

Healey ALS Platform Trial CNM-Au8 Results Webcast October 2022 Exhibit 99.2
Introduction & CLNN Program Update Rob Etherington, President and Chief Executive Officer I Clene Inc. HEALEY ALS Platform Trial Results for CNM-Au8 Merit Cudkowicz, M.D., MSc I Chief of Neurology at Massachusetts General Hospital Director of the Sean M. Healey & AMG Center for ALS Clene Milestones Q&A session Rob Etherington, President and Chief Executive Officer I Clene Inc. Robert Glanzman M.D., FAAN, Chief Medical Officer I Clene Inc. Michael Hotchkin, Chief Development Officer I Clene Inc. Merit Cudkowicz, M.D., MSc I Massachusetts General Hospital 01 02 03 Healey ALS Platform Trial Results Webcast

Forward Looking Statements This presentation contains “forward-looking statements” within the meaning of Section 21E of the Securities Exchange Act of 1934, as amended, and Section 27A of the Securities Act of 1933, as amended, which are intended to be covered by the “safe harbor” provisions created by those laws. Clene’s forward-looking statements include, but are not limited to, statements regarding our or our management team’s expectations, hopes, beliefs, intentions or strategies regarding our future operations. In addition, any statements that refer to projections, forecasts or other characterizations of future events or circumstances, including any underlying assumptions, are forward-looking statements. The words “anticipate,” “believe,” “contemplate,” “continue,” “estimate,” “expect,” “intends,” “may,” “might,” “plan,” “possible,” “potential,” “predict,” “project,” “should,” “will,” “would,” and similar expressions may identify forward-looking statements, but the absence of these words does not mean that a statement is not forward-looking. These forward-looking statements represent our views as of the date of this presentation and involve a number of judgments, risks and uncertainties. We anticipate that subsequent events and developments will cause our views to change. We undertake no obligation to update forward-looking statements to reflect events or circumstances after the date they were made, whether as a result of new information, future events or otherwise, except as may be required under applicable securities laws. Accordingly, forward-looking statements should not be relied upon as representing our views as of any subsequent date. As a result of a number of known and unknown risks and uncertainties, our actual results or performance may be materially different from those expressed or implied by these forward-looking statements. Some factors that could cause actual results to differ include our substantial dependence on the successful commercialization of our drug candidates, if approved, in the future; our inability to maintain the listing of our common stock on Nasdaq; our significant net losses and net operating cash outflows; our ability to demonstrate the efficacy and safety of our drug candidates; the clinical results for our drug candidates, which may not support further development or marketing approval; actions of regulatory agencies, which may affect the initiation, timing and progress of clinical trials and marketing approval; our ability to achieve commercial success for our drug candidates, if approved; our ability to obtain and maintain protection of intellectual property for our technology and drugs; our reliance on third parties to conduct drug development, manufacturing and other services; our limited operating history and our ability to obtain additional funding for operations and to complete the licensing or development and commercialization of our drug candidates; the impact of the COVID-19 pandemic on our clinical development, commercial and other operations; changes in applicable laws or regulations; the effects of inflation; the effects of staffing and materials shortages; the possibility that we may be adversely affected by other economic, business, and/or competitive factors; and other risks and uncertainties set forth in “Risk Factors” in our most recent Annual Report on Form 10-K and any subsequent Quarterly Reports on Form 10-Q. In addition, statements that “we believe” and similar statements reflect our beliefs and opinions on the relevant subject. These statements are based upon information available to us as of the date of this presentation, and while we believe such information forms a reasonable basis for such statements, such information may be limited or incomplete, and our statements should not be read to indicate that we have conducted an exhaustive inquiry into, or review of, all potentially available relevant information. These statements are inherently uncertain and you are cautioned not to rely unduly upon these statements. All information in this presentation is as of the date of this presentation. The information contained in any website referenced herein is not, and shall not be deemed to be, part of or incorporated into this presentation.

