation until a balance is reached between import and export of medical cannabis. In July 2022, a committee appointed by the Director General of the MOH for the purpose of examination of the professional and regulatory framework that will allow the transition from a licensing regime to a prescriptions’ regime with respect to the use of medical cannabis (the “Committee”), published its recommendations. The Committee concluded that it will be advisable to transit from the grant of personal patient licenses to the issuance of prescriptions available through public healthcare services (the “Reform“). The new regulations adapting this Reform (the “Reform Regulations“) have been presented for the public comments. The Reform Regulations propose to amend the Dangerous Drugs Ordinance to allow medical cannabis to be prescribed by physicians, trained and certified as stipulated in the Reform Regulations, and to be held and distributed by pharmacies. The Reform Regulations will take effect 180 days following the adoption by the Israeli Parliament of the HMOs collection plan.
German Regulatory Framework
The Company, through its wholly-owned subsidiary 10870277 Canada Inc., has acquired a 25% interest in the capital of alpha-cannabis Pharma GmbH (“ACG”). In addition, the Company has entered into two supply agreements with ACG: one for the supply of CBD isolate from ACG, and the other for the supply of dried cannabis flower from Organigram Inc. to ACG, for which the Company has provided notice of termination.
On March 10, 2017, significant changes to the German Federal Law on Narcotic Drugs (Betäubungsmittelgesetz, “BtMG”) as well as changes of other related legal rules entered into force. The new standards now allow the prescription of medical cannabis in Germany. This changes the overall legal framework for importing, trading and cultivating cannabis as well as the import and trade of cannabinoids such as CBD into and within Germany.
Cannabis itself is subject to German drug and narcotics law. The question of whether CBD is also subject to German drug law depends on the intended use and the corresponding dosage of the CBD. In any case, the narcotics law is not applicable to CBD. For the import of cannabis into the EU, various permits under German drug and narcotics law are required. For the trade and export of CBD, if classified as a drug or active pharmaceutical ingredient, permits under German drug law or at least notifications to authorities are required.
Furthermore, based on the United Nations Single Convention on Narcotic Drugs (1961) and Sec. 19 Para. 2aBtMG, the Bundesinstitut fur Arzneimittel und Medizinprodukte (“BfArM”) established a so-called “Cannabis Agency” (Cannabisagentur) as soon as the latest changes of the law on narcotic drugs entered into force. The purpose of this agency is solely to control the future cultivation of medical cannabis in Germany. This includes the Cannabis Agency’s competence for the actual cultivation as well as for harvesting, processing, quality control, storage, packaging and distribution of cannabis to pharmaceutical wholesalers.
The Cannabis Agency only distributes cannabis that is grown in accordance with the “Good Agricultural and Collection Practice” for drugs and other relevant guidelines. The cultivation and distribution of cannabis is not technically executed by the Cannabis Agency. The agency therefore enters into “supply contracts and distribution contracts” with agricultural businesses and distributors. The supply contracts are limited in respect of their duration and the quantity of cannabis the business is allowed to grow. Cultivation of medical cannabis is therefore not allowed by granting general licenses. To enter into a supply contract or distribution contract as mentioned before, businesses must first be selected in a public call for tender procedure. Such tender procedures shall be open to be entered by suppliers in the whole European Union. The necessity of a tender process is based on the fact that medical cannabis must be bought as part of a public procurement procedure. The first allowances for the growing of medical cannabis in Germany based on a tender procedure were issued on April 17, 2019. Organigram and ACG jointly submitted a tender for domestic cultivation in that process, but were unsuccessful.
Australian Regulatory Framework
Under Australia’s federal system, activities related to medical cannabis are regulated at both the Commonwealth (national/federal) level and at the individual state and territory level. In October 2016, the Australian Government introduced amendments to the Narcotic Drugs Act 1967 (Cth) (the “ND Act”), through the Narcotic Drugs Amendment Act 2016 (Cth), and a new Narcotic Drugs Regulation 2016 (Cth) (the “ND Regulation”) which introduced a Commonwealth (national) licensing and permit scheme for the cultivation, production and manufacture of medical cannabis and medical cannabis products. The scheme is administered primarily by the Office of Drug Control (ODC) within the Commonwealth Department of Health and Aged Care. The scheme was amended in 2021 by the passage of the Narcotic Drugs Amendment (Medicinal Cannabis) Act 2021 (Cth), to streamline the current Commonwealth licensing and permit scheme.
The Therapeutic Goods Act 1989 (Cth) (the “TG Act”) and its subordinate legislation (particularly the Therapeutic Goods Regulations 1990 (Cth) (the “TG Regulations”) also operate at the Commonwealth level in parallel to the ND Act and ND Regulation to more generally regulate therapeutic goods, including medical cannabis products. Such regulation covers their import into or export from Australia, and their manufacture, advertisement and supply in Australia. Further, for medical cannabis products that are imported into Australia, there is an additional import regime that applies under the Customs Act 1901 (Cth), and the Customs (Prohibited Imports) Regulations 1956 (Cth) (the “CPI Regulations”). Although the regimes under the ND Act and TG Act described above touch on import and/or export activities, the import of medical cannabis products also requires compliance with the additional import-specific requirements of the CPI Regulations.
As ‘prohibited drugs’ under those regulations, cannabis and medical cannabis products (whether raw/starting materials, refined active ingredients or finished dosage forms) can only be imported by a person holding an import licence and import permit under the CPI Regulations. A licence must be obtained from the Drug Control Section of the ODC, which requires, among other things, establishing the qualifications and experience of the applicant, whether they are a fit and proper person to hold and fitness of the applicant to hold the licence and undertake the proposed activities, and that adequate security arrangements will be implemented in respect of the goods.
All medical cannabis products manufactured in Australia (or imported for human therapeutic use in Australia) must also meet all mandatory standards applicable to such goods under the TG Act. Key among these are the standards set out in Therapeutic Goods Order No. 93 (Standard for Medicinal Cannabis) (“TGO 93”). Amendments to TGO 93 were made in March 2022, which are subject to transition periods to allow industry to make changes to their products, with all medical cannabis products released for supply in Australia on or after July 1, 2023 needing to comply with these requirements.
The states and territories regulate lawful dealings in medicines and poisons primarily by reference to their scheduling status. Medicines and poisons are categorized into different schedules depending on their intended use(s) and potential for harm, with the intention that different levels of control will be applied by reference to the different schedules. There is a measure of national uniformity due to the fact that the categorization of substances into schedules occurs at the Commonwealth level, through promulgation and regular amendment of the Standard for the Uniform Scheduling of Medicines and Poisons (the “Poisons Standard”). However, each state and territory adopts the Poisons Standard (with occasional jurisdictional modifications) and through its own laws to a similar (but not identical) extent the various intended controls. Medical cannabis products for human therapeutic use are mostly in Schedule 8, a category which the Poisons Standard describes as ‘controlled drugs’, being substances which should be available for use but require restrictions on manufacture, supply, distribution, possession and use to reduce abuse, misuse and physical or psychological dependence. A limited class of medical cannabis products containing predominantly cannabidiol are also listed in Schedule 4 (being ‘prescription-only medicines) and in Schedule 3 (‘pharmacist-only medicines’). Cannabis products for non-human research are in Schedule 9 – such ‘prohibited substances’ are susceptible to abuse or misuse and their manufacture, possession, sale or use should be prohibited by law except when required for medical or scientific research or for analytical, teaching or training purposes with approval of Commonwealth and/or state or territory health authorities.
RISK FACTORS
There are a number of risk factors that could cause future results to differ materially from those described herein. The risks and uncertainties described herein are not the only ones the Company faces. Additional risks and uncertainties, including those that the Company does not know about now or that it currently deems immaterial, may also adversely affect the Company’s business.
If any of the following risks actually occur, the Company’s business may be harmed and its financial condition and results of operations may suffer significantly.
Competition
There is potential that the Company will face intense competition from other companies, some of which having longer operating histories and more financial resources and production and marketing experience than the Company.
The cannabis industry and businesses ancillary to and directly involved with cannabis businesses are undergoing rapid growth and substantial change, which has resulted in an increase in competitors, consolidation and formation of strategic relationships. As such, we face competition from companies that may have greater capitalization, access to public equity markets, more experienced management or more maturity as a business. We are likely to continue to face increasing and intense competition from these companies. Increased competition by larger and better financed competitors could materially and adversely affect our business, financial condition and results of operations. We expect that competition will become more intense as current and future competitors begin to offer an increasing number of diversified products to respond to such increased demand. To remain competitive, we will require a continued investment in research and development, marketing, sales and client support. We may not have sufficient resources to maintain sufficient levels of investment in research and development, marketing, sales and client support efforts to remain competitive, which could materially and adversely affect our business, financial condition and results of operations.
Acquisitions or other consolidating transactions in the cannabis industry could harm us in a number of ways, including losing customers, revenue and market share, or forcing us to expend greater resources to meet new or additional competitive threats, all of which could harm our operating results. As competitors enter the market and become increasingly sophisticated, competition in our industry may intensify and place downward pressure on retail prices for our products and services, which could negatively impact our profitability.
Reliance on Key Personnel
The success of the Company is dependent upon the ability, expertise, judgment, discretion and good faith of its executive and senior management. The Company’s future success depends on its continuing ability to attract, develop, motivate and retain highly qualified and skilled employees. Qualified individuals are in high demand, and the Company may incur significant costs to attract and retain them. The loss of the services of A member of the Company’s executive and senior management, or an inability to attract other suitably qualified persons when needed, could have a material adverse effect on the Company’s ability to execute on its business plan and strategy, and the Company may be unable to find adequate replacements on a timely basis, or at all. Further, as designated individuals of a licensee under the Cannabis Act, key personnel of the Company are subject to a security clearance by Health Canada. There is no assurance that any of the Company’s key personnel who presently or may in the future require a security clearance will be able to obtain or renew such clearances or that new personnel who require a security clearance will be able to obtain one. A failure by any of those individuals to maintain or renew his or her security clearance, could result in a material adverse effect on the Company’s business, financial condition and results of operations. In addition, if any such individual leaves the Company, and the Company is unable to find a suitable replacement that has a security clearance required by the Cannabis Act in a timely manner, or at all, there could occur a material adverse effect on the Company’s business, financial condition and results of operations.
Changes in Laws, Regulations and Guidelines
The Company’s business is subject to a variety of laws, regulations and guidelines relating to marketing, acquisition, manufacture, management, transportation, storage, sale and disposal of cannabis but also laws and regulations relating to health and safety, the conduct of operations and the protection of the environment. Changes to such laws, regulations and guidelines may cause adverse effects to the Company’s operations.
The legislative framework pertaining to the Canadian adult-use recreational cannabis market is subject to significant provincial and territorial regulation, which varies across provinces and territories and results in an asymmetric regulatory and market environment, different competitive pressures and significant additional compliance and other costs and/or limitations on the Company’s ability to participate in such markets.
The laws, regulations and guidelines applicable to the cannabis industry domestically and internationally, including in Germany, Australia and Israel, may change in ways currently unforeseen by the Company. The Cannabis Act became effective on October 17, 2018, however, continued uncertainty exists with respect to the future implementation, interpretation and evolution of the Cannabis Act, federal regulations thereunder as well as the various provincial and territorial regimes governing the distribution and sale of cannabis for adult-use recreational purposes.
The laws and regulations may change both federally and provincially, with new rules and regulations arising regularly. The Cannabis Regulations were amended effective October 17, 2019, to allow for cannabis edibles to be introduced into the market and expand the use of cannabis derivatives commercially. However, the amendments are highly restrictive, and include restrictions on adding caffeine, nicotine, or alcohol to cannabis edibles.
Additional restrictions on edible and other cannabis derivative based products may also be introduced by the Provincial and Territorial governments. Effective January 1, 2020, the legal age to buy adult-use recreational cannabis increased to 21 in Quebec and the Quebec government banned the sale of certain edible cannabis products in the form of chocolate, candy and other desserts. Staying on side of regularly changing rules and regulations will require ongoing time and attention from Company management. In addition, the Company’s derivative product strategy includes vaporizable products which may be subject to negative consumer perception and may be subject to additional regulation and restriction over and above the current regulatory requirements in place under the Cannabis Act. This may include governmental restriction of the sale of such products and/or imposition of additional costs.
While the Company does not currently have a license issued under Section 9 of the Industrial Hemp Regulations, it may purchase industrial hemp from such licensees. Any change to the Cannabis Act or the Industrial Hemp Regulations promulgated thereunder that impacts suppliers’ ability to cultivate, produce, or sell industrial hemp to the Company could adversely impact the Company’s ability to deliver its products or services, should the Company depend on such supply to meet its product production goals or obligations.
Wholesale Price of Cannabis Volatility
The Company’s revenues are in a large part derived from the production, sale, and distribution of cannabis. The cost of production, sale, and distribution of cannabis is dependent on a number of key inputs and their related costs, including equipment and supplies, labour and raw materials related to our growing operations, as well other overhead costs such as electricity, water, and utilities. Any significant interruption or negative change in the availability or economics of the supply chain for key inputs could materially impact our financial condition and operating results. Any inability to secure required supplies and services or to do so on appropriate terms could have a materially adverse impact on our business, financial condition, results of operations and prospects. This includes any change in the selling price of products set by the applicable province or territory. There is currently no established market price for cannabis and the price of cannabis is affected by numerous factors beyond our control. Any price decline may have a material adverse effect on our business, financial condition and operations.
Reliance Primarily on a Single Cultivation Facility
To date, the Company’s cultivation activities have been primarily focused on the Moncton Campus in Moncton, New Brunswick and the Company will continue to primarily rely on it for the foreseeable future. Adverse changes or developments affecting the Moncton Campus could have a material and adverse effect on the Company’s business, financial condition and prospects, including impacting the quantity of product produced by the Company.
Acquisition and Integration Risk
The Company has in the past made and may in the future make acquisitions and investments that could divert management’s attention, result in operating difficulties and dilution to shareholders and otherwise disrupt the operations of the Company. The Company may have difficulty integrating any such acquisitions successfully or realising the anticipated benefits therefrom, any of which could have a material adverse effect on the Company’s business, financial condition, results of operations and cash flows. See “Developments during the financial year ended August 31, 2021” and “Developments during the financial year ended August 31, 2022” as it relates to the Company’s acquisitions of EIC and LAU, respectively.
Pursuing potential strategic acquisitions or investment opportunities is one possible growth strategy. Any transactions that the Company enters into could be material to its business, financial condition, results of operations, cash flows and prospects. The process of acquiring and integrating another company or technology could create unforeseen operating difficulties and expenditures. Acquisitions and investments involve a number of risks, including:
•diversion of management time and focus from operating the Company’s business;
•use of resources that are needed in other areas of the Company’s business;
•integration of the acquired company;
•implementation or remediation of controls, procedures and policies of the acquired company;
•difficulty integrating the accounting systems and operations of the acquired company;
•coordination of product, engineering and selling and marketing functions, including difficulties and additional expenses associated with supporting legacy services and products and hosting infrastructure of the acquired company;
•retention and integration of employees from the acquired company, and preservation of our corporate culture;
•the potential loss of key employees;
•unforeseen costs or liabilities, including the use of substantial portions of our available cash to consummate the acquisition;
•adverse effects to our existing business relationships with customers as a result of the acquisition or investment;
•the possibility of adverse tax consequences;
•litigation or other claims arising in connection with the acquired company or investment; and
•the need to integrate potential operations across different cultures and languages and to address the particular economic, currency, political and regulatory risks associated with specific countries.
The Company May Not be Able to Successfully Develop New Products or Find a Market for Their Sale
The medical and adult-use recreational cannabis industries are in the early stages of development and it is likely that the Company, and its competitors, will seek to introduce new products in the future, including edible cannabis products and cannabis derivatives. In attempting to keep pace with any new market developments, the Company may need to expend significant amounts of capital in order to successfully develop and generate revenues from new products introduced by the Company. As well, the Company may be required to obtain and maintain additional regulatory approvals from Health Canada and any other applicable regulatory authority, which may take significant amounts of time. The Company may not be successful in developing effective and safe new products, bringing such products to market in time to be effectively commercialized, or obtaining any required regulatory approvals, which, together with any capital expenditures made in the course of such product development and regulatory approval processes,
may have a material adverse effect on the Company’s business, financial condition and results of operations.
Volatile Market Price of the Company’s Securities
The market price of the Company’s securities may be volatile and subject to wide fluctuations in response to numerous factors, many of which are beyond the Company’s control. This volatility may affect the ability of holders of Company’s securities to sell their securities at an advantageous price. Market price fluctuations in the Company’s securities may be due to the Company’s operating results, failing to meet expectations of securities analysts or investors in any period, downward revision in securities analysts’ estimates, adverse changes in general market conditions, economic trends, acquisitions, dispositions, or material public announcements by government and regulatory authorities, the Company or its competitors, along with a variety of additional factors. These broad market fluctuations may adversely affect the market price of the Company’s securities.
Financial markets have at times historically experienced significant price and volume fluctuations that have particularly affected the market prices of equity securities of companies and that have often been unrelated to the operating performance, underlying asset values or prospects of such companies. Accordingly, the market price of the Company’s securities may decline even if the Company’s operating results, underlying asset values or prospects have not changed. Additionally, these factors, as well as other related factors, may cause decreases in asset values that are deemed to be other than temporary, which may result in impairment losses. There can be no assurance that continuing fluctuations in price and volume will not occur. If such increased levels of volatility and market turmoil continue, the Company’s operations could be adversely impacted and the trading price of the Company’s securities may be materially adversely affected.
