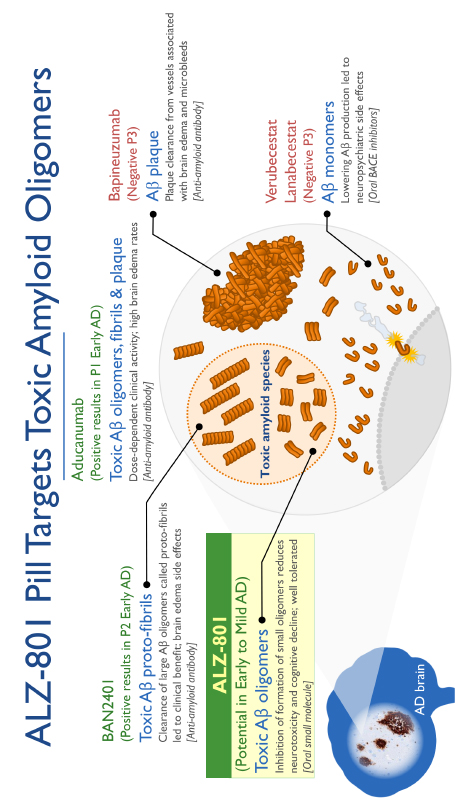
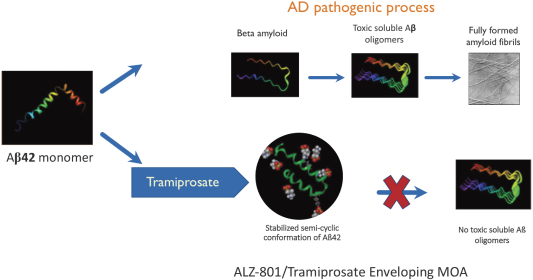
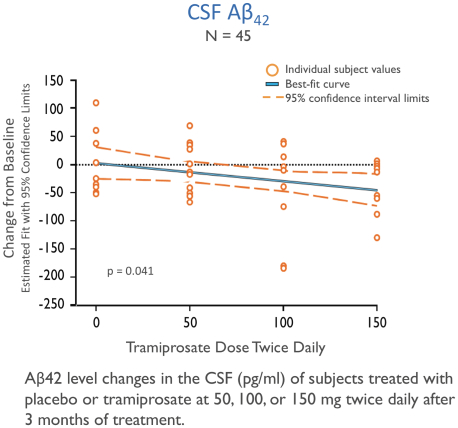
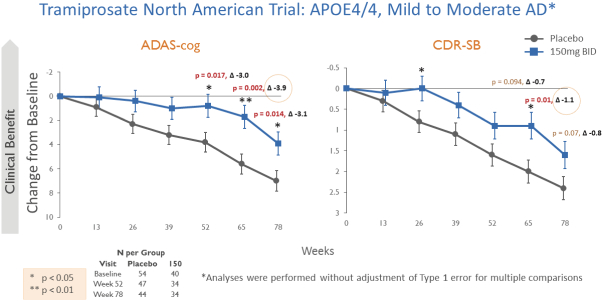
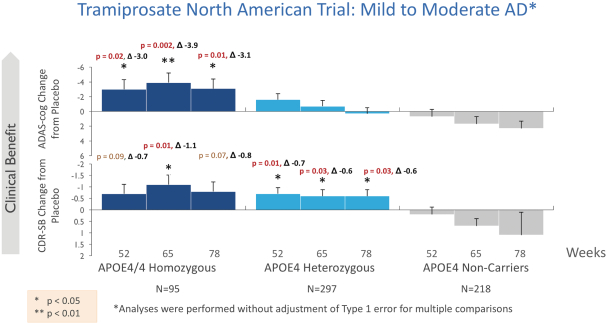
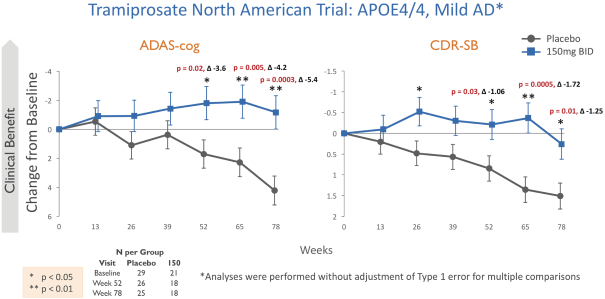
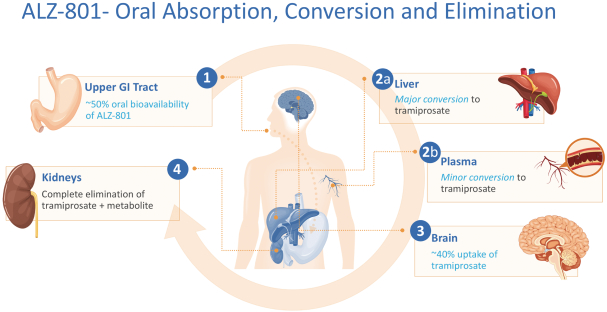
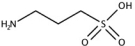
The information in this prospectus is not complete and may be changed. We may not sell these securities until the registration statement filed with the Securities and Exchange Commission is effective. This prospectus is not an offer to sell these securities and we are not soliciting offers to buy these securities in any state where the offer or sale is not permitted.
| PRELIMINARY PROSPECTUS | SUBJECT TO COMPLETION | DATED AUGUST 27, 2018 |
Shares
Common Stock

ALZHEON, INC.
This is a firm commitment initial public offering of shares of common stock of Alzheon, Inc. No public market currently exists for our shares. We anticipate that the initial public offering price of our shares will be between $ and $ and for calculation purposes herein, we assume a mid-point of $ per share.
We have applied to list our shares of common stock for trading on the Nasdaq Global Market under the symbol “ALZH.” No assurance can be given that our application will be approved.
We are an emerging growth company under the Jumpstart our Business Startups Act of 2012, or JOBS Act, and, as such, may elect to comply with certain reduced public company reporting requirements for future filings.
Investing in our common stock is highly speculative and involves a high degree of risk. See “Risk Factors” beginning on page 12 of this prospectus for a discussion of information that should be considered in connection with an investment in our common stock.
Neither the Securities and Exchange Commission nor any state securities commission has approved or disapproved of these securities or passed upon the accuracy or adequacy of this prospectus. Any representation to the contrary is a criminal offense.
| Per Share | Total | |||||||
| Initial public offering price |
$ | $ | ||||||
| Underwriting discounts and commissions(1) |
$ | $ | ||||||
| Proceeds to Alzheon, Inc. (before expenses) |
$ | $ | ||||||
| (1) | Underwriting discounts and commissions do not include a non-accountable expense allowance equal to 1.0% of the public offering price payable to the underwriters. In addition, the representative of the underwriters may, under certain circumstances, receive warrants to purchase shares of our common stock as compensation for this offering. We refer you to “Underwriting” beginning on page 151 for additional information regarding underwriters’ compensation. |
We have granted the underwriters a 30-day over-allotment option to purchase up to additional shares of common stock at the initial public offering price less underwriting discounts and commissions.
The underwriters expect to deliver our shares to purchasers in the offering on or about , 2018
ThinkEquity
A division of Fordham Financial Management, Inc.
The date of this prospectus is , 2018