CLENE | Growing Clinical Evidence Across ALS and MS Supports CNM-Au8 Therapeutic Potential Data on File, Clene Nanomedicine, Inc. 1Robinson et al. Sci Rep. 2020 Feb 11;10(1):1936.2https://clinicaltrials.gov/ct2/show/NCT04414345. 70% decreased risk of death in ALS CNM-Au8® a gold nanocrystal suspension, in development as the first cellular energetic catalyst to remyelinate1 & protect neurological function Strong IP: 150+ patents on Clean-Surface- Nanocrystal technology (CSN®) platform >400 patient years of CNM-Au8 clinical exposure Demonstrated global neurological improvement in MS patients on adjunctive DMT standard of care Proprietary Nanotherapeutic Manufacturing
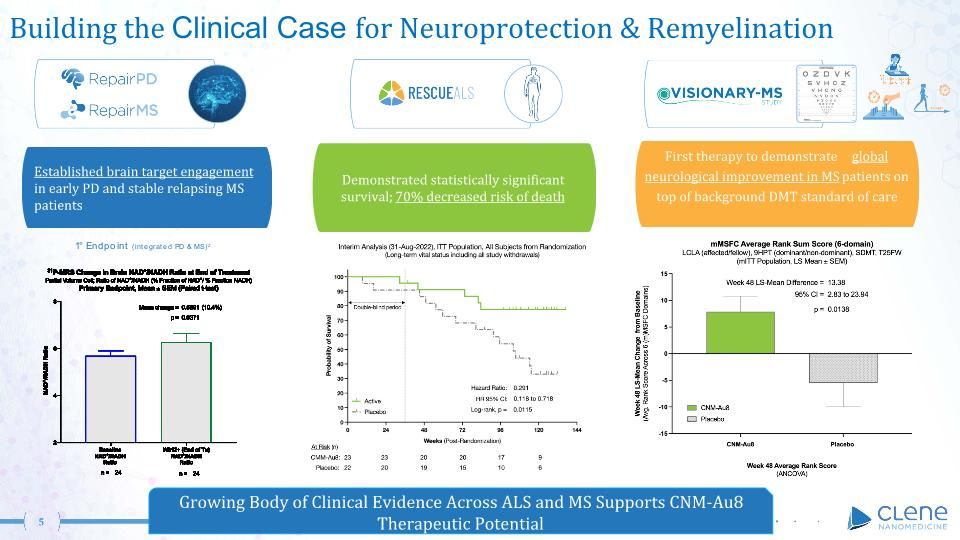
Building the Clinical Case for Neuroprotection & Remyelination Demonstrated statistically significant survival; 70% decreased risk of death Established brain target engagement in early PD and stable relapsing MS patients First therapy to demonstrate global neurological improvement in MS patients on top of background DMT standard of care Growing Body of Clinical Evidence Across ALS and MS Supports CNM-Au8 Therapeutic Potential
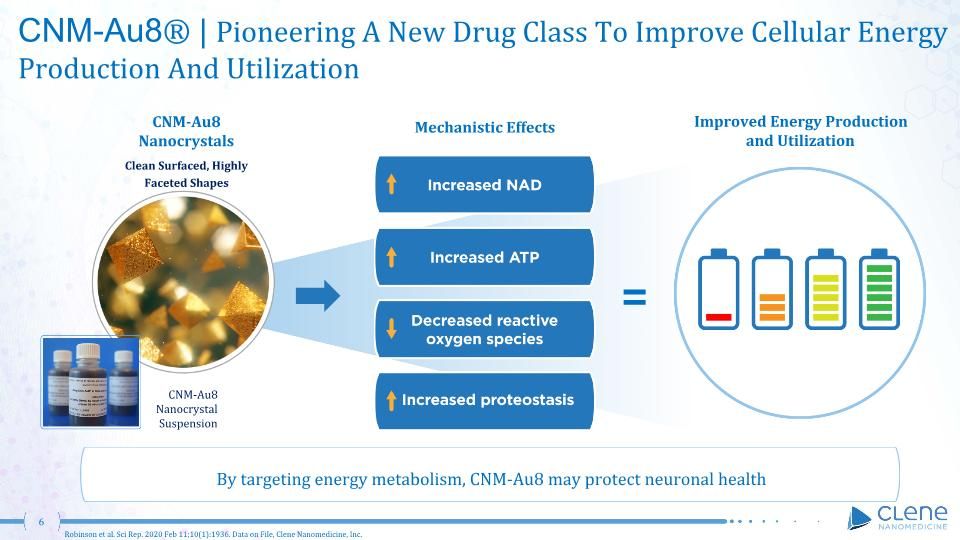