Failure to Develop and Maintain Effective Internal Controls for Reliable Financial Results and to Prevent Fraud (SOX)
Under Section 404 of the Sarbanes-Oxley Act (“SOX”) and SEC rules promulgated thereunder, the Company is required to design, document and test the effectiveness of our internal controls over financial reporting (“ICFR”) during the fiscal year ended August 31, 2022. There is no assurance that our efforts to design, develop and maintain our internal controls will be successful or sufficient to meet our obligations under SOX. Effective internal controls are required for the Company to accurately and reliably report our financial results and other financial information. Any failure to design, develop or maintain effective controls, or difficulties encountered in implementing, improving or remediating lapses in internal controls, may affect the Company’s ability to prevent fraud, detect material misstatements, and fulfill its reporting obligations. We do not know the specific time frame needed to fully remediate the material weaknesses identified below.
The design of any system of internal controls is based in part upon certain assumptions about the likelihood of future events, and there can be no assurance that any design will succeed in achieving the stated goals under all potential future conditions. In addition, regardless of how well controls are designed, internal controls have inherent limitations and can only provide reasonable assurance that the controls are meeting the Company’s objectives in providing reliable financial reporting information in accordance with International Financial Reporting Standards, as issued by the International Accounting Standards Board.
The Company has limited the scope of its evaluation of ICFR to exclude controls, policies and procedures over entities that were acquired by the Company not more than 365 days before the end of the financial period. The only entity controlled by the Company but that was scoped out of the evaluation of ICFR was LAU (acquired on December 21, 2021). Excluding goodwill and intangible assets, LAU constitutes approximately 2% of the Company’s current assets, 5% of total assets, 7% of current liabilities and 11% of total liabilities, as well as 5% of net revenue and 3% of net loss as at and for the year ended August 31, 2022.
The Company’s management, under the supervision and with the participation of its CEO and CFO, conducted an evaluation of the effectiveness of the Company’s ICFR as of August 31, 2022, using the criteria set forth by the COSO 2013 Framework. Based on this evaluation, management concluded that
internal control over financial reporting was not effective as of August 31, 2022, due to the following material weaknesses in internal control over financial reporting.
A material weakness is a deficiency, or a combination of deficiencies, in internal control over financial reporting, such that there is a reasonable possibility that a material misstatement of the Company’s annual or interim financial statements will not be prevented or detected on a timely basis. Management has identified the following material weaknesses:
•An ineffective control environment resulting from an insufficient number of trained financial reporting and accounting, information technology (IT) and operational personnel with the appropriate skills and knowledge and with assigned responsibility and accountability related to the design, implementation and operation of ICFR.
•The insufficient number of personnel described above contributed to an ineffective risk assessment process necessary to identify all relevant risks of material misstatement and to evaluate the implications of relevant risks on its ICFR.
•An ineffective information and communication process resulting from: (i) insufficient communication of internal control information, including objectives and responsibilities, such as delegation of authority; and (ii) ineffective general IT controls, ineffective controls related to complex spreadsheets, and ineffective controls over information from service organizations, resulting in insufficient controls to ensure the relevance, timeliness and quality of information used in control activities.
•As a consequence of the above, the Company had ineffective control activities related to the design, implementation and operations of process level and financial statement close controls which had a pervasive impact on the Company’s ICFR.
Cyber Security
The Company has entered into agreements with third parties for hardware, software, telecommunications and other information technology services in connection with its operations. The Company’s operations depend, in part, on how well it and its suppliers protect networks, equipment, information technology systems and software against damage from a number of threats, including, but not limited to, cable cuts, damage to physical plants, natural disasters, intentional damage and destruction, fire, power loss, hacking, computer viruses, vandalism and theft. The Company’s operations also depend on the timely maintenance, upgrade and replacement of networks, equipment, information technology systems and software, as well as pre-emptive expenses to mitigate the risks of failures. Any of these and other events could result in information system failures, delays, and/or increase in capital expenses. The failure of information systems or a component of information systems could, depending on the nature of any such failure, adversely impact the Company’s reputation and results of operations.
The Company has not experienced any material losses to date relating to cyber-attacks or other information security breaches, but there can be no assurance that the Company will not incur such losses in the future. The Company’s risk and exposure to these matters cannot be fully mitigated because of, among other things, the evolving nature of these threats. As a result, cyber security and the continued development and enhancement of controls, processes and practices designed to protect systems, computers, software, data and networks from attack, damage or unauthorized access is a priority. As cyber threats continue to evolve, the Company may be required to expend additional resources to continue to modify or enhance protective measures or to investigate and remediate any security vulnerabilities.
Competition from the Illicit Market
The Company also faces competition from unlicensed and unregulated market participants, including individuals or groups that process cannabis without a license under the Cannabis Act, including illicit medical dispensaries and other illicit participants selling cannabis in Canada. These competitors may be able to offer products with higher concentrations of active ingredients than the Company would be authorized to produce and sell. The competition presented by these participants, and any unwillingness by consumers currently using these illicit distribution channels to begin purchasing from the regulated market for any reason, or any inability of law enforcement authorities to enforce existing laws prohibiting the unlicensed cultivation, production and sale of cannabis, could adversely affect our market share, result
in increased competition through the illicit market for cannabis or have an adverse impact on the public perception of cannabis use, and of Canadian federal license holders.
Management of Growth
The Company may be subject to growth-related risks, including capacity constraints and pressure on its internal systems and controls. The ability of the Company to manage growth effectively will require it to continue to implement and improve its operational and financial systems and to expand, train and manage its employee base. If the Company is unable to deal with this growth, it may have a material adverse effect on the Company’s business, financial condition, results of operations and prospects.
Reliance on License Renewal
The Company’s ability to produce, store and sell cannabis, cannabis oil extracts and derivative products in Canada is dependent on its License from Health Canada. Failure to comply with the requirements of the License or any failure to maintain this License would have a material adverse impact on the business, financial condition and operating results of the Company. The OGI License expires on March 20, 2025, the EIC License expires December 11, 2023, and the LAU License expires on April 17, 2023. The Company intends to renew its licenses.
Although management believes it will meet the requirements of the Cannabis Act for extension of its licenses, there can be no guarantee that Health Canada will extend or renew the licenses or, if it is extended or renewed, that it will be extended or renewed on the same or similar terms. Should Health Canada not extend or renew the licenses, or should it renew the licenses on different terms or not provide the amendments as requested for anticipated capacity increases, the business, financial condition and results of the operations of the Company will be materially adversely affected. OGI, EIC and LAU have also been issued cannabis licenses under the Excise Act which is required to package and distribute cannabis and a research license as described herein.
Risks Inherent in an Agricultural Business
The Company’s business involves the growing of cannabis, an agricultural product. As such, the business is subject to the risks inherent in the agricultural business, such as insects, plant diseases and similar agricultural risks that may create crop failures, lower THC or less desirable products and supply interruptions for the Company’s customers. Although the Company grows its products indoors under climate-controlled conditions and carefully monitors the growing conditions with trained personnel, there can be no assurance that natural elements will not have a material adverse effect on the production of its products.
Negative Cash Flow from Operations
During the year ended August 31, 2022, the Company had negative cash flow from operating activities. The Company’s cash and short-term investments as at August 31, 2022, were approximately $98,607,000.
Although the Company anticipates it will have positive cash flow from operating activities in future periods, the Company cannot guarantee it will have a cash flow positive status in the future due to its desire to increase the number of employees and its level of participation in the adult-use recreational cannabis market in Canada. To the extent that the Company has negative cash flow in any future period, certain of the proceeds from its offerings may be used to fund such negative cash flow from operating activities.
Limited Operating History and History of Losses
The Company began its business in 2013 and generated minimal revenue until fiscal 2017 and incurred losses since inception. The Company’s adult-use recreational cannabis business has only been operative since legalization in October 2018 and the sale of its edible and derivative products is in its infancy. The Company is therefore subject to many of the risks common to early-stage enterprises, including limitations with respect to personnel and other resources and lack of revenues. There is no assurance that
the Company will be successful in achieving a return on shareholders’ investments and the likelihood of success must be considered in light of the early stage of operations.
Product Liability
As a manufacturer and distributor of products designed to be ingested or vaporized by humans, the Company faces an inherent risk of exposure to product liability claims, regulatory action and litigation if its products are alleged to have caused significant loss or injury. In addition, the manufacture and sale of the Company’s products involve the risk of injury to consumers due to tampering by unauthorized third parties or product contamination. Previously unknown adverse reactions resulting from human consumption of the Company’s products alone or in combination with other medications or substances could occur. The Company may be subject to various product liability claims, including, among others, that the Company’s products caused injury or illness, include inadequate instructions for use or include inadequate warnings concerning possible side effects or interactions with other substances. A product liability claim or regulatory action against the Company could result in increased costs, could adversely affect the Company’s reputation with its clients and consumers generally, and could have a material adverse effect on our results of operations and financial condition of the Company.
There can be no assurances that the Company will be able to obtain or maintain product liability insurance on acceptable terms or with adequate coverage against potential liabilities. Such insurance is expensive and may not be available in the future on acceptable terms, or at all. The inability to obtain sufficient insurance coverage on reasonable terms or to otherwise protect against potential product liability claims could prevent or inhibit the commercialization of the Company’s potential products. As of the current date, the Company has a small amount of insurance coverage for product liabilities.
Sufficiency of Insurance
The Company maintains various types of insurance which may include financial institution bonds; errors and omissions insurance; directors’ and officers’ insurance; property coverage; product liability recall insurance; and, general commercial and liability insurance. There is no assurance that claims will not exceed the limits of available coverage, if any, that any insurer will remain solvent or willing to continue providing insurance coverage with sufficient limits or at a reasonable cost, or that any insurer will not dispute coverage of certain claims. There is also no assurance that coverage will be available to cover any or all claims. A judgment against the Company or any member of the Company in excess of available coverage could have a material adverse effect on the Company in terms of damages awarded and the impact on the reputation of the Company. There can also be no assurance that the Company will be able to secure insurance coverage on commercially reasonable terms, or at all, as it may require to implement its business objectives, including with respect to derivative products.
Global Economic Risk
An economic downturn of global capital markets has been shown to make the raising of capital by equity or debt financing more difficult. The Company will be dependent upon the capital markets to raise additional financing in the future, while it establishes a user base for its products. As such, the Company is subject to liquidity risks in meeting its development and future operating cost requirements in instances where cash positions are unable to be maintained or appropriate financing is unavailable. These factors may impact the Company’s ability to raise equity or obtain loans and other credit facilities in the future and on terms favorable to the Company and its management. If uncertain market conditions persist, the Company’s ability to raise capital could be jeopardized, which could have an adverse impact on the Company’s operations and the trading price of the Company’s shares on the TSX and Nasdaq.
TSX and Nasdaq Requirements
The Common Shares commenced trading on the TSX on August 22, 2019 following the Company’s graduation from the TSX-V. The Company’s Common Shares also began trading on Nasdaq on May 21, 2019.
The Company is required to comply with TSX and Nasdaq rules, policies and guidelines, especially when pursuing business internationally. As a public company, the business is subject to corporate governance
and public disclosure requirements that may at times increase the Company’s compliance costs and risk of non-compliance. These regulations, rules, policies and guidelines may change over time, and failure to continue to meet them could result in significant material adverse consequences.
The TSX has issued guidance directed at cannabis companies, and specifically with respect to any company operating in the United States. In addition, in connection with its listing on Nasdaq, the Company certified that neither it nor any of its subsidiaries will conduct any business activities in the U.S., or utilize any employees, facilities or operations in the U.S. Presently, the Company has no business in the U.S., but this could present additional barriers in the future should the Company seek to do business in any form in the U.S. Any violation of U.S. federal law regarding cannabis could result in delisting of the Company from TSX and Nasdaq.
As a public company in the U.S., the Company is subject to additional legal, insurance, accounting, administrative and other costs and expenses which, in the aggregate, can be substantial.
Financing
There is no guarantee that the Company will be able to achieve its business objectives. The continued development of the Company may require additional financing. The failure to raise such capital could result in the delay or indefinite postponement of current business objectives or the Company ceasing to carry on business. There can be no assurance that additional capital or other types of financing will be available if needed or that, if available, the terms of such financing will be favorable to the Company. In addition, from time to time, the Company may enter into transactions to acquire assets or the shares of other corporations. These transactions may be financed wholly or partially with debt, which may increase the Company’s debt levels above industry standards.
Litigation
On January 18, 2019, the Nova Scotia Supreme Court certified a class action lawsuit by a class of persons and entities who purchased medical cannabis that was the subject of the Company’s product recalls in December 2016 and January 2017, as such products may have contained trace elements of the pesticides myclobutanil and bifenazate, which are not approved for use in cannabis production. On April 30, 2020, the Nova Scotia Court of Appeal partially overturned the certification of the class action, significantly reducing the scope of the claim against the Company. On June 23, 2022, the Company announced that it had reached the Settlement. As part the Settlement, the Company has agreed to pay a settlement amount in the aggregate of $2,310,000. On August 31, 2022, the Court held a hearing and approved the Settlement. See “Legal Proceedings and Regulatory Actions”.
A legal action has been commenced in the Court of Queen’s Bench in Alberta, which action seeks damages against many of the largest Canadian cannabis companies (including the Company). A certification hearing has not yet been scheduled.
In addition to ongoing litigation, the Company may become party to litigation from time to time in the ordinary course of business which could adversely affect its business. Should any litigation in which the Company becomes involved be determined against the Company, such a decision could adversely affect the Company’s ability to continue operating and the market price for the Common Shares, and could require the use of significant resources. Even if the Company is involved in litigation and wins, litigation can redirect significant Company resources.
Expansion into New Markets
The Company’s expansion into jurisdictions outside of Canada is subject to risks. In jurisdictions outside of Canada, there can be no assurance that any market for the Company’s products will develop. The Company may face new or unexpected risks or significantly increase its exposure to one or more existing risk factors, including economic instability, changes in laws and regulations, and the effects of competition. These factors may limit the Company’s ability to successfully expand its operations into such jurisdictions and may have a material adverse effect on the Company’s business, financial condition and results of operations. The Company has no investment or ownership in any U.S. entity nor does it provide any products or services to U.S. entities.
Product Recalls
On January 9, 2017, the Company expanded its voluntary recall to a further 69 lots of product in addition to the recall of five lots of product initiated on December 28, 2016. The recalled products included dried cannabis and cannabis oil supplied between February and December 2016, after testing revealed the presence of low levels of myclobutanil and/or bifenazate in some of the lots, which are unapproved pesticides not registered for use in cannabis production under the Pest Control Products Act. While the initial recall had classified the recall as a Type III recall (not likely to cause harm), the second recall elevated this classification to a Type II recall (product exposure may cause temporary adverse health consequences). There can be no assurance that additional adverse reaction reports will not be filed with Health Canada. To the extent any additional adverse reaction reports are filed, such an occurrence could have an adverse impact on the business, results of operations and financial condition of the Company. A class action lawsuit has also been filed, certified and now settled, as more particularly described in these risk factors under the subheadings “Product Liability”, “Litigation” and “Legal Proceedings and Regulatory Actions”.
Manufacturers and distributors of products are sometimes subject to the recall or return of their products for a variety of reasons, including product defects, such as contamination, unintended harmful side effects or interactions with other substances, packaging safety and inadequate or inaccurate labeling disclosure. Undiscovered product liability claims are always a potential risk. However, moving forward, if any of the Company’s products are recalled in the future due to an alleged product defect or for any other reason, the Company would be required to incur the unexpected expense of the recall and any legal proceedings that might arise in connection with the recall. The Company may lose a significant amount of sales and may not be able to replace those sales at an acceptable margin or at all. In addition, a product recall may require significant management attention thereby reducing the amount of time members of management would otherwise have focused towards managing the Company. Although the Company has detailed procedures in place for testing finished products, there can be no assurance that any quality, potency or contamination problems will be detected in time to avoid unforeseen product recalls, regulatory action or lawsuits. Additionally, if one of the Company’s significant brands were subject to recall, the image of that brand and the Company could be harmed. A recall for any of the foregoing reasons could lead to decreased demand for the Company’s products and could have a material adverse effect on the results of operations and financial condition of the Company. Additionally, product recalls may lead to increased scrutiny of the Company’s operations by Health Canada or other regulatory agencies, requiring further management attention and potential legal fees and other expenses.
Governmental Regulation
The business and activities of the Company are heavily regulated in all jurisdictions where the Company carries on business. The Company’s operations are subject to various laws, regulations and guidelines by governmental authorities, particularly Health Canada, relating to the manufacture, marketing, management, transportation, storage, sale, pricing and disposal of cannabis, cannabis extracts, and cannabis derivatives. The Company is also subject to laws and regulations relating to health and safety, insurance coverage, the conduct of operations and the protection of the environment. Laws and regulations, applied generally, grant government agencies and self-regulatory bodies broad administrative discretion over the activities of the Company, including the power to limit or restrict business activities as well as impose additional disclosure requirements on the Company regarding its products and services.
Achievement of the Company’s business objectives are contingent, in part, upon compliance with regulatory requirements enacted by governmental authorities and obtaining all regulatory approvals, where necessary, for the production and sale of its products. The Company cannot predict the time required to secure all appropriate regulatory approvals for its products, or the extent of testing and documentation that may be required by governmental authorities. Any delays in obtaining, or failure to obtain regulatory approvals would significantly delay the development of markets and products and could have a material adverse effect on the business, results of operations and financial condition of the Company.
