
EXHIBIT 99.2

NASDAQ: ABUS www.arbutusbio.com November 9, 2022 Corporate Presentation

Forward - Looking Statements This presentation contains forward - looking statements within the meaning of the U . S . Private Securities Litigation Reform Act of 1995 and Canadian securities laws . All statements that are not historical facts are hereby identified as forward - looking statements for this purpose and include, among others, statements relating to : the potential market opportunity for HBV ; Arbutus’ ability to meet a significant unmet medical need ; the sufficiency of Arbutus’ cash and cash equivalents for the anticipated durations ; the expected cost, timing and results of Arbutus’ clinical development plans and clinical trials, including its clinical collaborations with third parties ; the potential for Arbutus’ product candidates to achieve their desired or anticipated outcomes ; Arbutus’ expectations regarding the timing and clinical development of Arbutus’ product candidates, including its articulated clinical objectives ; the timeline to a combination cure for HBV ; Arbutus’ coronavirus strategy ; Arbutus’ expectations regarding its technology licensed to third parties ; the expected timing and payments associated with strategic and/or licensing agreements ; the patent infringement lawsuit against Moderna ; and other statements relating to Arbutus’ future operations, future financial performance, future financial condition, prospects or other future events . With respect to the forward - looking statements contained in this presentation, Arbutus has made numerous assumptions regarding, among other things : the timely receipt of expected payments ; the effectiveness and timeliness of pre - clinical studies and clinical trials, and the usefulness of the data ; the timeliness of regulatory approvals ; the continued demand for Arbutus’ assets ; and the stability of economic and market conditions . While Arbutus considers these assumptions to be reasonable, these assumptions are inherently subject to significant business, economic, competitive, market and social uncertainties, and contingencies including uncertainties and contingencies related to the ongoing COVID - 19 pandemic and patent litigation matters . Forward - looking statements herein involve known and unknown risks, uncertainties and other factors that may cause the actual results, events or developments to be materially different from any future results, events or developments expressed or implied by such forward - looking statements . Such factors include, among others : anticipated pre - clinical and clinical trials may be more costly or take longer to complete than anticipated, and may never be initiated or completed, or may not generate results that warrant future development of the tested drug candidate ; changes in Arbutus’ strategy regarding its product candidates and clinical development activities ; Arbutus may not receive the necessary regulatory approvals for the clinical development of Arbutus' products ; economic and market conditions may worsen ; uncertainties associated with litigation generally