CNM-Au8® | Pioneering A New Drug Class To Improve Cellular Energy Production And Utilization Improved Energy Production and Utilization CNM-Au8 Nanocrystals Clean Surfaced, Highly Faceted Shapes Robinson et al. Sci Rep. 2020 Feb 11;10(1):1936. Data on File, Clene Nanomedicine, Inc. Mechanistic Effects By targeting energy metabolism, CNM-Au8 may protect neuronal health CNM-Au8 Nanocrystal Suspension =
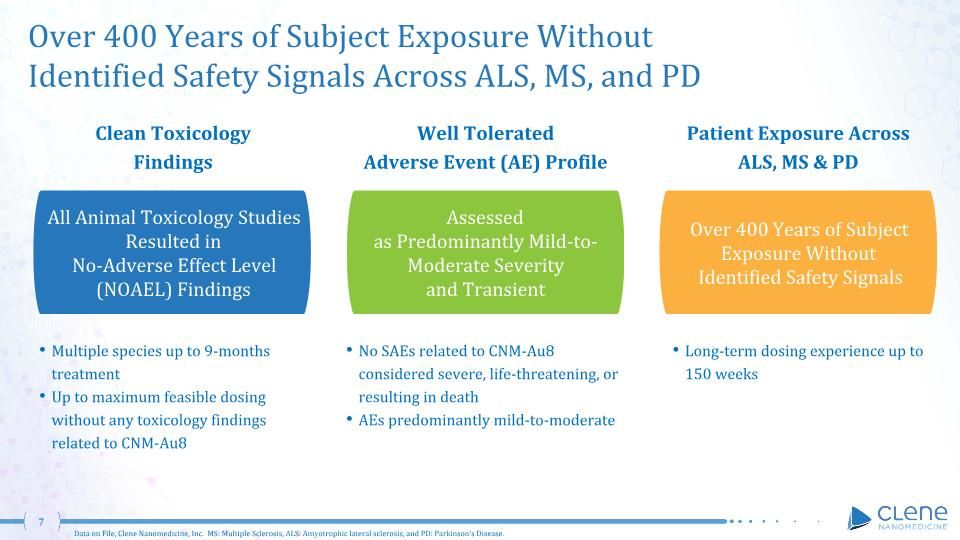
Over 400 Years of Subject Exposure Without Identified Safety Signals Across ALS, MS, and PD Over 400 Years of Subject Exposure Without Identified Safety Signals Patient Exposure Across ALS, MS & PD Long-term dosing experience up to 150 weeks All Animal Toxicology Studies Resulted in No-Adverse Effect Level (NOAEL) Findings Clean Toxicology Findings Multiple species up to 9-months treatment Up to maximum feasible dosing without any toxicology findings related to CNM-Au8 Assessed as Predominantly Mild-to-Moderate Severity and Transient Well Tolerated Adverse Event (AE) Profile No SAEs related to CNM-Au8 considered severe, life-threatening, or resulting in death AEs predominantly mild-to-moderate Data on File, Clene Nanomedicine, Inc. MS: Multiple Sclerosis, ALS: Amyotrophic lateral sclerosis, and PD: Parkinson's Disease.