Failure to comply with the laws and regulations applicable to the Company’s operations may lead to possible sanctions including the revocation or imposition of additional conditions on licenses to operate the Company’s business; the suspension or expulsion from a particular market or jurisdiction or of its key
personnel; the imposition of additional or more stringent inspection, testing and reporting requirements; and, the imposition of fines and censures. To the extent that there are changes to the existing laws and regulations or the enactment of future laws and regulations that affect the sale or offering of the Company’s products or services in any way, the Company’s revenues may be adversely affected.
In light of the illegality of cannabis under U.S. federal law (other than measures to legalize hemp) any engagement in cannabis-related activities, both in Canada as well as in foreign jurisdictions, may lead to heightened scrutiny by regulatory bodies and other authorities that could negatively impact the Company and/or its personnel. The Company does not have U.S. cannabis-related activities. Specifically, the Company has no investment or ownership in any U.S. entity nor does it provide any products or services to U.S. entities.
Recent Developments in the Canadian Cannabis Industry and Market
As a licence holder authorized to process, formulate and manufacture cannabinoid-based products, the Company is operating its business in a relatively new industry and market, and the Company’s success in the cannabis market will depend in part on its ability to attract and retain customers, develop and maintain commercial relationships with Canadian and international cannabis brands and develop innovative products. In addition to being subject to general business risks applicable to a business involving an agricultural product and a regulated consumer product, the Company will need to make significant investments in its business strategy. These investments include the procurement of raw material, equipment relating to the distillation, extraction and formulation of cannabis products, site improvements and research and development projects. The Company expects that competitors will undertake similar investments to compete with it. Competitive conditions, consumer preferences, customer requirements and spending patterns in this industry and market are relatively unknown and may have unique circumstances that differ from other existing industries and markets and cause the Company’s future efforts to develop its business to be unsuccessful or to have undesired consequences for it. As a result, the Company may not be successful in its efforts to attract customers, leverage its commercial partnerships or to develop new cannabis products and produce and distribute these cannabis products, or these activities may require significantly more resources than it currently anticipates in order to be successful.
Securities Class Action
Securities class action litigation is often brought against companies following a period of volatility in the market price of their securities. Litigation can result in significant costs and damages and divert management attention and resources.
Third Party Transportation
In order for customers of the Company to receive their product, the Company must rely on third-party transportation services. This can cause logistical problems with and delays in patients, government entities and private retailers obtaining their orders and cannot be directly controlled by the Company. Any delay by third party transportation services may adversely affect the Company’s financial performance.
Moreover, security of the product during transportation to and from the Moncton Campus, the Winnipeg Facility and the Lac Supérieur Facility is critical due to the nature of the product. A breach of security during transport could have material adverse effects on the Company’s business, financials and prospects. Any such breach could impact the Company’s ability to continue operating under its licenses or the prospect of renewing its licenses.
COVID-19 and Other Infectious Diseases
The Company’s business, including its operations, supply chains and interactions with counterparties, and its financial condition may be adversely impacted by the effects of COVID-19 and other infectious diseases. On March 11, 2020, the World Health Organization declared the outbreak of a novel strain of coronavirus (including its new variants “COVID-19”) a global pandemic.
The extent to which COVID-19 and other infectious diseases may impact the Company’s business, including its operations and the market for its securities and its financial condition, will depend on future developments, which are highly uncertain and cannot be predicted at this time. These include the duration, severity and scope of the outbreak and the actions taken by applicable governmental entities to address and mitigate COVID-19 or any other infectious diseases. In particular, the continued spread of COVID-19 globally could materially and adversely impact the Company’s business including, without limitation, employee health, workforce productivity, increased insurance premiums, limitations on travel, disruption to supply chains and the ability to deliver the Company’s products to end customers. In addition, government efforts to curtail the spread of COVID-19 or other infectious diseases may result in temporary or long-term suspensions or shut-downs of our operations, impact our customers and affect our supply chain. Such suspensions and disruptions may have a material and adverse effect on the Company’s business, financial condition and results of operations.
Rising Energy Costs
The Company’s extraction and manufacturing operations consume considerable energy, making the Company vulnerable to rising energy costs. Rising or volatile energy costs may adversely impact the business of the Company and its ability to operate profitably.
Difficulties with Forecasts
The Company must rely largely on its own market research to forecast sales as detailed forecasts are not generally obtainable from other sources at this early stage of the cannabis industry. A failure in the demand for its products to materialize as a result of competition, technological change or other factors could have a material adverse effect on the business, results of operations and financial condition of the Company.
Uninsured or Uninsurable Risks
While we may have insurance to protect our assets, operations, and employees, such insurance is subject to coverage limits and exclusions and may not be available for the risks and hazards to which we are exposed. No assurance can be given that such insurance will be adequate to cover our liabilities or that it will be available in the future or at all, and that it will be commercially justifiable. We may be subject to liability for risks against which we cannot insure or against which we may elect not to insure due to the high cost of insurance premiums or other factors. The payment of any such liabilities would reduce the funds available for our normal business activities. Payment of liabilities for which we do not carry insurance may have a material adverse effect on our financial position and operations.
Unknown Health Impact of Use of Cannabis and Derivatives
There is little in the way of longitudinal studies on the short-term and long-term effects of cannabis use on human health, whether used for recreational or medical purposes. As such, there are inherent risks associated with using the Company’s cannabis and derivative products. Previously unknown or unforeseeable adverse reactions arising from human consumption of cannabis products may occur which could adverse affect social acceptance of cannabis and the demand for the Company’s products.
Ability to Meet Production Targets
The Company sets production targets on dried flower, extracted oil and formulated oil. Actual production amounts may not achieve targeted production figures as a result of many factors including but not limited to: genetic drift in the strains of cannabis plants grown, shift in strains grown as a result of competitive pressure, natural variations in plant development, inability to precisely influence growth measures as a result of numerous variables that may influence the plant growth that are varied from one growth cycle to another, product that does not meet quality assurance specifications including, but not limited to, pesticide or heavy metals testing, tetrahydrocannabinol and cannabidiol specifications, terpene profile or visual appearance, operational inefficiencies from extraction processes or in production of formulated oil for sale.
Scale of Operations
The Company has implemented supplier arrangements that it believes will adequately meet demand for its product. Should demand for the Company’s products increase there exists the risk of the Company being unable to fulfil demand. Although the Company is currently on track to meet its intended capacity goals, delays in meeting its capacity goals could result in unfulfilled purchase orders and the Company may lose a significant amount of sales. Any inability to secure the required supply of cannabis to meet the demands of supplier agreements either by means of internal generation or through acquisition could have a materially adverse impact on operating results of the Company.
Continuance of Contractual or Other Relations with Provincial and Territorial Governments Cannot be Guaranteed
The Company expects to derive a significant portion of its future revenues from its supply arrangements with the various Canadian provinces and territories. There are many factors which could impact the Company’s contractual arrangements with the provinces and territories, including but not limited to availability of supply, product selection and the popularity of the Company’s products with retail customers. If the Company’s supply arrangements with certain Canadian provinces and territories are amended, terminated or otherwise altered, the Company’s sales and results of operations could be adversely affected, which could have a material adverse effect on the Company’s business, financial condition and results of operations. Some provinces and territories have letters of intent or have moved to purchase orders or other listing agreements to form the basis of their distribution arrangements.
In addition, not all of the Company’s supply arrangements with the various Canadian provinces and territories contain purchase commitments or otherwise obligate the provincial or territorial wholesaler to buy a minimum or fixed volume of cannabis products from the Company. The amount of cannabis that the provincial and territorial wholesalers may purchase under the supply arrangements may therefore vary from what the Company expects or has planned for. As a result, the Company’s revenues could fluctuate materially in the future and could be materially and disproportionately impacted by the purchasing decisions of the provincial and territorial wholesalers. If any of the provincial or territorial wholesalers decide to purchase lower volumes of products from the Company than the Company expects, alters its purchasing patterns at any time with limited notice, decides to return product or decides not to continue to purchase the Company’s cannabis products at all, the Company’s revenues could be materially adversely affected, which could have a material adverse effect on the Company’s business, financial condition, results of operations and prospects.
No Assurance That Listing Standards of TSX & Nasdaq Will Continue to be Met
The Company must meet continuing listing standards to maintain the listing of the Common Shares on the TSX and Nasdaq, including minimum price of such Common Shares. If the Company fails to comply with listing standards and the TSX or Nasdaq delists the Common Shares, the Company and its shareholders could face significant material adverse consequences, including: (i) a limited availability of market quotations for the Common Shares; (ii) reduced liquidity for the Common Shares; (iii) a determination that the Common Shares are “penny stock,” which would require brokers trading in the Common Shares to adhere to more stringent rules and possibly result in a reduced level of trading activity in the secondary trading market for the Common Shares; (iv) a limited amount of news about the Company and analyst coverage; and (v) a decreased ability for the Company to issue additional equity securities or obtain additional equity or debt financing in the future.
Intellectual Property
The ownership and protection of trademarks, patents, if any, trade secrets and intellectual property rights, if any, are significant aspects of the Company’s future success. Unauthorized parties may attempt to replicate or otherwise obtain and use the Company’s products and technology or the Company may not be able to secure required protection. Policing the unauthorized use of the Company’s current or future trademarks, patents, trade secrets or intellectual property rights could be difficult, expensive, time- consuming and unpredictable, as may be enforcing these rights against unauthorized use by others.
In addition, other parties may claim that the Company’s products infringe on their proprietary or patent protected rights. Such claims, whether or not meritorious, may result in the expenditure of significant financial and managerial resources, legal fees, injunctions, temporary restraining orders and/or require the payment of damages. As well, the Company may need to obtain licenses from third parties who allege that the Company has infringed on their lawful rights. Such licenses, however, may not be available on terms acceptable to the Company or at all. In addition, the Company may not be able to obtain or utilize on terms that are favorable to it, or at all, licenses or other rights with respect to intellectual property that it does not own.
Canadian Company and Shareholder Protection may Differ from Shareholder Protection in US or Elsewhere
The Company is organized and exist under the laws of Canada and, accordingly, are governed by the Canada Business Corporations Act (the “CBCA”). The CBCA differs in certain material respects from laws generally applicable to United States corporations and shareholders, including the provisions and proceedings relating to interested directors, mergers, amalgamations, restructuring, takeovers, shareholders’ suits, indemnification of directors, and inspection of corporation records.
Increased Volatility for Dual Listed Shares
The Company’s listing on both the TSX and Nasdaq may increase volatility due to the ability to buy and sell Common Shares in two places, different market conditions in different capital markets, and different trading volumes. This may result in less liquidity on both exchanges, different liquidity levels, and different prevailing trading prices.
Limited Market for Securities
The Common Shares are listed on the TSX and on Nasdaq, however, there can be no assurance that an active and liquid market for the Common Shares will be maintained and an investor may find it difficult to resell any securities of the Company. The market price for Common Shares may be volatile and subject to wide fluctuations in response to numerous factors, many of which are outside of the Company’s control.
Risks Inherent in Investments
The Company is not directly involved in the ownership or operation of and may have limited contractual rights relating to the operations of its current and future investee entities. An investee generally has the power to determine the manner in which its business is developed, expanded and operated, and the Company’s interest in an investee is subject to the risks applicable to the business carried on by the investee, and the Company may fail to realize all of the potential benefits from its investments. The interests of the Company and its investees may not always be aligned. As a result, any cash flows of the Company from investees will be dependent upon the activities of the investees, which creates the risk that at any time those investees may: (i) have business interests or targets that are inconsistent with those of the Company; (ii) take action contrary to the Company’s policies or objectives; (iii) be unable or unwilling to fulfill their obligations under their agreements with the Company; (iv) experience financial, operational or other difficulties, including insolvency, which could limit or suspend an investee’s ability to perform its obligations under agreements with the Company or (v) fail to comply with applicable laws or best practices.
The Company is a Foreign Private Issuer Within the Meaning of the U.S. Securities Exchange Act of 1934
The Company is a foreign private issuer under the United States Securities Exchange Act of 1934, as amended (the “Exchange Act”), and exempt from certain securities rules applicable to U.S. domestic issuers. Some of these rules require reduced reporting and disclosure requirements. As a result, the shareholder may not receive as much information or information as frequently from the Company as would otherwise be made available by a U.S. domestic issuer. The Company’s status as a foreign private issuer under the Exchange Act would be lost if a majority of our Common Shares were held by persons in the United States and the Company failed to meet any of the additional requirements necessary to avoid
loss of foreign private issuer status. The regulatory and compliance costs to us under U.S. securities laws as a U.S. domestic issuer would be greater than the cost incurred as a Canadian foreign private issuer.
Foreign Investment
In relation to investments in international operations, in addition to the foregoing, there is also the risk of increased disclosure requirements; currency fluctuations; restrictions on the ability of local operating companies to hold Canadian dollars or other foreign currencies in offshore bank accounts; import and export regulations; increased regulatory requirements and restrictions; limitations on the repatriation of earnings; and increased financing costs.
These risks may limit or disrupt the Company’s strategic alliances or investments, restrict the movement of funds, cause the Company to have to expend more funds than previously expected or required, or result in the deprivation of contract rights or the taking of property by nationalization or expropriation without fair compensation, and may materially adversely affect the Company’s financial position and/or results of operations. In addition, the enforcement by the Company of its legal rights in foreign countries, including rights to exploit properties or utilize permits and licenses and contractual rights may not be recognized by the court systems in such foreign countries or enforced in accordance with the rule of law.
The Company’s Operations in Emerging Markets are Subject to Political and Other Risks Associated with Operating in a Foreign Jurisdiction
The Company’s investments have operations in various emerging and foreign markets and the Company will be seeking to grow its operations through prudent synergistic acquisitions or development of international operations.
Such operations expose the Company to the socioeconomic conditions as well as the laws governing the cannabis industry in such countries. Inherent risks with conducting foreign operations include, but are not limited to: high rates of inflation; extreme fluctuations in currency exchange rates, military repression; war or civil war; social and labour unrest; organized crime; hostage taking; terrorism; violent crime; expropriation and nationalization; renegotiation or nullification of existing licenses, approvals, permits and contracts; changes in taxation policies; restrictions on foreign exchange and repatriation; and changing political norms, banking and currency controls and governmental regulations that favour or require the Company to award contracts in, employ citizens of, or purchase supplies from, the jurisdiction.
Governments in certain foreign jurisdictions intervene in their economies, sometimes frequently, and occasionally make significant changes in policies and regulations. Changes, if any, in cannabis industry or investment policies or shifts in political attitude in the countries in which the Company operates may adversely affect the Company’s operations or profitability. Operations may be affected in varying degrees by government regulations with respect to, but not limited to, restrictions on production, price controls, export controls, currency remittance, importation of product and supplies, income and other taxes, royalties, the repatriation of profits, expropriation of property, foreign investment, maintenance of concessions, licenses, approvals and permits, environmental matters, land use, land claims of local people, water use and workplace safety. Failure to comply strictly with applicable laws, regulations and local practices could result in loss, reduction or expropriation of licenses, or the imposition of additional local or foreign parties as joint venture partners with carried or other interests.
The Company continues to monitor developments and policies in the emerging and foreign markets in which it operates or invests and assess the impact thereof to its operations; however such developments cannot be accurately predicted and could have an adverse effect on the Company’s operations or profitability.
Corruption and Fraud in Certain Emerging Markets Relating to Ownership of Real Property
There are uncertainties, corruption and fraud relating to title ownership of real property in certain emerging markets in which the Company may invest. Property disputes over title ownership are frequent in emerging markets, and, as a result, there is a risk that errors, fraud or challenges could adversely affect the Company’s ability to successfully invest in such jurisdictions.
Credit Risk
Credit risk arises from deposits with banks, short term investments and outstanding receivables. For trade receivables, the Company does not hold any collateral as security but mitigates this risk by dealing only with what management believes to be financially sound counterparties and, accordingly, does not anticipate significant loss for non-performance. For other receivables, out of the normal course of business, management may obtain guarantees and general security agreements.
Liquidity Risk
The Company’s liquidity risk is the risk the Company will not be able to meet its financial obligations as they become due. The Company manages its liquidity risk by reviewing on an ongoing basis its capital requirements.
Market Risk
Market risk is the risk that the fair value or future cash flows of a financial instrument will fluctuate because of changes in market prices. Market risk comprises two types of risk: currency rate risk and interest rate risk.
Currency risk is the risk to the Company’s earnings that arise from fluctuations of foreign exchange rates. The Company is not exposed to foreign currency exchange risk as the number of financial instruments denominated in a foreign currency held by the Company is not material.
Interest risk is the risk that the fair value or future cash flows of a financial instrument will fluctuate because of changes in market interest rates. The Company may be exposed to interest rate risk pursuant to any long-term debt that it incurs from time to time.
Concentration Risk
The Company’s accounts receivable are primarily due from the Government of Canada, provincial government agencies, legal trusts and patients covered under group insurance, and, thus, the Company believes that the accounts receivable balance is collectible.
Significant Shareholder
The Company has a significant shareholder that could significantly influence matters submitted to the shareholders for approval, including the election of directors and the approval of certain corporate transactions. In some cases, the significant shareholder’s interests may not be the same as those of the other shareholders.
Dividends
The Company has no dividend record and may not pay any dividends on the Common Shares in the foreseeable future. Dividends paid by the Company could be subject to tax and, potentially, withholdings.