and patent litigation specifically ; market shifts may require a change in strategic focus ; the parties may never realize the expected benefits of the collaborations ; and the ongoing COVID - 19 pandemic could significantly disrupt Arbutus’ clinical development programs . A more complete discussion of the risks and uncertainties facing Arbutus appears in Arbutus' Annual Report on Form 10 - K, Quarterly Report on Form 10 - Q and Arbutus' periodic disclosure filings, which are available at www . sec . gov and at www . sedar . com . All forward - looking statements herein are qualified in their entirety by this cautionary statement, and Arbutus disclaims any obligation to revise or update any such forward - looking statements or to publicly announce the result of any revisions to any of the forward - looking statements contained herein to reflect future results, events or developments, except as required by law . 2

Our Strategy Leverage the proven track record of success established with our team's expertise in understanding and treating viral infections by discovering and developing a broad, differentiated pipeline of therapies targeting chronic HBV, COVID - 19, and future coronavirus outbreaks. Develop a combination therapy that includes antivirals and immunologics to provide a finite duration treatment for people with cHBV that results in >20% functional cure rate. Develop novel oral pan coronavirus antivirals targeting essential viral proteins with the goal of reducing hospitalizations and providing pre - exposure prophylactic therapy. HBV: Hepatitis B Virus | cHBV : chronic HB V 3
Investment Highlights Strong financial position Indications with significant unmet medical need & large market opportunities Patented LNP technology Broad portfolio of internally discovered assets with distinct MOAs Lead HBV compound – AB - 729 RNAi therapeutic in multiple Phase 2a combination clinical trials Team with virology expertise and proven track record Focused on developing functional cure for HBV and oral pan - coronavirus therapeutics Cash runway into Q2 2024 Data shows AB - 729 is generally safe and well tolerated and has shown meaningful suppression of HBsAg while on - or off - treatment RNAi therapeutic PD - L1 inhibitor RNA destabilizer M pro inhibitor Nsp12 polymerase inhibitor Receiving licensing royalties arising from Alnylam’s Onpattro ® and seeking damages for Moderna - COVID - 19 vaccine sales Discovered, developed & commercialized multiple drugs MOA: Mechanism of Action | PD - L1: Programmed death - ligand 1 | M pro : Main protease | NSP12 : N on - structural protein | HBsAg: Hepatitis B surface antigen 4
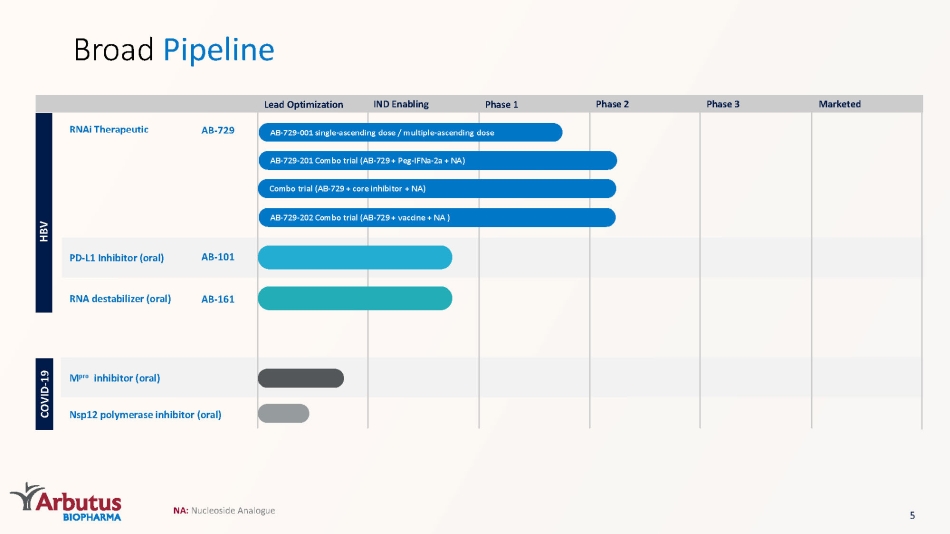
Broad Pipeline HBV AB - 729 - 001 single - ascending dose / multiple - ascending dose RNAi Therapeutic AB - 729 AB - 729 - 201 Combo trial (AB - 729 + Peg - IFNa - 2a + NA) Combo trial (AB - 729 + core inhibitor + NA) AB - 729 - 202 Combo trial (AB - 729 + vaccine + NA ) PD - L1 Inhibitor (oral) AB - 101 RNA destabilizer (oral) AB - 161 COVID - 19 M pro inhibitor (oral) Nsp12 polymerase inhibitor (oral) Lead Optimization IND Enabling Phase 1 Phase 2 Phase 3 Marketed NA: Nucleoside Analogue 5