Merit Cudkowicz, M.D., MSc Director of the Sean M. Healey & AMG Center for ALS Chief of the Department of Neurology at MGH, and the Julieanne Dorn Professor of Neurology at Harvard Medical School Principal Investigator and Sponsor of the Healey ALS Platform Trial Results for CNM-Au8 Regimen
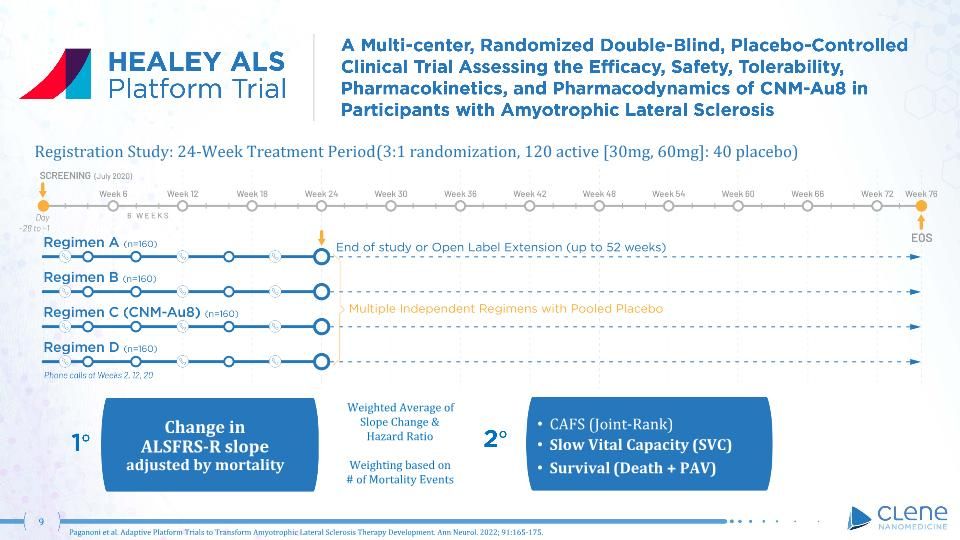
CAFS (Joint-Rank) Slow Vital Capacity (SVC) Survival (Death + PAV) Change in ALSFRS-R slope adjusted by mortality Registration Study: 24-Week Treatment Period (3:1 randomization, 120 active [30mg, 60mg]: 40 placebo) 1 2 Paganoni et al. Adaptive Platform Trials to Transform Amyotrophic Lateral Sclerosis Therapy Development. Ann Neurol. 2022; 91:165-175. . Weighted Average of Slope Change & Hazard Ratio Weighting based on # of Mortality Events
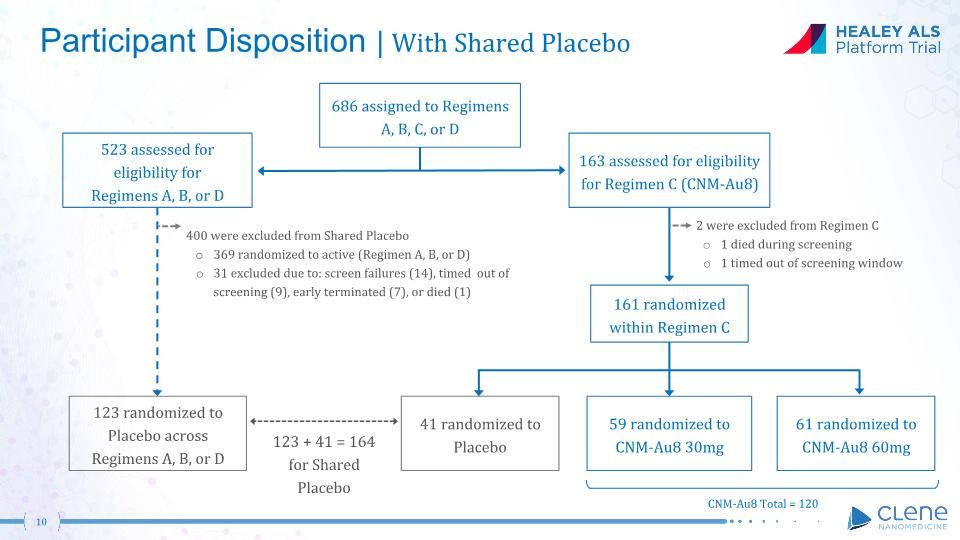
Participant Disposition | With Shared Placebo 523 assessed for eligibility for Regimens A, B, or D 163 assessed for eligibility for Regimen C (CNM-Au8) 400 were excluded from Shared Placebo 369 randomized to active (Regimen A, B, or D) 31 excluded due to: screen failures (14), timed out of screening (9), early terminated (7), or died (1) 