Publicity or Consumer Perception
The Company believes the cannabis industry is highly dependent upon consumer perception regarding the safety, efficacy and quality of the cannabis and other products produced by the Company from time to time. Consumer perception of the Company’s products can be significantly influenced by scientific research or findings, regulatory investigations, litigation, media attention and other publicity regarding the consumption of cannabis products. There can be no assurance that future scientific research, findings, regulatory proceedings, litigation, media attention or other research findings or publicity will be favourable to the cannabis market or any particular product, or consistent with earlier publicity. Future research reports, findings, regulatory proceedings, litigation, media attention or other publicity that are perceived as less favourable than, or that question, earlier research reports, findings or publicity could have a material adverse effect on the demand for the Company’s products and the business, results of
operations, financial condition and the Company’s cash flows. The Company’s dependence upon consumer perceptions means that adverse scientific research reports, findings, regulatory proceedings, litigation, media attention or other publicity, whether or not accurate or with merit, could have a material adverse effect on the Company, the demand for the Company’s products, and the business, results of operations, financial condition and cash flows of the Company.
Any product recall affecting the cannabis industry more broadly could lead consumers to lose confidence in the safety and security of the products sold by licensed producers generally, which could have a material adverse effect on the Company’s business, financial condition and results of operations. Adverse publicity reports or other media attention regarding the safety, efficacy and quality of cannabis and derivative products in general, or the Company’s products specifically, or associating the consumption of cannabis or use of derivative products with illness or other negative effects or events, could have such a material adverse effect. Such adverse publicity reports or other media attention could arise even if the adverse effects associated with such products resulted from consumers’ failure to consume such products appropriately or as directed.
Research in Canada, the U.S. and internationally regarding the benefits, viability, safety, efficacy, dosing and social acceptance of cannabis or isolated cannabinoids (such as CBD and THC) remains in early stages. There have been relatively few clinical trials on the benefits of cannabis or isolated cannabinoids (such as CBD and THC). Although the Company believes that the articles, reports and studies support its beliefs regarding the medical benefits, viability, safety, efficacy, dosing and social acceptance of cannabis, future research and clinical trials may prove such statements to be incorrect, or could raise concerns regarding, and perceptions relating to, cannabis. Given these risks, uncertainties and assumptions, prospective purchasers of securities should not place undue reliance on such articles and reports. Future research studies and clinical trials may draw opposing conclusions to those stated in this Annual Information Form or reach negative conclusions regarding the medical benefits, viability, safety, efficacy, dosing, social acceptance or other facts and perceptions related to medical cannabis, which could have a material adverse effect on the demand for the Company’s products with the potential to lead to a material adverse effect on the Company’s business, financial condition and results of operations.
The Company is No Longer an Emerging Growth Company
Based on the market value of our equity securities held by non-affiliates as of February 28, 2021, we became a “large accelerated filer”, and are no longer an “emerging growth company” (as each of those terms are defined in Rule 12b-2 under the United States Securities Exchange Act of 1934, as amended), as of August 31, 2021. As such, we are no longer permitted to rely on an exemption from the auditor attestation requirements of Section 404(b) of the Sarbanes-Oxley Act of 2002. As a result, we may incur significant additional expenses that we did not previously incur. In particular, we expect to incur substantial accounting expense and expend significant management time on additional compliance-related issues. If we or our independent registered public accounting firm continues to identify deficiencies in our internal control over financial reporting as material weaknesses, we may be required to make prospective or retroactive changes to our financial statements, consider other areas for further attention or improvement, or be unable to obtain the required attestation in a timely manner, if at all.
Privacy
In addition, the Company collects and stores personal information about its patients and customers, and is responsible for protecting that information from privacy breaches. A privacy breach may occur through procedural or process failure, information technology malfunction, or deliberate unauthorized intrusions. Theft of data for competitive purposes is an ongoing risk whether perpetrated via employee collusion or negligence or through deliberate cyber-attack. Any such theft or privacy breach would have a material adverse effect on the Company’s business, financial condition and results of operations.
In addition, there are a number of federal and provincial laws protecting the privacy and confidentiality of certain patient health information, including patient records, and restricting the collection, use and disclosure of that protected information. In particular, the privacy rules under the Personal Information Protection and Electronics Documents Act (Canada) (“PIPEDA”) and provincial statutes regulating the collection, use and disclosure of personal information, protect medical records and other personal health information by limiting their use and disclosure of health information to the minimum level reasonably
necessary to accomplish the intended purpose. If the Company was found to be in violation of the privacy or security rules under PIPEDA or other laws protecting the privacy and confidentiality of patient health information, it could be subject to sanctions and civil or criminal penalties, which could increase its liabilities, harm its reputation and have a material adverse effect on the business, results of operations and financial condition of the Company.
Product Security
Given the nature of the Company’s products and the lack of legal availability of such products outside of channels approved by the Government of Canada, as well as the concentration of inventory at the Moncton Campus, Winnipeg Facility and Lac Supérieur Facility, despite meeting or exceeding legislated security requirements, there remains a risk of shrinkage as well as theft. A security breach at the Moncton Campus, Winnipeg Facility or Lac Supérieur Facility could expose the Company to additional liability and to potentially costly litigation, increased expenses relating to the resolution and future prevention of these breaches and may deter potential patients or recreational adult-users from choosing the Company’s products.
Environmental and Employee Health and Safety Regulations
The Company’s operations are subject to environmental and safety laws and regulations concerning, among other things, emissions and discharges to water, air and land, the handling and disposal of hazardous and non-hazardous materials and wastes, and employee health and safety. The Company will incur ongoing costs and obligations related to compliance with environmental and employee health and safety matters. Failure to comply with environmental and safety laws and regulations may result in additional costs for corrective measures, penalties or in restrictions on the Company’s manufacturing operations. In addition, changes in environmental, employee health and safety or other laws, more vigorous enforcement thereof or other unanticipated events could require extensive changes to the Company’s operations or give rise to material liabilities, which could have a material adverse effect on the business, results of operations and financial condition of the Company.
Government approvals and permits are currently and may in the future be required in connection with, the Company’s operations. To the extent such approvals are required and not obtained, the Company may be curtailed or prohibited from its proposed production of medical and/or adult-use recreational cannabis or from proceeding with the development of its operations as currently proposed.
Reliance on Key Inputs
The Company’s business is dependent on a number of key inputs and their related costs including raw materials and supplies related to its growing operations, as well as electricity, water and other local utilities. Any significant interruption or negative change in the availability or economics of the supply chain for key inputs could materially impact the business, financial condition and operating results of the Company. Some of these inputs may only be available from a single supplier or a limited group of suppliers. If a sole source supplier was to go out of business, the Company might be unable to find a replacement for such source in a timely manner or at all. If a sole source supplier were to be acquired by a competitor, that competitor may elect not to sell to the Company in the future. Any inability to secure required supplies and services or to do so on appropriate terms could have a materially adverse impact on the business, financial condition and operating results of the Company.
Regulatory Proceedings, Investigations, and Audits
The Company’s business requires compliance with many laws and regulations. Failure to comply with these laws and regulations could subject the Company to regulatory proceedings or investigations and could also lead to damage awards, fines and penalties. The Company may become involved in a number of government proceedings, investigations and audits. The outcome of any regulatory or agency proceedings, investigations, audits, and other contingencies could harm the Company’s reputation, require the Company to take, or refrain from taking, actions that could harm its operations or require the Company to pay substantial amounts of money, harming its financial condition. There can be no assurance that any pending or future regulatory proceedings, investigations and audits will not result in
substantial costs or a diversion of management’s attention and resources or have a material adverse impact on the Company’s business, financial condition and results of operation.
Catastrophic Events
Natural disasters, such as earthquakes, tsunamis, floods or wildfires, public health crises, such as epidemics and pandemics, political instability, acts of terrorism, war or other conflicts and other events outside of the Company’s control, may adversely impact our business and operating results. In addition to the direct impact that such events could have on our facilities and workforce, these types of events could negatively impact consumer spending in the impacted regions or depending on the severity, globally, which would impact our strategic partners and in turn impact on demand for our products and services.
Fraudulent or Illegal Activity by the Company’s Employees, Contractors and Consultants
The Company is exposed to the risk that its employees, independent contractors and consultants may engage in fraudulent or other illegal activity. Misconduct by these parties could include intentional, reckless and/or negligent conduct that violates: (i) government regulations; (ii) manufacturing standards; (iii) federal and provincial healthcare fraud and abuse laws and regulations; or (iv) laws that require the true, complete and accurate reporting of financial information or data. It is not always possible for the Company to identify and deter misconduct by its employees and other third parties, and the precautions taken by the Company to detect and prevent this activity may not be effective in controlling unknown or unmanaged risks or losses or in protecting the Company from governmental investigations or other actions or lawsuits stemming from a failure to comply with such laws or regulations. If any such actions are instituted against the Company, and it is not successful in defending itself or asserting its rights, those actions could have a significant impact on the Company’s business, including the imposition of civil, criminal and administrative penalties, damages, monetary fines, contractual damages, reputational harm, diminished profits and future earnings, and curtailment of the Company’s operations, any of which could have a material adverse effect on the Company’s business, financial condition and results of operations.
The Company’s Operations may be Impaired as a Result of Restrictions on the Acquisition or Use of Properties by Foreign Investors or Local Companies under Foreign Control
Non-resident individuals and non-domiciled foreign legal entities may be subject to restrictions on the acquisition or lease of properties in certain emerging markets. Limitations also apply to legal entities domiciled in such countries which are controlled by foreign investors, such as the entities through which the Company may make investments. Accordingly, the Company’s current and future operations may be impaired as a result of such restrictions on the acquisition or use of property, and the Company’s ownership or access rights in respect of any property it owns or leases in such jurisdictions may be subject to legal challenges, all of which could result in a material adverse effect on the Company’s business, results of operations, financial condition and cash flows.
The Company May Expand into Other Geographic Areas, which could Increase the Company’s Operational, Regulatory and Other Risks
In addition to the jurisdictions described elsewhere in this Annual Information Form, the Company may in the future expand into other geographic areas, which could increase the Company’s operational, regulatory, compliance, reputational and foreign exchange rate risks. The failure of the Company’s operating infrastructure to support such expansion could result in operational failures and regulatory fines or sanctions. Future international expansion could require the Company to incur a number of up-front expenses, including those associated with obtaining regulatory approvals, as well as additional ongoing expenses, including those associated with infrastructure, staff and regulatory compliance. The Company may not be able to successfully identify suitable acquisition and expansion opportunities or integrate such operations successfully with the Company’s existing operations.
The Company Relies on International Advisors and Consultants in Order to Keep Abreast of Material Legal, Regulatory and Government Developments that Impact the Company’s Business and Operations in the Jurisdictions in Which it Operates
The legal and regulatory requirements in the foreign countries in which the Company may invest or operate with respect to the cultivation and sale of cannabis, banking systems and controls, as well as local business culture and practices are different from those in Canada. The Company’s officers and directors must rely, to a great extent, on local legal counsel and consultants in order to keep abreast of material legal, regulatory and governmental developments as they pertain to and affect the Company’s business operations, and to assist with governmental relations. The Company must rely, to some extent, on those members of management and the board of directors who have previous experience working and conducting business in these countries, if any, in order to enhance our understanding of and appreciation for the local business culture and practices. The Company also relies on the advice of local experts and professionals in connection with current and new regulations that develop in respect of the cultivation and sale of cannabis as well as in respect of banking, financing, labour, litigation and tax matters in these jurisdictions. Any developments or changes in such legal, regulatory or governmental requirements or in local business practices are beyond the Company’s control. The impact of any such changes may adversely affect the Company’s business.
Anti-Money Laundering Laws and Regulation Risks
The Company is subject to a variety of laws and regulations pertaining to money laundering, financial recordkeeping and proceeds of crime, including the Proceeds of Crime (Money Laundering) and Terrorist Financing Act (Canada), as amended and the rules and regulations thereunder, the Criminal Code (Canada) and any related or similar rules, regulations or guidelines, issued, administered or enforced by governmental authorities internationally.
In the event that any of the Company’s operations or investments, any proceeds thereof, any dividends or distributions therefrom, or any profits or revenues accruing from such operations or investments were found to be in violation of money laundering legislation, such transactions may be viewed as proceeds of crime under one or more of the statutes noted above or any other applicable legislation. This could restrict or otherwise jeopardize the Company’s ability to declare or pay dividends, effect other distributions or subsequently repatriate such funds back to Canada. Furthermore, while the Company has no current intention to declare or pay dividends in the foreseeable future, in the event that a determination was made that proceeds obtained by the Company could reasonably be shown to constitute proceeds of crime, the Company may decide or be required to suspend declaring or paying dividends without advance notice and for an indefinite period of time.
Corruption and Anti-bribery Law Violation Risks
The Company’s business is subject to the Corruption of Foreign Public Officials Act (Canada) (the “CFOPA”), which generally prohibits companies and employees from engaging in bribery or other prohibited payments to foreign officials for the purpose of obtaining or retaining business. In addition, the Company is subject to the Foreign Corrupt Practices Act (United States) (the “FCPA”), and is or may become subject to anti-bribery laws of any other countries in which it conducts business now or in the future. The Company’s employees or other agents may, without its knowledge and despite its efforts, engage in prohibited conduct under the Company’s policies and procedures and anti-bribery laws for which the Company may be held responsible. The CFPOA and the FCPA also require companies to maintain accurate books and records and internal controls, including at foreign controlled subsidiaries. The Company’s policies mandate compliance with these anti-corruption and anti-bribery laws. However, there can be no assurance that the Company’s internal control policies and procedures will always protect it from recklessness, fraudulent behaviour, dishonesty or other inappropriate acts committed by its affiliates, employees, contractors or agents. If the Company’s employees or other agents are found to have engaged in such practices, the Company could suffer severe penalties and other consequences that may have a material adverse effect on its business, financial condition and results of operations.
Conflicts of Interest
The Company may be subject to various potential conflicts of interest because of the fact that some of its officers and directors may be engaged in a range of business activities. In some cases, the executive officers and directors may have fiduciary obligations associated with these business interests that interfere with their ability to devote time to the Company and its affairs, and that could adversely affect Company operations. These business interests could require significant time and attention of the Company’s executive officers and directors. In addition, the Company may also become involved in other transactions which conflict with the interests of the Company’s directors and officers who may from time to time deal with persons, firms, institutions or corporations with which the Company may be dealing, or which may be seeking investments similar to those the Company desires. The interests of these persons could conflict with the Company’s interests. In addition, from time to time, these persons may be competing with the Company for available investment opportunities. Conflicts of interest, if any, will be subject to the procedures and remedies provided under applicable laws. In particular, in the event that such a conflict of interest arises at a meeting of directors, a director who has such a conflict will abstain from voting for or against the approval of such participation or such terms. In accordance with applicable laws, directors are required to act honestly, in good faith and in the Company’s best interests.
Future Acquisitions or Dispositions and Management of the Impact of Such Transactions on the Company’s Operations
In the event that the Company proceeds with a material acquisition, disposition or other strategic transaction, such transaction would be subject to a number of risks, including: (i) potential disruption of the Company’s ongoing business; (ii) distraction of management; (iii) the Company may become more financially leveraged; (iv) the anticipated benefits and cost savings of those transactions may not be realized fully or at all or may take longer to realize than expected; (v) increasing the scope and complexity of the Company’s operations, and (vi) loss or reduction of control over certain of the Company’s assets.
The presence of one or more material liabilities of an acquired company that are unknown to the Company at the time of acquisition could have a material adverse effect on the results of operations, business prospects and financial condition of the Company. A strategic transaction may result in a significant change in the nature of the Company’s business, operations and strategy. In addition, the Company may encounter unforeseen obstacles or costs in implementing a strategic transaction or integrating any acquired business into the Company’s operations.
General Business Risk and Liability
Given the nature of the Company’s business, it may from time to time be subject to claims or complaints from investors or others in the normal course of business. The legal risks facing the Company, its directors, officers, employees or agents in this respect include potential liability for violations of securities laws, breach of fiduciary duty and misuse of investors’ funds. Some violations of securities laws and breach of fiduciary duty could result in civil liability, fines, sanctions, or the suspension or revocation of the Company’s right to carry on its existing business. The Company may incur significant costs in connection with such potential liabilities.
Risk Factors Related to Dilution
The Company may issue additional securities in the future, which may dilute a shareholder’s holdings, or a holder of a convertible security’s underlying relative interest, in the Company. The Company’s articles permit the issuance of an unlimited number of Common Shares and preferred shares, and shareholders, other than BAT, will have no pre-emptive rights in connection with any such further issuance. The directors of the Company have discretion to determine the price and the terms of further issuances. Moreover, additional Common Shares will be issued by the Company on the exercise of options under its stock option plan and pursuant to the 2020 Equity Incentive Plan (as defined below) pursuant to which the Company has issued options, restricted share units and performance share units.
Constraints on Marketing Products
In view of the restrictions on marketing, advertising and promotional activities set forth in the Cannabis Act and related regulations, the Company’s business and operating results may be hindered by applicable restrictions on sales, branding and marketing activities. If the Company is unable to effectively brand and market its products and compete for market share, or if the costs of compliance with government legislation and regulation cannot be absorbed through increased selling prices for its products, the Company’s sales and operating results could be adversely affected.
Marketing Risks Arising from Provincial Legislative Controls
The provincial and territorial adult-use recreational cannabis markets are end consumer driven. It is not possible to predict the quantities of product that will be purchased and made available to the end consumer in such adult-use recreational cannabis markets. Further, regulations like those currently implemented in Quebec may limit the marketability of some of the Company’s products and the Company’s number of end consumers. These factors may have an adverse effect on the Company’s business.