HBV Overview Life - threatening liver infection caused by hepatitis B virus (HBV) Transmitted through body fluids and from mother to child Long - term chronic infection ( cHBV ) leads to higher risk of cirrhosis and/or liver cancer Cause & Symptoms Diagnosis HBsAg detection Additional biomarkers necessary to determine stage of disease Treatments NA therapy – lifelong daily therapy, aimed at reducing HBV DNA and risk of cirrhosis and/or HCC Peg - IFN α – administered weekly; poorly tolerated <5% of patients achieve functional cure Rationale Need for finite and more efficacious HBV treatments that further improve long - term outcomes and increase functional cure rate Combination therapy with different MOAs will be required to reduce HBsAg, suppress HBV DNA, and boost immune system Sources for all data on slide: 1 Hepatitis B Fact Sheet, WHO https://www.who.int/news - room/fact - sheets/detail/hepatitis - b ; Hep B Foundation link https://www.hepb.org/what - is - hepatitis - b/what - is - hepb/facts - and - figures/ ; Kowdley et al. Hepatology (2012) Prevalence of Chronic Hepatitis B Among Foreign - Born Persons Living in the US by Country of Origin 2 Pegasys , PEG - Intron, Baraclude and Viread Package Inserts HBsAg : HBV Surface Antigen | HCC: Hepatocellular carcinoma 6
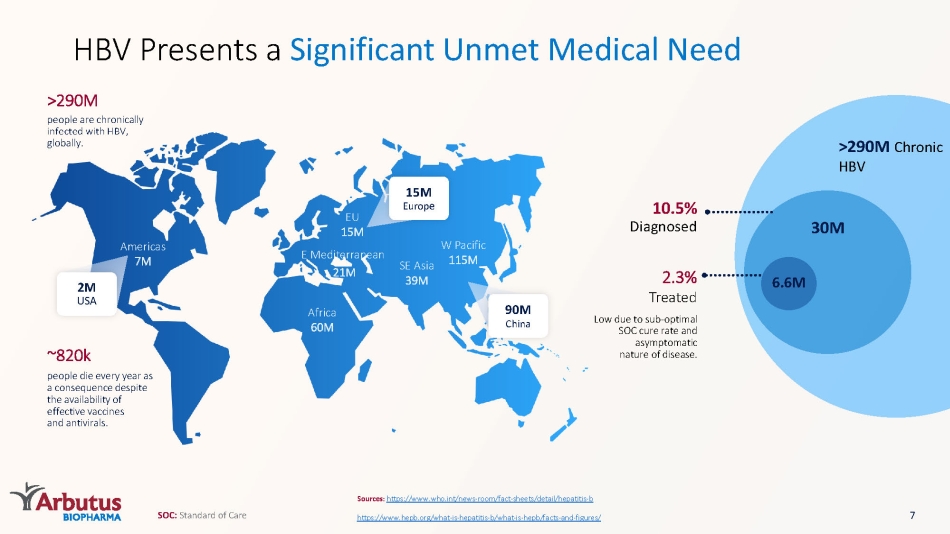
Africa 60M E Mediterranean 21M SE Asia 39M W Pacific 115M EU 15M Americas 7M ~820k people die every year as a consequence despite the availability of effective vaccines and antivirals. people are chronically infected with HBV, globally. >290M >290M Chronic HBV Sources: https://www.who.int/news - room/fact - sheets/detail/hepatitis - b https://www.hepb.org/what - is - hepatitis - b/what - is - hepb/facts - and - figures/ HBV Presents a Significant Unmet Medical Need 30M 6.6M 2.3% Treated Low due to sub - optimal SOC cure rate and asymptomatic nature of disease. 10.5% Diagnosed 2M USA 15M Europ e 90M China 7 SOC: Standard of Care
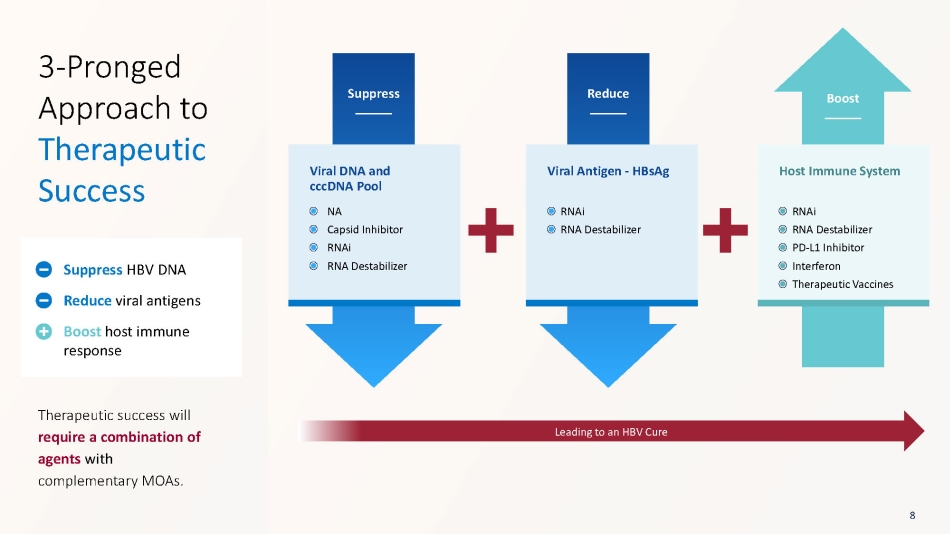
Suppress Reduce Boost Viral DNA and cccDNA Pool Viral Antigen - HBsAg Host Immune System Leading to an HBV Cure 3 - Pronged Approach to Therapeutic Success Therapeutic success will require a combination of agents with complementary MOAs. Suppress HBV DNA Reduce viral antigens Boost host immune response 8 NA Capsid Inhibitor RNAi RNA Destabilizer RNAi RNA Destabilizer RNAi RNA Destabilizer PD - L1 Inhibitor Interferon Therapeutic Vaccines
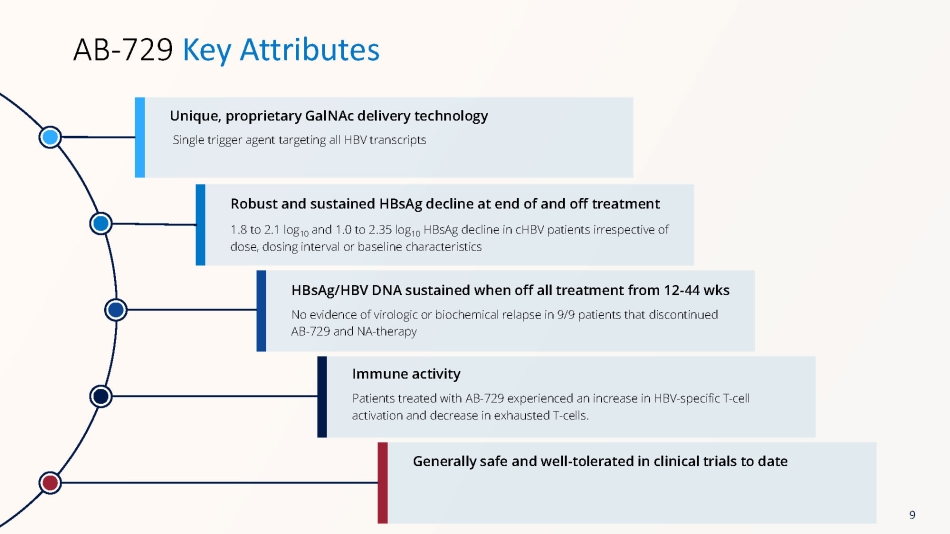
Single trigger agent targeting all HBV transcripts Unique, proprietary GalNAc delivery technology AB - 729 Key Attributes 1.8 to 2.1 log 10 and 1.0 to 2.35 log 10 HBsAg decline in cHBV patients irrespective of dose, dosing interval or baseline characteristics Robust and sustained HBsAg decline at end of and off treatment No evidence of virologic or biochemical relapse in 9/9 patients that discontinued AB - 729 and NA - therapy HBsAg/HBV DNA sustained when off all treatment from 12 - 44 wks Patients treated with AB - 729 experienced an increase in HBV - specific T - cell activation and decrease in exhausted T - cells. Immune activity Generally safe and well - tolerated in clinical trials to date 9