123 randomized to Placebo across Regimens A, B, or D 2 were excluded from Regimen C 1 died during screening 1 timed out of screening window 161 randomized within Regimen C 686 assigned to Regimens A, B, C, or D 59 randomized to CNM-Au8 30mg 61 randomized to CNM-Au8 60mg 41 randomized to Placebo 123 + 41 = 164 for Shared Placebo CNM-Au8 Total = 120
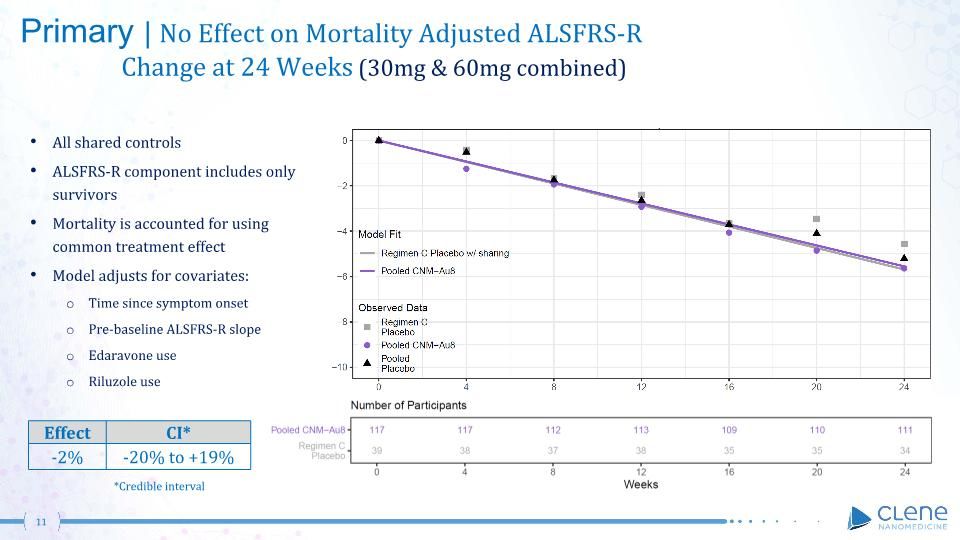
Primary | No Effect on Mortality Adjusted ALSFRS-R Change at 24 Weeks (30mg & 60mg combined) All shared controls ALSFRS-R component includes only survivors Mortality is accounted for using common treatment effect Model adjusts for covariates: Time since symptom onset Pre-baseline ALSFRS-R slope Edaravone use Riluzole use Effect CI* -2% -20% to +19% *Credible interval
No Effect on Key Secondary Endpoints at 24 Weeks (30mg & 60mg combined) Combined Assessment of Function and Survival (CAFS) change at 24 weeks was not significant No effect on Slow Vital Capacity (SVC) at 24 weeks
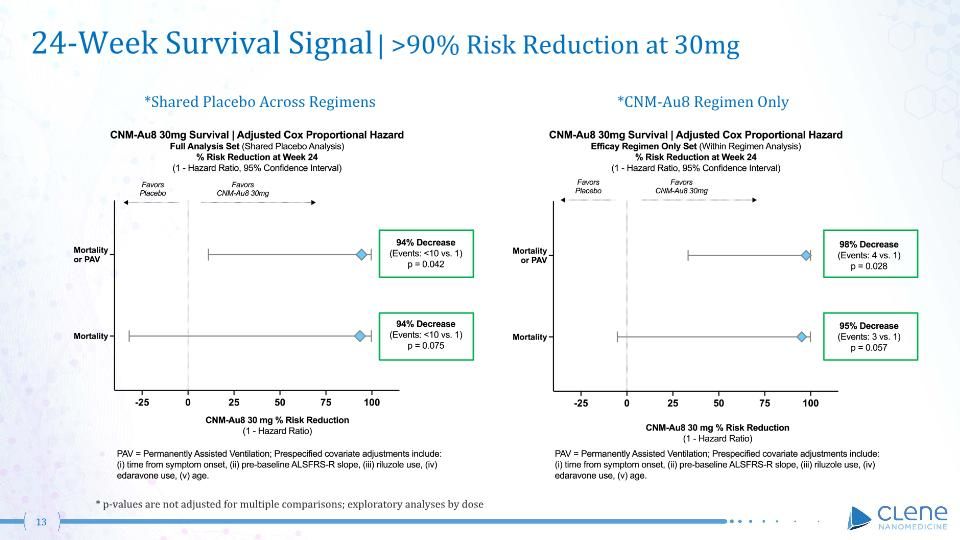
24-Week Survival Signal | >90% Risk Reduction at 30mg *Shared Placebo Across Regimens * p-values are not adjusted for multiple comparisons; exploratory analyses by dose *CNM-Au8 Regimen Only