Suppliers and Skilled Labour
The Company’s ability to compete and grow will be dependent on having access, at a reasonable cost and in a timely manner, to skilled labor, equipment, parts and components. No assurances can be given that the Company will be successful in maintaining its required supply of skilled labor, equipment, parts and components. It is also possible that the final costs of the major equipment contemplated by the Company’s capital expenditure program may be significantly greater than anticipated by management, and may be greater than funds available to the Company, in which circumstance the Company may curtail, or extend the timeframes for completing, capital expenditure plans. This could have an adverse effect on the Company’s financial results.
The Company’s success will depend on its directors’ and officers’ ability to develop and execute on the Company’s business strategies and manage its ongoing operations, and on the Company’s ability to attract and retain key quality assurance, scientific, sales, public relations and marketing staff or consultants now that production and selling operations have begun. The loss of any key personnel or the inability to find and retain new key persons could have a material adverse effect on the Company’s business. Competition for qualified technical, sales and marketing staff, as well as officers and directors can be intense and no assurance can be provided that the Company will be able to attract or retain key personnel in the future, which may adversely impact the Company’s operations.
Holding Company Status
The Company is a holding company and essentially all of its operating assets are the capital stock of its primary subsidiaries, OGI, EIC, LAU and 10870277 Canada Inc. As a result, investors in the Company are subject to the risks attributable to its subsidiaries. As a holding company, the Company conducts substantially all of its business through its subsidiaries, which generates substantially all of its revenues. Consequently, the Company’s cash flows and ability to complete current or desirable future enhancement opportunities are dependent on the earnings of the subsidiary and the distribution of those earnings to the Company.
DIVIDENDS
As of the date of this Annual Information Form, the Company has no current intention to declare dividends on its Common Shares in the foreseeable future. Any decision to pay dividends on its Common Shares in the future will be at the discretion of the Company’s board of directors and will depend on, among other things, the Company’s results of operations, current and anticipated cash requirements and surplus, financial condition, any contractual restrictions and financing agreement covenants, including those in the Credit Agreement, solvency tests imposed by corporate law, and other factors that the Company’s board of directors may deem relevant.
CAPITAL STRUCTURE
Common Shares
The Company is authorized to issue an unlimited number of Common Shares and an unlimited number of preferred shares. As of August 31, 2022, there were 313,815,503 Common Shares issued and outstanding and as of November 24, 2022, there are 313,856,912 Common Shares issued and outstanding. There are no preferred shares issued and outstanding. The holders of the Common Shares are entitled to one vote per share at all meetings of the shareholders of the Company. The holders of Common Shares are also entitled to dividends, if and when declared by the Company’s board of directors, and to the distribution of the residual assets of the Company in the event of a liquidation, dissolution or winding up of the Company. Should the Company issue preferred shares, the holders would be entitled to receive, before any distribution is made to holders of Common Shares, the amount required to be paid in accordance with the special rights and restrictions attached to the series of shares held by them, including any fixed premium and any accrued and unpaid preferential dividends. Following any such payment, preferred shareholders would not, as such, be entitled to share in any further distribution of the property or assets of the Company except as may be specifically provided in the special rights and restrictions attached to any particular series. Preferred shareholders would only be entitled to receive notice of and/or attend and/or vote at any general meeting of shareholders as provided in any special rights and restrictions that may attach to any particular series if and when issued.
The Company has three equity compensation plans in place: (a) the 2011 stock option plan (the “SOP”), (b) the 2017 equity incentive plan (the “2017 Plan”), and (c) a long term-omnibus equity incentive plan adopted on February 25, 2020 (the “2020 Equity Incentive Plan”). The 2020 Equity Incentive Plan permits the Company to grant equity-based incentive awards in the form of options, restricted share units, performance share units and deferred share units. Following the adoption of the 2020 Equity Incentive Plan, all future grants of equity-based awards will be made pursuant to, or as otherwise permitted by, the 2020 Equity Incentive Plan and no further equity-based awards will be made pursuant to the SOP or the 2017 Plan. The maximum number of Common Shares that may be issued upon exercise of awards granted under the 2020 Equity Incentive Plan shall not exceed 10% of the Company’s issued and outstanding Common Shares from time to time, combined with any equity securities granted under all other compensation plans previously adopted by the Company, including the SOP and 2017 Plan.
On March 10, 2021, in connection with the strategic investment from BAT, the Company entered into an investor rights agreement with BAT (the “Investor Rights Agreement”). Pursuant to the Investor Rights Agreement, the Company granted BAT certain rights, including pre-emptive rights, to participate in distributions of Common Shares to maintain its proportionate ownership in certain circumstances, as well as other rights (“Top-up Rights”) to subscribe for additional Common Shares in specified circumstances where the pre-emptive rights are not applicable (referred to in the Investor Rights Agreement as “Exempt Distributions”) and in specified circumstances where pre-emptive rights were not exercised (referred to in the Investor Rights Agreement as “bought deal Distributions”). The price per Common Share to be paid by BAT pursuant to the exercise of its Top-Up Rights will equal the price paid by other participants in the Exempt Distribution or bought deal Distribution, subject to certain restrictions (including, if such price is not permitted pursuant to securities laws, at the lowest price permitted thereunder).
As of August 31, 2022, there were 11,050,939 options issued and outstanding. As of November 24, 2021, there are 11,997,539 options issued and outstanding. As of August 31, 2022, there were 2,345,777 restricted share units issued and outstanding. As of November 24, 2022, there are 3,796,681 restricted share units issued and outstanding. As of August 31, 2022, there were 264,871 performance share units issued and outstanding. As of November 24, 2022, there are 1,103,099 performance share units issued and outstanding. As of August 31, 2022, there were nil deferred share units issued and outstanding. As of November 24, 2022, there are nil deferred share units issued and outstanding.
Warrants
As of August 31, 2022, the Company had 16,943,650 Warrants outstanding. As of November 24, 2022, the Company has 16,943,650 Warrants outstanding, exercisable to purchase up to 16,943,650 Warrant Shares (as defined herein) at an exercise price of $2.50 per Warrant Share.
The following is a summary of certain of the rights, privileges, restrictions and conditions attaching to the Warrants. For a complete description of the Warrants, please refer to the Warrant Indenture (as defined herein), which has been filed on the Canadian Securities Administrators’ SEDAR website at www.sedar.com and on the United States Securities and Exchange Commission’s EDGAR website at www.sec.gov.
The outstanding Warrants were issued under and are governed by a warrant indenture (the “Warrant Indenture”) entered into on November 12, 2020 between the Company and TSX Trust Company, as warrant agent. The Company has appointed the principal transfer office of the TSX Trust Company in Toronto, Ontario as the location at which the Warrants may be surrendered for exercise, transfer or exchange. A register of holders is maintained at the primary offices of the warrant agent in Toronto, Ontario.
Each Warrant is transferrable, and entitles the holder thereof to acquire one Warrant Share at an exercise price of $2.50 per Warrant Share, until 5:00 p.m. (Eastern Time) on November 12, 2023, subject to adjustment in certain customary events, after which time the Warrants will expire and become null and void. Pursuant to the Warrant Indenture, and subject to applicable securities legislation and approval of applicable regulatory authorities, the Company is entitled to purchase in the market, by private contract or otherwise, any of the Warrants then outstanding and any Warrants so purchased will be cancelled.
The Warrant Indenture provides for adjustment in the number of Warrant Shares issuable upon the exercise of the Warrants and/or the exercise price per Warrant Share upon the occurrence of certain events, including:
(a)the issuance of Common Shares or securities exchangeable for or convertible into Common Shares to all or substantially all of the holders of the Common Shares by way of a stock dividend or other distribution (other than a distribution of Warrant Shares upon the exercise of any Warrants);
(b)the subdivision, redivision or change of the Common Shares into a greater number of Common Shares;
(c)the consolidation, reduction or combination of the Common Shares into a lesser number of Common Shares;
(d)the issuance to all or substantially all of the holders of the Common Shares of rights, options or warrants under which such holders are entitled, during a period expiring not more than 45 days after the record date for such issuance, to subscribe for or purchase Common Shares, or securities exchangeable for or convertible into Common Shares; and
(e)the issuance or distribution to all or substantially all of the holders of the Common Shares of (i) securities of any class, whether of the Company or any other trust (other than Common Shares), (ii) rights, options or warrants to subscribe for or purchase Common Shares (or other securities convertible into or exchangeable for Common Shares), other than pursuant to a “Rights Offering” (as defined in the Warrant Indenture); (iii) evidences of its indebtedness or (iv) any property or other assets.
The Warrant Indenture also provides for adjustment in the class and/or number of securities issuable upon the exercise of the Warrants and/or exercise price per security in the event of the following additional events:
(a)reclassifications of the Common Shares or a capital reorganization of the Company;
(b)consolidations, amalgamations, arrangements or mergers of the Company with or into any other corporation or other entity; or
(c)the transfer of the undertaking or assets of the Company as an entirety or substantially as an entirety to another corporation or other entity.
No adjustment in the exercise price or the number of Warrant Shares issuable upon the exercise of the Warrants will be required to be made unless the cumulative effect of such adjustment or adjustments would result in a change of at least 1% in the exercise price or a change in the number of Warrant Shares issuable upon exercise by at least one one-hundredth of a Warrant Share, as the case may be. Furthermore, no adjustment will be made in the right to acquire Warrant Shares if an issue of Common Shares of the Company is being made in connection with a share incentive plan, restricted share plan or
share purchase plan for the benefit of directors, officers, employees, consultants or other service providers, or the satisfaction of existing instruments issued as of the date of the Warrant Indenture.
Pursuant to the Warrant Indenture, the Company has covenanted that, during the period in which the Warrants are exercisable, it will give notice to TSX Trust Company and to the holders of the Warrants of certain stated events, including events that would result in an adjustment to the exercise price for the Warrants or the number of Warrant Shares issuable upon exercise of the Warrants, at least 14 days prior to the record date of such event, if any.
No fractional Warrant Shares will be issuable upon the exercise of any Warrants and no cash or other consideration will be paid in lieu of fractional Warrant Shares. Holders of Warrants will not have any voting or pre-emptive rights or any other rights which a holder of Common Shares would have.
The Company may provide certain buy-in rights to a holder if it fails to cause the warrant agent to deliver the Warrant Shares by three trading days after the delivery to the Company of the notice of exercise and the aggregate exercise price (or notice of cashless exercise). The buy-in rights apply if after the trading day after the date of such delivery by the holder, the holder purchases (in an open market transaction or otherwise) Common Shares to deliver in satisfaction of a sale by the holder of the Warrant Shares that the holder anticipated receiving from the Company upon exercise of the Warrant. In this event, the Company will: (i) pay in cash to the holder the amount equal to the excess (if any) of the buy-in price over the product of (A) such number of Warrant Shares, times (B) the price at which the sell order giving rise to holder’s purchase obligation was executed; and (ii) at the election of the holder, either (A) reinstate the portion of the Warrant as to such number of Warrant Shares, or (B) deliver to the holder a certificate or certificates representing such number of Warrant Shares that would have been issued to the holder had the Company complied with its delivery obligations under the Warrant Indenture.
The Warrant Indenture includes certain beneficial ownership limitations under which Warrants are not exercisable to the extent that, after giving effect to the issuance of the Warrant Shares issuable upon such exercise of the Warrants, the holder, together with its affiliates and other persons acting as a group with the holder or any of its affiliates, would beneficially own in excess of 4.99% of the number of Common Shares outstanding immediately after giving effect to such issuance. Such beneficial ownership limitation may be increased or decreased by the holder upon notice to the Company, to a maximum of 9.99%. Except as provided in the Warrant Indenture, beneficial ownership will be calculated in accordance with Section 13(d) of the Exchange Act and the rules and regulations promulgated thereunder. To the extent the beneficial ownership limitations apply, the determination of whether a Warrant is exercisable and of which portion of a Warrant is exercisable shall be in the sole discretion and at the sole responsibility of the holder, and the submission of an exercise notice in respect of any Warrants shall be deemed to be the holder’s determination of whether the Warrants are exercisable, and neither the warrant agent nor the Company will have any obligation to verify or confirm the accuracy of such determination.
The Warrant Indenture provides that the Company will use its reasonable best efforts to maintain the terms of the registration statement on Form F-10 (File No. 333-234564) (the “Registration Statement”) or another registration statement under the United States Securities Act of 1933, as amended (the “U.S. Securities Act”) relating to the Warrant Shares effective until the earlier of the expiration date of the Warrants and the date on which no Warrants remain outstanding (provided, however, that nothing shall prevent the Company’s amalgamation, arrangement, merger or sale, including any take-over bid, and any associated delisting or deregistration or ceasing to be a reporting issuer, provided that, so long as the Warrants are still outstanding and represent a right to acquire securities of the acquiring corporation, the acquiring corporation shall assume the Company’s obligations under the Warrant Indenture). The Canadian short form base shelf prospectus forming part of the Registration Statement has expired, with the result that the Registration Statement is no longer effective, and the holders of the Warrants have been so notified in accordance with the provisions of the Warrant Indenture. Accordingly, no person holding Warrants is currently permitted to exercise Warrants, unless an exemption or a safe harbor from the registration requirements of the U.S. Securities Act, as amended, and applicable state securities laws is available. During any such period, any person holding Warrants may give notice of their desire to exercise the Warrants, at which time the Company will permit the cashless exercise of the Warrants and issue such number of Warrant Shares calculated pursuant to the provisions of the Warrant Indenture, provided that such Warrant Shares shall not be subject to any transfer restrictions in the United States or Canada.
The Warrant Indenture provides that, from time to time, the Company may amend or supplement the Warrant Indenture for certain purposes, without the consent of the holders of the Warrants, including curing defects or inconsistencies or making any change that does not prejudice the rights of any holder. Any amendment or supplement to the Warrant Indenture that would prejudice the interests of the holders of Warrants may only be made by “extraordinary resolution”, which will be defined in the Warrant Indenture as a resolution either: (i) passed at a meeting of the holders of Warrants at which there are holders of Warrants present in person or represented by proxy representing at least 20% of the aggregate number of the then outstanding Warrants by the affirmative vote of the holders of Warrants representing not less than 662/3% of the aggregate number of Warrants represented at the meeting and voted on the poll upon such resolution; or (ii) adopted by an instrument in writing signed by the holders of Warrants representing not less than 66 2/3% of the aggregate number of all the then outstanding Warrants.
MARKET FOR SECURITIES
Common Shares
Common Shares are listed and traded on the TSX under the trading symbol “OGI”. The following table sets forth the price range per share and trading volume for the Common Shares on the TSX for the periods indicated.
| | | | | | | | | | | |
| Period | High Trading Price ($) | Low Trading ($) | Volume (#) |
| August 2022 | $ 1.63 | $ 1.30 | 13,572,697 |
| July 2022 | $ 1.55 | $ 1.17 | 9,570,382 |
| June 2022 | $ 1.48 | $ 1.17 | 10,354,813 |
| May 2022 | $ 1.93 | $ 1.43 | 14,996,089 |
| April 2022 | $ 2.29 | $ 1.74 | 19,339,219 |
| March 2022 | $ 2.32 | $ 1.65 | 26,281,479 |
| February 2022 | $ 2.21 | $ 1.69 | 26,381,293 |
| January 2022 | $ 2.29 | $ 1.66 | 29,403,270 |
| December 2021 | $ 2.69 | $ 2.19 | 40,496,473 |
| November 2021 | $ 3.19 | $ 2.33 | 56,280,100 |
| October 2021 | $ 3.12 | $ 2.72 | 23,254,979 |
| September 2021 | $ 3.49 | $ 2.89 | 28,242,361 |
Common Shares are listed and traded on the Nasdaq under the trading symbol “OGI”. The following table sets forth the price range per share and trading volume for the Common Shares on the Nasdaq for the periods indicated.
| | | | | | | | | | | |
| Period | High Trading Price (USD) | Low Trading (USD) | Volume (#) |
| August 2022 | $ 1.27 | $ 1.00 | 45,861,582 |
| July 2022 | $ 1.20 | $ 0.90 | 45,356,380 |
| June 2022 | $ 1.18 | $ 0.91 | 47,680,000 |
| May 2022 | $ 1.51 | $ 1.10 | 129,621,805 |
| April 2022 | $ 1.82 | $ 1.35 | 112,695,830 |
| March 2022 | $ 1.87 | $ 1.27 | 120,100,450 |
| February 2022 | $ 1.74 | $ 1.30 | 84,071,766 |
| January 2022 | $ 1.86 | $ 1.30 | 119,198,910 |
| December 2021 | $ 2.13 | $ 1.71 | 139,115,750 |
| November 2021 | $ 2.55 | $ 1.83 | 221,208,420 |
| October 2021 | $ 2.53 | $ 2.17 | 109,366,016 |
| September 2021 | $ 2.78 | $ 2.26 | 106,314,290 |
PRIOR SALES
The following table summarizes details of the following securities that are not listed or quoted on a marketplace issued by the Company during the period between September 1, 2021 and August 31, 2022:
| | | | | | | | | | | |
| Date of Issuance | Security | Issuance/Exercise Price Per Security ($) | Number of Securities |
| October 14, 2021 | Restricted Share Units | 2.82 | 11,754 |
| October 14, 2021 | Options | 2.85 | 520,000 |
| October 22, 2021 | Performance Share Units | 2.90 | 169,843 |
| October 22, 2021 | Restricted Share Units | 2.90 | 379,494 |
| May 24, 2022 | Performance Share Units | 1.61 | 18,430 |
| May 24, 2022 | Restricted Share Units | 1.61 | 18,430 |
| May 24, 2022 | Options | 1.61 | 280,000 |
| July 21, 2022 | Restricted Share Units | 1.40 | 1,035,000 |
| July 21, 2022 | Options | 1.40 | 4,677,000 |
ESCROWED SECURITIES AND SECURITIES SUBJECT TO CONTRACTUAL RESTRICTION ON TRANSFER
The following table summarizes details of the Company’s securities of each class held, to the Company’s knowledge, in escrow or that were subject to a contractual restriction on transfer as of August 31, 2022:
| | | | | | | | |
| Designation of Class | Number of securities held in escrow or that are subject to a contractual restriction on transfer1 | Percentage of Class |
| Common Shares | 60,996,108 | 19.44%2 |
1 The Common Shares acquired by BAT on March 10, 2021 are subject to transfer restrictions outlined in the investor rights agreement between the Company and BAT, including certain restrictions on prearranged trades.