Proprietary GalNAc - conjugate delivery technology provides liver targeting and enables subcutaneous dosing Single trigger RNAi agent targeting all HBV transcripts Inhibits HBV replication and lowers all HBV antigens Pan - genotypic activity across HBV genotypes Demonstrated complementarity with capsid inhibitors Actively targets the liver Active against cccDNA derived and integrated HBsAg transcripts Clean profile in long term preclinical safety studies RNAi Therapeutic AB - 729 GalNAc n Linker Polymerase, Core Ag, eAg , pgRNA sAg sAg HBx 10
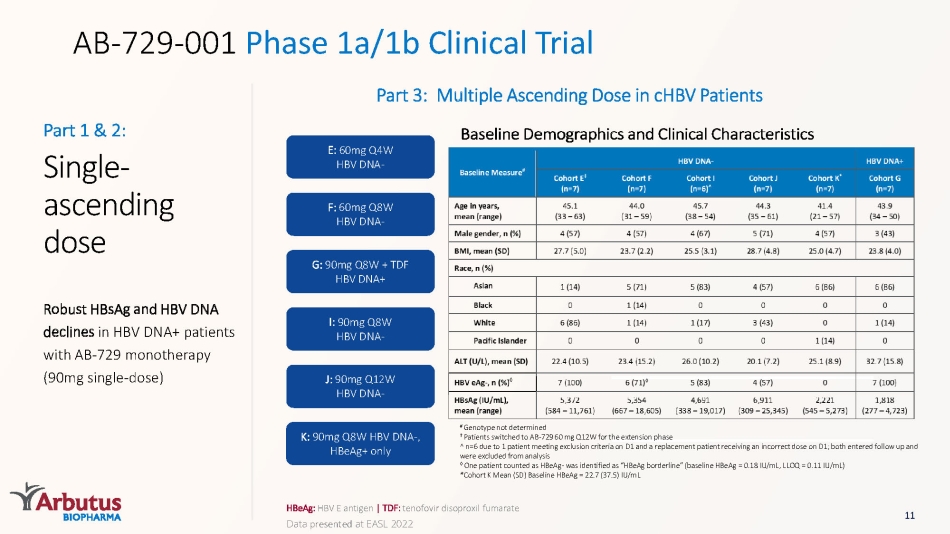
AB - 729 - 001 Phase 1a/1b Clinical Trial HBeAg : HBV E antigen | TDF: tenofovir disoproxil fumarate Part 3: Multiple Ascending Dose in cHBV Patients E: 60mg Q4W HBV DNA - F: 60mg Q8W HBV DNA - G: 90mg Q8W + TDF HBV DNA+ I: 90mg Q8W HBV DNA - J: 90mg Q12W HBV DNA - K: 90mg Q8W HBV DNA - , HBeAg + only Single - ascending dose Robust HBsAg and HBV DNA declines in HBV DNA+ patients with AB - 729 monotherapy (90mg single - dose) Part 1 & 2: 11 Baseline Demographics and Clinical Characteristics # Genotype not determined ‡ Patients switched to AB - 729 60 mg Q12W for the extension phase ^ n =6 due to 1 patient meeting exclusion criteria on D1 and a replacement patient receiving an incorrect dose on D1; both entered follow up and were excluded from analysis ◊ One patient counted as HBeAg - was identified as “ HBeAg borderline” (baseline HBeAg = 0.18 IU/mL, LLOQ = 0.11 IU/mL) *Cohort K Mean (SD) Baseline HBeAg = 22.7 (37.5) IU/mL Data presented at EASL 2022
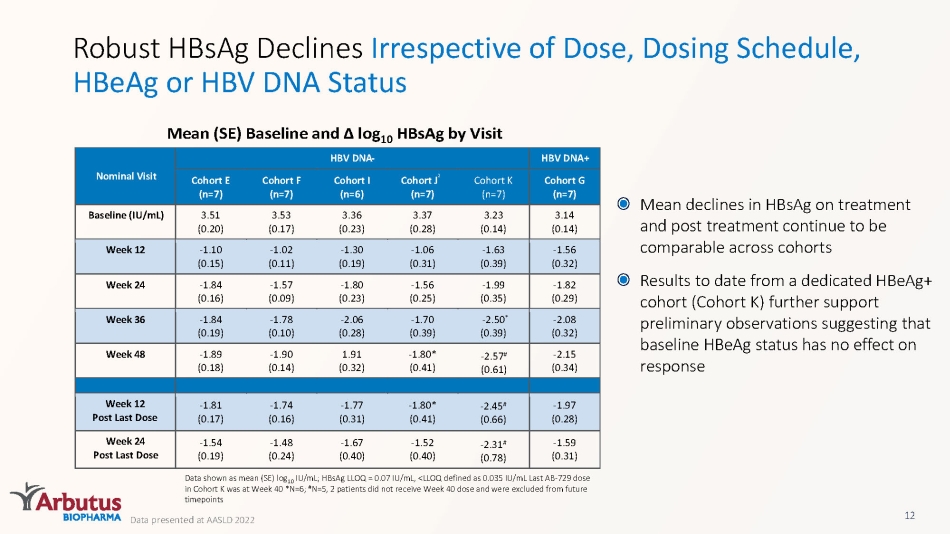
Robust HBsAg Declines Irrespective of Dose, Dosing Schedule, HBeAg or HBV DNA Status Mean (SE) Baseline and Δ log 10 HBsAg by Visit Data shown as mean (SE) log 10 IU/mL; HBsAg LLOQ = 0.07 IU/mL, <LLOQ defined as 0.035 IU/mL Last AB - 729 dose in Cohort K was at Week 40 *N=6; # N=5, 2 patients did not receive Week 40 dose and were excluded from future timepoints 12 Mean declines in HBsAg on treatment and post treatment continue to be comparable across cohorts Results to date from a dedicated HBeAg + cohort (Cohort K) further support preliminary observations suggesting that baseline HBeAg status has no effect on response Data presented at AASLD 2022 Nominal Visit HBV DNA - HBV DNA+ Cohort E ( n =7) Cohort F ( n =7) Cohort I ( n =6) Cohort J ² ( n =7) Cohort K ( n =7) Cohort G ( n =7) Baseline (IU/mL) 3.51 (0.20) 3.53 (0.17) 3.36 (0.23) 3.37 (0.28) 3.23 (0.14) 3.14 (0.14) Week 12 - 1.10 (0.15) - 1.02 (0.11) - 1.30 (0.19) - 1.06 (0.31) - 1.63 (0.39) - 1.56 (0.32) Week 24 - 1.84 (0.16) - 1.57 (0.09) - 1.80 (0.23) - 1.56 (0.25) - 1.99 (0.35) - 1.82 (0.29) Week 36 - 1.84 (0.19) - 1.78 (0.10) - 2.06 (0.28) - 1.70 (0.39) - 2.50 * (0.39) - 2.08 (0.32) Week 48 - 1.89 (0.18) - 1.90 (0.14) 1.91 (0.32) - 1.80* (0.41) - 2.15 (0.34) Week 12 Post Last Dose - 1.81 (0.17) - 1.74 (0.16) - 1.77 (0.31) - 1.80* (0.41) - 1.97 (0.28) Week 24 Post Last Dose - 1.54 (0.19) - 1.48 (0.24) - 1.67 (0.40) - 1.52 (0.40) - 1.59 (0.31) - 2.57 # (0.61) - 2.45 # (0.66) - 2.31 # (0.78)