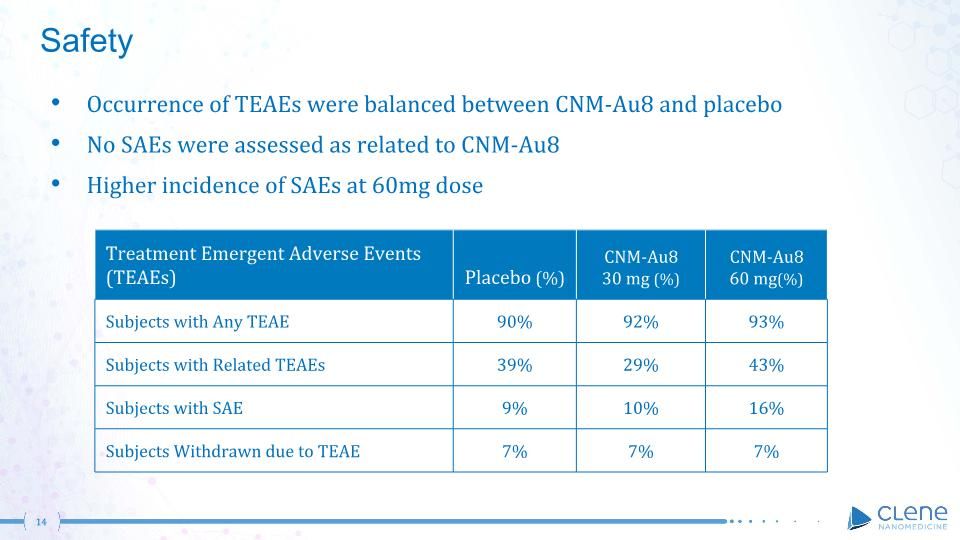
Safety Occurrence of TEAEs were balanced between CNM-Au8 and placebo No SAEs were assessed as related to CNM-Au8 Higher incidence of SAEs at 60mg dose Treatment Emergent Adverse Events (TEAEs) Placebo (%) CNM-Au8 30 mg (%) CNM-Au8 60 mg(%) Subjects with Any TEAE 90% 92% 93% Subjects with Related TEAEs 39% 29% 43% Subjects with SAE 9% 10% 16% Subjects Withdrawn due to TEAE 7% 7% 7%
Healey ALS Platform Trial CNM-Au8 Summary No evidence for treatment effect at 24 weeks for either adjusted ALSFRS-R, CAFS, or SVC (combined doses) Potential survival signal: >90% decreased risk at 30mg Mortality/PAV, p=0.028; Mortality = 0.057 (Regimen only) Mortality/PAV, p=0.042; Mortality = 0.075 (Shared placebo) Well tolerated with no definitive safety signals

Robert Glanzman, M.D., FAAN Clene Chief Medical Officer
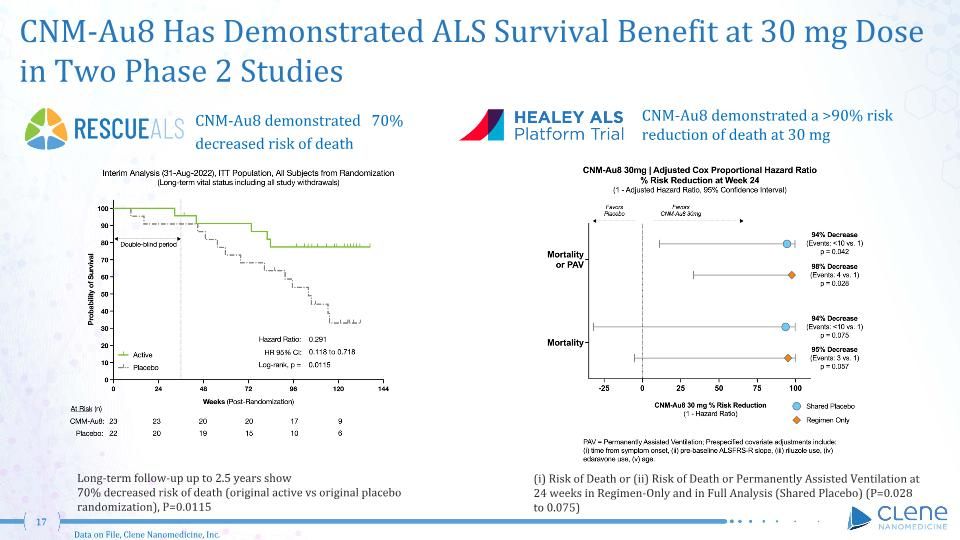
CNM-Au8 Has Demonstrated ALS Survival Benefit at 30 mg Dose in Two Phase 2 Studies Long-term follow-up up to 2.5 years show 70% decreased risk of death (original active vs original placebo randomization), P=0.0115 (i) Risk of