2 Based on 313,815,503 Common Shares outstanding as of August 31, 2022.
DIRECTORS AND EXECUTIVE OFFICERS
Name, Occupation and Security Holding
Below are the names, province and country of residence, principal occupation and periods of service of the directors and executive officers of the Company as of November 24, 2022.
| | | | | | | | | | | |
| Name, Province and Country of Residence | Principal Occupation for the Past Five Years | Position and Offices held with the Company(1) | Number of Shares Beneficially Owned or Controlled(2) |
Beena Goldenberg
Toronto, Ontario
Canada | May 2005 to April 2020 – Chief Executive Officer of Hain Celestial Canada; April 2020 to August 2021 – President and CEO of The Supreme Cannabis Company Inc.; September 2021 to present – Chief Executive Officer of the Company | Chief Executive Officer since September 9, 2021; and Director since November 19, 2021 | 5,000 (~0.0015%) |
Derrick West, CPA Oakville, Ontario
Canada | March 2008 to June 2012 – Chief Financial Officer of Landdrill International Inc.; March 2014 to December 2019 – Chief Financial Officer of Partners Real Estate Investment Trust; March 2020 to present – Chief Financial Officer of the Company | Chief Financial Officer since March 4, 2020 | 13,505 (~0.004%) |
Paolo De Luca, CPA, CFA Woodbridge, Ontario Canada | December 2013 to December 2017 – Chief Financial Officer, Meridian LNG (a West Face Capital portfolio company); December 2017 to March 2020 – Chief Financial Officer of the Company; March 2020 to present – Chief Strategy Officer of the Company | Chief Strategy Officer since March 4, 2020; and Chief Financial Officer from December 19, 2017 to March 3, 2020 | 55,950 (~0.017%) |
| | | | | | | | | | | |
Timothy Emberg
Ottawa, Ontario
Canada | April 2012 to September 2017 – Executive Director of Marketing of Roche Diabetes Care-Canada; October 2017 to July 2021 – Senior Vice President, Sales and Commercial Operations of the Company; July 2021 to October 2022 - Chief Revenue Officer of the Company; October 2022 to present – Chief Commercial Officer | Chief Commercial Officer since October 6, 2022; Chief Revenue Officer from July 15, 2021 to October 5, 2022; Senior Vice President, Sales and Commercial Operations from September 9, 2018 to July 14, 2021; Vice President of Sales and Commercial Operations from October 2, 2017 to September 8, 2018 | Nil (0%) |
Helen Martin
Toronto, Ontario
Canada | November 2014 to October 2018 – Chief Operating Officer of Crosswinds Holdings Inc.; November 2018 to July 2021 – Vice-President, Strategic Initiatives and Legal Affairs of the Company; July 2021 to present – Chief Legal Officer of the Company | Chief Legal Officer since July 15, 2021; Senior Vice President, Strategic and Legal Affairs from April 5, 2019 to July 14, 2021; Vice President, Strategic Initiatives and Legal Affairs from November 26, 2018 to April 4, 2019; Corporate Secretary since March 4, 2019 | Nil (0%) |
Megan McCrae
Toronto, Ontario
Canada | August 2016 to December 2017 – Director of Marketing & Communications, Aphria Inc.; January 2018 - September 2019- Vice-President, Marketing, Aphria Inc.; September 2019 - May 2020 -Chief Marketing Officer, Aphria Inc.; May 2021 to present – SVP Marketing and Communications of the Company | Senior Vice President of Marketing and Communications since May 31, 2021 | Nil (0%) |
| | | | | | | | | | | |
Nathalie Batten
Moncton, New Brunswick Canada | January 2014 to May 2020 – Director of Sales and Operations of Irving Oil Limited; November 2020 to March 2021 – President of Infinite Impact Consulting Corp.; March 2021 to June 2021 – Plant Manager of the Company; June 2021 to October 2022 – VP Operations of the Company; October 2022 to present – Senior VP Operations | Senior Vice President of Operations since October 6, 2022; Vice President of Operations from June 1, 2021 to October 5, 2022 | Nil (0%) |
James Fletcher
Winnipeg, Manitoba
Canada | 2012 to present – President of Cavalier Candies Ltd; 2018 to present – President of EIC | President of EIC since April 6, 2021 | 8,824 (~0.002%) |
Katrina McFadden Milton, Ontario Canada | November 2014 to January 2019 - VP People and Culture and CHRO of ArcelorMittal Dofasco; January 2019 to July 2021 - VP People and Culture of Telus; July 2021 to January 2022 - VP Human Resources of Weston Foods; August 2022 to Present - Chief People Officer of the Company | Chief People Officer since August 29, 2022 | 1,070 (~0.000%) |
Geoff Riggs Montreal, Quebec Canada | March 1998 to June 2018 – IBM Canada, Global Business Services; July 2018 to Aug 2020 Chief Information Officer of The Green Organic Dutchman; October 2020 to April 2021 - Director of Systems of The Cronos Group; October 2021 to 2022 Senior Manager, Tech Strategy of Deloitte Canada; August 2022 to Present, Chief Information Officer of the Company. | Chief Information Officer since August 15, 2022 | Nil (0%) |
| | | | | | | | | | | |
Peter Amirault(6)
Mississauga, Ontario
Canada | 2009 to present – President of BML Group Limited. | Director since June 2, 2016; Executive Chair from May 3, 2021 to October 31, 2021 | 160,000 (~0.050%) |
Geoffrey Machum(4)(5) Halifax, Nova Scotia
Canada | 1985 to present – Commercial Litigation Partner at Stewart McKelvey LLP; 2016 to present – Director of WildBrain Ltd. | Director since February 25, 2020 Lead Independent Director from May 3, 2021 to October 31, 2021 Chair of the Governance, Nominating and Sustainability Committee | 4,500 (~0.001%) |
Ken Manget(3)(5)(6) Toronto, Ontario
Canada | 2014 to 2019 - Global Head of Relationship Investing at Ontario Teachers’ Pension Plan Board; 2019 to present – Director at Canadian Ditchley Foundation; 2020 to present – CFO & Director, Northern Genesis Acquisition Corp. | Director since February 25, 2020 | Nil (0%) |
Stephen Smith(3)(6) Etobicoke, Ontario, Canada | 2018 to present – Director of MAV Beauty Brands Inc.; 2018 to 2019 – Director of Newstrike Brands Ltd.; 2020 to present – Director of Freshii Inc.; 2014 to 2018 - EVP and Advisory Board Director, Jackman Reinvention Inc. | Director since February 25, 2020 Chair of the Audit Committee | 10,300 (~0.003%) |
Sherry Porter, CM(4)(5) Halifax, Nova Scotia
Canada | 2010 to 2017 – Board member of the Nova Scotia Liquor Corporation; 2014 to present - Board member of the Halifax International Airport Authority; March 2015 to present – Board member of Pharmasave Drugs (Atlantic) Limited | Director since December 17, 2018 Chair of the Compensation Committee | 19,500 (~0.006%) |
| | | | | | | | | | | |
Dexter John(3)(4)(6)
Whitby, Ontario
Canada | June 2014 to April 2019 – Executive Vice President of D.F. King (Canada); April 2019 – present – President and CEO of Gryphon Advisors Inc. | Director since December 17, 2018 Chair of the Investment Committee | 6,120 (~0.019%) |
Marni Wieshofer(3)(6)
Santa Monica, California
USA | June 2015 to November 2019 – Managing Director, Head of Media at Houlihan Lokey; December 2019 to present – Director of several companies including Thunderbird Entertainment, Hycroft Mining Holding Corporation, and Acceso Impact, Inc. | Director since January 12, 2021 | Nil (0%) |
Simon Ashton(6)
Staines-upon-Thames England | September 2015 to August 2019 – Area head of Finance (Middle East) at BAT; August 2019 to August 2021 - Area head of Finance (North West Europe) at BAT; August 2021 to present – Group Head of New Categories and Combustibles Finance at BAT. | Director since February 23, 2022 | Nil (0%) |
Notes:
(1)The previous term of the current directors of the Company expired at the conclusion of the annual meeting of the shareholders held on February 23, 2022 (the “2022 Shareholder Meeting”), excluding the new directors elected at or after the 2022 Shareholder Meeting. All of the directors noted above were re-elected and their terms will expire at the conclusion of the next annual meeting of shareholders other than Simon Ashton who was appointed to the Company’s board of directors immediately after the 2022 Shareholder Meeting.
(2)As of November 24, 2022, all directors and executive officers noted above of the Company, as a group, beneficially own, directly or indirectly, or exercise control or direction over 284,769 Common Shares of the Company, representing ~1% of the Company’s outstanding Common Shares. The total number of issued and outstanding shares as of November 24, 2022 is 313,856,912 Common Shares.
(3)Member of the Audit Committee.
(4)Member of the Governance, Nominating and Sustainability Committee.
(5)Member of the Compensation Committee.
(6)Member of the Investment Committee.
DIRECTOR & EXECUTIVE OFFICER BIOGRAPHIES
Beena Goldenberg – Director and Chief Executive Officer
Ms. Goldenberg has more than 30 years of experience in consumer-packaged goods manufacturing and marketing. Most recently, Ms. Goldenberg was President and CEO of The Supreme Cannabis Company Inc., where she achieved significant growth in the first year of her tenure through a focus on distribution, innovation, and brand portfolio development. Ms. Goldenberg also served as Chief Executive Officer, President and General Manager at Hain-Celestial Canada, ULC where, among other notable accomplishments, she led the strategic growth of the company through the organic growth of existing brands by increasing distribution, launching on-trend innovations, integrating newly acquired US brands, and completing two Canadian acquisitions. During this time, Ms. Goldenberg also served two years as the Chief Executive Officer of Cultivate Ventures, the growth venture platform for The Hain Celestial Group, where she was responsible for investment in small portfolio brands and incubator opportunities with a focus on health and wellness. Before Hain-Celestial Canada, Ms. Goldenberg held senior leadership and
marketing roles at other leading Canadian packaged goods companies, including Catelli Foods Corporation, Parmalat Canada and Pillsbury Company Limited. She also served on the Board of Food and Consumer Products of Canada, the largest CPG industry association, from 2008 to 2020. She holds a Bachelor and Master of Engineering (Chemical) from McGill University.
Derrick West – Chief Financial Officer
Mr. West was formerly the Chief Financial Officer and Corporate Secretary of Partners Real Estate Investment Trust (TSX:PAR.UN), an open end real estate investment trust focused on managing a portfolio of retail community centres across Canada. Mr. West previously served as the Chief Financial Officer of Landdrill International Inc. (TSXV:LDI), an international mining services corporation with operations in Canada, Russia, Mongolia, and Mexico. Additionally, Mr. West served as the Vice-President of Accounting and Administration for Plazacorp Retail Properties Limited (renamed Plaza Retail REIT, TSX:PLZ.UN). Mr. West has 30 years business experience, the last 18 in senior positions with publicly traded enterprises. He has financial reporting, finance, treasury management, internal control, human resource, strategy and business development experience. Mr. West is a Chartered Professional Accountant, obtaining his Chartered Accountancy designation in New Brunswick with Grant Thornton LLP. He holds a Bachelor of Commerce degree from Mount Allison University.
Paolo De Luca, CPA, CA, CFA – Chief Strategy Officer
Mr. De Luca assumed the role as the Company’s Chief Strategy Officer on March 4, 2020, having previously held the position of Chief Financial Officer. With more than 25 years of diversified financial business experience, Mr. De Luca has held senior financial, investor relations, and accounting leadership roles at companies, including West Face Capital, one of Canada’s leading alternative asset management firms; Meridian LNG; Potash Ridge; C.A. Bancorp; and TD Securities. With this diverse industry and international background, he has extensive experience with both traditional and non-traditional financings and debt offerings as well as M&A activities. Mr. De Luca is a graduate of York University’s Schulich School of Business, is a Chartered Professional Accountant and a member of the Chartered Professional Accountants of Ontario, and is a CFA Charter holder.
Timothy Emberg – Chief Commercial Officer
Mr. Emberg, Chief Commercial Officer, is an accomplished, bilingual, senior sales and marketing leader with a proven track record in healthcare, over-the-counter and consumer packaged goods organizations including Roche Canada, Jamieson Laboratories and Frito-Lay Canada. Mr. Emberg also brings an extensive knowledge of the Canadian market access and regulatory environments to the role, which will be an asset moving forward. As Chief Commercial Officer, his role is to lead both adult-use recreational and medical cannabis sales while ensuring that the Company is well established and strongly represented nationally. Mr. Emberg will also play a key role in other commercially driven initiatives that will help set the stage for future growth and development of the organization while further enhancing the Company’s position as an industry leader in Canada.
Helen Martin – Chief Legal Officer, and Corporate Secretary
Ms. Martin joined the Company as its Vice President Strategic Initiatives and Legal Affairs in November 2018 and was appointed Corporate Secretary in March 2019. She was promoted to Senior Vice President, Strategic and Legal Affairs, in April 2019, and to Chief Legal Officer in July 2021. Prior to joining Organigram, she was the Chief Operating Officer of Crosswinds Holdings Inc. from November 2014 to October 2018. She was Senior Legal Counsel at AUM Law Professional Corporation where she held various legal roles since 2011. Ms. Martin was employed as General Counsel and Corporate Secretary of C.A. Bancorp Inc. from 2009 to 2011 and In-House Counsel at Sentry Select Capital Corp. from 2007 to 2008. Prior to joining Sentry Select, Ms. Martin was a lawyer in the securities group at Blake, Cassels & Graydon LLP from 2005 to 2007. Ms. Martin is a member of the Law Society of Ontario. She received her law degree from the University of Toronto and a Bachelor of Arts (Honours) from the University of Victoria.
Megan McCrae – Senior Vice President of Marketing and Communications
Ms. McCrae is a seasoned marketing professional with 17 years of consumer packaged goods marketing & sales management, communications, brand building, and consumer insights experience. Ms. McCrae is a cannabis industry veteran having spent nearly four years with Aphria Inc. where she led the company’s brand and portfolio management, consumer insights, innovation, and digital strategy. Ms. McCrae also spent ten years in various global progressive consumer and trade marketing management roles with global tobacco giant, Japan Tobacco International (JTI) as well as holding the position of Board Chair on the Cannabis Council of Canada.
Nathalie Batten – Senior Vice President of Operations
Ms. Batten has recently held senior-level positions, including Director, Fleet Sales and Operations, and Director, Blending and Packaging at Irving Oil, an international refining and marketing company that operates Canada’s largest refinery and more than 1,200 fuelling locations. There, Nathalie built high-performance teams and led the development and execution of strategic business plans. Before Irving Oil, she held various roles in business development and strategy at Keyera Corporation. Nathalie is a professional industrial engineer, has a Master of Business Administration from the University of Calgary, a Bachelor of Mechanical Engineering from Queen’s University, and is an Executive Coach from Royal Roads University.
James Fletcher – President of EIC
Mr. Fletcher has extensive experience in the confectionary manufacturing space having been President of Cavalier Candies Ltd. since 2012 and having founded the Edibles and Infusions Corporation in 2018. Prior to this, Mr. Fletcher was the Director of Commodities Trading at RBC Capital Markets where he specialized in natural gas and crude oil trading. Mr. Fletcher holds a Bachelor of Applied Science in Computer Engineering from the University of Waterloo.
Katrina McFadden – Chief People Officer
Ms. McFadden is an experienced Human Resources executive who has worked across several industries including telecommunications, manufacturing and consumer goods. Throughout her 20-year career she held senior leadership positions with organizations such as ArcelorMittal Dofasco, TELUS and most recently Weston Foods, supporting these organizations in earning accolades for innovative people and culture programs focused on enhancing the employee experience. Ms. McFadden holds a Bachelor of Applied Science in Chemical Engineering from the University of Waterloo and a Masters of Business Administration from McMaster University.
Geoff Riggs – Chief Information Officer
Mr. Riggs has spent 25 years in technology and business strategy including four (4) years in the cannabis industry, starting as the CIO at The Green Organic Dutchman in 2018, then Director of Systems at Cronos and most recently as the cannabis technology strategy leader with Deloitte Canada. Previously he spent 20 years with IBM in a variety of roles including project management, business development, and strategy consulting. He has operated across Canada, the US and Europe and has experience in numerous sectors including finance, oil and gas, transportation, natural resources, and government. He has a track record of successful complex systems implementations and driving innovation into emerging market spaces. Mr. Riggs holds a Bachelor of Commerce in Management Information Systems from Memorial University and a Masters of Business Administration from Antioch University with focus on Sustainability. He holds designations of PMP and ITIL, and numerous IT-related training certifications.
Peter Amirault – Chairman of the Board
Mr. Amirault is currently the President of BML Group Limited in Toronto, a holding company with interests in real estate development and private investments. Prior to joining BML Group, Mr. Amirault held varying executive roles including: President of Swiss Chalet North America for the Cara Group of Companies, CEO of Creemore Springs Brewery Ltd, Senior Vice President of Molson Coors Canada,
Managing Director of Sleeman Brewing Ltd, along with senior roles at Nestle Canada and The Premium Beer Company of Toronto. Mr. Amirault holds a Bachelor of Business Administration from Acadia University and a Master of Business Administration from The Schulich School of Business. Mr. Amirault’s previous board experience and roles at senior management levels will bring a wealth of knowledge to the corporate director team at the Company.