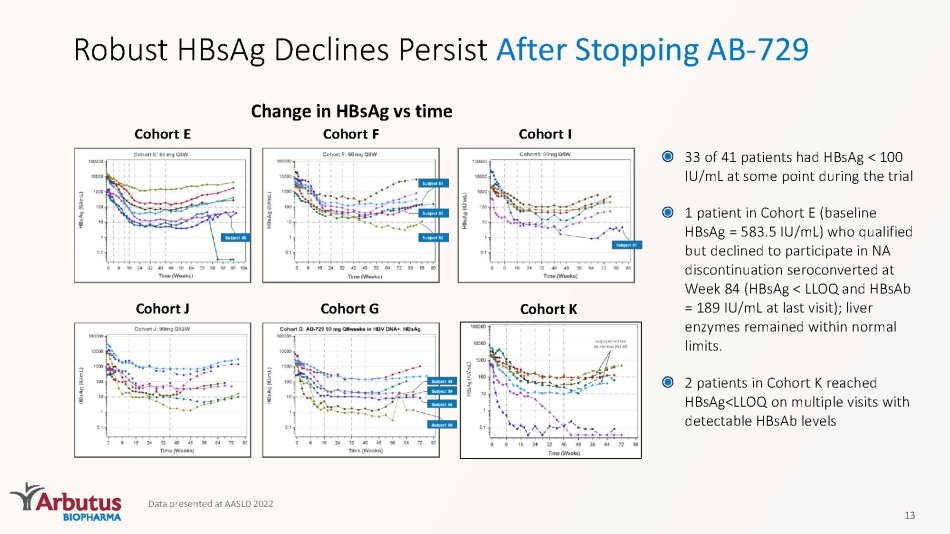
Data presented at AASLD 2022 13 Change in HBsAg vs time Robust HBsAg Declines Persist After Stopping AB - 729 33 of 41 patients had HBsAg < 100 IU/mL at some point during the trial 1 patient in Cohort E (baseline HBsAg = 583.5 IU/mL) who qualified but declined to participate in NA discontinuation seroconverted at Week 84 (HBsAg < LLOQ and HBsAb = 189 IU/mL at last visit); liver enzymes remained within normal limits. 2 patients in Cohort K reached HBsAg<LLOQ on multiple visits with detectable HBsAb levels Cohort I Cohort F Cohort E Cohort J Cohort G Cohort K
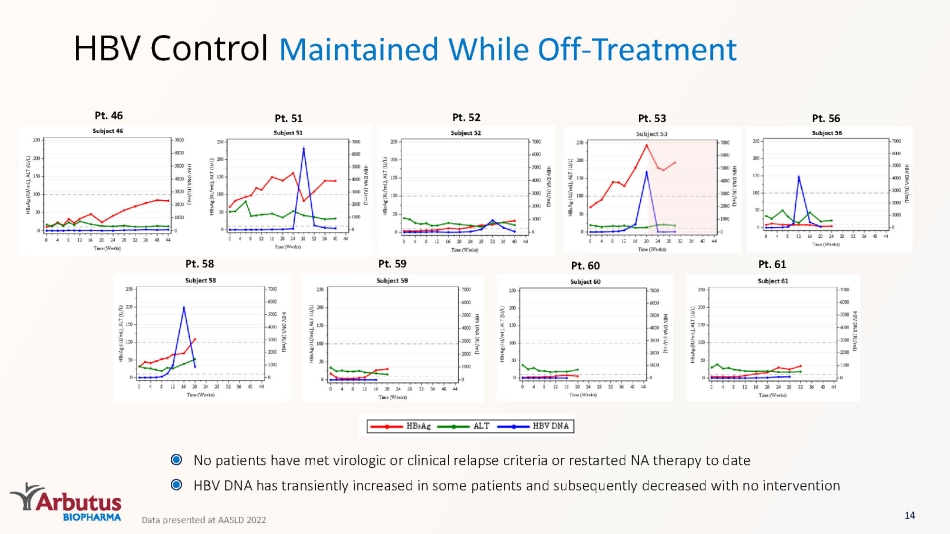
HBV Control Maintained While Off - Treatment Pt. 46 Pt. 51 Pt. 52 Pt. 53 Pt. 56 Pt. 58 Pt. 59 Pt. 60 Pt. 61 No patients have met virologic or clinical relapse criteria or restarted NA therapy to date HBV DNA has transiently increased in some patients and subsequently decreased with no intervention 14 Data presented at AASLD 2022
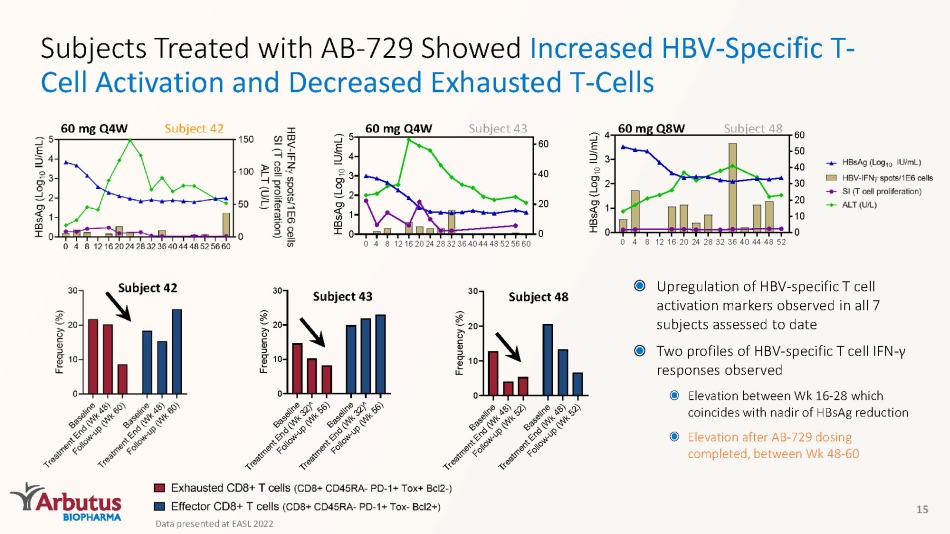
Subjects Treated with AB - 729 Showed Increased HBV - Specific T - Cell Activation and Decreased Exhausted T - Cells 15 Subject 42 60 mg Q4W 0 4 812162024283236404448525660 0 1 2 3 4 5 0 20 40 60 H B s A g ( L o g 1 0 I U / m L ) Subject 43 60 mg Q4W 0 4 8 1216202428323640444852 0 1 2 3 4 0 10 20 30 40 50 60 H B s A g ( L o g 1 0 I U / m L ) Subject 48 60 mg Q8W Subject 42 B a s e l i n e T r e a t m e n t E n d ( W k 4 8 ) F o l l o w - u p ( W k 5 2 ) B a s e l i n e T r e a t m e n t E n d ( W k 4 8 ) F o l l o w - u p ( W k 5 2 ) 0 10 20 30 F r e q u e n c y ( % ) Subject 48 B a s e l i n e T r e a t m e n t E n d ( W k 3 2 ) ^ F o l l o w - u p ( W k 5 6 ) B a s e l i n e T r e a t m e n t E n d ( W k 3 2 ) ^ F o l l o w - u p ( W k 5 6 ) 0 10 20 30 F r e q u e n c y ( % ) Subject 43 Upregulation of HBV - specific T cell activation markers observed in all 7 subjects assessed to date Two profiles of HBV - specific T cell IFN - γ responses observed Elevation between Wk 16 - 28 which coincides with nadir of HBsAg reduction Elevation after AB - 729 dosing completed, between Wk 48 - 60 Data presented at EASL 2022

AE: Adverse Event | TEAE: Treatment Emergent Adverse Event AB - 729 - 001 Safety Summary *1 patient (Cohort A) with rapid decline in HBsAg of ~2.0 log10 IU/mL had an unrelated Gr 2 AE of food poisoning resulting in unrelated transient Grade 3 AEs of ALT/AST elevation (without bilirubin changes) AB - 729 generally safe and well - tolerated in clinical trials after single and repeat doses No treatment - related SAEs or discontinuations due to AEs No treatment - related Grade 3 or 4 AEs * No treatment - related Grade 3 or 4 laboratory abnormalities * Grade 1 and Grade 2 ALT elevations have improved or stabilized with continued treatment Injection site TEAEs were mostly mild (erythema, pain, bruising, pruritis) No clinically meaningful changes in ECGs or vital signs 16