Death or (ii) Risk of Death or Permanently Assisted Ventilation at 24 weeks in Regimen-Only and in Full Analysis (Shared Placebo) (P=0.028 to 0.075) CNM-Au8 demonstrated a >90% risk reduction of death at 30 mg CNM-Au8 demonstrated 70% decreased risk of death Data on File, Clene Nanomedicine, Inc.
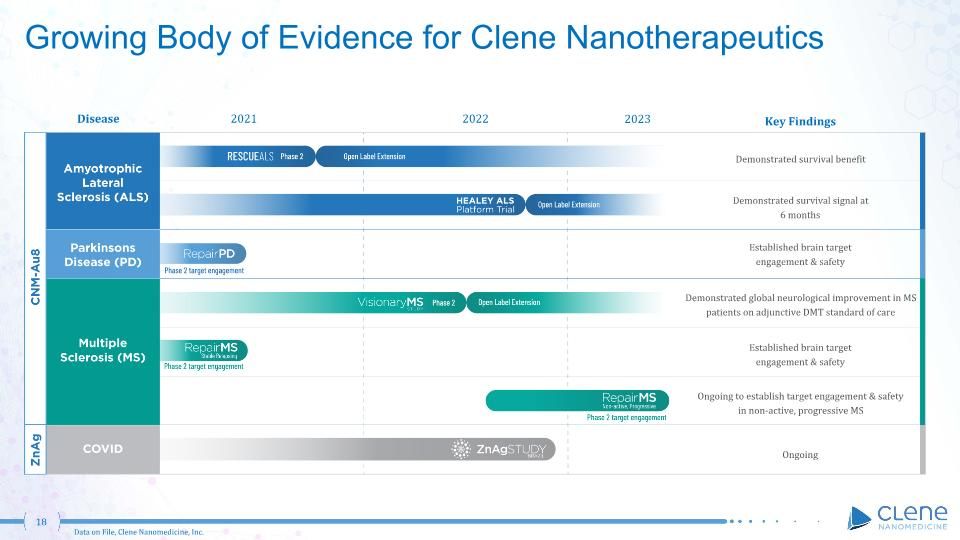
Growing Body of Evidence for Clene Nanotherapeutics Data on File, Clene Nanomedicine, Inc. Disease 2022 2021 2023 Demonstrated survival benefit Demonstrated survival signal at 6 months Established brain target engagement & safety Demonstrated global neurological improvement in MS patients on adjunctive DMT standard of care Established brain target engagement & safety Key Findings Ongoing to establish target engagement & safety in non-active, progressive MS Ongoing

CNM-Au8 Question and Answers Merit Cudkowicz, M.D., MSc Chief, Department of Neurology at MGH Director of the Sean M. Healey & AMG Center for ALS Robert Glanzman, M.D., FAAN Chief Medical Officer Clene Nanomedicine, Inc. Rob Etherington Chief Executive Officer Clene Nanomedicine, Inc. Michael Hotchkin Chief Development Officer Clene Nanomedicine, Inc.