Geoffrey Machum – Director
Mr. Machum is a commercial litigation partner at Stewart McKelvey LLP, Atlantic Canada’s largest law firm and one of the top 15 largest firms in Canada. He currently serves on the firm’s Compensation Committee, and previously served as Chairman of the firm’s Regional Partnership Board and on its Human Resources and Governance Committee, and its Audit and Finance Committee. Mr. Machum was awarded Kings Counsel in 2003, and has received repeated recognition by Lexpert, Best Lawyers, and Benchmark Canada for his extensive experience in practice areas including commercial litigation, directors and officers’ liability, corporate governance, insurance, construction law, and products liability. Mr. Machum currently serves on the board of WildBrain Ltd., where he is the Chair of its Governance and Nomination Committee and member of its Human Resources and Compensation Committee and previously served on the Board’s Special Strategic Review Committee. Previously, he chaired the board of Halifax Port Authority, and served on the Governance, Human Resources and Audit and Finance Committees. Mr. Machum holds a BA in Economics Political Science from Dalhousie University, and University of New Brunswick. Mr. Machum also received ICD.D designation from the University of Toronto Rotman School of Management in 2015.
Ken Manget – Director
Mr. Manget is the former Global Head of Relationship Investing at Ontario Teachers’ Pension Plan Board where he was responsible for a global team in Hong Kong, London & Toronto and ran a multi-billion portfolio of pre-IPO, public and private equity investments. Mr. Manget started his career at Schlumberger Limited as a Field Engineer in Venezuela. His finance background includes stints at Salomon Brothers in London and New York, at BMO Capital Markets where he has had exposure to all facets of the capital markets including: M&A, equities, fixed income, derivatives and securitization and at Desjardins Capital Markets where he was Head of Investment Banking. Mr. Manget holds a Mechanical Engineering degree from the University of Toronto and a Master of Business Administration from the Harvard Business School. Mr. Manget is a past board member of St. Joseph’s Health Centre Foundation, the Heart and Stroke Foundation and currently serves as a member of the Board of the Canadian Ditchley Foundation and as an alumnus volunteer for Harvard University. In August 2020, he was appointed CFO and Director of Northern Genesis Acquisition Corp.
Dexter John – Director
Mr. John is currently the President and CEO of Gryphon Advisors Inc. Prior to that he was the Executive Vice President of D.F. King (Canada) and was responsible for sales across the Canadian enterprise. Mr. John has over 20 years of experience in the capital markets and has spent six years in structured finance where he executed over $4 billion in transactions. He has worked at a major Canadian law firm as a securities associate, focusing on the public equities market with emphasis on mergers and acquisitions. In addition, Mr. John also has regulatory experience through his tenure at Investment Industry Regulatory Organization of Canada, the Ontario Securities Commission and the Toronto Stock Exchange. Mr. John holds a Bachelor of Laws degree from Queens University and the ICD.D designation.
Sherry Porter, CM – Director
Ms. Porter is a seasoned executive with 30 years of experience with a myriad of organizations in Canada. She has held senior corporate roles with Sobeys Inc., Nova Scotia Power, Shoppers Drug Mart and The Caldwell Partners. She also has experience with trade associations in the grocery and retail drug area. She was the founding President and CEO of the Canadian Association of Chain Drug Stores, working with the chief executive officers of the traditional drug chains, mass merchants and grocery operations in Canada. Ms. Porter chaired the board of directors of the Nova Scotia Liquor Corporation from 2010-2017 and is currently a board member of the Halifax International Airport Authority and Pharmasave Atlantic. She is a past Vice Chair of Dalhousie University and a past chair of Human Resources, Governance and
Nominating, and she also serves as a board member of the QEII Health Sciences Centre Foundation and the Symphony Nova Scotia Foundation.
Stephen Smith – Director
Mr. Smith is an accomplished executive with extensive leadership and managerial experience in complex, low margin and highly competitive retail environments. He currently serves on the board of directors of MAV Beauty Brands Inc. (Audit Committee Chair), Freshii Inc. (Lead Director and Audit Committee Chair) and CE Brands Inc. (Lead Director). From 2018 to 2019, Mr. Smith served on the board of directors of Newstrike Brands Ltd. (Lead Director and Audit Committee Chair). From 2013 to 2017, Mr. Smith served on the board of directors of CST Brands Inc., an SEC registrant (Audit Committee and Executive Committee). From 2014 to 2018, Mr. Smith held the position of Executive Vice President and Advisory Board Director of Jackman Reinvention, Inc., a privately held brand and strategy consulting firm in Toronto. From 2007 until 2013, Mr. Smith served as Co-Chief Executive Officer and Chief Financial Officer of Cara Operations Limited (now Recipe Unlimited), Canada’s oldest and largest full-service restaurant company. From 1985 to 2007, Mr. Smith held various senior and executive level positions, including Executive Vice President, from 1999 to 2006, with Loblaw Companies Limited, the leading food and pharmacy retailer in Canada. Mr. Smith is a Chartered Professional Accountant (CPA, CA) and holds a Bachelor of Commerce degree from the University of Toronto.
Marni Wieshofer – Director
Ms. Wieshofer has more than thirty years of diverse experience, including Board membership at public and private companies, particularly in the U.S., international M&A, and finance. She was recognized by Variety magazine in the 2018 Dealmakers Impact Report. Previous roles have included CFO and EVP of Corporate Development at Lions Gate Entertainment Corporation, a multi-billion dollar global entertainment company, where she oversaw the company’s M&A and other strategic financial initiatives including the acquisitions and integration of Trimark Pictures, Artisan Entertainment and Redbus Films Distribution U.K. Her background also includes being a Managing Director in Houlihan Lokey’s TMT Corporate Finance Group, based out of Los Angeles, providing M&A, capital markets, financial restructuring, and financial advisory services. Before joining Houlihan Lokey, Ms. Wieshofer was a Managing Director at MESA, a boutique advisory investment bank and prior to MESA, she was the SVP of M&A and CFO at Media Rights Capital. Ms. Wieshofer also holds the ICD.D designation.
Simon Ashton – Director
Mr. Ashton has extensive expertise in finance and business leadership and is currently Group Head of New Categories and Combustibles Finance at BAT. Throughout his nearly 30-year career with BAT, Mr. Ashton has led various Finance teams across Europe, Asia, the Middle East and Africa driving revenue growth, leading business transformation initiatives and finding innovative solutions to economic challenges. In addition, he also spent time in M&A, Operations Finance, and Audit.
CEASE TRADE ORDERS, BANKRUPTCIES, PENALTIES OR SANCTIONS
Other than as set out below, to the knowledge of the Company, no director or executive officer of the Company is, as of the date of this Annual Information Form or within ten years prior to the date of this Annual Information Form has been, a director, chief executive officer of chief financial officer of any company (including the Company) that:
(i)was subject to a cease trade order, an order similar to a cease trade order or an order that denied the relevant company access to any exemption under securities legislation, that was in effect for a period of more than 30 consecutive days, and was issued while the director or executive officer was acting in the capacity as director, chief executive officer or chief financial officer; or
(ii)was subject to a cease trade order, an order similar to a cease trade order or an order that denied the relevant company access to any exemption under securities
legislation, that was in effect for a period of more than 30 consecutive days, that was issued after the director or executive officer ceased to be a director, chief executive officer or chief financial officer and which resulted from an event that occurred while that person was acting in the capacity as director, chief executive officer or chief financial officer.
Other than as set out below, to the knowledge of the Company, no director or executive officer of the Company, or a shareholder holding a sufficient number of securities of the Company to affect materially the control of the Company:
(iii)is, or within ten years prior to the date of this Annual Information Form has been, a director or executive officer of any company (including the Company) that, while that person was acting in that capacity, or within a year of that person ceasing to act in that capacity, became bankrupt, made a proposal under any legislation relating to bankruptcy or insolvency or was subject to or instituted any proceedings, arrangement or compromise with creditors or had a receiver, receiver manager or trustee appointed to hold its assets; or
(iv)has, within ten years prior to the date of this Annual Information Form, become bankrupt, made a proposal under any legislation relating to bankruptcy or insolvency, or become subject to or instituted any proceedings, arrangement or compromise with creditors, or had a receiver, receiver manager or trustee appointed to hold the assets of the director, executive officer or shareholder.
No director or executive officer of the Company, or a shareholder holding a sufficient number of securities of the Company to affect materially the control of the Company, has, to the knowledge of the Company, been subject to (i) any penalties or sanctions imposed by a court relating to securities legislation or by a securities regulatory authority or has entered into a settlement agreement with a securities regulatory authority; or (ii) any other penalties or sanctions imposed by a court or regulatory body that would likely be considered important to a reasonable investor in making an investment decision.
CONFLICTS OF INTEREST
The Company may from time to time become involved in transactions which conflict with the interests of the directors and the officers of the Company. The interest of these persons could conflict with those of the Company. Conflicts of interest, if any, will be subject to the procedures and remedies provided under applicable laws.
In particular, in the event that such a conflict of interest arises at a meeting of the directors of the Company, a director who has such a conflict will abstain from voting for or against the approval of such participation or such terms. In accordance with applicable laws, the board of directors of the Company are required to act honestly, in good faith and in the best interest of the Company.
LEGAL PROCEEDINGS AND REGULATORY ACTIONS
On March 3, 2017, a claim (the “Claim”) in connection with a proposed class action lawsuit was filed with the Nova Scotia Supreme Court seeking to represent a class of persons and entities who purchased medical cannabis that was the subject of the Company’s product recalls in December 2016 and January 2017, as it may have contained trace elements of the pesticides myclobutanil and bifenazate which are not approved for use in cannabis production. The Claim identifies several causes of action including, among others: (i) negligent design, development and testing, (ii) negligent manufacturing, (iii) negligent distribution, marketing and sale, (iv) breach of contract, (v) unjust enrichment, and (vi) breach of the Competition Act, the Consumer Protection Act, and the Sale of Goods Act, and is seeking remedy in the form of, among other things, the disgorgement of profits accrued to the Company for the sale of contaminated products, exemplary or punitive damages and certain costs. The Claim was amended on November 16, 2017, to include a claim for alleged adverse health consequences caused as a result of
using the recalled product. As at the date hereof, the Company has not received any medical information demonstrating adverse health effects caused as a result of using the recalled product.
The class action alleges damages for two main types of claims: 1) consumer based claims relating to the transaction costs for the product and 2) claims for damages resulting from adverse health effects of the recalled product. The Nova Scotia Supreme Court certified the Claim as a class action on January 18, 2019. The Company filed an appeal of the certification of the claims alleging adverse health effects with the Nova Scotia Court of Appeal. On April 30, 2020, the Nova Scotia Court of Appeal partially overturned the certification, thereby significantly reducing the scope of the claim against the Company. On June 26, 2020, the plaintiff filed an application for leave to appeal the Nova Scotia Court of Appeal’s decision to the Supreme Court of Canada. On November 5, 2020, the Supreme Court of Canada dismissed the leave to appeal with costs. On June 23, 2022, the Company announced that it had reached a settlement with respect to the Claim (the “Settlement”). As part the Settlement, the Company has agreed to pay an aggregate of $2,310,000, which amount has been accrued as of August 31, 2022. On August 31, 2022, the Court held a hearing and approved the Settlement. The Settlement Amount is being used to provide class members a refund of the amounts paid to purchase the voluntarily recalled product, less any refunds they have already received, as well as the payment of legal fees. In addition, the Company has agreed to pay the third-party claims administration costs.
On June 16, 2020, a legal action was commenced in the Court of Queen’s Bench in Alberta, which action seeks damages against many of the largest Canadian cannabis companies, including the Company. A certification hearing has not yet been scheduled. The Company has reported the claim to its insurers.
INTEREST OF MANAGEMENT AND OTHERS IN MATERIAL TRANSACTIONS
No director, executive officer, or principal shareholder of the Company and no associate or affiliate of the foregoing have had a material interest, direct or indirect, in any transaction in which the Company has participated within the three most recently completed financial years or during the current financial year, which has materially affected or is reasonably expected to materially affect the Company, other than as set forth below.
James Fletcher is the President of EIC, a wholly-owned subsidiary of the Company. In April 2021, the Company purchased all of the issued and outstanding shares of SUHM Investments Inc. and Quality Confections Canada Inc., which in turn owned all of the issued and outstanding shares of EIC. James Fletcher was a shareholder of SUHM Investments Inc., and his spouse was the sole shareholder of Quality Confections Canada Inc. As such, James Fletcher and his spouse received or are entitled to receive a 0.849% and 20.00% allocation respectively of: (i) the $22.0 million purchase price for EIC; (ii) the $3.5 million paid in Organigram shares on August 13, 2021 on achievement of the first earnout milestone; and (iii) up to an additional $9.5 million in Organigram shares payable upon the EIC business achieving certain further earnout milestones. In July 2022, EIC exercised its option to purchase the facility at 160 Eagle Drive, Winnipeg, Manitoba, from 10026308 Manitoba Ltd., a company controlled by James Fletcher, for $4,000,000. The decision to exercise the option was made by the Company without the involvement of Mr. Fletcher.
TRANSFER AGENT AND REGISTRAR
The transfer agent and registrar of the Company is TSX Trust Company at its offices in Vancouver, British Columbia and Toronto, Ontario. VStock Transfer, LLC is the Company’s co-transfer agent in the United States.
MATERIAL CONTRACTS
Except for contracts entered into in the ordinary course of business, there are no contracts entered into by the Company during the twelve-month period ending August 31, 2022, which are material or entered into before the twelve-month period ending August 31, 2022, but are still in effect which are material, except as disclosed below:
•the Investor Rights Agreement (as described under “Capital Structure – Common Shares”);
•the Collaboration Agreement (as described under “Three-Year History”);
•the Subscription Agreement between the Company and BAT dated March 10, 2021, pursuant to which BAT subscribed for 58,336,392 Common Shares of the Company for total proceeds of approximately $221,000,000; and
•the Warrant Indenture (as described under “Capital Structure – Warrants”)
Copies of the Investor Rights Agreement, the Collaboration Agreement, the Subscription Agreement, and the Warrant Indenture are available under the Company’s corporate profile on the Canadian Securities Administrators’ SEDAR website at www.sedar.com and on the United States Securities and Exchange Commission’s EDGAR website at www.sec.gov.
INTERESTS OF EXPERTS
KPMG LLP are the auditors of the Company and have confirmed with respect to the Company that they are independent within the meaning of the relevant rules and related interpretations prescribed by the relevant professional bodies in Canada and any applicable legislation or regulations, and within the meaning of the United States Securities Act of 1933, as amended and the applicable rules and regulations thereunder adopted by the Securities Exchange Commission and the Public Company Accounting Oversight Board (United States).
AUDIT COMMITTEE INFORMATION
Audit Committee Charter
The charter of the Company’s Audit Committee is attached to this Annual Information Form as Appendix “A”.
Composition of Audit Committee & Relevant Education and Experience
As of August 31, 2022 and the date hereof, the members of the Audit Committee are Stephen Smith (Chair), Dexter John, Ken Manget, and Marni Wieshofer, each of whom is independent and financially literate within the meaning of National Instrument 52-110. The education and experience of each Audit Committee member are described in this Annual Information Form under the section entitled “Directors and Executive Officers”.
Each of the Audit Committee members has an understanding of the accounting principles used to prepare the Company’s financial statements, experience preparing, auditing, analyzing or evaluating comparable financial statements and experience as to the general application of relevant accounting principles, as well as an understanding of the internal controls and procedures necessary for financial reporting.
The Company’s board of directors has determined that Stephen Smith qualifies as an “audit committee financial expert” (as defined in paragraph (8)(b) of General Instruction B to Form 40-F under the Exchange Act). The United States Securities and Exchange Commission has indicated that the designation of a director as an audit committee financial expert does not make such director an “expert” for any other purpose, impose any duties, obligations or liability on such director that are greater than those imposed on members of the Audit Committee and Board who do not carry this designation or affect the duties, obligations or liability of any other member of the Audit Committee.
The Audit Committee has the primary function of fulfilling its responsibilities in relation to reviewing the integrity of the Company’s financial statements, financial disclosures and internal controls over financial reporting; monitoring the system of internal control; monitoring the Company’s compliance with legal and regulatory requirements, selecting the external auditor for shareholder approval; reviewing the qualifications, independence and performance of the external auditor; and reviewing the qualifications, independence and performance of the Company’s internal auditors. The Audit Committee has specific responsibilities relating to the Company’s financial reports; the external auditor; the internal audit function; internal controls; regulatory reports and returns; legal or compliance matters that have a material impact on the Company; and the Company’s whistleblowing procedures. In fulfilling its responsibilities, the Audit Committee meets regularly with the internal and external auditor and key management members. Information concerning the relevant education and experience of the Audit Committee
members can be found in “Directors and Executive Officers” above. The full text of the Audit Committee’s charter is disclosed in Appendix “A”.
Audit Committee Oversight
At no time since the commencement of the Company’s most recently completed financial year have any recommendations by the Audit Committee respecting the appointment and/or compensation of the Company’s external auditor not been adopted by the board of directors of the Company.