AB - 729 - 001 Clinical Trial Key Takeaways AB - 729 provided robust and comparable HBsAg declines regardless of dose, dosing interval, HBeAg or DNA status ~75% (26 of 34) patients had HBsAg levels <100 at some point during the trial 50% (16 of 32) patients maintained HBsAg <100 IU/mL for 24 weeks after stopping AB - 729 treatment Discontinuation of both AB - 729 and NA - therapy results in a sustained reduction in HBsAg No evidence of virologic or biochemical relapse detected in 9 patients who discontinued all therapy from 12 to 44 weeks. No patient met protocol - defined criteria to restart NA - therapy as of date data was presented.* AB - 729 was generally safe and well - tolerated after completing dosing in 41 patients AB - 72 9 continues to result in HBV - specific T - cell immune restoration and decrease of exhausted T - cells *Data presented at EASL 2021 17 * Data presented at AASLD 2022
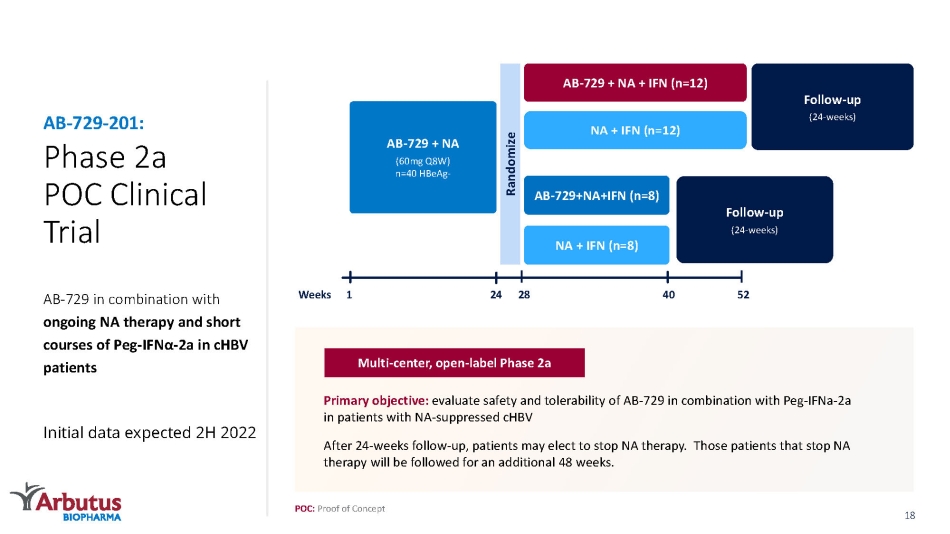
Phase 2a POC Clinical Trial AB - 729 in combination with ongoing NA therapy and short courses of Peg - IFN α - 2 a in cHBV patients Initial data expected 2H 2022 AB - 729 - 201: Follow - up (24 - weeks) AB - 729 + NA + IFN (n=12) NA + IFN (n=12) AB - 729+NA+IFN (n=8) NA + IFN (n=8) AB - 729 + NA (60mg Q8W) n=40 HBeAg - Randomize Follow - up (24 - weeks) 1 52 28 24 40 Weeks POC: Proof of Concept Primary objective: evaluate safety and tolerability of AB - 729 in combination with Peg - IFNa - 2a in patients with NA - suppressed cHBV After 24 - weeks follow - up, patients may elect to stop NA therapy. Those patients that stop NA therapy will be followed for an additional 48 weeks. Multi - center, open - label Phase 2a 18
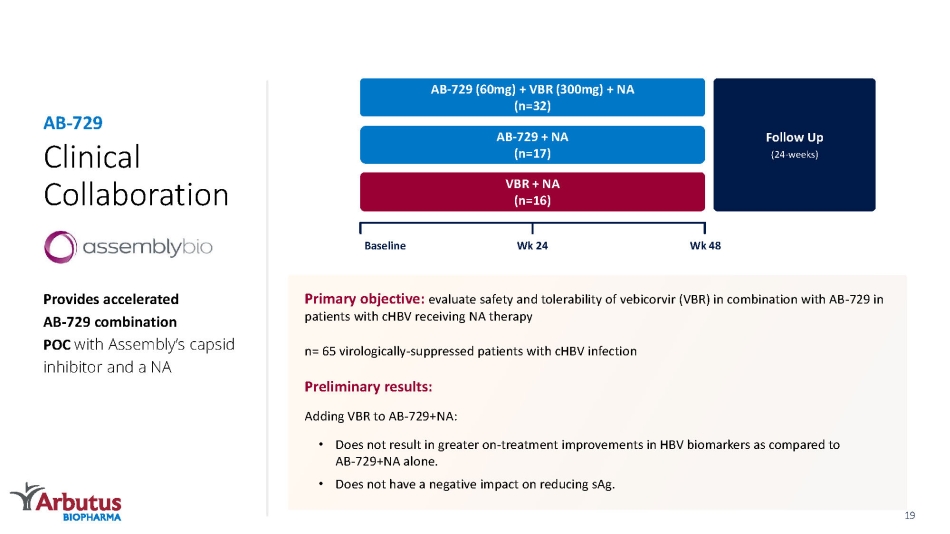
Clinical Collaboration Provides accelerated AB - 729 combination POC with Assembly’s capsid inhibitor and a NA AB - 729 Follow Up (24 - weeks) AB - 729 (60mg) + VBR (300mg) + NA (n=32) AB - 729 + NA (n=17) VBR + NA (n=16) Baseline Wk 48 Wk 24 Primary objective: evaluate safety and tolerability of vebicorvir (VBR) in combination with AB - 729 in patients with cHBV receiving NA therapy n= 65 virologically - suppressed patients with cHBV infection Preliminary results: Adding VBR to AB - 729+NA: • Does not result in greater on - treatment improvements in HBV biomarkers as compared to AB - 729+NA alone. • Does not have a negative impact on reducing sAg. 19

Phase 2a POC Clinical Trial POC Phase 2a clinical trial evaluating AB - 729 in combination with Vaccitech’s immunotherapeutic, VTP - 300, and a NA First patient dosed AB - 729 - 202: Follow - up (24 - 48 weeks) VTP - 300 + NA (n=20) NA + sham (n=20) 1 48 26 AB - 729 + NA (60mg Q8W) n=40 24 Randomize Weeks Primary objective: evaluate safety and reactogenicity of AB - 729 followed by VTP - 300 or placebo At week 48 all participants who are eligible to discontinue NA therapy will be followed for 48 - weeks Full rights retained by the Companies of their respective product candidates and all costs split equally 20
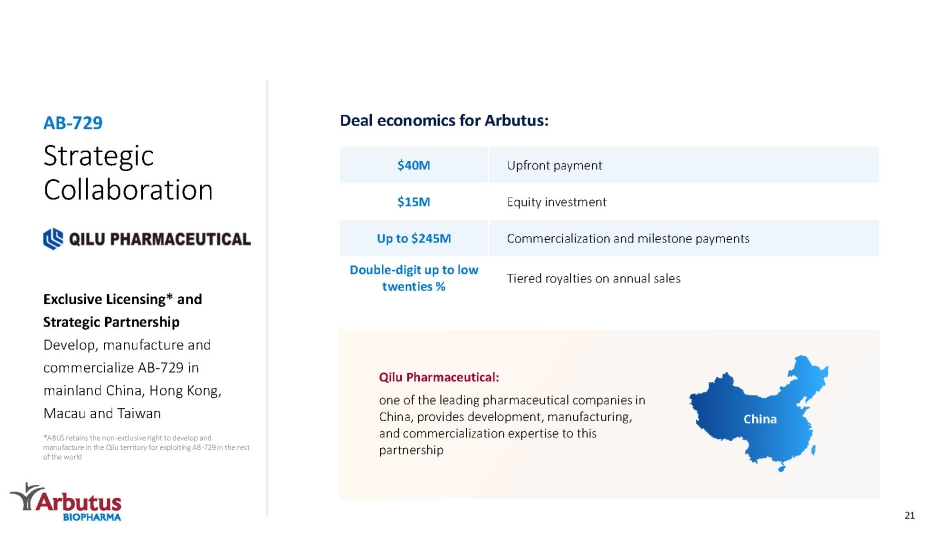
Strategic Collaboration Exclusive Licensing* and Strategic Partnership Develop, manufacture and commercialize AB - 729 in mainland China, Hong Kong, Macau and Taiwan AB - 729 $40M Upfront payment $15M Equity investment Up to $245M Commercialization and milestone payments Double - digit up to low twenties % Tiered royalties on annual sales *ABUS retains the non - exclusive right to develop and manufacture in the Qilu territory for exploiting AB - 729 in the rest of the world Deal economics for Arbutus: Qilu Pharmaceutical: one of the leading pharmaceutical companies in China, provides development, manufacturing, and commercialization expertise to this partnership China 21
AB - 161: Next Generation Oral RNA Destabilizer AB - 161 is currently in IND - enabling studies Next generation small molecule anticipated to circumvent non - clinical safety findings with first generation molecule Offers a novel mechanism of action to reduce HBsAg, other viral proteins and viral RNA Potential for an oral HBsAg reducing agent and all oral combination therapy Safety Novelty Convenience 22
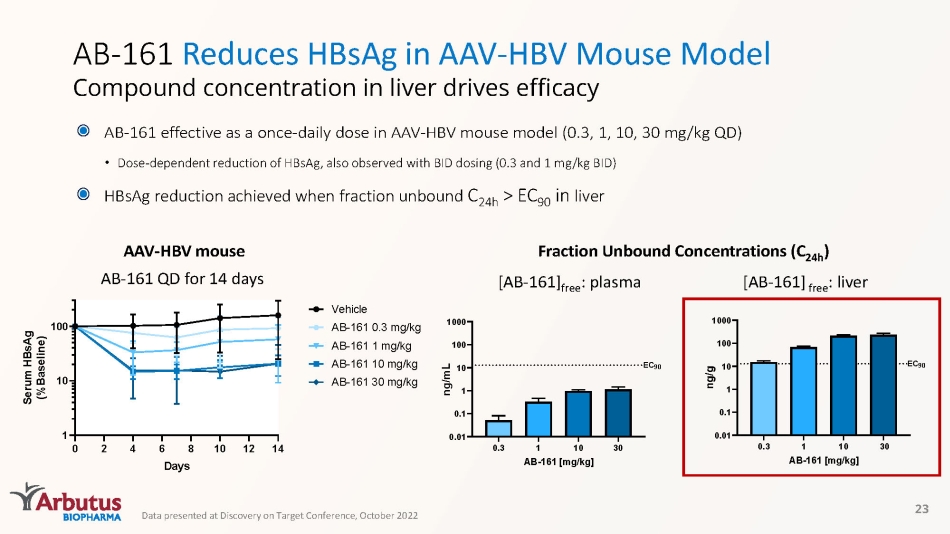
AB - 161 Reduces HBsAg in AAV - HBV Mouse Model Compound concentration in liver drives efficacy 23 Fraction Unbound Concentrations (C 24h ) 0 2 4 6 8 10 12 14 1 10 100 Days S e r u m H B s A g ( % B a s e l i n e ) Vehicle AB-161 0.3 mg/kg AB-161 1 mg/kg AB-161 10 mg/kg AB-161 30 mg/kg [AB - 161] free : plasma [AB - 161] free : liver AAV - HBV mouse AB - 161 QD for 14 days 0.3 1 10 30 0.01 0.1 1 10 100 1000 AB-161 [mg/kg] n g / m L EC 90 Data presented at Discovery on Target Conference, October 2022 AB - 161 effective as a once - daily dose in AAV - HBV mouse model (0.3, 1, 10, 30 mg/kg QD) • Dose - dependent reduction of HBsAg, also observed with BID dosing (0.3 and 1 mg/kg BID) HBsAg reduction achieved when fraction unbound C 24h > EC 90 in liver
AB - 101: Oral PD - L1 Inhibitor for HBV Immune Reactivation PD - 1: Programmed death ligand protein | Abs: Antibodies AB - 101 is currently in IND - enabling studies Rationale • HBV immune tolerance is a critical driver of cHBV infection • PD - 1:PD - L1 checkpoint axis plays a key role in immune tolerization in cHBV • PD - L1 expression upregulated during HBV infection • PD - 1 upregulated on HBV - specific T - and B - cells • Inhibition associated with HBsAg loss in some cHBV patients AB - 101 • Blocks PD - L1/PD - 1 interaction at sub - nM concentrations • Activates HBV - specific immune responses in T - cells from cHBV patients in vitro • Novel MOA identified • Demonstrates a robust checkpoint mediated in vivo effect • Improves HBV - specific T - and B - cell responses ex vivo Small - Molecule Inhibitor Approach • Allows controlled checkpoint blockade • Enables oral dosing • Designed to reduce systemic safety issues seen with Abs 24
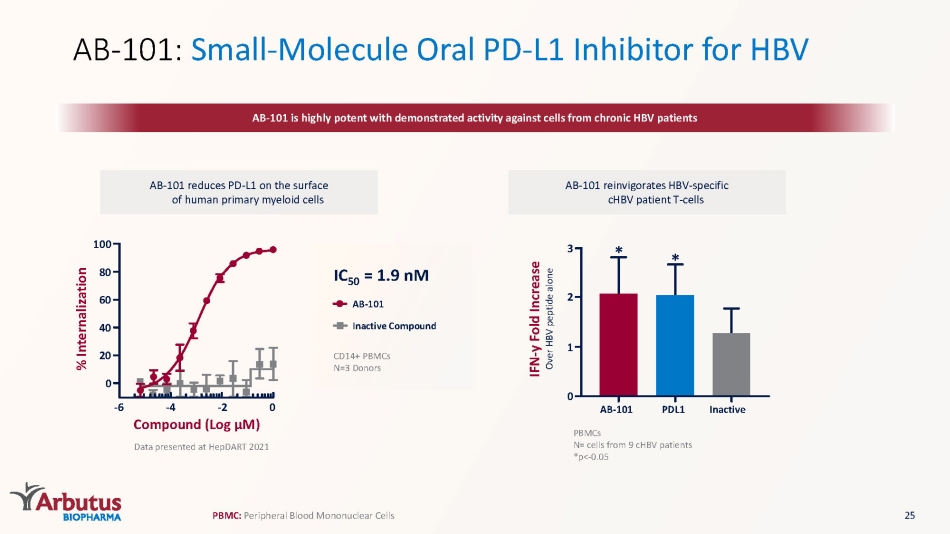
AB - 101: Small - Molecule Oral PD - L1 Inhibitor for HBV AB - 101 is highly potent with demonstrated activity against cells from chronic HBV patients AB - 101 reduces PD - L1 on the surface of human primary myeloid cells AB - 101 reinvigorates HBV - specific cHBV patient T - cells Data presented at HepDART 2021 0 20 40 60 80 100 - 6 - 4 - 2 0 Compound (Log μ M) % Internalization PBMCs N= cells from 9 cHBV patients *p< - 0.05 PBMC: P eripheral Blood Mononuclear Cells PDL1 * Inactive AB - 101 * 0 3 2 1 IFN - y Fold Increase Over HBV peptide alone CD14+ PBMCs N=3 Donors IC 50 = 1.9 nM AB - 101 Inactive Compound 25