Pre-Approval Policies and Procedures
The Audit Committee will pre-approve all non-audit services to be provided to the Company or any subsidiary entities by its external auditors or by the external auditors of such subsidiary entities. The Audit Committee may delegate to one or more of its members the authority to pre-approve non- audit services but preapproval by such member or members so delegated shall be presented to the full Audit Committee at its first scheduled meeting following such pre-approval.
External Auditor Service Fees
The following table sets forth, by category, the fees for all services rendered by the Company’s current external auditors, KPMG LLP for the financial years ended August 31, 2022 and 2021 (including estimates).
| | | | | | | | | | | | | | |
| Type of Work | Year ended August 31, 2022 | Year ended August 31, 2021 |
| Fees | Percentage | Fees | Percentage |
Audit fees(1) | $1,407,780(3) | 95% | $ 1,224,420 | 95% |
| Audit-related fees | Nil | Nil | Nil | Nil |
Tax fees(2) | $82,200 | 5% | $60,900 | 5% |
| All other fees | Nil | Nil | Nil | Nil |
| Total | $1,489,980 | 100% | $1,285,320 | 100% |
Notes:
(1)For the year ended August 31, 2021 (“FY’2021”) audit fees were comprised of quarterly reviews, the annual audit (including the audit of internal controls over financial reporting) and a securities engagement. For the year ended August 31, 2022 (“FY’2022”) audit fees were comprised of quarterly reviews and the annual audit (including the audit of internal controls over financial reporting).
(2)Includes fees for all tax services other than those included in “Audit Fees” and “Audit-Related Fees”. This category includes fees for tax compliance and advisory in FY’2022 and FY’2021. Tax advice includes advice related to mergers and acquisitions, and a captive insurance structure.
(3)Of these fees, $181,560 relates to the FY’2021 audit fees but was only invoiced in FY’2022.
ADDITIONAL INFORMATION
Additional information, including directors’ and officers’ remuneration and indebtedness, principal holders of securities of the Company and securities authorized for issuance under equity compensation plans, is contained in the Company’s management information circular relating to the most recent annual meeting of shareholders of the Company. Additional financial information is contained in the Company’s financial statements and management discussion and analysis for the year ended August 31, 2022 Additional information relating to the Company may also be found on the Canadian Securities Administrators’ SEDAR website at www.sedar.com and on the United States Securities and Exchange Commission’s EDGAR website at www.sec.gov. Copies of all of these documents may be obtained upon request from Organigram’s Investor Relations department at 333 Bay Street, Suite 1250, Toronto, ON M5H 2R2.
Appendix “A” – AUDIT COMMITTEE CHARTER
ORGANIGRAM HOLDINGS INC.
(THE “CORPORATION”)
CHARTER OF THE AUDIT COMMITTEE
This Charter of the Audit Committee (the “Charter”) was adopted by the board of directors of the Corporation (the “Board”) on August 26, 2019, and last reviewed on August 26, 2022.
1.Purpose
The Audit Committee (the “Committee”) is a committee of the Board. The members of the Committee and the chair of the Committee (the “Chair”) are appointed by the Board on an annual basis (or until their successors are duly appointed) for the purpose of overseeing the Corporation’s financial controls and reporting and monitoring whether the Corporation complies with financial covenants and legal and regulatory requirements governing financial disclosure matters and financial risk management.
2.Composition
(a)The Committee should be comprised of a minimum of three directors of the Corporation.
(b)All members of the Committee must meet the independence and audit committee composition requirements promulgated by all governmental and regulatory bodies having jurisdiction over the Corporation as may be in effect from time to time, including Rule 10A-3 of the United States Securities Exchange Act of 1934, as amended (the “Exchange Act”), Rule 5605 of the Nasdaq Marketplace Rules, National Instrument 52-110 – Audit Committees (“NI 52-110”), and the relevant rules of any other stock exchanges on which the Corporation’s securities are listed. In general, each member of the Committee must be free from any relationship that, in the view of the Board, could be reasonably expected to interfere with the exercise of his or her independent judgment as a member of the Committee.
(c)All members of the Committee must be financially literate (which is defined as the ability to read and understand a set of financial statements that present a breadth and level of complexity of accounting issues that are generally comparable to the breadth and complexity of the issues that can reasonably be expected to be raised by the Corporation’s financial statements). At least one member of the Committee must satisfy the definition of “financial expert” as set out in Item 407(d)(5)(ii) of Regulation S-K under the United States Securities Act of 1933, as amended, and the Exchange Act.
(d)The Board shall designate the Chair of the Committee, who shall have responsibility for overseeing that the Committee fulfills its mandate and duties effectively. If the Board does not designate a Chair, the Committee will elect a Chair from among their members.
(e)Any member of the Committee may be removed or replaced at any time by the Board and will cease to be a member of the Committee on ceasing to be a director of the Corporation. The Board may fill vacancies on the Committee by election from among the Board. If and whenever a vacancy will exist on the Committee, the remaining members may exercise all powers of the Committee so long as a quorum remains.
(f)No members of the Committee shall receive, directly or indirectly, other than for service on the Board or the Committee or other committees of the Board, any consulting, advisory, or other compensatory fee from the Corporation or any of its related parties or subsidiaries.
(g)Prior to any member of the Committee or the Board engaging the services of the Corporation’s auditor in a personal capacity, the consent of the Chair of the Committee shall be obtained.
3.Limitations on Committee’s Duties
In contributing to the Committee’s discharge of its duties under this Charter, each member of the Committee will be obliged only to exercise the care, diligence and skill that a reasonably prudent person would exercise in comparable circumstances. Nothing in this Charter is intended or may be construed as imposing on any member of the Committee a standard of care or diligence that is in any way more onerous or extensive than the standard to which any member of the Board may be otherwise subject.
Members of the Committee are entitled to rely, absent actual knowledge to the contrary, on (a) the integrity of the persons and organizations from whom they receive information, (b) the accuracy and completeness of the information provided, (c) representations made by management of the Corporation (“Management”) as to the non-audit services provided to the Corporation by the external auditor, (d) financial statements of the Corporation represented to them by a member of Management or in a written report of the external auditors to present fairly the financial position of the Corporation in accordance with applicable generally accepted accounting principles, and (e) any report of a lawyer, accountant, engineer, appraiser or other person whose profession lends credibility to a statement made by any such person.
4.Meetings
The Committee shall meet regularly, but not less frequently than quarterly. A quorum for the transaction of business at any meeting of the Committee will be a majority of the members of the Committee or such greater number as the Committee will by resolution determine. The Committee will keep minutes of each meeting of the Committee. A copy of the minutes will be provided to each member of the Committee.
Meetings of the Committee will be held from time to time and at such place as any member of the Committee will determine upon two days’ prior notice to each of the other Committee members. The members of the Committee may waive the requirement for notice. In addition, each of the Chief Executive Officer, the Chief Financial Officer and the external auditor will be entitled to request that the Chair call a meeting.
The Committee may ask members of Management and employees of the Corporation (including, for greater certainty, its affiliates and subsidiaries) or others (including the external auditor) to attend meetings and provide such information as the Committee requests. Members of the Committee will have full access to information of the Corporation (including, for greater certainty, its affiliates, subsidiaries and their respective operations) and will be permitted to discuss such information and any other matters
relating to the results of operations and financial position of the Corporation with Management, employees, the external auditor and others as they consider appropriate.
The Committee or its Chair should meet at least once per year with Management and the external auditor in separate sessions to discuss any matters that the Committee or either of these groups desires to discuss privately. In addition, the Committee or its Chair should meet with Management quarterly in connection with the Corporation’s interim financial statements.
The Committee will determine any desired agenda items.
5.Committee Responsibilities
As part of its function in assisting the Board in fulfilling its oversight responsibilities (and without limiting the generality of the Committee’s role), the Committee is mandated to carry out the following responsibilities:
External Auditors
(a)Subject to applicable law, appointing, compensating, overseeing and terminating the external auditors. The external auditors shall report directly to the Committee and shall be accountable to the Board and the Committee as representatives of the shareholders.
(b)Pre-approving all non-audit mandates and fees for services the external auditor shall undertake, and considering whether the nature of such services will harm the firm’s independence in carrying out its audit function.
(c)Reviewing, negotiating and either signing or recommending to the Board the execution of all engagement letters of the external auditors, both for audit and non-audit services.
(d)Satisfying itself, on behalf of the Board, that the external auditor is independent of Management. In assessing such independence, the Committee shall discuss with the external auditors, and may require a letter from the external auditor outlining any relationships between the external auditors and the Corporation or its affiliates.
(e)Reviewing the audit plan of the external auditors, the integration of the external audit with the internal control program, and the results of the audit, which shall include reviewing the external auditor’s letter to Management and Management’s response thereto and other material written communications between Management and the external auditors.
(f)Reviewing the performance of the external auditors, including the compensation, scope, and timeliness of the audits and all other related services and any non-audit services provided by the external auditors.
(g)Satisfying itself, annually or more frequently as the Committee considers appropriate, as to the external auditors’
internal quality control procedures and any material issues raised by the most recent internal quality control review, or peer review, of the external auditor, or by any public enquiry, review, or investigation by governmental, professional or other regulatory authorities.
(h)Periodically reviewing and discussing with Management and the external auditors the quality and acceptability of the Corporation’s accounting policies and practices, the materiality levels which the external auditors propose to employ, any significant changes in the accounting policies and any proposed changes in accounting or financial reporting that may have a significant impact on the Corporation.
(i)Discussing with Management and the external auditors of the Corporation all alternative treatments of financial information within International Financial Reporting Standards (“IFRS”) accounting principles that have been discussed with Management by the external auditors, the ramifications of these alternative treatments and the treatment preferred by the external auditors.
(j)Reviewing, where there is to be a change of external auditors, all issues related to the change, including the information to be included in the notice of change of auditor called for under National Instrument 51-102 – Continuous Disclosure Obligations or any successor legislation (“NI 51-102”), and the planned steps for an orderly transition. The Committee shall further review all reportable events, including disagreements, unresolved issues and consultations, as defined in NI 51-102 or any successor legislation, on a routine basis, whether or not there is to be a change of external auditor.
(k)Establishing and overseeing policies with regards to the hiring by the Corporation of any partners, employees, and any former partners or employees of any present or former firms that acted as external auditors of the Corporation.
Financial Information
(l)Ensuring, through discussions with Management and the external auditors, that the audited annual financial statements and the unaudited quarterly financial statements, as applicable, present fairly (in accordance with IFRS) in all material respects the financial condition, results of operations and cash flows of the Corporation as of and for the periods presented and, where appropriate, recommending for approval to the Board such financial statements of the Corporation.
(m)Reviewing any errors or omissions in the current or prior year’s financial statements.
(n)Reviewing with the external auditors the level of co-operation they received from Management, employees and personnel of the Corporation during the audit process, any issues encountered by the auditors and any impediments on the external auditor’s work.
(o)Reviewing and resolving any disagreements between Management and the external auditors with respect to accounting practices and principles.
(p)Monitoring the objectivity and credibility of the Corporation’s financial reports.
(q)Reviewing the status of material contingent liabilities as reported to the Committee by the Corporation’s Management, and the manner in which any material contingent liability has been disclosed in the Corporation’s financial statements.
(r)Reviewing any legal matters or claims that could have a material impact on the financial statements of the Corporation, and the manner in which any such legal matters or claims have been disclosed in the Corporation’s financial statements.
(s)Reviewing any reserves, accruals, provisions, estimates or adopted programs and policies, including factors that affect asset and liability carrying values and the timing of revenue and expense recognition that may have a material effect upon the financial statements of the Corporation.
(t)Reviewing the use of special purpose entities and the business purpose and economic effect of off-balance sheet transactions, arrangements, obligations, guarantees and other relationships of the Corporation and their impact on the reported financial results of the Corporation.
(u)Reviewing the treatment for financial reporting purposes of any significant transactions which are not a normal part of the Corporation’s operations.
(v)Reviewing Management’s determination of tangible or intangible asset impairment, if any, as required by applicable accounting standards.
(w)Reviewing the annual report to shareholders and other financial information (including the annual and quarterly management’s discussion and analysis, the annual information form and any prospectus, offering circular or other disclosure document issued by the Corporation or on behalf of the Corporation) prepared by the Corporation with Management and, where appropriate, recommending such documents for approval to the Board and for filing with regulatory bodies.
(x)Reviewing any news releases and reports to be issued by the Corporation containing earnings guidance or financial information for research, analysts and rating agencies. The Committee shall also review the Corporation’s policies relating to financial disclosure and the release of earnings guidance and the Corporation’s compliance with financial disclosure rules and regulations.
(y)Remaining apprised, through discussions with Management and the external auditors, of important trends and developments in financial reporting practices and requirements and their effect on the Corporation’s financial statements, including consolidated financial statements.
(z)Reviewing the financial statements and other financial information of material subsidiaries of the Corporation and any auditor recommendations concerning such subsidiaries.
(aa)Reviewing the financial reporting obligations of the Corporation pursuant to its by-laws, its borrowing covenants, the Canada Business Corporations Act and applicable securities regulation and monitor the Corporation’s compliance thereunder.
Internal Control
(ab)Annually and in advance of each respective fiscal period, completing a financial review of the Corporation’s strategic plan and annual budget, and reporting to the Board the results of its review.
(ac)Overseeing the adequacy and effectiveness of the Corporation’s internal control systems, through discussions with the Corporation’s external auditors and Management, and reporting its findings to the Board on an annual basis.
(ad)Reviewing and comparing Management’s quarterly report of operating against its budget variances and reporting the results of such review and comparison to the Board.
(ae)Establishing procedures for:
(i)The receipt, retention, and treatment of complaints received by the Corporation regarding accounting, internal accounting controls, or auditing matters; and
(ii)The confidential, anonymous submission by employees of the Corporation of concerns regarding questionable accounting or auditing matters.
(af)Annually reviewing the Corporation’s Whistleblower Policy and its effectiveness and enforcement.
Compliance with Legal and Regulatory Requirements
(ag)Reviewing with Management, and/or any internal or external counsel as the Committee considers appropriate, any legal
matters (including the status of pending litigation) that may have a material impact on the Corporation and any material reports.
(ah)Reviewing with Management and the Board any issues with regulatory agencies that are likely to have a significant financial impact on the Corporation.
(ai)Reviewing with counsel the adequacy and effectiveness of the Corporation’s procedures to ensure compliance with the legal and regulatory responsibilities.
(aj)Reviewing the status of income tax returns and any significant tax issues as they are reported to the Committee by Management or the Board.
(ak)Reviewing any inquiries, investigations, or audits of a financial nature by any government, regulatory, or taxation authorities.
(al)Reviewing any legal matters or claims that could have a material impact on the Corporation’s compliance policies or any material reports, inquiries, or other correspondence received from regulators or governmental agencies.
Other
(am)Assisting the Board in the discharge of its duties relating to the Corporation’s accounting policies and practices, reporting practices and internal controls, including under its by-laws, securities regulations and otherwise.
(an)Reviewing the appointments of the Corporation’s Chief Financial Officer, internal auditor (or persons appointed to perform the internal audit function), and any key financial executives involved in the financial reporting process of the Corporation and any material subsidiary.
(ao)Establishing and overseeing the effectiveness of procedures for the receipt, retention and treatment of complaints regarding accounting, internal accounting controls or auditing under the Corporation’s Whistleblower Policy.
(ap)Ensuring that this Charter or an appropriate summary of it which has been granted approval by the Committee is properly disclosed in accordance with any securities laws or regulatory requirements.
(aq)Reviewing the integrity of the Corporation’s financial reporting processes, both internal and external, in consultation with the external auditor.
(ar)Periodically assessing the Corporation’s need for an internal audit function, if not present.
(as)Reviewing all material balance sheet issues, material contingent obligations and material related party transactions.
(at)Taking such other actions within the general scope of its responsibilities as the Committee shall deem appropriate or as directed by the Board.
6.Resources
(a)The Committee shall have the authority, in its sole discretion, to retain independent legal, accounting and other consultants to advise the Committee at the expense of the Corporation. The Committee shall be provided with the necessary funding to compensate the external auditors and any other advisors they engage.
(b)The Committee shall have access to such officers and employees of the Corporation and to the Corporation’s external auditors and legal counsel, and any information with regards to the Corporation as it considers necessary in order to discharge its duties under this Charter.
(c)The Committee, through the Chair, may contact any director, member of Management or other officer or employee of the Corporation as it deems necessary, and any director, member of Management or other officer or employee of the Corporation may bring any matter before the Committee involving illegal, questionable, improper, or unethical practices or transactions.
(d)The external auditors shall be entitled to communicate directly with the Chair of the Committee and may meet separately with the Committee and any member of the Committee.
(e)The Committee may request any director, member of Management or other officer or employee of the Corporation or the Corporation’s external counsel or external auditors to attend a meeting of the Committee or to meet with any member of, or consultants to, the Committee. The Committee shall have full access to all of the Corporation’s books, records, properties, facilities and personnel, subject to compliance with any leases or similar contracts governing same.
(f)The Committee may delegate its authority and duties to subcommittees or individual members of the Committee as it deems appropriate from time to time.
7.Annual Evaluation
At least annually, the Committee shall, in a manner it determines to be appropriate:
(a)Perform a review and evaluation of the performance of the Committee and its members, including the compliance of the Committee with this Charter.
(b)Review and assess the adequacy of this Charter and recommend to the Board any improvements to this Charter that the Committee believes to be appropriate.
8.Inconsistencies with Applicable Laws
In the event of any conflict or inconsistency between this Charter and the applicable laws, in each case as amended, restated or amended and restated from time to time, the provisions hereof shall be ineffective and shall be superseded by the provisions of such applicable laws to the extent necessary to resolve such conflict or inconsistency.