Pan - Coronavirus Overview 1 https://www.healthdata.org/special - analysis/ estimation - excess - mortality - due - covid - 19 - and - scalars - reported - covid - 19 - deaths Coronavirus Infections, such as COVID - 19 caused by SAR - CoV - 2 Spreads through breathing out droplets and small particles that contain the virus Older adults and people with severe underlying conditions at higher risk of developing serious complications Virus continues to mutate with variant strains developing Cause & Symptoms Population ~6.9M deaths globally 1 In US: ~80M cases; 1M deaths (as of March 2022) Treatments Vaccines Durability of effect uncertain, boosters required, limited efficacy on variant strains Therapies Sub - optimal Rationale Pan - coronavirus focused: need for effective and safe therapies to combat COVID - 19 and future coronavirus outbreaks Address essential viral targets – nsp12 viral polymerase and nsp5 viral protease Potential for combo therapy to enhance efficacy and reduce symptomology 26
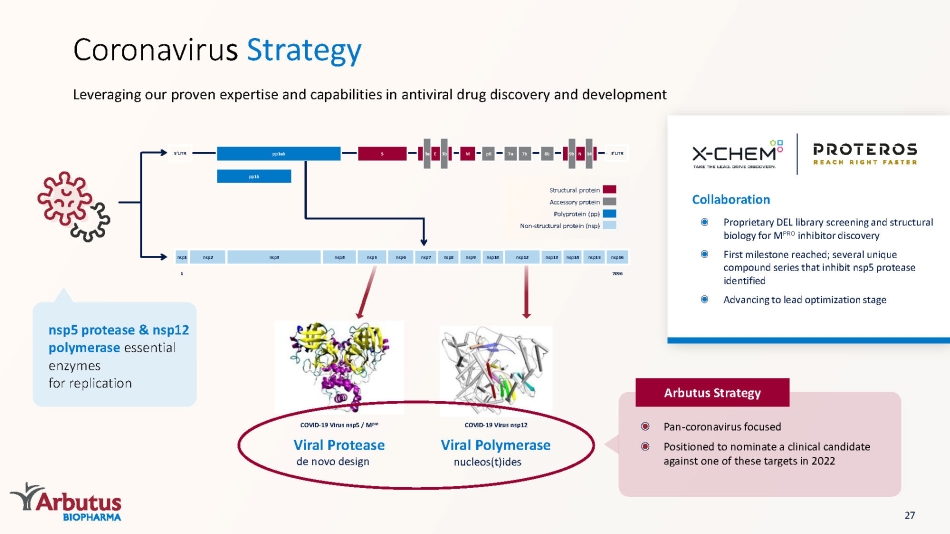
Viral Polymerase nucleos (t)ides COVID - 19 Virus nsp12 Viral Protease de novo design COVID - 19 Virus nsp5 / M pro Leveraging our proven expertise and capabilities in antiviral drug discovery and development Coronaviru s Strategy Arbutus Strategy nsp5 protease & nsp12 polymerase essential enzymes for replication Collaboration Proprietary DEL library screening and structural biology for M PRO inhibitor discovery First milestone reached; several unique compound series that inhibit nsp5 protease identified Advancing to lead optimization stage Structural protein Accessory protein Polyprotein (pp) Non - structural protein ( nsp ) nsp1 nsp2 nsp3 nsp4 nsp5 nsp6 nsp7 nsp8 nsp9 nsp10 nsp12 nsp13 nsp14 nsp15 nsp16 7096 1 pp1b pp1ab S E M p6 7a 7b 8b 3a 3b N 9b 14 5’UTR 3’UTR 27 Pan - coronavirus focused Positioned to nominate a clinical candidate against one of these targets in 2022

Milestone Anticipated Timing 2022 AB - 836, next generation oral capsid inhibitor: Data from Phase 1a/1b clinical trial in patients with chronic HBV 1H AB - 729, RNAi therapeutic: Initiate a triple combination Phase 2a POC clinical trial with VTP - 300 ( Vaccitech ) and a NA 1H AB - 729: Follow - up data (long - term on - and off - treatment) from Phase 1a/1b, evaluating multiple doses and dosing schedules 1H / 2H AB - 729: Initial data from Phase 2a combination trial with NA therapy and Peg - IFNα - 2a 2H AB - 729: Initial data from Phase 2a combination trial with VBR (Assembly) and a NA 2H AB - 101, oral PD - L1 inhibitor compound: Complete IND - enabling studies 2H AB - 161, next generation oral RNA destabilizer: Complete IND - enabling studies 2H COVID M pro : Advance clinical candidate that inhibits the SARS - CoV - 2 nsp5 main protease into IND - enabling studies 2H 2022 Key Milestones Cash balance * of $190.2M as of September 30, 2022, cash runway into Q2 2024 *Consists of cash, cash equivalents and marketable securities 28

Thank You 29